Abstract
Background
A dysfunction in glutamate neurotransmission is critical for seizure. Glutamate is the major excitatory drive in the cerebral cortex, where seizures occur. Glutamate acts via (i) ionotropic (iGlu) receptors, which are ligand-gated ion channels mediating fast excitatory synaptic transmission; and (ii) G proteins coupled metabotropic (mGlu) receptors.
Objective
To overview the evidence on the role of iGlu receptors in the onset, duration, and severity of convulsive and non-convulsive seizures to lay the groundwork for novel strategies for drug-resistant epilepsy.
Methods
We used PubMed crossed-search for “glutamate receptor and epilepsy” (sorting 3,170 reports), searched for “ionotropic glutamate receptors”, “AMPA receptors”, “NMDA receptors”, “kainate receptors”, “convulsive seizures”, “absence epilepsy”, and selected those papers focusing this Review’s scope.
Results
iGlu receptor antagonists inhibit, whereas agonists worsen experimental seizures in various animal species. Clinical development of iGlu receptor antagonists has been limited by the occurrence of adverse effects caused by inhibition of fast excitatory synaptic transmission. To date, only one drug (perampanel) selectively targeting iGlu receptors is marketed for the treatment of focal epilepsy. However, other drugs, such as topiramate and felbamate, inhibit iGlu receptors in addition to other mechanisms.
Conclusion
This review is expected to help dissect those steps induced by iGlu receptors activation, which may be altered to provide antiepileptic efficacy without altering key physiological brain functions, thus improving the safety and tolerability of iGlu-receptor directed antiepileptic agents. This effort mostly applies to drug resistant seizures, which impact the quality of life and often lead to status epilepticus, which is a medical urgency.
Keywords: Ionotropic glutamate receptors, AMPA, NMDA, kainate, epilepsy, convulsive seizures, absence epilepsy
1. INTRODUCTION
1.1. Glutamate, Excitotoxicity and Epileptic Seizures
The word “excitotoxicity” as introduced by Olney [1] meant the “excitation to death” produced by the acidic amino acids glutamate, aspartate and their analogs. These compounds, indeed, were known to damage neurons due to an over-depolarization since the early 1960s [2]. At present, the concept of excitotoxicity pervaded almost all fields in neuroscience research and mostly in neurological disorders [3-5]. This concept, which refers to the presence of endogenous neurotoxins was anticipated by the concept of exogenous neurotoxins-induced cell death. In both cases, it was moststruggling the outcome of a “selective neurotoxicity”, which suggested the presence of a sort of selective binding to limit the damage to a specific neuronal cluster. When applied in the field of epilepsy such a specificity was related to the selective regions being recruited by seizure spreading. On the other hand, this phenomenon needs the presence of specific receptors on post-synaptic neurons, which are able to selectively bind and transduce the input provided by glutamate neurotransmission. The fast post-synaptic alterations provided by the activation of ionotropic glutamate (iGlu) receptors offers the basis of such a selective and massive effect. In the present review, the anatomical and pharmacological basis for the selectivity produced by iGlu receptors activation in the course of epilepsy is provided. At the same time, we discuss the bias which may be induced when a systemic excitotoxin is administered. In fact, in these experimental conditions, the neuronal selectivity for excitotoxicity simply relies on the presence of ionotropic receptors. This condition produces an artifact since the selective pattern of the anatomical circuitry, which is indeed recruited, is lost. Thus, two conditions generate the selective excitotoxicity in seizures and epilepsy: (i) the presence of specific glutamate receptors; (ii) the brain sites selectively recruited during the natural seizure spreading.
The occurrence of excitotoxicity requires an altered status which may be simplified by a few items which lose their balance: (i) excessive and/or prolonged release of glutamate into the synaptic cleft, (ii) impaired clearance of glutamate from the extracellular space and (iii) impairment of surrounding GABAergic inhibition. This imbalance between glutamate and GABA seems to be critical. For instance, in a seminal paper, Sloviter et al. [6] showed the co- existence of GABA within glutamatergic hippocampal mossy fibers and demonstrated an activity-dependent (compensatory?) increase in GABA synthesis. The preferential zonal representations of glutamate receptors could be a critical factor for the selective vulnerability of different classes of neurons to different insults [7]. Interestingly, when acute insults are repeated, the neuronal loss progresses downstream to synaptically linked neurons. This trans-synaptic progression of neuronal death after repeated acute insults (e.g. epilepsy) resembles what slowly occurs in neurodegenerative diseases in which the “systemic degeneration” consists of spreading cell loss to neurons interconnected in functional circuits. This was postulated in 1997 by Fornai et al. [8], and clearly established by Freire [9], who reported the occurrence of primary traumatic events followed by secondary pathological events contributing to tissue damage where glutamate release plays a leading role, and recently by Kaur and Sharma [10] who posed the role of excitotoxicity in the course of events which sustain the natural course of neuronal damage following an acute traumatic event.
2. IONOTROPIC GLUTAMATE RECEPTORS AS TARGET IN EPILEPSY
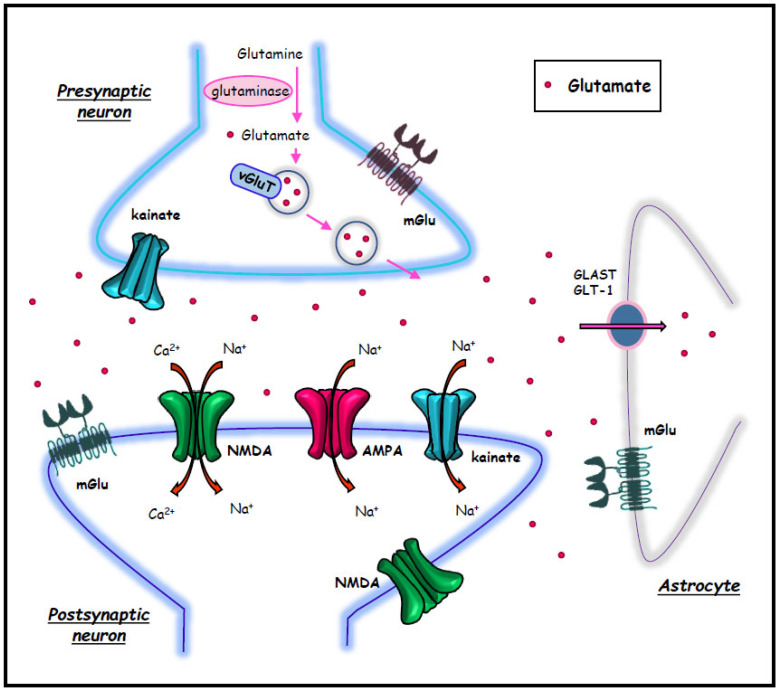
An imbalance between excitatory and inhibitory neurotransmission lies at the core of the pathophysiology of epilepsies, independently of the specific syndrome and disease. This is expected to modulate seizure onset, spreading, spontaneous resolution, along with seizure-induced sensitization (kindling) and seizure-induced brain damage. Apart from the literature concerning the inhibitory effect played by GABA, when the excitatory over-activity is considered, most data refer to the excitatory role of glutamic acid, which represents the prevalent excitatory neurotransmitter in the mammalian brain (Fig. 1). This explains why drugs acting at glutamate receptors potentially alter all seizure-related events; similarly, drugs acting as agonists and (or) antagonists at specific glutamate receptors are strong candidates to treat epilepsies. Despite a plethora of findings and review articles on the role of glutamate receptors in seizures, the present review is an attempt to provide novel insights to finely dissect the role of iGlu receptors in the context of specific, still questionable, seizure-related events [11-16]. In keeping with this introductory statement, it is mandatory to distinguish three classic types of iGlu receptors: AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), NMDA (N-Methyl-D-aspartic acid), and kainate receptors. All these receptor types are structured as ligand-gated ion channels; thus, upon binding with endogenous glutamate, specific inward cation currents are produced due to channel openings. This contrasts with metabotropic glutamate (mGlu) receptors, which, once binding extracellular glutamate, activate specific intracellular biochemical pathways through an intramembrane G protein. The role of mGlu receptors in the pathophysiology of convulsive and absence epilepsy has been extensively discussed in a number of review articles [12, 15, 17-24].
Fig. (1).
Glutamate synapse components. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Glutamate is the principal excitatory neurotransmitter of the mammalian brain. The major source of glutamate in the brain is glutamine, which is converted in glutamate via glutaminase enzyme. The vesicular glutamate transporter, vGluT, mediates the increased concentration of glutamate in synaptic vesicles in the axon terminals. Glutamate acts via ionotropic receptors (NMDA, AMPA, and kainate) and metabotropic (mGlu) receptors. Astrocytes play a major role in glutamate reuptake through GLAST and GLT-1 transporters.
All iGlu receptors are heterotetramers, each subunit being formed by three trans-membrane (TM) domains (TM1, -3 and -4) and an incomplete TM2 domain, which does not cross the membrane but kinks back on itself within the membrane. This allows the intracellular C-terminus receptor domain to interact with a number of scaffolding and adaptor proteins. The incomplete TM2 domain contributes to build the wall of an ion channel, and its amino acid composition confers specific ion selectivity (i.e., the Na+ to Ca2+ ratio) to the three iGlu receptors. This feature considerably varies depending on the combination of receptor subunits that contribute to forming the whole iGlu receptor subtype. In fact, in spite of three classic receptor types, iGlu receptors may vary depending on specific subunit composition and assembly, which in turn may be altered by ongoing synaptic activity via epigenetic alterations. In most cases, AMPA receptors are impermeable to Ca2+ in most of the synapses due to the presence of a positively charged arginine residue in the TM2 of the GluA2 subunit (see paragraph 3) [25-28]. AMPA receptor permeability may vary depending on several conditions. Similarly, the overall impact of each iGlu receptors in the pathophysiology of epilepsy depends on a high number of variables, such as their surface expression, opening frequency and dissociation kinetics, cation selectivity, expression of interacting proteins, regulation of intracellular transduction mechanisms. For instance, S-palmitoylation of the receptor allows a reversible covalent link of the receptor protein to membrane lipids, which regulates a variety of iGlu receptors properties such as: synaptic and membrane expression, intracellular placement, and the type of scaffolding proteins. In general, it is reported that a defective iGlu receptor palmitoylation may cause neuronal hyperexcitability and fosters the onset of epileptic seizures [29].
Here, we will discuss the specific role of each iGlu receptors in the pathophysiology of seizures and the potential treatment of epilepsy.
In order to provide a vision “at a glance” of those concepts developed in the review, we built a summarizing table (Table 1- Summary of specific ionotropic glutamate receptors, their ligands and mechanisms of action).
Table 1.
Summary of specific ionotropic glutamate receptors, their ligands and mechanisms of action. AMPA receptors and convulsive seizures. Competitive antagonists of AMPA receptors.
| Compound | Subject | Effect | Refs. |
|---|---|---|---|
| NBQX | DBA/2 mice | protects against sound-induced seizures | [60] |
| rat amygdala-kindling model | decreases the duration of after discharge activity | [62] | |
| mouse model of mesial temporal lobe epilepsy | attenuates spontaneous seizures and occludes limbic seizure-induced brain damage | [64] | |
| Long-Evans rats | suppression of behavioral alterations and the onset of spontaneous secondary seizures | [65] | |
| YM872 | rat amygdala kindling model | suppressed fully kindled seizures | [68] |
| YM90K | DBA/2 mice | potent suppressive activity against audiogenic seizure | [69] |
| rat amygdala-kindling model | suppressed fully kindled seizures | [70] | |
| LY293558 | mouse | protection versus maximal electroshock seizures and decreases in spontaneous motor activity | [73] |
| RPR117824 | mouse or rat | blocker of convulsions induced by supramaximal electroshock or chemoconvulsive agents | [74] |
| NS1209 | mouse | increased the seizure threshold for electroshock-induced tonic seizures | [76] |
| rat | protects against status epilepticus induced by electrical stimulation of the amygdala | [77] |
3. AMPA RECEPTORS, SEIZURES AND EPILEPSY
AMPA receptors consist in the heterotetrameric combination of GluA1-4 subunits, encoded by the GRIA1-4 genes [30, 31]. The incomplete TM2 domain of the GluA2 subunit contains an arginine residue in position 607, which restrains Ca2+ influx. Thus, AMPA receptors typically own a high Na+ to Ca2+ ratio concerning channel permeability [32]. Remarkably, when AMPA receptors constitutively lack GluA2, Ca2+ permeability is present and a similar effect occurs within the context of activity-dependent synaptic plasticity that diminishes the expression of GluA2 [30]. This witnesses how iGlu receptor subunits composition may result from inherited constitutive structure or is the consequence of specific micro-environmental stimuli, which eventually alter subunit gene expression and synaptic plasticity [33]. This may explain why Ca2+-permeable AMPA receptors are mainly expressed on the surface of inhibitory interneurons, which routinely receive a significant excitatory input [34, 35]. AMPA receptors are predominantly expressed at the core of postsynaptic targets, to produce fast excitatory postsynaptic events all over the central nervous system (CNS) [36]. Changes in membrane expression of AMPA receptor subunits are critical to produce long-term potentiation (LTP) and long-term depression (LTD), which represent plastic phenomena concerning excitatory synaptic transmission [37]. These changes are partly due to in-and-out movements of AMPA receptors, which are driven by nanoscale dynamics of receptor organization [36, 37]. GluA1, GluA2, and GluA3 are the most abundant subunits in the forebrain, with the exception of some thalamic nuclei, where GluA4 is also abundant [38].
AMPA receptors are intimately associated with numerous auxiliary proteins, like the transmembrane AMPA receptor regulatory proteins (TARPs), γ-2 (stargazin), γ-3, γ-4, γ-5, γ-7 and γ-8 [39]. TARPs may stabilize AMPA receptors on the cell surface of synaptic areas [40]. A loss of TARPs, reduces the stability and surface expression of AMPA receptors [41, 42]. TARPs also influence the kinetics of AMPA receptor currents and responses to receptor ligands [43-45]. Impairments in the regulation of AMPA receptor function, trafficking, and signalling may produce neuronal hyperexcitability and epileptogenesis, which suggests how AMPA receptors may represent a potential target for epilepsy treatment [46]. The critical role of AMPA receptors in the pathophysiology of epileptic seizures and anti-epileptic treatment is highlighted by elegant reviews [47, 48].
