Abstract
Background and Objective
An inconsistent association between exposure to SSRIs and SNRIs and the risk for ASD and ADHD in the Offspring was observed in observational studies. Some suggest that the reported association might be due to unmeasured confounding. We aimed to study this association and to look for sources of bias by performing a systematic review and meta-analysis.
Methods
Medline, Embase, and the Cochrane Library were searched up to June 2019 for studies reporting on ASD and ADHD in the Offspring following exposure during pregnancy. We followed the PRISMA 2009 guidelines for data selection and extraction. Outcomes were pooled using random-effects models and odds ratios (OR), and 95% confidence intervals (CI) were calculated for each outcome using the adjusted point estimate of each study.
Results
Eighteen studies were included in the meta-analysis. We found an association between SSRIs/SNRIs prenatal use and the risk for ASD and ADHD (OR=1.42, 95% CI: 1.23–1.65, I2=58%; OR=1.26, 95% CI: 1.07-1.49, I2=48%, respectively). Similar findings were obtained in women who were exposed to SSRIs/SNRIs before pregnancy, representing statistically significant association with ASD (OR=1.39, 95% CI: 1.24-1.56, I2=33%) and ADHD (OR=1.63, 95% CI: 1.50-1.78, I2=0%) in the Offspring, although they were not exposed to those medications in utero.
Conclusions
Although we found an association between exposure to SSRIs/SNRIs during pregnancy and the risk for ASD and ADHD, an association with those disorders was also present for exposure pre-pregnancy, suggesting that the association might be due to unmeasured confounding. We are aiming to further assess the role of potential unmeasured confounding in the estimation of the association and perform a network meta-analysis.
Keywords: SSRI’s, SNRI’s, antidepressants, ASD, ADHD, prenatal exposure, pregnancy
1. INTRODUCTION
ASD (Autism spectrum disorder) and ADHD (Attention-deficit hyperactivity disorder) are rapidly increasing over the past decade [1, 2].
ASD has an estimated worldwide prevalence of about 1.5%, ADHD has a worldwide combined prevalence of about 5.3% in childhood [3].
Many risk factors might cause neurodevelopmental abnormalities. These risk factors include maternal age, parity, gestational age at birth, maternal smoking status, medications or drug abuse during pregnancy, psychiatric and mental disorders, as well as other maternal and paternal medical conditions and comorbidities. The effects of maternal antidepressant therapy on cognitive and behavioral development in childhood were studies as well [4-8]. Recent studies reported an association between exposure to SSRIs and SNRIs during pregnancy and ASD and ADHD in Offspring [1, 9-13]. However, other studies did not find these associations [2, 14-24].
Selective serotonin reuptake inhibitors (SSRIs) (fluoxetine, paroxetine, sertraline, citalopram, escitalopram, fluvoxamine), and serotonin-norepinephrine reuptake inhibitors (SNRI's) (venlafaxine) are increasingly used since 1990 for the treatment of depression and anxiety disorders. They are considered the preferred first-line treatment during pregnancy [25]. It has been suggested that 7-13% of women are exposed to antidepressant therapy (SSRI's and SNRI's), at any trimester of pregnancy [4]. However, SSRIs and SNRIs are known to cross the placenta, and it is estimated that fetal exposure for fluoxetine, citalopram, escitalopram, and sertraline are 65%, 70%, 50%, and 30%, respectively [5]. The use of SSRIs/SNRIs during pregnancy was reported to be associated with adverse pregnancy outcomes, including birth defects such as cardiac malformations (although controversial), neonatal adaptation syndrome, and increased rate of persistent pulmonary hypertension of the newborn. Despite the possible risk for the fetus, discontinuation of antidepressants during pregnancy can increase the risk of relapse, and maternal depression during pregnancy is associated with health complications for both the mother and infant, such as premature delivery and decreased breastfeeding initiation [26, 27]. As with any drug treatment in pregnancy, the benefits to the mother should be considered versus the possible hazards to the developing embryo/fetus [7].
Presently, there is inadequate evidence for an association between antidepressant therapy in pregnancy and those neurodevelopmental disorders in the Offspring, especially when controlling possible confounding factors. One of the possible explanations for the differences in the results of the studies may lie in genetic and epigenetic differences among the populations. SSRI's may have epigenetic effects, and epigenetic changes are known to be associated with neurodevelopmental disorders [7].
Due to the conflicting and an uncertain data, regarding the association between prenatal exposure to SSRIs/SNRIs, and ADHD or ASD in offspring [7], we aimed to perform a systematic review and meta-analysis to study the association between exposure to SSRIs/SNRI's during pregnancy and the risk for ASD and ADHD in the Offspring.
2. MATERIAL AND METHODS
2.1. Data Sources
This systematic review followed the Meta-analysis for Observational Studies in Epidemiology (MOOSE) checklist and the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) 2009 guidelines (Appendixes A and B) [28]. MEDLINE, Embase, and Cochrane databases were searched up to June 2019 to identify all published cohort and case-control studies, assessing the association between exposure to SSRIs or SNRIs during pregnancy and ASD or ADHD. The following keywords, in different combinations and Medical Subject Heading terms were used to identify relevant studies: “pregnancy”, “pregnant”, ” prenatal”, “female”, ” fetus”, “offspring”, “newborn”, “embryos”, “infant”, ” neonatal”, ” selective serotonin reuptake inhibitors”, “SSRI”, “selective norepinephrine reuptake inhibitors”, “SNRI”, ” antidepressant”, “fluoxetine”, “sertraline”, “paroxetine”, “citalopram”, “escitalopram”, “fluvoxamine”, “venlafaxine”, “autism”, “ASD”, “autistic spectrum disorder”, “ADHD”, “attention-deficit hyperactivity disorder “, “neurodevelopment”, “risk”, “outcome”. In addition, we searched and evaluated published systematic reviews, online resources, and conference abstracts, to ensure the identification of all studies. No language or date restrictions were applied. The review protocol was registered at the PROSPERO registry: CRD42019138483. Retrospective cohort studies and case-control studies reporting the risk for ASD or ADHD in the Offspring of women exposed to selective SSRI's or SNRI's during pregnancy, were extracted. Two independent researchers identified relevant data (Siham Nashef and Jessica Sliman). Random-effects meta-analysis was used to pool results. Odds ratios were calculated with subsequent 95% confidence intervals (CI). Network meta-analysis was conducted, incorporating direct and indirect comparisons among different selective serotonin reuptake inhibitors. The primary outcome was the risk for ASD or/and ADHD of the Offspring after maternal exposure to SSRI's or SNRI's during pregnancy.
