Abstract
The neuroinflammatory hypothesis of Alzheimer’s disease (AD) was proposed more than 30 years ago. The involvement of the two main types of glial cells microglia and astrocytes, in neuroinflammation, was suggested early on. In this review, we highlight that the exact contributions of reactive glia to AD pathogenesis remain difficult to define, likely due to the heterogeneity of glia populations and alterations in their activation states through the stages of AD progression. In the case of microglia, it is becoming apparent that both beneficially and adversely activated cell populations can be identified at various stages of AD, which could be selectively targeted to either limit their damaging actions or enhance beneficial functions. In the case of astrocytes, less information is available about potential subpopulations of reactive cells; it also remains elusive whether astrocytes contribute to the neuropathology of AD by mainly gaining neurotoxic functions or losing their ability to support neurons due to astrocyte damage. We identify L-type calcium channel blocker, nimodipine, as a candidate drug for AD, which potentially targets both astrocytes and microglia. It has already shown consistent beneficial effects in basic experimental and clinical studies. We also highlight the recent evidence linking peripheral inflammation and neuroinflammation. Several chronic systemic inflammatory diseases, such as obesity, type 2 diabetes mellitus, and periodontitis, can cause immune priming or adverse activation of glia, thus exacerbating neuroinflammation and increasing risk or facilitating the progression of AD. Therefore, reducing peripheral inflammation is a potentially effective strategy for lowering AD prevalence.
Keywords: Neurodegenerative diseases, microglia, astrocytes, neurotoxicity, systemic inflammation, neuroprotective drugs, L-type calcium channel blockers
1. NEUROINFLAMMATORY HYPOTHESIS OF ALZHEIMER’S DISEASE
The hypothesis proposing neuroinflammation as one of the key mechanisms of Alzheimer’s disease (AD) pathogenesis was introduced more than 30 years ago. Since then, neuroinflammation research has become a major field of neuroscience. The initial support for the neuroinflammatory hypothesis of AD came from immunohistochemical studies demonstrating reactive microglia and astrocytes in brain areas known to exhibit the two main biochemical markers of AD pathology: amyloid plaques and neurofibrillary tangles. Reactive phenotypes of microglia and astrocytes were defined by upregulated expression of the major histocompatibility complex (MHC) class II protein human leukocyte antigen (HLA)-DR and glial fibrillary acidic protein (GFAP), respectively. Reactive microglia were also shown to have altered amoeboid-shaped morphology and to be phagocytic [1, 2]. Subsequent biochemical and molecular biology studies discovered upregulated expression of inflammatory cytokines and complement activation in AD brain tissue as markers of neuroimmune responses. Epidemiological research identified a reduced risk of AD in chronic users of anti-inflammatory drugs [3-5]. Early in vitro studies demonstrated that microglia and other macrophages could be activated by synthetic human amyloid-β peptides (Aβ), leading to secretion of cytotoxins and neuronal cell death [6, 7]. Even though such observations implied that the reactive microglia seen in AD tissues could represent Aβ-activated cells that contribute to neurodegeneration [3], this hypothesis was not universally accepted. An alternative view of microglia in AD was that these cells merely alter their phenotype in response to neuronal loss without actively contributing to the disease pathology [8]. More recently, it has become clear that the contribution of microglia to AD pathogenesis is very complex, involving both protective and deleterious actions, which most likely depend on the activation states of microglia as well as the stage of the disease process [9, 10].
Significant support for the neuroinflammatory hypothesis and active role of microglia in AD was provided by genome-wide association studies, which identified several inflammation-linked genes as risk factors for the late-onset form of AD [11]. All these factors have low to moderate effect sizes. Unlike the genes linked to the early-onset familial AD cases, which are inherited in an autosomal dominant fashion, none of these risk factors alone can accurately predict the development of AD; however, if multiple factors are present, they may have a cumulative effect on disease susceptibility. The most prominent examples of genes potentially linked to inflammatory processes in AD include TREM2 (triggering receptor expressed on myeloid cells 2), CD33 (SIGLEC-3, sialic acid-binding Ig-like lectin 3), CR1 (complement receptor 1), PLCG2 (phospholipase C gamma 2), and INPP5D (inositol polyphosphate-5-phosphatase D). In the central nervous system (CNS) these genes are expressed mainly by microglia, which implicates them as key players in AD pathogenesis [12]. TREM2, in particular, has become a focus of intense research due to its ability to regulate the phagocytic activity of microglia, even though its expression by human microglia is still controversial. While human microglia from AD brains have upregulated TREM2 mRNA expression [13], this increase cannot be readily confirmed at the protein level [14, 15]. For example, an extensive immunohistochemistry study involving 299 post-mortem cases (including 83 with AD pathology) shows that most microglia do not express TREM2 protein in the human brain [16], while a study on 11 AD cases reported TREM2 immunoreactive microglia associated with plaques [17]. Furthermore, the fact that monocytes recruited to the human brain express high TREM2 protein levels may indicate the contribution of the peripheral inflammatory conditions, such as metabolic syndrome and periodontitis, to the onset and progression of AD (see section 4 below) [14, 16].
Initially, it was only possible to investigate glial cell involvement in AD by studying human post-mortem tissues, in vitro cell and tissue systems, or animal models; however, advances in positron emission tomography (PET) imaging using ligands that bind to the mitochondrial translocator protein (TSPO) have enabled in vivo imaging of microglia activation in clinical studies. These studies have confirmed disease-specific anatomical distribution of reactive microglia in the brains of patients with probable AD as well as demonstrated that it is possible to detect activated microglia at very early stages of AD [18, 19]. These new imaging techniques could provide new insights into the early stages and clinical progression of neuroimmunopathology in AD patients.
2. CELLULAR MECHANISMS OF NEURO-INFLAMMATION: MICROGLIA
2.1. Microglia Origin and Physiological Functions
Microglia are the most prevalent professional immune cells in a healthy human brain. They have been considered the CNS resident macrophages belonging to the mononuclear phagocyte system [20]. Recent tracing studies have challenged this long-established view by demonstrating that microglia are an ontogenetically distinct population of mononuclear phagocytes that are not derived from hematopoietic stem cells but originate from yolk sac progenitors at the early stages of embryogenesis [21, 22]. The central role of microglia in CNS immune defense and various brain pathologies, including infection, trauma, and autoimmune or neurodegenerative diseases, has long been recognized; however, these cells also perform essential support functions in the physiological processes of CNS development, maturation, maintenance, and decline [23]. Microglia populate the embryonic brain very early, which allows them to contribute to key neurodevelopmental processes, such as neurogenesis, oligodendrogenesis, myelinogenesis, vascularization, and establishment of brain cytoarchitecture [24, 25]. Microglia have been shown to regulate numbers of neurons and neural precursors through two opposing mechanisms: 1) support of cell proliferation and survival by secreting growth factors, such as insulin-like growth factor-1 (IGF-1), glial cell line-derived neurotrophic factor (GDNF), and granulocyte-macrophage colony-stimulating factor (GM-CSF) [23, 26, 27]; and 2) elimination of neurons by phagocytosing these cells through the process termed neurophagy, or by promoting the death of unwanted cells through secretion of reactive oxygen species and other cytotoxins [28]. Microglia can also exert opposing effects on synapses: they have been shown to provide trophic support to newly established neuronal connections as well as participate in synaptic elimination by the mechanism termed synaptic pruning [29]. The latter involves phagocytosis of synapses primarily mediated by microglia complement receptor 3 (CR3) recognizing inactive synapses marked with complement proteins C1q and C3 [30, 31]. Microglia are also essential for vascular remodelling during CNS maturation and help maintain vasculature in the adult brain [32, 33].
2.2. Microglia Heterogeneity
To fulfill diverse physiological functions during various developmental stages and within different brain environments, microglia must change their function and morphology. Our understanding of this process has advanced significantly over the last thirty years; the initial descriptions of the two-state microglia (resting or activated) have evolved due to 1) recent observations that “resting” microglia are in constant motion, actively sampling their surrounding environment; and 2) recognition that there are multiple activation states of microglia characterized by distinct morphological features and functional parameters. Microglia phenotype and functions depend on their anatomical location, the proximity of synapses and apoptotic cells, and age and sex of the animal [34]. For example, the initial reports demonstrating region-specific morphology and density of microglia in the murine brain [35] were followed by studies identifying differences in cell surface protein expression in microglia from different brain regions [36]. These observations have recently been supplemented by studies documenting differences in RNA expression profiles in microglia populations isolated from several mouse brain regions and across the animal life span [37, 38], as well as through single-cell sequencing of microglia and myeloid cells from mouse brains [39]. Our understanding of microglia heterogeneity is mainly based on studies with murine cells and tissues; however, recent investigations of microglia extracted from human post-mortem brain tissues indicate brain region-specific protein and gene expression of microglia [40, 41] as well as age-associated changes in human microglia gene expression [42]. Heterogeneity within the human microglia population and brain region-specific expression of microglia phenotypic markers have also been documented by studying single cells [43, 44].
Microglia most likely acquire diverse functional and morphological phenotypes in response to environmental cues that are brain-region specific and vary between different developmental stages. Microglia express a range of cell surface receptors that interact selectively with diverse CNS molecules, including catecholamines (norepinephrine and dopamine), excitatory amino acids (glutamate), nucleotides (adenine di- and tri-phosphates), neuropeptides (substance P and vasoactive intestinal peptide), hormones (corticotropin-releasing hormone), and growth factors (nerve growth factor and GDNF) [26, 45]. In addition, direct contact with other cells has been proposed as one of the mechanisms regulating microglia functions; for example, the interaction between CD200 expressed on neurons and CD200 receptor (CD200R) on microglia is considered critical for regulating microglia functions [46].
Immune activation of microglia can be induced and regulated by a broad set of cytokines and chemokines, including several interleukins (IL), fractalkine, tumor necrosis factor (TNF)-α, and chemokine (C-C motif) ligand 2 (CCL2). Microglia also express pattern-recognition receptors (PRRs), such as receptor for advanced glycation end products (RAGE), nucleotide‐binding oligomerization domain‐like receptors (NLRs), and toll-like receptors (TLRs) 2 and 4, which interact with damage-associated molecular patterns (DAMPs) and resolution-associated molecular patterns (RAMPs) released from dying and damaged cells [47-50]. High-mobility group box 1 (HMGB1) is likely the most studied DAMP in the context of microglia activation; it is a non-histone DNA binding nuclear protein, which can be passively released from dying cells or actively secreted from various cell types by several different pathways. HMGB1 activates microglia TLR4 and RAGE [51-53]. Several mitochondrial proteins, including cytochrome C and mitochondrial transcription factor A (TFAM), once released extracellularly, have also been shown to regulate select microglia immune functions [54-56].
2.3. Microglia Activation in AD
The original description of reactive microglia in cortical structures of AD brains included observations of swollen, phagocytosing cells that stained intensely with antibodies against HLA-DR protein [1]. Since these reactive microglia congregated around senile plaques, it was hypothesized first and later demonstrated experimentally that microglia are activated by Aβ, which is the main constituent of the plaques. It was hypothesized that microglia in AD brains are adversely activated, which leads to the secretion of pro-inflammatory mediators and neurotoxins partially responsible for the excessive death of neurons observed in AD [4, 9, 57]. Intense research efforts were launched aimed at pinpointing the receptors, mediating the interaction of microglia with Aβ, and identifying the neurotoxin(s) released by activated microglia in AD brains (see section 2.4. below). More than a dozen distinct microglia receptors have now been shown to interact with different forms of Aβ (e.g., Aβ1-40, Aβ1-42) at various aggregation states; they include several scavenger receptors (scavenger receptor A-1, SCARA-1; scavenger receptor B-1, SCARB-1; CD36; RAGE), toll-like receptors (TLR2; TLR4) and the G protein-coupled receptors (formyl peptide receptor 2, FPR2; chemokine-like receptor 1, CMKLR1). Functionally, binding of Aβ to these receptors induces secretion of inflammatory mediators, changes in cell morphology, and/or uptake of Aβ [58, 59].
As mentioned above, the adversely activated amoeboid microglia, often forming clusters around amyloid plaques, were initially contrasted to resting cells, which displayed ramified morphology with thin, highly branched processes. Resting microglia were shown to form a tile-like network evenly covering the CNS parenchyma without touching each other or overlapping. This initial concept of on-off activation states of microglia was later challenged by observations demonstrating resting microglia surveying and sampling CNS environment actively by extending and retracting their processes and touching neighbouring cells, including other microglia, astrocytes, and neurons, as well as blood vessels; thus, the term “resting” has been replaced with “surveying” or “homeostatic” when describing microglia in their highly ramified state [9, 60-62].
