Abstract
The mammalian immune system packs serious punch against infection but can also cause harm: for example, coronavirus disease 2019 (COVID-19) made headline news of the simultaneous power and peril of human immune responses. In principle, natural selection leads to exquisite adaptation and therefore cytokine responsiveness that optimally balances the benefits of defense against its costs (e.g., immunopathology suffered and resources expended). Here, we illustrate how evolutionary biology can predict such optima and also help to explain when/why individuals exhibit apparently maladaptive immunopathological responses. Ultimately, we argue that the evolutionary legacies of multicellularity and life-history strategy, in addition to our coevolution with symbionts and our demographic history, together explain human susceptibility to overzealous, pathology-inducing cytokine responses. Evolutionary insight thereby complements molecular/cellular mechanistic insights into immunopathology.
Keywords: evolutionary biology, immunopathology, cytokines, optimality, Goldilocks
Mammalian cytokine responses confer power but pose peril
The simultaneous power and peril of the cells and molecules of the mammalian immune system are well documented in biomedical immunology. No sooner is an enthralling cytokine or effector discovered and its role in defense elucidated than its dangers are revealed: for example, interleukin (IL)-33 was first described in 2005 when its superpower in gut defense against nematodes was apparent [1], but by 2010 the perils of IL-33 dysregulation had been discovered, including potential roles in inflammatory bowel disease [2].
Indeed, for every unit of power to kill invaders, the mammalian immune system also possesses power to kill our own cells, or even to kill us outright, such as during septic or anaphylactic shock. This pairing of power with peril helps to explain why phrases about the need to balance protection and pathology (e.g., a recent 'Cytokines in the balance' special issue [3]) and stories such as that of Goldilocks (e.g., the perfect immune response is not too hot, not too cold, but 'just right' [4]) permeate the immunological literature. Likewise, images of see-saws or Yin-Yang symbols often appear. For example, both were used on the cover of Trends in Immunology in 2017 to visualize the balancing acts surrounding IL-17 (e.g., [5]). The implication is that an optimal response is of sufficient magnitude to slay the pathogens, but not so great as to risk immunopathological damage to cells, tissues, or even the life of the host. Such logic arguably best applies to the cytokines and chemokines that chart the magnitude, type, and location of the effector response. Cytokines and chemokines, after all, are the signaling molecules that make the 'system' of the immune system.
How does natural selection (see Glossary) actually operate on cytokine responsiveness, and why do so many individuals mount apparently maladaptive responses that lead to immunopathology? To answer such questions it is essential to move beyond intuition about individual-scale optimality to evolutionary explanations for not only the mean but also the variance in cytokine responses among hosts. We offer such explanations in this article. We contend that application of evolutionary biology to immunology is fruitful, although rare. In particular, mechanistic insights from molecular and cellular studies of cytokine biology and immunopathology are enriched by placement into an evolutionary context, and our understanding of the evolution of immune systems is enhanced by probing with tools from evolutionary biology, as follows.
Optimality analysis provides an evolutionary lens
Optimality analysis is foundational to evolutionary biology. The theory of natural selection hinges on the idea that competition among individuals exhibiting differential survival and reproduction favors phenotypes that best match the current environment [6]. Those who survive and reproduce best in that environment attain the highest fitness, in other words, the greatest genetic representation in subsequent generations. The mean phenotype is expected to approximate the optimum, although complexities of biology lead individual phenotypes to vary around the mean and potentially generate maladaptation to that environment, resulting in poor survival or reproduction.
Evolutionary biologists draw upon economic cost–benefit analysis to make quantitative predictions about optimal phenotypes, to test those predictions, and to explain why the realized phenotypes of individuals deviate from the optimal ideal [7., 8., 9.]. Such frameworks enable rigorous investigation of maladaptation and the persistence of suboptimal phenotypes in a population (e.g., [10]). Causes of maladaptation include mismatch between an organism and its environment, or excessive variance of a trait [10]. Both are arguably of particular concern in mammalian cytokine biology, given that cytokines are such powerful tuners of the systemic response [11] and are also the most variably expressed genes in mammals [12].
We examine apparently maladaptive immunopathologies and autoimmune and inflammatory diseases (hereafter shorthanded as 'immunopathology') in light of such evolutionary analysis. To frame our arguments, we begin by dissecting the fundamentals of quantitative cost–benefit analysis and its predictions for optimal cytokine responses. We then consider several categories of evolutionary legacy that keep hosts from achieving such optima, especially coevolution with parasites, multicellularity, and life history. Throughout, we draw on recent advances and add to arguments offered in past reviews of the evolutionary biology of immunopathology (e.g., [13., 14., 15., 16., 17., 18.]). Ultimately, we argue that discovering the causes of individual, population, and species divergence in susceptibility to immunopathology requires an evolutionary approach.
Cost–benefit analysis of cytokine responses
What makes a host able to fend off infection (e.g., a cytokine response that rapidly mobilizes a potent effector response) can be exactly what makes the host risk immunopathology. The power of the immune system thus poses tradeoffs. The optimal immune response is hypothesized to be the response that best balances such a tradeoff, given the challenge at hand [14,15,17]. For example, optimal rates of IL-13-driven epithelial cell turnover might purge gastrointestinal nematodes such as whipworms (Trichuris spp.) [19] down to a tolerable burden, below which the costs of further clearance might outweigh the benefits [20]. Costs of complete clearance of gastrointestinal nematodes by this mechanism might include the nutritionally dangerous rapidity of gut transit – for example, if the 50% acceleration in movement of digesta through the small intestine associated with self-cure of Ascaris suum in piglets [21] caused sufficient malnutrition to impair growth or survival. Another potential cost could arise if sustained, rapid stem cell division is oncologically dangerous – for example, if the doubled rate of movement of epithelial cells out of the colonic crypts of Trichuris muris-resistant BALB/c mice [19] were associated with sufficient additional stem cell division to put the hosts at elevated risk of colon cancer (paralleling the observation that the number of stem cell divisions predicts variation among human tissues in risk of cancer [22]). Such logic suggests that type 2 cytokine responses against helminth infections must be balanced so that the costs do not outweigh the benefits. Similar logic arguably applies to type 1 cytokine responses [17]; for example, a tightly regulated tumor necrosis factor (TNF)-α response by neutrophils in wounded rats controls Pseudomonas aeruginosa infection without incurring intolerable inflammatory damage [23]. Other aspects of immune responses pose tradeoffs and are in principle optimized (e.g., specificity or accuracy versus the speed of a response [24., 25., 26.]), but we focus here on optimization of cytokine response magnitude.
A 'Goldilocks' intermediate optimum [4] that balances the costs and benefits of cytokines can in principle be quantified: formal cost–benefit analyses can be used to predict the magnitude that maximizes the benefit while minimizing the cost of a cytokine response (Figure 1A). Individual hosts mounting responses that deviate from such targets are predicted to exacerbate either parasitemia (at the low-magnitude end of the spectrum) or immunopathology (at the high-magnitude end) [14].
Figure 1.
Dissecting the Goldilocks optimum.
