Abstract
Background
Endometriosis is a condition characterised by the presence of ectopic deposits of endometrial‐like tissue outside the uterus, usually in the pelvis. The impact of laparoscopic treatment on overall pain is uncertain and a significant proportion of women will require further surgery. Therefore, adjuvant medical therapies following surgery, such as the levonorgestrel‐releasing intrauterine device (LNG‐IUD), have been considered to reduce recurrence of symptoms.
Objectives
To determine the effectiveness and safety of post‐operative LNG‐IUD in women with symptomatic endometriosis.
Search methods
We searched the following databases from inception to January 2021: The Specialised Register of the Cochrane Gynaecology and Fertility Group, CENTRAL (which now includes records from two trial registries), MEDLINE, Embase, PsycINFO, LILACS and Epistemonikos. We handsearched citation lists of relevant publications, review articles, abstracts of scientific meetings and included studies. We contacted experts in the field for information about any additional studies.
Selection criteria
We included randomised controlled trials (RCTs) comparing women undergoing surgical treatment of endometriosis with uterine preservation who were assigned to LNG‐IUD insertion, versus control conditions including expectant management, post‐operative insertion of placebo (inert intrauterine device), or other medical treatment such as gonadotrophin‐releasing hormone agonist (GnRH‐a) drugs.
Data collection and analysis
Two review authors independently selected studies for inclusion, and extracted data to allow for an intention‐to‐treat analysis. For dichotomous data, we calculated the risk ratio (RR) and 95% confidence interval (CI) using the Mantel‐Haenszel fixed‐effect method. For continuous data, we calculated the mean difference (MD) and 95% CI using the inverse variance fixed‐effect method.
Main results
Four RCTs were included, with a total of 157 women. Two studies are ongoing. The GRADE certainty of evidence was very low to low. The certainty of evidence was graded down primarily for serious risk of bias and imprecision.
LNG‐IUD versus expectant management
Overall pain: No studies reported on the primary outcome of overall pain.
Dysmenorrhoea: We are uncertain whether LNG‐IUD improves dysmenorrhoea at 12 months. Data on this outcome were reported on by two RCTs; meta‐analysis was not possible (RCT 1: delta of median visual analogue scale (VAS) 81 versus 50, P = 0.006, n = 55; RCT 2: fall in VAS by 50 (35 to 65) versus 30 (25 to 40), P = 0.021, n = 40; low‐certainty evidence).
Quality of life: We are uncertain whether LNG‐IUD improves quality of life at 12 months. One trial demonstrated a change in total quality of life score with postoperative LNG‐IUD from baseline (mean 61.2 (standard deviation (SD) 14.8) to 12 months (mean 70.3 (SD 16.2) compared to expectant management (baseline 55.1 (SD 17.0) to 57.0 (SD 33.2) at 12 months) (n = 55, P = 0.014, very low‐certainty evidence).
Patient satisfaction: Two studies found higher rates of satisfaction with LNG‐IUD compared to expectant management; however, combining the studies in meta‐analysis was not possible (n = 95, very low‐certainty evidence). One study found 75% (15/20) of those given post‐operative LNG‐IUD were "satisfied" or "very satisfied", compared to 50% (10/20) of those in the expectant management group (RR 1.5, 95% CI 0.90‐2.49, 1 RCT, n=40, very low‐certainty evidence). The second study found that fewer were "very satisfied" in the expectant management group when compared to LNG, but there were no data to include in a meta‐analysis.
Adverse events: One study found a significantly higher proportion of women reporting melasma (n = 55, P = 0.015, very low‐certainty evidence) and bloating (n = 55, P = 0.021, very low‐certainty evidence) following post‐operative LNG‐IUD. There were no differences in other reported adverse events, such as weight gain, acne, and headaches.
LNG‐IUD versus GnRH‐a
Overall pain: No studies reported on the primary outcome of overall pain.
Chronic pelvic pain: We are uncertain whether LNG‐IUD improves chronic pelvic pain at 12 months when compared to GnRH‐a (VAS pain scale) (MD ‐2.0, 95% CI ‐20.2 to 16.2, 1 RCT, n = 40, very low‐certainty evidence).
Dysmenorrhoea: We are uncertain whether LNG‐IUD improves dysmenorrhoea at six months when compared to GnRH‐a (measured as a reduction in VAS pain score) (MD 1.70, 95%.CI ‐0.14 to 3.54, 1 RCT, n = 18, very low‐certainty evidence).
Adverse events: One study suggested that vasomotor symptoms were the most common adverse events reported with patients receiving GnRH‐a, and irregular bleeding in those receiving LNG‐IUD (n = 40, very low‐certainty evidence)
Authors' conclusions
Post‐operative LNG‐IUD is widely used to reduce endometriosis‐related pain and to improve operative outcomes. This review demonstrates that there is no high‐quality evidence to support this practice. This review highlights the need for further studies with large sample sizes to assess the effectiveness of post‐operative adjuvant hormonal IUD on the core endometriosis outcomes (overall pain, most troublesome symptom, and quality of life).
Plain language summary
Use of a levonorgestrel‐releasing intrauterine device (LNG‐IUD) for reducing pain in women who have had surgery for endometriosis
What is endometriosis?
Endometriosis is a chronic pain syndrome associated with the presence of endometrial (womb‐like) tissue outside the womb, usually in the pelvis, that can lead to infertility and pelvic pain (pain below the belly button).
How is endometriosis treated?
Endometriosis is usually managed with hormonal medications, surgery, or a combination of both. The progestogen levonorgestrel is one such hormonal medication that is believed to stop the growth of endometrial tissue outside the womb.
What is the aim of the review?
The aim of this review was to assess whether the use of a LNG‐IUD was beneficial for managing associated painful symptoms and improving the quality of life and patient satisfaction in women who have recently had surgery for endometriosis.
What did the review find?
At this stage, there is not enough evidence to support the use of LNG‐IUD after surgery to reduce pain caused by endometriosis. Although there was some evidence of a benefit in reducing painful periods and improving quality of life and satisfaction when using LNG‐IUD after surgery, the certainty of evidence was low to very low, due to the small numbers of studies and study participants, as well as flaws in study design. This suggests that further studies are required before a recommendation can be made for its use.
Summary of findings
Summary of findings 1. Postoperative use of LNG‐IUD compared to expectant management for symptomatic endometriosis following surgery.
| Postoperative use of LNG‐IUD compared to no postoperative treatment for symptomatic endometriosis following surgery | ||||||
| Patient or population: Patients with symptomatic endometriosis following surgery Intervention: Postoperative use of LNG‐IUD Comparison: Postoperative expectant management | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Expectant management | Postoperative use of LNG‐IUD | |||||
| Overall pain at 12 months | No studies reported on this outcome. | |||||
| Chronic pelvic pain at 12 months | No studies reported on this outcome. | |||||
| Dysmenorrhoea at 12 months |
Tanmahasamut 2012 found a median reduction of 50 mm (0‐78) (pain scores on a 100mm VAS) P < .001 Vercellini 2003 found a median reduction of 30 mm (25‐40) (pain scores on a 100mm VAS) P = 0.021 |
Tanmahasamut 2012 found a median reduction of 81 mm (51.5 ‐87.5) (pain scores on a 100mm VAS) P < .001. Vercellini 2003 found a median reduction of 50 mm (35–65) (pain scores on a 100mm VAS) P = 0.021 |
‐ | 95 (two studies) | ⊕⊝⊝⊝ VERY LOWa,b | We are uncertain whether post‐operative LNG‐IUD reduces dysmenorrhoea in women with symptomatic endometriosis. |
| Improvement of the most troublesome symptom at 12 months | No studies reported on this outcome. | |||||
| Quality of life at 12 months | Measured using Thai version SF‐12 scoring. Baseline score 56.1 (SD 16.5) to 57.0 (SD 33.2) at 12 months . | Measured using Thai version SF‐12 scoring. Baseline 61.3 (SD 16.4) to 70.3 (SD 16.2) at 12 months. | ‐ | 55 participants (1 study) | ⊕⊝⊝⊝ VERY LOWa,b | We are uncertain whether post‐operative LNG‐IUD improves quality of life in women with symptomatic endometriosis. |
| Patient satisfaction at 12 months | Measured using the Likert scale. Vercellini 2003 found 50% (10/20) of those in the expectant management group were satisfied or very satisfied (RR 1.5, 95% CI 0.90‐2.49). Tanmahasamut 2012 found that fewer were very satisfied in the expectant management group when compared to LNG‐IUD (RR 0.64, 95% CI 0.33–1.24; P = .184 as reported by trial authors). |
Measured using the Likert scale. Vercellini 2003 found 75% (15/20) of those given post‐operative LNG‐IUD were satisfied or very satisfied (RR 1.5, 95% CI 0.90‐2.49). | ‐ | 95 participants (2 studies) | ⊕⊝⊝⊝ VERY LOWa,b | We are uncertain whether post‐operative LNG‐IUD improves patient satisfaction in women with symptomatic endometriosis. |
| Adverse events | There was a significant increase in women reporting bloating (P = .021) and melasma (P = .15) in those who received post‐operative LNG‐IUD compared to those receiving no post‐operative treatment. | ‐ | 55 participants (1 study) | ⊕⊝⊝⊝ VERY LOWa,b | We are uncertain of whether post‐operative LNG‐IUD increases adverse events following surgery for symptomatic endometriosis. | |
|
The risk in the intervention group (and its 95% confidence interval (CI)) is based on theassumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). §Follow‐up duration was 12 months in all 3 trials except one (Gomes 2007), which was 6 months. CI: Confidence interval; LNG‐IUD: levonorgestrel intrauterine device; RR: Risk ratio; SD: Standard deviation; VAS: Visual analogue scale | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
a we downgraded twice for very serious imprecision (wide confidence intervals, small sample size)
b we downgraded once for serious risk of bias (high risk of performance bias)
Summary of findings 2. Postoperative use of LNG‐IUD compared to postoperative GnRH‐a for symptomatic endometriosis after surgery.
| Outcome | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (Studies) |
Certainty of the evidence
|
Comments |
|
| Assumed risk | Corresponding risk | |||||
| No postoperative treatment | Postoperative use of LNG‐IUD | |||||
| Overall pain at 12 months | No studies reported on this outcome | |||||
| Chronic pelvic pain at 12 months | The mean chronic pelvic pain score (VAS) in the control group was 37 |
MD 2 lower (20.23 lower to 16.23 higher) | ‐ | 40 participants (1 study) | ⊕⊝⊝⊝ VERY LOWa,b |
We are uncertain of the difference in efficacy between LNG‐IUD and GnRH‐a with regard to chronic pelvic pain. The evidence was downgraded due to the high risk of bias and the small sample size of the one trial included. |
| Dysmenorrhoea at 12 months | The mean score (reduction in VAS) in the control group was 0.4 |
MD 1.7 higher (0.14 lower to 3.54 higher) | ‐ | 18 participants (1 study) | ⊕⊝⊝⊝ VERY LOWa,b |
We are uncertain of the difference in efficacy between LNG‐IUD and GnRH‐a with regard to dysmenorrhoea. The evidence was downgraded due to the high risk of bias and the small sample size of the one trial included. |
| Improvement of the most troublesome symptom at 12 months | No studies reported on this outcome | |||||
| Quality of life at 12 months | No studies reported on this outcome | |||||
| Patient satisfaction at 12 months | No studies reported on this outcome | |||||
| Adverse events | The most common side effects were irregular menstrual bleeding and abdominal pain. | The most common side effects in the GnRH‐a group were vasomotor symptoms and amenorrhoea. | ‐ | 40 participants (1 study) | ⊕⊝⊝⊝ VERY LOWa,b |
We are uncertain on whether there is a difference in adverse events in women treated with post‐operative LNG‐IUD compared with GnRH‐a. |
|
The risk in the intervention group (and its 95% confidence interval (CI)) is based on theassumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). §Follow‐up duration was 12 months in both trials. CI: Confidence interval; GnRH‐a: Gonadotrophin‐releasing hormone agonist; LNG‐IUD: Levonorgestrelintrauterine device; MD: Mean difference; RR: Risk ratio;VAS: Visual Analogue Scale; | ||||||
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. | ||||||
a we downgraded twice for very serious imprecision (wide confidence interval, data from one small trial)
b we downgraded once for serious risk of bias (unclear risk of selection bias; high risk of performance bias)
Background
Description of the condition
Endometriosis is a common gynaecological disease affecting 10% of the female population (Eskenazi 1997). It is defined as the presence of ectopic deposits of endometrial‐like tissue found outside the uterus in women of reproductive age (Johnson 2017). These deposits usually, but not exclusively, occur in the pelvis and induce a chronic inflammatory reaction which can lead to infertility and pelvic pain (Ballard 2008; Kennedy 2005). Although the exact pathogenesis is uncertain, the disease is thought to be oestrogen‐driven and due to retrograde menstruation (migration of endometrial deposits into the peritoneal cavity during menstruation) (Henderson 1991; Sampson 1927; Tal 2019).