With regard to microglia activation, it is now apparent that more than one activation state exists [63]. Advances in immunohistochemistry, molecular biology, and single-cell characterization techniques have identified numerous novel activation markers of microglia beside the classical HLA-DR upregulation, including F4/80, CD68, CD45, and ionised calcium-binding adapter molecule 1 (IBA1), also known as the allograft inflammatory factor-1 (AIF-1) [33]. Based on already introduced activation phenotypes of peripheral macrophages [64], the M1/M2 polarization axis was proposed for microglia. The classical pro-inflammatory M1 activation phenotype is induced by such potent immune stimuli as lipopolysaccharide (LPS) and interferon (IFN)-γ, while the alternative, anti-inflammatory/resolution M2 activation phenotype is induced by IL-4 and IL-10 [65]. The M1/M2 microglia activation phenotypes are characterized by partially overlapping sets of cell surface markers and secretory products. The list of microglia M1/M2 polarization markers has been growing steadily [66, 67], which, combined with more detailed functional studies, has led to the identification of several subtypes of the M2 activation phenotype [68]. It is also becoming apparent that microglia shift their activation states and acquire intermediate phenotypes along the M1/M2 polarization axis [69].
Recent studies considering multiple molecular markers have identified additional microglia functional and phenotypic states that do not align well with the M1/M2 polarization axis. For example, disease-associated microglia (DAM) have been identified in AD model mice brains and human post-mortem AD brain tissues. These cells accumulate around amyloid plaques and display enhanced phagocytic activity internalizing Aβ, thus potentially restricting neurodegeneration [70]. Another distinct subtype of microglia has been termed proliferative-region associated microglia (PAM). These cells, which appear transiently in regions of developing white matter and are essential for phagocytosis of the large number of oligodendrocytes that die during the process of myelination, share a characteristic gene signature with DAM [39]. It has been suggested that this subset of microglia can be specifically targeted in neurodegenerative diseases, including AD [63, 70].
Studies with AD model mice and post-mortem human AD brain tissues have identified microglia exhibiting both M1 and M2 phenotypes [68, 69]. In the hippocampus of the double-transgenic PS1M146L/APP751SL mouse model of AD, a switch of microglia from M2 to M1 phenotype was demonstrated as the disease pathology progressed [71]. The presence of M1 microglia in the late stages of AD is consistent with the original hypothesis of these cells having adverse effects on CNS homeostasis due to secretion of pro-inflammatory cytokines and cytotoxins. The presence of M2 microglia at early stages of AD, as well as the appearance of DAM, indicate the protective role these cells may have, which is aimed at phagocytosing Aβ deposits and degrading extracellular Aβ by secreting several different proteases [72]. Interestingly, microglia committed to a pro-inflammatory response have lower phagocytic capacity because pro-inflammatory cytokines downregulate their phagocytic activity [73].
The various activation states and phenotypes of microglia in AD are most likely induced and regulated by a combination of physiological mediators (see 2.3.) and AD-specific stimuli. As discussed above, microglia recognize Aβ, but depending on its aggregation state, functional consequences of this interaction could be very different; thus, the oligomeric Aβ is a stronger inducer of M1 phenotype than the fibrillar form of Aβ [74], while microglia surrounding amyloid plaques generally manifest M2 phenotype, including enhanced phagocytic capacity [71]. It has also been suggested that molecules associated with the amyloid plaque, including complement proteins and chromogranin A, regulate microglia activation and facilitate Aβ phagocytosis [49, 75].
Due to increased cellular injury and necrosis, AD brain tissue most likely has elevated levels of various DAMPs and RAMPs, which activate microglial PRRs. While the majority of these molecules (DAMPs) are believed to induce pro-inflammatory activation of microglia, some of them (RAMPs) have also been suggested to downregulate microglia and other mononuclear phagocytes facilitating the resolution of inflammation [48, 49, 54, 76, 77]. Since the blood-brain barrier (BBB) becomes compromised in AD, peripheral inflammatory mediators typically not present in the brain parenchyma, such as fibrinogen and immunoglobulins, could enter the brain tissue and modulate microglia activation state [78-80]. Recent studies have also highlighted peripheral hormones, like insulin, incretins, and steroids, as well as gut bacteria metabolites, as candidate regulators of microglia activation (see section 4 below and [26, 78, 81-87]).
2.4. Adverse Effects of Microglia in AD
Even though a broad range of protective functions of microglia in AD has been identified, their adverse contribution to the AD pathology, especially during the mid- to late-stages of the disease process, is likely [10, 58]. In addition to the data obtained by using animal models, strong support to this conclusion comes from clinical imaging studies showing a high correlation between activated microglia and cognitive decline in AD subjects [88]. The most studied aspect of the adverse activation of microglia is their ability to secrete neurotoxins, including glutamate, quinolinic acid, reactive oxygen, and nitrogen species, as well as a range of proteases capable of killing neurons directly [28, 89, 90]. It is important to note that significant species differences exist between murine and human microglia, with the latter, for example, unable to produce high levels of nitric oxide, implicated as one of the key neurotoxins of murine microglia [23]. Other direct mechanisms of microglia-induced neurodegeneration include the recently discovered phagocytosis of stressed, but live neurons and complement-dependent stripping of dendritic spines by overactivated microglia [10, 91].
Microglia can also cause neuronal injury indirectly. A combination of IL-1α, TNF-α, and C1q secreted by activated microglia has been recently shown to transform astrocytes into a neurotoxic A1 phenotype, which is also observed in AD brains [92]. It is well established that the classical M1 activation phenotype of microglia is characterized by increased secretion of IL-1β, IL-6, TNF-α, and chemokine (C-X-C motif) ligand 1 (CXCL1), which recruit and activate other microglia and astrocytes; thus, microglia could cooperate with astrocytes to mediate neurotoxicity. Microglia have also been implicated as one of the key cellular sources of the amyloid plaques in AD tissues [93]; depleting microglia with a selective colony-stimulating factor 1 receptor (CSF1R) inhibitor demonstrated that they could be critical for the initiation of Aβ aggregation [94]. Microglia can also convert Aβ aggregates they have internalized into neurotoxic species that are subsequently released extracellularly with microglial microvesicles [95]. Studies using a mouse model expressing mutant tau protein, the main component of the neurofibrillary tangles seen in AD, have shown that microglia depletion limits the spread of tau deposits across the brain; thus, microglia can contribute to AD pathology through uptake and exosomal release of tau [96].
3. CELLULAR MECHANISMS OF NEURO-INFLAMMATION: ASTROCYTES
3.1. Physiological Functions of Astrocytes
Astrocytes are the most abundant glial cell type in the mammalian CNS and constitute up to 50% of its volume [97]. The existence of astrocytes was first inferred by Virchow, in the mid-nineteenth century, with his observation of a type of connective tissue that contains cellular elements in the human brain. The term astrocyte was introduced by Lenhossek in 1895 and was greatly popularized by Cajal, who developed gold and mercury chloride (sublimate) staining techniques to label glial filaments and astrocyte end-feet [98]. Astrocytes have traditionally been thought to form a homogenous cell population and function as passive housekeepers to preserve the optimal microenvironment in the CNS. However, evidence is emerging that astrocytes are highly heterogeneous in form and function regardless of whether the cells are in the homeostatic or activated state [99, 100]. Moreover, astrocytes are active players demonstrating remarkable adaptive plasticity essential for the functional maintenance of the CNS in development and aging [98]. The key physiological functions of astrocytes include: 1) regulating normal functions of the BBB [101]; 2) supplying energy-carrying molecules to neurons; 3) secreting neurotrophic factors, antioxidant molecules, including glutathione, and synaptic activity modulators; 4) forming tripartite synapse to regulate synaptic function and plasticity with preservation of the extracellular balance of ions and neurotransmitters, such as glutamate and gamma-aminobutyric acid (GABA); 5) mediating glymphatic drainage of toxic metabolites out of the CNS during sleep [102], and 6) contributing to the CNS innate immunity [103]. Considering the link between astrocytes and AD pathology, it is noteworthy that astrocytes participate in the clearance of the interstitial solute Aβ out of the brain parenchyma by glymphatic drainage [102]. Astrocytes also phagocytose and degrade Aβ. This astrocyte ability is apolipoprotein E (ApoE) dependent [104] and is more prominent in young compared to aged individuals [105, 106]. Recently, astrocytes have also shown to engulf synapses by using a phagocytic mechanism that is independent of C1q but involves the phagocytic receptors multiple epidermal growth factors (EGF)-like domains 10 (MEGF10) and MER tyrosine kinase (MERTK) [30, 107]. Phagocytosis of apoptotic cells, tissue debris, and synapses by both microglia and astrocytes are clearly essential during CNS development when excessive numbers of cells appear, many of which need to be eliminated. Recent evidence, however, indicates that phagocytic processes continue to be critical for CNS homeostasis throughout adulthood, for example, by supporting hippocampal neurogenesis [32]. The astrocyte functions mentioned above are crucial for the homeostasis and physiology of the CNS. Accordingly, impairment of astrocyte functions contributes to AD pathophysiology.
3.2. Dysfunction of Astrocytes in AD
Along with activated/reactive astrocytes, damaged astrocytes have been identified in post-mortem AD brain tissues [108]. Although the exact AD-specific features of astrocyte damage and subsequent astrocyte dysfunction remain unknown, several possible mechanisms have been postulated. For example, it has been observed that extracellular Aβ triggers elevation of intracellular calcium ([Ca2+]i) in astrocytes. Aβ is preferentially incorporated into astrocyte membranes, which have higher cholesterol content compared to neuronal membranes. This leads to the formation of Ca2+-permeable channels allowing Ca2+ influx from the extracellular space [109]. A resulting increase in [Ca2+]i causes mitochondrial depolarization and astrocyte damage [110]. Increased [Ca2+]i also leads to the release of glutamate from astrocytes [111] and activation of nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase, resulting in astrocyte production of the oxygen free radical superoxide [110]. Glutamate and free radicals, in excess, are potentially toxic to neighboring cells, including neurons.
It is likely that damage caused to astrocytes by the microenvironment in AD brains leads to their compromised or even lost physiological functions. It is also plausible that the compromise/loss of normal astrocyte functions directed at protecting and supporting neurons contributes to neurodegeneration occurring in AD. For instance, impaired uptake and conversion of glutamate into glutamine, using astrocyte-specific glutamate transporters and glutamine synthase, respectively, have been shown to elevate extracellular glutamate levels up to excitotoxic levels [98, 112]. In addition, the inability of astrocytes to phagocytose and degrade Aβ, and to facilitate the clearance of interstitial Aβ from the brain parenchyma, can exacerbate the neurodegenerative pathology of AD and accelerate its progression.
3.2.1. Activated Astrocytes in AD
The presence of activated astrocytes, also referred to as reactive astrocytes or astrogliosis [99], in affected brain regions is a highly reproducible immunohistochemical finding in post-mortem brains from patients with common neurodegenerative diseases, such as AD [1, 113], Parkinson’s disease (PD) [113], amyotrophic lateral sclerosis (ALS) [114, 115], and multiple sclerosis (MS) [113]. Specifically, in AD, activated astrocytes are gathered around the periphery of the Aβ plaques, and their processes surround and invade the plaques [1, 113]. Activated astrocytes are characterized by cellular hypertrophy (i.e., hypertrophy of soma and main processes) and a marked increase in immunoreactivity for GFAP, S100B, and vimentin. A post-mortem quantitative immunohistochemistry study has demonstrated that the relative proportion of Aβ plaques colocalized with GFAP immunoreactivity in the absence of dystrophic neurites increases in mild AD but significantly decreases in severe AD [116]. This observation indicates that astrocyte activation may be an early and contributory event in the AD pathology rather than merely a response to neuronal death [116]. While astrocyte activation is not limited to AD, Perez-Nievas and Serrano-Pozo postulate that the relationship between activated astrocytes and the typical hallmarks of pathology, especially Aβ plaques, in AD is more intimate compared to astrocyte interaction with pathological structures in other neurodegenerative diseases [117]. It is also plausible that in the AD brain, not all astrocytes are uniformly activated since a seminal population-based neuropathology study showed that increased immunoreactivity for GFAP did not correlate with either Aβ or tau immunoreactivity [118]. This finding indicates that astrocytes are activated through complex interactions with not only Aβ and tau but also with other pathological factors in AD brains. Several such “alternative pathological factors” have been identified in the AD brain.
