Abstract
IL-17-secreting CD8+ T cells (Tc17) have been implicated in immunity to infections, cancer and autoimmune diseases. Thus far, studies on Tc17 cells have investigated primarily their development from naïve precursors, while biology of committed Tc17 cells has been less characterized. Hence, studies focused on the latter cells are needed to understand their regulation during the effector phase of immune responses. IL-27 is an important regulator of inflammation through induction of regulatory Tr1 cells, as well as a potent suppressor of Th17 cell development. IL-27 suppresses development of Tc17 cells, but its effects on committed Tc17 cells are unknown. We demonstrate that even though IL-27 completely inhibited their development, it had little effect on already committed Tc17 cells. Although committed Tc17 cells were capable of responding to IL-27, it had no effect on expression of RORγt and RORα, or production of various cytokines. Committed Tc17 cells did not express granzyme B and lacked cytotoxicity in vitro, features that remained unaltered by IL-27 treatment. Nonetheless, they efficiently induced diabetes, irrespective of treatment with IL-27 prior to transfer into RIP-mOVA mice. These findings suggest that use of IL-27 to modulate autoimmune diseases might have limited therapeutic efficacy if autoaggressive Tc17 cells have already developed.
Keywords: IL-27, Tc17, IL-23, RORγt, T-bet, STAT1, Diabetes
INTRODUCTION
A growing body of evidence implicates IL-17-producing CD8+ T (Tc17) cells in various diseases such as infections, cancer and autoimmunity [1–3]. For example, Tc17 cells are found in the microenvironment of several mouse and human tumors [4] and in mice responding to influenza A infection as well [5]. In psoriasis and in an experimental model of this disease, increased numbers of Tc17 cells are present in blood and in skin lesions [6, 7]. Tc17 cells also constitute a high proportion of T cells infiltrated into active brain lesions in multiple sclerosis [8]. Overall, current knowledge strongly supports the view of Tc17 cells as an important element of both beneficial and harmful immunity, similar to other T cell lineages.
Activation by antigen-presenting cells (APCs) initiates differentiation of naïve CD8+ T cell into one of several effector Tc lineages, which is a process largely directed by cytokine milieu [9]. We and others have shown that, analogous to CD4+ T cells, naïve CD8+ T cells also acquire IL-17-producing phenotype in the presence of TGF-β and IL-6 or IL-21 [5, 10, 11]. In addition to IL-17A, Tc17 cells secrete IL-17F and IL-22, and express IL-23R together with Th17 lineage-specific transcription factors retinoid-related orphan receptors gamma t (ROR)γt and RORα [11]. In addition to phenotypic similarities between Th17 and Tc17 cells, we have shown that differentiated Tc17 cells also require IL-23 signaling to become pathogenic in an adoptive transfer model of autoimmune diabetes [11], as has been demonstrated for Th17 cells [12]. Type I CD8+ cytotoxic T (Tc1) cells that secrete IFN-γ are cytolytic and kill their targets by either perforin/granzyme- or Fas-mediated mechanisms [13]. Studies on the in vitro cytotoxic capacity of Tc17 cells have reported inconsistent results, ranging from its complete absence to a slightly reduced cytolytic activity compared to Tc1 cells [5, 10, 11, 14–16]. Nonetheless, Tc17 eventually became as cytotoxic as Tc1 cells when injected in mice and were efficient in the control of both acute infections and tumor growth [15, 17]. Several groups have reported that Tc17 cells demonstrate phenotypic plasticity similar to Th17 cells [18, 19].
IL-27 belongs to the IL-6/IL-12 family of cytokines and has both anti- and proinflammatory functions [reviewed in [20]]. IL-27 is secreted by activated APCs as a heterodimer of non-covalently associated EBV-induced gene 3 (EBI-3) and p28 subunits [21], and it signals via heterodimeric receptor, comprising gp130 and IL-27RA subunits [22]. IL-27R signaling in CD8+ T cells induces T-bet expression, resulting in IL-12Rβ2 expression, and an increase in cell proliferation and IFN-γ production [23–25]. IL-27 activates multiple STATs, most notably STAT1 and STAT3. While STAT1 phosphorylation leads to a decrease in Gata-3 and RORγt expression, phosphorylation of STAT3 is involved in the expression of IL-10 [26, 27]. Furthermore, IL-27 promotes CD8+ T cell cytotoxicity by inducing expression of granzyme B and perforin [23]. On the other hand, IL-27 induces expression of the anti-inflammatory cytokine IL-10 by CD4+ and CD8+ T cells [26], and treatment with IL-27 attenuates EAE by induction of IL-10 [28]. Overall, the findings presented above highlight the complex and pleiotropic role of IL-27 in immune responses.
We and others have previously reported that IL-27 inhibits the development of Th17 cells but has much less effect on established Th17 cell responses [29, 30]. However, the role played by IL-27 in shaping CD8+ T cell-mediated immunity remains less well characterized. While it is known that IL-27 potently inhibits de novo differentiation of Tc17 cells from naïve precursors [26], the effects of IL-27 on committed Tc17 cells are not known.
In the present study we show that in contrast to its profound inhibition of Tc17 cell development, IL-27 had little effect on committed Tc17 cells. Although committed Tc17 cells expressed functional IL-27R, IL-27 failed to suppress their expression of RORγt and production of IL-17 and other cytokines. Unlike the case of developing Tc17 cells, IL-27 did not convert Tc17 cells to Tc1 cells expressing IFN-γ, and did not suppress pathogenicity of committed Tc17 cells in an adoptive transfer model of diabetes. Taken together, our data clearly demonstrate that phenotype and function of committed Tc17 cells are not substantially altered by IL-27.
RESULTS
IL-27 suppresses Tc17 development in a STAT1- and T-bet-dependent manner.
