Abstract
Chronic Pulmonary Aspergillosis (CPA) is a destructive pulmonary disease caused by a fungal infection, affecting mainly individuals with prior or concurrent pulmonary conditions. It has a global prevalence of 42 per 100,000 population, but in the US and Europe, prevalence is less than 1 per 100,000. The clinical definition of CPA is based on various factors accounting for comorbidities, clinical presentation, and duration. It may be categorized into five subtypes that the disease may evolve between over time. Based on global consensus covering the spectrum of low-resource to high-resource settings, diagnosis is a multi-factorial process that involves a combination of clinical presentation persisting over 3 months, radiological findings, positive culture growth, and serological tests. CPA remains underdiagnosed due to a lack of awareness and is often misdiagnosed due to the comorbidities present. Treatment options are limited due to a lack of research. Furthermore, associated comorbidities and drug interactions further complicate treatment plans. Follow-up throughout treatment should be based on understanding the predictors of mortality. Identification of potential relapse or resistance to antifungal therapy is crucial to limit the low long-term survival rate. Awareness surrounding this devastating disease needs to be raised further to enable earlier identification, improve understanding of patient factors associated with prognosis, and the future potential for targeted therapies. This review aims to raise awareness of this rare condition among practitioners, by providing an overview of common risk factors influencing the prevalence and incidence of the disease. We further discuss current approaches and recent advancements in CPA diagnosis and treatment.
Keywords: chronic pulmonary aspergillosis, fungal pulmonary disease, tuberculosis, fungal infection comorbidities, global aspergillosis burden
Introduction
Chronic Pulmonary Aspergillosis (CPA) is a destructive disease caused by a fungal infection of the lung, by members of the Aspergillus genus, most notably Aspergillus fumigatus. CPA is particularly rare in the West but is also likely to be under-diagnosed, although the prevalence of the disease is more significant in lower-to-middle income countries where risk factors are more prevalent. As CPA is a disease that develops following or alongside other pulmonary diseases such as pulmonary TB, there has been a history of misdiagnosis of this disease, particularly in resource-limited settings, although this is improving with the help of concrete clinical guidelines being published in recent years [1]. The purpose of this review is to determine and evaluate the depth of current knowledge concerning CPA and to identify areas for further research and improvement of diagnosis and treatment.
Risk Factors
Many prior or concurrent diseases, predominantly pulmonary insults, present a risk of developing CPA [2]. Primarily, tuberculosis, non-TB mycobacterial infection (NTM), and allergic bronchopulmonary aspergillosis (ABPA) have been reported to be the major primary underlying conditions in the development of CPA [3]. Based on a 7-year study at the UK National Aspergillosis Centre (NAC) for 126 patients diagnosed with CPA, the frequency of underlying conditions as the primary conditions (as many patients presented with multiple underlying co-morbidities) for CPA development are shown in Table 1.
Table 1. Frequency and Percentage of Primary Underlying Condition in CPA Patients. Adapted from Smith and Denning, 2011 [3].
| Underlying condition | Frequency (percentage) of 126 CPA cases as primary underlying condition |
| Classical TB | 20 (15.9) |
| NTM | 18 (14.3) |
| ABPA+asthma | 15 (11.9) |
| COPD and/or emphysema+bullae | 12 (9.5) |
| Pneumothorax+bullae | 12 (9.5) |
| Lung cancer survivor | 12 (9.5) |
| Pneumonia | 10 (7.9) |
| Sarcoidosis | 9 (7.1) |
| Thoracic surgery | 6 (4.8) |
| Rheumatoid arthritis, no immunosuppression | 4 (3.2) |
| Asthma (no ABPA or SAFS) | 3 (2.4) |
| SAFS+asthma | 2 (1.6) |
| Bullae, no COPD, and no pneumothorax | 1 (0.8) |
| SAIA | 1 (0.8) |
| Ankylosing spondylitis | 0 (0.0) |
| Other | 0 (0.0) |
| None | 1 (0.8) |
To date, Smith and Denning’s summary is the most comprehensive report of incidence of underlying diseases for CPA. Various other studies have reported the frequency of underlying conditions, albeit not for all as outlined in Table 1, in CPA [4-10]. There are disparities in the reported frequencies to those shown in Table 1. This may be due to the retrospective nature of the study, leading to inconsistencies in identifying primary conditions. Furthermore, no study matched the sample size nor duration of data collection. The study is limited as it excluded immunosuppressed patients, however, they only formed a negligible proportion of the NAC cohort. Given the length of time over which the data was collected at a national scale, it may be assumed that the reported frequencies are the best available estimates for Western populations – applicability is limited for other populations due to greater prevalence of conditions such as TB (eg, in Taiwan, South Korea, or India where TB has been reported as the primary underlying cause for up to 93% of CPA cases) [9-11].
