Abstract
In accordance with Article 6 of Regulation (EC) No 396/2005, the applicant Nissan Chemical Europe S.A.S. submitted a request to the competent national authority in Finland to modify the existing maximum residue level (MRL) for quizalofop in caraway to accommodate the intended NEU use of quizalop‐P‐ethyl for this commodity. The data submitted in support of the request were found to be sufficient to derive an MRL proposal for caraway. Adequate analytical methods for enforcement are available to control the residues of quizalofop, resulting from the use of quizalofop‐P‐ethyl, on the commodity under consideration at the validated limit of quantification (LOQ) of 0.01 mg/kg. Based on the risk assessment results, EFSA concluded that the short‐term and long‐term intake of residues resulting from the use of quizalofop‐P‐ethyl according to the reported agricultural practice is unlikely to present a risk to consumer health.
Keywords: Quizalofop, quizalofop‐P‐ethyl, caraway, herbicide, MRL, consumer risk assessment
Summary
In accordance with Article 6 of Regulation (EC) No 396/2005, Nissan Chemical Europe S.A.S. submitted an application to the competent national authority in Finland (evaluating Member State, EMS) to modify the existing maximum residue level (MRL) for the active substance quizalofop (resulting from the use of quizalofop‐P‐ethyl) in caraway. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 13 April 2021. To support the intended NEU use of quizalofop‐P‐ethyl in caraway, the EMS proposed to lower the existing MRL from 0.05* to 0.04 mg/kg based on new residues trials and analytical data.
It is noted that several tentative MRL proposals (including one for caraway) have been implemented in the MRL legislation by Commission Regulation (EU) No Reg. (EU) 2019/973 for ‘quizalofop, its salts, its esters (including propaquizafop) and its conjugates’; including footnotes, indicating the type of confirmatory data that should be provided by a party having an interest in maintaining the proposed tentative MRLs by 14 June 2021. In view of the above, EFSA initially put the assessment of the present application on hold to clarify first if such confirmatory data were already submitted to the Rapporteur Member State (RMS) or in the process of being submitted. After a clarification from the RMS and the Commission that such confirmatory data were not available, and considering that the deadline for their submission already expired, EFSA resumed the assessment of the current application and the evaluation report, as required by Article 10 of the MRL regulation on 7 October 2021.
Based on the conclusions derived by EFSA in the framework of Directive 91/414/EEC, the data evaluated under previous MRLs assessments, including the review of the existing MRLs for all quizalofop‐P ester variants according to Article 12 of Regulation (EC) No 396/2005 (MRL review), and the additional data provided by the EMS in the framework of this application, the following conclusions are derived.
The metabolism of quizalofop‐P‐ethyl following foliar applications was investigated in the framework of the peer review and the MRL review in crops belonging to the groups of fruit crops, root crops and pulses/oilseeds. Additionally, a new metabolism study in cereals was investigated in a subsequent MRL application. Considering the available metabolism studies of quizalofop‐P‐ethyl as well as metabolism studies of the other ester variants of quizalofop‐P (quizalofop‐P‐tefuryl and propaquizafop), it was concluded that the metabolic patterns of all ester variants in plants were similar with the parent ester rapidly hydrolysed to the corresponding acid (quizalofop) which was always present at harvest. Other phenoxy metabolites than quizalofop were identified at harvest in lower amounts. The MRL review did not propose the inclusion of these metabolites in the residue definition for the time being but recommended to reconsider the toxicological relevance of these phenoxy metabolites under the renewal process.
Studies investigating the effect of processing on the nature of quizalofop‐P‐ethyl (hydrolysis studies) showed no degradation under conditions representative of pasteurisation and baking/brewing/boiling while for conditions simulating sterilisation, quizalofop‐P‐ethyl was partly hydrolysed to quizalofop. Since studies addressing the effect of processing on the nature of quizalofop demonstrated that quizalofop is hydrolytically stable, it was confirmed that the residue definitions for primary crops are also applicable to processed commodities.
From the available rotational crop metabolism studies performed with quizalofop‐P‐ethyl in sugar beets, lettuces, cotton seeds, peanuts and wheat, the MRL review concluded that metabolism in rotational crops proceeds in a similar pathway as in primary crops. No further data were required for the intended use on caraway assessed under this application.
Based on the metabolic pattern identified in metabolism studies with quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop, the results of hydrolysis studies with quizalofop‐P‐tefuryl and quizalofop‐P‐ethyl, the toxicological significance of metabolites and the capabilities of enforcement analytical methods, the residue definitions for plant products were proposed for all quizalofop ester variants as ‘the sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers)’ for both enforcement and risk assessment. These residue definitions are applicable to primary crops (all groups), rotational crops and processed products. The residue definition for enforcement set in Regulation (EC) No 396/2005 is identical with the above‐mentioned residue definition.
EFSA concluded that for the crop assessed in this application, metabolism of quizalofop‐P‐ethyl in primary and in rotational crops, and the possible degradation in processed products has been sufficiently addressed and that the previously derived residue definition is applicable.
A sufficiently validated analytical method based on high‐performance liquid chromatography with tandem mass spectrometry (HPLC‐MS/MS) is available to quantify quizalofop‐P‐ethyl residues in caraway according to the enforcement and risk assessment residue definition. The method enables quantification of residues at or above 0.01 mg/kg in caraway (limit of quantification (LOQ)). As part of the current application, the applicant also provided a new cross‐validation study which was deemed sufficient to demonstrate the extraction efficiency and hydrolysis of conjugates in high water and high oil matrices.
The available residue trials are sufficient to derive an MRL proposal of 0.04 mg/kg for caraway.
Specific studies investigating the magnitude of quizalofop‐P‐ethyl residues in processed commodities are not required for the present application, as significant residues are not expected for caraway and the contribution of caraway to the overall chronic exposure is below 0.01% of the acceptable daily intake (ADI).
The occurrence of quizalofop‐P‐ethyl residues in rotational crops was investigated in the framework of the EU pesticides peer review and the MRL review. Based on available confined rotational crop studies conducted at twice the intended application rate of quizalofop‐P‐ethyl in caraway, it is concluded that no residues are expected in rotational crops if quizalofop‐P‐ethyl is applied on caraway according to the intended good agricultural practice (GAP).
Residues of quizalofop‐P‐ethyl in commodities of animal origin were not assessed since caraway is not fed to livestock.
The toxicological profiles for the different quizalofop‐P‐ester variants (quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop) were derived in the framework of the EU pesticides peer review. Since all these different ester variants share the same residue definition based on quizalofop, EFSA considered for the consumer risk assessment the lowest toxicological reference values available (respectively, the ADI set for quizalofop‐P‐ethyl and the acute reference dose (ARfD) set for quizalofop‐P‐tefuryl) expressed as ‘quizalofop’, by correcting them by the different molecular weights. Consequently, the resulting values of 0.0083 mg/kg body weight (bw) per day and 0.08 mg/kg bw were used in the chronic and acute dietary exposure assessments, respectively. The metabolites included in the residue definition are deemed of similar toxicity than the parent active substance.
The consumer risk assessment was performed with revision 3.1 of the EFSA Pesticide Residues Intake Model (PRIMo).
The short‐term exposure did not exceed the ARfD for the crop assessed in this application. It should be, however, noted that an establishment of an ARfD to quizalofop‐P‐ethyl was not considered necessary by the peer review (EFSA, 2009) and the ARfD used to evaluate the acute risk was the one set for quizalofop‐P‐tefuryl, recalculated as quizalofop equivalents.
In the framework of the MRL review, a comprehensive long‐term exposure assessment was performed, taking into account the existing uses at EU level of all quizalofop‐P ester variants. EFSA updated the calculation with the STMR value derived from the residue trials submitted in support of this MRL application for caraway and other STMRs derived in another EFSA opinion published after the MRL review. The estimated long‐term dietary intake accounted for 26% of the ADI (NL toddler diet). The contribution of residues expected in caraway to the overall long‐term exposure is below 0.01% of the ADI.
