Abstract
Goldenseal has been used for the treatment of a wide variety of ailments including gastrointestinal disturbances, urinary tract disorders, and inflammation. The five major alkaloid constituents in goldenseal are berberine, palmatine, hydrastine, hydrastinine, and canadine. When goldenseal was evaluated by the National Toxicology Program (NTP) in the standard 2-year bioassay, goldenseal induced an increase in liver tumors in rats and mice; however, the mechanism of goldenseal-associated liver carcinogenicity remains unknown. In this study, the toxicity of the five goldenseal alkaloid constituents was characterized, and their toxic potencies were compared. As measured by the Comet assay and the expression of γ-H2A.X, berberine, followed by palmatine, appeared to be the most potent DNA damage inducer in human hepatoma HepG2 cells. Berberine and palmatine suppressed the activities of both topoisomerase (Topo) I and II. In berberine-treated cells, DNA damage was shown to be directly associated with the inhibitory effect of Topo II, but not Topo I by silencing gene of Topo I or Topo II. In addition, DNA damage was also observed when cells were treated with commercially available goldenseal extracts and the extent of DNA damage was positively correlated to the berberine content. Our findings suggest that the Topo II inhibitory effect may contribute to berberine- and goldenseal-induced genotoxicity and tumorigenicity.
Keywords: Berberine, Comet assay, DNA damage, Goldenseal, γ-H2A.X, Topoisomerase
1. Introduction
Goldenseal (Hydrastis canadensis) is available in a wide array of herbal products on the international market. According to the American Herbal Products Association (AHPA), over 90% of its member businesses sell goldenseal-containing products (CITES, 1997; NatureServe), with sales close to $50 million annually in the United States alone (Govindan and Govindan, 2000). Pharmacological studies of goldenseal have focused on its alkaloid constituents, particularly on berberine. Because of their purported beneficial effects, goldenseal and berberine have been used for a wide range of remedies, both internally and externally, for their anti-catarrhal, anti-inflammatory, anti-diabetes, antiseptic, astringent, bitter tonic, laxative, and muscular stimulant properties (Hamon, 1990; NTP, 2009).
Goldenseal was nominated to the National Toxicology Program (NTP) for toxicity evaluation based on the potential high level of human exposure and the lack of carcinogenicity data. The NTP has conducted 2-year carcinogenicity bioassays on goldenseal powder in rodents. The primary finding was that goldenseal increases the incidence of liver tumors in rats and mice (Dunnick et al., 2011): as summarized in an NTP report (NTP, 2009), “there was clear evidence of carcinogenic activity of goldenseal root powder in male F344/N rats based on the increased incidences of hepatocellular adenoma and carcinoma. There was clear evidence of carcinogenic activity of goldenseal root powder in female F344/N rats based on the increased incidence of hepatocellular adenoma. There was some evidence of carcinogenic activity of goldenseal root powder in male B6C3F1 mice based on the increased incidences of hepatoblastoma and multiple hepatocellular adenomas”. Since the mechanism of goldenseal-associated liver carcinogenicity is unknown, it is of great interest to investigate the potential mechanisms that may contribute to its carcinogenicity.
DNA topoisomerases are evolutionally conserved nuclear enzymes that are able to break and rejoin the sugar-phosphate backbone of DNA and modulate the topology of DNA helix (Champoux, 2001; Vos et al., 2011). Cells have two types of DNA topoisomerases: Topo I acts by introducing a transient breakage of a single strand of DNA, while Topo II transiently breaks both DNA strands; both types of breaks are essential for DNA replication during cell proliferation. Inhibition of topoisomerase activity also results in significant genotoxicity and jeopardizes cellular functions (McClendon and Osheroff, 2007).
Unrepaired DNA breaks trigger chromosomal translocations, multiloci deletions, and loss of heterozygosity, all of which contribute to carcinogenicity. Clinically, an association between exposure to topoisomerase inhibitors and secondary leukemia has been reported (Boos and Stopper, 2000; Hijiya et al., 2009; Seiter et al., 2001; Tebbi et al., 2007). Previous studies have indicated that berberine, berberubine (one of berberine’s metabolites), and some protoberberine alkaloids could inhibit the activities of topoisomerases (Kang and Chung, 2002; Kettmann et al., 2004; Kim et al., 1998; Kobayashi et al., 1995; Li et al., 2000; Qin et al., 2007); however, it remains unclear if the inhibitory effect occurs on Topo I or II or both.
In the present study, we examined five goldenseal constituents for their inhibitory effect on Topo I and Topo II in a cell free system and for their ability to induce DNA damage in HepG2 cells. Our results indicate that berberine and, to a lesser degree, palmatine, cause profound DNA strand breakage. Berberine and palmatine inhibit the activity of both Topo I and II. In addition, berberine modulates the G1 and G2/M checkpoint responses. Also, in HepG2 cells, the Topo II inhibitory effect of berberine is involved in generating excessive DNA damage which may contribute to berberine- and goldenseal-induced genotoxicity and tumorigenicity.
2. Materials and methods
2.1. Chemicals and reagent
Berberine was purchased from TCI America (Portland, OR); palmatine, hydrastine, and hydrastinine were from Sigma-Aldrich (St. Louis, MO); canadine (Fig. 1) was purchased from Ryan Scientific, Inc. (Mount Pleasant, SC). Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Lawrenceville, GA). William’s E medium, penicillin, streptomycin, hydrogen peroxide (H2O2), and dimethysulfoxide (DMSO) were purchased from Sigma-Aldrich. Purified human Topo I and II enzymes and Topo I and II assay kits were from TopoGen, Inc. (Port Orange, FL).
Fig. 1.
Structures of five goldenseal constituents.
2.2. Cell culture
The human hepatoma cell line HepG2 was obtained from the American Type Culture Collection (ATCC; Manassas, VA). The cells were grown in Williams’s E medium supplemented with 10% FBS and antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin) at 37 °C in a humidified atmosphere with 5% CO2. Unless otherwise specified, HepG2 cells were seeded at a concentration of 2–5 × 105 cells/ml in volumes of 100 μl in the wells of 96-well plates, in volumes of 5 ml in 60 mm tissue culture plates, or in volumes of 10 ml in 100 mm tissue culture plates. Cells were cultured for about 24 h prior to treatment with the indicated concentrations of goldenseal constituents or the vehicle control, DMSO (final concentration 0.1%).
The 293T cell line used for lentivirus packaging was purchased from Biosettia (San Diego, CA) and maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS in the presence of 1 mM sodium pyruvate, non-essential amino acids, and antibiotics.
2.3. Vector construction and stable cell lines establishment
Two specific target sites for silencing of human Topo I and two sites for silencing of Topo II were identified using RNAi Designer available at the Invitrogen’s website (https://rnaidesigner.invitrogen.com/rnaiexpress/index.jsp). Small hairpin RNA (shRNA) sequences were designed according to the structure of a Doxcyclin (Dox) inducible shRNA lentiviral expression vector (Biosettia Inc., San Diego, CA). The following shRNAs-encoding DNA oligos containing inner palindromic sequences were synthesized (Biosythesis, Inc., Lewisville, Texas): sh1-topo I (5′-AAAAGCATAGCAACAGTGAACATTTGGATCCAAATGTTCACTGTTGCTATGC-3′) and sh2- topo I (5′-AAAAGGATGATGCTGATTATAAATTGGATCCAATTTATAATCAGCATCATCC-3′) were used for silencing of Topo I. sh1-topo II (5′-AAAAGGAAACAGCCAGTAGAGAATTGGATCCAATTCTCTACTGGCTGTTTCC-3′) and sh2-topo II (5′-AAAAGCCATCCACTTCTGATGATTTGGATCCAAATCATCAGAAGTGGATGGC-3′) were used for silencing of Topo II. The scramble shRNA (5′-AAAAGCTACACTATCGAGCAATTTTGGATCCAAAATTGCTCGATAGTGTAGC-3′), which did not contain significant homology to known genes, was used as control in silencing experiments. DNA oligos were annealed to each other to form a double-stranded oligo and then ligated into linearized vector pLV-H1TetO-GFP-Bsd following the manufacturer’s instructions (Biosettia Inc.). The generated lenti-shRNA vectors and viral packaging system were co-transfected into 293T cells to produce lenti-shRNA viral stocks. HepG2 cells were then infected with lentivirus carrying scramble control shRNA or shRNA targeting either Topo I or II transcript, followed by colony selection in the presence of 6 μg/ml Blasticidin (Bsd) to generate a polyclone of HepG2 cells with stable expression of Dox-inducible scramble control or stable sh-topo I or sh-topo II expression. In total, five stable cell lines were generated, namely, SC (scramble control), sh1-topo I (Topo I silencing cell line 1), sh2-topo I (Topo I silencing cell line 2), sh1-topo II (Topo II silencing cell line 1), and sh2-topo II (Topo II silencing cell line 2).
