Abstract
Many water systems are challenged with uncertainty regarding service line material type. This work investigated using a simple drinking water flushed sampling approach and a more complicated and invasive sequential profile sampling approach to predict whether homes had an existing lead service line (LSL). Homes that never had an LSL (control groups) and homes with LSLs (study groups) in two communities having different degrees of corrosion control were sampled. Using control groups’ results, community-specific “threshold” lead levels were determined and compared to results from study groups. The flushed sampling maximum lead concentration (FMC) of lead accurately predicted 100% and 60% of LSL sites for the community with poor and good corrosion control, respectively. The weighted average sequential profile lead concentration (WASLC) increased the 40% not identified as LSL sites by fully flushed samples to 100%. The WASLC closely followed by the maximum sequential profile lead concentration were most reliable in identifying LSLs.
Keywords: drinking water, flush profile, identification, lead service line, sequential profile
1 |. INTRODUCTION
Lead is a hazardous neurotoxin that can irreversibly affect the intellectual development in children, even at blood lead levels (BLLs) as low as 5 μg/dl (Deshommes et al., 2013; Lanphear et al., 2002). Recent studies have identified increases in BLLs with the ingestion of average daily water concentrations of under 5 μg/L (Ngueta et al., 2014, 2015, 2016). Studies have also linked waterborne lead exposure even at low levels with an increase in blood pressure in the general population, as well as increased cardiovascular disease mortality (Fewtrell et al., 2003; Menke et al., 2006; Triantafyllidou & Edwards, 2012).
Direct sources of lead in drinking water include lead service lines (LSLs; lead communication pipes or lead service branches; nearly 100% lead by weight), galvanized iron pipe zinc coating (<1.4% lead by weight), lead-tin solder (40–50% lead by weight), and plumbing fixtures and fittings made from or which include various brass alloys (<8% lead by weight) (ASTM, 2017a, 2017b; AWWARF, 1990; Sandvig et al., 2008; Schock, 1990; McFarren et al., 1977; Trussell & Wagner, 1996). Recent work has shown that lead in water could also indirectly come from the release of lead accumulated within corrosion byproduct scales (Maynard & Wasserstrom, 2017; McFadden et al., 2011). The accumulated lead may potentially be an even bigger source of lead than the fresh zinc coating when the old galvanized pipes are downstream of LSLs or goosenecks (Maynard & Wasserstrom, 2017; McFadden et al., 2011).
The concentration of lead measured in drinking water collected from a residential faucet depends on many factors (Britton & Richards, 1981; Schock, 1990; Schock & Lytle, 2011; Triantafyllidou et al., 2021). These factors include water volume, location, and exposed internal pipe lead surfaces; duration and frequency of stagnation periods; the amount of water used between stagnation periods; the configuration of the plumbing network; the types and locations of different types of service line (SL) piping; the type of connections between sections of pipe; the location of the sampled tap and volume of water sampled; the water corrosivity and rate of metal mobilization and release (such as oxidation rates or radial and longitudinal diffusion of ions); the age of the plumbing water contacting surfaces; water temperature; tendency of pipe scales to release particulate materials; and the amount and timing of physical/hydraulic disturbances.
Given that multiple lead sources may be present, and the complex set of factors that can influence lead release, it is necessary to understand drinking water lead source inventories within a community. LSLs, when present, almost always represent the largest source of lead mass to drinking water. As a result, there are many important reasons for a water system or a public health agency to know how many LSLs are present in a drinking water system and where they are located. Aside from current and potential state or federal regulatory drivers, there is the important public health goal of having the ability to inform a resident of the existence of an actual LSL that is a contamination risk for their drinking water. LSLs can contribute as much as 50%–75% of the total lead at the tap after an extended stagnation time (Cartier et al., 2011; Lytle et al., 2019; Sandvig et al., 2008), and logically, bottle-fed infants will potentially have a very high relative exposure from drinking water used to make formula where LSLs are present (Stanek et al., 2020; Zartarian et al., 2017). LSL replacement remains the most effective long-term solution to eliminate likely the largest risk of lead exposure from drinking water (Cornwell et al., 2021; Environmental Protection Agency, 2021; Stanek et al., 2020). However, replacing the existing legacy of the estimated 6–10 million or more LSLs (Cornwell et al., 2016; e-CFR Electronic Code of Federal Regulations, 2019; Weston, 1990) is expected to come with significant expense over a long period of time. Thus, identifying locations of LSLs is an important priority for any drinking water system and community. Some states require water systems to catalog the location of LSLs (Environmental Defense Fund, 2019), and effectively identifying LSLs will improve the efficiency of LSL removal programs.
Sequential (profile) sampling can be used to identify maximum lead concentrations after stagnation, validate corrosion control efficacy, and improve exposure assessment (AWWARF, 1990; Del Toral et al., 2013; Doré et al., 2019; Health Canada, 2009; Lytle et al., 2019; MDDELCC, 2014; Schock et al., 2019; Schock & Lytle, 2011; Vaccari, 1994). Sequential sampling has also been used to confirm the presence of an LSL as well as identifying other in-line lead sources, when coupled with plumbing size and length information (Cardew, 2006; Clark et al., 2015; Deshommes et al., 2016, 2018; Doré et al., 2019; Lytle et al., 2019; Pieper et al., 2015; Sandvig et al., 2008; Schock et al., 2019; Schock & Lytle, 2011; Trueman et al., 2016). Sequential sampling in a home consists of the collection of a series of consecutive samples (typically 500–1000 mL) from the kitchen tap following flushing and a standardized stagnation period (>6 h). Water volumes correlate to pipe and plumbing component lengths provided associated diameters are known. The number of sequential samples needed depends upon the length and inside diameter of piping from the kitchen tap to the main and the level of resolution desired in detecting in-line lead sources. The smaller the sample volumes, the more detail; smaller volumes necessitate more samples and more time at the residence to perform sample collection. Typically, observed peak lead concentrations correspond to water having stagnated in the LSL and other in-line lead sources. Sequential sampling is relatively invasive to the resident, and complex to accomplish, but may be most informative when used to identify lead plumbing sources.
Notably, several simpler and less invasive water sampling and analysis approaches have been demonstrated to predict whether a home has an LSL. For example, what has come to be known as the “Montreal protocol” combines characteristics of a fully flushed sample as well as some short-term amplification of the lead concentration through the collection of the second liter after a 15-min stagnation time (Cartier et al., 2012). A community-specific threshold lead concentration based on baseline sampling of homes having LSLs was established above which there was a high certainty of the presence of an LSL. The LSL identification threshold concentration level for the Montreal protocol was 3 μg/L. Other examples of LSL identification protocols include a flowing sampling protocol used by the cities of Ottawa and Guelph that used an LSL identification threshold concentration of 1 and 5 μg/L, respectively (Douglas et al., 2007; Muylwyk et al., 2011). Alternatively, a protocol that consists of random daytime sampling that includes a 2.5 μg/L LSL identification threshold concentration (Hayes, 2009) was developed in the United Kingdom. Finally, a French protocol that used a 5 μg/L LSL identification threshold upon a 1 L water sample collected after a 30-min stagnation period (MDDELCC, 2014) was identified.