2.2. Selection Criteria
The following screening criteria were applied to assess eligibility: manuscripts and abstracts of cohort or case-control studies reporting the risk for ASD/ADHD in Offspring of women exposed to SSRIs or SNRIs during pregnancy, using risk ratio (RR), incidence rate ratio, or odds ratio (OR). Outcomes reported by the same cohort in different publications were included only once in each analysis (most recent publication). We excluded cross-sectional studies, case reports and case series, guidelines, expert opinion, editorials, letters to the editor, and comments.
2.3. Data Extraction
Data were identified by 2 investigators (Siham Nashef and Jessica Sliman). Titles and abstracts were independently screened by the 2 investigators. Disagreements were resolved by consensus or referral to a third investigator. Full text was retrieved by the 2 investigators. The primary outcome of this analysis was ASD and/or ADHD. Network meta-analysis was conducted to compare the risk of ASD or ADHD with the different SSRIs.
2.4. Quality Assessment
Risk of bias and quality were assessed using the Newcastle-Ottawa scale (NOS) for assessing quality of nonrandomized studies [29]. The scale is based on 8 criteria and provides a star rating score ranging from 0 (high risk for bias) to 9 (low risk for bias). Summary assessments of risk of bias were derived for each study. Assessments were carried out independently by 2 investigators (Siham Nashef and Jessica Sliman).
2.5. Publication Bias
Publication bias was assessed by visual inspection of the funnel plot and Egger test [30]. The nonparametric trim-and-fill technique was used to identify and correct funnel plot asymmetry if found. We used CMA Software Version 3.3.070 [31] and R Version 3.4.3 and the “metafor” package Version 1.9-9 [32], respectively [33].
2.6. Data Synthesis and Statistical Analysis
To estimate ASD and ADHD risk, we used CMA Software, applying random-effects meta-analysis (Mantel Haenszel) for the results [31]. We used a random-effects model, because the effect size varies across the studies due to a real difference in the exposure effect and sampling variability [34, 35]. Pooled adjusted ORs and 95% confidence intervals (CIs) for ASD and ADHD were collected from the data in relevant studies, including in the meta-analysis. Since both our outcomes are relatively rare, we assumed that RRs and ORs are expected to be equal. I2 statistic was used to assess the heterogeneity, while the low, medium, and high heterogeneity expressed by I2 values of 25%, 50%, and 75%, respectively [36]. We defined a 2-sided α of < 0.05 for statistical significance, and confidence intervals (CIs) that did not include OR value of “1” considered clinically significant.
2.7. Network Meta-analysis
To investigate the differences in the risk for ASD or ADHD between various SSRI's agents, we performed a pairwise network meta-analysis, using random effects. Agents compared included sertraline, citalopram, fluoxetine, fluvoxamine, paroxetine and venlafaxine, and the network incorporated data on results relative to no treatment and head-to-head comparisons. ORs and 95% CIs were modeled with the pairwise method. The risk of ASD or ADHD was ranked using P scores derived from network point estimates and SE. The P score is a frequentist equivalent to the Bayesian network surface under the cumulative ranking curve. The P score of treatment can be interpreted as the mean extent of certainty that the treatment is better than another treatment, and can be used to rank a treatment within a range of treatments, measured on a scale from 0 (worst) to 1 (best) [37]. Analysis was performed using R Version 3.4.3 and the “netmeta” package Version 0.9-8 [38].
3. RESULTS
3.1. Search Process
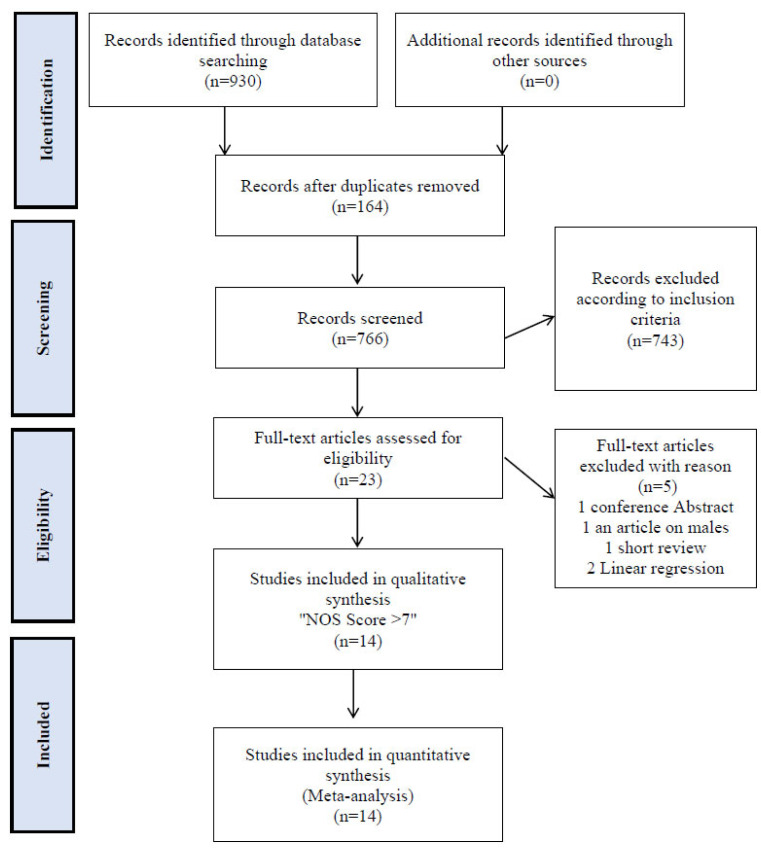
The systematic search yielded 930 citations. Preliminary screening excluded 164 duplicate citations. The 766 remaining titles were reviewed by abstract. A total of 743 citations were excluded according to inclusion criteria, leaving 23 records for full-text review. Full review excluded 5 additional citations (1 - conference Abstract, 1 - an article on males, 1 - short review and 2 linear regression), leaving 18 records for analysis. The search flow process is illustrated in Fig. (1).
