Abstract
Peripheral nerve injury involves complex molecular, cellular, and genetic events that help in ultimate regeneration of nerve. Some key factors are upregulated and some downregulated in the process of regeneration of nerve to attain almost perfect architecture. This review renders short overview about how the injured neurons and supporting cells like Schwann cells help in creating ample microenvironment for regeneration of peripheral nerve and their maturation.
KEYWORDS: Nerve regeneration, neuroscience, peripheral nerve
INTRODUCTION
Peripheral nervous system (PNS) is made up of complex tissue encompassing two main parts, the root end which conveys motor or sensory information and to and from the central nervous system through soma (neuronal cell bodies) and the main trunk encompassing axons. The continuous flow of molecules happens between axons (synapses) and neuronal cell body (soma). Soma is located at the end of trunk having active nucleus and Golgi apparatus helping in the conduction of molecule.[1] The cytoskeleton of PNS encompasses neuronal membrane and neurofilaments having layer of lipids that facilitate exchange of impulses between exterior and interior axon. The peripheral nerve injury (PNI) repair involves complex stochastic growth signaling events of different cell populations. They are guided by the same process found in embryonic development.[2] The nonneuronal cells such as Schwann cells (SCs) play active role in regeneration, by creating ambient microenvironment for injured axons.[3]
REGENERATION OF NERVE
Nerve regeneration involves three phases or 3Rs, the first phase is called regeneration of axons, which is divided into two steps, the sprouting from the transected axons toward the injury site is the first step called latency period of staggered growth. There will be elongation of axons from the distal stump of nerve for some distance. Axons emit many regenerative sprouts, which cross injury site and try to reach the distal stump at the rate of 1–3 mm/day.[4] SCs and basal membrane help in this process, finding the suitable target tissue, thereby establishing synapses with the glands or muscle fibers or to connect sensory receptors. The direction of the growth sprout toward distal stump is critical as misdirectional growth may end up in mismatched targets, causing uninvited reaction. On reaching appropriate target and reinnervations, the axons mature by increasing in its size and forming more myelin to revive normal conduction properties of nerve. The restoration of complex functions such as sensory discrimination and fine motor control is restituted by central connection.[2,5]
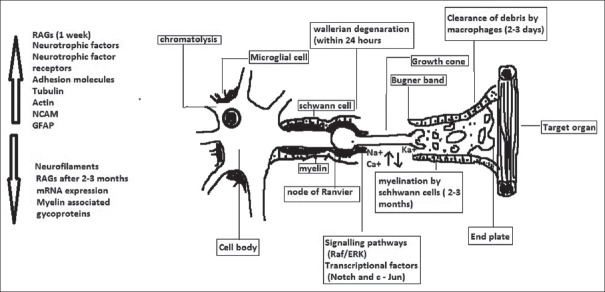
Immediately of PNI proximal and distal stumps of the nerve undergo molecular and structural changes thereby initiating axonal regeneration. The proximal stump degenerates up to node of Ranvier, and other injured axon also elaborates more daughter axons. Most of the daughter axons get pruned, the axon that survived, start the process of elongation through distal stumps forming regenerating units.[6] The distal nerve stump undergoes Wallerian degeneration after injury, which is essential process in axonal regeneration. After PNI, within 1 h, die back in proximal and distal stump happens, which is mediated by calcium influx and upregulation of calpains.[7] This leads to cleavage of neurofilaments and microtubule-associated components such as tubulin and spectrin. The die back happens up to first node of Ranvier, in the proximal stump. Action potentials are seen up to 24 h after injury, after which disintegration of cytoskeletal proteins is initiated by calpains and ubiquitin–proteasome system.[8] The axonal regeneration initially is sustained by locally generated cytoskeletal matters and also by neuronally generated, anterogradely transported cytoskelatal proteins such as actin and tubulin. Further regeneration of distal stump is dependent on microenviroment provided by SCs. The paucity of SC–laden endoneurial channels otherwise called bands of Bungner can lead to misdirected regeneration.[9,10]
SCHWANN CELLS IN NERVE REGENERATION
The SCs help in the formation of regeneration tracks, myelin cells that are seen as flattened sheath encircling axons adopt elongated bipolar architecture. This type of arrangement allows SCs to form column called as Bungner bands which is enclosed in basal lamina. The column helps in forming tracks from site of injury to the target areas in which SCs provide necessary substrate and guidance cues for axonal regeneration.[11] The immature SC is characterized by upregulation of molecules such as p75NTR, NCAM, and glial fibrillary acidic protein whereas factors such as key myelin transcription factor, Egr2, and membrane-associated proteins are downregulated. Invading macrophages help in scavenging dead myelin and also vascularization of the distal nerve stump.[12] SCs play a paramount role by the phagocytosis of myelin and axonal debris. SCs also secrete chemoattractant proteins-1 and interleukin-1 which recruit macrophages that scavenge axon and myelin debris. The mitogens released from axon debris help in SC division and promoting secretion of cytokines, eventually helping in myelin phagocytosis.[13] The migratory behavior of SCs is orchestrated by set of genes such as sox2 and N-cadherin.