As part of the response of the U.S. Environmental Protection Agency (U.S. EPA) to the water crisis in Flint, MI, the U.S. EPA recommended a set of research studies to develop important tools to address the contributions of different leaded plumbing materials to drinking water lead exposure risk, including development of LSL detection methodology (U. S. EPA Flint Task Force, 2015). Furthermore, the need to identify where LSLs exist is of great national importance in efforts to reduce public health risks associated with lead exposure from drinking water. As a result, the primary objective of this study was to evaluate and refine an approach for identifying LSLs, based on a combination of the tap water sampling LSL identification protocols in two communities with different water chemistries and treatment histories. Specifically, this work investigated the feasibility of using simple flushed sampling (evaluated under low, medium, and high flow rates) to predict whether a given site had an existing LSL. Second, a more complicated and invasive but potentially more accurate sequential sampling approach was also explored to identify the presence of LSLs that were not captured by the flushed sampling methodology. Finally, the accuracy of flushed and sequential samples to identify the presence of an LSL was compared to first-draw samples and water samples collected under a modified Montreal protocol. Comparing multiple water sampling approaches will ultimately lead to the proposal of a water sampling-based LSL identification methodology that considers accuracy, complexity, costs, and other factors. The outcome will help water utilities develop a water sampling approach that facilitates relatively rapid and cost-effective city-wide screening to detect LSLs in locations where the SL makeup is unknown and potentially avoid the need to employ excavation to conclusively identify unknown SL materials.
2 |. MATERIALS AND METHODS
2.1 |. Sampling sites and schedule
Water samples were collected at homes in City 1, IL and City 2, MI, both of which provided the research team with a list of houses that never had a lead service line (control groups, Table S1) and a priority list of houses scheduled for LSL replacement (study groups with known LSLs, Table 1). The general background water chemistry of the two communities was provided in Table S2. In City 1, seven control houses (copper SLs to copper or plastic internal home plumbing) were sampled in the fall of 2017. Twelve study houses with LSLs scheduled for full replacement were sampled in the summer of 2017 and 11 of those same study houses were resampled about 7.5 weeks after the LSL replacement. Flushed sampling in 4 of the 12 study houses were conducted during the winter of 2017 (described in Section 2.2) to see the impact of seasonal water factors. Collecting the control set of samples in City 1 in winter months when water is colder and water quality is expected to be less corrosive may result in relatively lower lead levels. In City 2, 12 control houses were sampled in the spring of 2017. Ten study houses with LSLs scheduled for full replacement were sampled in the summer of 2017 and seven of those same houses were resampled about 7.5 weeks after the LSL replacement. Although the ideal procedure is to collect data from the control groups during the same season in proximity to collecting samples from the study groups, it was not logistically possible in this project to synchronize the sampling.
TABLE 1.
Summary of house plumbing information for both the City 1, IL (house ID: GS#) and City 2, MI (house ID: FS#) sites that had lead service lines
| House ID | Private-side galvanized replaced (ft/m) | Total LSL replaced (ft/m) | Internal plumbing |
|
|---|---|---|---|---|
| Basement | Under kitchen sink | |||
| GS1 | NA | 37.5/11.4 | Pb pipe to galvanized steel fittings to brass meter to Cu fittings to brass fittings to PVC pipe | PVC pipe to Cu/chrome tubing |
| GS2 | NA | 36/11 | Pb pipe to Pb fixtures to brass meter to GSP | PVC pipe to flex poly tubing |
| GS3 | NA | 88/27 | Pb pipe to brass fittings to brass meter to PVC pipe | Cu pipe to flex plastic hose |
| GS4 | NA | 44/13 | Pb pipe to brass meter to galvanized steel fixtures to Cu pipe | Cu pipe to metal poly braided tubing |
| GS5 | NA | 40/12 | Pb pipe to galvanized steel fittings to brass fittings to brass meter to Cu pipe | Metal flex poly tubing |
| GS6 | NA | 36/11 | Pb pipe to brass fittings to brass meter to GSP | Cu pipe to plastic poly tubing |
| GS7 | NA | 85/26 | Pb pipe to brass fittings to brass meter to galvanized steel pipe | Cu pipe to metal poly tubing |
| GS8 | NA | 41/12.5 | Pb pipe to brass fittings to GSP | Cu pipe to plastic flex tubing |
| GS9 | NA | 28/8.5 | Pb pipe to brass fittings to new meter to brass fittings to PVC pipe | Cu pipe to poly metal braided tubing |
| GS10 | NA | 78/24 | Pb pipe to galvanized steel fittings to brass meter to GSP | Cu pipe to plastic flex tubing |
| GS11 | NA | 82/25 | Pb pipe to brass fittings to galvanized steel pipe | Cu pipe to plastic flex tubing |
| GS12 | NA | 78/24 | Pb pipe to brass fittings to GSP | PVC pipe to plastic flex tubing |
| FS1 | 0/0 | 83/25 | Cu pipe to new plastic meter to blue PVC pipe | Cu pipe to plastic tubing |
| FS2 | 41/12 | 55/17 | GSP to Cu fittings to old brass meter to PVC pipe | PVC pipe to braided poly tubing |
| FS3 | 32/10 | 76/23 | GSP to brass fittings to plastic meter to Cu pipe | Cu pipe to plastic flex tubing |
| FS4 | 40/12 | 48/15 | GSP to galvanized fixtures to plastic meter to PVC pipe | PVC pipe to Cu pipe |
| FS5 | 42/13 | 48/15 | GSP to plastic meter to brass fittings to Cu pipe | Cu pipe to metal braided poly tubing |
| FS6 | 0/0 | 2/0.6 | Cu pipe to old brass meter to GSP | Cu pipe to plastic flex tubing |
| FS7 | 0/0 | 36/11 | Cu pipe to old brass meter to Cu pipe | PVC pipe to plastic braided poly tubing |
| FS8 | 0/0 | 12/4 | Cu pipe to brass fittings to new plastic meter to PVC pipe | PVC pipe to metal braided poly tubing |
| FS9 | 46/14 | 59/18 | GSP to galvanized fixtures to brass meter to PVC pipe | PVC pipe to smaller PVC pipe |
| FS10 | 0/0 | 8/2 | Cu pipe to brass meter to GSP | GSP to plastic braided poly tubing |
Abbreviations: Cu, copper; GSP, galvanized steel pipe; LSL, lead service line; NA, not available; Pb, lead; PVC, polyvinyl chloride.
2.2 |. Sequential and flushed sampling protocol
The research team set up appointments with residents to solicit involvement, perform sampling, and provide written and verbal presampling instructions. The instructions included bypassing all point-of-entry filters, removing the point-of-use filter at the kitchen tap, removing the aerator, and then performing a 5-min prestagnation flush before the start of the minimum 6-h stagnation period.
Residents were instructed not to use any water throughout the household (no showering, washing clothes/dishes, flushing toilets, use of outdoor hose bibs or irrigation systems, etc.) during the stagnation period. To ensure the instructions were followed and the house was occupied prior to the sampling event, the field sampling team dropped off written instructions at the homes 24 h before the appointment.
