Abstract
The human digestive system is embedded with trillions of microbes of various species and genera. These organisms serve several purposes in the human body and exist in symbiosis with the host. Their major role is involved in the digestion and conversion of food materials into many useful substrates for the human body. Apart from this, the gut microbiota also maintains healthy communication with other body parts, including the brain. The connection between gut microbiota and the brain is termed as gut-brain axis (GBA), and these connections are established by neuronal, endocrine and immunological pathways. Thus, they are involved in neurophysiology and neuropathology of several diseases like Parkinson’s disease (PD), Alzheimer’s disease (AD), depression, and autism. There are several food supplements such as prebiotics and probiotics that modulate the composition of gut microbiota. This article provides a review about the role of gut microbiota in depression and supplements such as probiotics that are useful in the treatment of depression.
Keywords: Gut microbiota, gut-brain axis (GBA), depression, probiotics, prebiotics, food supplement
1. INTRODUCTION
1.1. Gut and Brain
One of the most striking features of vertebrae is the coordination between various cells to function as a whole. These complex systems, which arose through evolution, continue to amaze mankind in several ways. At a higher level, the human brain coordinates many organs together to help in functioning, perception, speech and writing, and several phenomena for existence. This integration between brain and body parts is extensively studied. One of such synergistic biological functioning exists between the brain and the gut. Though there is a range of established facts between these two vital organs, there might be many more points left unknown that connect neuro-functioning with gut physiology.
Orexigenic and anorexigenic effects in the gastrointestinal tract are promoted and mediated to the brain via major gastrointestinal hormones known as ghrelin and leptin. The endothelial lining of the stomach and pancreas are responsible for the synthesis of the peptide ghrelin, which travels all the way long to the blood-brain barrier, crosses it and reaches the hypothalamus, stimulating the signal of hunger.
On the contrary, adipose tissue is responsible for the secretion of leptin, the appetite-suppressing hormone that stimulates the hypothalamus to cease food intake. The facts about the mechanism of action of these amines are well-established and considered to be involved in metabolism and maintaining energy balance [1].
Other than hormonal connection, there exist several other types of connections between the digestive system and neurological system. Among these, few were explored, and few are left to explore.
1.2. Association between Digestive and Neurological Systems
The connection between gut and brain can be broadly classified into two types - anatomical connection and physiological connection. Anatomical connection involves the structural framework of nerves between these two organ systems, whereas physiological connection is further classified into immunological and biochemical connections.
1.2.1. Neuronal Connection
The enteric nervous system (ENS), which is a part of the autonomic nervous system (ANS), is the major pathway through which the guts and brain communicate. The most crucial part of ENS is the vagus nerve that contains millions of neuronal synapses. The colonic part of the gut is studded by layers of enteroendocrine cells, which secrete hormones that help in digestion and hunger suppression. These cells communicate with foot-like projections, which resemble neuronal synapses [1]. Bohórquez and his colleagues conducted a study in mice by injecting fluorescent rabies virus into colons of mice and found that they lighted up in the brain stem region. Thus, he concluded that the enteroendocrine cells synapse with the vagal neurons and communicate using the secreted hormones which are carried through electrical signals to the brain stem within 100 milliseconds, i.e., less than an eye blink [1].
Many studies show that individuals suffering from gastrointestinal problems also suffer from neurological problems such as stress, anxiety, and depression, and vice-versa. Few conditions such as irritable bowel syndrome (IBS) [2] and Crohn’s disease [3] cause a decline in the vagal tone, indicating reduced vagal nerve function. In certain stress conditions, the vagal nerve signals are reduced, which leads to gastrointestinal problems such as abdominal pain and constipation [4]. In a study conducted in male rats, sub-diaphragmatic vagal deafferentation (SDA) was performed to study the variations in behavioral patterns. It was proved that SDA rats consistently displayed anhedonic behavior and increased immobility [5]. Also, to check the connectivity between the gastrointestinal tract and the parts of the brain, such as the hippocampus, which are responsible for episodic and spatial memory, a study was conducted in rats by using the saporin-based lesioning (SAP) procedure. It revealed that ablation of selective gastrointestinal vagal afferent neurons impaired hippocampus-dependent spatial and episodic memory. This establishes the neuronal connectivity between gut and brain [6]. Furthermore, genetically guided mapping of GPR65 and GLP1R neurons, in vivo imaging, and optogenetics directly proved that vagal sensory neurons detect stretch and nutrients in the digestive system [7].
1.2.2. Biochemical Connection
Communication between gut and brain through biochemical systems occurs via the use of chemical messengers called “neurotransmitters”. They include nitric Oxide (NO), tryptamine, GABA, and monoamines such as dopamine, serotonin, and noradrenaline.
Few of these neurotransmitters, such as NO, are produced by few species of gut microbes. These bacteria produce nitric oxide synthase, which is involved in the conversion of dietary nitrite to NO. Thus, the produced microbial NO is involved in many functions of the brain, but its exact role is not clearly understood.
Tryptamine is a neurotransmitter produced by bacteria known as Clostridium sporogenes, which convert dietary amino acid tryptophan to tryptamine using the enzyme tryptophan decarboxylase. Tryptamine causes the release of serotonin from enterochromaffin cells. Serotonin is implicated in many neurological functions, such as stimulation of enteric neurons, which further stimulate gastric motility [8]. Few types of enteric bacteria, such as Lactobacillus and Bifidobacterium, produce GABA. This compound is one of the major types of inhibitory neurotransmitters in the brain. Its effect in the gut is multiplied because it is involved in the control of several functions such as gastric acid secretion, gastric motility, and emptying.
2.3. Immunological Connection
The immunological relationship between the brain and gut majorly involves immune cells such as microglia that function upon the presence or absence of microbial barrier along the length of the intestine, which facilitates the exchange of water, nutrients, and electrolytes. It prevents the entry of toxic substances, pathogens, and antigens into the bloodstream from GI lumen [9]. The activation of pro-inflammatory mediators further activates immune cells such as microglia, T-cells, and macrophages, upon disruption of this microbial barrier in the intestine that causes activation of cytokines. Excessive activation of these cytokines, which are BBB permeable, leads to the stimulation of brain microbial cells that may cause inflammation in the brain. If this persists for a long duration, it may lead to brain stroke and autoimmune disorders such as multiple sclerosis [10]. In the mucosal immune system, the infiltration process of lymphocytes into the intraepithelial compartment of the intestine occurs as a defense mechanism against invading pathogens. The ‘stressor’ conditions invoked in the intestine are carried to the brain by paraventricular nuclei (PVN),which activate the sympathetic response against it [11]. Therefore, immune cells play a vital role in maintaining integrity between gut and brain and are involved in maintaining homeostasis.
2. ROLE OF GUT MICROBIOTA IN NEURO-PHYSIOLOGY
Gut microbiota is a community of microbes that exist in a symbiotic relationship with its host in the intestine. This flora consists of unique assemblies of microorganisms such as bacteria, archaea, fungi, and viruses that colonize in the gut after birth [12]. Evidences show the maternal inheritance of these gut flora and postnatal development of these organisms [13]. Few types of flora are acquired during the ingestion of certain foods. It was once believed that every individual possesses a unique set of commensal bacteria, which is a myth. After several studies of deep sequencing on stool samples of hundreds of individuals, it was proved that bacteria are dominated by three classes of enterotypes, i.e., Bacteroides, Prevotella, and Ruminococcus [14]. Functions of these enterotypes vary depending on strain but all over, they perform multiple functions such as modulating gut motility, maintaining the intestinal barrier, distribution of visceral fat, development of innate and adaptive immunity, etc. [15].
Apart from these functions, these commensal bacteria are also involved in regulating neurophysiology by being a part of the gut-brain axis (GBA). Gut-brain axis (GBA), also referred to as Brain-Gut-Microbiome Axis (BGM), is a bi-directional communication system that integrates nervous, gastrointestinal, and immune systems [16]. It involves all the mechanisms previously discussed, such as neuronal, bio-chemical, and immunological connections. In addition, few other compounds such as secondary bile acids, dopamine, and short-chain fatty acids (SCFAs) are also involved in signaling between the brain and gut [17, 18]. The mechanisms of these compounds in signaling are complex and not clearly understood.
2.1. Potential Clinical Evidence for the Role of Gut Microbiota in Neurophysiology
The postnatal colonization of commensal bacteria has a long-lasting effect on the host’s brain physiology and neural plasticity. The impact continues even during brain development and behavior and has persistent implications on health. This was proved both in rodents as well as in humans. Listed below are a few of the clinical and pre-clinical evidence for the role of gut flora in neurophysiology (Table 1).
3. DIETARY SUBSTITUENTS WHICH CHANGE THE COMPOSITION OF GUT MICROBIOTA
It is a well-established fact that the human gastrointestinal tract harbors trillions of microbes. It is also proved that these organisms take part in several physiological functions in our body. Evidence mentioned above in Table 2 also encourages the fact that few genera are capable of performing certain particular bodily functions. When the average count of those genera is disrupted, these functions also show variations. Therefore, the gut microbiota composition needs to be maintained for the normal health of an individual [26, 27]. In this context, certain dietary substituents and nutrients should be ingested to conserve a healthy host-microorganism balance, consequently maintaining the intestinal barrier and preventing disease development. Diet is one of the key modulators of gut microbiota. Let us now look into a few dietary substituents and supplements and their effect on gut microbiota.
