Abstract
Neurodegenerative diseases are characterized by the increasing dysfunction and death of neurons, resulting in progressive impairment of a person’s mobility and/or cognition. Protein misfolding and aggregation are commonly hypothesized to cause neurotoxicity and, eventually, neuronal degeneration that are associated with these diseases. Emerging experimental evidence, as well as recent findings from human studies, reveal that the C-terminus of Hsp70 Interacting Protein (CHIP), or STIP1 Homology and U-box containing Protein 1 (STUB1), is a quality control protein involved in neurodegeneration. Here, we review evidence that CHIP interacts with and plays a role in regulating proteins implicated in the pathogenesis of Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and polyglutamine diseases, including Huntington’s disease and spinocerebellar ataxias. We also review clinical findings identifying mutations in STUB1 as a cause of both autosomal recessive and autosomal dominant forms of cerebellar ataxia. We propose that CHIP modulation may have therapeutic potential for the treatment of multiple neurodegenerative diseases.
Keywords: Alzheimer’s disease, amyotrophic lateral sclerosis, cerebellar ataxia, huntington disease, neurodegenerative diseases, parkinson’s disease, polyglutamine diseases, STUB1, STUB1-associated disease
1. INTRODUCTION
Neurodegenerative diseases are chronic neurological conditions that include disorders such as Parkinson’s disease (PD), Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), and inherited cerebellar ataxias. These diseases are all incurable at present, with no available treatments to modify the neurodegenerative process or provide neuroprotection. Age is a major risk factor for most neurodegenerative diseases and thus, without such disease-modifying or neuroprotective treatments, neurodegenerative diseases will represent an increasingly significant medical and public health concern as life expectancy increases and populations age worldwide [1, 2].
All neurodegenerative diseases are defined by progressive neuronal dysfunction and death. They are associated with a gradual decline in a person’s mobility and/or cognition. Specific symptoms of each disease reflect the neuronal populations affected and, while an explanation for the selective vulnerability of discrete neuronal populations in different disorders remains to be elucidated, mounting evidence indicates that certain cellular and molecular mechanisms are shared across these diseases. Importantly, protein misfolding and aggregation are associated with neurotoxicity and eventual neuronal loss [3].
Quality control systems exist within each cell to maintain protein homeostasis, or ‘proteostasis’. Proteostatic systems include the chaperone system, the ubiquitin-proteasome system (UPS), and autophagy-lysosomal pathway (ALP). Together these systems serve to reduce or prevent the accumulation of misfolded proteins and aggregates. Deciphering the role of quality control proteins is especially important for understanding the pathogenesis of neurodegenerative diseases and developing disease-modifying therapies. This review discusses the role of one such quality control protein, the co-chaperone C-terminus of Hsp70 Interacting Protein (CHIP). Emerging experimental evidence, together with clinical findings, suggest that CHIP may play a critical neuroprotective role in multiple neurodegenerative diseases.
2. C-TERMINUS OF HSP70 INTERACTING PROTEIN (CHIP)
The chaperone system is comprised of a family of proteins, including molecular chaperones and co-chaperones, that work together to facilitate de novo protein folding as well as refolding of misfolded proteins. Chaperones, such as Heat Shock Protein 70 (Hsp70) and Heat Shock Protein 90 (Hsp90), directly interact with newly synthesized peptides to assist with proper folding and to prevent aggregation into potentially cytotoxic assemblies [4]. Co-chaperones also assist with folding by interacting with chaperones to modulate chaperone activity or by linking chaperones to other proteostatic systems [5]. When proteins are irreversibly misfolded or aggregated, they are targeted for degradation via the UPS or ALP, which often occurs in a chaperone-dependent manner [4, 6]. The activity of chaperones and co-chaperones is therefore, critical to multiple cellular pathways due to their central role in maintaining proteostasis.
CHIP, or STIP1 Homology and U-box containing Protein 1 (STUB1), is a 34.5 kDa co-chaperone encoded by the STUB1 gene. STUB1 is located on chromosome 16 in humans, contains 7 exons, and has 2 alternative splicing transcript variants. STUB1 is highly conserved across species and is expressed in most tissues [7]. CHIP contains an N-terminal tetratricopeptide (TPR) domain and a C-terminal U-box domain. The TPR domain interacts with the chaperones Hsp70 and Hsp90 [7], whereas the U-box domain confers E3 ubiquitin ligase activity [7, 8]. The TPR and U-box domains are connected by a central, highly-charged, coiled- coil domain that mediates the homodimerization of CHIP [9, 10]. CHIP homodimers exhibit conformational flexibility, allowing for both symmetrical and asymmetrical dimeric conformations [11-13], with the asymmetric conformation being necessary for the catalytic activity of the U-box domain [12, 9]. The flexible conformation of CHIP homodimers also allows interaction with a diverse range of substrates [11]. Through these domains, CHIP has a range of functions it can exert through chaperone protein networks and, by extension, various cell systems.
Ubiquitin is a small protein that can be covalently linked to proteins to facilitate a number of diverse functions [14-17]. Substrates can be mono- or poly-ubiquitinated, and the location of attachment between conjoined ubiquitin molecules ultimately determines the type of chain as well as the ultimate fate of the substrate [14, 18]. CHIP regulates the ubiquitination of chaperone-bound proteins by directly engaging chaperones via its TPR domain. This interaction results in the formation of a dynamic complex that promotes conformational changes in the CHIP protein, allowing it to interact with and ubiquitinate chaperone-bound proteins. Specifically, the U-box domain mediates monoubiquitination or polyubiquitination of chaperone-bound targets or even chaperones themselves [19]. In addition to ubiquitinating chaperone-bound targets, CHIP can also ubiquitinate itself, in a process referred to as auto-ubiquitination [20], which may represent an important capacity of the protein to self-regulate. Polyubiquitination requires cooperation between CHIP and E2 ubiquitin-conjugating enzymes. The E2 enzymes that co-ordinate with CHIP have an important role in determining whether CHIP will ubiquitinate itself or other targets [20, 21] and the type of ubiquitin chain formed [22]. CHIP can catalyze lysine-27 (Lys-27), Lys-48, and Lys-63 linked polyubiquitin chains [9, 23-26]. Lys-48 polyubiquitin chains are canonically associated with targeting proteins to the UPS for proteasomal degradation [14, 15, 27], although non-proteolytic functions of these chains have also been reported [17]. Lys-63 chains are largely associated with non-proteolytic functions, including DNA damage response, kinase activation, endocytosis, signal transduction, and selective autophagy, but targeting for proteasomal degradation has also been reported [17, 28]. Lys-27 chains have been associated with mitochondrial damage response, signal transduction, and proteasomal degradation [14, 23, 29, 30]. As CHIP can catalyze the formation of different types of polyubiquitin chains, CHIP-mediated ubiquitination of substrates can serve both proteolytic and non-proteolytic functions [23].
In addition to its function in the UPS, CHIP has an important regulatory role in autophagy. CHIP has been demonstrated to promote the degradation of the inactive phosphorylated form of transcription factor EB (TFEB), a pro-autophagy transcription factor [31, 32], increasing TFEB activity and autophagy [33]. CHIP-deficient cells exhibit impaired chaperone-mediated autophagy (CMA) [34]. Specifically, autophagosome accumulation and a lack of autophagosome-lysosome fusion have been observed in these cells. CHIP has been shown to direct the degradation of some proteins that contain a KFERQ-like motif through interactions with the chaperone Hsp70 protein 8 (HSPA8) [34]. These interactions are necessary to degrade proteins in the lysosome because they are required to target substrates to the lysosomal receptor, lysosomal-associated membrane protein 2A (LAMP2A) [34]. Since Lys-63 linked polyubiquitin chains can target substrates for lysosomal degradation via the ALP pathway [17, 35], CHIP-mediated ubiquitination may also be targeting substrates for lysosomal degradation. Indeed, CHIP has been shown to target Hypoxia-Inducible Factor 1 (HIF1) for lysosomal degradation via the ALP pathway through the addition of Lys-63 linked ubiquitin chains [36]. CHIP has also been demonstrated to localize to mitochondria within primary neurons following stress, and has been proposed to enhance stress-induced mitochondrial autophagy (mitophagy) [37]. In CHIP knockout mice, expression of genes involved in the initiation of autophagy and mitophagy were found to be decreased in cardiac muscle following treatment with fenofibrate (a PPARα agonist which stimulates oxidative metabolism), but not in wild-type controls [38], while voluntary exercise increased cardiac autophagy in CHIP null mice compared to controls [39], indicating that CHIP may influence autophagy differently in response to different stressors. With its connections to chaperones, the UPS, and the ALP, CHIP is well positioned to triage misfolded or aggregated proteins for refolding or degradation [40].
The autophagic activity has been found to decline in aging animal models [41-43], and age-related changes in CHIP protein levels have also been observed in vivo. CHIP protein levels, but not mRNA levels, were found to decrease in the brains of male wild-type C57BL mice with age [44]. CHIP knockout mice also exhibit accelerated aging [45] and senescence-accelerated mouse models have decreased CHIP mRNA and protein levels compared to senescence-resistant controls [46, 47]. These findings suggest anti-aging functions for CHIP and are consistent with a role in regulating autophagic pathways.
CHIP has additional functions that appear to be independent of ubiquitination and its E3 ubiquitin ligase activity. For example, CHIP has been found to function as an autonomous chaperone that promotes conformational changes of adenosine monophosphate-activated protein kinase (AMPK), resulting in increased AMPK protein stability and kinase activity [48]. CHIP was also shown to promote the trimerization, activation, and nuclear localization of heat shock factor-1 (HSF1) [49-51], a transcription factor that promotes the transcription of various heat shock proteins in response to misfolded protein accumulation. Whether these functions of CHIP are related to neurodegeneration, remain to be elucidated. Regardless, they demonstrate that the CHIP function is not restricted to its E3 ligase activity.
Post-translational modifications of CHIP have been demonstrated to impact its function (Fig. 1). Cyclin-Dependent Kinase 5 (CDK5) was found to phosphorylate murine CHIP at serine 20, which is located N-terminal to the TPR domain. Phosphorylation at this site reduced turnover of truncated Apoptosis-Inducing Factor (tAIF) by CHIP and the UPS via a mechanism that involved an alteration of CHIP’s binding affinity to the protein without affecting its E3 ligase activity. In contrast, aurora kinase A (AURKA) phosphorylates CHIP at serine 273 and appears to regulate its E3 ligase activity, promoting the ubiquitination and proteasomal degradation of androgen receptor (AR) [52]. The Auto-ubiquitination of CHIP has been shown to assist with translocation to the proteasome. This did not cause the degradation of CHIP but helped to facilitate the degradation of the substrate bound to it [53]. CHIP can also monoubiquitinate itself with the help of Ubiquitin-conjugating enzyme E2 W (UBE2W) following cellular stress, promoting its interaction with and ubiquitination of ataxin-3 [54]. The full breadth and functional significance of these and other potentially undescribed post-translational modifications (PTMs) of CHIP remain to be elucidated as they could be important regulators of CHIP activity and substrate preference.
Fig. (1).
Post-translational modifications of CHIP. A) CDK5 mediated phosphorylation of mouse CHIP at serine 20 (S20) reduced CHIP- mediated ubiquitylation and proteasomal degradation of tAIF. B) AURKA phosphorylates CHIP at serine 273 (S273), promoting the ubiquitination and proteasomal degradation of AR. C) The ubiquitin-conjugating enzyme E2, UBE2W, promotes CHIP self-monoubiquitination, which enhances its ability to ubiquitinate and promote proteasomal degradation of polyQ expanded ataxin-3. AR: androgen receptor; AURKA: Aurora Kinase A; CDK5: cyclin-dependent kinase 5; CHIP: C-terminus of Hsp70 interacting protein; P: phosphorylation; polyQ: polyglutamine expansion; tAIF; truncated apoptosis inducing factor; Ub: ubiquitin; UBE2W: ubiquitin-conjugating enzyme E2 W.
CHIP is expressed in many different tissues, but it is most highly expressed in the brain, skeletal muscle, and cardiac muscle, suggesting its importance in systems with high metabolic activity [40, 55]. While CHIP is widely expressed throughout the brain, there is a particular abundance within the Purkinje layer of the cerebellum [55]. Stub1 knockout mice show partial lethality during the perinatal development stage [45]. Knockout mice were significantly smaller than wild-type mice and showed an accelerated aging phenotype that resulted in a significantly decreased lifespan [45]. Stub1 knockout mice also displayed severe ataxia, cognitive impairment, hypogonadism, and severe impairments in mitochondrial stress response [55, 56]. Brain lysates from Stub1 knockout mice showed a significant increase in misfolded proteins compared to age-matched or older wild-type controls [45]. Examination of the cerebella of these animals demonstrated a specific and drastic loss of Purkinje cells with increased pyknotic nuclei and severe dendritic swelling [57]. This evidence indicates that aberrations in CHIP can cause significant impairments in multiple physiological processes, including development and neurodegeneration.
In this review, we discuss evidence of the involvement of CHIP within various neurodegenerative diseases, each associated with abnormal aggregation of endogenous macromolecules. Given that CHIP is an E3 ligase that promotes the elimination of mutated, misfolded, or aggregated proteins via the UPS and ALP, we hypothesize that CHIP may be involved in each of these neurodegenerative diseases as a common downstream effector, facilitating the removal of a variety of disease-associated proteins. Consequently, CHIP modulation may have therapeutic potential for the treatment of multiple neurodegenerative diseases.
3. CHIP AND ITS MOLECULAR INTERACTIONS RELEVANT TO NEURODEGENERATIVE DISEASES
3.1. Parkinson’s Disease (PD)
PD is the most common neurodegenerative movement disorder and is associated with the classical ‘parkinsonian’ motor features (bradykinesia, rigidity, tremor, and postural instability) as well as multiple non-motor features, including cognitive impairment. PD is characterized by prominent neuronal loss in multiple brain regions, primarily the dopaminergic neurons of the substantia nigra pars compacta (SNpc). Within degenerating areas of the brain, there is a presence of intraneuronal inclusions, called Lewy bodies and Lewy neurites, which are primarily composed of aggregated α-synuclein (α-syn) protein [5]. The normal turnover of α-syn utilizes UPS and CMA-mediated degradation [58]. Aggregation of α-syn, due to excessive and/or misfolded proteins, typically follows a process in which misfolding is followed by dimerization, oligomerization, protofibril formation, fibrilization and finally, tightly packed Lewy body formation [59]. Aggregated α-syn has been shown to bind to and inhibit the action of the proteasome, an early-onset event which contributes to neurodegeneration [5, 60]. As a consequence, the ALP is the predominant pathway that cells utilize to degrade α-syn aggregates [58].
Experiments performed in cell culture indicate that CHIP, Hsp70, and α-syn form a complex [8, 61], and examination of post-mortem PD brain tissue demonstrates that they also co-localize within Lewy bodies [8]. Several lines of evidence suggest that Hsp70 can inhibit α-syn aggregation and thereby prevent the neuronal loss, including findings in cell culture models, transgenic Drosophila melanogaster, and mouse models [8, 59, 62]. Overexpression of CHIP has been shown to increase the rate of α-syn clearance from cells [8, 61] and reduce α-syn aggregation in rat brains in vivo [63]. CHIP appears to preferentially target specific oligomeric confirmations for degradation [64] via both proteasomal and lysosomal mechanisms [8]. Through direct or indirect interactions mediated by Hsp70, CHIP can ubiquitinate α-syn in vitro [8, 61, 64]. The co-chaperone BCL2 Associated Athanogene 5 (BAG5) can interact with CHIP via Hsp70 and inhibit the E3 ubiquitin ligase activity of CHIP. As a result, BAG5 causes a reduction in α-syn ubiquitination and mitigates the ability of CHIP to reduce α-syn oligomers [61]. Taken together, these data suggest that Hsp70 and CHIP may be important regulators of α-syn pathology.
In addition to α-syn, endonuclease G (EndoG) has been identified as a substrate of CHIP. EndoG is a mitochondrial localised DNase that initiates apoptosis following its nuclear relocalization [65]. EndoG levels have been found to be elevated and increasingly localized within the nucleus in post- mortem PD SNpc tissue compared to healthy controls. Furthermore, EndoG has been found to be crucial for α-syn mediated dopaminergic degeneration in vitro and in vivo [66]. CHIP has been shown to ubiquitinate and promote proteasomal degradation of EndoG, resulting in reduced EndoG protein levels, protecting against cell death [67].
While most PD cases are sporadic, some forms are inherited, such as those caused by mutations in parkin, PTEN-induced protein kinase 1 (PINK1), or leucine-rich repeat kinase-2 (LRRK2) [68]. Mutations in parkin cause autosomal recessive PD [69]. CHIP plays a role in positively regulating the activity of parkin, an E3 ligase that mediates ubiquitination via a RING finger domain [70]. Parkin is part of the PINK1-parkin mitochondrial clearance pathway which acts to remove damaged mitochondria from cells in a selective manner via mitophagy [69]. Parkin acts to ubiquitinate proteins on the outer-mitochondrial membrane to facilitate the degradation of depolarized mitochondria through the ALP [70]. In Drosophila, knockout of Parkin leads to loss of dopaminergic neurons, abnormal wing posture, thoracic indentation, muscle degeneration, and locomotor deficits. Parkin-null Drosophila also exhibit mitochondrial abnormalities including excessive fusion, reduced ATP production, and disorganized crista. Overexpression of CHIP in Parkin-null Drosophila suppressed dopaminergic neuron loss, as well as locomotor and mitochondrial defects. Knockout of CHIP in Parkin-null Drosophila exacerbated Parkin-mediated defects and resulted in a significantly reduced Drosophila lifespan [71]. Together, these findings suggest that CHIP has a critical protective role in the context of parkin dysfunction.
Parkin interacts with probable G-protein coupled receptor 37 (Pael-R), a transmembrane protein that can accumulate inside cells and can promote SNpc cell death, independent of Lewy body pathology [72]. Through this interaction, parkin ubiquitinates Pael-R, promoting its proteasomal degradation [72]. Overexpression of parkin reduced both Pael-R protein levels and aggregate formation [72], whereas parkin inactivation enhanced ER stress and dopamine toxicity-mediated dopaminergic neuron death in the SNpc [73]. CHIP has been shown to outperform Hsp70 for binding to parkin in vitro, potentiating the E3 ligase activity of parkin and promoting parkin-mediated ubiquitination of Pael-R, further supporting a role for CHIP in enhancing the protective effects of parkin [72].
Similar to parkin mutations, PINK1 mutations also cause autosomal recessive PD [74-77]. PINK1 is a serine-threonine protein kinase that, together with Parkin, is known to be highly involved in regulating mitophagy [78]. In Drosophila, knockout of Pink1, similar to knockout of Parkin, leads to loss of dopaminergic neurons, abnormal wing posture, thoracic indentation, locomotor deficits, muscle degeneration, and mitochondrial defects. These mitochondrial defects include excessive fusion, reduced ATP content, and crista disorganisation. Overexpression of CHIP in Pink1-null Drosophila suppressed movement and mitochondrial dysfunctions. Unlike in Parkin-null Drosophila, knockout of CHIP in Pink1-null Drosophila did not exacerbate the phenotype, indicating that CHIP may act downstream of PINK1 [71]. CHIP-null Drosophila exhibit impairments in climbing ability as well as reduced thoracic ATP, reduced thoracic mitochondrial DNA, and abnormal mitochondrial morphology [71]. Importantly, CHIP interacts with PINK1, promoting its polyubiquitylation and proteasomal degradation. Changes in CHIP protein levels are inversely correlated with PINK1 protein levels [79]. CHIP-mediated PINK1 degradation increased cytotoxicity in dopaminergic neuroblastoma SH-SY5Y cells following treatment with staurosporine, an apoptosis inducer [79]. Consistent with this finding, CHIP knockout mice have significantly increased levels of PINK1 protein in whole brain lysates [56].
Mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common cause of autosomal dominant PD [80]. LRRK2 is a large multidomain protein believed to play several roles, including maintenance of neuronal polarity [81, 82]. LRRK2 contains a serine/threonine kinase domain and several PD-causing mutations enhance its kinase activity [72]. CHIP has been shown to interact with LRRK2 in vitro in a manner requiring the TPR domain of CHIP, suggesting that Hsp70 or Hsp90 may mediate this binding [82], as well as weakly binding to the charged coiled-coil domain of CHIP [83]. The ubiquitination of wild-type and mutant forms of LRRK2 by CHIP has been shown to cause proteasomal degradation of LRRK2 [81-83]. Furthermore, levels of CHIP and Hsp90 were found to regulate LRRK2-related cytotoxicity [81]. Taken together, work to date demonstrates a beneficial role of CHIP in modulating the molecular pathogenesis of PD, which may occur, in part, through its interactions with α-syn, EndoG, parkin, PINK1, and LRRK2 (Fig. 2).
Fig. (2).