(A) The graphs depict the optimality analysis of cytokine response magnitude under different conditions. The relative risks posed by infection and immunopathology differ across the range of variation in cytokine responses. Specifically, as the cytokine response (x axis) varies from weaker ('cold') to stronger ('hot'), the risk of mortality associated with immunopathology increases (increasing curve) while the risk of mortality associated with infectious disease declines (declining curve). At the scale of the population, fitness (y axis, thick line) is low where either source of mortality is high. The optimum corresponding to the highest fitness (red point) corresponds to the balance of cytokines that minimizes both sources of mortality. The optimal cytokine response for a given environment (e.g., the intermediate optimum of Goldilocks) thus minimizes the joint risks and maximizes host health/fitness. (B) If the risk of mortality due to immunopathology is steeper than the risk of death due to infection, then the evolutionarily optimal cytokine responsiveness will tend toward 'hot' but risks overshooting. (C) Uncertainty in genetic or environmental context that causes uncertainty in the risk of immunopathology or infection will lead to uncertainty in the optimal cytokine responsiveness. (D) Even when the optimal cytokine responsiveness is relatively certain, it may not be precisely attained.
However, the health/fitness risks posed by infection and immunopathology are not necessarily symmetrical. For example, if the health/fitness cost of infection is higher than the cost of immunopathology, rigorous mathematical analysis – the calculus that identifies the optimum – reveals that natural selection will favor strong defenses, even if such responses verge on catastrophic overshooting and possibly lead to host death [27] (Figure 1B). In general, homeostatic circuits, including those governing inflammation, are vulnerable to such dysregulation [28]. The benefits of speedy upregulation of cytokine responses upon infection [29] and the propensity of parasites to evolve negative regulators [25] [e.g., the capacity of the protozoan Leishmania donovani to suppress IL-12p40 production by human monocytes despite Toll-like receptor (TLR)-2 or TLR-4 stimulation [30]] may help to explain the evolution of immune systems that are prone to overshooting.
Empirical evidence is consistent with a Goldilocks optimum that is intermediate between the extremes of cytokine response magnitude. For example, knockout and monoclonal antibody-treated mice routinely show that hosts fare poorly against infections when deficient in proinflammatory cytokine signaling [e.g., IL-17 receptor α knockout (Il17ra −/−) mice are acutely susceptible to oral Candida albicans [31] and Porphyromonas gingivalis [32] infections], but suffer severe immunopathology when the mice are instead deficient in anti-inflammatory cytokines [e.g., IL-10 knockout (Il10 −/−) mice suffer extreme periodontal bone loss during P. gingivalis infections following excessive production of IL-17 [33]]. Indeed, such studies provide the empirical basis for inferring that protection–pathology balancing acts are required for optimal deployment of cytokines such as IL-17 [5]. Such inferences are supported by more formal quantitative investigation of the optimal mix of pro- and anti-inflammatory cytokines that maximizes host survival. For example, in wild-type SWISS mice responding to lipopolysaccharide (LPS) challenge, mice mounting strong systemic IL-6 responses (measured in plasma) only survived if they also mounted a potent systemic IL-10 response [34]; the authors infer that natural selection must therefore operate simultaneously on IL-6 and IL-10 production [34]. Nonetheless, evolutionary analysis also supports the idea that evolved expression of cytokines will err on the side of overshooting: meta-analysis of dozens of studies of cytokine knockout mice suggests that infection is more likely to kill the host than is immunopathology [35]. On average, the benefits of powerful cytokine responses are thus likely to outweigh immunopathological costs over evolutionary time [14,35].
Irrespective of the precise shape of the tradeoff between risks of infection and immunopathology, realized cytokine responses of hosts often differ from the optimum for a variety of reasons that are interesting and important to dissect – for example, one might consider whether altered environments modulate the optimal response [17], or whether genetic constraint keeps hosts from attaining optimal cytokine signaling [36]. In general, environmental and genetic contexts combine to determine the optimal cytokine response that would maximize host health/fitness. However, because populations face a range of environmental conditions – even over short periods of evolutionary time – and contain substantial genetic variation, an individual organism cannot 'know' the exact context it will face. This uncertainty prevents evolution from achieving the optimal cytokine responsiveness in every individual organism (Figure 1C) (indeed, uncertainty can promote qualitatively different cytokine signaling networks in addition to simply tuning the balance [36].) Moreover, even if the optimal cytokine responsiveness were to be relatively certain, it may not always be physiologically or evolutionarily achievable [36] (Figure 1D). In the following sections we therefore argue that susceptibility to immunopathology, including the propensity to mount excessive proinflammatory cytokine responses, is the result of uncertainty and constraints arising from such evolutionary legacies as coevolution with symbionts, the evolution of multicellularity, and divergent life-history strategies.
Coevolutionary legacies that confer susceptibility to immunopathology
Coevolution with immunomodulatory symbionts has left a lasting imprint on mammalian immune function. Since Strachan's initial proposal that lack of exposure to microbes might promote the development of allergies [37], abundant evidence has emerged to suggest that lack of exposure to such symbionts can unmask immunopathology by altering the strength and type of realized cytokine responses. Influential symbionts include gastrointestinal bacteria, with which mammalian immune systems coevolved [38] and on which our immune systems depend for balance among T helper cell subsets [39] – for example to avoid inflammatory bowel disease [40].
Parasitic helminths, or worms, soon joined the germs in colonizing vertebrate bodies: first flatworms in the phylum Platyhelminthes [41,42], and then roundworms in the phylum Nematoda [43,44], evolved parasitism of vertebrate hosts 400–500 million years ago. Exactly as for germs, the legacy of vertebrate coevolution with these 'old friends' is that the cytokine responsiveness of mammals is attuned to the immunomodulatory prowess of helminths [45]. A vivid example of that prowess is the functional molecular mimetic of transforming growth factor (TGF)-β that is produced by the murine gastrointestinal nematode Heligmosomoides polygyrus [46]. Indeed, excretory/secretory and other products of helminths that promote chronic infection, and thus parasite fitness, are among the most promising immunomodulatory agents in the pharmaceutical pipeline [47].
In the presence of helminths, natural selection is thought to have favored hosts with powerful proinflammatory cytokine responses (to protect the host against microbial coinfections), while helminth-derived modulators such as the TGF-β mimetic [46] take off the inflammatory edge and protect against immunopathology (Figure 2A); in the absence of helminths, such protection is lost [45] (Figure 2B). Although it is difficult to test hypotheses about historical natural selection directly, these ideas are supported by experimental, epidemiological, and population genetic evidence. For example, in modern human populations, the frequency of alleles conferring susceptibility to dysregulated cytokine responses (e.g., the risk allele of the IL-18 receptor accessory protein gene, Il18RAP, that is associated with inflammatory bowel disease, among other conditions [48]) is positively associated with the diversity and abundance of parasites, including helminths, circulating in the population [49,50].
