Abstract
Introduction
Programmed death-ligand 1 (PD-L1) expression is used for treatment prediction in non-small cell lung cancer (NSCLC). While cytology may be the only available material in the routine clinical setting, testing in clinical trials has mainly been based on biopsies.
Methods
We included 2 retrospective cohorts of paired, concurrently sampled, cytological specimens and biopsies. Also, the literature on PD-L1 in paired cytological/histological samples was reviewed. Focus was on the cutoff levels ≥1 and ≥50% positive tumor cells.
Results
Using a 3-tier scale, PD-L1 was concordant in 40/47 (85%) and 66/97 (68%) of the paired NSCLC cases in the 2 cohorts, with kappa 0.77 and 0.49, respectively. In the former cohort, all discordant cases had lower score in cytology. In both cohorts, concordance was lower in samples from different sites (e.g., biopsy from primary tumor and cytology from pleural effusion). Based on 25 published studies including about 1,700 paired cytology/histology cases, the median (range) concordance was 81–85% (62–100%) at cutoff 1% for a positive PD-L1 staining and 89% (67–100%) at cutoff 50%.
Conclusions
The overall concordance of PD-L1 between cytology and biopsies is rather good but with significant variation between laboratories, which calls for local quality assurance.
Keywords: 22C3, 28-8, Cell block, Cytology, CytoLyt, Histology, PreservCyt
Introduction
In non-small cell lung cancer (NSCLC), immunohistochemical (IHC) staining for programmed death-ligand 1 (PD-L1) is used to predict response to immune checkpoint inhibitors [1, 2, 3, 4, 5]. Different PD-L1 assays have been used for the various programmed death-1 (PD-1)/PD-L1 inhibitors, and a lot of studies have presented data on comparison of assays [6, 7, 8]. Essentially, the PD-L1 clones 22C3, 28-8, and SP263 (especially the former two) exhibit a similar staining pattern, while SP142 differs from the others. Laboratory-developed tests may give the same results as approved assays, but a highly varying number of laboratory-developed tests have shown inadequate quality [9, 10, 11].
Other technical factors may also potentially affect PD-L1 staining results. Short fixation time, some decalcification procedures, older paraffin blocks, and long storage time of slides before staining have been linked to reduced staining of tumor cells [12, 13, 14, 15, 16]. In contrast, delayed or prolonged formalin fixation has not shown any significant effect on PD-L1 staining [13, 17].
Several biological factors that may affect PD-L1 staining results have also been investigated. PD-L1 expression may differ between primary tumor and metastasis with either gain or loss of expression in the metastasis [18, 19, 20, 21, 22]. A longer time interval between sampling has been reported to correlate with greater intertumor discrepancy (indicating a change in PD-L1 expression over time) [19], while the metastatic site has been reported not to influence PD-L1 expression [23]. Impact of intratumor heterogeneity has also been explored, and, for example, concordance of PD-L1 expression between paired biopsies and resected tumors has been reported to be 63–92% for <1 versus ≥1% positive tumor cells [24, 25, 26, 27, 28].
As immune checkpoint inhibitors are used in advanced and recurrent lung cancer, biopsies have been the main specimen type for PD-L1 evaluation in treatment studies (although resected tumor tissue was also allowed in most) [1, 2, 4, 5, 29, 30, 31] and is regarded as the gold standard. However, cytology is not seldom the only available material in the clinical setting, and some (e.g., technical) factors may differ between cytological and histological specimens. The aim of the present study was to explore if PD-L1 testing in NSCLC is comparable for cytological specimens and biopsies based on the standard procedures in southern Sweden and to review the current literature.
Materials and Methods
In the present study, paired cytology/biopsy specimens from NSCLC cases stained with PD-L1 were included from 2 sites in southern Sweden: The Department of Genetics and Pathology in Lund and the Department of Pathology and Cytology in Halmstad.
Study Material
The 2 cohorts included all available NSCLC cases from 2017 to 2019 in Lund and 2003 to 2019 in Halmstad (the Halmstad cases were all stained in 2016–2020), respectively, with a paired cell block and biopsy stained for PD-L1. For the Lund cohort, all PD-L1 stains were from the clinical setting, while for the Halmstad cohort, stains were either from the clinical setting or identically stained as part of the present study.
For both cohorts, only cases where the paired specimens were part of the same diagnostic workup were included (<4 weeks between the samples). Only 1 cell block and 1 biopsy were stained with PD-L1 for each individual, but sometimes the cell block contained material from >1 cytological sample, for example, both bronchial brush and endobronchial ultrasound (EBUS)-guided lymph node aspiration combined into a single cell block. Cases with <100 evaluable tumor cells in either sample were excluded. Also, 2 cases were excluded where the biopsy and cytology showed different histological types, namely, squamous cell carcinoma and adenocarcinoma (presumably adenosquamous carcinoma) and large cell neuroendocrine carcinoma and adenocarcinoma (either combined large cell neuroendocrine carcinoma or 2 synchronous tumors), respectively. Comparisons of preparation of cytology and PD-L1 staining (including assays) for the 2 cohorts are summarized in Table 1 and are presented in detail in online suppl. material (for all online suppl. material, see www.karger.com/doi/10.1159/000517078) together with images of control tissue stained for PD-L1 (online suppl. Fig. 1).
Table 1.