CHIP regulation of PD-related proteins. 1) CHIP ubiquitinates α-synuclein oligomers for proteasomal degradation, thereby preventing their accumulation into fibrils and Lewy bodies. This process occurs in cooperation with the chaperones Hsp70 or Hsp90 and can be inhibited by BAG5. 2) CHIP positively regulates parkin-mediated ubiquitination of misfolded α-synuclein monomers, preventing their incorporation into oligomers. CHIP enhances parkin-mediated degradation of Pael-R by displacing Hsp70, which when bound to Pael-R, prevents its parkin-mediated ubiquitination. This prevents pathological accumulation of Pael-R into aggregates. 3) With Hsp90, CHIP has been shown to facilitate proteasomal degradation of LRRK2. 4) With Hsp70, CHIP has been shown to facilitate proteasomal degradation of PINK1. 5) In response to oxidative stress and genetic polymorphisms, abnormal mitochondria are targeted for degradation in a PINK1- and Parkin-dependent manner via mitophagy. CHIP inhibits nuclear translocation of the mitochondrial protein EndoG, a process that is accelerated in the presence of misfolded α-synuclein and results in dopaminergic cell death. 6) CHIP promotes degradation of Lewy bodies by autophagy. α-syn: α-synuclein; BAG5: BCL2 associated athanogene 5; CHIP; C-terminus of Hsp70 interacting protein; EndoG: endonuclease G; Hsp70: Heat shock protein 70; Hsp90: Heat shock protein 90; LRRK2: Leucine rich repeat kinase 2; PINK1: PTEN-induced protein kinase; Pael-R: Parkin-associated endothelin receptor-like receptor; Ub: Ubiquitin.
3.2. Alzheimer’s Disease (AD)
AD is the most prevalent neurodegenerative disease and is characterized by progressive cognitive decline, primarily affecting memory, which eventually leads to impairment of a person’s ability to function in daily life and the emergence of behavioural symptoms. All forms of AD appear to share two molecular pathological hallmarks: the presence of intra-neuronal neurofibrillary tangles composed of tau and the deposition of extra-neuronal amyloid β (Aβ) plaques [84]. CHIP protein levels have been shown to be increased in AD patients [85].
Tau stabilizes microtubules, a critical component of the cytoskeleton, and is enriched in neuronal axons. Due to this important structural function, impairment in tau can be highly deleterious and hence its tight regulation is critical for proper neuronal function [86]. Hyper-phosphorylation of tau can cause its detachment from the axonal microtubules and induce aggregation [86]. In AD patient samples, CHIP levels were found to be inversely proportional to sarkosyl-insoluble tau accumulation, and CHIP knockout mice were found to have increased levels of insoluble tau accumulation [85] and soluble phosphorylated tau [87], indicating that CHIP may protect against neurofibrillary tangle formation in AD. Indeed, overexpressing chaperones, such as Hsp70 or Hsp90, has also been shown to reduce tau aggregation [88], and CHIP levels in brains of both AD patients and healthy controls have been found to be highly correlated with Hsp90 protein levels [85]. CHIP co-immunoprecipitated with tau and Hsp70, and CHIP has been shown to ubiquitinate tau to target it to the proteasome for degradation [26]. Hsp90 and CHIP have also been shown to work in concert to refold or degrade aberrant tau [89]. Histone deacetylase 6 (HDAC6) is a deacetylase of Hsp90, which reduced Hsp90-mediated refolding activity, promoting degradation of Hsp90 substrate proteins [90, 91]. Overexpression of HDAC6 has been shown to promote tau accumulation in HeLa cells. Conversely, decreased HDAC6 increased Hsp90-mediated clearance of tau in primary mouse neurons [92]. CHIP has been shown to bind, ubiquitinate, and regulate the expression of HDAC6 [92]. Brain homogenates from CHIP knockout mice exhibited both increased tau accumulation and levels of HDAC6 92]. Thus, HDAC appears to be involved in the interplay between Hsp90 and CHIP in regulating tau accumulation.
Tau phosphorylation by microtubule affinity regulating kinase 2 (MARK2) prevents its recognition by CHIP or Hsp90 [93]. Protein Kinase B (Akt), which can be degraded by Hsp90, has been shown to enhance the activity of MARK2 [89]. Akt knockout mice have decreased levels of CHIP, indicating that CHIP expression may be tied to Akt levels [93]. Interestingly, a reduction in Akt levels still enhanced CHIP-mediated degradation of tau, due to Akt having a higher binding affinity for tau than CHIP [93]. The understanding of CHIP’s regulation of tau requires further investigation and presents an interesting avenue to explore how co-chaperones regulate this protein.
Aβ is formed from its precursor protein, amyloid precursor protein (APP), which is a transmembrane protein enriched at neuronal synapses [94, 95]. Functional roles for APP include signalling, gene transduction, neurite growth, and synaptogenesis [96]. APP has multiple cleavage sites, including sites recognized by α-, β-, and γ-secretases. Cleavage of APP by β-secretase 1 (BACE1) leads to the production of the pathogenic 42 amino acid form of Aβ (Aβ42), an insoluble product that can aggregate to form Aβ plaques [96]. Multiple lines of evidence demonstrate that CHIP can reduce Aβ aggregation either by regulating levels of APP or BACE1. Evidence for the effects of CHIP on APP includes a demonstration that CHIP co-immunoprecipitated with APP from human brain lysates, indicating a possible in vivo interaction [97, 98]. This interaction was shown to either be direct or in a complex with Hsp70 [97]. The ubiquitination of APP by CHIP was shown to target it to the proteasome for degradation [97]. Depletion of CHIP or Hsp70 was associated with an increase in cellular APP levels [97], whereas overexpression of CHIP in an inducible Aβ-producing cell line was found to decrease Aβ accumulation. When primary cortical neurons were challenged with Aβ accumulation, CHIP overexpression increased their survival, supporting a role for CHIP in mitigating Aβ-mediated cytotoxicity [97]. Regulation of BACE1 by CHIP is suggested by the finding of increased expression of BACE1 under CHIP knockdown conditions [98] and potentially indicated by the reduced expression of CHIP in neurons in human AD brains [99]. CHIP knockdown and overexpression have been shown to be associated with increased and decreased BACE1 expression, respectively [98]. Deletion constructs of CHIP lacking either the TPR or U-box domain did not affect BACE1 expression [98], indicating that full-length CHIP protein is required for regulation of BACE1 levels. Similarly, CHIP can ubiquitinate BACE1 but requires both the TPR and U-box domain for this action [98]. Furthermore, CHIP is capable of stabilizing p53, a transcription factor that negatively regulates BACE1, in an active conformation and thereby reduces BACE1 expression [98]. Overall, these findings indicate that CHIP plays a protective role in modulating AD through its interactions with tau, HDAC6, APP, BACE1, and Akt (Fig. 3).
Fig. (3).
CHIP regulation of AD-related proteins. 1) CHIP together with Hsp70 or Hsp90 can recognize hyperphosphorylated tau, promote its ubiquitination and target it for proteasomal degradation. Akt enhances MARK2 activity, which prevents hyperphosphorylated tau recognition by CHIP. 2) HDAC6 inhibits HSP90-mediated refolding of tau, promoting aggregate formation. CHIP can promote the proteasomal degradation of HDAC6, enhancing HSP90 activity and reducing tau accumulation. 3) CHIP interacts with and ubiquitinates APP, targeting it for proteasomal degradation and is also able to decrease amyloid beta accumulation. 4) BACE1 promotes cleavage of APP into the pathogenic Aβ42 form, which promotes amyloid β plaque formation. CHIP is able to reduce BACE1 levels through ubiquitination of BACE1, through its stabilization of the transcription factor p53 which negatively regulates BACE1 transcription. AAP: amyloid precursor protein; Aβ42; 42 amino acid form of amyloid beta; Akt: Protein kinase B; BACE-1: beta-secretase-1; CHIP: C-terminus of Hsp70 interacting protein; Hsp70: Heat shock protein 70; Hsp90: Heat shock protein 90; HDAC6: Histone deacetylase 6; MARK2: Microtubule affinity regulating kinase 2; p53: tumor protein 53; Ub: Ubiquitin.
3.3. Amyotrophic Lateral Sclerosis (ALS)
ALS is a rapidly progressive neurodegenerative disease that causes muscle weakness, disability, and, ultimately death due to selective loss of motor neurons. Pathological protein inclusions, frequently containing TAP DNA binding protein 43 (TDP-43), are found in upper and lower motor neurons as well as glial cells [100]. Patients typically present with a combination of upper and lower motor neuron signs and symptoms, which progress to life-threatening respiratory failure and dysphagia [100]. The median survival from the time of diagnosis is 3 to 5 years [101]. While most cases of ALS are sporadic with no identified cause, approximately 5 to 10 percent of cases are familial with an underlying genetic cause, including mutations in genes such as TDP-43, C9orf72, NIMA-related kinase 1(NEK1), Fused in Sarcoma (FUS), and ubiquilin-2 (UBQLN2). At the time of writing this review, there were no reports of an association between CHIP and these ALS-associated genes. However, an interaction between CHIP and superoxide dismutase 1 (SOD1) has been identified. Mutations in SOD1 account for up to 2 percent of ALS cases [102, 103].
SOD1 is a Cu/Zn superoxide dismutase which prevents damage caused by free radical oxygen molecules. Hsp70 has been shown to interact with SOD1 and to favour interaction with mutant forms over wild-type SOD1 [104]. CHIP expression reduced SOD1 levels, and this effect was attenuated by a proteasome inhibitor, suggesting that CHIP may be mediating proteasomal degradation of SOD1 [104]. Indeed, CHIP has been shown to interact with mutant SOD1 indirectly through Hsp70, which promoted UPS-mediated degradation of mutant SOD1 [104]. Aggregated mutant SOD1 may impair the normal function of the proteasome [104, 105]. In this scenario, SOD1 aggregates can be cleared by Heat Shock Protein beta-8 (HSPB8), a chaperone molecule that can be induced in response to proteasomal inhibition, resulting in reduced size and number of aggregates [105]. HSPB8, together with the co-chaperone BCL2 Associated Athanogene 3 (BAG3), was reported to induce the creation of an autophagosome around the SOD1 aggregate, promoting ALP- mediated degradation [105]. When HSPB8-linked autophagosomes were co-immunoprecipitated, Hsp70, BAG3, and CHIP were present within the protein complex [106]. A CHIP-Hsp70-BAG3-HSPB8 complex has been shown to recruit p62, an autophagy scaffolding protein, promoting autophagosome development around misfolded proteins (Fig. 4), highlighting a possible role for CHIP in assisting in the clearance of SOD1 aggregates via both proteasomal and lysosomal mechanisms [106].
Fig. (4).
CHIP regulation of ALS-related proteins. CHIP together with Hsp70 can target mutant SOD1 for proteasomal degradation. Aggregation of mutant SOD1 may impair proteasomal function. CHIP together with Hsp70, BAG3 and HSPB8 can mediate SOD1 mutant aggregate degradation independent of the proteasome, by facilitating autophagosome formation, leading to autophagy-lysosome mediated degradation of mutant SOD1 aggregates. BAG3; BCL2-associated athanogene 3; CHIP: C-terminus of Hsp70-interacting protein; Hsp70: Heat shock protein 70; SOD1: Superoxide dismutase; HSPB8; Heat shock protein Beta-8; p62: nucleoporin p62; Ub: ubiquitin.
CHIP’s ability to degrade mutant SOD1 via two independent mechanisms suggests it could have a beneficial role in SOD1-mutant forms of ALS and has led to the investigation of CHIP as a potential ALS therapeutic. Dorfin is an E3 ubiquitin ligase that degrades mutant SOD1 and reduces mutant SOD1-mediated toxicity [107]. Because of its ability to degrade mutant SOD1, Dorfin has been of interest as a potential ALS therapeutic, but in vivo investigations have only shown modest benefit, and Dorfin has been found to have an extremely short half-life. To increase the efficacy of Dorfin as a therapeutic, engineered chimeric proteins have been generated, combining the substrate-binding domain of Dorfin and the U-box domain of CHIP [108]. This chimeric protein has been shown to have an increased half-life, to increase ubiquitination of mutant SOD1, to reduce aggregate formation, and to rescue neurons from mutant SOD1 toxicity. However, in vivo results have yet to be reported and will be required to support further development of this potential therapeutic approach.
Necroptosis is a pro-inflammatory form of programmed cell death which has been implicated in neuroinflammation and the pathogenesis of neurodegenerative diseases, most notably ALS, but also AD and PD [109-111]. Elevated levels of key necroptosis mediator proteins, receptor-interacting protein kinase 1 (RIPK1) and receptor-interacting protein kinase 3 (RIPK3), have been observed in ALS mouse models and patient samples [110, 111], raising the possibility of inhibition of these proteins as a potential treatment strategy for ALS. CHIP ubiquitinates and decreases levels of both RIPK1 and RIPK3 in vitro and in vivo, reducing necroptosis in response to various stressors [112, 113]. These findings indicate that CHIP could also provide therapeutic benefit for ALS treatment, and potentially other neurodegenerative diseases, through its ability to decrease RIPK1 and RIPK3 protein levels and reduce necroptosis.
3.4. Lafora Disease (LD)
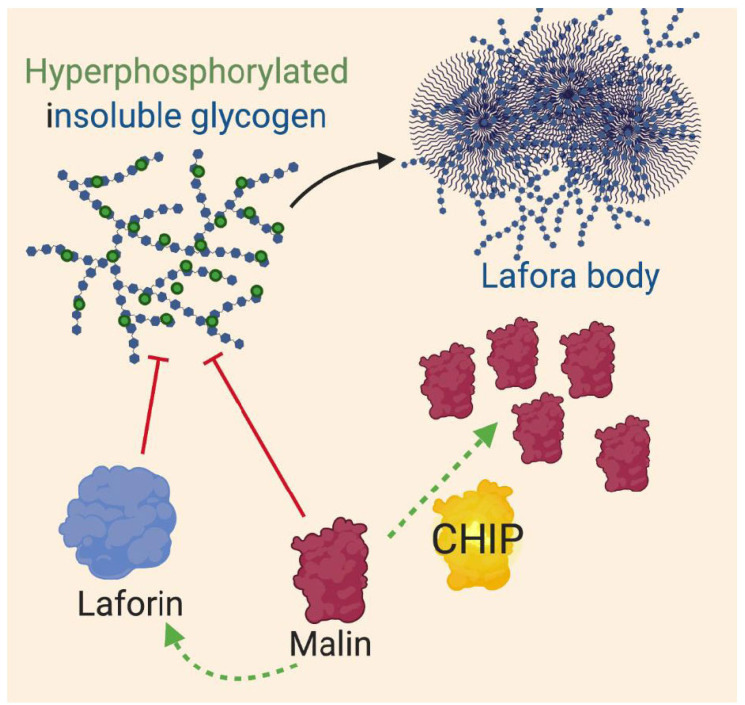
LD is an autosomal recessive neurodegenerative disorder, leading to defects in the development of cerebral cortical neurons and is most commonly associated with epileptic seizures [114, 115]. LD is caused by loss of function mutations in the genes encoding laforin, a phosphatase, and malin, an E3 ubiquitin ligase that is responsible for regulating laforin protein levels [116]. LD is characterized by the presence of Lafora bodies, which are cytoplasmic polyglucosan aggregates, not protein aggregates. Lafora bodies exist within both neuronal and non-neuronal tissues. Laforin and malin are both critical proteins involved in glycogen production whose loss of function results in hyperphosphorylated, insoluble glycogen [117, 118]. Overexpression of mutant or wild-type malin can lead to polyglucosan aggregation, proteasomal dysfunction, and cell death. CHIP does not associate with mutant or aggregated malin, but does interact with wild-type malin through Hsp70 and increases malin protein levels in a concentration-dependent manner (Fig. 5) [119]. The mechanism by which CHIP increases malin protein levels is currently unknown is independent of the U-box domain and thus could be due to the stabilisation of malin [119]. It is also unknown whether CHIP promotes malin aggregation or cellular dysfunction. While, the role of CHIP in LD is not well understood, current findings suggest CHIP could potentiate LD.
Fig. (5).
CHIP regulation of proteins involved in LD. Loss of function of malin or laforin results in hyperphosphorylated insoluble glycogen which promotes the formation of polyglucosan aggregates termed Lafora bodies. CHIP increases wildtype malin proteins in a concentration dependent manner through an unknown mechanism. CHIP: C-terminus of Hsp70 interacting protein.
3.5. Huntington Disease (HD) and other Polyglutamine (PolyQ) Diseases
Polyglutamine (polyQ) diseases are inherited neurodegenerative disorders caused by the expansion of cytosine-adenine-guanine (CAG) in disease-causing genes. Currently, there are nine clinically described polyQ diseases: HD, spinal-bulbar muscular atrophy (SBMA), spinocerebellar ataxia 1 (SCA1), SCA2, SCA3, SCA6, SCA7, SCA17, and dentatorubral-pallidoluysian atrophy (DRPLA). Protein aggregation is present in all of these diseases, and none have therapies for prevention or cure [120, 121].
HD is a neurodegenerative disease characterized by the progressive movement, behavioural, and psychiatric symptoms. HD is caused by polyQ expansion in the gene encoding the huntingtin protein (HTT) [122]. CHIP has been shown to reduce polyQ-HTT protein aggregation and toxicity [123, 124], but did not promote the degradation of wild- type HTT [125]. Knockdown of CHIP in mice overexpressing neuronal polyQ-HTT resulted in reduced lifespan, accelerated neuronal dysfunction, behavioural abnormalities, and increased cerebellar granular cell HTT inclusions [123]. It has also been shown that high levels of Hsp70 binding protein 1 (HSPBP1) expression in neurons inhibits CHIP activity, reducing CHIP-mediated elimination of neuronal polyQ-HTT, while HSPBP1 knockdown has been shown to increase CHIP activity (Fig. 6). Using an AAV CRISPR/Cas9 to target and knockdown HSPBP1 in a HD mouse model prevented accumulation and aggregation of HTT protein, and decreased loss of striatal presynaptic neurons [124].
Fig. (6).
CHIP regulation of polyQ expanded proteins. A) Within HD disease models, CHIP reduces polyQ-HTT aggregation. High levels of HSPBP1 inhibits CHIP-mediated reductions in polyQ-HTT aggregation. B) Within a model of SBMA, CHIP overexpression promotes proteasomal degradation of polyQ-AR and reduced polyQ-AR nuclear accumulation. C) Within a SCA3 model of disease, self-monoubiquitination of CHIP with UBE2W promotes ubiquitination and proteasomal degradation of polyQ-ataxin-3. PolyQ-ataxin-3, a DUB, promotes deubiquitination of CHIP, reducing polyQ-ataxin-3 ubiquitination and degradation. D) Within a SCA1 model of disease, CHIP is able to reduce polyQ-ataxin-1 aggregation. AR: androgen receptor; CHIP: C-terminus of Hsp70 interacting protein; polyQ: polyglutamine expansion; HSPBP1: Hsp70 binding protein 1; HTT; huntingtin protein; SBMA; spinal and bulbar muscular atrophy; Ub: ubiquitin; UBE2W; ubiquitin- conjugating enzyme E2 W.
SBMA is an X-linked genetic neurodegenerative disease caused by polyQ expansion within the gene encoding AR. SMBA is only present in males and is characterized by muscle weakness and progressive muscular atrophy [126]. CHIP has been shown to preferentially target polyQ-AR mutant proteins for proteasomal degradation compared to wild-type AR (Fig. 6) [127]. Overexpression of CHIP in neuronal cells reduced mutant AR monomers, and overexpression in a SBMA mouse model improved movement defects and reduced polyQ-AR nuclear accumulation [127].
PolyQ expansion of the protein ataxin-3, a deubiquitinating enzyme (DUB), causes SCA3, also known as Machado-Joseph disease [128]. SCA3 is an autosomal dominant form of inherited cerebellar ataxia (see below). CHIP has been shown to preferentially bind and degrade mutant or polyQ-ataxin-3, but not wild-type ataxin-3, in neuronal cell lines. CHIP-mediated protection was improved by Hsc70 overexpression [123]. Using SCA3 transgenic mouse models, knockout and/or knockdown of CHIP was shown to worsen SCA3 motor defects, increase neuronal ataxin-3 microaggregation, and result in early death in a concentration-dependent manner [129]. PolyQ-ataxin-3 interacts with both monoubiquitinated and non-ubiquitinated CHIP, but the monoubiquitylation of CHIP by UBE2W has been shown to stabilize CHIP’s interaction with ataxin-3 and to enhance ataxin-3 ubiquitination (Fig. 6) [53]. It was also shown that ataxin-3, as a DUB, can cleave monoubiquitin from CHIP, reducing its ubiquitylation activity. SCA3 transgenic mice have significantly decreased CHIP levels, while ataxin-3 knockout mice have no difference in CHIP levels compared to wild-type mice [53]. These results suggest a complex interplay between CHIP and polyQ-ataxin-3.
PolyQ expansion of ataxin-1 causes SCA1, another autosomal dominant form of inherited cerebellar ataxia [130]. CHIP associates with both wild-type and polyQ-ataxin-1 in a TPR domain-dependent manner [131]. Overexpression of CHIP in SCA1 mutant Drosophila results in decreased wild- type and polyQ-ataxin-1 protein levels (Fig. 6) and significantly suppresses polyQ-ataxin-1 mediated toxicity within Drosophila eyes [132]. CHIP overexpression has also been shown to reduce wild-type ataxin-1 solubility, increasing aggregate formation [131].
CHIP has been shown to associate with and promote degradation of polyQ expanded but not wild-type forms of HTT, AR, ataxin-3, and CHIP is also able to promote degradation of polyQ expanded repeat short peptides [123, 133]. These results indicate that CHIP can specifically recognize polyQ expansions and warrants investigation into the role of CHIP in other polyQ diseases, including other SCAs (SCA 2, 6, 7, and 17) and DRPLA. CHIP appears to have a more complicated role in polyQ-ataxin-1 SCA1, as it may promote ataxin-1 aggregation, but has been shown to have a promising protective role in mouse models of HD, SBMA, and SCA3.