2.4. Cell cycle analysis
Cells (1 × 106/ml) were trypsinized and fixed on ice in 1 ml 70% cold ethanol for 1 h. After washing twice with cold PBS, cells were resuspended in 0.25 ml of ice-cold PBS containing 0.2 μg/μl RNase A (Qiagen, Valencia, CA) and incubated at 37 °C for 1 h. Propidium iodide (PI; Sigma-Aldrich) was added to the cells at a final concentration of 10 μg/ml and the cells were further incubated at 4 °Covernight. The analysis of DNA content (cell cycle analysis) was performed the following day on a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA). Data were acquired using FACSDiva software (BD Biosciences) and the cell cycle distribution was determined using ModFit LT 2.0 (Verity Software House Inc., Topsham, ME).
2.5. High-performance liquid chromatography (HPLC) determination
HPLC analysis of berberine and other goldenseal alkaloids content in commercial goldenseal products was performed using a refrigerated Varian 920LC system. A Phenomenex (Torrance, CA. USA) Luna C18(2) (5 μm particles 100 A pore) HPLC Column (150 mm × 3 mm), equipped with a Phenomenex Secure Guard cartridge, was used. The mobile phase composition for the first 3.5 min was 10% methanol and 90% of an aqueous solution of 0.1% formic acid. The methanol percentage increased to 20% at 5 min, 30% at 10 min, 35% at 15 min, and held at 35% for 5 min before returning to the initial 10% at 22 min. An equilibration time of 18 min was included. The flow rate was initially 0.4 ml/min and was increased to 0.6 ml/min over 0.5 min and held at that rate for 40.5 min.
The commercial goldenseal extracts were initially diluted 1/100 in 0.1% formic acid running buffer and a mix containing the five standards was included with each run. Berberine was detected with a Varian PDA (wavelength range from 200 to 450 nm 4 nm slits) and eluted at approximately 17 min under these conditions. The chromatogram was monitored at wavelengths of 265 and 347 nm. The other elution times were: hydrastinine, approximately 3 min; hydrastine, approximately 4.0 min (a second peak was present in the hydrastine standard, with an elution time of approximately 12 min); canadine, approximately 15.5 min; and palmatine, approximately 18 min. Linear regression was performed for calculating concentration of berberine in each extract.
2.6. Assay of Topo I-mediated relaxation of supercoiled plasmid
The Topo I enzyme assay was conducted by measuring the relaxation of negative supercoiled plasmid DNA pBR322 (TopoGen). A 20 μl mixture reaction containing 10 mM Tris HCl (pH 7.9), 150 mM NaCl, 1 mM EDTA, 0.1 mM spermidine, 0.1% BSA, 5% glycerol, and 250 ng supercoiled plasmid DNA was incubated with or without the test compound (berberine or other goldenseal constituents). Camptothecin (CPT) was used a positive control. The enzyme reaction was initiated by addition of 2 units of purified human Topo I (TopoGen) at 37 °C for 30 min.
2.7. Assay of Topo II-mediated kDNA decatenation
Topo II enzymatic activity was assayed by measuring decatenation of kinetoplast DNA (kDNA) (TopoGen). A total of 20 μl reaction containing 50 mM Tris HCl (pH 8.0), 150 mM NaCl, 10 mM MgCl2, 2 mM ATP, 0.5 mM DTT, 30 μg/ml BSA, and 125 ng kDNA was incubated with or without test compound; the enzyme reaction was initiated by the addition of 1 unit purified human Topo II (TopoGen) at 37 °C for 30 min.
2.8. Comet assay
The Comet assays were performed under alkaline condition to detect DNA single and double strand breaks. HepG2 cells were seeded in a 100 mm tissue culture plate, and the cells were exposed to goldenseal constituents up to 50 μM, vehicle control (0.1% DMSO), or positive control (200 μM H2O2 or 0.1 μg/ml 4-NQO) for 2 h or 6 h. Cells were washed twice with PBS (Ca2+ Mg2+ free), collected by trypsin digestion, and resuspended in PBS (Ca2+ Mg2+ free) at a concentration of 1 × 105 cells/ml. Then 50 μl of cell suspension was combined with 500 μl molten 0.75% low melting point agarose (Trevigen Inc., Gaithersburg, MD), and 50 μl was immediately pipetted onto a Trevigen CometSlide™. After solidification, the slides were immersed in 4°C prechilled lysis solution for 1 h. The slides were then placed in a electrophoresis tank filled with ice-cold electrophoretic buffer (200 mM NaOH and 1 mM EDTA, pH > 13) for 1 h to allow DNA unwinding. Electrophoresis was performed in the same buffer for 45 min at 33 V and 260 mA. After electrophoresis, the slides were removed and rinsed by placing them in ice-cold ddH2O twice, 5 min each time, and then in ice-cold 70% ethanol for 5 min, then stained with 100 μl of SYBR® Gold dye (Life technologies, Grand Island, NY) just before analysis. The slides were scored using an imaging analysis system consisting of a Nikon 501 fluorescent microscope (Melville, NY) and a Comet Assay IV digital analysis system (Perceptive Instruments Ltd., Edmunds, UK). A total of 100 cells per sample was scored and data was pooled together as an endpoint. The percentage of DNA in tails was used as the parameter for evaluation of DNA breakage.
2.9. RNA isolation and real-time TaqMan PCR assay
Total RNA from cells was isolated using the RNeasy system (Qiagen, Germantown MD). Determination of quality and quantity was as described previously (Li et al., 2011).
Real-time TaqMan PCR assays for Topo I and Topo II were conducted using a Bio-Rad CFX96™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Reactions were prepared according to the manufacturer’s instructions for TaqMan® Gene Expression Assays (Applied Biosystems by Life Technologies, Foster City, CA). TaqMan assays were Topo I (Hs00243257_m1), Topo II (Hs00172214_m1), and endogenous control β-actin (ACTB, Hs01060665_g1). Assays were run with Applied Biosystems’ Universal Master Mix (2×) without AmpErase UNG, using universal cycling conditions (10 min at 95 °C; 15 s at 95 °C, 1 min 60 °C, 40 cycles). Data normalization and analysis were conducted as described previously (Guo et al., 2009).
2.10. Western blot analysis
Cells were grown and treated with berberine and commercial goldenseal extracts in 100 mm tissue culture plates or 60 mm tissue culture plates. Standard Western blots were performed using antibodies against total H2A.X, γ-H2A.X, p21Waf1/Cip1, p-ChK1 (Ser-345), p-ChK2 (Thr-68) (Cell Signaling Technology, Danvers, MA), and β-actin (internal control, Santa Cruz Biotechnology, Santa Cruz, CA) followed by a secondary antibody conjugated with horseradish peroxidase (HRP). The intensity of each band was quantified with UVP BioSpectrum Imaging System (UVP, LLC; Upland, CA).
2.11. Statistical analysis
Data are presented as mean ± standard deviation (SD) of at least three independent experiments. Analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA). Statistical significance was determined by one way analysis of variance (ANOVA) followed by the Dunnett’s tests for pairwise-comparisons or two way ANOVA followed by the Bonferroni post-test. The difference was considered statistically significant when p < 0.05.
3. Results
3.1. Berberine induces DNA damage in HepG2 cells
To assess DNA damaging potential of goldenseal constituents, a single-cell gel electrophoresis procedure, often termed the “Comet assay”, was used. HepG2 cells were treated with berberine, palmatine, hydrastine, hydrastinine, or canadine at 50 μM for 24 h. Of the five constituents tested, berberine caused a strong induction of Comet tails and palmatine caused moderate induction, whereas the other three compounds did not increase the tail intensity (data not shown). Berberine-induced DNA strand breakage was further examined at various berberine concentrations. As shown in Fig. 2A, treatment with berberine resulted in a dose- and time-dependent increase in the Comet tail intensity. Significant DNA damage was observed as early as at 2 h; a 16.6% tail intensity was observed with 6.25 μM berberine treatment, whereas only a 7.7% tail intensity appeared with the control. At 6 h and 24 h, berberine caused extensive DNA breakage starting at 3.125 μM with tail intensities of 14.6% and 17.9%, respectively.
Fig. 2.
