Abstract
OBJECTIVE
To examine changes in and the relationships between diabetes management and rural and urban residence.
RESEARCH DESIGN AND METHODS
Using National Health and Nutrition Examination Survey (1999–2018) data from 6,372 adults aged ≥18 years with self-reported diagnosed diabetes, we examined poor ABCS: A1C >9% (>75 mmol/mol), Blood pressure (BP) ≥140/90 mmHg, Cholesterol (non-HDL) ≥160 mg/dL (≥4.1 mmol/L), and current Smoking. We compared odds of urban versus rural residents (census tract population size ≥2,500 considered urban, otherwise rural) having poor ABCS across time (1999–2006, 2007–2012, and 2013–2018), overall and by sociodemographic and clinical characteristics.
RESULTS
During 1999–2018, the proportion of U.S. adults with diabetes residing in rural areas ranged between 15% and 19.5%. In 1999–2006, there were no statistically significant rural-urban differences in poor ABCS. However, from 1999–2006 to 2013–2018, there were greater improvements for urban adults with diabetes than for rural for BP ≥140/90 mmHg (relative odds ratio [OR] 0.8, 95% CI 0.6–0.9) and non-HDL ≥160 mg/dL (≥4.1 mmol/L) (relative OR 0.45, 0.4–0.5). These differences remained statistically significant after adjustment for race/ethnicity, education, poverty levels, and clinical characteristics. Yet, over the 1999–2018 time period, minority race/ethnicity, lower education attainment, poverty, and lack of health insurance coverage were factors associated with poorer A, B, C, or S in urban adults compared with their rural counterparts.
CONCLUSIONS
Over two decades, rural U.S. adults with diabetes have had less improvement in BP and cholesterol control. In addition, rural-urban differences exist across sociodemographic groups, suggesting that efforts to narrow this divide may need to address both socioeconomic and clinical aspects of care.
Rural-urban disparities have been described for a wide variety of illnesses and mortality rates (1,2). Since 1999, rural residents generally have had higher age-adjusted mortality rates (3,4), including for the five leading causes of death (heart disease, stroke, cancer, unintentional injury, and chronic lower respiratory disease) (5). Although national mortality rates have generally declined in the past few decades, the urban-rural disparity in life expectancy has widened, with greater improvements in urban areas than rural (4,6). Many rural communities experience greater prevalence of chronic health conditions and complications (6,7) and less access to comprehensive health care (8).
Diabetes complications, such as microvascular (nephropathy, neuropathy, and retinopathy) and macrovascular diseases (atherosclerosis of coronary, cerebrovascular, and peripheral vasculatures) increase morbidity, disability, and mortality (9,10). As such, poor glycemic control and cardiovascular health are culprits in increasing risk for complications, declining quality of life, and greater financial burden (11-14). This has led the American Diabetes Association and other organizations to emphasize clinical care guidelines and health care quality metrics for more than three decades. The optimal levels of some of the ABCS (A1C, blood pressure [BP], cholesterol, and smoking), key risk factors for the development of diabetes complications, have evolved over time. The most recent evolution of these guidelines is as follows: A1C <7.0% (<53 mmol/mol); BP <140/90 mmHg; the lower the non-HDL cholesterol levels, the better; and avoidance of cigarette and other tobacco product or e-cigarette use (15,16). Previous analyses have documented substantial improvements in the management of ABCS until 2010 (17,18) with stagnation thereafter (19). Disparities by age, race/ethnicity, and socioeconomic status have also persisted (17-19). Furthermore, after a 20-year decline, recent national increases in selected diabetes-related complications, particularly in young and middle-aged adults, emphasize the importance of improving poor ABCS measures and addressing disparities (20).
Rural areas have higher age-adjusted prevalence of diabetes than urban areas, and adults with diagnosed diabetes in rural areas report less adherence to some preventive measures, such as dilated eye or foot examinations, and more complications, such as diabetic retinopathy and foot sores, than their urban counterparts (21). However, there have been no comparisons of ABCS management among adults with diabetes in rural and urban areas. Since the definition of optimal ABCS control has changed over time and currently varies based on life expectancy or the presence of comorbid conditions, in this report, we assessed trends in poor ABCS among adults with diagnosed diabetes and disparities between rural and urban areas using updated national data from 1999 to 2018.
RESEARCH DESIGN AND METHODS
Data Source
The National Health and Nutrition Examination Survey (NHANES) was designed to investigate the health and nutritional status of the noninstitutionalized U.S. civilian population through a complex multistage sampling design (22,23). Since 1999, NHANES has been conducted continuously with administration of questionnaires to obtain sociodemographic and health information (e.g., diabetes and smoking status), collection of blood samples for laboratory tests (e.g., glycohemoglobin and lipid profiles), and physical exams including measures of BP and anthropometry (e.g., BMI). Data are released in 2-year cycles.
The National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention administers NHANES, and its Research Ethics Review Board approved the NHANES protocol. Data used in this study are publicly available (https://wwwn.cdc.gov/nchs/nhanes/default.aspx) with the exception of urban/rural residential status, true sampling stratum, and true probability sampling unit, which were analyzed at the restricted-access NCHS Research Data Center (https://www.cdc.gov/rdc/). NHANES publicly releases pseudo sampling strata and pseudoprobability sampling unit information to minimize disclosure risk.
