Abstract
Aims
A 5-year survival of patients with unprotected left main (ULM) stenosis according to the choice of revascularization (percutaneous vs. surgical) remains to be defined.
Methods and results
Randomized controlled trials (RCTs) comparing percutaneous coronary intervention (PCI) vs. coronary artery bypass graft (CABG) with a follow-up of at least 5 years were included. All-cause death was the primary endpoint. MACCE [a composite endpoint of all-cause mortality, myocardial infarction (MI), stroke, and repeat revascularization] along with its single components and cardiovascular (CV) death were the secondary ones. Analyses were stratified according to the use of first- vs. last-generation coronary stents. Subgroup comparisons were performed according to SYNTAX score (below or above 33) and to age (using cut-offs of each trial’s subgroup analysis). Four RCTs with 4394 patients were identified: 2197 were treated with CABG, 657 with first generation, and 1540 with last-generation stents. At 5-year rates of all-cause death did not differ [odds ratio (OR) 0.93, 95% confidence interval (CI) 0.71–1.21], as those of CV death and stroke. Coronary artery bypass graft reduced rates of MACCE (OR 0.69, 95% CI 0.60–0.79), mainly driven by MI (OR 0.48, 95% CI 0.36–0.65) and revascularization (OR 0.53, 95% CI 0.45–0.64). Benefit of CABG for MACCE was consistent, although with different extent, across values of SYNTAX score (OR 0.76, 95% CI 0.59–0.97 for values < 32 and OR 0.63, 95% CI 0.47–0.84 for values ≥ 33) while was not evident for ‘younger’ patients (OR 0.83, 95% CI 0.65–1.07 vs. OR 0.65, 95% CI 0.51–0.84 for ‘older’ patients).
Conclusion
For patients with ULM disease followed-up for 5 years, no significant difference was observed in all-cause and cardiovascular death between PCI and CABG. Coronary artery bypass graft reduced risk of MI, revascularization, and MACCE especially in older patients and in those with complex coronary disease and a high SYNTAX score.
Keywords: Coronary artery disease, Unprotected left main, Percutaneous coronary intervention, Coronary artery bypass graft, Meta-analysis
Introduction
Stenosis of unprotected left main coronary artery (ULMCA) is associated with high morbidity and mortality rates due to the great amount of supplied myocardium. About 6% of patients undergoing coronary angiography both for acute coronary syndromes and stable angina are diagnosed with ULMCA stenosis.1
Coronary artery bypass grafting (CABG) has been the standard of care for patients suffering from ULMCA stenosis for several decades because of an established survival benefit as compared to medical therapy.2 However, the introduction of coronary stents along with the improvements in technology and pharmacological management has encouraged the use of percutaneous coronary intervention (PCI) in these high-risk patients with satisfactory results, demonstrating similar rates in terms of death and stroke with a benefit of CABG in terms of lower subsequent revascularization.3–7 Current guidelines for myocardial revascularization recommend PCI as an alternative to CABG in patients with low to intermediate complexity of ULMCA stenosis (SYNTAX score ≤ 32), with a class of recommendation I and IIa, respectively, whereas CABG is still the preferred option in patients with high complexity lesions (namely SYNTAX score ≥ 33) due to a reduced incidence of major adverse cardiovascular and cerebrovascular events (MACCE).8
However, none of the most recent randomized controlled trials (RCTs) that compared the effectiveness of PCI with CABG in the treatment of ULMCA stenosis was adequately powered to detect differences in low-frequency events, such as mortality.9–12 Analyses of two large contemporary RCTs comparing PCI with CABG for ULMCA stenosis11,12 using second-generation drug-eluting stent (DES) and cutting-edge surgical techniques reported conflicting results, especially regarding mortality. Moreover, pooled data about long-term outcomes of patients revascularized on ULMCA according to the strategy of revascularization are not yet available.
Give from these premises, we performed this meta-analysis collecting data from RCTs with at least 5 years of follow-up comparing PCI with CABG in patients with ULMCA stenosis.
Methods
Trials to be included in this review were retrieved from a previous meta-analysis published by our group on this topic including both RCTs and observational studies with propensity score/multivariate adjustments and performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses statements (PRISMA).6 However, for the purpose of the present meta-analysis, only RCTs were considered and an additive inclusion criterion concerning follow-up duration (at least 5 years) was adopted. After selecting RCTs, updated follow-up data of such trials were searched across most relevant sources including PubMed, Cochrane database, Google Scholar www.tctmd.com, www.clinicaltrials.gov, www.clinicaltrialresults.org, www.cardiosource.com, and abstracts and presentations from major cardiovascular (CV) meetings by two investigators (F.D.A. and O.D.F.). In the case of duplicate reporting, the manuscript with the largest sample of patients meeting inclusion criteria was selected. Disagreements were resolved by consensus. For trials that updated baseline and procedural features of patients considered for the long-term outcome reporting, these data were abstracted on prespecified electronic forms. However, as data extraction was partly available as a result of our previously published meta-analysis, this project was not registered on PROSPERO. This work was not supported by extramural funding.
