Abstract
Background
The development of novel malaria vaccines and antimalarial drugs is limited partly by emerging challenges to conduct field trials in malaria endemic areas, including unknown effects of existing immunity and a reported fall in malaria incidence. As a result, Controlled Human Malaria Infection (CHMI) has become an important approach for accelerated development of malarial vaccines and drugs. We conducted a systematic review of the literature to establish aggregate evidence on the reproducibility of a malaria sporozoite challenge model.
Methods
A systematic review of research articles published between 1990 and 2018 on efficacy testing of malaria vaccines and drugs using sporozoite challenge and sporozoite infectivity studies was conducted using Pubmed, Scopus, Embase and Cochrane Library, ClinicalTrials.gov and Trialtrove. The inclusion criteria were randomized and non-randomized, controlled or open-label trials using P. falciparum or P. vivax sporozoite challenges. The data were extracted from articles using standardized data extraction forms and descriptive analysis was performed for evidence synthesis. The endpoints considered were infectivity, prepatent period, parasitemia and safety of sporozoite challenge.
Results
Seventy CHMI trials conducted with a total of 2329 adult healthy volunteers were used for analysis. CHMI was induced by bites of mosquitoes infected with P. falciparum or P. vivax in 52 trials and by direct venous inoculation of P. falciparum sporozoites (PfSPZ challenge) in 18 trials. Inoculation with P. falciparum-infected mosquitoes produced 100% infectivity in 40 studies and the mean/median prepatent period assessed by thick blood smear (TBS) microscopy was ≤ 12 days in 24 studies. On the other hand, out of 12 infectivity studies conducted using PfSPZ challenge, 100% infection rate was reproduced in 9 studies with a mean or median prepatent period of 11 to 15.3 days as assessed by TBS and 6.8 to 12.6 days by PCR. The safety profile of P. falciparum and P.vivax CHMI was characterized by consistent features of malaria infection.
Conclusion
There is ample evidence on consistency of P. falciparum CHMI models in terms of infectivity and safety endpoints, which supports applicability of CHMI in vaccine and drug development. PfSPZ challenge appears more feasible for African trials based on current evidence of safety and efficacy.
Keywords: Controlled human malaria infection, Sporozoite challenge, Malaria vaccine, Antimalarial drug, Systematic review
Background
Plasmodium falciparum and Plasmodium vivax are the most common plasmodium species causing human malaria worldwide. Currently, RTS,S is the first and the only licensed malaria vaccine shown to provide partial protection against malaria in young children in three sub-Saharan African countries [1] The emergence of artemisinin resistance, unfavorable pharmacokinetic or toxicological profile of available drugs and other unmet medical needs highlight the need for new antimalarial drugs with novel mechanisms of action [2]. There are promising antimalarial molecules in clinical development, including KAF156 [3], KAE609 [4], DSM265 [5] and M5717, formerly DDD498 [6]. After successful completion of phase 1 safety and pharmacokinetic studies, phase 2 trials have been undertaken or are being planned in malaria-endemic areas. However, ethical and logistical problems, unknown effects of existing immunity and a reported fall in malaria incidence in endemic areas are hampering the execution of both drug and vaccine trials [2, 7]. As a result, Controlled Human Malaria Infection (CHMI) models are being used as emerging approaches for testing efficacy of investigational medicinal products against malaria.
CHMI entails a deliberate infection of healthy volunteers either by inoculation of plasmodium sporozoites (sporozoite challenge) by mosquito bite or direct injection of Plasmodium falciparum sporozoites (PfSPZ Challenge) or Plasmodium-infected erythrocytes [8]. Since the first deliberate infection of volunteers with malaria as a treatment for neurosyphilis in the 1920s, CHMI has increasingly been used to understand parasite biology and as a framework in the assessment of novel vaccine, drug, and diagnostic candidates [7, 9–11]. Indeed, CHMI studies have become a vital tool to accelerate vaccine and drug development and are integrated into First-in-Human trials. CHMI studies provide a cost-effective way to investigate efficacy and characterize the potential therapeutic dose range in early clinical development to gain exploratory proof of concept [12], and to provide an early alert for clinical safety signals.
Mosquito bite-induced CHMI has been shown to be safe and effective for efficacy testing of anti-malarial drug and vaccine candidates for more than 25 years, including early studies of subunit malaria vaccines and atovaquone efficacy [10]. There is an exponential increase in the use of CHMI models worldwide due to the availability of cryopreserved infectious P. falciparum sporozoites (PfSPZ Challenge), and the need to test more vaccine and drug candidates [11]. Heterogenous study designs and procedures have been used in malaria sporozoite-challenge trials that resulted in variable study outcomes. Moreover, comparative reproducibility of various modalities of sporozoite-challenge trials are lacking in the literature. The objective of this study was, therefore, to conduct a systematic review of current literature on reproducibility of efficacy, such as infection rate, prepatent period, parasitemia and safety clinical endpoints of P. falciparum and P. vivax sporozoite challenge model in humans. In addition, we evaluated the study designs, variables influencing study outcomes, optimal and standardized procedures, ethical and regulatory considerations, and limitations in the conduct of sporozoite challenge studies.
Methods
A systematic review of the literature published between 1990 and 2018 was undertaken using public databases including Pubmed, Scopus, Embase, Cochrane Library, ClinicalTrials.gov and Trialtrove. The search was conducted from 30 September 2018 to 10 November 2018. Randomized or non-randomized and controlled or open-label trials testing efficacy of antimalaria drug and vaccine candidates conducted using P. falciparum or P. vivax sporozoite challenge through mosquito bites or needle injection and sporozoite infectivity human studies were included. Ex-vivo studies, nonclinical studies and studies based on induced blood-stage malaria using infected erythrocytes were excluded. The search was conducted using key words, including sporozoite challenge, sporozoite challenge studies, controlled human malaria infection, clinical trials, human, P. falciparum and P. vivax. Abstracts, articles and conference papers were searched without time restriction. Additionally, the grey literature was used to complement evidence synthesis. Only healthy volunteers receiving placebo but not any of the investigational vaccine or drug, and those who participated in sporozoite infectivity studies were considered for analysis of reproducibility. Infection rate, prepatent period and parasitemia were extracted as efficacy end points, while adverse events and laboratory abnormalities as safety endpoints for sporozoite challenge model. The data were extracted using standardized data extraction forms, and descriptive analysis was conducted for evidence synthesis.
Results
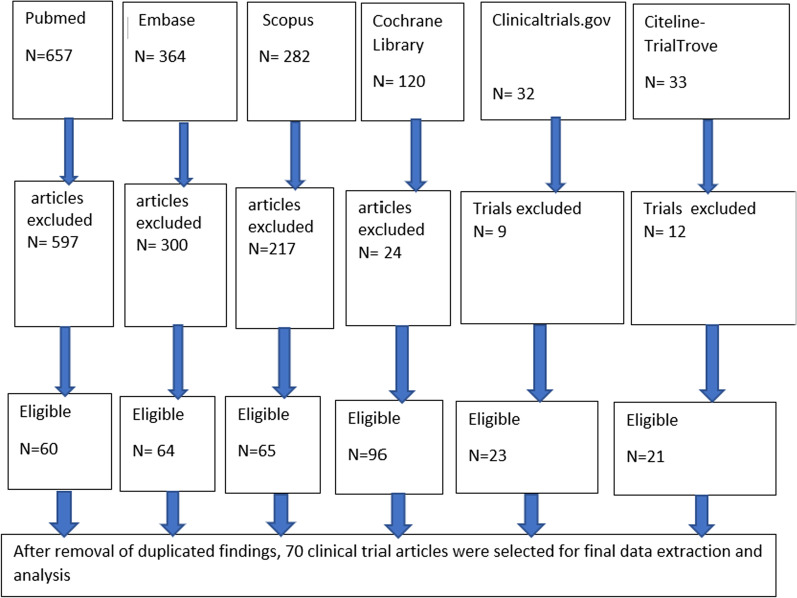
The procedure for screening of articles is shown in Fig. 1. We identified 1488 articles from all searched databases and 70 of them were found to be eligible, out of which 44 were vaccine trials, 19 sporozoite infectivity studies, 5 prophylactic drug trials and 2 assessments of natural immunity. The CHMI trials were conducted from 1990 to 2018 and included a total of 2329 adult healthy volunteers, out of which 1221 were on either placebo treatment used in vaccine and drug efficacy evaluation or in sporozoite infectivity trials. Table 1 summarizes the global landscape of malaria sporozoite challenge trials. The majority of P. falciparum challenge studies were conducted in the USA, the Netherlands, UK, Germany, and Spain; while P. vivax challenge studies were conducted only in Colombia and the USA. Few P. falciparum studies were also carried out in African countries, particularly in Tanzania, Kenya, Mali, Equatorial Guinea and Gabon.
Fig. 1.
Flowchart of search results for sporozoite challenge model based studies from different databases
Table.1.
