Abstract
Summary
Feminizing estrogen-secreting adrenocortical carcinomas (ACCs) are exceedingly rare and carry a poor prognosis. The most common presenting trait is gynecomastia, but enlarged breasts are also a frequent clinical finding in healthy men. Biochemical evaluation may be challenging. As such, there is a high risk of delayed diagnosis and treatment opportunity. Here, we present a case with an estrogen-producing ACC where the abnormal steroid profile obtained at the time of initial workup was essential for the prompt diagnosis. Wider adoption of liquid chromatography mass spectrometry-based steroid assays has potential to improve early diagnosis of feminizing estrogen-secreting ACC.
Learning points
Feminizing estrogen-secreting adrenocortical carcinomas (ACCs) are a rare, but an important differential diagnosis in men with rapidly developing gynecomastia.
Biochemical evaluation is essential for a prompt diagnosis.
Steroid hormone profiling using liquid chromatography mass spectrometry technology has the potential to improve early diagnosis of feminizing estrogen-secreting ACC.
Keywords: Adult, Male, White, Norway
Keywords: Adrenal, Adrenal
Keywords: Surgery
Keywords: Novel diagnostic procedure, November, 2021
Background
Gynecomastia is defined as a benign proliferation of subareolar glandular breast tissue in men. It is caused by increased estrogen activity relative to testosterone activity. Several medical conditions can disrupt the ratio through mechanisms of altered hormone production (1, 2). The increasingly adopted analytical technique, liquid chromatography mass spectrometry (LCMS), offers key features that could reduce time-to-treatment, which is critical in rare, but diagnostically challenging cases of adrenocortical estrogen-secreting carcinomas.
In this case report, we present a male with gynecomastia caused by excess endogenic estrogen production.
Case presentation
A 58-year-old male was referred to our endocrinology department for evaluation of gynecomastia. During the last 6 months, he had experienced increasing growth of breast tissue, most pronounced on the right side. Both breasts were tender and painful, and this bothered him especially during physical activity. Libido was also reduced. There was no report of galactorrhea, and his weight was stable. Several years earlier, he had been treated for a minor cardiac infarction with percutaneous coronary intervention, and had been on unaltered medication since then (75 mg acetylsalicylic acid, 25 mg metoprolol and 80 mg atorvastatin daily). He reported no drug abuse or excessive alcohol consumption.
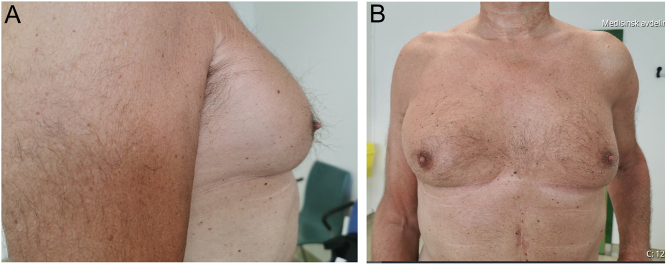
Physical examination confirmed bilateral gynecomastia with tender, firm mounds of tissue subareolar bilaterally, predominantly on the right side (Fig. 1). No palpable lymph nodes in the axillae, collum or groin were found. The testes had no palpable nodules and were of normal size, the right testicle slightly larger than the left, 5.3 × 1.6 × 3 vs 4.3 × 1.6 × 3 cm, confirmed by ultrasound. His weight was 78 kg and height was 179 cm (BMI 24.3 kg/m2) with normal fat distribution. Blood pressure was increased to 170/90 mmHg, otherwise the patient had no clinical features of cortisol excess.
Figure 1.
Gynecomastia. Picture taken at primary consultation.
Investigation
Multiplex liquid chromatography tandem mass spectrometry (LC-MS/MS) steroid analysis enables easy, highly accurate determination of multiple hormones simultaneously. In this patient, high levels of estradiol (208 pmol/L) and the steroid precursor hormones, 11-deoxyxortisol (23.5 nmol/L), DHEAS (10.6 µmol/L) and androstenedione (18.1 nmol/L) were detected. Cortisol obtained at 10:44 h was in the normal range (284 nmol/L), but a 1 mg overnight dexamethasone suppression test showed less than expected suppression to 110 nmol/L, suggesting cortisol overproduction. Luteinizing hormone and follicle stimulating hormone were in the lower reference range, and he had normal levels of sex hormone-binding globulin (SHBG). Adrenocorticotropic hormone (ACTH), growth hormone and prolactin were also in the normal range. Test results are summarized in Table 1.
