Abstract
Fragile X syndrome is the most common monogenetic cause of inherited intellectual disability and syndromic autism spectrum disorder. Fragile X syndrome is caused by an expansion (full mutation ≥200 CGGs repeats, normal 10-45 CGGs) of the fragile X mental retardation 1 (FMR1) gene, epigenetic silencing of the gene, which leads to reduction or lack of the gene’s product: the fragile X mental retardation protein. In this cross-sectional study, we assessed general and pharmacotherapy knowledge (GK and PTK) of fragile X syndrome and satisfaction with education in neurodevelopmental disorders (NDDs) among senior medical students in Serbia (N=348), Georgia (N=112), and Colombia (N=58). A self-administered 18-item questionnaire included GK (8/18) and PTK (7/18) components and self-assessment of the participants education in NDDs (3/18). Roughly 1 in 5 respondents had correct answers on half or more facts about fragile X syndrome (GK>PTK), which ranged similarly 5-7 in Serbia, 6-8 in Georgia, and 5-8 in Colombia, respectively. No cohort had an average value greater than 9 (60%) that would represent passing score “cut-off.” None of the participants answered all the questions correctly. More than two-thirds of the participants concluded that they gained inadequate knowledge of NDDs during their studies, and that their education in this field should be more intense. In conclusion, there is a major gap in knowledge regarding fragile X syndrome among senior medical students in these three developing countries. The finding could at least in part be generalized to other developing countries aimed toward increasing knowledge and awareness of NDDs and fostering an institutional collaboration between developed and developing countries.
Keywords: Fragile X syndrome, developing countries, knowledge and awareness, medical collaboration
Introduction
Fragile X Syndrome (FXS) is a global neurodevelopmental disorder (NDD) caused by the full mutation (FM, ≥200 CGG repeats) of the fragile X mental retardation 1 (FMR1) gene and epigenetic silencing of the gene, which results in a deficiency or absence of fragile X mental retardation protein (FMRP) [1-4]. With an estimated prevalence of 1:4000 in males and 1:6000 in females, FXS is the most known single gene cause of inherited intellectual disability (ID) and autism spectrum disorder (ASD), which accounts for up to 5% of all ASD [3-7]. Reduced levels of FMRP are not only a basis for FXS since it leads to ID but also a contributor to the ASD phenotype [5,8-13]. Indeed, FMRP expression in the brain is the ultimate factor determining the severity of the neurobehavioral phenotype [11] and males with severe ID or severe ASD have the lowest FMRP values [10]. This is not surprising since FMRP is a RNA binding protein involved in the synaptic and dendritic maturity as well as synaptic plasticity [14]. Individuals with FXS present with a broad range of physical and neurobehavioral abnormalities including prominent ears, long face, hyperextensible finger joints, macroorchidism with puberty, stereotypies, aggression, poor eye contact, excessive shyness, tactile defensiveness, and hyperarousal. Common comorbid psychiatric conditions include attention deficit hyperactivity disorder (ADHD), social anxiety, and ASD [5,10,15-17].
The field of FXS leads the way as the most common monogenic form of ASD and the most translated among NDDs into clinical trials [18,19]. Yet, questions remain as to whether these trials were conducted with the optimal outcome endpoints or in the most appropriate age group [19-21]. While there remains a great need for safe and effective treatments for FXS, particularly for targeted treatments that surpass the symptom-based management in FXS, no medication is approved by the US regulatory agency for the treatment of FXS [22-24].
Since the discovery of the gene in 1991, many studies have focused on the molecular diagnoses of FXS and other fragile X-associated disorders (FXAD), including the fragile X-associated tremor ataxia syndrome (FXTAS) and the fragile X-associated primary ovarian insufficiency (FXPOI) experienced by premutation (PM; 55 to 200 CGG repeats) carriers. The genetic/medical diagnosis of FXS is determined by polymerase chain reaction (PCR) and Southern blotting. Furthermore, the next generation FMR1 gene-specific PCR technology (Amplidex) detects the full range of fragile X expanded alleles and minimizes the need for Southern blot (SB) analysis [25-27]. The availability of the sensitive and precise assays, which includes quantification of FMRP, allowed more accurate FMR1–FMRP correlations; thus, detecting other phenotypical correlates of FMRP deficiency not reported in previous relevant studies [8,10,13,28]. There are three general directions in which fragile X testing should be recommended: (i) clinical symptoms that suggest FXAD, (ii) family history of FXAD, intellectual or learning disabilities, ASD, or infertility and (iii) family or personal history of a fragile X genetics and inheritance (ie, carrier) [29]. A current recommendation of the American Academy of Pediatrics is to test individuals with ID, global developmental delay, ASD, and/or family history of the FMR1 FM or PM [15,30]. Thus, the fragile X testing provides important information for the diagnosis, treatment and prevention of FXAD [24] to allow an early diagnosis of FXS with or without ASD [31]. Therapeutic development has been on a rapid pace since the early 2000s and experts in this field believe that treatment needs to be implemented very early (for example, within the first years of life) for the most effectiveness in improving long-term outcomes for individuals with FXS [32]. While the genetic testing has been widely used in developed countries such as the US, such testing is infrequently used in developing countries, due to high costs and the lack of trained local genetic laboratories conducting PCR and SB analysis. Consequently, prevalence of FXAD and conditions associated with them in the later countries remains unknown. For example, according to results from previous studies, medical professionals in Serbia were barely familiar with disorders associated with the PM of the FMR1 gene (FXTAS and FXPOI) [33]. Nevertheless, their knowledge of the FM of the FMR1 gene remains unknown [33,34]. Together, similar studies of knowledge and practices related to FXAD should be carried out in other developing countries.