The proinflammatory cytokine IFN-γ is one such alternative pathological factor. IFN-γ is predominantly secreted by the professional antigen-presenting cells, T cells, and natural killer (NK) cells, and it exerts antimicrobial, anti-tumor, and immunoregulatory effects [119]. While in the normal brain T-cell traffic across the BBB is minimal, and IFN-γ is generally undetectable, post-mortem studies have shown the infiltration of both T cells and NK cells in the human AD brain [120, 121]. In addition, it has been demonstrated that the cerebrospinal fluid levels of IFN-γ in AD subjects are significantly higher than those in healthy age-matched subjects [122]. A post-mortem genetic association study has shown upregulation of IFN-γ in the brains of AD patients who carry the R47H TREM2 variant [123]. It remains elusive whether astrocytes under neuropathological conditions lose neurosupportive functions or gain neurotoxic functions. Our previous in vitro studies showed that adult human astrocytes became potently neurotoxic when activated by IFN-γ through secreting soluble neurotoxins [124]. Using post-mortem brain tissues, we showed sharp upregulation of the IFN-γ receptor on activated astrocytes in areas affected by AD, PD, ALS, and MS [124]. We also demonstrated that the IFN-γ-induced neurotoxicity of astrocytes was mediated by signal transducer and activator of transcription (STAT) 3 phosphorylation at tyrosine-705 residue (Tyr705) [125]. The drugs capable of inhibiting the STAT3 phosphorylation attenuated this neurotoxicity [125-127] (see sections 3.2.2 and 3.3 below).
Another pathological factor causing astrocyte activation in AD is reactive microglia. Activated microglia have been implied to play a pivotal role in directing the fate of astrocytes to become neurotoxic. Liddelow et al. demonstrated that activated microglia induced neurotoxic astrocytes, termed A1 astrocytes, by secreting IL-1α, TNF-α, and C1q. Together, these three factors were both necessary and sufficient to induce the A1 astrocyte phenotype in rodents with systemic inflammation, which was induced by intraperitoneal injection of lipopolysaccharide [92]. Their finding of microglia-induced A1 astrocytes is consistent with a post-mortem quantitative neuropathological study, which demonstrated that microglia activation preceded astrocyte activation in the AD pathogenesis [128]. On the other hand, it has been implied that astrocyte activation occurs in the early stage of the disease [116, 129]. A1 astrocytes can be identified by the expression of complement component C3, and they cause neuronal death by secreting soluble neurotoxins. Importantly, A1 astrocytes are abundant in several neurodegenerative disorders, namely AD, PD, ALS, MS, and Huntington’s disease [92]. A1 astrocytes and their neurotoxicity have also been observed in transgenic mice overexpressing both human tau and human ApoE4 [130] during normal aging [131] and in mice intrastriatally injected with preformed fibrils of α-synuclein [132]. It appears that IFN-γ-activated astrocytes are not identical to A1 astrocytes since the latter are induced by different stimuli, which originate from different cell types (i.e., T cell/NK cell vs microglia). However, due to the similarity of their neurotoxic characteristics, the gene expression profiles of IFN-γ-activated astrocytes and A1 astrocytes may be comparable.
Analogous to imaging microglia activation in vivo, advances in PET technology have also enabled the detection of astrocyte activation in the living human brain. Since the early 1990s, the majority of PET studies to detect neuroinflammation in the brains of AD patients have aimed to visualize microglia activation as measured by increased binding of radioactive tracers, such as 11C-PK11195 and 11C-PBR28, to the 18-kDa TSPO. Relatively fewer PET studies have focused on detecting astrocyte activation in the human brain. In AD, activated astrocytes express high levels of monoamine oxidase-B (MAO-B), which is localized on the outer mitochondrial membrane and catalyzes the oxidative deamination of catecholamine neurotransmitters [133]. At present, the only well-established radioligand available for PET imaging of astrogliosis is 11C-deuterium-L-deprenyl (11C-DED), which binds specifically to MAO-B. Since 2009, when the first study using 11C-DED was performed in AD patients [134], several other PET studies have demonstrated a significantly higher 11C-DED retention in AD compared to healthy control brains [129, 135]. For example, Carter et al. showed that 11C-DED retention was increased in 11C-Pittsburgh compound-positive (a marker of amyloid plaques) patients with mild cognitive impairment relative to healthy controls and even AD patients [129]. Their finding indicates that astrogliosis occurs in the earlier prodromal stages of AD.
3.2.2. Astrocyte Neurotoxicity and STAT3 in AD
The Janus kinase (JAK)-STAT cascade is one of the pivotal signaling pathways mediating neuroinflammation. Specifically, the JAK/STAT3 pathway is activated by extracellular cytokines, including IFN-γ. Although multiple signaling cascades are involved in astrocyte activation, the JAK/STAT3 cascade has been regarded as the key regulator of astrocyte activation in several different neuropathological conditions [99, 136, 137]. A recent study demonstrated that the immunoreactivity for STAT3 was increased in the nuclei of GFAP- and vimentin-immunopositive astrocytes in the APPswe/PS1dE9 and 3xTg mouse models of AD [136]. The STAT3 activation is also considered as a putative marker for the activity of neuroinflammatory conditions, such as MS [138] and chronic inflammatory demyelinating polyradiculoneuropathy [139].
Interestingly, astrocyte activation mediated by the same STAT3 pathway has different effects on specific disease neuropathologies. Thus, STAT3 signaling in activated astrocytes has been implicated in neurotoxicity observed in mouse models of AD [140] and hypoxia [141] but has also been associated with neuroprotective effects in mouse models of spinal cord injury [142]. In line with the involvement of the STAT3 pathway in the activated astrocytes exerting neurotoxicity in AD, our previous in vitro studies have shown that activated STAT3 with phosphorylation at Tyr705 mediates the neurotoxicity of adult human astrocytes stimulated with IFN-γ [125-127, 143].
It is well known that the biological characteristics and functions, including gene expression, of glial cells show fundamental differences between species and are age-dependent [144]. For instance, fetal glial cells are not immunologically developed compared to adult glia [145]. In addition, the IFN-γ receptor is expressed on astrocytes, but not on microglia, in the adult human brain, while the receptor is expressed on microglia, but not on astrocytes, in the adult rodent brain [144, 146]. Accordingly, studies using primary cultures of adult human glial cells are of prime importance to maximize the relevance of experimental research to clinical applications and to decipher the basis of the neuropathologies in the aged human brains affected by neurodegenerative diseases, including AD.
3.3. Therapeutic Molecules Inhibiting Astrocyte Neurotoxicity
Using primary cultures of adult human astrocytes, we have previously demonstrated that several compounds with anti-inflammatory potential significantly reduce the neurotoxicity of IFN-γ-activated astrocytes. They include the preclinically studied STAT3 inhibitors [125], as well as the clinically used L-type calcium channel blockers (CCBs) nimodipine and verapamil [126], the histone deacetylase (HDAC) inhibitor suberoylanilide hydroxamic acid (SAHA) [127], and the proton pump inhibitors lansoprazole and omeprazole [143]. All these compounds attenuate phosphorylation of STAT3 at Tyr705, but not STAT1, in IFN-γ-activated astrocytes without affecting their viability at the concentrations tested. While these compounds show robust anti-neurotoxic effects by inhibiting astrocyte activation in vitro, the following sections summarize their activity in AD reported by clinical and epidemiological studies. Of the compounds discussed, nimodipine currently appears to be the strongest candidate as a novel therapeutic and/or preventive drug for AD, showing consistent beneficial effects in both experimental and clinical studies.
3.3.1. L-type Calcium Channel Blockers (CCBs)
It is well known that hypertension in midlife is a major risk factor for the development of all types of dementia [147, 148]. Several observational studies have reported the beneficial effect of antihypertensive treatment in preventing cognitive decline; however, randomized controlled trials (RCTs) have produced controversial results (reviewed in [148]). Clinical trials to determine the effects of L-type CCBs on dementia have mainly tested dihydropyridine molecules that were originally developed for the treatment of hypertension. Specifically, nimodipine seems to be the most tested dihydropyridine molecule. A Cochrane review updated in 2010 analyzed 15 double-blind RCTs from 1985 to 2005, involving 2492 subjects in total. This systematic review concluded that nimodipine (90 mg/day for 12 weeks) had significant efficacy in treating dementia compared to placebo, with similar benefits for AD and vascular dementia [149]. Since the lipophilic CCBs penetrate the BBB effectively, the beneficial effect of nimodipine in AD could be attributed to its anti-neurotoxic effects via inhibition of both astrocyte [126] and microglia activation [126, 150], rather than its antihypertensive effects. Contradicting data were obtained by a prospective cohort study (n=1092) with a long follow-up period (up to 19 years), which indicated that neither dihydropyridine-type nor non-dihydropyridine-type CCBs, including verapamil, significantly reduced the risk of AD [151].
3.3.2. Histone Deacetylase (HDAC) Inhibitors
While accumulating evidence indicates that HDAC inhibitors have the potential to ameliorate AD pathology, there is discordance in their effectiveness between experimental studies and clinical trials. Specifically, HDAC inhibitors have shown to decrease tau phosphorylation and accumulation and to improve cognitive memory performance in several different transgenic mice models of AD [152, 153]. To our knowledge, four RCTs have investigated the efficacy of valproate, the most-prescribed HDAC inhibitor in clinical psychiatry, on behavioral and psychological symptoms of dementia (BPSD) and cognitive function of individuals who meet clinical criteria for probable or possible AD (n=391 in total) [154-157]. It is noteworthy that all four RCTs concluded that valproate did not significantly improve BPSD or slow cognitive decline of AD patients. Using magnetic resonance imaging (MRI), one of these trials demonstrated that valproate even accelerated brain atrophy of AD patients [154].
SAHA, also known as vorinostat, was the first HDAC inhibitor approved by the United States Food and Drug Administration (FDA) in 2006 as an anti-lymphoma drug. SAHA inhibits various subclasses of HDAC [158] and attenuates the neurotoxicity of adult human astrocytes in vitro [127]. A phase 1 clinical trial has been initiated to determine the tolerable doses of SAHA in patients with mild AD [159].
3.3.3. Proton Pump Inhibitors (PPIs)
Currently, there is no consensus on the effects of PPIs on AD risk, owing to controversial results from several observational studies. Zhang et al. performed a meta-analysis on six prospective cohort studies and found that the use of PPIs was a risk factor for dementia, including AD (hazard ratio, HR=1.29; 95% confidence interval, CI=1.12-1.49) [160]. On the other hand, Li et al. conducted a meta-analysis on six prospective cohort studies and observed no statistical association between the use of PPIs and increased risk of dementia or AD [161]. Interestingly, there were five cohort studies that overlapped between these two meta-analyses. An additional meta-analysis made by Song et al., which included five case-control studies and five cohort studies, concluded that PPI usage did not increase the risk of dementia or AD [162]. Several observational studies even reported a negative correlation between PPI usage and the risk of dementia or AD [163-165]. These inconsistencies may stem from the variations in methods of assessment of PPI usage, the criteria used to diagnose dementia, the cohort type, the number of subjects, the follow-up duration, and controlling for variables across the studies. Well-designed RCTs are therefore warranted to further evaluate exact relationships between the use of PPIs and AD risk/progression.
4. SYSTEMIC INFLAMMATION & NEUROINFLAMMATION
Systemic inflammation caused by, for example, infection or chronic inflammatory diseases leads to increased release of pro-inflammatory mediators from peripheral organs into the bloodstream [166]. Crosstalk between systemic inflammation and neuroinflammation has become a well-accepted concept. Enhanced transduction of the peripheral inflammatory signals into the CNS can induce microglia-driven neuroimmune responses [167, 168]. This interplay can manifest as “sickness behavior” characterized by a set of clinical symptoms similar to those of major depression, including loss of appetite, sleep disturbance, reduced physical activity, and decreased social interest [169, 170].
4.1. Communication between Systemic Inflammation and Neuroinflammation
Several routes of transduction of the systemic inflammatory signals into the brain have been postulated [167, 168]. Of these, the brain vasculature, and BBB in particular, is considered to play prominent roles. Intact BBB is not freely permeable to cells and large biomolecules; thus, it tightly regulates the movement of peripheral molecules and immune cells into the brain parenchyma. While certain molecules can freely cross the BBB either transcellularly or paracellularly, there are several additional routes of immune signaling from the periphery into CNS. First, certain inflammatory mediators can be transferred into the brain parenchyma by specific transporters (e.g., IL-1α, IL-1β, TNF-α) [171]. Second, transcytosis of inflammatory mediators across endothelial cells (ECs) has been reported (e.g., TNF-α, chemokines CCL2 and CCL5) [172, 173]. Third, certain molecules interact with the receptors on the luminal surface of ECs (on the “blood” side of cells), inducing release of inflammatory mediators into the brain parenchyma (e.g., adiponectin-induced secretion of cytokines) [174]. Fourth, the circumventricular organs (CVOs), specialized brain structures lining the third and the fourth ventricles in which capillaries lack continuous BBB, can be involved. These structures allow interaction of microglia and astrocytes with circulating inflammatory cytokines, including IL-1β and TNF-α [175]. Fifth, the autonomic nervous system route involves direct stimulation of vagus nerve afferents by inflammatory mediators, such as IL-1β, which stimulates the hypothalamic–pituitary–adrenal (HPA) axis and induces illness symptoms [176, 177]. All these pathways could ultimately lead to an increase of pro-inflammatory cytokines in the brain, as well as microglia activation [169, 178].
