Abstract
Per- and polyfluoroalkyl substances (PFAS), which are present in many waters, have detrimental impacts on human health and the environment. Reverse osmosis (RO) and nanofiltration (NF) have shown excellent PFAS separation performance in water treatment; however, these membrane systems do not destroy PFAS but produce concentrated residual streams that need to be managed. Complete destruction of PFAS in RO and NF concentrate streams is ideal, but long-term sequestration strategies are also employed. Because no single technology is adequate for all situations, a range of processes are reviewed here that hold promise as components of treatment schemes for PFAS-laden membrane system concentrates. Attention is also given to relevant concentration processes because it is beneficial to reduce concentrate volume prior to PFAS destruction or sequestration. Given the costs and challenges of managing PFAS in membrane concentrates, it is critical to evaluate both established and emerging technologies in selecting processes for immediate use and continued research.
Keywords: brine management, concentrate management, membranes, per- and polyfluoroalkyl substances, water treatment
1 |. INTRODUCTION
For the past several years, per- and polyfluoroalkyl substances (PFAS) have been a high-profile issue for the water sector and the public. Certain PFAS have been associated with adverse health effects, including cancer, immune system dysfunction, liver damage, and hormone disruption (Agency for Toxic Substances and Disease Registry [ATSDR], 2018). US EPA has set oral noncancer reference doses for both perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) (US EPA, 2016a, 2016b) and issued drinking water health advisories of 70 ppt (parts per trillion, or ng/L) for combined concentrations of PFOA and PFOS (US EPA, 2020). To date, a number of states have established advisories and regulations for select PFAS (“California’s OEHHA intends to add PFOA and PFOS to Prop 65 list,” Legislation column, 2016; Garnick et al., 2021).
PFAS have been manufactured and used in a wide variety of industries since the 1940s, and, as shown in Figure 1, they have been found in many wastewaters, environmental waters, and drinking water sources (Crone et al., 2019). Reported PFAS concentrations in water vary between sources and locations. In the United States, the mean concentrations of PFOA were found to be 10–2305 ng/L and <5–821 ng/L for groundwater and surface water, respectively, and those for PFOS were found to be 4–59 ng/L and <1–69 ng/L for groundwater and surface water, respectively (Crone et al., 2019). The presence of PFAS in drinking water and reuse water influents have created pressure to find treatment processes that can remove them without causing unintended consequences, such as by-product generation, release of concentrated PFAS into the environment, increased corrosion in the distribution system, or difficulty in maintaining a disinfectant residual. Taking these issues and treatment effectiveness into account, the technologies that have been widely accepted as applicable for PFAS removal are granular activated carbon (GAC), anion exchange (AIX) resins, and reverse osmosis (RO) and nanofiltration (NF) membranes (Crone et al., 2019; Dickenson & Higgins, 2016; USEPA, 2016a, 2016b). Although treatment efficacy has been studied, much less research has been done on handling residual streams such as spent adsorbents and membrane concentrates, which become concentrated with PFAS. The management of concentrate streams from membrane systems that treat PFAS-contaminated waters, which is the focus of this study, has received little attention thus far.
FIGURE 1.
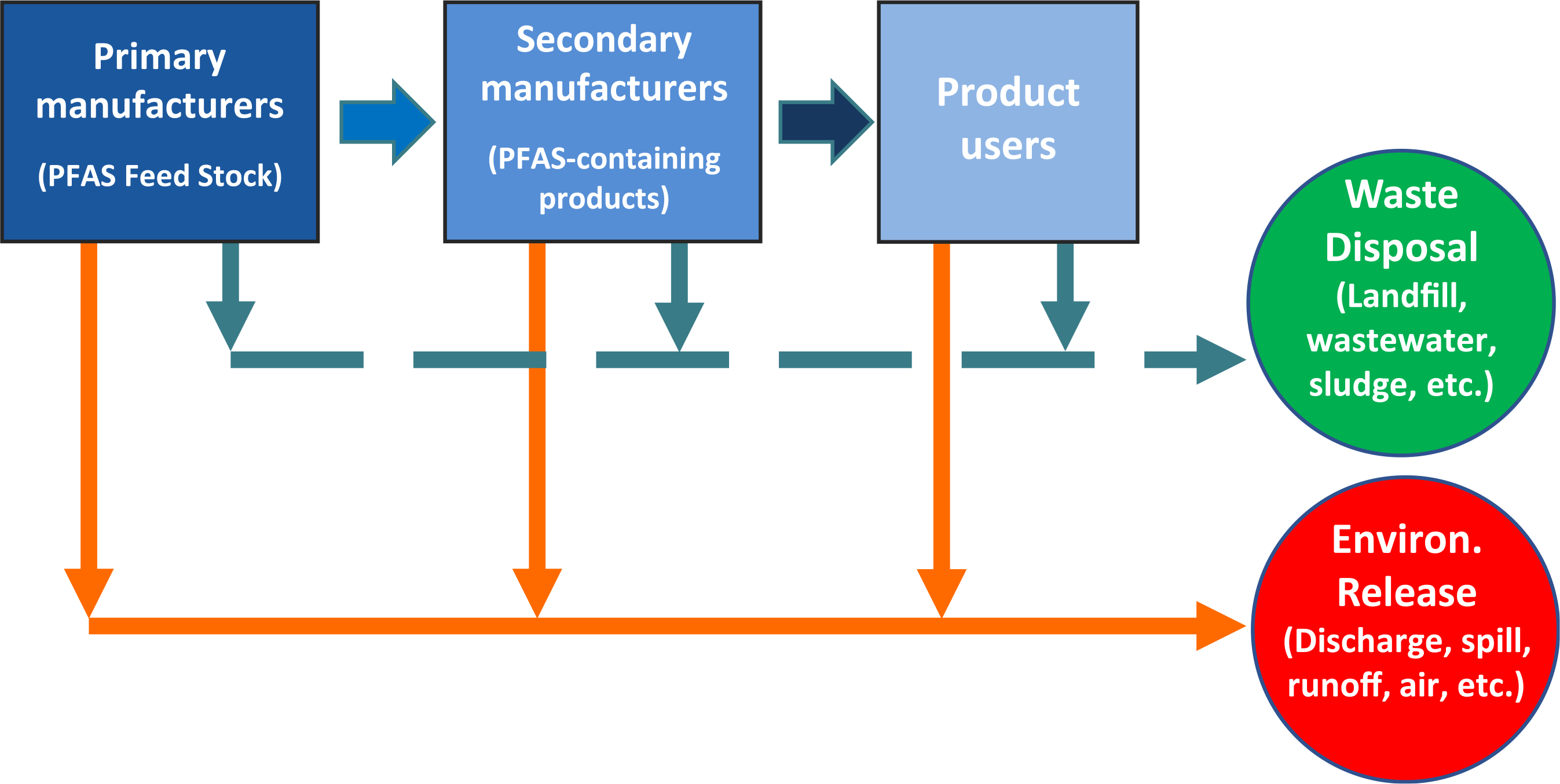
Conceptual sketch of PFAS transport pathways from source to disposal or environmental release. Disposed streams and environmental waters may be subjected to further treatment for PFAS
Membrane processes are used for many purposes in water treatment. They are generally divided into two categories: filtration and desalination systems. Although filtration (“low-pressure”) membrane systems (i.e., microfiltration and ultrafiltration) can be paired with adsorbent technologies (e.g., powdered activated carbon [PAC]) to remove specific dissolved contaminants by themselves, they are effective only for particulate or colloidal control (Dickenson & Higgins, 2016; Van Der Bruggen et al., 2003). Desalination (RO and NF) systems are utilized for removing dissolved constituents including PFAS.
Numerous studies have shown that RO and NF membrane systems effectively remove a wide range of PFAS with rejections generally above 90% (Appleman et al., 2014; Busch et al., 2010; Flores et al., 2013; Lipp et al., 2010; Quiñones & Snyder, 2009; Steinle-Darling et al., 2010; Steinle-Darling & Reinhard, 2008; Tang et al., 2006; Tang et al., 2007; Thompson et al., 2011; Yan et al., 2015; Zeng et al., 2017). Specific issues that impact the performance of membrane treatment of PFAS-laden waters include the synthesis of the membrane and the resulting molecular weight cutoff and membrane charge (Appleman et al., 2013; Rahman et al., 2014; Steinle-Darling et al., 2010; Steinle-Darling & Reinhard, 2008); molecular size and charge of the individual PFAS (Steinle-Darling et al., 2010; Steinle-Darling & Reinhard, 2008); pH (Kwon et al., 2012; Wang, Zhao, et al., 2015); background constituents (e.g., calcium, sodium, humic acids) (Appleman et al., 2013; Zhao et al., 2016); PFAS adsorption into the membrane matrix (Kwon et al., 2012; Tang et al., 2007); and membrane fouling (Appleman et al., 2013; Rahman et al., 2014; Steinle-Darling & Reinhard, 2008).
Once a membrane is chosen for use, a water system must consider not only the membrane system operation but also the approach to residual stream management (Figure 2). Residual management is complicated by the presence of contaminants of concern such as PFAS, which have an unclear final regulatory outcome as related to both human and ecological health. Although RO and NF membranes are extremely effective at separating PFAS, the difficulty treating or disposing of the waste concentrate stream remains a major challenge.
FIGURE 2.
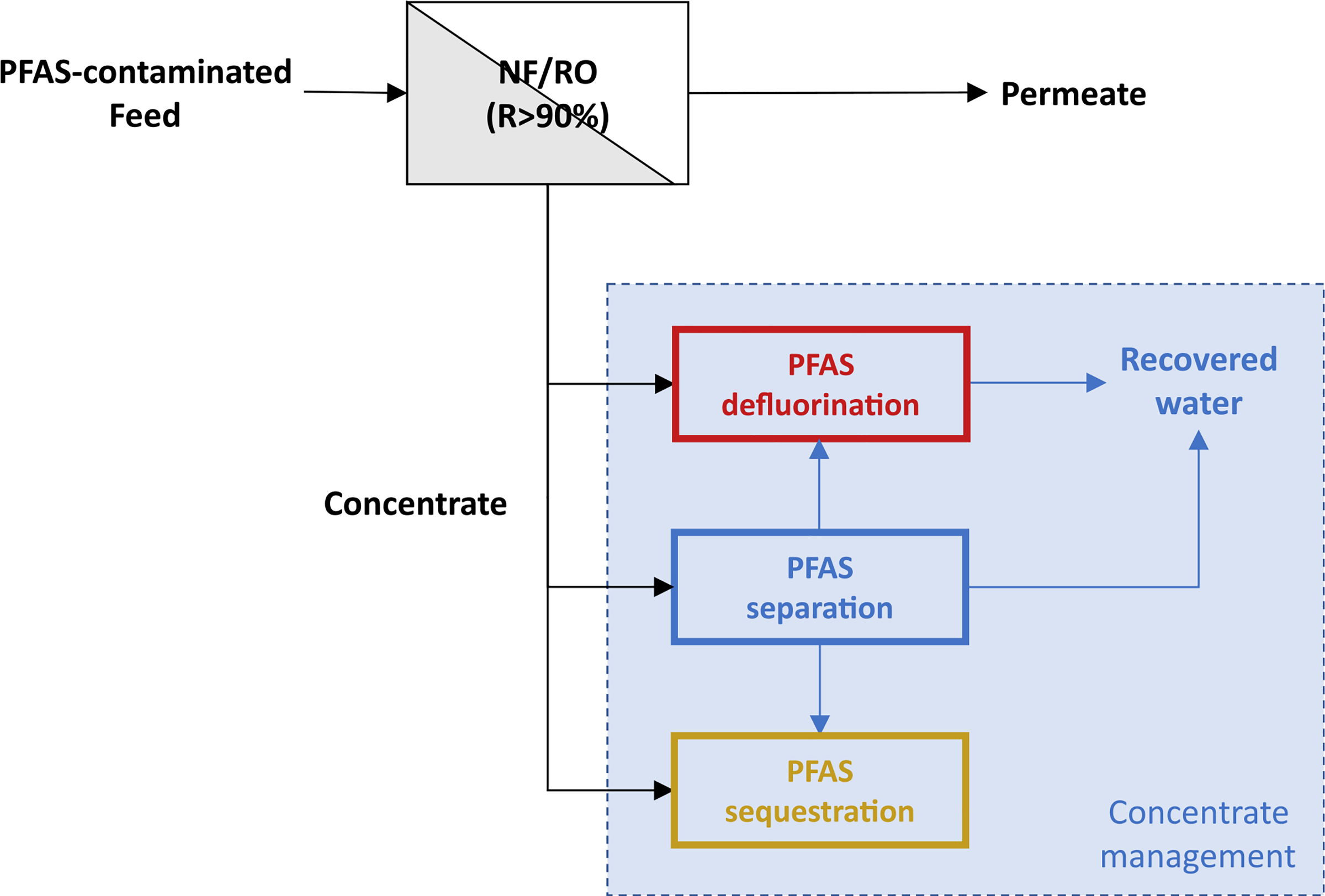
Conceptual sketch of membrane and concentrate treatment unit processes for PFAS-contaminated feed water
The concentrate residual flow rate of the membrane system is dictated by the system recovery, defined as the percentage of influent water that passes through the membrane as permeate. The system recovery is typically limited to approximately 90% for low-salinity water sources (Bonn et al., 2000; Schafer et al., 2005) and between 50% and 60% for seawater (Mickley, 2009). As a result, the volumetric flow rate of the concentrate stream can still be significant (greater than 10% of the feed flow rate). Because membrane systems separate contaminants by a rejection mechanism that does not destroy the contaminants, the PFAS concentrations in the residual stream are a function of the percent rejection and the system recovery. When the percent rejection for PFAS approaches 100%, as it tends to in RO treatment, the concentration of PFAS in the concentrate, Cc, can be expressed as Cf/(1–R), where Cf is the feed concentration and R is the system recovery. The management of large volumes of membrane concentrate streams at high PFAS concentration requires careful thought and ongoing research.
Typically, membrane concentrates are disposed of via surface discharge, sewer, deep well injection, land application, or evaporation ponds (Mickley, 2009). Of these, surface or sewer discharge is the most prevalent (Mickley, 2018) due to their overall cost and ability to handle higher flows. Surface water discharge is often the easiest and least expensive disposal approach; however, it falls within the National Pollutant Discharge Elimination System (NPDES) permitting process and future regulatory determinations may necessitate PFAS management. Disposal into the local sewer system does not fall within the NPDES program, but the local wastewater treatment utility may not agree to handle the stream or may require a pretreatment system. Deep well injection (into Class 1 wells) is occasionally used for membrane concentrate streams. The ability to utilize this approach is a function of the proximity of the well, the volume of concentrate produced, the capacity of the well, whether the well can be used for the lifetime of the membrane facility, and whether there is an alternate approach for when the well is taken offline such as for mechanical integrity testing. Because of these constraints, deep-well injection is not commonly used outside Florida (Mickley, 2018). Evaporation ponds are also occasionally used for concentrate disposal, but their performance is a function of the climate (warmer/drier), land accessibility, and cost, which is the driving factor. Finally, land application is occasionally used. This option also requires warmer climates or the ability to manage the concentrates during episodes of cold weather. None of these disposal options destroy PFAS, and they each risk introducing PFAS back into water sources.