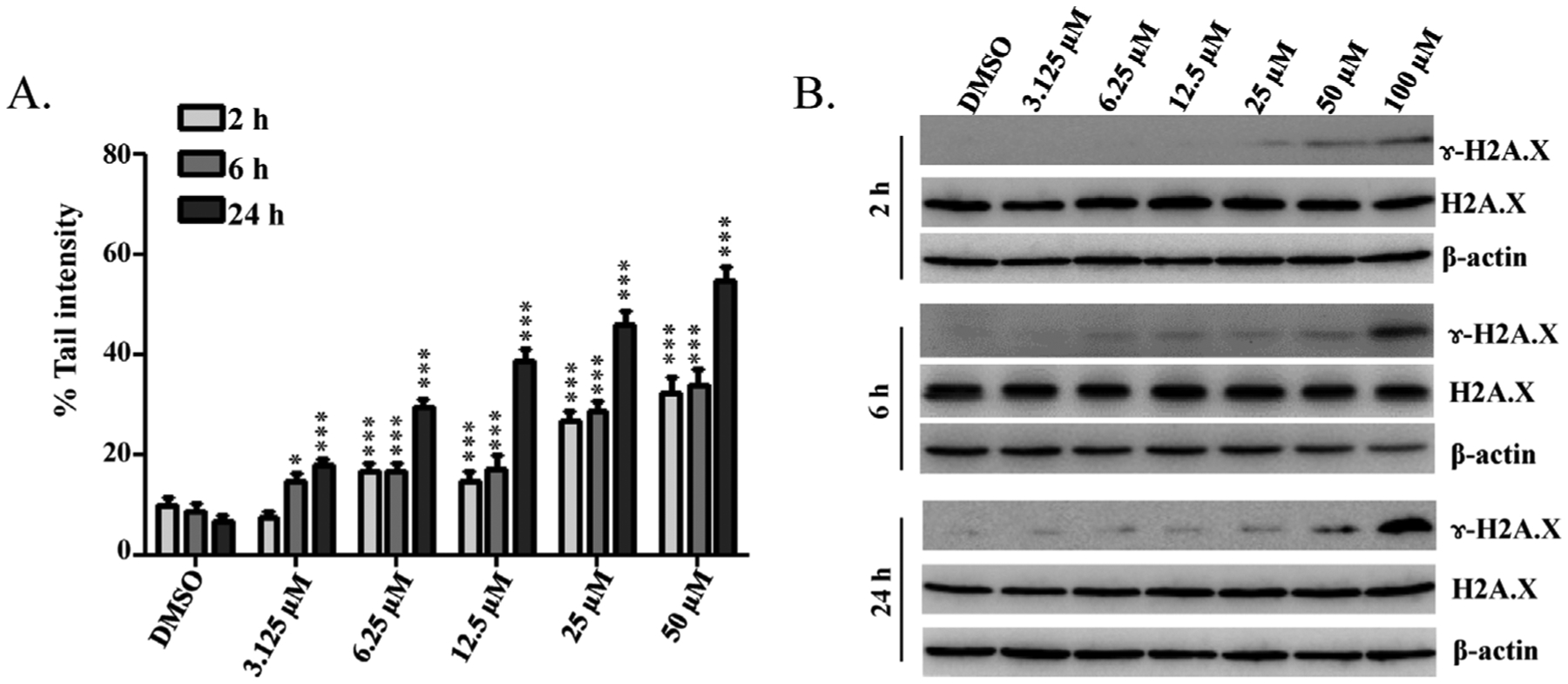
Berberine induces DNA damage in HepG2 cells. HepG2 cells were exposed to increased concentrations (0, 3.125, 6.25, 12.5, 25, 50, or 100 μM) of berberine for 2, 6, or 24 h. (A) Quantification of the percentages of cells with Comet tails at different concentrations and time points. Data are the means ± S.D. (n = 100 cells in each of three trials; * p < 0.05, ***p < 0.001). (B) Total cellular protein was extracted at the indicated times, and levels of phosphorylated (γ-H2A.X) and total histones H2A.X were detected by Western blotting. β-actin was used as a loading control. Similar results were obtained from three independent experiments.
The presence of DNA strand breaks was confirmed by measuring histone H2A.X phosphorylation at Serine139 (γ-H2A.X), a hallmark of DNA strand breakage in cells (Rogakou et al., 1998). As shown in Fig. 2B, phosphorylated γ-H2A.X was detected in cells treated with 100 μM berberine at 2 h and was dramatically elevated in cells treated at 24 h. The levels of total H2A.X protein were not different between berberine treated cells and the vehicle control, indicating that berberine is critical for the efficient induction of phosphate H2A.X (γ-H2A.X) after DNA damage.
3.2. Commercial goldenseal products cause DNA damage in HepG2 cells and the extent of the damage correlates with berberine content
Next, whether or not eight commercial goldenseal extract products produce DNA damage in liver cells was determined. We evaluated DNA damage capacity using γ-H2A.X as the indicator of DNA strand breaks. Berberine content in each goldenseal extract ranged from 5.3 to 49.5 mM as determined by HPLC (Fig. 3A). HepG2 cells were exposed to a constant volume of eight goldenseal extracts on 100 mm dishes for 6 h (20 μl in 10 ml medium). The resulting berberine final concentrations in cultures were from 10.6 to 99 μM. As depicted in Fig. 3B, Western blot analysis showed that γ-H2A.X was induced by most extracts, and the levels of γ-H2A.X were dependent on berberine concentration, i.e. the higher the berberine content in the goldenseal extract, the stronger the induction of γ-H2A.X. For example, Extract #1 (E1), with a final berberine concentration of 99 μM in the cell culture medium, showed the greatest γ-H2A.X induction, whereas Extract #8 (10.6 μM berberine) did not induce a detectable increase in γ-H2A.X (Fig. 3B). A linear relationship was observed when densitometric quantitation of the levels of normalized γ-H2A.X expression (the ratio of the γ-H2A.X to β-actin) were plotted against berberine contents in the eight commercial goldenseal extracts (correlation coefficient = 0.9758 and p < 0.001) (Fig. 3C). These results indicate that the goldenseal products induce DNA damage in liver cell cultures and DNA damage may be attributed to the berberine contained in them.
Fig. 3.
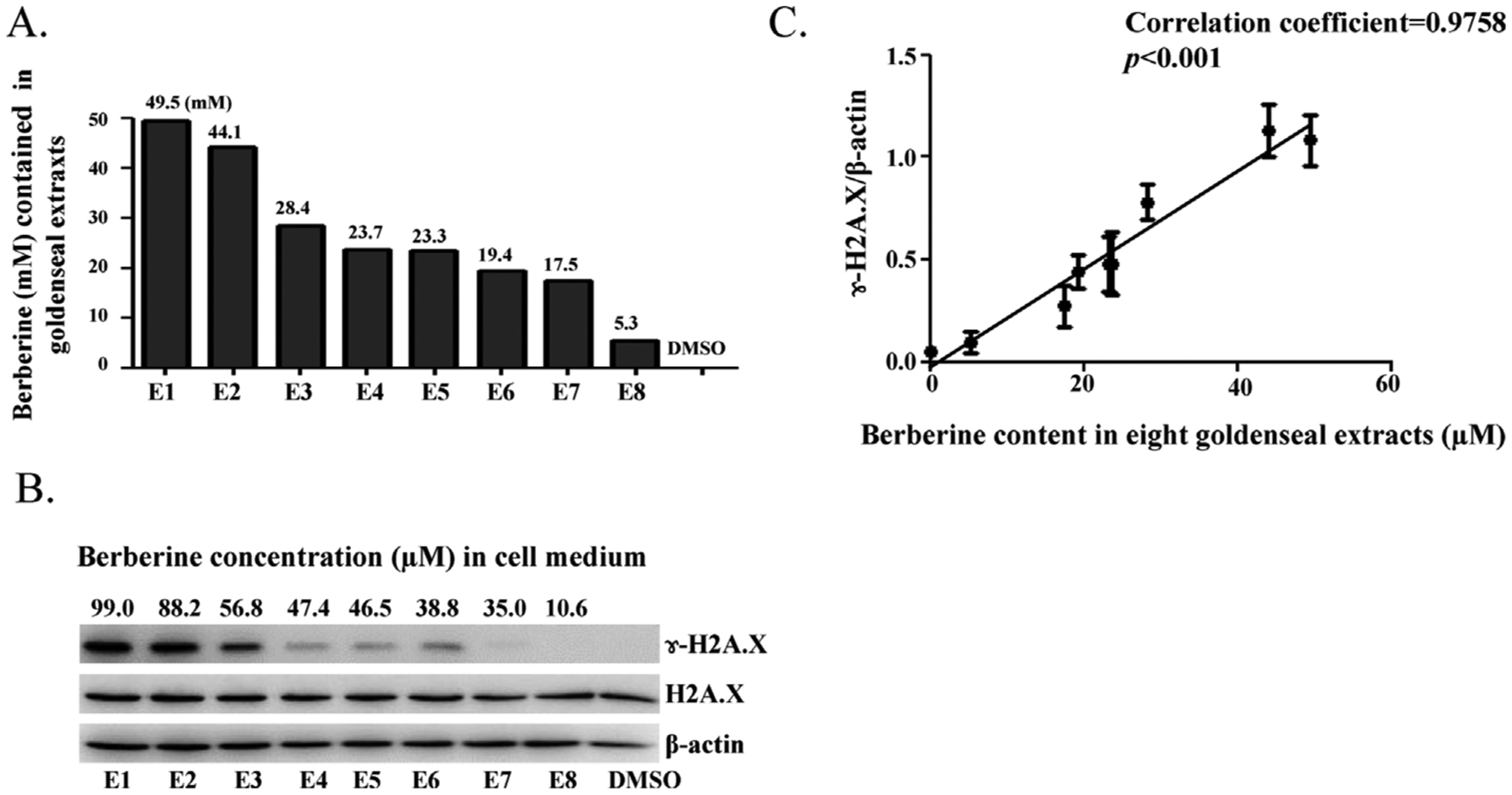
Commercial goldenseal extracts induce DNA damage and the extent of DNA damage correlates with berberine content in the extracts. (A) The bar graph represents the berberine concentrations of eight commercially available goldenseal products as measured by HPLC. (B) HepG2 cells were treated with 20 μl of commercial goldenseal extracts for 6 h in 10 ml culture medium and analyzed for γ-H2A.X by Western blotting. β-actin was used as a loading control. (C) Statistical analysis of the relationship between the expression levels of γ-H2A.X and the concentration of berberine in eight commercial goldenseal extracts. Plot represents quantitative expression levels, shown in (B), of γ-H2A.X normalized by β-actin. The band intensities in the Western blots were determined by Image J software. Data were expressed as means ± S.D. of three separate experiments.