EFSA concluded that the proposed use of quizalofop‐P‐ethyl on caraway will not result in a consumer exposure exceeding the toxicological reference values and therefore is unlikely to pose a risk to consumers’ health.
However, it should be noted that the MRL review recommended to reconsider the toxicological relevance of the phenoxy metabolites identified in primary and rotational crop metabolism studies under the renewal process, and therefore, the residue definitions and the consumer risk assessment may be reconsidered following the evaluation of this information. In addition, EFSA also highlighted that metabolism studies did not investigate the possible impact of plant metabolism on the isomer ratio of the active substance. Further investigation on this matter would in principle be required. EFSA would therefore recommend reconsidering also this point in the framework of the renewal of approval of the active substance.
EFSA proposes to amend the existing MRL as reported in the summary table below.
Full details of all end points and the consumer risk assessment can be found in Appendices B–D.
| Code( a ) | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Quizalofop (sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers)) | ||||
| 0820030 | Caraway | 0.05* (Ft) | 0.04/Further risk managers’ consideration |
The submitted data are sufficient to derive an MRL proposal for the NEU use. Risk for consumers unlikely. EFSA notes that the current MRL for quizalofop in caraway is set as tentative at 0.05* mg/kg, a higher level than the MRL of 0.04 mg/kg proposed in the present application. It is noted that an application to address all the Art.12 confirmatory data set for quizalofop has not been submitted yet and the deadline to provide such data is now expired. Nevertheless, the confirmatory data requirements on residue trials, analytical methods and storage stability for caraway could be considered as sufficiently addressed and the footnote can be removed for this crop. EFSA therefore considers the proposed MRL derived in the context of the present application as acceptable, also taking into account the availability of an enforcement method with an LOQ at 0.01 mg/kg for caraway. |
MRL: maximum residue level; NEU: northern Europe; SEU: southern Europe; GAP: Good Agricultural Practice.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
(ft): Footnote in Commission Regulation (EU) 2019/973 for caraway: The European Food Safety Authority identified some information on residue trials, analytical methods and storage stability as unavailable for quizalofop‐P‐ethyl. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
Assessment
The European Food Safety Authority (EFSA) received an application to modify the existing maximum residue level (MRL) for quizalofop (resulting from the use of quizalofop‐P‐ethyl) in caraway. The detailed description of the intended use of quizalofop‐P‐ethyl, which is the basis for the current MRL application, is reported in Appendix A.
Quizalofop‐P‐ethyl is the ISO common name for ethyl (2R)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy) phenoxy] propionate (IUPAC). It is an ester variant of quizalofop‐P. Quizalofop‐P is the ISO common name for (R)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionic acid (IUPAC). The unresolved isomeric mixture of this substance has the common name quizalofop. Quizalofop‐P belongs to the class of aryloxyphenoxypropionic herbicides which are taken up via leaves and hinder the synthesis of fatty acids by inhibition of the enzyme Acetyl‐CoA carboxylase (ACCase). The chemical structures of the active substance, of other quizalofop ester variants and their main metabolites are reported in Appendix E.
Quizalofop‐P (considered variants quizalofop‐P‐ethyl and quizalofop‐P‐tefuryl) was evaluated in the framework of Directive 91/414/EEC1 with Finland designated as rapporteur Member State (RMS) for the representative uses as a foliar treatment on oilseed rape, sugar/fodder beet, potatoes, peas, beans, linseed and sunflower. The draft assessment report (DAR) prepared by the RMS has been peer reviewed by EFSA (EFSA, 2009). Quizalofop‐P was approved2 for the use as herbicide on 1 December 2009.
The EU MRLs for quizalofop are established in Annex II of Regulation (EC) No 396/20053. The review of existing MRLs according to Article 12 of Regulation (EC) No 396/2005 (MRL review) has been performed on all quizalofop‐P‐ester variants (quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop) (EFSA, 2017) and the proposed modifications have been implemented in the MRL legislation. After completion of the MRL review, EFSA has issued one reasoned opinion on the modification of MRLs for quizalofop‐P‐ethyl (EFSA, 2018b). The proposals from these reasoned opinions have been considered in recent MRL regulations.4
In accordance with Article 6 of Regulation (EC) No 396/2005, Nissan Chemical Europe S.A.S. submitted an application to the competent national authority in Finland (evaluating Member State, EMS) to modify the existing maximum residue level (MRL) for quizalofop (resulting from the use of quizalofop‐P‐ethyl) in caraway. The EMS drafted an evaluation report in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to the European Food Safety Authority (EFSA) on 13 April 2021. To accommodate for the intended use of quizalofop‐P‐ethyl, the EMS proposed to lower the existing MRL from 0.05* mg/kg (ft) to 0.04 mg/kg based on new residues data supporting the intended NEU use of quizalofop‐P‐ethyl in caraway.
It is noted that several tentative MRL proposals (including one for caraway) have been implemented in the MRL legislation by Commission Regulation (EU) No Reg. (EU) 2019/9736 for ‘quizalofop, its salts, its esters (including propaquizafop) and its conjugates’; including footnotes, indicating the type of confirmatory data that should be provided by a party having an interest in maintaining the proposed tentative MRL by 14 June 2021. In view of the above, EFSA initially put the assessment of the present application on hold in order to clarify first if such confirmatory data were already submitted to the rapporteur Member State (RMS) or were in the process of being submitted. After a clarification from the RMS and the Commission that such confirmatory data were not available, and considering that the deadline for their submission already expired, EFSA resumed the assessment of the current application and the evaluation report, as required by Article 10 of the MRL regulation on 7 October 2021.
EFSA based its assessment on the evaluation report submitted by the EMS (Finland, 2021), the DAR and its addendum (Finland, 2007, 2008) prepared under Council Directive 91/414/EEC, the Commission review report on quizalofop‐P‐ethyl (European Commission, 2010c), the conclusion on the peer review of the pesticide risk assessment of the active substance quizalofop‐P‐ethyl (EFSA, 2009), as well as the conclusions from the reasoned opinion on the MRL review according to Article 12 of Regulation No 396/2005 performed on all quizalofop‐P‐ester variants (quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop) (EFSA, 2017) and another EFSA opinion on quizalofop‐P‐ethyl (EFSA, 2018b).
For this application, the data requirements established in Regulation (EU) No 544/20117 and the guidance documents applicable at the date of submission of the application to the EMS are applicable (European Commission, 1997a–g, 2000, 2010a,b; OECD, 2011). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/20118.
A selected list of end points of the studies assessed by EFSA in the framework of this MRL application including the end points of relevant studies assessed previously is presented in Appendix B.
The evaluation report submitted by the EMS (Finland, 2021) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
The metabolism of quizalofop‐P‐ethyl in primary crops belonging to the groups of fruit crops (tomatoes), root crops (sugar beets and potatoes) and pulses/oilseeds (cotton and soya beans) has been investigated in the framework of the EU pesticides peer review (EFSA, 2009) and the MRL review (EFSA, 2017). Additionally, a new metabolism study in cereals (GM maize, containing the aryloxyalkanoate dioxygenase (aad‐1) gene) was investigated in a subsequent MRL application (EFSA, 2018b). No additional metabolism studies were submitted in the framework of this MRL application.
Additionally, metabolism studies performed with other quizalofop‐P ester variants (quizalofop‐P‐tefuryl and propaquizafop) have been considered in previous assessments and in the MRL review. The metabolic patterns of all three different ester variants in plants were similar with the parent ester rapidly hydrolysed to the corresponding acid (quizalofop), which was always present at harvest. In most cases, the amount of other metabolites than quizalofop was low at harvest, with exception of the metabolites phenoxy acid (PPA), phenoxy propionate (EPP), quizalofop‐phenol (CQOP) and hydroxy‐quizalofop‐phenol (CQOPOH). The MRL review did not propose the inclusion of these metabolites in the residue definition for the time being but recommended to reconsider the toxicological relevance of these phenoxy metabolites under the renewal process.