Destruction of PFAS in the concentrate stream without creating by-products would be ideal. Such a stream could be reintroduced to the water supply at a number of points and would obviate concern about ecological impacts or human health impacts via water reuse. In this light, the intent of this article is to cover the state of the science for technologies that may be suitable for treating RO and NF membrane concentrates containing PFAS, including concentration processes prior to PFAS defluorination or sequestration, as shown in Figure 2. For each technology, the mechanism of removal will be discussed with regard to potential efficacy, the formation of breakdown products (if applicable), and the impact of background water constituents that could affect the technology applicability.
2 |. FURTHER CONCENTRATION OF MEMBRANE CONCENTRATES
Further concentration of PFAS-laden concentrates may be beneficial to reduce the volume of wastewater for PFAS destruction or sequestration. This section describes a range of established and emerging technologies for the additional concentration of PFAS from membrane concentrates. These processes may be used individually or in sequence to achieve the desired level of concentration; for example, RO or NF concentrate could undergo foam fractionation. The PFAS-enriched coalesced foam could be solidified by evaporation to achieve a form that could be managed effectively via long-term sequestration or destruction.
2.1 |. RO and NF
RO and NF membrane systems have successfully demonstrated targeted long-chain PFAS rejection from approximately 90%–99% and targeted short-chain PFAS from approximately 50%–99% (Appleman et al., 2013; Lipp et al., 2010; Tang et al., 2007; Wang et al., 2018) against background aqueous matrices of varying strength, including synthetic high total organic carbon water (Wang et al., 2018; Zhao et al., 2016), semiconductor wastewater (Tang et al., 2006), and groundwater (Franke et al., 2019).
The volume of concentrate from primary membrane treatment processes (or any residual waste stream) may be significantly reduced via the application of additional RO and NF membrane treatment processes in batch or semi-batch configurations, which are optimized for higher recovery (Bond & Veerapaneni, 2008; Tong & Elimelech, 2016). This reduction of volume and the resulting increase in concentration may provide benefits for downstream PFAS treatment technologies, which are discussed in more detail later. Specifically, closed-circuit desalination (CCD) is a commercially available semi-batch desalination configuration with potential for broad applicability due to its capabilities for high recovery and its operational flexibility relative to conventional configurations (Lin & Elimelech, 2015).
In a CCD system, feed water is continuously pumped to a membrane pressure vessel at the same flow rate that permeate is discharged. The resulting concentrate is continuously recirculated within the system until a user-defined recovery setpoint has been achieved, at which point the concentrate is purged from the system and the process restarts (Warsinger et al., 2016). The CCD configuration is designed to operate at constant flux and can be outfitted with either NF or RO membrane elements (Riley et al., 2018). CCD systems have achieved product water recoveries as high as 97% and performance models predict marked energy efficiency advantages of CCD when compared to other RO and NF membrane system configurations (Lin & Elimelech, 2015; Stover, 2013; Sutariya & Raval, 2021; Warsinger et al., 2016). However, solute retention may occur in the feed channel even after the concentrate purging since the convective residence time in commercial NF or RO membrane elements may differ from that of an ideal plug-flow (Li, 2021). As a result, the energy efficiency of CCD systems is largely affected by the purging (i.e., flushing) efficacy (Lee, Rahardianto, & Cohen, 2019). Additionally, excessive concentrate recirculation for high-recovery CCD operation may promote the formation of mineral scaling or biofouling due to the high concentration factor in the feed stream (Lee, Choi, & Cohen, 2019). Therefore, refreshing the feed channel and freeing the membrane surface from residual solutes at the end of each CCD cycle remains a key challenge.
As with many applications of RO and NF membrane systems, membrane fouling remains a concern for performance longevity; particularly considering the implementation of batch or semi-batch configurations operating at high recoveries for the purpose of primary membrane concentrate residuals’ management. Although each situation demands its own assessment, research suggests that membrane fouling within batch and semi-batch configurations may be mitigated by modifying the membrane system operational parameters (e.g., increasing cross-flow velocity) (Lin & Elimelech, 2015), rapid salinity cycling (Warsinger et al., 2018), pH adjustment, the addition of antiscalants (Franke et al., 2019), and the development of tailored membrane polymers (Boo et al., 2018; Wang, Zhao, et al., 2015).
2.2 |. Emerging membrane processes
Membrane distillation (MD) technologies (e.g. direct contact membrane distillation [DCMD]), exploit the difference in volatility between compounds in the contaminated feed solution for separation. Given the low vapor pressure of PFAS, MD allows selective permeation of water vapor across the porous membrane, which is made of hydrophobic polymers such as polyvinylidene difluoride to prevent pore wetting. Chen et al. (2020) studied the suitability of DCMD to treat PFAS-contaminated waters by evaluating the DCMD performance for concentrating and removing perfluoropentanoic acid (PFPeA) on a bench-scale DCMD system using a poly(tetrafluoroethylene) (PTFE) membrane. The highest removal rate of PFPeA in this study was 85% at a feed temperature of 50C. However, the study reported severe surface fouling leading to a decrease in rejection due to transportation of PFPeA via surface diffusion. Therefore, it is necessary to develop surface modification or coating technologies to prevent surface diffusion by limiting interactions between PFAS and the membrane surface. Another limitation is that there are no available cost data for PFAS concentration via MD.
Forward osmosis (FO) technology uses a semipermeable membrane for selective transport of water via osmosis, driven by a concentration difference between two liquid bodies (i.e., a feed solution and a draw solution) separated by a membrane. Although experimental study of PFAS separation with FO to date has been limited, a bench-scale investigation (Joshua, 2020) showed that cellulose triacetate FO membranes rejected 98% of PFOS and 97% of PFOA, which is comparable to RO membrane performance. FO has also been successfully demonstrated for the treatment of wastewater from a sewage treatment plant, the Ngong Ping Sewage Treatment Works, in Hong Kong. PFOA and PFOS were separated from water using a widely available thin-film composite membrane with a polyamide barrier layer (Choi et al., 2021). To overcome the challenge from regeneration of the draw solution, Choi et al. adopted a highly soluble, high molecular weight (>70 kDa) poly (styrenesulfonate) (PSS) as a draw solute. The abovementioned approach allowed the application of a low-pressure ultrafiltration process for regeneration of the draw solution to maintain the overall energy efficiency of the FO system. The study successfully demonstrated continuous production of permeate above 3 Lm−2h−1 on a bench-scale test with PFAS rejections as high as 93% and 99% for PFOA and PFOS, respectively. However, it is noted that the system required an extended pretreatment period (up to 6 days) for ultrafiltration to attain high PSS rejection efficiency for clean permeate production. The specific energy consumption for the osmotic dilution step of FO is significantly lower than that of desalination processes (Shaffer et al., 2014). However, for permeate production and continuous operation, draw solution regeneration is required. Given that the regeneration of the draw solution can be more energy intensive than RO (McGovern & Lienhard, 2014), further research would be necessary to develop effective and affordable draw solution regeneration methods for FO-based concentration of PFAS-laden concentrate.
2.3 |. Electrodialysis–RO hybrid systems
Electrodialysis (ED) is a proven water treatment process that separates charged constituents from feed water and has more than half a century of application at a large industrial scale (Strathmann, 2010). ED separates charged compounds in the solution through anion and cation exchange membranes via electric potential. The application of ED to RO concentrate has been studied to improve the overall water recovery of the system (Oren et al., 2010; Zhang et al., 2011; Zhao et al., 2019). Although ED has not yet been applied to PFAS separation, ED may directly separate anion PFAS compounds, such as perfluoroalkyl acids (PFAAs) in RO concentrate or fluoride ions from the PFAS destruction system (Takeuchi et al., 2012). If the majority of PFAS compounds in the RO concentrate are neutral, ED would not be an applicable process to separate the PFAS compounds. However, ED may help further consolidation of RO concentrate because the application of ED to RO concentrate can produce fewer charged ions but the same concentration of PFAS compounds in the ED dilute stream (deionized stream), which could be recycled as an RO feed for further PFAS concentration.
2.4 |. Foam fractionation
As surfactants, PFAS tend to accumulate at air–water interfaces, including in foams present on PFAS-contaminated natural waters (Schwichtenberg et al., 2020). Their surfactant qualities enable a class of remediation processes based on increasing the interfacial area density through the creation of bubbles and foams and collection or destruction of interface-bound PFAS.
Through degassing experiments, nanobubbles bound to the hydrophobic sections of carbonaceous nanomaterials were shown to play a role in PFAS removal (Liu et al., 2020; Meng et al., 2014). This finding was developed into a process for concentrating PFAS that takes advantage of their affinity for the air–water interface: aeration–foam collection (Meng et al., 2018). In aeration–foam collection, also known as foam fractionation, air is bubbled through PFAS-containing liquids, and the foam that rises to the top is collected and allowed to settle and break (Meng et al., 2018) (Figure 3). Aeration–foam collection was tested with aqueous film-forming foam (AFFF) solution and optimized to remove 99.9% of PFOS at a concentration 8400 times that of the original solution (Meng et al., 2018); however, the process was less effective for short-chain PFAS such as perfluorobutyl sulfonate (PFBS) due to their lower surface activity, a finding also noted in Dai et al. (2019).
FIGURE 3.

(a) Foam fractionation column treating PFAS-laden water (photo courtesy of Evocra Pty Ltd). (b) Foam fractionation system (photo courtesy of OPEC systems and Dora Chiang, CDM Smith)
Several variants on foam fractionation have been tested with generally high effectiveness at separating at least some PFAS. Aeration with foam collection has been tested on PFAS-laden landfill leachate with an overall PFAS separation efficiency of 92% (Robey et al., 2020). When aeration bubbles burst at the free surface of the PFAS-laden liquid, rather than being captured as a foam, they can create a PFAS-enriched aerosol that can be captured for further treatment (Ebersbach et al., 2016). Ozonated air fractionation has showed higher PFAS removal than air alone (Dai et al., 2019), and foam fractionation has also been combined with ultraviolet (UV) photodegradation to concentrate and destroy PFOS (Lyu et al., 2020). Meng et al. (2018) found that higher ionic strength (up to 5 mM NaCl) slightly enhanced PFAS separation efficiency in aeration–foam collection, which suggests that the elevated salt content expected in PFAS-laden concentrate streams could be conducive to aeration-foam collection.
2.5 |. Electrocoagulation
Electrocoagulation (EC) has been successfully applied to the removal of various contaminants from water, offering multiple advantages including high levels of removal, ease of operation, decreased sludge production, and lower energy consumption. Although there is limited literature available on the removal of PFAS by EC, the complete removal of PFAS, primarily PFOA and PFOS, has been reported (Lin, Wang, et al., 2015). The proposed removal mechanism of PFAS by EC is hypothesized to be the hydrophobic interaction of PFAS with metal hydroxide flocs, which results in the enmeshment of PFAS with flocs and their subsequent removal by precipitation. Therefore, the type of anode/cathode used in the system is critical for the removal of PFAS by the formation of hydroxide flocs. Many factors including applied current–voltage density, stirring speed, electrolyte type (NaNO3, NaCl, Na2SO4, Na2CO3), the presence of inorganics (Cl−, SO42−, CO32−, HCO32−, NO3−), and pH have been shown to impact the efficiency and cost of the process. Previous literature studies have indicated that the combined application of different anode/cathode metals improved the removal efficiency of PFOA and lowered the process cost (Liu et al., 2018). However, while the practical implementation of EC treatment can be impacted by the dissolution and passivation of the anode plate, the source water-specific optimization of operating conditions is also required to achieve efficient use of the technology at full scale.
2.6 |. Evaporation ponds
Evaporation ponds are shallow, artificially constructed, lined ponds that enhance water evaporation from aqueous waste solutions using solar energy and wind (Sahu, 2021). Maintenance is necessary, including the periodic removal of solids. Evaporation ponds require large areas of land and tend to be most effective and generally more affordable in arid regions with inexpensive land. Although they are used for inland dewatering of RO brine (Katal et al., 2020), no studies were found on the use of evaporation ponds for PFAS-laden wastes (from RO or other PFAS concentration processes). Due to lack of research, it is not known whether gas-phase secondary emission of PFAS from evaporation ponds and other open-air evaporation techniques would be substantial (US EPA, 2020).
Previous studies estimated the total cost per unit volume reduction (including amortized capital and O&M) to range between $0.14/m3 and $0.25/m3 (based on modeling [Foldager, 2003]) and between $3.3/m3 and $10.0/m3 (based on a review of several studies [Panagopoulos et al., 2019]), depending on local weather conditions; underlying groundwater aquifers; and the costs of the appropriate pond liner, local land, and energy.
2.7 |. Brine concentrators and crystallizers
Thermal brine concentrators can be used to reduce RO concentrate volume or to eliminate discharge of liquid concentrate. Thermal brine concentration systems require significant thermal and/or electrical energy input (tens of kWhe/m3 for mechanical vapor compression to hundreds of kWhth/m3 for heat-driven distillation methods) (Thiel et al., 2015), so brine recovery RO systems (see previous sections) are often used first. To further reduce the concentrate volume containing PFAS, zero liquid discharge (ZLD) and near-ZLD strategies can be used (Appleman et al., 2013; Rahman et al., 2014; Steinle-Darling & Reinhard, 2008). The minimum liquid volume is achieved with a near-ZLD process using a falling film evaporator to concentrate brine. Recovery of the RO concentrate in a brine concentrator from a reclamation facility typically ranges between 95% and 98%. If further reduction or elimination of the concentrate volume is required, the concentrate discharged from the brine concentrator can be treated with a crystallizer or spray dryer to evaporate remaining liquid and precipitate the most soluble salts (e.g., sodium sulfate, sodium chloride, and calcium chloride). Reduction of concentrate volume using a brine concentrator and subsequent crystallizer increases the water recovery to nearly 100% (Appleman et al., 2013; Rahman et al., 2014; Steinle-Darling & Reinhard, 2008).
2.8 |. Adsorption
Adsorption of PFAS by GAC is the most commonly adopted PFAS removal technology because it is easy to operate and has been an established technology in drinking water production for decades (Merino et al., 2016). However, GAC shows limited sorption potential, especially for short-chain PFAS compounds (i.e., <6 carbon skeleton). GAC also shows a rapid decrease in efficiency, allowing for the breakthrough of PFAS after varying periods of operation (Flores et al., 2013; McCleaf et al., 2017). Therefore, operating GAC for PFAS management requires close monitoring of the GAC for PFAS breakthrough and may require frequent regeneration and/or replacement of saturated adsorbents (Belkouteb et al., 2020).
Adsorption technologies, such as GAC, PAC, and AIX, are often coupled with membranes to separate PFAS (Franke et al., 2019, 2020; Murray et al., 2019). GAC and AIX show up to fourfold improved PFAS removal efficiency when applied to treat NF concentrate streams compared with GAC or AIX treatment of the raw water (Franke et al., 2019). The cost of GAC and AIX treatment for NF concentrate depends on discharge volume and drinking water treatment goals in the drinking water treatment plant (Franke et al., 2020). Franke et al. reported that AIX resins may be more cost-effective than GAC to treat NF concentrate for many discharge goals. Super-fine powdered activated carbon was combined with ceramic microfiltration for enhanced PFAS adsorption and performed better than GAC due to high specific surface area and faster adsorption kinetics (Franke et al., 2019).