Endpoints
All-cause death was the primary endpoint, while MACCE a composite of all-cause death, myocardial infarction (MI), stroke, and repeat revascularization along with its single components and CV death were the secondary ones. All analyses were stratified according to the use of first- vs. last-generation coronary stents. Each trial’s definition of each single adverse events was used. Definition of endpoints in each trial has been reported in Supplementary material online, Appendix Table S3. For most of the trials, definitions of death and stroke were similar; however, one trial performed with second-generation DES did not collect data about periprocedural MI, thus only non-procedural MI events were considered in all trials using these devices. Studies presenting subgroup analysis according to SYNTAX score, to age and diabetes were included in prespecified sub-analyses (with SYNTAX score and age cut-offs as established by each trial) with respect to a composite endpoint of ‘trial-defined MACCE’ (i.e. MACCE according to each study definition, mainly representing the primary endpoint of included RCTs).
Quality study evaluation
The quality of included studies was independently appraised by two reviewers (O.D.F. and F.D.A.), with disagreements resolved by consensus. For each RCT, we evaluated the risk of bias (low, moderate, unclear, or high) for random-sequence generation, allocation concealment, blinding of patients and physicians, blinding during assessment of follow-up, incomplete outcome evaluation, and selective reporting, in keeping with the Cochrane Collaboration approach.
Statistical analysis
Continuous variables are reported as mean (SD) or median (first and third quartile). Categorical variables are expressed as n (%). Statistical pooling for incidence estimates was performed according to a random-effect model with generic inverse-variance weighting, computing risk estimates with 95% confidence intervals (CIs), using RevMan 5.2 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). Hypothesis testing for superiority was set at the two-tailed 0.05 level. Hypothesis testing for statistical homogeneity was set at the two-tailed 0.05 level and based on the Cochran Q test, with I2 values of 25%, 50%, and 75% representing mild, moderate, and severe heterogeneity, respectively.
Results
Studies and patients
Out of 26 studies included in a previous meta-analysis of our group, 6 RCTs comparing CABG vs. PCI on ULMCA were identified, of which 4 trials with a follow-up duration of at least 5 years were finally considered for this analysis (Supplementary material online, Figure S1). Globally 2197 patients assigned to PCI (namely with first-generation DES in SYNTAX and PRECOMBAT, and with second-generation DES in NOBLE and EXCEL) and 2197 patients assigned to CABG were involved. Main characteristics of these studies are listed in Table 1. Inclusion and exclusion criteria, patient clinical, angiographic, and procedural features along with definitions of clinical endpoints in the individual RCTs considered for this meta-analysis are reported in Supplementary material online, Appendix Table S2–S4. Study quality was high across all the four studies (Supplementary material online, Table S5) with a low estimated risk of bias.
Table 1.
Trials characteristics and major inclusion and exclusion criteria
| SYNTAX | PRECOMBAT | EXCEL | NOBLE | |
|---|---|---|---|---|
| Publication year | 2014 | 2015 | 2019 | 2019 |
| Number of patients after 5-year follow-up | 705 (PCI, n = 357; CABG, n = 348) | 600 (PCI, n = 300; CABG, n = 300) | 1905 (PCI, n = 948; CABG, n = 957) | 1184 (PCI, n = 592; CABG, n = 592) |
| Major inclusion criteria | Symptomatic stenosis >50% of ULMCA or with assessed myocardial ischaemia | Symptomatic or asymptomatic ULMCA stenosis >50% regardless of other significant lesions | ULMCA stenosis >70% or >50% if haemodynamically significant | ULMCA stenosis >50% or FFR ≤0.80 without more than three non-complex lesions |
| Major exclusion criteria | Previous PCI or CABG, acute MI, need for concomitant cardiac surgery | MI in the previous week, PCI in the previous year, LVEF <30%, cardiogenic shock, stroke in the previous 6 months, CKD, severe hepatic dysfunction, life expectancy <1 year | PCI or CABG of ULMCA in the previous year, need of concomitant cardiac surgery, SYNTAX score ≥33,a life expectancy <3 years | Patients considered too high risk for PCI or CABG, STEMI <24 h, life expectancy <1 year |
| Primary endpoint | Death, MI, stroke, or UR | Death, MI, stroke, or TVR | Death, stroke, or MI | Death, non-procedural MI, stroke, or UR |
CABG, coronary artery bypass graft; CKD, chronic kidney disease; FFR, fractional flow reserve; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; TVR, target vessel revascularization; ULMCA, unprotected left main coronary artery; UR, unplanned revascularization.
About 25% of the enrolled population met these criteria after laboratory analysis without being excluded.