3.3. Berberine induces cell cycle arrest and activates checkpoints related protein in HepG2 cells
HepG2 cells were treated with various concentrations of berberine for 24 h (under these conditions, no significant cytotoxicity was observed with the lactate dehydrogenase (LDH) assay as described previously (Li et al., 2012), Harker et al., 1991; Supplemental Fig. 1), and cell cycle profiles were analyzed by measuring the DNA content. Flow cytometric cell cycle distribution analysis indicated that the percentage of cells in S-phase decreased with increasing concentration of berberine, with parallel increases in the percentage of cells in G1-phase and G2/M-phase, compared to DMSO-treated cells (Fig. 4A and B). These results implied that berberine blocks cell cycle progression by arresting cells in G1 and G2/M phases.
Fig. 4.
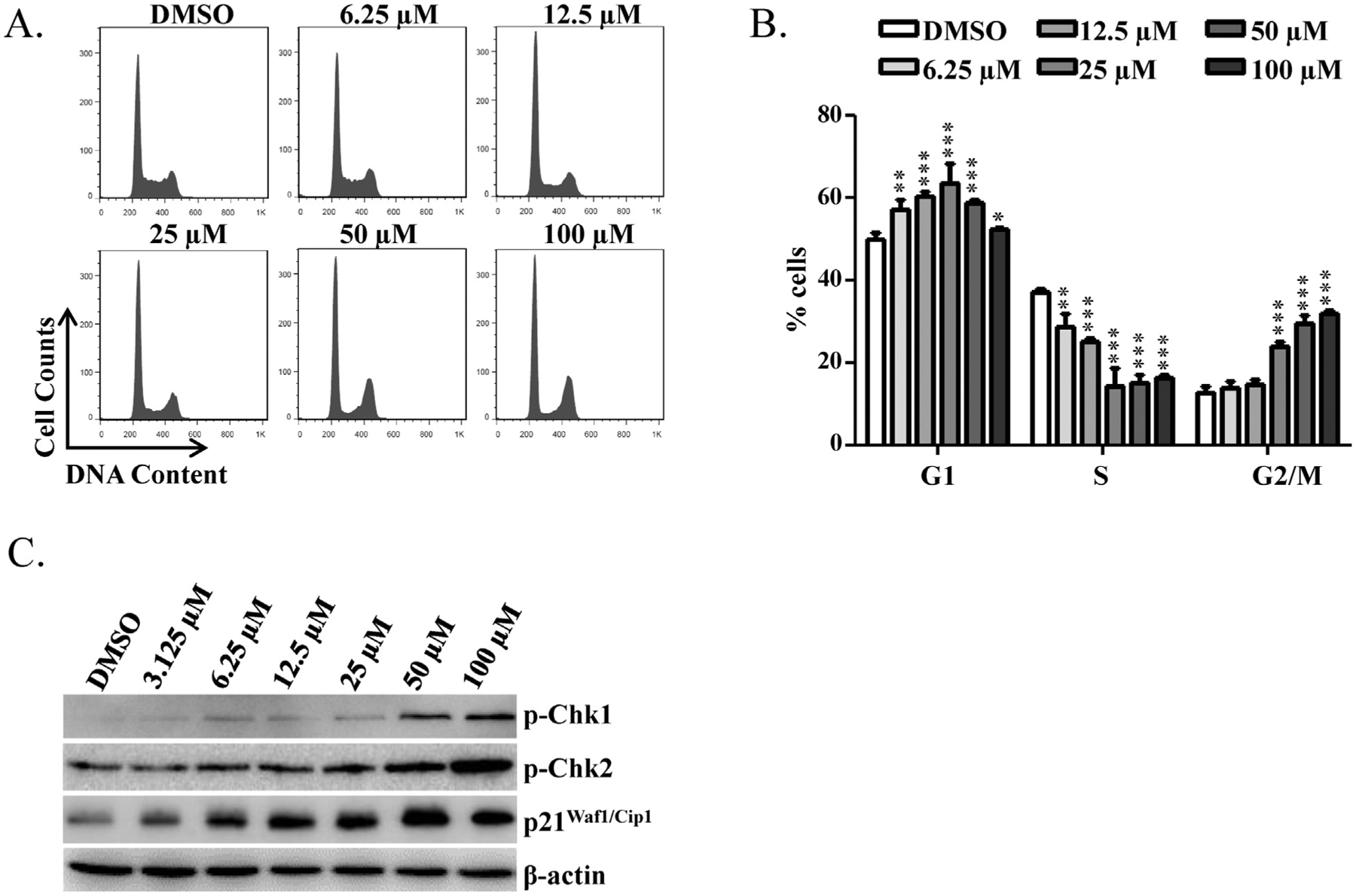
Effects of berberine on cell cycle of HepG2 cells. (A) Flow cytometric analysis for cell cycle distribution. Histograms shown are DNA content analyses for HepG2 cells treated with the indicated concentrations of berberine for 24 h. Treated cells were stained with propidium iodide (PI) and processed for cell cycle analysis. (B) The bar graph depicts the mean percentage of each cell cycle phase ± S.D. from four independent experiments. * p < 0.05, ** p < 0.005, and ***p < 0.001 versus the respective G1, S, or G2/M phase of DMSO-treated cells. (C) Expression of cell cycle checkpoint-related proteins. HepG2 cells were treated with the indicated concentrations of berberine for 6 h. Treated cells were lysed and subjected to Western blotting analyses with antibodies against phospho-Chk1, phosphor-Chk2, and p21Waf1/Clp1. β-actin was used as a loading control.
To confirm further that berberine induces cell cycle arrest, cell cycle checkpoint-related proteins, specifically phosphorylated-Chk1 (p-Chk1), phosphorylated-Chk2 (p-Chk2), and p21Waf1/Cip1, were examined with Western blots. It is known that activated Chk2 and p21Waf1/Cip1 lead to cell cycle arrest in G1 phase (Chehab et al., 2000; Gartel and Radhakrishnan, 2005; Matsuoka et al., 1998). Activated Chk1 phosphorylates and inhibits Cdc25C, thus arresting cells at G2/M (Matsuoka et al., 1998; Sanchez et al., 1997). Starting at the 3.125 μM, treatment of HepG2 cells with berberine for 6 h displayed dose-dependent increase in p-Chk1, p-Chk2, and p21Waf1/Cip1 (Fig. 4C). Taken together, these data indicate that berberine induces cell cycle arrest at G1 and G2/M through activation of Chk1, Chk2, and p21Waf1/Cip1.