2.9 |. Coagulant aids
The specialized coagulant PerfluorAd® uses cationic surfactants to remove anionic PFAS (such as the PFOS found in AFFF-contaminated water) as a pretreatment to other processes such as GAC to extend the lifetime of that downstream process (Cornelsen et al., 2021) and reduce environmental harm (Maga et al., 2021). PerfluorAd® is mixed with PFAS-containing liquid in a stirred tank and solids are removed by settling or filtration (Edel et al., 2018). At a dose of approximately 2 g/L, PerfluorAd® was found to remove over 99% of total PFAS from diluted AFFF solutions that had a PFAS concentration of 66 μg/L (Cornelsen et al., 2021). The optimal PerfluorAd® dose depends approximately linearly on anionic surfactant (PFAS and others) concentration (Maga et al., 2021), and should be determined carefully, as both over- and underdosing lead to reduced PFAS removal. In a life cycle analysis of AFFF solution treatment methods, using PerfluorAd® to reduce PFAS concentration before treatment with activated carbon and incineration reduced the environmental impact of PFAS destruction significantly (Maga et al., 2021). The authors are unaware of published studies using PerfluorAd® for PFAS-laden membrane concentrates, and although it may be similarly effective at removing anionic PFAS from those streams, the effects of high salt concentration merit future investigation.
Other additives, such as RemBind™ and matCARE™, are also emerging as potentially effective PFAS separation technologies; however, no peer-reviewed studies of their effectiveness are known to the authors at this time.
3 |. DEFLUORINATION OF PFAS IN CONCENTRATES
A range of options for degrading PFAS through defluorination are discussed in this section, including both established and emerging technologies. Some of the possibilities reviewed herein are not effective—at least not yet—at defluorinating PFAS in waste streams; however, relatively ineffective technologies for defluorination are included here for completeness and to inform future research directions.
3.1 |. Biological treatment
To date, microbial degradation of PFAS has been largely unsuccessful (Coyle et al., 2021). The dense hydrophobic layer surrounding the carbon–carbon bonds prevents microorganisms from easily using the carbon as their energy source (Kucharzyk et al., 2017). Some limited success has been demonstrated in the partial defluorination of PFAA precursors (i.e., fluorotelomers) (Horst et al., 2020); however, there are concerns this could result in the generation of new PFAAs. A promising recent study demonstrated microbial defluorination of PFAAs using Acidimicrobium sp. strain A6 via the Feammox process in which Fe (III) serves as the electron acceptor and ammonia as the electron donor (Huang & Jaffé, 2019). This research is still at the bench scale and appears to be more applicable to contaminated iron-rich soil than for direct treatment of membrane concentrate.
3.2 |. UV irradiation
Combined chemical/UV light-based processes have been developed to produce a range of reactive species for the degradation of organic compounds (Miklos et al., 2018). Although oxidation-based UV processes (discussed later) are ineffective for PFAA degradation, UV photosensitizer processes that generate hydrated electrons have been effective for the degradation of a diverse set of PFAS (Bentel et al., 2020). The use of bisulfite as a UV sensitizer has recently garnered attention as a viable PFAS destruction technology due to the availability of UV reactors, availability of bisulfite, and benign nature of the sulfate by-product (Tenorio et al., 2020). To date, most research evaluating this technology has been at the laboratory scale using synthetic PFAS solutions consisting mainly of PFOA and PFOS, and there are questions regarding energy requirements, compatibility with real water matrices, and effectiveness for short-chain PFAAs.
3.3 |. Photocatalysis
To overcome the slow decomposition kinetics of organic compounds, photoactive catalysts (e.g., TiO2, titanate nanotubes [TNTs], Fe/TNTs@AC, and boron nitride) have been tested to accelerate PFAS degradation using ultraviolet C (UVC, wavelength 200–280 nm) irradiation (Chen et al., 2011; Duan et al., 2020; Li et al., 2020; Sansotera et al., 2014; Wang & Zhang, 2011). Recently, photocatalysts that worked under visible light (VL, wavelength over 400 nm) were used in a proof-of-concept study carried out with model solutions (Wang, Cao, et al., 2020; Wang, Chen, et al., 2020; Xu et al., 2020). The hydrophobicity of photocatalyst surfaces holds promise to enhance PFAS interaction with the catalyst and was a reason for explaining superior (i.e., lower energy requirements) defluorination of PFOA on boron nitride than TiO2 photocatalysts with UV light (Duan et al., 2020). TiO2 with peroxymonosulfate activation under VL degraded 100% of PFOA within 8 h; however, intermediates such as perfluoroheptanoic acid, perfluorohexanoic acid (PFHxA), PFPeA, pentafluorobenzoic acid (PFBA), perfluoropropanoic acid (PFPA), and trifluoroacetic acid (TFA) were detected during the PFOA photocatalytic process (Xu et al., 2020). The Pt-Bi2O4 photocatalyst with KI as an electron donor decomposed 45% of PFOA after 6 h of VL irradiation and nearly 99% of decomposed PFOA was defluorinated (Wang, Cao, et al., 2020). The BiOI@Bi5O7I photocatalyst proposed by Wang, Cao, et al. (2020) provided about 60% degradation of PFOA under VL in 6 h of irradiation and the PFOA was decomposed to CF2 units. Photocatalysts working under VL have the potential to be applied to PFAS photocatalysis in RO concentrate. Pilot- and full-scale demonstrations are needed to vet this technology for RO concentrate management.
Although it is still under development due to its potentially high cost, one of the most recent PFAS removal advancements is based on single-atom catalysis (Huang et al., 2018). This approach involves UV illumination of a highly reduced form of a single metal (e.g., platinum) supported on Si C to facilitate defluorination of PFAS compounds to achieve Si–H/ C–F to Si–F/C–H conversion (i.e., defluorination of PFAS) (Huang et al., 2018). Currently, the feasibility of regenerating the exhausted Si–F material and its environmental implications remain questionable.
3.4 |. Advanced oxidation
Studies have investigated the efficacy of advanced oxidation processes (AOPs) toward PFAS degradation using UV radiation with hydrogen peroxide, ozone, peroxide, or heat-activated persulfate (Anumol et al., 2016). However, AOPs tend to be limited in the extent of degradation of PFAS because of the high oxidation potential of C–F bonds. Barisci and Suri (2021) have recently demonstrated effective removal of fluorotelomer alcohols (FTOHs, precursors to PFAS compounds such as PFOA) by UV/H2O2, solar irradiation with or without H2O2, ozonation, and O3/H2O2. Ozonation has effectively oxidized 75.9% of FTOHs; however, the degradation efficiency declines in the presence of H2O2 in ozonation due to the scavenging effect of hydroxyl radicals (Barisci & Suri, 2021). Sulfate radical anions are highly oxidative and more efficient than hydroxyl radicals for the mineralization of PFAS to fluoride ion and CO2. Beyond H2O2, many other chemical additives upon UV irradiation have shown the ability to degrade PFAS. PFAS compounds transform into short-chain PFAS derivatives through photochemical reaction upon irradiation of sulfite (SO32−), persulfate anion (S2O82−), and other sulfur-based chemicals by producing sulfur oxide radicals, hydroxyl radicals, and hydrated electrons (Hori et al., 2005; Tenorio et al., 2020).
3.5 |. Hydrated electrons
Hydrated electrons (eaq−) can be used as reducing agents for effective cleavage of C–F bonds due to extremely reactive oxidation–reduction potential. As discussed in other sections (Sections 3.2 and 3.9), hydrated electrons can be generated by various technologies including but not limited to radiolysis, sonolysis of water by strong laser pulses or UV radiation in the presence of certain chemicals such as sulfite and iodide ions and indole (Park et al., 2009; Song et al., 2013; Tian et al., 2016). Degradation/defluorination of legacy PFAS (e.g., PFOA, PFOS, and GenX) has been accomplished by hydrated electrons generated from a sulfite/UV process at ambient temperature and slightly basic medium (i.e., pH 9–10). Despite the efficient performance of the sulfite/UV process for PFAS removal, the formation of sulfate by-products and the high energy requirement (for UV, microwave radiation, ultrasound, etc.) remain major challenges hindering its commercial application (Song et al., 2013). Studies reported novel approaches for efficient generation of hydrated electrons using plasma technology to overcome the above challenges (Lewis et al., 2020; Rumbach et al., 2015); however, plasma use for this application still remains at an early developmental stage because formation mechanisms of hydrated electrons and their behaviors under various chemical events or strong electrical fields (in plasma systems) remain subjects for further investigation.
3.6 |. Plasma-based treatment
Plasma-based water treatment (PWT) is a relatively new technology that uses electrical discharge for the production of plasma and subsequent reactive aqueous species (e.g., radicals, ions, and high-energy electrons) capable of degrading a variety of organic compounds (Mededovic Thagard et al., 2017). A promising PWT reactor approach was recently developed that achieved rapid defluorination of a variety of PFAAs in aqueous solutions (Stratton et al., 2017) and led to a pilot-scale (1–10 gpm) PWT reactor consisting of gas electrical discharge plasma coupled with argon gas bubbling. Effective PFAA treatment using this PWT approach has been demonstrated on various PFAS-impacted water sources (e.g., AIX regenerant waste, landfill leachate, groundwater) with a wide range of water quality and PFAA concentrations (Nau-Hix et al., 2021; Singh et al., 2020; Singh, Multari, et al., 2019). Reportedly, rapid and effective removal of long-chain PFAA has been achieved (>99% in 10 min) with relatively low fluorinated by-product generation (Singh et al., 2020; Singh, Fernando, et al., 2019; Singh, Multari, et al., 2019); however, treatment of short-chain PFAA removal was less effective. Reported energy consumptions for this process range from 1.7 to 36 kWh/m3 depending on the source water quality and treatment goals (Singh et al., 2020; Tenorio et al., 2020).
3.7 |. Electron beam
Electron beam (e-beam) is a radiation-based treatment technology that has been shown to be capable of PFAS destruction through both oxidation and reduction processes. Electricity is accelerated to simultaneously produce highly oxidizing species (hydroxyl radicals) and reducing species (aqueous electrons and hydrogen radicals) (Tetra tech EM Inc., 2003). The primary mechanism for PFAS destruction via e-beam technology (Horst et al., 2020) is proposed to be the attack of the positive dipole end of the fluorine–carbon bond by aqueous electrons. E-beam for water treatment is shown in Figure 4. The technology was first introduced and implemented in the 1970s and 1980s, respectively, to irradiate sludge at wastewater treatment plants in Boston and Miami (Waite et al., 1998). Pilot plants in Brazil, Korea, and China have used e-beam technology to treat industrial wastewater (Lu et al., 2020) and it has been investigated for groundwater remediation of methyl t-butyl ether (MtBE) (Tetra tech EM Inc., 2003). Direct application to membrane concentrates has not been found in the literature; however, a recent laboratory study of PFOA and PFOS degradation in distilled water by e-beam found that at low doses of 50 kGy, PFOA was degraded by 87% whereas only 16% of PFOS was removed (Pillai, 2020). PFOS removal was improved at higher e-beam doses (48.6% removal efficiency at 500 kGy and 96.6% removal efficiency at 2000 kGy). Adjustment of pH to 13 and amendment with sodium nitrate and sodium bicarbonate negatively impacted the degradation of PFOS (22% PFOS reduction at a 500 kGy dose and 88.8% PFOS reduction at a 2000 kGy dose). Membrane concentrates are complex solutions that may contain high levels of confounding constituents (e.g., salts, organic matter, colloids, and antiscalants), so studies conducted in water matrices containing a range of constituents are needed to ascertain whether direct application of e-beam technology to membrane concentrates is viable. Fortunately, e-beam technology has been already commercially used for food pasteurization and medical device sterilization. Challenges related to the water industry include energy requirements, capital costs, and flow volume capacities. A 2003 technology evaluation for e-beam remediation of MtBE estimated that costs ranged from $40 per thousand gallons for small-scale remedial applications to about $1 per thousand gallons for larger-scale drinking water applications of 10 MGD (Tetra tech EM Inc., 2003). Pillai (2020) estimated capital costs for a 10 MeV, 560-kW fixed e-beam system operating at 1500 kGy to be $7.7 million US dollars and that the price to treat water would range from $0.10 (500 kGy treatment) to $0.80 (3000 kGy treatment) per gallon.
FIGURE 4.
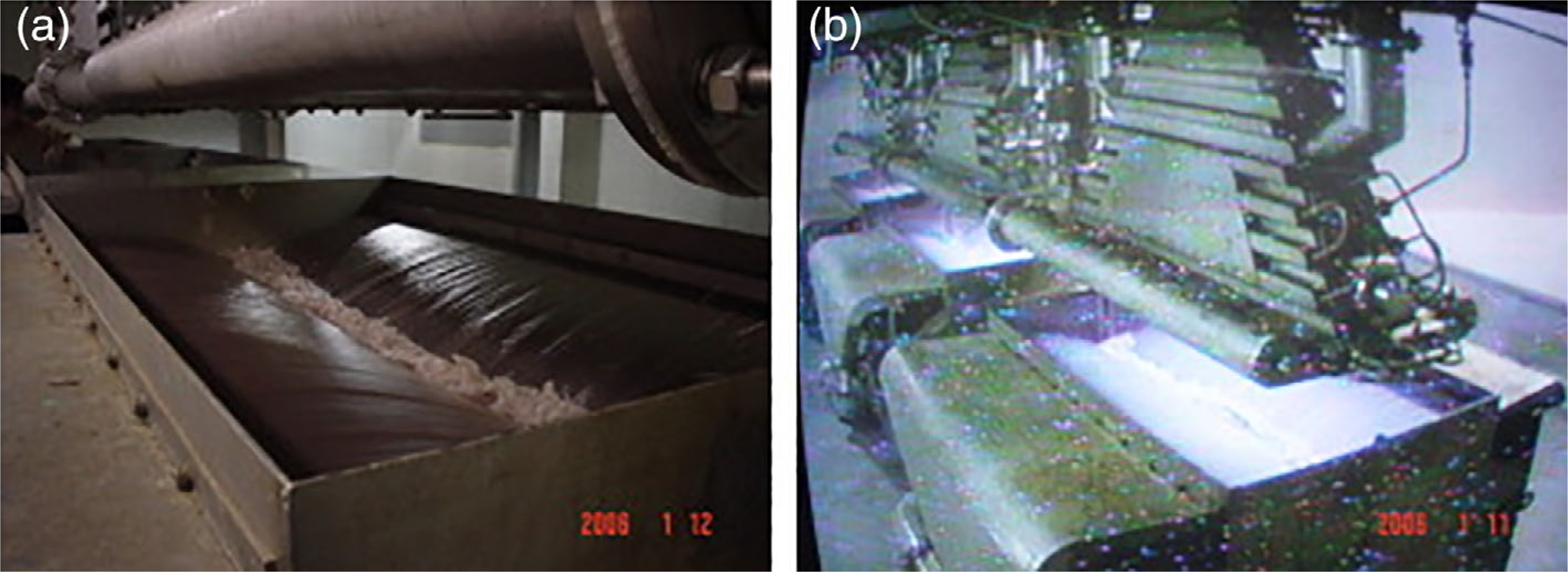
Operation of industrial wastewater plant with electron beam. (a) Injection of wastewater through nozzles. (b) Wastewater under treatment (reprinted from [Han et al., 2012] with permission from Elsevier)
3.8 |. Zero-valent iron
Nanometals are affordable and widely available alternative reducing agents. Decomposition of organic contaminants in soil and water systems has been successfully demonstrated using nanometals. Nanoscale zero-valent iron (nZVI) (Masud et al., 2021) is one of the most promising nanometals with high reductive capacity (standard reduction potential, Eo = –0.44 V (Adusei-Gyamfi & Acha, 2016). Recently, PFAS decomposition was demonstrated in batch experiments using nZVI conjugated with oxidants such as hydrogen peroxide (Parenky et al., 2020). The reactivity of nZVI was further improved by employing graphene oxide (GO) as a solid support to prevent nZVI agglomeration during reductive PFAS decomposition in water (Masud et al., 2021). The abovementioned approach was effective for accelerating PFAS decomposition kinetics (particularly for longer-chain PFAS) due to the presence of delocalized electrons on GO enhancing the contact/interaction between nZVI and PFAS (Gu et al., 2018). However, sulfonate PFAS were found to be more resistive to nZVI-based defluorination processes than carboxylic PFAS (Parenky et al., 2020). In addition, the formation of shorter-chain PFAS in the presence of oxidants remains a lingering challenge that has yet to be investigated.
