Abstract
Curcumin is a spice derived nutraceutical which gained tremendous attention because of its profound medicinal values. It alters a number of molecular pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF‐κB), signal transducer and activator of transcription 3 (STAT3), nuclear factor erythroid 2-related factor 2 (Nrf2) and cyclooxygenases-2 (COX‐2), which make it potential therapeutic choice in treating multiple disorders. It also possesses the potential to prevent protein aggregation and thus protect against degeneration of neurons in neurodegenerative disorders including Huntington’s disease (HD). HD is an autosomal dominant disorder linked with altered gene expression which leads to an increase in the size of cytosine, adenine and guanine (CAG) trinucleotide repeats, aids in protein aggregation throughout the brain and thus damages neurons. Upstream regulation of oxidative stress and inflammatory cascade are two important factors that drive HD progression. Available therapies just suppress the severity of symptoms with a number of side effects. Curcumin targets multiple mechanisms in treating or preventing HD including antioxidant and anti-inflammatory potential, metal ion chelation, transcriptional alterations and upregulating activity of molecular chaperons, heat shock proteins (HSPs). Having a favorable safety profile, curcumin can be an alternative therapeutic choice in treating neurodegenerative disorders like HD. This review will focus on mechanistic aspects of curcumin in treating or preventing HD and its potential to arrest disease progression and will open new dimensions for safe and effective therapeutic agents in diminishing HD.
Keywords: Curcumin, huntington’s disease, therapeutic potential, degenerative diseases, oxidative stress, neuroinflammation, underlying mechanisms
1. INTRODUCTION
Curcumin is a polyphenolic compound, chemically known as 1,7-bis (4-hydroxy, 3-methoxyphenyl) 1,6-heptadiene-3,5-dione [1]. The botanical source of curcumin is Turmeric or Curcuma longa which belongs to the Zingiberaceae family [2]. Other sources of curcumin include Curcuma phaeocaulis, Curcuma aromatic, Curcumacaesia and Curcuma zedoaria [3-5]. Curcumin gained a tremendous attention among nutraceuticals because of its profound medicinal values [6]. Pharmacologically, curcumin is one of the most active polyphenolic compounds and its various targets in the treatment of several disorders have been reported so far [7]. The first use of curcumin in the treatment of human diseases was published in 1937 [8]. Polyphenolic compounds have been demonstrated to possess anti-oxidant and anti-inflammatory potential as well as altering several molecular pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells(NF‐κB), signal transducer and activator of transcription 3 (STAT3), nuclear factor erythroid 2-related factor 2 (Nrf2) and cyclooxygenases-2 (COX‐2), which makes them vulnerable choice in the management of multiple disorders including diabetes, cardiovascular, neurologic, metabolic, inflammatory, and skin disorders, hepatotoxicity, respiratory tract infections, and diseases of infectious origin [9].
Despite of advances in the field of drug discovery, currently no therapy is available to cure HD, while some therapeutic options may attenuate the severity of the symptoms [10]. Deutetrabenazine and tetrabenazine are two drugs, approved by the Food and Drug Administration (FDA) for the treatment of chorea, but both are themselves associated with depression and suicidal ideation [11]. Thus, it is essential to identify safe and effective alternatives for HD treatment, without undesirable effects. The present review is focused on the therapeutic potential of curcumin in the management of HD and highlights its mechanistic perspectives.
2. HUNTINGTON’S DISEASE AND DEGENERATIVE DISEASES
Neurodegenerative diseases are disabling diseases, often incurable, with a strong connection to age. Such disorders have a deceitful onset and their differentiation is often difficult, due to the overlap of the clinical manifestations [12]. The pathogenesis of neurodegenerative diseases has still left unclear. They are characterized by progressive neuronal death that may involve different brain areas, depending on the disease, and it is affected by both genetic and environmental factors. In the early neurodegenerative disorders’ onset, the accumulation of mutant or misfolded proteins in brain cells, as well as amyloid in Alzheimer’s, α-synuclein in Parkinson’s, huntingtin in Huntington’s, seems to play a crucial role [13, 14].
In addition, the disease’s occurrence is accompanied by the production of pro-inflammatory mediators and neurotoxic factors leading to oxidative stress that generates a neuroinflammatory answer [15]. As regards Huntington’s disease (HD), an autosomal dominant inherited neurodegenerative disease, a mutation in the CAG triplet repeat number, in exon 1 of huntingtin (HTT) gene, represents the main protagonist of the disease onset [16]. This gene, placed on chromosome 4p16.3 [17] leads to the synthesis of a mutant huntingtin (mHTT) protein, characterized by a long polyglutamine stretch. Other diseases caused by triplet expansion comprise the fragile X syndrome, characterized by abnormal repeats of CGG, myotonic dystrophy by CTG repeats, and Friedreich ataxia by GAA repeats [18].
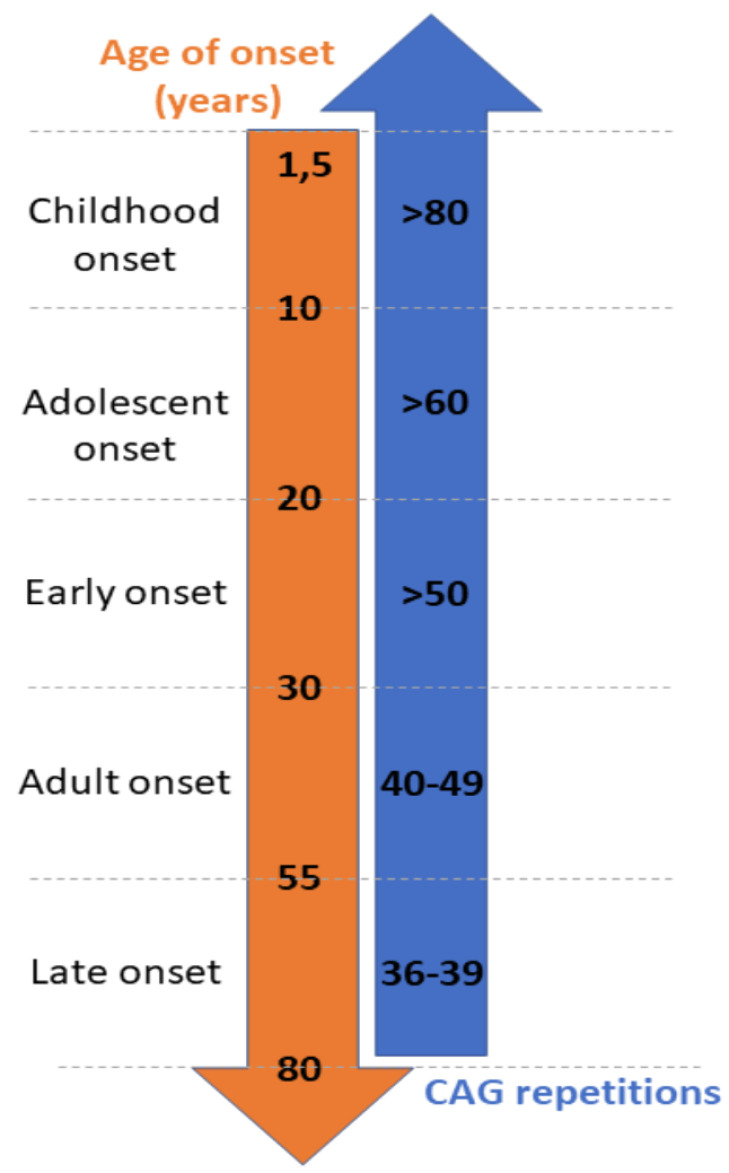
The number of CAG repetitions in HD is inversely propotional to the age-onset resulting in an earlier onset for more significant expansion (Fig. 1) [18]. Data about HD epidemiology reveal that it is an exceptionally rare pathology, with a relatively long clinical course (10-20 years) [19].