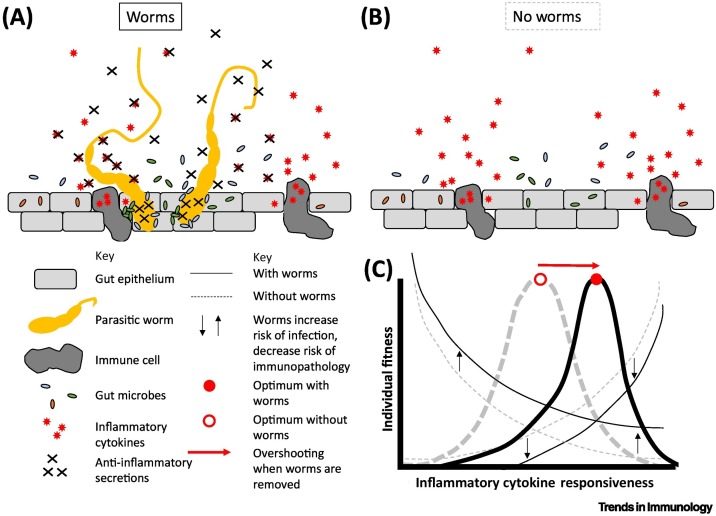
Figure 2.
Optimality analysis of proinflammatory cytokine responsiveness in the presence versus absence of immunomodulatory symbionts such as parasitic helminths.
(A) In the presence of parasitic helminths (e.g., gastrointestinal nematodes), large releases of proinflammatory cytokines are partially offset by potent anti-inflammatory molecules secreted by the worms; this is expected to decrease the risk of immunopathology but may increase the risk of microbial infection. (B) In the absence of parasitic worms, large releases of proinflammatory cytokines are not offset, thus increasing the risk of immunopathology but likely decreasing the risk of microbial infection. (C) Together, these risks determine differing optimal cytokine responsiveness in differing environments. Because hygiene and other factors reduce worm burden in some areas, the new optimal cytokine response may be overshot as a result of a coevolutionary legacy.
Given these coevolutionary legacies, hygiene that precludes exposure to particularly influential worm and germ species in some populations constitutes an environmental shift that arguably makes human hosts more prone to cytokine overshooting because of the loss of anti-inflammatory activity [17,45] (Figure 2B). By this same logic, evolved dependence on immunomodulatory symbionts might help to explain seemingly maladaptive exacerbation of immunopathology when the presence of those symbionts becomes less certain under hygiene (Figure 2C).
For a different set of reasons, malaria parasites have likewise been highly influential in the history of hominins [51]. This is not only because malaria has driven the evolution of the sickle-cell trait and other red blood cell polymorphisms [52], but selection for malaria resistance may also have enhanced susceptibility to cytokine-driven immunopathology. For example, resistance against malaria is observed when an immune inhibitory receptor, Fcγ receptor IIb, is deficient in mice or impaired in humans (owing to enhanced TNF-α and phagocytic effector activity against Plasmodium chabaudi and P. falciparum by murine and human monocyte-derived macrophages, respectively), but this phenotype is also associated with systemic lupus erythematosus (SLE; lupus) susceptibility [53]. By controlling the magnitude of proinflammatory cytokine responses, this receptor therefore appears to mediate a tradeoff between susceptibility to infection and to immunopathology. We might thus understand lupus partly as a legacy of our coevolutionary history with malaria.
Many other infectious agents are likely to have left legacies of strong cytokine responses and thus of immunopathology. For example, natural selection by Yersinia pestis infection (the agent of plague) may have left a legacy of familial Mediterranean fever in modern populations [54]. Furthermore, as discussed above, any rapidly replicating [29] or sabotaging [25] parasite might favor the evolution of overshooting cytokine responses. Risk of sabotage by parasites may even be a primary reason for the structural redundancies of the cytokine network, despite the overshooting risks that redundancies may generate [13,36].
These coevolutionary legacies with old friends as well as with old and pernicious enemies offer a rich vein for further research. However, several other fundamental evolutionary legacies affect the odds of immunopathology.
Legacies of multicellularity that confer susceptibility to immunopathology
In all organisms, unicellular or multicellular, natural selection is expected to favor defenses that maximize the benefit while minimizing the cost in a given environment. Indeed, optimality models accurately predict resource- and dose-dependent deployment of inducible defenses (such as CRISPR-Cas) of unicellular organisms such as Pseudomonas aeruginosa against bacteriophages [55] as well as the CRISPR repertoire size that maximizes anti-phage defense but minimizes dangerous self-reactivity across prokaryotes [56].
Multicellular organisms face an additional obstacle because their defenses arise from a multitude of cells, each of which has both limited information and limited agency [4,57,58], but which together form the powerful, perilous collective. TLR signaling and induced cytokine secretion, for example, occur at a cellular scale [e.g., TLR-2, -3, or -4 agonists induce interferon (IFN)-γ production by human M1-like inflammatory macrophages in vitro [59]], but affect fitness at the organismal scale (e.g., strong IFN-γ responsiveness is essential for controlling chronic pulmonary aspergillosis and extending the life of human patients [60]). Similarly, sensitive cellular detection of LPS by a wide array of host species [61] may translate into disastrously potent cytokine responses and life-threatening septic shock at the organismal scale (e.g., in C57BL/6 mice injected with LPS [62]). 'Negative regulators' might rein in such danger – for example as seen for soluble receptors such as sIL-6R from dendritic cells that bind plasma IL-6 and thereby reduce its concentration and persistence in mouse blood [63], or various inhibitory receptors encoded by the human genome that are hypothesized to dampen the rates of cell activation [64]; however, these regulators are often too slow to prevent immunopathology at the organismal scale [25].
Why has more precise cross-scale control not evolved? Might imprecision actually have benefits for hosts – for example if it accelerates the speed [65] or agility [57] of immune decision-making? Might discordant activity of different immune cells within a host be a form of bet-hedging to ensure that at least some cells are well-matched to combat uncertain threats? [66]. In addition, to what extent is imprecise control of cytokine response magnitude an inevitable consequence of a decentralized system that depends upon signaling feedbacks among autonomous cells? [58]. To answer such questions and explore the opportunities and engineering constraints posed by mammalian immune systems, mathematical modeling tools have proved to be invaluable in recent years.
Indeed, mathematical analysis of collective decision-making [4] and quorum sensing [67] by immune cells may be especially relevant for understanding immunopathology. For example, data-driven mathematical models reveal how a T helper cytokine bias at the organismal level arises from cellular- and molecular-scale signaling feedback loops if cell density is sufficiently high [66], as well as how regulatory T cells (Tregs) can jointly compute a receptor repertoire to optimize responsiveness and avoid autoimmune disease [68]. In each of these studies the authors draw upon previous empirical studies in which T cell phenotypes of inbred mice are characterized by conventional methods in cellular immunology, such as flow cytometry or immunofluorescence staining (e.g., [69,70]). The authors of mathematical studies then capture details of the relevant T helper (Th) cell population biology (whether Th1/Th2 differentiation [66] or Treg receptor repertoire development [68]) in a system of differential equations that enable rigorous analysis of the organismal-scale behavior of the system.