AMPA receptors play a key role in seizure generation and spreading, as demonstrated in human brain regions highly involved in epilepsy, such as the isocortex [49, 50] and limbic regions, such as the entorhinal mesocortex [51].
Egbenya et al. [52] examined long-term changes in synaptic contents of AMPA receptor subunits, which could influence calcium regulation in chronic epilepsy. A relative increase in the expression of GluA2-lacking (Ca2+-permeable) AMPA receptors was found in hippocampal synaptosomes prepared from rats treated with kainic acid, which model temporal lobe epilepsy (TLE, the most common form of refractory focal epilepsy).
3.1. AMPA Receptors and Convulsive Seizures
AMPA receptor antagonists have been developed for the treatment of convulsive epilepsy showing good efficacy in preclinical models [53, 54]. Negative allosteric modulators (NAMs) have a greater clinical potential compared with competitive antagonists due to high PK compatibility. However, a concern was raised by the occurrence of CNS depression with some of these drugs due to the key role played by AMPA receptors in fast excitatory synaptic transmission in the CNS [55]. Accordingly, AMPA receptor antagonists were found to induce neurological side effects at anticonvulsant doses in animal models, although this depends on specific seizure models and testing conditions [56, 57].
3.1.1. Preclinical Data with Competitive Antagonists of AMPA Receptors
Quinoxaline derivatives CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) and NBQX (2,3-dihydroxy-6-nitro-7-sulfamoylbenzo (F) quinoxaline) represent the most common AMPA receptor antagonists [58-63].
NBQX is a competitive AMPA receptor antagonist that showed anti-seizure but not antiepileptogenic activity in the mouse model of mesial temporal lobe epilepsy induced by intrahippocampal injection of kainate [64]. Acute treatment with NBQX soon after hypoxia-induced postnatal seizures attenuates spontaneous seizures and occludes limbic seizure-induced brain damage, as evidenced by hippocampal mossy fiber sprouting [64]. Occlusion of such a pathological alteration, which is reminiscent of medial temporal lobe sclerosis and correlates with secondary epileptogenesis is confirmed by data showing suppression of behavioral alterations and the onset of spontaneous secondary seizures following NBQX in adult Long-Evans rats [65]. This suggests that persistent alterations of AMPA receptors play a role in maladaptive plasticity underlying epilepsy-induced secondary seizures and kindling. In humans, this may be relevant when considering how epileptic seizures in children may promote a later in life occurrence of epilepsy. As expected, NBQX consistently reduces seizure-induced motor activity (convulsions) and, when tested in the experimental model of amygdala kindling, it decreases the duration of after discharge activity [62]. As a direct effect of the antagonism on AMPA receptors, NBQX powerfully occludes AMPA-induced seizures [60]. It is remarkable that NBQX also powerfully mitigates hindbrain seizures induced by sound in DBA/2 mice [60]. Despite being routinely considered as a specific AMPA receptor antagonist, NBQX indeed binds with a noticeable affinity to kainate receptors; thus, the data reported above cannot be considered as necessary due to the role of AMPA receptors, unless the role of kainate receptor is ruled out. This is a common problem with a number of non-NMDA receptor antagonists as it leaves unanswered the specific role of AMPA and kainate receptor as non-NMDA seizure determinants.
In fact the issue remains with additional quinoxalinediones with in vivo anticonvulsant activity, such as YM872 [66-68], YM90K [69, 70], ZK 200775 (MPQX, fanapanel) [71], AMP397 (becampanel) [63, 72], and LY293558 (tezampanel = (3S,4aR,6R,8aR)-6-[2-(1(2)H-Tetrazole-5-yl) ethyl]decahydroisoquinoline-3-carboxylic acid) [73]. Some other competitive AMPA receptor antagonists with in vivo anticonvulsant activity were identified, including the pyrazine derivative RPR117824 [74, 75], and isatin oximes, such as NS1209 (SPD 502) [76, 77], which inhibits GluK1- containing kainate receptors. NS1209 was investigated as an intravenous agent for the treatment of status epilepticus because it is not orally active [78]. Pitkänen et al. [77] demonstrated that NS1209 potently protects against status epilepticus induced by electrical stimulation of the amygdala.
In spite of the promising preclinical findings, competitive antagonists have not advanced to clinical development because of their sub-optimal PK profile (water-soluble molecules, such as CNQX, poorly penetrate the blood-brain barrier, whereas lipophilic molecules, such as NBQX, precipitate in the kidney [79]).
3.1.2. Pharmacological Probing of AMPA vs. Kainate Selectivity in non-NMDA iGlu Receptor-induced Seizures
There are only a few pharmacological tools that can efficiently discriminate between AMPA and kainate receptors, and, therefore, the role played by AMPA or kainate receptors in several seizure models are either over- or underestimated. Within current literature, this mostly applies to AMPA receptors and it is the consequence of the early overestimation of NBQX as a potential selective AMPA receptor antagonist. This led to a number of reports, which attributed to AMPA receptors a number of effects in the field of epilepsies; in contrast, these effects were due to a combined activation of AMPA and kainate receptors [47, 48, 52, 80-82].
At the experimental level, such an issue was solved by using a class of compounds known as desensitization blockers, which allow to discriminate between AMPA and kainate receptors or the ratio of one receptor vs. the other. These probes were validated by patch clamp recording and they are fully disclosed in their molecular mechanisms. Thus, in order to produce an overstimulation, which is purely due to AMPA receptors, we need to administer cychlotiazide, which is a selective blocker of AMPA desensitization. Conversely, when the kainate receptor needs to be overactivated, the kainate desensitization blocker Concanavalin A can be administered. These compounds were used in experimental models to assert and validate the role of AMPA receptor in seizures [83].
In particular, in order to avoid the bias due to systemic administration, the AMPA receptor desensitization blocker, cychlotiazide, was focally administered in the rat within the rostral piriform allocortex, alone or in combination with the GABAA antagonist, bicuculline [83]. In these experimental conditions, the proconvulsant effects of focally administered bicuculline, induced by the relief from baseline GABAergic inhibition, were enhanced by the concomitant microinfusion of cychlotiazide [83].
Noteworthy, while the seizure produced by bicuculline could be suppressed by either D-2-amino-7-phosphonoheptanoic acid (AP7, an NMDA receptor antagonist,) or NBQX (a non-NMDA receptor antagonist), seizures induced by cychlotiazide plus bicuculline were refractory to AP7 and were either prevented or suppressed by NBQX administration [83]. This suggests that, while serial seizures induced by bicuculline were sustained by the combined activity of NMDA and non-NMDA receptors, seizures produced by cychlotiazide plus bicuculline fully depend on over-activation of non-NMDA receptors. Since cychlotiazide is a pure ligand for AMPA receptor subtypes, these seizures fully depend on over-activation of AMPA receptors. Remarkably, these seizures induced by combined drug administration were not merely an amplification phenomenon consisting of more robust seizures compared with that induced by bicuculline alone. In fact, in the presence of bicuculline, serial seizures starting around 5 min following microinjection progressively became more frequent up to a point in which a single seizing episode was no longer discernible, and a continuous persistent long-acting and self-sustaining status epilepticus developed [83]. The duration of such a status epilepticus far exceeded the persistence of bicuculline in the brain, showing that a self-sustaining phenomenon was taking place where the epileptic circuitry downstream the infusion site took a leading role as seizure pacemaker. This demonstrates that, while NBQX was effective to prevent seizure onset and still effective to suppress serial seizures, when limbic status epilepticus is manifest the drug is no longer effective when infused in the same site. Still, some anticonvulsant effect could be obtained by micro-infusing NBQX in brain areas placed downstream of the piriform allocortex in the limbic seizure circuitry [84-86]. Thus, a novel, important role of AMPA receptors in seizure consisted of switching serial seizure episodes (reminiscent of limbic seizures in humans) into self-sustaining status epilepticus (reminiscent of the most common form of status epilepticus in humans, which affects limbic regions associated with mesial temporal lobe sclerosis). This condition is often refractory to drug administration and is associated with brain damage, which may be implicated in secondary epileptogenesis leading to cognitive impairment. The duration of such a status epilepticus may last for days, and while no convulsive activity is detected in response to diazepam administration, electroencephalography (EEG) abnormalities are not suppressed, as convulsions reappear when diazepam-induced sedation vanishes. AMPA receptors are critically involved in two phenomena that influence the clinical outcome of epilepsy, i.e., drug resistance and the onset of status epilepticus. From a physiological perspective, AMPA receptors are likely to induce a spontaneously epileptic circuitry in which the ongoing epileptic activity increases synaptic strength as a consequence of maladaptive plasticity. Accordingly, GluA2 subunit-containing AMPA receptors show a transition to a higher conductance state in the presence of cyclothiazide, which stabilizes the open configuration of the receptor prolonging the depolarization phase [87].
3.1.3. Negative Allosteric Modulators of AMPA Receptors: From the Bench to the Clinic
In line with the previous data, administering GYKI 52466 (1-(4-aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine), the prototypic compound of the 2,3-benzodiazepine family, a highly selective, non-competitive AMPA receptor antagonist produces similar evidence concerning the significance AMPA receptors and seizures. Fritsch et al. [88] evaluated the effect of GYKI 52466 on early and late kainate-induced SE, demonstrating that GYKI 52466 inhibited both seizure activity and recurrence.
Yamaguchi et al. [56] compared the anticonvulsant activities of GYKI 52466 and NBQX in the maximal electroshock seizure (MES) test, where both were found to be protective. GYKI 52466 was also able to protect against seizures and lethality induced by 4-aminopyridine, kainate and AMPA, whereas NBQX was ineffective [56]. Talampanel (GYKI 53773, LY300164 = 7-acetyl-5-(4-aminophenyl)-8,9-dihydro-8 methyl-7H-1, 3 dioxolo(4, 5H)-2,3-benzodiazepine), a selective non-competitive antagonist of AMPA receptors, showed protective activity in the kainate-induced neonatal status epilepticus model [89].
Topiramate is an antiepileptic drug approved for the treatment of partial seizures, primarily generalized seizures, and seizures associated with Lennox-Gastaut syndrome in children [90, 91]. Its mechanisms of action include blockade of voltage-sensitive sodium channels, enhancement of GABAergic transmission, and antagonism of AMPA/kainate receptors [92, 93]. Topiramate showed antiepileptic and neuroprotective properties inhibiting responses to kainate and AMPA in cultured neurons [94, 95]. Topiramate also reduced high basal concentrations of extracellular glutamate in the hippocampus of spontaneously epileptic rats [96]. The drug was found to selectively inhibit pharmacologically isolated GluK1 kainate receptor-mediated postsynaptic currents, whereas AMPA receptor-mediated currents were only modestly reduced [97]. Topiramate afforded protection against seizures induced by intravenous infusion of the selective GluK1-containing kainate receptor agonist, ATPA, but was less effective on clonic seizures induced by AMPA or NMDA. This indicates that the selective interaction of topiramate with GluK1-containing kainate receptors is relevant to its anticonvulsant properties [98].
Consistently with the effects of AMPA receptors reported in paragraph 2, topiramate dose-dependently reduces spike-and-wave discharges (SWDs) in Wistar Audiogenic Sensitive (AS) rats, an audiogenic model of convulsive seizures [99].
There is evidence that topiramate can be clinically effective in treating different forms of status epilepticus [100]. A recent study showed that topiramate reduces seizures when administered as an add-on treatment for drug-resistant focal epilepsy [101].
Perampanel 2-(2-oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydropyridin-3-yl)benzonitrile, a potent, selective, and orally-active AMPA receptor NAM, has been approved by FDA and EMA for the treatment of focal epilepsy and generalized tonic-clonic epilepsy [102-106]. Perampanel selectively inhibits AMPA receptors at concentrations falling within the therapeutic range, although it can also inhibit NMDA and kainate receptors at higher concentrations [107]. Interestingly, perampanel also restrains myoclonic or absence seizures [108, 109], and, in animals, shows protection against audiogenic seizures [47], pentylenetetrazole (PTZ)-induced clonic seizures and psychomotor seizures in the 6-Hz stimulation protocol [110].
Perampanel shortens after-discharge activity and prolongs latency to onset of generalized seizures in the amygdala kindling [111, 112], while blocking pilocarpine-induced status epilepticus [113]. Augustin et al. [114] demonstrated that the combined use of perampanel and dietary decanoic acid (also acting as a non-competitive AMPA receptor antagonist) act synergistically in restraining seizures in human brain slices.
Perampanel is well tolerated in children and adolescents with focal epilepsy [115]. Perampanel also showed good efficacy and tolerability in young children with intractable epilepsy [116].
Experience from clinical trials indicates that AMPA receptor antagonists show a good tolerability profile, although they may cause transient sedation, an effect that is also observed in animal models [56]. AMPA receptor antagonists were found to be safe and effective in patients with partial-onset seizures [105].
Clinical studies have also shown that AMPA antagonists can be effective in the acute treatment of benzodiazepine-resistant status epilepticus [77, 88]. This is consistent with what was reported previously about the powerful induction of status epilepticus, which follows the potentiation of AMPA receptors in the presence of baseline glutamate activity.
3.2. AMPA Receptors in Models of Absence Epilepsy
Mice with genetic deletion of stargazin (stargazer mice) show ataxia and absence-like seizure [117]. Stargazer mice show a selective loss of AMPA receptors at synapses between cortical afferent fibers and GABAergic neurons of the reticular thalamic nucleus (nRT) [118]. Interestingly, these mice possess lower levels of GluA1, 3 and 4 subunits of AMPA receptors, which anticipate seizure onset. In contrast, after seizure activity, there is a reduced expression of GluA2- containing AMPA receptors. Therefore, one might argue that a loss of GluA4-containing AMPA receptors (likely GluA1/4 and GluA3/4 receptors) is linked to seizure induction, whereas a loss of GluA2-containing AMPA receptors contributes to seizure maintenance [82]. A specific loss in cortical-nRT excitation, leading to a reduced feed-forward inhibition of thalamic relay nuclei, characterizes the absence of epilepsy model of Gria4 knockout mice, which lack the GluA4 subunit [119].