The worldwide HD prevalence is estimated to be 2.7 cases per 100,000 persons, with an incidence of 0.38 cases per 100,000 persons per year [20]. Marked differences are evident in the global occurrence of the disease, ranging from 0.17-0.72 cases/100,000 persons in Asia to 7.33-13.7 in North America [21, 22]. Several studies have tried to assess the distribution of HD among different parts of the world, but many inconsistencies may be found between the various authors. Probably, it may be due to the heterogeneity in study methods, as well as to underestimated cases in underdeveloped areas because of the lack of an effective sanitary system or to previously misdiagnosed cases, as only in 1993 the identification of the underlying disease mutation led to the availability of diagnostic tests [23]. Possible explanations of differential worldwide HD epidemiology are correlated to the CAG repeat length and the mutability of CAG on different haplotypes [23, 24]. In HD two models of neural degeneration have been proposed. In the first, m-HTT accumulates in the cell, compromising intracellular signaling. The second model is an unclassical theory that assumes the induction of mutant neurons in a steady-state by the m-HTT that progressively degenerate during maturation under the toxicity of m-HTT [18].
Wild-type HTT is a large protein, ubiquitously expressed in the body. In particular, higher toxicity is associated with the nuclear accumulation of fragments deriving from the mutant form of this protein [25]. Many in vivo and in vitro evidence have reported high levels of fragments’ aggregates in cortical neurons, suggesting their deep implication in HD pathogenesis [25]. These N-terminal HTT fragments formed from a specific cleavage include the poly-Q stretch that can interact with many transcriptional regulators, altering neurodevelopmental pathways. Indeed, the presence of many HEAT domains and CAG repeats in HTT enhances the interaction with protein partners which are modulators in various stages of the neural growth. Thus the increment of repeats leads to a higher possibility of interaction, mediating the toxic activity of m-HTT in neuron degeneration [26]. In microglia, mutant huntingtin is able to promote the macrophage differentiation autonomousl, through the expression of the myeloid lineage-determining factors [27]. The greater vulnerability of specific types of neurons rather than others may be explained by their content in glutamate-receptor subtypes that leads to a greater vulnerability to glutamate-mediated excitotoxic damages[28]. HD involves striatum brain area [29]. The reason for which HD patients showed the involvement of this particular area may be found in the alteration in neuro-developmental processes of specific neural subsets [30]. Soluble signals, morphogens, and transcription factors expressed during progressive stages of neural development, confer exclusive features to each neural subpopulation. Aberrations of such mediators, together with mutations occurring in genes encoding proteins normally expressed during neural development, as well as HTT in HD, may be responsible for regio-selective death [31-33]. It involves the caudate [17], neocortex, and the white matter, but in the worst cases, the neuronal loss extends to other areas, as well as the hippocampus, thalamus, cerebellum, and brainstem nuclei with an impairment of the severity of clinical signs [20]. The involvement of these areas leads to typical HD symptoms, including motor, cognitive and psychiatric dysfunctions. Probably, the first ones represent the distinctive features of the HD, to have made it known as the ‘Chorea of Huntington’ because of the involuntary dance-like movements that characterize the patients [18].
From the analysis of postmortem brain tissues in HD patients, it emerged that the most HD-affected brain regions showed alterations in Ca2+ homeostasis [34], electron transport chains and the mitochondrial tricarboxylic acid cycle. As these mechanisms are broadly involved in the generation of ATP, tissues with high energy consumption are more prone to oxidative stress. Indeed, in brain cells, the high membrane lipid concentration, together with the high energy expenditure, lead to an increased susceptibility to oxidative damages, as demonstrated by the increment of oxidative stress markers in HD patients with respect to non-HD patients [35]. Lipid peroxidation and its related by-products and endoperoxides and malondialdehyde, increase with the worsening of the disease. During alterations in the mitochondrial electron transport chain, superoxide anions may leak out and damage lipids, proteins, and DNA. In vivo studies confirmed the radical-mediated DNA damage in mice that is associated with transcription errors, misfolded protein synthesis, altered HTT-protein interactions and defects in neuronal trafficking [36].
Recently, several natural substances, acting as antioxidants, have been studied for their effectiveness in reducing neuron death or protein aggregation in several in vitro or in vivo studies. It has been demonstrated that these molecules not only act as antioxidants, but several other mechanisms of action have been recognized, especially at the genomic or the transcriptional level. Curcumin is one of the most investigated polyphenolic compounds and, as described below, it has been demonstrated to exert neuroprotective effects [37-39].
3. CURCUMIN AND DEGENERATIVE DISEASES
Curcumin is the most abundant [40] and biologically active polyphenolic compound [15], deriving from the rhizome of Curcuma longa, belonging to the Zingiberaceae family. Also known as diferuloylmethane, it was purified from the plant rhizome for the first time in 1815, while its chemical structure was established only in 1910 [15]. Chemically, curcumin is a diarylheptanoid, existing in several tautomeric forms, among which the enolic one is the more stable. Curcuma longa rhizome, better known as turmeric, is a very common and widespread used South Asian spice, thanks to its well-recognized health-promoting activity, including hepatoprotective, nephroprotective, antimicrobial, antioxidant and anti-inflammatory properties. Its characteristic bright yellow color, due to the presence of curcumin and other two curcuminoids, desmethoxycurcumin, and bis-desmethoxycurcumin, explains its employment as a food coloring [41, 42]. Importantly, epidemiological studies have revealed an inverse correlation between curcumin consumption and the incidence of neurodegenerative diseases including Huntington’s disease, with a negative trend in the Indian population [43]. This may be explained by the capability of curcumin to interact with the Wnt/β-catenin pathway, which is involved in cortex neurodevelopment, neural precursor revival, and adult hippocampal neurogenesis. Under neuropathologic conditions, indeed, this pathway results under-expressed. Curcumin, by interacting with the Wnt inhibitory factor, can activate it, enhancing proliferation and differentiation of brain cells [44].
Curcumin has been shown to possess a positive impact on a broad range of brain cells, providing proof of its neuroprotective activity in both cellular and animal models [45, 46]. Research has been focused on the potential of curcumin against neurodegenerative disorders characterized by toxic proteins’ aggregates accumulation [47]. A recent study on HT22 cells reported that curcumin revealed a higher anti-aggregation activity among 214 antioxidant compounds tested [48]. The ability of curcumin to inhibit aggregation and disaggregate mutant proteins’ complexes results in the reduction of huntingtin aggregates in HD [49]. Although significant differences in the inflammatory mechanisms between invertebrates and humans occur, some evidence in transgenic worms suggested that curcumin is able to increase lifespan through the maintenance of the protein homeostasis, by the regulation of heat-shock factors (HSPs) [39].
Molecular chaperones that are part of the highly conserved defense system of cells are involved in protein folding, avoiding protein aggregation as well as correcting misfolded and degrading damaged proteins. It appears reasonable to suppose that the dysfunction of these systems may leads to the accumulation of insoluble aggregates with progressive neural loss of function and death. In particular, HSP40 and HSP70 enhance the degradation of HTT protein aggregates by binding precursors of mutant protein complexes and by activation of a specialized chaperone-mediated autophagy mechanism, which targets and addresses aggregates to degradation [13, 44]. In view of this, ever-increasing attention is given to the research of phytochemical molecules that may be able to increase neuroprotection by maintaining protein homeostasis through the restoration of endogenous control mechanisms. Curcumin, as a natural inducer of HSPs, arose as a valuable candidate. It re-establishes HSPs levels in transgenic animal models for neurodegenerative disorders, even if molecular mechanisms underlying curcumin’s effects remain unclear [13]. As already mentioned, neuro-inflammation is a key factor in neurodegenerative diseases. Therefore, all compounds that are able to interfere with inflammation-related pathways and mediators are of utmost interest. In this context, 24h treatment with 20 µM of curcumin is associated with a dose-dependent reduction of the expression of different types of interleukins and tumor necrosis factor-alpha (TNF-α) in lipopolysaccharide-stimulated microglia [50]. Particularly, intracellular levels of interleukins (IL-12 p40/p70 and IL-12 p70) have been found to be lowered after curcumin exposure, together with an under expression of the IL-1 and IL-6 pro-inflammatory cytokines [51].
