Abstract
This study identified ecological and human health risks exposure of COVID-19 pharmaceuticals and their metabolites in environmental waters. Environmental concentrations in aquatic species were predicted using surface water concentrations of pharmaceutical compounds. Predicted No-Effect Concentrations (PNEC) in aquatic organisms (green algae, daphnia, and fish) was estimated using EC50/LC50 values of pharmaceutical compounds taken from USEPA ECOSAR database. PNEC for human health risks was calculated using the acceptable daily intake values of drugs. Ecological PNEC revealed comparatively high values in algae (Chronic toxicity PNEC values, high to low: ribavirin (2.65 × 105 μg/L) to ritonavir (2.3 × 10−1 μg/L)) than daphnia and fish. Risk quotient (RQ) analysis revealed that algae (Avg. = 2.81 × 104) appeared to be the most sensitive species to pharmaceutical drugs followed by daphnia (Avg.: 1.28 × 104) and fish (Avg.: 1.028 × 103). Amongst the COVID-19 metabolites, lopinavir metabolites posed major risk to aquatic species. Ritonavir (RQ = 6.55) is the major drug responsible for human health risk through consumption of food (in the form fish) grown in pharmaceutically contaminated waters. Mixture toxicity analysis of drugs revealed that algae are the most vulnerable species amongst the three trophic levels. Maximum allowable concentration level for mixture of pharmaceuticals was found to be 0.53 mg/L.
Abbreviations: BCF, bio-concentration factor; BAF, bio-accumulation factor; ECOSAR, Ecological Structure Activity Relationship; EMEA, European Medicines Evaluation Agency; NOEL, no observable effect level; NOAEL, no observed adverse effect level; OECD, The Organization for Economic Co-operation and Development; PEC, predicted environmental concentrations; PNEC, predicted no-effect concentrations; RQ, risk quotient; QSAR, Quantitative Structure-Property Relationships; TU, toxicity units; US EPA, United States Environmental Protection Agency; WHO, World Health Organization
Keywords: COVID-19 pandemic, Aquatic organisms, Ecological risk, Human health effects
Graphical abstract
1. Introduction
Pharmaceutical compounds are being continuously discharged into the aquatic environment where they can cause toxic effects on underlying organisms, even if present in low concentrations (ng/L) level (Pereira et al., 2015; Simazaki et al., 2015). Their presence raises serious concerns for the quality of water resources (Taylor and Senac, 2014; He et al., 2018). Possible undesirable effects, even at lower concentrations, to aquatic organisms has been a reason to be concerned since they were first reported in 1985 (Silva et al., 2012). Illustrating pollution characteristics of antibiotics and ecological hazard of surface waters is important for ecological safety and human health (Wang et al., 2017).
Continuous use of drugs has increased the existing pollution load of water and can show adverse effects on the underlying aquatic ecosystem (Bopp et al., 2018). Chemical contamination of the environment is definitely not restricted to short-term or acute exposures but long-term and low-level chronic exposures can be equally detrimental (Saaristo et al., 2018). Any compound either man-made or natural, is a possible source of concern in the field of ecological toxicology (USEPA, 1998). Ecological hazards posed by contaminants such as pharmaceuticals need to be addressed to understand possible adverse effects to aquatic organisms, if any. Subsequent consumption of contaminated food (taken in the form of fish) grown in aquatic environment can also show adverse effects on human health. Hence, the health hazard of human exposure by ingestion of contaminated foods should also be taken into account (Zenker et al., 2014).
Eco-toxicological evaluation investigates and assess the potential harmful effects caused by chemical contaminants on organisms, inhabitants, societies and environments (Li et al., 2019). When no experimental data are available, ecological risk assessment (ERA) is generally estimated using recommended EC50 or LC50 values from the U.S. Environmental Protection Agency (EPA) Ecological Structure Activity Relationships Class Program (ECOSAR database). This program estimates data through the molecule structure, sometimes underestimating toxic effects (Raimondo et al., 2010). The data derived on the toxicity of the compounds are used to define the predicted-no-effect-concentration (PNEC) with the application of an assessment factor (AF) for the lowest effective concentration. The calculation of a risk quotient (RQ) on the basis of the PEC/PNEC ratio can then be used to characterize the risk in relation to aquatic ecosystems (Zalęska-Radziwill et al., 2017). The measure of the predicted environmental concentration (PEC) and the PNEC has in fact become a standard for the ERA and characterization of chemical substances such as biocides and pharmaceuticals (Backhaus and Faust, 2012).
Besides individual risk of pharmaceuticals, mixture of contaminants might also pose unacceptable risk to aquatic habitats (Zhang et al., 2018) and human health. Study reported that the actual exposure scenario with respect to multiple pharmaceutical drugs would be different from that of individual drugs (Kumari and Kumar, 2020a). If several pharmaceuticals having similar mode of action are present in environmental waters, then the toxicity of this mixture could be higher than any one pharmaceutical present. This could result in risk underestimation, as the typical exposure is toward multicomponent chemicals (Oliver et al., 2015; Backhaus and Faust, 2012; Jones-Lepp et al., 2012). Therefore, it is important to determine mixture toxicity of contaminants in ecological risk analysis. Mixture toxicity risk assessment of compounds is also important due to high discharge and shock loading.
To address the above mentioned aspects, this study considered five frequently used pharmaceutical drugs (lopinavir, ritonavir, chloroquine, ribavirin, and rapamycin) in treating COVID-19 infection, Since, the past two years, the world is facing COVID-19 pandemic so it would be interesting to identify whether the drugs show any adverse effects on ecological and human health. Huge amount of research has been conducted on SARS-COV-2, the causative agent of COVID-19 (Fung and Liu, 2019; Liu et al., 2020; Singh et al., 2020), but none of the studies reported has addressed the ecological/human risk aspects. Amongst the drugs, lopinavir-ritonavir was found to be effective in treating COVID-19 based on preclinical and empirical studies (Horby et al., 2020). Ribavirin is an antiviral agent, which is used in concoction with lopinavir-ritonavir and minimizes the risk of horrible clinical side-effects besides diminishing viral load in patients infected with SARS (Rabi et al., 2020). Chloroquine is an antimalarial drug, and reportedly served as an effective tool against SARS CoV-1 and CoV-2 variants (Colson et al., 2020; Yao et al., 2020). Rapamycin immuno-therapeutic capacity was found to be successful against COVID-19 infection (Omarjee et al., 2020). Detailed information is provided in supplementary table, T1.
The present study estimates the overall ecological risk of mixture of compounds including ecological and human health risk hazards due to the exposure of individual pharmaceuticals in environmental waters. ERA is determined for three trophic levels i.e. green algae, daphnia, and fishes while human health risk is estimated for the consumption of contaminated food (in the form of fishes) grown in pharmaceutically contaminated waters. The results presented in this study can help in understanding the overall implications of presence of COVID-19 drugs in environmental waters and the risk associated.
2. Methodology
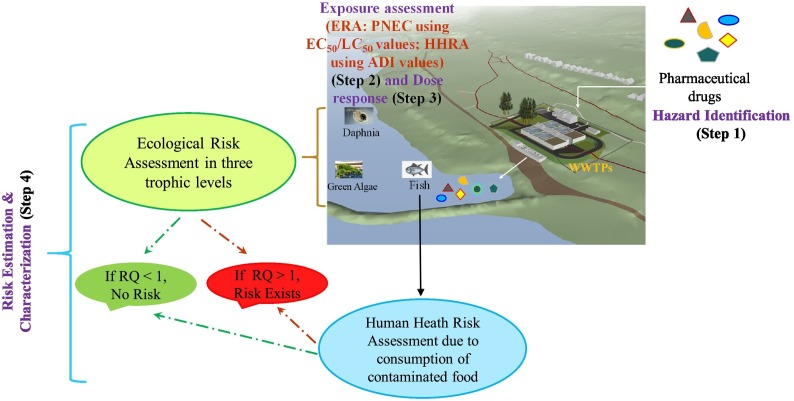
This study uses a six-step approach comprising of hazard identification, dose-response assessment, exposure assessment, risk estimation, risk characterization and management to determine risk estimates (Sohaili et al., 2017; Kumari and Kumar, 2021). The methodology adopted to determine risk estimates is widely used worldwide and has previously been used to predict the concentration levels of chemical compounds like pharmaceutical drugs (Schwab et al., 2005; Kumar and Xagoraraki, 2010), antibiotics (Kumari and Kumar, 2020a) and nanomaterials (Parsai and Kumar, 2020) in drinking water, ground water, lakes and streams. Briefly, the study assumed that the overuse of repurposed drugs in treating COVID-19 infection has increased the existing pollution load of water which might enter into underlying aquatic organisms through food chain showing possible risks and concerns. Fishes are the major source of food taken by human beings. There is a possibility of drugs accumulating within the fish muscles and tissues which on consumption by humans might show possible adverse effects to human health and subsequent risks. This study estimated human health risks for children since they have been recognized as the most sensitive sub-population compared to adults (Kumar and Xagoraraki, 2010). Fig. 1 shows the methodology adopted for determining ecological and human health risks of COVID-19 pharmaceutical compounds.
