Abstract
Some previous studies have shown that increased stress hormone levels have beneficial effects on memory encoding; however, there is no clear consensus on which encoding-related processes are affected by stress hormones. In the present study, we investigated the relationship between interindividual differences in neuroendocrine response to acute stress and interference resolution (i.e., mnemonic discrimination). Participants were healthy young adults who were exposed to physical and psychological stressors (Socially Evaluated Cold Pressor Test). Then participants completed the modified version of the Mnemonic Similarity Task. Specifically, they were presented with photographs of emotionally arousing (negative and positive) and nonarousing (neutral) scenes followed by a recognition memory test where they saw a mixture of old and new stimuli. Crucially, participants were also presented with critical lure items, that is, visually similar stimuli to ones presented at encoding. We found that participants who had higher cortisol response to the stressors were better in discriminating between the studied items and their visually similar lures. This effect was present for the arousing and nonarousing materials as well. These findings suggest that increased hormonal response to acute stress has a beneficial impact on the formation of distinct, nonoverlapping, unique memory representations, and consequently, on episodic memory encoding processes.
Cortisol effects on hippocampus-related memory functions
Stressful experiences are those situations that are novel, unpredictable, and uncontrollable (Mason 1968). Stress exposure leads to a long line of physiological changes, including the secretion of stress hormones, such as cortisol in humans. Interestingly, there is a great interindividual variability in the way people react to stressors. Some individuals show large increase in cortisol levels, whereas others show little or no such elevation in response to the same stressors (Pruessner et al. 1997; Buchanan and Tranel 2008; Miller et al. 2013). This difference between individuals seems to be especially important, because cortisol secretion is known to affect a wide range of cognitive functions (for review, see Lupien et al. 2007), including learning and memory (for review, see Roozendaal 2002; Wolf 2009; Wingenfeld and Wolf 2014).
It has been demonstrated that cortisol increase before retrieval impairs hippocampus-related memory performance, in particular for emotionally arousing materials (Kuhlmann et al. 2005a,b). For the impact of cortisol on memory encoding, the empirical findings are more controversial. Some studies found that cortisol elevation impairs memory encoding (e.g., Maheu et al. 2005; Payne et al. 2007; Schwabe and Wolf 2010; see also the meta-analysis of Shields et al. 2017), whereas others failed to find any significant relationship between cortisol increase and the encoding of new information (e.g., De Quervain et al. 2000; Maheu et al. 2005; Henckens et al. 2012). There have been studies, however, showing that memory encoding benefits from the elevation of cortisol (Buchanan and Lovallo 2001; Nater et al. 2007; Smeets et al. 2007; Schwabe et al. 2008a; Vogel and Schwabe 2016). These controversial findings indicate that there are crucial factors that can alter the impact of cortisol on memory encoding (for review, see Joëls et al. 2006). For example, Nater et al. 2007 showed that memory encoding is better in those individuals who show larger cortisol response following stress induction (but see Shields et al. 2017). Furthermore, Schwabe et al. (2008a) found that the beneficial effect of cortisol increase on memory encoding is more pronounced for emotionally arousing learning materials (see also Payne et al. 2007; but see Shields et al. 2017).
Opposing effects of cortisol on different memory processes (encoding and retrieval) were further supported by neuroimaging studies reporting reduced activation in the hippocampus during retrieval and increased hippocampal activity at encoding (Weerda et al. 2010; see also Wingenfeld and Wolf 2014). However, enhanced memory encoding is not necessarily associated with increased hippocampal activity as a result of stress-induced cortisol elevation (Henckens et al. 2009). Some studies found that if hippocampal activity is lower following hydrocortisone administration/stress induction, there is no improvement in memory encoding (Henckens et al. 2012; Schwabe and Wolf 2012; but see Henckens et al. 2009; for the effect of stress induction on hippocampal activation, see also Pruessner et al. 2008).
In sum, there have been behavioral and neuroimaging studies that investigated stress/cortisol effects on encoding-related processes. Nevertheless, as there are conflicting results, there is a need to further examine and map the complex relationship between cortisol increase and memory encoding. Specifically, there is a need to systematically investigate different encoding-related processes by using tasks that were developed to assess one specific aspect of memory encoding.
Memory encoding and pattern separation
Strongly related to the encoding of new information, one crucial feature of human memory is the ability of forming distinct, unique memory representations (Tulving 1993; Conway 2009). Relatedly, it has been suggested that a computational mechanism, called pattern separation, plays a key role in the encoding of nonoverlapping memory representations (Yassa and Stark 2011; Hunsaker and Kesner 2013). Specifically, this process is responsible for the reduction of interference effects between sensory inputs that share similar features. A long line of research pointed out that pattern separation is supported by specific subregions of the hippocampus, including the dentate gyrus (DG) and the CA3 (for reviews, see Yassa and Stark 2011; Rolls 2013). In fact, the process of pattern separation is temporally constrained, since the reduction of overlap between sensory inputs occurs at the time of encoding (Hunsaker and Kesner 2013). However, with behavioral methods, there is no opportunity to directly examine pattern separation. Instead, there is a variety of recognition memory tasks developed to assess the behavioral outcome of pattern separation, that is, the retrieval of unique, distinct memory representations.
The Mnemonic Similarity Task (MST) is a frequently used tool to assess the behavioral consequence of putative hippocampal processes enabling pattern separation (e.g., Kirwan et al. 2012; Stark et al. 2013; Stark et al. 2015; Keresztes et al. 2017; for review, see Stark et al. 2019). In the test phase of this task, participants are shown studied old stimuli, unstudied new stimuli (targets and foils, respectively), and critical lure items as well that are visually similar images to ones presented at encoding. A frequently used measure for assessing the behavioral outcome of pattern separation is the so-called Lure Discrimination Index (or Mnemonic Discrimination Index), that is, when one correctly identifies a lure stimulus as “Similar.” This index is more sensitive to hippocampal integrity as compared with the correct recognition of a studied old item. Specifically, the selective damage of the DG impairs lure discrimination (Baker et al. 2016), hippocampal maturity (including the volume of the DG/CA3) correlates with lure discrimination performance in children (Keresztes et al. 2017), DG/CA3 activity and the volumetric decrease of these regions are associated with impaired lure discrimination in healthy older adults (Yassa et al. 2011; Doxey and Kirwan 2015; Stark and Stark 2017) and in pathological ageing (Yassa et al. 2010).