Curcumin dietary interventions in rats are associated with an improvement of neurodegenerative disease-related clinical manifestations, as well as cognitive impairments and memory deficit [52]. It improves mood changes and depressive behavior by activating the ERK-Bcl-2-BDNF neurotrophic pathway [41], as well as promoting in vitro and in vivo neurogenesis through the MAPK/ERK pathway activation. An increment of the number of neurons was observed in chronically stressed rats [45]. In mice, likewise, decreased oxidative damage and lower oxygen and nitrogen reactive species production may be observed [53]. As oxidative stress plays an important role in the physiopathology of neurodegenerative disorders, antioxidant compounds deriving from plants may be promising therapeutic candidates. The antioxidant power of curcuminoids makes these compounds able to inhibit free radical chain reactions and lipid peroxidation, as demonstrated by the reduction of lipofuscin, a lipid peroxidation marker. On the basis of these beneficial effects, there is a general improvement in the endogenous antioxidant system, with the elevation of superoxide dismutase and glutathione levels [51], as well as those of cytochrome c oxidase and NADH dehydrogenase [54]. The cytoprotective effects exerted by curcumin against oxidative stress also pass through the induction of the hemoxygenase protein by upregulating the Nrf2 which enhances the transcription of genes involved in detoxification [55]. In microglia brain cells, curcumin induces hemoxygenase activity that reduces proinflammatory markers decreasing neuroinflammation and oxidative stress [15].
Besides, the almost total absence of side effects of curcumin that makes it a valuable neuroprotective candidate, the sole limit of its use in neuroprotection being represented by its poor brain bioavailability. As a lipophilic compound, it should normally have no problem crossing the brain- blood barrier. However, due to selective permeability constraints, curcumin levels are nor very high with serum levels of 0.5 µmol/L at doses of 4 g/day [56] being the latter further decreased by rapid metabolization of curcumin in to its glucuronide derivatives [45]. The focus on curcumin, although other antioxidants like tocopherol are more bioavailable, derives from the observation that curcumin had stronger anti-aggregation properties against Q-rich and non Q-rich aggregates rather than other antioxidants [57]. Some strategies, as well as the use of curcumin nanoparticles [58], chemical analogs or liposomal formulation [45] have been implemented during the last years in order to improve the bioavailability [57]. The ability of curcumin to cross the brain-blood barrier into brain regions is closely linked with its hydrophobic property. Only molecules with high lipophilicity and low molecular weight can cross the tight junctions of capillary endothelial cells that form this high-selective control system of the brain. Thus, in these curcumin-based alternative formulations, the application of lipophilic materials as well as polyvinylpyrrolidone [59] and poly(lactic-co-glycolic acid) [60] improves the retention time in the brain [59], also reducing the distribution to metabolizing organs [60]. Indeed, when administered orally, curcumin undergoes an extensive liver and intestinal metabolism to tetrahydrocurcumin and glucuronides or sulfates [59]. By contrast, in tg2576 transgenic mice, it has been demonstrated that nanoparticles are liver metabolism resistant, increasing detectable plasma concentrations and, consequently, brain levels [59]. Likewise, in rats, the administration of 10 and 20 mg/kg body weight of curcumin nanoparticles increased by up then 2-fold curcumin brain levels with respect to free curcumin [45]. This property should be due to the small size that might allow nanoparticles to bypass metabolism and be directly absorbed in the blood, crossing brain blood barrier or to avoid uptake by the reticuloendothelial system, is not metabolized by the liver, extending circulation times [59]. After intravenous administration, curcumin retention time in the brain was markedly increased (from 20.4 to 27.1) rather than curcumin [60]. The pharmacokinetic parameters showed an increment of 96% in the cerebral cortex and 83% in the hippocampus when curcumin is administered as nanoparticles [60]. Given the central role of the hippocampus in memory and learning, the ability of curcumin nanoparticles to accumulate in this brain region is an important added value.
4. HUNTINGTON’S DISEASE AND CURCUMIN
In view of the above, many pathways are involved in neurodegenerative disorders (Fig. 2). The way in which curcumin can modulate them and thus explaining its therapeutic effects remains an open issue for researchers. As there is no cure available for this disease, the discovery of the mechanisms of the action of curcumin, together with an improvement of its bioavailability, may be the starting point towards alternative therapeutic strategies.
4.1. Misfolding (Heat Shock Proteins and Vacuolar Protein Sorting Protein 36)
Misfolded HTT protein accumulation is critical in HD [61, 62]. Curcumin, as a booster of the endogenous HSPs checking systems, may be a valuable tool in HD therapy. Experimental studies revealed that curcumin reduces HTT aggregate accumulation in HD [63, 64] by modulating HSP70 and HSP90 expression at doses of 0.01 µM in human bone marrow neuroblast cells. The mechanism underlying the anti-amyloidogenic activity of HSP70 involves the binding to the poly-Q stretch of m-HTT, preventing the formation of aggregates. Thus the curcumin-mediated induction of HSP70 [64] is associated with an improvement of the pathology. Interestingly, curcumin does not affect the activity of HSP90 on the serine/threonine kinase Akt, reducing the apoptotic stimulus [13]. Reduction in cell death has indeed been observed in curcumin feeding Drosophila melanogaster [65]. In addition, in vivo studies have suggested that treatment with 555 ppm of curcumin in CAG140 KI mice reduces the formation of aggregates, with a concomitant decrement of severity in neuropathology [66].
Curcumin exhibits an inhibitory action against the formation of both Q-rich and nonQ-rich aggregates in yeast expression vectors [57]. It prevents HTT aggregation by the dose-dependently downregulation of the Vacuolar Protein Sorting 36 (Vps36), a component of the endosomal sorting complex required for transport (ESCRT-II). Indeed, treatment with 20 µM and 40 µM of curcumin for 16 hours, is associated with a Vps36mRNA reduction by 1.5- and 4-fold, respectively [57]. In this way, protein trafficking is altered and the recruitment of m-HTT from various cellular compartments becomes difficult. In addition, curcumin can disaggregate the already formed m-HTT complexes [57, 67].
4.2. p21-activated Kinase (PAK)
Dysregulation of the p21-activated kinase (PAK) is associated with several neurodegenerative disorders [68, 69], as well as HD. Since it is involved in learning and memory processes, the PAK-inhibitory potential of curcumin has been assessed. HD’s severity is exacerbated not only by the progressive accumulation of m-HTT, but also by the loss of function of the wild-type HTT that becomes unable to prevent the cleavage of PAK2 by caspases, leading to cellular death [70]. Furthermore, HD toxicity seems to be also mediated by PAK1 which enhances HTT aggregation by promoting both self- and wild-type HTT-aggregation. Curcumin may mitigate neural toxicity by the inhibition of the HTT aggregate accumulation [71].
4.3. Oxidative Stress
As previously discussed, oxidative stress plays a critical role in the late stages of HD pathogenesis. The partial purification of m-HTT reveals a considerable amount of oxidized proteins which represents a harmful source of ROS [72, 73]. The beneficial effects of curcumin are related to its well-recognized antioxidant power. It is able to up-regulate endogenous anti-oxidative defense systems, as well as superoxide dismutase and glutathione enzyme expression [51]. Curcumin doses of 25 and 50 mg/kg are able to inhibit lipid peroxidation and re-establish physiological nitrite levels [74]. Curcumin activates the Keap1/Nrf2/ARE-dependent cytoprotective pathway [55] which is associated with the expression of glutathione S-transferase and heme oxygenase-1 proteins. This ability is related to the curcumin chemical structures: hydroxyl groups in ortho-position at the aromatic rings may interact with cysteine residues of Keap1, causing a conformational change that leads to the release of Nrf-2 [75]. This factor then moves into the nucleus for binding antioxidant response elements (ARE) to induce the transcription of genes involved in cytoprotective responses [76]. In this way, curcumin is revealed to decrease oxidative stress, measured as the protein carbonyl content, and mediate in vivo neuroprotection, reducing striatal morphological alterations induced by quinolinic acid which reproduces the oxidative status that may be found in HD [54].
4.4. Mitochondrial Dysfunction
m-HTT has been shown to have a negative implication in ATP production, by impairing oxidative phosphorylation in striatal cells [35]. To assess the effect of curcumin in restoring the mitochondrial dynamics, an in vivo study has been performed, by using the reducing sugar D-galactose which induces oxidative stress and which is involved in the aging process. Therefore, it replies the mitochondrial damage that could occur in neurodegenerative disorders, due to the energetic dysfunctions in mitochondrial respiration. The treatment of rats with different oral doses of curcumin (50 and 100 mg/kg) was effective in elevating the levels of succinate dehydrogenase and aconitase, improving the activity of complexes I, II and III of the respiratory chain as well as being effective in mitochondrial membrane stabilization [72].
