Abstract
Background:
Anastomotic leak is a feared complication after left-sided colectomy, but its risk can potentially be reduced with the use of a diverting ostomy. However, an ostomy has its own associated negative sequelae, therefore, it is critical to appropriately identify patients to divert. This is difficult in practice since many risk factors for anastomotic leak exist and outside factors bias this decision. We aimed to develop and validate a risk score to predict an individual’s risk of anastomotic leak and aid in the decision.
Methods:
The American College of Surgeons National Surgical Quality Improvement Program Colectomy Targeted PUF was queried from 2012–2016 for patients undergoing elective left-sided resection for malignancy, benign neoplasm, or diverticular disease. Multivariable logistic regression identified predictors of anastomotic leak in non-diverted patients, and a risk score was developed and validated.
Results:
38,475 patients underwent resection with an overall anastomotic leak rate of 3%. Independent risk factors for anastomotic leak included younger age, male sex, tobacco use, and omission of combined bowel preparation. A risk score incorporating independent predictors demonstrated excellent calibration. There was strong visual correspondence between predicted and observed anastomotic leak rates. 3,960 patients underwent resection with diversion, yet over half of these patients had a predicted leak rate of less than 4%.
Conclusion:
A novel risk score can be used to stratify patients according to anastomotic leak risk after elective left-sided resection. Intraoperative calculation of scores for patients can help guide surgical decision-making in both diverting the highest risk patients and avoiding diversion in low-risk patients.
Keywords: anastomotic leak, risk score, temporary stoma, colectomy
INTRODUCTION
An anastomotic leak complicates 3–20% of left-sided colorectal resections [1–5] and is associated with increased morbidity, mortality, and healthcare costs [6, 7]. Temporary intestinal diversion can reduce both the overall risk of anastomotic leak and the severity of an anastomotic leak when one does occur [5, 8, 9]. However, a diverting stoma has its own associated morbidity, including an increased risk of readmission for dehydration and the requirement of an additional operation to restore intestinal continuity [10, 11].
Consequently, there is a need to accurately estimate an individual patient’s risk of anastomotic leak so that high-risk patients can be diverted, while simultaneously, overutilization of diversion in patients at low risk of anastomotic leak is avoided. This is difficult in practice for several reasons, including that there are a wide variety of identified risk factors for anastomotic leak [12]. Additionally, the availability heuristic and the operating surgeon’s personality influence the perceived risk of anastomotic leak and the decision to divert a patient [13, 14]. Lastly, existing decision tools either were developed to apply to all colorectal resections [15, 16], to only oncologic resections [15], or omit clinically relevant risk factors from the calculation of anastomotic leak probability [15–17].
Therefore, our aim was to develop and validate a risk score composed of objective preoperative and intraoperative factors that a surgeon can use to both predict an individual’s risk of anastomotic leak and to aid their decision-making process of whether a patient warrants temporary diversion after elective left-sided resection.
METHODS
Data Source
The American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) 2012–2016 participant user files (PUFs) were utilized and merged with the ACS NSQIP Colectomy Targeted PUFs from the same years. Trained data abstractors at each participating hospital collect the requisite data based on protocols as defined by ACS NSQIP. The ACS performs site audits to ensure reliability of the data. Since ACS NSQIP data is de-identified, it is exempt from review by our institutional review board.
Cohort and Primary Outcome
Patients undergoing an elective left-sided resection with anastomosis for a diagnosis of a left-sided cancer, benign neoplasm, or diverticular disease were identified (Table 1). Since our aim was to develop an anastomotic leak risk score that could be used to help estimate risk preoperatively, patients undergoing non-elective surgery were excluded (full cohort development depicted in Figure 1). Consistent with prior work, patients who underwent concurrent intestinal diversion as defined by a secondary CPT for ostomy construction (CPTs 44187, 44188, and 44310) were excluded from the cohort utilized to develop the anastomotic leak risk score [15, 16]. However, leak scores were calculated for these patients (n=3,960) post hoc to determine their estimated probability of anastomotic leak at the time the decision was made to divert them. The primary outcome was the development of an anastomotic leak within 30 days of surgery that required treatment with antibiotics, percutaneous drainage, or reoperation.
Table 1.
Operative CPTs and Diagnosis Codes Included in the Study
| Current Procedural Terminology Code | Anastomosis |
|---|---|
| 44140, 44204 | Colocolostomy |
| 44145, 44207 | Coloproctostomy (low pelvic) |
| ICD 9/10 Code | Diagnosis Group |
| 153.1, 153.2, 153.3, 153.7, 154.0, 154.1, C18.4, C18.5, C18.6, C18.7, C19, C20 | Malignancy |
| 211.3, D12.3, D12.4, D12.5 | Benign neoplasm |
| 562.1x, K57.2x, K57.3x | Diverticular disease |
Figure 1.
