Abstract
Initiation of ribosomal DNA (rDNA) transcription by RNA polymerase I (Pol I) in the yeast Saccharomyces cerevisiae involves upstream activation factor (UAF), core factor, the TATA binding protein (TBP), and Rrn3p in addition to Pol I. We found previously that yeast strains carrying deletions in the UAF component RRN9 switch completely to the use of Pol II for rRNA transcription, with no residual Pol I transcription. These polymerase-switched strains initially grow very slowly, but subsequent expansion in the number of rDNA repeats on chromosome XII leads to better growth. Recently, it was reported that TBP overexpression could bypass the requirement of UAF for Pol I transcription in vivo, producing nearly wild-type levels of growth in UAF mutant strains (P. Aprikian, B. Moorefield, and R. H. Reeder, Mol. Cell. Biol. 20:5269–5275, 2000). Here, we demonstrate that deletions in the UAF component RRN5, RRN9, or RRN10 lead to Pol II transcription of rDNA. TBP overexpression does not suppress UAF mutation, and these strains continue to use Pol II for rRNA transcription. We do not find evidence for even low levels of Pol I transcription in UAF mutant strains carrying overexpressed TBP. In diploid strains lacking both copies of the UAF component RRN9, Pol II transcription of rDNA is more strongly repressed than in haploid strains but TBP overexpression still fails to activate Pol I. These results emphasize that UAF plays an essential role in activation of Pol I transcription and silencing of Pol II transcription of rDNA and that TBP functions to recruit the Pol I machinery in a manner completely dependent on UAF.
Initiation of rRNA transcription in the yeast Saccharomyces cerevisiae requires four factors in addition to RNA polymerase I (Pol I): upstream activation factor (UAF), core factor (CF), TATA binding protein (TBP), and Rrn3p. UAF is a multisubunit transcription factor consisting of proteins encoded by RRN5, RRN9, and RRN10, core histones H3 and H4, and protein p30 (9, 12). CF is another Pol I-specific complex, consisting of subunits encoded by RRN6, RRN7, and RRN11 (11, 14, 15).
TBP is generally required for transcription by all three nuclear RNA polymerases (8). In the case of yeast Pol I transcription, we have previously studied the function of TBP by both genetic and biochemical approaches. TBP was found to interact with components of both UAF and CF but most strongly with the Rrn9p subunit of UAF (19), suggesting that TBP may act as a bridge to recruit CF onto a UAF-bound promoter template. In vitro, TBP and UAF, as well as the upstream promoter element, are dispensable for low-level, basal transcription of rRNA from the core ribosomal DNA (rDNA) promoter. When these components are present, however, transcription is greatly stimulated. In an in vitro system using purified proteins, addition of TBP without UAF fails to stimulate basal transcription (10). Thus, TBP, in association with UAF, mediates activated transcription from the rDNA promoter.
The role of TBP in Pol I transcription has become a matter of some debate due to a recently published paper on the effects of TBP overexpression. Aprikian and coworkers (1) reported that overexpression of TBP can suppress UAF mutation by directly stimulating CF-directed transcription. They claimed that UAF mutant strains, which grow on galactose using GAL7-35S rDNA helper plasmids, could grow to “nearly wild-type levels,” even on glucose, when transformed with high-copy-number plasmids carrying TBP. They further demonstrated that, in vitro, increased concentrations of TBP led to stimulation of rRNA transcription in the absence of UAF or the upstream element. Thus, they concluded that high levels of TBP can bypass the requirement for UAF and that TBP directly stimulates transcription from the core transcriptional machinery (1).
We found previously that mutations in UAF components immediately lead to a polymerase switch in rRNA transcription from Pol I to Pol II (17, 20). This state is called N-PSW. Subsequent adaptation events result in rDNA repeat number expansion, allowing these strains to grow well on glucose in the absence of GAL7-35S rDNA plasmids. This state is called PSW. It is therefore possible that the UAF mutant strains described by Aprikian and coworkers (1), when growing on glucose with overexpressed TBP, used Pol II for rRNA transcription from the chromosomal rDNA repeats. Perhaps TBP overexpression facilitates Pol II transcription, leading to better growth. In this study, we more thoroughly investigated the effects of TBP overexpression in UAF mutant strains and found that, contrary to the results reported by Aprikian and coworkers (1), TBP overexpression cannot bypass the essential requirement of UAF for either activation of Pol I transcription or silencing of Pol II transcription of rDNA. We discuss some published experimental results obtained in vertebrate systems that are relevant to the conclusions obtained with the yeast system.
MATERIALS AND METHODS
Yeast strains and plasmids.
