Abstract
Aims:
The study aimed to determine the inhibition activity of a combination of Noni fruit extract and Pandan leaves extract toward bacterial isolates that cause dandruff and to determine the potential of extract combination as an anti-dandruff shampoo.
Subjects and Methods:
The test bacteria were isolated from dandruff. The inhibition test of the extract combination was done by paper disc and well diffusion method. The identification of bacterial isolates was done in Balai Laboratorium Kesehatan Yogyakarta.
Results:
The study showed that the density of bacteria isolated from dandruff was 3.4 × 107 CFU/g. There were 28 pure bacteria isolates from purification process. The combination of Noni and Pandan extracts (1:1 v/v) could inhibit the growth of 92.9% bacterial isolates. There were seven species of bacteria from a total of 28 pure isolates, namely: Bacillus licheniformis, Bacillus megaterium, Bacillus alvei, Bacillus subtilis, Micrococcus sp, Stomatococcus sp, and Pseudomonas sp.
Conclusions:
Based on the research, it can be concluded that a combination of Noni fruit extract and Pandan leaves extract inhibited the growth of bacteria isolated from dandruff, so the combination of the extracts has potential as an anti-dandruff shampoo.
KEYWORDS: Antibacterial, anti-dandruff shampoo, extract, noni, pandan
INTRODUCTION
Anti-dandruff shampoo is a shampoo specially made for dealing with dandruff on the scalp. Dandruff is one of the hair issues on the scalp that may cause discomfort due to the emergence of itching and can cause hair loss if not treated immediately. Dandruff is often complained, even 50% of the world's population, especially people with the age of 15–50 years old have dandruff on their scalp.[1] Dandruff is dead skin exfoliation results on the scalp that occurs excessively, constantly, and sometimes is followed by skin irritation. Dandruff is often characterized by itchy scalp and can cause hair loss.
Noni (Morinda citrifolia) and Pandan (Pandanus amaryllifolius Roxb.) are known to have an antibacterial effect. All parts of Noni– stem, leaf, fruit, and seeds– can inhibit the growth of some species of bacteria.[2] Noni has inhibition activity against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus),[3,4] Bacillus subtilis (B. subtilis),[4] and Klebsiella pneumoniae (K. pneumoniae).[5]
The previous study shows that the ethanol extract and ethyl acetate extract of combination of Urang Aring (Eclipta prostrata) leaves and Noni leaves could inhibit the growth of seven test bacteria, namely Bacillus cereus (B. cereus), Micrococcus luteus (M. luteus), S. aureus, E. coli, Pseudomonas aeruginosa (P. aeruginosa), K. pneumoniae, and Salmonella typhimurium (S. typhimurium).[6] Other research shows that the combination of leaves and seeds of Noni could inhibit the growth of five test bacteria, namely E. coli, Pseudomonas spp, Salmonella spp, S. aureus, and Klebsiella spp. It was also found that the inhibition of Noni seed extracts was greater than the leaf extracts. While Noni fruit extracts could only inhibit the growth of E. coli and Pseudomonas spp.[7]
Other studies have shown that Morinda citrifolia L. extract mixed with irreversible hydrocolloids could reduce the percentage of microorganisms in dental impression, and did not affect the surface quality or dimensional stability of dental impression. This study also noted that mixing of M. citrifolia L. extract with irreversible hydrocolloid powder was an alternative method to prevent contamination without sacrificing the quality of the dental impression.[8]
Pandan (Pandanus amaryllifolius) has many medicinal properties such as anti-seizure, anti-oxidant, anti-virus, anti-diabetes, anti-inflammatory, neuroprotective, analgesic, anti-neoplastic, anti-microbial, and anti-diuretic.[9] Pandan (P. amaryllifolius Roxb) is known to have inhibitory properties against bacteria S. aureus and E. coli.[10,11] It also inhibited P. aeruginosa growth.[10] Pandan leaves have been known and used by the ancestors of Indonesia as coloring and flavoring in food. Pandan leaves have a distinctive and attractive scent so these leaves are often added to food processing to enhance the aroma and provide the green color to the food. According to,[11] Pandan leaves are used in food preparation in Asian countries as a flavoring agent. It is mainly used to impart a certain aroma to rice preparations. It is then used in bread preparation to give a characteristic spicy taste.