Here, we aim to assess: (i) general knowledge of FXS, (ii) knowledge of pharmacotherapy of FXS, and (iii) satisfaction with education in NDDs among senior medical students in developing countries such as Serbia, Georgia, and Colombia. NDDs include a broad spectrum of disorders that disrupt the normal brain development. Thus, we also aim to initiate a universal and widely used action plan in these countries that may support a pathway towards raising knowledge and awareness of NDDs, focusing here on FXS. The research is applicable to all developing countries.
Materials and Methods
Sample
This was a cross-sectional study conducted among senior medical students by investigators at the Faculty of Medicine at the University of Belgrade in Serbia, the Faculty of Medicine at the Tbilisi State Medical University in Georgia, and at the Universidad del Valle, School of Medicine in Cali, Colombia. Participation response rate ranged from 16.52% in Georgia, 31.87% in Colombia, to 99.43% in Serbia. The sample distribution of the study participants was as follows (mean age in years): (i) 348 in Serbia (24.44 ± 1.18, 227 females); (ii) 112 in Georgia (24.55 ± 0.90, 77 females), and (iii) 58 in Colombia (24.57 ± 2.74, 33 females). Anonymity of data was carried out for all participants. It was emphasized that the collected data would serve exclusively for statistical analysis, and it would be published only in a summary form as a group to establish a baseline of their knowledge related to FXS.
Measurement Tool
The instrument used for this study was a self-administered 18-item questionnaire survey. Design of the questionnaire was based on an extensive database search that included MEDLINE/PubMed, Web of Science, PsycINFO and Embase. A combination of the following keywords was used during the search: “fragile X syndrome,” “fragile X related disorders,” “drug development,” “clinical studies,” “preclinical studies,” and “pharmacological treatment.” If the data were limited or not available, an additional search included other fields of relevance (eg, “neurodevelopmental disorders,” “psychopharmacology,” etc.). In addition to the aforementioned systematic searches and the authors’ clinical experiences in the disorders related to fragile X, the questionnaire was developed by consulting a range of relevant literature involving FXS [5,9,33,35,36]. Testing of the questionnaire’s content validity was performed by a panel of three experts, who validated all items before the final version of the survey was distributed. The survey also included basic socio-demographic items (age, gender, and academic year of medical study). The complete questionnaire survey is available as supplementary material (Appendix A), which was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [37].
The survey consisted of 18 total questions, which were divided into three sections:
1. General knowledge of FXS. This section had eight items that aimed to assess general knowledge of: (i) FXS, including its frequency and symptoms; (ii) the availability of pharmacotherapy and non-pharmacological treatments in FXS; and (iii) drug development, ie, preclinical and clinical studies in FXS.
2. Knowledge of pharmacotherapy of FXS. This section had seven questions that aimed to assess the participants practical knowledge of pharmacotherapy of FXS. For example, they were asked to choose the best medicine for treating symptoms such as attention deficit, hyperactivity, sleep problems, anxiety, aggressiveness, etc. The experts who composed the questions were child psychiatrists and it is assumed they follow the psychiatric guidelines for the treatment approach.
3. Self-assessment of education in NDDs during their medical studies. Finally, the participants were asked to assess their education in NDDs during medical studies.
As presented above, two sections (General and Pharmacotherapy knowledge) were designed to assess knowledge of FXS (15 questions in total). Score higher than 9 (60%) would represent passing score “cut-off” [38,39]. The last section assessed the participants’ attitudes towards their education in NDDs during medical studies. Overall, this survey could assess their familiarity with NDDs in general.
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 22 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). The data were analyzed using descriptive and analytical statistics. Descriptive statistics included frequency (percent) of nominal variables and the measures of dispersion focusing on standard deviation (SD) for continuous variables. Parametric and nonparametric tests were used to test differences between variables. As for the latter, chi-square and Mann-Whitney U (M-W) tests were used to examine for the differences between nominal and ordinal variables, respectively. Kruskal-Wallis H test (K-W, “one-way ANOVA on ranks”), a rank-based nonparametric test, was also used to determine if there are statistically significant differences between two or more groups of an independent variable on a continuous or ordinal dependent variable. Finally, the one-way analysis of variance (ANOVA) is used to determine whether there are any statistically significant differences between the means of three independent groups. Significance was indicated by p ≤ 0.05 and internal consistency – “reliability” of the survey by Cronbach’s Alpha.
The study was approved by the Faculty of Medicine University of Belgrade Institutional Review Board (IRB) (reference number 1322/III-9).
Results
General Knowledge of FXS
Table 1 depicts the number of correct answers among the participants to all questions in Section I.
