Abstract
Background:
The present study was conducted to assess level of cytokine and herpesvirus in peri-implantitis and healthy patients.
Methodology:
Fifty patients with healthy dental implant (Group I) and dental implant with peri-implantitis (Group II) were enrolled. The level of interleukin (IL)-1 β, IL-2, IL-4, IL-6, MCP-1 and tumor necrosis factor-α (TNF-α), and herpesvirus was estimated.
Results:
The mean IL-1 β level was 1.54 in Group I and 5.12 in Group II, IL-2 was 0.05 in Group I and 0.02 in Group II, IL-4 was 0.018 in Group I and 0.0021 in Group II, IL-6 was 0.83 in Group I and 0.92 in Group II, MCP-1 was 64.5 in Group I and 23.1 in Group II, and TNF-alpha was 1.54 in Group I and 1.05 in Group II. There was a significant higher level of TNF-alpha in virus + patients (P < 0.05).
Conclusion:
The cytokine level was increased in patients with peri-implantitis as compared to patients with healthy dental implants. Virus-positive patients exhibited higher cytokine levels than virus-negative patients.
KEYWORDS: Cytokine, dental implants, herpes
INTRODUCTION
Dental implants are regarded as a successful treatment modality for missing teeth. Although it has resulted in significant advancement in the field of dentistry, few complications such as peri-implantitis cannot be avoided. Peri-implantitis is the leading cause of destruction of soft and hard peri-implant tissues, ultimately leading to bone loss.[1] Patients who receive dental implants need to be clinically as well as radiographically assessed at routine follow-up. The diagnosis of peri-implant diseases seeks careful and extensive clinical measurements such as probing depth, clinical attachment levels (CALs), and bone loss. Diagnostic radiographs provide useful information regarding peri-implant status. In last few years, numerous researches have demonstrated less invasive diagnostic tools to notice periodontal and peri-implant diseases.[2]
Saliva is an oral fluid, biological in nature, noninvasive, and easily collected. It is useful in diagnosis of various diseases. Bacteria and the inflammatory processes together lead to the pathogenesis of peri-implantitis.[3] Human herpesvirus (HHV)-1 can result in the pathogenesis of periodontitis. Till date, eight different types of HHV have been recognized: herpes simplex viruses' Types 1 and 2, varicella-zoster virus, Epstein–Barr virus (EBV), cytomegalovirus (CMV), Roseoloviruses HHV-6 and HHV-7, and Kaposi sarcoma-associated herpesvirus (HHV-8).[4] It is found that EBV and HHV-8 are carcinogenic. HHV persists in human body and remains latent in the ganglion and can lead to infection in the presence of various precipitating factors. HHV has an active role in the progression of periodontitis. The viruses can provoke the inflammatory process and subsequently peri-implantitis.[5] The present study was conducted to assess the level of cytokine and herpesvirus in peri-implantitis and healthy patients.
METHODOLOGY
In this study, we enrolled 50 patients who received dental implants in the last 3 years. Only those patients who had at least one healthy dental implant (Group I) and one dental implant with peri-implantitis (Group II) were enrolled. Ethical approval was obtained before starting the study.
The presence of radiographic evidence of more than 3 mm bone loss, suppuration, bleeding on probing, and >4 mm probing depth stated peri-implantitis. Parameters such as bleeding on probing, probing depth, gingival bleeding, CAL, and suppuration were determined at six sites around each implant. DNA extraction of the saliva samples was performed using a DNA Blood Mini kit. HHV estimation was performed using polymerase chain reaction. The levels of interleukin (IL)-1 β, IL-2, IL-4, IL-6, MCP-1, and tumor necrosis factor-α (TNF-α) were measured using a high-sensitivity human cytokine 8-plex by Millipore. The results of the study were clubbed and analyzed using Mann–Whitney U test using a significant value below 0.05.