Outcomes
After 5 years, there were no significant differences in the risk of all-cause mortality [odds ratio (OR) 0.93, 95% CI 0.71–1.21, P = 0.58, I2 = 40%]. This result was mainly driven by studies using first-generation DES (OR 1.19, 95% CI 0.83–1.71, P = 0.93, I2 = 0%), whereas latest RCTs using last-generation DES showed a borderline significant lower risk of global mortality with CABG (OR 0.78, 95% CI 0.62–0.99, P = 0.04, I2 = 0%; Figure 1). The risk of MACCE (including repeated revascularization) was significantly lower in patients assigned to CABG as compared to patients assigned to PCI (OR 0.69, 95% CI 0.60–0.79, P < 0.00001, I2 = 0%; Figure 2A). This result was then mainly driven by a reduction of risk of MI (OR 0.48, 95% CI 0.36–0.65, P < 0.0001, I2 = 43%) and revascularization (OR 0.53, 95% CI 0.45–0.64, P < 0.0001, I2 = 0%; Figure 2B and C). In particular, a comparable lower risk of repeated revascularization was observed both when CABG was tested against both first-generation (OR 0.50, 95% CI 0.36–0.68, P < 0.0001, I2 = 0%) and second-generation DES (OR 0.55 95% CI 0.45–0.69, P < 0.0001, I2 = 0%). In a sensitivity analysis, evaluating both procedural and spontaneous MI across three studies reporting on such aggregate data, difference between CABG and PCI was no longer significant (OR 0.80, 95% CI 0.61–1.05, P = 0.10, I2 = 0%, see Supplementary material online, Appendix Figure S2).
Figure 1.
Five-year rates of all-cause death in coronary artery bypass graft vs. percutaneous coronary intervention. Random effect risk estimates with 95% confidence intervals for all-cause death and stroke. CABG, coronary artery bypass graft; CI, confidence intervals; DES, drug-eluting stents; PCI, percutaneous coronary intervention.
Figure 2.
Five-year major adverse cardiovascular and cerebrovascular events (A), myocardial infarction (B), and revascularization (C) rates in patients treated on unprotected left main coronary artery with coronary artery bypass graft vs. percutaneous coronary intervention. Random effect risk estimates with 95% confidence intervals for major adverse cardiovascular and cerebrovascular events, myocardial infarction, and revascularization. Major adverse cardiovascular and cerebrovascular events included the original primary endpoint of PRECOMBAT, NOBLE, and SYNTAX LM (all-cause death, myocardial infarction, stroke, and revascularization), whereas, for EXCEL, a secondary prespecified endpoint was adopted to include revascularization. In NOBLE trial data about periprocedural myocardial infarction were not collected, PRECOMBAT and SYNTAX LM provided only aggregate data of procedural and non-procedural myocardial infarction; thus the myocardial infarction outcome is shown in B embrace all myocardial infarction occurring during follow-up for studies with first-generation drug-eluting stents (PRECOMBAT and SYNTAX LM) and only non-procedural myocardial infarction for randomized controlled trials with second-generation drug-eluting stents (NOBLE and EXCEL). CABG, coronary artery bypass graft; CI, confidence intervals; DES, drug-eluting stents; PCI, percutaneous coronary intervention.
There were no significant between-groups differences in the risk of CV mortality (OR 0.95, 95% CI 0.68–1.32, P = 0.75, I2 = 34%). This result was consistent both across studies using first-generation DES (OR 1.16, 95% CI 0.49–2.76, P = 0.74, I2 = 71%) and latest RCTs using last-generation DES (OR 0.85, 95% CI 0.62–1.17, P = 0.31, I2 = 0%; Figure 3A). The non-significant two-fold risk of stroke in the CABG group highlighted by RCTs performed with older DES (OR 2.34, 95% CI 0.94–5.83, P = 0.07, I2 = 0%), was counterbalanced by latest trials (OR 0.88, 95% CI 0.40–1.94, P = 0.75, I2 = 69%), resulting globally in a similar risk of cerebrovascular events (OR 1.17, 95% CI 0.59–2.31, P = 0.66, I2 = 58%; Figure 3B).
Figure 3.
Five-year cardiovascular death (A) and stroke (B) in patients with unprotected left main coronary disease treated with coronary artery bypass graft vs. percutaneous coronary intervention. Random effect risk estimates with 95% confidence intervals for cardiovascular death. CABG, coronary artery bypass graft; CI, confidence intervals; DES, drug-eluting stents; PCI, percutaneous coronary intervention.
Impact of the SYNTAX score
All the four studies considered in this analysis reported clinical outcomes for their primary endpoint (i.e. excluding revascularization for EXCEL) stratified according to tertiles of SYNTAX score (PRECOMBAT, NOBLE, and EXCEL) or according to lower-intermediate SYNTAX score (0–32) vs. high SYNTAX score (≥33) (SYNTAX trial) assessed by core angiographic laboratories of each trial. Globally, data of 4283 randomized patients (2132 assigned to CABG and 2151 assigned to PCI) contributed to this sub-analysis. In the low and intermediate SYNTAX score pooled tertiles (score 0–32, 1684 patients and 1643 patients assigned to PCI and CABG group, respectively), CABG was associated with a borderline significant lower risk of trial-defined MACCE (OR 0.76 95% CI 0.59–0.97, P = 0.03, I2 = 48%; Figure 4A). This benefit was driven by the results of NOBLE trial, whereas non-significant between-group differences were reported in other RCTs. The benefit of CABG was instead more evident among 956 patients (467 PCI, 489 CABG) in the highest SYNTAX score tertile (OR 0.63, 95% CI 0.47–0.84, P = 0.002, I2 = 0%; Figure 4B).