Comparison of fixation and preparation of cytological cell blocks and routine PD-L1 staining for NSCLC for the 2 involved pathology departments (for further details, see online suppl. material)
| Issue | Lund | Halmstad |
|---|---|---|
| Cell block preparation of pleural effusions | Washed 1–2 times in CytoLyt®, then PreservCyt® 1–3 days, then Cellient™ cell block | Formalin fixation of centrifuged pellet in cassette (bloody samples first washed ≤3 times in CytoLyt®) |
|
| ||
| Cell block preparation of other cytology | CytoLyt® a few hours, then PreservCyt® 1–3 days, then CellientTM cell block | CytoLyt® 1–24 h, then formalin fixation of centrifuged pellet in cassette (nodal EBUS aspirations first in sodium chloride for less than a few hours) |
|
| ||
| PD-L1 staining | 22C3 (Agilent/pharmDx) | 28–8 (Agilent/pharmDx) |
|
| ||
| Staining platform | Ventana Benchmark Ultra | Dako Autostainer Link 48 |
|
| ||
| Control tissue | Tonsil and placenta (on each slide) | Tonsil, placenta, and either small intestine or appendix (on each slide); separate slide stained with negative control reagent; positive and negative cell lines (in each run) |
|
| ||
| PD-L1 analysis of small specimens | Always performed, biopsy preferred in the clinical setting (parallel staining of cell blocks common in 2017–2019) | Always performed, biopsy preferred in the clinical setting |
|
| ||
| PD-L1 analysis of resected tumors | Sometimes performed but not mandatory | Not applicable (no resections) |
PD-L1, programmed death-ligand 1; NSCLC, non-small cell lung cancer.
PD-L1 Evaluation
All evaluations were performed using a conventional light microscope. All involved pathologists assess PD-L1 in daily practice. Hematoxylin-eosin-stained sections (and diagnostic IHC staining when performed) were available at time of evaluation of PD-L1. PD-L1 was scored <1%, 1–49%, and ≥50%, based on any intensity of partial or complete linear membranous staining of tumor cells in line with assessment manuals (Agilent/pharmDx, Santa Clara, CA, USA) [32, 33].
For the Lund cohort, all PD-L1 slides were assessed by at least 2 pathologists. Review was performed as part of the study by pathologists D.V. or H.B. and in most cases also by the pathology resident R.M. Cases were reviewed by all 3 and/or an additional pathologist (K.E.L.) for confirmation when needed.
For the Halmstad cohort, all PD-L1 slides were assessed as part of the study by the certified cytotechnologist M.S.I.M., and one or, when needed, both the pathologists T.S. and H.B. Cases with discordant PD-L1 between biopsy and cytology were also assessed by the pathologist A.D.
As all cases were primarily assessed by 2 investigators, interobserver agreement could be evaluated, although not representing the concordance between the same 2 investigators for all 144 cases. PD-L1 was scored the same by the 2 investigators for 138 biopsies and 131 cytological specimens (no significant difference with Fisher's exact test; kappa 0.93 and 0.86, respectively).
Statistical Analysis
Kappa and Wilcoxon signed-rank test were used for diagnostic concordance and comparison of PD-L1 expression, respectively, in the paired cases. Fisher's exact test was used for comparison of concordance between cohorts. The analyses were performed with MedCalc 14.12.0 (MedCalc Software bvba, Ostend, Belgium).
Literature Search
PubMed was searched using “PD-L1 cytology lung cancer” as the search term. Original articles in English until December 2020 that included NSCLC cases with PD-L1 analyzed on cytological material and paired histological specimens were selected. Further articles were identified through the “similar articles” utility of PubMed and individual journals as well as cited references in the retrieved articles. Studies not presenting data for pure cytological versus pure histological specimens were excluded [23, 28, 34, 35, 36, 37].
Results
PD-L1 Concordance
Concordance of PD-L1 expression in paired biopsies and cytological specimens from NSCLC cases in Lund 2017–2019 and Halmstad 2003–2019 (stained 2016–2020), respectively, is found in Table 2. As evident, 40 (85%) of 47 cases (from 47 different individuals) were concordant in the Lund cohort. All discordant cases had lower PD-L1 score in cytology, and the difference in score was significant between biopsies and cytology (Wilcoxon test p = 0.02). Unweighted kappa was 0.77 (95% CI: 0.62–0.93). The concordance was 94% (44/47) for the 1% cutoff (i.e., <1 or ≥1% positive tumor cells) and 89% (42/47) for the 50% cutoff.
Table 2.
Concordance of PD-L1 expression in 47 and 97 paired biopsies and cytological specimens from NSCLC, from Lund and Halmstad, respectively
| Cytology, % | <1 | 1-49 | ≥50 |
|---|---|---|---|
| Lund cohort | Biopsy | ||
| <1 | 11 | 2 | 1 |
| 1–49 | 0 | 11 | 4 |
| ≥50 | 0 | 0 | 18 |
|
| |||
| Halmstad cohort | Biopsy | ||
| <1 | 40 | 12 | 2 |
| 1–49 | 4 | 10 | 7 |
| ≥50 | 0 | 6 | 16 |
PD-L1, programmed death-ligand 1; NSCLC, non-small cell lung cancer.
In the Halmstad cohort, 66 (68%) of 97 cases (from 97 different individuals) were concordant, and unweighted kappa was 0.49 (95% CI: 0.35–0.63). The concordance was 81% (79/97) for the 1% cutoff (i.e., <1 or ≥1% positive tumor cells) and 85% (82/97) for the 50% cutoff. As evident from Table 2, there were more cases with lower PD-L1 score in cytology in the Halmstad cohort, with a nonsignificant trend (Wilcoxon test p = 0.055).
The number of concordant cases was significantly lower in the Halmstad cohort compared to the Lund cohort (Fisher's exact test p = 0.043). See Figures 1 and 2 for examples of a concordant and a discordant case (using the 3-tier scale <1%/1–49%/≥50%), respectively, and online suppl. Figures 2 and 3 for additional cases.
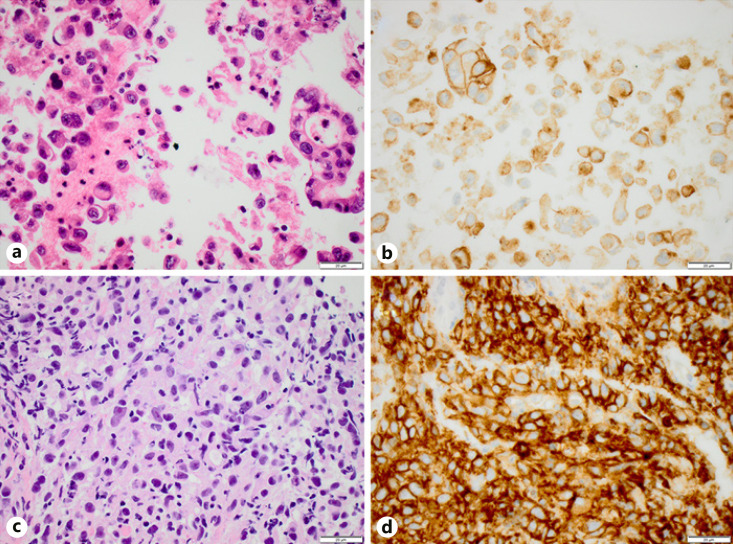
Fig. 1.