Prevalence and Incidence
There is an estimated prevalence of only 3 million cases of CPA worldwide (therefore based on the 2011 (date of model) global population of 7 billion, this places global prevalence at 42 per 100,000), of which 1.2 million are estimated to be complications of pulmonary tuberculosis [2]. However, this proportion can vary widely from region to region. Prevalence ranges from <1 (0.6) per 100,000 population in Western Europe and the US, to 42.9 in the Democratic Republic of Congo and Nigeria [2]. A study conducted in the United Kingdom found that previous tuberculosis accounted for 15.9% of cases [3]. Alternatively, a study based in Karachi, Pakistan found that tuberculosis was the underlying cause of CPA in 86.6% of patients [12]. Therefore, it is very difficult to pinpoint the exact number of cases of CPA that develop from pulmonary TB, and any study which attempts to do so will have a significant caveat in that it is only applicable for the region in which the study was conducted. Additionally, there are up to 411,000 cases of CPA complicating ABPA [13] and 72,000 cases of CPA complicating sarcoidosis [14]. A potential criticism of the methodology in these prevalence and incidence studies is that they utilize many assumptions and rely upon extrapolation, such as the estimated prevalence of 1,238,000 cases of sarcoidosis worldwide and an estimated 6% complication rate of CPA. Thus, any estimates of worldwide cases of CPA complicating sarcoidosis as a function of these inputs decreases the reliability. However, given the low prevalence of this disease and this particular risk factor, this is one of the few viable methods of conducting studies of this nature.
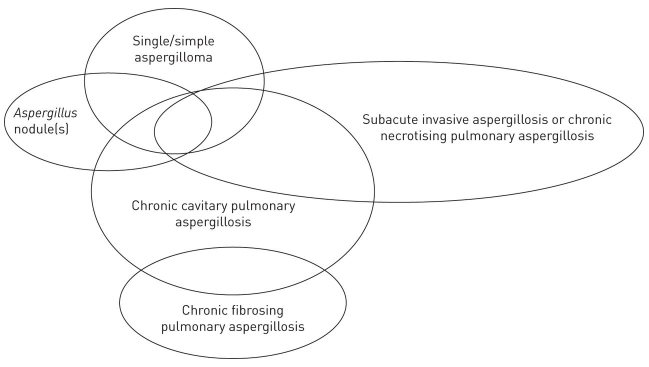
CPA may be further categorized into five subtypes: single aspergilloma, chronic cavitary pulmonary aspergillosis (CCPA), chronic fibrosing pulmonary aspergillosis (CFPA), Aspergillus nodule(s), or subacute invasive aspergillosis (SAIA) (see Figure 1) [15]. Note that these are overlapping presentations that the disease can evolve between over time, rather than distinct subtypes. The differentials are associated with patient populations affected (eg, SAIA common in immunocompromised patients), disease progression (eg, single aspergilloma shows no progression over prolonged periods), nature and extent of pulmonary or systemic symptoms (eg, CFPA can present with major loss of lung function, unlike simple aspergilloma), and methods of diagnosis (eg, Aspergillus nodule can only be definitively diagnosed based on histological findings) [15]. An in-depth discussion of the subtypes and their clinical management is beyond the scope of this review.
Figure 1.