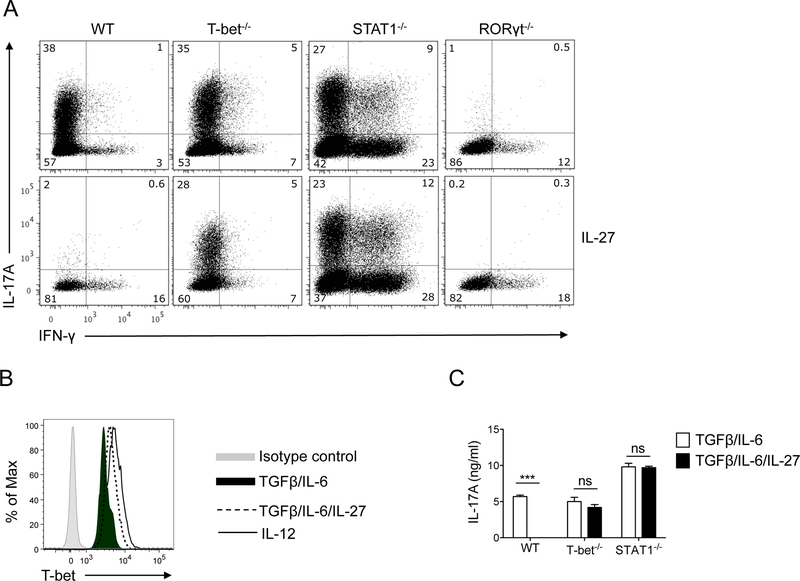
IL-27 potently antagonizes Th17-polarizing effects of TGF-β and IL-6 on naïve CD4+ T cells, and diverts their differentiation toward Th1 lineage [31, 32]. We first sought to determine if IL-27 has similar effects on development of Tc17 cells from naïve CD8+ T cells. Inclusion of IL-27 in cell culture media abrogated the Tc17-promoting effect of TGF-β and IL-6, resulting in development of IFN-γ-producing Tc1 cells instead of IL-17-producing Tc17 cells (Fig. 1A). In contrast to wild-type (WT) cells, IL-27 only modestly suppressed development of T-bet−/− Tc17 cells, demonstrating that T-bet plays a role in IL-27-mediated suppression of Tc17 development. In agreement with this observation, we found that T-bet was upregulated when IL-27 was added to the culture, although not as potently as by IL-12 (Fig. 1B). RT-PCR analyses also show that IL-27 upregulated T-bet expression, although its capacity to do so was reduced by Tc17-polarizing cytokines (Supplementary Fig. 1), most likely by TGF-β [33]. The diminished capacity of IL-27 to suppress polarization of T-bet−/− CD8+ T cells into Tc17 lineage was not related to defective induction of IFN-γ production by IL-27, as both WT and T-bet−/− cultures had a similar small percentage of IFN-γ+ cells. Furthermore, neutralizing anti-IFN-γ Ab was included in the culture media, precluding potential effects of IFN-γ on Tc17 development. Similar to T-bet, the capacity of IL-27 to affect Tc17 development was dependent on STAT1, as STAT1−/− CD8+ T cells remained unaffected by IL-27 (Fig. 1A). Consistent with the crucial role of RORγt in Th17 cell differentiation [34], RORγt−/− CD8+ T cells had impaired polarization into Tc17 cells, which was further suppressed by IL-27 (Fig. 1A), possibly through suppression of RORα. In agreement with flow cytometric data, measurement of IL-17A concentrations in cell culture supernatants confirmed a strong IL-27-mediated suppression of Tc17 differentiation of WT CD8+ T cells, a less potent inhibition of T-bet−/− cells, and no effect on STAT1−/− cells (Fig. 1C). Taken together, these data demonstrate that IL-27 potently inhibits Tc17 cell differentiation in a STAT1- and T-bet-dependent manner. In the above experiments we tested the effect of IL-27 on purified CD8+ T cells activated with mitogenic stimuli. Development of Tc17 cells in the system where CD8+ T cells were activated with their cognate antigen in the presence of other immune (splenic) cells was also efficiently suppressed by IL-27 (Fig. 2), demonstrating that this effect is not an artifact.
Figure 1: The suppressive effect of IL-27 on Tc17 cell differentiation is dependent on STAT1 and T-bet.
CD8+ T cells from WT C57BL/6, T-bet−/−, STAT1−/−, and RORγt−/− mice were activated with anti-CD3 and anti-CD28 Abs in the presence of IL-6+TGF-β ± IL-27 and anti-IFN-γ and anti-IL-4 Abs. Seventy-two hours later, cells were stimulated with PMA and ionomycin in the presence of Golgiplug for 4 h, stained, and analyzed by flow cytometry for IL-17A and IFN-γ. Gating strategy is shown in Supplementary Fig. 2 (A). T-bet expression in CD8+ T cells from WT C57BL/6 mice activated as described above and analyzed by flow cytometry. Expression of T-bet in WT CD8+ T cells activated in the presence of IL-12 is shown as positive control (B). Concentrations of IL-17A in supernatants of cell culture described above were quantified by ELISA (C). Levels of IL-17A in RORγt−/− cultures were below the detection limit (not shown). Data are representative of three experiments (error bars, s.e.m; ns = not significant; ***p<0.001).
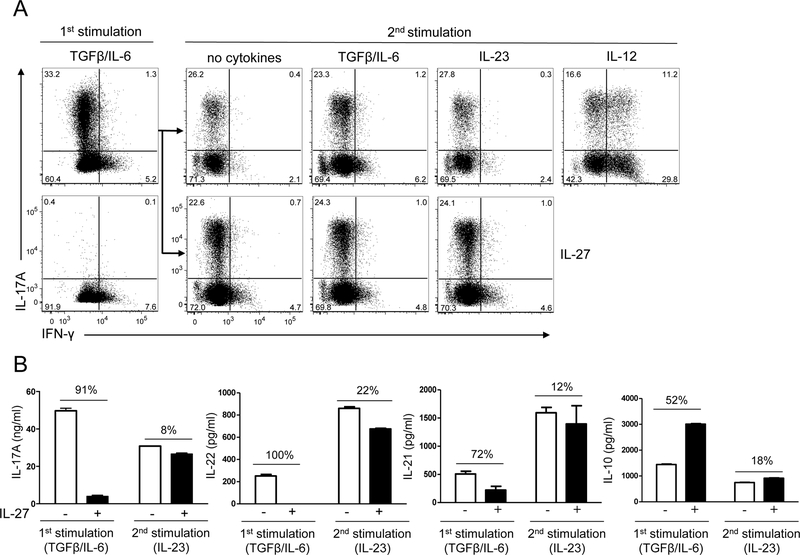
Figure 2: Committed Tc17 cells are resistant to suppression by IL-27.