Table 1. Level of General Knowledge of Fragile X Syndrome Among Senior Medical Students in Serbia, Georgia, and Colombia.
| Correct answers, N (%) |
|||||
| Question (1-8) related to: | Serbia | Georgia | Colombia | χ² | p |
| 1. Onset of FXSa symptoms | 276 (79.32) | 81 (72.32) | 39 (67.24) | 5.37 | 0.068 |
| 2. Early treatment in FXS | 213 (61.21) | 71 (63.39) | 30 (51.72) | 3.14 | 0.208 |
| 3. Beginning of pharmacotherapy in FXS | 67 (19.25) | 27 (24.11) | 15 (25.86) | 2.12 | 0.347 |
| 4. Frequency of pharmacotherapy in FXS | 34 (9.77) | 27 (24.11) | 8 (13.79) | 15.10 | 0.001* |
| 5. Types of pharmacotherapy in FXS | 277 (79.60) | 88 (78.57) | 53 (91.38) | 4.84 | 0.089 |
| 6. FXS symptoms that could be modified by pharmacotherapy | 286 (82.18) | 97 (86.61) | 54 (93.10) | 5.04 | 0.080 |
| 7. Preclinical research in FXS | 42 (12.07) | 15 (13.39) | 9 (15.52) | 0.57 | 0.752 |
| 8. Conduct of clinical trials in FXS | 217 (62.36) | 61 (54.46) | 44 (75.86) | 7.46 | 0.024* |
Abbreviation: afragile X syndrome; χ²: value of Chi-square test; *statistically significant p value: p<0.05.
The highest number of correct answers among the students was obtained for “symptoms in FXS that could be modified by pharmacotherapy” that ranged from 93% for students in Colombia to 82% for students in Serbia (q6 in Section I; Table 1). In contrast, the lowest number of correct answers was regarding “frequency of pharmacotherapy in FXS” that ranged from ~10% to 14% for students in Serbia and in Colombia, respectively (q4 in Section I; Table 1), which was the only correct answer from Georgian participants that was significantly higher (24%) compared to the two other country participants (p = 0.001, Table 1). On the other hand, students from Georgia had the fewest correct answers regarding “preclinical research in FXS” (q7 in Section I; Table 1). Finally, students from Colombia had statistically significant higher number of correct answers regarding “conduct of clinical trials in FXS” than students from the two other countries (q8 in Section I; p < 0.05).
The median of correct answers among the three groups of participants was 4 (range 3-5) in Section I. Only one (0.9%) participant from Georgia and none from Serbia or Colombia had all 8 correct answers in the General knowledge section, whereas two participants from Serbia (0.57%), three from Georgia (2.68%), and none from Colombia answered all the items incorrectly. There was a strong internal consistency of the study for all three sites (Cronbach’s Alpha 0.996 for Serbian, 0.978 for Georgian, and 0.977 for Colombian participants, respectively).
Knowledge of Pharmacotherapy of FXS Among Senior Medical Students
Table 2 depicts the number of correct answers among the participants to questions in Section II.
Table 2. Level of Knowledge of Pharmacotherapy of Fragile X Syndrome Among Medical Students in Serbia, Georgia, and Colombia.
| Correct answers, N (%) |
|||||
| Question (1-7) related to: | Serbia | Georgia | Colombia | χ² | p |
| 1. Treatment of ADHDa in FXSb | 91 (26.15) | 42 (37.50) | 19 (32.76) | 5.63 | 0.600 |
| 2. Treatment of sleep problems in FXS | 112 (32.18) | 78 (69.64) | 26 (44.83) | 49.17 | <.0001* |
| 3. Use of alpha-adrenergic agonists in FXS | 53 (15.23) | 18 (16.07) | 8 (13.79) | 0.02 | 0.926 |
| 4. Use of guanfacine in FXS | 151 (43.39) | 63 (56.25) | 36 (62.07) | 10.60 | 0.005* |
| 5. Use of SSRIc in FXS | 90 (25.86) | 38 (33.93) | 18 (31.03) | 2.99 | 0.225 |
| 6. Treatment of anxiety in FXS | 78 (22.41) | 45 (40.18) | 37 (63.79) | 45.65 | <.0001* |
| 7. Treatment of aggressive behavior in FXS | 111 (31.89) | 33 (29.46) | 24 (41.38) | 2.61 | 0.271 |
Abbreviation: aAttention Deficit Hyperactivity Disorder; bfragile X syndrome; cSelective Serotonin Reuptake Inhibitors; χ²: value of Chi-square test; *statistically significant p value: p<0.05.
The highest number of correct answers among the three countries was recorded for “treatment of sleep problems in FXS” in Georgia (~70%), “treatment of anxiety in FXS” in Colombia (~64%), and “use of guanfacine in FXS” in Serbia (43%), respectively. The lowest number of correct answers regarding “use of alpha-adrenergic agonists in FXS” was quite comparable among the three groups of students and ranged from 14% to 16% for students in Colombia and Georgia, respectively (q3 in Section II; Table 2, p > 0.05). Participants from Georgia had a statistically significant highest number (~70%) of correct answers to “treatment of sleep problems in FXS” (q2 in Section II, Table 2; p < 0.0001), whereas students from Colombia had a statistically significant highest number (62%) when asked about “guanfacine’s usage in FXS” (q4 in Section II; Table 2; p < 0.01) and (~ 64%) “treatment of anxiety in FXS” (q6 in Section II; Table 2; p < 0.0001). Only two (1.79%) participants in Georgia and none in the two other countries answered all the questions correctly.
The median number of correct answers in this section among participants 3 (range 2-4) in both Georgia and Colombia and 2 in Serbia (range 1-3). Overall, participants from Georgia and Colombia had statistically significant higher number of correct answers to questions in section II than the students from Serbia (K-W test, H = 44,349, p < .0001; post hoc M-W U test: Serbia vs. Georgia: U = 89.880, p < .0001, Serbia vs. Colombia: U = 93.099, p < .0001). There was a strong internal consistency for those set of questions of the survey for all three sites (Cronbach’s Alpha ranged from 0.888 for Colombian to 0.930 for Serbian to 0.942 for Georgian participants, respectively.