In the framework of the MRL review, it was also highlighted that metabolism studies did not investigate the possible impact of plant metabolism on the isomer ratio of the active substance. Further investigation on this matter would in principle be required. It is noted that the EFSA guidance on the risk assessment of compounds that may have stereoisomers has been issued (EFSA, 2019b). EFSA would therefore recommend reconsidering this point in the framework of the renewal of approval of the active substance.
For the intended use, the metabolic behaviour in primary crops is sufficiently addressed.
1.1.2. Nature of residues in rotational crops
Caraway can be grown in rotation with other crops. According to the soil degradation studies evaluated in the framework of the peer review, quizalofop‐P‐ethyl has a low persistence in soil with a DT90 value under aerobic laboratory conditions of 1.1–3.5 days (Finland, 2007, EFSA, 2009). However, a significantly higher DT90 (603 days) was obtained for the free acid metabolite quizalofop. Therefore, further studies investigating the nature and magnitude of residues in rotational crops are required.
No new studies are submitted with the present application, but the metabolism of quizalofop‐P‐ethyl in rotational crops has been previously investigated in sugar beets, lettuces, cotton seeds, peanuts and wheat sown at 30 and 60 days after bare soil application at 308 g/ha (1.2 N compared to the maximum dose rate authorised for quizalofop‐P‐ethyl) of racemate quizalofop‐P‐ethyl labelled on either the quinoxaline or phenyl rings (Finland, 2007). According to the results of this study, residue levels were not significant (< 0.05 mg eq./kg) regardless of ageing period in soil in all crop parts analysed. It was also concluded that all compounds detected in the rotational crops were also present in primary crops suggesting a similar metabolic pathway between primary and rotational crops.
The same conclusion is also applicable to the present application since it is based on a use with an application rate of 150 g a.s./ha which is lower than the critical GAP considered in the MRL review.
1.1.3. Nature of residues in processed commodities
The effect of processing on the nature of quizalofop was investigated in the framework of the MRL review for the variant quizalofop‐P‐tefuryl (EFSA, 2017) and a new study investigating the effect of processing for the variant quizalofop‐P‐ethyl was submitted with the current application (Finland, 2021).
The standard hydrolysis study investigated in the MRL review, showed that quizalofop is hydrolytically stable under conditions representative for pasteurisation, baking/brewing/boiling and sterilisation (EFSA, 2017).
In the new study provided as part of this MRL application, the hydrolytic stability of quizalofop‐P‐ethyl was investigated with 14C‐phenoxy and 14C‐quinoxalin radiolabelled active substance under conditions representative for pasteurisation, baking/brewing/boiling and sterilisation (Finland, 2021). No degradation of quizalofop‐P‐ethyl was detected under conditions representative of pasteurisation, and baking/brewing/boiling while for conditions simulating sterilisation, quizalofop‐P‐ethyl was partly hydrolysed to quizalofop at maximum of 24% applied radioactivity with quizalofop being the remaining 76% of AR. Since quizalofop‐P‐ethyl and quizalofop were the main compounds detected, it was confirmed that the residue definitions for primary crops are also applicable to processed commodities.
1.1.4. Methods of analysis in plants
Analytical methods for the determination of quizalofop residues from the use of the different quizalofop‐P‐ester variants were assessed during the EU peer review and in the framework of the MRL review (EFSA, 2009, 2017).
An analytical method for enforcement of quizalofop‐P‐ethyl (method AN41) was evaluated during the combined MRL review for quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop (EFSA, 2017). The method was considered as sufficiently validated for residues of quizalofop‐P‐ethyl and quizalofop for dry/high starch/high protein plant matrices (wheat grain and dry beans) and for plant matrices with high acid (oranges), high water (tomatoes) and high oil (cotton seeds) content. The method allows quantifying residues at or above the limit of quantification (LOQ) of 0.01 mg/kg for the total residue (sum of quizalofop‐P‐ethyl and quizalofop). An independent laboratory validation (ILV) for this method was successfully performed for the same four representative plant matrices. However, in the framework of the MRL review, it was noted that extraction efficiency and hydrolysis of conjugates was not demonstrated and that a validated analytical method for enforcement in complex matrices (such as herbal infusions and spices) was not available and it was required (EFSA, 2017).
As part of this application, the applicant provided a cross‐validation study to demonstrate the extraction efficiency and hydrolysis of conjugates. The extraction efficiency was investigated in samples of oilseed rape whole plants (high water content) and seeds (high oil content). An exaggerated application rate (600 g a.s./ha) was applied to oilseed rape to generate treated samples with sufficient incurred residues in the whole plant and in seeds. Different solvents systems used in several analytical methods were applied showing comparable extractions of all analytical methods for the oilseed rape whole plant (high water matrix). Similar extraction was also observed in oilseed rape seeds (high oil matrix) for the enforcement method AN41 compared with the metabolism studies methods RD051/RD2 and the pre‐registration method C43766 (75% of the mean residues detected from the RD051/RD2 and C43766 methods before hydrolysis and 67% after hydrolysis) therefore demonstrating the equivalency of these methods.
Additionally, this new study demonstrates that conjugates are efficiently released upon hydrolysis using the analytical method for enforcement AN41. As shown by the study, higher levels of quizalofop were detected in seeds after hydrolysis with a 39.6% increase compared to the quizalofop levels measured before hydrolysis (average of three trials). This increase following the hydrolysis step is in line with the one observed in the two methods used in metabolism studies RD051/RD2 (average of 39.5% increase of quizalofop after hydrolysis). It is therefore concluded that the enforcement method AN41 will hydrolyse conjugates with the same efficiency as the methods used in the metabolism studies.
Regarding the confirmatory data request of a validated analytical method for enforcement in complex matrices (such as herbal infusions and spices), the EMS is of the opinion that such method is not indispensable within the framework of this application since the analytical method AN41 has been successfully validated in four different matrices and taking into account the insignificant contribution of caraway seeds to the overall dietary exposure. EFSA agrees with the EMS’ view, also considering that caraway seeds are mostly composed of fatty acids and oils, hence the validation of the analytical method AN41 in four different matrices (including high oil matrix) – in addition to caraway being a minor crop with insignificant contribution to the dietary exposure – is sufficient reasons to consider the present analytical method AN41 sufficiently suitable for the crop under assessment.
Regarding the achievable LOQ for caraway, EFSA notes that a validated analytical method for enforcement in complex matrices is still not available. However, additional recovery data were generated for caraway with the enforcement method AN41, which was also used for the analysis of the residue trials, achieving an LOQ of 0.01 mg/kg for this commodity (Finland, 2021). EFSA considered the confirmatory data requirements on analytical methods as sufficiently addressed for caraway.
1.1.5. Storage stability of residues in plants
The storage stability of residues of the different quizalofop‐P‐ester variants, including quizalofop‐P‐ethyl, in plants stored under frozen conditions was investigated in the framework of the MRL review (EFSA, 2017). Stability of quizalofop‐P‐ethyl and quizalofop residues was demonstrated for at least 12 months when stored at −18°C in samples of crops classified as matrices with high water content (snap beans), high acid content (oranges), high oil content (cotton seeds and rape seeds) and dry matrices (wheat grain, GM maize).
EFSA notes that storage stability in complex matrices (such as herbal infusions and spices) has not been demonstrated yet. However, considering that in the available residue trials on caraway, samples of caraway seeds were stored frozen for a maximum of 62 days prior to extraction and storage stability was demonstrated in four different main matrices for at least 12 months (including high oil matrix), it is very unlikely that any degradation would occur in such a short time.