Upon arrival at the house for sampling, the sampling team confirmed with the homeowner that the prestagnation flush was performed and that the minimum 6-h stagnation period was followed before sampling. The sampling team documented the SL material entering the house, the material of the exposed internal plumbing, and the meter reading. The plumbing material types under the kitchen sink were also recorded. Sequential sampling was conducted, consisting of collecting 13–18 bottles; two 125 ml wide-mouth HDPE [Thermo Scientific Nalgene] bottles at the start followed by up to sixteen 1-L wide-mouth HDPE bottle [Thermo Scientific Nalgene] samples representing the pipe length extending from the kitchen tap to the water main in the street. Fifteen samples were collected if the water main was on the same side of the street and 18 samples were collected if the water main was across the street. The cold water tap was slowly opened to collect the 125-ml samples then opened all the way to collect the 1-L samples. The sequential bottles were carefully filled so that no water was spilled from the tap.
After sequential sampling, the tap was adjusted to fully opened for the collection of fully flushed samples. After flushing for 5 min, a 1-L sample was collected at a high flow of approximately 6 L/min, representing the high flush (or fully flushed) sample. The flow rate was then reduced to a medium flow of approximately 4 L/min and a 1-L sample (medium flow sample) was collected after flushing for 5 min. The flow rate was finally reduced to a low flow of approximately 3 L/min and a 1-L sample (low flow sample) was collected after flushing for 6 min.
2.3 |. Water sample analytical analysis
Samples were field preserved by the sampling team using 0.15% v/v nitric acid (Ultra-Pure Fisher Scientific). After preservation, the pH was checked with pH paper strips (Fisher Scientific, 13–640–511) to confirm that the pH was less than 2.0. Samples were analyzed using inductively coupled plasma mass spectrometry for total recoverable lead per EPA Method 200.8 (EPA, 1994). The laboratory’s reporting limit (RL) for the total lead was 0.5 μg/L.
2.4 |. Statistical analysis
The figures and statistics were generated in the SigmaPlot 14.0 Notebook (Systat Software, Inc, San Jose, CA). An alpha value (α) of 0.05 was used to calculate the statistical significance. For water samples containing lead levels below RL, the RL of 0.5 μg/L was used for graphic and data analysis purposes. To compare the lead concentrations among different groups (e.g., different flow rates, control and study houses, houses before and after LSL removal, different seasons), t-test, Mann–Whitney, or Wilcoxon signed-rank test was used depending on the normality of the data. A p-value of >.05 from a Shapiro–Wilk test indicates normally distributed data and t-test was used; otherwise, Mann–Whitney or Wilcoxon test was used for non-normally distributed data (Shapiro–Wilk test, p < .05).
3 |. RESULTS
For water sampling to be useful as an LSL identification tool, lead concentration differences between water samples collected from homes with and without LSLs must be as large as possible to increase confidence in LSL presence prediction. Drinking water lead concentrations in flushed samples collected at three flow rates from homes with known LSLs and homes that never had LSLs were compared to identify differences. The flow rate could be important for two reasons. First, if the interior pipe scales are physically stable and the lead release is radial diffusion controlled, lower lead concentrations would be generated by increasing flow rates, because of the decreased contact time between the water and the scale (Kuch & Wagner, 1983; Van Der Leer et al., 2002). However, if the scale material has the tendency to release colloidal or particulate material during water flow, an inverse pattern of higher total lead with an increasing flow rate would be observed. Which effect dominates may be specific to an individual water system and its pipe scales.
The following sections first present the flushed sampling results at low- to high flow rates for both City 1 and City 2, followed by the sequential sampling results during LSL pre-replacement and post-placement for both communities. Within the city-specific results, results from the control homes that never had LSLs were also compared.
3.1 |. Flushed sampling water lead levels and LSL detection
Flushed samples were collected at high (6 L/min), medium (4 L/min), and low (3 L/min) flow rates, in both control and study groups, to determine whether flow rate impacted water lead levels. The statistical examination of the winter (colder weather) and summer (warmer weather) flushed sampling for four houses in City 1’s study group showed that the lead concentrations in cold weather were considerably lower than those in warmer weather (Mann–Whitney rank sum test, p ≤ .012; data not shown). Consequently, the winter flushed sample results were excluded from analyses and discussions below. The observation supports the importance of seasonal considerations to the success of water sampling-based LSL identifications.
3.1.1 |. City 1 flushed sample water lead levels
In City 1, the control group of seven homes that had never had an LSL was sampled and all of them had flushed samples containing lead below the RL (BRL) of 0.5 μg/L at any flow rate tested (Figure 1a). The flushed sampling before and after LSL replacement was performed at 11 LSL-containing houses (an additional house was only sampled pre-LSL replacement due to concerns over adherence to sampling protocol). In comparison, the LSL pre-replacement lead concentrations ranged from 0.8 to 25 μg/L and were always detectable regardless of flow rate (Figure 1b). The data support the conclusion that locations with flushed water samples above the lead RL indicated the presence of an LSL, whereas flushed samples below 0.5 μg/L indicated a house that likely did not currently have an LSL, and possibly never had one. Flushed samples collected from the same homes for all flow rates following LSL replacement ranged from BRL to 4.7 μg/L (Figure 1c). Despite full LSL removal, low lead levels in nearly all fully flushed water samples were still detected, although at considerably lower levels than pre-LSL removal (Wilcoxon signed-rank test, p < .001) but greater than the control group (Mann–Whitney rank sum test, p < .001). On average, there was an approximately 80% decrease in lead concentrations for 10 out of the 11 homes after LSL removal across all flow rates with each flow rate demonstrating similar decreases. The only exception was home GS10 in which the lead levels increased by 187% and 34% at medium and high flow, respectively. The results suggest that after the LSL was removed, accumulated remnant particulate lead from the LSL remained in the premise plumbing or other lead sources including lead solders, leaded fixtures, and/or galvanized iron pipes may have been disturbed during extraction contributing lead to water. The contamination from remnant particulate lead or other lead sources could place some limitations on the accuracy of determining if an LSL was present at one time and the records documenting the replacement had been lost by creating a “false positive” conclusion.
FIGURE 1.