3.4. The inhibitory effect of goldenseal constituents on Topo I and II activities
Previously, it was reported that berberine can inhibit topoisomerases (Gatto et al., 1996; Kang and Chung, 2002; Kobayashi et al., 1995), but whether Topo I, Topo II, or both can be so inhibited remained unclear. In addition, it was not known if the other goldenseal alkaloid constituents exert similar effects. Thus, we investigated the effect of goldenseal constituents on the enzymatic activities of Topo I and Topo II in cell free systems. The effect of five goldenseal constituents on the Topo I-mediated DNA relaxation of supercoiled plasmid DNA pBR322 was studied. As shown in Fig. 5A, berberine and palmatine at 500 μM inhibited the DNA relaxation activity of Topo I (as judged by a decrease in the fraction of relaxed DNA and an increase in supercoiled DNA), whereas the other three did not. The inhibitory effects of both berberine and palmatine were also dose-dependent (Fig. 5B and C).
Fig. 5.
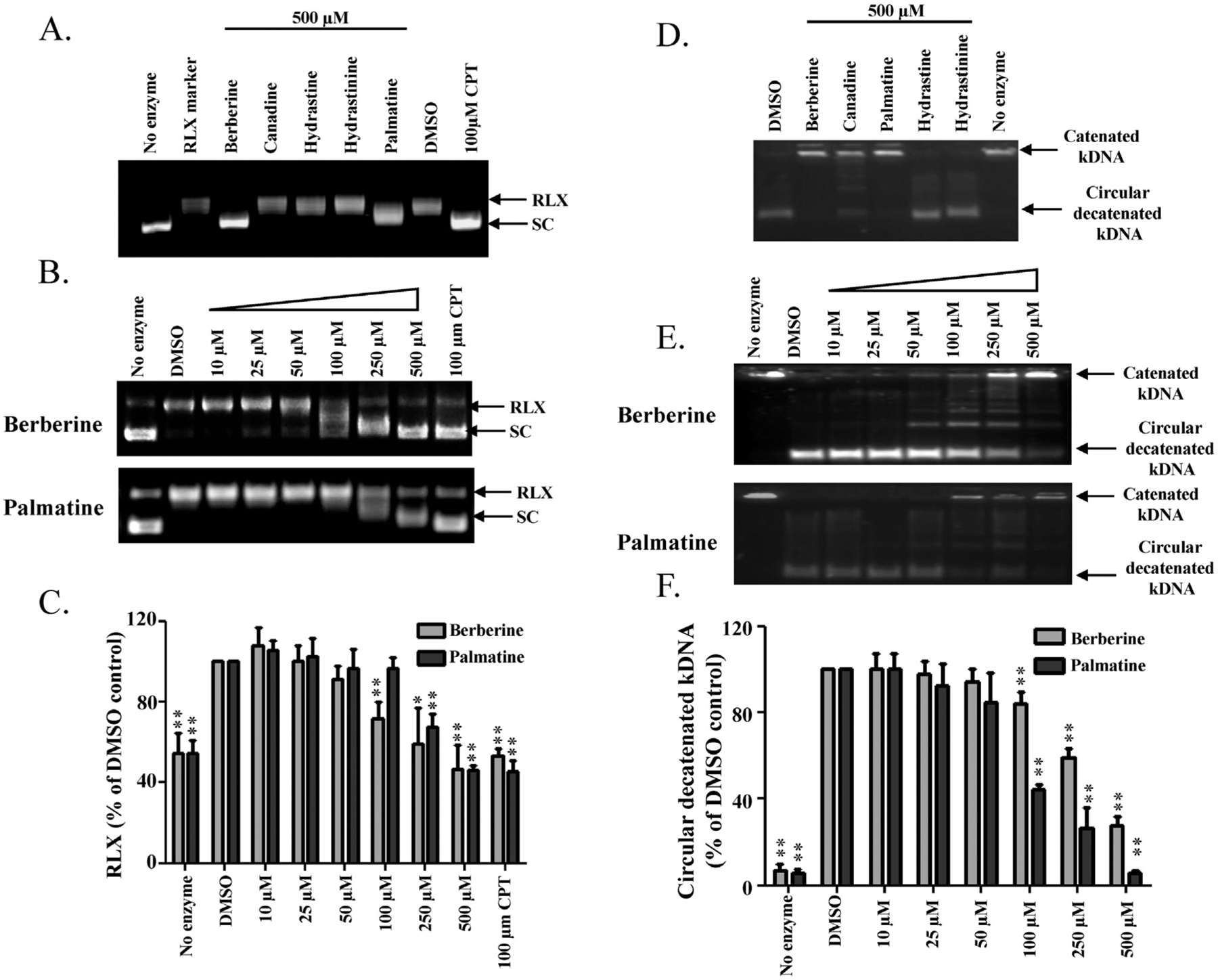
Inhibitory effects of goldenseal constituents on topoisomerase activities. Supercoiled DNA relaxation assay for Topo I (A, B) and kDNA decatenation assay for Topo II (D, E). Supercoiled pBR322 plasmid DNA or kDNA was incubated at 37 °C for 30 min with or without Topo I or Topo II enzyme in the presence of the indicated goldenseal constituents (A, D) or increasing concentrations of berberine and palmatine (B, E); CPT (camptothecin) was used as a Topo I positive control inhibitor. DNA samples were separated by electrophoresis on 1% agarose gel, stained with ethidium bromide, and visualized by UV light. RLX, relaxed DNA; SC, supercoiled DNA. Bar graphs C and F represent the densitometric analyses of the results from B and E. Values are means ± S.D. of three separate experiments. * p < 0.05, ** p < 0.005 versus the DMSO control.
The effect of goldenseal constituents on Topo II-mediated decatenation of double-stranded catenated kinetoplast DNA (kDNA) was also assessed. At 500 μM, berberine and palmatine significantly inhibited Topo II activity as evidenced by reducing the amount of circular decatenated kDNA and increasing the amount of catenated kDNA, while canadine showed marginal Topo II inhibition (Fig. 5D). The inhibitory effects of berberine and palmatine were dose-dependent (Fig. 5E and F). Taken together, these results clearly demonstrated that berberine and palmatine are inhibitors of both Topo I and II.
3.5. The DNA damaging effect of berberine is Topo II dependent but Topo I independent
To investigate the role of Topo I and II in berberine-induced DNA damage, a lentivirus system was used to establish doxycycline (DOX)-induced silencing of Topo I and II in HepG2 cells. Two sets of shRNAs, targeting Topo I (sh1-Topo I and sh2-Topo I) or Topo II (sh1-Topo II and sh2-Topo II), were designed, and all were shown to down-regulate transcription of their respective targets, Topo I or Topo II, with efficiencies of 67% and 89% for Topo I and 94% and 83% for Topo II (Fig. 6A and B). DNA damage was investigated by measuring levels of γ-H2A.X in HepG2 cells exposed to 0, 50, or 100 μM berberine for 6 h after the silencing of Topo I or II. The Western blotting results showed that silencing Topo II alleviated DNA damage (Fig. 6D). In contrast, no significant change in γ-H2A.X was observed in berberine-treated cells after Topo I was silenced, compared to the scrambled control (Fig. 6C). These data suggest that berberine causes DNA breakage via a Topo II-mediated, may not a Topo I-mediated, mechanism.
Fig. 6.