NHANES urban/rural residential status, used in this study, is determined in partnership with the U.S. Department of Housing and Urban Development, which geocodes addresses of NHANES participants to the 2010 census (https://wwwn.cdc.gov/Nchs/Nhanes/limited_access/GEO_2000.htm#UR). Census tracts with at least 2,500 people were considered “urban,” including both urban clusters (2,500–<50,000 people) and urban areas (≥50,000 people). All areas not included within the urban definition were considered “rural.” For the proportion of county residence in an urban census tract based on 2010 census, please refer to Supplementary Fig. 1. Since rural/urban status was missing for almost 10% of participants in NHANES 1999–2018, we used multiple imputation to estimate rural/urban status for missing values including the following covariates: true sampling stratum and primary sampling unit, age, sex, and race/ethnicity.
Study Population
We identified the study population using NHANES data from ten 2-year cycles (from 1999–2000 to 2017–2018). Eligibility criteria included the following: having a physical exam at the Mobile Examination Center, age ≥18 years, and responding “yes” to the question, “Other than during pregnancy, have you ever been told by a health professional that you have diabetes or sugar diabetes?” Of the 6,393 adults with diagnosed diabetes, 21 pregnant women were excluded from the analysis for a total sample size of 6,372. For this study, we grouped survey cycles into three time periods: 1999–2006 (n = 1,897), 2007–2012 (n = 2,121), and 2013–2018 (n = 2,354). We also stratified by rural (n = 872) and urban (n = 5,500) residence.
Study Variables
Considering the evolving clinical guidelines from both the American Diabetes Association and the American Heart Association during 1999–2018, for the purpose of this study, we focused on poor ABCS measures. Poor ABCS measures were defined as values above cut points based on which patients with diabetes with or without comorbidities would be universally considered to have poor control, as diagnosis criteria, or threshold for treatment initiation during this time period. For this study, poor ABCS was A1C >9.0% (>75 mmol/mol), BP ≥140/90 mmHg (systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg), non-HDL cholesterol ≥160 mg/dL (≥4.1 mmol/L) (equivalent to LDL ≥130 mg/dL, ≥3.4 mmol/L), and being a current smoker. Non-HDL cholesterol was calculated by subtracting values for HDL cholesterol from total cholesterol. Participants were considered current smokers if they 1) self-reported smoking at least 100 cigarettes in their life and now smoking cigarettes every day or some days or 2) had serum cotinine levels >10 ng/mL.
Data Analysis
We described characteristics and demographics of adults with diagnosed diabetes across three time periods (1999–2006, 2007–2012, and 2013–2018) and by rural/urban residence as percentages and tested differences in distributions across time periods or rural/urban residence using a design-based Pearson χ2 test. The demographics considered were age-group (18–44, 45–64, and ≥65 years), sex, and race/ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American, and other race/multiracial). Other characteristics included socioeconomic variables, such as education attainment (less than high school diploma, high school diploma, some college, and college degree) and poverty-to-income ratio (PIR) (<100%, 100–299%, 300–499%, and ≥500%). Medical or clinical characteristics were also included (health insurance coverage [yes/no], time since diabetes diagnosis [0 to <5, 5 to <15, and ≥15 years], age at diabetes diagnosis [<30, 30 to <45, 45 to <60, and ≥60 years], and BMI [<25.0, 25.0–29.9, and ≥30 kg/m2]).
Differences between rural and urban residence in the distribution of ABCS categories were assessed—ranging from A1C <6.0% to ≥10.0% (<42 to ≥86 mmol/mol), BP <120/80 to ≥160/100 mmHg, non-HDL cholesterol <130 to ≥220 mg/dL (<3.4 to ≥5.7 mmol/L), and smoking status of never, former, and current smoker—with a design-based Pearson χ2 test. We used weighted logistic regression models accounting for survey design to examine the relationship between rural/urban residence and poor ABCS. For each of the ABCS, a model was used with the poor ABCS measure as the dependent variable. For odds ratio (OR) estimates between rural and urban residence at each time period and changes over time, the regression model contained a two-way interaction for rural/urban residence * time period, including lower-order variables and covariates. Independent variables included as covariates in the model were analyzed in a staged approach beginning with model 1, which was unadjusted (no covariates). Model 2 included demographic covariates (age [years], sex, race/ethnicity). The covariates included in model 3 were socioeconomic factors (education attainment and PIR [%]). Model 4 adjusted for clinical characteristics such as BMI (kg/m2), age at diabetes diagnosis (years), and years since diabetes diagnosis. Model 5 was the full model and included all covariates from models 2–4. Linear combinations (STATA lincom command) of estimated parameters were used to estimate adjusted ORs for each time period and relative ORs between time periods (1999–2006 vs. 2013–2018 and 2007–2012 vs. 2013–2018) to assess changes over time. For examining sociodemographic and clinical characteristics and disparities in poor ABCS between rural and urban residence, the regression model contained a two-way interaction for rural/urban residence * characteristics with lower-order variables. Linear combinations of estimated parameters were used to estimate ORs between rural and urban residence for each sociodemographic and clinical characteristics subgroup. Sociodemographic and clinical characteristics considered were age, sex, race/ethnicity, education attainment, PIR, health insurance coverage, time since diabetes diagnosis, age at diabetes diagnosis, and BMI.
Results with P values <0.05 were determined to be statistically significant. We performed all analyses using STATA 14.0 accounting for the NHANES complex sampling design. Examination sample weights were used for all analyses.
