Abstract
The aim was to localize chemokine ligand twelve (CXCL12) in sheep placental tissues during early gestation and after assisted reproductive technologies (ART). Uteri were collected from naturally (NAT) mated ewes and ewes receiving embryo transfer (ET), in vitro fertilization (IVF) or in vitro activation (IVA). CXCL12 was immunolocalized to endometrial stroma, glands, and trophoblast. Greater CXCL12 immunoreactivity was present in trophoblast on day 22 and 24 and in NAT ewes compared to IVF and IVA. Increased CXCL12 expression suggests CXCL12 promotes implantation and placentation. Decreased CXCL12 in IVF and IVA embryos, may compromise pregnancy establishment when utilizing ART methods.
Keywords: CXCL12, implantation, placentation, assisted reproductive technologies
Introduction
CXCL12 binding to its receptor, CXCR4 influences pathways important for embryo attachment and placentation, leading to embryo survival [1–3]. To enhance our understanding of CXCL12 at the fetal-maternal interface in livestock, determining cell types that express CXCL12 is imperative. Our objectives were to localize CXCL12 within ovine utero-placenta from normal and assisted reproductive technologies (ART). We hypothesized CXCL12 would localize to trophoblast cells based on day of gestation and ART.
Materials and Methods
Animals
All procedures were approved by North Dakota State University Institutional Animal Care and Use Committee as previously reported [4–7].
Experiment 1
Uteri were obtained from ewes (n=3 per day) on days 18, 20, 22, 24, 26, and 28 after mating. Cross sections of gravid uterus were obtained followed by immersion in 10% neutral buffered formalin, and paraffin embedded according to standard histological procedures [8].
Experiment 2
Methods for experiment two are published [6, 7, 9]. Briefly, gravid uteri were collected on day 22 of gestation from control group (NAT), ewes in which pregnancy was achieved through embryo transfer after natural mating (NAT-ET), in vitro fertilization (IVF) or in vitro activation (IVA; parthenotes-production of embryo from female gamete only). Cross sections of gravid uterus were obtained followed by immersion in 10% neutral buffered formalin and paraffin embedded according to standard histological procedures [8].
Immunohistochemistry
Tissues sections (5μm) were mounted onto glass slides, and de-paraffinized (Histoclear; National Diagnostics, Atlanta, GA, USA) and rehydrated with a series of ethanol washes. Antigen retrieval was performed in 10 mM sodium citrate buffer, pH 6, with 0.05% Tween 20 in a 2100 retriever (Electron Microscopy Sciences, Hatfield, PA). Slides were rinsed twice in Tris-buffered saline with Triton X-100 (TBST; 0.05 M Tris, 0.15 M NaCl, 0.1% TritonX-100). To block nonspecific binding, slides were treated for 20 minutes with blocking buffer (10% normal goat serum). Tissue sections were incubated with anti CXCL12 antibody (1:50 dilution in 1X TBS, R&D Systems, Minneapolis, MN) followed by incubation with Alexa 647-labeled antibody (1:200 dilution, Invitrogen A21235, Grand Island, NY). Slides were mounted with Pro-Long Gold with 4,6-diamidino-2-phenylindole (DAPI; Life Technologies, Grand Island, NY) to counterstain nuclei. Control sections were incubated with normal goat serum in place of CXCL12 antibody.
Microscopy
Photomicrographs were taken at same exposure time with a Zeiss Imager.M2 epifluorescence microscope using a 10x objective and AxioCam HRm camera, as well as a Zeiss piezo automated stage controlled by MosaiX module of Zeiss AxioVision software (Carl Zeiss Microscopy, LLC; 1 Zeiss Dr., Thornwood, NY).
Image and Statistical Analyses
MosaiX images were analyzed using Fiji Is Just Image J software program [10]. Image background was subtracted and ten separate areas analyzed for CXCL12 intensity for each ewe. Significant differences (P<0.05) in CXCL12 intensity were determined using one-way ANOVA analysis in Prism software (version 5, GraphPad Software, Inc.). Figures were designed using FigureJ [11].