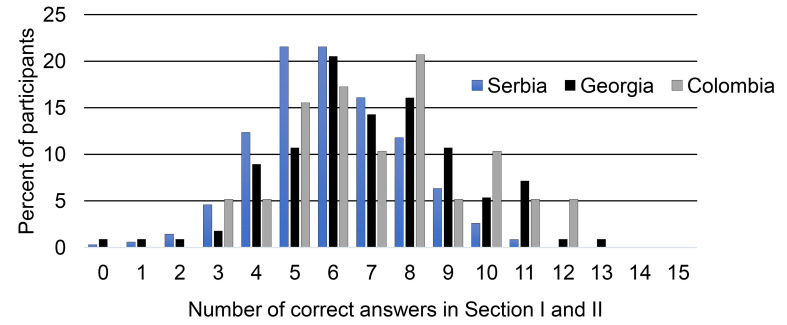
Figure 1 depicts the number of correct answers to all questions included in both Sections related to FXS among senior medical students in Serbia, Georgia, and Colombia.
Figure 1.
Total number of correct answers in Section I (General knowledge of FXS) and II (Knowledge of pharmacotherapy of FXS) among senior medical students in Serbia, Georgia, and Colombia.
As presented in Figure 1, the students from Serbia had in total 5 to 7 correct answers representing on average 1 in 5 of all their sample answering correctly (21%, 21%, and 16% of their cohort of students, respectively). In Georgia, similarly, their students had 6 to 8 correct answers (20.5%, 14.3% and 16.1% of their students, respectively), while students from Colombia most frequently had 5 to 8 correct answers (from 10% to 21% of their total students). We further compared distributions of the students with a top frequency of correct answers. As presented in Figure 1, the most frequent numbers of correct answers ranged from 5 to 10, capturing 1/3 (5/15) - 2/3 (10/15) of the survey’s total number of questions. This category-based approach found no statistically significant difference among the cohorts (278/348, 87/112, and 46/58 in Serbia, Georgia and Colombia, respectively; p = 0.88, Chi-square test value: 0.25).
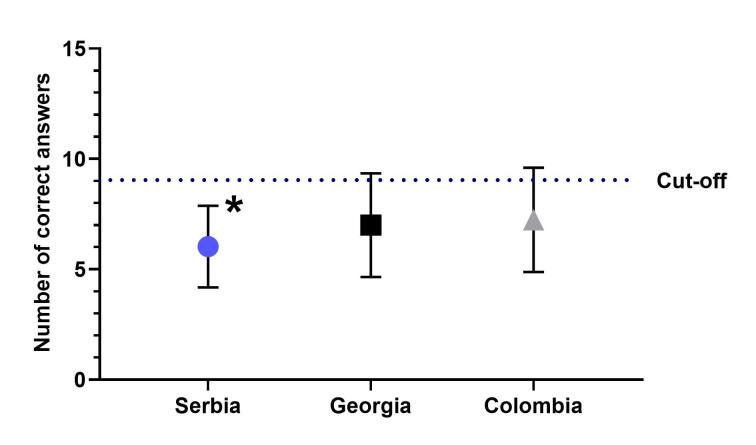
As presented in Figure 2, while there was a statistically significant difference among the average numbers of total correct answers (mean ± SD, 6.03 ± 1.85 vs. 7.00 ± 2.35 vs. 7.24 ± 2.36, p < 0.05; ANOVA) among the cohorts in Serbia, Georgia, and Colombia, respectively, neither cohort had an average value greater than 9 (60%) that would represent passing score “cut-off.” None of the participants answered all the questions in Sections I and II correctly.
Figure 2.
The average numbers of total correct answers in Section I (General knowledge of FXS) and II (Knowledge of pharmacotherapy of FXS) among senior medical students in Serbia, Georgia, and Colombia. There was statistically significant difference among the average values (* p < 0.05; ANOVA) among the cohorts in Serbia, Georgia, and Colombia. Error bars represent standard deviations.
Self-assessment of Education in NDDs During Medical Studies Among Senior Medical Students
Table 3 depicts a self-assessment of medical students’ knowledge of FXS.
Table 3. Self-assessment of Participants from Serbia, Georgia, and Colombia of Knowledge of Fragile X Syndrome.
| Answers, N (%) |
|||||
| During studies: | Serbia | Georgia | Colombia | χ² | p |
| 1. I have never heard about FXSa | 14 (4.02) | 13 (11.61) | 1 (1.72) | 11.26 | 0.003 |
| 2. I have heard about FXS, but don’t know much about FXS | 168 (48.28) | 56 (50.00) | 33 (56.90) | 1.49 | 0.47 |
| 3. I gained basic knowledge of FXS | 162 (46.55) | 39 (34.82) | 25 (39.66) | 4.74 | 0.09 |
| 4. I learned about FXS in detail. | 4 (1.15) | 4 (3.51) | 1 (1.72) | 2.91 | 0.23 |
Abbreviation: afragile X syndrome; χ²: value of Chi-square test; *statistically significant p value: p<0.05.
Table 3 shows that roughly half of the students answered that they “have heard about FXS, but don’t know much about FXS” (range from 48% in Serbia to 56% in Colombia; Table 3; p > 0.05) or “have basic knowledge of FXS” (range from 34% in Georgia to 46% in Serbia; Table 3; p > 0.05). The fewest number of students in the three countries claimed that they “learned about FXS in detail” (from 1.15% in Serbia to 3.51% in Georgia; Table 3; p > 0.05). In addition, most students (more than two-thirds of participants in each group, p > 0.05) concluded that, during studies, they gained insufficient knowledge of pharmacotherapy of NDDs, including FXS and ASD (data not shown). Finally, almost all included participants from the three countries (p > 0.05) thought that education in the field of NDDs should be more intense (data not shown).
