Abstract
Introduction
As base excess had shown superiority over lactate as a prognostic parameter in intensive care unit (ICU) surgical patients we aimed to evaluate course of lactate, base excess and pH for prediction of mortality of medical ICU patients.
Materials and methods
For lactate, pH and base excess, values at the admission to ICU, at 24 ± 4 hours, maximum or minimum in the first 24 hours and in 24–48 hours after admission were collected from all patients admitted to the Medical ICU of the University Hospital Tübingen between January 2016 until December 2018 (N = 4067 at admission, N = 1715 with ICU treatment > 48 h) and investigated for prediction of in-hospital-mortality.
Results
Mortality was 22% and significantly correlated with all evaluated parameters. Strongest predictors of mortality determined by ROC were maximum lactate in 24 h (AUROC 0.74, cut off 2.7 mmol/L, hazard ratio of risk group with value > cut off 3.20) and minimum pH in 24 h (AUROC 0.71, cut off 7.31, hazard ratio for risk group 2.94). Kaplan Meier Curves stratified across these cut offs showed early and clear separation. Hazard ratios per standard deviation increase were highest for maximum lactate in 24 h (HR 1.65), minimum base excess in 24 h (HR 1.56) and minimum pH in 24 h (HR 0.75).
Conclusion
Lactate, pH and base excess were all suitable predictors of mortality in internal ICU patients, with maximum / minimum values in 24 and 24–48 h after admission altogether stronger predictors than values at admission. Base excess and pH were not superior to lactate for prediction of mortality.
Introduction
Estimation of the mortality of patients at intensive care unit (ICU) is necessary for treatment planning and treatment decisions as well as for support and advice for the patient’s relatives. Various surrogate parameters and their significance for the assessment of mortality risk have been evaluated, in particular lactate as read-out of anaerobic metabolism and tissue perfusion [1, 2]. Elevated lactate level is common in patients admitted to ICU and a strong predictor of mortality in unselected ICU patients [3, 4] and lactate clearance was recently discovered as an even stronger parameter than initial lactate level for assessing mortality risk of critically ill patients [5, 6].
To account for the ability to buffer a metabolic (lactate) acidosis, parameters of acid-base balance, such as base excess or pH, could represent the body’s conditions as more general parameters than lactate. Acid-base parameters have recently been evaluated as parameters for estimation of mortality in different subgroups of patients: In patients after cardiac surgery, base excess at ICU admission was a stronger parameter for prediction of ICU mortality than lactate-levels [7]. Lactate, anion gap and base excess were interchangeable biomarkers of traumatic shock [8] and base excess was a strong predictor of mortality in a large cohort of trauma patients [9]. Bicarbonate and anion gap were associated with higher mortality in sepsis patients even if lactate levels were low [10]. Metabolic acidosis at admission to ICU and early pH changes correlated with higher mortality in a small Indian cohort of critically ill patients [11]. However, for evaluation of acid base parameters as predictors of mortality of patients requiring treatment at a medical ICU, there is still a lack of data. We therefore aimed to evaluate parameters of acid-base balance obtainable by blood gas analysis as predictors of mortality in critically ill medical patients.
Materials and methods
Patients and blood gas analysis
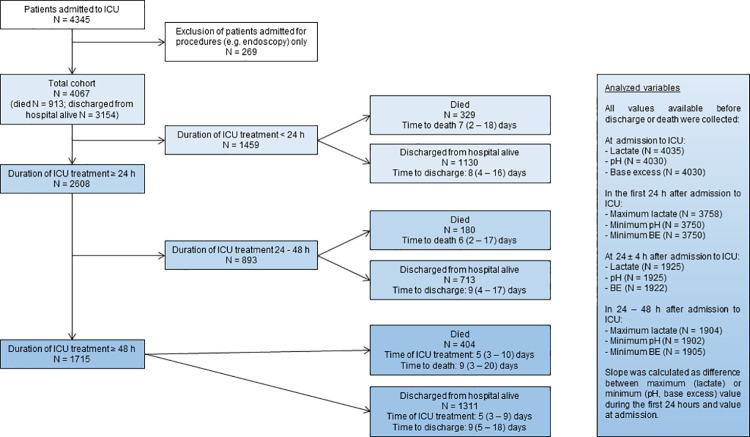
The study was approved by the local ethics committee of the University of Tuebingen and the need for consent was waived for this retrospective analysis (139/2019B02). Data from all patients admitted to the Medical ICU of the University Hospital Tübingen between January 2016 until December 2018 was collected from the patient data management system (ICCA, Philips GmbH) of the University Hospital Tübingen and evaluated retrospectively. Age, gender and SAPS II score at admission to ICU and need for invasive ventilation or dialysis during the treatment at ICU were documented (we did not use SAPS III score due to too many missing values). Laboratory data obtained included base excess, pH and lactate from arterial or venous blood gas analysis at admission to ICU and during the first 48 hours after admission to ICU. All blood gas analyses were performed with a Radiometer ABL90 FLEX. The following parameters were evaluated as predictors of mortality (Fig 1): value at admission, value after 24 ± 4 hours, maximum (lactate) or minimum (pH, base excess) value in the first 24 hours and between 24–48 hours after admission and slope of lactate, pH and base excess. Slope of the variables was calculated as difference between maximum (lactate) or minimum (pH, base excess) value during the first 24 hours and value at admission. All analyses were performed with all available patient data at the respective time points.