Subtypes of CPA. Overlaps demonstrate the potential for forms to evolve over the course of disease evolution. For example, ongoing immunosuppression may see CCPA evolve into SAIA while this may reverse after antifungal therapy [37]. Adapted from Denning et al., 2016 [15]
Diagnosis
The diagnosis of CPA is a multi-factorial process that involves a combination of clinical presentation, radiological findings, positive culture growth, and serological tests [16]. Furthermore, the disease will have to have been present for at least 3 months and with little/no immunocompromise, to distinguish from SAIA [17]. The worldwide consensus of clinicians who have practiced from low- to middle- and high-income settings across various populations allows for this robust clinical definition [18]. The respiratory symptoms are unspecific and commonly include shortness of breath, sputum production, a chronic cough, and hemoptysis, while systemic symptoms include weight loss and fatigue [19]. However, as the presented symptoms could be a result of comorbidities, symptoms alone cannot be used to distinguish CPA from other diseases [20].
The radiological findings can be presented through a simple aspergilloma (also known as a fungal ball), an Aspergillus nodule, or through the presence of cavities that may contain fungal material in addition to pleural thickening [19]. Initial analysis of CPA can be achieved through chest X-rays, with additional CT scans giving insight toward the distribution and characteristics of the infection, be that fibrosing or cavitary disease presentation [21]. Imaging alone can also not be diagnostic as the findings are not distinctive to CPA. For example, Aspergillus nodules may look like malignancy or rheumatoid nodules [15]. The literature cannot give an extensive list on the possible misdiagnoses, as most of these cases are likely still unknown.
Definitive diagnosis can be achieved through sampling of the sputum or bronchoalveolar lavage and attempting to grow the fungus in culture, though this has negligible success rates, with studies quoting positivity rates as low as 26% and, therefore, this cannot be used to rule out disease [22]. However, the presence of A. fumigatus is significantly more common in CPA infection rather than colonization, and high-volume cultures could boost positivity rates of these tests threefold [17,23]. However, one cannot assume these levels of positivity without directly observing the specific culturing techniques used alongside this claim. Culture growth is hence a useful diagnostic tool for positive results but cannot be used to rule out CPA if a negative result is presented, particularly as results are interpreted subjectively. Consequently, the combination of symptoms, radiological findings and culture techniques may still not provide adequate information on the diagnosis of CPA. Thankfully for clinicians, this void is filled through the detection of raised anti-aspergillus IgG which is seen in nearly all CPA patients [20].
Testing methods for IgG levels have evolved over the years, with a precipitin test that developed into counterimmunoelectrophoresis (CIE) to increase speed of diagnosis through applying a current. These tests provide a positive result when immune complexes are formed which are visible to the naked eye [20]. Contrastingly, Aspergillus specific IgG ELISAs are quantitative, can be fully automated (reducing labor costs), and can be performed in as little as 2 hours [20]. These tests exhibit robust performance metrics, for example, a leading immunoassay test has a sensitivity between 92.9-96.0% and a specificity of 98.0% up to 99.3% [17,24]. Nevertheless, these publications are limited to small samples and these numbers cannot be taken as standard.
For clinics in resource-poor settings, a more affordable alternative can be offered through a lateral flow device, for example LDBIO ICT test. This test offers competitive performance metrics – sensitivity at 88.9-91.6% and specificity at 96.3-98.0% – while giving results in under an hour. While not quantitative, the ease of use and low cost of this test makes it well suited for definitive diagnosis in these areas [25,26]. At this point, there has been little research into the benefits of a lower price test compared to the limitations through lack of quantification and how this may affect patient outcomes.
Despite a myriad of testing apparatus with acceptable performance, CPA is still underdiagnosed due to a lack of awareness of the disease and misdiagnosis due to comorbidities that are often present.
Treatment
Treatment options for CPA are fairly limited and invariable, partly due to a lack of current research on developing new treatments for the disease, and research on the progression of the disease itself, to target future therapy development. However, the treatment of the disease itself is far from straightforward due to the associated comorbidities and drug interactions.