3.9 |. Sonochemical treatment
Sonochemical treatment (sonolysis) has been effective for degrading PFAS through pyrolysis and the formation of highly reactive free radicals (Cao et al., 2020). The technology uses low-to high-frequency (20 kHz to 1 MHz) ultrasound waves to create cavitation bubbles in which pollutants are destroyed due to a high bubble interface temperature (1000–1500 K) and vapor temperature (4000–10,000 K) and reaction with free hydroxy radicals (OH, IO3, Br2, SO4−) formed in the process (Cao et al., 2020). During the sonochemical reaction, PFAS could be oxidized and degraded to their alkyl species as well as complete mineralization by-products, CO, CO2, F–, and SO42−. Previous studies evaluated the operating factors that impact the performance of sonolysis on PFAS destruction. Factors include reactor size, power density/frequency, number/location of transducers, concentration, the chain length of PFAS, operating pH, time and temperature, sparging gas type, and additives that promote radical formation or reduce surface energy.
Because of the power and cost-related scalability issues (e.g., electrical energy per order of approximately 2600 kWh/m3; Crittenden et al., 2012), the demonstration of sonolysis treatment of PFAS has typically been limited to small reactor volumes ranging between 0.2 and 12 L (Fernandez et al., 2016; Rodriguez-Freire et al., 2016). In a unique study conducted by Gole et al., a large-scale reactor (91 L), which occupied multiple transducers and was capable of providing single/dual frequencies (1 MHz and 500 kHz), was used to evaluate the degradation of AFFFs into F− and SO42− (Gole et al., 2018). The authors reported that the F− and SO42− release from the degradation of AFFFs was inversely proportional to dilution ratio (900× to 100×) and pH of the aqueous phase. These results suggest that sonochemical treatment can be an alternative treatment technique for the management of PFAS concentrate waste streams. The energy cost of the process for AFFF degradation in this large reactor at 500× dilution (at pH 4) was estimated to range between $0.015 and $0.019 per liter of the solution. Fernandez et al. demonstrated that while the defluorination rate of per-/polyfluorinated compounds increased with the increasing chain length of PFAS, perfluoroalkyl compounds are more amenable to sonolysis than the polyfluoroalkyls (Fernandez et al., 2016). In another literature study, researchers reported that the degradation rate of nine PFAS species (PFOS, PFHxS, PFBS, PFOA, PFHxA, PFPeA, PFPA, fluorotelomer sulfonate [6:2 FTS], and PFEES) increased with increasing hydrophobicity or chain length (Vecitis et al., 2008). Other studies have also shown enhancement of PFOA degradation by the addition of persulfate, periodate, permanganate, salts, and surfactants during the sonochemical treatment (Cao et al., 2020). Phan et al. demonstrated that an increase in the concentration of NaHCO3 (0–30 mM) in the reactor enhanced the destruction of PFOA up to 75%; in the same study, increasing the reaction time from 15 min to 4 h also increased removal efficiency from 18% to ~98% (Phan Thi et al., 2014). In another study, Lin, Lo, et al. (2015) investigated the effect of sulfate (25 mM) addition on the sonochemical removal of PFOA, reporting an increase in PFAS removal up to 99% after 2 h of ultrasound irradiation. The combination of additional treatment factors increases the formation of reactive hydroxyl radicals and promotes the formation of cavities by increasing ionic strength, hydrophobicity, and surface tension. The degradation rate of PFOA was also shown to decrease in the presence of dissolved oxygen during the sonolytic reaction (Lee et al., 2016). Researchers have tested different atmospheric conditions (e.g. various Ar, N2, and O2 concentrations) on the degradation efficiency and reported that degradation efficiency is a function of the polytropic index of the gases (Moriwaki et al., 2005; Phan Thi et al., 2014).
The abovementioned studies successfully demonstrated the effectiveness of sonochemical treatment on PFAS degradation under specific operating conditions on bench-scale systems with simple, synthetic water matrices. Further investigation is necessary to understand the effectiveness and cost of this technology in full-scale applications and complex water matrices such as RO and NF concentrates.
3.10 |. Incineration
RO/NF brines containing PFAS can be directly incinerated or post-concentrated on adsorbents, which can then be thermally treated. Factors influencing thermal reactor efficiency include the properties of waste, such as the presence of phosphates and silicates, which were shown to improve the mineralization of PFAS. Operating conditions (e.g., residence time, operating temperature, mixing/turbulence, and presence of excess oxygen) or addition of coadditives (CaO, ZVI, KOH, Al2O3, La2O3, Na2FeO4, Na2S2O8, etc.) are also important (Cagnetta et al., 2017; Deng et al., 2020; Lu et al., 2017; Lv et al., 2018; Wang et al., 2013; Wang, Lu, et al., 2015). Typically, incineration is performed in a reactor containing primary and secondary chambers. In these chambers, the waste is initially heated and volatilized at temperatures >800ºC (~1500ºF) in the first chamber. The by-products from incomplete combustion are further treated in the second chamber at temperatures >1100ºC (~2000ºF) with excess oxygen. Liquid wastes can also be sprayed into a secondary chamber. During the thermal regeneration of spent GAC media, the primary chamber is kept at lower temperatures (<815ºC or ~1500ºF) and supplied with inert gases to eliminate oxidation and changes to the pore structure. The current literature is still lacking information on the thermal behavior of PFAS-laden wastes during high-temperature combustion or GAC reactivation.
Aleksandrov et al. (2019) treated PTFE in a pilot-scale incinerator, showing that only PFOA formed at detectable but insignificant levels (Aleksandrov et al., 2019). Similar results were also reported by Taylor et al. (2014), who investigated incineration of fluorotelomer-based polymers. These studies reported complete mineralization of fluorinated compounds at high temperatures (>1000ºC) with a residence time >2 s and excess oxygen. The main by-product formed as a result of incineration was hydrofluoric acid (HF). In other studies, authors investigated the effect of calcium supplement addition on the mineralization of PFAS during incineration and showed faster mineralization rates at lower temperatures (Wang et al., 2011; Wang et al., 2013; Wang, Lu, et al., 2015). Xiao et al. (2020) showed that the thermal resistance of PFAS was different based on their chemical structure. For example, while the decomposition of perfluorinated carboxylic acids (PFCAs, e.g., PFOA) on GAC occurred at temperatures ~200ºC, perfluoroalkane sulfonates (PFSAs, e.g., PFOS) decomposed at higher temperatures (≥450ºC). Above 700ºC, the complete mineralization of PFOA and PFOS to fluoride (>80%) was observed (Xiao et al., 2020). To accomplish successful thermal mineralization of wastes (e.g., single-use AIX resins, brines, soil) and regeneration of spent GAC, research is needed to understand the temperatures that volatilize and mineralize PFAS. Analytical methods to track PFAS transformations in solid and gas phases are needed to quantify and verify fluorine mass balances.
3.11 |. Supercritical water oxidation
Supercritical water oxidation (SCWO) successfully degrades organic compounds including PFAS, so it may have potential for treating PFAS-laden brines and adsorbents (e.g., GAC) used to remove PFAS from concentrates; however, the scaling propensity of SCWO and the high salinity of membrane concentrates pose a challenge. Since the 1980s, SCWO has been used successfully to treat halogenated waste materials including PCBs (Abeln et al., 2001; Kim et al., 2010). Supercritical water occurs when the water temperature is above 374ºC (705ºF) and the pressure is higher than 221.1 bar (US EPA, 2021). Under these conditions, water can change from liquid to gas without undergoing a discrete phase transition. Organic compounds that are usually insoluble in water may be highly soluble in supercritical water. In the presence of an oxidizing agent such as oxygen, supercritical water dissolves and oxidizes various hazardous organic pollutants. SCWO destroys PFAS by breaking the C–F bonds and decomposing the material into a nontoxic waste stream. Some studies have reported greater than 99% destruction of 12 PFAS (from 3.6 to 0.036 ppb) from landfill leachate (US EPA, 2021).
Some of the SCWO technologies that have been commercialized to treat sewage sludge include the Aqua Reci® Technology (Stendahl & Jäfverström, 2004), Aqua Citrox® Technology (Gidner & Stenmark, 2001), and theAthos® Technology (Veolia Water). More recently, the Danish company Aquarden Technologies developed a full-scale solution (Figure 5) to capture and destroy PFAS. The use of SCWO for PFAS is new, but a non-peer-reviewed article (Mikkelsen, 2020) reported up to 99% destruction of PFAS in contaminated drinking water and wastewater; the PFAS were broken down into water, nitrogen gas, fluoride, and carbon dioxide. The full-scale system is based on modular components where contaminated water is treated by adsorption columns, and the spent adsorbent with PFAS is destroyed in an on-site or off-site SCWO plant. The technology has been installed at several locations in Denmark, Norway, and Sweden primarily to treat PFAS in groundwater and landfill leachate (Mikkelsen, 2020).
FIGURE 5.

Supercritical water oxidation reactor (photo courtesy of Aquarden Technologies)
General operational challenges when implementing SCWO are high temperatures and pressures that can lead to system degradation and maintenance issues (Vadillo et al., 2013), including scaling and corrosion (Pinkard et al., 2021), and high energy demands. Fluoride salts, a by-product of SCWO of PFAS, can lead to reactor plugging, decreasing overall performance (Voisin et al., 2017). Also, fluorine readily converts to HF, so protection for equipment and staff is critical.
A challenge particular to SCWO-based defluorination of PFAS in membrane concentrates, which tend to be saline, is the scaling propensity of SCWO (Pinkard et al., 2021) arising from the low solubility of most salts in supercritical water (Leusbrock et al., 2010). Processes such as ED might be used to remove salt from membrane concentrates before PFAS destruction by SCWO. Alternatively, adsorbents could be used as an intermediary between membrane concentrates and SCWO. Further study on SCWO of PFAS-laden RO and NF concentrates is needed to mitigate scaling and corrosion issues, investigate process hybridizations that prevent scaling, develop life cycle costs, and address other operation and maintenance challenges (US EPA, 2021).
4 |. SEQUESTRATION OF PFAS WASTE
When sequestration of PFAS-laden waste, rather than defluorination, is the goal, two disposal methods are currently considered as best practices: deep well injection (for liquid wastes, such as RO concentrate) and landfill (for solids, such as spent adsorbents) (US EPA, 2020).
4.1 |. Deep well injection
PFAS-laden liquids including membrane process concentrate and aqueous solutions used for resin regeneration can both be sequestered within confined aquifers by deep well injection (Crone et al., 2019). In deep well injection, a well is drilled into a deep (typically about 500–3000 m) confined aquifer that is isolated from underground drinking water sources and then waste fluid is injected at high pressure (Sahu, 2021; US EPA, 2020). U.S. EPA Class I wells are commonly used for sequestering both hazardous and nonhazardous waste streams. Although concerns about groundwater contamination and earthquakes persist (Tsang et al., 2008), the risk of Class I well failure is considered low due to careful permitting requirements and the redundancy of multiple impermeable rock layers (US EPA, 2001).
No records of deep well injection of PFAS-laden membrane system concentrate are known to the authors; however, because perfluorinated compounds are ubiquitous, it is reasonable to expect that concentrate streams will have relatively higher levels of the compounds than in the membrane feed waters. Fouling (scaling) of injection wells may be an issue with highly saline PFAS-laden concentrate streams (e.g., from RO), which may be near or above the saturation concentration of scale-forming salts. However, PFAS-laden leachate and industrial wastewater have been disposed by deep well injection at two sites in the United States. (US EPA, 2020; Koropeckyj-Cox, 2019).
Geologic and permitting requirements and high construction costs limit the availability of injection wells and in turn increase associated costs for projects. The pervolume cost of deep well injection varies widely and has been estimated using cost models to be between $0.93/m3 and $4.58/m3 for an injection stream of 0.25 MGD and between $0.15/m3 and $0.48/m3 for 2.5 MGD (Foldager, 2003); another study puts the range between $0.54/m3 and $2.65/m3 (Panagopoulos et al., 2019); and a recent report estimated the price for third-party disposal of PFAS liquid waste as $48/m3 to $66/m3 (DeSilva, 2019).
4.2 |. Landfill
Landfills, which are engineered sites for the storage and gradual breakdown of solid waste, can be used to store PFAS-laden solids from water treatment including non-regenerated GAC or PAC, spent AIX resins, or solids from zero- or minimal-liquid-discharge treatment processes (e.g., from an evaporation pond), but the efficacy with which landfills sequester PFAS is not well understood. The U.S. EPA provides an overview of the potential and issues with landfilling PFAS waste (US EPA, 2020). PFAS in solids contaminate the landfill liquid waste stream (leachate), which is typically difficult to treat (Kamaruddin et al., 2015). Leachate wastewater treatment plants are rarely equipped for PFAS treatment (USEPA, 2020). PFAS may also escape within landfill gas, both captured and fugitive (US EPA, 2020), and has been detected in air around landfills (Hamid et al., 2018). Landfill barrier systems (liners and caps) will ultimately fail, so PFAS sequestration in landfills cannot be considered permanent (US EPA, 2020). In light of these issues, many conventional landfills may not accept PFAS-contaminated waste streams. Hazardous waste landfills, with their double liners and leachate collection, are better equipped for disposal of PFAS-laden solids from water treatment, but the effectiveness of leachate treatment for PFAS still varies.
