Abstract
Degradation of collagenous extracellular matrix by collagenase 1 (also known as matrix metalloproteinase 1 [MMP-1]) plays a role in the pathogenesis of various destructive disorders, such as rheumatoid arthritis, chronic ulcers, and tumor invasion and metastasis. Here, we have investigated the role of distinct mitogen-activated protein kinase (MAPK) pathways in the regulation of MMP-1 gene expression. The activation of the extracellular signal-regulated kinase 1 (ERK1)/ERK2 (designated ERK1,2) pathway by oncogenic Ras, constitutively active Raf-1, or phorbol ester resulted in potent stimulation of MMP-1 promoter activity and mRNA expression. In contrast, activation of stress-activated c-Jun N-terminal kinase and p38 pathways by expression of constitutively active mutants of Rac, transforming growth factor β-activated kinase 1 (TAK1), MAPK kinase 3 (MKK3), or MKK6 or by treatment with arsenite or anisomycin did not alone markedly enhance MMP-1 promoter activity. Constitutively active MKK6 augmented Raf-1-mediated activation of the MMP-1 promoter, whereas active mutants of TAK1 and MKK3b potently inhibited the stimulatory effect of Raf-1. Activation of p38 MAPK by arsenite also potently abrogated stimulation of MMP-1 gene expression by constitutively active Ras and Raf-1 and by phorbol ester. Specific activation of p38α by adenovirus-delivered constitutively active MKK3b resulted in potent inhibition of the activity of ERK1,2 and its upstream activator MEK1,2. Furthermore, arsenite prevented phorbol ester-induced phosphorylation of ERK1,2 kinase-MEK1,2, and this effect was dependent on p38-mediated activation of protein phosphatase 1 (PP1) and PP2A. These results provide evidence that activation of signaling cascade MKK3-MKK3b→p38α blocks the ERK1,2 pathway at the level of MEK1,2 via PP1-PP2A and inhibits the activation of MMP-1 gene expression.
Matrix metalloproteinases (MMPs) play an important role in the pathogenesis of disorders in which excessive degradation of extracellular matrix (ECM) occurs, such as rheumatoid arthritis, osteoarthritis, autoimmune blistering skin diseases, and tumor cell invasion and metastasis (44). The MMP gene family consists of at least 20 structurally related zinc-dependent neutral endopeptidases, collectively capable of degrading essentially all components of the ECM. According to their substrate specificities and structure, MMPs are often divided into subgroups of collagenases, stromelysins, gelatinases, membrane-type MMPs, and other MMPs (25, 52). Collagenase 1 (henceforth designated MMP-1) is expressed by several types of normal and malignant cells, and it is one of the few proteolytic enzymes capable of degrading native fibrillar collagens. Increased expression of MMP-1 has been shown to correlate with poor prognosis of malignant tumors, including gastric and colon carcinomas (22, 35).
MMP-1 gene expression is stimulated at the transcriptional level by various growth factors, cytokines, and tumor promoters via a promoter segment located between −95 to −72 bp upstream of the transcription initiation site, which contains adjacent binding sites for AP-1 and ETS transcription factors (52, 54). The expression and transactivation capacities of AP-1 and ETS transcription factors are regulated by mitogen-activated protein kinase (MAPK) pathways, a large network of signaling modules activated by a variety of stimuli (17, 28). Three distinct MAPK pathways have been characterized in detail: extracellular signal-regulated kinase 1 (ERK1)/ERK2 (designated ERK1,2), c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), and p38. The ERK1,2 pathway (Raf→MEK1,2→ERK1,2) is activated by mitogens via Ras and by phorbol esters via protein kinase C. The stress-activated MAPK pathways JNK/SAPK (MEK kinase 1,3→MAPK kinase 4,7 [MKK4,7]→JNK1,2,3) and p38 (MAPK kinase kinase [MAPKKK]→MKK3,6→p38α,β,γ,δ) are activated by cellular stress, e.g., UV light, osmotic and oxidative stress, and inflammatory cytokines (17, 28). The activation of MAPKs requires phosphorylation of conserved tyrosine and threonine residues by dual-specificity MAPK kinases, which in turn are activated by phosphorylation of two serine residues by upstream MAPKKKs. Phosphorylation of MAPKs results in their translocation to the nucleus, where they activate transcription factors by phosphorylation. Activity of MAPK kinases and MAPKs is inhibited by dephosphorylation of the regulatory serine, threonine, and tyrosine residues by serine/threonine, tyrosine, and dual-specificity phosphatases, respectively (see reference 26). Serine/threonine protein phosphatase 1 (PP1) and PP2A inhibit the activity of the ERK1,2 pathway by dephosphorylation of MEK1,2 and ERK1,2 (26, 32, 48). Furthermore, inhibition of PP1 and PP2A activity results in activation of ERK1,2 and in enhancement of AP-1-dependent gene expression (14, 32, 48).
Recent studies have shown that the ERK1,2 pathway mediates the activation of the MMP-1 promoter via an AP-1 element by Ras, serum, phorbol ester, insulin, and oncostatin M (15, 27, 42) and that specific activation of ERK1,2 induces MMP-1 production by fibroblasts (39). On the other hand, activity of p38 MAPK is required for induction of MMP-1 gene expression by interleukin-1, ceramide, and the tumor promoter okadaic acid (40, 41, 51). Furthermore, blocking the JNK or ERK1,2 pathway also inhibits ceramide- and okadaic acid-elicited induction of MMP-1 expression, suggesting that coordinate activation of multiple MAPK pathways determines the rate of MMP-1 gene transcription (40, 51). However, the interplay between distinct MAPK pathways in the regulation of MMP-1 gene expression is not clear. Here, we show that in contrast to the ERK1,2 pathway, activation of the JNK and p38 pathways alone is not sufficient to markedly activate MMP-1 gene transcription. Furthermore, we show that activation of the MKK3→p38α pathway inhibits phorbol ester-, Ras-, and Raf-1-elicited MMP-1 promoter activation and that this involves PP1- and PP2A-mediated MEK1,2 inactivation. These results provide evidence for a novel role of p38α in the inhibition of ERK1,2-mediated induction of MMP-1 gene expression.
MATERIALS AND METHODS
Cell cultures
Human skin fibroblast cultures were established from a healthy male volunteer donor (aged 28 years). Mouse NIH 3T3 fibroblasts, human neonatal foreskin fibroblasts, and HeLa cells were obtained from the American Type Culture Collection (Rockville, Md.). Establishment of KMS-6 and KMST-6/Ras fibroblasts (24) and embryonal fibroblasts from JNK2−/− mice (40) has been described previously. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS), 2 mM glutamine, 100 IU of penicillin G/ml, and 100 μg of streptomycin/ml, except NIH 3T3 cells, which were cultured in a similar medium supplemented with 10% calf serum.
Reagents and antibodies.
12-O-tetradecanoyl-13-phorbol acetate (TPA), anisomycin, sodium m-arsenite, and human recombinant transforming growth factor β1 (TGF-β1) were purchased from Sigma Chemical Co. (St. Louis, Mo.). Calyculin A, okadaic acid, p38 inhibitor SB203580, and MEK1,2 inhibitor PD98059 were from Calbiochem (San Diego, Calif.). Phospho-specific MEK1,2, ERK1,2, JNK, and p38 antibodies and antibodies against total MEK1,2, ERK1,2, and p38 antibodies were obtained from New England Biolabs (Beverly, Mass.). Antibody against c-Raf-1 was from Santa Cruz Biotechnology (Santa Cruz, Calif.). Antibody against the catalytic subunit of PP2A (6) was kindly provided by David Brautigan (University of Virginia, Charlottesville). Antibody against the catalytic subunit of PP1 was kindly provided by John Eriksson, University of Turku, Turku, Finland.
RNA analysis.
Total cellular RNA was isolated from cells using the RNAeasy kit (Qiagen). Aliquots of total RNA (5 to 15 μg) were fractionated on 0.8% agarose gels containing 2.2 M formaldehyde, transferred to a Zeta probe filter (Bio-Rad, Richmond, Calif.) by vacuum transfer (VacuGene XL; LKB, Bromma, Sweden), and immobilized by heating at 80°C for 30 min. The filters were prehybridized for 2 h and subsequently hybridized for 20 h with a 2.0-kb human MMP-1 cDNA (18) or 1.3-kb rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA (12) labeled by [α-32P]dCTP by random priming. The filters were washed, with a final stringency of 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) at 60 or 53°C. The cDNA-mRNA hybrids were visualized by autoradiography, and the levels of MMP-1 mRNA were quantitated by scanning densitometry with MCID software (Imaging Research, Inc., St. Catharines, Ontario, Canada), and corrected for the levels of GAPDH mRNA in the same samples.