Therefore, the integrity of the caraway seed samples is considered sufficiently demonstrated for the present application. EFSA considered the confirmatory data requirements on storage stability as sufficiently addressed for caraway.
1.1.6. Proposed residue definitions
Based on the metabolic pattern identified in metabolism studies, the results of hydrolysis studies, the toxicological significance of metabolites and the capabilities of enforcement analytical methods, the following residue definition was proposed in the framework of the MRL review, for both enforcement and risk assessment ‘sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers)’.
The same residue definition is applicable to rotational crops and processed products for all groups. The residue definition for enforcement set in Regulation (EC) No 396/2005 is identical with the above‐mentioned residue definition. Taking into account the proposed use assessed in this application, EFSA concluded that this residue definition is appropriate, and no modification is required.
However, EFSA notes that the MRL review recommended to reconsider the toxicological relevance of the phenoxy metabolites identified in primary and rotational crop metabolism studies under the renewal process, and therefore, the above residue definition may be reconsidered following the evaluation of this information. In addition, EFSA highlighted that the metabolism studies did not investigate the possible impact of plant metabolism on the isomer ratio of the active substance. Further investigation on this matter would in principle be required. EFSA would therefore recommend reconsidering also this point in the framework of the renewal of approval of the active substance.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
In support of this MRL application, the applicant submitted residue trials performed in caraway seeds. The residue trial samples were analysed for the parent compound and the metabolites included in the residue definitions for enforcement and risk assessment.
According to the assessment of the EMS (Finland, 2021), the methods used were sufficiently validated and fit for purpose. The samples of these residue trials were stored under conditions for which integrity of the samples can be considered as sufficiently demonstrated (see Section 1.1.5).
Caraway
NEU outdoor GAP: 1 × 150 g a.s/ha, BBCH = 11–60, PHI = 56 days
The applicant provided four residue trials conducted in Finland over the period 2015–2016 to determine the residues of quizalofop in caraway after the application of quizalofop‐P‐ethyl according to the intended GAP as reported in Appendix A. All residue trials were considered independent as they were performed in different geographical locations and over two different growing seasons.
EFSA notes that sampling of seeds occurred at 57, 66, 67 and 73 days after the application. Therefore, for three of these trials, the preharvest interval (PHI) is within the ±25% tolerance rule (42–70 days for a PHI of 56 days) while for one trial only (trial number ONE0316‐02), the PHI of 73 days slightly exceeds this tolerance rule. This minor deviation was justified since a PHI of 73 days was considered necessary for this trial to ensure the sampling at harvest matches the maturity of the crop. EFSA agrees to accept this minor deviation and considers the independency and number of trials sufficient to derive an MRL proposal of 0.04 mg/kg for caraway in support of the intended NEU use of quizalofop‐P‐ethyl.
EFSA considered the confirmatory data on residue trials as sufficiently addressed for caraway.
1.2.2. Magnitude of residues in rotational crops
The possible transfer of quizalofop residues to crops that are grown in crop rotation has been assessed in the EU pesticides peer review and in the MRL review (EFSA, 2009, 2017) for the different ester variants of quizalofop‐P, including quizalofop‐P‐ethyl.
The MRL review concluded that significant residues of quizalofop‐P ester variants (including quizalofop‐P‐ethyl) and their metabolites are not expected to be present in rotational crops based on the confined rotational crop studies conducted at 2.8N (propaquizafop), 1.2N (quizalofop‐P‐ethyl) and 2.5N (quizalofop‐P‐tefuryl) and the maximum application rates supported in the framework of the MRL review, provided that these quizalofop ester variants are applied according to the existing GAPs considered in the MRL review.
Since the maximum annual application rate for the crop under consideration (i.e. 150 g a.s./ha) is lower than the application rate tested in the rotational crop study, it is concluded that no residues are expected, provided that the active substance is applied according to the intended GAP.
1.2.3. Magnitude of residues in processed commodities
Specific processing studies for the crop under assessment are not available and are not required since residues in caraway do not exceed the trigger value of 0.1 mg/kg and the contribution of caraway to overall chronic exposure is less than 0.01% of the ADI.
1.2.4. Proposed MRLs
The available data are considered sufficient to derive an MRL proposal for quizalofop in caraway in support of the intended NEU use of quizalofop‐P‐ethyl as well as risk assessment values for the commodity under evaluation (see Appendix B.4).
EFSA notes that the current MRL for quizalofop in caraway is set as tentative at 0.05* mg/kg (Ft) 5 , a higher level than the MRL of 0.04 mg/kg proposed in the present application. It is noted that an application to address all the Art.12 confirmatory data set for quizalofop has not been submitted yet and the deadline to provide such data is now expired. Nevertheless, the confirmatory data requirements on residue trials, analytical methods and storage stability for caraway could be considered as sufficiently addressed and the footnote can be removed for this crop. EFSA, therefore, considers the proposed MRL derived in the context of the present application as acceptable, also taking into account the availability of an enforcement method with an LOQ at 0.01 mg/kg for caraway.
In Section 3, EFSA assessed whether residues on this crop resulting from the intended use are likely to pose a consumer health risk.
2. Residues in livestock
Not relevant as caraway seeds are not used for feed purposes.
3. Consumer risk assessment
EFSA performed a dietary risk assessment using revision 3.1 of the EFSA PRIMo (EFSA, 2018a, 2019a). This exposure assessment model contains food consumption data for different sub‐groups of the EU population and allows the acute and chronic exposure assessment to be performed in accordance with the internationally agreed methodology for pesticide residues (FAO, 2016).
The toxicological reference values for the different quizalofop‐P‐ester variants (quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop) were derived in the framework of the EU pesticides peer review (EFSA, 2009; European Commission, 2010c). The metabolites included in the risk assessment residue definition were deemed of similar toxicity than the parent active substance. Since all these different ester variants share the same residue definition based on quizalofop, EFSA considered for the consumer risk assessment the lowest toxicological reference values available (respectively, the ADI set for quizalofop‐P‐ethyl and the ARfD set for quizalofop‐P‐tefuryl) expressed as ‘quizalofop’ by correcting them by the different molecular weights. Consequently, the resulting values of 0.0083 mg/kg bw per day and 0.08 mg/kg bw were used in the chronic and acute dietary exposure assessment, respectively.
Short‐term (acute) dietary risk assessment
The short‐term exposure assessment was performed only for the commodity assessed in this application in accordance with the internationally agreed methodology (FAO, 2016). The calculations were based on the highest residue (HR) derived from supervised field trials and the complete list of input values can be found in Appendix D.1.
The short‐term exposure did not exceed the ARfD for the crop assessed in this application (see Appendix B.3). It should be, however, noted that an establishment of an ARfD for quizalofop‐P‐ethyl was not considered necessary by the peer review (EFSA, 2009) and the ARfD used in this application to evaluate the acute risk was the one set for quizalofop‐P‐tefuryl, recalculated as quizalofop equivalent (0.08 mg/kg bw).
Long‐term (chronic) dietary risk assessment
In the framework of the MRL review, a comprehensive long‐term exposure assessment was performed, taking into account the existing uses at EU level of all quizalofop‐P ester variants (EFSA, 2017). EFSA updated the calculation with the STMR value derived from the residue trials submitted in support of this MRL application for caraway and other STMRs derived in an EFSA opinion published after the MRL review (EFSA, 2018b). The input values used in the exposure calculations are summarised in Appendix D.1. The estimated long‐term dietary intake accounted for 26% of the ADI (NL toddler diet). The contributions of residues expected in caraway assessed in the present MRL application to the overall long‐term exposure is insignificant. The ADI used in this application to evaluate the chronic risk is based on the lowest ADI of 0.009 mg/kg bw per day derived for quizalofop‐P‐ethyl (EFSA, 2009) and recalculated as quizalofop equivalent (0.0083 mg/kg bw per day).