Fig. 1.
Diagrammatic representation of methodology used to determine ecological and human health exposure of pharmaceutical drugs. PNEC; predicted o-effect concentration of drugs, ERA; ecological risk assessment, HHRA; is the human health risk assessment, ADI; acceptable daily intake, and RQ; risk quotient.
2.1. Hazard identification
The study selected five commonly used pharmaceuticals (lopinavir, ritonavir, chloroquine, ribavirin, and rapamycin) administered to COVID-19 patients. The compounds were selected based on their use and efficacy in treating SARS-CoV-2 infection as reported by clinical trial studies (WHO, 2020). Following human consumption, pharmaceuticals are excreted from human body and released into wastewater as unaltered drugs or metabolites, which are only partially removed in conventional wastewater treatment plants (WWTPs) (Nannou et al., 2020). These residues present in receiving environmental waters can pose ecotoxicological concerns (Al Aukidy et al., 2012; Godoy and Kummrow, 2017; Santos et al., 2010). In particular, during pandemic events, high amounts of antiviral drugs and their metabolites were released into environmental waters which are likely to pose high risks to aquatic ecosystem (Jain et al., 2013; Nannou et al., 2020). Thus, it is important to determine the risk effects of COVID-19 pharmaceuticals and their metabolites.
2.1.1. Environmental concentration of pharmaceuticals in surface water, PECSW
Environmental concentration of pharmaceuticals in surface water, PECSW was predicted in accordance with the Technical Guidance Document on Risk Assessment part II, TGD (EC, 2003) using Eq. (1). Drug dose data of pharmaceutical (Drug dose population) was extracted from drug sites (www.drugs.com and www.drugbank.ca). Table 1 list the parameters used in determining PECSW values.
| (1) |
where, Drug dosepopulation (mg/person/day); Consumption rate of pharmaceutical drug administered per person per day in USA, Fpen; Market penetration and represents the fraction of the total population that consumes the pharmaceutical on any given day, FExec; Fraction of parent drug excreted through urine and feces, 1-FWWTP; Fraction of pharmaceutical's emission from WWTPs to surface waters, WWpopulation (L/person/day); Amount of wastewater per person per day, N; number of person infected.
Table 1.
Values of parameters used for estimating PECSW.
| Parameters | Value | References |
|---|---|---|
| Drug dosepopulation (mg/person/day) | Taken from literature | www.drugbank.ca and www.drugs.com |
| FPen | 0.47 | Elflein, 2020 |
| FExec (Both urine and feces considered) | For ritonavir, chloroquine and ribavirin, the values were taken from Drug bank database. For lopinavir and rapamycin, a default value of 0.5 was applied |
www.drugbank.ca |
| FWWTP | 0.50 | Gómez-Canela et al., 2019 |
| WWpopulation (L/person/day) | 200 | EC, 2003 |
| DF (mg/L) | 10 | Gómez-Canela et al., 2019 |
2.1.2. Predicted environmental concentration of metabolites (PECW, M)
Predicted environmental concentration of metabolites (PECW, M) is estimated using Eq. (2). Excretion rate of metabolites (FExec, M) and removal of metabolites by wastewater plant (FWWTP, M) is taken from Kuroda et al. (2021), rest of the parameters are similar to that described in Eq. (1).
| (2) |
2.1.3. Predicted environmental concentration of pharmaceuticals in fish, PECFish
Concentration level of pharmaceuticals in fish, PECFish was predicted using Eq. (3).
| (3) |
where, PECfish; Predicted environmental concentration (mg/Kg), BCF; Bio-concentration factor value is calculated using the equation given by Al-Khazrajy and Boxall (2016). BMF: Bio-magnification factor (dimensionless), value was taken from TGD (EC, 2003).
2.2. Dose-response and exposure assessment
2.2.1. Ecological risks
2.2.1.1. Pharmaceutical compounds
To estimate ecological risks of pharmaceuticals in three different trophic levels i.e. algae, crustaceans (daphnia) and fish, firstly the PNEC values were calculated using EC50/LC50 values and assessment factor (AF). However, when no experimental toxicological studies are reported in published literature, recommended effect concentration 50 (EC50) and lethal concentration 50 (LC50) EC50/LC50 values from U.S. EPA ECOSAR database have been used to determine PNEC (ECHA, 2014; Sanderson et al., 2004). ECOSAR is a pragmatic approach to Quantitative structure-activity relationships (QSAR) and have been used by the U.S. EPA and REACH guidelines to predict the aquatic toxicity of chemicals in the absence of test data. Ecotoxicity data of pharmaceuticals for algae, crustaceans and fish were obtained from the ECOTOX database of U.S. EPA using SMILES notations (USEPA, 2009). The value of PNEC is often extrapolated from acute/chronic toxicity data divided by an assessment factor, AF (Chen et al., 2018). AF were selected according to Technical Guidance Document from the European Commission (EC, 2003), OECD and REACH (http://www.chemsafetypro.com/Topics/CRA/ecotox_aquatic_toxicity.html) (ECHA, 2014; ECHA, 2008; EC, 1996; Sanderson et al., 2003; Brock et al., 2006). Ecotoxicological data are presented in Table 2 , besides being accessible on the U.S. EPA ECOTOX database (2020) (https://comptox.epa.gov/dashboard/) (USEPA, 2009).
Table 2.
Ecotoxicological data of pharmaceutical drugs in three trophic levels.
| Drugs | EC50/LC50, green algae (mg/L) | EC50, daphnia (mg/L) | LC50, fish (mg/L) |
|---|---|---|---|
| Lopinavir | 2.7 × 10−3 | 7.5 × 10−3 | 9.90 × 10−2 |
| Ritonavir | 2.3 × 10−3 | 4.0 × 10−3 | 6.20 × 10−2 |
| Chloroquine | 1.04 × 10−1 | 2.24 × 10−1 | 1.41 |
| Ribavirin | 2.65 × 103 | 1.70 × 105 | 9.48 × 104 |
| Rapamycin | 1.18 | 4.31 | 2.8 |
PNEC of pharmaceutical drugs in three trophic levels were calculated for both acute and chronic toxicity conditions using Eq. (4). Research investigations indicates that several pharmaceutical compounds show bioaccumulation and bio magnification potential, thus, chronic effects on ecosystems cannot be ignored for animals at the higher end of the food web (Crouse et al., 2012).
| (4) |
where, AF; Assessment factor (1000 for acute toxicity, and 10 for chronic toxicity).
2.2.1.2. PNEC, pharmaceutical metabolites
PNEC of pharmaceutical metabolites (lopinavir, ritonavir, chloroquine, and ribavirin) is taken from Kuroda et al. (2021) study. Rapamycin metabolites has not been reported in literature (to the authors' best knowledge) and thus were not estimated.
2.2.2. Human health risks
Human health risk due to exposure of pharmaceuticals through the consumption of food (in the form of fishes) cultivated in pharmaceutically contaminated water was estimated in Eq. (5). The acceptable daily intake (ADI) value of pharmaceuticals is taken from our previously published work (Kumari and Kumar, 2021).
| (5) |
where, ADI; Acceptable daily intake (mg/kg-day), estimated using NOAEL values in rats; BW is the body weight of children (Kg), AT; Average lifetime (days), BCF; Bio-concentration factor in fishes (Al-Khazrajy and Boxall, 2016), EF; Exposure frequency (days/year), ED; Exposure duration (year), and CRFish; Rate of fish consumption in children (mg/day), the value is taken from recommended US FDA guidelines.
2.3. Risk estimation and characterization
2.3.1. Environmental risk assessment
2.3.1.1. Pharmaceutical compounds
Environmental Risk Assessment (ERA) of pharmaceuticals in aquatic environment was performed in accordance with the Technical Guidance Document on risk assessment from European Commission (EC, 2003) using the Risk Quotient (RQ). RQ is estimated as a ratio of PEC to PNEC (Eq. (6)).
| (6) |
where, i indicates the trophic level analyzed. If the PEC/PNEC ratio is greater than 1, the compound is of concern and further risk characterization and risk management steps are required to reduce the risk anticipated (Ma et al., 2015; EU Commission, 2003).
2.3.1.2. Pharmaceutical metabolites
Risk of pharmaceutical metabolites was conducted using an approach similar to that used for determining risk of pharmaceutical compounds as mentioned in Section 2.3.1.1.