Transient transfections.
Confluent NIH 3T3 cells were transiently transfected with calcium phosphate-DNA coprecipitation, followed by 2 min of glycerol shock as previously described (53). Human newborn skin fibroblasts were transiently transfected with FuGene6 reagents (Roche Biochemicals, Mannheim, Germany). In cotransfection experiments, the cells were transiently transfected with 2 μg of the MMP-1 promoter-chloramphenicol transferase (CAT) construct -2278CLCAT (13) (kindly provided by Steven Frisch, Washington University, St. Louis, Mo.) in combination with the expression plasmids for constitutively active forms of Raf-1 (RafBXB) (2) (kindly provided by U. Rapp, University of Würzburg, Würzburg, Germany), Rac (RacQL) (7) (kindly provided by J. Lacal, University of Madrid, Madrid, Spain), TGF-β-activated kinase 1 (TAK1) (ΔNTAK1) (58) (kindly provided by E. Nishida, Kyoto University, Kyoto, Japan), MKK6 [MKK6(E)](38) (kindly provided by R. Davis, University of Massachusetts, Worcester), MKK3 [MKK3(E)], MKK3b [MKK3b(E)], and wild-type p38α (20). Control cultures were cotransfected in parallel with the respective empty expression vectors. As an indicator of promoter activity, CAT activity was assayed as described previously (54). The transfection efficiency was monitored by cotransfecting the cells with 4 μg of Rous sarcoma virus–β-galactosidase construct and correcting the CAT activities for β-galactosidase activity. The expression of constitutively active Raf-1 in cells transfected with RafBXB was determined by Western blot analysis of cell lysates with a specific antibody.
Determination of MAPK activity.
The activation of MEK1,2, ERK1,2, JNK, and p38 was determined by Western blotting with antibodies specific for phosphorylated, activated forms of these kinases. The cultures were maintained for 18 h in medium supplemented with 1% FCS, treated as indicated, and lysed in 100 μl of Laemmli sample buffer. Samples were then sonicated, fractionated by SDS–10% polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech). Western blotting was performed as described previously (39, 40), with phospho-specific antibodies with peroxidase-conjugated secondary antibodies visualized by enhanced chemiluminescence (Amersham Pharmacia Biotech).
To determine the activation of p38 by constitutively active forms of TAK1 and Rac, NIH 3T3 cells were transiently transfected with 4 μg (each) of the expression vectors for constitutively active TAK1 (ΔNTAK1) and Rac [Rac(QL)] together with the expression vector for p38α containing a Flag tag. To assay the activation of different p38 isoforms by constitutively active MKK3b and MKK6b, NIH 3T3 cells were transiently transfected with expression vectors MKK3b(E) and MKK6b(E) (19) in combination with expression vectors for Flag-tagged wild-type p38α, p38β, p38γ, and p38δ (37). Control cultures were transfected with the corresponding empty expression vectors. The cultures were maintained for 36 h in 1% FCS–DMEM and harvested in 300 μl of lysis buffer (phosphate-buffered saline [pH 7.4], 1% NP-40, 0.5% sodium deoxycholate, 1 mM Na3VO4, 0.1% SDS, 1 mM EDTA, 1 mM EGTA, 20 mM NaF, 1 mM phenylmethylsulfonyl fluoride, and 1 μg [each] of aprotinin, leupeptin, and pepstatin per ml), p38 isoforms were immunoprecipitated with anti-Flag M2 monoclonal antibody (Sigma) and coupled to protein G-Sepharose (Pharmacia), and their activity was determined in a kinase assay with [γ-32P]ATP with glutathione S-transferase (GST)–activating transcription factor 2 (ATF-2) as a substrate, as described previously (23). The samples were resolved by SDS–12.5% polyacrylamide gel electrophoresis, and phosphorylated GST–ATF-2 was visualized by autoradiography.
Determination of PP1-PP2A activity and expression.
Confluent human skin fibroblasts were maintained for 24 h in medium supplemented with 1% FCS, treated as indicated, and harvested in phosphatase lysis buffer (20 mM HEPES [pH 7.4], 10% glycerol, 0.1% NP-40, 30 mM β-mercaptoethanol, 1 mM EGTA). Cell lysate was homogenized by being passed five times through a 20-gauge needle, and equal amounts of the protein were used to determine the PP1-PP2A phosphatase activity with 32P-labeled glycogen phosphorylase as a substrate with the Protein Phosphatase Assay system (Life Technologies, Paisley, United Kingdom). The levels of PP1 and PP2A catalytic subunits were determined by Western blot analysis using specific antibodies (see above).
Infection of fibroblasts with recombinant adenoviruses.
Recombinant replication-deficient adenovirus RAdlacZ (RAd35) (55), which contains the Escherichia coli β-galactosidase (lacZ) gene under the control of the cytomegalovirus immediate-early promoter, and empty adenovirus RAd66 (55) were kindly provided by Gavin W.G. Wilkinson (University of Cardiff, Cardiff, Wales). Construction and characterization of recombinant adenoviruses containing the coding region of mutated, constitutively active human MKK3b [RAdMKK3b(E)] and MKK6b [RAdMKK6b(E)], and wild-type p38α genes driven by the cytomegalovirus immediate-early promoter have been described previously (49). In our experiments, 5 × 105 KMST-6/Ras fibroblasts in suspension were infected as previously described (39) with recombinant adenoviruses at a multiplicity of infection of 500, which was found to give 100% transduction efficiency with RAdlacZ, plated, and incubated for 18 h. The culture medium (DMEM with 1% FCS) was changed, and the cultures were incubated for an additional 24 h. The cell layers were harvested and used for determination of MAPK activation by Western blot analysis with phospho-specific antibodies, as described above.
RESULTS
Differential regulation of MMP-1 promoter activity by mitogen- and stress-activated MAPKs.
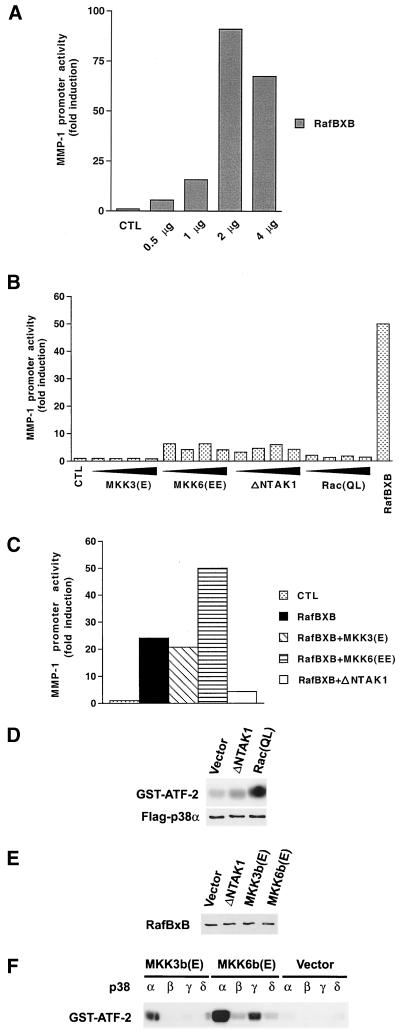
To study the roles of distinct MAPK pathways in the regulation of MMP-1 promoter activity, we initially cotransfected murine NIH 3T3 fibroblasts with -2278CLCAT reporter construct, which contains 2.278 kb of the 5′-flanking region of the human MMP-1 gene linked to the CAT reporter gene, in combination with expression plasmids for constitutively active mutants of different upstream kinases of the ERK1,2, JNK, and p38 pathways. As shown in Fig. 1A, expression of constitutively active Raf-1 (RafBXB) resulted in dose-dependent enhancement of MMP-1 promoter activity, indicating that activation of the ERK1,2 pathway alone is sufficient to induce MMP-1 gene transcription. In contrast, expression of constitutively active MKK3 or Rac had no marked effect on MMP-1 promoter activity (Fig. 1B). In comparison, constitutively active MKK6 and TAK1 enhanced MMP-1 promoter activity, but clearly less potently than constitutively active Raf-1.
FIG. 1.
