Abstract
The preprotein translocase of the yeast mitochondrial outer membrane (TOM) consists of the initial import receptors Tom70 and Tom20 and a ∼400-kDa (400 K) general import pore (GIP) complex that includes the central receptor Tom22, the channel Tom40, and the three small Tom proteins Tom7, Tom6, and Tom5. We report that the GIP complex is a highly stable complex with an unusual resistance to urea and alkaline pH. Under mild conditions for mitochondrial lysis, the receptor Tom20, but not Tom70, is quantitatively associated with the GIP complex, forming a 500K to 600K TOM complex. A preprotein, stably arrested in the GIP complex, is released by urea but not high salt, indicating that ionic interactions are not essential for keeping the preprotein in the GIP complex. Under more stringent detergent conditions, however, Tom20 and all three small Tom proteins are released, while the preprotein remains in the GIP complex. Moreover, purified outer membrane vesicles devoid of translocase components of the intermembrane space and inner membrane efficiently accumulate the preprotein in the GIP complex. Together, Tom40 and Tom22 thus represent the functional core unit that stably holds accumulated preproteins. The GIP complex isolated from outer membranes exhibits characteristic TOM channel activity with two coupled conductance states, each corresponding to the activity of purified Tom40, suggesting that the complex contains two simultaneously active and coupled channel pores.
Mitochondria import several hundred different proteins from the cytosol. Upon synthesis on cytosolic polysomes, targeting signals of the preproteins are recognized by receptors on the mitochondrial surface. The preproteins are then translocated across the outer mitochondrial membrane through a general import pore (GIP) and are transferred to the further mitochondrial subcompartments (32, 35, 37, 45). Three proteins of the translocase of the outer membrane (TOM) have been identified as receptors for preproteins. Tom20 and Tom70 are the initial receptors for preproteins with N-terminal targeting signals and internal targeting signals, respectively. Tom22 functions as central receptor and is associated with the channel-forming subunit Tom40. Coimmunoprecipitation and blue native polyacrylamide gel electrophoresis (BN-PAGE) of mitochondria from the yeast Saccharomyces cerevisiae led to the identification of a ∼400-kDa (400K) complex, termed the GIP complex, that contains Tom22, Tom40, and three small Tom proteins, Tom5, Tom6, and Tom7 (9, 10, 52). Tom5 mediates transfer of preproteins from Tom22 to Tom40 (10), while Tom6 and Tom7 modulate assembly steps of the GIP complex (3, 9, 19, 40). The TOM complex was also isolated from Neurospora crassa mitochondria, and its holo form was shown to contain not only Tom40, Tom22, Tom7, and Tom6 but also Tom20 and some Tom70; however, no Neurospora homolog of Tom5 has been found (1, 27). Electron microscopic analysis and assessment of the stoichiometry of TOM complexes have suggested that the TOM complex contains two or three translocation channels (1, 9, 27, 52). Electrophysiological studies with the Neurospora TOM complex resolved conductance states suggesting the presence of two independent channels (27, 28).
Little is known about the functional architecture of the TOM machinery and the interactions of its subunits. It is unknown which Tom proteins and type of interactions are responsible for keeping a preprotein in the import channel. Moreover, it is an open question whether translocase components of the intermembrane space or inner membrane (TIM) are needed for stable accumulation of preproteins in the GIP (30, 31) or whether the TOM complex is functional by itself. Possible candidates are the TIM23 complex for preproteins containing N-terminal targeting signals or the small Tim proteins of the intermembrane space and inner membrane, such as Tim10 and Tim12, for preproteins containing internal targeting signals (4, 22, 26, 56).
In this study we attempted to address these questions regarding the functional architecture of the mitochondrial TOM machinery. We found that the GIP complex is of unusual high stability, with a Tom40-Tom22 core structure responsible for stably holding a preprotein by nonionic forces. Tim proteins are not needed for accumulation of a preprotein in the GIP complex, and evidence for a coupled function of two channel pores in a GIP complex is presented.
MATERIALS AND METHODS
In vitro import of preproteins into mitochondria.
We used the haploid S. cerevisiae strain PK82 (his4-713 lys2 ura3-52 Δtrp1 leu2-3,112) (13). Mitochondria were isolated essentially as described by Daum et al. (6), resuspended in SEM buffer (250 mM sucrose, 1 mM EDTA, 10 mM morpholinepropanesulfonic acid [MOPS] [pH 7.2]), and stored at −80°C in 10-mg/ml aliquots. Preproteins were synthesized by in vitro transcription and translation in rabbit reticulocyte lysate (Amersham) in the presence of [35S]methionine-cysteine (Amersham). Mitochondria were diluted in import buffer (3% [wt/vol] bovine serum albumin, 250 mM sucrose, 5 mM MgCl2, 80 mM KCl, 10 mM MOPS-KOH [pH 7.2]) containing 2 mM ATP and 2 mM NADH. After adding the labeled preprotein, the mixture was incubated at 25°C for various times. To arrest the fusion protein ADP/ATP carrier (AAC)-dihydrofolate reductase (DHFR) in the TOM complex, 20 μM methotrexate was added (42). For trypsin pretreatment, mitochondria were incubated in the presence of trypsin (20 μg/ml) for 20 min on ice. Trypsin was inactivated by addition of a 30-fold excess of soybean pancreatic trypsin inhibitor and incubation for 10 min on ice. The import reactions were subjected to BN-PAGE, and labeled proteins were detected using the PhosphorImager storage technology (digital autoradiography; Molecular Dynamics).
BN-PAGE.
Mitochondrial pellets (50 to 100 μg of protein) or mitochondrial outer membrane vesicles (1 to 5 μg of protein) were lysed in 50 μl of ice-cold solubilization buffer (20 mM Tris-HCl [pH 7.4], 0.1 mM EDTA, 50 mM NaCl, 10% [vol/vol] glycerol, 1 mM phenylmethylsulfonyl fluoride, either 0.1 to 1% digitonin or 0.5% Triton X-100). After a clarifying spin, 5 μl of sample buffer (100 mM bis-Tris [pH 7.0], 500 mM 6-aminocaproic acid, 5% [wt/vol] Coomassie brilliant blue G250) was added to the supernatant, and the samples were loaded onto a 6 to 13% or 6 to 16.5% polyacrylamide gradient gel (43). After electrophoresis, the gels were soaked in transfer buffer (25 mM Tris, 150 mM glycine, 0.02% [wt/vol] sodium dodecyl sulfate [SDS], 20% [vol/vol] methanol) and then transferred via semidry blotting onto polyvinylidene difluoride (PVDF) membranes (Millipore). Immunodecoration was performed by standard techniques using the Amersham enhanced chemiluminescence system. Radiolabeled proteins were detected by digital autoradiography using either dried gels or PVDF membranes. For two-dimensional gel electrophoresis, lanes from the first-dimension BN-PAGE were excised and polymerized into the stacking gel of a second-dimension SDS-polyacrylamide gel. After electrophoresis, the gels were blotted onto PVDF membranes and analyzed by immunodecoration.
Isolation of the 400K GIP complex from mitochondrial outer membrane vesicles and electrophysiological characterization.
Mitochondria (100 mg of protein) were resuspended in swelling buffer (5 mM potassium phosphate [pH 7.4], 1 mM phenylmethylsulfonyl fluoride) at a concentration of 4 mg/ml and incubated on ice for 20 min. Following treatment by 20 strokes in a glass-Teflon potter, the outer membrane vesicles were recovered by two consecutive ultracentrifugation steps on sucrose gradients as described previously (2). The membranes were stored at −80°C in EM buffer (1 mM EDTA, 10 mM MOPS [pH 7.2]). For isolation of the TOM complex, mitochondrial outer membranes (200 μg of protein) were pelleted at 100,000 × g for 15 min and then solubilized in 200 μl of solubilization buffer containing 1% digitonin (with or without 4 M urea) for 30 min on ice. After separation by BN-PAGE, the 400K band was excised from the gel, and the TOM complex was electroeluted overnight in elution buffer (25 mM Tricine, 7.5 mM bis-Tris [pH 7.0], 1% N-heptyl-β-thioglucopyranoside) using the BIOTRAP system (Schleicher & Schuell). The integrity of the eluted complex was confirmed by BN-PAGE, and the components were analyzed by SDS-PAGE after trichloroacetic acid (TCA) precipitation.