The Descriptive summary of global landscape of malaria sporozoite challenge studies conducted from 1990 to 2018
| Characteristics | Number of studies |
|---|---|
| Sporozoite challenge study based on purpose | |
| Sporozoite infectivity studies | 19 |
| Malaria vaccine efficacy studies | 44 |
| Drug evaluation (chemoprophylaxis) | 5 |
| Acquired immunity assessment | 2 |
| Country trial conducted | |
| USA | 31 |
| Netherlands | 11 |
| UK | 9 |
| Germany | 3 |
| Colombia | 5 |
| Spain | 2 |
| Tanzania | 2 |
| Kenya | 3 |
| Gabon | 1 |
| Equatorial Guinea | 1 |
| Mali | 2 |
| Allocation to treatment | |
| Randomized placebo-controlled | 39 |
| Non-randomized controlled | 16 |
| Open-label | 2 |
| Not known | 9 |
| Masking type | |
| Double-blind | 26 |
| Single-blind | 6 |
| Open-label | 35 |
| Not known | 3 |
| Intervention model | |
| Parallel assignment | 54 |
| Single group assignment | 8 |
| Factorial assignment | 2 |
| Not known | 5 |
| Phase of study | |
| Phase 1 | 32 |
| Phase 1/2a | 20 |
| Phase 2 | 6 |
| Not applicable | 6 |
| Not known | 5 |
| Sporozoite challenge study type | |
| Mosquito bite based | 52 |
| PfSPZ challenge based | 18 |
| Strain/clone of plasmodium used | |
| PfNF54 | 29 |
| Pf3D7 | 24 |
| PfNF166.C8 | 3 |
| PfNF135.C10 | 3 |
| Pf7G8 | 4 |
| Pv clinical isolate | 7 |
| Mosquito species used | |
| A.stephensi | 44 |
| A.albimanus | 5 |
| A. gambiae | 1 |
| A.dirus | 1 |
Analysis of the data by method of infection indicated that 45 (64.3%) studies used P. falciparum-infected mosquito bites (n = 1286), 7 (10%) P. vivax-infected mosquito bites (n = 189), and 18 (25.7%) injection of cryopreserved P. falciparum sporozoites (n = 854). It is of note that only 56% (39/70) of the studies were randomized placebo-controlled trials with blinding. The sporozoite challenge studies were conducted using various P. falciparum strains or clones including NF54, 3D7, NF166.C8, NF135.C10, or 7G8 induced through either sporozoite inoculation or via bite of laboratory-reared mosquitoes (A. Stephensi or A. gambiae). However, only seven sporozoite challenge studies were conducted through the bite of P.vivax -infected mosquitoes (A. dirus or A. albimanu).
Detection and treatment of malaria challenge infections
Following inoculation of sporozoites to healthy volunteers, parasitemia was monitored by clinical signs and symptoms, microscopic examination of thick blood smears (TBS) and DNA amplification methods (PCR). Out of the 70 studies, 53 used PCR methods with different sensitivity for parasite detection, where 18 studies used PCR, 23 qPCR and 12 RT-qPCR, but 17 studies did not use any PCR method. Rarely, thin blood smears, quantitative buffy coat and blood culture were also used for parasite detection.
In all studies, parasitemia was monitored 5 to 8 days following sporozoite inoculation until day 21 or 28. Parasitemia was treated using standard antimalarial drugs; chloroquine, atovaquone-proguanil, artemether-lumefantrine, mefloquine, or primaquine; based on sensitivity of strains of the parasite used. Subjects who continued to have negative results on blood smear from the day of sporozoite inoculation until the last date of the study (21 or 28) were prophylactically treated. The studies used different criteria for treatment initiation. Clinical signs and symptoms of malaria with confirmed TBS was used in 56 studies and detection of > 500 parasites/mL of blood with qPCR in 3 studies. In smear negative subjects, > 100–500 parasites/mL of blood with qPCR was used as criteria to initiate treatment.
Efficacy of antimalarial drugs against sporozoite challenge
Only five studies evaluated chemoprophylactic activity of candidate antimalarial drugs against mosquito-bite- or PfSPZ challenge-induced infection. The drug molecules evaluated were DSM265 [5, 14]), atovaquone [15], atovaquone-proguanil [16] and pafuramidine [17]. A single dose of DMS265 had 100% (5/5) chemoprophylactic activity when given a day before CHMI. However, dosing 3 or 7 days before CHMI showed partial efficacy (33%, 2/6), presumably due to insufficient exposure to DMS265 at the time of sporozoite challenge.
Atovaquone was also found to be 100% chemoprophylactic when given a day before CHMI and atovaquone-proguanil was 100% protective given 4 days after CHMI. Pafuramidine administration 1 or 8 days before sporozoite challenge was not effective, as it conferred protection to 6.3% (1/16) of the participants.
Efficacy of malarial candidate vaccines against sporozoite challenge
This review indicated that sporozoite challenge has been largely used for evaluating efficacy of vaccine candidates against P. falciparum infection, mainly induced through bite of infected mosquitoes. The outcome of efficacy studies of vaccines is summarized in Table 2. Immunization with irradiated mosquito bite showed over 90% efficacy in P. falciparum [18] and 42% in P. vivax infections [19]. PfNF54 immunization, with mosquito bite under chloroquine prophylaxis (NF54 -CPS), showed up to 100% protective efficacy against homologous PfNF54 and Pf3D7 strains [20–24]. This approach of immunization was, however, less effective for heterologous strains [22, 25]. The protective efficacy of chemo-attenuated PfSPZ challenge (CVac) was 33–100% against PfSPZ challenge [26], while that of irradiated PfSPZ vaccines was 20–100% against PfSPZ challenge [27] and 66–100% against mosquito-bite including heterologous strains [28, 29].
Table.2.
The protective efficacy of various types of sporozoite-based malaria vaccines against homologous and heterologous sporozoite—induced malaria infections
| Vaccine category | Vaccination delivery | CHMI-strain | Vaccine efficacy (%,n) | References |
|---|---|---|---|---|
| NF54 -CPS | NF54-infected mosquitoes | PfNF54 | 100(10/10) | [20] |
| NF54 -CPS | NF54-infected mosquitoes | Pf3D7 | 100 (5/5) | [21] |
| NF54 -CPS | NF54-infected mosquitoes | NF54, NF166.C8, NF135.C10 |
100(5/5)-for NF54 20(2/10)-forNF135.C10 11(1/9)- for NF166.C8 |
[22] |
| NF54 -CPS | NF54-infected mosquitoes | NF135.C10 | 15(2/13) | [25] |
| NF54 -CPS | NF54-infected mosquitoes | PfNF54 | 70(7/10) | [23] |
| NF54 -CPS | NF54-infected mosquitoes | PfNF54 | 66(4/6) | [24] |
| NF54-irradiated | NF54-infected mosquitoes | NF54, Pf3D7, Pf7G8 | 92(24/26) | [18] |
| NF54-irradiated | Infected mosquito | PfNF54, 3D7 | 33(1/3) | [33] |
| X-irradiated Pv-SPZ | Infected mosquito | Pv | 42(5/12) | [19] |
|
RTS, S /AS01B, RTS,S/AS02A |
IM injection | Pf3D7 | 32–50 | [32] |
| RTS,S | IM injection | Pf3D7 | 41(9/22) | [34] |
| RTS,S | IM injection | Pf3D7 | 29(2/7) | [35] |
| RTS,S/SBAS2 | IM injection | Pf3D7 | 43(18/41) | [36] |
| RTS,S/AS02A | IM injection | Pf3D7 | 42(8/19) | [31] |
| Ad35.CS.01- RTS,S/AS01 | IM injection | Pf3D7 | 44 and 52 | [37] |
| RTS,S/AS02A and MVA-CS | IM/ID injection | Pf3D7 | 33(4/12) | [38] |
| RTS,S | IM injection | Pf7G8 | 14(2/14) | [39] |
| RTS,S/AS01B with ME-TRAP | IM injection | Pf3D7 | 82(14/17) & 75(12/16) | [40] |
| RTS,S/AS01 | IM injection | Pf3D7 | 63(10/16) & 87(26/30) | [41] |
| R32N51-81 | IM injection | Pf3D7 | 36(4/11) | [42] |
| ChAd63-MVA CS, & ChAd63-MVA ME-TRAP | IM injection | Pf3D7 | 7(1/15) &13(2/15) | [43] |
| PfSPZ vaccine | IV injection | PfNF54, 3D7 | 66(6/9) &100(6/6) | [28] |
| PfSPZ vaccine | IV injection | NF54, NF166.C8, NF135.C10 |
92(12/13)-homologous 80(4/5)-heterologous |
[29] |
| PfSPZ challenge | DVI | PfSPZchallenge injection by DVI | 33(3/9), 100(9/9) | [26] |
| PfSPZ vaccine | IM injection | PfSPZ challenge injection by DVI | 20(4/20) & 100(4/4) | [27] |
CPS Chemoprophylaxis
Infectivity of malaria sporozoite challenge in healthy volunteers
The analysis of reproducibility of infectivity parameters for sporozoite challenge was done in subjects who received sporozoite challenge as infectivity controls, implemented as part of placebo-controlled vaccine or antimalarial compound efficacy trials. The subjects enrolled in the experimental sporozoite infectivity studies did not receive any investigational compound. Once infectivity of the experimental challenge (parasitemia) was confirmed, all participants had received mandatory rescue medication with confirmation of cure. Table 3 depicts a summary of the efficacy endpoints measured by different methods. A sample size of usually 6 subjects per group (range 2–18) was used in sporozoite challenge studies. The P. falciparum infected mosquito challenge was 100% infective in 88.9% (40/45) of the studies. In 9 studies, the infectivity was, however, came down to 88.3%, whereby one subject per group did not develop parasitemia [9, 19, 24, 28, 36, 44–46].
Table.3.