Yeast extract-peptone (YEP)-glucose (YEPD) medium, YEP-galactose medium, synthetic galactose medium, and synthetic glucose medium were used to grow yeast cells, as described previously (11, 16). Yeast strains and plasmids used in this study are listed in Table 1. Diploid strain NOY1002 was constructed by crossing an rrn9Δ N-PSW strain carrying pNOY103 (GAL7-35S rDNA, URA3) with an rrn9Δ N-PSW strain carrying pNOY199 (GAL7-35S rDNA, TRP1) and selecting diploids on media lacking uracil and tryptophan.
TABLE 1.
Yeast strains and plasmids
| Strain or plasmid |
Description |
|---|---|
| Strains | |
| NOY388 | (= W303-1a) MATa ade2-1 ura3-1 trp1-1 leu2-3,112 his3-11 can1-100 |
| NOY677 | MATa/MATα ade2/ade2 ade3/ade3 leu2/leu2 ura3/ura3 trp1/trp1 can1/can1 RRN10/rrn10Δ::LEU2 |
| NOY678 | MATa/α ade2-1/ade2-1 ura3-1/ura3-1 leu2-3,112/leu2-3,112 trp1-1/trp1-1 his3-11/his3-11 can1-100/can1-100 RRN9/rrn9Δ::HIS3 |
| NOY679 | MATa/α ade2-1/ade2-1 ura3-1/ura3-1 leu2-3,112/leu2-3,112 trp1-1/trp1-1 his3-11/his3-11 can1-100/can1-100 RRN5/rrn5Δ::LEU2 |
| NOY703 | MATaade2-1 ura3-1 trp1-1 leu2-3,112 his3-11 can1-100 rrn9Δ::HIS3 pNOY103; N-PSW |
| NOY751 | MATa/α ade2-1/ade2-1 ura3-1/ura3-1 leu2-3,112/leu2-3,112 trp1-1/trp1-1 his3-11/his3-11 can1-100/can1-100 spt15Δ::HIS3/SPT15 |
| NOY902 | Same as NOY703, but PSW and pNOY103 removed by 5-fluoroorotic acid selection |
| NOY921 | Same as NOY703, but fob1Δ::LEU2 |
| NOY1000 | MATaade2-1 ura3-1 trp1-1 leu2-3,112 his3-11 can1-100 rrn9Δ::HIS3 pNOY103 pNOY419; N-PSW |
| NOY1001 | MATa ade2-1 ura3-1 trp1-1 leu2-3,112 his3-11 can1-100 rrn9Δ::HIS3 pNOY103 pRS425; N-PSW |
| NOY1002 | MATa/α ade2-1/ade2-1 ura3-1/ura3-1 leu2-3,112/leu2-3,112 trp1-1/trp1-1 his3-11/his3-11 can1-100/can1-100 rrn9Δ::HIS3/rrn9Δ::HIS3 pNOY103 pNOY199 |
| Plasmids | |
| pNOY103 | High-copy-number plasmid carrying GAL7-35S rDNA, URA3, 2μm, and amp |
| pNOY199 | High-copy-number plasmid carrying GAL7-35S rDNA, TRP1, 2μm, and amp |
| pNOY347 | SPT15 under its own promoter cloned into pRS315 (CEN6 LEU2 amp) |
| pNOY419 | SPT15 under its own promoter cloned into YEp351 (2μm LEU2 amp) |
| pRS415 | Escherichia coli-yeast shuttle vector carrying CEN6 ARS4 LEU2 (4) |
| pRS425 | E. coli-yeast shuttle vector carrying 2μm ARS4 LEU2 (4) |
Western blot analysis.
Extracts were prepared from 15-ml cultures of strains grown in synthetic galactose medium lacking leucine (to maintain selection for plasmids), as described previously (10). Total protein in the extracts was determined by Lowry assay, and Western blot analysis was carried out using affinity-purified TBP antibodies, as described previously (10).
Primer extension analysis.
Total RNA was prepared and primer extension analysis was carried out using a γ-32P-labeled primer which hybridizes to the 35S rRNA external transcribed spacer, as described previously (20). Autoradiograms were quantitated by a PhosphorImager.
RESULTS
Like rrn9Δ strains, rrn5Δ and rrn10Δ strains undergo polymerase switching.
