Abstract
Background
In a review and meta‐analysis conducted in 1993, psychological preparation was found to be beneficial for a range of outcome variables including pain, behavioural recovery, length of stay and negative affect. Since this review, more detailed bibliographic searching has become possible, additional studies testing psychological preparation for surgery have been completed and hospital procedures have changed. The present review examines whether psychological preparation (procedural information, sensory information, cognitive intervention, relaxation, hypnosis and emotion‐focused intervention) has impact on the outcomes of postoperative pain, behavioural recovery, length of stay and negative affect.
Objectives
To review the effects of psychological preparation on postoperative outcomes in adults undergoing elective surgery under general anaesthetic.
Search methods
We searched the Cochrane Register of Controlled Trials (CENTRAL 2014, Issue 5), MEDLINE (OVID SP) (1950 to May 2014), EMBASE (OVID SP) (1982 to May 2014), PsycINFO (OVID SP) (1982 to May 2014), CINAHL (EBESCOhost) (1980 to May 2014), Dissertation Abstracts (to May 2014) and Web of Science (1946 to May 2014). We searched reference lists of relevant studies and contacted authors to identify unpublished studies. We reran the searches in July 2015 and placed the 38 studies of interest in the `awaiting classification' section of this review.
Selection criteria
We included randomized controlled trials of adult participants (aged 16 or older) undergoing elective surgery under general anaesthesia. We excluded studies focusing on patient groups with clinically diagnosed psychological morbidity. We did not limit the search by language or publication status. We included studies testing a preoperative psychological intervention that included at least one of these seven techniques: procedural information; sensory information; behavioural instruction; cognitive intervention; relaxation techniques; hypnosis; emotion‐focused intervention. We included studies that examined any one of our postoperative outcome measures (pain, behavioural recovery, length of stay, negative affect) within one month post‐surgery.
Data collection and analysis
One author checked titles and abstracts to exclude obviously irrelevant studies. We obtained full reports of apparently relevant studies; two authors fully screened these. Two authors independently extracted data and resolved discrepancies by discussion.
Where possible we used random‐effects meta‐analyses to combine the results from individual studies. For length of stay we pooled mean differences. For pain and negative affect we used a standardized effect size (the standardized mean difference (SMD), or Hedges' g) to combine data from different outcome measures. If data were not available in a form suitable for meta‐analysis we performed a narrative review.
Main results
Searches identified 5116 unique papers; we retrieved 827 for full screening. In this review, we included 105 studies from 115 papers, in which 10,302 participants were randomized. Mainly as a result of updating the search in July 2015, 38 papers are awaiting classification. Sixty‐one of the 105 studies measured the outcome pain, 14 behavioural recovery, 58 length of stay and 49 negative affect. Participants underwent a wide range of surgical procedures, and a range of psychological components were used in interventions, frequently in combination. In the 105 studies, appropriate data were provided for the meta‐analysis of 38 studies measuring the outcome postoperative pain (2713 participants), 36 for length of stay (3313 participants) and 31 for negative affect (2496 participants). We narratively reviewed the remaining studies (including the 14 studies with 1441 participants addressing behavioural recovery). When pooling the results for all types of intervention there was low quality evidence that psychological preparation techniques were associated with lower postoperative pain (SMD ‐0.20, 95% confidence interval (CI) ‐0.35 to ‐0.06), length of stay (mean difference ‐0.52 days, 95% CI ‐0.82 to ‐0.22) and negative affect (SMD ‐0.35, 95% CI ‐0.54 to ‐0.16) compared with controls. Results tended to be similar for all categories of intervention, although there was no evidence that behavioural instruction reduced the outcome pain. However, caution must be exercised when interpreting the results because of heterogeneity in the types of surgery, interventions and outcomes. Narratively reviewed evidence for the outcome behavioural recovery provided very low quality evidence that psychological preparation, in particular behavioural instruction, may have potential to improve behavioural recovery outcomes, but no clear conclusions could be reached.
Generally, the evidence suffered from poor reporting, meaning that few studies could be classified as having low risk of bias. Overall,we rated the quality of evidence for each outcome as ‘low’ because of the high level of heterogeneity in meta‐analysed studies and the unclear risk of bias. In addition, for the outcome behavioural recovery, too few studies used robust measures and reported suitable data for meta‐analysis, so we rated the quality of evidence as `very low'.
Authors' conclusions
The evidence suggested that psychological preparation may be beneficial for the outcomes postoperative pain, behavioural recovery, negative affect and length of stay, and is unlikely to be harmful. However, at present, the strength of evidence is insufficient to reach firm conclusions on the role of psychological preparation for surgery. Further analyses are needed to explore the heterogeneity in the data, to identify more specifically when intervention techniques are of benefit. As the current evidence quality is low or very low, there is a need for well‐conducted and clearly reported research.
Plain language summary
The effect of psychological preparation on pain, behavioural recovery, negative emotion and length of stay after surgery
Background
The way people think and feel before surgery can affect how they feel and what they do after surgery. For example, research shows that people who feel more anxious before their surgery experience more pain after it. A review conducted in 1993 looked at the impact of psychological preparation on outcomes after surgery. The term `psychological preparation' includes a range of techniques that aim to change what people think, how they feel or what they do. This 1993 review found that psychological preparation techniques reduced pain after surgery, improved behavioural recovery (how quickly people return to activities), decreased length of stay in hospital and reduced negative emotion (e.g. feelings of anxiety or depression). We aimed to carry out an up‐to‐date review using Cochrane methodology to learn whether there are helpful (or harmful) effects of psychological preparation for people undergoing surgery, and which outcomes (pain after surgery, behavioural recovery, negative emotion or length of stay) are improved.
Study characteristics
We included studies of adults who received planned surgery with general anaesthesia. We looked at seven psychological preparation techniques: procedural information (information about what, when and how processes will happen); sensory information (what the experience will feel like and what other sensations they may have, e.g. taste, smell); behavioural instruction (telling patients what they need to do); cognitive intervention (techniques that aim to change how people think); relaxation techniques; hypnosis; and emotion‐focused interventions (techniques that aim to help people to manage their feelings). The psychological preparation had to be delivered before surgery for the study to be included in the review. We included studies that looked at the effect of psychological preparation on pain, behavioural recovery, length of stay and negative emotion after surgery (within one month). Studies were included in the review up to the search date of 4 May 2014. We updated the search on 7 July 2015 and will incorporate the 38 studies found in this later search when the review is updated. We included 105 studies from 115 papers, with 10,302 participants taking part. Sixty‐one studies measured the outcome pain, 14 behavioural recovery, 58 length of stay and 49 negative emotion. In accordance with the review protocol, we did not record details about funding sources.
Key results
In this review we included 105 studies, which were reported in 115 papers. A total of 10,302 participants were randomized in these studies. For pain, length of stay and negative emotion we combined numerical findings from the studies. We found that psychological preparation before surgery seemed to reduce pain and negative emotion after the operation and may reduce the time spent in hospital by around half a day but the quality of the evidence was low. Also, the studies used many different psychological preparation techniques (often in different combinations) so it was not possible to discover which techniques were better. We could not statistically combine numerical findings for behavioural recovery because few studies provided sufficient details and studies used different ways of measuring how quickly people returned to usual activities. In reviewing the studies, we found that psychological preparation, in particular behavioural instruction, may have the potential to improve behavioural recovery. However, the quality of this evidence was very low. We looked at the effect of psychological preparation on pain, behavioural recovery, length of stay and negative emotion in this review and did not find evidence to suggest that psychological preparation might lead to harm in these outcomes. However, as we did not look at other outcomes it is possible that we did not identify potential harm.
Quality of the evidence
Many studies were poorly reported, so we could not be confident that findings were reliable. For this reason and because of the large variation in psychological techniques, types of surgery and measures used, we graded the quality of the evidence as `low' for the outcomes pain, negative emotion and length of stay; we cannot be confident that these techniques help patients to recover from surgery. For behavioural recovery, we further downgraded the quality of the evidence to `very low' because of problems with measurement and reporting of the outcome.
Summary of findings
Background
Many people experience anxiety and negative cognitions when approaching surgery (Mathews 1981). There is good evidence that how people think and feel before surgery affects their outcomes after surgery. Negative psychological factors such as anxiety, depression and catastrophizing have been found to predict postoperative pain (Arpino 2004; Bruce 2012; Granot 2005; Munafó 2001). Catastrophizing has been defined as "an exaggerated negative orientation toward noxious stimuli" (Sullivan 1995).
A range of mechanisms exist by which psychological variables could affect recovery after surgery. First, negative emotions can enhance pain sensations (Rainville 2005; van Middendorp 2010). Second, cognitions and emotions influence behaviour (for example doing physiotherapy exercises, taking analgesics) and are likely to influence pain and return to usual activities. Third, stress has been linked to the slower healing of wounds through psychoneuroimmunological mechanisms (mechanisms whereby psychology interacts with the nervous and immune systems) (Maple 2015; Marucha 1998; Walburn 2009). It is therefore likely that psychological interventions that reduce negative emotions such as anxiety, worry about surgery and perceptions of stress, or that change patients' recovery‐related behaviour, may lead to positive postoperative outcomes.
Psychological preparation for surgery has been demonstrated to improve outcomes. In a review and meta‐analysis (Johnston 1993), psychological preparation was found to be beneficial for a range of outcome variables that included negative affect, pain, pain medication, length of hospital stay, behavioural recovery, clinical recovery, physiological indices and satisfaction.
Since the 1993 review (Johnston 1993), this research field has continued to develop. Standards of conducting randomized controlled trials have improved, technology has advanced to permit more detailed bibliographic searching and new studies testing psychological preparation procedures have been published. The present review tested, using modern review techniques, analysis methods and a larger research base, a) whether there is evidence for beneficial (or harmful) effects of psychological preparation for surgery, and b) which outcomes of pain, behavioural recovery, length of stay and negative affect are improved (or worsened) following preparation.
Description of the condition
Surgery is carried out for a range of health conditions either as a diagnostic or treatment intervention. While surgery may lead to health improvements, it also negatively impacts on a range of health outcomes including pain, activity limitations and anxiety, at least in the short term (Johnston 1980).
Elective surgery differs from emergency surgery in that patients have time to prepare themselves and to be prepared for surgery. Preparation for emergency surgery is much more difficult to provide in a controlled manner and the effectiveness of such interventions is likely to differ because of that difference in context. Thus, emergency surgery should be considered separately and we only included participants undergoing elective surgery in this review.
Different psychological threats and coping mechanisms can be involved for the patient depending on whether procedures are undertaken using general anaesthetic or local anaesthetic. For example in some procedures that are performed under local anaesthetic the patients are required to be actively involved, and so effective preparation will have different components compared with preparation for a procedure where the patient is unconscious. Therefore, following Johnston 1993, we only included procedures involving general anaesthetic.
Description of the intervention
Psychological preparation incorporates a range of strategies designed to influence how a person feels, thinks or acts (emotions, cognitions or behaviours). Johnston 1993 found that the following types of intervention benefited patients, on at least one postoperative outcome: procedural information, sensory information, behavioural instruction, cognitive intervention, relaxation techniques, hypnosis and emotion‐focused interventions.
Procedural information
Procedural information describes the process the patient will undergo in terms of what will happen, when it will happen and how it will happen.
Sensory information
Sensory information describes the experiential aspects of the procedure, that is, what it will feel like and any other relevant sensations (for example taste, smell).
Behavioural instruction
Behavioural instruction consists of telling patients what they should do to facilitate either the procedure or their recovery from the procedure (Mathews 1984). For example a patient could be told how to use equipment, such as a patient‐controlled analgesia pump.
Cognitive interventions
Cognitive interventions aim to change how an individual thinks, especially about negative aspects of the procedure. Cognitive techniques include cognitive reframing and distraction. Cognitive reframing involves developing a different perspective that enables a positive or neutral rather than negative thought, for example focusing on the number of people who do well after a surgical procedure rather than the number who fare badly. Distraction leads to focusing thoughts on other things (and could include relaxation).
Relaxation techniques
These involve "systematic instruction in physical and cognitive strategies to reduce sympathetic arousal, and to increase muscle relaxation and a feeling of calm" (Michie 2008). Relaxation techniques can be used before surgery to reduce tension and anxiety and include progressive muscle relaxation (where each muscle group is tensed and then relaxed), simple relaxation (each muscle group is relaxed in turn), breathing techniques (for example the practice of diaphragmatic breathing) and guided imagery (for example imagining a pleasant, relaxing environment).
Hypnosis
A range of procedures are used for hypnotic induction, including suggestions to relax. During hypnosis "one person (the subject) is guided by another (the hypnotist) to respond to suggestions for changes in subjective experience, alterations in perception, sensation, emotion, thought or behavior" (APA 2005).
Emotion‐focused interventions
Emotion‐focused interventions aim to enable the person to regulate or manage their feelings or emotions. Emotion‐focused methods include: enabling the discussion, expression or acceptance of emotions; facilitating contextualization (putting emotions into context, e.g. of life, relationships, past experiences); and enabling the understanding of emotions (e.g. giving them meaning). In this review, if the focus of the intervention was to change how someone thinks, we coded it as a ‘cognitive intervention’.
How the intervention might work
Studies have shown that psychological preparation for surgery can have a beneficial effect upon a range of postoperative outcomes (Johnston 1993). Likely mechanisms for these processes vary depending upon the intervention used. Some intervention types focus on reducing negative emotions, such as anxiety, and negative thought processes. Providing procedural information is expected to reduce anxiety because it helps the patient to know what to expect when they undergo surgery. It reduces uncertainty, and ensures that concern is not caused by events that are part of normal hospital procedures (Ridgeway 1982). Similarly to providing procedural information, providing sensory information is expected to reduce anxiety by reducing the discrepancy between the sensation expected by the patient and the sensation actually experienced (Johnson 1973). For example, if a patient expects to experience discomfort after surgery in a particular bodily location, when this discomfort is experienced it is understood as being part of the normal surgical experience rather than an indication that something has gone wrong. Cognitive interventions aim to reduce negative emotions and thoughts related to the surgical process by either changing negative thoughts or refocusing attention elsewhere, and emotion‐focused interventions target an individual's emotions directly. Relaxation and hypnosis interventions aim to make an individual feel more relaxed, both psychologically and physiologically, and may effectively act as distraction techniques, so reducing both negative emotions and negative thoughts. As noted earlier, negative thoughts and emotions influence wound healing (Kiecolt‐Glaser 1998), perceptions of pain and also behaviour. Finally, behavioural instruction aims to directly influence behaviours that are important in enabling the surgical procedure to go well and to enhance recovery, for example teaching people how to manage their own analgesia, or instructing them as to when they should return to usual activities for optimal recovery.
Why it is important to do this review
Improving outcomes after surgery has a range of benefits both for the individual and for the healthcare service. Individuals will benefit from reduced postoperative pain and a quicker return to activity. Economic benefits include shorter stays in hospital, reduced use of pain medication and quicker return to work.
Objectives
To review the effects of psychological preparation on postoperative outcomes in adults undergoing elective surgery under general anaesthetic.
Methods
Criteria for considering studies for this review
Types of studies
We included both published and unpublished randomized controlled trials (RCTs). We excluded quasi‐randomized trials. We included, and narratively described, cluster‐randomized controlled trials but did not include them in the meta‐analyses.
Types of participants
We included studies with adult participants (aged 16 years or older) undergoing elective surgery under general anaesthesia. If information about anaesthesia was not provided we contacted the study authors for confirmation. If no response was received, we took advice from a clinician (either a surgeon or anaesthesiologist) who assessed whether that type of surgery would usually be performed under general anaesthesia. We included or excluded studies on this basis. Some surgical procedures are carried out under either general or local anaesthesia (for example inguinal hernia repair surgery). We included studies containing a mixture of participants undergoing general and local anaesthesia but excluded studies where all participants underwent, or were expected to have undergone, local (or no) anaesthesia (with or without sedation).
We included studies of people who have received premedicative sedative prior to general anaesthesia. Different issues are encountered with children undergoing surgery (for example their developmental stage) and different psychological techniques are used (Johnston 1993). Studies tend to focus either on adults or children. We excluded participants aged less than 16 years from this current review.
We excluded studies focusing on patient groups with clinically diagnosed psychological morbidity. However, we did not exclude studies that included participants with mental disorders or subclinical symptoms co‐existing with the condition that led to the operation.
Types of interventions
Psychological preparation, including:
procedural information;
sensory information;
behavioural instruction;
cognitive interventions;
relaxation techniques;
hypnosis;
emotion‐focused interventions.
‘Psychological preparation’ was defined as interventions where the intervention was entirely provided before surgery (this preparation could include, for example, instructions for the participant for after surgery, but the implementation of the intervention had to be pre‐surgery). We were interested in the psychological content of the intervention in this review rather than how it is delivered. There are studies that compare different formats (e.g. leaflet versus video) or timings, but the actual content of the intervention is the same. We excluded these papers. Where the control group also received an element of preoperative preparation (for example, procedural information), the intervention group was required to receive that element beyond that received by the control group (for example, more detailed procedural information, or procedural information about additional aspects of surgery) to be considered as an `intervention'.
Types of outcome measures
We included studies that collected data on two primary and two secondary outcomes. We only included outcomes measured within 30 days/one month post‐surgery. We excluded studies that did not measure these outcomes for pragmatic reasons: because of the size of the review and available research team resources, including all studies measuring any outcome was not manageable (see Differences between protocol and review). Where repeated measurements of outcomes were taken postoperatively, we used the earliest measure for the main meta‐analysis. This is because, while the longest follow‐up is important for longer‐term recovery, it was likely that most studies would include short‐term outcome data but only a few would also include longer time frames.
Primary outcomes
1. Postoperative pain
1a. Postoperative pain intensity: there are a range of well‐used measures for pain and some studies report pain as an outcome using more than one measure. We extracted all reported postoperative pain outcomes from each study.
We used the following hierarchy when deciding which postoperative pain measure to use in the meta‐analysis:
the pre‐specified postoperative pain outcome (if given);
a visual analogue scale (VAS), for example from 0 to 100 (or 0 to 10);
McGill Pain Questionnaire (MPQ) (Melzack 1975) intensity rating, Present Pain Intensity;
other MPQ ratings: i) Pain Rating Index (weighted or unweighted), ii) Number of Words Counted;
Short Form‐36 (SF‐36) pain (Ware 2000);
Nottingham Health Profile pain (Hunt 1983);
other pain intensity scale.
We analysed pain at rest over pain at movement; moving in bed over pain when standing or walking; average pain over pain at rest or current pain; current pain over retrospective pain; worst pain over least pain or current pain. We prioritized sensory over affective measures, and self‐report over observer‐report pain measures.
1b. Proportion of participants in pain postoperatively as defined by the authors of included studies.
2. Behavioural recovery* (defined as: resumption of performance of tasks and activities).
Where multiple measures were used, we made the following decisions in prioritizing measures:
SF‐36 physical function (Ware 2000);
Nottingham Health Profile: Physical mobility (Hunt 1983);
Barthel Index (Mahoney 1965);
Western Ontario and McMaster Osteoarthritis Index (WOMAC) functional status (Bellamy 1988).
Secondary outcomes
1 Negative affect*
Where multiple measures were used, we used the following hierarchy when deciding which measures to use in meta‐analysis:
State Trait Anxiety Inventory (STAI) state (Spielberger 1983);
STAI trait (Spielberger 1983);
Profile of Mood States (POMS) tension/anxiety (McNair 1971);
POMS global (McNair 1971);
Multiple Affect Adjective Check List (MAACL) Anxiety/fear (Zuckerman 1965);
MAACL total (Zuckerman 1965);
Mood Adjective Checklist (MACL) (Radloff 1968);
Hospital Anxiety and Depression Scale (HADS) anxiety (Zigmond 1983);
HADS depression (Zigmond 1983);
General Health Questionnaire 28 (Goldberg 1978);
Perceived Stress Scale (Cohen 1983);
Hospital Anxiety Scale (Lucente 1972);
SF‐36 mental health (Ware 2000);
Nottingham Health Profile: Emotional Reaction (Hunt 1983);
Psychologic Global Well‐being Scale (Dupuy 1984);
BSKE (EWL) (Befindlichkeitsskalierung durch Kategorien und Eigenschaftswörter): Psychological Global Well‐being/mood (Janke 1994);
Structured interview: Modified Present State Examination schedule (Tait 1982) and the Diagnostic and Statistical Manual of Mental Disorders, third edition (DSM‐III) (APA 1980).
2. Length of stay in hospital (days)
*For the outcomes of behavioural recovery and negative affect we included only studies that used measures with published psychometric properties, including reliability and validity. We recorded the timing of outcome assessment.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 5); MEDLINE (Ovid SP) (1950 to 4 May 2014); EMBASE (Ovid SP) (1982 to 4 May 2014); PsycINFO (Ovid SP) (1982 to 4 May 2014); CINAHL (EBSCOhost) (1980 to 4 May 2014); Dissertation Abstracts and ISI Web of Science (1946 to 4 May 2014). We reran the search on 7 July 2015; the additional studies identified (after screening titles and abstracts to exclude any obviously irrelevant studies) are listed under Characteristics of studies awaiting classification.
We used the following subject search terms for searching the databases:
`psychological preparat*', education, information, instruction, cognitive interven*, `cognitive behavio?ral therapy', `cognitive therapy', `behavio*ral therapy', hypnosis, relaxation, guided imagery, surgery, operat*, surgical procedure, general an*esthetic, elective surgery, cholecystectomy, hysterectomy, hernia repair, herniorrhaphy, hernioplasty, joint replacement surgery, arthroplasty.
We combined our subject search terms with the Cochrane highly sensitive search strategy for identifying RCTs as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The full search strategies are provided in the Appendices (Appendix 1 for CENTRAL, The Cochrane Library; Appendix 2 for MEDLINE (OvidSP); Appendix 3 for EMBASE (OvidSP); Appendix 4 for CINAHL (EBSCOhost); Appendix 5 for ISI Web of Science).
Searching other resources
We searched the reference lists of relevant papers for additional sources where references were provided in the English language. We contacted the authors of relevant studies to identify unpublished studies and dissertations.
We did not limit the search by language or publication status. Where papers were in a non‐English language, we asked a speaker of that language to screen the paper. A member of the review team went over the screening, checking each decision with the screener's description of what happened in the paper. Where the paper was deemed to fit the review criteria, if a member of the review team spoke the language, that individual extracted the data, with a second member of the review team (RP) then checking, by discussion with the first extractor, that decisions made and data extracted were correct. Where no member of the review team spoke the language of the paper, we gained English translations and extracted data in the same way as for English language papers.
Data collection and analysis
Selection of studies
One review author (RP) checked titles and abstracts of retrieved studies to exclude obviously irrelevant reports. A small, random sample was double‐checked by a second researcher (research assistant Yvonne Cooper, or authors MU and JB). Where the title and abstract indicated that a paper had the potential to fit inclusion criteria, copies of the trial were independently assessed for inclusion by two researchers (RP and one other member of the team: research assistant Louise Pike or authors MU, AM, CV, JB, NS, MJ or LBD). We resolved any disagreements by discussion with a third researcher (a member of the authorship team who had not assessed the paper).