3.1. Carbohydrates
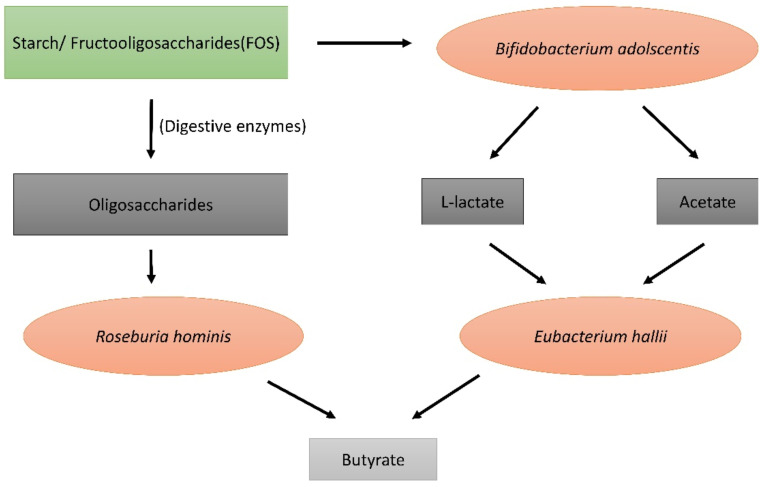
Carbohydrates obtained from food can be categorized into 3 types: simple sugars, soluble polysaccharides, and insoluble complex carbohydrates. Simple sugars such as glucose, fructose, galactose, and soluble polysaccharides such as starch are enzymatically degraded in the upper part of the intestinal tract. The insoluble complex carbohydrates such as indigestible starch, xylan, pectin, inulin, and cell-wall derived polysaccharides and oligosaccharides reach the lower part of the gastrointestinal tract (lower colon), where they undergo bacterial fermentation to yield certain partially absorbed gases such as hydrogen and methane and short-chain fatty acids (SCFAs) which are highly absorbed in the lower colonic region of the intestinal tract [28]. These SCFAs are produced by fermentation by various species of gut microbes residing in the colonic region. They include species such as Eubacterium, Roseburia intestinalis, Ruminococcus, Coprococcus, Butyrovibrio fibrosolvens, and Clostridium saccharolyticum [29]. Few species such as Eubacterium ferment only particular types of polysaccharides such as fructooligosaccharides (FOS). Here is an example of how these species convert complex carbohydrates into simple SCFAs (Fig. 1).
The SCFAs thus produced are L-lactate, acetate, propionate, and butyrate. They have their own roles once absorbed into the blood [30].
Bacterial composition in the gut varies with the type of carbohydrate intake. Digestible carbohydrates like glucose, fructose and sucrose reduce the levels of species like Bacteroides and Clostridia. Non-digestible carbohydrates like starch consistently increase lactic acid-producing bacteria such as E.rectale, Roseburia, Ruminococcus, etc., while reducing Clostridium and Enterococcus species. Bifidobacterium species are increased by both digestible and non-digestible carbohydrates. This occurs only in people who do not consume alcohol. In individuals with high alcohol intake, species of Bifidobacterium are significantly lower compared to normal individuals even though they consume both digestible and non-digestible carbohydrates.
3.2. Proteins
Protein consumption is shown to correlate with microbial diversity in the intestine. Individuals consuming only plant proteins exhibit an increase in species such as Bifidobacterium and Lactobacillus and also a decline in pathogenic species such as Bacteroides fragilis and Clostridium perfringes. Likewise, individuals consuming animal protein such as meat and beef along with plant protein showed an increase in species such as Bacteriodes, Alistipes, and Bilophila [31]. This was proved in a study conducted on Italian children who consume a lot of animal protein-based diet compared to rural African children [32]. The fermentation of amino acids in the distal colon is done by genera such as Firmicutes, Bacteriodetes, and Proteobacteria. Proteolytic fermentation also produces SCFAs but lesser than that by saccharolytic fermentation.
Consumption of a high protein diet is also a detriment to health. It leads to a reduction in carbohydrate consumption which causes weight loss but reduces butyrate-producing bacterial number in the intestine leading to a pro-inflammatory state. At times, it may lead to colorectal cancer [33].
3.3. Fats
Fat quantity and quality can also affect the diversity of gut microbiota. Diets high in fat interact with gut microbiota in various ways. This interaction facilitates the translocation of lipopolysaccharides (LPS) which causes inflammatory action, and it may persist [34]. Firmicutes increase lipid absorption in the form of droplets of various sizes through intestinal epithelia. An increase in LPS content in the diet may lead to the incorporation of it into the lipid droplets and may interfere with regular lipid absorption.
As plant-derived diet is generally low in fat content, it favors beneficial butyrate-producing bacteria such as Bifidobacteria. This diet contains mono and polyunsaturated fatty acids, which increase Bacteriodetes and Firmicutes species, and also lactic acid bacteria such as Akkermansia muciniphilia. However, on the whole, plant-derived fat decreases the level of Clostridium species. Saturated fat found exclusively in animal sources increases Faecalibacterium prausnitzii and decreases Bifidobacteria [35]. This may cause an increase in the levels of inflammatory mediators such as IL-1, IL-6, and TNF-α. This may lead to metabolic disorders. Saturated fat consumption is known to increase the risk of cardiovascular and metabolic diseases. It also reduces the levels of Bacteroides, Bacteriodetes, Prevotella, Lactobacillus, and Bifidobacterium species [36].
3.4. Probiotics
Probiotics are defined as ‘live microbial food ingredient beneficial to health.’ Mostly, the probiotics consumed as food supplements belong to Lactobacillus or Bifidobacterium genera as they have highly beneficial effects and safety and also have been investigated for effectiveness against a lot of gastrointestinal disorders [37]. In diseases such as lactose indigestion, diarrhoea, inflammatory bowel disease (IBD), and Crohn’s disease, the use of probiotic supplements helps regrow the compromised gut microbial composition, thus restoring the physiology of the intestinal tract. In the case of lactose intolerance, the non-fermented type of lactose consumed by certain individuals is not digested in the intestine leading to symptoms like nausea, bloating, and diarrhoea [38]. If these individuals consume fermented lactose (in dairy products like yoghurt), lactase-producing bacteria are present, which release lactase enzyme when lysed by bile secretions in the gut. The lactase thus released acts on lactose present in the food. Therefore, there are no symptoms of lactose maldigestion. However, in the case of consumption of non-fermented dairy products (such as raw milk), there is no lactase enzyme produced by the bacteria. Hence, the lactose remains in the gut, causing intolerance. In such cases, the intake of Lactobacillus or Bifidobacterium genera helps in the digestion of lactose as they produce the enzyme lactase [39].
In chronic diseases like inflammatory bowel disease (IBD), there is persistent and recurrent mucosal inflammation. The plausible mechanism of inflammation of gut mucosa may be due to the immunological reaction to some members of gut microbiota. Probiotic administration may help reduce the severity either by regulating the inflammatory response or by altering the gut microbial composition. The majorly known mechanism is the interaction of probiotics with mucosal T-reg cells and regulation of cytokine transcription factors in response to foreign bacteria. Certain strains of probiotics used for the treatment of IBD include E.coli, which maintains the remission of ulcerative colitis [40]. Streptococcus boulardii reduces bowel movements, incidents of relapse [41], and VSL#3, which reduces inflammation and also the risk of relapse. VSL#3 is a combination of 8 species of bacterial strains, which are mentioned in Table 2 below [42].
Diarrhoea is another common digestive problem associated with symptoms like loose stools and abdominal discomfort. Diarrhoea can occur due to multiple reasons. It is therefore classified into several types such as infantile diarrhoea, antibiotic-associated diarrhoea, inflammation-associated diarrhoea, and travellers’ diarrhoea. In the case of acute infantile diarrhoea, the infection is mainly due to rotavirus. Probiotic treatment containing Lactobacillus rhamnosus GG is shown to reduce diarrhoea up to 50%. The mechanism of action is still unclear, but it might be due to the stimulation of anti-rotavirus specific immunoglobulin A (Ig A). Other combinations of probiotics to reduce rotavirus infection include Streptococcus thermophiles with Bifidobacterium bifidum [43]. In diarrhoea caused due to antibiotic treatment, species such as Lactobacillus rhamnosus GG are used especially for triple therapy of antibiotics (Rabeprazole, Clarithromycin, and Tinidazole), which is used to treat H. pylori infection. Other strains such as Lactobacillus acidophilus, Lactobacillus johnsonii, Bifidobacteria, and Streptococcus boulardii also show similar effects [44]. Even in the case of travellers’ diarrhoea, Lactobacillus rhamnosus GG showed effective results [45].
3.5. Prebiotics
A prebiotic is ‘a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth/activity of one or a limited number of bacteria in the colon, thus improving host health.’ The most commonly used prebiotics are fructooligosaccharides (FOS) and inulin. These are fermentable fructans that stimulate the growth of Bifidobacteria in the intestine. Bifidobacteria release butyrate as the end product, which is highly useful for fortification of the gut epithelial wall and stimulation of immune response [46].
In the case of colon cancer, there is a high rate of genotoxicity and mutagenesis. Certain types of dietary fibres such as inulin and FOS are present in foodstuff like onion, garlic, and asparagus. They undergo bacterial fermentation in the gut as they convert into soluble fibres, which have several activities like the growth of Bifidobacteria, reducing the growth of Bacteroides, Clostridia, and coliforms. In individuals having colon cancer, these fibers are administered in the form of food supplements. The protective effect of these prebiotics in colon cancer is proved in a study conducted on rats [43]. In this study, male F344 rats were induced with colon cancer using a colon-specific carcinogen, AOM, subcutaneously. This carcinogen induces ACF (Aberrant Crypt Foci), early preneoplastic lesions in the colon. Except for the control group, all other groups were fed either with experimental diets of 10% oligofructose or 10% inulin. After the 7-week study, the animals were necropsied, and the colon region was studied for ACF lesions. The groups administered with oligofructose and inulin showed a reduced number of ACF lesions compared to the control group, which did not receive any prebiotic supplementation. Out of both prebiotics, the degree of ACF inhibition was more pronounced in rats fed with inulin compared to oligofructose. This proves that prebiotics help reduce the mutagenic ability of the APC gene and ras oncogene caused due to ACF lesions, and the SCFAs and butyric acid reduced by fermentation of these prebiotics increase apoptosis in the colonic region [47, 48].
In the case of inflammatory diseases like inflammatory bowel disease (IBD) and ulcerative colitis (UC) [49], there is a lot of mucosal damage due to bacterial translocation. To reduce the severity, high levels of butyrate are required, which can maintain remission of IBD by accelerating the healing process. Dietary fibres upon fermentation produce butyrate. Glutamine present especially in germinated barley and other hemicellulose-rich fibres can alleviate the symptoms both in animal models and human studies [50]. The process of healing generally occurs in 3 steps: decreasing stool frequency, increasing concentration of butyrate, and increasing the number of Bifidobacteria and Eubacteria.