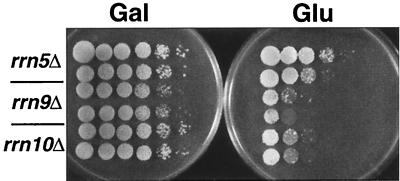
We originally discovered the polymerase switching phenomenon in strains carrying deletions in the UAF component RRN9 (20). These strains grow well on galactose, using GAL7-35S rDNA helper plasmids for transcription of rRNA, but produce a low frequency of variants that can also grow on glucose, where transcription of the GAL7 promoter is repressed. We called these variants PSW, for polymerase switched, and we called the original galactose-dependent strain N-PSW, for no good growth, polymerase switched, and we demonstrated Pol II transcription (and no Pol I transcription) of chromosomal rDNA in both of these strains (17, 20). As with rrn9Δ, disruptions in genes for the UAF components RRN5 and RRN10 give similar results. As shown in Fig. 1, rrn5Δ, rrn9Δ, and rrn10Δ strains carrying GAL7-35S rDNA plasmids grow well on galactose but also produce some PSW variants on glucose. The different frequencies of PSW variants arising in these three strains, which are apparent in Fig. 1, have been confirmed by analysis of many independent clones by spot tests on glucose plates. The results suggest that RRN5 is somehow the most important component for silencing Pol II transcription of chromosomal rDNA, followed by RRN10 and then RRN9.
FIG. 1.
Polymerase switch phenotype in rrn5Δ, rrn9Δ, and rrn10Δ strains. Diploid strains NOY679 (RRN5/rrn5Δ), NOY678 (RRN9/rrn9Δ), and NOY677 (RRN10/rrn10Δ) were transformed with the GAL7-35S rDNA plasmid pNOY199 and sporulated, and then tetrads were dissected on galactose plates. Tetrads corresponding to rrn5Δ, rrn9Δ, and rrn10Δ (as indicated by slow growth) were restreaked once onto another galactose plate (lacking tryptophan, to maintain pNOY199), and then aliquots of 10-fold serial dilutions of two independent colonies from these strains were spot tested on YEP-galactose (Gal) and YEPD (Glu) plates, as shown. Plates were incubated for 6 days at 30°C.
High-copy-number TBP plasmids lead to overexpression of functional TBP.
We wanted to determine whether excess TBP could suppress the polymerase-switched phenotype of UAF mutant strains. To overexpress TBP in our UAF mutant strains, we used high-copy-number (2μm) plasmids carrying SPT15, the gene encoding TBP, under control of its endogenous promoter, which is similar to the plasmid used by Aprikian and coworkers (1) in their experiments. We transformed this plasmid, or a vector control, into an rrn9Δ N-PSW strain carrying GAL7-35S rDNA plasmids (NOY703).
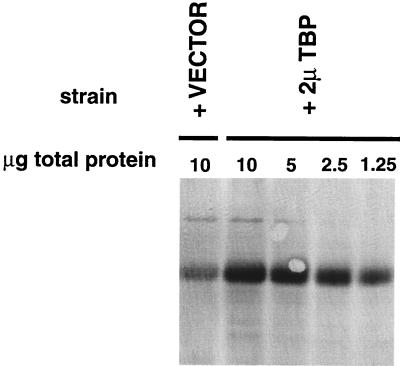
We first confirmed that this strain overexpressed TBP by analyzing TBP levels in extracts by Western blot analysis using affinity-purified TBP antibody. As shown in Fig. 2, strain NOY703 carrying the 2μm-TBP plasmids contains roughly eight times as much TBP as the same strain carrying a control 2μm vector.
FIG. 2.
Overexpression of TBP in an rrn9Δ strain carrying a 2μm (2μ)-TBP plasmid (pNOY419). Protein extracts prepared from rrn9Δ N-PSW strains carrying the vector control (NOY1001) or carrying 2μm-TBP (NOY1000) were analyzed by Western blotting using affinity-purified TBP antibody. Ten micrograms of total protein from both strains and 5, 2.5, and 1.25 μg of protein from the strain carrying 2μm-TBP were run in parallel, demonstrating that 2μm-TBP elevates the levels of TBP approximately eightfold over levels produced by the vector control.
We also wanted to confirm that the TBP expressed by these plasmids was functional. Accordingly, we transformed the 2μm-TBP plasmid, or a 2μm vector control, into strain NOY751, which is a diploid strain carrying a deletion of the TBP-encoding gene (spt15Δ::HIS3) on one chromosome. Sporulation of the diploid transformants, followed by tetrad dissection, resulted in a 2:2 (viable-to-inviable) segregation pattern in the strain carrying the 2μm vector control, as expected. Strains carrying 2μm-TBP plasmids gave rise to four viable haploid segregants, and haploids carrying the spt15Δ::HIS3 allele could survive due to the presence of the 2μm-TBP plasmid (data not shown). Thus, strains carrying 2μm-TBP plasmids overproduce functional TBP.