Data extraction and management
Two review authors (RP and either MU, AM, JB, CV, MJ, JB, NS or research assistant Louise Pike) independently carried out data extraction using a data extraction form (see Appendix 6). We resolved any disagreement by discussing the matter with a third author (an author who had not previously extracted data from that paper). We extracted the following data:
Study participants: age, gender, total number of participants, location, setting, surgery type.
Study methods: study design, study duration.
Interventions: theoretical nature of intervention, number of intervention groups, specific intervention, intervention details (including delivery method), integrity of intervention, timing of intervention, control groups, usual care description, adherence to intervention and control, attrition rate, loss to follow‐up rate.
Outcomes: outcomes and time points a) collected, and b) reported; outcome definition, author's definition of outcome; measurement tool details (including, for example, upper and lower limits, whether high or low score is good outcome).
Results: number of participants allocated to each intervention group, missing participants, means, standard deviations, proportions, estimate of effect with confidence interval, P value, subgroup analysis information when appropriate (e.g. monitors and blunters (information seekers or avoiders), see Miller 1983).
Study withdrawals or losses to follow‐up.
We described interventions according to whether they contained procedural information, sensory information, behavioural instruction, cognitive intervention, relaxation techniques, hypnosis or emotion‐focused interventions. We coded preparation received by control group participants in the same way.
We (RP) contacted study authors for additional data. We used a two‐stage approach. A first email asked for key information: whether (if not stated) general anaesthesia was used, whether they measured any outcomes not reported in the paper and whether they knew of other (e.g. unpublished) studies. We also asked the study authors if they would be happy for us to contact them with additional questions. If the study authors replied and were happy for us to ask them for further information, we sent them a more detailed email if further information was required.
Assessment of risk of bias in included studies
Two review authors (RP and either MU, AM, JB, CV, MJ, JB, NS or research assistant Louise Pike) independently assessed studies' risk of bias using the tool described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool requires the review authors to assess risk of bias in the following domains: sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias. In addition, the review authors noted whether the study used intention‐to‐treat analysis methods (Hollis 1999) (see Appendix 7 for table). We used a single criterion to classify studies as following the intention‐to‐treat principle: participants needed to be kept in the intervention groups to which they were randomized, regardless of the intervention they received (i.e. analysis was not according to per‐protocol or treatment‐received).
Studies with high or unclear risk of bias were to be given reduced weight in the meta‐analysis compared with studies at low risk of bias. We anticipated that meta‐analysis would be restricted to studies at low (or lower) risk of bias, as per Section 8.8.3.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to conduct sensitivity analyses to determine whether excluding studies at a high risk of bias affected the results but we did not do so because of the low number of studies deemed to be at `low risk' of bias (see Risk of bias in included studies). We did not expect blinding of participants or personnel administering the intervention because of the interactive nature of the interventions. We described any blinding that was carried out, and rated the risk of bias following the Cochrane guidelines, but high risk of bias for performance bias was not seen to diminish the quality of the paper. We recorded the adequacy of the blinding of outcome assessors (returning data by post was deemed acceptable).
Measures of treatment effect
We performed meta‐analyses according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For dichotomous variables, we planned to calculate risk ratios (RR) with 95% confidence intervals (CI). For continuous data where each study used the same units (i.e. for length of stay), we calculated mean differences for each study and their 95% CIs.
For the postoperative pain and negative affect outcomes a variety of scales were used so we calculated a standardized effect size ‐ the standardized mean difference (SMD), or Hedges' g. We used final scores as standard. However, some studies only reported mean (SD) change from baseline; for these studies we used the difference in mean change scores as the effect size. If no continuous postoperative pain data were available but dichotomous data were presented, we used the log odds ratio instead as the effect size. It was only necessary to do this for one study (Coslow 1998).
If necessary, we reversed the sign of the effect size so that values below zero always indicated that the intervention group was favoured.
Unit of analysis issues
We included only patient‐randomized studies in the meta‐analyses. We reported the results of cluster‐randomized studies as part of the narrative review.
Dealing with missing data
If any necessary data were missing, when we contacted authors about their studies we specifically asked them about the missing data (see Data extraction and management for procedure taken with contacting authors). Missing standard deviations (SD) was a common situation in this review. We were able to calculate (or estimate) standard deviations in a variety of ways. These included calculating the SD from the standard error of the mean (SEM), 95% confidence intervals or from t or F statistics. If the majority of studies in a meta‐analysis still had missing SDs we did not impute these. Otherwise, we used an unweighted average of SDs from other studies in the review. We used identical imputed values for both intervention and control groups.
Assessment of heterogeneity
We considered and tested heterogeneity between trials, where appropriate. To test for gross statistical heterogeneity between all trials, we used Chi2 tests for heterogeneity and quantified heterogeneity using the I2 statistic (Higgins 2011).
Assessment of reporting biases
We did not plan to assess reporting biases using, for example, funnel plots, because of the probable heterogenous nature of the studies and probable small number of studies appropriate for comparison. However, there proved sufficient studies to examine funnel plots for the overall, `omnibus' analyses.
Data synthesis
We entered quantitative data into Cochrane RevMan 5.3 software and, where appropriate, statistically aggregated the data. We pooled data for all outcomes using an inverse variance approach. We used random‐effects models for all analyses because of expected heterogeneity in interventions and outcomes.
Where it was not possible to pool data, or if summary measures were medians (with range or interquartile range (IQR)), we presented these details in table format and discussed the results.
For each outcome we performed an initial `omnibus' meta‐analysis. We use the term `omnibus' to describe an overall analysis, including all of the psychological preparation interventions (whatever the types of interventions used) and compared these (any psychological preparation intervention) versus controls.
Many studies in the review contained two or more randomized arms. We classified the interventions in each arm separately. To avoid double counting of control groups, for the omnibus analysis we pooled the data in all intervention arms using the standard pooling formula and classified the study as administering any of the interventions included in any of the pooled arms.
The only non‐standard design (i.e. non individually randomized controlled trial) that met the inclusion criteria was a clustered randomized controlled trial design. We narratively synthesized these studies ‐ they were not included in meta‐analysis.
`Summary of findings' table
We included each outcome (postoperative pain, behavioural recovery, negative affect and length of stay) in a `Summary of findings' table (Table 1). For each outcome, the table indicated the effect for the control group and corresponding effect for the intervention group as appropriate, with the number of studies and participants included in analyses. We assessed the quality of the body of evidence for each outcome (postoperative pain, behavioural recovery, negative affect, length of stay) using the GRADE approach, as described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). As we only included RCTs, our start point for grading the evidence was `high quality'. We downgraded by one level for serious factors, and two levels for very serious factors in: limitations in design or implementation of studies (risk of bias); indirectness of evidence; heterogeneity or inconsistency of results; imprecision of results; or high likelihood of publication bias.
Summary of findings for the main comparison. Any intervention compared to control for adults undergoing surgery under general anaesthesia.
| Any psychological preparation intervention compared to control for adults undergoing surgery under general anaesthesia | ||||||
|
Patient or population: adults undergoing elective surgery under general anaesthesia Setting: pre‐surgical contexts (typically hospitals/preoperative clinic settings); setting was not limited by country/language/type of hospital Intervention: psychological preparation interventions presented to participants preoperatively; interventions contained one or more of the following components: procedural information; sensory information; behavioural instruction; cognitive intervention; relaxation techniques; hypnosis; emotion‐focused intervention Comparison: control group (typically standard care and/or attention control) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Any intervention | |||||
| Postoperative pain ‐ measured with a range of tools and placed on a standardized scale Higher scores = higher pain |
‐ | The mean pain in the intervention group was 0.2 (95% confidence interval 0.35 to 0.06) standard deviations lower | ‐ | 2713 (38 RCTs) | ⊕⊕⊝⊝ LOW1 | ‐ |
| Behavioural recovery ‐ measured with a range of tools | Insufficient data were available to calculate standardized scores | Findings suggested that psychological preparation has potential to improve behavioural recovery outcomes, but no clear conclusions could be reached | ‐ | 1441 participants were randomized (14 RCTs) | ⊕⊝⊝⊝ VERY LOW2 | Data from studies were not combined in meta‐analysis because of a low number of studies containing suitable data and a wide range of outcome measures |
| Length of stay in hospital (days) | The mean length of stay for the control groups ranged from 2.11 to 18.6 days | The mean length of stay (days) in the intervention group was 0.52 days fewer (95% confidence interval 0.82 to 0.22) | ‐ | 3313 (36 RCTs) | ⊕⊕⊝⊝ LOW3 | ‐ |
| Negative affect ‐ measured with a range of tools and placed on a standardized scale Higher scores = higher negative affect (e.g. more anxiety) |
‐ | The mean negative affect in the intervention group was 0.35 (95% confidence interval 0.54 to 0.16) standard deviations lower | ‐ | 2496 (31 RCTs) | ⊕⊕⊝⊝ LOW4 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Many studies reported insufficient methodological details to ascertain risk of bias (rated `serious', see Figure 1), and heterogeneity was high (71%, also rated `serious'). We therefore downgraded the overall quality of evidence by two points.
2We downgraded the quality of evidence as `risk of bias' was rated as `very serious' ‐ there were a high proportion of `uncertain' ratings for risk of bias categories, and the number of studies with robust measures meeting our inclusion criteria and reporting suitable data for meta‐analysis was low. We made a further downgrade for high heterogeneity (treated as `serious'). We therefore downgraded the overall quality of evidence by three points.
3Many studies reported insufficient methodological details to ascertain risk of bias (rated `serious', see Figure 1), and heterogeneity was high (74%, also rated `serious'). We therefore downgraded the overall quality of evidence by two points.
4Many studies reported insufficient methodological details to ascertain risk bias (rated `serious', see Figure 1), and heterogeneity was high (81%, also rated `serious'). We therefore downgraded the overall quality of evidence by two points.
Subgroup analysis and investigation of heterogeneity
We planned to carry out subgroup analyses to compare trials of high methodological quality with trials of low methodological quality but did not do so because of the small number of studies judged to be at `low risk' of bias (see Risk of bias in included studies).
Following the omnibus analysis we carried out additional separate meta‐analyses corresponding to the seven intervention categories (procedural information, sensory information, behavioural instruction, cognitive interventions, relaxation techniques, hypnosis and emotion‐focused interventions). We divided studies into those with that intervention category only (referred to as `pure' studies, e.g. procedural information only) and those including that intervention category in combination with other intervention types (referred to as `mixed', e.g. procedural information + sensory information + behavioural instruction) and conducted subgroup analyses so that the effect of both all studies including procedural information and of `pure' procedural information studies could be evaluated. For multi‐arm studies, by including only data from relevant arms we were often able to include different data to those included in the omnibus analysis.
Sensitivity analysis
Jüni recommends consideration of the important quality components of a given meta‐analysis when conducting sensitivity analyses (Jüni 2001). We planned to perform sensitivity analyses to evaluate the effect on the overall result of removing trials with low methodological quality (as identified using the Cochrane tool) (Appendix 6), but did not do so because of the small number of studies judged to be at low risk of bias (see Risk of bias in included studies). Low methodological quality studies were those where: a) sequence generation or allocation concealment was judged as high risk or unclear, b) there was no or unclear blinding of outcome assessors, c) incomplete outcome data were not adequately addressed (assessed as high risk or unclear), d) the study appeared to be at risk of selective outcome reporting (high risk or unclear), d) the study did not appear to have been conducted according to intention‐to‐treat (i.e. it was not clear that participants were kept in the group to which they were allocated, regardless of the intervention they received) (high risk or unclear), d) the study appeared to be at risk of other sources of bias (high risk or unclear).
Results
Description of studies
Results of the search
Electronic searches identified 6781 papers; we identified an additional 151 papers through contact with authors and screening reference lists. We removed 1816 duplicate papers, leaving 5116 whose titles and abstracts we screened for broad relevance. This led to us retrieving 827 papers for full screening. We were unable to locate 24 references (2.9% of papers to be retrieved for full screening). See Figure 2 for the flow chart of studies included and excluded from the review.
2.
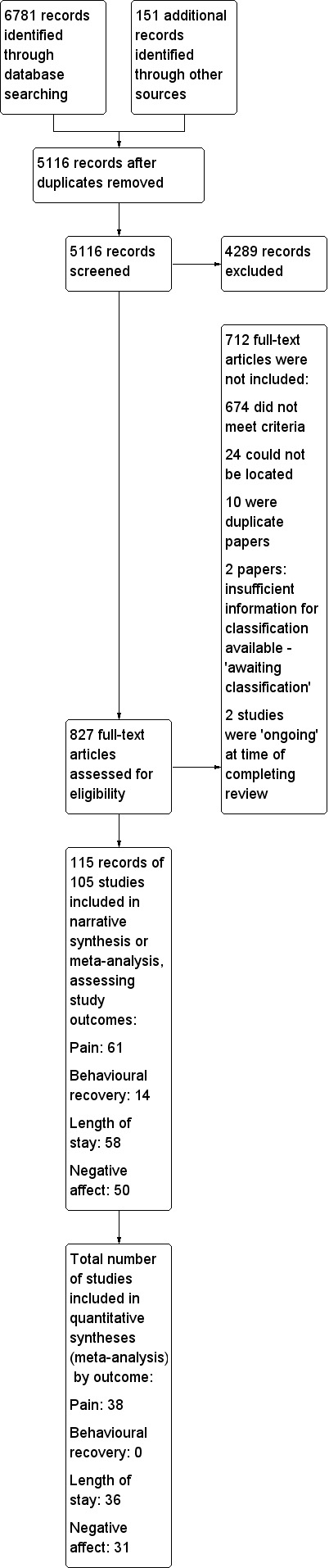
Study flow diagram.
Included studies
We included 105 studies (from 115 papers) in which 10,302 participants were randomized (see Characteristics of included studies). Sixty‐one papers measured the outcome postoperative pain, 58 length of stay, 50 negative affect and 14 behavioural recovery. We attempted to contact all authors, with the exception of five studies' authors where the study reports were retrieved late in the review process (Barbalho‐Moulim 2011; Done 1998; McGregor 2004; Rajendran 1998; Rosenfeldt 2011). The publication dates of the included studies ranged from 1970 to 2014 and studies were conducted in a wide range of countries (36 in the USA, 13 in the UK, nine in Canada, seven in China, six in Australia, five in the Netherlands, four in Germany, three in Sweden, and one or two studies in each of: Austria, Brazil, Denmark, Egypt, France, India, Iran, Ireland, Italy, New Zealand, Nigeria, Romania, Serbia, Singapore, Spain, Switzerland, Taiwan and Turkey).
The study participants underwent a wide range of surgical procedures. Twenty‐seven studies investigated participants undergoing cardiothoracic surgery (including 17 exclusively containing participants undergoing coronary artery bypass graft surgery). Hip or knee joint surgery was examined in 22 studies (four knee replacement only, 10 hip replacement only, eight both hip and knee replacement surgery). Seven studies considered cholecystectomy, seven hysterectomy and two breast surgery. The following procedures were considered in a single study each: urinary diversion surgery, colorectal resection, laparoscopic tubal ligation, minimally invasive radio‐guided parathyroidectomy, rectal cancer surgery, periodontal surgery, inguinal hernia and gastric bypass surgery. Thirty‐one studies addressed a mixture of procedures and one study did not state the surgical procedure(s).
The included studies used a range of intervention components, and intervention content was rarely `pure', consisting of a single intervention. Procedural information was reported in 59 interventions (`pure' procedural information content in eight), sensory information in 38 (`pure' sensory information in one), behavioural instruction in 71 (`pure' in 28), cognitive interventions in 27 (`pure' in eight), relaxation techniques in 35 (`pure' in 13), hypnosis in six (`pure' in one) and emotion‐focused interventions in 12 (`pure' in one). Studies generally contained fairly small sample sizes.
We found that control group content was generally poorly reported. Pure procedural information content was reported in 17 control groups, pure behavioural instruction in 11 and combinations of interventions in 23 studies. Fifty‐six studies provided insufficient information for us to categorize control content ‐ for example, authors frequently described the control group as consisting of `usual care' without describing what usual care was. It is highly likely that intervention content is missing from these descriptions because if participants were provided with absolutely no procedural information or behavioural instruction prior to their surgery they would not know when to arrive for their surgery or what to do (e.g. when to fast prior to their anaesthetic).
As per our protocol (Powell 2010), we did not extract funding sources from papers in this review.
Excluded studies
We excluded 674 papers on full screening of retrieved papers. Details of 27 key excluded papers are provided (Anderson 1987; Blay 2005; Boore 1978; Burton 1991; Burton 1994; Croog 1994; Domar 1987; Enqvist 1995; Eremin 2009; Huang 2012; Johnson 1978a; Lengacher 2008; Liu 2013; Manyande 1995; Manyande 1998; Mitchell 2000; Montgomery 2002; Montgomery 2007; Sheard 2006; Shelley 2009; Stergiopoulou 2006; Sugai 2013; Surman 1974; Timmons 1993; Voshall 1980; Wang 2002; Wells 1986). For further details of the excluded studies see the Characteristics of excluded studies.
Ongoing studies
We did not include two papers, Jong 2012 and Hansen 2013, as the research was complete but authors were reluctant to share study details with us prior to publication.
Studies awaiting classification
On full screening of retrieved papers in May 2014, two provided insufficient information to determine whether or not they met the review's inclusion criteria and our attempts to contact the authors for further information were not successful (Johansson 2007; Lookinland 1998).
We reran the searches in July 2015. These searches identified a further 753 papers. On removing duplicates across databases, 614 papers remained. We checked these references for overlap with searches previously conducted and identified a further 96 duplicates. These searches therefore identified 518 new papers. RP screened the titles and abstracts of these papers for relevance (with JB checking a randomly selected 5% of titles and abstracts); 482 papers were excluded. The remaining 36 papers appear to potentially have relevance and should be retrieved and screened in detail when this review is updated (Akinci 2015; Angioli 2014; Attias 2014; Bergin 2014b; Calsinski Assis 2014; Chevillon 2014; Chow 2014; Dathatri 2014; Eckhouse 2014; El Azem 2014; Ellett 2014; Foji 2015; Fraval 2015; Furuya 2015; Gade 2014; Gillis 2014; Gyulaházi 2015; Hansen 2015; Henney 2014; Heras 2014; Hoppe 2014; Huber 2015; Johansson 2007; Kol 2014; Lai Ngor 2014; Louw 2014; Mohammadi 2014; Novick 2014; Paul 2015; Rolving 2014; Saleh 2015; Shahmansouri 2014; Umpierres 2014; Van Acker 2014; West 2014; Würtzen 2015; Xin 2015). Details of these papers can be found in Characteristics of studies awaiting classification.
Risk of bias in included studies
Details of `Risk of bias' assessments for each study are provided in Characteristics of included studies, with summaries across studies being presented in Figure 1 and Figure 3. We did not expect many studies in this review to be rated as `low risk' for performance bias. However, even ignoring this category, only three studies received `low risk' ratings on all other items (Crowe 2003; Goodman 2008; Mahler 1998). We therefore did not carry out the planned sensitivity analyses to compare meta‐analyses including only high quality, `low risk' studies with analyses including all available data, nor the planned subgroup analyses to compare findings of high quality, `low risk' studies with findings of low quality, `high risk' studies.
1.
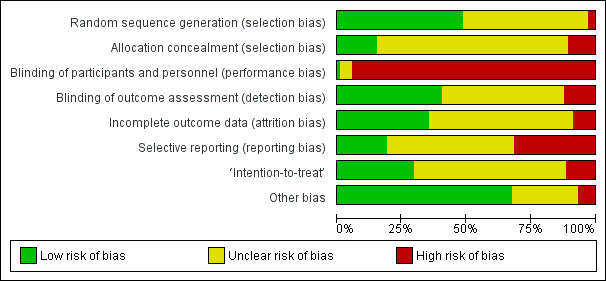
`Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
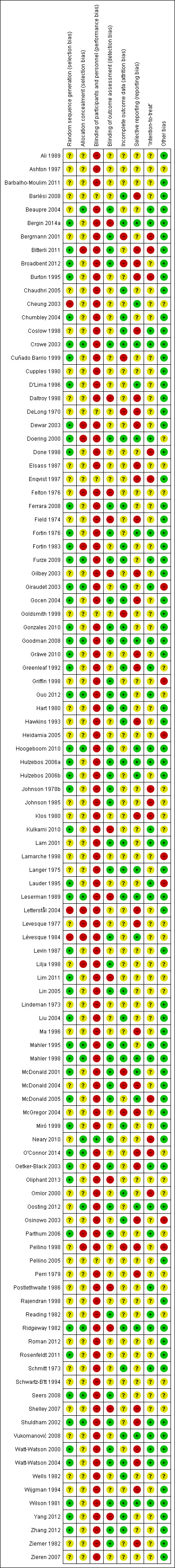
`Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
As shown in Figure 1, we rated very few studies as ‘high risk’ for random sequence generation. This is because, following our protocol (Powell 2010), we only included RCTs ‐ where a non‐random approach was described (such as alternation), or where there was no mention of randomization in the study description, studies were excluded. This meant that studies that could be rated as `high risk' would usually be excluded from the review. Despite this inclusion criterion, the randomization procedure was sufficiently described to rate the study as ‘low risk of bias’ in only about half of studies (51 of 105, see Figure 3) – giving insufficient information to ascertain the procedure used for allocation was common.
Clear descriptions of allocation concealment were even more rare, with only 16 (of 105) studies being judged as `low risk of bias' (Beaupre 2004; Crowe 2003; Furze 2009; Giraudet 2003; Goodman 2008; Guo 2012; Hoogeboom 2010; Leserman 1989; Mahler 1995; Mahler 1998; Neary 2010; O'Connor 2014; Oosting 2012; Ridgeway 1982; Schwartz‐B'tt 1994; Shuldham 2002) – this was an aspect that was simply not mentioned in most studies. Awarding the designation of `low risk' tended to depend on information that we were able to gain directly from authors themselves.
Blinding
Studies' poorest risk of bias ratings were for performance bias: blinding of participants and personnel. We rated most studies in the review as being at ‘high risk of bias’ in this category. We anticipated this and did not expect to see blinding of participants or of the personnel administering the intervention because many psychological interventions are interactive in nature. It was therefore rare to find a study where the person administering the intervention could be blind to the participant’s group allocation and, if participants were fully informed about the nature of the study, they would also tend not to be blinded to treatment condition. One study did report blinding of both participants and personnel, using an intervention delivered via a website (Neary 2010). Studies rated as `unclear' for performance bias (n = 5: Barlési 2008; DeLong 1970; Enqvist 1997; Goldsmith 1999; Pellino 2005) used a limited range of intervention formats, administered on paper (Barlési 2008), by audiorecording (DeLong 1970; Enqvist 1997), information on paper and tape (Pellino 2005), or via a website (Goldsmith 1999).