Modulation of Raf-1-induced MMP-1 promoter activity by stress-activated MAPK pathways. (A to C) After transfection and glycerol shock, the cultures were maintained for 36 h in 1% FCS, and CAT activity was measured from cell lysates. Transfection efficiency was monitored by cotransfecting the cells with 4 μg of RSV–β-galactoside construct. The values represent the mean of at least two independent experiments, each performed in duplicate. (A) NIH 3T3 fibroblasts were transiently transfected with indicated amounts of the expression vector for the constitutively active form of Raf-1 (RafBXB) together with the -2278CLCAT construct (2 μg), containing 2,278 bp of the human MMP-1 gene promoter linked to the CAT gene. (B) NIH 3T3 fibroblasts were transiently transfected with increasing amounts (0.5, 1, 2, and 4 μg) of expression vectors for constitutively active MKK3 [MKK3(E)], MKK6 [MKK6(EE)], TAK1 (ΔNTAK1), and Rac (RacQL), or with Raf-1 (RafBXB) (2 μg) in combination with MMP-1 promoter-CAT construct -2278CLCAT (2 μg). (C) NIH 3T3 fibroblasts were transiently transfected with MMP-1 promoter-CAT construct -2278CLCAT (1 μg) with the expression vector for constitutively active Raf-1 (RafBXB) alone or in combination with expression vectors for constitutively active MKK3, MKK6, and TAK1 (1 μg each). (D) NIH 3T3 fibroblasts were transiently transfected with 4 μg of expression vectors for the constitutively active form of TAK1 (ΔNTAK1) and Rac [Rac(QL)] together with the expression vector for Flag-tagged p38α. Activity of p38α immunoprecipitated from cell lysates with anti-Flag antibody was determined with GST–ATF-2 as substrate. The levels of p38α in cell lysates were determined by Western blot analysis with anti-Flag antibody. (E) NIH 3T3 fibroblasts were transiently transfected with expression vectors for the constitutively active form of TAK1 (ΔNTAK1) (2 μg), MKK3b [MKK3b(E)] and MKK6b [MKK6b(E)] (4 μg), together with the expression vector for constitutively active Raf-1 (RafBxB) (2 μg). The level of RafBxB in cell lysates was determined by Western blot analysis with c-Raf-1 antibody. (F) NIH 3T3 fibroblasts were transiently transfected with 4 μg of expression vectors for constitutively active forms of MKK3b [MKK3b(E)] and MKK6b [MKK6b(E)], together with expression vectors for wild-type p38 isoforms (p38α, -β, -γ, and -δ) (4 μg each). Control cultures were transfected with the corresponding empty expression vector. Cultures were then maintained for 36 h in 1% FCS–DMEM, and the activity of p38 isoforms immunoprecipitated from cell lysates with anti-Flag antibody was determined using GST–ATF-2 as substrate. CTL, empty expression vector.
We have previously shown that maximal activation of MMP-1 expression by okadaic acid and ceramide requires coordinate activation of the ERK1,2, JNK, and/or p38 pathways (40, 51). In this context, we studied the effect of simultaneous activation of these pathways on MMP-1 promoter activity. As shown in Fig. 1C, expression of constitutively active MKK3 had no marked effect on RafBXB-induced MMP-1 promoter activity, whereas activated MKK6 clearly potentiated the effect of RafBXB. Interestingly, expression of active TAK1 potently inhibited RafBXB-elicited MMP-1 promoter activation (Fig. 1C), indicating that activation of distinct stress-activated MAPKs may have opposite effects on MMP-1 gene expression.
To confirm the activation of p38 by constitutively active Rac and TAK1, we cotransfected the corresponding expression vectors with an expression vector coding for p38α with Flag tag. A kinase assay with p38α immunoprecipitated with the anti-Flag antibody with ATF-2 as a substrate corroborated the activation of p38α by Rac(QL) and ΔNTAK1 (Fig. 1D). No differences in the levels of Flag-p38α in cell lysates were detected in Western blots with anti-Flag antibody (Fig. 1D). Furthermore, Western blot analysis of cotransfected fibroblasts revealed no differences in the levels of constitutively active Raf-1 (RafBXB) between cells cotransfected with expression vectors for constitutively active TAK1, MKK3b, and MKK6b (Fig. 1E). Expression vectors for distinct p38 isoforms p38α, p38β, p38γ, and p38δ with Flag tags were also cotransfected with expression vectors for constitutively active MKK3b and MKK6b. Kinase assays with immunoprecipitated p38 isoforms showed that MKK3b potently activated p38α, but not other p38 isoforms (Fig. 1F). MKK6b potently activated p38α and also p38β, -γ, and -δ isoforms (Fig. 1F). No activation of p38 isoforms was detected in cells cotransfected with empty expression vector (Fig. 1F).
Arsenite inhibits ERK1,2-mediated enhancement of MMP-1 expression.
Next, we studied the regulation of the endogenous MMP-1 gene in human skin fibroblasts by arsenite and anisomycin, two well-known activators of p38 and JNK/SAPK pathways, in combination with the phorbol ester TPA, an activator of the ERK1,2 pathway. Treatment with TPA (60 ng/ml) potently induced MMP-1 mRNA expression in human skin fibroblasts (Fig. 2A), whereas treatment of cells with arsenite (80 μM) or anisomycin (25 ng/ml) alone did not stimulate MMP-1 mRNA abundance. Interestingly, treatment of cells with arsenite in combination with TPA completely abrogated the TPA-elicited induction of MMP-1 mRNA abundance, whereas anisomycin treatment had no effect (Fig. 2A). As shown in Fig. 2B, the inhibitory effect of arsenite on TPA-elicited induction of MMP-1 mRNA was dose dependent, the maximal inhibition noted with concentration being 80 μM.
FIG. 2.
Arsenite inhibits TPA-elicited induction of MMP-1 expression. Total RNA was isolated and analysed for MMP-1 and GAPDH mRNA abundance by Northern blot hybridizations. Quantitations of MMP-1 mRNA levels corrected for GAPDH mRNA levels are shown below panel C relative to untreated control cultures. (A) Human skin fibroblasts were treated with TPA (60 ng/ml), arsenite (80 μM), and anisomycin (25 ng/ml) alone and in combination for 12 h. (B) Human skin fibroblasts were treated for 12 h with TPA (60 ng/ml) alone or in combination with increasing concentrations of arsenite. (C) HeLa cells were treated with TPA (60 ng/ml) and arsenite (50 μM) alone or in combination for 12 h.
It has been shown that treatment of transformed epithelial HeLa cells by arsenite (50 μM) results in activation of the minimal MMP-1 promoter construct containing the proximal AP-1 binding site (4). To examine whether the inhibitory effect of arsenite on MMP-1 gene expression is specific for fibroblasts, we studied the regulation of MMP-1 expression by TPA and arsenite in HeLa cells. Treatment of HeLa cells with arsenite (50 μM) also potently inhibited TPA-elicited induction of MMP-1 mRNA expression and alone did not induce MMP-1 mRNA levels (Fig. 2C). These results show that the inhibitory effect of arsenite on MMP-1 gene expression is not specific for fibroblasts.
Arsenite blocks ERK1,2 pathway at the level of MEK1,2.
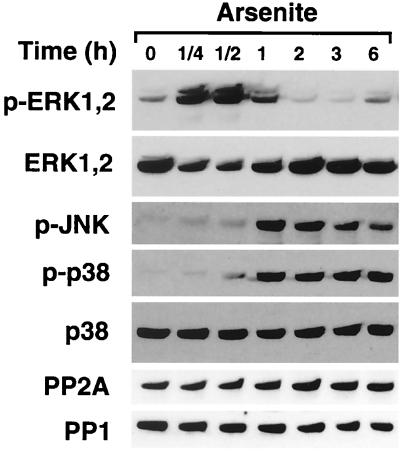
Next, we treated fibroblasts with arsenite for different periods of time and determined the activation of ERK1,2, JNK, and p38 kinases by Western blotting with antibodies against the phosphorylated forms of the kinases. Interestingly, arsenite treatment activated ERK1,2 potently and transiently between 15 min and 1 h, after which ERK1,2 phosphorylation declined below the basal level and was restored at 6 h (Fig. 3). In contrast to ERK1,2, activation of JNK and p38 by arsenite persisted until 6 h (Fig. 3). Interestingly, onset of p38 activation at the time range of 30 min to 1 h clearly preceded the decline of ERK1,2 activation, first noted at the 1-h time point. Treatment of fibroblasts transfected with the p38α expression vector with arsenite activated Flag-tagged p38α, as determined by an immunocomplex assay with GST–ATF-2 as a substrate (data not shown).
FIG. 3.
Arsenite inhibits activation of ERK1,2. Human skin fibroblasts were treated with arsenite (80 μM) for indicated periods of time. Thereafter, cells were lysed and the levels of phosphorylated ERK1,2 (p-ERK1,2), JNK (p-JNK), and p38 (p-p38) were determined by Western blot analysis with phospho-specific antibodies. Protein loading was studied with antibodies against total p38 and ERK1,2. The cellular levels of catalytic subunits for PP1 and PP2A were determined by Western blot analysis with specific antibodies.