Electrophysiological measurements were performed on planar lipid bilayers, produced by the painting technique (16, 18). The electroeluted 400K complex was reconstituted into liposomes by the dialysis technique (18). The liposomes were added to the cis chamber below the bilayer at asymmetrical buffer concentrations (cis chamber, 250 mM KCl, 10 mM CaCl2, 10 mM MOPS-Tris [pH 7.0]; trans chamber, 20 mM KCl, 10 mM MOPS-Tris [pH 7.0]). After fusion, the buffers on both sides of the membrane were changed to the final composition by perfusion. The membrane potentials indicated refer to the trans compartment.
Purification of the TOM complex via Tom22-His10.
For isolation of the TOM complex on Ni-nitrilotriacetic acid (NTA) (Qiagen), a yeast strain was modified to contain Tom22 with a C-terminal 10-histidine residue tag. The vector pFA-GFP-HIS3MX5 (57) was digested with the restriction enzymes BamHI and AscI to release the green fluorescent protein open reading frame (ORF). The complementary primers HIS-A (5′ GAT CCC CCG GGC ACC ACC ATC ATC ACC ATC ATC ATC ATC ATT AAG G 3′) and HIS-B (5′ CGC GCC TTA ATG ATG ATG ATG ATG GTG ATG ATG GTG GTG CCC GGG G 3′) encoding the amino acid sequence GSPGHHHHHHHHHH plus stop codon were then annealed and ligated into the linearized pFA vector, creating the vector pFA-His10-HIS3MX5. The primers 5-Tom22 (5′GAA TAA CAA GCT TTG TTC CTG TTT ATT 3′) and 3-Tom22 (5′ GGC GGA TCC ATT GGC TGT TGC TGC AGC ATC 3′) were employed in PCR using Vent polymerase and yeast genomic DNA as template to amplify the ORF of Tom22 without the stop codon. The PCR product was digested with the restriction enzymes HindIII and BamHI and subsequently cloned into pFA-His10-HIS3MX5 previously digested with HindIII and BamHI, creating constructs encoding Tom22 with a C-terminal histidine tag. The primers TOM22-5 (5′ ATG GTC GAA TTA ACT GAA ATT 3′) and TOM22-P1 (5′ ATC GCT CGA CAC GAT TGA AAG GAA TAT GTA AAG GTT CAAACA TCG ATG AAT TCG AGC TCG 3′) were then used in a PCR to amplify the TOM22 ORF and the downstream HIS3 marker and additionally contained a 3′ region complementary to a region downstream of the TOM22 ORF. The PCR product was subsequently transformed into the yeast strain YPH499 (46) by the method of Philippsen et al. (38). Transformants were selected for growth on medium lacking histidine, and the presence of a histidine tag at the C terminus of Tom22 was verified by PCR and immunodecoration.
Tom22-His10-containing mitochondria (10 mg of protein) were incubated in EM buffer at a protein concentration of 2 mg/ml for 20 min on ice. After sonication, the membranes were pelleted for 15 min at 100,000 × g and subsequently solubilized in 1 ml of solubilization buffer containing 0.5% digitonin. After a clarifying spin, the supernatant was incubated with 0.5 ml of Ni-NTA in solubilization buffer containing 30 mM imidazole and 250 mM NaCl. For washing, the imidazole concentration was increased to 50 or 80 mM and the digitonin concentration was set to 0.2%. Elution occured at a final imidazole concentration of 200 mM.
DHFR folding assay.
The folding state of DHFR was determined by the resistance of the folded domain against treatment with proteinase K. Radiolabeled AAC-DHFR was imported into mitochondria (25 μg of protein) for 25 min at 25°C in the presence of 20 μM methotrexate. The mitochondria were solubilized in solubilization buffer containing 1% digitonin and 0 to 8 M urea. After a clarifying spin, the supernatant was diluted 40-fold into assay buffer (80 mM KCl, 60 mM MOPS-KOH [pH 7.2]) containing proteinase K (100 μg/ml). After 10 min of incubation on ice, the reaction was stopped by adding 2 mM phenylmethylsulfonyl fluoride. The samples were subjected to TCA precipitation and SDS-PAGE. Protease-resistant DHFR was detected by digital autoradiography.
RESULTS
The 400K GIP complex is highly resistant to treatment with urea, salt, or alkaline pH.
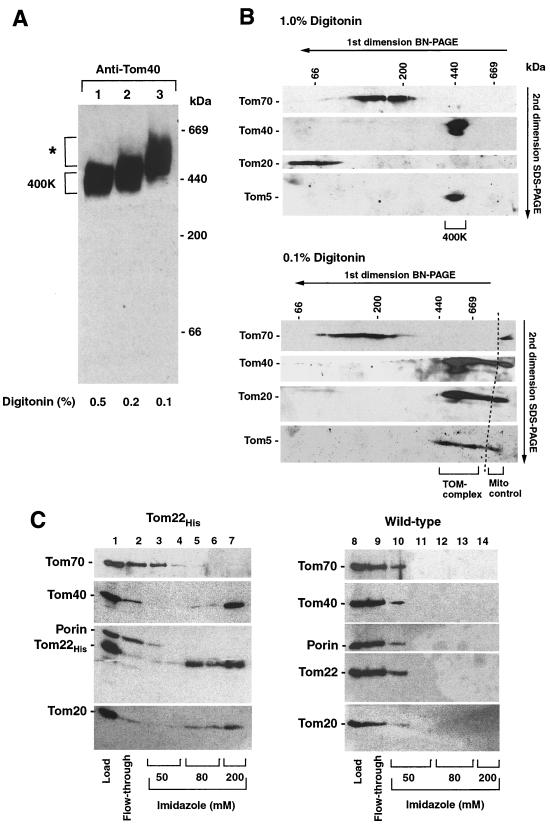
When isolated yeast mitochondria were solubilized in digitonin and subjected to BN-PAGE, the GIP complex, detected by immunodecoration with antibodies directed against Tom40, migrated as a characteristic band in the 400K region (Fig. 1A, top, lane 1) (7–10, 52). To analyze the stability of the 400K complex, we lysed the yeast mitochondria in the presence of different concentrations of urea. Surprisingly, the 400K complex was still visible up to a urea concentration of 6 M (Fig. 1A, top, lanes 2 to 4). A slight shift to a higher mobility was observed with increasing urea concentration (2 to 6 M). At 6 M urea, the amount of GIP complex was decreased; at 8 M urea, no GIP complex was detectable (Fig. 1A, top, lanes 4 and 5). To exclude that the decreased amount of GIP complex was caused by a degradation of Tom40, aliquots of the samples were separated by SDS-PAGE and Tom40 was detected by immunodecoration. The amount of Tom40 was unchanged under all conditions (Fig. 1A, bottom). The lack of detection of a Tom40 signal on BN-PAGE after treatment with 8 M urea can thus be attributed to a dissociation of the complex and migration of Tom40 below the detection range of the native gel system used. Immunodecoration for Tom22 performed in parallel gave a similar result (lanes 6 to 10), demonstrating that the two major components of the GIP complex, Tom40 and Tom22, show an unusual resistance to urea. For comparison, we analyzed the most abundant outer membrane protein porin that is found in three complexes on BN-PAGE in the range from 440 to 200 kDa (Fig. 1A, top, lane 11). These porin complexes were completely dissociated by 4 M urea (lane 13).
FIG. 1.