Based on the risk assessment results, EFSA concluded that the long‐term and short‐term intake of residues occurring in food from the existing uses of quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop and from the intended use of quizalofop‐P‐ethyl in caraway, is unlikely to present a risk to consumer health.
However, it should be noted that the MRL review recommended to reconsider the toxicological relevance of the phenoxy metabolites identified in primary and rotational crop metabolism studies under the renewal process, and therefore, the residue definitions and the consumer risk assessment may be reconsidered following the evaluation of this information. In addition, EFSA also highlighted that metabolism studies did not investigate the possible impact of plant metabolism on the isomer ratio of the active substance. Further investigation on this matter would in principle be required. EFSA would therefore recommend reconsidering also this point in the framework of the renewal of approval of the active substance.
For further details on the exposure calculations, a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
4. Conclusion and Recommendations
The data submitted in support of this MRL application were found to be sufficient to derive an MRL proposal for caraway.
EFSA concluded that the proposed use of quizalofop‐P‐ethyl on caraway will not result in a consumer exposure exceeding the toxicological reference values and therefore is unlikely to pose a risk to consumers’ health.
However, it should be noted that the MRL review recommended to reconsider the toxicological relevance of the phenoxy metabolites identified in primary and rotational crop metabolism studies under the renewal process, and therefore, the residue definitions and the consumer risk assessment may be reconsidered following the evaluation of this information. In addition, EFSA also highlighted that metabolism studies did not investigate the possible impact of plant metabolism on the isomer ratio of the active substance. Further investigation on this matter would in principle be required. EFSA would therefore recommend reconsidering also this point in the framework of the renewal of approval of the active substance.
EFSA considered the confirmatory data requirements on residue trials, analytical methods and storage stability could be considered as sufficiently addressed for caraway and the related footnote in Regulation can be removed for this crop.
The MRL recommendations are summarised in Appendix B.4.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- Bw
body weight
- CAC
Codex Alimentarius Commission
- CAS
Chemical Abstract Service
- CCPR
Codex Committee on Pesticide Residues
- CEN
European Committee for Standardisation (Comité Européen de Normalisation)
- CF
conversion factor for enforcement to risk assessment residue definition
- DAR
draft assessment report
- DAT
days after treatment
- DT90
period required for 90% dissipation (define method of estimation)
- EMS
evaluating Member State
- Eq
residue expressed as a.s. equivalent
- EURL
EU Reference Laboratory (former Community Reference Laboratory (CRL))
- FAO
Food and Agriculture Organization of the United Nations
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GC‐MS
gas chromatography with mass spectrometry
- HPLC
high performance liquid chromatography
- HPLC‐MS
high performance liquid chromatography with mass spectrometry
- HPLC‐MS/MS
high performance liquid chromatography with tandem mass spectrometry
- HPLC‐UVD
high performance liquid chromatography with ultra‐violet detector
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- LOQ
limit of quantification
- MRL
maximum residue level
- MS
Member States
- MS
mass spectrometry detector
- MS/MS
tandem mass spectrometry detector
- MW
molecular weight
- NEU
northern Europe
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PHI
preharvest interval
- Pow
partition coefficient between n‐octanol and water
- PRIMo
(EFSA) Pesticide Residues Intake Model
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged, and Safe (analytical method)
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- RPF
relative potency factor
- SANCO
Directorate‐General for Health and Consumers
- SC
suspension concentrate
- SCPAFF
Standing Committee on Plants, Animals, Food and Feed (formerly: Standing Committee on the Food Chain and Animal Health; SCFCAH)
- SEU
southern Europe
- SG
water‐soluble granule
- SL
soluble concentrate
- SP
water‐soluble powder
- STMR
supervised trials median residue
- WHO
World Health Organization
Appendix A – Summary of intended GAP triggering the amendment of existing EU MRLs
1.
| Crop and/or situation | NEU, SEU, MS or country | F, G or I b , a | Pests or Group of pests controlled | Preparation | Application | Application rate per treatment | PHI (days) d | Remarks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type b | Conc. a.s | Method kind | Range of growth stages & season c | number min–max | Interval between application (min) | g a.s./hL min–max | Water L/ha min–max | Rate | Unit | ||||||
| Caraway | NEU | F | Annual and perennial grasses | EC | 50 g/L | Foliar treatment – broadcast spraying | BBCH 11–60 | 1 | n.a. | 25–75 | 200–300 | 0.075–0.150 | kg a.i./ha | 56 | |
MRL: maximum residue level; GAP: Good Agricultural Practice; NEU: northern European Union; SEU: southern European Union; MS: Member State; a.s.: active substance; EC: Emulsifiable concentrate.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 7th Edition. Revised March 2017. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI – minimum preharvest interval.
Appendix B – List of end points
B.1 Residues in plants
B.1.1 Nature of residues and methods of analysis in plants
B.1.1.1 Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) a | Comment/Source |
|---|---|---|---|---|---|
| Fruit crops | Tomatoes b | Foliar, 1 × 167–173 g a.s./ha | 0, 12 and 105 | (EFSA, 2017; EFSA, 2018b for a study in GM maize) | |
| Root crops | Sugar beets b | Foliar, 1 × 280 g a.s./ha | 31, 60 and 90 | ||
| Sugar beets c | Foliar, 1 × 6 g a.s./ha | 28 | |||
| Potatoes c | Foliar, 1 × 6 g a.s./ha | 14 | |||
| Sugar beets d | Foliar, 1 × 316 g a.s./ha | 31 | |||
| Pulses/oilseeds | Cotton e | Foliar, 1 × 260 g a.s./ha | 0, 7, 21 and 42 | ||
| Soya beans e | Foliar, 1 × 273–287 g a.s./ha | 0, 7, 21 and 42 | |||
| Soya beans f | Foliar, 1 × 280 g a.s./ha | 0, 7, 14, 29 and 63 | |||
| Soya beans g |
Foliar, 1 × 340 g a.s./ha (R/S); 1 × 160 g a.s./ha (R + S) |
1, 14 and 105 | |||
| Cereals | GM maize b (aad‐1 gene) | 1 × 98 g a.s./ha | 48 (forage); 72 (grain, cobs, stover/fodder) |
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Root/tuber crops | Sugar beets e | Bare soil, 308 g a.s./ha | 30, 60 | EFSA (2017) | |
| Leafy crops | Lettuces e | Bare soil, 308 g a.s./ha | 30, 60 | ||
| Pulses and oilseeds |
Cotton seeds e Peanuts e |
Bare soil, 308 g a.s./ha | 30, 60 | ||
| Cereal (small grain) | Wheat e | Bare soil, 308 g a.s./ha | 30, 60 |
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source |
|---|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Yes | Finland (2021) | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Yes | ||
| Sterilisation (20 min, 120°C, pH 6) | No (quizalofop‐P‐ethyl was partly hydrolysed to quizalofop) | ||
|
A standard hydrolysis study performed with quizalofop‐P‐terfuryl was assessed in the framework of the MRL review demonstrating that quizalofop is not expected to degrade hydrolytically under conditions representative of three different processing practices (EFSA, 2017). A new study performed with quizalofop‐P‐ethyl was also submitted with this application. As quizalofop‐P‐ethyl and quizalofop were the main compounds detected in the buffer solutions in conditions simulating pasteurisation, baking/brewing/boiling and sterilisation, it can be concluded that the residue definitions for primary crops are also applicable to processed commodities |
|||
DAT: days after treatment.
Phenyl‐ and quinoxaline‐labelled quizalofop‐P‐ethyl (R‐enantiomer).
Phenyl‐labelled quizalofop‐ethyl (Racemate (R/S)). Study results used for information only considering the low application rate.