Splenocytes from OT-I mice were activated with OVA257–264 peptide in the presence of IL-6+TGF-β ± IL-27, and anti-IFN-γ and anti-IL-4 Abs (1st stimulation). Cells were stimulated 72 h later with PMA and ionomycin in the presence of Golgiplug for 4 h and analyzed by flow cytometry for IL-17A and IFN-γ in CD8+ cells (A; left panel). Cells that were not exposed to IL-27 in the 1st stimulation were rested for 2 days in the presence of IL-2 and then restimulated with the peptide in the presence of cytokines indicated in each panel (2nd stimulation). Seventy-two hours later, cells were stimulated with PMA, ionomycin in the presence of Golgiplug for the final 4 h, stained and analyzed by flow cytometry for IL-17A and IFN-γ in CD8+ cells (A; right panel). IL-17A, IL-22, IL-10 and IL-21 concentrations in supernatants of cell culture described in A were quantified by ELISA (B). Changes in cytokine concentrations (%) when IL-27 was added to the culture are indicated above the bars. Data are representative of three experiments (error bars, s.e.m).
IL-27 has no effect on committed Tc17 cells
We have shown previously that IL-27 has surprisingly little effect on phenotype of committed Th17 cells, in stark contrast to its profound effect on developing cells [29]. Here we tested the effect of IL-27 on committed Tc17 cells after one round of polarization with TGF-β and IL-6 (1st stimulation). To this end, we reactivated Tc17 OT-I cells with OVA257–264 peptide (2nd stimulation) in the presence of IL-27. IL-27 had no effect on the percentage of IL-17+CD8+ T cells, irrespective of whether in the 2nd stimulation other exogenous cytokines were added (Fig. 2A). IL-27 also did not promote IFN-γ production, as the percentages of IFN-γ+CD8+ and IL-17+IFN-γ+CD8+ T cells were similar in samples with and without added IL-27. In contrast, addition of IL-12 potently induced production of IFN-γ, as the majority of CD8+ cells became IFN-γ+ (Fig. 2A).
We next measured IL-17A concentrations in the supernatants of OT-I splenic cultures after the 1st stimulation and after reactivation in the presence of IL-23 ± IL-27. IL-27 suppressed production of IL-17A, IL-21 and IL-22 during the 1st stimulation (Fig. 2B). However, production of the aforementioned cytokines in the 2nd stimulation was only slightly downregulated by IL-27 (Fig. 2B). We and others have demonstrated that IL-27 induces expression of IL-10 in both Th1 and Th2 cells but not in developing and committed Th17 cells [26, 28, 29]. In contrast, IL-27 upregulated production of IL-10 in developing but not in committed Tc17 cells (Fig. 2B). These results clearly show that the phenotype of committed Tc17 cells is largely resistant to IL-27.
The presence of large proportions of IL-17A and IFN-γ double negative CD8+ T cells in Tc17 polarized cultures prompted us to analyze the effect of IL-27 on these cells during restimulation. The double negative cells did not express T-bet or RORγt, and IL-27 did not modify their expression. IL-27 also had no effect on expression of T-bet, RORγt and IL-23R in subpopulations of IL-17A+IFN-γ- and IL-17+IFN-γ+ Tc17 cells as well (Supplementary Fig. 3).
We and others have recently shown that GM-CSF production by Th1 and Th17 cells, as well as by CD8+ T cells, is essential to their effector functions [35–38] and that GM-CSF plays a role in diabetes development, an experimental in vivo model that was used in this study [39]. In addition, we have shown that IL-23 enhances GM-CSF production by Th17 cells [35]. It has also been shown that IL-27 inhibits GM-CSF production by Th1 cells, but it did not suppress GM-CSF production by committed Th17 cells [40]. We sought to determine whether IL-27 affects GM-CSF expression by committed Tc17 cells. Addition of IL-27 to Tc17 cells restimulated in the presence of TGF-β and IL-6 or IL-23 did not suppress GM-CSF expression by Tc17 cells (Supplementary Fig. 4A). In agreement with our flow cytometric data, measurement of GM-CSF concentrations in Tc17 culture supernatants confirmed that IL-27 had no suppressive effect (Supplementary Fig. 4B). These data demonstrate that, similar to other cytokines, IL-27 does not alter GM-CSF production by committed Tc17 cells.
Committed Tc17 cells express functional IL-27 receptor
Given that IL-27 had minimal effects on several features of committed Tc17 cells, we tested whether they expressed a functional IL-27R. We measured IL-27RA mRNA expression by Tc17 cells by quantitative PCR after the 1st and 2nd activation. Both developing and committed Tc17 cells expressed significant levels of IL-27RA mRNA and exposure to IL-27 further augmented its expression (Fig. 3A and 3B). To test if IL-27R is functional, we analyzed phosphorylation of STAT1 and STAT3 in response to IL-27 by flow cytometry. To increase cell survival, 2 ng/ml of IL-2 was routinely added to Tc17 cell culture media during a resting period between stimulations. Given that IL-2 can affect expression of IL-27R on activated T cells [41], Tc17 cells that were rested with and without addition of IL-2 were examined in parallel. After the 1st stimulation, committed Tc17 cells were rested for two days and then reactivated in the presence of either IL-6 or IL-27. Treatment with IL-6 was a positive control for phosphorylation of STAT3 [29]. As shown in Figure 3C and 3D, IL-27 induced phosphorylation of both STAT1 and STAT3 in committed Tc17 cells to a similar, if not greater, extent than in naïve cells. Addition of IL-2 to the culture did not modify the response of Tc17 cells to IL-27. These data demonstrate that committed Tc17 cells express functional IL-27R.
Figure 3: Developing and committed Tc17 cells express functional IL-27R and phosphorylate both STAT1 and STAT3 in response to IL-27.
Real-time PCR analysis of IL-27Rα expression in developing (A) and committed Tc17 cells differentiated from naïve WT CD8+ T cells, then rested for two days and restimulated with indicated cytokines (B). Freshly isolated WT CD8+ T cells (C) or committed Tc17 cells rested for 2 days, with or without IL-2 (D), were stimulated or not (NS) with anti-CD3 in the presence of IL-6 or IL-27 for 30 minutes. Cells were fixed, permeabilized and analyzed by flow cytometry for phosphorylated STAT1 (pSTAT1) and STAT3 (pSTAT3). Data are representative of three experiments. (error bars, s.e.m.; *p<0.05; **p<0.01; ***p<0.001).