Of relevance to cytokine-driven immunopathology, a key emerging insight from such work is that reciprocal feedback loops among Th cell populations within the host ultimately generate 'alternative stable states' such that tiny stochastic differences in early induced responses [4] lead, via cytokine feedback, to huge differences at the organismal scale (Figure 3A). This bifurcation behavior is commonly observed in complex systems, and is arguably to be expected from all systems driven by feedback loops, including homeostatic circuits [11,28]; nevertheless, detailed examples obtained from host defense datasets are only beginning to emerge. For example, in fruit flies [71] and flour beetles [72], subtly divergent early dynamics ultimately determine whether the host will survive or die; in the fruit flies (Drosophila melanogaster) the relative rates of bacterial proliferation versus Imd-driven antimicrobial peptide production [73] immediately following Providencia rettgeri injection determined whether or not the fly would survive [71]. Mathematical modeling of mammalian immune dynamics suggests that such bifurcations also determine organism-scale Th cell phenotype [66], type I IFN responsiveness [74], and ultimately the chronicity of infection [75,76]. We eagerly look forward to experimental tests of these predictions in mammals.
Figure 3.
Cytokine signaling feedback loops can generate a system of alternative stable states.
(A) Phase diagrams of cytokine-driven feedbacks show that, through time (increasingly dark arrows), initial responses are amplified toward stable attractors (thick dots) guided by the overall system structure (captured in the signaling diagram and broken line). In such a system, small stochastic variations in the initial response can propagate to enormous differences in the proinflammatory versus anti-inflammatory nature of the fully engaged immune response. (B,C) Genetic variants (such as mutations that affect promoter efficiency, the DNA-binding domains of transcription factors, etc.) can strengthen or weaken different cytokine-driven feedbacks. In turn, this alters the structure of the alternative stable states that favor proinflammatory (B) or anti-inflammatory (C) responses, thus potentiating even greater variation among individuals in their response to similar stimuli.
We expect that the evolutionary legacy of multicellularity will affect susceptibility to immunopathology due to such system dynamics. In particular, genetics and environmental conditions, although important, are insufficient predictors of immunopathology: perfect matching to the optimum (striking the perfect balance between protection and immunopathology) for every individual becomes impossible because small stochastic fluctuations propagated through feedback loops in the cellular communication circuits generate variation in the realized cytokine response [66,74]. Furthermore, genetic variants that even subtly modulate feedback strengths (such as the Imd-deficient and other fruit fly variants in a seminal study [71]) can also drastically alter the probabilistic cytokine output in response to similar stimuli (Figure 3B,C) [77]. Thus, cellular collectives form a powerful defense system but can become locked into non-optimal behavior in the face of uncertainty, and when coupled with stochasticity.
How human immunopathology depends upon such dynamics remains to be revealed, although the COVID-19 pandemic might provide a timely example: it is plausible that the divergent severity of COVID-19 disease arises due to quantifiable alternative stable states in induced cytokine dynamics because of the cellular stochasticity and emergent feedback loops outlined above [78]. Indeed, recent data suggest that such feedback loops are a feature of COVID-19-associated alveolar inflammation and immunopathology: chemokine and cytokine gene expression by T cells and macrophages in bronchoalveolar lavage from COVID-19 patients with differing disease severity revealed that escalating cycles of inflammation within alveoli may cause the most severe pneumonia [79]. Therefore, further pairing of theory and data to dissect the contributions of cellular swarms to the ensuing immunopathology of multicellular organisms, including human hosts, are needed and are keenly awaited.
Legacies of life-history strategy that confer susceptibility to immunopathology
To an evolutionary biologist, the term life-history strategy encompasses the rules by which organisms regulate their growth, somatic maintenance, and reproduction, and thereby achieve evolutionary fitness (i.e., genetic representation in future generations [80]). Resource limitation is expected to impose tradeoffs such that no organism can grow large, live long, and reproduce rapidly – and must instead prioritize a subset of these traits. Indeed, different species have strikingly different life-history strategies: for example, contrast the body sizes, lifespans, and reproductive rates of blue whales versus mice (among mammals) or albatrosses versus sparrows (among birds); the world is rich with such diverse strategies.
Diversity in immune defense strategy, and therefore susceptibility to immunopathology, is predicted to mirror and even mediate this life-history diversity. This is because the immune system is a primary contributor to somatic maintenance (fighting infections, repairing wounds, etc.), but immune responses deplete the amount of resources available for growth and reproduction [81]. Theory therefore suggests that the evolutionarily optimal immune response arises from the epidemiological setting, life history, and demography of the whole organism – for example, whether a host is at greater risk of infectious or inflammatory disease, and how those risks vary with age, sex, and reproductive effort [82., 83., 84] (Figure 4A). Optimal immune responses are expected to be further shaped by parasite life-history strategy (e.g., the characteristic virulence or chronicity of infection [85,86]), as well as by the associated immunomodulatory factors considered in the coevolution section above.
Figure 4.
Life history can modify optimal cytokine responsiveness.
(A) The heat map depicts hypothesized optimal investments in cytokine-driven immunity that depend on the timing and magnitude of other investments such as reproduction and/or parental care (x axis) or survival more broadly (y axis). Species with different life-history strategies might therefore have different optima in terms of cytokine-responsiveness. (B) Even within a single species, there is life-history variation (e.g., between males and females) that could drive variation in cytokine-responsiveness.
The evolutionary vantage point thus adds further challenges to the protection–immunopathology balancing act (e.g., [5]). Risking immunopathology by investing in a powerful cytokine response carries an additional cost if the response depletes resources that could otherwise be put into fighting for mates or gestating a fetus. Hosts with life-history strategies geared for rapid reproduction are therefore predicted to exhibit immunosuppression [81,87] and, arguably, a low risk of immunopathology.
Empirical evidence of interspecific variation in propensity to suffer immunopathology is only slowly emerging. Veterinarians have reported that captive polar bears can exhibit human-like immunopathology of the brain [88], and that stranded northern elephant seals exhibit shock-like reactions [89], suggesting that wild animal species do suffer immunopathology and/or overzealous cytokine responses. In some species the causes may be genetic (e.g., via TLR5 polymorphisms that confer greater IBD susceptibility in maned wolves and red wolves than in domesticated dogs [90]). However, environmental factors are also likely to be important [91]. It has even been suggested that only individuals in captivity experience immunopathology as a result of dysbiosis associated with the artificial environment (e.g., in marmosets [92], red wolves [93], and cheetahs [94]). This idea in turn suggests that a wide variety of mammals might be prone to immunopathology in certain environments, due to their own legacies of coevolution with symbionts (as outlined above). Nonetheless, the incidence and causes of immunopathology in wild animals remain an important area for further research [14].
In the meantime, reported associations between immune phenotypes (e.g., white blood cell differential counts or cytokine gene expression data) and divergent life-history strategies are emerging. For example, neutrophil densities in the blood increase more rapidly with body size than do lymphocyte densities across terrestrial mammals, such that elephants have ~13 million-fold more neutrophils than a mouse, even though their body masses differ by only a factor of ~250 000 [95]. This is intriguing because body size is an important correlate of survival and reproductive strategies, in that larger organisms generally live longer and reproduce more slowly [80]. It will be fascinating to discover whether body size, or perhaps defensible surface area, is a strong general predictor of neutrophilia, and how body size or surface area (as proxies for parasite exposure risks [95]) and slow reproductive rate might interact [84] to shape the susceptibility of species such as elephants to cytokine storms or other immunopathologies. Likewise, variation among nonhuman primate species in cytokine responses to TLR stimulation [96] is intriguing but awaits evolutionary explanation.