An adenocarcinoma with concordant PD-L1 expression (both ≥50%) between paired EBUS cytology (a, b) and bronchial biopsy (c, d). Hematoxylin-eosin (a, c) and PD-L1 clone 22C3 (b, d). Scale bar, 20 μm. PD-L1, programmed death-ligand 1; EBUS, endobronchial ultrasound.
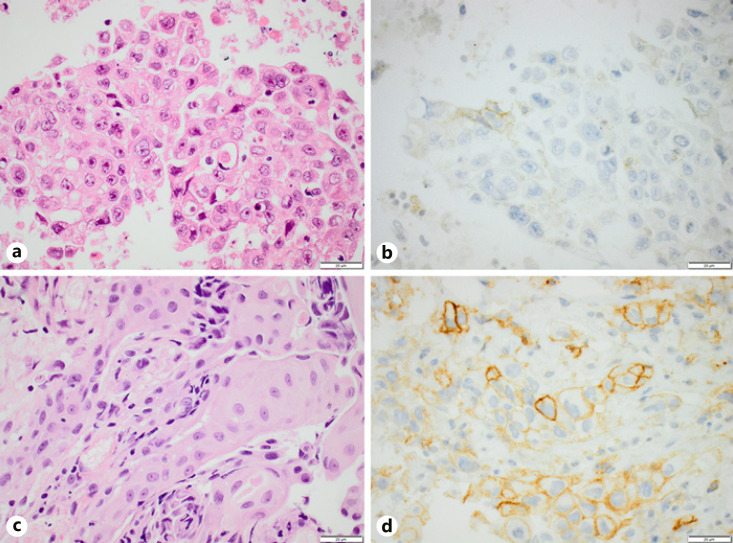
Fig. 2.
A squamous cell carcinoma with discordant PD-L1 expression (<1% vs. ≥50%) between paired EBUS cytology (a, b) and bronchial biopsy (c, d). Hematoxylin-eosin (a, c) and PD-L1 clone 22C3 (b, d). Scale bar, 20 μm. PD-L1, programmed death-ligand 1; EBUS, endobronchial ultrasound.
Characteristics of the Specimens
The diagnosis of the 47 cases in the Lund cohort was adenocarcinoma in 32 (whereof 29 concordant PD-L1), squamous cell carcinoma in 13 (9 concordant), and NSCLC not otherwise specified in 2 cases (both concordant). The biopsies were bronchial biopsies in 42 cases, transthoracic core biopsies in 3 cases, liver biopsy in 1 case, and biopsy from a cervical lymph node in 1 case. The cytological specimens were bronchial brush in 13 cases, other bronchial cytology including suction catheter, bronchoalveolar lavage (BAL), or mix of any of the two with bronchial brush in 5 cases, EBUS-guided lymph node aspirations in 15 cases, mix of EBUS from lymph nodes and bronchial cytology in 12 cases, and pleural effusions in 2 cases.
The samples were from the same site in 18 cases (whereof 16 concordant PD-L1), partly from the same site in 12 cases (11 concordant), or from different sites in 17 cases (13 concordant), respectively. The samples from the same site were all from the primary tumor. The samples partly from the same site were biopsies from the primary tumor and mixed cytological specimens (all mixed bronchial cytology and EBUS from lymph nodes).
The diagnosis of the 97 cases in the Halmstad cohort was adenocarcinoma in 67 (whereof 43 concordant PD-L1) and squamous cell carcinoma in 30 cases (23 concordant). The biopsies were bronchial biopsies in 62 cases and transthoracic core biopsies in 35 cases. The cytological specimens were bronchial brush in 13 cases, BAL in 53 cases, both bronchial brush and BAL in 7 cases, pleural effusion in 17 cases, EBUS-guided lymph node aspirations in 2 cases, fine-needle aspiration (FNA) of the lymph node in 2 cases, and both BAL and either pleural effusion, EBUS, or FNA of the lymph node in 1 case each.
The samples were from the same site in 73 cases (whereof 54 concordant PD-L1), partly from the same site in 3 cases (all concordant), or from different sites in 21 cases (9 concordant), respectively. The samples partly from the same site were biopsies from the primary tumor and mixed cytological specimens (mixed BAL and either pleura, EBUS, or FNA of the lymph node). The paraffin blocks had been stored for at least 3 years before PD-L1 staining in 36 of the cases (whereof 25 concordant PD-L1) and shorter than 3 years in the remaining 61 cases (41 concordant).
Literature Data
Based on 25 published studies, the mean, median, and range for PD-L1 concordance in studies on paired cytology/histology cases were 83–85%, 81–85%, and 62–100%, respectively, at cutoff 1% for a positive PD-L1 staining and 87–89%, 89%, and 67–100%, respectively, at cutoff 50% [38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62]. The reason for the intervals (here and below) is that some studies presented separate data for >1 cytological preparation, PD-L1 assay, or histological specimen type. The numbers remained the same if the data from the present study were included. For further details on individual studies, see online suppl. Table 1.
Summation of all paired cytology/histology cases in the studies (including the cases from the present study) resulted in a concordance of 82–83% (1,465/1,785–1,488/1,792 cases) at cutoff 1% for a positive PD-L1 staining and 88–89% (1,398/1,587–1,408/1,580 cases) at cutoff 50% [38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62]. If excluding studies with resections, 6 studies (including the present) remained, reporting a concordance in paired cytology/biopsy cases of 81–86% (228/283–248/290 cases) at cutoff 1% for a positive PD-L1 staining and 86–90% (249/290–254/283 cases) at cutoff 50% [41, 43, 46, 49, 59].