Stability of the 400K GIP complex. (A) Mitochondria (100 μg of protein) were lysed in solubilization buffer containing 1% digitonin and the indicated concentration of urea for 10 min on ice. After a clarifying spin, protein complexes were separated by BN-PAGE and blotted onto PVDF membranes, followed by immunodecoration using antisera against Tom40, Tom22, and porin. As control, 25% of the samples were directly subjected to SDS-PAGE and immunodecoration. (B) Mitochondria (100 μg of protein) were resuspended in 100 mM Na2CO3 (pH 11.5) and incubated for 30 min on ice. After centrifugation at 100,000 × g, the nonextractable pellet was lysed in digitonin buffer and subjected to BN-PAGE, followed by immunodecoration against Tom40 and Tom22 (lane 1); the control represents mitochondria without carbonate treatment. A control sample, the nonextractable pellet, and the TCA-precipitated supernatant (Sn.) were also analyzed by SDS-PAGE, showing the correct fractionation patterns (lanes 6 to 8). BN-PAGE of control samples and the carbonate-resistant fractions, followed by Western blot analysis using antisera against Tim22, Tim23, the β subunit of the F1-ATPase, and Hsp60, revealed no carbonate-resistant complexes (lanes 9 to 12). For salt extraction (lanes 3 to 5), mitochondria were resuspended and incubated in SEM buffer containing the indicated NaCl concentrations for 10 min on ice. After the salt was washed off with SEM buffer, the mitochondria were lysed in digitonin-containing buffer and subjected to BN-PAGE.
As a second means to analyze the stability of the 400K GIP complex, isolated mitochondria were extracted with sodium carbonate at pH 11.5. By this procedure, protein-protein interactions are typically disrupted and peripheral membrane proteins as well as soluble proteins are extracted, whereas integral membrane proteins are retained in the membrane sheets (5, 12). Carbonate-extracted membranes are usually analyzed by SDS-PAGE (Fig. 1B, lanes 6 to 8). The mitochondrial integral membrane proteins Tom40 and Tom22, and inner membrane AAC remain in the membrane sheets (lane 7), while the peripheral inner membrane protein Tim44 is extracted (lane 8). To determine if the subunit interations within the 400K complex were disrupted by the carbonate treatment, the nonextractable fraction was solubilized by digitonin and separated by BN-PAGE. Surprisingly, the 400K complex, detected with antibodies against Tom40 or Tom22, was largely unaffected by the carbonate treatment, and only a small shift to a higher mobility was observed (lane 1). We thus analyzed several other mitochondrial protein complexes, the two translocases of the inner membrane (TIM22 complex and TIM23 complex), the F1F0-ATPase, and the chaperonin Hsp60. None of these complexes was resistant to treatment at alkaline pH (lanes 9 to 12), indicating that the association of Tom proteins in the GIP complex shows a remarkable resistance to alkaline treatment. Finally, we analyzed the sensitivity of the GIP complex to a treatment with salt and found a complete resistance to all concentrations of NaCl tested (up to 1.5 M) (lanes 3 to 5).
How stable is the association of the three small Tom proteins, Tom5, Tom6, and Tom7, with the GIP complex? The presence of the small Tom proteins in the complex was assayed after import of the 35S-labeled proteins into mitochondria and digital autoradiography of the BN-gels (Fig. 2A, lanes 1, 6, and 12) (9). Solubilization of the mitochondria in the presence of urea revealed that all small Tom proteins were present in the 400K complex up to 4 M urea, albeit at different yields (lanes 3, 8, and 14). While the amount of Tom5 was reduced more than 50% at 4 M urea, the amounts of Tom6 and Tom7 were less affected (Fig. 2B). Treatment with 6 M urea led to a complete loss of Tom5 from the 400K complex and a reduction of Tom6 and Tom7 to 20 to 30% (Fig. 2A, lanes 4, 9, and 15; Fig. 2B). A comparison of the urea resistance of Tom40 and Tom22 revealed that the small Tom proteins are released from the GIP complex before Tom40 and Tom22 (Fig. 2B), explaining the slight shift of Tom40 and Tom22 to a higher mobility on BN-PAGE at increasing urea concentration (Fig. 1A, lanes 2 to 4 and 7 to 9).
FIG. 2.
Association of the small Tom proteins with the 400K GIP complex. (A) Stability of the small Tom proteins in the 400K complex following urea treatment. Mitochondria (50 μg of protein) were incubated with the radiolabeled preproteins of the small Tom proteins in import buffer for 10 min at 25°C. After lysis in solubilization buffer containing 1% digitonin and the indicated concentrations of urea, the samples were subjected to BN-PAGE and digital autoradiography. (B) Quantitation of the urea-resistant Tom proteins in the 400K complex (as described for Fig. 1A and panel A). The total amount of each Tom protein in the 400K complex in the absence of urea was set to 100% (control). (C) Carbonate resistance of the small Tom proteins in the 400K complex. Radiolabeled preproteins of the small Tom proteins were imported into mitochondria (50 μg of protein) for 10 min at 25°C. Mitochondrial pellets were resuspended in 100 mM Na2CO3 (pH 11.5) and incubated on ice for 30 min. After centrifugation at 100,000 × g for 30 min, the carbonate-resistant pellets were solubilized in 1% digitonin and separated by BN-PAGE (lanes 4 to 6). As a control, mitochondria containing imported Tom proteins were directly lysed and separated by BN-PAGE (lanes 1 to 3). Tom40 was detected by immunodecoration in a control sample and a carbonate-resistant pellet (lanes 7 and 8, respectively). The amount of carbonate-resistant Tom protein was quantified and compared to the total amount of the respective Tom protein in the 400K complex (control) (columns 9 to 12). For comparison, radiolabeled small Tom proteins were imported into mitochondria (lane 13), separated into carbonate-resistant pellet (lane 14) and carbonate-extractable supernatant (Sn.; TCA precipitated; lane 15), and subjected to SDS-PAGE.
Upon extraction with carbonate, the major fraction of the small Tom proteins remained stably integrated within the 400K complex (Fig. 2C, lanes 4 to 6). Quantification demonstrated that ∼75% of the small Tom proteins were still present in the 400K complex after carbonate extraction (Fig. 2C, columns 9 to 11), while Tom40 was almost completely resistant to the alkaline treatment (Fig. 2C, column 12). Thus, not only are the three small Tom proteins resistant to an alkaline treatment when analyzed individually by SDS-PAGE (Fig. 2C, lanes 13 to 15), but also their association with the GIP complex is highly stable.
These results indicate that the five Tom proteins forming the 400K GIP complex must be associated in an unusually tight manner. A Tom40-Tom22 core structure shows the highest stability, whereas the association of the small Tom proteins is slightly less stable.
Quantitative association of Tom20, but not Tom70, with the yeast GIP complex under mild conditions.
Different results have been reported on the relationship of the two peripheral receptors Tom20 and Tom70 to the GIP complex. Coprecipitation experiments suggested that Tom20 and a fraction of Tom70 were associated with Tom40 and the GIP complex (1, 9, 19, 24, 27, 33). The purified cytosolic domains of Tom20 and Tom70 interacted with that of Tom22, suggesting a transient interaction of the receptor domains (52). In particular, a TOM holo complex was isolated from N. crassa mitochondria that contained Tom20 and some Tom70 (27). However, analysis of yeast mitochondria by BN-PAGE identified the stable 400K GIP complex that neither contained Tom20 nor Tom70 (9, 52). It has thus been suggested that the negatively charged Coomassie blue dye used in BN-PAGE exerts a destabilizing effect on the TOM complex, leading to a release of the peripheral receptors (1).
We examined whether a larger yeast TOM complex could be identified by BN-PAGE and finally found that the mobility of Tom40 on BN-PAGE was significantly slower when the mitochondria were lysed by very low concentrations of digitonin. Compared to the standard conditions of 1 to 0.5% digitonin (Fig. 1; Fig. 3A, lane 1) (9), the complex was shifted up to ∼450 kDa at 0.2% digitonin (Fig. 3A, lane 2) and even to ∼500 to 600 kDa at 0.1% digitonin (Fig. 3A, lane 3). A further reduction of the concentration of digitonin was not applicable since it led to an insufficient extraction of Tom proteins from the mitochondrial outer membrane. By two-dimensional electrophoresis, i.e., BN-PAGE followed by SDS-PAGE, we analyzed if the peripheral receptors were associated with the GIP complex at very low digitonin concentrations. While Tom70 migrated in the 100- to 250-kDa range independently of the concentration of digitonin (Fig. 3B), the mobility of Tom20 was dramatically changed. At 1% digitonin, all Tom20 molecules migrated below 100 kDa and the subunits of the GIP complex, analyzed by immunodecoration for Tom40 and Tom5, migrated at 400 kDa (Fig. 3B, top). At 0.1% digitonin, Tom20 completely shifted to the high-molecular-mass range, with a peak at 500 to 600 kDa, and thereby comigrated with Tom40 and Tom5 (Fig. 3B, bottom). These results indicate that Tom20, but not Tom70, is quantitatively associated with the GIP complex under mild conditions, leading to a large TOM complex of ∼500 to 600 kDa.
