Abstract
Introduction: Schwannoma of the male genital system is very uncommon and is mostly treated by surgery. However, prostatic schwannoma presenting with elevated prostate-specific antigen (PSA) level and treated conservatively are extremely rare. Case presentation: Herein, we present a rare case of a prostatic schwannoma in a 65-year-old man who initially presented with an elevated PSA level. Digital rectal examination revealed an enlarged prostate with a palpable hard nodule on the left side. Transrectal ultrasonography revealed an enlarged prostate with a well-defined homogeneously hypoechoic nodule in the left peripheral lobe. Biopsy was done, and histopathology revealed a prostatic schwannoma. Conservative treatment with regular image follow-up was done per the patient’s preference. Mild PSA progression but no worsening of symptoms was found in 6 years of follow-up. Conclusions: PSA elevation could be a rare presentation of prostatic schwannoma. Treatment options other than surgery, such as conservative treatment with close observation, could be feasible for these rare tumors and long-term survivorship can be achieved.
Keywords: Schwannoma, Prostate, Prostate-specific antigen
Introduction
Schwannomas are rare tumors that originate from Schwann cells of peripheral nerve sheaths, and they are generally located in the head, neck, extremities, mediastinum, and retroperitoneum but very rarely affect the male genital system [1]. Most cases are sporadic, but some are related to inheritable disorders, such as neurofibromatosis [2]. These tumors are mostly benign and solitary, usually grow slowly, and therefore are usually asymptomatic until a space-occupying lesion causes a mass effect [3]. Because of their rarity and non-specific clinical and radiographic findings, delayed or missed diagnoses are very common [4]. Herein, we report a case of a schwannoma of the prostate, initially suspected as prostate cancer due to an elevated prostate-specific antigen (PSA) level and palpable hard nodule.
Case Presentation
A 65-year-old man initially presented with an elevated PSA level (up to 12.6 ng/ml) noted during a regular health check-up. The free PSA level was 2.6 ng/ml, and the free to total ratio was about 20%. The patient only complained of a weak urine stream and straining to void less than half of the time. Lower urinary tract symptoms (LUTSs) were mild with a total International Prostate Symptoms Score (IPSS) of 4. A digital rectal examination revealed a grade 3 enlarged prostate with elasticity but a palpable hard nodule on the left side with a regular border [5]. Transrectal ultrasonography (TRUS) revealed an estimated prostate volume of 65.1 cm3 and a homogenously hypoechoic nodule over the left peripheral lobe (Figure 1A). Multiparametric magnetic resonance imaging (mpMRI) of the pelvis revealed a 15.4-mm mass in the peripheral zone of the left lobe of the prostate with mild hypointensity in T2-weighted sequence (Figure 1B) and increased signal intensity on diffusion-weighted imaging (Figure 1C), but without an extraprostatic extension or seminal vesicle invasion. There were neither enlarged regional or para-aortic lymph nodes nor evidence of other lesions elsewhere. The TRUS biopsy revealed a spindle cell tumor with alternating cellularity, which was immunohistochemically positive for S-100, validating a schwannoma of the prostate (Figure 2). Benign prostatic tissue was found in all other biopsied sections with no evidence of cancer. Both neurological and dermatological examinations revealed no signs attributable to neurofibromatosis. The patient also denied having any familial history of neurofibromatosis. He was not operated on and was placed under close observation after shared decision making. Routine follow-up with clinical symptom evaluation, biochemical PSA level determination, and TRUS imaging was maintained throughout a total follow-up period of 6 years to date. Dutasteride was used, and the prostate size decreased (from 65.1 to 45.6 cm3 6 years after the initial diagnosis) with a mild increase in the tumor size (15.4 to 18.7 mm 6 years after the initial diagnosis). However, no worsening of LUTSs was found. A serial follow-up of the PSA level revealed a decrease to a nadir of 5.2 ng/ml. A repeated biopsy was done at 3 years after the initial diagnosis due to an increase in the tumor size and pathological report revealed the same diagnosis. The patient was regularly followed-up with at our hospital.
Figure 1.
A. Transrectal ultrasonography revealed a homogenously hypoechoic nodule with maximal tumor diameter 14.2 mm over the left peripheral lobe. B. T2-weighted magnetic resonance imaging showed a mildly hypointense tumor with maximal tumor diameter of 15.4 mm, without extraprostatic extension or seminal vesicle invasion in the left prostatic lobe. C. The tumor showed increased signal intensity on diffusion-weighted imaging.
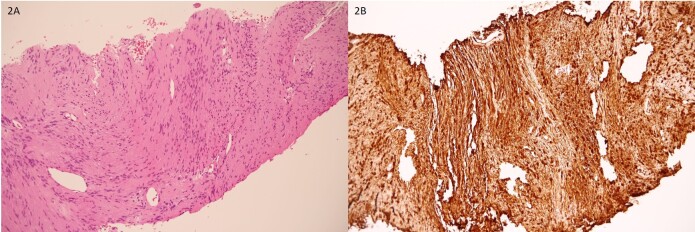
Figure 2.
A. Histopathological finding with hematoxylin and eosin stain (x200) revealed spindle-shaped cells arranged in fascicle pattern. B. The cells were strongly positively stained after immunostaining for S100 (x200).
