Abstract
Williams Syndrome (WS) is a rare genetic multisystem disorder that occurs because of a deletion of approximately 25 genes in the 7q11.23 chromosome region. This causes dysmorphic facial appearances, multiple congenital cardiovascular defects, delayed motor skills, and abnormalities in connective tissues and the endocrine system. The patients are mostly diagnosed with mild to moderate mental retardation, however, they have a hyper sociable, socially dis-inhibited, and outgoing personality, empathetic behavior, and are highly talkative. Oxytocin (OT), a neuropeptide synthesized at the hypothalamus, plays an important role in cognition and behavior, and is thought to be affecting WS patients’ attitudes at its different amounts. Oxytocin receptor gene (OXTR), on chromosome 3p25.3, is considered regulating oxytocin receptors, via which OT exerts its effect. WS is a crucial disorder to understand gene, hormone, brain, and behavior associations in terms of sociality and neuropsychiatric conditions. Alterations to the WS gene region offer an opportunity to deepen our understandings of autism spectrum disorder, schizophrenia, anxiety, or depression. We aim to systematically present the data available of OT/OXTR regulation and expression, and the evidence for whether these mechanisms are dysregulated in WS. These results are important, as they predict strong epigenetic control over social behavior by methylation, single nucleotide polymorphisms, and other alterations. The comparison and collaboration of these studies may help to establish a better treatment or management approach for patients with WS if backed up with future research.
Keywords: Williams syndrome, Williams-Beuren syndrome, oxytocin, oxytocin receptor, oxytocin receptor gene, sociality, sociobehavior, neuromodulation, epigenetic regulation
Introduction
Williams syndrome (WS) or Williams-Beuren syndrome (WBS) is a rare genetic multisystem disorder with an estimated prevalence of 1 in 7500 to 20000 live births [1,2]. A deletion of approximately 25 genes causes this neuro-developmental condition in one copy of chromosome 7q11.23 [3]; which potentially causes dysmorphic facial appearances, multiple congenital cardiovascular defects, delayed motor skills, and abnormalities in connective tissues and endocrine system [1,4]. People with WS usually have a borderline intelligence quotient (IQ) between 70-79 or low intelligence IQ < 70 scores, who mostly are diagnosed with mild to moderate mental retardation and intellectual disability [5]. They also are severely weak at visuospatial construction [6].
However, WS patients present a hyper sociable, socially dis-inhibited, and outgoing personality, a befriending highly empathetic behavior, and are highly talkative [7,8]. They are abnormally expressive, insensitive to negative emotional signals, lack shyness, and are socially fearless [7,9]. The patients’ interest in unfamiliar people is clear from infancy as they do not express stranger anxiety [10]. They are driven to strangers and prefer, and better process, social over non-social stimuli [11,12]. They are relatively strong in language, grammar, nonverbal reasoning, and verbal short-term memory compared to patients with similar IQ levels [8,13]. However, patients often exhibit inappropriate social behavior, are deficient in social skills, and have a hard time sustaining relationships [14]. Their personality exposes them to risks, such as abuse and social isolation [15].
WS usually has a sporadic occurrence, however, in some cases autosomal inheritance was reported, mostly without the confirmation of a cytogenetic test [16]. Family haplotype analysis showed that 2/3 of cases arose from a mis-intrachromosomal shuffling between homologous chromosomes 7, and 1/3 from a disarrangement between sister chromatids [17]. Copy number variants (CNV), which are deletion or duplication of genes, account for approximately 5% of human genome and are closely related to disease and disease susceptibility [18-20].
WS is caused by a 1.55 mb microdeletion in 7q11.23 which is due to a misalignment of the gametes during meiosis, resulting in non-allelic homologous recombination (NAHR), flanked by three low copy repeats (LCRs) A, B, and C [21,22]. Close distance and high similarity of the LCR blocks facilitate NAHR at the region [23]. The most common deletion in WBS locus is thought to be between a point at the centromeric and medial LCR B, one A-B-C complex being 320 mb and 1.2 mb being the single copy gene region in-between [24].
The paradoxical WS social phenotype, unlike typical extraversion, may imply dysregulation of multiple pathways in the brain. These socio-behavioral outcomes are guided by the neural system, and the deleted genes are thought to be regulating this pathway. To this date, studies identified genes CYLN2, FZD9, LIMK1, STX1A, GTF2I, GTF2IRD1, and collectively TFII-I being responsible for cognitive and behavioral attitudes of WS patients [25,26]. A study with mice of GTF2I deletion reported hyper-social behaviors compared to duplicated GTF2I mice or wild-type mice [27]. This solidifies that social motivation has some roots in GTF2I.
NAHR can also generate microduplications in the same area of 7q11.23 [28]. This CNV identifies a neurodevelopmental disorder and is common amongst children who are diagnosed with autism spectrum disorder (ASD) and thus, is a risk factor for disease progression.
Even though ASD symptoms vary from patient to patient, they have two features in common: restricted social communication and repetitive sensory-motor behaviors [29]. They present with intellectual disability, pay reduced attention to faces, have a hard time understanding people’s actions, have limited expressive vocabulary, and are hyposociable [30-33]. Patients have a heightened sense of threat in processing emotions. It can therefore be concluded that ASD is the social opposite of WS, however, they have commonalities if closer attention is paid. They both exhibit irregular social skills and have difficulties in maintaining relationships [34].
The physiological, neurobiological, and genetic factors which control normal human behavior and how they work are not exactly clear, but some mechanisms shed light on the issue. Animal studies showed metabolism and locomotion are parts of the physiological aspect of socio-behavioral function as they predict the capability of cohesion to a group by providing energy and capacity for movement. Translating environmental information into motor responses and therefore following particular rules of interaction in a group depends on individual’s neurophysiological characteristics [35,36]. Genetics and epigenetics are crucial aspects of human behavior. Genes encode molecular products, neuropeptides, through which behavior is expressed in the brain. Brain plasticity and social behavior are both inherited and environmentally impacted. There is a strong assumption that social information can in turn affect gene regulation, by changing its epigenetic functioning.
Oxytocin (OT), a neuropeptide synthesized at the hypothalamus, form the hypothalamo-neuro-hypophysial system alongside with arginine vasopressin and play an important role in cognition and behavior. Studies implicated that the neuro-behavioral axis influenced human emotions in a neuro-endocrine way, by using neuropeptides such as OT as a behavioral and emotional modulator [37].
To support this, a trial showed OT administration increased sociality, outgoing and friendly behavior, and lessened social anxiety in subjects [38]. Another 4-week trial found that intranasal OT administration to patients with ASD between the ages 6-12, 24 international units total, twice a day enhanced their social skills [39].
Rodent mice studies of fragile X syndrome, Prader-Willi syndrome, cortical dysplasia, and focal epilepsy syndrome reported lower amounts of OT producing cells in hypothalamus and therefore social impairments. Their symptoms have ameliorated with OT treatment [40-43]. However, it is still unknown if endogenous OT would produce a similar or as powerful effect as exogenously administered OT. WS models may lead the way to identify this situation by tracking the patients’ bodily OT levels, and if elevated, whether endogenously upregulated OT levels provoke social behavior similar to exogenous administration and contribute to social functioning.