Pelvic pain is the primary complaint of women suffering from endometriosis and presents in numerous forms (Bellelis 2010; Hirsch 2016). Dysmenorrhoea is by far the most frequent symptom and form of pain reported by women with endometriosis (Bellelis 2010; Vercellini 1996). However, depending on the location of the lesions, women may also experience deep dyspareunia, and dyschezia (Seracchioli 2008; Thomassin 2004).
Laparoscopy with histological sampling is the gold standard for diagnosing endometriosis (Dunselman 2014). A clinical staging system has been developed to allow researchers and clinicians during laparoscopy to describe the extent of the disease (ASRM 2006). However, grading systems have been criticised for their inability to provide clinically relevant disease and treatment prognostication (Johnson 2017). Other modalities such as transvaginal ultrasound can aid in the diagnosis of endometriosis, however, it is less accurate for uterosacral, vaginal, and rectovaginal septum involvement (Bazot 2004).
Various treatment options exist for endometriosis, including ovarian suppression therapy, surgical treatment, or a combination of these strategies. Surgical treatment of endometriosis aims to remove visible areas of endometriosis and restore the anatomy by dividing adhesions. Surgically, there are several techniques by which endometriosis can be removed or destroyed, each with potential advantages, disadvantages, and differences in efficacy (Bafort 2020; Duepree 2002; Fedele 2004; Ford 2004; Leonardi 2020). The role of surgery differs depending upon the site of the endometriotic deposits, the extent of disease, and reproductive status of the women (Bafort 2020; NICE 2004; Nicklin 1999). Surgery may be as conservative as laparoscopic cauterisation of endometriotic deposits or as radical as hysterectomy with bilateral oophorectomy and resection of portions of the bowel.
It was previously proposed that laparoscopic treatment may improve pelvic pain associated with endometriosis, but this assertion has recently been brought into question (Bafort 2020).
Certainly, a proportion of women continue to experience pain following laparoscopic surgery or the pain recurs within one to two years after surgery (Crosignani 1996; Guo 2009; Sutton 1994; Vercellini 2000). The lesion subtype does not appear to affect the rate of recurrence and the lesions tend to re‐present as the same subtype excised in the initial surgery (Nirgianakis 2020). It is estimated that up to 50% of women will require further surgery within the first five years due to the recurrence of pain (Shakiba 2008). Therefore, adjuvant medical therapies have been considered in addition to surgery to reduce surgical treatment failures and recurrence rates. Postoperative medical therapy should theoretically induce resorption of microscopic foci and lesions that could not be surgically removed, reduce the risk of iatrogenic dissemination of endometriotic cells, and improve pain relief (ASRM 2006; Vercellini 2000). Medical therapy aims to inhibit the growth of endometriotic implants by suppression of ovarian steroids and induction of a hypo‐estrogenic state (Vercellini 1998). Anti‐oestrogens and regimens that induce either medical menopause (such as gonadotrophin‐releasing hormone agonists (GnRH‐a)) or a pseudo‐pregnant state (e.g. continuous combined oral contraceptive or progestogens) are among the systemic medical therapeutic options used (Luciano 1988). Danazol was the main agent used in the 1980s, and GnRH‐a were the standard treatment in the 1990s (Vercellini 1998). In recent years, both of these have been superseded by progestogen, with or without oestrogen treatment, due to their superior side‐effect profile.
Description of the intervention
The levonorgestrel intrauterine device (LNG‐IUD), also known as the levonorgestrel intrauterine system (LNG‐IUS), provides a mechanism of local delivery of progestogen to the uterus and pelvis. Levonorgestrel is a 19‐nortestosterone that prevents decidualisation of the stroma inducing endometriotic atrophy (Vigano 2007; Beatty 2009).
How the intervention might work
Endometriosis is an oestrogen‐dependent condition; thus, treatment strategies often involve hormonal suppression. Locally administered levonorgestrel has a profound effect on the endometrium, which becomes atrophic and inactive, while ovulation is usually not suppressed (Crosignani 1997). Its effects are predominantly localised to the endometrium with the high concentrations of levonorgestrel inducing atrophy and pseudo‐decidualisation (Maruo 2001; Nilsson 1978; Silverberg 1986). The systemic levels following LNG‐IUD administration are less than those achieved with therapeutic oral or parenteral doses of progestogens (Du 1999; Fedele 2001; Luciano 1988; Moghissi 1999; Nilsson 1978) hence side effects should theoretically be less. When provided immediately or very soon after surgical removal or ablation of endometriotic patches, levonorgestrel is expected to suppress the regeneration of endometriosis (Tanmahasamut 2012).
Why it is important to do this review
A recent Cochrane Review (Chen 2020) found inconclusive evidence on the effect of timing (pre‐operative versus post‐operative) when using systemic hormonal therapies to improve endometriosis symptoms. As LNG‐IUD is a non‐systemic hormonal therapy, it was excluded from Chen 2020. Our Cochrane Review is an update (Abou‐Setta 2013) assessing whether there is evidence for the widely used practice of post‐operative LNG‐IUD to improve surgical outcomes and to prevent secondary recurrence.
Objectives
To determine if the LNG‐IUD improves pain symptoms, quality of life, and patient satisfaction when inserted postoperatively in women undergoing surgery for endometriosis, compared with expectant management, postoperative placebo (inert IUD), or alternative postoperative medical treatment (e.g. GnRH‐a).
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) with a parallel design. We excluded quasi‐randomised and cross‐over trials.
Types of participants
Women with any stage of endometriosis who had undergone any type of surgery for endometriosis that preserved their uterus (including diagnostic laparoscopy), with surgery no more than three months prior to randomisation, were eligible for inclusion. The diagnosis of endometriosis was clinical, based on laparoscopy or laparotomy findings, with or without histology. Studies were considered for inclusion if they incorporated a subgroup of patients who met the inclusion criteria and for whom data were available for analysis.
Types of interventions
Intervention
Post‐operative insertion of LNG‐IUD
Comparison
Post‐operative expectant management
Placebo (inert IUD)
Any other medical treatment (e.g. GnRH‐a)
Types of outcome measures
In 2020, a minimum set of core outcomes was developed to assess research reporting on endometriosis outcomes. This was generated using a formal consensus method involving endometriosis specialists, researchers, and endometriosis sufferers across the globe (Duffy 2020). The Cochrane Gynaecology and Fertility Group as supported the implementation of these core outcome set for endometriosis research. As such, we assessed these outcomes in this review.
Primary outcomes
1) Overall pain
Effectiveness of treatment: pain measured by validated pain scales, for example, visual analogue pain scale (VAS) scores, the McGill Pain Questionnaire (MPQ), a pain improvement rating scale, general pain experience, and a gynaecological pain questionnaire. All time‐points were measured, with a preference of outcomes reported at 12 months post‐randomisation.
Secondary outcomes
1) Chronic pelvic pain (CPP)
2) Dysmenorrhoea
3) Improvement of most troublesome symptom
4) Quality of life, as described by women
5) Patient satisfaction with treatment, as described by women
6) Adverse outcomes (e.g. treatment intolerance, side effects of intervention)
Outcome measures
Outcomes were reported using continuous or dichotomous values. If more than one measurement of the outcome was presented, we gave priority to the binary outcome. If binary outcomes were not reported, we selected the most frequently used validated scale across the included studies.
Effectiveness of treatment with regard to pain was measured by validated pain scales, for example, visual analogue scale (VAS) pain scores, the McGill Pain Questionnaire (MPQ), a pain improvement rating scale, general pain experience, and a gynaecological pain questionnaire. VAS pain scales involved a zero to100 mm horizontal line, with two descriptors, i.e. 'no pain' at the left end and 'intolerable pain' at the right end.
Psychological outcomes were indicated by scores such as depression scores (Hamilton Depression Rating Scale (HAM‐D) score, Hospital Anxiety Depression Scale (HADS) score), and mood scores.
Quality of life outcomes was indicated by, for example, the Medical Outcomes Study Short Form 36 (SF‐36), the Social Adjustment Survey (SAS‐WR), the Sickness Impact Profile (SIP), a general health questionnaire (GHQ), the revised Sabbatsberg Sexual Rating Scale (rSSRS) and EuroQOL‐5D (EQ‐5D).
Patient satisfaction outcomes were indicated with validated scales such as the Likert‐scale questionnaire, whereby participants would select from the options of 'very satisfied', 'satisfied', 'uncertain', 'dissatisfied', or 'very dissatisfied'.
Primary and secondary outcome time points
All time points for outcome measures were extracted. Studies were expected to assess outcomes beyond six months, to exclude a placebo effect. Priority was given to measures 12 months post‐randomisation. Where this was not available, the nearest time was chosen between six and 18 months, with a preference for outcomes reported later than 12 months.
Search methods for identification of studies
In consultation with the Cochrane Gynaecology and Fertility Group Information Specialist, we searched for all published and unpublished RCTs conducted to date, with no language restriction.
Electronic searches
We searched the following electronic databases for relevant trials:
The Cochrane Gynaecology and Fertility Group (CGF) Specialised Register of Controlled Trials, ProCite platform, searched on 12 January 2021 (Appendix 1);
CENTRAL via the Cochrane Register of Studies Online (CRSO), Web platform, searched on 12 January 2021 (Appendix 2). CENTRAL now contains records from CINAHL as well as from the World Health Organization's International Clinical Trials Registry Portal (ICTRP) and the ClinicalTrials.gov trials registry at the US National Institutes of Health;
MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations), Ovid platform, searched from 1946 to 12 January 2021 (Appendix 3);
Embase, Ovid platform, searched from 1980 to 12 January 2021 (Appendix 4);
PsycINFO, Ovid platform, searched from 1806 to 12 January 2021 (Appendix 5).
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying randomised trials, which is described in the Cochrane Handbook (Version 5.1.0 chapter 6, 6.4.11).
We handsearched reference lists of relevant trials and systematic reviews retrieved by the search and contacted experts in the field to obtain additional trials. We also handsearched relevant journals and conference abstracts that are not covered in the CGF Specialised Register, in liaison with the Information Specialist.
Searching other resources
Other electronic sources of trials included:
LILACS (Latin American and Caribbean Health Science Information database), Web platform, searched on 18 January 2021;
Google Scholar, for recent trials not yet indexed in the major databases, Web platform, searched on 18 January 2021;
Epistemonikos database (www.epistemonikos.org), a multilingual database of health evidence, Web platform, searched on 18 January 2021.
Data collection and analysis
Data collection and analysis were conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Selection of studies
All relevant trials identified by the search strategy were considered for inclusion in the review, irrespective of how outcome data were reported. Two review authors (TG and EG) independently screened titles and abstracts retrieved by the search using the Covidence platform (Covidence). We then retrieved the full texts of all potentially eligible studies and contacted authors for missing texts if necessary. Two review authors (TG and EG) independently examined full‐text articles for compliance with the inclusion criteria and selected eligible studies. Any missing information that was needed to make a decision about eligibility was sought from the principal investigators of the trials. Disagreements were initially discussed between the review authors (TG and EG), any disagreements that could not be resolved were discussed with the full team (TG, EG, YC and MW). A PRISMA flow chart (Liberati 2009) documents our selection process.
Data extraction and management
Two review authors (TG and EG) independently extracted data from eligible studies. Data extracted included study characteristics and outcome data. Where studies had multiple publications, we collated the reports of the same under a single study ID with multiple references. Any disagreements within the data extraction process were resolved by discussion between the two review authors (TG and EG). Any disagreements that could not be resolved were discussed with the other review authors (YC and MW).