In studies with cytology fixed in formalin, that is, excluding studies with nonformalin or mixed formalin/nonformalin fixation, the concordance with paired histological specimens was 81–82% (333–336/410 cases) at cutoff 1% and 88–89% (316–317/358 cases) at cutoff 50% based on 7 studies [38, 39, 48, 50, 58, 60, 62]. If instead, only including studies with cytology fixed in nonformalin fixatives, excluding mixed formalin/nonformalin fixation, the concordance with paired histological specimens was 79–81% (500–512/633 cases) at cutoff 1% and 88–89% (554–565/633 cases) at cutoff 50% based on 9 studies including the Lund cohort from the present study [41, 45, 46, 49, 52, 55, 56, 62].
The concordance in studies using only cytological cell blocks compared to histology was 82–84% (1,181/1,436–1,217/1,443 cases) at cutoff 1% and 88–89% (1,085/1,238–1,091/1,231 cases) at cutoff 50% based on 23 studies including the present [38, 39, 40, 41, 42, 43, 44, 47, 48, 49, 50, 51, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62]. Correspondingly, the concordance for cytological smears versus histology was 79–80% (327–329/413 cases) at cutoff 1% and 93% (383–384/413) cases at cutoff 50% based on 5 studies [43, 45, 46, 49, 52].
Discussion
The paired cytology/biopsy NSCLC cases of our study support that PD-L1 expression is lower in cytological specimens, with significance in one and trend in the other of our 2 cohorts. Indeed, the PD-L1 score was lower in the cytological specimen for all discordant cases in the Lund cohort. It is noteworthy that the concordance was quite limited in the Halmstad cohort, with a kappa value of 0.49. Although discordant expression was more common in paired samples from different sites (e.g., biopsy from the primary tumor and cytology from pleura or lymph nodes) with 58% concordance (22/38 cases) in our 2 cohorts combined, discordant cases were also observed in paired samples from the same site (concordance 77%; 70/91). Concordance was similar for adenocarcinomas and squamous cell carcinomas and for new and archival blocks.
An important difference between, but also within, our 2 cohorts was the time in alcohol-based fixative (typically 1–3 days vs. <24 h, but also some with only formalin fixation) and the use of CellientTM versus manual cell blocks. While fixation and processing of biopsies is essentially the same at all pathology departments, there are substantial differences in handling of cytological specimens for IHC. Apart from formalin, cells may be fixed in various ethanol- or methanol-containing liquids (e.g., CytoLyt®, CytoRichTM Red, Novaprep®, and PreservCyt®), while different techniques such as direct smears, CytospinTM preparations, or cell blocks (e.g., plasma-thrombin, agar, CellientTM, and ShandonTM) may be used [63], also evident from the published studies compiled in online suppl. Table 1.
Alcohol fixation of tissue has been suggested to affect PD-L1 staining negatively [14], and some studies, like our Lund cohort, report a lower PD-L1 score for alcohol-fixed cytology in all discordant paired cytology/histology cases [45, 64]. Also, Koomen et al. [62] clearly demonstrated that alcohol-fixed CellientTM cell blocks result in lower concordance with histology compared to formalin-fixed agar-based cell blocks. However, the compiled literature data did not show any obvious difference between formalin and nonformalin fixation. Also, Lou et al. [60] presented a very good concordance for formalin-fixed cell blocks with or without prefixation with CytoLyt® [60], while a perfect concordance was seen in the study by Gosney et al. [65] with EBUS cytology fixed in formalin versus alcohol. Fixation in, for example, CytoLyt®, is very rapid, and it has been suggested that <1-h fixation has no effect on IHC staining (personal communication). The area thus merits future investigation.
Furthermore, specimen types differed between our cohorts, with more bronchial biopsies, brushes, and EBUS-guided lymph node aspirations in the Lund cohort, while the Halmstad cohort contained more transthoracic core biopsies, BAL, and pleural effusions. Both, but especially the Lund cohort, contained cell blocks mixed from different cytological samples (for increased tumor cell content). This complicates analyses of sample types but reflects the real-world diagnostic situation in our setting.
Grosu et al. [50] and Zou et al. [57] showed a higher concordance for PD-L1 in pleural effusions compared to matched histological samples (87–97% at 1% cutoff) than did Yoshimura et al. [37] and Jug et al. [58] for EBUS-guided samples (70–84%). Such a trend is not seen when comparing our 2 cohorts but may be concealed by other differences with greater impact. There were too few pleural effusions in the Lund cohort and too few EBUS-guided aspirations in the Halmstad cohort for comparisons of these 2 sample types within each cohort.
Interestingly, in the Halmstad cohort, biopsy/cytology PD-L1 concordance was lower for bronchial brushes and pleural effusions than for BAL specimens, while there was no obvious difference between bronchial and transthoracic core biopsies. However, our cohorts do not contain enough cases for adequate multivariable analyses, and ideally future studies may include multiple samples from the same patient for further analysis of potential effect of sample type on PD-L1 expression.
Still, the overall concordance of PD-L1 expression between cytology and histology was rather good based on our results and previously published studies. In the literature, discordant cases typically show either higher score in cytology or in histology [39, 42, 43, 55, 66], which is expected due to heterogeneity of PD-L1 expression [24, 25, 26, 27, 28, 67]. However, the range of concordance was quite broad, 62–100% at cutoff 1% and 67–100% at cutoff 50% based on 25 publications. Also, several studies present a positive PD-L1 expression (≥1%) in <40% of NSCLC cases when using cytology [41, 42, 44, 52, 54, 61, 68, 69], which is notably lower than what has been reported in both early treatment studies and large studies with real-life data, with PD-L1 ≥1% in 53–86% [70] and 56–63% [71, 72] of NSCLC, respectively (61% for cytology [71]). Furthermore, for example, the large study by Kuempers et al. [52] showed a high concordance (93%) at the 50% cutoff for a positive staining, but still no case with >50% in both cytology (mainly imprints from resections) and the paired resected tumors, which makes the applicability of the results questionable. We believe it would be reasonable for any department that use cytology for PD-L1 to (a) investigate the proportion of PD-L1-positive NSCLC in 50–100 nonselective cytological specimens and (b) evaluate the concordance between biopsies and cytological specimens in 20–30 paired cases. A lab with <55% PD-L1-positive cases and/or concordance <85% should consider investigating possible causes and potential improvements.