Discussion
To our knowledge, this is the first study describing both general and more specific evidence-based level of knowledge currently recommended for the treatment of behavior problems in individuals with FXS conducted in senior medical students in Serbia, Georgia, and Colombia. In general, the study revealed a rather low level of knowledge of FXS among future rising medical doctors in these three developing countries as neither cohort reached an average value greater than 60% of the questions answered correctly as a passing score’s “cut-off” [38,39]. Thus, we concluded that the students from three cohorts had insufficient knowledge of FXS. To illustrate, roughly 1 in 5 of the study respondents scored correctly on half or more facts about FXS from the survey. Moreover, some of the participants scored even lower when asked about if they have heard about preclinical studies in FXS. Importantly, many of those senior medical students did not have enough knowledge of pharmacotherapy of FXS, which was worse than their general knowledge of FXS. Indeed, unfamiliarity with the pharmacotherapy of symptoms associated with FXS was dominant, which prevailed in all aspects of pharmacotherapy covered by the survey. The results were corroborated by strong reliability analysis (Cronbach’s α > 0.95) for each part of the survey, which was examined separately for those three countries. Together, most participants have shown a low level of general and specific knowledge of FXS.
This study was conducted in the three developing countries with economies in transition [40,41], which could be at least in part generalized worldwide to countries with similar economies. There is a lack of data of frequency and services provided for individuals with NDDs in many low- and middle-income countries [42]. Barriers to access and adequate care of those individuals with NDDs and their families include lack of knowledge, presence of stigma, systemic failures, and consequent poor quality of current services. The latter is in line with literature as 40%-80% of individuals with different mental disorders worldwide do not receive any kind of screening, treatment, or intervention [43]. For example, in Colombia and Serbia, clinical testing for FMR1 mutations is rare resulting in an older age of diagnosis of FXS when compared to developed countries. These issues might be caused by restricted access to molecular testing through national health systems, a presence of negative stereotypes towards NDDs, and lack of knowledge among healthcare professionals about FXS and disorders related to FMR1 mutations [33,34,44].
As widely reported, there is a major knowledge gap about NDDs such as ASD in different communities of those low- and middle-income countries [45,46]. A knowledge, attitude, and practice (KAP) survey conducted in Serbia in 2016 revealed a major gap in knowledge regarding the FXAD among medical professionals [33]. As FXS could be evaluated and treated by different medical specialties (ie, pediatrics, genetics, neurology, psychiatry, ophthalmology, orthopedics, ENT specialties), one might assume that would help with exposing senior medical students to the field during their medical studies and increase their knowledge about FXS and NDDs in general. The current study reveals that the education of medical professionals in the field of FXS, as a proxy of the field of NDDs, during their training is limited. Specialists that treat patients with NDDs come from different medical disciplines: psychiatry, neurology, pediatrics, genetics, etc. Each medical professional may have a different approach to treat those conditions. The knowledge of medical students about pharmacotherapy gets influenced by the source of knowledge, their personal preference for professional advancement, or the common use of available or less expensive medication. Importantly, the senior medical students in all three countries clearly indicated that they need additional education regarding FXS.
It is well-known that there is a cultural influence, at the macro- and micro-levels, on NDDs diagnoses, treatments, and treatment goals [47]. There is a possibility that the results of the applied survey in the current study could indicate the presence of stigma related to NDDs in these three different cultures/countries and a bias against the use of medications in children with FXS. Clinicians in those countries ought to be familiar with their own cultural biases of NDDs assessment and treatment. They need to have skills to deal with cultural norms in clinical practices [47]. According to results published by Mascayano and colleagues (2020), stigma toward mental illness could present a crucial limit for implementation of mental health services in low- and middle-income countries. They analyzed interventions to reduce stigma toward mental illness that has been implemented in these countries through interpretation of articles published from 1990 to 2017. Based on their study, interventions are mostly based on improving attitudes and knowledge through the education of community members, consumers, as well as healthcare practitioners [48]. However, there are limited investigations on the cultural influences in this area and further research is needed [47].
Institutional collaborations between developed and developing countries could be crucial in education, research, provision of training and personnel in the field of NDDs, such as FXS. To illustrate, an excellent example is a collaborative agreement between the Kennedy Krieger Institute in Baltimore (https://www.kennedykrieger.org), an internationally recognized institution dedicated to improving the lives of individuals with disorders of the brain, spinal cord, musculoskeletal system, and the Faculty of Medicine in Belgrade in the field of FXAD. Similarly, the University of California Davis MIND Institute in Sacramento (https://health.ucdavis.edu/mindinstitute/) offers opportunities for international medical professionals through training programs such as The International Training Program in Neurodevelopmental Disorders (ITPND). In addition, the US-based National Fragile X Foundation dedicates a portion of its resources towards building international collaborations and holds a biennial international conference for families and professionals. The international collaboration between the world-renowned MIND Institute and relevant institutions in developing countries has inspired and helped promote education and research around the world. For example, in Colombia, the Ricaurte district contained a genetic cluster of FXS, which was uncovered and studied by the Universidad del Valle [49] in conjunction with the MIND Institute. The collaborative efforts included symposiums and academic events on FXAD in order to share information with the Ricaurte community, health professionals, and medical students. In addition to Serbia, Georgia, and Colombia, the field of FXAD is also currently developing in a number of countries including India, Mexico, the Republic of the Philippines, and Brazil [15,50-54]. The effort serves as a good example of an action plan towards a focus on increasing knowledge and awareness about FXS, with a potential for improving research, teaching, and education while increasing resources for patients with NDDs. Nevertheless, the current study reveals that the effort is not enough per se and ought to be expanded to include more of institutional support. Finally, this study might be applicable to other developing countries as a “jump-start” towards raising awareness about NDDs and improving the education of treatment and intervention professionals in this field.