RESULTS
Characteristics of U.S. adults with diagnosed diabetes significantly changed across time periods (Table 1). The population of adults with diagnosed diabetes shifted toward older ages (mean age changed from 58.8 to 60.5 years, P = 0.01), more men (from 47.7% to 53.1%, P = 0.024), higher education attainment (some college, from 26.9% to 33.7%, and college degree or more, from 14.9% to 21.4%; P < 0.001), and higher PIR (mean, from 2.6 to 2.8; P = 0.039). There were also changes in having health insurance coverage (from 89.6% to 91.9%; P = 0.003), longer duration of diabetes since diagnosis (≥15 years, from 26.1% to 32.3%; P < 0.001), and greater BMI (mean, from 32.1 to 33.0 kg/m2; P = 0.014). However, the race/ethnicity and rural/urban distributions did not statistically significantly differ across the time periods.
Table 1—
Characteristics of U.S. adults with diagnosed diabetes: NHANES 1999–2018
| 1999–2006 (n = 1,897) |
2007–2012 (n = 2,121) |
2013–2018 (n = 2,354) |
Year groups P value* |
Rural (n = 872) |
Urban (n = 5,500) |
Rural vs urban P value† |
|
|---|---|---|---|---|---|---|---|
| Age (years) | 58.8 (0.5) | 60.0 (0.4) | 60.5 (0.4) | 0.010 | 61.3 (0.6) | 59.5 (0.3) | 0.010 |
| Women | 52.3 | 51.0 | 46.9 | 0.024 | 48.0 | 50.1 | 0.353 |
| Race/ethnic group | |||||||
| Non-Hispanic White | 62.6 | 60.5 | 60.5 | 0.332 | 83.1 | 56.2 | <0.001 |
| Non-Hispanic Black | 16.6 | 17.7 | 13.5 | 6.9 | 17.7 | ||
| Mexican American | 7.4 | 8.3 | 10.2 | 4.0 | 9.9 | ||
| Other | 13.4 | 13.5 | 15.8 | 6.1 | 16.2 | ||
| Education (among those aged ≥25 years) | |||||||
| <High school diploma | 32.1 | 29.5 | 19.9 | <0.001 | 24.2 | 27.0 | 0.007 |
| High school diploma | 26.0 | 24.5 | 25.1 | 31.0 | 23.9 | ||
| Some college | 26.9 | 27.9 | 33.7 | 30.6 | 29.8 | ||
| ≥College degree | 14.9 | 18.1 | 21.4 | 14.2 | 19.4 | ||
| PIR | 2.6 (0.1) | 2.6 (0.1) | 2.8 (0.1) | 0.039 | 2.8 (0.1) | 2.6 (0.1) | 0.051 |
| Health insurance coverage | 89.6 | 87.9 | 91.9 | 0.003 | 91.0 | 89.8 | 0.394 |
| Time since diabetes diagnosis (years) | |||||||
| 0 to <5 | 35.2 | 33.2 | 27.5 | <0.001 | 31.5 | 31.5 | 0.983 |
| 5 to <15 | 38.7 | 38.8 | 40.2 | 39.7 | 39.3 | ||
| ≥15 | 26.1 | 28.0 | 32.3 | 28.9 | 29.2 | ||
| BMI (kg/m2) | 32.1 (0.3) | 33.0 (0.3) | 33.0 (0.3) | 0.014 | 33.8 (0.4) | 32.5 (0.2) | 0.001 |
| Residential status | |||||||
| Rural | 15.0 | 19.9 | 19.5 | 0.414 | — | — | |
| Urban | 85.0 | 80.1 | 80.5 | — | — | ||
Data are % or means (SE).
Design-based Pearson χ2 test of characteristic by year groups.
Design-based Pearson χ2 test of characteristic by rural/urban area.
Over 1999–2018, adults with diagnosed diabetes that reside in urban areas were more likely to be younger (mean age 59.5 years for urban and 61.3 years for rural, P = 0.010), a lower proportion were non-Hispanic White (56.2% urban and 83.1% rural, P < 0.001), there was a higher proportion of education attainment at the extremes (<high school, 27.0% urban and 24.2% rural; college degree or higher, 19.4% urban, and 14.2% rural; P = 0.007), and individuals in urban areas on average had lower BMI than those in rural areas (mean 32.5 kg/m2 urban and 33.8 kg/m2 rural; P = 0.001). There was no significant difference in sex, PIR, health insurance coverage, or time since diabetes diagnosis between rural and urban residence of adults with diagnosed diabetes.
In 1999–2006 (1st time period), there were no significant associations between rural/urban residence and each poor ABCS measure (models 1–5) (Table 2). Adults with diagnosed diabetes residing in urban areas were less likely to have non-HDL cholesterol ≥160 mg/dL (≥4.1 mmol/L) compared with those in rural areas (unadjusted OR 0.7, 95% CI 0.4–0.9) in 2007–2012 (2nd time period) and in 2013–2018 (3rd time period) (unadjusted OR 0.5, 0.3–0.8). There were significant differences between the 1st and 3rd time periods in the OR for BP ≥140/90 mmHg (relative OR 0.8, 0.6–0.9) and non-HDL cholesterol ≥160 mg/dL (≥4.1 mmol/L) (relative OR 0.45, 0.4–0.5). These significant differences from the 1st and 3rd time periods signify that rural residents were more likely than urban residents to have poor BP and poor cholesterol by 2013–2018, which persisted even after adjustment for socioeconomic, demographic, and clinical measures. There were no statistically significant associations of rural/urban residence with current smoking status or A1C >9.0% (>75 mmol/mol) in adults with diagnosed diabetes at any time period or over time.