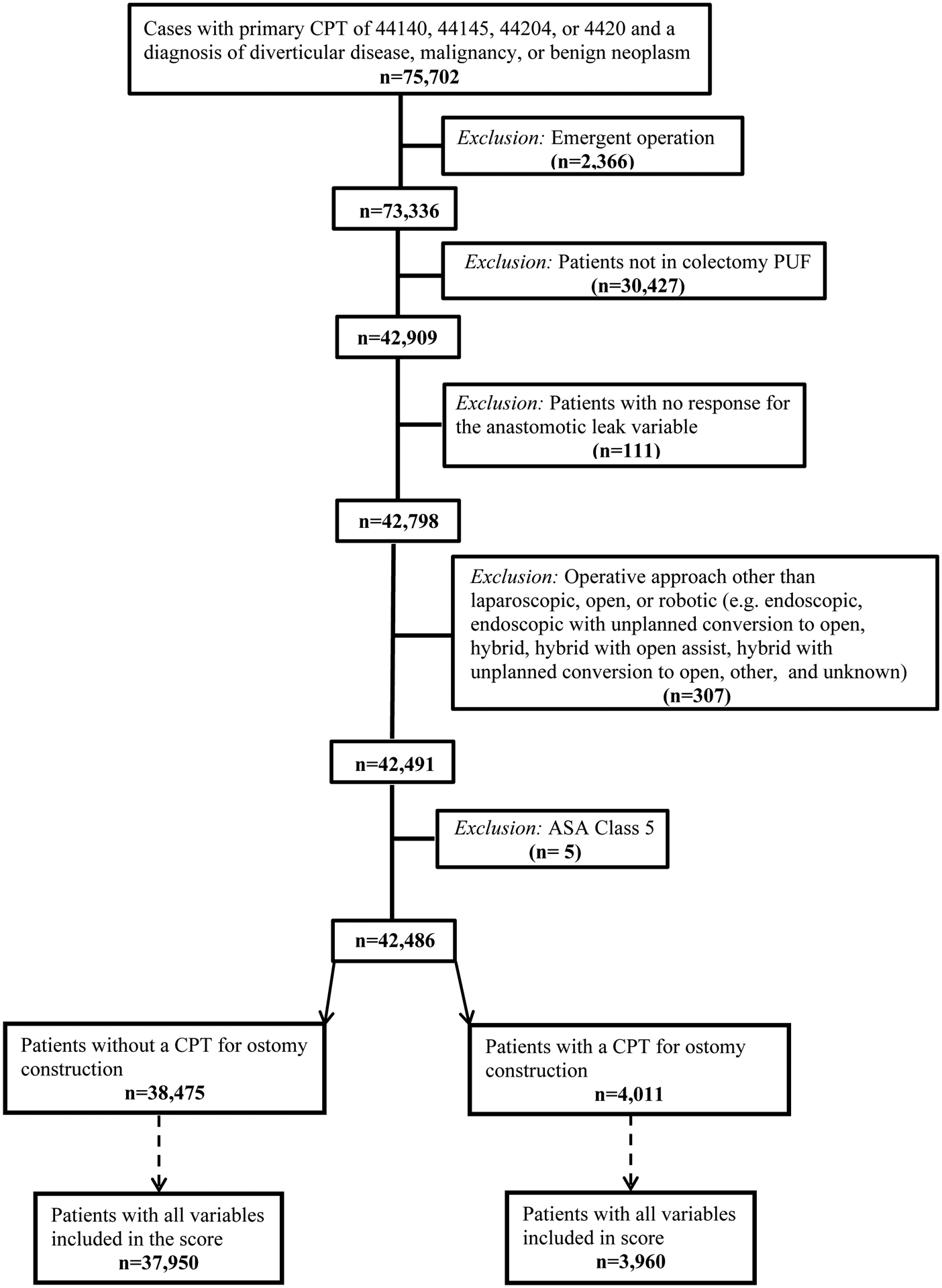
Flow chart of cohort development
Covariates
Demographic variables collected by ACS NSQIP include age, sex, and race/ethnicity. Comorbidities abstracted included hypertension requiring medication, diabetes mellitus treated with oral medications or insulin, preoperative requirement of dialysis, steroid/immunomodulator use for a chronic condition, smoking status (positive if patient smoked cigarettes at any point in the prior 12 months), preoperative weight loss > 10% in the preceding 6 months, and receipt of chemotherapy within 90 days before surgery. Preoperative laboratories assessed included hematocrit (anemia if hematocrit < 30%) and albumin (abnormal if < 3.5 g/dL). For preoperative bowel preparation, a multi-tiered variable was created based on the responses to the receipt of mechanical bowel preparation and receipt of oral antibiotic bowel preparation variables. If a patient received both, they were classified as “combined,” receipt of neither was considered “none,” and receipt of one but not the other was considered either “mechanical only” or “antibiotic only.” If a patient was missing a response for both oral antibiotic preparation and mechanical antibiotic preparation, they were classified as “missing.” If a patient had a response for either mechanical or antibiotic preparation but was missing a response for the other preparation type, the missing preparation was considered to be not administered. Operative characteristics included patient American Society of Anesthesiologists (ASA) class, diagnostic indication (cancer, benign neoplasm, or diverticular disease), surgical approach, anastomosis location (coloproctostomy versus colocolostomy), wound classification, and operative time. Operative approach was obtained from the ACS NSQIP Colectomy Targeted PUF and anastomosis location was defined by CPT code. These variables were combined to create an “operative approach with anastomosis location” variable.
Statistical Analysis
Categorical data are presented as number (percent) and continuous data are presented as median (interquartile range [IQR]). Univariate comparisons were performed with either Chi-square or Fisher’s exact test depending on expected sample counts for the categorical data. Backward elimination multivariable logistic regression with an entry criteria of p < 0.05 on univariate analysis of anastomotic leak and a stay criteria of p < 0.05 was used to construct a parsimonious model for anastomotic leak. Covariates present in less than 0.7% of included patients were not in the model to avoid overfitting. The discrimination of the model was assessed via the c-index. Both 10-fold cross validation and bootstrapping (200 replications) were performed to assess the internal validity of the model. For covariates with missing data greater than 5% of the time, a “missing” variable was created and included as a term in the univariate and multivariable analysis. Otherwise, missing data was excluded from analysis.
Anastomotic Leak Score Development and Application
The maximum likelihood estimates of the predictors in the final multivariable model were multiplied by 10 and rounded to the nearest integer to assign point values to each predictor that are more easily applicable [18]. This resulted in a range of possible scores from −10 to 43 points. The predicted anastomotic leak rate was then calculated for each point value via the equation [19]:
Where is the estimated probability of anastomotic leak, β0 is the logistic regression intercept (−4.6525) and Xi is the total number of points divided by 10. An anastomotic leak risk score was then calculated for each patient who underwent a primary anastomosis without diversion. Risk scores of included patients ranged from a minimum of −10 to a maximum of 34. Ten deciles of risk were then created based on the potential total point scores (point possibilities within each decile are available in Figure 2 and Table 6; the range of values is 5 points in decile 9 and 6 points in deciles 1 and 10 to account for the sparsity of observations at the extremes). The predicted anastomotic leak rate was then calculated by averaging the equation-generated anastomotic leak rate for each score within a risk decile. The observed anastomotic leak rate and its continuity-corrected 95% confidence interval (CI) was then calculated for all patients included in each risk decile (i.e., 7,424 patients had a score of between 4 and 7 points and 122 experienced an anastomotic leak, so the observed anastomotic leak rate was 1.64%). Visual correspondence was assessed between the predicted anastomotic leak rate and the observed anastomotic leak rate within each risk decile. Lastly, anastomotic leak risk scores were calculated for the cohort of patients excluded from model development, secondary to intestinal diversion. This allowed assessment of the use of intestinal diversion based on predicted probability of anastomotic leak. All analysis was performed using SAS 9.4 software (SAS Institute, Cary, NC) with significance set at p<0.05.
