Abstract
In Bangladesh and West Bengal cholera is seasonal, transmission occurs consistently annually. By contrast, in most African countries, cholera has inconsistent seasonal patterns and long periods without obvious transmission. Transmission patterns in Africa occur during intermittent outbreaks followed by elimination of that genetic lineage. Later another outbreak may occur because of reintroduction of new or evolved lineages from adjacent areas, often by human travelers. These then subsequently undergo subsequent elimination.
The frequent elimination and reintroduction has several implications when planning for cholera’s elimination including: a) reconsidering concepts of definition of elimination, b) stress on rapid detection and response to outbreaks, c) more effective use of oral cholera vaccine and WASH, d) need to readjust estimates of disease burden for Africa, e) re-examination of water as a reservoir for maintaining endemicity in Africa. This paper reviews major features of cholera’s epidemiology in African countries which appear different from the Ganges Delta.
Keywords: cholera, Bangladesh, Africa, epidemiology, Cameroon, Uganda, refugee, emergencies GTFCC, roadmap
Cholera continues to affect many countries around the world, having spread from its homeland in the Indian subcontinent to other countries in Asia, Africa, and Hispaniola. The disease, caused by intestinal infection with Vibrio cholerae, serotype O1 or O139, is characterized by acute watery diarrhea. In severe cases, this leads to rapidly progressing severe dehydration and, if not treated promptly, could lead to death within a few hours. Two recent estimates of cholera disease burden concluded that between 95 000 and 107 000 deaths result from the 2.86 to 2.88 million episodes of cholera annually [1, 2]. The disease is transmitted by the fecal-oral route and is more common in areas with poor water and sanitation. Much of the research on cholera has taken place in the Ganges Delta where the disease occurs consistently, including in Bangladesh at the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) and in West Bengal at the National Institute of Cholera and Enteric Diseases. Thus, many of the concepts of its epidemiology, transmission, methods for treatment and prevention, and its persistence in environmental reservoirs were developed from this research in the Ganges Delta.
Many cases occur in Asia, though they are rarely reported to the World Health Organization (WHO). Since 1970, most of the cases that are reported are from Africa [3]. With an emphasis on cholera control in Africa, increasing efforts are being carried out in Africa to understand the true burden, transmission patterns, and methods to document impact of vaccine and water-sanitation-hygiene (WASH) interventions. The studies in Africa benefit from the methods and concepts developed in Asia; however, differences in disease patterns suggest that some of these concepts and methods from Asia need to be reevaluated when applied to most countries in Africa.
CHOLERA EPIDEMIOLOGY IN THE GANGES DELTA
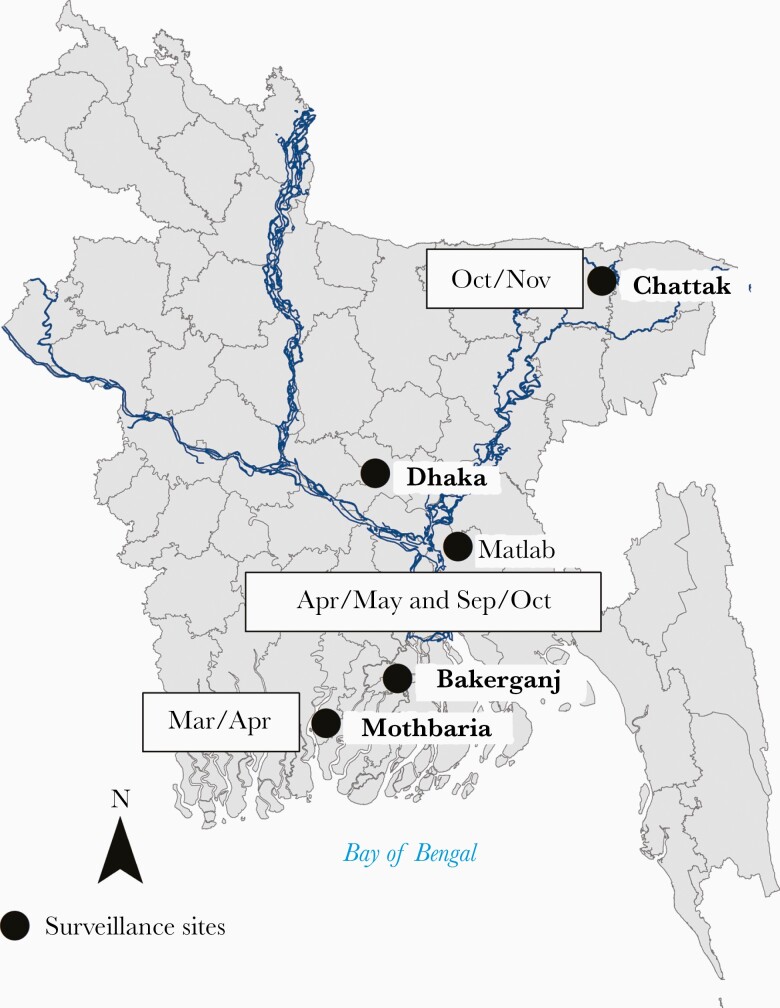
Cholera is endemic in the Ganges Delta. The WHO defines cholera as being endemic when it occurs in country during 3 of 5 years [4]. In Bangladesh cases occur throughout the year, every year. Rates of cholera vary during the season, but cases are documented every month of the year at the icddr,b hospital in Dhaka [5]. As illustrated in Figure 1, in Dhaka, the high season occurs before and after the monsoon (June to August) and numbers decrease during the cooler season (January to February) and during the monsoon. In the northern part of the country, the high season is October to November and in the southern part, the season is March to April [6]. Thus, even though the country is geographically small, being only 500 miles from north to south, these distinct seasonal differences are key features of the disease. Epidemiologists often refer to cholera in terms of outbreaks, but for Bangladesh the outbreak continues indefinitely without an end.