RESULTS
Table 1 shows that mean probing depth in Group I was 3.1 mm and in Group II was 5.2 mm, CAL in Group I was 0.16 mm and in Group II was 5.38 mm, % plaque site in Group I was 43.5 and in Group II was 32.7, gingival bleeding was 27.4 in Group I and 58.2 in Group II, bleeding on probing was 51.4 in Group I and 82.4 in Group II, and suppuration was 11.4 in Group II. The difference was significant (P < 0.05).
Table 1.
Assessment of parameters
| Parameters | Group I | Group II | P |
|---|---|---|---|
| Probing depth (mm) | 3.1 | 5.2 | 0.014 |
| CAL (mm) | 0.16 | 5.38 | 0.021 |
| Percentage plaque site | 43.5 | 32.7 | 0.09 |
| Gingival bleeding | 27.4 | 58.2 | 0.01 |
| Bleeding on probing | 51.4 | 82.4 | 0.035 |
| Suppuration | 0 | 11.4 | 0.017 |
CAL: Clinical attachment level
Table 2 shows that mean IL-1 β level was 1.54 in Group I and 5.12 in Group II, IL-2 was 0.05 in Group I and 0.02 in Group II, IL-4 was 0.018 in Group I and 0.0021 in Group II, IL-6 was 0.83 in Group I and 0.92 in Group II, MCP-1 was 64.5 in Group I and 23.1 in Group II, and TNF-alpha was 1.54 in Group I and 1.05 in Group II. The difference was nonsignificant (P > 0.05).
Table 2.
Assessment of cytokine level in both groups
| Cytokine | Group I | Group II | P |
|---|---|---|---|
| IL-1β | 1.54 | 5.12 | 0.72 |
| IL-2 | 0.05 | 0.02 | 0.13 |
| IL-4 | 0.018 | 0.0021 | 0.08 |
| IL-6 | 0.83 | 0.92 | 0.06 |
| MCP-1 | 64.5 | 23.1 | 0.001 |
| TNF-alpha | 1.54 | 1.05 | 0.72 |
MCP: Monocyte chemoattractant protein, TNF: Tumor necrosis factor, IL: Interleukin
Table 3 shows that in Group I, 19 were negative for viruses and in Group II, 16 were negative for viruses. Two patients were positive for EBV and HHV 7 and four for HHV in Group I. Two patients were positive for EBV in Group I, where as six patients were found positive for HHV 7. In Group II, four patients were EBV positive, one patient was HHV 6 positive, whereas nine patients were HHV 7 positive.
Table 3.
Assessment of virus in both groups
| EBV | HHV 6 | HHV 7 | CMV, VZV, HSV1, HSV2, HHV8 | |
|---|---|---|---|---|
| Group I | ||||
| Patient 1 | + | − | + | − |
| 5 | + | − | + | − |
| 8 | − | − | + | − |
| 10 | − | − | + | − |
| 16 | − | − | + | − |
| 23 | − | − | + | − |
| Group II | ||||
| Patient 2 | + | − | + | − |
| 4 | − | − | + | − |
| 7 | − | − | + | − |
| 11 | − | − | + | − |
| 14 | − | − | + | − |
| 17 | − | − | + | − |
| 20 | + | − | + | − |
| 22 | + | − | + | − |
| 23 | + | + | + | − |
+: Positive, −: Negative, EBV: Epstein-Barr virus, HHV: Human herpesvirus, CMV: Cytomegalovirus, VZV: Varicella-zoster virus, HSV: Human herpesvirus
Table 4 shows a significant higher level of TNF-alpha in virus + patients (P < 0.05).
Table 4.
