Abstract
Multiple studies on the pathomechanisms of depressive disorder and antidepressants have been reported. However, literature involving scientometric analysis of depressive disorder is sparse. Here, we use scientometric analysis and a historical review to highlight recent research on depression. We use the former to examine research on depressive disorders from 1998 to 2018. The latter is used to identify the most frequent keywords in keyword analysis, as well as explore hotspots and depression trends. Scientometric analysis uncovered field distribution, knowledge structure, research topic evolution, and topics emergence as main explorations in depressive disorder. Induction factor, comorbidity, pathogenesis, therapy and animal models of depression help illustrate occurrence, development and treatment of depressive disorder. Scientometric analysis found 231,270 research papers on depression, a 4-fold increase over the last 20 years. These findings offer a vigorous roadmap for further studies in this field.
Keywords: Scientometrics analysis, visualization, depressive disorder, citespace, VOSviewer, depression, depressive disorder
1. INTRODUCTION
Depressive disorder is a primary public health challenge that appears at all ages. This chronic disorder substantially weakens patients’ psychosocial and occupational function, affecting patients’ feelings, as well as their perception of themselves and the world around them [1]. Recent years have seen growing research interest in depressive disorder, as evidenced by the high number of research and review articles on this topic. For example, systematic review and meta-analysis of longitudinal studies on overweight, obesity, and depression, found a reciprocal link between depression and obesity, and that obesity may enhance the risk of depression [2]. A patient-level meta-analysis of antidepressant effects on depression severity found that while the benefits of antidepressants relative to placebo may be minimal or nonexistent in patients with mild or moderate symptoms, the opposite was true in cases of very severe depression [3]. However, comprehensive reviews on depression are lacking. There are some scientometric articles on depression, including center of epidemiological studies of depression scale [4], biological treatments for major depressive disorder [5], and artificial intelligence application in the management of depressive disorders [6]. While these studies offer preliminary insight into the field of depression, a more comprehensive scientometric analysis of depression is not available in literature.
In this article, scientometric analysis is mainly reflected in bibliometric and manual analysis. Bibliometric analysis isan emerging tool for quickly exploring structures and trends of a subject or domain via statistical methods and visualization. This approach identifies relevant nodes and extracts useful information from large volumes of information [7, 8]. Bibliometric software, including CiteSpace [9], VOSviewer [10], bibExcel [11], Science of Science (SCI2) [12] and HistCite [13] have been used in scientometric analysis. Of these, CiteSpace and VOSviewer are the most popular. CiteSpace is a freely available Java application that can visualize and analyze scientific literature trends and patterns. CiteSpace was designed by Professor Chaomei Chen for progressive knowledge domain visualization and identification of critical points in the development of a field, especially intellectual turning points and pivotal points [14]. VOSviewer was created by Centre for Science and Technology Studies, Leiden University and is used to visualize science mapping. VOSviewer is freely available and has been used to visualize bibliometric maps [15].
Despite methodological limitations, scientometrics is useful for evaluating scientific research in a given subject or field [16]. Relative to descriptive literature reviews (conventional reviews), systematic reviews like scientometrics have advantages that can quickly identify critical issues in the field of interest and guide future research. For example, a bibliometric analysis of research on the topic “cancer rehabilitation”, led to the conclusions that National Cancer Institute was the most prominent funder, and that cognitive and behavioral therapies, as well as psychological interventions were major topics, followed by depression and exercise therapy [17]. Bibliometric analysis of global research on PD-1 and PD-L1 in cancer concluded that, after 2014, PD-1 and PD-L1 studies rose significantly, most publications came from USA, and future research should develop new topics, like boosting anti-cancer therapeutic strategies [18]. Bibliometric analysis of traditional Chinese medicine formula (TCMF) concluded the most journal, disease types, the hottest category and keywords of TCMF [19]. Bibliometric analysis of journal trends, such as Sustainability offered new directions for the development of periodicals [20]. Thus, bibliometric analysis of depressive disorder may uncover valuable insights. Generally, science mapping can effectively demonstrate bibliometric visualization, illuminating the discipline’s current situation and development trends [21].
Here, we sought to put forward a scientometric method for depressive disorder and suggested future research directions [21]. Statistical results of keyword analysis were analyzed and summarized by manual classification, which included induction factor, comorbidity, pathogenesis, therapy and animal models of depression. Combining scientometric analysis and historical review will uncover critical evidence and highlight emerging depressive disorder trends.
2. DATA AND METHODS
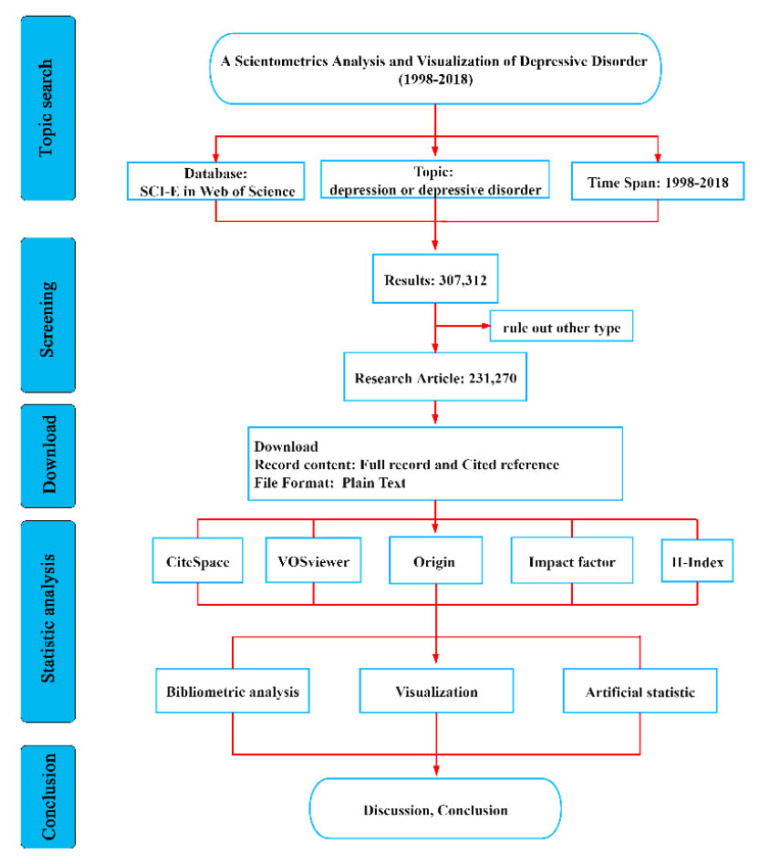
Citation data used in bibliometric research was downloaded from science citation index expanded (SCI-E) database on Web of Science (WOS). Relative to general databases like Scopus, Derwent, China national knowledge infrastructure (CNKI) and Chinese social sciences citation index (CSSCI), WOS includes more scientific publications and provides overall data sources for bibliometric software. Thus, WOS is the database most frequently used for bibliometric research [22-24]. The scientometric research process is shown in (Fig. 1). WOS’s core collection database as of July 22, 2019 was used for this bibliometric analysis. We took “depression” or “depressive disorder” as topical retrieval terms and time span limited to 1998-2018. The literature data used in this study were downloaded from WOS. CiteSpace was initially used for bibliometric analysis, including country, institute, keyword, category, reference, and cited journal. Parameter settings included the time slicing (1998-2018), node types, and selection criteria (top 50 levels of the most cited or occurring items) from each slice. VOSviewer was used to optimize and supplement the unaesthetic map. Impact factor (IF) and H-index were added to the table for a comprehensive, scientometric, results analysis.
Fig. (1).
Flow chart of scientometric analysis. SCI-E = Science citation index expand; WOS = Web of science; IF = impact factor; H index = high citation index. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. RESULTS AND DISCUSSION
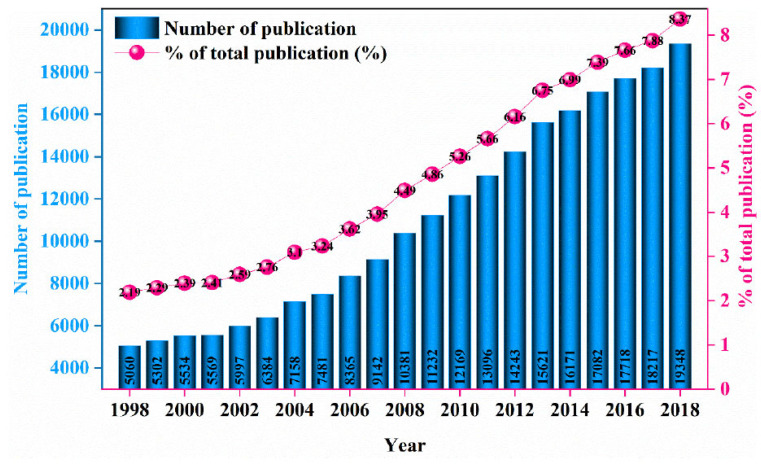
231,270 articles related to “depressive disorder” or “depression” were identified. Based on the search results for depressive disorder or depression in WOS, publication growth on this topic can be divided into 2 stages, a slow phase from 1998-2003 and a rapid growth phase from 2004-2018 (Fig. 2). Rapid growth in depression research may be explained by 2 factors. First, depression affects >320 million people worldwide [25, 26], and has been associated with increased cancer risk and shortened survival [27]. The big data research and development initiative [28], and the precision medicine initiative [29], launched by the Obama administration in 2012 and 2015, respectively, may also contribute to the increased rate of depression research. From 1998-2018, the average annual publication was 11,012, a very high publication rate. For bibliometric analysis on the topic of depression, the more the data, the more reliable the results.
Fig. (2).
Annual publications between 1998-2018. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.1. Cooperation Map of Country/region and Institute
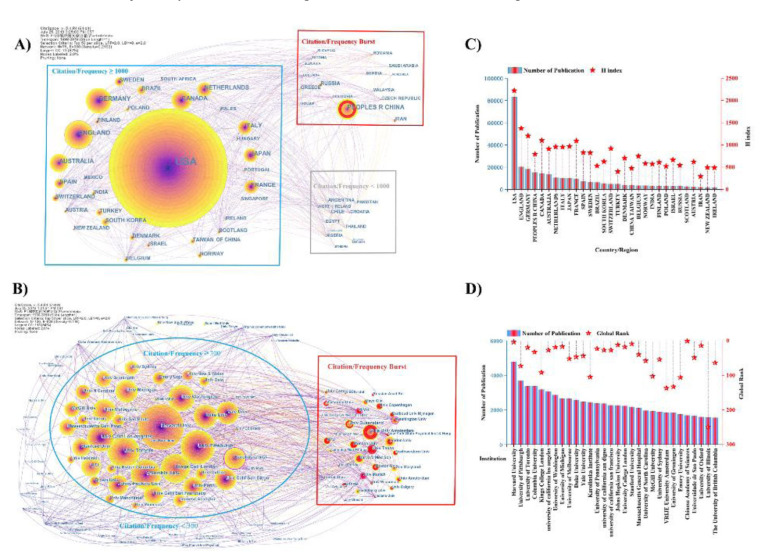
Citespace offers 3 levels of scientific cooperation network analysis: microcosmic author cooperation network, mesoscopic institutional cooperation network, and macroscopical national cooperation network. In the cooperative network obtained via CiteSpace, node size represents the number of publications published by an author, institution, or country/region [30, 31]. It is observed that the US ranks first and has >80,000 publications (36.082% of the total) (Fig. 3A), (Fig. 3C). The US is followed by England, Germany, China, Canada, Australia, Netherlands, Italy, and Japan, with >100,000 publications (48.762% of the total). For depression research, there is close cooperation between countries, highlighting depression as a global challenge. Developed countries have a greater share of total publications, which may be explained by objective and subjective factors. Objectively, developed countries dedicate more funding to scientific research. Subjectively, common chronic pain conditions related to depression are more prevalent in developed countries. Making it a problem in need of urgent solutions [32]. From an institutional perspective, the US has 7 institutes in the top-10, with Harvard University (4773) ranking first (Fig. 3B, 3D) The ranking of the US country/region first reflects its stronger scientific prowess. This analysis also ranked University of Toronto (3192) (Canada) and Kings College London (3181) (England) in the top 10. Citation frequency bursts indicate that a paper is highly cited in a certain period. Vrije University (Amsterdam), University of Texas, and Harvard medical school are speculated to have made novel discoveries in depression research. The H-index and global rank (Fig. 3C, 3D) are trustworthy indicators aimed at measuring an individual’s scientific achievements
Fig. (3).
Cooperation map of country/region and institute. A) Country/region co-occurrence network. 75 nodes and 600 connection lines emerged. Node and line size represent the number of publications from a country/region and the cooperative relationship in the country/region, respectively. Red nodes indicate that publications of the country/region have a citation or frequency burst. Connecting lines of different colors represent different years. Blue rectangles show a citation/frequency of >1000 for a country/region. Gray rectangles indicate citation/frequency of <1000 for a country/region. Red rectangles indicate a burst in the citation/frequency of country/region. B) Number of publications and H-Index scores for countries/regions. C) Institute co-occurrence network. 120 nodes and 828 connection lines emerged. Node size and lines represent the number of publications from institutes and their cooperative relationship, respectively. Red nodes indicate a citation or frequency burst in publications of the institutes. Connecting lines of different color represent different years. Blue ellipses show that the citation/frequency of institute is >300. The outside of the blue ellipses indicates that the citation/frequency of the institute is <300. Red rectangles indicate burst in the citation/frequency of the institute. D) Number of publications and global institutional rank. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
or influence [33] and to offer a reliable metric for a scientist’s academic evaluation [34]. H-index (high citations index) shows an individual’s citation rate. For example, a researcher’s H-index shows that they have at most H papers cited at least H times [35]. By combining countries with institutes, the US has a H-index of 2222 and is home to several world-famous institutions. Relative to other countries/regions, it has an obvious advantage and makes outstanding contributions to depression research. England (1373), Germany (1203) and China (794) also make prominent contributions to depression research.
