Abstract
Three new fungal species in the Clavicipitaceae (Hypocreales, Ascomycota) associated with plants were collected in Thailand. Morphological characterisation and phylogenetic analyses based on multi-locus sequences of LSU, RPB1 and TEF1 showed that two species belong to Aciculosporium and Shimizuomyces. Morakotia occupies a unique clade and is proposed as a novel genus in Clavicipitaceae. Shimizuomyces cinereus and Morakotia fusca share the morphological characteristic of having cylindrical to clavate stromata arising from seeds. Aciculosporium siamense produces perithecial plates and occurs on a leaf sheath of an unknown panicoid grass.
Keywords: new taxa, phylogeny, taxonomy
INTRODUCTION
Clavicipitaceae is one of the most heterogeneous fungal families in the order Hypocreales (Ascomycota) that is associated with insects, plants, fungi and invertebrates (Gams & Zare 2003, Spatafora et al. 2007, Sung et al. 2007, Steiner et al. 2011, Kepler et al. 2012b). For instance, Metarhizium species are well-known entomopathogens in the Clavicipitaceae and are associated with both insects and plants. Furthermore, they play roles as endophytes and rhizosphere-inhabiting fungi (Greenfield et al. 2016, Nishi & Sato 2019, Mongkolsamrit et al. 2020). Tyrannicordyceps was proposed as a new genus associated with fungi, producing yellow or bright red stromata attacking the sclerotia of Claviceps (Kepler et al. 2012b). Clavicipitaceous fungi associated with plants have been described for species in Aciculosporium, Atkinsonella, Balansia, Claviceps, Epichloë, Heteroepichloë, Myriogenospora, Periglandula, Shimizuomyces and Ustilaginoidea. Species in Aciculosporium, Atkinsonella, Balansia, Epichloë, Myriogenospora and Parepichloë have been documented as fungal endophytes of grasses, and in their life cycle they can form ascomata (sexual morph) on these host plants (Cheplick & Faeth 2009, Torres & White 2009). Macro-morphology and habitats of several species associated with plants within these genera are epibiotic and produce ascomata on stems, leaves or culms, and inflorescences of plants. Most species of these genera produce pale brown to black coloured stromata such as B. aristidae, C. purpurea, M. atramentosa, P. cinerea, except for Epichloë typhina which produces white to yellow stromata (Bischoff & White 2003, Górzyńska et al. 2017).
The genus Aciculosporium was established by Miyake (1908) with A. take as type species. Almost a century later, a second species, A. sasicola, was reported from Japan (Oguchi 2001). Aciculosporium take and A. sasicola were documented as causative agents of the economically important witches’ broom disease of bamboo in Japan, China, and Taiwan (Tsuda et al. 1997, Oguchi 2001, Tanaka et al. 2003). Recently, Píchová et al. (2018) combined Cepsiclava phalaridis and Neoclaviceps monostipa in Aciculosporium. To date, this genus comprises only four fungal species viz. A. take, A. monostipum, A. phalaridis, and A. sasicola. Aciculosporium phalaridis (= Cepsiclava phalaridis) produces stromata on sclerotia of commercial phalaris (Phalaris aquatica) seeds in southern New South Wales and Victoria (Walker 2004). Neoclaviceps monostipum was discovered in Costa Rica from unknown panicoid grasses (Sullivan et al. 2001).
Kobayasi (1981) established the genus Shimizuomyces from Japanese collections, comprising two species, namely S. paradoxus (type species) growing on Smilax sieboldii fruits and S. kibianus growing on Smilax china seeds (Kobayasi 1984). Besides Japan, S. paradoxus was also reported from Korea by Sung et al. (2010). Based on the morphological characters in the natural specimens, Shimizuomyces resembles Cordyceps in possessing brightly coloured, fleshy stromata with cylindrical stipes and enlarged apical heads (Sung et al. 2007).
During field surveys for arthropod-pathogenic fungi in central and western regions of Thailand, we collected two unidentified species producing brown cylindrical to clavate stromata and another species with grey stromata occurring on seeds. Based on the macro- and micro-morphological characteristics of all collected strains, they were preliminarily identified as members of Shimizuomyces. Additionally, we also found one species that morphologically resembles Aciculosporium by producing brown ascomata on the leaf sheath of an unknown panicoid grass. The aims of this study were to clarify the placement and name these collections through molecular phylogenetic studies combined with observations of diagnostic micro-morphological characters.
MATERIALS AND METHODS
Collection and isolation
Fungal specimens occurring on seeds of dicot plants and the leaf sheath of an unknown panicoid grass were collected from Ban Phaothai community forest and Khao Yai National Park, Thailand. The specimens were collected carefully so as not to damage either host or stipe, and were placed in small plastic boxes before returning to the laboratory for isolation. The protocol for the isolation from stromata containing mature perithecia followed previous studies (Luangsa-ard et al. 2018, Mongkolsamrit et al. 2018). Ascospores were discharged on potato dextrose agar (PDA; freshly diced potato 200 g, dextrose 20 g, agar 15 g, in 1 L distilled water) and placed in a plastic box with moist tissue paper overnight to create a humid chamber with 99 % humidity at 25 °C. The following morning, plates were examined with an Olympus SZ61 dissecting microscope to observe discharged ascospores that were then transferred to fresh PDA plates. Pure cultures were deposited at the BIOTEC Culture Collection (BCC), National Center for Genetic Engineering and Biotechnology, Thailand. Specimens were dried in an electric food dryer (50–55 °C) overnight and stored in plastic boxes before storage at the BIOTEC Bangkok Fungarium (BBH), National Biobank of Thailand.
Morphological study
Fungal structures, such as perithecia, asci and ascospores were mounted in lactophenol cotton blue solution and measured using a compound microscope. Twenty to fifty perithecia, asci, ascospores, phialides and conidia were measured and the range and standard deviation calculated. Morphological characters of these structures were photographed using an Olympus DP70 Digital Camera mounted on an Olympus BX51 compound microscope and SZX12 (Olympus) dissecting microscope. Colour changes of stromata were monitored in 3 % potassium hydroxide (KOH). For detailed morphological comparisons of conidia, phialides and colony colours, cultures were grown on PDA agar plates and 2 % malt extract agar (2 % MEA Difco; malt extract, 20 g; agar, 15 g in 1 L distilled water) at 25 °C under a zeitgeber 14:10 light : dark cycle for 21 to 30 d, depending on fungal sporulation. Protocols for culture observations and comparisons followed Mongkolsamrit et al. (2018). Colours of fresh specimens and cultures incubated on PDA and MEA were described following the Sixth Royal Horticultural Society (R.H.S.) Colour Chart.