FIG. 3.
Relation of Tom20 and Tom70 to the yeast GIP complex. (A) BN-PAGE of mitochondria (50 μg of protein) lysed in different concentrations of digitonin, followed by immunodecoration with Tom40 antiserum. The high-molecular-weight complex observed at 0.1% digitonin is indicated by an asterisk. (B) Two-dimensional electrophoresis, BN-PAGE followed by SDS-PAGE, of mitochondria (100 μg of protein) solubilized in 1% digitonin (top) or 0.1% digitonin (bottom). As a control, mitochondria (20 μg of protein) were loaded directly onto the SDS-PAGE and electrophoresed in one dimension (bottom, Mito control). Immunodecoration was performed with antiserum directed against Tom70, Tom40, Tom20, or Tom5. (C) Binding of Tom proteins from Tom22-His mitochondria (left) or wild-type mitochondria (right) onto Ni-NTA. Mitochondrial membranes were isolated and solubilized as described in Materials and Methods. After binding and collection of the flowthrough fraction, the column was washed with 50 mM imidazole followed by 80 mM imidazole, and the TOM complex was eluted at 200 mM imidazole. Aliquots of each fraction were subjected to SDS-PAGE and immunoblotting.
To analyze the difference in behavior of yeast Tom20 and Tom70 in a BN-PAGE-independent approach, we inserted a region coding for a His10 tag at the C-terminal end of Tom22 into the chromosomal TOM22 gene of the haploid yeast strain YPH499. After solubilization of the mitochondria by digitonin, Tom22-His and the associated proteins were purified by Ni-NTA affinity chromatography (Fig. 3C). Upon incubation of the Ni-NTA resin with the lysed mitochondria, increasing concentrations of imidazole were added. Some Tom22-His as well as Tom20 were released by 80 mM imidazole (Fig. 3C, lanes 5 and 6), but the major fraction of Tom22-His, Tom40, and Tom20 were eluted at 200 mM imidazole (lane 7). A possible nonspecific binding of these Tom proteins to the Ni-NTA column was excluded by a parallel experiment using wild-type mitochondria without a His-tagged Tom22 where the Tom proteins were already released by washing with the lowest concentration of imidazole applied (lanes 10 to 14). Moreover, the most abundant outer membrane protein porin was released from the Ni-NTA at the lowest concentration of imidazole with both wild-type mitochondria and Tom22-His-mitochondria (Fig. 3C). Thus, Tom20 was specifically bound to Ni-NTA via the Tom22-His. When the digitonin concentration was increased to more than 0.7%, Tom20, but not Tom40, was released from Tom22-His (not shown). Tom70, however, was not associated with the Tom22-His–Tom40 complex under all conditions tested (Fig. 3C, lanes 3 to 7). Moreover, we tested several other detergents and varied salt, temperature, and incubation time but were not able to detect Tom70 associated with the yeast TOM complex to a significant extent under any of those conditions (not shown).
Thus, two independent approaches, BN-PAGE and Ni-NTA affinity chromatography, demonstrate that Tom20, but not Tom70, is efficiently associated with the yeast GIP complex when mild conditions for solubilization of mitochondria are applied.
Arrested AAC-DHFR is stably kept in the GIP complex despite the release of Tom20 and the small Tom proteins.
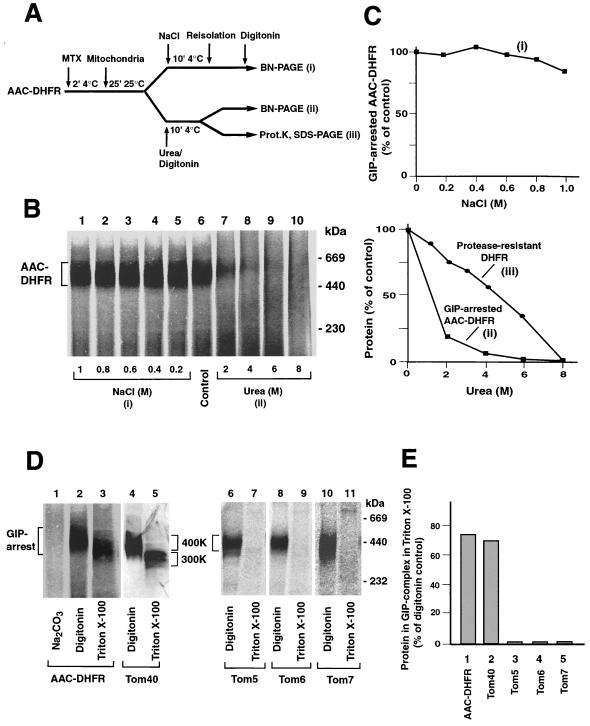
We then examined how stable a preprotein is kept in the GIP. We used the preprotein of AAC in a chimeric form, carrying the passenger protein DHFR at its C terminus (Fig. 4A). In the presence of the specific ligand methotrexate the DHFR domain is stably folded and cannot be unfolded and imported by mitochondria (11, 41, 42). AAC-DHFR is thereby arrested in the GIP complex, migrating at 450 kDa in BN-PAGE, i.e., 400 kDa of the GIP complex plus 52 kDa of the preprotein (Fig. 4B, lane 6) (42). We used 1% digitonin, where Tom20 is completely released from the GIP complex (Fig. 3 and data not shown), demonstrating that Tom20 is not required to keep the preprotein in the GIP. The accumulated preprotein was not released by treatment of the mitochondria with NaCl at all concentrations used (Fig. 4B, lanes 1 to 5; Fig. 4C, top), indicating that ionic forces were not critical for keeping AAC-DHFR in the GIP. Similarly, a matrix-targeted soluble preprotein, a fusion protein between a part of cytochrome b2 and DHFR, accumulated in the outer and inner membrane translocases (7) was not released by a treatment with up to 1.5 M NaCl (not shown).
FIG. 4.
Accumulation and stability of a preprotein in the GIP complex. (A) Schematic diagram depicting the experimental procedures for analysis of AAC-DHFR arrested in the TOM complex. MTX, methotrexate. (B) Arrested AAC-DHFR dissociates from the complex in the presence of urea but not high salt concentrations. Radiolabeled AAC-DHFR was incubated with mitochondria (50 μg of protein) in the presence of methotrexate for 25 min at 25°C. The mitochondria were either treated with NaCl (as indicated) in SEM buffer followed by solubilization in 1% digitonin or directly solubilized in digitonin in the presence of the indicated urea concentrations. After BN-PAGE, AAC-DHFR was detected by digital autoradiography. (C) Quantitation of GIP-arrested AAC-DHFR after high-salt (top) or urea (bottom) treatment. In the lower graph, the quantitation of proteinase K-resistant DHFR after treatment with urea is also shown (see Materials and Methods). (D) The small Tom proteins are not needed to keep a preprotein in the TOM complex. Mitochondria containing arrested radiolabeled AAC-DHFR were solubilized in either 1% digitonin or 0.5% Triton X-100 and subjected to BN-PAGE (lanes 2 and 3). The TOM complex lacking arrested preprotein is shown in lanes 4 and 5. The pellet arising from carbonate-treated mitochondria containing arrested AAC-DHFR was solubilized in 1% digitonin (lane 1). Mitochondria containing imported small Tom proteins were lysed in 1% digitonin or 0.5% Triton X-100 (lane 6 to 11). The blue native gels were blotted onto PVDF membranes, and radiolabeled proteins were detected by digital autoradiography. Lanes 4 and 5 were immunodecorated with Tom40 antiserum. (E) The amount of individual Tom proteins retained in the complex following Triton X-100 solubilization and BN-PAGE (as described for panel D) were quantitated and compared to the amount of protein found in digitonin-lysed mitochondria (set to 100%).