Fig 1. Flow chart study cohort and evaluated parameters.
Fig 1 shows a flow chart on the outcome of patients with different duration of ICU treatment with an overview of available numbers of examined parameters at the respective time points.
Mortality was defined by the outcome at discharge from hospital to one of two categories: patients who died in hospital and patients who were discharged from hospital alive. Primary diagnosis and cause of death were classified into groups according to the recorded ICD-10 (International Statistical Classification of Diseases and Related Health Problems) classification.
Statistical analysis
Statistical analyses were performed using R version 3.6.1, SAS JMP Pro 14.2.0 and MedCalc 19.1. Distributions are reported as number (n) and percent for categorical parameters. Median and interquartile range (IQ) are provided for continuous parameters. χ2 test (nominal variables) and Mann Whitney U test (continuous variables) were performed to test for differences between groups.
Computation of receiver operating characteristics (ROC, C-statistics) was performed to evaluate the ability of parameters to predict mortality, with determination of the cut off value by Youden index (J = sensitivity + (specificity– 1)), and the area under the receiver operating characteristics curve (AUROC) is reported. Hazard ratios were determined from Cox regression for risk groups divided by cut offs from ROC, or per increase of the variable of 1 standard deviation (SD). Kaplan Meier curves were constructed for groups stratified by cut-offs from C-statistics using log-rank test to test for differences. Statistical significance was determined by two-sided tests with an alpha of 0.05 (p < 0.05).
Results
Study cohort
A total number of 4067 patients was admitted to intensive care treatment at the medical ICU of the University Hospital Tübingen between January 2016 until December 2018 and included in the analysis as shown in Fig 1. N = 913 patients (22%) died after a median of 8 (interquartile range 2–18) days.
Causes of death in the cohort classified by ICD-10 category were I (‘diseases of circulatory system’, including stroke, intracranial bleeding, pulmonary embolism, myocardial infarction, cardiomyopathy, valvular diseases, cardiac arrhythmias; 21%), J (‘diseases of the respiratory system’, including pneumonia, chronic obstructive pulmonary disease; 16%), A + B (‘certain infectious and parasitic disease’, including sepsis; 16%), R57.0 (‘cardiogenic shock’; 13%), C + D (‘neoplasms and diseases of the blood and hematopoietic organs and certain disorders involving the immune system’; 11%), K (‘diseases of the digestive system’, including alcoholic cirrhosis of the liver; 7%), and R57.2 (‘septic shock’; 6%); in 10% of patients, cause of death could not be classified into one of these categories.
The full characteristics of the total study cohort and of the patients who died or were discharged from hospital alive are listed in Table 1. Patients who died or were discharged from hospital alive were not different regarding age and gender, but showed significant differences in SAPS II Score, need for invasive ventilation or dialysis, and base excess, lactate and pH values (Table 1). Patients could be assigned into groups based on primary diagnoses as follows (Table 1): Infectious = ICD R57.2 + A + B (n = 290), cardiac = ICD R57.0 + I (n = 1482), respiratory = ICD J (n = 726), malignant = ICD C + D (n = 421) and other / uncertain (n = 1148). In our ICU cohort, there was a relevant number of patients with a short duration of ICU treatment, N = 1715 patients (42% of the total cohort) required ICU treatment > 48 h. Table 2 compares characteristics of patients with duration of ICU treatment >48 h compared to patients with short term ICU treatment <48 h. Patients who required ICU treatment > 48 h had higher SAPS II score, and more often required invasive ventilation or dialysis. There was no significant difference in mortality, age, gender, primary diagnosis group, and base excess, lactate and pH at ICU admission between patients with ICU treatment duration > 48 h or < 48 h.
Table 1. Characteristics of study cohort.