The aim of this research were to determine the inhibition activities of combination of 96% ethanol extract of Noni fruit (EM) and 96% ethyl acetate extract of Pandan leaves (EPW) in the ratio 1:1 (v/v) against the bacteria isolated from dandruff and to found the potential combination of extracts as an anti-dandruff shampoo.
The content of chemical compounds in Noni includes scopoletin, octanoic acid, potassium, Vitamin A and C, terpenoids, anthraquinone, and carotene.[12] On the other hand, Pandan contains lignans, isoflavones, phenolic compounds, steroids, saponins, terpenoids, glycosides, tannins, and flavonoids.[9]
SUBJECTS AND METHODS
Subjects
The subjects in this study were bacterial isolates isolated from dandruff.
Isolation of bacteria from dandruff
Isolation was conducted by as much as 1 g dandruff diluted in 9 mL of sterile distilled water (dilution 10−1). One mL from 10−1 dilution was taken and put in 9 mL of sterile distilled water (dilution 10−2). In the same way, it made up to 10−7 dilution rate. From 10−2 to 10−7 dilution rate, 1 mL was taken and placed in a petri dish respectively. Then, in each petri dish as much as 15 mL Nutrient Agar (NA) (Oxoid, CM0003B, UK) liquid were added. After that, it was incubated at 37° C for 24 h.
Purification of bacterial isolates
Purification of bacterial isolates was carried out by colonies grown on the NA medium observed. Each colony with a different appearance was then isolated on NA medium to obtain pure isolate. The purification was used the streak plate method.
Extraction method
Extraction was done by the maceration method; the solvent used was ethyl acetate for Noni extraction and ethanol for Pandan extraction.
Antibacterial activity of the extract
Primary screening test for antibacterial activities of extract was carried out using paper disc dilution method with a diameter of paper 6 mm. The first, crude extracts 96% of ethanol Noni fruit extract (EM) and 96% ethyl acetate of Pandan leaves extract (EPW) diluted by adding Dimethyl Sulfoxide Solvent (DMSO) (1:1 w/v), respectively. After that, the combination of Noni extract and Pandan extract was made with a concentration ratio EM: EPW (3:1 v/v), (1:1 v/v), and (1:3 v/v), respectively. Paper disc was then dropped with extract volume of 10 μl respectively and placed on one of the quadrants on a petri dish containing NA media inoculated with a bacteria isolate. DMSO was used as the negative control, while antibiotic chloramphenicol 100 μg/mL (PA with 98% concentration) was used as the positive control. All cultures were then incubated at 37° C for 24 h. The level of inhibition was categorized by the diameter of inhibition zone. The diameter of the inhibition zone is categorized 7–15 mm (weak), 16–25 mm (moderate), while more than 25 mm (strong).[13]
Further screening
Further screening was done by testing the activity of the extract combinations of EM: EPW which have the highest activity to inhibit the bacterial isolates. It was conducted using a well dilution method. The concentration levels of extract combination made are 5%, 6.25%, 12.5%, 25%, 50%, and 100%. A well on one of the quadrants on a petri dish containing NA media inoculated with a test bacteria (the pure bacteria isolated from dandruff) was then dropped with extract combination (for example EM: EPW = 3:1 v/v) volume of 10 μl, respectively.
Identification of the bacteria isolates
The identification of bacteria isolated from dandruff was done by: First, Gram staining to determine cell morphology and Gram category. Second, identification down to the species level was carried out at Balai Laboratorium Kesehatan (BLK) Yogyakarta, Indonesia.
RESULTS
Isolation and purification of bacterial isolates
Based on isolation, it was known that the density of bacteria isolated from the dandruff sample of 3.4 × 107 CFU/g. The density of bacteria in dandruff indicates the level of microorganism contamination on the scalp. In fact, the cause of dandruff is fungi, yet bacteria can be present on the scalp when secondary infection occurs, for example, sores on the scalp due to scratching. A total of 28 bacterial isolates was successfully purified from the dandruff sample.