2.3.2. Risk calculation for co-occurrence of pharmaceutical drugs
Research investigations revealed that pharmaceuticals often occur in water as multi-component mixtures, and co-occurrence data has also been reported (Kumari and Kumar, 2020a; Mahmood et al., 2019). Assessing risk exposure effects of co-occurring pharmaceuticals in different aquatic compartments requires more effort than individual ones (Paíga et al., 2016; Kumari and Kumar, 2020b). This study used two concentration addition (CA)-based risk assessment models for determining comprehensive risk hazards of COVID-19 pharmaceutical compounds (i) summation of Toxic Units (MHQSTU, Eq. (7)) and (ii) summation of risk quotients (MRQPEC/PNEC) of the individual mixture components.
| (7) |
If risk is identified (i.e., RQmix > 1), the MRA is refined in the second calculation step, based on the sum of toxic units (MHQSTU) (Eq. (8)).
| (8) |
where, TU; the toxic unit (PEC/EC50 or LC50), STU; the sum of toxic unit. The values of PEC, EC50/LC50, and AF are the same as in individual HQ approaches.
2.4. Maximum allowable concentrations (Callowable)
2.4.1. Callowable for co-occurrence of pharmaceuticals in water
Maximum allowable concentration (C allowable) signifies the levels beyond which no adverse effects are expected to occur. Several model approaches have been developed and are available in literature for determining risks of co-occurring pollutants, of which CA model is reported to be the most effective one (Kumari and Kumar, 2020b). CA model ignores the possible antagonistic and synergistic interactions of chemical compounds and can serve as an essential tool for assessing mixture risk and identifying dominant pollutants and threatened taxonomic groups (Backhaus and Faust, 2012; Molnar et al., 2020).
2.5. Sensitivity analysis
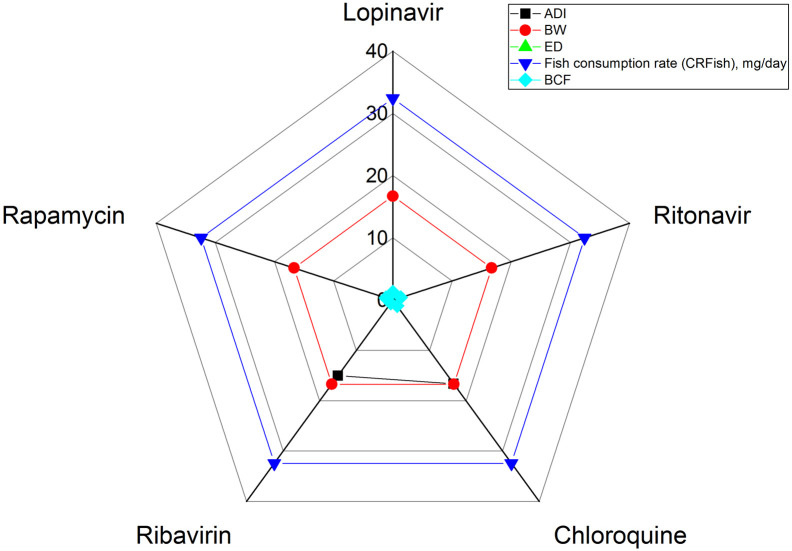
Sensitivity analysis helps in identifying the main input parameter governing risk estimates so that better management options can be implemented to reduce risks, if any. Various input parameters are applied to calculate risk exposure effects of contaminants on human health (Kumari and Gupta, 2018). It is quite possible that the influence of one variable on the risk estimation process would be different than others. Therefore, it is important to determine the effect of input variables on overall risk estimation process. This study used the sensitivity analysis tool to identify the major parameter responsible for risk to human health. The study was carried out using Origin, 2021 software and the results are depicted by means of radar plots.
3. Results and discussion
3.1. Predicted environmental concentrations, PEC
3.1.1. Predicted environmental occurrence of pharmaceuticals in environmental waters, PECSW
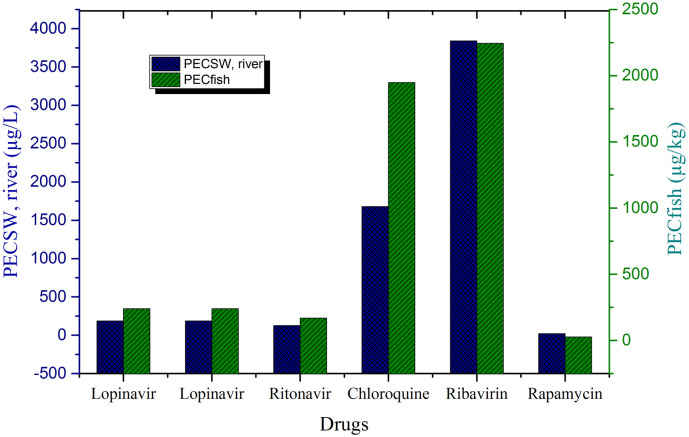
PEC of pharmaceutical in environmental waters (PECSW) ranged from 3840 μg/L (ribavirin) to 22 μg/L (rapamycin) (Fig. 2 ). The observed high levels of substances in environmental waters indicates the excessive use of compounds in treating COVID-19 infected patients leading to their increased concentration. Hence, there is a very strong probability that they can be identified in exceedingly high concentration under real conditions. Study shows that ribavirin is one of the most frequently administered HIV drug to human (Pradat et al., 2014), and there is a chance that the untreated wastewater effluent is discharged into the nearby rivers or lakes resulting in high levels as observed in this study. Moreover, conventional wastewater treatment plants are not designed for the removal of these (Markert et al., 2020) compounds leading to their incessant discharge in surface water bodies. Lopinavir has been detected in wastewater at an average concentration of 0.239 μg/L (Wood et al., 2015). Ritonavir has been detected in wastewater in South Africa (3.20 μg/L) (Abafe et al., 2018) and surface water in France (Aminot et al., 2015). Concentration of ribavirin were below the quantifiable limit in wastewater in Germany (Prasse et al., 2010) and China (Peng et al., 2014). The estimated PEC of pharmaceuticals (lopinavir, ritonavir, and ribavirin) in environmental waters were several times higher than aforementioned pharmaceuticals in wastewater however, their occurrence in environmental waters have not been reported (as per authors best knowledge).
Fig. 2.
PEC values of pharmaceutical drugs.
3.1.2. Predicted environmental occurrence of pharmaceuticals in fish, PECFish
PECFish were predicted using the surface water concentration of pharmaceuticals and BCF values. BCF values of all the substances except ribavirin was observed to be greater than 1.0 L/kg, indicating their possible bio-accumulation within fish tissues/organs. BCF indicates the bio-accumulative potential of a chemical compound (Landis et al., 2011). PECFish values ranged from 2240 μg/kg (for ribavirin) to 20 μg/kg (for rapamycin). The average values of compounds in environmental waters (1171.2 μg/L) is more than that observed in fish (723.8 μg/kg). Contrary to the results presented in this study, Fent et al. (2006) reported high concentration of drugs in fishes than in water. Pharmaceuticals have a fairly similar mode of action in target organisms, and provided that fish and invertebrates share more drug targets with humans, it would be anticipated that they would also react to drugs in a similar fashion (Pereira et al., 2020). The study observed that the calculated estimated PEC of pharmaceuticals exceeded more than 0.01 mg/L, hence further risk assessment studies were conducted as per the recommended guidelines (OECD, 2013; Zalęska-Radziwill et al., 2017).
3.1.3. Predicted environmental occurrence of metabolites (PEC,M) in environmental waters
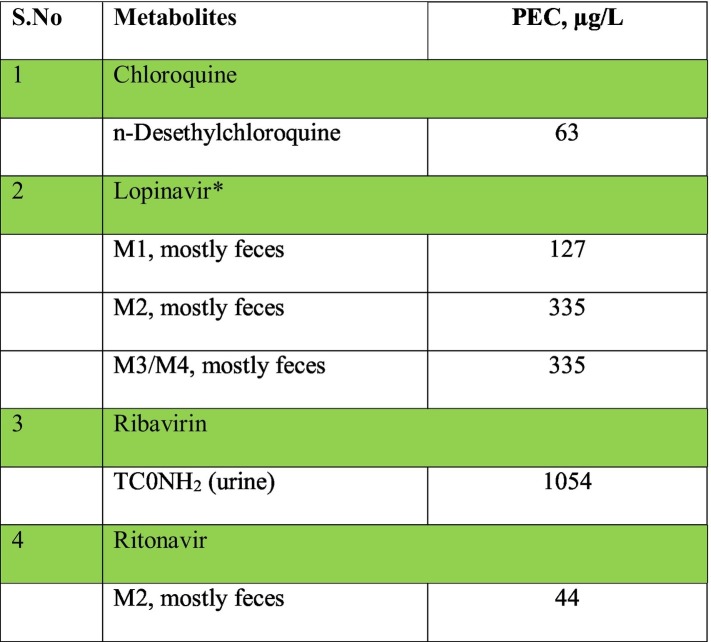
Environmental concentration of four pharmaceutical metabolites (PEC,M) i.e. lopinavir, ritonavir, chloroquine and ribavirin was predicted however, for rapamycin metabolites, the value was not predicted due to lack of data in published literature. High concentration was predicted for TC0NH2 (1054 μg/L), the major metabolite of ribavirin, followed by M1/M2 combined (462 μg/L) as shown in Table 3 . On the contrary, low values were predicted for n-desethylchloroquine (63 μg/L), chloroquine metabolite and M2 (44 μg/L), a ritonavir metabolite because of low dose and high removal rates. High values of metabolites has been reported in a previous study (Kuroda et al., 2021), in which the values were 3 times higher than those observed in this study The values of all the pharmaceuticals in environmental waters are lowered by a factor of 10, the assumed dilution factor.