DNA extraction, sequencing and phylogenetic analysis
Genomic DNA was harvested from mycelia on PDA plates and small pieces of fresh stromatal tissue using a modified cetyltrimethyl-ammonium bromide (CTAB) method as described previously (Mongkolsamrit et al. 2009). Nuclear loci, including nuc 28 rDNA (Large Subunit Ribosomal DNA: LSU), the partial gene regions of the RNA polymerase II largest subunit (RPB1) and the translation elongation factor-1α gene (TEF1), were sequenced. The primer pairs and thermocycler conditions for PCR amplifications used in this study followed the method described in Mongkolsamrit et al. (2019). The purified PCR products were sequenced with PCR amplification primers for Sanger dideoxy sequencing. The PCR amplicon sequences were examined for ambiguous base calls using BioEdit v. 7.2.5 (Hall 2004). Verified sequences were submitted to GenBank. Multi-locus sequences of closely-related taxa for analyses were taken from previous studies as shown in Table 1. The final alignment was deposited in TreeBASE (www.treebase.org) under accession number ID 26949. Phylogenetic analysis was performed using RAxML-VI-HPC2 v. 8.2.12 (Stamatakis 2014) on XSEDE (http://www.phylo.org/), with 1 000 bootstrap iterations. Bayesian inference (BI) analysis was performed in MrBayes v. 3.2.7a (Ronquist et al. 2012) on XSEDE, with the GTR + I + G model (General Time Reversible model with a proportion of invariable sites and a gamma-shaped distribution of rates across sites). Markov chain Monte Carlo (MCMC) simulations were run for 5 000 000 generations, sampling every 1 000, and discarding the first 10 % as burn-in. RAxML output was imported into TreeView v. 1.6.6 to view the phylogenetic tree (Page 1996; http://taxonomy.zoology.gla.ac.uk/rod/treeview.html).
RESULTS
Molecular phylogeny
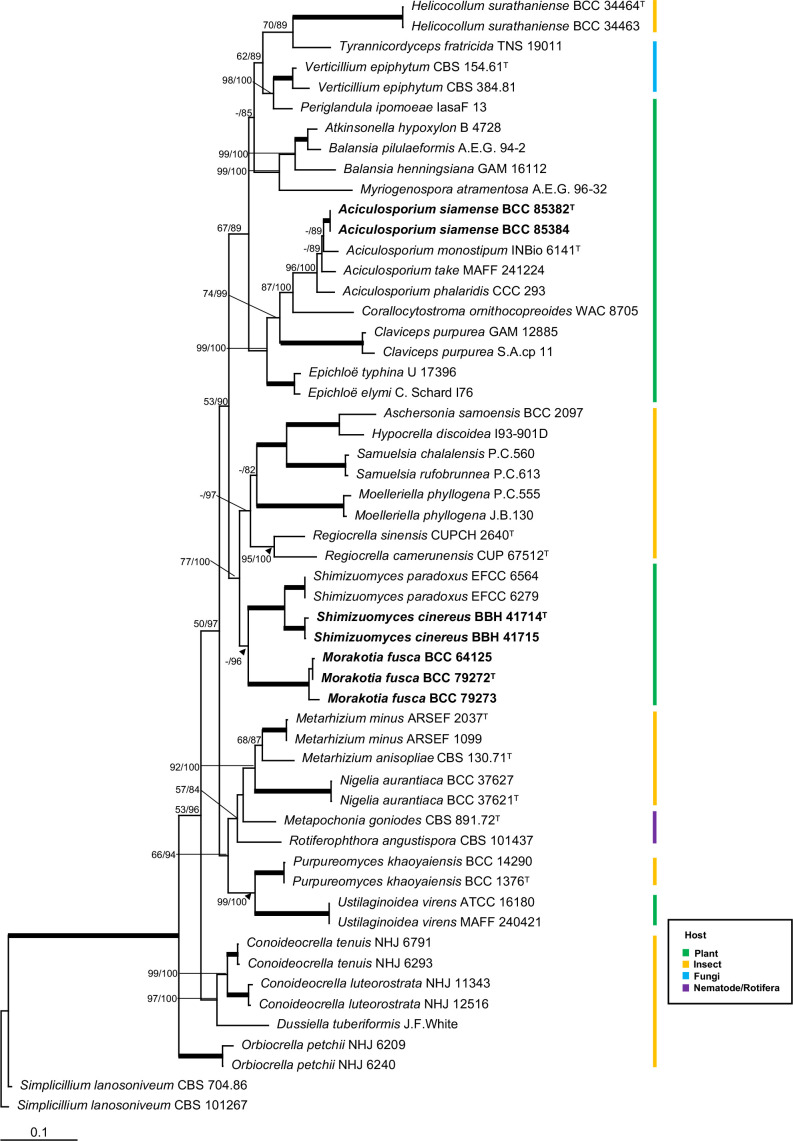
We generated seven LSU, five RPB1 and six TEF1 sequences in this study from living cultures and fresh stromata (Table 1). The combined dataset of 55 taxa with multi-locus sequences had a total alignment length of 2 286 characters. Sequences of Simplicillium lanosoniveum CBS 704.86 and Simplicillium lanosoniveum CBS 101267 in the Cordycipitaceae were used as outgroups. The RAxML analysis resulted in a single tree which is shown in Fig. 1. The phylogenetic tree strongly supports Aciculosporium, Morakotia and Shimizuomyces as monophyletic clades. The descriptions based on morphological characters of two new species belong to Aciculosporium and Shimizuomyces, and a new genus Morakotia are provided below.
Fig. 1.
RAxML tree of Aciculosporium siamense, Morakotia fusca and Shimizuomyces cinereus with other genera in the Clavicipitaceae from a combined LSU, RPB1 and TEF1 dataset. Numbers at the major nodes represent maximum likelihood bootstrap values (MLBP) and Bayesian posterior probabilities (BPP) multiplied by 100. Fully-supported (MLBP/BPP = 100/100) branches are thickened.