City 1, IL flushed sample lead concentrations at high, medium, and low flow rates for the control group (a), pre-lead service line replacement (b), and post-lead service line replacement (c). The dashed reference line was the reporting limit of 0.5 μg/L. NA indicated no post-replacement flush sampling for house GS12
3.1.2 |. City 2 flushed sample water lead levels
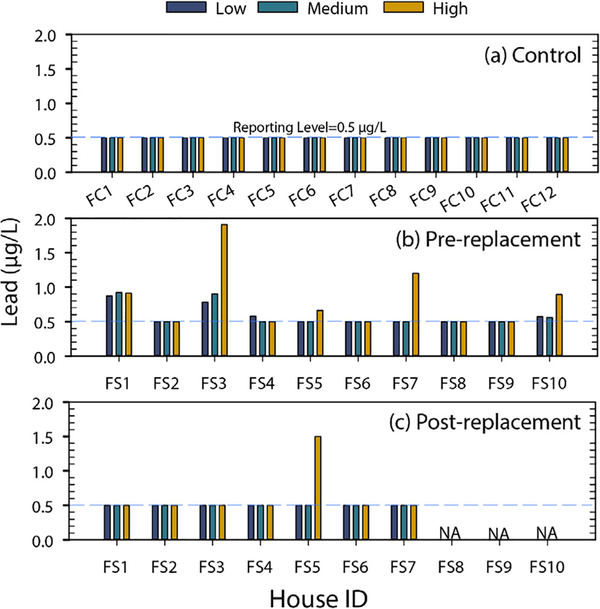
In City 2, the control group of 12 houses that had never had an LSL and no visible galvanized pipe had water lead levels of BRL in all but one sample (Figure 2a). The sample that contained lead above the RL was just 0.56 μg/L at high flow for house FC8. Flush profile sampling before and after LSL replacement was performed at seven LSL-containing houses (three additional houses were only sampled pre-LSL replacement due to limited participation from homeowners). The water lead levels in LSL pre-replacement flushed samples at all flow rates were generally low; only 2 of the 30 samples had greater than 1 μg/L lead and 18 of the 30 samples were BRL (Figure 2b). For the seven post-LSL replacement houses, only 1 of the 21 samples had a detectable lead concentration of 1.5 μg/L in house FS5 at a high flow (Figure 2c). Across the three categories of houses sampled in City 2, lead concentrations were either below or just above that RL (>0.5 μg/L). Due to very few detectable lead results in collected water samples, it is difficult to draw qualitative and meaningful conclusions from statistical analyses regarding the potential impact of LSL replacement and/or flow rate. The lead levels in City 2 were lower compared to City 1 during even challenging water flow rates, reflecting a relatively stable scale and more effective corrosion control treatment. Consequently, this study illustrated the site specificity of the approach and predictable potential shortcoming of the flush profile to detect LSLs under conditions of very good corrosion control.
FIGURE 2.
City 2, MI flushed sample lead concentrations at high, medium, and low flow rates for the control group (a), pre-lead service line replacement (b), and post-lead service line replacement (c). The dashed reference line was the reporting limit of 0.5 μg/L. NA indicated no post-replacement flush sampling for houses FS8, FS9, and FS10
3.1.3 |. Impact of flow rate on flushed sample water lead levels
Graphical and statistical examination of the data at low to high flow rates in both cities’ test homes showed that when LSLs were present, most often the trend was toward higher lead levels in water collected under higher flow rates (Figures 1 and 2). Conceptually, the lead level should decrease with a higher flow rate if the lead release is in the dissolved form and controlled by radial diffusion (Kuch & Wagner, 1983; Van Der Leer et al., 2002). This observation was not consistent with the diffusion-controlled dissolved lead release but rather indicative of the probable contribution of particulate lead, originating from shearing or sloughing of scale material from the LSLs, other premise plumbing lead-containing materials, or both. Therefore, the most sensitive analysis for detecting LSLs in these systems was through the use of the highest flow rate data of the fully flushed samples to define the flushed sampling maximum lead concentration (FMC); (Tables S4 and S5). Furthermore, the statistical examination of the winter versus summer sampling events as previously noted suggested that lead concentrations in warmer weather were significantly higher, and it is important to assure that the sampling-based screening study must conduct both control group and study group analyses as contemporaneously as possible to achieve optimal results.
3.2 |. Sequential sampling water lead levels and LSL detection
Sequential samples (including two 125 mL samples, and eleven, thirteen, or sixteen 1-L samples depending on the house) were collected at control and study houses from City 1 (Figures S1, 3) and City 2 (Figures S2, 4). In each sequential profile, the amplitude of the signal was the lead concentration for each incremental interval of the sample volumes (AWWARF, 1990; Del Toral et al., 2013; Lytle et al., 2019; Vaccari, 1994).
3.2.1 |. Sequential sample water lead levels in control and study houses in City 1 and City 2
For the 16 sampled control houses in both communities, all sequential samples were relatively low; 86% (191 out of 222) of the water samples were BRL (<0.5 μg/L), with a maximum level at 4.65 μg/L in the first draw of 125-mL sample from house FC4 (Figures S1 and S2). Control sequential samples were very similar between City 1 and City 2 samples despite differences in flushed sample lead concentration results.
In contrast, very few sequential sampling samples were BRL in pre-replacement LSL homes and many lead concentrations were elevated. In City 1, only 0.5% (1 out of 210) of the pre-LSL replacement sequential samples were below 0.5 μg/L lead with a maximum level at 233 μg/L (Figure 3). The state of system corrosion control was clearly revealed in the sequential sampling of LSL houses as City 1 lead detections were notably higher than in City 2 (Figures 3 and 4). In City 2, 9% of the 159 pre-LSL replacement sequential samples still measured BRL with a maximum level at 122 μg/L (Figure 4). Caution must be exercised when interpreting the sequential profile data, particularly when samples contained >100 μg/L. The lead in these samples is likely associated with the random particulate lead release that could have originated from any part of the lead-containing plumbing or deposited lead particles. The data from City 1 and City 2 suggest that implementation and degree of effective corrosion control treatment influence the lead concentrations in both the fully flushed and sequential samples. For example, the average lead concentration of all pre-LSL removal fully flushed samples in City 2 was 0.8 μg/L, compared with 6.3 μg/L in City 1.
FIGURE 3.
Lead service line pre- and post-replacement sequential profiles for study houses GS1–GS12 in City 1, IL
FIGURE 4.
Lead service line pre- and post-replacement sequential profiles for study houses FS1–FS10 in City 2, MI
Finally, sequential profile parameters in post-replacement LSL homes were much lower than pre-replacement values (for City 1, p = .325 for GS10, p < .001 for others; for City 2, p ≤ .004 for all), but not as low as control homes (Figures 3 and 4). For post-replacement sequential water samples, 15% (31 out of 205) and 44% (68 out of 153) of water samples had BRL lead for City 1 and City 2, respectively. This observation was consistent with the flushed sampling results in that following LSL removal, lead levels in the water were greatly reduced but remained greater than levels in homes that never had an LSL.
The benefit of LSL removal was obvious from comparing the total lead mass reduction of lead pre- and post-LSL replacement as calculated from the sequential profile water data before and after removal. There was a reduction of 4 mg lead mass in water totaled across all homes in City 1 (or 83%) following the replacement of LSLs. It is worth noting that one 1-L post-LSL replacement water sample in City 2 had 1150 μg/L lead, which contained even more than the total 659 μg lead mass from all the pre-replacement water samples in City 2. The observation reinforces one potential conclusion drawn from the flushed samples—that remnant lead, likely particulate in form and originally associated with the LSL, contaminated the premise plumbing over time. After excluding this home, there was a reduction of 0.5 mg lead mass in water across the remaining six homes in City 2 (or 93%) following the replacement of LSLs.
3.2.2 |. Quantitative methods to detect the presence of LSLs
For this study, the sequential sampling results were evaluated as a complementary means of identifying the presence of LSLs particularly in cases where the FMC values were less sensitive. Sequential profiles were examined in multiple quantitative ways to establish the most effective approach to distinguishing between control and study groups and further to detect the presence of LSL for each study community. Specifically, the following parameters were determined from all control, pre-, and post-LSL profiles: first draw equivalent (FDE), modified Montreal equivalent (MME; second liter sample after 6 h stagnation rather than 15 min), sequential profile sampling maximum lead concentration (SPMC), and weighted average sequential lead concentration (WASLC). The FDE was calculated to capture the lead in the first draw 1-L sample and the WASLC represents an average level of lead in the water from the tap to the distribution system main in contact with the premise plumbing and service line. The details and derivations of the calculations were demonstrated in Calculation S1, and Tables S3 and S4.