IL-27 does not down-regulate RORγt and other Tc17 markers
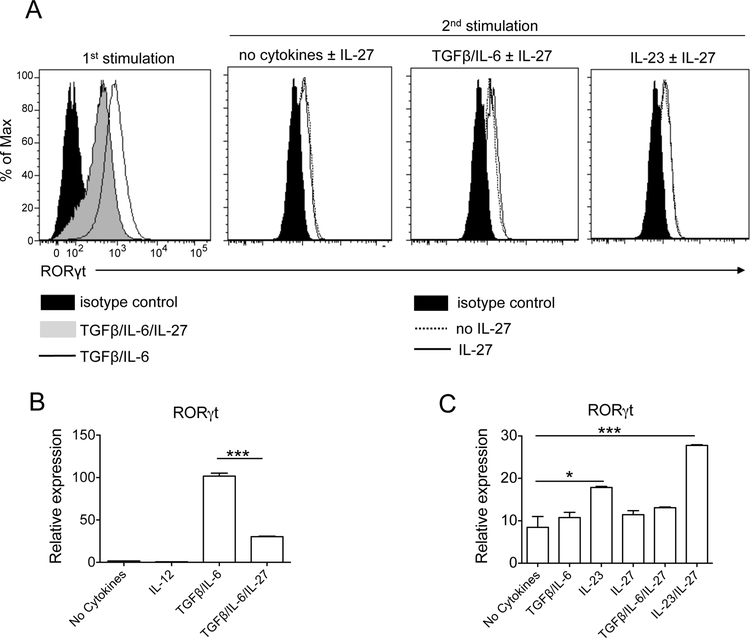
We have previously reported that IL-27 down-regulated expression of RORγt in developing but not in committed Th17 cells [29]. Thus we tested the effect of IL-27 on its expression in Tc17 cells. IL-27 substantially reduced expression of RORγt in developing Tc17 cells, but did not abrogate it, as IL-27-treated cells still contained readily detectable RORγt (Fig. 4A; 1st stimulation). Quantitative PCR analysis of RORγt mRNA in developing Tc17 cells confirmed its limited expression in the presence of increasing concentrations of IL-27 (Fig. 4B and Supplementary Fig. 5A). In addition, a small number of Tc17 cells that had been exposed to IL-27 during the 1st stimulation, upon reactivation in the presence of TGF-β and IL-6, started expressing IL-17A (Supplementary Fig. 5B). Together, these data demonstrate that IL-27 suppresses RORγt expression incompletely and that in a portion of cells the suppressive effect of IL-27 on Tc17 development program is reversible.
Figure 4: IL-27 does not suppress RORγt expression in committed Tc17 cells.
CD8+ T cells of WT C57BL/6 mice were activated with anti-CD3 and anti-CD28 Abs in the presence of IL-6+TGF-β ± IL-27 and blocking Abs to IL-4 and IFN-γ (1st stimulation). After 72 h cells were rested for 2 days in the presence of IL-2 and then restimulated during three days with Abs in the presence of the cytokines indicated in each panel (2nd stimulation). Cells were then stained and analyzed by flow cytometry for RORγt expression (A). Real-time PCR analysis of RORγt expression in developing (B) and committed Tc17 cells (C) differentiated from naïve WT CD8+ T cells and reactivated in the presence of indicated cytokines. Data are representative of three (flow cytometry) and two qPCR experiments. (error bars, s.e.m.; *p< 0.05; ***p<0.001).
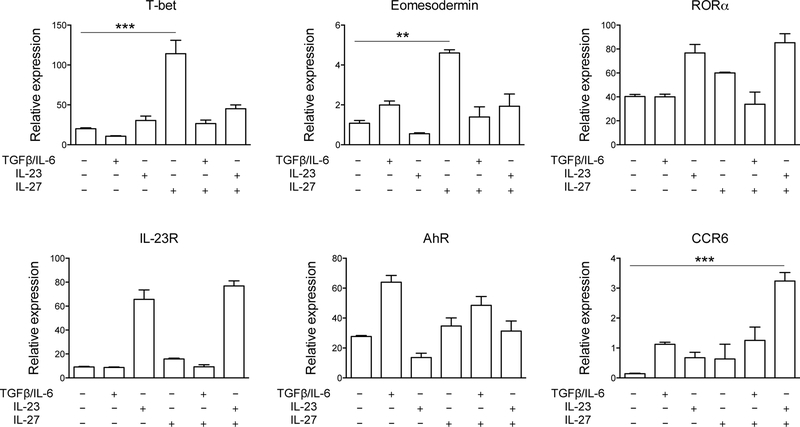
Contrary to the 1st stimulation, RORγt expression in committed Tc17 cells was not affected by IL-27 [Fig. 4A (2nd stimulation); Fig. 4C; Supplementary Fig. 3). We then analyzed the expression of other Tc17 markers, namely T-bet, eomesodermin, RORα, IL-23R, AhR and CCR6 in differentiating and in committed Tc17 cells. During Tc17 development, IL-27 suppressed expression of RORα, Ahr and IL-23R while inducing T-bet (Supplementary Fig. 1). In committed Tc17 cells, IL-27 significantly increased expression of T-bet (~6 fold) and eomesodermin (~5 fold), and synergized with IL-23 to increase expression of CCR6 (~6 fold) (Fig. 5). Levels of RORα, IL-23R, and AhR expression remained unaffected by IL-27. Furthermore, IL-27 had a modest effect on expression of Runx1 in developing and committed Tc17 cells (Supplementary Fig. 6A), but it strongly upregulated Blimp1 expression in committed Tc17 cells (Supplementary Fig. 6B). Taken together, these data demonstrate that IL-27 has certain effects on committed Tc17 cells, while it fails to modulate expression of factors (i.e. RORγt) that are perhaps the most important for maintenance of the Tc17 phenotype.
Figure 5: Expression of Tc17 markers is little affected by IL-27.
Real-time PCR analysis of T-bet, eomesodermin, RORα, IL-23R, AhR, and CCR6 in committed Tc17 cells differentiated from naïve WT CD8+ T cells, then rested for two days and reactivated in the presence of TGF-β+IL-6 or IL-23, ± IL-27; results are presented relative to the expression of 18S ribosomal RNA. Data are representative of two experiments (error bars, s.e.m.; **p<0.01; ***p<0.001).
Tc17 cells do not express granzyme B and lack cytotoxic capacity, irrespective of IL-27
Given the importance of cytotoxicity in the effector function of Tc cells, we analyzed the effect of IL-27 on in vitro cytotoxic capacity of OT-I cells that had first been polarized into Tc1 and Tc17 lineages and subsequently reactivated in the presence of several cytokines (± IL-27). Committed Tc17 cells did not express granzyme B in any conditions tested irrespective of the presence of IL-27 (Fig. 7A). In contrast, most Tc1 cells expressed high levels of granzyme B, and IL-27 and IL-12 increased the proportion of Tc1 cells expressing it (Fig 6A). In agreement with these data, committed Tc17 cells treated with various cytokines were uniformly non-cytotoxic to E.G7-OVA target cells, whereas committed Tc1 cells displayed substantial lytic activity, regardless of IL-27 treatment (Fig. 6B). These results show that committed Tc17 cells are not cytotoxic in vitro, likely because of their lack of granzyme B expression; and that IL-27 does not induce its expression in committed Tc17 cells.