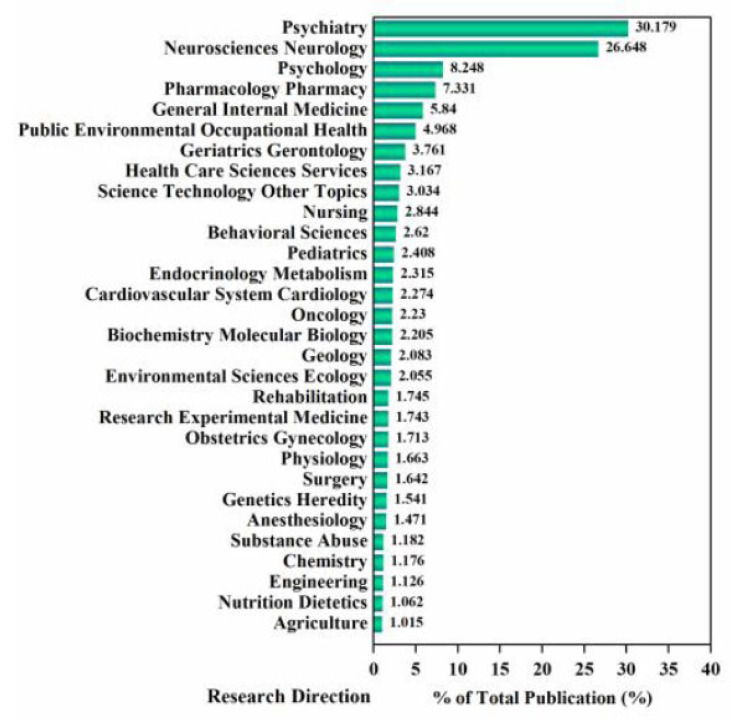
56.827% of the total publications regard psychiatry and neurosciences (Fig. 4). Psychiatric disorders account for 22.8% of the disease burden worldwide [36]. Depression, a major cause of disability, has considerably surged since 1990, mostly driven by population growth and aging [26, 37]. Depression is treated using physiologic and psychologic means. Thus, there are a higher number of publications on psychiatry and neurosciences, followed by pharmacological pharmacy, due to the need for antidepressants. For instance, given that oral antidepressants have poor bioavailability, nanoparticles given via nose-to-brain delivery have emerged as novel promising candidates for evaluation of the antidepressant activity of original drugs [38]. Psychology accounts for 8.248% of total publications, suggesting that psychological therapies, including psychological consultation, are therapeutic effects against depression [39]. There are numerous categories, including public environmental occupational health, health care sciences services, nursing, environmental sciences ecology, physiology, surgery, nutrition dietetics, and agriculture, indicating that depression is a major disorder, often accompanying other diseases. For instance, there is post-stroke depression [40], and postpartum depression [41]. These results highlight the complexity of depression, and indicate that depression treatment should have broad and multi- factorial considerations.
Fig. (4).
Category exploration map. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.2. Journal and Co-cited Journal
Table (1) presents the top 30 journals in the field of depression. Journal of affective disorders (6058, 2.619%) ranks first, followed by PLOS One (4060, 1.756%) and psychiatry research (2930, 1.267%). Of the top 10 journals, 5 are from the US, 3 from England, and 2 from Netherlands. The number of publications in the top 20 is >1000. Among the top 30, there are 7 journals with impact factors >5 that include Journal of neuroscience (6.074), psychological medicine (5.641), biological psychiatry (11.501), American journal of psychiatry (13.655), neuropsychopharmacology (7.16), British journal of psychiatry (7.233), Journal of the American Academy of child and adolescent psychiatry (6.391). Journal of nervous and mental disease (1.859) has the lowest impact factor among the top 30 journals Table (2). Based on the cited number of publications, archives of general psychiatry (71,718) ranked first, followed by American journal of psychiatry (70,801), journal of affective disorders (52,623), biological psychiatry (51,404), and British journal of psychiatry (51,126). There are 7 Journals with impact factors >10, including American journal of psychiatry (13.655), biological psychiatry (11.501), JAMA (51.273), Lancet (59.102), Science (41.037), Nature (43.07), NEJM (70.67), British medical journal (27.604), Neuron (14.403), and molecular psychiatry (11.973). Many publications on depression are published in low impact factor journals, suggesting that researchers demand further research in this field. Research on depression includes studies on novel preparations [42], and exploration of pathological mechanisms [43-45]. Compared with journal analysis, the co-cited top journal has a surge, demonstrating that the study of this journal is closer to basics or has a stronger theory.
Table 1.
The top 30 journals related to depression research.
| Ranking | Number of Publication |
% of Total Publication |
Journal | |||
|---|---|---|---|---|---|---|
| Name | Country / Region | Impact Factor (2018) | H-Index (2018) | |||
| 1 | 6058 | 2.619 | Journal of Affective Disorders | Netherlands | 4.084 | 165 |
| 2 | 4060 | 1.756 | PLOS One | USA | 2.776 | 268 |
| 3 | 2930 | 1.267 | Psychiatry Research | Netherlands | 2.208 | 118 |
| 4 | 1777 | 0.768 | Journal of Clinical Psychiatry | USA | 4.023 | 189 |
| 5 | 1722 | 0.745 | Journal of Neuroscience | USA | 6.074 | 422 |
| 6 | 1691 | 0.731 | Psychological Medicine | England | 5.641 | 188 |
| 7 | 1587 | 0.686 | Biological Psychiatry | USA | 11.501 | 290 |
| 8 | 1502 | 0.649 | BMC Psychiatry | England | 2.666 | 79 |
| 9 | 1441 | 0.623 | Journal of Psychiatric Research | England | 3.917 | 123 |
| 10 | 1342 | 0.58 | International Journal of Geriatric Psychiatry | USA | 3.141 | 116 |
| 11 | 1339 | 0.579 | Journal of Psychosomatic Research | Netherlands | 2.722 | 141 |
| 12 | 1197 | 0.518 | Depression and Anxiety | USA | 4.935 | 110 |
| 13 | 1171 | 0.506 | American Journal of Psychiatry | USA | 13.655 | 325 |
| 14 | 1127 | 0.487 | Comprehensive Psychiatry | England | 2.586 | 94 |
| 15 | 1119 | 0.484 | Psycho neuroendocrinology | England | 4.013 | 155 |
| 16 | 1061 | 0.459 | Neuroscience Letters | Netherlands | 2.173 | 155 |
| 17 | 1058 | 0.457 | Neuropsychopharmacology | England | 7.16 | 204 |
| 18 | 1034 | 0.447 | Journal of Nervous and Mental Disease | USA | 1.859 | 113 |
| 19 | 1010 | 0.437 | American Journal of Geriatric Psychiatry | USA | 3.488 | 108 |
| 20 | 1010 | 0.437 | Neuroscience | England | 3.244 | 204 |
| 21 | 995 | 0.43 | Psychosomatic Medicine | USA | 3.937 | 171 |
| 22 | 965 | 0.417 | Behavioural Brain Research | Netherlands | 2.77 | 154 |
| 23 | 963 | 0.416 | Psychopharmacology | Germany | 3.424 | 181 |
| 24 | 943 | 0.408 | British Journal of Psychiatry | England | 7.233 | 209 |
| 25 | 938 | 0.406 | Acta Psychiatrica Scandinavica | England | 4.694 | 132 |
| 26 | 929 | 0.402 | Psycho-Oncology | USA | 3.43 | 123 |
| 27 | 914 | 0.395 | Brain Research | Netherlands | 2.929 | 188 |
| 28 | 884 | 0.382 | General Hospital Psychiatry | Netherlands | 3.22 | 94 |
| 29 | 825 | 0.357 | Journal of The American Academy of Child and Adolescent Psychiatry | Netherlands | 6.391 | 222 |
| 30 | 819 | 0.354 | BMJ Open | England | 2.376 | 69 |
Table 2.
The top 30 co-cited journals related to depression research.
| Rank | Cited Number | Journal | |||
|---|---|---|---|---|---|
| Name | Country/Region | Impact Factor (2018) | H index (2018) | ||
| 1 | 71718 | Archives of General Psychiatry | USA | / | / |
| 2 | 70801 | American Journal of Psychiatry | USA | 13.655 | 325 |
| 3 | 52623 | Journal of Affective Disorders | Netherlands | 4.084 | 165 |
| 4 | 51404 | Biological Psychiatry | USA | 11.501 | 290 |
| 5 | 51126 | British Journal of Psychiatry | England | 7.233 | 209 |
| 6 | 47113 | Jama-Journal of The American Medical Association | USA | 51.273 | 622 |
| 7 | 43381 | Psychological Medicine | England | 5.641 | 188 |
| 8 | 40554 | Journal of Clinical Psychiatry | USA | 4.023 | 189 |
| 9 | 40328 | Lancet | England | 59.102 | 700 |
| 10 | 38753 | Acta Psychiatrica Scandinavica | England | 4.694 | 132 |
| 11 | 36545 | Science | USA | 41.037 | 1058 |
| 12 | 36321 | Proceedings of The National Academy of Sciences of The United States of America | USA | 9.58 | 699 |
| 13 | 32946 | Journal of Neuroscience | USA | 6.074 | 422 |
| 14 | 32807 | Nature | England | 43.07 | 1096 |
| 15 | 30766 | New England Journal of Medicine | USA | 70.67 | 933 |
| 16 | 29052 | Psychiatry Research | Netherlands | 2.208 | 118 |
| 17 | 27297 | PLOS One | USA | 2.776 | 268 |
| 18 | 26371 | Journal of Psychosomatic Research | England | 2.722 | 141 |
| 19 | 26027 | Neuropsychopharmacology | England | 7.16 | 204 |
| 20 | 25219 | Psychosomatic Medicine | USA | 3.937 | 171 |
| 21 | 24788 | Brain Research | Netherlands | 2.929 | 188 |
| 22 | 22634 | Neurology | USA | 8.689 | 331 |
| 23 | 21937 | Journal of Neurology Neurosurgery and Psychiatry | England | 8.272 | 188 |
| 24 | 21698 | Journal of Consulting and Clinical Psychology | USA | 4.358 | 218 |
| 25 | 21123 | British Medical Journal | England | 27.604 | 392 |
| 26 | 20212 | Psychopharmacology | Germany | 3.424 | 181 |
| 27 | 20164 | Neuroscience | Netherlands | 3.244 | 204 |
| 28 | 18959 | Neuron | USA | 14.403 | 430 |
| 29 | 18946 | Archives of Internal Medicine | USA | / | / |
| 30 | 17068 | Molecular Psychiatry | England | 11.973 | 196 |
“/”: The magazine is not included in the latest SCI-E database, so the impact factor and H-index are missing.
3.3. Co-cited Reference
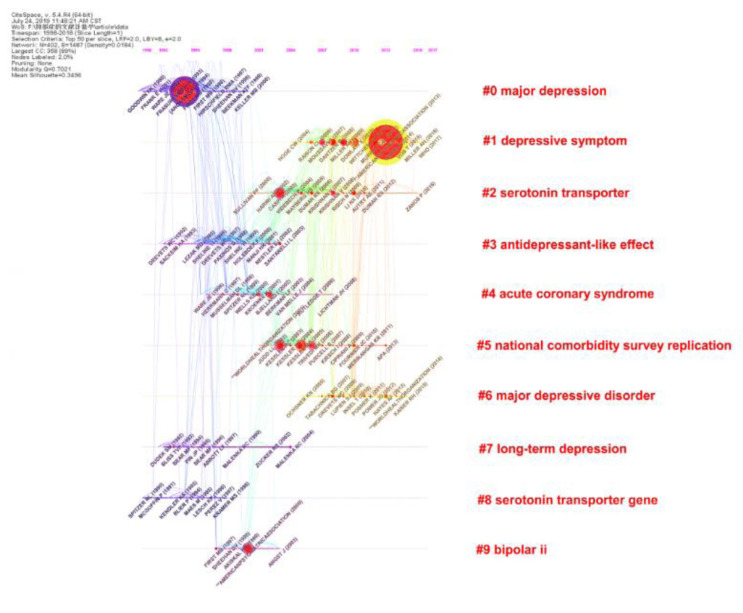
Co-cited reference means that one or more references have been co-cited in one or more publications. The top 30 co-cited references regarding depression research are presented in Table (3). One reference is co-cited over 1000 times, eighteen references are co-cited between 500 and 1000 times, and others are co-cited between 300 and 500 times. Topics covered by highly co-cited papers include survey [46], meta-analysis [47], epidemiology [48], moderation by gene polymorphisms, such as serotonin transporter [49], inflammation pathophysiology [50-52], use of depression scale [53], antidepressants, such as citalopram [54], and reviews neurotrophic models of stress-related mood disorders [55]. 19 papers were in top journals (IF >10) of the top 30: Science (4), Lancet (3), JAMA (3), Biological psychiatry (3), Neuron (2), Nature (1), American journal of psychiatry (1), PLOS medicine (1), and Trends in immunology (1). The co-cited reference timeline view is shown in (Fig. 5). The reference network map has 402 nodes and 1604 links. All references form 10 clusters and there is active co-citation between references, including the serotonin transporter (#2) and serotonin transporter gene (#8) clusters, suggesting that the serotonin transporter is critical for regulating serotonin function in the brain [56]. Because serotonin regulates excitatory and inhibitory neurotransmitters, its transporters are used as indexes in depression studies [57-59].
Table 3.