Among these quantitative methods, the SPMCs were always the highest, capturing potentially the worst case at the time of sampling in each house. In all but three of the 22 houses with LSLs still present, SPMCs were measured at least 5 μg/L, and in most cases, the levels were several to more than 45 times that level. In contrast, the WASLCs were the most conservative (lowest) measure of lead concentration for most houses with the FDEs and MMEs in between.
It is also noteworthy that while there often was a signal of elevated lead likely from within the premise, caused by lead accumulation in the scales, leaded brass components, galvanized iron pipes, and sometimes lead: tin soldered joints, peaks presumably attributable to the LSLs varied widely by length and position along the line of plumbing from tap to main. Unfortunately, detailed plumbing configuration maps were not available to precisely match lead profile patterns to lead sources. Nonetheless, this dramatically shows that only a full sequential profile, and not an assumed fixed volume location, is appropriate for LSL identification.
In comparison with the sequential profiles of the control groups, identifying LSLs simply by sample result inspection is straightforward, as the lead levels in the controls are typically a small fraction of the lead levels in the profiles from LSL homes, and even post-LSL replacement homes in the two cities in this study. Estimates can easily be made for the volume contained within the premise plumbing using fairly straightforward relationships to length and inside diameter of different pipe materials as noted previously (Schock & Lytle, 2011; Triantafyllidou et al., 2021), with adjustments made to compensate for the decrease in effective inside diameter and flow blockage from scale buildup and tuberculation.
3.3 |. Practical implications from flushed and sequential sampling efforts
The collection of flushed samples and sequential samples has several practical implications when considering an LSL identification program. Most importantly is the confidence level at which the approach accurately predicts the presence of an LSL. Other practical factors including the number of samples collected, the stability of water quality between the determination of the threshold concentration and the screening sampling, the time it takes to collect the samples, the level of intrusiveness on residents, the project cost, and the level of training of the sampler must be considered (Hensley et al., 2021). However, since the accuracy of the methods discussed is in large part dictated by the completeness of the number and types of plumbing configurations chosen for the control group, cost considerations should not be allowed to compromise the necessary robustness and statistical validity of this fundamental task, or it will seriously undermine the accuracy and confidence of the screening results.
Fully flushed samples offer advantages over other LSL identification schemes because they require only a single sample without a stagnation requirement and only take approximately 5 min to collect. Fully flushed samples have a robust collection protocol, which is conducive to the collection by either utility sampling personnel or residents of homes in the LSL verification pool. Only minimal written or verbal directions are necessary to describe the sampling protocol. In contrast, for sequential sampling, the stagnation requirement is very invasive to the routine of the home resident(s), and the number of consecutive samples that must be collected in a very short time frame makes it more challenging for someone without training to perform.
Control group sampling and screening programs for LSLs should take place in as proximate a time frame as possible, so that seasonal or other factors that could induce differences in the amount of lead solubility or lead release rate would be controlled and optimized. Seasonal water quality changes, including temperature, have been shown to impact lead corrosion and release. Thus, the sensitivity of the fully flushed or any other water sampling-based LSL presence prediction will vary with water quality and temperature season, resulting in important detectability differences between fully flushed water samples from homes with and without LSLs. This was reflected in City 1 by fully flushed water sample results collected pre-LSL replacement in the Summer (August) and Winter (January and February). Due to a limited dataset, it is difficult to draw any definitive conclusions about seasonal variation. It is also possible that the time delay between the two sampling periods allowed for increased effectiveness of any corrosion control measures that had been implemented in City 1 during that period. Given this data, it is advisable to perform sampling in warm weather months to observe a worst-case scenario for lead release. Additionally, if a water system has multiple water quality zones containing LSLs, which vary in likely lead release or exposure potential, control groups and thresholds for predicting the presence of an LSL, must be carried out on a zonal basis.
Sensitivity of predicting LSLs will depend on length of LSLs, the corrosivity of the water, and the volumes of sequential samples. Smaller volumes give more locational precision of lead sources through the home and SL. Other important influences are the number, type, and age of leaded brass interior plumbing devices; accumulation of lead in the premise plumbing scales; and the number and quality of the soldered joints. In the control group, choosing homes that never had an LSL is of utmost importance, because the inclusion of homes that previously had an LSL will almost certainly reduce the sensitivity of the water sampling results in detecting current LSL homes. As houses vary in size, types of plumbing materials (likely relating to age and construction practice changes), plumbing layout, and water usage patterns, particular care must be taken to include a statistically reasonable number of sites representing the range of configurations present that are of similar age and location to housing with suspected or with a reasonable possibility of containing LSLs.
Fully flushed samples also have limitations because they are only a single sample that provides an aggregate lead concentration assuming minimal water line stagnation. Sequential sampling provides a more detailed view of potential maximum lead exposure because stagnation reflects the approximate location of lead sources within the plumbing line from the main to the house. For the purposes of LSL detection, there is no specific stagnation time required. The primary purpose is signal amplification for the lead sources. Thus, in relatively corrosive waters, even 30 min would provide better resolution (Deshommes et al., 2016). The longer the stagnation time, up to the typical quasi-equilibrium lead saturation level, will provide a progressively more sensitive determination of whether there is an LSL or other strong lead-emitting plumbing component.
3.4 |. Proposed framework for using water samples for LSL identification
Based on data collected from City 1 and City 2, and considering past research efforts, a four-step screening approach based on water sampling that municipalities can use to identify whether an LSL is present is proposed: (1) establish baseline threshold lead concentrations for fully flushed and sequential sample sets from homes that have never had LSLs, (2) collect fully flushed samples and sequential samples from homes with known LSLs, (3) collect fully flushed samples from homes with unknown and suspected LSLs, and (4) collect sequential profiles from homes with unknown or suspected LSLs if fully flushed samples do not clearly indicate the presence of an LSL. Each step is described in more detail in the following sections.
3.4.1 |. Establish baseline threshold lead concentrations from homes that have never had LSLs
Once a city has determined the neighborhoods that may have LSLs (based on the age of home, records, and construction practices), the first step is to perform a control investigation to establish a control pool of houses that are used to determine the threshold lead concentration for predicting the presence of an LSL with fully flushed samples. To do this step, control samples should be collected from a robust number of homes that have never had an LSL, but that are in or located near the LSL neighborhoods. The control site characteristics should cover a representation of the configurations of interior plumbing to that in the areas where LSLs would be present. The number of homes will depend on the amount of variability and level of lead observed in the control group fully flushed samples, which in turn may be a function of the range of configurations observed in a neighborhood or community. The more variation in site plumbing types, and in lead levels, the more control locations will be needed, and numbers in the 20–50 range would not be unreasonable. The baseline samples should include a full set of sequential samples as well as a fully flushed sample, to provide a baseline for the worst-case situation where corrosion control treatment is very good and lead release from LSLs is low. Knowledge of pipe inside diameters would also aid in cross-checking the accuracy of the profiles. Threshold values would be calculated for the fully flushed samples, and the lead release trends for the non-LSL houses would be inspected. The fully flushed threshold lead concentration is defined as the average of all control samples. Alternatively, the threshold could be defined as a safety factor (e.g., 2, 3, 5) times the average to increase confidence an LSL is present. A safety factor would minimize false-positive LSL determinations and associated costs. Similarly, thresholds should be established for sequential profile parameters (FDE, MME, SPMC, and WASLC). Note that more than one control pool threshold sampling event may become necessary if the overall screening to detect and enumerate LSLs continues through multiple seasons or multiple water quality zones.