Treatment with curcumin nanoparticles is associated with a remarkable improvement of the mitochondrial activity and cytochrome levels [54]. Curcumin, acting as a hydrogen donor, reduces protein oxidation induced by galactose thus maintaining the cellular redox balance [72].
4.5. Neuroinflammation
In HD, inflammatory cytokines, like IL-1β and TNF-α, activate the NF-κB pathway, resulting in pro-inflammatory and pro-apoptotic responses and leading to neural damage and death. In fact, TNF-α can bind its TNFr1-type receptor on neurons and promote the expression of many inflammatory mediators, as well as phospholipases and cyclooxygenases [77], stimulating an inflammatory process associated with striatum neurodegeneration. In rats, curcumin pre-treatment with two different oral daily doses (25 and 50 mg/kg) has shown a substantial decrease in IL-1β and TNF-α concentrations [74]. Interestingly, the association of curcumin with piperidine, an alkaloid deriving from black pepper, enhances the anti-inflammatory answer [74]. This may be due to a synergistic action that improves the curcumin brain bioavailability. Curcumin combined with piperidine is also associated with an increment of the catecholaminergic tone which is also suppressed in HD, thanks to an inhibitory activity on monoamine oxidases [78].
4.6. Neurodegeneration
The progressive degeneration of neural cells could be attenuated by curcumin. The phosphatidylinositol 3-kinase /Akt pathway could be modulated by curcumin, mediating brain cell survival [54], while neurogenesis could be enhanced by the inhibition of the glycogen synthase kinase-3β [45]. Proof comes from different dietary interventions performed on animal models. Chronic pre-treatment with oral daily doses of curcumin (25 and 50 mg/kg) was correlated to beneficial results towards motility alterations, bio- and neuro-chemical variations in 3-nitropropionic acid-induced HD rats [74]. In the same way, curcumin and its combination with piperidine also improve behavioral and neurological disorders in quinolinic acid-induced HD rats [78]. Ina Drosophila HD transgenic model, curcumin (5 and 10 μM) reduces poly-Q-mediated photoreceptor degeneration and progressive motoneuron loss of function in a dose-dependent manner [65].
5. MECHANISTIC ASPECTS
Oxidative stress is the most important mechanism as a pathological cause of neurodegenerative disorders including Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, and Amyotrophic Lateral Sclerosis. Polyphenols in various cell and animal cultures showed neuroprotective effects attributed to their potential to combat oxidative and inflammatory stresses as well as modulating several cell signaling pathways [79-82]. Considering the pathological features of HD, curcumin can halt disease progression by altering multiple targets such as oxidative stress, inflammatory cascade pathways, mitochondrial dysfunction and transcriptional dysregulation [65]. Table 1 and Fig. 3 summarized the mechanistic aspects of curcumin activity in treating and preventing neurotoxicity and HD.
5.1. Antioxidant and Anti-inflammatory Potential
Curcumin is the most widely studied polyphenolic compound which is well known for its anti-oxidant properties through reactive oxygen species (ROS) scavenging, nitric oxide-based free radicals neutralization and decrease of the expression of inflammatory mediators including tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β) [83-85]. Further studies have investigated the anti-oxidant and iron (Fe) binding properties of curcumin, capsaicin and S-allylcysteine in rat brain homogenates [86]. It was observed that these compounds potently scavenge free radicals such as superoxide anions decreasing lipid peroxidation and Fe ion chelation. Metal chelation and reducing oxidative stress are indeed one of the key mechanisms for the prevention or the treatment of neurodegenerative disorders.
Sandhir et al. (2014) studied the neuroprotective effects of curcumin encapsulated in solid lipid nanoparticles (C-SLNs) in experimental models of HD induced by 3-nitropropionic acid (3-NP) [58]. C-SLN in oral (p.o.) dose of 40 mg/kg body weight results in ROS decrease, glutathione levels and activity of superoxide dismutase (SOD) restoration, mitochondrial activity increase, lipid peroxidation reduction thus ameliorating 3-NP-induced neurotoxicity in rats. In another study, it was concluded that a chronic treatment with curcumin at doses of 10, 20 and 50 mg/kg p.o. reduces oxidative stress and restored succinate dehydrogenase activity in 3-NP treated rats [87].
While investigating the neuroprotective effects of curcumin in a model study of quinolinic acid-induced neurotoxicity, Carmona-Ramírez et al. (2013) observed that curcumin at a dose of400 mg/kg p.o. for 10 days prevents the onset and progression of HD by activating the striatal intra-nuclear Nrf2 cytoprotective pathway and increasing total SOD and glutathione peroxidase activities [55]. Further experiments also revealed that the anti-oxidant and anti-inflammatory properties of curcumin constitute one of the most potent mechanisms in preventing and treating HD [88].
5.2. Metal Ion Chelation
Heavy metals such as copper (Cu), aluminum (Al), iron (Fe), lead (Pb), cadmium (Cd), zinc (Zn) and manganese (Mn) can cause aggregation of misfolded proteins and induction of oxidative stress in various neurodegenerative disorders [89]. The presence of one active methylene (CH2) group and two phenolic (OH) groups in curcumin makes it an excellent ligand to chelate heavy metals [90]. The neuroprotection activity of curcumin through chelation of Cd, Pb, Fe, Cu and Zn has been reported as a mechanism that is more likely to halt protein aggregation [51, 91, 92]. It has been documented that metal ion chelation by curcumin can also decrease oxidative stress as well as neuroinflammation leading to the protection of neurons from degeneration [93].
5.3. Transcriptional Alterations
Being an autosomal dominant disorder unstable elongated polyglutamine repeats near the N terminus of huntingtin gene forming HTT protein which may aggregate throughout the brain [94, 95]. Hickey et al. studied the effect of curcumin on the improvement of transcriptional deficits using CAG 140 knock-in mice model of HD [66]. It was observed that curcumin at a dose of 555 pm causes an improvement in transcriptional dysregulation decreasing HTT protein aggregation and improved rearing deficits.
5.4. Molecular Chaperones and Protein Vibration
Accumulation and aggregation of misfolded proteins is a common feature of all neurodegeneration disorders playing a crucial role in disease progression and severity [96]. Depending on the size and the degree of misfolding, most of the misfolded proteins are degraded by lysosomes. In contrast, heat shock proteins (HSPs) or molecular chaperons selectively tagged the rest of the proteins which are degraded by phagosomes or proteasomes [97-99]. Therefore, failure of SHPs to properly perform their function can lead to neuronal degeneration such as in Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, and Amyotrophic Lateral Sclerosis [100]. Naturally-occurring compounds are now known to activate HSPs including celastrol, withaferin-A and gambogic acid [101, 102].
Curcumin has been reported to induce HSPs such as HSP40, HSP60, HSP70 and HSP90 and thus it can reverse the misfolding of proteins which leads to protection against the degeneration of neurons [103]. Majumdar studied the upregulation of HSPs by curcumin in the prevention of HD [104]. The results of this study revealed that curcumin administration leads to disaggregation of aggregated HTT proteins and an upregulation of the activity of HSP-70 and HSP-90. A protein vibration approach was also explained as a possible source for the disaggregation of glutamic acid molecules from aggregated HTT proteins and modulation of HSP activity to slow down amyloid formation.
CONCLUSION
Neurodegenerative disorders such as Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, and Amyotrophic Lateral Sclerosis could not be cured completely with the available therapies, rather this latter can only decrease the severity of disease symptoms and are associated with a number of undesirable side effects. Curcumin is a polyphenolic compound isolated from the rhizome of turmeric. It has a wide range of pharmacological properties and multiple therapeutic uses of curcumin have been reported so far. The role of curcumin in preventing and treating neurological disorders has also been investigated and numerous studies support the successful use of curcumin in neurodegenerative disorders including HD. Curcumin possesses the potential to halt the progression of neurodegeneration in HD by targeting multiple mechanisms including reducing oxidative and inflammatory stresses, metal ion chelation, transcriptional alterations and disaggregation of aggregated proteins by increasing the activity of HSPs. Having a favorable safety profile, curcumin could be an alternative to conventional therapies in treating and preventing HD. However, data from human studies are lacking and clinical trials should be highly encouraged in this regard.