Motivation and aims
In brief, there have been studies showing that cortisol administration (Buchanan and Lovallo 2001) and acute stress-induced elevation of cortisol levels (Nater et al. 2007; Schwabe et al. 2008a) have beneficial impacts on declarative memory encoding. In accordance with these behavioral findings, the results of some neuroimaging studies show that cortisol increase is associated with increased hippocampal activity at the encoding of new information (Weerda et al. 2010). Computational models of the hippocampal formation suggest that the hippocampus is essential in domain-general pattern separation (Yassa and Stark 2011; Rolls 2013) and that pattern separation is temporally constrained, since it occurs during memory encoding and not at retrieval (Hunsaker and Kesner 2013). Based on these observations it seems plausible that cortisol affects memory encoding by facilitating pattern separation in the hippocampus.
Mnemonic discrimination is the behavioral outcome of pattern separation, that is, when one becomes able to discriminate between a previously studied item and its visually similar lure (Stark et al. 2019). Therefore, we can expect a positive relationship between mnemonic discrimination and cortisol response. This assumption is in line with the idea of Roozendaal (2002) who raised the possibility that cortisol facilitates memory encoding and consolidation processes by reducing interference effects between overlapping information. Our aim was to test this idea by investigating the relationship between interindividual differences in cortisol response to acute stress and mnemonic discrimination performance.
Cortisol effects on memory is more prominent for emotionally arousing as compared with nonarousing stimuli (Buchanan and Lovallo 2001; Kuhlmann et al. 2005a,b; Schwabe et al. 2008a). Therefore, we used a modified version of the MST (Leal et al. 2014; see also Szőllősi and Racsmány 2020) that uses nonarousing (neutral) and arousing (negative and positive) stimuli. At first, participants were exposed either to physical and psychological stressors in laboratory settings (Socially Evaluated Cold Pressor Test) (see Schwabe et al. 2008b) or to a nonstressful control task (for the procedure of the experimental session, see Fig. 1A). Then all participants were presented with images of negative, positive, and neutral scenes, followed by a recognition memory test. In the test phase, within each of the three conditions (negative, positive, and neutral), there were studied old stimuli as well as unstudied new stimuli, and critical lure items as well (see Fig. 1B). We expected a higher “Similar” response ratio for the lures as a result of cortisol increase following stress induction.
Figure 1.
The procedure of the experimental session (A) and the emotional memory task (B). (S1) Saliva sample 1 (minute 0), (S2) saliva sample 2 (minute 20), (S3) saliva sample 3 (minute 40), (MST) Mnemonic Similarity Task, (targets) exact repetitions of images presented at encoding, (lures) visually similar images to ones presented at encoding, (foils) completely new images not presented at all before.
In addition to the short-term effects of cortisol increase on memory performance, there is a more general relationship between cortisol reactivity and memory functions (for review, see Kim and Diamond 2002). There is a difference between individuals with and without higher cortisol reactivity in memory performance not only at the time of cortisol increase or shortly after that. Moreover, this relationship is shown to be present not only in patient populations, but also in healthy young adults (see, e.g., Szőllősi et al. 2017). Accordingly, there is a relation between cortisol reactivity and the volume of the hippocampus (e.g., Lindauer et al. 2006), including the CA3 (McEwen 2000) that is thought to play an important role in pattern separation (Yassa and Stark 2011). Therefore, we tested participants in another (control) session when no stress induction occurred. Participants performed the original version of the MST (Stark et al. 2019) and two other tasks that were developed to measure cognitive functions known to contribute to mnemonic discrimination (see Ly et al. 2013; Ngo et al. 2021). Specifically, participants performed a perceptual discrimination task and a verbal recognition memory task. We aimed at analyzing the relationship between performance in this control session (when no stress induction occurred) and the magnitude of cortisol response to the stress induction (in the experimental session).
Although we randomly assigned the participants into one of the experimental groups, the other rationale for this control session was to compare the two experimental groups when no stress induction occurred. We aimed to verify that there was no group difference in processes that are known to contribute to mnemonic discrimination. Since it has been demonstrated that there is a relationship between cortisol reactivity and symptom severity of depression (e.g., Burke et al. 2005), anxiety (e.g., Furlan et al. 2001), and posttraumatic stress (e.g., Steudte-Schmiedgen et al. 2015), we aimed to verify that there is no difference between the groups in levels of symptoms of depression, anxiety, and posttraumatic stress. Therefore, participants completed a set of mood questionnaires at the end of the control session.
Results
Control session
We found no significant differences between the groups in the three control tasks (the MST, the perceptual discrimination task, and the verbal recognition memory task) in the control session (when no stress induction occurred). There were no significant group differences in scores of the mood questionnaires either (Beck Depression Inventory [BECK], the trait form of the State-Trait Anxiety Inventory [STAI-Trait], and the civilian version of the Posttraumatic Stress Disorder Checklist [PCL-C]). The descriptive statistics and the results of the comparative statistics are shown in Table 1. (Although there was no stress induction in this session, we continue to refer to the two experimental groups as the stress and control groups.)
Table 1.
Performance (percentage) in the control tasks and scores of the mood questionnaires: comparison between the experimental groups
For cortisol levels in the control session, we found no significant main effects of Group (F(1,70) = 0.658, P = 0.420, ηp2 = 0.009) and Time (F(2,140) = 2.545, P = 0.082, ηp2 = 0.035). The interaction between these variables was also not significant (F(2,140) = 0.655, P = 0.521, ηp2 = 0.009). The descriptive statistics for cortisol levels are seen in Table 2 for the two groups separately. Altogether, this pattern of findings indicates that the two experimental groups did not differ in cognitive performance known to contribute to mnemonic discrimination (see Ly et al. 2013; Ngo et al. 2021), in levels of symptoms of depression, trait anxiety, and posttraumatic stress, as well as in salivary cortisol levels when no stress induction occurred.
Table 2.
Cortisol levels at minute 0 (baseline), minute 15, and minute 30 in the control session
Validation of the stress induction: cortisol levels
For cortisol levels in the experimental session (see Table 3), the ANOVA indicated a significant main effect of Time (F(2,140) = 70.789, P < 0.001, ηp2 = 0.503) and a significant Stress × Time interaction (F(2,140) = 76.943, P < 0.001, ηp2 = 0.524). The main effect of Stress was not significant (F(1,70) = 3.738, P = 0.057, ηp2 = 0.051).
Table 3.