| Total cohort | Patients who died | Patients discharged from hospital alive | p value | |
|---|---|---|---|---|
| Age | 68 (55–78) | 71 (60–80) | 67 (53–77) | <0.0001 |
| Gender (m, male; f, female) | m 2270 (56%) | m 511 (56%) | m 1757 (56%) | 0.9151 |
| f 1797 (44%) | f 402 (44%) | f 1395 (44%) | ||
| SAPS II score, points | 42 (28–54) | 44 (29–57) | 41 (28–53) | 0.0383 |
| SAPS II estimated mortality rate, % | 29 (9–55) | 32 (10–62) | 27 (9–53) | 0.0377 |
| Invasive ventilation | 1894 (47%) | 480 (53%) | 1416 (45%) | <0.0001 |
| Dialysis | 531 (13%) | 143 (16%) | 388 (12%) | 0.0079 |
| Primary diagnosis, | <0.0001 | |||
| • Infectious | 290 (7%) | 103 (11%) | 187 (6%) | |
| • Cardiac | 1482 (36%) | 298 (33%) | 1184 (38%) | |
| • Respiratory | 726 (18%) | 140 (15%) | 586 (19%) | |
| • Malignant | 421 (10%) | 168 (18%) | 253 (8%) | |
| • Other / uncertain | 1148 (28%) | 204 (22%) | 944 (30%) | |
| Duration to death or discharge, days | 8 (2–18) | 9 (4–17) | ||
| Base excess at admission, mmol/L | 0.2 (-4.2–3.9) | -3.4 (-9.2–2.3) | 0.8 (-2.9–4.3) | <0.0001 |
| Base excess at 24h, mmol/L | 1.1 (-2.2–5.0) | -0.3 (-4.0–3.4) | 1.8 (-1.4–5.5) | <0.0001 |
| Base excess minimum in 24h, mmol/L | -1.0 (-5.8–2.8) | -5.7 (-12.1–0.4) | -0.3 (-4.2–3.1) | <0.0001 |
| Base excess minimum in 24-48h, mmol/L | 3.2 (0.1–7.2) | 1.85 (-1.5–5.8) | 3.7 (0.6–7.5) | <0.0001 |
| Base excess slope, mmol/L | -0.3 (-2.0–0) | -0.9 (-3.2–0) | -0.1 (-1.8–0) | <0.0001 |
| Lactate at admission, mmol/L | 1.4 (0.9–2.4) | 2.3 (1.2–5.8) | 1.2 (0.8–2.0) | <0.0001 |
| Lactate at 24h, mmol/L | 1.1 (0.8–1.7) | 1.5 (0.9–2.5) | 1.0 (0.7–1.5) | <0.0001 |
| Lactate maximum in 24h, mmol/L | 1.7 (1.1–2.9) | 3.0 (1.6–9.0) | 1.5 (1.0–2.3) | <0.0001 |
| Lactate maximum in 24-48h, mmol/L | 1.4 (0.9–2.1) | 2.0 (1.3–3.6) | 1.2 (0.8–1.8) | <0.0001 |
| Lactate slope, mmol/L | -0.3 (-1.0–0) | -0.5 (-1.6–0) | -0.3 (-0.9–0) | <0.0001 |
| pH at admission | 7.39 (7.33–7.44) | 7.35 (7.23–7.43) | 7.40 (7.35–7.44) | <0.0001 |
| pH at 24h | 7.42 (7.37–7.47) | 7.40 (7.33–7.45) | 7.43 (7.39–7.47) | <0.0001 |
| pH minimum in 24h | 7.37 (7.28–7.41) | 7.28 (7.13–7.37) | 7.38 (7.32–7.42) | <0.0001 |
| pH minimum in 24-48h | 7.45 (7.41–7.49) | 7.43 (7.39–7.49) | 7.46 (7.42–7.5) | <0.0001 |
| pH slope | 0 (-0.05–0) | -0.03 (-0.09–0) | 0 (-0.04–0) | <0.0001 |
Values are n (%) for categorical variables and median (interquartile range) for continuous variables. Differences of groups of patients who died, and patients discharged from hospital alive were tested and p values are reported from χ2 test for nominal variables and Mann Whitney U test for continuous variables.
Definition of primary diagnosis groups: Infectious = ICD R57.2 + A + B; Cardiac = ICD R57.0 + I; Respiratory = ICD J; Malignant = ICD C + D; Other / uncertain
ICD-10:
A + B = Certain infectious and parasitic diseases
C + D = Neoplasms and Diseases of the blood and hematopoietic organs and certain disorders involving the immune system
E = Endocrine, nutritional and metabolic diseases
F = Mental and behavioral disorders
G = Diseases of the nervous system
I = diseases of circulatory system
J = Diseases of the respiratory system
K = Diseases of the digestive system
R57.0 = Cardiogenic shock; R57.1 = Hypovolemic shock; R57.2 = Septic shock.
Note: Pneumonia and ARDS classified in category J (disease of respiratory system)
Table 2. Characteristics of complete cases (patients with duration of ICU treatment >48 h) compared to patients with ICU treatment <48 h.
| Patients with ICU treatment >48 h | Patients with ICU treatment <48 h | p value | |
|---|---|---|---|
| Duration of ICU treatment | 5 (3–9) days | 21 (14–28) h | |
| Mortality | 404 (24%) | 509 (22%) | n.s. |
| Age | 67 (55–78) | 68 (55–78) | n.s. |
| Gender (m, male; f, female) | m 984 (57%) | m 1286 (55%) | n.s. |
| f 731 (43%) | f 1066 (45%) | ||
| SAPS II score, points | 43 (29–55) | 39 (27–52) | 0.0001 |
| SAPS II estimated mortality rate, % | 31 (10–58) | 23 (8–51) | 0.0002 |
| Invasive ventilation | 1177 (69%) | 719 (31%) | <0.0001 |
| Dialysis | 364 (21%) | 167 (17%) | <0.0001 |
| Primary diagnosis, | n.s. | ||
| • Infectious | 126 (7%) | 164 (7%) | |
| • Cardiac | 596 (35%) | 886 (38%) | |
| • Respiratory | 323 (19%) | 403 (17%) | |
| • Malignant | 195 (11%) | 226 (10%) | |
| • Other / uncertain | 475 (28%) | 673 (29%) | |
| Base excess at admission, mmol/L | 0.3 (-4.3–4.0) | 0.2 (-4.1–3.8) | n.s. |
| AUC for mort. 0.643 | AUC for mort. 0.655 | ||
| Lactate at admission, mmol/L | 1.4 (0.9–2.4) | 1.4 (0.9–2.5) | n.s. |
| AUROC for mort. 0.679 | AUC for mort. 0.713 | ||
| pH at admission | 7.39 (7.33–7.44) | 7.39 (7.32–7.44) | n.s. |
| AUROC for mort. 0.617 | AUC for mort. 0.639 |
Values are n (%) for categorical variables and median (interquartile range) for continuous variables. Differences of groups of patients with duration of ICU treatment >48 h or < 48 h were tested and p values are reported from χ2 test for nominal variables and Mann Whitney U test for continuous variables.