Table 3.
Predicted environmental concentration of pharmaceutical metabolites. Coloured cells indicates COVID-19 drugs.
*The fraction of each of the four metabolites of lopinavir (M1 to M4) was not available, thus the sum of the four metabolites is shown.
3.2. PNEC estimation
3.2.1. Ecological PNEC
PNEC of pharmaceuticals were calculated in three trophic levels for both (a) acute toxicity, and (ii) chronic toxicity conditions (Table 4 ). For algae, ribavirin showed the highest PNEC, whereas lopinavir the lowest. In case of daphnia, similar to algae, ribavirin has the maximum observed PNEC with ritonavir showing the minimum values. PNEC in fish showed a trend similar to that observed for green algae and daphnia. Amongst the three trophic levels, fish showed the highest PNEC followed by daphnia and green algae. Previous studies have also reported high PNEC in fish for antibiotics like azithromycin, ciprofloxacin and erythromycin (Isidori et al., 2005; Li et al., 2019). Based on the results obtained, algae emerged as the most sensitive trophic level species in aquatic environment and are in line with those reported in literature (De Lange et al., 2006; Johnson et al., 2007). Sensitivity of algae to chemical compounds (Tang et al., 2015; Deng et al., 2016), antibiotics and herbicide (Machado and Soares, 2019) has been reported. Amongst the pharmaceuticals, ribavirin has the highest observed PNEC in all the three trophic levels whereas ritonavir the lowest.
Table 4.
PNEC values in three different trophic levels (highest values are shown in bold and are in italics).
| Drugs | PNEC, μg/L (acute toxicity) |
PNEC, μg/L (chronic toxicity) |
||||
|---|---|---|---|---|---|---|
| Green algae | Daphnia | Fishes | Green algae | Daphnia | Fishes | |
| Lopinavir | 2.70 × 10−3 | 7.50 × 10−3 | 9.90 × 10−5 | 2.70 × 10−1 | 7.50 × 10−1 | 9.90 |
| Ritonavir | 2.30 × 10−3 | 4.0 × 10−3 | 6.20 × 10−5 | 2.30 × 10−1 | 4.0 × 10−1 | 6.20 |
| Chloroquine | 1.04 × 10−1 | 2.24 × 10−1 | 1.41 × 10−3 | 1.04 × 10−1 | 22.4 | 141 |
| Ribavirin | 2.65 × 103 | 1.70 × 105 | 94.8 × 103 | 2.65 × 105 | 1.70 × 107 | 94.8 × 105 |
| Rapamycin | 1.18 | 4.31 | 2.8 | 118 | 431 | 280 |
Ecological PNEC under acute toxicity condition was observed to be less than that obtained for chronic toxicity in three trophic levels (Table 4). This is due to difference in AF used for determining acute and chronic toxicity. Previous study has also reported lower acute toxicity values in fish (Kienzler et al., 2016). To determine the effects of long term exposure of pharmaceutical compounds on aquatic organisms, further risk assessment studies were carried out using the chronic toxicity PNEC.
3.2.2. Human health PNEC
PNEC were also calculated to determine risk exposure effects on human health due to consumption of food (in the form of fish) cultivated in pharmaceutically contaminated waters. Health risk estimation was conducted for the most sensitive human sub-population, children. The study observed that rapamycin has the lowest PNEC (4.3 μg/L) which is followed by ritonavir (19.52 μg/L), lopinavir (40 μg/L), chloroquine (7.36 × 103 μg/L), and ribavirin (13.23 × 103 μg/L), respectively. Previous studies have reported high PNEC of pharmaceuticals such as fluoroquinolones, sulfamethoxazole for drinking water (Schwab et al., 2005) and fish consumption exposure (Schwab et al., 2005; Kumari and Kumar, 2021). Lower PNEC concentration of rapamycin in surface waters poses possible risks to human health. The lower the PNEC values, higher is the risk due to drug-of-concern for a given exposure pathway.
3.3. Risk characterization or risk quotient analysis
Risk Characterization or risk quotient analysis was carried out using the PEC and PNEC values of pharmaceuticals for assessing ecological and human health risks.
3.3.1. Ecological risk assessment (ERA)
3.3.1.1. Pharmaceutical compounds
ERA estimates ecological hazard for the worst-case scenario (Thomaidi et al., 2015). For ERA, PNEC of pharmaceuticals were calculated using the chronic toxicity data as they are considered to be more reliable (Zhang et al., 2017). EMEA also recommends the use of chronic toxicity data for PNEC estimation (EMEA, 2006), and gives much better insight into the “true” risk of chemical. In algae, high RQ values were obtained for lopinavir (6.88 × 102), ritonavir (5.56 × 102), and chloroquine (1.61 × 102) (Table 5 ). No risk was obtained for the other two compounds as evident by low RQ values. In daphnia, comparatively low RQ values was obtained for lopinavir (2.48 × 102), ritonavir (3.20 × 102), chloroquine (7.49 × 101) whereas ribavirin and rapamycin does not pose any risk (RQ < 1). Even lower RQ values were observed in fish, in which the values ranged from 2.06 × 101 (for ritonavir, maximum risk) to 4.05 × 10−4 (for ribavirin, no risk). Overall, RQ values of ritonavir, lopinavir, and chloroquine in three trophic levels falls within “very high” category of acceptable risk level (RQ > 1). High ecological risk (RQ > 1) of pharmaceutically active compounds in freshwater ecosystems has been reported (Molnar et al., 2020). Overall, ritonavir emerged as the major compound responsible for risk in the three trophic levels, followed by lopinavir and chloroquine. High toxicity of ritonavir, lopinavir and chloroquine can be attributed to low EC50/LC50 values which is less than 0.1 mg/L, and as per the EU-directive 93/67/EEC, 1996 (EC, 2003), compounds with EC50 < 0.1 mg/L are considered to be extremely toxic to aquatic organisms. EC50/LC50 values of other substances were more than 0.1 mg/L which resulted in low toxicity.
Table 5.
Risk quotients in aquatic organisms (the value in italics show high risk).
| Pharmaceuticals | Risk quotient |
||
|---|---|---|---|
| Green algae | Daphnia | Fishes | |
| Lopinavir | 6.88 × 102 | 2.48 × 102 | 1.87 × 101 |
| Ritonavir | 5.56 × 102 | 3.20 × 102 | 2.06 × 101 |
| Chloroquine | 1.61 × 102 | 7.49 × 101 | 1.19 × 101 |
| Ribavirin | 1.4 × 10−2 | 2.25 × 10−4 | 4.05 × 10−4 |
| Rapamycin | 1.86 × 10−1 | 5.10 × 10−2 | 7.85 × 10−2 |
The order of susceptibility amongst the three trophic levels was: algae ≥ daphnia ≥ fish. Past studies have also reported algae to be the most sensitive species to pharmaceuticals (antibiotics) in aquatic environment (Jeram et al., 2005; Li et al., 2015). Algae have high sensitivities to environmental pollution (Bi et al., 2018) and can accumulate several contaminants from water which can be transferred to species at higher trophic levels (Xie et al., 2008, Xie et al., 2010). The aforementioned observation is also supported by Margin of Safety, MoS (1/HQ) which in case of algae was relatively low ranging from 1.45 × 10−3 (low) to 6.9 × 101 (high). MoS value was observed to be 33 times lower in algae compared to fishes which showed maximum values. Lopinavir has the minimum MoS values (1.43 × 10−3) amongst all the drugs, which is a cause of possible concern for aquatic organisms. Ecological risks of drugs are a cause of concern and thorough monitoring and analysis must be carried out to know their actual concentrations in water environment. Uncontrolled and irregular discharge of pharmaceutical effluents has been the main source of these micro-pollutants in the water environment. Aquatic organisms in the water bodies receiving untreated or partially treated sewage are exposed to a combination of drugs residues, and not just as individual compounds. Therefore, better control and management strategies are required to reduce harmful effects posed by the contaminants under study.