Fig. (1).
Flow diagram of studies through review process.
3.2. Study Characteristics
Eighteen studies were included in the meta-analysis. Study characteristics are summarized in Tables 1 (a, b) and 2. Of which, six studies were case-control studies [1, 9-11, 16, 21] and twelve studies were historical cohorts [2, 12-15, 17-20, 22-24]. In total, 133,799 women and their offspring were exposed to SSRIs or SNRIs during pregnancy; ASD was detected among 1373 exposed offspring (rate of 10.3/1000 live births) and ADHD among 1240 (rate of 9.3/1000 live births).
Table 1a.
Characteristics of studies on ASD included in analysis.
| Study, Year | No. of Cases of ASD | Exposure Assessment | Exposure Timing and Type |
|---|---|---|---|
| Croen et al, [9] 2011 | Exposed: 15/49Not exposed: 283/1756 | Prescription dispensing | All trimesters: SSRI's, SNRI's |
| Hviid et al, [14] 2013 | Exposed: 52/6068Not exposed: 3752/620807 | DBL | All trimesters: citalopram, fluoxetine, sertraline, escitalopram, fluvoxamine. |
| Rai et al, [11] 2013 | Exposed: 14/85Not exposed: 1665/18439 | DBL and maternal reports. | SSRI's and Non-SSRI's (monoamine reuptake inhibitors) |
| Sorensen et al, [15] 2013 | Exposed: 104/8833Not exposed: 5333/646782 | Prescription dispensing | All trimesters: SSRI's, SNRI's and TCAs. |
| Gidaya et al, [10] 2014 | Exposed: 76/441Not exposed: 5139/56924 | DBL | All trimesters: fluoxetine, citalopram sertraline, fluvoxamine, paroxetine, escitalopram. |
| Harrington et al, [16] 2014 | Exposed: 29/40Not exposed: 463/772 | Telephone interviews and DBL (when available). | All trimesters: fluoxetine, sertraline, paroxetine, citalopram, escitalopram. |
| Clements et al, [1] 2015 | Exposed: 40/121Not exposed: 1337/5278 | Prescription dispensing | All trimesters: Serotonergic and non-SSRI's antidepressants. |
| Boukhris et al, [12] 2016 | Exposed: 46/4724Not exposed: 1008/140732 | DBL and maternal reports. | All trimesters: SSRI's, SNRI's, TCAs, MAOIs and other. |
| Malm et al, [13] 2016 | Exposed: 88/15729Not exposed: 100/31394 | Prescription dispensing | All trimesters: fluoxetine, citalopram, paroxetine, sertraline, fluvoxamine, escitalopram. |
| Brown et al, [17] 2017 | Exposed: 58/2837Not exposed: 335/33069 | DBL and prescription dispensing. | First to third trimester: SSRI's or SNRI's. |
| Rai et al, [18] 2017 | Exposed: 136/3342Not exposed: 353/12352 | DBL and maternal reports. | All trimesters: SSRI's and others. |
| Sujan et al, [19] 2017 | Exposed: 299/22544Not exposed: 14318/1558085 | DBL and maternal reports. | First trimester: Any antidepressant |
| Viktorin et al, [20] 2017 | Exposed: 85/3982Not exposed: 1524/172646 | DBL | All trimesters: SSRI's, SNRI's and others. |
| Wilcox-Hagberg et al, [21] 2017 | Exposed: 324/20355Unexposed: 1696/168797 | DBL | All trimesters: SSRI's, SNRI's, TCA's and others. |
| Yamamoto-Sasaki et al, [22] 2019 | Exposed: 7/195Not exposed: 423/26730 | DBL | All trimesters: SSRI's, SNRI's and others. |
Table 1b.
Characteristics of studies on ADHD included in analysis.
| Study, Year | No. of Cases of ADHD | Exposure Assessment | Exposure Timing and Type |
|---|---|---|---|
| Laugesen et al, [23] 2013 | Exposed: 79/348Not exposed: 270/519 | DBL | All trimesters: SSRI's, SNRI's, TCAs and others. |
| Clements et al, [1] 2015 | Exposed: 63/131Not exposed: 2180/7743 | Prescription dispensing | All trimesters: Serotonergic and non-SSRI's antidepressants. |
| Malm et al, [13] 2016 | Exposed: 160/15729Not exposed: 124/31394 | Prescription dispensing | All trimesters: fluoxetine, citalopram, paroxetine, sertraline, fluvoxamine, escitalopram. |
| Boukhris et al, [2] 2017 | Exposed: 267/4678Not exposed: 4297/139728 | DBL and prescription dispensing. | Second and third trimester:SSRIs, SNRIs, MAOIs, TCAs other antidepressants. |
| Man et al, [24] 2017 | Exposed: 58/1024Not exposed: 5564/189002 | DBL and prescription dispensing. | All trimesters: SSRI's and Non-SSRI's. |
| Sujan et al, [19] 2017 | Exposed: 613/22544Not exposed: 32311/1558085 | DBL and maternal reports. | First trimester: Any antidepressant. |
Baseline characteristics of mothers and their Offspring were used to adjust for potential confounders in the studies included in our meta-analysis. Eight studies in our meta-analysis [ 9, 15, 17-19, 21-23] performed an additional analysis. The risk for ASD and ADHD was compared between two siblings, one exposed to SSRIs/SNRIs during pregnancy while the other was not. Furthermore, four studies [2, 14, 20, 21] adjusted for an ASD/ADHD diagnosis in either the mother and or the father. The remaining seven studies included in the meta-analysis did not refer to the family history of ASD/ADHD.