Blinding of outcome assessment (to avoid detection bias) was feasible in the types of studies we assessed – by ensuring that the person administering postoperative measures was blind to allocation. However, this was frequently not reported, allowing us to rate 42 (of 105) studies as `low risk of bias' (Beaupre 2004; Bergmann 2001; Bitterli 2011; Broadbent 2012; Crowe 2003; Doering 2000; Ferrara 2008; Fortin 1976; Furze 2009; Gocen 2004; Gonzales 2010; Goodman 2008; Griffin 1998; Guo 2012; Hart 1980; Hoogeboom 2010; Hulzebos 2006a; Hulzebos 2006b; Johnson 1978b; Johnson 1985; Lam 2001; Langer 1975; Lévesque 1984; Lilja 1998; Lin 2005; Mahler 1995; Mahler 1998; McDonald 2001; McDonald 2004; McDonald 2005; Neary 2010; Oetker‐Black 2003; Oosting 2012; Parthum 2006; Reading 1982; Seers 2008; Shuldham 2002; Watt‐Watson 2000; Watt‐Watson 2004; Wilson 1981; Zhang 2012; Ziemer 1982).
Incomplete outcome data
Attrition was frequently poorly reported in the studies, leading to ratings of `unclear risk of bias'. Sufficient information was provided, demonstrating good practice, in 37 `low risk' studies (Barlési 2008; Bergin 2014a; Chaudhri 2005; Chumbley 2004; Coslow 1998; Crowe 2003; Doering 2000; Ferrara 2008; Fortin 1983; Furze 2009; Giraudet 2003; Gocen 2004; Gonzales 2010; Goodman 2008; Greenleaf 1992; Guo 2012; Hart 1980; Hawkins 1993; Hulzebos 2006a; Lam 2001; Langer 1975; Leserman 1989; Lin 2005; Liu 2004; Mahler 1995; Mahler 1998; Miró 1999; Omlor 2000; Osinowo 2003; Ridgeway 1982; Schmitt 1973; Vukomanović 2008; Watt‐Watson 2004; Wells 1982; Wilson 1981; Yang 2012; Zhang 2012).
Selective reporting
The proportion of studies rated as `low risk' for selective reporting was low (20 of 105) (Bergin 2014a; Cheung 2003; Crowe 2003; D'Lima 1996; Doering 2000; Fortin 1976; Goodman 2008; Hoogeboom 2010; Hulzebos 2006a; Hulzebos 2006b; Langer 1975; Leserman 1989; Levesque 1977; Mahler 1998; McDonald 2001; McDonald 2005; Oosting 2012; Ridgeway 1982; Vukomanović 2008; Wilson 1981). Thirty‐three were designated `high risk'. This may reflect our strict application of the Cochrane guidelines (Cochrane Handbook for Systematic Reviews of Interventions, Table 8.5.d.; Higgins 2011), which stated that for a judgement of `low risk' either "the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way" or "the study protocol is not available but it is clear that the published reports included all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon)". It was extremely rare to find studies with reference to protocol documents, and only a very small number of trials had been registered. To provide a rating of `low risk' we tended to be dependent on authors responding to our queries as to whether any outcomes were measured but not reported.
Other potential sources of bias
We evaluated studies for analysis according to the principles of intention‐to‐treat (whether participants were analysed in the group to which they were allocated, regardless of the intervention they received). This was often not reported, leading to the evaluation of 31 studies as `low risk of bias' (Beaupre 2004; Bergin 2014a; Coslow 1998; Crowe 2003; Doering 2000; Fortin 1976; Furze 2009; Giraudet 2003; Goodman 2008; Greenleaf 1992; Guo 2012; Hoogeboom 2010; Hulzebos 2006a; Kulkarni 2010; Lam 2001; Lauder 1995; Leserman 1989; Mahler 1995; Mahler 1998; Oetker‐Black 2003; Oosting 2012; Parthum 2006; Postlethwaite 1986; Reading 1982; Ridgeway 1982; Schmitt 1973; Shuldham 2002; Vukomanović 2008; Watt‐Watson 2000; Watt‐Watson 2004; Wilson 1981), 12 as `high risk' and the remainder as `unclear'.
Most studies were not found to have additional sources of bias, with concerns being raised for seven studies rated as `high risk' and 27 as `unclear'.
Effects of interventions
See: Table 1
A summary of key findings, with quality gradings, is provided in Table 1.
Findings by outcome
Primary outcomes
1.Postoperative pain
Studies included in meta‐analysis
Sixty‐one studies assessed the outcome postoperative pain. It was possible to include data for 38 studies (36% of 105 studies) (Barbalho‐Moulim 2011; Bergin 2014a; Bitterli 2011; Cheung 2003; Coslow 1998; D'Lima 1996; Doering 2000; Fortin 1983; Giraudet 2003; Gocen 2004; Goldsmith 1999; Gonzales 2010; Gräwe 2010; Griffin 1998; Guo 2012; Heidarnia 2005; Lam 2001; Lauder 1995; Leserman 1989; Levin 1987; Lin 2005; Ma 1996; McDonald 2001; McDonald 2004; McDonald 2005; McGregor 2004; Miró 1999; Neary 2010; Omlor 2000; Pellino 2005; Postlethwaite 1986; Reading 1982; Ridgeway 1982; Roman 2012; Schwartz‐B'tt 1994; Seers 2008; Watt‐Watson 2000; Zieren 2007), with analysis of 2713 participants' data (26% of 10,302 participants randomized across all studies), in the omnibus meta‐analysis, which included studies comparing any intervention versus control (Analysis 1.1; Figure 4). As a variety of scales were used to measure postoperative pain, we used standardized scores to pool data using the SMD (Hedges' g). Higher scores indicate higher pain; effect scores below zero indicate that the intervention group had lower pain. Overall, the pooled effect size (SMD) was ‐0.20 (95% confidence interval (CI) ‐0.35 to ‐0.06), suggesting a statistically significant effect in favour of the intervention groups. There were, however, high levels of statistical heterogeneity between studies (I2 statistic = 71%).
1.1. Analysis.
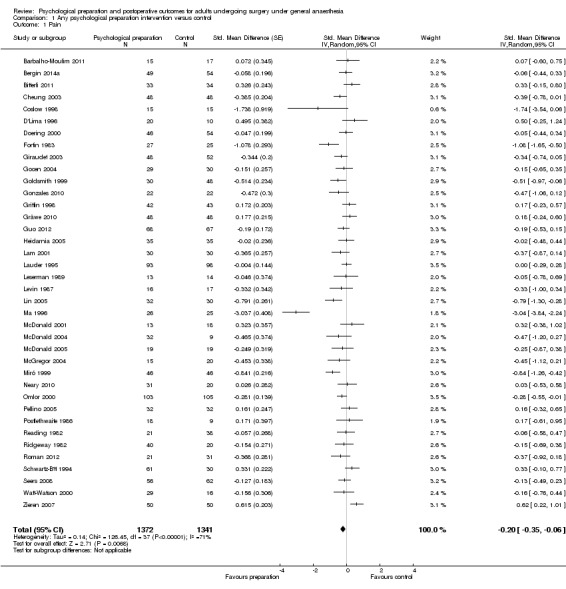
Comparison 1 Any psychological preparation intervention versus control, Outcome 1 Pain.
4.
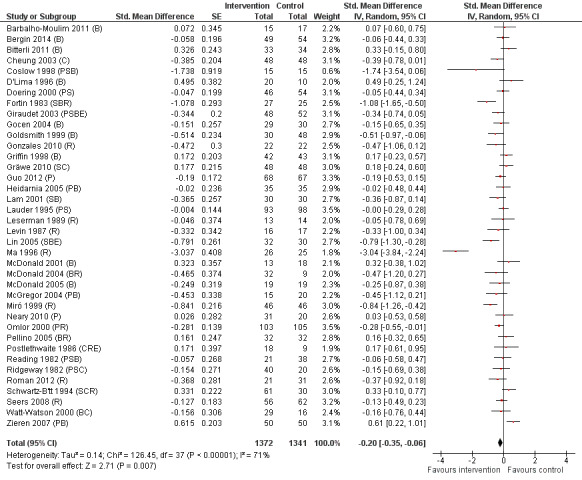
Pain (any psychological preparation intervention versus control). B: behavioural instruction; C: cognitive interventions; E: emotion‐focused interventions; H: hypnosis; P: procedural information; R: relaxation; S: sensory information.
One study appeared to be an outlier (Ma 1996). We assumed statistics in the paper to represent mean and standard deviation as the notation "x bar +/‐ s" was used but this was not explicitly stated and it is possible that `s' represented standard error. Excluding this study did not affect the interpretation of the outcome, but reduced the observed statistical heterogeneity (I2 statistic = 53%). Excluding the single study where an effect size had been derived from categorical data, Coslow 1998, also had no effect on the results.
Subsequent forest plots show the results for the individual types of intervention (Analysis 2.1; Analysis 3.1; Analysis 4.1; Analysis 5.1; Analysis 6.1; Analysis 8.1; no studies used the intervention hypnosis). Most studies included more than one intervention type and, except for behavioural instruction and relaxation, there were no more than two `pure' studies that included just that particular intervention type. This makes it very difficult to separate the effect of a particular intervention category from other types of intervention also administered. For most intervention types the pattern of results was similar to the omnibus analysis and results for `pure' and `mixed' studies were also similar. The analyses for behavioural instruction showed a somewhat different pattern, however, with a relatively consistent effect size for the `pure' behavioural instruction studies suggesting no difference between intervention and control (SMD 0.01, 95% CI ‐0.19 to 0.21, I2 statistic = 27%). The meta‐analysis results for individual intervention types were statistically significant for the meta‐analysis of the two studies including cognitive intervention (Cheung 2003; Ridgeway 1982; SMD ‐0.34, 95% CI ‐0.68 to ‐0.01, I2 statistic = 0%; Analysis 5.1.1) and the meta‐analysis of seven `pure' relaxation studies (Gonzales 2010; Leserman 1989; Levin 1987; Ma 1996; Miró 1999; Roman 2012; Seers 2008; SMD ‐0.71, 95% CI ‐1.29 to ‐0.13, I2 statistic = 87%; Analysis 6.1.1). No data for studies investigating postoperative pain after hypnosis could be included in the meta‐analyses. The funnel plot showed no clear evidence of publication bias.
2.1. Analysis.
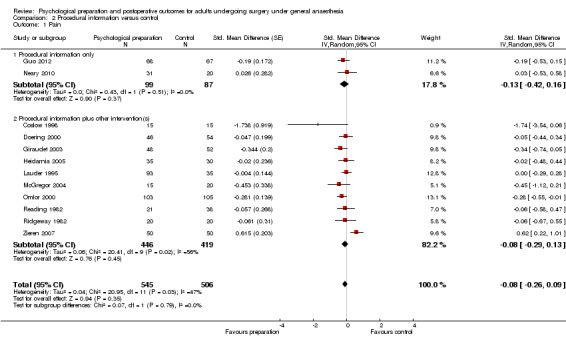
Comparison 2 Procedural information versus control, Outcome 1 Pain.
3.1. Analysis.
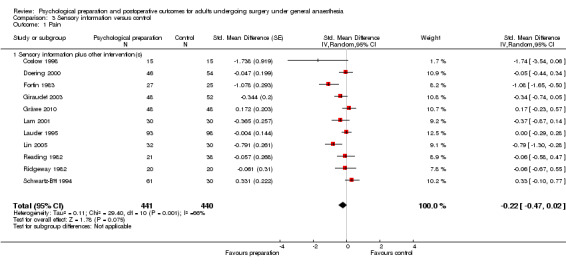
Comparison 3 Sensory information versus control, Outcome 1 Pain.
4.1. Analysis.
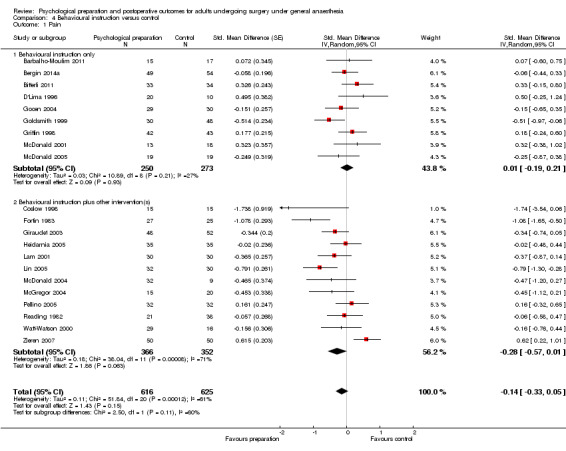
Comparison 4 Behavioural instruction versus control, Outcome 1 Pain.
5.1. Analysis.
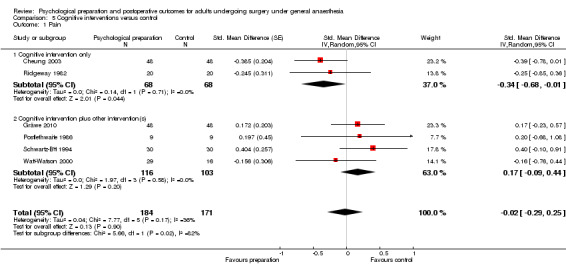
Comparison 5 Cognitive interventions versus control, Outcome 1 Pain.
6.1. Analysis.
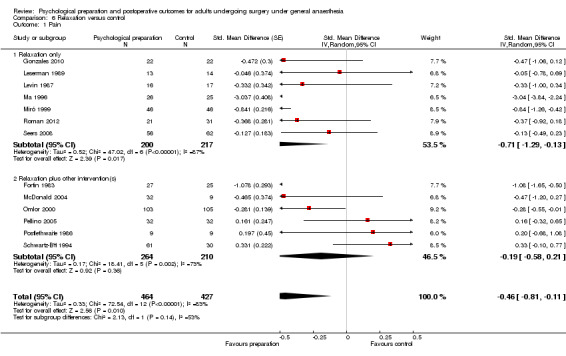
Comparison 6 Relaxation versus control, Outcome 1 Pain.
8.1. Analysis.
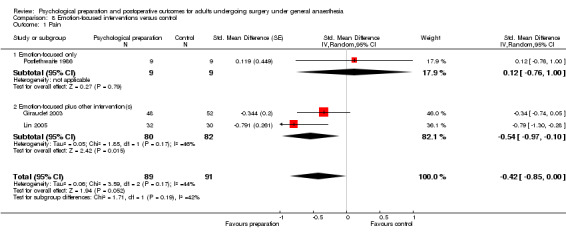
Comparison 8 Emotion‐focused interventions versus control, Outcome 1 Pain.
Studies not included in meta‐analysis
Twenty‐three studies addressing the postoperative pain outcome did not contain data appropriate for meta‐analysis (Chumbley 2004; Daltroy 1998; Dewar 2003; Enqvist 1997; Ferrara 2008; Field 1974; Gilbey 2003; Hawkins 1993; Johnson 1978b; Johnson 1985; Kulkarni 2010; Lilja 1998; Liu 2004; Oetker‐Black 2003; Parthum 2006; Perri 1979; Shelley 2007; Shuldham 2002; Vukomanović 2008; Watt‐Watson 2004; Wells 1982; Wijgman 1994; Ziemer 1982) (Table 2). Three of these were not eligible for meta‐analysis as they reported cluster‐randomized trials (Chumbley 2004; Parthum 2006; Vukomanović 2008). Median scores were provided in two studies (Kulkarni 2010; Wijgman 1994); most studies in this group lacked sufficient detail to be entered into meta‐analysis.
1. Findings of studies that examined the outcome pain but could not be included in meta‐analyses.
| Author, year | Surgery type and sample size (randomized) | Intervention categories |
Pain measure(s) The first measure listed is that prioritized in this review |
Pain findings (as available) |
| Chumbley 2004 | Mixed: surgeries that would receive PCA routinely N = 246 |
Intervention 1: Behavioural instruction (delivered in leaflet) Intervention 2: Behavioural instruction (delivered in interview) |
1) Visual analogue scale (VAS) days 1 to 5 post‐surgery 2) Word rating on 5‐point scale; days 1 to 5 post‐surgery |
Cluster‐randomized VAS day 1 postoperatively mean (95% CI): Control: 3.7 (2.93 to 4.45); Intervention 1: 2.8 (2.04 to 3.56); Intervention 2: 3.2 (2.43 to 6.21). ANOVA, repeated measures: for VAS pain scores, between‐groups effect: F = 1.88, P value = 0.23 |
| Daltroy 1998 | Total hip or knee arthroplasty N = 12 |
Procedural and sensory information | Day 4 post‐surgery Measure not clearly described, assume same as preoperatively: mean of 3 x 5‐point scales assessing pain at night, resting and when active |
Intervention did not affect pain in general linear model (P value = 0.16) |
| Dewar 2003 | Mixed surgeries N = 254 |
Procedural information, behavioural instruction, cognitive intervention, relaxation | Evening after surgery (day 0) Brief Pain Inventory: numerical rating scale from 0 to 10 |
Control n = 118; intervention n = 104 No significant difference |
| Enqvist 1997 | Breast reduction N = 50 |
Relaxation, hypnosis | Days 1 to 5 post‐surgery, measured with `10‐degree VAS’. Not clear exactly what was asked, or if measured once in this period or daily | Control n = 25; intervention n = 23 No significant differences |
| Ferrara 2008 | Total hip replacement N = 23 |
Behavioural instruction | 15 days and 4 weeks post‐surgery: VAS Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) subscale |
Control n = 12; intervention n = 11 VAS pain scores: significantly lower in intervention group at 4 weeks (not at 15 days apparently) |
| Field 1974 | Mixed orthopaedic surgery N = 60 |
Procedural information, hypnosis | Between 2 and 7 days post‐surgery; no further information | Control n = 30; intervention n = 30 No significant difference |
| Gilbey 2003 | Total hip arthroplasty N = 76 |
Behavioural instruction | 3 weeks post‐surgery Pain domain of WOMAC |
Control n = 25; intervention n = 32 Significant difference (P value < 0.01) for total WOMAC (pain, physical function and stiffness) and physical function domain. Reports surgery had such beneficial effect on pain that impact of intervention only marginal. |
| Hawkins 1993 | Gynaecological surgery N = 60 |
Behavioural instruction | 48 hours post‐surgery: VAS of average pain; categorical scale (5 categories from no pain to unbearable pain); nurse ratings of pain (collected hourly pain reports when not sleeping for first 48 hours after surgery) |
Control n = 40 (standard care and attention control); intervention n = 20 No significant differences (VAS ANOVA F = 0.06, df = 2, P value = 0.93) |
| Johnson 1978b | Sample 1: cholecystectomy, N = 81 Sample 2: inguinal hernia repair, N = 68 |
Intervention 1: ‘Instruction’: Behavioural instruction (deep breathing, coughing, leg exercises) Intervention 2: ‘Procedure information’: focus procedural information, also some sensory information and behavioural instruction Intervention 3: `Sensation information’: focus: sensory information, also some procedural information and behavioural instruction 2 x 3 factorial design: no instruction/instruction (Intervention 1; no information/information (Interventions 2 and 3) |
Pain: days 1, 2 and 3 post‐surgery: intensity of sensations on 10‐point scale Scores totaled over the 3 days in analysis | Sample 1 No main effect of condition Sample 2 MANOVA with DVs pain and distress of pain sensation: for first postoperative day: significant main effects for information level (F(4, 104) = 2.55, P value < 0.05), trend for an effect for instruction (F(2, 52) = 3.07, P value = 0.055), but only a main effect for distress scores reported (no univariate findings reported for pain – so seems no significant effects) |
| Johnson 1985 | Abdominal hysterectomy N = 199 |
Intervention 1: Procedural and sensory information Intervention 2: ‘Cognitive‐coping technique’ – cognitive intervention Intervention 3: ‘Behavioural‐coping technique’ – behavioural instruction 2x3 factorial design: no information/information (Intervention 1); no coping technique/coping technique (Interventions 2 and 3) |
Day 3 post‐surgery Pain scale from 1 to 10 |
MANOVA, controlling for covariates, with various outcomes including pain: ‘significant’ at P value < 0.10: coping technique, F (16, 286) = 1.59, P value =0.07. However, pain does not appear to be one of the outcomes responsible for this. |
| Kulkarni 2010 | Major abdominal surgery N = 80 |
Intervention 1: Behavioural instruction (deep breathing training) Intervention 2: Behavioural instruction (incentive spirometry) Intervention 3: Behavioural instruction (specific inspiratory muscle training) |
Pain (no information of how measured/when) | Control n = 17; intervention 1 n = 17; intervention 2 n = 15; intervention 3 n = 17. Median pain score for all groups is 3 (no ranges/IQRs) |
| Lilja 1998 | Breast cancer (BC) surgery N = 46 Total hip replacement (THR) N = 55 |
Procedural information, behavioural instruction | First 3 days post‐surgery: VAS | Control: n = 22, mode = 1 (BC day 1); intervention n = 22 No significant differences groups for either BC or THR patients (analysed separately) |
| Liu 2004 | Mixed orthopaedic surgery N = 74 |
Cognitive intervention | Pain: 0 to 10 VAS; timing not stated | Control n = 35, mean (SD)= 2.5 (0.52); intervention n = 39, mean = 2.85 (0.33) Significant difference (t = 2.61, P value < 0.05). Discussion: authors state “patients from the experimental group…had…low scores on pain compared to the control group with statistical significance” (p5). This appears to be at odds with mean scores, suggesting error in paper. |
| Oetker‐Black 2003 | Total abdominal hysterectomy N = 108 |
Behavioural instruction, cognitive intervention, relaxation | Day 1 post‐surgery: VAS At discharge: bodily pain (Health Status Questionnaire) |
No significant differences (VAS: t(1,105) = ‐0.54, P value = 0.591) |
| Parthum 2006 | Cardiac surgery N = 93 |
Procedural information, sensory information, behavioural instruction | 1. Pain intensity: VAS as part of modified McGill Pain Questionnaire (MPQ), day 1 postoperative and retrospective rating of pain while on ICU 2. Proportion of patients in pain postoperatively (cut off: VAS > 3 on above measures) |
Cluster‐randomized Control n = 36, median (VAS current, at rest) = 4.0. Intervention: n = 37, median = 3.0 No significant differences between groups |
| Perri 1979 | Vaginal hysterectomy N = 26 |
Relaxation | Self report. 1 and 3 days postoperation; ‘McGill‐Melzack Pain Questionnaire’ Observed. 1 and 3 days postoperation – observed pain behaviour – Chambers‐Price Rating Scale for Pain |
Control n = 13; intervention mean = 13. No significant differences between groups (P value < 0.05) |
| Shelley 2007 | Coronary artery bypass surgery N = 90 |
Cognitive intervention | At discharge (4 days post‐surgery): 10 cm VAS | Control n = 43; intervention n = 37 Significant interaction between group, self efficacy and external health locus of control (F(1,71) = 4.06, P value < 0.05). Post hoc analysis: trend‐level effects: smaller increase in pain for prepared patients than controls if high external health locus of control and low self efficacy. Matched control appraisal patients: increased pain in intervention group compared with controls (controlling for baseline pain). |
| Shuldham 2002 | Coronary artery bypass surgery N = 356 |
Procedural information, behavioural instruction | Questionnaires presented on day 3 post‐surgery (or 3rd day after transfer to ward if still in intensive care unit on day 3 post‐surgery) Composite measure (including VAS, body map and categorical rating scale), authors used VAS in analysis |
No significant differences (using Mann‐Whitney U): U = 10,197.5; Z = ‐0.72, P value = 0.47 |
| Vukomanović 2008 | Total hip arthroplasty N = 45 |
Procedural information, behavioural instruction | VAS at discharge: pain at rest and movement |
Cluster‐randomized Control n = 20, mean (SD) = 6.2 (14.95); Intervention n = 20, mean (SD) = 3.95 (13.08) No significant difference in pain |
| Watt‐Watson 2004 | Coronary artery bypass surgery N = 406 |
Behavioural instruction, cognitive intervention | Days 1 to 5 post‐surgery: McGill Short‐form. Scores: Present Pain Intensity: most severe pain in previous 24 hours Pain Rating Index (sensory, affective and total); Numerical Rating Scale (on moving and worst pain in previous 24 hours) |
No main effect of group |
| Wells 1982 | Cholecystectomy N = 12 |
No control group Intervention 1: ‘Control’: Sensory information; behavioural instruction Intervention 2: (do not appear to receive ‘control’ intervention) Relaxation |
Rated on 10 cm line on evening on day of surgery, and days 1 and 2 post‐surgery | Intervention 1: n = 6, mean (SD) eve of operation = 5.4 (3.39); intervention 2: n = 6, mean (SD) = 5.65 (1.6) No main effect for treatment (F(1,7) = 3.0, P value = 0.13), time (F(7,2) = 3.3, P value = 0.07) or interaction between treatment and time (F(2,4) = 1.0, P value = 0.4) |
| Wijgman 1994 | Total knee arthroplasty N = 64 |
No control group Intervention 1: Procedural information Intervention 2: Behavioural instruction |
2, 5, 7, 10, 14 days post‐surgery and at discharge. VAS where 100 = worst pain | Overall n at day 2 = 63. Medians (IQRs) presented in Figure 1, not clear. No significant differences between groups |
| Ziemer 1982 | Gynaecologic or gastrointestinal N = 111 |
Intervention 1: Sensory information Intervention 2: Sensory information, behavioural instruction, cognitive intervention, relaxation |
2 to 4 days post‐surgery: 5‐point pain intensity rating scale | Control n = 40; intervention 1 n = 34; intervention 2 n = 37 Focus: correlation of pain with coping scales |
ANOVA = analysis of variance
BC = breast cancer
F = F statistic (ANOVA)
ICU = intensive care unit
IQR = interquartile range
MANOVA = multivariate analysis of variance
MPQ = McGill Pain Questionnaire (Melzack 1975)
N = number of participants in sample
PCA = patient‐controlled analgesia
SD = standard deviation
THR = total hip replacement
VAS = visual analogue scale
Fourteen of these studies reported no statistically significant differences between intervention and control conditions (Chumbley 2004; Daltroy 1998; Dewar 2003; Enqvist 1997; Field 1974; Gilbey 2003; Hawkins 1993; Lilja 1998; Oetker‐Black 2003; Parthum 2006; Perri 1979; Shuldham 2002; Vukomanović 2008; Watt‐Watson 2004). A further two studies did not clearly report postoperative pain findings, but this appears to be because comparisons were not significant (Johnson 1978b; Johnson 1985). These studies used a range of intervention techniques: procedural and sensory information (one study), procedural information and behavioural instruction (three), procedural information, sensory information, behavioural instruction (two); behavioural instruction (three); procedural information, behavioural instruction, cognitive interventions, relaxation techniques (one), procedural information, hypnosis (one); procedural and sensory information/cognitive interventions/behavioural instruction (one); behavioural instruction, relaxation techniques, cognitive interventions (one); relaxation (one); behavioural instruction, cognitive interventions (one); relaxation techniques, hypnosis (one).