Peeters et al. [120] have studied the involvement of AMPA receptors in WAG/Rij rats, which develop spontaneous absence seizures after 2/3 months of age [121]. In these rats, intracerebroventricular (i.c.v.) injection of AMPA increases SWDs (the EEG hallmark of absence epilepsy). In contrast, i.c.v. injection of glutamic acid diethyl ester (GDEE), a non- subtype selective kainate/AMPA receptor antagonist, decreases absence seizures dose-dependently [120]. The AMPA receptor antagonist, CNQX, also dose-dependently reduces the number of SWDs in WAG/Rij rats after i.c.v. injection [122].
Jakus et al. [123] examined the effect of GYKI 52466 in WAG/Rij rats, showing that i.p. administration fast increases in a dose-dependent manner, both number and duration of SWDs. Two non-competitive AMPA receptor antagonists, CFM-2 (1-(4-aminophenyl)-3,5-dihydro-7,8-dimethoxy-4H- 2,3-benzodiazepin-4-one) and THIQ-10c (N-acetyl-1-(4-chlorophenyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline) reduce dose-dependently both frequency and duration of SWDs, when locally injected within the peri-oral region of the primary somatosensory cortex (S1po) of WAG/Rij rats, whereas they were inactive when locally injected within thalamic nuclei [124].
Russo et al. [125] tested the potential effect of THIQ-10c alone or in combination with the anti-absence drug, ethosuximide in WAG/Rij rats. THIQ-10c did not affect the number and duration of SWDs when administered alone, but it was able to enhance the antiepileptic activity of ethosuximide [125].
Both perampanel and telampanel reduced the number of SWDs in WAG/Rij rats after systemic administration [126, 127]. Finally, topiramate dose-dependently reduced SWDs in GAERS (Genetic Absence Epilepsy Rat from Strasbourg), which are widely used as an experimental animal model of absence epilepsy [99].
4. NMDA RECEPTORS AND EPILEPSY
NMDA receptors are receptor-operated channels (ROC) consisting of non-specific cation channels with a high permeability to Ca2+ (as well as Na+ and K+) [128, 129]. At neuronal resting membrane potential, the channel is blocked by Mg2+ ions and this block is released by membrane depolarization [130, 131]. NMDA receptors mediate most of the slow excitatory postsynaptic potentials (EPSP) essential to global information processing [11]. NMDA receptor activation is important in signal transduction, as well as synapse formation and maintenance [132-138].
Functional NMDA receptors are tetramers composed of GluN1, GluN2A-D, and/or GluN3A-B [26]. The genes encoding NMDA receptor subunits are called GRIN1, GRIN2A, GRIN2B, GRIN2C, GRIN2D, GRIN3A, and GRIN3B [26]. NMDA is mainly postsynaptic, but they have also been found at pre-synaptic sites on axon terminals. The distribution of NMDA receptors surpasses neuronal membrane being abundant also in cortical astrocytes [139].
GRIN2D variations have been associated with drug-refractory epileptic encephalopathy (which does not improve following conventional antiepileptic drug treatment [140]).
In 2017, Gao et al. [141] identified a de novo missense mutation (D731N) of GRIN2A gene in a patient with childhood focal epilepsy and acquired epileptic aphasia. The mutation, which is located in a portion of the agonist binding domain of GluN2A, reduces the potency of glutamate in activating NMDA receptors by > 3,000-fold.
Marwick et al. [142] investigated a de novo genetic variant found in patients with epileptic encephalopathy, which changes a residue located in the ion channel pore of GluN2A-containing NMDA receptors. In cultured primary mouse cortical neurons, this variant (GluN2A, N615K) markedly reduces Mg2+ blockade and increases channel conductance even when expressed alongside wild-type subunits.
4.1. NMDA Receptors and Convulsive Seizures
Recent studies show that blockade of GluN2B-containing NMDA receptors reduces short-term brain damage induced early-in-life by status epilepticus [143].
Inhibition of brain-specific microRNA-134, which is involved in NMDA receptor-dependent spine remodeling [144], can prevent spontaneous recurrent seizures, which appear several months after status epilepticus in >90% of animals, suggesting a new strategy to prevent TLE [145]. It has been reported that microRNA-139-5p negatively regulates NR2A-containing NMDA receptors in the rat pilocarpine model and patients with TLE [146].
Hamamoto et al. [147] recently studied the modulation of NMDA receptors in the amygdala and hippocampus of patients with mesial TLE with hippocampal sclerosis (which represents the most frequent form of focal epilepsy in adults). The authors observed an inverse relationship between miR-219 (that target GluN1) [148] and NMDA receptor expression, suggesting the importance of the regulatory role of miR-219 in excitatory neurotransmission in patients with epilepsy.
It is well known that agonists acting on NMDA receptors can elicit seizures in animal or human subjects, while antagonists inhibit seizures in animal models, indicating that NMDA receptors are candidate targets for antiepileptic drugs [16]. However, NMDA receptor antagonists are largely inactive against fully kindled seizures and may produce adverse effects in kindled animals, such as hyperlocomotion and stereotypies [149, 150]. In some rodent models of epilepsy, competitive and non-competitive NMDA receptor antagonists have demonstrated weak efficacy [151, 152].
Competitive NMDA receptor antagonists binding at the glutamate recognition site, such as D-2-amino-5-phosphonopen-tanoate (AP5) [153] and 3-[(±)-2-carboxypiperazin-4-yl]propyl-l-phosphonate (CPP) [154], have a poor brain penetration, and their action may be overcome by the high concentrations of synaptic and extra-synaptic glutamate during epileptic seizures. Slow NMDA channel blockers, such as dizocilpine (MK-801) and the dissociative anesthetics phencyclidine and ketamine, which act as non-competitive antagonists overcome these limitations [155], but their use is limited by psychotomimetic effects and other adverse effects.
Rundfeldt et al. [156] demonstrated that injection of drugs acting as antagonists (7-chlorokynurenic acid), or partial agonists (R(+)-3-amino-l-hydroxypyrrolid-2-one, D-cycloserine) at the glycine site of NMDA receptors elicit potent anticonvulsant effects in fully kindled rats, causing an increase in the threshold for after-discharge activity in focal seizures.
Long-lasting status epilepticus may produce glutamate-dependent synaptic plasticity. This phenomenon in turn is believed to foster secondary spontaneous epileptogenesis. In this way, we should consider that when administering NMDA receptors, apart from producing symptomatic seizure occlusion, an altered maturation in the epileptic circuitry is promoted. This may prevent the generation of a self-sustaining, self-propagating, drug-refractory seizure activity from producing a disease modifying event, preventing later seizures [157]. In addition, the occlusion of glutamate overactivity produced by these drugs is supposed to prevent from potential seizure-induced brain damage. In fact, MK-801 and ketamine suppress seizures while protecting neuronal damage induced by long-lasting status epilepticus [158, 159].
Therefore, these drugs may be useful to disrupt epileptic circuitries when they become refractory to classic antiepileptic activity due to maladaptive plasticity. In line with this, ketamine has been reported to produce beneficial effects (seizure blockade within 24-48 hours) in children with severe refractory epilepsy (Lennox-Gastaut Syndrome, myoclonic-astatic epilepsy, progressive myoclonic epilepsy and Pseudo-Lennox Syndrome) [160]. Similarly, clinical studies extended the use of these drugs to long-lasting status epilepticus [160], where the non-competitive antagonist MK-801 dose-dependently prevents orphenadrine-induced status epilepticus [161].
The use of these compounds eventually leads to the addition of non-epilepsy related events, which may sustain unbearable side effects. In fact, MK-801apart from increasing seizure-threshold impairs motor activity, and combined administration of D-cycloserine with MK-801 potentiates MK-801–induced motor impairments without improving anticonvulsant effects, suggesting a deleterious enhancement of side- vs. therapeutic effects [162].
When referring to the modulation of NMDA receptors in seizures, it is critical to consider the glycine B site. In detail, the relevance of the glycine B site was reported as follows: The glycine B site possesses a key role in the ionotropic mediated glutamate-induced epileptic activity. This might be relevant also to convert seizures into status epilepticus. For instance, in DBA/2 mice, the administration of the glycine B site antagonist MNQX exerts protective effects against kindling-induced status epilepticus [163]. In fact, it is a common belief that at least part of the anticonvulsant effects exerted by felbamate, remacemide and riluzole is mediated by their antagonism at glycine B site [164]. Conversely, glycine B partial antagonists gifted with a weak intrinsic activity may exert a pro-convulsant effect.
This is further confirmed by the use of a highly selective glycine B antagonist SM-31900 [165], which occludes seizures induced by systemic administration of NMDA.
A number of drugs owing to multiple actions behave as the ligand for the glycine B site [166-172].
For instance, remacemide ((+/-)-2-amino-N-(1-methyl-1,2-diphenylethyl)acetamide), a weak inhibitor of NMDA receptors, and its active des-glycine metabolite, (+/-)-1-methyl-1,2-diphenylethylamine, displayed anticonvulsant and neuroprotective activities by blocking NMDA receptors [173].
Remacemide shows greater efficacy than the sodium channel blocker, carbamazepine, in patients with two or more focal or generalized tonic-clonic seizures [174]; however, remacemide demonstrated efficacy as adjunctive therapy in patients with refractory epilepsy [175].
The antiepileptic activity of felbamate has been ascribed to the inhibition of NMDA receptors [176]. Felbamate is effective in a variety of pediatric seizure types, including seizures associated with Lennox-Gastaut syndrome [177]. This makes it useful in the treatment of refractory epilepsy in children [178, 179], although there is no indication for the use of felbamate as an add-on therapy in people with drug-resistant focal epilepsy [180].
What is not consistent with the anti-seizure activity of NMDA receptor antagonists in animal models [181-184] is the occurrence of epileptic seizures in patients with limbic autoimmune encephalopathy caused by anti-NMDA receptor antibodies [185]. These antibodies target both GluN1 and GluN2 subunits, causing the internalization of NMDA receptors from plasma membrane to the cytosol [185-188]. Although in most animal studies NMDA receptor antagonists did not elicit seizures [189, 190], the competitive NMDA receptor antagonist, D-CPP-ene, worsened seizures in 3/8 patients with epilepsy [191]. The difference between animal and clinical studies may reflect the different distribution of NMDA receptors in cortical inhibitory interneurons and pyramidal neurons. Parvalbumin-positive neurons, which represent the largest population of cortical interneurons, are constitutively activated by NMDA receptors, and inhibition of these interneurons may cause seizures in patients with anti-NMDA receptor antibodies.
4.2. NMDA Receptors in Models of Absence Epilepsy
Lacey et al. [192] observed a trafficking defect in synaptic AMPA receptors in nRT cells of stargazer mice, which leads to a compensatory increase in synaptic NMDA receptors and enhanced thalamic excitability. In a more recent study, however, Barad et al. [193] found that the defective AMPA receptor trafficking in the thalamus of stargazer mice does not cause compensatory increases in total and synaptic NMDA receptor expression. Thus, the role played by NMDA receptors in the pathophysiology of SWDs in stargazer mice is controversial.
MK-801 reduces the number and mean duration of SWDs in WAG/Rij rats [194], and attenuates gamma-hydroxybutyrate (GHB)-induced SWDs [195, 196].
Intraperitoneal or i.c.v. injections or bilateral infusion of MK-801 in the thalamus of GAERS rats, dose-dependently suppress SWDs [197].
Remacemide and its active metabolite, FPL 12495, were tested in the GAERS and WAG/Rij rat models of absence epilepsy. Both drugs reduce the frequency of SWDs [198, 199], and, in WAG/Rij rats, FPL 12495 has greater potency compared with remacemide [199].
5. KAINATE RECEPTORS AND EPILEPSY
Kainate receptors are homo- or heterotetramers formed by GluK1-5 subunits encoded by the GRIK1-5 genes [26, 200], and they are placed at pre- and post-synaptic membranes, where they contribute to excitatory synaptic transmission and modulate network excitability by regulating neurotransmitter release [201-203]. Kainate receptors are structurally related to AMPA receptors but functionally different [204] and exert metabotropic/non-canonical actions [205, 206].
High densities of kainate receptors were found in the hippocampus, a key structure in TLE. Kainate receptors are up-regulated in astrocytes in response to status epilepticus [207]. Most of the studies on kainate receptors in epilepsy models used the prototype non-specific non-NMDA agonist, kainic acid, to induce seizures [208-211]. For example, kainic acid is used to model human TLE and status epilepticus in animals [212]. However, it should be highlighted that kainic acid can also activate AMPA receptors without inducing receptor desensitization [213]. This is an important confounding element because, in some studies, the pro-convulsant action of kainic acid was maintained in mice with genetic deletion of GluK1 subunit-containing kainate receptors [211].
Nonetheless, kainate injection produces acute epileptogenesis, which at least in part is mediated by kainate receptor- mediated suppression of pre-synaptic GABA release, combined with the activation of post-synaptic kainate receptors [208]. There is evidence that GluK2 represents a major target for kainate-induced seizures and status epilepticus [209]. Presynaptic GluK2-containing kainate receptors modulate glutamate release also via metabotropic activity [201, 214].
The epileptogenic effects of pre-synaptic kainate receptors may also be due to disinhibition since kainate, acting on interneurons, reduces hippocampal GABA release [204, 215, 216]. In fact, studies carried out in GluK2-over-expressing or knock-out mice suggest that hippocampal GluK2 is critical for seizure activity [215, 217].
Kainate receptors containing the GluK2 subunit are linked to limbic epilepsy due to a widespread distribution within CA3 pyramidal neurons [218]. Consistently, deletion of GluK2 reduces seizure developments after kainate injection in mice [209].