Antibacterial activity of the extract combinations
Primary screening
Based on the primary screening test for antibacterial activities of both extract combinations, it was found that the extract combination of EM: EPW (1:1 v/v) had the best inhibition activities compared to extract combinations with a ratio EM: EPW (3:1 v/v) and a ratio (1:3, v/v). The inhibition test results of EM: EPW (1:1, v/v) are presented in Table 1.
Table 1.
The inhibition test of noni fruit extract combined with pandan leaves extract against bacteria isolated from dandruff
| Number | Code of bacteria isolates | Inhibition zone diameter (mm) on bacterial isolates results of extract purification | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control (−) DMSO | Control (+) cloramphenicol 100 µg/ml | EM: EPW (3:1, v/v) | EM: EPW (1:1, v/v) | EM: EPW (1: 3, v/v) | ||
| 1 | B1a | - | 30* | 15 | 15 | 15 |
| 2 | B1b | - | 28* | 16 | 16 | 16 |
| 3 | B2 | - | 7 | 18 | 19 | 18 |
| 4 | B3 | - | 16 | 13 | 13 | 13 |
| 5 | B4a | - | 9 | 30* | 29* | 30* |
| 6 | B4b | - | 29* | 30* | 35* | 35* |
| 7 | B4c | - | 17 | 31* | 31* | 30* |
| 8 | B4d | - | 28* | 16 | 13 | 12 |
| 9 | B5 | - | 17 | 12 | 14 | 13 |
| 10 | B6a | - | 15 | 11 | 20 | 16 |
| 11 | B7 | - | 7 | 15 | 15 | 13 |
| 12 | B8 | - | 18 | 15 | 16 | 15 |
| 13 | B9 | - | 30* | 18 | 18 | 18 |
| 14 | B10a | - | 14 | 23 | 24 | 23 |
| 15 | B11 | - | 18 | 13 | 15 | 15 |
| 16 | B13 | - | 9 | 20 | 20 | 20 |
| 17 | B14a | - | 35* | 33* | 35* | 35* |
| 18 | B14b | - | 20 | 18 | 17 | 16 |
| 19 | B14c | - | - | - | - | - |
| 20 | B16a | - | 15 | 16 | 15 | 15 |
| 21 | B16b | - | - | 23 | 24 | 24 |
| 22 | B16c | - | 16 | - | - | - |
| 23 | B16d | - | 21 | 13 | 13 | 11 |
| 24 | B17a | - | 24 | 11 | 14 | 15 |
| 25 | B17b | - | 31* | 13 | 14 | 15 |
| 26 | B18 | - | 15 | 15 | 15 | 15 |
| 27 | B19 | - | 19 | 19 | 18 | 17 |
| 28 | B20 | - | 17 | 16 | 17 | 17 |
*Categories strong inhibition zone.[13] DMSO: Dimethyl Sulfoxide, EM: Noni fruit extract, EPW: Pandan leaves extract
Further screening
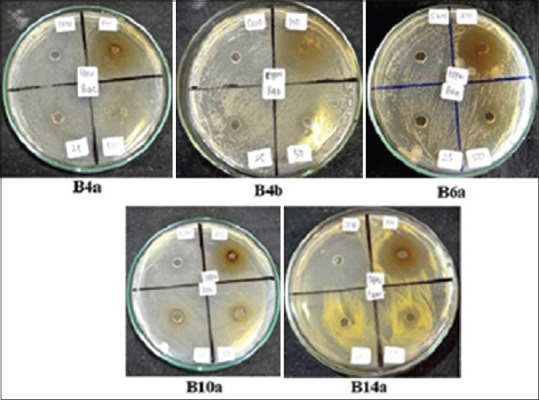
The inhibition test results of EM: EPW (1:1, v/v) are presented in Table 2 and Figure 1.
Table 2.