AUTHORS’ CONTRIBUTIONS
Fabiana Labanca, Hammad Ullah, Haroon Khan, Luigi Milella wrote the initial draft of the article, including figures and tables. Haroon Khan, Jianbo Xiao, Philippe Jeandet and Zora Dajic-Stevanovic corrected the final version of the article.
Fig. (1).
Correlation between the number of CAG repetition and the age of onset of HD [18]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (2).
Curcumin improves HD clinical course by modulation of several pathways. The figure summarizes the main activity of curcumin on HD-related mechanisms. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
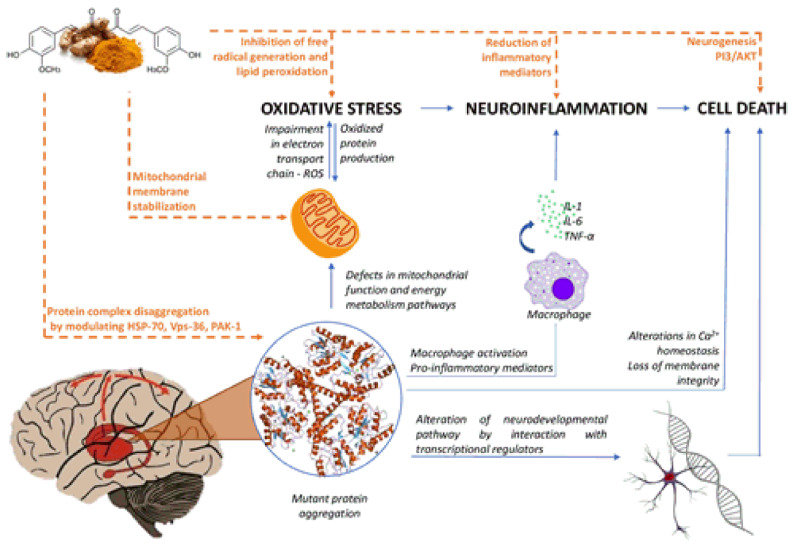
Fig. (3).
Mechanisms of Curcumin in Huntington's disease. Curcumin possesses the potential to treat or prevent HD by targeting multiple mechanisms: (1) Curcumin can decrease production of reactive oxygen species (ROS) and increase the production of antioxidant enzymes such as glutathione which leads to reduction of oxidative stresses (2) Metal ion toxicity also enhances oxidative stress in neuronal tissues and curcumin can chelate metal ions thus reducing metal ion toxicity. Both these mechanisms help in reduction of oxidative stresses which leads to the downregulation of inflammatory mediators thus decreasing neuroinflammation and resulting in neuroprotection (3) Curcumin can upregulate the activity of heat shock proteins (HSPs) which reverses the misfolding of proteins and disaggregates the aggregated proteins (4) Curcumin can also alter genes responsible for causing HD including genes for maintaining mitochondrial activity. It can improve transcriptional dysregulation and decrease HTT protein aggregation. All these mechanisms directly result in neuroprotection. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
Mechanistic insights of curcumin in different models of neurotoxicity.
| Experimental Model | Dose | Effects | Refs. |
|---|---|---|---|
| 3-NP-induced HD in rats | p.o. 40 mg/kg BW, 7 days (C-SLN) | ↓ROS and lipid peroxidation, Restored glutathione levels and SOD activity, ↑ mitochondrial activity |
[58] |
| 3-NP-induced neurotoxicity in rats |
p.o. 10, 20 and 50 mg/kg, 8 days(curcumin) | Improved motor and cognitive impairment, ↓ lipid peroxidation, ↑ glutathione levels and succinate dehydrogenase activity |
[87] |
| Quinolinic acid-induced neurotoxicity in rats |
p.o. 400 mg/kg, 10 days(curcumin) | Upregulated striatal intra-nuclear Nrf2 cytoprotective pathway, ↑ SOD and glutathione peroxidase activities |
[55] |
| CAG 140 mice | Diet, 555 ppm (curcumin) | ↓ Protein aggregation, Improved rearing, ↓ climbing |
[66] |
| Cultured Mouse Neuroblastoma Cells |
Diet, 0.01 µM, 0.1 µM, 1 µM, 10 µM (curcumin and SLCP)1 | ↓ ROS production and GSK-3𝛽 levels, ↑HSPs expression (HSP40, HSP60, HSP70, HSP90), Reversed misfolding of proteins |
[103] |
1SLCP provides greater neuroprotection comparatively to dietary curcumin 3-nitropropionic acid (3-NP), Huntington's disease (HD), curcumin- solid lipid nanoparticles (C-SLN), reactive oxygen species (ROS), superoxide dismutase (SOD), Nuclear factor erythroid 2-related factor 2(Nrf2), parts per million (ppm), heat shock proteins (HSPs), solid lipid curcumin particles (SLCP).
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Padhye S., Chavan D., Pandey S., Deshpande J., Swamy K.V., Sarkar F.H. Perspectives on chemopreventive and therapeutic potential of curcumin analogs in medicinal chemistry. Mini Rev. Med. Chem. 2010;10(5):372–387. doi: 10.2174/138955710791330891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao W., Li K., Rong S., Yao P., Hao L., Ying C., Zhang X., Nussler A., Liu L. Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J. Ethnopharmacol. 2010;128(2):549–553. doi: 10.1016/j.jep.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 3.Lobo R., Prabhu K.S., Shirwaikar A., Shirwaikar A. Curcuma zedoaria Rosc. (white turmeric): a review of its chemical, pharmacological and ethnomedicinal properties. J. Pharm. Pharmacol. 2009;61(1):13–21. doi: 10.1211/jpp.61.01.0003. [DOI] [PubMed] [Google Scholar]
- 4.Rajamma A.G., Bai V., Nambisan B. Antioxidant and antibacterial activities of oleoresins isolated from nine Curcuma species. Phytopharmacology. 2012;2:312–317. [Google Scholar]
- 5.Tohda C., Nakayama N., Hatanaka F., Komatsu K. Comparison of anti-inflammatory activities of six Curcuma rhizomes: a possible curcuminoid-independent pathway mediated by Curcuma phaeocaulis extract. Evid. Based Complement. Alternat. Med. 2006;3(2):255–260. doi: 10.1093/ecam/nel008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad S., Gupta S.C., Tyagi A.K., Aggarwal B.B. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol. Adv. 2014;32(6):1053–1064. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Zhou H., Beevers C.S., Huang S. The targets of curcumin. Curr. Drug Targets. 2011;12(3):332–347. doi: 10.2174/138945011794815356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oppenheimer A. Turmeric (curcumin) in biliary diseases. Lancet. 1937;229:619–621. doi: 10.1016/S0140-6736(00)98193-5. [DOI] [Google Scholar]
- 9.Khan H., Ullah H., Nabavi S.M. Mechanistic insights of hepatoprotective effects of curcumin: Therapeutic updates and future prospects. Food Chem. Toxicol. 2019;124:182–191. doi: 10.1016/j.fct.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Martin B., Golden E., Keselman A., Stone M., Mattson M.P., Egan J.M., Maudsley S. Therapeutic perspectives for the treatment of Huntington’s disease: treating the whole body. Histol. Histopathol. 2008;23(2):237–250. doi: 10.14670/hh-23.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA Approves Drug for Huntington's Disease 2017.
