Abstract
Recruitment of modifiers and remodelers to specific DNA sites within chromatin plays a critical role in controlling gene expression. The study of globin gene regulation provides a convergence point within which to address these issues in the context of tissue-specific and developmentally regulated expression. In this regard, erythroid Krüppel-like factor (EKLF) is critical. EKLF is a red cell-specific activator whose presence is crucial for establishment of the correct chromatin structure and high-level transcriptional induction of adult β-globin. We now find, by metabolic labeling-immunoprecipitation experiments, that EKLF is acetylated in the erythroid cell. EKLF residues acetylated by CREB binding protein (CBP) in vitro map to Lys-288 in its transactivation domain and Lys-302 in its zinc finger domain. Although site-specific DNA binding by EKLF is unaffected by the acetylation status of either of these lysines, directed mutagenesis of Lys-288 (but not Lys-302) decreases the ability of EKLF to transactivate the β-globin promoter in vivo and renders it unable to be superactivated by coexpressed p300 or CBP. In addition, the acetyltransferase function of CBP or p300 is required for superactivation of wild-type EKLF. Finally, acetylated EKLF has a higher affinity for the SWI-SNF chromatin remodeling complex and is a more potent transcriptional activator of chromatin-assembled templates in vitro. These results demonstrate that the acetylation status of EKLF is critical for its optimal activity and suggest a mechanism by which EKLF acts as an integrator of remodeling and transcriptional components to alter chromatin structure and induce adult β-globin expression within the β-like globin cluster.
Recent advances in reconstructing transcriptional regulatory events have relied on biochemical and genetic studies that identified the basal transcription machinery and its activators, along with functional studies that delineated how these molecules work together to activate transcription, both on naked DNA and on DNA packaged into chromatin. A major insight into this mechanism has been that the dynamic range of transcription is greatly accentuated by the use of chromatinized templates, which are fully repressed compared to naked DNA, and that optimal induction begins from this repressed state rather than from the basal (or ground) state observed on naked DNA (7, 35, 66, 73). It is within this system that chromatin modifiers and remodelers play a critical role (36, 80). Chromatin modifiers acetylate (e.g., CREB binding protein [CBP], p300, P/CAF) or deacetylate (e.g., histone deacetylases) histones at specific lysines within their amino termini, resulting in altered DNA binding affinities and a looser or tighter chromatin structure (15, 67, 80). Chromatin remodelers are multiprotein complexes (e.g., SWI-SNF and NURF) that utilize the energy from ATP hydrolysis to reorganize chromatin to a more open and accessible structure and do not covalently modify histones in the process (68, 70, 74). Transcriptional activators or repressors may play an active role in recruiting these activities to discrete sites when needed to induce or shut off adjacent gene expression (49, 76).
However, modification of histones is not the only way that modifiers exerts their effect on transcription, as an ever-growing number of transcription factors are also substrates for acetylation by some of these same proteins (4). The effects of these modifications are only beginning to be understood, but they appear able to alter site-specific DNA binding and protein-protein properties, providing yet another potential level of cellular control upon genetic expression in addition to protein phosphorylation.
In this context, regulation of the β-like globin cluster provides an extremely fertile paradigm within which to study the role of chromatin in gene regulation. The details of how transcriptional, tissue-specific, and developmental control of globin gene expression occurs has followed from convergence of genetic studies of β-thalassemias, structural analyses of chromatin within and surrounding the β locus, and molecular studies that identified the major players required for its erythroid-specific expression and the sequences to which they bind (3, 11, 20, 21, 52, 64, 69, 71).
However, whether the erythroid-specific transcription factors play any role in forming or maintaining the higher-order chromatin structure known to form at the the β-like globin locus is only beginning to be understood. Of particular interest in this regard is erythroid Krüppel-like factor (EKLF or KLF1) (47). EKLF is a red cell-specific transcriptional activator that is critical for switching on high-level adult β-globin expression during erythroid ontogeny (reviewed in references 5 and 55). It accomplishes this by binding, via its three C2H2 zinc fingers, to the CACCC element located at position −90 of the β-globin promoter (18, 22). Genetic studies reveal that the absence of EKLF leads to embryonic death at the time of the switch due to a profound β-thalassemia (43, 50, 57). In addition, analysis of compound transgenic embryos show that fetal γ-globin transcripts persist beyond their normal shutoff and are expressed at a level fivefold higher than in the presence of EKLF, indicating that EKLF plays a role in completion of the fetal-to-adult globin transition (56, 78).
Absence of EKLF also leads to alteration of the chromatin structure at the β-like globin locus, as the DNase-hypersensitive site at the adult β-globin promoter was lost, and hypersensitive site 3 within the distal upstream locus control region was diminished (23, 78). A potential mechanism to account for these effects was revealed by two sets of experiments. First, EKLF was shown to associate with p300 and CBP (81). p300 and CBP are coactivators that utilize multiple mechanisms to increase transcription, including acting as bridging molecules between activators and the basal transcription machinery (29), and utilizing their associated histone acetyltransferase (HAT) activity to modify histones and disrupt higher-order chromatin structure (79). EKLF interaction with these coactivators led to its own acetylation and to an enhancement of EKLF transcriptional activity at the β-globin promoter. Second, EKLF, along with an MEL cell-derived protein complex called E-RC1, were both required for formation of a DNase-hypersensitive site and transcription at the β-globin gene in an in vitro chromatin assembly-transcription system (2, 33). Purification of E-RC1 revealed that it is enriched for the mammalian homologues of the SWI-SNF chromatin remodeling family (36, 68, 74, 80). As a result, EKLF action is associated with both chromatin modifiers and remodelers, providing a means to explain the biological effects of its absence upon chromatin structure and transcription.
It is with this background in mind that we determined, more precisely, the role that HAT coactivators play in EKLF activity by mapping the acetylation sites and by determining the effects that these modifications have upon EKLF DNA binding and transcriptional activity at the β-globin promoter in vitro and in the erythroid cell. These results have general implications for the linkage between the action of modifiers and remodelers in chromatin.
MATERIALS AND METHODS
Cell culture and manipulations.
K562 cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum. MEL, MEL-derived M4D3 and COS7 cells were maintained in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum. Transfections, immunoprecipitations, Western blot analysis, and HAT immunoprecipitation assays were as previously described (81).
Antibodies.
Anti-CBP rabbit polyclonal antibody raised against amino acids (aa) 1736 to 2179 was purchased from Upstate Biotechnology. 4B9 and 6B3 are two mouse monoclonal antibodies against EKLF generated in this lab. We used a rabbit antipeptide polyclonal antibody (63) or antibody 4B9 for Western blot analysis and antibody 6B3 for immunoprecipitation of EKLF. Anti-BRG1 antibodies were a kind gift from G. Crabtree.
Plasmid constructions and mutagenesis.
pSG5/EKLF (47), pCMVβ-HA-p300 (19), pSG5/CBP (81), and pHS2/β/luc (12) were as described elsewhere. pSG5/CBP(HAT−) was made by insertion of the BamHI piece of pRc/RSV/CBP(HAT−) (F1541A mutation; kind gift from T. Kouzarides [44]) into pSG5. pCL/p300(ΔH) was a kind gift from Y. Nakatani (10). Glutathione S-transferase (GST)-CBP/HAT (aa 1196 to 1718) was a kind gift from G. Blobel (27).