The inhibition of extract combination of noni fruit extract with pandan leaves extract (1:1, v/v) against bacteria isolated from dandruff
| Number | Code of bacteria isolates | Inhibition zone diameter (mm) on bacterial isolates on levels of extract combination EM: EPW (1: 1, v/v) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Control (−) DMSO | Control (+) chloramphenicol 100 µg/ml | EM + EPW (100%) | EM + EPW (50%) | EM + EPW (25%) | EM + EPW (12.5%) | EM + EPW (6.25%) | EM + EPW (5%) | ||
| 1 | B1a | - | 28* | 15 | 10 | 8 | 8 | - | - |
| 2 | B1b | - | 22 | 13 | 11 | 8 | 7 | - | - |
| 3 | B2 | - | - | 8 | 8 | - | - | - | - |
| 4 | B3 | - | 15 | 13 | 7 | 8 | - | - | - |
| 5 | B4a | - | 10 | 30* | 15 | 8 | 10 | - | - |
| 6 | B4b | - | 20 | 30* | 13 | 10 | 9 | - | - |
| 7 | B4c | - | - | 17 | 15 | 15 | 10 | - | - |
| 8 | B4d | - | 20 | 13 | - | - | - | - | - |
| 9 | B5 | - | 18 | 11 | 7 | - | - | - | - |
| 10 | B6a | - | 15 | 30* | 20 | 15 | 10 | - | - |
| 11 | B7 | - | 9 | 14 | 10 | 9 | 8 | 8 | - |
| 12 | B8 | - | 20 | 12 | - | - | - | - | - |
| 13 | B9 | - | 30* | 20 | - | - | - | - | - |
| 14 | B10a | - | 15 | 29* | 13 | 10 | 8 | - | - |
| 15 | B11 | - | 18 | 15 | 8 | 7 | 7 | - | - |
| 16 | B13 | - | - | 19 | 10 | 7 | - | - | - |
| 17 | B14a | - | - | 29* | 10 | 9 | - | - | - |
| 18 | B14b | - | 18 | 11 | 10 | 9 | 9 | 8 | |
| 19 | B14c | - | - | - | - | - | - | - | - |
| 20 | B16a | - | 14 | 14 | 11 | 8 | 7 | - | - |
| 21 | B16b | - | - | 20 | 13 | 10 | 8 | - | - |
| 22 | B16c | - | - | - | - | - | - | - | - |
| 23 | B16d | - | 23 | 14 | 12 | 9 | - | - | - |
| 24 | B17a | - | 18 | 15 | 9 | 7 | 7 | - | - |
| 25 | B17b | - | - | - | - | - | - | - | - |
| 26 | B18 | - | 14 | 15 | 11 | 6 | - | - | - |
| 27 | B19 | - | 15 | 10 | 8 | - | - | - | - |
| 28 | B20a | - | 15 | 15 | 10 | 8 | - | - | - |
*Categories strong inhibition zone.[13] DMSO: Dimethyl Sulfoxide, EM: Noni fruit extract, EPW: Pandan leaves extract
Figure 1.
The inhibition of extract combination of EM: extract of Pandan leaves (1: 1 v/v) against bacteria isolated from dandruff
Identification of the bacteria isolates
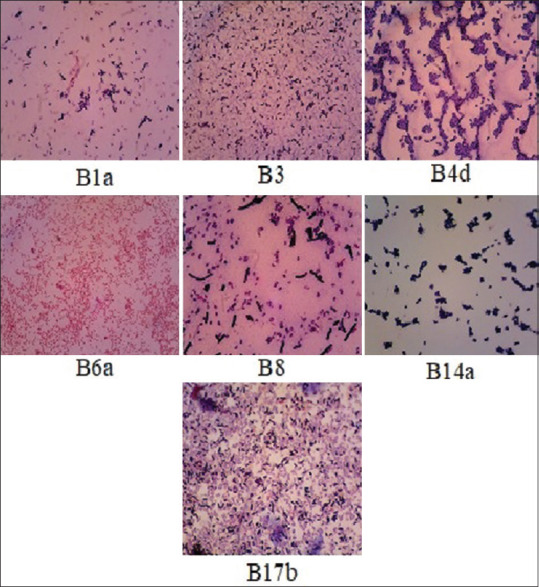
Before the identification of the isolates to the species level, the initial identification was first performed by Gram staining. The Gram staining is intended to determine cell morphology of the bacteria. The identification results are presented in Table 3 and Figure 2.
Table 3.