3.4.2 |. Collect fully flushed samples and sequential samples from homes with known LSLs
In the second step, the procedure to establish baseline threshold lead concentrations from homes that have never had LSLs in step 1 should be repeated for a pool of homes known to have LSLs. The analysis of lead levels in this group is to be compared to the control group to ensure that differences between the two groups are in fact distinguishable. If for some reason (e.g., excellent corrosion control program in place, adherent lead scale, or lead sources in control group release elevated lead), the two sets are not different, water sampling-based approaches to identify LSLs in the community may not be viable. If differences are apparent, the comparisons can help identify an appropriate safety factor that maximizes the identification of LSLs while minimizing the number of false-negative determinations.
3.4.3 |. Collect fully flushed samples from study homes
The third step is to have a fully flushed sample collected at every home that is in the suspected LSL neighborhood where the service material is uncertain. Either have a city staff member go out and perform the sample collection or coordinate the residents of the neighborhood to perform their own sampling after providing them a sample container and informing them how to collect the sample. Using the baseline threshold determined from the first step, eliminate all high-probability LSL homes (i.e., measured fully flushed sample is greater than the threshold) from future sampling and log them as most likely an LSL. If homes are ambiguous in terms of showing lead levels near the threshold or below, but having service connection records, or historical plumbing code or construction factors that would strongly suggest that having an LSL would be likely, define a follow-up pool for sequential sampling verification. It is important to keep in mind that if the LSLs are in the range of 0.63–0.75-in ID (16–19 mm), the length of pipe per liter of sample would range from about 17–12 feet (5.1–3.5 m). Thus, it may be necessary to use sample volumes less than 1 L to obtain enough resolution to accurately estimate the presence of an LSL or segment of lead pipe.
3.4.4 |. Collect sequential profile samples from selected study homes
The fourth and final step would be to implement the short stagnation time sequential sampling of homes having unknown SL materials where flushed samples fall below the fully flushed threshold. Identify or calculate sequential profile parameters (FDE, MME, SPMC, and WASLC) and compare to corresponding threshold levels to try to verify or refute the presence of an LSL.
3.4.5 |. Application of the proposed framework to study communities
Fully flushed samples and sequential profile lead parameters from homes with LSLs in the two test cities were compared to the respective community-specific threshold concentrations to test the predictive power of each approach. Control threshold lead concentrations for flushed samples (FMC) and sequential profile parameters (FDE, MME, SPMC, and WASLC) were calculated with safety factors of 1–5, and 10 times the respective averages (Table S3). Similarly, comparison tables (Tables S4 and S5) for pre- and post-replacement of LSLs are summarized in Figure 5. In City 1, 100% of the LSL sites were accurately above the 1x thresholds for all flushed and sequential profile parameters, whereas in City 2, only 60% of LSL homes were predicted by fully flushed samples (FMC; Figure 5). The predictiveness of water sampling could be improved to 100% by comparing the SPMC or WASLC parameter determined from sequential sampling. In practice, flushed samples would be collected from the homes initially. A return visit to the 40% of homes that failed the flushed test could receive repeat sequential sampling to improved prediction confidence. Increasing to a 2x threshold slightly decreased the City 1 flushed outcome, however, SPMC and WASLC parameter still accurately predicted the LSL home 100% of the time. The FDE was no better than the fully flushed sample, and the MME predicted LSL homes 90% of the time. As the factor of safety increased, the predictability of all parameters decreased. For example, at a 5x threshold, the fully flushed sample (FMC) accurately predicted 75% of the LSL homes in City 1, and the accuracy was increased to 100% by performing sequential sampling and examining the SPMC or WASLC in the homes that failed the fully flushed sample test. In City 2, the fully flushed sample (FMC) accurately predicted 0% of the LSL homes at a 5x threshold, and the accuracy was increased to 70% by performing sequential sampling and examining the WASLC in the homes that failed the fully flushed sample test. When the same approach was applied post-replacement sites, the tendency to falsely predict LSL homes decreased as the safety factor increased (Figure 5, Tables S4 and S5).
FIGURE 5.
Accurately predicted fraction of houses that have lead service line (LSL) before LSL removal (a,c), and that do not have LSL after LSL removal (b,d) in City 1, IL and City 2, MI using multiple quantitative methods. (a) City 1, pre-replacement. (b) City 1, post-replacement. (c) City 2, pre-replacement. (d) City 2, post-replacement. FDE, first draw equivalent; FMC, flushing sampling maximum concentration; MME, modified montreal equivalent; SPMC, sequential profile maximum concentration; WASLC, weighted average sequential lead concentration
4 |. CONCLUSIONS
State and local governments that are dealing with concerns over lead in drinking water, especially when the likely source is LSLs, face a difficult problem of accurately and effectively identifying LSLs. It has been nearly a decade since the general principles of the use of fully flushed samples as a screening tool to find LSLs were proposed and were thoroughly evaluated in Montreal, Quebec (Cartier et al., 2012). By revisiting the approach in two water systems with different water chemistry characteristics from Montreal, and each other, this study validates the considerable promise for a four-pronged approach for finding LSLs quickly and effectively. In the two water systems in this study, with somewhat different corrosion control histories and likely types of lead pipe scales, the fully flushed protocol was an effective and robust first-level screening tool to detect LSLs in the pools of houses tested.
A combined fully flushed and sequential sampling approach to municipality LSL identification was found to be robust in predicting the presence of LSLs under varied housing configurations and water qualities. This approach consists of initially using a large-scale screening program composed of inexpensive and noninvasive fully flushed sampling to screen high-probability LSLs that would allow LSL replacement to happen more quickly and leave the lower probably homes to be evaluated more closely by a profiling confirmatory technique. One of the ancillary benefits of flushed samples for LSL detection is that it provides a powerful database of the lowest background lead release that would be encountered by residents. This is important information for risk assessment and to communicate to residents their best-case exposure scenario, to inform their decision on whether supplemental lead removal filtration needs to be acquired for the household.
Some additional research would be helpful to create a widely applicable national or regional guidance for designing and carrying out LSL detection programs based on water sampling. Whether or not sensitivity is better using high velocity (fully flushed) sampling or slower flow sampling is almost certainly dependent upon the nature of the pipe scales formed in each system. Any water system using the fully flushed sampling method should test a subset of houses with LSLs and different interior plumbing to determine whether, for that system, there is higher lead release at higher velocity or lower. When there are poorly adherent depositional coatings, it is likely that small particulates (down to nanoparticle sizes) containing lead will be sheared off during sampling and thus increase the sensitivity of the technique. Similarly, accumulated lead in premise plumbing scales will enhance sensitivity by essentially serving as extensions of the LSLs. If the pipe scales are in more of a stable, adherent, passivating film nature, radial diffusion of the lead during flow would be the dominant release mechanism, which would be favored by the longer contact time of the slower flowing water.
Supplementary Material
Article Impact Statement.
An approach that uses flush and sequential profile drinking water samples to identify whether a lead service line is present is outlined.