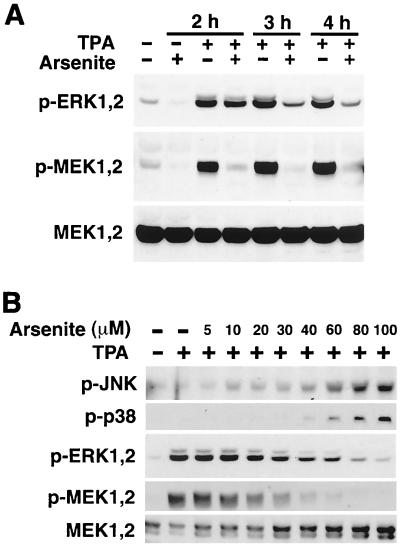
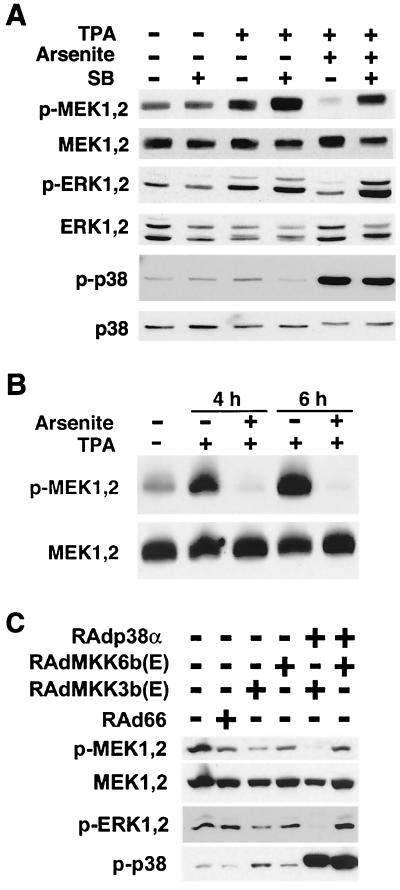
These results suggest that the activation of JNK and/or p38 inhibits MMP-1 expression by blocking the ERK1,2 pathway. To study this possibility, we treated fibroblasts simultaneously with TPA and arsenite and determined the activation of ERK1,2 and MEK1,2. Interestingly, simultaneous arsenite treatment clearly inhibited TPA-induced ERK1,2 phosphorylation at 3- and 4-h time points, and this was associated with marked reduction in the levels of phosphorylated MEK1,2 in the time range from 30 min (data not shown) to 4 h (Fig. 4A). In addition, arsenite treatment resulted in reduction in the basal levels of activated ERK1,2 and MEK1,2 (Fig. 4A). Furthermore, dose-dependent activation of JNK and p38 by arsenite correlated with the inactivation of ERK1,2 and MEK1,2 (Fig. 4B) and with the down-regulation of TPA-induced MMP-1 mRNA expression (Fig. 2B).
FIG. 4.
Treatment of fibroblasts with arsenite prevents activation of MEK1,2 and ERK1,2. Cells were lysed, and the levels of phosphorylated ERK1,2 (p-ERK1,2), MEK1,2 (p-MEK1,2), JNK (p-JNK), and p-38 (p-p38) were determined by Western blot analysis with corresponding phospho-specific antibodies. Protein loading was determined using antibodies against total MEK1,2. (A) Human skin fibroblasts were treated with TPA (60 ng/ml) alone or in combination with arsenite (80 μM) for indicated periods of time. (B) Human skin fibroblasts were treated with TPA (60 ng/ml) alone or in combination with increasing concentrations of arsenite for 2 h.
Blocking MEK1,2 activation by arsenite inhibits ERK1,2-mediated enhancement of MMP-1 expression.
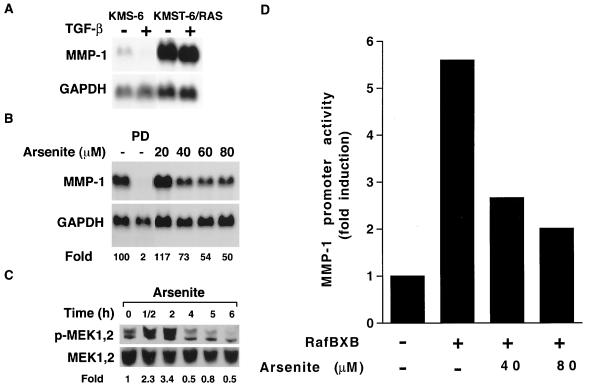
The results presented above provide evidence that treatment of fibroblasts with arsenite blocks the ERK1,2 pathway at the level of MEK1,2. To confirm that the effect of arsenite occurs downstream of Ras, we treated human Ha-Ras-transformed fibroblasts (KMST-6/Ras) (24) with arsenite and studied MEK1,2 activation and the regulation of MMP-1 expression. As shown in Fig. 5A, Ras transformation resulted in a marked increase in MMP-1 mRNA abundance, compared with that of the parental cell line, KMS-6. In both cell lines, expression of MMP-1 mRNA was suppressed by TGF-β to a certain extent (Fig. 5A), indicating that both cell lines respond to signals previously shown to negatively regulate MMP-1 gene expression. In addition, treatment of KMST-6/Ras cells with specific MEK1,2 inhibitor PD98059 potently suppressed expression of MMP-1 mRNA, indicating that high levels of basal expression of MMP-1 in these cells are dependent on MEK1,2 activity (Fig. 5B). Treatment of KMST-6/Ras cells with arsenite also resulted in dose-dependent down-regulation of MMP-1 expression (Fig. 5B), and the maximal down-regulation of MMP-1 mRNA expression correlated with the maximal inhibition of MEK1,2 activity first noted at 4 h (Fig. 5C).
FIG. 5.
Arsenite treatment blocks the ERK1,2 pathway downstream of Ras and Raf-1. (A) The Ras-transformed human embryonal fibroblast line (KMST-6/RAS) and the parental fibroblasts (KMS-6) were treated for 24 h with TGF-β (10 ng/ml). The expression of MMP-1 and GAPDH mRNAs was determined by Northern blot hybridizations. (B) KMST-6/RAS cells were incubated for 12 h with MEK1,2 inhibitor PD98059 (PD; 20 μM) or with arsenite in concentrations indicated. Thereafter, expression of MMP-1 and GAPDH mRNAs was determined by Northern blot hybridizations. Quantitation of MMP-1 mRNA levels corrected for GAPDH mRNA levels is shown below the panel relative to untreated control cultures (100). (C) KMST-6/RAS cells were incubated with arsenite (80 μM) for indicated periods of time, and the levels of phosphorylated MEK1,2 (p-MEK1,2) and total MEK1,2 were determined by Western blot analysis. Quantitation of p-MEK1,2 levels is shown below the panel relative to the levels in cultures at time point 0 h (lane 1). (D) Neonatal human skin fibroblasts were transiently transfected with expression vector for the constitutively active form of Raf-1 (RafBXB) together with MMP-1 reporter gene construct -2278CLCAT (2 μg). Twelve hours after the transfection, cells were treated with arsenite (40 or 80 μM), incubation was continued for 12 h, cells were harvested, and CAT activity was measured. The values represent the mean of three experiments each performed in duplicate.
To exclude the possibility that arsenite could block the activation of the ERK1,2 pathway at the level of Ras or Raf-1, we transfected human neonatal foreskin fibroblasts with the expression vector for constitutively active Raf-1 (RafBXB) and studied the effect of arsenite on Raf-1-induced activation of the MMP-1 promoter. As shown in Fig. 5D, RafBXB increased MMP-1 promoter activity up to sixfold, compared to cells cotransfected with the respective empty expression vector, and this effect was potently and dose dependently inhibited by treatment with arsenite (Fig. 5D). Taken together, these results clearly show that activation of the Ras→Raf-1→MEK1,2→ERK1,2 pathway is inhibited by arsenite at the level of MEK1,2, resulting in the inhibition of MMP-1 gene expression in fibroblasts.
Inhibition of MEK1,2 activation by arsenite is mediated by p38 MAPK.
The results above show that activation of JNK or p38 MAPK results in inactivation of MEK1,2. To study the role of p38 MAPK in this process, human skin fibroblasts were pretreated for 2 h with a specific inhibitor of p38 activity, SB203580, followed by exposure to TPA and arsenite alone and in combination for 4 h. Blocking p38 activity by SB203580 had no effect on basal MEK1,2 or ERK1,2 phoshorylation, but it augmented the activation of MEK1,2 and ERK1,2 by TPA (Fig. 6A). As before (see above), arsenite inhibited the activation of MEK1,2 and ERK1,2 by TPA (Fig. 6A). Interestingly, treatment of cells with SB203580 abrogated the inhibitory effect of arsenite on TPA-elicited activation of MEK1,2 and ERK1,2 (Fig. 6A).
FIG. 6.