First line treatment for CPA involves one of the antifungal triazole drugs itraconazole or voriconazole, administered orally. The aim of the treatment in the case of Aspergillus nodule formation is to cure the disease, which can be achieved through surgically removing the aspergilloma. Conversely, in the cases of CCPA and CFPA, the aim is to improve symptoms, with the aim of reducing the morbidity (and mortality) of the disease to as great an extent as possible, through antifungal therapy [19].
Itraconazole has been the first line treatment for the past 30 years due to its moderate cost and good availability. Itraconazole has been shown to cause improvement in 76.5% of patients, compared to just 35.7% of patients undergoing standard supportive care, which involves treatment such as antitussives [27]. One shortcoming of this study was that the sample was taken from a single treatment center, and given the regional variability in the underlying cause of CPA based on region; these results may not be globally representative. A shortcoming of itraconazole itself is that it is a known inhibitor of the CYP3A4 metabolic enzyme and therefore can be a potent source of drug-drug interactions and negative side effects, such as rhabdomyolysis when combined with statins [28].
As such, voriconazole is also used as a second-line treatment for CPA, with usage beginning in the 1990s, with a 6-month overall positive response rate of 61% [29]. Voriconazole is an inhibitor of the CYP2C9 enzyme, and thus is susceptible to the same pitfalls as itraconazole. Therefore, as a first-line, one of itraconazole or voriconazole is administered based on the patient profile and any other medications they may currently be taking. In the case of drug intolerance or drug toxicity (which is not uncommon), patients may then be prescribed posaconazole (similar efficacy but higher cost) or isavuconazole (recently FDA approved). In rare cases of pan-azole resistance, intravenous amphotericin B is often administered, with response rates of 77% for 2-4 courses with interval periods of 6 months due to risk of nephrotoxicity [30]. An alternative treatment in the case of pan-azole resistance is micafungin, which has similar efficacy to voriconazole but with lower risk of side-effects and drug-drug interactions [31]. Figure 2 summarizes the treatment algorithm [19].
Figure 2.
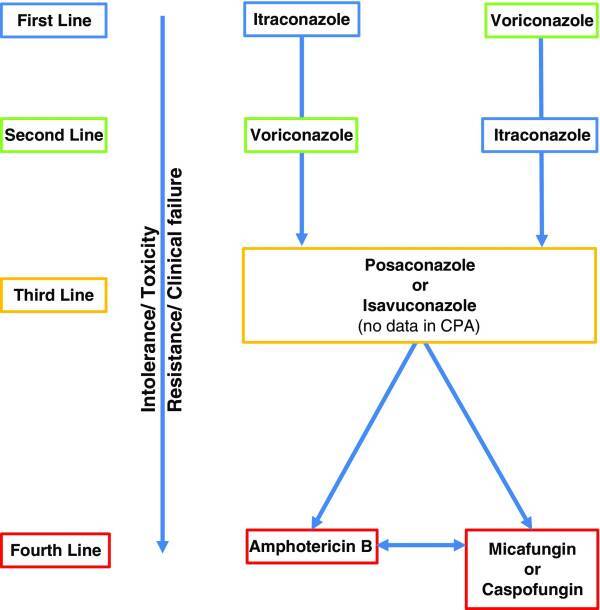
CPA Treatment stratified by toxicity and resistance. Adapted from Maghrabi and Denning, 2017 [19]
If further research is undertaken in the future to trial new drugs and investigate the pathophysiology of CPA, there is potential for more specific therapies to be developed in the future. However, considering the rarity of the disease and sufficient efficacy of current treatments, it may be difficult to garner support for such programs.
Assessing Progress
Following treatment, it is vital to assess progress to determine disease evolution and identify necessary changes in the treatment plan.
Various treatment studies have utilized different follow-up periods. The range is from 11 days to >10 years [32]. As evidenced from collating the literature, it is recommended that imaging to assess progress should be quarterly or biannually [15]. The lower bound is derived given that very little change is otherwise visible radiographically if the follow-up period is less than 3 months. During follow-ups, it is also crucial to determine any potential relapses, indicated by a sharply rising antibody titer, which can be common following the discontinuation of antifungal therapy [15]. Evidence of therapeutic failure or relapse can be indicative of the need to transition to second-line treatment.