The identification of bacteria isolated from dandruff
| Group | Isolates code | Identification results |
|---|---|---|
| 1 | B1a=B4b=B4c=B13=B14b=B14c=B17a | Bacillus licheniformis |
| 2 | B3=B4a=B16a=B16b=B16d=B18=B20 | Bacillus megaterium |
| 3 | B2=B4d=B7=B10a=B16c | Micrococcus sp |
| 4 | B8=B9=B11 | Bacillus alvei |
| 5 | B14a | Stomatococcus sp |
| 6 | B1b=B5=B17b=b19 | Bacillus subtilis |
| 7 | B6a | Pseudomonas sp |
Figure 2.
The Gram staining of bacteria isolated from dandruff
DISCUSSION
Antibacterial activity of the extract combinations
Primary screening
Based on Table 1, it was found that the extract combination of EM: EPW (1:1, v/v) was able to inhibit 26 of 28 bacteria isolates (92,9%) with a diameter of the inhibition zone ranging from 13 mm to 35 mm. Besides that, it was discovered that as many as four isolates could be strongly inhibited, namely B4a with inhibition zone diameter of 29 mm, B4b and B14a (35 mm respectively), and B4c (31 mm). Therefore, a further test used combination of EM: EPW (1:1, v/v) with a ratio of various concentrations: 5%, 6.25%, 12.5%, 25%, 50%, and 100%.
Further screening
Based on Table 2, it was noted that 89.3% of bacterial isolates were inhibited by the combination of EM: EPW = 1: 1 (v/v). The diameter of the inhibition zone formed ranged from 8 to 30 mm. Among five isolates from 25 isolates inhibited by extract combinations, those strongly inhibited were B4a, B4b, and B6a (30 mm respectively), B10a, and B14a (29 mm respectively).
Positive control used in antibacterial test was chloramphenicol 100 μg/ml where negative control was DMSO. The method used was a well method diffusion of the extract combination as much as 10 mL/well. When compared to the positive control, it was revealed that 10 isolates were inhibited more strongly by a combination of Noni fruit and Pandan leaves extracts than chloramphenicol [Table 2].
The result was consistent with the previous study by[14] revealing that the methanol extract, ethanol, and ethyl acetate from Noni fruit and Pandan leaves acted as antibacterial and inhibited 12 bacteria test, it was also known that methanol extracts had the greatest inhibition. Based on research by,[15] it was noted that the ether and ethanol extracts of Noni showed antibacterial activities and inhibited the growth of E. coli, B. subtilis, and S. aureus with inhibition zone diameter ranging from 17 to 20 mm.
Other study concluded that the hydroethanolic of Noni fruit extract inhibited 10 bacteria test.[5] Besides that, ethanol extract of Noni fruit exhibited antibacterial effects against Fusobacterium nucleatum.[16]
On the other hand, extracts of Pandan leaves comprising ethanol or ethyl acetate extract could inhibit the growth of P. aeruginosa and B. subtilis.[17] Another study concludes that crude extract of P. amaryllifolius leaves showed antibacterial activities toward S. aureus ATCC 25923 but not to E. coli ATCC 25922 and P. aeruginosa ATCC 27853.[10]
Identification of the bacteria isolates
Table 3 shows that all of 28 isolates of bacteria isolated from dandruff could be grouped into seven categories. Furthermore, each representative further isolates was identified in BLK Yogyakarta. The identification results of the seven group bacteria were Bacillus licheniformis, Bacillus megaterium, Bacillus alvei, B. subtilis, Micrococcus sp, Stomatococcus sp, and Pseudomonas sp.
