Abstract
Background
The standard treatment of hypothyroidism is levothyroxine (LT-4). However, there are several controversies regarding treatment of hypothyroid patients.
Aim
To investigate the Swedish endocrinologists’ use of thyroid hormones in hypothyroid and euthyroid individuals.
Methods
Physician members of the Swedish Endocrine Society (SEF) were invited by e-mail to participate in an online survey investigating this topic.
Results
Out of the eligible 411 members, 116 (28.2%) responded. The majority (98.9%) stated that L-T4 is the treatment of choice. However, around 50% also prescribed liothyronine (L-T3) or a combination of L-T4+L-T3 in their practice. Combination therapy was mostly (78.5%) used in patients with persistent hypothyroid symptoms despite biochemical euthyroidism on L-T4 treatment. Most respondents prescribed L-T4 tablets and did not expect any major changes with alternative formulations such as soft-gel capsules or liquid formulations in situations influencing the bioavailability of L-T4. In euthyroid patients, 49.5% replied that treatment with thyroid hormones was never indicated, while 47.3% would consider L-T4 for euthyroid infertile women with high thyroid peroxidase (TPO) antibody levels.
Conclusion
The treatment of choice for hypothyroidism in Sweden is L-T4 tablets. Combination therapy with L-T4+L-T3 tablets was considered for patients with persistent symptoms despite biochemical euthyroidism. Soft-gel capsules and liquid solutions of L-T4 were infrequently prescribed. Swedish endocrinologists’ deviation from endocrine society guidelines merits further study.
Keywords: levothyroxine, liothyronine, desiccated thyroid extract (DTE), hypothyroidism, euthyroidism, survey, Swedish Endocrine Society
Introduction
Hypothyroidism, overt or subclinical, affects approximately 5% of the adult population (1). The standard treatment for hypothyroidism is L-thyroxine (L-T4) and treatment is monitored by measuring the serum levels of thyroid-stimulating hormone (TSH) (2). Different L-T4 formulations are commercially available, including tablets, soft-gel capsules, and liquid solutions. Soft-gel capsules and liquid solutions were introduced to overcome potential problems due to intolerance and bioavailability, as the latter may be influenced by L-T4 administration together with foods and beverages or certain medications, and by the presence of concurrent gastrointestinal diseases (3, 4).
In Sweden, only L-T4 tablets were widely available at the time of the survey whereas other formulations could only be obtained with an individual application by the physician and approval by the Swedish Medical Products Agency (Läkemedelsverket). As in other countries (5, 6), the number of L-T4 prescriptions has recently increased in Sweden (7). The reason for this seems to be a decreasing TSH threshold for initiating of treatment for hypothyroidism (8–10) and an increase in disease awareness by both physicians and patients.
Approximately 10% of patients treated with L-T4 for hypothyroidism report persisting hypothyroid symptoms despite biochemical euthyroidism (11). Several hypotheses have been proposed to explain this phenomenon; they include individual variation in the ability to transport L-T4 and synthesize triiodothyronine from L-T4 in peripheral tissues of hypothyroid patients treated with L-T4, possibly leading to intracellular hypothyroidism in one or several tissues and incomplete resolution of symptoms [reviewed in (12)]. According to this hypothesis, persistent symptoms could be relieved by adding synthetic triiodothyronine, liothyronine (L-T3). Many unsubstantiated claims of the positive effects of L-T3 treatment are aired online and via patient organizations, resulting in an increasing number of patients requesting treatment with L-T3 (13, 14). As a consequence the L-T3 prescriptions have increased dramatically worldwide, including in Sweden (7). To date, a number of randomized controlled trials have studied the effect of combined L-T4+L-T3 and five systemic reviews/meta-analyses have concluded that the combined treatment is not superior to standard L-T4 treatment with respect to hypothyroid symptoms and quality of life (15–18) or patient preference (19). Data on long-term safety are limited (20, 21). Therefore, European guidelines restrict the recommendation of L-T3 to that of experimental treatment (2). Desiccated thyroid extract (DTE), based on porcine thyroid and containing both L-T4 and L-T3, among other substances, is, in Sweden, only available with an individual license approved by the Swedish Medical Products Agency due to limited scientific evidence for its benefit (22, 23).
Sweden does not have national guidelines for treatment of hypothyroidism but local guidelines generally follow international recommendations and are a result of a collaboration between endocrinologists and general practitioners. Most Swedish patients with hypothyroidism are treated by general practitioners. Patients referred to endocrinologists include those with concomitant other thyroid or endocrine disorders treated by endocrinologists, some pregnant patients or patients preparing for or undergoing treatment for infertility, and complicated cases of hypothyroidism.
This survey is part of an ongoing international initiative referred to as THESIS (Treatment of Hypothyroidism in Europe by Specialists: An International Survey) investigating current attitudes of European endocrinologists towards the treatment of hypothyroidism in 28 countries. To date, the Italian (24), Bulgarian (25), Romanian (26), Polish (27), Spanish (28), and Danish (29) national surveys have been published. The aim was to examine the opinions of members of the Swedish Endocrine Society (Svenska Endokrinologföreningen, SEF) on the treatment of hypothyroid and euthyroid patients with thyroid hormones.
Material and Methods
Survey
We used a web-based survey constructed with Survey Monkey, an open-access platform that provides various questionnaire templates. The survey was developed in English to ensure comparability between respondents from different European countries and consisted of 23 questions, which could be completed in 10-15 minutes. Eight questions on demographic data (A1-A8) were followed by 23 questions on the practice of treating hypothyroid and euthyroid patients (B1-B23). Space for free text was available at the end of the questionnaire (B24). The survey questionnaire is presented in Supplement 1 .