Figure 1.
Identification of the months with high rate of cholera in Bangladesh (source of map https://gadm.org/maps/BGD.html) [6].
As is typical of other endemic diseases, young children have the highest rates of cholera [7]. The decreasing rates of disease with age are thought related to acquired immunity, and this is supported by the increasingly elevated vibriocidal antibody titers by age in the population [8, 9]. While rates are highest in young children, in fact, more older children and adults are affected because these older age groups make up a larger proportion of the population.
In Bangladesh, sentinel surveillance conducted in preselected facilities in different parts of the country is a useful method to characterize cholera seasonality and disease burden. Two key sites for such surveillance are the hospitals of the icddr,b in urban Dhaka and rural Matlab [5]. Other sentinel sites were established in other parts of the country based on convenience, logistical considerations, and specific scientific questions of interest [6, 7, 10].
With knowledge of the catchment population around the sentinel site, one can determine rates of disease from season to season and year to year. One such area used to determine precise rates of cholera is the demographically and geographically defined Matlab area [11]. With a population of more than 200 000, the etiology of diarrhea of those seeking care at the icddr,b hospital in Matlab is confirmed by microbial culture. Until recently, rates of cholera regularly exceeded 1 per 1000 every year. The detailed information on cholera cases by time and place has allowed for many epidemiological studies, vaccine field trials, and water interventions [12, 13]. Vaccine trials were also possible by creating demographically defined urban areas in Dhaka and Kolkata where there have been high and consistent rates of disease [14–16].
The consistently high rates of cholera also make it possible to understand transmission through family studies in which household members of cholera cases are studied prospectively to detect secondary cases and asymptomatic infections and to determine risk factors for these symptomatic and asymptomatic infections. These studies show that only about 20% of infected persons in Bangladesh develop severe disease [17–19]. They also provide evidence that markers of immunity [20] are protective and that intensive WASH interventions can prevent transmission within the household [19]. Additional studies are being planned to determine if an intensive, vaccine and/or WASH intervention will prevent transmission to the immediate neighbor households.
Vibrio species, including V. cholerae, naturally inhabit environmental waters [21] and identification of a persistent, viable but nonculturable (VBNC) form of V. cholerae O1 provides a hypothesis for an environmental reservoir for cholera [22]. Their presence in the environment suggested that, under certain climatic or other environmental conditions, the VBNC V. cholerae might infect people, leading to human disease and onward transmission. Because VBNC V. cholerae cannot be cultured, it is difficult to establish its true role as a reservoir for initiating clinical disease and outbreaks.
Environmental studies also showed that V. cholerae O1 become associated with plankton on which they may persist for a prolonged period [23]. The association with plankton suggested that people might ingest a large inoculum of bacteria if they consumed water with Vibrio-contaminated plankton, and that filtering drinking water might reduce the inoculum and reduce risk of cholera. In fact, cholera rates were decreased by about 50% in Matlab communities when cloth sari material was used to filter water [13].
The role of water in cholera transmission was also shown by detecting V. cholerae in household water, including source water being collected for the household. Often, but not always, the genotypes of the V. cholerae in the water were like those isolated from the stools of cholera patients, suggesting that the contaminated water was, in fact, the source of the infection. When the genotype of the isolates in source water from multiple households is the same as the outbreak isolates, it seems likely the source water initiated the outbreak. However, when the genotype of the V. cholerae in the water was not the same as the patient stool specimens, then the water could not be the source [24].
Persons infected with V. cholerae O1 develop an immune response, which protects them for several years from subsequent disease [25, 26]. Similarly, oral cholera vaccine (OCV) stimulates immunity for 3 to 5 years [16, 27]. Because both natural infections and vaccine stimulate immune protection, persons who were vaccinated and then experience natural exposure are likely to develop a boost in their protective immunity. Similarly, persons who were previously naturally exposed will develop an enhanced immune response if they are vaccinated. The resulting effectiveness of vaccine is likely the result of this interaction between vaccination and natural exposure.