4. ROLE OF PROBIOTICS ON BRAIN HEALTH
It is already a well-established fact that brain health depends upon the composition of gut microbiota. There is a growing appreciation of the role of gut microbes in brain health and disease. Depletion or modulation of gut microbes can severely affect central pathology or behavioral patterns observed in a variety of brain disorders [51]. Additionally, both preclinical and clinical evidence show that targeting gut microbial composition with prebiotics, probiotics, and dietary interventions may be an effective ‘psychobiotic’ strategy for treating neurodegenerative and neurodevelopmental disorders.
In stress-related disorders like depression and anxiety, consumption of dietary supplements such as omega-3-fatty acids proved to reduce the severity, especially in animal models. In a study conducted on C57BL/6 mice, pregnant animals were fed with omega-3-deficient (O3-) or omega-3 supplemented (O3+) diet, and cognitive, depressive, and social behaviors were assessed by a battery of behavioral tests in the male offspring at both adolescence and adulthood. The O3 intervention induced behavioral changes in offspring both during early-life and adolescence. O3- animals displayed deprived communication, social and depression-related behaviors, whereas O3+ animals displayed enhanced cognition. Fecal microbiotal composition was analyzed by 16S RNA sequencing, and O3+ animals showed greater fecal Bifidobacterium and Lactobacillus count compared to O3- mice. Also, there was dampened HPA activity in O3+ mice compared to O3- mice indicating reduced corticosterone production and reduced stress [52]. In another study conducted on female Sprague-Dawley rats, animals were administered with omega-3-fatty acids like eicosapentaenoic acid (EPS) and docosahexaenoic acid (DHA) (80% EPA and 20%DHA). Initially, animals were subjected to early-life stress, maternal separation (MS) procedure from postnatal days 2 to 12. Non-separated (NS) and MS rats were administered with saline and EPA/DHA, respectively. In adulthood, EPA/DHA treated rats had a dose-dependent reduction in anxiety compared to NS rats. EPA/DHA decreased behavioral despair in the forced swim test [53].
Change in the dietary pattern has also shown improvement in social behavior as it is a less costly and safer type of therapy. In a randomized control study conducted by Jacka et al., it was demonstrated that a modified Mediterranean style of diet was beneficial in treating mild depression. The symptomology of depression was improved substantially when assessed by Montgomery-Åsberg Depression Rating Scale (MADRS) after 12 weeks of the dietary trial [54]. Similarly, the modifications in the dietary patterns also helped in improving autism-related behavior. Treating BTBR mice as well as environmental models of autism with a high-fat, low-carbohydrate ketogenic diet helped improve deficits in social behavior.
5. NEUROPATHOLOGIES AND THEIR ASSOCIATION WITH GUT
Many neuropathological and neurodegenerative diseases are associated with the Gut-Brain Axis (GBA). Several preclinical and clinical investigations suggested that diseases like Alzheimer’s disease (AD) and Parkinson’s diseases (PD) are associated with this GBA via immunological, neuroendocrine, and direct neural connections [4]. The gut microbiota is able to synthesize numerous neurotransmitters and neuromodulators such as acetylcholine, ϒ-aminobutyric acid (GABA), histamine, dopamine, serotonin, or SCFAs. The production of these neurochemicals enables intracellular communication between members of the microbiota. Therefore, the existence of ‘microbiotal organ-specific nervous system’ based on the species of microbes present in the intestine is hypothesized. This microbial endocrinology-based pathway is a loop i.e., gut microbiota affects the brain health, and the brain, in return affects the microbiotal composition [55]. Firstly, food ingested by the host contains compounds required for neurochemical production directly by the host or by the gut microbes, or else, fully functional neuroactive components may be present in the food itself. The gut microbiota may get influenced by the compounds in the food or by the neuroactive chemicals produced by the host’s enteric nervous system (ENS). Otherwise, the microbiota is capable of producing neurochemicals from the substrates of host’s food. Thus produced neurochemicals have two ways of influencing the hosts. One, the neurochemicals enter the portal circulation and reach the brain. Two, they directly act upon the receptors found on synaptic endings of ENS, which are present all over the lining of GIT, which in turn influences components of the nervous system and the brain. The result of this pathway is an alteration in brain behavior and cognition as well as appetite and food preference. As mentioned earlier, this, in return, influences the composition of the gut microbiome through the specific release of neurochemicals in the gut lumen [56].
As stated in the above Fig. 2, the same type of mechanism is observed even when the host is supplemented with prebiotics or probiotics [57-59].
Neuropathologies of degenerative diseases such as PD and AD have a direct connection with GBA, especially through ENS. As ENS has many branches that innervate all regions of GIT, any alteration in the gut can directly stimulate the synapsis of ENS, which further leads to an effect on the brain [60].
PD is usually characterized by α-synucleinopathy, i.e., misfolding of a component called α-synuclein, which keeps aggregating in regions of CNS such as substantia nigra. This leads to symptoms such as tremors, bradykinesia, rigidity, and postural instability. Excessive stimulation of the innate immune system resulting from bacterial overgrowth in the gut or gut dysbiosis may cause increased intestinal permeability, which further leads to systemic inflammation. The systemic inflammation or activation of enteric neurons and glial cells may cause misfolding of α-synuclein [55].
The integrity of the intestine and brain is vital for brain development and function. Entire GIT is lined with a layer of epithelial cells that form a mucosal barrier to protect from the entry of harmful chemicals, bacteria, and other toxins into the bloodstream. The layer of cells is connected by tight junctions that maintain permeability across the gut. Disruption in this intestinal barrier due to gut microbial dysbiosis or infections may result in increased inflammatory levels. This process is referred to as ‘leaky-gut’ [61]. The cytokines produced during inflammation permeate into the bloodstream provoking systemic inflammation accompanied by several diseases. Intestinal changes gradually lead to the disruption of the integrity of the blood-brain barrier (BBB), allowing the entry of toxic substances into the brain. This process is referred to as ‘leaky-brain’, and this disrupts brain development and function, causing many neurodegenerative disorders [61]. One such toxic component is lipopolysaccharide (LPS). LPS is a major constituent of the cell wall of gram-negative bacteria. It is an endotoxin and a serious inducer of inflammatory response. In the case of ‘leaky-gut’, it translocates into the bloodstream provoking a systemic inflammatory response. In the case of ‘leaky-brain’, it permeates the BBB to reach the brain and causes prolonged elevation of hippocampal amyloid-β (Aβ). Aggregation of Aβ in the brain leads to cognitive deficits and further progresses into AD [62]. Thus, increased influx-efflux of LPS between blood and brain causes loss of memory and AD progression [63].
Apart from neurodegenerative disorders like AD and PD, other neuro-functional disorders like depression, psychosis, anxiety, schizophrenia, etc., which occur due to stress, are also linked to the composition of gut microbiota and the activities associated with them. In general, both physical and psychological stress activates the hypothalamic-pituitary-adrenal axis (HPA), resulting in the release of corticotrophin-releasing hormone (CRH), which is a principal regulator of the HPA axis itself [64]. This CRH induces the release of an adrenocorticotropic hormone (ACTH) into the bloodstream. This ACTH acts on the adrenal cortex and stimulates glucocorticoid synthesis. Glucocorticoids, thus produced in turn, downregulate the HPA axis [65, 66]. Both glucocorticoids and catecholamine (adrenaline and noradrenaline) are released into the circulation during physical and psychological stress. Prolonged stress causes the continuous release of glucocorticoids and catecholamines into the blood leading to disorders related to stress-like depression and anxiety. It is already a well-established fact that GIT is sensitive to stress mediators like catecholamines [67]. The enteric bacteria can respond to stress-related neurochemical mediators released by the host. As few species of enteric bacteria are capable of producing several neuro-active chemicals that act on synapses of ENS present in the intestinal wall, the activation of ENS by stress-related neuroactive compounds in the bloodstream can be known by the enteric bacteria.
In several preclinical studies conducted, it is evident that gut microbiota is responsible for the expression of anxiety-like behavior and behavioral despair. Germ-free (GF) maternally separated mice showed higher levels of corticosterone in blood and also cholinergic nerve dysfunction compared to specific pathogen-free (SPF) maternally separated mice [68]. This indicates that the occurrence of behavioral changes alters in the presence of gut microbiota [69].
The scope of this article is only depression. Therefore, only the neuropathology of depression and the association of gut microbiota with it are discussed in this article.
6. PATHOPHYSIOLOGY OF DEPRESSION
Depression is a common psychiatric disorder ranging from a mild condition which is almost near normal to a severe condition which is accompanied by hallucinations, delusions, anxiety and suicidal tendency. Depression is a heterogeneous disorder with few or more core symptoms like eating disorders, addiction, sleep disturbance, retardation of thought and action. There are two types of depression- unipolar and bipolar depression. In unipolar depression, all the mood changes are in the same direction. However, in bipolar depression, depression alternates with mania. Unipolar depression is common and is clearly associated with stressful events of life and usually accompanied by symptoms like anxiety and agitation. Therefore, it is also termed as ‘reactive depression’. Bipolar depression is usually rare and is observed in early adult life and results in oscillating depression and mania over a period of a few weeks.
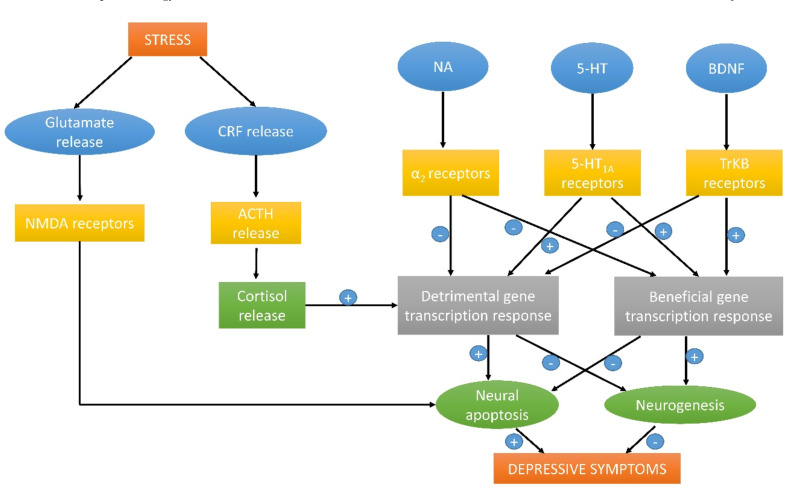
Depression cannot be attributed to altered neuronal activity within a single brain region. Rather, the circuitry linking different parts of the brain may be affected. Brain imaging studies show that areas like the prefrontal cortex, amygdala, and hippocampus may be involved in different components of these disorders. Several theories manifest the pathophysiology of depression (Fig. 3).