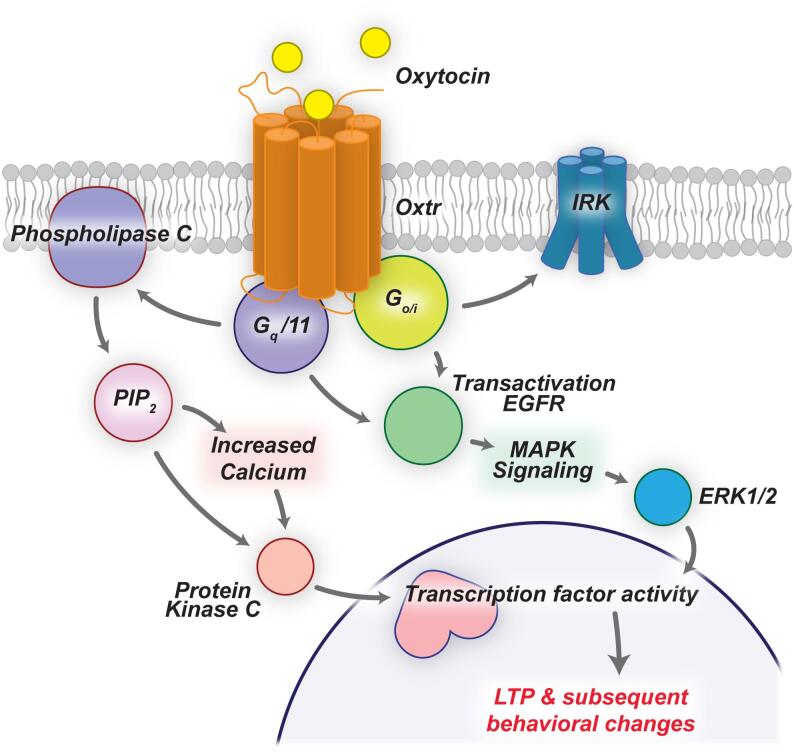
The oxytocin receptor is a 7-transmembrane G-protein coupled receptor which can bind to Gq or Go/Gi subunits, which activate cascades MAPK, PKC, PLC, EGFR, or CaMK or inward rectifying potassium channels which assemble transcription factors such as CREB or MEF-2. These factors regulate neurite growth, viability and survival. Oxytocin enhances excitatory connections and promotes a disinhibitory effect in hippocampus which consolidates long-term-potentiation (LTP) and lead to behavioral changes [44,45].
Oxytocin receptor gene (OXTR), on chromosome 3p25.3, is considered to regulate oxytocin receptors, via which OT exerts its prosocial effects (Figure 1).
Figure 1.
Oxytocin signaling cascades [44]. From the article “Oxytocin and Sensory Network Plasticity” by Pekarek et al. in Front Neurosci, 2020
Single nucleotide polymorphisms (SNPs) in OXTR have been linked to socio-behavioral outcomes of ASD, schizophrenia, or depression, indicating the role of an epigenetic modification [46,47]. These preliminary studies have led the way to inquire if OT or OXTR is related to social outcomes in WS.
Comparing the deletion and duplication syndromes as well as mood disorders, in means of OT regulation and OXTR expression, may help us find common altered epigenetic mechanisms, methylations, SNPs, or microRNAs underlying such disorders. The findings of multi-disease trials may offer an opportunity to treat the psychosocial adversities in a gene-based, epigenetic manner.
We aim to systematically present the data available of OT/OXTR regulation and expression, and the evidence for whether these mechanisms are dysregulated in WS with different cohorts of human and animal subjects. Four different subject trial studies had been run, to the best of our knowledge, to further investigate the levels in blood or brain OT and its implications, or to correlate social behaviors with OT and/or OXTR up/downregulation. However, the outcomes of these studies have been contra-indicatory. The comparison and collaboration of these studies may help to establish a better treatment or management approach for patients with WS if backed up with future research.
Materials and Methods
We used Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines while running this study [48] (Figure 2).
Figure 2.
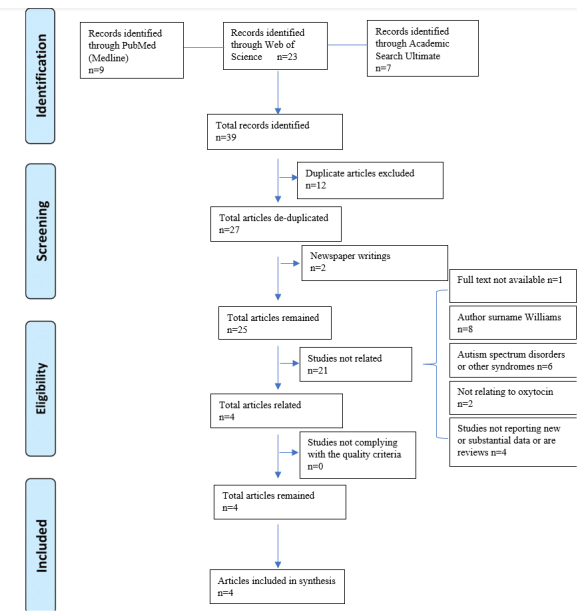
PRISMA study flow diagram
Data Sources and Searching
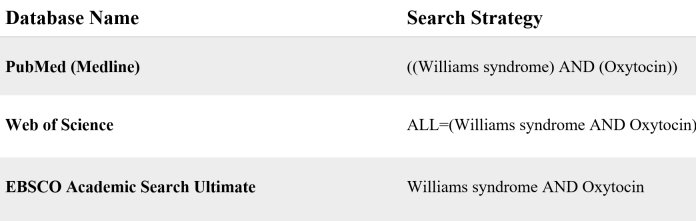
Systematic research was conducted. MEDLINE (PubMed), Web of Science Core Collection, and EBSCO Academic Search Ultimate were searched from inception, until 12 May 2021 for articles focusing on oxytocin regulating mechanisms and related hyper-sociability response in subjects with William’s syndrome. All databases were automatically searched using the advanced search tool with terms “Williams syndrome” and “oxytocin.” The search strategies were determined: (Williams syndrome) AND (oxytocin). Figure 3 formulates all search strategies used for this study. No date, document type, category, or language filter was administered.
Figure 3.
Search strategies
Screening
After a thorough search, results were exported to Endnote X9 citation software in RIS format. The abstracts and bibliometrics of the studies were screened by authors. The following inclusion criteria were determined:
1. Subjects of study being affected by William’s syndrome.
2. Study published in a peer-reviewed journal or available as a preprint or is gray literature.
3. Study reporting substantive data.
4. Study published in English.
5. Full-length article available for the study.
6. Study complying with a certain quality standard.
The data were tabulated using Microsoft Excel and were reviewed by all three authors to check for accuracy. Editorials, commentaries, and manuscripts not reporting new data were eliminated. Duplicated studies were considered only once. Figure 3 shows a flowchart depicting the article selection process, from identification to inclusion. We decided four manuscripts were eligible for the synthesis.