4. CHIP MUTATIONS IMPLICATED IN NEURODEGENERATIVE DISEASE
4.1. Inherited Cerebellar Ataxias
Inherited cerebellar ataxias are neurodegenerative diseases characterized by a progressive loss of cerebellar Purkinje neurons. Cerebellar neurodegeneration leads to a variety of symptoms, including decreased voluntary muscle control and incoordination, as well as clinical signs and symptoms that often reflect the anatomic site of damage. For example, damage to midline structures of the cerebellum often results in the development of gait ataxia, imbalance, truncal ataxia (inability to sit unsupported by their arms), vertigo, and nystagmus (rhythmic oscillations of the eyes). In contrast, damage to the cerebellar hemispheres is usually associated with limb ataxia, intention tremor (a tremor that increases in severity as the hand moves near its target), and ataxic dysarthria (difficulty articulating speech with a scanning quality) [134]. Increasingly, cerebellar lesions have also been suggested to have effects on cognitive and emotional processing [135].
Inherited cerebellar ataxias are phenotypically heterogenous and can demonstrate various modes of inheritance: autosomal dominant (such as the SCAs described above), autosomal recessive, X-linked, or even mitochondrial [136]. Autosomal dominant cerebellar ataxias are estimated to have a prevalence of around 1-5 per 100,000 people and autosomal recessive cerebellar ataxias have a prevalence of around 3 per 100,000 people [137]. A subset of autosomal recessive cerebellar ataxia includes Gordon Holmes syndrome (GHS), a rare autosomal recessive disease characterized by progressive cerebellar ataxia and hypogonadotropic hypogonadism.
Genetic testing of cerebellar ataxia patients of unknown cause has identified 132 patients with disease-associated STUB1 mutations. To date, 69 different disease-associated STUB1 mutations have been identified and confirmed. Of the identified disease-associated STUB1 mutations, 6 mutations map to intronic sequences, 21 mutations affect the TPR domain, 18 affect the coiled-coil domain, 23 map to the U-box domain, and 1 maps to the 3’ UTR (Fig. 7). Thirty- eight of the identified mutations are associated with an autosomal recessive inheritance pattern of the disease, while 35 are associated with an autosomal dominant inheritance pattern. Our current understanding of autosomal recessive STUB1-associated disease and autosomal dominant STUB1-associated disease from these cases is described below.
Fig. (7).
Mutations identified among individuals with STUB1-associated disease. CHIP is a 303 amino acid protein that contains an N-terminal tetratricopeptide (TPR) domain (which interacts with Hsp70 and Hsp90), a coiled-coil domain (which mediates asymmetric homodimerization of CHIP), and a C-terminal U-box domain (which confers E3 ubiquitin ligase activity). Sixty-nine different STUB1 mutations have been reported to be associated with ataxia, 62 of which are present within exons. The locations of each mutation in the protein are shown in the schematic figure with an arrow. Arrows of the same colour indicate compound heterozygous mutations which have been identified together. The most frequent observed mutation is L275Dfs*16 (Mutation #64). Additional details regarding each mutation are listed in Table (1), including results from computational in silico prediction programs. Where the number of patients with this mutation is listed as NA, mutations were reported in a WES study which did not report patient numbers. cDNA: coding DNA; NA: not available.
4.2. Autosomal Recessive STUB1-associated Disease
Autosomal recessive spinocerebellar ataxia 16 (SCAR16) is a type of cerebellar ataxia caused by autosomal recessive mutations in the STUB1 gene. To date, 42 patients with autosomal recessive forms of STUB1-associated disease have been identified from 23 kindreds, 38 of which were diagnosed with cerebellar ataxia (20 male, 17 female, and 1 unspecified case) [55, 57, 138-150]. Four additional STUB1 patients were identified from a screen of patients with nervous system abnormalities [151]. In more than half of the cases, multiple siblings were affected. Out of all 42 patients identified, no generational family disease history was reported, as is typically observed in autosomal recessive conditions. Patients were ethnically diverse; of those kindreds for which ethnicity was specified, four families were of Chinese descent, two were Taiwanese, ten were European, three were Middle Eastern, and one was Sri Lankan. The age of onset for ataxia ranged from infancy to 57 years, with a mean of 22.3 years. All patients exhibited motor dysfunction and, for those who had neuroimaging, all demonstrated cerebellar atrophy Table (1); however, there was large variation in the reported clinical phenotypes. For example, patients exhibited large differences in cognitive status ranging from no impairment to severe impairment/dementia. Additional neurological findings included epilepsy, eye movement abnormalities, chorea, dysarthria, urinary incontinence, and pyramidal symptoms, amongst others. Nerve conduction studies were abnormal in 3 out of 12 patients tested, hyperreflexia was observed in 15 of out 24 patients, and cognitive impairment was observed within 24 out of 35 patients. In addition, impaired sexual development/endocrine function was found in 5 out of 27 patients Table (1). The diversity of symptoms exhibited by SCAR16 patients suggest involvement of multiple brain regions Table (1). Indeed, one SCAR16 patient (Patient #25, Table 1) was shown, with diffusion tensor imaging (DTI), to have degeneration throughout the brain, not solely limited to the cerebellum [144]. Additionally, neuropathological analysis of another SCAR16 patient (Patient #21, Table 1) displayed abnormalities that were not restricted to the cerebellum. Within this patient, dramatic cerebellar cortical, Purkinje, and granular cell loss with reactive Bergmann gliosis was observed. Within the frontal cortex, swollen axonal processed we observed, and CHIP staining within neurons was reported to be diffuse and cytoplasmic with intense nuclear staining in neurons within deep layers of the frontal cortex. Ubiquitin and p62 positive neuronal intranuclear inclusions (NII) were also observed within frontal cortex neurons [142].
Table 1.
Summary of all reported individuals with STUB1-associated disease.
| Kindred | Patient |
AAO (y)/
Sex |
Ethnicity |
Clinical
diagnosis |
STUB1 mutation | CHIP mutation | Ataxia | Cognitive status | Eye movement abnormalities | Upper motor neuron features | Other movement disorders | Sexual development | Additional clinical features | Neuroimaging | Nerve conduction studies |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A [55] | 1 | 17/F | Chinese | SCAR16 | c.493C>T c.493C>T |
L165F L165F |
Severe truncal/limb ataxia, dysarthria | Severe impairment | Ophthalmoplegia | Normal tendon reflexes | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported |
| 2 | 17/F | SCAR16 | c.493C>T c.493C>T |
L165F L165F |
Moderate truncal/limb ataxia, dysarthria | Normal | Nystagmus | Increased tendon reflexes | No abnormalities reported | Not reported | No abnormalities reported | Severe cerebellar atrophy | Slowed NCV (motor and sensory) | ||
| 3 | 14/F | SCAR16 | c.493C>T c.493C>T |
L165F L165F |
Moderate truncal/limb ataxia, dysarthria | Moderate impairment | Ophthalmoplegia | Increased tendon reflexes | No abnormalities reported | Not reported | No abnormalities reported | Severe cerebellar atrophy | Slowed NCV (motor and sensory) | ||
| 4 | 19/M | SCAR16 | c.493C>T c.493C>T |
L165F L165F |
Moderate truncal/limb ataxia, dysarthria | Normal | Nystagmus | Increased tendon reflexes | No abnormalities reported | Not reported | No abnormalities reported | Severe cerebellar atrophy | Slowed NCV (motor and sensory) | ||
| B [55] | 5 | 20/M | Chinese | SCAR16 | c.389A>T c.441G>T |
N130I W147C |
Subtle/mild truncal/limb ataxia, dysarthria | Normal | Normal | Normal tendon reflexes | No abnormalities reported | Not reported | No abnormalities reported | Severe cerebellar atrophy | Not reported |
| C [55] |
6 | 16/F | Chinese | SCAR16 | c.621C>G c.707G>C |
Y207* S236T |
Moderate /severe truncal/limb ataxia, dysarthria | Normal | Normal | Increased tendon reflexes | No abnormalities reported | Not reported | No abnormalities reported | Severe cerebellar atrophy | Not reported |
| D [57] | 7 | 19/F | Chinese | SCAR16 | c.737C>T c.737C>T |
T246M T246M |
Severe ataxia, dysarthria | Cognitive impairment | Nystagmus | Normal tendon reflexes | No abnormalities reported | Hypogonadism | No abnormalities reported | Cerebellar atrophy | Normal |
| 8 | 17/F | c.737C>T c.737C>T |
T246M T246M |
Severe ataxia | Cognitive impairment | No abnormalities reported | Pyramidal features, increased tendon reflexes | Tremor | Hypogonadism | No abnormalities reported | Cerebellar atrophy | Not reported | |||
| E [138] | 9 | 2/M | German | SCAR16 | c.367C>G c.367C>G |
L123V L123V |
Ataxia | Normal | No abnormalities reported | Pyramidal features | No abnormalities reported | Normal | UI | Cerebellar atrophy | Normal |
| F [138] | 10 | 16/F | Turkish | SCAR16 | c.719T>C c.719T>C |
M240T M240T |
Ataxia | Cognitive impairment | No abnormalities reported | Pyramidal features | No abnormalities reported | Normal | UI | Cerebellar atrophy | Normal |
| G [138] | 11 | 29/M | Saudi Arabian | SCAR16 | c.235G>A c.236C>A |
A79T A79D |
Ataxia | Normal | No abnormalities reported | Pyramidal features, increased tendon reflexes | No abnormalities reported | Normal | No abnormalities reported | Cerebellar atrophy | Not reported |
| 12 | 49/M | SCAR16 | c.235G>A c.236C>A |
A79T A79D |
Ataxia | Normal | No abnormalities reported | Pyramidal features, increased tendon reflexes | No abnormalities reported | Normal | UI | Cerebellar atrophy | Not reported | ||
| H [139] | 13 | 2/M | Saudi Arabian | SCAR16 | c.194A>G c.194A>G |
N65S N65S |
Ataxia, dysarthria | Cognitive impairment, delayed development, dyspraxia | Nystagmus | No abnormalities reported | No abnormalities reported | Normal | Aged appearance, alopecia, T1D | Cerebellar hypoplasia | Not reported |
| 14 | 0/M | SCAR16 | c.194A>G c.194A>G |
N65S N65S |
Ataxia, dysarthria | Cognitive impairment | Chronic iridocyclitis with secondary glaucoma, oculomotor dyspraxia with saccadic pursuit | No abnormalities reported | Tremor | Normal | Dysphagia, aged appearance, ulcerative colitis | Severe cerebellar atrophy | Not reported | ||
| 15 | 0.67/F | SCAR16 | c.194A>G c.194A>G |
N65S N65S |
Ataxia | Cognitive impairment, delayed development, dyspraxia | Nystagmus | No abnormalities reported | No abnormalities reported | Normal | Epilepsy (until 2 yrs), aged appearance, facial dysmorphism | Cerebellar hypoplasia | Not reported | ||
| I [139] | 16 | 33/F | Sri Lankan | SCAR16 | c.82G>A c.430A>T |
E28K K144* |
Gait ataxia, dysarthria | No abnormalities reported | Not reported | No abnormalities reported | No abnormalities reported | Oligomenorrhea, secondary infertility | Dysphagia | Cerebellar hypoplasia | Not reported |
| J [140] | 17 | 23/M | Belgian | SCAR16 | c.433A>C c.687-690del CTAC |
K145Q I227P |
Gait/limb ataxia, dysarthria | Cognitive impairment | No abnormalities reported | Increased tendon reflexes | No abnormalities reported | Normal | Dysphagia | Cerebellar atrophy | Normal |
| 18 | 25/M | c.433A>C c.687-690del CTAC |
K145Q I227P |
Gait/limb ataxia, dysarthria | Cognitive impairment | No abnormalities reported | Increased tendon reflexes | No abnormalities reported | Normal | Dysphagia | Cerebellar atrophy | Not reported | |||
| K [141] | 19 | 15/M | Not reported | SCAR16 | c.612+1G>C c.823C>G |
Intronic L275V |
Truncal/limb ataxia, dysarthria | Cognitive impairment, attention failures, executive dysfunction | Nystagmus, fractionated pursuit, hypometric saccades | Pyramidal features, increased tendon reflexes | Myoclonus | Normal | Dysphagia | Cerebellar atrophy | Normal |
| L [142] | 20 | 20/F | Spanish | SCAR16 | c.633G>A c.712G>T |
M211I E238* |
Generalized ataxia, dysarthria | Severe impairment/dementia | Saccadic ocular pursuit | Pyramidal features, increased tendon reflexes | Myoclonus | Normal | No abnormalities reported | Cerebellar atrophy | Normal |
| 21 | 22/M | c.633G>A c.712G>T |
M211I E238* |
Generalized ataxia, dysarthria | Severe impairment/dementia | No abnormalities reported | Spasticity, increased tendon reflexes | No abnormalities reported | Normal | No abnormalities reported | Cerebellar atrophy | Normal | |||
| M [143] | 22 | 15/F | Not reported | Unconfirmed | Unconfirmed | Unconfirmed | Ataxia, dysarthria | Cognitive impairment | Oculomotor dysfunction | Spastic equinovarus deformity | Myoclonic and tremulous movement | Not reported | No abnormalities reported | Not reported | Not reported |
| 23 | 19/M | SCAR16 | c.724G>A c.724G>A |
E242K E242K |
Truncal/limb ataxia, dysarthria | Cognitive impairment | Nystagmus | No abnormalities reported | Choreoathetosis, dystonia, myoclonus | Not reported | No abnormalities reported | Severe cerebellar atrophy | Not reported | ||
| 24 | 24/M | c.724G>A c.724G>A |
E242K E242K |
Ataxia, dysarthria | Cognitive impairment | Vertical ocular flutter | No abnormalities reported | Dystonia, myoclonic and tremulous movement | Not reported | No abnormalities reported | Severe cerebellar atrophy | Not reported | |||
| N [144] | 25 | 12/M | German | SCAR16 | c.355C>T c.880A>T |
R119* I294F |
Ataxia | Severe impairment/ dementia | Nystagmus | Spastic tetraparesis, increased tendon reflexes, Babinski sign | Dystonia | Hypogonadism | Generalized tonic-clonic seizures, hypomimia, UI | Cerebellar atrophy, atrophy of mesencephalon and parieto-occipital cortex | Normal |
| O [144] | 26 | 12/M | Belgian | SCAR16 | c.433A>C c.728C>T |
K145Q P243L |
Ataxia | Severe impairment/ dementia, mutism | No abnormalities reported | Spastic tetraparesis, increased tendon reflexes, Babinski sign | Dystonia | Normal | Generalized tonic-clonic seizures, UI | Cerebellar atrophy | Normal |
| 27 | 20/F | c.433A>C c.728C>T |
K145Q P243L |
Ataxia | Severe impairment/ dementia, mutism | Nystagmus | Spastic tetraparesis, increased tendon reflexes, Babinski sign | No abnormalities reported | Normal | Generalized tonic-clonic seizures, UI | Cerebellar atrophy | Normal | |||
| P [151] | 28 | -- | Not reporter | Nervous system abnormality | c.268G>T c.268G>T |
D90Y D90Y |
Not reported -WES study | ||||||||
| Q [151] | 29 | -- | Not reported | Nervous system abnormality | c.358+2T>G c.358+2T>G |
Intronic Intronic |
Not reported -WES study | ||||||||
| R [151] | 30 | -- | Not reporter | Nervous system abnormality | c.670-11_670-10delCT c.670-11_670-10delCT |
Intronic Intronic |
Not reported -WES study | ||||||||
| S [151] | 31 | -- | Not reported | Nervous system abnormality | c.844C>G c.844C>G |
P282A P282A |
Not reported -WES study | ||||||||
| T [145] | 32 | -- | European | SCAR16 | c.433A>C c.502C>T |
K145Q L168F |
Ataxia | Not reported -WES study | |||||||
| U [171] | 33 | 53/F | Spanish | SCA48 | c.823_824delCT | L275Dfs*16 | Ataxia, dysarthria | CCAS | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | Anxiety, dysphagia, cachexia, UI | Cerebellar atrophy | Not reported |
| 34 | 52/F | c.823_824delCT | L275Dfs*16 | Ataxia, dysarthria | CCAS | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | Anxiety, dysphagia, cachexia, UI | Cerebellar atrophy | Not reported | |||
| 35 | 41/F | c.823_824delCT | L275Dfs*16 | Ataxia, dysarthria | CCAS, aphasia | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | Anxiety, dysphagia, stroke | Cerebellar atrophy, cerebellar and left cerebral hemisphere hypoperfusion | Not reported | |||
| 36 | 53/M | c.823_824delCT | L275Dfs*16 | No ataxia | CCAS | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Cerebellar atrophy, preserved cerebral perfusion | Not reported | |||
| 37 | 42/F | c.823_824delCT | L275Dfs*16 | Ataxia, dysarthria | CCAS | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | Anxiety, organic personality disorder, dysphagia, UI | Cerebellar atrophy, atrophy of frontal lobe, cerebellar hypoperfusion | Not reported | |||
| 38 | 56/M | c.823_824delCT | L275Dfs*16 | No ataxia | CCAS | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Cerebellar atrophy, preserved cerebral perfusion | Not reported | |||
| 39 | F | c.823_824delCT | L275Dfs*16 | Ataxia, dysarthria | CCAS | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | Anxiety | Cerebellar atrophy | Not reported | |||
| 40 | 42/F | c.823_824delCT | L275Dfs*16 | Ataxia, dysarthria | CCAS | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | Organic personality disorder, dysphagia | Cerebellar atrophy | Not reported | |||
| 41 | 33/M | c.823_824delCT | L275Dfs*16 | No ataxia | Normal | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Cerebellar atrophy | Not reported | |||
| V [146] | 42 | 54/F | Not reported | SCAR16 | c.103C>A c.678_679del |
R35S I227Pfs*11 |
Ataxia, dysarthria | Severe impairment | Nystagmus, abnormal ocular pursuit | No abnormalities reported | Chorea | Normal blood hormone levels | No abnormalities reported | Cerebellar atrophy, hypoperfusion of fronto-parietal cortex | Not reported |
| W [147] | 43 | 57/M | Not reported | SCAR16 | c.*204T>C c.*204T>C |
3’UTR 3’UTR |
Ataxia, dysarthria | Normal | Normal | Pyramidal features, increased tendon reflexes | No abnormalities reported | Normal | No abnormalities reported | Cerebellar atrophy | Not reported |
| 44 | 37/F | c.*204T>C c.*204T>C |
3’UTR 3’UTR |
Ataxia, dysarthria | Normal | Normal | Pyramidal features, increased tendon reflexes | No abnormalities reported | Normal | No abnormalities reported | Cerebellar atrophy | Not reported | |||
| 45 | 31/F | c.*204T>C c.*204T>C |
3’UTR 3’UTR |
Ataxia, dysarthria | Cognitive impairment | Nystagmus | Pyramidal features, increased tendon reflexes | No abnormalities reported | Normal | Depression | Cerebellar atrophy | Not reported | |||
| X [172] | 46 | 50/F | Italian | SCA48 | c.97G>A | G33S | Ataxia, dysarthria | Mild cognitive impairment, memory impairment, executive dysfunction | No abnormalities reported | No abnormalities reported | Chorea, parkinsonism | Not reported | Psychiatric symptoms, UI | Not reported | Not reported |
| 47 | 12/F | c.97G>A | G33S | Ataxia, dysarthria | Moderate cognitive impairment, memory impairment, executive dysfunction | No abnormalities reported | Increased tendon reflexes | Parkinsonism | Not reported | Generalized tonic-clonic seizures, dysphagia, psychiatric symptoms, UI | Cerebellar atrophy | Not reported | |||
| 48 | 5/M | c.97G>A | G33S | Ataxia, dysarthria | Moderate cognitive impairment, memory impairment, executive dysfunction | No abnormalities reported | Increased tendon reflexes | Chorea, dystonia, parkinsonism | Not reported | Generalized tonic-clonic seizures, dysphagia, psychiatric symptoms | Cerebellar atrophy | Not reported | |||
| 49 | 28/F | c.97G>A | G33S | Ataxia, dysarthria | Moderate cognitive impairment, memory impairment, executive dysfunction | No abnormalities reported | Increased tendon reflexes | Chorea, dystonia, parkinsonism, tremor | Not reported | Seizures, dysphagia, psychiatric symptoms | Cerebellar atrophy | Not reported | |||
| 50 | 45/M | c.97G>A | G33S | Ataxia, dysarthria | Moderate cognitive impairment, memory impairment, executive dysfunction | No abnormalities reported | Increased tendon reflexes | Dystonia, parkinsonism, tremor | Not reported | Generalized tonic-clonic seizures, dysphagia, psychiatric symptoms | Cerebellar atrophy | Normal | |||
| 51 | 44/M | c.97G>A | G33S | Ataxia, dysarthria | Mild cognitive impairment, memory impairment, executive dysfunction | No abnormalities reported | Increased tendon reflexes | No abnormalities reported | Not reported | Seizures, dysphagia, hearing loss, psychiatric symptoms, UI | Cerebellar atrophy | Normal | |||
| Y [172] | 52 | 55/M | Italian | SCA48 | c.682C>T | P228S | Ataxia, dysarthria | Moderate cognitive impairment | No abnormalities reported | No abnormalities reported | Blepharospasm, chorea, tongue tremor | Not reported | Dysphagia, psychiatric symptoms | Cerebellar atrophy | Normal |
| 53 | 35/M | c.682C>T | P228S | Ataxia, dysarthria | Mild cognitive impairment | No abnormalities reported | Increased tendon reflexes | Tremor | Not reported | Dysphagia, hearing loss | Cerebellar atrophy | Not reported | |||
| Z [173] | 54 | 31/M | Italian | SCA48 | c.689_692delACCT | Y230Cfs*9 | Gait ataxia, dysarthria | Normal | Nystagmus, broken smooth pursuit | Increased tendon reflexes | Dystonia | Low testosterone | No abnormalities reported | Cerebellar atrophy | Normal |