The cost of landfilling spent GAC from PFAS removal has been estimated at $0.04/lb ($0.09/kg) (US EPA, 2020). For an additional cost, stabilization and solidification of PFAS-contaminated waste streams with appropriate additives can reduce leaching of PFAS; for example, adding 2% activated carbon to PFAS-contaminated soil during stabilization and solidification reduced leaching significantly (up to 99.9% for PFOS) (Sörengård et al., 2019).
5 |. SUMMARY AND CONCLUSION
When treating drinking water to remove PFAS, it is important to manage residual streams in a way that minimizes negative health impacts and environmental damage. The common technologies used for PFAS removal currently include adsorbents (activated carbon or ion exchange) and membranes (RO and NF). RO and NF membranes have been shown to have excellent removal properties for a wide range of PFAS. Like adsorbents, RO and NF membranes do not destroy PFAS, but rather produce a concentrated residual stream that is high in PFAS and other dissolved constituents. Therefore, there is a need to manage the concentrated residual stream via either a long-term sequestration or a destructive defluorination approach.
The available concentrate residual treatment technologies and management approaches were categorized as concentration, defluorination, or sequestration. The advantages and disadvantages of the technologies are summarized in Table 1 and their technology readiness levels (TRL) are categorized in Figure 6 in accordance with those promulgated by the U.S. Department of Energy (2011). TRL group 1–3 indicates the lowest level of readiness, where research is still being done to establish a proof of concept and determine feasibility. TRL group 4–6 indicates that a component or system has been validated in a laboratory environment or at a pilot scale. TRL group 7–9 indicates that full-scale systems have already been operated with relevant water streams.
TABLE 1.
High-level summary of various PFAS management technologies
| Technology | Advantages | Disadvantages | |
|---|---|---|---|
| Concentration | Reverse osmosis and nanofiltration | • Effective removal • Small footprint • Relatively energy-efficient |
• Membrane scaling limits recovery |
| Membrane distillation | • Effective removal of PFPeA | • Membrane fouling • Very early research stage • High thermal energy use |
|
| Forward osmosis | • High rejection and recovery possible | • Draw regeneration difficult | |
| RO-electrodialysis hybrids | • Possibly concentrate charged PFAS compounds | • No validation with PFAS | |
| Foam fractionation | • Effective removal of long-chain PFAS, including PFOS | • Less effective for short-chain PFAS | |
| Electrocoagulation | • Ease of operation • Effective removal of PFOA and PFOSwith Zn anode |
• Requires optimization ofoperational conditions • Passivation of electrode material |
|
| Evaporation ponds | • Compatible with solar energy | • High land area requirement | |
| • ZLD possible | • PFAS emissions to air possible | ||
| Brine concentrators and crystallizers | • Significant concentration and ZLD possible | • High energy requirement • PFAS emissions to air possible |
|
| Adsorption | • Reliable and easy to operate | • May require frequent regeneration and/or replacement of saturatedadsorbents | |
| Coagulant aids | • Early data show high effectiveness | • Proprietary formulations | |
| Defluorination | Biological treatment | • Could scale well | • Largely unsuccessful so far |
| Ultraviolet irradiation | • Availability of equipment | • Ineffective for PFAA | |
| Photocatalysis | • Effective degradation of PFOA • Reduce energy consumption by using visible light |
• May generate intermediates, such as PFHpA, PFHxA, PFPeA, PFBA, PFPA, and TFA • Proof-of-concept stage |
|
| Advanced oxidation | • Moderate success with FTOHs | • Limited success with PFAS | |
| Hydrated electrons | • Effective destruction of at least some PFAS | • Early stage | |
| Plasma-based treatment | • Early evidence of effective defluorination | • Early stage | |
| Electron beam | • High technology readiness level: previously used at full and pilotscales for wastewater and groundwater and commercially available in medical and pasteurization industries | • Large capital cost and high priceper volume treated • Needs further research onmembrane concentrate to understand matrix interferences |
|
| • Effective at PFOA and PFOS degradation | |||
| Zero-valent iron | • Effective with carbonate PFAS | • Less effective with sulfonate PFAS | |
| Sonochemical treatment | • Complete mineralization of PFAS can be achieved without any pretreatment | • High energy input and scalability issues | |
| Incineration | • Effective in desorption/destruction of PFAS | • Formation of by-products | |
| Supercritical water oxidation | • Effective with many PFAS • Demonstrated at scale with PFAS-laden groundwater and leachate |
• High temperature and pressure create operational challenges | |
| • Salts in membrane concentrates must be removed to prevent severescaling | |||
| Sequestration | Deep-well injection | • Reliable sequestration | • Nondestructive |
| Landfill | • Endpoint for solids | • Nondestructive • Impermanent sequestration due toPFAS-laden leachate production |
Note: For references, see the relevant section of the narrative.
Abbreviations: FTOHs, fluorotelomer alcohols; PFAA, perfluoroalkyl acid; PFAS, per- and polyfluoroalkyl substances; PFBA, pentafluorobenzoic acid; PFHpA, perfluoroheptanoic acid; PFHxA, perfluorohexanoic acid; PFOS, perfluorooctane sulfonic acid; PFPeA, perfluoropentanoic acid; PFPA, perfluoropropanoic acid; RO, reverse osmosis; TFA, trifluoroacetic acid.
FIGURE 6.
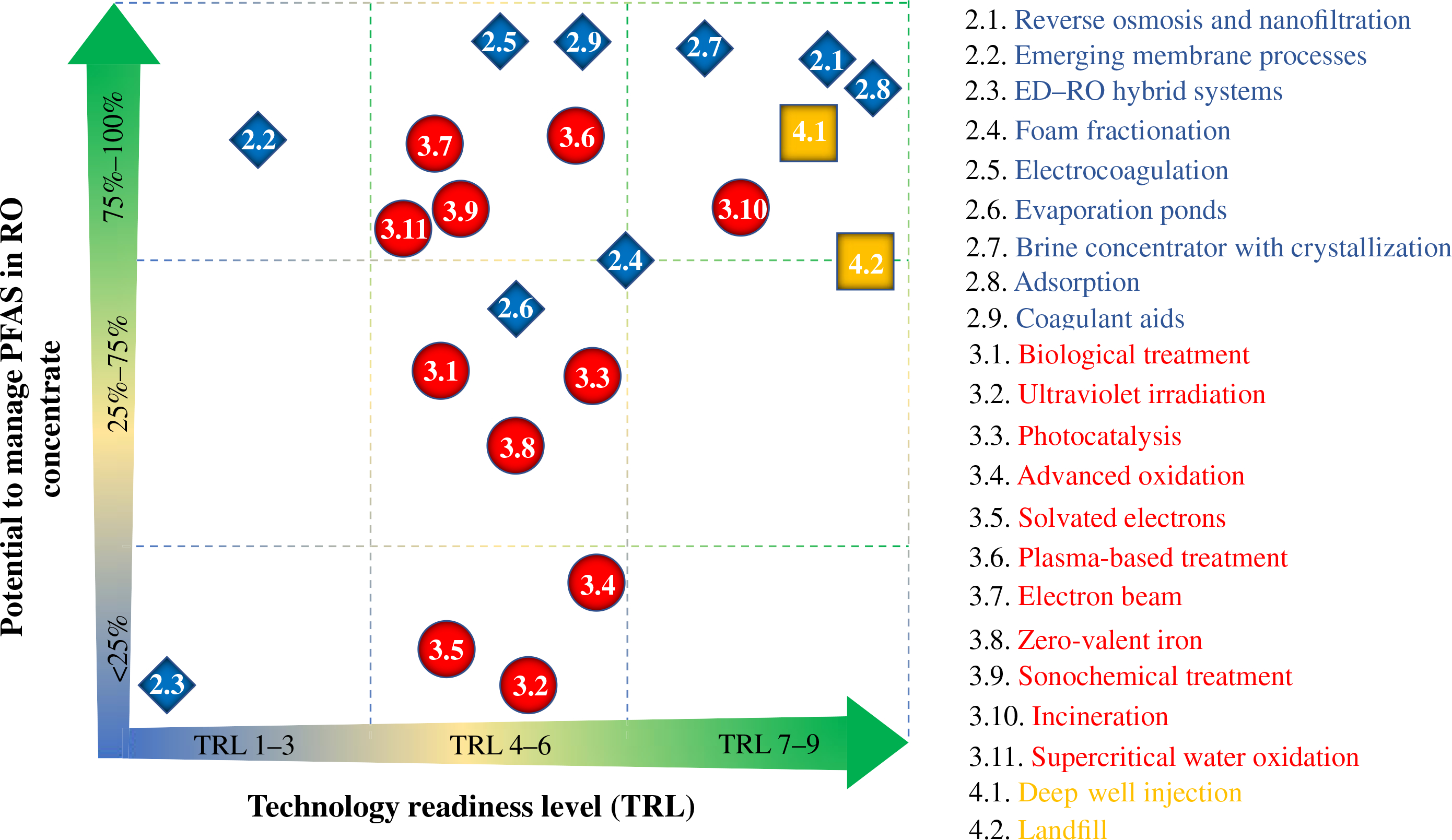
Graphical summary of technology readiness levels (ITRC, 2020) and nominal reported potential to manage (separate, defluorinate, or sequester) PFAS from reverse osmosis concentrate. The relative placement of processes in the plot does not consider capital and operating costs, in part because these are challenging to estimate for early-stage technologies. Numbers correspond to the sections of this article where each technology is discussed
Assignment of each technology to a TRL group was done through consensus among the authors, but it is recognized that others may have different opinions about the readiness of each technology so the placement of technologies in Figure 6 is not meant to be more granular than these three TRL groups.
Like the TRL grouping, the “potential to manage PFAS in RO concentrate” on the vertical-axis of Figure 6 has been broken into three groups. The “<25%” group indicates that the defluorination technologies can destroy roughly 25% or less of the PFAS in an RO concentrate and the separation technologies can separate out less than roughly 25% of the PFAS. The “25%–75%” group indicates that the separation or defluorination is more effective than the 25% group, but has not been shown to achieve more than roughly 75% separation or defluorination. The “75%–100%” group comprises the most effective technologies, achieving the best separation or defluorination.
Concentration technologies are relevant because minimizing volumes and increasing concentrations can lead to increased efficiency or lower cost for the chosen final management tactic (defluorination or sequestration). The concentration technologies identified as particularly viable include semi-batch RO, foam fractionation, evaporation, adsorption, and additive-enhanced coagulation; however, further research is needed to identify the optimal process for a given application.
Long-term sequestration of liquid concentrates (with limited risk of environmental release) is limited to deep well injection. For solid residuals from concentrate treatment (e.g., spent adsorbents, precipitates, or ash), the best sequestration option, which itself is not without environmental risk, is landfilling. These limited options and the associated environmental concerns motivate further development of defluorination technologies to degrade PFAS and prevent the contamination of environmental waters. Deep well injection and landfill both fall in the high TRL and high potential to manage PFAS groups in Figure 6, but because there is potential for escape from landfills and deep injection wells, these are not regarded as 100% effective.
Among possible approaches to PFAS destruction or degradation, this review focused on defluorination. Although it is possible to break the parent PFAS molecule at the terminal functional group, a defluorination approach was addressed herein anticipating that removing a functional end moiety would not be sufficient to eliminate future risks to health and the environment. Defluorination technologies that have been investigated for concentrate treatment include biological, UV, advanced oxidation, plasma, hydrated electrons, zero-valent iron, sonochemical, e-beam, incineration, and SCWO. Of these, incineration is the most mature technology; however, it is most appropriate for highly concentrated streams or solid wastes from concentration efforts such as spent media, evaporated solids, precipitates, and foams. Other technologies are largely considered emerging for this application. Some of them have shown high percent destructions for parent PFAS compounds, but none to date have shown complete mineralization with membrane concentrate water qualities and treatment conditions at the full scale, and costs at scale are largely unknown. Defluorination of PFAS in concentrated waste streams remains an open area of research.
This review aims to advance the field of PFAS management in membrane concentrates to enable permanent elimination of PFAS in water sources. Readers are encouraged to evaluate the advantages and disadvantages of the various technologies summarized in Table 1 and expanded upon in the narrative. Research efforts that result in mitigating the disadvantages listed can result in movement of a technology into a higher TRL. For example, EC has a disadvantage of “passivation”; research is needed on new materials that are more resistant to passivation.
Further studies ought to address both individual defluorination technologies and system-level approaches to PFAS destruction. Research into the efficacy, environmental impact, and cost of PFAS defluorination in membrane concentrates—particularly using technologies that have already shown significant promise—is urgently needed to improve the state of the art in PFAS destruction. Additionally, due to the anticipated high energy requirements and costs of defluorination processes (per unit volume), it is worth investigating sequences of concentration and defluorination processes that will minimize the environmental footprint and cost of responsibly managing PFAS-laden concentrates.
Article Impact Statement.
Readers will benefit from learning about established and emerging management approaches for per- and polyfluoroalkyl substances and the peculiarities of their application to reverse osmosis and nanofiltration concentrates.
ACKNOWLEDGMENTS
This work was partially funded by the National Science Foundation (EEC-1449500) Nanosystems Engineering Research Center on Nanotechnology-Enabled Water Treatment. We thank Evocra Pty Ltd (Riverside, Tasmania, Australia), Sheau-Yun (Dora) Chiang (Vice President, CDM Smith, Marietta, Georgia, USA), OPEC Systems (Belrose, New South Wales, Australia), and Ea Dehn (Business Development, Aquarden Technologies, Skævinge, Denmark) for providing photographs of treatment systems. This work has been subjected to the United States Environmental Protection Agency’s review and has been approved for publication. The views expressed in this manuscript are those of the authors and do not necessarily represent the views or policies of the Agency. Any mention of trade names, products, or services does not imply an endorsement by the Agency. The Agency does not endorse any commercial products, services, or enterprises.
Funding information
National Science Foundation: EEC-1449500
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
DATA AVAILABILITY STATEMENT
No original data are presented.