We addressed two literature reviews mentioning WS, with a very light touch, on the potential effect of OT or OXTR regulation in Williams syndrome [1,2]. Those studies were mostly focused on the socio-behavioral profile of WBS, not the predicted underlying mechanisms or OT/OXTR release. Thus, they were not overlapping with this study’s purposes and were excluded from the analyses. We could not find a review with the primary concern on OT/OXTR study in WS or a study that contrasts the previous findings extensively. This study is the very first review of WS treatment.
Data Extraction and Quality Assessment and Risk of Bias
The data extracted was publicly available; Ethics Committee approval was unnecessary. Once the studies met our first five inclusion criteria, each study was reviewed by two author-investigators, EÇ and MNŞ, for compliance with the outlines of study quality independently. We assessed study quality using the evaluating tool by the National Institutes of Health (NIH), Quality Assessment Tool for Case-Control Studies with criteria including but not limited to: study’s method and properties, research topic, study type, aims and implications, method, and sampling, data analysis, results and findings, ethics and bias, and usefulness (Table 1).
Table 1. Quality Analysis of Studies Using NIH Quality Assessment Tool for Case-Control Studies.
| Dai et al. | Kimura et al. | Haas et al. | Nygaard et al. | |
| Research question or aim clearly stated and appropriate | Yes | Yes | Yes | Yes |
| Study population specified and defined | Yes | Yes | Yes | Yes |
| Includes sample size justification | Not reported | Not reported | Not reported | Not reported |
| Control selected from the same or similar population | Yes | Yes | Yes | Yes |
| Definitions, inclusion and exclusion criteria, algorithms, or processes to identify or select cases and controls are valid, reliable, and implemented consistently. | Yes | Yes | Not reported | Yes |
| Cases clearly defined and differentiated from controls | Yes | Yes | Yes | Yes |
| If less than 100 percent of eligible cases and/or controls were selected for the study, cases and/or controls randomly selected from those eligible | Not reported | Not reported | Not reported | Not reported |
| Use of concurrent controls | Yes | Yes | Yes | Yes |
| Investigators can confirm that the exposure/risk occurred before the development of the condition or event that defined a participant as a case | Yes | Yes | Yes | Yes |
| The measures of exposure clearly defined are valid, reliable, and implemented consistently across all study participants | Yes | Yes | Yes | Yes |
| The assessors of exposure/risk are blinded to the case or control status of participants | Not reported | Not reported | Not reported | Not reported |
| Statistical analysis accurate | Yes | Yes | Yes | Yes |
| Quality rating (Good, Fair, and Poor) | Good | Good | Good | Good |
Two authors, EÇ and MNŞ independently assessed the risk of bias in included studies. Disagreements were discussed with all authors and the decision was made via a consensus. Clarity Group Risk of Bias Tool for Case-Control Studies by McMaster University was used [49] (Table 2). We did not set a different evaluation approach towards animal studies, as there is no available paradigm for case-control quality assessment in animals.
Table 2. Risk of bias analysis using Clarity Group Risk of Bias Tool for Case-Control Studies by McMaster University.
| Dai et al. | Kimura et al. | Haas et al. | Nygaard et al. | |
| Can we be confident in the assessment of exposure? | Definitely yes | Definitely yes | Definitely yes | Definitely yes |
| Can we be confident that cases had developed the outcome of interest and controls had not? | Definitely yes | Definitely yes | Definitely yes | Definitely yes |
| Were the cases properly selected? | Definitely yes | Definitely yes | Probably yes | Definitely yes |
| Were the controls properly selected? | Definitely yes | Definitely yes | Probably yes | Probably yes |
| Were cases and controls matched according to important prognostic variables or was statistical adjustment carried out for those variables? | Definitely yes | Definitely yes | Definitely yes | Definitely yes |
| Overall | Low risk | Low risk | Low risk | Low risk |
Results
The title and the type of study, the properties of subject groups, the experiential paradigm, data analysis, and results were investigated. However, given the heterogeneity of the included studies, different key concepts were addressed in the results for each study when significant. The purpose was to present data relevant to OT, OXTR, and gene regulation in WS in the selected studies. This meant, at times, we had to exclude irrelevant study structures, subject groups, and findings of the listed studies not concerning OT/OXTR mechanisms.
Search Results
The initial library search identified potentially relevant citations from PubMed, Web of Science, and EBSCO. We also checked gray literature and addressed 39 articles in total. We excluded two newspaper articles. Subsequently, 12 duplicates were removed. A full-text article was not available for one study. Out of 27 remaining articles, eight manuscripts were excluded because of the author’s surname being Williams and not concentrating on OT effects on WS. Six articles focused on ASD and other syndromes, two other articles were not related to OT or OXTR. Four articles did not report new or substantive data. Out of the remaining four articles, none failed to comply with the quality criteria, all being case-control studies however, one was an animal case-control study (Table 3).
Table 3. An Overview of the Research Items.
| Author | Type of Study | Purpose/Topic | Subjects | Key Findings |
| Dai et al. | Case-control human trial by comparing cohorts. | -Basal OT levels are higher in WS. -An increase in basal OT is related to social function. -WS may cause exaggerated OT release to emotional stimuli. |
-WS cohort = 13 individuals (7 females aged 22–42, and 6 males aged
19–38). -Typical controls = 9 individuals (4 females aged 20–45, 4 males aged 19–40). |
-The median basal OT level is 3-times higher in WS. -OT levels are higher in WS at all-time points during interventions. -Approachability correlates significantly with basal OT. -Maladaptive behaviors significantly negatively correlate with basal OT levels. -Basal OT does not correlate with FSIQ in WS. -Changes in OT, to emotional stimulus exhibit both greater variability and greater increases in WS. |
| Kimura et al. | Case-control human trial by comparison of cohorts and existing data. | -Show irregular OXTR levels in WS. -Investigate OXTR expression in WS compared to healthy controls. |
1. For gene expression analysis: -WS cohort = 15 patients TC=15 age–sex–race balanced Japanese controls. 2. For DNA methylation analysis: -Set A: WS cohort = 34 patients TC = 34 age–sex–race balanced Japanese controls -Set B: WS cohort = 20 pediatric patients TC = 15 age-balanced controls of European origin. |
-WS patients showed significantly lower expression of OXTR. -No
significant correlation between the expression levels of OXTR and total
SRS-T scores. -OXTR expression was downregulated in WS compared to controls, although no clear correlation between expression levels and severity of social function was found. -WS patients have significantly higher methylation at three CpG sites around the transcriptional start site. -The methylation observed in the blood correlated with the brain. Three CpG sites (cg25140571, cg00247334, and cg17036624) were significantly correlated with the superior temporal gyrus. |
| Haas et al. | Case-control human trial with comparison to existing patient data. | -Show if the irregular expression of OXTR alters OT functioning in WS. | -WS Cohort = 8 patients (mean age = 4.87 years; standard deviation =
2.10). -Control Cohort = 9 age and sex-matched healthy individuals. |
-WS subjects exhibited greater expression of OXTR. -The variance between groups was statistically significant for OXTR expression. -The abnormal function of the OT system in WS, may in part be influenced by overexpression of OXTR. |
| Nygaard et al. | Case-control animal trial by comparing cohorts. | -Compare blood OT levels of WS with the control group. -Investigate whether OXTR expression differs in WS versus wild-type mice across the brain. |
1. The cohort for blood OT test: WS = 13 mice, TC = 11 WT male mice from
8 independent litters. 2. The cohort to evaluate OXTR expression: WS = 14 mice (5 female, 9 male) TC = 22 (12 female, 10 male) mice from 11 independent litters. |
-No significant differences in OT levels between genotypes. -Measured areas of where OT has been shown to affect sociability or memory, no significant differences in OXTR binding between genotypes within regions of interest. |
All case-control studies were included in the qualitative synthesis. Due to a variety of data and the limited number of studies, the overall findings were not suitable for further quantitative synthesis.