CPA is a serious sequela to various serious pulmonary conditions resulting in high morbidity and mortality. The largest study to date of CPA and its patient outcomes of 387 patients between a 20-year period from 1992 to 2012 reports 1-year, 5-year, and 10-year survival rates to be 86.0%, 62.0%, and 47.0%, respectively [22]. This is broadly in line with ranges from prior studies from the same time period [11,33-35]. Two studies, from Japan (n=42) and South Korea (n=43), report consistently lower rates of 65.0-70.0%, 17.5-50.0%, and 30.0%, respectively [16,36]. A higher median age of the Asian cohorts alongside a skewed distribution of underlying pulmonary conditions with higher cases of TB and NTM may explain the deviation observed [37]. The large sample size and length of period of data collection further strengthen the reports, although the study design is retrospective in nature which limits full data collection, as evidenced by the authors’ own admission of needing to use incomplete death certificates [32]. Arbitrary judgement may have decreased the reliability of the reporting. In addition, since the patients were UK-based, this limits applicability of the findings to mainly Western, high-income populations. As a result, it fails to provide insights into the predictors of mortality in low- and middle-income countries where lack of access to resources due to unavailability or unaffordability may require adjustment of treatment options [32]. Understanding predictors of mortality allow for appropriate prognostic risk assessment of patients to identify necessary adjustments to progress assessment, for example, the need for more frequent follow-ups for higher-risk patients to ultimately improve outcomes and reduce mortality.
Conclusions and Outlook
There is a drastic underappreciation for the severity and level of disease burden of CPA due to a lack of awareness, resulting in underdiagnosis and misdiagnosis. Furthermore, the intrinsic features of the disease lean itself towards small study sizes with limited applicability to other environments. In addition to this, the settings where complex disease studies can be conducted is likely the exact place where disease incidence is the lowest, while lower income countries would benefit greater if these studies were carried out in their region. Though there are a variety of high performing diagnostic techniques suitable for differing settings, these are underutilized due to the seemingly low-level incidence rates, which further exacerbates the misdiagnosis issue. The combination of these diagnostic obstacles leads to a stagnant approach to the disease. Additionally, recognizing that most cases of CPA occur in middle-income regions, the findings cited here cannot be universally applied, and are driven by a small number of professional opinions. Consequentially, this review has highlighted a profound need for investigation and detection of patients with suspected CPA in order to increase the breadth of the current literature. However, this is only achievable through a higher level of understanding and recognition of this disease which will continue to be underappreciated until these studies have been released. This generates a reinforcing cycle and a call for new advocates for the disease, in order to accelerate the mission for improved CPA diagnosis and to save lives.
Acknowledgments
We would like to express our gratitude to Prof. David W. Denning, Prof. Dimitrios P. Kontoyiannis, and Dr. Felix Bongomin for taking the time out of their busy schedules to introduce us to CPA and for providing a comprehensive overview of the field as a starting point for our review.
Glossary
- CPA
Chronic Pulmonary Aspergillosis
- TB
tuberculosis
- NTM
non-tuberculous mycobacterial infection
- ABPA
allergic bronchopulmonary aspergillosis
- NAC
UK National Aspergillosis Centre
- CCPA
chronic cavitary pulmonary aspergillosis
- CFPA
chronic fibrosing pulmonary aspergillosis
- SAIA
subacute invasive aspergillosis
- SAFS
severe asthma with fungal sensitization
Author Contributions
AZ conceptualized the article. All authors contributed equally to the writing, reviewing, and editing of the manuscript.
