Abstract
Background
Among nonpregnant individuals, diabetes mellitus and high body mass index increase the risk of COVID-19 and its severity.
Objective
This study aimed to determine whether diabetes mellitus and high body mass index are risk factors for COVID-19 in pregnancy and whether gestational diabetes mellitus is associated with COVID-19 diagnosis.
Study Design
INTERCOVID was a multinational study conducted between March 2020 and February 2021 in 43 institutions from 18 countries, enrolling 2184 pregnant women aged ≥18 years; a total of 2071 women were included in the analyses. For each woman diagnosed with COVID-19, 2 nondiagnosed women delivering or initiating antenatal care at the same institution were also enrolled. The main exposures were preexisting diabetes mellitus, high body mass index (overweight or obesity was defined as a body mass index ≥25 kg/m2), and gestational diabetes mellitus in pregnancy. The main outcome was a confirmed diagnosis of COVID-19 based on a real-time polymerase chain reaction test, antigen test, antibody test, radiological pulmonary findings, or ≥2 predefined COVID-19 symptoms at any time during pregnancy or delivery. Relationships of exposures and COVID-19 diagnosis were assessed using generalized linear models with a Poisson distribution and log link function, with robust standard errors to account for model misspecification. Furthermore, we conducted sensitivity analyses: (1) restricted to those with a real-time polymerase chain reaction test or an antigen test in the last week of pregnancy, (2) restricted to those with a real-time polymerase chain reaction test or an antigen test during the entire pregnancy, (3) generating values for missing data using multiple imputation, and (4) analyses controlling for month of enrollment. In addition, among women who were diagnosed with COVID-19, we examined whether having gestational diabetes mellitus, diabetes mellitus, or high body mass index increased the risk of having symptomatic vs asymptomatic COVID-19.
Results
COVID-19 was associated with preexisting diabetes mellitus (risk ratio, 1.94; 95% confidence interval, 1.55–2.42), overweight or obesity (risk ratio, 1.20; 95% confidence interval, 1.06–1.37), and gestational diabetes mellitus (risk ratio, 1.21; 95% confidence interval, 0.99–1.46). The gestational diabetes mellitus association was specifically among women requiring insulin, whether they were of normal weight (risk ratio, 1.79; 95% confidence interval, 1.06–3.01) or overweight or obese (risk ratio, 1.77; 95% confidence interval, 1.28–2.45). A somewhat stronger association with COVID-19 diagnosis was observed among women with preexisting diabetes mellitus, whether they were of normal weight (risk ratio, 1.93; 95% confidence interval, 1.18–3.17) or overweight or obese (risk ratio, 2.32; 95% confidence interval, 1.82–2.97). When the sample was restricted to those with a real-time polymerase chain reaction test or an antigen test in the week before delivery or during the entire pregnancy, including missing variables using imputation or controlling for month of enrollment, the observed associations were comparable.
Conclusion
Diabetes mellitus and overweight or obesity were risk factors for COVID-19 diagnosis in pregnancy, and insulin-dependent gestational diabetes mellitus was associated with the disease. Therefore, it is essential that women with these comorbidities are vaccinated.
Key words: body mass index, COVID-19, diabetes mellitus, gestational diabetes mellitus, obesity, overweight, pregnancy, SARS-CoV-2
Introduction
Pregnant women with COVID-19 are at increased risk of severe illness compared with other pregnant and nonpregnant women.1, 2, 3, 4 Data from the multinational INTERCOVID study showed that neonates born to women with COVID-19 are at increased risk of morbidity and mortality.3 As with nonpregnant individuals, pregnant women with comorbidities, such as diabetes mellitus (DM) and overweight or obesity, are at risk of more severe COVID-19 outcomes,1 , 4, 5, 6 including mortality. In nonpregnant individuals, DM, particularly among people who use insulin,7 , 8 and high body mass index (BMI)9, 10, 11 increase not only the risk of severe COVID-19 outcomes but also the risk of SARS-CoV-2 infection.
AJOG at a Glance.
Why was this study conducted?
We quantified the risk of COVID-19 in pregnancy for women with gestational diabetes mellitus (GDM), preexisting diabetes mellitus (DM), or overweight or obesity by prepregnancy or early pregnancy body mass index.
Key findings
A COVID-19 diagnosis in pregnancy was associated with preexisting DM (risk ratio [RR], 1.94; 95% confidence interval [CI], 1.55–2.42) and high body mass (≥25 kg/m2) (RR, 1.20; 95% CI, 1.06–1.37), and GDM (RR, 1.21; 95% CI, 0.99–1.46), specifically among women who were insulin dependent whether they were of normal weight (RR, 1.79; 95% CI, 1.06–3.01) or overweight or obese (RR, 1.77; 95% CI, 1.28–2.45).
What does this add to what is known?
Pregnant women with DM or overweight or obesity were at high risk of COVID-19. Moreover, insulin-dependent GDM was associated with COVID-19 diagnosis.
As pregnant women were initially excluded from COVID-19 vaccine trials,12 there are little data on the safety of the COVID-19 vaccines during pregnancy.13 Concerns about safety have contributed to the lower levels of vaccine acceptance among pregnant women than among nonpregnant women.14 , 15 As of 6 November 2021, only 35.3% of pregnant women in the United States are vaccinated according to the most recent Centers for Disease Control and Prevention report, with rates as low as 20.6% among African Americans.16 The vaccination rates are low despite the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommendations of COVID-19 vaccination for pregnant and lactating women.17 , 18 Most recently, the Centers for Disease Control and Prevention urged pregnant women to be vaccinated,19 citing evidence from the v-safe pregnancy registry on safety of the messenger RNA vaccines13 and recent data from safety monitoring systems.20
Herein, we explored in the INTERCOVID study the association in pregnant women between a COVID-19 diagnosis and preexisting DM or high BMI and the diagnosis of gestational DM (GDM). Establishing the impact of comorbidities on the risk of infection in pregnant women can provide additional impetus for COVID-19 vaccination among those who remain hesitant.
Materials and Methods
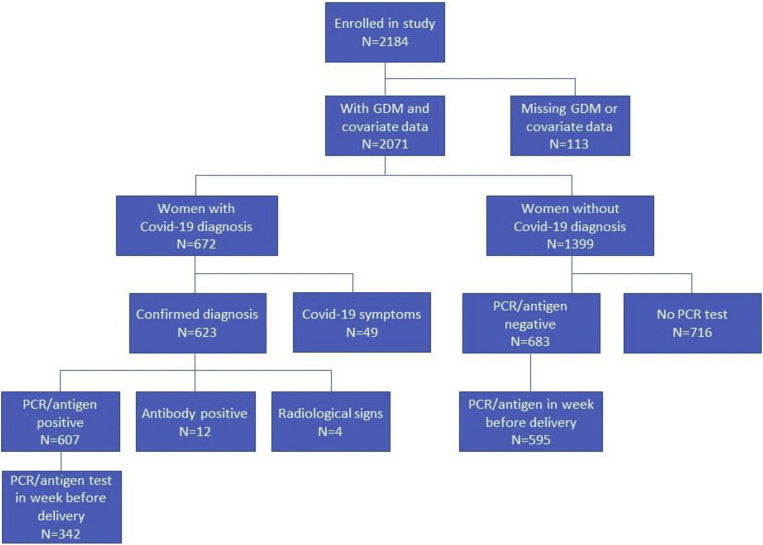
INTERCOVID was a multinational study assessing the effects of COVID-19 in pregnancy on mothers and neonates up to the time of their hospital discharge.3 , 21 Briefly, 2184 pregnant women aged ≥18 years, including 725 diagnosed and 1429 not diagnosed with COVID-19, were prospectively enrolled at 43 institutions in 18 countries (Argentina, Brazil, Egypt, France, Ghana, India, Indonesia, Italy, Japan, Mexico, Nigeria, North Macedonia, Pakistan, Russia, Spain, Switzerland, the United Kingdom, and the United States) between March 2, 2020, and February 2, 2021 (Supplemental Figure). Missing information on covariates reduced the sample size from 2184 to 2071 (approximately 5% of the total sample), which included 672 diagnosed with COVID-19 and 1399 not diagnosed.