Assessment of risk of bias in included studies
Two review authors (TG and EG) independently assessed the included studies for risk of bias, using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). We assessed the risk of selection bias (random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting of outcomes); and other bias. Judgments were assigned as recommended in the Cochrane Handbook Section 8.5 (Higgins 2011). Disagreements were initially between TG and EG; any disagreements that could not be resolved were discussed with the other review authors (YC and MW).
Measures of treatment effect
For binary outcomes, data were extracted to allow for the calculation of the risk ratio (RR). For continuous outcomes, data were extracted to allow for the calculation of the mean difference (MD). If continuous outcome data were presented in differing formats (for example, severity of painful periods) the standardised mean difference (SMD) was calculated. Ordinal data (for example, quality of life scores) were treated as continuous data. We present the 95% confidence intervals (CI) for all outcomes.
Unit of analysis issues
The primary analysis was per woman randomised. Priority was given to measures reported 12 months post‐randomisation.
Dealing with missing data
To the extent possible, we analysed the data on an intention‐to‐treat basis (i.e. including all randomised participants in analysis, in the groups to which they were randomised). Attempts were made to obtain missing participant data from the original investigators. Where participant data were unobtainable, imputation was planned for the primary outcome only.
Missing summary and study design data were sought from the original investigators.
Assessment of heterogeneity
We assessed statistical heterogeneity by the measure of the I² statistic. An I² measurement greater than 50% was taken to indicate substantial heterogeneity (Higgins 2021). We also assessed homogeneity by visual inspection of the outcomes tables and by using the Chi2 test for heterogeneity with a 10% level of statistical significance; a P value of 0.1 was the cut‐off point for rejection of the null hypothesis of study homogeneity to limit type II errors.
Assessment of reporting biases
Reporting bias was assessed by comparing the study protocols, when available, and the methods sections to the results presented in the trial publications. If there were ten or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We planned to perform a meta‐analysis on the results where at least two studies were suitable for inclusion. If a meta‐analysis was not possible due to an insufficient number of studies, we provided only a narrative description of the results. We pooled data from studies that were sufficiently similar to make meta‐analysis appropriate. We extracted and analysed data on an intention‐to‐treat basis. For binary outcomes, the overall RR (that is, the risk of having clinical symptoms) and 95% CI were calculated using a fixed‐effect model, as per the protocol. A fixed‐effect model was chosen at the protocol stage due to the assumption that the underlying effect size would be the same for all the trials in the analysis, and that there would likely be fewer than ten included studies, increasing imprecision in the random‐effects model. For continuous data, the overall MD and 95% CI were calculated using a fixed‐effect model. When only computed effect sizes were reported, they were combined using the generic inverse variance method. Statistical analysis was performed using Review Manager Web (RevMan Web 2021).
Subgroup analysis and investigation of heterogeneity
No subgroup analysis was planned. We planned to investigate heterogeneity where found, to determine the possible sources of heterogeneity (e.g. baseline severity grading, age, or LNG‐IUD dosage) to explain the observations. If the source was located, we planned to performed subgroup analyses to determine the effect of the heterogeneity on the outcome measures. Differences between subgroups would have been assessed using the formal Test for Subgroup Differences in Review Manager Web (RevMan Web 2021).
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcome to determine whether the conclusions were robust to arbitrary decisions made regarding the eligibility and analysis. These analyses would have included consideration of whether the review conclusions would have differed if:
eligibility had been restricted to studies at low risk of bias (i.e. no high risk of selection bias);
a random‐effects model had been adopted;
alternative imputation strategies had been implemented (for example imputation of a mean rather than the last time‐point observation carried forward); or
the summary effect measure had been odds ratio rather than relative risk ratio.
Summary of findings and assessment of the certainty of the evidence
We followed Cochrane methods (Higgins 2021) to prepare 'Summary of findings' tables (Table 1; Table 2), using the GRADEpro GDT software (GRADEpro GDT). These tables evaluated the overall certainty of the body of evidence for each of the main review outcomes. We assessed the certainty of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. Judgments about evidence certainty (high, moderate, low, or very low) were made by two review authors (TG and EG) working independently, with disagreements resolved by discussion. Judgments were justified, documented, and incorporated into reporting of results for each outcome.
As per the review protocol, we extracted study data, formatted our comparisons in data tables, and prepared 'Summary of findings' tables before writing the results and conclusions of our review. The key considerations of our 'Summary of findings' tables included:
The main comparisons (LNG‐IUD versus expectant management or LNG‐IUD versus placebo IUD and LNG‐IUD versus any other medical treatment) were planned to appear at the front of the published review
We planned to present one table per comparison (LNG‐IUD versus expectant management or LNG‐IUD versus placebo IUD and LNG‐IUD versus any other medical treatment).
The outcomes presented are as follows.
Overall pain
Chronic pelvic pain
Dysmenorrhoea
Improvement of the most troublesome symptom
Quality of life
Patient satisfaction
Adverse effects
The same outcomes were presented for each comparison. The same comparisons and outcomes were reported in the abstract as in the 'Summary of findings' tables. All GRADE considerations were clearly described and were used to rank the certainty of evidence. For each assumed risk cited in the 'Summary of findings' tables we provided a source and rationale.
If a meta‐analysis was not possible, we planned to present the results narratively in a ‘Summary of findings’ table format.
Results
Description of studies
See Included studies and Characteristics of excluded studies.
Results of the search
Electronic searches and handsearches produced 531 citations, 80 of which were duplicates (Figure 1). After screening the titles and abstracts, 30 citations were considered to be potentially relevant to this review and were screened using the full‐text manuscripts. After the full‐text screening, 21 studies were excluded (10 duplicates, 11 with the wrong study design; see Figure 1). Two trials are still ongoing (Daoudom 2014; Lee 2017). We included four studies (Bayoglu 2011; Gomes 2007; Tanmahasamut 2012; Vercellini 2003). We placed three studies in Studies awaiting classification; Xu 2011 (awaiting translation from Chinese), Wang 2018, and Magos 2004 (awaiting full text).
1.
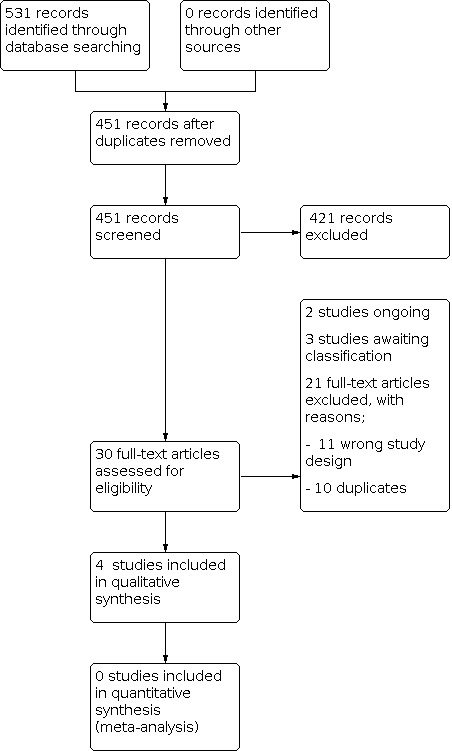
Included studies
Study design and setting
The included studies all had a parallel design. One was undertaken in Brazil (Gomes 2007), one in Turkey (Bayoglu 2011), one in Thailand (Tanmahasamut 2012), and one (Vercellini 2003) in Italy.
Participants
The included studies involved 153 participants. The trials were all small with the number of women randomised ranging from 22 to 55. The age distribution of the participants in this review ranged from 18 to 45 and there were no significant differences in baseline demographics between groups.
Intervention
Two studies (Tanmahasamut 2012; Vercellini 2003) investigated post‐operative LNG‐IUD versus expectant management. Two studies (Bayoglu 2011; Gomes 2007) investigated post‐operative LNG‐IUD versus GnRH‐a.
Outcome measures
No studies reported on overall pain, the primary outcome.
Three of four studies (Gomes 2007; Tanmahasamut 2012; Vercellini 2003) reported on the secondary outcome, dysmenorrhoea. Gomes 2007 reported chronic pelvic pain which was cyclical, which the study authors confirmed is equivalent to dysmenorrhoea.
One study (Bayoglu 2011) reported on chronic pelvic pain.
One study reported on quality of life (Tanmahasamut 2012).
Three studies reported patient satisfaction (Bayoglu 2011; Tanmahasamut 2012; Vercellini 2003). However, one study (Bayoglu 2011) did not publish their results, and we were not able to obtain the missing data from the authors. We were thus unable to include data from this study in the analysis.
Two studies reported on adverse events (Bayoglu 2011; Tanmahasamut 2012).
No studies reported on the most troublesome symptom.
Apart from one study (Gomes 2007), which reported outcomes up to six months, all studies reported outcomes up to 12 months.
In studies reporting improvement in pain outcomes (Tanmahasamut 2012; Vercellini 2003), a VAS pain scale was used with the baseline score being taken prior to surgical or medical management. The VAS scales were graded from zero to 100 mm (a score of zero representing no pain, a score of 100 representing the worst pain).
Participant satisfaction was measured in two studies (Tanmahasamut 2012; Vercellini 2003) with a Likert‐scale questionnaire, whereby participants would select from the options of 'very satisfied', 'satisfied', 'uncertain', 'unsatisfied' or 'very dissatisfied'. One study (Bayoglu 2011) recorded patient satisfaction after 12 months; however, the method of collecting data and numerical results were not published.
Quality of life was measured in one study (Tanmahasamut 2012) using a Thai version Short Form‐56. This form provided data on physical and mental domains, as well as providing a total quality of life score. Unpublished data were retrieved from the authors to allow reporting on the difference in baseline to 12‐month scores.
Additional outcomes to those pre‐specified as our primary and secondary outcomes were reported by all four studies. Bayoglu 2011 reported on the total endometriosis severity profile (TESP) at baseline and 12 months following surgery, with or without LNG‐IUD. Gomes 2007 reported the change in American Society for Reproductive Medicine (ASRM) score at first look and second‐look laparoscopy, following six months of postoperative hormonal therapy (GnRH‐a or LNG‐IUD). Tanmahasamut 2012 commented on non‐cyclical pain and dyspareunia. Vercellini 2003 reported on dyspareunia and non‐menstrual pelvic pain at 12 months following surgery with (or without) postoperative LNG‐IUD.
Excluded studies
Thirty full‐text articles were assessed for eligibility; we excluded 21 from the review (Figure 1). Ten studies were excluded for having the wrong study design. Ten were excluded as they were found to be duplicates, after the initial exclusion of duplicates. Two studies (Daoudom 2014; Lee 2017) were not included in this review, as they are ongoing. These studies meet the inclusion criteria and should be considered for the update of this review.
Out of the 11 studies excluded for the incorrect study design, one study (Acien 2019) was excluded as the participants did not have surgically confirmed endometriosis and not all participants had surgical intervention as part of the trial. Two studies (Margatho 2018; Petta 2005) were excluded as participants had over three months between their surgeries and randomisation to LNG‐IUD. One study (Alhamdan 2010) was excluded as it included women with endometriosis and/or chronic pelvic pain. Despite attempts to contact the author, we were unable to obtain the data for women with only endometriosis and as the study was over 11 years old, the raw data was likely unobtainable. Two studies (Chen 2017; Seo 2018) were excluded as GnRH‐a were used in both the intervention and control arms, so the effectiveness of solely post‐operative LNG‐IUD could not be ascertained. Three studies (Lee 2018; Lockhat 2005; Taneja 2017) were excluded as they were not RCTs. One study (Yagamuti 2014) was excluded as it did not report any of the outcomes specified in our protocol. Finally, one study (Qiu 2017) was excluded as it was a letter to the editor (Chen 2017).
Risk of bias in included studies
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation in all included studies was performed using a computer‐generated randomisation sequence in a 1:1 ratio. Treatment allocation and allocation concealment were performed in accordance with the randomisation sequence using sealed envelopes with two of the trials (Tanmahasamut 2012; Vercellini 2003), describing them as sealed and opaque envelopes, whilst two trials (Bayoglu 2011; Gomes 2007) only reported that sealed envelopes were used without reporting any further details on opacity or sequential numbering of the envelopes.