Less clear findings were reported in two studies. Ferrara 2008 reported that postoperative pain scores were significantly lower in the intervention group than the control group at four weeks after surgery, but a comparison at 15 days was not clearly reported – it is possible that authors were choosing to not report non‐significant findings. Shelley 2007 reported a significant interaction between intervention group, self‐efficacy and external health locus of control (EHLC), but post‐hoc analyses revealed only trend level effects, such that intervention participants had a smaller pain increase than controls if they had high EHLC and low self‐efficacy. Participants with high self‐efficacy and high EHLC, or low self‐efficacy and low EHLC, reported increased pain for intervention participants compared with controls.
Five studies' findings were difficult to interpret in the context of our review questions. Kulkarni 2010 reported that median postoperative pain scores for all three groups was `3', but no information was provided as to how pain was measured, and information about range/interquartile range or analyses were reported. Liu 2004's findings were puzzling because the authors stated in their Discussion that intervention participants had significantly lower pain scores compared with the control group, but the mean scores provided in the Results section suggested their findings were in the opposite direction. Wells 1982 and Wijgman 1994 had no control group – in each case two different interventions were compared, meaning that it was not possible to determine what the effect of the intervention was over standard care or an attention control. Ziemer 1982 did not report postoperative pain as an outcome and instead focused on how pain correlated with coping scales.
Summary: postoperative pain
In summary, the pattern of evidence from the meta‐analyses suggests that psychological preparation may reduce postoperative pain in the first month after surgery, although this finding should be treated with caution since it is based on pooling studies with diverse types of psychological interventions and because the size of the pooled effect (‐0.20) would generally be considered of low magnitude (Cohen 1988). Of the narratively synthesized studies, most found no significant difference between intervention and control groups. It is of interest that, while none of these studies contained `pure' behavioural instruction, 12 of the 16 studies reporting non‐significant differences contained behavioural instruction as a component (Chumbley 2004; Dewar 2003; Hawkins 1993; Johnson 1978b; Johnson 1985; Lilja 1998; Oetker‐Black 2003; Parthum 2006; Shuldham 2002; Vukomanović 2008; Watt‐Watson 2004; Wijgman 1994). This would be consistent with the meta‐analysis findings suggesting that behavioural instruction does not impact postoperative pain. However, similarly to the studies in the meta‐analyses, there is a high degree of heterogeneity in these studies in terms of the types of surgery and intervention content. Due to the high heterogeneity, and the high number of studies reporting sufficient methodological details to ascertain risk of bias, we downgraded the overall quality of evidence for the outcome postoperative pain by two points to `low' (see Table 1).
2.Behavioural recovery
Fourteen studies (13% of 105 studies) were included that measured a behavioural recovery outcome, in which 1135 participants were randomized (11% of 10,302 participants randomized across all studies): D'Lima 1996; Ferrara 2008; Fortin 1976; Gilbey 2003; Heidarnia 2005; Hoogeboom 2010; Lévesque 1984; Mahler 1998; McGregor 2004; Oetker‐Black 2003; Oosting 2012; Ridgeway 1982; Watt‐Watson 2004; Zieren 2007. One study was cluster‐randomized and therefore not eligible for inclusion in meta‐analysis (Lévesque 1984). Suitable continuous data for meta‐analysis were available in only three studies (Mahler 1998; McGregor 2004; Zieren 2007), and dichotomous data in two studies (Fortin 1976; Oosting 2012). As there was also a range of different behavioural recovery outcome measures, we decided that a narrative synthesis would be more appropriate for this outcome than meta‐analysis.
Behavioural recovery findings are summarized in Table 3. Behavioural instruction was a common intervention type for these studies (included in all interventions except that of Ridgeway 1982). Statistically significant beneficial effects of the intervention over control conditions were reported in five studies (Fortin 1976; Gilbey 2003; Heidarnia 2005; McGregor 2004; Oetker‐Black 2003). Ridgeway 1982 reported a trend effect, such as that participants in their cognitive intervention group were carrying out more household activities (P value = 0.10), and Watt‐Watson 2004 found mixed results: behaviours of deep breathing and coughing were experienced as being less affected by pain in the intervention group, but no significant differences were seen for other activities (general activities, sleep, walking). Differences between groups were not significant in three studies (Hoogeboom 2010; Lévesque 1984; Mahler 1998), and analyses were not reported in three studies (Ferrara 2008; Oosting 2012; Zieren 2007).
2. Findings of studies that examined the outcome behavioural recovery.
| Author, year | Surgery type and sample size (randomized) | Intervention categories |
Behavioural recovery measure(s) The first measure listed is that prioritized in this review |
Behavioural recovery findings (as available) |
| D'Lima 1996 | Total knee replacement N = 30 |
Intervention 1: Behavioural instruction Intervention 2: Behavioural instruction |
3 weeks post‐surgery Function scale from Hospital for Special Surgery Knee Rating; high score = better function |
Control mean = 35, n= 10 Intervention 1 mean = 32, n = 10 Intervention 2 mean = 30.5, n = 10 "in the immediate postoperative period both exercise groups showed a steeper decline in function than the control group"; statistics not provided |
| Ferrara 2008 | Total hip replacement N = 23 |
Behavioural instruction | 15 days and 4 weeks post‐surgery: Disability (Barthel Index) (high scores: less disabled) Functional status (from WOMAC); high scores = worse function |
Intervention n = 11, control n = 12 No data/findings reported for these time points (study focus: 3 months postoperation) |
| Fortin 1976 | Herniorraphy, cholecystectomy, intra‐pelvic surgery (primarily hysterectomies) n = 69 |
Procedural information, behavioural instruction | Day 2 postoperation: "inpatient ambulatory activity" (IAA). Ability to do physical activities at hospital in immediate postoperative period – e.g. movements in bed, get up, walk. Higher level (max = 3) = can do more. Day 10 post‐surgery: ‘Activities of Daily Living’ (ADL). Capacity to perform tasks appropriate to normal life at home. Higher level (max = 3) = more independent. |
Authors combined levels 1 and 2 in analysis 2 days IAA: Intervention n at level 3/total N = 27/37, control group = 5/32 10 days ADL: Intervention n at level 3/total N = 27/36, control group = 8/31 Better function in intervention than control group with both assessments Analysing 29 matched pairs, significant difference at 2 and 10 days (P value < 0.01 for each, Wilcoxon matched pairs) Full sample: also significantly different at both time points (Mann‐Whitney U, P value < 0.05 for each) |
| Gilbey 2003 | Total hip arthroplasty N = 76 |
Behavioural instruction | Week 3 post‐surgery: Physical function domain of WOMAC |
Intervention n = 32; control n = 25 Means/SDs presented only for total WOMAC scale, not for physical function domain. Significant difference (P value < 0.01) for physical function domain reported (intervention group scoring better). |
| Heidarnia 2005 | Coronary artery bypass surgery N = 80 |
Procedural information, behavioural instruction | 1 month post‐surgery: SF‐36 Physical Function (high scores = more active) Nottingham Health Profile (NHP) Physical Mobility (high scores = greater dysfunction) |
Intervention n = 35; control n = 35 SF‐36 Physical Function: Intervention mean = 25.3, control mean = 21.8 NHP Physical Mobility: Intervention mean = 32.97, control mean = 26.1 Independent t‐tests. Intervention group better than control group on both outcomes: SF‐36 Physical Function (P value < 0.00001); NHP Physical mobility P value < 0.00001) |
| Hoogeboom 2010 | Total hip replacement N = 21 |
Behavioural instruction | Iowa Level of Assistance Scale ‐ taken each postoperative day in hospital; authors used this to measure "time needed to reach functional independence": lower scores = more independent | Intervention: time to reach functional independence median 4 days (range 3 to 6, n = 8(?)); control group median 4 days (range 3 to 5, n = 10) Difference in time to reach functional independence not significant (P value = 0.963) |
| Lévesque 1984 | Cholecystectomy N = 125 |
Intervention 1: Procedural information, sensory information, behavioural instruction, emotion‐focused (at pre‐admission, 15 days before surgery) Intervention 2: Procedural information, sensory information, behavioural instruction, emotion‐focused (afternoon before surgery) |
First 2 post‐surgery days: A postoperative recovery index; dimension "physical functional ability". Believe high scores = better outcome (not clear). |
Cluster‐randomized trial. Data = mean (SD). Intervention 1: day 1: 14.26 (3.4); day 2: 20.7 (2.5), n = 40 Intervention 2: day 1: 15.45 (3.16); day 2: 20.87 (2.43), n = 42 Control: day 1: 14.65 (3.02); day 2: 20.85 (2.17), n = 43 The 2 intervention groups were combined for analyses. Carried out multiple regressions to control for other independent variables (including study group), and used these to select covariates to enter into MANOVAs. For physical function recovery, no covariates entered for day 1; state anxiety on eve of surgery for day 2. Both day 1 and day 2: F ratios not significant. |
| Mahler 1998 | Coronary artery bypass surgery N = 268 |
Intervention 1: Procedural and sensory information; behavioural instruction Intervention 2: Procedural and sensory information; cognitive intervention Intervention 3: Procedural and sensory information; cognitive intervention |
Monitoring of ambulation with device that counts movements using mercury tilt switch. Worn on days 2, 3 and 4 at one hospital; days 3, 4, 5 post‐surgery at second hospital. Worn from morning to late afternoon/early evening. | Intervention 1: mean (SD) = 11.01 (1.02), n = 65 Intervention 2: 10.77 (1.02), n = 65 Intervention 3: 11.41 (1.12), n = 60 Control: 9.69 (0.85), n = 67 ANOVA and planned orthogonal comparisons. No significant effects by study group (P values < 0.60) |
| McGregor 2004 | Total hip arthroplasty N = 39 |
Procedural information, behavioural instruction | Before discharge: Barthel Index: high score = less limited WOMAC function (high scores = worse functional limitations) |
Intervention n = 15; control n = 20 Barthel index: Intervention mean (SD): 19.8 (.4); Control: 18.7 (1.4) WOMAC function: Intervention mean (SD): 25.7 (8.3); Control: 28.3 (12.1) Barthel Index: better improvement in older adults in intervention group (P value < 0.005). Trend to reduction in WOMAC scores for older adults in intervention group. Does not report analysis of a simple comparison by group alone. |
| Oetker‐Black 2003 | Total abdominal hysterectomy N = 108 |
Behavioural instruction, cognitive intervention, relaxation | At discharge: Health Status Questionnaire (HSQ): Physical Functioning Subscale: high scores = better outcome Length of time ambulated on first post‐surgery day |
Mean (SD) not reported for HSQ Ambulation: Intervention mean (SD): 330 (615); control 156 (97) HSQ analyses are not presented by subscale Ambulation: intervention participants ambulated longer than controls (F(1,105) = 2.05, P value = 0.043) |
| Oosting 2012 | Total hip arthroplasty N = 30 |
Behavioural instruction | 4 days post‐surgery: Iowa Level of Assistance Scale (ILAS), ability to function in daily life. Low scores = more independent. Split scores: < 6 (for "functional mobility" or ≥ 6 |
Intervention: 10 of n = 12 rated "functionally mobile"; control: 11 of n = 13 rated "functionally mobile" No reported test of significance for this outcome |
| Ridgeway 1982 | Abdominal hysterectomy N = 60 |
Intervention 1: Procedural and sensory information Intervention 2: Cognitive intervention |
Diary record – days when performed 10 household activities over 3 post‐surgery weeks. For score: summed across tasks and no. days each was performed. | Intervention 1 mean = 6.6, n = 20 Intervention 2 mean = 6.9, n = 20 Control mean = 5.9, n = 20 Report trend, Intervention 2 doing most (ANOVA F = 2.2, df = 3.66, P value = 0.10). NOTE: included a 4th group in ANOVA – patients who refused information (not relevant to review as not randomized) |
| Watt‐Watson 2004 | CABG N = 406 |
Intervention: Behavioural instruction, cognitive intervention | Days 3 and 5 post‐surgery: pain interference with general activities, sleep, walking, deep breathing and coughing (modified Interference Subscale of Brief Pain Inventory) | Behavioural recovery: controls: more pain interference related to deep breathing and coughing (mean 3.8 (SD 3.1) versus mean 2.7 (SD 3.1); t(355) = 2.54; P value < 0.01). Other activities not significant. |
| Zieren 2007 | Inguinal hernia surgery N = 100 |
Procedural information, behavioural instruction | DAy 1 post‐surgery: SF‐36 physical functioning (high scores: less disability) |
Intervention n = 50; control n = 50 No statistics presented. Observed that differences were visible on first postoperative day, with physical and psychological functions being less affected in intervention than control group. |
ADL = activities of daily living
ANOVA = analysis of variance
CABG = coronary artery bypass graft
F = F statistic (ANOVA)
HSQ = Health Status Questionnaire
IAA = inpatient ambulatory activity
ILAS = Iowa Level of Assistance Scale
N = number of participants in sample
NHP = Nottingham Health Profile
SD = standard deviation
SF = Short Form
T = T statistic value (t‐test)
WOMAC = Western Ontario and McMaster Osteoarthritis Index
Finally, D'Lima 1996 reported more negative outcomes for intervention groups, with the control group having the highest function score. It is of concern that an intervention could lead to a worse outcome, so it is helpful to examine this study further. D'Lima 1996 used two intervention groups, both focused on exercise prior to knee replacement surgery: intervention 1 consisted of physical therapy sessions designed to strengthen muscles and improve range of motion; intervention 2 consisted of cardiovascular conditioning to improve fitness. The mean outcome function scores were 35 for the control group, 32 for intervention 1 and 30.5 for intervention 2, with higher scores indicating better function. The authors do not present any direct comparison information across groups, simply stating that the intervention groups showed a decline in function. It is therefore not clear whether these scores are significantly different across groups, and with only 10 participants in each group it is unclear how reliable these findings are. Other studies in this group also used preoperative exercises to strengthen muscles, improve range of motion and/or improve cardiac fitness in people undergoing joint replacement surgery (Ferrara 2008; Gilbey 2003; Hoogeboom 2010; Oosting 2012). Ferrara 2008 and Oosting 2012 did not report significance of findings for this outcome (at time points relevant to the review). Hoogeboom 2010 reported no difference between intervention and control groups in time to reach "functional independence", but Gilbey 2003 reported that the intervention group had significantly better scores than the control group on the physical function domain of the WOMAC at three weeks after the operation. Thus, while variance in measure types and timings makes it difficult to determine whether, or how, this type of behavioural instruction is of benefit to patients undergoing joint replacement surgery, it is not clear that the intervention is harmful.
The five studies that reported statistically significant effects in favour of the intervention group address a range of surgical procedures (one coronary artery bypass surgery, two total hip arthroplasty, one hysterectomy and one mixed surgical types), and a range of interventions (behavioural instruction about pre and postoperative behaviours, procedural information) (Fortin 1976; Gilbey 2003; Heidarnia 2005; McGregor 2004; Oetker‐Black 2003). One study incorporated relaxation and cognitive intervention alongside behavioural instruction (Oetker‐Black 2003). The three studies that found differences to be non‐significant similarly addressed various procedures (total hip replacement, cholecystectomy, coronary artery bypass surgery) and interventions (behavioural instruction about pre‐ and postoperative behaviour, procedural information, sensory information and emotion‐focused interventions) (Hoogeboom 2010; Lévesque 1984; Mahler 1998). There were also no obvious differences in the types of outcome measures used by studies that did, and did not, find effects: the studies with significant differences used measures of inpatient ambulatory activity and activities of daily living, the physical function domain of WOMAC, SF‐36 physical function, the Barthel Index and the Health Status Questionnaire. The studies that did not find significant differences used the Iowa Level of Assistance Scale (Shields 1995), a postoperative recovery index measuring physical functional ability and ambulation monitoring.
Summary: behavioural recovery
Thus, while there were some promising findings suggesting that psychological preparation, in particular behavioural instruction, may improve behavioural recovery outcomes, there is a need for agreement on the outcome measures used to be able to more directly compare findings across studies, and for studies to consistently report findings in sufficient detail to allow data to be pooled across studies in meta‐analysis. We rated the overall quality of evidence as `very low' (downgraded by three points). We rated the risk of bias as `very serious', leading to downgrading by two points, because of the high proportion of `uncertain' ratings and because the number of studies with sufficiently robust measurement to meet our inclusion criteria and reporting suitable data for meta‐analysis was low. We made a further downgrade for high heterogeneity (see Table 1).
Secondary outcomes
1.Negative affect
Studies included in meta‐analysis
Fifty studies reported the outcome negative affect. We included 31 (30% of 105 studies) in the omnibus meta‐analysis (Ali 1989; Ashton 1997; Bergmann 2001; Bitterli 2011; Broadbent 2012; Cheung 2003; Cuñado Barrio 1999; Cupples 1990; Doering 2000; Done 1998; Felton 1976; Fortin 1983; Giraudet 2003; Guo 2012; Hart 1980; Heidarnia 2005; Lamarche 1998; Leserman 1989; Levesque 1977; Lim 2011; Ma 1996; Oliphant 2013; Pellino 2005; Postlethwaite 1986; Reading 1982; Ridgeway 1982; Schwartz‐B'tt 1994; Seers 2008; Yang 2012; Zhang 2012; Zieren 2007), with data from 2496 participants analysed (24% of 10,302 participants randomized across all studies) (Analysis 1.3; Figure 5). As a variety of scales were used to measure negative affect, we used standardized scores (SMD (Hedges' g)) to pool data. Higher scores indicate higher negative affect; effect scores below zero indicate that the intervention group had lower negative affect. Overall, there was evidence of lower negative affect in the intervention groups compared with the control groups (SMD ‐0.35, 95% CI ‐0.54 to ‐0.16). Although once again there were very high levels of statistical heterogeneity (I2 statistic = 81%), which suggests extreme caution needs to be taken when interpreting the result of the meta‐analysis, the results of the forest plot show a consistent pattern of results in favour of lower negative affect after psychological preparation.
1.3. Analysis.
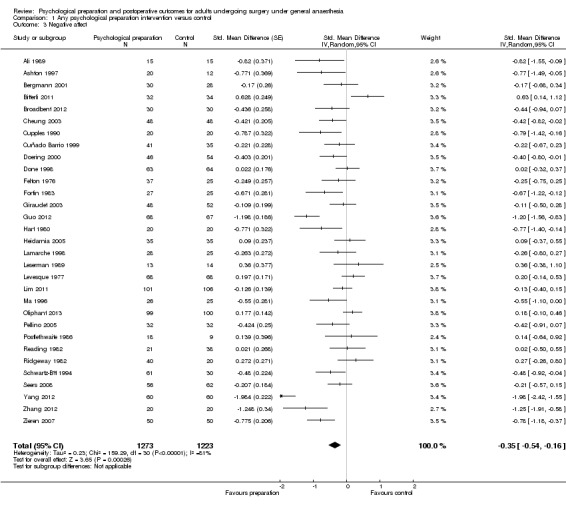
Comparison 1 Any psychological preparation intervention versus control, Outcome 3 Negative affect.