Inhibitory effect of arsenite on MEK1,2 activation is mediated by p38 MAPK. (A) Human skin fibroblasts were incubated for 4 h with TPA (60 ng/ml) and arsenite (80 μM) alone or in combination. Where indicated, cells were pretreated for 2 h with specific inhibitor of p38 activity SB203580 (SB; 20 μM). Thereafter, cells were lysed and the levels of phosphorylated MEK1,2 (p-MEK1,2), ERK1,2 (p-ERK1,2), and p38 (p-p38) and total MEK1,2, ERK1,2, and p38 were determined by Western blot analysis. (B) Embryonal fibroblasts from JNK2−/− mice were incubated with TPA (60 ng/ml) alone or in combination with arsenite (80 μM) for the time period indicated. Cells were lysed, and levels of phosphorylated MEK1,2 (p-MEK1,2) and total MEK1,2 were determined by Western blotting. (C) Ras-transformed human embryonal fibroblasts (KMST-6/Ras) were infected with recombinant adenoviruses for constitutively active MKK3b [RAdMKK3b(E)] and MKK6b [RAdMKK6b(E)] alone or in combination with adenovirus harboring wild-type p38α (RAdp38α) at a multiplicity of infection of 500. Control cultures were infected with empty adenovirus RAd66. After 18 h in DMEM with 1% FCS, the medium was changed and the incubations were continued for 48 h. Cells were lysed, and the levels of phosphorylated MEK1,2 (p-MEK1,2), ERK1,2 (p-ERK1,2), and p38 (p-p38) and total MEK1,2 were determined by Western blot analysis.
To study the role of the JNK signaling cascade in mediating arsenite-elicited MEK1,2 inactivation, we examined whether arsenite exerts a similar effect in embryonal fibroblasts derived from JNK2−/− mice. As shown in Fig. 6B, TPA potently activated MEK1,2 in JNK2−/− fibroblasts, and as in human skin fibroblasts, MEK1,2 activation was entirely prevented by arsenite treatment, showing that arsenite-elicited inactivation of MEK1,2 is not mediated by JNK2.
To directly examine the inhibitory role of p38 MAPK on the ERK1,2 cascade, we infected KMST-6/Ras fibroblasts with adenoviruses harboring constitutively active MKK3b and MKK6b, alone and together with adenovirus for wild-type p38α. Activation of endogenous p38α by MKK3b(E) resulted in reduction in the levels of activated MEK1,2 and ERK1,2, compared with uninfected cells or cells infected with empty control virus RAd66 (Fig. 6C). Furthermore, simultaneous overexpression of p38α clearly augmented the inhibitory effect of MKK3b(E) on MEK1,2 and ERK1,2 activation (Fig. 6C). In contrast, although constitutively active MKK6b clearly activated p38α, this had no effect on MEK1,2 and ERK1,2 activity (Fig. 6C). These results provide evidence that MKK3 and MKK6 play a different role in controlling MEK1,2 activity, possibly due to their different p38 isoform activation profiles. Nevertheless, these results clearly show that specific activation of p38α by MKK3b results in potent inhibition of MEK1,2 and ERK1,2 activity.
Inhibition of MMP-1 promoter activity by p38 MAPK.
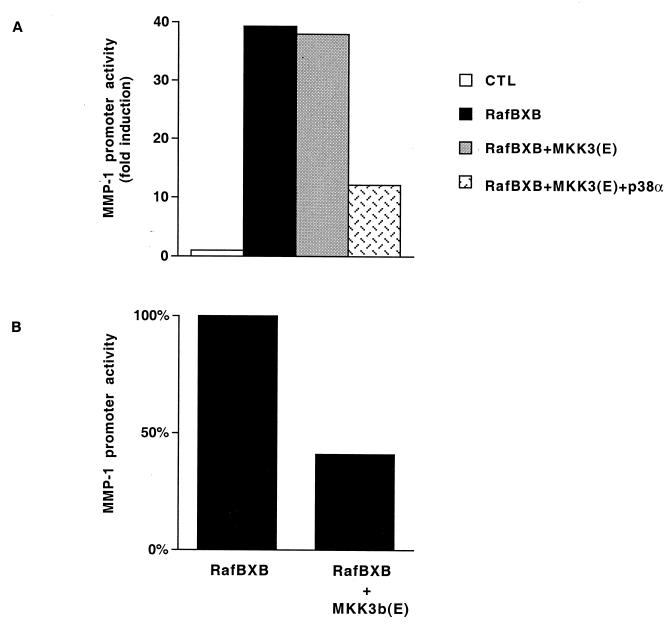
The results above suggest that signaling via p38 MAPK mediates the inhibitory effect of arsenite or TAK1 on the ERK1,2 signaling cascade. Our cotransfections showed that expression of constitutively active MKK6, a potent and specific activator of p38 (10), enhanced the effect of RafBXB on MMP-1 promoter activity, whereas MKK3 had no effect (Fig. 1A). However, it has been shown that the constitutively active mutant of MKK3, MKK3(E), alone does not stimulate ATF-2-dependent transcription but requires simultaneous overexpression of p38 (20). In this respect, we studied the effect of coexpression of MKK3(E) and p38α on MMP-1 promoter activity. Expression of RafBXB in NIH 3T3 cells potently enhanced MMP-1 promoter activity, and this effect was not modulated by coexpression of MKK3(E) alone (Fig. 7A). However, overexpression of MKK3(E) in combination with p38α potently inhibited the effect of RafBXB on the MMP-1 promoter (Fig. 7A). Moreover, as shown in Fig. 7, the stimulatory effect of RafBXB on MMP-1 promoter activity was potently inhibited by expression of MKK3b(E), a predominant MKK3 isoform which potently activates p38α (Fig. 1F and 6C), and ATF-2-dependent transcription (20). Taken together, these results suggest that MKK3/MKK3b and MKK6 play opposite roles in the regulation of MMP-1 gene expression and that signaling pathway MKK3/MKK3b→p38α mediates arsenite-elicited inhibition of MEK1,2 activity.
FIG. 7.
(A) NIH 3T3 fibroblasts were transiently transfected with MMP-1 promoter-reporter gene construct -2278CLCAT (1 μg) and the expression vector for constitutively active Raf-1 (RafBXB) alone or in combination with expression vectors for constitutively active MKK3 [MKK3(E)] and wild-type p38α (1 μg each). Control cultures were transfected with the corresponding empty expression vectors. (B) NIH 3T3 fibroblasts were transiently transfected with -2278CLCAT reporter construct and 1 μg of Raf-1 (RafBXB) alone or in combination with the constitutively active form of MKK3b [MKK3b(E)]. After 36 h, cells were lysed, and CAT activity was measured as an indicator of promoter activity. Transfection efficiency was monitored by cotransfecting the cells with 4 μg of the RSV–β-galactosidase construct. The values represent the mean of two independent experiments each performed in duplicate.
Activation of PP1-PP2A via p38 is required for arsenite-elicited inactivation of MEK1,2.
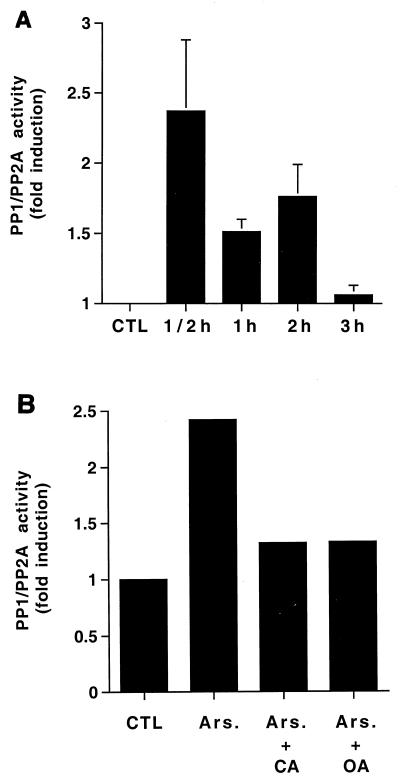
The results above suggest that arsenite-elicited MEK1,2 inactivation is mediated by direct MEK1,2 dephosphorylation. Previous studies have shown that PP1 and PP2A dephosphorylate MEK1,2 and ERK1,2 and that inhibition of PP1-PP2A activity results in activation of ERK1,2 and expression of MMP-1 in fibroblasts (3, 51, 53). Therefore, we studied the role of PP1-PP2A in arsenite-elicited inactivation of MEK1,2. Interestingly, treatment of human skin fibroblasts with arsenite resulted in a persistent increase in cellular PP1-PP2A activity, starting at 30 min and extending until 2 h (Fig. 8A). Arsenite had no effect on the cellular levels of catalytic subunits of PP1 and PP2A, as determined by Western blot analysis (Fig. 3). Arsenite-elicited increase of PP1-PP2A activity was potently blocked by pretreatment of cells with calyculin A (2.5 nM) or okadaic acid (20 ng/ml), specific inhibitors of PP1 and PP2A activity (Fig. 8B) (11).