Comparison of cytokine level in virus-positive and negative patients
| Cytokine | Virus + | Virus − | P |
|---|---|---|---|
| IL-1β | 17.2 | 6.9 | 0.61 |
| IL-2 | 0.06 | 0.04 | 0.15 |
| IL-4 | 0.017 | 0.009 | 0.06 |
| IL-6 | 0.85 | 0.81 | 0.09 |
| MCP-1 | 31.2 | 62.3 | 0.08 |
| TNF-alpha | 1.82 | 0.46 | 0.04 |
+: Positive, −: Negative, IL: Interleukin, MCP: Monocyte chemoattractant protein, TNF: Tumor necrosis factor
DISCUSSION
Saliva and crevicular fluid are frequently used fluids for the assessment of biomarkers for the diagnosis of periodontal and peri-implant diseases. It is established fact that 90% of individuals are infected with herpesvirus in their life span. Hence, the occurrence of herpes viruses in the body is not uncommon.[6] They remain inactive in the ganglion and become active in the presence of precipitating factors leading to an active infection. Further, it is ascertained that herpesviruses can aggravate periodontal breakdown. Thus, detection of viruses in patients having dental implants may be helpful in assessing peri-implantitis.[7] Considering this, this study assessed cytokine levels as well as herpes virus in patients with peri-implantitis and healthy dental implant patients with no signs of peri-implantitis.
For the study, we enrolled 50 patients, which were classified into Group I (healthy dental implant) and Group II (peri-implantitis). Marques et al.[8] in their study on 42 patients found no statistically significant differences in cytokine levels between patients with healthy implants and those with peri-implantitis (P > 0 05). Patients with peri-implantitis exhibited a 1.97-fold higher occurrence of herpesvirus as compared to healthy subjects. The association of the presence or absence of herpesvirus with the salivary markers was statistically significant for MIP-1 β and TNF-α (P = 0 0437) only in the peri-implantitis group.
We observed that the mean probing depth in Group I was 3.1 mm and in Group II was 5.2 mm, CAL in Group I was 0.16 mm and in Group II was 5.38 mm, % plaque site in Group I was 43.5 and in Group II was 32.7, gingival bleeding was 27.4 in Group I and 58.2 in Group II, bleeding on probing was 51.4 in Group I and 82.4 in Group II, and suppuration was 11.4 in Group II. The study of Nowzari et al.[9] revealed that there is an increase level of cytokines around teeth and implants in the presence of periodontal pathogens. Jankovic et al.[10] signified a significant CMV and EBV role in the progression of peri-implantitis. The authors found a high incidence of both viruses in subgingival plaque at peri-implantitis sites.
In this study, the mean IL-1 β level found to be 1.54 in Group I and 5.12 in Group II, IL-2 was 0.05 in Group I and 0.02 in Group II, IL-4 was 0.018 in Group I and 0.0021 in Group II, IL-6 was 0.83 in Group I and 0.92 in Group II, MCP-1 was 64.5 in Group I and 23.1 in Group II, and TNF-alpha was 1.54 in Group I and 1.05 in Group II [Graph 1]. Teles et al.[11] in their study detected cytokine levels in 74 periodontitis patients and 44 healthy subjects. The authors found no statistically significant differences in cytokines level between the groups.
Graph 1.
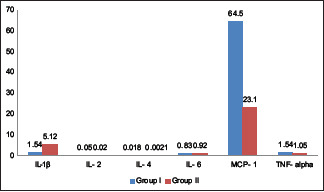
Assessment of cytokine level in both the groups
We found that in Group I, 19 were negative for viruses and in Group II, 16 were negative for viruses. In Group I, two patients were EBV positive and seven patients were HHV 7 positive. But in Group II, four were EBV positive, one was HHV 6 positive and nine were HHV 7 positive. Miranda et al.[12] compared cytokine level between healthy and diseased sites in patients with untreated periodontitis in 112 patients having 448 greatest common factor (GCF) samples. The GCF levels of cytokines were significantly higher in the diseased in comparison to healthy sites (P < 0.05). Levels of IL-8 and MIP-1α were significantly higher in the healthy than in the diseased sites (P < 0.05). In the healthy sites, IL-8 and MIP-1α formed an independent cluster of cytokines and MIP-1α positively correlated with Porphyromonas gingivalis (P < 0.05).
The shortcoming of the study is a small sample size.
