Abstract
Glioblastoma multiforme (GBM) is the most common primary malignant Central Nervous System cancer, responsible for about 4% of all deaths associated with neoplasia, characterized as one of the fatal human cancers. Tumor resection does not possess curative character, thereby radio and/or chemotherapy are often necessary for the treatment of GBM. However, drugs used in GBM chemotherapy present some limitations, such as side effects associated with non-specific drug biodistribution as well as limited bioavailability, which limits their clinical use. To attenuate the systemic toxicity and overcome the poor bioavailability, a very attractive approach is drug encapsulation in drug delivery nanosystems. The main focus of this review is to explore the actual cancer global problem, enunciate barriers to overcome in the pharmacological treatment of GBM, as well as the most updated drug delivery nanosystems for GBM treatment and how they influence biopharmaceutical properties of anti-GBM drugs. The discussion will approach lipid-based and polymeric nanosystems, as well as inorganic nanoparticles, regarding their technical aspects as well as biological effects in GBM treatment. Furthermore, the current state of the art, challenges to overcome and future perspectives in GBM treatment will be discussed.
Keywords: Brain cancer, pharmaceutical nanotechnology, cancer therapy, lipid-based systems, polymeric systems, inorganic nanoparticles
1. INTRODUCTION
According to the American Cancer Society, cancer is the common name given to a group of more than 100 diseases triggered by uncontrolled growth and spread of abnormal cells. Although the exact and precise cause of cancer remains unknown, several risk factors related to unhealthy lifestyles, mainly, are well-known and preventable, like high alcohol consumption, tobacco use, obesity, physical inactivity, and poor nutrition. Some other cancer risk factors aren’t preventable, such as aging, inherited genetic predisposition, as well as immune and hormonal conditions. Less commonly, cancer can also be caused by exposure to ionizing radiation (i.e., x-rays, gamma rays, alpha particles, beta particles, and neutrons), cancer-causing substances (i.e., aflatoxins, benzene, formaldehyde, vinyl chloride) and infectious agents such as human papillomavirus (HPV), hepatitis B virus (HBV), hepatitis C virus (HCV) and Helicobacter pylori (H. pylori) [1, 2].
Nowadays, cancer is a global problem and do not present uniform neither statistical pattern, varying worldwideaccording to the prevalence of the previously mentioned risk factors. With better life quality and longer life expectancy, cancer incidence could rise fast. In 2018, the International Agency for Research on Cancer (IARC) expected an annual incidence of 18.1 million cancer cases, while 29.4 million new cases are expected for 2040 [3]. The association of health (i.e., higher demands on health systems), social (i.e., better and top-technology medicines are expansive, limiting the access by some social classes despite others) and economic factors (i.e., cancer can considerably weaken patients, so they’re unable to work and generate incomes for their countries, depending, many times, on social assistance programs) make cancer a global burden, that caught the attention of the scientific community, concentrating efforts to obtain better and effective medicines and diffuse as maximum as possible correct preventive actions, to make the cancer impact around the world less significant.
In this review, we will mainly focus on summarizing updated works and the main results related to the use of nanosystems as deliver platforms in GBM treatment. Bibliographical research was done in the following databases: Web of Science, Science Direct, PubMed and Google Scholar, using combinations of “nano*particle”, “glioblastoma multiforme” as keywords (Access May 2020 June 2020), selecting the most relevant articles for each abovementioned nanosystem, published in the last 10 years.
1.1. Glioblastoma Multiforme
Glioblastoma multiforme (GBM) is a stage IV astrocytoma [4] - a type of cancer originated from astrocytes, one type of glial cells found in the brain, varying from stages I (less aggressive and small tumor) to IV (more aggressive, like GBM) - and also the most common and invasive central neoplasia, responsible for about 60% of all primary brain tumors in adults [5]. Around 4% of deaths by neoplasias are attributed to GBM. Although GBM occurs mainly in the brain (60% cases), it can also affect other Central Nervous System (CNS) structures, like brainstem, cerebellum, and spinal cord [6]. GBM occurs mostly from 60 to 64 years of age, more in men than women (1.6:1), as well as Caucasian people despite other ethnicities [7]. The most frequent GBM clinical manifestations are higher intracranial pressure, headaches, and focal or progressive neurological deficits; 25% of patients present seizures, a characteristic symptom of a later stage GBM [8]. Initial diagnoses are made through digital imaging techniques, such as computed tomography and magnetic resonance imaging.
GBM's current therapeutic guidelines recommend a multidisciplinary approach, mainly with surgical removal, associated with radio and chemotherapy, when convenient. However, these treatments are quite invasive and end up weakening patients’ health considerably. Moreover, due to its high invasiveness, the surgical removal of GBM is not curative: invariably, some persistent cells will remain in the brain, which can lead to disease progression or recurrence, and it is necessary to associate with this technique, very often, radio and/or chemotherapy [9]. Although new technologies are being developed for the treatment of GBM, this neoplasm is still characterized as quite lethal and with poor prognosis, since the currently available treatments do not have a curative character, only the role of prolonging patients’ survival time [10]. Despite all advances in the medical field and understanding of GBM, after diagnosis, only 8% of patients can survive up to 2.5 years, and less than 5% of patients survive 5 years [11]. As GBM exhibits high resistance to conventional therapies, mortality rates are usually high for this type of cancer [12].
1.2. Pharmaceutical Nanotechnology
Nanotechnology is a multidisciplinary science born at the early ’20s, and can be defined as new technologies or devices employing components with at least one dimension in nanoscale, usually from 1 to 100 nm; for health destinated products like medicines and diagnostic assays, this size can range up to 1000 nm [13]. Nowadays, nanotechnology represents a promising field and, in the last few years, has revolutionized the health quality with many remarkable applications in the pharmaceutical field, thereby originating a hybrid science known as pharmaceutical nanotechnology. Currently, tools from pharmaceutical nanotechnology are highlighted by improving the pharmacological treatment and repurposing the use of medicines [14].
Recent studies have shown that drug delivery nanosystems (DDN) have been gaining attention in cancer treatment, especially in GBM, because they demonstrated to be efficient alternatives to conventional formulations currently available in the market, besides being capable to optimize the drug delivery to cancer cells, improving the toxicity profile and adverse effects, reducing the systemic toxicity of formulations containing anti-cancer agents [15, 16]. As can be seen in (Fig. 1), a large variety of DDN can be found in literature as a possible therapeutic approach for GBM treatment, reiterating the importance of further studies on this topic. Thus, this review will focus on the analysis and critical discussion of the actual trends in DDN designed to treat GBM and their main advantages, as well as future perspectives and general conclusions.
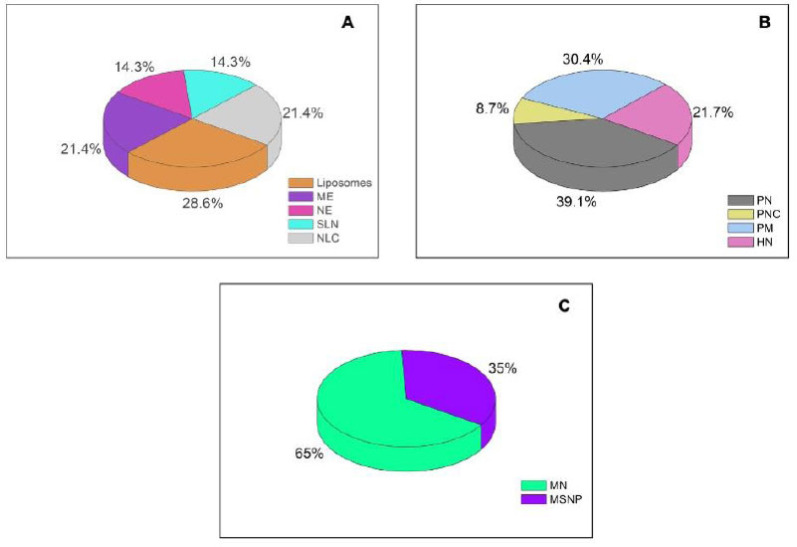
Fig. (1).
Number of publications based on the bibliographical results found in this review, according to the type of nanosystem: A (Lipid-based systems); B (polymeric nanosystems) and C (inorganic nanosystems); ME = microemulsions; NE = nanoemulsions; SLN = solid lipid nanoparticles; NLC = nanostructured lipid carriers; PN = polymeric nanoparticles; PM = polymeric micelles; HN = hybrid nanoparticles, MN = metallic nanoparticles and; MSNP = mesoporous silica nanoparticles. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2. LIPID-BASED DRUG DELIVERY NANOSYSTEMS
Lipidic systems have been widely investigated as DDN to deliver therapeutic molecules across the blood-brain barrier (BBB) on GBM treatment. Their application in the nanomedicine field is own to several advantageous features, such as their biocompatibility and biodegradability (promoted by the materials used in its’ composition), capacity to enhance the solubility and bioavailability of water-insoluble chemotherapeutics, as well as great physicochemical stability [17]. Also, these nanosystems have shown high penetrability in tumor cells, which can be even greater due to their easy surface modification using ligands with high affinity and specificity to some receptors, capable of triggering mediated endocytosis in BBB and receptors overexpressed on tumor cell membranes [18, 19]. Thus, the main lipidic systems designed for this purpose will be focused in this review, such as liposomes, solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), nanoemulsions, and microemulsions. Figure 2 summarizes the main lipid-based DDN approached in this article.
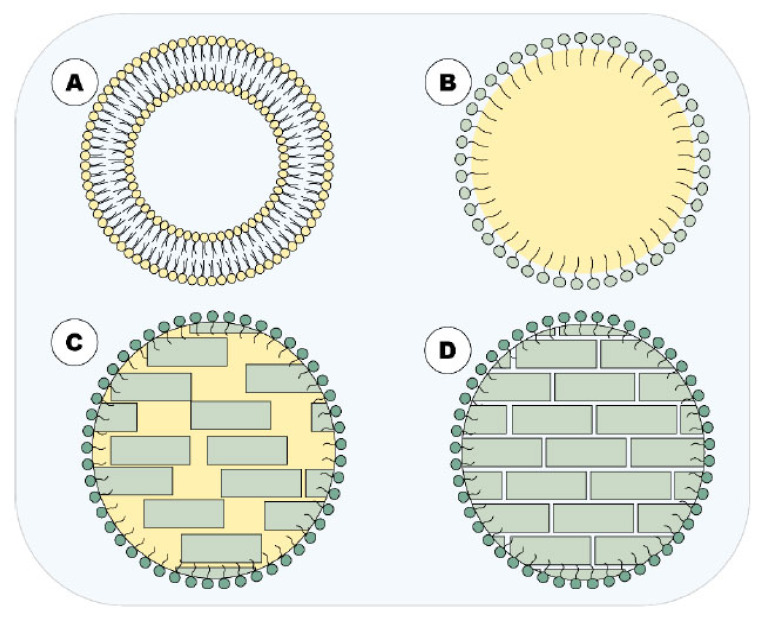
Fig. (2).
Main lipid-based DDN approached in this article. A = liposome; B = NE; C = NLC and; D = SLN. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.1. Liposomes
Liposomes are phospholipid-bilayer vesicles used to deliver drugs into the brain due to their capacity to entrap both hydrophilic and hydrophobic molecules, as well as biocompatibility, biodegradability and mainly by their potential to permeate biological membranes [17, 20, 21].To improve blood circulation time and active targeting they have, they can have their surface modified with polyethylene glycol (PEG) and other targeting ligands, respectively. There are many ways to produce theses nanoparticles, among then the solvent injection and the thin-film hydration followed by either sonication or extrusion methods, which are the most common techniques used to obtain liposomes with small size and polydispersity index [22-24].
Lakkadawala et al. [25] proposed the development of two liposomes containing cell-penetrating peptide (TAT or QLPVM peptides) and transferrin (Tf) for codelivery of doxorubicin and erlotinib (Tf-TAT-LIP and Tf-QLPVM-LIP, respectively) to treat GBM. Transferrin (Tf) is a serum glycoprotein that has been widely used to functionalize nanosystems to improve their transport across BBB and promote increased drug uptake by GBM cells through specific receptors overexpressed in cellular membranes [26]. Both liposomes were produced by thin-film hydration method and presented a mean size of 174.90 ± 4.45 nm and 175.57 ±, polydispersity index (PDI) of 0.254 ± 0.03 and 0.246 ± 0.02 and zeta potential of 15.03 ± 3.94 mV and 14.87 ± 0.53 mV for Tf-TAT-LIP and Tf-QLPVM-LIP, respectively. The authors investigated the antitumoral efficacy of these liposomes using an in vivo brain tumor model and biodistribution assay. After intravenous administration was observed, a higher brain distribution of both dual-functionalized liposomes, with no significant difference between the use of TAT and QLPVM as a penetrating agent. Moreover, these liposomes showed about a 10- to 2.7-fold increase in doxorubicin and erlotinib accumulation. Thus, dual-functionalized liposomes can improve the delivery of chemotherapeutics across the BBB into tumor cells viaa receptor and adsorptive mediated transcytosis pathways.
Guo and co-workers [27] also developed functionalized liposomes to improve doxorubicin transport across the BBB and its accumulation into the tumor microenvironment. The authors used an integrin α-2 (ITGA-2) as a target to overexpressed receptors in GBM cell lines. The liposomes were produced by a thin-film hydration method followed by the extrusion technique, which promoted particle sizes about 110 nm and narrow PDI. Aiming to investigate the anti-GBM potential of this formulation, in vitro cellular uptake, and transportation across the blood-brain tumor barrier (BBTB), which is a disrupted blood-tumor barrier that difficult the drug delivery and accumulation in cancer cells, were performed. ITGA-2 modified liposomes showed higher cellular uptake, approximately 75% to 150%, in GBM cell lines (A172, U87, and U118) than non-specific liposomes. Whereas, functionalized liposomes showed a reduced uptake by normal cells (SVG-P12), indicating their high affinity by receptor overexpressed in GBM cells. Finally, the in vitro BBTB model indicates the capacity of this formulation to bypass BBTB due to the angiogenesis effects induced by GBM.
Jhaveri et al. [28] proposed a way to enhance the delivery of resveratrol using liposomes. For this purpose, the liposomes were produced by a thin-film hydration technique followed by extrusion in 200 nm membranes. The extrusion technique was important to produce liposomes with low sizes (211.2 ± 0.8 nm) and PDI (0.09), besides a high resveratrol entrapment efficiency. In vitro assays using GBM tumor (U87) and normal (HA) cells indicated the higher cytotoxicity capacity of transferrin-targeted resveratrol-loaded liposomes when compared with the unmodified formulation. Besides, they showed a greater selectivity effect on cancer cells. Their selectivity to U87 cells was also observed during cellular uptake by confocal microscopy. The tumor-inhibition study corroborates with the in vitro assay through the significant control of tumor growth when compared with all control groups, suggesting the great potential of this formulation to treat GBM.
A complex formulation was developed by Anilkumar et al. [29] to combine photothermal (PTT) and photodynamic therapies (PDT) in functionalized liposomes with hyaluronic acid for specific bind to CD44 overexpressed receptors in GBM cells. For this purpose, magnetic nanoparticles (photothermal therapy agent) and the photosensitizer indocyanine green (ICG) were incorporated in liposomes during the re-hydration step of the thin-film hydration technique. After the sonication and extrusion process, magnetic liposomes with a particle size of 221.9 ± 16.9 nm and low PDI (0.29 ± 0.03) were obtained. The in vitro and in vivo antitumoral efficacy of this formulation was evaluated with and without irradiation using 808 nm NIR laser at 2W/cm2 at 4 and 5 min, respectively. The laser irradiation process is important to generate cytotoxic reactive oxygen species (ROS) by ICG and the local heating by magnetic nanoparticles, which are the mechanism of action of PDT and PTT, respectively. The in vitro assay showed an enhanced reduction in tumor cell viability after the laser light incidence. Besides, in vivo studies with xenograft mice, the tumor model indicated the high accumulation in tumor tissue and tumor growth inhibition after laser light exposure.
Belhadj and colleagues [30] developed multifunctional doxorubicin-loaded liposomes composed of p-hydroxybenzoic acid and cyclic RGD (c(RGDyK)) peptide to promote specific target to the brain by recognition of dopamine and integrin αvβ3 receptors expressed on the BBB, respectively. The liposomes were developed using the thin-film hydration technique followed by the extrusion method and presented narrow size distribution. In vitro assays indicated the capacity of this multifunctional liposome to increase the doxorubicin cytotoxicity and besides being highly internalized into brain capillary endothelial cells (bEnd.3), GBM cell line (U-87MG) and umbilical vein endothelial cells (HUVECs). Furthermore, in vivo study also indicated the potential of this system to improve the active targeting to GBM and enhance the median survival time, 2.31 folds more than unmodified liposomes.
In another study, Papachristodoulou and colleagues produced liposomes to carry a small molecule, an O6-methylguanine-DNA methyltransferase (MGMT) gene inactivator, to sensitize GBM cells to temozolomide [31]. To improve the brain targeting, the BBB was opened using magnetic resonance image-guided low-intensity pulsed focused ultrasound (LIFU). The liposomes were produced by thin-film hydration technique and extruded to obtain vesicles with 75 nm with narrow size distribution. In vivo study indicated the potential of these liposomes to promote depletion of MGMT gene, reduce the tumor growth and prolonged survival time when temozolomide was used in combination
2.2. Micro and Nanoemulsions
Micro (ME) and nanoemulsions (NE) are colloidal dispersions composed of an aqueous phase mixed with an organic phase and stabilized by surfactants to decrease interfacial tensions between both phases. Although “micro-” terminology is used to designate the micrometric dimension, in the nanomedicine field both micro and nanoemulsions are defined with particle sizes below 500 nm, being ME smaller than NE. The main differences between them are related to their free energy. NE isis produced under energy supply because they are kinetically stable while ME is thermodynamic stable and formed spontaneously [32-36]. They are considered to be used as DDN to target molecules across the BBB to tumor cells owing to their stability, sustained release, and permeability through biological barriers [37-39].