Study Characteristics and Subjects
Included research was all conducted on two different populations. One WS cohort was compared with a healthy, age-sex-ethnicity matched cohort. A total of 90 WS patients and 27 WS mice were monitored, to 82 typical human controls and 33 typical control (TC) mice.
Dai et al. [50] conducted their searches on an adult population, recruited 13 individuals for the WS cohort, females aged between 22-42 and males between 19-40, and nine individuals for TC cohort, four females aged 20-45, four males aged 19-40. While Haas et al. [51] aimed their research to a pediatric group with a mean age of 4.87 for eight WS individuals and nine TC, Kimura et al. [52] concentrated on both adult and pediatric patients and conducted their search with both Japanese subjects and subjects of European ancestry. They utilized three different datasets, a WS cohort with 15 patients and age-sex-ethnicity matched TC for gene expression analysis. For gene methylation analysis, their WS cohort comprised 34 WS individuals and 34 controls as in their generated dataset. Their pediatric WS cohort consisted of 20 WS patients to 15 TC from a publicly available dataset Gene Expression Omnibus. Nygaard et al. [53] focused on 13 male WS mice and 11 male wild-type mice for blood OT level analysis and 14 WS (5 females, 9 males) to 22 TC mice (12 female, 10 male) for OXTR expression analysis, at the mice’s second postnatal week of life.
Studies of Kimura et al. and Haas et al. also used an existing gene dataset on WS to combine the findings related to OT modulation and contrasted it with a recruited control group from publicly available gene data. The gene data proposed by Henrichsen et al. [54] was used for the study of Haas et al. Except for Haas et al., all three studies investigated blood OT levels in subjects, and except Dai et al. all three studies aimed to determine the OXTR gene expressions’ epigenetic modifications in subjects.
Dai et al. and Kimura et al. looked for a relationship between social function and OT levels amongst the WS population by using the Social Responsiveness Scale (SRS-2), Adolph’s Approachability Test, Salk Institute Sociability Questionnaire (SISQ), and the Scales of Independent Behavior Revised (SIB-R). Dai et al. also used The Salk-McGill Music Inventory Questionnaire of Music Ability and Interest test as they looked into OT production concerning music. Nonetheless, OT releases of negative and positive emotional stimuli were questioned by the same group of researchers.
Experimental Paradigms
Dai et al. confirmed WS diagnosis by fluorescent in situ hybridization, the WS group had typical WS range IQ. Socio-behavioral tests were run prior to investigation which are SISQ, SIB-R. They instructed the subjects to bring their favorite piece of music, as this is a method thought to produce the greatest amount of positive emotional stimulus. They drew samples in a socially prohibited room, only allowing the same nurse to enter the room for a blood draw at -5, 0, 1, 5, 10, 15, 20, 25, 30, 45 mins. Samples collected at -5 and 0 mins, 0 min being the initiation point of music, were averaged, and determined as the baseline points. The music stopped at 5-8 mins, and the subjects dipped their hands in cold water (15°C) at min 19 for less than 45 seconds.
Kimura et al. confirmed WS diagnosis by fluorescent in situ hybridization and determined the social severity using SRS-2. For gene expression analysis, they extracted total RNA samples from peripheral blood, performed reverse transcription. They measured the OXTR expression level and normalized it relative to GAPDH expression. For DNA methylation analysis, they analyzed their generated dataset with publicly available datasets using the GEO2R online tool (https://www.ncbi.nlm.nih.gov/geo/geo2r/). To compare blood methylation levels with brain tissue samples, Kimura et al. used The Blood-Brain DNA Methylation Comparison Tool (https://epigenetics.essex.ac.uk/bloodbrain/) which includes four different regions of the brain.
Haas et al. utilized the gene expression data from a previous study about WS by using GEO. They analyzed the results using the GEO2R online tool. They extracted and analyzed data for two transcripts accordingly with their WS and TC cohorts.
Nygaard et al. experimented with mice with hemizygous complete deletion for Williams syndrome gene region and wild-type TC mice. They maintained the mice on a 12-hour dark and 12-hour light basis. They used four different WS cohorts for their experiments, however, two of them were used for a fear-associated behavior response study that is out of this study’s scope. For blood oxytocin level analysis, blood was drawn from anesthetized mice’s retro-orbital sinuses, and an ELISA test was run with those samples. For OXTR receptor research, they euthanized mice with cervical dislocation. They retrieved brain pieces and stored them at -80°C, sliced into sections with a cryostat at -16°C and -18°C. To assess OXTR availability in the brain, they injected iodinated ornithine vasotocin analog ([125I] OVT). After preparation procedures, OVT binding was measured at the anterior olfactory nucleus, lateral septal nucleus, anterior cingulate cortex, striatum, hippocampal CA2, and CA3 regions, para-ventricular hypothalamic region, piriform cortex, and the combined basolateral and lateral amygdala.
Key Findings
Dai et al. reported that basal OT level in the WS cohort was 3 times higher compared to TC (WS median = 538 pg/mL, TC median = 181 pg/mL, P < 0.001). At all-time points, OT levels of the WS population were higher than TC (p = < 0.007). They did not report a significant difference between ages or genders for OT (t-test, p = 0.11). They noted the small number of controls reduced their statistical power without compromising the validity of inferences whatsoever. They found out that basal OT was correlated with Adolph’s Approachability test (r = 0.85; P = 0.0008). In contrast, they reported decreased adaptive social behaviors to increased basal OT levels (r = -0.62, P = 0.04). They did not find any relations between FSIQ and basal OT levels (Spearman’s rho, p = 0.36). The WS population showed a greater response to stimuli compared to TC (38% versus 24% median increase, P = 0.21, with music; 26% versus 4% increase, P = 0.01), and a greater variability (P = 0.025 with music; P = 0.007 with cold pressor, using permutation test). They found positive correlations in WS for OT responses to music and cold administration both (R = 20.25, P = 0.59 for the TC, and R = 0.77, P = 0.004 for WS patients). They could not identify a correlation between The Salk-McGill Music Inventory Questionnaire of Music Ability and Interest and OT levels, increased OT changes were found to be related to prior music-related activities (r = 0.57, P = 0.06, Spearman’s rho, not corrected).
Kimura et al. reported that OXTR expression was lower in the WS cohort compared to TCs (p < 0.05). However, they did not find any correlations between SRS-T scores or social function and OXTR expression. They indicated that both set A and set B WS cohorts showed higher methylation at three CpG sites (cg25140571, cg00247334, and cg17036624) at the transcriptional start site (FDR < 0.05), regardless of age or ethnicity. Utilizing the Blood-Brain DNA Methylation Comparison Tool, they noted that the mentioned CpG sites were strongly related to the superior temporal gyrus in the brain. They especially noted cg25140571’s association to the prefrontal cortex.