An invitation e-mail containing the description of the study, including an electronic link leading to the questionnaire, was sent to the 411 members of the SEF registered as physicians (nurses, medical students, and other professional categories were excluded) on 19 November 2020, followed by two reminders on 4 December 2020 and 14 January 2021, respectively. The survey closed on 31 January 2021. Anonymized survey responses were collected and electronically stored by the Survey Monkey service. Repeat submissions from the same IP-address were automatically blocked.
Statistical Analysis
Only data from respondents who had completed all questions about demographic data were considered valid for statistical analysis. In all analyses, respondents who did not know the answer to a question were pooled with respondents who did not provide an answer to that question. The Pearson goodness of fit chi2-test was used to compare frequencies between the categorical variables. Pearson chi2-test was used to test if variables in the demographic data (section A) were independent of the outcome in questions in section B. If any variable was not independent of the outcome in any question in section B, a logistic regression analysis was done. A two-sided p-value of <0.05 was considered statistically significant. All analyses were performed using IBM SPSS statistics software version 26 (SPSS Inc., Chicago, IL, USA).
Results
Sample Characteristics
One hundred sixteen (28.2%) of the eligible members of SEF responded and 93 (22.6%) completed all the questions of the survey. The demographic data of the respondents are summarized in Table 1 . All respondents had at least one medical specialty; 106 (91.4%) were endocrinologists.
Table 1.
Characteristics of the 116 respondents.
| N (%) | |
|---|---|
| Gender | |
| Male | 61 (52.6) |
| Female | 55 (47.4) |
| Age | |
| 20-30 | 2 (1.7) |
| 31-40 | 24 (20.7) |
| 41-50 | 35 (30.2) |
| 51-60 | 26 (22.4) |
| 61-70 | 21 (18.1) |
| 70+ | 8 (6.9) |
| Years in medical practice | |
| 0-10 | 10 (8.6) |
| 11-20 | 49 (42.2) |
| 21-30 | 29 (25.0) |
| 31-40 | 15 (12.9) |
| 40+ | 13 (11.2) |
| Specialization* | |
| Endocrinology | 106 (91.4) |
| Internal medicine | 86 (74.1) |
| Other | 4 (3.4) |
| Society mebership* | |
| SEF | 116 (100) |
| ETA | 10 (8.6) |
| ATA | 1 (0.9) |
| Additional national scientific societies | 80 (69.0) |
| Place of practice* | |
| University centre | 55 (47.4) |
| Regional hospital | 58 (50.0) |
| Private clinic | 4 (3.4) |
| General practice | 1 (0.9) |
| Specialist practice | 9 (7.8) |
| Clinically active | |
| Yes | 109 (94.0) |
| No | 7 (6.0) |
| Management of patients with thyroid disease | |
| Daily | 57 (49.1) |
| Weekly | 49 (42.2) |
| Rarely | 10 (8.6) |
| Management of patients with hypothyroidism | |
| >100 patients/year | 18 (15.5) |
| 51-100 patients/year | 34 (29.3) |
| 10-50 patients/year | 57 (49.1) |
| Rarely | 7 (6.0) |
SEF, Swedish Endocrine Society; ETA, European Thyroid Association; ATA, American Thyroid Association.
*Total is >100% as responders were allowed to tick more than one options in the questionnaire.
Treatment of Choice for Hypothyroidism
Concerning the first choice for treatment of hypothyroidism, the vast majority (92; 79.3% corresponding to 98.9% of those who responded to the question) stated that their preference was L-T4. One (0.9%) suggested L-T4 and L-T3 combination therapy, while no responder chose L-T3 monotherapy or DTE. Although combination therapy was not the initial treatment of choice for nearly all respondents, a significant proportion of responders who answered this question used L-T3-containing treatments in their clinical practice for specific indications [48 (51.6%) L-T3, 46 (49.5%) L-T4 + L-T3 combination, 16 (17.2%) DTE].
Use of Different L-T4 Formulations
The majority of those who answered this question indicated that the formulation of L-T4 they recommended was the one dispensed (83, 89.2%), 2 (2.2%) that they had control over the type of L-T4 dispensed but had to justify it to the regulatory authorities. Finally, 5 (5.4%) had no control, and 3 (3.2%) stated that general practitioners mostly chose the dispensed type of L-T4 (L-T4 dispensed as recommended + need to justify to authorities vs. no control + chosen by general practitioners; p<0.001). Table 2 shows the respondents’ preferences for different L-T4 formulations in several clinical scenarios. The majority preferred tablets and did not expect any major effect of switching to a different formulation such as liquid L-T4 or soft-gel capsules, or tablets from a different manufacturer in these situations (p<0.001).
Table 2.
Use of different L-T4 formulations in various clinical situations.
| Question | I expect no major changes with the different formulations, n (%) | Tablets/tablets from another manufacturer, n (%) | Soft-gel capsules, n (%) | Liquid solutions, n (%) |
|---|---|---|---|---|
| B5- Interfering drugs may influence the stability of therapy. Which L-T4 preparation is in your experience least likely to be subject to variable absorption?* | 53 (57.0) | 30 (32.3) | 8 (8.6) | 2 (2.2) |
| B6- Which of the following preparations of L-T4 would you prescribe in case of first diagnosis of hypothyroidism when the patient self-reports intolerance to various foods raising the possibility of celiac disease, malabsorption, lactose intolerance, or intolerance to common excipients?* | 24 (25.5) | 61 (65.6) | 7 (7.5) | 1 (1.1) |
| B7- Which of the following preparations of L-T4 would you prescribe for a patient established on L-T4 who has unexplained poor biochemical control of hypothyroidism?* | 30 (32.3) | 49 (52.7) | 12 (12.9) | 2 (2.2) |
| B8- Which of the following preparations of L-T4 would you prescribe for a patient with poor biochemical control who is unable (due to busy lifestyle) to take L-T4 fasted and separate from food/drink?* | 35 (37.6) | 50 (53.8) | 5 (5.4) | 3 (3.2) |
| B9- Which of the following preparations of L-T4 would you prescribe for a patient established on L-T4 tablets who has good biochemical control of hypothyroidism but continues to have symptoms?* | 66 (71.0) | 23 (24.7) | 4 (4.3) | 0 (0) |
*In all of the above scenarios choices for soft-gel capsules + liquid solutions were significantly fewer than other options (p < 0.001).