Blinding
Blinding of participants
One trial was reported as an open‐label study (Vercellini 2003); one trial (Tanmahasamut 2012) attempted to blind participants, but there were no comments on whether dummy IUDs had been used, therefore it was possible that participants could have felt their IUD strings. Two trials did not report on blinding (Bayoglu 2011; Gomes 2007), but it is likely the participants were aware of their treatment as the use of dummy IUD or injections were not reported.
Blinding of outcome assessors
One trial was reported as an open‐label study (Vercellini 2003); one trial (Tanmahasamut 2012) was reported as double‐blind and two trials (Bayoglu 2011; Gomes 2007) did not comment on outcome assessor blinding.
Incomplete outcome data
In one trial (Vercellini 2003) displacement of the LNG‐IUD was observed in one woman in the LNG‐IUD group five months after insertion. In addition, one participant in each group was lost to follow‐up (at nine months in the LNG‐IUD group, and seven months in the control group). In the second trial (Bayoglu 2011), no drop‐outs were reported. In the third trial (Tanmahasamut 2012), four women (three in the control group and one in the LNG‐IUD group) were lost to follow‐up and one woman was removed from the control group due to protocol violation. Nevertheless, as all three trials used an intention‐to‐treat analysis, and the number of withdrawals was small, they were all considered to be at low risk of bias.
In the fourth trial (Gomes 2007), four patients were excluded (one in the LNG‐IUS group and three in the GnRH‐a group) because they declined second‐look laparoscopy. There was no evidence to suggest that the analysis corrected for bias, or that sensitivity analyses were performed showing that the missing data made little difference to the outcome; as such, the missing data could have influenced the true value, therefore was deemed at high risk of bias.
Selective reporting
Trial protocols were not available, but no evidence of selective reporting was evident from the trial reports.
Other potential sources of bias
No other potential source of bias was identified for these trials.
Effects of interventions
LNG‐IUD versus expectant management
Primary outcome
Overall pain
No studies reported on the primary outcome, overall pain.
Secondary outcomes
Chronic pelvic pain
No studies reported on this outcome.
Dysmenorrhoea
Two trials (Tanmahasamut 2012; Vercellini 2003) comprising 95 participants assessed the effect of post‐operative LNG‐IUD on dysmenorrhoea; however, meta‐analysis was not possible. In both trials, participants were asked to report pain scores using a VAS scoring system. Due to the abnormal distribution of results, the studies were unable to report mean scores for meta‐analysis. As such, the median reduction scores were reported.
One study (Tanmahasamut 2012) found a median reduction of 81 mm with post‐operative LNG‐IUD compared to 50 mm in the expectant management group (P < 0.001). The second study (Vercellini 2003) found a reduction in VAS pain score by 50 mm (interquartile range (IQR) 35 mm to 65 mm) with post‐operative LNG‐IUD compared to 30 mm (IQR 25 mm to 40 mm) in the expectant group (P = .012).
Most troublesome symptom
No studies reported on this outcome.
Quality of life
One study of 55 participants (Tanmahasamut 2012) assessed quality of life (Table 3) using the Thai version SF‐36 form, and found an increase (P = 0.014) in the change in total quality of life mean score from baseline (61.2 (standard deviation (SD) 14.8) to 12 months (70.3 (SD 16.2)) with LNG‐IUD, compared to expectant management (from baseline 55.1 (SD 17.0) to 12 months 57.0 (SD 33.2)). There was a significant increase in the physical subscale score (P = .015) with post‐operative LNG‐IUD (baseline mean 56.8 (SD 17.5) to 68.0 (SD 16.1) at 12 months) compared to expectant management (baseline 55.1 (SD 17.0) to 54.9 (SD 32.1) at 12 months), but not the mental subscale score (P = .229).
1. Post‐operative LNG‐IUD versus no post‐operative treatment quality of life scores.
| Quality of life | LNG‐IUD | Control | P value |
| Physical health at 0 months | 56.8 +/‐17.5 | 55.1 +/‐ 17.0 | |
| Mental health at 0 months | 61.2 +/‐ 14.8 | 53.7 +/‐ 15.1 | |
| Total score at 0 months | 61.3 +/‐ 16.4 | 56.1 +/‐ 16.5 | |
| Physical health at 6 months | 63.4 +/‐ 15.3 | 56.1 +/‐ 29.6 | |
| Mental health at 6 months | 65.6 +/‐ 13.2 | 52.5 +/‐ 28.4 | |
| Total score at 6 months | 66.6 +/‐ 12.8 | 57.2 +/‐ 30.1 | |
| Physical health at 12 months | 68.3 +/‐ 16.1 | 54.9 +/‐ 32.1 | .229 |
| Mental health at 12 months | 68.0 +/‐ 16.4 | 53.9 +/‐ 32.1 | 0.36 |
| Total score at 12 months | 70.3 +/‐ 16.2 | 57.0 +/‐ 33.2 | .014* |
Quality of life scores from Tanmahasamut 2012.
Statistical significance was performed for 12‐month results only.
* indicates statistical significance
Patient satisfaction
Two trials (Tanmahasamut 2012; Vercellini 2003) comprising 95 participants, assessed satisfaction, however combining the studies in meta‐analysis was not possible. We are uncertain of the benefits of LNG‐IUD on satisfaction. Although, both studies found that more women were satisfied or very satisfied with their treatment results in the LNG‐IUD group, the confidence intervals (CI) include the line of no effect.
Tanmahasamut 2012 found that the proportion of participants reporting that they were very satisfied was lower in the expectant management group when compared to LNG‐IUD, but there were no available data to include in the meta‐analysis. The trial authors reported that the CI included the line of no effect (RR 0.64, 95% CI 0.33 to 1.24; P = .184 as reported by trial authors, with no further information). Vercellini 2003 found that 75% (15/20) of those given post‐operative LNG‐IUD were satisfied or very satisfied compared with 50% (10/20) of those in the expectant management group; however, the CIs include the line of no effect (RR 1.5, 95% CI 0.90 to 2.49, 1 RCT, n=40, very low‐certainty evidence; Analysis 1.1). Care should be taken when interpreting these results given the small sample size, imprecision, and risk of bias due to insufficient participant blinding. The certainty of evidence has been downgraded to very low‐certainty evidence and the results should be interpreted as such.
1.1. Analysis.

Comparison 1: Postoperative use of LNG‐IUD compared with expectant treatment in women with endometriosis, Outcome 1: Patient satisfaction
Adverse events
One study of 55 participants (Tanmahasamut 2012) reported adverse events (Table 4). More women reported bloating (P = .021) and melasma (P = .015) in those who received post‐operative LNG‐IUD. There were no differences in other reported adverse events such as weight gain, acne, and headaches.
2. Comparing adverse events in post‐operative IUD versus no treatment post‐operatively .
| Adverse event | LNG‐IUD group n =2 7 (%) | Expectant group n = 23 (%) | P‐value |
| Bloating | 10 (37.0) | 16 (69.6) | 0.021* |
| Acne | 16 (59.3) | 13 (56.5) | 0.849 |
| Oily skin | 20 (74.1) | 16 (69.6) | 0.730 |
| Melasma | 6 (22.2) | 0 (0) | 0.015* |
| Weight gain | 17 (62.9) | 13 (56.5) | 0.651 |
| Breast tenderness | 18 (66.7) | 9 (39.1) | 0.053 |
| Headache | 13 (48.1) | 17 (73.9) | 0.066 |
| Nausea | 11 (40.7) | 9 (39.1) | 0.910 |
| Leukorrhoea | 1 (3.7) | 3 (13.0) | 0.233 |
Adverse events from Tanmahasamut 2012
*Indicates statistical significance
LNG‐IUD versus GnRH‐a
Primary outcome
Overall pain
No studies reported on the primary outcome, overall pain.
Secondary outcomes
Chronic pelvic pain
We are uncertain whether LNG‐IUD improves chronic pelvic pain (VAS) at 12 months when compared to GnRH‐a (MD ‐2.0, 95% CI ‐20.2 to 16.2, 1 RCT, n = 40, very low‐certainty evidence; Analysis 2.1; Figure 4).
2.1. Analysis.

Comparison 2: Postoperative use of LNG‐IUD compared with GnRH‐a in women with endometriosis, Outcome 1: Chronic pelvic pain
4.

Chronic pelvic pain outcome ‐ LNG‐IUD Vs GnRH‐a
Dysmenorrhoea
One study reported on dysmenorrhoea measured as a reduction in VAS pain score (Gomes 2007). We are uncertain whether LNG‐IUD improves dysmenorrhoea at 6 months when compared to GnRH‐a. (MD 1.70, 95%.CI ‐0.14 to 3.54, 1 RCT, n = 22, very low‐certainty evidence; Analysis 2.2; Figure 5).
2.2. Analysis.

Comparison 2: Postoperative use of LNG‐IUD compared with GnRH‐a in women with endometriosis, Outcome 2: Dysmenorrhoea
5.

Most troublesome symptom
No studies reported on this outcome.
Quality of life
No studies reported on this outcome.
Patient satisfaction
One study recorded this outcome; however, in the published report, no data was reported for analysis. It was reported, however, that GnRH‐a resulted in a higher patient satisfaction score than LNG‐IUD.
Adverse events
One study of 40 participants (Bayoglu 2011) reported on adverse events. Vasomotor symptoms were the most common symptoms reported with patients receiving GnRH‐a, and irregular bleeding was most common in those receiving LNG‐IUD (Table 5).
3. Comparing adverse events with post‐operative LNG‐IUD versus post‐operative GnRH‐a.
| Adverse event | LNG‐IUD n = 20 (%) | GnRH n = 20 (%) |
| Irregular bleeding | 13 (65) | 0 (0) |
| One‐sided lower abdominal pain | 8 (40) | 0 (0) |
| Weight gain | 2 (10) | 1 (5) |
| Amenorrhoea | 0 (0) | 6 (30) |
| Vasomotor symptoms | 0 (0) | 10 (50) |
| Simple ovarian cysts | 11 (55) | 0 (0) |
Adverse events from Bayoglu 2011
Discussion
Summary of main results
No studies reported on the primary outcome of overall pain.
With respect to secondary outcomes, we are uncertain whether post‐operative LNG‐IUD improves dysmenorrhoea at 12 months, compared to expectant management, as data on this outcome were reported by two small RCTs providing low‐certainty evidence. Similarly, we are uncertain whether post‐operative LNG‐IUD improves quality of life (one small trial) or satisfaction (two small trials) at 12 months (very low‐certainty evidence).
Finally, compared to post‐operative GnRH‐a, we are uncertain whether post‐operative LNG‐IUD improves dysmenorrhoea or chronic pelvic pain.
Overall completeness and applicability of evidence
The uncertainty of this review is due to the small number of RCTs reporting on the endometriosis core outcomes (Duffy 2020); no trials reported on the primary outcome (overall pain), nor the secondary outcome, most troublesome symptom. Moreover, the studies that were included were small and lacked appropriate data for meta‐analysis. Furthermore, the included trials lacked proper reporting on all aspects of potential biases, thereby limiting the internal validity of the results. Additional large trials are needed, reporting the suggested endometriosis outcomes (overall pain, most troublesome symptom, and quality of life) in order to produce clinically relevant effect estimates.
Quality of the evidence
The available data came from four small trials that included 153 women in total. All four were at high risk of bias due to lack of blinding. One (Vercellini 2003) was an open‐label study; three (Bayoglu 2011; Gomes 2007; Tanmahasamut 2012) did not report dummy injections or IUDs, so it is possible the participants were aware of their allocated group.
Unfortunately, there were too few studies reporting the pre‐specified outcomes for most of the planned comparisons; as such, meta‐analysis was not possible. Using GRADE methods of assessment, as shown in Table 1, the certainty of the evidence was graded as very low due to the inclusion of only two small trials, with a high risk of bias and imprecision.
Potential biases in the review process
Numerous steps were taken during the process of this review to reduce bias. Firstly, the search was developed and run by the Cochrane Gynaecology and Fertility group with no limitations in language or date. Secondly, screening and extraction were conducted independently by two review authors. Any conflicts that could not be resolved were discussed with the other review authors. Despite efforts to minimise bias, there were multiple outcomes assessed using evidence from a small number of trials, with a small sample size, which may have introduced bias and resulted in difficulties extrapolating clinically relevant conclusions. Furthermore, three studies remain in 'awaiting classification' as we were unable to translate the Chinese text for one of the studies, despite many attempts to recruit a translator. The second study is complete according to trial registries, but there are no published results. It was not clear whether the right population was included in the third study. These studies introduce the possibility of publication bias in this review.