Gadhave and co-workers [40] developed an intranasal teriflunomide mucoadhesive ME for the treatment of GBM. Aiming to understand and optimize the main formulation variables (oil, water, and surfactant mixture volumes) involved in the quality of ME produced by progressive aqueous phase titration method, the authors used a Quality by Design approach, known as Box-Behnken design. This tool made it possible to determine the optimal production condition for desirable features. The optimized formulation showed a particle size of 22.81 ± 0.48 nm, PDI of 0.049 ± 0.01, transmittance percentage of 99.70 ± 0.2% and drug content of 98.88 ± 0.39%. Chitosan was added in ME to promote mucoadhesive properties, and its feature was confirmed by ex vivo. In addition, in vivo studies comparing intranasal and intravenous routes indicated that intranasal drug delivery of ME is also safer and more effective to GBM treatment with reduced risks of kidney and liver toxicity.
The potential of ME to deliver drugs into the brain tumor has also been studied by Shinde et al. [41]. The author developed curcumin-loaded ME functionalized with docosahexaenoic acid (DHA)-rich oil that presents receptor-mediated endocytosis in BBB. ME was prepared through emulsification and showed sizes lower than 20 nm with high stability. In vivo studies were conducted using intranasal or intravenous administration. As noted by Gadhave and co-workers [40], the authors also observed higher drug accumulation of functionalized curcumin-loaded microemulsions into the brain using the intranasal route, about 2.09-fold more than intravenous. This result can be explained by drug delivery through the olfactory pathway and trigeminal nerves. Besides, the formulation improved curcumin accumulation into the brain both by intravenous and intranasal administration when compared with curcumin solution, which indicates great promise for GBM treatment.
As reported, the intranasal route has been studied as a strategic route to deliver nanomedicines into the CNS since the olfactory pathway and transport through trigeminal nerves provide rapid and direct transport to the brain [39-41]. Colombo and co-workers [42] evaluated the potential of this route to delivery kaempferol-loaded NE as an alternative GBM therapy. Thus, to decrease the mucociliary clearance and increase the residence time in the nasal cavity, chitosan was added to form mucoadhesive NE. Thus, the kaempferol-NE was produced by a high-pressure homogenization technique with subsequent chitosan incubation. This system showed a low globule size (180.48 ± 8.37 nm) and PDI (0.211 ± 0.023). In vitro cytotoxicity assay showed a higher reduction of C6 glioma cell viability when compared with non-mucoadhesive NE. Ex vivo study indicated a significant increase in permeation capacity with the addition of chitosan. Furthermore, this high permeation of modified NE was confirmed by in vivo study with high brain levels of kaempferol, about 5- and 4.5-fold higher than free kaempferol and unmodified NE.
Paula and co-workers [43] developed nanoemulsions to combine hyperthermia therapy and PDT to treat GBM. For this purpose, citrate-coated maghemite nanoparticles and chloroaluminum-phthalocyanine were added into NE during their production through the spontaneous emulsification process. Particle size analysis showed low narrow size values (220 ± 20) and PDI of 0.25. The in vitro cytotoxicity assay using U-87MG, BM-MSC, and T98G cell lines showed a reduction of about 15% in cell viability when only hyperthermia therapy was stimulated. When only PDT light treatment was applied, a cell death average of 52% was detected. However, stimulating both PDT and hyperthermia therapy, a total reduction of about 70% was reached. So, the developed lipid system using both therapies represents an interesting strategy for improving GBM therapy.
Azambuja and co-workers [44] developed a cationic NE to deliver CD73-siRNA (NE-siRNA CD73) for GBM treatment via the intranasal route. CD73 is an enzyme overexpressed in cancer cells that induces tumor immune escape. Thus, the inhibition of this enzyme production by small interfering RNA (siRNA) can inhibit tumor progression. Cationic NE was prepared by microfluidization followed by siRNA complexation by adsorption. The developed lipid system showed a particle size of 262.7–601.9 nm and an evidenced siRNA complexation. In vitro assays detected that NE-siRNA CD73 was uptaken by the C6 cell line and reduced cell viability about 30-50%. Moreover, in vivo assays confirmed CD73 silence by siRNA and tumor growth reduction about 60%. Thus, the delivery of siRNA by a lipidic nanosystem through the nasal route demonstrated a great potential to treat GBM.
2.3. Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLN) were developed to overcome some limitations of other lipid-based systems. These nanostructures are composed of a variety of lipids in a solid-state at room and body temperature (e.g., stearyl alcohol, monostearin, Compritol® 888 ATO, and glycerol monostearate) and surfactants (e.g., Tween® 80 and poloxamer 188 [45-48].This composition allows them higher physical stability, sustained release profile, and the capacity to escape from the reticuloendothelial system [49]. Due to their advantages, SLN with and without functionalization have been studied for GBM treatment [18, 50, 51].
Küçüktürkmen and Bozkir [52] developed a cationic SLN to deliver pemetrexed and anti-miRNA for drug and genetic-based GBM treatment. The anti-miRNA used was anti-miR-21 to silence the miRNA that regulates genes responsible for tumor cell proliferation. Also, pemetrexed was used to inhibit tumor proliferation by disrupting folate-dependent metabolic processes. Cationic SLN was prepared using a high-pressure homogenization method in which anti-miR-21 and pemetrexed were added in the lipid phase. Characterization studies confirmed the low particle size 124.9 ± 1.6 nm, PDI of 0.302 ± 0.002, zeta potential of 27.3 ± 1.6 mV, and prolonged-release profile. In vitro studies using the U-87MG cell line demonstrated higher cellular uptake of this system when compared with free pemetrexed, which indicates the ability of these nanoparticles to permeate biological membranes for GBM treatment. However, the author suggested further in vivo studies with higher miRNA complexation to better comprehend its action.
Another SLN for brain tumor treatment was developed by Grillone and co-workers [53]. The author produced a nutlin-loaded magnetic SLN to use an external magnetic field to magnetically deliver the nutlin-loaded SLN across the BBB. So, SLN was produced through solvent evaporation technique, and nutlin-3a and superparamagnetic iron oxide nanoparticles (SPIONs) were incorporated into the organic phase. Characterization studies indicated colloidal stability, the particle size of 180 ± 40 nm, and negative zeta potential (-40.0 ± 1.4 mV). The subsequent analysis using BBB in vitro models demonstrated the capacity of the SLN to transpose the barrier when an external magnetic field was applied, which allowed the greater nutlin-3a cytotoxicity towards U-87MG cells than the free drug. Thus, nutlin- loaded magnetic SLN represented a potential lipid-based system to treat GBM.
2.4. Nanostructured Lipid Carriers
Although SLNs show advantageous features, their main limitations are the low entrapment efficiency and the tendency to expel molecules from their structure during crystallization. This crystallization process does not allow empty spaces for drug storage that makes it difficult to keep drugs inside the SLNs. To overcome this limitation, the second generation of SLNs was developed and named as nanostructured lipid carriers (NLC). NLCs are composed of solid and liquid lipids at room and body temperature as well as lipids with different chain lengths that promote the formation of an imperfect crystal structure [54-60]. Thus, this structure avoids crystallization and allows free spaces for drug storage and improves drug loading, which makes them suitable for drug delivery into brain tumor cells.
To improve the oral bioavailability of CAT-3, an anti-GBM pro-drug, Wang and co-workers [61] investigated its delivery through an NLC system. To enhance CAT3 lipid solubility and increase its entrapment efficiency in NLC, CAT3 was conjugated with oleic acid, a liquid lipid also used to formulate NLC with a less-ordered crystalline structure. The system was developed using hot homogenization, followed by the hot ultra-sonication method. NLC had low particle size (151.3 ± 17.51 nm) with narrow distribution and high values of drug loading and entrapment efficiency, suitable to transpose the BBB. In vitro analysis demonstrated the higher cytotoxicity of this formulation when compared with all controls, with a reduction of C6 cell viability to 29.77% and 10.75% after 24 and 48 hours of treatment, respectively. Furthermore, in vivo pharmacokinetic studies indicated a greater concentration of CAT-3 active metabolites in plasm, being a promising system to deliver antitumoral agents through a non-invasive route.
Optimized paclitaxel loaded NLC functionalized with transferrin was produced by Emami and coworkers [62] using Box-Behnken design, a Quality by Design approach capable of providing information about variables involved with the final NLC quality. The author prepared NLC through the emulsification-solvent evaporation method and investigated the influence of the amount of cholesterol, triolein, and poloxamer 188 in particle size, zeta potential, entrapment efficiency, drug loading and mean release rate. The optimized nanocarriers showed a size of 205.4 nm, zeta potential of 25.7 mV, good entrapment efficiency and mean release rate of 29.3 hours. This formulation allowed a high transferrin coupling efficiency, which is important for its recognition by the transferrin receptor in BBB and tumor cells. In vitro cytotoxicity assay indicated the higher cytotoxic effect of functionalized NLC when compared to unmodified NLC and free paclitaxel, suggesting its potential to GBM therapy.
Another study that used NLC to improve GBM treatment was performed by Zhang and co-workers [63]. The authors produced a dual functionalized NLC to deliver temozolomide and vincristine by modifying the NLC surface with lactoferrin and RGD peptide, to provide specific recognition by receptors overexpressed in BBB and GBM cells. For this purpose, nanocarriers were developed using the solvent diffusion method. The developed formulation showed a size of 139.3 nm, PDI of 0.187, and demonstrated a high ability to load both temozolomide and vincristine. In vitro assays indicated sustained-release behavior, high cytotoxicity, synergy effects, and greater cellular uptake than unmodified NLC. Furthermore, in vivo tissue biodistribution and antitumor effect studies corroborated with cell cultures assays, demonstrating higher accumulation and tumor reduction when dual functionalized NLC was administered intravenously. Moreover, low systemic toxicity was observed during the treatment with this nanocarrier, suggesting its potential to improve brain cancer treatment with low side effects for patients.
Table (1) summarizes the studies found in the literature about lipid-based nanosystems for GBM treatment and their main characteristics.
Table 1.
Studies designing lipid-based drug delivery nanosystems for glioblastoma multiforme treatment and their main characteristics.
| Nanosystem | Composition | Surface Modification | Size (nm) | Therapeutic Molecule/Drug | In vitro Model | In vivo Model | Route of Administration | Refs. |
|---|---|---|---|---|---|---|---|---|
| Liposomes | DSPE-PEG(2000)-NHS and cholesterol | 1) TAT and transferrin2) QLPVM and transferrin | 1) 174.90 ± 4.452) 175.57 ± 4.57 | Doxorubicin and Erlotinib | U-87MG, bEnd.3 and glial cells | Orthotopic brain tumor model in nude mice | Intravenous | [25] |
| Liposomes | DOPC and DSPE-PEG(2000)-COOH | ITGA-2 | 110 | Doxorubicin | A172, U-87MG, U118, SVG-P12 and HBMVEC | - | - | [27] |
| Liposomes | DSPE-PEG(2000), DOPE and CHEMS | Transferrin | 211.2 ± 0.8 | Resveratrol | U-87MG and HA | Subcutaneous xenograft model in Athymic NCr-nu/nu nude mice | Intravenous | [28] |
| Liposomes | DSPE, Cholesterol, DDAB, and PEG(5000) | Hyaluronic acid | 221.9 ± 16.9 | Magnetic nanoparticles and photosensitizer indocyanine green | U-87MG | Subcutaneous xenograft model BALB/c nude mice | Intravenous | [29] |
| Liposomes | HSPC, cholesterol, mPEG-DSPE, c(RGDyK)-PEG-DSPE, pHA-PEG-DSPE | C(RGDyK) | - | Doxorubicin | U-87MG | Orthotopic brain tumor model in mice | [30] | |
| Liposomes | DOPC, cholesterol, DSPE-PEG | - | 72 nm | O6-methylguanine-DNA methyltransferase | SMA-497 | Orthotopic brain tumor model in mice | [31] | |
| ME | Maisine 35–1, Labrasol and Transcutol HP | - | 22.81 ± 0.48 | Teriflunomide | U-87MG | Biodistribuion studies in Swiss albino mice | Intranasal | [40] |
| ME | Tween 80, Capmul MCM | docosahexaenoic acid | < 20 nm | Curcumin | U-87MG | Pharmacokinetic and brain uptake studies in Sprague-Dawley rats | Intranasal and intravenous | [41] |
| NE | Egg-lecithin, polysorbate 80, medium-chain triglycerides and chitosan | - | 180.53 ± 4.90 | Kaempferol | C6 | Brain distribution Wistar rats | Intranasal | [42] |
| NE | Medium-chain triglycerides, soy phospholipids and poloxamer 188 | - | 220 ± 20 nm | citrate-coated maghemite nanoparticles and chloroaluminumphthalocyanine | BM-MSC, U-87MG, and T98G | - | - | [43] |
| NE | Lecithin, medium-chain triglycerides, DOTAP | - | 392.7 ± 19.1 and 273.9 ± 13.6 | CD73-siRNA | C6 and rat astrocyte primary cultures | OrthotopicWistar rats | Intranasal | [44] |
| SLN | Trimyristin, DDAB and Tween 80 | - | 124.9 ± 1.6 | Pemetrexed and anti-miR-21 | U-87MG | - | - | [52] |
| SLN | Cetyl palmitate and DSPE-PEG(5000) | - | 180 ± 40 | Superparamagnetic iron oxide nanoparticles and Nutlin-3a | U-87MG and bEnd.3 | - | - | [53] |
| NLC | Oleic acid, Compritol 888 ATO, Lipoid S75, Tween 80 and Poloxamer 188 | - | 151.3 ± 17.51 | CAT3 | C6 | Bioavailability studies in Sprague Dawley rats | Oral | [61] |
| NLC | Cholesterol, triolein, stearyl amine, soy lecithin and poloxamer 188 | Transferrin | 205.4 ± 11 | Paclitaxel | U-87MG | - | - | [62] |
| NLC | SPC, Compritol 888 ATO, chemophor ELP, soya lecithin, DEPE-PEG(2000), DDAB | Lactoferrin and RGD | 139.3±4.9 | Temozolomide and vincristine | U-87MG, A549 and T98G | Xenograft model on Balb/c nude mice | Intravenous | [63] |
Abbreviations: ME – microemulsion; NE – nanoemulsion; SLN – solid lipid nanoparticle; NLC – nanostructured lipid carrier; DSPE-PEG(2000)- polyethyleneglycol-carbamyl distearoylphosphatidyl-ethanolamine; DOPC - 1,2-dioleoyl-sn-glycero-3-phosphocholine; TAT – cell penetrating peptide ; ITGA-2 - integrin alpha-2; DOPE - 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; CHEMS - cholesteryl hemisuccinate; DDAB - dimethyldioctadecyl ammonium bromide; DOTAP -, 2-dioleoyloxy-3-(trimethylammonium)propane; DDAB - dimethyldioctadecylammonium bromide; SPC - soybean phosphatidylcholine. RGD - Arg-Gly-Asp, CAT-3 - 13a-(S)-3-pivaloyloxyl-6,7-dimethoxyphenanthro[9, 10-b]-indolizidine;
3. POLYMERIC NANOSYSTEMS
Polymer-based DDNs can be obtained using polymers or monomers of synthetic as well as the natural origin, preferably with biodegradable characteristics, thus, they originate biocompatible by-products, a feature that led them to be extensively explored as pharmaceutical DDNs. The most frequent biodegradable polymers used in development of polymeric nanoparticles for GBM treatment are chitosan [64, 65], poly lactic-co-glycolic acid (PLGA) [66] and poly(ɛ- caprolactone) (PLC) [67]. There are different methods for obtaining polymeric nanocarriers such as nanoprecipitation, salting-out, emulsification (solvent evaporation and solvent diffusion), supercritical fluid technology, and ionic gelation. The choice of method depends on the properties of the encapsulated drug and the desired application [17, 68, 69]. Additionally, polymer-based nanocarriers can have their biopharmaceutical properties modulated through surface modification by adding ligands such as antibodies, proteins, which collaborate in specific tissue targeting and drug deposition [70, 71]. (Fig. 3) summarizes the main polymeric DDN as an approach to GBM treatment, discussed in this article.
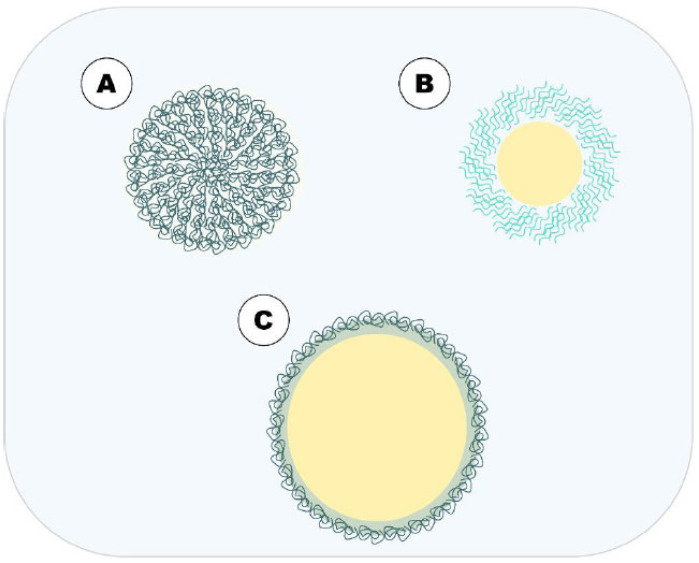
Fig. (3).
Main polymer-based DDN approached in this article. A = polymeric nanoparticles; B = polymeric micelles and; C = HN.(A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.1. Polymeric Nanoparticles
Polymeric nanoparticles are colloidal systems, characterized by its small particles size ranging from 1 to 1000 nm [72, 73]. According to their composition, they are presented as nanospheres or nanocapsules [68], where the drug is dispersed in the polymeric matrix or encapsulated in polymeric capsules of lipid core, respectively [69]. These systems have been used mainly due to the controlled release of drugs, the ability of specific targeting tissues, besides improving physical-chemical properties inherent to the drug such as stability, solubility, bioavailability, and reduction of systemic toxicity [69, 72-76]. In the treatment of GBM, polymeric nanoparticles show advantages in terms of size and safety, having a small size, and can be made of biodegradable polymers that facilitate the drug release from these particles, that promote higher bioaccumulation in the tumor through the enhanced permeation and retention effect (EPR) as well as safe use. Another remarkable property that made polymer-based nanosystems be extensively explored in anti-GBM therapy is the ease of modifying its surface by adding ligands that can improve interaction with specific cells through structures like receptors and other molecules expressed on the GBM cell membranes. However, the main disadvantage of polymer-based DDN against other DDN – for example, lipid-based ones - is the difficulty of crossing BBB by passive diffusion, since the brain matrix is quite dense, preventing the diffusion of the drug that leads to insufficient drug concentrations for a correct treatment [18, 77, 78].