| 55 | 48/F | c.689_692delACCT | Y230Cfs*9 | Gait ataxia, dysarthria | Moderate cognitive impairment | Broken smooth pursuit | Normal | Chorea, dystonia, parkinsonism | Normal | Depression, UI | Cerebellar atrophy | Not reported | |||
| AA [173] | 56 | 42/F | Italian | SCA48 | c.818_819dupGC | P274Afs*3 | Gait ataxia, dysarthria | Moderate cognitive impairment | Normal | Normal | Anxiety, chorea, dystonia | Normal | Dysphagia, diabetes mellitus, UI | Cerebellar atrophy | Not reported |
| 57 | 34/F | c.818_819dupGC | P274Afs*3 | Gait ataxia, dysarthria | Moderate cognitive impairment | Broken smooth pursuit | Increased tendon reflexes | Chorea | Normal | No abnormalities reported | Cerebellar atrophy | Not reported | |||
| AB [173] | 58 | 56/M | Italian | SCA48 | c.791_792delTG | V264Gfs*4 | Gait ataxia, dysarthria | Moderate cognitive impairment | Broken smooth pursuit, impersistence | Normal | Parkinsonism | Normal | Dysphagia | Cerebellar atrophy | Normal |
| 59 | 40/F | c.791_792delTG | V264Gfs*4 | Gait ataxia, dysarthria | Moderate cognitive impairment | Broken smooth pursuit, slow saccades | Normal | Chorea | Normal | Frontal meningioma | Not reported | Not reported | |||
| AC [173] | 60 | 37/F | Italian | SCA48 | c.199G>A | A67T | Gait ataxia, dysarthria | Normal | Impersistence | Increased tendon reflexes | Chorea, dystonia | Normal | Thyroid cancer, UI | Cerebellar atrophy | Normal |
| AD [173] | 61 | 50/F | Italian | SCA48 | c.673C>T | R225* | Gait ataxia, dysarthria | Normal | Impersistence | Normal | No abnormalities reported | Normal | Dysphagia | Cerebellar atrophy | Normal |
| AE [173] | 62 | 46/F | Italian | SCA48 | c.721C>T | R241W | Mild ataxia, dysarthria | Cognitive impairment | Abnormalities | Normal | No abnormalities reported | Hypogonadism | Hashimoto’s thyroiditis | Cerebellar atrophy | Normal |
| AF [173] | 63 | 30/F | Italian | SCA48 | c.823_824delCT | L275Dfs*16 | Gait ataxia, dysarthria | Normal | Nystagmus | Increased tendon reflexes | Chorea, parkinsonism | Normal | Anxiety, depression, dysphagia, UI | Cerebellar atrophy | Not reported |
| AG [173] | 64 | 43/M | Italian | SCA48 | c.170C>T | P57L | Gait ataxia, dysarthria | No abnormalities reported | Broken smooth pursuit, hypometric saccades | Increased tendon reflexes | No abnormalities reported | Normal | Self-harm, dysphagia, tongue atrophy | Cerebellar atrophy | Normal |
| AH [174] | 65 | 51/F | Turkish | SCA48 | c.823_24delCT | L275Dfs*16 frameshift |
Gait ataxia, dysarthria | CCAS, apraxia | No abnormalities reported | Pyramidal features, Babinski sign | Chorea, dystonia, parkinsonism |
Not reported | Anxiety, palilalia, dysphagia, cachexia, UI | Cerebellar atrophy, hypoperfusion in frontal, parietal and temporal lobes |
Not reported |
| 66 | 60/F | Unconfirmed | Unconfirmed | Unconfirmed | Ataxia | CCAS, apraxia | No abnormalities reported | No abnormalities reported | Chorea, dystonia, parkinsonism | Not reported | Palilalia, cachexia, UI | Not reported | Not reported | ||
| 67 | 60/F | Unconfirmed | Unconfirmed | Unconfirmed | Ataxia | CCAS, apraxia | No abnormalities reported | No abnormalities reported | Chorea, dystonia, parkinsonism | Not reported | Palilalia, cachexia, UI | Not reported | Not reported | ||
| AI [175] | 68 | 41/M | Not reported | SCA48 | c.158T>C | I53T | Gait ataxia, dysarthria | Cognitive impairment/ dementia |
Saccadic eye movements | Increased tendon reflexes | Parkinsonism | Not reported | Diabetes mellitus, sleep apnea | Cerebellar atrophy, mild cortical atrophy | Not reported |
| 69 | 63/M | c.158T>C | I53T | Gait ataxia, dysarthria | Cognitive impairment | Saccadic eye movements | Increased tendon reflexes | No abnormalities reported | Not reported | No abnormalities reported | Cerebellar atrophy at superior vermis and dorsal region | Not reported | |||
| 70 | 26/M | c.158T>C | I53T | Gait ataxia, dysarthria | Dementia | Saccadic smooth pursuit, hypermetric saccades | Increased tendon reflexes | No abnormalities reported | Not reported | Drug and alcohol abuse | Marked cerebellar atrophy | Not reported | |||
| 71 | -/F | c.158T>C | I53T | Gait ataxia | Severe cognitive impairment, childlike affect | Occasional saccadic interruptions | Increased tendon reflexes | No abnormalities reported | Not reported | Autism spectrum disorder, UI | Severe cerebellar atrophy, moderate cortical atrophy | Not reported | |||
| AJ [175] | 72 | 41/F | Not reported | SCA48 | c.111C>G | F37L | Gait ataxia, dysarthria | Cognitive impairment, confusion, personality change | Nystagmus | Normal | Unusual wing-beating tremor | Not reported | No abnormalities reported | Marked cerebellar atrophy, basis pontis flattening | Not reported |
| 73 | -/M | Unconfirmed | Unconfirmed | Unconfirmed | Ataxia | No abnormalities reported | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported | ||
| 74 | -/F | Unconfirmed | Unconfirmed | Unconfirmed | Ataxia | Cognitive impairment | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | Psychosis | Not reported | Not reported | ||
| AK [148] | 75 | 45/F | European | SCAR16 | c.358+1G>A c.566A>C |
-- D189A |
Ataxia, dysarthria | Normal | Mild pursuit abnormalities | Increased tendon reflexes | Dystonia | Normal | No abnormalities reported | Cerebellar hemisphere and vermis atrophy, normal basal ganglia | Normal |
| 76 | 46/F | Unconfirmed | Unconfirmed | Unconfirmed | Not reported | Memory impairment | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | Dysarthria | Not reported | Not reported | ||
| AL [176] | 77 | 71/- | Dutch | SCA48 | c.731_732delGC | C244Yfs*24 | Gait ataxia | Cognitive impairment | No abnormalities reported | No abnormalities reported | Chorea | Not reported | Social withdrawal | Not reported | Not reported |
| 78 | 66/- | SCA48 | c.731_732delGC | C244Yfs*24 | Gait/limb ataxia, dysarthria | Cognitive impairment | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Cerebellar atrophy | Not reported | ||
| 79 | 72/- | SCA48 | c.731_732delGC | C244Yfs*24 | Gait ataxia, dysarthria | Cognitive impairment, memory defects | Saccadic pursuit | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Cerebellar atrophy, generalized atrophy | Not reported | ||
| 80 | 69/- | SCA48 | c.731_732delGC | C244Yfs*24 | Gait ataxia, dysarthria | Cognitive impairment, memory defects, apraxia | Saccadic pursuit | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Cerebellar atrophy | Not reported | ||
| 81 | 65/- | SCA48 | c.731_732delGC | C244Yfs*24 | No ataxia | Cognitive impairment, memory defects, impulsivity | Saccadic pursuit, gaze palsy | No abnormalities reported | Parkinsonism | Not reported | Dysarthria | Cerebellar atrophy, generalized atrophy | Not reported | ||
| 82 | 67/- | SCA48 | c.731_732delGC | C244Yfs*24 | Gait/limb ataxia, dysarthria | Cognitive impairment, executive dysfunction, apraxia | Normal | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Generalized atrophy | Not reported | ||
| 83 | 61/- | SCA48 | c.731_732delGC | C244Yfs*24 | Gait ataxia, dysarthria | Cognitive impairment, memory defects | Gaze palsy | No abnormalities reported | Chorea, parkinsonism | Not reported | No abnormalities reported | Cerebellar atrophy | Not reported | ||
| 84 | 50/- | SCA48 | c.731_732delGC | C244Yfs*24 | Gait ataxia, dysarthria | Cognitive impairment, aggressiveness, childish behavior | Gaze palsy | No abnormalities reported | Chorea | Not reported | No abnormalities reported | Cerebellar atrophy | Not reported | ||
| 85 | 67/- | SCA48 | c.731_732delGC | C244Yfs*24 | Gait/limb ataxia, dysarthria | Cognitive impairment, executive dysfunction, impulsivity, memory defects | Saccadic pursuit, gaze palsy | No abnormalities reported | Chorea, parkinsonism | Not reported | No abnormalities reported | Generalized atrophy | Not reported | ||
| 86 | -- | Unconfirmed | Unconfirmed | Unconfirmed | Motor abnormalities | Cognitive abnormalities | Not reported | ||||||||
| 87 | |||||||||||||||
| 88 | |||||||||||||||
| 89 | |||||||||||||||
| 90 | |||||||||||||||
| 91 | |||||||||||||||
| AM [149] | 92 | 29/M | Taiwanese | SCAR16 | c.433A>C C.721C>T |
K145Q R241W |
Gait ataxia, dysarthria | Normal | Cogwheel pursuit | No abnormalities reported | No abnormalities reported | Normal | No abnormalities reported | Cerebellar atrophy | Not reported |
| AN [149] | 93 | 22/M | Taiwanese | SCAR16 | C.433A>C C.694T>G |
K145Q C232G |
Gait ataxia, dysarthria | Mild cognitive impairment | Cogwheel pursuit | No abnormalities reported | No abnormalities reported | Normal | No abnormalities reported | Cerebellar atrophy | Normal |
| 94 | 39/M | SCAR16 | C.433A>C C.694T>G |
K145Q C232G |
Gait unsteadiness, dysarthria | No abnormalities reported | No abnormalities reported | No abnormalities reported | No abnormalities reported | Normal | No abnormalities reported | Cerebellar atrophy | Not reported | ||
| 95 | -/M | Unconfirmed | Unconfirmed | Unconfirmed | Gait difficulties | No abnormalities reported | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported | ||
| AO [150] | 96 | 44/M | European | SCA48 | c.338 C>A | A113D | Ataxia | Normal | Saccadic pursuit | No abnormalities reported | Myoclonus | Not reported | Weight loss | Not reported | Not reported |
| 97 | 40/F | SCA48 | c.338 C>A | A113D | Ataxia | Cognitive impairment | Not reported | Pyramidal features | Chorea | Not reported | Lower limb wasting | Not reported | Not reported | ||
| AP [150] | 98 | 30/M | European | SCA48 | c.728C>T | P243L | Ataxia | Normal | Nystagmus, saccadic pursuit | No abnormalities reported | No abnormalities reported | Not reported | Lower limb wasting | Not reported | Not reported |
| AQ [150] | 99 | 27/F | European | SCA48 | c.787-1G>C | Splice acceptor | Ataxia, dysarthria | Cognitive impairment, apathy | No abnormalities reported | Babinski sign | Myoclonus | Not reported | No abnormalities reported | Not reported | Not reported |
| AR [150] | 100 | 51/F | European | SCA48 | c.194A>G | N65S | Ataxia | Normal | Nystagmus, ophthalmoplegia | No abnormalities reported | No abnormalities reported | Not reported | Hearing loss | Not reported | Not reported |
| 101 | 55/F | SCA48 | c.194A>G | N65S | Ataxia | Cognitive impairment | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported | ||
| 102 | 30/F | SCA48 | c.194A>G | N65S | Ataxia | Normal | Nystagmus, saccadic pursuit | Babinski sign | Dystonia, retrocollis | Not reported | Scoliosis | Not reported | Not reported | ||
| AS [150] | 103 | 29/F | European | SCA48 | c.596G>T* c.635A>G* |
C199F D212G |
Ataxia, dysarthria | Cognitive impairment | Nystagmus, saccadic pursuit | Pyramidal features, Babinski sign | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported |
| 104 | 23/F | SCA48 | c.596G>T* c.635A>G* |
C199F D212G |
Ataxia, dysarthria | Cognitive impairment | Nystagmus, saccadic pursuit | Pyramidal features, Babinski sign | No abnormalities reported | Not reported | Decreased vibration sense | Not reported | Not reported | ||
| 105 | 29/M | SCA48 | c.596G>T* c.635A>G* |
C199F D212G |
Ataxia | Normal | Saccadic pursuit | No abnormalities reported | Tremor | Not reported | No abnormalities reported | Not reported | Not reported | ||
| AT [150] | 106 | 60/F | European | SCA48 | c.136G>C | A46P | Ataxia | Normal | Nystagmus | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported |
| 107 | 38/F | SCA48 | c.136G>C | A46P | Ataxia, dysarthria | Cognitive impairment | Saccadic pursuit | No abnormalities reported | Hypokinesia, facial chorea | Not reported | Scoliosis | Cerebellar, cortical, and pons atrophy | Not reported | ||
| 108 | 47/F | SCA48 | c.136G>C | A46P | Ataxia, dysarthria | Cognitive impairment, aggressive | No abnormalities reported | No abnormalities reported | Chorea, facial dystonia | Not reported | No abnormalities reported | Not reported | Not reported | ||
| AU [150] | 109 | 30/F | European | SCA48 | c.460C>T | R154C | Ataxia | Cognitive impairment, frontal syndrome, pseudobulbar affect | Saccadic pursuit | No abnormalities reported | Hypokinesia | Not reported | No abnormalities reported | Not reported | Not reported |
| AV [150] | 110 | 30/M | European | SCA48 | c.358+2T>C | Splice donor | Ataxia, spastic gait | Cognitive impairment, frontal syndrome | No abnormalities reported | No abnormalities reported | Chorea | Not reported | No abnormalities reported | Not reported | Not reported |
| AW [150] | 111 | 52/F | European | SCA48 | c.426_428del | K143del | Ataxia | CCAS, FTD- like | Nystagmus | No abnormalities reported | Hypokinesia, myokymia | Not reported | No abnormalities reported | Not reported | Not reported |
| AX [150] | 112 | 27/F | European | SCA48 | c.194A>G | N65S | Ataxia | No abnormalities reported | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported |
| AY [150] | 113 | 53/F | European | SCA48 | c.440G>A | W147* | Ataxia, dysarthria | Cognitive impairment | No abnormalities reported | Babinski sign | Axial hypertonus | Not reported | No abnormalities reported | Not reported | Not reported |
| AZ [150] | 114 | 40/M | European | SCA48 | c.136G>C | Y49C | Ataxia, dysarthria, dysmetria | Cognitive impairment, FTD-like, apraxia | Saccadic pursuit, nystagmus | No abnormalities reported | Dystonia | Not reported | Weight loss, lower limb wasting | Cerebellar atrophy | Not reported |
| 115 | 51/F | SCA48 | c.136G>C | Y49C | Ataxia, dysarthria | CCAS, pseudobulbar affect | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | Dysphonia | Not reported | Not reported | ||
| 116 | 38/F | SCA48 | c.136G>C | Y49C | Ataxia, dysarthria | Cognitive impairment, frontal syndrome | Diplopia, ptosis | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Cerebellar atrophy | Not reported | ||
| BA [150] | 117 | 41/F | European | SCA48 | c.194A>G | N65S | Ataxia, dysarthria | Cognitive impairment, frontal syndrome | Oculomotor apraxia, slow saccades | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported |
| BB [150] | 118 | -/F | European | SCA48 | c.301G>C | G101R | Ataxia | Normal | Slow saccades | No abnormalities reported | Neck and shoulder dystonia | Not reported | Fasciculations | Not reported | Not reported |
| 119 | 25/M | SCA48 | c.301G>C | G101R | Ataxia | Normal | Diplopia, nystagmus, saccadic pursuit | Babinski sign | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported | ||
| 120 | 30/M | SCA48 | c.301G>C | G101R | Ataxia, dysarthria | Normal | Nystagmus, saccadic pursuits, ophthalmoplegia | Pyramidal features | Head tremor | Not reported | No abnormalities reported | Not reported | Not reported | ||
| 121 | 50/F | SCA48 | c.301G>C | G101R | Ataxia | Cognitive impairment, apathy | Slow saccades, saccadic pursuit | Babinski sign | Tremor, hypokinesia | Not reported | Epilepsy | Not reported | Not reported | ||
| 122 | -/F | SCA48 | c.301G>C | G101R | Ataxia | Normal | Saccadic pursuit, ophthalmoplegia | Babinski sign | No abnormalities reported | Not reported | Decreased vibration sense | Not reported | Not reported | ||
| BC [150] | 123 | -/F | European | SCA48 | c.824delT | L275Rfs*11 | Ataxia | Normal | Slow saccades, oculomotor apraxia | No abnormalities reported | Tremor | Not reported | No abnormalities reported | Not reported | Not reported |
| BD [150] | 124 | 40/F | European | SCA48 | c.235G>C | A79P | Ataxia, dysarthria | Cognitive impairment | No abnormalities reported | Babinski sign | No abnormalities reported | Not reported | Epilepsy | Not reported | Not reported |
| BE [150] | 125 | 33/F | European | SCA48 | c.728C>T | P243L | Ataxia, dysarthria | Normal | Nystagmus | Pyramidal features | Facial dystonia | Not reported | No abnormalities reported | Not reported | Not reported |
| BF [150] | 126 | 50/M | European | SCA48 | c.665G>A | R222K | Ataxia, dysarthria | Normal | Slow saccades, saccadic pursuit | Babinski sign | Tremor | Not reported | Orthostatic hypotension | Not reported | Not reported |
| BG [150] | 127 | 48/F | European | SCA48 | c.849C>G | N283K | Ataxia, dysarthria | Normal | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported |
| BH [150] | 128 | 65/F | European | SCA48 | c.544C>T | R182* | Ataxia | CCAS, frontal syndrome | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | CJD-like | Not reported | Not reported |
| BI [150] | 129 | 65/F | European | SCA48 | c.221A>C | Q74P | Ataxia, dysarthria | Cognitive impairment | Oculomotor apraxia | No abnormalities reported | No abnormalities reported | Not reported | Facial dysmorphism | Not reported | Not reported |
| BJ [150] | 130 | 44/M | European | SCA48 | c.206G>A | C69Y | Ataxia | Cognitive impairment | Saccadic pursuit | No abnormalities reported | No abnormalities reported | Not reported | Cataract | Not reported | Not reported |
| BK [150] | 131 | 67/F | European | SCA48 | c.794G>A | G265D | Ataxia, dysarthria | Cognitive impairment | Slow saccades, saccadic pursuit | No abnormalities reported | FXTAS-like | Not reported | Cataract | Not reported | Not reported |
| BL [150] | 132 | 60/M | European | SCA48 | c.170C>T | P57L | Ataxia | Cognitive impairment, impulsive, aggressive | Diplopia, ptosis | Babinski sign | Tremor, rigidity | Not reported | No abnormalities reported | Not reported | Not reported |
| 133 | 40/F | SCA48 | c.170C>T | P57L | Ataxia, dysarthria | Normal | Diplopia, ptosis | No abnormalities reported | Hypokinesia | Not reported | No abnormalities reported | Not reported | Not reported | ||
| 134 | 40/F | SCA48 | c.170C>T | P57L | Ataxia, dysarthria | Normal | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported | ||
| BM [150] | 135 | 27/F | European | SCA48 | c.426_428del | K143del | Ataxia | Normal | Nystagmus, saccadic pursuit | No abnormalities reported | Cervical dystonia | Not reported | No abnormalities reported | Not reported | Not reported |
| 136 | M | SCA48 | c.426_428del | K143del | No ataxia | Normal | Saccadic pursuit | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported | ||
| BN [150] | 137 | 51/W | European | SCA48 | c.146A>G | Y49C | Ataxia, dysarthria | Cognitive impairment, frontal syndrome | No abnormalities reported | Increased tendon reflexes | Chorea, dystonia, rigidity | Not reported | No abnormalities reported | Not reported | Not reported |
| 138 | 37/M | SCA48 | c.146A>G | Y49C | Ataxia, dysarthria | Cognitive impairment, behavior change | Slow saccades, saccadic pursuit, oculomotor apraxia | No abnormalities reported | Chorea, myoclonus | Not reported | No abnormalities reported | Not reported | Not reported | ||
| 139 | 37/F | SCA48 | c.146A>G | Y49C | Ataxia, dysarthria | Cognitive impairment, behavior change | Slow saccades, saccadic pursuit, oculomotor apraxia | No abnormalities reported | Chorea, myoclonus, rigidity | Not reported | No abnormalities reported | Not reported | Not reported | ||
| 140 | -/W | SCA48 | c.146A>G | Y49C | Ataxia | No abnormalities reported | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported | ||
| BO [150] | 141 | 74/W | European | SCA48 | c.206G>A | C69Y | Ataxia | Cognitive impairment | Nystagmus, slow saccades, ophthalmoplegia | No abnormalities reported | Tremor, hypokinesia | Not reported | No abnormalities reported | Not reported | Not reported |
| BP [150] | 142 | -/W | European | SCA48 | c.502C>T | L168F | Ataxia | Mild cognitive impairment | No abnormalities reported | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported |
| BQ [150] | 143 | 20/M | European | SCAR16 | c.359C>T c.632T>C |
A120V M211T |
Ataxia | Cognitive impairment | Nystagmus, saccadic pursuit | No abnormalities reported | No abnormalities reported | Not reported | No abnormalities reported | Not reported | Not reported |
| 144 | 21/F | SCAR16 | c.359C>T c.632T>C |
A120V M211T |
Ataxia | Cognitive impairment | No abnormalities reported | Babinski sign | Voice and neck dystonia | Not reported | No abnormalities reported | Not reported | Not reported | ||
| BR [150] | 145 | 17/M | European | SCAR16 | c.691C>G c.691C>G |
L231V L231V |
Ataxia | Cognitive impairment | No abnormalities reported | Babinski sign | No abnormalities reported | Hypogonadism | Dysphagia, UI | Not reported | Not reported |
Grey boxes indicate patients with disease symptoms who did not have genetic testing. These patients without a confirmed genotype were not included in patient numbers indicated within the manuscript text. Where only one STUB1 mutation/CHIP mutation is indicated, the patient is heterozygous with one mutant allele and one wild-type allele. AAO: age at disease onset; CCAS: cerebellar cognitive affective syndrome; CJD: Creutzfeldt-Jakob disease; FTD: frontotemporal dementia; FXTAS: fragile X-associated tremor/ataxia syndrome; NCV: nerve conduction velocity; SCA48: spinocerebellar ataxia autosomal dominant type 48; SCAR16: spinocerebellar ataxia autosomal recessive type 16; T1D: type 1 diabetes; UI: urinary incontinence; UTR: untranslated region; WES: whole exome sequencing. *Variants in cis.