The AMPA/kainate receptor antagonist, NS1209, is effective against refractory status epilepticus in phase 2 clinical trials [219]. CNQX, another AMPA/kainate receptor antagonist, prevents high-frequency hippocampal oscillations associated with seizures [220].
Fritsch et al. [211] demonstrate that selective activation of GluK1 kainate receptors elicit seizures in vivo, and epileptiform discharges in the BLA (basolateral amygdala) in vitro; however, whether or not GluK1-containing kainate receptors can be targeted by therapeutic intervention is still a matter of debate.
Although LY293558 was originally believed to be a selective AMPA receptor antagonist, it inhibits GluK1 (formerly known as GluR5)-containing kainate receptors with even greater potency. This compound is effective in status epilepticus, even when administered after seizure onset [221]. LY293558 has been clinically tested and found to be safe and well tolerated [222, 223]. Inhibition of GluK1-containing kainate receptors could also represent one of the mechanisms underlying the anticonvulsant activity of topiramate [224].
The role of kainate receptors in the pathophysiology of TLE remains elusive. Some studies have focused on the role of heteromeric GluK2/GluK5 kainate receptors in the generation of recurrent seizures in TLE: the inter-ictal and ictal events were reduced in mice lacking the GluK2 subunit or in the presence of a GluK2/GluK5 antagonist [215]. In tissue from patients with refractory TLE, an increase in GluK4 and GluK5 subunits has been reported [225]. GluK1 and GluK5 are up-regulated chronically during post-ictal spontaneous seizures [207].
Subtype-selective GluK5 kainate receptor antagonism prevents pilocarpine-induced limbic seizures in rats and epileptiform activity induced by electrical stimulation, both in vitro and in vivo [210].
Recent studies reported that GluK2 undergoes post-translational modifications such as S-nitrosylation (SNO), which is involved in neuronal injury in epileptic rats by forming a calcium-dependent GluK2-PSD95-nNOS cytosolic complex, which may represent a therapeutic target for epilepsy [226].
Orav et al. [227] described neuropilin tolloid-like protein 1 (NETO1) as a potential therapeutic target for the treatment of both adult and early life seizures: it acts as kainate receptor auxiliary protein necessary for dendritic delivery of kainate receptor subunits and formation of kainate receptor- containing synapses in cultured GABAergic neurons.
CONCLUSION
Despite being apparently well-known, the role of iGlu receptors in epilepsies and seizures bring a number of confounding issues. In the present review, apart from summarizing the classic knowledge about the key role of these glutamate receptors in seizure onset, severity and duration, we presented findings on the various role played by these receptors depending on the kind of epileptic seizures. Again, we tried to elucidate the confounding role of non-NMDA receptors based on the experimental tools used to study their role in seizure activity. With the aim to overview critically the plethora of reports about the role of iGlu receptors in epilepsies, we faced novel issues that may be considered in re-evaluating ionotropic ligands as useful therapeutic agents in epilepsy. This mainly applies to refractory epilepsy and life-threatening conditions such as self-sustaining long-lasting status epilepticus. The development of new antiepileptic drugs with a better profile of tolerability and safety is an urgent medical need. Thus, we aimed to highlight the necessity of further research on this topic that would be useful to unravel the mechanisms underlying the development of epilepsies and to promote the development of drugs targeting the iGlu receptors.
Negative allosteric modulators of AMPA receptors.
| Compound | Subject | Effect | Refs. |
|---|---|---|---|
| GYKI52466 | NIH Swiss mice | protective against seizures in maximal electroshock seizure test | [56] |
| mouse | inhibition of seizure activity and recurrence in kainic acid-induced status epilepticus | [88] | |
| Telampanel | rat | protective activity in the kainate-induced neonatal status epilepticus model | [89] |
| Topiramate | human | approved for the treatment of partial seizures, primarily generalized seizures, and seizures associated with Lennox-Gastaut syndrome | [90-91] |
| mouse | protection against seizures induced by infusion of ATPA | [98] | |
| human | reduces seizures when administered as an add-on treatment for drug-resistant focal epilepsy | [101] | |
| Perampanel | human | approved for the treatment of focal epilepsy and generalized tonic-clonic epilepsy; restrains myoclonic or absence seizures | [102-109] |
| mouse | protection against PTZ-induced clonic seizures and psychomotor seizures in the 6-Hz stimulation protocol | [110] | |
| rat amygdala kindling model | shortens after-discharge activity and prolongs latency to onset of generalized seizures | [111, 112] | |
| rat | block of pilocarpine-induced status epilepticus | [113] | |
| human | good efficacy and tolerability in young children with intractable epilepsy | [116] |
AMPA receptors in models of absence epilepsy.
| Compound | Subject | Effect | Refs. |
|---|---|---|---|
| Topiramate | GAERS rats | dose-dependently reduces SWDs | [99] |
| GDEE | WAG/Rij rats | dose-dependently decreases absence seizures | [120] |
| CNQX | WAG/Rij rats | dose-dependently reduces the number of SWDs | [122] |
| CFM-2 THIQ-10c |
WAG/Rij rats | dose-dependently reduce frequency and duration of SWDs | [124] |
| Telampanel | WAG/Rij rats | reduces the number of SWDs | [126] |
| Perampanel | WAG/Rij rats | reduces the number of SWDs | [127] |
NMDA receptors and convulsive seizures.
| Compound | Subject | Effect | Refs. |
|---|---|---|---|
| 7-chlorokynurenic acid D-cycloserine |
kindled rats | anticonvulsant effects in focal seizures | [156] |
| ketamine | human | seizure blockade in children with severe refractory epilepsy | [160] |
| MK-801 | rat | dose-dependently prevents orphenadrine-induced status epilepticus | [161] |
| MNQX | DBA/2 mice | protective effects against kindling-induced status epilepticus | [163] |
| Remacemide | rat | anticonvulsant by blocking NMDA receptors | [173] |
| human | efficacy in patients with two or more focal or generalized tonic-clonic seizures | [174] | |
| human | efficacy as adjunctive therapy in patients with refractory epilepsy | [175] | |
| Felbamate | human | effective in a variety of pediatric seizure types including Lennox-Gastaut syndrome | [177] |
| human | useful in the treatment of refractory epilepsy in children | [178, 179] |
NMDA receptors in models of absence epilepsy.
Kainate receptors and epilepsy.
ACKNOWLEDGEMENTS
Roberta Celli and Francesco Fornai wrote the paper and revised the final manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
REFERENCES
- 1.Olney J.W. In: Experimental and clinical neurotoxicology. Spencer P.S., Shaumberg H.H., editors. Baltimore: Williams and Wilkins; 1980. Excitotoxic mechanisms of neurotoxicity. pp. 272–294. [Google Scholar]
- 2.Lucas D.R., Newhouse J.P. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch. Opthalmol. 1957;58(2):193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- 3.Choi D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 4.Choi D.W. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11(10):465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- 5.Lipton S.A., Rosenberg P.A. Excitatory amino acids as a final common pathway for neurologic disorders. New Engl. J. Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 6.Sloviter R.S., Sollas A.L., Dean E., Dichter M.A. Immunocy- tochemical localization of 65 and 67 kDa glutamic acid decarbox- ylase (GAD) and GABA-like immunoreactivities in the rat hippocampal formation; GAD 67 and GABA are present in normal dentate granule cells and are rapidly induced by afferent stimulation, kainic acid or pilocarpine. Soc. Neurosci. Abs. 1995;771:2. [Google Scholar]
- 7.Banmgarten H.G., Zimmermann B. In: Selective neurotoxicity. Herken H., Hucho F., editors. Berlin: Springer-Verlag; 1992. Cellular and subcellular targets of neurotoxins: the concept of selective vulnerability. pp. 1–27. [Google Scholar]
- 8.Fornai F., Vaglini F., Maggio R., Bonuccelli U., Corsini G.U. Species differences in the role of excitatory amino acids in experimental parkinsonism. Neurosci. Biobehav. Rev. 1997;21(4):401–415. doi: 10.1016/S0149-7634(96)00042-5. [DOI] [PubMed] [Google Scholar]
- 9.Freire M.A.M. Pathophysiology of neurodegeneration following traumatic brain injury. West Indian Med. J. 2012;61(7):751–755. [PubMed] [Google Scholar]
- 10.Kaur P., Sharma S. Recent advances in pathophysiology of traumatic brain injury. Curr. Neuropharmacol. 2018;16(8):1224–1238. doi: 10.2174/1570159X15666170613083606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker-Haliski M., White H.S. Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harb. Perspect. Med. 2015;5(8):a022863. doi: 10.1101/cshperspect.a022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ngomba R.T., van Luijtelaar G. Metabotropic glutamate receptors as drug targets for the treatment of absence epilepsy. Curr. Opin. Pharmacol. 2018;38:43–50. doi: 10.1016/j.coph.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Leo A., Giovannini G., Russo E., Meletti S. The role of AMPA receptors and their antagonists in status epilepticus. Epilepsia. 2018;59(6):1098–1108. doi: 10.1111/epi.14082. [DOI] [PubMed] [Google Scholar]
- 14.Mihály A. The reactive plasticity of hippocampal ionotropic glutamate receptors in animal epilepsies. Int. J. Mol. Sci. 2019;20(5):1030. doi: 10.3390/ijms20051030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Celli R., Santolini I., Van Luijtelaar G., Ngomba R.T., Bruno V., Nicoletti F. Targeting metabotropic glutamate receptors in the treatment of epilepsy: rationale and current status. Expert Opin. Ther. Targets. 2019;23(4):341–351. doi: 10.1080/14728222.2019.1586885. [DOI] [PubMed] [Google Scholar]
- 16.Hanada T. Ionotropic Glutamate Receptors in Epilepsy: A Review Focusing on AMPA and NMDA Receptors. Biomolecules. 2020;10(3):464. doi: 10.3390/biom10030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong R.K., Bianchi R., Taylor G.W., Merlin L.R. Role of metabotropic glutamate receptors in epilepsy. Adv. Neurol. 1999;79:685–698. [PubMed] [Google Scholar]
- 18.Doherty J., Dingledine R. The roles of metabotropic glutamate receptors in seizures and epilepsy. Curr. Drug Targets CNS Neurol. Disord. 2002;1(3):251–260. doi: 10.2174/1568007023339355. [DOI] [PubMed] [Google Scholar]
- 19.Moldrich R.X., Chapman A.G., De Sarro G., Meldrum B.S. Glutamate metabotropic receptors as targets for drug therapy in epilepsy. Eur. J. Pharmacol. 2003;476(1-2):3–16. doi: 10.1016/S0014-2999(03)02149-6. [DOI] [PubMed] [Google Scholar]
- 20.Ure J., Baudry M., Perassolo M. Metabotropic glutamate receptors and epilepsy. J. Neurol. Sci. 2006;247(1):1–9. doi: 10.1016/j.jns.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Alexander G.M., Godwin D.W. Metabotropic glutamate receptors as a strategic target for the treatment of epilepsy. Epilepsy Res. 2006;71(1):1–22. doi: 10.1016/j.eplepsyres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Tang F.R., Bradford H.F., Ling E.A. Metabotropic glutamate receptors in the control of neuronal activity and as targets for development of anti-epileptogenic drugs. Curr. Med. Chem. 2009;16(17):2189–2204. doi: 10.2174/092986709788612710. [DOI] [PubMed] [Google Scholar]
- 23.Ngomba R.T., Santolini I., Salt T.E., Ferraguti F., Battaglia G., Nicoletti F., van Luijtelaar G. Metabotropic glutamate receptors in the thalamocortical network: strategic targets for the treatment of absence epilepsy. Epilepsia. 2011;52(7):1211–1222. doi: 10.1111/j.1528-1167.2011.03082.x. [DOI] [PubMed] [Google Scholar]
- 24.Qian F., Tang F.R. Metabotropic glutamate receptors and interacting proteins in epileptogenesis. Curr. Neuropharmacol. 2016;14(5):551–562. doi: 10.2174/1570159X14666160331142228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassel B., Dingledine R. In: Basic Neurochemistry. 7th ed. Siegel G.J., Albers R.W., Brady S.T., Price D.L., editors. London, UK: Elsevier; 2006. Glutamate. pp. 267–290. [Google Scholar]
- 26.Collingridge G.L., Olsen R.W., Peters J., Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56(1):2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheefhals N., MacGillavry H.D. Functional organization of postsynaptic glutamate receptors. Mol. Cell. Neurosci. 2018;91:82–94. doi: 10.1016/j.mcn.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiner A., Levitz J. Glutamatergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron. 2018;98(6):1080–1098. doi: 10.1016/j.neuron.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi T. Post-translational palmitoylation of ionotropic glutamate receptors in excitatory synaptic functions. Br J Pharmacol. 2020 doi: 10.1111/bph.15050. [DOI] [PubMed] [Google Scholar]