Biopsies are currently the standard for PD-L1 testing. However, based on histology, PD-L1 is not considered an optimal predictor of immunotherapy response [1, 2, 3, 4, 5, 29, 30, 31]. Also, there is a variation of positive PD-L1 expression between studies for biopsies as well (e.g., see online suppl. Table 1, but also seen in treatment studies [70]). Thus, it may be argued that the predictive value of PD-L1 in cytology (and preferably also the concordance with biopsies) should be evaluated in a population of patients treated with immunotherapy. Such studies are missing today.
PD-L1 expression in immune cells, relevant for a different PD-L1 assay (SP142), was not a focus of the present study. The representation of tumor-infiltrating immune cells in small samples has been discussed, and the interobserver concordance for PD-L1 has been shown to be low for immune cells [6, 7]. We noted in our Halmstad cohort that >1% positive lymphocytes were seen in 7 (7%) of 94 and >1% positive macrophages in 60 (65%) of 93 cytological specimens.
Some strengths and weaknesses of the present study must be discussed. Importantly, our paired samples were from the same diagnostic workup, which eliminates any potential effect of systemic therapy and time on PD-L1 expression, and only biopsies (not resections) and cases with >100 evaluable tumor cells were included. We did not limit the material to cases with sampling from the same site to eliminate another potentially confounding factor, but data for samples from the same and different sites, respectively, are presented. All cases in each cohort were stained with the same marker, 28-8 or 22C3, respectively. As very similar staining patterns have been reported for these 2 PD-L1 clones [6, 7, 8], the staining is probably comparable. The Halmstad cohort contained some old archival cases (which is linked to lower PD-L1 expression and not recommended [7, 15]), but this was about equal for the cell blocks and biopsies, which probably limits the impact on the results. In the Lund cohort, there was a slight selection bias for PD-L1-positive cases as a cytological cell block was not ordered as often in the clinical setting when a biopsy was PD-L1 negative. However, this probably does not affect the results significantly. The inclusion of cell blocks with mixed material from different sites limits the possibility to fully evaluate any possible impact of the sampling site on PD-L1. Also, it may be of note that interobserver discrepancy was 17% (3/18) for these cell blocks compared to 8% (10/126) for the remaining cases.
In conclusion, there is a rather good concordance for PD-L1 expression in NSCLC between cytology and histology, but with quite substantial variation between studies. As fixatives and preparation techniques for cytology may probably affect PD-L1 expression, it is of importance that each laboratory's quality of PD-L1 staining in cytology is kept high, preferably by ascertaining a good correlation with biopsies.
Statement of Ethics
The study was conducted in adherence to the Declaration of Helsinki and was approved by the Regional Ethical Review Board in Lund (Dnr 2019-04782 and Dnr 2006-399 with addition 2017-708, respectively).
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Funding Sources
The study was supported by the Regional Agreement on Medical Training and Clinical Research (ALF), Regional Research and Development Funding (FoU), and the Swedish Cancer Society. The funding sources had no role in the design or conduct of the study.
Author Contributions
M.S.I.M., T.S., D.V., K.D., A.D., M.P., and H.B. planned the study. L.T. and K.H. performed staining. M.S.I.M., K.E.L., T.S., U.M., R.M., B.H., D.V., A.D., and H.B. collected data. M.S.I.M. and H.B. analyzed the data and wrote the manuscript, and all other authors revised the manuscript for its intellectual content.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgment
The authors wish to thank the cytology units at the Department of Genetics and Pathology, Lund, and at the Department of Pathology and Cytology, Halmstad. The study was supported by the Regional Agreement on Medical Training and Clinical Research (ALF), Regional Research and Development Funding (FoU), and the Swedish Cancer Society.
References
- 1.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 3.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–30. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 4.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 5.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379:2342–50. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 6.Lantuejoul S, Damotte D, Hofman V, Adam J. Programmed death ligand 1 immunohistochemistry in non-small cell lung carcinoma. J Thorac Dis. 2019;11:S89–101. doi: 10.21037/jtd.2018.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lantuejoul S, Sound-Tsao M, Cooper WA, Girard N, Hirsch FR, Roden AC, et al. PD-L1 testing for lung cancer in 2019: perspective from the IASLC pathology committee. J Thorac Oncol. 2020;15:499–519. doi: 10.1016/j.jtho.2019.12.107. [DOI] [PubMed] [Google Scholar]
- 8.Koomen BM, Badrising SK, van den Heuvel MM, Willems SM. Comparability of PD-L1 immunohistochemistry assays for non-small cell lung cancer: a systematic review. Histopathology. 2020;76:793–802. doi: 10.1111/his.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheel AH, Baenfer G, Baretton G, Dietel M, Diezko R, Henkel T, et al. Interlaboratory concordance of PD-L1 immunohistochemistry for non-small-cell lung cancer. Histopathology. 2018;72:449–59. doi: 10.1111/his.13375. [DOI] [PubMed] [Google Scholar]