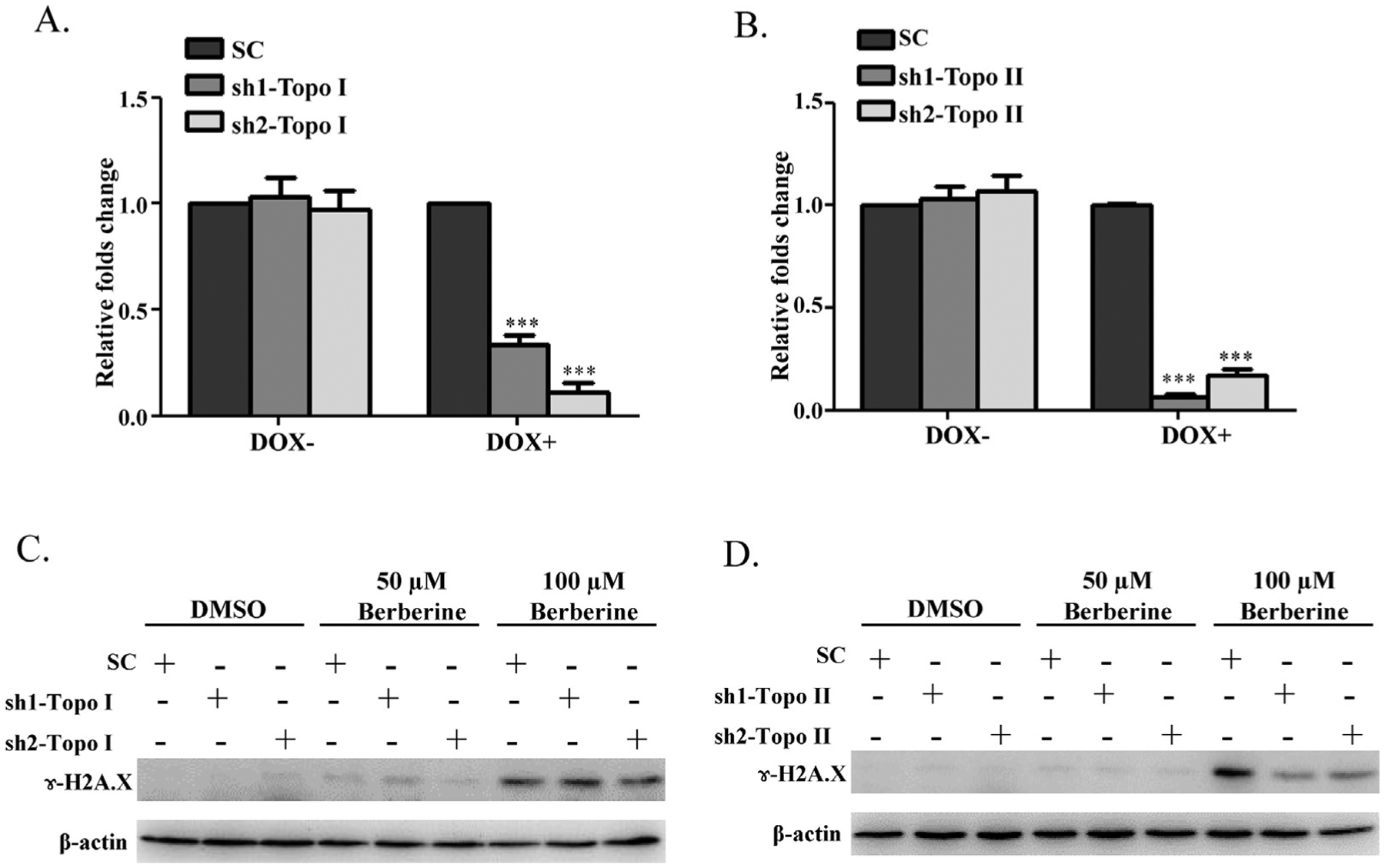
Effect of down-regulation of Topo I or Topo II on berberine induced DNA damage. (A, B) Establish HepG2 cells stably express doxycycline (DOX) inducible sh1-Topo I, sh2-Topo I, sh1-Topo II, sh2-Topo II, or a scrambled (SC) control. These five cell lines were incubated with DOX for 3 days followed by continued culture for another 6 h without DOX; then the decrease in Topo I or Topo II mRNA was assessed by real-time PCR. The results indicated that the mRNA expression levels of Topo I (A) or Topo II (B) were significantly decreased by DOX induction of the shRNAs. Values were means ± S.D. of three separate experiments. *** p < 0.001 versus the SC controls. (C) sh1-Topo I, sh2-Topo I, (D) sh1-Topo II, sh2-Topo II, and SC cells were incubated with DOX for 3 days and then treated with berberine at 50 μM or 100 μM for another 6 h without DOX. Treated cells were then lysed and subjected to Western blot analyses with an antibody against γ-H2A.X. β-actin was used as a loading control. Similar results were obtained from three repeated experiments.
4. Discussion
The use of herbal dietary supplements has become popular in the United States since the US Congress passed the Dietary Supplement Health and Education Act (DSHEA) in 1994, but despite reports of human toxicity associated with their use, concerns about the safety of herbal products have not been adequately addressed. The mechanisms of toxicity remain unclear for many herbal products and data on the identification of toxic ingredients in herbal products are also largely lacking. For such reasons, a number of herbal dietary supplements have been assessed by the NTP for toxicity and tumorigenicity (Fu et al., 2008; Guo et al., 2008, 2009, 2010). Goldenseal root powder was one of the herbal dietary supplements selected for assessment, and the primary finding of the NTP’s study was that chronic exposure to goldenseal causes an increase in liver tumors in rodents (Dunnick et al., 2011; NTP, 2009). However, the mechanism of goldenseal-associated tumorigenicity was entirely unknown and the toxic ingredients in goldenseal remained to be identified.
In the present study, we evaluated DNA damaging capacity of five alkaloid constituents in goldenseal and explored potential molecular mechanisms of induction of the damage. We used hepatic cells in our study because the primary target organ of goldenseal-induced carcinogenicity is liver. We chose HepG2 cells because they demonstrated increased values in toxicological studies (Dykens et al., 2008; Guo et al., 2006; Liu and Zeng, 2009; Nguyen et al., 2013; Wei et al., 2009), and importantly, the capability of genetic modification (in our case, silencing genes of interest) provides more opportunities for studying in-depth mechanisms. Although the lack of some drug metabolizing genes (as we demonstrated previously (Guo et al., 2011)) could be problematic for certain specific studies, it was not a major concern for us because this current study was not designed to study the impact of metabolism on berberine or other constituents. Our data demonstrated that out of the five goldenseal alkaloid constituents tested in vitro, berberine has a relatively high DNA damaging capacity as measured with the Comet assay (Fig. 2A). Further study showed that berberine induced DNA double strand breaks evidenced by the enhancement of cellular levels of γ-H2A.X (Fig. 2B). Previous studies suggested that berberine and its metabolites may inhibit activity of topoisomerases and that there may be a link between berberine-induced DNA damage and topoisomerase inhibition (Kang and Chung, 2002; Kettmann et al., 2004; Kim et al., 1998; Kobayashi et al., 1995; Li et al., 2000; Qin et al., 2007).
Topo I and II-targeted inhibitors are commonly classified as catalytic inhibitors and poisons (Bartek and Lukas, 2003; Larsen et al., 2003; Wilstermann and Osheroff, 2003). Topoisomerases catalytic inhibitors interact with the free enzyme and inhibit the formation of topo-DNA complexes, thus blocking all subsequent steps in the catalytic cycle of the enzymes. In contrast, the topoisomerase poisons freeze the normally transient topo-DNA complexes; act after the cleavage of DNA by the enzyme and inhibit the religation. Topoisomerase poisons act by blocking the ligation step of the cell cycle, thus generating single and double stranded breaks that destroy the integrity of the genome. Although some topoisomerase inhibitors have therapeutic efficacy in human cancer, the use of topoisomerase inhibitors can also cause secondary tumor formation by interfering with DNA repair processes and inducing chromosomal aberrations (Ezoe, 2012). Our studies with Topo I-mediated pBR322 relaxation and Topo II-mediated kDNA decatenation clearly demonstrated that berberine suppressed the activities of both Topo I and II (Fig. 5).
Berberine possess low affinity of direct binding DNA (Mazzini et al., 2003; Park et al., 2004), in addition, our data also showed that berberine does not act as a DNA intercalator (Supplemental Fig. 2), suggesting that berberine’s DNA damaging effect is unlikely through the direct interaction with DNA.
Most Topo II inhibitors, such as etoposide (Fortune and Osheroff, 2000) and emodin (Li et al., 2010), interfere the cleavage and religation step in the normal action of the enzymes, stabilize cleavable Topo-DNA complexes, and the trigger DNA double strand breaks. Berberine has demonstrated distinct enzyme poisoning activities by stabilizing topoisomerase II-DNA cleavable complexes (Jeon et al., 2002). Moreover, when silencing Topo II in HepG2 cells, DNA double strand breaks were effectively suppressed (Fig. 6D), suggesting that inhibition of Topo II activity and stabilization of Topo II-DNA complexes play a critical role in the induction of DNA double strand breaks and DNA damage may not be associated with inhibition of Topo I, although berberine inhibited the activities of both Topo I and II in cell free system (Fig. 5).
DNA damage triggers the activation of checkpoint responses that arrest the cell cycle and stimulate the transcription of genes that facilitate DNA repair. Ongoing DNA damage leads to genomic instability that is involved in initiation and promotion of cancer. Ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad-3-related (ATR), members of the phoshpoinositide kinases-related protein family, are two most important kinases responding to DNA double strand breaks and subsequent cell cycle arrest (Mei et al., 2003; Shiloh, 2001). Once activated by DNA damage, ATM and ATR phosphorylate a number of substrates including Chk1 and Chk2, which in turn target other proteins to induce cell cycle arrest and facilitate DNA repair. Chk1 activation requires phosphorylation at Ser-317 and Ser-345 catalyzed by ATM and/or ATR (Gatei et al., 2003; Zhao and Piwnica-Worms, 2001). Chk2 is activated by ATM-mediated phosphorylation at Thr-68 (Bartek and Lukas, 2003). Activation of Chk1 and Chk2 further phosphorylate downstream proteins such as Cdc25 family and p53. Phosphorylation of Cdc25 family contributes to G2/M arrest, while phosphorylation of p53 enhances the transcription of p21waf1/cip1 and result in G1 arrest and/or G2/M arrest (Matsuoka et al., 1998; Niculescu et al., 1998; Sanchez et al., 1997; Waldman et al., 1995). Most importantly, once ATM and ATR activated, these kinases rapidly phosphorylate multiple effector molecules at the sites of damage, such as H2A.X, a variant of the core histone H2A family, to produce γH2A.X, which contributes to nucleosome-formation and serves as a biomarker of DNA double strand breaks (Ivashkevich et al., 2012). Our results include induction of p-Chk1, p-Chk2, p21Waf1/Cip1 (Fig. 4C), and γ-H2A.X (Fig. 2B), and cell cycle arrest (Fig. 4A and B) indicate there is a cascaded cellular response in response to the exposure of berberine.
