Abstract
We performed an exploratory analysis to evaluate the effects of a treadmill exercise program on brachial artery (BA) intima–media thickness (IMT) and three BA grayscale ultrasound measures that may indicate subclinical arterial injury. Data were from a clinical trial in individuals with peripheral artery disease who were randomly assigned to treadmill exercise training or attention control. B-mode ultrasonography was performed at baseline and after 26 weeks. BA IMT, grayscale median (GSM), entropy, and gray-level difference statistic-contrast (GLDS-CON) were measured by a single reader. The 184 participants were (mean (SD)) 66.7 (8.2) years old and had an ankle–brachial index of 0.70 (0.18). Exercise training was associated with a 0.01 (0.06) mm (p = 0.025) reduction in BA IMT compared to 0.00 (0.05) mm (p = 0.807) in the control group (between-group p = 0.061). BA GSM, entropy, and GLDS-CON did not change significantly with exercise. Improvements in the 6-minute walk distance correlated with increases in resting BA blood flow (r = 0.23, p = 0.032), flow-mediated dilation (r = 0.24, p = 0.022), diameter (r = 0.29, p = 0.005), entropy (r = 0.21, p = 0.047), and GLDS-CON (r = 0.22, p = 0.041). In a post hoc analysis, BA IMT improved significantly with treadmill exercise training but did not change with attention control; however, the between-group difference did not reach statistical significance. With exercise, improvements in the 6-minute walk distance were associated with improved endothelial function, increased resting blood flow, and BA dilation, as well as higher grayscale entropy and GLDS-CON, indicating that lower extremity exercise is associated with salutary changes in upper-extremity arterial wall structure and function. ClinicalTrials.gov Identifier: NCT01408901
Keywords: brachial artery intima-media thickness, clinical trial, duplex ultrasound, supervised exercise therapy, peripheral artery disease (PAD), ultrasound, vascular biology
Introduction
Supervised treadmill exercise (STE) improves walking performance in people with peripheral artery disease (PAD); however, its effects on peripheral arterial endothelial function in patients with PAD have been mixed.1,2 Bone marrow and splenic-derived endothelial progenitor cells released into the circulation during or after STE may affect peripheral arterial structure and function.3,4 In the PROPEL randomized clinical trial (NCT01408901), individuals with PAD were randomly assigned in a 2 × 2 factorial fashion to STE or attention control and to granulocyte-macrophage colony stimulating factor (GM-CSF) or placebo.2,5 STE but not GM-CSF improved 6-minute walk distance compared with attention control.2 In PROPEL, brachial artery (BA) flow-mediated vasodilation (FMD; a measure of endothelial function) and peak hyperemic flow velocity (a measure of microvascular function) did not improve with treadmill exercise or GM-CSF.2
Advances in imaging technology and image analysis software permit measurement of BA intima–media thickness (IMT), a measure of subclinical arterial injury associated with increased cardiovascular disease (CVD) risk,6,7 as well as novel grayscale ultrasound measures that describe grayscale pixel brightness, variation, and density, which may reflect tissue structure and composition. Parameters such as grayscale median (GSM) and entropy are derived from the image pixel brightness histogram and describe the echogenicity and randomness of pixel brightness in the arterial wall, respectively.8-11 Arterial contrast, calculated using gray-level difference statistics (GLDS-CON), describes spatial relationships between two ultrasound pixels.8-11 Grayscale parameters of lower GSM (darker or echolucent walls) and lower contrast (walls with more pixels that have the same grayscale value) have been associated with atherosclerotic cardiovascular disease (ASCVD) risk factors and future ASCVD events when measured in the carotid arterial bed.12-17 In the BA, GSM has been associated with CVD risk factors in a single cross-sectional study of older adults,6 but associations of BA entropy and GLDS-CON have not been described previously. Changes in carotid artery IMT and GSM have been described with statin therapy;18-22 however, BA IMT, GSM, and other novel grayscale texture measures have not been described previously in people with PAD or with CVD risk-reducing therapies such as exercise.
We performed an exploratory analysis to evaluate the biological effects of treadmill exercise training on BA IMT as well as three novel BA grayscale ultrasound measures that may indicate subclinical arterial injury: GSM, entropy, and GLDS-CON. We hypothesized that BA IMT would improve with a treadmill exercise program and that improvements in walking performance would be related to changes in BA diameter, GSM, entropy, and GLDS-CON, indicating structural effects on the BA wall related to walking. We also sought to describe baseline associations of these novel markers with PAD severity and CVD risk factors.
Methods
Participants
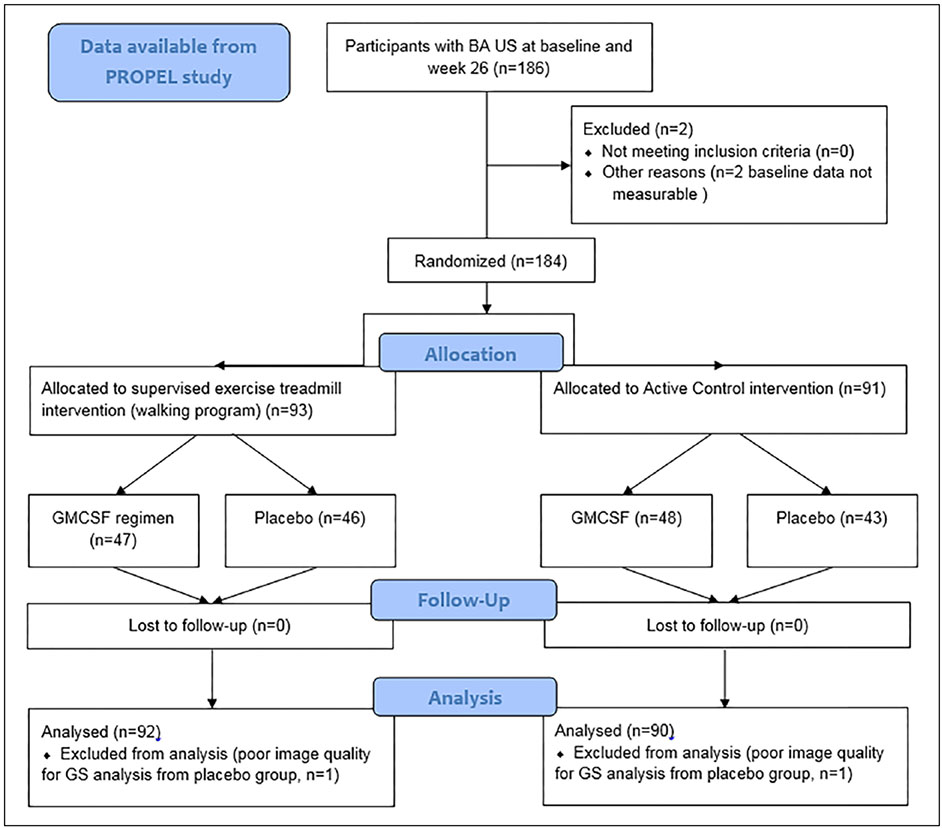
This was an exploratory analysis of data from the PROPEL clinical trial.2,5 PAD was an inclusion criterion and was defined as: (i) an ankle–brachial index (ABI) ⩽ 0.90; (ii) an ABI > 0.90 if PAD was confirmed by a lower extremity angiogram or by a hospital affiliated vascular laboratory; (iii) an ABI between 0.90 and 1.00 with a ⩾ 20% decline following a heel-rise test; or (iv) a normal ABI with prior history of lower extremity revascularization but a ⩾ 20% decline following a heel-rise test.2,5,23 The exclusion criteria have been reported and were focused on safe implementation of the exercise intervention and excluded participants that already were exercising at a level similar to the intervention.2 Randomization was stratified by the presence of diabetes mellitus. The STE intervention (walking program), GM-CSF regimen, and active control regimens, as well as the primary study’s findings, have been described previously.2,5 The CONSORT diagram (Figure 1) starts with participants from the original PROPEL results publication.2 Baseline data were available for 186 individuals; grayscale images were measurable on 184 baseline and 182 week 26 scans.