Taxonomy
Aciculosporium siamense Mongkolsamrit, Noisripoom & Luangsa-ard, sp. nov. MycoBank MB 838347. Fig. 2A–S.
Fig. 2.

Aciculosporium siamense (BBH 43077, BCC 85382). A–C. Ascomata on leaf sheaths of grass. D. Perithecia. E. Asci. F. Ascus tip. G. Whole ascospores. H. Whole ascospore with septations. I, J. Colonies on PDA. K–M. Holoblastic conidia on PDA. N, O. Colonies on 2 % MEA. P–S. Holoblastic conidia on 2 % MEA. Scale bars: I, N = 20 mm; A, J = 5 mm; B, C, O = 1 mm; D = 100 μm; E, G = 20 μm; F, H, P–S = 10 μm; K–M = 5 μm.
Etymology: The specific epithet refers to the old name of the Kingdom of Thailand, Siam.
Typus: Thailand, Saraburi Province, Chet Kot Waterfall, Khao Yai National Park, on leaf sheath (Poaceae), 8 Jan. 2017, U. Pinruan (UP), S. Mongkolsamrit (SM) & P. Srikitikulchai (PS), SM 2081 (holotype BBH 43077, ex-type culture BCC 85382).
Ascomata hemispherical perithecial plates, singly or composed of multiple perithecial plates, pale brownish orange (N167A), 2–8 mm in diam, 2–3 mm high. Perithecia immersed, obovate, 420–550(–600) × (160–)180–220(–230) μm, with dark brown ostioles. Asci cylindrical, (165–)203–347(–400) × 4–(4.5–5) μm with caps 4–5 μm thick. Ascospores hyaline, filiform with one end blunt and narrow at the other end, 3-septate, (60–)71.5–95.5(–125) × (1–) 1.5–2 μm. Colour change of stromata in 3 % KOH not observed.
Culture characteristics: Colonies on PDA attaining 8–10 mm diam in 14 d, yellowish white (N158C), reverse uncoloured. Dimorphic with mass of conidia, yeast-like and vegetative hyphae smooth, wet, 3–4 μm diam. Conidia holoblastic, hyaline, cylindrical to filiform, narrower at one end than the other, 1–3-septate, (22–)33–57.5(–70) × 1.5–2 μm, with dichotomously branched appendages, usually appearing at the narrow end of conidia, 1–5 × 0.5 μm.
Colonies on 2 % MEA attaining 4–5 mm diam in 14 d, light yellow (163D), reverse uncoloured. Colonies dimorphic, producing a cerebriform yeast-like mass of conidia at the centre and smooth vegetative hyphae on the edges of the colony, wet, 3–4 μm diam. Conidia holoblastic, hyaline, cylindrical to filiform, narrower at one end than the other, 1–2-septate, (24–)30–50(–60) × 1.5–2 μm, with dichotomously branched appendages, usually appearing at the narrow end of conidia, 1–6 × 0.5 μm.
Distribution: Found in the central and western regions of Thailand.
Additional materials examined: Thailand, Chet Kot Waterfall, Khao Yai National Park, on leaf sheath (Poaceae), 8 Jan. 2017, UP, SM & PS, SM 2080 (BBH 43076 paratype), ex-paratype culture BCC 85381, SM 2082 (BBH 43078), culture BCC 85383, SM 2083 (BBH 43079), culture BCC 85384; Pi Tu Kro Waterfall, Umphang Wildlife Sanctuary, on leaf sheath (Poaceae), 26 Jun. 2008, SM, K. Tasanathai (KT), B. Thongnuch (BT), PS, AK & J.J. Luangsa-ard (JJL), SM 517 (BBH 24722), culture BCC 32351.
Notes: Aciculosporium siamense is a rare species in Thailand, found only in Chet Kot Waterfall and Pi Tu Kro Waterfall. This species produces single to multiple hemispherical perithecial plates similar to the sexual morph of Aschersonia luteola and A. badia by producing crowded perithecia immersed in stromata (Mongkolsamrit et al. 2009). However, Aciculosporium siamense differs from Aschersonia luteola and A. badia on the basis of their hosts. Aciculosporium siamense occurs on leaf sheaths (Poaceae), while Aschersonia luteola and A. badia occur on scale insects (Hemiptera) and are found on the underside of leaves.
Morakotia Mongkolsamrit, Noisripoom, Khonsanit, Thanakitpipattana & Luangsa-ard, gen. nov. MycoBank MB 838348.
Etymology: In honour of Prof. Dr Morakot Tanticharoen, for her support of invertebrate-pathogenic fungi research in BIOTEC, Thailand.
Stromata solitary or multiple, unbranched, tough, arising from seed plant, cylindrical to clavate, moderate orange yellow to brown orange (164A–164B). Fertile part clavate. Perithecia crowded, densely packed, ovoid to long ovoid, ordinal in arrangement, completely immersed, with a reddish brown ostioles. Asci cylindrical. Ascospores hyaline, whole, filiform, elongate clavate with septations.
Type species: Morakotia fusca Mongkolsamrit, Noisripoom, Khonsanit, Thanakitpipattana & Luangsa-ard
Morakotia fusca Mongkolsamrit, Noisripoom, Khonsanit, Thanakitpipattana & Luangsa-ard, sp. nov. MycoBank MB 838349. Fig. 3A–P.
Fig. 3.

Morakotia fusca (BBH 41710, BCC 79272). A. Stroma on seed. B. Fertile part of stroma. C. Immersed perithecia. D–F. Asci with asci caps. G, H. Whole ascospores with septations (arrows). I, J. Colony with synnema on PDA. K. Conidiophores consisting of verticillate phialides on PDA. L. Conidia on PDA. M. Colony on 2 % MEA. N, O. Conidiophores consisting of verticillate phialides on 2 % MEA. P. Conidia on 2 % MEA. Scale bars: I, M = 10 mm; A = 5 mm; B, J = 1 mm; C = 200 μm; D, E = 50 μm; G, K, O = 20 μm; F, H, L, N, P = 10 μm.
Etymology: The specific epithet is from the Latin “fuscus”, referring to brown colour of fresh stromata.