5.
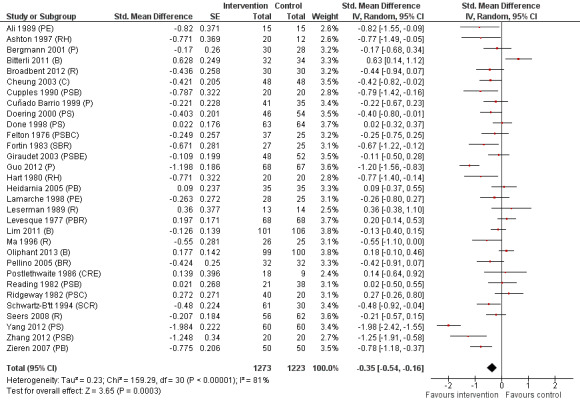
Negative affect (any psychological preparation intervention versus control). B: behavioural instruction; C: cognitive interventions; E: emotion‐focused interventions; H: hypnosis; P: procedural information; R: relaxation; S: sensory information.
The results for individual intervention types again tended to be similar to the omnibus meta‐analysis. When considering both `pure' and `mixed' studies together, there were statistically significant results for the procedural information (Analysis 2.3), sensory information (Analysis 3.3), relaxation techniques (Analysis 6.3) and hypnosis (Analysis 7.1) analyses. There was no clear evidence of an effect for the behavioural instruction (Analysis 4.3), cognitive (Analysis 5.3) or emotion‐focused (Analysis 8.3) interventions. The funnel plot showed no clear evidence of publication bias.
2.3. Analysis.
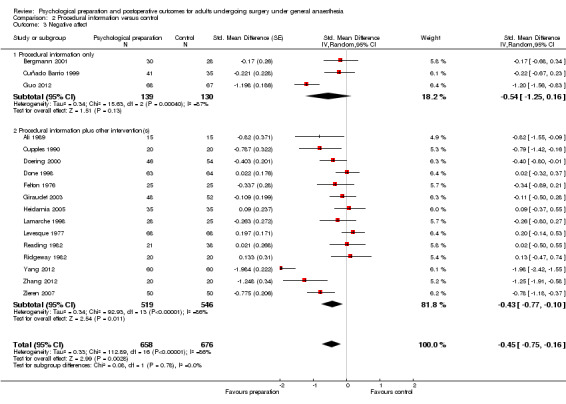
Comparison 2 Procedural information versus control, Outcome 3 Negative affect.
3.3. Analysis.
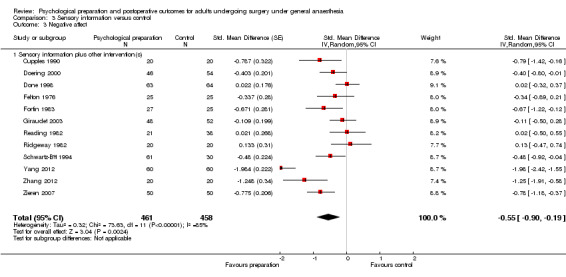
Comparison 3 Sensory information versus control, Outcome 3 Negative affect.
6.3. Analysis.
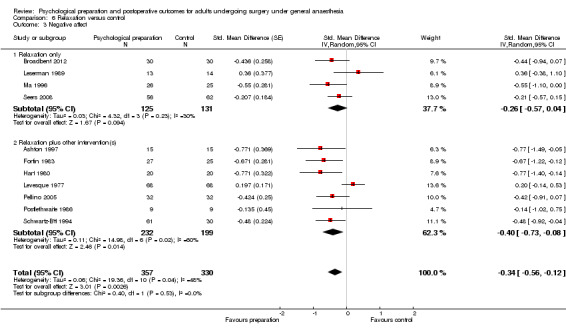
Comparison 6 Relaxation versus control, Outcome 3 Negative affect.
7.1. Analysis.
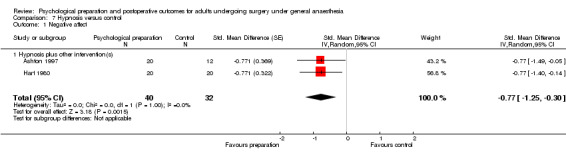
Comparison 7 Hypnosis versus control, Outcome 1 Negative affect.
4.3. Analysis.
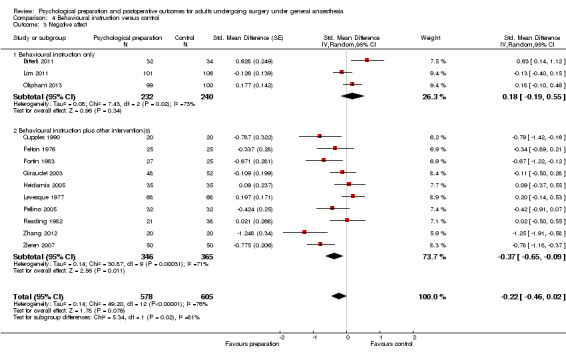
Comparison 4 Behavioural instruction versus control, Outcome 3 Negative affect.
5.3. Analysis.
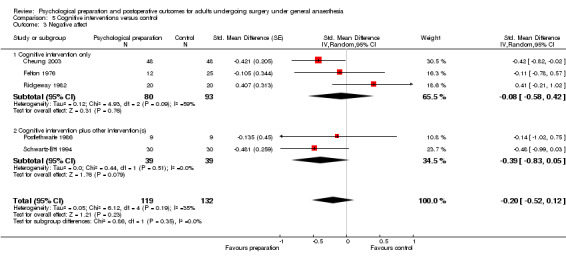
Comparison 5 Cognitive interventions versus control, Outcome 3 Negative affect.
8.3. Analysis.
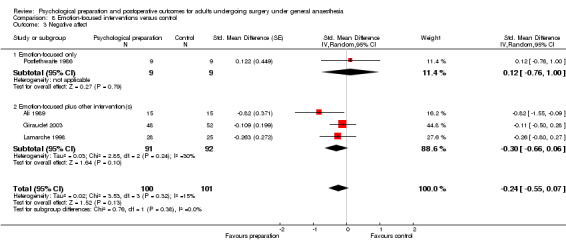
Comparison 8 Emotion‐focused interventions versus control, Outcome 3 Negative affect.
Studies not included in meta‐analysis
Nineteen studies contained appropriate data for narrative synthesis only: Barlési 2008; Burton 1995; Chumbley 2004; Daltroy 1998; DeLong 1970; Elsass 1987; Gräwe 2010; Hawkins 1993; Johnson 1978b; Johnson 1985; Klos 1980; Lévesque 1984; McGregor 2004; O'Connor 2014; Oetker‐Black 2003; Osinowo 2003; Shelley 2007; Shuldham 2002; Watt‐Watson 2004 (see Table 4). We excluded one of these studies from meta‐analysis because it was a cluster‐randomized trial (Lévesque 1984); we excluded the remainder from meta‐analysis because they provided insufficient information.
3. Findings of studies that examined the outcome negative affect but could not be included in meta‐analyses.
| Author, year | Surgery type and sample size (randomized) | Intervention categories |
Negative affect measure(s) The first measure listed is that prioritized in this review |
Negative affect findings (as available) |
| Barlési 2008 | Thoracic surgery for non‐small cell lung cancer N = 102 |
Procedural information | Timing unclear: at time of surgery (postoperative period) or 1 month post‐surgery Psychologic Global Well‐being Scale; components include Anxiety, Depressed Mood and Positive Well‐being (also self control, general health, vitality) |
Control n = 34; intervention n = 41 Mean/SD provided only for total scale (including non‐negative affect components). For the individual elements, no significant differences (no details provided). |
| Burton 1995 | Mastectomy/sector mastectomy for breast cancer N = 215 |
Intervention 1: Cognitive intervention and emotion‐focused (preoperative interview) Intervention 2: Cognitive intervention and emotion‐focused (preoperative interview + 30 minute ‘chat’ on unrelated matters) Intervention 3. Cognitive intervention and emotion‐focused (preoperative interview + 30‐minute brief psychotherapeutic intervention – additional emotion‐focused content) |
Day 4 post‐surgery: Hospital Anxiety and Depression Scale (HADS) Anxiety and Depression. Also General Health Questionnaire ‐28 and modified Present State Examination schedule and the Diagnostic and Statistical Manual of Mental Disorders, 3rd Ed (DSM‐III) but results are not reported. |
Only report mean HADS scores for the overall sample, not by group at 4 days postoperation. Other negative affect also not reported by group at this time point. |
| Chumbley 2004 | Mixed: surgeries that would receive PCA routinely N = 246 |
Intervention 1: Behavioural instruction (leaflet) Intervention 2: Behavioural instruction (interview) |
24‐72 hours post‐surgery: HADS Anxiety Profile of Mood States (POMS) Tension/anxiety |
Cluster‐randomized trial HADS Anxiety: Control mean (95% CI) = 6.17 (5.34 to 8.00, n = 73); Intervention 1 mean (95% CI) = 6.03 (4.94 to 7.12, n = 75); Intervention 2 mean (95% CI) = 6.52 (5.59 to 7.45, n = 72) No significant difference across groups (HADS anxiety, P value = 0.31; POMS tension/anxiety P value = 0.28) |
| Daltroy 1998 | Total hip or knee arthroplasty N = 222 |
Procedural and sensory information | Day 4 after surgery: State Trait Anxiety Inventory (STAI) state anxiety |
Intervention did not affect anxiety in general linear model (P value = 0.94). No interaction between intervention and denial, anxiety or desire for information. No main effects mentioned. |
| DeLong 1970 | Gall bladder removal and removal of uterus N = 70 |
Procedural information, sensory information, behavioural instruction | Day 5 or 6 after surgery: STAI (state and trait anxiety) |
No differences in anxiety scores across groups (no statistics provided) |
| Elsass 1987 | Inguinal hernia or varicose vein surgery N = 90 |
Procedural information | 1 ½ hours after surgery and day after surgery STAI state anxiety |
Control n = 40; intervention n = 40. Anxiety scores are presented but unclear whether mean or median: Control score = 52; intervention score = 42 (reading off Figure 1). Difference in scores between groups "increased significantly" at 1 ½ hrs after operation (P value < 0.05, Mann Whitney); intervention group less anxious |
| Gräwe 2010 | Mixed: abdominal or vascular surgery N = 96 |
Sensory information, cognitive intervention | Days 1 to 3 post‐surgery: STAI state anxiety BSKE – general psychological well‐being |
Comparisons by group not reported for this outcome |
| Hawkins 1993 | Gynaecological surgery (mixed) N = 60 |
Behavioural instruction | 48 hours after surgery: Hospital Anxiety Scale |
Control n = 40 (combining standard care and attention controls); intervention n = 20 No report of comparisons for this outcome |
| Johnson 1978b | Sample 1: cholecystectomy, N = 81 Sample 2: inguinal hernia repair, N = 68 |
Intervention 1: ‘Instruction’: Behavioural instruction (deep breathing, coughing, leg exercises) Intervention 2: ‘Procedure information’: focus procedural information, also some sensory information and behavioural instruction Intervention 3: `Sensation information’: focus: sensory information, also some procedural information and behavioural instruction 2 x 3 factorial design: no instruction/instruction (Intervention 1; no information/information (Interventions 2 and 3) |
Scores totaled over days 1, 2 and 3: Mood Adjective Checklist (fear, well‐being, happiness, helplessness, anger) | Sample 1 Negative affect: no main effect of interventions but interactions between instruction and preoperative fear (F(5, 61) = 4.69, P value < 0.001) and information and preoperative fear (F(10,122) = 2.07, P value < 0.05) Low fear group: `instruction' tended to increase negative moods and decrease positive moods compared with no‐instruction, and tendency for ‘procedure information’ to decrease and ‘sensation information’ to increase negative mood compared with no information, but these comparisons were not significant High fear group: `instruction' tended to decrease negative mood and increase positive mood compared with no instruction; significant for anger and happiness (Dunnett’s t(1,65) = 3.32, P value < 0.001; t(1,65) = 3.35, P value < 0.001). Those receiving ‘procedure information’: higher means for fear and positive moods, and lower means for helplessness and anger, but only anger significant (Dunnett’s t(2,65) = 2.00, P value < 0.05). ‘Sensation information’: positive moods tended to be higher and negative moods lower than no information group; only anger significant (Dunnett’s t(2,65) = 2.43, P value < 0.025). Sample 2 Interaction between instruction and information (F(10,96) = 1.93, P value < 0.05) but no significant univariate findings, difficult to interpret |
| Johnson 1985 | Abdominal hysterectomy N = 199 |
Intervention 1: procedural and sensory information Intervention 2: ‘cognitive‐coping technique’ – cognitive intervention Intervention 3: ‘Behavioural‐coping technique’ – behavioural instruction 2 x 3 factorial design: no information/information (Intervention 1); no coping technique/coping technique (Interventions 2 and 3) |
Day 3 post‐surgery (and 1st and 4th weeks post‐discharge): Profile of Mood States (POMS: anxiety, confusion, anger, depression, fatigue, vigour). 3rd postoperative day and 1st and 4th week post‐discharge. |
Outcomes entered into MANOVA included anxiety. Significant at P value < 0.10: coping technique, F (16, 286) = 1.59, P value = 0.07 (outcomes physical recovery, narcotic doses and length of stay seem to be responsible for this effect). Included race as factor; interaction between race and coping technique (F16, 286) = 1.58, P value = 0.07). For white patients, ‘behavioural coping’ reduced anxiety (Dunnett’s t(3,150) = 3.45, P value < 0.001); ‘cognitive’ and ‘behavioural’ techniques reduced confusion (Dunnett’s t(3,150) = 2.75, P value < 0.025); non‐significant for black participants. |
| Klos 1980 | Cholecystectomy N = 50 |
Intervention 1: Procedural information, behavioural instruction (pamphlet) Intervention 2: Procedural information, behavioural instruction (nurse visit) Intervention 3: Procedural information, behavioural instruction (pamphlet and nurse visit) |
2nd post‐surgery day: Mood Adjective Checklist: 15 adjectives describing 5 mood dimensions: fear, well‐being, happiness, helplessness, anger |
Authors did not report analyses by whole intervention group; instead, analyses are reported after median split into high‐ preoperative‐fear and low‐preoperative fear groups 2 x 2 factorial design: pamphlet/no pamphlet versus nurse visit/no nurse visit Significant differences between means of intervention 2 (nurse‐visit) and no‐nurse visit for high‐preoperative‐fear group for well‐being [F(1,20) = 6.57, P value < 0.10] and happiness (F (1,20) = 11.89, P value < 0.05). Patients with the nurse visit scored higher on positive moods than those who did not receive it. |
| Lévesque 1984 | Cholecystectomy N = 125 |
Intervention 1: Procedural information, sensory information, behavioural instruction, emotion focused (15 days before surgery) Intervention 2: Procedural information, sensory information, behavioural instruction, emotion focused (afternoon before surgery) |
First 3 days after surgery STAI (French version) state anxiety |
Cluster‐randomized Day 1 Control mean (SD) = 37.5 (8.51, n = 43); intervention 1 mean (SD) = 35.34 (9.34, n = 40); intervention 2 mean (SD) = 37.38 (8.29, n = 42) No significant difference between groups for postoperative state anxiety |
| McGregor 2004 | Total hip arthroplasty N = 39 |
Procedural information, behavioural instruction | Positive & Negative Affect Schedule (PANAS) | Control n = 20; intervention n = 15 No mention of findings for analysis by group. May only be presenting positive findings – if so, this would suggest null result. |
| O'Connor 2014 | Surgery for rectal cancer N = 85 |
Procedural information | Prior to discharge: HADS anxiety and depression |
Numerical data not reported for this outcome Control group: slightly higher anxiety score but not significantly different; depression – similar means, not significantly different |
| Oetker‐Black 2003 | Total abdominal hysterectomy N = 108 |
Behavioural instruction, cognitive intervention, relaxation | Day 1 post‐surgery day and at discharge: STAI state anxiety |
Only analyses at later time points reported |
| Osinowo 2003 | Not stated – participants from surgical and gynaecological wards N = 33 |
Intervention 1: Cognitive intervention (Rational Emotive Therapy) Intervention 2: Cognitive intervention (Self‐Instructional Training) |
24 hours post‐surgery: STAI state anxiety HADS Anxiety HADS Depression |
STAI scores: Control mean unclear (2 possible scores), n = 11; intervention 1 mean (SD) = 30.91 (6.61, n = 11); intervention 2 mean (SD) = 33.82 (6.21, n = 11). Intervention 2 (SIT): decrease in anxiety from pre‐intervention to postoperation. HADS anxiety: decreased for both intervention groups; changes in control group ns (Intervention 1: t(10) = 3.62, P value < 0.01; Intervention 2: t(10) = 2.06, P value < 0.05; control t(10) = 1.13, non‐significant. HADS depression: no significant changes across time Paper generally written unclearly |
| Shelley 2007 | Coronary artery bypass surgery N = 90 |
Cognitive intervention | Day 4 post‐surgery: Distress (Depression, Anxiety and Stress Scales, DASS) |
Control n = 43; intervention n = 37 Direct effect of group not significant; 3‐way interaction was significant (intervention x external health locus of control x self efficacy, F(1,71) = 6.20, P value < 0.05). Fig 1 suggests, for intervention participants: lower distress than controls if EHLC and self efficacy either both high or both low. If EHLC low and self efficacy high, appears to be little change; if high EHLC and low self efficacy then lower distress for Control group. |
| Shuldham 2002 | Coronary artery bypass surgery N = 356 |
Procedural information and behavioural instruction | Day 3 post‐surgery: Anxiety – HADS Depression – HADS ‘tense and uptight’ – General Well‐being Questionnaire ‘worn out’ – General Well‐being Questionnaire |
Control n = 156; intervention n = 173 No significant differences between variables at 3 days post‐surgery (using Mann‐Whitney U): Anxiety: U = 11,636, Z = ‐0.28, P value = 0.78 Depression: U = 10,756; Z = ‐1.24, P value = 0.22 Tense and uptight: U = 10,008, Z = ‐1.27, P value = 0.21 Worn out: U = 9,717.5, Z = ‐1.49, P value = 0.14 |
| Watt‐Watson 2004 | CABG N = 406 |
Behavioural instruction, cognitive intervention | Days 3 and 5 post‐surgery Pain interference with mood; modified version of Interference Subscale of the Brief Pain Inventory (BPI‐I) |
Findings are not reported for this outcome – it would appear that authors are only reporting significant findings so it seems likely that group differences were not significant |
BPI‐I = Interference Subscale of the Brief Pain Inventory
BSKE (EWL) = Befindlichkeitsskalierung durch Kategorien und Eigenschaftswörter (measuring general psychological well‐being)
CABG = coronary artery bypass graft
CI = confidence interval
DASS = Depression, Anxiety and Stress Scales
DSM‐III = Diagnostic and Statistical Manual (of Mental Disorders), version 3
EHLC = external health locus of control
F = F statistic (analysis of variance)
HADS = Hospital Anxiety and Depression Scale
N = number of participants in sample
PANAS = Positive and Negative Affect Schedule
PCA = patient‐controlled analgesia
POMS = Profile of Mood States
SD = standard deviation
SIT = Self‐Instructional Training
STAI = State Trait Anxiety Inventory
U = U statistic (Mann‐Whitney test)
A statistically significant impact of the intervention over control was reported by Elsass 1987, who found a procedural information intervention led to less anxiety in the intervention group than the control group 1½ hours after surgery. Unclear findings were reported by Osinowo 2003: it would seem that participants receiving a cognitive intervention experienced a decrease in anxiety while a control group did not, but groups do not appear to have been directly compared.
Mixed findings were reported by authors of four papers that examined interactions in their data. Johnson 1978b reported interactions in their sample of patients undergoing cholecystectomy. In their low preoperative fear group, differences between groups were not significant. For the high fear group, participants receiving behavioural instruction tended to have decreased negative mood and increased positive mood (significant for anger and happiness, not fear, helplessness or well‐being). In the high fear group, those receiving interventions focusing on procedural and sensory information also had significantly lower anger scores. In a second sample (inguinal hernia repair patients), an interaction was discovered between behavioural instruction and procedural/sensory information‐focused groups but no significant comparisons were identified. Klos 1980 also compared high and low fear groups: participants with high preoperative fear receiving procedural information and behavioural instruction via a nurse visit had higher scores for happiness than a control group; other analyses were not reported (including for the outcome fear, which this review would prioritize), suggesting that other findings were not significant. Johnson 1985 reported that a behavioural instruction intervention reduced postoperative anxiety, although only for white participants (intervention effects were not significant for black participants). An interaction of intervention (cognitive intervention) x external health locus of control (EHLC) x self‐efficacy was examined by Shelley 2007. There was no significant direct effect of group allocation, but the interaction between the three factors was significant. It seemed likely that lower distress was reported for intervention than control participants if EHLC and self‐efficacy were either both high or both low. If participants had high EHLC and low self‐ efficacy, then the control group seemed to be less distressed.
Seven studies reported no significant differences between groups (Barlési 2008; Chumbley 2004; Daltroy 1998; DeLong 1970; Lévesque 1984; O'Connor 2014; Shuldham 2002). These studies included the interventions procedural information (two studies), procedural and sensory information (one), behavioural instruction (one), procedural information, sensory information and behavioural recovery (one), procedural information and behavioural instruction (one), procedural information, sensory information, behavioural instruction and emotion‐focused intervention (one). As such, all but one contained the component (procedural information) that was contained in the study that found significant effects (Elsass 1987). In this narrative synthesis, only one study reported findings using a cognitive intervention (Osinowo 2003); it is unfortunate that the findings were not more clearly reported.
Six studies did not report analyses for the negative affect outcome of relevance to the review even though authors reported measuring it (Burton 1995; Gräwe 2010; Hawkins 1993; McGregor 2004; Oetker‐Black 2003; Watt‐Watson 2004). In some cases it may be because studies reported significant findings only, and findings were not significant, but this is not clear.
Summary: negative affect
In summary, there was some evidence from the meta‐analyses of a beneficial effect of psychological preparation techniques on postoperative negative affect, although once again the high levels of unexplained statistical heterogeneity make it difficult to accept this result with confidence. The pooled effect size from the omnibus analysis was ‐0.35, often considered to represent a small effect (Cohen 1988). There did not appear to be evidence that certain techniques performed better than others in reducing negative affect. Overall, it would seem that psychological preparation techniques may have beneficial effects of postoperative outcomes but the high level of heterogeneity in the data makes it difficult to determine the circumstances and intervention content that would consistently improve outcomes. There is also some suggestion that individual characteristics (e.g. level of preoperative fear) may affect the way that psychological preparations impact on postoperative outcomes. Due to the high heterogeneity and the high number of studies reporting sufficient methodological details to ascertain risk of bias, we downgraded the overall quality of evidence for the outcome negative affect by two points to `low' (see Table 1).