Supplemental Figure.
Enrollment flowchart for the INTERCOVID study
GDM, gestational diabetes mellitus; PCR, polymerase chain reaction.
Eskenazi et al. COVID-19 and gestational diabetes, diabetes, and body mass index in pregnant women. Am J Obstet Gynecol 2022.
Women received a COVID-19 diagnosis based on a positive real-time polymerase chain reaction (RT-PCR) or antigen test (90.3%), a positive antibody test (1.8%), radiological pulmonary findings suggestive of COVID-19 (0.6%), or ≥2 predefined COVID-19 symptoms (7.3%) at any time during pregnancy, with approximately 80% diagnosed at delivery. Supplemental Table 1 shows the COVID-19 tests, and Supplemental Table 2 shows the symptoms recorded and their frequencies. Two “nondiagnosed” women were enrolled for each woman diagnosed with COVID-19. The nondiagnosed women had to be of similar gestational age (±2 weeks), to be receiving standard prenatal care from the same institution, and to be the patients who were provided care immediately following the diagnosed women. If a nondiagnosed woman was later diagnosed with COVID-19, she was maintained as nondiagnosed, using an “intention-to-treat approach.”
The Oxford Tropical Research Ethics Committee and all local ethics committees approved the study. Women provided informed consent (oral or written) according to local requirements, except if a waiver or exemption of such consent was granted by a local committee.
Diagnosis of diabetes mellitus, high body mass, and gestational diabetes mellitus
Information on preexisting DM, BMI, and GDM was abstracted from medical records using standardized forms. Women’s height was measured in duplicate using an adult stadiometer and recorded in centimeters to 1 decimal place. Women’s first-trimester weight was measured; if unavailable, women were asked to approximate weight before pregnancy. The woman’s prepregnancy or first-trimester weight was recorded in kilograms to 1 decimal place. BMI was calculated and categorized as normal weight (18.50–24.99 kg/m2), overweight (25.00–29.99 kg/m2), and obese (≥30 kg/m2) according to the World Health Organization definition.22 Moreover, from medical records, we collected information on the presence or absence of GDM during the index pregnancy and whether the women had been prescribed insulin for GDM and whether there was a previous diagnosis of DM. Women with preexisting DM were considered not to have GDM (per the definition of GDM).
Data management and analysis
We used the same centrally coordinated data management system established for the International Fetal and Newborn Growth Consortium for the 21st Century Project (INTERGROWTH-21st; MedSciNet, London, United Kingdom).23 All data were entered locally into the online system with its comprehensive, built-in, quality control facility. Queries could be dispatched immediately to the study sites, which provided continuously cleaned datasets for intermediate analysis (see https://intergrowth21.tghn.org/intercovid/intercovid-study-documents/ and previous publications for more details on study protocol and methods).3 , 24
Statistical analysis
We constructed 2 primary models for the analysis. The first model examined the association of COVID-19 diagnosis and GDM during the index pregnancy and preexisting DM; women with no history of either GDM or DM served as the reference group. Furthermore, BMI was included in this model. Initially, BMI was included as a 3-level variable, but because there was no clear dose response in the upper 2 levels, the categories of overweight or obese were collapsed to maximize sample size in that statum. Women with a BMI ≥25 kg/m2 were compared with those with a BMI <25 kg/m2 as the reference group. Covariates for the adjusted models were selected using a directed acyclic graph and included maternal age (continuous), parity (nulliparous vs parous), and tobacco use during pregnancy (yes vs no).
Second, we examined the association of COVID-19 diagnosis and GDM, DM, and BMI in 8 subgroups of women, including those with (1) no GDM or DM and normal weight (reference), (2) no GDM or DM and overweight or obese, (3) GDM not using insulin and normal weight, (4) GDM not using insulin and overweight or obese, (5) GDM using insulin and normal weight, (6) GDM using insulin and overweight or obese, (7) preexisting DM and normal weight, and (8) preexisting DM and overweight or obese. Furthermore, maternal age, parity, and tobacco use were included in this model.
Relationships of exposures and COVID-19 diagnosis were assessed using generalized linear models with a Poisson distribution and log link function, with robust standard errors to account for model misspecification. Analyses were performed using Stata (version 15.0; StataCorp, College Station, TX).25
Sensitivity analyses
As women with GDM are more likely to have closer clinical surveillance during pregnancy, including for COVID-19 symptoms, we included in sensitivity analyses only those who had an RT-PCR or antigen test within the last 7 days of pregnancy or on the day of delivery (n=937; 342 tested positive and 595 tested negative) (Supplemental Figure). Because of the proximity of testing to delivery, we assumed in these cases that the diagnosis of GDM, which typically develops in the second half of pregnancy, very likely preceded the COVID-19 diagnosis. Women who tested negative for COVID-19 on an RT-PCR or antigen test but were diagnosed by other means (n=12) were excluded from the analysis.
In other sensitivity analyses, we restricted the sample to women who had received an RT-PCR or antigen test at any time during pregnancy (607 who tested positive and 683 who tested negative) (Supplemental Figure). Furthermore, because the pandemic underwent fluctuations in cases in the course of the study, we conducted sensitivity analyses controlling for month of entry into the study.
As noted above, missing values reduced the sample size by 113 women (33 for GDM diagnosis, 88 for BMI category, 22 for tobacco use during pregnancy, and 7 for previous parity). To include these participants in sensitivity analyses, we generated values for all missing data employing multiple imputation using chained equations with 10 iterations, which brought the sample size back to 2184 women. We examined the same associations as above of COVID-19 diagnosis concerning GDM, DM, and BMI and by subgroups (eg, by insulin use and body mass).
We recorded symptomatology, but we did not record information on the severity of COVID-19. Hence, we could only consider whether GDM, DM, and BMI were more likely to be related to asymptomatic or symptomatic disease based on the women’s self-report. We restricted these models to women diagnosed with COVID-19 (n=672). We examined the association of having COVID-19 symptoms vs not having symptoms with GDM and DM (vs women with neither medical condition) and women who were overweight or obese (vs women of normal BMI). We repeated these analyses restricting the sample to those diagnosed by a positive RT-PCR or antigen test within the last 7 days of pregnancy or on the day of delivery (n=342).
Results
Women enrolled in these analyses averaged 30.2 years of age (standard deviation, ±6.1), and 43.4% of women were nulliparous (Table 1 ). GDM and DM were diagnosed in 194 (9.4%) and 53 (2.6%) participants, respectively; of note, 43.0% were overweight or obese. Overall, after adjusting for potential confounders, women with preexisting DM had nearly double the risk of COVID-19 (risk ratio [RR], 1.94; 95% confidence interval [CI], 1.55–2.42) (Table 2 ), and those who were overweight or obese had a 20% increase in risk (RR, 1.20; 95% CI, 1.06–1.37). Women who developed GDM had a 21% increased risk of COVID-19 (RR, 1.21; 95% CI, 0.99–1.46).
Table 1.