GST-EKLF constructs were as described elsewhere (47, 63, 81) except as follows. GST-EKLF(20–185) was made by digesting GST-EKLF(20–376) with NruI and EcoRI, followed by fill-in and ligation. GST-EKLF(20–195) was produced by digesting GST-EKLF(20–376) with EcoRI and SacII and ligation of the filled-in ends. GST-EKLF(280–301), GST-EKLF(254–287), GST-EKLF/ZF1(293–317), GST-EKLF/ZF2(318–347), and GST-EKLF/ZF3(348–376) were generated by PCR, utilizing pSG5-EKLF as a template with the following primers: 5′-GGCCGGATCCCGCAGCCGGCGAACTTTG-3′ and 5′-CGCGGAATTCCCCGCAGCCTTCGTGCCC-3′ for GST-EKLF(280–301), 5′-GGCCGGATCCGGGACTGTGGCCACAGAA-3′ and 5′-CGCGGAATTCAGGTGCCAAAGTTCGCCG-3′ for GST-EKLF(254–287), 5′-GGCCGGATCCCATACGTGCGGGCACGAA-3′ and 5′-CGCGGAATTCGTGCGTGCGCAGGTGCGC-3′ for GST-EKLF/ZF1, 5′-GGCCGGATCCACGGGAGAGAAGCCTTAT-3′ and 5′-CGCGGAATTCGTGCTTCCGGTAGTGGCG-3′ for GST-EKLF/ZF2, and 5′-GGCCGGATCCACTGGACATCGTCCCTTC-3′ and 5′-CGCGGAATTCTCACTCAGAGGTGACGCTTC-3′ for GST-EKLF/ZF3. All of the PCR products were digested with BamHI and EcoRI and directionally inserted into pGEX-2TK vector. All PCR-based constructs were confirmed by DNA sequencing.
Site-directed mutants were generated with a Stratagene Quickchange kit using the following primer pairs. Mutation GST-EKLF(287–376) 288K to E was produced by utilizing GST-EKLF(287–376) as template and oligonucleotides 5′-GGTCGTGGGCCCCCTGAGAGGCAGGCG-3′ and 5′-CGCCTGCCTCTCAGGGGGCCCACGACCTTC-3′ as primers. Mutation GST-EKLF(20–376) K279 to E was made by using GST-EKLF(20–376) as template and oligonucleotides 5′-CTGCGCCGCCCGAACGCAGCCGG-3′ and 5′-CCGGCTGCGTTCGGGCGGCGCAG-3′ as primers. GST-EKLF(20–376) K288-to-E mutation was generated by using GST-EKLF(20–376) as template and oligonucleotides 5′-CTTTGGCACCTGAGAGGCAGGCGGC-3′ and 5′-GCCGCCTGCCTCTCAGGTGCCAAAG-3′ as primers. Double mutation GST-EKLF(20–376) K279 to E, K288 to E was generated by using GST-EKLF(20–376) K279E as template and 5′-CTTTGGCACCTGAGAGGCAGGCGGC-3′ and 5′-GCCGCCTGCCTCTCAGGTGCCAAAG-3′ as primers. pSG5-EKLF(K288A) was produced by using pSG5-EKLF as template and 5′-ACTTTGGCACCTGCGAGGCAGGCGGCA-3′ and 5′-TGCCGCCTGCCTCGCAGGTGCCAAAGT-3′ as primers. pSG5-EKLF(K288R) was generated by utilizing PSG5-EKLF as template and 5′-ACTTTGGCACCTAGAAGGCAGGCGGCA-3′ and 5′-TGCCGCCTGCCTTCTAGGTGCCAAAGT-3′ as primers. pSG5-EKLF(K302A) was made by using pSG5-EKLF as template and 5′-GAAGGCTGCGGGGCGAGCTACTCCAAG-3′ and 5′-CTTGGAGTAGCTCGCCCCGCAGCCTTC-3′ as primers. pSG5-EKLF(K302R) was produced by using pSG5-EKLF as template and 5′-GAAGGCTGCGGGAGGAGCTACTCCAAG-3′ and 5′-CTTGGAGTAGCTCCTCCCGCAGCCTTC-3′ as primers. Double mutation pSG5-EKLF(288R/302R) was produced by using PSG5-EKLF(K288R) as template and 5′-GAAGGCTGCGGGAGGAGCTACTCCAAG-3′ and 5′-CTTGGAGTAGCTCCTCCCGCAGCCTTC-3′ as primers. Mutation pGEX-EKLF(287–376) 288K to E was checked with BamHI and ApaI, as the mutated construct should gain the ApaI site and lose the BamHI site. All other mutated constructs were verified by DNA sequencing.
His-EKLF constructs were as described elsewhere (2). Site-directed mutations (K288A and K302A) were generated in the same way as the analogous GST-EKLF mutations.
In vivo labeling of endogenous EKLF in erythroid cells.
Cell lines K562, MEL, and MEL-derived M4D3 were grown to 2 × 108 cells, washed twice with cold phosphate-buffered saline, resuspended in DMEM labeling medium (1 mCi of 3H-sodium acetate/ml and 2 μM trichostatin A in 5 ml of DMEM), and incubated at 37°C for 1 h. Cells were pelleted and washed twice with cold phosphate-buffered saline, and extracts were prepared, processed for immunoprecipitation with monoclonal antibody 6B3, and analyzed as previously described (81). The final, dried gel was exposed to film at −80°C for 5 to 10 days.
Peptide acetylation assay.
Acetyllysine peptides were synthesized by the Hunter College Protein Core Facility as follows (an asterisk indicates that an acetylated lysine was incorporated into the sequence). Peptide K302(o) is N′-HTCGHEGCGK[302]SYSK∗SSHLK∗AHLRTH-C′; peptide K306(o) is N′-HTCGHEGCGK∗SYSK[306]SSHLK∗AHLRTH-C′; peptide K311(o) is N′-HTCGHEGCGK∗SYSK∗SSHLK[311]AHLRTH-C′; peptide K288(o) is N′-GTAPPKRSRRTLAPK[288]RQAAH-C′; peptide 288K∗ is N′-RRTLAPK∗RQAAHTC-C′.
GST-CBP/HAT (aa 1196 to 1718) fusion protein was prepared from transformed Escherichia coli BL-21; 1 μg of GST-CBP bound on the beads was used as enzyme and 5 mM each peptide was used as substrate in the presence of 2 μl of 0.25 mCi of 3H-labeled acetyl coenzyme A (acetyl-CoA; Amersham) per ml. The mixture was incubated at 30°C for 45 min. After the acetylation reaction, the GST-CBP beads were pelleted, and the supernatants were spotted onto a Whatman P81 phosphocellulose paper disk, processed, and counted (26). The experiment was repeated three times, and the results were averaged.
Gel shift assay.
Five micrograms of GST-EKLF(76–376), GST-EKLF(Δ172–272), or GST-EKLF(287–376) fusion protein was acetylated in vitro with immunoprecipitated CBP complex in the presence of 20 μM acetyl-CoA (Sigma). A parallel set of reactions was performed in the absence of acetyl-CoA to serve as unacetylated controls. After acetylation, reaction samples were spun down to remove the CBP-bound beads. The supernatant (100 ng of GST-EKLF fusions) was used for the gel shift assay. The β-globin CAC site oligonucleotides were labeled (47), and gel shift binding reactions were performed with 250 pg of labeled double-stranded DNA oligonucleotides and 100 ng of acetylated or unacetylated GST-EKLF fusion in 20 μl of 25 mM HEPES, (pH 7.5)–16 mM KCl–50 mM NaCl–2 μM ZnCl2–0.6 mM β-mercaptoethanol–8% glycerol on ice for 30 min.
To test the DNA binding activities of different EKLF mutants, pSG5-EKLF, pSG5-EKLF/K288A, pSG5-EKLF/K288R, pSG5-EKLF/K302A, and pSG5-EKLF/K302R were transfected into COS7 cells with DMRIE-C reagent as described elsewhere (81). Extracts were prepared after 48 h (63) and analyzed by gel shift analysis as above. Anti-EKLF antibody 4B9 was used to identify the novel EKLF band shift. Equal amounts of cell extracts were checked by Western blot analysis for the expression levels of wild-type or mutant EKLF. Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Cotransfections, luciferase, growth hormone, and ECF protein expression level assays.
K562 cells were transfected with desired plasmids using DMRIE-C as described elsewhere (81). All transfections were normalized by cotransfecting growth hormone plasmid pXGH5 as an internal control. At 36 h posttransfection, cells were harvested for the luciferase assay (Promega kit) using equivalent amounts of extracted protein; medium supernatants were used for the growth hormone assay (Nichols Institute). Luciferase levels are plotted after normalization to cotransfected growth hormone levels. All experiments were repeated three times in duplicate.