Figure 1.
CONSORT diagram.
Interventions
Treadmill exercise was performed three times weekly and was supervised by an exercise physiologist. Walking exercise duration was increased gradually up to 50 minutes of exercise per session. Participants were asked to exercise to maximal ischemic leg symptoms. Participants randomized to attention control attended weekly 1-hour educational sessions. GM-CSF or placebo were administered subcutaneously three times weekly for 2 weeks in a double-blinded fashion. The effect of the intervention was measured by changes in the 6-minute walk distance24 and the maximal treadmill walking time between baseline and week 26 following standardized protocols.1,24-27
Brachial Artery (BA) ultrasound imaging
All studies were performed by a single registered diagnostic cardiac sonographer in participants who held vasoactive medications and were instructed not to smoke cigarettes or exercise prior to testing.1,2 After a 15-minute supine rest, left arm brachial blood pressure was measured by oscillometric sphygmomanometry (OMRON Model HEM-907XL; Omron Healthcare Inc., Kyoto, Japan). The right BA was imaged using a linear array vascular ultrasound transducer (ACUSON Sequoia Model C512, 8L5c transducer; Siemens Medical Solutions, Malvern, PA, USA). Extra-vascular landmarks were labeled to ensure consistent imaging within and between studies over time. Overall gain, time-gain compensation settings, and contrast were the same across studies for each participant. After baseline B-mode and Doppler images were obtained, a blood pressure cuff was placed proximal to the visualized BA segment, was inflated for 4 minutes at 50 mmHg above systolic pressure, and adjusted upwards if necessary to ensure complete occlusion using a Hokanson E20 Rapid Cuff Inflation System (D.E. Hokanson Inc., Bellevue, WA, USA). After cuff release, longitudinal images of the BA and Doppler blood flow were obtained up to 90 seconds after cuff deflation.
Brachial artery image analysis for flow and diameter measurements
Ultrasound instrumentation settings for data acquisition are provided in Table 1. Digital Imaging and Communications in Medicine images were sent electronically to the core lab for quality assessment and interpretation by a single, experienced technician using AccessPoint Web software (Freeland Systems, Alpharetta, GA, USA). BA diameters and spectral Doppler flow velocities were measured in triplicate before and after reactive hyperemia at both time points. FMD was defined as the ratio of the maximum BA diameter obtained within 90 seconds after reactive hyperemia to the resting diameter, expressed as a percent.
Table 1.
Ultrasound image instrumentation settings.
| Imaging parameter | Instrumentation setting |
|---|---|
| Imaging frequency | 8.0 MHz |
| Dynamic range | 70 dB |
| Space/time | T2 |
| Edge enhancement | + 3 |
| Persistence | 0 |
| Grayscale map | 5 |
| Delta | 1 |
| Overall gain | Adjusted as needed per patient |
| Time-gain compensation | Adjusted as needed per patient |
All images were acquired with a Siemens Medical Solutions ACUSON Sequoia with an 8L5c transducer.