Cortisol levels at minute 0 (baseline), minute 20 (15 min after the stress/control task), and minute 40 (at the end of the session) in the experimental session
Participants in the stress group had higher cortisol levels at minute 20 (F(1,35) = 105.779, P < 0.001, ηp2 = 0.751) and minute 40 (F(1,35) = 24.711, P < 0.001, ηp2 = 0.414), as compared with the baseline (minute 0). There was no significant cortisol increase (as compared with the baseline) in the control group either at minute 20 (F(1,35) = 0.859, P = 0.360, ηp2 = 0.024) or at minute 40 (F(1,35) = 1.104, P = 0.301, ηp2 = 0.031).
At minute 20 (15 min after the stress/control task), cortisol level was higher in the stress group than it was in the control group (t(70) = 4.450, P < 0.001, d = 1.064). Baseline cortisol levels did not differ between the groups (t(70) = 0.647, P = 0.520, d = 0.155), and there was no significant group difference at minute 40 either (t(70) = 1.311, P = 0.194, d = 0.313). It should be also highlighted that only one participant did not show a cortisol response following the stress induction. Altogether these results confirm the success of the stress induction (i.e., the Socially Evaluated Cold Pressor Test).
Memory performance: the emotional Mnemonic Similarity Task
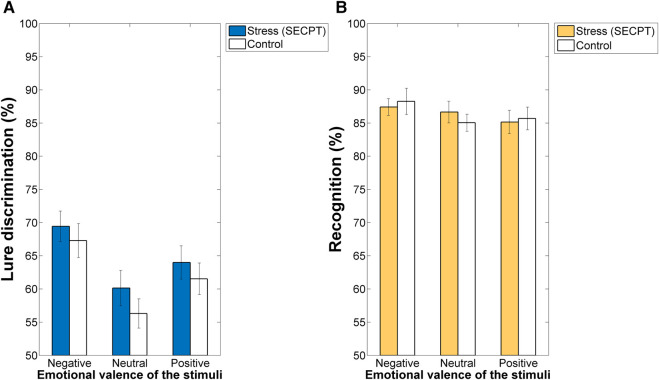
For lure discrimination performance (see Fig. 2A), the ANOVA revealed a significant main effect of Valence (F(2,140) = 23.289, P < 0.001, ηp2 = 0.250). The post hoc analyses showed that lure discrimination was better for the negative stimuli as compared with the neutral (F(1,70) = 47.694, P < 0.001, ηp2 = 0.405) and positive (F(1,70) = 12.846, P < 0.01, ηp2 = 0.155) conditions. Additionally, lure discrimination was better for the positive images than it was for the neutral stimuli (F(1,70) = 10.059, P < 0.01, ηp2 = 0.126). The main effects of Stress (F(1,70) = 0.880, P = 0.351, ηp2 = 0.012) and the Stress × Valence interaction (F(2,140) = 0.184, P = 0.832, ηp2 = 0.003) were not significant.
Figure 2.
Memory performance for the emotional and neutral stimuli. Note(s). Lure discrimination was sensitive to the emotional arousal of the stimuli (negative > positive > neutral; all Ps < 0.01) in both groups (A), whereas standard recognition memory hit rate was not (all Ps > 0.05) (B). (SECPT) Socially Evaluated Cold Pressor Test. Error bars indicate the standard errors of the mean.
For standard recognition memory performance (see Fig. 2B), no significant main effects of Valence (F(2,140) = 1.911, P = 0.152, ηp2 = 0.027) and Stress (F(1,70) = 0.002, P = 0.966, ηp2 = 2.6 × 10−5) were found. The Stress × Valence interaction was also not significant (F(2,140) = 0.515, P = 0.599, ηp2 = 0.007).
Finally, we found significant positive correlations between cortisol response and lure discrimination in the stress group in each of the three conditions (Fig. 3): negative (r(36) = 0.329, P < 0.05, 95% CI = [0.001, 0.593]), neutral (r(36) = 0.372, P < 0.05, 95% CI = [0.050, 0.624]), and positive (r(36) = 0.416, P < 0.05, 95% CI = [0.102, 0.655]). There was no significant correlation between cortisol response and the standard recognition memory hit rate: negative (r(36) = 0.014, P = 0.936, 95% CI = [−0.315, 0.340]), neutral (r(36) = 0.054, P = 0.754, 95% CI = [−0.279, 0.375]), and positive (r(36) = 0.117, P = 0.498, 95% CI = [−0.219, 0.429]).
Figure 3.
The relationship between cortisol response and lure discrimination for the negative (A), neutral (B), and positive stimuli (C). Linear regression lines (and 95% confidence intervals of the regression lines) are indicated.
Relationship between cortisol response and performance in the control tasks
We analyzed the relationship between the magnitude of cortisol response (in the experimental session) and performance in the control tasks (in the control session). Cortisol response did not correlate with the ratio of “Old” responses given to the targets (in the original version of the MST, r(36) = 0.210, P = 0.218, 95% CI = [−0.127, 0.504]; in the perceptual discrimination task, rs(36) = −0.245, P = 0.151, 95% CI = [−0.530, 0.091]; and in the word recognition memory task, r(36) = 0.256, P = 0.132, 95% CI = [−0.080, 0.539]). Cortisol response did not correlate with the ratio of “Similar” responses given to the lures either (in the original version of the MST, r(36) = 0.042. P = 0.806, 95% CI = [−0.290, 0.366], and in the perceptual discrimination task, rs(36) = −0.034, P = 0.844, 95% CI = [−0.358, 0.298]).
Additional analyses
We collected data on whether female participants were on hormonal contraceptives. There were 25 female participants in the stress group and only three participants were on contraceptives. It has been shown that there is a relationship between oral contraceptive medication and cortisol response to acute stress induction (e.g., Kirschbaum et al. 1995); therefore, we reanalyzed our data. Specifically, we excluded these three participants from the sample, and then we reanalyzed the relationship between cortisol response and lure discrimination performance in the emotional memory task. The significant results remained significant, that is, there was a positive linear correlation between cortisol response and lure discrimination in each of the three valence conditions: negative (r(33) = 0.423, P = 0.014, 95% CI = [0.093, 0.669]), neutral (r(33) = 0.354, P = 0.043, 95% CI = [0.012, 0.622]), and positive (r(33) = 0.465, P = 0.006, 95% CI = [0.145, 0.697]).