Because some of the EKLF mutant proteins are more stable than the wild type, EKLF expression levels were directly normalized after transfection. Each extract was subjected to Western blot analysis by using 4B9 as the primary antibody and anti-mouse antibody conjugated with alkaline phosphatase as the secondary antibody. The filter was incubated with Vistra ECF substrate (Amersham) at room temperature for 10 min and scanned with a Storm PhosphorImager scanner (Molecular Dynamics). The intensities of the wild-type and mutated EKLF bands were quantified. The luciferase activity was then further normalized to the wild-type or mutated EKLF protein level in each extract in those experiments in which EKLF mutants were tested.
In vitro chromatin assembly.
Purification of His-tagged recombinant EKLF proteins and Flag-tagged SWI-SNF complexes, DNA assembly into chromatin, in vitro transcription, and pull-down assays were as described elsewhere (2, 33, 58). When needed, recombinant EKLF was incubated with immunopurified p300 for 30 min at 30°C in the presence of acetyl-CoA prior to its use in transcription or pull-down assays. Transcript or protein levels were quantitated by PhosphorImager analysis.
RESULTS
EKLF is acetylated within its transactivation domain at a single lysine.
There are five conserved lysines that may be acetylation targets within the EKLF transactivation region: Lys-47, -74, -177, -279, and -288. Our earlier studies had excluded Lys-47 and Lys-74 as potential substrates for in vitro acetylation by CBP (81). We therefore generated GST-EKLF fusions that contained the other sites and directly tested these for substrate suitability by CBP (Fig. 1). Neither a fragment that contains Lys-47, -74, and -177 nor one with only Lys-279 provide a suitable substrate for acetylation; however, a fragment that contains Lys-288 is readily acetylated by CBP, allowing us to conclude Lys-288 in the EKLF transactivation domain is the primary site of acetylation by CBP.
FIG. 1.
Acetylation of EKLF transactivation domain by CBP in vitro. Endogenous CBP from COS7 cells was immunoprecipitated, and HAT immunoprecipitation assays were performed with various GST-EKLF fusion proteins as diagrammed on the right, which also shows the locations of lysines conserved between mouse and human EKLF (positons 47, 74, 177, 279, 288; mouse numbering is based on initiator methionine being residue 19 [47]). Proteins were resolved and subjected to autoradiography (top left) or stained for protein (bottom left). Labeled CBP is as indicated and is a positive control. Asterisks show locations of nondegraded GST-EKLF fusion proteins. Molecular weight markers (on the left) are 70, 55, and 33 kDa (top to bottom).
We had previously shown that EKLF is acetylated in vivo after transfection into nonerythroid cells (81), but we wished to determine if endogenous EKLF from an erythroid cell was also acetylated. Immunoprecipitation of EKLF from metabolically labeled MEL cells indicates that it is acetylated (Fig. 2). In addition, the M4D3 MEL cell line, which contains a stably integrated, inducible EKLF construct that includes only the proline-rich transactivation region (aa 20 to 291 [53]), is also acetylated, appearing as a faster-migrating band on the autoradiograph (Fig. 2). Labeled K562 cells served as a negative control, as they are erythroid but do not express EKLF. These data demonstrate that EKLF is an acetylated protein in the erythroid cell and that, consistent with the in vitro acetylation data in Fig. 1, its transactivation region is the site of one of the modified lysines.
FIG. 2.
Acetylation status of EKLF in erythroid cells in vivo. K562, MEL, and M4D3 MEL cells were labeled with 3H-sodium acetate, and extracts were immunoprecipitated with anti-EKLF monoclonal antibody 6B3. Immunoprecipitated samples were resolved by SDS-PAGE, processed, and exposed to autoradiography. Locations of molecular weight markers (70, 55, and 33 kDa, from top to bottom), full-length EKLF, and EKLFΔZF are shown.
The EKLF zinc finger domain is also acetylated by CBP.
Although these studies focused on lysine modification within the EKLF transactivation domain, the three zinc fingers also contain lysines that might be suitable substrates for acetylation. This became apparent when full-length GST-EKLF fusions that contain mutated Lys-288 were still able to be acetylated by CBP in vitro (Fig. 3A, lanes 1 to 4). As in the earlier studies (81), our smallest zinc finger construct (aa 287 to 376) contains Lys-288 and is acetylated (lane 5). However, mutation of this lysine resulted in a substrate that remained readily acetylated (lane 6), allowing us to conclude that the EKLF zinc fingers domain can be modified independently of the transactivation domain.
FIG. 3.
Acetylation of EKLF zinc finger domain by CBP in vitro. HAT assays were performed using immunoprecipitated CBP from COS7 cells (A, B, and D) or bacterially expressed GST-CBP (C) with various GST-EKLF fusion proteins or synthetic peptides. (A) Autoradiograph (top) or protein stain (bottom) of EKLF (lane 1), EKLF(K288E) (lane 2), EKLF(K279E) (lane 3), EKLF(K279E/K288E) (lane 4), EKLF-ZnF (lane 5) and EKLF-ZnF(K288E) (lane 6). Labeled CBP is as indicated and is a positive control. (B) Autoradiograph (top) or protein stain (bottom) of EKLF-ZnF1, -2, or -3, as indicated. Labeled CBP is as indicated and is a positive control. (C) Peptides spanning the K288 site or the three lysines of ZnF1 (K302, K306, and K311) were synthesized using blocked (i.e., acetylated) or unmodified lysines and used as substrate for in vitro acetylation assays. Samples from three experiments were processed, and radioactivity was measured and averaged. (D) Autoradiograph (top) or protein stain (bottom) of EKLF (lane 1) and EKLF(K288R/K302R) (lane 2). Labeled CBP is as indicated and is a positive control.
There are six potential lysine targets within the EKLF zinc fingers: three in finger 1, two in finger 2, and one in finger 3. GST fusions to individual fingers, followed by testing for CBP acetylation, revealed that only zinc finger 1 was acetylated (Fig. 3B). To determine which of the three lysines (at positions 302, 306, and 311) within finger 1 can be acetylated, three peptides that spanned all three sites were synthesized. These differ only by the fact that each has one of its three lysines singly available for acetylation [Fig. 3C, 302(o), 306(o), 311(o)]. In each case, the other two lysine positions are blocked. As controls, blocked (288Ac) and unmodified [288(o)] peptides that span Lys-288 were also included in these tests. These latter constructs demonstrated the validity of the assay, as only 288(o) was able to be acetylated by CBP in vitro. Comparison of the finger 1 peptides demonstrates that only 302(o) attains acetylation levels as high as 288(o), whereas 306(o) and 311(o) levels are not significantly above that of 288Ac (Fig. 3C). We conclude from these experiments that EKLF can be acetylated within its zinc finger domain, specifically at Lys-302 within finger 1. Consistent with the data in this and the previous figures, a full-length GST-EKLF fusion that contains both of the K288 and K302 mutations is no longer a substrate for acetylation by CBP in vitro (Fig. 3D).
Acetylated EKLF is unaltered in its DNA binding properties.
The DNA binding properties of transcription factors can be affected by acetylation (4). We performed two experiments to test whether DNA binding by EKLF is altered after acetylation. First, GST-EKLF fusions were incubated with CBP in vitro in the presence or absence of acetyl-CoA, and DNA binding was monitored by electrophoretic mobility shift assay (EMSA) of a radiolabeled β-globin CACCC element oligonucleotide. These conditions led to acetylation of the GST-EKLF substrates (data not shown), but there was no discernible effect on DNA binding in any of the constructs (Fig. 4A). This remained true even with the smallest construct tested, which retained both Lys-288 and Lys-302.
FIG. 4.