6.1. Monoamine Theory
Neurotransmitters like 5-HT (5- Hydroxytryptamine) and nor-adrenaline are considered monoamines as their chemical structure contains a single amino group. According to the monoamine theory proposed by Schildkraut in 1965, depression results from functionally deficient monoaminergic transmission in the CNS. Deficiency of these monoamines in parts of the brain may cause neurodegeneration and also reduced neurogenesis in the hippocampus.
6.2. Neuroendocrine Mechanisms
Hypothalamic neurons in the brain that control pituitary function receive nor-adrenaline and 5-HT inputs, which control the discharge of these neurotransmitters. Hypothalamic neurons release corticotrophin-releasing hormone (CRH), stimulating pituitary neurons to secrete ACTH, in turn leading to cortical secretion. Usually, in depressed patients, cortisol levels are high in the blood, which leads to translocation of it into the brain through BBB. Once in the brain, high cortisol levels cause depressive symptoms like diminished neural activity, loss of appetite, and increased signs of anxiety. Furthermore, hypoactivity of monoamines and hyperactivity of cortisol lead to chronic stress and prolonged depression [70].
6.3. Trophic Effects and Neuroplasticity
It is believed that lower levels of a brain-derived neurotrophic factor (BDNF) or malfunction of its receptor TrKB play a vital role in the pathology of depression. Also, glycogen synthase kinase 3 (GSK3β) is also implicated in the pathology of depression.
Glutamatergic neurotransmission is also involved in depression. Changes in glutamate levels in the cerebral cortex are an important implication of depression. Other evidence is that depression is majorly associated with neuronal loss in the hippocampal and prefrontal cortex [71].
7. ROLE OF CHOLINERGIC SYSTEM AND ITS INTERPLAY WITH GABAERGIC SYSTEM IN DEVELOPMENT OF DEPRESSION
7.1. Cholinergic Neurotransmission in Depression
In the cholinergic system, the major neurotransmitter is acetylcholine, and the receptors involved are muscarinic and nicotinic receptors. Muscarinic receptors are of 5 sub-types, and they belong to the category of G-protein coupled receptors [72]. Nicotinic receptors belong to the family of ligand-gated ion channels and are divided into 3 groups (α1, β1, δ, ε, γ), (α2-α6, β2-β4), (α7-α9) [73].
According to the physiological evidence, depression involves over activity of the cholinergic system. Increased level of choline, the precursor of acetylcholine in the brains of individuals with depression was identified through neuroimaging studies [74]. Targeting the muscarinic receptors and blockade of M1 receptors in the nucleus accumbens region of brain caused anti-depressant effect in rats [75].
7.2. GABAergic Neurotransmission in Depression
There are three classes of GABA receptors (A, B, C). GABAA,C receptors are ligand-gated ionotropic whereas GABAB are metabotropic transmembrane receptors. GABAA,C are post-synaptic inhibitory receptors, and thus when GABA is bound to them, they cause hyperpolarization of nerve membrane, increasing threshold depolarization potential for excitatory neurotransmitters. Consequently, the neuron will not be able to generate action potentials [76].
Major observation in patients of depression was a low level of GABA in cerebrospinal fluid in comparison with healthy individuals. In subsequent research, it was proved that regions of the brain such as the prefrontal cortex and hippocampus expressed a lesser number of GABA receptors in individuals with depression [77]. Also, reduced expression of somatostatin, a commonly associated neurotransmitter with GABAergic interneurons, was observed in the brains of depressive patients, especially in regions of the amygdala and subgenual cingulate [78].
7.3. Relation between Cholinergic and GABAergic Systems in the Development of Depression
Hippocampus plays a vital role in this relation as it contains cholinergic and GABAergic receptors. It produces theta waves which are linked to emotional behavior in animals. There are two types of theta waves produced by the hippocampus- T1 and T2 [79]. Emotional excitement or suppression increases or decreases T1/T2 ratio. The regions of the hippocampus, such as the medial, septal nucleus, and the vertical limb of the diagonal band of Broca, are involved in the generation and modulation of theta waves [80] as they have neuronal innervations from GABAergic, cholinergic and glutamatergic systems. It was found that septohippocampal cholinergic afferents cause a reduction in the inhibitory postsynaptic potentials (IPSPs) which are already evoked. GABAergic neurons are also implicated in mediating inhibition of action potentials. However, the blockade caused by GABA causes stimulation of their afferent inputs. This effect is termed as ‘discriminative inhibition’ whereby inhibition of one region causes excitation of another [81].
Both activities are equally necessary to elicit the theta waves. Increase in cholinergic activity is required to elicit the waves along with an increase in cholinergic activity accompanied by subsequent reduction in GABAergic activity. Both activities occur in a reciprocal fashion. Theta waves are implicated in maintaining memory, emotion, and stress. The cholinergic input to the hippocampus mediates the slow disinhibitory effect by inactivating several K+ conducting channels. Increased disinhibition of K+ by excessive acetylcholine relays the rhythmic activity of theta waves. This disinhibition is coupled with rhythmic inhibition by GABAergic neurons. Therefore, any dysregulation in levels of acetylcholine or GABA may lead to asynchrony of theta waves which is known as large irregular amplitude (LIA) [81]. Increase in acetylcholine and a decline in GABA and their neuronal activity is seen in the pathophysiology of depression, as discussed earlier [74,77]. Thus to conclude, the cholinergic and GABAergic system and their activation in the hippocampal region is a potential variant in depression development.
8. LINK BETWEEN PATHOPHYSIOLOGY OF DEPRESSION AND GBA
As mentioned earlier, stress is associated with HPA hyperactivity and also increased intestinal permeability. It is evident that, indirectly, the gut microbiota has an influence on the HPA axis and intestinal permeability in a study conducted on GF mice [82]. The direct influence of the gut microbiome on stress circuits in CNS is also observed lately. In a study conducted on CF-1 male mice, it was demonstrated that bacteria residing in GIT could activate stress circuits through vagal pathways [83]. This was proved when CF-1 male mice were ingested with food-borne pathogens like Camphylobacter jejuni [84]. When a neural activation marker cFOS was observed in vagal sensory neurons during the early phase of C.jejuni infection, it was proved that gut bacteria stimulated the stress response even before the presence of systemic immune response. Apart from vagal neurons, even central brain regions showed cFOS activation. This establishes that commensal bacteria communicate the stress response via the neuronal pathway [85].
Sensory neurons of the myenteric plexus in the ENS are the first point of contact for intestinal microbiota residing in the gut lumen. These sensory neurons synapse with enteric motor neurons, which control gut motility. Therefore, in the case of the stress response, gut microbiota activates the sensory myenteric plexus neurons, which in turn activate enteric motor neurons. Altogether, there are alterations in electrophysiological properties in ENS neurons due to changes in commensal bacteria. ENS is connected with higher regions of the brain. Thus, the brain is informed about changes occurring in the gut lumen.
Intestinal microbes have the ability to alter levels of neurotransmitters by modifying their precursor metabolism. Tryptophan is one such precursor. Its metabolism occurs in three major pathways: 1) direct metabolism by gut microbes, 2) the kynurenine pathway in immune cells, and 3) serotonin production pathway in enterochromaffin cells. In the case of the direct metabolic pathway, the gut microbes produce few ligands for the aryl hydrocarbon receptor (AhR). These ligands include indole-3-acetic acid (IAA), indole-3-propionic acid (IPA), indole-3-acetaldehyde (IAAld), and indole arylic acid [86]. AhR signaling is a key component in triggering an immune response and may communicate with the brain to activate the sympathetic system against it. Otherwise, commensal bacteria such as Clostridium sporogens have the capability to decarboxylase tryptophan into neurotransmitter tryptamine [87]. An increase in dietary consumption of tryptophan elevated mood in normal human subjects, whereas tryptophan-deficient diet caused mild deterioration in mood [88]. As tryptophan is a precursor of tryptamine and tryptamine acts as a neuromodulator in the serotonergic system in the brain, alterations in levels of dietary tryptophan can have an effect on mood. In healthy individuals, it might not cause major changes, but in patients with major depression, it can have a huge impact. Neuroinflammation plays a key role in depression development, and gut bacteria play a key role in the promotion of neuroinflammation [89]. A study conducted in patients with major depression proved that increased levels of serum IgA and IgM were observed in patients with depression compared to controls. The results showed increased mucosal dysfunction caused increased LPS (Lipopolysaccharide) translocation into systemic circulation evoked inflammatory response [90]. Thus, serum values IgA and IgM were high. Therefore, patients with depression should be checked for gut mucosal dysfunction and treated accordingly.
9. EFFECT OF PROBIOTICS ON DEPRESSION - PRECLINICAL AND CLINICAL EVIDENCE
Combining all the studies in animals and humans, probiotics have a positive effect on improving the central nervous system function. As mentioned earlier, most studies used Bifidobacterium and Lactobacillus preparations and all of them were effective in improving CNS functions. Few species like B.longum, B.breve, B.infantis, L.helveticus, L.rhamnosus, L.plantarum, and L.casei were most effective in treating psychiatric disorders like depression, anxiety, mood and stress response, etc. Usually, 2 weeks of probiotic supplementation showed sufficient effects. Many studies on probiotics were conducted on animals. More studies should be conducted on humans. Also, the translation of animal studies to humans may be applicable. Human studies can be conducted using the same probiotics, but due to ethical regulations, they are not conducted extensively. Still, there is a need for the development of more neuroimaging studies rather than using only psychological questionnaires or scales. Therefore, there is more preclinical data available than clinical data for the effect of probiotics on depression. Table 3 contains few studies on animals and humans for the effect of probiotics on depression.
In the preclinical study performed by Y.-W. et al., 2016 [91], early-life stressed (ELS) mice and naïve mice were used. ELS mice showed an increase in levels of IL-6. Increased serum IL-6 is associated with stress-related disorders in humans [98]. Also, a clinical study reported increased IL-6 and lowered IL-10 levels in depressed patients compared to normal individuals [99]. Therefore, immune changes are the major cause of depression, and a complex cross-regulation between HPA and the immune system may lead to modulation of animal behavior. Dosing of PS128 for 4 weeks reduced depression-like behavior and normalized the HPA-immune system cross-regulation. This happened through the modulation of gut microbiota and strengthening of gut barrier. The increased levels of IL-6 in serum were also alleviated by treatment with PS128. Thus, it is positively correlated with depression.