Brachial artery intima-media thickness and grayscale analysis
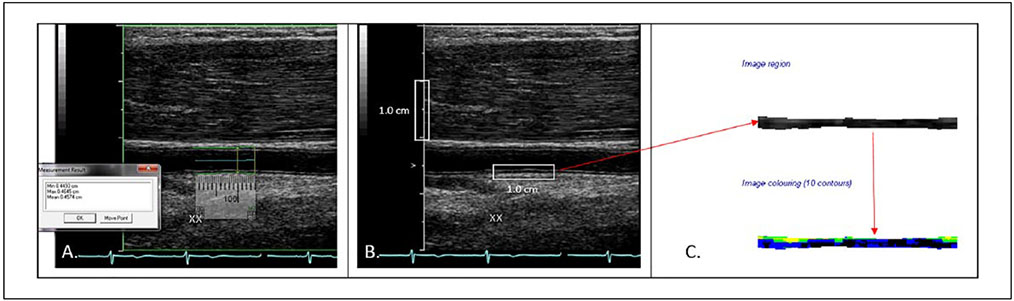
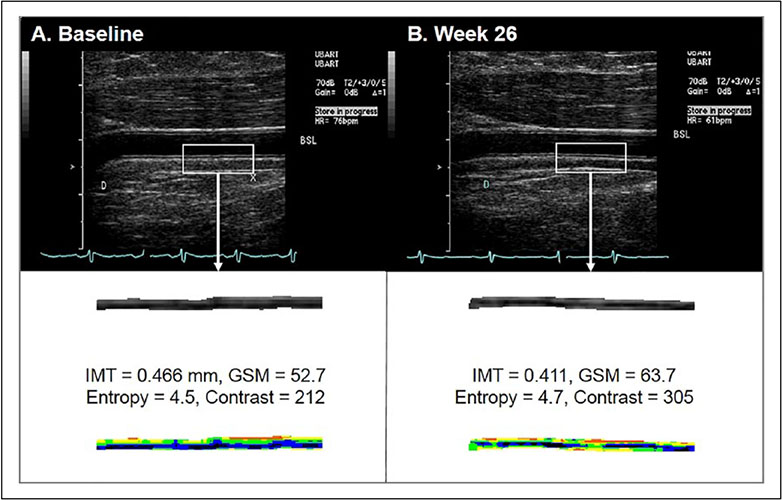
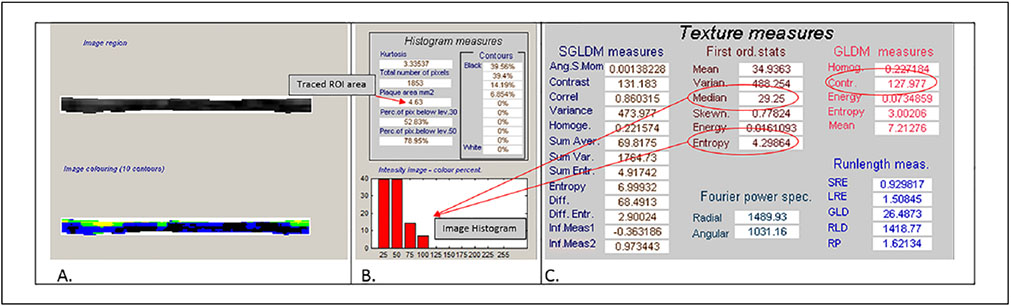
DICOM images of the proximal BA at end-diastole while participants were resting (prior to induction of hyperemia) were converted to bitmaps for IMT measurement and grayscale analysis with LifeQ Medical software (Nicosia, Cyprus) (Figures 2-5).28 Images were normalized by assigning the blackest area of the blood a grayscale value of 0 and the whitest area of the middle two-fourths of the adventitia a grayscale value of 190, then were standardized to a uniform pixel density of 20 mm.11,17,28,29 A reproducible 10 mm segment of the BA far wall that was present in both scans within and between visits was segmented by placing the caliper on the blood–intima and media–adventitia interfaces. From this segmentation process, the area (mm2) and grayscale texture features were measured. BA IMT was calculated by dividing the area by 10 mm (Figure 2). The first order grayscale texture features extracted for this study were the GSM (a measure of overall echogenicity) and entropy (a measure of randomness of the pixels in the far wall of the BA) based on measures from the image histogram (Figures 3-5).17,28 The texture feature contrast was extracted using the GLDS method, which describes differences in grayscale values between pixels at different distances and directions (Figures 3-5).11,28,30
Figure 2.
Measurement technique for grayscale analysis. (A) Brachial artery diameter measurement at baseline using AccessPoint software. (B) Baseline grayscale image bitmap selected for grayscale analysis. The white rectangles demonstrate the distance of 1.0 cm on the vertical scale, and the 1.0 cm segment of the far wall of the brachial artery to the segment for grayscale analysis. Note the same segment was used for measurement of diameter and grayscale analysis across all visits. (C) The cropped grayscale and colorized segmented brachial arterial wall.
Figure 5.
Representative brachial artery B-mode images (upper frames) with extracted segment images and values below: (A) baseline, week 0; (B) after 26 weeks of treadmill exercise intervention. The lower frames demonstrate extracted and traced segments that were used for measurement of IMT and grayscale analysis with colorization below.
GSM, grayscale median; IMT, intima–media thickness.
Figure 3.
Cropped images from the grayscale LifeQ Medical software features extraction mode. (A) Cropped grayscale and colorized arterial wall. (B) Histogram measures and image histogram data for this brachial artery segment. The area for this tracing is 4.63 mm2, the IMT is 0.463 mm. (C) The grayscale median value is 29.25 and the entropy value is 4.30. The gray-level difference statistic method contrast measure is 127.977 units.
Measurement reproducibility
For BA IMT and grayscale measures, a single reader (JB) measured images from 24 participants twice, blinded to the first measurement. Intra-class correlation coefficients for IMT = 0.88, GSM = 0.96, GLDS-CON = 0.87, and entropy = 0.90 were excellent. The intra-class correlation coefficient for BA diameter also was excellent (0.99) as measured blindly from a subset of 10 participants by a different single reader (JMW).
Ankle–brachial index and walking outcomes
These have been described previously in the main PROPEL outcomes paper.2 For ABI testing, a handheld Doppler probe (Nicolet Vascular Pocket-Dop II; Golden, CO, USA) was used to measure systolic blood pressures after the participant had rested supine for 5 minutes. Pressures were measured in the right brachial, dorsalis pedis, and posterior tibial arteries and left dorsalis pedis, posterior tibial, and brachial arteries and repeated. The ABI was calculated by dividing the average pressures in each leg by the average of the four brachial pressures.2,31 For the 6-minute walk test, participants walked up and down a 100-foot (30.5 meters) hallway for 6 minutes after instructions to cover as much distance as possible. The distance completed after 6 minutes was recorded.2,25 Maximal treadmill walking distance was assessed by the Gardner–Skinner protocol.2,27
Statistical analysis
All analyses were performed using XLSTAT 2017: Data Analysis and Statistical Solution for Microsoft Excel (Addinsoft, Paris, France) and SigmaPlot 13.0 (Systat Software, San Jose, CA, USA). Baseline participant characteristics were described as means (SD) overall and by treatment group, focusing on the exercise or control intervention. Differences were tested with t-tests for continuous measures or chi-squared tests for binary and categorical measures with significance set at p < 0.05. Pair-wise Pearson correlation coefficients were calculated between baseline measures and between their 26-week changes. For the primary outcome of changes in BA IMT and the three grayscale measures (GSM, entropy, and GLDS-CON) after 26 weeks, we compared within-group changes using paired t-tests and between-group changes using linear regression by group. Similar analyses were performed for GM-CSF and placebo. Baseline analyses required measureable BA parameters at baseline. The complete case analysis was performed by deleting observations with missing outcomes at either baseline or follow-up visit.