Monitoring of the Treatment With Thyroid Hormone
After starting L-T4 treatment, 61 (65.6%) of those who responded to this question recommended checking the TSH after 4-6 weeks and 32 (34.4%) after 8 weeks (4-6 weeks vs. 8 weeks; p=0.003). None suggested control after 2 weeks or relying mostly on clinical evaluation. After switching to a different formulation or changing from one manufacturer’s L-T4 tablets to another’s, 48 (51.6%) would re-check TSH after 4-6 weeks and 34 (36.6%) after 8 weeks (4-6 weeks vs. 8 weeks; p=0.12). Six (6.5%) did not think it was necessary to check TSH if the dosage was the same and five (5.4%) would rely on clinical evaluation only.
Treatment of Euthyroid Patients With Thyroid Hormones
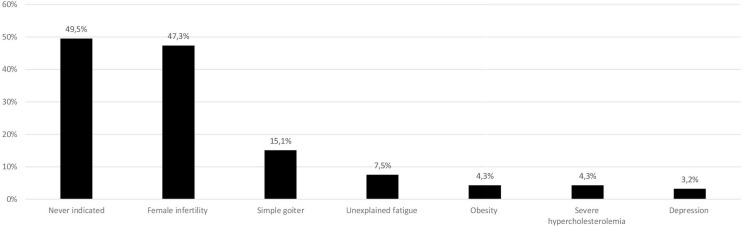
Participants were asked to respond whether thyroid hormones may be indicated in biochemically euthyroid patients with: unexplained fatigue; obesity resistant to life-style interventions; severe hypercholesterolemia, as a complementary treatment; depression resistant to anti-depressant medications; female infertility with high level of thyroid antibodies; simple goiter growing over time; or whether treatment is never indicated for these patients. Of those who answered the question, in biochemically euthyroid patients, 46 (49.5%) indicated that treatment with thyroid hormones was never indicated, 44 (47.3%) would consider treatment in case of female infertility with high levels of thyroid peroxidase (TPO) antibodies, and 14 (15.1%) in case of simple goiter growing over time. Only a minority would consider treatment for unexplained fatigue (7.5%), obesity resistant to lifestyle interventions (4.3%), as a complementary treatment for severe hypercholesterolemia (4.3%), or depression resistant to antidepressants (3.2%) (never use vs. use; p=0.92) ( Figure 1 ).
Figure 1.
Use of thyroid hormones in euthyroid subjects. Total is >100% as responders were allowed to tick more than one options in the questionnaire (data from 93 respondents, 23 did not answer this question). The same 23 individuals did not respond in Figures 1 – 3 .
Use of Combination Therapy With L-T4 and L-T3
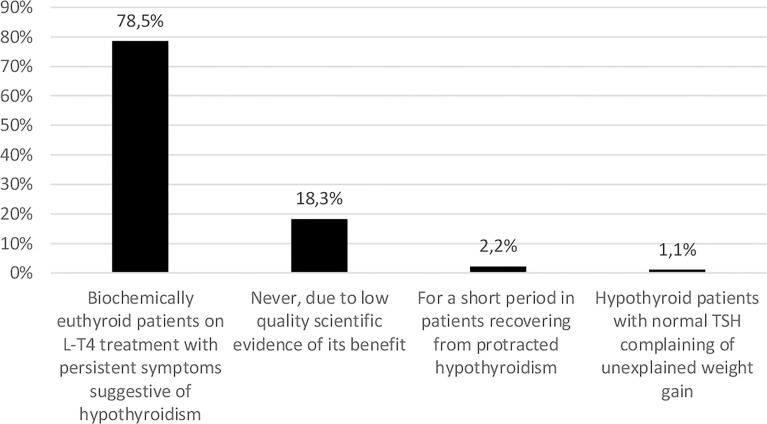
The majority of respondents who answered this question (73; 78.5%) considered combination therapy in biochemically euthyroid patients on L-T4 treatment with persistent symptoms suggestive of hypothyroidism. Seventeen (18.3%) would never use combination therapy due to low quality scientific evidence for benefit. Two (2.2%) would consider combination therapy for a short period in patients recovering from protracted hypothyroidism, while one (1.1%) would suggest it in hypothyroid patients with normal TSH complaining of unexplained weight gain (combination therapy vs. never use; p<0.001). ( Figure 2 ).
Figure 2.
Use of levothyroxine (L-T4) and liothyronine (L-T3) combination therapy in patients with hypothyroidism (data from 93 respondents, 23 did not answer this question). The same 23 individuals did not respond in Figures 1 – 3 .
Persistent Symptoms in L-T4 Treated Patients
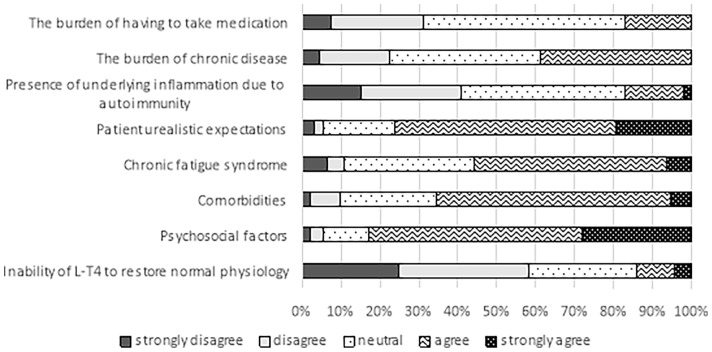
Thirty-four (35.6%) respondents to this question indicated that persistent symptoms despite normal serum TSH were observed in their practice in 6-10% of patients, 22 (23.7%) in 11-30% of patients, 21 (22.6%) in less than 5% of patients, 5 (5.4%) in more than 30% patients, and 11 (11.8%) were not sure ( Table 3 ). As for the trend over the past five years, 54 (58.1%) respondents saw more such cases, 24 (25.8%) experienced no change, three (3.2%) responded fewer, and eleven (11.8%) were not sure ( Table 3 ). Only a minority (14%) responded that persistent symptoms might be due to L-T4’s inability to restore normal physiology while the majority agreed that the cause could be patients’ unrealistic expectations (76.4%), psychosocial factors (82.8%), or comorbidities (65.6%). Many (56%) also suspected chronic fatigue syndrome ( Figure 3 ).