The infectivity, prepatent period and parasitemia measurements in healthy volunteers in malaria sporozoite challenge participated as controls in vaccine or drug trials and in infectivity studies
| Plasmodium spp/strain | CHMI type | Infectivity % (n) | TBS-Prepatent period (mean or median) days | PCR-Prepatent period (mean, median) days | PCR-parasitemia (n/µl) Mean or median |
References |
|---|---|---|---|---|---|---|
| PfNF54 | PfSPZ challenge | 100(4/4) | 11.7* | 7.8 | 19.55–28.756 | [5] |
| PfNF54 | PfSPZ challenge | 100(13/13) | 11** | [26] | ||
| PfNF54 | PfSPZ challenge | 100 (5/5) | 12.7* | 10.3 | 25.6 | [71] |
| PfNF54 | PfSPZ challenge | 100(6/6) | 11.4/12.2* | 6.8 | [69] | |
| PfNF54 | PfSPZ challenge | 84(5/6) | 13/12.7/13* | 10.6/10.3/ 9.9 | 0.07/0.2 | [46] |
| PfNF54 | PfSPZ challenge | 100 (28/28) | 12.6/11.8/12/ 16.4/13.3/11.9 ** | [67] | ||
| PfNF54 | PfSPZ challenge | 100(18/18) | 12.5/13/12** | 0.5 | [27] | |
| PfNF54 | PfSPZ challenge | 92/91 | 15.4/13.5** | 12.6 /11.1 | 0.11/0.16 | [9] |
| PfNF54 | PfSPZ challenge | 100(6/6) | 12.7** | [66] | ||
| PfNF54 | Mosquito bite/PfSPZ | 100(6/6) | 8.3–9.4** | ≥ 0.25 | [14] | |
| PfNF54 | PfSPZ challenge | 100(9/9) | 11.2* | [68] | ||
| PfNF54 | PfSPZ challenge |
64%(7/11) 56(5/9) |
16.9/19.1* | [72] | ||
| PfNF54 | Mosquito bite | 100(5/5) | 13.5** | 7 | [54] | |
| Pf3D7 | Mosquito bite | 100(12/12) | [37] | |||
| PfNF54 | Mosquito bite | 100(6/6) | 11.2* | 7.5 | 33.712 | [53] |
| Pf | Mosquito bite | 100(15/15) | 11.34/ 12.54* | 7/6.38 | 23.92/35.74 | [13] |
| PfNF54 | Mosquito bite | 100(13/13) | 11.2* | – | – | [78] |
| PfNF54 | Mosquito bite | 94.4(17/18) | 10.9* | 7.5 | [45] | |
| PfNF54 | Mosquito bite | 100(19/19) | 10.9* | 7.5 | 1.7 | [52] |
| PfNF54 | Mosquito bite | 100(6/6) | 9** | 6.7 | [30] | |
| Pf3D7 | Mosquito bite | 100(6/6) | 9–13 | [79] | ||
| PfNF54 | Mosquito bite | 100(4/4) | 9–18 | [15] | ||
| PfNF54 | Mosquito bite | 100(4/4) | 8.5** | 6.3 | > 0.035 | [23] |
| Pf3D7 | Mosquito bite | 100(6/6) | 9–13 | 2.7 | [16] | |
| Pf3D7 | Mosquito bite | 100(6/6) | 12.8* | [80] | ||
| PfNF54 | Mosquito bite | 100(5/5) | 9.2* | [20] | ||
| PfNF54 | Mosquito bite | 100(4/4) | 12.5 * | 10.8 | [17] | |
| Pf3D7 | Mosquito bite | 100(6/6) | 12.3* | [31] | ||
| Pf3D7 | Mosquito bite | 100 | 10.7 | [38] | ||
| Pf3D7 | Mosquito bite | 100(5/5) | 11* | > 1 | [56] | |
| Pf3D7 | Mosquito bite | 100(6/6) | 11.6 * | 10.8 | > 1 | [57] |
| PfNF54 | Mosquito bite | 80 (4/5) | 7–12 | [24] | ||
| Pf3D7 | Mosquito bite | 100 (6/6) | 12.9* | [81] | ||
| Pf3D7 | Mosquito bite | 100(5/5) | 11.8 * | [55] | ||
| Pf3D7 | Mosquito bite | 100% (6/6) | 11–13* | [34] | ||
| Pf3D7 | Mosquito bite | 95(22/23) | 12 | [36] | ||
| Pf3D7 | Mosquito bite | 100(5/5) | 12.3* | 9 | [21] | |
| Pf3D7 | Mosquito bite | 100(6/6) | 9–12* | 7 | [51] | |
| Pf | Mosquito bite | 100(6/6) | 11.8* | 11.1 | > 1 | [58] |
| Pf3D7 | Mosquito bite | 100(6/6) | 10.8 | [35] | ||
| Pf3D7 | Mosquito bite | 100(16/16) | – | > 0.5 | [82] | |
| Pf3D7 | Mosquito bite | 100(11/11) |
12.25 ** |
7.4 | > 0.5 | [41] |
| Pf3D7/7G8 | Mosquito bite | 100(4/4) | 10–12 | [39] | ||
| Pf3D7 | Mosquito bite | 100(36/36) | 11–14 | [18] | ||
| Pf3D7 | Mosquito bite | 100(11/11) | 11–14 | [42] | ||
| Pf3D7 | Mosquito bite | 100 (13/13) | 13* | [41] | ||
| PfNF54/3D7 | Mosquito bite | 100 (5/5) | 11–14 | [33] | ||
| Pf7G8 | Mosquito bite | 100(4/4) | 9–12 | [49] | ||
| Pf | Mosquito bite | 100(36/36) | 10.8/11.8* | [32] | ||
| Pf3D7 | Mosquito bite | 100(6/6) | 10.3** | 7.5 | > 0.5 | [67] |
| Pf | Mosquito bite | 91(11/12) | 7–13 | [28] | ||
| Pf3D7/Pf7G8 | Mosquito bite | 100(22/22) | 10.9/11.6/11.9** | [29] | ||
| PfNF54/NF166.C8/ NF135.C10 | Mosquito bite | 100(23/23) | 10.2/7.2/7.4* | 7.5/6.5/6.5 | [47] | |
| PfNF135.C10 | Mosquito bite | 100 (5/5/) | 8.5** | - | > 0.05 | [25] |
| PfNF54/NF166.C8/ NF135.C10 | Mosquito bite | 100(15/15) | - | - | > 0.1 | [22] |
| NF166.C8/ NF135.C10 | Mosquito bite | 100(4/4/) | 7.5** | - | > 0.5 | [48] |
| Pv | Mosquito bite | 100(17/17) | 12 * | - | [62] | |
| Pv | Mosquito bite | 94(15/16) | 12.5/12.8 * | 9.2–9.4 | 17.5 | [64] |
| Pv | Mosquito bite | 100(2/2) | 12–13* | 8–11 | 12.5–1050 | [19] |
| Pv | Mosquito bite | 100 | 11–13 | – | – | [83] |
| Pv | Mosquito bite | 94.4(17/18) | 11* | 9–13 | [44] | |
| PV | Mosquito bite | 100(6/6) | 10.7** | [65] | ||
| PV | Mosquito bite | 100(18/18) | 11, 11, 9 * | [63] |
*Geometric mean, **Median
The infection outcomes of sporozoite challenge studies conducted through either bite of plasmodium- infected mosquito or parenteral injection of purified P. falciparum sporozoites are described below.
Mosquito-based P. falciparum challenge studies
Our review indicates that mosquito-based P. falciparum sporozoite challenge studies were conducted in well-established research centers, including Walter Reed Army Institute of Research (WRAIR) and Naval Medical Research Center, Sanaria Inc (Biotechnology company), University of Maryland (USA), Seattle Biomedical (USA), Oxford University (UK), and Radboud University Medical Centre (Netherlands). The main strains used were NF54 and 3D7, while the mosquito infection rates and sporozoite load in salivary glands of mosquitoes varies from 40 to 100%.
Malaria infections were induced by 1–13 bites of laboratory-reared P. falciparum–infected female anopheles’ mosquitoes. Five mosquito bites were used by 39 out of 52 studies (75%) as standard challenge dose, which reproducibly resulted in optimal (100%) infection rates in healthy volunteers challenged with parasite clones such as NF54, 3D7, NF135.C10 and NF166.C8 and 7G8 [22, 25, 29, 39, 47–49].
The review indicated that 100% infectivity of P. falciparum-infected mosquitoes was reproduced in 88.9% (41/45) of the studies (Table 3). The mean or median prepatent period for P. falciparum infection with TBS was ≤ 12 days in 24 studies, and between 7 and 13 days in all studies. Similarly, the mean prepatent period with qPCR was between 6 and 11 days in 9 studies. PCR detected parasites, on average, 2–6 days earlier than TBS [12, 13, 17, 21, 23, 30, 40, 44, 46, 52–54].
The studies reported variable average parasite growth rates of 8–16.8 fold per 48 h [13, 21, 51, 55] while the qPCR detected parasite densities were ranging from 0.2 to 33.7 parasites/µl of blood [13, 16, 51]. The parasite detection limits of PCR ranged from 35 to 1000 parasites/mL of blood [12, 14, 22, 23, 25, 40, 49, 56–58]. Most CHMI studies resulted in 100% infectivity through standardized five bites from P. falciparum infected mosquitoes. CHMI conducted using three aseptically reared mosquitoes, produced by Sanaria Inc under current GMP, however, resulted in 100% (25/25) infectivity with prepatent period of 9–12 days and fewer adverse events compared to the standard CHMI by mosquito bite [45, 52].