Phenyl‐labelled quizalofop‐P‐ethyl (R‐enantiomer). Residues analysed in foliage only.
Phenyl‐ and quinoxaline‐labelled quizalofop‐ethyl (racemate (R/S)).
Phenyl‐ and quinoxaline‐labelled quizalofop‐ethyl (racemate (R/S) and R‐enantiomer).
Quinoxaline‐labelled quizalofop‐ethyl (racemate (R/S) and R‐ and S‐enantiomer).

B.1.1.2 Stability of residues in plants
| Plant product (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/Source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Snap beans | –20 | 28 a | Months | quizalofop‐P‐ethyl and quizalofop‐P | Since conjugates may only degrade to the acid form, the reported storage stability studies are expected to cover all compounds included in the residue definition, including conjugates. (EFSA, 2017) | |
| High oil content |
Cotton seeds Rape seeds |
–20 | 28 a | Months | |||
| Dry/High starch | Wheat grain | –18 | 12 a | Months | |||
| GM maize grain | –20 | 13 a | Months | ||||
| High acid content | Oranges | –18 | 12 a | Months | |||
| Processed products | GM maize oil | –20 | 13 b | Months | |||
| GM maize flour | –20 | 13 a | Months | ||||
| GM maize starch | –20 | 13 b | Months | ||||
| Others | GM maize stover | –20 | 13 a | Months | |||
| GM maize forage | –20 | 13 a | Months | ||||
GM: genetically modified.
Storage stability refers to the total residues of quizalofop‐P‐ethyl and quizalofop.
Storage stability demonstrated individually for quizalofop‐P‐ethyl and quizalofop.
B.1.2 Magnitude of residues in plants
B.1.2.1 Summary of residues data from the supervised residue trials
| Commodity | Region/ a | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HR b (mg/kg) | STMR c (mg/kg) |
|---|---|---|---|---|---|---|
| Caraway seeds | NEU | Mo/RA: 2 × < 0.01; 2 × 0.02 |
Residue trials on caraway compliant with GAP. MRLOECD = 0.04 |
0.04 | 0.02 | 0.02 |
MRL: maximum residue level; GAP: Good Agricultural Practice; Mo: monitoring; RA: risk assessment.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, EU: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment refers to the whole commodity and not to the edible portion.
Indicates that the MRL is proposed at the limit of quantification.
B.1.2.2 Residues in rotational crops

B.1.2.3 Processing factors
No processing studies were submitted in the framework of the present MRL application and not required.
B.2 Residues in livestock
Not relevant.
B.3 Consumer risk assessment

B.4 Recommended MRLs
| Code( a ) | Commodity | Existing EU MRL (mg/kg) | Proposed EU MRL (mg/kg) | Comment/justification |
|---|---|---|---|---|
| Enforcement residue definition: Quizalofop (sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers)) | ||||
| 0820030 | Caraway | 0.05* (Ft) | 0.04/Further risk managers’ considerations |
The submitted data are sufficient to derive an MRL proposal for the NEU use. Risk for consumers unlikely. EFSA notes that the current MRL for quizalofop in caraway is set as tentative at 0.05* mg/kg, a higher level than the MRL of 0.04 mg/kg proposed in the present application. It is noted that an application to address all the Art.12 confirmatory data set for quizalofop has not been submitted yet and the deadline to provide such data is now expired. Nevertheless, the confirmatory data requirements on residue trials, analytical methods and storage stability for caraway could be considered as sufficiently addressed and the footnote can be removed for this crop. EFSA therefore considers the proposed MRL derived in the context of the present application as acceptable, also taking into account the availability of an enforcement method with an LOQ at 0.01 mg/kg for caraway. |
MRL: maximum residue level; NEU: northern Europe; SEU: southern Europe; GAP: Good Agricultural Practice.
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.

Appendix D – Input values for the exposure calculations
D.1 Consumer risk assessment
| Commodity | Existing/proposed MRL (mg/kg) | Source | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment a | |||
| Risk assessment residue definition: Quizalofop (sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers)) | ||||||
| Grapefruits | 0.02* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Oranges | 0.02* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Lemons | 0.02* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Limes | 0.02* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Mandarins | 0.02* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Other citrus fruit | 0.02* | EFSA (2017) | 0.01 | STMR‐RAC | ||
| Almonds | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Brazil nuts | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Cashew nuts | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Chestnuts | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Coconuts | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Hazelnuts/cobnuts | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Macadamia | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Pecans | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Pine nut kernels | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Pistachios | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Walnuts | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Other tree nuts | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | ||
| Apples | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Pears | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Quinces | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Medlar | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Loquats/Japanese medlars | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Other pome fruit | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | ||
| Apricots | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Cherries (sweet) | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Peaches | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Plums | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Other stone fruit | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | ||
| Table grapes | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Wine grapes | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Strawberries | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Blackberries | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Dewberries | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Raspberries (red and yellow) | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Kumquats | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Potatoes | 0.1 | EFSA (2017) | 0.04 | STMR‐RAC | 0.08 | HR‐RAC |
| Beetroots | 0.06 | EFSA (2017) | 0.04 | STMR‐RAC | 0.05 | HR‐RAC |
| Carrots | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | 0.1 | HR‐RAC |
| Celeriacs/turnip‐rooted celeries | 0.08 | EFSA (2017) | 0.02 | STMR‐RAC | 0.06 | HR‐RAC |
| Horseradishes | 0.08 | EFSA (2017) | 0.02 | STMR‐RAC | 0.06 | HR‐RAC |
| Jerusalem artichokes | 0.08 | EFSA (2017) | 0.02 | STMR‐RAC | 0.06 | HR‐RAC |
| Parsnips | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | 0.1 | HR‐RAC |
| Parsley roots/Hamburg roots parsley | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | 0.1 | HR‐RAC |
| Radishes | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | 0.1 | HR‐RAC |
| Salsifies | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | 0.1 | HR‐RAC |
| Swedes/rutabagas | 0.06 | EFSA (2017) | 0.04 | STMR‐RAC | 0.05 | HR‐RAC |
| Turnips | 0.08 | EFSA (2017) | 0.03 | STMR‐RAC | 0.04 | HR‐RAC |
| Other root and tuber vegetables | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | ||
| Garlic | 0.04 | EFSA (2017) | 0.04 | STMR‐RAC | 0.04 | HR‐RAC |
| Onions | 0.04 | EFSA (2017) | 0.04 | STMR‐RAC | 0.04 | HR‐RAC |
| Shallots | 0.04 | EFSA (2017) | 0.04 | STMR‐RAC | 0.04 | HR‐RAC |
| Tomatoes | 0.05 | EFSA (2017) | 0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Aubergines/egg plants | 0.05 | EFSA (2017) | 0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Broccoli | 0.4 | EFSA (2017) | 0.06 | STMR‐RAC | 0.26 | HR‐RAC |
| Cauliflowers | 0.4 | EFSA (2017) | 0.06 | STMR‐RAC | 0.26 | HR‐RAC |
| Other flowering brassica | 0.4 | EFSA (2017) | 0.06 | STMR‐RAC | ||
| Head cabbages | 0.6 | EFSA (2017) | 0.05 | STMR‐RAC | 0.2 | HR‐RAC |
| Lamb's lettuce/corn salads | 0.2 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | HR‐RAC |
| Lettuces | 0.2 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | HR‐RAC |
| Escaroles/broad‐leaved endives | 0.2 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | HR‐RAC |
| Cress and other sprouts and shoots | 0.2 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | HR‐RAC |
| Land cress | 0.2 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | HR‐RAC |