Assessment of exposure during pregnancy was carried out by interviews in 1 study [11], by prescription dispensing and database linkage in 13 studies [1, 2, 9, 10, 13-17, 20-24] or by both these sources in 3 studies [12, 18, 19]. Seventeen studies [1, 2, 9-18, 20-24] reported on SSRI or SNRI exposure during all pregnancy (some of them were separated into three different trimesters), and 1 study reported on exposure during only the first trimester [19]. Fourteen studies reached a NOS score of 7 [1, 2, 9, 12-16, 18, 20-24] and 4 studies reached NOS scores of 6 [10, 11, 17, 19]. Quality assessment scores and adjustments made for potential confounders in each study are detailed in Supplementary Tables 1 and 2.
3.3. Meta-analysis
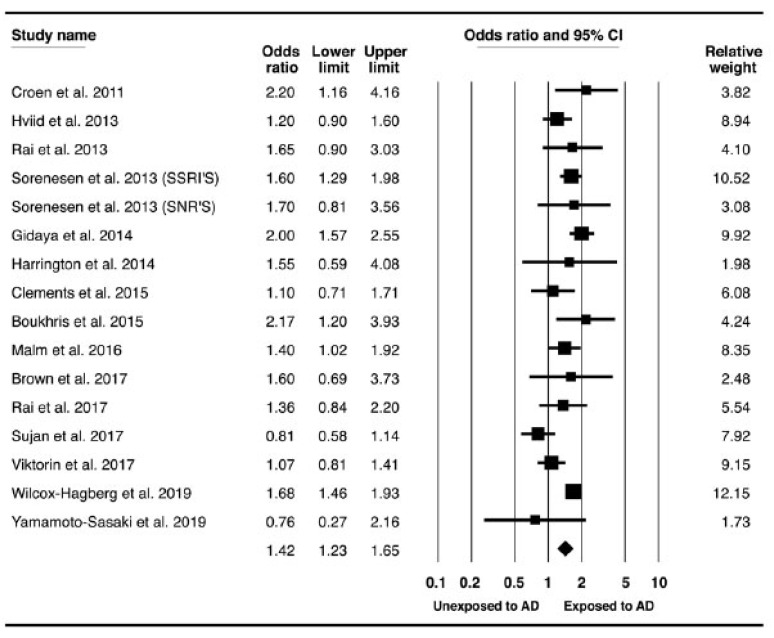
Most studies evaluated maternal exposure to antidepressant therapy during all pregnancy, except for two works [11, 19] that assessed only the first trimester. Some of the articles [1, 9, 13-16, 18, 19, 21, 23] also examined the association between pre-conception maternal exposure and the primary outcomes. Nine cohort, four case-control and two nested case-control studies evaluated the risk for ASD. Five cohort and one case-control studies evaluated the risk for ADHD. Using random-effects model, SSRI or SNRI exposure during pregnancy (any trimester) was significantly associated with an increased risk for ASD, with moderate heterogeneity (OR, 1.26; 95% CI, 1.07-1.49; I2= 48%). (Fig. 2) However, when pre-pregnancy exposure to SSRI or SNRI, was studied, we found a statistically significant association with ASD, with a similar point estimate of the relative risk (OR, 1.39; 95% CI, 1.24-1.56; I2= 33%). (Supplementary Fig. 1). Similar findings were obtained with ADHD, revealing slightly increased risk in a prenatal maternal exposure group (OR, 1.26; 95% CI, 1.07-1.49; I2= 48%) (Fig. 3). Exposure to SSRIs/SNRIs before pregnancy is associated with elevated risk for ADHD as well (OR, 1.63; 95% CI, 1.50-1.78; I2= 0%) (Supplementary Fig. 2).
Fig. (2).
Association between maternal exposure to anti-depressant and the risk for ASD in offspring.Forest plot of OR for ASD in offspring. Black boxes represent point estimates for OR surrounded by 95% CI. *OR, odds ratio; ASD, autistic spectrum disorder; CI, confidence interval; AD, antidepressants; EXP, exposure. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (3).
Meta-analysis of all studies: Association between maternal exposure to anti-depressant and the risk for ADHD in offspring.Forest plot of OR for ADHD in offspring. Black boxes represent point estimates for OR surrounded by 95% CI. *OR, odds ratio; ADHD, attention-deficit hyperactivity disorder; CI, confidence interval; AD, antidepressants; EXP, exposure. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.4. Network Meta-analysis
In a network meta-analysis, the risk of ASD was compared between specific SSRI agents. Based on the results of this network, Venlafaxine was found to have the highest P score, indicating the lowest probability for ASD (P score = .65); while Fluvoxamine was associated with a higher risk of ASD (P score = .17). (Table 3) Nevertheless, no statistically significant difference in ASD risk was found among any of the comparison pairs of the SSRI agents (Fig. 4). In addition, that was no correlation between the placental transfer rate of a SSRI-specific agent and its risk for that adverse event, as was demonstrated in previous works [4, 5].
Table 3.
Treatment ranks for selective serotonin reuptake inhibitors safety (P scores) and probability for ASD in offspring.
| Medication | P score |
|---|---|
| Venlafaxine | 0.65 |
| Sertraline | 0.50 |
| Citalopram | 0.50 |
| Sertraline | 0.46 |
| Fluoxetine | 0.37 |
| Paroxetine | 0.32 |
| Fluvoxamine | 0.17 |
Fig. (4).