Twenty-two of the patients with autosomal recessive forms of STUB1-associated disease carried homozygous mutations and 20 carried compound heterozygous mutations (Fig. 7). Patients with compound heterozygous mutations had either two different missense mutations or one missense and one nonsense or frameshift mutation. In total, 37 different autosomal recessive associated STUB1 mutations have been identified, 4 map to an intronic region, 9 affect the TPR domain, 10 affect the coiled coil domain, 13 affect the U-box domain, and 1 was found within the 3’ UTR region. In silico prediction tools have mostly supported the STUB1 variants associated with autosomal recessive disease to date as being pathogenic (Fig. 7). MutationTaster [152] predicted all identified mutations as disease-causing while SIFT [153] and PolyPhen2 [154] predicted that all but 10 and 2 of the reported mutations would be disease causing, respectively. Most SCAR16 patients tested have been reported to have reduced levels of steady-state CHIP protein [139, 147, 155]. Multiple in vitro studies have been conducted to examine 13 different autosomal recessive associated mutations: E28K, N65S, A79T, A79D, L123V, N130I, K145Q, W147C, L165F, M211I, S236T, M240T, and T246M. Multiple mutants were shown to have reduced cellular expression following transfection [156]. These mutations have also been shown to reduce CHIP’s ability to interact with Hsc70 to varying degrees in vitro, with TPR domain mutants showing the most significant defects [156]. U-box mutations have been found to have increased binding capacity, but not affinity, for Hsp70 [157]. All tested mutations, other than N65S, showed decreased thermal stability and increased propensity for oligomerization [155, 156, 158]. U-box mutations were significantly more likely to form dimers than TPR or coiled- coil mutants, which had a higher propensity to form higher order oligomers [155, 156, 158]. In addition, U-box mutants, as well as the N65S TPR mutation, were all shown to retain their ability to recruit E2s but lacked E3 ligase activity [156], while other TPR and coiled-coil mutants retained E3 ligase activity [157]. CHIP mutants that had more E3 ligase activity and normal tertiary structure were associated with worsened disease outcomes than CHIP mutations lacking E3 ligase activity, indicating that the inhibition of mutant CHIP activity may be beneficial to STUB1-associated disease patients [157]. Interestingly, an earlier report demonstrated that within SCAR16 patients, U-box domain mutations were associated with a higher incidence of cognitive impairment than TPR or coiled-coil domain mutations [157]. Since that report, additional SCAR16 cases have been published and the higher incidence of cognitive impairment with U-box domain mutations is still observed: 62.5% (10/16) of TPR mutated alleles, 59% (13/22) of coiled-coiled mutated alleles, and 92% (22/24) of U-box mutant alleles (P = 0.024, Fisher’s exact test).
To date, the most intensively studied CHIP mutation associated with SCAR16 is T246M. This missense mutation occurs in CHIP’s U-box domain and has been identified in two homozygous patients with GHS [57]. T246M-CHIP has been shown in vitro to possess no E3 ubiquitin ligase activity and to have an increased propensity for oligomerization, but it maintains normal chaperone interaction. Rats and mice expressing homozygous T246M-CHIP exhibited Purkinje cell degeneration, decreased survival, cognitive defects, gonadal atrophy, and reduced body weight and brain mass [155]. Mutant mice also exhibited age-dependent progressive motor dysfunction. In contrast, Stub1 null mice display severe motor impairment at the time of weaning with little progression over time. Thus, the progression observed with T246M-CHIP mice more accurately models symptoms observed in STUB1-associated disease patients and also allows for the study of earlier or pre-clinical symptoms. Tissue lysates from the T246M-CHIP mouse cerebellum, whole brain, and testes, as well as embryonic fibroblasts, all displayed decreased CHIP protein levels compared to controls. Interestingly, proteomics analysis of T246M-CHIP rat cerebella and whole brain, compared to controls, showed increased levels of tau and decreased levels of both α-syn and PINK1 [155]. While whole-brain lysates from Stub1 null mice also showed increased levels of tau, there was no difference in α-syn protein levels [87] and increased PINK1 levels [56], compared to controls. The differences between CHIP mutant and null animal phenotypes support the observation that STUB1-associated disease is not solely the result of the loss of E3 ligase function. Further study is still required to confirm that all of the STUB1-disease associated mutations are indeed disease-causing and not just variants of unknown significance, but the rodent model recapitulation of STUB1-associated disease symptoms, as well as in vitro studies and in silico predictions, provide convincing evidence that CHIP mutations can cause multisystemic symptoms including neurodegeneration and hypogonadism.
The specific molecular pathways affected by CHIP mutations in SCAR16 remain an area open for investigation. SCAR16 patients have been shown to exhibit cyclic AMP (cAMP) signaling dysregulation [159]. cAMP is an ATP derivative involved in intracellular signaling [160, 161]. cAMP signaling involves activation of various protein kinases, including protein kinase A (PKA), and can function to regulate ion channels [162, 163]. Dysregulation of cAMP-PKA signaling has been observed in many other neurodegenerative diseases, including PD [164], AD [165], HD [166], and SCA1 [167-169]. CHIP has been shown to attenuate cAMP-PKA signaling through Hsp70-mediated interaction with the catalytic subunit PKAc, leading to its proteasomal degradation [159, 170]. Further research is necessary to determine how mutant CHIP may lead to cAMP-PKA signaling dysregulation observed within SCAR16 patients. Understanding how this pathway could contribute to disease pathogenesis may provide insights into the development of treatments for SCAR16 as well as into the role of CHIP in other neurodegenerative diseases.
4.3. Autosomal Dominant STUB1-associated Disease
Spinocerebellar ataxia 48 (SCA48) is an autosomal dominant form of cerebellar ataxia caused by a heterozygous STUB1 mutation. At the time of this review, 90 patients (26 male, 55 female, and 9 unspecified) from 43 kindreds with autosomal dominant STUB-associated mutations have been described in the literature [150, 171-176]. Interestingly, when sex is specified, 67.9% of identified SCA48 patients are female, while a difference in sex-based prevalence of SCAR16 is not apparent. Sex-based differences in disease prevalence, onset, and outcome have been observed in a number of neurodegenerative diseases [177, 178]. It has been reported that cerebellar CHIP protein levels are lower in females than males, which may play a role in the observed sex-based difference in SCA48 prevalence [150].
Out of the 43 reported kindreds, 34 had a positive family history of disease. An absence of family history may be explained by spontaneous mutations, incomplete penetrance, or later onset of disease with some family members with a STUB1 mutation not living long enough to display symptoms. For cases in which ethnicity was indicated, all patients were of European descent. The age of symptom onset among SCA48 patients varied from age 5 to 74 years; the mean age of onset for these patients was 44.6 years, which was older than the average age at onset of SCAR16 patients. Symptom onset typically begins during mid-adulthood and includes cognitive impairment, including cerebellar cognitive affective syndrome (CCAS), and/or motor disturbances. Patients presenting with primarily cognitive impairment may not display motor features for years after the age of initial disease onset, but most patients eventually develop both cognitive and motor impairments. All but five patients were described as having ataxia. Two non-ataxic patients had no symptoms (Patient #41 & 136, Table 1). Two other non-ataxic patients (Patient #36 & 38, Table 1) presented with CCAS without motor dysfunction. As mentioned previously, cognitive impairment precedes movement abnormalities for many SCA48 patients, so these patients may go on to develop ataxia. The last non-ataxia patient (Patient #81, Table 1) presented with parkinsonism without ataxia. Of the patients reported, 15 out of 22 displayed hyperreflexia, 63 out of 86 presented with cognitive impairment, and 2 out of 11 displayed sexual development disturbances. Additional reported symptoms included but were not limited to anxiety, generalized tonic-clonic seizures, chorea, parkinsonism, dystonia, chorea, and urinary incontinence, among others. One patient had been diagnosed with autism spectrum disorder (Patient #71, Table 1). Among patient who had neuroimaging, all showed significant cerebellar atrophy, including an asymptomatic patient. Some patients also exhibited hypoperfusion to various brain regions including the cerebellum as well as frontal, parietal, and temporal lobes. One patient displayed severe frontal lobe atrophy and some displayed additional cortical atrophy. Additionally, T2-weighted imaging (T2WI) revealed bilateral dentate nuclei hyperintensity in 9/10 SCA48 patients [179]. Dentate nuclei hyperintensity by T2WI is not typical of other SCAs [180], and this hyperintensity combined with atrophy of the postero-lateral portions of the cerebellar hemisphere has been termed the “crab sign” [179].
Thirty-two different autosomal dominant mutations were observed in these patients and were reported to segregate with the disease: 12 affected the TPR domain, 8 affected the coiled-coil domain, and 10 mapped to the U-box, and 2 were intronic. Twenty mutations were missense, 3 were nonsense, 8 caused frameshift mutations, and 1 was an in-frame deletion. The most common mutation reported to date is a dominant heterozygous frameshift truncation mutations located within the U-box domain (ENST00000219548: c.823_824delCT, ENSP00000219548: p.L275Dfs*16). This mutation has been identified within 3 unrelated kindreds of 3 different ethnicities. In silico prediction tools for identifying disease causing mutations were preformed using all described autosomal dominant STUB1 mutations (Fig. 7). MutationTaster predicted all identified mutations would be disease-causing while SIFT and PolyPhen2 predicted that all but 2 of the reported mutations would be disease causing. To date, no further in vitro or in vivo analysis has been performed to further investigate any of the identified autosomal dominant STUB1 mutations.
Neuropathologic examination of 5 SCA48 patients with missense mutations located within the TPR domain exhibited significant loss of cerebellar Purkinje cells with increased astrogliosis and Bergmann gliosis [150, 175]. One patient displayed mild frontal lobe atrophy. Chen et al. found ubiquitinated inclusions within the cerebellum which were negative for p62, TDP-43, and α-syn [175]. Neurofibrillary tangles were present but Braak staging was low and consistent with the age of the patient. No signs of basal ganglia abnormalities were observed in their 4 cases, despite one patient presenting with mild parkinsonism. Roux et al., 2020 reported one SCA48 case with mild neuronal loss within the SNpc [150]. This case demonstrated only infrequent pretangles and neuropil threads with tau immunostaining in the hippocampus, as well as rare p62 nuclear inclusions in the frontal cortex and hippocampus. No inclusions were seen with immunostaining for ubiquitin, TDP-43, α-syn, polyQ, or prion protein (PrP). Confocal microscopy of healthy controls showed CHIP expression to be prominent within Purkinje cells and localised within the cell bodies and proximal dendrites, with little staining in distal dendritic compartments. In contrast, CHIP localised throughout somatodendritic and distal dendritic regions of Purkinje cells in the brains of SCA48 patients. This may suggest that the mislocalisation of CHIP, not the absolute level of CHIP, is relevant to disease pathogenesis. Purkinje cells from SCA3 and SCA5 patients displayed CHIP localisation consistent with healthy controls, while SCA7 patients showed CHIP mislocalisation consistent with SCA48 patients [175], indicating that the CHIP mislocalisation is not solely due to CHIP mutations and may be attributed to other aspects of Purkinje neurodegeneration.
Neuropathological examinations of 3 related SCA48 patients with a U-box frameshift mutation displayed an almost complete loss of cerebellar Purkinje cells in all cases and Bergmann gliosis. Braak staging was low and consistent with the age of the patient [176]. Significant neuronal loss was also observed within the mesencephalon, medulla oblongata, and subthalamic nucleus. NIIs positive for ubiquitin and p62 were present within the cerebellum, SNpc, and deep pontine nucleus. p62 positive inclusions were also observed within the hippocampus. CHIP staining within neurons was observed to be diffuse throughout but not different from controls. Within one patient with atypical parkinsonism (Patient #81, Table 1), tau pathology was observed within many brain regions, including the temporal cortex, hippocampus, SNpc, locus coeruleus, cerebellar dentate nucleus, and spinal motor neurons. None of the patients displayed α-syn or TDP-43 positive inclusions. Some faint diffuse nuclear polyglutamine immunoreactivity was observed within the cerebellum of the 2 brains tested [176].
How CHIP mutations cause disease and the multitude of complex variable symptoms observed within SCAR16 and SCA48 patients still require further elucidation. Variability between patients, even those carrying the same mutation, indicates that other genetic or environmental factors may play a role in the pathophysiology of the disease. Sex-based differences in the prevalence of SCA48 but not SCAR16 may be due to differences in cerebellar CHIP expression between males and females but could also point to additional mechanisms underlying disease pathology, such as microglia function, which has been implicated in sex-based differences in the prevalence of many neurodegenerative diseases [177, 178]. In vitro and in vivo analysis of multiple SCAR16 mutations have reported that mutations result in decreased CHIP protein expression [139, 147, 155, 156]. However, differences between the T246M mouse model and Stub1 null mice suggest that the effects of CHIP mutations are the result of more than solely loss of function. Additionally, post- mortem brain tissue from SCA48 patients were absent of tau pathology [175], aside from one patient with atypical parkinsonism, while Stub1 knockout mice and C. elegans showed substantial levels of tau pathology [93], indicating that mutant forms of CHIP may still be able to eliminate tau, further suggesting the cause of the disease is more than merely CHIP loss of function. Furthermore, the association between cognitive impairment and U-box domain mutations suggests that there may be different mechanisms contributing to specific STUB1-associated disease symptoms. Interestingly, other causative mutations identified in patients with GHS affect Ring Finger Protein 216 (RNF216), which is an E3 ubiquitin ligase, Patatin Like Phospholipase Domain Containing 6 (PNPLA6), which is a phospholipase [181, 182], and OTU Deubiquitinase 4 (OTUD4), which is a DUB [135], all of which play important roles in proteostasis, like CHIP, and may point to a converging molecular pathway. Additionally, multiple genes associated with autosomal dominant cerebellar ataxias, including ataxin-1 and ataxin-3, are known interactors of CHIP (described above), which may also point to the converging pathway among autosomal dominant cerebellar ataxias.
5. THERAPEUTIC POTENTIAL OF CHIP
As discussed above, CHIP has the potential to promote the degradation of proteins associated with multiple neurodegenerative disorders, including PD, AD, ALS, HD, SBMA, SCA1 and SCA3. Thus, increasing CHIP levels or activity could present a promising treatment strategy for these diseases. To date, a limited number of compounds have been found to increase CHIP protein levels in vitro, which include trehalose, anisomycin, and YL-109. Trehalose, a disaccharide of glucose and known autophagy enhancer, has been implicated as a potential STUB1-associated disease therapeutic requiring further investigation. Treatment of SCAR16 patient fibroblasts with trehalose was shown to increase CHIP and Hsp70 protein levels, increase autophagy [183], and reduce oxidative stress [184]. It has also been shown that anisomycin treatment increases CHIP protein levels and protects cells against OGD-induced necroptosis in in vitro models of cerebellar ischemia [112]. The agent 2-(4-Hydroxy-3-methoxyphenyl)-benzothiazole (YL-109) has been shown to suppress triple-negative breast cancer cell growth in vitro and in vivo [185]. YL-109 was also shown to increase CHIP transcription through aryl hydrocarbon receptor (AhR) recruitment upstream of the STUB1 gene. YL-109 treatment of cells with knockdown of AhR or CHIP rescued proliferation [185]. While evidence to date suggests that increasing CHIP expression or activity could be beneficial for various neurodegenerative diseases, there may be undesirable effects that need to be considered. For instance, as discussed above, CHIP can promote degradation of polyQ-ataxin-1, but it can also promote degradation and decreased solubility of wild-type ataxin-1. Additionally, while CHIP may reduce α-syn aggregation, reduce Pael-R levels, and protect against LRRK2 mutations in PD models, one of the substrates of CHIP may be tyrosine hydroxylase [63], an enzyme critical in the production of dopamine, which could make non-specifically increasing CHIP expression or activity unsuitable as a therapy for PD.
For each neurodegenerative disease, taking different approaches to CHIP modulation may be necessary to optimise efficacy and limit side effects. One interesting avenue warranting investigation is the use of protein-protein interaction (PPI) modulators. Certain small molecules have been shown to stabilise specific PPIs [186], including E3 ligase-substrate interactions [187]. PPI modulators, which could stabilise the interaction between CHIP and specific aggregated, overexpressed, or mutated disease-associated proteins, could be employed to enhance the removal of specific proteins without broadly increasing CHIP-mediated protein degradation. Influencing PTMs of CHIP could also provide another interesting therapeutic treatment avenue that may reduce unwanted effects. For instance, increasing CHIP monoubiquitylation via UBE2W increases degradation of polyQ-ataxin-3, and could potentially be useful for SCA3 treatment. To date, PTMs of CHIP are not well understood and warrant further research, as they may provide insight into improving the therapeutic benefit or target specificity of CHIP. High-throughput mutagenesis of E3 ubiquitin ligases has been used to determine activity enhancing mutations, including mutations that enhance U-box domain interaction with specific E2s, allowing for the enhancement of degradation of certain proteins and not others [188]. High-throughput mutagenesis could also be used to identify mutations that could enhance specific interactions between CHIP and disease-associated proteins.
The generation of chimeric proteins utilising either the TPR or U-box domain CHIP could present another method for developing the therapeutic potential of CHIP, potentially increasing disease-associated protein degradation while decreasing undesirable interactions. As described above, CHIP is able to promote proteasomal degradation of mutant SOD1 through an indirect interaction mediated by Hsp70 [104]. To improve on the therapeutic potential, a Dorfin-CHIP chimeric protein [108] described above, was generated utilised the E3 activity of the U-box domain of CHIP linked to the substrate-binding domain of Dorfin, which is able to interact directly with mutant SOD1. This Dorfin-CHIP chimeric protein was shown to increase mutant SOD1 degradation. The TPR domain of CHIP is able to interact with many different substrates, including polyQ expanded proteins, and may also be useful for the generation of chimeric protein therapeutics. The utility of a chimeric protein containing either the TPR or U-box domain would likely depend on the specific disease and protein being targeted.
For the treatment of STUB1-associated diseases, it is currently unknown how CHIP modulation may impact disease symptoms and progression. Increasing expression of CHIP via trehalose treatment in SCAR16 patient fibroblasts showed benefits in vitro [184], but multiple lines of evidence suggest that STUB1-associated disease may be due to more than just loss of CHIP function. Several SCAR16 mutations have been shown to have increased propensity to form higher-order oligomers in vitro, so increasing CHIP expression may exacerbate disease in these patients. Further investigation is required to determine whether upregulating or downregulating CHIP within STUB1-associated diseases may provide therapeutic benefit for patients. As with other genetic diseases, major therapeutic breakthroughs for STUB1-associated diseases will likely require precision or personalised medicine in which the intervention targets the individual patient’s CHIP defect. And, as with all neurodegenerative disorders, the timing of the intervention during the disease course will be an important consideration.
CONCLUSION
The co-chaperone CHIP plays a major role in proteostasis and hence is emerging as an important regulator of neurodegeneration. Direct evidence for its importance in neurodegenerative disease comes from the identification of disease-associated STUB1 mutations in individuals with both autosomal recessive and autosomal dominant forms of cerebellar ataxia. Multiple lines of evidence also demonstrate that CHIP can reduce protein aggregation in neurodegenerative diseases characterised as proteinopathies, including PD, AD, HD, SBMA, SCA1, SCA3 and ALS, and could be a promising therapeutic target for the treatment of these diseases. Future research will need to elucidate how CHIP levels, CHIP functions, and CHIP interactions and networks are affected in these diseases. Understanding the interplay between CHIP and other contributors to disease pathogenesis, such as genetic and environmental factors, will be needed to explain how CHIP dysfunction may manifest as different neurodegenerative diseases in different individuals. This will also be important in informing disease-specific treatment strategies. Such insights will assist in deciphering how CHIP can most effectively be modified in vivo to enhance its specific degradation of disease-associated proteins.
ACKNOWLEDGEMENTS
Figures 1 to 6 were created using BioRender.com.