Table 1.
Biochemical evaluation.
| Hormone (reference range) | Preoperative | Postoperative (2 weeks) | |
|---|---|---|---|
| Mineralocorticoids | S-aldosterone (20–620 pmol/L620 pmol/L) | 48 | 28 |
| P-Renin concentration (4.4–46.1 mIE/L | 17 | ||
| Glucocorticoids | S-Cortisol morning (120–600 nmol/L600 nmol/L) | 373 | 222 |
| S-11-Deoxycortisol (<5.0 nmol/L) | 23.5 | 2.2 | |
| Sex hormones | S-DHEAS (<2.5 umol/L) | 10.6 | <0.4 |
| S-androstendione (0.8-3.1 nmol/L) | 18.1 | 58 | |
| S-testosterone (6.7-31.9 nmol/L) | 8.5 | 9.6 | |
| S-estradiol (<107 pmol/L) | 258 | 32 | |
| S-progesterone (<2.5 nmol/L) | <1.0 | 1.8 | |
| Catecholamines | P-metanefrine (<0.45 nmol/L) | 0.23 | |
| P-normetanefrine (<1.10 nmol/L) | 0.27 | ||
| Pitutiary hormones | S-ACTH morning (2.0–11-6 pmol/L) | 7.9 | |
| S-FSH (0.7–11.1 IU/L) | 1.6 | 6.5 | |
| S-LH (0.8–7.6 UI/L) | 1.1 | 3.1 | |
| S-prolactin (53–360 mIU/L) | 312 | ||
| S-TSH (0.4–4.5 mIU/L) | 1.68 | ||
| Other | s-FT4 (9.5–22.0 pmol/L) | 15.9 | |
| HbA1c (<53 mmol/mol) | 35 | ||
| s-K (3.5–5.0 mmol/L) | 4.2 | ||
| s-Na (137–145 mmol/L145 mmol/L) | 138 | ||
| S-SHBG (13–71 nmol/L1 nmol/L) | 76 | 58 |
ACTH, adrenocorticotropic hormone; FSH, follicle stimulating hormone; LH, luteinizing hormone; TSH, thyroid-stimulating hormone.
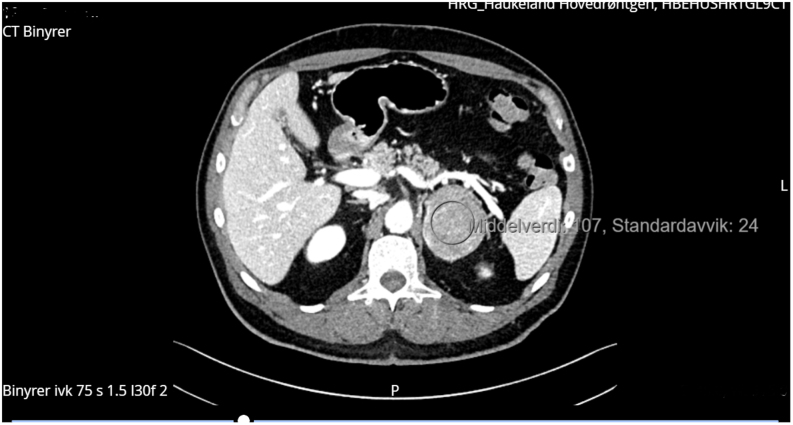
The hormone assays raised immediate suspicions of a hormone-producing adrenal tumor, which was confirmed by CT imaging. CT revealed a large tumor in the left adrenal gland (65 mm × 52 mm) with a pre-contrast density of 32 HU (Fig. 2). In the portal venous contrast phase, density was 100 HU, falling to 56 HU after 10 min. Absolute wash-out density was 60%. The contrast enhancement pattern was described as inhomogeneous. The right adrenal gland was normal. There were no radiological signs of local invasive growth or enlarged paraaortic lymph nodes. A CT of the thorax was performed to locate metastases and it revealed a small pulmonary embolus in the periphery of the right lung and a 9 mm peribronchovascular nodule suspicious of a mucocele, centrally in the right upper lobe. There were no enlarged lymph nodes in the axillae, mediastinum or hila, and there was no evidence of metastasis in the spleen and liver.
Figure 2.