3.3.1.2. Pharmaceutical metabolites
High RQ values of pharmaceutical metabolites was obtained which ranged from 3.27 × 104 (for lopinavir metabolites combined) to 1.15 × 103 (for n-desethylchloroquine, a chloroquine metabolite). As can been seen high ecotoxicological risk was predicted for pharmaceutical metabolites in environmental waters. The predicted high risk posed by lopinavir and ritonavir is similar to those reported in a previous study, wherein the two drugs predominated RQ in hospital effluents (Escher et al., 2011). High risk predicted for chloroquine and ribavirin metabolites is in contrast to those reported by Kuroda et al. (2021), in which low risk was predicted for the compounds studied. On the whole, detrimental ecological consequences can be posed in river waters accepting discharge of pharmaceutical compounds and metabolites.
3.3.2. Human health risk assessment (HHRA) of pharmaceuticals through food (in the form of fishes) grown in pharmaceutically contaminated waters
RQ values of pharmaceuticals were estimated using PEC and PNEC values. RQ values for fish consumption exposure ranged from 4.7 (for lopinavir) to 0.22 (for chloroquine). RQ obtained for ritonavir (RQ = 6.55), rapamycin (RQ = 5.08), and lopinavir (RQ = 4.65), is higher than the prescribed risk level (RQ > 1). This revealed that significant concern exists on human health by consuming fishes cultivated in these pharmaceutically contaminated waters. No significant concern exists due to the presence of chloroquine and ribavirin as indicated by low RQ values (RQ < 1). The above-mentioned observation is supported by MoS values which is less than 1 for lopinavir (0.215), ritonavir (0.152), and rapamycin (0.196). The values for chloroquine (4.39) and ribavirin (3.44) were greater than 1 and found to be protective. Bigger the MoS values, better it is (https://www.chemsafetypro.com/Topics/CRA/margin_of_safety_MOS_margin_of_exposure_MOE_difference_chemical_risk_assessment.html). High risk values of pharmaceuticals necessities the need of their regular monitoring and analysis in wastewater treatment plants as well as in the effluents discharged so as to protect the human beings from their harmful consequences.
3.4. Comprehensive risk hazard analysis
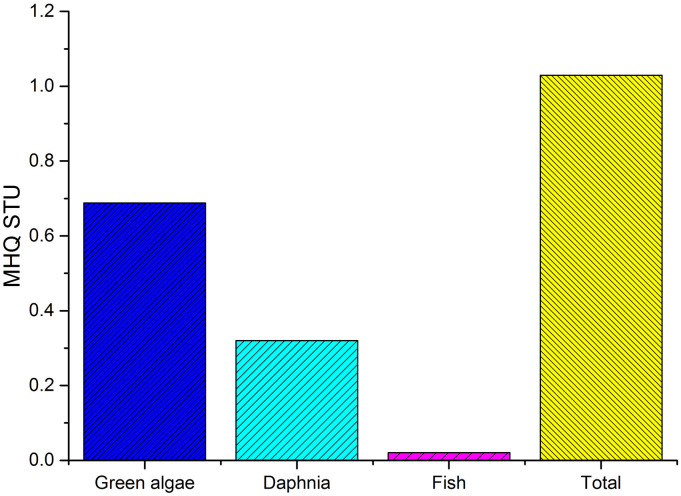
The study revealed high MRQPEC/PNEC values for the three trophic level of which maximum value was observed in green algae (14.06 × 102) followed by daphnia (6.43 × 102) and fish (5.1 × 101). The results demonstrated high ecological risk for mixture of pharmaceutical drugs in environmental waters. High MRQ values can be mainly attributed to high individual RQ values of ritonavir and lopinavir, which accounted for 73.50%–62.35% and 35.41%–26.17% of the total MRQ. The results revealed that ritonavir and lopinavir are the two major drugs contributing to the overall RQ, and needs to be regulated and controlled discharge of effluents must be carried out in order to protect the aquatic ecosystem. The study also observed that the calculated MRQPEC/PNEC is greater than 1, therefore, a refinement of MRQPEC/PNEC is required to investigate the cause of concern (Kienzler et al., 2019). As mentioned in the Methodology section, improvement of MRQPEC/PNEC is carried out by calculating MRQSTU. MRQSTU values in algae (0.68), daphnia (0.32), and fish (0.02) were less than 1, of which fish has the lowest MRQSTU values. Environmental risks were considered as low when RQs were less than 1 (Markert et al., 2020). Hence, no significant ecological risk exists for mixture of pharmaceuticals in environmental waters. The total MRQSTU (1.029) values was greater than 1 indicating possible concern on ecological ecosystem (Fig. 3 ). It was observed that lopinavir, ritonavir, and chloroquine are the major contributors to the overall estimated mixture risks. These substances need to be studied in order to obtain a comprehensive overview of the mixture risks in surface waters.
Fig. 3.
MRQ STU in three trophic levels.
3.5. Maximum allowable concentrations (Callowable)
3.5.1. Callowable for co-existing pharmaceutical compounds in aquatic environment
It is essential to determine the maximum allowable concentrations, C allowable for co-existing pharmaceutical compounds in environmental waters since the substances are usually present as mixture combinations, and not as individual compounds (Kienzler et al., 2019). Mixture toxicity assessment of pharmaceuticals would be helpful in providing information about the maximum allowable level which can be tolerated by aquatic species. C allowable values of pharmaceutical mixture was found to be 0.53 mg/L. This indicates the level below which no adverse effects are expected to occur in underlying aquatic organisms and is also considered to be safe for human consumption. The estimated C allowable values are low which calls for strict regulation formulations and their subsequent implementation in environmental waters to protect the underlying ecosystem. The results presented in this study can be used by regulatory agencies in making guidelines so that step can be taken for implementing proper control measures in wastewater treatment plants and effluents discharged. This would help in restricting the exposure of pharmaceutical compounds in aquatic ecosystems and can also be used for human consumption.
3.6. Sensitivity analysis and uncertainty in risk
Fig. 4 shows the radar plot of sensitivity analysis for determining the major governing parameter responsible for risk estimates. Amongst the input parameters considered, ED was found to be most influential parameter followed by fish consumption rate, BW and ADI values for all the pharmaceutical drugs. The overall results of sensitivity analysis indicated that ED is the most dominant parameter governing risk estimates. Fish consumption rate also showed significant effects on risk estimates. The parameters followed the order of: ED > fish consumption rate > BW > ADI of pharmaceutical compounds > BCF.
Fig. 4.
Sensitivity analysis (via radar plot) to show the major parameters governing risk estimates.
It is imperative to ascertain the uncertainty associated in risk estimation to arrive at proper conclusions (Kumari and Gupta, 2018). It is believed that uncertainty in risk estimation arises because of the variation in input parameters such as BW, ED, EF, BCF, toxicity values etc., used in determining risk estimates. Uncertainty might occur due to lack of evidence on the environment-relevant concentrations of the drugs considered. Looking into the potential health benefits and economic importance of pharmaceutical drugs, the most appropriate information must be used. Use of the predicted environmental concentration of drugs to determine risk estimates might have added uncertainty in risk estimates thus regular monitoring and analysis of drugs in water matrices is needed to attain more clarity on the data presented. Besides, BCF is also a vital parameter which would lead to uncertainty. BCF values for majority of drugs are available in literature but since the drugs considered in this study are new and no specific information is available, therefore, the values were calculated using Al-Khazrajy and Boxall (2016) equation. Fish consumption rate in children is taken as per USFDA recommendations however, the values might differ depending upon the geo-climatic and geographical locations where the study is conducted. Looking into the above mentioned aspects, the values must be considered as per the need and requirements of the study conducted.
4. Conclusions and implications
This study predicted the environmental concentration of COVID-19 pharmaceutical compounds and their metabolites in environmental waters and also derived risk of individual pharmaceutical compounds and in mixture to identify possible exposure effects on ecological and human health.
-
•
High PEC of pharmaceutical compounds in environmental waters indicates the excessive use of drugs and incompetence of wastewater treatment plants in removing COVID-19 substances. The effluents are discharged in the receiving water without any proper treatment resulting in high levels. Similar observations were made for pharmaceutical metabolites.
-
•
Ecological PNEC revealed that algae are the most sensitive species to pharmaceutical compounds in the aquatic environment owing to their low values (Average: 0.53 μg/L) compared to daphnia and fish. PNEC were derived for a hypothetical situation which can ultimately be refined or accompanied with evidence on experimentally derived selective concentrations in microbial communities as such data become available.