GROWTH FACTORS AND GENETIC FACTORS IN NERVE REGENERATION
Neurotrophic factors such as brain-derived neurotrophic factor (BDNF) modulate regeneration of axon. The BDNF plays a crucial role in axon regeneration during electric excitation and optogenetic stimulation. The single nucleotide polymorphism human Va166Met and bdnf gene can help in personalized regenerative treatment in nerve injuries.[14,15] Neurotrophic receptor P75NTR is main transmembrane protein involved in nerve regeneration, and other proteins such as growth factor neuregulin-1 and the receptor Notch create excellent microenvironment for regeneration of injured axons. After nerve injury, mitochondrial DNA fragment will be released from axon terminals along with “mitochondrial damage-associated molecular pattern” which might have positive factor in SC proliferation.[16] Immediately after PNI, the loss of axonal contact induces proliferation of SCs thereby switching them to nonmyelinating growth to supportive phenotype. There will be upregulation of regenerative-associated genes (RAGs) such as neurotrophins, e.g., nerve growth factor, their receptors, e.g., P75 and neural cell adhesion molecules.[17,18] On the other hand, mRNA expression, myelin-associated glycoproteins, and myelin-associated proteins are downregulated in Figure 1.
Figure 1.
Events in the regeneration of injured peripheral nerve
Action potentials in nerve regeneration
Injured neuron downregulates protein needed for neurotransmission and upregulates protein needed for regeneration thereby changing the action potential. The proteins such as actin, GAP-43, and tubulin are upregulated after injury. RAG cannot be sustained for more periods, (<6 months in experimental animal); they are downregulated thereby reducing the regenerative capacity of nerve. Deformation of axon due to trauma results in higher sodium influx through mechanically sensitive sodium channels. This leads to increased calcium concentration through the opening of voltage-gated calcium channels and Na+-Ca2+ exchanger reversal.[19]
Signaling pathways in nerve regeneration
The signaling pathways such as Raf/ERK and transcriptional factors such as Notch and c-Jun influence myelin cells at some trigger points to change its phenotype. Raf/ERK pathway activation plays a major role in demyelination and proliferation of SCs.[11] It also helps in breaking blood-nerve barrier and trigger inflammatory response. The response can be reversed by switching off the ERK pathway signal pathway leading to remyelination of SCs and restoring inflammatory response. c-Jun which is upregulated in SCs has great role in transcription of myelin SCs into Bugner repair cells.[20]
CONCLUSION
The regenerated PNS neurons after injury return only to suboptimal function especially the nerve trunk which is close to spinal cord and far from target organ have guarded prognosis. In nerve injury, injured neurons regenerate at rate of 1 mm/1 day, at this rate reestablishment of sensory organ innervations or functional motor unit might take many months or even years. The late reestablishment of regeneration is called chronic axotomy, which may lead to chronic muscle denervation leading to irreversible atrophy of muscles and sensory organ, causing fat tissue replacement. Another failure of functional recovery of nerve is due to regeneration of axon into different end organ and endoneurial tubes. Physical exercise has shown to improve motor recovery of transected nerve, during its repair process. Besides exercise, ascorbic acid, and neurotrophic factors have shown positive neuritic outgrowth. Mesenchymal stem cells have shown good regenerative potential, in nerve regeneration, which will be an excellent tool for the same. Combinations of new strategies such as using regenerative medicine technologies can accentuate nerve regeneration process.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Petcu EB, Midha R, McColl E, Popa-Wagner A, Chirila TV, Dalton PD. 3D printing strategies for peripheral nerve regeneration. Biofabrication. 2018;10:032001. doi: 10.1088/1758-5090/aaaf50. [DOI] [PubMed] [Google Scholar]