CHMI with P. vivax-infected mosquitoes
Methods for P. vivax CHMI were also developed at WRAIR (USA) in collaboration with institutions in Thailand and Colombia. The P. vivax CHMI studies were conducted through the use of infected A. albimaus or A. dirus mosquitoes from the malaria endemic area in Colombia or Thailand. P. vivax sporozoite challenge studies were conducted on Duffy ( +) subjects and Duffy (−) were used as controls. Our review (Table 3) indicated that 100% infectivity of P. vivax-infected mosquitoes was reproduced in five studies and infectivity was 94% in one study [44]. In three of the studies, 100% infectivity was achieved through 2–4 mosquito bites [19, 62, 63]. There was no difference in rate of infection and prepatent period when different batches of infected mosquitoes were used to infect healthy volunteers [63]. The mean or median prepatent period detected by microscopy was 9–13 days [19, 44, 62, 64, 65]. The PCR detected mean prepatent time ranged 8–13 days.
The safety review of P. vivax CMHI indicates mild to moderate local reactogenicity due to mosquito bites. Signs and symptoms typical of uncomplicated malaria were observed but serious adverse events were not reported. Most laboratory abnormalities were associated with mild to moderate increase in liver transaminases, alkaline phosphatase and leukopenia.
PfSPZ challenge-mediated CHMI studies
Aseptic, purified, cryopreserved P. falciparum sporozoites (PfSPZ challenge) was developed from NF54 strain of P. falciparum by Sanaria Inc in Maryland, USA. PfSPZ challenge is fully infectious and stable over time when stored in liquid nitrogen vapor phase (LNVP) below a temperature of -150 °C. To establish optimal infectivity and safety of PfSPZ challenge, several CHMI studies were conducted in healthy volunteers using different doses, injection volumes and routes of administration. These clinical studies were conducted at Radboud University Medical Center [46], the University of Oxford [66], Tanzanian Ifakara Health Institute [9], University of Maryland [45], Kenya Medical Research Institute [12, 67], University of Tübingen [68] and Barcelona Centre for International Health [69]. These studies successfully defined the reproducible regimen for administration of PfSPZ challenge resulting in 100% infection rate, with a prepatent period of ≤ 12 days and enabled replication of outcomes from mosquito-induced CHMI. Additional quality control studies to assess the reproducibility and parameters affecting the prepatent period and percentage of infectivity have been conducted and established a dose of 3,200 PfSPZ as an optimal dose [46, 59, 68, 70].
The ultimate goal of using sporozoite-based malaria vaccines such as chemo-attenuated PfSPZ challenge and irradiated PfSPZ vaccine is to vaccinate population in endemic countries and induce protective immunity. In that regard, several studies have reported optimization of PfSPZ Challenge injections given Intra-muscularly (IM) and intra-dermally (ID) [9, 46, 71]. Additional studies were conducted in low, moderate, and high exposure to P. falciparum population to assess the impact of naturally acquired immunity on prepatent period and infectivity rate of malaria induced by PfSPZ challenge [67, 72]. For example, the Kenyan study explored the unknown effects of prior exposure to malaria on CHMI using IM PfSPZ Challenge at doses of 25,000, 75,000 and 125,000. This study screened participants’ prior exposure to P. falciparum using anti-schizont and anti-merozoite surface protein 2 (MSP2) antibody assay. The study included 14 volunteers with minimum exposure (antibody negative) and 14 participants with definite exposure (antibody positive) to P. falciparum. The results indicated that all participants developed malaria infection with the exception of one volunteer who remained blood film negative, but qPCR positive. This volunteer had reduced parasite multiplication rate, but had the highest anti-schizont and MSP2 antibody. There was no significant difference in adverse events between minimally exposed and definitely exposed subjects. Moreover, the safety profile was similar to that reported in malaria-naïve subjects, with the exception that Kenyan participants experienced adverse events of longer duration.
Similarly, another study [72] used CHMI to investigate infection rates, parasite kinetics, and malaria symptoms in lifelong malaria–exposed (semi-immune) Gabonese adults with and without sickle cell trait. Eleven semi-immune Gabonese with normal hemoglobin, nine with sickle cell trait, and five nonimmune European controls with normal hemoglobin received 3,200 PfSPZ by direct venous inoculation (DVI). Malaria infection rates detected by qPCR were 82% (9/11), 78% (7/9), and 100% (5/5), respectively. All lifelong malaria-exposed adults controlled parasite multiplication. Adverse events were more severe in non-immune volunteers, but no other differences between the study groups were found.
The studies indicated that the minimum infectious PfSPZ challenge dose for 100% infection rate in malaria naïve healthy volunteers, with prepatent period comparable to five mosquito bites, is 3200 Pf sporozoites inoculated DVI [27, 68], 75,000 IM [69], or 50,000 ID [71]. Nevertheless, it should be noted that a 3200 sporozoite dose given by DVI was not optimal in semi-immune African population [72], although this was reported in few studies. After standardization, DVI of 3200 PfSPZ has been implemented for testing of PfSPZ vaccine efficacy in Tanzania [27], Mali [73], Germany [26], and for efficacy testing of antimalarial drugs [5, 14]. Additional similar studies in Africa are ongoing at Mali [74, 75], Equatorial Guinea [76], and Kenya [77]. The details of outcomes of PfSPZ challenge studies i.e. infection rate, prepatent period and parasitemia are presented in Table 3.
Safety and tolerability of CHMI by Sporozoite challenge
Analysis of the safety profiles of PfSPZ challenge from all studies reviewed indicates that the safety findings included the local and systemic signs and symptoms and laboratory safety signals. The safety profiles following P. falciparum infections by mosquito bites indicated consistent adverse events related to mosquito bite, clinical malaria and its treatment.
Intradermal injection of PfSPZ challenge was generally safe and well tolerated, only with mild to moderate local reactogenicity such as pruritus, erythema, swelling, and systemic malaria symptoms [9, 71]. Laboratory abnormalities, such as elevations in ALT/AST, thrombocytopenia and leukopenia, were infrequent and self-limited with similar incidence rates among all subjects and normalized following treatment with antimalarial drugs and at the end of follow up. Roestenberg et al. [46] reported an incidence of Grade 3 adverse events (AEs) in 44% of the subjects without clinically significant laboratory abnormalities before initiation of anti-malarial treatment.
In another study [66], frequencies and severities of laboratory abnormalities during malaria infection were reported to be in concordance with those expected following P. falciparum infection. Similarly, IV inoculation of PfSPZ Challenge was generally well tolerated with no local solicited AEs. However, three systemic solicited Grade1AEs [27] during IV and IM administration as well as Grade 2 headache, fever, fatigue; and Grade 3 lymphopenia and neutropenia, laboratory abnormalities expected of malaria were recorded, with no serious adverse outcomes [69]. Lell et al. [72] showed that DVI of PfSPZ Challenge in semi-immune African population was well tolerated, without SAE and clinically significant laboratory abnormalities. Generally, ID, IM, and IV inoculation of PfSPZ challenge in healthy malaria naïve and semi-immune subjects was safe and well tolerated, and SAEs were very limited. The mild to moderate AEs and malaria signs and symptoms as well as laboratory abnormalities consistently observed were expected of P. falciparum infection and treatment, and usually normalized after treatment.
Discussion
Our systematic review attempted to establish evidence on the reproducibility of P. falciparum and P. vivax malaria sporozoite challenge model using search of literature from several databases. Seventy studies were identified and used for analysis of data reported. The efficacy endpoints of the sporozoite challenge model were infectivity, prepatent period and parasitemia; while the safety endpoints were adverse events and laboratory abnormalities.
The majority of P. falciparum and P. vivax challenge studies were conducted in developed countries (USA, the Netherlands, UK, Germany, and Spain) while only few P. falciparum challenge studies were also conducted in African countries. The lack of phase 1 clinical trial centers and mosquito laboratory facilities were reasons for the small number of CHMI trials conducted in Africa [9].
Analysis of detection of malaria challenge infections in healthy volunteers indicated that following inoculation of sporozoites, parasitemia was monitored by clinical signs and symptoms, microscopic examination of thick blood smears and PCR techniques. The use of various methodologies with different sensitivity for parasite detection was a source of variability in assessing clinical endpoints. The sensitivity of thick blood film is reported to be 1–10 parasites/µl, which is roughly one-tenth of qPCR [2]. Using qPCR in sporozoite-initiated CHMI has an advantage of shortening the duration of parasitemia due to lower absolute number of parasites so as to avoid or at least reduce the appearance of clinical symptoms in participants [8, 13].
Few studies evaluated chemoprophylactic activity of candidate antimalarial molecules against mosquito-bite- or PfSPZ challenge-induced infections. The studies [5, 14–17] indicate that the variability in chemoprophylactic activity of candidate molecules was observed due to differences in dosing regimen, time of administration, pharmacokinetics and pharmacodynamics of the compounds. Nevertheless, the results reaffirm the use of the sporozoite challenge model for evaluating prophylactic activity of new chemical entities, though work related to standardization remains to be done.
The review of available evidence indicated that sporozoite challenge model has been used to assess the efficacy of several vaccine candidates. The level of protective efficacy of sporozoite-based vaccines varied depending on the type of vaccine-adjuvant system, vaccination regimen, previous exposure status, and type of strain. This model showed positive infectivity in the control arm receiving placebo, although a wide range of protective responses were observed with the different antigens. The reasons for high variability observed in the protective response are largely unknown. Some of them could be directly related to the nature of the sporozoite challenge model, such as the high intensity of the challenge compared to natural infection [30], lack of genetic diversity coverage, and sub-optimal vaccination regimens during the experimental phase. The CHMI model demonstrated its ability to predict efficacy of malaria vaccines in the field as indicated by development of the RTS, S vaccine [7, 31, 32]. However, so far, only limited vaccine candidates have been tested in population at risk of malaria infection. The translation of the results obtained in the CHMI to malaria exposed patients thus remains to be confirmed.