Acknowledgments
This research was supported by the Science Fund of the Republic of Serbia, Program DIASPORA, Grant No 6431806, Acronym PREMED-FRAX. This article was published with the financial support of the Science Fund of the Republic of Serbia. The authors of the publication are responsible for the content of this publication and this content does not express the attitudes of the Science Fund of the Republic of Serbia. We thank the Faculty of Medicine at the University of Belgrade, Faculty of Medicine at the Tbilisi State Medical University in Georgia, and the School of Medicine and Surgery at the Universidad del Valle for allowing us to conduct this study with their students.
Glossary
- ADHD
attention deficit hyperactivity disorder
- ASD
autism spectrum disorder
- FMR1
fragile X mental retardation 1
- FMRP
fragile X mental retardation protein
- FXAD
fragile X-associated disorders
- FXPOI
fragile X-associated primary ovarian insufficiency
- FXS
fragile X syndrome
- FXTAS
fragile X-associated tremor/ataxia syndrome
- ID
intellectual disability
- IRB
Institutional Review Board
- KAP
knowledge, attitude, and practice
- K-W
Kruskal-Wallis test
- M-W
Mann-Whitney U test
- NDDs
neurodevelopmental disorders
- PCR
polymerase chain reaction
- PM
premutation
- SB
Southern blot
- SD
standard deviation
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
Appendix A.
Author Contributions
DP: Conception, organization and execution of the research project. Writing a first draft of the manuscript. MJSA: Conception, organization and execution of the research project. Writing of the first draft of the manuscript. MS: Statistical analyses and preparation of tables and figures. WS: Execution of the research project. Writing of the first draft of the manuscript. LA: Execution of the research project. Writing of the first draft of the manuscript. RM: Review and critique of the manuscript. NT: Execution of the research project. MP: Execution of the research project. RH: Critically reviewed the manuscript. DBB: Conception and organization of the research project. Critically reviewed the manuscript, determining its final content.
References
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991. May;65(5):905–14. 10.1016/0092-8674(91)90397-h [DOI] [PubMed] [Google Scholar]
- Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7(1):219–45. 10.1146/annurev-pathol-011811-132457 [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Abrams MT, Chen W, Reiss AL. Genotype, molecular phenotype, and cognitive phenotype: correlations in fragile X syndrome. Am J Med Genet. 1999. Apr;83(4):286–95. [DOI] [PubMed] [Google Scholar]
- Tassone F, Iong KP, Tong TH, Lo J, Gane LW, Berry-Kravis E, et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012. Dec;4(12):100. 10.1186/gm401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DB Jr, Moine H, Kooy RF, et al. Fragile X syndrome. Nat Rev Dis Primers. 2017. Sep;3(1):17065. 10.1038/nrdp.2017.65 [DOI] [PubMed] [Google Scholar]
- Budimirovic D, Haas-Givler B, Blitz R, Esler A, Kaufmann W, Sudhalter V, et al. Autism Spectrum Disorder in Fragile X Syndrome: National Fragile X Foundation; 2014. (updated 2020) [cited 2021. May 18.]. Available from: https://fragilex.org/wp-content/uploads/2012/08/Autism-Spectrum-Disorder-in-Fragile-X-Syndrome-2014-Nov.pdf
- Budimirovic D, Subramanian M. Neurobiology of Autism and Intellectual Disability: Fragile X Syndrome. In: Johnston M, Adams H, Fatemi A. Neurobiology of Disease. New York, USA. New York: Oxford University Press; 2016. pp. 375–84. [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev. 2004;10(1):31–41. 10.1002/mrdd.20006 [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Kidd SA, Andrews HF, Budimirovic DB, Esler A, Haas-Givler B, et al. Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics. 2017. Jun;139 Suppl 3:S194–206. 10.1542/peds.2016-1159F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budimirovic DB, Schlageter A, Filipovic-Sadic S, Protic DD, Bram E, Mahone EM, et al. A Genotype-Phenotype Study of High-Resolution FMR1 Nucleic Acid and Protein Analyses in Fragile X Patients with Neurobehavioral Assessments. Brain Sci. 2020. Sep;10(10):E694. 10.3390/brainsci10100694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Hessl D, Randol JL, Espinal GM, Schneider A, Protic D, et al. Association between IQ and FMR1 protein (FMRP) across the spectrum of CGG repeat expansions. PLoS One. 2019. Dec;14(12):e0226811. 10.1371/journal.pone.0226811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25(1):315–38. 10.1146/annurev.neuro.25.112701.142909 [DOI] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Dissanayake C, Clifford S, Gould E, Bulhak-Paterson D, et al. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neurosci Biobehav Rev. 2007;31(3):315–26. 10.1016/j.neubiorev.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile V, Pucci C, Chiurazzi P, Neri G, Tabolacci E. DNA Methylation, Mechanisms of FMR1 Inactivation and Therapeutic Perspectives for Fragile X Syndrome. Biomolecules. 2021. Feb;11(2):296. 10.3390/biom11020296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo-Arellano MJ, Hagerman RJ, Martínez-Cerdeño V. Fragile X syndrome: clinical presentation, pathology and treatment. Gac Med Mex. 2020;156(1):60–6. 10.24875/GMM.19005275 [DOI] [PubMed] [Google Scholar]