In the preclinical study performed by Liang et al., 2015 [92], adult rats were subjected to stress for 3-4 weeks and showed depressive behavior (less sucrose preference and memory impairment). BDNF content reduction in the hippocampus is observed. BDNF decline can induce hippocampus atrophy [100]. After treatment with probiotic L.helvecticus NS8, spatial memory improvement was observed in adult mice which may be a result of the restoration of BDNF levels in the hippocampus. BDNF content is influenced by HPA activity and monoamine transmission. Controlled stress hormone and monoamine levels may be the cause of normalized BDNF levels.
In the study conducted by Savignac et al., 2015 [93], it was demonstrated that the amygdala and hippocampus are two major regions that are affected positively by probiotic treatment. Both these regions are involved in mood, learning, and memory. Through the battery of tests performed, it was concluded that the effect produced by the commensal bacteria highly strains specific because B.longum 1714 induced positive modulation of memory in BALB/c mice, whereas treatment with B.breve 1205 had a lower significant impact.
In the preclinical study conducted by Bravo et al., 2011 [94], vagotomy was performed in mice. GABA acts as a major inhibitory neurotransmitter, and L.rhamnosus was found to modulate the levels of GABA. GABAB1b mRNA levels increased in cortical regions, whereas mRNA levels GABAAα2 mRNA expression reduced in prefrontal cortex and amygdala. However, these levels were unaffected even though the vagotamized mice were supplemented with the probiotic. This demonstrates that L.rhamnosus modulated GABA levels through vagal sensory neurons.
In the clinical study conducted by Steenbergen et al., 2015 [95], a multispecies prebiotic supplementation (B.bifidum, B.lactis, L.acidophilus, L.brevis, L.casei, L.salivarius, and Lactococcus lactis) was given to patients with depression for 4 weeks which increased mood and ameliorated overall cognitive reactivity to depressed mood. This is probably because of tryptophan metabolism, which increases the serotonin levels through the serotonin production pathway [101]. Serotonin is generally termed as ‘happy hormone’ and has been implicated in improving mood [102]. Therefore, the cognitive reactivity to the sad mood in this study may be due to serotonin level-rise in the brain.
In the clinical study conducted by Pinto-Sanchez et al., 2017 [96], psychiatric comorbidity in patients with chronic bowel disorders was found to improve their condition after treatment with B.longum NCC3001. In a sensitivity analysis performed, improved depression scores were more likely in those patients who reported significant relief in chronic bowel disorder (IBD) symptoms. This establishes a co-existing bidirectional connection between gut and brain. fMRI patterns revealed reduced engagement of regions of the amygdala, frontal and temporal cortices in negative stimuli such as fear. It was also correlated to reduced depression scores. Therefore, the decline in amygdala overactivity may help ameliorate depression [103] and also pain perception, thus relieving symptoms of bowel disorder (IBD).
In the clinical study conducted by Slykerman et al., 2017 [97], L. rhamnosus HN001 was supplemented to women with depression during pregnancy and postpartum. Reduced depression scores were observed in women supplemented with probiotics during pregnancy and postpartum compared to placebo. This effect is possible because of GABAergic activation through vagal neurons in the hippocampal region.
10. PLAUSIBLE MECHANISMS BEHIND EFFECT OF PROBIOTICS ON DEPRESSION
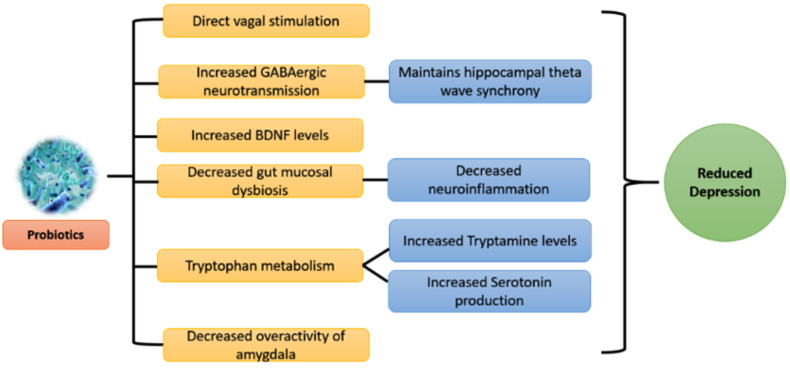
Many plausible mechanisms can be derived from the preclinical and clinical evidence available for the effect of probiotics on brain health. Especially in the case of depression, several factors such as neurotransmitters, immune cells, and neuronal pathways come into way between the gut microbiome and brain. The above-mentioned factors are not individually responsible for decreasing depression but happen collectively. Preclinical evidence is more clear about underlying mechanisms of the prebiotic effect compared to clinical evidence because of ethical considerations. Also, few mechanisms are species and strain-specific. Therefore, the underlying cause of depression should be evaluated in detail before supplementation of appropriate strain. Combinations of strains can also be administrated, but the resultant overall effect should be cautiously studied (Fig. 4).
11. CORRELATIONS AND DIFFERENCES
Correlation between human and murine systems in the effect of probiotics on depression is a topic that is debatable and lacks complete understanding. Few facts such as immune responses (increased serum IL-6 levels) during mucosal dysfunction can lead to depression. They are established in both preclinical and clinical studies. Probiotic supplementation showed positive effects on the human and murine system, but only extensive preclinical data is available currently for future studies. As interventional and postmortem studies reveal a lot more information about the mechanisms involved, it is not conducted extensively in humans. Also, the gut microbial composition varies between animals and humans, which is a potential factor to be considered. Neonatal growth and development depend on gut microbial composition. This can be studied using murine strains such as germ-free mice and specific-pathogen free mice, but it is not possible in the case of humans. Therefore, a lot of information remains unclear. Comparison of preclinical data with clinical data can reveal few mechanistic effects of probiotics. For example, hippocampal expression of genes related to HPA regulation was observed in animals supplemented with probiotics [104]. A similar kind of effect was observed in humans [105]. Also, the tryptophan metabolism pathway was well-established in both preclinical and clinical studies [106]. Thus to conclude, the probiotic effect is similar in animals and humans though there exist few mechanistic variations.
Gender is one of the major factors in depression development. The fact that women are more prone to depression compared to men [107] is proven. According to the available theories, men are more likely to engage themselves in distractive activities, decreasing the depressive mood, whereas women are more likely to amplify this mood by ruminating the thoughts that caused it [108]. The factors leading to this type of behavior are environmental, social, cultural, and parental. These factors include social expectations, lack of health care needs, childbirth-related needs, history of medical problems, physical problems during pregnancy and postpartum, etc. [109]. From the evidence available, probiotic supplementation to depressed women can help relieve their condition to some extent [97].
CONCLUSION
All the body functions are directly or indirectly connected and monitored by the brain. It is also proved that the reverse is possible. Many processes of the brain, such as behavior, cognition, neurogenesis, and development, are controlled by other body organs such as the gut. The microbes residing in the gut play a major role in gut-brain axis (GBA) activity which helps regulate brain functions via neural, hormonal and immunological pathways, thus maintaining homeostasis. Therefore, if the composition of commensal bacteria is altered, the overall brain function is also altered. In neurological diseases such as depression, autism, Alzheimer’s disease, Parkinson’s disease, etc., where brain function is disrupted, targeting gut microbiotal composition may show beneficial effects in ameliorating the disease condition. Therefore, several approaches like prebiotics, probiotics, synbiotics, etc., which strengthen the gut microbial function, are used that help in reducing disease severity and improving brain function [110]. Depression is one of the psychiatric disorders which in severe cases leads to neurodegeneration. Therefore, probiotics like Bifidobacterium and Lactobacillus are proved to be effective in the treatment of depression [111]. Lactobacillus and Bifidobacterium are two major families of microbiota residing in the gut. Several strains of these two genera occupy the major part of commensal bacteria through families such as Clostridium and E.coli. The co-existence of Lactobacillus and Bifidobacterium species can act in optimum synergy to restore intestinal integrity and overall health of the host [112]. Therefore, these two species are extensively available in the market as prebiotic supplements. However, strain specificity in these two genera should be cautiously evaluated to obtain appropriate results.
Fig. (1).
Figure depicting saccharolytic fermentation by few species of gut microbiota.
Fig. (3).
Overall mechanism involved in the pathophysiology of depression.
Fig. (4).
Plausible mechanisms underlying the effect of probiotics on depression.
Table 1.
Clinical evidence for the role of Gut flora in neurophysiology.