2.Length of stay
Studies included in meta‐analysis
Of the 58 studies with length of stay as an outcome, sufficient data were available for meta‐analysis in 36 (34% of 105 studies: Ashton 1997; Barbalho‐Moulim 2011; Beaupre 2004; Bergin 2014a; Bitterli 2011; Chaudhri 2005; Crowe 2003; Cuñado Barrio 1999; D'Lima 1996; Daltroy 1998; Doering 2000; Felton 1976; Fortin 1976; Furze 2009; Giraudet 2003; Hulzebos 2006a; Lam 2001; Langer 1975; Leserman 1989; Levin 1987; Lin 2005; Lindeman 1973; Mahler 1995; Mahler 1998; McGregor 2004; Oosting 2012; Rajendran 1998; Ridgeway 1982; Schmitt 1973; Shuldham 2002; Watt‐Watson 2000; Watt‐Watson 2004; Wilson 1981; Zhang 2012; Ziemer 1982; Zieren 2007), with data from 3313 participants (32% of 10,302 participants randomized across all studies). Overall, when considering all types of psychological intervention, there was evidence of shorter length of stay in the intervention groups compared with the control groups (mean difference (MD) ‐0.52 days, 95% CI ‐0.82 to ‐0.22) (Analysis 1.2; Figure 6). There were, however, high levels of statistical heterogeneity between studies (I2 statistic = 74%).
1.2. Analysis.
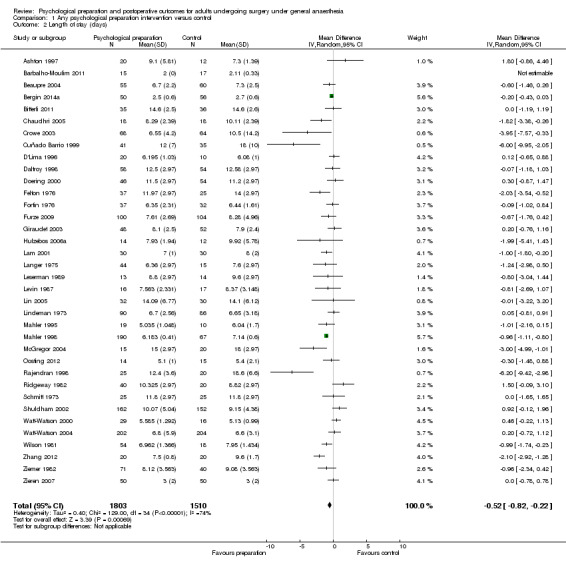
Comparison 1 Any psychological preparation intervention versus control, Outcome 2 Length of stay (days).
6.
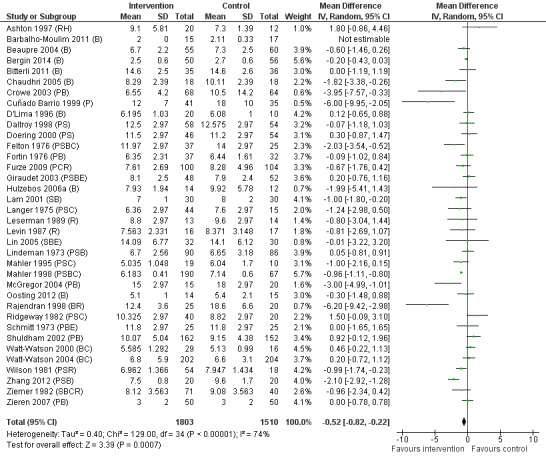
Length of stay (any psychological preparation intervention versus control). B: behavioural instruction; C: cognitive interventions; E: emotion‐focused interventions; H: hypnosis; P: procedural information; R: relaxation; S: sensory information.
The meta‐analysis results for individual intervention types were generally similar. When looking at all studies including a particular intervention type as one of the intervention components there were statistically significant results for the procedural information (Analysis 2.2), sensory information (Analysis 3.2), behavioural instruction (Analysis 4.2) and relaxation (Analysis 6.2) intervention types, although there was not always evidence of an effect when including just the `pure' studies. There was no evidence of an effect on length of stay for cognitive intervention studies (Analysis 5.2). There were few studies evaluating hypnosis (one study, Ashton 1997, MD 1.80, 95% CI ‐0.86 to 4.46, P value = 0.19) or emotion‐focused (Analysis 8.2) interventions. The funnel plot showed no clear evidence of publication bias.
2.2. Analysis.
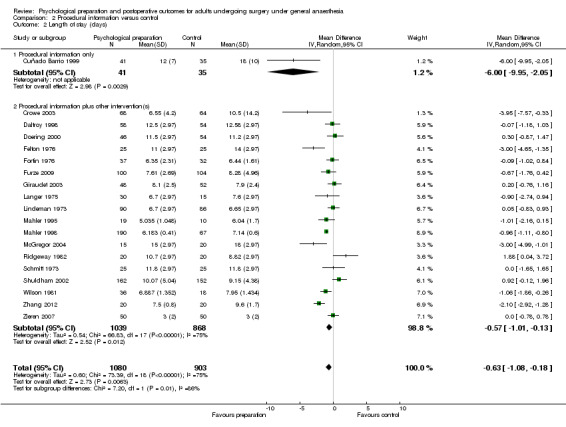
Comparison 2 Procedural information versus control, Outcome 2 Length of stay (days).
3.2. Analysis.
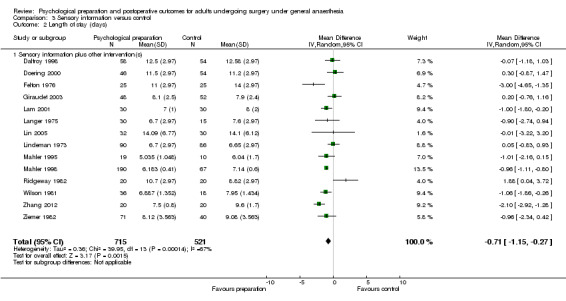
Comparison 3 Sensory information versus control, Outcome 2 Length of stay (days).
4.2. Analysis.
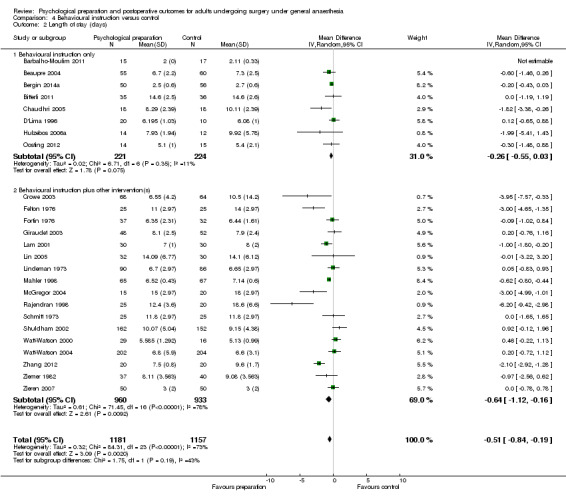
Comparison 4 Behavioural instruction versus control, Outcome 2 Length of stay (days).
6.2. Analysis.
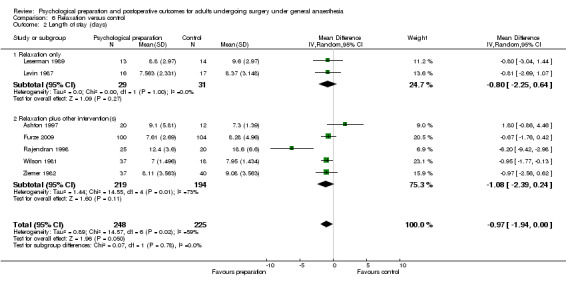
Comparison 6 Relaxation versus control, Outcome 2 Length of stay (days).
5.2. Analysis.
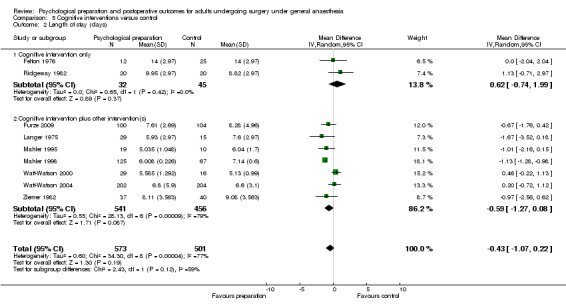
Comparison 5 Cognitive interventions versus control, Outcome 2 Length of stay (days).
8.2. Analysis.
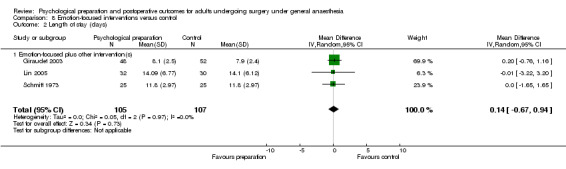
Comparison 8 Emotion‐focused interventions versus control, Outcome 2 Length of stay (days).
Studies not included in meta‐analysis
Twenty‐two studies contained sufficient data for narrative synthesis only: Coslow 1998; DeLong 1970; Field 1974; Gocen 2004; Goodman 2008; Greenleaf 1992; Guo 2012; Hoogeboom 2010; Hulzebos 2006b; Johnson 1978b; Johnson 1985; Klos 1980; Kulkarni 2010; Letterstål 2004; Levesque 1977; Lévesque 1984; Oetker‐Black 2003; Oliphant 2013; Omlor 2000; Pellino 1998; Rosenfeldt 2011; Vukomanović 2008 (see Table 5).
4. Findings of studies that examined the outcome length of stay but could not be included in meta‐analyses.
| Author, year | Surgery type and sample size (randomized) | Intervention categories | Length of stay findings (as available) |
| Coslow 1998 | Laparoscopic tubal ligation N = 30 |
Procedural information, sensory information and behavioural instruction | Intervention n = 15; control n = 15 No significant difference |
| DeLong 1970 | Gall bladder removal and removal of uterus N = 70 |
Procedural information, sensory information, behavioural instruction | Intervention n = 31; control n = 33 Intervention significantly decreased no. days in hospital (F = 4.70, df = 1/62, P value < 0.05). Intervention mean standardized days 47.06; control mean standardized days 52.32. When analysed by coping style: intervention reduced length of stay for copers (F = 6.43, df =1/20, P value < 0.05), but not avoiders or non‐specific defenders. |
| Field 1974 | Mixed orthopaedic surgery N = 60 |
Procedural information, hypnosis | Intervention n = 30; control n = 30 No significant difference |
| Gocen 2004 | Total hip replacement N = 59 |
Behavioural instruction | Intervention n = 29; control n = 30 No significant difference (P value > 0.05) |
| Goodman 2008 | Cardiac bypass surgery N = 188 |
Behavioural instruction, relaxation, emotion‐focused | Intervention median 8.5 (IQR 3.25, range 4 to 50 days, n = 91) Control median 9 (IQR 3, range 2 to 170 days, n = 90) No significant difference (Mann‐Whitney U = 0.29, P value not provided) |
| Greenleaf 1992 | Coronary artery bypass surgery N = 32 |
Intervention 1: Hypnosis and relaxation Intervention 2: Hypnosis |
No significant difference between the groups |
| Guo 2012 | Cardiac surgery N = 153 |
Procedural information | Intervention median 14.0 days (IQR 9.3 to 19.8, n = 68) Control median 12.0 days (IQR 10 to 17, n = 67) No significant difference (P value = 0.17) |
| Hoogeboom 2010 | Primary total hip replacement due to osteoarthritis N = 21 |
Behavioural instruction | Intervention median: 6 days (range 5 to 22, n = 0) Control median: 6 days (range 4 to 7, n = 10) No significant difference (P value = 0.228) |
| Hulzebos 2006b | CABG N = 279 |
Procedural information, behavioural instruction | Intervention median 7 days (range 5 to 41, n = 139) Control median 8 days (range 6 to 70, n = 137) Intervention group: significantly shorter stay. Mann‐Whitney U (z = ‐2.42, P value = 0.02). |
| Johnson 1978b | Sample 1: cholecystectomy, N = 81 Sample 2: inguinal hernia repair, N = 68 |
Intervention 1: ‘Instruction’: Behavioural instruction (deep breathing, coughing, leg exercises) Intervention 2: ‘Procedure information’: focus procedural information, also some sensory information and behavioural instruction Intervention 3: `Sensation information’: focus: sensory information, also some procedural information and behavioural instruction 2 x 3 factorial design: no instruction/instruction (Intervention 1; no information/information (Interventions 2 and3) |
Sample 1 (Cholecystectomy) Length of stay: patients in Intervention 2 (‘Procedure information’) and Intervention procedure and Intervention 3 (‘Sensation information’): shorter postoperative stays than no‐information participants; only significant for sensation information (Dunnett’s t(3,64) = 3.45, P value < 0.001). Control (no instruction or information intervention): mean stay = 6.36, n = 10; Intervention 1 only: mean stay = 6.20, n = 14; Intervention 2 only: mean = 5.97, n = 14; Intervention 3 only: mean = 5.78, n = 12; Intervention 1 and Intervention 2: mean = 5.84, n = 14; Intervention 1 and Intervention 3: mean = 5.29, n = 13 Sample 2 (Hernia repair) No significant effects of interventions for length of stay |
| Johnson 1985 | Abdominal hysterectomy N = 199 |
Intervention 1: procedural and sensory information Intervention 2: ‘cognitive‐coping technique’ – cognitive intervention Intervention 3: ‘behavioural‐coping technique’ – behavioural instruction 2 x 3 factorial design: no information/information (Intervention 1); no coping technique/coping technique (Interventions 2 and 3) |
Outcomes entered into MANOVA included length of stay. Coping technique was significant using a P value < 0.10 criterion (F (16, 286) = 1.59, P value = 0.07). Cognitive‐coping group: longer hospitalization than control group (Dunnett’s t (3,150) = 2.52, P value < 0.025) Adjusted mean scores and sample size according to coping groups: Control mean = 6.56, n = 72; Intervention 2 mean = 6.97, n = 48; Intervention 3 mean = 6.50, n = 47 |
| Klos 1980 | Cholecystectomy N = 50 |
Intervention 1: procedural information, behavioural instruction (pamphlet) Intervention 2: procedural information, behavioural instruction (nurse visit) Intervention 3: procedural information, behavioural instruction (pamphlet and nurse visit) |
Authors did not report analyses by whole intervention group; instead, analyses are reported after median split into high‐preoperative‐fear and low‐preoperative‐fear groups. An interaction effect was reported between preoperative fear and receiving the pamphlet (F(1,39) = 4.14, P value < 0.05). If high preoperative fear and received pamphlet, shorter stay than those with high fear who did not receive pamphlet (but difference in means non‐significant: 5.09 versus 5.79 days). If low preoperative fear and pamphlet: significantly longer postoperative stay than those who did not receive pamphlet (F(1,18) = 4.84, P value < 0.05; means = 5.64 and 4.45). Observations are made about length of stay in the nurse visit groups, but no statistical tests are reported. Low preoperative fear: means for stay length for Interventions 1, 2, 3 and Control respectively are: 5.64, 4.61, 5.05, 4.45 High preoperative fear: means for stay length for Interventions 1, 2, 3 and Control respectively are: 5.18, 6.02, 5.33, 5.91 |
| Kulkarni 2010 | Major abdominal surgery N = 80 |
Intervention 1: behavioural instruction (deep breathing training) Intervention 2: behavioural instruction (incentive spirometry) Intervention 3: behavioural instruction (specific inspiratory muscle training) |
Intervention 1 (Deep breathing): median stay = 5 days (range 1 to 10, n=17); Intervention 2 (Incentive spirometry): median = 4 (range 2 to 22, n = 15); Intervention 3 (Inspiratory muscle training); median = 4 (range 1 to 13, n = 17) Control median stay = 6 (range 1 to 14, n = 17) No analysis is reported |
| Letterstål 2004 | Abdominal aortic aneurysm open repair N = 52 |
Procedural and sensory information | Intervention: median = 11 days (range 4 to 34, n = 18) Control: median = 9 days (range 6 to 42, n = 17) Mann‐Whitney: no difference between groups (P value = 0.14) |
| Levesque 1977 | Cholecystectomy (n = 82); hysterectomy (n = 54) Total N = 136 |
Procedural information, behavioural instruction, relaxation | No significant difference |
| Lévesque 1984 | Cholecystectomy N = 125 |
Intervention 1: procedural information, sensory information, behavioural instruction, emotion‐focused (at pre‐admission, 15 days before surgery) Intervention 2: procedural information, sensory information, behavioural instruction, emotion‐focused (afternoon before surgery |
Cluster‐randomized trial Intervention 1 mean (SD) = 5.85 (1.19), n = 40 Intervention 2 mean (SD) = 5.94 (1.42), n = 42 Control mean (SD) = 5.60 (1.05), n = 43 No analyses are reported for length of stay |
| Oetker‐Black 2003 | Total abdominal hysterectomy N = 108 |
Behavioural instruction, cognitive intervention, relaxation | No significant difference: t(1,93) = ‐0.77, P value = 0.444) |
| Oliphant 2013 | Pelvic reconstructive and/or urinary incontinence surgery N = 199 |
Behavioural instruction | Intervention median = 1 day (IQR 0 to 2, n = 93); control median = 1 day (IQR 0 to 2, n = 93) No significant difference (Mann‐Whitney U, P value = 0.63) |
| Omlor 2000 | Inguinal hernia surgery or thyroidectomy N ≥ 211 |
Procedural information, relaxation | Intervention n = 103; control n = 105 No significant difference. The paper presents medians (ranges) for control and intervention groups, by each type of surgery and combined, but there appears to be an error as these are contradictory: Inguinal hernia, intervention: 7.5 (1 to 11); control: 8 (3 to 22) Thyroidectomy, intervention: 7.2 (2 to 16); control: 7.9 (4 to 13) Groups combined: intervention median 7.95; control median 7.4 |
| Pellino 2005 | Orthopaedic surgery procedures. 90 randomized; 83 consented (consent post‐randomization). | Procedural information, behavioural instruction | No significant difference Data reported: expected length of stay minus actual length of stay (days): Intervention mean = ‐0.46 (SD 1.00, n = 39) Control mean = ‐0.29 (SD 1.19, n = 35) |
| Rosenfeldt 2011 | CABG and/or valve surgery N = 119 |
Behavioural instruction, cognitive intervention, relaxation | Intervention median = 6 days (IQR 5 to 8, n = 60) Control median = 6 days (IQR 5 to 8, n = 57) No significant difference (Wilcoxon, P value = 0.54) |
| Vukomanović 2008 | Total hip arthroplasty N = 45 |
Procedural information, behavioural instruction | Cluster‐randomized trial Intervention mean (SD) = 9.8 (2.4), n = 20 Control mean (SD) = 10.2 (1.7), n = 20 No significant difference, P value ≤ 0.67 |
CABG = coronary artery bypass graft
F = F statistic (analysis of variance)
IQR = inter‐quartile range
MANOVA = multivariate analysis of variance
N = number of participants in sample
SD = standard deviation
Two studies were not included in the meta‐analysis because they were cluster‐randomized trials (Lévesque 1984; Vukomanović 2008). Both of these studies found no statistically significant difference in length of stay between groups.
In nine cases, data were unavailable for meta‐analysis because data were presented in the form of medians and interquartile ranges rather than means and standard deviations (Goodman 2008; Guo 2012; Hoogeboom 2010; Hulzebos 2006b; Kulkarni 2010; Letterstål 2004; Oliphant 2013; Omlor 2000; Rosenfeldt 2011). Seven of these studies reported no significant difference between the groups for length of stay, one found a significantly shorter stay in the intervention group (Hulzebos 2006b), and one study did not report any analysis (Kulkarni 2010).
Complex factorial designs were used by Johnson 1978b and Johnson 1985 with mixed results. Johnson 1978b examined two levels of instruction (no instruction versus behavioural instruction) over three information levels (no instruction versus `sensation information' (focus: sensory information, also containing procedural information and behavioural instruction) versus `procedure information' (focus: procedural information, also containing sensory information and behavioural instruction). In a sample of cholecystectomy patients, patients receiving `sensation information' had a shorter stay than `no information' participants, but no significant effects were seen in their sample of patients receiving inguinal hernia repair. Using a similar design, Johnson 1985 compared two levels of information (no information versus procedural and sensory information) and three `coping levels' (no `coping' intervention versus cognitive intervention versus behavioural instruction) in patients undergoing hysterectomy. The cognitive intervention group had a significantly longer stay than the control group.
Of the remaining studies, no statistically significant difference between groups was reported by Coslow 1998, Field 1974, Gocen 2004, Greenleaf 1992, Levesque 1977 and Oetker‐Black 2003. Pellino 1998 also reported no significant difference but conducted an unusual analysis, comparing expected minus actual length of stay across the two study groups. Two studies looked at interaction effects.
DeLong 1970 found that, for their overall sample, the intervention significantly decreased the number of days in hospital (intervention mean standardized days 47.06; control mean standardized days 52.32). Analysing findings by coping style, it was seen that the intervention reduced length of stay for `copers', but not for `avoiders' or `non‐specific defenders'.
Klos 1980 analysed data by level of preoperative fear (low or high) for four groups (control and three intervention groups: pamphlet, nurse, pamphlet and nurse, all containing procedural information and behavioural instruction), and did not report analyses conducted by whole intervention groups. An interaction was found such that those low in fear who received the pamphlet had a significantly longer stay than control participants low in fear who did not receive the pamphlet containing procedural information and behavioural instruction.
A range of intervention types were used within the 16 studies that did not find an effect of intervention on length of stay, including many using procedural information or behavioural instruction ‐ intervention types suggested to be beneficial by the meta‐analysis. The two studies that did report statistically significant benefits for length of stay were consistent with meta‐analysis findings: the components included were procedural information, sensory information and behavioural instruction (DeLong 1970; Hulzebos 2006b).
Summary: length of stay
In summary, the meta‐analyses suggested that psychological preparation led to a reduction in mean length of stay of around half a day. This effect might be considered important to patients and clinicians and to represent savings in healthcare resources. The meta‐analysis, however, had high statistical heterogeneity, which needs to be considered when interpreting this pooled effect. Although no clear explanations for the heterogeneity could be found, there was clearly considerable variation between the studies in the types of interventions administered. The pattern of results did, however, suggest a similar benefit of psychological preparation for all intervention types. The results of the studies included in the narrative review were generally not statistically significant, but would not contradict a pattern of a modest reduction of length of stay in the intervention group. The high heterogeneity and the high number of studies reporting sufficient methodological details to ascertain risk of bias meant that we downgraded the overall quality of evidence for the outcome length of stay by two points to `low' (see Table 1).