In vitro DNA binding assay of EKLF. (A) Purified GST-EKLF fusions were incubated with CBP in the presence (+) or absence (−) of acetyl-CoA prior to incubation with a radiolabeled β-globin CACCC element and analysis by EMSA. EKLF(287–376) contains only the zinc finger domain, EKLF(Δ172-272) has an internal portion of the transactivation region removed, and EKLF(76–376) is missing part of the amino terminus. (B) Extracts were prepared from COS7 cells that had been transfected with full-length EKLF (wild type or mutant) and monitored for binding to a radiolabeled β-globin CACCC element by EMSA; position of the novel EKLF band (not present in empty vector [EV]-transfected cells) is indicated. Plus signs indicate that samples were incubated in the presence of 4B9 anti-EKLF monoclonal antibody (Ab) prior to analysis. Variation in the level of the EKLF shift is due to differing expression levels of mutated EKLF relative to the wild type (not shown).
Second, full-length EKLF or its site-directed mutants were transfected into COS7 cells, and extracts were monitored for DNA binding ability. We have previously shown that transfected EKLF is acetylated in vivo in COS7 cells (81). Transfected EKLF leads to a novel band shift that is not present in extracts from cells transfected with empty vector; in addition, this band can be supershifted with anti-EKLF antibody (Fig. 4B). This novel gel shift band remained whether EKLF Lys-288 or Lys-302 was altered to alanine or arginine (Fig. 4B). The differences in gel shift intensities observed simply mirror the different amounts of EKLF mutant protein expressed in each of the extracts (data not shown; also see below). We conclude that acetylation of EKLF has little effect on its ability to interact with DNA.
An intact acetylation function is required for optimal EKLF activity in vivo.
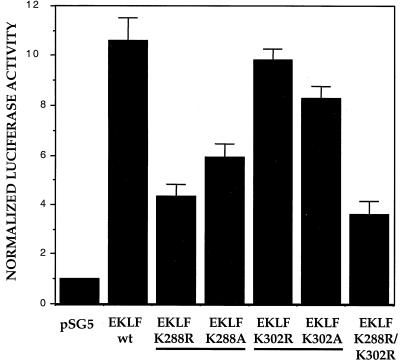
We next tested the functional consequences of site-directed mutants at selected lysines by monitoring EKLF's ability to activate the β-globin promoter, which is its normal activation target. We used the K562 erythroleukemic cell line, as these cells express neither EKLF nor the endogenous or a transfected adult β-globin gene (6). However, cotransfection of EKLF with an exogenous β-globin reporter switches on its expression, dependent on EKLF binding to the CACCC promoter element (18). We had found that steady-state expression levels of the EKLF mutants vary considerably after transfection (data not shown). As a result, for these experiments we quantitated the protein expression level for each transfection and included this additional normalization in our analysis (see Materials and Methods).
Mutagenesis of EKLF to arginine or alanine had little effect when directed at Lys-302, but transactivation dropped by 50 to 60% when these substitutions were directed at Lys-288 (Fig. 5). As the double mutant had little additional effect beyond that of the single Lys-288 mutant (Fig. 5), we conclude that Lys-288, but not Lys-302, is required for full EKLF activity at the β-globin promoter in these transient assays.
FIG. 5.
Effects of site-directed mutants of EKLF upon activation of the β-globin promoter. K562 erythroleukemic cells were transfected with pHS2/β/luciferase reporter and wild-type (wt) or mutant EKLF expression or vector (pSG5) plasmids, and extracts were processed for luciferase activity. Multiple experiments were averaged after normalization of luciferase activity to quantitated EKLF protein levels in each extract and cotransfected growth hormone.
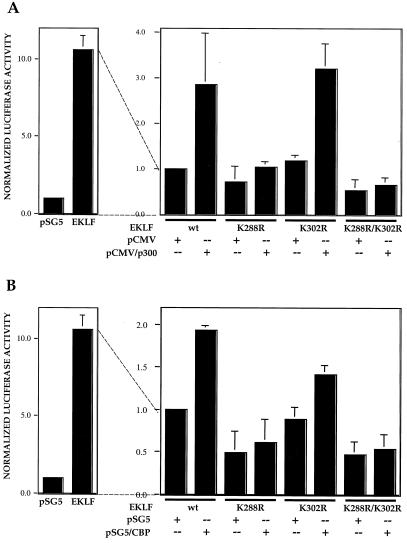
Our earlier studies had demonstrated that EKLF activity in vivo at the β-globin promoter in the erythroid cell can be superactivated in a dose-dependent manner by cotransfection with CBP or p300 (81). We repeated these experiments with the EKLF K288R and K302R single mutants, as well as with the double mutant. We found that neither the Lys-288 mutant nor the double mutant could be superactivated by either p300 (Fig. 6A) or CBP (Fig. 6B). However, the Lys-302 mutant was superactivated as well as wild-type EKLF in either case. These results demonstrate that the Lys-288 mutant is not competent for activation by the HAT coactivator and likely explains the lowered transfection capability of mutant EKLF observed in Fig. 5. In addition, these results are consistent with the idea that acetylation by p300 or CBP at Lys-288 is critical for optimal EKLF function.
FIG. 6.
Superactivation of the β-globin promoter by coactivators. K562 erythroleukemic cells were transfected with pHS2/β/luciferase reporter and wild-type (wt) EKLF expression or vector (pSG5) plasmids (left). In the panel on the right, all cells received an equal amount of wild-type or mutant EKLF expression vector and p300 (A) or CBP (B) expression or vector (pCMV or pSG5) plasmid. Extracts were processed for luciferase activity. Multiple experiments were averaged after normalization of luciferase activity to quantitated EKLF protein levels in each extract and cotransfected growth hormone. To simplify the comparison, the level of activity of wild-type EKLF plus vector alone (on the right) was given an arbitrary value of 1 although its absolute level was as high as that seen in the panel on the left.
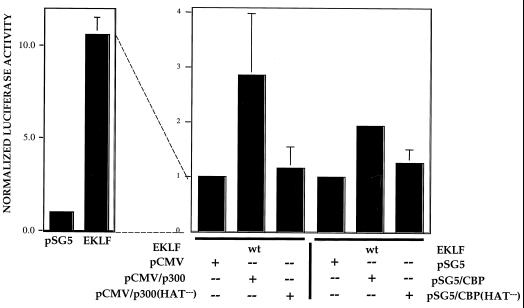
To test this idea further, a site-directed mutant of CBP (44) and a small deletion mutant of p300 (10) which renders them inactive for acetylation activity were tested for the ability to superactivate EKLF on the β-globin promoter in K562 cells. The results (Fig. 7) show that neither mutant can superactivate EKLF. This demonstrates that the associated acetyltransferase activity of p300 or CBP is critical for generating an optimally active EKLF that induces maximal transcript levels at the β-globin promoter.
FIG. 7.
Effects of mutant coactivators on superactivation. K562 erythroleukemic cells were transfected with pHS2/β/luciferase reporter and wild-type (wt) EKLF expression or vector (pSG5) plasmids (left). In the panel on the right, all cells received an equal amount of EKLF expression vector and wild-type or HAT-defective p300 or CBP expression plasmids or vector-alone (pCMV or pSG5) plasmid. Extracts were processed for luciferase activity. Multiple experiments were averaged after normalization of luciferase activity to cotransfected growth hormone. To simplify the comparison, the level of activity of wild-type EKLF plus vector alone (on the right) was given an arbitrary value of 1 although its absolute level was as high as that seen in the panel on the left.
Finally, we excluded the possibility that these results could be explained by a deficient interaction of mutant EKLF with p300 or CBP. As EKLF interactions with these coactivators were originally monitored by cotransfection-coimmunoprecipitation assays (81), we used the same protocol and monitored the ability of anti-CBP to coimmunoprecipitate wild-type, K288R, and/or K302R EKLF. The results (Fig. 8) demonstrate that EKLF association with CBP is equivalent and proportional to the EKLF expression level.
FIG. 8.