The mosquito-based P. falciparum sporozoite challenge studies were also conducted in well-established research centers in developed nations using parasite clones such as NF54, 3D7, NF135.C10 and NF166.C8. The analysis of efficacy (infectivity) of malaria sporozoite challenge in healthy volunteers shows that P. falciparum infected mosquito challenge was 100% infective in 88.9% of the studies with five mosquito bites resulting in 100% reproducible infections. Although infectivity exhibited a decreasing trend in some studies, the data collectively indicate very good performance of the CHMI experiments. In 84.6% (44/52) of the studies, A. stephensi mosquitoes, a major vector for malaria infections in urban areas and established in laboratories [50, 51], were used for infection with P. falciparum. The outcomes of these studies indicated that optimum infection could be obtained with standardized five bites of P. falciparum infected A. stephensi mosquitoes, regardless of the geographic origin of the infecting parasite, strongly justifying the use of CHMI in testing efficacy of drug and vaccine candidates.
The mean or median prepatent period for P. falciparum infection with TBS was ≤ 12 days in 24 studies, and between 7 and 13 days in all studies. Similarly, the mean prepatent period with qPCR was between 6 and 11 days in 9 studies. PCR detected parasites, on average; 2–6 days earlier than TBS. The parasite detection limits of PCRs ranged from 35 to 100 parasites/mL of blood and average parasite growth rates were also variable. This variability in PCR-detection sensitivity may influence the comparability of study outcomes across trial sites and study designs, justifying the need for standardization of PCR procedures.
The CHMI studies resulted in 100% infectivity through standardized five bites from P. falciparum infected mosquitoes, however, using three aseptically reared mosquitoes also resulted in 100% infectivity. This observation indicates the possibility of setting aseptic model as a new standard for CHMI trials in non-endemic areas with the advantage of reducing adverse events.
Malaria infection with bite of mosquitoes, where the probability of infection increases with the number of infectious bites, reliably reflects the natural infection process. The studies indicated that the pre-patent period depends on strain of parasite, biting dose and number of mosquitoes used. Even though five mosquito bites consistently produced 100% infectivity, impact of the number of sporozoites inoculated in those bites was not clear until recently. Statistical analysis of > 1000 experimental infections by Churcher et al. [59] indicated that the probability of infection and pre-patent period depends on the number of residual-sporozoites in salivary glands of mosquitoes. The salivary gland residual-sporozoite load per fed mosquito was scored as 0 (no sporozoites), 1 (1–10), 2 (11–100), 3 (101–1000), 4 (> 1000) [60]. However, the number of sporozoites injected into each participant in mosquito bite-initiated CHMI was highly variable. As a result, the number of sporozoites counted in mosquito salivary glands is a poor predictor of the number of sporozoites injected [7].
Few studies on CHMI with P. vivax-infected mosquitoes were conducted on Duffy ( +) subjects. The Duffy antigen, a glycosylated membrane protein located on the surface of red blood cells and functioning as a multi-specific receptor for several chemokines, is also the obligate trans-membrane receptor for P. vivax invasion of red blood cells. The low endemicity of P. vivax infection in sub-Saharan Africa is perceived to be due to high prevalence of Duffy (−) phenotype, which confers resistance to P. vivax infection. However, evidence emerging from P. vivax infections confirmed by molecular diagnostic tests in Duffy (−) hosts indicates an additional Duffy-independent -mechanism of transmission across sub-Saharan Africa [61].
There was similar infection outcomes in CHMI with P. vivax with same five mosquito bites however, P. vivax infected mosquitoes appear more potent compared to P. falciparum infection, which requires five bites for reliable infection, although this needs to be validated with more studies.
The standardization of P. vivax challenge is proven to be more difficult, due to unavailability of long-term in-vitro culture, however, an alternative approach has been using fresh gametocytes from infected patients to infect mosquitoes. The P. vivax sporozoites were generated by feeding gametocyte-infected blood to laboratory-reared mosquitoes from residents areas in Colombia and Thailand [7, 8]) or by shipping infected mosquitoes to USA for challenge study [65]. The use of fresh P. vivax isolates may have limitations including variability in drug sensitivity, parasite multiplication rates and prepatent periods [8]. Potential hypnozoite formation and infection relapse are additional complications from P. vivax sporozoite-initiated CHMI studies. In addition, screening for CYP mutations associated with increased metabolism of primaquine is required for CHMI studies involving P. vivax to provide a terminal treatment of liver stages of P. vivax infection. Generally, the current methods for sporozoite challenge through bite of P. vivax-infected mosquitoes is validated for its infectivity and safety in small number of studies. Hence, more studies may be needed for procedures optimization.
Review of PfSPZ challenge-mediated CHMI studies indicated that this method is well established and standardized. The studies have successfully defined the reproducible regimen for administration of PfSPZ challenge resulting in 100% infection rate, with a prepatent period of ≤ 12 days which is 3200 P.falciparum sporozoites given by direct venous inoculation. However, natural adaptive immunity to malaria significantly prolonged the time to parasitemia. Sickle cell trait seemed to prolong it further, whereas 20% (4/20) semi-immunes demonstrated sterile protective immunity. The observed differences in infection outcomes following PfSPZ Challenge injection in Kenyan [67] and Gabonese [72] population could be attributed to population differences in malaria exposure and induction of natural adaptive immunity. However, due to small sample size, more studies in malaria endemic populations are needed to confirm infectivity of PfSPZ challenge in malaria exposed individuals in support of the future use of PfSPZ challenge for testing efficacy of vaccines and anti-malaria drugs.
Analysis of the safety and tolerability of CHMI by Sporozoite challenge indicates local and systemic signs and symptoms and laboratory safety signals that are features of malaria infection which normalize following treatment with antimalarial drugs. The safety profiles following P. falciparum infections by mosquito bites indicated consistent adverse events related to mosquito bite, clinical malaria and its treatment but serious adverse events were rare. Two previous studies [84, 85] reported cardiac-related serious AEs (SAEs) (acute coronary syndrome/myocarditis), whose causality was not definitely established, following a mosquito-bite CHMI study with P. falciparum infection. As a result, CHMI studies recommend assessment of markers for cardiac damage and coagulation such as troponin, lactate dehydrogenase (LDH), platelets, and D-dimer and ECG [9, 43, 46].
The sporozoite infection model with infectious mosquitoes mimics the natural route of infection and allows the occurrence of natural and artificial conditions impacting the development of natural malaria infection, such as naturally or vaccine-induced immunity and drugs. The model has been used in the development and early clinical validation of vaccines [21, 24] and more recently has been established as a translational model for prophylactic drug development [5, 14–17]. Historically, the reliability of the model to support the development of drugs or vaccines has been impaired by the complexity and reproducibility of infective bites by mosquitoes; uncontrolled number of sporozoites inoculated by biting mosquitoes were limiting the interpretation of results from experimental challenge models [7]. The conduct of mosquito-challenge trials was complicated by the need for a mosquito insectary and entomological expertise and risk of adventitious infections [45, 52]. The recent availability of aseptically manufactured PfSPZ Challenge has changed the landscape for CHMI trials with sporozoites and expanded the global capacity to conduct CHMI trials [86], particularly in endemic countries. It also allowed establishing standardized CHMI conditions for drug development [27, 68].
The development of cryopreserved sporozoites helped to produce large scale vaccination in endemic countries following the initial observation that irradiated sporozoites could induce fully protective immune response [87]. With the industrial success of Sanaria Inc to produce large quantity of cryopreserved sporozoites, several CHMI studies have been conducted in malaria endemic countries in Africa to prepare the ground for vaccination campaigns [9, 12, 27, 67, 72–77]. These studies have demonstrated the feasibility of CHMI studies in Africa and allowed to assess the influence of pre-established immunity and malaria related genetic factors such as G6PD and sickle cell trait in the readout of the results [72]. This study indicated that naturally adaptive immunity to malaria and sickle cell trait prolonged prepatent time. The African CHMI studies have also demonstrated the need for addressing the technical and scientific as well as ethical and regulatory challenges [8, 12, 88]. These include prior consultation with key stakeholders, community sensitization, extensive exclusion criteria, consenting issues, challenges of screening of previous exposure, screening asymptomatic parasitemia, confounding of haemoglobinopathies and natural immunity, clinical & laboratory capacity and multitiered system of ethical review.
This systematic review lacks meta-analysis of data on CHMI trials conducted in healthy malaria naïve population and those in endemic countries due to heterogeneous design of trials conducted. However, the data clearly emphasize that these types of experiments can now be conducted in a reliable and systematic manner in well-equipped clinical settings in Africa or other endemic countries. It also provides a comprehensive qualitative evidence on reliability of sporozoite challenge for testing novel therapeutic and prophylactic interventions in healthy volunteers and guide further clinical development. Altogether, the data advocate for further capacity building initiatives to malaria-endemic country based clinical research centers.
Conclusion and future perspectives
Current evidence indicates that mosquito-based sporozoite challenge of healthy volunteers has been validated for several strains of P. falciparum, with five infective mosquito bites being the optimal dose resulting in reproducible infectivity, prepatent period and safety profiles. Mosquito-based sporozoite challenge is a gold standard as it mimics natural infection, however, the method is not yet evaluated in the African setting.
The consistent safety and infectivity of PfSPZ challenge is verified among populations in Europe, America, and Africa. Infective doses are optimized, with DVI of PfSPZ challenge being a new emerging gold standard, including in malaria endemic African population, although there was variability in infection outcomes. Both methods of sporozoite challenge are well characterized for consistent safety profiles. The P. vivax CHMI model using infected mosquitoes has also been validated in few studies, with some limitations including lack of optimization of long-term culture and CHMI procedures.