ACKNOWLEDGMENTS
The project was funded by U.S. EPA Contract No. EP-C-15-010, Work Assignment No. WA 2-22 with Battelle (Columbus, OH) working as a subcontractor to Pegasus Technical Services. This project was supported in part by appointment to the Research Participation Program at the U.S. Environmental Protection Agency Office of Research and development, administered by the Oak Ridge Institute for Science and Education (ORISE), Oak Ridge, Tennessee, through an interagency agreement between the U.S. Department of Energy and U.S. EPA. We acknowledge Maria Caballero (former ORISE participant) for her help with graphical and data analyses.
Funding information
U.S. EPA, Grant/Award Number: EP-C-15-010
Footnotes
CONFLICT OF INTEREST
The authors declare that this manuscript does not contain conflicts of interest.
DISCLAIMER
The information in this article has been reviewed in accordance with the U.S. Environmental Protection Agency’s policy and approved for publication. The views expressed in this article are those of the authors and do not necessarily represent the views or the policies of EPA. Any mention of trade names, manufacturers, or products does not imply an endorsement by the U.S. Government or EPA; EPA and its employees do not endorse any commercial products, services, or enterprises.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in U.S. EPA’s Science Inventory Website upon publication.
REFERENCES
- ASTM (2017a). Standard specification for zinc (hot-dip galvanized) coatings on iron and steel products. volume 01.06, A123/A123M–17. American Society for Testing and Materials, West Conshohocken, PA. [Google Scholar]
- ASTM. (2017b). Standard specification for zinc. volume 01.06, B6–18. American Society for Testing and Materials, West Conshohocken, PA. [Google Scholar]
- AWWARF. (1990). Lead control strategies. AWWA Research Foundation and AWWA. [Google Scholar]
- Britton A, & Richards WN (1981). Factors influencing plumbosolvency in Scotland. Journal of the Institution of Water Engineers and Scientists, 35(5), 349–364. [Google Scholar]
- Cardew PT (2006). Development of a convective diffusion model for lead pipe rigs operating in laminar flow. Water Research, 40(11), 2190–2200. http://www.ncbi.nlm.nih.gov/pubmed/16704873 [DOI] [PubMed] [Google Scholar]
- Cartier C, Bannier A, Pirog M, Nour S, & Prévost M (2012). A rapid method for lead service line detection. Journal of American Water Works Association, 101(11), E582–E595. [Google Scholar]
- Cartier C, Laroche L, Deshommes E, Nour S, Richard G, Edwards M, & Prevost M (2011). Investigating dissolved lead at the tap using various sampling protocols. Journal of American Water Works Association, 103(3), 55–67. [Google Scholar]
- Clark BN, Masters SV, & Edwards MA (2015). Lead release to drinking water from galvanized steel pipe coatings. Environmental Engineering Science, 32(8), 713–721. [Google Scholar]
- Cornwell DA, Brown RA, & Via SH (2016). National survey of lead service line occurrence. Journal American Water Works Association, 108, E182–E191. 10.5942/jawwa.2016.108.0086 [DOI] [Google Scholar]
- Cornwell DA, Crawford-Brown D, Wagner JR, & Brown R (2021). Measurements and modeling of lead reduction strategies. AWWA Water Science, 3(4), e1232. 10.1002/aws2.1232 [DOI] [Google Scholar]
- Del Toral MA, Porter A, & Schock MR (2013). Detection and evaluation of elevated lead release from service lines: A field study. Environmental Science & Technology, 47(16), 9300–9307. 10.1021/es4003636 [DOI] [PubMed] [Google Scholar]
- Deshommes E, Bannier A, Laroche L, Nour S, & Prevost M (2016). Monitoring-based framework to detect and manage lead water service lines. Journal of American Water Works Association, 108, E555–E570. [Google Scholar]
- Deshommes E, Prévost M, Levallois P, Lemieux F, & Nour S (2013). Application of lead monitoring results to predict 0–7 year old children’s exposure at the tap. Water Research, 47(7), 2409–2420 http://www.sciencedirect.com/science/article/pii/S0043135413001012 [DOI] [PubMed] [Google Scholar]
- Deshommes E, Trueman B, Douglas I, Huggins D, Laroche L, Swertfeger J, Spielmacher A, Gagnon GA, & Prévost M (2018). Lead levels at the tap and consumer exposure from legacy and recent lead service line replacements in six utilities. Environmental Science & Technology, 52(16), 9451–9459. 10.1021/acs.est.8b02388 [DOI] [PubMed] [Google Scholar]
- Doré E, Deshommes E, Laroche L, Nour S, & Prévost M (2019). Lead and copper release from full and partially replaced harvested lead service lines: Impact of stagnation time prior to sampling and water quality. Water Research, 150, 380–391. 10.1016/j.watres.2018.11.076 [DOI] [PubMed] [Google Scholar]
- Douglas I, Campbell A & Muylwyk Q, (2007). Lead control in soft water: Experience from a Canadian utility, Proceedings AWWA annual conference and exhibition, Toronto, ON, June 24–28. [Google Scholar]
- e-CFR Electronic Code of Federal Regulations. (2019). Title 40: Protection of environment, PART 141—National primary drinking water regulations, Subpart I—Control of lead and copper. https://www.ecfr.gov/cgi-bin/text-idx?SID=e2518c71584f661373492ec68f3f6660&mc=true&node=sp40.25.141.i&rgn=div6
- Environmental Defense Fund. (2019). State efforts to support LSL replacement. https://www.edf.org/health/state-efforts-supportlsl-replacement
- Environmental Protection Agency. (2021). National primary drinking water regulations: Lead and copper rule revisions, 40 Parts 141 and 142, 86 FR 4198, p. 4198, January 15, 2021, https://www.regulations.gov/document?D=EPA-HQ-OW-2017-0300-1550
- Fewtrell L, Kaufmann R, & Prüss-Üstün A (2003). Lead: Assessing the environmental burden of disease at national and local levels. World Health Organization. [Google Scholar]
- Hayes CR (2009). Computational modelling to investigate the sampling of lead in drinking water. Water Research, 43(10), 2647–2656. http://www.sciencedirect.com/science/article/B6V73-4VXB8S2-1/2/e5f8ac33fe8181eede30460c5e4580a8 [DOI] [PubMed] [Google Scholar]
- Health Canada, 2009. Guidance on controlling corrosion in drinking water distribution systems, Report No. Catalogue No. H128–1/09–595E, Health Canada, Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety & Branch, Ottawa, ON, Canada. [Google Scholar]
- Hensley K, Bosscher V, Triantafyllidou S, & Lytle D (2021). Lead service line identification: A review of strategies and approaches. AWWA Water Science, 3(3), e1226. 10.1002/aws2.1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuch A, & Wagner I (1983). Mass transfer model to describe lead concentrations in drinking water. Water Research, 17(10), 1303. [Google Scholar]
- Lanphear BP, Hornung R, Ho M, Howard CR, Eberly S, & Knauf K (2002). Environmental lead exposure during early childhood. The Journal of Pediatrics, 140(1), 40–47. [DOI] [PubMed] [Google Scholar]