Findings by intervention
1. Procedural information
Of the meta‐analysed studies, procedural information was a component in interventions of 12 studies with the outcome pain (1051 participants); 19 studies with the outcome length of stay (1983 participants) and 17 studies with the outcome negative affect (1334 participants). There was no evidence that interventions containing procedural information improved postoperative pain outcomes (pooled effect size (SMD) ‐0.08, 95% CI ‐0.26 to 0.09, Analysis 2.1), but procedural information was statistically significantly beneficial for length of stay (MD ‐0.63 days, 95% CI ‐1.08 to ‐0.18 days, Analysis 2.2). Procedural information was also beneficial for negative affect (SMD ‐0.45 days, 95% CI ‐0.75 to ‐0.16, Analysis 2.3), although analyses examining interventions containing procedural information alone (`pure' studies) did not reach statistical significance. Procedural information was included in four studies that found a benefit of the intervention on behavioural recovery (Fortin 1976; Heidarnia 2005; McGregor 2004; Zieren 2007 (statistics not presented)) but was also in three studies with non‐significant findings for this outcome (Lévesque 1984; Mahler 1998; Ridgeway 1982; Table 3).
2. Sensory information
Of the meta‐analysed studies, sensory information was a component in interventions of 11 studies with the outcome pain (881 participants); 14 studies with the outcome length of stay (1236 participants) and 12 studies with the outcome negative affect (919 participants). No interventions contained purely sensory information – it was always presented with other intervention components. For the outcome postoperative pain, there was no clear evidence that intervention patients benefited when receiving interventions containing sensory information (SMD ‐0.22, 95% CI ‐0.47 to 0.02, Analysis 3.1). Statistically significant beneficial effects of sensory information were seen for length of stay (MD ‐0.71, 95% CI ‐1.15 to ‐0.27, Analysis 3.2) and negative affect outcomes (SMD ‐0.55, 95% CI ‐0.90 to ‐0.19, Analysis 3.3). Sensory information was not included in any studies finding statistically significant effects for behavioural recovery, but was included in two non‐significant studies (Table 3).
3. Behavioural instruction
Of the meta‐analysed studies, behavioural instruction was a component in interventions of 21 studies with the outcome pain (1241 participants); 25 studies with the outcome length of stay (2338 participants) and 13 studies with the outcome negative affect (1183 participants). There was no evidence that behavioural instruction had an effect on postoperative pain or negative affect (SMD ‐0.14, 95% CI ‐0.33 to 0.05, Analysis 4.1; SMD ‐0.22, 95% CI ‐0.46 to 0.02, Analysis 4.3 respectively). A significantly beneficial effect of behavioural instruction was seen for length of stay (MD ‐0.51 days, 95% CI ‐0.84 to ‐0.19, Analysis 4.2), although findings were not statistically significant when only `pure' studies were included. Behavioural instruction appears to be of greatest potential for behavioural recovery outcomes – it featured as a component in all five studies reporting statistically significant benefits of the intervention (in pure form in one), but it also featured in many studies that did not find significant effects, and was the only component in the one study that reported more negative outcomes for intervention groups (D'Lima 1996; Table 3).
4. Cognitive interventions
Of the meta‐analysed studies, cognitive interventions were a component in interventions of six studies with the outcome pain (355 participants); nine studies with the outcome length of stay (1074 participants) and five studies with the outcome negative affect (251 participants). Thus, a relatively small number of studies contributed to the meta‐analyses. Cognitive interventions were not significantly beneficial overall for the outcome postoperative pain (SMD ‐0.02, 95% CI ‐0.29 to 0.25, Analysis 5.1), although combining the two `pure' studies did indicate a benefit for participants receiving the cognitive intervention (Cheung 2003; Ridgeway 1982). There was no evidence for an effect on length of stay (MD ‐0.43, 95% CI ‐1.07 to 0.22, Analysis 5.2) or negative affect (SMD ‐0.20, 95% CI ‐0.52 to 0.12, Analysis 5.3). Cognitive interventions were a component in one statistically significant and two non‐significant interventions for behavioural recovery (Table 3).
5. Relaxation techniques
Of the meta‐analysed studies, relaxation techniques were a component in interventions of 13 studies with the outcome pain (891 participants); seven studies with the outcome length of stay (473 participants) and 11 studies with the outcome negative affect (687 participants). Relaxation techniques had statistically significant beneficial effects on postoperative pain (SMD ‐0.46, 95% CI ‐0.81 to ‐0.11, Analysis 6.1) and negative affect (SMD ‐0.34, 95% CI ‐0.56 to ‐0.12, Analysis 6.3), although the effect on negative affect was not statistically significant when only `pure' studies were meta‐analysed. For length of stay, the mean difference was high (‐0.97 days – almost a day's shorter stay for intervention participants) but the 95% CI was ‐1.94 to ‐0.00 (P value = 0.05, Analysis 6.3), indicating that caution is needed in interpreting this finding. Relaxation was included in one significantly effective behavioural recovery intervention and in no non‐significant studies (Table 3).
6. Hypnosis
Of the meta‐analysed studies, hypnosis was a component in interventions of no studies with the outcome pain; one study with the outcome length of stay (32 participants) and two studies with the outcome negative affect (72 participants). Thus, hypnosis was rarely seen in the studies eligible for inclusion in the meta‐analyses. No studies used hypnosis for the outcome postoperative pain; for length of stay one study used hypnosis (combined with relaxation, non‐significant; Ashton 1997), and two studies addressing negative affect used hypnosis combined with relaxation, with statistically significant benefits (Ashton 1997; Hart 1980; SMD ‐0.77, 95% CI ‐1.25 to ‐0.30, Analysis 7.1). No studies addressing behavioural recovery incorporated hypnosis (Table 3).
7. Emotion‐focused interventions
Of the meta‐analysed studies, emotion‐focused interventions were a component in interventions of three studies with the outcome pain (180 participants); three studies with the outcome length of stay (212 participants) and four studies with the outcome negative affect (201 participants). For postoperative pain, the three studies meta‐analysed suggested potential for a beneficial impact on pain (SMD ‐0.42, 95% CI ‐0.85 to 0.00, P value = 0.05, Analysis 8.1; Giraudet 2003; Lin 2005; Postlethwaite 1986), although the study including emotion‐focused intervention in `pure' form showed no evidence of benefit (Postlethwaite 1986). Emotion‐focused interventions provided no benefit for length of stay (MD 0.14 days, 95% CI ‐0.67 to 0.94, Analysis 8.2) or negative affect (SMD ‐0.24, 95% CI ‐0.55 to 0.07, Analysis 8.3). For behavioural recovery, emotion‐focused interventions were a component of no significantly beneficial studies, but featured in two non‐significant studies (Table 3). The numbers of studies using emotion‐focused techniques were small for all outcomes.
Discussion
Summary of main results
Summary by outcome
1. Postoperative pain
The meta‐analysis suggested that psychological preparation may reduce postoperative pain (SMD ‐0.20, 95% CI ‐0.35 to ‐0.06), although these findings should be treated with caution because of the high heterogeneity (Figure 4). For most intervention types, results were similar to the omnibus analysis over all intervention types, with the exception of the analyses for behavioural instruction – there was no evidence that behavioural instruction reduced pain. Most studies included in the narrative synthesis found no statistically significant difference between intervention and control groups. While none of the narratively synthesized studies contained `pure' behavioural instruction, 12 of the 16 studies reporting non‐significant differences contained behavioural instruction as a component (Chumbley 2004; Dewar 2003; Hawkins 1993; Johnson 1978b; Johnson 1985; Lilja 1998; Oetker‐Black 2003; Parthum 2006; Shuldham 2002; Vukomanović 2008; Watt‐Watson 2004; Wijgman 1994).
2. Behavioural recovery
We did not conduct meta‐analyses for this outcome as there were few studies, there was large variation in reported outcomes and usable data were often not reported. The evidence was promising, suggesting that psychological preparation, in particular behavioural instruction, has potential to improve behavioural recovery outcomes, but no clear conclusions could be reached. We identified a need for more consistent use of outcome measures and clearer reporting so that findings can be compared across studies.
3. Negative affect
In meta‐analysis, there was some evidence of a beneficial effect of psychological preparation techniques on postoperative negative affect (SMD ‐0.35, 95% CI ‐0.54 to ‐0.16), although high statistical heterogeneity reduces the confidence that can be placed in this finding (Figure 5). While the pooled effect size of ‐0.35 would be regarded as a `small' effect (Cohen 1988), it could still be clinically important. There did not appear to be evidence that certain techniques performed better than others in reducing negative affect. In the narrative synthesis, many studies either reported null effects, or did not report analyses for the negative affect outcome of relevance to the review, even though authors reported measuring it. There was some suggestion that individual characteristics (e.g. level of preoperative fear) may affect the way that psychological preparations impact on postoperative outcomes. Overall, psychological preparation may benefit postoperative negative affect but the high level of heterogeneity in the data makes it difficult to determine the circumstances and intervention content that would consistently improve outcomes.
4. Length of stay
The meta‐analyses suggested that psychological preparation led to a reduction in mean length of stay of around half a day (MD ‐0.52 days, 95% CI ‐0.82 to ‐0.22), an effect size that could have considerable impact for patients and clinicians and represent savings in healthcare resources (Figure 6). However, the effect must be interpreted with caution because of high statistical heterogeneity. There appeared to be a similar benefit of psychological preparation for all intervention types. Studies included in the narrative review generally reported findings that were not statistically significant.
Summary by intervention
The number of studies using each intervention for each outcome varied. In general, pooled effect sizes tended to be similar regardless of the intervention types used and there was no clear evidence that results differed according to intervention. However, a different pattern did seem to emerge for behavioural instruction, for which there was no evidence of an effect for the outcomes postoperative pain or negative affect, but which was a component in all studies that successfully improved the outcome behavioural recovery. This difference may relate to the mechanism by which interventions are expected to take effect. Most of the intervention techniques included in the review are anticipated to improve recovery by reducing negative emotions (such as anxiety, worry about surgery, perceptions of stress) or enhancing relaxation, or both. Behavioural instruction is different: its goal is to help people to change their behaviour in such a way that their recovery is facilitated. Thus, it may be that reducing negative emotion before surgery is key to patients experiencing lower pain and lower negative affect after surgery, but when it comes to supporting patients' return to usual activities then behavioural instruction is more important. However, this is a cautious explanation as the behaviours targeted by behavioural instruction vary widely ‐ for example Chumbley 2004 addressed use of patient‐controlled analgesia ‐ such behavioural instruction might be expected to reduce pain, even though Chumbley 2004's findings were not statistically significant.
Overall completeness and applicability of evidence
This review addressed elective surgery where at least some patients underwent general anaesthesia. Findings cannot, therefore, be generalized to non‐elective procedures, or those where local anaesthesia is routinely used. It also cannot be assumed that similar findings would result in research with children rather than adults. However, as we did not limit the surgical procedures further, these findings would potentially be generalizable across elective surgical procedures, although the high degree of heterogeneity causes some concern. In future work, we plan to carry out secondary analyses to examine the impact of psychological preparation by surgery type.
The effect in our meta‐analyses for postoperative pain and negative affect may appear to be small according to Cohen 1988 (SMD = ‐0.20 and ‐0.35 respectively). However, given the high prevalence of surgery, such effect sizes may still be of clinical or cost significance, or both. There were 4.7 million surgical admissions in 2013‐14 in England alone, with common procedures being operations of the type included in the present review (120,198 hernia repairs, 197,348 hip or knee replacements, 76,497 gall bladder removals) (Royal College of Surgeons 2016).
The focus of the present review was the content of intervention – the types of psychological techniques used. We did not examine how the interventions were delivered – whether the timing or the format of the intervention is important. These are issues addressed by Nicholson 2013 (published Cochrane protocol). We would expect Nicholson 2013's findings to complement those of our review; together the reviews will evidence the current state of knowledge for the preparation of surgical patients.
Some types of intervention included as psychological preparation techniques in the review, for example procedural information and behavioural instruction, might be considered to be `common sense' rather than `psychological'. However, the term `psychological' encompasses what we think, what we feel and what we do, so any intervention that is designed to change what we think (for example, changing our expectations about what will happen) or what we do (for example, deep breathing after surgery) are effectively psychological techniques. An approach may appear to be `common sense' but still have a strong theoretical and evidence base to support it. For example, it may seem obvious that providing procedural information will help people to know what to expect and to feel prepared and less anxious about an event. However, it may seem equally obvious that giving someone procedural information in advance of surgery could increase anxiety by increasing thinking and worrying about a procedure. Thus, even such apparently unsophisticated procedures need to be considered and rigorously evaluated in the same way as more complex intervention techniques.
Studies included in the review measured outcomes at various time points, and some studies measured outcomes at multiple time points. In analysing the data, we used the earliest outcome measure reported by each study (see Characteristics of included studies for details of time points). It is possible that in the earliest times after surgery, within 48 hours, an outcome such as pain may be more influenced by biological factors such as analgesia intake and acute postoperative complications than by psychological aspects. Thus, by focusing on the earliest outcomes we may be under‐estimating the impact of psychological preparation. However, it is likely that psychological and biological factors interact ‐ for example, an intervention may include instruction in the use of using patient‐controlled analgesia (e.g. Chumbley 2004).
Quality of the evidence
We graded the quality of evidence as `low' for the outcomes postoperative pain, negative affect and length of stay and `very low' for the outcome behavioural recovery (see Table 1). The two main problems with studies in the review were risk of bias ratings and heterogeneity. As seen in Figure 1, with the exception of performance bias (blinding of participants and personnel), a low proportion of studies received `high' risk of bias ratings. However, a large number of studies in each category received `unclear' ratings resulting from the poor reporting of studies. It is therefore not clear whether such studies were actually poorly conducted ‐ and therefore at high risk of bias ‐ or whether they were well designed and implemented but poorly reported. Improving reporting should be a primary aim for researchers ‐ and journal editors ‐ in this field. For the outcome behavioural recovery there was the further problem of a small number of studies including outcomes that were a) assessed with measures with demonstrated validity and reliability and b) reported in a form that could be included in meta‐analysis, meaning that only narrative synthesis could be conducted. High heterogeneity was also a problem, particularly in the varying content of interventions. Rather than simply label all interventions as being `psychological' we classified them into seven groups, which has enabled us to demonstrate the high level of variation across interventions. There was also heterogeneity in the wide range of surgery types participants underwent. Nevertheless, we did not find evidence of publication bias and all our outcomes were directly measured. For each outcome, there were some studies with small sample sizes and wide confidence intervals but overall imprecision does not appear to have been a problem.
In this review, we did not consider intervention fidelity ‐ whether interventions were delivered in accordance with the study protocol. This is particularly important where a complex psychological preparation technique is delivered by an individual rather than in a standard format such as a DVD or leaflet. It is important that the individual delivering the intervention is fully trained in the content and technique of delivery, and that this is evaluated during the study to ensure that interventions are indeed delivered as intended. If a fidelity check is not conducted then it is possible that important elements are missed, or that individuals add elements and it is unclear exactly what is being evaluated. We did record intervention fidelity processes on our data extraction forms, and observed that many studies do not seem to have addressed the issue. However, in accordance with our protocol, we did not formally include this when assessing risk of bias.
A limitation of our omnibus meta‐analyses is that they assume that diverse interventions have similar effects, whether separately or in combination with other types of interventions. In addition, most of the evidence for the separate meta‐analyses of individual intervention types came from studies judged to comprise at least one other intervention category. The present analyses can therefore only give a broad indication of the effectiveness of individual intervention types and we are unable to comment on how intervention types may interact with each other.
Potential biases in the review process
We conducted this large review in a careful and thorough manner: we not only carried out detailed searches of databases but also systematically sought to contact every included study's author to ask about additional research, and we searched studies' reference lists to ensure as complete coverage as possible. We also set high standards for the review, by only including randomized trials (excluding any work where we knew a random allocation method was not used), and by only including negative affect and behavioural recovery outcomes where measures with published psychometric properties were used. However, while we included papers in non‐English languages our search was limited in that we only conducted the searches in English, and we did not check the reference lists of non‐English papers. In addition, it is possible that unpublished studies exist of which we did not learn, but the funnel plots did not show evidence of asymmetry.
A relatively low proportion of the identified trials could be included in the meta‐analyses, however. This was sometimes because medians were presented, but there were also many studies that did not report any usable data for our outcomes of interest, despite having collected this. Extracting data from the publications was often challenging and we often had to calculate standard deviations from other statistics or use pooling formulae when authors chose to present data for subgroups only.
For the outcome length of stay, it should be considered that a number of studies appeared to regard length of stay not as an outcome, but as a descriptive statistic that was measured, reported and compared across groups. For example, Oliphant 2013 reported length of stay and compared this across groups, as one of many patient characteristics rather than as a specified outcome measure. The standard of reporting in papers was generally low, and it was often not possible to determine which outcomes were intended, a priori, to be treated as study outcomes, therefore we included any measure reports that fitted the definitions of our review. This was a conservative approach, as time to discharge may be short and largely determined by system factors. This is likely to have resulted in our findings erring on the side of over‐reporting non‐significant length of stay data (particularly in the narrative synthesis, where we could not pool studies to increase power).
We have reported meta‐analysis findings despite high levels of heterogeneity, which limits the confidence that can be placed in the findings. We believe that this is, however, helpful, as this is a large review and summarizing data in this way allows the findings to be more easily interpreted than placing so many studies in a table. In addition, as many studies contained small samples and individual results were often not statistically significant, combining studies allows a helpful picture of the potential of interventions. This practice has been followed in other Cochrane reviews, for example Gurusamy 2014 conducted meta‐analyses despite finding I2 statistics of 75% and 87%. Although we did not identify specific reasons for this heterogeneity, it is clear that the studies were very diverse in terms of interventions, surgery types and outcomes used.
Combining interventions allows us to compare our findings with the earlier review (Johnston 1993), and we have, in the main, followed the analysis process as outlined in our protocol (Powell 2010). However, secondary analyses would be helpful in unpicking the cause of the heterogeneity and in identifying where benefits to patients may be obtained. A primary source of heterogeneity is in the varied way in which studies combined the intervention components included in the review. While we have conducted subgroup analyses examining `pure' and mixed' interventions for each outcome, we have not unpicked the value of each intervention component further. In future work we plan to carry out secondary analyses to explore how each individual component contributes to variance for each outcome.
A more challenging aspect of intervention content is that, within each intervention component, studies varied widely. For example, procedural information might focus on what will happen before surgery, the surgical process or what will happen after surgery. This issue was particularly pertinent with behavioural instruction – this could target a range of issues: exercises to be carried out before surgery to enhance strength, fitness or lung capacity (e.g. D'Lima 1996), exercises or movements to be carried out after surgery (e.g. deep breathing, how to turn in bed, e.g. Levesque 1977), behaviours to be carried out to gain effective pain relief (e.g. using patient‐controlled analgesia or asking for pain medication, e.g. Chumbley 2004). In future research we plan to carry out a secondary analysis to compare behavioural instruction targeting behaviours to be carried out before surgery with behavioural instruction targeting behaviours to be carried out after surgery. A further challenge in analysing the detail of psychological preparation interventions is the inconsistency between studies in how the interventions were reported. It was rare to find a sufficiently detailed description for the intervention to be replicated. A barrier to this would appear to be the lack of a standard language to describe intervention content in this context. A Behaviour Change Technique Taxonomy has been developed to enable researchers to describe, and code, interventions designed to effect behaviour change (Michie 2013), and the subset of studies in this review that target behaviour change could be recoded according to this taxonomy. However, many of the interventions do not explicitly aim to change behaviour – focusing instead on outcomes of perception (pain), emotion (negative affect) or a complex outcome that results from an interaction between patient clinical status, patient behavioural recovery and hospital strategy (length of stay). A taxonomy is needed that addresses a wider range of psychological interventions than those focused only on changing participant behaviour.
In future work we also plan to incorporate date of publication into secondary analysis (network meta‐synthesis). We did not exclude studies on the basis of date, ensuring the completeness of this review. However, the inclusion of studies over a wide period of time means that early studies may not reflect what would be found should the same interventions be used in modern practice. In particular, length of stay is now typically much shorter than that at the time of early studies such as DeLong 1970. The reporting of control condition content was generally very poor, but it is likely that, with approaches to patient care changing with psychological input into training of health professionals increasing and patient satisfaction gaining prominence, `standard care' in modern studies would contain a higher level of psychological preparation than in earlier studies. Thus, by assuming the absence of psychological techniques in `usual care' interventions unless stated, it is likely that we are over‐estimating the difference in treatment between patient groups within studies, and underestimating effect sizes. Evidence from other types of psychological intervention have indeed found that the prevalence of psychological techniques in the management of patients in the control groups reduced the effect sizes for trialled interventions (de Bruin 2009). In addition, management and clinical practices have changed, for example with hospitals seeking to discharge patients sooner. It might be expected, for example, that more recent studies would be less likely to show differences between groups in length of stay if length of stay has reduced over time.
As per our protocol, we did not extract studies' funding sources from papers. This is a limitation as we cannot comment on the potential impact of funding source on review outcomes. Similarly, we did not extract information about any conflicts of interest reported.
Potential biases resulting from differences between the protocol and the review
In the review, we more tightly defined the intervention types and what we meant by `psychological preparation for surgery'. We believe that this resulted in a stronger review, with interventions more clearly specified, but it may have led to the exclusion of some studies, which is likely to have affected the results.
In the review, we restricted inclusion to studies that reported one of the four outcomes postoperative pain, behavioural recovery, negative affect or length of stay. This was a pragmatic decision given the large size of the review, but excluding other outcomes means that we may have missed important impacts of psychological preparation. Importantly, if harm were identified on an outcome other than those we included, this review would not have detected it. We also refined our search criteria such that we only searched the reference lists of papers published in English. It is therefore possible that we missed studies in other languages, which may have resulted in bias.
We limited the subgroup analyses (as described in Differences between protocol and review). This has led to a more restricted range of findings but did not affect the planned analyses that we conducted.
In the review, we further specified the way we selected outcome measures where multiple measures were reported. While we conducted this process carefully and as objectively as possible, it would have been better to have pre‐specified the process to eliminate any potential for bias in the measures used.
We did not anticipate all the forms in which data might be presented in the protocol. We made the following decisions after seeing the data set: some studies only reported mean (SD) change from baseline (rather than absolute mean (SD)); for these studies we used the difference in mean change scores as the effect size. If no continuous pain data were available but dichotomous data were presented, we used the log odds ratio as the effect size. As we made these decisions after the data were available there is potential for bias.
