Summary
Background
The use of rosuvastatin plus colchicine and emtricitabine/tenofovir in hospitalized patients with SARS-CoV-2 disease (COVID-19) has not been assessed. The objective of this study was to assess the effectiveness and safety of rosuvastatin plus colchicine, emtricitabine/tenofovir, and their combined use in these patients.
Methods
This was a randomized, controlled, open-label, multicentre, parallel, pragmatic study conducted in six referral hospitals in Bogotá, Colombia. The study enrolled hospitalized patients over 18 years of age with a confirmed diagnosis of COVID-19 complicated with pneumonia, not on chronic treatment with the study medications, and with no contraindications for their use. Patients were assigned 1:1:1:1. 1) emtricitabine with tenofovir disoproxil fumarate (FTC/TDF, 200/300 mg given orally for 10 days); 2) colchicine plus rosuvastatin (COLCH+ROSU, 0.5 mg and 40 mg given orally for 14 days); 3) emtricitabine with tenofovir disoproxil plus colchicine and rosuvastatin at the same doses and for the same period of time (FTC/TDF+COLCH+ROSU); or 4) the Colombian consensus standard of care, including a corticosteroid (SOC). The primary endpoint was 28-day all-cause mortality. A modified intention-to-treat analysis was used together with a usefulness analysis to determine which could be the best treatment. The trial was registered at ClinicalTrials.gov: NCT04359095
Findings
Out of 994 candidates considered between August 2020 and March 2021, 649 (65.3%) patients agreed to participate and were enrolled in this study; among them, 633 (97.5%) were included in the analysis. The mean age was 55.4 years (SD ± 12.8 years), and 428 (68%) were men; 28-day mortality was significantly lower in the FTC/TDF+COLCH+ROSUV group than in the SOC group, 10.7% (17/159) vs. 17.4% (28/161) (hazard ratio [HR] 0.53; 95% CI 0.29 to 0.96). Mortality in the FTC/TDF group was 13.8% (22/160, HR 0.68, 95% CI 0.39 to 1.20) and 14.4% in the COLCH+ROSU group (22/153) (HR 0.78, 95% CI 0.44 to 1.36). A lower need for invasive mechanical ventilation was observed in the FTC/TDF+COLCH+ROSUV group than in the SOC group (risk difference [RD] - 0.08, 95% CI 0.11 to 0.04). Three patients presented severe adverse events, one severe diarrhoea in the COLCH+ROSU and one in the FTC/TDF+COLCH+ROSU group and one general exanthema in the FTC/TDF group.
Interpretation
The combined use of FTC/TDF+COLCH+ROSU reduces the risk of 28-day mortality and the need for invasive mechanical ventilation in hospitalized patients with pulmonary compromise from COVID-19. More randomized controlled trials are needed to compare the effectiveness and cost of treatment with this combination versus other drugs that have been shown to reduce mortality from SARS-CoV-2 infection and its usefulness in patients with chronic statin use.
Research in context.
Evidence before this study
Antiviral and anti-inflammatory drugs have been used based on COVID-19 pathophysiology. Emtricitabine/tenofovir (FTC/TDF) inhibits RNA polymerase, and anti-inflammatory drugs such as colchicine (COLCH) regulate the release of inflammatory mediators that lead to lung injury, and statins such as rosuvastatin (ROSUV) have a protective action on the body's endothelial cells. In a search in Medline via PubMed using the terms “COVID-19”, “statins”, “colchicine”, “tenofovir”, and “emtricitabine,” no published studies exploring a combination of these drugs were found. The only studies identified included 1 RCT that assessed the effects of statins in critically ill patients, an RCT that assessed the use of FTC/TDF for reducing viral load in outpatients with COVID-19, and 3 RCTs assessing the effectiveness of COLCH in COVID-19 patients, albeit with conflicting results. No studies assessing their combination was found on the clinicaltrials.gov website. Only 2 RCTs assessing FTC/TDF and 8 studies exploring the use of statins in COVID-19 were identified.
Added value of this study
In this open-label, parallel-group, randomized controlled trial, we found that in hospitalized adult patients with mild, moderate or severe pneumonia, the FTC/TDF+COLCH+ROSUV combination was associated with a reduced risk of dying within the first 28 days and a lower need for invasive mechanical ventilation than the standard of care with dexamethasone. We did not find effects on mortality or other clinical outcomes in the COLCH and ROSUV group or the FTC/TDF group. To our knowledge, this is the first trial to study a combination of these medications in patients with moderate to severe COVID-19 pneumonia. These medications are safe in patients with COVID-19 pneumonia
Implications of all the available evidence
These results are highly relevant, especially for medium- and low-income countries, considering the lower cost of this treatment compared to other far more expensive alternatives that have been shown to be effective. More randomized controlled trials are needed to compare the effectiveness and cost of treatment with this combination versus other drugs with good effectiveness in terms of mortality and its usefulness in patients with chronic statin use.
Alt-text: Unlabelled box
Introduction
The SARS-CoV-2 or COVID-19 pandemic has created a great challenge in multiple realms throughout the world but is particularly significant for health systems.1 Several vaccines have been developed and are being applied worldwide in an attempt to improve the prognosis of the disease, albeit with variable effectiveness, which is still under review.2 Considering that a large proportion of the population has not been vaccinated and given the presence of new SARS-CoV-2 variants that could have an impact on vaccine effectiveness, it may well be that we will continue to see COVID-19 patients who develop pneumonia or become critically ill, requiring hospitalization and treatments to reduce the frequency of respiratory failure and mortality. At the start of the pandemic, based on experience with similar situations in the past, drugs such as hydroxychloroquine, lopinavir/ritonavir, beta interferon, remdesivir, azithromycin and ivermectin were assessed but ultimately shown not to be effective.3,4 To date, only dexamethasone has been shown to conclusively reduce 28-day mortality in hospitalized patients on mechanical ventilation (MV) or oxygen supplementation.5
Colchicine (COLCH) was selected because of its fast anti-inflammatory effect mediated by neutrophil chemotaxis and NLRP3 inflammasome signalling inhibition, potentially preventing cytokine storm development.6 Statins (Rosuvastatin -ROSU-) were also selected on the grounds of their anti-inflammatory, immunomodulatory and antithrombotic properties with a potential protective effect against COVID-19-related complications.7 Emtricitabine/Tenofovir disoproxil fumarate (FTC/TDF) nucleotide analogues widely used as anti-retroviral therapy in HIV, were selected because of potential inhibition of SARS-CoV-2 ribonucleic acid-dependent RNA polymerase, an enzyme that plays a key role in viral transcription and replication.8 At that time, there was a paucity of clinical information on the impact of each of these drugs on COVID-19 patients, with only one published randomized clinical trial (RCT) assessing colchicine9 and only observational studies regarding the use of the other two medications.10,11
In view of the lack of randomized studies on the effects of FTC/TDF in slowing the initial rapid viral replication phase, the potential action of the combined use of ROSUV plus COLCH in reducing COVID-19 effects on endothelial cells and the increased risk of organ thrombosis and controlling the exaggerated inflammatory host response, our aim was to assess the effectiveness and safety of the COLCH+ROSUV combination, the effects of FTC/TDF and, finally, the combined effect of antiviral and anti-inflammatory drugs versus the standard of care in hospitalized COVID-19 patients in the context of a pragmatic randomized controlled trial.12
Methods
Study design
A randomized, controlled, open-label, multicentre, parallel, pragmatic trial was conducted in six high complexity referral hospitals in Bogotá, Colombia. The protocol was published in Clinicaltrials.gov under identifier NCT04359095. Both the study protocol and the informed consent form were approved by the ethics committees of the National University of Colombia School of Medicine and Pontificia Universidad Javeriana and of each of the participating hospitals (the details of the protocol are found in the Supplementary Materials).