However, when the mitochondria carrying AAC-DHFR were treated with urea, more than 75% of the preprotein molecules were released from the GIP complex at 2 M urea (Fig. 4B, lanes 7 to 10; Fig. 4C, bottom). In contrast, the association of Tom40, Tom22, and the three small Tom proteins showed a significantly higher resistance to treatment with urea (Fig. 2B). Of concern was the possibility that the DHFR moiety was unfolded by the low-urea treatment and thus the preprotein slipped through the import channel. We therefore analyzed the folding state of DHFR by its resistance to proteinase K since only fully folded DHFR is resistant to the protease treatment (53, 55). A fraction of the urea-treated mitochondria was thus incubated with proteinase K and the stability of DHFR analyzed by SDS-PAGE (Fig. 4A, iii). Quantification by digital autoradiography demonstrated that methotrexate-bound DHFR of the chimeric AAC preprotein exhibited a markedly higher resistance to urea (Fig. 4C, lower panel, iii) than the association of the preprotein with the GIP (Fig. 4C, bottom, ii). Release of the preprotein from the GIP complex is thus not caused by unfolding of the DHFR moiety. We conclude that the preprotein accumulated in the GIP is readily released by urea. Moreover, treatment of mitochondria with sodium carbonate at pH 11.5 led to a complete dissociation of the preprotein from the GIP complex (Fig. 4D, lane 1). Thus, under two conditions, urea and alkaline pH, the accumulated preprotein is released from the GIP complex before the small Tom proteins.
Triton X-100 causes a release of all three small Tom proteins from the GIP complex, leaving the Tom40-Tom22 core complex with a size of ∼300 kDa (9), as evidenced in Fig. 4D by immunodecoration for Tom40 (lane 5) and by the loss of the small Tom proteins from the high-molecular-weight region (lanes 7, 9, and 11). AAC-DHFR accumulated in the presence of methotrexate remained in the GIP complex upon lysis with digitonin (lane 2) or Triton X-100 (lane 3); in the presence of Triton X-100 it just shifted to a lower molecular weight similar to the GIP complex (compare lanes 2 and 3 to lanes 4 and 5). Quantification of the Triton X-100-lysed mitochondria in comparison to the digitonin-lysed mitochondria showed that the accumulated preprotein was efficiently retained in the GIP complex, comparable to the efficiency of retention of Tom40 (Fig. 4E, columns 1 and 2), while Tom5, Tom6, and Tom7 were completely released. We conclude that neither the receptors Tom20 and Tom70 nor the three small Tom proteins are needed to stably keep an accumulated preprotein in the GIP complex.
Purified outer membrane vesicles efficiently accumulate AAC-DHFR in the GIP complex.
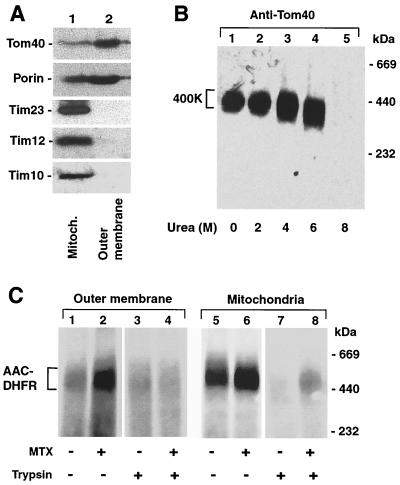
To test if components of the inner membrane or intermembrane space were needed for the accumulation of AAC-DHFR in the GIP, we purified outer membrane vesicles (16). The vesicles were enriched in Tom40 and porin and devoid of proteins of the inner membrane and intermembrane space, as assessed by immunodecoration for Tim23, Tim12, and Tim10 (Fig. 5A). The GIP complex of purified outer membranes showed the characteristic migration on BN-PAGE (Fig. 5B, lane 1) and high resistance to urea (lanes 2 to 4). The outer membrane vesicles were then incubated with AAC-DHFR. When preincubated with methotrexate, the preprotein accumulated in the GIP complex (Fig. 5C, lane 2) like it did in total mitochondria (lane 6). In the absence of methotrexate, the accumulation in the GIP complex was reduced with both outer membrane vesicles and mitochondria (lanes 1 and 5), demonstrating that the accumulation depended on the folded state of the preprotein. Moreover, pretreatment with trypsin inhibited the accumulation of AAC-DHFR in outer membrane vesicles (lanes 3 and 4), similar to the situation in mitochondria (lanes 7 and 8), revealing a requirement for surface receptor domains for generation of the intermediate in both cases. We conclude that stoichiometric amounts of translocase components of the intermembrane space or inner membrane are not essential for accumulation of the preprotein in the GIP.
FIG. 5.
Accumulation of a preprotein in purified outer membrane vesicles. (A) Purity of outer membrane vesicles. Mitochondria (Mitoch.; 25 μg of protein) and outer membrane vesicles (2.5 μg of protein) were separated by SDS-PAGE, blotted on PVDF membranes, and probed with antisera against proteins of the outer membrane (Tom40 and porin), the inner membrane (Tim23), and the intermembrane space (Tim12 and Tim10). (B) Stability of the 400K GIP complex of outer membrane vesicles against treatment with urea. Outer membrane vesicles (5 μg of protein) were lysed in 1% digitonin in the presence of the indicated urea concentrations and subjected to BN-PAGE, followed by immunodecoration of Tom40. (C) Accumulation of AAC-DHFR in outer membrane vesicles. Radiolabeled AAC-DHFR was imported into outer membrane vesicles (5 μg of protein; lanes 1 to 4) or mitochondria (50 μg of protein; lanes 5 to 8) in the presence or absence of methotrexate (MTX). Where indicated, the outer membrane vesicles and the mitochondria were pretreated with trypsin prior to the import reaction to remove the surface receptor domains.
Reconstitution of electroeluted GIP complex and evidence for two coupled channel pores.
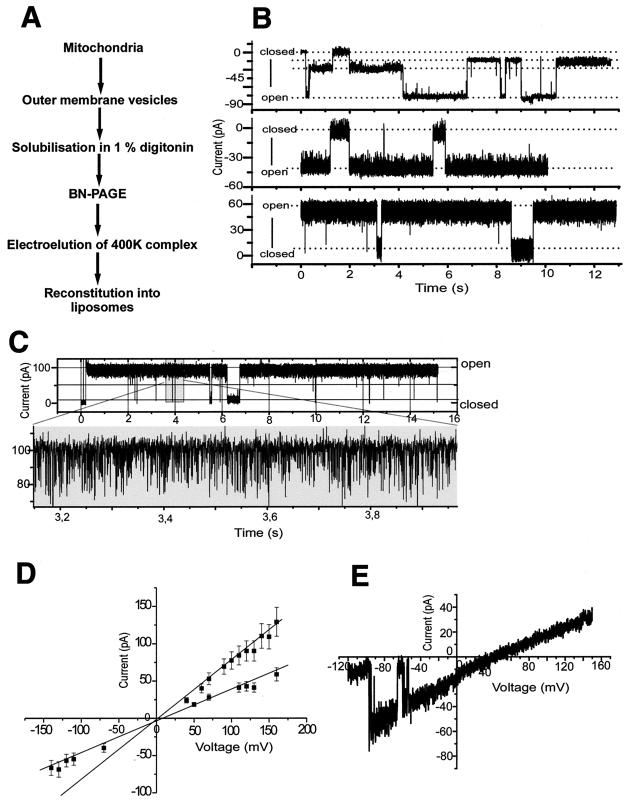
Does the 400K GIP complex separated by BN-PAGE still contain a functional channel? We purified outer membrane vesicles from isolated yeast mitochondria, lysed them with digitonin, and separated the complexes by BN-PAGE. The 400K band was excised; the complex was released by electroelution and reconstituted into liposomes (Fig. 6A). The integrity of the electroeluted complex was confirmed by a further BN-PAGE and analysis of the components on SDS-PAGE (not shown). Liposomes containing the 400K complex were fused with planar lipid bilayers (16, 52). We detected cation-selective channel activity with distinct subconductance states (Fig. 6B and C). The current-voltage relationship of the reconstituted 400K complex revealed a maximal conductance of Λ = 810 ± 30 pS (Fig. 6D). Closer examination revealed two main conductance states of Λ = 400 ± 40 pS (Fig. 6D). From the time course of the current traces, additional infrequent conductances with smaller amplitudes could be attributed to subconductance states of each of the two main conductances (Fig. 6B and C). A K+/Cl− permeability ratio of 10:1 was calculated from the reversal potential under asymmetric conditions (Fig. 6E).