The suggested maximum human oral dose of berberine for the treatment of diabetes (http://examine.com/supplements/Berberine) or cardiac dysfunction is 2000 mg daily (Zeng et al., 2003). Berberine is absorbed from gastrointestinal tract following oral administration (Deng et al., 2008; Qiu et al., 2008) and it can be actively transported to the liver (Tsai and Tsai, 2004). Although bioavailability of berberine is low (0.68%), some co-administered drugs can enhance its absorption (Chen et al., 2011). Clinical studies have shown that the plasma concentration of berberine can reach to 0.39 mg/l (1.2 μM) after a single oral dose of 300 mg berberine (Ye et al., 2009) and 0.3 mg/l (0.9 μM) after multi-dose oral administration of 1200–2000 mg berberine per day for eight weeks (Zeng et al., 2003). In our studies, toxic effect of berberine became apparent at 3.125 μM (Fig. 2), which is close to the plasma concentration detected in human blood samples. Thus, the use of berberine at the suggested clinically relevant levels may carry a risk of DNA damage.
It is worth mentioning that DNA damage was also observed with commercial goldenseal products and the extent of DNA damage was positively correlated with the berberine content, with a correlation coefficient of 0.98 (Fig. 3). Berberine was detected as the major alkaloid component in commercial goldenseal extracts. Our data support the notion that berberine is the major contributor to goldenseal-induced DNA damage and monitoring the concentration of berberine in goldenseal products can be used to predict the toxicity of goldenseal.
In conclusion, we demonstrated that berberine, the major goldenseal alkaloid constituent, induced DNA damage in liver cells, and confirmed that DNA damage effect is via its interaction with topoisomerase. We suspect that the DNA damaging ability and tumorigenicity of berberine and goldenseal may be due in part to the inhibition of topoisomerase by berberine. The use of goldenseal or berberine products may be not without risk due to this “off-target” toxicity.
Supplementary Material
HIGHLIGHTS.
The toxic potencies of five goldenseal alkaloid constituents were compared.
Berberine appeared to be the most potent DNA damage inducer.
DNA damage was directly associated with the inhibitory effect of Topo II.
The extent of DNA damage was positively correlated to the berberine content in goldenseal products.
Acknowledgements
SC, HL, and YL were supported by appointments to the Postgraduate Research Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science Education through an interagency agreement between the U.S. Department of Energy and the U.S. FDA. LW was supported by the U.S. FDA’s International Scientist Exchange Program. We thank Dr. William Melchior for his critical review of this manuscript.
This article is not an official guidance or policy statement of the U.S. FDA. No official support or endorsement by the U.S. FDA is intended or should be inferred.
Footnotes
Conflicts of interest
None.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxlet.2013.05.641.
References
- Bartek J, Lukas J, 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3, 421–429. [DOI] [PubMed] [Google Scholar]
- Boos G, Stopper H, 2000. Genotoxicity of several clinically used topoisomerase II inhibitors. Toxicol. Lett 116, 7–16. [DOI] [PubMed] [Google Scholar]
- Champoux JJ, 2001. DNA topoisomerases: structure, function, and mechanism. Annu. Rev. Biochem 70, 369–413. [DOI] [PubMed] [Google Scholar]
- Chehab NH, Malikzay A, Appel M, Halazonetis TD, 2000. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 14, 278–288. [PMC free article] [PubMed] [Google Scholar]
- Chen W, Miao YQ, Fan DJ, Yang SS, Lin X, Meng LK, Tang X, 2011. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech 12, 705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CITES, 1997. Amendments to Appendices I and II of the Convention on International Trade in Endangered Species and Wild Fauna and Flora.
- Deng Y, Liao Q, Li S, Bi K, Pan B, Xie Z, 2008. Simultaneous determination of berberine, palmatine and jatrorrhizine by liquid chromatography-tandem mass spectrometry in rat plasma and its application in a pharmacokinetic study after oral administration of coptis-evodia herb couple. J. Chromatogr. B: Analyt. Technol. Biomed. Life Sci 863, 195–205. [DOI] [PubMed] [Google Scholar]
- Dunnick JK, Singh B, Nyska A, Peckham J, Kissling GE, Sanders JM, 2011. Investigating the potential for toxicity from long-term use of the herbal products, goldenseal and milk thistle. Toxicol. Pathol 39, 398–409. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Jamieson JD, Marroquin LD, Nadanaciva S, Xu JJ, Dunn MC, Smith AR, Will Y, 2008. In vitro assessment of mitochondrial dysfunction and cytotoxicity of nefazodone, trazodone, and buspirone. Toxicol. Sci 103, 335–345. [DOI] [PubMed] [Google Scholar]
- Ezoe S, 2012. Secondary leukemia associated with the anti-cancer agent, etoposide, a topoisomerase II inhibitor. Int. J. Environ. Res. Public Health 9, 2444–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune JM, Osheroff N, 2000. Topoisomerase II as a target for anticancer drugs: when enzymes stop being nice. Prog. Nucleic Acid Res. Mol. Biol 64, 221–253. [DOI] [PubMed] [Google Scholar]
- Fu PP, Xia Q, Guo L, Yu H, Chan PC, 2008. Toxicity of kava kava. J. Environ. Sci. Health C: Environ. Carcinog. Ecotoxicol. Rev 26, 89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartel AL, Radhakrishnan SK, 2005. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 65, 3980–3985. [DOI] [PubMed] [Google Scholar]
- Gatei M, Sloper K, Sorensen C, Syljuasen R, Falck J, Hobson K, Savage K, Lukas J, Zhou BB, Bartek J, Khanna KK, 2003. Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation. J. Biol. Chem 278, 14806–14811. [DOI] [PubMed] [Google Scholar]
- Gatto B, Sanders MM, Yu C, Wu HY, Makhey D, LaVoie EJ, Liu LF, 1996. Identification of topoisomerase I as the cytotoxic target of the protoberberine alkaloid coralyne. Cancer Res. 56, 2795–2800. [PubMed] [Google Scholar]
- Govindan M, Govindan G, 2000. A convenient method for the determination of the quality of goldenseal. Fitoterapia 71, 232–235. [DOI] [PubMed] [Google Scholar]
- Guo L, Zhang L, Sun Y, Muskhelishvili L, Blann E, Dial S, Shi L, Schroth G, Dragan YP, 2006. Differences in hepatotoxicity and gene expression profiles by anti-diabetic PPAR gamma agonists on rat primary hepatocytes and human HepG2 cells. Mol. Divers 10, 349–360. [DOI] [PubMed] [Google Scholar]
- Guo L, Shi Q, Fang JL, Mei N, Ali AA, Lewis SM, Leakey JE, Frankos VH, 2008. Review of usnic acid and Usnea barbata toxicity. J. Environ. Sci. Health C: Environ. Carcinog. Ecotoxicol. Rev 26, 317–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Li Q, Xia Q, Dial S, Chan PC, Fu P, 2009. Analysis of gene expression changes of drug metabolizing enzymes in the livers of F344 rats following oral treatment with kava extract. Food Chem. Toxicol 47, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Mei N, Xia Q, Chen T, Chan PC, Fu PP, 2010. Gene expression profiling as an initial approach for mechanistic studies of toxicity and tumorigenicity of herbal plants and herbal dietary supplements. J. Environ. Sci. Health C: Environ. Carcinog. Ecotoxicol. Rev 28, 60–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Dial S, Shi L, Branham W, Liu J, Fang JL, Green B, Deng H, Kaput J, Ning B, 2011. Similarities and differences in the expression of drug-metabolizing enzymes between human hepatic cell lines and primary human hepatocytes. Drug Metab. Dispos 39, 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon NW, 1990. Goldenseal. Can. Pharm. J 123, 508–510. [Google Scholar]