Since it has been demonstrated that there is a difference between females and males in cortisol response following the Socially Evaluated Cold Pressor Test (see Schwabe and Schächinger 2018), we compared the magnitude of cortisol response between female (M = 9.8 nmol/L, SE = 1.8) and male participants (M = 9.9 nmol/L, SE = 1.2). We found no significant group difference in our sample (t(34) = 0.029, P = 0.977, d = 0.010).
Finally, we analyzed the relationship between indices in the neutral condition of the emotional MST and the control tasks (i.e., hit rate: the ratio of “Old” responses given to the targets; lure discrimination: the ratio of “Similar” responses given to the lures), and we found significant correlations between the MSTs for both indices (r(72) = 0.343, P = 0.003, 95% CI = [0.121, 0.532]; r(72) = 0.425, P < 0.001, 95% CI = [0.214, 0.598]), respectively. However, the analysis did not show a significant correlation between the emotional MST and the perceptual discrimination task: rs(72) = 0.191, P = 0.109, 95% CI = [−0.043, 0.404] (hit rate), and rs(72) = 0.205, P = 0.085, 95% CI = [−0.028, 0.416] (lure discrimination).
Discussion
The aim of the present study was to investigate the relationship between interindividual differences in cortisol response to acute stress and hippocampus-related memory encoding processes. Specifically, we examined the formation of distinct, nonoverlapping memory representations. For this purpose, we used a modified recognition memory task that allows the examination of mnemonic discrimination, that is, the behavioral outcome of pattern separation.
Our first major finding was that mnemonic discrimination was better for the emotional stimuli than it was for the neutral images. Additionally, mnemonic discrimination was better for the negative stimuli than it was for the positive images. We found exactly the same pattern of results in one of our previous works, and we showed that this effect was due to arousal and not the valence of the study material (Szőllősi and Racsmány 2020). These findings are in line with studies showing that memory is better for stimuli that evoked arousal (e.g., Cahill and McGaugh 1995; Kensinger and Corkin 2004) and that interference resolution benefits from emotional arousal (Levens and Phelps 2008; Levens et al. 2011). In the present study, we extended these findings by demonstrating that mnemonic discrimination was sensitive to emotional arousal not only in the control condition but also in stressed participants.
Previous studies have shown that there is a great interindividual variability in cortisol response to stress (see Dedovic et al. 2009; Miller et al. 2013) and that the magnitude of this response is associated with memory performance (e.g., Nater et al. 2007; Schwabe et al. 2008a). In accordance with these results, our second major finding is the positive linear relationship between mnemonic discrimination performance and cortisol response in stressed participants. Moreover, we found a dissociation; while mnemonic discrimination correlated with cortisol response, standard recognition memory hit rate (the correct recognition of studied old items) did not.
For the relationship between cortisol increase and memory encoding, the empirical findings are fairly controversial. There have been studies reporting beneficial cortisol effects on memory encoding (e.g., Buchanan and Lovallo 2001; Domes et al. 2002; Nater et al. 2007; Smeets et al. 2007; Schwabe et al. 2008a), whereas others found no such a relationship (e.g., De Quervain et al. 2000; Maheu et al. 2005; Henckens et al. 2012). Based on this line of research and the results of the present study (the dissociation of cortisol effects on mnemonic discrimination and correct recognition), we can assume that cortisol affects memory encoding when the task requires interference resolution. This assumption is in accordance with the idea of Roozendaal (2002) who raised the possibility that cortisol might facilitate memory encoding (and consolidation) process(es) by reducing interference effects between overlapping information. A previous study corroborated this idea by showing improved interference resolution (in a working memory task) as a result of hydrocortisone administration (Oei et al. 2009).
It should be also noted that the measure of mnemonic discrimination is more sensitive to hippocampal integrity as compared with the correct recognition of studied old items (Stark et al. 2013; Stark et al. 2015; Keresztes et al. 2017). Furthermore, a series of studies have demonstrated that there is a relationship between cortisol increase and hippocampal functioning (for review, see Wingenfeld and Wolf 2014). Specifically, cortisol increase is shown to be associated with increased hippocampal activation during memory encoding (Weerda et al. 2010). Therefore, it can be assumed that cortisol selectively facilitates hippocampus-related memory processes during encoding (or at least those processes that are associated with specific subregions of the hippocampus, including the DG and the CA3) resulting in better mnemonic discrimination together with no change in correct recognition. The results of previous neuroimaging findings are controversial regarding the relationship between hippocampal activity and stress/cortisol effects on memory encoding (Henckens et al. 2009, 2012; Schwabe and Wolf 2012). There is a need for future studies to further investigate this question with a focus on specific subregions and not the whole hippocampus. It seems plausible that cortisol increase has opposing effects on different hippocampal subregions, and consequently, has opposing effects on different encoding-related memory processes.
Our further important result is the lack of significant correlation between cortisol response (in the experimental session when stress induction occurred) and performance in the control session (when no stress induction occurred in either groups). In the control session, participants completed the original version of the MST (Stark et al. 2019) and two other control tasks that were developed to measure cognitive functions known to contribute to lure discrimination (see Ly et al. 2013; Ngo et al. 2021). These control tasks were a perceptual discrimination task and a verbal recognition memory task. The lack of significant correlation between performance in these control tasks and cortisol response suggests no general relationship between cortisol reactivity and cognitive performance in our sample. Instead, the elevation of cortisol had an exclusive short-term effect on lure discrimination performance in the experimental session shortly after the stress induction. Importantly though, task difficulty is a crucial factor (see, e.g., Pishdadian et al. 2020), and maybe the perceptual discrimination task we used was not sensitive enough to detect differences in a sample of healthy, young adults. Along with other authors, we believe that there is a need to develop tasks that are sensitive to perceptual discrimination abilities in different populations.
Importantly, a recent study also investigated mnemonic discrimination following stress induction (Jiang et al. 2019). The authors used the long-term variant of the MST with a 24-h retention interval between study and test. Stress induction occurred either after the encoding phase or before retrieval. There were only emotionally neutral stimuli (photographs of everyday objects) in this study. Cortisol elevation showed a positive linear relationship with mnemonic discrimination performance only in that condition when the encoding was followed by stress induction. Continuing this line of research, we found better mnemonic discrimination performance as a result of cortisol increase when stress induction occurred 15 minutes before encoding. It should be highlighted that the encoding phase was immediately followed by the recognition memory test in our study. Therefore, this aspect of the study design makes it difficult to conclude whether cortisol elevation affected encoding-, consolidation-, or retrieval-related processes. However, the findings of Jiang et al. (2019) and our results together make it probable that cortisol promotes the encoding and consolidation of distinct, nonoverlapping representations, whereas it has no effect on retrieval processes in the MST.