For base excess, lactate and pH at admission, AUROC for prediction of mortality is reported additionally (all p <0.0001).
Abbreviations: ICU, intensive care unit; h, hours; n.s., not significant; mort., mortality.
Univariate analysis: ROC and Cox regression
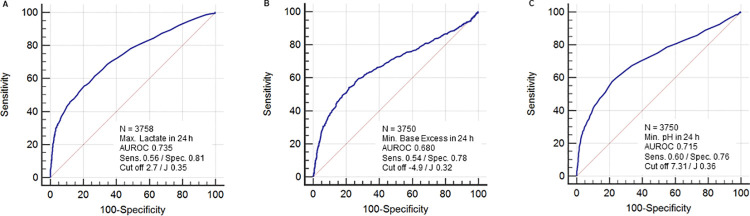
All evaluated variables (value at admission, value after 24 ± 4 hours, maximum (lactate) or minimum (pH, base excess) value in the first 24 hours and in 24–48 hours after admission and slope of lactate, pH and base excess) were associated significantly with mortality in univariate analysis (Table 3). The variables with the highest area under the receiver operating characteristics curve (AUROC) were maximum lactate in the first 24 hours after admission (AUROC 0.74, sensitivity 0.56 and specificity 0.81 at a cut off value of 2.7 mmol/L) and minimum pH in the first 24 hours after admission (AUROC 0.72, sensitivity 0.60 and specificity 0.76 at a cut off value of 7.31, Fig 2). Lactate, base excess and pH at ICU admission predicted mortality in both subgroups of patients with ICU treatment durations >48 h or <48 h (AUROC in Table 2).
Table 3. Univariate correlations with mortality: ROC and Cox regression.
| Parameter | N | AUROC | Risk group (Cut off) | HR of risk group | HR per SD(95% CI) |
|---|---|---|---|---|---|
| (95% CI) | (95% CI) | ||||
| Age, years | 4067 | 0.575 | > 58 | 1.61 | 1.35 |
| (0.560–0.591) | (1.37–1.90) | (1.26–1.46) | |||
| SAPS II estimated mortality rate, % | 2481 | 0.529 | > 52 | 1.33 | 1.10 |
| (0.509–0.548) | (1.12–1.59) | (1.01–1.19) | |||
| Base excess at admission, mmol/L | 4030 | 0.649 | < -3.8 | 2.27 | 0.69 |
| (0.635–0.664) | (1.99–2.59) | (0.65–0.74) | |||
| Base excess at 24h, mmol/L | 1922 | 0.604 | < -1.2 | 1.57 | 0.79 |
| (0.583–0.628) | (1.32–1.86) | (0.72–0.86) | |||
| Base excess min in 24h, mmol/L | 3750 | 0.680 | < -4.9 | 2.47 | 0.64 |
| (0.665–0.695) | (2.15–2.83) | (0.61–0.68) | |||
| Base excess min in 24-48h, mmol/L | 1905 | 0.602 | < 2.2 | 1.53 | 0.76 |
| (0.580–0.625) | (1.28–1.82) | (0.69–0.84) | |||
| Base excess slope, mmol/L | 3750 | 0.589 | < -2.4 | 1.52 | 0.84 |
| (0.572–0.604) | (1.31–1.75) | (0.81–0.89) | |||
| Lactate at admission, mmol/L | 4035 | 0.698 | > 2.1 | 2.93 | 1.39 |
| (0.683–0.712) | (2.57–3.34) | (1.34–1.44) | |||
| Lactate at 24h, mmol/L | 1925 | 0.652 | > 1.4 | 2.06 | 1.26 |
| (0.632–0.675) | (1.74–2.44) | (1.21–1.32) | |||
| Lactate max in 24h, mmol/L | 3758 | 0.735 | > 2.7 | 3.20 | 1.40 |
| (0.721–0.749) | (2.79–3.67) | (1.35–1.44) | |||
| Lactate max in 24-48h, mmol/L | 1904 | 0.702 | > 1.7 | 2.20 | 1.30 |
| (0.683–0.724) | (1.84–2.64) | (1.24–1.36) | |||
| Lactate slope, mmol/L | 3758 | 0.574 | < -1.0 | 1.62 | 0.84 |
| (0.557–0.589) | (1.40–1.86) | (0.80–0.88) | |||
| pH at admission | 4030 | 0.630 | < 7.31 | 2.60 | 0.72 |
| (0.614–0.645) | (2.28–2.97) | (0.68–0.75) | |||
| pH at 24h | 1925 | 0.640 | < 7.36 | 1.89 | 0.76 |
| (0.617–0.661) | (1.59–2.26) | (0.71–0.81) | |||
| pH min in 24h | 3750 | 0.715 | < 7.31 | 2.94 | 0.64 |
| (0.700–0.729) | (2.56–3.38) | (0.61–0.67) | |||
| pH min in 24-48h | 1902 | 0.592 | < 7.43 | 1.73 | 0.77 |
| (0.569–0.614) | (1.45–2.07) | (0.71–0.83) | |||
| pH slope | 3751 | 0.612 | < -0.05 | 1.54 | 0.95 |
| (0.597–0.628) | (1.36–1.80) | (0.93–0.98) |
Hazard ratios are of risk group defined by cut off from ROC (e.g. risk group with age ≥ 58 years compared to group with age < 58 years) and per standard deviation increase.