Pairwise network meta-analysis for different SSRI's and the risk for ASD.Blue boxes represent point estimates for OR surrounded by 95% CI. * SSRI's, selective serotonin reuptake inhibitors ASD, autistic spectrum disorder; OR, odds ratio; CI, confidence interval. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.5. Sensitivity Analysis
An analysis restricted to fully published studies reaching a NOS score of 7, the association with ASD remained significant, with a similar point estimate (OR, 1.43; 95% CI, 1.25-1.64; I2=38%) and ADHD (OR, 1.34; 95% CI, 1.15-1.55; I2=23%) (Supplementary Fig. 3 & 4, respectively).
3.6. Publication Bias
Publication bias was calculated for ASD analysis, including all 14 studies. Visual inspection of the funnel plot (Fig. 5) shows slight asymmetry. When applying the trim-and-fill technique, no studies were missing in the plot. Egger test was not statistically significant (P = .37). Therefore, we conclude no publication bias was detected in our analysis.
Fig. (5).
Funnel plot for publication bias assessment.* OR, odds ratio; SE, standard error. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. DISCUSSION
Our results suggest that the association between exposure to SSRIs/SNRIs during pregnancy and ASD/ADHD may be due to residual confounding, mainly confounding by indication. We showed that this association is statistically significant even in mothers who were exposed to SSRIs/SNRIs before pregnancy, and the Offspring were not exposed to SSRIs/SNRIs, hence suggesting confounding by indication. These drugs are indicated for the treatment of depression and/or anxiety.
Maternal depression and stress are associated with birth and neurodevelopmental problems, suggesting that antidepressant associations could be attributable to confounding by indication for such treatment. Physiologic changes related to a mother’s stress or depression during pregnancy. A recently reported experimental model demonstrates that the combined effect of maternal serotonin transporter genotype and prenatal stress may contribute to autistic-like behaviors in Offspring. Whether the combined effects of prenatal SSRI exposure and prenatal stress are etiologically related to ASD in humans remains to be elucidated [9, 19].
Furthermore, the etiology of autism spectrum disorder and attention-deficit/hyperactivity disorder involves genetic, epigenetic and environmental factors. Heritability of ASD is estimated to be ~80% [40]. ADHD has an underlying genetic component, with heritability estimated at ~76% [41, 42].
The sex of the Offspring is a well-documented risk factor for ASD and ADHD, providing further confidence in the data quality. For example, boys were more likely to have a diagnosis of ASD than girls, with a ratio about 4:1. Although not all studies indicated the exact sex distribution in research groups, all of their models performed sex adjustment. That data is summarized in supplementary Table 3 .
Heritability is known to be a strong risk factor for ASD/ADHD. Thus, the increase in ASD/ADHD in Offspring of women with depression and other mental diseases may reflect genetic predisposition. Examining any mental illness diagnosis in the parents’ lifetime allowed detailed adjustment for confounding due to, e.g ., genetic liability. Sibling study designs offer a way to control for genetics as well as other unmeasured time-invariant factors. Sorensen et al. [ 15 ] reported that, among 2,765 families in which at least one child had ASD, the risk of ASD in children exposed to antidepressants for any indication during pregnancy was 1.1 (95% CI 0.5–2.3) compared to their unexposed siblings. In their sibling comparison, Sujan et al. [ 19 ] reported that the risk of ASD in children exposed to antidepressants in the first trimester was 0.83 (95% CI 0.62–1.13 (compared to unexposed siblings. Brown et al. [ 17 ] estimated that the risk of ASD in children exposed to two or more antidepressant prescriptions was 1.60 (95% CI 0.69–3.74) compared to unexposed siblings. Wilcox-Hagberg et al. [ 21 ] sibling analysis based on 136 discordant sibling pairs reported that the risk of having a child with ASD among women with untreated depression (RR =1.18, 95% CI 0.64–2.20) was similar to that of the Sorensen et al [ 15 ] study. However, the magnitude of the effect was slightly higher among women with treated depression (RR =1.53, 95% CI 0.89,(2.62- an estimate similar to that of the Brown et al [ 17 ] study. These sibling analyses suggest that after controlling for genetics and time-invariant confounders, prenatal SSRIs/SNRIs use does not significantly increase the risk of ASD in Offspring. However, there may remain a difference in risk by the severity of depression during pregnancy.
In addition, the observed association between prenatal use of SSRIs and risk of ASD or ADHD in Offspring can be partially explained by confounding by indication, because the results from sibling-matched analyses in some studies including in our meta-analysis do not support an increased risk of those disorders in discordant exposed siblings [15, 43].
This highlights the potential effect of confounding by indication of SSRIs and the importance of being able to adequately take this confounding into account in the study's designs. Although all studies in our meta-analysis performed variable statistical models for estimation of adjusted odds ratio, but as with all observational studies, the possible presence of residual and unmeasured confounding or ascertainment bias with respect to exposure and outcome adds to the imprecision of our estimates. Thus, the interpretation of our results should take this factor into account.
The serotonin transporter, which is blocked by SSRIs, is expressed transiently in many brain areas during fetal life and serotonin plays a key role in neural development and maturation [23]. Hyperserotonemia is found in approximately one-third of children with autism. Altered serotonin levels during early development are speculated to lead to abnormal brain circuitry and autism symptoms. SSRIs, which increase extracellular serotonin, given the central role of serotonin in brain development through maternal-fetal and placental interactions [16].
The results of this meta-analysis were consistent with numerous observational studies that have demonstrated associations between prenatal antidepressant exposure and neurodevelopmental problems [1, 9-13]. Some published observational studies prompted concerns that the prenatal antidepressant exposure is associated with birth and neurodevelopmental problems, including shorter gestation and reduced fetal growth [39], autism spectrum disorder and attention-deficit/hyperactivity disorder [9, 12, 16, 43]. However, other publications didn't support this finding, particularly after fully adjusted analysis, taking into the account main confounders, such as maternal psychiatric disorders and their severity, mother's age and sibling diagnosed with these disorders [1, 15, 18, 20, 24].