In relation with memory consolidation processes, the findings of some animal and human studies indicate that increased glucocorticoid levels have beneficial effects on memory generalization (e.g., Bahtiyar et al. 2020; for review, see Roozendaal and Mirone 2020). In fact, generalization is related to another computational process of the hippocampus (and not to the process of pattern separation), pattern completion, which refers to the process when memories turn into accessible in response to partial/degraded cues (Hunsaker and Kesner 2013; Rolls 2013; Keresztes et al. 2018). Importantly, pattern separation and pattern completion are suggested to intimately interact with each other, since one must retrieve a previously perceived stimulus when an overlapping item is presented to detect the differences between them (see, e.g., Kirwan and Stark 2007). Therefore, it seems possible that glucocorticoids support both pattern separation and completion.
As a final remark, there are examples of studies showing that cortisol effects on memory is more prominent for emotional stimuli (Buchanan and Lovallo 2001; Kuhlmann et al. 2005a,b) and that this effect is due to increased arousal and not the valence of the study material (Kuhlmann et al. 2005b). In the present study, the relationship between cortisol response and mnemonic discrimination was present for the arousing and nonarousing stimuli as well. This finding and the results of Jiang et al. (2019) suggest that cortisol effects on memory encoding and consolidation processes can be extended to nonarousing stimuli at least when the task requires the discrimination between overlapping information.
Conclusions
In sum, we have shown that cortisol improves hippocampus-related memory encoding processes related to interference resolution. The encoding of nonoverlapping representations is better for stimuli that evoke arousal. Furthermore, the encoding of nonoverlapping representations is better when one shows a large increase of cortisol levels as the consequence of stress exposure. Future studies are needed, however, to specify the boundary conditions and the exact mechanisms of this relationship, such as the role of different (physical, psychological) stressors and the contribution of noradrenergic activity in addition to cortisol. Also, there is a need to include further measures to assess stress-reactivity (e.g., blood pressure, pulse, and affective states) and to investigate sex differences.
Materials and Methods
Participants
Required sample size was calculated on the basis of a pilot study (n = 18). We focused on group comparisons (stress vs. control) and on the main indices of the emotional memory task (i.e., lure discrimination index in each of the three conditions). We found the lowest effect size value for the negative condition of the task. Based on these parameters, we used G*Power (version 3.1.9.2) (see Faul et al. 2007) to calculate required sample size with an α error probability of 0.05 and a power of 0.95. Based on the output parameters, the required sample size was n = 76. Expecting some drop out, we invited 80 individuals to participate in the study, 72 of them volunteered to participate.
Participants were 72 undergraduate students with no history of psychiatric and neurological disorders or any other chronic medical problems. Participants all had normal or corrected-to-normal vision. Participants were randomly assigned to either the stress group (n = 36, 25 women; Mage = 21.3 yr, SE = 0.3) or the control group (n = 36, 27 women; Mage = 22.3 yr, SE = 0.7).
Since menstrual cycle phase is shown to influence cortisol response to laboratory-based stress induction (see, e.g., Montero-López et al. 2018), we checked for those female participants whose regular menstrual cycles were within the range of 26–35 d when the stress induction occurred. No participant met this criterion.
The study was approved by the Hungarian United Ethical Review Committee for Research in Psychology and was carried out in accordance with the code of ethics for human experiments (Declaration of Helsinki). All participants gave written informed consent. They received either extra course credit or money for participation. Compensation was divided equally between the two experimental groups.
General procedure: control session
All participants were tested in a control session. At first, they completed the original version of the MST that uses only nonarousing stimuli (Stark et al. 2019). Additionally, they completed two other control tasks measuring cognitive functions known to affect mnemonic discrimination performance (Ly et al. 2013; Ngo et al. 2021). These tasks were a perceptual discrimination task and a word recognition memory task. Finally, the levels of symptoms of trait anxiety, depression, and posttraumatic stress were assessed by three questionnaires (BECK, STAI-Trait, and PCL-C). Saliva samples were collected three times during this session. The samples were collected at the beginning of the session, and then 15 and 30 min after the beginning. For the detailed description of the procedure of this session and data analysis, see Supplemental Material S1.
General procedure: experimental session
The experimental session started with the stress induction in the stress group and with the control version of this task in the control group. It was followed by a 15-min delay (while participants were given magazines to read) in order to reach the cortisol peak (in the stress group) or at least to get close to it (Kirschbaum and Hellhammer 1994). Following the delay, participants completed the emotional MST.
To eliminate the effect of circadian-dependent change in cortisol levels (Clow et al. 2010), the experiment was run between 12:00 p.m. and 5:00 p.m. The level of cortisol can be affected by several external factors including physical exercise and smoking (Kirschbaum and Hellhammer 1994), alcohol intake (Badrick et al. 2008), and caffeine intake, as well as meals (Lovallo et al. 2006). Therefore, participants were asked to abstain from these activities 2 h prior to the experiment.
Stress induction
At the beginning of the experimental session, participants in the stress group performed the Socially Evaluated Cold Pressor Test (Schwabe et al. 2008b), which was developed to induce acute combined (physical and psychosocial) stress in laboratory settings. As a physical stress factor, participants were instructed to keep their nondominant hand in ice-cold (0°C–3°C) water for 3 min. As a psychosocial stress factor, participants performed the task in front of a (female) observer who monitored their behavior with no feedback. Although participants were told that video recording would be taken for later analyzing their behavior, no recording was actually made. Participants were informed that they could have removed their hand from the water if the procedure would be too painful or uncomfortable.
There were no stress factors (cold water, observer, and camera) in the control group. Control participants were asked to put their nondominant hand into warm water (35°C–37°C) for three minutes. Although the (female) experimenter stayed in the experimental room, contrary to the stress condition, this time she did not observe participants’ behavior.
To assess cortisol levels, saliva samples were collected at the beginning of the experimental session, and then 20 and 40 min after the beginning. The first two samples were collected immediately before and 15 min after the stress/control task. The last sample was collected immediately after the emotional MST (at the end of the experimental session). (We expected a difference in cortisol levels between the groups at minute 20.) The samples were collected using Eppendorf Safe-Lock Tubes (1.5 mL) and were kept at −8°C until the analysis. Salivary cortisol levels were measured by immunoassay kits (Salimetrics, LLC). Intra- and interassay coefficients of variation were 1.6% and 1.4%, respectively.