Figure 2.
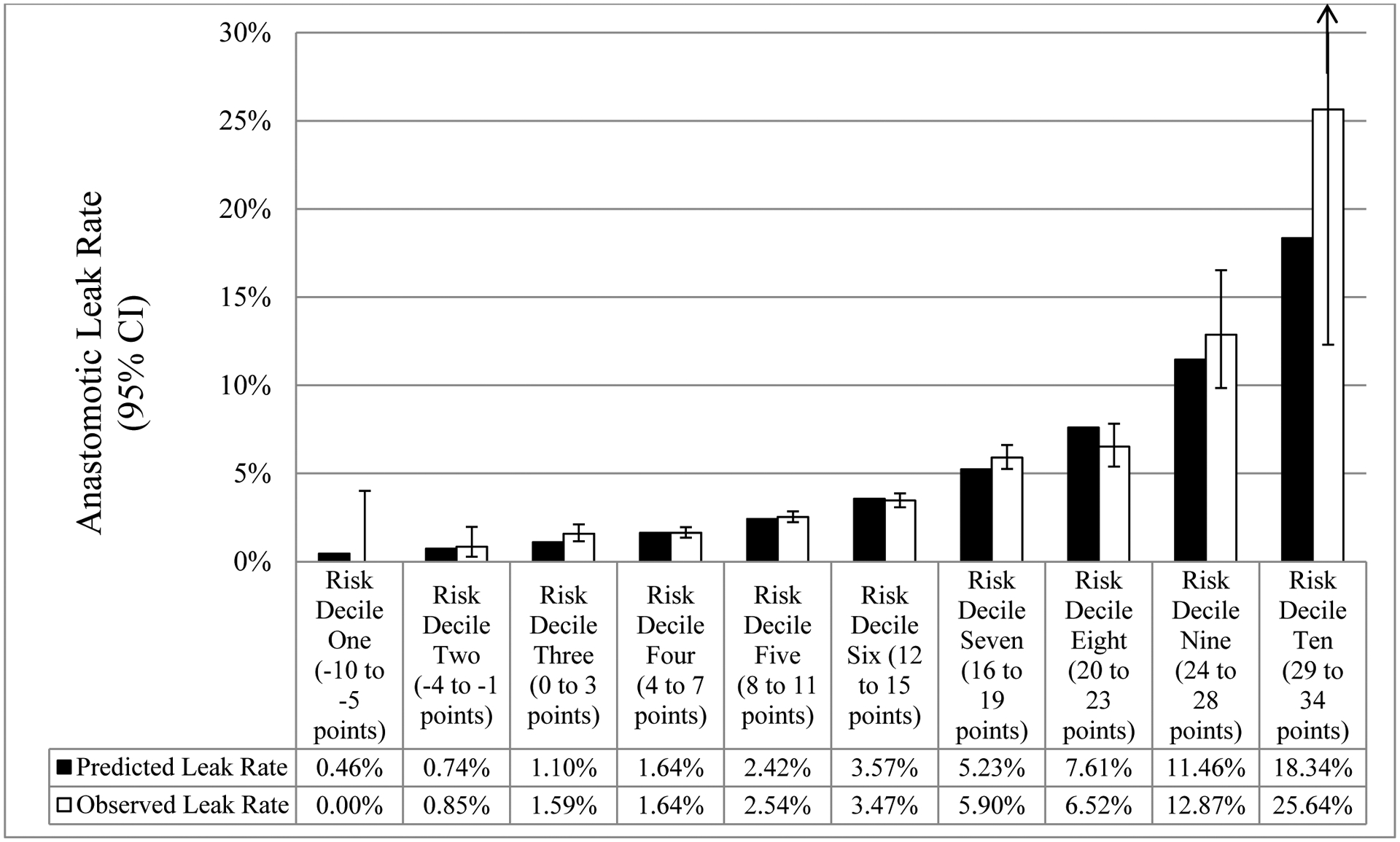
Comparison between mean model-predicted and observed (95% confidence interval) anastomotic leak rate across the risk score deciles in non-diverted patients
a Upper limit of 95% confidence interval for risk decile 10 is 47.15%
Table 6.
Distribution of patients by risk score stratified by diverted versus nota
| Total operations, n (column % / row %) | ||
|---|---|---|
| Risk Decile: Point Range | Number of Patients Not Diverted (n=37,950) | Number of Patients Diverted (n=3,960) |
| Risk Decile 1: −10 to −5 points | 92 (<1% / 100%) | 0 (0% / 0%) |
| Risk Decile 2: −4 to −1 points | 589 (2% / 100%) | 0 (0% / 0%) |
| Risk Decile 3: 0 to 3 points | 2,898 (8% / 98%) | 56 (1% / 2%) |
| Risk Decile 4: 4 to 7 points | 7,424 (20% / 97%) | 252 (6% / 3%) |
| Risk Decile 5: 8 to 11 points | 10,514 (28% / 93%) | 783 (20% / 7%) |
| Risk Decile 6: 12 to 15 points | 9,056 (24% / 89%) | 1138 (29% / 11%) |
| Risk Decile 7: 16 to 19 points | 5,085 (13% / 84%) | 975 (25% / 16%) |
| Risk Decile 8: 20 to 23 points | 1,779 (5% / 77%) | 545 (14% / 23%) |
| Risk Decile 9: 24 to 28 points | 474 (1% / 71%) | 190 (5% / 29%) |
| Risk Decile 10: 29 to 34 points | 39 (<1% / 65%) | 21 (1% / 35%) |
First thickened line indicates where predicted anastomotic leak rate crosses 5% and second thickened black line indicates where predicted anastomotic leak rate crosses 10%
RESULTS
A total of 38,475 patients (51% female) with a median age of 61 (IQR, 52–70) years underwent left-sided resection without diversion. The most common diagnostic indication was diverticular disease (n=18,328; 48%), followed by malignancy (n=15,546; 40%), and benign neoplasm (n=4,601; 12%). Over 75% of the operations were completed minimally invasively and a coloproctostomy was performed in 47% of cases (Table 2). The overall 30-day anastomotic leak rate was 3.3%.
Table 2.