Values with p < 0.05 are listed only. There was no significant correlation of gender and mortality. Highest AUROC and highest or lowest hazard ratios are marked in bold.
Abbreviations: AUROC, Area under the receiver operating characteristic curve; HR, hazard ratio; CI, confidence interval; n.s., not significant; min, minimum; max, maximum.
Fig 2.
ROC analysis of mortality by maximum lactate (A), minimum base excess (B) and minimum pH (C) in the first 24 h after admission. Abbreviations: AUROC, Area under the receiver operating characteristics curve; max., maximum; min., minimum; sens., sensitivity; spec., specificity; J, Youden-Index.
In proportional hazard analyses using the cut-offs from ROC analyses for stratification of risk groups, the highest hazard ratios were found for base excess, lactate and pH at admission and for minimum base excess, minimum pH and maximum lactate in 24 hours after admission (Table 3), with hazard ratio for minimum or maximum values in the first 24 h overall higher than for values at admission. In proportional hazards determined per standard deviation, maximum lactate in 24 h, lactate at admission, minimum base excess in 24 h and minimum pH in 24 h showed highest or lowest hazard ratio per SD (Table 3).
Results of proportional hazard analyses in the subgroups of primary diagnoses overall resembled the results in the total cohort (Table 4): Maximum lactate in 24 h was a strong predictor of mortality in all groups; lactate values were overall strong predictors of mortality, and the interval-related maximum or minimum values of all markers were overall stronger predictors of mortality than values at admission. Additionally, base excess at admission and minimum base excess in 24 h were strong predictors of mortality in the cardiac disease group; and age was a strong predictor of mortality in the respiratory disease group (Table 4).
Table 4. Univariate hazard ratios for subgroups of primary diagnosis.
| Primary diagnosis | Infectious | Cardiac | Respiratory | Malignant | Uncertain / other |
|---|---|---|---|---|---|
| n = 290 | n = 1482 | n = 726 | n = 421 | n = 1148 | |
| Age | 1.24 | 1.27 | 2.13 | n.s. | 1.61 |
| (1.00–1.56) | (1.09–1.49) | (1.65–2.79) | (1.40–1.85) | ||
| SAPS II score | n.s. | n.s. | n.s. | 1.28 | n.s. |
| (1.05–1.57) | |||||
| Base excess at admission | 0.76 | 0.54 | 0.77 | 0.75 | 0.75 |
| (0.62–0.93) | (0.49–0.61) | (0.66–0.90) | (0.63–0.90) | (0.67–0.85) | |
| Base excess at 24h | n.s. | 0.76 | 0.79 | n.s. | 0.72 |
| (0.62–0.93) | (0.69–0.93) | (0.59–0.87) | |||
| Base excess min in 24h | 0.75 | 0.53 | 0.73 | 0.71 | 0.63 |
| (0.63–0.90) | (0.47–0.59) | (0.62–0.86) | (0.60–0.84) | (0.56–0.72) | |
| Base excess min in 24-48h | n.s. | n.s. | 0.79 | n.s. | 0.65 |
| (0.65–0.95) | (0.52–0.80) | ||||
| Base excess slope | 0.86 | 0.85 | 0.83 | n.s. | 0.83 |
| (0.77–0.97) | (0.77–0.95) | (0.73–0.96) | (0.77–0.90) | ||
| Lactate at admission | 1.37 | 1.46 | 1.49 | 1.40 | 1.33 |
| (1.23–1.51) | (1.38–1.54) | (1.20–1.79) | (1.21–1.59) | (1.23–1.42) | |
| Lactate at 24h | 1.40 | 1.30 | 1.17 | 1.18 | 1.28 |
| (1.25–1.55) | (1.16–1.42) | (1.00–1.32) | (1.03–1.37) | (1.19–1.37) | |
| Lactate max in 24h | 1.32 | 1.53 | 1.52 | 1.56 | 1.46 |
| (1.20–1.45) | (1.44–1.62) | (1.28–1.76) | (1.01–1.26) | (1.36–1.57) | |
| Lactate max in 24-48h | 1.34 | 1.29 | 1.48 | 1.14 | 1.41 |
| (1.19–1.50) | (1.18–1.39) | (1.13–1.85) | (1.00–1.25) | (1.29–1.52) | |
| Lactate slope | n.s. | 0.79 | 0.78 | n.s. | n.s. |
| (0.69–0.93) | (0.73–0.84) | ||||
| pH at admission | 0.72 | 0.64 | 0.76 | 0.85 | 0.73 |
| (0.63–0.84) | (0.60–0.69) | (0.66–0.87) | (0.76–0.96) | (0.65–0.82) | |
| pH at 24h | 0.70 | 0.69 | 0.81 | 0.78 | 0.72 |
| (0.59–0.85) | (0.60–0.80) | (0.72–0.93) | (0.65–0.94) | (0.63–0.85) | |
| pH min in 24h | 0.71 | 0.60 | 0.66 | 0.76 | 0.61 |
| (0.62–0.82) | (0.55–0.64) | (0.58–0.75) | (0.68–0.86) | (0.54–0.69) | |
| pH min in 24-48h | 0.82 | 0.75 | 0.75 | 0.76 | 0.73 |
| (0.69–0.98) | (0.64–0.88) | (0.63–0.91) | (0.63–0.93) | (0.61–0.88) | |
| pH slope | 0.80 | n.s. | 0.54 | 0.64 | 0.58 |
| (0.65–1.00) | (0.44–0.69) | (0.50–0.84) | (0.49–0.70) |
Values are Hazard ratio per standard deviation increase and 95% confidence interval. Highest or lowest hazard ratios for every group of primary diagnosis are marked in bold. For definition of primary diagnosis groups see Table 1.