The emotional mnemonic similarity task
We used the stimulus set of Leal et al. (2014) to make our findings and the results of previous studies more comparable. The stimuli were color photographs of scenes. There were three stimulus types: negative, neutral, and positive images. For stimulus validation (subjective ratings of emotional valence/arousal level as well as similarity level between targets and the corresponding lure images), see Leal et al. (2014) and Szőllősi and Racsmány (2020).
The task consisted of an incidental encoding phase and a recognition memory test. In the encoding phase, participants saw 156 images in the middle of the computer screen on a white background (2500 msec/stimulus, prestimulus interval [PSI] = 500 msec). There were 52 negative, 52 neutral, and 52 positive images. Participants were instructed to rate the emotional valence of the stimuli on a nine-point scale (where 1 = “Negative” and 9 = “Positive”). The response options (i.e., the scale) remained at the bottom of the screen for the duration of the encoding phase. The encoding phase was preceded by 10 practice trials with four negative, two neutral, and four positive images.
The encoding phase was followed by a surprise memory test while participants were presented with 234 images (2500 msec/stimulus, PSI = 500 msec). A 3 × 3 experimental design was used with Valence and Stimulus type as within-subject independent factors. Specifically, participants saw negative, neutral, and positive images; within each valence condition, there were 26 targets, 26 lures, and 26 foils. Targets were exact repetitions of images presented at encoding, whereas foils were completely new items not presented at all before. Crucially, lure items were visually similar images to ones presented at encoding. For each stimulus (presented at encoding) either a target image or corresponding lure images was presented in the test phase. Participants’ task was to make “Old”/“Similar”/“New” decisions. The response options (F = “Old,” H = “Similar,” and K = “New”) remained at the bottom of the screen for the duration of the memory test.
Data analysis
For statistical analyses we used the Matlab computing environment (version 2014a, The MathWorks, Inc.). To verify the success of the stress induction, salivary cortisol levels were analyzed by conducting a 2 × 3 mixed-design ANOVA with Stress (stress and control) as a between-subjects variable and Time (minute 0, minute 20, and minute 40) as a within-subject factor. Sphericity and distributions of the residuals for normality were tested. As post hoc tests, a list of simple contrast analyses was conducted. Cortisol levels were compared between the groups by conducting independent samples t-tests.
For the emotional MST, two indices were calculated: a standard recognition memory hit rate (i.e., the ratio of “Old” responses given to the targets) and a Lure Discrimination Index (i.e., the ratio of “Similar” responses given to the lures). For both indices, a 2 × 3 mixed-design ANOVA was conducted with Stress (stress and control) as a between-subjects variable and Valence (negative, neutral, and positive) as a within-subject factor. As post hoc tests, simple contrast analyses were conducted.
For participants in the stress group, a cortisol response value was determined by calculating the difference between cortisol levels at minute 20 (15 min after the stress/control task) and minute 0 (baseline). Since it has been demonstrated that there is a difference between females and males in cortisol response following the Socially Evaluated Cold Pressor Test (see Schwabe and Schächinger 2018), we compared the magnitude of cortisol response between females and males by conducting an independent-samples t-test. A series of Pearson's correlation analyses was conducted between this cortisol response value and memory performance in the emotional MST (i.e., standard recognition memory hit rate and lure discrimination performance in the three valence conditions). It has been shown that there is a relationship between oral contraceptive medication and cortisol response to acute stress induction (e.g., Kirschbaum et al. 1995); therefore, we conducted an additional analysis. Specifically, we excluded those participants from the sample who were on hormonal contraceptives (n = 3), and then we reanalyzed the relationship between cortisol response and lure discrimination performance in the emotional memory task by conducting Pearson's correlation analyses. Finally, we analyzed the relationship between the magnitude of cortisol response (in the experimental session) and performance in the control tasks (in the control session) by conducting a list of Pearson's/Spearman's correlation analyses.
Supplementary Material
Acknowledgments
This work was supported by the 2017-1.2.1-NKP-2017-00002 research grant (National Brain Research Program, Hungary) and by the National Research, Development, and Innovation Office K124098 research grant. Á.S. was supported by the New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (ÚNKP-20-4-II-BME-13). We thank Anita Lencsés, Zsófia Miklós, and Edina Török for the help in data collection. We thank Dorottya Bencze for the help in data analysis and preparing the figures.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053452.121.
References
- Badrick E, Bobak M, Britton A, Kirschbaum C, Marmot M, Kumari M. 2008. The relationship between alcohol consumption and cortisol secretion in an aging cohort. J Clin Endocrin Metab 93: 750–757. 10.1210/jc.2007-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahtiyar S, Karaca KG, Henckens MJ, Roozendaal B. 2020. Norepinephrine and glucocorticoid effects on the brain mechanisms underlying memory accuracy and generalization. Mol Cell Biol 108: 103537. 10.1016/j.mcn.2020.103537 [DOI] [PubMed] [Google Scholar]
- Baker S, Vieweg P, Gao F, Gilboa A, Wolbers T, Black SE, Rosenbaum RS. 2016. The human dentate gyrus plays a necessary role in discriminating new memories. Curr Biol 26: 2629–2634. 10.1016/j.cub.2016.07.081 [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Lovallo WR. 2001. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology 26: 307–317. 10.1016/S0306-4530(00)00058-5 [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D. 2008. Stress and emotional memory retrieval: effects of sex and cortisol response. Neurobiol Learn Mem 89: 134–141. 10.1016/j.nlm.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. 2005. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology 30: 846–856. 10.1016/j.psyneuen.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. 1995. A novel demonstration of enhanced memory associated with emotional arousal. Conscious Cogn 4: 410–421. 10.1006/ccog.1995.1048 [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. 2010. The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev 35: 97–103. 10.1016/j.neubiorev.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Conway MA. 2009. Episodic memories. Neuropsychologia 47: 2305–2313. 10.1016/j.neuropsychologia.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, Pruessner JC. 2009. The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage 47: 864–871. 10.1016/j.neuroimage.2009.05.074 [DOI] [PubMed] [Google Scholar]