Sporozoite challenge model is extensively used for testing vaccine efficacy, although few vaccines have been advanced to date. The applicability of the method is also verified in a few prophylactic drug efficacy studies. However, for efficacy studies in African population, PfSPZ challenge is recommended based on current evidence on safety, infectivity and ease of logistical requirement.
The understanding of sporozoite CHMI is advancing, however, gaps in current scientific knowledge to be addressed by future CHMI studies include further assessment of genetic and antigenic diversity of P. falciparum, use of bolus dose of sporozoites in CHMI compared to multiple exposures occurring naturally, comparative studies on wild and lab-cultivated parasites and mosquito vectors, CHMI-mosquito studies in malaria endemic settings in Africa, impact of natural immunity and hemoglobinopathies on CHMI outcomes, development of long-term in-vitro culture of P. vivax and cryopreserved Plasmodium vivax sporozoites (PvSPZ).
Acknowledgements
The authors acknowledge European and Developing Countries Clinical Trials Partnership (EDCTP) and European Union for granting fellowship support to WS. We thank also Dr. Jutta Reinhard-Rupp, Patricia Schaffner and Vanessca Bohling at Merck Healthcare KGaA, Darmstadt, Germany for facilitating hosting of the fellow (WS) and overall support and mentorship, and Addis Ababa University for supporting the fellowship and granting sabbatical leave for fellow.
Abbreviations
- CHMI
Controlled human malaria infection
- PfSPZ
Plasmodium falciparum sporozoite
- PvSPZ
Plasmodium vivax sporozoite
- P.falciparum
Plasmodium falciparum
- P. vivax
Plasmodium vivax
- RCT
Randomized controlled trial
- TBS
Thick blood smears
- CPS
Chemoprophylaxis
- qPCR
Quantitative polymerase chain reaction
- G6PD
Glucose -6-phosphate dehydrogenase
- DVI
Direct venous inoculation
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ID
Intraderma
- IM
Intramuscular
- SAE
Serious adverse event
Authors' contributions
WS developed proposal, involved in data collection, entry, analysis, and writing the manuscript. WB, OY and AT were involved in proposal development, and read final manuscript. EE was involved in manuscript preparation and editing. CO conceived proposal, and proofread final manuscript. All authors read and approved the final manuscript.
Authors' information
WS (PhD) EDCTP is a fellow at Merck Healthcare KGaA, Darmstadt, Germany, and an associate professor of Pharmacology at Department of Pharmacology & Clinical Pharmacy, College of Health Sciences, Addis Ababa University, Ethiopia. WB (PhD) is Clinical Pharmacology Director, Translational Medicine, is an employee of Merck Serono S.A., an affiliate of Merck KGaA. OY (MD, PhD) is Medical Lead, Translational Medicine, is an employee of Merck Healthcare KGaA, Darmstadt, Germany. AT (MD) is Global Patient Safety Lead, is an employee of Merck Healthcare KGaA, Darmstadt, Germany. EE (PhD) is a professor of Pharmacology at Department of Pharmacology at Department of Pharmacology & Clinical Pharmacy, College of Health Sciences, Addis Ababa University, Ethiopia. CO (PhD) is an employee of the Global Health Institute of Merck, Ares Trading S.A., a subsidiary of Merck KGaA, Switzerland.
Funding
This study was supported by a grant from European and Developing Countries Clinical Trials Partnership (EDCTP) with Grant Number TMA 2016IF-1778. The study was also supported by Merck Healthcare KGaA, Darmstadt, Germany. The EDCTP did not have a role in the design of the study, collection, analysis, and interpretation of data or in writing the manuscript. The co-authors WB, ÖY, AT and CO are employees of Merck Healthcare KGaA, Darmstadt, Germany or its affiliates who contributed to the study design, analysis, and interpretation of data as well as writing the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Malaria: The malaria vaccine implementation programme (MVIP), Q&A on the RTS, S malaria vaccine. WHO News letter. 2020. https://www.who.int/news-room/q-a-detail/malaria-vaccine-implementation-programme.
- 2.Engwerda CR, Minigo G, Amante FH, McCarthy JS. Experimentally induced blood stage malaria infection as a tool for clinical research. Trends Parasitol. 2012;28(11):515–521. doi: 10.1016/j.pt.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Koller R, Mombo-Ngoma G, Grobusch MP. The early preclinical and clinical development of ganaplacide (KAF156), a novel antimalarial compound. Expert Opin Investig Drugs. 2018;27(10):803–810. doi: 10.1080/13543784.2018.1524871. [DOI] [PubMed] [Google Scholar]
- 4.Stein DS, Jain JP, Kangas M, Lefèvre G, Machineni S, Griffin P, Lickliter J. Open-label, single-dose, parallel-group study in healthy volunteers to determine the drug-drug interaction potential between KAE609 (cipargamin) and piperaquine. Antimicrob Agents Chemother. 2015;59(6):3493–3500. doi: 10.1128/AAC.00340-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sulyok M, Rückle T, Roth A, Mürbeth RE, Chalon S, Kerr N, et al. DSM265 for Plasmodium falciparum chemoprophylaxis: a randomised, double blinded, phase 1 trial with controlled human malaria infection. Lancet Infect Dis. 2017;17(6):636–644. doi: 10.1016/S1473-3099(17)30139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baragaña B, Hallyburton I, Lee MC, Norcross NR, Grimaldi R, Otto TD, Proto WR, et al. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature. 2015;522(7556):315–320. doi: 10.1038/nature14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol. 2011;11(1):57–64. doi: 10.1038/nri2902. [DOI] [PubMed] [Google Scholar]
- 8.Stanisic DI, McCarthy JS, Good MF. Controlled human malaria infection: applications, advances, and challenges. Infect Immun. 2017 doi: 10.1128/IAI.00479-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shekalaghe S, Rutaihwa M, Billingsley PF, Chemba M, Daubenberger CA, James ER, et al. Controlled human malaria infection of tanzanians by intradermal injection of aseptic, purified, cryopreserved plasmodium falciparum sporozoites. Am J Trop Med Hyg. 2014;91(3):471–480. doi: 10.4269/ajtmh.14-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein JE, Rao S, Williams F, Freilich D, Luke T, Sedegah M, et al. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: an update. J Infect Dis. 2007;196(1):145–154. doi: 10.1086/518510. [DOI] [PubMed] [Google Scholar]
- 11.Roestenberg M, Mordmüller B, Ockenhouse C, Mo A, Yazdanbakhsh M, Kremsner PG. The frontline of controlled human malaria infections: a report from the controlled human infection models Workshop in Leiden University Medical Centre 5 May 2016. Vaccine. 2017;35(51):7065–7069. doi: 10.1016/j.vaccine.2017.10.093. [DOI] [PubMed] [Google Scholar]
- 12.Hodgson SH, Juma E, Salim A, Magiri C, Njenga D, Molyneux S, et al. Lessons learnt from the first controlled human malaria infection study conducted in Nairobi, Kenya. Malar J. 2015;28(14):182. doi: 10.1186/s12936-015-0671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamau E, Alemayehu S, Feghali KC, Komisar J, Regules J, Cowden J, Ockenhouse CF. Measurement of parasitological data by quantitative real-time PCR from controlled human malaria infection trials at the Walter Reed Army Institute of Research. Malar J. 2014;13:288. doi: 10.1186/1475-2875-13-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy SC, Duke ER, Shipman KJ, Jensen RL, Fong Y, Ferguson S, et al. A randomized trial evaluating the prophylactic activity of DSM265 against preerythrocytic Plasmodium falciparum infection during controlled human malarial infection by mosquito bites and direct venous inoculation. J Infect Dis. 2018;217:693–702. doi: 10.1093/infdis/jix613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro TA, Ranasinha CD, Kumar N, Barditch-Crovo P. Prophylactic activity of atovaquone against Plasmodium falciparum in humans. Am J Trop Med Hyg. 1999;60(5):831–836. doi: 10.4269/ajtmh.1999.60.831. [DOI] [PubMed] [Google Scholar]
- 16.Deye GA, Miller RS, Miller L, Salas CJ, Tosh D, Macareo L, et al. Prolonged protection provided by a single dose of atovaquone-proguanil for the chemoprophylaxis of Plasmodium falciparum malaria in a human challenge model. CID. 2012 doi: 10.1093/cid/cir770. [DOI] [PubMed] [Google Scholar]
- 17.Nyunt MM, Hendrix CW, Bakshi RP, Kumar N, Shapiro TA. Phase I/II evaluation of the prophylactic antimalarial activity of pafuramidine in healthy volunteers challenged with Plasmodium falciparum sporozoites. Am J Trop Med Hyg. 2009;80(4):528–535. [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman SL, Goh LM, Luke TC, Schneider I, Le TP, Doolan DL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 19.Arévalo-Herrera M, Vásquez-Jiménez JM, Lopez-Perez M, Vallejo AF, Amado-Garavito AB, Céspedes N, et al. Protective efficacy of Plasmodium vivax radiation-attenuated sporozoites in colombian volunteers: a randomized controlled trial. PLoS Negl Trop Dis. 2016;10(10):e0005070. doi: 10.1371/journal.pntd.0005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:5. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 21.Bijker EM, Bastiaens GJ, Teirlinck AC, van Gemert GJ, Graumans W, van de Vegte-Bolmer M, et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A. 2013;110(19):7862–7867. doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walk J, Reuling IJ, Behet MC, Meerstein-Kessel L, Graumans W, van Gemert GJ, et al. Modest heterologous protection after Plasmodium falciparum sporozoite immunization: a double-blind randomized controlled clinical trial. BMC Med. 2017;15(1):168. doi: 10.1186/s12916-017-0923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bijker EM, Schats R, Obiero JM, Behet MC, van Gemert GJ, van de Vegte-Bolmer M, et al. Sporozoite immunization of human volunteers under mefloquine prophylaxis is safe, immunogenic and protective: a double-blind randomized controlled clinical trial. PLoS ONE. 2014;9(11):e112910. doi: 10.1371/journal.pone.0112910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roestenberg M, Teirlinck AC, McCall MB, Teelen K, Makamdop KN, Wiersma J, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet. 2011;377:1770–1776. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 25.Schats R, Bijker EM, van Gemert GJ, Graumans W, van de Vegte-Bolmer M, van Lieshout L, et al. Heterologous protection against malaria after immunization with plasmodium falciparum sporozoites. PLoS ONE. 2015;10(5):e0124243. doi: 10.1371/journal.pone.0124243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mordmüller B, Surat G, Lagler H, Chakravarty S, Ishizuka AS, Lalremruata A, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542(7642):445–449. doi: 10.1038/nature21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jongo SA, Shekalaghe SA, Church LWP, Ruben AJ, Schindler T, Zenklusen I, et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am J Trop Med Hyg. 2018;99(2):338–349. doi: 10.4269/ajtmh.17-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seder RA, Chang LJ, Enama ME, Zephir KL, Sarwar UN, Gordon IJ, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341(6152):1359–1365. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 29.Epstein JE, Paolino KM, Richie TL, Sedegah M, Singer A, Ruben AJ, et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight. 2017;2(1):e89154. doi: 10.1172/jci.insight.89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genton B, D'Acremont V, Lurati-Ruiz F, Verhage D, Audran R, Hermsen C, et al. Randomized double-blind controlled Phase I/IIa trial to assess the efficacy of malaria vaccine PfCS102 to protect against challenge with P. falciparum. Vaccine. 2010;28:6573–6580. doi: 10.1016/j.vaccine.2010.07.067. [DOI] [PubMed] [Google Scholar]