Demographic and Operative Characteristics
| Characteristic | Value |
|---|---|
| Age, years, median (IQR) | 61 (52–70) |
| Female, n (%) | 19,515 (51) |
| Diagnostic indication, n (%) | |
| Diverticular disease | 18,328 (48) |
| Malignancy | 15,546 (40) |
| Benign neoplasm | 4,601 (12) |
| Approach with anastomosis location, n (%) | |
| Laparoscopic with colocolostomy | 15,205 (40) |
| Laparoscopic with coloproctostomy | 12,743 (33) |
| Open with colocolostomy | 3,769 (10) |
| Open with coloproctostomy | 3,295 (9) |
| Robotic with coloproctostomy | 1,988 (5) |
| Robotic with colocolostomy | 1,475 (4) |
On univariate analysis of anastomotic leak, younger age and male sex were both significant predictors. Patient characteristics/comorbidities that were significantly different between those with an anastomotic leak and those without included tobacco use, hypertension, and anemia. Perioperative factors associated with anastomotic leak included receipt of neoadjuvant chemotherapy, increasing ASA class, omission of combined oral antibiotic and mechanical bowel preparation, operative approach with anastomosis location, wound classification, and operative time (Table 3).
Table 3.
Univariate analysis of potential risk factors for anastomotic leak
| Characteristic, n (%) | Anastomotic leak - yes (n=1,260) | Anastomotic leak - no (n=37,215) | p-value |
|---|---|---|---|
| Age, years | 0.03 | ||
| 18–39 | 86 (7) | 1857 (5) | |
| 40–49 | 178 (14) | 5,031 (14) | |
| 50–59 | 336 (27) | 10,166 (27) | |
| 60–69 | 353 (28) | 10,557 (28) | |
| 70–79 | 203 (16) | 6,752 (18) | |
| 80+ | 104 (8) | 2,852 (8) | |
| Sex, male | 731 (58) | 18,229 (49) | <0.01 |
| Body mass index, kg/m2 | 0.02 | ||
| < 18.5 | 25 (2) | 521 (1) | |
| 18.5–24.9 | 284 (23) | 9,054 (25) | |
| 25.0–29.9 | 418 (34) | 13,184 (36) | |
| 30.0+ | 522 (42) | 14,244 (39) | |
| Missing | 11 | 212 | |
| Tobacco use | 311 (25) | 6,125 (17) | <0.01 |
| ASA Class | <0.01 | ||
| I/II | 574 (46) | 20,676 (56) | |
| III | 635 (50) | 15,462 (42) | |
| IV | 50 (4) | 1,033 (3) | |
| Missing | 1 | 44 | |
| Diagnosis | <0.01 | ||
| Diverticular disease | 522 (44) | 17,776 (48) | |
| Cancer | 576 (46) | 14,970 (40) | |
| Benign neoplasm | 132 (11) | 4,469 (12) | |
| Hypertension | 636 (51) | 17,683 (48) | 0.04 |
| Diabetes mellitus treatment | 0.36 | ||
| Insulin | 52 (4) | 1,479 (4) | |
| Oral | 139 (11) | 3,666 (10) | |
| None | 1,069 (85) | 32,070 (86) | |
| Chronic obstructive pulmonary disease | 74 (6) | 1,473 (4) | <0.01 |
| Bleeding disorder | 38 (3) | 823 (2) | 0.06 |
| Steroid/immunomodulator use | 50 (4) | 1,108 (3) | 0.04 |
| >10% weight loss in prior 6 months | 60 (5) | 1,044 (3) | <0.01 |
| Hematocrit < 30% | <0.01 | ||
| No | 1,104 (88) | 33,412 (90) | |
| Yes | 85 (7) | 1629 (4) | |
| Missing | 71 (6) | 2,174 (6) | |
| Albumin < 3.5 g/dL | <0.01 | ||
| Yes | 177 (14) | 3,818 (10) | |
| No | 650 (52) | 20,332 (55) | |
| Missing | 433 (34) | 13,065 (35) | |
| Neoadjuvant chemotherapy | <0.01 | ||
| Yes | 88 (7) | 1,376 (4) | |
| No | 1,154 (93) | 35,379 (96) | |
| Missing | 18 | 460 | |
| Preoperative systemic inflammatory response syndrome or sepsis or septic shock | 28 (2) | 497 (1) | <0.01 |
| Bowel preparation | <0.01 | ||
| Combined | 281 (22) | 12,761 (34) | |
| Mechanical only | 411 (33) | 11,456 (31) | |
| Antibiotic only | 50 (4) | 1,743 (5) | |
| None | 386 (31) | 7,281 (20) | |
| Missing | 132 (11) | 3,974 (11) | |
| Approach with anastomosis location | <0.01 | ||
| Laparoscopic with colocolostomy | 457 (36) | 14,748 (40) | |
| Laparoscopic with coloproctostomy | 395 (31) | 12,348 (33) | |
| Open with colocolostomy | 190 (15) | 3,579 (10) | |
| Open with coloproctostomy | 120 (10) | 3,175 (9) | |
| Robotic with colocolostomy | 22 (2) | 1,453 (4) | |
| Robotic with coloproctostomy | 76 (6) | 1,912 (5) | |
| Operative time, minutes | <0.01 | ||
| ≤ 122 | 226 (18) | 8,381 (23) | |
| 123–168 | 278 (22) | 9,506 (26) | |
| 169–225 | 333 (26) | 9,736 (26) | |
| ≥ 226 | 423 (34) | 9,590 (26) | |
| Missing | 0 | 2 | |
| Wound classification | <0.01 | ||
| I/II | 945 (75) | 30,351 (82) | |
| III/IV | 315 (25) | 6,864 (18) |
On multivariable analysis, younger age, male sex, tobacco use, anemia, receipt of neoadjuvant chemotherapy, a diagnostic indication of cancer, bowel preparation other than combined, ASA class III and IV, wound class of III/IV, an open operative approach, and increasing operative time were independent risk factors for anastomotic leak (Table 4). The model demonstrated excellent calibration on validation (raw c-index 0.66, 10-fold cross validation c-index 0.65, and bootstrap 200-replication c-index 0.65). The maximum likelihood estimates of these risk factors were used to develop an anastomotic leak risk calculator, and individual point values ranged from a low of −8 for robotic colectomy with colocolostomy to a high of 8 for omission of combined mechanical and oral antibiotic bowel prep (Table 4).