FIG. 6.
The GIP complex eluted from outer membrane vesicles after BN-PAGE is functional. (A) Schematic diagram showing isolation of the 400K GIP complex. (B and C) Current traces from a bilayer fused with 400K complex containing liposomes under symmetric conditions (250 mM KCl, 10 mM MOPS-Tris [pH 7.0] on both sides of the membrane). The bottom trace of panel C shows a time scale-expanded current recording with high time resolution. (D) Current-voltage relationship of the two most frequent conductance states with the same symmetric KCl concentrations as in panel B. (E) Current-voltage relationship of the main conductance level at asymmetric buffers (cis, 250 mM KCl; trans, 20 mM KCl) for determination of reverse potential.
The basic characteristics of a single conductance state, including the relatively high cation selectivity, were similar to those observed for purified Tom40 (16). Indeed, no direct transitions between the two main conductance states were found, supporting the presence of two channels. Interestingly, both conductance states were observed simultaneously but not individually (Fig. 6B and C), indicating that two channels acted in a coupled manner. We observed comparable channel characteristics when the 400K complex was isolated from mitochondria treated with 4 M urea. Under these conditions, the high-molecular-weight complexes of porin quantitatively dissociate (Fig. 1A, lane 13). This finding and the high cation selectivity of the channel exclude a contamination with the anion-selective porin. We conclude that the GIP complex separated on BN-PAGE (with or without prior treatment with urea) contains characteristic TOM channel activity with two main conductance states, indicating that the total GIP complex contains two simultaneously active coupled channel pores.
The purified GIP complex was subjected to an extensive mass spectrometric analysis in order to identify possible new Tom components; however, we did not detect any unknown component. We therefore conclude that the 400K GIP complex is made up of Tom40, Tom22, Tom7, Tom6, and Tom5; Tom20 is loosely associated with this complex.
DISCUSSION
We report a systematic analysis of the interaction of Tom proteins and preproteins in the GIP complex of the yeast mitochondrial outer membrane. We find that the GIP complex is of unusually high stability, with resistance to treatment with salt and high concentrations of urea and alkaline pH where most other protein complexes dissociate. The Tom proteins are not covalently linked to form the complex since all can be dissociated by 8 M urea or heating in SDS. According to their stability of association, the subunits of the yeast TOM machinery can be grouped as follows. (i) Tom40 and Tom22 form the core of the GIP complex with a very stable mode of interaction. (ii) The three small Tom proteins are attached to this core, with Tom5 being slightly less stable than Tom6 and Tom7. (iii) Tom20 is very loosely associated with the yeast GIP complex. Under standard conditions of lysis of mitochondria (1% digitonin), Tom20 is not associated with the GIP complex independently of the analysis method used. Only when the detergent concentration is significantly lowered does Tom20 remain associated with the GIP complex in a quantitative manner, forming a 500K-600K TOM complex. (iv) Under none of the conditions did we find Tom70 in association with the other Tom proteins isolated from yeast mitochondria. A significant interaction between yeast Tom70 and Tom22 had been observed only when the expressed and purified cytosolic domains of the receptors were incubated in the absence of detergent, suggesting a loose and transient interaction between the peripheral receptor Tom70 and the central receptor Tom22 (52). Interestingly, the TOM complexes of other organisms show a higher stability of association of the two peripheral receptors. When lysed with 1% digitonin, the TOM complex from N. crassa contains Tom20 and some Tom70 (27); only by using harsher detergent conditions are these receptors released (1, 50). The TOM complex from potato mitochondria shows an even higher stability (21). No Tom5 homolog has been identified in either N. crassa or potato mitochondria, raising the possibility that yeast Tom5 may substitute in part for the lack of tight interaction with the peripheral receptors. In fact, it has been found that Tom5 can perform receptor-like functions by binding preproteins and facilitating their insertion into the Tom40 channel (10, 29).
Knowing the stability of associations between the different Tom proteins, it was now possible to address the central question of how a preprotein is kept in the GIP. A preprotein arrested in the GIP complex was readily released by treatment at alkaline pH or with urea but not with high salt. Thus, urea-sensitive interactions, such as hydrogen bonds and hydrophobic interactions, are important for keeping the preprotein in the GIP, while solvent-accessible ionic interactions are not essential. The acid chain hypothesis predicted that preproteins are directed across the mitochondrial outer membrane by ionic interactions with a number of charged patches of Tom proteins (10, 44). We propose to extend it to the binding chain hypothesis where the TOM machinery provides multiple weak binding sites for preproteins, including each type of noncovalent interactions, to form a guidance system for preproteins across the outer membrane.
Moreover, we could examine which Tom proteins are required to hold a preprotein in the import channel. Triton X-100 caused a quantitative release of the three small Tom proteins as well as Tom20 from the GIP during solubilization; however, the accumulated preprotein remained stably associated with the complex. Previous studies showed that the peripheral receptors Tom20 and Tom70 and the three small Tom proteins are required at distinct stages of translocation of a preprotein across the outer mitochondrial membrane, including targeting, insertion into the GIP and transfer to the further subcompartments (3, 10, 15, 17, 19, 34, 39, 48, 49, 51). Stan et al. (50) reported that the isolated TOM core complex of Neurospora mitochondria, consisting of Tom40, Tom22, Tom7, and Tom6, was able to interact with preproteins, yet they observed a high salt sensitivity of interaction (complete inhibition by 250 mM salt). They concluded that their system promoted only partial translocation of the preprotein, indicating that the preprotein was not stably inserted into the GIP, and thus the factors required for keeping the preprotein in the GIP could not be analyzed. The results presented here demonstrate that neither the three small Tom proteins nor the two peripheral receptors are needed for stably holding the preprotein in the GIP. Only the Tom40-Tom22 core structure is necessary to efficiently perform this task. However, a possibility was that translocase components of the intermembrane space and inner membrane were involved in keeping a preprotein in the GIP. In particular, the preprotein used here, the AAC, interacts with the small Tim proteins of the intermembrane space upon passage through the GIP (4, 25, 26, 47). We thus prepared highly pure outer membrane vesicles that are devoid of components of the intermembrane space and inner membrane. These vesicles accumulated the preprotein in the GIP in a manner comparable to total mitochondria. We conclude that accumulation of a preprotein in the GIP complex requires only Tom proteins, while stoichiometric amounts of Tim proteins are not essential.
BN-PAGE is now widely used to study mitochondrial preprotein translocases. Thus, an important finding is that the GIP complex eluted from the native gel is indeed functional since, like the purified Tom40, it shows channel activity with high conductance and cation selectivity. Interestingly, the purified GIP complex reveals two coupled states of subconductance, with each single subconductance state resembling the properties of purified Tom40 activity. The two channel activities do not function independently of each other but are always observed simultaneously. Therefore, the channels in the TOM complex do not act as independent units as previously assumed (27, 28), but the complex contains two simultaneously active channel pores whose activity is coupled. Since a sensitive mass spectrometry analysis of the GIP complex did not identify any further Tom protein, we conclude that only the Tom proteins described above are crucial for the formation of the GIP.