- 31.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Jr, Hall T, et al. A phase I/IIa safety, immunogenicity, and efficacy bridging randomized study of a two-dose regimen of liquid and lyophilized formulations of the candidate malaria vaccine RTS, S/AS02A in malaria-naïve adults. Vaccine. 2007;25:5359–5366. doi: 10.1016/j.vaccine.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Kester KE, Cummings JF, Ofori-Anyinam O, Ockenhouse CF, Krzych U, Moris P, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS, S /AS01B and RTS, S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200(3):337–346. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 33.Edelman R, Hoffman SL, Davis JR, Beier M, Sztein MB, Losonsky G, et al. Long-term persistence of sterile immunity in a volunteer immunized with X- irradiated Plasmodium falciparum sporozoites. J Infect Dis. 1993;168(4):1066–1070. doi: 10.1093/infdis/168.4.1066. [DOI] [PubMed] [Google Scholar]
- 34.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N Engl J Med. 1997;336(2):86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 35.Stoute JA, Kester KE, Krzych U, Wellde BT, Hall T, White K, et al. Long-term efficacy and immune responses following immunization with the RTS. S Malaria Vaccine J Infect Dis. 1998;178(4):1139–1144. doi: 10.1086/515657. [DOI] [PubMed] [Google Scholar]
- 36.Kester KE, McKinney DA, Tornieporth N, Ockenhouse CF, Heppner DG, Hall T, et al. Malaria Vaccine Evaluation Group. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J Infect Dis. 2001;183(4):640–647. doi: 10.1086/318534. [DOI] [PubMed] [Google Scholar]
- 37.Ockenhouse CF, Regules J, Tosh D, Cowden J, Kathcart A, Cummings J, et al. Ad35.CS.01—RTS, S/AS01 heterologous prime-boost vaccine efficacy against sporozoite challenge in healthy malaria-naïve adults. PLoS ONE. 2015;10(7):e0131571. doi: 10.1371/journal.pone.0131571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunachie SJ, Walther M, Vuola JM, Webster DP, Keating SM, Berthoud T, et al. A clinical trial of prime-boost immunisation with the candidate malaria vaccines RTS, S/AS02A and MVA-CS. Vaccine. 2006;24:2850–2859. doi: 10.1016/j.vaccine.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 39.Gordon DM, McGovern TW, Krzych U, Cohen JC, Schneider I, LaChance R, et al. Safety, immunogenicity, and efficacy of a recombinantly produced Plasmodium falciparum circumsporozoite protein-hepatitis B surface antigen subunit Vaccine. J Infect Dis. 1995;171(6):1576–1585. doi: 10.1093/infdis/171.6.1576. [DOI] [PubMed] [Google Scholar]
- 40.Rampling T, Ewer KJ, Bowyer G, Bliss CM, Edwards NJ, Wright D, et al. Safety and high level efficacy of the combination malaria vaccine regimen of RTS, S/AS01B with chimpanzee adenovirus 63 and modified vaccinia ankara vectored vaccines expressing ME-TRAP. J Infect Dis. 2016;214(5):772–781. doi: 10.1093/infdis/jiw244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, et al. Fractional third and fourth dose of RTS, S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J Infect Dis. 2016;214(5):762–771. doi: 10.1093/infdis/jiw237. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman SL, Edelman R, Bryan JP, Schneider I, Davis J, Sedegah M, et al. Safety, immunogenicity, and efficacy of a malaria sporozoite vaccine administered with monophosphoryl lipid A, cell wall skeleton of mycobacteria, and squalane as adjuvant. Am J Trop Med Hyg. 1994;51(5):603–612. doi: 10.4269/ajtmh.1994.51.603. [DOI] [PubMed] [Google Scholar]
- 43.Hodgson SH, Ewer KJ, Bliss CM, Edwards NJ, Rampling T, Anagnostou NA, et al. Evaluation of the efficacy of ChAd63-MVA Vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Dis. 2015;211(7):1076–1086. doi: 10.1093/infdis/jiu579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herrera S, Fernández O, Manzano MR, Murrain B, Vergara J, Blanco P, et al. Successful sporozoite challenge model in human volunteers with Plasmodium vivax strain derived from human donors. Am J Trop Med Hyg. 2009;81(5):740–746. doi: 10.4269/ajtmh.2009.09-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyke KE, Laurens M, Adams M, Billingsley PF, Richman A, Loyevsky M, et al. Plasmodium falciparum malaria challenge by the bite of aseptic Anopheles stephensi mosquitoes: results of a randomized infectivity trial. PLoS ONE. 2010 doi: 10.1371/journal.pone.0013490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roestenberg M, Bijker EM, Sim BK, Billingsley PF, James ER, Bastiaens GJ, et al. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am J Trop Med Hyg. 2013;88(1):5–13. doi: 10.4269/ajtmh.2012.12-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCall MBB, Wammes LJ, Langenberg MCC, van Gemert GJ, Walk J, Hermsen CC, et al. Infectivity of Plasmodium falciparum sporozoites determines emerging parasitemia in infected volunteers. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aag2490. [DOI] [PubMed] [Google Scholar]
- 48.Langenberg MCC, Wammes LJ, McCall MBB, Bijker EM, van Gemert GJ, Graumans W, et al. Controlled human malaria infection with graded numbers of Plasmodium falciparum NF135.C10- or NF166.C8-infected mosquitoes. Am J Trop Med Hyg. 2018;99(3):709–712. doi: 10.4269/ajtmh.18-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heppner DG, Gordon DM, Gross M, Wellde B, Leitner W, et al. Safety, immunogenicity, and efficacy of Plasmodium falciparum repeatless circumsporozoite protein vaccine encapsulated in liposomes. J Infect Dis. 1996;174(2):361–366. doi: 10.1093/infdis/174.2.361. [DOI] [PubMed] [Google Scholar]
- 50.Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89. doi: 10.1186/1756-3305-4-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spring MD, Cummings JF, Ockenhouse CF, Dutta S, Reidler R, Angov E, et al. Phase 1/2a study of the malaria vaccine candidate apical membrane antigen-1 (AMA-1) administered in adjuvant system AS01B or AS02A. PLoS ONE. 2009;4(4):e5254. doi: 10.1371/journal.pone.0005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laurens MB, Billingsley P, Richman A, Eappen AG, Adams M, Li T, et al. Successful human infection with P. falciparum using three aseptic Anopheles stephensi mosquitoes: a new model for controlled human malaria infection. PLoS ONE. 2013;8(7):e68969. doi: 10.1371/journal.pone.0068969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talley KA, Healy SA, Finney SO, Murphy SC, Kublin J, Salas CJ, et al. Safety and comparability of controlled human plasmodium falciparum infection by mosquito bite in malaria-naïve subjects at a new facility for sporozoite challenge. PLoS ONE. 2014 doi: 10.1371/journal.pone.0109654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bastiaens GJ, van Meer MP, Scholzen A, Obiero JM, Vatanshenassan M, van Grinsven T, et al. Safety, immunogenicity, and protective efficacy of intradermal immunization with aseptic, purified, cryopreserved Plasmodium falciparum sporozoites in volunteers under chloroquine prophylaxis: a randomized controlled trial. Am J Trop Med Hyg. 2016;94(3):663–673. doi: 10.4269/ajtmh.15-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thompson FM, Porter DW, Okitsu SL, Westerfeld N, Vogel D, Todryk S, et al. Evidence of blood stage efficacy with a virosomal malaria vaccine in a phase iia clinical trial. PLoS ONE. 2008;3(1):e1493. doi: 10.1371/journal.pone.0001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, et al. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge. Infect Immun. 2006 doi: 10.1128/IAI.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walther M, Dunachie S, Keating S, Vuola JM, Berthoud T, Schmidt A, et al. Safety, immunogenicity and efficacy of a pre-erythrocytic malaria candidate vaccine, ICC-1132 formulated in Seppic ISA 720. Vaccine. 2005;23(7):857–864. doi: 10.1016/j.vaccine.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 58.Walther M, Thompson FM, Dunachie S, Keating S, Todryk S, Berthoud T, et al. Safety, immunogenicity, and efficacy of prime-boost immunization with recombinant poxvirus FP9 and modified vaccinia virus ankara encoding the full-length Plasmodium falciparum circumsporozoite protein. Infect Immun. 2006;74(5):2706–2716. doi: 10.1128/IAI.74.5.2706-2716.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Churcher TS, Sinden RE, Edwards NJ, Poulton ID, Rampling TW, Brock PM, et al. Probability of transmission of malaria from mosquito to human is regulated by mosquito parasite density in naïve and vaccinated hosts. PLoS Pathog. 2017;13(1):e1006108. doi: 10.1371/journal.ppat.1006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beier JC, Davis JR, Vaughan JA, Noden BH, Beier MS. Quantitation of Plasmodium falciparum sporozoites transmitted in vitro by experimentally infected Anopheles gambiae and Anopheles stephensi. Am J Trop Med Hyg. 1991;44(5):564–570. doi: 10.4269/ajtmh.1991.44.564. [DOI] [PubMed] [Google Scholar]
- 61.Howes RE, Reiner RC, Jr, Battle KE, Longbottom J, Mappin B, Ordanovich D, Tatem AJ, et al. Plasmodium vivax transmission in Africa. PLoS Negl Trop Dis. 2015;9(11):e0004222. doi: 10.1371/journal.pntd.0004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herrera S, Solarte Y, Jordán-Villegas A, Echavarría JF, Rocha L, Palacios R, et al. Consistent safety and infectivity in sporozoite challenge model of Plasmodium vivax in malaria-naive human volunteers. Am J Trop Med Hyg. 2011;84(Suppl 2):4–11. doi: 10.4269/ajtmh.2011.09-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evaluation of reproducibility of a sporozoite challenge model for Plasmodium vivax in human volunteers. Trial No. NCT00367380.