Participants
Adults 18 years of age or older with a clinical picture of COVID-19 infection diagnosed by real-time polymerase chain reaction (RT–PCR) or viral antigen testing and with mild, moderate or severe pneumonia requiring in-hospital treatment were enrolled. Mild pneumonia was defined based on chest X-ray findings plus 2 or more risk factors for COVID-19 complications, including age over 60 years, pre-existing cardiovascular disease, diabetes mellitus, chronic obstructive pulmonary disease (COPD), or cancer. Moderate pneumonia was defined based on X-ray findings, such as, bilateral air-space consolidation, usually ground glass opacities with peripheral and basal distribution, in accordance with local guidelines.13 and hospitalization criteria on the simplified severity scale (CRB-65) > 1 or ambient oxygen saturation under 90%. Severe pneumonia was defined using the same criteria as for moderate pneumonia plus any of the following: respiratory rate of more than 30 breaths per minute; or the need for mechanical ventilation (invasive or non-invasive); or sepsis identified by a score of 2 or more on the Sequential Organ Failure Assessment (SOFA) scale; or 2 out of 3 of a Glasgow score of 13 or less, systolic blood pressure of 100 mmHg or less, and respiratory rate of 22 breaths per minute or higher; or a diagnosis of septic shock or multiple organ failure or adult respiratory distress syndrome. Pregnant women; patients taking any of the study drugs within the last 7 days; patients with known allergies to any of the drugs; patients with a history of myopathy, rhabdomyolysis, liver or renal failure or lung fibrosis; patients with advanced or metastatic cancer; and patients with a score greater than 3 on the frailty scale were excluded.
Randomization and masking
Patients were randomly assigned using a balanced design of 4 and 8 patient-block sizes, stratified by participating centres (six). Randomization was carried out in R, version 4.0.2, using the Blockrand software package, to one of four arms using a Web-based randomization system (UNcovApp®), which allows us to maintain concealment closest to the start of treatment. At each hospital, consenting eligible patients received the standard of care (SoC) based on the recommendations of the Colombian consensus for hospitalized patients with COVID-19 that includes the use of dexamethasone, ivermectin or albendazole as prophylaxis for Strongyloides infection, enoxaparin, acetaminophen, oxygen as needed, and mechanical ventilation, or dialysis, if required.13 Patients were assigned 1:1:1:1 to receive 1) emtricitabine with tenofovir disoproxil fumarate (FTC/TDF, 200/300 mg PO for 10 days); 2) colchicine plus rosuvastatin (COLCH+ROSU, 0.5 mg and 40 mg PO for 14 days); 3) emtricitabine with tenofovir disoproxil fumarate plus colchicine and rosuvastatin at the same dose and during the same time period (FTC/TDF+COLCH+ROSU); or 4) only the SoC. Assigned treatments were prescribed by the treating physicians at each centre; the patients and the local researchers who assessed the results were aware of the treatment received, but the statisticians were blinded to the treatment by means of treatment arm re-coding.
Procedures
At each hospital, two general practitioners identified candidates for the study. After verifying the inclusion and exclusion criteria, patients were invited to participate and sign informed consent forms. In those cases, in which the patients could not receive information or were in the intensive care unit (ICU), a family member or witness was asked to sign on behalf of the patient and provide the relevant information. The data were then entered into a Web-based electronic database (Research Electronic Data Capture [REDCap], Ver. 6.16, Vanderbilt University). Data collection included sociodemographic characteristics, pre-existing comorbidities, functional status, care setting, and respiratory support level on the day of assignment to the treatment.
Patients were followed on a daily basis until discharge, death or Day 28 after assignment. Data on the clinical course, clinical condition, need for respiratory function or other organ support, laboratory test results, adherence to treatment and primary and secondary outcomes were entered in the Web-based study forms. Definitive discontinuation of any medication after 24 hours of treatment was considered non-adherence. Discharged patients were followed by a telephone call on post discharge Days 7 and 28. No information was collected after Day 28.
Outcomes
The primary effectiveness and usefulness outcome was all-cause mortality within 28 days after treatment assignment. In terms of safety, serious adverse events (SAEs) were assessed. To determine that anyone SAE was attributable more to any of the study drugs or the standard of care than to the Covid-19 infection or its complications, we used the causality criteria utilized in patient safety research by Wilson et al.14 Therefore, it was considered that an SAE was caused by the study drugs when we ranked with a score equal or higher than 4 (> 50% probability to be associated to management causation). Secondary outcomes were 7-day mortality, the proportion of admissions to the ICU, the proportion with mechanical ventilation requirement, time to death, length of stay, and any study treatment-related adverse event.