Table 2—
Trends in ORs of poor ABCS for urban versus rural residence in U.S. adults aged ≥18 years with diagnosed diabetes: NHANES, 1999–2018
| Urban vs. rural: reference | 1999–2006 | 2007–2012 | 2013–2018 | Relative OR 1999–2006 and 2013–2018 |
Relative OR 2007–2012 and 2013–2018 |
|---|---|---|---|---|---|
| Model 1, unadjusted | |||||
| A1C >9.0% (>75 mmol/mol) | 1.8 (0.8–4.0) | 1.6 (0.7–3.5) | 1.4 (0.7–3.2) | 0.8 (0.6–1.0) | 0.9 (0.7–1.2) |
| BP ≥140/90 mmHg | 1.0 (0.7–1.5) | 0.8 (0.5–1.1) | 0.8 (0.6–1.2) | 0.8 (0.6–0.9) | 1.1 (0.9–1.4) |
| Non-HDL cholesterol ≥160 mg/dL (≥4.1 mmol/L) | 1.1 (0.8–1.7) | 0.7 (0.4–0.9) | 0.5 (0.3–0.8) | 0.45 (0.4–0.5) | 0.8 (0.6–0.9) |
| Current smoker | 0.8 (0.5–1.4) | 0.8 (0.5–1.2) | 0.7 (0.4–1.2) | 0.8 (0.6–1.1) | 0.9 (0.7–1.1) |
| Model 2 covariates: age, sex, race/ethnicity | |||||
| A1C >9.0% (>75 mmol/mol) | 1.5 (0.7–3.2) | 1.3 (0.6–2.7) | 1.2 (0.6–2.6) | 0.8 (0.6–1.0) | 0.9 (0.7–1.2) |
| BP ≥140/90 mmHg | 1.0 (0.7–1.5) | 0.7 (0.5–0.9) | 0.8 (0.5–1.1) | 0.7 (0.6–0.9) | 1.1 (0.9–1.4) |
| Non-HDL cholesterol ≥160 mg/dL (≥4.1 mmol/L) | 1.0 (0.7–1.6) | 0.6 (0.4–0.9) | 0.5 (0.3–0.7) | 0.5 (0.4–0.6) | 0.8 (0.6–0.9) |
| Current smoker | 0.8 (0.5–1.4) | 0.8 (0.5–1.3) | 0.7 (0.4–1.3) | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) |
| Model 3 covariates: educationand PIR | |||||
| A1C >9.0% (>75 mmol/mol) | 1.9 (0.9–3.9) | 1.7 (0.8–3.4) | 1.6 (0.8–3.3) | 0.8 (0.6–1.1) | 1.0 (0.7–1.2) |
| BP ≥140/90 mmHg | 1.0 (0.7–1.5) | 0.8 (0.5–1.1) | 0.9 (0.6–1.3) | 0.8 (0.6–1.0) | 1.1 (0.9–1.4) |
| Non-HDL cholesterol ≥160 mg/dL (≥4.1 mmol/L) | 1.2 (0.8–1.8) | 0.7 (0.5–1.1) | 0.6 (0.4–0.9) | 0.5 (0.4–0.6) | 0.8 (0.7–1.0) |
| Current smoker | 0.9 (0.5–1.6) | 0.9 (0.5–1.6) | 0.8 (0.5–1.5) | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) |
| Model 4 covariates: BMI, age at diabetes diagnosis, and years since diabetes diagnosis | |||||
| A1C >9.0% (>75 mmol/mol) | 2.0 (0.9–4.3) | 1.8 (0.8–3.8) | 1.6 (0.7–3.5) | 0.8 (0.6–1.1) | 0.9 (0.7–1.2) |
| BP ≥140/90 mmHg | 1.1 (0.8–1.5) | 0.8 (0.5–1.1) | 0.8 (0.6–1.1) | 0.7 (0.6–0.9) | 1.0 (0.8–1.3) |
| Non-HDL cholesterol ≥160 mg/dL (≥4.1 mmol/L) | 1.1 (0.7–1.8) | 0.7 (0.4–1.0) | 0.5 (0.3–0.8) | 0.5 (0.4–0.6) | 0.8 (0.6–0.99) |
| Current smoker | 0.8 (0.5–1.4) | 0.8 (0.5–1.3) | 0.7 (0.4–1.2) | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) |
| Model 5 covariates: all from models 2–4 | |||||
| A1C >9.0% (>75 mmol/mol) | 1.9 (0.8–4.2) | 1.7 (0.8–3.7) | 1.6 (0.8–3.6) | 0.9 (0.7–1.2) | 1.0 (0.7–1.3) |
| BP ≥140/90 mmHg | 1.1 (0.8–1.6) | 0.8 (0.5–1.1) | 0.8 (0.6–1.2) | 0.8 (0.6–0.97) | 1.1 (0.8–1.4) |
| Non-HDL cholesterol ≥160 mg/dL (≥4.1 mmol/L) | 1.1 (0.7–1.7) | 0.7 (0.4–1.0) | 0.6 (0.4–0.9) | 0.5 (0.4–0.6) | 0.9 (0.7–1.1) |
| Current smoker | 0.9 (0.5–1.6) | 0.9 (0.5–1.5) | 0.8 (0.5–1.5) | 1.0 (0.7–1.3) | 0.9 (0.7–1.2) |
Data are OR (95% CI).