In conclusion, the TOM complex contains seven major components that are involved in distinct stages of the translocation of preproteins. Tom40 and Tom22 form the highly stable core structure that is responsible for holding preproteins and forms two coupled channels. The further Tom proteins are associated with this core structure, the three small Tom proteins with quite high stability. The receptor Tom20 is quantitatively but loosely associated with the core structure, while Tom70 only transiently contacts this TOM complex. The question arises as to why the core of the TOM complex shows such an unusually high stability compared to other complexes. The following considerations may provide a possible framework to explain this surprising property. The TOM machinery represents the initial entry gate into mitochondria where different preproteins must be unfolded by being pulled against the import channel through the action of the inner membrane potential and the matrix protein Hsp70 (11, 14, 20, 22, 23, 36, 41, 54). At the same time, however, the GIP complex must be firmly embedded in the outer membrane, and its disintegration must be avoided to prevent unspecific leakage of components of the intermembrane space. The stable core structure may thus provide the essential basis that the mitochondrial TOM machinery can perform two distinct tasks, providing both high flexibility for the passage of hundreds of different preproteins and a strong barrier against the unspecific leakage of proteins.
ACKNOWLEDGMENTS
We thank C. Koehler and G. Schatz for Tim12 antiserum and P. Philippsen for the pFA vector.
This work was supported by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 388, and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Ahting U, Thun C, Hegerl R, Typke D, Nargang F E, Neupert W, Nussberger S. The TOM core complex: the general protein import pore of the outer membrane of mitochondria. J Cell Biol. 1999;147:959–968. doi: 10.1083/jcb.147.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alconada A, Gärtner F, Hönlinger A, Kübrich M, Pfanner N. Mitochondrial receptor complex from Neurospora crassa and Saccharomyces cerevisiae. Methods Enzymol. 1995;260:263–286. doi: 10.1016/0076-6879(95)60144-9. [DOI] [PubMed] [Google Scholar]
- 3.Alconada A, Kübrich M, Moczko M, Hönlinger A, Pfanner N. The mitochondrial receptor complex: the small subunit Mom8b/Isp6 supports association of receptors with the general insertion pore and transfer of preproteins. Mol Cell Biol. 1995;15:6196–6205. doi: 10.1128/mcb.15.11.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer M F, Hofmann S, Neupert W, Brunner M. Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol. 2000;10:25–31. doi: 10.1016/s0962-8924(99)01684-0. [DOI] [PubMed] [Google Scholar]
- 5.Blom J, Kübrich M, Rassow J, Voos W, Dekker P J T, Maarse A C, Meijer M, Pfanner N. The essential yeast protein MIM44 (encoded by MPI1) is involved in an early step of preprotein translocation across the mitochondrial inner membrane. Mol Cell Biol. 1993;13:7364–7371. doi: 10.1128/mcb.13.12.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daum G, Böhni P C, Schatz G. Import of proteins into mitochondria. cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982;257:13028–13033. [PubMed] [Google Scholar]
- 7.Dekker P J T, Martin F, Maarse A C, Bömer U, Müller H, Guiard B, Meijer M, Rassow J, Pfanner N. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 1997;16:5408–5419. doi: 10.1093/emboj/16.17.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dekker P J T, Müller H, Rassow J, Pfanner N. Characterization of the preprotein translocase of the outer mitochondrial membrane by blue native electrophoresis. Biol Chem. 1996;77:535–538. [PubMed] [Google Scholar]
- 9.Dekker P J T, Ryan M T, Brix J, Müller H, Hönlinger A, Pfanner N. Preprotein translocase of the outer mitochondrial membrane: molecular dissection and assembly of the general import pore complex. Mol Cell Biol. 1998;18:6515–6524. doi: 10.1128/mcb.18.11.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietmeier K, Hönlinger A, Bömer U, Dekker P J T, Eckerskorn C, Lottspeich F, Kübrich M, Pfanner N. Tom5 functionally links mitochondrial preprotein receptors to the general import pore. Nature. 1997;388:195–200. doi: 10.1038/40663. [DOI] [PubMed] [Google Scholar]
- 11.Eilers M, Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986;322:228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- 12.Fujiki Y, Hubbard A L, Fowler S, Lazarow P B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982;93:97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gambill B D, Voos W, Kang P J, Miao B, Langer T, Craig E A, Pfanner N. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J Cell Biol. 1993;123:109–117. doi: 10.1083/jcb.123.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glick B S. Can Hsp70 proteins act as force-generating motors? Cell. 1995;80:11–14. doi: 10.1016/0092-8674(95)90444-1. [DOI] [PubMed] [Google Scholar]
- 15.Haucke V, Ocana C S, Hönlinger A, Tokatlidis K, Pfanner N, Schatz G. Analysis of the sorting signals directing NADH-cytochrome b5 reductase to two locations within yeast mitochondria. Mol Cell Biol. 1997;17:4024–4032. doi: 10.1128/mcb.17.7.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill K, Model K, Ryan M T, Dietmeier K, Martin F, Wagner R, Pfanner N. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature. 1998;395:516–521. doi: 10.1038/26780. [DOI] [PubMed] [Google Scholar]
- 17.Hines V, Brandt A, Griffiths G, Horstmann H, Brütsch H, Schatz G. Protein import into yeast mitochondria is accelerated by the outer membrane protein MAS70. EMBO J. 1990;9:3191–3200. doi: 10.1002/j.1460-2075.1990.tb07517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinnah S C, Hill K, Wagner R, Schlicher T, Soll J. Reconstitution of a chloroplast protein import channel. EMBO J. 1997;16:7351–7360. doi: 10.1093/emboj/16.24.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hönlinger A, Bömer U, Alconada A, Eckerskorn C, Lottspeich F, Dietmeier K, Pfanner N. Tom7 modulates the dynamics of the mitochondrial outer membrane translocase and plays a pathway-related role in protein import. EMBO J. 1996;15:2125–2137. [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S, Ratliff K S, Schwartz M P, Spenner J M, Matouschek A. Mitochondria unfold precursor proteins by unraveling them from their N-termini. Nat Struct Biol. 1999;6:1132–1138. doi: 10.1038/70073. [DOI] [PubMed] [Google Scholar]
- 21.Jänsch L, Kruft V, Schmitz U K, Braun H P. Unique composition of the preprotein translocase of the outer mitochondrial membrane from plants. J Biol Chem. 1998;273:17251–17257. doi: 10.1074/jbc.273.27.17251. [DOI] [PubMed] [Google Scholar]
- 22.Jensen R E, Johnson A E. Protein translocation: is Hsp70 pulling my chain? Curr Biol. 1999;9:R779–R782. doi: 10.1016/S0960-9822(00)80012-3. [DOI] [PubMed] [Google Scholar]
- 23.Kang P J, Ostermann J, Shilling J, Neupert W, Craig E A, Pfanner N. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature. 1990;348:137–143. doi: 10.1038/348137a0. [DOI] [PubMed] [Google Scholar]
- 24.Kiebler M, Pfaller R, Söllner T, Griffiths G, Horstmann H, Pfanner N, Neupert W. Identification of a mitochondrial receptor complex required for recognition and membrane insertion of precursor proteins. Nature. 1990;348:610–616. doi: 10.1038/348610a0. [DOI] [PubMed] [Google Scholar]