Etoposide acts by inhibition of topoisomerase II and has been explored in nanosystems to improve its anticancer activity, as well as facilitate its permeation across BBB for the treatment of GBM [79-81]. PLGA nanoparticles prepared by emulsification and solvent diffusion method crosslinked with lactoferrin (Lf) and folic acid (FA) were used for transporting etoposide [79]. The Lf/FA/PLGA nanoparticles (NPs) demonstrated in vitro that etoposide release was more sustained than that of FA/PLGA NPs and PLGA NPs, possibly due to the surface modification. All nanoparticles tested presented considerable toxicity for human brain-microvascular endothelial cells (HBMEC) human astrocyte (HA), reducing cell viability by 83.5 ± 3.6% and 88.7 ±2.7%, respectively; what can be explained by etoposide high toxicity and the surface modification improved cellular internalization. For U87MG cells, the Lf/FA/PLGA NPs showed great internalization, reducing cell viability compared to free etoposide, 18.94 ± 5.4%, and 37.3 ± 3.2%, respectively; this antiproliferative activity of nanoparticles may be due to the binding in folate receptors overexpressed by U87MG cells. Thus, the crosslinking of PLGA NPs with lactoferrin and folic acid improved the anti-cancer activity of etoposide, providing targeted delivery to GBM cells and sustained drug release.
MPEG-PCL nanoparticles were used to encapsulate different anticancer drugs as etoposide, carmustine, and doxorubicin, and crosslinked with wheat germ agglutinin (WGA) and folic acid (FA) to anti-GBM activity against U87MG cell in vitro [80]. These nanoparticles were obtained by the solvent emulsification-evaporation method. Two polymer chain lengths (PCL), denominated PEPC1 NP (shorter chain) and PEPC2 NP (longer chain), were evaluated, showing particle size 157.5 ± 3.1 and 166 ± 9.4 nm, respectively. The drugs had better encapsulation efficiency and cumulative release (pH 5.0) in PEPC1. The process of crosslinking with WGA and FA was performed with PEPC1 (WFNPs): this process increased particle size to 223.7 ± 14.1 nm and zeta potential suffered no significant changes. After the development of WFNPs, the etoposide, carmustine, and doxorubicin were encapsulated and the best-controlled release profile was found at pH 5.0 with release up to 30 days. In human endothelial cell lines, free drugs showed a decrease in viability, while drugs loaded WFNPs showed increased viability, demonstrating that WFNPs reduce drug toxicity. Additionally, WFNPs showed increased permeability in these cells. In U87MG cells, the drugs-loaded WFNPs lead to an increased antiproliferative activity, accentuated by WGA and FA. Thus, dual targeting on the surface of NPs can be promising in targeting therapy of GBM.
Malinovskaya et al. [82] and Maksimenko et al. [83] evaluated doxorubicin (Dox)-loaded PLGA nanoparticles coated with poloxamer 188 (Dox-PLGA + P188) for the treatment of GBM in vitro/in vivo, and Pereverzeva [84] evaluated acute/chronic toxicity of these nanoparticles. The Dox-PLGA nanoparticles were prepared by double emulsification (w/o/w) and solvent evaporation methods and marked with Cyanine 5.5 amine (Cy5.5) fluorescent dye, which is not caused physical-chemical changes to these nanoparticles. Dox-PLGA-Cy5.5 showed better release in pH 4.5 than pH 7.4. These NPs lead to concentration-dependent cytotoxicity: the higher the Dox concentration, the lower the U87MG cell viability. The Dox-PLGA-Cy5.5 showed better cell internalization when compared to NPs without Dox, which is possibly attributed to the presence of P188 surfactant on these nanoparticles’ surface [82]. These Dox-PLGA produced by Maksimenko [83] in the pilot-scale were not considered haemotoxic in assays of blood coagulation time, platelet activation, and hemolysis. In anti-tumor activity tests using the 101.8 GBM model, Dox-PLGA pilot-scale + P188 presented a better performance when compared to the free drug. In the acute toxicity test, no statistical difference was observed between free Dox and Dox-PLGA nanoparticles. In chronic toxicity assay, these Dox-PLGA nanoparticles could decrease the toxic effects of free Dox, and the crosslinking with P188 leads to a reduction in cardiotoxicity when compared to free Dox. In this way, the safety profile of Dox could be improved by the nanoencapsulation process [84].
Temozolomide (TMZ) is the first-line chemotherapeutic drug used for GBM treatment and presents high toxicity, systemic side effects, and low permeability through BBB. The use of TMZ-loaded poly (lactic-co-glycolic acid) (PLGA) nanoparticles, functionalized with a monoclonal antibody (OX26) for transferrin receptor (TfR) has been described [12]. These TMZ-PLGA NPs were prepared by the solvent emulsion-evaporation technique and had its surface modified by maleimide-PEG to allow the covalent bonding of OX26 mAb. These nanoparticles presented particle size below 200 nm, with homogeneous size distribution and negative surface charge, characteristics that can contribute to the passage through BBB associated with functionalization for binding specific to TfR (active transportation). The mAb-PLGA NPs encapsulated 48% of TMZ, while 44% for non- functionalized NPs. In in vitro release by dialysis bag method, TMZ release rates were higher for non-functionalized NPs (98 ± 2% after 9 days) when compared to mAb-PLGA NPs (78 ± 2% at 20 days). The mAb-PLGA NPs were internalized in cells (U251, U87MG, and NHA), probably by endocytosis mechanism, preventing TMZ efflux from cells. The use of a mAb improved cellular uptake due to the TfR target, suggesting that nanoparticles with mAb can permeate more easily without causing damage to the BBB, as well as a reduction in cytotoxicity compared to non-functionalized NPs, which can be justified by the more controlled release of TMZ from mAb-PLGA NPs.
Sayiner et al. [85] also evaluated TMZ-loaded PLGA nanoparticles incorporated in a thermoreversible hydrogel. The preparation was done by the solvent emulsification-evaporation method. The smallest particle size obtained was 164.40 ± 4.24 nm and the largest 235.50 ± 24.96 nm; all formulations showed negative zeta potential values, in agreement with previous reports [12]. The free TMZ incorporated in the hydrogel was released 93% in 8h, while the TMZ-PLGA nanoparticles released only 10% in 12h, followed by sustained release up to 60 days, with 50% of TMZ released at the end of experiments. Among the three nanoparticles tested (A2, A2-5, A2-10), A2-10 demonstrated greater cytotoxicity to RG2 cells – the formulation with higher encapsulation efficiency and smallest particle size. When the cytotoxicity of the nanoparticles incorporated in the Pluronic 127 hydrogel was evaluated, formulation A2 reduced cell viability to 32 ± 3%, showing to be more cytotoxic. Thus, the thermoreversible hydrogel was useful in modulating the release of TMZ and its cytotoxicity and can be an adjuvant alternative in treatment for GBM.
Polymeric nanoparticles functionalized with folic acid (FA) encapsulating TMZ were coated using magnetite (Fe3O4 - SPION). The copolymer used was poly (ethylene glycol)-poly (butylene adipate)-poly (ethylene glycol) (PEG-PBA-PEG) to better protect the drug and promote stability in biological fluids. These TMZ-SPION-PEG-PBA-PEG nanoparticles were prepared by the nanoprecipitation method. TMZ showed sustained release above 70% after 48h for both nanoparticles functionalized or not with FA. Cellular uptake was evaluated through intracellular ferric iron concentration; control nanoparticles showed around 60 pg/cell on both cell lines and TMZ-SPION-PEG-PBA-PEG- FA showed 157.39 ± 3.4 (C6) and 67.7 ± 1.57 (OLN-93) pg/cell and exhibited higher cytotoxicity when compared to the than without FA. The TMZ encapsulated in polymeric FA- functionalized nanoparticles, as evidenced by different assays with the C6 cell line, shown increased cytotoxicity, decreased anti-proliferative activity of tumor cells, increased levels of apoptosis and necrosis compared to free TMZ. The nanoparticles without TMZ showed no damage to the tested strains [86].
Another monoclonal antibody tested on GBM was bevacizumab (BEV), which exhibits anti-angiogenic activity by binding to the extracellular portion of vascular endothelial growth factor (VEGF) receptor, expressed in tumor cells and frequently overexpressed in aggressive and metastatic GBM [87, 88]. BEV-loaded PLGA nanoparticles were obtained as a possible nose-to-brain administration. The nanoparticles presented particle size below 200 nm, monodispersed size distribution as well as negative surface charge, in addition to functionalization efficiency of 82.47 ± 0.56% and BEV release around 14% [89]. This administration aims to improve the brain bioavailability of the drug and decrease systemic toxicity. After 7 days of IN treatment, BEV was quantified, showing greater accumulation in the brain (5400 ± 2313 ng/g tissue) from PLGA NP and 1346 ± 391 ng/g tissue for free BEV. Only free BEV was found in the blood, lung, and liver at significant levels. BEV-loaded PLGA nanoparticles exhibited greater area under the curve to the last concentration (AUClast), maximum concentration (Cmax) and the last concentration (Clast) compared to free BEV brain tissue. These results suggest that the nanoparticles presented a greater capacity for penetration and improved BEV bioavailability in the brain. Both BEV-PLGA nanoparticles and free BEV showed a similar reduction in tumor growth during the treatment after two IN administrations. However, the quantification of human VEGF was reduced after BEV-PLGA nanoparticle treatment and only these were found in brain tissue after the 14-day treatment, whereas free BEV was found in the lung and liver in high concentrations. The absence of toxicity in non-target organs (lung and liver) was confirmed by histological analysis. The results suggested that the use of BEV as an anti-angiogenic agent was effective in reducing GBM through a non-invasive route [87].
Polymeric nanocapsules (PNC) were used to deliver methotrexate (MTX) against two GBM cell lines, C6 rat glioma [90], and GL261 murine glioma [91]. MTX is a drug that acts as an enzyme inhibitor of purine precursors and is used as a chemotherapeutic drug for brain tumors. In both models, MTX-loaded PNC was prepared by the solvent emulsification-evaporation method and showed similar physical- chemical characteristics, with particle size below 200 nm, negative zeta potential, and PDI ≤ 0.1. After 24h, MTX-PNC showed a reduction in C6 cells viability of 43%, against 30% for MTX in solution at the same concentration. Both samples were able to interfere in the C6 cell cycle, reducing the formation of G2/M, . The antiproliferative effect was also evaluated against the GL261 cell line, with an IC50 of 0.21 ± 0.004 μmol/L. MTX-PNC demonstrated an increase in cell death due to early apoptosis through caspase-3 enzymatic activation and by inhibition of the anti-apoptotic protein BCL2. In in vivo assays, the formulations were applied intraperitoneally for C6 induced model; MTX-PNC proved to be more efficient in reducing the tumor volume (98 ± 32 mm3) compared to MTX in solution (200 ± 37 mm3). When administered orally in GL261 in vivo model, MTX-PNC showed a greater reduction in tumor volume compared to MTX in solution. This improvement seen on MTX-PNC may be correlated with its ability to cross the blood-brain barrier more easily [90, 91].
3.2. Polymeric Micelles
Polymeric micelles are formed by the spontaneous association of amphiphilic block copolymers, forming a hydrophilic core-shell with hydrophobic nucleus structure [92, 93]. They can be obtained by the methods of direct dissolution, solvent evaporation, film-hydration, and dialysis, mostly [94, 95]. They have a small particle size from 10 to 1000 nm, can improve the water solubility of drugs [94, 96], modulate the release kinetic, and decrease the toxicity of chemotherapeutic drugs often used in GBM treatment [93, 96]. Micelles can have very small particle sizes, even smaller than other lipid-based colloidal carriers like liposomes and polymeric nanoparticles. Due to this, micelles are the DDN that can better exhibit the EPR effect in solid tumor tissues, such as GBM, and deliver the drug to the tumor more effectively. Furthermore, lipids or surfactants used to obtain micelles can be conjugated with ligands to perform brain-targeting and active transport across BBB, which contributes to obtaining an innovative and effective alternative to currently available treatments of GBM. However, micelles have technical limitations such as encapsulation of hydrophobic drugs only; when they do not have a modified surface, they have a higher chance of being opsonized in the bloodstream and trapped in the spleen or liver, which would make it difficult to release the drug in the brain and even cause some side effects decurrent to lipid deposition in this organs [18, 94, 97].
Micelles encapsulating two tyrosine kinase inhibitors (TIKs), Dasatinib and Crizotinib, were proposed for GBM treatment, due to the difficulty of these drugs in permeating the BBB [98]. These micelles were obtained by direct dissolution of poly (styrene-co-maleic acid) (SMA) in an aqueous phase containing the TIKs; the drugs were encapsulated isolated (SMA-D or SMA-Cr) and associated (SMA-Co). The SMA-D presented smaller particle size than SMA-Cr (below 100 nm), both had negative surface charge and sustained release at pH 7.4, SMA-D showing less release compared to SMA-Cr (39.7 and 53.6%, respectively). The micellar formulations did not demonstrate better cytotoxic performance against the 6 cell lines (U87, LN-18, U373, A172, NZG0906, and NZG1003) tested; when using SMA-Co, the cytotoxicity was accentuated. However, the combination of the two mentioned free TIKs was more cytotoxic. Against U87 cells, Cr free, SMA-Cr, and SMA-Co reduced cell proliferation by 50%, causing cell death by apoptosis. The combined free TIKs and SMA-Co showed antiangiogenic action in all tested cells and were more efficient in preventing the migration and invasion of the cells, especially the highly invasive LN-18 cell line. Although SMA-D has not shown the best in vitro results, it was the one that most reduced tumor growth (GL261) in vivo, around 80%, followed by SMA-Co and free combined TIKs. Micellar formulations containing TIKs proved to be more efficient than their free forms (combined or isolated) for the treatment of GBM, justifying that the encapsulation process can improve the anti-tumor efficiency of these drugs.
Honokiol (HNK) is a polyphenol found in Magnolia Officinalis, with apoptosis-inducing properties. HNK has been associated with Gem-C12 in surface-modified hyaluronic acid (HA-M) micelles for synergistic effect as anti-GBM [99]; HA was used as a ligand to GB44 glycoprotein, overexpressed in GBM. It was found that cellular uptake by U87MG cells was more intense for HA-M than free drugs or HA alone. In all evaluated concentrations (0.1 to 50 μM), HA-M containing GemC12 and HNK and conventional micelles showed greater cytotoxicity compared to free drugs, with IC50 values 5.31, 8.32 and 17.15 μM respectively. HA-M also demonstrated greater tumor growth inhibition in vivo, followed by conventional micelles and free drugs; Additionally, greater accumulation in the tumor region was found for HA-M, suggesting that the modification with HA attributed effectiveness in crossing to BBB and in penetrating the GBM tumor cells. Also, HA-M improved the pharmacokinetic parameters of GemC12 and HNK.
Verteporfin (VP) is a derivative of benzoporphyrin, a very hydrophobic molecule, and Yes-associated protein (YAP) inhibitor, expressed in tumors like GBM. VP was encapsulated in micelles of poly(ethylene glycol)-poly(β-amino ester)- poly(ethylene glycol) (PEG-PBAE-PEG) triblock copolymer [100] and tested against GBM cell lines. Two types of micelles were prepared to encapsulate the PV, spherical (sVPM) and filamentous (fVPM) micelles, using the nanoprecipitation method. For tumor cells, both micellar formulations showed high uptake efficiency, with uptake concentration-dependent. In cytotoxicity assays, sVPM showed greater cytotoxicity at concentrations 62.5 and 125 nM for both tumor cell lines; fVPM was not considered cytotoxic for GBM1A at 62.5 nM, only at the concentration of 125. Although it did not demonstrate greater cytotoxicity in vitro, fVPM showed a longer half-life and higher accumulation in GBM tumor in vivo.
Epirubicin (Epi) is an anthracycline, a potent anti-GBM drug, however, it has high efflux mediated by P-glycoprotein and low penetration through BBB. Thus, Epi was encapsulated in polymeric micelles (Epi/m) by dialysis method, which was functionalized with cyclic Arg-Gly-Asp (cRGD) peptide for integrin targeting [101]. cRGD-Epi/m showed less cytotoxicity compared to free Epi after 48h of contact with U87MG cells monolayer; although cRGD-Epi/m demonstratED high internalization by endocytosis, slow drug release may occur. In U87MG spheroids, it was observed that cRGD-Epi/m showed greater fluorescence intensity due to higher penetration into cells than Epi/m, in all 3 evaluated times. Functionalized Epi-micelles was able to reach the nucleus of the cells, differently from the free form of Epi that was accumulated in the cytoplasm. cRGD-Epi/m further reduced tumor growth in vivo, as verified by bioluminescence and fluorescence. This result can be correlated to the cRGD peptide, that promoted transcellular penetration mediated by αvβ3/αvβ5 integrin. Thus, cRGD conjugation in the micelles contributed to enhanced penetration and accumulation of Epi, increasing its anti-tumor efficiency.
cRGD was also conjugated in PM encapsulating (1,2-diaminocyclohexane) platinum (II) (DACHPt), a platinum-based drug used against GBM, that have high systemic toxicity. These micelles were obtained by the dialysis method [102]. Functionalized micelles containing the drug were denominated cRGD/m and demonstrated rapid cellular uptake in U87MG cells, and concentration-dependent cytotoxicity, possibly due to the αvβ3/αvβ5 integrins target, compared to micelles not labeled with peptide. cRGD/m demonstrated a greater reduction of tumor volume in vivo and lower bioluminescence in the cranial region compared to oxaliplatin, the antitumor drug used as a standard. The better performance of cRGD/m may be due to the overexpression of integrins in U87MG cells. Thus, the modification of the surface of the PM with cRGD promoted better antitumor action in vitro and in vivo in doses lower than oxaliplatin, being a potent candidate for anti-GBM DDN.