n.s. = not significant; for all other tests p-value was < 0.05.
Abbreviations: min, minimum; max, maximum.
Kaplan Meier curves
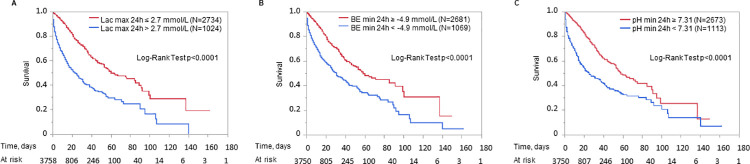
Kaplan Meier curves for groups stratified by cut offs from C-statistics are shown as an example for maximum lactate in 24 h after admission and minimum base excess and minimum pH in 24 h after admission (Fig 3). Kaplan Meier curves for the cut offs maximum lactate in 24 h > 2.7 mmol/l, minimum base excess in 24 h < -4.9 mmol/L and minimum pH in 24 h < 7.31 showed a clear separation particularly in the first 20 days after admission to ICU (Fig 3).
Fig 3.
Kaplan Meier curve of mortality by maximum lactate (A), minimum base excess (B) and minimum pH (C) in the first 24 h after admission. Cut off values used for stratification in risk groups were determined by ROC analysis. Abbreviations: Lac, lactate; BE, base excess.
Discussion
In our cohort of medical ICU patients, all investigated parameters, lactate, pH and base excess, were suitable predictors of ICU mortality. Cut off values at admission to ICU for prediction of mortality were with 2.1 mmol/L for lactate and -3.8 mmol/L for base excess in a range consistent with the values determined in other studies (around 1.5–2.5 mmol/L for lactate and -4 - -6 mmol/L for base excess) [4, 12–14].
However, base excess and pH were not superior to lactate for prediction of mortality in this unselected cohort of medical ICU patients. This is in contrast to patients after heart surgery at admission to ICU, where base excess was superior to lactate for prediction of mortality [7] and trauma patients, where base excess has been found a strong predictor of mortality [9, 14]. Interestingly, in our subgroup of cardiac patients, base excess in addition to lactate was also a strong predictor of mortality. In our analyses of the total cohort of medical ICU patients, lactate was the strongest predictor of ICU mortality, followed by pH: Lactate values showed the highest AUROC in ROC analysis and the highest Hazard ratio per standard deviation increase; pH values had the second highest AUROC in ROC analysis; Kaplan Meier curve stratified by maximum lactate over the first 24 h and minimum pH over the first 24 h showed the clearest separations.
For lactate, prognostic significance of the course or clearance has been evaluated [3, 13, 15–17]. In sepsis patients, lactate at 24 hours was found to be strongest predictor of mortality in serial lactate measurements [17] and early lactate clearance was associated with improved outcome [16]. In other unselected cohorts of ICU patients, mortality was higher in patients developing high lactate levels after more than 24 hours following ICU admission or missing lactate clearance in the first 12 hours [3] and lactate at 24 hours after admission to ICU was strongest for prediction of mortality [18]. There are systematic reviews available, that found that across different ICU cohorts lactate clearance was associated with a better outcome [19, 20]; the significance of the course of lactate was thereby independent of the initial value and it was recommended to monitor the lactate level by measurements every 1 to 2 hours [21]. Lactate-guided therapy with monitoring the course of lactate levels after admission to ICU has been suggested to improve treatment outcome [22]. Our findings are consistent with these reports: In our cohort of medical ICU patients, from all lactate values, maximum lactate during the first 24 hours and during 24 to 48 hours after admission to ICU were strongest predictors for mortality in the total cohort and in primary diagnosis subgroups. Altogether, lactate values both at admission and during 48 hours after admission to the ICU are valuable indicators for prognosis assessment.