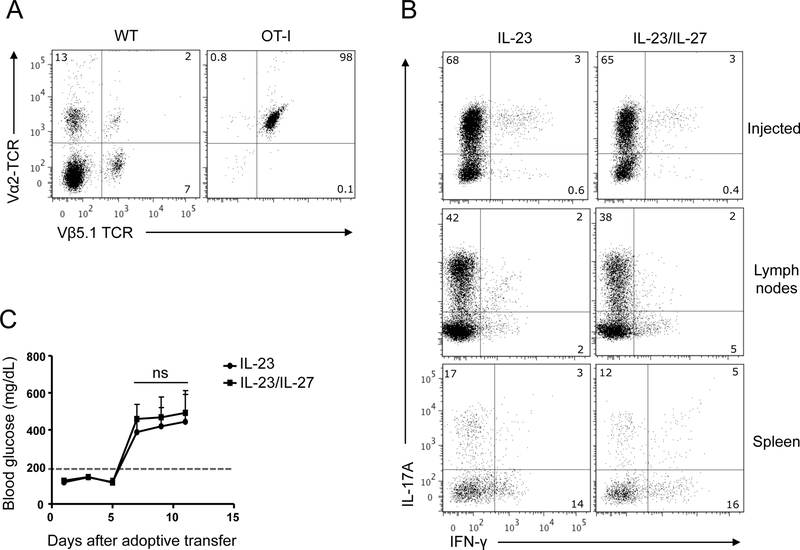
Figure 7: IL-27 does not change pathogenicity of OVA-reactive Tc17 cells.
Flow cytometric analysis of transgenic TCR expression on CD8+ T cells from C57BL/6 and OT-I mice (Vα2+Vβ5.1+ TCR is marker of OT-I T cells) (A). Splenic OT-I Tc17 cells were initially differentiated with TGF-β+IL-6 and OVA257–264 peptide for 3 days and then reactivated in the presence of IL-23 ± IL-27. CD8+ T cells from culture were enriched with magnetic beads and injected i.p. into RIP-mOVA C57BL/6 mice. A portion of Tc17 cells used for the injection was stimulated with PMA and ionomycin in the presence of Golgiplug for 4 h, stained and analyzed by flow cytometry for IL-17A and IFN-γ (B; upper panel). Blood glucose levels >200 mg/dL (dotted line on plot) were considered a sign of hyperglycemia (C). Five days after the transfer, RIP-mOVA C57BL/6 mice were sacrificed and pancreas-draining LN cells or splenocytes were stimulated with PMA and ionomycin in the presence of Golgiplug for 4 h, then stained and analyzed by flow cytometry for IL-17A and IFN-γ on gated OT-I cells identified by Vα2/Vβ5.1 TCR expression, as shown in A (B; medium and lower panels). Data are representative of two experiments with at least 6 mice per group (error bars, s.e.m; ns = not significant).
Figure 6: Tc17 cells do not express granzyme B and are non-cytotoxic irrespective of IL-27.
Tc1 and Tc17 cells differentiated from OT-I splenocytes in the presence of IL-12 or TGF-β+IL-6, respectively, were reactivated with OVA257–264 peptide in the presence of the indicated cytokines. After 72 h, cells were stimulated with PMA, ionomycin in the presence of Golgiplug for 4 h, stained and analyzed by flow cytometry for IL-17A, IFN-γ and granzyme B (A). For cytotoxicity assays, EL4 and EL4-OVA cells, used as target cells, were labeled with two concentrations of CFSE, mixed in a 1:1 ratio (10,000 cells of each per sample), and then combined with OT-I Tc1 (B) or Tc17 cells (C) that were reactivated as described above. After 4 h of incubation at 37° C, cells were analyzed by flow cytometry in the presence of DAPI. Live cells (DAPI-negative) were gated and the percentage of specific cell lysis was calculated from the ratio of EL4 and EL4-OVA cells. Data are representative of three experiments.
IL-27 does not alter pathogenicity of OVA-reactive Tc17 cells
To examine the effect of IL-27 on Tc17 effector function in vivo, we used an IL-23-driven adoptive transfer model of diabetes in which Tc17 cells differentiated from TCR transgenic OT-I OVA-specific mice are injected into RIP-mOVA mice [11]. OVA-specific OT-I cells express a TCR comprising Vα2 and Vβ5.1 variable regions, allowing identification of these cells by immunostaining and flow cytometry (Fig. 7A). Differentiated OT-I Tc17 cells were reactivated in the presence of OVA257–264 and IL-23 ± IL-27. CD8+ T cells were then enriched by magnetic bead separation and injected i.p. into RIP-mOVA mice. Clinical follow-up of the recipient mice showed that mice injected with either IL-23-treated or IL-23 + IL-27-treated Tc17 OT-I cells developed hyperglycemia with similar kinetics and severity (Fig. 7C). We analyzed expression of IL-17A and IFN-γ in Vα2+Vβ5.1+CD8+ cells harvested from pancreatic draining lymph nodes (dLNs) and spleens of recipient mice five days post adoptive transfer, at the time immediately before disease onset. Fewer OT-I cells were found in spleen when compared to dLNs (data not shown). The percentage of OT-I cells expressing IL-17A was 2–3 times higher in dLNs than in spleen, irrespective of IL-27 treatment before cell transfer into RIP-mOVA mice (Fig. 7B). In addition, OT-I cells in dLNs did not start expressing IFN-γ and changed phenotype into IL-17A+IFN-γ+ cells within five days post transfer, while in the spleen a larger percentage of IFN-γ+ OT-I cells was found. Taken together, these results demonstrate that IL-27 did not alter effector functions of committed Tc17 cells in vivo, and did not promote the loss of Tc17 phenotype or accelerated conversion into Th1 cells.