- 2.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulaiman W, Gordon T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: From bench top research to bedside application. Ochsner J. 2013;13:100–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Geuna S, Raimondo S, Fregnan F, Haastert-Talini K, Grothe C. In vitro models for peripheral nerve regeneration. Eur J Neurosci. 2016;43:287–96. doi: 10.1111/ejn.13054. [DOI] [PubMed] [Google Scholar]
- 5.Navarro X. Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models: A critical overview. Eur J Neurosci. 2016;43:271–86. doi: 10.1111/ejn.13033. [DOI] [PubMed] [Google Scholar]
- 6.Ertürk A, Hellal F, Enes J, Bradke F. Disorganized microtubules underlie the formation of retraction bulbs and the failure of axonal regeneration. J Neurosci. 2007;27:9169–80. doi: 10.1523/JNEUROSCI.0612-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhai Q, Wang J, Kim A, Liu Q, Watts R, Hoopfer E, et al. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–25. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 8.Wang JT, Medress ZA, Barres BA. Axon degeneration: Molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sulaiman OA, Gordon T. Role of chronic Schwann cell denervation in poor functional recovery after nerve injuries and experimental strategies to combat it. Neurosurgery. 2009;65:A105–14. doi: 10.1227/01.NEU.0000358537.30354.63. [DOI] [PubMed] [Google Scholar]
- 10.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 11.Jessen KR, Mirsky R, Lloyd AC. Schwann cells: Development and role in nerve repair. Cold Spring Harb Perspect Biol. 2015;7:a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulaiman OA, Gordon T. Cellular and molecular interactions after peripheral and central nerve injury. Biomed Rev. 2003;14:51–62. [Google Scholar]
- 13.Brushart TM. Nerve Repair. New York, NY: Oxford University Press; 2011. [Google Scholar]
- 14.Gambarotta G, Raimondo S, Udina E, Phillips JB, Haastert-Talini K. Editorial: Peripheral nerve regeneration. Front Cell Neurosci. 2019;13:464. doi: 10.3389/fncel.2019.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGregor CE, English AW. The role of BDNF in peripheral nerve regeneration: Activity-dependent treatments and Val66Met. Front Cell Neurosci. 2018;12:522. doi: 10.3389/fncel.2018.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korimová A, Klusáková I, Hradilová-Svíženská I, Kohoutková M, Joukal M, Dubový P. Mitochondrial damage-associated molecular patterns of injured axons induce outgrowth of Schwann cell processes. Front Cell Neurosci. 2018;12:457. doi: 10.3389/fncel.2018.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duraikannu A, Krishnan A, Chandrasekhar A, Zochodne DW. Beyond trophic factors: Exploiting the intrinsic regenerative properties of adult neurons. Front Cell Neurosci. 2019;13:128. doi: 10.3389/fncel.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan A, Duraikannu A, Zochodne DW. Releasing 'brakes' to nerve regeneration: Intrinsic molecular targets. Eur J Neurosci. 2016;43:297–308. doi: 10.1111/ejn.13018. [DOI] [PubMed] [Google Scholar]
- 19.Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci. 2001;21:1923–30. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–47. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]