Other biological factors have also been found to affect the risk for cholera. Vibrio is rapidly killed when exposed to gastric acid and persons with hypochlorhydria have increased risk [28, 29]. Persons with blood group O have higher rates of severe cholera compared to persons with other blood groups [30–33] and Lewis blood group antigen may also affect susceptibility to severe cholera [34]. Key features of cholera’s epidemiology in Bangladesh are summarized in the middle column of Table 1.
Table 1.
Comparison of Cholera in the Ganges Delta and Many Countries in Africa
| Feature | Ganges Delta | Africa |
|---|---|---|
| Endemicity | Cholera cases are reported every year, throughout the year | Sporadic outbreaks |
| Seasonality | Different regions within the country have peak rates depending on season The seasonal peaks are consistent, year to year |
Some countries have a strong seasonality (eg, Burundi), but outbreaks may occur during different seasons |
| Geographic consistency | The same areas are affected from year to year | Hotspots identified but variable from year to year for most countries |
| Outbreaks | In Bangladesh, cases never stop; thus, it is difficult to define the end of an outbreak | Cholera occurs during well-defined outbreaks with a clear start and end |
| Risk by age group | The highest rates occur among young children aged 2–5 y | Similar rates across the age groups |
| Risk by sex | Higher number of cases in young boys compared to girls Higher numbers in women aged 15–45 y compared to men |
Similar to Asia, but needs more study |
| Asymptomatic infections | Most infections are asymptomatic or mildly symptomatic | Needs further study |
| Biological susceptibility to severe disease | Persons with hypochlorhydria, with blood group O and possibly Lewis blood group Le(a+ b−) have higher rates | Not known |
| Methods to monitor disease burden | Sentinel surveillance at preselected sites is efficient when monitoring rates of disease and disease burden | Sentinel surveillance has limited value, but broad-based detection with rapid reporting is needed |
| Relation between endemic disease and vaccine effectiveness | Preexisting immunity affects vaccine response Vaccine response affects response to future natural exposures Protection results from combination of vaccine and natural exposure |
Vaccine stimulates immune protection, but natural exposure has limited effect |
| Detection of Vibrio cholerae in environmental water | V. cholerae can be identified frequently in ponds and rivers as well as drinking water at the source and in the household |
V. cholerae is rarely detected More studies are needed to determine optimal methods |
| Viable but not culturable (VBNC) V. cholerae | VBNC forms of V. cholerae can be identified throughout the year in pond water | Not yet studied |
| Genetic characteristics of V. cholerae | Multiple genotypes circulating in the country | A single genotype, or few types spread through an area |
STUDIES IN AFRICAN COUNTRIES WITH CHOLERA
After studying cholera in Bangladesh for many years, our group at Johns Hopkins University initiated studies in several African countries, starting in Cameroon. Cameroon was identified as a cholera hotspot in Africa, especially the Far North and Littoral Regions of the country [35, 36]. These areas experienced a very large cholera outbreak in 2010–2011, during which 33 192 cases with 1440 deaths (case fatality rate = 4.3%) were reported to WHO. The Lake Chad area seemed to be an ideal site to study the clinical, epidemiological, and ecological aspects of cholera because Lake Chad is a large shallow lake where people live in close association with the lake, many of whom subsist on fishing.
Based on methods for studying cholera’s epidemiology in Bangladesh, we established 9 sentinel surveillance sites in hospitals and clinics near Lake Chad although the numbers of reported cases had decreased since the major outbreak in 2011. When designing the surveillance, we made several assumptions based on findings from Bangladesh.
First, because the area was already defined as a major hotspot in Africa, we assumed that cholera was endemic and that cases would be identified readily. Second, as described in the Cholera Fact Sheet from the WHO [4], cholera surveillance methods are insensitive in Africa but we assumed that an intensive surveillance system would detect an accurate count, including cases that might not be recognized by a routine system. Third, we assumed that some cases, as in Bangladesh, would be mild or asymptomatic, requiring inclusion of mild as well as severe cases in the surveillance. Fourth, we assumed that cholera may be seasonal, requiring an extended period of surveillance, at least 3 years, to fully understand its seasonality. Fifth, we expected that a cholera outbreak may start with a few mild cases with higher numbers subsequently. Detection of these mild, early cases might provide an early warning for an impending outbreak. Finally, we hoped that by testing environment water for cholera, one might find an early warning for an outbreak.
Although these sentinel sites had reported many cholera cases previously, and these sites did report many cases of diarrhea during the intensive surveillance, both mild and severe, none were confirmed as cholera until an outbreak occurred in the Far North Region in 2014 [37]. The other diarrhea cases, except during the outbreak, had other etiologies, but were not cholera. Thus, this intensive surveillance for 3 years was not able to confirm the presence of any cases of cholera, which may have been occurring at a low rate or with mild symptoms, except cases that occurred during the 2014 outbreak, and this outbreak appeared to spread from nearby Nigeria. Also, monthly water samples from 30 various water collection sites in Cameroon did not (except for 1) detect any V. cholerae O1. The water sampling did detect many (approximately 20%) specimens positive for V. cholerae non-O1. The 1 positive water sample for V. cholerae O1 was collected during an outbreak from a well for drinking water on an island in Lake Chad. After detecting this positive sample using a rapid diagnostic test (RDT) method [38], the contaminated well was closed the next day and case numbers decreased on the island. A similar study of multiple environmental water sources in Uganda also found many samples positive for non-O1 V. cholerae O1 but no toxigenic V. cholerae O1 [39].