TBP overexpression does not suppress UAF mutation and does not increase frequency of N-PSW to PSW changes.
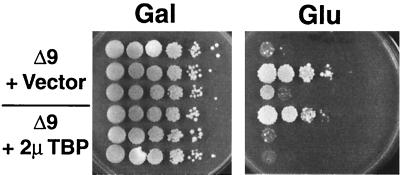
Aprikian and coworkers (1) claimed that TBP overexpression suppressed UAF mutations and led to nearly wild-type levels of growth by activating Pol I transcription. We tried to duplicate this finding by analyzing growth phenotypes of the rrn9Δ N-PSW strain NOY703 carrying either the 2μm-TBP plasmid or a vector control (the same strains used for Western analysis [Fig. 2]). This strain has not undergone the N-PSW to PSW transition and is therefore mostly galactose dependent, using a GAL7-35S rDNA helper plasmid for growth. If TBP overexpression suppresses UAF mutation by activating Pol I transcription, we should expect to see better growth on glucose from strains carrying the high-copy-number TBP plasmids than from the vector control strains. Alternatively, if TBP overexpression increases the frequency of N-PSW to PSW changes, then we would expect to see more colonies on glucose when TBP is overexpressed in this strain. As shown in Fig. 3, however, the TBP-overexpressing strains do not grow better, nor do they consistently produce more colonies on glucose. It is clear that the frequency of PSW variants arising from a given N-PSW strain differs depending on the particular colony picked from the transformation plate, probably depending on how early the rDNA repeats became expanded during formation of that colony. This clonal variation could lead to misinterpretation of the effects of TBP overexpression. By comparing the median values for the frequency of PSW variants produced by nine different colonies, we found no significant difference between strains carrying 2μm-TBP plasmids or the vector control; both showed median frequencies of approximately 10−2 to 10−3 in this experiment. We conclude that TBP overexpression does not suppress UAF mutation by activating Pol I transcription and, in addition, does not significantly increase the frequency of N-PSW to PSW changes.
FIG. 3.
TBP overexpression does not suppress UAF mutation and does not increase the frequency of PSW variants. Leu+ colonies were picked directly from transformation plates containing strain NOY703 (rrn9Δ N-PSW) transformed with either the vector control (NOY1001) or 2μm-TBP (NOY1000). Colonies were suspended in water, and aliquots of 10-fold serial dilutions were then spotted on YEP-galactose (Gal) and YEPD (Glu). A total of nine independent colonies of each strain were analyzed, and three representative colonies of each transformation are shown here. The top three rows contain the control vector (pRS425), and the bottom three contain the 2μm-TBP plasmid (pNOY419). Plates were incubated for 8 days at 30°C.
PSW strains carrying high-copy-number TBP plasmids do not use Pol I for rRNA transcription.
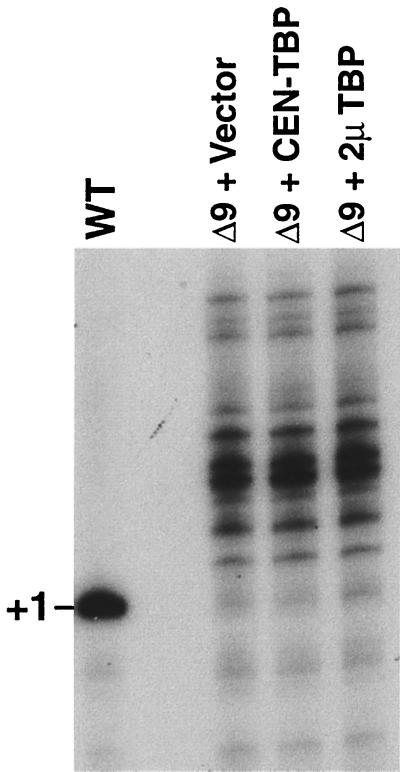
We demonstrated previously that Pol I-mediated transcription of rRNA can be distinguished from Pol II-mediated transcription of rRNA by primer extension, since Pol I and Pol II use different start sites (20). PSW strains grow well by switching completely to Pol II transcription of rRNA and undergoing reversible rDNA repeat expansion, but they still grow slower than wild-type strains. Reversibility of switching from the PSW state to the N-PSW state has been demonstrated previously, for example, after complementation of the UAF defect in the PSW state with a plasmid-borne wild-type UAF subunit gene, resulting in restoration of Pol I-mediated transcription of rRNA (17, 20). Similarly, if TBP overexpression could suppress the UAF defect and allow Pol I transcription to occur, then we would expect to detect Pol I-specific rRNA transcripts in UAF mutant strains carrying overexpressed TBP.