Figure 4.
Major adverse cardiovascular and cerebrovascular events in patients with ULMCAD treated with coronary artery bypass graft or percutaneous coronary intervention according to SYNTAX score. Random effect risk estimates with 95% confidence intervals for trial-defined major adverse cardiovascular and cerebrovascular events in low and intermediate SYNTAX score (SYNTAX score 0–32, A, above) and high SYNTAX score (SYNTAX score ≥ 33, B, below). Primary endpoints of each included trial were adopted for the outcome ‘trial-defined MACCE’ (i.e. not including revascularization in EXCEL trial). CABG, coronary artery bypass graft; CI, confidence intervals; DES, drug-eluting stents; PCI, percutaneous coronary intervention.
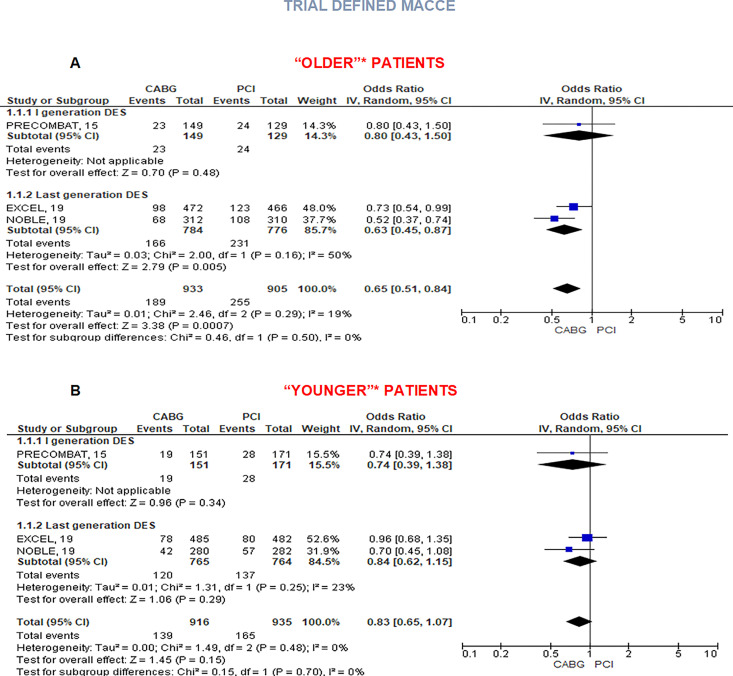
Impact of age and diabetes
Three RCTs (NOBLE, PRECOMBAT, and EXCEL) performed a prespecified subgroup analysis according to the age of enrolled patients with respect to trial-defined MACCE. In particular, NOBLE and EXCEL trial compared outcomes in patients older or younger than 67 years old, whereas a cut-off of 65 years old was chosen in the PRECOMBAT trial. The benefit for CABG in terms of MACCE was consistent for older patients (OR 0.65, 95% CI 0.51–0.84, P < 0.001), but disappeared for ‘younger’ ones (OR 0.83, 95% CI 0.65–1.07, P = 0.15, I2 = 0%), consistently in both recent trials and PRECOMBAT (Figure 5A and B). All the four included trials tested the potential interaction between revascularization strategy and diabetes. Coronary artery bypass graft benefit in reducing trial-defined MACCE was confirmed regardless of diabetic status (OR 0.74, 95% CI 0.56–0.97, P = 0.03, I2 = 0% in diabetic patients and OR 0.70, 95% CI 0.61–0.90, P = 0.002 in non-diabetic patients, Supplementary material online, Appendix Figure S3).
Figure 5.
Major adverse cardiovascular and cerebrovascular events in patients with ULMCAD treated with coronary artery bypass graft or percutaneous coronary intervention according to age. Random-effect risk estimates with 95% confidence intervals for major adverse cardiovascular and cerebrovascular events in ‘older’ patients (A) and ‘younger’ patients (B). aSubgroup analysis for age was not provided in SYNTAX LM. NOBLE and EXCEL compared outcomes in patients younger or older than 67 years old, whereas PRECOMBAT used age cut-off of 65 years old. CABG, coronary artery bypass graft; CI, confidence intervals; DES, drug-eluting stents; PCI, percutaneous coronary intervention.
Discussion
The main findings of this study are as follows:
No significant difference in all-cause death and CV death was found between CABG and PCI in patients revascularized on ULMCA over a 5-year follow-up.