Statistical analysis
A sample size of 816 patients (204 patients per treatment arm) was calculated based on a 10% difference in 28-day mortality, with 15% in standard care based on the mortality reported by Wang15 and Borba16 and 5% for the three medication groups to be compared against standard care (we took the same effect for the three intervention groups based on their biological plausibility and assuming there was no synergy between the antiviral and the anti-inflammatory medications). We considered a 10% difference in 28-day mortality based on the notion that it was clinically significant and allowed us to have a sample size that was feasible considering the available funds. The sample size was adjusted on the basis of Bonferroni multiple comparisons (each treatment versus standard treatment) and 10% withdrawals.17 We used the Bonferroni correction when calculating sample size by adjusting alpha, dividing it by the number of comparisons that we planned (3), for an alpha of 0.0167 (0.05/3); this enabled us to maintain the family-wise error rate and use a significant level of 0.05 in the analysis. We interrupted the recruitment of patients on February 28, 2021, due to lack of funds, and the study coordinating committee ended the study in May 2021 as we were not able to obtain additional funding.
Baseline conditions at the time of treatment assignment, adverse events and reasons for non-adherence were described for each treatment arm. We used the modified Intention to Treat (ITT) analysis according to Gravel's definition, excluding from the analysis patients for whom the final primary outcome is unknown because they were either lost to follow-up, withdrew, or did not meet the eligibility criteria, based on the fact that inclusion of those patients could bias the effect of the intervention.18 For the 28-day mortality outcome, the mortality rate ratio was estimated as the hazard ratio (HR). Because of the existing correlation among individuals in the same hospital and heterogeneity in the number of patients and mortality proportion by hospital (Table S1), a Cox regression model with shared frailty factor was used for HR estimation (i.e., hospital random effects) following the recommendations by Austin.19 Moreover, the absolute difference in mortality proportion (RD) was estimated using a generalized estimation equation (GEE) with a log-binomial link function, assuming an interchangeable correlation structure with each hospital as a cluster and robust standard errors derived from the delta method. RDs were estimated for secondary outcomes using GEE. All estimators were described with their 95% confidence intervals (95% CI) and the modified intention-to-treat analysis approach.20 Crude analyses were carried out for all previous estimators assuming interobservation independence (i.e., not taking into account hospital clusters), and age-, sex- and pneumonia severity-adjusted analyses were conducted based on the fact that these variables are associated with death from COVID-19,5 and they were slightly imbalanced between treatment groups in measuring baseline characteristics of patients. Additionally, an RD sensitivity analysis was performed including the 13 losses as deaths. An unplanned analysis to estimate RD by the pneumonia severity group was also carried out. Finally, as part of the pragmatic approach, the treatment effect classification was estimated using an empirical bootstrap with 10 000 repetitions, and the surface under the cumulative ranking curve (SUCRA) was calculated for each arm.21
Role of the funding source
This project was approved by grant 374–2020 from the Colombian Ministry of Science and Technology to Universidad Nacional de Colombia. The funding agency did not play any role in the study design, data collection, data analysis, data interpretation or writing of the report. HGGD, CJRR, VARR, SLVS, GB, MMQ and NRM had full access to all clinical data and all authors agreed with the final decision to submit for publication.
Results
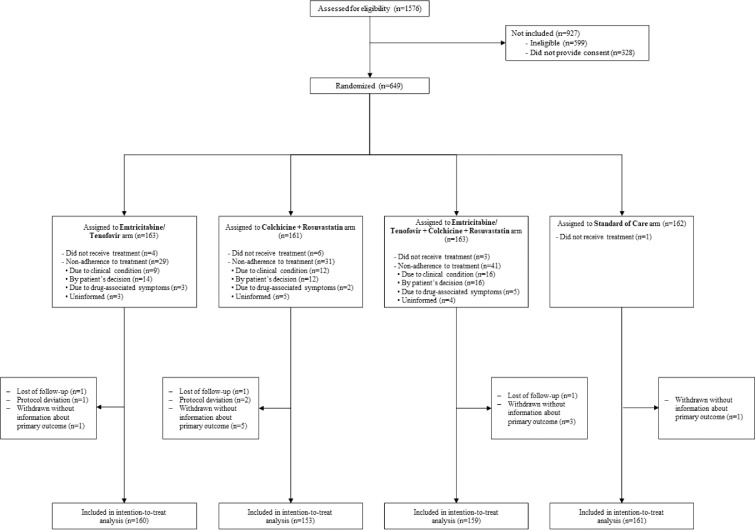
A total of 1576 patients were assessed between 24 August 2020 and 20 March 2021 to verify eligibility. Among them, 599 (38%) had at least one exclusion criterion (the use of statins within the previous 7 days was the main exclusion criterion in 587 (98%) cases). Overall, 994 patients were invited to participate in the study, 328 (33%) declined, and ultimately, 649 were randomized and assigned to one of the four treatment arms. Among them, 3 (0.46%) subjects did not meet the eligibility criteria (they were taking statins chronically, but only one took 1 dose of the study medications). In the end, 633 (97.5 %) patients were considered in the modified ITT analysis (Figure 1). There were variations among hospitals in terms of the distribution of the number of patients enrolled and the proportion of mortality (see the Supplemental material, Table S1).
Figure 1.
Enrolment, Randomisation, and Inclusion in the intention-to-treat analysis.
1576 patients were seen in the six hospitals; 599 (38%) had some exclusion criteria, mainly chronic use of statins in 582. Overall, 994 patients were invited to participate in the study, 328 (33%) refused to sign the informed consent, 649 were randomised and assigned to one of four arms of treatment. Of them, 3 subjects did not meet protocol selection criteria, 3 subjects were lost of follow-up and 10 patients withdrew; consequently, the primary outcome was unknown in 13 patients, so they were not included in the analysis. Finally, 633 patients were considered in the modified ITT analysis.
Regarding the patient baseline characteristics, their mean age was 55.4 (SD ± 12.8 years), and 205 (32%) were women. There was a history of diabetes in 76 (12%), hypertension in 176 (28%), COPD in 28 (4%) and smoking in 104 (16.4%). The median time between the onset of symptoms and hospitalization was 10 days. At the time of enrolment, 45 (7%) cases were classified as mild pneumonia, 425 (67%) as moderate and 163 (26%) as severe; 49 (8%) patients were on invasive mechanical ventilation, 57 (9%) on high flow nasal cannula, 418 (66%) were receiving oxygen alone, and 109 (17%) were not on supplemental oxygen (Table 1). Nonadherence to the assigned treatment varied between 29 (18%) and 41 (25%) patients per arm (Figure 1). In all four arms of the study, 98% of patients did not receive other antiviral or anti-inflammatory treatments, except for the use of dexamethasone as part of the standard of care (Table S2).
Table 1.