-
•
Ecological risk of pharmaceutical compounds revealed that ritonavir showed major effects in three trophic level. The study observed that algae has the highest average RQ values (2.81 × 102) followed by daphnia (1.28 × 102) and fish (1.02 × 101). On the basis of results presented algae are the most sensitive species followed by daphnia and fish. The aquatic biota could be vulnerable to the presence of pharmaceuticals in their environment, and toxic effects are expected to occur with unexpected outcomes.
-
•
Ecological risk of metabolites revealed high RQ values. The environmental waters receiving effluents discharged from wastewater treatment plants pose risk to aquatic species affecting them.
-
•
Human health risks through consumption of contaminated food revealed that maximum effects of risk exposure come through ritonavir followed by rapamycin and lopinavir as indicated by high RQ values (RQ > 1). Risk due to other compounds was found to be insignificant and negligible.
-
•
Comprehensive risk assessment analysis revealed that total estimated MHQPEC/PNEC values were greater than 1, suggesting possible risk on the trophic level studied.
-
•
The approach used for assessing comprehensive risk is conceptually different from concentration addition model because the PNEC might be based on different (groups of) species and also using different AFs; however, summation of PEC/PNEC ratios can be used as a screening-level approach (Backhaus and Faust, 2012).
-
•
Callowable values of co-existing pharmaceutical compounds was found to be 530 μg/L which indicates that efforts are required in assessing mixture toxicity of contaminants and guidelines/standards needs to be formulated for their control in environmental waters.
-
•
Sensitivity analysis revealed that for human health, exposure duration is the main parameter governing risk estimates followed by fish consumption rate.
Environmental concentrations of COVID-19 pharmaceuticals at various temporal scales is required in environmental waters for accurately predicting ecological and human health risks. Looking into the potential mixture toxicity of compounds, strict control measures can be implemented by adopting stringent effluent discharge standard in the receiving water bodies so as to protect the underlying aquatic ecosystem and also for reducing human health risk exposure by consuming food grown in pharmaceutically contaminated waters. Co-existence of pharmaceutical compounds has been reported in different water matrices, however, the type of interactions (synergistic/antagonistic) has not been reported for majority of substances. Due to lack of information available in published literature, we have used concentration addition approach to delineate mixture toxicity of pharmaceutical compounds. Interaction based studies (either in vivo or in vitro) are required and needs to be conducted for upcoming risk assessment studies involving COVID-19 pharmaceuticals. The findings of this study can be used by (i) environment researchers for understanding and recognizing the possible pollution of pharmaceutical substances, (ii) risk due to exposure of (a) pharmaceuticals, (b) metabolites, and (c) mixture of drugs so that strict control measures can be adopted to regulate their discharge in receiving water bodies, and (iii) regulatory agencies for guideline development of pharmaceutical drugs in water. Societies can also provide a helping hand by restricting the use of pharmaceutical drugs beyond the prescribed limit.
The following is the supplementary data related to this article.
Information of selected drugs.
CRediT authorship contribution statement
Minashree Kumari: Conceptualization, Organization, Data collection and analysis, Writing - original draft and editing.
Arun Kumar: Conceptualization, Writing - review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Department of Civil Engineering, Indian Institute of Technology (Delhi, India) for supporting this study. The work carried as a part of the corresponding author (Dr. Minashree Kumari) research from her Post-Doctoral work.
Editor: Damià Barceló
References
- Abafe O.A., Späth J., Fick J., Jansson S., Buckley C., Stark A., et al. LC-MS/MS determination of antiretroviral drugs in influents and effluents from wastewater treatment plants in KwaZulu-Natal, South Africa. Chemosphere. 2018;200:660–670. doi: 10.1016/j.chemosphere.2018.02.105. [DOI] [PubMed] [Google Scholar]
- Al Aukidy M., Verlicchi P., Jelic A., Petrovic M., Barcelo D. Monitoring release of pharmaceutical compounds: occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci. Total Environ. 2012;438:15–25. doi: 10.1016/j.scitotenv.2012.08.061. [DOI] [PubMed] [Google Scholar]
- Al-Khazrajy O.S.A., Boxall A.B.A. Risk-based prioritization of pharmaceuticals in the natural environment in Iraq. Environ. Sci. Pollut. Res. 2016;23:15712–15726. doi: 10.1007/s11356-016-6679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminot Y., Litrico X., Chambolle M., Arnaud C., Pardon P., Budzindki H. Developmentand application of amulti-residue method for the determination of pharmaceuticals in water, sediment, and suspended solids using liquid chromatographytandem mass spectrometry. Anal. Bioanal. Chem. 2015;407:8585–8604. doi: 10.1007/s00216-015-9017-3. [DOI] [PubMed] [Google Scholar]
- Backhaus T., Faust M. Predictive environmental risk assessment of chemical mixtures: a conceptual framework. Environ. Sci. Technol. 2012;46:2564–2573. doi: 10.1021/es2034125. [DOI] [PubMed] [Google Scholar]
- Bi R., Zeng X., Mu L., Hou L., Liu W., Li P., Chen H., Li D., Bouchez A., Tang J., Xie L. Sensitivities of seven algal species to triclosan, fluoxetine and their mixtures. Sci Rep. 2018;8(1):15361. doi: 10.1038/s41598-018-33785-1. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bopp S.K., Barouki R., Brack W., Dalla Costa S., Dorne J.-L.C.M., Drakvik P.E., Faust M., Karjalainen T.K., Kephalopoulos S., van Klaveren J. Current EU research activities on combined exposure to multiple chemicals. Environ. Int. 2018;120:544–562. doi: 10.1016/j.envint.2018.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T.C.M., Arts G.H.P., Maltby L., Van den Brink P.J. Aquatic risks of pesticides, ecological protection goals, and common aims in European Union legislation. Integr. Environ. Assess. Manag. 2006;2(4):20–46. [Google Scholar]
- Chen H., Jing L., Teng Y., Wang J. Characterization of antibiotics in a large-scale river system of China: occurrence pattern, spatiotemporal distribution and environmental risks. Sci. Total Environ. 2018;618:409–418. doi: 10.1016/j.scitotenv.2017.11.054. [DOI] [PubMed] [Google Scholar]
- Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse B.A., Ghoshdastidar A.J., Tong A.Z. The presence of acidic and neutral drugs in treated sewage effluents and receiving waters in the Cornwallis and Annapolis River watersheds and the Mill CoveSewage Treatment Plant in Nova Scotia, Canada. Environ Res. 2012;112:92–99. doi: 10.1016/j.envres.2011.11.011. [DOI] [PubMed] [Google Scholar]
- De Lange H.J., Noordoven W., Murk A.J., Lürling M., Peeters E.T.H.M. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquat. Toxicol. 2006;78:209–216. doi: 10.1016/j.aquatox.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Deng W., Li N., Zheng H., Lin H. Occurrence and risk assessment of antibiotics in river water in Hong Kong. Ecotoxicol. Environ. Saf. 2016;125:121–127. doi: 10.1016/j.ecoenv.2015.12.002. [DOI] [PubMed] [Google Scholar]
- EC (European Commission) Joint Research Center, Ispra; 1996. Technical guidance document in support of Commission directive 93/67/EEC on risk assessment for new notified substances and Commission regulation (EC) no 1488/94 on risk assessment for existing substances. [Google Scholar]
- EC (European Commission) 2nd ed. European Commission; Luxembourg: 2003. Technical guidance document in support of Commission Directive 93/67/EEC on Risk assessment for new notified substances and Commission Regulation (EC) No 1488/94 on Risk assessment for existing substances and Commission Directive (EC) 98/ 8 on Biocides. Part 1, 2 and 3, 760p. [Google Scholar]
- ECHA . 2008. Guidance on information requirements and chemical safety assessment Chapter R.10: Characterization of dose [concentration]- response for environment, Helsinki. [Google Scholar]
- ECHA . 2014. Guidance on information requirements and chemical safety assessment Chapter R.7b: Endpoint specific guidance, Helsinki. [Google Scholar]