Haas et al. reported that WS subjects express OXTR greater (M = 10.22, SD = 0.68) compared to TCs (M = 7.86, SD = 1.79) (t = 3.51, p = 0.003, 95% CI = 0.93-3.81). They found a significant variance between the two groups expression (Levine’s test for Equality of Variances: F = 7.87, p = 0.013), the two-sample t-test was run and the variance remained statistically notable (t = 3.68, p = 0.004).
Nygaard et al. reported that they did not find a remarkable difference in OT amounts between WS mice and wild-type mice (t=0.003, df=21.664, p=0.9976). The autoradiography study with labeled OVT ligands revealed no significant difference in OXTR binding between WS and wild-type mice brains at regions concerning hyper-sociability and cognitive impairments.
Discussion
A hemizygous deletion in approximately 25 gene areas on 7q11.23 results in a neuro-socio-behaviorally complex disorder, WS, which manifests as atypical social and behavioral responses in patients [24,55].
Duplication of this area is thought to provoke autistic-like behaviors or possible autism, which deserve future research [56,57]. The exploratory research by Sanders et al. found that ASD subjects with duplication in chromosome 7 scored markedly better on ADOS Social and Communication Total and worse on the Child Behavior Checklist scale compared to ASD subjects with no duplication at the same region [29,30]. Mervis et al. run the ASD Social Communication Questionnaire with duplication 7 patients’ parents, and found the score were below the cutoff for 66.7% of the patients. Thirty-three-point-three % scored well above, indicating the need for a diagnostic evaluation for ASD [31,32]. Thus, studying regions that are key to disrupted social behavior may guide us to understand the pathophysiology of other neurodevelopmental conditions, such as autism, as this condition is also thought to be linked with a high OXTR methylation [58,59].
Currently, a few genes at the single copy gene region are known to be related to cognitive phenotype of WS. In a study, deletion of FZD9 caused developmental problems in hippocampus and defects in visuospatial learning in knockout mice [60]. STX1A contributes to exocytosis in neural and neuroendocrine cells [61]. LIMK1 is a protein kinase responsible for brain development, visuospatial cognition, synapse maintenance, and formation [62]. CLIP2 protein is highly expressed in hippocampus, piriform cortex, and inferior olive [63]. Recent advancements concentrating on deleted genes individually pointed out what WS patients’ sociability and cognition may depend on are GTF2I, GTF2IRD1, and GTF2IRD2. As an example, GTF2IRD1 knockout mice has shown to display craniofacial dysmorphisms and issues in brain development [63].
The molecular communicants in the brain, the neuropeptides, are neuromodulators of neural signals in means of timing and strength. They, for example, coordinate behavioral response to stress as corticotropin releasing factor (CRF) does by regulating the activity of hypothalamo-pituitary-adrenal (HPA) axis which controls the body’s fight-or-flight response. Arginine-vasopressin peptide (AVP) facilitates the activity of CRF in HPA [64].
Inappropriate activation of HPA axis is harmful to physiological and psychological well-being, as a study reveals the elevated CRF levels and other points a reduced release of AVP to cerebrospinal fluid in depressed patients [64]. An animal trial observed, after an hour of immobilization stress to female prairie voles, they recovered better if they were returned to their partner, a process which is facilitated by OT [65]. As this species have an abundant amount of OT and AVP receptors in the brain and are pair-bonded, they showed depressed-like behaviors during the loss of their partners during laboratory tests [66]. These trials demonstrate the evidence of an association between CRF, AVP, and OT in regulating social and emotional behavior [64].
OT, a neuropeptide synthesized in the hypothalamus at the paraventricular nucleus and supraoptic nucleus, is released to stressors and a variety of stimuli which includes suckling, lactation, parturition-related events, birth, mating and sexual stimulation, physical and emotional stress, and exercise [42,45,67]. OT activities can be blocked by OXTR agonists, such as atosiban, dnalovt or carbetocin. OT is believed to be a hormone, regulating maternal behavior, and a neuromodulator, responsible for approach behavior, cognition, reading emotions, engaging with peers, social processing, social anxiety, and fear [68-70]. Historically considered as a pro-social hormone, today we know OT promotes both positive and negative social interactions within context. Manipulations to OT levels suggest greater attention and devotion to social cues [71].
Procyshyn et al. proposed that OT reactivity and SNP of Williams syndrome-associated gene GTF2I are closely related, SNP s13227433 being a common type of polymorphism in GTF2I and modulating socio-behavioral outcome [72]. Individuals with a higher OT reactive genotype, report having less social anxiety as in WS. Researchers believe this condition is at least partially affected by GTF2I downregulation, deletion, or epigenetic mutations. Besides, some research proposes the predicted outcomes are because of changes in brain plasticity of patients and altered structure of it at places where it relates to a social function [73]. Meyer-Lindenberg et al. found WS patients had an altered amygdala reactivity and disruption in the orbitofrontal cortex [74]. This evidence suggests GTF2I gene expression variations mediate cognitive phenotypes and affect the amygdala and OT reactivity [75]. A prediction is that GTF2I influences levels of oxytocin in individuals healthy or affected [76].
WS is a crucial disorder to understand gene, hormone, brain, and behavior associations in terms of sociality and neuropsychiatric conditions. Alterations to the Williams syndrome gene region offer an opportunity to deepen our understandings of ASD, schizophrenia, anxiety, or depression [77,78]. Single nucleotide polymorphisms (SNPs) in OXTR have been linked to socio-behavioral outcomes of ASD, schizophrenia, or depression, indicating the role of an epigenetic modification [46,47].
The research discussing the relationship between OXTR SNPs and ASD has been controversial. A meta-analysis was conducted with 16 OXTR SNPs and 3941 ASD patients to reveal if these epigenetic changes are related to ASD symptomatology. SNPs, rs7632287A, rs237887A, rs2268491T, and rs2254298A were found to be significantly related to the severity of ASD [79]. Another study marked OXTR SNPs and blood OT levels are correlated with social impairments in all children, regardless of ASD presence [80]. Studies pointed out that, a G to A change in intronic SNP rs53576, is linked to detrimental psychosocial traits, even though this change to A is silent. The A allele presenters showed a higher rate of impaired sociability, antisocial behavior, decreased empathy, and feelings of loneliness [81-83].
In this article we have drawn OT and OXTR research together, linking endocrinological, psychological, neuroscience, and gene-related studies across disciplines. Evidence mostly supported OT and OXTR are dysregulated in WS. Dai et al. claimed an increase in basal OT amongst the human WS population when Nygaard et al. showed indifferent levels of OT amongst the mice WS population. Two studies looked for a correlation between social presentations and OT levels Dai et al. noted a correlation, Kimura et al. did not. While Kimura et al. marked a lower expression of OXTR and greater methylation at the transcriptional sites, Haas et al. proposed an overexpression. And Nygaard et al. noted no difference in OXTR binding regions of WS in the brain. Reasons for these inconsistent findings could be limited sample sizes, sex bias, RNA quality, ethnicity differences, age ranges, different experimental paradigms, or properties of populations coupled with scarce literature on the topic. Preferred social cue determining tools may be inappropriate to use directly to WS if they are not standardized for the disorder. Some studies lacked RNA quality data or preferred samples of same sex only. And for some studies, we cannot understand if the samples were drawn at the peak time or not, from blood.