Computer tomography of the adrenal glands demonstrating a large inhomogenous tumor on the left side (65 mm × 52 mm).
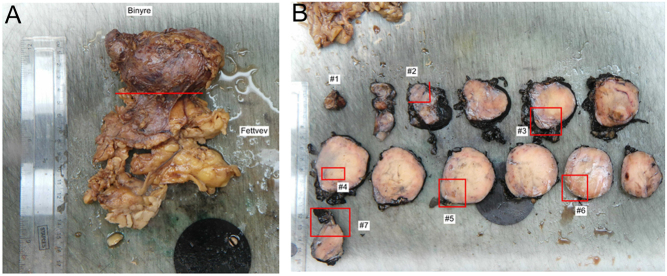
The tumor and the paraaortic lymph nodes were excised by transabdominal laparoscopic approach with no sign of local invasion (R0) (Fig. 3). The postoperative course was complicated by a bleeding in the operating area leading to reoperation and splenectomy.
Figure 3.
Adrenal tumor.
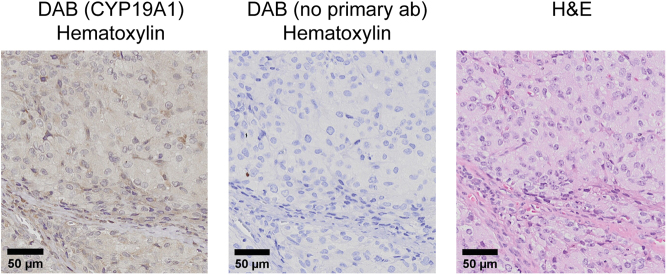
Histology of the left adrenal gland (90 mm) showed no infiltration of surrounding tissue or lymph nodes. The tumor was classified as a high-grade adrenocortical carcinoma (ACC) with a Weiss score of 7 and modified Lin–Weiss–Biscealia criteria with >20 mitoses/50 HPF, Stage II, T2N0M0. Immunohistochemistry revealed cells positive for inhibin, synaptophysin, CD31 (PECAM-1) and aromatase (CYP19A1) (Fig. 4). The Ki-67 proliferation index was overall >5% with >50% in hotspots. Microsatellite instability was not found. The tumor was negative for p53, ACTH, estrogen-, progesterone- and androgen-receptors, CK AE1/AE3, CK7, CK20, CK cam5.2, EMA, PAX8, CEA, chromogranin A, calretinin and melan A.
Figure 4.
Immunohistochemical staining of adrenal tumor. 3,3′-diaminobenzidine staining with (left) and without (middle) primary anti-aromatase antibody (hematoxylin as counterstain). Hematoxylin and eosin staining (right).
Most ACCs are sporadic, although they can be part of hereditary genetic syndromes associated with elevated cancer risks (2). In this case, there was no family history of cancer, but our patient received genetic counseling and screening with negative findings.
Adjuvant treatment, outcome and follow-up
The day after surgery, blood tests showed normalized levels of serum estradiol (58 pmol/L) and androstenedione (1.0 nmol/L), confirmed by repeated testing at follow-up the next year (Table 1). His resting blood pressure was stabilized, and he experienced orthostatic hypotension and fatigue after the operation. A cosytropin test was performed 2 weeks postoperatively with a basal ACTH of 6.0 pmol/L and cortisol 222 nmol/L. Cortisol increased to 380 nmol/L after 60 min, suggesting an attenuated cortisol response. He was therefore prescribed cortisone acetate replacement therapy.
Adjuvant therapy with the adrenolytic drug mitotane was started 8 weeks after resection, gradually increasing the dose to 1.5 g/day with plasma-mitotane in the range 44–62μmol/L. Treatment was intended to be continued for 2 years, but due to increasing liver steatosis and asthenia it was discontinued after 9 months treatment.
His gynecomastia had not improved significantly 1 year after surgery, and therefore liposuction and perioareolar incision was planned.
Discussion
Feminizing estrogen-producing ACCs are exceedingly rare with fewer than 100 cases reported, accounting for less than 1–2% of all ACCs (3). On the other hand, 90% of all feminizing tumors stem from adrenal origin, actualizing ACC as an important differential diagnosis of gynecomastia (4).