Zoonoses offer 'natural experiments' in this type of comparative immunology. For example, deer mice are better at avoiding hantavirus-induced pulmonary immunopathology than humans are [97], and they also exhibit remarkable endotoxin tolerance [98]. As another example, researchers have hypothesized that bats may be especially tolerant of viruses because of their life history-strategies that are characterized by long lifespans despite small body sizes [99]. Indeed, bats have unusual immune systems that offer them constitutive IFN production [100] and avoidance of cytokine storms, even during Marburg virus infections [101]. We look forward to further research to explain these patterns.
Even within a species, individuals diverge markedly in their life-history strategy, and much of that heterogeneity is thought to be mediated by the immune system. A key axis to consider here is biological sex [102] (Figure 4B) because women are more prone to many immunopathologies than are men [103]. For example, at least 90% of patients treated for lupus are women [104], and Crohn's disease also disproportionately affects women, although incidence and severity of ulcerative colitis is greatest in men – an exception to the general rule of female-biased susceptibility to autoimmune disease [105]. Infection-induced immunopathology presents a more complex pattern of sex-dependence. This is partly because immunopathology can arise during persistent infections, and males are, in general, more susceptible to persistent infections [103]. For example, visceral leishmaniasis is found primarily in men [106], but experimental work in hamsters suggests that the inflammatory syndrome may ultimately be due to the failure of males to control parasite replication [107]. COVID-19 is another infectious disease that affects men more severely than women [108], though differences in the induced immune response rather than persistent viral load appear to be associated with differential susceptibility of the sexes to lung hyperinflammation [109]. Several mechanisms, including hormones and genetics, have been proposed to explain the differential susceptibility of the sexes to infections and immunopathology. For example, many immune genes are found on the X chromosome [103], and escape from X-chromosome inactivation necessary for dosage compensation has been implicated in lupus [110], although why immune genes should be concentrated on the X chromosome remains unclear.
Whatever the manifestations in contemporary populations, differences in immune function between sexes across the tree of life are likely to reflect different selection pressures associated with competing versus caring [111]. Mathematical analysis of sex-specific immunological optima in light of these life-history differences suggests that risk of infection during reproductive years and transfer of maternal antibodies are key explanations for sex differences in the sensitivity and specificity of immune responses in mammals [83]. Much work remains to be done, however, to discover the mechanistic causes of the divergence – for example, to better understand the sex differences in the acute and chronic immunopathologies of COVID-19 [109]. Furthermore, it is possible that the shapes of the trade-off curves between parasite clearance and immunopathology (e.g., as depicted in Figure 1) are not only sex-dependent but are also age- or lifestage-dependent. We look forward to the elucidation of these possibilities. Indeed, to obtain deeper evolutionary understanding of human susceptibility to immunopathology, it will be important to dissect the relative contributions of life-history strategies to the cytokine responsiveness of great apes in general [96], of humans in particular, and of males versus females across the lifespan.
Concluding remarks
Synthesis: simultaneous evolutionary legacies to explain human immunopathology
Evolutionary biology aims, above all, to explain variation, such as the origins and maintenance of biodiversity, including the causes of heterogeneity among species, populations, and individuals in crucial fitness-determining traits such as immune function. We have argued that legacies of multicellularity, history of coevolution with parasites, and life history may explain much of mammalian susceptibility to immunopathology.
In human history, natural selection for pathogen resistance is thought to have profound effects on modern human immune function [18,112], but the role of contingencies (e.g., population bottlenecks), chance, and other non-selective processes in the evolution of human immune function cannot be ignored. Especially compelling examples relevant to immunopathology include introgression from other hominins, small effective population sizes leading to persistence of deleterious mutations, and recent population expansions that amplify the frequency of previously rare variants; the impact of such demographic legacies on our susceptibility to immunopathology has been reviewed recently [51].
Indeed, adaptation by natural selection, chance, and history are fundamental drivers of biological variation, and robust analysis requires consideration of all three. We therefore posit that heterogeneity among organisms in susceptibility to immunopathology can best be explained in light of adaptation (e.g., to minimize the cost–benefit ratio for cytokine responses across different epidemiological settings), chance (e.g., the demographic history of hominins that affects the genes governing cytokine responsiveness), and history (e.g., the legacies of coevolution, multicellularity and life history that constrain optimization of cytokine response magnitude to maximize organismal survival). Evolutionary biology is arguably as central as molecular and cellular immunology to explaining the occurrence of human immunopathologies. We therefore propose a research vision that encompasses multiple biological scales of multiple species (Figure 5, Key figure) to maximize insight into the evolutionary origins and thus the varied occurrence of immunopathology across mammals. Solving the types of mysteries captured in the Outstanding questions might elucidate our fundamental understanding of evolutionary immunology in this context, and may ultimately inform medical interventions to maximize the power and minimize the peril of cytokine responses.
Figure 5.
Key figure. A vision for the value of investigating multiple biological scales across multiple species to elucidate the power and peril of mammalian immune systems.
Studying the cells of the mammalian immune system (e.g., neutrophils, dendritic cells, and B cells, from top left to bottom left) in model species such as mice (top middle) is essential for understanding the proximate causes of cytokine storms and other immunopathologies. However, we advocate several additions to this paradigm. Cross-scale analysis across host species to address not only organismal (middle column) but also population-scale (right column) factors will deepen insight considerably by revealing how history and chance as well as adaptation by natural selection have shaped the propensity of mammals to immunopathology. Watercolor interpretation provided by Matilda Luk.
Outstanding questions.
Are human hosts special in our predisposition to immunopathology, and are bats special in their avoidance of immunopathology? If so, why? Full evaluation and explanation of both inter- and intra-specific variation in mammalian susceptibility to immunopathology will not only elucidate basic biology but also inform management of zoonotic infections.
How has simultaneous selection by multiple parasite species (e.g., helminths and malaria parasites) shaped susceptibility to immunopathology? This could shed light on the net coevolutionary effect of opposing infectious pressures on the cytokine responsiveness of mammals.
To what extent might tissue-specific deployment of immune cells (e.g., resident macrophages) mitigate the peril posed by potent cytokine responses via spatial containment? Spatial compartmentalization within multicellular organisms may be an important check on the risk of overzealous cytokine responses.
Given that cell lifespans are nested within the lifespans and life-history strategies of the organisms that harbor those cells, what are the consequences of this nesting for cytokine responsiveness? Do the attributes of cytokine-producing cells scale consistently with size or age across mammalian species? Scaling laws have explanatory power in ecology and evolution in general, and thus may also inform evolutionary immunology.
What insights into the development of successful medical interventions arise from consideration of our long-lived, sexually dimorphic life history and our multicellularity?
Alt-text: Outstanding questions
Declaration of interests
The authors declare no conflicts of interest.
Glossary
- Bet-hedging
a multi-pronged strategy to mitigate risk in a variable environment; the opposite of putting all your eggs in one basket.
- Biological scales
units of organization in biology, ranging from molecules and cells at smaller spatial scales through organismal to the population scale and beyond.
- Collective decision-making
process of deciding among options (e.g., fight or flight; T helper cells 1 or 2) that involves multiple organisms or multiple cells rather than individual decision-makers.
- Constraint
an obstacle to achieving an end; for example genetic, resource, or engineering constraints can preclude achieving an optimal cytokine response.