- Elflein J. Canada: COVID-19 cases by province. Statistica. 2020 [Google Scholar]
- EMEA Guideline on the Limits of Genotoxic Impurities. Committee for Medicinal Products for Human Use (CPMP), London. 2006. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-limits-genotoxic-impurities_en.pdf Available from:
- Escher B.I., Baumgartner R., Koller M., Treyer K., Lienert J., McArdell C.S. Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res. 2011;45:75–92. doi: 10.1016/j.watres.2010.08.019. https://www.statista.com/statistics/1107066/covid19-confirmed-cases-by-province-territory-canada/ [DOI] [PubMed] [Google Scholar]
- Fent K., Weston A., Caminada D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006;76:122–159. doi: 10.1016/j.aquatox.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Fung T.S., Liu D.X. Human coronavirus: host-pathogen interaction. Annu. Rev. Microbiol. 2019;73(2019):29–57. doi: 10.1146/annurev-micro-020518-115759. [DOI] [PubMed] [Google Scholar]
- Godoy A.A., Kummrow F. What do we know about the ecotoxicology of pharmaceutical and personal care product mixtures? A critical review. Crit. Rev. Environ. Sci. Technol. 2017;47:1453–1496. [Google Scholar]
- Gómez-Canela C., Pueyo V., Barata C., Lacorte S., Maria Marcé R. Development of predicted environmental concentrations to prioritize the occurrence of pharmaceuticals in rivers from Catalonia. Sci. Total Environ. 2019:57–67. doi: 10.1016/j.scitotenv.2019.02.078. [DOI] [PubMed] [Google Scholar]
- He K., Asada Y., Echigo S., Itoh S. Biodegradation of pharmaceuticals and personal care products in the sequential combination of activated sludge treatment and soil aquifer treatment. Environ. Technol. 2018:1–11. doi: 10.1080/09593330.2018.1499810. [DOI] [PubMed] [Google Scholar]
- Horby P., Mafham M., Linsell L., et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. medRxiv. 2020 doi: 10.1101/2020.07.15.20151852. published online July 15. (preprint) [DOI] [Google Scholar]
- Isidori M., Lavorgna M., Nardelli A., Pascarella L., Parrella A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 2005;346:87–98. doi: 10.1016/j.scitotenv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Jain S., Kumar P., Vyas R.K., Pandit P., Dalai A.K. Occurrence and removal of antiviral drugs in environment: a review. Water Air Soil Pollut. 2013;224:1410. [Google Scholar]
- Jeram S.J., Sintes M., Halder J., Fentanes B., Sokull-Klüttgen T., Hutchinson A. A strategy to reduce the use of fish in acute ecotoxicity testing of new chemical substances notified in the European Union. Regul. Toxicol. Pharmacol. 2005;42:218–224. doi: 10.1016/j.yrtph.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Johnson D.J., Sanderson H., Brain R.A., Wilson C.J., Solomon K.R. Toxicity and hazard of selective serotonin reuptake inhibitor antidepressants fluoxetine, fluvoxamine, and sertraline to algae. Ecotoxicol. Environ. Saf. 2007;67:128–139. doi: 10.1016/j.ecoenv.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Jones-Lepp T.L., Sanchez C., Alvarez D.A., Wilson D.C., Taniguchi-Fu R.L. Point sources of emerging contaminants along the Colorado River basin: source water for the arid southwestern United States. Sci. Total Environ. 2012;430:237–245. doi: 10.1016/j.scitotenv.2012.04.053. [DOI] [PubMed] [Google Scholar]
- Kienzler A., Bopp S.K., van der Linden S., Berggren E., Worth A. Regulatory assessment of chemical mixtures: requirements, current approaches and future perspectives. Regul. Toxicol. Pharmacol. 2016;80:321–334. doi: 10.1016/j.yrtph.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Kienzler A., Bopp S., Halder M., Embry M., Worth A. Application of new statistical distribution approaches for environmental mixture risk assessment: a case study. Sci. Total Environ. 2019;693 doi: 10.1016/j.scitotenv.2019.07.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Xagoraraki I. Human health risk assessment of pharmaceuticals in water: an uncertainty analysis for meprobamate, carbamazepine, and phenytoin. Regul. Toxicol. Pharmacol. 2010;57(2–3):146–156. doi: 10.1016/j.yrtph.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Kumari M., Gupta S.K. Age dependent adjustment factor (ADAF) for the estimation of cancer risk through trihalomethanes (THMs) for different age groups- a innovative approach. Ecotox. Environ Saf. 2018;148:960–968. [Google Scholar]
- Kumari M., Kumar A. Human health risk assessment of antibiotics in binary mixtures for finished drinking water. Chemosphere. 2020;240 doi: 10.1016/j.chemosphere.2019.124864. [DOI] [PubMed] [Google Scholar]
- Kumari M., Kumar A. Identification of component-based approach for prediction of joint chemical mixture toxicity risk assessment with respect to human health: a critical review. Food Chem. Toxicol. 2020;143 doi: 10.1016/j.fct.2020.111458. [DOI] [PubMed] [Google Scholar]
- Kumari M., Kumar A. Can pharmaceutical drugs used to treat Covid-19 infection leads to human health risk? A hypothetical study to identify potential risk. Sci. Total Environ. 2021;778 doi: 10.1016/j.scitotenv.2021.146303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., Li C., Dhangar K., Kumar M. Predicted occurrence, ecotoxicological risk and environmentally acquired resistance of antiviral drugs associated with COVID-19 in environmental waters. Sci. Total Environ. 2021;776 doi: 10.1016/j.scitotenv.2021.145740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis W.G., Sofield R.M., Ming-Ho Yu. Fifth edition. CRC Press; 2011. Introduction to Environmental Toxicology: Molecular substructures to Ecological Landscapes. ISBN 9781498750448, 1498750443. [Google Scholar]
- Li W.H., Gao L.H., Shi Y.L., Liu J.M., Cai Y.Q. Occurrence, distribution and risks of antibiotics in urban surface water in Beijing, China. Environ. Sci. Process. Impacts. 2015;17:1611–1619. doi: 10.1039/c5em00216h. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang L., Liu X., Ding J. Ranking and prioritizing pharmaceuticals in the aquatic environment of China. Sci. Total Environ. 2019;658:333–342. doi: 10.1016/j.scitotenv.2018.12.048. [DOI] [PubMed] [Google Scholar]
- Liu Xi, Chen H., Shang Y., Zhu H., Chen G., Chen Y., Liu S., Zhou Y., Huang M., Hong Z., Xia J. Efficacy of chloroquine versus lopinavir/ritonavir in mild/general COVID19 infection: a prospective, open-label, multicenter, randomized controlled clinical study. Trials. 2020;21:622. doi: 10.1186/s13063-020-04478-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Li M., Wu M., Li Z., Liu X. Occurrence ad regional distributions of 20 antibiotics in water bodies during groundwater recharge. Sci. Total Environ. 2015;518–519:498–506. doi: 10.1016/j.scitotenv.2015.02.100. [DOI] [PubMed] [Google Scholar]
- Machado M.D., Soares E.V. Sensitivity of freshwater and marine green algae to three compounds of emerging concern. J. Appl. Phycol. 2019;31:399–408. doi: 10.1007/s10811-018-1511-5. [DOI] [Google Scholar]
- Mahmood A.R., Al-Haideri H.H., Hassan F.M. Detection of Antibiotics in Drinking Water Treatment Plants in Baghdad City, Iraq. Adv. Public Health. 2019 doi: 10.1155/2019/7851354. [DOI] [Google Scholar]
- Markert N., Rhiem S., Trimborn M., Guhl B. Mixture toxicity in the Erft River: assessment of ecological risks and toxicity drivers. Environ. Sci. Europe. 2020;32(51) doi: 10.1186/s12302-020-00326-5. [DOI] [Google Scholar]
- Molnar E., Maasz G., Pirger Z. Environmental risk assessment of pharmaceuticals at a seasonal holiday destination in the largest freshwater shallow lake in Central Europe. Environ Sci. Pollut. Res. 2020 doi: 10.1007/s11356-020-09747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannou C., Ofrydopoulou A., Evgenidou E., Heath D., Heath E., Lambropoulou D. Antiviral drugs in aquatic environment and wastewater treatment plants: a review on occurrence, fate, removal and ecotoxicity. Sci. Total Environ. 2020;699 doi: 10.1016/j.scitotenv.2019.134322. [DOI] [PubMed] [Google Scholar]
- OECD . OECD Publishing; Paris: 2013. Fish, early-life stage toxicity test. [Google Scholar]
- Oliver A.L.S., Munoz-Olivas R., Sanz Landaluze J., Rainieri S., Camara C. Bioaccumulation of ionic titanium and titanium dioxide nanoparticles in zebrafish eleutheroembryos. Nanotoxicology. 2015;9(7):835–842. doi: 10.3109/17435390.2014.980758. [DOI] [PubMed] [Google Scholar]