This results in the question, whether the course of values in the first hours after initiation of intensive care treatment should also be considered for other markers used for evaluation of mortality risk. In patients with extracorporeal life support after out of hospital cardiac arrest, lactate and base excess both showed best predictive power for values measured 3 h after initiation of extracorporeal life support [23]. In our cohort of medical ICU patients, initial values of pH and base excess were less predictive than values in the first 24 to 48 hours of the ICU stay. The strongest predictors were the maximum or minimum values during the first 24 hours after admission. These were also superior to the slope between value at admission and maximum or minimum value in the first 24 hours. Our study therefore corroborates the prognostic significance of the values of all parameters, lactate, pH and base excess, in the first 24 to 48 hours after admission to intensive care unit compared to the single value at admission.
In out cohort, SAPS II predicted mortality with a low precision (AUROC only 0.529) compared to the original AUROC of 0.86 in the generation cohort from 1993 [24]. The actual mortality rate in our cohort was with 22% lower than the mortality rate estimated by SAPS II (which was 29%). This could be due to improved treatment, highlighting that mortality remains difficult to predict as it is dependent on many influenceable and non-influenceable factors. Estimators used to assess mortality risk should be reviewed repeatedly (as happened by developing SAPS III). Blood gas analysis represents a feasible tool to estimate mortality in clinical routine as it is easy to perform (in contrast to scores using multiple parameters) and can be re-evaluated continuously.
We had a relevant number of patients with short term ICU treatment <48 h. Patients who needed treatment at ICU longer than 48 h had a higher SAPS II estimated mortality rate. However, lactate, base excess and pH at admission to ICU were all suitable to predict mortality irrespective of duration of ICU treatment. Course of lactate, base excess and pH also predicted mortality in all primary diagnosis subgroups. This undermines the value of blood gas analysis for estimation of mortality in all critically ill medical patients.
The study is limited by the retrospective and single-center design. Additionally, the influence of treatment on the investigated biomarkers could only be assessed to a limited extent, whereby the response to therapy is likely to be reflected in the course of the parameters: A better prediction of mortality by biomarkers assessed during the course of ICU treatment, compared with values at admission to ICU, reflects the association of poor response to therapy, and thus poor recovery of organ function and normalization of acid-base balance, with mortality. The study complements previous studies in the field of mortality prediction in critically ill patients by highlighting the analytes pH and base excess in addition to lactate and their course over the first 48 hours after admission to ICU in medical ICU patients.
In conclusion, lactate, pH and base excess proved to be consistently valid parameters for estimating mortality in medical critically ill patients, and monitoring changes in these parameters during the first hours of intensive care treatment can improve the accuracy of mortality estimates.
Supporting information
(XLSX)
Acknowledgments
We thank Dr. Bernhard N. Bohnert for the valuable assistance during the analyses. We acknowledge support by Open Access Publishing Fund of University of Tübingen.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. 2014;371(24):2309–19. Epub 2014/12/11. doi: 10.1056/NEJMra1309483 . [DOI] [PubMed] [Google Scholar]
- 2.Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med. 2009;37(10):2827–39. Epub 2009/08/27. doi: 10.1097/CCM.0b013e3181a98899 . [DOI] [PubMed] [Google Scholar]
- 3.Haas SA, Lange T, Saugel B, Petzoldt M, Fuhrmann V, Metschke M, et al. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive care medicine. 2016;42(2):202–10. Epub 2015/11/12. doi: 10.1007/s00134-015-4127-0 . [DOI] [PubMed] [Google Scholar]
- 4.Juneja D, Singh O, Dang R. Admission hyperlactatemia: causes, incidence, and impact on outcome of patients admitted in a general medical intensive care unit. J Crit Care. 2011;26(3):316–20. Epub 2011/01/25. doi: 10.1016/j.jcrc.2010.11.009 . [DOI] [PubMed] [Google Scholar]
- 5.Masyuk M, Wernly B, Lichtenauer M, Franz M, Kabisch B, Muessig JM, et al. Prognostic relevance of serum lactate kinetics in critically ill patients. Intensive care medicine. 2019;45(1):55–61. Epub 2018/11/28. doi: 10.1007/s00134-018-5475-3 . [DOI] [PubMed] [Google Scholar]