| Roman rocket/rucola | 0.2 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | HR‐RAC |
| Red mustards | 0.2 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | HR‐RAC |
| Baby leaf crops (including brassica species) | 0.2 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | HR‐RAC |
| Other lettuce and other salad plants | 0.2 | EFSA (2017) | 0.2 | STMR‐RAC | ||
| Spinaches | 0.2 | EFSA (2017) | 0.02 | STMR‐RAC | 0.12 | HR‐RAC |
| Chards/beet leaves | 0.04 | EFSA (2017) | 0.04 | STMR‐RAC | 0.04 | HR‐RAC |
| Chervil | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Chives | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Celery leaves | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Parsley | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Sage | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Rosemary | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Thyme | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Basil and edible flowers | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Laurel/bay leaves | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Tarragon | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Other herbs | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | ||
| Beans (with pods) | 0.3 | EFSA (2017) | 0.02 | STMR‐RAC | 0.17 | HR‐RAC |
| Beans (without pods) | 0.2 | EFSA (2017) | 0.04 | STMR‐RAC | 0.07 | HR‐RAC |
| Peas (with pods) | 0.03 | EFSA (2017) | 0.01 | STMR‐RAC | 0.02 | HR‐RAC |
| Peas (without pods) | 0.2 | EFSA (2017) | 0.03 | STMR‐RAC | 0.11 | HR‐RAC |
| Lentils (fresh) | 0.2 | EFSA (2017) | 0.03 | STMR‐RAC | 0.11 | HR‐RAC |
| Florence fennels | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Beans | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | STMR‐RAC |
| Lentils | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | STMR‐RAC |
| Peas | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | STMR‐RAC |
| Linseeds | 0.3 | EFSA (2017) | 0.1 | STMR‐RAC | 0.1 | STMR‐RAC |
| Poppy seeds | 0.7 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | STMR‐RAC |
| Sunflower seeds | 0.8 | EFSA (2017) | 0.12 | STMR‐RAC | 0.12 | STMR‐RAC |
| Rapeseeds/canola seeds | 2 | EFSA (2017) | 0.23 | STMR‐RAC | 0.23 | STMR‐RAC |
| Soya beans | 0.2 | EFSA (2017) | 0.04 | STMR‐RAC | 0.04 | STMR‐RAC |
| Mustard seeds | 0.7 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | STMR‐RAC |
| Cotton seeds | 0.1 | EFSA (2017) | 0.04 | STMR‐RAC | 0.04 | STMR‐RAC |
| Maize/corn | 0.02 | EFSA (2018b) | 0.02 | STMR‐RAC | 0.02 | STMR‐RAC |
| Rice | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | STMR‐RAC |
| Chamomille | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Hibiscus/roselle | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Rose | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Jasmine | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Lime/linden | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Other herbal infusions (dried flowers) | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | ||
| Strawberry leaves | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Rooibos | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Mate/maté | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Other herbal infusions (dried leaves) | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | ||
| Anise/aniseed | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Black caraway/black cumin | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Celery seed | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Coriander seed | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Cumin seed | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Dill seed | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Fennel seed | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Fenugreek | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Nutmeg | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Other spices (seeds) | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | ||
| Allspice/pimento | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Sichuan pepper | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Caraway | 0.04 | Proposed | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Cardamom | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Juniper berry | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Peppercorn (black, green and white) | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Vanilla pods | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Tamarind | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Other spices (fruits) | 0.05* | EFSA (2017) | 0.05 | STMR‐RAC | ||
| Sugar beet roots | 0.06 | EFSA (2017) | 0.04 | STMR‐RAC | 0.05 | HR‐RAC |
| Chicory roots | 0.08 | EFSA (2017) | 0.02 | STMR‐RAC | 0.06 | HR‐RAC |
| Swine: Muscle/meat | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Swine: Fat tissue | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Swine: Liver | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Swine: Kidney | 0.1 | EFSA (2017) | 0.07 | STMR‐RAC | 0.1 | HR‐RAC |
| Swine: Edible offals (other than liver and kidney) | 0.1 | EFSA (2017) | 0.07 | STMR‐RAC | 0.1 | HR‐RAC |
| Bovine: Muscle/meat | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Bovine: Fat tissue | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Bovine: Liver | 0.03 | EFSA (2017) | 0.02 | STMR‐RAC | 0.03 | HR‐RAC |
| Bovine: Kidney | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Bovine: Edible offals (other than liver and kidney) | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Sheep: Muscle/meat | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Sheep: Fat tissue | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Sheep: Liver | 0.03 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Sheep: Kidney | 0.3 | EFSA (2017) | 0.17 | STMR‐RAC | 0.24 | HR‐RAC |
| Sheep: Edible offals (other than liver and kidney) | 0.3 | EFSA (2017) | 0.17 | STMR‐RAC | 0.24 | HR‐RAC |
| Goat: Muscle/meat | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Goat: Fat tissue | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Goat: Liver | 0.03 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Goat: Kidney | 0.3 | EFSA (2017) | 0.17 | STMR‐RAC | 0.24 | HR‐RAC |
| Goat: Edible offals (other than liver and kidney) | 0.3 | EFSA (2017) | 0.17 | STMR‐RAC | 0.24 | HR‐RAC |
| Equine: Muscle/meat | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Equine: Fat tissue | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Equine: Liver | 0.03 | EFSA (2017) | 0.02 | STMR‐RAC | 0.03 | HR‐RAC |
| Equine: Kidney | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Equine: Edible offals (other than liver and kidney) | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Poultry: Muscle/meat | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Poultry: Fat tissue | 0.04 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Poultry: Liver | 0.04 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Poultry: Kidney | 0.04 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Poultry: Edible offals (other than liver and kidney) | 0.04 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Other farmed animals: Muscle/meat | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Other farmed animals: Fat tissue | 0.02* | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Other farmed animals: Liver | 0.03 | EFSA (2017) | 0.02 | STMR‐RAC | 0.03 | HR‐RAC |
| Other farmed animals: Kidney | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Other farmed animals: Edible offals (other than liver and kidney) | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Milk: Cattle | 0.015 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Milk: Sheep | 0.015 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Milk: Goat | 0.015 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Milk: Horse | 0.015 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Milk: Others | 0.015 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Eggs: Chicken | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Eggs: Duck | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Eggs: Goose | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Eggs: Quail | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Eggs: Others | 0.01* | EFSA (2017) | 0.01 | STMR‐RAC | ||
STMR‐RAC: supervised trials median residue in raw agricultural commodity; HR‐RAC: highest residue in raw agricultural commodity; PeF: Peeling factor.
Input values for the commodities which are not under consideration for the acute risk assessment are reported in grey.
Appendix E – Used compound codes
1.