Table 4.
Multivariable logistic regression analysis of risk factors for anastomotic leak and their associated point values
| Variable | Odds Ratio (95% CI) | Points Assigned |
|---|---|---|
| Age, years | ||
| 18–39 | 1.6 (1.2–2.1) | 5 |
| 40–49 | 1.3 (1.0–1.6) | 3 |
| 50–59 | 1.2 (1.0–1.4) | 2 |
| 60–69 | 1.2 (1.0–1.4) | 1 |
| 70–79 | Reference | 0 |
| 80+ | 1.1 (0.9–1.4) | 1 |
| Sex | ||
| Female | Reference | 0 |
| Male | 1.4 (1.2–1.5) | 3 |
| Tobacco use | ||
| No | Reference | 0 |
| Yes | 1.5 (1.3–1.8) | 4 |
| Hematocrit < 30% | ||
| No | Reference | 0 |
| Yes | 1.3 (1.0–1.7) | 3 |
| Missing | 1.1 (0.9–1.5) | 1 |
| Diagnostic indication | ||
| Cancer | Reference | 0 |
| Benign neoplasm | 0.9 (0.7–1.1) | −1 |
| Diverticular disease | 0.8 (0.7–1.0) | −2 |
| Neoadjuvant chemotherapy with last dose within 90 days | ||
| No | Reference | 0 |
| Yes | 1.6 (1.2–2.0) | 4 |
| Bowel Prep | ||
| Combined | Reference | 0 |
| No prep | 2.3 (2.0–2.7) | 8 |
| Mechanical only | 1.6 (1.4–1.9) | 5 |
| Oral antibiotic only | 1.3 (1.0–1.8) | 3 |
| Missing | 1.5 (1.2–1.8) | 4 |
| ASA class | ||
| I/II | Reference | 0 |
| III | 1.4 (1.2–1.6) | 3 |
| IV | 1.4 (1.0–2.0) | 4 |
| Approach with anastomosis location | ||
| Laparoscopic with colocolostomy | Reference | 0 |
| Robotic with colocolostomy | 0.5 (0.3–0.7) | −8 |
| Laparoscopic with coloproctostomy | 1.0 (0.8–1.1) | −1 |
| Open with coloproctostomy | 1.0 (0.8–1.2) | 0 |
| Robotic with coloproctostomy | 1.1 (0.9–1.5) | 1 |
| Open with colocolostomy | 1.5 (1.3–1.8) | 4 |
| Operative time, minutes | ||
| ≤ 122 | Reference | 0 |
| 123–168 | 1.1 (0.9–1.3) | 1 |
| 169–225 | 1.3 (1.1–1.5) | 3 |
| ≥ 226 | 1.6 (1.3–1.9) | 4 |
| Wound class | ||
| I/II | Reference | 0 |
| III/IV | 1.5 (1.3–1.7) | 4 |
Predicted leak rates ranged across the risk deciles, from a low of 0.5% for a score of −10 to −5 points (risk decile 1) to a high of 18.3% for a score of 29–34 points (risk decile 10). There was strong visual correspondence between the calculated anastomotic leak rates and the predicted anastomotic leak rates. The predicted anastomotic leak rates were within the 95% CI of the actual anastomotic leak rate across all risk deciles except risk decile 3 (predicted: 1.10% versus 95% CI lower limit: 1.16%) and risk decile 7 (predicted: 5.23% versus 95% CI lower limit: 5.25%). Of note, risk deciles 9 and 10 had significantly higher anastomotic leak rates than the other deciles (Figure 2).
Comparing patients who were diverted versus not diverted at the time of surgery revealed that all independent risk factors for anastomotic leak were also significantly associated with the use of diversion (Table 5). However, the presence of multiple risk factors did not substantially alter the decision to divert with only 35% of all patients with a predicted anastomotic leak rate of greater than 18% receiving a temporary ostomy. Further, of the 3,960 patients who were diverted at the time of left-sided colectomy, 56% would have been in risk decile 6 or less, indicating a predicted anastomotic leak rate of 0.5%−3.6% (Table 6).
Table 5.
Univariate analysis of the use of diversion by significant risk factors for anastomotic leak
| Characteristic, n (%) | Not Diverted (n=37,950) | Diverted (n=3,960) | p-value |
|---|---|---|---|
| Age, years | <0.01 | ||
| 18–39 | 1,917 (5) | 220 (6) | |
| 40–49 | 5,149 (14) | 569 (14) | |
| 50–59 | 10,385 (27) | 1,219 (31) | |
| 60–69 | 10,758 (28) | 1,136 (29) | |
| 70–79 | 6,844 (18) | 617 (16) | |
| 80+ | 2,897 (8) | 199 (5) | |
| Sex, male | 18,697 (49) | 2,311 (58) | <0.01 |
| Tobacco use | 6,351 (17) | 810 (21) | <0.01 |
| Hematocrit < 30% | <0.01 | ||
| No | 34,045 (90) | 3,665 (93) | |
| Yes | 1,686 (4) | 178 (5) | |
| Missing | 2,219 (6) | 117 (3) | |
| Diagnostic indication | <0.01 | ||
| Cancer | 15,242 (40) | 3,112 (79) | |
| Benign neoplasm | 4,548 (12) | 19 (1) | |
| Diverticular disease | 18,160 (48) | 829 (21) | |
| Neoadjuvant chemotherapy with last dose within 90 days | <0.01 | ||
| Yes | 1,463 (4) | 1,771 (45) | |
| Bowel Prep | <0.01 | ||
| Mechanical + antibiotic | 12,936 (34) | 1,538 (39) | |
| No prep | 11,779 (31) | 1,200 (30) | |
| Mechanical only | 1,749 (5) | 153 (4) | |
| Oral antibiotic only | 7,634 (20) | 710 (18) | |
| Missing | 3,852 (10) | 359 (9) | |
| ASA class | <0.01 | ||
| I/II | 21,013 (55) | 1,784 (45) | |
| III | 15,875 (42) | 2,069 (52) | |
| IV | 1,062 (3) | 107 (3) | |
| Approach with anastomosis location | <0.01 | ||
| Laparoscopic with colocolostomy | 15,036 (40) | 194 (5) | |
| Robotic with colocolostomy | 1,424 (4) | 18 (1) | |
| Laparoscopic with coloproctostomy | 12,599 (33) | 1,886 (48) | |
| Open with coloproctostomy | 3,235 (9) | 1,130 (29) | |
| Robotic with coloproctostomy | 1,948 (5) | 572 (14) | |
| Open with colocolostomy | 3,708 (10) | 160 (4) | |
| Operative time (minutes) | <0.01 | ||
| ≤ 122 | 8,493 (22) | 210 (5) | |
| 123–168 | 9,663 (26) | 401 (10) | |
| 169–225 | 9,934 (26) | 916 (23) | |
| ≥ 226 | 9,860 (26) | 2,433 (61) | |
| Wound class | <0.01 | ||
| I/II | 30,854 (81) | 3,082 (78) | |
| III/IV | 7,096 (19) | 878 (22) |
DISCUSSION
Anastomotic leak is a feared complication after colectomy and potentially preventable with the use of temporary intestinal diversion. However, ileostomies have their own associated morbidity, so it is critical to accurately identify patients at high risk of anastomotic leak so that intestinal diversion is not under or overused. We therefore developed a validated anastomotic leak risk calculator that uses eleven readily available variables to preoperatively predict the risk of anastomotic leak following elective left-sided resections. Surgeons can use this risk score to calculate the potential risk of anastomotic leak and inform both their preoperative discussion of risks with the patient and their own intraoperative surgical decision-making on which patients warrant diversion.