Baseline characteristics among women in the INTERCOVID study (n=2071)
| Characteristic | |
|---|---|
| Maternal age (y) | 30.2±6.1 |
| Parity | |
| Nulliparous | 898 (43.4) |
| Parous | 1173 (56.6) |
| Prepregnancy BMI (kg/m2) | 25.3±5.8 |
| Normal weight, <25 kg/m2 | 1180 (57.0) |
| Overweight, 25 to <30 kg/m2 | 535 (25.8) |
| Obese, ≥30 kg/m2 | 356 (17.2) |
| Tobacco use during pregnancy | |
| Yes | 72 (3.5) |
| No | 1999 (96.5) |
| Preexisting diabetes mellitus | |
| Yes | 53 (2.6) |
| No | 2018 (97.4) |
| Preexisting hypertension | |
| Yes | 56 (2.7) |
| No | 2012 (97.3) |
| Gestational diabetes mellitus during index pregnancy | |
| Yes | 194 (9.4) |
| No | 1877 (90.6) |
| Gestational age at delivery (wk) | 38.3±3.3 |
Data are presented as mean±standard deviation or number (percentage).
BMI, body mass index.
Eskenazi et al. COVID-19 and gestational diabetes, diabetes, and body mass index in pregnant women. Am J Obstet Gynecol 2022.
Table 2.
Associations between gestational diabetes mellitus, preexisting diabetes mellitus, and body mass index and COVID-19 diagnosis in the INTERCOVID study (n=2071)
| Variable | n (%) | COVID-19 diagnosis, n (%) | No COVID-19 diagnosis, n (%) | Unadjusted RR (95% CI) | Adjusted RR (95% CI) |
|---|---|---|---|---|---|
| GDM or DM | |||||
| No GDM, no DM | 1824 (88.1) | 564 (30.9) | 1260 (69.1) | Ref | Ref |
| GDM, no DM | 194 (9.4) | 75 (38.7) | 119 (61.3) | 1.19 (0.98–1.44)a | 1.21 (0.99–1.46)a |
| Preexisting DM | 53 (2.6) | 33 (62.3) | 20 (37.7) | 1.88 (1.51–2.36)b | 1.94 (1.55–2.42)b |
| BMI | |||||
| Normal weight (<25 kg/m2) | 1180 (57.0) | 344 (29.2) | 836 (70.8) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 891 (43.0) | 328 (36.8) | 563 (63.2) | 1.21 (1.06–1.37)b | 1.20 (1.06–1.37)b |
Data were adjusted for maternal age, parity, and tobacco use during pregnancy.
CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; GDM, gestational diabetes mellitus, RR, risk ratio.
Eskenazi et al. COVID-19 and gestational diabetes, diabetes, and body mass index in pregnant women. Am J Obstet Gynecol 2022.
P<.1
P<.05.
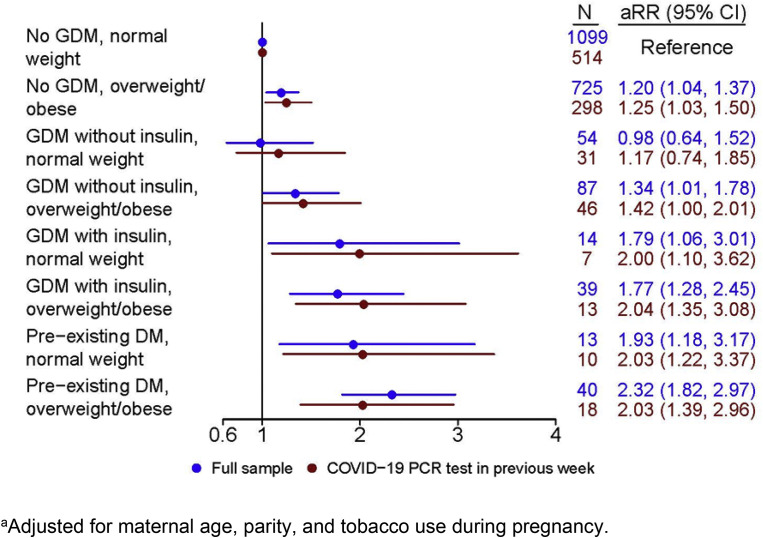
In the Figure , we presented the association of COVID-19 diagnosis among women in various exposure strata compared with women with a normal BMI (<25 kg/m2) who did not have preexisting DM or GDM. There was no association of GDM and COVID-19 diagnosis in women who were of normal weight and not using insulin (RR, 0.98; 95% CI, 0.64–1.52), However, COVID-19 diagnosis was associated with being overweight or obese among women who did not have GDM (RR, 1.20; 95% CI, 1.04–1.37), which was only slightly, but not significantly (P=.44), stronger among those with GDM (no insulin) who were also overweight or obese (RR, 1.34; 95% CI, 1.01–1.78). There was approximately an 80% increased risk of being diagnosed with COVID-19 among women with GDM using insulin whether they were of normal BMI (RR, 1.79; 95% CI, 1.06–3.01) or overweight or obese (RR, 1.77; 95% CI, 1.28–2.45). We observed the strongest association with COVID-19 diagnosis among women with preexisting DM, which was only slightly higher (P=.50) if they were overweight or obese (RR, 2.32; 95% CI, 1.82–2.97) than if they were of normal weight (RR, 1.93; 95% CI, 1.18–3.17).
Figure.
Risk of COVID-19 diagnosis according to gestational diabetes mellitus, insulin use, body mass index, and preexisting history of diabetes mellitus
The figure displays the risk of COVID-19 diagnosis according to GDM, insulin use, BMI, and preexisting history of DM for participants in the INTERCOVID study, in the full population (n=2071) and the subset of women with an RT-PCR or antigen test in the week before or on the day of delivery (n=937). The results were adjusted for maternal age, previous parity, and tobacco use during pregnancy.
BMI, body mass index; GDM, gestational diabetes mellitus; RT-PCR, real-time polymerase chain reaction.
Eskenazi et al. COVID-19 and gestational diabetes, diabetes, and body mass index in pregnant women. Am J Obstet Gynecol 2022.
In sensitivity analyses, when the sample was restricted to participants who had a COVID-19 RT-PCR or antigen test in the week before delivery, although the sample size was reduced by more than 50%, the observed associations were similar (Figure and Supplemental Table 3). Results were comparable when participants were restricted to those who received an RT-PCR or antigen tests at any time during pregnancy (Supplemental Table 4), when we controlled for month of enrollment (Supplemental Table 5), or when we performed multiple imputation on missing values (Supplemental Table 6).
When considering only the 672 women who were diagnosed with COVID-19 (Supplemental Table 7), having neither GDM (RR, 0.96; 95% CI, 0.88–1.03) nor preexisting DM (RR, 0.97; 95% CI, 0.87–1.07) was associated with the presence of COVID-19 symptoms, suggesting that women with these conditions were not more likely to have symptomatic infection than asymptomatic infection. However, women who were overweight or obese were more likely to report COVID-19 symptoms than women with normal weight (RR, 1.06; 95% CI, 1.01–1.11). The results were comparable when restricting the analysis to the 342 women with a positive RT-PCR or antigen test in the week before delivery.
Comment
Principal findings
In the INTERCOVID multinational study of more than 2000 pregnant women from 18 countries, preexisting DM and higher BMI were each associated with a higher risk of being diagnosed with COVID-19, after controlling for other potential confounders. Although women with GDM overall were marginally at higher risk of COVID-19 diagnosis, we did observe a significantly higher risk of COVID-19 diagnosis associated with GDM in people who use insulin; the strength of the association approached that for DM, regardless of a woman’s BMI. High BMI, but not GDM or DM, was related to more symptomatic disease among those with COVID-19.