Our previous studies indicate that, in addition to the above five “classical” routes, the leptomeninges can serve as an alternative site of communication between the periphery and the resident microglia of the brain [179, 180]. Leptomeningeal cells cover the surface of the brain and spinal cord, forming the cerebrospinal fluid-blood barrier. Leptomeningeal cells express TLR4, which can be activated by circulating LPS, triggering the release of pro-inflammatory cytokines into the brain parenchyma. Similarly, leptomeningeal cells can be activated by systemically circulating cytokines to produce and release inflammatory mediators into the brain parenchyma. The pro-inflammatory mediators released from leptomeningeal cells subsequently activate microglia to evoke neuroinflammation. Accordingly, the leptomeninges could be critical for the transduction of the peripheral inflammatory signals into the CNS, leading to modulation of neuroinflammatory responses [179, 180].
4.2. Impacts of Lifestyle-related Systemic Inflammation on Neuroinflammation
Lifestyle-related diseases, including obesity, type 2 diabetes mellitus (T2DM), and periodontitis, are characterized by a common feature of chronic, low-grade systemic inflammation. Studies on subjects without dementia demonstrate that systemic inflammation is associated with cognitive decline [181] and reduced hippocampal volume [182, 183]; therefore, the onset of neuroinflammatory processes due to systemic inflammation can be considered an important causal mechanism in cognitive decline. For example, considerable evidence points to increased systemic inflammation as a risk factor for developing AD, and neuroinflammatory mechanisms can be at least partially responsible for this link [184].
Metabolic syndrome is a cluster of conditions, which includes obesity, lipid abnormalities, high blood sugar, and increased blood pressure. It is associated with an increased risk of T2DM, stroke, and heart disease. The systemic inflammation accompanying metabolic syndrome, obesity and T2DM can cause BBB breakdown and glia activation leading to neuroinflammation and neuron death, which are the key elements of several different neurodegenerative diseases, including AD. Neuroinflammation is observed in normal animals after a high-fat diet, as well as in genetic animal models of metabolic syndromes, such as obese Zucker rats [185-187]. A high-fat diet is associated with glia activation and chronic increase of inflammatory mediators in the CNS [188, 189]. Interestingly, the hippocampus and the cortex, brain regions implicated in cognitive processing, learning, and memory, are particularly vulnerable to inflammation caused by obesity [187]. In animal models of obesity, glial cells acquire reactive phenotype in a brain region-specific manner [190]. The disruption of BBB by systemic inflammation in obesity/T2DM can be caused by several different mechanisms, including modification of tight junctions, endothelial damage, and changes in astrocyte end-feet [191].
The role of gut and its microbiota in the regulation of normal cognitive functions is increasingly recognized. One of the possible mechanisms responsible for this link often termed the “gut-brain axis”, is the interaction between factors produced by gut microbiota and microglia [192]. Recent studies demonstrate that the gut microbiota plays a pivotal role in regulating microglia maturation and function [192, 193]. The genes that regulate cell activation and immune system defense pathways, including Mapk8, IL-1α, Ly86, Jak3, and Stat1, are downregulated in microglia from adult germ-free mice; such an immature gene expression profile contributes to an abnormal response of these animals to LPS challenge [83]. Interestingly, it has been proposed that manipulation of gut microbiota composition can be used to modulate neuroinflammation; for example, consumption of prebiotics, probiotics, and synbiotics has been shown to restore cognition in obese, insulin resistant rodents, leading to improved hippocampal plasticity and decreased microglia activation [194].
Periodontitis is a common chronic oral inflammatory disease, which leads to the upregulation of systemic inflammatory mediators. Periodontitis is known to exacerbate peripheral diseases, including T2DM. Its impact on neurodegenerative diseases has been suggested but is less understood [166]. The available evidence linking periodontitis and AD includes studies showing impaired oral health in AD patients and data indicating that uncontrolled periodontal disease triggers or exacerbates neuroinflammation, possibly leading to AD [195]. Several constituents of periodontal pathogens, including LPS and gingipain from Porphyromonas gingivalis, have been found in the post-mortem brain tissues from AD patients [196, 197]. We have recently demonstrated that chronic systemic exposure of mice to LPS from P. gingivalis induces microglia-dependent neuroinflammation and AD-like pathological changes, including intracellular accumulation of Aβ in neurons and impairment of learning and memory by the time these animals reach middle age [198]. Cerebrovascular accumulation of Aβ after P. gingivalis infection is mediated by upregulated receptor for glycation end products (RAGE) in cerebral ECs [199]. Interestingly, Aβ production is induced in the inflammatory macrophages from the gingival tissues of patients with periodontitis [200]. Periodontitis can also lead to altered gut microbiota composition with a subsequent switch in the gut immune profile to the pro-inflammatory Th17 causing exacerbation of systemic inflammatory responses [201].
Clinical studies also support a causal link between periodontitis and AD. Prospective cohort studies show that IgG antibody levels to periodontitis bacteria, such as P. gingivalis, Tannerella forsythia, and Treponema denticola (the so-called “red complex”), are significantly increased in sera of individuals who are diagnosed with AD several years later, compared to normal control subjects. In addition, a recent meta-analysis concludes that periodontitis is significantly associated with an increased risk of AD (odds ratio, OR 1.69, 95% CI 1.21-2.35) [202]. The same study demonstrates an even stronger association between severe forms of periodontitis and AD (OR 2.98, 95% CI 1.58-5.62). The above observations indicate that periodontitis may contribute to the increased risk of AD and to the progression of this disease [203, 204].
4.3. Systemic Inflammation and Age-dependent Neuroinflammation
Chronic systemic inflammation has an age-dependent effect on microglia activation status. Perry (2004) introduced the concept of “primed microglia,” which, similar to activated cells, are characterized by shortened processes and increased expression of cell surface antigens; however, unlike the classically activated microglia, primed cells do not secrete inflammatory cytokines [205]. It is plausible that primed microglia are not only a result of systemic inflammation, but they also are sensitized to respond excessively to peripheral inflammatory signals, which can provoke exaggerated neuroinflammation.
Starting from middle age (~50 and older for humans), the brain undergoes significant structural and functional changes, including its response to peripheral inflammatory stimuli [206]. We show that chronic systemic inflammation caused by adjuvant arthritis (AA) in young rats induces microglia activation, characterized by the production of anti-inflammatory mediators, including IL-10 and transforming growth factor-β1 (TGF-β1). In contrast, AA in middle-aged rats leads to activated microglia that produce pro-inflammatory mediators, including IL-1β and prostaglandin E2 (PGE2) [207]. This study demonstrates significant age dependence of microglia responses to chronic systemic inflammation. Chronic systemic exposure to LPS from P. gingivalis is associated with increased microglia production of IL-1β in middle-aged mice but not in young animals. In addition, only mice middle-aged experience decline in learning and memory [198]. The long-term potentiation (LTP) in neurons provides the functional basis for learning and memory. It has been possible to demonstrate that chronic AA significantly impairs hippocampal LTP in middle-aged rats but not in young animals [208]. Since systemic administration of minocycline, a well-known inhibitor of microglia activation, significantly restores the impaired LTP in middle-aged rats with AA, glia-mediated neuroinflammation has been suggested as the mechanistic link between chronic peripheral inflammation and cognitive impairment [208].
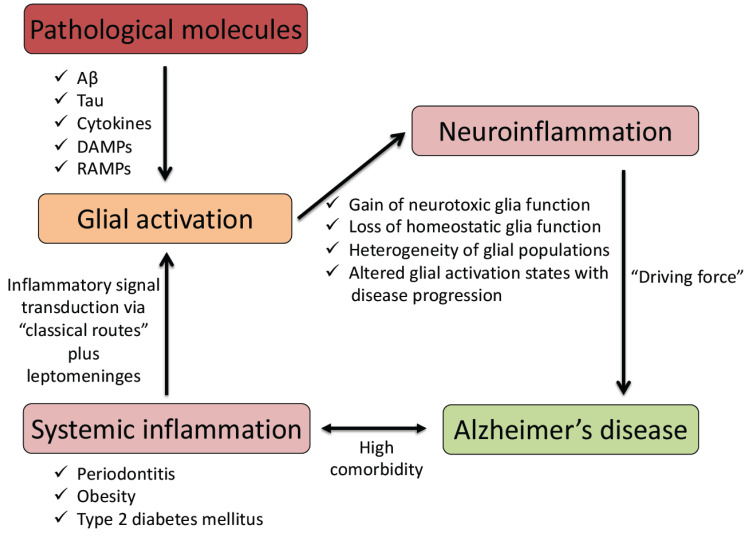
It can be concluded that systemic inflammation affects the onset and progression of neurodegenerative diseases, including AD, by initiating and promoting several neuroinflammatory mechanisms, including inflammatory priming and adverse activation of glia (see Fig. 1). Therefore, preventing and reducing systemic inflammation could be beneficial for AD and other neurodegenerative diseases driven by neuroinflammatory processes.
CONCLUSION
Both microglia and astrocytes support a broad range of physiological processes throughout the development, maturation, and decline of the CNS. Glia facilitates CNS cell proliferation and survival by releasing specific growth factors, but they also promote the death of unwanted cells through the secretion of cytotoxins. Microglia and astrocyte populations within the brain are heterogeneous, reflecting brain region specificity of their functions as well as changes in the pathophysiological status of the CNS. With onset of pathological processes, such as neurodegenerative disorders, both types of glial cells undergo phenotypic changes in brain regions affected by the disease processes. While significant recent advances have been made in our understanding of the various phenotypes of reactive glia, such as M1/M2 and A1/A2 axes for microglia and astrocytes, respectively, it remains to be determined conclusively whether the loss of trophic support by glia or their excessive release of cytotoxins is the critical factor contributing to the degeneration of neurons associated with CNS diseases. In AD, it has been demonstrated that glial heterogeneity and phenotypes evolve as the disease progresses. These complex, brain region- and disease stage-specific transformations in glial phenotype and function require further studies. Since most of this evidence has been collected using animal models of AD, and given the increasingly recognized differences between glia from different species, further studies of glial contribution to AD pathology using human-specific models and clinical approaches are essential. Development and application of microglia- and astrocyte-specific PET tracers that recognize different reactive phenotypes may provide new, clinically relevant information.
Recent evidence also indicates that systemic inflammation can regulate neuroinflammatory processes in an age-dependent manner. There have been considerable advances in our understanding of the routes of immune communication between the periphery and CNS. Modulation of neuroinflammation is one of the possible mechanisms linking peripheral immune processes and diseases with altered risk and progression of AD. Therefore, it may be possible to regulate glial cell phenotype and activation status through peripheral immune interventions or by adjusting gut microbiota composition. Overall, further phenotypic and functional characterization of glial cell subpopulations in AD brains and mechanisms of glia regulation by peripheral immune signals could lead to the identification of novel pharmacological targets and therapeutic approaches for controlling immune functions of glia in a disease stage-specific manner.
Fig. (1).