- Familial Mediterranean fever
an inflammatory syndrome characterized by recurrent fever and pain that especially affects people of Mediterranean ancestry.
- Interspecific variation
differences among species; contrasts with intraspecific variation which describes differences among individuals within a species.
- Introgression from other hominins
DNA hybridization that brought allelic variants from Neanderthals and Denisovans into the genomes of modern humans as a result of interbreeding.
- Life-history strategy
the reproductive and survival schedule by which organisms of a species complete the life cycle; this is distinct from the exposure history or experience of individuals.
- Maladaptation
an apparent mismatch between the phenotype of an organism and its environment that is associated with reduced survival, reproduction, or both (i.e., reduced fitness).
- Mathematical modeling
the use of equations to capture the mechanisms, dynamics, and assumptions of complex biological systems. Modeling is used to test immunological hypotheses in silico that are too complex to test easily in vitro or in vivo in most empirical systems (including mammals).
- Natural selection
the evolutionary process by which organisms best adapted to their environment survive and reproduce best; contrasts with other mechanisms of evolution (i.e., gene frequency change) such as genetic drift, mutation, or organismal migration.
- Optimality analysis
formal, quantitative identification of the phenotype that maximizes the benefits while minimizing costs (of immune defense) in a given environment.
- Quorum sensing
a collective behavior in which groups of organisms (e.g., fish) or cells (e.g., bacteria or T cells) make decisions and express phenotypes if their density is sufficient; requires signaling mechanisms such as cytokine production.
- Reproductive effort
the energy, time, or other resources invested in generating and/or caring for offspring.
- Symbionts
multiple species that 'live together' closely; the ecological definition includes the full spectrum of relationships including parasites, commensals, mutualists, and more.
- X-chromosome inactivation
a mechanism whereby female mammals shut down one X chromosome in all cells except eggs, leading to equal expression of X chromosome gene products in XY males and XX females.
References
- 1.Schmitz J., et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Hodzic Z., et al. IL-33 and the intestine: the good, the bad, and the inflammatory. Cytokine. 2017;100:1–10. doi: 10.1016/j.cyto.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Editorial Cytokines in the balance. Nat. Immunol. 2019;20:1557. doi: 10.1038/s41590-019-0557-0. [DOI] [PubMed] [Google Scholar]
- 4.Germain R.N. The art of the probable: system control in the adaptive immune system. Science. 2001;293:240–245. doi: 10.1126/science.1062946. [DOI] [PubMed] [Google Scholar]
- 5.Amatya N., et al. IL-17 signaling: the Yin and the Yang. Trends Immunol. 2017;38:310–322. doi: 10.1016/j.it.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams G.C. Oxford University Press; 1992. Natural Selection: Domains, Levels, and Challenges. [Google Scholar]
- 7.Lande R. Quantitative genetic-analysis of multivariate evolution, applied to brain–body size allometry. Evolution. 1979;33:402–416. doi: 10.1111/j.1558-5646.1979.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 8.Parker G.A., Maynard-Smith J. Optimality theory in evolutionary biology. Nature. 1990;348:27–33. [Google Scholar]
- 9.Lynch M., Walsh B. Sinauer; 1998. Genetics and Analysis of Quantitative Traits. [Google Scholar]
- 10.Brady S.P., et al. Causes of maladaptation. Evol. Appl. 2019;12:1229–1242. doi: 10.1111/eva.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Germain R.N. Maintaining system homeostasis: the third law of Newtonian immunology. Nat. Immunol. 2012;13:902–906. doi: 10.1038/ni.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagai T., et al. Gene expression variability across cells and species shapes innate immunity. Nature. 2018;563:197–202. doi: 10.1038/s41586-018-0657-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergstrom C.T., Antia R. How do adaptive immune systems control pathogens while avoiding autoimmunity? Trends Ecol. Evol. 2006;21:22–28. doi: 10.1016/j.tree.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 14.Graham A.L., et al. Evolutionary causes and consequences of immunopathology. Ann. Rev. Ecol. Evol. Sys. 2005;36:373–397. [Google Scholar]
- 15.Viney M.E., et al. Optimal immune responses: immunocompetence revisited. Trends Ecol. Evol. 2005;20:665–669. doi: 10.1016/j.tree.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Sorci G., Faivre B. Inflammation and oxidative stress in vertebrate host–parasite systems. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2009;364:71–83. doi: 10.1098/rstb.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okin D., Medzhitov R. Evolution of inflammatory diseases. Curr. Biol. 2012;22:R733–R740. doi: 10.1016/j.cub.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinkworth J.F., Barreiro L.B. The contribution of natural selection to present-day susceptibility to chronic inflammatory and autoimmune disease. Curr. Opin. Immunol. 2014;31:66–78. doi: 10.1016/j.coi.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cliffe L.J., et al. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 20.Behnke J.M., et al. Understanding chronic nematode infections: evolutionary considerations, current hypotheses and the way forward. Int. J. Parasitol. 1992;22:861–907. doi: 10.1016/0020-7519(92)90046-n. [DOI] [PubMed] [Google Scholar]
- 21.Masure D., et al. The intestinal expulsion of the roundworm Ascaris suum is associated with eosinophils, intra-epithelial T cells and decreased intestinal transit time. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomasetti C., Vogelstein B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanno E., et al. Neutrophil-derived tumor necrosis factor-alpha contributes to acute wound healing promoted by N-(3-oxododecanoyl)-L-homoserine lactone from Pseudomonas aeruginosa. J. Dermatol. Sci. 2013;70:130–138. doi: 10.1016/j.jdermsci.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Oyler-Yaniv J., et al. TNF controls a speed–accuracy tradeoff in the cell death decision to restrict viral spread. Nat. Commun. 2021;12:2992. doi: 10.1038/s41467-021-23195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frank S.A., Schmid-Hempel P. Evolution of negative immune regulators. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shudo E., Iwasa Y. Dynamic optimization of host defense, immune memory, and post-infection pathogen levels in mammals. J. Theor. Biol. 2004;228:17–29. doi: 10.1016/j.jtbi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Urban M.C., et al. Asymmetric selection and the evolution of extraordinary defences. Nat. Commun. 2013;4:2085. doi: 10.1038/ncomms3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotas M.E., Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank S.A. Immune response to parasitic attack: evolution of a pulsed character. J. Theor. Biol. 2002;219:281–290. doi: 10.1006/jtbi.2002.3122. [DOI] [PubMed] [Google Scholar]
- 30.Chandra D., Naik S. Leishmania donovani infection down-regulates TLR2-stimulated IL-12p40 and activates IL-10 in cells of macrophage/monocytic lineage by modulating MAPK pathways through a contact-dependent mechanism. Clin. Exp. Immunol. 2008;154:224–234. doi: 10.1111/j.1365-2249.2008.03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conti H.R., et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J.J., et al. An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood. 2007;109:3794–3802. doi: 10.1182/blood-2005-09-010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun L., et al. IL-10 dampens an IL-17-mediated periodontitis-associated inflammatory network. J. Immunol. 2020;204:2177–2191. doi: 10.4049/jimmunol.1900532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerreiro R., et al. Correlational selection on pro- and anti-inflammatory effectors. Evolution. 2012;66:3615–3623. doi: 10.1111/j.1558-5646.2012.01708.x. [DOI] [PubMed] [Google Scholar]