Table 3.
Respondents’ (n = 93) perceptions about persistence of hypothyroid symptoms despite normal serum TSH. 23 did not answer this question.
| Physicians’ perception | Response | N (%) |
|---|---|---|
| Frequency of persistent symptoms despite normal serum TSH | >30% | 5 (5.4) |
| 11-30% | 22 (23.7) | |
| 6-10% | 34 (35.6) | |
| <5% | 21 (22.6) | |
| Not sure | 11 (11.8) | |
| Trend in the past five years | More such cases | 54 (58.1) |
| No change | 24 (25.8) | |
| Fewer | 3 (3.2) | |
| Not sure | 11 (11.8) |
Figure 3.
Possible factors explaining persistent hypothyroid symptoms despite biochemical euthyroidism in patients treated with L-T4 (data from 93 respondents, 23 did not answer this question). The same 23 individuals did not respond in Figures 1 – 3 .
Use of Dietary Supplements
Of those who answered the question, 33 (35.5%) would use dietary supplements such as selenium and iodine upon request from the patient or as a complementary treatment; four (4.3%) would suggest use in case of coexisting autoimmune thyroiditis; none for subclinical hypothyroidism, while 56 (60.2%) answered that they should never be used (use vs. never use; p=0.049).
Endocrinologists With Hypothyroidism
Seven (7.5%) participants had hypothyroidism. One (1.1%) reported excessive tiredness and the same respondent tried DTE treatment, which led to overtreatment. One respondent had tried combination of L-T4 and L-T3 but did not experience any improvement in well-being and switched back to L-T4. Seventy-two (78.3%) of those who answered the question would not consider combination therapy with L-T4 and L-T3 for themselves if they were to develop hypothyroidism, whereas 20 (21.7%) would consider this option (would consider vs. would not consider combination therapy; p<0.001).
Discussion
This study, first of its kind in the Swedish medical literature, summarizes the opinions of 116 Swedish medical specialists, mostly endocrinologists, on different aspects of treatment of hypothyroidism.
Response Rate
The Swedish response rate (28.2%) was slightly lower than the average of the other published national THESIS studies (33%) which has so far varied between 25.8% (Spain) (28) and 54.4% (Poland) (27). The fact that both the invitation email and the reminder were sent at the time of the peaking second wave of the covid-19 pandemic in Sweden offers a possible explanation. Additionally, some of the questions, such as those regarding different L-T4 formulations, might have been considered irrelevant as these formulations are rarely used in Sweden. Moreover, and importantly, most hypothyroid patients in Sweden are treated in the primary care setting.
Treatment of Hypothyroid and Euthyroid Patients With L-T4
In accordance with international guidelines (30, 31) and with the results of all the national THESIS studies published so far (24–29), L-T4 was the treatment of choice for the Swedish endocrinologists. The majority of Swedish endocrinologist preferred L-T4 tablets and did not expect any major effect of switching to different formulations such as soft-gel capsules or liquid L-T4, or tablets from a different manufacturer, in situations such as concomitant ingestion of interfering drugs, intolerance to various foods, unexplained poor biochemical control, poor biochemical control due to inability to take L-T4 separate from food/drink, or persistent symptoms despite good biochemical control. This is in line with the results of the Danish (29), Polish (27), Bulgarian (25), Romanian (26), and Spanish (28) studies but in sharp contrast to the Italian study (24). The vast majority of the Italian endocrinologist would recommend soft-gel capsules or liquid L-T4 solutions in all these scenarios except for “persistent symptoms despite good biochemical control”. This may be because liquid L-T4 and/or soft-gel capsules were not widely available outside of Italy at time of survey. Moreover, most studies on soft-gel capsules have been performed in Italy and the products have been available in the Italian market for a longer time than in other countries, rendering the Italian physicians more familiar with these products. Additionally, the requirement for case-by-case approval by the national medical products agencies possibly makes physicians more reluctant to prescribe these formulations. Finally, the scientific evidence favoring use of soft-gel capsules and liquid preparation over L-T4 tablets needs to be tested in large-scale randomized studies, as recently reviewed by Nagy et al. (4).
Swedish respondents did not recognize unexplained fatigue, obesity, severe hypercholesterolemia, or depression as indications for thyroid hormone treatment, which is in accordance with current guidelines (30, 31). Interestingly, 49.5% reported that treatment with thyroid hormone was never indicated for biochemically euthyroid patients, while almost the same proportion (47.3%) of the Swedish respondents would consider L-T4 treatment in case of high levels of TPO-antibodies and female infertility. Similar proportions of Romanian (36.4%) (26), Italian (37.3%) (24), Danish (42.1%) (29) and Spanish (48.5%) (28) and even a larger proportion of the Polish (63.4%) (27) respondents indicated that they would do likewise. Anti-TPO positivity has been associated with increased risk of miscarriage (32) and adverse obstetrical outcomes (33, 34), however, recent evidence has not supported these findings (35). According to the latest guidelines of the American Thyroid Association (ATA) (36) and guidelines of the Swedish Society of Obstetrics and Gynecology (SFOG) (37), treatment with L-T4 might be considered in euthyroid anti-TPO positive women with TSH within the upper normal reference range (2.5-4.0 mIU/L), especially in those with a history of miscarriage or undergoing fertility treatment. The rationale is to secure euthyroidism in case of pregnancy, although current evidence from randomized trials (38, 39) suggests that L-T4 treatment neither increases fertility rates nor reduces the risk of adverse pregnancy outcomes in these individuals.