Results
Experiment 1
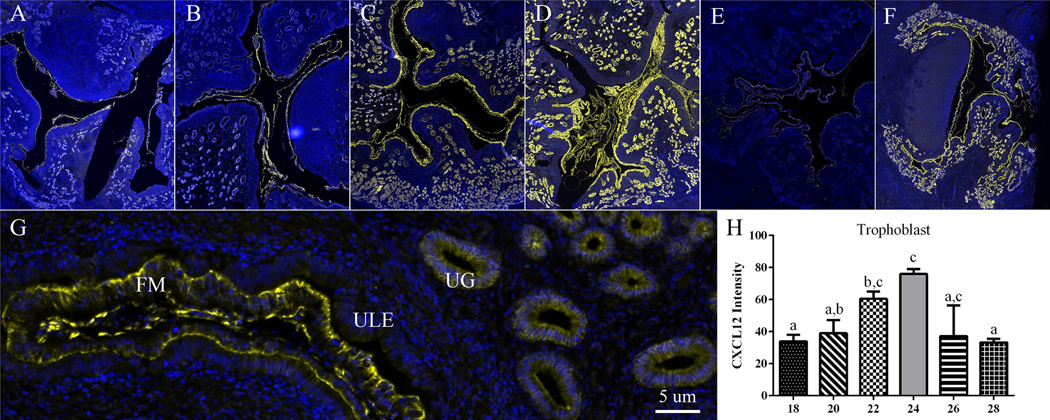
CXCL12 was immunolocalized to endometrial stroma, glands, and trophoblast on each day of gestation (Fig. 1A–G). Intensity of CXCL12 staining in trophoblast was greater (P<0.05) on day 22 compared to days 18 and 28, and day 24 was greater (P<0.05) than days 18, 20 and 28 (Fig. 1H). The greatest intensity of CXCL12 immunofluorescence in trophoblast was on days 22 and 24, and the least on days 18 and 28, with staining intensity intermediate on days 20 and 26.
Figure One. CXCL12 intensity increases in trophoblast cells on day 22 and 24 of gestation in sheep.
Representative MosaiX and 40x magnification (5 μm) images of CXCL12 in sheep uterine tissue on days 18, 20, 22, 24, 26 and 28 (A-F respectively). CXCL12 is localized in the endometrial stroma (ES), endometrial glands (EG), and trophoblast cells of fetal membrane (FM) (G). On day 22 and 24 CXCL12 intensity increased in FM compared to day 18 and 28 and day 18, 20 and 28 respectively (H). Different letters above each bar denote a significant (P < 0.05) difference. Yellow fluorescence signifies CXCL12 localization and blue represents 4,6-diamidino-2-phenylindole (DAPI) counterstaining of nuclei.
Experiment 2
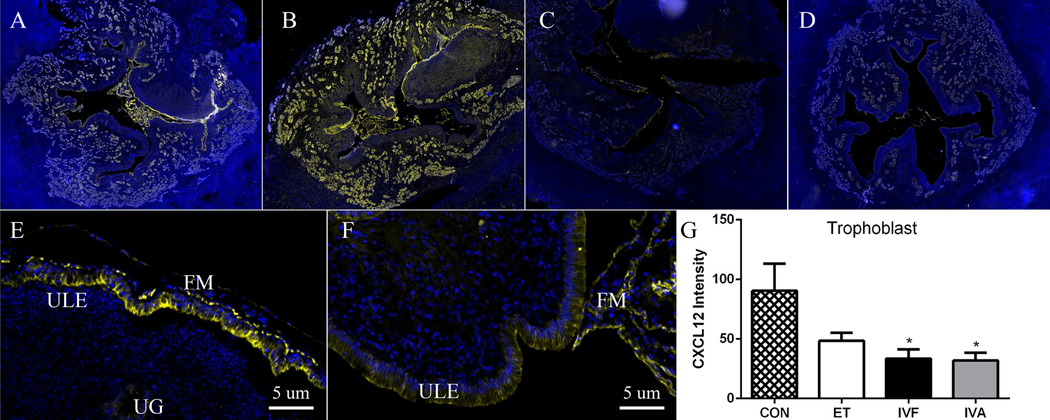
Similar to experiment one, CXCL12 was localized to endometrial stroma, glands, and trophoblast in NAT and ART uterine cross sections (Fig. 2A–F). Intensity of CXCL12 immunofluorescence differed in ewes exposed to ART compared to NAT, with less trophoblast CXCL12 intensity in IVF and IVA ewes than NAT (Fig. 2G).
Figure Two. CXCL12 intensity is less in fetal membranes (FM) in IVF and IVA groups compared to NAT control (natural breeding).
Representative MosaiX and 40x magnification (5 μm) images of CXCL12 immunolocalization in sheep uteroplacental tissues from NAT control (A, E) and after transfer of embryos created though in vivo fertilization (NAT-ET; B), in vitro fertilization (IVF; C,F) and in vitro activation (IVA; D). CXCL12 is localized in the endometrial stroma (ES), endometrial glands (EG), and FM in control (E) and ART ewes (F). Greater (P<0.05) CXCL12 intensity is present in FM from NAT control compared to IVF and IVA (G). Asterisks denote a significant difference compared to NAT control. Yellow fluorescence signifies CXCL12 localization and blue represents 4,6-diamidino-2-phenylindole (DAPI) counterstaining of nuclei.