REFERENCES
- Abeln J, Kluth M, Petrich G, & Schmieder H (2001). Supercritical water oxidation (SCWO): A process for the treatment of industrial waste effluents. High Pressure Research, 20(1–6), 537. 10.1080/08957950108206202 [DOI] [Google Scholar]
- Adusei-Gyamfi J, & Acha V (2016). Carriers for nano zerovalent iron (nZVI): Synthesis, application and efficiency. RSC Advances, 6(93), 91025–91044. 10.1039/c6ra16657a [DOI] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). (2018). Toxicological profile for perfluoroalkyls (draft for public comment). 10.15620/cdc:59198 [DOI] [PubMed]
- Aleksandrov K, Gehrmann HJ, Hauser M, Mätzing H, Pigeon D, Stapf D, & Wexler M (2019). Waste incineration of polytetrafluoroethylene (PTFE) to evaluate potential formation of per- and poly-fluorinated alkyl substances (PFAS) in flue gas. Chemosphere, 226, 898–906. 10.1016/j.chemosphere.2019.03.191 [DOI] [PubMed] [Google Scholar]
- Anumol T, Dagnino S, Vandervort DR, & Snyder SA (2016). Transformation of polyfluorinated compounds in natural waters by advanced oxidation processes. Chemosphere, 144, 1780. 10.1016/j.chemosphere.2015.10.070 [DOI] [PubMed] [Google Scholar]
- Appleman TD, Dickenson ERV, Bellona C, & Higgins CP (2013). Nanofiltration and granular activated carbon treatment of perfluoroalkyl acids. Journal of Hazardous Materials, 260, 740–746. 10.1016/j.jhazmat.2013.06.033 [DOI] [PubMed] [Google Scholar]
- Appleman TD, Higgins CP, Quiñones O, Vanderford BJ, Kolstad C, Zeigler-Holady JC, & Dickenson ERV (2014). Treatment of poly- and perfluoroalkyl substances in U.S. full-scale water treatment systems. Water Research, 51, 246–255. 10.1016/j.watres.2013.10.067 [DOI] [PubMed] [Google Scholar]
- Barisci S, & Suri R (2021). Removal of polyfluorinated telomer alcohol by advanced oxidation processes (AOPs) in different water matrices and evaluation of degradation mechanisms. Journal of Water Process Engineering, 39(October 2020), 101745. 10.1016/j.jwpe.2020.101745 [DOI] [Google Scholar]
- Belkouteb N, Franke V, McCleaf P, Köhler S, & Ahrens L (2020). Removal of per- and polyfluoroalkyl substances (PFASs) in a full-scale drinking water treatment plant: Long-term performance of granular activated carbon (GAC) and influence of flow-rate. Water Research, 182, 115913. 10.1016/j.watres.2020.115913 [DOI] [PubMed] [Google Scholar]
- Bentel MJ, Yu Y, Xu L, Kwon H, Li Z, Wong BM, Men Y, & Liu J (2020). Degradation of perfluoroalkyl ether carboxylic acids with hydrated electrons: Structure-reactivity relationships and environmental implications. Environmental Science and Technology, 54, 2489. 10.1021/acs.est.9b05869 [DOI] [PubMed] [Google Scholar]
- Bond P, & Veerapaneni S (2008). Zeroing in on ZLD technologies for inland desalination. Journal/American Water Works Association, 100, 76–89. 10.1002/j.1551-8833.2008.tb09722.x [DOI] [Google Scholar]
- Bonn PAC, Hofman JAMH, & van Der Hoek JP (2000). Scaling control of RO membranes and direct treatment of surface water. Desalination, 132(October), 109–119. [Google Scholar]
- Boo C, Wang Y, Zucker I, Choo Y, Osuji CO, & Elimelech M (2018). High performance nanofiltration membrane for effective removal of perfluoroalkyl substances at high water recovery. Environmental Science and Technology, 52, 7279–7288. 10.1021/acs.est.8b01040 [DOI] [PubMed] [Google Scholar]
- Busch J, Ahrens L, Sturm R, & Ebinghaus R (2010). Polyfluoroalkyl compounds in landfill leachates. Environmental Pollution, 158, 1467. 10.1016/j.envpol.2009.12.031 [DOI] [PubMed] [Google Scholar]
- Cagnetta G, Zhang Q, Huang J, Lu M, Wang B, Wang Y, Deng S, & Yu G (2017). Mechanochemical destruction of perfluorinated pollutants and mechanosynthesis of lanthanum oxyfluoride: A waste-to-materials process. Chemical Engineering Journal, 316, 1078–1090. 10.1016/j.cej.2017.02.050 [DOI] [Google Scholar]
- Cao H, Zhang W, Wang C, & Liang Y (2020). Sonochemical degradation of poly- and perfluoroalkyl substances—A review. Ultrasonics Sonochemistry, 69(July), 105245. 10.1016/j.ultsonch.2020.105245 [DOI] [PubMed] [Google Scholar]
- Chen X, Vanangamudi A, Wang J, Jegatheesan J, Mishra V, Sharma R, Gray SR, Kujawa J, Kujawski W, Wicaksana F, & Dumée LF (2020). Direct contact membrane distillation for effective concentration of perfluoroalkyl substances – Impact of surface fouling and material stability. Water Research, 182, 1–10. 10.1016/j.watres.2020.116010 [DOI] [PubMed] [Google Scholar]
- Chen YC, Lo SL, & Kuo J (2011). Effects of titanate nanotubes synthesized by a microwave hydrothermal method on photocatalytic decomposition of perfluorooctanoic acid. Water Research, 45(14), 4131–4140. 10.1016/j.watres.2011.05.020 [DOI] [PubMed] [Google Scholar]
- Choi PJ, Lao JY, Lam PKS, Im SJ, Jang A, & An AK (2021). Low-pressure volume retarded osmosis for removal of per-and polyfluoroalkyl substances. Water Research, 194, 116929. 10.1016/j.watres.2021.116929 [DOI] [PubMed] [Google Scholar]
- Cornelsen M, Weber R, & Panglisch S (2021). Minimizing the environmental impact of PFAS by using specialized coagulants for the treatment of PFAS polluted waters and for the decontamination of firefighting equipment. Emerging Contaminants, 7, 63–76. 10.1016/j.emcon.2021.02.001 [DOI] [Google Scholar]
- Coyle C, Ghosh R, Leeson A, & Thompson T (2021). US Department of defense–funded research on treatment of per- and polyfluoroalkyl substance–laden materials. Environmental Toxicology and Chemistry, 40(1), 44–56. 10.1002/etc.4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JC, Trussell RR, Hand DW, Howe KJ, & Tchobanoglous G (2012). MWH’s water treatment principles and design (3rd ed.). John Wiley & Sons. [Google Scholar]
- Crone BC, Speth TF, Wahman DG, Smith SJ, Abulikemu G, Kleiner EJ, & Pressman JG (2019). Occurrence of per- and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water. Critical Reviews in Environmental Science and Technology, 49(24), 2359–2396. 10.1080/10643389.2019.1614848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Xie Z, Dorian B, Gray S, & Zhang J (2019). Comparative study of PFAS treatment by UV, UV/ozone, and fractionations with air and ozonated air. Environmental Science: Water Research and Technology, 5(11), 1897–1907. 10.1039/c9ew00701f [DOI] [Google Scholar]
- Deng S, Bao Y, Cagnetta G, Huang J, & Yu G (2020). Mechanochemical degradation of perfluorohexane sulfonate: Synergistic effect of ferrate(VI) and zero-valent iron. Environmental Pollution, 264, 114789. 10.1016/j.envpol.2020.114789 [DOI] [PubMed] [Google Scholar]
- DeSilva V (2019). PFAS in landfill leachate. http://www.mowastecoalition.org/resources/Documents/2019conference/MWCC2019-deSilva(SCS)-PFAS-July16,2019.pdf
- Dickenson E, & Higgins C (2016). Treatment mitigation strategies for poly-and Perfluoroalkyl substances. Water Research Foundation Web Report, 4322. [Google Scholar]
- Duan L, Wang B, Heck K, Guo S, Clark CA, Arredondo J, Wang M, Senftle TP, Westerhoff P, Wen X, Song Y, & Wong MS (2020). Efficient photocatalytic PFOA degradation over boron nitride. Environmental Science and Technology Letters, 7(8), 613–619. 10.1021/acs.estlett.0c00434 [DOI] [Google Scholar]
- Ebersbach I, Ludwig SM, Constapel M, & Kling HW (2016). An alternative treatment method for fluorosurfactant-containing wastewater by aerosol-mediated separation. Water Research, 101, 333–340. 10.1016/j.watres.2016.05.063 [DOI] [PubMed] [Google Scholar]
- Edel H-G, Klopp D, Drubel J, Korte D, Kellner C, & Rehnig U (2018). English translation reprint from Handbuch Altlastensanierung und Flächenmanagement (HdA) PFAS groundwater remediation: State of the art technology and comparison of costs. https://www.zueblin-umwelttechnik.com/databases/internet/_public/files30.nsf/SearchView/49C26B9C0D98CED1C125835F0030D11D/$File/HdAbroschur-englisch.pdf [Google Scholar]
- Fernandez NA, Rodriguez-Freire L, Keswani M, & Sierra-Alvarez R (2016). Effect of chemical structure on the sonochemical degradation of perfluoroalkyl and polyfluoroalkyl substances (PFASs). Environmental Science: Water Research and Technology, 2(6), 975–983. 10.1039/c6ew00150e [DOI] [Google Scholar]
- Flores C, Ventura F, Martin-Alonso J, & Caixach J (2013). Occurrence of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in N.E. Spanish surface waters and their removal in a drinking water treatment plant that combines conventional and advanced treatments in parallel lines. Science of the Total Environment, 461–462, 618–626. 10.1016/j.scitotenv.2013.05.026 [DOI] [PubMed] [Google Scholar]
- Foldager RA (2003). Economics of desalination concentrate disposal methods in inland regions: Deep-well injection, evaporation ponds, and salinity gradient solar ponds. New Mexico State University. [Google Scholar]
- Franke V, McCleaf P, Lindegren K, & Ahrens L (2019). Efficient removal of per- and polyfluoroalkyl substances (PFASs) in drinking water treatment: Nanofiltration combined with active carbon or anion exchange. Environmental Science: Water Research and Technology, 5(11), 1836–1843. 10.1039/c9ew00286c [DOI] [Google Scholar]
- Franke V, Ullberg M, Mccleaf P, Wålinder M, Ko SJ, & Ahrens L (2021). The price of really clean water: Combining nanofiltration with granular activated carbon and anion exchange resins for the removal of per- and polyfluoralkyl substances (PFASs) in drinking water production. ACS EST Water, 1(4), 782–795. 10.1021/acsestwater.0c00141 [DOI] [Google Scholar]
- Garnick L, Massarsky A, Mushnick A, Hamaji C, Scott P, & Monnot A (2021). An evaluation of health-based federal and state PFOA drinking water guidelines in the United States. Science of the Total Environment, 761, 144107. 10.1016/j.scitotenv.2020.144107 [DOI] [PubMed] [Google Scholar]
- Gidner A, & Stenmark L (2001). Supercritical water oxidation of sewage sludge – State of the art. Proceedings of the IBC’s Conference on Sewage Sludge and Disposal Options, Birmingham, England [Google Scholar]
- Gole VL, Sierra-Alvarez R, Peng H, Giesy JP, Deymier P, & Keswani M (2018). Sono-chemical treatment of per- and polyfluoroalkyl compounds in aqueous film-forming foams by use of a large-scale multi-transducer dual-frequency based acoustic reactor. Ultrasonics Sonochemistry, 45(February), 213–222. 10.1016/j.ultsonch.2018.02.014 [DOI] [PubMed] [Google Scholar]
- Gu M, Farooq U, Lu S, Zhang X, Qiu Z, & Sui Q (2018). Degradation of trichloroethylene in aqueous solution by rGO supported nZVI catalyst under several oxic environments. Journal of Hazardous Materials, 349, 35–44. 10.1016/j.jhazmat.2018.01.037 [DOI] [PubMed] [Google Scholar]
- Hamid H, Li LY, & Grace JR (2018). Review of the fate and transformation of per- and polyfluoroalkyl substances (PFASs) in landfills. Environmental Pollution, 235, 74–84. 10.1016/j.envpol.2017.12.030 [DOI] [PubMed] [Google Scholar]
- Han B, Kyu Kim J, Kim Y, Seung Choi J, & Young Jeong K (2012). Operation of industrial-scale electron beam wastewater treatment plant. Radiation Physics and Chemistry, 81(9), 1475–1478. 10.1016/j.radphyschem.2012.01.030 [DOI] [Google Scholar]
- Hori H, Yamamoto A, Hayakawa E, Taniyasu S, Yamashita N, Kutsuna S, Kiatagawa H, & Arakawa R (2005). Efficient decomposition of environmentally persistent perfluorocarboxylic acids by use of persulfate as a photochemical oxidant. Environmental Science and Technology, 39(7), 2383–2388. 10.1021/es0484754 [DOI] [PubMed] [Google Scholar]
- Horst J, McDonough J, Ross I, & Houtz E (2020). Understanding and managing the potential by-products of PFAS destruction. Groundwater Monitoring and Remediation, 40(2), 17–27. 10.1111/gwmr.12372 [DOI] [Google Scholar]
- Huang D, De Vera GA, Chu C, Zhu Q, Stavitski E, Mao J, Xin H, Spies JA, Schmuttenmaer CA, Niu J, & Haller GL (2018). Single-atom Pt catalyst for effective C-F bond activation via Hydrodefluorination. ACS Catalysis, 8(10), 9353–9358. 10.1021/acscatal.8b02660 [DOI] [Google Scholar]
- Huang S, & Jaffé PR (2019). Defluorination of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) by Acidimicrobium sp. strain A6. Environmental Science and Technology, 53, 11410–11419. 10.1021/acs.est.9b04047 [DOI] [PubMed] [Google Scholar]
- ITRC. (2020). PFAS technical and regulatory guidance document. https://pfas-1.itrcweb.org/tables_april_2020/ITRCPFASTable12-1LiquidTechnologiesApr2020.pdf
- Joshua F (2020). Water reclamation using functionalized forward osmosis membrane. University of Central Florida. https://stars.library.ucf.edu/etd2020/444/ [Google Scholar]
- Kamaruddin MA, Yusoff MS, Aziz HA, & Hung Y-T (2015). Sustainable treatment of landfill leachate. Applied Water Science, 5(2), 113–126. 10.1007/s13201-014-0177-7 [DOI] [Google Scholar]
- Katal R, Shen TY, Jafari I, Masudy-Panah S, & Farahani MHDA (2020). An overview on the treatment and management of the desalination brine solution. In Desalination - challenges and opportunities. Intech Open. 10.5772/intechopen.92661 [DOI] [Google Scholar]
- Kim K, Son SH, Kim KS, Kim K, & Kim YC (2010). Environmental effects of supercritical water oxidation (SCWO) process for treating transformer oil contaminated with polychlorinated biphenyls (PCBs). Chemical Engineering Journal, 165(1), 170–174. 10.1016/j.cej.2010.09.012 [DOI] [Google Scholar]
- Koropeckyj-Cox LJ (2019). Quantifying the transport of per- and polyfluoroalkyl substances (PFAS) from groundwater to surface water near the chemours property in Bladen County, NC (MS Thesis). North Carolina State University. https://repository.lib.ncsu.edu/handle/1840.20/36724?show=full [Google Scholar]