- Omarjee L., Janin A., Perrot F., Laviolle B., Meilhac O., Mahe G., et al. Targeting Tcell senescence and cytokine storm with rapamycin to prevent severe progression in COVID-19. Clin. Immunol. 2020;216:08464. doi: 10.1016/j.clim.2020.108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paíga P., Santos L.H.M.L.M., Ramos S., Jorge S., Silva J.G., Delerue-Matos C. Presence of pharmaceuticals in the Lis river (Portugal): sources, fate and seasonal variation. Sci. Total Environ. 2016;573:164–177. doi: 10.1016/j.scitotenv.2016.08.089. [DOI] [PubMed] [Google Scholar]
- Parsai P., Kumar A. Tradeoff between risks through ingestion of nanoparticle contaminated water or fish: human health perspective. Sci. Total Environ. 2020;740 doi: 10.1016/j.scitotenv.2020.140140. [DOI] [PubMed] [Google Scholar]
- Peng X., Wang C., Zhang K., Wang Z., Huang Q., Yu Y., et al. Profile and behavior of antiviral drugs in aquatic environments of the Pearl River Delta, China. Sci. Total Environ. 2014;466–467:755–761. doi: 10.1016/j.scitotenv.2013.07.062. [DOI] [PubMed] [Google Scholar]
- Pereira A.M.P.T., Silva L.J.G., Meisel L.M., Lino C.M., Pena A. Environmental impact of pharmaceuticals from Portuguese wastewaters: geographical and seasonal occurrence, removal and risk assessment. Environ. Res. 2015;136:108–119. doi: 10.1016/j.envres.2014.09.041. [DOI] [PubMed] [Google Scholar]
- Pereira A., Silva L., Laranjeiro C., Lino C., Pena A. Selected pharmaceuticals in different aquatic compartments: part II-toxicity and environmental risk assessment. Molecules (Basel, Switzerland) 2020;25(8):1796. doi: 10.3390/molecules25081796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradat P., Virlogeux V., Gagnieu M.-C., Zoulim F., Bailly F. Ribavirin at the era of novel direct antiviral agents for the treatment of hepatitis C virus infection: Relevance of pharmacological monitoring. Adv. Hepatol. 2014;13 doi: 10.1155/2014/493087. [DOI] [Google Scholar]
- Prasse C., Schlüsener M.P., Schulz R., Ternes T.A. Antiviral drugs in wastewater and surface waters: a new pharmaceutical class of environmental relevance? Environ. Sci. Technol. 2010;44:1728–1735. doi: 10.1021/es903216p. [DOI] [PubMed] [Google Scholar]
- Rabi F.A., Zoubi M.S., Kasasbeh G.A., Salameh D.M., Al-Nasser A.D. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9(3):231. doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo S., Vivian D.N., Barron M.G. Web-based Interspecies Correlation Estimation (Web-ICE) for Acute Toxicity: User Manual. Version 1.1. EPA/600/R-10/004. Gulf Breeze, FL. 2010. http://www.epa.gov/ceampubl/fchain/webice/index.html Available at:
- Saaristo M., Brodin T., Balshine S., Bertram M.G., Brooks B.W., Ehlman S.M., McCallum E.S., Sih A., Sundin J., Wong B., Arnold K.E. Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife. Proc. Biol. Sci. 2018;285(1885):20181297. doi: 10.1098/rspb.2018.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson H., Johnson D.J., Wilson C.J., Brain A.R., Solomon K.R. Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol. Lett. 2003;144:383–395. doi: 10.1016/s0378-4274(03)00257-1. [DOI] [PubMed] [Google Scholar]
- Sanderson H., Johnson D.J., Reitsma T., et al. Ranking and prioritization of environmental risks of pharmaceuticals in surface waters. Regul. Toxicol. Pharmacol. 2004;39:158–183. doi: 10.1016/j.yrtph.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Santos L.H.M.L.M., Araújo A.N., Fachini A., Pena A., Delerue-Matos C., Montenegro M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010;175:45–95. doi: 10.1016/j.jhazmat.2009.10.100. [DOI] [PubMed] [Google Scholar]
- Schwab B.W., Hayes E.P., Fiori J.M., Mastrocco F.J., Roden N.M., Cragin D., Meyerhoff R.D., D’Aco V.J., Anderson P.D. Human pharmaceuticals in US surface waters: a human health risk assessment. Regul. Toxicol. Pharmacol. 2005;42:296–312. doi: 10.1016/j.yrtph.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Silva L.J.G., Lino C.M., Meisel L.M., Pena A. Selective serotonin re-uptake inhibitors (SSRIs) in the aquatic environment: an ecopharmacovigilance approach. Sci. Total Environ. 2012;437:185–195. doi: 10.1016/j.scitotenv.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Simazaki D., Kubota R., Suzuki T., Akiba M., Nishimura T., Kunikane S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015;76:187–200. doi: 10.1016/j.watres.2015.02.059. [DOI] [PubMed] [Google Scholar]
- Singh T.U., Parida S., Lingaraju M.C., Kesevan M., Kumar D., Singh R.K. Drug repurposing approach to fight COVID-19. Pharmacol. Rep. 2020;5:1–30. doi: 10.1007/s43440-020-00155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohaili J., Muniyandi S.K., Mohamad R. In: book Household hazardous waste management. Mmereki Daniel., editor. 2017. Chapter Dose response and exposure assessment of household hazardous waste. [DOI] [Google Scholar]
- Tang J., Shi T.Z., Wu X.W., Cao H.Q., Li X.D., Hua R.M., Tang F., Yue Y. The occurrence and distribution of antibiotics in Lake Chaohu, China: seasonal variation, potential source and risk assessment. Chemosphere. 2015;122:154–161. doi: 10.1016/j.chemosphere.2014.11.032. [DOI] [PubMed] [Google Scholar]
- Taylor D., Senac T. Human pharmaceutical products in the environment-the “problem” in perspective. Chemosphere. 2014;115:95–99. doi: 10.1016/j.chemosphere.2014.01.011. [DOI] [PubMed] [Google Scholar]
- Thomaidi V.S., Stasinakis A.S., Borova V.L., et al. Is there a risk for the aquatic environment due to the existence of emerging organic contaminants in treated domestic wastewater? Greece as a case study. J. Hazard. Mater. 2015;283:740–747. doi: 10.1016/j.jhazmat.2014.10.023. [DOI] [PubMed] [Google Scholar]
- US EPA (United States Environmental Protection Agency) Office of Prevention, Pesticides and Toxic Substances; Washington, DC: 1998. Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC) Report. [Google Scholar]
- US EPA (United States Environmental Protection Agency) 2009. ECOSAR (Ecological Structure Activity Relationships) v. 1.00. OPPT – Risk Assessment Division. Available from: [Google Scholar]
- Wang Z., Du Y., Yang C., Liu X., Zhang J., Li E., Zhang Q., Wang X. Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci Total Environ. 2017;609:1423–1432. doi: 10.1016/j.scitotenv.2017.08.009. [DOI] [PubMed] [Google Scholar]
- WHO “Solidarity” clinical trial for COVID-19 treatments. Latest update on treatment arms. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/globalresearch-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments
- Wood T.P., Duvenage C.S.J., Rohwer E. The occurrence of anti-retroviral compounds used for HIV treatment in South African surface water. Environ. Pollut. 2015;199:235–243. doi: 10.1016/j.envpol.2015.01.030. [DOI] [PubMed] [Google Scholar]
- Xie L., Flippin J.L., Deighton N., Funk D.H., Dickey D.A., Buchwalter D.B. Mercury (II) bioaccumulation and antioxidant physiology in four aquatic insects. Environ. Sci. Technol. 2008;43:934–940. doi: 10.1021/es802323r. [DOI] [PubMed] [Google Scholar]
- Xie L., Funk D.H., Buchwalter D.B. Trophic transfer of cd from natural periphyton to the grazing mayfly Centroptilum triangulifer in a life cycle test. Environ. Pollut. 2010;158:272–277. doi: 10.1016/j.envpol.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalęska-Radziwill M., Affek K., Doskocz N. Ecotoxicological risk assessment of chosen pharmaceuticals detected in surface waters. J. Environ. Sci. Health A. 2017;10(13):1233–1239. doi: 10.1080/10934529.2017.1356199. [DOI] [PubMed] [Google Scholar]
- Zenker A., Cicero M.R., Prestinaci F., Bottoni P., Carere M. Bioaccumulation and biomagnification potential of pharmaceuticals with a focus to the aquatic environment. J. Environ. Manag. 2014;133:378–387. doi: 10.1016/j.jenvman.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang T., Guo C., et al. Drugs of abuse and their metabolites in the urban rivers of Beijing, China: occurrence, distribution, and potential environmental risk. Sci. Total Environ. 2017;579:305–313. doi: 10.1016/j.scitotenv.2016.11.101. [DOI] [PubMed] [Google Scholar]
- Zhang P.-W., Zhou H.-D., Li K., Zhao X.-H., Liu Q.-N., Liu D.-J., Zhao G.-F. Occurrence of pharmaceuticals and personal care products, and their associated environmental risks in a large shallow lake in North China. Environ. Geochem. Health. 2018;3:1–15. doi: 10.1007/s10653-018-0069-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Information of selected drugs.