Over the entire time period from 1999 to 2018, the distribution of each ABCS measure did not statistically significantly differ between adults with diagnosed diabetes residing in urban and rural areas (Fig. 1). However, the associations between rural/urban residence and poor ABCS varied by socioeconomic and clinical characteristics (Table 3). Adults with diagnosed diabetes in urban areas were more likely to have A1C >9.0% (>75 mmol/mol) than those in rural areas if they were non-Hispanic Black (OR 2.2, 95% CI 1.6–3.2), Mexican American (2.8, 1.9–4.1), other race or multiracial (1.9, 1.3–2.9). Similarly, A1C >9.0% (>75 mmol/mol) was more likely in adults with diabetes residing in urban than rural areas if they had less than a high school diploma (2.2, 1.2–4.0), PIR <100% (1.9, 1.1–3.4) or no health insurance coverage (3.7, 2.6–5.5) or if there was 5 to <15 years since diabetes diagnosis (2.1, 1.1–3.8). Urban adults with diagnosed diabetes were more likely than their rural counterparts to have BP ≥140/90 mmHg if they were ≥65 years old (4.4, 2.1–9.2), if they were non-Hispanic Black (1.5, 1.1–1.9), if there was 5 to <15 years (1.5, 1.03–2.2) or ≥15 years (1.9, 1.3–2.9) since diabetes diagnosis, or if they were age ≥60 years at diabetes diagnosis (2.5, 1.2–5.2). Compared with adults with diagnosed diabetes residing in rural areas, those in urban areas were less likely to have non-HDL cholesterol ≥160 mg/dL (≥4.1 mmol/L) if they were ≥65 years old (0.4, 0.3–0.8), if they had had a college degree or higher (0.6, 0.4–0.9), or if there was ≥15 years since diabetes diagnosis (0.6, 0.4–0.95). However, adults with diagnosed diabetes residing in urban areas, as compared with those in rural areas, were more likely to have non-HDL cholesterol ≥160 mg/dL (≥4.1 mmol/L) if they were Mexican American (1.5, 1.1–2.1) or other race or multiracial (1.4, 1.0–2.1), if they had no health insurance coverage (2.2, 1.6–3.1), if they were age 30 to <45 years at diabetes diagnosis (2.6, 1.3–5.4), or if they had BMI 25–29.9 kg/m2 (2.1, 1.1–3.7) or ≥30 kg/m2 (2.3, 1.3–4.1). Also, adults with diagnosed diabetes and no health insurance coverage in urban areas were more likely to be current smokers than those in rural areas (2.0, 1.4–2.8). However, adults with diabetes in urban areas were less likely to be current smokers than adults in rural areas if they were 45–64 years old (0.5, 0.3–0.9), ≥65 years old (0.2, 0.1–0.3), or female (0.6, 0.5–0.9); had college degree or higher (0.4, 0.3–0.6), PIR 100%–299% (0.6, 0.4–0.9), PIR 300%–499% (0.6, 0.3–0.9), or PIR ≥500% (0.5, 0.3–0.8); or were age ≥60 years at diabetes diagnosis (0.4, 0.2–0.8).
Figure 1—
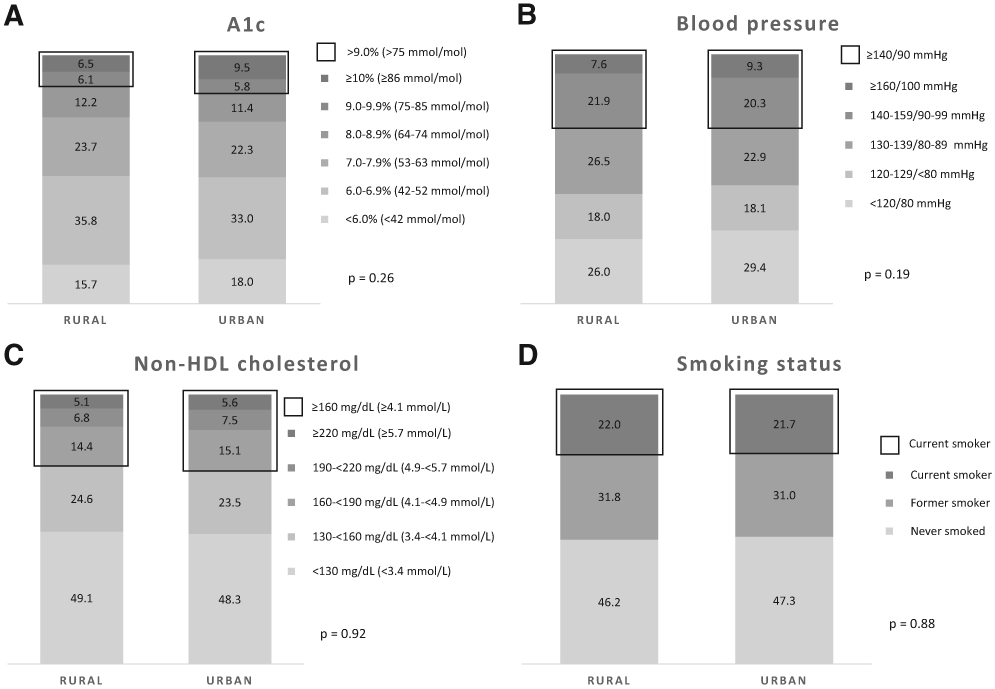
Distribution of ABCS risk factors for rural and urban residence in U.S. adults with diagnosed diabetes, 1999–2018.