LIST OF ABBREVIATIONS
- Aβ
Amyloid Beta
- AD
Alzheimer’s Disease
- AhR
Aryl Hydrocarbon Receptor
- Akt
Protein Kinase B
- ALP
Autophagy Lysosome Pathway
- ALS
Amyotrophic Lateral Sclerosis
- AMPK
Adenosine Monophosphate-Activated Protein Kinase
- APP
Amyloid Precursor Protein
- AR
Androgen Receptor
- AURKA
Aurora Kinase A
- BACE1
β-Secretase 1
- BAG
BCL-2 Associated Athanogene
- CAG
Cytosine-Adenosine-Guanine
- cAMP
Cyclic AMP
- CCAS
Cerebellar Cognitive Affective Syndrome
- CDK5
Cyclin Dependent Kinase 5
- CHIP
C-Terminus of Hsp70 Interacting Protein
- CMA
Chaperone Mediated Autophagy
- DRPLA
Dentatorubral-Pallidoluysian Atrophy
- DTI
Diffusion Tensor Imaging
- DUB
Deubiquitinase
- EndoG
Endonuclease G
- ERAD
Endoplasmic Reticulum Associated Protein Degradation
- FUS
Fused in Sarcoma
- GHS
Gordon Holmes Syndrome
- HD
Huntington’s Disease
- HDAC6
Histone Deacetylase 6
- HIF1
Hypoxia-Inducible Factor 1
- HSF1
Heat Shock Factor-1
- Hsp
Heat Shock Protein
- HSPA8
Heat Shock Protein 8
- HSPB8
Heat Shock Protein Beta-8
- HSPBP1
Hsp70 Binding Protein 1
- HTT
Huntingtin
- LAMP2A
Lysosomal-Associated Membrane Protein 2
- LD
Lafora Disease
- LRRK2
Leucine Rich Repeat Kinase 2
- MARK2
Microtubule Affinity Regulating Kinase 2
- NEK1
NIMA-Related Kinase 1
- NII
Neuronal Intranuclear Inclusion
- OTUD4
OTU Deubiquitinase 4
- Pael-R
Probable G-protein Coupled Receptor 37
- PD
Parkinson’s Disease
- PINK1
PTEN-Induced Protein Kinase 1
- PKA
Protein Kinase A
- PNPLA6
Patatin Like Phospholipase Domain Containing 6
- PolyQ
Polyglutamine
- PPI
Protein-Protein Interaction
- PrP
Prion Protein
- PTM
Post-Translational Modification
- RIPK1
Receptor-Interacting Protein Kinase 1
- RIPK3
Receptor-Interacting Protein Kinase 3
- RNF216
Ring Finger Protein 216
- SBMA
Spinal-Bulbar Muscular Atrophy
- SCA
Spinocerebellar Ataxia
- SCAR16
Spinocerebellar Ataxia Autosomal Recessive 16
- SCA1
Spinocerebellar Ataxia Type 1
- SCA3
Spinocerebellar Ataxia Type 3
- SCA48
Spinocerebellar Ataxia Type 48
- SNpc
Substantia Nigra Pars Compacta
- SOD1
Superoxide Dismutase 1
- STUB1
STIP1 Homology and Ubox Containing Protein 1
- tAIF
Truncated Apoptosis Inducing Factor
- TDP43
TAR DNA Binding Protein
- TFEB
Transcription Factor EB
- TPR
Tetratricopeptide
- T2WI
T2-Weighted Imaging
- UBE2W
Ubiquitin-Conjugating Enzyme E2 W
- UBQLN2
Ubiquilin 2
- UPS
Ubiquitin Proteasome System
- WES
Whole Exome Sequencing
- YL-109
2-(4-Hydroxy-3-methoxyphenyl)-benzothiazole
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no conflicts of interest, financial or otherwise.
References
- 1.Ferri C.P., Prince M., Brayne C., Brodaty H., Fratiglioni L., Ganguli M., Hall K., Hasegawa K., Hendrie H., Huang Y., Jorm A., Mathers C., Menezes P.R., Rimmer E., Scazufca M. Alzheimer’s Disease International. Global Prevalence of Dementia: A Delphi Consensus Study. Lancet Lond. Engl. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wimo A., Jönsson L., Bond J., Prince M., Winblad B. Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9(1):1–11.e3. doi: 10.1016/j.jalz.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Lindberg I., Shorter J., Wiseman R.L., Chiti F., Dickey C.A., McLean P.J. Chaperones in Neurodegeneration. J. Neurosci. 2015;35(41):13853–13859. doi: 10.1523/JNEUROSCI.2600-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mayer M.P., Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005;62(6):670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson T.M., Dawson V.L. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302(5646):819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 6.Guo D., Ying Z., Wang H., Chen D., Gao F., Ren H., Wang G. Regulation of autophagic flux by CHIP. Neurosci. Bull. 2015;31(4):469–479. doi: 10.1007/s12264-015-1543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballinger C.A., Connell P., Wu Y., Hu Z., Thompson L.J., Yin L.Y., Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol. Cell. Biol. 1999;19(6):4535–4545. doi: 10.1128/MCB.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin Y., Klucken J., Patterson C., Hyman B.T., McLean P.J. The co-chaperone carboxyl terminus of Hsp70-interacting protein (CHIP) mediates alpha-synuclein degradation decisions between proteasomal and lysosomal pathways. J. Biol. Chem. 2005;280(25):23727–23734. doi: 10.1074/jbc.M503326200. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M., Windheim M., Roe S.M., Peggie M., Cohen P., Prodromou C., Pearl L.H. Chaperoned ubiquitylation--crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol. Cell. 2005;20(4):525–538. doi: 10.1016/j.molcel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Nikolay R., Wiederkehr T., Rist W., Kramer G., Mayer M.P., Bukau B. Dimerization of the human E3 ligase CHIP via a coiled-coil domain is essential for its activity. J. Biol. Chem. 2004;279(4):2673–2678. doi: 10.1074/jbc.M311112200. [DOI] [PubMed] [Google Scholar]
- 11.Qian S-B., Waldron L., Choudhary N., Klevit R.E., Chazin W.J., Patterson C. Engineering a ubiquitin ligase reveals conformational flexibility required for ubiquitin transfer. J. Biol. Chem. 2009;284(39):26797–26802. doi: 10.1074/jbc.M109.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye Z., Needham P.G., Estabrooks S.K., Whitaker S.K., Garcia B.L., Misra S., Brodsky J.L., Camacho C.J. Symmetry breaking during homodimeric assembly activates an E3 ubiquitin ligase. Sci. Rep. 2017;7(1):1789. doi: 10.1038/s41598-017-01880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z., Devlin K.I., Ford M.G., Nix J.C., Qin J., Misra S. Structure and interactions of the helical and U-box domains of CHIP, the C terminus of HSP70 interacting protein. Biochemistry. 2006;45(15):4749–4759. doi: 10.1021/bi0601508. [DOI] [PubMed] [Google Scholar]
- 14.Akutsu M., Dikic I., Bremm A. Ubiquitin chain diversity at a glance. J. Cell Sci. 2016;129(5):875–880. doi: 10.1242/jcs.183954. [DOI] [PubMed] [Google Scholar]
- 15.Meyer H-J., Rape M. Enhanced protein degradation by branched ubiquitin chains. Cell. 2014;157(4):910–921. doi: 10.1016/j.cell.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clague M.J., Urbé S. Ubiquitin: same molecule, different degradation pathways. Cell. 2010;143(5):682–685. doi: 10.1016/j.cell.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Li W., Ye Y. Polyubiquitin chains: functions, structures, and mechanisms. Cell. Mol. Life Sci. 2008;65(15):2397–2406. doi: 10.1007/s00018-008-8090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu P., Duong D.M., Seyfried N.T., Cheng D., Xie Y., Robert J., Rush J., Hochstrasser M., Finley D., Peng J. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137(1):133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian S-B., McDonough H., Boellmann F., Cyr D.M., Patterson C. CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature. 2006;440(7083):551–555. doi: 10.1038/nature04600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soss S.E., Yue Y., Dhe-Paganon S., Chazin W.J. E2 conjugating enzyme selectivity and requirements for function of the E3 ubiquitin ligase CHIP. J. Biol. Chem. 2011;286(24):21277–21286. doi: 10.1074/jbc.M111.224006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Windheim M., Lang C., Peggie M., Plater L.A., Cohen P. Molecular mechanisms involved in the regulation of cytokine production by muramyl dipeptide. Biochem. J. 2007;404(2):179–190. doi: 10.1042/BJ20061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blessing N.A., Brockman A.L., Chadee D.N. The E3 ligase CHIP mediates ubiquitination and degradation of mixed-lineage kinase 3. Mol. Cell. Biol. 2014;34(16):3132–3143. doi: 10.1128/MCB.00296-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S., Li Y., Hu Y-H., Song R., Gao Y., Liu H-Y., Shu H-B., Liu Y. STUB1 is essential for T-cell activation by ubiquitinating CARMA1. Eur. J. Immunol. 2013;43(4):1034–1041. doi: 10.1002/eji.201242554. [DOI] [PubMed] [Google Scholar]
- 24.Page R.C., Pruneda J.N., Amick J., Klevit R.E., Misra S. Structural insights into the conformation and oligomerization of E2~ubiquitin conjugates. Biochemistry. 2012;51(20):4175–4187. doi: 10.1021/bi300058m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Z., Kohli E., Devlin K.I., Bold M., Nix J.C., Misra S. Interactions between the quality control ubiquitin ligase CHIP and ubiquitin conjugating enzymes. BMC Struct. Biol. 2008;8(1):26. doi: 10.1186/1472-6807-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrucelli L., Dickson D., Kehoe K., Taylor J., Snyder H., Grover A., De Lucia M., McGowan E., Lewis J., Prihar G., Kim J., Dillmann W.H., Browne S.E., Hall A., Voellmy R., Tsuboi Y., Dawson T.M., Wolozin B., Hardy J., Hutton M. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 2004;13(7):703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 27.Adams J. The proteasome: structure, function, and role in the cell. Cancer Treat. Rev. 2003;29(Suppl. 1):3–9. doi: 10.1016/S0305-7372(03)00081-1. [DOI] [PubMed] [Google Scholar]
- 28.Ohtake F., Tsuchiya H., Saeki Y., Tanaka K. K63 ubiquitylation triggers proteasomal degradation by seeding branched ubiquitin chains. Proc. Natl. Acad. Sci. USA. 2018;115(7):E1401–E1408. doi: 10.1073/pnas.1716673115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geisler S., Holmström K.M., Skujat D., Fiesel F.C., Rothfuss O.C., Kahle P.J., Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010;12(2):119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 30.Swatek K.N., Komander D. Ubiquitin modifications. Cell Res. 2016;26(4):399–422. doi: 10.1038/cr.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sardiello M., Palmieri M., di Ronza A., Medina D.L., Valenza M., Gennarino V.A., Di Malta C., Donaudy F., Embrione V., Polishchuk R.S., Banfi S., Parenti G., Cattaneo E., Ballabio A. A gene network regulating lysosomal biogenesis and function. Science. 2009;325(5939):473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 32.Settembre C., Di Malta C., Polito V.A., Garcia Arencibia M., Vetrini F., Erdin S., Erdin S.U., Huynh T., Medina D., Colella P., Sardiello M., Rubinsztein D.C., Ballabio A. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332(6036):1429–1433. doi: 10.1126/science.1204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sha Y., Rao L., Settembre C., Ballabio A., Eissa N.T. STUB1 regulates TFEB-induced autophagy-lysosome pathway. EMBO J. 2017;36(17):2544–2552. doi: 10.15252/embj.201796699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreira J.V., Fôfo H., Bejarano E., Bento C.F., Ramalho J.S., Girão H., Pereira P. STUB1/CHIP is required for HIF1A degradation by chaperone-mediated autophagy. Autophagy. 2013;9(9):1349–1366. doi: 10.4161/auto.25190. [DOI] [PubMed] [Google Scholar]
- 35.Korolchuk V.I., Menzies F.M., Rubinsztein D.C. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2010;584(7):1393–1398. doi: 10.1016/j.febslet.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira J.V., Soares A.R., Ramalho J.S., Pereira P., Girao H. K63 linked ubiquitin chain formation is a signal for HIF1A degradation by chaperone-mediated autophagy. Sci. Rep. 2015;5(1):10210. doi: 10.1038/srep10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lizama B.N., Palubinsky A.M., Raveendran V.A., Moore A.M., Federspiel J.D., Codreanu S.G., Liebler D.C., McLaughlin B. Neuronal preconditioning requires the mitophagic activity of C-terminus of HSC70-interacting protein. J. Neurosci. 2018;38(31):6825–6840. doi: 10.1523/JNEUROSCI.0699-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravi S., Parry T.L., Willis M.S., Lockyer P., Patterson C., Bain J.R., Stevens R.D., Ilkayeva O.R., Newgard C.B., Schisler J.C. Adverse effects of fenofibrate in mice deficient in the protein quality control regulator, CHIP. J. Cardiovasc. Dev. Dis. 2018;5(3):E43. doi: 10.3390/jcdd5030043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willis M.S., Min J-N., Wang S., McDonough H., Lockyer P., Wadosky K.M., Patterson C. Carboxyl terminus of Hsp70-interacting protein (CHIP) is required to modulate cardiac hypertrophy and attenuate autophagy during exercise. Cell Biochem. Funct. 2013;31(8):724–735. doi: 10.1002/cbf.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McDonough H., Patterson C. CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones. 2003;8(4):303–308. doi: 10.1379/1466-1268(2003)008<0303:CALBTC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ott C., König J., Höhn A., Jung T., Grune T. Macroautophagy is impaired in old murine brain tissue as well as in senescent human fibroblasts. Redox Biol. 2016;10:266–273. doi: 10.1016/j.redox.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saha S., Ash P.E.A., Gowda V., Liu L., Shirihai O., Wolozin B. Mutations in LRRK2 potentiate age-related impairment of autophagic flux. Mol. Neurodegener. 2015;10:26. doi: 10.1186/s13024-015-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonsen A., Cumming R.C., Brech A., Isakson P., Schubert D.R., Finley K.D. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult Drosophila. Autophagy. 2008;4(2):176–184. doi: 10.4161/auto.5269. [DOI] [PubMed] [Google Scholar]
- 44.Tsvetkov P., Adamovich Y., Elliott E., Shaul Y. E3 ligase STUB1/CHIP regulates NAD(P)H:quinone oxidoreductase 1 (NQO1) accumulation in aged brain, a process impaired in certain Alzheimer disease patients. J. Biol. Chem. 2011;286(11):8839–8845. doi: 10.1074/jbc.M110.193276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Min J-N., Whaley R.A., Sharpless N.E., Lockyer P., Portbury A.L., Patterson C. CHIP deficiency decreases longevity, with accelerated aging phenotypes accompanied by altered protein quality control. Mol. Cell. Biol. 2008;28(12):4018–4025. doi: 10.1128/MCB.00296-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng X-R., Zhou W-X., Zhang Y-X., Zhou D-S., Yang R-F., Chen L-F. Differential gene expression profiles in the hippocampus of senescence-accelerated mouse. Neurobiol. Aging. 2007;28(4):497–506. doi: 10.1016/j.neurobiolaging.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Zhang G-R., Cheng X-R., Zhou W-X., Zhang Y-X. Age-related expression of STUB1 in senescence-accelerated mice and its response to anti-Alzheimer’s disease traditional Chinese medicine. Neurosci. Lett. 2008;438(3):371–375. doi: 10.1016/j.neulet.2008.04.075. [DOI] [PubMed] [Google Scholar]
- 48.Schisler J.C., Rubel C.E., Zhang C., Lockyer P., Cyr D.M., Patterson C. CHIP protects against cardiac pressure overload through regulation of AMPK. J. Clin. Invest. 2013;123(8):3588–3599. doi: 10.1172/JCI69080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai Q., Zhang C., Wu Y., McDonough H., Whaley R.A., Godfrey V., Li H-H., Madamanchi N., Xu W., Neckers L., Cyr D., Patterson C. CHIP activates HSF1 and confers protection against apoptosis and cellular stress. EMBO J. 2003;22(20):5446–5458. doi: 10.1093/emboj/cdg529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang C-Y., Kuo W-W., Lo J-F., Ho T-J., Pai P-Y., Chiang S-F., Chen P-Y., Tsai F-J., Tsai C-H., Huang C-Y. Doxorubicin attenuates CHIP-guarded HSF1 nuclear translocation and protein stability to trigger IGF-IIR-dependent cardiomyocyte death. Cell Death Dis. 2016;7(11):e2455. doi: 10.1038/cddis.2016.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S-A., Yoon J-H., Kim D-K., Kim S-G., Ahn S-G. CHIP interacts with heat shock factor 1 during heat stress. FEBS Lett. 2005;579(29):6559–6563. doi: 10.1016/j.febslet.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 52.Sarkar S., Brautigan D.L., Larner J.M. Aurora Kinase A Promotes AR Degradation via the E3 Ligase CHIP. Mol. Cancer Res. 2017;15(8):1063–1072. doi: 10.1158/1541-7786.MCR-17-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beyer K., Domingo-Sàbat M., Humbert J., Carrato C., Ferrer I., Ariza A. Differential expression of alpha-synuclein, parkin, and synphilin-1 isoforms in Lewy body disease. Neurogenetics. 2008;9(3):163–172. doi: 10.1007/s10048-008-0124-6. [DOI] [PubMed] [Google Scholar]
- 54.Scaglione K.M., Zavodszky E., Todi S.V., Patury S., Xu P., Rodríguez-Lebrón E., Fischer S., Konen J., Djarmati A., Peng J., Gestwicki J.E., Paulson H.L. Ube2w and ataxin-3 coordinately regulate the ubiquitin ligase CHIP. Mol. Cell. 2011;43(4):599–612. doi: 10.1016/j.molcel.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi Y., Wang J., Li J-D., Ren H., Guan W., He M., Yan W., Zhou Y., Hu Z., Zhang J., Xiao J., Su Z., Dai M., Wang J., Jiang H., Guo J., Zhou Y., Zhang F., Li N., Du J., Xu Q., Hu Y., Pan Q., Shen L., Wang G., Xia K., Zhang Z., Tang B. Identification of CHIP as a novel causative gene for autosomal recessive cerebellar ataxia. PLoS One. 2013;8(12):e81884. doi: 10.1371/journal.pone.0081884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palubinsky A.M., Stankowski J.N., Kale A.C., Codreanu S.G., Singer R.J., Liebler D.C., Stanwood G.D., McLaughlin B. CHIP Is an essential determinant of neuronal mitochondrial stress signaling. Antioxid. Redox Signal. 2015;23(6):535–549. doi: 10.1089/ars.2014.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi C-H., Schisler J.C., Rubel C.E., Tan S., Song B., McDonough H., Xu L., Portbury A.L., Mao C-Y., True C., Wang R-H., Wang Q-Z., Sun S-L., Seminara S.B., Patterson C., Xu Y-M. Ataxia and hypogonadism caused by the loss of ubiquitin ligase activity of the U box protein CHIP. Hum. Mol. Genet. 2014;23(4):1013–1024. doi: 10.1093/hmg/ddt497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebrahimi-Fakhari D., McLean P.J., Unni V.K. Alpha-synuclein’s degradation in vivo: opening a new (cranial) window on the roles of degradation pathways in Parkinson disease. Autophagy. 2012;8(2):281–283. doi: 10.4161/auto.8.2.18938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klucken J., Shin Y., Masliah E., Hyman B.T., McLean P.J. Hsp70 Reduces alpha-synuclein aggregation and toxicity. J. Biol. Chem. 2004;279(24):25497–25502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- 60.McKinnon C., De Snoo M.L., Gondard E., Neudorfer C., Chau H., Ngana S.G., O’Hara D.M., Brotchie J.M., Koprich J.B., Lozano A.M., Kalia L.V., Kalia S.K. Early-onset impairment of the ubiquitin-proteasome system in dopaminergic neurons caused by α-synuclein. Acta Neuropathol. Commun. 2020;8(1):17. doi: 10.1186/s40478-020-0894-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalia L.V., Kalia S.K., Chau H., Lozano A.M., Hyman B.T., McLean P.J. Ubiquitinylation of α-synuclein by carboxyl terminus Hsp70-interacting protein (CHIP) is regulated by Bcl-2-associated athanogene 5 (BAG5). PLoS One. 2011;6(2):e14695. doi: 10.1371/journal.pone.0014695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Auluck P.K., Chan H.Y.E., Trojanowski J.Q., Lee V.M.Y., Bonini N.M. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295(5556):865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 63.Dimant H., Zhu L., Kibuuka L.N., Fan Z., Hyman B.T., McLean P.J. Direct visualization of CHIP-mediated degradation of alpha-synuclein in vivo: implications for PD therapeutics. PLoS One. 2014;9(3):e92098. doi: 10.1371/journal.pone.0092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tetzlaff J.E., Putcha P., Outeiro T.F., Ivanov A., Berezovska O., Hyman B.T., McLean P.J. CHIP targets toxic alpha-Synuclein oligomers for degradation. J. Biol. Chem. 2008;283(26):17962–17968. doi: 10.1074/jbc.M802283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li L.Y., Luo X., Wang X., Endonuclease G. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412(6842):95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 66.Büttner S., Habernig L., Broeskamp F., Ruli D., Vögtle F.N., Vlachos M., Macchi F., Küttner V., Carmona-Gutierrez D., Eisenberg T., Ring J., Markaki M., Taskin A.A., Benke S., Ruckenstuhl C., Braun R., Van den Haute C., Bammens T., van der Perren A., Fröhlich K-U., Winderickx J., Kroemer G., Baekelandt V., Tavernarakis N., Kovacs G.G., Dengjel J., Meisinger C., Sigrist S.J., Madeo F., Endonuclease G. Endonuclease G mediates α-synuclein cytotoxicity during Parkinson’s disease. EMBO J. 2013;32(23):3041–3054. doi: 10.1038/emboj.2013.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J.S., Seo T.W., Yi J.H., Shin K.S., Yoo S.J. CHIP has a protective role against oxidative stress-induced cell death through specific regulation of endonuclease G. Cell Death Dis. 2013;4(6):e666. doi: 10.1038/cddis.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferreira M., Massano J. An updated review of Parkinson’s disease genetics and clinicopathological correlations. Acta Neurol. Scand. 2017;135(3):273–284. doi: 10.1111/ane.12616. [DOI] [PubMed] [Google Scholar]