We transformed an rrn9Δ PSW strain with either a 2μm-TBP plasmid, a CEN-TBP plasmid, or an empty vector as a control. The three resultant strains showed equivalent growth rates (data not shown). We then isolated total RNA from these strains and analyzed the rRNA precursor start sites by primer extension. As is evident in Fig. 4, all three strains showed Pol II-specific start sites; no Pol I-specific sites were detectable. Thus, TBP overexpression does not lead to any transcription by Pol I in UAF mutant strains.
FIG. 4.
Primer extension analysis of rRNA precursors in UAF mutant strains overexpressing TBP. RNA was isolated from a wild-type (WT) strain (NOY388) and from rrn9Δ PSW strains (NOY902) carrying the vector control (pRS415), a CEN-TBP plasmid (pNOY347), or a 2μm (2μ)-TBP plasmid (pNOY419). Primer extension was then carried out on 3 μg of total RNA from the wild-type strain or 7 μg of total RNA from the other strains by using a 32P-labeled rDNA primer, as described previously (20). The Pol II-derived rRNA precursors (with multiple start sites from upstream of the Pol I start site, +1; see reference 20) are present in all three rrn9Δ strains, and a Pol I-specific start site (+1) is not detectable.
TBP overexpression in N-PSW/N-PSW diploid strains.
The discrepancies between our results and those of Aprikian and coworkers (1) are based in part on different interpretations of the growth on glucose seen with UAF mutants carrying GAL7-rDNA plasmids. They concluded that growth on glucose of these strains with TBP overexpression was due to suppression of the UAF defect and a return to high levels of Pol I transcription. In our hands, however, TBP overexpression did not lead to wild-type levels of growth; only a minor fraction of cells showed growth on glucose, and the frequency of these colonies varied among different independent isolates, supporting the conclusion that it was due to polymerase switching.
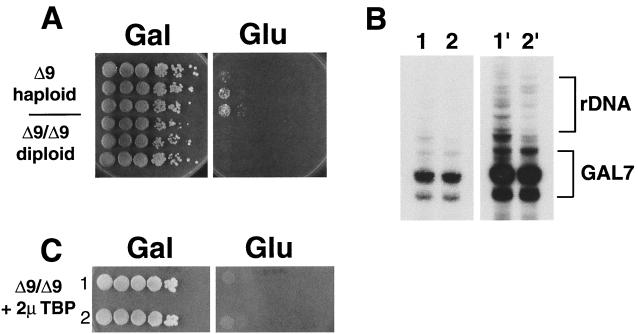
To demonstrate more convincingly that TBP cannot suppress UAF defects, we relied on a discovery we made while examining the phenotypes of diploids and haploid segregants obtained in various crosses among PSW and N-PSW strains. An rrn9Δ N-PSW strain produces some colonies on glucose after extended incubation, due to expansion of the number of rDNA repeats and a shift to the PSW state (Fig. 5A, top three rows). The frequency of switching for the haploid strain was estimated to be 10−3 to 10−4. Crossing two such N-PSW strains results in diploids that do not produce any PSW variants at all on glucose (Fig. 5A, bottom three rows). The frequency of switching for the N-PSW diploid was less than 10−5.
FIG. 5.
Decreased frequency of polymerase switching in rrn9Δ/rrn9Δ diploids and effects of TBP overexpression. (A) Aliquots of 10-fold serial dilutions of an rrn9Δ N-PSW strain (NOY703) and an rrn9Δ/rrn9Δ diploid strain (NOY1002) were spotted on YEP-galactose (Gal) and YEPD (Glu). The plates were incubated for 10 days at 30°C. (B) Primer extension analysis of rrn9Δ haploid and rrn9Δ/rrn9Δ diploid strains for measuring amounts of Pol II-derived rRNA transcripts. RNA was isolated from an rrn9Δ fob1Δ strain stably carrying ∼80 copies of rDNA (strain 1, NOY921) and from an rrn9Δ/rrn9Δ diploid strain (strain 2, NOY1002) grown on galactose medium (note that a fob1Δ derivative of the haploid rrn9Δ strain was used to stabilize ∼80 rDNA copy numbers because the fob1 deletion is known to prevent rDNA copy number expansion and contraction; see reference 17). Five micrograms of total RNA was subjected to primer extension analysis, as described previously (20). The rRNA transcripts derived from the GAL7-rDNA plasmid (GAL7) and from Pol II transcription of the chromosomal repeats (rDNA) are indicated. It should be noted that no Pol I-specific start site is detectable. The left panel (lanes 1 and 2) is from a 3-h exposure, and the right panel (lanes 1′ and 2′) is the same gel after a 16-h exposure. PhosphorImager quantitation of the Pol II-transcribed chromosomally derived bands showed an ∼3-fold decrease in the diploid strain relative to the haploid strain, per total amount of RNA (and per GAL7-35S rDNA-derived rRNA bands, which were approximately the same in both lanes). (C) TBP overexpression does not activate Pol I (or Pol II) transcription in rrn9Δ/rrn9Δ diploids. The rrn9Δ/rrn9Δ diploid strain (NOY1002) was transformed with the 2μm-TBP plasmid (pNOY419). Two independent transformants were then picked and aliquots of 10-fold serial dilutions were spotted on YEP-galactose and YEPD media. The plates were incubated for 11 days at 30°C.