The national cholera surveillance for Cameroon [40] shows that cases are reported during many years, but in some years the numbers were either zero or very low, suggesting that cholera was present in Cameroon intermittently but not continuously (Figure 2) [41]. A cholera distribution pattern, different from that of Bangladesh, was also observed in a multicountry study in 7 enhanced surveillance zones and 4 outbreak sites in Togo, Democratic Republic of the Congo (DRC), Guinea, Uganda, Mozambique, and Cote d’Ivoire [42] and from the national reports from other African countries, including Guinea-Bissau, Ghana, and Zambia [43]. Cholera was reported frequently but years with many cases were interspersed by other years with no or few cases.
Figure 2.
Yearly number of cholera cases (columns) and case fatality ratio (circles) in Cameroon 1990–2016 (data from [41]).
Examining data at a subnational level, countries where cholera is deemed to be endemic do report cholera within their national borders often, but the cases do not necessarily occur in the same districts year to year, as they do in Bangladesh, and countries that report cholera annually identify cases in different districts from year to year. Hotspot districts, where cases are seen more frequently, can be identified but even these hotspot districts do not report cholera every year and often have no cases for several consecutive years [36, 44]. Between these outbreaks, even within hotspot districts, cholera appears to have disappeared.
Most Outbreaks in Africa Are Short
In contrast to the cholera seasons in Bangladesh, which persist indefinitely, most outbreaks (with a few exceptions) in Africa are relatively short. An example is the outbreak in Uganda that lasted 10 weeks [45]. No cases were seen during the other weeks in the year. Similar short outbreaks are documented in Tanzania (unpublished data) and Burundi [46].
Implications for Surveillance and Disease Burden Estimates
The sentinel surveillance model, which detects cases at preselected sites, adapted from Bangladesh, was found to be insufficient, as illustrated in Cameroon. Although we expected to find cases in a defined hotspot, in fact, cholera was just as likely to appear in a different area that was not selected. Outbreaks seem to occur sporadically and not in specific, predetermined sites. Thus, an attempt to determine rates of disease and disease burden through sentinel surveillance was not helpful in Africa. Rather, a surveillance system needs to be alert for cholera outbreaks whenever and wherever they may occur and not be limited to a specific location.
When estimating disease burden, the consistent pattern seen in Bangladesh, where one could estimate an average incidence of disease, was not seen in Africa. Numbers of cases varied widely from year to year and an average or a median rate varied considerably from the observed rates. This calls into question the current estimates of about 2.86 or 2.88 million cases, which assumed rates of cholera between 2 and 4 per 1000 for sub-Saharan Africa [1]. If these relatively high rates occur only from time to time, or if the high rates apply to only some limited areas of the country, the average rate is actually much lower. Similarly, if cholera occurs during defined outbreaks and not as an endemic infection, the numbers will also be much lower. Although surveillance systems in African countries may underestimate the true number of cases during an outbreak, the severity of the undercount is likely much less than has been assumed and this underestimate is partially compensated by overcounting diarrhea cases that are not cholera. If most cases only occur during outbreaks, the actual number of cases occurring in Africa is likely to be much lower than previously estimated.
Cholera Elimination as Applied to Africa
One of the goals of the Global Task Force on Cholera Control (GTFCC) is to eliminate cholera from > 20 countries by the year 2030 [47]. Elimination means no cases in an area for at least 3 years. While only a few African countries would qualify as having eliminated cholera, in fact, many districts within the countries would qualify. The pattern of cholera in most African districts (subnational areas) is one of repeated elimination. Countries are considered cholera endemic not because of continued transmission, as in Bangladesh, but rather because cholera frequently occurs in some district(s) within the country. We hypothesize that the pattern of cholera in most African countries (not including DRC and perhaps Mozambique) is for repeated elimination within a district but then with subsequent reintroduction. This suggests that cholera elimination within a country should focus on eliminating cholera from each district and monitor the number of districts where elimination has been achieved. The goal of the national control programs would be to prevent cholera reintroduction into these districts and to gauge success by maintaining district-level elimination.
Recent studies using whole-genome sequencing (WGS) on transmission of cholera to Africa from South Asia reinforces this concept that cholera is repeatedly introduced into Africa. As shown by Weill et al [48], 12 transmission events took place between 1970 and 2014. The V. cholerae genetic lineages transmitted to Africa were termed T1 to T12. Later, T13 was identified in Uganda [49], Zambia [50], and Yemen [51]. Interestingly, most of the earlier genetic lineages are no longer seen in Africa, so the pattern seems to be one of introduction of genetic lineages followed by their elimination, but also followed by introductions of new genetic lineages through transmission from outside the continent.