These preliminary studies have led the way to inquire if OT or OXTR is related to social outcomes in WS. These results are important, as they predict strong epigenetic control over social behavior by methylation, SNPs, and other alterations. Which potentially have a regulating effect on OT metabolism and therefore a pathway for social outcomes to occur. These studies shed light on the gene-behavior axis and might lead the way to define a solid regulating system of the effect of genes on behavior changes using modulatory peptides. Combined human and animal model studies may give us clues about the importance of OT on development.
However, there may possibly be some limitations to this study. The studies, if any, that do not take place on search databases MEDLINE (PubMed), Web of Science Core Collection, and EBSCO Academic Search Ultimate, and search engines Google©, Yahoo!, and Bing may not have been accessed.
Future studies are needed to confirm the findings of previous studies and to resolve this controversy. Origins, predictors, and precursors of WS social phenotype are to be explored. Studies should recruit people with a diversity of ages and investigate multiple levels of function. The gained data should establish specific interventions to better manage and treat patients. Specific patterns should be linked with different neurodevelopmental conditions, considering genetic and environmental variations, and find out what differs these populations from normal human behavior.
Glossary
- WS
Williams Syndrome
- OT
Oxytocin
- OXTR
Oxytocin receptor gene
- WBS
Williams-Beuren syndrome
- CNV
Copy number variants
- NAHR
non-allelic homologous recombination
- ASD
autism spectrum disorder
- LTP
long-term-potentiation
- SNPs
Single nucleotide polymorphisms
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- TC
typical control
- SRS-2
Social Responsiveness Scale
- SISQ
Salk Institute Sociability Questionnaire
- SIB-R
Scales of Independent Behavior Revised
- [125I] OVT
iodinated ornithine vasotocin analog
- AVP
arginine-vasopressin peptide
Author Contributions
EÇ, MNŞ, MAG: Concept, Design, Supervision, Resources, Materials, Data collection and/or processing, Analysis and/or Interpretation, Literature Search, Writing Manuscript, Critical Review.
References
- Morris CA. Introduction: williams syndrome. Am J Med Genet C Semin Med Genet. 2010. May;154C(2):203–8. 10.1002/ajmg.c.30266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol. 2002. Apr;17(4):269–71. 10.1177/088307380201700406 [DOI] [PubMed] [Google Scholar]
- Dai L, Bellugi U, Chen XN, Pulst-Korenberg AM, Järvinen-Pasley A, Tirosh-Wagner T, et al. Is it Williams syndrome? GTF2IRD1 implicated in visual-spatial construction and GTF2I in sociability revealed by high resolution arrays. Am J Med Genet A. 2009. Mar;149A(3):302–14. 10.1002/ajmg.a.32652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer CM, Morrison N, Tolmie JL. Clinical and molecular cytogenetic (FISH) diagnosis of Williams syndrome. Arch Dis Child. 1996. Jan;74(1):59–61. 10.1136/adc.74.1.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauna-Aho O, Bjelogrlic-Laakso N, Sirén A, Kangasmäki V, Arvio M. Cognition in adults with Williams syndrome-A 20-year follow-up study. Mol Genet Genomic Med. 2019;7(6):e695-e. doi: 10.1002/mgg3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF, Bertrand J, Morris CA, Klein-Tasman BP, Armstrong SC. The Williams syndrome cognitive profile. Brain Cogn. 2000. Dec;44(3):604–28. 10.1006/brcg.2000.1232 [DOI] [PubMed] [Google Scholar]
- Doyle TF, Bellugi U, Korenberg JR, Graham J. “Everybody in the world is my friend” hypersociability in young children with Williams syndrome. Am J Med Genet A. 2004. Jan;124A(3):263–73. 10.1002/ajmg.a.20416 [DOI] [PubMed] [Google Scholar]
- Mervis CB, John AE. Cognitive and behavioral characteristics of children with Williams syndrome: implications for intervention approaches. Am J Med Genet C Semin Med Genet. 2010. May;154C(2):229–48. 10.1002/ajmg.c.30263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonherz Y, Davidov M, Knafo A, Zilkha H, Shoval G, Zalsman G, et al. Shyness discriminates between children with 22q11.2 deletion syndrome and Williams syndrome and predicts emergence of psychosis in 22q11.2 deletion syndrome. J Neurodev Disord. 2014;6(1):3-. doi: 10.1186/1866-1955-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Dida J, Lam E, Crawford-Zelli NA, Young EJ, Henderson DR, et al. Duplication of GTF2I results in separation anxiety in mice and humans. Am J Hum Genet. 2012. Jun;90(6):1064–70. 10.1016/j.ajhg.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Klein-Tasman BP. Williams syndrome: cognition, personality, and adaptive behavior. Ment Retard Dev Disabil Res Rev. 2000;6(2):148–58. [DOI] [PubMed] [Google Scholar]
- Järvinen-Pasley A, Vines BW, Hill KJ, Yam A, Grichanik M, Mills D, et al. Cross-modal influences of affect across social and non-social domains in individuals with Williams syndrome. Neuropsychologia. 2010. Jan;48(2):456–66. 10.1016/j.neuropsychologia.2009.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Caselli MC, Gagliardi C, Tonucci F, Volterra V. Language acquisition in special populations: a comparison between Down and Williams syndromes. Neuropsychologia. 2002;40(13):2461–70. 10.1016/s0028-3932(02)00083-0 [DOI] [PubMed] [Google Scholar]
- Klein-Tasman BP, Li-Barber KT, Magargee ET. Honing in on the social phenotype in Williams syndrome using multiple measures and multiple raters. J Autism Dev Disord. 2011. Mar;41(3):341–51. 10.1007/s10803-010-1060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawaid A, Riby DM, Owens J, White SW, Tarar T, Schulz PE. ‘Too withdrawn’ or ‘too friendly’: considering social vulnerability in two neuro-developmental disorders. J Intellect Disabil Res. 2012. Apr;56(4):335–50. 10.1111/j.1365-2788.2011.01452.x [DOI] [PubMed] [Google Scholar]
- Pankau R, Siebert R, Kautza M, Schneppenheim R, Gosch A, Wessel A, et al. Familial Williams-Beuren syndrome showing varying clinical expression. Am J Med Genet. 2001. Feb;98(4):324–9. [DOI] [PubMed] [Google Scholar]
- Cuscó I, Corominas R, Bayés M, Flores R, Rivera-Brugués N, Campuzano V, et al. Copy number variation at the 7q11.23 segmental duplications is a susceptibility factor for the Williams-Beuren syndrome deletion. Genome Res. 2008. May;18(5):683–94. 10.1101/gr.073197.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain LV, Armour JA, Tobin MD. Genomic copy number variation, human health, and disease. Lancet. 2009. Jul;374(9686):340–50. 10.1016/s0140-6736(09)60249-x [DOI] [PubMed] [Google Scholar]
- Bailey JA, Gu Z, Clark RA, Reinert K, Samonte RV, Schwartz S, et al. Recent segmental duplications in the human genome. Science. 2002. Aug;297(5583):1003–7. 10.1126/science.1072047 [DOI] [PubMed] [Google Scholar]