- 10.Adam J, Le Stang N, Rouquette I, Cazes A, Badoual C, Pinot-Roussel H, et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann Oncol. 2018;29:953–8. doi: 10.1093/annonc/mdy014. [DOI] [PubMed] [Google Scholar]
- 11.NordiQC PD-L1. Available from: https://www.nordiqc.org/epitope.php?id=107: NordiQC; 2018 (Accessed 2021 Jan 13)
- 12.Sato Y, Fujimoto D, Uehara K, Kawachi H, Nagata K, Nakagawa A, et al. Reduced tumour proportion scores for programmed cell death ligand 1 in stored paraffin tissue sections. Anticancer Res. 2018;38:1401–5. doi: 10.21873/anticanres.12363. [DOI] [PubMed] [Google Scholar]
- 13.Forest F, Cote G, Laville D, Da Cruz V, Dal Col P, Camy F, et al. Impact of delayed fixation and decalcification on PD-L1 expression: a comparison of two clones. Virchows Arch. 2019;475:693–9. doi: 10.1007/s00428-019-02613-w. [DOI] [PubMed] [Google Scholar]
- 14.Roche VENTANA PD-L1 (SP263) Assay staining of non-small cell lung cancer interpretation guide. Available from: https://www.rochebiomarkers.be/content/media/Files/PD-L1_SP263_interpretation_guide_NSCLC.pdf: 2019 (Accessed 2021 Jan 13)
- 15.Gagne A, Wang E, Bastien N, Orain M, Desmeules P, Page S, et al. Impact of specimen characteristics on PD-L1 testing in non-small cell lung cancer: validation of the IASLC PD-L1 testing recommendations. J Thorac Oncol. 2019;14:2062–70. doi: 10.1016/j.jtho.2019.08.2503. [DOI] [PubMed] [Google Scholar]
- 16.Giunchi F, Degiovanni A, Daddi N, Trisolini R, Dell'Amore A, Agostinelli C, et al. Fading with time of PD-L1 immunoreactivity in non-small cells lung cancer tissues: a Methodological Study. Appl Immunohistochem Mol Morphol. 2018;26:489–94. doi: 10.1097/PAI.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 17.Kawachi H, Fujimoto D, Yamashita D, Fukuoka J, Kitamura Y, Hosoya K, et al. Association between formalin fixation time and programmed cell death ligand 1 expression in patients with non-small cell lung cancer. Anticancer Res. 2019;39:2561–7. doi: 10.21873/anticanres.13378. [DOI] [PubMed] [Google Scholar]
- 18.Kim MY, Koh J, Kim S, Go H, Jeon YK, Chung DH. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;88:24–33. doi: 10.1016/j.lungcan.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Mansfield AS, Aubry MC, Moser JC, Harrington SM, Dronca RS, Park SS, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 2016;27:1953–8. doi: 10.1093/annonc/mdw289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takamori S, Toyokawa G, Okamoto I, Takada K, Kozuma Y, Matsubara T, et al. Discrepancy in programmed cell death-ligand 1 between primary and metastatic non-small cell lung cancer. Anticancer Res. 2017;37:4223–8. doi: 10.21873/anticanres.11813. [DOI] [PubMed] [Google Scholar]
- 21.Kim HR, Cha YJ, Hong MH, Gandhi M, Levinson S, Jung I, et al. Concordance of programmed death-ligand 1 expression between primary and metastatic non-small cell lung cancer by immunohistochemistry and RNA in situ hybridization. Oncotarget. 2017;8:87234–43. doi: 10.18632/oncotarget.20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Dong Z, Jiang T, Hou L, Wu F, Gao G, et al. Heterogeneity of PD-L1 expression among the different histological components and metastatic lymph nodes in patients with resected lung adenosquamous carcinoma. Clin Lung Cancer. 2018;19:e421–30. doi: 10.1016/j.cllc.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Agulnik J, Kasymjanova G, Fiset PO, Camilleri-Broet S, Redpath M, et al. The metastatic site does not influence PD-L1 expression in advanced non-small cell lung carcinoma. Lung Cancer. 2019;132:36–8. doi: 10.1016/j.lungcan.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Kitazono S, Fujiwara Y, Tsuta K, Utsumi H, Kanda S, Horinouchi H, et al. Reliability of small biopsy samples compared with resected specimens for the determination of programmed death-ligand 1 expression in non-small-cell lung cancer. Clin Lung Cancer. 2015;16:385–90. doi: 10.1016/j.cllc.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Ilie M, Long-Mira E, Bence C, Butori C, Lassalle S, Bouhlel L, et al. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016;27:147–53. doi: 10.1093/annonc/mdv489. [DOI] [PubMed] [Google Scholar]
- 26.Gradecki SE, Grange JS, Stelow EB. Concordance of PD-L1 expression between core biopsy and resection specimens of non-small cell lung cancer. Am J Surg Pathol. 2018;42:1090–4. doi: 10.1097/PAS.0000000000001085. [DOI] [PubMed] [Google Scholar]
- 27.Elfving H, Mattsson JSM, Lindskog C, Backman M, Menzel U, Micke P. Programmed cell death ligand 1 immunohistochemistry: a concordance study between surgical specimen, biopsy, and tissue microarray. Clin Lung Cancer. 2019;20:258–e1. doi: 10.1016/j.cllc.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Tsunoda A, Morikawa K, Inoue T, Miyazawa T, Hoshikawa M, Takagi M, et al. A prospective observational study to assess PD-L1 expression in small biopsy samples for non-small-cell lung cancer. BMC Cancer. 2019;19:546. doi: 10.1186/s12885-019-5773-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–65. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agilent PD-L1 IHC 28-8 pharmDx Interpretation Manual. Available from: https://www.agilent.com/en/product/pharmdx/pd-l1-ihc-28-8-overview: 2020 (Accessed 2021 Jan 13)
- 33.Agilent PD-L1 IHC 22C3 pharmDx Testing for NSCLC. Available from: https://www.agilent.com/en/product/pharmdx/pd-l1-ihc-22c3-pharmdx-testing-for-nsclc: 2020 (Accessed 2021 Jan 13)