- De Quervain D, Roozendaal B, Nitsch R, McGaugh J, Hock C. 2000. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci 3: 313–314. 10.1038/73873 [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Reichwald U, Hautzinger M. 2002. Hypothalamic–pituitary–adrenal axis reactivity to psychological stress and memory in middle-aged women: high responders exhibit enhanced declarative memory performance. Psychoneuroendocrinology 27: 843–853. 10.1016/S0306-4530(01)00085-3 [DOI] [PubMed] [Google Scholar]
- Doxey CR, Kirwan CB. 2015. Structural and functional correlates of behavioral pattern separation in the hippocampus and medial temporal lobe. Hippocampus 25: 524–533. 10.1002/hipo.22389 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- Furlan PM, DeMartinis N, Schweizer E, Rickels K, Lucki I. 2001. Abnormal salivary cortisol levels in social phobic patients in response to acute psychological but not physical stress. Biol Psychiatry 50: 254–259. 10.1016/S0006-3223(00)01126-4 [DOI] [PubMed] [Google Scholar]
- Henckens MJ, Hermans EJ, Pu Z, Joëls M, Fernández G. 2009. Stressed memories: how acute stress affects memory formation in humans. J Neurosci 29: 10111–10119. 10.1523/JNEUROSCI.1184-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, Pu Z, Hermans EJ, van Wingen GA, Joëls M, Fernández G. 2012. Dynamically changing effects of corticosteroids on human hippocampal and prefrontal processing. Hum Brain Mapp 33: 2885–2897. 10.1002/hbm.21409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Kesner RP. 2013. The operation of pattern separation and pattern completion processes associated with different attributes or domains of memory. Neurosci Biobehav Rev 37: 36–58. 10.1016/j.neubiorev.2012.09.014 [DOI] [PubMed] [Google Scholar]
- Jiang A, Tran TT, Madison FN, Bakker A. 2019. Acute stress-induced cortisol elevation during memory consolidation enhances pattern separation. Learn Mem 26: 121–127. 10.1101/lm.048546.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. 2006. Learning under stress: how does it work? Trends Cog Sci 10: 152–158. 10.1016/j.tics.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. 2004. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci 101: 3310–3315. 10.1073/pnas.0306408101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes A, Bender AR, Bodammer NC, Lindenberger U, Shing YL, Werkle-Bergner M. 2017. Hippocampal maturity promotes memory distinctiveness in childhood and adolescence. Proc Natl Acad Sci 114: 9212–9217. 10.1073/pnas.1710654114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes A, Ngo CT, Lindenberger U, Werkle-Bergner M, Newcombe NS. 2018. Hippocampal maturation drives memory from generalization to specificity. Trends Cog Sci 22: 676–686. 10.1016/j.tics.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. 2002. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci 3: 453–462. 10.1038/nrn849 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. 1994. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 19: 313–333. 10.1016/0306-4530(94)90013-2 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. 1995. Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology 20: 509–514. 10.1016/0306-4530(94)00078-O [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. 2007. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learn Mem 14: 625–633. 10.1101/lm.663507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan C, Hartshorn A, Stark SM, Goodrich-Hunsaker NJ, Hopkins RO, Stark CEL. 2012. Pattern separation deficits following damage to the hippocampus. Neuropsychologia 50: 2408–2414. 10.1016/j.neuropsychologia.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann S, Kirschbaum C, Wolf OT. 2005a. Effects of oral cortisol treatment in healthy young women on memory retrieval of negative and neutral words. Neurobiol Learn Mem 83: 158–162. 10.1016/j.nlm.2004.09.001 [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Piel M, Wolf OT. 2005b. Impaired memory retrieval after psychosocial stress in healthy young men. J Neurosci 25: 2977–2982. 10.1523/JNEUROSCI.5139-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal SL, Tighe SK, Yassa MA. 2014. Asymmetric effects of emotion on mnemonic interference. Neurobiol Learn Mem 111: 41–48. 10.1016/j.nlm.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens SM, Phelps EA. 2008. Emotion processing effects on interference resolution in working memory. Emotion 8: 267–280. 10.1037/1528-3542.8.2.267 [DOI] [PubMed] [Google Scholar]
- Levens SM, Devinsky O, Phelps EA. 2011. Role of the left amygdala and right orbital frontal cortex in emotional interference resolution facilitation in working memory. Neuropsychologia 49: 3201–3212. 10.1016/j.neuropsychologia.2011.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer RJ, Olff M, Van Meijel EP, Carlier IV, Gersons BP. 2006. Cortisol, learning, memory, and attention in relation to smaller hippocampal volume in police officers with posttraumatic stress disorder. Biol Psychiatry 59: 171–177. 10.1016/j.biopsych.2005.06.033 [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Vincent AS, Thomas TL, Wilson MF. 2006. Cortisol responses to mental stress, exercise, and meals following caffeine intake in men and women. Pharmacol Biochem Behav 83: 441–447. 10.1016/j.pbb.2006.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. 2007. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn 65: 209–237. 10.1016/j.bandc.2007.02.007 [DOI] [PubMed] [Google Scholar]
- Ly M, Murray E, Yassa MA. 2013. Perceptual versus conceptual interference and pattern separation of verbal stimuli in young and older adults. Hippocampus 23: 425–430. 10.1002/hipo.22110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu F, Collicutt P, Kornik R, Moszkowski R, Lupien S. 2005. The perfect time to be stressed: a differential modulation of human memory by stress applied in the morning or in the afternoon. Prog Neuropsychopharmacol Biol Psychiatry 29: 1281–1288. 10.1016/j.pnpbp.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Mason JW. 1968. A review of psychoendocrine research on the sympathetic-adrenal medullary system. Psychosom Med 30: 631–653. 10.1097/00006842-196809000-00022 [DOI] [PubMed] [Google Scholar]
- McEwen BS. 2000. Effects of adverse experiences for brain structure and function. Biol Psychiatry 48: 721–731. 10.1016/S0006-3223(00)00964-1 [DOI] [PubMed] [Google Scholar]
- Miller R, Plessow F, Kirschbaum C, Stalder T. 2013. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: evaluation of salivary cortisol pulse detection in panel designs. Psychosom Med 75: 832–840. 10.1097/PSY.0000000000000002 [DOI] [PubMed] [Google Scholar]