On a subnational basis a similar pattern persists. Clonal complexes identified using multiple locus variable-number tandem repeat analysis or WGS-defined genetic lineages move through an area and then die out [49]. This suggests that large areas of a cholera-endemic country eliminate cholera but then it is reintroduced from a neighboring area. An example is that of Tanzania in which different clonal complexes were detected as they moved through different parts of the country, some of which overlapped and one of which moved on to cause the recent outbreaks in Zanzibar [52]. A second example from Lusaka, Zambia identified 3 successive outbreaks in 2009, 2016, and 2017 each of which were caused by genetically distinct V. cholerae O1 that were more closely related to isolates from Tanzania or Uganda than to isolates from the other Zambian outbreaks [50]. The characterization of cholera, not just as an infection caused by V. cholerae O1 but rather as a specific genetic lineage of V. cholerae O1, illustrates that genetic lineages spread through a region within and between countries, and then die out. These findings suggest that cholera lineages appear to have been repeatedly eliminated from many areas of Africa, but then new or evolved lineages are introduced from outside the area, leading to subsequent outbreaks.
A concept that has intrigued cholera epidemiologists is the potential for cholera to reside in an environmental reservoir and then to emerge, based on suitable climatic conditions, to begin spreading from person to person. Our studies did not identify environmental, culturable V. cholerae O1 in either Cameroon or Uganda and the molecular data suggest that the outbreaks were caused by person-to-person spread (through fecal-oral transmission) leading to spread through an area, likely by movement of people, rather than emerging from an environmental reservoir. If V. cholerae was to emerge from an environmental reservoir, it would likely be of the same genetic lineage as the previous outbreak in that area. Instead, isolates generally are genetically closely related to isolates from other locations and then evolve new variation during the outbreak, as expected [53].
Implications for the Cholera Roadmap
The GTFCC hopes to eliminate cholera from > 20 countries by 2030. Our studies suggest that this goal is obtainable because cholera lineages have repeatedly been eliminated from many countries and from many districts within these countries over the years. The major problem for cholera control is stopping reintroduction of cholera from outside an area. The same interventions identified in the roadmap [47] , including early identification and control of outbreaks, use of OCV, and improvement of WASH in hotspots, are still suitable for preventing transmission. However, increased emphasis is needed for broad-based surveillance to identify outbreaks at the earliest stage to prevent these outbreaks from spreading to new areas. The wide-scale use of RDT and a rapid reporting system will greatly facilitate the type of comprehensive and intensive surveillance that is required [54, 55]. Microbial culture is still needed, especially for determining antimicrobial sensitivity, but RDTs should be widely available at the district or ward level so that cases can be detected very early in an outbreak and an effective response can rapidly be mounted. Waiting for a culture result, which may take days or weeks [56], before declaring an outbreak may delay a rapid response that is needed. RDTs can be used to declare outbreaks quickly, especially if more than 1 patient is found to be positive. Preliminary studies suggest that the RDTs can also be saved in a plastic bag, sent to a laboratory, and the DNA from the dipstick can be extracted to detect V. cholerae using polymerase chain reaction (PCR). The DNA from the RDT may also be used for molecular characterization of the Vibrio, providing even more epidemiological information about disease transmission.
While vaccination of hotspot areas remains a key strategy, its use should also focus on routes of transmission, as suggested for Uganda [57] and Burundi [46], which focus on persons, including refugees, arriving from neighboring countries where cholera is common. Specific interventions for migrants must, however, be cognizant of the need to avoid stigma, yet still be effective.
Because cholera is transmitted by people when traveling, reintroduction needs to be considered as a cross-national border as well as a cross-district issue. Current methods for detecting hotspots focus on the district as the unit of analysis; however, within districts, microhotspots may better define outbreaks at the ward level and will provide a critical understanding when attempting to interrupt transmission (Ngwa, unpublished).
The definition of elimination may also need to be adjusted for susceptible countries that continue to be at risk. A country without cholera for 3 consecutive years may still be at high risk if it borders countries with continued transmission.
Other factors defined in Bangladesh need to be reexamined for Africa. For example, family studies, which revealed high rates of mild and asymptomatic infection in Bangladesh, need to be undertaken for countries in Africa. These mild infections in Bangladesh may be related to preexisting immunity and a higher proportion of infections in Africa might be severe because of lack of this immunity. Biological risk factors, such as hypochlorhydria and blood group, have not been studied in Africa. Initially, there was concern that OCV may be less effective in Africa compared to Bangladesh because the population had less natural exposure; however, studies have found the vaccine to be equally effective in Africa [27].