The types of active compounds in Noni fruit extract were alkaloids (1.97% w/w), phenol (12.50% w/w), and flavonoids (8.61% w/w), while the contents of the Pandan leaves extract are phenol (9.42% w/w) and flavonoids (4.39% w/w). Thus, the contents of phenols and flavonoids were higher in Noni fruit extract.[18]
This research supports the previous study which concluded that Noni leaves extract contains various compounds including tannins, phenols, alkaloids, flavonoids, glycosides, steroids, and terpenoids.[10]
This research supports the previous study which concludes that Noni leaves extract contains various compounds including tannins, phenols, alkaloids, flavonoids, glycosides, steroids, and terpenoids.[19] Meanwhile, Pandan leaves water extract contains bioactive compounds of tannins, alkaloids, flavonoids, saponins, and polyphenols.[20]
The alkaloid was capable of inhibiting cell wall synthesis which will cause cell lysis so that the cell dies. The mechanism happens by way of disrupting the peptidoglycan constituent components on a bacterial cell so that the layer of the cell wall is not perfectly formed, thus causing cell lysis either prone to physical or osmotic and cause cell death.[21] Besides that, alkaloids showed antibacterial activity as well as inhibiting ATP-dependent transport of compounds across the cell membrane.[22]
Other study showed that the phenolic and flavonoid content in P. amaryllifolius (Roxb.) leaves extract could act as antibacterial.[23] Phenolic compounds had antimicrobial activity because they possess antioxidant effects, possibly involving proton exchange processes in the antimicrobial activity.[24] Inhibition mechanisms of phenolics include inhibiting enzymes. It was suggested that this inhibition takes place through reactions with sulfhydryl groups on the proteins.[25]
Flavonoid compound tends to bind the proteins, thus disrupting the process of metabolism. Compounds of flavonoids may damage the cytoplasmic membrane, which causes leaking of important metabolites and disabling bacterial enzyme systems. This damage allows the nucleotides and amino acids on bacteria seeped out and prevents the inclusion of the active substances into the cell and causes cell death.[26]
CONCLUSIONS
Based on the study, it can be concluded that the combination of Noni fruit extract and Pandan leaves extract can inhibit the growth of bacteria isolated from dandruff. Therefore, combination of both extracts potential as an anti-dandruff shampoo.
Financial support and sponsorship
The authors would like to thank the Indonesian Ministry of Research, Technology and Higher Education, for providing research fund with “Hibah Bersaing” program with the Research Agreement Letter No. 007/K6/KM/SP2H/PENELITIAN_BATCH 1/2015, dated March 30th, 2015.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Niharika A, Aquicio JM, Anand A. Antifungal properties of Neem (Azardirachta indica) leaves extract to treat hair dandruff. E-Int Sci Res J. 2010;2:244–52. [Google Scholar]
- 2.Pamungkas BT, Muktiwardojo M, Rostinawati T. Review: Antibacterial activities of various parts of Mengkudu (Morinda citrifolia L.) plants on some species of bacteria. J Trop Pharm Chem. 2019;4:244–9. [Google Scholar]
- 3.Usha R, Sashidharan S, Palaniswamy M. Antimicrobial activity of a rarely known species, Morinda citrifolia L. Ethnobot Leaf. 2010;14:306–11. [Google Scholar]
- 4.Kumar KT, Panda DS, Nanda UN, Khuntia S. Evaluation of antibacterial, antifungal and anthelmintic activity of Morinda citrifolia L.(Noni) Int J Pharmtech Res. 2010;2:1030–2. [Google Scholar]
- 5.Srinivasahan V, Durairaj B. Antimicrobial activities of hydroethanolic extract of Morinda citrifolia fruit. Int J Curr Microbiol App Sci. 2014;3:26–33. [Google Scholar]
- 6.Sharma MC, Sharma S. Phytochemical screening of methanolic extract and antibacterial activity of Eclipta alba and Morinda citrifolia L. Middle East J Sci Res. 2010;6:445–9. [Google Scholar]
- 7.Sunder J, Singh DR, Jeyakumar S, Kundu A, De AR. Antibacterial activity in solvent extract of different parts of Morinda citrifolia plant. J Pharm Sci Res. 2011;3:1404–7. [Google Scholar]