- Salcedo-Arellano MJ, Dufour B, McLennan Y, Martinez-Cerdeno V, Hagerman R. Fragile X syndrome and associated disorders: clinical aspects and pathology. Neurobiol Dis. 2020. Mar;136:104740. 10.1016/j.nbd.2020.104740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Capone GT, Clarke M, Budimirovic DB. Autism in Genetic Intellectual Disability: Insights into Idiopathic Autism. In: Aw Z. Autism: Current Theories and Evidence. Totowa, NJ, USA: The Humana Press Inc; 2008. pp. 81–108. 10.1007/978-1-60327-489-0_4 [DOI] [Google Scholar]
- Budimirovic DB, Protic D, Toma AE. Fragile X syndrome: leading the way as the most common monogenic form of autism spectrum disorder and the most translated among neurodevelopmental disorders in clinical trials. J Clin Gen Genomics. 2017;1(1):1–2. [Google Scholar]
- Budimirovic DB, Berry-Kravis E, Erickson CA, Hall SS, Hessl D, Reiss AL, et al. Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. J Neurodev Disord. 2017. Jun;9(1):14. 10.1186/s11689-017-9193-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duy PQ, Budimirovic DB, Fragile X. Fragile X Syndrome: Lessons Learned from the Most Translated Neurodevelopmental Disorder in Clinical Trials. Transl Neurosci. 2017. Mar;8(1):7–8. 10.1515/tnsci-2017-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis EM, Lindemann L, Jønch AE, Apostol G, Bear MF, Carpenter RL, et al. Drug development for neurodevelopmental disorders: lessons learned from fragile X syndrome. Nat Rev Drug Discov. 2018. Apr;17(4):280–99. 10.1038/nrd.2017.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AW, Ventola P, Budimirovic D, Berry-Kravis E, Visootsak J. Clinical Development of Targeted Fragile X Syndrome Treatments: An Industry Perspective. Brain Sci. 2018. Dec;8(12):E214. 10.3390/brainsci8120214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Mathur S. Clinic-based retrospective analysis of psychopharmacology for behavior in fragile x syndrome. Int J Pediatr. 2012;2012:843016. 10.1155/2012/843016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protic D, Salcedo-Arellano MJ, Dy JB, Potter LA, Hagerman RJ. New Targeted Treatments for Fragile X Syndrome. Curr Pediatr Rev. 2019;15(4):251–8. 10.2174/1573396315666190625110748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic-Sadic S, Sah S, Chen L, Krosting J, Sekinger E, Zhang W, et al. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin Chem. 2010. Mar;56(3):399–408. 10.1373/clinchem.2009.136101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Hadd A, Sah S, Filipovic-Sadic S, Krosting J, Sekinger E, et al. An information-rich CGG repeat primed PCR that detects the full range of fragile X expanded alleles and minimizes the need for southern blot analysis. J Mol Diagn. 2010. Sep;12(5):589–600. 10.2353/jmoldx.2010.090227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F. Advanced technologies for the molecular diagnosis of fragile X syndrome. Expert Rev Mol Diagn. 2015;15(11):1465–73. 10.1586/14737159.2015.1101348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFauci G, Adayev T, Kascsak R, Kascsak R, Nolin S, Mehta P, et al. Fragile X screening by quantification of FMRP in dried blood spots by a Luminex immunoassay. J Mol Diagn. 2013. Jul;15(4):508–17. 10.1016/j.jmoldx.2013.02.006 [DOI] [PubMed] [Google Scholar]
- NFXF. Fragile X Syndrome Testing & Diagnosis 2017. [cited 2020. Dec 20.]. Available from: https://fragilex.org/understanding-fragile-x/fragile-x-101/testing-diagnosis/
- AAP. Fragile X Syndrome: Resources for Pediatric Clinicians 2021. [cited 2021. April 22.]. Available from: https://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/Pages/Fragile-X-Syndrome.aspx
- Hinton R, Budimirovic DB, Marschik PB, Talisa VB, Einspieler C, Gipson T, et al. Parental reports on early language and motor milestones in fragile X syndrome with and without autism spectrum disorders. Dev Neurorehabil. 2013;16(1):58–66. 10.3109/17518423.2012.704414 [DOI] [PubMed] [Google Scholar]
- Okoniewski KC, Wheeler AC, Lee S, Boyea B, Raspa M, Taylor JL, et al. Early Identification of Fragile X Syndrome through Expanded Newborn Screening. Brain Sci. 2019. Jan;9(1):E4. 10.3390/brainsci9010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budimirovic DB, Cvjetkovic S, Bukumiric Z, Duy PQ, Protic D. Fragile X-Associated Disorders in Serbia: Baseline Quantitative and Qualitative Survey of Knowledge, Attitudes and Practices Among Medical Professionals. Front Neurosci. 2018. Sep;12:652. 10.3389/fnins.2018.00652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budimirovic DB, Protic D. FMR1 gene mutations cause neurodevelopmental-degenerative disorders: importance of fragile X testing in Serbia. Vojnosanit Pregl. 2016. Dec;73(12):1089–93. 10.2298/VSP161006315B [DOI] [PubMed] [Google Scholar]