Coronary artery bypass graft is associated with a significant reduction of the risk of adverse CV events, due to a reduction of the rates of revascularization and myocardial infarction.
The benefit of CABG as compared to PCI is consistent regardless of diabetic status, whereas it resulted more evident in older patients and those with complex coronary atherosclerotic disease (namely patients with SYNTAX score ≥ 33).
According to ESC guidelines for myocardial revascularization,8 PCI is an alternative to CABG in low- and intermediate-risk patients with ULMCA disease, although there is a linear trend towards better survival rates in high SYNTAX score patients treated with CABG.5,13 Despite these elements, the comparison of these revascularization strategies has been matters of debate in recent years without definitive evidences especially regarding long-term outcomes. So far, this is the first analysis presenting pooled outcome data of four high-quality RCTs performed to compare PCI vs. CABG for the treatment of ULMCA disease over a follow-up of 5 years.
Cardiovascular and all-cause mortality
Percutaneous and surgical revascularization strategies proved comparable effectiveness respect to survival rates, both looking at all-cause death and CV mortality. Of note, a previous meta-analysis by Palmerini et al.,4 including all the four RCTs considered in the present analysis but with a shorter follow-up (3 years) reported a significant interaction between SYNTAX score and mortality, such that the risk of death tended to be reduced with PCI compared with CABG in patients in the lowest SYNTAX score tertile, similar in those with a SYNTAX score of 23–32, and higher in those with the highest SYNTAX score. However, this finding diverges from the recently reported 10-year follow-up outcomes of the SYNTAX trial,14 showing no interaction between the SYNTAX score and all-cause mortality. As trials considered in this meta-analysis reported only their composite outcomes stratified according to tertiles of SYNTAX score, a potential interaction between coronary atherosclerotic disease complexity and our primary endpoint could not be tested. However, in line with our results and the ones of SYNTAX trial at 10 years, a pooled analysis of individual patient data comparing mortality outcomes after CABG vs. PCI in coronary artery disease showed no interaction of survival with the SYNTAX score.5
Major cardiac and cerebrovascular events and SYNTAX score stratification
Looking at the data of the composite endpoint of MACCE, CABG was superior both in the overall cohort or in first- and second-generation DES-treated population. This result was driven by revascularization and MI. From a physiopathological point of view,15 CABG has been described to provide ‘a collateralization effect’ over PCI by bypassing diseased territories and preventing symptoms in case of plaque rupture and vessel occlusion. The higher revascularization rate paid by PCI-treated patients was expected and confirmed over a long-term follow-up.4,6,16,17 Despite the global revascularization rate accounted also revascularization not on the left main, even RCTs reporting more accurate endpoint as target vessel (PRECOMBAT), or target lesion revascularizations (NOBLE) showed a consistent benefit of CABG. On the other hand, this is one of the first studies in ULM setting reporting a benefit of CABG with respect to MI and can be explained with the longer follow-up of studies. In accordance, a landmark analysis of the EXCEL trial showed higher incidence of MI in the CABG group in the first 30 days after revascularization, similar rates between 30 days and 1 year and a significant lower rate in patients treated with CABG between 1 and 5 years. It must be pointed out that our result was mainly driven by RCTs performed with second-generation DES, and that for the purpose of this meta-analysis, only non-procedural MI of these two trials was considered (while including both procedural and non-procedural MI of PRECOMBAT and SYNTAX). Data about procedural MI were not routinely collected in NOBLE, whereas a numerically higher number of these events were recorded in EXCEL in the CABG arm. In the same context and with the same potential limitation, we report a borderline significant superiority of CABG over PCI with respect to trial-defined MACCE in low–intermediate SYNTAX score tertiles that was mainly driven by NOBLE trial. On the other hand, a more consistent benefit of CABG was found in patients with high coronary atherosclerotic disease complexity. Surgical revascularization offers the advantage to overcome the overall burden of atherosclerotic disease and to achieve higher rates of complete revascularization as compared to PCI.18–20 Percutaneous coronary intervention instead is usually restricted to flow-limiting lesions and can be technically challenging in patients with diffuse coronary disease, thus resulting often in incomplete revascularization. These differences could account for the superiority of CABG in preventing revascularization, myocardial infarction, and thus the overall major adverse CV events especially in patients with the highest SYNTAX score.
Finally, we reported comparable outcomes between PCI and CABG in younger patients and the benefit of surgical revascularization over PCI in older patients. However, this result must be taken in the context of the low surgical risk of patients enrolled in these trials (i.e. mean EuroSCORE 2% in PRECOMBAT and 2.8% in NOBLE), thus poorly reflecting ordinary clinical practice where older patients are usually the less suitable candidates to cardiac surgery due to their higher comorbidities burden.19 On the other hand, the equivalence of PCI vs. CABG in younger patients may be theoretically ascribed to lower rates of CV risk factors compared to older ones, consequently leading to a reduced atherosclerotic burden. The benefit of CABG according to trial-defined MACCE was confirmed irrespectively of diabetic status. Of note, in both diabetic and non-diabetic patients, this result was mainly driven by the NOBLE trial, where the high incidence of revascularizations occurring at follow-up accounted for such composite endpoint.