FIG. 8.
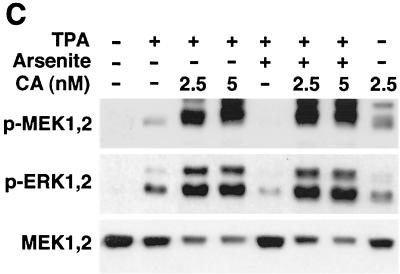
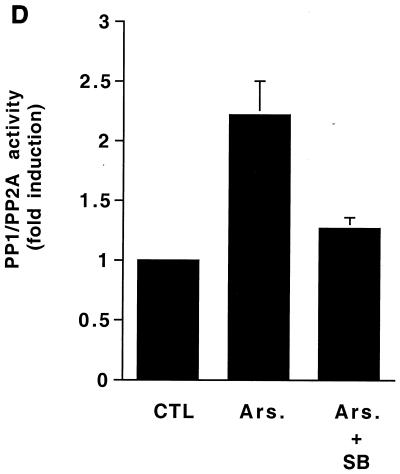
Activation of PP1-PP2A is required for arsenite-elicited MEK1,2 dephosphorylation. (A) Human skin fibroblasts were treated with arsenite (80 μM) for the time periods indicated. Thereafter, cells were lysed and equal amounts of protein were assayed for PP1-PP2A phosphatase activity using 32P-labeled glycogen phosphorylase as a substrate. The mean and standard deviation of values from three experiments are shown. x axis, length of arsenite treatment. (B) Human skin fibroblasts were treated with arsenite (Ars; 80 μM) for 30 min. Where indicated, cells were pretreated for 2 h with okadaic acid (OA) (20 ng/ml) or for 15 min with calyculin A (CA) (5 nM). Thereafter, cells were lysed and equal amounts of total protein were assayed for PP1-PP2A activity as described in the legend to panel A. The results of a representative experiment of two experiments with similar results are shown. (C) Human skin fibroblasts were treated for 2 h with TPA (60 ng/ml) and arsenite (80 μM) alone or in combination. Where indicated, cells were pretreated for 15 min with calyculin A (CA) in concentrations shown. The levels of phosphorylated MEK1,2 (p-MEK1,2) and ERK1,2 (p-ERK1,2) were determined by Western blotting using phospho-specific antibodies. The filter was stripped and protein loading was determined by using an antibody against total MEK1,2. A representative blot of two independent experiments with similar results is shown. (D) Human skin fibroblasts were incubated for 30 min with arsenite (Ars; 80 μM). Where indicated cells were pretreated for 2 h with p38 inhibitor SB203580 (SB; 20 μM). Cells were then lysed and PP1-PP2A phosphatase activity was measured as described in the legend to panel A. The mean plus standard deviation of two independent experiments are shown. CTL, untreated control cultures.
To study whether PP1-PP2A activity is required for an inhibitory effect of arsenite on MEK1,2 activation, skin fibroblasts were pretreated with calyculin A (2.5 and 5 nM) and exposed to TPA and arsenite alone and in combination for 2 h. Treatment of cells with calyculin A activated MEK1,2 and ERK1,2 as potently as TPA and augmented the effect of TPA on MEK1,2 and ERK1,2 phosphorylation (Fig. 8C). As noted above, arsenite treatment potently inhibited TPA-elicited MEK1,2 and ERK1,2 phosphorylation, but this effect was absent in cells in which PP1-PP2A activity was blocked by calyculin A (Fig. 8C). These results clearly show that PP1-PP2A activity is required for arsenite-elicited inactivation of MEK1,2. Finally, blocking p38 activity by SB203580 potently inhibited the activation of PP1-PP2A by arsenite (Fig. 8D), showing that p38 activity is required for both activation of PP1-PP2A and for the inactivation of MEK1,2 by arsenite.
DISCUSSION
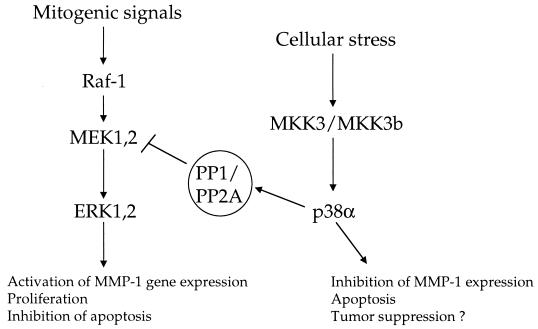
The results of the present study show that activation of p38 MAPKs by MKK3 or MKK6 results in opposite effects on MMP-1 promoter activity. Based on our results, it appears that activation of p38 by MKK6 results in synergistic enhancement of MMP-1 promoter activity in combination with the activation of the ERK1,2 pathway. This suggests that the MKK6→p38 module could mediate the induction of MMP-1 gene expression, e.g., by ceramide and interleukin-1 (40, 41). The results of our cotransfections are in accordance with our recent findings that adenovirus-mediated expression of constitutively active MKK6b potentiates the stimulatory effect of constitutively active MEK1 on the expression of the endogenous MMP-1 gene (39). In contrast to MKK6, activation of the signaling cascade downstream of TAK1 and MKK3/MKK3b appears to inhibit ERK1,2-mediated induction of MMP-1 gene transcription. TAK1 has been shown to activate both MKK4→JNK and MKK3/MKK6→p38 signaling pathways (34, 45). Although we have not dissected in detail the signaling cascade activated by TAK1 in fibroblasts, our results clearly show that TAK1-activated downstream signaling in fibroblasts mimics the effects of arsenite and increased MKK3→p38α activity, providing evidence for p38-mediated inhibition of MMP-1 gene expression. The opposite roles of MKK3 and MKK6 in the regulation of MMP-1 gene expression may be based on differences in their binding affinity and kinase activity toward distinct p38 isoforms (8, 10). Our results show that in fibroblasts, an active mutant of MKK3b potently activates p38α, whereas the activation of other p38 isoforms is negligible. In contrast, constitutively active MKK6b also activates p38β, -γ, and -δ isoforms in addition to p38α. Based on studies with knockout mice, MKK3 and MKK6 have nonredundant functions in vivo (10, 30, 56). These knockout mice provide interesting models to examine the different roles of MKK3 and MKK6 in the regulation of MMP gene expression.
The p38 MAPK-mediated inhibition of ERK1,2 activation provides a functional link between these two signaling pathways, previously shown to have opposite roles, e.g., in cell proliferation, survival, and apoptosis (33, 57). Based on our results, activation of the MKK3→p38α pathway in response to proapoptotic signals would result in inhibition of ERK1,2-mediated survival signals, hence favoring apoptosis (Fig. 9). In accordance with this, arsenite treatment and specific activation of p38α have been shown to induce apoptosis (5, 36). Regarding the role of stress-activated MAPKs in cell growth and malignant transformation, it has been reported that the MKK4 gene is inactivated in various types of malignant tumors, suggesting a role for MKK4 as a tumor suppressor gene (46, 47). Moreover, activation of p38 MAPKs is impaired in MKK4−/− fibroblasts (16), raising the interesting possibility that inactivation of the MKK4 gene during cancer progression could abrogate negative signaling from p38 MAPK to MEK1,2 and result in enhanced ERK1,2 activity, cell proliferation, and MMP-1 expression. In view of the results of the present study, it is tempting to speculate that MKK3 and/or MKK3b might also function as a tumor suppressor (Fig. 9).
FIG. 9.
Schematic presentation of the proposed opposite roles of ERK1,2 and MKK3/MKK3b→p38α pathways in the regulation of cell behavior.
The effects of arsenite on MAPK activity appear to be cell type and dose dependent. It has been shown that low doses of arsenite (<50 μM) specifically activate the ERK1,2 pathway, whereas higher doses (>50 μM) seem to preferentially activate JNK and p38 MAPKs (4, 21, 29). Interestingly, a previous study showed that arsenite treatment results in MKK6→p38 cascade-mediated delayed activation of ERK1,2 and that triggering the MKK6→p38 pathway results in activation of ERK1,2 (31). This suggests a possible mechanism for the synergistic effect of MKK6 and Raf-1 observed in the present study with low amounts of constitutively active Raf-1 expression vector, resulting in submaximal activation of the MMP-1 promoter (Fig. 1A and B).