The top 30 cited articles related to depression research.
| Rank | Cited Number |
The Title of Article | Year | Journal | |||
|---|---|---|---|---|---|---|---|
| Name | Country | Impact Factor (2018) | H-Index (2018) | ||||
| 1 | 1111 | Lifetime prevalence and age-of-onset distributions ' of DSM-IV disorders in the national comorbidity survey replication | 2005 | Archives of general psychiatry | USA | / | / |
| 2 | 950 | A Meta-Analysis of Cytokines in Major Depression | 2010 | Biological psychiatry | Netherlands | 11.501 | 290 |
| 3 | 927 | The epidemiology of major depressive disorder - Results from the National Comorbidity Survey Replication (NCS -R). | 2003 | JAMA-Journal of The American Medical Association | USA | 51.273 | 622 |
| 4 | 869 | Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene | 2003 | Science | USA | 41.037 | 1058 |
| 5 | 815 | Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication | 2005 | Archives of general psychiatry | USA | / | / |
| 6 | 788 | From inflammation to sickness and depression: when the immune system subjugates the brain | 2008 | Nature reviews Neuroscience | England | 33 | 375 |
| 7 | 699 | Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression | 2009 | Biological psychiatry | Netherlands | 11.501 | 290 |
| 8 | 672 | The validity of the Hospital Anxiety and Depression Scale An updated literature review | 2002 | Journal of psychosomatic research | Netherlands | 2.722 | 141 |
| 9 | 666 | Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice | 2006 | Science | USA | 41.037 | 1058 |
| 10 | 636 | Overweight, Obesity, and Depression A Systematic Review and Meta-analysis of Longitudinal Studies | 2010 | Archives of general psychiatry | USA | / | / |
| 11 | 586 | A neurotrophic model for stress-related mood disorders | 2006 | Biological psychiatry | Netherlands | 11.501 | 290 |
| 12 | 561 | Associations of Depression With C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis | 2009 | Psychosomatic medicine | USA | 3.937 | 171 |
| 13 | 546 | mTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists | 2010 | Science | USA | 41.037 | 1058 |
| 14 | 517 | Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report | 2006 | American Journal of Psychiatry | USA | 13.655 | 325 |
| 15 | 512 | The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review | 2010 | General hospital psychiatry | Netherlands | 3.22 | 94 |
| 16 | 511 | Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants | 2003 | Science | USA | 41.037 | 1058 |
| 17 | 510 | Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010 | 2013 | PLOS medicine | USA | 11.048 | 197 |
| 18 | 504 | Depression, chronic diseases, and decrements in health: results from the World Health Surveys | 2007 | The Lancet | England | 59.102 | 700 |
| 19 | 500 | Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010 | 2013 | The Lancet | ENGLAND | 59.102 | 700 |
| 20 | 493 | The molecular neurobiology of depression | 2008 | Nature | ENGLAND | 43.07 | 1096 |
| 21 | 444 | The PHQ-9 - Validity of a brief depression severity measure | 2001 | Journal of general internal medicine | Germany | 4.606 | 161 |
| 22 | 442 | Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression | 2008 | Brain structure and function | Germany | 3.622 | 82 |
| 23 | 414 | Cytokines sing the blues: inflammation and the pathogenesis of depression | 2006 | Trends in immunology | Netherlands | 13 | 209 |
| 24 | 411 | Neurobiology of Depression | 2002 | Neuron | USA | 14.403 | 430 |
| 25 | 405 | The World Mental Health (WMH) Survey Initiative version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) | 2004 | International journal of methods in psychiatric research | USA | 2.276 | 66 |
| 26 | 396 | Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models | 2008 | Behavior research methods | Germany | 3.579 | 114 |
| 27 | 395 | Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study | 2012 | The Lancet | ENGLAND | 59.102 | 700 |
| 28 | 385 | Deep brain stimulation for treatment-resistant depression | 2005 | Neuron | USA | 14.403 | 430 |
| 29 | 345 | Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial | 2002 | JAMA-Journal of The American Medical Association | USA | 51.273 | 622 |
| 30 | 344 | Antidepressant drug effects and depression severity: a patient-level meta-analysis | 2010 | JAMA-Journal of The American Medical Association | USA | 51.273 | 622 |
Fig. (5).
Reference timeline view. Node size and color represents total number of citations and a single time slice, respectively. Lines of different colors show that the two articles were co-cited in an article. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.4. Keywords Analysis
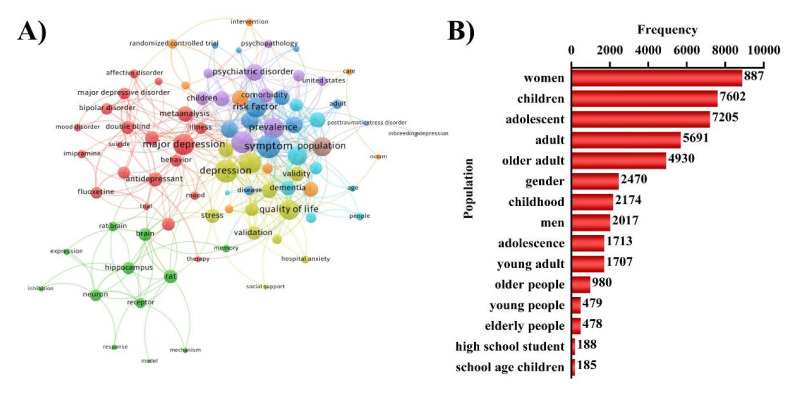
According to bibliometrics theory, keywords indicate hotspots and trends in a research field [60]. Keyword analysis also provided a typical overview of study trends that stood for the journal, as they reflect the focus of an article or an author [61]. Based on (Fig. 6A), the biggest node is depression, which is same as research topic. This includes 3 main aspects: basic scientific research, social science research and clinical study, that exhibit a complex relationship. (Fig. 6B). shows that the group on depression mainly includes women, children, adolescent, and adult, suggesting that these groups are at high risk of depression. These keywords also show that depression has far-ranging distribution in all ages.
Fig. (6).
Keywords map. (A) Keywords co-occurrence network. Node size and color represents the number of keywords and cluster. Lines of different colors show that the 2 keywords appear in an article. (B) Population distribution. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
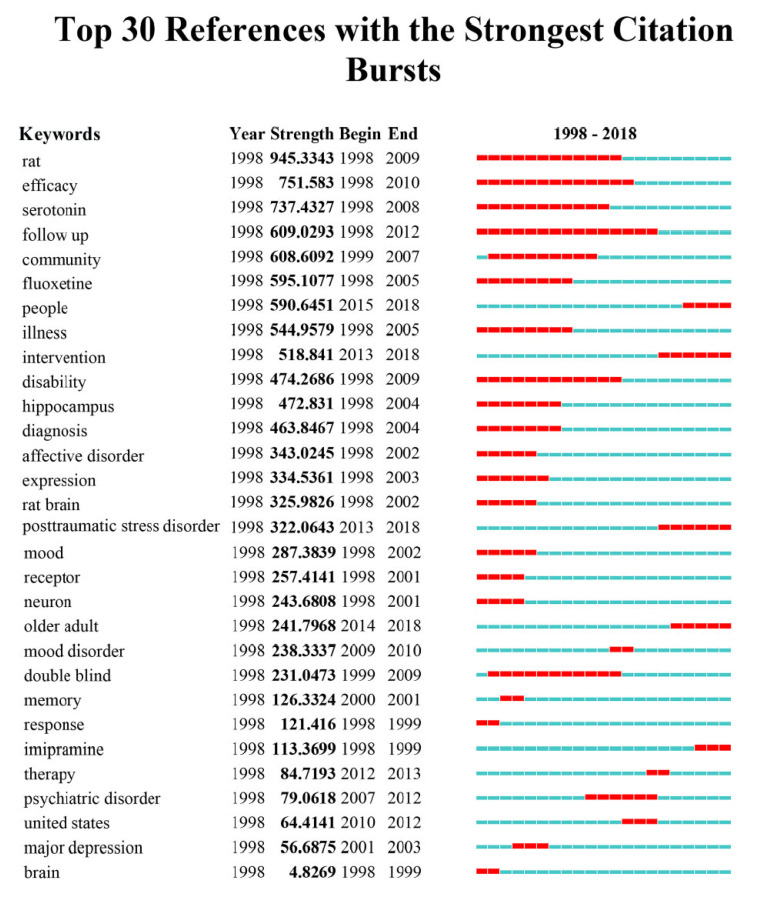
3.5. Keywords with Citation Bursts
Keywords with citation bursts indicate that keywords have higher citations in a given period, which can test whether a field of research is hot or not over a given period [62], as well as highlight emerging topics. Through the observation of keywords such as rat (strength, 949.2387; time span, 1998-2009), serotonin (739.1515, 1998-2008), fluoxetine (596.0059, 1998-2005), hippocampus (473.3706, 1998-2004), rat brain (316.2198, 1998-2002), and receptor (257.5575, 1998-2001) (Fig. 7), it is inferred that animal experiments, particularly hippocampus studies in rat brain, such as brain-derived neurotrophic factor (BDNF) studies [63], was a hotspot in the first ten years. Keywords like United States (64.6058, 2010-2012) indicated that the US is one of the most studied countries with regards to depression, which is consistent with results from country, institute, and journal analysis.
Fig. (7).
Top 30 keywords with strongest citation bursts. Blue bars and red bars mean that some keywords are cited frequently in a certain period. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. MAJOR FINDING
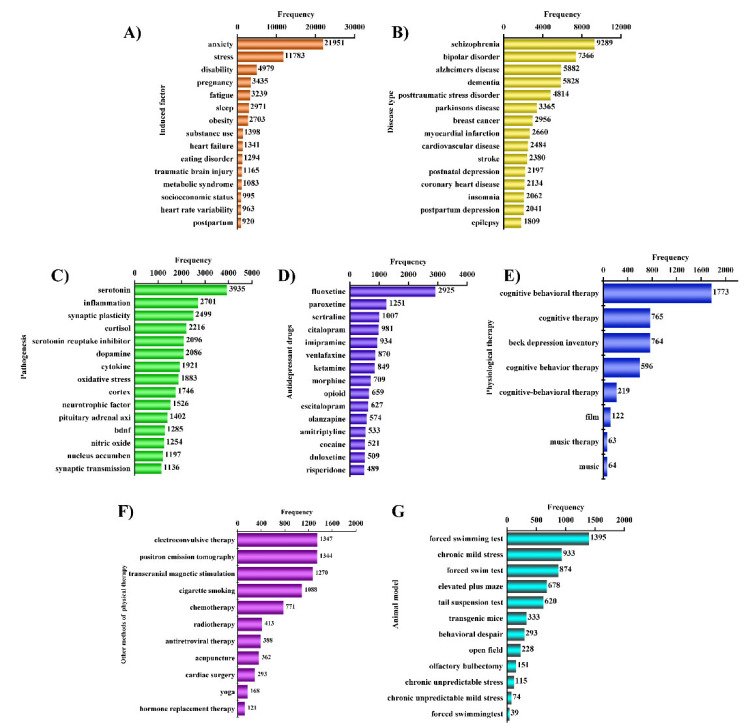
Based on keyword analysis, we summarized the top 15 keywords for each classification in (Fig. 8) so as to systematically understand depression and inform future research direction.
Fig. (8).
Manual analysis results. (A) Induction factor. (B) Disease type. (C) Disease mechanism. (D) Antidepressant drugs. (E) Physiological therapy. (F) Other antidepressive ways. (G) Animal models. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4.1. Induction Factor of Depression
The major causes of depressive disorder include long-term, chronic, and low-intensity stress. It is thought that depression is induced by various biological factors, including psychosocial factors, genetic factors, and pathogenic factors [64]. The top 15 keywords related to the induction factor are shown in (Fig. 8A).
Physical illness usually accompanies biological abnormalities, such as metabolic abnormalities of neurotransmitters. The lack of neurotransmitters in the central nervous system directly affects brain function and may induce depression [65]. Physical illnesses also affect neuroimmune and neuroendocrine processes, causing emotional disorders. Many physical diseases can cause brain chemical imbalance, which may trigger or accompany depression [66], which has 6 main types; endocrine diseases such as hyperthyroidism or hypothyroidism [67]; metabolic diseases such as diabetes [68]; infectious diseases such as AIDS, encephalitis, pneumonia, and hepatitis [69]; internal organ diseases such as pulmonary encephalopathy, hepatic encephalopathy, coronary atherosclerotic heart disease, heart failure, and renal failure [70]; organic brain diseases such as traumatic brain injury, stroke [71], Alzheimer's disease [72], Parkinson's disease [73]; obesity [74], sexual dysfunction [75], cancer [76], disability [77], systemic lupus erythematosus, rheumatoid arthritis, autism, as well as malnutrition and eating disorders. Side effects of drugs such as opioids and substance abuse may also induce depression [78].
Survival pressures and competition greatly increase depression incidence. Various social stresses may cause feelings of frustration and helplessness [79], contributing to depression. Such life stresses include childhood trauma, divorce, loss of love, pregnancy, postpartum stress, abortion and economic burden [80]. Natural and social factors, such as earthquakes, accidents, rape, robbery, and catastrophic emergencies [81, 82] contribute to depressive disorder.
Multiple studies show that stressful life events are correlated with depression. If there were stressful life events, the incidence of depression is high. However, not all people facing the same life pressures get depression, suggesting that psychological factors are involved [83]. Individuals with different psychological qualities have different tolerances to external stimuli. Individuals who suffered from childhood trauma are more likely to suffer depression. Also, negative life events such as disability and postpartum stress may cause different degrees of psychological trauma. Depression may also be influenced by genetic factors. People from families with depression histories tend to have the same disease more often than those without family depression history [84].
4.2. Disease Type Accompanying Depression
Studies on mental disorder comorbidity have increased in recent years. For example, depression is often accompanied by various disorders, including nervous system disorders, cardiovascular diseases, and cancer (Fig. 8B). Depression comorbidities aggravate negative emotions, greatly reduce the quality of life, and also increase difficulty in disease treatment.
Comorbid depression is very common in nervous system diseases, especially schizophrenia, one of the most serious mental disorders. Depression worsens schizophrenia and increases the risk of suicide [85]. A systematic review and meta-analysis showed that antidepressants may relieve depression with schizophrenia [86]. Bipolar disorder is a combination of mania and depression, characterized by manic and depressive phases of varying recurrence rates and episode duration [87, 88]. Relative to depression, bipolar patients have longer durations of depressive symptoms. Most people who have experienced trauma exhibit post-traumatic stress disorder (PTSD) symptoms, partly resulting from depression complications [89]. PTSD and depression patients are harder to treat, and suffer severer mental and physical trauma. Chronic acquired progressive mental retardation syndromes, including dementia, are characterized by a slow decline in intelligence and varying degrees of personality change. Although cognitive impairment and social dysfunction are the core symptoms of dementia, depression is also common in dementia and may increase patient mortality and burden to caregivers [90].
Alzheimer’s disease is a progressive neurodegenerative disease with hidden onset, and is accompanied by symptoms like the decline in cognitive function, behavioral disorders, and a decline in living ability [91]. Studies indicate that depression is one of the most common symbiotic mental disorders in Alzheimer’s disease. These patients suffer losses in quality of life and cognitive ability, significantly increasing morbidity and mortality [92].