Results in the context of what is known
Our results supported the evidence in nonpregnant individuals, demonstrating that preexisting DM is associated with risk of SARS-CoV-2 infection. Our findings were biologically plausible given higher rates of other types of infection, for example, pneumonia,26 urinary tract infections,27 and vaginitis,28 in individuals with DM, including during pregnancy.29
Accili30 posed the question as to the direction of the relationship between COVID-19 and DM. He concluded that although it is possible that SARS-CoV-2 can cause DM because of in vitro evidence demonstrating the susceptibility of β cells to SARS-CoV-2 and that any inflammatory state can lead to insulin resistance, the more likely causal direction is that DM is a risk factor for infection. Supporting this hypothesis, other researchers have shown that pancreatic islet cells express the angiotensin-converting enzyme 2 (ACE2) receptor and that SARS coronaviruses depend on the ACE2 receptor for attachment and invasion into cells.31 Zhao et al32 underlined the essential roles for glycosylation in mediating ACE2 receptor binding and antigenic shielding of SARS-CoV-2 spikes. Specifically, the SARS-CoV-2 spike protein, necessary for cell adhesion and invasion, and ACE2 receptors are glycosylated or glycated. These glycan-protein interactions in the SARS-CoV-2 spike protein and ACE2 receptor complex are important for cell invasion and infection.32 DM is associated with increased glycation or glycosylation in a variety of cells and tissues.33 Moreover, Hill et al34 have suggested that hyperglycemia increases viral replication and suppresses the antiviral immune response as evidenced by animal models of DM showing several structural changes to the lung, including increased permeability of the vasculature and breakdown of the alveolar epithelium.35 Similar hypotheses have been proposed for DM and GDM and COVID-19 during pregnancy.36
In our study of COVID-19 in pregnancy, the women with DM had this condition diagnosed before pregnancy and thus before COVID-19 diagnosis. Although the directionality of the association between COVID-19 and GDM was less clear in the larger cohort, the confirmation of results in the subgroup of women who received a COVID-19 test during the time of delivery provided reassurance that GDM diagnoses preceded infection and supported the hypothesis that the hyperglycemic state decreased the immune response to infection.26, 27, 28 , 37 The fact that preexisting DM poses a higher risk than GDM, except in women who are insulin dependent, is also biologically plausible given that women with preexisting disease have been in this hyperglycemic state for a longer period. That GDM was only associated with COVID-19 diagnosis in women who were using insulin indicated that they had more severe GDM.
As in other observational epidemiologic studies, our findings could be explained by uncontrolled confounding. However, the findings persisted after we controlled for potential confounders identified by a directed acyclic graph, and the women not diagnosed with COVID-19 and women diagnosed with COVID-19 were selected from the same hospital or country, gestation duration, and date, assuring similar clinical practices by location during the COVID-19 pandemic.
Strengths and limitations
We employed data from a large-scale multinational study that was specifically conducted to assess the symptoms and effects of COVID-19 during pregnancy on maternal and neonatal outcomes compared with pregnant women not diagnosed with COVID-19 and enrolled concomitantly in the same facility, in the same level of care, and at the same gestational age to minimize any selection bias. The study abstracted information on maternal and neonatal outcomes and used rigorous data collection procedures, employing structured forms and stringent quality control across 43 institutions to record morbidity.
The proportions of women diagnosed on the basis of COVID-19 symptoms alone and those diagnosed by RT-PCR or antigen tests changed during the study in each country as laboratory testing became more available. It remains possible that some of those not diagnosed with COVID-19 may have included women with SARS-CoV-2 infection who were asymptomatic and not identified, either because routine testing was not available or because they became infected after enrollment. This potential for misclassification would have led to more conservative estimates.
Although DM and high BMI preceded infection in all cases, GDM very likely preceded infection for roughly half of those who were diagnosed by RT-PCR or antigen tests around the time of delivery. It remains possible that women admitted to the hospital with severe complications of pregnancy, such as uncontrolled GDM or DM, were more likely to be tested for, or diagnosed with, COVID-19. However, the study design avoided such systematic bias by selecting 2 women immediately after a diagnosed woman at the same level of care as the reference group. In addition, when we restricted the analysis to those women who were PCR or antigen tested, the results were similar, suggesting that this bias was minimal.
In contrast to women of high BMI with COVID-19, women with GDM or DM were not more likely to have symptomatic COVID-19. Although we had information on ICU hospitalization and death, we could not attribute whether COVID-19 was the cause3; thus, we could not conduct analyses according to the COVID-19 severity classification proposed by the National Institutes of Health, because we did not have the complete information required by these relatively recent criteria.39
Other limitations of this observational study were that the medical record abstractions did not indicate the criteria for GDM diagnosis or the time during the pregnancy at which it was diagnosed. We did not interfere with the usual clinical care provided by the hospitals or clinicians provided across the 43 hospitals and clinical practices, including the protocols for prescribing insulin to women with GDM, which may have varied across these institutions.40 In addition, protocols for GDM screening may have changed during the pandemic to prevent infection.41, 42, 43, 44 However, our study design, in which women diagnosed with COVID-19 and women not diagnosed with COVID-19 were matched by hospital and time, should have controlled for these changes in screening protocols.
Research implications
The limitations and strengths of our study noted above can inform future studies. For example, prospective multinational cohort studies in which pregnant women are enrolled early in pregnancy, routinely and frequently tested for COVID-19 using RT-PCR, monitored carefully for onset of symptoms and severity of disease, and screened uniformly for GDM would be important to confirm our findings. In addition, future research should study the long-term sequelae of COVID-19 during pregnancy on both the woman and her child. Lastly, given the risk of COVID-19 to pregnant women, especially in those with comorbidities, public health programs should be implemented to overcome vaccine hesitancy and barriers to access.
Clinical implications
COVID-19 during pregnancy is known to increase severe maternal morbidity and death,2 particularly intubation and intensive care unit (ICU) admission.3 Our data provided additional information about the increased risk of infection associated with preexisting comorbidities, such as DM and high body mass, and the association with insulin-dependent GDM. Women with these conditions should be monitored carefully for COVID-19, glycemic control, and weight gain.38 Most importantly, unvaccinated pregnant women and those considering pregnancy with these risk factors should be strongly encouraged to be vaccinated.
Conclusions
Our findings suggested that DM and overweight or obesity are more prevalent in women diagnosed with COVID-19 in pregnancy than in women not diagnosed with COVID-19 in pregnancy, suggesting that these risk factors make infection more likely. Moreover, COVID-19 diagnosis was associated with GDM among women who were using insulin. This information can help guide decision-making for those women who still may be hesitant to receive COVID-19 vaccination.
Acknowledgments
We are very grateful to the contributing institutions and local researchers involved in the study. The Appendix contains their details and details of the study committees.
Footnotes
J.V. and A.T.P. contributed equally to this work.
B.E., R.B.G., and S.R. reported receiving grants from the Oxford University during the conduct of the study and the US National Institutes of Health. A.T.P. reported receiving grants from the National Institute for Health ResearchBiomedical Research Centre and support from Intelligent Ultrasound as director outside the submitted work. The other authors report no conflict of interest.
The study was supported by the COVID-19 Research Response Fund from the University of Oxford (reference number 0009083). The investigators acknowledge the philanthropic support of the donors to the University of Oxford’s COVID-19 Research Response Fund. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Cite this article as: Eskenazi B, Rauch S, Iurlaro E, et al. Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: the INTERCOVID study. Am J Obstet Gynecol 2022;227:74.e1-16.
Appendix
Contributors and members of the International Study on the Effects of COVID-19 in pregnancy on maternal and newborn outcomes in the International Fetal and Newborn Growth Consortium for the 21st Century global network (the INTERCOVID study)
x
Members of the International Study on the Effects of COVID-19 in pregnancy on maternal and newborn outcomes in the International Fetal and Newborn Growth Consortium for the 21st Century global network (the INTERCOVID study) and its Committees
Scientific Advisory Committee: Ana Langer (Chair), Cesar Victora, Jose Cordero, Brenda Eskenazi, Enrico Ferrazzi, Zulfiqar Bhutta, Julian Robinson, and Jim Thornton.