Table 3—
Disparities in ORs of poor ABCS for urban versus rural residence: NHANES, 1999–2018
| Urban vs. rural (reference) | A1C >9.0% (>75 mmol/mol) |
BP ≥140/90 mmHg | Non-HDL cholesterol ≥160 mg/dL (≥4.1 mmol/L) |
Current smoker |
|---|---|---|---|---|
| Age (years) | ||||
| 18–44 | 2.3 (0.9–5.9) | 1.3 (0.6–2.8) | 1.0 (0.6–1.8) | 0.7 (0.4–1.2) |
| 45–64 | 1.3 (0.5–3.2) | 2.1 (0.9–4.4) | 0.9 (0.5–1.5) | 0.5 (0.3–0.9) |
| ≥65 | 0.5 (0.2–1.2) | 4.4 (2.1–9.2) | 0.4 (0.3–0.8) | 0.2 (0.1–0.3) |
| Sex | ||||
| Male | 1.2 (0.8–1.8) | 0.9 (0.7–1.2) | 1.0 (0.7–1.5) | 1.0 (0.7–1.4) |
| Female | 1.0 (0.7–1.5) | 1.1 (0.8–1.4) | 1.4 (0.97–2.0) | 0.6 (0.5–0.9) |
| Race/ethnicity | ||||
| Non-Hispanic White | 1.0 (0.7–1.5) | 1.0 (0.7–1.3) | 1.1 (0.8–1.5) | 1.0 (0.8–1.4) |
| Non-Hispanic Black | 2.2 (1.6–3.2) | 1.5 (1.1–1.9) | 1.1 (0.8–1.5) | 1.3 (0.98–1.7) |
| Mexican American | 2.8 (1.9–4.1) | 0.9 (0.7–1.2) | 1.5 (1.1–2.1) | 0.8 (0.6–1.1) |
| Other race or multiracial | 1.9 (1.3–2.9) | 0.9 (0.6–1.2) | 1.4 (1.0–2.1) | 0.8 (0.6–1.2) |
| Education (among those aged ≥25 years) | ||||
| <High school diploma | 2.2 (1.2–4.0) | 1.2 (0.9–1.5) | 1.3 (0.9–1.8) | 0.8 (0.6–1.1) |
| High school diploma | 1.8 (0.9–3.5) | 1.0 (0.7–1.3) | 1.0 (0.7–1.5) | 0.8 (0.6–1.2) |
| Some college | 1.8 (0.9–3.5) | 0.8 (0.6–1.1) | 1.1 (0.8–1.5) | 0.8 (0.5–1.0) |
| ≥College degree | 1.1 (0.6–2.2) | 0.7 (0.5–1.0) | 0.6 (0.4–0.9) | 0.4 (0.3–0.6) |
| PIR | ||||
| <100% | 1.9 (1.1–3.4) | 1.1 (0.8–1.5) | 1.2 (0.8–1.9) | 1.1 (0.7–1.7) |
| 100–299% | 1.1 (0.6–1.9) | 1.1 (0.8–1.5) | 0.9 (0.6–1.3) | 0.6 (0.4–0.9) |
| 300–499% | 0.8 (0.4–1.5) | 0.8 (0.5–1.2) | 0.6 (0.4–1.0) | 0.6 (0.3–0.9) |
| ≥500% | 1.1 (0.6–1.9) | 0.8 (0.6–1.2) | 0.7 (0.4–1.1) | 0.5 (0.3–0.8) |
| Health insurance | ||||
| Yes | 1.0 (0.7–1.5) | 1.0 (0.8–1.2) | 1.1 (0.9–1.4) | 1.0 (0.8–1.4) |
| No | 3.7 (2.6–5.5) | 1.0 (0.8–1.3) | 2.2 (1.6–3.1) | 2.0 (1.4–2.8) |
| Time since diabetes diagnosis (years) | ||||
| 0 to <5 | 1.2 (0.6–2.3) | 1.2 (0.8–1.8) | 1.1 (0.7–1.6) | 1.0 (0.6–1.5) |
| 5 to <15 | 2.1 (1.1–3.8) | 1.5 (1.03–2.2) | 0.9 (0.6–1.4) | 0.8 (0.5–1.2) |
| ≥15 | 1.5 (0.8–2.7) | 1.9 (1.3–2.9) | 0.6 (0.4–0.95) | 0.6 (0.4–1.0) |
| Age at diabetes diagnosis (years) | ||||
| <30 | 1.5 (0.5–4.4) | 1.0 (0.5–2.0) | 2.0 (0.9–4.5) | 1.1 (0.5–2.3) |
| 30 to <45 | 1.8 (0.6–5.2) | 1.4 (0.7–2.8) | 2.6 (1.3–5.4) | 1.1 (0.5–2.2) |
| 45 to <60 | 0.8 (0.3–2.3) | 1.6 (0.8–3.1) | 1.8 (0.8–3.8) | 0.8 (0.4–1.6) |
| ≥60 | 0.4 (0.1–1.1) | 2.5 (1.2–5.2) | 1.4 (0.7–3.1) | 0.4 (0.2–0.8) |
| BMI (kg/m2) | ||||
| <25.0 | 0.9 (0.3–2.3) | 0.8 (0.4–1.5) | 1.8 (0.99–3.3) | 0.8 (0.5–1.5) |
| 25.0–29.9 | 0.9 (0.3–2.4) | 0.8 (0.4–1.4) | 2.1 (1.1–3.7) | 0.6 (0.3–1.1) |
| ≥30.0 | 0.9 (0.4–2.4) | 0.7 (0.4–1.2) | 2.3 (1.3–4.1) | 0.7 (0.3–1.3) |
Data are unadjusted OR (95% CI); each model was unadjusted for covariates.