Results
Participant characteristics
Participants in this study were (mean (SD)) 66.7 (8.2) years old, 61% were males, 67% were African-American, and they had an ABI of 0.70 (0.18) (Table 2). Comorbidities included diabetes mellitus in 37%, hypertension in 84%, history of myocardial infarction in 24%, and stroke history in 15%; 54% were current smokers, 33% were previous smokers, and 13% never smoked cigarettes regularly. At baseline, there were no significant differences between participants randomized to the exercise or control interventions in these parameters or in any of the BA measures (Table 2).
Table 2.
Baseline participant characteristics.
| All n = 184 |
Control n = 91 |
Exercise n = 93 |
Between- groupa |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean / n (%) | SD | Mean / n (%) | SD | Mean / n (%) | SD | p-value | ||
| Age, years | 66.7 | 8.2 | 66.3 | 7.6 | 67.1 | 8.9 | 0.489 | |
| Male, n (%) | 113 (61) | 60 (66) | 53 (57) | 0.271 | ||||
| Race, n (%) | 0.628 | |||||||
| African American | 123 (67) | 63 (69) | 60 (65) | |||||
| White | 56 (30) | 24 (26) | 32 (34) | |||||
| Asian | 2 (> 1) | 1 (> 1) | 1 (1) | |||||
| Native Hawaiian/Pacific Islander | 2 (> 1) | 2 (> 2) | 0 (0) | |||||
| Heart rate, bpm | 65.7 | 10.8 | 65.7 | 10.5 | 65.7 | 11.0 | 0.969 | |
| Systolic blood pressure, mmHg | 141.1 | 17.3 | 141.6 | 17.3 | 140.5 | 17.4 | 0.671 | |
| Diastolic blood pressure, mmHg | 78.0 | 9.8 | 78.2 | 8.4 | 77.8 | 11.1 | 0.788 | |
| Body mass index, kg/m2 | 30.8 | 6.5 | 30.9 | 6.9 | 30.7 | 6.2 | 0.864 | |
| Ankle–brachial index | 0.70 | 0.18 | 0.70 | 0.18 | 0.69 | 0.19 | 0.617 | |
| Total treadmill time, minutes | 7.67 | 4.62 | 8.03 | 4.12 | 7.31 | 5.07 | 0.297 | |
| 6-minute walk test, meters | 342.9 | 99.4 | 345.2 | 95.9 | 340.8 | 103.2 | 0.765 | |
| Brachial artery measures | ||||||||
| FMD, % | 5.9 | 3.6 | 6.1 | 3.3 | 5.6 | 3.8 | 0.386 | |
| Diameter, mm | 4.4 | 0.7 | 4.4 | 0.7 | 4.4 | 0.8 | 0.553 | |
| Resting blood flow, mL/min | 148.4 | 70.8 | 145.8 | 70.6 | 150.9 | 71.3 | 0.627 | |
| IMT, mm | 0.34 | 0.06 | 0.34 | 0.06 | 0.34 | 0.07 | 0.934 | |
| GSM, units | 78.1 | 16.3 | 78.0 | 15.3 | 78.2 | 17.3 | 0.959 | |
| Entropy, units | 4.32 | 0.24 | 4.31 | 0.24 | 4.33 | 0.24 | 0.773 | |
| GLDS-CON, units | 179.1 | 90.6 | 185.6 | 94.6 | 172.8 | 86.5 | 0.339 | |
The p-value from t-test or chi-squared test.
FMD, flow-mediated vasodilation; GLDS-CON, gray-level difference statistic-contrast; GSM, grayscale median; IMT, intima–media thickness.
Baseline correlations of brachial artery measures
At baseline, higher values for BA IMT were associated with older age, higher systolic blood pressure, larger BA diameter, resting BA blood flow, BA GLDS-CON, and BA entropy (Table 3). Higher BA IMT was inversely correlated with BA FMD and BA GSM (Table 3). BA IMT was higher in men than in women (0.35 [0.06] vs 0.32 [0.06] mm, p = 0.002) and in participants with diabetes mellitus (0.36 [0.07] vs 0.33 [0.06] mm, p = 0.021). In addition, BA GSM increased with BA GLDS-CON but was inversely related to body mass index, BA diameter, and resting BA blood flow. BA GSM also was lower in men than in women (75.9 [16.6] vs 81.6 [15.4] units, p = 0.021). Additional significant positive correlates of BA GLDS-CON were systolic blood pressure, BA diameter, BA IMT, and BA GSM; GLDS-CON also was higher in participants with diabetes mellitus (200.6 [102.9] vs 166.5 [80.4] units, p = 0.013). Additional significant positive correlates of BA entropy were age, systolic blood pressure, BA diameter, resting BA blood flow, and BA GLDS-CON; BA entropy also was higher in men than in women (4.35 [0.23] vs 4.27 [0.25] units, p = 0.023) and in participants with diabetes mellitus (4.38 [0.23] vs 4.29 [0.24] units, p = 0.017). None of these parameters had a significant correlation with ABI, total treadmill walking time, 6-minute walk test distance, heart rate, or diastolic blood pressure.
Table 3.
Baseline correlations of brachial artery measures (n = 184).
| BA IMT |
BA GSM |
BA entropy |
BA GLDS-CON |
|||||
|---|---|---|---|---|---|---|---|---|
| r | p-value | r | p-value | r | p-value | r | p-value | |
| Age | 0.25 | 0.001 | −0.07 | 0.349 | 0.25 | 0.001 | 0.04 | 0.566 |
| Body mass index | 0.08 | 0.272 | −0.19 | 0.009 | 0.02 | 0.804 | −0.12 | 0.108 |
| Heart rate | −0.10 | 0.162 | 0.11 | 0.134 | −0.12 | 0.104 | 0.01 | 0.942 |
| Systolic blood pressure | 0.18 | 0.016 | −0.11 | 0.145 | 0.20 | 0.006 | 0.17 | 0.024 |
| Diastolic blood pressure | −0.01 | 0.875 | 0.01 | 0.926 | −0.01 | 0.866 | 0.04 | 0.563 |
| Ankle–brachial index | 0.03 | 0.715 | −0.04 | 0.582 | −0.02 | 0.767 | 0.03 | 0.698 |
| 6-minute walk distance | −0.09 | 0.253 | 0.14 | 0.068 | −0.02 | 0.815 | 0.11 | 0.125 |
| Total treadmill time | −0.09 | 0.205 | 0.02 | 0.753 | −0.05 | 0.487 | 0.12 | 0.118 |
| BA FMD | −0.18 | 0.013 | 0.03 | 0.669 | −0.11 | 0.122 | −0.09 | 0.233 |
| BA diameter | 0.48 | < 0.0001 | −0.18 | 0.016 | 0.39 | < 0.0001 | 0.20 | 0.006 |
| BA resting blood flow | 0.26 | < 0.0001 | −0.17 | 0.021 | 0.23 | 0.002 | 0.14 | 0.051 |
| BA IMT | −0.26 | < 0.0001 | 0.55 | < 0.0001 | 0.18 | 0.014 | ||
| BA GSM | −0.06 | 0.400 | 0.22 | 0.002 | ||||
| BA entropy | 0.63 | < 0.0001 | ||||||
Bold type represents p-value <0.05.