Which revascularization strategy in unprotected left main patients?
Results of this meta-analysis are somewhat new and anachronistic at the same time. Actually, revascularization techniques made relevant steps forwards. This refers to both CABG with the affirmation of arterial grafts and diffusion of no-touch ascending aorta and off-pump techniques,21–23 and PCI with the widespread of ancillary technologies that demonstrated to improve outcomes. In particular, current revascularization guidelines recommend invasive physiological assessment of intermediate coronary stenosis including left main, driven by RCTs demonstrating the superiority of physiology over angiography-guided revascularization even in ULM8 with reassuring results also for patients with deferred lesions in this setting.24 Similarly, the utilization of intravascular coronary imaging recently received a Class IIa recommendation due to its reliability in assessing ULM stenosis severity and optimizing stents deployment, although only a limited number of procedures were IVUS-guided in such trials.25 Further, most of patients undergoing PCI in these trials were treated with a double-antiplatelet therapy (DAPT) consisting of acetylsalicylic acid and clopidogrel, and only a small percentage of patients were still on DAPT at 12 months. Nowadays more potent DAPT strategies are available for patients with acute coronary syndromes and a prolonged DAPT regimen (≥12 months) proved a favourable benefit–risk profile in high-risk settings and in patients treated on ULM.26–29 In conclusion, the optimal revascularization strategy for patients with ULM stenosis should always rely on a multidisciplinary assessment30 evaluating the objective to achieve complete revascularization, local expertise, available ancillary tools along with patients’ surgical risk and personal preferences.
Limitations
As with all meta-analysis, this study shares the limitations of each single trial included. Individual-level data were not available; thus, the potential effect of patient heterogeneity could not be examined. However, the four trials considered for this analysis were all RCTs characterized by an estimated low risk of bias, although the present analysis is intrinsically limited by the reduced number of included studies. The definition of clinical endpoints differed slightly across trials. In particular, NOBLE and SYNTAX considered all revascularization occurring during follow-up; in EXCEL trial, only ‘ischaemia driven’ revascularizations accounted for a composite secondary endpoint that was considered in our MACCE analysis. Finally, PRECOMBAT authors’ adopted a more specific outcome of ‘ischaemia-driven target-vessel revascularization’; however, only 2 (out of 38) and 3 (out of 21) non-target vessel revascularizations in PCI and CABG groups were respectively recorded, thus the bias of such divergence in our MACCE analysis is expected to be minimal. Further, the definition of MI, and consequently each trial definition of MACCE, differed among the four studies. In NOBLE data about periprocedural MI were not collected, whereas, in PRECOMBAT and SYNTAX, all kind of MI occurring during follow-up accounted for MI and MACCE endpoints. EXCEL authors provided both the data about total and non-periprocedural MI, but only the former accounted for MACCE. For the purpose of this meta-analysis, and counting on available data, we considered global incidence of MI in PRECOMABT and SYNTAX and only non-procedural MI in NOBLE and EXCEL, chasing a major homogeneity at least between trials adopting similar devices (first- vs. second-generation DES) and with similar sample size. However, as the rate of procedural MI was numerically higher in the CABG group of EXCEL, this choice could have overestimated the benefit of CABG with respect to MI. Sub-analysis that are presented in this study according to age and SYNTAX score are conceived as hypothesis-generating in order to give the reader more insights about the included studies and their results; while they lie on prespecified subgroup analyses of RCTs, age and SYNTAX score were not randomization criteria in any of the trials included. Finally, despite our results indicate a possible major benefit of CABG in patients with high SYNTAX score coronary artery disease, data about outcomes in relation to completeness of revascularization were not systematically collected across the RCTs included in this meta-analysis; and therefore, we could not address the impact of such variable on long-term prognosis.
Conclusions
Over a 5-year follow-up, no significant differences in all-cause death emerged between percutaneous and surgical revascularization in patients with ULM disease. Nevertheless, CABG was associated with a lower risk of major adverse CV events, in particular, revascularization and myocardial infarction.
Supplementary material
Supplementary material is available at European Heart Journal – Quality of Care and Clinical Outcomes online.
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Fabrizio D’Ascenzo, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Ovidio De Filippo, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Edoardo Elia, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Mattia Paolo Doronzo, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Pierluigi Omedè, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Antonio Montefusco, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Mauro Pennone, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Stefano Salizzoni, Division of Cardiac Surgery, Department of Surgical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Federico Conrotto, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Guglielmo Gallone, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Filippo Angelini, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Luca Franchin, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Francesco Bruno, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Massimo Boffini, Division of Cardiac Surgery, Department of Surgical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Mario Gaudino, Department of Cardiothoracic Surgery, Cornell Medicine, 1300 York Ave, New York, NY 10065, USA.