Discussion
Schwannomas of the prostate are extremely rare. To our knowledge, this is the seventh case of a prostatic schwannoma in the literature [1,3,6-9]. The rarity of schwannomas of the prostate may be due to their clinically asymptomatic features [1]. This case has shown several unique features which have not been reported in previous cases [1,3,6-9]. First of all, none of the other cases presented with elevated PSA level except in one case, but it was in conjunction with prostate adenocarcinoma [9]. Secondly, none of the other cases had palpable nodules upon DRE, likely consistent with their location which is mostly intraprostatic. The lesion in this case is located peripherally, probably arising from the posterolateral neurovascular bundle and is palpable with hard consistency. Lastly, most of the other cases presented with severe LUTSs, unlike this patient who only had mild LUTSs and is discovered due to elevated PSA along with a palpable hard nodule, suggesting an initial high suspicion of prostate cancer. The reason for PSA elevation in our patient is unclear. Benign prostatic hyperplasia (BPH) may play an important role due to prostatic enlargement, because the PSA level decreased by about 50% (from 12.6 to 5.2 ng/ml) after use of dutasteride and decrease of total prostate volume. The possibility of prostatic cancer in our patient was low, because no cancer was detected after prostatic biopsies twice.
Schwannomas pose a difficult diagnostic challenge to urologists due to their non-specific clinical and radiological findings [10]. Ultrasonography may be the initial diagnostic tool, but either computed tomography or mpMRI provides more information in determining the size, location, local involvement, and distant spread of the tumor. The only definite diagnostic investigation is through histopathologic and immunohistochemical examinations of either biopsied or excised specimens [4]. Since schwannomas are not sensitive to either radiation therapy or chemotherapy, surgical excision remains the mainstay treatment of choice [4]. The efficacy of conservative treatment for these benign tumors is still unclear. Observation was preferred in our patient not only due to the benign nature of the tumor, its relatively small size, and lack of bothersome symptoms but also the patient’s preference was taken into account. Follow-up in the present case showed only a mild increase in the tumor size, with mild PSA elevation but no clinical progression, warranting this approach being feasible for these rare tumors.
Conclusion
To our knowledge, this report is the first documented observation of a schwannoma of the prostate presenting with elevated PSA level and with the longest follow-up period of 6 years since initial diagnosis. Treatment with conservative management instead of surgical management could be feasible for these rare tumors and long-term survivorship can be achieved.
Glossary
- PSA
prostate-specific antigen
- IPSS
International Prostate Symptoms Score
- LUTSs
Lower urinary tract symptoms
- TRUS
Transrectal ultrasonography
- mpMRI
Multiparametric magnetic resonance imaging
- BPH
Benign prostatic hyperplasia
Author Contributions
BCHL was a major contributor in writing the manuscript. KHL provided the initial idea. BCHL and HJS performed initial analysis and interpretation of the data. BCHL and HJS reviewed the literature and drafted the literature review and discussion. HJS and SHS revised the final manuscript and gave some effective advances. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This manuscript was approved by the Institutional Review Board(ethics committee) of Taipei Medical University (Protocol Number: N201906045).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Funding
No funding was obtained for this study.
References
- Jiang R, Chen JH, Chen M, Li QM. Male genital schwannoma, review of 5 cases. Asian J Androl. 2003. Sep;5(3):251–4. [PubMed] [Google Scholar]
- Rodriguez FJ, Stratakis CA, Evans DG. Genetic predisposition to peripheral nerve neoplasia: diagnostic criteria and pathogenesis of neurofibromatoses, Carney complex, and related syndromes. Acta Neuropathol. 2012. Mar;123(3):349–67. 10.1007/s00401-011-0935-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francica G, Bellini S, Miragliuolo A. Schwannoma of the prostate: ultrasonographic features. Eur Radiol. 2003. Aug;13(8):2046–8. 10.1007/s00330-002-1699-1 [DOI] [PubMed] [Google Scholar]
- Shelat VG, Li K, Naik S, Ng CY, Rao N, Rao J, et al. Abdominal schwannomas: case report with literature review. Int Surg. 2013. Jul-Sep;98(3):214–8. 10.9738/INTSURG-D-13-00019.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederico RR, Antonio WR, Thadeu BF, et al. The prostate exam. Health Educ J. 2011;71(2):239–50. [Google Scholar]
- Rane A, Juhasz A, McEwan A, Mene A. A urological diagnostic conundrum: schwannoma masquerading as an enlarged prostate. Br J Urol. 1995. May;75(5):683–4. 10.1111/j.1464-410X.1995.tb07440.x [DOI] [PubMed] [Google Scholar]
- Oktay Ü, Mehmet FZ, Mehmet FK, et al. Schwannoma of the prostate: a rare case report and literature review. Gen Med (Los Angel). 2015;3:6. [Google Scholar]
- Dietrick B, Friedes C, White MJ, Allaf ME, Meyer AR. Incidental periprostatic schwannoma discovered during evaluation for prostatic adenocarcinoma. Urol Case Rep. 2020. Feb;31:101150. 10.1016/j.eucr.2020.101150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JW, Velaga J, Yuen JS, Cheng XM, Law YM. Periprostatic schwannoma mimicking metastatic lymphadenopathy in a case of multifocal prostate adenocarcinoma. J Radiol Case Rep. 2021. Mar;15(3):9–18. 10.3941/jrcr.v15i3.4210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe K, Shimizu T, Akahane T, Kato H. Imaging of ancient schwannoma. AJR Am J Roentgenol. 2004. Aug;183(2):331–6. 10.2214/ajr.183.2.1830331 [DOI] [PubMed] [Google Scholar]