| S. No. | Investigators | Species used for Experimentation | Method(s)/Treatment(s) Employed |
Effect on Neurofunction/
Behavioral Alterations |
Conclusion | ||
|---|---|---|---|---|---|---|---|
| 1. | Degroote et al., 2016 [19] |
Wistar rats (male and female) | - Abx diet (Diet containing 1% succinyl sulfathiazole (SST) for parent female Wistar rats) | - Reduced social interactions, exploration, startle inhibition, and increased anxiety in infants born | - Maternal gut microbiota alterations affect infants’ behavioral patterns | ||
| 2. | Heijtz et al., 2011 [20] | GF mice, SPF mice, Normal mice (NMRI Strain) | - Behavioral tests: Open field test, elevated plus maze, light dark box test - Neurochemical analysis of brain tissue: RP-HPLC, Western immunoblotting |
- Increased motor activity and reduced anxiety-like behavior in GF mice compared to SPF mice - Increased NA, DA, 5-HT turnover in the striatum of GF mice compared to SPF mice - Altered expression in synaptic plasticity-related genes in GF mice - Reduced protein expression of synaptophysin and PSD-95 in the striatum of Gf mice due to gut microbiota colonization |
- Altered expression profiles of canonical signalling pathways, neurotransmitter turnover, and synaptic related proteins may contribute to behavioral differences observed between GF and SPF mice. | ||
| 3. | Addolorato et al., 2008 [21] |
Humans (clinical study, outpatients with gastrointestinal disorders) | - State and trait anxiety, current depression were assessed by state and anxiety inventory and Zung self-rating depression scale, respectively. | - Varying percentages of anxiety and depression were observed in patients with gastrointestinal disorders such as IBS, Coeliac disease, ulcerative colitis, Helicobacter pylori infection, food allergies, etc. | The study supports that patients infected with gastrointestinal disorders also suffer from anxiety/ depression at varying levels, and they should be treated by a team of gastroenterologists and psychologists or alternatively by a gastroenterologist having expertise in treating psychological disorders. | ||
| 4. | Desbonnet et al., 2008 [22] |
20 adult male SD rats divided into treatment group (n=12) and control group (n=8) |
- Bifidobacterium infantis in powdered form was dissolved in drinking water (dose = 1 x 1010 live bacterial cells in 100 ml water) and was given every morning for 14 days to the treatment group. The control group received normal drinking water. -Tests such as whole blood culture, cytokine analysis by flow cytometry, Plasma tryptophan pathway analysis by HPLC were performed after 14 days of treatment. |
- Marked increase in tryptophan levels in the treatment group of rats compared to the control group. - Significant suppression of pro-inflammatory cytokine release (IL-6, INF-ү) following stimulation was observed in blood samples from rats that received B.infantis. |
- Ingestion of B.infantis showed an elevation in levels of tryptophan, a precursor of 5-HT, a major neurotransmitter in the Gut-Brain Axis. This concludes that this strain of microbes plays some role in the regulation of ENS as well as in 5-HT synthesis. | ||
| 5. | Cattaneo et al., 2017 [23] |
Humans (241 patients with cognitive complaints and Alzheimer’s disease and 26 cognitively healthy volunteers) | - Treatment with antibiotics and anti-inflammatory drugs - Sample stool and blood collection and examination before and after the drug treatment - Amyloid imaging with Positron-Emission Tomography (PET) - Neuropsychological assessment, MRI and CSF analyses for Aβ and total phosphorylated tau assessment before and after drug treatment. |
- High abundance of pro-inflammatory Escherichia/ Shigella and low abundance of anti-inflammatory E.rectale in stools of subjects with positive amyloidosis compared to amyloid negative subjects. - Increased levels of pro-inflammatory cytokines (IL-6, CXCL2, NLRP3, and IL-1β) and reduced levels of anti-inflammatory cytokines (IL-10) in subjects with AD pathogenesis compared to control subjects. |
- Genera Escherichia/ Shigella may significantly increase the formation of Aβ aggregates via activation of inflammatory processes and the release of several pro-inflammatory cytokines and chemokines. - A significant decrease in E.rectale species abundance may cause a reduction in levels of butyrate (produced by E.rectale itself), which is an anti-inflammatory compound and may cause a decline in the protective role against chronic inflammation leading to Aβ accumulation. |
||
| S. No. | Investigators | Species used for Experimentation | Method(s)/Treatment(s) Employed | Effect on Neurofunction/ Behavioral Alterations | Conclusion | ||
| 6. | Keshavarzian et al., 2015 [24] | Humans (38 Parkinson’s patients and 34 healthy subjects) | - Sigmoid mucosal biopsy - Fecal sample collection - Predictive assessment of fecal microbial community by High through-put ribosomal RNA gene amplicon sequencing. - Data collected was correlated with clinical measures of PD to assess the functional potential of the microbial community. |
- Marked difference in the fecal bacterial community between PD patients and control subjects. - Reduced levels of anti-inflammatory butyrate-producing genera of bacteria such as Blautia, Coprococcus, Roseburia and a significant increase in pro-inflammatory Proteobacteria of genus Ralstonia in PD patients compared to control subjects. |
- This study reports evidence that increased pro-inflammatory dysbiosis and reduced protective anti-inflammatory action could trigger misfolding of α-synuclein and development of PD pathology. |
||
| 7. | Tamtaji et al., 2017 [25] | - 40 human subjects suffering from multiple sclerosis (MS) were assigned in a randomized, double-blinded, placebo-controlled clinical trial | - 40 humans were divided into two groups (n=20) to receive a probiotic capsule or placebo for 12 weeks - Gene expression related to inflammation, insulin, and lipids was assessed in blood samples of MS patients via the RT-PCR method. |
- Down-regulation of gene expression of IL-8 and TNF-α mRNA in peripheral blood mononuclear cells in MS patients who received probiotic supplementation compared to patients who received placebo. | - Probiotic supplementation may decrease the pro-inflammatory cytokine activation by reducing their gene expression, thus leading to amelioration of MS-related disorders such as mortality, morbidity, and insulin resistance. | ||
Table 2.
Composition of VSL#3 for IBD.
| Strain | Species in the Composition of VSL#3 |
|---|---|
| Lactobacilli strains | L.plantarum, L.acidophilus, L.delbrucckii and L.bulgaricus |
| Bifidobacterium strains | B.longum, L.breve, B.infantis |
| Streptococcus strain | Streptococcus salivarius sub.sp thermophiles |
Table 3.
Preclinical and clinical evidence for the effect of probiotics in treating depression.
| Refs. | Species Used | Tests Performed | Probiotics Used | Result/Outcome |
|---|---|---|---|---|
| Y.-W. et al., 2016 [91] | ELS mice, Naïve mice | Sucrose- preference test, forced-swim test | L.plantarum PS128 for 28 days | Decreased depression in ELS mice, increased 5-HT and Dopamine. |
| Liang et al., 2015 [92] | SPF CRS rats | Sucrose- preference test | L.helvectivus NS8 for 21 days | Decreased depression, increase in 5-HT and NA levels. |
| Savignac et al., 2015 [93] | mice | Tail-suspension test, forced- swim test |
B.longum 1714/ B.breve 1205 for 21 days |
Decreased depression in mice. |
| Bravo et al., 2011 [94] | mice | Forced-swim test | L.rhamnosus JB-1 for 28 days | Decreased depression, probiotic effect via vagal neurons. |
| Steenbergen et al., 2015 [95] | 40 Human volunteers | Leiden index of the depression sensitivity scale | B.bifidum W23, B.lactis W52, L.acidophilus W37, L.brevis W63, L.casei W56, L.salivarius W24 and Lactococcus lactis (W19 and W58) for 4 weeks | Reduced overall cognitive reactivity to sad mood |
| Pinto-Sanchez et al., 2017 [96] | 44 Human volunteers | Hospital Anxiety and Depression scale, Functional MRI | B.longum NCC3001 for 6 weeks | Reduced depression and reduced response to negative stimuli in multiple regions of brain-like amygdala and fronto-limbic regions |
| Slykerman et al., 2017 [97] | 423 female human volunteers who are either pregnant or in postpartum period | Edinburgh postnatal depression scale | L.rhamnosus HN001 was given from 14-16weeks of gestation to 6 months postpartum | Reduced depression scores in women postpartum |
ACKNOWLEDGEMENTS
We thank Manipal Academy of Higher Education, Manipal for providing access to journal.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Underwood E. Your gut is directly connected to your brain, by a newly dis-covered neuron circuit. Science (80- ), 2018;359(6380),1083. [Google Scholar]
- 2.Brenner D.M., Moeller M.J., Chey W.D., Schoenfeld P.S. The utility of probiotics in the treatment of irritable bowel syndrome: a systematic review. Am. J. Gastroenterol. 2009;104(4):1033–1049. doi: 10.1038/ajg.2009.25. [DOI] [PubMed] [Google Scholar]
- 3.Brown A.C., Rampertab S.D., Mullin G.E. Existing dietary guidelines for Crohn’s disease and ulcerative colitis. Expert Rev. Gastroenterol. Hepatol. 2011;5(3):411–425. doi: 10.1586/egh.11.29. [DOI] [PubMed] [Google Scholar]
- 4.Greene J.G. Causes and consequences of degeneration of the dorsal motor nucleus of the vagus nerve in Parkinson’s disease. Antioxid. Redox Signal. 2014;21(4):649–669. doi: 10.1089/ars.2014.5859. [DOI] [PubMed] [Google Scholar]
- 5.Klarer M., Weber-Stadlbauer U., Arnold M., Langhans W., Meyer U. Abdominal vagal deafferentation alters affective behaviors in rats. J. Affect. Disord. 2019;252:404–412. doi: 10.1016/j.jad.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Suarez A.N., Hsu T.M., Liu C.M., Noble E.E., Cortella A.M., Nakamoto E.M., Hahn J.D., de Lartigue G., Kanoski S.E. Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat. Commun. 2018;9(1):2181. doi: 10.1038/s41467-018-04639-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams E.K.K., Chang R.B.B., Strochlic D.E.E., Umans B.D.D., Lowell B.B.B., Liberles S.D.D. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166(1):209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sikander A., Rana S.V., Prasad K.K. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin. Chim. Acta. 2009;403(1-2):47–55. doi: 10.1016/j.cca.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Yano J.M., Yu K., Donaldson G.P., Shastri G.G., Ann P., Ma L., Nagler C.R., Ismagilov R.F., Mazmanian S.K., Hsiao E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Aidy S., Dinan T.G., Cryan J.F. Immune modulation of the brain-gut-microbe axis. Front. Microbiol. 2014;5:146. doi: 10.3389/fmicb.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nick P., Marjorie M.W., Nicholas J.T. The mucosal immune system: Master regulator of bidirectional gut-brain communications. Nat. Rev. Gastroenterol. Hepatol. 2017;14(3):143–159. doi: 10.1038/nrgastro.2016.191. [DOI] [PubMed] [Google Scholar]
- 12.Bercik P., Collins S.M., Verdu E.F. Microbes and the gut-brain axis. Neurogastroenterol. Motil. 2012;24(5):405–413. doi: 10.1111/j.1365-2982.2012.01906.x. [DOI] [PubMed] [Google Scholar]