CONCLUSIONS
To our knowledge, this is the first study to investigate trends and disparities in measures of the ABCS in adults with diagnosed diabetes by rural versus urban residence in the U.S. Over 1999–2018, there was no significant difference in ABCS distributions between rural and urban adults with diagnosed diabetes. Yet, over the period, U.S. adults with diagnosed diabetes who resided in urban areas had greater improvements in non-HDL cholesterol and BP than their rural counterparts; there were no significant rural-urban differences in improvement of poor A1C levels or current smoking status. However, there were significant socioeconomic and clinical disparities in the association between poor ABCS management and rural/urban residence. Adults with diagnosed diabetes residing in urban areas were more likely than rural residents to have A1C levels >9.0% (>75 mmol/mol) if they were of race/ethnicity other than non-Hispanic White, had lowest education attainment or PIR or had no health insurance coverage or if there had been 5 to <15 years since diabetes diagnosis. These rural-urban disparities varied in terms of how different sociodemographic and clinical characteristics were associated with poor BP, and poor non-HDL cholesterol, and current smoking.
Although adults residing in rural areas have greater prevalence of chronic conditions and associated risk factors than those in urban areas (6,24), we found that overall, among adults with diagnosed diabetes, there were no significant differences between rural and urban areas in the distribution of these ABCS measures. In considering changes across time, there were no statistically significant differences in A1C levels >9.0% (>75 mmol/mol) between rural and urban areas among adults with diagnosed diabetes at each time period during 1999–2018, suggesting similar poor glycemic control in adults with diagnosed diabetes regardless of residence. Additionally, current smoking status in adults with diagnosed diabetes did not significantly change over 1999–2018 and did not differ between those residing in urban areas and those in rural areas. Our overall data from adults with diagnosed diabetes contrast with data showing greater prevalence of smoking in the general population in rural versus urban areas (25,26) and greater declines in smoking in urban than in rural areas (24). However, the sociodemographic and clinical subgroup results in adults with diagnosed diabetes elicited a greater likelihood of smoking in rural versus urban among a few subgroups.
Adults with diagnosed diabetes in urban areas experienced greater improvements in BP and non-HDL cholesterol from 1999–2006 to 2013–2018 than their rural counterparts. During the past decades, BP and lipid medication use has increased among adults with diabetes (27-29). Reasons for the difference in improvement of BP and non-HDL cholesterol may be in medication prescribing or adherence among adults with diagnosed diabetes in rural and urban areas that need to be further investigated.
Our data also highlighted critical sociodemographic disparities, emphasizing the multidimensional nature of diabetes management. Of note, minority race/ethnicity and socioeconomically disadvantaged urban adults with diagnosed diabetes were more likely to have poor ABCS measures than rural counterparts. This is in keeping with recent reports (30) and with the association between geographic structural racial and socioeconomic inequities with health and health outcomes (31-33). With the results varying across subgroups in which residential status was more likely to be associated with poor ABCS, epidemiologic research needs to go beyond adjusting regression analysis for the covariates of socioeconomic, demographic, and clinical characteristics, which cannot highlight these disparities. For achievement of health equity in diabetes management, examination of the specificities in the social determinants of health pathways and the impact they have on different populations is needed (30). Together, our findings suggest that clinical and public health efforts to improve diabetes management will both require tailored strategies that consider the multidimensional contexts of adults with diagnosed diabetes, including socioeconomic barriers to care and to health opportunities.
Since this study was limited in categorization of urban and very rural categories, there were limited numbers of rural adult participants with diagnosed diabetes in each time period. As a result, the rural/urban analyses may have lacked the power to show true associations. Similarly, disparities between sociodemographic and clinical subgroups in poor ABCS between rural and urban residence may not have been detected due to even smaller sample sizes; that said, we combined NHANES cycles to provide stable estimates. Although the impact of multiple comparisons cannot be ignored, this exploratory study had significant findings that seem to be consistent with expectations based on the published literature.
There were a few additional limitations in this study. First, these findings were representative of adults who self-reported being diagnosed with diabetes and not the entire adult population with diabetes. Awareness of diabetes status may vary by rural and urban residence, and adults aware of their diabetes diagnosis may have characteristics and ABCS measures that are different from those of adults unaware of their condition.
Second, this study was of cross-sectional design, and we were able to investigate population-level associations in poor ABCS measures but were unable to examine or quantify individual temporal changes of ABCS measures in a cohort of adults with diagnosed diabetes.
Third, since we encountered differential missingness for rural/urban residence status for each survey cycle (ranging from 3% to 12%), we imputed missing values based on the sampling design true primary sampling unit and strata to minimize any bias that may have been introduced. Nonetheless, it is possible that our limited rural sample size within subgroup analyses may produce compromised U.S. representative estimates. Additionally, we were also limited by the dichotomous definition of rural/urban available from NHANES data and unable to further investigate the different levels of metropolitan areas that can provide a clearer picture of the associations with diabetes care management.
Lastly, NHANES Mobile Examination Center response rates have steadily declined from 80% to the most recent response rate at 49%. While data are not available for response rates by rural/urban residence, it is possible that this decline in response rate may have potentially affected rural residents more so than urban residents. Although weights accounting for nonresponse were used, the ability to calculate estimates representative of the U.S. noninstitutionalized population may become compromised with declining response rates.
Although a few rural-urban differences were observed overall and over time, we noted important differences in poor ABCS in socioeconomically affected groups and other clinical subgroups between adults residing in urban versus rural areas. There is room for improvement when it comes to complete comprehensive care. Clinical and public health professionals need to incorporate evidence-based approaches (30,34) in addressing sociodemographic barriers to achieve better ABCS measures across the nation but especially in disproportionately affected groups living in urban and rural areas.