| Code/trivial name a | Chemical name/SMILES notation/ InChiKey b | Structural formula c |
|---|---|---|
| Quizalofop‐P‐ethyl |
ethyl (2R)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionate O = C(OCC)[C@@H](C)Oc1ccc(cc1)Oc2cnc3cc(Cl)ccc3n2 OSUHJPCHFDQAIT‐GFCCVEGCSA‐N |

|
| Quizalofop‐P‐tefuryl |
(RS)‐tetrahydrofurfuryl (R)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionate O = C(OCC1CCCO1)[C@@H](C)Oc4ccc(Oc2cnc3cc(Cl)ccc3n2)cc4 BBKDWPHJZANJGB‐IKJXHCRLSA‐N |

|
|
Propaquizafop |
2‐isopropylideneaminooxyethyl (R)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionate C/C(C)=N\OCCOC(=O)[C@@H](C)Oc1ccc(cc1)Oc2cnc3cc(Cl)ccc3n2 FROBCXTULYFHEJ‐OAHLLOKOSA‐N |

|
| Quizalofop‐P |
(R)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionic acid O = C(O)[C@@H](C)Oc1ccc(cc1)Oc2cnc3cc(Cl)ccc3n2 ABOOPXYCKNFDNJ‐SNVBAGLBSA‐N |

|
| Quizalofop |
(RS)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionic acid O = C(O)C(C)Oc1ccc(cc1)Oc2cnc3cc(Cl)ccc3n2 ABOOPXYCKNFDNJ‐UHFFFAOYSA‐N |

|
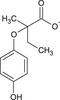
| Phenoxy propionate (EPP) |
2‐(4‐hydroxyphenoxy)‐2‐methylbutanoate [O‐]C(=O)C(C)(CC)Oc1ccc(O)cc1 CFECBIHTYUULLL‐UHFFFAOYSA‐M |
|
|
Phenoxy acid Hydroxyphenoxypropionic acid (PPA) |
(R)‐2‐(4‐hydroxyphenoxy)propionic acid C[C@@H](Oc1ccc(O)cc1)C(=O)O AQIHDXGKQHFBNW‐ZCFIWIBFSA‐N |

|
|
Quizalofop‐phenol Hydroxy ether (CQOP) |
4‐(6‐chloroquinoxalin‐2‐yloxy)phenol Oc1ccc(cc1)Oc2cnc3cc(Cl)ccc3n2 UVYFSLAJRJHGJB‐UHFFFAOYSA‐N |

|
|
Hydroxy‐quizalofop‐phenol (CQOPOH) Dihydroxy ether |
7‐chloro‐3‐(4‐hydroxyphenoxy)quinoxalin‐2(1H)‐one Oc1ccc(cc1)Oc2nc3ccc(Cl)cc3nc2O SUDISTHTCZHOSE‐UHFFFAOYSA‐N |

|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2020.2.1 ACD/Labs 2020 Release (File version N15E41, Build 116563, 15 Jun 2020).
ACD/ChemSketch 2020.2.1 ACD/Labs 2020 Release (File version C25H41, Build 121153, 22 Mar 2021).
Suggested citation: EFSA (European Food Safety Authority) , Bellisai G, Bernasconi G, Brancato A, Carrasco Cabrera L, Ferreira L, Giner G, Greco L, Jarrah S, Leuschner R, Oriol Magrans J, Miron I, Nave S, Pedersen R, Reich H, Ruocco S, Santos M, Pia Scarlato A, Theobald A, Vagenende B and Verani A, 2021. Reasoned Opinion on the modification of the existing maximum residue level for quizalofop (resulting from the use of quizalofop‐P‐ethyl) in caraway. EFSA Journal 2021;19(12):6957, 32 pp. 10.2903/j.efsa.2021.6957
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00211
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to thank Stathis Anagnos, Laszlo Bura, Aija Kazocina, Andrea Mioč, Marta Szot, Aikaterini Vlachou for the support provided to this scientific output.
Adopted: 15 November 2021
Notes
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.08.1991, p. 1–32.
Commission Directive 2009/37/EC of 23 April 2009 amending Council Directive 91/414/EEC to include chlormequat, copper compounds, propaquizafop, quizalofop‐P, teflubenzuron and zeta‐cypermethrin as active substances OJ L 104, 24.4.2009, p. 23–32.
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.03.2005, p. 1–16.
For an overview of all MRL Regulations on this active substance, please consult: https://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐database/active‐substances/?event=search.as
Footnote in Commission Regulation (EU) 2019/973 for caraway: The European Food Safety Authority identified some information on residue trials, analytical methods and storage stability as unavailable for quizalofop‐P‐ethyl. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
Commission Regulation (EU) 2019/973 of 13 June 2019 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for bispyribac, denatonium benzoate, fenoxycarb, flurochloridone, quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl, propaquizafop, tebufenozide in or on certain products.C/2019/4258. OJ L 157, 14.6.2019, p. 3–27.
Commission Regulation (EU) No 544/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the data requirements for active substances. OJ L 155, 11.6.2011, p. 1–66.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
MW quizalofop = 344.8; MW quizalofop‐P‐ethyl = 372,8; MW quizalofop‐P‐tefuryl = 428.8.
References
- EFSA (European Food Safety Authority) , 2009. Conclusion on the peer review of the pesticide risk assessment of the active substance quizalofop‐P (considered variants quizalofop‐P‐ethyl and quizalofop‐P‐tefuryl). EFSA Journal 2009;7(7):205r, 216 pp. 10.2903/j.efsa.2009.205r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato A, Brocca D, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Nougadere A, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Reasoned opinion on the review of the existing maximum residue levels for quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2017;15(12):5050, 119 pp. 10.2903/j.efsa.2017.5050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato A, Brocca D, Ferreira L, Greco L, Jarrah S, Leuschner R, Medina P, Miron I, Nougadere A, Pedersen R, Reich H, Santos M, Stanek A, Tarazona J, Theobald A and Villamar‐Bouza L, 2018a. Guidance on use of EFSA Pesticide Residue Intake Model (EFSA PRIMo revision 3). EFSA Journal 2018;16(1):5147, 43 pp. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato A, Brocca D, Carrasco Cabrera L, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Pedersen R, Reich H, Riemenschneider C, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B and Villamar‐Bouza L, 2018b. Reasoned opinion on the setting of import tolerance for quizalofop‐P‐ethyl in genetically modified maize. EFSA Journal 2018;16(3):5250, 23 pp. 10.2903/j.efsa.2018.5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou M, Brancato A, Carrasco Cabrera L, Ferreira L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Pedersen R, Raczyk M, Reich H, Ruocco S, Sacchi A, Santos M, Stanek A, Tarazona J, Theobald A and Verani A, 2019a. Pesticide Residue Intake Model‐ EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA supporting publication 2019;EN‐1605, 15 pp. 10.2903/sp.efsa.2019.EN-1605 [DOI]
- EFSA (European Food Safety Authority) , Bura L, Friel A, Magrans JO, Parra‐Morte JM and Szentes C, 2019b. Guidance of EFSA on risk assessments for active substances of plant protection products that have stereoisomers as components or impurities and for transformation products of active substances that may have stereoisomers. EFSA Journal 2019;17(8):5804, 33 pp. 10.2903/j.efsa.2019.5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 1997a. Appendix A. Metabolism and distribution in plants. 7028/VI/95‐rev.3, 22 July 1997.
- European Commission , 1997b. Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the Appendix of Council Directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission , 1997c. Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission , 1997d. Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997e. Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission , 1997f. Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission , 1997g. Appendix I. Calculation of maximum residue level and safety intervals. 7039/VI/95 22 July 1997. As amended by the document: classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2000. Residue analytical methods. For pre‐registration data requirements for Annex II (part A, section 4) and Annex III (part A, section 5) of Directive 91/414. SANCO/3029/99‐rev. 4. 11 July 2000.
- European Commission , 2010a. Classes to be used for the setting of EU pesticide Maximum Residue Levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission , 2010b. Residue analytical methods. For post‐registration control. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2010c. Review report for the active quizalofop‐P. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 23 January 2009 in view of the inclusion of quizalofop‐P in Annex I of Council Directive 91/414/EEC. SANCO/169/08 final. (revised), 28 September 2012.
- FAO (Food and Agriculture Organization of the United Nations) , 2016. Submission and evaluation of pesticide residues data for the estimation of Maximum Residue Levels in food and feed. Pesticide Residues. 3rd Edition. FAO Plant Production and Protection Paper 225, 298 pp.
- Finland , 2007. Draft assessment report on the active substance quizalofop‐P‐ethyl prepared by the rapporteur Member State Finland in the framework of Council Directive 91/414/EEC, January 2007. Available online: www.efsa.europa.eu
- Finland , 2008. Final addendum to the draft assessment report on the active substance quilazofop‐P‐ethyl, compiled by EFSA, September 2008. Available online: www.efsa.europa.eu
- Finland , 2021. Evaluation report on the modification of MRLs for quizalofop‐P‐ethyl in caraway. April 2021, 39 pp. Available online: www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development) , 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: http://www.oecd.org