Fourteen percent of the respondents recommended L-T4 treatment in euthyroid individuals with simple goiter growing over time, a rate similar to their Danish (12.5%) (29) and Italian (18%) (24) colleagues, while Spanish (21.2%) (28), Romanian (24%) (26), Polish (40.3%) (27), and Bulgarian (55%) (25) physicians would recommend it even more frequently. This practice is against current guidelines, based on modest effects and significant risks associated with TSH suppression, such as osteoporosis (40), cardiovascular morbidity (41), and increased mortality (42). Ongoing use of L-T4 in simple goiter growing over time probably represents the European endocrinologists´ reluctance to abandon old practices despite long-established evidence (43).
Use of Combination Therapy With L-T4 and L-T3
Combination treatment with L-T3 + L-T4 is recommended by neither the European nor the American Guidelines (2, 31) on hypothyroidism, based on insufficient evidence of its superiority over L-T4 treatment and lack of long-term data. According to the European guidelines, L-T4 + L-T3 combination therapy might be considered as a trial in L-T4-treated patients who have persistent complaints despite biochemical euthyroidism after exclusion of other autoimmune diseases and comorbidities (2), the latter being very prevalent in patients with hypothyroidism (44, 45). While the vast majority considered L-T4 the initial treatment of choice for hypothyroidism, 78.5% of the respondents would prescribe L-T4 + L-T3 combination therapy in the presence of persistent symptoms suggestive of hypothyroidism. This number was similar to the results of the Danish (71%) (29) but a lot higher than in the Italian (40%) (24), Romanian (35.9%) (26), and Polish (32.2%) (27) surveys. A survey by the American Thyroid Association revealed similar findings to ours, with a high percentage of physicians willing to prescribe combination therapy under specific circumstances such as persisting hypothyroid symptoms (46). These results suggest that this topic is still highly controversial and balancing patient dissatisfaction and demands and lack of scientific evidence remains a challenge. Well-designed randomized clinical trials in patients with persistent symptoms after exclusion of comorbidities are required to address this issue. Although our recent large register-based study (21) provided reassuring evidence regarding the risk of cancer and mortality in L-T3 treated Swedish patients, there could still be other health risks associated with long-term L-T3 therapy warranting randomized trials examining the safety of the combined treatment. Moreover, the cost-effectiveness of the combined treatment also remains an issue, as it is more expensive than L-T4 treatment alone (47) and usually requires more frequent monitoring.
As to the cause of patients´ dissatisfaction with L-T4 treatment, the majority (86%) stated other factors than the inability of L-T4 to achieve tissue euthyroidism. DTE treatment was not popular among Swedish physicians, probably due to limited scientific evidence (22) and the need of approval by the Swedish Medical Products Agency. Interestingly, while 78.5% of the respondents would prescribe L-T4 + L-T3 combination therapy to their patients in the presence of persistent symptoms suggestive of hypothyroidism, the same proportion (78.3%) would not consider combination therapy for themselves should they develop hypothyroidism. This potential discrepancy can be better explored from the aggregate data of all 28 countries that contributed to THESIS. However, it is of interest that in all national surveys so far published, the proportion of physicians who would consider combination therapy for themselves was lower than for their patients.
Use of Dietary Supplements
More than a half (60.2%) of the respondents suggested that dietary supplements, including selenium and iodine, should never be used. Approximately one third suggested that they could be used upon request from the patient or as a complementary treatment, while only few recommended them in the context of coexisting autoimmune thyroiditis. This result is probably based on insufficient evidence for clinical efficacy of selenium supplementation (48), despite evidence of an effect on TPOAb concentrations (49), and the fact that the Swedish population is considered iodine sufficient from iodine fortification of salt since 1936 (50, 51).
Strengths and Limitations
All of the participants being medical specialists and nearly all of them endocrinologists is a strength, as they represent key opinion leaders. While one can assume that endocrinologists’ practrices may reflect choices made by the physicians in general practice, where the majority of patients are treated, direct evidence supporting this is lacking. The main limitation is the low response rate, which questions whether the conclusions of the study can be considered representative for all Swedish endocrinologists. However, it is likely that most of the non-responders were not clinically active, or did not treat patients with hypothyroidism thus leading to a falsely low response rate.
In conclusion, L-T4 tablets were the primary treatment of choice for hypothyroidism among Swedish endocrinologists. In general, alternative L-T4 formulations, like soft-gel capsules and liquid solutions, were not recommended. The majority would consider L-T3 + L-T4 combination treatment for patients with a diagnosis of hypothyroidism who are biochemically euthyroid on L-T4 and complain of persistent hypothyroid symptoms. In a biochemically euthyroid patient, the only scenario where L-T4 was considered by a significant number of participants was female infertility with high TPO antibody levels. This deviation from endocrine society guidelines merits further study. Overall, the responses of the Swedish endocrinologists did not deviate from the recommendations of the current international guidelines and were very similar to the responses of their Danish colleagues (29). Compared with the other national THESIS studies, country-specific factors such as the availability and cost of various preparations and local traditions most probably influenced the choice of treatment. In the future, the joint analysis of the entire THESIS cohort summarizing data from 28 European countries will provide us with a more universal view of this topic.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
PP, EP, RA, EN, and LH contributed to the study conception, design and creation of the survey. TP and ML were responsible for writing of the invitation letter and reminder. TP was responsible for data analysis. TP wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The first author was supported by grants from Lundgrens stiftelse and SUS fonder. The THESIS study received funding from IBSA, Institute Biochimique SA. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. All authors declare no other competing interests.