Parkinson’s disease, a neurodegenerative disease common in middle-aged and elderly people, is characterized by symptoms like static tremor, ankylosis, and bradykinesia [93]. Up to 35% of Parkinson’s patients have depression symptoms [94]. Depression in Parkinson's disease accelerates the loss of patients’ cognitive abilities, lowering quality of life [95].
Stroke survivors often suffer from psychological distress and neuropsychiatric disorders. A third of stroke survivors experience depression, anxiety or apathy, the most common neuropsychiatric sequela. Post-stroke depression affects about 33% of stroke survivors.
Depressive seizures in epilepsy are the most common comorbidities. One to sixty-two percent of epilepsy patients suffer depression. Epilepsy patients have higher depression and suicide rates relative to healthy people. Depression is often seen as a response to epilepsy stigma and associated poor quality of life [96].
Migraine, characterized by recurrent pulsatile headaches, is a common psychosomatic disease associated with psychosocial factors. Patients with migraine are 2.2 to 4 more likely to suffer from depression relative to those without [97]. Headache is a depression predisposing factor and depressive symptoms are indicators of increased headache and disability [98]. The degree of headache positively correlates with depression severity.
Postpartum depression is a subtype of depression that typically occurs within 4 weeks of delivery and is accompanied by puerperal mental illness, including postpartum restlessness and postpartum psychosis [99]. Patients with postpartum depression experience persistent depression, insomnia, excessive sleep, fatigue, low self-evaluation, low confidence, and suicidal thoughts.
Obsessive-compulsive disorder (OCD) is a common psychological condition affecting 1-3% of the general population [100] and is characterized by obsessive-compulsive concepts and behavior, conscious self-compulsion, and self-anti-obsessive-compulsive coexistence. A multinational study found that people with OCD are at higher risk of severe depression relative to the general population [101]. Depression makes the treatment of OCD more difficult.
Depression also raises the risk of cardiovascular disorders, which are the leading cause of death [102]. Acute myocardial infarction is the main cause of cardiovascular disease, and depression patients are at greater risk of cardiovascular diseases, including myocardial infarction. Depression adversely affects cardiovascular disease prognosis and has been shown to be an independent risk factor for death from arrhythmia and heart failure [103]. Coronary heart disease (CHD) and mental illness are the main causes of global morbidity and mortality. Decades of research suggest that the 2 might lead to each other but the nature of these links are unclear [104].
Depression is the most common psychiatric problem in cancer patients. Because symptoms range from sadness to severe emotional disorders, the study of depression has always been a challenge. When patients suffer from repeated life threats, cancer treatment, and fatigue, it is often difficult to assess emotional changes. Depression highly correlates with oropharyngeal, pancreatic, breast [105], ovarian [106], prostate [107] and lung cancer. It is reported that patients with other cancers, such as colon cancer, gynecological cancer and lymphoma, have lower depression rates, and that depression in cancer patients occurs throughout the course of the disease [108].
Other diseases, including rheumatoid arthritis, a major cause of disability worldwide, are associated with depression [109]. A systematic review and meta-analysis of the strength of association between rheumatoid arthritis and depression found that depression is more common in patients with rheumatoid arthritis relative to healthy individuals. This difference was not due to the sociodemographic differences between the population but the degree of pain experienced [110].
Insomnia is also an important diagnostic criterion for depression. About 90% of depression patients have insomnia symptoms [111] and 30% of insomnia patients have varying mental disorders [112]. Depression has been reported as a potential cause of insomnia, the most common clinical complaint and consistent biological change in depression patients. The relationship between mood and glycemic control suggests that diabetes may double the risk of comorbid depression. Clinically, depression is very common in type I and type 2 diabetes and has a significant impact on the course and outcome of this disease. Patients with diabetes mellitus experience greater difficulty in glycemia control and greater complications, including microvascular and macrovascular complications [113]. Therefore, the combination of conventional antidepressants with hypoglycemics may be effective, but such treatment should be individualized. Glycemia control may also help improve mood and well-being [114].
Depression is also very common in HIV/AIDS patients. The prevalence of depression in HIV+ patients ranges from 18-81%. Depression might alter lymphocyte function in HIV patients and reduce activity of natural killer cells, thereby increasing mortality in these patients. Depression treatment can improve the quality of life for HIV-positive patients [115].
Depression is considered a predisposing factor for irritable bowel syndrome (IBS), an intestinal dysfunction caused by structural and biochemical abnormalities in the gastrointestinal tract. IBS is associated with a high prevalence of mental illness. Thus, the level of anxiety and depression in IBS patients is significantly higher than in healthy people. Thus, IBS patients should be actively monitored and treated for anxiety and depression symptoms, because psychological factors influence symptom severity, persistence, decision to seek treatment, and treatment responses [116].
4.3. Pathogenesis of Depression
The etiology of depression is unclear and its pathogenesis is complicated. Several hypotheses have been proposed to explain its pathogenesis. Bibliometric analysis results shown in (Fig. 8C) revealed that monoamine transmitters and receptors, glutamate and its receptors, neuroendocrine systems, neuropeptides, neurotrophic factors, hormones and inflammatory response doctrines play a role in the pathogenesis of depression.
It was earlier proposed that depression is caused by insufficient levels of noradrenaline in the central nervous system, particularly in the brain [117]. Hypersensitivity of presynaptic membrane α2 receptor reduces the release of noradrenaline [118], which eventually affect brain functions, increase the compensatory sensitivity of noradrenaline β-receptor leading to the development of depression [119]. Serotonin is also a major factor the contributes to depression. Clinical studies have demonstrated that cell-mediated immune activation increases indoleamine-2, 3-dioxygenase (IDO) activity in serum. This, in turn, can accelerate the breakdown of the tryptophan kynurenine pathway, reduce the metabolism of serotonin, and decrease the level of serotonin between synapses. These processes contribute to the pathogenesis of depression [59]. Other monoamine neurotransmitters such as dopamine potently alter the neural encoding of depressive behavior in the downstream nucleus accumbens of experimental animals which exhibit depressive symptoms [120].
Evidence collected over the past decade supports the role of the excitatory amino acid (EAA) neurotransmitter glutamate, and its receptors in depressive disorder and antidepressant activity [121]. Studies have shown that increased glutamate in the frontal lobe may induce depression, mainly through the ionotropic glutamate receptor NMDA receptors [122]. Antidepressants modulate the release or uptake of glutamate through binding to NMDA receptors and outcompeting the binding of NMDA receptor ligands [123-125]. Chronic unpredictable stress process may reduce expression of glutamate receptor in the medial prefrontal cortex, increase nitric oxide synthase (NOS) expression and excessive NO production by causing over-activation of hippocampal NMDA receptors. This may induce depression [126].
Depression may also be caused by various pathogenic processes such as hypothalamic-pituitary-adrenalin (HPA) axis dysregulation that includes corticotropin-releasing hormone, peripheral adrenocorticotropic hormone, and glucocorticoids levels. Stress signals are transmitted to the hypothalamus and to the cerebral cortex, which then elevate serum glucocorticoid (GC) levels as a result of enhanced HPA axis [127]. Overexcitation of HPA axis impairs hypothalamic-pituitary-thyroid (HTP) axis function. This explains the recurrence rate of depression in patients with hyperthyroidism is higher than in those with non-thyroid function. Dysregulation of HPA axis affects the hippocampal glutamate and NMDA receptors and glucocorticoid receptors, which mediate the close association of depression with HPA axis [128].
Depression is associated with the function of neuropeptides and neurotrophic factors, substance P antagonists (SPA) and substance P (SP). These factors regulate the secretion of serotonin in the dorsal raphe nucleus by altering the functional activities of the habenular nucleus, causing antidepressant effects [129]. Animal models show that neuroplastic markers brain-derived neurotrophic factor (BDNF) modify the structure and function of the hippocampus. Long-term use of antidepressant therapy promotes synthesis and signaling pathways of BDNF. In mouse with BDNF knocked-out, decreased hippocampal dentate gyrus neurons regeneration was observed, indicating that neurotrophic factors affect depression [130-132].
Changes in hormone levels have been shown to influence the development of depression. Activation of paraventricular nucleus neurons affects the corticotropin-releasing hormone (CRH), the key regulator of the HPA axis via the hypothalamic paraventricular nucleus (PVN). Elevated CRH increases Crhr1, estrogen receptor alpha (Esr-α), vasopressin receptor (Avpr1a) and glucocorticoid receptor gene expression leading to the development of depression in response to stress [133]. Increased estrogen levels have been implicated in the occurrence of depression. A survey study demonstrated that more women develop depression, twice the number of men, such as postpartum and menopausal mood disorders [134]. In addition, abnormal glucocorticoid levels have been linked to depression. Genetic variations of the glucocorticoid receptor gene (Nuclear Receptor Subfamily 3, Group C, Member 1; NR3C1) affects the development of depression. Polymorphisms in the 5' region of the NR3C1 gene may play a role in the genetic vulnerability for major depressive disorder [135].
Inflammatory cytokines regulate not only inflammatory responses but also neurogenesis and neuroprotection [136]. High levels of pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), interferon-γ (IFN-γ) and tumor necrosis factor α (TNF-α) lead to chronic neuroinflammation and eventually depression. In addition, inflammatory cytokines trigger peripheral immune activation, and this in turn causes neuroendocrine and immune system dysfunction that lead to depression. Multiple inflammatory-cytokines and oxygen radicals have been found in patients with depression as well as animal models of depression [137].
4.4. Antidepressant Therapy
Currently, depression is treated with drugs which improve depression by regulating neurotransmitter and hormone levels in the brain. Common antidepressants include traditional tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), noradrenergic and specific serotonergic antidepressants (NaSSAs) as well as reversible inhibitors of monoamine oxidase-A (RIMAs). The top 15 antidepressant medicines are showed in (Fig. 8D).
Clomipramine, one of the TCAs, has been widely used in Europe [138]. TCAs exert antidepressant and anxiolytic effects by restraining norepinephrine and serotonin reuptake in the central nervous system, which may also improve the compliance of patients when toxic reactions are produced. Amitriptyline is another drug used to treat depression [139]. It improves the emotional state of depressed patients, therapy anxiety or agitated depression.
SSRIs are also mainstream antidepressants used in clinical practice. They include fluoxetine, paroxetine, sertraline, fluvoxamine and citalopram, known as the five golden flowers of new antidepressants. Fluoxetine elevates serotoninergic neurotransmission by restraining neuronal reuptake of serotonin [140]. However, fluoxetine alone has a slow effect, and its early effect on the 5-HT2 receptor also causes anxiety and uneasiness in patients. Some atypical antipsychotics such as olanzapine reduce the discharge of dopaminergic neurons in the inter-brain limbic system. They can control the affinity of dopamine, block central 5-HT2A and 5-HT2C receptors to lower symptoms of depression. Moreover, olanzapine may be applied to alleviate bipolar disorder after using serotonin reuptake inhibitors, placating the sentiment of patients [141]. A combination of olanzapine and fluoxetine has shown benefits in bipolar depression [142]. Paroxetine is a widely prescribed and reliable antidepressant [143], which effectively inhibits the uptake of serotonin. Sertraline is used as the first SSRIs to limit in vivo ingestion of dopamine by reducing the liveness of the presynaptic terminal [144]. Fluvoxamine increases the concentration of serotonin in the synapse and fortifies the effects of serotonin on nerve conduction, thereby exerts antidepressant and anxiolytic effects [145]. Citalopram plays a feeble role in the regulation of norepinephrine, epinephrine receptors, dopamine, histamine, muscarine, and benzodiazepines in depressive disorder [146, 147]. It is highly selective for serotonin receptor, hence can treat depression by decreasing serotonin levels.
As one representative drug of SNRIs and an emblematic phenylethylamine derivative, venlafaxine exerts antidepressant effects. Venlafaxine differs from general antidepressants. It relieves depression symptoms by increasing the concentration of norepinephrine and serotonin, slightly inhibiting dopamine uptake, and decreasing the number of adrenaline receptors [148].
Mirtazapine, as a member of NaSSAs, is generally used to treat depression [149]. It is however, associated with side- effects such as sexual dysfunction, nausea, and anorexia. Mirtazapine can block 5-HT2 and 5-HT3 receptors to improve 5-HT1-mediated serotonin neurotransmission, thereby mitigating depression and anxiety.
Moclobemide is a newly developed reversible MAO-A subtype inhibitor and the key metabolic enzyme of norepinephrine, dopamine and serotonin. It reduces the degradation of central monoamine mediators. The broad-spectrum antidepressant effect of moclobemide may heal endogenous and exogenous depression, there improve the sleep quality of patients. Compared to other antidepressants, moclobemide does not cause significant hepatotoxicity or adverse effects in cardiovascular tissues. It is therefore a safe medicine, especially on the autonomic nervous system [150].
The currently used first- and second-generation antidepressants are not sufficiently effective. For instance, agomelatine and bupropion have a selective action on melatonin receptors and dopamine receptors making them ineffective antidepressants. Agomelatine is a melatonin agonist and selective serotonin antagonist [151]. It is suitable for severe depression complicated with insomnia. Bupropion controls depression by inhibiting noradrenaline and dopamine secretion [152]. Given the side effects of bupropion, it is generally contraindicated in patients with epilepsy.
In addition to antidepressants, other interventions have been developed to be used as adjuncts to drug therapy, some of them are shown in (Fig. 8E). It has been reported that patients put on cognitive behavioral-therapy regained confidence [153]. Cognitive-behavioral therapy is not different from second-generation antidepressants. On the basis of medication, cognitive therapy, an effective auxiliary tool, improved the condition of depression and reduced the recurrence rate [154]. Electroconvulsive therapy is another effective intervention applied in most psychiatric diseases, but it cannot be used more than thrice a week because of its side effects like headaches and nausea [155]. Therefore, electroconvulsive therapy is often used as second-line therapy [156].
In addition to conventional treatments, acupuncture has been proposed as a complementary therapy. The data shown in (Fig. 8F). Indicates that it improves depression in a number of clinical trials [157]. There is evidence that acupuncture regulates hormones such as norepinephrine, dopamine, endorphins and affects the endocrine system and the nervous system thereby modulates depression symptoms [158]. Therapies combining acupuncture and other treatment are more effective than single therapies [159]. In addition, music therapy, yoga, and meditation are used to manage emotions and alleviate anxiety and passivity symptoms [160]. Music therapy improves patients’ condition and enhances their conversational and social skills. Aerobic exercise, such as yoga can make depressive patients relax and improve depression symptoms [161]. Yoga reduces the harmful side effects of drugs and can be combined with drugs to improve the treatment of depression [162].