- McCarroll SA, Kuruvilla FG, Korn JM, Cawley S, Nemesh J, Wysoker A, et al. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat Genet. 2008. Oct;40(10):1166–74. 10.1038/ng.238 [DOI] [PubMed] [Google Scholar]
- Peoples R, Franke Y, Wang YK, Pérez-Jurado L, Paperna T, Cisco M, et al. A physical map, including a BAC/PAC clone contig, of the Williams-Beuren syndrome—deletion region at 7q11.23. Am J Hum Genet. 2000. Jan;66(1):47–68. 10.1086/302722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayés M, Magano LF, Rivera N, Flores R, Pérez Jurado LA. Mutational mechanisms of Williams-Beuren syndrome deletions. Am J Hum Genet. 2003. Jul;73(1):131–51. 10.1086/376565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR, Stankiewicz P. Genomic disorders: molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet. 2005. Dec;1(6):e49. 10.1371/journal.pgen.0010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert C. The genomic basis of the Williams-Beuren syndrome. Cell Mol Life Sci. 2009. Apr;66(7):1178–97. 10.1007/s00018-008-8401-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat Rev Neurosci. 2006. May;7(5):380–93. 10.1038/nrn1906 [DOI] [PubMed] [Google Scholar]
- Morris CA, Mervis CB, Hobart HH, Gregg RG, Bertrand J, Ensing GJ, et al. GTF2I hemizygosity implicated in mental retardation in Williams syndrome: genotype-phenotype analysis of five families with deletions in the Williams syndrome region. Am J Med Genet A. 2003. Nov;123A(1):45–59. 10.1002/ajmg.a.20496 [DOI] [PubMed] [Google Scholar]
- Martin LA, Iceberg E, Allaf G. Consistent hypersocial behavior in mice carrying a deletion of Gtf2i but no evidence of hyposocial behavior with Gtf2i duplication: implications for Williams-Beuren syndrome and autism spectrum disorder. Brain Behav. 2017. Dec;8(1):e00895. 10.1002/brb3.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski JR. Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet. 1998. Oct;14(10):417–22. 10.1016/s0168-9525(98)01555-8 [DOI] [PubMed] [Google Scholar]
- Khan NZ, Gallo LA, Arghir A, Budisteanu B, Budisteanu M, Dobrescu I, et al. Autism and the grand challenges in global mental health. Autism Res. 2012. Jun;5(3):156–9. 10.1002/aur.1239 [DOI] [PubMed] [Google Scholar]
- Howlin P. Autism and intellectual disability: diagnostic and treatment issues. J R Soc Med. 2000. Jul;93(7):351–5. 10.1177/014107680009300704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. J Autism Dev Disord. 1999. Dec;29(6):499–508. 10.1023/A:1022299920240 [DOI] [PubMed] [Google Scholar]
- Friedman L, Sterling A. A Review of Language, Executive Function, and Intervention in Autism Spectrum Disorder. Semin Speech Lang. 2019. Aug;40(4):291–304. 10.1055/s-0039-1692964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak B, Feng G. Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat Neurosci. 2016. Apr;19(6):647–55. 10.1038/nn.4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niego A, Benítez-Burraco A. Autism and Williams syndrome: Dissimilar socio-cognitive profiles with similar patterns of abnormal gene expression in the blood. Autism. 2021;25(2):464-89. doi: 10.1177/1362361320965074. [DOI] [PubMed] [Google Scholar]
- Seebacher F, Krause J. Physiological mechanisms underlying animal social behaviour. Phil Trans R Soc B. 2017;372(1727):20160231. doi: doi: 10.1098/rstb.2016.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killen SS, Marras S, Nadler L, Domenici P. The role of physiological traits in assortment among and within fish shoals. Phil Trans R Soc B. 2017;372(1727):20160233. doi: doi: 10.1098/rstb.2016.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011. Aug;12(9):524–38. 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- Jones C, Barrera I, Brothers S, Ring R, Wahlestedt C. Oxytocin and social functioning. Dialogues Clin Neurosci. 2017. Jun;19(2):193–201. 10.31887/DCNS.2017.19.2/cjones [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci USA. 2017. Jul;114(30):8119–24. 10.1073/pnas.1705521114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller F, Watrin F, Sturny R, Massacrier A, Szepetowski P, Muscatelli F. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum Mol Genet. 2010. Dec;19(24):4895–905. 10.1093/hmg/ddq424 [DOI] [PubMed] [Google Scholar]
- Peñagarikano O, Lázaro MT, Lu XH, Gordon A, Dong H, Lam HA, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015. Jan;7(271):271ra8. 10.1126/scitranslmed.3010257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SM, Sagar A, Levin-Decanini T, Liu W, Carter CS, Jacob S. Oxytocin and vasopressin systems in genetic syndromes and neurodevelopmental disorders. Brain Res. 2014. Sep;1580:199–218. 10.1016/j.brainres.2014.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meziane H, Schaller F, Bauer S, Villard C, Matarazzo V, Riet F, et al. An Early Postnatal Oxytocin Treatment Prevents Social and Learning Deficits in Adult Mice Deficient for Magel2, a Gene Involved in Prader-Willi Syndrome and Autism. Biol Psychiatry. 2015. Jul;78(2):85–94. 10.1016/j.biopsych.2014.11.010 [DOI] [PubMed] [Google Scholar]
- Pekarek BT, Hunt PJ, Arenkiel BR. Oxytocin and Sensory Network Plasticity. Front Neurosci. 2020. Jan;14(30):30. 10.3389/fnins.2020.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurek B, Neumann ID. The Oxytocin Receptor: From Intracellular Signaling to Behavior. 2018. Physiol Rev. 98(3):1805-908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- Kraaijenvanger EJ, He Y, Spencer H, Smith AK, Bos PA, Boks MP. Epigenetic variability in the human oxytocin receptor (OXTR) gene: A possible pathway from early life experiences to psychopathologies. Neurosci Biobehav Rev. 2019. Jan;96:127–42. 10.1016/j.neubiorev.2018.11.016 [DOI] [PubMed] [Google Scholar]
- Tops S, Habel U, Radke S. Genetic and epigenetic regulatory mechanisms of the oxytocin receptor gene (OXTR) and the (clinical) implications for social behavior. Horm Behav. 2019. Feb;108:84–93. 10.1016/j.yhbeh.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021. Mar;372(71):n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi BJ, Procyshyn TL. Williams syndrome deletions and duplications: genetic windows to understanding anxiety, sociality, autism, and schizophrenia. Neurosci Biobehav Rev. 2017. Aug;79:14–26. 10.1016/j.neubiorev.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Dai L, Carter CS, Ying J, Bellugi U, Pournajafi-Nazarloo H, Korenberg JR. Oxytocin and vasopressin are dysregulated in Williams Syndrome, a genetic disorder affecting social behavior. PLoS One. 2012;7(6):e38513. 10.1371/journal.pone.0038513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Smith AK. Oxytocin, vasopressin, and Williams syndrome: epigenetic effects on abnormal social behavior. Front Genet. 2015. Feb;6:28. 10.3389/fgene.2015.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R, Tomiwa K, Inoue R, Suzuki S, Nakata M, Awaya T, et al. Dysregulation of the oxytocin receptor gene in Williams syndrome. Psychoneuroendocrinology. 2020;115. doi: 10.1016/j.psyneuen.2020.104631. [DOI] [PubMed] [Google Scholar]