Currently, the operating surgeon decides whether an anastomosis should have a diverting ostomy either before the operation is performed or after performance of the anastomosis intraoperatively. This decision is based on his or her estimate of the risk of anastomotic leakage, which is informed by the presence of established preoperative risk factors, intraoperative findings, and their own personal experience. Unfortunately, surgeons are poor predictors of anastomotic leak risk [20], and their poor estimates are likely secondary to the influence of external biases that do not actually affect the anastomotic leak risk in an individual patient. While the study of the use of heuristic techniques for surgical decision-making is a developing field, it has recently been shown that having a patient recently die secondary to an anastomotic leak and being criticized for a recent anastomotic leak both influence the decision on whether to divert a patient [13]. Additionally, surgeon personality influences the decision to divert with a propensity for risk-taking in a surgeon’s everyday life reducing the probability of defunctioning an anastomosis [14].
Given the influence of these outside forces, grounding the estimated risk by using the developed anastomotic risk score may help optimize surgical decision-making on diversion and help inform preoperative risk counseling. By making this decision more objective, the patients at highest risk of an anastomotic leak who have the most to gain from temporary diversion can be readily identified. Simultaneously, patients whose score puts them at low risk of anastomotic leak could potentially avoid having a temporary ostomy. Although most of the focus after surgery is the morbidity and cost associated with the development of an anastomotic leak, the creation of an ostomy is accompanied by its own associated cost and major morbidity. This is secondary to ileostomy-related complications that require reoperation [21], an increased need for readmission secondary to fluid and electrolyte imbalances [11], and the requirement for an additional operation for reversal that has its own associated morbidity [21, 22]. Taken together the risk of readmission and reoperation related to a temporary ileostomy is likely around 12–15% [11, 21, 22]. In the current study, over half of the patients who were diverted had a predicted anastomotic leak risk of less than 4%, and may have reasonably been able to undergo primary anastomosis alone without diversion. Lastly, in the preoperative setting, using the developed anastomotic leak score can enable patient-specific counseling on the postoperative risk of anastomotic leak, instead of providing a subjective assessment of risk that is likely influenced by recent personal experiences and other outside factors.
In the risk score we developed, well-established risk factors for anastomotic leak including younger age, male sex, increasing ASA class, preoperative tobacco use, anemia, and omission of combined bowel prep were included and are readily available at the time of preoperative consultation [23–27]. Receipt of chemotherapy within the 90 days before surgery was also found to be an independent risk factor for anastomotic leakage. However, this may not represent chemotherapy alone. Since NSQIP does not currently collect data on preoperative radiation, some of this observed effect for chemotherapy is likely secondary to concomitant radiation therapy, which is a well-established risk factor for anastomotic leakage [28], and is likely indirectly captured by our model. Interestingly, having a coloproctostomy only marginally affected the risk of leak in our cohort, unlike previous studies where lower anastomoses had significantly higher leak rates [29, 30]. This is likely secondary to selection bias in favor of diversion when a coloproctostomy was performed compared to a colocolostomy, as we observed.
Of all identified risk factors, bowel preparation had the single greatest impact with omission of combined preparation increasing the predicted anastomotic leak risk five-fold in the absence of other risk factors. The use of bowel preparation has not been included in prior iterations of anastomotic leak risk calculators [15–17, 31, 32]. It is unclear if patients were not routinely prepped in these studies, or if bowel preparation was not included for another reason, such as lack of significance. Though the use of combined bowel preparation before surgery was controversial in the past, the American Society of Colon and Rectal Surgeons Clinical Practice Guidelines now recommend the use of combined bowel preparation before all elective colorectal resections [33]. Therefore, it is likely that combined bowel preparation will increasingly be used routinely in the future, and this leads to a question of the applicability of prior anastomotic leak risk calculators given this change in treatment paradigm. Due to bowel preparation’s outsized impact on anastomotic leak, our risk score should provide better risk estimates than prior risk calculators, since it includes bowel preparation.
Additional drawbacks of prior risk scores for anastomotic leak encompass their inclusion of a combination of left-sided and right-sided resections despite differences in leak rates and leak risk factors [16], their development in only oncologic resections [34], or both of these limitations [15, 32]. Our score has the advantage of being specific to left-sided resections while accounting for anastomosis type and being applicable to the three most common indications for left-sided colectomy of malignancy, diverticular disease, and benign neoplasm. By including multiple pathologies, the same risk score can be utilized for multiple indications, thereby enhancing the ease of use. Lastly, the point system allows quick calculation of a patient’s point total that can be translated to a predicted anastomotic leak rate using the accompanying risk deciles in a more facile way than the using a nomogram.