DISCUSSION
It is now clear that, like other T cell lineages, Tc17 cells play an important role in infectious diseases, cancer and autoimmunity. To date, however, studies have focused mainly on their development and only minimally on the impact of various factors, including cytokines, on their long-term function and persistence. Given that IL-27 greatly influences Th17 cells, we sought to investigate whether it also has an effect on development and function of Tc17 cells. We show that developing Tc17 cells are highly susceptible to suppression by IL-27, while already committed Tc17 cells are largely resistant to its effects. IL-27 did not affect RORγt expression or cytokine production by committed Tc17 cells. Importantly, IL-27 did not alter their effector function in an adoptive transfer model of diabetes. Committed Tc17 cells expressed functional IL-27 receptor, as evidenced by STAT1 and STAT3 phosphorylation, and T-bet, eomesodermin, and Blimp1 induction upon treatment with IL-27. These data demonstrate a differential response of Tc17 cells to the same stimuli depending on their stage of development. Molecular mechanisms underlying these differences remain unknown.
RORγt, the lineage-specific transcription factor that directs development of Th17 cells, is also expressed in Tc17 cells [42]. As shown previously for Th17 cells [34], we demonstrate here that RORγt is also a key transcription factor for differentiation of Tc17 cells given that a very small number of CD8+ T cells derived from RORγt-deficient mice developed into Tc17 cells. Addition of IL-27 further inhibited Tc17 cell differentiation, likely by suppressing RORα expression. In contrast to the inhibitory effect of IL-27 on RORγt expression during de novo differentiation of Tc17 cells, committed Tc17 cells maintained similar RORγt levels regardless of IL-27 signaling, providing evidence that committed Tc17 cells exhibit little susceptibility to modulation by IL-27. These findings are in agreement with previous reports demonstrating the limited capacity of IL-27 to downregulate IL-17A production and RORγt in memory T cells [29, 30, 43].
Although IL-27 completely suppressed development of Tc17 cells, expression of RORγt in these cells was only partially reduced, even at very high concentrations of IL-27 (200 ng/ml). Yang et al. also found, in agreement with our results, that RORγt in developing Th17 cells is only partially suppressed by IL-27 [44]. This is in contrast to findings by Kastelein’s group, who showed that IL-27 almost completely suppressed RORγt expression in developing Th17 cells [30], while we previously found that IL-27 only partially suppressed RORγt expression in those cells [29]. Nonetheless, it is worth noting that even incomplete reduction of RORγt levels corresponded to complete inhibition of Tc17 development, indicating the existence of additional mechanisms by which IL-27 suppresses Tc17 cell development.
One such mechanism is likely to be induction of T-bet expression in developing Tc17 cells, as we have shown here. In addition, development of T-bet−/− CD8+ T cells into Tc17 cells was minimally affected by IL-27, demonstrating that T-bet mediates the suppressive effect of IL-27 on Tc17 development. T-bet suppresses development of Th17 cells by interacting with the transcription factor Runx1, blocking Runx1-mediated transactivation of Rorc and subsequent RORγt expression [45]. It is likely that T-bet suppresses development of Tc17 cells by the same mechanism, given that Runx1 expression in Tc17 cells is modestly impacted by IL-27. An added layer of complexity is that IL-27 can induce expression of eomesodermin in CD8+ T cells, a functional homologue of T-bet [23]. Findings by Intlekofer et al. that T-bet and eomesodermin double-deficient, but not single-deficient, mice develop exaggerated Tc17 responses indicate that T-bet and eomesodermin have redundant roles in suppressing Tc17 development [46]. However, given that IL-27 had no effect either on T-bet−/− CD8+ T cells or on eomesodermin expression during Tc17 differentiation, it appears that eomesodermin does not play a role in IL-27-mediated suppression of Tc17 cell development.
STAT1 phosphorylation is a critical downstream effect of IL-27 signaling in CD4+ T cells [27], and we found that the same applies to CD8+ T cells, as STAT1 was required for inhibition of Tc17 development by IL-27. Thus, our results demonstrate that both STAT1 and T-bet are required for IL-27-mediated inhibition of Tc17 differentiation. Nevertheless, data obtained with CD4+ T cells indicate that both transcription factors act independently, with STAT1 not being required for T-bet-mediated suppression and T-bet being dispensable for STAT-1 induced suppression of Th17 development by IL-27 [47].
Committed Tc17 cells had preserved IL-27R expression and function, and treatment with IL-27 modified certain phenotypic characteristics of these cells, such as increased T-bet and Blimp1 expression in the absence of IL-23 or TGF-β/IL-6, but it did not impact RORγt levels in IL-17A+ cells. IL-17A-IFN-γ- cells that developed under Tc17-polarizing conditions, and were often the most abundant population in the culture, did not express RORγt or T-bet, irrespective of IL-27 treatment. It remains unclear why a large portion of cells did not acquire any distinct lineage characteristic.
In contrast to differentiated Tc1 cells, we found that committed Tc17 cells are not cytotoxic in vitro. Although IL-27 can augment the cytotoxic phenotype of CD8+ T cells [24, 48], exposure of committed Tc17 cells to IL-27 neither increased their cytotoxic potential, nor induced granzyme B expression; while it moderately increased the percentage of Tc1 cells expressing granzyme B, it did not change their net cytotoxicity. Despite being non-cytotoxic in vitro, Tc17 cells induced diabetes when injected into mRIP-OVA mice, similar to Tc1 cells, and irrespective of IL-27 treatment. Lack of expression of granzyme B in Tc17 cells suggests that cytotoxicity of Tc1 and Tc17 is mediated by different mechanisms. Indeed, it has been shown that cytotoxicity of in vivo transferred Tc17 cells is independent of perforin expression [5], but it is significantly decreased by blocking FasL [15]. We have shown previously that both IL-17A and IL-17F are necessary, but not sufficient, for diabetes induction by Tc17 cells, and that Tc17 cells likely initiate tissue damage by attracting other immune cells [11]. Collectively, these data suggest that although granzyme and perforin are likely the major cytotoxic effector molecules expressed by Tc1 cells, FasL and possibly other molecules have an important function in Tc17 cytotoxicity. Alternatively, Tc17 cell cytotoxicity does not play an important role in our adoptive transfer diabetes model, and these cells induce diabetes through other mechanisms, such as attracting inflammatory cells that subsequently kill beta cells.
A known feature of Tc17 cells that have developed in vitro is that upon transfer in vivo they can convert into IFN-γ-producing Tc17 cells that act as pathogenic effector cells [5, 14, 16, 17]. In our experimental model Tc17 cells largely retained their phenotype in vivo, at least during the period prior to disease onset. Approximately a third of transferred Tc17 cells stopped producing IL-17A, but only a small proportion of the cells produced IFN-γ, and prior exposure to IL-27 had no IFN-γ-inducing effect. This contrasts with the dramatic effect of IL-12 on committed Tc17 cells, by readily converting them into Tc1 cells. Thus, our data support the idea that Tc17 cells do not need to express IFN-γ to be pathogenic, and are in agreement with published findings from a similar diabetes model where Tc17 cells generated from IFN-γ-deficient mice were pathogenic [49].