- Nygaard KR, Swift RG, Glick RM, Wagner RE, Maloney SE, Gould GG, et al. Oxytocin receptor activation does not mediate associative fear deficits in a Williams Syndrome model. 2021. [DOI] [PMC free article] [PubMed]
- Henrichsen CN, Csárdi G, Zabot MT, Fusco C, Bergmann S, Merla G, et al. Using transcription modules to identify expression clusters perturbed in Williams-Beuren syndrome. PLOS Comput Biol. 2011. Jan;7(1):e1001054. 10.1371/journal.pcbi.1001054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen-Pasley A, Bellugi U, Reilly J, Mills DL, Galaburda A, Reiss AL, et al. Defining the social phenotype in Williams syndrome: a model for linking gene, the brain, and behavior. Dev Psychopathol. 2008;20(1):1–35. 10.1017/S0954579408000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beunders G, van de Kamp JM, Veenhoven RH, van Hagen JM, Nieuwint AW, Sistermans EA. A triplication of the Williams-Beuren syndrome region in a patient with mental retardation, a severe expressive language delay, behavioural problems and dysmorphisms. J Med Genet. 2010. Apr;47(4):271–5. 10.1136/jmg.2009.070490 [DOI] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011. Jun;70(5):863–85. 10.1016/j.neuron.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009. Oct;7(1):62. 10.1186/1741-7015-7-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijlaarsdam J, van IJzendoorn MH, Verhulst FC, Jaddoe VW, Felix JF, Tiemeier H, et al. Prenatal stress exposure, oxytocin receptor gene (OXTR) methylation, and child autistic traits: the moderating role of OXTR rs53576 genotype. Autism Res. 2017. Mar;10(3):430–8. 10.1002/aur.1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Avilés C, Abel RA, Almli CR, McQuillen P, Pleasure SJ. Hippocampal and visuospatial learning defects in mice with a deletion of frizzled 9, a gene in the Williams syndrome deletion interval. Development. 2005. Jun;132(12):2917–27. 10.1242/dev.01871 [DOI] [PubMed] [Google Scholar]
- Osborne LR, Soder S, Shi XM, Pober B, Costa T, Scherer SW, et al. Hemizygous deletion of the syntaxin 1A gene in individuals with Williams syndrome. Am J Hum Genet. 1997. Aug;61(2):449–52. 10.1086/514850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RW, Olson MF. LIM kinases: function, regulation and association with human disease. J Mol Med (Berl). 2007. Jun;85(6):555–68. 10.1007/s00109-007-0165-6 [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Eussen BH, Langeveld A, van Haperen R, Winterberg S, Wouters CH, et al. The murine CYLN2 gene: genomic organization, chromosome localization, and comparison to the human gene that is located within the 7q11.23 Williams syndrome critical region. Genomics. 1998. Nov;53(3):348–58. 10.1006/geno.1998.5529 [DOI] [PubMed] [Google Scholar]
- Hammock EA. Neuropeptides in Mental Health: CRF, AVP, and OXT. Encyclopedia of Mental Health. 2016:193-6. [Google Scholar]
- Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014. Aug;76(4):281–8. 10.1016/j.biopsych.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009. May;34(6):1406–15. 10.1038/npp.2008.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009. Oct;30(4):548–57. 10.1016/j.yfrne.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: implications for autism spectrum disorders? Behav Brain Res. 2007. Jan;176(1):170–86. 10.1016/j.bbr.2006.08.025 [DOI] [PubMed] [Google Scholar]
- Järvinen A, Korenberg JR, Bellugi U. The social phenotype of Williams syndrome. Curr Opin Neurobiol. 2013. Jun;23(3):414–22. 10.1016/j.conb.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol. 2009. Oct;30(4):442–59. 10.1016/j.yfrne.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Love TM. Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav. 2014. Apr;119:49–60. 10.1016/j.pbb.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procyshyn TL, Spence J, Read S, Watson NV, Crespi BJ. The Williams syndrome prosociality gene GTF2I mediates oxytocin reactivity and social anxiety in a healthy population. Biol Lett. 2017. Apr;13(4):20170051. 10.1098/rsbl.2017.0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV. Developmental disorders of activity dependent neuronal plasticity. Indian J Pediatr. 2001. May;68(5):423–6. 10.1007/bf02723021 [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, et al. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat Neurosci. 2005. Aug;8(8):991–3. 10.1038/nn1494 [DOI] [PubMed] [Google Scholar]
- Jabbi M, Chen Q, Turner N, Kohn P, White M, Kippenhan JS, et al. Variation in the Williams syndrome GTF2I gene and anxiety proneness interactively affect prefrontal cortical response to aversive stimuli. Transl Psychiatry. 2015;5(8):e622-e. doi: 10.1038/tp.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Waller R, Bogdan R, Knodt AR, Sabhlok A, Hyde LW, et al. A Common Polymorphism in a Williams Syndrome Gene Predicts Amygdala Reactivity and Extraversion in Healthy Adults. Biol Psychiatry. 2017. Feb;81(3):203–10. 10.1016/j.biopsych.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle JG, Pulver AE, McGrath JA, Wolyniec PS, Dodd AF, Cutler DJ, et al. Molecular Genetics of Schizophrenia Consortium. Reciprocal duplication of the Williams-Beuren syndrome deletion on chromosome 7q11.23 is associated with schizophrenia. Biol Psychiatry. 2014. Mar;75(5):371–7. 10.1016/j.biopsych.2013.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm K, Lin A, Abu-Akel A, Wood SJ. The association between autism and schizophrenia spectrum disorders: A review of eight alternate models of co-occurrence. Neurosci Biobehav Rev. 2015. Aug;55:173–83. 10.1016/j.neubiorev.2015.04.012 [DOI] [PubMed] [Google Scholar]
- LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry. 2015. May;20(5):640–6. 10.1038/mp.2014.77 [DOI] [PubMed] [Google Scholar]
- Parker KJ, Garner JP, Libove RA, Hyde SA, Hornbeak KB, Carson DS, et al. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci USA. 2014. Aug;111(33):12258–63. 10.1073/pnas.1402236111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roekel E, Verhagen M, Scholte RH, Kleinjan M, Goossens L, Engels RC. The oxytocin receptor gene (OXTR) in relation to state levels of loneliness in adolescence: evidence for micro-level gene-environment interactions. PLoS One. 2013. Nov;8(11):e77689. 10.1371/journal.pone.0077689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci USA. 2010. Aug;107(31):13936–41. 10.1073/pnas.1003296107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci USA. 2009. Dec;106(50):21437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]