- 12.Mestre T.A. Recent advances in the therapeutic development for Huntington disease. Parkinsonism Relat. Disord. 2019;59:125–130. doi: 10.1016/j.parkreldis.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Maiti P., Manna J., Veleri S., Frautschy S. Molecular chaperone dysfunction in neurodegenerative diseases and effects of curcumin. BioMed Res. Int. 2014;2014:495091. doi: 10.1155/2014/495091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fusilli C., Migliore S., Mazza T., Consoli F., De Luca A., Barbagallo G., Ciammola A., Gatto E.M., Cesarini M., Etcheverry J.L., Parisi V., Al-Oraimi M., Al-Harrasi S., Al-Salmi Q., Marano M., Vonsattel J.G., Sabatini U., Landwehrmeyer G.B., Squitieri F. Biological and clinical manifestations of juvenile Huntington’s disease: a retrospective analysis. Lancet Neurol. 2018;17(11):986–993. doi: 10.1016/S1474-4422(18)30294-1. [DOI] [PubMed] [Google Scholar]
- 15.Ullah F., Liang A., Rangel A., Gyengesi E., Niedermayer G., Münch G. High bioavailability curcumin: an anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch. Toxicol. 2017;91(4):1623–1634. doi: 10.1007/s00204-017-1939-4. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y.M., Zhang Y.B., Wu Z.Y. Huntington’s disease: relationship between phenotype and genotype. Mol. Neurobiol. 2017;54(1):342–348. doi: 10.1007/s12035-015-9662-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee J.M., Gillis T., Mysore J.S., Ramos E.M., Myers R.H., Hayden M.R., Morrison P.J., Nance M., Ross C.A., Margolis R.L., Squitieri F., Griguoli A., Di Donato S., Gomez-Tortosa E., Ayuso C., Suchowersky O., Trent R.J., McCusker E., Novelletto A., Frontali M., Jones R., Ashizawa T., Frank S., Saint-Hilaire M.H., Hersch S.M., Rosas H.D., Lucente D., Harrison M.B., Zanko A., Abramson R.K., Marder K., Sequeiros J., MacDonald M.E., Gusella J.F. Common SNP-based haplotype analysis of the 4p16.3 Huntington disease gene region. Am. J. Hum. Genet. 2012;90(3):434–444. doi: 10.1016/j.ajhg.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nopoulos P.C. Huntington disease: a single-gene degenerative disorder of the striatum. Dialogues Clin. Neurosci. 2016;18(1):91–98. doi: 10.31887/DCNS.2016.18.1/pnopoulos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manoharan S., Guillemin G.J., Abiramasundari R.S., Essa M.M., Akbar M., Akbar M.D. The role of reactive oxygen species in the pathogenesis of Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease: A mini review. Oxid. Med. Cell. Longev. 2016;2016:8590578. doi: 10.1155/2016/8590578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erkkinen M.G., Kim M.O., Geschwind M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018;10(4):10. doi: 10.1101/cshperspect.a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.s MD, Wexler NS, Wexler AR, Tabrizi SJ, Douglas I, Evans SJ, Smeeth L. The prevalence of Huntington’s disease. Neuroepidemiology. 2016;46:144–153. doi: 10.1159/000443738. [DOI] [PubMed] [Google Scholar]
- 22.Adachi Y., Nakashima K. Population genetic study of Huntington’s disease--prevalence and founder’s effect in the San-in area, western Japan. Nihon Rinsho. 1999;57(4):900–904. [PubMed] [Google Scholar]
- 23.Kay C., Hayden M.R., Leavitt B.R. Epidemiology of Huntington disease. Handb. Clin. Neurol. 2017;144:31–46. doi: 10.1016/B978-0-12-801893-4.00003-1. [DOI] [PubMed] [Google Scholar]
- 24.Baig S.S., Strong M., Quarrell O.W. The global prevalence of Huntington’s disease: a systematic review and discussion. Neurodegener. Dis. Manag. 2016;6(4):331–343. doi: 10.2217/nmt-2016-0008. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Severiano F., Ríos C., Segovia J. Striatal oxidative damage parallels the expression of a neurological phenotype in mice transgenic for the mutation of Huntington’s disease. Brain Res. 2000;862(1-2):234–237. doi: 10.1016/S0006-8993(00)02082-5. [DOI] [PubMed] [Google Scholar]
- 26.Mehler M.F., Gokhan S. Mechanisms underlying neural cell death in neurodegenerative diseases: alterations of a developmentally-mediated cellular rheostat. Trends Neurosci. 2000;23(12):599–605. doi: 10.1016/S0166-2236(00)01705-7. [DOI] [PubMed] [Google Scholar]
- 27.Crotti A., Benner C., Kerman B.E., Gosselin D., Lagier-Tourenne C., Zuccato C., Cattaneo E., Gage F.H., Cleveland D.W., Glass C.K. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat. Neurosci. 2014;17(4):513–521. doi: 10.1038/nn.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young A.B., Greenamyre J.T., Hollingsworth Z., Albin R., D’Amato C., Shoulson I., Penney J.B. NMDA receptor losses in putamen from patients with Huntington’s disease. Science. 1988;241(4868):981–983. doi: 10.1126/science.2841762. [DOI] [PubMed] [Google Scholar]
- 29.Morigaki R., Goto S. Striatal Vulnerability in Huntington’s disease: neuroprotection versus neurotoxicity. Brain Sci. 2017;7(6):63. doi: 10.3390/brainsci7060063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim R.G., Salazar L.L., Wilton D.K., King A.R., Stocksdale J.T., Sharifabad D., Lau A.L., Stevens B., Reidling J.C., Winokur S.T. HD iPSC Consortium. Developmental alterations in Huntington’s disease neural cells and pharmacological rescue in cells and mice. Nat. Neurosci. 2017;20(5):648–660. doi: 10.1038/nn.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bizat N., Hermel J-M., Boyer F., Jacquard C., Créminon C., Ouary S., Escartin C., Hantraye P., Kajewski S., Brouillet E. Calpain is a major cell death effector in selective striatal degeneration induced in vivo by 3-nitropropionate: implications for Huntington’s disease. J. Neurosci. 2003;23(12):5020–5030. doi: 10.1523/JNEUROSCI.23-12-05020.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cattaneo E., Rigamonti D., Goffredo D., Zuccato C., Squitieri F., Sipione S. Loss of normal huntingtin function: new developments in Huntington’s disease research. Trends Neurosci. 2001;24(3):182–188. doi: 10.1016/S0166-2236(00)01721-5. [DOI] [PubMed] [Google Scholar]
- 33.Reddy P.H., Williams M., Charles V., Garrett L., Pike-Buchanan L., Whetsell W.O., Jr, Miller G., Tagle D.A. Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nat. Genet. 1998;20(2):198–202. doi: 10.1038/2510. [DOI] [PubMed] [Google Scholar]
- 34.Essa M.M., Moghadas M., Ba-Omar T., Walid Qoronfleh M., Guillemin G.J., Manivasagam T., Justin-Thenmozhi A., Ray B., Bhat A., Chidambaram S.B., Fernandes A.J., Song B.J., Akbar M. Protective effects of antioxidants in Huntington’s disease: an extensive review. Neurotox. Res. 2019;35(3):739–774. doi: 10.1007/s12640-018-9989-9. [DOI] [PubMed] [Google Scholar]
- 35.Tabrizi S.J., Workman J., Hart P.E., Mangiarini L., Mahal A., Bates G., Cooper J.M., Schapira A.H. Mitochondrial dysfunction and free radical damage in the Huntington R6/2 transgenic mouse. Ann. Neurol. 2000;47(1):80–86. doi: 10.1002/1531-8249(200001)47:1<80:AID-ANA13>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 36.Chang D.T., Rintoul G.L., Pandipati S., Reynolds I.J. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol. Dis. 2006;22(2):388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Ng T-P., Chiam P-C., Lee T., Chua H-C., Lim L., Kua E-H. Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 2006;164(9):898–906. doi: 10.1093/aje/kwj267. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira N., Santos S.A., Domingues M.R.M., Saraiva M.J., Almeida M.R. Dietary curcumin counteracts extracellular transthyretin deposition: insights on the mechanism of amyloid inhibition. Biochim. Biophys. Acta. 2013;1832(1):39–45. doi: 10.1016/j.bbadis.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Hsu A-L., Murphy C.T., Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300(5622):1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 40.Vallianou N.G., Evangelopoulos A., Schizas N., Kazazis C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015;35(2):645–651. [PubMed] [Google Scholar]