- Montero-López E, Santos-Ruiz A, García-Ríos MC, Rodríguez-Blázquez M, Rogers HL, Peralta-Ramírez MI. 2018. The relationship between the menstrual cycle and cortisol secretion: daily and stress-invoked cortisol patterns. Int J Psychophysiol 131: 67–72. 10.1016/j.ijpsycho.2018.03.021 [DOI] [PubMed] [Google Scholar]
- Nater UM, Moor C, Okere U, Stallkamp R, Martin M, Ehlert U, Kliegel M. 2007. Performance on a declarative memory task is better in high than low cortisol responders to psychosocial stress. Psychoneuroendocrinology 32: 758–763. 10.1016/j.psyneuen.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Ngo CT, Michelmann S, Olson IR, Newcombe NS. 2021. Pattern separation and pattern completion: behaviorally separable processes? Mem Cogn 49: 193–205. 10.3758/s13421-020-01072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei NY, Tollenaar MS, Spinhoven P, Elzinga BM. 2009. Hydrocortisone reduces emotional distracter interference in working memory. Psychoneuroendocrinology 34: 1284–1293. 10.1016/j.psyneuen.2009.03.015 [DOI] [PubMed] [Google Scholar]
- Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. 2007. Stress administered prior to encoding impairs neutral but enhances emotional long-term episodic memories. Learn Mem 14: 861–868. 10.1101/lm.743507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishdadian S, Hoang NV, Baker S, Moscovitch M, Rosenbaum RS. 2020. Not only memory: investigating the sensitivity and specificity of the mnemonic similarity task in older adults. Neuropsychologia 149: 107670. 10.1016/j.neuropsychologia.2020.107670 [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Gaab J, Hellhammer DH, Lintz D, Schommer N, Kirschbaum C. 1997. Increasing correlations between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology 22: 615–625. 10.1016/S0306-4530(97)00072-3 [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. 2008. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry 63: 234–240. 10.1016/j.biopsych.2007.04.041 [DOI] [PubMed] [Google Scholar]
- Rolls E. 2013. The mechanisms for pattern completion and pattern separation in the hippocampus. Front Syst Neurosci 7: 74. 10.3389/fnsys.2013.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B. 2002. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem 78: 578–595. 10.1006/nlme.2002.4080 [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Mirone G. 2020. Opposite effects of noradrenergic and glucocorticoid activation on accuracy of an episodic-like memory. Psychoneuroendocrinology 114: 104588. 10.1016/j.psyneuen.2020.104588 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Schächinger H. 2018. Ten years of research with the socially evaluated cold pressor test: data from the past and guidelines for the future. Psychoneuroendocrinology 92: 155–161. 10.1016/j.psyneuen.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. 2010. Learning under stress impairs memory formation. Neurobiol Learn Mem 93: 183–188. 10.1016/j.nlm.2009.09.009 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. 2012. Stress modulates the engagement of multiple memory systems in classification learning. J Neurosci 32: 11042–11049. 10.1523/JNEUROSCI.1484-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Bohringer A, Chatterjee M, Schachinger H. 2008a. Effects of pre-learning stress on memory for neutral, positive and negative words: different roles of cortisol and autonomic arousal. Neurobiol Learn Mem 90: 44–53. 10.1016/j.nlm.2008.02.002 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, Schächinger H. 2008b. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology 33: 890–895. 10.1016/j.psyneuen.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, McCullough AM, Yonelinas AP. 2017. The effects of acute stress on episodic memory: a meta-analysis and integrative review. Psychol Bull 143: 636–675. 10.1037/bul0000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets T, Giesbrecht T, Jelicic M, Merckelbach H. 2007. Context-dependent enhancement of declarative memory performance following acute psychosocial stress. Biol Psychol 76: 116–123. 10.1016/j.biopsycho.2007.07.001 [DOI] [PubMed] [Google Scholar]
- Stark SM, Stark CEL. 2017. Age-related deficits in the mnemonic similarity task for objects and scenes. Behav Brain Res 333: 109–117. 10.1016/j.bbr.2017.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, Stark CEL. 2013. A task to assess behavioral pattern separation (BPS) in humans: data from healthy aging and mild cognitive impairment. Neuropsychologia 51: 2442–2449. 10.1016/j.neuropsychologia.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Stevenson R, Wu C, Rutledge S, Stark CEL. 2015. Stability of age-related deficits in the mnemonic similarity task across task variations. Behav Neurosci 129: 257–268. 10.1037/bne0000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Kirwan CB, Stark CEL. 2019. Mnemonic Similarity Task: a tool for assessing hippocampal integrity. Trends Cogn Sci 23: 938–951. 10.1016/j.tics.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S, Stalder T, Schönfeld S, Wittchen HU, Trautmann S, Alexander N, Miller R, Kirschbaum C. 2015. Hair cortisol concentrations and cortisol stress reactivity predict PTSD symptom increase after trauma exposure during military deployment. Psychoneuroendocrinology 59: 123–133. 10.1016/j.psyneuen.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Szőllősi Á, Racsmány M. 2020. Enhanced mnemonic discrimination for emotional memories: the role of arousal in interference resolution. Mem Cogn 48: 1032–1045. 10.3758/s13421-020-01035-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szőllősi Á, Keresztes A, Novák B, Szászi B, Kéri S, Racsmány M. 2017. The testing effect is preserved in stressful final testing environment. Appl Cogn Psychol 31: 615–622. 10.1002/acp.3363 [DOI] [Google Scholar]
- Tulving E. 1993. What is episodic memory? Curr Dir Psychol Sci 2: 67–70. 10.1111/1467-8721.ep10770899 [DOI] [Google Scholar]
- Vogel S, Schwabe L. 2016. Stress in the zoo: tracking the impact of stress on memory formation over time. Psychoneuroendocrinology 71: 64–72. 10.1016/j.psyneuen.2016.04.027 [DOI] [PubMed] [Google Scholar]
- Weerda R, Muehlhan M, Wolf OT, Thiel CM. 2010. Effects of acute psychosocial stress on working memory related brain activity in men. Hum Brain Mapp 31: 1418–1429. 10.1002/hbm.20945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingenfeld K, Wolf OT. 2014. Stress, memory, and the hippocampus. Front Neurol Neurosci 34: 109–120. 10.1159/000356423 [DOI] [PubMed] [Google Scholar]
- Wolf OT. 2009. Stress and memory in humans: twelve years of progress? Brain Res 1293: 142–154. 10.1016/j.brainres.2009.04.013 [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. 2011. Pattern separation in the hippocampus. Trends Neurosci 34: 515–525. 10.1016/j.tins.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CEL. 2010. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage 51: 1242–1252. 10.1016/j.neuroimage.2010.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. 2011. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus 21: 968–979. 10.1002/hipo.20808 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.