Characteristics of the Patients at Baseline, According to Treatment Assignment
| Characteristic* | Treatment assignment |
|||
|---|---|---|---|---|
| Emtricitabine/Tenofovir | Colchicine+Rosuvastatin | Emtricitabine/ Tenofovir + Colchicine + Rosuvastatin | Standard of Care | |
| (N=160) | (N=153) | (N=159) | (N=161) | |
| Female sex — no. (%) | 60 (37.5) | 50 (32.7) | 47 (29.6) | 48 (29.8) |
| Age | ||||
| Mean age±SD — yr | 56.6±13.1 | 56.1±13.2 | 53.6±12.6 | 55.3±12.3 |
| Over 60 years — no. (%) | 66 (41.2) | 62 (40.5) | 47 (29.6) | 61 (37.9) |
| Urban Residency — no. (%) | 152 (96.8) | 149 (98.0) | 152 (96.8) | 156 (96.9) |
| Marital status — no. (%) | ||||
| Single | 25 (16.2) | 21 (13.9) | 29 (18.5) | 24 (15.4) |
| Married | 91 (59.1) | 99 (65.6) | 94 (59.9) | 94 (60.3) |
| Other | 38 (24.7) | 31 (20.5) | 34 (21.7) | 38 (24.4) |
| Schooling — no. (%) | ||||
| Primary or less | 13 (8.6) | 17 (11.9) | 13 (8.6) | 20 (13.2) |
| Secondary or Middle | 54 (35.5) | 52 (36.4) | 48 (31.6) | 47 (30.9) |
| Technical and technological | 22 (14.5) | 19 (13.3) | 18 (11.8) | 17 (11.2) |
| Professional and specialized | 63 (41.4) | 55 (38.5) | 73 (48.0) | 68 (44.7) |
| Social stratification (%)† | ||||
| Medium-Low | 110 (71.0) | 110 (74.3) | 102 (68.0) | 102 (65.8) |
| Medium-High | 45 (29.0) | 38 (25.7) | 48 (32.0) | 53 (34.2) |
| Previous coexisting conditions — no. (%) | ||||
| Smoking | 24 (15.3) | 21 (14.2) | 26 (16.9) | 33 (20.9) |
| Alcoholism | 7 (4.5) | 3 (2.0) | 4 (2.6) | 7 (4.5) |
| Cardiovascular disease | 9 (5.6) | 4 (2.6) | 2 (1.3) | 2 (1.2) |
| Diabetes mellitus 1-2 | 21 (13.1) | 24 (15.7) | 19 (11.9) | 12 (7.5) |
| Chronic respiratory disease | 9 (5.6) | 6 (3.9) | 5 (3.1) | 8 (5.0) |
| Arterial hypertension | 46 (28.7) | 51 (33.3) | 50 (31.4) | 29 (18.0) |
| Cancer | 9 (5.6) | 8 (5.2) | 6 (3.8) | 5 (3.1) |
| Obesity | 58 (38.2) | 48 (34.0) | 58 (39.2) | 45 (30.2) |
| Glomerular Filtration Rate — mean±SD | 88.3±19.4 | 91.7±17.4 | 91.4±19.6 | 92.8±16.6 |
| Length of stay in previous month — no. (%) | 0 (0.0) | 3 (2.0) | 3 (1.9) | 7 (4.4) |
| Charlson CCI — mean±SD | 0.3±0.7 | 0.4±0.9 | 0.3±0.7 | 0.2±0.6 |
| NEWS2 Classification — no. (%) | ||||
| Low | 66 (41.2) | 59 (38.6) | 69 (43.7) | 74 (46.0) |
| Medium | 55 (34.4) | 54 (35.3) | 55 (34.8) | 51 (31.7) |
| High | 39 (24.4) | 40 (26.1) | 34 (21.5) | 36 (22.4) |
| Epidemiologic contact history — no. (%) | ||||
| Known contact history | 58 (36.2) | 53 (34.6) | 63 (39.6) | 66 (41.0) |
| Community contact | 55 (94.8) | 50 (94.3) | 57 (90.5) | 60 (90.9) |
| Transportation contact | 0 (0.0) | 1 (1.9) | 0 (0.0) | 1 (1.5) |
| Healthcare staff | 3 (5.2) | 2 (3.8) | 6 (9.5) | 5 (7.6) |
| Service/Unit of current stay — no. (%) | ||||
| General ward | 112 (70.0) | 109 (71.2) | 110 (69.2) | 114 (70.8) |
| ICU-Intensive | 44 (27.5) | 37 (24.2) | 44 (27.7) | 44 (27.3) |
| ICU-Step-down | 4 (2.5) | 7 (4.6) | 5 (3.1) | 3 (1.9) |
| Pneumonia diagnosis — no. (%) | ||||
| Mild | 11 (6.9) | 12 (7.8) | 14 (8.8) | 8 (5.0) |
| Moderate | 109 (68.1) | 101 (66.0) | 102 (64.2) | 113 (70.2) |
| Severe | 40 (25.0) | 40 (26.1) | 43 (27.0) | 40 (24.8) |
| Median of days since onset of symptoms (Q1-Q3) | 9 (7-12) | 10 (6-12) | 10 (7-12) | 10 (7-12) |
| Median of days since admission (Q1-Q3) | 2 (1-3) | 2 (1-3) | 2 (1-3) | 2 (1-3) |
| Sepsis — no. (%) | 27 (65.9) | 24 (60.0) | 29 (67.4) | 28 (70.0) |
| Septic shock — no. (%) | 9 (22.5) | 8 (20.0) | 9 (20.9) | 10 (25.0) |
| ARDS — no. (%) | 39 (97.5) | 39 (97.5) | 43 (100.0) | 39 (97.5) |
| ARDS classification — no. (%) | ||||
| Mild | 0 (0.0) | 1 (2.6) | 0 (0.0) | 1 (2.6) |
| Moderate | 19 (48.7) | 18 (46.2) | 14 (32.6) | 13 (33.3) |
| Severe | 20 (51.3) | 20 (51.3) | 29 (67.4) | 25 (64.1) |
| Oxygen delivery method — no. (%) | ||||
| Non-invasive support | 100 (62.5) | 103 (67.3) | 107 (67.3) | 108 (67.1) |
| High-flow cannula | 16 (10.0) | 12 (7.8) | 13 (8.2) | 16 (9.9) |
| Mechanical ventilation | 14 (8.8) | 12 (7.8) | 12 (7.5) | 11 (6.8) |
| No oxygen | 30 (18.8) | 26 (17.0) | 27 (17.0) | 26 (16.1) |
Plus–minus values are means ±SD, ICU intensive care unit, ARDS Acute Respiratory Distress Syndrome, SD Standard Deviation, and Q1-Q3 25th percentile and 75th percentile.