- 13.Gritz E.C., Bhandari V. The human neonatal gut microbiome: a brief review. Front Pediatr. 2015;3:17. doi: 10.3389/fped.2015.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu G.D., Chen J., Hoffmann C., Bittinger K., Chen Y.Y., Keilbaugh S.A. Linking long-term dietary patterns with gut microbial entero-types. Science (80-), . 2011;334(6052):105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer E.A. Gut feelings: the emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011;12(8):453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 2018;6(2):133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Haq R., Schlachetzki J.C.M., Glass C.K., Mazmanian S.K. Microbiome-microglia connections via the gut-brain axis. J. Exp. Med. 2019;216(1):41–59. doi: 10.1084/jem.20180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skonieczna-Żydecka K., Marlicz W., Misera A., Koulaouzidis A., Łoniewski I. Microbiome-the missing link in the gut-brain axis: focus on its role in gastrointestinal and mental health. J. Clin. Med. 2018;7(12):E521. doi: 10.3390/jcm7120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Degroote S., Hunting D.J., Baccarelli A.A., Takser L. Maternal gut and fetal brain connection: Increased anxiety and reduced social interactions in Wistar rat offspring following peri-conceptional antibiotic exposure. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;71:76–82. doi: 10.1016/j.pnpbp.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz Heijtz R., Wang S., Anuar F., Qian Y., Björkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Addolorato G., Mirijello A., D’Angelo C., Leggio L., Ferrulli A., Abenavoli L., Vonghia L., Cardone S., Leso V., Cossari A., Capristo E., Gasbarrini G. State and trait anxiety and depression in patients affected by gastrointestinal diseases: psychometric evaluation of 1641 patients referred to an internal medicine outpatient setting. Int. J. Clin. Pract. 2008;62(7):1063–1069. doi: 10.1111/j.1742-1241.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 22.Desbonnet L., Garrett L., Clarke G., Bienenstock J., Dinan T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008;43(2):164–174. doi: 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C., Ferrari C., Guerra U.P., Paghera B., Muscio C., Bianchetti A., Volta G.D., Turla M., Cotelli M.S., Gennuso M., Prelle A., Zanetti O., Lussignoli G., Mirabile D., Bellandi D., Gentile S., Belotti G., Villani D., Harach T., Bolmont T., Padovani A., Boccardi M., Frisoni G.B. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Keshavarzian A., Green S.J., Engen P.A., Voigt R.M., Naqib A., Forsyth C.B., Mutlu E., Shannon K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015;30(10):1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 25.Tamtaji O.R., Kouchaki E., Salami M., Aghadavod E., Akbari E., Tajabadi-Ebrahimi M., Asemi Z. The effects of probiotic supplementation on gene expression related to inflammation, insulin, and lipids in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. J. Am. Coll. Nutr. 2017;36(8):660–665. doi: 10.1080/07315724.2017.1347074. [DOI] [PubMed] [Google Scholar]
- 26.Wong J.M.W. Gut microbiota and cardiometabolic outcomes: influence of dietary patterns and their associated components. Am. J. Clin. Nutr. 2014;100(Suppl. 1):369S–377S. doi: 10.3945/ajcn.113.071639. [DOI] [PubMed] [Google Scholar]
- 27.Rinninella E., Cintoni M., Raoul P., Lopetuso L.R., Scaldaferri F., Pulcini G., Miggiano G.A.D., Gasbarrini A., Mele M.C. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients. 2019;11(10):E2393. doi: 10.3390/nu11102393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.den Besten G., van Eunen K., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013;54(9):2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flint H.J., Duncan S.H., Scott K.P., Louis P. Interactions and competition within the microbial community of the human colon: links between diet and health. Environ. Microbiol. 2007;9(5):1101–1111. doi: 10.1111/j.1462-2920.2007.01281.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y-K., Shin C. The microbiota-gut-brain axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Curr. Neuropharmacol. 2018;16(5):559–573. doi: 10.2174/1570159X15666170915141036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomova A., Bukovsky I., Rembert E., Yonas W., Alwarith J., Barnard N.D., Kahleova H. The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 2019;6:47. doi: 10.3389/fnut.2019.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., Collini S., Pieraccini G., Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh R.K., Chang H.W., Yan D., Lee K.M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T.H., Bhutani T., Liao W. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017;15(1):73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cani P.D., Bibiloni R., Knauf C., Neyrinck A.M., Delzenne N.M. Changes in gut microbiota control metabolic diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 35.Sheflin A.M., Melby C.L., Carbonero F., Weir T.L. Linking dietary patterns with gut microbial composition and function. Gut Microbes. 2017;8(2):113–129. doi: 10.1080/19490976.2016.1270809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y.K. Effects of Diet on Gut Microbiota Profile and the Implications for Health and Disease. Biosci. Microbiota Food Health. 2013;32(1):1–12. doi: 10.12938/bmfh.32.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuohy K.M., Probert H.M., Smejkal C.W., Gibson G.R. Using probiotics and prebiotics to improve gut health. Drug Discov. Today. 2003;8(15):692–700. doi: 10.1016/S1359-6446(03)02746-6. [DOI] [PubMed] [Google Scholar]
- 38.Oak S.J., Jha R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019;59(11):1675–1683. doi: 10.1080/10408398.2018.1425977. [DOI] [PubMed] [Google Scholar]
- 39.Vesa T.H., Marteau P., Korpela R. Lactose intolerance. J. Am. Coll. Nutr. 2000;19(2) Suppl.:165S–175S. doi: 10.1080/07315724.2000.10718086. [DOI] [PubMed] [Google Scholar]
- 40.Rembacken B.J., Snelling A.M., Hawkey P.M., Chalmers D.M., Axon A.T.R. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354(9179):635–639. doi: 10.1016/S0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- 41.Malchow H.A. Crohn’s disease and Escherichia coli. A new approach in therapy to maintain remission of colonic Crohn’s disease? J. Clin. Gastroenterol. 1997;25(4):653–658. doi: 10.1097/00004836-199712000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Gionchetti P., Rizzello F., Helwig U., Venturi A., Lammers K.M., Brigidi P., Vitali B., Poggioli G., Miglioli M., Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124(5):1202–1209. doi: 10.1016/S0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 43.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 44.Armuzzi A., Cremonini F., Bartolozzi F., Canducci F., Candelli M., Ojetti V., Cammarota G., Anti M., De Lorenzo A., Pola P., Gasbarrini G., Gasbarrini A. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment. Pharmacol. Ther. 2001;15(2):163–169. doi: 10.1046/j.1365-2036.2001.00923.x. [DOI] [PubMed] [Google Scholar]
- 45.Oksanen P.J., Salminen S., Saxelin M., Hämäläinen P., Ihantola-Vormisto A., Muurasniemi-Isoviita L., Nikkari S., Oksanen T., Pörsti I., Salminen E. Prevention of travellers’ diarrhoea by Lactobacillus GG. Ann. Med. 1990;22(1):53–56. doi: 10.3109/07853899009147242. [DOI] [PubMed] [Google Scholar]
- 46.Guglielmetti S., Fracassetti D., Taverniti V., Del Bo’ C., Vendrame S., Klimis-Zacas D., Arioli S., Riso P., Porrini M. Differential modulation of human intestinal bifidobacterium populations after consumption of a wild blueberry (Vaccinium angustifolium) drink. J. Agric. Food Chem. 2013;61(34):8134–8140. doi: 10.1021/jf402495k. [DOI] [PubMed] [Google Scholar]
- 47.Hague A., Manning A.M., Hanlon K.A., Huschtscha L.I., Hart D., Paraskeva C. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p53-independent pathway: implications for the possible role of dietary fibre in the prevention of large-bowel cancer. Int. J. Cancer. 1993;55(3):498–505. doi: 10.1002/ijc.2910550329. [DOI] [PubMed] [Google Scholar]
- 48.Vivona A.A., Shpitz B., Medline A., Bruce W.R., Hay K., Ward M.A., Stern H.S., Gallinger S. K-ras mutations in aberrant crypt foci, adenomas and adenocarcinomas during azoxymethane-induced colon carcinogenesis. Carcinogenesis. 1993;14(9):1777–1781. doi: 10.1093/carcin/14.9.1777. [DOI] [PubMed] [Google Scholar]
- 49.Derikx L.A.A.P., Dieleman L.A., Hoentjen F. Probiotics and prebiotics in ulcerative colitis. Best Pract. Res. Clin. Gastroenterol. 2016;30(1):55–71. doi: 10.1016/j.bpg.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Bamba T., Kanauchi O., Andoh A., Fujiyama Y. A new prebiotic from germinated barley for nutraceutical treatment of ulcerative colitis. J. Gastroenterol. Hepatol. 2002;17(8):818–824. doi: 10.1046/j.1440-1746.2002.02709.x. [DOI] [PubMed] [Google Scholar]
- 51.Sherwin E., Dinan T.G., Cryan J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2018;1420(1):5–25. doi: 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- 52.Pusceddu M.M., Kelly P., Ariffin N., Cryan J.F., Clarke G., Dinan T.G. n-3 PUFAs have beneficial effects on anxiety and cognition in female rats: Effects of early life stress. Psychoneuroendocrinology. 2015;58:79–90. doi: 10.1016/j.psyneuen.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 53.Robertson R.C., Seira Oriach C., Murphy K., Moloney G.M., Cryan J.F., Dinan T.G., Paul Ross R., Stanton C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017;59:21–37. doi: 10.1016/j.bbi.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 54.Jacka F.N., O’Neil A., Opie R., Itsiopoulos C., Cotton S., Mohebbi M., Castle D., Dash S., Mihalopoulos C., Chatterton M.L., Brazionis L., Dean O.M., Hodge A.M., Berk M. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017;15(1):23. doi: 10.1186/s12916-017-0791-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mulak A., Bonaz B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015;21(37):10609–10620. doi: 10.3748/wjg.v21.i37.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyte M. The microbial organ in the gut as a driver of homeostasis and disease. Med. Hypotheses. 2010;74(4):634–638. doi: 10.1016/j.mehy.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 57.Lyte M. Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014;817:3–24. doi: 10.1007/978-1-4939-0897-4_1. [DOI] [PubMed] [Google Scholar]
- 58.Norris V., Molina F., Gewirtz A.T. Hypothesis: bacteria control host appetites. J. Bacteriol. 2013;195(3):411–416. doi: 10.1128/JB.01384-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Furness J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012;9(5):286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 60.Iyer L.M., Aravind L., Coon S.L., Klein D.C., Koonin E.V. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004;20(7):292–299. doi: 10.1016/j.tig.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Hu X., Wang T., Jin F. Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 2016;59(10):1006–1023. doi: 10.1007/s11427-016-5083-9. [DOI] [PubMed] [Google Scholar]