Our results clearly show that inactivation of the ERK1,2 pathway by arsenite occurs at the level of MEK1,2. In addition, our results with recombinant adenoviruses show that activation of the p38α isoform by constitutively active MKK3b results in potent inhibition of MEK1,2 and ERK1,2 activation. This provides direct evidence that inhibition of MEK1,2 and ERK1,2 activity as a result of p38α activation serves as a negative regulatory mechanism for ERK1,2 activation. To our knowledge this is the first evidence for a direct functional link between p38 and MEK1,2. Previously, high-dose (500 μM) arsenite treatment has been shown to block growth factor-mediated activation of the ERK1,2 pathway upstream of Ras (9). Although we cannot exclude the possibility that inhibition of TPA-elicited activation of MMP-1 mRNA expression by arsenite would also involve inactivation of signaling at the level or upstream of Ras, our results clearly show that an additional mechanism exists that prevents the activation of MEK1,2 by Raf-1. Our observations also show that arsenite-elicited activation of p38 results in increased PP1-PP2A activity, and when PP1-PP2A activity is inhibited, arsenite treatment cannot inactivate MEK1,2.
Recent studies have shown that although PP2A negatively regulates signaling from Ras to Raf, the catalytic activity of Raf-1 is stimulated by PP2A (1, 50). These observations, together with our results showing that arsenite treatment inhibits MMP-1 promoter activation by constitutively activated Ras and Raf-1, support the view that increased PP1-PP2A activity by arsenite directly inhibits MEK1,2 phosphorylation. Furthermore, our results, showing that ERK1,2 inactivation occurs somewhat later than MEK1,2 inactivation, indicate that MEK1,2 is the primary target for PP1-PP2A in arsenite-treated fibroblasts. This notion is also supported by our previous results showing that inhibition of PP1-PP2A activity by okadaic acid is not sufficient to activate MMP-1 gene expression when MEK1,2 activation is blocked (51).
The mechanism by which p38 activates PP1-PP2A is not known at present. The formation of complexes between catalytic and regulatory subunits of PP1 and PP2A holoenzymes regulates the substrate specificity of PP1 and PP2A (26). Furthermore, the activity and complex formation of the catalytic subunits of PP1 and PP2A are regulated by phosphorylation (26, 32). This together with the rapid kinetics of PP1-PP2A activation following p38 activation suggests that PP1 and/or PP2A complexes are direct targets for p38-mediated phosphorylation. In this context, it will be of great interest to study which subunits target PP1 or PP2A complexes toward MEK1,2 and how these complexes are activated by p38.
In conclusion, our results show that the stimulatory effect of the ERK1,2 pathway in the regulation of MMP-1 gene expression is differentially modulated by the p38 pathway depending on the upstream activator of p38 MAPKs. These results provide evidence that stress-activated MAPKs may serve as physiological negative regulators of the activity of mitogenic signaling via the ERK1,2 cascade and may also play a negative role in the regulation of MMP-1 gene expression. In addition, our results suggest that blocking the MKK3/MKK3b→p38α→PP1-PP2A pathway may result in increased expression of MMP-1 and subsequent degradation of collagenous ECM in pathological conditions, such as tumor invasion and metastasis.
ACKNOWLEDGMENTS
This study was supported by grants from the Academy of Finland (projects 30985 and 45996), Sigrid Jusélius Foundation, Cancer Research Foundation of Finland, Turku University Central Hospital (EVO grant 13336), and Finnish Culture Foundation and by a research contract from the Finnish Life and Pension Insurance Companies.
We thank Hanna Haavisto and Tarja Heikkilä for skillful technical assistance. We also thank the Bohmann lab for helpful discussions and Dirk Bohmann for critically reading the manuscript.
J. Westermarck and S. Li contributed equally to this work.
REFERENCES
- 1.Abraham D, Podar K, Pacher M, Kubicek M, Welzel N, Hemmings B A, Dilworth S M, Mischak H, Kolch W, Baccarini M. Raf-1-associated PP2A as a positive regulator of kinase activation. J Biol Chem. 2000;275:22300–22304. doi: 10.1074/jbc.M003259200. [DOI] [PubMed] [Google Scholar]
- 2.Bruder J T, Heidecker G, Rapp U R. Serum, TPA-, and Ras-induced expression from Ap-1/Ets driven promoters requires Raf-1 kinase. Genes Dev. 1992;6:545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- 3.Casillas A M, Amaral K, Chegini Farahani S, Nel A E. Okadaic acid activates p42 mitogen-activated protein kinase (MAP kinase; ERK-2) in B-lymphocytes but inhibits rather than augments cellular proliferation: contrast with phorbol 12-myristate 13-acetate. Biochem J. 1993;290:545–550. doi: 10.1042/bj2900545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavigelli M, Li W W, Lin A, Su B, Yoshioka K, Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Lin-Shiau S, Lin J. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J Cell Physiol. 1998;177:324–333. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Chung H, Brautigan D. Protein phosphatase 2A suppresses MAP kinase signalling and ectopic protein expression. Cell Signal. 1999;11:575–580. doi: 10.1016/s0898-6568(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 7.Coso O A, Chiariello M, Yu J C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 8.Cuenda A, Cohen P, Buee Scherrer V, Goedert M. Activation of stress-activated protein kinase-3 (SAPK3) by cytokines and cellular stresses is mediated via SAPKK3 (MKK6); comparison of the specificities of SAPK3 and SAPK2 (RK/p38) EMBO J. 1997;16:295–305. doi: 10.1093/emboj/16.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doza Y, Hall-Jackson C, Cohen P. Arsenite blocks growth factor induced activation of MAP kinase cascade, upstream of Ras and downstream of Grb2-Sos. Oncogene. 1998;17:19–24. doi: 10.1038/sj.onc.1202168. [DOI] [PubMed] [Google Scholar]
- 10.Enslen H, Brancho D M, Davis R J. Molecular determinants that mediate selective activation of p38 MAP kinase isoforms. EMBO J. 2000;19:1301–1311. doi: 10.1093/emboj/19.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favre B, Turowski P, Hemmings B A. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem. 1997;272:13856–13863. doi: 10.1074/jbc.272.21.13856. [DOI] [PubMed] [Google Scholar]
- 12.Fort P, Marty L, Piechaczyk M, el Sabrouty S, Dani C, Jeanteur P, Blanchard J M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frisch S M, Reich R, Collier I E, Genrich L T, Martin G, Goldberg G I. Adenovirus E1A represses protease gene expression and inhibits metastasis of human tumor cells. Oncogene. 1990;5:75–83. [PubMed] [Google Scholar]
- 14.Frost J A, Alberts A S, Sontag E, Guan K, Mumby M C, Feramisco J R. Simian virus 40 small t antigen cooperates with mitogen-activated kinases to stimulate AP-1 activity. Mol Cell Biol. 1994;14:6244–6252. doi: 10.1128/mcb.14.9.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost J A, Geppert T D, Cobb M H, Feramisco J R. A requirement for extracellular signal-regulated kinase (ERK) function in the activation of AP-1 by Ha-Ras, phorbol 12-myristate 13-acetate, and serum. Proc Natl Acad Sci USA. 1994;91:3844–3848. doi: 10.1073/pnas.91.9.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganiatsas S, Kwee L, Fujiwara Y, Perkins A, Ikeda T, Labow M, Zon L. SEK1 deficiency reveals mitogen-activated protein kinase cascade crossregulation and leads to abnormal hepatogenesis. Proc Natl Acad Sci USA. 1998;95:6881–6886. doi: 10.1073/pnas.95.12.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrington T P, Johnson G L. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg G I, Wilhelm S M, Kronberger A, Bauer E A, Grant G A, Eisen A Z. Human fibroblast collagenase. Complete primary structure and homology to an oncogene transformation-induced rat protein. J Biol Chem. 1986;261:6600–6605. [PubMed] [Google Scholar]
- 19.Goldberg Y. Protein phosphatase 2A: who shall regulate the regulator? Biochem Pharmacol. 1999;57:321–328. doi: 10.1016/s0006-2952(98)00245-7. [DOI] [PubMed] [Google Scholar]
- 20.Han J, Wang X, Jiang Y, Ulevitch R J, Lin S. Identification and characterization of a predominant isoform of human MKK3. FEBS Lett. 1997;403:19–22. doi: 10.1016/s0014-5793(97)00021-5. [DOI] [PubMed] [Google Scholar]