Social stratification is a government classification that groups areas of residential property where the study subjects live. Under this classification, Medium-Low corresponds to subject who benefit from utility subsidies and Medium-High are subjects who either do not receive subsidies and do not have to pay contributions or must pay a premium on the cost of utilities. DANE, La estratificación socioeconómica en el régimen de los servicios públicos domiciliarios, retrieved on June 16, 2021 at https://www.dane.gov.co/files/geoestadistica/Estratificacion_en_SPD.pdf
Primary outcome
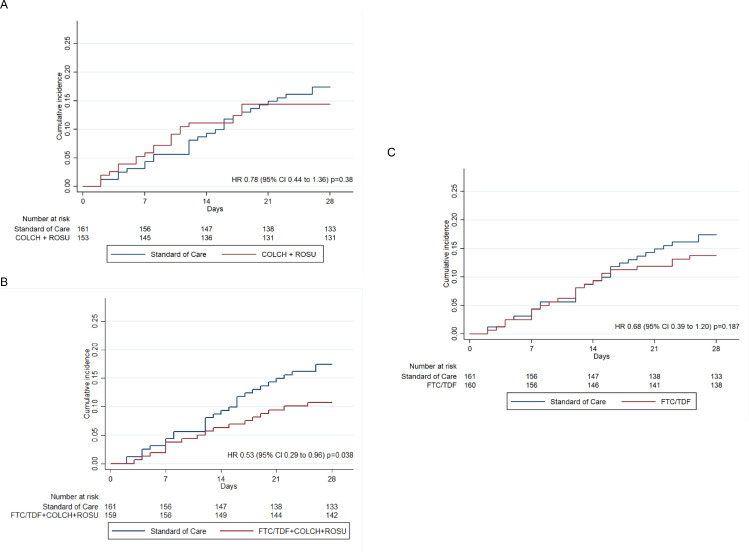
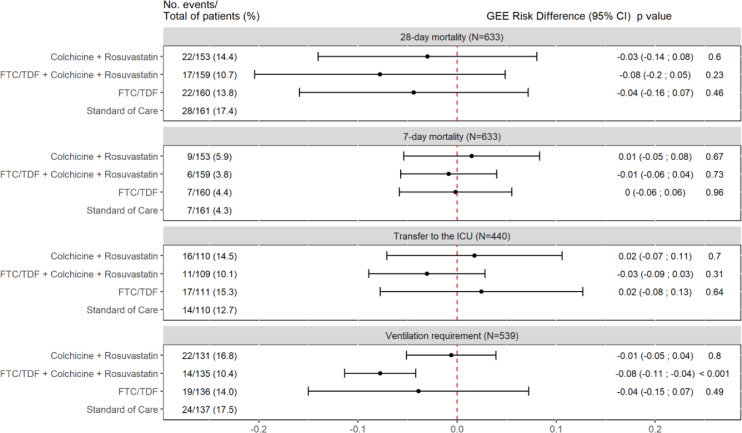
In the FTC/TDF+COLCH+ROSUV group, 28-day mortality was significantly lower than in the SOC group, with a mortality of 17 out of 159 patients (10.7%) and 28 out of 161 patients (17.4%), respectively (HR 0.53; 95% CI 0.29 to 0.96). No differences were found among the FTC/TDF group, with a mortality of 22 out of 153 patients (14.4%), the SOC group (HR 0.68; 95% CI 0.39 to 1.20), the COLCH+ ROSUV group, with a mortality of 22 out of 160 patients (13.8%), and the SOC group (HR 0.78; 95% CI 0.44 to 1.36) (Figure 2). These results are similar to those found with crude models adjusted by age, sex and type of pneumonia (Table 3). Additionally, according to the planned analyses, the absolute effect between the SOC and FTC/TDF+COLCH+ROSUV groups was assessed, showing a trend towards a lower risk of dying for the latter (RD -0.08; 95% CI -0.2 to 0.05). This effect was not shown for the FTC/TDF and COLCH+ROSUV groups versus the SOC (RD -0.04; 95% CI -0.16 to 0.07, and RD -0.03; 95% CI -0.14 to 0.08, respectively) (see Figure 3). These results were similar to those obtained using crude RD and RD adjusted for age, sex and pneumonia severity (see Supplementary Material, Figures S1 and S2). Finally, in the unplanned exploratory analysis based on pneumonia severity, 28-day mortality was lower in the FTC/TDF+COLCH+ROSUV group than in the SOC group in patients with mild-to-moderate pneumonia (RD -0.05; 95% CI -0.07 to -0.04), and, also, in severe pneumonia however this result is imprecise (RD -0.17; 95% CI -0.38 to 0.03) (Figure S3). The sensitivity analysis showed similar results in terms of direction and magnitude of the effect (Figure S4). In terms of safety, three patients with SAEs were identified: 1 patient in the FTC/TDF+COLCH+ROSUV group (severe diarrhoea) and 1 patient in the FTC/TDF group (drug-related exanthema).
Figure 2.
Time to death analysis
Kaplan-Meier plots of the cumulative estimate of the outcome of death from any cause. HR: Hazard Ratio. 95% CI: 95% confidence interval. COLCH: Colchicine. ROSU: Rosuvastatin. FTC/TDF: Emtricitabine/Tenofovir disoproxil fumarate. (A) COLCH+ROSUV compared with Standard of Care, (B) FTC/TDF + COLCH + ROSUV compared with Standard of Care, and (C) FTC/TDF compared with Standard of Care. Hazard Ratios (HR) estimated from shared-frailty (i.e., hospital random-effects) Cox models. The global test of proportional-hazards assumption on the basis of Schoenfeld residuals is chi2=5.76 (p=0.12).
Table 3.