Tests of EKLF interaction with CBP in vivo. Wild-type EKLF (lane 1) or K288R (lane 2), K302R (lane 3), or K288R/K302R (lane 4) mutant EKLF was transfected into COS7 cells, and whole-cell extracts (400 μl) were subjected to immunoprecipitation with anti-CBP antibodies. Immunoprecipitated proteins were resolved, blotted, and probed with 4B9 anti-EKLF antibody. Analysis of the input lysates (20 μl) used for the immunoprecipitation is on the top, and that of the pellet is on the bottom. The location of EKLF is shown; the asterisk indicates nonspecific (immunglobulin heavy chain) signals from the immunoprecipitating antibodies.
Acetylation alters EKLF interactions with the SWI-SNF complex in vitro.
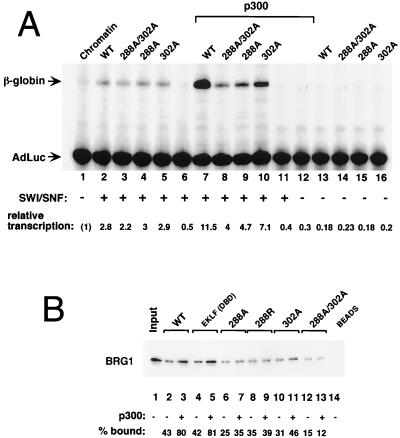
Given that acetylation of EKLF does not affect its ability to bind DNA yet modifies is transactivation capability in vivo, we addressed whether protein interactions might be altered. Of particular interest was to assess whether EKLF–E-RC1 (SWI-SNF) interactions were affected by the acetylation status of EKLF. This idea was tested by using the coupled in vitro chromatin assembly-transcription system that contains purified EKLF (or its mutated derivatives), the human SWI-SNF chromatin remodeling complex, and the β-globin promoter template (2, 33). The data (Fig. 9A) show that the individual EKLF lysine mutants (at K288 and K302) are equally capable of reconstituting accurate transcription on chromatinized templates (lanes 2 to 5) dependent on inclusion of the SWI-SNF complex (compare to lanes 13 to 16). However, acetylation of EKLF by preincubation with p300 superactivates transcription fourfold, an effect partially observed with the p300-treated K302 mutant (twofold) and greatly diminished in the p300-treated K288 mutants (lanes 7 to 10). The results are consistent with the in vivo transfection data and demonstrate that acetylation of EKLF, particularly at K288, can stimulate its transactivation properties to optimal levels within the context of chromatin in vitro.
FIG. 9.
In vitro transcription and protein-protein interactions by recombinant EKLF. (A) In vitro transcription of chromatin-assembled β-globin templates was performed in the presence (lanes 2 to 11) or absence (lanes 1 and 12 to 16) of SWI-SNF (E-RC1) complex and 5 pmol of wild-type (WT; lanes 2, 7, and 13) or mutated (lanes 3 to 5, 8 to 10, and 14 to 16) His-EKLF. EKLF samples used in lanes 7 to 12 were in vitro acetylated by p300 prior to addition to the chromatin-assembled template. Primer extension products derived from the β-globin transcript or the adeno-luciferase (AdLuc) internal control are indicated by arrows. Fold transcription activation was determined by subtracting a background value for each lane from the β-globin and AdLuc signals. The control AdLuc signal for lane 1 was set at 1, and the relative AdLuc values were divided into the appropriate β-globin signal. Repressed β-globin transcription (lane 1) was then set at 1. (B) Interactions between His-EKLF [wild type, DNA binding domain alone (DBD), K288 and/or K302 mutant] and E-RC1 were monitored by Ni-resin pull-down assay followed by SDS-PAGE, blotting, and probing with anti-BRG1 antibodies. EKLF protein was acetylated by p300 as indicated prior to incubation with E-RC1. Percent bound, relative to input signal, is shown below each lane.
One way in which this stimulation may occur is if EKLF's physical interaction with the SWI-SNF complex is increased by acetylation. This idea was tested by in vitro pull-down assays using recombinant EKLF and Flag-tagged SWI-SNF. These data (Fig. 9B) show that in vitro acetylation of either full-length EKLF or truncated EKLF (containing aa 287 to the end, which overlaps the zinc finger domain) by p300 leads to a twofold increase in its ability to interact with SWI-SNF (as judged by monitoring the presence of BRG1 in the recovered complex). However, the EKLF K288 and/or K302 mutants do not show this increase in binding affinity for SWI-SNF. These data suggest that acetylated EKLF's ability to potentiate transcription on chromatinized templates is due to enhanced protein-protein interactions with the SWI-SNF complex, which provides a plausible explanation for the transcription results.
DISCUSSION
EKLF plays critical roles in transcriptional activation (2, 18, 33, 50, 57) and chromatin integrity (2, 23, 46, 78) at the β-globin locus. Its interaction with coactivators that harbor acetyltransferase activity (81), in addition to its own modification by these activators, raises a number of scenarios that place EKLF within a central role in integrating these functions at the appropriate time in erythroid differentiation.
Maximal activity of EKLF is dependent on its acetylation status.
Our results demonstrate that EKLF is positively regulated by acetylation, as mutagenesis of its modified lysine residues, and use of acetylation-deficient coactivators, leads to suboptimal EKLF transactivational activity in vivo and in vitro. Although the bridging functions (16, 29, 30) of the coactivators used in this study are likely important, a critical conclusion is that this is not sufficient and that their associated acetylation activity plays a necessary role in generating the most efficient level of EKLF activity. These two properties are not always linked, as transcriptional activation by MyoD can be superactivated when acetylase-defective p300 (but not P/CAF) is used (59).
It is of interest that the modified lysines mapped in the present study are located close together at the basic region adjacent to and within EKLF finger 1. In vitro assays using EKLF and E-RC1 proteins showed that correct chromatin assembly, as judged by localized DNase-hypersensitive formation at the β-globin promoter, could still form when EKLF proteins that are deleted at the amino terminus are used (2). As expected from other molecular analyses (14), these EKLF amino-terminal mutants could no longer stimulate transcription. These data localized the region of EKLF that is minimally important for chromatin assembly to the carboxyl, zinc finger-containing domain. The present analyses suggest that EKLF's effectiveness in this function can be influenced by the modification status of the two lysines.
Molecular events at the β-globin locus.
Recent studies have demonstrated that lysine modification of DNA binding proteins alters their interactions with DNA (4). For example, modifications at the amino-terminal tails of histones are thought to decrease their affinity for DNA, thereby loosening their higher-order structure, resulting in more open chromatin domain (75). In addition, acetylation of HMG1 reduces its binding to DNA, leading to disruption of the beta interferon enhanceosome (48). On the other hand, the DNA binding affinity of p53 is increased 20- to 30-fold upon acetylation by either p300 or CBP (24, 60). Although one study saw a similar effect on GATA-1 (10), this was not observed in another study (27); thus, the effect of acetylation on GATA-1 remains controversial. In the present case, we did not find any major effect of acetylation on EKLF binding to the CACCC element, neither after it was acetylated in vitro nor after site-directed mutants of lysines shown to be acetylated by p300 or CBP were isolated and directly tested after in vivo transfection. Rather, EKLF protein-protein interactions, particularly with the SWI-SNF complex, were modulated by acetylation. There is precedent for this idea, as the ACTR coactivator's ability to interact with the estrogen receptor is destabilized after its own acetylation by p300 (13). In addition, acetylation of the drosophila TCF transcription factor by CBP lowers its affinity for Armadillo (77). Finally, the ability of the P/CAF bromodomain to interact with a peptide derived from the histone H4 amino-terminal tail is significantly altered by H4 acetylation status (17). As a result, EKLF may be integrating signals from both histone modifiers and chromatin remodelers to the β-globin locus. This hypothesis is further strengthened by recent studies demonstrating that recruitment of a marked BRG1 to the β-globin locus in vivo is not observed when the EKLF binding site (CACCC element) is mutated (42).
Conservation with other KLF family members.