Typus: Thailand, Phitsanulok Province, Ban Phaothai community forest, on seed (Smilacaceae), in leaf litter, 10 Oct. 2015, A. Khonsanit (AK), D. Thanakitpipattana (DT), S. Lamlertthon (SL), SM & W. Noisripoom (WN), MY 10972 (holotype BBH 41710, ex-type culture BCC 79272).
Stromata solitary or multiple, unbranched, tough, 20–75 mm long, 0.5–2 mm broad, cylindrical to enlarging apically, arising from the seed buried approximately 5–10 mm underground. Fertile part moderate orange yellow to brown orange (164A–164B), cylindrical to clavate, 5–20 mm long, 1.5–2.5 mm broad. Perithecia completely immersed, ordinal in arrangement, narrow flask-shaped, (320–)380–510(–570) × (120–)130–165(–180) μm, ostioles darker reddish orange (175B). Asci cylindrical, 8-spored, (105–)160–240(–245) × (7–)8–9(–10) μm with caps 4–5 μm thick. Ascospores hyaline, whole, filiform, 7–8-septate, (70–)80–95(–105) × 2–4 μm. Colour change of stromata in 3 % KOH not observed.
Culture characteristics: Colonies on PDA attaining 30–35 mm diam in 30 d, cottony with high mycelial density, pale yellow (11C–D) to strong orange yellow (163B), reverse pale brown. Synnemata deep orange yellow (163A), 5–20 × 2–2.5 mm. Conidiogenous structures consisting of erect conidiophores arising from the vegetative hyphae. Conidiophores consist of verticillate phialides, singly or in whorls of two. Phialides awl-shaped, (20–)22–35(–40) × 1.5–2.5 μm. Conidia hyaline, globose, not in chains, (4–)4.5–5.5(–6) μm diam. Chlamydospores not observed.
Colonies on 2 % MEA attaining 20–25 mm diam in 30 d, cottony, white, scarce mycelial density, reverse brownish orange (N167B). Conidiogenous structures consisting of erect conidiophores arising from the vegetative hyphae or monophialidic arising along the hyphae. Conidiophores consist of verticillate phialides, singly or in whorls of two. Phialides awl-shaped, (20–)25–35(–40) × (1–)1.5–2(–2.5) μm. Conidia hyaline, globose, singly not in chains, (4–)4.5–6 μm diam. Chlamydospores not observed.
Distribution: Found in the central and northeastern regions of Thailand.
Additional materials examined: Thailand, Phitsanulok Province, Ban Phaothai community forest, on seed (Smilacaceae), in the leaf litter, 10 Oct. 2015, AK, DT, SL, SM & W. Noisripoom (WN); MY 10973 (BBH 41711 paratype), ex-paratype culture BCC 79273, MY 10974 (BBH 41712), culture BCC 79274; idem., 4 Sep. 2016, SM, WN, R. Somnuk (RS), PS, KT, DT, S. Wongkanoun (SW), MY 11425 (BBH 41790), culture BCC 82798; Nakhon Ratchasima, Khao Yai National Park, on seed (Palmae), in the leaf litter, 24 Jun. 2012, AK, SM, WN, RS, PS & KT, MY 8554 (BBH 37585), culture BCC 64124, MY 8555 (BBH 37740) culture BCC 64125, idem., 26 Jun. 2012, AK, SM, WN, RS, PS & KT, MY 8624 (BBH 37745), culture BCC 64172.
Notes: Based on the natural specimen, Morakotia fusca is similar to Tolypocladium ophioglossoides in the colour and shape of its stromata. Both Morakotia fusca and T. ophioglossoides have brown orange and cylindrical stromata. However, Morakotia fusca differs from T. ophioglossoides on the basis of their hosts. Morakotia fusca occurs on seeds, whereas T. ophioglossoides occurs on truffles.
Shimizuomyces cinereus Mongkolsamrit, Noisripoom, Khonsanit, Thanakitpipattana & Luangsa-ard, sp. nov. MycoBank MB 838350. Fig. 4A–H.
Fig. 4.
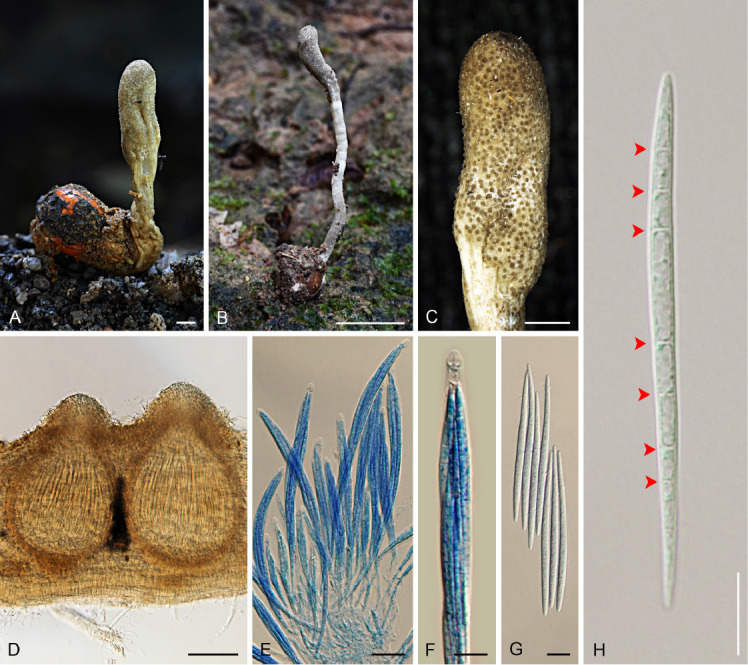
Shimizuomyces cinereus (BBH 41714). A, B. Stroma on seed. C. Fertile part of stroma. D. Perithecia. E, F. Asci and asci caps. G, H. Whole ascospores with septations (arrows). Scale bars: B = 5 mm; A, C = 1 mm; D = 100 μm; E = 20 μm; F–H = 10 μm.
Etymology: The specific epithet is from the Latin “cinereus”, referring to the grey colour of the stroma.
Typus: Thailand, Phitsanulok Province, Ban Phaothai community forest, on seed (Smilacaceae), in the leaf litter, 10 Oct. 2015, AK, DT, SL, SM & WN, MY 10976 (holotype BBH 41714).