All estimates of time to event analysis
| HR | 95% CI | p-val | |
|---|---|---|---|
| Colchicine + Rosuvastatin | |||
| Crude | 0.839 | (0.480 - 1.466) | 0.537 |
| Frailty | 0.776 | (0.443 - 1.360) | 0.376 |
| Frailty-Adjusted | 0.719 | (0.408 - 1.265) | 0.252 |
| FTC/TDF + Colchicine + Rosuvastatin | |||
| Crude | 0.598 | (0.328 - 1.093) | 0.095 |
| Frailty | 0.527 | (0.288 - 0.964) | 0.038 |
| Frailty-Adjusted | 0.483 | (0.263 - 0.887) | 0.019 |
| FTC/TDF | |||
| Crude | 0.786 | (0.449 - 1.373) | 0.397 |
| Frailty | 0.685 | (0.390 - 1.201) | 0.187 |
| Frailty-Adjusted | 0.605 | (0.343 - 1.065) | 0.081 |
HR: Hazard Ratio. FTC/TDF: Emtricitabine/Tenofovir disoproxil fumarate. Crude: HR estimated from Cox regression models. Frailty: HR estimated from shared-frailty (ie, hospital random-effects) Cox models. Frailty-Adjusted: HR estimated from shared-frailty (i.e., hospital random-effects) Cox models adjusted by age, sex, and pneumonia severity. The global tests of proportional-hazards assumption on the basis of Schoenfeld residuals for each model are: Crude model chi2=5.34 (p=0.15); Frailty model chi2=5.76 (p=0.12); Frailty-Adjusted model chi2=9.74 (p=0.14).
Figure 3.
Secondary Outcomes with Unadjusted Estimates (GEE models)
Unadjusted Risk Differences were estimated using Log-binomial General Estimating Equation (GEE) models, assuming exchangeable correlation structure with each centre as a cluster. ICU: intensive care unit. FTC/TDF: Emtricitabine/Tenofovir disoproxil fumarate. 95% CI: 95% confidence interval. Patients who were in the ICU at the time of randomisation or before are excluded of the analysis of transfer to ICU outcome. Patients who required ventilation at the time of randomisation or before are excluded of the analysis of ventilation requirement outcome.
Secondary outcomes
In terms of the need for invasive mechanical ventilation, it was found to be lower in the FTC/TDF+COLCH+ROSUV group than in the SOC group (RD -0.08; 95% CI -0.11 to -0.04) (Figure 3). This result is consistent with that obtained with the adjusted GEE (see Figure S2). There were no differences between FTC/TDF and the SOC or between COLCH+ROSUV and the SOC in terms of the need for invasive mechanical ventilation. For 7-day mortality and the need for intensive care, no differences were found between the treatment groups and the SOC (Figure 3, and Figures S1 and S2). These results were similar to those obtained when using crude and adjusted estimators (see Supplementary Material, Figures S1 and S2). Finally, there were no differences in median length of stay (Q1-Q3) among the COLCH+ROSUV arm (4.0 days [2.0–9.0]), the FTC/TDF arm (5 days [3–10]), the FTC/TDF+COLCH+ROSUV arm (5.0 days [2.5–8.0]) and the SOC (4.0 days [2.0–9.0]). The most frequent nonserious adverse events are shown in Table 2.
Table 2.
Frequency of non-serious adverse events
| Description of non-serious adverse events* | Total | Emtricitabine/Tenofovir | Colchicine+Rosuvastatin | Emtricitabine/ Tenofovir + Colchicine + Rosuvastatin | Standard of Care |
|---|---|---|---|---|---|
| Gastrointestinal (nausea, diarrhoea, epigastralgia) | 88 | 22 | 24 | 38 | 4 |
| Hepatic (elevation of transaminases, alkaline phosphatase, and bilirubin) | 67 | 15 | 17 | 24 | 11 |
| Non-specific (asthenia, cramps, diaphoresis) | 23 | 6 | 6 | 9 | 2 |
| Neurologic (headache, delirium, seizure episode) | 22 | 5 | 2 | 10 | 5 |
| Cardiovascular (hypertension, bradycardia, atrial fibrillation) | 21 | 6 | 4 | 6 | 5 |
| Renal (kidney injury, hematuria, and increased creatine phosphokinase) | 20 | 7 | 7 | 1 | 5 |
| Hematologic (Anemia, thrombocytopenia, thrombocytosis) | 16 | 2 | 5 | 5 | 4 |
| Allergic (Exanthema, rash, and allergic reaction) | 14 | 5 | 6 | 3 | 0 |
| Metabolic (diabetes, hyperglycemia and cholelithiasis) | 12 | 6 | 2 | 3 | 1 |
| Osteomuscular (contracture, weakness, myalgia) | 6 | 2 | 1 | 3 | 0 |
| Infectious (bacteremia, herpes zoster and catheter site infection) | 5 | 1 | 2 | 0 | 2 |
| Electrolytes (Hyperkalemia and hypokalemia) | 4 | 1 | 2 | 0 | 1 |
| Psychiatric (Anxiety, panic, and psychosis) | 3 | 1 | 0 | 1 | 1 |
| Respiratory (Dyspnea and pneumothorax) | 3 | 1 | 2 | 0 | 0 |
| Otolaryngological (Otorrhagia) | 2 | 1 | 1 | 0 | 0 |
| Dental (Tooth loss) | 1 | 0 | 1 | 0 | 0 |
We do not present information with relative frequencies (i.e., percentages) because some events occurred simultaneously in the same patients; therefore, it is difficult to have a denominator.
In the SUCRA analysis for 28-day mortality, the RD classification from the SUCRA analysis also revealed that the FTC/TDF+COLCH+ROSUV combination had the highest probability of being the best among the four treatment options (SUCRA 76.2%) (Figure S5).
Discussion
This pragmatic randomized controlled trial (RCT) shows that in hospitalized adult patients with COVID-19 diagnosed by RT–PCR or viral antigen testing with mild, moderate, or severe pneumonia and receiving dexamethasone, the FTC/TDF+COLCH+ROSUV combination is associated with a reduced risk of dying within the first 28 days and a lower need for invasive mechanical ventilation than the standard of care with dexamethasone. No differences were found between the treatment with FTC/TDF or COLCH+ROSUV compared with the SOC in terms of the need for transfer to the intensive care unit or mortality within the first 7 days of hospital stay.