- Kucharzyk KH, Darlington R, Benotti M, Deeb R, & Hawley E (2017). Novel treatment technologies for PFAS compounds: A critical review. Journal of Environmental Management, 204, 757–764. 10.1016/j.jenvman.2017.08.016 [DOI] [PubMed] [Google Scholar]
- Kwon YN, Shih K, Tang C, & Leckie JO (2012). Adsorption of perfluorinated compounds on thin-film composite polyamide membranes. Journal of Applied Polymer Science, 124, 1042. 10.1002/app.35182 [DOI] [Google Scholar]
- Lee T, Choi JY, & Cohen Y (2019). Gypsum scaling propensity in semi-batch RO (SBRO) and steady-state RO with partial recycle (SSRO-PR). Journal of Membrane Science, 588, 117106. 10.1016/j.memsci.2019.05.030 [DOI] [Google Scholar]
- Lee T, Rahardianto A, & Cohen Y (2019). Multi-cycle operation of semi-batch reverse osmosis (SBRO) desalination. Journal of Membrane Science, 588, 117090. 10.1016/j.memsci.2019.05.015 [DOI] [Google Scholar]
- Lee YC, Chen MJ, Huang CP, Kuo J, & Lo SL (2016). Efficient sonochemical degradation of perfluorooctanoic acid using periodate. Ultrasonics Sonochemistry, 31, 499–505. 10.1016/j.ultsonch.2016.01.030 [DOI] [PubMed] [Google Scholar]
- Legislation column. (2016). California’s OEHHA intends to add PFOA and PFOS to prop 65 list. Focus on Surfactants, 12, 6. 10.1016/j.fos.2016.12.026 [DOI] [Google Scholar]
- Leusbrock I, Metz SJ, Rexwinkel G, & Versteeg GF (2010). The solubilities of phosphate and sulfate salts in supercritical water. Journal of Supercritical Fluids, 54(1), 1–8. 10.1016/j.supflu.2010.03.003 [DOI] [Google Scholar]
- Lewis AJ, Joyce T, Hadaya M, Ebrahimi F, Dragiev I, Giardetti N, Yang J, Fridman G, Rabinovich A, Fridman AA, McKenzie ER, & Sales CM (2020). Rapid degradation of PFAS in aqueous solutions by reverse vortex flow gliding arc plasma. Environmental Science: Water Research and Technology, 6(4), 1044–1057. 10.1039/c9ew01050e [DOI] [Google Scholar]
- Li F, Wei Z, He K, Blaney L, Cheng X, Xu T, Liu W, & Zhao D (2020). A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst. Water Research, 185, 1–14. 10.1016/j.watres.2020.116219 [DOI] [PubMed] [Google Scholar]
- Li M (2021). Residence time distribution in RO channel. Desalination, 506, 115000. 10.1016/j.desal.2021.115000 [DOI] [Google Scholar]
- Lin H, Wang Y, Niu J, Yue Z, & Huang Q (2015). Efficient sorption and removal of Perfluoroalkyl acids (PFAAs) from aqueous solution by metal hydroxides generated in situ by electrocoagulation. Environmental Science and Technology, 49(17), 10562–10569. 10.1021/acs.est.5b02092 [DOI] [PubMed] [Google Scholar]
- Lin JC, Lo SL, Hu CY, Lee YC, & Kuo J (2015). Enhanced sonochemical degradation of perfluorooctanoic acid by sulfate ions. Ultrasonics Sonochemistry, 22, 542–547. 10.1016/j.ultsonch.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Lin S, & Elimelech M (2015). Staged reverse osmosis operation: Configurations, energy efficiency, and application potential. Desalination, 366, 9. 10.1016/j.desal.2015.02.043 [DOI] [Google Scholar]
- Lipp P, Sacher F, & Baldauf G (2010). Removal of organic micro-pollutants during drinking water treatment by nanofiltration and reverse osmosis. Desalination and Water Treatment, 13, 226. 10.5004/dwt.2010.1063 [DOI] [Google Scholar]
- Liu L, Liu Y, Gao B, Ji R, Li C, & Wang S (2020). Removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from water by carbonaceous nanomaterials: A review. Critical Reviews in Environmental Science and Technology, 50 (22), 2379–2414. 10.1080/10643389.2019.1700751 [DOI] [Google Scholar]
- Liu Y, Hu XM, Zhao Y, Wang J, Lu MX, Peng FH, & Bao J (2018). Removal of perfluorooctanoic acid in simulated and natural waters with different electrode materials by electrocoagulation. Chemosphere, 201, 303–309. 10.1016/j.chemosphere.2018.02.129 [DOI] [PubMed] [Google Scholar]
- Lu D, Sha S, Luo J, Huang Z, & Zhang Jackie X (2020). Treatment train approaches for the remediation of per- and polyfluoroalkyl substances (PFAS): A critical review. Journal of Hazardous Materials, 386(October 2019), 121963. 10.1016/j.jhazmat.2019.121963 [DOI] [PubMed] [Google Scholar]
- Lu M, Cagnetta G, Zhang K, Huang J, & Yu G (2017). Mechanochemical mineralization of “very persistent” fluorocarbon surfactants 6:2 fluorotelomer sulfonate (6:2FTS) as an example. Scientific Reports, 7(1), 1–10. 10.1038/s41598-017-17515-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H, Wang N, Zhu L, Zhou Y, Li W, & Tang H (2018). Alumina-mediated mechanochemical method for simultaneously degrading perfluorooctanoic acid and synthesizing a polyfluoroalkene. Green Chemistry, 20(11), 2526–2533. 10.1039/c8gc00854j [DOI] [Google Scholar]
- Lyu XJ, Liu Y, Chen C, Sima M, Lyu JF, Ma ZY, & Huang S (2020). Enhanced use of foam fractionation in the photodegradation of perfluorooctane sulfonate (PFOS). Separation and Purification Technology, 253(July), 117488. 10.1016/j.seppur.2020.117488 [DOI] [Google Scholar]
- Maga D, Aryan V, & Bruzzano S (2021). Environmental assessment of various end-of-life pathways for treating per- and polyfluoroalkyl substances in spent fire-extinguishing waters. Environmental Toxicology and Chemistry, 40(3), 947–957. 10.1002/etc.4803 [DOI] [PubMed] [Google Scholar]
- Masud A, Guardian MGE, Travis SC, Chavez Soria NG, Jarin M, Aga DS, & Aich N (2021). Redox-active rGO-nZVI nanohybrid-catalyzed chain shortening of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS). Journal of Hazardous Materials Letters, 2(November 2020), 100007. 10.1016/j.hazl.2020.100007 [DOI] [Google Scholar]
- McCleaf P, Englund S, Östlund A, Lindegren K, Wiberg K, & Ahrens L (2017). Removal efficiency of multiple poly- and perfluoroalkyl substances (PFASs) in drinking water using granular activated carbon (GAC) and anion exchange (AE) column tests. Water Research, 120, 77–87. 10.1016/j.watres.2017.04.057 [DOI] [PubMed] [Google Scholar]
- McGovern RK, & Lienhard VJH (2014). On the potential of forward osmosis to energetically outperform reverse osmosis desalination. Journal of Membrane Science, 469, 245–250. 10.1016/j.memsci.2014.05.061 [DOI] [Google Scholar]
- Mededovic Thagard S, Stratton GR, Dai F, Bellona CL, Holsen TM, Bohl DG, Paek E, & Dickenson ERV (2017). Plasma-based water treatment: Development of a general mechanistic model to estimate the treatability of different types of contaminants. Journal of Physics D: Applied Physics, 50(1), 014003. 10.1088/1361-6463/50/1/014003 [DOI] [Google Scholar]
- Meng P, Deng S, Lu X, Du Z, Wang B, Huang J, Wang Y, Yu G, & Xing B (2014). Role of air bubbles overlooked in the adsorption of perfluorooctanesulfonate on hydrophobic carbonaceous adsorbents. Environmental Science and Technology, 48(23), 13785–13792. 10.1021/es504108u [DOI] [PubMed] [Google Scholar]
- Meng P, Deng S, Maimaiti A, Wang B, Huang J, Wang Y, Cousins IT, & Yu G (2018). Efficient removal of perfluorooctane sulfonate from aqueous film-forming foam solution by aeration-foam collection. Chemosphere, 203, 263–270. 10.1016/j.chemosphere.2018.03.183 [DOI] [PubMed] [Google Scholar]
- Merino N, Qu Y, Deeb RA, Hawley EL, Hoffmann MR, & Mahendra S (2016). Degradation and removal methods for Perfluoroalkyl and Polyfluoroalkyl substances in water. Environmental Engineering Science, 33(9), 615–649. 10.1089/ees.2016.0233 [DOI] [Google Scholar]
- Mickley. (2009). Treatment of concentrate, DWPR report no., 155. U. S. Dept. of interior, Bureau of Reclamation. [Google Scholar]
- Mickley M (2018). Updated and extended survey of U.S. Municipal desalination plants, DWPR report no., 207. U.S. Dept. of interior, Bureau of Reclamation. [Google Scholar]
- Mikkelsen FR (2020). PFAS destruction through supercritical water oxidation. Water Online. https://www.wateronline.com/doc/pfas-destruction-through-supercritical-water-oxidation-0001 [Google Scholar]
- Miklos DB, Remy C, Jekel M, Linden KG, Drewes JE, & Hübner U (2018). Evaluation of advanced oxidation processes for water and wastewater treatment – A critical review. Water Research, 139, 118. 10.1016/j.watres.2018.03.042 [DOI] [PubMed] [Google Scholar]
- Moriwaki H, Takagi Y, Tanaka M, Tsuruho K, Okitsu K, & Maeda Y (2005). Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environmental Science and Technology, 39(9), 3388–3392. 10.1021/es040342v [DOI] [PubMed] [Google Scholar]
- Murray CC, Vatankhah H, McDonough CA, Nickerson A, Hedtke TT, Cath TY, Higgins CP, & Bellona CL (2019). Removal of per- and polyfluoroalkyl substances using super-fine powder activated carbon and ceramic membrane filtration. Journal of Hazardous Materials, 366(June 2018), 160–168. 10.1016/j.jhazmat.2018.11.050 [DOI] [PubMed] [Google Scholar]
- Nau-Hix C, Multari N, Singh RK, Richardson S, Kulkarni P, Anderson RH, Holsen TM, & Mededovic Thagard S (2021). Field demonstration of a pilot-scale plasma reactor for the rapid removal of poly-and perfluoroalkyl substances in groundwater. ACS ES&T Water, 1, 680–687. 10.1021/acsestwater.0c00170 [DOI] [Google Scholar]
- Oren Y, Korngold E, Daltrophe N, Messalem R, Volkman Y, Aronov L, Weismann M, Bouriakov N, Glueckstern P, & Gilron J (2010). Pilot studies on high recovery BWRO-EDR for near zero liquid discharge approach. Desalination, 261(3), 321–330. 10.1016/j.desal.2010.06.010 [DOI] [Google Scholar]
- Panagopoulos A, Haralambous KJ, & Loizidou M (2019). Desalination brine disposal methods and treatment technologies - A review. Science of the Total Environment, 693, 133545. 10.1016/j.scitotenv.2019.07.351 [DOI] [PubMed] [Google Scholar]
- Parenky AC, Gevaerd De Souza N, Asgari P, Jeon J, Nadagouda MN, & Choi H (2020). Removal of perfluorooctanesulfonic acid in water by combining zerovalent iron particles with common oxidants. Environmental Engineering Science, 37(7), 472–481. 10.1089/ees.2019.0406 [DOI] [Google Scholar]
- Park H, Vecitis CD, Cheng J, Choi W, Mader BT, & Hoffmann MR (2009). Reductive defluorination of aqueous perfluorinated alkyl surfactants: Effects of ionic headgroup and chain length. Journal of Physical Chemistry A, 113(4), 690–696. 10.1021/jp807116q [DOI] [PubMed] [Google Scholar]
- Phan Thi LA, Do HT, & Lo SL (2014). Enhancing decomposition rate of perfluorooctanoic acid by carbonate radical assisted sonochemical treatment. Ultrasonics Sonochemistry, 21 (5), 1875–1880. 10.1016/j.ultsonch.2014.03.027 [DOI] [PubMed] [Google Scholar]
- Pillai SD (2020). Ex situ remediation of investigation-derived wastes containing PFAS by electron beam technology distribution. Strategic Environmental Research and Development Program (SERDP). [Google Scholar]
- Pinkard BR, Shetty S, Stritzinger D, Bellona C, & Novosselov IV (2021). Destruction of perfluorooctanesulfonate (PFOS) in a batch supercritical water oxidation reactor. Chemosphere, 279(May), 130834. 10.1016/j.chemosphere.2021.130834 [DOI] [PubMed] [Google Scholar]
- Quiñones O, & Snyder SA (2009). Occurrence of perfluoroalkyl carboxylates and sulfonates in drinking water utilities and related waters from the United States. Environmental Science and Technology, 43, 9089–9095. 10.1021/es9024707 [DOI] [PubMed] [Google Scholar]
- Rahman MF, Peldszus S, & Anderson WB (2014). Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review. Water Research, 50, 318–340. 10.1016/j.watres.2013.10.045 [DOI] [PubMed] [Google Scholar]
- Riley SM, Ahoor DC, Oetjen K, & Cath TY (2018). Closed circuit desalination of O&G produced water: An evaluation of NF/RO performance and integrity. Desalination, 442, 51. 10.1016/j.desal.2018.05.004 [DOI] [Google Scholar]
- Robey NM, Da Silva BF, Annable MD, Townsend TG, & Bowden JA (2020). Concentrating per- and polyfluoroalkyl substances (PFAS) in municipal solid waste landfill leachate using foam separation. Environmental Science and Technology, 54(19), 12550–12559. 10.1021/acs.est.0c01266 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Freire L, Abad-Fernández N, Sierra-Alvarez R, Hoppe-Jones C, Peng H, Giesy JP, Snyder S, & Keswani M (2016). Sonochemical degradation of perfluorinated chemicals in aqueous film-forming foams. Journal of Hazardous Materials, 317, 275–283. 10.1016/j.jhazmat.2016.05.078 [DOI] [PubMed] [Google Scholar]
- Rumbach P, Bartels DM, Sankaran RM, & Go DB (2015). The solvation of electrons by an atmospheric-pressure plasma. Nature Communications, 6, 7248. 10.1038/ncomms8248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu P (2021). A comprehensive review of saline effluent disposal and treatment: Conventional practices, emerging technologies, and future potential. Journal of Water Reuse and Desalination, 11(1), 33–65. 10.2166/wrd.2020.065 [DOI] [Google Scholar]
- Sansotera M, Persico F, Pirola C, Navarrini W, Di Michele A, & Bianchi CL (2014). Decomposition of perfluorooctanoic acid photocatalyzed by titanium dioxide: Chemical modification of the catalyst surface induced by fluoride ions. Applied Catalysis B: Environmental, 148–149, 29–35. 10.1016/j.apcatb.2013.10.038 [DOI] [Google Scholar]
- Schafer A, Fane AG, & Waite TD (2005). Nanofiltration principles and applications. Elsevier. [Google Scholar]
- Schwichtenberg T, Bogdan D, Carignan CC, Reardon P, Rewerts J, Wanzek T, & Field JA (2020). PFAS and dissolved organic carbon enrichment in surface water foams on a northern U.S. freshwater Lake. Environmental Science and Technology, 54(22), 14455–14464. 10.1021/acs.est.0c05697 [DOI] [PubMed] [Google Scholar]
- Shaffer DL, Werber JR, Jaramillo H, Lin S, & Elimelech M (2014). Forward osmosis: Where are we now? Desalination, 356, 271–284. 10.1016/j.desal.2014.10.031 [DOI] [Google Scholar]