Primer extension analysis of these strains confirmed that there is less Pol II-mediated rRNA transcription in diploid N-PSW strains than in haploid N-PSW strains. Growth rates of these haploid and diploid strains on YEP-galactose medium are about the same, and their growth is dependent on the synthesis of rRNA from the GAL7-35S rDNA fusion gene. As shown in Fig. 5B, transcription of chromosomal rDNA repeats by Pol II, normalized to transcription from the GAL7 promoter, was significantly lower in the diploid rrn9Δ/rrn9Δ strain (lanes 2 and 2′) than in a haploid rrn9Δ N-PSW strain (lanes 1 and 1′). (By PhosphorImager analysis, we found an ∼3-fold decrease in the amount of Pol II-derived rRNA transcripts in the diploid strain relative to the haploid strain per equal amounts of total RNA, whereas levels of transcription from the GAL7 promoter were approximately equal for both strains.) Although we have not studied the reason for the decreased Pol II transcription of chromosomal rDNA in diploid N-PSW strains, this decrease may be responsible for the lower frequency of switching to the PSW state in the diploid strain (see Discussion).
If TBP overexpression can suppress UAF defects and allow Pol I transcription to occur, then we should be able to see growth of the N-PSW diploid on glucose when it carries high-copy-number TBP plasmids. We transformed the rrn9Δ/rrn9Δ diploid strain with the 2μm-TBP plasmid. Western blot analysis of this strain confirmed that TBP was overproduced (data not shown). Several transformant colonies were picked and spotted on either galactose or glucose plates. As shown in Fig. 5C, the presence of 2μm-TBP plasmids does not cause any growth of this strain on glucose, nor does it increase the frequency of PSW variants (compare to Fig. 5A); this indicates that TBP cannot activate Pol I transcription of rDNA in this strain, nor does it increase the frequency of switching to the PSW state.
DISCUSSION
Our inability to reproduce the results recently shown by Aprikian and coworkers (1) suggests that the growth on glucose they observed in UAF mutants carrying overexpressed TBP was perhaps due to polymerase switching. In fact, in some of their experiments, UAF mutants overexpressing TBP showed colony sizes on glucose that were quite heterogeneous, suggesting selection of PSW variants, as we observe in our UAF mutants, rather than the homogeneous colony size expected with genetic suppression. Aprikian and coworkers dismissed this possibility by claiming that temperature-sensitive mutations in Pol I (rpa190ts) led to a loss of the stimulating effect of TBP overexpression in UAF mutant strains specifically at the nonpermissive temperature. They concluded that TBP overexpression stimulates Pol I transcription, and not Pol II transcription, in UAF mutant strains (1). However, we were unable to reproduce the published results, even when using their rrn5Δ, rrn9Δ, and rrn5Δ rpa190ts strains and the TBP overexpression plasmid used in their experiments (experimental data not shown), thus ruling out the possibility of strain variations. Introduction of the TBP overexpression plasmid into these strains by transformation did not suppress the UAF defects; the transformants remained galactose dependent. Thus, we cannot satisfactorily explain how Aprikian and coworkers obtained the results published in their paper.
Aprikian and coworkers also published results of in vitro experiments using unfractionated crude extracts showing that TBP stimulates basal transcription from template DNA containing only the core promoter. The results supported their conclusion that rDNA transcription by Pol I can take place in the absence of UAF, if the TBP concentration is increased, but are in conflict with the results and conclusions of in vitro experiments using both crude extracts (19) and purified components (10) obtained in our laboratory. Their published data are difficult for us to explain. However, even if they obtained such stimulation of basal transcription by TBP under their experimental conditions, we believe that their results are probably not relevant to the transcription of rDNA in vivo, because there is no reliable evidence to support stimulation of rDNA transcription by TBP in UAF mutants in vivo.