Coordinating Unit: Jose Villar, Aris Papageorghiou, Adele Winsey, Rachel Craik, Montserrat Izquierdo Renault, Stephen Kennedy, Ricardo Nieto, Albertina Rego, Josephine and Agyeman-Duah.
Data Management Group: Carmen Condon, Robert Gunier, Stephen Rauch, Rachel Craik, and Adele Winsey.
Participating institutions and investigators
Clinica Otamendi, Buenos Aires, Argentina: Carmen Vecciarell (principal investigator), Cristina Osio, Cecilia Baston, Marcela Volpe, Zenith Guzman, Hernan Jensen, Fernando Tami, and Federico Crispin.
Hospital Materno Infantil Ramón Sarda, Buenos Aires, Argentina: Ricardo Nieto and Constanza Soto Conti (co-principal investigators), Eugenia Luque, Jimena Melisa Vargas, Marta Isabel Lopez, and Karen Zelada.
Hospital Universitario Austral, Buenos Aires, Argentina: Maria Carola Capelli and Milagros Risso (co-principal investigators), and Josefina Maria Bran.
Hospital Magdalena V de Martinez, Buenos Aires, Argentina: Eduardo A Duro (principal investigator).
Hospital Nacional Profesor Alejandro Posadas, Buenos Aires, Argentina: Roberto Casale (principal investigator), Silvia Garcia, Alberto Ferreiros, Lucio Ribola, Grethelm Ferrufino, Karell Rojas, Dafne Sidiropulos, and Silvana Varela.
Hospital Municipal O. B de Lavignole, Buenos Aires, Argentina: Mónica Savorani (principal investigator), Silvana Aguirre, and Maria Rocio Tozzini.
Hospital Universitário da Universidade Federal do Maranhão, São Luís, Brazil: Marynéa Silva do Vale (principal investigator), Ana Claudia Garcia Marques, Patricia Franco Marques, and Rebeca Aranha Arrais Santos Almeida.
Tanta University Hospital, Tanta, Egypt: Sherief Abd-Elsalam (principal investigator), Mohamed Elbahnasawy, Mai Khalaf, Mohamed Samir Abd El Ghafar, and Eslam Saber Esmail.
Bordeaux University Hospital, Bordeaux, France: Loïc Sentilhes (principal investigator), Amaury Brot, Aurélien Mattuizzi, and Clémence Houssin.
AP-HP, Hôpital Universitaire Necker-Enfants Malades, Paris, France: Laurent J Salomon (principal investigator), Joanna Sichitiu, and Laurence Bussières.
Hôpitaux Universitaires de Strasbourg, Strasbourg, France: Philippe Deruelle (principal investigator), Fanny De Marcillac, Mary Pontvianne, Georges-Emmanuel Roth, Charlotte Jouffrieau, Sylvain Hufschmitt-Henry, Valentine Bergthold, Mathilde Airoldi, Olivier Behra, Julie Delplanque, abd Coraline Schutz.
Fr. Thomas Alan Rooney Memorial Hospital, Asankragua, Ghana: Vincent Bizor Nachinab (principal investigator), Bright Sandow, Priscilla Baffour Kyeremeh, Rita Akpene Kumi, Thomas Asechaab, and Grace Hanson
National Catholic Health Service, Hwidiem, Ghana: George Adjei, Anita Appiah, Roberta Ama Asiedu, and Ivan Essegbey.
Holy Family Hospital, Nkawkaw, Ghana: Eric Baafi (principal investigator) and Genevieve Insaidoo.
Employee's State Insurance Corporation Medical College and Hospital and affiliated Badshah Khan Civil Hospital, Faridabad, Haryana, India: Ramachandran Thiruvengadam and Shinjini Bhatnagar (co-principal investigators), Vandita Bhartia, Mudita Wahi, Anil K. Pandey, Jagadish Chandra Sharma, Rajesh Dhiman, and GARBH-Ini- DBT India Consortium for COVID Research Collaboration.
Medical Faculty Universitas Airlangga, Surabaya, Indonesia - Dr Soetomo General Academic Hospital: Ernawati Ernawati (principal investigator), Hendy Hendarto, Erry Gumilar, and Aditiawarman.
Spedali Civili and University of Brescia, Brescia, Italy: Federico Prefumo (principal investigator), Roberta Castellani, and Marta Papaccio.
Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy: Paola Roggero and Enrico Ferrazzi (co-principal investigators), Camilla Menis, Michela Perrone, and Enrico Iurlaro.
Ospedale Luigi Sacco, Milan, Italy: Valeria Savasi (principal investigator), Silvia Corti, and Francesca Rana.
IRCCS San Raffaele Hospital and University, Milan, Italy: Paolo Ivo Cavoretto (principal investigator), Massimo Candiani, and Giulia Bonavina.
Ospedale Vittore Buzzi, Milan, Italy: Irene Cetin (principal investigator), Alice Zavatta, and Stefania Livio.
University of Pavia and Fondazione IRCCS Policlinico San Matteo, Pavia, Italy: Hellas Cena and Rosa Maria Cerbo (co-principal investigators), Debora Porri, Rachele De Giuseppe, Arsenio Spinillo, Annachiara Licia Scatigno, Francesca Perotti, and Marco Zecca.
Ospedale Infantile Regina Margherita-Sant'Anna, Città della Salute e della Scienza di Torino, Turin, Italy: Francesca Giuliani and Manuela Oberto (co-principal investigators), Enrico Bertino, Pietro Gaglioti, Cristian Romolo Macchione, and Francesca Carpano Maglioli.
The Jikei University School of Medicine, Tokyo, Japan: Ken Takahashi (principal investigator), and Osamu Samura.
Keio University School of Medicine, Tokyo, Japan: Mamoru Tanaka and Satoru Ikenoue (co-principal investigators), Daigo Ochiai, Yoshifumi Kasuga, and Miho Iida.
General Hospital Borka Taleski, Prilep, North Macedonia: Gabriela Tavchioska (principal investigator).
General Hospital Kumanovo, Prilep, North Macedonia: Aleksandra Hristova (principal investigator).
Instituto Nacional de Perinatología Isidro Espinosa de los Reyes, Mexico City, Mexico: Jorge Arturo Cardona-Perez (principal investigator), Sandra Acevedo-Gallegos, Irma Alejandra Coronado-Zarco, Brenda Ivette Frías, Addy Cecilia Helguera-Repetto, Maria José Rodriguez Sibaja, Isabel Villegas-Mota, and Maria Yolotzin Valdespino.
Lic. Adolfo López Mateos ISSSTE, Mexico City, Mexico: Perla K García-May (principal investigator), Alberto Almanza Aguilar, Sanjuanita Gonzalez Garza, Lorena Gomez Aldape, Alejandra Loera Olvera, and Paulina Soriano Cabelo.
Abubakar Tafawa Balewa University Teaching Hospital, Bauchi, Nigeria: Muhammad Baffah Aminu (principal investigator), and Tiamiyu Ismail.
University of Calabar Teaching Hospital, Calabar, Nigeria: Saturday Etuk (principal investigator), Chinyere Akpanika, Komommo Okpebri, Etim Ekanem, and Ubong Akpan.
Federal Teaching Hospital Gombe, Gombe, Nigeria: Babagana Bako (principal investigator) and Yahaya Musa Suleiman.
University College Hospital, Ibadan, Nigeria: Adejumoke Idowu Ayede and Yetunde John-Akinola (co-principal investigators), Oladapo OlayemI, and Olufisayo Christopher Ologunore.
Mainland Hospital Yaba, Lagos, Nigeria: Abimbola Bowale (principal investigator) and Tope Ogunniyan.