- 69.Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 70.Dawson T.M., Dawson V.L. The role of parkin in familial and sporadic Parkinson’s disease. Mov. Disord. 2010;25(Suppl. 1):S32–S39. doi: 10.1002/mds.22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen J., Xue J., Ruan J., Zhao J., Tang B., Duan R. Drosophila CHIP protects against mitochondrial dysfunction by acting downstream of Pink1 in parallel with Parkin. FASEB J. 2017;31(12):5234–5245. doi: 10.1096/fj.201700011R. [DOI] [PubMed] [Google Scholar]
- 72.Imai Y., Soda M., Hatakeyama S., Akagi T., Hashikawa T., Nakayama K.I., Takahashi R. CHIP is associated with Parkin, a gene responsible for familial Parkinson’s disease, and enhances its ubiquitin ligase activity. Mol. Cell. 2002;10(1):55–67. doi: 10.1016/S1097-2765(02)00583-X. [DOI] [PubMed] [Google Scholar]
- 73.Kitao Y., Imai Y., Ozawa K., Kataoka A., Ikeda T., Soda M., Nakimawa K., Kiyama H., Stern D.M., Hori O., Wakamatsu K., Ito S., Itohara S., Takahashi R., Ogawa S. Pael receptor induces death of dopaminergic neurons in the substantia nigra via endoplasmic reticulum stress and dopamine toxicity, which is enhanced under condition of parkin inactivation. Hum. Mol. Genet. 2007;16(1):50–60. doi: 10.1093/hmg/ddl439. [DOI] [PubMed] [Google Scholar]
- 74.Valente E.M., Bentivoglio A.R., Dixon P.H., Ferraris A., Ialongo T., Frontali M., Albanese A., Wood N.W. Localization of a novel locus for autosomal recessive early-onset parkinsonism, PARK6, on human chromosome 1p35-p36. Am. J. Hum. Genet. 2001;68(4):895–900. doi: 10.1086/319522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valente E.M., Brancati F., Ferraris A., Graham E.A., Davis M.B., Breteler M.M.B., Gasser T., Bonifati V., Bentivoglio A.R., De Michele G., Dürr A., Cortelli P., Wassilowsky D., Harhangi B.S., Rawal N., Caputo V., Filla A., Meco G., Oostra B.A., Brice A., Albanese A., Dallapiccola B., Wood N.W. European consortium on genetic susceptibility in Parkinson’s Disease. PARK6-linked parkinsonism occurs in several European families. Ann. Neurol. 2002;51(1):14–18. doi: 10.1002/ana.10053. [DOI] [PubMed] [Google Scholar]
- 76.Bentivoglio A.R., Cortelli P., Valente E.M., Ialongo T., Ferraris A., Elia A., Montagna P., Albanese A. Phenotypic characterisation of autosomal recessive PARK6-linked parkinsonism in three unrelated Italian families. Mov. Disord. 2001;16(6):999–1006. doi: 10.1002/mds.10034. [DOI] [PubMed] [Google Scholar]
- 77.Zhang B.R., Hu Z.X., Yin X.Z., Cai M., Zhao G.H., Liu Z.R., Luo W. Mutation analysis of parkin and PINK1 genes in early-onset Parkinson’s disease in China. Neurosci. Lett. 2010;477(1):19–22. doi: 10.1016/j.neulet.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 78.Geisler S., Holmström K.M., Treis A., Skujat D., Weber S.S., Fiesel F.C., Kahle P.J., Springer W. The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy. 2010;6(7):871–878. doi: 10.4161/auto.6.7.13286. [DOI] [PubMed] [Google Scholar]
- 79.Yoo L., Chung K.C. The ubiquitin E3 ligase CHIP promotes proteasomal degradation of the serine/threonine protein kinase PINK1 during staurosporine-induced cell death. J. Biol. Chem. 2018;293(4):1286–1297. doi: 10.1074/jbc.M117.803890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Healy D.G., Falchi M., O’Sullivan S.S., Bonifati V., Durr A., Bressman S., Brice A., Aasly J., Zabetian C.P., Goldwurm S., Ferreira J.J., Tolosa E., Kay D.M., Klein C., Williams D.R., Marras C., Lang A.E., Wszolek Z.K., Berciano J., Schapira A.H., Lynch T., Bhatia K.P., Gasser T., Lees A.J., Wood N.W. International LRRK2 Consortium. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7(7):583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ko H.S., Bailey R., Smith W.W., Liu Z., Shin J-H., Lee Y-I., Zhang Y-J., Jiang H., Ross C.A., Moore D.J., Patterson C., Petrucelli L., Dawson T.M., Dawson V.L. CHIP regulates leucine-rich repeat kinase-2 ubiquitination, degradation, and toxicity. Proc. Natl. Acad. Sci. USA. 2009;106(8):2897–2902. doi: 10.1073/pnas.0810123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rudenko I.N., Kaganovich A., Langston R.G., Beilina A., Ndukwe K., Kumaran R., Dillman A.A., Chia R., Cookson M.R. The G2385R risk factor for Parkinson’s disease enhances CHIP-dependent intracellular degradation of LRRK2. Biochem. J. 2017;474(9):1547–1558. doi: 10.1042/BCJ20160909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ding X., Goldberg M.S. Regulation of LRRK2 stability by the E3 ubiquitin ligase CHIP. PLoS One. 2009;4(6):e5949. doi: 10.1371/journal.pone.0005949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mufson E.J., Ikonomovic M.D., Counts S.E., Perez S.E., Malek-Ahmadi M., Scheff S.W., Ginsberg S.D. Molecular and cellular pathophysiology of preclinical Alzheimer’s disease. Behav. Brain Res. 2016;311:54–69. doi: 10.1016/j.bbr.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sahara N., Murayama M., Mizoroki T., Urushitani M., Imai Y., Takahashi R., Murata S., Tanaka K., Takashima A. In vivo evidence of CHIP up-regulation attenuating tau aggregation. J. Neurochem. 2005;94(5):1254–1263. doi: 10.1111/j.1471-4159.2005.03272.x. [DOI] [PubMed] [Google Scholar]
- 86.Ballatore C., Lee V.M-Y., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007;8(9):663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 87.Dickey C.A., Yue M., Lin W-L., Dickson D.W., Dunmore J.H., Lee W.C., Zehr C., West G., Cao S., Clark A.M.K., Caldwell G.A., Caldwell K.A., Eckman C., Patterson C., Hutton M., Petrucelli L. Deletion of the ubiquitin ligase CHIP leads to the accumulation, but not the aggregation, of both endogenous phospho- and caspase-3-cleaved tau species. J. Neurosci. 2006;26(26):6985–6996. doi: 10.1523/JNEUROSCI.0746-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fontaine S.N., Martin M.D., Akoury E., Assimon V.A., Borysov S., Nordhues B.A., Sabbagh J.J., Cockman M., Gestwicki J.E., Zweckstetter M., Dickey C.A. The active Hsc70/tau complex can be exploited to enhance tau turnover without damaging microtubule dynamics. Hum. Mol. Genet. 2015;24(14):3971–3981. doi: 10.1093/hmg/ddv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dickey C.A., Kamal A., Lundgren K., Klosak N., Bailey R.M., Dunmore J., Ash P., Shoraka S., Zlatkovic J., Eckman C.B., Patterson C., Dickson D.W., Nahman N.S., Jr, Hutton M., Burrows F., Petrucelli L. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Invest. 2007;117(3):648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bali P., Pranpat M., Bradner J., Balasis M., Fiskus W., Guo F., Rocha K., Kumaraswamy S., Boyapalle S., Atadja P., Seto E., Bhalla K. Inhibition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: a novel basis for antileukemia activity of histone deacetylase inhibitors. J. Biol. Chem. 2005;280(29):26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 91.Kovacs J.J., Murphy P.J.M., Gaillard S., Zhao X., Wu J-T., Nicchitta C.V., Yoshida M., Toft D.O., Pratt W.B., Yao T-P. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol. Cell. 2005;18(5):601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 92.Cook C., Gendron T.F., Scheffel K., Carlomagno Y., Dunmore J., DeTure M., Petrucelli L. Loss of HDAC6, a novel CHIP substrate, alleviates abnormal tau accumulation. Hum. Mol. Genet. 2012;21(13):2936–2945. doi: 10.1093/hmg/dds125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dickey C.A., Koren J., Zhang Y-J., Xu Y-F., Jinwal U.K., Birnbaum M.J., Monks B., Sun M., Cheng J.Q., Patterson C., Bailey R.M., Dunmore J., Soresh S., Leon C., Morgan D., Petrucelli L. Akt and CHIP coregulate tau degradation through coordinated interactions. Proc. Natl. Acad. Sci. USA. 2008;105(9):3622–3627. doi: 10.1073/pnas.0709180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sisodia S.S., Price D.L. Amyloidogenesis in Alzheimer’s disease: basic biology and animal models. Curr. Opin. Neurobiol. 1992;2(5):648–652. doi: 10.1016/0959-4388(92)90033-H. [DOI] [PubMed] [Google Scholar]
- 95.Slunt H.H., Thinakaran G., Von Koch C., Lo A.C., Tanzi R.E., Sisodia S.S. Expression of a ubiquitous, cross-reactive homologue of the mouse beta-amyloid precursor protein (APP). J. Biol. Chem. 1994;269(4):2637–2644. [PubMed] [Google Scholar]
- 96.Müller U.C., Deller T., Korte M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017;18(5):281–298. doi: 10.1038/nrn.2017.29. [DOI] [PubMed] [Google Scholar]
- 97.Kumar P., Ambasta R.K., Veereshwarayya V., Rosen K.M., Kosik K.S., Band H., Mestril R., Patterson C., Querfurth H.W. CHIP and HSPs interact with beta-APP in a proteasome-dependent manner and influence Abeta metabolism. Hum. Mol. Genet. 2007;16(7):848–864. doi: 10.1093/hmg/ddm030. [DOI] [PubMed] [Google Scholar]
- 98.Singh A.K., Pati U. CHIP stabilizes amyloid precursor protein via proteasomal degradation and p53-mediated trans-repression of β-secretase. Aging Cell. 2015;14(4):595–604. doi: 10.1111/acel.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liang W.S., Dunckley T., Beach T.G., Grover A., Mastroeni D., Ramsey K., Caselli R.J., Kukull W.A., McKeel D., Morris J.C., Hulette C.M., Schmechel D., Reiman E.M., Rogers J., Stephan D.A. Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: a reference data set. Physiol. Genomics. 2008;33(2):240–256. doi: 10.1152/physiolgenomics.00242.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Blokhuis A.M., Groen E.J.N., Koppers M., van den Berg L.H., Pasterkamp R.J. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125(6):777–794. doi: 10.1007/s00401-013-1125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chiò A., Logroscino G., Hardiman O., Swingler R., Mitchell D., Beghi E., Traynor B.G. Eurals Consortium. Prognostic factors in ALS: A critical review. Amyotroph. Lateral Scler. 2009;10(5-6):310–323. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaur S.J., McKeown S.R., Rashid S. Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene. 2016;577(2):109–118. doi: 10.1016/j.gene.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 103.Borchelt D.R., Lee M.K., Slunt H.S., Guarnieri M., Xu Z.S., Wong P.C., Brown R.H., Jr, Price D.L., Sisodia S.S., Cleveland D.W. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. Natl. Acad. Sci. USA. 1994;91(17):8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Urushitani M., Kurisu J., Tateno M., Hatakeyama S., Nakayama K., Kato S., Takahashi R. CHIP promotes proteasomal degradation of familial ALS-linked mutant SOD1 by ubiquitinating Hsp/Hsc70. J. Neurochem. 2004;90(1):231–244. doi: 10.1111/j.1471-4159.2004.02486.x. [DOI] [PubMed] [Google Scholar]
- 105.Crippa V., Sau D., Rusmini P., Boncoraglio A., Onesto E., Bolzoni E., Galbiati M., Fontana E., Marino M., Carra S., Bendotti C., De Biasi S., Poletti A. The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum. Mol. Genet. 2010;19(17):3440–3456. doi: 10.1093/hmg/ddq257. [DOI] [PubMed] [Google Scholar]
- 106.Arndt V., Dick N., Tawo R., Dreiseidler M., Wenzel D., Hesse M., Fürst D.O., Saftig P., Saint R., Fleischmann B.K., Hoch M., Höhfeld J. Chaperone-assisted selective autophagy is essential for muscle maintenance. Curr. Biol. 2010;20(2):143–148. doi: 10.1016/j.cub.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 107.Niwa J., Ishigaki S., Doyu M., Suzuki T., Tanaka K., Sobue G. A novel centrosomal ring-finger protein, dorfin, mediates ubiquitin ligase activity. Biochem. Biophys. Res. Commun. 2001;281(3):706–713. doi: 10.1006/bbrc.2001.4414. [DOI] [PubMed] [Google Scholar]
- 108.Ishigaki S., Niwa J., Yamada S., Takahashi M., Ito T., Sone J., Doyu M., Urano F., Sobue G. Dorfin-CHIP chimeric proteins potently ubiquitylate and degrade familial ALS-related mutant SOD1 proteins and reduce their cellular toxicity. Neurobiol. Dis. 2007;25(2):331–341. doi: 10.1016/j.nbd.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 109.Zhang S., Tang M.B., Luo H.Y., Shi C.H., Xu Y.M. Necroptosis in neurodegenerative diseases: a potential therapeutic target. Cell Death Dis. 2017;8(6):e2905. doi: 10.1038/cddis.2017.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ito Y., Ofengeim D., Najafov A., Das S., Saberi S., Li Y., Hitomi J., Zhu H., Chen H., Mayo L., Geng J., Amin P., DeWitt J.P., Mookhtiar A.K., Florez M., Ouchida A.T., Fan J.B., Pasparakis M., Kelliher M.A., Ravits J., Yuan J. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science. 2016;353(6299):603–608. doi: 10.1126/science.aaf6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu D., Jin T., Zhu H., Chen H., Ofengeim D., Zou C., Mifflin L., Pan L., Amin P., Li W., Shan B., Naito M.G., Meng H., Li Y., Pan H., Aron L., Adiconis X., Levin J.Z., Yankner B.A., Yuan J. TBK1 Suppresses RIPK1-Driven Apoptosis and Inflammation during development and in aging. Cell. 2018;174(6):1477–1491.e19. doi: 10.1016/j.cell.2018.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang M-B., Li Y-S., Li S-H., Cheng Y., Zhang S., Luo H-Y., Mao C-Y., Hu Z-W., Schisler J.C., Shi C-H., Xu Y-M. Anisomycin prevents OGD-induced necroptosis by regulating the E3 ligase CHIP. Sci. Rep. 2018;8(1):6379. doi: 10.1038/s41598-018-24414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seo J., Lee E-W., Sung H., Seong D., Dondelinger Y., Shin J., Jeong M., Lee H-K., Kim J-H., Han S.Y., Lee C., Seong J.K., Vandenabeele P., Song J. CHIP controls necroptosis through ubiquitylation- and lysosome-dependent degradation of RIPK3. Nat. Cell Biol. 2016;18(3):291–302. doi: 10.1038/ncb3314. [DOI] [PubMed] [Google Scholar]
- 114.Minassian B.A. Lafora’s disease: towards a clinical, pathologic, and molecular synthesis. Pediatr. Neurol. 2001;25(1):21–29. doi: 10.1016/S0887-8994(00)00276-9. [DOI] [PubMed] [Google Scholar]
- 115.Delgado-Escueta A.V. Advances in lafora progressive myoclonus epilepsy. Curr. Neurol. Neurosci. Rep. 2007;7(5):428–433. doi: 10.1007/s11910-007-0066-7. [DOI] [PubMed] [Google Scholar]
- 116.Singh S., Ganesh S. Lafora progressive myoclonus epilepsy: a meta-analysis of reported mutations in the first decade following the discovery of the EPM2A and NHLRC1 genes. Hum. Mutat. 2009;30(5):715–723. doi: 10.1002/humu.20954. [DOI] [PubMed] [Google Scholar]
- 117.Tagliabracci V.S., Turnbull J., Wang W., Girard J-M., Zhao X., Skurat A.V., Delgado-Escueta A.V., Minassian B.A., Depaoli-Roach A.A., Roach P.J. Laforin is a glycogen phosphatase, deficiency of which leads to elevated phosphorylation of glycogen in vivo. Proc. Natl. Acad. Sci. USA. 2007;104(49):19262–19266. doi: 10.1073/pnas.0707952104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vilchez D., Ros S., Cifuentes D., Pujadas L., Vallès J., García-Fojeda B., Criado-García O., Fernández-Sánchez E., Medraño-Fernández I., Domínguez J., García-Rocha M., Soriano E., Rodríguez de Córdoba S., Guinovart J.J. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat. Neurosci. 2007;10(11):1407–1413. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- 119.Rao S.N.R., Sharma J., Maity R., Jana N.R. Co-chaperone CHIP stabilizes aggregate-prone malin, a ubiquitin ligase mutated in Lafora disease. J. Biol. Chem. 2010;285(2):1404–1413. doi: 10.1074/jbc.M109.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paulson H.L., Bonini N.M., Roth K.A. Polyglutamine disease and neuronal cell death. Proc. Natl. Acad. Sci. USA. 2000;97(24):12957–12958. doi: 10.1073/pnas.210395797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jana N.R., Nukina N. Recent advances in understanding the pathogenesis of polyglutamine diseases: involvement of molecular chaperones and ubiquitin-proteasome pathway. J. Chem. Neuroanat. 2003;26(2):95–101. doi: 10.1016/S0891-0618(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 122.Gusella J.F., MacDonald M.E. Huntington’s disease. Semin. Cell Biol. 1995;6(1):21–28. doi: 10.1016/1043-4682(95)90011-X. [DOI] [PubMed] [Google Scholar]
- 123.Miller V.M., Nelson R.F., Gouvion C.M., Williams A., Rodriguez-Lebron E., Harper S.Q., Davidson B.L., Rebagliati M.R., Paulson H.L. CHIP suppresses polyglutamine aggregation and toxicity in vitro and in vivo. J. Neurosci. 2005;25(40):9152–9161. doi: 10.1523/JNEUROSCI.3001-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao T., Hong Y., Yin P., Li S., Li X-J. Differential HspBP1 expression accounts for the greater vulnerability of neurons than astrocytes to misfolded proteins. Proc. Natl. Acad. Sci. USA. 2017;114(37):E7803–E7811. doi: 10.1073/pnas.1710549114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jana N.R., Dikshit P., Goswami A., Kotliarova S., Murata S., Tanaka K., Nukina N. Co-chaperone CHIP associates with expanded polyglutamine protein and promotes their degradation by proteasomes. J. Biol. Chem. 2005;280(12):11635–11640. doi: 10.1074/jbc.M412042200. [DOI] [PubMed] [Google Scholar]
- 126.Kennedy W.R., Alter M., Sung J.H. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18(7):671–680. doi: 10.1212/WNL.18.7.671. [DOI] [PubMed] [Google Scholar]