Mauro Rinaldi, Division of Cardiac Surgery, Department of Surgical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
Gaetano Maria De Ferrari, Division of Cardiology, Department of Medical Sciences, Città della Salute e della Scienza, Corso Bramante 88, 10126, Turin, Italy.
References
- 1. D'Ascenzo F, Presutti DG, Picardi E, Moretti C, Omedè P, Sciuto F et al. Prevalence and noninvasive predictors of left main or three-vessel coronary disease: evidence from a collaborative international meta-analysis including 22 740 patients. Heart 2012;98:914–919. [DOI] [PubMed] [Google Scholar]
- 2. Yusuf S, Zucker D, Passamani E, Peduzzi P, Takaro T, Fisher LD et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists collaboration. Lancet 1994;344:563–570. [DOI] [PubMed] [Google Scholar]
- 3. Lee PH, Ahn J-M, Chang M, Baek S, Yoon S-H, Kang S-J et al. Left main coronary artery disease: secular trends in patient characteristics, treatments, and outcomes. J Am Coll Cardiol 2016;68:1233–1246. [DOI] [PubMed] [Google Scholar]
- 4. Palmerini T, Serruys P, Kappetein AP et al. Clinical outcomes with percutaneous coronary revascularization vs coronary artery bypass grafting surgery in patients with unprotected left main coronary artery disease: a meta-analysis of 6 randomized trials and 4,686 patients. Am Heart J 2017;190:54–63. [DOI] [PubMed] [Google Scholar]
- 5. Head SJ, Milojevic M, Daemen J, Ahn J-M, Boersma E, Christiansen EH et al. Mortality after coronary artery bypass grafting versus percutaneous coronary intervention with stenting for coronary artery disease: a pooled analysis of individual patient data. Lancet 2018;391:939–948. [DOI] [PubMed] [Google Scholar]
- 6. Bertaina M, De Filippo O, Iannaccone M, Colombo A, Stone G, Serruys P et al. Percutaneous coronary intervention or coronary artery bypass graft in left main coronary artery disease: a comprehensive meta-analysis of adjusted observational studies and randomized controlled trials. J Cardiovasc Med 2018;19:554–563. [DOI] [PubMed] [Google Scholar]
- 7. D’Ascenzo F, Iannaccone M, Giordana F, Chieffo A, Connor SO, Napp LC et al. Provisional vs. two-stent technique for unprotected left main coronary artery disease after ten years follow up: a propensity matched analysis. Int J Cardiol 2016;211:37–42. [DOI] [PubMed] [Google Scholar]
- 8. Neumann F-J, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U et al. ; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165.30165437 [Google Scholar]
- 9. Morice M-C, Serruys PW, Kappetein AP, Feldman TE, Ståhle E, Colombo A et al. Five-year outcomes in patients with left main disease treated with either percutaneous coronary intervention or coronary artery bypass grafting in the Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery trial. Circulation 2014;129:2388–2394. [DOI] [PubMed] [Google Scholar]
- 10. Ahn J-M, Roh J-H, Kim Y-H, Park D-W, Yun S-C, Lee PH et al. Randomized trial of stents versus bypass surgery for left main coronary artery disease: 5-year outcomes of the PRECOMBAT study. J Am Coll Cardiol 2015;65:2198–2206. [DOI] [PubMed] [Google Scholar]
- 11. Stone GW, Kappetein AP, Sabik JF, Pocock SJ, Morice M-C, Puskas J et al. Five-year outcomes after PCI or CABG for left main coronary disease. N Engl J Med 2019;381:1820–1830. [DOI] [PubMed] [Google Scholar]
- 12. Holm NR, Mäkikallio T, Lindsay MM et al. Percutaneous coronary angioplasty versus coronary artery bypass grafting in the treatment of unprotected left main stenosis: updated 5-year outcomes from the randomised, non-inferiority NOBLE trial. Lancet 2020;395:191–199. [DOI] [PubMed] [Google Scholar]
- 13. Giacoppo D, Colleran R, Cassese S, Frangieh AH, Wiebe J, Joner M et al. Percutaneous coronary intervention vs coronary artery bypass grafting in patients with left main coronary artery stenosis: a systematic review and meta-analysis. JAMA Cardiol 2017;2:1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thuijs D, Kappetein AP, Serruys PW, Mohr FW, Morice MC, Mack MJ et al. ; SYNTAX Extended Survival Investigators. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019;394:1325–1334. [DOI] [PubMed] [Google Scholar]
- 15. Doenst T, Haverich A, Serruys P, Bonow RO, Kappetein P, Falk V et al. PCI and CABG for treating stable coronary artery disease: JACC review topic of the week. J Am Coll Cardiol 2019;73:964–976. [DOI] [PubMed] [Google Scholar]