- Erickson CA, Davenport MH, Schaefer TL, Wink LK, Pedapati EV, Sweeney JA, et al. Fragile X targeted pharmacotherapy: lessons learned and future directions. J Neurodev Disord. 2017. Jun;9(1):7. 10.1186/s11689-017-9186-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaratnam A, Shergill J, Salcedo-Arellano M, Saldarriaga W, Duan X, Hagerman R. Fragile X syndrome and fragile X-associated disorders. F1000 Res. 2017. Dec;6:2112. 10.12688/f1000research.11885.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019. Apr;13(5 Suppl 1):S31–4. 10.4103/sja.SJA_543_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcini JJ. Setting standards on educational tests. Med Educ. 2003. May;37(5):464–9. 10.1046/j.1365-2923.2003.01495.x [DOI] [PubMed] [Google Scholar]
- Tekian A, Norcini J. Overcome the 60% passing score and improve the quality of assessment. GMS Z Med Ausbild. 2015. Oct;32(4):Doc43. 10.3205/zma000985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meetings WB. Moving the Needle: Mental Health Stories from Around the World. World Bank Group; 2018. [Google Scholar]
- World Population Review. Developing Countries 2021. Available from: https://worldpopulationreview.com/country-rankings/developing-countries
- Bitta M, Kariuki SM, Abubakar A, Newton CR. Burden of neurodevelopmental disorders in low and middle-income countries: A systematic review and meta-analysis. Wellcome Open Res. 2017. Dec;2:121. 10.12688/wellcomeopenres.13540.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Dantés O, Frenk J. Neither myth nor stigma: mainstreaming mental health in developing countries. Salud Publica Mex. 2018. Mar-Apr;60(2):212–7. 10.21149/9244 [DOI] [PubMed] [Google Scholar]
- Saldarriaga-Gil W, Cabal-Herrera AM, Fandiño-Losada A, Vásquez A, Hagerman R, Tassone F. Inequities in diagnosis of Fragile X syndrome in Colombia. J Appl Res Intellect Disabil. 2021. May;34(3):830–9. 10.1111/jar.12863 [DOI] [PubMed] [Google Scholar]
- Low HM, Wong TP, Lee LW, Makesavanh S, Vongsouangtham B, Phannalath V, et al. A grassroots investigation of ASD knowledge and stigma among teachers in Luang Prabang, Lao PDR. Res Autism Spectr Disord. 2021;80:101694. 10.1016/j.rasd.2020.101694 [DOI] [Google Scholar]
- Durkin MS, Elsabbagh M, Barbaro J, Gladstone M, Happe F, Hoekstra RA, et al. Autism screening and diagnosis in low resource settings: challenges and opportunities to enhance research and services worldwide. Autism Res. 2015. Oct;8(5):473–6. 10.1002/aur.1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier R, Mao A, Yen J. Psychopathology, families, and culture: autism. Child Adolesc Psychiatr Clin N Am. 2010. Oct;19(4):855–67. 10.1016/j.chc.2010.07.005 [DOI] [PubMed] [Google Scholar]
- Mascayano F, Toso-Salman J, Ho YC, Dev S, Tapia T, Thornicroft G, et al. Including culture in programs to reduce stigma toward people with mental disorders in low- and middle-income countries. Transcult Psychiatry. 2020. Feb;57(1):140–60. 10.1177/1363461519890964 [DOI] [PubMed] [Google Scholar]
- Saldarriaga W, Forero-Forero JV, González-Teshima LY, Fandiño-Losada A, Isaza C, Tovar-Cuevas JR, et al. Genetic cluster of fragile X syndrome in a Colombian district. J Hum Genet. 2018. Apr;63(4):509–16. 10.1038/s10038-017-0407-6 [DOI] [PubMed] [Google Scholar]
- Sachdeva A, Jain P, Gunasekaran V, Mahay SB, Mukherjee S, Hagerman R, et al. Indian Academy of Pediatrics Consensus in Diagnosis and Management of Fragile X Syndrome Committee. Indian Academy of Pediatrics Consensus in Diagnosis and Management of Fragile X Syndrome Committee. Consensus Statement of the Indian Academy of Pediatrics on Diagnosis and Management of Fragile X Syndrome in India. Indian Pediatr. 2019. Mar;56(3):221–8. 10.1007/s13312-019-1504-8 [DOI] [PubMed] [Google Scholar]
- Saldarriaga-Gil WH, Salcedo MJ, Tassone F, Ramírez-Cheyne J, Silva M, Fragile X. Syndrome in a Colombian Family. Latreia. 2018;31(1):76–85. 10.17533/udea.iatreia.v31n1a07 [DOI] [Google Scholar]
- Miller R. The Philippines Join the International Fragile X Movement: National Fragile X Foundation; 2019. [cited 2020. Dec 18.]. Available from: https://fragilex.org/international/philippines-join-fx-movement/
- Griffith D. Healing and Hope: Treating Fragile X in Serbia: UC Davis MIND Institute; 2020. [cited 2020. Dec 18.]. Available from: https://www.ucdavis.edu/health/news/spreading-healing-and-hope/
- Griffith D. In Brazil, a long history of fragile X testing, but too few with access to treatment: UC Davis Health; 2019. [cited 2020. Dec 18.]. Available from: https://health.ucdavis.edu/blog/randi-on-the-road/in-brazil-a-long-history-of-fragile-x-testing-but-too-few-with-access-to-treatment/2019/11