The peptide c(RGDyK) (cyclic Arg–Gly–Asp-D- Tyr-Lys) was associated with PM encapsulating another peptide sPMI (Ac-1Thr-2Ser-3Phe-cyclo(4R8-5Glu-6Tyr-7Trp-8 Ala-9Leu-10Leu-11S5)-NH2 [103], an antagonist of MDM2 and MDMX, deregulated proteins that inhibit p53, triggering oncogenesis [104]. The PM (RGD-M/sPMI micelle) was prepared by the film-hydration method. RGD-M/sPMI micelles showed better release in acidic medium and were stable in the in vivo simulated extracellular medium, with no sPMI leakage. RGD-M/sPMI micelles showed antiproliferative action against U87MG cells similar to the MDM2 inhibitor, Nutlin-3, used as a standard, possibly due to the interruption of the cell cycle and increased induction of apoptosis. These effects were lower in isolated sPMI and Nutlin-3. In vivo assays using RGD-M/sPMI + TMZ (10 mg/kg + 50 mg/kg) showed more reduction in tumor volume and longer survival time (70 days) compared with the other groups, that were less than 59 days. Besides, 10 mg/kg RGD-M/sPMI micelles showed greater expression of p53. The combined therapy of RGD-M / sPMI peptides as p53 activators, together with TMZ, was able to promote a remarkable anti-GBM effect by reducing the effective dose of TMZ, which would possibly reduce its systemic toxic effects.
The cyclic peptide c(RGDyK) was conjugated to micelles containing PTX (c(RGDyK)-PEG-PLA) for GBM treatment, prepared by the film-hydration method. This system showed a small particle size (35 nm) along with PTX sustained release of 80% in 72h [95]. c(RGDyK)-PEG-PLA micelles were able to increase the cytotoxicity of PTX by 2.5 times and through the enhanced permeability and retention (EPR) effect, they accumulate in the tumor tissue, which contributes to better anti-tumor effect of PTX and increases in vivo survival rates, compared to non-targeted micelles and non-encapsulated PTX. As in other studies, c(RGDyK) showed higher binding affinity to U87MG cells due to the high expression of integrins by these cells, which contributes to the targeting and controlled release of the drug [102, 103].
Xu et al. [105] evaluated the synergy of two anti-cancer drugs, PTX and TMZ, encapsulated in mPEG-PLGA NPs (PTX/TMZ-NPs) against GBM cell lines. The NPs were prepared by double emulsification/solvent evaporation and had a particle size of 206.3 nm and sustained release. In the in vitro cytotoxicity assay, PTX/TMZ-NPs showed greater inhibition for U87 and C6 cells in 48 h after administration, extended until 72 h, as well as a higher apoptosis index compared to NPs with isolated drugs or with the mixture of free drugs. According to the authors, the inhibition time is probably correlated with the sustained drug release from the NPs. The xenographic model in BALB/c nude mice was used to assess the antitumor effect in vivo and PTX/TMZ-NPs demonstrated inhibition growth and volume of the tumor. In this way, the PTX and TMZ drugs act synergistically in the treatment of GBM and this synergy can be improved through encapsulation in NPs.
Chu et al. [106] developed TMZ butyl ester (TBE)-loaded NPs, functionalized with ephrin type-A receptor 3 tyrosine kinase antibody (anti-EPHA3) as a non-invasive – intranasal - administration and GBM-targeting treatment. This formulation was prepared by the solvent emulsion/evaporation method originating particles with a size of 145.9 nm, negative zeta potential, and sustained release profile for up to 48 h. To assess the in vitro targeting of NPs, C6 cells expressing the EPHA3 receptor were used and data shows an increase in EPHA3-TBE-NP cellular uptake, demonstrating a reduction in cell viability (25.76%) while conventional TBE-NP showed cell viability greater than 40%. In the in vivo assays, functionalized NPs accumulated mostly in the brain region after intranasal administration and showed increased survival rates of animals with GBM for 26 days. Thus, the functionalized formulation is a promising alternative for the treatment of GBM by the nose-to-brain route that reduces the systemic distribution of the drug, fact that can reduce its side effects due to systemic toxicity.
In different types of tumors, microRNAs (miRNA) expression dysregulation occurs, and in GBM, miR-21 and miR-10b are correlated with tumor progression, proliferation, and resistance to conventional marketed treatments. It is known that using antisense miRNA (antagomiRs) can assist in tumor suppression. In this way, antagomiR-21 and antogomiR-10b were encapsulated in NPs - to prevent their degradation in plasma -, that were functionalized using the cRGD peptide to improve their internalization in tumor cells via the integrin receptors [107]. These NPs were obtained by double emulsion/solvent evaporation technique and showed a smaller size than 200 nm, with a negative surface charge. In the in vitro assay using U87MG and Ln229 cells, there was an increase in cellular internalization of functionalized NP. Also, it was verified in xenograft tumor in mice model that pretreatment with functionalized NPs + TMZ considerably reduced the tumor volume, demonstrating that NPs containing antagomiRs can chemosensitize cells to reduce doses of TMZ in GBM.
3.3. Hybrid Nanoparticles
The association of delivery systems such as polymeric nanoparticles and lipid-based systems allowed the formation of hybrid nanoparticles (HN) [108, 109]. They usually have a polymeric hydrophobic nucleus coated with a lipid layer, which can add to the surface different ligands that aim at specific targeting. In this way, they take advantage of both systems to provide improvements in the delivery system for the treatment of GBM [110-112].
HN – as obvious as it can sound - have advantages of both systems that originated them – polymeric nanoparticles and liposomes – aiming to reduce the limitations of these isolated systems in the treatment of cancers. This system can encapsulate both hydrophilic and hydrophobic drugs, more than one drug. HN can also improve the release profile and bioavailability of some drugs that haven’t possess good in vitro properties in isolated polymeric nanoparticles or liposomes. As happens for lipid and polymer-based systems isolated, HN can be combined with one or more ligands to promote active targeting and assist in the BBB penetration [110, 113, 114]. For example, among the works about functionalized polymeric systems loaded with PTX, in vivo tests showed that micelles presented the highest survival rate (48 days), followed by hybrid nanoparticles (42 days) and polymeric nanoparticles (nanocapsules: 34 days and nanospheres: 28 days) [47, 64, 72, 83] demonstrating that systems that exhibited particle size less than 100 nm and those that have a lipid phase in their composition showed better biological performance in reaching the tumor for PTX delivery, increasing the survival rates of animal models with GBM.Dox was encapsulated in cRGD-targeted hybrid nanoparticles (cRGD-HN-Dox). The developed HN were composed of gold nanorods (AuNR) with PEG-b-PCL-LA copolymers, for specific targeting of integrins in GBM tumor cells [115]. cRGD-HN-Dox showed a small particle size, below 100 nm, it was observed that NIR irradiation modified the release profile and decreased the cell viability of U87 cells. For cellular internalization, HN containing cRGD showed greater uptake than those non-targeted. It was observed that cRGD-HN- Dox with NIR irradiation showed a marked distribution in the tumor and inhibited its growth considerably compared to free dox and HN-dox without NIR. Also, there was an increase in survival at 48 days, while controls varied from 15 to 40 days. Thus, it was found that the use of cRGD promotes specific targeting and NIR irradiation contributed to intensifying Dox anti-GBM activity.
Another model containing Dox incorporated in RGD-lipid-polymer (RGD-LP) HN was described by Shi et al. [112]. This HN was prepared by nanoprecipitation method with self-assembly and presented a great particle size (110 nm), and controlled Dox release around 65% in 6 days, due to the difficulty of water penetrating in the hydrophobic nucleus containing the drug. After 2h in contact with C6 and bEnd.3 cells, the RGD-LP and LP formulations showed higher fluorescence intensity due to internalization, however, RGD-LP was more efficient in the antiproliferative effect of C6, with a reduction of approximately 40% in viability at the lowest concentration evaluated 0.3 μg/mL, due to the high expression of integrins as a target for RGD in C6 and in normal bEnd.3 cells, due to the similarity of the membrane structure with LP. As expected, RGD-LP showed greater penetration into tumor spheroids as well as higher growth inhibition in vitro. The affinity of RGD-LP for tumor cells was confirmed by observing the fluorescence intensity in the tumor region and by increasing the animals' survival to 57 days, suggesting a potential use for GBM treatment.
Paclitaxel has been linked to cyclo-[Arg-Gly-Asp-D- Phe-Lys] (cRGDfK) and encapsulated in HN surface-modified with folic acid [110]. This formulation was prepared by the association of nanoprecipitation and self-assembly methods, denominated PtxR-FPLNs. It was observed that the modification with FA improved the cytotoxic performance of the formulations and the PtxR-FPLNs showed a lower IC50 of 0.054 μg/mL after 72h of contact with T98G cells. Besides, they exhibited the best cellular uptake results with 97.7% after 2 hours of treatment and better performance in antitumor activity in vivo with a smaller tumor volume of 76.5 ± 1.3 mm3 compared to PtxR and PtxR-PLNs (both above 100 mm3), as well as improving survival at 42 days. Thus, it is suggested that the association of FA and cRGDfK mediate for folate and integrin receptor targets, contributing to the best antitumor efficiency.
Farnesylthiosalicylic acid (FTA) is a new antitumor drug, which has difficulty crossing BBB, already associated with nanosystems, to overcome this problem in GBM treatment [116]. HN was used as DDN, composed of PLGA, 1,2- -distearoyl-glycerol-3-phosphoethanolamine-N [meth- oxy (polyethylene glycol)-2000] (ammonium salt) (DSPE) and PEG associated with 1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP) (HN+D), which can improve the internalization of HN. HN+D was prepared by the emulsion method and showed a particle size of 127 nm and zeta potential of -11 mV. HNP+D demonstrated greater cytotoxicity for RG2 cells than HNP without DOTAP and less significant toxicity for non-cancerous cells. These results show that DOTAP provides HN targeting for RG2 cells. For the in vivo anti-GBM study, HN without DOTAP were evaluated by comparing two routes of administration, which demonstrated that the FTA encapsulated in HN was effective in reducing the volume of the tumor, from ~ 22mm2 to 5.93 and 3.15 mm2 for intravenous and intratumoral routes, respectively. Noting that HN contributes to potentiate the anti-tumor effect of FTA.
Similarly to the previously mentioned HN, FTA was evaluated for its action by intravenous and a non-invasive (intranasal - IN) administrations [111]. These FTA-HN obtained 97.7% encapsulation with 45.8% release in 12h, with cytotoxic potential, reducing cell viability (~ 40%) for RG2 and absence of cytotoxicity for L929 cells. After treatment with a single dose, there was a reduction of the tumor area by 57.3 and 31.0% for FTA-HN IV and IN, respectively. However, treatment with repeated doses was able to equally reduce 55% of the tumor area for both routes of administration of FTA-HN. There was an accumulation in the brain of FTA-HN by the two routes and in the liver/spleen only those administered by IV, demonstrating that the IN can be an alternative route for anti-GBM treatment by delivering the drug directly to the brain through the olfactory bulb, which would decrease systemic side effects.
Table (2) summarizes the studies found in the literature about polymeric nanosystems for GBM treatment and their main characteristics.
Table 2.
Studies designing polymeric drug delivery nanosystems for glioblastoma multiforme treatment and their main characteristics.
| Nanosystem | Composition | Surface Modification | Size (nm) | Therapeutic Molecule/Drug | In vitro Model | In vivo Model | Route of Administration | Refs. |
|---|---|---|---|---|---|---|---|---|
| NP | Sodium cholate,, PLC | MPEG | 72.5 ± 2.2 | Paclitaxel | C6 | Balb/c nude mice | intravenous | [47] |
| NP | DSPE-PEG (2000)- COOH, DMAB, PLGA | Lactoferrin,folic acid | - | Etoposide | U87MG | - | - | [79] |
| NP | DSPE-PEG (2000)- COOH, Pluronic F127, N-hydroxy succinimide sodium salt, and PCL | Wheat germ agglutinin, folic acid, MPEG | - | Etoposide, carmustine, and doxorubicin | U87MG | - | - | [80] |
| NP | PVA, mannitol, PLGA | poloxamer 188 | 114 ± 10 | Doxorubicin | U87MG | - | - | [82] |
| NP | PVA, mannitol, PLGA | poloxamer 188 | 108 ± 60 | Doxorubicin | - | Orthotopic model in Wistar rats | intravenous | [83] |
| NP | PLGA, PVA and PLGA | Maleimide-PEG-OX26 mAb | 194 ± 10 | Temozolomide | U251/U87 | - | - | [12] |
| NP | PVA andPLGA | Hydrogel | 169.30 ± 4.05 | Temozolomide | RG2 | - | - | [85] |
| NP | SPION-PEG-PBA | Folic acid | 48.6 | Temozolomide | C6 | - | - | [86] |
| NP | PVA, PLGA | - | 185 ± 30 | Bevacizumab | - | Xenograft model in nude mice | intranasal | [87] |
| NP | PLGA, Pluronic F-68 | mPEG | 206.3±14.7 | Paclitaxel and temozolomide | U87MG and C6 | Subcutaneous xenograft model in BALB/c nude mice | intravenous | [105] |
| NP | PLGA, N-trimethylated chitosan, maleimide, polyvinyl alcohol | Antibody-EPHA3 | 145.9±8.7 | Temozolomide butyl ester | C6 | Orthotopic model in Sprague-Dawley rats | intravenous | [106] |
| NP | PLGA-PEG, polyvinyl alcohol | cRGD | 184.2±1.99 | antagomiR-21 and antagomiR10b | U87MG and Ln299 | Subcutaneous xenograft model in nude mice (nu/nu) | intravenous | [107] |
| PNC | Span 60, CCT, Tween 80, PCL | - | 187 ± 8 | Methotrexato | C6 | Orthotopic xenograft model in Wistar rats | intraperitoneal | [90] |
| PNC | Span 60, CCT, Tween 80, PCL | - | 192 ± 6 | Methotrexate | GL261 | Orthotopic xenograft model in C57BL/6 mice | oral | [91] |
| PM | SMA, EDAC | - | 121 ± 59.9 and 89.14 ± 55.3 | Crizotinib and Dasatinib | A172, GL261, LN-18, U373, U87, NZG1003, NZG0906 | Subcutaneous xenograft model in C57BL/6 mice | intravenous | [98] |
| PM | Deoxycholic acid, sodium deoxycholate, Lipoid E8 | Hyaluronic acid | 53.36 | Gem-C12 and Honokiol | U87MG | Orthotopic xenograft model ni Balb/c nude mice | intravenous | [99] |
| PM | PEG-PBAE-PEG | - | 156 ± 2 and 350 ± 20 | Verteporfin | GBM1A, JHGBM612 | Pharmacokinetics and biodistribution studies in Athymic nude mice | intravenous | [100] |
| PM | PEG-PBLA-Ac, MeO-PEG-PBLA-Ac | cRGD | 30 | Epirubicin | U87MG | Orthotopic tumor model in Balb/c nude mice | intravenous | [101] |
| PM | MeO-PEG-b-P(Glu), Mal-PEG-b-P(Glu) | cRGD | 29 | DACHPt | U87MG | Subcutaneous tumor model in Balb/c nude mice | intravenous | [102] |
| PM | PEG3000-PLA2000, mPEG2000-PLA2000 | c(RGDyK) | 22.4 ± 0.3 | sPMI | U87MG | Subcutaneous and orthotopic model in Balb/c nude mice | intravenous | [103] |
| PM | mPEG-PLA, mal-PEG- PLA | c(RGDyK) | 35 | Paclitaxel | U87MG | Orthotopic tumor model in Balb/c nude mice | intravenous | [95] |
| HN | AuNR, DMF, PEG-b-PCL-LA, PB. | cRGD | 90 ± 2 | Doxorubicin | U87MG | Orthotopic model in nude mice | intravenous | [115] |
| HN | PLGA, soybean lecithin, DSPE-PEG | RGD | 110 ± 13.5 | Doxorubicin | C6 | Orthotopic model in Sprague-Dawley rats | intravenous | [112] |
| HN | PLGA, soybean, DSPE-PEG | Folic acid | - | Paclitaxel | T98G | Orthotopic model in Balb/c mice | intravenous | [110] |
| HN | PLGA-DSPE-PEG, lecithin | DOTAP | 127 ± 2.0 | Farnesylthiosalicylic acid | RG2 | Orthotopic model in Wistar rats | intravenous and intratumor | [116] |
| HN | PLGA, lecithin, DSPE-PEG | DOTAP | 164.3 ± 10.3 | Farnesylthiosalicylic acid | RG2 | Orthotopic model Wistar rats | intravenous and intranasal | [111] |
Abbreviations: NP – nanoparticle; PNC – polymeric nanocapsule; PM – polymeric micelle; HN – Hybrid nanoparticle; DSPE-PEG (2000)-COOH - 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)-2000]; DMAB - didodecyldimethylammonium bromide; PLGA - poly(lactide-co-glycolide); PCL - poly(ε-caprolactone); PLGA - poly(lactic-co-glycolic acid); PVA - Polyvinyl alcohol; SPION-PEG-PBA - poly (ethylene Glycol)–Poly (Butylene Adipate)–Poly (ethylene Glycol); CCT - Caprylic/capric triglyceride; SMA - poly(styrene-co-maleic acid); EDAC - carbodiimide hydrochloride; PEG-PBAE-PEG - poly(ethylene glycol)-poly(β-amino ester)-poly(ethylene glycol); cRGD - cyclic-Arg-Gly-Asp; c(RGDyK) - cyclic Arginine–Glycine–Aspartic acid-d-Tyrosine-Lysine; DOTAP - 1,2 Dioleoyl 3 trimethylammonium propane.