CONCLUSION
The authors found increased cytokine level in patients with peri-implantitis patients as compared to patients with healthy dental implants. Virus-positive patients exhibited higher cytokine levels than virus-negative patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Verdugo F, Castillo A, Castillo F, Uribarri A. Epstein-Barr virus associated peri-implantitis: A split-mouth study. Clin Oral Investig. 2015;2:535–43. doi: 10.1007/s00784-014-1250-1. [DOI] [PubMed] [Google Scholar]
- 2.Verdugo F, Castillo A, Simonian K, Castillo F, Farez-Vidal E, D'Addona A. Periodontopathogen and Epstein-Barr virus-associated periapical periodontitis may be thesource of retrograde infectious peri-implantitis. Clin Implant Dent Relat Res. 2015;17:199–207. doi: 10.1111/cid.12083. [DOI] [PubMed] [Google Scholar]
- 3.Fonseca FJ, Junior MM, Lourenço EJ, deMoraes Teles D, Figueredo CM. Cytokines expression insaliva and peri-implant crevicular fluid of patients with peri-implantdisease. Clin Oral Implants Res. 2014;25:68–72. doi: 10.1111/clr.12052. [DOI] [PubMed] [Google Scholar]
- 4.Faot F, Nascimento GG, Bielemann AM, Campão TD, Leite FR, Quirynen M. Can peri-implant crevicularfluid assist in the diagnosis of peri-implantitis A systematicreview and meta-analysis. J Periodontol. 2015:631–45. doi: 10.1902/jop.2015.140603. [DOI] [PubMed] [Google Scholar]
- 5.Holmlund A, Hänström L, Lerner UH. Bone resorbing activity and cytokine levels in gingival crevicular fluid before and after treatment of periodontal disease. J Clin Periodontol. 2004;31:475–82. doi: 10.1111/j.1600-051X.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 6.Reinhardt RA, Masada MP, Kaldahl WB, DuBois LM, Kornman KS, Choi JI, et al. Gingival fluid IL-1 and IL-6 levels in refractory periodontitis. J Clin Periodontol. 1993;20:225–31. doi: 10.1111/j.1600-051x.1993.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 7.Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol. 2000;71:1535–45. doi: 10.1902/jop.2000.71.10.1535. [DOI] [PubMed] [Google Scholar]
- 8.Marques Filho JS, Gobara J, Jr, da Silva Salomao GV, Sumita LM, Shibli JA, Viana RG, et al. Cytokine levels and human herpesviruses in saliva from clinical periodontal healthy subjects with peri-implantitis: A case-control study. Mediators Inflamm. 2018;2018:6020625. doi: 10.1155/2018/6020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowzari H, Botero JE, DeGiacomo M, Villacres MC, Rich SK. Microbiology and cytokine levels around healthydental implants and teeth. Clin Implant Dent Relat Res. 2008;10:166–73. doi: 10.1111/j.1708-8208.2007.00076.x. [DOI] [PubMed] [Google Scholar]
- 10.Jankovic S, Aleksic Z, Dimitrijevic B, Lekovic V, Milinkovic I, Kenney B. Correlation between differentgenotypes of human cytomegalovirus and Epstein-Barr virusand peri-implant tissue status. Aust Dent J. 2011;4:382–8. doi: 10.1111/j.1834-7819.2011.01360.x. [DOI] [PubMed] [Google Scholar]
- 11.Teles RP, Likhari V, Socransky SS, Haffajee AD. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: A cross-sectional study. J Periodontal Res. 2009;44:411–7. doi: 10.1111/j.1600-0765.2008.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miranda TS, Figueiredo NF, Figueiredo LC, Silva HDPD, Rocha FR, Duarte PM. Cytokine profiles of healthy and diseased sites in individuals with periodontitis. Arch Oral Biol. 2020;120:104957. doi: 10.1016/j.archoralbio.2020.104957. [DOI] [PubMed] [Google Scholar]