- 6.Nichol A, Bailey M, Egi M, Pettila V, French C, Stachowski E, et al. Dynamic lactate indices as predictors of outcome in critically ill patients. Crit Care. 2011;15(5):R242. Epub 2011/10/22. doi: 10.1186/cc10497 ; PubMed Central PMCID: PMC3334793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zante B, Reichenspurner H, Kubik M, Kluge S, Schefold JC, Pfortmueller CA. Base excess is superior to lactate-levels in prediction of ICU mortality after cardiac surgery. PloS one. 2018;13(10):e0205309. Epub 2018/10/06. doi: 10.1371/journal.pone.0205309 ; PubMed Central PMCID: PMC6173442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caputo ND, Kanter M, Fraser R, Simon R. Comparing biomarkers of traumatic shock: the utility of anion gap, base excess, and serum lactate in the ED. Am J Emerg Med. 2015;33(9):1134–9. Epub 2015/06/02. doi: 10.1016/j.ajem.2015.04.085 . [DOI] [PubMed] [Google Scholar]
- 9.Jin WYY, Jeong JH, Kim DH, Kim TY, Kang C, Lee SH, et al. Factors predicting the early mortality of trauma patients. Ulusal travma ve acil cerrahi dergisi = Turkish journal of trauma & emergency surgery: TJTES. 2018;24(6):532–8. Epub 2018/12/06. doi: 10.5505/tjtes.2018.29434 . [DOI] [PubMed] [Google Scholar]
- 10.Mitra B, Roman C, Charters KE, O’Reilly G, Gantner D, Cameron PA. Lactate, bicarbonate and anion gap for evaluation of patients presenting with sepsis to the emergency department: A prospective cohort study. Emergency medicine Australasia: EMA. 2019. Epub 2019/06/12. doi: 10.1111/1742-6723.13324 . [DOI] [PubMed] [Google Scholar]
- 11.Samanta S, Singh RK, Baronia AK, Mishra P, Poddar B, Azim A, et al. Early pH Change Predicts Intensive Care Unit Mortality. Indian journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2018;22(10):697–705. Epub 2018/11/09. doi: 10.4103/ijccm.IJCCM_129_18 ; PubMed Central PMCID: PMC6201653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith I, Kumar P, Molloy S, Rhodes A, Newman PJ, Grounds RM, et al. Base excess and lactate as prognostic indicators for patients admitted to intensive care. Intensive care medicine. 2001;27(1):74–83. Epub 2001/03/31. doi: 10.1007/s001340051352 . [DOI] [PubMed] [Google Scholar]
- 13.Filho RR, Rocha LL, Corrêa TD, Pessoa CM, Colombo G, Assuncao MS. Blood Lactate Levels Cutoff and Mortality Prediction in Sepsis-Time for a Reappraisal? a Retrospective Cohort Study. Shock. 2016;46(5):480–5. Epub 2016/10/19. doi: 10.1097/SHK.0000000000000667 ; PubMed Central PMCID: PMC5058781 of interest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamed R, Mekki I, Aouni H, Hedhli H, Zoubli A, Maaref A, et al. Base Excess usefulness for prediction of immediate mortality in severe trauma patients admitted to the Emergency department. Tunis Med. 2019;97(12):1357–61. Epub 2020/03/17. . [PubMed] [Google Scholar]
- 15.Ryoo SM, Lee J, Lee YS, Lee JH, Lim KS, Huh JW, et al. Lactate Level Versus Lactate Clearance for Predicting Mortality in Patients With Septic Shock Defined by Sepsis-3. Crit Care Med. 2018;46(6):e489–e95. Epub 2018/02/13. doi: 10.1097/CCM.0000000000003030 . [DOI] [PubMed] [Google Scholar]
- 16.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32(8):1637–42. Epub 2004/08/03. doi: 10.1097/01.ccm.0000132904.35713.a7 . [DOI] [PubMed] [Google Scholar]
- 17.Mahmoodpoor A, Shadvar K, Saghaleini SH, Koleini E, Hamishehkar H, Ostadi Z, et al. Which one is a better predictor of ICU mortality in septic patients? Comparison between serial serum lactate concentrations and its removal rate. J Crit Care. 2018;44:51–6. Epub 2017/10/25. doi: 10.1016/j.jcrc.2017.10.019 . [DOI] [PubMed] [Google Scholar]
- 18.Hayashi Y, Endoh H, Kamimura N, Tamakawa T, Nitta M. Lactate indices as predictors of in-hospital mortality or 90-day survival after admission to an intensive care unit in unselected critically ill patients. PloS one. 2020;15(3):e0229135. Epub 2020/03/10. doi: 10.1371/journal.pone.0229135 ; PubMed Central PMCID: PMC7062275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Xu X. Lactate clearance is a useful biomarker for the prediction of all-cause mortality in critically ill patients: a systematic review and meta-analysis*. Crit Care Med. 2014;42(9):2118–25. Epub 2014/05/07. doi: 10.1097/CCM.0000000000000405 . [DOI] [PubMed] [Google Scholar]
- 20.Chertoff J, Chisum M, Garcia B, Lascano J. Lactate kinetics in sepsis and septic shock: a review of the literature and rationale for further research. J Intensive Care. 2015;3:39. Epub 2015/10/09. doi: 10.1186/s40560-015-0105-4 ; PubMed Central PMCID: PMC4594907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vincent JL, Quintairos ESA, Couto L Jr., Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care. 2016;20(1):257. Epub 2016/08/16. doi: 10.1186/s13054-016-1403-5 ; PubMed Central PMCID: PMC4983759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(6):752–61. Epub 2010/05/14. doi: 10.1164/rccm.200912-1918OC . [DOI] [PubMed] [Google Scholar]
- 23.Jouffroy R, Lamhaut L, Guyard A, Phillipe P, Deluze T, Jaffry M, et al. Base excess and lactate as prognostic indicators for patients treated by extra corporeal life support after out hospital cardiac arrest due to acute coronary syndrome. Resuscitation. 2014;85(12):1764–8. Epub 2014/12/03. doi: 10.1016/j.resuscitation.2014.10.012 . [DOI] [PubMed] [Google Scholar]
- 24.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. Jama. 1993;270(24):2957–63. Epub 1993/12/22. doi: 10.1001/jama.270.24.2957 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.