- Lytle DA, Schock MR, Wait K, Cahalan K, Bosscher V, Porter A, & Del Toral M (2019). Sequential drinking water sampling as a tool for evaluating lead in Flint, Michigan. Water Research, 157, 40–54. https://www.sciencedirect.com/science/article/pii/S0043135419302647?via%3Dihub [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard JB & Wasserstrom L (2017). Premise plumbing scales that can release lead after a lead service line replacement: Galvanized pipes from three cities. Proceedings AWWA Annual Conference and Exposition, Philadelphia, PA, June 11–14. [Google Scholar]
- McFadden M, Giani R, Kwan P, & Reiber SH (2011). Contributions to drinking water lead from galvanized iron corrosion scales. Journal of American Water Works Association, 103(4), 76–89. [Google Scholar]
- McFarren EF, Buelow RW, Thurnau RC, Gardels MC, Sorrell RK, Snyder P, & Dressman RC (1977). Water quality deterioration in the distribution system, Proceedings AWWA Water Quality Technology Conference, Kansas City, MO. [Google Scholar]
- MDDELCC (2014). Guide d’Évaluation et d’Intervention Relatif au Suivi du Plomb et du Cuivre dans l’Eau Potable, Ministère du Développement Durable de l’Environnement et Lutte Contre les Changements Climatiques, Quebec, Canada. [Google Scholar]
- Menke A, Muntner P, Batuman V, Silbergeld EK, & Guallar E (2006). Blood lead below 0.48 {micro}mol/L (10 {micro}g/dL) and mortality among US adults. Circulation, 114(13), 1388–1394. http://circ.ahajournals.org/content/114/13/1388.full.pdf [DOI] [PubMed] [Google Scholar]
- Muylwyk Q, Waller M, Spielmacher A, Olesiuk J, & Suffoletta V (2011). Full versus partial lead service line replacement and lead release in a well-buffered groundwater, Proceedings AWWA Water Quality Technology Conference, Phoenix, AZ, November 13–17. [Google Scholar]
- Ngueta G, Abdous B, Tardif R, St-Laurent J, & Levallois P (2016). Use of a cumulative exposure index to estimate the impact of tap-water lead concentration on blood lead levels in 1- to 5-year-old children (Montreal, Canada). Environmental Health Perspectives, 124(3), 388–395. http://www.ncbi.nlm.nih.gov/pubmed/26080391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngueta G, Gonthier C, & Levallois P (2015). Colder-to-warmer changes in children’s blood lead concentrations are related to previous blood lead status: Results from a systematic review of prospective studies. Journal of Trace Elements in Medicine and Biology, 29, 39–46. http://www.sciencedirect.com/science/article/pii/S0946672X14001357 [DOI] [PubMed] [Google Scholar]
- Ngueta G, Prévost M, Deshommes E, Abdous B, Gauvin D, & Levallois P (2014). Exposure of young children to household water lead in the Montreal area (Canada): The potential influence of winter-to-summer changes in water lead levels on children’s blood lead concentration. Environment International, 73, 57–65. [DOI] [PubMed] [Google Scholar]
- Pieper KJ, Krometis LA, Gallagher D, Benham B, & Edwards M (2015). Profiling private water systems to identify patterns of waterborne lead exposure. Environmental Science & Technology, 49(21), 12697–12704. http://www.ncbi.nlm.nih.gov/pubmed/26426487 [DOI] [PubMed] [Google Scholar]
- Sandvig A, Kwan P, Kirmeyer G, Maynard B, Mast D, Trussell RR, Trussell S, Cantor AF, & Prescott A (2008). Contribution of service line and plumbing fixtures to lead and copper rule compliance issues, Report No. 91229, American Water Works Association Research Foundation and U. S. Environmental Protection Agency, Denver, CO. [Google Scholar]
- Schock MR (1990). Causes of temporal variability of lead in domestic plumbing systems. Environmental Monitoring and Assessment, 15(1), 59–82, http://link.springer.com/article/10.1007%2FBF00454749 [DOI] [PubMed] [Google Scholar]
- Schock MR, & Lytle DA (2011). Internal corrosion and deposition control. In Edzwald JK (Ed.), Water quality and treatment: A handbook of community water supplies (chap. 20) (6th ed.). American Water Works Association and McGraw Hill. [Google Scholar]
- Schock MR, Triantafyllidou S, Tully J, DeSantis M, & Lytle D (2019). Diagnostic sampling tools for lead in drinking water, Proceedings AWWA Annual Conference and Exposition, Denver, CO, June 9–13. [Google Scholar]
- Stanek LW, Xue J, Lay CR, Helm EC, Schock M, Lytle DA, Speth TF, & Zartarian VG (2020). Modeled impacts of drinking water pb reduction scenarios on children’s exposures and blood lead levels. Environmental Science & Technology, 54(15), 9474–9482. 10.1021/acs.est.0c00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllidou S, Burkhardt J, Tully J, Cahalan K, DeSantis M, Lytle D, & Schock M (2021). Variability and sampling of lead (Pb) in drinking water: Assessing potential human exposure depends on the sampling protocol. Environment International, 146, 106259. https://www.ncbi.nlm.nih.gov/pubmed/33395926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafyllidou S, & Edwards M (2012). Lead (Pb) in tap water and in blood: Implications for lead exposure in the United States. Critical Reviews in Environmental Science and Technology, 42, 1297–1352. 10.1080/10643389.2011.556556 [DOI] [Google Scholar]
- Trueman BF, Camara E, & Gagnon GA (2016). Evaluating the effects of full and partial lead service line replacement on lead levels in drinking water. Environmental Science & Technology, 50(14), 7389–7396, http://www.ncbi.nlm.nih.gov/pubmed/27337040 [DOI] [PubMed] [Google Scholar]
- Trussell RR, & Wagner I (1996). Corrosion of galvanized pipe. In Internal corrosion of water distribution systems (chap. 3) (3rd ed., pp. 71–129). AWWA Research Foundation/TZW. [Google Scholar]
- U. S. EPA Flint Task Force. (2015). Lead in drinking water—Preliminary assessment. https://www.epa.gov/mi/City2-safe-drinking-water-task-force-draft-lead-drinking-water-preliminary-assessment
- U.S. EPA. (1994). Methods for the determination of metals in environmental samples, EPA-600/R-94/111. Office of Research and Development, U.S. EPA, May 1994. [Google Scholar]
- Vaccari DA (1994). Spatial distribution of lead in plumbing systems. Proceedings AWWA Annual Conference & Exposition, New York, NY. [Google Scholar]
- Van Der Leer D, Weatherill NP, Sharp RJ, & Hayes CR (2002). Modelling the diffusion of lead into drinking water. Applied Mathematical Modelling, 26(6), 681–699, http://www.sciencedirect.com/science/article/B6TYC-45KNF9Y-2/1/9c7274026f20bae89c27bf7652b31896 [Google Scholar]
- Weston RF, & Economic & Engineering Services, Inc. (1990). Lead service line replacement: A benefit-to-cost analysis. Denver, CO. [Google Scholar]
- Zartarian V, Xue J, Tornero-Velez R, & Brown J (2017). Children’s lead exposure: A multimedia modeling analysis to guide public health decision-making. Environ Health Perspect, 125(9), 097009, https://www.ncbi.nlm.nih.gov/pubmed/28934096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in U.S. EPA’s Science Inventory Website upon publication.