Stroma solitary, unbranched, 10–28 mm in long and 0.5–2 mm broad, cylindrical to enlarging apically, arising from the seed buried approximately 5–10 mm in the leaf litter. Fertile part yellowish grey (A–B), 3–8 × 1.5–3 mm, cylindrical to clavate. Perithecia immersed, with ostioles slightly projecting, ordinal in arrangement, pyriform, (310–)320–370(–380) × (150–)190–250 μm, ostioles greyish yellow green (197D), ca. 100 μm diam. Asci cylindrical, 8-spored, (125–)160–235(–250) × (8–)7–9(–10) μm, with caps 4–5 μm thick. Ascospores hyaline, whole, filiform, 7–8 septate, (65–)70–85 × (3–) 3.5–4 μm. Colour change of stromata in 3 % KOH not observed.
Distribution: Found in the central region of Thailand.
Additional materials examined: Thailand, Phitsanulok Province, Ban Phaothai community forest, on seed (Smilacaceae), in the leaf litter, 10 Oct. 2015, AK, DT, SL, SM, WN, MY 10979 (paratype BBH 41715), MY 10959 (BBH 41709), MY 10975 (BBH 41713).
Notes: Shimizuomyces cinereus is only recorded from Ban Phaothai community forest in Phitsanulok Province. This species is easy to find in the leaf litter or on the ground due to the abundance of natural specimens located around the area producing bright grey stromata. Shimizuomyces cinereus and Morakotia fusca occur on seeds (Smilacaceae) and these two species can be found at the same site in Ban Phaothai community forest. Shimizuomyces cinereus differs from Morakotia fusca in having pale grey stromata meanwhile M. fusca has an orange and tough stroma.
DISCUSSION
Phylogenetic analyses combined with morphology classified Thai specimens associated with plants as new species in Aciculosporium and Shimizuomyces, and a novel genus, Morakotia. This study has contributed to our knowledge on the taxonomy, morphology and geographical distribution of fungi in Clavicipitaceae (Hypocreales).
Aciculosporium siamense from Thailand can be easily recognised by its host – an unknown panicoid grass found in the rainforest that was also reported for A. monostipum from Costa Rica (South America) and A. phalaridis from Phalaris aquatica in Australia and New Zealand. Aciculosporium take and A. sasicola can be found on several genera of bambusoid grasses in Japan (Tsuda et al. 1997). We compared the morphological characters of A. siamense with known species in Aciculosporium and found that A. siamense is morphologically similar to A. take and A. sasicola in the formation of astipitate ascomatal stromata (Tsuda et al. 1997). Aciculosporium monostipum produces stipitate ascomatal stromata arising directly from parasitised plant ovaries (Sullivan et al. 2001), whereas A. phalaridis produces a discrete sclerotium with stalked ascostromata (Walker 2004) on seeds. Although the asexual morph of Aciculosporium siamense was not seen in the natural habitat, cream-coloured, yeast-like masses of conidia were produced on PDA and 2 % MEA. From our microscopic observation of the conidia on cultures, we found that Aciculosporium siamense produces holoblastic appendaged conidia on both media. Our results reveal that species in Aciculosporium share this unique character (apomorphies) in having a holoblastic appendaged conidia, which was also reported from A. monostipum, A. phalaridis, A. take and A. sasicola (Oguchi 2001, Sullivan et al. 2001, Walker 2004, Píchová et al. 2018).
Multi-gene phylogenetic analyses presented in Fig. 1 fully support (MLBP/BPP = 100/100) Morakotia as a distinct clade from Shimizuomyces. So far, only one species, Morakotia fusca, has been proposed in Morakotia. Considering the morphology of natural specimens and ecology, these two genera share similarity of having cylindrical stroma arising directly from seeds, the fertile parts are cylindrical to clavate, and can be found on the ground. Microscopic observation between species in the two genera showed the synapomorphic character of producing filiform ascospores with distinct septations. However, Morakotia fusca differs from Shimizuomyces spp. (Table 2) in that the perithecia in M. fusca are completely immersed and narrow flask-shaped, whereas the perithecia in all species of Shimizuomyces are immersed with slightly projecting ostioles and are pyriform in shape. The Shimizuomyces clade itself is fully supported (MLBP/BPP = 100/100) with the Thai specimens as a new member in Shimizuomyces. Shimizuomyces cinereus differs from S. paradoxus in having grey stroma, whereas stroma in S. paradoxus is bright yellow. Shimizuomyces kibianus was not included in the phylogenetic analysis because no sequence data was available for this taxon. According to the description and illustration given by Kobayasi (1984) and Shimizu (1994), S. kibianus also has grey stroma similar to S. cinereus. However, S. cinereus possesses a larger fertile part and perithecia, and longer asci than reported for S. kibianus. Shimizuomyces is thus distributed in Japan, Korea and Thailand.
Table 2.
Morphological comparisons of Morakotia fusca and species in Shimizuomyces.
| Species | Stroma (mm) | Fertile part (mm) | Perithecia (μm) | Asci (μm) | Ascospores (μm) | References |
|---|---|---|---|---|---|---|
| Morakotia fusca | 20–75 × 0.5–2 | 5–20 × 1.5–2.5 | narrow flask-shaped, 320–570 × 120–180 | 105–245 × 7–10 | 70–105 × 2–4, with septation | This study |
| Shimizuomyces cinereus | 10–28 × 0.5–2 | 3–8 × 1.5–3 | pyriform, 310–380 × 150–250 | 125–250 × 8–10 | 65–85 × 3–4, with septation | This study |
| S. kibianus | 15–18 mm long | 2.5 × 2 | pyriform, 300–320 × 230–240 | 140–160 × 6 | 30–80 × 1.5, with septation | Kobayasi (1984), Shimizu (1994) |
| S. paradoxus | 10–30 × 0.5–1.2 | 5–15 × 1–2 | pyriform, 350–400 × 200–250 | 100–130 × 6–7 | 60–75 × 2–2.5, with septation | Kobayasi (1981) |
Table 1.