A schematic diagram showing links between systemic inflammation, glial activation, and neuroinflammation contributing to the pathogenetic mechanism of Alzheimer’s disease. Aβ, amyloid-β peptides; DAMPs, damage-associated molecular patterns; RAMPs, resolution-associated molecular patterns. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was supported by JSPS KAKENHI grants 19K08018 (SH) and 16K11478 (ZW); the OBT research center grant from the Kyushu University (ZW); the Natural Sciences and Engineering Research Council of Canada, and Jack Brown and Family Alzheimer's Disease Research Foundation (AK).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.McGeer P.L., Itagaki S., Tago H., McGeer E.G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 1987;79(1-2):195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 2.McGeer P.L., Itagaki S., McGeer E.G. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 1988;76(6):550–557. doi: 10.1007/BF00689592. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G.M., Cooper N.R., Eikelenboom P., Emmerling M., Fiebich B.L., Finch C.E., Frautschy S., Griffin W.S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I.R., McGeer P.L., O’Banion M.K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F.L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21(3):383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGeer P.L., Rogers J., McGeer E.G. Neuroimmune mechanisms in Alzheimer disease pathogenesis. Alzheimer Dis. Assoc. Disord. 1994;8(3):149–158. doi: 10.1097/00002093-199408030-00001. [DOI] [PubMed] [Google Scholar]
- 5.McGeer P.L., Schulzer M., McGeer E.G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47(2):425–432. doi: 10.1212/WNL.47.2.425. [DOI] [PubMed] [Google Scholar]
- 6.Klegeris A., Walker D.G., McGeer P.L. Activation of macrophages by Alzheimer beta amyloid peptide. Biochem. Biophys. Res. Commun. 1994;199(2):984–991. doi: 10.1006/bbrc.1994.1326. [DOI] [PubMed] [Google Scholar]
- 7.Meda L., Cassatella M.A., Szendrei G.I., Otvos L., Jr, Baron P., Villalba M., Ferrari D., Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374(6523):647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 8.Graeber M.B., Li W., Rodriguez M.L. Role of microglia in CNS inflammation. FEBS Lett. 2011;585(23):3798–3805. doi: 10.1016/j.febslet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 9.ElAli A., Rivest S. Microglia in Alzheimer’s disease: A multifaceted relationship. Brain Behav. Immun. 2016;55:138–150. doi: 10.1016/j.bbi.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Spangenberg E.E., Green K.N. Inflammation in Alzheimer’s disease: Lessons learned from microglia-depletion models. Brain Behav. Immun. 2017;61:1–11. doi: 10.1016/j.bbi.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takatori S., Wang W., Iguchi A., Tomita T. Genetic risk factors for Alzheimer disease: Emerging roles of microglia in disease pathomechanisms. Adv. Exp. Med. Biol. 2019;1118:83–116. doi: 10.1007/978-3-030-05542-4_5. [DOI] [PubMed] [Google Scholar]
- 12.Efthymiou A.G., Goate A.M. Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Mol. Neurodegener. 2017;12(1):43. doi: 10.1186/s13024-017-0184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosselin D., Skola D., Coufal N.G., Holtman I.R., Schlachetzki J.C.M., Sajti E., Jaeger B.N., O’Connor C., Fitzpatrick C., Pasillas M.P., Pena M., Adair A., Gonda D.D., Levy M.L., Ransohoff R.M., Gage F.H., Glass C.K. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356(6344):eaal3222. doi: 10.1126/science.aal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satoh J., Tabunoki H., Ishida T., Yagishita S., Jinnai K., Futamura N., Kobayashi M., Toyoshima I., Yoshioka T., Enomoto K., Arai N., Arima K. Immunohistochemical characterization of microglia in Nasu-Hakola disease brains. Neuropathology. 2011;31(4):363–375. doi: 10.1111/j.1440-1789.2010.01174.x. [DOI] [PubMed] [Google Scholar]
- 15.Satoh J., Kawana N., Yamamoto Y., Ishida T., Saito Y., Arima K. A survey of TREM2 antibodies reveals neuronal but not microglial staining in formalin-fixed paraffin-embedded postmortem Alzheimer’s brain tissues. Alzheimers Res. Ther. 2013;5(4):30. doi: 10.1186/alzrt184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahrenhold M., Rakic S., Classey J., Brayne C., Ince P.G., Nicoll J.A.R., Boche D., Mrc C. MRC-CFAS. TREM2 expression in the human brain: a marker of monocyte recruitment? Brain Pathol. 2018;28(5):595–602. doi: 10.1111/bpa.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lue L.F., Schmitz C.T., Serrano G., Sue L.I., Beach T.G., Walker D.G. TREM2 Protein expression changes correlate with Alzheimer’s Disease neurodegenerative pathologies in post-mortem temporal cortices. Brain Pathol. 2015;25(4):469–480. doi: 10.1111/bpa.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parbo P., Ismail R., Sommerauer M., Stokholm M.G., Hansen A.K., Hansen K.V., Amidi A., Schaldemose J.L., Gottrup H., Brændgaard H., Eskildsen S.F., Borghammer P., Hinz R., Aanerud J., Brooks D.J. Does inflammation precede tau aggregation in early Alzheimer’s disease? A PET study. Neurobiol. Dis. 2018;117:211–216. doi: 10.1016/j.nbd.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Passamonti L., Rodríguez P.V., Hong Y.T., Allinson K.S.J., Bevan-Jones W.R., Williamson D., Jones P.S., Arnold R., Borchert R.J., Surendranathan A., Mak E., Su L., Fryer T.D., Aigbirhio F.I., O’Brien J.T., Rowe J.B. [11C]PK11195 binding in Alzheimer disease and progressive supranuclear palsy. Neurology. 2018;90(22):e1989–e1996. doi: 10.1212/WNL.0000000000005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., Mehler M.F., Conway S.J., Ng L.G., Stanley E.R., Samokhvalov I.M., Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez Perdiguero E., Klapproth K., Schulz C., Busch K., Azzoni E., Crozet L., Garner H., Trouillet C., de Bruijn M.F., Geissmann F., Rodewald H.R. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518(7540):547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng J., Ruedl C., Karjalainen K. Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity. 2015;43(2):382–393. doi: 10.1016/j.immuni.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Wolf S.A., Boddeke H.W., Kettenmann H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- 24.Bar E., Barak B. Microglia roles in synaptic plasticity and myelination in homeostatic conditions and neurodevelopmental disorders. Glia. 2019;67(11):2125–2141. doi: 10.1002/glia.23637. [DOI] [PubMed] [Google Scholar]
- 25.Lenz K.M., Nelson L.H. Microglia and beyond: Innate immune cells as regulators of brain development and behavioral function. Front. Immunol. 2018;9:698. doi: 10.3389/fimmu.2018.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watters J.J., Pocock J.M. In: Microglial physiology. Microglia in Health and Disease; Tremblay, M-È. Sierra A., editor. New York, NY, USA: Springer-Verlag; 2014. pp. 47–80. [Google Scholar]
- 27.Hanisch U.K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 28.Brown G.C., Vilalta A. How microglia kill neurons. 2015. [DOI] [PubMed]
- 29.Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., III, Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung Y.J., Chung W.S. Phagocytic roles of glial cells in healthy and diseased brains. Biomol. Ther. (Seoul) 2018;26(4):350–357. doi: 10.4062/biomolther.2017.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Y., Dissing-Olesen L., MacVicar B.A., Stevens B. Microglia: Dynamic mediators of synapse development and plasticity. Trends Immunol. 2015;36(10):605–613. doi: 10.1016/j.it.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reemst K., Noctor S.C., Lucassen P.J., Hol E.M. The indispensable roles of microglia and astrocytes during brain development. Front. Hum. Neurosci. 2016;10:566. doi: 10.3389/fnhum.2016.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tay T.L., Savage J.C., Hui C.W., Bisht K., Tremblay M.E. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J. Physiol. 2017;595(6):1929–1945. doi: 10.1113/JP272134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stratoulias V., Venero J.L., Tremblay M.E., Joseph B. Microglial subtypes: diversity within the microglial community. EMBO J. 2019;38(17):e101997. doi: 10.15252/embj.2019101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawson L.J., Perry V.H., Dri P., Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–170. doi: 10.1016/0306-4522(90)90229-W. [DOI] [PubMed] [Google Scholar]
- 36.de Haas A.H., Boddeke H.W., Biber K. Region-specific expression of immunoregulatory proteins on microglia in the healthy CNS. Glia. 2008;56(8):888–894. doi: 10.1002/glia.20663. [DOI] [PubMed] [Google Scholar]
- 37.Grabert K., Michoel T., Karavolos M.H., Clohisey S., Baillie J.K., Stevens M.P., Freeman T.C., Summers K.M., McColl B.W. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat. Neurosci. 2016;19(3):504–516. doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammond T.R., Dufort C., Dissing-Olesen L., Giera S., Young A., Wysoker A., Walker A.J., Gergits F., Segel M., Nemesh J., Marsh S.E., Saunders A., Macosko E., Ginhoux F., Chen J., Franklin R.J.M., Piao X., McCarroll S.A., Stevens B. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50(1):253–271.e6. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q., Cheng Z., Zhou L., Darmanis S., Neff N.F., Okamoto J., Gulati G., Bennett M.L., Sun L.O., Clarke L.E., Marschallinger J., Yu G., Quake S.R., Wyss-Coray T., Barres B.A. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron. 2019;101(2):207–223.e10. doi: 10.1016/j.neuron.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizee M.R., Miedema S.S., van der Poel M. Adelia; Schuurman, K.G.; van Strien, M.E.; Melief, J.; Smolders, J.; Hendrickx, D.A.; Heutinck, K.M.; Hamann, J.; Huitinga, I. Isolation of primary microglia from the human post-mortem brain: effects of ante- and post-mortem variables. Acta Neuropathol. Commun. 2017;5(1):16. doi: 10.1186/s40478-017-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Poel M., Ulas T., Mizee M.R., Hsiao C.C., Miedema S.S.M. Adelia.; Schuurman, K.G.; Helder, B.; Tas, S.W.; Schultze, J.L.; Hamann, J.; Huitinga, I. Transcriptional profiling of human microglia reveals grey-white matter heterogeneity and multiple sclerosis-associated changes. Nat. Commun. 2019;10(1):1139. doi: 10.1038/s41467-019-08976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galatro T.F., Holtman I.R., Lerario A.M., Vainchtein I.D., Brouwer N., Sola P.R., Veras M.M., Pereira T.F., Leite R.E.P., Möller T., Wes P.D., Sogayar M.C., Laman J.D., den Dunnen W., Pasqualucci C.A., Oba-Shinjo S.M., Boddeke E.W.G.M., Marie S.K.N., Eggen B.J.L. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 2017;20(8):1162–1171. doi: 10.1038/nn.4597. [DOI] [PubMed] [Google Scholar]
- 43.Böttcher C., Schlickeiser S., Sneeboer M.A.M., Kunkel D., Knop A., Paza E., Fidzinski P., Kraus L., Snijders G.J.L., Kahn R.S., Schulz A.R., Mei H.E., Hol E.M., Siegmund B., Glauben R., Spruth E.J., de Witte L.D., Priller J., Priller J. NBB-Psy. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat. Neurosci. 2019;22(1):78–90. doi: 10.1038/s41593-018-0290-2. [DOI] [PubMed] [Google Scholar]
- 44.Masuda T., Sankowski R., Staszewski O., Böttcher C., Amann L. Sagar.; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; Coenen, V.A.; Reinacher, P.C.; Michel, A.; Sure, U.; Gold, R.; Grün, D.; Priller, J.; Stadelmann, C.; Prinz, M. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566(7744):388–392. doi: 10.1038/s41586-019-0924-x. [DOI] [PubMed] [Google Scholar]
- 45.Kettenmann H., Hanisch U.K., Noda M., Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 46.Walker D.G., Lue L.F. Understanding the neurobiology of CD200 and the CD200 receptor: a therapeutic target for controlling inflammation in human brains? 2013. [DOI] [PMC free article] [PubMed]
- 47.Hanisch U.K. Proteins in microglial activation--inputs and outputs by subsets. Curr. Protein Pept. Sci. 2013;14(1):3–15. doi: 10.2174/1389203711314010003. [DOI] [PubMed] [Google Scholar]
- 48.Klegeris A. Regulation of neuroimmune processes by damage- and resolution-associated molecular patterns. Neural Regen. Res. 2021;16(3):423–429. doi: 10.4103/1673-5374.293134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venegas C., Heneka M.T. Danger-associated molecular patterns in Alzheimer’s disease. J. Leukoc. Biol. 2017;101(1):87–98. doi: 10.1189/jlb.3MR0416-204R. [DOI] [PubMed] [Google Scholar]
- 50.Wenzel T.J., Kwong E., Bajwa E., Klegeris A. Resolution-associated molecular patterns (RAMPs) as endogenous regulators of glia functions in neuroinflammatory disease. CNS Neurol. Disord. Drug Targets. doi: 10.2174/1871527319666200702143719. [DOI] [PubMed] [Google Scholar]
- 51.Andersson U., Yang H., Harris H. High-mobility group box 1 protein (HMGB1) operates as an alarmin outside as well as inside cells. Semin. Immunol. 2018;38:40–48. doi: 10.1016/j.smim.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 52.Bianchi M.E. HMGB1 loves company. J. Leukoc. Biol. 2009;86(3):573–576. doi: 10.1189/jlb.1008585. [DOI] [PubMed] [Google Scholar]