- 30.Lodge D. The history of the pharmacology and cloning of ionotropic glutamate receptors and the development of idiosyncratic nomenclature. Neuropharmacology. 2009;56(1):6–21. doi: 10.1016/j.neuropharm.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Sobolevsky A.I., Rosconi M.P., Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462(7274):745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isaac J.T., Ashby M.C., McBain C.J. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54(6):859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Buldakova S.L., Vorobjev V.S., Sharonova I.N., Samoilova M.V., Magazanik L.G. Characterization of AMPA receptor populations in rat brain cells by the use of subunit-specific open channel blocking drug, IEM-1460. Brain Res. 1999;846(1):52–58. doi: 10.1016/S0006-8993(99)01970-8. [DOI] [PubMed] [Google Scholar]
- 35.Talos D.M., Follett P.L., Folkerth R.D., Fishman R.E., Trachtenberg F.L., Volpe J.J., Jensen F.E. Developmental regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J. Comp. Neurol. 2006;497(1):61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anggono V., Huganir R.L. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012;22(3):461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choquet D. Linking Nanoscale Dynamics of AMPA Receptor Organization to Plasticity of Excitatory Synapses and Learning. J. Neurosci. 2018;38(44):9318–9329. doi: 10.1523/JNEUROSCI.2119-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beneyto M., Meador-Woodruff J.H. Expression of transcripts encoding AMPA receptor subunits and associated postsynaptic proteins in the macaque brain. J. Comp. Neurol. 2004;468(4):530–554. doi: 10.1002/cne.10981. [DOI] [PubMed] [Google Scholar]
- 39.Tomita S., Chen L., Kawasaki Y., Petralia R.S., Wenthold R.J., Nicoll R.A., Bredt D.S. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol. 2003;161(4):805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bats C., Groc L., Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53(5):719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Fukaya M., Tsujita M., Yamazaki M., Kushiya E., Abe M., Akashi K., Natsume R., Kano M., Kamiya H., Watanabe M., Sakimura K. Abundant distribution of TARP gamma-8 in synaptic and extrasynaptic surface of hippocampal neurons and its major role in AMPA receptor expression on spines and dendrites. Eur. J. Neurosci. 2006;24(8):2177–2190. doi: 10.1111/j.1460-9568.2006.05081.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen L., Chetkovich D.M., Petralia R.S., Sweeney N.T., Kawasaki Y., Wenthold R.J., Bredt D.S., Nicoll R.A. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408(6815):936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 43.Menuz K., Stroud R.M., Nicoll R.A., Hays F.A. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science. 2007;318(5851):815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- 44.Cokić B., Stein V. Stargazin modulates AMPA receptor antagonism. Neuropharmacology. 2008;54(7):1062–1070. doi: 10.1016/j.neuropharm.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Kato A.S., Gill M.B., Yu H., Nisenbaum E.S., Bredt D.S. TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci. 2010;33(5):241–248. doi: 10.1016/j.tins.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Charsouei S., Jabalameli M.R., Karimi-Moghadam A. Molecular insights into the role of AMPA receptors in the synaptic plasticity, pathogenesis and treatment of epilepsy: therapeutic potentials of perampanel and antisense oligonucleotide (ASO) technology. Acta Neurol Belg. 2020 doi: 10.1007/s13760-020-01318-1. [DOI] [PubMed] [Google Scholar]
- 47.Rogawski M.A. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr. 2011;11(2):56–63. doi: 10.5698/1535-7511-11.2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogawski M.A. AMPA receptors as a molecular target in epilepsy therapy. Acta Neurol. Scand. Suppl. 2013;(197):9–18. doi: 10.1111/ane.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwa G.G.C., Avoli M. The involvement of excitatory amino acids in neocortical epileptogenesis: NMDA and non-NMDA receptors. Exp. Brain Res. 1991;86(2):248–256. doi: 10.1007/BF00228949. [DOI] [PubMed] [Google Scholar]
- 50.Hwa G.G.C., Avoli M., Oliver A., Villemure J.G. Bicuculline-induced epileptogenesis in the human neocortex maintained in vitro. Exp. Brain Res. 1991;83(2):329–339. doi: 10.1007/BF00231156. [DOI] [PubMed] [Google Scholar]
- 51.Jones R.S., Lambert J.D. Synchronous discharges in the rat entorhinal cortex in vitro: site of initiation and the role of excitatory amino acid receptors. Neuroscience. 1990;34(3):657–670. doi: 10.1016/0306-4522(90)90172-Z. [DOI] [PubMed] [Google Scholar]
- 52.Egbenya D.L., Hussain S., Lai Y.C., Xia J., Anderson A.E., Davanger S. Changes in synaptic AMPA receptor concentration and composition in chronic temporal lobe epilepsy. Mol. Cell. Neurosci. 2018;92:93–103. doi: 10.1016/j.mcn.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Anovadiya A.P., Sanmukhani J.J., Tripathi C.B. Epilepsy: Novel therapeutic targets. J. Pharmacol. Pharmacother. 2012;3(2):112–117. doi: 10.4103/0976-500X.95505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El Desoky E.S. The AMPA receptor antagonist perampanel is a new hope in the treatment for epilepsy. Fundam. Clin. Pharmacol. 2014;28(5):473–480. doi: 10.1111/fcp.12081. [DOI] [PubMed] [Google Scholar]
- 55.Russo E., Gitto R., Citraro R., Chimirri A., De Sarro G. New AMPA antagonists in epilepsy. Expert Opin. Investig. Drugs. 2012;21(9):1371–1389. doi: 10.1517/13543784.2012.705277. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi S., Donevan S.D., Rogawski M.A. Anticonvulsant activity of AMPA/kainate antagonists: comparison of GYKI 52466 and NBOX in maximal electroshock and chemoconvulsant seizure models. Epilepsy Res. 1993;15(3):179–184. doi: 10.1016/0920-1211(93)90054-B. [DOI] [PubMed] [Google Scholar]
- 57.Katsumori H., Minabe Y., Osawa M., Ashby C.R., Jr Acute effects of various GABA receptor agonists and glutamate antagonists on focal hippocampal seizures in freely moving rats elicited by low-frequency stimulation. Synapse. 1998;28(1):103–109. doi: 10.1002/(SICI)1098-2396(199801)28:1<103::AID-SYN12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 58.Honoré T., Davies S.N., Drejer J., Fletcher E.J., Jacobsen P., Lodge D., Nielsen F.E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- 59.Drejer J., Honoré T. New quinoxalinediones show potent antagonism of quisqualate responses in cultured mouse cortical neurons. Neurosci. Lett. 1988;87(1-2):104–108. doi: 10.1016/0304-3940(88)90153-X. [DOI] [PubMed] [Google Scholar]
- 60.Chapman A.G., Smith S.E., Meldrum B.S. The anticonvulsant effect of the non-NMDA antagonists, NBQX and GYKI 52466, in mice. Epilepsy Res. 1991;9(2):92–96. doi: 10.1016/0920-1211(91)90018-B. [DOI] [PubMed] [Google Scholar]
- 61.Taylor C.P., Vartanian M.G. Probenecid pretreatment enhances anticonvulsant action of NBQX in mice. Eur. J. Pharmacol. 1992;213(1):151–153. doi: 10.1016/0014-2999(92)90247-2. [DOI] [PubMed] [Google Scholar]
- 62.Namba T., Morimoto K., Sato K., Yamada N., Kuroda S. Antiepileptogenic and anticonvulsant effects of NBQX, a selective AMPA receptor antagonist, in the rat kindling model of epilepsy. Brain Res. 1994;638(1-2):36–44. doi: 10.1016/0006-8993(94)90630-0. [DOI] [PubMed] [Google Scholar]
- 63.Catarzi D., Colotta V., Varano F. Competitive AMPA receptor antagonists. Med. Res. Rev. 2007;27(2):239–278. doi: 10.1002/med.20084. [DOI] [PubMed] [Google Scholar]
- 64.Twele F., Bankstahl M., Klein S., Römermann K., Löscher W. The AMPA receptor antagonist NBQX exerts anti-seizure but not antiepileptogenic effects in the intrahippocampal kainate mouse model of mesial temporal lobe epilepsy. Neuropharmacology. 2015;95:234–242. doi: 10.1016/j.neuropharm.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 65.Lippman-Bell J.J., Rakhade S.N., Klein P.M., Obeid M., Jackson M.C., Joseph A., Jensen F.E. AMPA receptor antagonist NBQX attenuates later-life epileptic seizures and autistic-like social deficits following neonatal seizures. Epilepsia. 2013;54(11):1922–1932. doi: 10.1111/epi.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kohara A., Okada M., Tsutsumi R., Ohno K., Takahashi M., Shimizu-Sasamata M., Shishikura J., Inami H., Sakamoto S., Yamaguchi T. In-vitro characterization of YM872, a selective, potent and highly water-soluble alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor antagonist. J. Pharm. Pharmacol. 1998;50(7):795–801. doi: 10.1111/j.2042-7158.1998.tb07142.x. [DOI] [PubMed] [Google Scholar]
- 67.Takahashi M., Kohara A., Shishikura J., Kawasaki-Yatsugi S., Ni J.W., Yatsugi S., Sakamoto S., Okada M., Shimizu-Sasamata M., Yamaguchi T. YM872: a selective, potent and highly water-soluble alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor antagonist. CNS Drug Rev. 2002;8(4):337–352. doi: 10.1111/j.1527-3458.2002.tb00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hara H., Yamada N., Kodama M., Matsumoto Y., Wake Y., Kuroda S. Effect of YM872, a selective and highly water-soluble AMPA receptor antagonist, in the rat kindling and rekindling model of epilepsy. Eur. J. Pharmacol. 2006;531(1-3):59–65. doi: 10.1016/j.ejphar.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu-Sasamata M., Kawasaki-Yatsugi S., Okada M., Sakamoto S., Yatsugi S., Togami J., Hatanaka K., Ohmori J., Koshiya K., Usuda S., Murase K. YM90K: pharmacological characterization as a selective and potent α-amino-3-hydroxy-5-methylisoxazole-4-propionate/kainate receptor antagonist. J. Pharmacol. Exp. Ther. 1996;276(1):84–92. [PubMed] [Google Scholar]
- 70.Kodama M., Yamada N., Sato K., Kitamura Y., Koyama F., Sato T., Morimoto K., Kuroda S. Effects of YM90K, a selective AMPA receptor antagonist, on amygdala-kindling and long-term hippocampal potentiation in the rat. Eur. J. Pharmacol. 1999;374(1):11–19. doi: 10.1016/S0014-2999(99)00295-2. [DOI] [PubMed] [Google Scholar]
- 71.Turski L., Huth A., Sheardown M., McDonald F., Neuhaus R., Schneider H.H., Dirnagl U., Wiegand F., Jacobsen P., Ottow E. ZK200775: a phosphonate quinoxalinedione AMPA antagonist for neuroprotection in stroke and trauma. Proc. Natl. Acad. Sci. USA. 1998;95(18):10960–10965. doi: 10.1073/pnas.95.18.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mattes H., Carcache D., Kalkman H.O., Koller M. α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) antagonists: from bench to bedside. J. Med. Chem. 2010;53(15):5367–5382. doi: 10.1021/jm901688m. [DOI] [PubMed] [Google Scholar]
- 73.Schoepp D.D., Lodge D., Bleakman D., Leander J.D., Tizzano J.P., Wright R.A., Palmer A.J., Salhoff C.R., Ornstein P.L. In vitro and in vivo antagonism of AMPA receptor activation by (3S, 4aR, 6R, 8aR)-6-[2-(1(2)H-tetrazole-5-yl) ethyl] decahydroisoquinoline-3-carboxylic acid. Neuropharmacology. 1995;34(9):1159–1168. doi: 10.1016/0028-3908(95)00099-R. [DOI] [PubMed] [Google Scholar]
- 74.Mignani S., Bohme G.A., Birraux G., Boireau A., Jimonet P., Damour D., Genevois-Borella A., Debono M.W., Pratt J., Vuilhorgne M., Wahl F., Stutzmann J.M. 9-Carboxymethyl-5H,10H-imidazo[1,2-a]indeno[1,2-e]pyrazin-4-one-2-carbocylic acid (RPR117824): selective anticonvulsive and neuroprotective AMPA antagonist. Bioorg. Med. Chem. 2002;10(5):1627–1637. doi: 10.1016/S0968-0896(01)00431-X. [DOI] [PubMed] [Google Scholar]
- 75.Krampfl K., Schlesinger F., Cordes A.L., Bufler J. Molecular analysis of the interaction of the pyrazine derivatives RPR119990 and RPR117824 with human AMPA-type glutamate receptor channels. Neuropharmacology. 2006;50(4):479–490. doi: 10.1016/j.neuropharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 76.Nielsen E.O., Varming T., Mathiesen C., Jensen L.H., Moller A., Gouliaev A.H., Wätjen F., Drejer J. SPD 502: a water-soluble and in vivo long-lasting AMPA antagonist with neuroprotective activity. J. Pharmacol. Exp. Ther. 1999;289(3):1492–1501. [PubMed] [Google Scholar]
- 77.Pitkänen A., Mathiesen C., Rønn L.C., Møller A., Nissinen J. Effect of novel AMPA antagonist, NS1209, on status epilepticus. An experimental study in rat. Epilepsy Res. 2007;74(1):45–54. doi: 10.1016/j.eplepsyres.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Ben-Menachem E. NS1209. In: Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Perucca E, Tomson T. Progress report on new antiepileptic drugs: a summary of the Eighth Eilat Conference (EILAT VIII). Epilepsy Res. 2007;73:1–52. doi: 10.1016/j.eplepsyres.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 79.Weiser T. AMPA receptor antagonists for the treatment of stroke. Curr. Drug Targets CNS Neurol. Disord. 2005;4(2):153–159. doi: 10.2174/1568007053544129. [DOI] [PubMed] [Google Scholar]
- 80.Rogawski M.A., Donevan S.D. AMPA receptors in epilepsy and as targets for antiepileptic drugs. Adv. Neurol. 1999;79:947–963. [PubMed] [Google Scholar]