- 34.Kim I, Kim A, Lee CH, Lee G, Kim A, Jo EJ, et al. Reliability of PD-L1 assays using small tissue samples compared with surgical specimens. Medicine. 2019;98:e14972. doi: 10.1097/MD.0000000000014972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lozano MD, Abengozar-Muela M, Echeveste JI, Subtil JC, Bertó J, Gúrpide A, et al. Programmed death-ligand 1 expression on direct Pap-stained cytology smears from non-small cell lung cancer: comparison with cell blocks and surgical resection specimens. Cancer Cytopathol. 2019;127:470–80. doi: 10.1002/cncy.22155. [DOI] [PubMed] [Google Scholar]
- 36.Bozzetti C, Squadrilli A, Nizzoli R, Lagrasta C, Gasparro D, Majori M, et al. Optimizing PD-L1 evaluation on cytological samples from advanced non-small-cell lung cancer. Immunotherapy. 2020;12:183–93. doi: 10.2217/imt-2019-0138. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimura K, Inoue Y, Karayama M, Tsuchiya K, Mori K, Suzuki Y, et al. Heterogeneity analysis of PD-L1 expression and copy number status in EBUS-TBNA biopsy specimens of non-small cell lung cancer: comparative assessment of primary and metastatic sites. Lung Cancer. 2019;134:202–9. doi: 10.1016/j.lungcan.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Sakakibara R, Inamura K, Tambo Y, Ninomiya H, Kitazono S, Yanagitani N, et al. EBUS-TBNA as a promising method for the evaluation of tumor PD-L1 expression in lung cancer. Clin Lung Cancer. 2017;18:527–534.e1. doi: 10.1016/j.cllc.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Skov BG, Skov T. Paired comparison of PD-L1 expression on cytologic and histologic specimens from malignancies in the lung assessed with PD-L1 IHC 28-8pharmDx and PD-L1 IHC 22C3pharmDx. Appl Immunohistochem Mol Morphol. 2017;25:453–9. doi: 10.1097/PAI.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 40.Heymann JJ, Bulman WA, Swinarski D, Pagan CA, Crapanzano JP, Haghighi M, et al. PD-L1 expression in non-small cell lung carcinoma: comparison among cytology, small biopsy, and surgical resection specimens. Cancer Cytopathol. 2017;125:896–907. doi: 10.1002/cncy.21937. [DOI] [PubMed] [Google Scholar]
- 41.Stoy SP, Rosen L, Mueller J, Murgu S. Programmed death-ligand 1 testing of lung cancer cytology specimens obtained with bronchoscopy. Cancer Cytopathol. 2018;126:122–8. doi: 10.1002/cncy.21941. [DOI] [PubMed] [Google Scholar]
- 42.Ilie M, Juco J, Huang L, Hofman V, Khambata-Ford S, Hofman P. Use of the 22C3 anti-programmed death-ligand 1 antibody to determine programmed death-ligand 1 expression in cytology samples obtained from non-small cell lung cancer patients. Cancer Cytopathol. 2018;126:264–74. doi: 10.1002/cncy.21977. [DOI] [PubMed] [Google Scholar]
- 43.Noll B, Wang WL, Gong Y, Zhao J, Kalhor N, Prieto V, et al. Programmed death ligand 1 testing in non-small cell lung carcinoma cytology cell block and aspirate smear preparations. Cancer Cytopathol. 2018;126:342–52. doi: 10.1002/cncy.21987. [DOI] [PubMed] [Google Scholar]
- 44.Sakata KK, Midthun DE, Mullon JJ, Kern RM, Nelson DR, Edell ES, et al. Comparison of programmed death ligand-1 immunohistochemical staining between endobronchial ultrasound transbronchial needle aspiration and resected lung cancer specimens. Chest. 2018;154:827–37. doi: 10.1016/j.chest.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Munari E, Zamboni G, Sighele G, Marconi M, Sommaggio M, Lunardi G, et al. Expression of programmed cell death ligand 1 in non-small cell lung cancer: comparison between cytologic smears, core biopsies, and whole sections using the SP263 assay. Cancer Cytopathol. 2019;127:52–61. doi: 10.1002/cncy.22083. [DOI] [PubMed] [Google Scholar]
- 46.Capizzi E, Ricci C, Giunchi F, Zagnoni S, Ceccarelli C, Gómez BUÁ, et al. Validation of the immunohistochemical expression of programmed death ligand 1 (PD-L1) on cytological smears in advanced non small cell lung cancer. Lung Cancer. 2018;126:9–14. doi: 10.1016/j.lungcan.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 47.Hernandez A, Brandler TC, Zhou F, Moreira AL, Schatz-Siemers N, Simsir A. Assessment of programmed death-ligand 1 (PD-L1) immunohistochemical expression on cytology specimens in non-small cell lung carcinoma. Am J Clin Pathol. 2019;151:403–15. doi: 10.1093/ajcp/aqy164. [DOI] [PubMed] [Google Scholar]
- 48.Xu H, Bratton L, Nead M, Russell D, Zhou Z. Comparison of programmed death-ligand 1 (PD-L1) immunostain for nonsmall cell lung carcinoma between paired cytological and surgical specimens. Cytojournal. 2018;15:29. doi: 10.4103/cytojournal.cytojournal_2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arriola AGP, Bashover E, Joseph C, Staerkel G, Wang WL, Roy-Chowdhuri S. The usefulness of various cytologic specimen preparations for PD-L1 immunostaining in non-small cell lung carcinoma. J Am Soc Cytopathol. 2018;7:324–32. doi: 10.1016/j.jasc.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Grosu HB, Arriola A, Stewart J, Ma J, Bassett R, Hernandez M, et al. PD-L1 detection in histology specimens and matched pleural fluid cell blocks of patients with NSCLC. Respirology. 2019;24:1198–203. doi: 10.1111/resp.13614. [DOI] [PubMed] [Google Scholar]
- 51.Pak MG, Roh MS. Cell-blocks are suitable material for programmed cell death ligand-1 immunohistochemistry: comparison of cell-blocks and matched surgical resection specimens in lung cancer. Cytopathology. 2019;30:578–85. doi: 10.1111/cyt.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuempers C, van der Linde LIS, Reischl M, Vogel W, Stellmacher F, Reck M, et al. Comparison of PD-L1 expression between paired cytologic and histologic specimens from non-small cell lung cancer patients. Virchows Arch. 2020;476:261–71. doi: 10.1007/s00428-019-02632-7. [DOI] [PubMed] [Google Scholar]