List of species and GenBank accession numbers of sequences used in this study. The novelties described here are in bold font.
| Species | Strain | Host/Substratum |
GenBank Accession no.
|
References | ||
|---|---|---|---|---|---|---|
| LSU | RPB1 | TEF1 | ||||
| Aciculosporium monostipum | INBio 6 141T | Poaceae | AF245293 | DQ000353 | AY986983 | Sullivan et al. (2001), Chaverri et al. (2005b) |
| Aciculosporium phalaridis | CCC 293 | Poaceae | – | – | LT216524 | Píchová et al. (2018) |
| Aciculosporium siamense | BCC 85382 T | Poaceae | MT743002 | – | MT762147 | This study |
| BCC 85384 | Poaceae | MT743003 | MT762149 | MT762148 | This study | |
| Aciculosporium take | MAFF 241224 | Plants | – | KC113319 | KP689550 | Schardl et al. (2013) |
| Aschersonia samoensis | BCC 2097 | Hemiptera | AF327381 | DQ000346 | AY986945 | Artjariyasripong et al. (2001), Chaverri et al. (2005b) |
| Atkinsonella hypoxylon | B4728 | Plants | – | – | KP689546 | Young et al. (2015) |
| Balansia henningsiana | GAM 16112 | Poaceae | AY545727 | AY489643 | AY489610 | Castlebury et al. (2004) |
| Balansia pilulaeformis | A.E.G. 94-2 | Poaceae | AF543788 | DQ522365 | DQ522319 | Currie et al. (2003), Spatafora et al. (2007) |
| Claviceps purpurea | GAM 12885 | Poaceae | AF543789 | AY489648 | AF543778 | Currie et al. (2003), Castlebury et al. (2004) |
| S.A. cp11 | Poaceae | EF469075 | EF469087 | EF469058 | Sung et al. (2007) | |
| Conoideocrella luteorostrata | NHJ 12516 | Hemiptera | EF468849 | EF468905 | EF468800 | Sung et al. (2007) |
| NHJ 11343 | Hemiptera | EF468850 | EF468906 | EF468801 | Sung et al. (2007) | |
| Conoideocrella tenuis | NHJ 6293 | Hemiptera | EU369044 | EU369068 | EU369029 | Johnson et al. (2009) |
| NHJ 6791 | Hemiptera | EU369046 | EU369069 | EU369028 | Johnson et al. (2009) | |
| Corallocytostroma ornithocopreoides | WAC 8705 | Plants | – | – | LT216546 | Píchová et al. (2018) |
| Dussiella tuberiformis | J.F. White | Hemiptera | – | JQ257015 | JQ257027 | Kepler et al. (2012b) |
| Epichloë elymi | C. Schardl760 | – | AY986924 | DQ000352 | AY986951 | Chaverri et al. (2005b) |
| Epichloë typhina | ATCC 56429 | Poaceae | U17396 | AY489653 | AF543777 | Rehner & Samuels (1995), Currie et al. (2003), Castlebury et al. (2004) |
| Helicocollum surathaniense | BCC 34463 | Hemiptera | KT222328 | – | KT222336 | Luangsa-ard et al. (2017a) |
| BCC 34464T | Hemiptera | KT222329 | – | KT222337 | Luangsa-ard et al. (2017a) | |
| Hypocrella discoidea | I93-901D | Hemiptera | EU392567 | EU392700 | EU392646 | Chaverri et al. (2008) |
| Metapochonia goniodes | CBS 891.72T | Nematoda | AF339550 | DQ522401 | DQ522354 | Sung et al. (2001), Spatafora et al. (2007) |
| Metarhizium anisopliae | CBS 130.71T | Avena sativa | MT078853 | MT07886 | MT078845 | Mongkolsamrit et al. (2020) |
| Metarhizium minus | ARSEF 1099 | Hemiptera | – | KJ398608 | KJ398799 | Kepler et al. (2014) |
| ARSEF 2037T | Hemiptera | AF339531 | DQ522400 | DQ522353 | Spatafora et al. (2007) | |
| Morakotia fusca | BCC 64125 | Plant | KY794862 | – | KY794857 | This study |
| BCC 79272 T | Plant | KY794861 | KY794865 | KY794856 | This study | |
| BCC 79273 | Plant | KY794860 | KY794866 | – | This study | |
| Moelleriella phyllogena | P.C.555 | Hemiptera | EU392610 | EU392726 | EU392674 | Chaverri et al. (2008) |
| J.B.130 | Hemiptera | EU392610 | EU392726 | EU392674 | Chaverri et al. (2008) | |
| Myriogenospora atramentosa | A.E.G.96-32 | Poaceae | AY489733 | AY489665 | AY489628 | Castlebury et al. (2004) |
| Nigelia aurantiaca | BCC 37621 | Lepidoptera | GU979946 | GU979964 | GU979955 | Luangsa-ard et al. (2017b) |
| BCC 37627 | Lepidoptera | GU979947 | GU979965 | GU979956 | Luangsa-ard et al. (2017b) | |
| Orbiocrella petchii | NHJ 6240 | Hemiptera | EU369038 | EU369060 | EU369022 | Johnson et al. (2009) |
| NHJ 6209 | Hemiptera | EU369039 | EU369061 | EU369023 | Johnson et al. (2009) | |
| Periglandula ipomoeae | IasaF 13 | Plant | – | JN587270 | – | Schardl et al. (2013) |
| Purpureomyces khaoyaiensis | BCC 1376T | Lepidoptera | KX983462 | – | KX983457 | Luangsa-ard et al. (2017b) |
| BCC 14290 | Lepidoptera | JF415970 | JN049888 | JF416012 | Kepler et al. (2012a) | |
| Regiocrella camerunensis | CUP 67512T | Hemiptera | DQ118735 | DQ127234 | DQ118743 | Chaverri et al. (2005a) |
| Regiocrella sinensis | CUP CH-2640T | Hemiptera | DQ118736 | DQ127235 | DQ118744 | Chaverri et al. (2005a) |
| Rotiferophthora angustispora | CBS 101437 | Rotifera | AF339535 | DQ522402 | AF543776 | Chaverri et al. (2005a), Currie et al. (2003), Spatafora et al. (2007) |
| Samuelsia chalalensis | P.C. 560 | Hemiptera | EU392637 | EU392743 | EU392691 | Chaverri et al. (2008) |
| Samuelsia rufobrunnea | P.C. 613 | Hemiptera | AY986918 | DQ000345 | AY986944 | Chaverri et al. (2005b) |
| Shimizuomyces cinereus | BBH 41714 T | Smilacaceae (Plant) | KY794864 | KY794867 | KY794859 | This study |
| BBH 41715 | Smilacaceae (Plant) | KY794863 | KY794868 | KY794858 | This study | |
| Shimizuomyces paradoxus | EFCC 6279 | Smilacaceae (Plant) | EF469084 | EF469100 | EF469071 | Sung et al. (2007) |
| EFCC 6564 | Smilacaceae (Plant) | EF469083 | EF469101 | EF469072 | Sung et al. (2007) | |
| Simplicillium lanosoniveum | CBS 704.86 | Hemileia vastatrix (Uredinales) | AF339553 | DQ522406 | DQ522358 | Sung et al. (2001), Spatafora et al. (2007) |
| CBS 101267 | Hemileia vastatrix (Uredinales) | – | DQ522405 | DQ522357 | Spatafora et al. (2007) | |
| Tyrannicordyceps fratricida | TNS 19011 | Fungi | JQ257023 | JQ257016 | JQ257028 | Kepler et al. (2012b) |
| Ustilaginoidea virens | ATCC 16180 | Plant | – | JQ257014 | JQ257026 | Kepler et al. (2012b) |
| MAFF 240421 | Plant | JQ257011 | – | JQ257024 | Kepler et al. (2012b) | |
| Verticillium epiphytum | CBS 154.61T | Hemileia vastatrix (Uredinales) | AF339548 | – | EF468802 | Sung et al. (2001), Sung et al. (2007) |
| CBS 384.81 | Hemileia vastatrix (Uredinales) | AF339547 | DQ522409 | DQ522361 | Sung et al. (2001), Spatafora et al. (2007) | |
T = Type species.