References
- Kwizera R, Katende A, Bongomin F, Nakiyingi L, Kirenga BJ. Misdiagnosis of chronic pulmonary aspergillosis as pulmonary tuberculosis at a tertiary care center in Uganda: a case series. J Med Case Reports. 2021. Mar;15(1):140. [cited 2021 May 26]. 10.1186/s13256-021-02721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011. [cited 2021 May 22]. Available from: 10.2471/BLT.11.089441 10.2471/BLT.11.089441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J. 2011. Apr;37(4):865–72. [cited 2021 May 22] Available from: https://pubmed.ncbi.nlm.nih.gov/20595150/ 10.1183/09031936.00054810 [DOI] [PubMed] [Google Scholar]
- Saraceno JL, Phelps DT, Ferro TJ, Futerfas R, Schwartz DB. Chronic necrotizing pulmonary aspergillosis: approach to management. Chest. 1997. Aug;112(2):541–8. [cited 2021 May 22] Available from: https://pubmed.ncbi.nlm.nih.gov/9266898/ 10.1378/chest.112.2.541 [DOI] [PubMed] [Google Scholar]
- Denning DW, Riniotis K, Dobrashian R, Sambatakou H. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis. 2003. Oct;37(s3 Suppl 3):S265–80. [cited 2021 May 22] Available from: https://sci-hub.st/https://academic.oup.com/cid/article-abstract/37/Supplement_3/S265/355125 [DOI] [PubMed] [Google Scholar]
- Camuset J, Nunes H, Dombret MC, Bergeron A, Henno P, Philippe B, et al. Treatment of chronic pulmonary aspergillosis by voriconazole in nonimmunocompromised patients. Chest. 2007. May;131(5):1435–41. [cited 2021 May 22] Available from: https://pubmed.ncbi.nlm.nih.gov/17400661/ 10.1378/chest.06-2441 [DOI] [PubMed] [Google Scholar]
- Jewkes J, Kay HH, Paneth M, et al. Pulmonary aspergilloma: Analysis of prognosis in relation to haemoptysis and survey of treatment. Thorax. 1983. [cited 2021 May 22]. Available from: http://thorax.bmj.com/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addrizzo-Harris DJ, Harkin TJ, McGuinness G, Naidich DP, Rom WN. Pulmonary aspergilloma and AIDS. A comparison of HIV-infected and HIV-negative individuals. Chest. 1997. Mar;111(3):612–8. [cited 2021 May 22] Available from: https://pubmed.ncbi.nlm.nih.gov/9118696/ 10.1378/chest.111.3.612 [DOI] [PubMed] [Google Scholar]
- Chen JC, Chang YL, Luh SP, Lee JM, Lee YC. Surgical treatment for pulmonary aspergilloma: a 28 year experience. Thorax. 1997. Sep;52(9):810–3. [cited 2021 May 22] Available from: http://thorax.bmj.com/ 10.1136/thx.52.9.810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R, Vaideeswar P, Pandit SP. Pathology of pulmonary aspergillomas. Indian J Pathol Microbiol. 2008. Jul-Sep;51(3):342–5. [cited 2021 May 22] Available from: https://pubmed.ncbi.nlm.nih.gov/18723954/ 10.4103/0377-4929.42507 [DOI] [PubMed] [Google Scholar]
- Nam HS, Jeon K, Um SW, Suh GY, Chung MP, Kim H, et al. Clinical characteristics and treatment outcomes of chronic necrotizing pulmonary aspergillosis: a review of 43 cases. Int J Infect Dis. 2010. Jun;14(6):e479–82. [cited 2021 May 22] Available from: https://pubmed.ncbi.nlm.nih.gov/19910234/ 10.1016/j.ijid.2009.07.011 [DOI] [PubMed] [Google Scholar]
- Iqbal N, Irfan M, Mushtaq A, Jabeen K. Underlying conditions and clinical spectrum of chronic pulmonary aspergillosis (CPA): an experience from a tertiary care hospital in Karachi, Pakistan. J Fungi (Basel). 2020. Mar;6(2):41. [cited 2021 May 26] Available from: www.mdpi.com/journal/jof 10.3390/jof6020041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013. [cited 2021 May 26]. Available from: https://academic.oup.com/mmy/article/51/4/361/1026656 [DOI] [PubMed] [Google Scholar]
- Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis complicating sarcoidosis. Eur Respir J. 2013. Mar;41(3):621–6. [cited 2021 May 26] Available from: www.erj.ersjournals.com 10.1183/09031936.00226911 [DOI] [PubMed] [Google Scholar]
- Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016. [cited 2021 May 22]. Available from: www.aspergillus.org.uk [DOI] [PubMed] [Google Scholar]
- Ohba H, Miwa S, Shirai M, Kanai M, Eifuku T, Suda T, et al. Clinical characteristics and prognosis of chronic pulmonary aspergillosis. Respir Med. 2012. May;106(5):724–9. 10.1016/j.rmed.2012.01.014 [DOI] [PubMed] [Google Scholar]
- Wilopo BAP, Richardson MD, Denning DW. Diagnostic Aspects of Chronic Pulmonary Aspergillosis: Present and New Directions. Curr Fungal Infect Rep. 2019. [cited 2021 May 26]. 13:292–300. Available from: https://doi.org/ 10.1007/s12281-019-00361-7. [DOI] [Google Scholar]
- Denning DW, Page ID, Chakaya J, Jabeen K, Jude CM, Cornet M, et al. Case definition of chronic pulmonary aspergillosis in resource-constrained settings. Emerg Infect Dis. 2018. Aug;24(8): [cited 2021 May 22] 10.3201/eid2408.171312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghrabi F, Denning DW. The Management of Chronic Pulmonary Aspergillosis: The UK National Aspergillosis Centre Approach. Curr Fungal Infect Rep. 2017;11(4):242–51. [cited 2021 May 26]. 10.1007/s12281-017-0304-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ID, Richardson M, Denning DW. Antibody testing in aspergillosis - Quo vadis? Med Mycol. 2015. Jun;53(5):417–39 [DOI] [PubMed] [Google Scholar]
- Godet C, Philippe B, Laurent F, Cadranel J. Chronic pulmonary aspergillosis: an update on diagnosis and treatment. Respiration. 2014;88(2):162–74. [cited 2021 Sep 6] Available from: https://www.karger.com/Article/FullText/362674 10.1159/000362674 [DOI] [PubMed] [Google Scholar]
- Jhun BW, Jeon K, Eom JS, Lee JH, Suh GY, Kwon OJ, et al. Clinical characteristics and treatment outcomes of chronic pulmonary aspergillosis. Med Mycol. 2013. Nov;51(8):811–7. [cited 2021 May 26] Available from: https://academic.oup.com/mmy/article/51/8/811/999366 10.3109/13693786.2013.806826 [DOI] [PubMed] [Google Scholar]
- Vergidis P, Moore CB, Novak-Frazer L, Rautemaa-Richardson R, Walker A, Denning DW, et al. High-volume culture and quantitative real-time PCR for the detection of Aspergillus in sputum. Clin Microbiol Infect. 2020. Jul;26(7):935–40. 10.1016/j.cmi.2019.11.019 [DOI] [PubMed] [Google Scholar]
- Page ID, Richardson MD, Denning DW. Comparison of six Aspergillus-specific IgG assays for the diagnosis of chronic pulmonary aspergillosis (CPA). J Infect. 2016. Feb;72(2):240–9. 10.1016/j.jinf.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Piarroux RP, Romain T, Martin A, Vainqueur D, Vitte J, Lachaud L, et al. Multicenter evaluation of a novel immunochromatographic test for anti-aspergillus IgG detection. Front Cell Infect Microbiol. 2019. Jan;9:12. [cited 2021 May 26] Available from: www.frontiersin.org 10.3389/fcimb.2019.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky Hunter E, Richardson MD, Denning DW. Evaluation of LDBio Aspergillus ICT lateral flow assay for IgG and IgM antibody detection in chronic pulmonary aspergillosis. J Clin Microbiol. 2019. Aug;57(9):e00538-19. [cited 2021 May 26] Available from: https://pubmed.ncbi.nlm.nih.gov/31217272/ 10.1128/JCM.00538-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R, Vishwanath G, Aggarwal AN, Garg M, Gupta D, Chakrabarti A. Itraconazole in chronic cavitary pulmonary aspergillosis: a randomised controlled trial and systematic review of literature. Mycoses. 2013. Sep;56(5):559–70. [cited 2021 May 26] Available from: www.statsdirect.com 10.1111/myc.12075 [DOI] [PubMed] [Google Scholar]