- 62.Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9(11):799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 63.Kelly J.R., Kennedy P.J., Cryan J.F., Dinan T.G., Clarke G., Hyland N.P. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith S.M., Vale W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sapolsky R.M., Romero L.M., Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 66.Heaney J. Pituitary-Adrenal Axis. Encyclopedia of Behavioral Medicine, 2013. [Google Scholar]
- 67.Lyte M., Vulchanova L., Brown D.R. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell Tissue Res. 2011;343(1):23–32. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- 68.Gareau M.G., Jury J., MacQueen G., Sherman P.M., Perdue M.H. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56(11):1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Palma G., Collins S.M., Bercik P., Verdu E.F. The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J. Physiol. 2014;592(14):2989–2997. doi: 10.1113/jphysiol.2014.273995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasler G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry. 2010;9(3):155–161. doi: 10.1002/j.2051-5545.2010.tb00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kugaya A., Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10(10):808–819. doi: 10.1017/S1092852900010403. [DOI] [PubMed] [Google Scholar]
- 72.Wess J. Novel insights into muscarinic acetylcholine receptor function using gene targeting technology. Trends Pharmacol. Sci. 2003;24(8):414–420. doi: 10.1016/S0165-6147(03)00195-0. [DOI] [PubMed] [Google Scholar]
- 73.Gotti C., Zoli M., Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol. Sci. 2006;27(9):482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 74.Meyerson L.R., Wennogle L.P., Abel M.S., Coupet J., Lippa A.S., Rauh C.E., Beer B. Human brain receptor alterations in suicide victims. Pharmacol. Biochem. Behav. 1982;17(1):159–163. doi: 10.1016/0091-3057(82)90279-9. [DOI] [PubMed] [Google Scholar]
- 75.Chau D.T., Rada P., Kosloff R.A., Taylor J.L., Hoebel B.G. Nucleus accumbens muscarinic receptors in the control of behavioral depression: antidepressant-like effects of local M1 antagonist in the Porsolt swim test. Neuroscience. 2001;104(3):791–798. doi: 10.1016/S0306-4522(01)00133-6. [DOI] [PubMed] [Google Scholar]
- 76.Sigel E., Steinmann M.E. Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 2012;287(48):40224–40231. doi: 10.1074/jbc.R112.386664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62(1):42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 78.Tripp A., Kota R.S., Lewis D.A., Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol. Dis. 2011;42(1):116–124. doi: 10.1016/j.nbd.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lega B., Burke J., Jacobs J., Kahana M.J. Slow-theta-to-gamma phase-amplitude coupling in human hippocampus supports the formation of new episodic memories. Cereb. Cortex. 2016;26(1):268–278. doi: 10.1093/cercor/bhu232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stewart M., Fox S.E. Do septal neurons pace the hippocampal theta rhythm? Trends Neurosci. 1990;13(5):163–168. doi: 10.1016/0166-2236(90)90040-H. [DOI] [PubMed] [Google Scholar]
- 81.Smythe J.W., Colom L.V., Bland B.H. The extrinsic modulation of hippocampal theta depends on the coactivation of cholinergic and GABA-ergic medial septal inputs. Neurosci. Biobehav. Rev. 1992;16(3):289–308. doi: 10.1016/S0149-7634(05)80203-9. [DOI] [PubMed] [Google Scholar]
- 82.Foster J.A., McVey Neufeld K.A. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 83.Powley T.L., Wang X.Y., Fox E.A., Phillips R.J., Liu L.W.C., Huizinga J.D. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol. Motil. 2008;20(1):69–79. doi: 10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 84.Goehler L.E., Park S.M., Opitz N., Lyte M., Gaykema R.P.A. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav. Immun. 2008;22(3):354–366. doi: 10.1016/j.bbi.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McVey Neufeld K.A., Mao Y.K., Bienenstock J., Foster J.A., Kunze W.A. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol. Motil. 2013;25(2):183–e88. doi: 10.1111/nmo.12049. [DOI] [PubMed] [Google Scholar]
- 86.Zelante T., Iannitti R.G., Cunha C., De Luca A., Giovannini G., Pieraccini G., Zecchi R., D’Angelo C., Massi-Benedetti C., Fallarino F., Carvalho A., Puccetti P., Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 87.Williams B.B., Van Benschoten A.H., Cimermancic P., Donia M.S., Zimmermann M., Taketani M., Ishihara A., Kashyap P.C., Fraser J.S., Fischbach M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16(4):495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Young S.N., Smith S.E., Pihl R.O., Ervin F.R. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology (Berl.) 1985;87(2):173–177. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- 89.Evrensel A., Ceylan M.E. The gut-brain axis: The missing link in depression. Clin. Psychopharmacol. Neurosci. 2015;13(3):239–244. doi: 10.9758/cpn.2015.13.3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maes M., Kubera M., Leunis J.C. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuroendocrinol. Lett. 2008;29(1):117–124. [PubMed] [Google Scholar]
- 91.Liu Y.W., Liu W.H., Wu C.C., Juan Y.C., Wu Y.C., Tsai H.P., Wang S., Tsai Y.C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016;1631:1–12. doi: 10.1016/j.brainres.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 92.Liang S., Wang T., Hu X., Luo J., Li W., Wu X., Duan Y., Jin F. Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience. 2015;310:561–577. doi: 10.1016/j.neuroscience.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 93.Savignac H.M., Tramullas M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- 94.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108(38):16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steenbergen L., Sellaro R., van Hemert S., Bosch J.A., Colzato L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015;48:258–264. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 96.Pinto-Sanchez M.I., Hall G.B., Ghajar K., Nardelli A., Bolino C., Lau J.T., Martin F.P., Cominetti O., Welsh C., Rieder A., Traynor J., Gregory C., De Palma G., Pigrau M., Ford A.C., Macri J., Berger B., Bergonzelli G., Surette M.G., Collins S.M., Moayyedi P., Bercik P. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–459.e8. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 97.Slykerman R.F., Hood F., Wickens K., Thompson J.M.D., Barthow C., Murphy R., Kang J., Rowden J., Stone P., Crane J., Stanley T., Abels P., Purdie G., Maude R., Mitchell E.A. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. 2017;24:159–165. doi: 10.1016/j.ebiom.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coelho R., Viola T.W., Walss-Bass C., Brietzke E., Grassi-Oliveira R. Childhood maltreatment and inflammatory markers: a systematic review. Acta Psychiatr. Scand. 2014;129(3):180–192. doi: 10.1111/acps.12217. [DOI] [PubMed] [Google Scholar]
- 99.Dhabhar F.S., Burke H.M., Epel E.S., Mellon S.H., Rosser R., Reus V.I., Wolkowitz O.M. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J. Psychiatr. Res. 2009;43(11):962–969. doi: 10.1016/j.jpsychires.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 100.Belmaker R.H., Agam G. Major depressive disorder. N. Engl. J. Med. 2008;358(1):55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 101.Jenkins T.A., Nguyen J.C.D., Polglaze K.E., Bertrand P.P. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8(1):E56. doi: 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barnes N.M. 5-HT: the promiscuous and happy hormone! Curr. Opin. Pharmacol. 2011;11(1):1–2. doi: 10.1016/j.coph.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 103.Chen C.H., Suckling J., Ooi C., Fu C.H.Y., Williams S.C.R., Walsh N.D., Mitterschiffthaler M.T., Pich E.M., Bullmore E. Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology. 2008;33(8):1909–1918. doi: 10.1038/sj.npp.1301593. [DOI] [PubMed] [Google Scholar]
- 104.Abildgaard A., Elfving B., Hokland M., Wegener G., Lund S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology. 2017;79:40–48. doi: 10.1016/j.psyneuen.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 105.de Weerth C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci. Biobehav. Rev. 2017;83:458–471. doi: 10.1016/j.neubiorev.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 106.Wang H., Lee I.S., Braun C., Enck P. Effect of probiotics on central nervous system functions in animals and humans: A systematic review. J. Neurogastroenterol. Motil. 2016;22(4):589–605. doi: 10.5056/jnm16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.a Morgan M.A., Pfaff D.W. Effects of Estrogen on Activity and Fear-Related Behaviors in Mice. Horm. Behav. 2001;40(4):472–482. doi: 10.1006/hbeh.2001.1716. [DOI] [PubMed] [Google Scholar]; b Azmitia E.C., Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 1978;179(3):641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]; c Belelli D., Lambert J.J. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005;6(7):565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]; d Yamaguchi-Shima N., Yuri K. Age-related changes in the expression of ER-β mRNA in the female rat brain. Brain Res. 2007;1155:34–41. doi: 10.1016/j.brainres.2007.04.016. [DOI] [PubMed] [Google Scholar]; e Thompson K.E., Sipes I.G., Greenstein B.D., Hoyer P.B. 17β-estradiol affords protection against 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in Fischer-344 Rats. Endocrinology. 2002;143(3):1058–1065. doi: 10.1210/endo.143.3.8665. [DOI] [PubMed] [Google Scholar]; f Munabi A.K., Cassorla F.G., Pfeiffer D.G., Albertson B.D., Loriaux D.L. The effects of estradiol and progesterone on rat ovarian 17-hydroxylase and 3β-hydroxysteroid dehydrogenase activities. Steroids. 1983;41(1):95–98. doi: 10.1016/0039-128x(83)90019-3. [DOI] [PubMed] [Google Scholar]; g Sex differences in rates of depression: cross-national per-spectives. J. Affect. Disord. 1993;29(2-3):77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- 108.Nolen-Hoeksema S. Sex differences in unipolar depression: evidence and theory. Psychol. Bull. 1987;101(2):259–282. doi: 10.1037/0033-2909.101.2.259. [DOI] [PubMed] [Google Scholar]
- 109.Sowa C.J., Lustman P.J. Gender differences in rating stressful events, depression, and depressive cognition. J. Clin. Psychol. 1984;40(6):1334–1337. doi: 10.1002/1097-4679(198411)40:6<1334:AID-JCLP2270400609>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 110.Rudzki L., Ostrowska L., Pawlak D., Małus A., Pawlak K., Waszkiewicz N., Szulc A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–222. doi: 10.1016/j.psyneuen.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 111.Aizawa E., Tsuji H., Asahara T., Takahashi T., Teraishi T., Yoshida S., Ota M., Koga N., Hattori K., Kunugi H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016;202:254–257. doi: 10.1016/j.jad.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 112.Sarkar A., Mandal S. Bifidobacteria-Insight into clinical outcomes and mechanisms of its probiotic action. Microbiol. Res. 2016;192:159–171. doi: 10.1016/j.micres.2016.07.001. [DOI] [PubMed] [Google Scholar]