In summary, we have failed to identify major changes in several prominent features of committed Tc17 cells caused by IL-27 signaling. This is in stark contrast to the profound effects of IL-27 on developing Tc17 cells. In this regard Tc17 and Th17 cells are highly similar. Overall, the effects of cytokines, including IL-27, on committed/memory/effector T cells have been studied markedly less than their effects on naïve cells. As a consequence, there is limited knowledge to draw upon when attempting to place our findings in the broader context of T cell regulation in the effector phase of an immune response. As we have pointed out earlier, insights into the effects of certain factors, such as cytokines, on mature T cell responses can be more important for designing novel therapies or at least to understanding why potential therapies have failed, even when studies using naïve T cells have indicated that they can be effective. This is especially relevant in the case of autoimmune diseases, because therapeutic interventions are always undertaken after autoimmune responses have already developed. The focus of this study was on Tc17 cells, and we did not thoroughly address the effects of IL-27 on other lineages of committed CD8+ T cells, such as Tc1 cells. Hence, we cannot extrapolate IL-27 effects in other lineages from findings on Tc17 cells and draw general conclusions about its effects on committed Tc cells. An indication that committed Tc17 and Tc1 cells may be differentially modulated by IL-27 can be found in lack of suppression of GM-CSF production by committed Th17 cells, while IL-27 suppressed GM-CSF production by Th1 cells [40]. Our data show that, similar to Th17 cells, IL-27 does not suppress GM-CSF production by committed Tc17 cells. Furthermore, IL-27 induced granzyme B expression in committed Tc1 cells, while it did not have that effect on Tc17 cells. The molecular basis of differential effects of IL-27 on developing and committed Tc17 cells, as well as on various Tc lineages, is unknown and merits further study.
MATERIALS AND METHODS
Mice
C57BL/6, OT-I transgenic (C57BL/6-Tg(TcraTcrb)1100Mjb/J), RIP-mOVA (C57BL/6-Tg (Ins2-TFRC/OVA)296Wehi/WehiJ) mice, Rorc−/− (B6.129P2(Cg)-Rorctm2Litt/J) and Tbx21−/− (B6.129S6-Tbx21tm1Glm/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). STAT1-deficient mice (129S6/SvEv-Stat1tm1Rds) were purchased from Taconic Farms (Cranbury, NJ). The Institutional Animal Care and Use Committee of Thomas Jefferson University approved all experimental procedures.
Reagents
Antibodies to CD3 (145–2C11) and CD28 (37.51) were purchased from BD Biosciences. Abs used for flow cytometry were from BD Biosciences (San Jose, CA): anti-CD8 (53–6.7), anti-IFN-γ (XMG1.2), anti-IL-17 (TC11–18H10), anti-GM-CSF (MP1–22E9), anti-CD16/32 (2.4G2) and anti-phosphorylated tyrosine residues of STAT1 (clone 4a) and STAT3 (clone 4/P-STAT3). Neutralizing Abs against IFN-γ and IL-4 and all cytokines used were from R&D Systems. Duoset ELISA kits used to quantify IL-17A, IL-10, IL-21 and IL-22 were from R&D Systems (Minneapolis, MN).
Cell preparation and culture
Tc17 cell differentiation and reactivation were performed as described in [11, 29].
Flow cytometry
Surface and intracellular staining of Tc17 cells prior to flow cytometry analysis was performed as described in [29]. Data were acquired on a FACSAria or FACSVerse (BD Biosciences) and analyzed with FlowJo software (Tree Star). The gating strategy used is described in supplementary Figure 2.
Intracellular staining for phosphorylated STAT1 and STAT3
Staining for phosphorylated STAT1 and STAT3 in differentiated Tc17 cells or in freshly isolated CD8+ T cells was performed as described in [29].
Real-time PCR
Quantitative measurement of gene transcription in Tc17 cells was performed as described in [29]. Primer pairs were purchased from Applied Biosystems (Supplementary Table 1).
Cytotoxic assay
Cytolitic activity of Tc17 cells was analyzed as described in [11]. Percentage of Ag-specific cytotoxicity was calculated by the formula: % cytotoxicity = 100 x [1 - (E.G7-OVA/EL4) experimental / (E.G7-OVA/EL4) control].
Adoptive transfer
Induction of diabetes was performed using adoptive transfer of OT-I Tc17 cells into RIP-mOVA mice as described in [11].
Statistics
An unpaired, two-tailed Student’s t test was used for statistical analysis. Differences with p values <0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the NIH/NINDS (2R01NS046782-05A2), the Groff Foundation, the Mary Gilbert Trust, and the National Multiple Sclerosis Society. MEB is recipient of a fellowship from the Fondation pour la Recherche Médicale and from the ARSEP. We thank Katherine Regan for editorial assistance.