EKLF is the founding member of the highly related Krüppel-like factor (KLF) family, which is different from Sp1 and which now includes at least 12 members (72). However, this family can be subdivided even further based on the conservation of the basic region adjacent to the zinc fingers (Fig. 10). Close inspection of this sequence reveals that only GKLF and LKLF share the sequence surrounding Lys-288 of EKLF, suggesting that this lysine may also be an acetylation site in GKLF and LKLF. GKLF is primarily expressed in epithelial cells of the gut (62) and is vital for skin barrier function (61), whereas LKLF, although enriched in lung tissues (1), is critical for T-cell viability (40) and blood vessel stabilization (39) during early development. Although their sequences are not preserved with EKLF at their basic regions, it is worth noting that BKLF, IKLF, UKLF, and FKLF also contain a lysine embedded within their own conserved sequences (72).
FIG. 10.
Conservation of acetylated lysine within KLF family members. The amino acid alignment at the start of the first zinc finger is shown for selected KLF family members. Numbering begins with the first amino acid in the sequence. Boxed regions emphasize the conservation with EKLF K288, C295, C300, and K302. EKLF, GKLF, and LKLF are the most highly conserved subfamily members (1, 72). Prefixes “h” and “m” denote human and murine, respectively.
Lys-302, on the other hand, is strictly conserved across all KLF family members (72), as it resides within the antiparallel β-sheet structure of the first (i.e., most amino-terminal) zinc finger (22, 54). As a result, it may be surprising that its change did not have a more drastic effect on DNA binding. Three explanations come to mind. First, unlike the α-helix portion of the finger, the β-sheet region does not directly interact with the DNA. Second, it is possible that any effect may be more subtle than detectable by a direct gel shift and would become apparent only by a competitive gel shift assay (22). Third, point mutations in the extended 9-bp EKLF binding site (5′-CCACACCCT-3′) have different relative binding affinities depending on their location in the sequence. In particular, point mutations located at sites where the second and third EKLF fingers interact have 40- to 100-fold-lower competitive ability than wild type for binding to EKLF (22). A point mutant located at the first finger interaction site, on the other hand, has only an eightfold-lower competitive ability (18). As a result, alteration of Lys-302 (in the EKLF first finger) may have a less drastic effect on EKLF binding than the analogous alteration of conserved amino acids in the second and third EKLF fingers would have.
Biological implications of EKLF acetylation.
The acetylation status of EKLF provides the cell with another point at which to control its activity, analogous to the way altered phosphorylation plays such an important role in controlling the activity of numerous transcription factors (28, 37). Acetylation of EKLF may play a directive role in the switch from fetal to adult β-like globin expression, particularly if protein-protein interactions between EKLF and chromatin remodelers such as SWI-SNF are altered by EKLF acetylation status. This may provide a resolution to the paradox that results from the fact that EKLF is expressed in early hematopoietic cells (83) and in both primitive and definitive cells (63), yet is functionally required only in definitive erythroid cells (50, 57). Monitoring EKLF acetylation status in these two cell populations, or in differentiating MEL cells or HOX11-immortalized cells (34), may provide evidence that partially resolves this paradox.
EKLF is also phosphorylated, and the two types of posttranslational modification may be interrelated; in particular, Thr-41 is critical for its activity (53). EKLF phosphorylation status may thus enable a more efficient association with p300 or CBP in a fashion similar to the way CREB (16), Smad3 (31), NF-κB (82), and p53 (41, 60) interactions with p300 or CBP are affected. As a result, one can envision a cascade (as discussed in reference 5) by which EKLF could be differentially modified, by phosphorylation and/or acetylation, depending on cellular signals present at different stages of development (i.e., primitive versus definitive) or at later stages of hematopoietic differentiation. The ultimate recruitment of HATs (p300 or CBP) and chromatin remodelers (E-RC1) would result in derepression of the closed chromatin structure at the β-globin cluster, leading to transcriptional activation of the β-globin gene at the correct time in erythroid ontogeny.
The importance of CBP for erythroid function has been tested in three ways. First, interference of CBP activity by forced expression of E1A leads to a block of differentiation and globin expression in MEL cells (9). Second, mice homozygous for a truncated form of CBP exhibited defective (although not ablated) primitive erythropoiesis and vasculogenesis and were embryonic lethal (51). Third, inactivation of a single CBP allele results in defective hematopoiesis and an increased incidence in hematologic malignancies (38).
It is particularly striking that a number of erythroid transcriptional activators of different types, including EKLF, GATA-1, and NF-E2 (G. Blobel, personal communication), are all acetylated and functionally altered as a result of their modification. One can therefore envision that these DNA binding factors could form a major transcription complex throughout the β-like globin locus that recruits coactivators with acetyltransferase activity (8), resulting in modification of the local histones and of themselves, and thus allowing the additional recruitment of chromatin remodelers, leading to the completely decondensed chromatin structure that is normally observed in red cells. This opening process would occur prior to transcriptional activation of the locus. Although speculative, such a scheme could help explain a number of intriguing observations: that the β-like globin locus is enriched in acetylated histones in erythroid cells (25) and is decondensed prior to transcription of any globin genes (32), that histone deacetylase inhibitors (butyrate or trichostatin A) alter the expression profile of the locus (45, 65), and that absence of only a single player (EKLF) leads to loss of DNase-hypersensitive site at the β-promoter and diminution of hypersensitive site 3 at the locus control region (23, 78).
ACKNOWLEDGMENTS
We thank Tony Kouzarides, Pat Nakatani, and Gerd Blobel for plasmids.
This work was supported by PHS grant DK46865 to J.J.B., who is a Scholar of the Leukemia Society of America, and by PHS grant GM38760 to B.M.E.
REFERENCES
- 1.Anderson K P, Kern C B, Crable S C, Lingrel J B. Isolation of a gene encoding a functional zinc finger protein homologous to EKLF: identification of a new multigene family. Mol Cell Biol. 1995;15:5957–5965. doi: 10.1128/mcb.15.11.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong J A, Bieker J J, Emerson B M. A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell. 1998;95:93–104. doi: 10.1016/s0092-8674(00)81785-7. [DOI] [PubMed] [Google Scholar]
- 3.Baron M H. Transcriptional control of globin gene switching during vertebrate development. Biochim Biophys Acta. 1997;1351:51–72. doi: 10.1016/s0167-4781(96)00195-9. [DOI] [PubMed] [Google Scholar]
- 4.Berger S L. Gene activation by histone and factor acetyltransferases. Curr Opin Cell Biol. 1999;11:336–341. doi: 10.1016/S0955-0674(99)80046-5. [DOI] [PubMed] [Google Scholar]
- 5.Bieker J J. EKLF and the development of the erythroid lineage. In: Ravid K, Licht J D, editors. Transcription factors: normal and malignant development of blood cells. New York, N.Y: Wiley-Liss; 1999. pp. 71–84. [Google Scholar]