The fetal cord blood and maternal plasma concentrations (C/M) distribution ration depends on the pharmacological properties of each antidepressant drug. In addition, genetic polymorphisms for CYPs may contribute to inter-individual variability of placenta transition within each SSRI [4]. The distribution of the SSRIs (citalopram, escitalopram, fluoxetine and fluvoxamine) and their metabolites across the placenta are generally high with median C/M values ranging from 0.7–0.86. However, the median C/M for sertraline, and for paroxetine is lower (0.36 and 0.15, respectively). Venlafaxine has a median C/M values of 0.72 [44, 45].
Tricyclic antidepressants (TCAs) and their metabolites cross readily into umbilical cord serum, but SSRIs have higher umbilical cord transfer rates than TCAs [46]. According to published observational studies, maternal TCAs use didn't increase the risk for ASD or ADHD in Offspring [2, 9, 11, 15, 23, 46].
In a network meta-analysis comparing the individual SSRI, no correlation between the specific SSRIs/SNRIs permeability through the placenta and the risk for ASD, thus corroborating our conclusion that the association we saw between these medications with ASD is not causal, but because a confounding by indication is present. Venlafaxine that has a higher placenta transfer, demonstrated the lowest risk for ASD in our founding. Nonetheless, no statistically significant difference in ASD risk was found among any of comparison pairs of the SSRI agents.
5. STRENGHTS AND WEAKNESSES
Strengths of our analysis include a thorough and systematic review of all available published studies, just cohort or case-control study design. We used a random-effects meta-analysis to overcome the heterogeneity in our analysis. We looked into pre-pregnancy exposure where the Offspring was not exposed to SSRIs/SNRIs, and we showed a similar pooled effect size as exposure during pregnancy, suggesting that the effect may be due to unmeasured variables, mainly confounding by indication. We conducted the network-meta analysis to explore the differences in the risk for ASD among specific SSRIs, and we found variable probability for that disorder among them, but the difference was not statistically significant. Finally, we conducted sensitivity analysis to assess the risk for ASD and ADHD in the preconception period, revealing the potential effect of confounding by indication of the SSRIS's and SNRI's treatment. We used the adjusted effect size from the studies included in the meta-analysis, thus lowering the risk for bias.
Limitations of our study include, firstly, the methodology of the studies included in the analysis is susceptible to recall and exposure bias. Another limitation is that meta-analysis does not enable adjustment to covariates, however, we used adjusted effect sizes meta-analysis. Data on medication exposure were collected by interviews or follow-up on prescription dispensing and databases linking. Mothers of infants with an ASD and ADHD diagnosis are more likely to remember and to associate between exposure to medications during pregnancy and offspring morbidity. Additionally, prescription dispensing does not necessarily indicate intrauterine exposure to SSRIs, which may contribute to the bias. Secondly, study-level meta-analysis does not allow for adjustment for all covariates that may affect the risk for ASD or ADHD. Moreover, some studies did not fully adjust the Odds ratio to SSRI's and SNRI's group, but only for all antidepressants medications. Furthermore, residual confounding by indication for SSRI use remains possible because we were unable to assess the severity of mental health symptoms. Unfortunately, no studies in our meta-analysis evaluated the possibility of dose-response of SSRIs and SNRIs. However, dosage may not correlate well with circulating SSRI levels, given differences in metabolism arising from, for example, metabolic gene polymorphisms. Moreover, the usually effective minimum dose of each SSRI produces comparable effects on the degree of serotonin reuptake inhibition, a surrogate for efficacy. Lastly, it is important to keep in mind the exploratory nature of network meta-analysis, which includes indirect comparisons of results obtained in different studies. In addition, the number of exposed children in each SSRIs group was small, and therefore, our analysis may not be robust enough.
CONCLUSION
Although we found an association between exposure to SSRIs/SNRIs during pregnancy and the risk for ASD and ADHD, an association with these neurodevelopment disorders was also present for exposure pre-pregnancy, suggesting that the associations might be due to unmeasured confounding. Moreover, the heterogeneity was high for the calculated pooled OR.
| Study, Year | Study Site | Study Type | Years (Duration) |
|---|---|---|---|
| Croen et al, [9] 2011 | Northern California | Case-control | 1999-1995 |
| Hviid et al, [14] 2013 | Denmark | Cohort | 2005-1996 |
| Laugesen et al, [23] 2013 | Denmark | Cohort | 2009-1996 |
| Rai et al, [11] 2013 | Sweden | Nested Case-control | 2001-2007 |
| Sorensen et al, [15] 2013 | Denmark | Cohort | 2006-1996 |
| Gidaya et al, [10] 2014 | Denmark | Case-control | 1997-2006 |
| Harrington et al, [16] 2014 | California | Case-control | 2013-2010 |
| Clements et al, [1] 2015 | --- | Case-control | 2010-1997 |
| Boukhris et al, [12] 2016 | Quebec | Cohort | 2009-1998 |
| Malm et al, [13] 2016 | Finland | Cohort | 1996-2010 |
| Boukhris et al, [2] 2017 | Canada | Cohort | 1998-2009 |
| Brown et al, [17] 2017 | Canada | Cohort | 2002-2010 |
| Man et al, [22] 2017 | Hong Kong | Cohort | 2001-2009 |
| Rai et al, [18] 2017 | Sweden | Cohort | 2001-2011 |
| Sujan et al, [19] 2017 | Sweden | Cohort | 1996-2012 |
| Viktorin et al, [20] 2017 | Sweden | Cohort | 2014-2006 |
| Wilcox-Hagberg et al, [21] 2017 | UK | Nested Case-control | 1989-2011 |
| Yamamoto-Sasaki et al, [24] 2019 | Japan | Cohort | 2005-2014 |
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- SSRI’s
Selective Serotonin Reuptake Inhibitors
- SNRI’s
Serotonin Norepinephrine Reuptake Inhibitors
- ASD
Autism Spectrum Disorder
- ADHD
Attention Deficit Hyperactivity Disorder
- TCAs
Tricyclic Antidepressants
- CYPs
Cytochrome P
CONSENT FOR PUBLICATION
Not applicable.