- 127.Adachi H., Waza M., Tokui K., Katsuno M., Minamiyama M., Tanaka F., Doyu M., Sobue G. CHIP overexpression reduces mutant androgen receptor protein and ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model. J. Neurosci. 2007;27(19):5115–5126. doi: 10.1523/JNEUROSCI.1242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Klinke I., Minnerop M., Schmitz-Hübsch T., Hendriks M., Klockgether T., Wüllner U., Helmstaedter C. Neuropsychological features of patients with spinocerebellar ataxia (SCA) types 1, 2, 3, and 6. Cerebellum. 2010;9(3):433–442. doi: 10.1007/s12311-010-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Williams A.J., Knutson T.M., Colomer Gould V.F., Paulson H.L. In vivo suppression of polyglutamine neurotoxicity by C-terminus of Hsp70-interacting protein (CHIP) supports an aggregation model of pathogenesis. Neurobiol. Dis. 2009;33(3):342–353. doi: 10.1016/j.nbd.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zoghbi H.Y., Orr H.T. Spinocerebellar ataxia type 1. Semin. Cell Biol. 1995;6(1):29–35. doi: 10.1016/1043-4682(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 131.Choi J.Y., Ryu J.H., Kim H-S., Park S.G., Bae K-H., Kang S., Myung P.K., Cho S., Park B.C., Lee D.H. Co-chaperone CHIP promotes aggregation of ataxin-1. Mol. Cell. Neurosci. 2007;34(1):69–79. doi: 10.1016/j.mcn.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 132.Al-Ramahi I., Lam Y.C., Chen H-K., de Gouyon B., Zhang M., Pérez A.M., Branco J., de Haro M., Patterson C., Zoghbi H.Y., Botas J. CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. J. Biol. Chem. 2006;281(36):26714–26724. doi: 10.1074/jbc.M601603200. [DOI] [PubMed] [Google Scholar]
- 133.Onodera O., Burke J.R., Miller S.E., Hester S., Tsuji S., Roses A.D., Strittmatter W.J. Oligomerization of expanded-polyglutamine domain fluorescent fusion proteins in cultured mammalian cells. Biochem. Biophys. Res. Commun. 1997;238(2):599–605. doi: 10.1006/bbrc.1997.7337. [DOI] [PubMed] [Google Scholar]
- 134.Anheim M., Tranchant C., Koenig M. The autosomal recessive cerebellar ataxias. N. Engl. J. Med. 2012;366(7):636–646. doi: 10.1056/NEJMra1006610. [DOI] [PubMed] [Google Scholar]
- 135.Margolin D.H., Kousi M., Chan Y-M., Lim E.T., Schmahmann J.D., Hadjivassiliou M., Hall J.E., Adam I., Dwyer A., Plummer L., Aldrin S.V., O’Rourke J., Kirby A., Lage K., Milunsky A., Milunsky J.M., Chan J., Hedley-Whyte E.T., Daly M.J., Katsanis N., Seminara S.B. Ataxia, dementia, and hypogonadotropism caused by disordered ubiquitination. N. Engl. J. Med. 2013;368(21):1992–2003. doi: 10.1056/NEJMoa1215993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klockgether T. Sporadic ataxia with adult onset: classification and diagnostic criteria. Lancet Neurol. 2010;9(1):94–104. doi: 10.1016/S1474-4422(09)70305-9. [DOI] [PubMed] [Google Scholar]
- 137.Ruano L., Melo C., Silva M.C., Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42(3):174–183. doi: 10.1159/000358801. [DOI] [PubMed] [Google Scholar]
- 138.Synofzik M., Schüle R., Schulze M., Gburek-Augustat J., Schweizer R., Schirmacher A., Krägeloh-Mann I., Gonzalez M., Young P., Züchner S., Schöls L., Bauer P. Phenotype and frequency of STUB1 mutations: next-generation screenings in Caucasian ataxia and spastic paraplegia cohorts. Orphanet J. Rare Dis. 2014;9:57. doi: 10.1186/1750-1172-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Heimdal K., Sanchez-Guixé M., Aukrust I., Bollerslev J., Bruland O., Jablonski G.E., Erichsen A.K., Gude E., Koht J.A., Erdal S., Fiskerstrand T., Haukanes B.I., Boman H., Bjørkhaug L., Tallaksen C.M.E., Knappskog P.M., Johansson S. STUB1 mutations in autosomal recessive ataxias - evidence for mutation-specific clinical heterogeneity. Orphanet J. Rare Dis. 2014;9:146. doi: 10.1186/s13023-014-0146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Depondt C., Donatello S., Simonis N., Rai M., van Heurck R., Abramowicz M., D’Hooghe M., Pandolfo M. Autosomal recessive cerebellar ataxia of adult onset due to STUB1 mutations. Neurology. 2014;82(19):1749–1750. doi: 10.1212/WNL.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 141.Cordoba M., Rodriguez-Quiroga S., Gatto E.M., Alurralde A., Kauffman M.A. Ataxia plus myoclonus in a 23-year-old patient due to STUB1 mutations. Neurology. 2014;83(3):287–288. doi: 10.1212/WNL.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 142.Bettencourt C., de Yébenes J.G., López-Sendón J.L., Shomroni O., Zhang X., Qian S-B., Bakker I.M.C., Heetveld S., Ros R., Quintáns B., Sobrido M-J., Bevova M.R., Jain S., Bugiani M., Heutink P., Rizzu P. Clinical and neuropathological features of spastic ataxia in a spanish family with novel compound heterozygous mutations in STUB1. Cerebellum. 2015;14(3):378–381. doi: 10.1007/s12311-014-0643-7. [DOI] [PubMed] [Google Scholar]
- 143.Kawarai T., Miyamoto R., Shimatani Y., Orlacchio A., Kaji R. Choreoathetosis, dystonia, and myoclonus in 3 siblings with autosomal recessive spinocerebellar ataxia type 16. JAMA Neurol. 2016;73(7):888–890. doi: 10.1001/jamaneurol.2016.0647. [DOI] [PubMed] [Google Scholar]
- 144.Hayer S.N., Deconinck T., Bender B., Smets K., Züchner S., Reich S., Schöls L., Schüle R., De Jonghe P., Baets J., Synofzik M. STUB1/CHIP mutations cause Gordon Holmes syndrome as part of a widespread multisystemic neurodegeneration: evidence from four novel mutations. Orphanet J. Rare Dis. 2017;12(1):31. doi: 10.1186/s13023-017-0580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Coutelier M., Coarelli G., Monin M-L., Konop J., Davoine C-S., Tesson C., Valter R., Anheim M., Behin A., Castelnovo G., Charles P., David A., Ewenczyk C., Fradin M., Goizet C., Hannequin D., Labauge P., Riant F., Sarda P., Sznajer Y., Tison F., Ullmann U., Van Maldergem L., Mochel F., Brice A., Stevanin G., Durr A. SPATAX network. A panel study on patients with dominant cerebellar ataxia highlights the frequency of channelopathies. Brain. 2017;140(6):1579–1594. doi: 10.1093/brain/awx081. [DOI] [PubMed] [Google Scholar]
- 146.Gazulla J., Izquierdo-Alvarez S., Sierra-Martínez E., Marta-Moreno M.E., Alvarez S. Inaugural cognitive decline, late disease onset and novel STUB1 variants in SCAR16. Neurol. Sci. 2018;39(12):2231–2233. doi: 10.1007/s10072-018-3545-5. [DOI] [PubMed] [Google Scholar]
- 147.Turkgenc B., Sanlidag B., Eker A., Giray A., Kutuk O., Yakicier C., Tolun A., Temel S.G. STUB1 polyadenylation signal variant AACAAA does not affect polyadenylation but decreases STUB1 translation causing SCAR16. Hum. Mutat. 2018;39(10):1344–1348. doi: 10.1002/humu.23601. [DOI] [PubMed] [Google Scholar]
- 148.Olszewska D.A., Kinsella J.A. Extending the phenotypic spectrum associated with STUB1 Mutations: A Case of Dystonia. Mov. Disord. Clin. Pract. (Hoboken) 2020;7(3):318–324. doi: 10.1002/mdc3.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chiu H-H., Hsaio C-T., Tsai Y-S., Liao Y-C., Lee Y-C., Soong B-W. Clinical and genetic characterization of autosomal recessive spinocerebellar ataxia type 16 (SCAR16) in Taiwan. Cerebellum. 2020;19(4):544–549. doi: 10.1007/s12311-020-01136-4. [DOI] [PubMed] [Google Scholar]
- 150.Roux T., Barbier M., Papin M., Davoine C.-S., Sayah S., Coarelli G., Charles P., Marelli C., Parodi L., Tranchant C., Goizet C., Klebe S., Lohmann E., Van Maldergen L., van Broeckhoven C., Coutelier M., Tesson C., Stevanin G., Duyckaerts C., Brice A., Durr A. SPATAX network. Clinical, Neuropathological, and Genetic Characterization of STUB1 Variants in Cerebellar Ataxias: A Frequent Cause of Predominant Cognitive Impairment. Genet. Med. Off. J. Am. Coll. Med. Genet. 2020 doi: 10.1038/s41436-020-0899-x. [DOI] [PubMed] [Google Scholar]
- 151.Retterer K., Juusola J., Cho M.T., Vitazka P., Millan F., Gibellini F., Vertino-Bell A., Smaoui N., Neidich J., Monaghan K.G., McKnight D., Bai R., Suchy S., Friedman B., Tahiliani J., Pineda-Alvarez D., Richard G., Brandt T., Haverfield E., Chung W.K., Bale S. Clinical application of whole-exome sequencing across clinical indications. Genet. Med. 2016;18(7):696–704. doi: 10.1038/gim.2015.148. [DOI] [PubMed] [Google Scholar]
- 152.Schwarz J.M., Cooper D.N., Schuelke M., Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat. Methods. 2014;11(4):361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 153.Vaser R., Adusumalli S., Leng S.N., Sikic M., Ng P.C. SIFT missense predictions for genomes. Nat. Protoc. 2016;11(1):1–9. doi: 10.1038/nprot.2015.123. [DOI] [PubMed] [Google Scholar]
- 154.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., Sunyaev S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shi C-H., Rubel C., Soss S.E., Sanchez-Hodge R., Zhang S., Madrigal S.C., Ravi S., McDonough H., Page R.C., Chazin W.J., Patterson C., Mao C-Y., Willis M.S., Luo H-Y., Li Y-S., Stevens D.A., Tang M-B., Du P., Wang Y-H., Hu Z-W., Xu Y-M., Schisler J.C. Disrupted structure and aberrant function of CHIP mediates the loss of motor and cognitive function in preclinical models of SCAR16. PLoS Genet. 2018;14(9):e1007664. doi: 10.1371/journal.pgen.1007664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kanack A.J., Newsom O.J., Scaglione K.M. Most mutations that cause spinocerebellar ataxia autosomal recessive type 16 (SCAR16) destabilize the protein quality-control E3 ligase CHIP. J. Biol. Chem. 2018;293(8):2735–2743. doi: 10.1074/jbc.RA117.000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Madrigal S.C., McNeil Z., Sanchez-Hodge R., Shi C.H., Patterson C., Scaglione K.M., Schisler J.C. Changes in protein function underlie the disease spectrum in patients with CHIP mutations. J. Biol. Chem. 2019;294(50):19236–19245. doi: 10.1074/jbc.RA119.011173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pakdaman Y., Sanchez-Guixé M., Kleppe R., Erdal S., Bustad H.J., Bjørkhaug L., Haugarvoll K., Tzoulis C., Heimdal K., Knappskog P.M., Johansson S., Aukrust I. In vitro characterization of six STUB1 variants in spinocerebellar ataxia 16 reveals altered structural properties for the encoded CHIP proteins. Biosci. Rep. 2017;37(2):BSR20170251. doi: 10.1042/BSR20170251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rinaldi L., Delle Donne R., Catalanotti B., Torres-Quesada O., Enzler F., Moraca F., Nisticò R., Chiuso F., Piccinin S., Bachmann V., Lindner H.H., Garbi C., Scorziello A., Russo N.A., Synofzik M., Stelzl U., Annunziato L., Stefan E., Feliciello A. Feedback inhibition of cAMP effector signaling by a chaperone-assisted ubiquitin system. Nat. Commun. 2019;10(1):2572. doi: 10.1038/s41467-019-10037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sutherland E.W., Rall T.W. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J. Biol. Chem. 1958;232(2):1077–1091. [PubMed] [Google Scholar]
- 161.Simonds W.F. G protein regulation of adenylate cyclase. Trends Pharmacol. Sci. 1999;20(2):66–73. doi: 10.1016/S0165-6147(99)01307-3. [DOI] [PubMed] [Google Scholar]
- 162.Scott J.D. Cyclic nucleotide-dependent protein kinases. Pharmacol. Ther. 1991;50(1):123–145. doi: 10.1016/0163-7258(91)90075-W. [DOI] [PubMed] [Google Scholar]
- 163.Colledge M., Scott J.D. AKAPs: from structure to function. Trends Cell Biol. 1999;9(6):216–221. doi: 10.1016/S0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- 164.Cash R., Raisman R., Ploska A., Agid Y. Dopamine D-1 receptor and cyclic AMP-dependent phosphorylation in Parkinson’s disease. J. Neurochem. 1987;49(4):1075–1083. doi: 10.1111/j.1471-4159.1987.tb09996.x. [DOI] [PubMed] [Google Scholar]
- 165.Yamamoto M., Götz M.E., Ozawa H., Luckhaus C., Saito T., Rösler M., Riederer P. Hippocampal level of neural specific adenylyl cyclase type I is decreased in Alzheimer’s disease. Biochim. Biophys. Acta. 2000;1535(1):60–68. doi: 10.1016/S0925-4439(00)00083-1. [DOI] [PubMed] [Google Scholar]
- 166.Gines S., Seong I.S., Fossale E., Ivanova E., Trettel F., Gusella J.F., Wheeler V.C., Persichetti F., MacDonald M.E. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington’s disease knock-in mice. Hum. Mol. Genet. 2003;12(5):497–508. doi: 10.1093/hmg/ddg046. [DOI] [PubMed] [Google Scholar]
- 167.Emamian E.S., Kaytor M.D., Duvick L.A., Zu T., Tousey S.K., Zoghbi H.Y., Clark H.B., Orr H.T. Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron. 2003;38(3):375–387. doi: 10.1016/S0896-6273(03)00258-7. [DOI] [PubMed] [Google Scholar]
- 168.Jorgensen N.D., Andresen J.M., Lagalwar S., Armstrong B., Stevens S., Byam C.E., Duvick L.A., Lai S., Jafar-Nejad P., Zoghbi H.Y., Clark H.B., Orr H.T. Phosphorylation of ATXN1 at Ser776 in the cerebellum. J. Neurochem. 2009;110(2):675–686. doi: 10.1111/j.1471-4159.2009.06164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pérez Ortiz J.M., Mollema N., Toker N., Adamski C.J., O’Callaghan B., Duvick L., Friedrich J., Walters M.A., Strasser J., Hawkinson J.E., Zoghbi H.Y., Henzler C., Orr H.T., Lagalwar S. Reduction of protein kinase A-mediated phosphorylation of ATXN1-S776 in Purkinje cells delays onset of Ataxia in a SCA1 mouse model. Neurobiol. Dis. 2018;116:93–105. doi: 10.1016/j.nbd.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Porpora M., Sauchella S., Rinaldi L., Delle Donne R., Sepe M., Torres-Quesada O., Intartaglia D., Garbi C., Insabato L., Santoriello M., Bachmann V.A., Synofzik M., Lindner H.H., Conte I., Stefan E., Feliciello A. Counterregulation of cAMP-directed kinase activities controls ciliogenesis. Nat. Commun. 2018;9(1):1224. doi: 10.1038/s41467-018-03643-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Genis D., Ortega-Cubero S., San Nicolás H., Corral J., Gardenyes J., de Jorge L., López E., Campos B., Lorenzo E., Tonda R., Beltran S., Negre M., Obón M., Beltran B., Fàbregas L., Alemany B., Márquez F., Ramió-Torrentà L., Gich J., Volpini V., Pastor P. Heterozygous STUB1 mutation causes familial ataxia with cognitive affective syndrome (SCA48). Neurology. 2018;91(21):e1988–e1998. doi: 10.1212/WNL.0000000000006550. [DOI] [PubMed] [Google Scholar]
- 172.De Michele G., Lieto M., Galatolo D., Salvatore E., Cocozza S., Barghigiani M., Tessa A., Baldacci J., Pappatà S., Filla A., De Michele G., Santorelli F.M. Spinocerebellar ataxia 48 presenting with ataxia associated with cognitive, psychiatric, and extrapyramidal features: A report of two Italian families. Parkinsonism Relat. Disord. 2019;65:91–96. doi: 10.1016/j.parkreldis.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 173.Lieto M., Riso V., Galatolo D., De Michele G., Rossi S., Barghigiani M., Cocozza S., Pontillo G., Trovato R., Saccà F., Salvatore E., Tessa A., Filla A., Santorelli F.M., De Michele G., Silvestri G. The complex phenotype of spinocerebellar ataxia type 48 in eight unrelated italian families. Eur. J. Neurol. 2019;27(3):498–505. doi: 10.1111/ene.14094. [DOI] [PubMed] [Google Scholar]
- 174.Palvadeau R., Kaya-Güleç Z.E., Şimşir G., Vural A., Öztop-Çakmak Ö., Genç G., Aygün M.S., Falay O., Başak A.N., Ertan S. Cerebellar cognitive-affective syndrome preceding ataxia associated with complex extrapyramidal features in a turkish SCA48 family. Neurogenetics. 2020;21(1):51–58. doi: 10.1007/s10048-019-00595-0. [DOI] [PubMed] [Google Scholar]
- 175.Chen D-H., Latimer C., Yagi M., Ndugga-Kabuye M.K., Heigham E., Jayadev S., Meabon J.S., Gomez C.M., Keene C.D., Cook D.G., Raskind W.H., Bird T.D. Heterozygous STUB1 missense variants cause ataxia, cognitive decline, and STUB1 mislocalization. Neurol. Genet. 2020;6(2):1–13. doi: 10.1212/NXG.0000000000000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mol M.O., van Rooij J.G.J., Brusse E., Verkerk A.J.M.H., Melhem S., den Dunnen W.F.A., Rizzu P., Cupidi C., van Swieten J.C., Donker K.L. Clinical and pathologic phenotype of a large family with heterozygous STUB1 mutation. Neurol. Genet. 2020;6(3):e417. doi: 10.1212/NXG.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hanamsagar R., Bilbo S.D. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Biol. 2016;160:127–133. doi: 10.1016/j.jsbmb.2015.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vegeto E., Villa A., Della Torre S., Crippa V., Rusmini P., Cristofani R., Galbiati M., Maggi A., Poletti A. The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr. Rev. 2020;41(2):bnz005. doi: 10.1210/endrev/bnz005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cocozza S., Pontillo G., De Michele G., Perillo T., Guerriero E., Ugga L., Salvatore E., Galatolo D., Riso V., Saccà F., Quarantelli M., Brunetti A. The “crab sign”: an imaging feature of spinocerebellar ataxia type 48. Neuroradiology. 2020;62(9):1095–1103. doi: 10.1007/s00234-020-02427-7. [DOI] [PubMed] [Google Scholar]
- 180.Heidelberg D., Ronsin S., Bonneville F., Hannoun S., Tilikete C., Cotton F. Main inherited neurodegenerative cerebellar ataxias, how to recognize them using magnetic resonance imaging? J. Neuroradiol. 2018;45(5):265–275. doi: 10.1016/j.neurad.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 181.Topaloglu A.K., Lomniczi A., Kretzschmar D., Dissen G.A., Kotan L.D., McArdle C.A., Koc A.F., Hamel B.C., Guclu M., Papatya E.D., Eren E., Mengen E., Gurbuz F., Cook M., Castellano J.M., Kekil M.B., Mungan N.O., Yuksel B., Ojeda S.R. Loss-of-function mutations in PNPLA6 encoding neuropathy target esterase underlie pubertal failure and neurological deficits in Gordon Holmes syndrome. J. Clin. Endocrinol. Metab. 2014;99(10):E2067–E2075. doi: 10.1210/jc.2014-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Synofzik M., Gonzalez M.A., Lourenco C.M., Coutelier M., Haack T.B., Rebelo A., Hannequin D., Strom T.M., Prokisch H., Kernstock C., Durr A., Schöls L., Lima-Martínez M.M., Farooq A., Schüle R., Stevanin G., Marques W., Jr, Züchner S. PNPLA6 mutations cause Boucher-Neuhauser and Gordon Holmes syndromes as part of a broad neurodegenerative spectrum. Brain. 2014;137(Pt 1):69–77. doi: 10.1093/brain/awt326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rusmini P., Cortese K., Crippa V., Cristofani R., Cicardi M.E., Ferrari V., Vezzoli G., Tedesco B., Meroni M., Messi E., Piccolella M., Galbiati M., Garrè M., Morelli E., Vaccari T., Poletti A. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy. 2019;15(4):631–651. doi: 10.1080/15548627.2018.1535292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Casarejos M.J., Perucho J., López-Sendón J.L., García de Yébenes J., Bettencourt C., Gómez A., Ruiz C., Heutink P., Rizzu P., Mena M.A. Trehalose improves human fibroblast deficits in a new CHIP-mutation related ataxia. PLoS One. 2014;9(9):e106931. doi: 10.1371/journal.pone.0106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hiyoshi H., Goto N., Tsuchiya M., Iida K., Nakajima Y., Hirata N., Kanda Y., Nagasawa K., Yanagisawa J. 2-(4-Hydroxy-3-methoxyphenyl)-benzothiazole suppresses tumor progression and metastatic potential of breast cancer cells by inducing ubiquitin ligase CHIP. Sci. Rep. 2014;4:7095. doi: 10.1038/srep07095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Andrei S.A., Sijbesma E., Hann M., Davis J., O’Mahony G., Perry M.W.D., Karawajczyk A., Eickhoff J., Brunsveld L., Doveston R.G., Milroy L-G., Ottmann C. Stabilization of protein-protein interactions in drug discovery. Expert Opin. Drug Discov. 2017;12(9):925–940. doi: 10.1080/17460441.2017.1346608. [DOI] [PubMed] [Google Scholar]
- 187.Simonetta K.R., Taygerly J., Boyle K., Basham S.E., Padovani C., Lou Y., Cummins T.J., Yung S.L., von Soly S.K., Kayser F., Kuriyan J., Rape M., Cardozo M., Gallop M.A., Bence N.F., Barsanti P.A., Saha A. Prospective discovery of small molecule enhancers of an E3 ligase-substrate interaction. Nat. Commun. 2019;10(1):1402. doi: 10.1038/s41467-019-09358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Starita L.M., Pruneda J.N., Lo R.S., Fowler D.M., Kim H.J., Hiatt J.B., Shendure J., Brzovic P.S., Fields S., Klevit R.E. Activity-enhancing mutations in an E3 ubiquitin ligase identified by high-throughput mutagenesis. Proc. Natl. Acad. Sci. USA. 2013;110(14):E1263–E1272. doi: 10.1073/pnas.1303309110. [DOI] [PMC free article] [PubMed] [Google Scholar]