4.5. Animal Models of Depression
(Fig. 8G). shows the four animal models related to depression, namely, behavioral despair in mice, chronic unpredictable mild model (CUMS), transgenic mice and olfactory bulbectomy. Behavioral despair in mice is used to screen the efficacy of antidepressants [163], including forced swimming (FST), tail suspension test (TST), open field test (OFT). The latter three models are used for pharmacology studies to explore the pathomechanisms of depression [164, 165]. These animal models of depression provide an important tool to develop more effective antidepressants.
5. LIMITATIONS AND ADVANTAGE
The data of this study are from SCI-E database in WOS. Although there are several databases, SCI-E provides the highest quality research findings of depression making it ideal for bibliometric analysis [166]. For author analysis of this literature, bibliometric software cannot distinguish the abbreviation of the author in the current and so it has no appearance for avoiding false conclusions in this paper. Although we only used the SCI-E database, this platform contains a large amount data to study the theme of depression. Therefore, the results obtained here are highly credible. Compared to other bibliometric analyses, the results of journals analysis were contained in this paper and they provided the selection of a journal for the submission of the article. Moreover, the data presented here reveal the trends in depression research to guide other researchers in the field of depression.
CONCLUSION
In this paper, scientometrics is used to analyze literature in the SCI-E database. Our results reveal research hotspots, key research directions, productive countries and institutions focusing on depression in the past 20 years. Research publications on depression vary across regions. The first reason is that people’s lives have been under great pressure in recent years. The second reason is that various factors can lead to the occurrence of depression. Hence there are more and more studies in the field of depression. In conclusion, it is found the most productive country, institute, category, journal, the most cited journal, reference and keywords through the statistical analysis of scientometrics. In addition, the traditional review concludes induction factor, comorbidity, pathogenesis, therapy and animal models of depression in the current by the most frequent keywords of keyword analysis. These not only show the current research hotspots but also reveals grey areas for further research.
ACKNOWLEDGEMENTS
Dong Xu contributed to the design and writing of manuscript. Yi-Lun Wang, Kun-Tang Wang, Yue Wang, Xin-Ran Dong, Jie Tang contributed to the writing of sectional manuscript. Yuan-lu Cui contributed to the design and review of manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by the National Natural Science Foundation of China (81741119).
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Gotlib I.H., Joormann J. Cognition and depression: current status and future directions. Annu. Rev. Clin. Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luppino F.S., de Wit L.M., Bouvy P.F., Stijnen T., Cuijpers P., Penninx B.W.J.H., Zitman F.G. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry. 2010;67(3):220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- 3.Fournier J.C., DeRubeis R.J., Hollon S.D., Dimidjian S., Amsterdam J.D., Shelton R.C., Fawcett J. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Forteza C., Echeagaray F.A.W., Tapia A.J. Center of Epidemiological Studies of Depression Scale (CES-D) in Mexico: bibliometric analysis. Salud Ment. 2012;35(1):13–20. [Google Scholar]
- 5.Tran B.X., Ha G.H., Vu G.T., Nguyen L.H., Latkin C.A., Nathan K., McIntyre R.S., Ho C.S., Tam W.W., Ho R.C. Indices of change, expectations, and popularity of biological treatments for major depressive disorder between 1988 and 2017: a Scientometric analysis. Int. J. Environ. Res. Public Health. 2019;16(13):2255. doi: 10.3390/ijerph16132255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran B.X., McIntyre R.S., Latkin C.A., Phan H.T., Vu G.T., Nguyen H.L.T., Gwee K.K., Ho C.S.H., Ho R.C.M. The current research landscape on the artificial intelligence application in the management of depressive disorders: A bibliometric analysis. Int. J. Environ. Res. Public Health. 2019;16(12):2150. doi: 10.3390/ijerph16122150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang M., Qi Y., Liu H., Chen Y. The role of nanomaterials and nanotechnologies in wastewater treatment: a bibliometric analysis. Nanoscale Res. Lett. 2018;13(1):233. doi: 10.1186/s11671-018-2649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moed H., De Bruin R., Van Leeuwen T. New bibliometric tools for the assessment of national research performance: Database description, overview of indicators and first applications. Scientometrics. 1995;33(3):381–422. doi: 10.1007/BF02017338. [DOI] [Google Scholar]
- 9.Wang W.H., Lu C. Visualization analysis of big data research based on Citespace. Soft Comput. 2020;24(11):8173–8186. doi: 10.1007/s00500-019-04384-7. [DOI] [Google Scholar]
- 10.Xie L., Chen Z., Wang H., Zheng C., Jiang J. Bibliometric and Visualized Analysis of Scientific Publications on Atlantoaxial Spine Surgery Based on Web of Science and VOSviewer. World Neurosurg. 2020;137:435–442.e4. doi: 10.1016/j.wneu.2020.01.171. [DOI] [PubMed] [Google Scholar]
- 11.Dai S.L., Duan X., Zhang W. Knowledge map of environmental crisis management based on keywords network and co-word analysis, 2005-2018. J. Clean. Prod. 2020;•••:121168. doi: 10.1016/j.jclepro.2020.121168. [DOI] [Google Scholar]
- 12.Wang M.H., Ho Y.S., Fu H.Z. Global performance and development on sustainable city based on natural science and social science research: A bibliometric analysis. Sci. Total Environ. 2019;666:1245–1254. doi: 10.1016/j.scitotenv.2019.02.139. [DOI] [PubMed] [Google Scholar]
- 13.Shah S.H.H., Lei S., Ali M., Doronin D., Hussain S.T. Prosumption: bibliometric analysis using HistCite and VOSviewer. Kybernetes. 2020;49(3):1020–1045. [Google Scholar]
- 14.Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006;57(3):359–377. doi: 10.1002/asi.20317. [DOI] [Google Scholar]
- 15.van Eck N.J., Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bordons M., Zulueta M.A. [Evaluation of the scientific activity through bibliometric indices]. Rev. Esp. Cardiol. 1999;52(10):790–800. doi: 10.1016/S0300-8932(99)75008-6. [DOI] [PubMed] [Google Scholar]
- 17.Stout N.L., Alfano C.M., Belter C.W., Nitkin R., Cernich A., Lohmann Siegel K., Chan L. A bibliometric analysis of the landscape of cancer rehabilitation research (1992–2016). J. Natl. Cancer Inst. 2018;110(8):815–824. doi: 10.1093/jnci/djy108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Y., Shi S., Ma W., Chen J., Cai Y., Ge L., Li L., Wu J., Tian J. Bibliometric analysis of global research on PD-1 and PD-L1 in the field of cancer. Int. Immunopharmacol. 2019;72:374–384. doi: 10.1016/j.intimp.2019.03.045. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y-B., Tong X-F., Ren J., Yu C-Q., Cui Y-L.J.E-B.C., Medicine A. Current research trends in traditional chinese medicine formula: a bibliometric review from 2000 to 2016. Evid. Based Complement. Alternat. Med. 2019;2019:3961395. doi: 10.1155/2019/3961395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang M., Liao H., Wan Z., Herrera-Viedma E., Rosen M. Ten years of sustainability (2009 to 2018): A bibliometric overview. Sustainability. 2018;10(5):1655. doi: 10.3390/su10051655. [DOI] [Google Scholar]
- 21.Powell T.H., Kouropalatis Y., Morgan R.E., Karhu P. Mapping knowledge and innovation research themes: Using bibliometrics for classification, evolution, proliferation and determinism. Int. J. Entrep. Innov. Manag. 2016;20(3-4):174–199. doi: 10.1504/IJEIM.2016.077960. [DOI] [Google Scholar]
- 22.Chen X., Yang K., Xu Y., Li K. Top-100 highest-cited original articles in inflammatory bowel disease: A bibliometric analysis. Medicine (Baltimore) 2019;98(20):e15718. doi: 10.1097/MD.0000000000015718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perazzo M.F., Otoni A.L.C., Costa M.S., Granville-Granville A.F., Paiva S.M., Martins-Júnior P.A. The top 100 most-cited papers in Paediatric Dentistry journals: A bibliometric analysis. Int. J. Paediatr. Dent. 2019;29(6):692–711. doi: 10.1111/ipd.12563. [DOI] [PubMed] [Google Scholar]
- 24.Dong R., Wang H., Ye J., Wang M., Bi Y. Publication Trends for Alzheimer’s Disease Worldwide and in China: A 30-Year Bibliometric Analysis. Front. Hum. Neurosci. 2019;13:259. doi: 10.3389/fnhum.2019.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari A.J., Charlson F.J., Norman R.E., Patten S.B., Freedman G., Murray C.J.L., Vos T., Whiteford H.A. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedrich M.J. Depression is the leading cause of disability around the world. JAMA. 2017;317(15):1517–1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- 27.Currier M.B., Nemeroff C.B. Depression as a risk factor for cancer: from pathophysiological advances to treatment implications. Annu. Rev. Med. 2014;65:203–221. doi: 10.1146/annurev-med-061212-171507. [DOI] [PubMed] [Google Scholar]
- 28.Trish B. Big Data under Obama and Trump: The Data-Fueled U.S. Presidency. Politics Gov. 2018;6(4):29–38. doi: 10.17645/pag.v6i4.1565. [DOI] [Google Scholar]
- 29.Mahomed S., Padayatchi N., Singh J., Naidoo K. Precision medicine in resistant Tuberculosis: Treat the correct patient, at the correct time, with the correct drug. J. Infect. 2019;78(4):261–268. doi: 10.1016/j.jinf.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X. A scientometric review of global BIM research: Analysis and visualization. Autom. Construct. 2017;80:37–47. doi: 10.1016/j.autcon.2017.04.002. [DOI] [Google Scholar]
- 31.Chen C., Hu Z., Liu S., Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin. Biol. Ther. 2012;12(5):593–608. doi: 10.1517/14712598.2012.674507. [DOI] [PubMed] [Google Scholar]
- 32.Tsang A., Von Korff M., Lee S., Alonso J., Karam E., Angermeyer M.C., Borges G.L.G., Bromet E.J., Demytteneare K., de Girolamo G., de Graaf R., Gureje O., Lepine J.P., Haro J.M., Levinson D., Oakley Browne M.A., Posada-Villa J., Seedat S., Watanabe M. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J. Pain. 2008;9(10):883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Esposito M. H-index: an index to quantifiy the impact of scientific research. Eur. J. Oral Implantology. 2010;3(1):3–4. [PubMed] [Google Scholar]
- 34.Bertoli-Barsotti L., Lando T. A theoretical model of the relationship between the h-index and other simple citation indicators. Scientometrics. 2017;111(3):1415–1448. doi: 10.1007/s11192-017-2351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bornmann L., Daniel H.D. What do we know about the h index? J. Am. Soc. Inf. Sci. Technol. 2007;58(9):1381–1385. doi: 10.1002/asi.20609. [DOI] [Google Scholar]