As with our patient, men with estrogen-secreting tumors often present with bilateral, painful gynecomastia without galactorrhea. Symptoms and clinical signs of hypoandrogenism with loss of libido, fatigue, reduction of body hair and testicular atrophy are the common findings (5). Under normal conditions, the steroid hormone-producing cells of the adrenal cortex do not express the steroidogenic enzyme aromatase (CYP19A1) and are therefore not capable of de novo biosynthesis of estrogen. Malignant cells may influence the steroid pathways causing excess hormones and disease through altered steroidogenic enzyme function. ACCs have the potential to express aromatase resulting in clinical feminization, as described here and in a few other case reports (6, 7). In addition, our patient had biochemical features of overproduction of other adrenal hormones and steroidogenic precursor hormones with hypertensive symptoms of Cushing and/or Conns syndrome. Hypercortisolism and/or hyperandrogenism are observed in more than half the cases of ACCs, while an excess of mineralocorticoids is very rare (2).
Biochemical evaluation of adrenal tumors has traditionally been investigated using immunoassay and guided by clinical presentation. Our endocrine center has used a multiplex LC-MS/MS steroid analyses for over 10 years which enables easy, highly accurate determination of multiple steroid hormones and precursors simultaneously. This assay has been described in detail previously (8). Briefly, it employs automated liquid–liquid extraction of 85 µL serum and subsequent ultra-high-pressure chromatography on a reverse phase column, and hormone detection by triple-quadrupole mass spectrometry.
LC-MS/MS is now becoming increasingly available to clinicians and offers key features that could facilitate quick diagnosis and reduced time-to-treatment. First, a single blood sample provides comprehensive data on common and rare steroid pathways which could alert the clinician on unusual, but serious differential diagnoses. In this case report, high levels of early precursors in the steroid pathways, especially 11-deoxyxortisol, was an important diagnostic clue in a patient without other predominant clinical signs of ACC. Notably, a few studies have shown that elevated levels of 11-deoxycortisol have a high predictive value for malignancy in males (9, 10). Secondly, immunoassays are prone to analytical interference by heterophilic antibodies and chemically resembling steroids while validated LCMSMS methods have analytical accuracy linked to internationally certified references enabling reliable comparison of test results. We believe that the adoption of steroid hormone profiling by LC-MS/MS will improve biochemical diagnostic accuracy of ACCs.
Distinguishing benign from malignant tumors is challenging, except when metastases are present (2). In our patient, the CT scan revealed radiological criteria indicating malignancy with a size >4 cm, a high HU-index and inhomogeneous density. Adrenalectomy was performed, and the evaluation of the biopsies using Weiss score (including the exact mitotic count) and Ki-67 index gave a clear indication of malignancy. Staining for melan A and synaptophysin are often positive in adrenal tumors, but it cannot differentiate between different types of ACCs. The positivity of aromatase immunostaining and normalization of serum estradiol after adrenalectomy confirmed that the elevated estrogen levels were secreted from the removed tumor tissue.
Treatment of hormone-producing ACCs should be directed toward both cancer and hormonal-related complications. Surgery is the only curative treatment for ACCs, but adjuvant medical treatment and radiotherapy should be considered (2). Specific steroidogenic enzyme inhibitors or hormone receptor antagonists are often required in patients with evidence of cortisol excess as Cushing’s syndrome is associated with increased risk of complications and mortality. ESMO 2020 guidelines recommend adjuvant treatment with mitotane in patients with high risk of recurrence (stage III or R1-Rx resection, and/or Ki-67 index >10%). Our patient had stage II, R0 and a Ki-67 >50% in hot spots, and was therefore given mitotane, a cytotoxic drug that reduces cortisol and aldosterone production by inhibiting cholesterol side-chain cleavage enzyme (CYP11A1), 11β-hydroxylase (CYP11B1) and 18-hydroxylase (CYP11B2) (2). The optimal duration of adjuvant therapy in such patients is not known, but guidelines recommend that mitotane should be administered for at least 2 years. However, the assessment of disease remission is difficult during ongoing mitotane treatment as this drug increases steroid binding proteins (corticosteroid binding globulin (CBG, SHBG) masquerading any decrease in cortisol and estradiol (2). In our patient, mitotane was discontinued after 9 months due to severe side effects.
Gynecomastia often regresses spontaneously after treatment of the underlying disorder, but surgical excision of the glandular tissue and liposuction of any coexisting adipose tissue can be considered (1).