- 64.Arévalo-Herrera M, Forero-Peña DA, Rubiano K, Gómez-Hincapie J, Martínez NL, Lopez-Perez M, et al. Plasmodium vivax sporozoite challenge in malaria-naïve and semi-immune Colombian volunteers. PLoS ONE. 2014;9(6):e99754. doi: 10.1371/journal.pone.0099754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bennett JW, Yadava A, Tosh D, Sattabongkot J, Komisar J, Ware LA, et al. Phase 1/2a trial of Plasmodium vivax malaria vaccine candidate VMP001/AS01B in malaria-naive adults: safety, immunogenicity, and efficacy. PLoS Negl Trop Dis. 2016;10(2):e0004423. doi: 10.1371/journal.pntd.0004423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheehy SH, Spencer AJ, Douglas AD, Sim BK, Longley RJ, Edwards NJ, et al. Optimising controlled human malaria infection studies using cryopreserved P. falciparum parasites administered by needle and syringe. PLoS ONE. 2013;8(6):e65960. doi: 10.1371/journal.pone.0065960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodgson SH, Juma E, Salim A, Magiri C, Kimani D, Njenga D, et al. Evaluating controlled human malaria infection in Kenyan adults with varying degrees of prior exposure to Plasmodium falciparum using sporozoites administered by intramuscular injection. Front Microbiol. 2014;5:686. doi: 10.3389/fmicb.2014.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mordmüller B, Supan C, Sim KL, Gómez-Pérez GP, Ospina Salazar CL, Held J, et al. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centers. Malar J. 2015;14:117. doi: 10.1186/s12936-015-0628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gómez-Pérez GP, Legarda A, Muñoz J, Sim BK, Ballester MR, Dobaño C, et al. Controlled human malaria infection by intramuscular and direct venous inoculation of cryopreserved Plasmodium falciparum sporozoites in malaria-naïve volunteers: effect of injection volume and dose on infectivity rates. Malar J. 2015;14:306. doi: 10.1186/s12936-015-0817-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sim BKL, James ER, Mordmüller B, Kremsner PG, Ruben AJ, Abebe Y, et al. Consistency of infection after controlled human malaria infection with PFSPZ challenge of different age and lots. Am J Trop Med Hyg. 2017;97:345–346. [Google Scholar]
- 71.Lyke KE, Laurens MB, Strauss K, Adams M, Billingsley PF, James E, et al. Optimizing intradermal administration of cryopreserved Plasmodium falciparum sporozoites in Controlled Human Malaria Infection. Am J Trop Med Hyg. 2015 doi: 10.4269/ajtmh.15-0341). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lell B, Mordmüller B, Dejon Agobe JC, Honkpehedji J, Zinsou J, Mengue JB, et al. Impact of sickle cell trait and naturally acquired immunity on uncomplicated malaria after Controlled Human Malaria Infection in adults in Gabon. Am J Trop Med Hyg. 2018;98(2):508–515. doi: 10.4269/ajtmh.17-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, Kamate B, et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomized, double-blind phase 1 trial. Lancet Infect Dis. 2017;17(5):498–509. doi: 10.1016/S1473-3099(17)30104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.PfSPZ Challenge With Prophylaxis in Mali. Trial No. NCT02996695.
- 75.Safety, Immunogenicity, and Protective Efficacy of Radiation Attenuated Plasmodium Falciparum NF54 Sporozoites (PfSPZ Vaccine) in Healthy African Adults in Mali. Trial No .NCT02627456
- 76.Safety, Tolerability and Immunogenicity of PfSPZ Vaccine in an Age De-escalation Trial in Equatorial Guinea. Trial No. NCT02859350.
- 77.Controlled Human Malaria Infection in Semi-Immune Kenyan Adults. (CHMI-SIKA) (CHMI-SIKA) Trial No. NCT02739763. [DOI] [PMC free article] [PubMed]
- 78.Richie TL, Charoenvit Y, Wang R, Epstein JE, Hedstrom RC, Kumar S, et al. Clinical trial in healthy malaria-naïve adults to evaluate the safety, tolerability, immunogenicity and efficacy of MuStDO5, a five-gene, sporozoite/hepatic stage Plasmodium falciparum DNA vaccine combined with escalating dose human GM-CSF DNA. Hum Vaccin Immunother. 2012 doi: 10.4161/hv.22129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cummings JF, Spring MD, Schwenk RJ, Ockenhouse CF, Kester KE, Polhemus ME, et al. Recombinant Liver Stage Antigen-1 (LSA-1) formulated with AS01 or AS02 is safe, elicits high titer antibody and induces IFN-gamma/IL-2 CD4+ T cells but does not protect against experimental Plasmodium falciparum infection. Vaccine. 2010;28:5135–5144. doi: 10.1016/j.vaccine.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 80.Porter DW, Thompson FM, Berthoud TK, Hutchings CL, Andrews L, Biswas S, et al. A human Phase I/IIa malaria challenge trial of a polyprotein malaria vaccine. Vaccine. 2011;29(43):7514–7522. doi: 10.1016/j.vaccine.2011.03.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tamminga C, Sedegah M, Regis D, Chuang I, Epstein JE, Spring M, et al. Adenovirus-5-Vectored P. falciparum vaccine expressing CSP and AMA1. Part B: safety, immunogenicity and protective efficacy of the CSP component. PLoS ONE. 2011;6(10):e25868. doi: 10.1371/journal.pone.0025868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reuling IJ, van de Schans L, Coffeng LE, Lanke K, Meerstein-Kessel L, Graumans W, et al. A randomized feasibility trial comparing four antimalarial drug regimens to induce Plasmodium falciparum gametocytemia in the controlled human malaria infection model. Elife. 2018 doi: 10.7554/eLife.31549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rieckmann KH. Human immunization with attenuated sporozoites. Bull World Health Org. 1990;13:13–16. [PMC free article] [PubMed] [Google Scholar]
- 84.Nieman AE, de Mast Q, Roestenberg M, Wiersma J, Pop G, Stalenhoef A, et al. Cardiac complication after experimental human malaria infection: a case report. Malar J. 2009;8:277. doi: 10.1186/1475-2875-8-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Meer MP, Bastiaens GJ, Boulaksil M, de Mast Q, Gunasekera A, Hoffman SL, Pop G, et al. Idiopathic acute myocarditis during treatment for controlled human malaria infection: a case report. Malar J. 2014;13:38. doi: 10.1186/1475-2875-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Richie TL, Billingsley PF, Sim BK, James ER, Chakravarty S, Epstein JE, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 2015;33(52):7452–7461. doi: 10.1016/j.vaccine.2015.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nussenzweig R. Use of radiation-attenuated sporozoites in the immunoprophylaxis of malaria. Int J Nucl Med Biol. 1980;7(2):89–96. doi: 10.1016/0047-0740(80)90026-1. [DOI] [PubMed] [Google Scholar]
- 88.Njue M, Njuguna P, Kapulu MC, Sanga G, Bejon P, Marsh V, Molyneux S, Kamuya D. Ethical considerations in Controlled Human Malaria Infection studies in low resource settings: experiences and perceptions of study participants in a malaria Challenge study in Kenya, Version 2. Wellcome Open Res. 2018;3:39. doi: 10.12688/wellcomeopenres.14439.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.