A summary of the epidemiological observations from Africa are shown in the right-hand column of Table 1 and are compared to those from the Ganges Delta. While our findings are based on studies from several African countries, they should not be applied to the DRC and perhaps not to Mozambique. DRC reports very high numbers of cases consistently, and so cholera is clearly endemic here, which seems unique among African countries. Similarly, the environmental conditions of the rivers and estuaries in Mozambique are more like Bangladesh and might facilitate persistence of Vibrio in this country. In Mozambique, genetically identical isolates of V. cholerae were collected 8 years apart with minimal evidence of clinical cases and no outbreaks during the intervening period [58].
The evidence seems to favor the hypothesis that cholera outbreaks are caused by reintroduction of V. cholerae O1 into an area rather than emerging from an environmental reservoir; however, it should be clear that we have not ruled out the possibility of an environmental reservoir in some areas of Africa. Studies to identify such a reservoir are needed but, with the possible exceptions of DRC and Mozambique, this seems unlikely. Even if cholera does not have an environmental reservoir, the association of cholera with season, temperature, rainfall, and flooding suggests an important role for climate in cholera’s transmission [59]. Whether this is a direct effect of Vibrio behavior and survival under different climatic and environmental conditions or results from changing behaviors during different seasons remains to be studied.
In summary, the epidemiology of cholera in most Africa countries is characterized by repeated outbreaks, most of which are relatively brief. These outbreaks result from the introduction of specific genetic lineages of V. cholerae into an area, following which cholera seems to disappear for a time until another outbreak occurs. The sporadic, inconsistent patterns of cholera outbreaks in Africa suggest that current estimates of disease burden overestimate the true numbers. An adjustment in the estimated disease burden should lead to a revision on cost effectiveness of various interventions. If cholera primarily spreads rather than emerges from the environment, this should lead to even more resources for early detection, reporting, and responding to outbreaks, including intervening with vaccine and WASH strategies to prevent its spread. There may be situations where a case area targeted interventions strategy, using vaccinations and intensive WASH in the neighborhood around the cases, will be appropriate [60, 61]. The wide-scale use of RDT at the ward level to rapidly detect outbreaks will facilitate the rapid response that is needed. Also, the routine inclusion of molecular characterization of outbreaks using DNA from isolates, filter paper, or RDTs will help understanding of the movement of specific genetic lineages within and between countries and help to refine interventions.
With many outbreaks that occur in Africa, we must assume that V. cholerae is often carried to areas outside the immediate outbreak zone; however, this does not always lead to a outbreak in the new area. Considerable effort is still needed to understand why, in the same country and even the same region of the country, some districts are rarely affected while others experience outbreaks, and to explore innovative methods to clarify the relationships between human factors, environment, climate, demography, and Vibrio biology that lead to the initiation, as well as the collapse, of an outbreak, and the factors that lead to the dying out of a specific Vibrio lineage.
Notes
Financial support. This work was supported in part by the Bill and Melinda Gates Foundation (grant number OPP1148763); and the National Institute of Allergy and Infectious Disease (grant number 5R01AI123422).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 15th Asian Conference on Diarrhoeal Disease and Nutrition, Dhaka, Bangladesh, 29 January 2020.
References
- 1. Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 2015; 9:e0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 2018; 18:1211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cholera vaccines: WHO position paper—August 2017. Wkly Epidemiol Rec 2017; 92:477–98. [PubMed] [Google Scholar]
- 4. World Health Organization. Cholera fact sheet, 2021. https://www.who.int/news-room/fact-sheets/detail/cholera. Accessed 22 May 2021.
- 5. International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b). Health and science bulletin: surveillance updates. Health and science bulletin. Vol. 12. Dhaka: icddr,b, 2014:22. [Google Scholar]
- 6. Sack RB, Siddique AK, Longini IM Jr, et al. A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. J Infect Dis 2003; 187:96–101. [DOI] [PubMed] [Google Scholar]
- 7. Azman AS, Lauer SA, Bhuiyan TR, et al. Vibrio cholerae O1 transmission in Bangladesh: insights from a nationally representative serosurvey. Lancet Microbe 2020; 1:e336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sack DA, Clemens JD, Huda S, et al. Antibody responses after immunization with killed oral cholera vaccines during the 1985 vaccine field trial in Bangladesh. J Infect Dis 1991; 164:407–11. [DOI] [PubMed] [Google Scholar]
- 9. Mosley WH, Benenson AS, Barui R. A serological survey for cholera antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera-vaccine field-trial area and the relation of antibody titre to the pattern of endemic cholera. Bull World Health Organ 1968; 38:327–34. [PMC free article] [PubMed] [Google Scholar]