Overall, the prognosis for ACCs is poor with a reported 5-year survival of approximately 50% for early stage disease and <25% for advanced stage disease. Feminizing estrogen-secreting ACCs with very high estrogen levels and large tumors are particularly associated with poor prognosis (2). However, several studies have highlighted considerable heterogeneity in survival, and there is reason for optimism regarding potential for faster diagnosis and more precise follow-up with increased use of LC-MS/MS technology. Postoperative normalization of hormone levels in our patient could indicate complete removal of the tumor. Due to high risk of recurrence, our patient will be followed-up every 3 months with biochemical and radiological markers of disease recurrence.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent has been obtained from the patient for publication of the submitted article and accompanying images.
Author contribution statement
Elinor Chelsom Vogt: Wrote the manuscript with support from the coauthors. Kathrin Hammerling: oncologist who treated the patient. Halfdan Sorbye: oncologist who treated the patient. Anette Heie: surgeon who treated the patient. Andre Sulen: Laboratory workup. Grethe Ueland: Expert discussion partner. Eystein Husebye: Expert discussion partner. Paal Methlie: Diagnosed and treated the patient. All authors reviewed the final version of the manuscript.
References
- 1.Sansone A, Romanelli F, Sansone M, Lenzi A, Di Luigi L. Gynecomastia and hormones. Endocrine 20175537–44. ( 10.1007/s12020-016-0975-9) [DOI] [PubMed] [Google Scholar]
- 2.Fassnacht M, Assie G, Baudin E, Eisenhofer G, de la Fouchardiere C, Haak HR, de Krijger R, Porpiglia F, Terzolo M, Berruti Aet al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2020311476–1490. ( 10.1016/j.annonc.2020.08.2099) [DOI] [PubMed] [Google Scholar]
- 3.Chentli F, Bekkaye I, Azzoug S. Feminizing adrenocortical tumors: literature review. Indian Journal of Endocrinology and Metabolism 201519332–339. ( 10.4103/2230-8210.152764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno S, Guillermo M, Decoulx M, Dewailly D, Bresson R, Proye CH. Feminizing adreno-cortical carcinomas in male adults. A dire prognosis. Three cases in a series of 801 adrenalectomies and review of the literature. Annales d’Endocrinologie 20066732–38. ( 10.1016/s0003-4266(0672537-9) [DOI] [PubMed] [Google Scholar]
- 5.Kidd MT, Karlin NJ, Cook CB. Feminizing adrenal neoplasms: case presentations and review of the literature. Journal of Clinical Oncology 201129e127–e130. ( 10.1200/JCO.2010.31.4799) [DOI] [PubMed] [Google Scholar]
- 6.Bouraïma H, Lireux B, Mittre H, Benhaim A, Herrou M, Mahoudeau J, Guillon-Metz F, Kottler ML, Reznik Y. Major hyperestrogenism in a feminizing adrenocortical adenoma despite a moderate overexpression of the aromatase enzyme. European Journal of Endocrinology 2003148457–461. ( 10.1530/eje.0.1480457) [DOI] [PubMed] [Google Scholar]
- 7.Phornphutkul C, Okubo T, Wu K, Harel Z, Tracy TF, Jr, Pinar H, Chen S, Gruppuso PA, Goodwin G. Aromatase p450 expression in a feminizing adrenal adenoma presenting as isosexual precocious puberty. Journal of Clinical Endocrinology and Metabolism 200186649–652. ( 10.1210/jcem.86.2.7201) [DOI] [PubMed] [Google Scholar]
- 8.Methlie P, Hustad SS, Kellmann R, Almas B, Erichsen MM, Husebye E, Løvås K. Multisteroid LC-MS/MS assay for glucocorticoids and androgens, and its application in Addison’s disease. Endocrine Connections 20132125–136. ( 10.1530/EC-13-0023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schweitzer S, Kunz M, Kurlbaum M, Vey J, Kendl S, Deutschbein T, Hahner S, Fassnacht M, Dandekar T, Kroiss M. Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma. European Journal of Endocrinology 2019180117–125. ( 10.1530/EJE-18-0782) [DOI] [PubMed] [Google Scholar]
- 10.Taylor DR, Ghataore L, Couchman L, Vincent RP, Whitelaw B, Lewis D, Diaz-Cano S, Galata G, Schulte KM, Aylwin Set al. A 13-steroid serum panel based on LC-MS/MS: use in detection of adrenocortical carcinoma. Clinical Chemistry 2017631836–1846. ( 10.1373/clinchem.2017.277624) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a