Conflict of Interest
EN, EP, PP, and LH have undertaken consultancy work for IBSA.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
IBSA had no role in the design of the survey, data analysis, data presentation, data interpretation or writing the manuscript; the authors did not receive remuneration by IBSA.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all members of the Swedish Endocrine Society who contributed to the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.795111/full#supplementary-material
Abbreviations
THESIS, Treatment of Hypothyroidism in Europe by Specialists: An International Survey.
References
- 1. Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, et al. Global Epidemiology of Hyperthyroidism and Hypothyroidism. Nat Rev Endocrinol (2018) 14(5):301–16. doi: 10.1038/nrendo.2018.18 [DOI] [PubMed] [Google Scholar]
- 2. Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MP. 2012 ETA Guidelines: The Use of L-T4 + L-T3 in the Treatment of Hypothyroidism. Eur Thyroid J (2012) 1(2):55–71. doi: 10.1159/000339444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fallahi P, Ferrari SM, Ruffilli I, Ragusa F, Biricotti M, Materazzi G, et al. Advancements in the Treatment of Hypothyroidism With L-T4 Liquid Formulation or Soft Gel Capsule: An Update. Expert Opin Drug Deliv (2017) 14(5):647–55. doi: 10.1080/17425247.2016.1227782 [DOI] [PubMed] [Google Scholar]
- 4. Nagy EV, Perros P, Papini E, Katko M, Hegedüs L. New Formulations of Levothyroxine in the Treatment of Hypothyroidism: Trick or Treat? Thyroid (2021) 31(2):193–201. doi: 10.1089/thy.2020.0515 [DOI] [PubMed] [Google Scholar]
- 5. Rodriguez-Gutierrez R, Maraka S, Ospina NS, Montori VM, Brito JP. Levothyroxine Overuse: Time for an About Face? Lancet Diabetes Endocrinol (2017) 5(4):246–8. doi: 10.1016/S2213-8587(16)30276-5 [DOI] [PubMed] [Google Scholar]
- 6. Cerqueira C, Knudsen N, Ovesen L, Laurberg P, Perrild H, Rasmussen LB, et al. Doubling in the Use of Thyroid Hormone Replacement Therapy in Denmark: Association to Iodization of Salt? Eur J Epidemiol (2011) 26(8):629–35. doi: 10.1007/s10654-011-9590-5 [DOI] [PubMed] [Google Scholar]
- 7. Socialstyrelsen . (2019). Available at: https://www.socialstyrelsen.se/statistik-och-data/register/alla-register/lakemedelsregistret/.
- 8. Taylor PN, Iqbal A, Minassian C, Sayers A, Draman MS, Greenwood R, et al. Falling Threshold for Treatment of Borderline Elevated Thyrotropin Levels-Balancing Benefits and Risks: Evidence From a Large Community-Based Study. JAMA Intern Med (2014) 174(1):32–9. doi: 10.1001/jamainternmed.2013.11312 [DOI] [PubMed] [Google Scholar]
- 9. Medici BB, Nygaard B, la Cour JL, Grand MK, Siersma V, Nicolaisdottir DR, et al. Changes in Prescription Routines for Treating Hypothyroidism Between 2001 and 2015: An Observational Study of 929,684 Primary Care Patients in Copenhagen. Thyroid (2019) 29(7):910–9. doi: 10.1089/thy.2018.0539 [DOI] [PubMed] [Google Scholar]
- 10. Petersen M, Knudsen N, Carlé A, Andersen S, Jørgensen T, Perrild H, et al. Increased Incidence Rate of Hypothyroidism After Iodine Fortification in Denmark: A 20-Year Prospective Population-Based Study. J Clin Endocrinol Metab (2019) 104(5):1833–40. doi: 10.1210/jc.2018-01993 [DOI] [PubMed] [Google Scholar]
- 11. Peterson SJ, Cappola AR, Castro MR, Dayan CM, Farwell AP, Hennessey JV, et al. An Online Survey of Hypothyroid Patients Demonstrates Prominent Dissatisfaction. Thyroid (2018) 28(6):707–21. doi: 10.1089/thy.2017.0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perros P, van der Feltz-Cornelis C, Papini E, Nagy EV, Weetman AP, Hegedüs L. The Enigma of Persistent Symptoms in Hypothyroid Patients Treated With Levothyroxine: A Narrative Review. Clin Endocrinol (Oxf) (2021) 00:1–8. doi: 10.1111/cen.14473 [DOI] [PubMed] [Google Scholar]
- 13. Michaelsson LF, Medici BB, la Cour JL, Selmer C, Roder M, Perrild H, et al. Treating Hypothyroidism With Thyroxine/Triiodothyronine Combination Therapy in Denmark: Following Guidelines or Following Trends? Eur Thyroid J (2015) 4(3):174–80. doi: 10.1159/000437262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mitchell AL, Hegedus L, Zarkovic M, Hickey JL, Perros P. Patient Satisfaction and Quality of Life in Hypothyroidism: An Online Survey by the British Thyroid Foundation. Clin Endocrinol (Oxf) (2021) 94(3):513–20. doi: 10.1111/cen.14340 [DOI] [PubMed] [Google Scholar]
- 15. Escobar-Morreale HF, Botella-Carretero JI, Escobar del Rey F, Morreale de Escobar G. REVIEW: Treatment of Hypothyroidism With Combinations of Levothyroxine Plus Liothyronine. J Clin Endocrinol Metab (2005) 90(8):4946–54. doi: 10.1210/jc.2005-0184 [DOI] [PubMed] [Google Scholar]
- 16. Grozinsky-Glasberg S, Fraser A, Nahshoni E, Weizman A, Leibovici L. Thyroxine-Triiodothyronine Combination Therapy Versus Thyroxine Monotherapy for Clinical Hypothyroidism: Meta-Analysis of Randomized Controlled Trials. J Clin Endocrinol Metab (2006) 91(7):2592–9. doi: 10.1210/jc.2006-0448 [DOI] [PubMed] [Google Scholar]
- 17. Joffe RT, Brimacombe M, Levitt AJ, Stagnaro-Green A. Treatment of Clinical Hypothyroidism With Thyroxine and Triiodothyronine: A Literature Review and Metaanalysis. Psychosomatics (2007) 48(5):379–84. doi: 10.1176/appi.psy.48.5.379 [DOI] [PubMed] [Google Scholar]