- 10. Khan AI, Rashid MM, Islam MT, et al. Epidemiology of cholera in Bangladesh: findings from nationwide hospital-based surveillance, 2014-2018. Clin Infect Dis 2020; 71:1635–42. [DOI] [PubMed] [Google Scholar]
- 11. Alam N, Ali T, Razzaque A, et al. Health and demographic surveillance system (HDSS) in Matlab, Bangladesh. Int J Epidemiol 2017; 46:809–16. [DOI] [PubMed] [Google Scholar]
- 12. Clemens JD, Sack DA, Harris JR, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet 1990; 335:270–3. [DOI] [PubMed] [Google Scholar]
- 13. Colwell RR, Huq A, Islam MS, et al. Reduction of cholera in Bangladeshi villages by simple filtration. Proc Natl Acad Sci U S A 2003; 100:1051–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nair GB, Ramamurthy T, Bhattacharya MK, et al. Emerging trends in the etiology of enteric pathogens as evidenced from an active surveillance of hospitalized diarrhoeal patients in Kolkata, India. Gut Pathog 2010; 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qadri F, Ali M, Lynch J, et al. Efficacy of a single-dose regimen of inactivated whole-cell oral cholera vaccine: results from 2 years of follow-up of a randomised trial. Lancet Infect Dis 2018; 18:666–74. [DOI] [PubMed] [Google Scholar]
- 16. Bhattacharya SK, Sur D, Ali M, et al. 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2013; 13:1050–6. [DOI] [PubMed] [Google Scholar]
- 17. Mosley WH. Epidemiology of cholera. Public Health Pap 1970; 40:23–7. [PubMed] [Google Scholar]
- 18. Weil AA, Khan AI, Chowdhury F, et al. Clinical outcomes in household contacts of patients with cholera in Bangladesh. Clin Infect Dis 2009; 49:1473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. George CM, Monira S, Sack DA, et al. Randomized controlled trial of hospital-based hygiene and water treatment intervention (CHoBI7) to reduce cholera. Emerg Infect Dis 2016; 22:233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris JB, LaRocque RC, Chowdhury F, et al. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis 2008; 2:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huq A, Sack RB, Nizam A, et al. Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl Environ Microbiol 2005; 71:4645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Colwell RR, Huq A. Environmental reservoir of Vibrio cholerae. The causative agent of cholera. Ann N Y Acad Sci 1994; 740:44–54. [DOI] [PubMed] [Google Scholar]
- 23. Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 1983; 45:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. George CM, Rashid M, Almeida M, et al. Genetic relatedness of Vibrio cholerae isolates within and between households during outbreaks in Dhaka, Bangladesh. BMC Genomics 2017; 18:903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clemens JD, van Loon F, Sack DA, et al. Biotype as determinant of natural immunising effect of cholera. Lancet 1991; 337:883–4. [DOI] [PubMed] [Google Scholar]
- 26. Levine MM, Black RE, Clements ML, Cisneros L, Nalin DR, Young CR. Duration of infection-derived immunity to cholera. J Infect Dis 1981; 143:818–20. [DOI] [PubMed] [Google Scholar]
- 27. Bi Q, Ferreras E, Pezzoli L, et al. ; Oral Cholera Vaccine Working Group of The Global Task Force on Cholera Control. Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17:1080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van Loon FP, Clemens JD, Shahrier M, et al. Low gastric acid as a risk factor for cholera transmission: application of a new non-invasive gastric acid field test. J Clin Epidemiol 1990; 43:1361–7. [DOI] [PubMed] [Google Scholar]
- 29. Sack GH Jr, Pierce NF, Hennessey KN, Mitra RC, Sack RB, Mazumder DN. Gastric acidity in cholera and noncholera diarrhoea. Bull World Health Organ 1972; 47:31–6. [PMC free article] [PubMed] [Google Scholar]
- 30. Glass RI, Holmgren J, Haley CE, et al. Predisposition for cholera of individuals with O blood group. Possible evolutionary significance. Am J Epidemiol 1985; 121:791–6. [DOI] [PubMed] [Google Scholar]
- 31. Anstee DJ. The relationship between blood groups and disease. Blood 2010; 115:4635–43. [DOI] [PubMed] [Google Scholar]
- 32. Harris JB, Khan AI, LaRocque RC, et al. Blood group, immunity, and risk of infection with Vibrio cholerae in an area of endemicity. Infect Immun 2005; 73:7422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levine MM, Nalin DR, Rennels MB, et al. Genetic susceptibility to cholera. Ann Hum Biol 1979; 6:369–74. [DOI] [PubMed] [Google Scholar]
- 34. Arifuzzaman M, Ahmed T, Rahman MA, et al. Individuals with Le(a+b−) blood group have increased susceptibility to symptomatic Vibrio cholerae O1 infection. PLoS Negl Trop Dis 2011; 5:e1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lessler J, Moore SM, Luquero FJ, et al. Mapping the burden of cholera in sub-Saharan Africa and implications for control: an analysis of data across geographical scales. Lancet 2018; 391:1908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ngwa MC, Liang S, Kracalik IT, et al. Cholera in Cameroon, 2000–2012: spatial and temporal analysis at the operational (health district) and sub climate levels. PLoS Negl Trop Dis 2016; 10:e0005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reliefweb. Cameroon: cholera outbreak—Jul 2014, 2021. https://reliefweb.int/disaster/ep-2014-000100-cmr. Accessed 22 May 2021.