Agreements and disagreements with other studies or reviews
In this updated review, one additional study was included (Gomes 2007), providing additional data on the comparison of postoperative LNG‐IUD vs GnRH‐a with regard to dysmenorrhoea and adverse events.
The current review findings contrast with those of the previous version (Abou‐Setta 2013). Although the findings of this review do demonstrate a possible benefit of post‐operative LNG‐IUD when compared with expectant management, there is a vast reduction in our level of certainty, and meta‐analysis was not possible. This is due to the difference in outcomes assessed, subsequent to the introduction of the core endometriosis outcome set (Duffy 2020). These outcomes were not routinely reported on in the included trials.
Authors' conclusions
Implications for practice.
Post‐operative levonorgestrel‐releasing intrauterine device (LNG‐IUD) is widely used to reduce endometriosis‐related pain and to improve operative outcomes. This review demonstrates that there is no high‐quality evidence to support this practice. No studies investigated the effect of post‐operative LNG‐IUD compared to expectant management on overall pain or chronic pelvic pain or the most troublesome symptom. In addition, we are uncertain of the benefits of post‐operative LNG‐IUD on dysmenorrhoea or satisfaction when compared to expectant management. The evidence was provided by one or two small trials and was deemed to be of very low to low certainty.
When comparing post‐operative LNG‐IUD to gonadotrophin‐releasing hormone agonists (GnRH‐a), no studies investigated the effects on overall pain, the most troublesome symptom, or quality of life. We are uncertain of the effect of LNG‐IUD on dysmenorrhoea, chronic pelvic pain, and satisfaction compared to GnRH‐a, as the evidence was of very low certainty and came from one or two small trials.
No conclusions can be drawn with regard to the safety of post‐operative LNG‐IUD as only two small studies commented on adverse events; however, no serious adverse events were reported. Given the findings of other Cochrane Reviews assessing the safety profile of LND‐IUD in treating conditions such as heavy menstrual bleeding, endometrial hyperplasia, and contraception, we do not anticipate that there are serious adverse events associated with LNG‐IUD (French 2004; Krashin 2015; Lopez 2015; Lou 2018; Rodriguez 2020). However, larger studies are needed to evaluate the safety of LNG‐IUD following surgery for endometriosis.
Implications for research.
Well‐designed and sufficiently powered RCTs are needed to investigate the comparative effectiveness of post‐operative LNG‐IUD with active systemic medical treatment such as GnRH‐a to assess the core endometriosis outcomes (overall pain, improvement of most troublesome symptom, and quality of life) (Duffy 2020). Researchers undertaking these studies need to consider randomising women pre‐operatively, to receive the allocated treatment at the time of surgery or in the few months immediately following, and to continue the follow‐up long‐term in order to evaluate important outcomes such as recurrence of endometriosis, need for further surgery, and fertility outcomes by preventing disease progression.
What's new
| Date | Event | Description |
|---|---|---|
| 12 January 2021 | New search has been performed | Updated search for clinical trials performed. New citations added including a new RCT. Conclusions changed; excluded data from previous edition as source could not be confirmed. |
| 12 January 2021 | New citation required and conclusions have changed | New trials added, conclusions now changed. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 5 November 2012 | New citation required and conclusions have changed | New trial added, conclusions now changed. |
| 13 June 2012 | New search has been performed | Updated search for clinical trials performed. New citations added including one new RCT. Conclusions changed. |
| 20 June 2011 | New search has been performed | Summary of findings tables added for primary outcome of pain. |
| 3 March 2011 | New citation required and conclusions have changed | Updated search for clinical trials performed. New citations added including a new RCT. Conclusions changed. |
| 22 February 2009 | New search has been performed | Updated search for clinical trials performed. New citations added but no new RCTs found. Conclusions not changed. |
| 20 April 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank all the staff members of the Cochrane Gynaecology and Fertility for their contributions to this review. We would also like to thank Dr Daoudom and Dr Middleton for responding to our correspondence with confirmation of their completion status. In addition, we would like to thank Dr Acien Alvarez for providing a full text of their unpublished work, and Dr Magatho, Dr Gomes and Dr Tanmahasamut for providing unpublished data.
The authors thank Dr Brett Houston, Prof Cindy Farquhar, Dr Ahmed Abou‐Setta, and Prof Hesham G Al‐Inany for their contribution to previous versions of this review.
We would like to thank Rik van Eekelen, Anne Lethaby, Claire Barker, and Roger Hart for the valuable peer review comments.
Appendices
Appendix 1. Gynaecology and Fertility specialised register search strategy
Searched 12 January 2021
ProCite platform
Keywords CONTAINS "endometriosis" or "endometriosis‐outcome" or "endometriosis scores" or "Endometriosis‐Symptoms" or "pelvic pain" or "dyschezia" or "dyspareunia" or "pain‐dyspareunia" or "pain‐endometriosis" or "The Endometriosis Health Profile" or Title CONTAINS "endometriosis" or "endometriosis‐outcome" or "endometriosis scores" or "Endometriosis‐Symptoms" or "pelvic pain" or "dyschezia" or "dyspareunia" or "pain‐dyspareunia" or "pain‐endometriosis" or "The Endometriosis Health Profile"
AND
Keywords CONTAINS "Levonorgestrel" or "levonorgestrel intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "Mirena" or "LNG‐IUS" or "intrauterine contraceptive devices" or "intrauterine devices" or "Intrauterine Devices, Medicated" or "intrauterine device" or "IUD" or "Intrauterine Releasing Devices" or "LNG20" or Title CONTAINS "Levonorgestrel" or "levonorgestrel intrauterine system" or "levonorgestrel‐releasing intrauterine device" or "Mirena" or "LNG‐IUS" or "intrauterine contraceptive devices" or "intrauterine devices" or "Intrauterine Devices, Medicated" or "intrauterine device" or "IUD" or "Intrauterine Releasing Devices" or "LNG20"
(32 records)
Appendix 2. CENTRAL via The Cochrane Register of Studies Online (CRSO) search strategy
Searched 12 January 2021
Web platform
#1 MESH DESCRIPTOR Endometriosis EXPLODE ALL TREES 833
#2 Endometrio*:TI,AB,KY 2734
#3 (pelvic adj2 pain*):TI,AB,KY 1877
#4 dyspareunia:TI,AB,KY 1085
#5 dyschezia:TI,AB,KY 41
#6 #1 OR #2 OR #3 OR #4 OR #5 4914
#7 MESH DESCRIPTOR Levonorgestrel EXPLODE ALL TREES 896
#8 MESH DESCRIPTOR Intrauterine Devices, Medicated EXPLODE ALL TREES 413
#9 levonorgestrel:TI,AB,KY 1809
#10 mirena:TI,AB,KY 154
#11 LNG‐IUS:TI,AB,KY 247
#12 LNG‐IUD:TI,AB,KY 89
#13 (LNG releasing):TI,AB,KY 14
#14 (progest* adj5 intrauterine):TI,AB,KY 57
#15 (progest* adj5 intra‐uterine):TI,AB,KY 2
#16 (intrauterine device*):TI,AB,KY 1357
#17 (intra‐uterine device*):TI,AB,KY 86
#18 (intra‐uterine system*):TI,AB,KY 13
#19 (intrauterine system*):TI,AB,KY 390
#20 (Skyla or Jaydess):TI,AB,KY 20
#21 (IUS or IUD):TI,AB,KY 1390
#22 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 3251
#23 #6 AND #22 178
Appendix 3. MEDLINE search strategy
Searched from 1946 to 12 January 2021
Ovid platform
1 exp Endometriosis/ (22196) 2 Endometrio$.tw. (31405) 3 (pelvic adj2 pain).tw. (10091) 4 dyspareunia.tw. (4043) 5 or/1‐4 (45461) 6 exp Levonorgestrel/ (4337) 7 exp Intrauterine Devices, Medicated/ (3417) 8 levonorgestrel.tw. (4789) 9 mirena.tw. (298) 10 LNG‐IUS.tw. (751) 11 LNG‐IUD.tw. (155) 12 LNG releasing.tw. (40) 13 (progest$ adj5 intrauterine).tw. (459) 14 (progest$ adj5 intra‐uterine).tw. (34) 15 intrauterine device$.tw. (5585) 16 intra‐uterine device$.tw. (411) 17 intra‐uterine system$.tw. (52) 18 intrauterine system.tw. (1153) 19 (Skyla or Jaydess).tw. (20) 20 (IUS or IUD).tw. (8210) 21 or/6‐20 (16388) 22 randomized controlled trial.pt. (520465) 23 controlled clinical trial.pt. (94008) 24 randomized.ab. (506731) 25 placebo.tw. (220343) 26 clinical trials as topic.sh. (194196) 27 randomly.ab. (349338) 28 trial.ti. (233182) 29 (crossover or cross‐over or cross over).tw. (87670) 30 or/22‐29 (1372540) 31 exp animals/ not humans.sh. (4775224) 32 30 not 31 (1262616) 33 5 and 21 and 32 (103)
Appendix 4. Embase search strategy
Searched from 1980 to 12 January 2021
Ovid platform
1 exp ENDOMETRIOSIS/ (37366) 2 Endometrio$.tw. (45712) 3 (pelvic adj2 pain).tw. (16597) 4 dyspareunia.tw. (7560) 5 or/1‐4 (69038) 6 exp LEVONORGESTREL/ (11976) 7 exp intrauterine contraceptive device/ (16636) 8 levonorgestrel.tw. (6188) 9 mirena.tw. (1613) 10 LNG‐IUS.tw. (1175) 11 LNG‐IUD.tw. (322) 12 (progest$ adj5 intrauterine).tw. (542) 13 (progest$ adj5 intra‐uterine).tw. (63) 14 intrauterine device$.tw. (6623) 15 intra‐uterine device$.tw. (492) 16 intra‐uterine system$.tw. (103) 17 intrauterine system$.tw. (1844) 18 (Skyla or Jaydess).tw. (118) 19 (IUS or IUD).tw. (8648) 20 or/6‐19 (28338) 21 Clinical Trial/ (989532) 22 randomized Controlled Trial/ (636916) 23 exp randomization / (89856) 24 Single Blind Procedure/ (41459) 25 Double Blind Procedure/ (177538) 26 Crossover Procedure/ (65742) 27 Placebo/ (348188) 28 Randomi?ed controlled trial$.tw. (247480) 29 Rct.tw. (40231) 30 random allocation.tw. (2127) 31 randomly allocated.tw. (37212) 32 allocated randomly.tw. (2622) 33 (allocated adj2 random).tw. (833) 34 Single blind$.tw. (25997) 35 Double blind$.tw. (210024) 36 ((treble or triple) adj blind$).tw. (1257) 37 placebo$.tw. (314719) 38 prospective study/ (653806) 39 or/21‐38 (2295998) 40 case study/ (75043) 41 case report.tw. (425262) 42 abstract report/ or letter/ (1138778) 43 or/40‐42 (1627724) 44 39 not 43 (2240296) 45 5 and 20 and 44 (399)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 12 January 2021
Ovid platform
1 exp Gynecological Disorders/ (1861) 2 Endometrio$.tw. (313) 3 (pelvic adj2 pain).tw. (681) 4 dyspareunia.tw. (600) 5 or/1‐4 (3100) 6 exp Intrauterine Devices/ (151) 7 levonorgestrel.tw. (125) 8 mirena.tw. (11) 9 LNG‐IUS.tw. (31) 10 LNG‐IUD.tw. (8) 11 (progest$ adj5 intrauterine).tw. (13) 12 (progest$ adj5 intra‐uterine).tw. (1) 13 levonorgestrel‐releasing intrauterine system.tw. (22) 14 levonorgestrel‐releasing.tw. (30) 15 or/6‐14 (260) 16 5 and 15 (13)
Data and analyses
Comparison 1. Postoperative use of LNG‐IUD compared with expectant treatment in women with endometriosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Patient satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 2. Postoperative use of LNG‐IUD compared with GnRH‐a in women with endometriosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Chronic pelvic pain | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Dysmenorrhoea | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bayoglu 2011.