- 81.Di Bonaventura C., Labate A., Maschio M., Meletti S., Russo E. AMPA receptors and perampanel behind selected epilepsies: current evidence and future perspectives. Expert Opin. Pharmacother. 2017;18(16):1751–1764. doi: 10.1080/14656566.2017.1392509. [DOI] [PubMed] [Google Scholar]
- 82.Adotevi N.K., Leitch B. Cortical expression of AMPA receptors during postnatal development in a genetic model of absence epilepsy. Int. J. Dev. Neurosci. 2019;73:19–25. doi: 10.1016/j.ijdevneu.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 83.Fornai F., Busceti C.L., Kondratyev A., Gale K. AMPA receptor desensitization as a determinant of vulnerability to focally evoked status epilepticus. Eur. J. Neurosci. 2005;21(2):455–463. doi: 10.1111/j.1460-9568.2005.03873.x. [DOI] [PubMed] [Google Scholar]
- 84.Halonen T., Tortorella A., Zrebeet H., Gale K. Posterior piriform and perirhinal cortex relay seizures evoked from the area tempestas: role of excitatory and inhibitory amino acid receptors. Brain Res. 1994;652(1):145–148. doi: 10.1016/0006-8993(94)90328-X. [DOI] [PubMed] [Google Scholar]
- 85.Tortorella A., Halonen T., Sahibzada N., Gale K. A crucial role of the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid subtype of glutamate receptors in piriform and perirhinal cortex for the initiation and propagation of limbic motor seizures. J. Pharmacol. Exp. Ther. 1997;280(3):1401–1405. [PubMed] [Google Scholar]
- 86.Cassidy R.M., Gale K. Mediodorsal thalamus plays a critical role in the development of limbic motor seizures. J. Neurosci. 1998;18(21):9002–9009. doi: 10.1523/JNEUROSCI.18-21-09002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carrillo E., Shaikh S.A., Berka V., Durham R.J., Litwin D.B., Lee G., MacLean D.M., Nowak L.M., Jayaraman V. Mechanism of modulation of AMPA receptors by TARP-γ8. J. Gen. Physiol. 2020;152(1):e201912451. doi: 10.1085/jgp.201912451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fritsch B., Stott J.J., Joelle Donofrio J., Rogawski M.A. Treatment of early and late kainic acid-induced status epilepticus with the noncompetitive AMPA receptor antagonist GYKI 52466. Epilepsia. 2010;51(1):108–117. doi: 10.1111/j.1528-1167.2009.02205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dhir A., Chavda V. Pre- and post-exposure talampanel (GYKI 53773) against kainic acid seizures in neonatal rats. Pharmacol. Rep. 2016;68(1):190–195. doi: 10.1016/j.pharep.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 90.Glauser T.A. Topiramate. Epilepsia. 1999;40(Suppl. 5):S71–S80. doi: 10.1111/j.1528-1157.1999.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 91.Jette N.J., Marson A.G., Hutton J.L. Topiramate add-on for drug-resistant partial epilepsy. Cochrane Database Syst. Rev. 2002;3(3):CD001417. doi: 10.1002/14651858.CD001417. [DOI] [PubMed] [Google Scholar]
- 92.Spritzer S.D., Bravo T.P., Drazkowski J.F. Topiramate for treatment in patients with migraine and epilepsy. Headache. 2016;56(6):1081–1085. doi: 10.1111/head.12826. [DOI] [PubMed] [Google Scholar]
- 93.Shank R.P., Gardocki J.F., Streeter A.J., Maryanoff B.E. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(s1) Suppl. 1:S3–S9. doi: 10.1111/j.1528-1157.2000.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 94.Gibbs J.W., III, Sombati S., DeLorenzo R.J., Coulter D.A. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41(Suppl. 1):S10–S16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 95.Poulsen C.F., Simeone T.A., Maar T.E., Smith-Swintosky V., White H.S., Schousboe A. Modulation by topiramate of AMPA and kainate mediated calcium influx in cultured cerebral cortical, hippocampal and cerebellar neurons. Neurochem. Res. 2004;29(1):275–282. doi: 10.1023/B:NERE.0000010456.92887.3b. [DOI] [PubMed] [Google Scholar]
- 96.Kanda T., Kurokawa M., Tamura S., Nakamura J., Ishii A., Kuwana Y., Serikawa T., Yamada J., Ishihara K., Sasa M. Topiramate reduces abnormally high extracellular levels of glutamate and aspartate in the hippocampus of spontaneously epileptic rats (SER). Life Sci. 1996;59(19):1607–1616. doi: 10.1016/0024-3205(96)00492-4. [DOI] [PubMed] [Google Scholar]
- 97.Gryder D.S., Rogawski M.A. Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J. Neurosci. 2003;23(18):7069–7074. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaminski R.M., Banerjee M., Rogawski M.A. Topiramate selectively protects against seizures induced by ATPA, a GluR5 kainate receptor agonist. Neuropharmacology. 2004;46(8):1097–1104. doi: 10.1016/j.neuropharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 99.Rigoulot M.A., Boehrer A., Nehlig A. Effects of topiramate in two models of genetically determined generalized epilepsy, the GAERS and the Audiogenic Wistar AS. Epilepsia. 2003;44(1):14–19. doi: 10.1046/j.1528-1157.2003.32902.x. [DOI] [PubMed] [Google Scholar]
- 100.Selvitelli M., Drislane F.W. Recent developments in the diagnosis and treatment of status epilepticus. Curr. Neurol. Neurosci. Rep. 2007;7(6):529–535. doi: 10.1007/s11910-007-0081-8. [DOI] [PubMed] [Google Scholar]
- 101.Bresnahan R., Hounsome J., Jette N., Hutton J.L., Marson A.G. Topiramate add-on therapy for drug-resistant focal epilepsy. Cochrane Database Syst. Rev. 2019;10:CD001417. doi: 10.1002/14651858.CD001417.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.French J.A., Krauss G.L., Biton V., Squillacote D., Yang H., Laurenza A., Kumar D., Rogawski M.A. Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304. Neurology. 2012;79(6):589–596. doi: 10.1212/WNL.0b013e3182635735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.French J.A., Krauss G.L., Steinhoff B.J., Squillacote D., Yang H., Kumar D., Laurenza A. Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia. 2013;54(1):117–125. doi: 10.1111/j.1528-1167.2012.03638.x. [DOI] [PubMed] [Google Scholar]
- 104.French J.A., Krauss G.L., Wechsler R.T., Wang X.F., DiVentura B., Brandt C., Trinka E., O’Brien T.J., Laurenza A., Patten A., Bibbiani F. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial. Neurology. 2015;85(11):950–957. doi: 10.1212/WNL.0000000000001930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krauss G.L., Serratosa J.M., Villanueva V., Endziniene M., Hong Z., French J., Yang H., Squillacote D., Edwards H.B., Zhu J., Laurenza A. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78(18):1408–1415. doi: 10.1212/WNL.0b013e318254473a. [DOI] [PubMed] [Google Scholar]
- 106.Nishida T., Lee S.K., Inoue Y., Saeki K., Ishikawa K., Kaneko S. Adjunctive perampanel in partial-onset seizures: Asia- Pacific, randomized phase III study. Acta Neurol. Scand. 2018;137(4):392–399. doi: 10.1111/ane.12883. [DOI] [PubMed] [Google Scholar]
- 107.Rudzinski L.A., Vélez-Ruiz N.J., Gedzelman E.R., Mauricio E.A., Shih J.J., Karakis I. New antiepileptic drugs: focus on ezogabine, clobazam, and perampanel. J. Investig. Med. 2016;64(6):1087–1101. doi: 10.1136/jim-2016-000151. [DOI] [PubMed] [Google Scholar]
- 108.Alsaadi T., Kassie S.A., Servano R. Efficacy and tolerability of perampanel in patients with genetic generalized epilepsy (GGE): A retrospective, single-center study from the United Arab Emirates (UAE). Epilepsy Behav. Rep. 2019;12:100330. doi: 10.1016/j.ebr.2019.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Villanueva V., Montoya J., Castillo A., Mauri-Llerda J.Á., Giner P., López-González F.J., Piera A., Villanueva-Hernández P., Bertol V., Garcia-Escrivá A., Garcia-Peñas J.J., Garamendi I., Esteve-Belloch P., Baiges-Octavio J.J., Miró J., Falip M., Garcés M., Gómez A., Gil-López F.J., Carreño M., Rodriguez-Uranga J.J., Campos D., Bonet M., Querol R., Molins A., Tortosa D., Salas-Puig J. Perampanel in routine clinical use in idiopathic generalized epilepsy: The 12-month GENERAL study. Epilepsia. 2018;59(9):1740–1752. doi: 10.1111/epi.14522. [DOI] [PubMed] [Google Scholar]
- 110.Bialer M., Johannessen S.I., Levy R.H., Perucca E., Tomson T., White H.S. Progress report on new antiepileptic drugs: a summary of the Tenth Eilat Conference (EILAT X). Epilepsy Res. 2010;92(2-3):89–124. doi: 10.1016/j.eplepsyres.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 111.Hanada T., Hashizume Y., Tokuhara N., Takenaka O., Kohmura N., Ogasawara A., Hatakeyama S., Ohgoh M., Ueno M., Nishizawa Y. Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia. 2011;52(7):1331–1340. doi: 10.1111/j.1528-1167.2011.03109.x. [DOI] [PubMed] [Google Scholar]
- 112.Wu T., Ido K., Ohgoh M., Hanada T. Mode of seizure inhibition by sodium channel blockers, an SV2A ligand, and an AMPA receptor antagonist in a rat amygdala kindling model. Epilepsy Res. 2019;154:42–49. doi: 10.1016/j.eplepsyres.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 113.Mohammad H., Sekar S., Wei Z., Moien-Afshari F., Taghibiglou C. Perampanel but not amantadine prevents behavioral alterations and epileptogenesis in pilocarpine rat model of status epilepticus. Mol. Neurobiol. 2019;56(4):2508–2523. doi: 10.1007/s12035-018-1230-6. [DOI] [PubMed] [Google Scholar]
- 114.Augustin K., Williams S., Cunningham M., Devlin A.M., Friedrich M., Jayasekera A., Hussain M.A., Holliman D., Mitchell P., Jenkins A., Chen P.E., Walker M.C., Williams R.S.B. Perampanel and decanoic acid show synergistic action against AMPA receptors and seizures. Epilepsia. 2018;59(11):e172–e178. doi: 10.1111/epi.14578. [DOI] [PubMed] [Google Scholar]
- 115.Operto F.F., Pastorino G.M.G., Mazza R., Di Bonaventura C., Matricardi S., Verrotti A., Carotenuto M., Viggiano A., Coppola G., Elia M. Perampanel tolerability in children and adolescents with focal epilepsy: Effects on behavior and executive functions. Epilepsy Behav. 2020;103(Pt A):106879. doi: 10.1016/j.yebeh.2019.106879. [DOI] [PubMed] [Google Scholar]
- 116.Chang F.M., Fan P.C., Weng W.C., Chang C.H., Lee W.T. The efficacy of perampanel in young children with drug-resistant epilepsy. Seizure. 2020;75:82–86. doi: 10.1016/j.seizure.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 117.Jackson A.C., Nicoll R.A. Stargazin (TARP gamma-2) is required for compartment-specific AMPA receptor trafficking and synaptic plasticity in cerebellar stellate cells. J. Neurosci. 2011;31(11):3939–3952. doi: 10.1523/JNEUROSCI.5134-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Adotevi N.K., Leitch B. Synaptic changes in ampa receptor subunit expression in cortical parvalbumin interneurons in the stargazer model of absence epilepsy. Front. Mol. Neurosci. 2017;10:434. doi: 10.3389/fnmol.2017.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Paz J.T., Bryant A.S., Peng K., Fenno L., Yizhar O., Frankel W.N., Deisseroth K., Huguenard J.R. A new mode of corticothalamic transmission revealed in the Gria4(-/-) model of absence epilepsy. Nat. Neurosci. 2011;14(9):1167–1173. doi: 10.1038/nn.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peeters B.W., Ramakers G.M., Vossen J.M., Coenen A.M. The WAG/Rij rat model for nonconvulsive absence epilepsy: involvement of nonNMDA receptors. Brain Res. Bull. 1994;33(6):709–713. doi: 10.1016/0361-9230(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 121.Coenen A.M., Van Luijtelaar E.L. Genetic animal models for absence epilepsy: a review of the WAG/Rij strain of rats. Behav. Genet. 2003;33(6):635–655. doi: 10.1023/A:1026179013847. [DOI] [PubMed] [Google Scholar]
- 122.Ramakers G.M., Peeters B.W., Vossen J.M., Coenen A.M. CNQX, a new non-NMDA receptor antagonist, reduces spike wave discharges in the WAG/Rij rat model of absence epilepsy. Epilepsy Res. 1991;9(2):127–131. doi: 10.1016/0920-1211(91)90023-9. [DOI] [PubMed] [Google Scholar]
- 123.Jakus R., Graf M., Ando R.D., Balogh B., Gacsalyi I., Levay G., Kantor S., Bagdy G. Effect of two noncompetitive AMPA receptor antagonists GYKI 52466 and GYKI 53405 on vigilance, behavior and spike-wave discharges in a genetic rat model of absence epilepsy. Brain Res. 2004;1008(2):236–244. doi: 10.1016/j.brainres.2004.01.087. [DOI] [PubMed] [Google Scholar]
- 124.Citraro R., Russo E., Gratteri S., Di Paola E.D., Ibbadu G.F., Curinga C., Gitto R., Chimirri A., Donato G., De Sarro G. Effects of non-competitive AMPA receptor antagonists injected into some brain areas of WAG/Rij rats, an animal model of generalized absence epilepsy. Neuropharmacology. 2006;51(6):1058–1067. doi: 10.1016/j.neuropharm.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 125.Russo E., Citraro R., De Fazio S., Marra R., Gitto R., Chimirri A., De Sarro G., Di Paola E.D. Enhancement of anti-absence effects of ethosuximide by low doses of a noncompetitive alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist in a genetic animal model of absence epilepsy. Epilepsy Behav. 2008;13(2):295–299. doi: 10.1016/j.yebeh.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 126.Kamiński R.M., Van Rijn C.M., Turski W.A., Czuczwar S.J., Van Luijtelaar G. AMPA and GABA(B) receptor antagonists and their interaction in rats with a genetic form of absence epilepsy. Eur. J. Pharmacol. 2001;430(2-3):251–259. doi: 10.1016/S0014-2999(01)01393-0. [DOI] [PubMed] [Google Scholar]