- 18. Ma C, Xie J, Huang X, Wang G, Wang Y, Wang X, et al. Thyroxine Alone or Thyroxine Plus Triiodothyronine Replacement Therapy for Hypothyroidism. Nucl Med Commun (2009) 30(8):586–93. doi: 10.1097/MNM.0b013e32832c79e0 [DOI] [PubMed] [Google Scholar]
- 19. Akirov A, Fazelzad R, Ezzat S, Thabane L, Sawka AM. A Systematic Review and Meta-Analysis of Patient Preferences for Combination Thyroid Hormone Treatment for Hypothyroidism. Front Endocrinol (Lausanne) (2019) 10:477. doi: 10.3389/fendo.2019.00477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leese GP, Soto-Pedre E, Donnelly LA. Liothyronine Use in a 17 Year Observational Population-Based Study - the Tears Study. Clin Endocrinol (Oxf) (2016) 85(6):918–25. doi: 10.1111/cen.13052 [DOI] [PubMed] [Google Scholar]
- 21. Planck T, Hedberg F, Calissendorff J, Nilsson A. Liothyronine Use in Hypothyroidism and its Effects on Cancer and Mortality. Thyroid (2021) 31(5):732–9. doi: 10.1089/thy.2020.0388 [DOI] [PubMed] [Google Scholar]
- 22. Hoang TD, Olsen CH, Mai VQ, Clyde PW, Shakir MK. Desiccated Thyroid Extract Compared With Levothyroxine in the Treatment of Hypothyroidism: A Randomized, Double-Blind, Crossover Study. J Clin Endocrinol Metab (2013) 98(5):1982–90. doi: 10.1210/jc.2012-4107 [DOI] [PubMed] [Google Scholar]
- 23. Shakir MKM, Brooks DI, McAninch EA, Fonseca TL, Mai VQ, Bianco AC, et al. Comparative Effectiveness of Levothyroxine, Desiccated Thyroid Extract, and Levothyroxine+Liothyronine in Hypothyroidism. J Clin Endocrinol Metab (2021) 106(11):e4400–e13. doi: 10.1210/clinem/dgab478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Negro R, Attanasio R, Nagy EV, Papini E, Perros P, Hegedüs L. Use of Thyroid Hormones in Hypothyroid and Euthyroid Patients; the 2019 Italian Survey. Eur Thyroid J (2020) 9(1):25–31. doi: 10.1159/000502057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borissova A-MI, Boyanov M, Attanasio R, Hegedüs L, Nagy E, Negro R, et al. Use of Thyroid Hormones in Hypothyroid and Euthyroid Patients: A THESIS* Questionnaire Survey of Bulgarian Physicians *THESIS: Treatment of Hypothyroidism in Europe by Specialists: An International Survey. Endocrinologia (2020) XXV(4):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Niculescu DA, Attanasio R, Hegedus L, Nagy EV, Negro R, Papini E, et al. Use of Thyroid Hormones in Hypothyroid and Euthyroid Patients: A Thesis* Questionnaire Survey of Romanian Physicians *Thesis: Treatment of Hypothyroidism in Europe by Specialists: An International Survey. Acta Endocrinol (Buchar) (2020) 16(4):462–9. doi: 10.4183/aeb.2020.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bednarczuk T, Attanasio R, Hegedus L, Nagy EV, Negro R, Papini E, et al. Use of Thyroid Hormones in Hypothyroid and Euthyroid Patients: A THESIS* Questionnaire Survey of Polish Physicians. *THESIS: Treatment of Hypothyroidism in Europe by Specialists: An International Survey. Endokrynol Pol (2021) 72(4):357–65. doi: 10.5603/EP.a2021.0048 [DOI] [PubMed] [Google Scholar]
- 28. Galofre JC, Attanasio R, Hegedus L, Nagy E, Negro R, Papini E, et al. Use of Thyroid Hormone in Hypothyroid Patients and Euthyroid Subjects in Spain: A THESIS* Questionnaire Survey. Endocrinol Diabetes Nutr (2021) S2530-0164(21)00190-7. doi: 10.1016/j.endinu.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 29. Riis KR, Frølich JS, Hegedüs L, Negro R, Attanasio R, Nagy EV, et al. Use of Thyroid Hormones in Hypothyroid and Euthyroid Patients: A 2020 THESIS Questionnaire Survey of Members of the Danish Endocrine Society. J Endocrinol Invest (2021) 44(11):2435–44. doi: 10.1007/s40618-021-01555-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical Practice Guidelines for Hypothyroidism in Adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract (2012) 18(6):988–1028. doi: 10.4158/EP12280.GL [DOI] [PubMed] [Google Scholar]
- 31. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the Treatment of Hypothyroidism: Prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid (2014) 24(12):1670–751. doi: 10.1089/thy.2014.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stagnaro-Green A, Roman SH, Cobin RH, El-Harazy E, Alvarez-Marfany M, Davies TF. Detection of At-Risk Pregnancy by Means of Highly Sensitive Assays for Thyroid Autoantibodies. JAMA (1990) 264(11):1422–5. doi: 10.1001/jama.1990.03450110068029 [DOI] [PubMed] [Google Scholar]
- 33. Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Thyroid Antibody Positivity in the First Trimester of Pregnancy Is Associated With Negative Pregnancy Outcomes. J Clin Endocrinol Metab (2011) 96(6):E920–E4. doi: 10.1210/jc.2011-0026 [DOI] [PubMed] [Google Scholar]
- 34. Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association Between Thyroid Autoantibodies and Miscarriage and Preterm Birth: Meta-Analysis of Evidence. BMJ (2011) 342:d2616. doi: 10.1136/bmj.d2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Plowden TC, Schisterman EF, Sjaarda LA, Zarek SM, Perkins NJ, Silver R, et al. Subclinical Hypothyroidism and Thyroid Autoimmunity Are Not Associated With Fecundity, Pregnancy Loss, or Live Birth. J Clin Endocrinol Metab (2016) 101(6):2358–65. doi: 10.1210/jc.2016-1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid (2017) 27(3):315–89. doi: 10.1089/thy.2016.0457 [DOI] [PubMed] [Google Scholar]
- 37. SFOG . (2019). Available at: https://www.sfog.se/start/raadriktlinjer/sfog-raad-obstetrik/antenatalt/ (Accessed October 2, 2021).