Discussion
Distinct CXCL12 localization in glandular endometrium implicates CXCL12 may be secreted by uterine glands and functions in a paracrine and/or autocrine fashion to support placental development and conceptus survival. Similar to CXCL12 staining in sheep, vascular endothelial growth factor (VEGF) localizes to uterine glandular endometrium during early gestation in cows [12]. Of note, the CXCL12-CXCR4 signaling axis stimulates VEGF synthesis [13] and in turn VEGF induces CXCR4 and CXCL12 production [14]. In support of CXCL12 and VEGF interplay, we demonstrated treatment of ovine trophoblast cells with CXCL12 results in greater VEGF and Fibroblast Growth Factor 2 (FGF2) expression [3]. Interestingly, expression of angiogenic factors in sheep utero-placental tissues is decreased after transfer of IVF and IVA embryos [9], which leads to poor placental development and embryo growth [9, 15]. Poor growth of embryos created through IVF and IVA may be due to decreased CXCL12 and angiogenic factor synthesis, leading to impaired placental vascularization.
In sheep, a primary requirement for successful pregnancy is remodeling of luminal epithelium to support trophoblast attachment [16, 17]. Staining of CXCL12 in apical regions of endometrial stroma follows similar patterns of integrin subunit localization, suggesting CXCL12 regulates endometrial stroma reorganization and embryo implantation [18]. The peak increase of CXCL12 in trophoblast on day 24 suggests CXCL12 signaling supports fetal-maternal communication and contributes to trophoblast attachment.
Results from this study established the expression pattern of CXCL12 in endometrial stroma, glands and trophoblast throughout early gestation and after application of ART. Based on localization of CXCL12 at the fetal-maternal interface, we suggest CXCL12 signaling impacts embryo survival and successful establishment of pregnancy. Reduced CXCL12 signaling in utero-placental tissues of IVF and IVA embryos may contribute to poor pregnancy outcomes noted in ART techniques [15, 19–23]. To better understand causes of early embryonic loss, additional research investigating chemokine signaling at the fetal maternal interface, specifically pathways associated with cell survival, migration, and vascularization is needed.
Acknowledgements
The authors are grateful to Dr. Sheri Dorsam for her excellent technical assistance.
Funding
This project was supported by Agriculture and Food Research Initiative Competitive grants 2009–65203-05717 and 2009–65203-30216 from the USDA National Institute of Food and Agriculture to R.L.A. and grant 2007–01215 to L.P.R. and A.T.G.-B.
Footnotes
Declaration of Interest
The authors declare that there is no conflict of interest.
References
- [1].Meng YH, Shao J, Li H, Hou YL, Tang CL, Du MR, Li MQ and Li DJ. CsA improves the trophoblasts invasiveness through strengthening the cross-talk of trophoblasts and decidual stromal cells mediated by CXCL12 and CD82 in early pregnancy. Int J Clin Exp Pathol. 2012;5(4):299–307. [PMC free article] [PubMed] [Google Scholar]
- [2].Li MQ, Tang CL, Du MR, Fan DX, Zhao HB, Xu B and Li DJ. CXCL12 controls over-invasion of trophoblasts via upregulating CD82 expression in DSCs at maternal-fetal interface of human early pregnancy in a paracrine manner. Int J Clin Exp Pathol. 2011;4(3):276–86. [PMC free article] [PubMed] [Google Scholar]
- [3].Quinn KE, Ashley AK, Reynolds LP, Grazul-Bilska AT and Ashley RL. Activation of the CXCL12/CXCR4 signaling axis may drive vascularization of the ovine placenta. Domest Anim Endocrinol. 2014;47:11–21. [DOI] [PubMed] [Google Scholar]
- [4].Grazul-Bilska AT, Borowicz PP, Johnson ML, Minten MA, Bilski JJ, Wroblewski R, Redmer DA and Reynolds LP. Placental development during early pregnancy in sheep: vascular growth and expression of angiogenic factors in maternal placenta. Reproduction. 2010;140(1):165–74. [DOI] [PubMed] [Google Scholar]
- [5].Grazul-Bilska AT, Johnson ML, Borowicz PP, Minten M, Bilski JJ, Wroblewski R, Velimirovich M, Coupe L, Redmer DA and Reynolds LP. Placental development during early pregnancy in sheep: Cell proliferation, global methylation and angiogenesis in the fetal placenta. Reproduction. 2011. [DOI] [PubMed] [Google Scholar]