- 53.Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim. Biophys. Acta. 2010;1799(1-2):101–113. doi: 10.1016/j.bbagrm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Bajwa E., Pointer C.B., Klegeris A. The role of mitochondrial damage-associated molecular patterns in chronic neuroinflammation. Mediators Inflamm. 2019;2019:4050796. doi: 10.1155/2019/4050796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gouveia A., Bajwa E., Klegeris A. Extracellular cytochrome c as an intercellular signaling molecule regulating microglial functions. Biochim. Biophys. Acta, Gen. Subj. 2017;1861(9):2274–2281. doi: 10.1016/j.bbagen.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 56.Schindler S.M., Frank M.G., Annis J.L., Maier S.F., Klegeris A. Pattern recognition receptors mediate pro-inflammatory effects of extracellular mitochondrial transcription factor A (TFAM). Mol. Cell. Neurosci. 2018;89:71–79. doi: 10.1016/j.mcn.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Sastre M., Klockgether T., Heneka M.T. Contribution of inflammatory processes to Alzheimer’s disease: molecular mechanisms. Int. J. Dev. Neurosci. 2006;24(2-3):167–176. doi: 10.1016/j.ijdevneu.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 58.Malm T.M., Jay T.R., Landreth G.E. The evolving biology of microglia in Alzheimer’s disease. Neurotherapeutics. 2015;12(1):81–93. doi: 10.1007/s13311-014-0316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu Y., Ye R.D. Microglial Aβ receptors in Alzheimer’s disease. Cell. Mol. Neurobiol. 2015;35(1):71–83. doi: 10.1007/s10571-014-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dubbelaar M.L., Kracht L., Eggen B.J.L., Boddeke E.W.G.M. The kaleidoscope of microglial phenotypes. Front. Immunol. 2018;9:1753. doi: 10.3389/fimmu.2018.01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 62.Sierra A., Beccari S., Diaz-Aparicio I., Encinas J.M., Comeau S., Tremblay M.E. Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014;2014:610343. doi: 10.1155/2014/610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prinz M., Jung S., Priller J. Microglia biology: One century of evolving concepts. Cell. 2019;179(2):292–311. doi: 10.1016/j.cell.2019.08.053. [DOI] [PubMed] [Google Scholar]
- 64.Martinez F.O., Gordon S., Locati M., Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 2006;177(10):7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 65.Colton C.A. Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 2009;4(4):399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Famenini S., Rigali E.A., Olivera-Perez H.M., Dang J., Chang M.T., Halder R., Rao R.V., Pellegrini M., Porter V., Bredesen D., Fiala M. Increased intermediate M1-M2 macrophage polarization and improved cognition in mild cognitive impairment patients on ω-3 supplementation. FASEB J. 2017;31(1):148–160. doi: 10.1096/fj.201600677rr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orihuela R., McPherson C.A., Harry G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016;173(4):649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker D.G., Lue L.F. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res. Ther. 2015;7(1):56. doi: 10.1186/s13195-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang Y., Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016;53(2):1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 70.Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., Itzkovitz S., Colonna M., Schwartz M., Amit I. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169(7):1276–1290. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 71.Jimenez S., Baglietto-Vargas D., Caballero C., Moreno-Gonzalez I., Torres M., Sanchez-Varo R., Ruano D., Vizuete M., Gutierrez A., Vitorica J. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: age-dependent switch in the microglial phenotype from alternative to classic. J. Neurosci. 2008;28(45):11650–11661. doi: 10.1523/JNEUROSCI.3024-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dionisio-Santos D.A., Olschowka J.A., O’Banion M.K. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J. Neuroinflammation. 2019;16(1):74. doi: 10.1186/s12974-019-1453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koenigsknecht-Talboo J., Landreth G.E. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 2005;25(36):8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michelucci A., Heurtaux T., Grandbarbe L., Morga E., Heuschling P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J. Neuroimmunol. 2009;210(1-2):3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Eikelenboom P., Hoozemans J.J., Veerhuis R., van Exel E., Rozemuller A.J., van Gool W.A. Whether, when and how chronic inflammation increases the risk of developing late-onset Alzheimer’s disease. Alzheimers Res. Ther. 2012;4(3):15. doi: 10.1186/alzrt118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pointer C.B., Wenzel T.J., Klegeris A. Extracellular cardiolipin regulates select immune functions of microglia and microglia-like cells. Brain Res. Bull. 2019;146:153–163. doi: 10.1016/j.brainresbull.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Shields A.M., Panayi G.S., Corrigall V.M. Resolution-associated molecular patterns (RAMP): RAMParts defending immunological homeostasis? Clin. Exp. Immunol. 2011;165(3):292–300. doi: 10.1111/j.1365-2249.2011.04433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newcombe E.A., Camats-Perna J., Silva M.L., Valmas N., Huat T.J., Medeiros R. Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflammation. 2018;15(1):276. doi: 10.1186/s12974-018-1313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ryu J.K., McLarnon J.G. A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain. J. Cell. Mol. Med. 2009;13(9A):2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zlokovic B.V. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brabazon F., Bermudez S., Shaughness M., Khayrullina G., Byrnes K.R. The effects of insulin on the inflammatory activity of BV2 microglia. PLoS One. 2018;13(8):e0201878. doi: 10.1371/journal.pone.0201878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duffy A.M., Hölscher C. The incretin analogue D-Ala2GIP reduces plaque load, astrogliosis and oxidative stress in an APP/PS1 mouse model of Alzheimer’s disease. Neuroscience. 2013;228:294–300. doi: 10.1016/j.neuroscience.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 83.Erny D., Hrabě de Angelis A.L., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., Schwierzeck V., Utermöhlen O., Chun E., Garrett W.S., McCoy K.D., Diefenbach A., Staeheli P., Stecher B., Amit I., Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Habib P., Beyer C. Regulation of brain microglia by female gonadal steroids. J. Steroid Biochem. Mol. Biol. 2015;146:3–14. doi: 10.1016/j.jsbmb.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 85.Spielman L.J., Bahniwal M., Little J.P., Walker D.G., Klegeris A. Insulin modulates in vitro secretion of cytokines and cytotoxins by human glial cells. Curr. Alzheimer Res. 2015;12(7):684–693. doi: 10.2174/1567205012666150710104428. [DOI] [PubMed] [Google Scholar]
- 86.Spielman L.J., Gibson D.L., Klegeris A. Incretin hormones regulate microglia oxidative stress, survival and expression of trophic factors. Eur. J. Cell Biol. 2017;96(3):240–253. doi: 10.1016/j.ejcb.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 87.Spielman L.J., Gibson D.L., Klegeris A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018;120:149–163. doi: 10.1016/j.neuint.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 88.Edison P., Archer H.A., Gerhard A., Hinz R., Pavese N., Turkheimer F.E., Hammers A., Tai Y.F., Fox N., Kennedy A., Rossor M., Brooks D.J. Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol. Dis. 2008;32(3):412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 89.Czeh M., Gressens P., Kaindl A.M. The yin and yang of microglia. Dev. Neurosci. 2011;33(3-4):199–209. doi: 10.1159/000328989. [DOI] [PubMed] [Google Scholar]
- 90.Madeira J.M., Little J.P., Klegeris A. 2012. [Google Scholar]
- 91.Brown G.C., Neher J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014;15(4):209–216. doi: 10.1038/nrn3710. [DOI] [PubMed] [Google Scholar]
- 92.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.S., Peterson T.C., Wilton D.K., Frouin A., Napier B.A., Panicker N., Kumar M., Buckwalter M.S., Rowitch D.H., Dawson V.L., Dawson T.M., Stevens B., Barres B.A. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wisniewski H.M., Wegiel J., Wang K.C., Kujawa M., Lach B. Ultrastructural studies of the cells forming amyloid fibers in classical plaques. Can. J. Neurol. Sci. 1989;16(4) Suppl.:535–542. doi: 10.1017/S0317167100029887. [DOI] [PubMed] [Google Scholar]
- 94.Spangenberg E., Severson P.L., Hohsfield L.A., Crapser J., Zhang J., Burton E.A., Zhang Y., Spevak W., Lin J., Phan N.Y., Habets G., Rymar A., Tsang G., Walters J., Nespi M., Singh P., Broome S., Ibrahim P., Zhang C., Bollag G., West B.L., Green K.N. Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer’s disease model. Nat. Commun. 2019;10(1):3758. doi: 10.1038/s41467-019-11674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Joshi P., Turola E., Ruiz A., Bergami A., Libera D.D., Benussi L., Giussani P., Magnani G., Comi G., Legname G., Ghidoni R., Furlan R., Matteoli M., Verderio C. Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ. 2014;21(4):582–593. doi: 10.1038/cdd.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Asai H., Ikezu S., Tsunoda S., Medalla M., Luebke J., Haydar T., Wolozin B., Butovsky O., Kügler S., Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015;18(11):1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brambilla L., Martorana F., Rossi D. Astrocyte signaling and neurodegeneration: new insights into CNS disorders. Prion. 2013;7(1):28–36. doi: 10.4161/pri.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Verkhratsky A., Nedergaard M. Physiology of Astroglia. Physiol. Rev. 2018;98(1):239–389. doi: 10.1152/physrev.00042.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Escartin C., Guillemaud O., Carrillo-de Sauvage M.A. Questions and (some) answers on reactive astrocytes. Glia. 2019;67(12):2221–2247. doi: 10.1002/glia.23687. [DOI] [PubMed] [Google Scholar]
- 100.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daneman R., Zhou L., Kebede A.A., Barres B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., Nagelhus E.A., Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farina C., Aloisi F., Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28(3):138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 104.Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J., Higgs R., Liu F., Malkani S., Bales K.R., Paul S.M. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat. Med. 2004;10(7):719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 105.Iram T., Trudler D., Kain D., Kanner S., Galron R., Vassar R., Barzilai A., Blinder P., Fishelson Z., Frenkel D. Astrocytes from old Alzheimer’s disease mice are impaired in Aβ uptake and in neuroprotection. Neurobiol. Dis. 2016;96:84–94. doi: 10.1016/j.nbd.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 106.Wyss-Coray T., Loike J.D., Brionne T.C., Lu E., Anankov R., Yan F., Silverstein S.C., Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat. Med. 2003;9(4):453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 107.Chung W.S., Clarke L.E., Wang G.X., Stafford B.K., Sher A., Chakraborty C., Joung J., Foo L.C., Thompson A., Chen C., Smith S.J., Barres B.A. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504(7480):394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mouser P.E., Head E., Ha K.H., Rohn T.T. Caspase-mediated cleavage of glial fibrillary acidic protein within degenerating astrocytes of the Alzheimer’s disease brain. Am. J. Pathol. 2006;168(3):936–946. doi: 10.2353/ajpath.2006.050798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abramov A.Y., Ionov M., Pavlov E., Duchen M.R. Membrane cholesterol content plays a key role in the neurotoxicity of β-amyloid: implications for Alzheimer’s disease. Aging Cell. 2011;10(4):595–603. doi: 10.1111/j.1474-9726.2011.00685.x. [DOI] [PubMed] [Google Scholar]
- 110.Abramov A.Y., Canevari L., Duchen M.R. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J. Neurosci. 2004;24(2):565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parpura V., Basarsky T.A., Liu F., Jeftinija K., Jeftinija S., Haydon P.G. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369(6483):744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 112.Rothstein J.D., Dykes-Hoberg M., Pardo C.A., Bristol L.A., Jin L., Kuncl R.W., Kanai Y., Hediger M.A., Wang Y., Schielke J.P., Welty D.F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/S0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 113.Yamada T., Kawamata T., Walker D.G., McGeer P.L. Vimentin immunoreactivity in normal and pathological human brain tissue. Acta Neuropathol. 1992;84(2):157–162. doi: 10.1007/BF00311389. [DOI] [PubMed] [Google Scholar]