REFERENCES
- 1.Girolomoni G, Mrowietz U and Paul C, Psoriasis: rationale for targeting interleukin-17. Br J Dermatol 2012. 167: 717–724. [DOI] [PubMed] [Google Scholar]
- 2.Nanjappa SG, Heninger E, Wuthrich M, Gasper DJ and Klein BS, Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PLoS Pathog 2012. 8: e1002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kryczek I, Wei S, Vatan L, Escara-Wilke J, Szeliga W, Keller ET and Zou W, Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J Immunol 2007. 179: 1423–1426. [DOI] [PubMed] [Google Scholar]
- 4.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A and Zou W, Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol 2007. 178: 6730–6733. [DOI] [PubMed] [Google Scholar]
- 5.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL and Dutton RW, Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol 2009. 182: 3469–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He D, Wu L, Kim HK, Li H, Elmets CA and Xu H, CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol 2006. 177: 6852–6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, Szeliga W, Wang Y, Liu Y, Welling TH, Elder JT and Zou W, Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol 2008. 181: 4733–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM and Fugger L, Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 2008. 172: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messi M, Giacchetto I, Nagata K, Lanzavecchia A, Natoli G and Sallusto F, Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat Immunol 2003. 4: 78–86. [DOI] [PubMed] [Google Scholar]
- 10.Huber M, Heink S, Grothe H, Guralnik A, Reinhard K, Elflein K, Hunig T, Mittrucker HW, Brustle A, Kamradt T and Lohoff M, A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol 2009. 39: 1716–1725. [DOI] [PubMed] [Google Scholar]
- 11.Ciric B, El-behi M, Cabrera R, Zhang GX and Rostami A, IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol 2009. 182: 5296–5305. [DOI] [PubMed] [Google Scholar]
- 12.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T and Cua DJ, TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol 2007. 8: 1390–1397. [DOI] [PubMed] [Google Scholar]
- 13.Croft M, Carter L, Swain SL and Dutton RW, Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med 1994. 180: 1715–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski P and Restifo NP, Type 17 CD8+ T cells display enhanced antitumor immunity. Blood 2009. 114: 596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh N, Glosson NL, Wang N, Guindon L, McKinley C, Hamada H, Li Q, Dutton RW, Shrikant P, Zhou B, Brutkiewicz RR, Blum JS and Kaplan MH, Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype. J Immunol 2010. 185: 2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yen HR, Harris TJ, Wada S, Grosso JF, Getnet D, Goldberg MV, Liang KL, Bruno TC, Pyle KJ, Chan SL, Anders RA, Trimble CL, Adler AJ, Lin TY, Pardoll DM, Huang CT and Drake CG, Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol 2009. 183: 7161–7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Hernandez Mde L, Hamada H, Reome JB, Misra SK, Tighe MP and Dutton RW, Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol 2010. 184: 4215–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bending D, De la Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B and Cooke A, Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest 2009. 119: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO and Weaver CT, Late developmental plasticity in the T helper 17 lineage. Immunity 2009. 30: 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stumhofer JS and Hunter CA, Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett 2008. 117: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D and Kastelein RA, IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity 2002. 16: 779–790. [DOI] [PubMed] [Google Scholar]
- 22.Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R and Kastelein RA, WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol 2004. 172: 2225–2231. [DOI] [PubMed] [Google Scholar]
- 23.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J and Yoshimoto T, Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol 2005. 175: 1686–1693. [DOI] [PubMed] [Google Scholar]
- 24.Schneider R, Yaneva T, Beauseigle D, El-Khoury L and Arbour N, IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol 2011. 41: 47–59. [DOI] [PubMed] [Google Scholar]
- 25.Morishima N, Mizoguchi I, Okumura M, Chiba Y, Xu M, Shimizu M, Matsui M, Mizuguchi J and Yoshimoto T, A pivotal role for interleukin-27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. J Biomed Biotechnol 2010. 2010: 605483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ and Hunter CA, Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol 2007. 8: 1363–1371. [DOI] [PubMed] [Google Scholar]
- 27.Lucas S, Ghilardi N, Li J and de Sauvage FJ, IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A 2003. 100: 15047–15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B and Rostami A, Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol 2007. 8: 1372–1379. [DOI] [PubMed] [Google Scholar]
- 29.El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC and Rostami A, Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol 2009. 183: 4957–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ and Kastelein RA, IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol 2009. 182: 5748–5756. [DOI] [PubMed] [Google Scholar]
- 31.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M and Hunter CA, Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 2006. 7: 937–945. [DOI] [PubMed] [Google Scholar]
- 32.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ and Ghilardi N, Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol 2006. 7: 929–936. [DOI] [PubMed] [Google Scholar]
- 33.Park IK, Shultz LD, Letterio JJ and Gorham JD, TGF-beta1 inhibits T-bet induction by IFN-gamma in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1. J Immunol 2005. 175: 5666–5674. [DOI] [PubMed] [Google Scholar]
- 34.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ and Littman DR, The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006. 126: 1121–1133. [DOI] [PubMed] [Google Scholar]
- 35.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN and Rostami A, The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol 2011. 12: 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min L, Mohammad Isa S. A., Shuai W, Piang CB, Nih FW, Kotaka M and Ruedl C, Cutting edge: granulocyte-macrophage colony-stimulating factor is the major CD8+ T cell-derived licensing factor for dendritic cell activation. J Immunol 2010. 184: 4625–4629. [DOI] [PubMed] [Google Scholar]
- 37.Kroenke MA, Chensue SW and Segal BM, EAE mediated by a non-IFN-gamma/non-IL-17 pathway. Eur J Immunol 2010. 40: 2340–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T and Becher B, RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 2011. 12: 560–567. [DOI] [PubMed] [Google Scholar]
- 39.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G and Amrani A, Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol 2007. 179: 3638–3647. [DOI] [PubMed] [Google Scholar]
- 40.Young A, Linehan E, Hams E, O’Hara Hall A. C., McClurg A, Johnston JA, Hunter CA, Fallon PG and Fitzgerald DC, Cutting edge: suppression of GM-CSF expression in murine and human T cells by IL-27. J Immunol 2012. 189: 2079–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Villarino AV, Larkin J 3rd, Saris CJ, Caton AJ, Lucas S, Wong T, de Sauvage FJ and Hunter CA, Positive and negative regulation of the IL-27 receptor during lymphoid cell activation. J Immunol 2005. 174: 7684–7691. [DOI] [PubMed] [Google Scholar]
- 42.Satoh T, Tajima M, Wakita D, Kitamura H and Nishimura T, The development of IL-17/IFN-gamma-double producing CTLs from Tc17 cells is driven by epigenetic suppression of Socs3 gene promoter. Eur J Immunol 2012. 42: 2329–2342. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, Yoshimura A and Yoshida H, Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol 2006. 177: 5377–5385. [DOI] [PubMed] [Google Scholar]
- 44.Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X, Sellati R, Jiang H, Zhang S, Li H, Zhao J, Ting AT, Mayer L, Unkeless JC, Labadia ME, Hodge M, Li J and Xiong H, Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and RORgamma t. Eur J Immunol 2008. 38: 1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK and Glimcher LH, T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol 2011. 12: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T and Reiner SL, Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 2008. 321: 408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villarino AV, Gallo E and Abbas AK, STAT1-activating cytokines limit Th17 responses through both T-bet-dependent and -independent mechanisms. J Immunol 2010. 185: 6461–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mittal A, Murugaiyan G, Beynon V, Hu D and Weiner HL, IL-27 induction of IL-21 from human CD8+ T cells induces granzyme B in an autocrine manner. Immunol Cell Biol 2012. 90: 831–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saxena A, Desbois S, Carrie N, Lawand M, Mars LT and Liblau RS, Tc17 CD8+ T cells potentiate Th1-mediated autoimmune diabetes in a mouse model. J Immunol 2012. 189: 3140–3149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.