- 36.Murray C.J., Barber R.M., Foreman K.J., Abbasoglu Ozgoren A., Abd-Allah F., Abera S.F., Aboyans V., Abraham J.P., Abubakar I., Abu-Raddad L.J., Abu-Rmeileh N.M., Achoki T., Ackerman I.N., Ademi Z., Adou A.K., Adsuar J.C., Afshin A., Agardh E.E., Alam S.S., Alasfoor D., Albittar M.I., Alegretti M.A., Alemu Z.A., Alfonso-Cristancho R., Alhabib S., Ali R., Alla F., Allebeck P., Almazroa M.A., Alsharif U., Alvarez E., Alvis-Guzman N., Amare A.T., Ameh E.A., Amini H., Ammar W., Anderson H.R., Anderson B.O., Antonio C.A., Anwari P., Arnlöv J., Arsic Arsenijevic V.S., Artaman A., Asghar R.J., Assadi R., Atkins L.S., Avila M.A., Awuah B., Bachman V.F., Badawi A., Bahit M.C., Balakrishnan K., Banerjee A., Barker-Collo S.L., Barquera S., Barregard L., Barrero L.H., Basu A., Basu S., Basulaiman M.O., Beardsley J., Bedi N., Beghi E., Bekele T., Bell M.L., Benjet C., Bennett D.A., Bensenor I.M., Benzian H., Bernabé E., Bertozzi-Villa A., Beyene T.J., Bhala N., Bhalla A., Bhutta Z.A., Bienhoff K., Bikbov B., Biryukov S., Blore J.D., Blosser C.D., Blyth F.M., Bohensky M.A., Bolliger I.W., Bora Başara B., Bornstein N.M., Bose D., Boufous S., Bourne R.R., Boyers L.N., Brainin M., Brayne C.E., Brazinova A., Breitborde N.J., Brenner H., Briggs A.D., Brooks P.M., Brown J.C., Brugha T.S., Buchbinder R., Buckle G.C., Budke C.M., Bulchis A., Bulloch A.G., Campos-Nonato I.R., Carabin H., Carapetis J.R., Cárdenas R., Carpenter D.O., Caso V., Castañeda-Orjuela C.A., Castro R.E., Catalá-López F., Cavalleri F., Çavlin A., Chadha V.K., Chang J.C., Charlson F.J., Chen H., Chen W., Chiang P.P., Chimed-Ochir O., Chowdhury R., Christensen H., Christophi C.A., Cirillo M., Coates M.M., Coffeng L.E., Coggeshall M.S., Colistro V., Colquhoun S.M., Cooke G.S., Cooper C., Cooper L.T., Coppola L.M., Cortinovis M., Criqui M.H., Crump J.A., Cuevas-Nasu L., Danawi H., Dandona L., Dandona R., Dansereau E., Dargan P.I., Davey G., Davis A., Davitoiu D.V., Dayama A., De Leo D., Degenhardt L., Del Pozo-Cruz B., Dellavalle R.P., Deribe K., Derrett S., Des Jarlais D.C., Dessalegn M., Dharmaratne S.D., Dherani M.K., Diaz-Torné C., Dicker D., Ding E.L., Dokova K., Dorsey E.R., Driscoll T.R., Duan L., Duber H.C., Ebel B.E., Edmond K.M., Elshrek Y.M., Endres M., Ermakov S.P., Erskine H.E., Eshrati B., Esteghamati A., Estep K., Faraon E.J., Farzadfar F., Fay D.F., Feigin V.L., Felson D.T., Fereshtehnejad S.M., Fernandes J.G., Ferrari A.J., Fitzmaurice C., Flaxman A.D., Fleming T.D., Foigt N., Forouzanfar M.H., Fowkes F.G., Paleo U.F., Franklin R.C., Fürst T., Gabbe B., Gaffikin L., Gankpé F.G., Geleijnse J.M., Gessner B.D., Gething P., Gibney K.B., Giroud M., Giussani G., Gomez Dantes H., Gona P., González-Medina D., Gosselin R.A., Gotay C.C., Goto A., Gouda H.N., Graetz N., Gugnani H.C., Gupta R., Gupta R., Gutiérrez R.A., Haagsma J., Hafezi-Nejad N., Hagan H., Halasa Y.A., Hamadeh R.R., Hamavid H., Hammami M., Hancock J., Hankey G.J., Hansen G.M., Hao Y., Harb H.L., Haro J.M., Havmoeller R., Hay S.I., Hay R.J., Heredia-Pi I.B., Heuton K.R., Heydarpour P., Higashi H., Hijar M., Hoek H.W., Hoffman H.J., Hosgood H.D., Hossain M., Hotez P.J., Hoy D.G., Hsairi M., Hu G., Huang C., Huang J.J., Husseini A., Huynh C., Iannarone M.L., Iburg K.M., Innos K., Inoue M., Islami F., Jacobsen K.H., Jarvis D.L., Jassal S.K., Jee S.H., Jeemon P., Jensen P.N., Jha V., Jiang G., Jiang Y., Jonas J.B., Juel K., Kan H., Karch A., Karema C.K., Karimkhani C., Karthikeyan G., Kassebaum N.J., Kaul A., Kawakami N., Kazanjan K., Kemp A.H., Kengne A.P., Keren A., Khader Y.S., Khalifa S.E., Khan E.A., Khan G., Khang Y.H., Kieling C., Kim D., Kim S., Kim Y., Kinfu Y., Kinge J.M., Kivipelto M., Knibbs L.D., Knudsen A.K., Kokubo Y., Kosen S., Krishnaswami S., Kuate Defo B., Kucuk Bicer B., Kuipers E.J., Kulkarni C., Kulkarni V.S., Kumar G.A., Kyu H.H., Lai T., Lalloo R., Lallukka T., Lam H., Lan Q., Lansingh V.C., Larsson A., Lawrynowicz A.E., Leasher J.L., Leigh J., Leung R., Levitz C.E., Li B., Li Y., Li Y., Lim S.S., Lind M., Lipshultz S.E., Liu S., Liu Y., Lloyd B.K., Lofgren K.T., Logroscino G., Looker K.J., Lortet-Tieulent J., Lotufo P.A., Lozano R., Lucas R.M., Lunevicius R., Lyons R.A., Ma S., Macintyre M.F., Mackay M.T., Majdan M., Malekzadeh R., Marcenes W., Margolis D.J., Margono C., Marzan M.B., Masci J.R., Mashal M.T., Matzopoulos R., Mayosi B.M., Mazorodze T.T., Mcgill N.W., Mcgrath J.J., Mckee M., Mclain A., Meaney P.A., Medina C., Mehndiratta M.M., Mekonnen W., Melaku Y.A., Meltzer M., Memish Z.A., Mensah G.A., Meretoja A., Mhimbira F.A., Micha R., Miller T.R., Mills E.J., Mitchell P.B., Mock C.N., Mohamed Ibrahim N., Mohammad K.A., Mokdad A.H., Mola G.L., Monasta L., Montañez Hernandez J.C., Montico M., Montine T.J., Mooney M.D., Moore A.R., Moradi-Lakeh M., Moran A.E., Mori R., Moschandreas J., Moturi W.N., Moyer M.L., Mozaffarian D., Msemburi W.T., Mueller U.O., Mukaigawara M., Mullany E.C., Murdoch M.E., Murray J., Murthy K.S., Naghavi M., Naheed A., Naidoo K.S., Naldi L., Nand D., Nangia V., Narayan K.M., Nejjari C., Neupane S.P., Newton C.R., Ng M., Ngalesoni F.N., Nguyen G., Nisar M.I., Nolte S., Norheim O.F., Norman R.E., Norrving B., Nyakarahuka L., Oh I.H., Ohkubo T., Ohno S.L., Olusanya B.O., Opio J.N., Ortblad K., Ortiz A., Pain A.W., Pandian J.D., Panelo C.I., Papachristou C., Park E.K., Park J.H., Patten S.B., Patton G.C., Paul V.K., Pavlin B.I., Pearce N., Pereira D.M., Perez-Padilla R., Perez-Ruiz F., Perico N., Pervaiz A., Pesudovs K., Peterson C.B., Petzold M., Phillips M.R., Phillips B.K., Phillips D.E., Piel F.B., Plass D., Poenaru D., Polinder S., Pope D., Popova S., Poulton R.G., Pourmalek F., Prabhakaran D., Prasad N.M., Pullan R.L., Qato D.M., Quistberg D.A., Rafay A., Rahimi K., Rahman S.U., Raju M., Rana S.M., Razavi H., Reddy K.S., Refaat A., Remuzzi G., Resnikoff S., Ribeiro A.L., Richardson L., Richardus J.H., Roberts D.A., Rojas-Rueda D., Ronfani L., Roth G.A., Rothenbacher D., Rothstein D.H., Rowley J.T., Roy N., Ruhago G.M., Saeedi M.Y., Saha S., Sahraian M.A., Sampson U.K., Sanabria J.R., Sandar L., Santos I.S., Satpathy M., Sawhney M., Scarborough P., Schneider I.J., Schöttker B., Schumacher A.E., Schwebel D.C., Scott J.G., Seedat S., Sepanlou S.G., Serina P.T., Servan-Mori E.E., Shackelford K.A., Shaheen A., Shahraz S., Shamah Levy T., Shangguan S., She J., Sheikhbahaei S., Shi P., Shibuya K., Shinohara Y., Shiri R., Shishani K., Shiue I., Shrime M.G., Sigfusdottir I.D., Silberberg D.H., Simard E.P., Sindi S., Singh A., Singh J.A., Singh L., Skirbekk V., Slepak E.L., Sliwa K., Soneji S., Søreide K., Soshnikov S., Sposato L.A., Sreeramareddy C.T., Stanaway J.D., Stathopoulou V., Stein D.J., Stein M.B., Steiner C., Steiner T.J., Stevens A., Stewart A., Stovner L.J., Stroumpoulis K., Sunguya B.F., Swaminathan S., Swaroop M., Sykes B.L., Tabb K.M., Takahashi K., Tandon N., Tanne D., Tanner M., Tavakkoli M., Taylor H.R., Te Ao B.J., Tediosi F., Temesgen A.M., Templin T., Ten Have M., Tenkorang E.Y., Terkawi A.S., Thomson B., Thorne-Lyman A.L., Thrift A.G., Thurston G.D., Tillmann T., Tonelli M., Topouzis F., Toyoshima H., Traebert J., Tran B.X., Trillini M., Truelsen T., Tsilimbaris M., Tuzcu E.M., Uchendu U.S., Ukwaja K.N., Undurraga E.A., Uzun S.B., Van Brakel W.H., Van De Vijver S., van Gool C.H., Van Os J., Vasankari T.J., Venketasubramanian N., Violante F.S., Vlassov V.V., Vollset S.E., Wagner G.R., Wagner J., Waller S.G., Wan X., Wang H., Wang J., Wang L., Warouw T.S., Weichenthal S., Weiderpass E., Weintraub R.G., Wenzhi W., Werdecker A., Westerman R., Whiteford H.A., Wilkinson J.D., Williams T.N., Wolfe C.D., Wolock T.M., Woolf A.D., Wulf S., Wurtz B., Xu G., Yan L.L., Yano Y., Ye P., Yentür G.K., Yip P., Yonemoto N., Yoon S.J., Younis M.Z., Yu C., Zaki M.E., Zhao Y., Zheng Y., Zonies D., Zou X., Salomon J.A., Lopez A.D., Vos T. GBD 2013 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–2191. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassebaum N.J., Arora M., Barber R.M., Bhutta Z.A., Brown J., Carter A., Casey D.C., Charlson F.J., Coates M.M., Coggeshall M. GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1603–1658. doi: 10.1016/S0140-6736(16)31460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh D., Rashid M., Hallan S.S., Mehra N.K., Prakash A., Mishra N. Pharmacological evaluation of nasal delivery of selegiline hydrochloride-loaded thiolated chitosan nanoparticles for the treatment of depression. Artif. Cells Nanomed. Biotechnol. 2016;44(3):865–877. doi: 10.3109/21691401.2014.998824. [DOI] [PubMed] [Google Scholar]
- 39.Pybis J., Saxon D., Hill A., Barkham M. The comparative effectiveness and efficiency of cognitive behaviour therapy and generic counselling in the treatment of depression: evidence from the 2nd UK National Audit of psychological therapies. BMC Psychiatry. 2017;17(1):215. doi: 10.1186/s12888-017-1370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y., Guo J.J., Zhan S., Patel N.C. Treatment effects of antidepressants in patients with post-stroke depression: a meta-analysis. Ann. Pharmacother. 2006;40(12):2115–2122. doi: 10.1345/aph.1H389. [DOI] [PubMed] [Google Scholar]
- 41.Amini S., Jafarirad S., Amani R. Postpartum depression and vitamin D: A systematic review. Crit. Rev. Food Sci. Nutr. 2019;59(9):1514–1520. doi: 10.1080/10408398.2017.1423276. [DOI] [PubMed] [Google Scholar]
- 42.Wu L., Qiao Y., Wang L., Guo J., Wang G., He W., Yin L., Zhao J. A Self-microemulsifying Drug Delivery System (SMEDDS) for a novel medicative compound against depression: a preparation and bioavailability study in rats. AAPS PharmSciTech. 2015;16(5):1051–1058. doi: 10.1208/s12249-014-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng G.J., Tian J.S., Gao X.X., Zhou Y.Z., Qin X.M. Research on the pathological mechanism and drug treatment mechanism of depression. Curr. Neuropharmacol. 2015;13(4):514–523. doi: 10.2174/1570159X1304150831120428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao H., Du H., Liu M., Gao S., Li N., Chao Y., Li R., Chen W., Lou Z., Dong X. Integrative Proteomics-Metabolomics Strategy for Pathological Mechanism of Vascular Depression Mouse Model. J. Proteome Res. 2018;17(1):656–669. doi: 10.1021/acs.jproteome.7b00724. [DOI] [PubMed] [Google Scholar]
- 45.Thomas A.J., Perry R., Barber R., Kalaria R.N., O’Brien J.T. Pathologies and pathological mechanisms for white matter hyperintensities in depression. Ann. N. Y. Acad. Sci. 2002;977(1):333–339. doi: 10.1111/j.1749-6632.2002.tb04835.x. [DOI] [PubMed] [Google Scholar]
- 46.Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 47.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 48.Kessler R.C., Berglund P., Demler O., Jin R., Koretz D., Merikangas K.R., Rush A.J., Walters E.E., Wang P.S. National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 49.Caspi A., Sugden K., Moffitt T.E., Taylor A., Craig I.W., Harrington H., McClay J., Mill J., Martin J., Braithwaite A., Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 50.Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 51.Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J. Psychosom. Res. 2002;52(2):69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 54.Trivedi M.H., Rush A.J., Wisniewski S.R., Nierenberg A.A., Warden D., Ritz L., Norquist G., Howland R.H., Lebowitz B., McGrath P.J., Shores-Wilson K., Biggs M.M., Balasubramani G.K., Fava M., Team S.S. STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 55.Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Kaufman J., Yang B.Z., Douglas-Palumberi H., Houshyar S., Lipschitz D., Krystal J.H., Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc. Natl. Acad. Sci. USA. 2004;101(49):17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olivier J.D.A., Van Der Hart M.G.C., Van Swelm R.P.L., Dederen P.J., Homberg J.R., Cremers T., Deen P.M.T., Cuppen E., Cools A.R., Ellenbroek B.A. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152(3):573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 58.Sharp T., Cowen P.J. 5-HT and depression: is the glass half-full? Curr. Opin. Pharmacol. 2011;11(1):45–51. doi: 10.1016/j.coph.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 59.Maes M., Leonard B.E., Myint A.M., Kubera M., Verkerk R. The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(3):702–721. doi: 10.1016/j.pnpbp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 60.Li H.J., An H.Z., Wang Y., Huang J.C., Gao X.Y. Evolutionary features of academic articles co-keyword network and keywords co-occurrence network: Based on two-mode affiliation network. Physica A. 2016;450:657–669. doi: 10.1016/j.physa.2016.01.017. [DOI] [Google Scholar]
- 61.Liu X.J., Zhan F.B., Hong S., Niu B.B., Liu Y.L. A bibliometric study of earthquake research: 1900-2010. Scientometrics. 2012;92(3):747–765. doi: 10.1007/s11192-011-0599-z. [DOI] [Google Scholar]
- 62.Yan W., Zheng K., Weng L., Chen C., Kiartivich S., Jiang X., Su X., Wang Y., Wang X. Bibliometric evaluation of 2000-2019 publications on functional near-infrared spectroscopy. Neuroimage. 2020;220:117121. doi: 10.1016/j.neuroimage.2020.117121. [DOI] [PubMed] [Google Scholar]
- 63.Shirayama Y., Chen A.C.H., Nakagawa S., Russell D.S., Duman R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schachter J., Martel J., Lin C-S., Chang C-J., Wu T-R., Lu C-C., Ko Y-F., Lai H-C., Ojcius D.M., Young J.D. Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav. Immun. 2018;69:1–8. doi: 10.1016/j.bbi.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 65.Villas Boas G.R., Boerngen de Lacerda R., Paes M.M., Gubert P., Almeida W.L.D.C., Rescia V.C., de Carvalho P.M.G., de Carvalho A.A.V., Oesterreich S.A. Molecular aspects of depression: A review from neurobiology to treatment. Eur. J. Pharmacol. 2019;851:99–121. doi: 10.1016/j.ejphar.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 66.Zhou Q., Li N. The impact of major physical diseases and its outcomes on depressive symptoms among Chinese population. J. Ment. Health. 2019;28(2):148–152. doi: 10.1080/09638237.2017.1417561. [DOI] [PubMed] [Google Scholar]
- 67.Cosci F., Fava G.A., Sonino N. Mood and anxiety disorders as early manifestations of medical illness: a systematic review. Psychother. Psychosom. 2015;84(1):22–29. doi: 10.1159/000367913. [DOI] [PubMed] [Google Scholar]
- 68.Fiore V., Marci M., Poggi A., Giagulli V.A., Licchelli B., Iacoviello M., Guastamacchia E., De Pergola G., Triggiani V. The association between diabetes and depression: a very disabling condition. Endocrine. 2015;48(1):14–24. doi: 10.1007/s12020-014-0323-x. [DOI] [PubMed] [Google Scholar]