- 114.Kamo H., Haebara H., Akiguchi I., Kameyama M., Kimura H., McGeer P.L. A distinctive distribution of reactive astroglia in the precentral cortex in amyotrophic lateral sclerosis. Acta Neuropathol. 1987;74(1):33–38. doi: 10.1007/BF00688335. [DOI] [PubMed] [Google Scholar]
- 115.Kawamata T., Akiyama H., Yamada T., McGeer P.L. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am. J. Pathol. 1992;140(3):691–707. [PMC free article] [PubMed] [Google Scholar]
- 116.Pike C.J., Cummings B.J., Cotman C.W. Early association of reactive astrocytes with senile plaques in Alzheimer’s disease. Exp. Neurol. 1995;132(2):172–179. doi: 10.1016/0014-4886(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 117.Perez-Nievas B.G., Serrano-Pozo A. Deciphering the astrocyte reaction in Alzheimer’s disease. Front. Aging Neurosci. 2018;10:114. doi: 10.3389/fnagi.2018.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Simpson J.E., Ince P.G., Lace G., Forster G., Shaw P.J., Matthews F., Savva G., Brayne C., Wharton S.B., MRC Cognitive Function and Ageing Neuropathology Study Group Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol. Aging. 2010;31(4):578–590. doi: 10.1016/j.neurobiolaging.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 119.Schroder K., Hertzog P.J., Ravasi T., Hume D.A. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 120.Rogers J., Luber-Narod J., Styren S.D., Civin W.H. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol. Aging. 1988;9(4):339–349. doi: 10.1016/S0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 121.Togo T., Akiyama H., Iseki E., Kondo H., Ikeda K., Kato M., Oda T., Tsuchiya K., Kosaka K. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 2002;124(1-2):83–92. doi: 10.1016/S0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 122.Llano D.A., Li J., Waring J.F., Ellis T., Devanarayan V., Witte D.G., Lenz R.A. Cerebrospinal fluid cytokine dynamics differ between Alzheimer disease patients and elderly controls. Alzheimer Dis. Assoc. Disord. 2012;26(4):322–328. doi: 10.1097/WAD.0b013e31823b2728. [DOI] [PubMed] [Google Scholar]
- 123.Roussos P., Katsel P., Fam P., Tan W., Purohit D.P., Haroutunian V. The triggering receptor expressed on myeloid cells 2 (TREM2) is associated with enhanced inflammation, neuropathological lesions and increased risk for Alzheimer’s dementia. Alzheimers Dement. 2015;11(10):1163–1170. doi: 10.1016/j.jalz.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hashioka S., Klegeris A., Schwab C., McGeer P.L. Interferon-gamma-dependent cytotoxic activation of human astrocytes and astrocytoma cells. Neurobiol. Aging. 2009;30(12):1924–1935. doi: 10.1016/j.neurobiolaging.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 125.Hashioka S., Klegeris A., Qing H., McGeer P.L. STAT3 inhibitors attenuate interferon-γ-induced neurotoxicity and inflammatory molecule production by human astrocytes. Neurobiol. Dis. 2011;41(2):299–307. doi: 10.1016/j.nbd.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 126.Hashioka S., Klegeris A., McGeer P.L. Inhibition of human astrocyte and microglia neurotoxicity by calcium channel blockers. Neuropharmacology. 2012;63(4):685–691. doi: 10.1016/j.neuropharm.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 127.Hashioka S., Klegeris A., McGeer P.L. The histone deacetylase inhibitor suberoylanilide hydroxamic acid attenuates human astrocyte neurotoxicity induced by interferon-γ. J. Neuroinflammation. 2012;9(1):113. doi: 10.1186/1742-2094-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vehmas A.K., Kawas C.H., Stewart W.F., Troncoso J.C. Immune reactive cells in senile plaques and cognitive decline in Alzheimer’s disease. Neurobiol. Aging. 2003;24(2):321–331. doi: 10.1016/S0197-4580(02)00090-8. [DOI] [PubMed] [Google Scholar]
- 129.Carter S.F., Schöll M., Almkvist O., Wall A., Engler H., Långström B., Nordberg A. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J. Nucl. Med. 2012;53(1):37–46. doi: 10.2967/jnumed.110.087031. [DOI] [PubMed] [Google Scholar]
- 130.Shi Y., Yamada K., Liddelow S.A., Smith S.T., Zhao L., Luo W., Tsai R.M., Spina S., Grinberg L.T., Rojas J.C., Gallardo G., Wang K., Roh J., Robinson G., Finn M.B., Jiang H., Sullivan P.M., Baufeld C., Wood M.W., Sutphen C., McCue L., Xiong C., Del-Aguila J.L., Morris J.C., Cruchaga C., Fagan A.M., Miller B.L., Boxer A.L., Seeley W.W., Butovsky O., Barres B.A., Paul S.M., Holtzman D.M. Alzheimer’s Disease Neuroimaging Initiative. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549(7673):523–527. doi: 10.1038/nature24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Clarke L.E., Liddelow S.A., Chakraborty C., Münch A.E., Heiman M., Barres B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA. 2018;115(8):E1896–E1905. doi: 10.1073/pnas.1800165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yun S.P., Kam T.I., Panicker N., Kim S., Oh Y., Park J.S., Kwon S.H., Park Y.J., Karuppagounder S.S., Park H., Kim S., Oh N., Kim N.A., Lee S., Brahmachari S., Mao X., Lee J.H., Kumar M., An D., Kang S.U., Lee Y., Lee K.C., Na D.H., Kim D., Lee S.H., Roschke V.V., Liddelow S.A., Mari Z., Barres B.A., Dawson V.L., Lee S., Dawson T.M., Ko H.S. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018;24(7):931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rodriguez-Vieitez E., Nordberg A. Imaging neuroinflammation: Quantification of astrocytosis in a multitracer PET approach. Methods Mol. Biol. 2018;1750:231–251. doi: 10.1007/978-1-4939-7704-8_16. [DOI] [PubMed] [Google Scholar]
- 134.Hirvonen J., Kailajärvi M., Haltia T., Koskimies S., Någren K., Virsu P., Oikonen V., Sipilä H., Ruokoniemi P., Virtanen K., Scheinin M., Rinne J.O. Assessment of MAO-B occupancy in the brain with PET and [11C]-L-deprenyl-D2: a dose-finding study with a novel MAO-B inhibitor, EVT 301. Clin. Pharmacol. Ther. 2009;85(5):506–512. doi: 10.1038/clpt.2008.241. [DOI] [PubMed] [Google Scholar]
- 135.Santillo A.F., Gambini J.P., Lannfelt L., Långström B., Ulla-Marja L., Kilander L., Engler H. In vivo imaging of astrocytosis in Alzheimer’s disease: an 11C-L-deuteriodeprenyl and PIB PET study. Eur. J. Nucl. Med. Mol. Imaging. 2011;38(12):2202–2208. doi: 10.1007/s00259-011-1895-9. [DOI] [PubMed] [Google Scholar]
- 136.Ben Haim L., Ceyzériat K., Carrillo-de Sauvage M.A., Aubry F., Auregan G., Guillermier M., Ruiz M., Petit F., Houitte D., Faivre E., Vandesquille M., Aron-Badin R., Dhenain M., Déglon N., Hantraye P., Brouillet E., Bonvento G., Escartin C. The JAK/STAT3 pathway is a common inducer of astrocyte reactivity in Alzheimer’s and Huntington’s diseases. J. Neurosci. 2015;35(6):2817–2829. doi: 10.1523/JNEUROSCI.3516-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hashioka S., McGeer E.G., Miyaoka T., Wake R., Horiguchi J., McGeer P.L. Interferon-γ-induced neurotoxicity of human astrocytes. CNS Neurol. Disord. Drug Targets. 2015;14(2):251–256. doi: 10.2174/1871527314666150217122305. [DOI] [PubMed] [Google Scholar]
- 138.Lu J.Q., Power C., Blevins G., Giuliani F., Yong V.W. The regulation of reactive changes around multiple sclerosis lesions by phosphorylated signal transducer and activator of transcription. J. Neuropathol. Exp. Neurol. 2013;72(12):1135–1144. doi: 10.1097/NEN.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 139.Madia F., Frisullo G., Nociti V., Conte A., Luigetti M., Del Grande A., Patanella A.K., Iorio R., Tonali P.A., Batocchi A.P., Sabatelli M. pSTAT1, pSTAT3, and T-bet as markers of disease activity in chronic inflammatory demyelinating polyradiculoneuropathy. J. Peripher. Nerv. Syst. 2009;14(2):107–117. doi: 10.1111/j.1529-8027.2009.00220.x. [DOI] [PubMed] [Google Scholar]
- 140.Reichenbach N., Delekate A., Plescher M., Schmitt F., Krauss S., Blank N., Halle A., Petzold G.C. Inhibition of Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer’s disease model. EMBO Mol. Med. 2019;11(2):e9665. doi: 10.15252/emmm.201809665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hristova M., Rocha-Ferreira E., Fontana X., Thei L., Buckle R., Christou M., Hompoonsup S., Gostelow N., Raivich G., Peebles D. Inhibition of Signal Transducer and Activator of Transcription 3 (STAT3) reduces neonatal hypoxic-ischaemic brain damage. J. Neurochem. 2016;136(5):981–994. doi: 10.1111/jnc.13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Okada S., Nakamura M., Katoh H., Miyao T., Shimazaki T., Ishii K., Yamane J., Yoshimura A., Iwamoto Y., Toyama Y., Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 2006;12(7):829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 143.Hashioka S., Klegeris A., McGeer P.L. Proton pump inhibitors reduce interferon-γ-induced neurotoxicity and STAT3 phosphorylation of human astrocytes. Glia. 2011;59(5):833–840. doi: 10.1002/glia.21157. [DOI] [PubMed] [Google Scholar]
- 144.Smith A.M., Dragunow M. The human side of microglia. Trends Neurosci. 2014;37(3):125–135. doi: 10.1016/j.tins.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 145.Watkins L.R., Hutchinson M.R. A concern on comparing ‘apples’ and ‘oranges’ when differences between microglia used in human and rodent studies go far, far beyond simply species: comment on Smith and Dragunow. Trends Neurosci. 2014;37(4):189–190. doi: 10.1016/j.tins.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 146.Hashioka S., Klegeris A., Schwab C., Yu S., McGeer P.L. Differential expression of interferon-gamma receptor on human glial cells in vivo and in vitro. J. Neuroimmunol. 2010;225(1-2):91–99. doi: 10.1016/j.jneuroim.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 147.Kennelly S.P., Lawlor B.A., Kenny R.A. Blood pressure and dementia - a comprehensive review. Ther. Adv. Neurol. Disord. 2009;2(4):241–260. doi: 10.1177/1756285609103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sierra C. Hypertension and the risk of dementia. Front. Cardiovasc. Med. 2020;7:5. doi: 10.3389/fcvm.2020.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.López-Arrieta J.M., Birks J. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst. Rev. 2002;2002(3):CD000147. doi: 10.1002/14651858.CD000147. [DOI] [PubMed] [Google Scholar]
- 150.Li Y., Hu X., Liu Y., Bao Y., An L. Nimodipine protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. Neuropharmacology. 2009;56(3):580–589. doi: 10.1016/j.neuropharm.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 151.Yasar S., Corrada M., Brookmeyer R., Kawas C. Calcium channel blockers and risk of AD: The baltimore longitudinal study of aging. Neurobiol. Aging. 2005;26(2):157–163. doi: 10.1016/j.neurobiolaging.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 152.Fernando W.M.A.D.B., Martins I.J., Morici M., Bharadwaj P., Rainey-Smith S.R., Lim W.L.F., Martins R.N. Sodium butyrate reduces brain amyloid-β levels and improves cognitive memory performance in an Alzheimer’s disease transgenic mouse model at an early disease stage. J. Alzheimers Dis. 2020;74(1):91–99. doi: 10.3233/JAD-190120. [DOI] [PubMed] [Google Scholar]
- 153.Janczura K.J., Volmar C.H., Sartor G.C., Rao S.J., Ricciardi N.R., Lambert G., Brothers S.P., Wahlestedt C. Inhibition of HDAC3 reverses Alzheimer’s disease-related pathologies in vitro and in the 3xTg-AD mouse model. Proc. Natl. Acad. Sci. USA. 2018;115(47):E11148–E11157. doi: 10.1073/pnas.1805436115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 154.Fleisher A.S., Truran D., Mai J.T., Langbaum J.B., Aisen P.S., Cummings J.L., Jack C.R., Jr, Weiner M.W., Thomas R.G., Schneider L.S., Tariot P.N. Alzheimer’s Disease Cooperative Study. Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology. 2011;77(13):1263–1271. doi: 10.1212/WNL.0b013e318230a16c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Herrmann N., Lanctôt K.L., Rothenburg L.S., Eryavec G. A placebo-controlled trial of valproate for agitation and aggression in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2007;23(2):116–119. doi: 10.1159/000097757. [DOI] [PubMed] [Google Scholar]
- 156.Tariot P.N., Raman R., Jakimovich L., Schneider L., Porsteinsson A., Thomas R., Mintzer J., Brenner R., Schafer K., Thal L. Alzheimer’s Disease Cooperative Study; Valproate Nursing Home Study Group. Divalproex sodium in nursing home residents with possible or probable Alzheimer Disease complicated by agitation: a randomized, controlled trial. Am. J. Geriatr. Psychiatry. 2005;13(11):942–949. doi: 10.1176/appi.ajgp.13.11.942. [DOI] [PubMed] [Google Scholar]
- 157.Tariot P.N., Schneider L.S., Cummings J., Thomas R.G., Raman R., Jakimovich L.J., Loy R., Bartocci B., Fleisher A., Ismail M.S., Porsteinsson A., Weiner M., Jack C.R., Jr, Thal L., Aisen P.S., Alzheimer’s Disease Cooperative Study Group Chronic divalproex sodium to attenuate agitation and clinical progression of Alzheimer disease. Arch. Gen. Psychiatry. 2011;68(8):853–861. doi: 10.1001/archgenpsychiatry.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lu X., Wang L., Yu C., Yu D., Yu G. Histone acetylation modifiers in the pathogenesis of Alzheimer’s disease. Front. Cell. Neurosci. 2015;9:226. doi: 10.3389/fncel.2015.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.ClinicalTrials.gov Clinical trial to determine tolerable dosis of Vorinostat in patients with mild Alzheimer disease. clinicaltrials.gov/ct2/show/NCT03056495?term=SAHA&cond=Dementia&draw=2&rank=2