- 25.Koehler C M, Jarosch K, Tokatlidis K, Schmid K, Schweyen R J, Schatz G. Import of mitochondrial carriers mediated by essential proteins of the intermembrane space. Science. 1998;279:369–373. doi: 10.1126/science.279.5349.369. [DOI] [PubMed] [Google Scholar]
- 26.Koehler C M, Merchant S, Schatz G. How membrane proteins travel across the mitochondrial intermembrane space. Trends Biochem Sci. 1999;24:428–432. doi: 10.1016/s0968-0004(99)01462-0. [DOI] [PubMed] [Google Scholar]
- 27.Künkele K P, Heins S, Dembowski M, Nargang F E, Benz R, Thieffry M, Walz J, Lill R, Nussberger S, Neupert W. The preprotein translocation channel of the outer membrane of mitochondria. Cell. 1998;93:1009–1019. doi: 10.1016/s0092-8674(00)81206-4. [DOI] [PubMed] [Google Scholar]
- 28.Künkele K P, Juin P, Pompa C, Nargang F E, Henry J P, Neupert W, Lill R, Thieffry M. The isolated complex of the translocase of the outer membrane of mitochondria: characterization of the cation-selective and voltage-gated preprotein-conducting pore. J Biol Chem. 1998;273:31032–31039. doi: 10.1074/jbc.273.47.31032. [DOI] [PubMed] [Google Scholar]
- 29.Kurz M, Martin H, Rassow J, Pfanner N, Ryan M T. Biogenesis of Tim proteins of the mitochondrial carrier import pathway: differential targeting mechanisms and crossing-over with the main import pathway. Mol Biol Cell. 1999;10:2461–2474. doi: 10.1091/mbc.10.7.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer A, Lill R, Neupert W. Translocation and insertion of precursor proteins into isolated outer membranes of mitochondria. J Cell Biol. 1993;121:1233–1243. doi: 10.1083/jcb.121.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer A, Neupert W, Lill R. Mitochondrial protein import: reversible binding of the presequence at the trans side of the outer membrane drives partial translocation and unfolding. Cell. 1995;80:127–137. doi: 10.1016/0092-8674(95)90457-3. [DOI] [PubMed] [Google Scholar]
- 32.Meisinger C, Brix J, Model K, Pfanner N, Ryan M T. The preprotein translocase of the outer mitochondrial membrane: receptors and a general import pore. Cell Mol Life Sci. 1999;56:817–824. doi: 10.1007/s000180050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moczko M, Dietmeier K, Söllner T, Segui B, Steger H F, Neupert W, Pfanner N. Identification of the mitochondrial receptor complex in Saccharomyces cerevisiae. FEBS Lett. 1992;310:265–268. doi: 10.1016/0014-5793(92)81345-m. [DOI] [PubMed] [Google Scholar]
- 34.Moczko M, Ehmann B, Gärtner F, Hönlinger A, Schäfer E, Pfanner N. Deletion of the receptor MOM19 strongly impairs import of cleavable preproteins into Saccharomyces cerevisiae mitochondria. J Biol Chem. 1994;269:9045–9051. [PubMed] [Google Scholar]
- 35.Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 36.Neupert W, Hartl F U, Craig E A, Pfanner N. How do polypeptides cross the mitochondrial membranes? Cell. 1990;63:447–450. doi: 10.1016/0092-8674(90)90437-j. [DOI] [PubMed] [Google Scholar]
- 37.Pfanner N, Craig E A, Hönlinger A. Mitochondrial preprotein translocase. Annu Rev Cell Dev Biol. 1997;13:25–51. doi: 10.1146/annurev.cellbio.13.1.25. [DOI] [PubMed] [Google Scholar]
- 38.Philippsen P, Stotz A, Scherf C. DNA of Saccharomyces cerevisiae. Methods Enzymol. 1991;194:169–182. doi: 10.1016/0076-6879(91)94014-4. [DOI] [PubMed] [Google Scholar]
- 39.Ramage L, Junne T, Hahne K, Lithgow T, Schatz G. Functional cooperation of mitochondrial protein import receptors in yeast. EMBO J. 1993;12:4115–4123. doi: 10.1002/j.1460-2075.1993.tb06095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapaport D, Künkele K P, Dembowski M, Ahting U, Nargang F E, Neupert W, Lill R. Dynamics of the TOM complex of mitochondria during binding and translocation of preproteins. Mol Cell Biol. 1998;18:5256–5262. doi: 10.1128/mcb.18.9.5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rassow J, Guiard B, Wienhues U, Herzog V, Hartl F U, Neupert W. Translocation arrest by reversible folding of a precursor protein imported into mitochondria. A means to quantitate translocation contact sites. J Cell Biol. 1989;109:1421–1428. doi: 10.1083/jcb.109.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan M T, Müller H, Pfanner N. Functional staging of ADP/ATP carrier translocation across the outer mitochondrial membrane. J Biol Chem. 1999;29:20619–20627. doi: 10.1074/jbc.274.29.20619. [DOI] [PubMed] [Google Scholar]
- 43.Schägger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 44.Schatz G. Just follow the acid chain. Nature. 1997;388:121–122. doi: 10.1038/40510. [DOI] [PubMed] [Google Scholar]
- 45.Schatz G, Dobberstein B. Common principles of protein translocation across membranes. Science. 1996;271:1519–1526. doi: 10.1126/science.271.5255.1519. [DOI] [PubMed] [Google Scholar]
- 46.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirrenberg C, Endres M, Fölsch H, Stuart R A, Neupert W, Brunner M. Carrier protein import into mitochondria mediated by the intermembrane proteins Tim10/Mrs11 and Tim12/Mrs5. Nature. 1998;391:912–915. doi: 10.1038/36136. [DOI] [PubMed] [Google Scholar]
- 48.Söllner T, Griffiths G, Pfaller R, Pfanner N, Neupert W. MOM19, an import receptor for mitochondrial precursor proteins. Cell. 1989;59:1061–1070. doi: 10.1016/0092-8674(89)90762-9. [DOI] [PubMed] [Google Scholar]
- 49.Söllner T, Pfaller R, Griffiths G, Pfanner N, Neupert W. A mitochondrial import receptor for the ADP/ATP carrier. Cell. 1990;62:107–115. doi: 10.1016/0092-8674(90)90244-9. [DOI] [PubMed] [Google Scholar]
- 50.Stan T, Ahting U, Dembowski M, Künkele K P, Nussberger S, Neupert W, Rapaport D. Recognition of preproteins by the isolated TOM complex of mitochondria. EMBO J. 2000;19:4895–4902. doi: 10.1093/emboj/19.18.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steger H F, Söllner T, Kiebler M, Dietmeier K A, Pfaller R, Trülzsch K S, Tropschug M, Neupert W, Pfanner N. Import of ADP/ATP carrier into mitochondria: two receptors act in parallel. J Cell Biol. 1990;111:2353–2363. doi: 10.1083/jcb.111.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Wilpe S, Ryan M T, Hill K, Maarse A C, Meisinger C, Brix J, Dekker P J T, Moczko M, Wagner R, Meijer M, Guiard B, Hönlinger A, Pfanner N. Tom22 is a multifunctional organizer of the mitochondrial preprotein translocase. Nature. 1999;401:485–489. doi: 10.1038/46802. [DOI] [PubMed] [Google Scholar]
- 53.Viitanen P V, Donaldson G K, Lorimer G H, Lubben T H, Gatenby A A. Complex interactions between the chaperonin 60 molecular chaperone and dihydrofolate reductase. Biochemistry. 1991;30:9716–9723. doi: 10.1021/bi00104a021. [DOI] [PubMed] [Google Scholar]
- 54.Voisine C, Craig E A, Zufall N, von Ahsen O, Pfanner N, Voos W. The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell. 1999;97:565–574. doi: 10.1016/s0092-8674(00)80768-0. [DOI] [PubMed] [Google Scholar]
- 55.von Ahsen O, Lim J H, Caspers P, Martin F, Schönfeld H J, Rassow J, Pfanner N. Cyclophilin-promoted folding of mouse dihydrofolate reductase does not include the slow conversion of the late-folding intermediate to the active enzyme. J Mol Biol. 2000;297:809–818. doi: 10.1006/jmbi.2000.3574. [DOI] [PubMed] [Google Scholar]
- 56.Voos W, Martin H, Krimmer T, Pfanner N. Mechanisms of protein translocation into mitochondria. Biochim Biophys Acta. 1999;1422:235–254. doi: 10.1016/s0304-4157(99)00007-6. [DOI] [PubMed] [Google Scholar]
- 57.Wach A, Brachat A, Alberti-Segui C, Rebischung C, Philippsen P. Heterologous HIS3 marker and GFP reporter modules for PCR-targeting in Saccharomyces cerevisiae. Yeast. 1997;13:1065–1075. doi: 10.1002/(SICI)1097-0061(19970915)13:11<1065::AID-YEA159>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]