- 35.Sorci G., et al. Benefits of immune protection versus immunopathology costs: a synthesis from cytokine KO models. Infect. Genet. Evol. 2017;54:491–495. doi: 10.1016/j.meegid.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Schrom E.C., et al. Immune signaling networks: sources of robustness and constrained evolvability during coevolution. Mol. Biol. Evol. 2018;35:676–687. doi: 10.1093/molbev/msx321. [DOI] [PubMed] [Google Scholar]
- 37.Strachan D.P. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moeller A.H., Sanders J.G. (2020) Roles of the gut microbiota in the adaptive evolution of mammalian species. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1808;375 doi: 10.1098/rstb.2019.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caruso R., et al. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 2020;20:411–426. doi: 10.1038/s41577-019-0268-7. [DOI] [PubMed] [Google Scholar]
- 41.Kearn G.C. Evolutionary expansion of the Monogenea. Int. J. Parasitol. 1994;24:1227–1271. doi: 10.1016/0020-7519(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 42.Park J.K., et al. A common origin of complex life cycles in parasitic flatworms: evidence from the complete mitochondrial genome of Microcotyle sebastis (Monogenea: Platyhelminthes) BMC Evol. Biol. 2007;7:11. doi: 10.1186/1471-2148-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark W.C. Origins of the parasitic habit in the nematoda. Int. J. Parasitol. 1994;24:1117–1129. doi: 10.1016/0020-7519(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 44.Blaxter M.L., et al. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- 45.Jackson J.A., et al. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology. 2009;126:18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smyth D.J., et al. TGF-beta mimic proteins form an extended gene family in the murine parasite Heligmosomoides polygyrus. Int. J. Parasitol. 2018;48:379–385. doi: 10.1016/j.ijpara.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maizels R.M., et al. Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity. 2018;49:801–818. doi: 10.1016/j.immuni.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hedl M., et al. The IL18RAP region disease polymorphism decreases IL-18RAP/IL-18R1/IL-1R1 expression and signaling through innate receptor-initiated pathways. J. Immunol. 2014;192:5924–5932. doi: 10.4049/jimmunol.1302727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fumagalli M., et al. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J. Exp. Med. 2009;206:1395–1408. doi: 10.1084/jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sironi M., Clerici M. The hygiene hypothesis: an evolutionary perspective. Microbes Infect. 2010;12:421–427. doi: 10.1016/j.micinf.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Dominguez-Andres J., Netea M.G. Impact of historic migrations and evolutionary processes on human immunity. Trends Immunol. 2019;40:1105–1119. doi: 10.1016/j.it.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goheen M.M., et al. The role of the red blood cell in host defence against falciparum malaria: an expanding repertoire of evolutionary alterations. Br. J. Haematol. 2017;179:543–556. doi: 10.1111/bjh.14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clatworthy M.R., et al. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park Y.H., et al. Ancient familial Mediterranean fever mutations in human pyrin and resistance to Yersinia pestis. Nat. Immunol. 2020;21:857–867. doi: 10.1038/s41590-020-0705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westra E.R., et al. Parasite exposure drives selective evolution of constitutive versus inducible defense. Curr. Biol. 2015;25:1043–1049. doi: 10.1016/j.cub.2015.01.065. [DOI] [PubMed] [Google Scholar]
- 56.Chen H., et al. Heterologous autoimmunity and prokaryotic immune defense. arXiv. 2021 arXiv:2101.01267. [Google Scholar]
- 57.Schrom E.C., Graham A.L. Instructed subsets or agile swarms: how T-helper cells may adaptively counter uncertainty with variability and plasticity. Curr. Opin. Genet. Dev. 2017;47:75–82. doi: 10.1016/j.gde.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 58.Segel L.A., Cohen I.R., editors. Design Principles for the Immune System and Other Distributed Autonomous Systems. Oxford University Press; 2001. [Google Scholar]
- 59.Gajanayaka N., et al. TLR-4 agonist induces IFN-gamma production selectively in proinflammatory human M1 macrophages through the PI3K-mTOR- and JNK-MAPK-activated p70S6K pathway. J. Immunol. 2021;207:2310–2324. doi: 10.4049/jimmunol.2001191. [DOI] [PubMed] [Google Scholar]
- 60.Colombo S.A.P., et al. Defective interferon-gamma production is common in chronic pulmonary aspergillosis. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab583. https://d10.1101/10.1093/infdis/jiab583 Published online November 29, 2021. [DOI] [PubMed] [Google Scholar]
- 61.Kagan J.C. Lipopolysaccharide detection across the kingdoms of life. Trends Immunol. 2017;38:696–704. doi: 10.1016/j.it.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kato Y., et al. Effects of thrombomodulin in reducing lethality and suppressing neutrophil extracellular trap formation in the lungs and liver in a lipopolysaccharide-induced murine septic shock model. Int. J. Mol. Sci. 2021;22:4933. doi: 10.3390/ijms22094933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yousif A.S., et al. The persistence of interleukin-6 is regulated by a blood buffer system derived from dendritic cells. Immunity. 2021;54:235–246. doi: 10.1016/j.immuni.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rumpret M., et al. Functional categories of immune inhibitory receptors. Nat. Rev. Immunol. 2020;20:771–780. doi: 10.1038/s41577-020-0352-z. [DOI] [PubMed] [Google Scholar]
- 65.Yates A., et al. Combining cytokine signalling with T-bet and GATA-3 regulation in Th1 and Th2 differentiation: a model for cellular decision-making. J. Theor. Biol. 2004;231:181–196. doi: 10.1016/j.jtbi.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 66.Schrom E.C., 2nd, et al. Quorum sensing via dynamic cytokine signaling comprehensively explains divergent patterns of effector choice among helper T cells. PLoS Comput. Biol. 2020;16 doi: 10.1371/journal.pcbi.1008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Antonioli L., et al. Rethinking communication in the immune system: the quorum sensing concept. Trends Immunol. 2019;40:88–97. doi: 10.1016/j.it.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marsland R., 3rd, et al. Tregs self-organize into a computing ecosystem and implement a sophisticated optimization algorithm for mediating immune response. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2011709118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang M., et al. Stochastic cytokine expression induces mixed T helper cell states. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferreira C., et al. Non-obese diabetic mice select a low-diversity repertoire of natural regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 2009;106:8320–8325. doi: 10.1073/pnas.0808493106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duneau D., et al. Stochastic variation in the initial phase of bacterial infection predicts the probability of survival in D. melanogaster. eLife. 2017;6 doi: 10.7554/eLife.28298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tate A.T., et al. The within-host dynamics of infection in trans-generationally primed flour beetles. Mol. Ecol. 2017;26:3794–3807. doi: 10.1111/mec.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Georgel P., et al. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev. Cell. 2001;1:503–514. doi: 10.1016/s1534-5807(01)00059-4. [DOI] [PubMed] [Google Scholar]