- 38. Debes AK, Ateudjieu J, Guenou E, et al. Clinical and environmental surveillance for Vibrio cholerae in resource constrained areas: application during a 1-year surveillance in the far north region of Cameroon. Am J Trop Med Hyg 2016; 94:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bwire G, Debes AK, Orach CG, et al. Environmental surveillance of Vibrio cholerae O1/O139 in the five African great lakes and other major surface water sources in Uganda. Front Microbiol 2018; 9:1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ngwa MC, Young A, Liang S, Blackburn J, Mouhaman A, Morris JG Jr. Cultural influences behind cholera transmission in the Far North Region, Republic of Cameroon: a field experience and implications for operational level planning of interventions. Pan Afr Med J 2017; 28:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. UNICEF. Cholera fact sheet Cameroun,2021. https://plateformecholera.info/index.php/country-monitoring/cameroun. Accessed 28 May 2021.
- 42. Sauvageot D, Njanpop-Lafourcade BM, Akilimali L, et al. Cholera incidence and mortality in sub-Saharan African sites during multi-country surveillance. PLoS Negl Trop Dis 2016; 10:e0004679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. UNICEF. Cholera platform, country monitoring. https://plateformecholera.info/index.php/country-monitoring/. Accessed 28 May 2021.
- 44. Bwire G, Ali M, Sack DA, et al. Identifying cholera “hotspots” in Uganda: an analysis of cholera surveillance data from 2011 to 2016. PLoS Negl Trop Dis 2017; 11:e0006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bwire G, Roskosky M, Ballard A, et al. Use of surveys to evaluate an integrated oral cholera vaccine campaign in response to a cholera outbreak in Hoima district, Uganda. BMJ Open 2020; 10:e038464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Debes AS, Ndikumana A, Liesse T, et al. Cholera hot-spots and contextual factors in Burundi, planning for elimination. Trop Med Infect Dis 2021; 6:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Global Task Force on Cholera Control. Ending cholera, a global roadmap to 2030. https://www.who.int/cholera/publications/global-roadmap-summary.pdf?ua=1. Accessed 28 May 2021.
- 48. Weill FX, Domman D, Njamkepo E, et al. Genomic history of the seventh pandemic of cholera in Africa. Science 2017; 358:785–9. [DOI] [PubMed] [Google Scholar]
- 49. Bwire G, Sack DA, Almeida M, et al. Molecular characterization of Vibrio cholerae responsible for cholera epidemics in Uganda by PCR, MLVA and WGS. PLoS Negl Trop Dis 2018; 12:e0006492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mwaba J, Debes AK, Murt KN, et al. Three transmission events of Vibrio cholerae O1 into Lusaka, Zambia. BMC Infect Dis 2021; 21:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weill FX, Domman D, Njamkepo E, et al. Genomic insights into the 2016–2017 cholera epidemic in Yemen. Nature 2019; 565:230–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bi Q, Abdalla FM, Masauni S, et al. The epidemiology of cholera in Zanzibar: implications for the Zanzibar comprehensive cholera elimination plan. J Infect Dis 2018; 218:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garg P, Aydanian A, Smith D, J Glenn M Jr, Nair GB, Stine OC. Molecular epidemiology of O139 Vibrio cholerae: mutation, lateral gene transfer, and founder flush. Emerg Infect Dis 2003; 9:810–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ontweka LN, Deng LO, Rauzier J, et al. Cholera rapid test with enrichment step has diagnostic performance equivalent to culture. PLoS One 2016; 11:e0168257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bwire G, Orach CG, Abdallah D, et al. Alkaline peptone water enrichment with a dipstick test to quickly detect and monitor cholera outbreaks. BMC Infect Dis 2017; 17:726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ngwa MC, Wondimagegnehu A, Okudo I, et al. The multi-sectorial emergency response to a cholera outbreak in internally displaced persons camps in Borno State, Nigeria, 2017. BMJ Glob Health 2020; 5:e002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bwire G, Orach CG, Aceng FL, et al. Refugee settlements and cholera risks in Uganda, 2016–2019. Am J Trop Med Hyg 2021; 104:1225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garrine M, Mandomando I, Vubil D, et al. Minimal genetic change in Vibrio cholerae in Mozambique over time: multilocus variable number tandem repeat analysis and whole genome sequencing. PLoS Negl Trop Dis 2017; 11:e0005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moore SM, Azman AS, Zaitchik BF, et al. El Niño and the shifting geography of cholera in Africa. Proc Natl Acad Sci U S A 2017; 114:4436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rebaudet S, Bulit G, Gaudart J, et al. The case-area targeted rapid response strategy to control cholera in Haiti: a four-year implementation study. PLoS Negl Trop Dis 2019; 13:e0007263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roskosky M, Ali M, Upreti SR, Sack D. Spatial clustering of cholera cases in the Kathmandu valley: implications for a ring vaccination strategy. Int Health 2021; 13:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]