ACKNOWLEDGEMENTS
This study was supported by the Platform Technology Management Section, National Center for Genetic Engineering and Biotechnology (BIOTEC), Grant No. P19-50231 and the Cluster and Program Management Office, NSTDA Grant No. P15-51452. We thank Mr. Witthaya Sangsawang and Ms. Wimonrat Guntang for assistance in collecting specimens. We are indebted to the Department of National Parks, Wildlife and Plant Conservation for their cooperation and support of our research project. We would like to thank Dr Philip James Shaw for thoughtful editing of the manuscript.
Footnotes
Citation: Mongkolsamrit S, Noisripoom W, Thanakitpipattana D, Khonsanit A, Lamlertthon S, Luangsa-ard JJ (2021). New species in Aciculosporium, Shimizuomyces and a new genus Morakotia associated with plants in Clavicipitaceae from Thailand. Fungal Systematics and Evolution 8: 27–37. doi: 10.3114/fuse.2021.08.03
Corresponding editor: P.W. Crous
Conflict of interest: The authors declare that there is no conflict of interest.
REFERENCES
- Artjariyasripong S, Mitchell JI, Hywel-Jones NL, et al. (2001). Relationship of the genus Cordyceps and related genera, based on parsimony and spectral analysis of partial 18S and 28S ribosomal gene sequences. Mycoscience 42: 503–517. [Google Scholar]
- Bischoff JF, White JF. (2003). The plant-infecting clavicipitaleans. In: Clavicipitalean fungi, evolutionary biology, chemistry, biocontrol and cultural impacts (White JF, Jr, Bacon CW, Hywel-Jones NL, et al., eds). Marcel Decker, Inc., New York: 125–149. [Google Scholar]
- Castlebury LA, Rossman AY, Sung GH, et al. (2004). Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycological Research 108: 864–872. [DOI] [PubMed] [Google Scholar]
- Chaverri P, Bischoff JF, Evans HC. et al. (2005a). Regiocrella, a new entomopathogenic genus with a pycnidial anamorph and its phylogenetic placement in the Clavicipitaceae. Mycologia 97: 1225–1237. [DOI] [PubMed] [Google Scholar]
- Chaverri P, Bischoff JF, Liu M. et al. (2005b). A new species of Hypocrella, H. macrostroma, and its phylogenetic relationships to other species with large stromata. Mycological Research 109: 1268–1275. [DOI] [PubMed] [Google Scholar]
- Chaverri P, Liu M, Hodge KT. (2008). A monograph of the entomopathogenic genera Hypocrella, Moelleriella, and Samuelsia gen. nov. (Ascomycota, Hypocreales, Clavicipitaceae), and their aschersonia-like anamorphs in the Neotropics. Studies in Mycology 60: 1–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheplick GP, Faeth S. (2009). Ecology and evolution of the grass endophyte symbiosis. Oxford University Press, New York. [Google Scholar]
- Currie CR, Wong B, Stuart AE. et al. (2003). Ancient tripartite coevolution in the attine ant-microbe symbiosis. Science 299: 386–388. [DOI] [PubMed] [Google Scholar]
- Gams W, Zare R. (2003). A taxonomic review of the clavicipitaceous anamorphs parasitizing nematodes and other microinvertebrates. In: Clavicipitalean fungi, evolutionary biology, chemistry, biocontrol and cultural impacts (White JF, Jr, Bacon CW, Hywel-Jones NL, et al., eds). Marcel Decker, Inc., New York: 17–73. [Google Scholar]
- Górzyńska K, Ryszka P, Anielska T, et al. (2017). Effect of Epichloë typhina fungal endophyte on the diversity and incidence of other fungi in Puccinellia distans wild grass seeds. Flora 228: 60–64. [Google Scholar]
- Greenfield M, Gómez-Jiménez MI, Ortiz V, et al. (2016). Beauveria bassiana and Metarhizium anisopliae endophytically colonize cassava roots following soil drench inoculation. Biological Control 95: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. (2004). BioEdit version 6.0.7. Department of Microbiology, North Carolina State University. Raleigh, NC, USA. [Google Scholar]
- Johnson D, Sung GH, Hywel-Jones NL, et al. (2009). Systematics and evolution of the genus Torrubiella (Hypocreales, Ascomycota). Mycological Research 113: 279–289. [DOI] [PubMed] [Google Scholar]
- Kepler RM, Humber RA, Bischoff JF. et al. (2014). Clarification of generic and species boundaries for Metarhizium and related fungi through multigene phylogenetics. Mycologia 106: 811–829. [DOI] [PubMed] [Google Scholar]
- Kepler RM, Sung GH, Ban S. et al. (2012a). New teleomorph combinations in the entomopathogenic genus Metacordyceps. Mycologia 104: 182–197. [DOI] [PubMed] [Google Scholar]
- Kepler RM, Sung GH, Harada Y. et al. (2012b). Host jumping onto close relatives and across kingdoms by Tyrannicordyceps (Clavicipitaceae) gen. nov. and Ustilaginoidea (Clavicipitaceae). American Journal of Botany 99: 552–561. [DOI] [PubMed] [Google Scholar]
- Kobayasi Y. (1981). Revision of the genus Cordyceps and its allies 1. Bulletin of the National Museum of Nature and Science. Series B 7: 1–13. [Google Scholar]
- Kobayasi Y. (1984). Miscellaneous notes of fungi (4). Journal of Japanese Botany 59: 31–32. [Google Scholar]
- Luangsa-ard J, Mongkolsamrit S, Noisripoom W, et al. (2017a). Helicocollum, a new clavicipitalean genus pathogenic to scale insects (Hemiptera) in Thailand. Mycological Progress 16: 419–431. [Google Scholar]