Supplementary Material
Acknowledgments
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
References
- 1.Cosby AG, McDoom-Echebiri MM, James W, Khandekar H, Brown W, Hanna HL. Growth and persistence of place-based mortality in the United States: the rural mortality penalty. Am J Public Health 2019;109:155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ely DM, Hoyert DL. Differences between rural and urban areas in mortality rates for the leading causes of infant death: United States, 2013-2015. NCHS Data Brief 2018:1–8 [PubMed] [Google Scholar]
- 3.Singh GK, Azuine RE, Siahpush M, Williams SD. Widening geographical disparities in cardiovascular disease mortality in the United States, 1969-2011. Int J MCH AIDS 2015;3:134–149 [PMC free article] [PubMed] [Google Scholar]
- 4.Singh GK, Siahpush M. Widening rural-urban disparities in life expectancy, U.S., 1969-2009. Am J Prev Med 2014;46:e19–e29 [DOI] [PubMed] [Google Scholar]
- 5.Moy E, Garcia MC, Bastian B, et al. Leading causes of death in nonmetropolitan and metropolitan areas-United States, 1999-2014. MMWR Surveill Summ 2017;66:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh GK, Daus GP, Allender M, et al. Social determinants of health in the United States: addressing major health inequality trends for the Nation, 1935-2016. International journal of MCH and AIDS. 2017;6:139–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh GK, Siahpush M, Azuine RE, Williams SD. Widening socioeconomic and racial disparities in cardiovascular disease mortality in the United States, 1969-2013. Int J MCH AIDS 2015;3:106–118 [PMC free article] [PubMed] [Google Scholar]
- 8.Lutfiyya MN, McCullough JE, Mitchell L, Dean LS, Lipsky MS. Adequacy of diabetes care for older U.S. rural adults: a cross-sectional population based study using 2009 BRFSS data. BMC Public Health 2011;11:940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naslafkih A, Sestier F. Diabetes mellitus related morbidity, risk of hospitalization and disability. J Insur Med 2003;35:102–113 [PubMed] [Google Scholar]
- 10.Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocrine reviews 2016;37:278–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 12.Kearney PM, Blackwell L, Collins R, et al. ; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–125 [DOI] [PubMed] [Google Scholar]
- 13.Turnbull F, Neal B, Algert C, et al. ; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med 2005;165:1410–1419 [DOI] [PubMed] [Google Scholar]
- 14.Hirsch JD, Morello CM. Economic impact of and treatment options for type 2 diabetes. Am J Manag Care 2017;23(Suppl.):S231–S240 [PubMed] [Google Scholar]
- 15.American Diabetes Association. 6. Glycemic targets: Standards of Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S73–S84 [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S125–S150 [DOI] [PubMed] [Google Scholar]
- 17.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med 2013;368:1613–1624 [DOI] [PubMed] [Google Scholar]
- 18.Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988-2014. Lancet Diabetes Endocrinol 2018;6:392–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ. Evaluation of the cascade of diabetes care in the United States, 2005-2016 JAMA Intern Med 2019;179:1376–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol 2016;4:537–547 [DOI] [PubMed] [Google Scholar]
- 21.Hale NL, Bennett KJ, Probst JC. Diabetes care and outcomes: disparities across rural America. J Community Health 2010;35:365–374 [DOI] [PubMed] [Google Scholar]
- 22.Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National Health and Nutrition Examination Survey: sample design, 2011-2014. Vital Health Stat 2 2014:1–33 [PubMed] [Google Scholar]
- 23.Zipf G, Chiappa M, Porter KS, et al. National Health and Nutrition Examination Survey: Plan and operations, 1999-2010. National Center for Health Statistics. Vital Health Stat 1 2013:1–37 [PubMed] [Google Scholar]
- 24.Doogan NJ, Roberts ME, Wewers ME, et al. A growing geographic disparity: rural and urban cigarette smoking trends in the United States. Prev Med 2017;104:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts ME, Doogan NJ, Kurti AN, et al. Rural tobacco use across the United States: how rural and urban areas differ, broken down by census regions and divisions. Health Place 2016;39:153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts ME, Doogan NJ, Stanton CA, et al. Rural versus urban use of traditional and emerging tobacco products in the United States, 2013-2014. Am J Public Health 2017;107: 1554–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong HK, Ong KL, Cheung CL, Cheung BM. Utilization of glucose, blood pressure, and lipid lowering medications among people with type II diabetes in the United States, 1999-2010. Ann Epidemiol 2014;24:516–21.e1 [DOI] [PubMed] [Google Scholar]
- 28.Kuznik A, Mardekian J. Trends in utilization of lipid- and blood pressure-lowering agents and goal attainment among the U.S. diabetic population, 1999-2008. Cardiovasc Diabetol 2011;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suh DC, Kim CM, Choi IS, Plauschinat CA, Barone JA. Trends in blood pressure control and treatment among type 2 diabetes with comorbid hypertension in the United States: 1988-2004. J Hypertens 2009;27:1908–1916 [DOI] [PubMed] [Google Scholar]
- 30.Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2020;44:258–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukachko A, Hatzenbuehler ML, Keyes KM. Structural racism and myocardial infarction in the United States. Soc Sci Med 2014;103:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell CN, Owens-Young JL. Self-rated health and structural racism indicated by county-level racial inequalities in socioeconomic status: the role of urban-rural classification. J Urban Health 2020;97:52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell CN, Kerr J, Young JL. Associations between obesity, obesogenic environments, and structural racism vary by county-level racial composition. Int J Environ Res Public Health 2019;16:861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powers MA, Bardsley JK, Cypress M, et al. Diabetes self-management education and support in adults with type 2 diabetes: a Consensus Report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Diabetes Care 2020;43:1636–1649 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