- 53.Wang G, Ionescu DN, Lee CH, Hiruki T, Myers R, Shaipanich T, et al. PD-L1 testing on the EBUS-FNA cytology specimens of non-small cell lung cancer. Lung Cancer. 2019;136:1–5. doi: 10.1016/j.lungcan.2019.07.033. [DOI] [PubMed] [Google Scholar]
- 54.Hendry S, Byrne DJ, Christie M, Steinfort DP, Irving LB, Wagner CA, et al. Adequate tumour cellularity is essential for accurate PD-L1 immunohistochemistry assessment on cytology cell-block specimens. Cytopathology. 2020;31:90–5. doi: 10.1111/cyt.12795. [DOI] [PubMed] [Google Scholar]
- 55.Song SG, Lee J, Koh J, Kim S, Chung DH, Jeon YK. Utility of PD-L1 immunocytochemistry using body-fluid cell blocks in patients with non-small-cell lung cancer. Diagn Cytopathol. 2020;48:291–9. doi: 10.1002/dc.24379. [DOI] [PubMed] [Google Scholar]
- 56.Dong Z, Liu Y, Jiang T, Hou L, Wu F, Gao G, et al. Cell block as a surrogate for programmed death-ligand 1 staining testing in patients of non-small cell lung cancer. J Cancer. 2020;11:551–8. doi: 10.7150/jca.35810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zou Y, Xu L, Tang Q, You Q, Wang X, Ding W, et al. Cytology cell blocks from malignant pleural effusion are good candidates for PD-L1 detection in advanced NSCLC compared with matched histology samples. BMC Cancer. 2020;20:344. doi: 10.1186/s12885-020-06851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jug R, Giovacchini CX, Liu B, Green CL, Clarke JM, Mahmood K, et al. EBUS-FNA cytologic-histologic correlation of PD-L1 immunohistochemistry in non-small cell lung cancer. J Am Soc Cytopathol. 2020;9:485–93. doi: 10.1016/j.jasc.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gagne A, Orain M, Ionescu D, Tsao MS, Joubert D, Joubert P. Comprehensive assessment of PD-L1 immunohistochemistry on paired tissue and cytology specimens from non-small cell lung cancer. Lung Cancer. 2020;146:276–84. doi: 10.1016/j.lungcan.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Lou SK, Ko HM, Kinoshita T, MacDonald S, Weiss J, Czarnecka-Kujawa K, et al. Implementation of PD-L1 22C3 IHC pharmDxTM in cell block preparations of lung cancer: concordance with surgical resections and technical validation of CytoLyt(R) prefixation. Acta Cytol. 2020;64:577–87. doi: 10.1159/000508628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bubendorf L, Conde E, Cappuzzo F, Langfort R, Schildhaus HU, Votruba J, et al. A noninterventional, multinational study to assess PD-L1 expression in cytological and histological lung cancer specimens. Cancer Cytopathol. 2020;128:928–38. doi: 10.1002/cncy.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koomen BM, van der Starre-Gaal J, Vonk JM, von der Thusen JH, van der Meij JJC, Monkhorst K, et al. Formalin fixation for optimal concordance of programmed death-ligand 1 immunostaining between cytologic and histologic specimens from patients with non-small cell lung cancer. Cancer Cytopathol. 2021 Apr;129((4)):304–17. doi: 10.1002/cncy.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain D, Mathur SR, Iyer VK. Cell blocks in cytopathology: a review of preparative methods, utility in diagnosis and role in ancillary studies. Cytopathology. 2014;25:356–71. doi: 10.1111/cyt.12174. [DOI] [PubMed] [Google Scholar]
- 64.Jain D, Sukumar S, Mohan A, Iyer VK. Programmed death-ligand 1 immunoexpression in matched biopsy and liquid-based cytology samples of advanced stage non-small cell lung carcinomas. Cytopathology. 2018;29:550–7. doi: 10.1111/cyt.12605. [DOI] [PubMed] [Google Scholar]
- 65.Gosney JR, Haragan A, Chadwick C, Giles TE, Grundy S, Tippett V, et al. Programmed death ligand 1 expression in EBUS aspirates of non-small cell lung cancer: is interpretation affected by type of fixation? Cancer Cytopathol. 2020;128:100–6. doi: 10.1002/cncy.22216. [DOI] [PubMed] [Google Scholar]
- 66.Russell-Goldman E, Kravets S, Dahlberg SE, Sholl LM, Vivero M. Cytologic-histologic correlation of programmed death-ligand 1 immunohistochemistry in lung carcinomas. Cancer Cytopathol. 2018;126:253–63. doi: 10.1002/cncy.21973. [DOI] [PubMed] [Google Scholar]
- 67.McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelekanou V, Rehman J, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016;2:46–54. doi: 10.1001/jamaoncol.2015.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandez-Bussy S, Pires Y, Labarca G, Vial MR. PD-L1 expression in a non-small cell lung cancer specimen obtained by EBUS-TBNA. Arch Bronconeumol. 2018;54:290–2. doi: 10.1016/j.arbres.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 69.Vigliar E, Malapelle U, Iaccarino A, Acanfora G, Pisapia P, Clery E, et al. PD-L1 expression on routine samples of non-small cell lung cancer: results and critical issues from a 1-year experience of a centralised laboratory. J Clin Pathol. 2019;72:412–7. doi: 10.1136/jclinpath-2019-205732. [DOI] [PubMed] [Google Scholar]
- 70.Gandini S, Massi D, Mandalà M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;100:88–98. doi: 10.1016/j.critrevonc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Evans M, O'Sullivan B, Hughes F, Mullis T, Smith M, Trim N, et al. The Clinicopathological and molecular associations of PD-L1 expression in non-small cell lung cancer: analysis of a series of 10,005 cases tested with the 22C3 assay. Pathol Oncol Res. 2020;26:79–89. doi: 10.1007/s12253-018-0469-6. [DOI] [PubMed] [Google Scholar]
- 72.Velcheti V, Patwardhan PD, Liu FX, Chen X, Cao X, Burke T. Real-world PD-L1 testing and distribution of PD-L1 tumor expression by immunohistochemistry assay type among patients with metastatic non-small cell lung cancer in the United States. PLoS One. 2018;13:e0206370. doi: 10.1371/journal.pone.0206370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data