No published RCTs testing the use of the FTC/TDF+COLCH+ROSUV combination were found in the literature search in PubMed. One published phase 2 RCT assessing the effect of the FTC/TDF combination in reducing the viral load in subjects with SARS-CoV-2-related nasopharyngeal infection was identified.22 As of 8 August 2021, 2 additional RCTs assessing these drugs were registered on the clinicaltrials.gov website (NCT04712357, NCT04890626). Regarding the use of statins in the treatment of COVID-19, one factorial RCT was carried out to assess the effect of two different doses of statins plus enoxaparin in preventing venous or arterial thrombosis, avoiding treatment with extracorporeal oxygenation membrane (ECMO) or reducing the 30-day mortality, based on the hypothesis that statins would mitigate poor outcomes in some subgroups of patients with hyperinflammatory states, however, those results are not available yet.23 Eight ongoing RCTs that explored the use of statins in this setting were found in clinicaltrials.gov (NCT04472611, NCT04813471, NCT04631536, NCT04348695, NCT04333407, NCT04380402, NCT04466241, and NCT04952350). Moreover, a meta-analysis showed a mortality risk reduction (OR 0.70, 95% CI: 0.55 to 0.88; 21 studies, I2 85%), albeit with a very low level of evidence and a very uncertain estimated effect. A subgroup analysis suggested that the effect was greater in the inpatient group receiving statins compared to prehospital statin use.24
Regarding colchicine, the evidence is conflicting. The COLCORONA-blinded RCT that included 4488 outpatients with COVID-19 showed a reduced incidence of the primary composite outcome of death or hospitalization compared to placebo in patients with positive PCR tests for COVID-19 (odds ratio [OR] 0.75; 95% CI 0.57 to 0,99) but not in the non-confirmed by COVID-19 PCR tests (OR 1.75; 95% CI (0.56–5.46).25 The authors of the GRECO study reported benefits in 105 hospitalized patients with COVID-19 who were treated with colchicine, with a reduction in the number of subjects with clinical deterioration: 7 patients (14%) in the control group and 1 patient (1.8%) in the colchicine group (OR 0.11; 95% CI 0.01 to 0.96).9 The RECOVERY trial with 2363 hospitalized patients with COVID-19 showed no association between the use of colchicine and lower mortality (RR 1.01; 95% CI 0.93 to 1.10) but reported no data on the need for invasive mechanical ventilation.26
Our results suggest a synergistic effect of using medications that act at different key time points in SARS-CoV-2 infection, from viral replication and cytopathic damage to immune hyperactivity resulting in hyperinflammatory states during the advanced stages of the disease due to sudden release of inflammatory mediators, explaining the so-called cytokine storm as a contributing factor to multiple organ damage, thrombosis, complications and death.27, 28, 29
Therefore, the action of the drug combination discussed in this study would begin with emtricitabine/tenofovir disoproxil fumarate, two nucleotide analogues, inhibiting SARS CoV-2 replication,8,30 an effect that has been described by Parienti et al., showing that early use of these antiviral agents reduces the viral load in the upper respiratory airway,22 a reduction that would result in a lower risk of hospitalization and death in patients with COVID-19.31 Besides, TDF has been shown to affect the cytokine profile, favouring the production of IL-12, which would increase the immune response against viral infections.32 The anti-inflammatory action of colchicine has been attributed to its ability to interfere with the inflammatory protein NLRP3 by modulating unregulated cytokine release, particularly IL-1β and IL-6, and inhibiting inflammatory chemokines, cell adhesion molecules, superoxide anion production and mast cell degranulation,33 among other actions that would diminish the severity of the lung injury associated with acute respiratory distress syndrome. Its combination with statins potentiates pleiotropic effects, counteracts endothelial dysfunction, stabilizes atherosclerotic plaques and regulates angiogenesis based on their antifibrotic, antithrombotic and antiviral action.34 This could have a potential effect against the apoptosis and pyroptosis processes that may cause damage to endothelial cells and lead to myocarditis-related manifestations in severe cases of SARS CoV2 infection.35 This effect could explain the separation of the mortality curves between the combined regimen and the SOC after 10 days of treatment for patients who received FTC/TDF (Figure 1).
Our results regarding the association between treatment with FTC/TDF+COLCH+ROSUV and reduced 28-day mortality open a window to continue research with this drug combination. The mortality rate ratio estimate takes into account the change in the mortality risk over time, providing a more accurate estimate than is achieved through mortality proportion and RD. On the other hand, the adjusted Cox regression model takes into consideration the imbalance in the distribution of age, sex, and severity of pneumonia between groups at the entrance to the study (Table 1). Interestingly, in frailty Cox model the estimated effect is very similar and more precise that the crude Cox model. The RD estimate, although imprecise, is stable in terms of effect direction, both in the GEE and adjusted GEE analyses, as well as in the sensitivity analysis, including the 13 patients lost to follow-up (Figures S1, S2 and S3), and the subgroup analysis of patients with moderate pneumonia.
The results in terms of the absolute effect (i.e., RD) are imprecise in this study because the sample size required for effectiveness assessment with 95% confidence was not achieved. This was due, on the one hand, to the high proportion of patients who refused to participate in this study (38%)—a cultural issue that needs to be analysed—and, on the other, to the number of subjects on chronic rosuvastatin use at the time of entering the study (33%), which was an exclusion criterion. Moreover, being an open-label study, it has a high risk of performance bias, accounting for the proportion of non-adherence to treatment, ranging between 18% and 25% in the different study medication groups (Figure 1). However, when considering this bias effect, it points towards a null hypothesis, and therefore, the estimated effect in terms of reduced mortality with the combined treatment is probably valid.
From a pragmatic point of view, the study was carried out in usual hospital conditions of significant workloads and standard medication administration systems, using an ITT approach to the analysis and aiming for the most useful treatment versus the standard of care to help guide clinical decisions. However, the generalization of the results is limited, considering that they are not applicable to chronic statin users.
The combined use of FTC/TDF+COLCH+ROSUV for 14 days reduces the risk of dying and the need for mechanical ventilation in patients with mild-moderate and severe SARS-CoV-2 infection that require hospitalization. These results are highly relevant for medium- and low-income countries with limited resources, considering the lower cost of this treatment compared to other far more expensive alternatives that have also been shown to be effective. More randomized controlled trials are needed to compare the effectiveness and cost of treatment with this combination versus other drugs that have been shown to reduce mortality from SARS-CoV-2 infection and its usefulness in patients with chronic statin use.
Declaration of Competing Interest
HGGD have received funding support for research (MinCiencias and Universidad Nacional de Colombia). CAM have received funding support for research (Pfizer, WHO, PAHO, Abbott and Merck), honoraria for lectures in educational events (Pfizer, Sanofi, Merck, Abbott, Biotoscana, Gilead, Roche), support for attending meeting and/or travel (Institute des Ameriques related to COVID-19), have participated on a Data Safety Monitoring Board or Advisory Board (Sanofi), and have participated in the National Committee of COVID-19 of Colombia and National COVID-19 Vaccines Committee (unpaid activities). JAC have received funding support for research (Pfizer). GB have received funding support for research (MinCiencias, Amgen Inc), and consulting fees (RTS-Baxter). All the other authors have no conflicts to declare.