BA, brachial artery; FMD, flow-mediated vasodilation; GLDS-CON, gray-level difference statistic-contrast; GSM, grayscale median; IMT, intima–media thickness.
Effects of supervised treadmill exercise
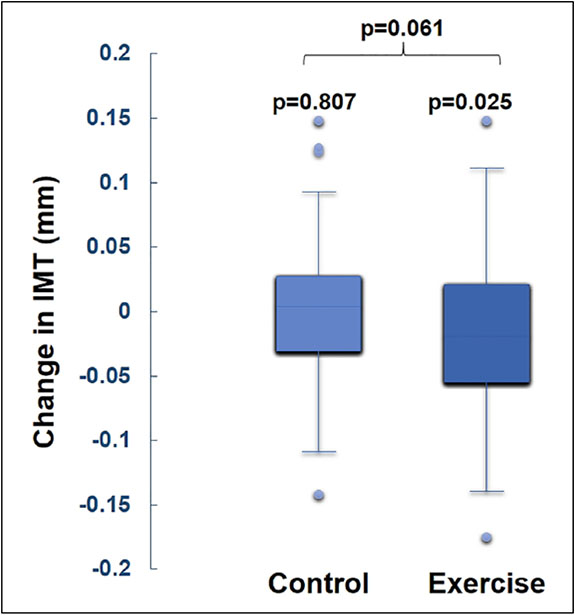
As reported previously, exercise training led to significant improvements in walking performance.2 In this exploratory analysis, exercise training was associated with a 0.01 (0.06) mm (p = 0.025) reduction in BA IMT compared to 0.00 (0.05) mm (p = 0.807) in the control group (between-group p = 0.061; Figure 6 and Table 4). BA IMT did not change significantly in the GM-CSF group (data not shown). Significant changes in BA GSM, entropy, and GLDS-CON were not identified in the exercise group or in the group that received GM-CSF (latter data not shown), but change in the 6-minute walk test in the exercise group was associated with improvements in BA FMD and increases in BA resting blood flow, diameter, entropy, and GLDS-CON (Table 5). Heart rate did not change significantly in any group. Diastolic blood pressure declined by 2.1 (9.2) mmHg (p = 0.039) in the exercise group but this was not different than seen in the control group (between-group p = 0.486). A similar reduction was observed for systolic blood pressure in the control group.
Figure 6.
Changes in brachial artery IMT after 26 weeks by intervention group.
IMT, intima–media thickness.
Table 4.
Within- and between-group changes from baseline after 26 weeks.
| All n = 182 |
Control n = 90 |
Exercise n = 92 |
Between groupa |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | Mean | SD | p-value | p-value | |
| 6-minute walk distance, m | 13.26 | 67.6 | −4.7 | 64.7 | 0.494 | 31.0 | 65.9 | < 0.0001 | 0.0002 |
| Total treadmill time, min | 2.5 | 4.7 | 0.63 | 3.2 | 0.062 | 4.0 | 5.7 | < 0.0001 | < 0.0001 |
| Heart rate, bpm | −1.0 | 13.3 | −0.3 | 13.4 | 0.712 | −1.7 | 13.2 | 0.483 | 0.407 |
| Systolic blood pressure, mmHg | −1.8 | 15.0 | −2.9 | 14.3 | 0.039 | −0.6 | 15.5 | 0.728 | 0.325 |
| Diastolic blood pressure, mmHg | −1.7 | 8.1 | −1.4 | 7.0 | 0.052 | −2.1 | 9.2 | 0.039 | 0.486 |
| BA FMD % | −0.3 | 2.8 | −0.5 | 2.7 | 0.117 | −0.1 | 2.8 | 0.680 | 0.451 |
| BA diameter, cm | 0.0 | 0.0 | 0.0 | 0.0 | 0.363 | 0.0 | 0.0 | 0.468 | 0.818 |
| BA resting blood flow, mL/min | 0.4 | 60.5 | −3.5 | 62.2 | 0.891 | 4.2 | 59.0 | 0.222 | 0.241 |
| BA IMT, mm | −0.01 | 0.06 | 0.00 | 0.05 | 0.807 | −0.01 | 0.06 | 0.025 | 0.061 |
| BA GSM, unitless | −1.7 | 14.6 | −1.5 | 13.1 | 0.294 | −2.0 | 16.0 | 0.246 | 0.846 |
| BA entropy, unitless | −0.02 | 0.20 | −0.01 | 0.20 | 0.562 | −0.02 | 0.21 | 0.298 | 0.755 |
| BA GLDS-CON, unitless | 1.8 | 99.0 | 1.3 | 101.5 | 0.902 | 2.2 | 97.0 | 0.829 | 0.725 |
Generalized linear model p-value.
Bold type represents p-value < 0.05.
BA, brachial artery; FMD, flow-mediated vasodilation; GLDS-CON, gray-level difference statistic-contrast; GSM, grayscale median; IMT, intima–media thickness.
Table 5.