| Study characteristics | ||
| Methods | Design: randomised controlled study. Setting: the reproductive endocrinology unit of a tertiary, research and education hospital. Randomisation: computer‐generated randomisation sequence. Follow‐up: 12 months |
|
| Participants | Women with severe endometriosis (revised the American Fertility Society classification > 40) and endometriosis‐related chronic pelvic pain (CPP). | |
| Interventions | Randomisation to Levonorgestrel‐Intrauterine system (LNG‐IUS) or depot Gonadotrophin releasing agonist (GnRH‐a) within 3 days after conservative laparoscopic surgery. GnRH‐a dose was repeated monthly for 6 months. | |
| Outcomes | Main outcome measure(s): Scores of CPP using a visual analogue scale (VAS) and total endometriosis severity profile. | |
| Notes | Corresponding author was contacted for clarification of data but no response was received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported the use of computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Authors only reported that sealed envelopes were used without any further details on opacity or sequential numbering of the envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not reported and use of a double‐dummy technique was not reported. One intervention is an intrauterine device and another an injection, so it is likely the patient would have been aware of their intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors was not reported. Use of a double‐dummy technique was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs were reported. Intention‐to‐treat principle was used for all analyses. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available but outcomes in methods and results are similar. |
| Other bias | Low risk | The authors reported that "there were no statistically significant differences between the two groups in terms of age, parity, gravity, and revised AFS scores (P > .05)". No other biases were evident from the trial report. |
Gomes 2007.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Women with endometriosis undergoing diagnostic laparoscopy to diagnose endometriosis | |
| Interventions | Postoperative Levonorgestrel‐Intrauterine Device (LNG‐IUD) or Gonadotrophin Releasing Hormone agonist (GnRH‐a) | |
| Outcomes | American Score for Reproductive Medicine (ASRM) score, chronic pelvic pain that is cyclical and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated system |
| Allocation concealment (selection bias) | Unclear risk | ‘sealed envelopes' but no other details if opaque and serially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of dummy coil or injections. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There is no mention if those assessing Visual Analogue Scale (VAS) scores knew the interventions the participants were assigned. However, they do comment that those carrying out second look laparoscopy did not know which treatment was given. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/22 patients excluded because they declined second look laparoscopy. |
| Selective reporting (reporting bias) | Low risk | No concerns, as all outcomes and time points reported |
| Other bias | Low risk | The authors have reported that there were no statistically significant differences between the two groups with respect to baseline data, including age, stage of endometriosis, smoking habits, parity, and use of medication before the study outset. |
Tanmahasamut 2012.
| Study characteristics | ||
| Methods | Design: double‐blind, parallel‐group, randomised controlled trial. Setting: Single centre gynaecologic endocrinology unit (University setting). Randomisation: Computer‐generated list of random numbers. Follow‐up: 12 months |
|
| Participants | Women (n = 55) with moderate to severe dysmenorrhoea, chronic pelvic pain, or both for more than 6 months and who were scheduled for laparoscopic surgery. | |
| Interventions | Randomisation to immediate Levonorgestrel‐Intrauterine Device (LNG‐IUD) insertion or no postoperative treatment (expectant management) after laparoscopic treatment of endometriotic lesions. | |
| Outcomes | Main outcome measures: severity of dysmenorrhoea. Secondary outcomes: severity of chronic pelvic pain and dyspareunia, changes in quality of life, overall satisfaction of the treatment, and side effects. | |
| Notes | Authors reported that the trial was "supported by the research fund of the Gynecologic Endocrinology Unit, Faculty of Medicine Siriraj Hospital, Mahidol University, Thailand" and that "Bayer Schering Pharma Company provided the levonorgestrel‐releasing intrauterine system". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported the use of computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Authors reported that "the codes were individually contained in a sealed opaque envelope, which was sequentially numbered and then chronologically opened in the operating room only after an eligible patient was identified". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Authors reported that "the patients and assessor nurse were blinded to the treatment groups" but not clear how patients were prevented from physically feeling the vaginally placed IUD strings. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors reported that "the patients and assessor nurse were blinded to the treatment groups". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors reported that one patient in the LNG‐IUD group was lost to follow‐up as compared with three in the control group. Also one patient was removed from the study due to a protocol violation. The authors analysed all the randomised patients with the exception of the patient with the protocol violation (e.g. 54/55) using last evaluation carried forward method. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available but outcomes in methods and results are similar. |
| Other bias | Low risk | Authors reported that "the two groups were comparable in age, weight, body mass index, obstetric history, and baseline pain scores" and provided statistical evidence of similarity. |
Vercellini 2003.
| Study characteristics | ||
| Methods | Design: open‐label, parallel‐group, randomised controlled trial. Setting: a tertiary care and referral centre for women with endometriosis. Randomisation: computer‐generated randomisation sequence. Follow‐up: 12 months |
|
| Participants | Parous women (n = 40) with moderate to severe dysmenorrhoea undergoing first‐line operative laparoscopy for symptomatic endometriosis. | |
| Interventions | Randomisation to immediate Levonorgestrel‐Intrauterine Device (LNG‐IUD) insertion or no postoperative treatment (expectant management) after laparoscopic treatment of endometriotic lesions. | |
| Outcomes | Main outcome measure(s): proportion of women with recurrence of moderate to severe dysmenorrhoea in the two study groups one year after surgery, and overall degree of satisfaction with treatment. | |
| Notes | Corresponding author was contacted for unpublished data but no response was received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported the use of computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Authors reported using serially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported as open‐label study (i.e. no blinding of participants and personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Reported as open‐label study (i.e. no blinding of outcome assessors). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors reported that "In one patient the LNG‐IUD was expelled after five months. One subject in each group was lost to follow‐up". Intention‐to‐treat analysis used for all analyses. |
| Selective reporting (reporting bias) | Low risk | Protocol was not available, but outcomes described in the methods section and results section match. |
| Other bias | Unclear risk | The authors reported that "the distribution of the study variables was similar in both groups" without providing any statistical support. No other biases were evident from the trial report. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acien 2019 | A diagnosis of endometriosis was not confirmed by surgery +/‐ histology. Not all participants had surgical intervention as part of the trial. Note: subsequent to the search being conducted a new publication of this study has been released (Acién 2021). |
| Alhamdan 2010 | Excluded as we were unable to get a response from authors to obtain data pertaining only to women with endometriosis. |
| Carvalho 2018 | Same patient cohort as Margatho 2018 and 2020, therefore excluded as it was a duplicate and had a large time interval between surgery and randomisation. |
| Chen 2017 | Participants from both groups had Gonadotrophin Releasing Hormone agonist (GnRH) and there was no published data or indication of completion. |
| de Sá Rosa e Silva 2006 | Surgery for endometriosis was performed 3 months to 2 years prior to enrolment in the study. Companion publication of another identified trial report (Petta 2005). |
| Lee 2018 | This study was a retrospective study not a randomised control trial. |
| Lockhat 2005 | Not a randomised trial. |
| Manetta 2008 | Time from surgery to randomisation not reported. Outcome measures of interest to this review not recorded by authors. Confirmed by contact with authors. |
| Margatho 2018 | The authors kindly provided data for participants with surgically diagnosed endometriosis only. However, it was excluded as there were over 3 months between surgery and randomisation for Levonorgestrel‐releasing device (LNG‐IUD) insertion. |
| Oh 2006 | Not a randomised trial. |
| Petta 2005 | Surgery for endometriosis was performed 3 months to 2 years prior to enrolment in the study. |
| Qiu 2017 | Letter to editor regarding a trial already reviewed. |
| Rosa e Silva 2005 | Surgery for endometriosis was performed 3 months to 2 years prior to enrolment in the study. |
| Seo 2018 | GnRH‐a in both the intervention and control arms |
| Taneja 2017 | Not a randomised control trial |
| Vieira 2007 | Surgery for endometriosis was performed 3 months to 2 years prior to enrolment in the study. |
| Wong 2010 | Surgery for endometriosis was performed up to five years before randomisation . |
| Yagamuti 2014 | Did not record any outcomes pre‐specified in our protocol. |
| Yucel 2018 | Did not have surgery 3 months prior to LNG insertion. |
ASRM: American Society for Reproductive Medicine
FIGO: International Federation of Gynecology and Obstetrics
GnRH‐a: Gonadotrophin Releasing Hormone agonist
LNG‐IUD/S: Levonorgestrel Releasing Intrauterine Device/System
MPA: Medroxyprogesterone acetate
Characteristics of studies awaiting classification [ordered by study ID]
Magos 2004.
| Methods | Randomised Control Trial (RCT) |
| Participants | Females with symptomatic endometriosis stage I to IV |
| Interventions | Post‐operative levonorgestrel‐releasing intra uterine system (IUS) treatment after conservative surgery for symptomatic endometriosis stage I to IV |
| Outcomes | Reduction in severity of dysmenorrhoea, pelvic pain and deep dyspareunia as assessed by multidimensional analogue questionnaire between the group treated with levonogestrel‐IUS and controls. |
| Notes | There are no published results from this trial, however, it is recorded as completed on the WHO Trials Registry (ICTRP Search Portal, 2021). Numerous attempts were made to retrieve a copy of the results, however, due to concerns with publication bias we did not exclude the trial and future efforts should be made to obtain a full text. |
Wang 2018.
| Methods | Randomised Control Trial (RCT) |
| Participants | Women with endometriosis diagnosed by laparoscopy |
| Interventions | Group 1:pretreatment without Levonorgestrel‐Releasing Intrauterine System Group 2:pretreatment with Levonorgestrel‐Releasing Intrauterine System |
| Outcomes | PRIMARY OUTCOME: implantation rate SECONDARY OUTCOME: Live birth rate |
| Notes | Unclear if pain outcomes were recorded and whether women received IUD within 3 months of surgery. Numerous attempts were made to retrieve the full text. |
Xu 2011.
| Methods | A total of 48 patients underwent randomization into two treatment groups. The regimens of LNG‐IUS (n = 24) and COC (n = 24) were offered. The volume of ovarian endometriotic cysts was recorded before treatment and at 6, 12, 18 and 24 months. The volume of ovarian endometriotic cysts, pain score of visual analogue scale (VAS), menstrual pattern, body weight, serum CA125, and serum lipids were compared to the pretreatment level within each treatment group, as well as between two treatment groups during the same period., |
| Participants | Women with recurrent ovarian endometriosis |
| Interventions | LNG‐IUS (n = 24) and COC (n = 24) |
| Outcomes | The volume of ovarian endometriotic cysts, pain score of visual analogue scale (VAS), menstrual pattern, body weight, serum CA125 and serum lipids were compared between groups. |
| Notes | Despite numerous attemps we were unable to obtain or translate a full text. |
COC Combined Oral Contraceptive
FET: Frozen Embryo Transfer
LNG‐IUD/S: Levonorgestrel‐Releasing Intrauterine Device/System
VAS: Visual Analogue Scale
Characteristics of ongoing studies [ordered by study ID]
Daoudom 2014.
| Study name | Effectiveness of levonorgestrel‐intrauterine system (LNG‐IUS) versus depot medroxyprogesterone acetate (DMPA) in treatment of pelvic pain in clinically diagnosed endometriotic patients |
| Methods | Randomised controlled trial |
| Participants | Women with endometriosis aged 18‐45 with moderate to severe pain. |
| Interventions | LNG‐IUS and DMPA |
| Outcomes | Primary outcome: Severity of pelvic pain: measured by visual analogue scale [Time Frame: 6 months] Secondary outcomes: 1. Quality of life measured by Quesionaire SF 36 Thai version [Time Frame: 6 months] 2. Lipid profile: total cholesterol, triglyceride, LDL, HDL [Time Frame: 6 months] Measured by blood collection in mg/dl |
| Starting date | August 2015 |
| Contact information | dr.kamolrat@safefertilitycenter.com |
| Notes | Author contacted and confirmed study complete but analysis and dissemination of results incomplete. |
Lee 2017.
| Study name | Preventing recurrence of endometriosis by means of long‐acting progestogen therapy (PRE‐EMPT): report of an internal pilot, multi‐arm, randomised controlled trial incorporating flexible entry design and adaption of design based on feasibility of recruitment |
| Methods | Four‐arm randomised control trial, assessing the effectiveness of post‐operative use of LARC (levonorgestrel intrauterine system (LNG‐IUS) or depot medroxyprogesterone acetate injection (DMPA)) or comparator (combined oral contraceptive pill (COCP) or no treatment) in the treatment of endometriosis. |
| Participants | Women undergoing surgery to treat their endometriosis. |
| Interventions | LARC (levonorgestrel intrauterine system (LNG‐IUS) or depot medroxyprogesterone acetate injection (DMPA)) or comparator (combined oral contraceptive pill (COCP) or no treatment). |
| Outcomes | Improvement in pain and quality of life (QoL) |
| Starting date | March 2014 |
| Contact information | Birmingham Clinical Trials Unit, Institute of Applied Health Research, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK. l.j.middleton@bham.ac.uk. |
| Notes | Contacted authors who confirmed trial still ongoing. |
LDL: Low Density Lipoprotein
HDL: High Density Lipoprotein
Differences between protocol and review
In this update, we implemented the core outcome set on endometriosis (Duffy 2020).