There are several limitations inherent to the current study based on its utilization of the data available within ACS NSQIP. First, ACS NSQIP does not collect data on preoperative radiation exposure specifically, which is a factor associated with anastomotic leak and is an indication for diversion for some surgeons. However, in rectal cancer, neoadjuvant chemotherapy typically accompanies radiation so the chemotherapy variable likely captures the patients who received radiation. Second, we cannot assess exact tumor location in the rectum, which would strengthen the predictive model. We may also be observing selection bias for low rectal tumors to be diverted. This likely explains part of the reason why colorectal anastomoses had a smaller effect size on anastomotic leak than a colocolostomy did. Additionally, patients with low tumors could be at a low predicted risk of anastomotic leak and still warrant diversion, since the consequence of an anastomotic leak in this situation is potentially a permanent ostomy. Third, we can only observe leak until 30-days postoperatively. It is possible that later leaks may have different risk factors, and that we are underestimating the risk of anastomotic leak compared to studies that looked at anastomotic leak at up to 90 days after surgery. However, by excluding diverted patients who typically present with a leak later [35], we likely reduced the potential impact. Fourth, we do not have information on performance or result of an air-leak test, which is an important factor in the decision to defunction an anastomosis. Fifth, the developed anastomotic leak score should be validated within individual hospitals when possible and perhaps especially for those that do not contribute data to the ACS NSQIP Colectomy PUF [36, 37]. Lastly, the decision to divert must be made by the operating surgeon, and a patient’s score alone should not dictate whether he or she is diverted or not. Despite these limitations, we developed an anastomotic risk score that clearly identifies patients at both high and low risk of anastomotic leak and can help guide surgical decision-making.
CONCLUSION
The overall anastomotic leak rate after left-sided resections was 3%, but this rate varied widely based on specific patient and intra-operative risk factors. The developed anastomotic leak risk score can be used to predict an individual patient’s risk of anastomotic leak intraoperatively in an objective manner free of outside biases that do not affect anastomotic leak occurrence in an individual patient. This would then help increase diversion in patients at high risk of anastomotic leak who can derive the most benefit, and equally importantly, reduce the use of diversion and the morbidity associated with an ileostomy in patients at low risk of anastomotic leak.
ACKNOWLEDGEMENTS
The Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery provides salary support for Dr. Habermann and Ms. Bews and in kind support for Dr. McKenna. Dr. McKenna receives salary support from the Mayo Clinic Clinical Investigator Training Program. These funding sources did not affect our investigation.
Funding:
This publication was made possible by CTSA Grant Number UL1 TR002379 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Presentation: The enclosed manuscript was presented at the 34th Annual SSAT Residents and Fellows Research Conference on May 18, 2019 in San Diego, CA and as a plenary presentation at the 2019 Digestive Diseases Week on May 19, 2019 in San Diego, CA.
Conflicts of interest: None of the authors have any conflicts of interest to disclose.
REFERENCES
- 1.Bakker IS, Grossmann I, Henneman D, Havenga K, Wiggers T. Risk factors for anastomotic leakage and leak-related mortality after colonic cancer surgery in a nationwide audit. Br J Surg 2014; 101: 424–32; discussion 32. [DOI] [PubMed] [Google Scholar]
- 2.Bretagnol F, Panis Y, Rullier E, Rouanet P, Berdah S, Dousset B, et al. Rectal cancer surgery with or without bowel preparation: The French GRECCAR III multicenter single-blinded randomized trial. Ann Surg 2010; 252: 863–8. [DOI] [PubMed] [Google Scholar]
- 3.Kream J, Ludwig KA, Ridolfi TJ, Peterson CY. Achieving low anastomotic leak rates utilizing clinical perfusion assessment. Surgery 2016; 160: 960–7. [DOI] [PubMed] [Google Scholar]
- 4.Levack M, Berger D, Sylla P, Rattner D, Bordeianou L. Laparoscopy decreases anastomotic leak rate in sigmoid colectomy for diverticulitis. Arch Surg 2011; 146: 207–10. [DOI] [PubMed] [Google Scholar]
- 5.Matthiessen P, Hallbook O, Rutegard J, Simert G, Sjodahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg 2007; 246: 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammond J, Lim S, Wan Y, Gao X, Patkar A. The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. J Gastrointest Surg 2014; 18: 1176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turrentine FE, Denlinger CE, Simpson VB, Garwood RA, Guerlain S, Agrawal A, et al. Morbidity, mortality, cost, and survival estimates of gastrointestinal anastomotic leaks. J Am Coll Surg 2015; 220: 195–206. [DOI] [PubMed] [Google Scholar]
- 8.Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg 2009; 96: 462–72. [DOI] [PubMed] [Google Scholar]
- 9.Mrak K, Uranitsch S, Pedross F, Heuberger A, Klingler A, Jagoditsch M, et al. Diverting ileostomy versus no diversion after low anterior resection for rectal cancer: A prospective, randomized, multicenter trial. Surgery 2016; 159: 1129–39. [DOI] [PubMed] [Google Scholar]
- 10.Chun LJ, Haigh PI, Tam MS, Abbas MA. Defunctioning loop ileostomy for pelvic anastomoses: predictors of morbidity and nonclosure. Dis Colon Rectum 2012; 55: 167–74. [DOI] [PubMed] [Google Scholar]
- 11.Messaris E, Sehgal R, Deiling S, Koltun WA, Stewart D, McKenna K, et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum 2012; 55: 175–80. [DOI] [PubMed] [Google Scholar]
- 12.Sciuto A, Merola G, De Palma GD, Sodo M, Pirozzi F, Bracale UM, et al. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol 2018; 24: 2247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moug SJ, Henderson N, Tiernan J, Bisset CN, Ferguson E, Harji D, et al. The colorectal surgeon’s personality may influence the rectal anastomotic decision. Colorectal Dis 2018; 20: 970–80. [DOI] [PubMed] [Google Scholar]