Correlations between changes in walking outcomes and brachial artery measures in the exercise group (n = 92).
| BA resting blood flow |
BA FMD |
BA diameter |
BA IMT |
BA GSM |
BA entropy |
BA GLDS-CON |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| 6-minute walk distance | 0.23 | 0.032 | 0.24 | 0.022 | 0.29 | 0.005 | 0.03 | 0.773 | 0.07 | 0.526 | 0.21 | 0.047 | 0.22 | 0.041 |
| Total treadmill time | −0.09 | 0.409 | 0.11 | 0.305 | 0.13 | 0.207 | −0.06 | 0.579 | −0.01 | 0.957 | −0.02 | 0.861 | 0.03 | 0.753 |
| BA resting blood flow | −0.02 | 0.872 | 0.42 | < 0.001 | −0.02 | 0.865 | 0.12 | 0.277 | 0.06 | 0.566 | 0.01 | 0.905 | ||
| BA FMD | −0.35 | 0.001 | 0.09 | 0.421 | 0.02 | 0.843 | 0.01 | 0.967 | −0.03 | 0.780 | ||||
| BA diameter | 0.12 | 0.272 | 0.01 | 0.988 | 0.14 | 0.194 | 0.18 | 0.095 | ||||||
| BA IMT | −0.14 | 0.197 | 0.14 | 0.199 | −0.12 | 0.266 | ||||||||
| BA GSM | 0.28 | 0.008 | 0.33 | 0.002 | ||||||||||
| BA entropy | 0.56 | < 0.0001 | ||||||||||||
Bold type represents p-value < 0.05.
BA, brachial artery; FMD, flow-mediated vasodilation; GLDS-CON, gray-level difference statistic-contrast; GSM, grayscale median; IMT, intima–media thickness.
Discussion
In this exploratory analysis of data from a randomized clinical trial of treadmill exercise, GM-CSF, or attention control that previously demonstrated the beneficial effects of treadmill exercise on walking performance in people with PAD,2 we explored the effects of treadmill exercise on BA structural measures such as wall thickness (IMT), echogenicity (GSM), and novel measures of grayscale texture (entropy and GLDS-CON) and their associations with CVD risk factors. We believe these measures reflect arterial tissue structure and composition related to biological factors that influence arterial injury and healing. In the carotid arteries, these ultrasound measures appear to be related to CVD risk.8,9,11,12,14-17,29
This report contains several novel imaging findings. First, we described, for the first time, associations of novel BA grayscale measures of echogenicity and grayscale ultrasound texture in people with PAD. Several of the BA parameters were correlated with each other and with CVD risk factors. BA GSM was inversely associated with increasing body mass index and was lower in men than in women, as previously described in the BAs of older adults and carotid arteries of smokers.6,17 Both BA GLDS-CON and entropy were associated with systolic blood pressure and were higher in participants with diabetes mellitus. BA entropy was further associated with increasing age and was higher in men. These findings suggest that grayscale texture features of the brachial arterial wall are associated with CVD risk factors and may represent tissue composition of the arterial wall. Previously, low GSM and GLDS-CON measures of the carotid arterial wall have been associated with CVD risk factors13,32 and CVD events.13,15 We did not observe significant differences between groups in any of the three BA grayscale measures with prescribed walking or GM-CSF compared to placebo; however, we identified significant positive correlations between improvements in walking performance (6-minute walk distance), BA entropy, and BA contrast. In previous work using ultrasound phantoms, we demonstrated that GLDS-CON varies based on scatterer properties of the medium that the sound wave is traveling in.11 The findings we presented above suggest that changes in the GLDS-CON and entropy measures may describe changes in arterial wall composition which alter ultrasound scatterer properties. Further studies that use histopathology are needed to evaluate these measures in actual human arterial tissue.
Second, we demonstrated that 26 weeks of a treadmill walking exercise program was associated with a reduction in BA IMT (within-group p = 0.025); however, the comparison with the placebo intervention did not cross the threshold for statistical significance (between-group p = 0.061). Previous reports suggested that BA IMT reflects CVD injury and risk. In a study of 388 participants in a Japanese screening population, higher BA IMT was associated with CVD risk factors such as increasing age, body mass index, systolic and diastolic blood pressures, glucose levels, and smoking burden, as well as carotid IMT, a strong, independent predictor of CVD events.7,20,33 We identified similar relationships between BA IMT, age, systolic blood pressure, male sex, and diabetes mellitus. Also, in a study of 1016 older adults in Sweden, BA IMT was associated with Framingham risk score and several CVD risk factors which we did not measure in our study.6 These findings suggest that walking exercise, in addition to improving treadmill performance in people with PAD, may reduce CVD risk in as short a time as 26 weeks; however, these findings need to be validated in a larger cohort.
Third, we identified positive relationships between increases in walking distance with improvements in BA endothelial function and BA dilation, as well as a significant reduction in diastolic blood pressure among those assigned to the exercise intervention. These observations suggest that walking exercise improves peripheral arterial function by endothelial adaptations in response to exposure to increased shear stress and cyclic strain,34 possibly via circulating factors released from adipose tissue and skeletal muscle,35,36 and/or endothelial progenitor cells released from the bone marrow and spleen.2-4 Our finding that walking exercise is associated with structural and functional improvements in an upper arm arterial bed reinforces previous findings of the systemic effects of exercise on peripheral arterial function in PAD.2-4 Importantly, changes in BA IMT were not correlated with changes in BA diameter, suggesting that a reduction in BA wall thickness was not simply due to arterial dilation (stretch) and conservation of mass.
Other than improvements in FMD that are known to indicate improved peripheral arterial health and indicate reduced CVD risk,37-40 the clinical implications of the effects of exercise on BA IMT, entropy, and contrast are not known; however, studies that have examined ultrasound features of carotid plaques and histopathology findings of carotid endarterectomy specimens suggest that ultrasound grayscale features are strongly associated with tissue composition. Carotid artery plaques with lower GSM, increased homogeneity, and black areas near the surface of the plaque have been associated with more lipid content, inflammation, and ulceration.29,41,42 Also, common carotid artery and carotid plaque echogenicity change with statin treatment,19,21,22 so it is plausible that changes in grayscale texture features seen in this study (increase in entropy and GLDS-CON) represent changes in arterial wall composition associated with walking exercise.
Study limitations
Our study had limitations. It was an exploratory, post hoc analysis of novel ultrasound measures. Several biomarkers of CVD risk, such as lipid or inflammatory markers, were not measured in the clinical trial, so our power to detect smaller effect sizes, significant relationships between more variable measurements, and to better understand the pathobiology of the observed BA changes we observed was limited. Furthermore, our primary outcome of change in BA IMT was statistically significant in the exercise group, but the p-value for the comparison with the control group did not achieve statistical significance, likely due to the small sample. Also, our study was only 26 weeks long, so a larger effect of walking and/or GM-CSF on BA measures over a longer duration is possible.