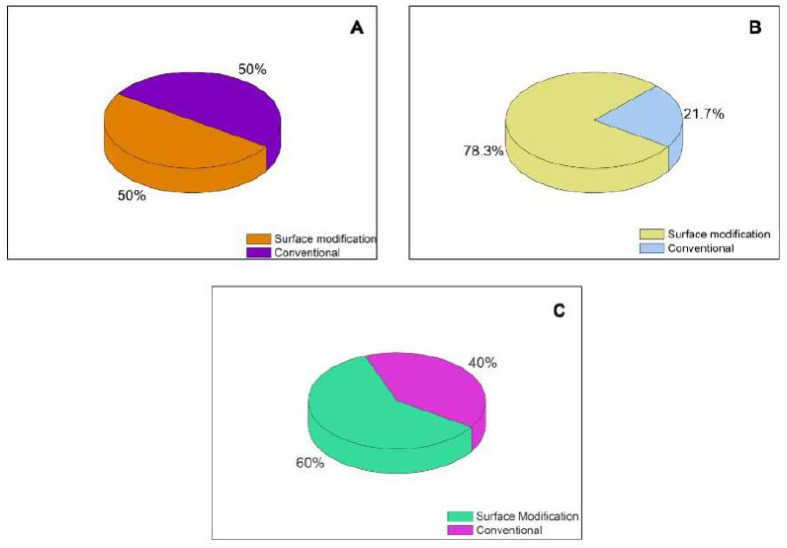
4. INORGANIC NANOPARTICLES
Inorganic nanoparticles (NP) are small structures that possess unique properties due to their nanometric size, as magnetic, electrical, and optical properties, usually in a range of 10-1000 nm [117, 118]. These NP can be manufactured by two approaches, top-down and bottom-up. The first one usually requires an input of energy into the system (mechanical/ball milling, thermal, laser ablation) for the generation of the NPs from bulk material. In the second one, the NPs are generated by atom-by-atom or molecule-by- molecule, often by self-assembly or self-organization [119, 120]. Because of these unique properties, inorganic nanoparticles can be employed for the diagnosis and treatment of several diseases and as drug delivery systems [121]. NPs for the treatment of cancer provide unique advantages for pharmacotherapy, as the possibility of surface functionalization leading to delivery of drugs on specifics sites, an increase of drug stability, controlled release among others [122-124]. (Fig. 4) summarizes the main founding regarding inorganic nanoparticles for GBM treatment.
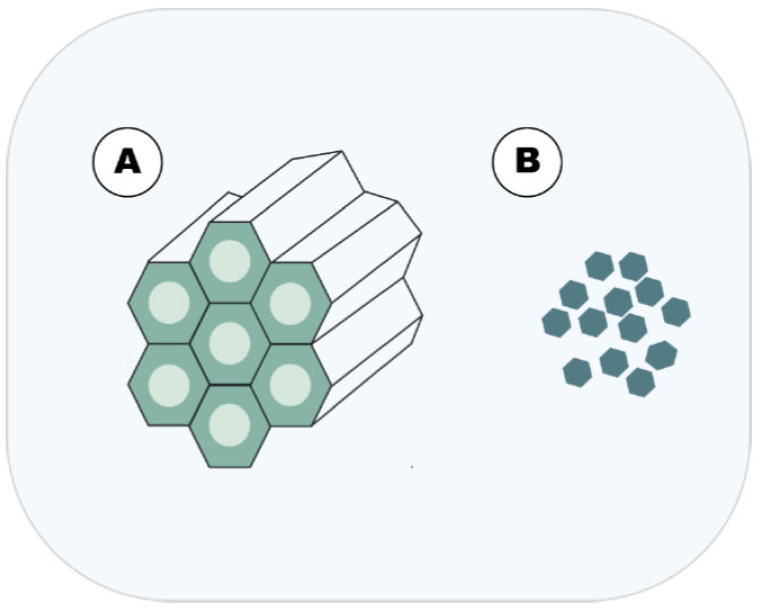
Fig. (4).
Main inorganic DDN approached in this article.A = MSNP and; B = metallic nanoparticles. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4.1. Metallic Nanoparticles
Several studies have shown the use of metallic nanoparticles as a potential tool for GBM treatment. Nanoparticles of TiO2-Pt, without a defined shape, lead to a tumor reduction on rats with peritoneal tumors induced by C6 cells when compared to rats that do not receive the nanoparticles [125]. Kim et al. evaluated the In vitro cytotoxicity of silicon dioxide and zinc oxide NPs on U373MG cells. It was shown that NPs leads to a reduction in cell viability, with NPs with smaller size presenting more toxicity. These NPs induce caspase-3 activation, leading to apoptosis, with the induction of chromosomal DNA fragmentation [126]. In another study, it was observed that zinc oxide nanostructures inhibited the T98G cell proliferation in a dose-dependent manner, reducing the colony capability formation of these cells. The exposure of cells to the nanostructures leads to the formation of reactive oxygen species (ROS) and activation of the caspase pathway. Also, these ZnO nanostructures suppress the invasiveness of GBM by the attenuation of N-cadherin and Zeb-1, factors associated with epithelial-to-mesenchymal transition (EMT) [127].
Surface modification of ZnO NPs with albumin, fibrinogen, and apo-transferrin covalently linked and nonspecifically adsorbed was evaluated against U373 cells. The cells submitted to a 300 µM of Zn2+ presented no viability after 24 hours exposure both in covalently linked and nonspecifically adsorbed proteins. The ZnO bounded with apo-transferrin at a concentration of 200 µM caused a significant decrease o cell viability, probably due to the overexpression of transferrin receptors on U373 cells. Also, the ZnO NPs leads to a modification of the cell cycle as well as altering the cell death mechanism, leading to a reduction in apoptosis and necrosis of the cells [128].
Mishra et al. obtained gold nanoparticles (AuNP) bio-synthetized by using Hibiscus sabdarifa extracts. U87 cells were exposed to AuNPs and it was shown that these NP induced cell death on 85%, also when exposing these AuNPs to a normal cell line (293), it was observed low toxicity (80% of cell viability). It was observed that the NP caused alteration on the nuclear morphology of U87 cells, with the degradation of DNA and uracil DNA glycosylase (GADPH), an enzyme responsible for DNA repair, indicating a possible mechanism of action of this ANPs. In another study, GBM02 cells were exposed to silver/silver chloride NPs. These NPs caused dose and time-dependent inhibition of cells as well as temozolomide (TMZ), a chemotherapy drug. Also, less toxicity was observed on astrocytes, indicating a possible safety effect on normal cells. The combination of NPs with TMZ leads to a similar reduction in cell proliferation both on normal astrocytes and GBM cells. A similar result was observed on the population doubling time experiments. Showing that the NPs presented less toxic effects to the cells when compared to the TMZ and the combination of NPs with TMZ [129].
Urbanska et al. implanted U-87MG cells on the chicken chorioallantoic membrane and exposed the tumors formed to silver nanoparticles (AgNPs). The treatment leads to a significant shrinking of GBM cells when compared to the control groups. The AgNPs leads to the activation of apoptosis by caspases activation [130]. Also, these NPs could lead to the formation of reactive oxygen species (ROS), a well-known mechanism of cell death [131-133].
Gold NPs capped with carboxymethyl xanthan gum (CMXGAuNPs) were used as drug delivery of doxorubicin (DOX). CMXGAuNPs presented a drug release on acidic conditions (pH 5.3 and 6.6) of 98 ± 4.2% and 89 ± 1.8%, respectively, and an insignificant release on pH 7.4. This is extremely important, considering the intended delivery of DOX in the tumor environment. These CMXGAuNPs significantly decreased the cell viability of LN229 cells when compared to control groups, with higher cell internalization of the NPs when compared to free DOX. These NPs lead to high levels of ROS, being following literature about the possible mechanism of action of metallic nanoparticles [134].
Radiotherapy is one of the main therapeutic strategies for GBM treatment. Silver (AgNPs) and gold NP (AuNP) were used to improve the radiotherapy efficacy in GBM treatment. Using Transmission electron microscopy, it was observed that the NPs were internalized by the cell, accumulating in endosomes. The AgNPs caused a significant reduction in U251 cell viability when compared to the AuNPs, as well as lead to an improvement in the radiation effect by the reduction of cell viability. The mean survival time of rats with induced GBM was longer than the control groups, showing an improvement of the GMB treatment, which could be related to the production of secondary electrons by the NPs induced by x-ray beams. The treatment of U251 cells with AgNPs enhanced apoptotic cell response when compared to control. Besides, the NPs increase the formation of acidic vesicular organelles, leading to autophagy upregulation, demonstrating the potential of these NPs as adjuvant treatment of GBM [135].
A similar study used iron-oxide NPs (IONPs) for potentializing the radiotherapy in a GBM model using the cell line U87MG overexpressing the EGFRvIII receptor. The IONPs were conjugated to cetuximab, a monoclonal antibody that binds the EGFRvIII. It was shown that the IONPs lead to a decrease in the viability of the U87MG cells when compared to the control and no toxicity was observed on human astrocytes, indicating a selective effect on GBM. Also, it was shown that the IONPs caused DNA damaged, expressed by the increase in the cH2AX foci, a DNA double-strand break marker, and increased the production of reactive oxygen species. The in vivo experiments showed that animals treated with IONPs retained the NPs on the brain and delayed xerograph growth. Also, it presented a statistically significant improvement in survival rate when compared to the control, demonstrating that the association of radiotherapy with NPs could be a good strategy on the GBM treatment [136]. Groysbeck et al, using gold nanoparticles (AuNPs) conjugated with cetuximab (Au-Cmab) against U87MG cells found similar results, showing that Au-Cmab attached and was successfully internalized by the cells, showing that the functionalization of NPs could be a promising strategy on GMB treatment.
4.2. Mesoporous Silica Nanoparticles
Mesoporous silica nanoparticles (MSNP) are an inorganic NP that has emerged as a drug delivery system, presenting desirable characteristics for this purpose as drug loading capacity, low toxicity, excellent biodistribution, good surface for functionalization leading to tumor-targeting among others [137]. Several studies have shown that MSNP possesses GBM therapeutic potential.
The use of RGD (Arg-Gly-Asp) has been explored as a nanoparticle for tumor-targeted delivery in GBM treatment [138, 139]. MSNPs with different sizes (20, 40, and 80 nm) functionalized with PEI-cR carrying Dox presenting loading efficiency of 10.57%, 9.53%, and 7.65%, respectively, were described by Mo et al. This MSNP presented higher cytotoxicity effects on glioma cells (U87, U251, and C6) and lower toxicity on normal cells (HBMEC) when compared to the free drug. The uptake of the MSNP was more pronounced on U87 cells than that in the other cell lineages, which can be related to the higher expression of the ανβ3 integrin receptor, that binds the RGD peptide, with the MSNP being localized in the cytoplasm of the cells. Also, the MSNP with 40 nm presented higher uptake than the other sizes. The exposure of the cells to the MSNPs leads to apoptosis in the GBM cell lineages, being more evident on U87 cells, with the MSNPs-40nm with the highest anti-glioma activity. This MSNPs-40nm presented great permeability across a blood-brain barrier (BBB) model when compared to the other MSNP and the free drug. Also, in a U87 tumor spheroid cell culture model, the MSNPs reduced the tumor volume, with higher permeability of Dox in the cells when compared to free Dox, showing a promise nanosystem for the treatment of GBM [140].
In a study using MSNPs-RGD as a carrier for BSeC, a selenium compound, it was shown that theses MSNPs-RGD-BSeC presented a permeability capacity of the BBB better than the free BSeC, with great cellular uptake and higher cell toxicity to U87 when compared to the free drug. This uptake was higher on cells overexpressing integrins (U87) when compared to cells that rarely express integrins (C6), showing the selective of the MSNPs-RGD-BSeC. These NPs entered the cells viaendocytosis through lysosomes. The release of the drug from the NPs was lower in pH 7.4 when compared to an acidic condition, with a release of 85.6% (pH 5.3) after 60 h, this is similar to the results found by the previous report and is important considering the tumor acidic environment. The MSNPs-RGD-BSeC high inhibition against U87 cells and low toxicity on normal cells (CHEM-5), inducing apoptosis in a dose-dependent way, with caspase viaactivation and mitochondrial dysfunction. Also, the MSNPs-RGD-BSeC triggered to ROS rise levels when compared to the BSeC. The U87 tumor spheroids showed that the treatment with the NPs caused a significant decrease in the tumor volume, similar to the results find by Mo et al. [141].
Li et al. studied the co-delivery of doxorubicin and camptothecin by MSNP using U87MG as in vitro model. The MSNP presented the drug loading capacity of 4.72% and 5.30% for Dox and Cpt, respectively. In a pH simulating the tumor acidic environment (pH 5.0), the NPs released 60% in 10h and 90% in 100 h of incubation of Dox. The exposure of U87MG cells to the NPs leads to a decrease in cell viability when compared to the control groups. In lower pH values (6.5), it was shown greater toxicity of the NPs, probably due to the faster release of Dox. Also, it was observed that the MSNP was internalized and found in acidic organelles, which can contribute positively to the Dox release [142]. This pH-responsive behavior was similar to MSNPs capped with chitosan (CMSNP) for the delivery of curcumin. After 96 h, the CMSNP released 42.72% of Cur in pH 5.5, higher than on pH 7.4 (19.54%), presenting a cytotoxicity effect on U87MG cells [143]. Oxidative stress and apoptosis are a well-known mechanism of action of Silica nanoparticles. Kusaczuk et al observed that LN229 cells exposed to these NPs presented loss on ell viability, with elevated levels early apoptotic cells, activation of caspase pathway, proinflammatory potential, with an increase on IL1B and COX2 [144, 145].
The effect of temozolomide loaded on mesoporous silica nanoparticles functionalized with NGR was assessed on C6 cells. It was shown that the NGR increase the uptake of the NPs by the cells and significantly reduced the cell viability when compared to the control group. The NPs induced autophagy and apoptosis on the cell, indicated by the detection of autophagosomes, autolysosomes, and the activation of caspases [146].
Table (3) summarizes the studies found in the literature about inorganic nanoparticles for GBM treatment and their main characteristics.
Table 3.
Studies designing inorganic nanoparticles for GBM treatment and their main characteristics.
| Nanosystem | Composition | Surface Modification | Size (nm) | Therapeutic Molecule/Drug | In vitro Model | In vivo model | Route of Administration | Refs. |
|---|---|---|---|---|---|---|---|---|
| SiO2 NP | SiO2 | Citrate, L-serine | 20 and 100 nm | - | U373MG cells | - | - | [126] |
| ZnO NP | ZnO | L-arginine | 20 and 100 nm | - | U373MG cells | - | - | [126] |
| ZnO NP | ZnO | - | ~ 200 nm long | - | T98G | - | - | [127] |
| ZnO NP | ZnO | Albumin, fibrinogen, and apo-transferrin | 220-1494 nm | - | U373MG cells | - | - | [128] |
| Au NP | Au | - | 10-60 nm | - | U87 cells | - | - | [147] |
| Ag/AgCl NP | Ag | - | 2-22 nm | - | GBM02 | - | - | [129] |
| Ag NP | Ag | - | 70 nm | - | U87MG | - | - | [130] |
| CMXGAuNPs | Au | carboxymethyl xantham gum | 8-10 nm | DOX | LN-229 | - | - | [134] |
| AuNP | Au | Citrate | 15 nm | - | U251 cells | Orthotopic mouse brain tumor model | Intratumorally | [135] |
| Ag NP | Ag | Citrate | 25 nm | - | U251 cells | Orthotopic mouse brain tumor model | Intratumorally | [135] |
| IO NO | Iron oxide | Cetuximab | 10 nm | - | U87-EGFRvIII | orthotopic xenografts model in Athymic nude mice | Convection-enhanced delivery | [136] |
| Pt/TiO2 NP | TiO2, Pt | - | 10 nm | - | - | Intraperitoneal xenograft model in Wistar rats | Intraperitoneal | [125] |
| AuNPs | Au | Cetuximab | 2.4 nm | - | U87 MG | - | - | [148] |
| MSNP | PEG, GO | PEG, IL-13 | DOX | U251 | - | - | [149] | |
| MSNP | Si | - | 140 nm | DOX, CPT | U87MG | - | - | [142] |
| MSNP | Si | PEI-cRGD | 20-80 nm | DOX | U87, U251, and C6 | - | - | [140] |
| MSNP | Si | RGD | 136 nm | BSeC | U87, C6 | - | - | [141] |
| MSNP | Si | NGR | 70 nm | TMZ | C6 | - | - | [146] |
| MSNP | Si | - | 180 nm | CUR | U87MG | - | - | [143] |
| MSNP | MPEG, Si | RGD | 148 nm | DOX | U87MG | - | - | [150] |
| - | - | - | 5-15 nm | - | LN229 | - | - |
Abbreviations: SiO2NP – Silicon dioxide nanoparticles; ZnONP – Zinc oxide nanoparticles; AuNP – gold nanoparticles; PEG – polyethyleneglycol; IL-13 – interleukin 13; PEI-cRGD - poly(ether imide)-cricoids Arg-Gly-Asp- Phe-Lys; DOX – doxorubicin; CPT – camptothecin; TMZ – temozolomide; CUR – curcumin.
CONCLUSION AND FUTURE PERSPECTIVES
Nowadays, cancer represents a global burden. GBM is one of the most aggressive human cancers that occur in CNS. Marketed medicines for GBM treatment today present some clinical limitations regarded as non-specific biodistribution, systemic toxicity, and often poor bioavailability. Many efforts has been made to achieve more efficient and less toxic anti-cancer medicines. An interesting approach is the use of DDN as platforms to deliver anti-cancer drugs, since they proved to have good biocompatibility, besides, to increase the bioavailability of poor-soluble drugs, with considerable safety. In this review, we showed that DDN is a promising tool to improve biopharmaceutical properties of drugs used in glioblastoma multiforme therapy, reducing adverse effects due to lower required doses, as well as improving the bioavailability of these drugs, making them more suitable for clinical use.
It is comprehensible that the major part of the works found in the literature is regarding polymeric and lipid-based systems (Fig. 1). Although many successful applications can be found, unfortunately, polymer-based DDN has some limitations regarding its low entrapment efficiency for hydrophobic drugs, particle-particle aggregation, as well as toxicity for some synthetic polymers used [107].