- 14.MacDermid E, Young CJ, Young J, Solomon M. Decision-making in rectal surgery. Colorectal Dis 2014; 16: 203–8. [DOI] [PubMed] [Google Scholar]
- 15.Frasson M, Flor-Lorente B, Rodriguez JL, Granero-Castro P, Hervas D, Alvarez Rico MA, et al. Risk Factors for Anastomotic Leak After Colon Resection for Cancer: Multivariate Analysis and Nomogram From a Multicentric, Prospective, National Study With 3193 Patients. Ann Surg 2015; 262: 321–30. [DOI] [PubMed] [Google Scholar]
- 16.Rencuzogullari A, Benlice C, Valente M, Abbas MA, Remzi FH, Gorgun E. Predictors of Anastomotic Leak in Elderly Patients After Colectomy: Nomogram-Based Assessment From the American College of Surgeons National Surgical Quality Program Procedure-Targeted Cohort. Dis Colon Rectum 2017; 60: 527–36. [DOI] [PubMed] [Google Scholar]
- 17.Dekker JW, Liefers GJ, de Mol van Otterloo JC, Putter H, Tollenaar RA. Predicting the risk of anastomotic leakage in left-sided colorectal surgery using a colon leakage score. J Surg Res 2011; 166: e27–34. [DOI] [PubMed] [Google Scholar]
- 18.Moons KG, Harrell FE, Steyerberg EW. Should scoring rules be based on odds ratios or regression coefficients? J Clin Epidemiol 2002; 55: 1054–5. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan LM, Massaro JM, D’Agostino RB Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med 2004; 23: 1631–60. [DOI] [PubMed] [Google Scholar]
- 20.Sammour T, Lewis M, Thomas ML, Lawrence MJ, Hunter A, Moore JW. A simple web-based risk calculator (www.anastomoticleak.com) is superior to the surgeon’s estimate of anastomotic leak after colon cancer resection. Tech Coloproctol 2017; 21: 35–41. [DOI] [PubMed] [Google Scholar]
- 21.Ihnat P, Gunkova P, Peteja M, Vavra P, Pelikan A, Zonca P. Diverting ileostomy in laparoscopic rectal cancer surgery: high price of protection. Surg Endosc 2016; 30: 4809–16. [DOI] [PubMed] [Google Scholar]
- 22.Phatak UR, Kao LS, You YN, Rodriguez-Bigas MA, Skibber JM, Feig BW, et al. Impact of ileostomy-related complications on the multidisciplinary treatment of rectal cancer. Ann Surg Oncol 2014; 21: 507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaimi I, Sparreboom CL, Lingsma HF, Doornebosch PG, Menon AG, Kleinrensink GJ, et al. The effect of age on anastomotic leakage in colorectal cancer surgery: A population-based study. J Surg Oncol 2018; 118: 113–20. [DOI] [PubMed] [Google Scholar]
- 24.Midura EF, Hanseman D, Davis BR, Atkinson SJ, Abbott DE, Shah SA, et al. Risk factors and consequences of anastomotic leak after colectomy: a national analysis. Dis Colon Rectum 2015; 58: 333–8. [DOI] [PubMed] [Google Scholar]
- 25.Richards CH, Campbell V, Ho C, Hayes J, Elliott T, Thompson-Fawcett M. Smoking is a major risk factor for anastomotic leak in patients undergoing low anterior resection. Colorectal Dis 2012; 14: 628–33. [DOI] [PubMed] [Google Scholar]
- 26.Kiran RP, Murray AC, Chiuzan C, Estrada D, Forde K. Combined preoperative mechanical bowel preparation with oral antibiotics significantly reduces surgical site infection, anastomotic leak, and ileus after colorectal surgery. Ann Surg 2015; 262: 416–25; discussion 23–5. [DOI] [PubMed] [Google Scholar]
- 27.Hayden DM, Mora Pinzon MC, Francescatti AB, Saclarides TJ. Patient factors may predict anastomotic complications after rectal cancer surgery: Anastomotic complications in rectal cancer. Ann Med Surg (Lond) 2015; 4: 11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin Q, Ma T, Deng Y, Zheng J, Zhou Z, Wang H, et al. Impact of Preoperative Radiotherapy on Anastomotic Leakage and Stenosis After Rectal Cancer Resection: Post Hoc Analysis of a Randomized Controlled Trial. Dis Colon Rectum 2016; 59: 934–42. [DOI] [PubMed] [Google Scholar]
- 29.Yeh CY, Changchien CR, Wang JY, Chen JS, Chen HH, Chiang JM, et al. Pelvic drainage and other risk factors for leakage after elective anterior resection in rectal cancer patients: a prospective study of 978 patients. Ann Surg 2005; 241: 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung SH, Yu CS, Choi PW, Kim DD, Park IJ, Kim HC, et al. Risk factors and oncologic impact of anastomotic leakage after rectal cancer surgery. Dis Colon Rectum 2008; 51: 902–8. [DOI] [PubMed] [Google Scholar]
- 31.Pasic F, Salkic NN. Predictive score for anastomotic leakage after elective colorectal cancer surgery: a decision making tool for choice of protective measures. Surg Endosc 2013; 27: 3877–82. [DOI] [PubMed] [Google Scholar]
- 32.Rojas-Machado SA, Romero-Simo M, Arroyo A, Rojas-Machado A, Lopez J, Calpena R. Prediction of anastomotic leak in colorectal cancer surgery based on a new prognostic index PROCOLE (prognostic colorectal leakage) developed from the meta-analysis of observational studies of risk factors. Int J Colorectal Dis 2016; 31: 197–210. [DOI] [PubMed] [Google Scholar]
- 33.Migaly J, Bafford AC, Francone TD, Gaertner WB, Eskicioglu C, Bordeianou L, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Use of Bowel Preparation in Elective Colon and Rectal Surgery. Dis Colon Rectum 2019; 62: 3–8. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Wan X, Wang G, Ren Y, Cheng Y, Zhao Y, et al. A scoring system to predict the risk of anastomotic leakage after anterior resection for rectal cancer. J Surg Oncol 2014; 109: 122–5. [DOI] [PubMed] [Google Scholar]
- 35.Leahy J, Schoetz D, Marcello P, Read T, Hall J, Roberts P, et al. What is the risk of clinical anastomotic leak in the diverted colorectal anastomosis? J Gastrointest Surg 2014; 18: 1812–6. [DOI] [PubMed] [Google Scholar]
- 36.Bergquist JR, Thiels CA, Etzioni DA, Habermann EB, Cima RR. Failure of Colorectal Surgical Site Infection Predictive Models Applied to an Independent Dataset: Do They Add Value or Just Confusion? J Am Coll Surg 2016; 222: 431–8. [DOI] [PubMed] [Google Scholar]
- 37.Cima RR, Bergquist JR, Hanson KT, Thiels CA, Habermann EB. Outcomes are Local: Patient, Disease, and Procedure-Specific Risk Factors for Colorectal Surgical Site Infections from a Single Institution. J Gastrointest Surg 2017; 21: 1142–52. [DOI] [PubMed] [Google Scholar]