- Dybro AM, Damkier P, Rasmussen TB, et al. Statin-associated rhabdomyolysis triggered by drug-drug interaction with itraconazole. BMJ Case Reports. 2016 [cited 2021 May 26];2016:bcr2016216457. Available from: http://group.bmj.com/group/rights-licensing/permissions https://doi.org/ 10.1136/bcr-2016-216457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain LR, Denning DW. The efficacy and tolerability of voriconazole in the treatment of chronic cavitary pulmonary aspergillosis. J Infect. 2006. May;52(5):e133–7. 10.1016/j.jinf.2005.08.022 [DOI] [PubMed] [Google Scholar]
- Newton PJ, Harris C, Morris J, Denning DW. Impact of liposomal amphotericin B therapy on chronic pulmonary aspergillosis. J Infect. 2016. Nov;73(5):485–95. 10.1016/j.jinf.2016.06.001 [DOI] [PubMed] [Google Scholar]
- Felton TW, Baxter C, Moore CB, Roberts SA, Hope WW, Denning DW. Efficacy and safety of posaconazole for chronic pulmonary aspergillosis. Clin Infect Dis. 2010. Dec;51(12):1383–91. [cited 2021 May 23] Available from: https://academic.oup.com/cid/article/51/12/1383/316574 10.1086/657306 [DOI] [PubMed] [Google Scholar]
- Lowes D, Al-Shair K, Newton PJ, Morris J, Harris C, Rautemaa-Richardson R, et al. Predictors of mortality in chronic pulmonary aspergillosis. Eur Respir J. 2017. Feb;49(2):1601062. [cited 2021 May 23]. 10.1183/13993003.01062-2016 [DOI] [PubMed] [Google Scholar]
- Nakamoto K, Takayanagi N, Kanauchi T, Ishiguro T, Yanagisawa T, Sugita Y. Prognostic factors in 194 patients with chronic necrotizing pulmonary aspergillosis. Intern Med. 2013;52(7):727–34. [cited 2021 May 23] Available from: https://pubmed.ncbi.nlm.nih.gov/23545666/ 10.2169/internalmedicine.52.9142 [DOI] [PubMed] [Google Scholar]
- Camara B, Reymond E, Saint-Raymond C, Roth H, Brenier-Pinchart MP, Pinel C, et al. Grenoble Aspergillus Committee. Characteristics and outcomes of chronic pulmonary aspergillosis: a retrospective analysis of a tertiary hospital registry. Clin Respir J. 2015. Jan;9(1):65–73. [cited 2021 May 23] Available from: https://pubmed.ncbi.nlm.nih.gov/24406138/ 10.1111/crj.12105 [DOI] [PubMed] [Google Scholar]
- Takeda K, Imamura Y, Takazono T, Yoshida M, Ide S, Hirano K, et al. The risk factors for developing of chronic pulmonary aspergillosis in nontuberculous mycobacteria patients and clinical characteristics and outcomes in chronic pulmonary aspergillosis patients coinfected with nontuberculous mycobacteria. Med Mycol. 2016. Feb;54(2):120–7. [cited 2021 May 23] Available from: https://pubmed.ncbi.nlm.nih.gov/26531100/ 10.1093/mmy/myv093 [DOI] [PubMed] [Google Scholar]
- Salzer HJF, Cornely OA. Awareness of predictors of mortality may help improve outcome in chronic pulmonary aspergillosis. Eur Respir J. European Respiratory Society; 2017. [cited 2021 May 23]. Available from: https://doi.org/ 10.1183/13993003.02520-2016. [DOI] [PubMed] [Google Scholar]
- Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015. Mar;70(3):270–7. 10.1136/thoraxjnl-2014-206291 [DOI] [PubMed] [Google Scholar]