Acknowledgments
Funding
This project was supported by grant 374–2020 from the Colombian Ministry of Science and Technology to Universidad Nacional de Colombia. Additionally, this was supported by Universidad Nacional de Colombia and Pontificia Universidad Javeriana.
Author's contributions
Hernando G. Gaitán-Duarte: leader researcher, design, contact with external entities, data collection, analysis and interpretation, funding, writing; Carlos Alvarez-Moreno: design, data collection, analysis and interpretation; Carlos Javier Rincon: design, analysis and interpretation, writing; Nancy Yomayusa-González: data collection, analysis and interpretation, writing; Jorge Alberto Cortés: data collection, analysis and interpretation, writing; Juan Carlos Villar: design, data collection; Juan Sebastián Bravo-Ojeda: data collection; Ángel García-Peña: design, data collection; Wilson Adarme-Jaimes: design, data collection; Viviana Rodríguez-Romero: design, analysis and interpretation; Steffany L Villate-Soto: supervisory role; Giancarlo Buitrago: design, data analysis and interpretation, writing; Julio Chacón-Sarmiento: data collection; Martín Macías-Quintero: data analysis; Claudia Patricia Vaca: analysis and interpretation; Carlos Gómez-Restrepo: analysis and interpretation; Nelcy Rodríguez-Malagón: design, data collection, analysis and interpretation.
Data sharing statement
In accordance with the protocol, no investigators will have access to participant data with identifiers. De-identified data will be shared with external investigators upon request. Proposals should be made to the corresponding author, Dr Gaitan-Duarte (hggaitand@unal.edu.co), and will require a data access agreement.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101242.
Appendix. Supplementary materials
References
- 1.World Bank . The World Bank; 2021. Global Economic Prospects, June 2021. [DOI] [Google Scholar]
- 2.Xing K, Tu X-Y, Liu M, et al. Efficacy and safety of COVID-19 vaccines: a systematic review. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23:221–228. doi: 10.7499/j.issn.1008-8830.2101133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siemieniuk RA, Bartoszko JJ, Ge L, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solidarity Trial Consortium WHO, Pan H, Peto R, et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collaborative Group RECOVERY, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitiello A, Ferrara F. Colchicine and SARS-CoV-2: Management of the hyperinflammatory state. Respir Med. 2021;178 doi: 10.1016/j.rmed.2021.106322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minz MM, Bansal M, Kasliwal RR. Statins and SARS-CoV-2 disease: Current concepts and possible benefits. Diabetes Metab Syndr. 2020;14:2063–2067. doi: 10.1016/j.dsx.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien M, Anderson TK, Jockusch S, et al. Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19. J Proteome Res. 2020;19:4690–4697. doi: 10.1021/acs.jproteome.0c00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Permana H, Huang I, Purwiga A, et al. In-hospital use of statins is associated with a reduced risk of mortality in coronavirus-2019 (COVID-19): systematic review and meta-analysis. Pharmacol Rep. 2021;73:769–780. doi: 10.1007/s43440-021-00233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Amo J, Polo R, Moreno S, et al. Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy : A Cohort Study. Ann Intern Med. 2020;173:536–541. doi: 10.7326/M20-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Clin Epidemiol. 2009;62:499–505. doi: 10.1016/j.jclinepi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Saavedra-Trujillo CH. Consenso Colombiano de atención, diagnóstico y manejo de la infección por SARS-COV-2/COVID-19 en establecimientos de atención de la salud: recomendaciones basadas en consenso de expertos e informadas en la evidencia ACIN-IETS. Segunda Edición. Infectio. 2020;24:262–292. [Google Scholar]
- 14.Wilson RM, Runciman WB, Gibberd RW, Harrison BT, Newby L, Hamilton JD. The Quality in Australian Health Care Study. Med J Aust. 1995;163:458–471. doi: 10.5694/j.1326-5377.1995.tb124691.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borba MGS, Val FFA, Sampaio VS, et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vickerstaff V, Omar RZ, Ambler G. Methods to adjust for multiple comparisons in the analysis and sample size calculation of randomised controlled trials with multiple primary outcomes. BMC Med Res Methodol. 2019;19:129. doi: 10.1186/s12874-019-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravel J, Opatrny L, Shapiro S. The intention-to-treat approach in randomized controlled trials: are authors saying what they do and doing what they say? Clin Trials. 2007;4:350–356. doi: 10.1177/1740774507081223. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. A Tutorial on Multilevel Survival Analysis: Methods, Models and Applications. Int Stat Rev. 2017;85:185–203. doi: 10.1111/insr.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. 2020 10.1002/9781119536604 (accessed Aug 18, 2021).
- 21.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parienti J-J, Prazuck T, Peyro-Saint-Paul L, et al. Effect of Tenofovir Disoproxil Fumarate and Emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: A pilot, randomized, open-label phase 2 trial. EClinicalMedicine. 2021 doi: 10.1016/j.eclinm.2021.100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.INSPIRATION Investigators. Sadeghipour P, Talasaz AH, et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vahedian-Azimi A, Mohammadi SM, Heidari Beni F, et al. Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: a systematic review and meta-analysis. Arch Med Sci. 2021;17:579–595. doi: 10.5114/aoms/132950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tardif J-C, Bouabdallaoui N, L'Allier PL, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00222-8. S2213-2600(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RECOVERY Collaborative Group, Horby PW, Campbell M, et al. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Infectious Diseases (except HIV/AIDS), 2021 DOI: 10.1101/2021.05.18.21257267. [DOI] [PMC free article] [PubMed]
- 27.Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370:eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370:eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clososki GC, Soldi RA, da Silva RM, et al. Tenofovir Disoproxil Fumarate: New Chemical Developments and Encouraging in vitro Biological Results for SARS-CoV-2. J Braz Chem Soc. 2020;31:1552–1556. [Google Scholar]
- 31.Néant N, Lingas G, Le Hingrat Q, et al. Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2017962118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melchjorsen J, Risør MW, Søgaard OS, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J Acquir Immune Defic Syndr. 2011;57:265–275. doi: 10.1097/QAI.0b013e3182185276. [DOI] [PubMed] [Google Scholar]
- 33.Perico N, Ostermann D, Bontempeill M, et al. Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation. J Am Soc Nephrol. 1996;7:594–601. doi: 10.1681/ASN.V74594. [DOI] [PubMed] [Google Scholar]
- 34.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol. 2001;21:1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 35.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.