- 160.Zhang Y., Liang M., Sun C., Song E.J., Cheng C., Shi T., Min M., Sun Y. Proton pump inhibitors use and dementia risk: a meta-analysis of cohort studies. Eur. J. Clin. Pharmacol. 2020;76(2):139–147. doi: 10.1007/s00228-019-02753-7. [DOI] [PubMed] [Google Scholar]
- 161.Li M., Luo Z., Yu S., Tang Z. Proton pump inhibitor use and risk of dementia: Systematic review and meta-analysis. Medicine (Baltimore) 2019;98(7):e14422. doi: 10.1097/MD.0000000000014422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Song Y.Q., Li Y., Zhang S.L., Gao J., Feng S.Y. Proton pump inhibitor use does not increase dementia and Alzheimer’s disease risk: An updated meta-analysis of published studies involving 642305 patients. PLoS One. 2019;14(7):e0219213. doi: 10.1371/journal.pone.0219213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Booker A., Jacob L.E., Rapp M., Bohlken J., Kostev K. Risk factors for dementia diagnosis in German primary care practices. Int. Psychogeriatr. 2016;28(7):1059–1065. doi: 10.1017/S1041610215002082. [DOI] [PubMed] [Google Scholar]
- 164.de Souto Barreto P., Lapeyre-Mestre M., Mathieu C., Piau C., Bouget C., Cayla F., Vellas B., Rolland Y. Prevalence and associations of the use of proton-pump inhibitors in nursing homes: a cross-sectional study. J. Am. Med. Dir. Assoc. 2013;14(4):265–269. doi: 10.1016/j.jamda.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 165.Goldstein F.C., Steenland K., Zhao L., Wharton W., Levey A.I., Hajjar I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J. Am. Geriatr. Soc. 2017;65(9):1969–1974. doi: 10.1111/jgs.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kamer A.R., Craig R.G., Dasanayake A.P., Brys M., Glodzik-Sobanska L., de Leon M.J. Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimers Dement. 2008;4(4):242–250. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 167.Hashioka S., Inoue K., Miyaoka T., Hayashida M., Wake R., Oh-Nishi A., Inagaki M. The possible causal link of periodontitis to neuropsychiatric disorders: More than psychosocial mechanisms. Int. J. Mol. Sci. 2019;20(15):3723. doi: 10.3390/ijms20153723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wu Z., Nakanishi H. Connection between periodontitis and Alzheimer’s disease: possible roles of microglia and leptomeningeal cells. J. Pharmacol. Sci. 2014;126(1):8–13. doi: 10.1254/jphs.14R11CP. [DOI] [PubMed] [Google Scholar]
- 169.Perry V.H., Cunningham C., Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 2007;7(2):161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- 170.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711(1-2):163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 171.Banks W.A., Lynch J.L., Price T.O. In: Cytokines and the blood–brain barrier. The Neuroimmunological Basis of Behavior and Mental Disorders; Siegel, A. Zalcman S.S., editor. Boston, MA, USA: Springer; 2009. pp. 3–17. [Google Scholar]
- 172.Minten C., Alt C., Gentner M., Frei E., Deutsch U., Lyck R., Schaeren-Wiemers N., Rot A., Engelhardt B. DARC shuttles inflammatory chemokines across the blood-brain barrier during autoimmune central nervous system inflammation. Brain. 2014;137(Pt 5):1454–1469. doi: 10.1093/brain/awu045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Osburg B., Peiser C., Dömling D., Schomburg L., Ko Y.T., Voigt K., Bickel U. Effect of endotoxin on expression of TNF receptors and transport of TNF-α at the blood-brain barrier of the rat. Am. J. Physiol. Endocrinol. Metab. 2002;283(5):E899–E908. doi: 10.1152/ajpendo.00436.2001. [DOI] [PubMed] [Google Scholar]
- 174.Spranger J., Verma S., Göhring I., Bobbert T., Seifert J., Sindler A.L., Pfeiffer A., Hileman S.M., Tschöp M., Banks W.A. Adiponectin does not cross the blood-brain barrier but modifies cytokine expression of brain endothelial cells. Diabetes. 2006;55(1):141–147. doi: 10.2337/diabetes.55.01.06.db05-1077. [DOI] [PubMed] [Google Scholar]
- 175.Ott D., Murgott J., Rafalzik S., Wuchert F., Schmalenbeck B., Roth J., Gerstberger R. Neurons and glial cells of the rat organum vasculosum laminae terminalis directly respond to lipopolysaccharide and pyrogenic cytokines. Brain Res. 2010;1363:93–106. doi: 10.1016/j.brainres.2010.09.083. [DOI] [PubMed] [Google Scholar]
- 176.Bonaz B., Sinniger V., Pellissier S. The vagus nerve in the neuro-immune axis: Implications in the pathology of the gastrointestinal tract. Front. Immunol. 2017;8:1452. doi: 10.3389/fimmu.2017.01452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Watkins L.R., Goehler L.E., Relton J.K., Tartaglia N., Silbert L., Martin D., Maier S.F. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 1995;183(1-2):27–31. doi: 10.1016/0304-3940(94)11105-R. [DOI] [PubMed] [Google Scholar]
- 178.Hashioka S., Inoue K., Hayashida M., Wake R., Oh-Nishi A., Miyaoka T. Implications of systemic inflammation and periodontitis for major depression. Front. Neurosci. 2018;12:483. doi: 10.3389/fnins.2018.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu Y., Wu Z., Zhang X., Ni J., Yu W., Zhou Y., Nakanishi H. Leptomeningeal cells transduce peripheral macrophages inflammatory signal to microglia in reponse to Porphyromonas gingivalis LPS. Mediators Inflamm. 2013;2013:407562. doi: 10.1155/2013/407562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wu Z., Zhang J., Nakanishi H. Leptomeningeal cells activate microglia and astrocytes to induce IL-10 production by releasing pro-inflammatory cytokines during systemic inflammation. J. Neuroimmunol. 2005;167(1-2):90–98. doi: 10.1016/j.jneuroim.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 181.Walker K.A., Gottesman R.F., Wu A., Knopman D.S., Gross A.L., Mosley T.H., Jr, Selvin E., Windham B.G. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology. 2019;92(11):e1256–e1267. doi: 10.1212/WNL.0000000000007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kraynak T.E., Marsland A.L., Wager T.D., Gianaros P.J. Functional neuroanatomy of peripheral inflammatory physiology: A meta-analysis of human neuroimaging studies. Neurosci. Biobehav. Rev. 2018;94:76–92. doi: 10.1016/j.neubiorev.2018.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Marsland A.L., Gianaros P.J., Abramowitch S.M., Manuck S.B., Hariri A.R. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol. Psychiatry. 2008;64(6):484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Holmes C. Review: systemic inflammation and Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2013;39(1):51–68. doi: 10.1111/j.1365-2990.2012.01307.x. [DOI] [PubMed] [Google Scholar]
- 185.De Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C., Saad M.J.A., Velloso L.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 186.Thaler J.P., Yi C-X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O., Zhao X., Sarruf D.A., Izgur V., Maravilla K.R., Nguyen H.T., Fischer J.D., Matsen M.E., Wisse B.E., Morton G.J., Horvath T.L., Baskin D.G., Tschöp M.H., Schwartz M.W. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tomassoni D., Martinelli I., Moruzzi M., Micioni Di Bonaventura M.V., Cifani C., Amenta F., Tayebati S.K. Obesity and age-related changes in the brain of the Zucker Lepr (fa/fa) rats. Nutrients. 2020;12(5):1356. doi: 10.3390/nu12051356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jeon B.T., Jeong E.A., Shin H.J., Lee Y., Lee D.H., Kim H.J., Kang S.S., Cho G.J., Choi W.S., Roh G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes. 2012;61(6):1444–1454. doi: 10.2337/db11-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pepping J.K., Freeman L.R., Gupta S., Keller J.N., Bruce-Keller A.J. NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2013;304(4):E392–E404. doi: 10.1152/ajpendo.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Baufeld C., Osterloh A., Prokop S., Miller K.R., Heppner F.L. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016;132(3):361–375. doi: 10.1007/s00401-016-1595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Varatharaj A., Galea I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017;60:1–12. doi: 10.1016/j.bbi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 192.Abdel-Haq R., Schlachetzki J.C.M., Glass C.K., Mazmanian S.K. Microbiome-microglia connections via the gut-brain axis. J. Exp. Med. 2019;216(1):41–59. doi: 10.1084/jem.20180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fung T.C., Olson C.A., Hsiao E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017;20(2):145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chunchai T., Thunapong W., Yasom S., Wanchai K., Eaimworawuthikul S., Metzler G., Lungkaphin A., Pongchaidecha A., Sirilun S., Chaiyasut C., Pratchayasakul W., Thiennimitr P., Chattipakorn N., Chattipakorn S.C. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflammation. 2018;15(1):11. doi: 10.1186/s12974-018-1055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Teixeira F.B., Saito M.T., Matheus F.C., Prediger R.D., Yamada E.S., Maia C.S.F., Lima R.R. Periodontitis and Alzheimer’s disease: A possible comorbidity between oral chronic inflammatory condition and neuroinflammation. Front. Aging Neurosci. 2017;9:327. doi: 10.3389/fnagi.2017.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dominy S.S., Lynch C., Ermini F., Benedyk M., Marczyk A., Konradi A., Nguyen M., Haditsch U., Raha D., Griffin C., Holsinger L.J., Arastu-Kapur S., Kaba S., Lee A., Ryder M.I., Potempa B., Mydel P., Hellvard A., Adamowicz K., Hasturk H., Walker G.D., Reynolds E.C., Faull R.L.M., Curtis M.A., Dragunow M., Potempa J. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019;5(1):eaau3333. doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Poole S., Singhrao S.K., Kesavalu L., Curtis M.A., Crean S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimers Dis. 2013;36(4):665–677. doi: 10.3233/JAD-121918. [DOI] [PubMed] [Google Scholar]
- 198.Wu Z., Ni J., Liu Y., Teeling J.L., Takayama F., Collcutt A., Ibbett P., Nakanishi H. Cathepsin B plays a critical role in inducing Alzheimer’s disease-like phenotypes following chronic systemic exposure to lipopolysaccharide from Porphyromonas gingivalis in mice. Brain Behav. Immun. 2017;65:350–361. doi: 10.1016/j.bbi.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 199.Zeng F., Liu Y., Huang W., Qing H., Kadowaki T., Kashiwazaki H., Ni J., Wu Z. Receptor for advanced glycation end products up-regulation in cerebral endothelial cells mediates cerebrovascular-related amyloid β accumulation after Porphyromonas gingivalis infection. J. Neurochem. doi: 10.1111/jnc.15096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nie R., Wu Z., Ni J., Zeng F., Yu W., Zhang Y., Kadowaki T., Kashiwazaki H., Teeling J.L., Zhou Y. Porphyromonas gingivalis infection induces amyloid-β accumulation in monocytes/macrophages. J. Alzheimers Dis. 2019;72(2):479–494. doi: 10.3233/JAD-190298. [DOI] [PubMed] [Google Scholar]
- 201.Sato K., Takahashi N., Kato T., Matsuda Y., Yokoji M., Yamada M., Nakajima T., Kondo N., Endo N., Yamamoto R., Noiri Y., Ohno H., Yamazaki K. Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis through modulation of the gut microbiota and gut immune system. Sci. Rep. 2017;7(1):6955. doi: 10.1038/s41598-017-07196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Leira Y., Domínguez C., Seoane J., Seoane-Romero J., Pías-Peleteiro J.M., Takkouche B., Blanco J., Aldrey J.M. Is periodontal disease associated with Alzheimer’s disease? A systematic review with meta-analysis. Neuroepidemiology. 2017;48(1-2):21–31. doi: 10.1159/000458411. [DOI] [PubMed] [Google Scholar]
- 203.Noble J.M., Scarmeas N., Celenti R.S., Elkind M.S., Wright C.B., Schupf N., Papapanou P.N. Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS One. 2014;9(12):e114959. doi: 10.1371/journal.pone.0114959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sparks Stein P., Steffen M.J., Smith C., Jicha G., Ebersole J.L., Abner E., Dawson D. III Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement. 2012;8(3):196–203. doi: 10.1016/j.jalz.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Perry V.H. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav. Immun. 2004;18(5):407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 206.Lindenberger U., Marsiske M., Baltes P.B. Memorizing while walking: increase in dual-task costs from young adulthood to old age. Psychol. Aging. 2000;15(3):417–436. doi: 10.1037/0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- 207.Wu Z., Tokuda Y., Zhang X.W., Nakanishi H. Age-dependent responses of glial cells and leptomeninges during systemic inflammation. Neurobiol. Dis. 2008;32(3):543–551. doi: 10.1016/j.nbd.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 208.Liu X., Wu Z., Hayashi Y., Nakanishi H. Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience. 2012;216:133–142. doi: 10.1016/j.neuroscience.2012.04.050. [DOI] [PubMed] [Google Scholar]