- 74.Van Eyndhoven L.C., et al. Decoding the dynamics of multilayered stochastic antiviral IFN-I responses. Trends Immunol. 2021;42:824–839. doi: 10.1016/j.it.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 75.van Leeuwen A., et al. (2019) Parasite resource manipulation drives bimodal variation in infection duration. Proc. Biol. Sci. 1902;286 doi: 10.1098/rspb.2019.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ellner S.P., et al. (2021) Host-pathogen immune feedbacks can explain widely divergent outcomes from similar infections. Proc. Biol. Sci. 1951;288 doi: 10.1098/rspb.2021.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oyler-Yaniv A., et al. A tunable diffusion-consumption mechanism of cytokine propagation enables plasticity in cell-to-cell communication in the immune system. Immunity. 2017;46:609–620. doi: 10.1016/j.immuni.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Metcalf C.J.E., et al. Disentangling the dynamical underpinnings of differences in SARS-CoV-2 pathology using within-host ecological models. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grant R.A., et al. Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia. Nature. 2021;590:635–641. doi: 10.1038/s41586-020-03148-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stearns S.C. Oxford University Press; 1992. The Evolution of Life Histories. [Google Scholar]
- 81.Sheldon B.C., Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- 82.Metcalf C.J.E., et al. Demographically framing trade-offs between sensitivity and specificity illuminates selection on immunity. Nat. Ecol. Evol. 2017;1:1766–1772. doi: 10.1038/s41559-017-0315-3. [DOI] [PubMed] [Google Scholar]
- 83.Metcalf C.J.E., Graham A.L. Schedule and magnitude of reproductive investment under immune trade-offs explains sex differences in immunity. Nat. Commun. 2018;9:4391. doi: 10.1038/s41467-018-06793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Downie, A.E. et al. Optimal immune specificity at the intersection of host life history and parasite epidemiology. BioRxiV. Published online March 12, 2021. https://d10.1101/10.1101/2021.03.11.434955 [DOI] [PMC free article] [PubMed]
- 85.Mayer A., et al. Diversity of immune strategies explained by adaptation to pathogen statistics. Proc. Natl. Acad. Sci. U. S. A. 2016;113:8630–8635. doi: 10.1073/pnas.1600663113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cressler C.E., et al. (2015) Evolution of hosts paying manifold costs of defence. Proc. Biol. Sci. 1804;282 doi: 10.1098/rspb.2015.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmid-Hempel P. Oxford University Press; 2012. Evolutionary Parasitology. [Google Scholar]
- 88.Pruss H., et al. Anti-NMDA receptor encephalitis in the polar bear (Ursus maritimus) Knut. Sci. Rep. 2015;5:12805. doi: 10.1038/srep12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gulland F.M.D., et al. Nematode (Otostrongylus circumlitus) infestation of northern elephant seals (Mirounga angustirostris) stranded along the central California coast. Mar. Mammal Sci. 1997;13:446–459. [Google Scholar]
- 90.Henson L.H., et al. Characterization of genetic variation and basis of inflammatory bowel disease in the Toll-like receptor 5 gene of the red wolf and the maned wolf. Endang. Species Res. 2017;32:135–144. [Google Scholar]
- 91.Schulte-Hostedde A.I., Mastromonaco G.F. Integrating evolution in the management of captive zoo populations. Evol. Appl. 2015;8:413–422. doi: 10.1111/eva.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wood J.D., et al. Colitis and colon cancer in cotton-top tamarins (Saguinus oedipus oedipus) living wild in their natural habitat. Dig. Dis. Sci. 1998;43:1443–1453. doi: 10.1023/a:1018842210330. [DOI] [PubMed] [Google Scholar]
- 93.Seeley K.E., et al. A survey of diseases in captive red wolves (Canis rufus), 1997–2012. J. Zoo Wildl. Med. 2016;47:83–90. doi: 10.1638/2014-0198.1. [DOI] [PubMed] [Google Scholar]
- 94.Terio K.A., et al. Characterization of the gastric immune response in cheetahs (Acinonyx jubatus) with Helicobacter-associated gastritis. Vet. Pathol. 2012;49:824–833. doi: 10.1177/0300985811412620. [DOI] [PubMed] [Google Scholar]
- 95.Downs C.J., et al. The effects of body mass on immune cell concentrations of mammals. Am. Nat. 2020;195:107–114. doi: 10.1086/706235. [DOI] [PubMed] [Google Scholar]
- 96.Hawash M.B.F., et al. Primate innate immune responses to bacterial and viral pathogens reveals an evolutionary trade-off between strength and specificity. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2015855118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schountz T., et al. Regulatory T cell-like responses in deer mice persistently infected with Sin Nombre virus. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15496–15501. doi: 10.1073/pnas.0707454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Balderrama-Gutierrez G., et al. An infection-tolerant mammalian reservoir for several zoonotic agents broadly counters the inflammatory effects of endotoxin. mBio. 2021;12 doi: 10.1128/mBio.00588-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Irving A.T., et al. Lessons from the host defences of bats, a unique viral reservoir. Nature. 2021;589:363–370. doi: 10.1038/s41586-020-03128-0. [DOI] [PubMed] [Google Scholar]
- 100.Zhou P., et al. Contraction of the type I IFN locus and unusual constitutive expression of IFN-α in bats. Proc. Natl. Acad. Sci. U. S. A. 2016;113:2696–2701. doi: 10.1073/pnas.1518240113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guito J.C., et al. Asymptomatic infection of Marburg virus reservoir bats is explained by a strategy of immunoprotective disease tolerance. Curr. Biol. 2021;31:257–270. doi: 10.1016/j.cub.2020.10.015. [DOI] [PubMed] [Google Scholar]
- 102.Zuk M. Reproductive strategies and disease susceptibility: an evolutionary viewpoint. Parasitol. Today. 1990;6:231–233. doi: 10.1016/0169-4758(90)90202-f. [DOI] [PubMed] [Google Scholar]
- 103.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 104.Weckerle C.E., Niewold T.B. The unexplained female predominance of systemic lupus erythematosus: clues from genetic and cytokine studies. Clin. Rev. Allergy Immunol. 2011;40:42–49. doi: 10.1007/s12016-009-8192-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goodman W.A., et al. Sex matters: impact on pathogenesis, presentation and treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020;17:740–754. doi: 10.1038/s41575-020-0354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cloots K., et al. Male predominance in reported visceral Leishmaniasis cases: nature or nurture? A comparison of population-based with health facility-reported data. PLoS Negl. Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0007995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Travi B.L., et al. Gender is a major determinant of the clinical evolution and immune response in hamsters infected with Leishmania spp. Infect. Immun. 2002;70:2288–2296. doi: 10.1128/IAI.70.5.2288-2296.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peckham H., et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takahashi T., et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Collison J. X-chromosome inactivation altered in SLE. Nat. Rev. Rheumatol. 2019;15:318. doi: 10.1038/s41584-019-0226-6. [DOI] [PubMed] [Google Scholar]
- 111.Stoehr A.M., Kokko H. Sexual dimorphism in immunocompetence: what does life-history theory predict? Behav. Ecol. 2006;17:751–756. [Google Scholar]
- 112.Quintana-Murci L. Human immunology through the lens of evolutionary genetics. Cell. 2019;177:184–199. doi: 10.1016/j.cell.2019.02.033. [DOI] [PubMed] [Google Scholar]