- 127.Citraro R., Leo A., Franco V., Marchiselli R., Perucca E., De Sarro G., Russo E. Perampanel effects in the WAG/Rij rat model of epileptogenesis, absence epilepsy, and comorbid depressive- like behavior. Epilepsia. 2017;58(2):231–238. doi: 10.1111/epi.13629. [DOI] [PubMed] [Google Scholar]
- 128.Hansen K.B., Yi F., Perszyk R.E., Furukawa H., Wollmuth L.P., Gibb A.J., Traynelis S.F. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 2018;150(8):1081–1105. doi: 10.1085/jgp.201812032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sibarov D.A., Antonov S.M. Calcium-Dependent Desensitization of NMDA Receptors. Biochemistry (Mosc.) 2018;83(10):1173–1183. doi: 10.1134/S0006297918100036. [DOI] [PubMed] [Google Scholar]
- 130.Mayer M.L., Westbrook G.L., Guthrie P.B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 131.Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 132.Barria A., Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35(2):345–353. doi: 10.1016/S0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- 133.Citri A., Malenka R.C. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33(1):18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 134.Li S.T., Ju J.G. Functional roles of synaptic and extrasynaptic NMDA receptors in physiological and pathological neuronal activities. Curr. Drug Targets. 2012;13(2):207–221. doi: 10.2174/138945012799201630. [DOI] [PubMed] [Google Scholar]
- 135.Paoletti P., Bellone C., Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 136.Granger A.J., Nicoll R.A. Expression mechanisms underlying long-term potentiation: a postsynaptic view, 10 years on. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;369(1633):20130136. doi: 10.1098/rstb.2013.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tovar K.R., Westbrook G.L. Modulating synaptic NMDA receptors. Neuropharmacology. 2017;112(Pt A):29–33. doi: 10.1016/j.neuropharm.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hansen K.B., Yi F., Perszyk R.E., Menniti F.S., Traynelis S.F. NMDA receptors in the central nervous system. Methods Mol. Biol. 2017;1677:1–80. doi: 10.1007/978-1-4939-7321-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Skowrońska K., Obara-Michlewska M., Zielińska M., Albrecht J. NMDA receptors in astrocytes: in search for roles in neurotransmission and astrocytic homeostasis. Int. J. Mol. Sci. 2019;20(2):309. doi: 10.3390/ijms20020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Camp C.R., Yuan H. GRIN2D/GluN2D NMDA receptor: Unique features and its contribution to pediatric developmental and epileptic encephalopathy. Eur. J. Paediatr. Neurol. 2020;24:89–99. doi: 10.1016/j.ejpn.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gao K., Tankovic A., Zhang Y., Kusumoto H., Zhang J., Chen W., XiangWei W., Shaulsky G.H., Hu C., Traynelis S.F., Yuan H., Jiang Y. A de novo loss-of-function GRIN2A mutation associated with childhood focal epilepsy and acquired epileptic aphasia. PLoS One. 2017;12(2):e0170818. doi: 10.1371/journal.pone.0170818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marwick K.F.M., Hansen K.B., Skehel P.A., Hardingham G.E., Wyllie D.J.A. Functional assessment of triheteromeric NMDA receptors containing a human variant associated with epilepsy. J. Physiol. 2019;597(6):1691–1704. doi: 10.1113/JP277292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Loss C.M., da Rosa N.S., Mestriner R.G., Xavier L.L., Oliveira D.L. Blockade of GluN2B-containing NMDA receptors reduces short-term brain damage induced by early-life status epilepticus. Neurotoxicology. 2019;71:138–149. doi: 10.1016/j.neuro.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 144.Schratt G.M., Tuebing F., Nigh E.A., Kane C.G., Sabatini M.E., Kiebler M., Greenberg M.E. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439(7074):283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 145.Jimenez-Mateos E.M., Engel T., Merino-Serrais P., McKiernan R.C., Tanaka K., Mouri G., Sano T., O’Tuathaigh C., Waddington J.L., Prenter S., Delanty N., Farrell M.A., O’Brien D.F., Conroy R.M., Stallings R.L., DeFelipe J., Henshall D.C. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat. Med. 2012;18(7):1087–1094. doi: 10.1038/nm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alsharafi W.A., Xiao B., Li J. MicroRNA-139-5p negatively regulates NR2A-containing NMDA receptor in the rat pilocarpine model and patients with temporal lobe epilepsy. Epilepsia. 2016;57(11):1931–1940. doi: 10.1111/epi.13568. [DOI] [PubMed] [Google Scholar]
- 147.Hamamoto O., Tirapelli D.P.D.C., Lizarte Neto F.S., Freitas-Lima P., Saggioro F.P., Cirino M.L.A., Assirati J.A., Jr, Serafini L.N., Velasco T.R., Sakamoto A.C., Carlotti C.G., Jr Modulation of NMDA receptor by miR-219 in the amygdala and hippocampus of patients with mesial temporal lobe epilepsy. J Clin Neurosci. 2020 doi: 10.1016/j.jocn.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 148.Zheng H., Tang R., Yao Y., Ji Z., Cao Y., Liu Z., Peng F., Wang W., Can D., Xing H., Bu G., Xu H., Zhang Y.W., Zheng W. MiR-219 Protects Against Seizure in the Kainic Acid Model of Epilepsy. Mol. Neurobiol. 2016;53(1):1–7. doi: 10.1007/s12035-014-8981-5. [DOI] [PubMed] [Google Scholar]
- 149.Löscher W., Hönack D. Anticonvulsant and behavioral effects of two novel competitive N-methyl-D-aspartic acid receptor antagonists, CGP 37849 and CGP 39551, in the kindling model of epilepsy. Comparison with MK-801 and carbamazepine. J. Pharmacol. Exp. Ther. 1991;256(2):432–440. [PubMed] [Google Scholar]
- 150.Dziki M., Hönack D., Löscher W. Kindled rats are more sensitive than non-kindled rats to the behavioural effects of combined treatment with MK-801 and valproate. Eur. J. Pharmacol. 1992;222(2-3):273–278. doi: 10.1016/0014-2999(92)90866-3. [DOI] [PubMed] [Google Scholar]
- 151.Löscher W., Rundfeldt C., Hönack D. Low doses of NMDA receptor antagonists synergistically increase the anticonvulsant effect of the AMPA receptor antagonist NBQX in the kindling model of epilepsy. Eur. J. Neurosci. 1993;5(11):1545–1550. doi: 10.1111/j.1460-9568.1993.tb00224.x. [DOI] [PubMed] [Google Scholar]
- 152.Löscher W., Nolting B., Hönack D. Evaluation of CPP, a selective NMDA antagonist, in various rodent models of epilepsy. Comparison with other NMDA antagonists, and with diazepam and phenobarbital. Eur. J. Pharmacol. 1988;152(1-2):9–17. doi: 10.1016/0014-2999(88)90830-8. [DOI] [PubMed] [Google Scholar]
- 153.Evans R.H., Francis A.A., Jones A.W., Smith D.A., Watkins J.C. The effects of a series of ω-phosphonic α-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br. J. Pharmacol. 1982;75(1):65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Davies J., Evans R.H., Herrling P.L., Jones A.W., Olverman H.J., Pook P., Watkins J.C. CPP, a new potent and selective NMDA antagonist. Depression of central neuron responses, affinity for [3H]D-AP5 binding sites on brain membranes and anticonvulsant activity. Brain Res. 1986;382(1):169–173. doi: 10.1016/0006-8993(86)90127-7. [DOI] [PubMed] [Google Scholar]
- 155.Rogawski M.A. Therapeutic potential of excitatory amino acid antagonists: channel blockers and 2,3-benzodiazepines. Trends Pharmacol. Sci. 1993;14(9):325–331. doi: 10.1016/0165-6147(93)90005-5. [DOI] [PubMed] [Google Scholar]
- 156.Rundfeldt C., Wlaź P., Löscher W. Anticonvulsant activity of antagonists and partial agonists for the NMDA receptor-associated glycine site in the kindling model of epilepsy. Brain Res. 1994;653(1-2):125–130. doi: 10.1016/0006-8993(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 157.Osterweil E.K., Chuang S.C., Chubykin A.A., Sidorov M., Bianchi R., Wong R.K., Bear M.F. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron. 2013;77(2):243–250. doi: 10.1016/j.neuron.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dorandeu F., Barbier L., Dhote F., Testylier G., Carpentier P. Ketamine combinations for the field treatment of soman-induced self-sustaining status epilepticus. Review of current data and perspectives. Chem. Biol. Interact. 2013;203(1):154–159. doi: 10.1016/j.cbi.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 159.Dorandeu F., Dhote F., Barbier L., Baccus B., Testylier G. Treatment of status epilepticus with ketamine, are we there yet? CNS Neurosci. Ther. 2013;19(6):411–427. doi: 10.1111/cns.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mewasingh L.D., Sékhara T., Aeby A., Christiaens F.J., Dan B. Oral ketamine in paediatric non-convulsive status epilepticus. Seizure. 2003;12(7):483–489. doi: 10.1016/S1059-1311(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 161.Rejdak K., Nieoczym D., Czuczwar M., Kiś J., Wlaź P., Turski W.A. Orphenadrine-induced convulsive status epilepticus in rats responds to the NMDA antagonist dizocilpine. Pharmacol. Rep. 2014;66(3):399–403. doi: 10.1016/j.pharep.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 162.Wlaź P., Baran H., Löscher W. Effect of the glycine/NMDA receptor partial agonist, D-cycloserine, on seizure threshold and some pharmacodynamic effects of MK-801 in mice. Eur. J. Pharmacol. 1994;257(3):217–225. doi: 10.1016/0014-2999(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 163.Sheardown M.J., Drejer J., Jensen L.H., Stidsen C.E., Honoré T. A potent antagonist of the strychnine insensitive glycine receptor has anticonvulsant properties. Eur. J. Pharmacol. 1989;174(2-3):197–204. doi: 10.1016/0014-2999(89)90312-9. [DOI] [PubMed] [Google Scholar]
- 164.McCabe R.T., Wasterlain C.G., Kucharczyk N., Sofia R.D., Vogel J.R. Evidence for anticonvulsant and neuroprotectant action of felbamate mediated by strychnine-insensitive glycine receptors. J. Pharmacol. Exp. Ther. 1993;264(3):1248–1252. [PubMed] [Google Scholar]
- 165.Katayama S., Ae N., Nagata R. Synthesis of tricyclic indole-2- carboxylic [correction of caboxylic] acids as potent NMDA-glycine antagonists. J. Org. Chem. 2001;66(10):3474–3483. doi: 10.1021/jo010002h. [DOI] [PubMed] [Google Scholar]
- 166.Croucher M.J., Collins J.F., Meldrum B.S. Anticonvulsant action of excitatory amino acid antagonists. Science. 1982;216(4548):899–901. doi: 10.1126/science.7079744. [DOI] [PubMed] [Google Scholar]
- 167.Parsons C.G., Danysz W., Quack G., Hartmann S., Lorenz B., Wollenburg C., Baran L., Przegalinski E., Kostowski W., Krzascik P., Chizh B., Headley P.M. Novel systemically active antagonists of the glycine site of the N-methyl-D-aspartate receptor: electrophysiological, biochemical and behavioral characterization. J. Pharmacol. Exp. Ther. 1997;283(3):1264–1275. [PubMed] [Google Scholar]
- 168.Dannhardt G., Kohl B.K. The glycine site on the NMDA receptor: structure-activity relationships and possible therapeutic applications. Curr. Med. Chem. 1998;5(4):253–263. [PubMed] [Google Scholar]
- 169.Krystal J.H., D’Souza D.C., Petrakis I.L., Belger A., Berman R.M., Charney D.S., Abi-Saab W., Madonick S. NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv. Rev. Psychiatry. 1999;7(3):125–143. doi: 10.3109/hrp.7.3.125. [DOI] [PubMed] [Google Scholar]
- 170.Sherwin A.L. Neuroactive amino acids in focally epileptic human brain: a review. Neurochem. Res. 1999;24(11):1387–1395. doi: 10.1023/A:1022580506443. [DOI] [PubMed] [Google Scholar]
- 171.Kohl B.K., Dannhardt G. The NMDA receptor complex: a promising target for novel antiepileptic strategies. Curr. Med. Chem. 2001;8(11):1275–1289. doi: 10.2174/0929867013372328. [DOI] [PubMed] [Google Scholar]
- 172.Jansen M., Dannhardt G. Antagonists and agonists at the glycine site of the NMDA receptor for therapeutic interventions. Eur. J. Med. Chem. 2003;38(7-8):661–670. doi: 10.1016/S0223-5234(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 173.Subramaniam S., Donevan S.D., Rogawski M.A. Block of the N-methyl-D-aspartate receptor by remacemide and its des-glycine metabolite. J. Pharmacol. Exp. Ther. 1996;276(1):161–168. [PubMed] [Google Scholar]
- 174.Brodie M.J., Wroe S.J., Dean A.D., Holdich T.A., Whitehead J., Stevens J.W. Efficacy and safety of remacemide versus carbamazepine in newly diagnosed epilepsy: comparison by sequential analysis. Epilepsy Behav. 2002;3(2):140–146. doi: 10.1006/ebeh.2002.0337. [DOI] [PubMed] [Google Scholar]
- 175.Chadwick D.W., Betts T.A., Boddie H.G., Crawford P.M., Lindstrom P., Newman P.K., Soryal I., Wroe S., Holdich T.A. Remacemide hydrochloride as an add-on therapy in epilepsy: a randomized, placebo-controlled trial of three dose levels (300, 600 and 1200 mg/day) in a Q.I.D. regimen. Seizure. 2002;11(2):114–123. doi: 10.1053/seiz.2002.0588. [DOI] [PubMed] [Google Scholar]
- 176.Kuo C.C., Lin B.J., Chang H.R., Hsieh C.P. Use-dependent inhibition of the N-methyl-D-aspartate currents by felbamate: a gating modifier with selective binding to the desensitized channels. Mol. Pharmacol. 2004;65(2):370–380. doi: 10.1124/mol.65.2.370. [DOI] [PubMed] [Google Scholar]
- 177.Hanrahan B., Abbasy M.S.U., Carson R.P. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020. [Google Scholar]