In the vertebrate Pol I transcription system, transcription from the core promoter requires transcription factor SL1, which contains TBP and three TBP-associated proteins. Thus, it has often been stated that TBP is required for transcription from the core promoter (e.g., reference 1). However, it should be noted that there has not been any experimental evidence for this statement. Data published by Tjian and coworkers have not demonstrated a TBP requirement for transcription from the core promoter. In their reconstitution experiments (5, 21), there were always weak but definitive transcripts visible in the absence of TBP, and TBP simply stimulated transcription. Importantly, these experiments were done with an intact promoter and in the presence of the upstream binding factor (UBF); the assay was therefore a measurement of activated Pol I transcription. An interaction of UBF with TBP has been reported by several investigators (2, 13), and it was suggested that this interaction might be a basis for recruitment of SL1 by UBF to the promoter. Thus, the previous results reported for vertebrate systems do not contradict our conclusion for the yeast system, i.e., that TBP is essential for activated rDNA transcription and that, in the absence of the activator UAF, TBP does not stimulate basal or core transcription. The question of whether TBP is required for core transcription in vertebrates has not been answered. In fact, transfection experiments carried out by Smale and Tjian (18) using cultured COS cells showed that, for mutant human rDNA promoters containing a partial deletion of the upstream control element, transcription was mostly carried out by Pol II from two initiation sites (−15 and −20) different from the Pol I start site (+1), even though the core promoter defined by in vitro experiments was intact. These results resemble the switching to Pol II transcription observed in yeast UAF mutant strains. In the yeast system, it was initially surprising to find the absence of basal transcription by Pol I in vivo (for example, in UAF mutants), which corresponds to that from the core promoter observed in vitro. However, the results obtained by Smale and Tjian (18) suggest that this might also be the case for higher eukaryotic cells. Further experiments may settle this question in a more definitive way.
We have observed that transcription of chromosomal rDNA repeats by Pol II in the absence of the UAF subunit Rrn9p is much lower in diploid cells than in haploid cells and that this is correlated with our inability to detect switching to the PSW state in diploid cells. In addition to the reduction in Pol II transcription, the rate of repeat expansion that is required for switching to the PSW state might also be affected in diploid cells, but we have not studied this possibility. Interactions between homologous chromosomes in diploid cells are known to take place in a variety of organisms, including yeast (3), and trans-sensing phenomena related to epigenetic alterations of gene expression have been documented (6, 7). Our observation of increased silencing of Pol II transcription in rrn9 diploids relative to haploids may be related to the pairing-dependent silencing of certain Drosophila genes (for reviews, see references 6 and 7) and may provide a system which could be used to study molecular mechanisms involved in this interesting phenomenon.
In summary, the results described here show that transcription factor UAF plays a unique role in silencing Pol II transcription of rDNA and activating Pol I transcription. Importantly, TBP overexpression cannot suppress defects in either of these functions of UAF. These results support our previously proposed model for the role of TBP as an activator of Pol I transcription that is completely dependent on the presence of UAF and the upstream element of the rDNA promoter (10, 19).
ACKNOWLEDGMENTS
We thank Ron Reeder for providing strains and plasmids used in some of the experiments published in reference 1.
This work was supported by Public Health Service grant GM 35949.