Contributions of authors
T Gibbons: responsible for the protocol development, screening of texts, data extraction of included studies, data analysis, and writing the full text.
E Georgiou: responsible for protocol development, screening of texts, data extraction of included studies and searched for studies, evaluated the methodological quality, extracted data from the included studies, and editing the full text.
Y Cheong: responsible for conflict resolution and editing the full text.
M Wise: responsible for conflict resolution and editing the full text.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
TG: nothing to declare
EG: nothing to declare
YC: lecturing Merck; minor share holdings Complete Fertility Ltd; previous advisory board contribution Merck, Ferring
MW: nothing to declare
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bayoglu 2011 {published data only}
- Bayoglu Tekin Y, Dilbaz B, Altinbas S K, Dilbaz S. Postoperative medical treatment of chronic pelvic pain related to severe endometriosis: Levonorgestrel-releasing intrauterine system versus gonadotropin-releasing hormone analogue. Fertility and Sterility 2011;95 (2):492-6. [DOI] [PubMed] [Google Scholar]
Gomes 2007 {published data only (unpublished sought but not used)}
- Gomes MK, Ferriani RA, Rosa e Silva JC, Japur de Sá Rosa e Silva AC, Vieira CS, Cândido dos Reis FJ. The levonorgestrel-releasing intrauterine system and endometriosis staging. Fertility and Sterility 2007;87(5):1231-1234. [DOI: ] [DOI] [PubMed] [Google Scholar]
Tanmahasamut 2012 {published data only}
- Tanmahasamut P, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Indhavivadhana S, Leerasiri P. Postoperative levonorgestrel-releasing intrauterine system for pelvic endometriosis-related pain: a randomized controlled trial. Obstetrics and Gynecology 2012;119(3):519-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vercellini 2003 {published data only}
- Frontino G, Vercellini P, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Levonorgestrel-releasing intrauterine device (Lng-IUD) versus expectant management after conservative surgery for symptomatic endometriosis. A pilot study. In: Fertility and Sterility. Vol. 77. 2002. [DOI] [PubMed]
- Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertility and Sterility 2003;80(2):305-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acien 2019 {unpublished data only}
- Acién P, Acién M. Anastrozole and levonorgrestrel-releasing intrauterine device in the treatment of endometriosis: a randomized clinical trial. Preprints 2019;1:1-2. [DOI: 10.20944/preprints201911.0010.v1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alhamdan 2010 {published data only (unpublished sought but not used)}
- Alhamdan D, Bignardi T, Hardas G, Merkur H, Condous G. Mirena intra-uterine system: does it improve long term symptoms in women with chronic pelvic pain and/or endometriosis after laparoscopy? A multicentre randomized controlled trial. Reviews on Recent Clinical Trials. 2010;5(3):143-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carvalho 2018 {published data only}
- Carvalho N, Margatho D, Cursino K, Benetti-Pinto CL, Bahamondes L. Control of endometriosis-associated pain with etonogestrel-releasing contraceptive implant and 52-mg levonorgestrel-releasing intrauterine system: randomized clinical trial.. Fertility and Sterility 2018;110(6):1129-1136. [DOI: 10.1016/j.fertnstert.2018.07.003] [DOI] [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen YJ. Postoperative maintenance levonorgestrel-releasing intrauterine system and endometrioma recurrence: a randomized controlled study. American Journal of Obstetrics and Gynecology 2017;216(6):582. [DOI: ] [DOI] [PubMed] [Google Scholar]
de Sá Rosa e Silva 2006 {published data only}
- Sá Rosa e Silva AC, Rosa e Silva JC, Nogueira AA, Petta CA, Abrão MS, Ferriani RA. The levonorgestrel-releasing intrauterine device reduces CA-125 serum levels in patients with endometriosis. Fertility and Sterility 2006;86(3):742-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2018 {published data only}
- Lee KH, Jung YW, Song SY, Kang BH, Yang JB, Ko YB, et al. Comparison of the efficacy of diegnogest and levonorgestrel-releasing intrauterine system after laparoscopic surgery for endometriosis. The Journal of Obstetric and Gynaecological Research 2018;44(9):1779-1786. [DOI: 10.1111/jog.13703] [DOI] [PubMed] [Google Scholar]
Lockhat 2005 {published data only}
- Lockhat FB, Emembolu JE, Konje JC. Serum and peritoneal fluid levels of levonorgestrel in women with endometriosis who were treated with an intrauterine contraceptive device containing levonorgestrel. Fertility and Sterility 2005;83:398-404. [PMID: ] [DOI] [PubMed] [Google Scholar]
Manetta 2008 {published data only}
- Manetta LA, Paula MW, Rosa e Silva JC, Sa Rosa e Silva ACJ, Nogueira AA, Ferriani RA. Uterine ultrasonographic changes during endometriosis treatment: a comparison between levonorgestrel-releasing intrauterine devices and a gonadotropin-releasing hormone agonist. Ultrasound in Medicine & Biology 2008;34(12):1914-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Margatho 2018 {published and unpublished data}
- Magartho D, Carvalho N, Eloy L, Bahmondes L. Assessment of biomarkers in women with endometriosis-associated pain using the ENG contraceptive implant or the 52 mg LNG-IUS: a non-inferiority randomised clinical trial. The European Journal of Contraception & Reproductive Health Care October 2018;23(5):344-350. [DOI: 10.1080/13625187.2018.1531117] [DOI] [PubMed] [Google Scholar]
Oh 2006 {published data only}
- Oh ST, Lim YT, Choi YM, Hur JY, Lee TH, Lee BS. The effect of combined treatment of Lng-ius and MPA for chronic pelvic pain of endometriosis. In: XVIII FIGO World Congress of Gynecology and Obstetrics. Vol. 2. 2006. [PNJ-47330788]
Petta 2005 {published data only}
- Petta CA, Ferriani RA, Abrao MS, Hassan D, Rosa E Silva JC, Podgaec S, Bahamondes L. Randomized clinical trial of a levonorgestrel-releasing intrauterine system and a depot GnRH analogue for the treatment of chronic pelvic pain in women with endometriosis. Human Reproduction 2005;20(7):1993-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Qiu 2017 {published data only}
- Qiu H, Yuan Z. Postoperative maintenance levonorgestrel-releasing intrauterine system and endometrioma recurrence: a randomized controlled study. American Journal of Obstetrics and Gynecology 2017;217(6):708. [DOI: ] [DOI] [PubMed] [Google Scholar]
Rosa e Silva 2005 {published data only}
- Rosa e Silva JC, Manetta LA, Rosa e Silva ACJS, Leite SR, Petta CA, Abrfio MS, et al. Effects of the levonorgestrel releasing intra-uterine system on uterine and ovarian volumes, endometrial thickness and dopplervelocimetry of uterinearteries, in women with pelvic endometriosis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2005;123:S39-40. [CENTRAL: CN-00600347] [Google Scholar]
Seo 2018 {published data only}
- Seo SK, Kim MK, Noe EB, Yun BH, Lee BS. Clinical effects of levonorgestrel-releasing intrauterine device in patients with endometrioma after surgery and 6 cycles of gonadotropin releasing hormone agonist. International Journal of Gynecology and Obstetrics 2018;25(1):39-43. [Google Scholar]
Taneja 2017 {published data only}
- Taneja A, Kaur S, Soni RK, Bhanupriya KJ, Singla L. Evaluating the efficacy of levonorgestrel intrauterine system and danazol for relief of postoperative pain in endometriosis. Journal of Clinical and Diagnostic Research 2017;11(7):10-12. [DOI: 10.7860/JCDR/2017/24126.10272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vieira 2007 {published data only}
- Vieira CS, Ferreira RA, Rosa e Silva JC, Rosa e Silva ACJS, Gomes MK, Ferriani RA. Comparative study of the influence of the levonorgestrel intra-uterine system and the GnRH analogues on cardiovascular risk markers in patients with endometriosis. Fertility and Sterility 2007;88:S211. [CENTRAL: CN-00635760] [Google Scholar]
Wong 2010 {published data only}
- Wong AYK, Tang LCH, Chin RKH. Levonorgestrel-releasing intrauterine system (Mirena) and depot medroxyprogesterone acetate (Depo-Provera) as long-term maintenance therapy for patients with moderate and severe endometriosis: a randomised controlled trial. Australian & New Zealand Journal of Obstetrics & Gynaecology 2010;50(3):273-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yagamuti 2014 {published data only}
- Yamaguti, Erciliene M M, Brito, Milena B, Ferriani, Rui A, Garcia, Andrea A, Rosa-e-Silva, Julio C, Vieira, Carolina S. Comparison of the hemostatic effects of a levonorgestrel-releasing intrauterine system and leuprolide acetate in women with endometriosis: a randomized clinical trial.. Thrombosis research 2014;134:1193-1197. [DOI: ] [DOI] [PubMed] [Google Scholar]
Yucel 2018 {published data only}
- Yucel N, Baskent E, Karamustafaoglu BC, Goynumer G. The levonorgestrel-releasing intrauterine system is associated with a reduction in dysmenorrhoea and dyspareunia, a decrease in CA 125 levels, and an increase in quality of life in women with suspected endometriosis. Australia Blackwell Publishing 2018;58(5):560-563. [DOI: 10.1111/ajo.12773] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Magos 2004 {published data only (unpublished sought but not used)}
- Magos AL. Post-operative levonorgestrel-releasing IUS treatment after conservative surgery for symptomatic endometriosis stage 1 to IV to reduce pelvic pain symptoms. The Royal Free Hampstead NHS Trust - National Research Register 2004.