- 178.Zupanc M.L., Roell Werner R., Schwabe M.S., O’Connor S.E., Marcuccilli C.J., Hecox K.E., Chico M.S., Eggener K.A. Efficacy of felbamate in the treatment of intractable pediatric epilepsy. Pediatr. Neurol. 2010;42(6):396–403. doi: 10.1016/j.pediatrneurol.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 179.Hussain S.A., Asilnejad B., Heesch J., Navarro M., Ji M., Shrey D.W., Rajaraman R.R., Sankar R. Felbamate in the treatment of refractory epileptic spasms. Epilepsy Res. 2020;161:106284. doi: 10.1016/j.eplepsyres.2020.106284. [DOI] [PubMed] [Google Scholar]
- 180.Shi L.L., Bresnahan R., Martin-McGill K.J., Dong J., Ni H., Geng J. Felbamate add-on therapy for drug-resistant focal epilepsy. Cochrane Database Syst. Rev. 2019;8:CD008295. doi: 10.1002/14651858.CD008295.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chapman A.G., Graham J., Meldrum B.S. Potent oral anticonvulsant action of CPP and CPPene in DBA/2 mice. Eur. J. Pharmacol. 1990;178(1):97–99. doi: 10.1016/0014-2999(90)94798-3. [DOI] [PubMed] [Google Scholar]
- 182.Fagg G.E., Olpe H.R., Pozza M.F., Baud J., Steinmann M., Schmutz M., Portet C., Baumann P., Thedinga K., Bittiger H., et al. CGP 37849 and CGP 39551: novel and potent competitive N-methyl-D-aspartate receptor antagonists with oral activity. Br. J. Pharmacol. 1990;99(4):791–797. doi: 10.1111/j.1476-5381.1990.tb13008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Leander J.D., Rathbun R.C., Zimmerman D.M. Anticonvulsant effects of phencyclidine-like drugs: relation to N-methyl-D-aspartic acid antagonism. Brain Res. 1988;454(1-2):368–372. doi: 10.1016/0006-8993(88)90839-6. [DOI] [PubMed] [Google Scholar]
- 184.McNamara J.O., Russell R.D., Rigsbee L., Bonhaus D.W. Anticonvulsant and antiepileptogenic actions of MK-801 in the kindling and electroshock models. Neuropharmacology. 1988;27(6):563–568. doi: 10.1016/0028-3908(88)90176-1. [DOI] [PubMed] [Google Scholar]
- 185.Dalmau J., Gleichman A.J., Hughes E.G., Rossi J.E., Peng X., Lai M., Dessain S.K., Rosenfeld M.R., Balice-Gordon R., Lynch D.R. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7(12):1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hughes E.G., Peng X., Gleichman A.J., Lai M., Zhou L., Tsou R., Parsons T.D., Lynch D.R., Dalmau J., Balice-Gordon R.J. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J. Neurosci. 2010;30(17):5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Moscato E.H., Peng X., Jain A., Parsons T.D., Dalmau J., Balice-Gordon R.J. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann. Neurol. 2014;76(1):108–119. doi: 10.1002/ana.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sharma R., Al-Saleem F.H., Panzer J., Lee J., Puligedda R.D., Felicori L.F., Kattala C.D., Rattelle A.J., Ippolito G., Cox R.H., Lynch D.R., Dessain S.K. Monoclonal antibodies from a patient with anti-NMDA receptor encephalitis. Ann. Clin. Transl. Neurol. 2018;5(8):935–951. doi: 10.1002/acn3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Manto M., Dalmau J., Didelot A., Rogemond V., Honnorat J. In vivo effects of antibodies from patients with anti-NMDA receptor encephalitis: further evidence of synaptic glutamatergic dysfunction. Orphanet J. Rare Dis. 2010;5:31. doi: 10.1186/1750-1172-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rosch R.E., Wright S., Cooray G., Papadopoulou M., Goyal S., Lim M., Vincent A., Upton A.L., Baldeweg T., Friston K.J. NMDA-receptor antibodies alter cortical microcircuit dynamics. Proc. Natl. Acad. Sci. USA. 2018;115(42):E9916–E9925. doi: 10.1073/pnas.1804846115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sveinbjornsdottir S., Sander J.W., Upton D., Thompson P.J., Patsalos P.N., Hirt D., Emre M., Lowe D., Duncan J.S. The excitatory amino acid antagonist D-CPP-ene (SDZ EAA-494) in patients with epilepsy. Epilepsy Res. 1993;16(2):165–174. doi: 10.1016/0920-1211(93)90031-2. [DOI] [PubMed] [Google Scholar]
- 192.Lacey C.J., Bryant A., Brill J., Huguenard J.R. Enhanced NMDA receptor-dependent thalamic excitation and network oscillations in stargazer mice. J. Neurosci. 2012;32(32):11067–11081. doi: 10.1523/JNEUROSCI.5604-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Barad Z., Grattan D.R., Leitch B. NMDA receptor expression in the thalamus of the stargazer model of absence epilepsy. Sci. Rep. 2017;7:42926. doi: 10.1038/srep42926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Peeters B.W., Van Rijn C.M., Van Luijtelaar E.L., Coenen A.M. Antiepileptic and behavioural actions of MK-801 in an animal model of spontaneous absence epilepsy. Epilepsy Res. 1989;3(2):178–181. doi: 10.1016/0920-1211(89)90047-8. [DOI] [PubMed] [Google Scholar]
- 195.Banerjee P.K., Snead O.C., III Involvement of excitatory amino acid mechanisms in gamma-hydroxybutyrate model of generalized absence seizures in rats. Neuropharmacology. 1992;31(10):1009–1019. doi: 10.1016/0028-3908(92)90102-U. [DOI] [PubMed] [Google Scholar]
- 196.Banerjee P.K., Snead O.C., III Thalamic NMDA receptors in the gamma-hydroxybutyrate model of absence seizures: a cerebral microinjection study in rats. Neuropharmacology. 1995;34(1):43–53. doi: 10.1016/0028-3908(94)00134-E. [DOI] [PubMed] [Google Scholar]
- 197.Koerner C., Danober L., Boehrer A., Marescaux C., Vergnes M. Thalamic NMDA transmission in a genetic model of absence epilepsy in rats. Epilepsy Res. 1996;25(1):11–19. doi: 10.1016/0920-1211(96)00015-0. [DOI] [PubMed] [Google Scholar]
- 198.Nehlig A., Boehrer A. Effects of remacemide in two models of genetically determined generalized epilepsy, the GAERS and the audiogenic Wistar AS. Epilepsy Res. 2003;52(3):253–261. doi: 10.1016/S0920-1211(02)00236-X. [DOI] [PubMed] [Google Scholar]
- 199.van Luijtelaar E.L., Coenen A.M. Effects of remacemide and its metabolite FPL 12495 on spike-wave discharges, electroencephalogram and behaviour in rats with absence epilepsy. Neuropharmacology. 1995;34(4):419–425. doi: 10.1016/0028-3908(95)00008-T. [DOI] [PubMed] [Google Scholar]
- 200.Pinheiro P., Mulle C. Kainate receptors. Cell Tissue Res. 2006;326(2):457–482. doi: 10.1007/s00441-006-0265-6. [DOI] [PubMed] [Google Scholar]
- 201.Jane D.E., Lodge D., Collingridge G.L. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology. 2009;56(1):90–113. doi: 10.1016/j.neuropharm.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 202.Møllerud S., Frydenvang K., Pickering D.S., Kastrup J.S. Lessons from crystal structures of kainate receptors. Neuropharmacology. 2017;112(Pt A):16–28. doi: 10.1016/j.neuropharm.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 203.Valbuena S., Lerma J. Kainate receptors, homeostatic gatekeepers of synaptic plasticity. Neuroscience. 2019;456:17–26. doi: 10.1016/j.neuroscience.2019.11.050. [DOI] [PubMed] [Google Scholar]
- 204.Crépel V., Mulle C. Physiopathology of kainate receptors in epilepsy. Curr. Opin. Pharmacol. 2015;20:83–88. doi: 10.1016/j.coph.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 205.Rodríguez-Moreno A., Sihra T.S. Kainate receptors with a metabotropic modus operandi. Trends Neurosci. 2007;30(12):630–637. doi: 10.1016/j.tins.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 206.Rodríguez-Moreno A., Sihra T.S. Metabotropic actions of kainate receptors in the CNS. J. Neurochem. 2007;103(6):2121–2135. doi: 10.1111/j.1471-4159.2007.04924.x. [DOI] [PubMed] [Google Scholar]
- 207.Vargas J.R., Takahashi D.K., Thomson K.E., Wilcox K.S. The expression of kainate receptor subunits in hippocampal astrocytes after experimentally induced status epilepticus. J. Neuropathol. Exp. Neurol. 2013;72(10):919–932. doi: 10.1097/NEN.0b013e3182a4b266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rodríguez-Moreno A., Herreras O., Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19(4):893–901. doi: 10.1016/S0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- 209.Mulle C., Sailer A., Pérez-Otaño I., Dickinson-Anson H., Castillo P.E., Bureau I., Maron C., Gage F.H., Mann J.R., Bettler B., Heinemann S.F. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392(6676):601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- 210.Smolders I., Bortolotto Z.A., Clarke V.R., Warre R., Khan G.M., O’Neill M.J., Ornstein P.L., Bleakman D., Ogden A., Weiss B., Stables J.P., Ho K.H., Ebinger G., Collingridge G.L., Lodge D., Michotte Y. Antagonists of GLU(K5)-containing kainate receptors prevent pilocarpine-induced limbic seizures. Nat. Neurosci. 2002;5(8):796–804. doi: 10.1038/nn880. [DOI] [PubMed] [Google Scholar]
- 211.Fritsch B., Reis J., Gasior M., Kaminski R.M., Rogawski M.A., Rogawski M.A. Role of GluK1 kainate receptors in seizures, epileptic discharges, and epileptogenesis. J. Neurosci. 2014;34(17):5765–5775. doi: 10.1523/JNEUROSCI.5307-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lévesque M., Avoli M. The kainic acid model of temporal lobe epilepsy. Neurosci. Biobehav. Rev. 2013;37(10 Pt 2):2887–2899. doi: 10.1016/j.neubiorev.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yamada K.A., Turetsky D.M. Allosteric interactions between cyclothiazide and AMPA/kainate receptor antagonists. Br. J. Pharmacol. 1996;117(8):1663–1672. doi: 10.1111/j.1476-5381.1996.tb15337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vincent P., Mulle C. Kainate receptors in epilepsy and excitotoxicity. Neuroscience. 2009;158(1):309–323. doi: 10.1016/j.neuroscience.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 215.Peret A., Christie L.A., Ouedraogo D.W., Gorlewicz A., Epsztein J., Mulle C., Crépel V. Contribution of aberrant GluK2- containing kainate receptors to chronic seizures in temporal lobe epilepsy. Cell Rep. 2014;8(2):347–354. doi: 10.1016/j.celrep.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 216.Paternain A.V., Herrera M.T., Nieto M.A., Lerma J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J. Neurosci. 2000;20(1):196–205. doi: 10.1523/JNEUROSCI.20-01-00196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tandon P., Yang Y., Stafstrom C.E., Holmes G.L. Downregulation of kainate receptors in the hippocampus following repeated seizures in immature rats. Brain Res. Dev. Brain Res. 2002;136(2):145–150. doi: 10.1016/S0165-3806(02)00358-9. [DOI] [PubMed] [Google Scholar]
- 218.Werner P., Voigt M., Keinänen K., Wisden W., Seeburg P.H. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature. 1991;351(6329):742–744. doi: 10.1038/351742a0. [DOI] [PubMed] [Google Scholar]
- 219.Swanson G.T. Targeting AMPA and kainate receptors in neurological disease: therapies on the horizon? Neuropsychopharmacology. 2009;34(1):249–250. doi: 10.1038/npp.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Medina-Ceja L., García-Barba C. The glutamate receptor antagonists CNQX and MPEP decrease fast ripple events in rats treated with kainic acid. Neurosci. Lett. 2017;655:137–142. doi: 10.1016/j.neulet.2017.06.056. [DOI] [PubMed] [Google Scholar]
- 221.Figueiredo T.H., Qashu F., Apland J.P., Aroniadou-Anderjaska V., Souza A.P., Braga M.F. The GluK1 (GluR5) Kainate/alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor antagonist LY293558 reduces soman-induced seizures and neuropathology. J. Pharmacol. Exp. Ther. 2011;336(2):303–312. doi: 10.1124/jpet.110.171835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gilron I., Max M.B., Lee G., Booher S.L., Sang C.N., Chappell A.S., Dionne R.A. Effects of the 2-amino-3-hydroxy-5-methyl-4-isoxazole-proprionic acid/kainate antagonist LY293558 on spontaneous and evoked postoperative pain. Clin. Pharmacol. Ther. 2000;68(3):320–327. doi: 10.1067/mcp.2000.108677. [DOI] [PubMed] [Google Scholar]
- 223.Sang C.N., Ramadan N.M., Wallihan R.G., Chappell A.S., Freitag F.G., Smith T.R., Silberstein S.D., Johnson K.W., Phebus L.A., Bleakman D., Ornstein P.L., Arnold B., Tepper S.J., Vandenhende F. LY293558, a novel AMPA/GluR5 antagonist, is efficacious and well-tolerated in acute migraine. Cephalalgia. 2004;24(7):596–602. doi: 10.1111/j.1468-2982.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 224.Dudek F.E., Bramley J.R. GluR5 kainate receptors and topiramate: a new site of action for antiepileptic drugs? Epilepsy Curr. 2004;4(1):17. doi: 10.1111/j.1535-7597.2004.04107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Das A., Wallace G.C., IV, Holmes C., McDowell M.L., Smith J.A., Marshall J.D., Bonilha L., Edwards J.C., Glazier S.S., Ray S.K., Banik N.L. Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience. 2012;220:237–246. doi: 10.1016/j.neuroscience.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang L., Liu Y., Lu R., Dong G., Chen X., Yun W., Zhou X. The role of S-nitrosylation of kainate-type of ionotropic glutamate receptor 2 in epilepsy induced by kainic acid. J. Neurochem. 2018;144(3):255–270. doi: 10.1111/jnc.14266. [DOI] [PubMed] [Google Scholar]
- 227.Orav E., Dowavic I., Huupponen J., Taira T., Lauri S.E. NETO1 regulates postsynaptic kainate receptors in CA3 interneurons during circuit maturation. Mol. Neurobiol. 2019;56(11):7473–7489. doi: 10.1007/s12035-019-1612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]