This being said, we can expect that there is a clear demand by the end of the 80s’ to develop new DDN that are capable to attend the needs of that time: successfully deliver poor-soluble molecules in enough amounts with safety, which couldn’t be achieved through conventional medicines. In a general way, lipid-based DDN represents an interesting alternative as delivery systems to treat GBM due to their excellent biodegradability, biocompatibility and ability to bypass the BBB even without any surface modification with specific ligands that mediate active transport across this barrier [18, 40, 42, 52, 61], besides having good entrapment efficiency for both hydrophilic and hydrophobic drugs. Later, it was found that, when appropriate, further modifications of these nanocarriers can be easily done and can increase the ability to transpose the BBB or BTBB [23, 29, 62]. However, the development of lipid-based nanosystems should be further established and optimized to overcome some limitations presented by the first generation of lipid carriers – like liposomes and microemulsion -, such as their low loading capacity when compared with other nanosystems – like SLN and NLC - and their tendency for gelation [18]. As can be seen in (Fig. 1), lipid-based systems are abundant in the literature. Liposomes and microemulsions represent the first generation of lipid carriers, thereby being more exploited, as shown by the higher number of works regarding these systems. In the last decade, although an increase in works bringing SLN and NLC as DDN can be seen as an alternative to correct some physical-chemical limitations of conventional colloidal carriers such as liposomes and microemulsions. Last but not the least, NLC has gained attention due to its promising biopharmaceutical features compared to SLN, that present some limitations that compromise its biological applications such as insufficient drug loading and drug leakage during storage, which does not occur for NLC, that presents high entrapment efficiency as well as good physical- chemical stability during storage. Nowadays, NLC is considered the most modern and superior lipid-based DDN over other lipid-based DDN [151].
Another DDS that can be used for drug delivery is inorganic nanoparticles. As can be seen in (Fig. 5C), metallic nanoparticles are the most used approach founded as anti-GBM among inorganic nanoparticles. When compared to the organic ones, these nanoparticles are more stable systems and, due to its higher density, could be more internalized by cells [117, 118]. In addition to drug delivery, these NP can be used as radiotherapy enhancers [123] and applied for brain imaging [152], presenting a good strategy as a theranostic.
Fig. (5).
Number of publications according to the type of nanosystem, using or not surface modifications: A (Lipid-based systems); B (polymeric nanosystems) and C (inorganic nanosystems). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Another remarkable finding was the high number of publications regarding DDN surface modifications, indicating a new trend in scientific research (Fig. 5). Surface functionalization came as a possibility to actively target tumor cells and deliver anti-cancer drugs selectively, which seems to improve even more these nanosystems’ target and bioaccumulation in the tumor tissue. Also, surface functionalization stands as an important strategy to overcome the BBB and BTBB, improving these DDN crossing through BBB and BTBB by active transportation. Together, these biopharmaceutical features can be related to an increase in the overall treatment efficiency, as well as decreased side effects due to non-selective systemic drug distribution. Although many in vitro studies had shown exciting results, further studies, including in vivo models as well as clinical trials, are needed to widely define these nanosystems’ properties as well as to determine their safety for human use.
Overall, this article has shown that nanomedicines for the GBM treatment are extensively studied, and many different promising DDN can be found. However, some challenges must be overcome to complement and obtain further data using translational research with human subjects. Considering only in vitro and in vivo models is not possible to assume that the results found on pre-clinical assays like in vivo and in vitro models will be reproducible on human subjects, mainly due to the heterogeneity of the disease [153, 154], what in GBM is a key factor. Another aspect is the difficulty of scale-up the production from small batches on research laboratories to industrial batches [155, 156]. Also, the lack of specific regulatory guidance for nanomedicines remains an obstacle to assure the safety and quality of innovative medicines, so it can be released in the market [153, 155, 157, 158].
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study was kindly funded by FAPESP #2018/ 18488-3, Conselho Nacional de Desenvolvimento Cientıfico e Tecnologico (CNPq), Programa de Apoio ao Desenvolvimento Cientıfico– FCF-UNESP (PADC) and Coordenação de Aperfeiçoamento de Pessoal de Nıvel Superior- Brasil (CAPES) - Finance Code 001.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2019. Am. Cancer Soc.; 2019. [Google Scholar]
- 2. National Cancer Institute. Risk Factors: National Cancer Institute https://www.cancer.gov/about-cancer/causes-prevention/risk/radiation (accessed Apr 13, 2020).
- 3.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Agnihotri S., Burrell K. E., Wolf A., Jalali S., Hawkins C., Rutka J. T., Zadeh G. Glioblastoma, a brief review of history, molecular genetics, animal models and novel therapeutic strategies. Archivum Immunologiae et Therapiae Experimentalis. Springer . 2013:25-41. doi: 10.1007/s00005-012-0203-0. [DOI] [PubMed] [Google Scholar]
- 5.Rock K., MCardle O., Forde P.M., Fitzpatrick D., O’Neill B., Faul C. A clinical review of treatment outcomes in glioblastoma multiforme—the validation in a non-trial population of the results of a randomised Phase III clinical trial: has a more radical approach improved survival? The British Journal of Radiology. 85 doi: 10.1259/bjr/83796755. https://www.birpublications.org/doi/full/10.1259/bjr/83796755 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blissitt P. A. Journal of Neuroscience Nursing. Lippincott Williams and Wilkins . Lippincott Williams and Wilkins; 2014. Clinical practice guideline series update. pp. 367–368. [DOI] [Google Scholar]
- 7.Ellor S.V., Pagano-Young T.A., Avgeropoulos N.G. Glioblastoma: background, standard treatment paradigms, and supportive care considerations. J. Law Med. Ethics. 2014;42(2):171–182. doi: 10.1111/jlme.12133. [DOI] [PubMed] [Google Scholar]
- 8.Lee E.Q., Reardon D.A., Schiff D., Drappatz J., Muzikansky A., Grimm S.A., Norden A.D., Nayak L., Beroukhim R., Rinne M.L., Chi A.S., Batchelor T.T., Hempfling K., McCluskey C., Smith K.H., Gaffey S.C., Wrigley B., Ligon K.L., Raizer J.J., Wen P.Y., Phase I.I. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro-oncol. 2015;17(6):862–867. doi: 10.1093/neuonc/nou350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson T.A., Karajannis M.A., Harter D.H. Glioblastoma multiforme: State of the art and future therapeutics. Surg. Neurol. Int. 2014;5(Suppl.):64. doi: 10.4103/2152-7806.132138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batash R., Asna N., Schaffer P., Francis N., Schaffer M. Glioblastoma multiforme, diagnosis and treatment; recent literature review. Curr. Med. Chem. 2017;24(27):3002–3009. doi: 10.2174/0929867324666170516123206. [DOI] [PubMed] [Google Scholar]
- 11.Bastiancich C., Danhier P., Préat V., Danhier F. Journal of Controlled Release. Elsevier B.V.; 2016. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. pp. 29–42. [DOI] [PubMed] [Google Scholar]
- 12.Ramalho M.J., Sevin E., Gosselet F., Lima J., Coelho M.A.N., Loureiro J.A., Pereira M.C. Receptor-mediated PLGA nanoparticles for glioblastoma multiforme treatment. Int. J. Pharm. 2018;545(1-2):84–92. doi: 10.1016/j.ijpharm.2018.04.062. [DOI] [PubMed] [Google Scholar]
- 13.Bhushan B. Introduction to Nanotechnology. Springer Handbooks. Springer; 2017. pp. 1–19. [DOI] [Google Scholar]
- 14.Rai M., Alves dos Santos C. Nanotechnology Applied to Pharmaceutical Technology. Springer International Publishing; 2017. [DOI] [Google Scholar]
- 15.Séhédic D., Cikankowitz A., Hindré F., Davodeau F., Garcion E. Nanomedicine to overcome radioresistance in glioblastoma stem-like cells and surviving clones. Trends Pharmacol Sci. 2015;36(4):236–252. doi: 10.1016/j.tips.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Mujokoro B., Adabi M., Sadroddiny E., Adabi M., Khosravani M. Nano-structures mediated co-delivery of therapeutic agents for glioblastoma treatment: a review. Mater. Sci. Eng. C. 2016;69:1092–1102. doi: 10.1016/j.msec.2016.07.080. [DOI] [PubMed] [Google Scholar]
- 17.Duwa R., Emami F., Lee S., Jeong J.H., Yook S. Polymeric and lipid-based drug delivery systems for treatment of glioblastoma multiforme. J. Ind. Eng. Chem. 2019;79:261–273. doi: 10.1016/j.jiec.2019.06.050. [DOI] [Google Scholar]
- 18.Ortiz R., Cabeza L., Perazzoli G., Jimenez-Lopez J., García-Pinel B., Melguizo C., Prados J. Nanoformulations for glioblastoma multiforme: a new hope for treatment. Future Med. Chem. 2019;11(18):2459–2480. doi: 10.4155/fmc-2018-0521. [DOI] [PubMed] [Google Scholar]
- 19.Sabir F., Ismail R., Csoka I. Nose-to-brain delivery of antiglioblastoma drugs embedded into lipid nanocarrier systems: status quo and outlook. Drug Discov. Today. 2020;25(1):185–194. doi: 10.1016/j.drudis.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Eloy J. O., Petrilli R., Trevizan L. N. F., Chorilli M. Immunoliposomes: a review on functionalization strategies and targets for drug delivery. Colloids Surf B Biointerfaces. 2017;159:454–467. doi: 10.1016/j.colsurfb.2017.07.085. [DOI] [PubMed] [Google Scholar]
- 21.Chorilli M., Calixto G., Rimério T.C., Scarpa M.V. Caffeine encapsulated in small unilamellar liposomes: characerization and in vitro release profile. J. Dispers. Sci. Technol. 2013;34(10):1465–1470. doi: 10.1080/01932691.2012.739535. [DOI] [Google Scholar]
- 22.Kang Y.J., Cutler E.G., Cho H. Therapeutic nanoplatforms and delivery strategies for neurological disorders. Nano Converg. 2018;5(1):35. doi: 10.1186/s40580-018-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakkadwala S., Dos Santos Rodrigues B., Sun C., Singh J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J. Control. Release. 2019;307:247–260. doi: 10.1016/j.jconrel.2019.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miretti M., Tempesti T.C., Prucca C.G., Baumgartner M.T. Zn phthalocyanines loaded into liposomes: Characterization and enhanced performance of photodynamic activity on glioblastoma cells. Bioorg. Med. Chem. 2020;28(7):115355. doi: 10.1016/j.bmc.2020.115355. [DOI] [PubMed] [Google Scholar]
- 25.Lakkadwala S., Dos Santos Rodrigues B., Sun C., Singh J. Biodistribution of TAT or QLPVM coupled to receptor targeted liposomes for delivery of anticancer therapeutics to brain in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2020;23:102112. doi: 10.1016/j.nano.2019.102112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandes M.A., Hanck-Silva G., Baveloni F.G., Oshiro J.A., Junior, de Lima F.T., Eloy J.O., Chorilli M. A Review of Properties, Delivery systems and analytical methods for the characterization of monomeric glycoprotein transferrin. Crit. Rev. Anal. Chem. 2020:1–12. doi: 10.1080/10408347.2020.1743639. [DOI] [PubMed] [Google Scholar]
- 27.Guo P., Moses-Gardner A., Huang J., Smith E.R., Moses M.A. ITGA2 as a potential nanotherapeutic target for glioblastoma. Sci. Rep. 2019;9(1):6195. doi: 10.1038/s41598-019-42643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jhaveri A., Deshpande P., Pattni B., Torchilin V. Transferrin- targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release. 2018;277:89–101. doi: 10.1016/j.jconrel.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anilkumar T.S., Lu Y.J., Chen H.A., Hsu H.L., Jose G., Chen J.P. Dual targeted magnetic photosensitive liposomes for photothermal/photodynamic tumor therapy. J. Magn. Magn. Mater. 2018;2019(473):241–252. doi: 10.1016/j.jmmm.2018.10.020. [DOI] [Google Scholar]
- 30.Belhadj Z., Zhan C., Ying M., Wei X., Xie C., Yan Z., Lu W. Multifunctional targeted liposomal drug delivery for efficient glioblastoma treatment. Oncotarget. 2017;8(40):66889–66900. doi: 10.18632/oncotarget.17976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papachristodoulou A., Signorell R.D., Werner B., Brambilla D., Luciani P., Cavusoglu M., Grandjean J., Silginer M., Rudin M., Martin E., Weller M., Roth P., Leroux J.C. Chemotherapy sensitization of glioblastoma by focused ultrasound-mediated delivery of therapeutic liposomes. J. Control. Release. 2019;295:130–139. doi: 10.1016/j.jconrel.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Oliveira M. B., Calixto G., Graminha M., Cerecetto H., González M., Chorilli M. Performance of fluconazole-loaded microemulsions for the topical treatment of Cutaneous Leishmaniasis. 2015;2015:396894. doi: 10.1155/2015/396894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvalho V.F.M., Salata G.C., de Matos J.K.R., Costa-Fernandez S., Chorilli M., Steiner A.A., de Araujo G.L.B., Silveira E.R., Costa-Lotufo L.V., Lopes L.B. Optimization of composition and obtainment parameters of biocompatible nanoemulsions intended for intraductal administration of piplartine (piperlongumine) and mammary tissue targeting. Int. J. Pharm. 2019;567:118460. doi: 10.1016/j.ijpharm.2019.118460. [DOI] [PubMed] [Google Scholar]
- 34.Dos Santos Ramos M.A., da Silva P.B., de Toledo L.G., Oda F.B., da Silva I.C., Dos Santos L.C., Dos Santos A.G., de Almeida M.T.G., Pavan F.R., Chorilli M., Bauab T.M. Intravaginal delivery of Syngonanthus nitens (Bong.) Ruhland fraction based on a nanoemulsion system applied to Vulvovaginal Candidiasis treatment. J. Biomed. Nanotechnol. 2019;15(5):1072–1089. doi: 10.1166/jbn.2019.2750. [DOI] [PubMed] [Google Scholar]
- 35.Medina-Alarcón K.P., L Singulani J., Dutra L.A., S Pitangui N., Pereira-da-Silva M.A., Dos Santos M.B., Ayusso G.M., Regasini L.O., Soares C.P., Chorilli M., Mendes-Giannini M.J.S., Fusco-Almeida A.M. Antifungal activity of 2′-hydroxychalcone loaded in nanoemulsion against Paracoccidioides spp. Future Microbiol. 2020;15(1):21–33. doi: 10.2217/fmb-2019-0095. [DOI] [PubMed] [Google Scholar]
- 36.C de Lima L., A S Ramos M., Toledo L.G., Rodero C.F., Hilário F., Dos Santos L.C., Chorilli M., Bauab T.M. Syngonanthus nitens (Bong.) ruhland derivatives loaded into a lipid nanoemulsion for enhanced antifungal activity against Candida parapsilosis. Curr. Pharm. Des. 2020;26(14):1556–1565. doi: 10.2174/1381612826666200317131041. [DOI] [PubMed] [Google Scholar]
- 37.Lago A.M.T., Neves I.C.O., Oliveira N.L., Botrel D.A., Minim L.A., de Resende J.V. Ultrasound-assisted oil-in-water nanoemulsion produced from pereskia aculeata miller mucilage. Ultrason. Sonochem. 2019;50:339–353. doi: 10.1016/j.ultsonch.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 38.Vladisavljević G.T. Preparation of microemulsions and nanoemulsions by membrane emulsification. Colloids Surf. A Physicochem. Eng. Asp. 2019;579:123709. doi: 10.1016/j.colsurfa.2019.123709. [DOI] [Google Scholar]
- 39.Chatterjee B., Gorain B., Mohananaidu K., Sengupta P., Mandal U.K., Choudhury H. Targeted drug delivery to the brain via intranasal nanoemulsion: available proof of concept and existing challenges. Int. J. Pharm. 2019;565:258–268. doi: 10.1016/j.ijpharm.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 40.Gadhave D., Gorain B., Tagalpallewar A., Kokare C. Intranasal teriflunomide microemulsion: an improved chemotherapeutic approach in glioblastoma. J. Drug Deliv. Sci. Technol. 2019;51:276–289. doi: 10.1016/j.jddst.2019.02.013. [DOI] [Google Scholar]
- 41.Shinde R.L., Devarajan P.V. Docosahexaenoic acid-mediated, targeted and sustained brain delivery of curcumin microemulsion. Drug Deliv. 2017;24(1):152–161. doi: 10.1080/10717544.2016.1233593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Colombo M., Figueiró F., de Fraga Dias A., Teixeira H.F., Battastini A.M.O., Koester L.S. Kaempferol-loaded mucoadhesive nanoemulsion for intranasal administration reduces glioma growth in vitro. Int. J. Pharm. 2018;543(1-2):214–223. doi: 10.1016/j.ijpharm.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 43.De Paula L.B., Primo F.L., Pinto M.R., Morais P.C., Tedesco A.C. Evaluation of a chloroaluminium phthalocyanine-loaded magnetic nanoemulsion as a drug delivery device to treat glioblastoma using hyperthermia and photodynamic therapy. RSC Adv. 2017;7(15):9115–9122. doi: 10.1039/C6RA26105A. [DOI] [Google Scholar]