Aminu Kano Teaching Hospital, Kano State, Nigeria: Hadiza Galadanci and Fatimah Hassan-Hanga (co-principal investigators), and Mahmoud Magashi.
Muhammad Abdullahi Wase Teaching Hospital, Kano State, Nigeria: Mustapha Ado Usman (principal investigator), Iman Haruna, Maryam Sulaiman, Rahila Garba, Badiyya Sayyidi, Hanifa Datti, Frank Akabudu, Abdurrahman Ali Bunawa, and Amira Aminu.
The Aga Khan University Hospital, Karachi, Pakistan: Shabina Ariff (principal investigator), Ghulam Zainab, Lumaan Shaikh, and Khalil Ahmed.
National Medical Research Center for Obstetrics, Gynecology & Perinatology, Moscow, Russia: Alexey Kholin (principal investigator), Irina Yakovleva, Aleksander Gus, Alexander Sencha, Roman Shmakov, and Gennady Sukhikh.
Hospital Universitari Vall d’Hebron, Barcelona, Spain: Nerea Maiz (principal investigator), Berta Serrano, Ester del Baco, Marta Miguez, Ana Perestelo, Lidia Barberán, Cristina Tusquets, Montserrat Capell, Clementina de Antonio, and Judit Gil.
Hospital Clínico Universitario Lozano Blesa Zaragoza, Zaragoza, Spain: Daniel Oros (principal investigator), Sara Ruiz-Martinez, Marta Fabre, and Cristina Paules.
Hôpitaux Universitaires de Genève, Geneva, Switzerland: Anne Caroline Benski (principal investigator), Martinez de Tejada Weber, Véronique Othenin-Girard, Monia Moreau, Dominique Deletraz, and David Baud.
St George's University Hospitals NHS Foundation Trust, London, United Kingdom: Aris T Papageorghiou (principal investigator), Becky Liu, Matthew Cauldwell, Yaa Acheampong, Danielle Hake, Sophie Robinson, and Rosemary Nyamboya.
University College Hospitals NHS Foundation Trust, London, United Kingdom: Raffaele Napolitano (principal investigator), Laura Salazar, Rohit Atre, Alex Fry, and Hilary Hewitt.
Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom: Jose Villar, Stephen Kennedy, Sonia Deantoni (co-principal investigators), Angelika Capp, Lotoyah Carty, Kate Dixon, Yvonne Nsiah, and Fenella Roseman.
Brigham and Women's Hospital, Boston, Massachusetts: Sarah Rae Easter (principal investigator).
Tufts Medical Center, Boston, Massachusetts: Mohak Mhatre (principal investigator), Jenny Koenig, and Arome Obende.
Mercy Hospital and Medical Center, Chicago, Illinois: Jagjit Singh Teji (principal investigator) and Michelle Firlit.
University of Washington, Seattle, Washington: Michael Gravett (principal investigator) and Lavone E Simmons.
Supplemental Table 1.
Real-time polymerase chain reaction, antigen tests, and antibody tests for SARS-CoV-2 used in the participating centers
| RT-PCR tests | Antibody tests |
|---|---|
|
|
IgG, immunoglobulin G; IgM, immunoglobulin M; RT-PCR, real-time polymerase chain reaction.
Eskenazi et al. COVID-19 and gestational diabetes, diabetes, and body mass index in pregnant women. Am J Obstet Gynecol 2022.
Supplemental Table 2.
Frequency of symptoms among pregnant women diagnosed with COVID-19
| Symptoms | Women “diagnosed” with COVID-19 (n=672) n (%) |
|---|---|
| Chest pain | 19 (2.8) |
| Diarrhea or vomiting | 48 (7.1) |
| Limb or joint pain | 54 (8.0) |
| Sore throat | 70 (10.4) |
| Flu-like symptoms | 76 (11.3) |
| Runny nose | 78 (11.6) |
| Breathlessness | 84 (12.5) |
| Headache | 92 (13.7) |
| Tiredness or lethargy | 110 (16.4) |
| Loss of smell | 116 (17.3) |
| Fever | 88 (28.0) |
| Cough | 232 (34.5) |
| 1 symptom | 66 (9.8) |
| 2 symptoms | 125 (18.6) |
| ≥3 symptoms | 209 (31.1) |
| Asymptomatic | 272 (40.5) |
Women could have ≥1 symptom.
Eskenazi et al. COVID-19 and gestational diabetes, diabetes, and body mass index in pregnant women. Am J Obstet Gynecol 2022.
Supplemental Table 3.
Associations between gestational diabetes mellitus, preexisting diabetes mellitus, and body mass index and COVID-19 diagnosis in the INTERCOVID study with a real-time polymerase chain reaction test or an antigen test in the week before or on day of delivery (n=937)
| Variable | n (%) | COVID-19 diagnosis, n (%) | No COVID-19 diagnosis, n (%) | Unadjusted RR (95% CI) | Adjusted RR (95% CI) |
|---|---|---|---|---|---|
| GDM or DM | |||||
| No GDM, no DM | 812 (86.7) | 282 (34.7) | 530 (65.3) | Ref | Ref |
| GDM, no DM | 97 (10.4) | 43 (44.3) | 54 (55.7) | 1.20 (0.94–1.54) | 1.27 (1.01–1.61)a |
| Preexisting DM | 28 (3.0) | 17 (60.7) | 11 (39.3) | 1.64 (1.19–2.25)a | 1.76 (1.29–2.39)a |
| BMI | |||||
| Normal weight (<25 kg/m2) | 562 (60.0) | 182 (32.4) | 380 (67.6) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 375 (40.0) | 160 (42.7) | 215 (57.3) | 1.27 (1.07–1.51)a | 1.23 (1.04–1.45)a |
Data were adjusted for maternal age, parity, and tobacco use during pregnancy.
CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; GDM, gestational diabetes mellitus, RR, risk ratio.
Eskenazi et al. COVID-19 and gestational diabetes, diabetes, and body mass index in pregnant women. Am J Obstet Gynecol 2022.
P<.05.
Supplemental Table 4.
Associations between gestational diabetes mellitus, preexisting diabetes mellitus, and body mass index and COVID-19 diagnosis in the INTERCOVID study with a real-time polymerase chain reaction or an antigen test at any time in the pregnancy (n=1290)
| Variable | N (%) | Crude RR (95% CI) | Adjusted RR (95% CI) |
|---|---|---|---|
| Model 1 | |||
| GDM or DM | |||
| No GDM, no DM | 1123 (87.1) | Ref | Ref |
| GDM, no DM | 123 (9.5) | 1.13 (0.95–1.35) | 1.18 (0.99–1.40)a |
| Preexisting DM | 44 (3.4) | 1.52 (1.27–1.83)b | 1.60 (1.33–1.91)b |
| BMI | |||
| Normal weight (<25 kg/m2) | 750 (58.1) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 540 (41.9) | 1.27 (1.13–1.43)b | 1.24 (1.11–1.40)b |
| Model 2 | |||
| No GDM or DM, normal weight | 696 (54.0) | Ref | Ref |
| No GDM or DM, overweight or obese | 427 (33.1) | 1.26 (1.11–1.43)b | 1.23 (1.09–1.40)b |
| GDM without insulin, normal weight | 34 (2.6) | 0.93 (0.60–1.44) | 0.96 (0.63–1.45) |
| GDM without insulin, overweight or obese | 58 (4.5) | 1.30 (1.01–1.68)b | 1.33 (1.04–1.72)b |
| GDM with insulin, normal weight | 9 (0.7) | 1.62 (1.01–2.60)b | 1.73 (1.13–2.64)b |
| GDM with insulin, overweight or obese | 22 (1.7) | 1.88 (1.47–2.40)b | 1.91 (1.52–2.41)b |
| Preexisting DM, normal weight | 11 (0.9) | 1.55 (0.98–2.44)a | 1.63 (1.05–2.53)b |
| Preexisting DM, overweight or obese | 33 (2.6) | 1.92 (1.57–2.34)b | 1.97 (1.62–2.38)b |
Data were adjusted for maternal age, parity, and tobacco use during pregnancy.
CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; GDM, gestational diabetes mellitus, RR, risk ratio.
Eskenazi et al. COVID-19 and gestational diabetes, diabetes, and body mass index in pregnant women. Am J Obstet Gynecol 2022.
P<.1
P<.05.
Supplemental Table 5.
Associations between gestational diabetes mellitus, preexisting diabetes mellitus, and body mass index and COVID-19 diagnosis in the INTERCOVID study (n=2071), with additional adjustment for month of enrollment
| Variable | n (%) | Adjusted RR (95% CI) |
|---|---|---|
| Model 1 | ||
| GDM or DM | ||
| No GDM, no DM | 1824 (88.1) | Ref |
| GDM, no DM | 194 (9.4) | 1.23 (1.01–1.49)a |
| Preexisting DM | 53 (2.6) | 1.93 (1.54–2.42)a |
| BMI | ||
| Normal weight (<25 kg/m2) | 1180 (57.0) | Ref |
| Overweight or obese (≥25 kg/m2) | 891 (43.0) | 1.21 (1.07–1.38)a |
| Model 2 | ||
| No GDM or DM, normal weight | 1099 (53.1) | Ref |
| No GDM or DM, overweight or obese | 725 (35.0) | 1.20 (1.05–1.38)a |
| GDM without insulin, normal weight | 54 (2.6) | 1.01 (0.65–1.55) |
| GDM without insulin, overweight or obese | 87 (4.2) | 1.37 (1.03–1.83)a |
| GDM with insulin, normal weight | 14 (0.7) | 1.81 (1.07–3.08)a |
| GDM with insulin, overweight/obese | 39 (1.9) | 1.81 (1.31–2.51)a |
| Preexisting DM, normal weight | 13 (0.6) | 1.94 (1.18–3.18)a |
| Preexisting DM, overweight or obese | 40 (1.9) | 2.33 (1.82–3.00)a |
Data were adjusted for maternal age, parity, tobacco use during pregnancy, and month of enrollment.
CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; GDM, gestational diabetes mellitus, RR, risk ratio.
Eskenazi et al. COVID-19 and gestational diabetes, diabetes, and body mass index in pregnant women. Am J Obstet Gynecol 2022.
P<.05.
Supplemental Table 6.
Associations between gestational diabetes mellitus, preexisting diabetes mellitus, and body mass index and COVID-19 diagnosis in the INTERCOVID study using multiple imputation of missing values (n=2184)
| Variable | n (%) | Crude RR (95% CI) | Adjusted RR (95% CI) |
|---|---|---|---|
| Model 1 | |||
| GDM or DM | |||
| No GDM, no DM | 1824 (88.1) | Ref | Ref |
| GDM, no DM | 194 (9.4) | 1.16 (0.96–1.40) | 1.18 (0.97–1.42)a |
| Preexisting DM | 53 (2.6) | 1.84 (1.47–2.30)b | 1.89 (1.52–2.36)b |
| BMI | |||
| Normal weight (<25 kg/m2) | 1180 (57.0) | Ref | Ref |
| Overweight or obese (≥25 kg/m2) | 891 (43.0) | 1.19 (1.05–1.35)b | 1.19 (1.05–1.34)b |
| Model 2 | |||
| No GDM or DM, normal weight | 1099 (53.1) | Ref | Ref |
| No GDM or DM, overweight or obese | 725 (35.0) | 1.18 (1.04–1.35)b | 1.18 (1.03–1.35)b |
| GDM without insulin, normal weight | 54 (2.6) | 0.94 (0.61–1.46) | 0.96 (0.62–1.48) |
| GDM without insulin, overweight or obese | 87 (4.2) | 1.27 (0.95–1.68) | 1.29 (0.97–1.71)a |
| GDM with insulin, normal weight | 14 (0.7) | 1.68 (0.99–2.87)a | 1.74 (1.03–2.92)b |
| GDM with insulin, overweight or obese | 39 (1.9) | 1.73 (1.26–2.38)b | 1.72 (1.25–2.37)b |
| Preexisting DM, normal weight | 13 (0.6) | 1.81 (1.09–3.02)b | 1.87 (1.14–3.08)b |
| Preexisting DM, overweight or obese | 40 (1.9) | 2.19 (1.71–2.80)b | 2.24 (1.76–2.87)b |
Data were adjusted for maternal age, parity, and tobacco use during pregnancy.
CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; GDM, gestational diabetes mellitus, RR, risk ratio.
Eskenazi et al. COVID-19 and gestational diabetes, diabetes, and body mass index in pregnant women. Am J Obstet Gynecol 2022.
P<.1
P<.05.
Supplemental Table 7.
Associations between gestational diabetes mellitus, preexisting diabetes mellitus, and body mass index and COVID-19 symptoms among all those diagnosed with COVID-19 (n=672) and among those diagnosed with a positive real-time polymerase chain reaction or antigen test in the week before or on day of delivery (n=342)
| Variable | COVID-19 without symptoms, n (%) | COVID-19 with symptoms, n (%) | RR (95% CI) |
|---|---|---|---|
| All women with COVID-19 | |||
| GDM or DM | |||
| No GDM, no DM | 226 (40.1) | 338 (59.9) | Ref. |
| GDM, no DM | 32 (42.7) | 43 (57.3) | 0.96 (0.88–1.03) |
| Preexisting DM | 14 (42.4) | 19 (57.6) | 0.97 (0.87–1.07) |
| BMI | |||
| Normal weight (<25 kg/m2) | 152 (44.2) | 192 (55.8) | Ref. |
| Overweight or obese (≥25 kg/m2) | 120 (36.6) | 208 (63.4) | 1.06 (1.01–1.11)a |
| Women with a positive RT-PCR or antigen test in the week before or on the day of delivery | |||
| GDM or DM | |||
| No GDM, no DM | 164 (58.2) | 118 (41.8) | Ref. |
| GDM, no DM | 25 (58.1) | 18 (41.9) | 0.97 (0.87–1.09) |
| Preexisting DM | 9 (52.9) | 8 (47.1) | 1.02 (0.87–1.20) |
| BMI | |||
| Normal weight (<25 kg/m2) | 114 (62.6) | 68 (37.4) | Ref. |
| Overweight or obese (≥25 kg/m2) | 84 (52.5) | 76 (47.5) | 1.08 (1.00–1.09)a |
Data were adjusted for maternal age, parity, and tobacco use during pregnancy.
CI, confidence interval; BMI, body mass index; DM, diabetes mellitus; GDM, gestational diabetes mellitus, RR, risk ratio; RT-PCR, real-time polymerase chain reaction.
Eskenazi et al. COVID-19 and gestational diabetes, diabetes, and body mass index in pregnant women. Am J Obstet Gynecol 2022.
P<.05.