- 6.Bieker J J. Isolation, genomic structure, and expression of human Erythroid Kruppel-like Factor (EKLF) DNA Cell Biol. 1996;15:347–352. doi: 10.1089/dna.1996.15.347. [DOI] [PubMed] [Google Scholar]
- 7.Bird A P, Wolffe A P. Methylation-induced repression—belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- 8.Blobel G A. CREB-binding protein and p300: molecular integrators of hematopoietic transcription. Blood. 2000;95:745–755. [PubMed] [Google Scholar]
- 9.Blobel G A, Nakajima T, Eckner R, Montminy M, Orkin S H. CREB-binding protein cooperates with transcription factor GATA-1 and is required for erythroid differentiation. Proc Natl Acad Sci USA. 1998;95:2061–2066. doi: 10.1073/pnas.95.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 11.Bulger M, Groudine M. Looping versus linking: toward a model for long-distance gene activation. Genes Dev. 1999;13:2465–2477. doi: 10.1101/gad.13.19.2465. [DOI] [PubMed] [Google Scholar]
- 12.Caterina J J, Ciavatta D J, Donze D, Behringer R R, Townes T M. Multiple elements in human beta-globin locus control region 5′ HS 2 are involved in enhancer activity and position-independent, transgene expression. Nucleic Acids Res. 1994;22:1006–1011. doi: 10.1093/nar/22.6.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Lin R J, Xle W, Wilpltz D, Evans R M. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell. 1999;98:675–686. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Bieker J J. Erythroid Krüppel-like factor (EKLF) contains a multifunctional transcriptional activation domain important for inter- and intramolecular interactions. EMBO J. 1996;15:5888–5896. [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung W L, Briggs S D, Allis C D. Acetylation and chromosomal functions. Curr Opin Cell Biol. 2000;12:326–333. doi: 10.1016/s0955-0674(00)00096-x. [DOI] [PubMed] [Google Scholar]
- 16.Chrivia J C, Kwok R P S, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 17.Dhalluin C, Carlson J E, Zeng L, He C, Aggarwal A K, Zhou M M. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 18.Donze D, Townes T M, Bieker J J. Role of erythroid Krüppel-like factor (EKLF) in human γ- to β-globin switching. J Biol Chem. 1995;270:1955–1959. doi: 10.1074/jbc.270.4.1955. [DOI] [PubMed] [Google Scholar]
- 19.Eckner R, Ewen M E, Newsome D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 20.Engel J D. Developmental regulation of human β-globin gene transcription: a switch of loyalties? Trends Genet. 1993;9:304–309. doi: 10.1016/0168-9525(93)90248-g. [DOI] [PubMed] [Google Scholar]
- 21.Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992;355:219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- 22.Feng W C, Southwood C M, Bieker J J. Analyses of β-thalassemia mutant DNA interactions with erythroid Krüppel-like factor (EKLF), an erythroid cell-specific transcription factor. J Biol Chem. 1994;269:1493–1500. [PubMed] [Google Scholar]
- 23.Gillemans N, Tewari R, Lindeboom F, Rottier R, de Wit T, Wijgerde M, Grosveld F, Philipsen S. Altered DNA-binding specificity mutants of EKLF and Sp1 show that EKLF is an activator of the beta-globin locus control region in vivo. Genes Dev. 1998;12:2863–2873. doi: 10.1101/gad.12.18.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu W, Roeder R G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 25.Hebbes T R, Clayton A L, Thorne A W, Crane-Robinson C. Core histone hyperacetylation comaps with generalized DNAsel sensitivity in the chicken beta-globin chromosomal domain. EMBO J. 1994;13:1823–1830. doi: 10.1002/j.1460-2075.1994.tb06451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horiuchi K, Fujimoto D. Use of phosphocellulose paper disks for the assay of histone acetyltransferase. Anal Biochem. 1975;69:491–496. doi: 10.1016/0003-2697(75)90151-7. [DOI] [PubMed] [Google Scholar]
- 27.Hung H L, Lau J, Kim A Y, Weiss M J, Blobel G A. CREB-binding protein acetylates hematopoietic transcription factor GATA- 1 at functionally important sites. Mol Cell Biol. 1999;19:3496–3505. doi: 10.1128/mcb.19.5.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter T, Karin M. The regulation of transcription by phosphorylation. Cell. 1992;70:375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- 29.Janknecht R, Hunter T. A growing coactivator network. Nature. 1996;383:22–23. doi: 10.1038/383022a0. [DOI] [PubMed] [Google Scholar]
- 30.Janknecht R, Hunter T. Nuclear fusion of signaling pathways. Science. 1999;284:443–444. doi: 10.1126/science.284.5413.443. [DOI] [PubMed] [Google Scholar]
- 31.Janknecht R, Wells N J, Hunter T. TGF-beta-stimulated cooperation of smad proteins with the coactivators CBP/p300. Genes Dev. 1998;12:2114–2119. doi: 10.1101/gad.12.14.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jimenez G, Griffiths S D, Ford A M, Greaves M F, Enver T. Activation of the beta-globin locus control region precedes commitment to the erythroid lineage. Proc Natl Acad Sci USA. 1992;89:10618–10622. doi: 10.1073/pnas.89.22.10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadam S, McAlpine G S, Phelan M L, Kingston R E, Jones K A, Emerson B M. Functional selectivity of recombinant mammalian SWI/SNF subunits. Genes Dev. 2000;14:2441–2451. doi: 10.1101/gad.828000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller G, Wall C, Fong A Z, Hawley T S, Hawley R G. Overexpression of HOX11 leads to the immortalization of embryonic precursors with both primitive and definitive hematopoietic potential. Blood. 1998;92:877–887. [PubMed] [Google Scholar]
- 35.Kingston R E, Bunker C A, Imbalzano A N. Repression and activation by multiprotein complexes that alter chromatin structure. Genes Dev. 1996;10:905–920. doi: 10.1101/gad.10.8.905. [DOI] [PubMed] [Google Scholar]
- 36.Kingston R E, Narlikar G J. ATP-dependent remodeling and acetylation as regulators of chromatin fluidity. Genes Dev. 1999;13:2339–2352. doi: 10.1101/gad.13.18.2339. [DOI] [PubMed] [Google Scholar]
- 37.Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 2000;19:1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kung A L, Rebel V I, Bronson R T, Ch'ng L E, Sieff C A, Livingston D M, Yao T P. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 2000;14:272–277. [PMC free article] [PubMed] [Google Scholar]
- 39.Kuo C T, Veselits M L, Barton K P, Lu M M, Clendenin C, Leiden J M. The LKLF transcription factor is required for normal tunica media formation and blood vessel stabilization during murine embryogenesis. Genes Dev. 1997;11:2996–3006. doi: 10.1101/gad.11.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuo C T, Veselits M L, Leiden J M. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 41.Lambert P F, Kashanchi F, Radonovich M F, Shiekhattar R, Brady J N. Phosphorylation of p53 serine 15 increases interaction with CBP. J Biol Chem. 1998;273:33048–33053. doi: 10.1074/jbc.273.49.33048. [DOI] [PubMed] [Google Scholar]
- 42.Lee C H, Murphy M R, Lee J S, Chung J H. Targeting a SWI/SNF-related chromatin remodeling complex to the beta-globin promoter in erythroid cells. Proc Natl Acad Sci USA. 1999;96:12311–12315. doi: 10.1073/pnas.96.22.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim S K, Bieker J J, Lin C S, Costantini F. A shortened life span of EKLF −/− adult erythrocytes, due to a deficiency of β-globin chains, is ameliorated by human γ-globin chains. Blood. 1997;90:1291–1299. [PubMed] [Google Scholar]
- 44.Martinez-Balbas M A, Bannister A J, Martin K, Haus-Seuffert P, Meisterernst M, Kouzarides T. The acetyltransferase activity of CBP stimulates transcription. EMBO J. 1998;17:2886–2893. doi: 10.1093/emboj/17.10.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCaffrey P G, Newsome D A, Fibach E, Yoshida M, Su M S S. Induction of γ-globin by histone deactylase inhibitors. Blood. 1997;90:2075–2083. [PubMed] [Google Scholar]