- 16. Capodanno D, Stone GW, Morice MC, Bass TA, Tamburino C. Percutaneous coronary intervention versus coronary artery bypass graft surgery in left main coronary artery disease: a meta-analysis of randomized clinical data. J Am Coll Cardiol 2011;58:1426–1432. [DOI] [PubMed] [Google Scholar]
- 17. Athappan G, Patvardhan E, Tuzcu ME, Ellis S, Whitlow P, Kapadia SR. Left main coronary artery stenosis: a meta-analysis of drug-eluting stents versus coronary artery bypass grafting. JACC Cardiovasc Interv 2013;6:1219–1230. [DOI] [PubMed] [Google Scholar]
- 18. Head SJ, Mack MJ, Holmes DR, Mohr FW, Morice M-C, Serruys PW et al. Incidence, predictors and outcomes of incomplete revascularization after percutaneous coronary intervention and coronary artery bypass grafting: a subgroup analysis of 3-year SYNTAX data. Eur J Cardiothorac Surg 2012;41:535–541. [DOI] [PubMed] [Google Scholar]
- 19. Gersh BJ, Frye RL. Methods of coronary revascularization—things may not be as they seem. N Engl J Med 2005;352:2235–2237. [DOI] [PubMed] [Google Scholar]
- 20. Quadri G, D’Ascenzo F, Moretti C, D’Amico M, Raposeiras-Roubín S, Abu-Assi E et al. Complete or incomplete coronary revascularisation in patients with myocardial infarction and multivessel disease: a propensity score analysis from the “real-life” BleeMACS (Bleeding complications in a Multicenter registry of patients discharged with diagnosis of Acute Coronary Syndrome) registry. Eurointervention 2017;13:407–414. [DOI] [PubMed] [Google Scholar]
- 21. Gaudino M, Bakaeen FG, Benedetto U, Di Franco A, Fremes S, Glineur D et al. ; ATLANTIC (Arterial Grafting International Consortium) Alliance members. Arterial grafts for coronary bypass: a critical review after the publication of ART and RADIAL. Circulation 2019;140:1273–1284. [DOI] [PubMed] [Google Scholar]
- 22. Glineur D, Rahouma M, Grau JB, Etienne PY, Fortier JH, Papadatos S et al. FFR cutoff by arterial graft configuration and location: IMPAG trial insights. JACC Cardiovasc Interv 2020;13:143–144. [DOI] [PubMed] [Google Scholar]
- 23. Lorusso R, Moscarelli M, Di Franco A, Grazioli V, Nicolini F, Gherli T et al. Association between coronary artery bypass surgical techniques and postoperative stroke. J Am Heart Assoc 2019;8:e013650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barbero U, D'Ascenzo F, Campo G, Kleczyński P, Dziewierz A, Menozzi M et al. Safety of FFR-guided revascularisation deferral in Anatomically prognostiC diseasE (FACE: CARDIOGROUP V STUDY): a prospective multicentre study. Int J Cardiol 2018;270:107–112. [DOI] [PubMed] [Google Scholar]
- 25. D'Ascenzo F, Omedè P, De Filippo O, Cerrato E, Autelli M, Trabattoni D et al. Impact of final kissing balloon and of imaging on patients treated on unprotected left main coronary artery with thin-strut stents (From the RAIN-CARDIOGROUP VII Study). Am J Cardiol 2019;123:1610–1619. [DOI] [PubMed] [Google Scholar]
- 26. Dellborg M, Bonaca MP, Storey RF, Steg PG, Im KA, Cohen M et al. Efficacy and safety with ticagrelor in patients with prior myocardial infarction in the approved European label: insights from PEGASUS-TIMI 54. Eur Heart J Cardiovasc Pharmacother 2019;5:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D’Ascenzo F, Barbero U, Abdirashid M, Trabattoni D, Boccuzzi G, Ryan N et al. Incidence of adverse events at 3 months versus at 12 months after dual antiplatelet therapy cessation in patients treated with thin stents with unprotected left main or coronary bifurcations. Am J Cardiol 2020;125:491–499. [DOI] [PubMed] [Google Scholar]
- 28. De Filippo O, D’Ascenzo F, Raposeiras-Roubin S, Abu-Assi E, Peyracchia M, Bocchino PP et al. P2Y12 inhibitors in acute coronary syndrome patients with renal dysfunction: an analysis from the RENAMI and BleeMACS projects. Eur Heart J Cardiovasc Pharmacother 2020;6:31–42. [DOI] [PubMed] [Google Scholar]
- 29. Bonaca MP, Storey RF, Theroux P, Steg PG, Bhatt DL, Cohen MC et al. Efficacy and safety of ticagrelor over time in patients with prior MI in PEGASUS-TIMI 54. J Am Coll Cardiol 2017;70:1368–1375. [DOI] [PubMed] [Google Scholar]
- 30. Patterson T, McConkey HZR, Ahmed-Jushuf F, Moschonas K, Nguyen H, Karamasis GV et al. Long-term outcomes following heart team revascularization recommendations in complex coronary artery disease. J Am Heart Assoc 2019;8:e011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.