- [6].Grazul-Bilska AT, Johnson ML, Borowicz PP, Baranko L, Redmer DA and Reynolds LP. Placental development during early pregnancy in sheep: effects of embryo origin on fetal and placental growth and global methylation. Theriogenology. 2013;79(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reynolds LP, Haring JS, Johnson ML, Ashley RL, Redmer DA, Borowicz PP and Grazul-Bilska AT. Placental development during early pregnancy in sheep: estrogen and progesterone receptor messenger RNA expression in pregnancies derived from in vivo-produced and in vitro-produced embryos. Domest Anim Endocrinol. 2015;53:60–9. [DOI] [PubMed] [Google Scholar]
- [8].Luna LG. Manual of Histological Staining Methods of the Armed Forces Institute Pathology 1968; New York: McGraw-Hill. [Google Scholar]
- [9].Grazul-Bilska AT, Johnson ML, Borowicz PP, Bilski JJ, Cymbaluk T, Norberg S, Redmer DA and Reynolds LP. Placental development during early pregnancy in sheep: effects of embryo origin on vascularization. Reproduction. 2014;147(5):639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P and Cardona A. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9(7):676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mutterer J and Zinck E. Quick-and-clean article figures with FigureJ. J Microsc. 2013;252(1):89–91. [DOI] [PubMed] [Google Scholar]
- [12].Pfarrer CD, Ruziwa SD, Winther H, Callesen H, Leiser R, Schams D and Dantzer V. Localization of vascular endothelial growth factor (VEGF) and its receptors VEGFR-1 and VEGFR-2 in bovine placentomes from implantation until term. Placenta. 2006;27(8):889–98. [DOI] [PubMed] [Google Scholar]
- [13].Rosenkilde MM and Schwartz TW. The chemokine system -- a major regulator of angiogenesis in health and disease. Apmis. 2004;112(7–8):481–95. [DOI] [PubMed] [Google Scholar]
- [14].Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S and Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99(8):2703–11. [DOI] [PubMed] [Google Scholar]
- [15].Fidanza A, Toschi P, Zacchini F, Czernik M, Palmieri C, Scapolo P, Modlinski JA, Loi P and Ptak GE. Impaired placental vasculogenesis compromises the growth of sheep embryos developed in vitro. Biol Reprod. 2014;91(1):21. [DOI] [PubMed] [Google Scholar]
- [16].Johnson GA, Burghardt RC, Bazer FW and Spencer TE. Osteopontin: roles in implantation and placentation. Biol Reprod. 2003;69(5):1458–71. [DOI] [PubMed] [Google Scholar]
- [17].Wooding FB. Role of binucleate cells in fetomaternal cell fusion at implantation in the sheep. Am J Anat. 1984;170(2):233–50. [DOI] [PubMed] [Google Scholar]
- [18].Johnson GA, Bazer FW, Jaeger LA, Ka H, Garlow JE, Pfarrer C, Spencer TE and Burghardt RC. Muc-1, integrin, and osteopontin expression during the implantation cascade in sheep. Biol Reprod. 2001;65(3):820–8. [DOI] [PubMed] [Google Scholar]
- [19].Farin PW, Piedrahita JA and Farin CE. Errors in development of fetuses and placentas from in vitro-produced bovine embryos. Theriogenology. 2006;65(1):178–91. [DOI] [PubMed] [Google Scholar]
- [20].Miglino MA, Pereira FT, Visintin JA, Garcia JM, Meirelles FV, Rumpf R, Ambrosio CE, Papa PC, Santos TC, Carvalho AF, Leiser R and Carter AM. Placentation in cloned cattle: structure and microvascular architecture. Theriogenology. 2007;68(4):604–17. [DOI] [PubMed] [Google Scholar]
- [21].Palmieri C, Loi P, Ptak G and Della Salda L. Review paper: a review of the pathology of abnormal placentae of somatic cell nuclear transfer clone pregnancies in cattle, sheep, and mice. Vet Pathol. 2008;45(6):865–80. [DOI] [PubMed] [Google Scholar]
- [22].Palmieri C, Loi P, Reynolds LP, Ptak G and Della Salda L. Placental abnormalities in ovine somatic cell clones at term: a light and electron microscopic investigation. Placenta. 2007;28(5–6):577–84. [DOI] [PubMed] [Google Scholar]
- [23].Reynolds LP, Borowicz PP, Palmieri C and Grazul-Bilska AT. Placental vascular defects in compromised pregnancies: effects of assisted reproductive technologies and other maternal stressors. Adv Exp Med Biol. 2014;814:193–204. [DOI] [PubMed] [Google Scholar]