- 46.McMorrow T, van Den Wijngaard A, Wollenschlaeger A, van De Corput M, Monkhorst K, Trimborn T, Fraser P, van Lohuizen M, Jenuwein T, Djabali M, Philipsen S, Grosveld F, Milot E. Activation of the beta globin locus by transcription factors and chromatin modifiers. EMBO J. 2000;19:4986–4996. doi: 10.1093/emboj/19.18.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller I J, Bieker J J. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Krüppel family of nuclear proteins. Mol Cell Biol. 1993;13:2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munshi N, Merika M, Yie J, Senger K, Chen G, Thanos D. Acetylation of HMG I(Y) by CBP turns off IFN beta expression by disrupting the enhanceosome. Mol Cell. 1998;2:457–467. doi: 10.1016/s1097-2765(00)80145-8. [DOI] [PubMed] [Google Scholar]
- 49.Neely K E, Hassan A H, Wallberg A E, Steger D J, Cairns B R, Wright A P, Workman J L. Activation domain-mediated targeting of the SWI/SNF complex to promoters stimulates transcription from nucleosome arrays. Mol Cell. 1999;4:649–655. doi: 10.1016/s1097-2765(00)80216-6. [DOI] [PubMed] [Google Scholar]
- 50.Nuez B, Michalovich D, Bygrave A, Ploemacher R, Grosveld F. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature. 1995;375:316–318. doi: 10.1038/375316a0. [DOI] [PubMed] [Google Scholar]
- 51.Oike Y, Takakura N, Hata A, Kaname T, Akizuki M, Yamaguchi Y, Yasue H, Arakl K, Yamamura K, Suda T. Mice homozygous for a truncated form of CREB-binding protein exhibit defects in hematopoiesis and vasculo-angiogenesis. Blood. 1999;93:2771–2779. [PubMed] [Google Scholar]
- 52.Orkin S H. Regulation of globin gene expression in erythroid cells. Eur J Biochem. 1995;231:271–281. doi: 10.1111/j.1432-1033.1995.tb20697.x. [DOI] [PubMed] [Google Scholar]
- 53.Ouyang L, Chen X, Bieker J J. Regulation of erythroid Kruppel-like factor (EKLF) transcriptional activity by phosphorylation of a protein kinase casein kinase II site within its interaction domain. J Biol Chem. 1998;273:23019–23025. doi: 10.1074/jbc.273.36.23019. [DOI] [PubMed] [Google Scholar]
- 54.Pavletich N P, Pabo C O. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991;252:809–816. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- 55.Perkins A. Erythroid Kruppel like factor: from fishing expedition to gourmet meal. Int J Biochem Cell Biol. 1999;31:1175–1192. doi: 10.1016/s1357-2725(99)00083-7. [DOI] [PubMed] [Google Scholar]
- 56.Perkins A C, Gaensler K M L, Orkin S H. Silencing of human fetal globin expression is impaired in the absence of the adult β-globin gene activator protein EKLF. Proc Natl Acad Sci USA. 1996;93:12267–12271. doi: 10.1073/pnas.93.22.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perkins A C, Sharpe A H, Orkin S H. Lethal β-thalassemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature. 1995;375:318–322. doi: 10.1038/375318a0. [DOI] [PubMed] [Google Scholar]
- 58.Phelan M L, Sif S, Narlikar G J, Kingston R E. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol Cell. 1999;3:247–253. doi: 10.1016/s1097-2765(00)80315-9. [DOI] [PubMed] [Google Scholar]
- 59.Puri P L, Sartorelli V, Yang X J, Hamamori Y, Ogryzko V V, Howard B H, Kedes L, Wang J Y J, Graessmann A, Nakatani Y, Levrero M. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell. 1997;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 60.Sakaguchi K, Herrera J E, Saito S, Miki T, Bustin M, Vassilev A, Anderson C W, Appella E. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Segre J A, Bauer C, Fuchs E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat Genet. 1999;22:356–360. doi: 10.1038/11926. [DOI] [PubMed] [Google Scholar]
- 62.Shields J M, Christy R J, Yang V W. Identification and characterization of a gene encoding a gut-enriched Kruppel-like factor expressed during growth arrest. J Biol Chem. 1996;271:20009–20017. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Southwood C M, Downs K M, Bieker J J. Erythroid Kruppel-like Factor (EKLF) exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny. Dev Dyn. 1996;206:248–259. doi: 10.1002/(SICI)1097-0177(199607)206:3<248::AID-AJA3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 64.Stamatoyannopoulos G, Nienhuis A W. Hemoglobin Switching. In: Stamatoyannopoulos G, Nienhuis A W, Majerus P W, Varmus H, editors. The molecular bases of blood diseases. 2nd ed. Philadelphia, Pa: W. B. Saunders Co.; 1994. pp. 107–155. [Google Scholar]
- 65.Stamatoyannopoulos G, Nienhuis A W. Therapeutic approaches to hemoglobin switching in treatment of hemoglobinopathies. Annu Rev Med. 1992;43:497–521. doi: 10.1146/annurev.me.43.020192.002433. [DOI] [PubMed] [Google Scholar]
- 66.Struhl K. Fundamentally different logic of gene regulation in eukaryotes and prokaryotes. Cell. 1999;98:1–4. doi: 10.1016/S0092-8674(00)80599-1. [DOI] [PubMed] [Google Scholar]
- 67.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 68.Sudarsanam P, Winston F. The Swi/Snf family nucleosome-remodeling complexes and transcriptional control. Trends Genet. 2000;16:345–351. doi: 10.1016/s0168-9525(00)02060-6. [DOI] [PubMed] [Google Scholar]
- 69.Townes T M, Behringer R R. Human globin locus activation region (LAR): role in temporal control. Trends Genet. 1990;6:219–223. doi: 10.1016/0168-9525(90)90182-6. [DOI] [PubMed] [Google Scholar]
- 70.Travers A. An engine for nucleosome remodeling. Cell. 1999;96:311–314. doi: 10.1016/s0092-8674(00)80543-7. [DOI] [PubMed] [Google Scholar]
- 71.Trimborn T, Gribnau J, Grosveld F, Fraser P. Mechanisms of developmental control of transcription in the murine alpha- and beta-globin loci. Genes Dev. 1999;13:112–124. doi: 10.1101/gad.13.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turner J, Crossley M. Mammalian Kruppel-like transcription factors: more than just a pretty finger. Trends Biochem Sci. 1999;24:236–240. doi: 10.1016/s0968-0004(99)01406-1. [DOI] [PubMed] [Google Scholar]
- 73.Tyler J K, Kadonaga J T. The “dark side” of chromatin remodeling: repressive effects on transcription. Cell. 1999;99:443–446. doi: 10.1016/s0092-8674(00)81530-5. [DOI] [PubMed] [Google Scholar]
- 74.Vignali M, Hassan A H, Neely K E, Workman J L. ATP-dependent chromatin-remodeling complexes. Mol Cell Biol. 2000;20:1899–1910. doi: 10.1128/mcb.20.6.1899-1910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wade P A, Pruss D, Wolffe A P. Histone acetylation: chromatin in action. Trends Biochem Sci. 1997;22:128–132. doi: 10.1016/s0968-0004(97)01016-5. [DOI] [PubMed] [Google Scholar]
- 76.Wallberg A E, Neely K E, Gustafsson J A, Workman J L, Wright A P, Grant P A. Histone acetyltransferase complexes can mediate transcriptional activation by the major glucocorticoid receptor activation domain. Mol Cell Biol. 1999;19:5952–5959. doi: 10.1128/mcb.19.9.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waltzer L, Bienz M. Drosophila CBP represses the transcription factor TCF to antagonize Wingless signalling. Nature. 1998;395:521–525. doi: 10.1038/26785. [DOI] [PubMed] [Google Scholar]
- 78.Wijgerde M, Gribnau J, Trimborn T, Nuez B, Philipsen S, Grosveld F, Fraser P. The role of EKLF in human β-globin gene competition. Genes Dev. 1996;10:2894–2902. doi: 10.1101/gad.10.22.2894. [DOI] [PubMed] [Google Scholar]
- 79.Wolffe A P, Pruss D. Targeting chromatin disruption: transcription regulators that acetylate histones. Cell. 1996;84:817–819. doi: 10.1016/s0092-8674(00)81059-4. [DOI] [PubMed] [Google Scholar]
- 80.Workman J L, Kingston R E. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- 81.Zhang W, Bieker J J. Acetylation and modulation of erythroid Kruppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc Natl Acad Sci USA. 1998;95:9855–9860. doi: 10.1073/pnas.95.17.9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhong H, Voll R E, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 83.Ziegler B L, Muller R, Valtieri M, Lamping C P, Thomas C A, Gabbianelli M, Giesert C, Buhring H J, Kanz L, Peschle C. Unicellular-unilineage erythropoietic cultures: molecular analysis of regulatory gene expression at sibling cell level. Blood. 1999;93:3355–3368. [PubMed] [Google Scholar]