Wang 2018 {published data only (unpublished sought but not used)}
- Wang Y. Effect of pretreatment with Levonorgestrel-Releasing Intrauterine System on FET outcomes in Women with endometriosis. Chinese Clinical Trials Registry 2018;1:1. [DOI: ] [Google Scholar]
Xu 2011 {published data only}
- Xu X-Wn, Wang L-D, Zhu X-Q, Yan L-Z, Guan Y-T, Zhu S-C, et al. [Levonorgestrel-releasing intrauterine system and combined oral contraceptives as conservative treatments for recurrent ovarian endometriosis: a comparative clinical study]. Chung-Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2011;91(15):1047-50. [PMID: ] [PubMed] [Google Scholar]
References to ongoing studies
Daoudom 2014 {published data only (unpublished sought but not used)}
- Daoudom K. Effectiveness of levonorgestrel-intrauterine system (LNG-IUS) versus depot medroxyprogesterone scetate (DMPA) in treatment of pelvic pain in clinically diagnosed endometriotic patients. ClinicalTrials.gov 2018. [CLINICALTRIALS.GOV IDENTIFIER: NCT02534688] [URL: https://clinicaltrials.gov/show/NCT02534688 2015;(): 2015]
Lee 2017 {published and unpublished data}
- Middleton LJ, Daniels JP, Weckesser A, Bhattacharya S. Preventing recurrence of endometriosis by means of long-acting progestogen therapy (PRE-EMPT): report of an internal pilot, multi-arm, randomised controlled trial incorporating flexible entry design and adaption of design based on feasibility of recruitment. Trials 11 March 2017;18:121. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Acién 2021
- Acién P, Velasco I, Acién M . Anastrozole and levonorgrestrel-releasing intrauterine device in the treatment of endometriosis: a randomized clinical trial.. BMC Womens Health. 2021;21(1):211. [DOI: 10.1186/s12905-021-01347-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
ASRM 2006
- American Society for Reproductive Medicine. Endometriosis and infertility: The Practice Committee of the American Society for Reproductive Medicine. Fertility and Sterility 2006;86(5 Suppl):156-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bafort 2020
- Bafort C, Beebeejaun Y, Tomassetti C, Bosteels J, Duffy JMN. Laparoscopic surgery for endometriosis. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD011031. [DOI: 10.1002/14651858.CD011031.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballard 2008
- Ballard KD, Seaman HE, Vries CS and Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG 2008;115:1382–1391. [DOI] [PubMed] [Google Scholar]
Bazot 2004
- Bazot M, Thomassin I, Hourani R, Cortez A, Darai E. Diagnostic accuracy of transvaginal sonography for deep pelvic endometriosis. Ultrasound in Obstetrics & Gynecology 2004;24(2):180-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Beatty 2009
- Beatty MN, Blumenthal PD. The levonorgestrel-releasing intrauterine system: Safety, efficacy, and patient acceptability. The Clinical Risk Manager 2009;5(3):561-574. [DOI: 10.2147/tcrm.s5624] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellelis 2010
- Beatty MN, Blumenthal PD. The levonorgestrel-releasing intrauterine system: Safety, efficacy, and patient acceptability.. Therapeutics and Clinical Risk Management 2009;5:561-574. [DOI: 10.2147/tcrm.s5624] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2020
- Chen I, Veth VB, Choudhry AJ, Murji A, Zakhari A, Black AY, et al. Pre‐ and postsurgical medical therapy for endometriosis surgery. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD003678. [DOI: 10.1002/14651858.CD003678.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence . Version accessed 20 January 2021. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Crosignani 1996
- Crosignani PG, Vercellini P, Biffignandi F, Costantini W, Cortesi I, Imparato E. Laparoscopy versus laparotomy in conservative surgical treatment for severe endometriosis. Fertility and Sterility 1996;66(5):706-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Crosignani 1997
- Crosignani PG, Vercellini P, Mosconi P, Oldani S, Cortesi I, De Giorgi O. Levonorgestrel-releasing intrauterine device versus hysteroscopic endometrial resection in the treatment of dysfunctional uterine bleeding. Obstetrics and Gynecology 1997;90(2):257-63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Du 1999
- Du M, Shao O, Zhou X. Serum levels of levonorgestrel during long-term use of Norplant. Chung-Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 1999;34(6):363-5. [PubMed] [Google Scholar]
Duepree 2002
- Duepree HJ, Senagore AJ, Delaney CP, Marcello PW, Brady KM, Falcone T. Laparoscopic resection of deep pelvic endometriosis with rectosigmoid involvement. Journal of the American College of Surgery 2002;195(6):754-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duffy 2020
- Duffy JMN, Hirsch M, Vercoe M, Abbott J, Barker C, Collura B, et al. Harmonising Outcomes and Outcome Measures for Endometriosis Research. A core outcome set for future endometriosis research: an international consensus development study. BJOG 2020;127(8):967-974. [DOI: 10.1111/1471-0528.16157] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dunselman 2014
- Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D'Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Human Reproduction 2014;29(3):400-412. [DOI: 10.1093/humrep/det457] [DOI] [PubMed] [Google Scholar]
Eskenazi 1997
- Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstetrics and Gynecology Clinics of North America 1997;24:235-238. [DOI] [PubMed] [Google Scholar]
Fedele 2001
- Fedele L, Bianchi S, Zancomoto G, Portuese A, Ricciardia R. Use of levonorgestrel-releasing intrauterine system in the treatment of rectovaginal endometriosis. Fertility and Sterility 2001;75:485-8. [DOI] [PubMed] [Google Scholar]
Fedele 2004
- Fedele L, Bianchi S, Zanconato G, Bettoni G, Gotsch F. Long-term follow-up after conservative surgery for rectovaginal endometriosis. American Journal of Obstetrics and Gynecology 2004;190(4):1020-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ford 2004
- Ford J, English J, Miles WA, Giannopoulos T. Pain, quality of life and complications following the radical resection of rectovaginal endometriosis. British Journal of Obstetrics and Gynaecology 2004;111(4):353-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
French 2004
- French R, Sorhaindo AM, Van Vliet H, Mansour DD, Robinson AA, Logan S, et al. Progestogen‐releasing intrauterine systems versus other forms of reversible contraceptives for contraception. Cochrane Database of Systematic Reviews 2004 , Issue 3. Art. No: CD001776. [DOI: 10.1002/14651858.CD001776.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 6 August 2021. Available at gradepro.org.
Guo 2009
- Guo SW. Recurrence of endometriosis and its control. Human Reproduction 2009;15(4):441-461. [DOI: 10.1093/humupd/dmp007] [PMID: ] [DOI] [PubMed] [Google Scholar]
Henderson 1991
- Henderson AF, Studd JWW. The role of definitive surgery and hormone replacement therapy in the treatment of endometriosis. In: Thomas E and Rock J, editors(s). Modern Approaches to Endometriosis. Dordrecht: Kulwer Academic Publishers, 1991:275-90. [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/. [DOI: 10.1136/bmj.d5928] [DOI]
Higgins 2021
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s) . Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hirsch 2016
- Hirsch M, Duy JM, Kusznir JO, Davis CJ, Plana MN, Khan KS. Variation in outcome reporting in endometriosis trials: a systematic review. American Journal of Obstetrics and Gynaecology 2016;214(4):452-464. [DOI] [PubMed] [Google Scholar]
Johnson 2017
- Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, et al. World Endometriosis Society consensus on the classification of endometriosis. Human Reproduction 2017;32:315-324. [DOI] [PubMed] [Google Scholar]
Kennedy 2005
- Kennedy S, Bergqvist A, Chapron C, D'Hooghe T, Dunselman G, Greb R, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Human Reproduction 2005;20(10):704-2698. [DOI: 10.1093/humrep/dei135] [DOI] [PubMed] [Google Scholar]
Krashin 2015
- Krashin J, Tang JH, Mody S, Lopez LM. Hormonal and intrauterine methods for contraception for women aged 25 years and younger. Cochrane Database of Systematic Reviews 2015 , Issue 3. Art. No: CD009805. [DOI: 10.1002/14651858.CD009805.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leonardi 2020
- Leonardi M, Gibbons T, Armour M, Wang R, Glanville E, Hodgson R, et al. When to Do Surgery and When Not to Do Surgery for Endometriosis: A Systematic Review and Meta-analysis. Journal of Minimally Invasive Gynecology 2020;27(2):390-407. [DOI: 10.1016/j.jmig.2019.10.014.] [PMID: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI: 10.1136/bmj.b2700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2015
- Lopez LM, Bernholc A, Hubacher D, Gretchen Stuart G, Van Vliet HA. Immediate postpartum insertion of intrauterine device for contraception. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD003036. [DOI: 10.1002/14651858.CD003036.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lou 2018
- Luo L, Luo B, Zheng Y, Zhang H, Jing L, Sidell N. Oral and intrauterine progestogens for atypical endometrial hyperplasia. Cochrane Database of Systematic Reviews 2018 , Issue 3. Art. No: CD009458. [DOI: 10.1002/14651858.CD009458.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luciano 1988
- Luciano AA, Turksoy RN, Carleo J. Evaluation of oral medroxyprogesterone acetate in the treatment of endometriosis. Obstetrics and Gynecology 1988;72:323-7. [PubMed] [Google Scholar]
Maruo 2001
- Maruo T, Laoag-Fernandez JB, Pakarinen P, Murakoshi H, Spitz IM, Johansson E. Use of a levonorgestrel intrauterine system on proliferation and apoptosis in the endometrium. Human Reproduction 2001;16:2103-8. [DOI] [PubMed] [Google Scholar]
Moghissi 1999
- Moghissi K. Medical treatment of endometriosis. Clinical Obstetrics and Gynecology 1999;42:620-32. [PMID: PMID: 10465472] [DOI] [PubMed] [Google Scholar]
NICE 2004
- National Collaborating Centre for Women's and Children's Health, National Institute for Clinical Excellence. Fertility: assessment and treatment for people with fertility problems. Royal College of Obstetrics and Gynaecology (RCOG) 2004.
Nicklin 1999
- Nicklin JL, Perrin L, Phillips G. Radical surgery for endometriosis. The Australian & New Zealand Journal of Obstetrics & Gynaecology 1999;39(1):68-74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nilsson 1978
- Nilsson CG, Luukkainen T, Arko H. Endometrial morphology of women using a D-norgestrel releasing intrauterine device. Fertility and Sterility 1978;29:397-401. [DOI] [PubMed] [Google Scholar]
Nirgianakis 2020
- Nirgianakis K, Ma L, McKinnon B, Mueller MD. Recurrence Patterns after Surgery in Patients with Different Endometriosis Subtypes: A Long-Term Hospital-Based Cohort Study. Journal of Clinical Medicine 2020;9(2):496. [DOI: 10.3390/jcm9020496] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2021 [Computer program]
- Review Manager Web (RevMan Web). Version 2.6.0. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Rodriguez 2020
- Rodriguez MB, Lethaby A, Jordan V. Progestogen‐releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD002126. [DOI: 10.1002/14651858.CD002126.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sampson 1927
- Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. The American Journal of Pathology 1927;3(143):93–110. [PMC free article] [PubMed] [Google Scholar]
Seracchioli 2008
- Seracchioli R, Mabrouk M, Guerrini M, Manuzzi L, Savelli L, Frascà C and Venturoli S. Dyschezia and posterior deep infiltrating endometriosis: analysis of 360 cases. Journal of Minimally Invasive Gynecology 2008;15:695-699. [DOI] [PubMed] [Google Scholar]
Shakiba 2008
- Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstetrics & Gynecology 2008;111(6):1285-1292. [PMID: ] [DOI] [PubMed] [Google Scholar]
Silverberg 1986
- Silverberg SG, Haukkamaa M, Arko H, Nilsson CG, Luukkainen T. Endometrial morphometry of women during long term use of levonorgestrel intrauterine system. International Journal of Gynecological Pathology 1986;5:235-41. [DOI] [PubMed] [Google Scholar]
Sutton 1994
- Sutton CJ, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertility and Sterility 1994;62(4):696-700. [DOI] [PubMed] [Google Scholar]
Tal 2019
- Tal A, Tal R, Pluchino N, and Taylor, H S. Endometrial cells contribute to preexisting endometriosis lesions in a mouse model of retrograde menstruation. Biology of Reproduction 2019;100:1453–1460. [DOI: doi: 10.1093/biolre/ioz039] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomassin 2004
- Thomassin I, Bazot M, Detchev R, Barranger E, Cortez A and Darai E. Symptoms before and after surgical removal of colorectal endometriosis that are assessed by magnetic resonance imaging and rectal endoscopic sonography. American Journal of Obstetrics and Gynecology 2004;190:1264-1271. [DOI] [PubMed] [Google Scholar]
Vercellini 1996
- Vercellini P, Trespidi L, De Giorgi O, Cortesi I, Parazzini F, Crosignani PG. Endometriosis and pelvic pain: relation to disease stage and localization. Fertility and Sterility 1996;65(2):299-304. [MEDLINE: ] [PubMed] [Google Scholar]
Vercellini 1998
- Vercellini P, De Giorgi O, Pesole A, Zaina B, Pisacreta A, Crosignani PG. Prevention of recurrences by postoperative medical treatment. In: Lemay A, Maheux R, editors(s). Understanding and Managing Endometriosis. Advances in research and practice. Quebec City: Parthenon Publishing, 1998:261-8. [Google Scholar]
Vercellini 2000
- Vercellini P, De Giorgi O, Pisacreta A, Pesole AP, Vicentini S, Crosignani PG. Surgical management of endometriosis. Best Practice & Research. Clinical Obstetrics & Gynaecology 2000;14(3):501-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vigano 2007
- Viganò P, Somigliana E, Vercellini P. Levonorgestrel-releasing intrauterine system for the treatment of endometriosis: biological and clinical evidence. Womens Health 2007;3(2):207-214. [DOI: 10.2217/17455057.3.2.207] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Abou‐Setta 2013
- Abou-Setta A, Houston B, Al-lnany H, Farquhar C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Systematic Reviews 2013;1:1-27. [DOI: 10.1002/14651858.CD005072.pub3] [DOI] [PubMed] [Google Scholar]