- 8.Ahmed AS, Charles PD, Cholan R, Russia M, Surya R, Jailance L. Antibacterial efficacy and effect of Morinda citrifolia L. mixed with irreversible hydrocolloid for dental impressions: A randomized controlled trial. J Pharm Bioallied Sci. 2015;7:S597–9. doi: 10.4103/0975-7406.163562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minh NP, Vo TT, Man LV, Phong TD, Toan NV, Nam VH. Green pigment extraction from Pandan (Pandanus amaryllifolius) and its application in food iIndustry. J Pharm Sci Res. 2019;11:925–9. [Google Scholar]
- 10.Dumaoal OS, Alaras LB, Sarah KG, Depadua AA, Pulmones CJ. In vitro activity of Pandan (Pandanus amaryllifolius Roxb.) leaves crude extract against selected bacterial isolates. Nat Peer Rev J. 2010;4:102–24. [Google Scholar]
- 11.Faras AF, Wadkar SS, Ghosh JS. 2014. Effect of extract of Pandanus amaryllifolius (Roxb.) on growth of Escherechia coli and Micrococcus (Staphylococcus) aureus. Int Food Res J. 2014;1:421–3. [Google Scholar]
- 12.Aruna MS, Rao NR, Deepthi B, Prasanna JL, Prabha MS. Ashyuka: A hub of medicinal values. IJKBR. 2013;4:1043–9. [Google Scholar]
- 13.Nedialkova D, Naidenova M. Screening the antimicrobial activity of Actinomycetes strains isolated from antarctica. J Cult Coll. 2005;4:29–35. [Google Scholar]
- 14.Natheer SE, Sekar C, Amutharaj P, Rahman MS, Khan KF. Evaluation of antibacterial activity of Morinda citrifolia, Vitex trifolia and Chromolaena odorata. Afr J Pharm Pharmaco. 2012;6:783–8. [Google Scholar]
- 15.Devi CH, Khrishna DG. Phytochemical screening, antibacterial, antifungal and antihelmintic activity of Morinda citrifolia stem. J Pharmacogn Phytochem. 2013;2:155–17. [Google Scholar]
- 16.Boel DT. Antibacterial effectiveness of noni extract (Morinda citrifolia L.) on the growth of Fusobacterium nucleatum (ATCC® 25586™) as an alternative material for root canal irrigation- An in vitro study. J Evolution Med Dent Sci. 2019;8:1046–8. [Google Scholar]
- 17.Murhadi M, Suharyono AS, Susilawati S. Antibacterial activity of (Syzygium Polyanta and amaryllifolius) leaf extracts. JTIP. 2007;18:17–24. [Google Scholar]
- 18.Ambarwati A, Sujono TA, Sintowati R. Combination extracts of Pandan Wangi Leaves (Pandanus amaryllifolius Roxb.) and Mengkudu Fruits (Morinda citrifolia) as Antifungal Against Fungi Causes Dandruff. JIFI. 2017;15:96–101. [Google Scholar]
- 19.Kakad SL, Pise SS, Dhembares AJ. Evaluation of phtochemical, antibacterial, antifungal activities of leaf extracts of Morinda citrifolia (Linn) Der Pharmacia Sinica. 2015;6:19–2. [Google Scholar]
- 20.Aini R, Mardiyaningsih A. Pandan leaves extract (Pandanus amaryllifolius Roxb) as a food preservative. JKKI. 2016;7:166–73. [Google Scholar]
- 21.Enwa FO. Mechanisms of antimicrobial actions of phytochemicals against enteric pathogens – A review. J Pharm Chem Biol Sci. 2014;2:77–85. [Google Scholar]
- 22.Mabhiza D, Chitemerere T, Mukanganyama S. Antibacterial properties of alkaloid extracts from callistemon citrinus and vernonia adoensis against staphylococcus aureus and pseudomonas aeruginosa. Int J Med Chem. 2016;2016:6304163. doi: 10.1155/2016/6304163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suwannakul S, Chaibenjawong P, Suwannakul S. Antioxidant anti-cancer and antimicrobial activitiesof ethanol Pandanus amaryllifolius Roxb. leaf extract (In vitro)-A potential medical application. J Int Dent Med Res. 2018;11:383–9. [Google Scholar]
- 24.Elo H, Kuure M, Pelttari E. Correlation of the antimicrobial activity of salicylaldehydes with broadening of the NMR signal of the hydroxyl proton. Possible involvement of proton exchange processes in the antimicrobial activity. Eur J Med Chem. 2015;92:750–3. doi: 10.1016/j.ejmech.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 25.Coppo E, Marchese A. Antibacterial activity of polyphenols. Curr Pharm Biotechnol. 2014;15:380–90. doi: 10.2174/138920101504140825121142. [DOI] [PubMed] [Google Scholar]
- 26.Levison ME. Pharmacodynamics of antimicrobial drugs. Infect Dis Clin North Am. 2004;18:451–65. doi: 10.1016/j.idc.2004.04.012. [DOI] [PubMed] [Google Scholar]