- Singh RK, Brown E, Mededovic Thagard S, & Holsen TM (2020). Treatment of PFAS-containing landfill leachate using an enhanced contact plasma reactor. Journal of Hazardous Materials, 408, 124452. 10.1016/j.jhazmat.2020.124452 [DOI] [PubMed] [Google Scholar]
- Singh RK, Multari N, Nau-Hix C, Anderson RH, Richardson SD, Holsen TM, & Mededovic Thagard S (2019). Rapid removal of poly- and perfluorinated compounds from investigation-derived waste (IDW) in a pilot-scale plasma reactor. Environmental Science and Technology, 53, 11375–11382. 10.1021/acs.est.9b02964 [DOI] [PubMed] [Google Scholar]
- Song Z, Tang H, Wang N, & Zhu L (2013). Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system. Journal of Hazardous Materials, 262, 332–338. 10.1016/j.jhazmat.2013.08.059 [DOI] [PubMed] [Google Scholar]
- Sörengård M, Kleja DB, & Ahrens L (2019). Stabilization and solidification remediation of soil contaminated with poly- and perfluoroalkyl substances (PFASs). Journal of Hazardous Materials, 367(January), 639–646. 10.1016/j.jhazmat.2019.01.005 [DOI] [PubMed] [Google Scholar]
- Steinle-Darling E, Litwiller E, & Reinhard M (2010). Effects of sorption on the rejection of trace organic contaminants during nanofiltration. Environmental Science & Technology, 44(7), 2592–2598. 10.1021/es902846m [DOI] [PubMed] [Google Scholar]
- Steinle-Darling E, & Reinhard M (2008). Nanofiltration for trace organic contaminant removal: Structure, solution, and membrane fouling effects on the rejection of perfluorochemicals. Environmental Science and Technology, 42, 5292–5297. 10.1021/es703207s [DOI] [PubMed] [Google Scholar]
- Stendahl K, & Jäfverström S (2004). Recycling of sludge with the aqua Reci process. Water Science and Technology, 49(10), 233–240. 10.2166/wst.2004.0652 [DOI] [PubMed] [Google Scholar]
- Stover RL (2013). Industrial and brackish water treatment with closed circuit reverse osmosis. Desalination and Water Treatment, 51, 1124. 10.1080/19443994.2012.699341 [DOI] [Google Scholar]
- Strathmann H (2010). Electrodialysis, a mature technology with a multitude of new applications. Desalination, 264(3), 268–288. 10.1016/j.desal.2010.04.069 [DOI] [Google Scholar]
- Stratton GR, Dai F, Bellona CL, Holsen TM, Dickenson ERV, & Mededovic Thagard S (2017). Plasma-based water treatment: Efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater. Environmental Science and Technology, 51, 1643. 10.1021/acs.est.6b04215 [DOI] [PubMed] [Google Scholar]
- Sutariya B, & Raval H (2021). Analytical study of optimum operating conditions in semi-batch closed-circuit reverse osmosis (CCRO). Separation and Purification Technology, 264, 118421. 10.1016/j.seppur.2021.118421 [DOI] [Google Scholar]
- Takeuchi N, Saito Y, Obo H, Kosugi A, & Yasuoka K (2012). Recovery of fluoride ions using electrodialysis from the plasmatreated solution of perfluoro compounds. IEEE Transactions on Plasma Science, 40(9), 2185–2190. 10.1109/TPS.2012.2205712 [DOI] [Google Scholar]
- Tang CY, Fu QS, Criddle CS, & Leckie JO (2007). Effect of flux (transmembrane pressure) and membrane properties on fouling and rejection of reverse osmosis and nanofiltration membranes treating perfluorooctane sulfonate containing wastewater. Environmental Science and Technology, 41, 2008. 10.1021/es062052f [DOI] [PubMed] [Google Scholar]
- Tang CY, Fu QS, Robertson AP, Criddle CS, & Leckie JO (2006). Use of reverse osmosis membranes to remove perfluorooctane sulfonate (PFOS) from semiconductor wastewater. Environmental Science and Technology, 40, 7343. 10.1021/es060831q [DOI] [PubMed] [Google Scholar]
- Taylor PH, Yamada T, Striebich RC, Graham JL, & Giraud RJ (2014). Investigation of waste incineration of fluorotelomer-based polymers as a potential source of PFOA in the environment. Chemosphere, 110, 17–22. 10.1016/j.chemosphere.2014.02.037 [DOI] [PubMed] [Google Scholar]
- Tenorio R, Liu J, Xiao X, Maizel A, Higgins CP, Schaefer CE, & Strathmann TJ (2020). Destruction of per-and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam (AFFF) with UV-sulfite photoreductive treatment. Environmental Science and Technology, 54(11), 6957–6967. 10.1021/acs.est.0c00961 [DOI] [PubMed] [Google Scholar]
- Tetra tech EM Inc. (2003). High energy electron injection (e-beam) technology for the ex-situ treatment of MtBE-contaminated groundwater, innovative technology evaluation report.
- Thiel GP, Tow EW, Banchik LD, Chung HW, & Lienhard V,. J. H.(2015). Energy consumption in desalinating produced water from shale oil and gas extraction. Desalination, 366, 94–112. 10.1016/j.desal.2014.12.038 [DOI] [Google Scholar]
- Thompson J, Eaglesham G, Reungoat J, Poussade Y, Bartkow M, Lawrence M, & Mueller JF (2011). Removal of PFOS, PFOA and other perfluoroalkyl acids at water reclamation plants in South East Queensland Australia. Chemosphere, 82, 9. 10.1016/j.chemosphere.2010.10.040 [DOI] [PubMed] [Google Scholar]
- Tian H, Gao J, Li H, Boyd SA, & Gu C (2016). Complete defluorination of perfluorinated compounds by hydrated electrons generated from 3-indole-acetic-acid in organomodified montmorillonite. Scientific Reports, 6(August), 1–9. 10.1038/srep32949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong T, & Elimelech M (2016). The global rise of zero liquid discharge for wastewater management: Drivers, technologies, and future directions. Environmental Science and Technology, 50, 6846. 10.1021/acs.est.6b01000 [DOI] [PubMed] [Google Scholar]
- Tsang CF, Birkholzer J, & Rutqvist J (2008). A comparative review of hydrologic issues involved in geologic storage of CO2 and injection disposal of liquid waste. Environmental Geology, 54 (8), 1723–1737. 10.1007/s00254-007-0949-6 [DOI] [Google Scholar]
- US EPA (2020). Interim guidance on the destruction and disposal of perfluoroalkyl and polyfluoroalkyl substances and materials containing perfluoroalkyl and polyfluoroalkyl substances (EPA-HQ-OLEM-2020–0527-0002). [Google Scholar]
- US Department of Energy. (2011). Technology readiness assessment guide (Vol. 73). Springer; https://www.directives.doe.gov/directives-documents/400-series/0413.3-EGuide-04a/@@images/file [Google Scholar]
- US EPA (2001). Class I underground injection control program: Study of the risks associated with class I underground injection wells. https://www.epa.gov/sites/production/files/2015-07/documents/study_uic-class1_study_risks_class1.pdf
- US EPA. (2016a). Drinking water health advisory for perfluorooctane sulfonate (PFOS). US EPA. [Google Scholar]
- US EPA. (2016b). Drinking water health advisory for perfluorooctanoic acid (PFOA). US EPA. [Google Scholar]
- US EPA. (2020). PFAS Laws and Regulations. US EPA. [Google Scholar]
- US EPA. (2021). Research brief: Potential PFAS destruction technology: Supercritical water oxidation. https://www.epa.gov/chemical-research/research-brief-potential-pfas-destruction-technology-supercritical-water-oxidation
- USEPA. (2016a). Drinking water health advisory for perfluorooctane sulfonate (PFOS). Office of Water Health and Ecological Criteria Division. [Google Scholar]
- USEPA. (2016b). Drinking water health advisory for perfluorooctanoic acid (PFOA). Office of Water Health and Ecological Criteria Division. [Google Scholar]
- Vadillo V, Sanchez-Oneto J, Portela JR, & Martínez De La Ossa EJ (2013). Problems in supercritical water oxidation process and proposed solutions. Industrial and Engineering Chemistry Research, 52(23), 7617–7629. 10.1021/ie400156c [DOI] [Google Scholar]
- Van Der Bruggen B, Vandecasteele C, Van Gestel T, Doyen W, & Leysen R (2003). A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environmental Progress, 22, 46–56. 10.1002/ep.670220116 [DOI] [Google Scholar]
- Vecitis CD, Park H, Cheng J, Mader BT, & Hoffmann MR (2008). Kinetics and mechanism of the sonolytic conversion of the aqueous perfluorinated surfactants, perfluorooctanoate (PFOA), and perfluorooctane sulfonate (PFOS) into inorganic products. Journal of Physical Chemistry A, 112(18), 4261–4270. 10.1021/jp801081y [DOI] [PubMed] [Google Scholar]
- Voisin T, Erriguible A, Ballenghien D, Mateos D, Kunegel A, Cansell F, & Aymonier C (2017). Solubility of inorganic salts in sub- and supercritical hydrothermal environment: Application to SCWO processes. Journal of Supercritical Fluids, 120, 18–31. 10.1016/j.supflu.2016.09.020 [DOI] [Google Scholar]
- Waite TD, Kurucz CN, Cooper WJ, & Brown D (1998). Full scale electron beam systems for treatment of water, wastewater and medical waste. In Radiation Technology for Conservation of the environment. Proceedings of a symposium. Zakopane, Poland: In Symposium on radiation technology for conservation of the environment; International Atomic Energy Agency (IAEA). https://www.osti.gov/etdeweb/servlets/purl/644018 [Google Scholar]
- Wang F, Lu X, Li XY, & Shih K (2015). Effectiveness and mechanisms of defluorination of perfluorinated alkyl substances by calcium compounds during waste thermal treatment. Environmental Science and Technology, 49(9), 5672–5680. 10.1021/es506234b [DOI] [PubMed] [Google Scholar]
- Wang F, Lu X, Shih K, & Liu C (2011). Influence of calcium hydroxide on the fate of perfluorooctanesulfonate under thermal conditions. Journal of Hazardous Materials, 192(3), 1067–1071. 10.1016/j.jhazmat.2011.06.009 [DOI] [PubMed] [Google Scholar]
- Wang F, Shih K, Lu X, & Liu C (2013). Mineralization behavior of fluorine in perfluorooctanesulfonate (PFOS) during thermal treatment of lime-conditioned sludge. Environmental Science and Technology, 47(6), 2621–2627. 10.1021/es305352p [DOI] [PubMed] [Google Scholar]
- Wang J, Wang L, Xu C, Zhi R, Miao R, Liang T, Yue X, Lv Y, & Liu T (2018). Perfluorooctane sulfonate and perfluorobutane sulfonate removal from water by nanofiltration membrane: The roles of solute concentration, ionic strength, and macromolecular organic foulants. Chemical Engineering Journal, 332, 787–797. 10.1016/j.cej.2017.09.061 [DOI] [Google Scholar]
- Wang J, Cao C, Wang Y, Wang Y, Sun B, & Zhu L (2020). In situ preparation of p-n BiOI@Bi5O7I heterojunction for enhanced PFOA photocatalytic degradation under simulated solar light irradiation. Chemical Engineering Journal, 391, 123530. 10.1016/j.cej.2019.123530 [DOI] [Google Scholar]
- Wang T, Zhao C, Li P, Li Y, & Wang J (2015). Fabrication of novel poly(m-phenylene isophthalamide) hollow fiber nanofiltration membrane for effective removal of trace amount perfluorooctane sulfonate from water. Journal of Membrane Science, 477, 74–85. 10.1016/j.memsci.2014.12.038 [DOI] [Google Scholar]
- Wang W, Chen Y, Li G, Gu W, & An T (2020). Photocatalytic reductive defluorination of perfluorooctanoic acid in water under visible light irradiation: The role of electron donor. Environmental Science: Water Research and Technology, 6(6), 1638–1648. 10.1039/d0ew00205d [DOI] [Google Scholar]
- Wang Y, & Zhang P (2011). Photocatalytic decomposition of perfluorooctanoic acid (PFOA) by TiO2 in the presence of oxalic acid. Journal of Hazardous Materials, 192(3), 1869–1875. 10.1016/j.jhazmat.2011.07.026 [DOI] [PubMed] [Google Scholar]
- Warsinger DM, Tow EW, Maswadeh LA, Connors GB, Swaminathan J, & Lienhard VJH (2018). Inorganic fouling mitigation by salinity cycling in batch reverse osmosis. Water Research, 137, 384–394. 10.1016/j.watres.2018.01.060 [DOI] [PubMed] [Google Scholar]
- Warsinger DM, Tow EW, Nayar KG, Maswadeh LA, & Lienhard V,. J. H. (2016). Energy efficiency of batch and semi-batch (CCRO) reverse osmosis desalination. Water Research, 106, 272–282. 10.1016/j.watres.2016.09.029 [DOI] [PubMed] [Google Scholar]
- Xiao F, Sasi PC, Yao B, Kubatova A, Golovko SA, Golovko MY, & Soli D (2020). Thermal stability and decomposition of perfluoroalkyl substances on spent granular activated carbon. Environmental Science and Technology Letters, 7(5), 343–350. 10.1021/acs.estlett.0c00114 [DOI] [Google Scholar]
- Xu B, Ahmed MB, Zhou JL, & Altaee A (2020). Visible and UV photocatalysis of aqueous perfluorooctanoic acid by TiO2 and peroxymonosulfate: Process kinetics and mechanistic insights. Chemosphere, 243, 125366. 10.1016/j.chemosphere.2019.125366 [DOI] [PubMed] [Google Scholar]
- Yan H, Cousins IT, Zhang C, & Zhou Q (2015). Perfluoroalkyl acids in municipal landfill leachates from China: Occurrence, fate during leachate treatment and potential impact on groundwater. Science of the Total Environment, 524, 23. 10.1016/j.scitotenv.2015.03.111 [DOI] [PubMed] [Google Scholar]
- Zeng C, Tanaka S, Suzuki Y, Yukioka S, & Fujii S (2017). Rejection of trace level perfluorohexanoic acid (PFHxA) in pure water by loose nanofiltration membrane. Journal of Water and Environment Technology, 15, 120. 10.2965/jwet.16-072 [DOI] [Google Scholar]
- Zhang Y, Ghyselbrecht K, Meesschaert B, Pinoy L, & Van der Bruggen B (2011). Electrodialysis on RO concentrate to improve water recovery in wastewater reclamation. Journal of Membrane Science, 378(1–2), 101–110. 10.1016/j.memsci.2010.10.036 [DOI] [Google Scholar]
- Zhao C, Tang CY, Li P, Adrian P, & Hu G (2016). Perfluorooctane sulfonate removal by nanofiltration membranethe effect and interaction of magnesium ion/humic acid. Journal of Membrane Science, 503, 31–41. 10.1016/j.memsci.2015.12.049 [DOI] [Google Scholar]
- Zhao D, Lee LY, Ong SL, Chowdhury P, Siah KB, & Ng HY (2019). Electrodialysis reversal for industrial reverse osmosis brine treatment. Separation and Purification Technology, 213, 339–347. 10.1016/j.seppur.2018.12.056 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No original data are presented.