Conclusions
In a post hoc analysis of data from a randomized clinical trial, we identified several baseline correlations between CVD risk factors and novel BA ultrasound measures such as IMT, GSM, entropy, and GLDS-CON. BA IMT improved significantly with treadmill exercise training but did not change with attention control; however, the between-group difference did not reach statistical significance. With exercise, improvements in walking distance were associated with improved endothelial function, increased resting blood flow, and BA dilation, as well as higher grayscale entropy and GLDS-CON, indicating that lower extremity exercise is associated with salutary changes in upper extremity arterial wall structure and function. These findings suggest that novel grayscale ultrasound measures are promising tools to describe arterial pathophysiology and associations with cardiovascular disease risk markers and prevention interventions.
Figure 4.
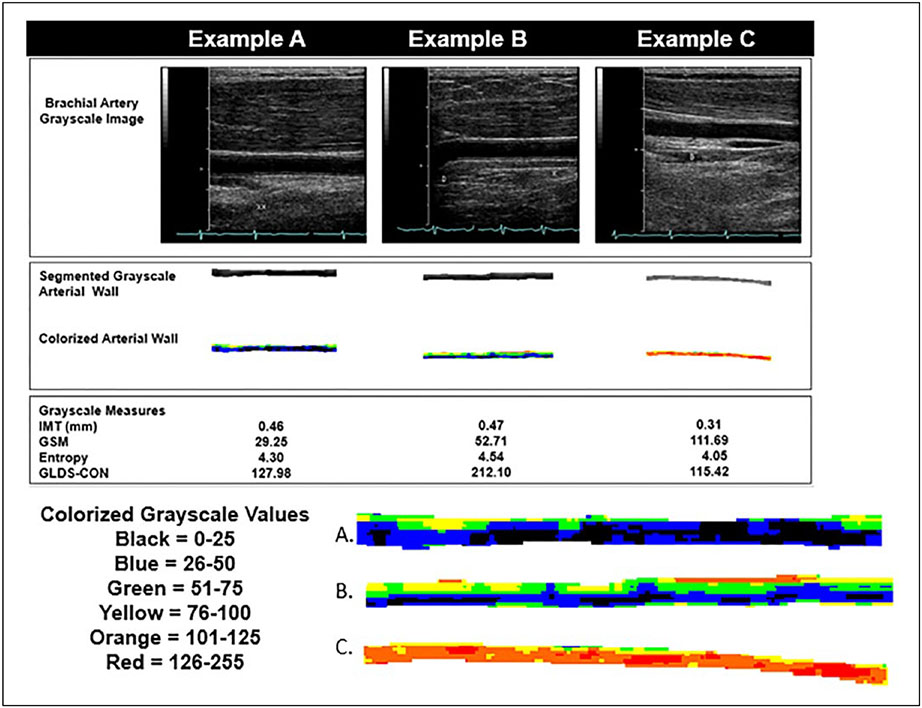
Three examples of brachial artery wall appearances from three participants. (A) The enlarged arterial wall segment demonstrates a wall with a lower grayscale median value. The image histogram would demonstrate more pixels with lower grayscale values. (B) This brachial artery wall segment demonstrates the highest entropy and contrast values of the examples. Note the variation in grayscale values for the colors depicted in the enlarged image of the segmented brachial artery wall. (C) This segmented brachial arterial wall has the highest grayscale median value (predominantly orange and red colorization) and the lowest contrast and entropy of the examples. This is a wall that is more uniform in grayscale values and has less variation in grayscale values. GLDS-CON, gray-level difference statistic-contrast; GSM, grayscale median; IMT, intima–media thickness.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the National Heart, Blood, and Lung Institute (R01-HL107510), intramural support was received from the National Institute on Aging, and from the Jesse Brown VA Medical Center.
Footnotes
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr McDermott reported research funding from Novartis and receipt of study drug for another peripheral artery disease study from Reserveage, Hershey’s, ChromaDex, and ViroMed. No other authors reported conflicts of interest.
References
- 1.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: A randomized controlled trial. JAMA 2009; 301: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDermott MM, Ferrucci L, Tian L, et al. Effect of granulocyte-macrophage colony-stimulating factor with or without supervised exercise on walking performance in patients with peripheral artery disease: The PROPEL randomized clinical trial. JAMA 2017; 318: 2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thijssen DH, Torella D, Hopman MT, et al. The role of endothelial progenitor and cardiac stem cells in the cardiovascular adaptations to age and exercise. Front Biosci 2009; 14: 4685–4702. [DOI] [PubMed] [Google Scholar]
- 4.Laufs U, Werner N, Link A, et al. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 2004; 109: 220–226. [DOI] [PubMed] [Google Scholar]
- 5.Domanchuk K, Ferrucci L, Guralnik JM, et al. Progenitor cell release plus exercise to improve functional performance in peripheral artery disease: The PROPEL study. Contemp Clin Trials 2013; 36: 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lind L, Andersson J, Ronn M, et al. Brachial artery intima-media thickness and echogenicity in relation to lipids and markers of oxidative stress in elderly subjects:-The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Lipids 2008; 43: 133–141. [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto Y, Maruhashi T, Fujii Y, et al. Intima-media thickness of brachial artery, vascular function, and cardiovascular risk factors. Arterioscler Thromb Vasc Biol 2012; 32: 2295–2303. [DOI] [PubMed] [Google Scholar]
- 8.Christodoulos IC, Kyriacoou E, Pattichis MS, et al. Plaque feature extraction. In: Nicolaides A, Beach KW, Kyriacou E, et al. (eds) Ultrasound and carotid bifurcation atherosclerosis. London: Springer, 2012, pp. 233–246. [Google Scholar]
- 9.Griffin M, Kyriacou E, Kakkos SK, et al. Image normalization, plaque typing, and texture feature extraction. In: Nicolaides A, Beach KW, Kyriacou E, et al. (eds) Ultrasound and carotid bifurction atherosclerosis. London: Springer, 2012, pp. 193–211. [Google Scholar]
- 10.Hall-Beyer M GLMC texture: A tutorial v. 3.0. http://hdl.handle.net/1880/51900 (March 2017, accessed 4 April 2017).