STANDARD OF REPORTING
PRISMA Guidelines and Methodologies were followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
REFERENCES
- 1.Clements C.C., Castro V.M., Blumenthal S.R., Rosenfield H.R., Murphy S.N., Fava M., Erb J.L., Churchill S.E., Kaimal A.J., Doyle A.E., Robinson E.B., Smoller J.W., Kohane I.S., Perlis R.H. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol. Psychiatry. 2015;20(6):727–734. doi: 10.1038/mp.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boukhris T., Sheehy O., Bérard A. Antidepressant use in pregnancy and the risk of attention deficit with or without hyperactivity disorder in children. Paediatr. Perinat. Epidemiol. 2017;31(4):363–373. doi: 10.1111/ppe.12378. [DOI] [PubMed] [Google Scholar]
- 3.Lipsker C.W., Bölte S., Hirvikoski T., Lekander M., Holmström L., Wicksell R.K. Prevalence of autism traits and attention-deficit hyperactivity disorder symptoms in a clinical sample of children and adolescents with chronic pain. J. Pain Res. 2018;11:2827–2836. doi: 10.2147/JPR.S177534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ewing G., Tatarchuk Y., Appleby D., Schwartz N., Kim D. Placental transfer of antidepressant medications: implications for postnatal adaptation syndrome. Clin. Pharmacokinet. 2015;54(4):359–370. doi: 10.1007/s40262-014-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masarwa R., Bar-Oz B., Gorelik E., Reif S., Perlman A., Matok I. Prenatal exposure to selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors and risk for persistent pulmonary hypertension of the newborn: a systematic review, meta-analysis, and network meta-analysis. Am. J. Obstet. Gynecol. 2019:57–66. doi: 10.1016/j.ajog.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 6.Gao S-Y., Wu Q-J., Sun C., Zhang T-N., Shen Z-Q., Liu C-X., Gong T-T., Xu X., Ji C., Huang D-H., Chang Q., Zhao Y-H. Selective serotonin reuptake inhibitor use during early pregnancy and congenital malformations: a systematic review and meta-analysis of cohort studies of more than 9 million births. BMC Med. 2018;16(1):205. doi: 10.1186/s12916-018-1193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ornoy A., Koren G. SSRIs and SNRIs (SRI) in Pregnancy: effects on the course of pregnancy and the offspring: how far are we from having all the answers? Int. J. Mol. Sci. 2019;20(10):2370. doi: 10.3390/ijms20102370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hermansen T.K., Melinder A. Prenatal SSRI exposure: Effects on later child development. Child Neuropsychol. 2015;21(5):543–569. doi: 10.1080/09297049.2014.942727. [DOI] [PubMed] [Google Scholar]
- 9.Croen L.A., Grether J.K., Yoshida C.K., Odouli R., Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch. Gen. Psychiatry. 2011;68(11):1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- 10.Gidaya N.B., Lee B.K., Burstyn I., Yudell M., Mortensen E.L., Newschaffer C.J. In utero exposure to selective serotonin reuptake inhibitors and risk for autism spectrum disorder. J. Autism Dev. Disord. 2014;44(10):2558–2567. doi: 10.1007/s10803-014-2128-4. [DOI] [PubMed] [Google Scholar]
- 11.Rai D., Lee B.K., Dalman C., Golding J., Lewis G., Magnusson C. Parental depression, maternal antidepressant use during pregnancy, and risk of autism spectrum disorders: population based case-control study. BMJ. 2013;346:f2059. doi: 10.1136/bmj.f2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boukhris T., Sheehy O., Mottron L., Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr. 2016;170(2):117–124. doi: 10.1001/jamapediatrics.2015.3356. [DOI] [PubMed] [Google Scholar]
- 13.Malm H., Brown A.S., Gissler M., Gyllenberg D., Hinkka-Yli-Salomäki S., McKeague I.W., Weissman M., Wickramaratne P., Artama M., Gingrich J.A., Sourander A. Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(5):359–366. doi: 10.1016/j.jaac.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hviid A., Melbye M., Pasternak B. Use of selective serotonin reuptake inhibitors during pregnancy and risk of autism. N. Engl. J. Med. 2013;369(25):2406–2415. doi: 10.1056/NEJMoa1301449. [DOI] [PubMed] [Google Scholar]
- 15.Sørensen M.J., Grønborg T.K., Christensen J., Parner E.T., Vestergaard M., Schendel D., Pedersen L.H. Antidepressant exposure in pregnancy and risk of autism spectrum disorders. Clin. Epidemiol. 2013;5:449–459. doi: 10.2147/CLEP.S53009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington R.A., Lee L-C., Crum R.M., Zimmerman A.W., Hertz-Picciotto I. Prenatal SSRI use and offspring with autism spectrum disorder or developmental delay. Pediatrics. 2014;133(5):e1241–e1248. doi: 10.1542/peds.2013-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown H.K., Ray J.G., Wilton A.S., Lunsky Y., Gomes T., Vigod S.N. Association between serotonergic antidepressant use during pregnancy and autism spectrum disorder in children. JAMA. 2017;317(15):1544–1552. doi: 10.1001/jama.2017.3415. [DOI] [PubMed] [Google Scholar]