- 44.Azambuja J.H., Schuh R.S., Michels L.R., Gelsleichter N.E., Beckenkamp L.R., Iser I.C., Lenz G.S., de Oliveira F.H., Venturin G., Greggio S., daCosta J.C., Wink M.R., Sevigny J., Stefani M.A., Battastini A.M.O., Teixeira H.F., Braganhol E. Nasal administration of cationic nanoemulsions as cd73-sirna delivery system for glioblastoma treatment: a new therapeutical approach. Mol. Neurobiol. 2020;57(2):635–649. doi: 10.1007/s12035-019-01730-6. [DOI] [PubMed] [Google Scholar]
- 45.Rigon R.B., Fachinetti N., Severino P., Durazzo A., Lucarini M., Atanasov A.G., El Mamouni S., Chorilli M., Santini A., Souto E.B. Quantification of trans-resveratrol-loaded solid lipid nanoparticles by a validated reverse-phase HPLC photodiode array. Appl. Sci. 2019;9(22):4961. doi: 10.3390/app9224961. [DOI] [Google Scholar]
- 46.Rigon R.B., Gonçalez M.L., Severino P., Alves D.A., Santana M.H.A., Souto E.B., Chorilli M. Solid lipid nanoparticles optimized by 22 factorial design for skin administration: Cytotoxicity in NIH3T3 fibroblasts. Colloids Surf. B Biointerfaces. 2018;171:501–505. doi: 10.1016/j.colsurfb.2018.07.065. [DOI] [PubMed] [Google Scholar]
- 47.Gonçalez M.L., Rigon R.B., Pereira-da-Silva M.A., Chorilli M. Curcumin-loaded cationic solid lipid nanoparticles as a potential platform for the treatment of skin disorders. Pharmazie. 2017;72(12):721–727. doi: 10.1691/ph.2017.7101. [DOI] [PubMed] [Google Scholar]
- 48.Rigon R.B., Fachinetti N., Severino P., Santana M.H.A., Chorilli M. Skin delivery and in vitro biological evaluation of trans-resveratrol-loaded solid lipid nanoparticles for skin disorder therapies. Molecules. 2016;21(1):E116. doi: 10.3390/molecules21010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tapeinos C., Battaglini M., Ciofani G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release. 2017;264(August):306–332. doi: 10.1016/j.jconrel.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sy Mohamad S.F., Mohd Said F., Abdul Munaim M.S., Mohamad S., Azizi Wan Sulaiman W.M. Application of experimental designs and response surface methods in screening and optimization of reverse micellar extraction. Crit. Rev. Biotechnol. 2020;40(3):341–356. doi: 10.1080/07388551.2020.1712321. [DOI] [PubMed] [Google Scholar]
- 51.Muntoni E., Martina K., Marini E., Giorgis M., Lazzarato L., Salaroglio I.C., Riganti C., Lanotte M., Battaglia L. Methotrexate-loaded solid lipid nanoparticles: protein functionalization to improve brain biodistribution. Pharmaceutics. 2019;11(2):1–18. doi: 10.3390/pharmaceutics11020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Küçüktürkmen B., Bozkır A. Development and characterization of cationic solid lipid nanoparticles for co-delivery of pemetrexed and miR-21 antisense oligonucleotide to glioblastoma cells. Drug Dev. Ind. Pharm. 2018;44(2):306–315. doi: 10.1080/03639045.2017.1391835. [DOI] [PubMed] [Google Scholar]
- 53.Grillone A., Battaglini M., Moscato S., Mattii L., de Julián Fernández C., Scarpellini A., Giorgi M., Sinibaldi E., Ciofani G. Nutlin-loaded magnetic solid lipid nanoparticles for targeted glioblastoma treatment. Nanomedicine (Lond.) 2019;14(6):727–752. doi: 10.2217/nnm-2018-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Souto E.B., Baldim I., Oliveira W.P., Rao R., Yadav N., Gama F.M., Mahant S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020;17(3):357–377. doi: 10.1080/17425247.2020.1727883. [DOI] [PubMed] [Google Scholar]
- 55.Gordillo-Galeano A., Mora-Huertas C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018;133:285–308. doi: 10.1016/j.ejpb.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Fachinetti N., Rigon R.B., Eloy J.O., Sato M.R., Dos Santos K.C., Chorilli M. Comparative study of glyceryl behenate or polyoxyethylene 40 stearate-based lipid carriers for trans-resveratrol delivery: development, characterization and evaluation of the in vitro tyrosinase inhibition. AAPS PharmSciTech. 2018;19(3):1401–1409. doi: 10.1208/s12249-018-0961-z. [DOI] [PubMed] [Google Scholar]
- 57.Sato M.R., Oshiro Junior J.A., Machado R.T.A., de Souza P.C., Campos D.L., Pavan F.R., da Silva P.B., Chorilli M. Nanostructured lipid carriers for incorporation of copper(II) complexes to be used against Mycobacterium Tuberculosis. Drug Des. Devel. Ther. 2017;11:909–921. doi: 10.2147/DDDT.S127048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sato M.R., Oshiro-Junior J.A., Souza P.C., Campos D.L., Pereira-Da-Silva M.A., Pavan F.R., Da Silva P.B., Chorilli M. Copper(II) complex-loaded castor oil-based nanostructured lipid carriers used against Mycobacterium tuberculosis : Development, characterisation, in vitro and in vivo biological assays. Pharmazie. 2019;74(12):715–720. doi: 10.1691/ph.2019.9110. [DOI] [PubMed] [Google Scholar]
- 59.Oshiro-Junior J.A., Sato M.R., Boni F.I., Santos K.L.M., de Oliveira K.T., de Freitas L.M., Fontana C.R., Nicholas D., McHale A., Callan J.F., Chorilli M. Phthalocyanine-loaded nanostructured lipid carriers functionalized with folic acid for photodynamic therapy. Mater. Sci. Eng. C. 2020;108:110462. doi: 10.1016/j.msec.2019.110462. [DOI] [PubMed] [Google Scholar]
- 60.do Prado A.H., Araújo V.H.S., Eloy J.O., Fonseca-Santos B., Pereira-da-Silva M.A., Peccinini R.G., Chorilli M. Synthesis and characterization of nanostructured lipid nanocarriers for enhanced sun protection factor of octyl p-methoxycinnamate. AAPS PharmSciTech. 2020;21(4):125. doi: 10.1208/s12249-019-1547-0. [DOI] [PubMed] [Google Scholar]
- 61.Wang H., Li L., Ye J., Wang R., Wang R., Hu J., Wang Y., Dong W., Xia X., Yang Y., Gao Y., Gao L., Liu Y. Improving the oral bioavailability of an anti-glioma prodrug cat3 using novel solid lipid nanoparticles containing oleic acid-cat3 conjugates. Pharmaceutics. 2020;12(2):E126. doi: 10.3390/pharmaceutics12020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Emami J., Rezazadeh M., Sadeghi H., Khadivar K. Development and optimization of transferrin-conjugated nanostructured lipid carriers for brain delivery of paclitaxel using Box-Behnken design. Pharm. Dev. Technol. 2017;22(3):370–382. doi: 10.1080/10837450.2016.1189933. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J., Xiao X., Zhu J., Gao Z., Lai X., Zhu X., Mao G. Lactoferrin- and RGD-comodified, temozolomide and vincristine- coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int. J. Nanomedicine. 2018;13:3039–3051. doi: 10.2147/IJN.S161163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Woensel M., Wauthoz N., Rosière R., Mathieu V., Kiss R., Lefranc F., Steelant B., Dilissen E., Van Gool S.W., Mathivet T., Gerhardt H., Amighi K., De Vleeschouwer S. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J. Control. Release. 2016;227:71–81. doi: 10.1016/j.jconrel.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 65.Gholami L., Tafaghodi M., Abbasi B., Daroudi M., Kazemi Oskuee R. Preparation of superparamagnetic iron oxide/doxorubicin loaded chitosan nanoparticles as a promising glioblastoma theranostic tool. J. Cell. Physiol. 2019;234(2):1547–1559. doi: 10.1002/jcp.27019. [DOI] [PubMed] [Google Scholar]
- 66.Velpurisiva P., Piel B.P., Lepine J., Rai P. GSK461364A, a Polo-Like Kinase-1 inhibitor encapsulated in polymeric nanoparticles for the treatment of Glioblastoma Multiforme (GBM). Bioengineering. 2018;5(4):83. doi: 10.3390/bioengineering5040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xin H., Chen L., Gu J., Ren X., Wei Z., Luo J., Chen Y., Jiang X., Sha X., Fang X. Enhanced anti-glioblastoma efficacy by PTX-loaded PEGylated poly(ɛ-caprolactone) nanoparticles: In vitro and in vivo evaluation. Int. J. Pharm. 2010;402(1-2):238–247. doi: 10.1016/j.ijpharm.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Crucho C.I.C., Barros M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C. 2017;80:771–784. doi: 10.1016/j.msec.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 69.George A., Shah P.A., Shrivastav P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019;561(561):244–264. doi: 10.1016/j.ijpharm.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 70.Tsai Y-C., Vijayaraghavan P., Chiang W-H., Chen H-H., Liu T-I., Shen M-Y., Omoto A., Kamimura M., Soga K., Chiu H-C. Targeted delivery of functionalized upconversion nanoparticles for externally triggered photothermal/photodynamic therapies of brain glioblastoma. Theranostics. 2018;8(5):1435–1448. doi: 10.7150/thno.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nam L., Coll C., Erthal L.C.S., de la Torre C., Serrano D., Martínez-Máñez R., Santos-Martínez M.J., Ruiz-Hernández E. Drug delivery nanosystems for the localized treatment of glioblastoma multiforme. Materials (Basel) 2018;11(5):779. doi: 10.3390/ma11050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prajapati S.K., Jain A., Jain A., Jain S. Biodegradable polymers and constructs: a novel approach in drug delivery. Eur. Polym. J. 2019;120:109191. doi: 10.1016/j.eurpolymj.2019.08.018. [DOI] [Google Scholar]
- 73.Sur S., Rathore A., Dave V., Reddy K.R., Chouhan R.S., Sadhu V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Structures & Nano-Objects. 2019;20:100397. doi: 10.1016/j.nanoso.2019.100397. [DOI] [Google Scholar]
- 74.Gatti T.H.H., Eloy J.O., Ferreira L.M.B., Da Silva I.C., Pavan F.R., Gremião M.P.D., Chorilli M. Insulin-loaded polymeric mucoadhesive nanoparticles: development, characterization and cytotoxicity evaluation. Brazilian J. Pharm. Sci. 2018;54(1) doi: 10.1590/s2175-97902018000117314. [DOI] [Google Scholar]
- 75.de Freitas L.M., Calixto G.M.F., Chorilli M., Giusti J.S.M., Bagnato V.S., Soukos N.S., Amiji M.M., Fontana C.R. Polymeric nanoparticle-based photodynamic therapy for chronic periodontitis in vivo. Int. J. Mol. Sci. 2016;17(5):E769. doi: 10.3390/ijms17050769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sakima V.T., Barbugli P.A., Cerri P.S., Chorilli M., Carmello J.C., Pavarina A.C., Mima E.G.O. Antimicrobial photodynamic therapy mediated by curcumin-loaded polymeric nanoparticles in a murine model of oral candidiasis. Molecules. 2018;23(8):E2075. doi: 10.3390/molecules23082075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao M., van Straten D., Broekman M.L.D., Préat V., Schiffelers R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics. 2020;10(3):1355–1372. doi: 10.7150/thno.38147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahmoud B.S., AlAmri A.H., McConville C. Polymeric nanoparticles for the treatment of malignant gliomas. Cancers (Basel) 2020;12(1):1–28. doi: 10.3390/cancers12010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuo Y-C., Chen Y-C. Targeting delivery of etoposide to inhibit the growth of human glioblastoma multiforme using lactoferrin- and folic acid-grafted poly(lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2015;479(1):138–149. doi: 10.1016/j.ijpharm.2014.12.070. [DOI] [PubMed] [Google Scholar]
- 80.Kuo Y-C., Chang Y-H., Rajesh R. Targeted delivery of etoposide, carmustine and doxorubicin to human glioblastoma cells using methoxy poly(ethylene glycol)-poly(ε-caprolactone) nanoparticles conjugated with wheat germ agglutinin and folic acid. Mater. Sci. Eng. C. 2019;96(96):114–128. doi: 10.1016/j.msec.2018.10.094. [DOI] [PubMed] [Google Scholar]
- 81.Karim R., Palazzo C., Evrard B., Piel G. Nanocarriers for the treatment of glioblastoma multiforme: Current state-of-the-art. J. Control. Release. 2016;227:23–37. doi: 10.1016/j.jconrel.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 82.Malinovskaya Y., Melnikov P., Baklaushev V., Gabashvili A., Osipova N., Mantrov S., Ermolenko Y., Maksimenko O., Gorshkova M., Balabanyan V., Kreuter J., Gelperina S. Delivery of doxorubicin-loaded PLGA nanoparticles into U87 human glioblastoma cells. Int. J. Pharm. 2017;524(1-2):77–90. doi: 10.1016/j.ijpharm.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 83.Maksimenko O., Malinovskaya J., Shipulo E., Osipova N., Razzhivina V., Arantseva D., Yarovaya O., Mostovaya U., Khalansky A., Fedoseeva V., Alekseeva A., Vanchugova L., Gorshkova M., Kovalenko E., Balabanyan V., Melnikov P., Baklaushev V., Chekhonin V., Kreuter J., Gelperina S. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int. J. Pharm. 2019;572:118733. doi: 10.1016/j.ijpharm.2019.118733. [DOI] [PubMed] [Google Scholar]
- 84.Pereverzeva E., Treschalin I., Treschalin M., Arantseva D., Ermolenko Y., Kumskova N., Maksimenko O., Balabanyan V., Kreuter J., Gelperina S. Toxicological study of doxorubicin-loaded PLGA nanoparticles for the treatment of glioblastoma. Int. J. Pharm. 2019;554:161–178. doi: 10.1016/j.ijpharm.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 85.Sayiner O., Arisoy S., Comoglu T., Ozbay F.G., Esendagli G. Development and in vitro evaluation of temozolomide-loaded plga nanoparticles in a thermoreversible hydrogel system for local administration in glioblastoma multiforme. J. Drug Deliv. Sci. Technol. 2020;57:101627. doi: 10.1016/j.jddst.2020.101627. [DOI] [Google Scholar]
- 86.Emamgholizadeh Minaei S., Khoei S., Khoee S., Karimi M.R. Tri-block copolymer nanoparticles modified with folic acid for temozolomide delivery in glioblastoma. Int. J. Biochem. Cell Biol. 2019;108(108):72–83. doi: 10.1016/j.biocel.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 87.Sousa F., Dhaliwal H.K., Gattacceca F., Sarmento B., Amiji M.M. Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles. J. Control. Release. 2019;309:37–47. doi: 10.1016/j.jconrel.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 88.Di Filippo L.D., dos Santos K.C., Hanck-Silva G., de Lima F.T., Gremião M.P.D., Chorilli M. A critical review of biological properties, delivery systems and analytical/bioanalytical methods for determination of bevacizumab. Crit Rev Anal Chem. 2020:1–9. doi: 10.1080/10408347.2020.1743641. [DOI] [PubMed] [Google Scholar]
- 89.Sousa F., Cruz A., Fonte P., Pinto I.M., Neves-Petersen M.T., Sarmento B. A new paradigm for antiangiogenic therapy through controlled release of bevacizumab from PLGA nanoparticles. Sci. Rep. 2017;7(1):3736. doi: 10.1038/s41598-017-03959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Figueiró F., de Oliveira C.P., Rockenbach L., Mendes F.B., Bergamin L.S., Jandrey E.H.F., Edelweiss M.I., Guterres S.S., Pohlmann A.R., Battastini A.M.O. Pharmacological improvement and preclinical evaluation of methotrexate-loaded lipid-core nanocapsules in a glioblastoma model. J. Biomed. Nanotechnol. 2015;11(10):1808–1818. doi: 10.1166/jbn.2015.2125. [DOI] [PubMed] [Google Scholar]
- 91.Pereira N.R.C., Loiola R.A., Rodrigues S.F., de Oliveira C.P., Büttenbender S.L., Guterres S.S., Pohlmann A.R., Farsky S.H. Mechanisms of the effectiveness of poly(ε-caprolactone) lipid- core nanocapsules loaded with methotrexate on glioblastoma multiforme treatment. Int. J. Nanomedicine. 2018;13:4563–4573. doi: 10.2147/IJN.S168400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aparicio-Blanco J., Torres-Suárez A-I. Glioblastoma multiforme and lipid nanocapsules: a review. J. Biomed. Nanotechnol. 2015;11(8):1283–1311. doi: 10.1166/jbn.2015.2084. [DOI] [PubMed] [Google Scholar]
- 93.Yokoyama M. Polymeric micelles as drug carriers: their lights and shadows. J. Drug Target. 2014;22(7):576–583. doi: 10.3109/1061186X.2014.934688. [DOI] [PubMed] [Google Scholar]
- 94.Reddy B.P.K., Yadav H.K.S., Nagesha D.K., Raizaday A., Karim A. Polymeric micelles as novel carriers for poorly soluble drugs--a review. J. Nanosci. Nanotechnol. 2015;15(6):4009–4018. doi: 10.1166/jnn.2015.9713. [DOI] [PubMed] [Google Scholar]
- 95.Zhan C., Gu B., Xie C., Li J., Liu Y., Lu W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J. Control. Release. 2010;143(1):136–142. doi: 10.1016/j.jconrel.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 96.Glaser T., Han I., Wu L., Zeng X. Targeted nanotechnology in glioblastoma multiforme. Front. Pharmacol. 2017;8(March):166. doi: 10.3389/fphar.2017.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seo J.W., Ang J., Mahakian L.M., Tam S., Fite B., Ingham E.S., Beyer J., Forsayeth J., Bankiewicz K.S., Xu T., Ferrara K.W. Self-assembled 20-nm (64)Cu-micelles enhance accumulation in rat glioblastoma. J. Control. Release. 2015;220(Pt A):51–60. doi: 10.1016/j.jconrel.2015.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greish K., Jasim A., Parayath N., Abdelghany S., Alkhateeb A., Taurin S., Nehoff H. Micellar formulations of Crizotinib and Dasatinib in the management of glioblastoma multiforme. J. Drug Target. 2018;26(8):692–708. doi: 10.1080/1061186X.2017.1419357. [DOI] [PubMed] [Google Scholar]