- 21.Huang C, Ma W-Y, Li J, Goranson A, Dong Z. Requirement of Erk, but not JNK, for arsenite-induced cell transformation. J Biol Chem. 1999;274:14595–14601. doi: 10.1074/jbc.274.21.14595. [DOI] [PubMed] [Google Scholar]
- 22.Inoue T, Yashiro M, Nishimura S, Maeda K, Sawada T, Ogawa Y, Sowa M, Chung K H. Matrix metalloproteinase-1 expression is a prognostic factor for patients with advanced gastric cancer. Int J Mol Med. 1999;4:73–77. doi: 10.3892/ijmm.4.1.73. [DOI] [PubMed] [Google Scholar]
- 23.Ivaska J, Reunanen H, Westermarck J, Koivisto L, Kähäri V-M, Heino J. Integrin α2β1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the α2 cytoplasmic tail. J Cell Biol. 1999;147:401–416. doi: 10.1083/jcb.147.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jahan I, Mihara K, Bai L, Namba M. Neoplastic transformation and characterization of human fibroblasts by treatment with 60Co gamma rays and the human c-Ha-ras oncogene. In Vitro Cell Dev Biol. 1993;29A:763–767. doi: 10.1007/BF02634342. [DOI] [PubMed] [Google Scholar]
- 25.Kähäri V-M, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumor invasion. Ann Med. 1999;31:34–45. doi: 10.3109/07853899909019260. [DOI] [PubMed] [Google Scholar]
- 26.Keyse S. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 27.Korzus E, Nagase H, Rydell R, Travis J. The mitogen-activated protein kinase and JAK-STAT signaling pathways are required for an oncostatin M-responsive element-mediated activation of matrix metalloproteinase 1 gene expression. J Biol Chem. 1997;272:1188–1196. doi: 10.1074/jbc.272.2.1188. [DOI] [PubMed] [Google Scholar]
- 28.Lewis T S, Shapiro P S, Ahn N G. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/s0065-230x(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 29.Lim C P, Jain N, Cao X. Stress-induced immediate-early gene, egr-1, involves activation of p38/JNK1. Oncogene. 1998;16:2915–2926. doi: 10.1038/sj.onc.1201834. [DOI] [PubMed] [Google Scholar]
- 30.Lu H T, Yang D D, Wysk M, Gatti E, Mellman I, Davis R J, Flavell R A. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999;18:1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig S, Hoffmeyer A, Goebeler M, Kilian K, Häfner H, Neufeld B, Han J, Rapp U. The stress inducer arsenite activates mitogen-activated protein extracellular signal-regulated kinases 1 and 2 via a MAPK kinase 6/p38-dependent pathway. J Biol Chem. 1998;273:1917–1922. doi: 10.1074/jbc.273.4.1917. [DOI] [PubMed] [Google Scholar]
- 32.Millward T A, Zolnierowicz S, Hemmings B A. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 33.Molnar A, Theodoras A, Zon L, Kyriakis J. Cdc42Hs, but not Rac1, inhibits serum-stimulated cell cycle progression at G1/S through a mechanism requiring p38/RK. J Biol Chem. 1997;272:13229–13235. doi: 10.1074/jbc.272.20.13229. [DOI] [PubMed] [Google Scholar]
- 34.Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida E, Hagiwara M. A. novel kinase cascade mediated by mitogen-activated protein kinase 6 and MKK3. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 35.Murray G I, Duncan M E, O'Neil P, Melvin W T, Fothergill J E. Matrix metalloproteinase-1 is associated with poor prognosis in colorectal cancer. Nat Med. 1996;2:461–462. doi: 10.1038/nm0496–461. [DOI] [PubMed] [Google Scholar]
- 36.Nemoto S, Xiang J, Huang S, Lin A. Induction of apoptosis by SB202190 through inhibition of p38β mitogen-activated protein kinase. J Biol Chem. 1998;273:16415–16420. doi: 10.1074/jbc.273.26.16415. [DOI] [PubMed] [Google Scholar]
- 37.New L, Jiang Y, Zhao M, Liu K, Zhu W, Flood L J, Kato Y, Parry G C, Han J. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 1998;17:3372–3384. doi: 10.1093/emboj/17.12.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravanti L, Häkkinen L, Larjava H, Saarialho-Kere U, Foschi M, Han J, Kähäri V-M. Transforming growth factor-β induces collagenase-3 expression by human gingival fibroblasts via p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:37292–37300. doi: 10.1074/jbc.274.52.37292. [DOI] [PubMed] [Google Scholar]
- 40.Reunanen N, Westermarck J, Häkkinen L, Holmström T H, Elo I, Eriksson J E, Kähäri V-M. Enhancement of fibroblast collagenase (matrix metalloproteinase-1) gene expression by ceramide is mediated by extracellular signal-regulated and stress-activated protein kinase pathways. J Biol Chem. 1998;273:5137–5145. doi: 10.1074/jbc.273.9.5137. [DOI] [PubMed] [Google Scholar]
- 41.Ridley S H, Sarsfield S J, Lee J C, Bigg H F, Cawston T E, Taylor D J, DeWitt D L, Saklatvala J. Actions of IL-1 are selectively controlled by p38 mitogen-activated protein kinase: regulation of prostaglandin H synthase-2, metalloproteinases, and IL-6 at different levels. J Immunol. 1997;158:3165–3173. [PubMed] [Google Scholar]
- 42.Rutter G A, White M R, Tavare J M. Involvement of MAP kinase in insulin signalling revealed by noninvasive imaging of luciferase gene expression in single living cells. Curr Biol. 1995;5:890–899. doi: 10.1016/s0960-9822(95)00179-5. [DOI] [PubMed] [Google Scholar]
- 43.Sabapathy K, Hu Y, Kallunki T, Schreiber M, David J P, Jochum W, Wagner E F, Karin M. JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development. Curr Biol. 1999;9:116–125. doi: 10.1016/s0960-9822(99)80065-7. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro S. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602–608. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 45.Shirakabe K, Yamaguchi K, Shibuya H, Irie K, Matsuda S, Moriguchi T, Gotoh Y, Matsumoto K, Nishida E. TAK1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-terminal kinase. J Biol Chem. 1997;272:8141–8144. doi: 10.1074/jbc.272.13.8141. [DOI] [PubMed] [Google Scholar]
- 46.Su G H, Hilgers W, Shekher M C, Tang D J, Yeo C J, Hruban R H, Kern S E. Alterations in pancreatic, biliary, and breast carcinomas support MKK4 as a genetically targeted tumor suppressor gene. Cancer Res. 1998;58:2339–2342. [PubMed] [Google Scholar]
- 47.Teng D H, Perry III W L, Hogan J K, Baumgard M, Bell R, Berry S, Davis T, Frank D, Frye C, Hattier T, Hu R, Jammulapati S, Janecki T, Leavitt A, Mitchell J T, Pero R, Sexton D, Schroeder M, Su P H, Swedlund B, Kyriakis J M, Avruch J, Bartel P, Wong A K, Oliphant A, Thomas A, Skolnick M H, Tavtigian S V. Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor. Cancer Res. 1997;57:4177–4182. [PubMed] [Google Scholar]
- 48.Virshup D M. Protein phosphatase 2A: a panoply of enzymes. Curr Opin Cell Biol. 2000;12:180–185. doi: 10.1016/s0955-0674(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Huang S, Sah V P, Ross J, Jr, Brown J H, Han J, Chien K R. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 50.Wassarman D A, Solomon N M, Chang H C, Karim F D, Therrien M, Rubin G M. Protein phosphatase 2A positively and negatively regulates Ras1-mediated photoreceptor development in Drosophila. Genes Dev. 1996;10:272–278. doi: 10.1101/gad.10.3.272. [DOI] [PubMed] [Google Scholar]
- 51.Westermarck J, Holmström T, Ahonen M, Eriksson J E, Kähäri V-M. Enhancement of fibroblast collagenase-1 (MMP-1) gene expression by tumor promoter okadaic acid is mediated by stress-activated protein kinases Jun N-terminal kinase and p38. Matrix Biol. 1998;17:547–557. doi: 10.1016/s0945-053x(98)90107-x. [DOI] [PubMed] [Google Scholar]
- 52.Westermarck J, Kähäri V-M. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 53.Westermarck J, Lohi J, Keski Oja J, Kähäri V-M. Okadaic acid-elicited transcriptional activation of collagenase gene expression in HT-1080 fibrosarcoma cells is mediated by JunB. Cell Growth Differ. 1994;5:1205–1213. [PubMed] [Google Scholar]
- 54.Westermarck J, Seth A, Kähäri V-M. Differential regulation of interstitial collagenase (MMP-1) gene expression by ETS transcription factors. Oncogene. 1997;14:2651–2660. doi: 10.1038/sj.onc.1201111. [DOI] [PubMed] [Google Scholar]
- 55.Wilkinson G W, Akrigg A. Constitutive and enhanced expression from the CMV major IE promoter in a defective adenovirus vector. Nucleic Acids Res. 1992;20:2233–2239. doi: 10.1093/nar/20.9.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wysk M, Yang D D, Lu H T, Flavell R A, Davis R J. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc Natl Acad Sci USA. 1999;96:3763–3768. doi: 10.1073/pnas.96.7.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]