- 69.Rueda S., Mitra S., Chen S., Gogolishvili D., Globerman J., Chambers L., Wilson M., Logie C.H., Shi Q., Morassaei S., Rourke S.B. Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: a series of meta-analyses. BMJ Open. 2016;6(7):e011453. doi: 10.1136/bmjopen-2016-011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eich D., Neuhaus C., Gamma A., Angst J., Rössler W., Ajdacic-Gross V., Opravil M. Is depression a risk factor for heart complaints? Longitudinal aspects in the Zurich study. Eur. Arch. Psychiatry Clin. Neurosci. 2007;257(7):396–401. doi: 10.1007/s00406-007-0747-x. [DOI] [PubMed] [Google Scholar]
- 71.Villa R.F., Ferrari F., Moretti A. Post-stroke depression: Mechanisms and pharmacological treatment. Pharmacol. Ther. 2018;184:131–144. doi: 10.1016/j.pharmthera.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Khundakar A.A., Thomas A.J. Neuropathology of depression in Alzheimer’s disease: current knowledge and the potential for new treatments. J. Alzheimers Dis. 2015;44(1):27–41. doi: 10.3233/JAD-148003. [DOI] [PubMed] [Google Scholar]
- 73.Borgonovo J., Allende-Castro C., Laliena A., Guerrero N., Silva H., Concha M.L. Changes in neural circuitry associated with depression at pre-clinical, pre-motor and early motor phases of Parkinson’s disease. Parkinsonism Relat. Disord. 2017;35:17–24. doi: 10.1016/j.parkreldis.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 74.Chaves Filho A.J.M., Lima C.N.C., Vasconcelos S.M.M., de Lucena D.F., Maes M., Macedo D. IDO chronic immune activation and tryptophan metabolic pathway: A potential pathophysiological link between depression and obesity. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;80(Pt C):234–249. doi: 10.1016/j.pnpbp.2017.04.035. [DOI] [PubMed] [Google Scholar]
- 75.Brotto L., Atallah S., Johnson-Agbakwu C., Rosenbaum T., Abdo C., Byers E.S., Graham C., Nobre P., Wylie K. Psychological and interpersonal dimensions of sexual function and dysfunction. J. Sex. Med. 2016;13(4):538–571. doi: 10.1016/j.jsxm.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 76.Chad-Friedman E., Coleman S., Traeger L.N., Pirl W.F., Goldman R., Atlas S.J., Park E.R. Psychological distress associated with cancer screening: A systematic review. Cancer. 2017;123(20):3882–3894. doi: 10.1002/cncr.30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shim E-J., Hahm B-J., Go D.J., Lee K-M., Noh H.L., Park S-H., Song Y.W. Modeling quality of life in patients with rheumatic diseases: the role of pain catastrophizing, fear-avoidance beliefs, physical disability, and depression. Disabil. Rehabil. 2018;40(13):1509–1516. doi: 10.1080/09638288.2017.1300691. [DOI] [PubMed] [Google Scholar]
- 78.Mazereeuw G., Sullivan M.D., Juurlink D.N. Depression in chronic pain: might opioids be responsible? Pain. 2018;159(11):2142–2145. doi: 10.1097/j.pain.0000000000001305. [DOI] [PubMed] [Google Scholar]
- 79.Köhler C.A., Evangelou E., Stubbs B., Solmi M., Veronese N., Belbasis L., Bortolato B., Melo M.C.A., Coelho C.A., Fernandes B.S., Olfson M., Ioannidis J.P.A., Carvalho A.F. Mapping risk factors for depression across the lifespan: An umbrella review of evidence from meta-analyses and Mendelian randomization studies. J. Psychiatr. Res. 2018;103:189–207. doi: 10.1016/j.jpsychires.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 80.Wosu A.C., Gelaye B., Williams M.A. History of childhood sexual abuse and risk of prenatal and postpartum depression or depressive symptoms: an epidemiologic review. Arch. Women Ment. Health. 2015;18(5):659–671. doi: 10.1007/s00737-015-0533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kraemer B., Wittmann L., Jenewein J., Schnyder U. 2004 Tsunami: long-term psychological consequences for Swiss tourists in the area at the time of the disaster. Aust. N. Z. J. Psychiatry. 2009;43(5):420–425. doi: 10.1080/00048670902817653. [DOI] [PubMed] [Google Scholar]
- 82.Learman L.A. Screening for Depression in pregnancy and the postpartum period. Clin. Obstet. Gynecol. 2018;61(3):525–532. doi: 10.1097/GRF.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 83.Cristea I.A., Karyotaki E., Hollon S.D., Cuijpers P., Gentili C. Biological markers evaluated in randomized trials of psychological treatments for depression: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2019;101:32–44. doi: 10.1016/j.neubiorev.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 84.Bromberger J.T., Epperson C.N. Depression during and after the perimenopause: impact of hormones, genetics, and environmental determinants of disease. Obstet. Gynecol. Clin. North Am. 2018;45(4):663–678. doi: 10.1016/j.ogc.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heilä H., Heikkinen M.E., Isometsä E.T., Henriksson M.M., Marttunen M.J., Lönnqvist J.K. Life events and completed suicide in schizophrenia: a comparison of suicide victims with and without schizophrenia. Schizophr. Bull. 1999;25(3):519–531. doi: 10.1093/oxfordjournals.schbul.a033398. [DOI] [PubMed] [Google Scholar]
- 86.Dauwan M., Begemann M.J.H., Heringa S.M., Sommer I.E. Exercise improves clinical symptoms, quality of life, global functioning, and depression in schizophrenia: a systematic review and meta-analysis. Schizophr. Bull. 2016;42(3):588–599. doi: 10.1093/schbul/sbv164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Purcell S.M., Wray N.R., Stone J.L., Visscher P.M., O’Donovan M.C., Sullivan P.F., Sklar P., Ruderfer D.M., McQuillin A., Morris D.W., O’Dushlaine C.T., Corvin A., Holmans P.A., Macgregor S., Gurling H., Blackwood D.H.R., Craddock N.J., Gill M., Hultman C.M., Kirov G.K., Lichtenstein P., Muir W.J., Owen M.J., Pato C.N., Scolnick E.M., St Clair D., Williams N.M., Georgieva L., Nikolov I., Norton N., Williams H., Toncheva D., Milanova V., Thelander E.F., Sullivan P., Kenny E., Quinn E.M., Choudhury K., Datta S., Pimm J., Thirumalai S., Puri V., Krasucki R., Lawrence J., Quested D., Bass N., Crombie C., Fraser G., Kuan S.L., Walker N., McGhee K.A., Pickard B., Malloy P., Maclean A.W., Van Beck M., Pato M.T., Medeiros H., Middleton F., Carvalho C., Morley C., Fanous A., Conti D., Knowles J.A., Ferreira C.P., Macedo A., Azevedo M.H., Kirby A.N., Ferreira M.A.R., Daly M.J., Chambert K., Kuruvilla F., Gabriel S.B., Ardlie K., Moran J.L. International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tondo L., Vázquez G.H., Baldessarini R.J. Depression and mania in bipolar disorder. Curr. Neuropharmacol. 2017;15(3):353–358. doi: 10.2174/1570159X14666160606210811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elhai J.D., Grubaugh A.L., Kashdan T.B., Frueh B.C. Empirical examination of a proposed refinement to DSM-IV posttraumatic stress disorder symptom criteria using the National Comorbidity Survey Replication data. J. Clin. Psychiatry. 2008;69(4):597–602. doi: 10.4088/JCP.v69n0411. [DOI] [PubMed] [Google Scholar]
- 90.Corbett A., Smith J., Creese B., Ballard C. Treatment of behavioral and psychological symptoms of Alzheimer’s disease. Curr. Treat. Options Neurol. 2012;14(2):113–125. doi: 10.1007/s11940-012-0166-9. [DOI] [PubMed] [Google Scholar]
- 91.Ownby R.L., Crocco E., Acevedo A., John V., Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Modrego P.J. Depression in Alzheimer’s disease. Pathophysiology, diagnosis, and treatment. J. Alzheimers Dis. 2010;21(4):1077–1087. doi: 10.3233/JAD-2010-100153. [DOI] [PubMed] [Google Scholar]
- 93.Martinez-Martin P., Rodriguez-Blazquez C., Kurtis M.M., Chaudhuri K.R., Group N.V. NMSS Validation Group. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov. Disord. 2011;26(3):399–406. doi: 10.1002/mds.23462. [DOI] [PubMed] [Google Scholar]
- 94.Reijnders J.S., Ehrt U., Weber W.E., Aarsland D., Leentjens A.F. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov. Disord. 2008;23(2):183–189. doi: 10.1002/mds.21803. [DOI] [PubMed] [Google Scholar]
- 95.Novais F., Starkstein S. Phenomenology of Depression in Alzheimer’s Disease. J. Alzheimers Dis. 2015;47(4):845–855. doi: 10.3233/JAD-148004. [DOI] [PubMed] [Google Scholar]
- 96.Błaszczyk B., Czuczwar S.J. Epilepsy coexisting with depression. Pharmacol. Rep. 2016;68(5):1084–1092. doi: 10.1016/j.pharep.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 97.Camarda C., Pipia C., Taglialavori A., Di Fiore P., Camarda R., Monastero R. Comorbidity between depressive symptoms and migraine: preliminary data from the Zabút Aging Project. Neurol. Sci. 2008;29(1) Suppl. 1:S149–S151. doi: 10.1007/s10072-008-0909-2. [DOI] [PubMed] [Google Scholar]
- 98.Venable V.L., Carlson C.R., Wilson J. The role of anger and depression in recurrent headache. Headache. 2001;41(1):21–30. doi: 10.1046/j.1526-4610.2001.111006021.x. [DOI] [PubMed] [Google Scholar]
- 99.Pitt B. “Atypical” depression following childbirth. Br. J. Psychiatry. 1968;114(516):1325–1335. doi: 10.1192/bjp.114.516.1325. [DOI] [PubMed] [Google Scholar]
- 100.Adam Y., Meinlschmidt G., Gloster A.T., Lieb R. Obsessive-compulsive disorder in the community: 12-month prevalence, comorbidity and impairment. Soc. Psychiatry Psychiatr. Epidemiol. 2012;47(3):339–349. doi: 10.1007/s00127-010-0337-5. [DOI] [PubMed] [Google Scholar]
- 101.Weissman M.M., Bland R.C., Canino G.J., Greenwald S., Hwu H-G., Lee C.K., Newman S.C., Oakley-Browne M.A., Rubio-Stipec M., Wickramaratne P-J., et al. The Cross National Collaborative Group. The cross national epidemiology of obsessive compulsive disorder. J. Clin. Psychiatry. 1994;55(3) Suppl.:5–10. [PubMed] [Google Scholar]
- 102.Liu H., Luiten P.G., Eisel U.L., Dejongste M.J., Schoemaker R.G. Depression after myocardial infarction: TNF-α-induced alterations of the blood-brain barrier and its putative therapeutic implications. Neurosci. Biobehav. Rev. 2013;37(4):561–572. doi: 10.1016/j.neubiorev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 103.Frasure-Smith N., Lespérance F., Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270(15):1819–1825. doi: 10.1001/jama.1993.03510150053029. [DOI] [PubMed] [Google Scholar]
- 104.De Hert M., Detraux J., Vancampfort D. The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin. Neurosci. 2018;20(1):31–40. doi: 10.31887/DCNS.2018.20.1/mdehert. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Burgess C., Cornelius V., Love S., Graham J., Richards M., Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330(7493):702–705. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hipkins J., Whitworth M., Tarrier N., Jayson G. Social support, anxiety and depression after chemotherapy for ovarian cancer: a prospective study. Br. J. Health Psychol. 2004;9(Pt 4):569–581. doi: 10.1348/1359107042304542. [DOI] [PubMed] [Google Scholar]
- 107.Erim D.O., Bensen J.T., Mohler J.L., Fontham E.T.H., Song L., Farnan L., Delacroix S.E., Peters E.S., Erim T.N., Chen R.C., Gaynes B.N. DO. Prevalence and predictors of probable depression in prostate cancer survivors. Cancer. 2019;125(19):3418–3427. doi: 10.1002/cncr.32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Massie M.J. Prevalence of depression in patients with cancer. J. Natl. Cancer Inst. Monogr. 2004;(32):57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- 109.Covic T., Cumming S.R., Pallant J.F., Manolios N., Emery P., Conaghan P.G., Tennant A. Depression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the hospital, Anxiety and Depression Scale (HADS). BMC Psychiatry. 2012;12(1):6. doi: 10.1186/1471-244X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dickens C., McGowan L., Clark-Carter D., Creed F. Depression in rheumatoid arthritis: a systematic review of the literature with meta-analysis. Psychosom. Med. 2002;64(1):52–60. doi: 10.1097/00006842-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 111.Hein M., Lanquart J-P., Loas G., Hubain P., Linkowski P. Similar polysomnographic pattern in primary insomnia and major depression with objective insomnia: a sign of common pathophysiology? BMC Psychiatry. 2017;17(1):273. doi: 10.1186/s12888-017-1438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sivertsen B., Krokstad S., Øverland S., Mykletun A. The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J. Psychosom. Res. 2009;67(2):109–116. doi: 10.1016/j.jpsychores.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 113.Semenkovich K., Brown M.E., Svrakic D.M., Lustman P.J. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75(6):577–587. doi: 10.1007/s40265-015-0347-4. [DOI] [PubMed] [Google Scholar]
- 114.Lustman P.J., Clouse R.E. Depression in diabetic patients: the relationship between mood and glycemic control. J. Diabetes Complications. 2005;19(2):113–122. doi: 10.1016/j.jdiacomp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 115.Arseniou S., Arvaniti A., Samakouri M. HIV infection and depression. Psychiatry Clin. Neurosci. 2014;68(2):96–109. doi: 10.1111/pcn.12097. [DOI] [PubMed] [Google Scholar]