REFERENCES
- 1.Aprikian P, Moorefield B, Reeder R H. TATA binding protein can stimulate core-directed transcription by yeast RNA polymerase I. Mol Cell Biol. 2000;20:5269–5275. doi: 10.1128/mcb.20.14.5269-5275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckmann H, Chen J-L, O'Brien T, Tjien R. Coactivator and promoter-selective properties of RNA polymerase I TAFs. Science. 1995;270:1506–1509. doi: 10.1126/science.270.5241.1506. [DOI] [PubMed] [Google Scholar]
- 3.Burgess S M, Kleckner N, Weiner B M. Somatic pairing of homologs in budding yeast: existence and modulation. Genes Dev. 1999;13:1627–1641. doi: 10.1101/gad.13.12.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christianson T W, Sikorski R S, Dante M, Shero J H, Hieter P. Multifunctional yeast high-copy shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- 5.Comai L, Tanese N, Tjian R. The TATA-binding protein and associated factors are integral components of the RNA polymerase I transcription factor, SL1. Cell. 1992;68:965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- 6.Henikoff S. Nuclear organization and gene expression: homologous pairing and long range interactions. Curr Opin Cell Biol. 1997;9:388–395. doi: 10.1016/s0955-0674(97)80012-9. [DOI] [PubMed] [Google Scholar]
- 7.Henikoff S, Comai L. Trans-sensing effects: the ups and downs of being together. Cell. 1998;93:329–332. doi: 10.1016/s0092-8674(00)81161-7. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez N. TBP, a universal eukaryotic transcription factor? Genes Dev. 1993;7:1291–1308. doi: 10.1101/gad.7.7b.1291. [DOI] [PubMed] [Google Scholar]
- 9.Keener J, Dodd J A, Lalo D, Nomura M. Histones H3 and H4 are components of upstream activation factor required for the high-level transcription of yeast rDNA by RNA polymerase I. Proc Natl Acad Sci USA. 1997;94:13458–13462. doi: 10.1073/pnas.94.25.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keener J, Josaitis C A, Dodd J A, Nomura M. Reconstitution of yeast RNA polymerase I transcription in vitro from purified components. J Biol Chem. 1998;273:33795–33802. doi: 10.1074/jbc.273.50.33795. [DOI] [PubMed] [Google Scholar]
- 11.Keys D A, Vu L, Steffan J S, Dodd J A, Yamamoto R T, Nogi Y, Nomura M. RRN6 and RRN7 encode subunits of a multiprotein complex essential for the initiation of rDNA transcription by RNA polymerase I in Saccharomyces cerevisiae. Genes Dev. 1994;8:2349–2362. doi: 10.1101/gad.8.19.2349. [DOI] [PubMed] [Google Scholar]
- 12.Keys D A, Lee B S, Dodd J A, Nguyen T T, Vu L, Fantino E, Burson L M, Nogi Y, Nomura M. Multiprotein transcription factor UAF interacts with the upstream element of the yeast RNA polymerase I promoter and forms a stable preinitiation complex. Genes Dev. 1996;10:887–903. doi: 10.1101/gad.10.7.887. [DOI] [PubMed] [Google Scholar]
- 13.Kwon H, Green M R. The RNA polymerase I transcription factor, upstream binding factor, interacts directly with the TATA box-binding protein. J Biol Chem. 1994;269:30140–30146. [PubMed] [Google Scholar]
- 14.Lalo D, Steffan J S, Dodd J A, Nomura M. RRN11 encodes the third subunit of the complex containing Rrn6p and Rrn7p that is essential for the initiation of rDNA transcription by yeast RNA polymerase I. J Biol Chem. 1996;271:21062–21067. doi: 10.1074/jbc.271.35.21062. [DOI] [PubMed] [Google Scholar]
- 15.Lin C W, Moorefield B, Payne J, Aprikian P, Mitomo K, Reeder R H. A novel 66-kilodalton protein complexes with Rrn6, Rrn7, and TATA-binding protein to promote polymerase I transcription initiation in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:6436–6443. doi: 10.1128/mcb.16.11.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogi Y, Vu L, Nomura M. An approach for isolation of mutants defective in 35S ribosomal RNA synthesis in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1991;88:7026–7030. doi: 10.1073/pnas.88.16.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oakes M, Siddiqi I, Vu L, Aris J, Nomura M. Transcription factor UAF, expansion and contraction of ribosomal DNA (rDNA) repeats, and RNA polymerase switch in transcription of yeast rDNA. Mol Cell Biol. 1999;19:8559–8569. doi: 10.1128/mcb.19.12.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smale S T, Tjian R. Transcription of herpes simplex virus tk sequences under the control of wild-type and mutant human RNA polymerase I promoters. Mol Cell Biol. 1985;5:352–362. doi: 10.1128/mcb.5.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steffan J S, Keys D A, Dodd J A, Nomura M. The role of TBP in rDNA transcription by RNA polymerase I in Saccharomyces cerevisiae: TBP is required for upstream activation factor-dependent recruitment of core factor. Genes Dev. 1996;10:2551–2563. doi: 10.1101/gad.10.20.2551. [DOI] [PubMed] [Google Scholar]
- 20.Vu L, Siddiqi I, Lee B S, Josaitis C A, Nomura M. RNA polymerase switch in transcription of yeast rDNA: role of transcription factor UAF (upstream activation factor) in silencing rDNA transcription by RNA polymerase II. Proc Natl Acad Sci USA. 1999;96:4390–4395. doi: 10.1073/pnas.96.8.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zomerdijk J C B M, Beckmann H, Comai L, Tjian R. Assembly of transcriptionally active RNA polymerase I initiation factor SL1 from recombinant subunits. Science. 1994;266:2015–2018. doi: 10.1126/science.7801130. [DOI] [PubMed] [Google Scholar]