- 99.Liu X., Li W., Chen T., Yang Q., Huang T., Fu Y., Gong T., Zhang Z. Hyaluronic acid-modified micelles encapsulating Gem-C12 and HNK for glioblastoma multiforme chemotherapy. Mol. Pharm. 2018;15(3):1203–1214. doi: 10.1021/acs.molpharmaceut.7b01035. [DOI] [PubMed] [Google Scholar]
- 100.Shamul J.G., Shah S.R., Kim J., Schiapparelli P., Vazquez-Ramos C.A., Lee B.J., Patel K.K., Shin A., Quinones-Hinojosa A., Green J.J. Verteporfin-loaded anisotropic poly(beta-amino ester)-based micelles demonstrate brain cancer-selective cytotoxicity and enhanced pharmacokinetics. Int. J. Nanomedicine. 2019;14:10047–10060. doi: 10.2147/IJN.S231167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Quader S., Liu X., Chen Y., Mi P., Chida T., Ishii T., Miura Y., Nishiyama N., Cabral H., Kataoka K. cRGD peptide-installed epirubicin-loaded polymeric micelles for effective targeted therapy against brain tumors. J. Control. Release. 2017;258:56–66. doi: 10.1016/j.jconrel.2017.04.033. [DOI] [PubMed] [Google Scholar]
- 102.Miura Y., Takenaka T., Toh K., Wu S., Nishihara H., Kano M.R., Ino Y., Nomoto T., Matsumoto Y., Koyama H., Cabral H., Nishiyama N., Kataoka K. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS Nano. 2013;7(10):8583–8592. doi: 10.1021/nn402662d. [DOI] [PubMed] [Google Scholar]
- 103.Chen X., Tai L., Gao J., Qian J., Zhang M., Li B., Xie C., Lu L., Lu W., Lu W. A stapled peptide antagonist of MDM2 carried by polymeric micelles sensitizes glioblastoma to temozolomide treatment through p53 activation. J. Control. Release. 2015;218:29–35. doi: 10.1016/j.jconrel.2015.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wade M., Li Y-C., Wahl G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer. 2013;13(2):83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu Y., Shen M., Li Y., Sun Y., Teng Y., Wang Y., Duan Y. The synergic antitumor effects of paclitaxel and temozolomide co-loaded in mPEG-PLGA nanoparticles on glioblastoma cells. Oncotarget. 2016;7(15):20890–20901. doi: 10.18632/oncotarget.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chu L., Wang A., Ni L., Yan X., Song Y., Zhao M., Sun K., Mu H., Liu S., Wu Z., Zhang C. Nose-to-brain delivery of temozolomide-loaded PLGA nanoparticles functionalized with anti-EPHA3 for glioblastoma targeting. Drug Deliv. 2018;25(1):1634–1641. doi: 10.1080/10717544.2018.1494226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malhotra M., Sekar T.V., Ananta J.S., Devulapally R., Afjei R., Babikir H.A., Paulmurugan R., Massoud T.F. Targeted nanoparticle delivery of therapeutic antisense microRNAs presensitizes glioblastoma cells to lower effective doses of temozolomide in vitro and in a mouse model. Oncotarget. 2018;9(30):21478–21494. doi: 10.18632/oncotarget.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Godara S., Lather V., Kirthanashri S.V., Awasthi R., Pandita D. Lipid-PLGA hybrid nanoparticles of paclitaxel: Preparation, characterization, in vitro and in vivo evaluation. Mater. Sci. Eng. C. 2020;109:110576. doi: 10.1016/j.msec.2019.110576. [DOI] [PubMed] [Google Scholar]
- 109.Khan M.M., Madni A., Torchilin V., Filipczak N., Pan J., Tahir N., Shah H. Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Deliv. 2019;26(1):765–772. doi: 10.1080/10717544.2019.1642420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Agrawal U., Chashoo G., Sharma P.R., Kumar A., Saxena A.K., Vyas S.P. Tailored polymer-lipid hybrid nanoparticles for the delivery of drug conjugate: dual strategy for brain targeting. Colloids Surf. B Biointerfaces. 2015;126(126):414–425. doi: 10.1016/j.colsurfb.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 111.Sekerdag E., Lüle S., Bozdağ Pehlivan S., Öztürk N., Kara A., Kaffashi A., Vural I., Işıkay I., Yavuz B., Oguz K.K., Söylemezoğlu F., Gürsoy-Özdemir Y., Mut M. A potential non-invasive glioblastoma treatment: Nose-to-brain delivery of farnesylthiosalicylic acid incorporated hybrid nanoparticles. J. Control. Release. 2017;261:187–198. doi: 10.1016/j.jconrel.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 112.Shi K., Zhou J., Zhang Q., Gao H., Liu Y., Zong T., He Q. Arginine-glycine-aspartic acid-modified lipid-polymer hybrid nanoparticles for docetaxel delivery in glioblastoma multiforme. J. Biomed. Nanotechnol. 2015;11(3):382–391. doi: 10.1166/jbn.2015.1965. [DOI] [PubMed] [Google Scholar]
- 113.Orunoğlu M., Kaffashi A., Pehlivan S.B., Şahin S., Söylemezoğlu F., Oğuz K.K., Mut M. Effects of curcumin-loaded PLGA nanoparticles on the RG2 rat glioma model. Mater. Sci. Eng. C. 2017;78:32–38. doi: 10.1016/j.msec.2017.03.292. [DOI] [PubMed] [Google Scholar]
- 114.Tahir N., Tahir Haseeb M., Madni A., Parveen F., Muzamil Khan M., Khan S., Jan N., Khan A. Lipid polymer hybrid nanoparticles: a novel approach for drug delivery. Role Nov. Drug Deliv. Veh. Nanobiomedicine. 2020 doi: 10.5772/intechopen.88269. [DOI] [Google Scholar]
- 115.Zhong Y., Wang C., Cheng R., Cheng L., Meng F., Liu Z., Zhong Z. cRGD-directed, NIR-responsive and robust AuNR/PEG-PCL hybrid nanoparticles for targeted chemotherapy of glioblastoma in vivo. J. Control. Release. 2014;195:63–71. doi: 10.1016/j.jconrel.2014.07.054. [DOI] [PubMed] [Google Scholar]
- 116.Kaffashi A., Lüle S., Bozdağ Pehlivan S., Sarısözen C., Vural İ., Koşucu H., Demir T., Buğdaycı K.E., Söylemezoğlu F., Karlı Oğuz K., Mut M. Farnesylthiosalicylic acid-loaded lipid-polyethylene glycol-polymer hybrid nanoparticles for treatment of glioblastoma. J. Pharm. Pharmacol. 2017;69(8):1010–1021. doi: 10.1111/jphp.12740. [DOI] [PubMed] [Google Scholar]
- 117.Pugazhendhi A., Edison T.N.J.I., Karuppusamy I., Kathirvel B. Inorganic nanoparticles: A potential cancer therapy for human welfare. Int. J. Pharm. 2018;539(1-2):104–111. doi: 10.1016/j.ijpharm.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 118.Evans E.R., Bugga P., Asthana V., Drezek R. Metallic nanoparticles for cancer immunotherapy. Mater Today. 2018;21(6):673–685. doi: 10.1016/j.mattod.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Salas G., Costo R., del Puerto Morales M. Synthesis of Inorganic Nanoparticles. 2012;4 doi: 10.1016/B978-0-12-415769-9.00002-9. [DOI] [Google Scholar]
- 120.Noah N. Green Synthesis: Characterization and Application of Silver and Gold Nanoparticles. Elsevier Inc.; 2019. [Google Scholar]
- 121.Carvalho A., Fernandes A.R., Baptista P.V. Nanoparticles as Delivery Systems in Cancer Therapy. Elsevier Inc.; 2019. [DOI] [Google Scholar]
- 122.Rizvi S.A.A., Saleh A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018;26(1):64–70. doi: 10.1016/j.jsps.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pinel S., Thomas N., Boura C., Barberi-Heyob M. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv. Drug Deliv. Rev. 2019;138:344–357. doi: 10.1016/j.addr.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 124.Huang H.C., Barua S., Sharma G., Dey S.K., Rege K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release. 2011;155(3):344–357. doi: 10.1016/j.jconrel.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 125.López T., Recillas S., Guevara P., Sotelo J., Alvarez M., Odriozola J.A. Pt/TiO2 brain biocompatible nanoparticles: GBM treatment using the C6 model in Wistar rats. Acta Biomater. 2008;4(6):2037–2044. doi: 10.1016/j.actbio.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 126.Kim J.E., Kim H., An S.S.A., Maeng E.H., Kim M.K., Song Y.J. In vitro cytotoxicity of SiO2 or ZnO nanoparticles with different sizes and surface charges on U373MG human glioblastoma cells. Int. J. Nanomedicine. 2014;9(Suppl. 2):235–241. doi: 10.2147/IJN.S57936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wahab R., Kaushik N., Khan F., Kaushik N.K., Choi E.H., Musarrat J., Al-Khedhairy A.A. Self-Styled ZnO Nanostructures promotes the cancer cell damage and supresses the epithelial phenotype of glioblastoma. Sci. Rep. 2015;2016(6):1–13. doi: 10.1038/srep19950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Altunbek M., Keleştemur S., Baran G., Çulha M. Role of modification route for zinc oxide nanoparticles on protein structure and their effects on glioblastoma cells. Int. J. Biol. Macromol. 2018;118(Pt A):271–278. doi: 10.1016/j.ijbiomac.2018.06.059. [DOI] [PubMed] [Google Scholar]
- 129.Eugenio M., Campanati L., Müller N., Romão L.F., de Souza J., Alves-Leon S., de Souza W., Sant’Anna C. Silver/silver chloride nanoparticles inhibit the proliferation of human glioblastoma cells. Cytotechnology. 2018;70(6):1607–1618. doi: 10.1007/s10616-018-0253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Urbańska K., Pająk B., Orzechowski A., Sokołowska J., Grodzik M., Sawosz E., Szmidt M., Sysa P. The effect of silver nanoparticles (AgNPs) on proliferation and apoptosis of in ovo cultured glioblastoma multiforme (GBM) cells. Nanoscale Res. Lett. 2015;10(1):98. doi: 10.1186/s11671-015-0823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mortezaee K., Najafi M., Samadian H., Barabadi H., Azarnezhad A., Ahmadi A. Redox interactions and genotoxicity of metal-based nanoparticles: A comprehensive review. Chem. Biol. Interact. 2019;312:108814. doi: 10.1016/j.cbi.2019.108814. [DOI] [PubMed] [Google Scholar]
- 132.Paunovic J., Vucevic D., Radosavljevic T., Mandić-Rajčević S., Pantic I. Iron-based nanoparticles and their potential toxicity: focus on oxidative stress and apoptosis. Chem. Biol. Interact. 2020;316 doi: 10.1016/j.cbi.2019.108935. [DOI] [PubMed] [Google Scholar]
- 133.Dayem A.A., Hossain M.K., Lee S. Bin; Kim, K.; Saha, S. K.; Yang, G. M.; Choi, H. Y.; Cho, S. G. The role of reactive oxygen species (ros) in the biological activities of metallic nanoparticles. Int. J. Mol. Sci. 2017;18(1):1–21. doi: 10.3390/ijms18010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Alle, M.; G, B, reddy.; Kim, reT. Hddy. Park, S. H.; Lee, S. H.; Kim, J. C. Doxorubicin-carboxymethyl xanthan gum capped gold nanoparticles: microwave synthesis, characterization, and anti-cancer activity. Carbohydr. Polym. 2019;229:115511. doi: 10.1016/j.carbpol.2019.115511. [DOI] [PubMed] [Google Scholar]
- 135.Liu P., Jin H., Guo Z., Ma J., Zhao J., Li D., Wu H., Gu N. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int. J. Nanomedicine. 2016;11:5003–5014. doi: 10.2147/IJN.S115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bouras A., Kaluzova M., Hadjipanayis C.G. Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibody-conjugated iron-oxide nanoparticles. J. Neurooncol. 2015;124(1):13–22. doi: 10.1007/s11060-015-1807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sábio R.M., Meneguin A.B., Ribeiro T.C., Silva R.R., Chorilli M. New insights towards mesoporous silica nanoparticles as a technological platform for chemotherapeutic drugs delivery. Int. J. Pharm. 2019;564:379–409. doi: 10.1016/j.ijpharm.2019.04.067. [DOI] [PubMed] [Google Scholar]
- 138.Hou J., Diao Y., Li W., Yang Z., Zhang L., Chen Z., Wu Y. RGD peptide conjugation results in enhanced antitumor activity of PD0325901 against glioblastoma by both tumor-targeting delivery and combination therapy. Int. J. Pharm. 2016;505(1-2):329–340. doi: 10.1016/j.ijpharm.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 139.Li X., Hou J., Wang C., Liu X., He H., Xu P., Yang Z., Chen Z., Wu Y., Zhang L. Synthesis and biological evaluation of RGD-conjugated MEK1/2 kinase inhibitors for integrin-targeted cancer therapy. Molecules. 2013;18(11):13957–13978. doi: 10.3390/molecules181113957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mo J., He L., Ma B., Chen T. Tailoring particle size of mesoporous silica nanosystem to antagonize glioblastoma and overcome blood-brain barrier. ACS Appl. Mater. Interfaces. 2016;8(11):6811–6825. doi: 10.1021/acsami.5b11730. [DOI] [PubMed] [Google Scholar]
- 141.You Y., Yang L., He L., Chen T. Tailored mesoporous silica nanosystem with enhanced permeability of the blood-brain barrier to antagonize glioblastoma. J. Mater. Chem. B Mater. Biol. Med. 2016;4(36):5980–5990. doi: 10.1039/C6TB01329E. [DOI] [PubMed] [Google Scholar]
- 142.Li Z.Y., Liu Y., Wang X.Q., Liu L.H., Hu J.J., Luo G.F., Chen W.H., Rong L., Zhang X.Z. One-pot construction of functional mesoporous silica nanoparticles for the tumor-acidity-activated synergistic chemotherapy of glioblastoma. ACS Appl. Mater. Interfaces. 2013;5(16):7995–8001. doi: 10.1021/am402082d. [DOI] [PubMed] [Google Scholar]
- 143.Ahmadi Nasab N., Hassani Kumleh H., Beygzadeh M., Teimourian S., Kazemzad M. Delivery of curcumin by a pH-responsive chitosan mesoporous silica nanoparticles for cancer treatment. Artif. Cells Nanomed. Biotechnol. 2018;46(1):75–81. doi: 10.1080/21691401.2017.1290648. [DOI] [PubMed] [Google Scholar]
- 144.Kusaczuk M., Krętowski R., Naumowicz M., Stypułkowska A., Cechowska-Pasko M. Silica nanoparticle-induced oxidative stress and mitochondrial damage is followed by activation of intrinsic apoptosis pathway in glioblastoma cells. Int. J. Nanomedicine. 2018;13:2279–2294. doi: 10.2147/IJN.S158393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Krętowski R., Kusaczuk M., Naumowicz M., Kotyńska J., Szynaka B., Cechowska-Pasko M. The effects of silica nanoparticles on apoptosis and autophagy of glioblastoma cell lines. Nanomaterials. 2017;7(8):230. doi: 10.3390/nano7080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang P., Tang M., Huang Q., Zhao G., Huang N., Zhang X., Tan Y., Cheng Y. Combination of 3-methyladenine therapy and Asn-Gly-Arg (NGR)-modified mesoporous silica nanoparticles loaded with temozolomide for glioma therapy in vitro. Biochem. Biophys. Res. Commun. 2019;509(2):549–556. doi: 10.1016/j.bbrc.2018.12.158. [DOI] [PubMed] [Google Scholar]
- 147.Mishra P., Ray S., Sinha S., Das B., Khan M.I., Behera S.K., Yun S. Il; Tripathy, S. K.; Mishra, A. Facile bio-synthesis of gold nanoparticles by using extract of hibiscus sabdariffa and evaluation of its cytotoxicity against u87 glioblastoma cells under hyperglycemic condition. Biochem. Eng. J. 2016;105:264–272. doi: 10.1016/j.bej.2015.09.021. [DOI] [Google Scholar]
- 148.Groysbeck N., Stoessel A., Donzeau M., da Silva E.C., Lehmann M., Strub J.M., Cianferani S., Dembélé K., Zuber G. Synthesis and biological evaluation of 2.4 nm thiolate-protected gold nanoparticles conjugated to Cetuximab for targeting glioblastoma cancer cells via the EGFR. Nanotechnology. 2019;30(18):184005. doi: 10.1088/1361-6528/aaff0a. [DOI] [PubMed] [Google Scholar]
- 149.Wang Y., Wang K., Zhao J., Liu X., Bu J., Yan X., Huang R. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 2013;135(12):4799–4804. doi: 10.1021/ja312221g. [DOI] [PubMed] [Google Scholar]
- 150.Xiao D., Jia H.Z., Zhang J., Liu C.W., Zhuo R.X., Zhang X.Z. A dual-responsive mesoporous silica nanoparticle for tumor-triggered targeting drug delivery. Small. 2014;10(3):591–598. doi: 10.1002/smll.201301926. [DOI] [PubMed] [Google Scholar]
- 151.Salvi V. R., Pawar P. Journal of drug delivery science and technology. Editions de Sante; 2019. Nanostructured Lipid Carriers (NLC) system: A Novel drug targeting carrier. pp. 255–267. [DOI] [Google Scholar]
- 152.Sintov A.C., Velasco-Aguirre C., Gallardo-Toledo E., Araya E., Kogan M.J. Metal Nanoparticles as Targeted Carriers Circumventing the Blood-Brain Barrier. 1st ed. Vol. 130. Elsevier Inc.; 2016. [DOI] [PubMed] [Google Scholar]
- 153.Hua S., de Matos M.B.C., Metselaar J.M., Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front. Pharmacol. 2018;9:790. doi: 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hare J.I., Lammers T., Ashford M.B., Puri S., Storm G., Barry S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017;108:25–38. doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 155.Wu L. P., Wang D., Li Z., Grand Challenges in Nanomedicine. Mater. Sci. Eng. C. 2020;106:110302. doi: 10.1016/j.msec.2019.110302. [DOI] [PubMed] [Google Scholar]
- 156.Wei A., Mehtala J.G., Patri A.K. Challenges and opportunities in the advancement of nanomedicines. J. Control. Release. 2012;164(2):236–246. doi: 10.1016/j.jconrel.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Martins J.P., das Neves J., de la Fuente M., Celia C., Florindo H., Günday-Türeli N., Popat A., Santos J.L., Sousa F., Schmid R., Wolfram J., Sarmento B., Santos H.A. The solid progress of nanomedicine. Drug Deliv. Transl. Res. 2020;10(3):726–729. doi: 10.1007/s13346-020-00743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mühlebach S. Regulatory challenges of nanomedicines and their follow-on versions: A generic or similar approach? Adv. Drug Deliv. Rev. 2018;131:122–131. doi: 10.1016/j.addr.2018.06.024. [DOI] [PubMed] [Google Scholar]