References
- 1.Allotey J., Stallings E., Bonet M., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jamieson D.J., Rasmussen S.A. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.08.054. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villar J., Ariff S., Gunier R.B., et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zambrano L.D., Ellington S., Strid P., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lassi Z.S., Ana A., Das J.K., et al. A systematic review and meta-analysis of data on pregnant women with confirmed COVID-19: clinical presentation, and pregnancy and perinatal outcomes based on COVID-19 severity. J Glob Health. 2021;11:05018. doi: 10.7189/jogh.11.05018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rancourt R.C., Schellong K., Plagemann A. Coronavirus disease 2019 and obesity: one pandemic meets another. Am J Obstet Gynecol. 2021;224:121–122. doi: 10.1016/j.ajog.2020.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun S.Y., Kim D.W., Lee S.A., et al. Does diabetes increase the risk of contracting COVID-19? A population-based study in Korea. Diabetes Metab J. 2020;44:897–907. doi: 10.4093/dmj.2020.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGurnaghan S.J., Weir A., Bishop J., et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9:82–93. doi: 10.1016/S2213-8587(20)30405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lusignan S., Dorward J., Correa A., et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raeisi T., Mozaffari H., Sepehri N., et al. The negative impact of obesity on the occurrence and prognosis of the 2019 novel coronavirus (COVID-19) disease: a systematic review and meta-analysis. Eat Weight Disord. 2021 doi: 10.1007/s40519-021-01269-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yates T., Razieh C., Zaccardi F., Davies M.J., Khunti K. Obesity and risk of COVID-19: analysis of UK Biobank. Prim Care Diabetes. 2020;14:566–567. doi: 10.1016/j.pcd.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riley L.E. mRNA Covid-19 vaccines in pregnant women. N Engl J Med. 2021;384:2342–2343. doi: 10.1056/NEJMe2107070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimabukuro T.T., Kim S.Y., Myers T.R., et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–2282. doi: 10.1056/NEJMoa2104983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blakeway H., Prasad S., Kalafat E., et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.08.007. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skjefte M., Ngirbabul M., Akeju O., et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36:197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention COVID data tracker. 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women Available at:
- 17.Riley L.E., Beigi R., Jamieson D.J., et al. COVID-19 vaccination considerations for obstetric–gynecologic care. The American College of Obstetricians and Gynecologists. 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care Available at:
- 18.Society for Maternal Fetal Medicine SMFM: provider considerations for engaging in COVID-19 vaccine counseling with pregnant and lactating patients. 2021. https://s3.amazonaws.com/cdn.smfm.org/media/3134/Provider_Considerations_for_Engaging_in_COVID_Vaccination_Considerations_10-1-21_%28final%29.pdf Available at:
- 19.Centers for Disease Control and Prevention COVID-19 vaccines while pregnant or breastfeeding. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html Available at:
- 20.Centers for Disease Control and Prevention COVID-19 vaccine monitoring systems for pregnant people. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/monitoring-pregnant-people.html Available at:
- 21.Global Health Network. Essential Study Documents • INTERGROWTH-21st. Available at: https://intergrowth21.tghn.org/intercovid/intercovid-study-documents/. Published May 14, 2020. Accessed April 18, 2021.
- 22.World Health Organization World Health Organization: Regional Office for Europe. Body mass index - BMI. 2021. https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi Available at: Accessed April 18, 2021.
- 23.Ohuma E.O., Hoch L., Cosgrove C., et al. Managing data for the international, multicentre INTERGROWTH-21st Project. BJOG. 2013;120(Suppl2):64–70. doi: 10.1111/1471-0528.12080. [DOI] [PubMed] [Google Scholar]
- 24.Papageorghiou A.T., Deruelle P., Gunier R.B., et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225:289.e1–289.e17. doi: 10.1016/j.ajog.2021.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.StataCorp . StataCorp LLC; College Station, TX: 2017. Stata statistical software: release 15. [Google Scholar]
- 26.Kornum J.B., Thomsen R.W., Riis A., Lervang H.H., Schønheyder H.C., Sørensen H.T. Type 2 diabetes and pneumonia outcomes: a population-based cohort study. Diabetes Care. 2007;30:2251–2257. doi: 10.2337/dc06-2417. [DOI] [PubMed] [Google Scholar]
- 27.Nitzan O., Elias M., Chazan B., Saliba W. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes. 2015;8:129–136. doi: 10.2147/DMSO.S51792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirji I., Andersson S.W., Guo Z., Hammar N., Gomez-Caminero A. Incidence of genital infection among patients with type 2 diabetes in the UK General Practice Research Database. J Diabetes Complications. 2012;26:501–505. doi: 10.1016/j.jdiacomp.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Stamler E.F., Cruz M.L., Mimouni F., et al. High infectious morbidity in pregnant women with insulin-dependent diabetes: an understated complication. Am J Obstet Gynecol. 1990;163:1217–1221. doi: 10.1016/0002-9378(90)90694-3. [DOI] [PubMed] [Google Scholar]
- 30.Accili D. Can COVID-19 cause diabetes? Nat Metab. 2021;3:123–125. doi: 10.1038/s42255-020-00339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J.K., Lin S.S., Ji X.J., Guo L.M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao P., Praissman J.L., Grant O.C., et al. Virus-receptor interactions of glycosylated SARS-CoV-2 spike and human ACE2 receptor. Cell Host Microbe. 2020;28:586–601.e6. doi: 10.1016/j.chom.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh V.P., Bali A., Singh N., Jaggi A.S. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18:1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill M.A., Mantzoros C., Sowers J.R. Commentary: COVID-19 in patients with diabetes. Metabolism. 2020;107:154217. doi: 10.1016/j.metabol.2020.154217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Philips B.J., Meguer J.X., Redman J., Baker E.H. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 2003;29:2204–2210. doi: 10.1007/s00134-003-1961-2. [DOI] [PubMed] [Google Scholar]
- 36.Eberle C., James-Todd T., Stichling S. SARS-CoV-2 in diabetic pregnancies: a systematic scoping review. BMC Pregnancy Childbirth. 2021;21:573. doi: 10.1186/s12884-021-03975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berbudi A., Rahmadika N., Tjahjadi A.I., Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. 2020;16:442–449. doi: 10.2174/1573399815666191024085838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Institute of Medicine (US), National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines . In: Weight gain during pregnancy: reexamining the guidelines. Rasmussen K.M., Yaktine A.L., editors. National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 39.National Institutes of Health Clinical spectrum of SARS-CoV-2 infection. National Institutes of Health. 2021. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/ Available at:
- 40.Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine. Hameed A.B., Combs C.A. Society for Maternal-Fetal Medicine Special Statement: updated checklist for antepartum care of pregestational diabetes mellitus. Am J Obstet Gynecol. 2020;223:B2–B5. doi: 10.1016/j.ajog.2020.08.063. [DOI] [PubMed] [Google Scholar]
- 41.Australasian Diabetes in Pregnancy Society, Australian Diabetes Society, Australian Diabetes Educators Association, Diabetes Australia. Diagnostic testing for gestational diabetes mellitus (GDM) during the COVID 19 pandemic: antenatal and postnatal testing advice. 2020. https://www.adips.org/documents/COVID-19GDMDiagnosis030420ADIPSADSADEADAforWebsite.pdf Available at:
- 42.Royal College of Obstetricians and Gynaecologists Guidance for maternal medicine services in the coronavirus (COVID-19) pandemic. 2020. https://www.rcog.org.uk/globalassets/documents/guidelines/2020-12-09-guidance-for-maternal-medicine-services-in-the-coronavirus-covid-19-pandemic.pdf Available at:
- 43.Royal College of Obstetricians and Gynaecologists Guidance for maternal medicine services in the evolving coronavirus (COVID-19) pandemic. 2021. https://www.rcog.org.uk/globalassets/documents/guidelines/2020-07-10-guidance-for-maternal-medicine.vpdf Available at:
- 44.Yamamoto J., Donovan L., Feig D., Berger H. Urgent update – temporary alternative screening strategy for gestational diabetes screening during the COVID-19 pandemic. 2020. https://els-jbs-prod-cdn.jbs.elsevierhealth.com/pb/assets/raw/Health%20Advance/journals/jcjd/JCJD_COVID_guidelines_020420.pdf Available at: [DOI] [PMC free article] [PubMed]