- 41.Tizabi Y., Hurley L.L., Qualls Z., Akinfiresoye L. Relevance of the anti-inflammatory properties of curcumin in neurodegenerative diseases and depression. Molecules. 2014;19(12):20864–20879. doi: 10.3390/molecules191220864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Gorro C., Garau-Rolandi M., Escrichs A., Rodriguez-Dechicha N., Vaquer I., Subira S., Calopa M., Martinez-Horta S., Perez-Perez J., Kulisevsky J., Muñoz E., Santacruz P., Ruiz-Idiago J., Mareca C., de Diego-Balaguer R., Camara E. An active cognitive lifestyle as a potential neuroprotective factor in Huntington’s disease. Neuropsychologia. 2019;122:116–124. doi: 10.1016/j.neuropsychologia.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 43.Darvesh A.S., Carroll R.T., Bishayee A., Novotny N.A., Geldenhuys W.J., Van der Schyf C.J. Curcumin and neurodegenerative diseases: a perspective. Expert Opin. Investig. Drugs. 2012;21(8):1123–1140. doi: 10.1517/13543784.2012.693479. [DOI] [PubMed] [Google Scholar]
- 44.Croce K.R., Yamamoto A. A role for autophagy in Huntington’s disease. Neurobiol. Dis. 2019;122:16–22. doi: 10.1016/j.nbd.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tiwari S.K., Agarwal S., Seth B., Yadav A., Nair S., Bhatnagar P., Karmakar M., Kumari M., Chauhan L.K.S., Patel D.K., Srivastava V., Singh D., Gupta S.K., Tripathi A., Chaturvedi R.K., Gupta K.C. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano. 2014;8(1):76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- 46.Monroy A., Lithgow G.J., Alavez S. Curcumin and neurodegenerative diseases. Biofactors. 2013;39(1):122–132. doi: 10.1002/biof.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spinelli K.J., Osterberg V.R., Meshul C.K., Soumyanath A., Unni V.K. Curcumin Treatment Improves Motor Behavior in α-Synuclein Transgenic Mice. PLoS One. 2015;10(6):e0128510. doi: 10.1371/journal.pone.0128510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim H., Park B-S., Lee K-G., Choi C.Y., Jang S.S., Kim Y-H., Lee S-E. Effects of naturally occurring compounds on fibril formation and oxidative stress of β-amyloid. J. Agric. Food Chem. 2005;53(22):8537–8541. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 49.Reddy P.H., Manczak M., Yin X., Grady M.C., Mitchell A., Tonk S., Kuruva C.S., Bhatti J.S., Kandimalla R., Vijayan M., Kumar S., Wang R., Pradeepkiran J.A., Ogunmokun G., Thamarai K., Quesada K., Boles A., Reddy A.P. Protective effects of Indian spice curcumin against amyloid-β in Alzheimer’s disease. J. Alzheimers Dis. 2018;61(3):843–866. doi: 10.3233/JAD-170512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho J.W., Lee K.S., Kim C.W. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int. J. Mol. Med. 2007;19(3):469–474. [PubMed] [Google Scholar]
- 51.Voulgaropoulou S.D., van Amelsvoort T.A.M.J., Prickaerts J., Vingerhoets C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019;1725:146476. doi: 10.1016/j.brainres.2019.146476. [DOI] [PubMed] [Google Scholar]
- 52.Frautschy S.A., Hu W., Kim P., Miller S.A., Chu T., Harris-White M.E., Cole G.M. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging. 2001;22(6):993–1005. doi: 10.1016/S0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 53.Lim G.P., Chu T., Yang F., Beech W., Frautschy S.A., Cole G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21(21):8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yavarpour-Bali H., Ghasemi-Kasman M., Pirzadeh M. Curcumin-loaded nanoparticles: a novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomedicine. 2019;14:4449–4460. doi: 10.2147/IJN.S208332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carmona-Ramírez I., Santamaría A., Tobón-Velasco J.C., Orozco-Ibarra M., González-Herrera I.G., Pedraza-Chaverrí J., Maldonado P.D. Retracted: Curcumin restores Nrf2 levels and prevents quinolinic acid-induced neurotoxicity. Elsevier; 2013. [DOI] [PubMed] [Google Scholar]
- 56.Cheng A.L., Hsu C.H., Lin J.K., Hsu M.M., Ho Y.F., Shen T.S., Ko J.Y., Lin J.T., Lin B.R., Ming-Shiang W., Yu H.S., Jee S.H., Chen G.S., Chen T.M., Chen C.A., Lai M.K., Pu Y.S., Pan M.H., Wang Y.J., Tsai C.C., Hsieh C.Y. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- 57.Verma M., Sharma A., Naidu S., Bhadra A.K., Kukreti R., Taneja V. Curcumin prevents formation of polyglutamine aggregates by inhibiting Vps36, a component of the ESCRT-II complex. PLoS One. 2012;7(8):e42923. doi: 10.1371/journal.pone.0042923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sandhir R., Yadav A., Mehrotra A., Sunkaria A., Singh A., Sharma S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. Neuromolecular Med. 2014;16(1):106–118. doi: 10.1007/s12017-013-8261-y. [DOI] [PubMed] [Google Scholar]
- 59.Cheng K.K., Yeung C.F., Ho S.W., Chow S.F., Chow A.H., Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013;15(2):324–336. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsai Y.M., Chien C.F., Lin L.C., Tsai T.H. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood-brain barrier penetration. Int. J. Pharm. 2011;416(1):331–338. doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 61.Muchowski P.J. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron. 2002;35(1):9–12. doi: 10.1016/S0896-6273(02)00761-4. [DOI] [PubMed] [Google Scholar]
- 62.Gu X., Greiner E.R., Mishra R., Kodali R., Osmand A., Finkbeiner S., Steffan J.S., Thompson L.M., Wetzel R., Yang X.W. Serines 13 and 16 are critical determinants of full-length human mutant huntingtin induced disease pathogenesis in HD mice. Neuron. 2009;64(6):828–840. doi: 10.1016/j.neuron.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cole G.M., Teter B., Frautschy S.A. Neuroprotective effects of curcumin.The molecular targets and therapeutic uses of curcumin in health and disease. Springer; 2007. pp. 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia C., Cai Y., Li S., Yang J., Xiao G. Curcumin increases HSP70 expression in primary rat cortical neuronal apoptosis induced by gp120 V3 loop peptide. Neurochem. Res. 2015;40(9):1996–2005. doi: 10.1007/s11064-015-1695-x. [DOI] [PubMed] [Google Scholar]
- 65.Chongtham A., Agrawal N. Curcumin modulates cell death and is protective in Huntington’s disease model. Sci. Rep. 2016;6:18736. doi: 10.1038/srep18736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hickey M.A., Zhu C., Medvedeva V., Lerner R.P., Patassini S., Franich N.R., Maiti P., Frautschy S.A., Zeitlin S., Levine M.S., Chesselet M.F. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington’s disease. Mol. Neurodegener. 2012;7:12. doi: 10.1186/1750-1326-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qadir F., Aziz M.A., Sari C.P., Ma H., Dai H., Wang X., Raithatha D., Da Silva L.G.L., Hussain M., Poorkasreiy S.P., Hutchison I.L., Waseem A., Teh M-T. Transcriptome reprogramming by cancer exosomes: identification of novel molecular targets in matrix and immune modulation. Mol. Cancer. 2018;17(1):97. doi: 10.1186/s12943-018-0846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao L., Ma Q-L., Calon F., Harris-White M.E., Yang F., Lim G.P., Morihara T., Ubeda O.J., Ambegaokar S., Hansen J.E., Weisbart R.H., Teter B., Frautschy S.A., Cole G.M. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat. Neurosci. 2006;9(2):234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- 69.Salminen A., Suuronen T., Kaarniranta K. ROCK, PAK, and Toll of synapses in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2008;371(4):587–590. doi: 10.1016/j.bbrc.2008.04.148. [DOI] [PubMed] [Google Scholar]
- 70.Canals J.M., Pineda J.R., Torres-Peraza J.F., Bosch M., Martín-Ibañez R., Muñoz M.T., Mengod G., Ernfors P., Alberch J. Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J. Neurosci. 2004;24(35):7727–7739. doi: 10.1523/JNEUROSCI.1197-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma Q.L., Yang F., Frautschy S.A., Cole G.M. PAK in Alzheimer disease, Huntington disease and X-linked mental retardation. Cell. Logist. 2012;2(2):117–125. doi: 10.4161/cl.21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Banji O.J., Banji D.Ch.K. Curcumin and hesperidin improve cognition by suppressing mitochondrial dysfunction and apoptosis induced by D-galactose in rat brain. Food Chem. Toxicol. 2014;74:51–59. doi: 10.1016/j.fct.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 73.Sorolla M.A., Rodríguez-Colman M.J., Tamarit J., Ortega Z., Lucas J.J., Ferrer I., Ros J., Cabiscol E. Protein oxidation in Huntington disease affects energy production and vitamin B6 metabolism. Free Radic. Biol. Med. 2010;49(4):612–621. doi: 10.1016/j.freeradbiomed.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 74.Singh S., Jamwal S., Kumar P. Piperine Enhances the Protective Effect of Curcumin Against 3-NP Induced Neurotoxicity: Possible Neurotransmitters Modulation Mechanism. Neurochem. Res. 2015;40(8):1758–1766. doi: 10.1007/s11064-015-1658-2. [DOI] [PubMed] [Google Scholar]