- 38. Dhillon-Smith RK, Middleton LJ, Sunner KK, Cheed V, Baker K, Farrell-Carver S, et al. Levothyroxine in Women With Thyroid Peroxidase Antibodies Before Conception. N Engl J Med (2019) 380(14):1316–25. doi: 10.1056/NEJMoa1812537 [DOI] [PubMed] [Google Scholar]
- 39. Wang X, Zhang Y, Tan H, Bai Y, Zhou L, Fang F, et al. Effect of Levothyroxine on Pregnancy Outcomes in Women With Thyroid Autoimmunity: A Systematic Review With Meta-Analysis of Randomized Controlled Trials. Fertil Steril (2020) 114(6):1306–14. doi: 10.1016/j.fertnstert.2020.06.034 [DOI] [PubMed] [Google Scholar]
- 40. Abrahamsen B, Jorgensen HL, Laulund AS, Nybo M, Bauer DC, Brix TH, et al. The Excess Risk of Major Osteoporotic Fractures in Hypothyroidism is Driven by Cumulative Hyperthyroid as Opposed to Hypothyroid Time: An Observational Register-Based Time-Resolved Cohort Analysis. J Bone Miner Res (2015) 30(5):898–905. doi: 10.1002/jbmr.2416 [DOI] [PubMed] [Google Scholar]
- 41. Lillevang-Johansen M, Abrahamsen B, Jorgensen HL, Brix TH, Hegedus L. Duration of Hyperthyroidism and Lack of Sufficient Treatment Are Associated With Increased Cardiovascular Risk. Thyroid (2019) 29(3):332–40. doi: 10.1089/thy.2018.0320 [DOI] [PubMed] [Google Scholar]
- 42. Thvilum M, Brandt F, Almind D, Christensen K, Hegedus L, Brix TH. Excess Mortality in Patients Diagnosed With Hypothyroidism: A Nationwide Cohort Study of Singletons and Twins. J Clin Endocrinol Metab (2013) 98(3):1069–75. doi: 10.1210/jc.2012-3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hegedus L, Bonnema SJ, Bennedbaek FN. Management of Simple Nodular Goiter: Current Status and Future Perspectives. Endocr Rev (2003) 24(1):102–32. doi: 10.1210/er.2002-0016 [DOI] [PubMed] [Google Scholar]
- 44. Thvilum M, Brandt F, Almind D, Christensen K, Brix TH, Hegedus L. Type and Extent of Somatic Morbidity Before and After the Diagnosis of Hypothyroidism. A Nationwide Register Study. PloS One (2013) 8(9):e75789. doi: 10.1371/journal.pone.0075789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thvilum M, Brandt F, Almind D, Christensen K, Brix TH, Hegedus L. Increased Psychiatric Morbidity Before and After the Diagnosis of Hypothyroidism: A Nationwide Register Study. Thyroid (2014) 24(5):802–8. doi: 10.1089/thy.2013.0555 [DOI] [PubMed] [Google Scholar]
- 46. Jonklaas J, Tefera E, Shara N. Physician Choice of Hypothyroidism Therapy: Influence of Patient Characteristics. Thyroid (2018) 28(11):1416–24. doi: 10.1089/thy.2018.0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taylor PN, Razvi S, Muller I, Wass J, Dayan CM, Chatterjee K, et al. Liothyronine Cost and Prescriptions in England. Lancet Diabetes Endocrinol (2019) 7(1):11–2. doi: 10.1016/S2213-8587(18)30334-6 [DOI] [PubMed] [Google Scholar]
- 48. Winther KH, Rayman MP, Bonnema SJ, Hegedüs L. Selenium in Thyroid Disorders - Essential Knowledge for Clinicians. Nat Rev Endocrinol (2020) 16(3):165–76. doi: 10.1038/s41574-019-0311-6 [DOI] [PubMed] [Google Scholar]
- 49. Wichman J, Winther KH, Bonnema SJ, Hegedus L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients With Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid (2016) 26(12):1681–92. doi: 10.1089/thy.2016.0256 [DOI] [PubMed] [Google Scholar]
- 50. Andersson M, Berg G, Eggertsen R, Filipsson H, Gramatkovski E, Hansson M, et al. Adequate Iodine Nutrition in Sweden: A Cross-Sectional National Study of Urinary Iodine Concentration in School-Age Children. Eur J Clin Nutr (2009) 63(7):828–34. doi: 10.1038/ejcn.2008.46 [DOI] [PubMed] [Google Scholar]
- 51. Nyström HF, Brantsæter AL, Erlund I, Gunnarsdottir I, Hulthén L, Laurberg P, et al. Iodine Status in the Nordic Countries - Past and Present. Food Nutr Res (2016) 60:31969. doi: 10.3402/fnr.v60.31969 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.