- Harker WG, Slade DL, Drake FH, Parr RL, 1991. Mitoxantrone resistance in HL-60 leukemia cells: reduced nuclear topoisomerase II catalytic activity and drug-induced DNA cleavage in association with reduced expression of the topoisomerase II beta isoform. Biochemistry 30, 9953–9961. [DOI] [PubMed] [Google Scholar]
- Hijiya N, Ness KK, Ribeiro RC, Hudson MM, 2009. Acute leukemia as a secondary malignancy in children and adolescents: current findings and issues. Cancer 115, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkevich A, Redon CE, Nakamura AJ, Martin RF, Martin OA, 2012. Use of the gamma-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 327, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YW, Jung JW, Kang M, Chung IK, Weontae L, 2002. NMR studies on antitumor drug candidates, berberine and berberrubine. Bull. Korean Chem. Soc 23, 391–394. [Google Scholar]
- Kang MR, Chung IK, 2002. Down-regulation of DNA topoisomerase II alpha in human colorectal carcinoma cells resistant to a protoberberine alkaloid, berberrubine. Mol. Pharmacol 61, 879–884. [DOI] [PubMed] [Google Scholar]
- Kettmann V, Kost’alova D, Holtje HD, 2004. Human topoisomerase I poisoning: docking protoberberines into a structure-based binding site model. J. Comput. Aided Mol. Des 18, 785–796. [DOI] [PubMed] [Google Scholar]
- Kim SA, Kwon Y, Kim JH, Muller MT, Chung IK, 1998. Induction of topoisomerase II-mediated DNA cleavage by a protoberberine alkaloid, berberrubine. Biochemistry 37, 16316–16324. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Yamashita Y, Fujii N, Takaboshi K, Kawakami T, Kawamura M, Mizukami T, Nakano H, 1995. Inhibitors of DNA topoisomerase I and II isolated from the Coptis rhizomes. Planta Med. 61, 414–418. [DOI] [PubMed] [Google Scholar]
- Larsen AK, Escargueil AE, Skladanowski A, 2003. Catalytic topoisomerase II inhibitors in cancer therapy. Pharmacol. Ther 99, 167–181. [DOI] [PubMed] [Google Scholar]
- Li TK, Bathory E, LaVoie EJ, Srinivasan AR, Olson WK, Sauers RR, Liu LF, Pilch DS, 2000. Human topoisomerase I poisoning by protoberberines: potential roles for both drug-DNA and drug-enzyme interactions. Biochemistry 39, 7107–7116. [DOI] [PubMed] [Google Scholar]
- Li Y, Luan Y, Qi X, Li M, Gong L, Xue X, Wu X, Wu Y, Chen M, Xing G, Yao J, Ren J, 2010. Emodin triggers DNA double-strand breaks by stabilizing topoisomerase II-DNA cleavage complexes and by inhibiting ATP hydrolysis of topoisomerase II. Toxicol. Sci 118, 435–443. [DOI] [PubMed] [Google Scholar]
- Li Y, Mei H, Wu Q, Zhang S, Fang JL, Shi L, Guo L, 2011. Methysticin and 7,8-dihydromethysticin are two major kavalactones in kava extract to induce CYP1A1. Toxicol. Sci 124, 388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Couch L, Higuchi M, Fang JL, Guo L, 2012. Mitochondrial dysfunction induced by sertraline, an antidepressant agent. Toxicol. Sci 127, 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZH, Zeng S, 2009. Cytotoxicity of ginkgolic acid in HepG2 cells and primary rat hepatocytes. Toxicol. Lett 187, 131–136. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Huang M, Elledge SJ, 1998. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282, 1893–1897. [DOI] [PubMed] [Google Scholar]
- Mazzini S, Bellucci MC, Mondelli R, 2003. Mode of binding of the cytotoxic alkaloid berberine with the double helix oligonucleotide d(AAGAATTCTT)(2). Bioorg. Med. Chem 11, 505–514. [DOI] [PubMed] [Google Scholar]
- McClendon AK, Osheroff N, 2007. DNA topoisomerase II, genotoxicity, and cancer. Mutat. Res 623, 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei N, Lee J, Sun X, Xing JZ, Hanson J, Le XC, Weinfeld M, 2003. Genetic predisposition to the cytotoxicity of arsenic: the role of DNA damage and ATM. FASEB J. 17, 2310–2312. [DOI] [PubMed] [Google Scholar]
- NatureServe, NatureServe Explorer: http://www.natureserve.org/explorer/servlet/NatureServe?searchName=Hydrastis+canadensis+.
- Nguyen KC, Willmore WG, Tayabali AF, 2013. Cadmium telluride quantum dots cause oxidative stress leading to extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2 cells. Toxicology 306C, 114–123. [DOI] [PubMed] [Google Scholar]
- Niculescu AB 3rd, Chen X, Smeets M, Hengst L, Prives C, Reed SI, 1998. Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol. Cell Biol 18, 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP, 2009. NTP technical report on the toxicity and carcinogenesis studies of goldenseal root power in F344/N rats and B6C3F1 mice. NTP TR 562, NIH Publication No 09–5903, National Toxicology Program. [Google Scholar]
- Park HS, Lim E, Sung YH, Kang M, Chung IK, Chaejoon C, Weontae L, 2004. DNA binding mode of the isoquinoline alkaloid berberine with the deoxyoligonucleotide d(GCCGTCGTTTTACA)2. Bull. Korean Chem. Soc 125, 539–544. [Google Scholar]
- Qin Y, Pang JY, Chen WH, Zhao ZZ, Liu L, Jiang ZH, 2007. Inhibition of DNA topoisomerase I by natural and synthetic mono- and dimeric protoberberine alkaloids. Chem. Biodivers 4, 481–487. [DOI] [PubMed] [Google Scholar]
- Qiu F, Zhu Z, Kang N, Piao S, Qin G, Yao X, 2008. Isolation and identification of urinary metabolites of berberine in rats and humans. Drug Metab. Dispos 36, 2159–2165. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM, 1998. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem 273, 5858–5868. [DOI] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ, 1997. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science 277, 1497–1501. [DOI] [PubMed] [Google Scholar]
- Seiter K, Feldman EJ, Sreekantaiah C, Pozzuoli M, Weisberger J, Liu D, Papageorgio C, Weiss M, Kancherla R, Ahmed T, 2001. Secondary acute myelogenous leukemia and myelodysplasia without abnormalities of chromosome 11q23 following treatment of acute leukemia with topoisomerase II-based chemotherapy. Leukemia 15, 963–970. [DOI] [PubMed] [Google Scholar]
- Shiloh Y, 2001. ATM and ATR: networking cellular responses to DNA damage. Curr. Opin. Genet. Dev 11, 71–77. [DOI] [PubMed] [Google Scholar]
- Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, Mendenhall NP, Sposto R, Chauvenet A, Schwartz CL, 2007. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J. Clin. Oncol 25, 493–500. [DOI] [PubMed] [Google Scholar]
- Tsai PL, Tsai TH, 2004. Hepatobiliary excretion of berberine. Drug Metab. Dispos 32, 405–412. [DOI] [PubMed] [Google Scholar]
- Vos SM, Tretter EM, Schmidt BH, Berger JM, 2011. All tangled up: how cells direct, manage and exploit topoisomerase function. Nat. Rev. Mol. Cell. Biol 12, 827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman T, Kinzler KW, Vogelstein B, 1995. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 55, 5187–5190. [PubMed] [Google Scholar]
- Wei W, Zhang C, Liu AL, Xie SH, Chen XM, Lu WQ, 2009. PCB126 enhanced the genotoxicity of BaP in HepG2 cells by modulating metabolic enzyme and DNA repair activities. Toxicol. Lett 189, 91–95. [DOI] [PubMed] [Google Scholar]
- Wilstermann AM, Osheroff N, 2003. Stabilization of eukaryotic topoisomerase II-DNA cleavage complexes. Curr. Top. Med. Chem 3, 321–338. [DOI] [PubMed] [Google Scholar]
- Yamashita Y, Fujii N, Murakata C, Ashizawa T, Okabe M, Nakano H, 1992. Induction of mammalian DNA topoisomerase I mediated DNA cleavage by antitumor indolocarbazole derivatives. Biochemistry 31, 12069–12075. [DOI] [PubMed] [Google Scholar]
- Ye M, Fu S, Pi R, He F, 2009. Neuropharmacological and pharmacokinetic properties of berberine: a review of recent research. J. Pharm. Pharmacol 61, 831–837. [DOI] [PubMed] [Google Scholar]
- Zeng XH, Zeng XJ, Li YY, 2003. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol 92, 173–176. [DOI] [PubMed] [Google Scholar]
- Zhao H, Piwnica-Worms H, 2001. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol. Cell. Biol 21, 4129–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