- 116.Fond G., Loundou A., Hamdani N., Boukouaci W., Dargel A., Oliveira J., Roger M., Tamouza R., Leboyer M., Boyer L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014;264(8):651–660. doi: 10.1007/s00406-014-0502-z. [DOI] [PubMed] [Google Scholar]
- 117.Schildkraut J.J., Gordon E.K., Durell J. Catecholamine metabolism in affective disorders. I. Normetanephrine and VMA excretion in depressed patients treated with imipramine. J. Psychiatr. Res. 1965;3(4):213–228. doi: 10.1016/0022-3956(65)90003-8. [DOI] [PubMed] [Google Scholar]
- 118.Fountoulakis K.N., Kelsoe J.R., Akiskal H. Receptor targets for antidepressant therapy in bipolar disorder: an overview. J. Affect. Disord. 2012;138(3):222–238. doi: 10.1016/j.jad.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 119.Yaniv S.P., Lucki A., Klein E., Ben-Shachar D. Dexamethasone enhances the norepinephrine-induced ERK/MAPK intracellular pathway possibly via dysregulation of the α2-adrenergic receptor: implications for antidepressant drug mechanism of action. Eur. J. Cell Biol. 2010;89(9):712–722. doi: 10.1016/j.ejcb.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 120.Tye K.M., Mirzabekov J.J., Warden M.R., Ferenczi E.A., Tsai H.C., Finkelstein J., Kim S.Y., Adhikari A., Thompson K.R., Andalman A.S., Gunaydin L.A., Witten I.B., Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paul I.A., Skolnick P. IA. Glutamate and depression: clinical and preclinical studies. Ann. N. Y. Acad. Sci. 2003;1003(1):250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- 122.Wang G.L., Wang Y.P., Zheng J.Y., Zhang L.X. Monoaminergic and aminoacidergic receptors are involved in the antidepressant-like effect of ginsenoside Rb1 in mouse hippocampus (CA3) and prefrontal cortex. Brain Res. 2018;1699:44–53. doi: 10.1016/j.brainres.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 123.Gołembiowska K., Zylewska A. Effect of antidepressant drugs on veratridine-evoked glutamate and aspartate release in rat prefrontal cortex. Pol. J. Pharmacol. 1999;51(1):63–70. [PubMed] [Google Scholar]
- 124.Bortolotto Z.A., Fitzjohn S.M., Collingridge G.L. Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr. Opin. Neurobiol. 1999;9(3):299–304. doi: 10.1016/S0959-4388(99)80044-0. [DOI] [PubMed] [Google Scholar]
- 125.Sills M.A., Loo P.S. Tricyclic antidepressants and dextromethorphan bind with higher affinity to the phencyclidine receptor in the absence of magnesium and L-glutamate. Mol. Pharmacol. 1989;36(1):160–165. [PubMed] [Google Scholar]
- 126.Jett J.D., Bulin S.E., Hatherall L.C., McCartney C.M., Morilak D.A. Deficits in cognitive flexibility induced by chronic unpredictable stress are associated with impaired glutamate neurotransmission in the rat medial prefrontal cortex. Neuroscience. 2017;346:284–297. doi: 10.1016/j.neuroscience.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hryhorczuk C., Sharma S., Fulton S.E. Metabolic disturbances connecting obesity and depression. Front. Neurosci. 2013;7:177. doi: 10.3389/fnins.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Duval F., Mokrani M-C., Monreal-Ortiz J.A., Fattah S., Champeval C., Schulz P., Macher J-P. Cortisol hypersecretion in unipolar major depression with melancholic and psychotic features: dopaminergic, noradrenergic and thyroid correlates. Psychoneuroendocrinology. 2006;31(7):876–888. doi: 10.1016/j.psyneuen.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 129.Yang L-M., Yu L., Jin H-J., Zhao H. Substance P receptor antagonist in lateral habenula improves rat depression-like behavior. Brain Res. Bull. 2014;100:22–28. doi: 10.1016/j.brainresbull.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 130.Cirulli F., Francia N., Berry A., Aloe L., Alleva E., Suomi S.J. Early life stress as a risk factor for mental health: role of neurotrophins from rodents to non-human primates. Neurosci. Biobehav. Rev. 2009;33(4):573–585. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grønli J., Bramham C., Murison R., Kanhema T., Fiske E., Bjorvatn B., Ursin R., Portas C.M. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol. Biochem. Behav. 2006;85(4):842–849. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 132.Murakami S., Imbe H., Morikawa Y., Kubo C., Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci. Res. 2005;53(2):129–139. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 133.Swaab D.F., Bao A-M., Lucassen P.J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005;4(2):141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 134.Li W., Li Q-J., An S-C. Preventive effect of estrogen on depression-like behavior induced by chronic restraint stress. Neurosci. Bull. 2010;26(2):140–146. doi: 10.1007/s12264-010-0609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.van West D., Van Den Eede F., Del-Favero J., Souery D., Norrback K-F., Van Duijn C., Sluijs S., Adolfsson R., Mendlewicz J., Deboutte D., Van Broeckhoven C., Claes S. Glucocorticoid receptor gene-based SNP analysis in patients with recurrent major depression. Neuropsychopharmacology. 2006;31(3):620–627. doi: 10.1038/sj.npp.1300898. [DOI] [PubMed] [Google Scholar]
- 136.Kim Y.K., Na K.S., Myint A.M., Leonard B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 137.Maes M., Yirmyia R., Noraberg J., Brene S., Hibbeln J., Perini G., Kubera M., Bob P., Lerer B., Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 2009;24(1):27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 138.Leinonen E., Lepola U., Koponen H., Mehtonen O.P., Rimon R. Long-term efficacy and safety of milnacipran compared to clomipramine in patients with major depression. Acta Psychiatr. Scand. 1997;96(6):497–504. doi: 10.1111/j.1600-0447.1997.tb09953.x. [DOI] [PubMed] [Google Scholar]
- 139.Guaiana G., Barbui C., Hotopf M. Amitriptyline for depression. Cochrane Database Syst. Rev. 2007;(3):CD004186. doi: 10.1002/14651858.CD004186.pub2. [DOI] [PubMed] [Google Scholar]
- 140.Benfield P., Heel R.C., Lewis S.P. Fluoxetine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness. Drugs. 1986;32(6):481–508. doi: 10.2165/00003495-198632060-00002. [DOI] [PubMed] [Google Scholar]
- 141.Silva M.T., Zimmermann I.R., Galvao T.F., Pereira M.G. Olanzapine plus fluoxetine for bipolar disorder: a systematic review and meta-analysis. J. Affect. Disord. 2013;146(3):310–318. doi: 10.1016/j.jad.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 142.Croxtall J.D., Scott L.J. Olanzapine/fluoxetine: a review of its use in patients with treatment-resistant major depressive disorder. CNS Drugs. 2010;24(3):245–262. doi: 10.2165/11203830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 143.Gibiino S., Serretti A. Paroxetine for the treatment of depression: a critical update. Expert Opin. Pharmacother. 2012;13(3):421–431. doi: 10.1517/14656566.2012.652085. [DOI] [PubMed] [Google Scholar]
- 144.Cipriani A., La Ferla T., Furukawa T.A., Signoretti A., Nakagawa A., Churchill R., McGuire H., Barbui C. Sertraline versus other antidepressive agents for depression. Cochrane Database Syst. Rev. 2010;(4):CD006117. doi: 10.1002/14651858.CD006117.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Omori I.M., Watanabe N., Nakagawa A., Akechi T., Cipriani A., Barbui C., McGuire H., Churchill R., Furukawa T.A. Meta-analysis of new generation Antidepressants (MANGA) Study Group. Efficacy, tolerability and side-effect profile of fluvoxamine for major depression: meta-analysis. J. Psychopharmacol. (Oxford) 2009;23(5):539–550. doi: 10.1177/0269881108089876. [DOI] [PubMed] [Google Scholar]
- 146.Ashby C.R., Jr, Kehne J.H., Bartoszyk G.D., Renda M.J., Athanasiou M., Pierz K.A., Seyfried C.A. Electrophysiological evidence for rapid 5-HT₁A autoreceptor inhibition by vilazodone, a 5-HT₁A receptor partial agonist and 5-HT reuptake inhibitor. Eur. J. Pharmacol. 2013;714(1-3):359–365. doi: 10.1016/j.ejphar.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 147.Cui M., Huang C.Y., Wang F. Efficacy and safety of citalopram for the treatment of poststroke depression: a meta-analysis. J. Stroke Cerebrovasc. Dis. 2018;27(11):2905–2918. doi: 10.1016/j.jstrokecerebrovasdis.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 148.Han D., Wang E.C. Remission from depression : a review of venlafaxine clinical and economic evidence. Pharmacoeconomics. 2005;23(6):567–581. doi: 10.2165/00019053-200523060-00004. [DOI] [PubMed] [Google Scholar]
- 149.Watanabe N., Omori I.M., Nakagawa A., Cipriani A., Barbui C., Churchill R., Furukawa T.A. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD006528.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Larsen J.K., Gjerris A., Holm P., Anderson J., Bille A., Christensen E.M., Høyer E., Jensen H., Mejlhede A., Langagergaard A., et al. Moclobemide in depression: a randomized, multicentre trial against isocarboxazide and clomipramine emphasizing atypical depression. Acta Psychiatr. Scand. 1991;84(6):564–570. doi: 10.1111/j.1600-0447.1991.tb03196.x. [DOI] [PubMed] [Google Scholar]
- 151.Norman T.R., Olver J.S. Agomelatine for depression: expanding the horizons? Expert Opin. Pharmacother. 2019;20(6):647–656. doi: 10.1080/14656566.2019.1574747. [DOI] [PubMed] [Google Scholar]
- 152.Patel K., Allen S., Haque M.N., Angelescu I., Baumeister D., Tracy D.K. Bupropion: a systematic review and meta-analysis of effectiveness as an antidepressant. Ther. Adv. Psychopharmacol. 2016;6(2):99–144. doi: 10.1177/2045125316629071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Eisendrath S.J., Gillung E., Delucchi K.L., Segal Z.V., Nelson J.C., McInnes L.A., Mathalon D.H., Feldman M.D. A randomized controlled trial of mindfulness-based cognitive therapy for treatment-resistant depression. Psychother. Psychosom. 2016;85(2):99–110. doi: 10.1159/000442260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Amick H.R., Gartlehner G., Gaynes B.N., Forneris C., Asher G.N., Morgan L.C., Coker-Schwimmer E., Boland E., Lux L.J., Gaylord S., Bann C., Pierl C.B., Lohr K.N. Comparative benefits and harms of second generation antidepressants and cognitive behavioral therapies in initial treatment of major depressive disorder: systematic review and meta-analysis. BMJ. 2015;351:h6019. doi: 10.1136/bmj.h6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jelovac A., Kolshus E., McLoughlin D.M. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467–2474. doi: 10.1038/npp.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smith C.A., Armour M., Lee M.S., Wang L.Q., Hay P.J. Acupuncture for depression. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD004046.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sallach K., Leonhardt M. Acupuncture for treatment of depressive disorders in pain diseases. Nervenarzt. 2018;89(9):986–993. doi: 10.1007/s00115-018-0576-3. [DOI] [PubMed] [Google Scholar]
- 158.Wang X.J., Wang L-L. A mechanism of endogenous opioid peptides for rapid onset of acupuncture effect in treatment of depression. J. Chin. Integr. Med. 2010;8(11):1014–1017. doi: 10.3736/jcim20101102. [DOI] [PubMed] [Google Scholar]
- 159.Chan Y-Y., Lo W-Y., Yang S-N., Chen Y-H., Lin J-G. The benefit of combined acupuncture and antidepressant medication for depression: A systematic review and meta-analysis. J. Affect. Disord. 2015;176:106–117. doi: 10.1016/j.jad.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 160.Sachs M.E., Damasio A., Habibi A. The pleasures of sad music: a systematic review. Front. Hum. Neurosci. 2015;9:404. doi: 10.3389/fnhum.2015.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Penman S., Cohen M., Stevens P., Jackson S. Yoga in Australia: Results of a national survey. Int. J. Yoga. 2012;5(2):92–101. doi: 10.4103/0973-6131.98217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Issakidis C., Andrews G. Service utilisation for anxiety in an Australian community sample. Soc. Psychiatry Psychiatr. Epidemiol. 2002;37(4):153–163. doi: 10.1007/s001270200009. [DOI] [PubMed] [Google Scholar]
- 163.Porsolt R.D., Bertin A., Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- 164.Forbes N.F., Stewart C.A., Matthews K., Reid I.C.J.P. Chronic mild stress and sucrose consumption: validity as a model of depression. Physiol. Behav. 1996;60(6):1481–1484. doi: 10.1016/S0031-9384(96)00305-8. [DOI] [PubMed] [Google Scholar]
- 165.Xu Y., Ku B.S., Yao H.Y., Lin Y.H., Ma X., Zhang Y.H., Li X.J. Antidepressant effects of curcumin in the forced swim test and olfactory bulbectomy models of depression in rats. Pharmacol. Biochem. Behav. 2005;82(1):200–206. doi: 10.1016/j.pbb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 166.Guo S., Wang L., Xie Y., Luo X., Zhang S., Xiong L., Ai H., Yuan Z., Wang J. Bibliometric and visualized analysis of stem cells therapy for spinal cord injury based on web of science and citespace in the last 20 years. World Neurosurg. 2019;132:e246–e258. doi: 10.1016/j.wneu.2019.08.191. [DOI] [PubMed] [Google Scholar]