- 75.Deck L.M., Hunsaker L.A., Vander Jagt T.A., Whalen L.J., Royer R.E., Vander Jagt D.L. Activation of anti-oxidant Nrf2 signaling by enone analogues of curcumin. Eur. J. Med. Chem. 2018;143:854–865. doi: 10.1016/j.ejmech.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 76.Levings D.C., Wang X., Kohlhase D., Bell D.A., Slattery M. A distinct class of antioxidant response elements is consistently activated in tumors with NRF2 mutations. Redox Biol. 2018;19:235–249. doi: 10.1016/j.redox.2018.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsieh H.L., Yang C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed Res. Int. 2013;2013:484613. doi: 10.1155/2013/484613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh S., Kumar P. Neuroprotective Activity of Curcumin in Combination with Piperine against Quinolinic Acid Induced Neurodegeneration in Rats. Pharmacology. 2016;97(3-4):151–160. doi: 10.1159/000443896. [DOI] [PubMed] [Google Scholar]
- 79.Skouta R., Morán-Santibañez K., Valenzuela C.A., Vasquez A.H., Fenelon K. Assessing the Antioxidant Properties of Larrea tridentata Extract as a Potential Molecular Therapy against Oxidative Stress. Molecules. 2018;23(7):1826. doi: 10.3390/molecules23071826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gutierrez-Merino C., Lopez-Sanchez C., Lagoa R., Samhan-Arias A.K., Bueno C., Garcia-Martinez V. Neuroprotective actions of flavonoids. Curr. Med. Chem. 2011;18(8):1195–1212. doi: 10.2174/092986711795029735. [DOI] [PubMed] [Google Scholar]
- 81.Solanki I., Parihar P., Parihar M.S. Neurodegenerative diseases: From available treatments to prospective herbal therapy. Neurochem. Int. 2016;95:100–108. doi: 10.1016/j.neuint.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 82.Ullah H., Khan H. Anti-parkinson potential of silymarin: Mechanistic insight and therapeutic standing. Front. Pharmacol. 2018;9:422. doi: 10.3389/fphar.2018.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scapagnini G., Caruso C., Calabrese V. Therapeutic potential of dietary polyphenols against brain ageing and neurodegenerative disorders.Bio-Farms for Nutraceuticals. Springer; 2010. pp. 27–35. [DOI] [PubMed] [Google Scholar]
- 84.Sumanont Y., Murakami Y., Tohda M., Vajragupta O., Matsumoto K., Watanabe H. Evaluation of the nitric oxide radical scavenging activity of manganese complexes of curcumin and its derivative. Biol. Pharm. Bull. 2004;27(2):170–173. doi: 10.1248/bpb.27.170. [DOI] [PubMed] [Google Scholar]
- 85.Kant V., Gopal A., Pathak N.N., Kumar P., Tandan S.K., Kumar D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014;20(2):322–330. doi: 10.1016/j.intimp.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 86.Dairam A., Fogel R., Daya S., Limson J.L. Antioxidant and iron-binding properties of curcumin, capsaicin, and S-allylcysteine reduce oxidative stress in rat brain homogenate. J. Agric. Food Chem. 2008;56(9):3350–3356. doi: 10.1021/jf0734931. [DOI] [PubMed] [Google Scholar]
- 87.Kumar P., Padi S.S., Naidu P.S., Kumar A. Possible neuroprotective mechanisms of curcumin in attenuating 3-nitropropionic acid-induced neurotoxicity. Methods Find. Exp. Clin. Pharmacol. 2007;29(1):19–25. doi: 10.1358/mf.2007.29.1.1063492. [DOI] [PubMed] [Google Scholar]
- 88.Elifani F., Crispi S., Filosa S., Castaldo S., Capocci L., Madonna M., Amico E., Pompeo G., Pompeo F., Brunetti A., Ruggieri S. L9 Curcumin: a natural compound to counteract the pathology of huntington’s disease? Journal of Neurology, Neurosurgery &. Psychiatry. 2016;87:A93–A93. [Google Scholar]
- 89.Song Z., Feng R., Sun M., Guo C., Gao Y., Li L., Zhai G. Curcumin-loaded PLGA-PEG-PLGA triblock copolymeric micelles: Preparation, pharmacokinetics and distribution in vivo. J. Colloid Interface Sci. 2011;354(1):116–123. doi: 10.1016/j.jcis.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 90.Wanninger S., Lorenz V., Subhan A., Edelmann F.T. Metal complexes of curcumin--synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015;44(15):4986–5002. doi: 10.1039/C5CS00088B. [DOI] [PubMed] [Google Scholar]
- 91.Eybl V., Kotyzová D., Bludovská M. The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicol. Lett. 2004;151(1):79–85. doi: 10.1016/j.toxlet.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 92.Shakibaei M., John T., Schulze-Tanzil G., Lehmann I., Mobasheri A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007;73(9):1434–1445. doi: 10.1016/j.bcp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Maiti P., Dunbar G.L. Use of curcumin, a natural polyphenol for targeting molecular pathways in treating age-related neurodegenerative diseases. Int. J. Mol. Sci. 2018;19(6):1637. doi: 10.3390/ijms19061637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Juenemann K., Schipper-Krom S., Wiemhoefer A., Kloss A., Sanz Sanz A., Reits E.A.J. Expanded polyglutamine-containing N-terminal huntingtin fragments are entirely degraded by mammalian proteasomes. J. Biol. Chem. 2013;288(38):27068–27084. doi: 10.1074/jbc.M113.486076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Juenemann K., Weisse C., Reichmann D., Kaether C., Calkhoven C.F., Schilling G. Modulation of mutant huntingtin N-terminal cleavage and its effect on aggregation and cell death. Neurotox. Res. 2011;20(2):120–133. doi: 10.1007/s12640-010-9227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Selkoe D.J. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat. Cell Biol. 2004;6(11):1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 97.Kakkar V., Meister-Broekema M., Minoia M., Carra S., Kampinga H.H. Barcoding heat shock proteins to human diseases: looking beyond the heat shock response. Dis. Model. Mech. 2014;7(4):421–434. doi: 10.1242/dmm.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dou F., Netzer W.J., Tanemura K., Li F., Hartl F.U., Takashima A., Gouras G.K., Greengard P., Xu H. Chaperones increase association of tau protein with microtubules. Proc. Natl. Acad. Sci. USA. 2003;100(2):721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wyttenbach A., Carmichael J., Swartz J., Furlong R.A., Narain Y., Rankin J., Rubinsztein D.C. Effects of heat shock, heat shock protein 40 (HDJ-2), and proteasome inhibition on protein aggregation in cellular models of Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2000;97(6):2898–2903. doi: 10.1073/pnas.97.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sherman M.Y., Goldberg A.L. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron. 2001;29(1):15–32. doi: 10.1016/S0896-6273(01)00177-5. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Y-Q., Sarge K.D. Celastrol inhibits polyglutamine aggregation and toxicity though induction of the heat shock response. J. Mol. Med. (Berl.) 2007;85(12):1421–1428. doi: 10.1007/s00109-007-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davenport J., Manjarrez J.R., Peterson L., Krumm B., Blagg B.S., Matts R.L. Gambogic acid, a natural product inhibitor of Hsp90. J. Nat. Prod. 2011;74(5):1085–1092. doi: 10.1021/np200029q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maiti P., Dunbar G.L. Comparative neuroprotective effects of dietary curcumin and solid lipid curcumin particles in cultured mouse neuroblastoma cells after exposure to Aβ42. Int. J. Alzheimers Dis. 2017;2017 doi: 10.1155/2017/4164872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Majumder B. Activation of Heat Shock Protein Induced by Curcumin to Prevent Huntington Disease- An Analytical Approach in the Context of Protein Vibration. International Journal of Biophysics. 2018;8:1–8. [Google Scholar]