- 18.Rai D., Lee B.K., Dalman C., Newschaffer C., Lewis G., Magnusson C. Antidepressants during pregnancy and autism in offspring: population based cohort study. BMJ. 2017;358:j2811. doi: 10.1136/bmj.j2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sujan A.C., Rickert M.E., Öberg A.S., Quinn P.D., Hernández-Díaz S., Almqvist C., Lichtenstein P., Larsson H., D’Onofrio B.M. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA. 2017;317(15):1553–1562. doi: 10.1001/jama.2017.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viktorin A., Uher R., Reichenberg A., Levine S.Z., Sandin S. Autism risk following antidepressant medication during pregnancy. Psychol. Med. 2017;47(16):2787–2796. doi: 10.1017/S0033291717001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagberg K.W., Robijn A.L., Jick S. Maternal depression and antidepressant use during pregnancy and the risk of autism spectrum disorder in offspring. Clin. Epidemiol. 2018;10:1599–1612. doi: 10.2147/CLEP.S180618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto-Sasaki M., Yoshida S., Takeuchi M., Tanaka-Mizuno S., Ogawa Y., Furukawa T.A., Kawakami K. Association between antidepressant use during pregnancy and autism spectrum disorder in children: a retrospective cohort study based on Japanese claims data. Matern. Health Neonatol. Perinatol. 2019;5:1. doi: 10.1186/s40748-018-0096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laugesen K., Olsen M.S., Telén A.A.B., Frøslev T., Sørensen H.T. In utero exposure to antidepressant drugs and risk of attention deficit hyperactivity disorder: a nationwide Danish cohort study. BMJ Open. 2013;3(9):e003507. doi: 10.1136/bmjopen-2013-003507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Man K.K.C., Chan E.W., Ip P., Coghill D., Simonoff E., Chan P.K.L., Lau W.C.Y., Schuemie M.J., Sturkenboom M.C.J.M., Wong I.C.K. Prenatal antidepressant use and risk of attention-deficit/hyperactivity disorder in offspring: population based cohort study. BMJ. 2017;357:j2350. doi: 10.1136/bmj.j2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrade S.E., Raebel M.A., Brown J., Lane K., Livingston J., Boudreau D., Rolnick S.J., Roblin D., Smith D.H., Willy M.E., Staffa J.A., Platt R. Use of antidepressant medications during pregnancy: a multisite study. Am. J. Obstet. Gynecol. 2008;198(2):194.e1–194.e5. doi: 10.1016/j.ajog.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 26.Cohen L.S., Altshuler L.L., Harlow B.L., Nonacs R., Newport D.J., Viguera A.C., Suri R., Burt V.K., Hendrick V., Reminick A.M., Loughead A., Vitonis A.F., Stowe Z.N. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499–507. doi: 10.1001/jama.295.5.499. [DOI] [PubMed] [Google Scholar]
- 27.Grigoriadis S. VonderPorten, E.H.; Mamisashvili. L.; Tomlinson, G.; Dennis, C-L.; Koren, G.; Steiner, M.; Mousmanis, P.; Cheung, A.; Radford, K.; Martinovic, J.; Ross, L.E. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J. Clin. Psychiatry. 2013;74:321. doi: 10.4088/JCP.12r07968. [DOI] [PubMed] [Google Scholar]
- 28.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 29.Wells G.A., Shea B., O’Connell D., Peterson J., Welch W., Losos M., Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quailty of nonrandomised studies in meta-analyses. 2011. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 30.Stuck A.E., Rubenstein L.Z., Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ. 1998;316(7129):469–477. doi: 10.1136/bmj.316.7129.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Available from: https://www.meta-analysis.com/pages/software_updates.php.
- 32.Viechtbauer W. Meta-analysis package for R. 2016. Available from: https://cran.r-project.org/web/packages/metafor/metafor.pdf.
- 33.Duval S., Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 34.Borenstein M., Hedges L., Rothstein H. Meta-analysis fixed effect vs. random effects, 2017. Test. doi: 10.1002/9780470743386. [DOI] [PubMed] [Google Scholar]
- 35.Riley R.D., Higgins J.P., Deeks J.J. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 36.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 37.Rücker G., Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rucker G., Schwarzer G., Krahn U., Konig J. Network Meta-Analysis using Frequentist Methods. 2018. Available from: https://cran.r-project.org/web/packages/netmeta/netmeta.pdf.
- 39.Huang H., Coleman S., Bridge J.A., Yonkers K., Katon W. A meta-analysis of the relationship between antidepressant use in pregnancy and the risk of preterm birth and low birth weight. Gen. Hosp. Psychiatry. 2014;36(1):13–18. doi: 10.1016/j.genhosppsych.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai D., Yip B.H.K., Windham G.C., Sourander A., Francis R., Yoffe R., Glasson E., Mahjani B., Suominen A., Leonard H., Gissler M., Buxbaum J.D., Wong K., Schendel D., Kodesh A., Breshnahan M., Levine S.Z., Parner E.T., Hansen S.N., Hultman C., Reichenberg A., Sandin S. Association of genetic and environmental factors with autism in a 5-Country Cohort. JAMA Psychiatry. 2019;76(10):1035–1043. doi: 10.1001/jamapsychiatry.2019.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsson H., Chang Z., D’Onofrio B.M., Lichtenstein P. The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychol. Med. 2014;44(10):2223–2229. doi: 10.1017/S0033291713002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faraone S.V., Larsson H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry. 2019;24(4):562–575. doi: 10.1038/s41380-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Marroun H., White T., Verhulst F.C., Tiemeier H. Maternal use of antidepressant or anxiolytic medication during pregnancy and childhood neurodevelopmental outcomes: a systematic review. Eur. Child Adolesc. Psychiatry. 2014;23(10):973–992. doi: 10.1007/s00787-014-0558-3. [DOI] [PubMed] [Google Scholar]
- 44.Rampono J., Simmer K., Ilett K.F., Hackett L.P., Doherty D.A., Elliot R., Kok C.H., Coenen A., Forman T. Placental transfer of SSRI and SNRI antidepressants and effects on the neonate. Pharmacopsychiatry. 2009;42(3):95–100. doi: 10.1055/s-0028-1103296. [DOI] [PubMed] [Google Scholar]
- 45.Homberg J.R., Schubert D., Gaspar P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol. Sci. 2010;31(2):60–65. doi: 10.1016/j.tips.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Fatima Z., Zahra A., Ghouse M., Wang X., Yuan Z. Maternal SSRIs experience and risk of ASD in offspring: a review. Toxicol. Res. (Camb.) 2018;7(6):1020–1028. doi: 10.1039/C8TX00102B. [DOI] [PMC free article] [PubMed] [Google Scholar]