- 11.Mitchell CC, Korcarz CE, Tattersall MC, et al. Carotid artery ultrasound texture, cardiovascular risk factors, and subclinical arterial disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Br J Radiol 2018; 91: 20170637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loizou CP, Georgiou N, Griffin M, et al. Texture analysis of the media-layer of the left and right common carotid artery. IEEE EMBS Int Conf Biomed Health Inform 2014; 684–687. [Google Scholar]
- 13.Mitchell C, Korcarz CE, Gepner AD, et al. Carotid artery echolucency, texture features, and incident cardiovascular disease events: The Multi-Ethnic Study of Atherosclerosis (MESA). (Under peer review, 2018) [Google Scholar]
- 14.Loizou CP, Pantziaris M, Pattichis MS, et al. Ultrasound image texture analysis of the intima and media layers of the common carotid artery and its correlation with age and gender. Comput Med Imaging Graph 2009; 33: 317–324. [DOI] [PubMed] [Google Scholar]
- 15.Wohlin M, Sundstrom J, Andren B, et al. An echolucent carotid artery intima-media complex is a new and independent predictor of mortality in an elderly male cohort. Atherosclerosis 2009; 205: 486–491. [DOI] [PubMed] [Google Scholar]
- 16.Andersson J, Sundstrom J, Gustavsson T, et al. Echogenecity of the carotid intima-media complex is related to cardiovascular risk factors, dyslipidemia, oxidative stress and inflammation: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. Atherosclerosis 2009; 204: 612–618. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell C, Piper ME, Korcarz CE, et al. Echogenicity of the carotid arterial wall in active smokers. J Diag Med Sonogr 2018; 34: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crouse JR III, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: The METEOR trial. JAMA 2007; 297: 1344–1353. [DOI] [PubMed] [Google Scholar]
- 19.Lind L, Peters SA, den Ruijter HM, et al. Effect of rosuvastatin on the echolucency of the common carotid intima-media in low-risk individuals: The METEOR trial. J Am Soc Echocardiogr 2012; 25: 1120–1127.e1121. [DOI] [PubMed] [Google Scholar]
- 20.Espeland MA, O’Leary DH, Terry JG, et al. Carotid intimalmedia thickness as a surrogate for cardiovascular disease events in trials of HMG-CoA reductase inhibitors. Curr Control Trials Cardiovasc Med 2005; 6: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadoglou NP, Gerasimidis T, Moumtzouoglou A, et al. Intensive lipid-lowering therapy ameliorates novel calcification markers and GSM score in patients with carotid stenosis. Eur J Vasc Endovasc Surg 2008; 35: 661–668. [DOI] [PubMed] [Google Scholar]
- 22.Della-Morte D, Moussa I, Elkind MS, et al. The short-term effect of atorvastatin on carotid plaque morphology assessed by computer-assisted gray-scale densitometry: A pilot study. Neurol Res 2011; 33: 991–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amirhamzeh MM, Chant HJ, Rees JL, et al. A comparative study of treadmill tests and heel raising exercise for peripheral arterial disease. Eur J Vasc Endovasc Surg 1997; 13: 301–305. [DOI] [PubMed] [Google Scholar]
- 24.McDermott MM, Guralnik JM, Criqui MH, et al. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014; 130: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: A randomized clinical trial. JAMA 2013; 310: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006; 54: 743–749. [DOI] [PubMed] [Google Scholar]
- 27.Gardner AW, Skinner JS, Cantwell BW, et al. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc 1991; 23: 402–408. [PubMed] [Google Scholar]
- 28.LifeQ Medical Ltd. Carotid Plaque Texture Analysis Research Software for Ultrasonic Arterial Wall and Atherosclerotic Plaques Measurements. Operation manual [computer program], Version 4.5. Nicosia, Cyprus, 2013. [Google Scholar]
- 29.Mitchell CC, Stein JH, Cook TD, et al. Histopathologic validation of grayscale carotid plaque characteristics related to plaque vulnerability. Ultrasound Med Biol 2017; 43: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skorton DJ, Collins SM, Nichols J, et al. Quantitative texture analysis in two-dimensional echocardiography: Application to the diagnosis of experimental myocardial contusion. Circulation 1983; 68: 217–223. [DOI] [PubMed] [Google Scholar]
- 31.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation 2012; 126: 2890–2909. [DOI] [PubMed] [Google Scholar]
- 32.Peters SA, Lind L, Palmer MK, et al. Increased age, high body mass index and low HDL-C levels are related to an echolucent carotid intima-media: The METEOR study. J Intern Med 2012; 272: 257–266. [DOI] [PubMed] [Google Scholar]
- 33.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc of Echocardiogr 2008; 21: 93–111; quiz 189–190. [DOI] [PubMed] [Google Scholar]
- 34.Tinken TM, Thijssen DH, Hopkins N, et al. Impact of shear rate modulation on vascular function in humans. Hypertension 2009; 54: 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Company JM, Booth FW, Laughlin MH, et al. Epicardial fat gene expression after aerobic exercise training in pigs with coronary atherosclerosis: Relationship to visceral and subcutaneous fat. J Appl Physiol (1985) 2010; 109: 1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev 2008; 88: 1379–1406. [DOI] [PubMed] [Google Scholar]
- 37.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007; 115: 1285–1295. [DOI] [PubMed] [Google Scholar]
- 38.Gokce N, Keaney JF Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 2003; 41: 1769–1775. [DOI] [PubMed] [Google Scholar]
- 39.Modena MG, Bonetti L, Coppi F, et al. Prognostic role of reversible endothelial dysfunction in hypertensive post-menopausal women. J Am Coll Cardiol 2002; 40: 505–510. [DOI] [PubMed] [Google Scholar]
- 40.Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol 2000; 86: 207–210. [DOI] [PubMed] [Google Scholar]
- 41.Doonan RJ, Gorgui J, Veinot JP, et al. Plaque echodensity and textural features are associated with histologic carotid plaque instability. J Vasc Surg 2016; 64: 671–677.e678. [DOI] [PubMed] [Google Scholar]
- 42.Sztajzel R, Momjian S, Momjian-Mayor I, et al. Stratified gray-scale median analysis and color mapping of the carotid plaque: Correlation with endarterectomy specimen histology of 28 patients. Stroke 2005; 36: 741–745. [DOI] [PubMed] [Google Scholar]