- Luangsa-ard JJ, Mongkolsamrit S, Thanakitpipattana D, et al. (2017b). Clavicipitaceous entomopathogens: new species in Metarhizium and a new genus Nigelia. Mycological Progress 16: 369–391. [Google Scholar]
- Luangsa-ard J, Tasanathai K, Thanakitpipattana D, et al. (2018). Novel and interesting Ophiocordyceps spp. (Ophiocordycipitaceae, Hypocreales) with superficial perithecia from Thailand. Studies in Mycology 89: 125–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake I. (1908). Witches’ broom disease of bamboo (Preliminary report). Botanical Magazine Tokyo 22: 305–307 (in Japanese). [Google Scholar]
- Mongkolsamrit S, Khonsanit A, Thanakitpipattana D. et al. (2020). Revisiting Metarhizium and the description of new species from Thailand. Studies in Mycology 95: 171–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongkolsamrit S, Luangsa-ard JJ, Spatafora JW, et al. (2009). A combined ITS rDNA and beta-tubulin phylogeny of Thai species of Hypocrella with non-fragmenting ascospores. Mycological Research 113: 684–699. [DOI] [PubMed] [Google Scholar]
- Mongkolsamrit S, Noisripoom W, Arnamnart N. et al. (2019). Resurrection of Paraisaria in the Ophiocordycipitaceae with three new species from Thailand. Mycological Progress 18: 1213–1230. [Google Scholar]
- Mongkolsamrit S, Noisripoom W, Thanakitpipattana D. et al. (2018). Disentangling cryptic species with isaria-like morphs in Cordycipitaceae. Mycologia 110: 230–257. [DOI] [PubMed] [Google Scholar]
- Nishi O, Sato H. (2019). Isolation of Metarhizium spp. from rhizosphere soils of wild plants reflects fungal diversity in soil but not plant specificity. Mycology 10: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguchi T. (2001). Aciculosporium sasicola sp. nov. on witches’ broom of Sasa senanensis. Mycoscience 42: 217–221. [Google Scholar]
- Page RD. (1996). TreeView: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357–358. [DOI] [PubMed] [Google Scholar]
- Píchová K, Pažoutová S, Kostovčík M, et al. (2018). Evolutionary history of ergot with a new infrageneric classification (Hypocreales: Clavicipitaceae: Claviceps). Molecular Phylogenetics and Evolution 123: 73–87. [DOI] [PubMed] [Google Scholar]
- Rehner SA, Samuels GJ. (1995). Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorphs. Canadian Journal of Botany 73: 816–823. [Google Scholar]
- Ronquist F, Teslenko M, Mark P. et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl CL, Young CA, Hesse U. et al. (2013). Plant-symbiotic fungi as chemical engineers: multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genetics 9: e1003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu D. (1994). Color iconography of vegetable wasps and plant worms (in Japanese). Seibundo Shinkosha, Tokyo. [Google Scholar]
- Spatafora JW, Sung GH, Sung JM. et al. (2007). Phylogenetic evidence for an animal pathogen origin of ergot and the grass endophytes. Molecular Ecology 16: 1701–1711. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner U, Leibner S, Schardl CL. et al. (2011). Periglandula, a new fungal genus within the Clavicipitaceae and its association with Convolvulaceae. Mycologia 103: 1133–1145. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Bergen MS, Patel R. et al. (2001). Features and phylogenetic status of an enigmatic clavicipitalean fungus Neoclaviceps monostipa gen. et sp. nov. Mycologia 93: 90–99. [Google Scholar]
- Sung GH, Hywel-Jones NL, Sung JM, et al. (2007). Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology 57: 5–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung GH, Shrestha B, Park KB. et al. (2010). Cultural characteristics of Shimizuomyces paradoxus collected from Korea. Mycobiology 38: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung GH, Spatafora JW, Zare R. et al. (2001) A revision of Verticillium sect. Prostrata. II. Phylogenetic analyses of SSU and LSU nuclear rDNA sequences from anamorphs and teleomorphs of the Clavicipitaceae. Nova Hedwigia 72: 311–328. [Google Scholar]
- Tanaka E, Tanaka C, Ishihara A. et al. (2003). Indole-3-acetic acid biosynthesis in Aciculosporium take, a causal agent of witches’ broom of bamboo. Journal of General Plant Pathology 69: 1–6. [Google Scholar]
- Torres MS, White JF., Jr (2009). Free-living and saprotrophs to plant endophytes. In: Encyclopedia of Microbiology 3rd edition. Schaechter Moselio, Ed., Elsevier: 422–430. [Google Scholar]
- Tsuda M, Shimizu K, Matsumura K. et al. (1997). Host range of Aciculosporium take, the causal agent of witches’ broom of bamboo plants. Bulletin of the National Science Museum, Series B 23: 25–34. [Google Scholar]
- Walker J. (2004). Claviceps phalaridis in Australia: biology, pathology and taxonomy with a description of the new genus Cepsiclava (Hypocreales, Clavicipitaceae). Australasian Plant Pathology 33: 211–239. [Google Scholar]
- Young CA, Schardl CL, Panaccione DG. et al. (2015). Genetics, genomics and evolution of ergot alkaloid diversity. Toxins 7: 1273–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]