Agreements and disagreements with other studies or reviews
Our review was based on that of Johnston 1993 but used different, more recently developed methods. We planned to carry out an up‐to‐date review, using modern techniques and standards. We used the same types of surgery and intervention categories, and examined four of the outcomes addressed by Johnston 1993. There are also some differences: Johnston 1993 required that patients have a postoperative night's stay – we did not make this a criterion as length of stay has reduced in recent years, and we adopted a different analysis strategy. We also had the additional criterion that behavioural recovery and negative affect measures needed to have published psychometric information for inclusion in the review. Johnston 1993 used the binomial test to pool data where studies did not provide the details for the calculation of pooled effect sizes, while we did not attempt to mathematically pool findings with insufficient details, according to standard Cochrane practice (Higgins 2011). Similarly to Johnston 1993, in omnibus analysis assessing whether psychological preparation has an impact on outcome (including all types of preparation), we found significant impact of preparation versus control for the outcomes of postoperative pain, length of stay and negative affect. For the outcome pain, Johnston 1993 reported relaxation, procedural information, cognitive interventions and behavioural instruction to be successful intervention components (assessed using the binomial test), whereas we found interventions other than behavioural instruction to generally appear to be beneficial. Effective preparations for negative affect in Johnston 1993 were procedural information, behavioural instruction, cognitive interventions and relaxation; our findings similarly suggested procedural information and relaxation could be beneficial, but also suggested that sensory information and hypnosis might be important, while we found no clear evidence for behavioural instruction or cognitive interventions. Johnston 1993 found all intervention methods other than cognitive interventions and hypnotic methods to be beneficial for the outcome length of stay; similarly, our meta‐analysis found most intervention types to be beneficial other than cognitive interventions, and we identified few studies for hypnosis or emotion‐focused interventions. Finally, Johnston 1993 found procedural information, sensory information and behavioural instruction to benefit behavioural recovery. We did not meta‐analyse data for this outcome, and we were more selective in which studies we included than were Johnston 1993 (we did not include measures without published psychometrics), but behavioural instruction was commonly used in successful interventions, and procedural information and sensory information also featured in some of these.
Other more recent reviews have addressed aspects of preparation that have also been covered in our review. Kekecs 2014 examined whether `suggestive interventions' (e.g. hypnosis and therapeutic suggestions) improved postoperative distress and pain intensity. They found evidence for suggestive interventions to reduce postoperative anxiety and pain intensity. Similarly, Tefikow 2013 found hypnosis to have positive effects on distress and pain in adults undergoing surgery or medical procedures, and found positive effects of the intervention for these outcomes. We also found studies including hypnosis (alongside relaxation) to be effective in reducing postoperative negative affect in our review. However, we did not include any studies with the component `hypnosis' in meta‐analysis for the outcome pain, and in meta‐analysis two studies with a hypnosis component reported non‐significant findings. This differences may be explained by Kekecs 2014 and Tefikow 2013 having different inclusion criteria to our review: surgical procedures under local anaesthesia, as well as general, were included in both reviews, and the search was limited to studies published after 1980 by Kekecs 2014.
Other reviews have focused on specific types of surgery. Louw 2013 and McDonald 2014 examined preparation for patients undergoing knee or hip replacement surgery. Both of these studies examined the effects of `education' interventions, which would appear to include both procedural information and behavioural instruction, on postoperative pain (Louw 2013 and McDonald 2014), and function, anxiety and length of stay (McDonald 2014 only). McDonald 2014 concluded that preoperative education may not be of benefit; Louw 2013 found benefits to pain to be limited (no meta‐analysis was conducted). McDonald 2014 focused on the latest time point studies included, while we used the first outcome time point assessed, Louw 2013 only included studies published between 1990 and 2011, and both included quasi‐randomized studies, unlike our review. However, our review does also include many studies with null findings – further subgroup analysis would help to establish for which interventions, and which types of surgery, preparation has most/least potential for benefit.
Gurusamy 2014 examined `information' interventions, whose content would appear to fit our categories of procedural information and potentially also behavioural instruction, with studies of patients undergoing day‐patient laparoscopic cholecystectomy. They evaluated the outcomes of pain, length of stay and anxiety, but found few studies addressing these outcomes (one for pain, none for length of stay, one for anxiety) and the authors concluded that the evidence had very low quality.
Hulzebos 2012 included both randomized and quasi‐randomized trials in adults undergoing elective cardiac surgery, with interventions described as "preoperative physical therapy with an exercise component" (p1). Such interventions would typically fit our category `behavioural instruction' where participants are instructed to carry out particular behaviours, such as exercise or incentive spirometry. They found that intervention participants had a significantly shorter length of stay but only one study included the outcome physical function (equivalent to `behavioural recovery'), finding a worse outcome for the intervention group.
A common finding across reviews is that studies are frequently small and of poor quality, consistent with our findings. Our review takes a broader approach than most reviews, including many more studies but of a higher quality, as we excluded quasi‐randomized trials. While heterogeneity of interventions is a problem in our review, we have been more specific in categorising and measuring components within interventions than some other reviews (i.e. those assessing the impact of `education' or `information'), allowing future secondary analyses to assess more precisely which components of interventions are effective.
Authors' conclusions
Implications for practice.
The evidence suggested that psychological preparation may be beneficial for the outcomes postoperative pain, behavioural recovery, negative affect and length of stay, and is unlikely to be harmful. However, as the quality of evidence was low or very low, the quality of evidence is insufficient to be used to make recommendations for practice. It is also not possible to be certain, at present, about which specific intervention types might be used to improve which post‐surgical outcomes.
Implications for research.
Further analyses are needed to explore the heterogeneity in the data, to identify more specifically when particular types of intervention are of benefit. The findings have shown that there is a paucity of well‐designed studies with a low risk of bias. There is a need for well‐conducted and clearly reported research and research that describes both intervention and control components in sufficient detail for replication. Researchers should follow the CONSORT Statement when designing studies and reporting findings (Schulz 2010), and use valid, reliable methods to assess outcomes. The review team plans to conduct further analyses using network meta‐synthesis to help determine which intervention types and other study characteristics are associated with more favourable postoperative outcomes. Future reviews should also consider conducting subgroup analyses of patients with chronic conditions, previous history of general anaesthesia, use of pharmacological premedication, length and type of surgery.
Acknowledgements
We wish to dedicate this work to the memory of Christian Osmer, a dedicated, caring doctor who was committed to achieving the best care for his patients and their relatives. He saw his contribution to this project as a way of advancing best care for surgical patients. We are very grateful for his valuable input to this work and the pleasure we had in working with him.
We are grateful to Karen Hovhanisyan (former Trials Search Co‐ordinator, Cochrane Anaesthesia, Critical and Emergency Care Group (ACE)) for carrying out the electronic database searches and to Jane Cracknell (Managing Editor, ACE) for her support throughout the review process. We would also like to thank W Alastair Chambers and Manjeet Shehmar for clinical advice relating to judgements about general anaesthesia usage, and Yvonne Cooper and Louise Pike who retrieved documents and screened papers as research assistants in earlier stages of the review. We are grateful to the following colleagues who helped us with foreign language papers ‐ either by screening papers or by providing translation: Stefano Carrubba, Chuan Gao, Chen Ji, Kate Rhie, Reza Roudsari and Alena Vasianovich.
We would like to thank Andy Smith (content editor), Nathan Pace (statistical editor), Michael Donnelly, Allan Cyna and Michael Wang (peer reviewers), and Shunjie Chua (consumer referee) for their help and editorial advice during the preparation of this systematic review. We would also like to thank Andrew Smith (content editor), Nathan Pace (statistical editor), Michael Wang and Allan Cyna (peer reviewers), and Lynda Lane (Cochrane Consumer Network representative) for their help and editorial advice during the preparation of the protocol (Powell 2010).
Appendices
Appendix 1. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor Patient Education as Topic explode all trees #2 MeSH descriptor Behavior Therapy explode all trees #3 MeSH descriptor Cognitive Therapy explode all trees #4 MeSH descriptor Relaxation Therapy explode all trees #5 MeSH descriptor Hypnosis, Anesthetic explode all trees #6 MeSH descriptor Imagery (Psychotherapy) explode all trees #7 (prevent* near (anxiety or stress or depression or catastrophizing or negative orientation or noxious stimuli or negative emotion*)) #8 physiotherapy exercise*:ti,ab or taking analgesic* or (Psychological near preparation*) or ((sensory or procedural) near information) or behavio?ral instruction* or ((emotion?focused or cognitive) near intervention*) or (relaxation or hypnosis):ti,ab or (cognitive near (reframing or distraction)) or guided imagery #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 MeSH descriptor Postoperative Care explode all trees #11 MeSH descriptor Pain, Postoperative explode all trees #12 MeSH descriptor Postoperative Complications explode all trees #13 MeSH descriptor General Surgery explode all trees #14 MeSH descriptor Cholecystectomy explode all trees #15 MeSH descriptor Hysterectomy explode all trees #16 MeSH descriptor Arthroplasty, Replacement explode all trees #17 MeSH descriptor Arthroplasty explode all trees #18 MeSH descriptor Anesthetics, General explode all trees #19 MeSH descriptor Anesthesia, General explode all trees #20 ((post?operative near (outcome* or pain)) or post?surgical pain) or (surgery or operat*):ti,ab or surgical procedure* #21 (cholecystectom* or hysterectom* or (hernia near repair*) or herniorrhaph* or hernioplasty or (joint replacement near surgery) or arthroplasty) or (general near an?esth*):ti,ab #22 (#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) #23 (#9 AND #22) #24 MeSH descriptor Economics explode all trees #25 MeSH descriptor Costs and Cost Analysis explode all trees #26 MeSH descriptor Cost‐Benefit Analysis explode all trees #27 MeSH descriptor Cost Savings explode all trees #28 MeSH descriptor Quality‐Adjusted Life Years explode all trees #29 (economic near evaluation):ti,ab or cost effectiveness analysis or cost utility analysis #30 (#24 OR #25 OR #26 OR #27 OR #28 OR #29) #31 (#23 AND #30)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. (prevent* adj3 (anxiety or stress or depression or catastrophizing or negative orientation or noxious stimuli or negative emotion*)).mp. 2. physiotherapy exercise*.ti,ab. or taking analgesic*.mp. or (Psychological adj3 preparation*).mp. or ((sensory or procedural) adj3 information).mp. or behavio?ral instruction*.mp. or ((emotion?focused or cognitive) adj3 intervention*).mp. or (relaxation or hypnosis).ti,ab. or (cognitive adj3 (reframing or distraction)).mp. or guided imagery.mp. 3. Patient Education as Topic/ or Behavior Therapy/ or Cognitive Therapy/ or Relaxation Therapy/ or Hypnosis, Anesthetic/ or Hypnosis/ or "Imagery (Psychotherapy)"/ 4. 1 or 2 or 3 5. ((post?operative adj3 (outcome* or pain)) or post?surgical pain).mp. or (surgery or operat*).ti,ab. or surgical procedure*.mp. 6. Postoperative Care/ or exp Pain, Postoperative/ or Postoperative Complications/ or General Surgery/ or Cholecystectomy/ or Hysterectomy/ or Arthroplasty, Replacement/ or Arthroplasty/ or Anesthetics, General/ or Anesthesia, General/ 7. (cholecystectom* or hysterectom* or (hernia adj5 repair*) or herniorrhaph* or hernioplasty or (joint replacement adj3 surgery) or arthroplasty).mp. or (general adj3 an?esth*).ti,ab. 8. 6 or 7 or 5 9. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 10. Economics/ or "Costs and Cost Analysis"/ or exp Cost‐Benefit Analysis/ or (economic adj3 evaluation).ti,ab. or cost effectiveness analysis.mp. or cost utility analysis.mp. or Cost minimisation.mp. or "Cost Savings"/ or QALY.mp. or Quality‐Adjusted Life Years/ 11. (10 or 9) not (child not (child and adult)).sh. 12. 8 and 11 and 4
Appendix 3. Search strategy for EMBASE (Ovid SP)
1. ((prevent* adj3 (anxiety or stress or depression or catastrophizing or negative orientation or noxious stimuli or negative emotion*)) or physiotherapy exercise* or taking analgesic* or (psychological adj3 preparation*) or ((sensory or procedural) adj3 information) or behavio?ral instruction* or ((emotion?focused or cognitive) adj3 intervention*) or (relaxation or hypnosis) or (cognitive adj3 (reframing or distraction)) or guided imagery).ti,ab. or patient education/ or behavior therapy/ or cognitive therapy/ or relaxation training/ or hypnosis/ or psychotherapy/ 2. ((post?operative adj3 (outcome* or pain)) or post?surgical pain or (surgery or operat*) or surgical procedure*).ti,ab. or postoperative care/ or postoperative pain/ or postoperative complication/ or general surgery/ or cholecystectomy/ or hysterectomy/ or arthroplasty/ or anesthetic agent/ or general anesthesia/ or (cholecystectom* or hysterectom* or (hernia adj5 repair*) or herniorrhaph* or hernioplasty or (joint replacement adj3 surgery) or arthroplasty).ti,ab. or (general adj3 an?esth*).ti,ab. 3. 1 and 2 4. ((((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.) or economics/ or "cost benefit analysis"/ or "cost effectiveness analysis"/ or (economic adj3 evaluation).ti,ab. or cost effectiveness analysis.mp. or "cost utility analysis"/ or "cost minimization analysis"/ or "cost control"/ or QALY.mp. or quality adjusted life year/) not (child not (child and adult)).sh. 5. 3 and 4
Appendix 4. Search strategy for CINAHL (EBSCOhost)
S1 ( (MH "Patient Education") OR (MH "Behavior Therapy") OR (MH "Cognitive Therapy") OR (MH "Hypnosis, Anesthetic") OR (MH "Guided Imagery") ) OR ( (prevent* and (anxiety or stress or depression or catastrophizing or negative orientation or noxious stimuli or negative emotion*)) ) OR ( physiotherapy exercise* or taking analgesic* or (Psychological and preparation*) or ((sensory or procedural) and information) or behavio?ral instruction* or ((emotion?focused or cognitive) and intervention*) or (relaxation or hypnosis) or (cognitive and (reframing or distraction)) or guided imagery ) S2 ( (MH "Postoperative Care") OR (MH "Postoperative Complications") OR (MH "Cholecystectomy") OR (MH "Hysterectomy") OR (MH "Arthroplasty, Replacement") OR (MH "Anesthetics, General") OR (MH "Anesthesia, General") OR (MH "Arthroplasty") ) OR ( ((post?operative and (outcome* or pain)) or post?surgical pain) or (surgery or operat*) or surgical procedure* ) OR ( (cholecystectom* or hysterectom* or (hernia repair*) or herniorrhaph* or hernioplasty or (joint replacement and surgery) or arthroplasty) or (general and an?esth*) ) S3 S1 and S2 S4 ( (MH "Random Assignment") OR (MH "Clinical Trials") OR (MH "Double‐Blind Studies") OR (MH "Intervention Trials") OR (MH "Randomized Controlled Trials") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") ) OR ( (MH "Economics") OR (MH "Costs and Cost Analysis") OR (MH "Cost Control") OR (MH "Quality‐Adjusted Life Years") ) S5 S3 and S4 S6 (child not (child and adult)) S7 S5 not S6
Appendix 5. Search strategy for ISI Web of Science
#1 TS=(prevent* SAME (anxiety or stress or depression or catastrophizing or negative orientation or noxious stimuli or negative emotion*)) or TS=(physiotherapy exercise* or taking analgesic* or (psychological SAME preparation*) or ((sensory or procedural) SAME information) or behavio?ral instruction* or ((emotion?focused or cognitive) SAME intervention*) or (relaxation or hypnosis) or (cognitive SAME (reframing or distraction)) or guided imagery) #2 TS=((post?operative SAME (outcome* or pain)) or post?surgical pain or surgery or operat* or surgical procedure*) or TS=(cholecystectom* or hysterectom* or (hernia SAME repair*) or herniorrhaph* or hernioplasty or joint replacement surgery or arthroplasty or (general SAME an?esth*)) #3 #2 AND #1 #4 TI=random* or TI=trial* or TS=(cost effectiveness analysis or cost utility analysis or cost minimisation or QALY or Quality‐Adjusted Life Years) #5 #4 AND #3
Appendix 6. Data extraction form
| Study details |
| Study ID: |
| Authors: |
| Year: |
| Journal/source: |
| Volume/page numbers: |
| Title: |
| Study location and setting: |
| Language: |
| Reviewer: Date of entry: |
| Participant characteristics |
| Age (mean, median, range etc): |
| Gender (no./%): |
| Surgery type(s): |
| % general anaesthetic: |
| % sedative prior to anaesthetic: |
| No. eligible patients: No. randomized: |
| No./% participants lost to follow‐up: |
|
Interventions. Please provide judgement of type of intervention according to systematic review categories (in addition to authors’ descriptions). |
|
Control group Components (as described by authors): Components (as per review definitions): Administration (including when, duration, by whom, how, materials): Fidelity (integrity of intervention delivery, participant adherence, attrition rate): Loss to follow‐up: |
|
Intervention 1: Theoretical basis of intervention: Components (as described by authors): Components (as per review definitions): Administration (including when, duration, by whom, how, materials): Fidelity (integrity of intervention delivery, participant adherence, attrition rate): Procedure‐specific (to this type of surgery) or general? Loss to follow‐up: |
|
Intervention 2: Theoretical basis of intervention: Components (as described by authors): Components (as per review definitions): Administration (including when, duration, by whom, how, materials): Fidelity (integrity of intervention delivery, participant adherence, attrition rate): Procedure‐specific (to this type of surgery) or general? Loss to follow‐up: |
|
Intervention 3: Theoretical basis of intervention: Components (as described by authors): Components (as per review definitions): Administration (including when, duration, by whom, how, materials): Fidelity (integrity of intervention delivery, participant adherence, attrition rate): Procedure‐specific (to this type of surgery) or general? Loss to follow‐up: |
|
Outcomes We are only considering outcomes measured within 30 days/1 month post‐surgery. For the outcomes of behavioural recovery and negative affect we are only including studies that use measures with published psychometric properties (including reliability and validity). |
|
Outcome 1: Outcome type (study definition): Outcome type (review definition – if different): Timing of outcome: Measurement tool (including upper/lower limits, whether high or low score desirable) Published psychometrics for measurement tool? Y / N / N/A |
|
Outcome 2: Outcome type (study definition): Outcome type (review definition – if different): Timing of outcome: Measurement tool (including upper/lower limits, whether high or low score desirable) Published psychometrics for measurement tool? Y / N / N/A |
|
Outcome 3: Outcome type (study definition): Outcome type (review definition – if different): Timing of outcome: Measurement tool (including upper/lower limits, whether high or low score desirable) Published psychometrics for measurement tool? Y / N / N/A |
|
Outcome 4: Outcome type (study definition): Outcome type (review definition – if different): Timing of outcome: Measurement tool (including upper/lower limits, whether high or low score desirable) Published psychometrics for measurement tool? Y / N / N/A |
|
Outcome 5: Outcome type (study definition): Outcome type (review definition – if different): Timing of outcome: Measurement tool (including upper/lower limits, whether high or low score desirable) Published psychometrics for measurement tool? Y / N / N/A |
|
Outcome 6 Outcome type (study definition): Outcome type (review definition – if different): Timing of outcome: Measurement tool (including upper/lower limits, whether high or low score desirable) Published psychometrics for measurement tool? Y / N / N/A |
| Any outcomes collected but not reported? Yes / No If yes, give details: |
| Were other outcomes measured? (i.e. study outcomes that do not meet our inclusion criteria (including timing requirements). If so, list names of all outcomes with time points below. |
| Continuous data | ||||
|
Outcome (add label) |
Intervention 1 (state) | Control | ||
| N | Mean (SD) | N | Mean (SD) | |
| 1. | ||||
| 2. | ||||
| 3. | ||||
|
Outcome (add label) |
Intervention 2 (state) | Control | ||
| N | Mean (SD) | N | Mean (SD) | |
| 1. | ||||
| 2. | ||||
| 3. | ||||
|
Outcome (add label) |
Intervention 3 (state) | Control | ||
| N | Mean (SD) | N | Mean (SD) | |
| 1. | ||||
| 2. | ||||
| 3. | ||||
| Dichotomous data | ||
|
Outcome (add label) |
Intervention 1 (n/N) n = no. participants with the outcome N = no. participants at risk of outcome |
Control (n/N) n = no. participants with the outcome N = no. participants at risk of outcome |
| 1. | ||
| 2. | ||
| 3. | ||
|
Outcome (add label) |
Intervention 2 (n/N) n = no. participants with the outcome N = no. participants at risk of outcome |
Control (n/N) n = no. participants with the outcome N = no. participants at risk of outcome |
| 1. | ||
| 2. | ||
| 3. | ||
|
Outcome (add label) |
Intervention 3 (n/N) n = no. participants with the outcome N = no. participants at risk of outcome |
Control (n/N) n = no. participants with the outcome N = no. participants at risk of outcome |
| 1. | ||
| 2. | ||
| 3. | ||
| Other outcome information: e.g. study’s estimation of effect sizes with confidence intervals & p values, any subgroup analyses, comments on analyses (e.g. use of multi‐level modelling/random effects regression). |
| Information on cost per outcome: If any information is given on cost per outcome, detail below (brief summary). |
| Information on resource use (Please list any outcomes measuring resource use – including any already listed in Outcomes above) (examples: length of stay, analgesia measures). |
|
Other relevant information Please indicate if there are gaps in the available data provided & where further information should be requested from the author. Indicate if any data were obtained from the primary author, if results estimated e.g. from graphs or calculated by you (give formula) – indicate any other methods of obtaining results other than reading in paper. |
| Any other comments – including writing actions e.g. contact with study authors. |
Appendix 7. Risk of bias form
The Cochrane tool for assessing risk of bias (with additional intention‐to‐treat item).
| Domain | Support for judgement | Review authors’ judgement |
| Selection bias | High risk, low risk or unclear | |
| Random sequence generation | Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. | Selection bias (biased allocation to interventions) due to inadequate generation of a randomized sequence. |
| Allocation concealment | Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment. | Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. |
| Performance bias | ||
| Blinding of participants and personnel | Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. |
| Detection bias | ||
|
Blinding of outcome assessment (please note if this differs with different outcomes) |
Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective. | Detection bias due to knowledge of the allocated interventions by outcome assessors. |
| Attrition bias | ||
|
Incomplete outcome data Outcome: (add a table line for each additional outcome, if differs) |
Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re‐inclusions in analyses performed by the review authors. | Attrition bias due to amount, nature or handling of incomplete outcome data |
| Reporting bias | ||
| Selective reporting | State how the possibility of selective outcome reporting was examined by the review authors, and what was found. | Reporting bias due to selective outcome reporting. |
| Other bias | ||
|
‘Intention‐to‐treat’ SeeHiggins 201116.2.1. |
Were participants kept in the intervention groups to which they were randomized, regardless of the intervention they received? Alternative possibilities: per‐protocol (only analysed if received some of allocated treatment) or treatment‐received (allocated according to the treatment received rather than that to which randomized). |
Bias due to analysis being per‐protocol or treatment‐received. |
| Other sources of bias e.g. contamination, clustering? | State any important concerns about bias not addressed in the other domains in the tool. | Bias due to problems not covered elsewhere in the table. |
Data and analyses
Comparison 1. Any psychological preparation intervention versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 38 | 2713 | Std. Mean Difference (Random, 95% CI) | ‐0.20 [‐0.35, ‐0.06] |
| 2 Length of stay (days) | 36 | 3313 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.82, ‐0.22] |
| 3 Negative affect | 31 | 2496 | Std. Mean Difference (Random, 95% CI) | ‐0.35 [‐0.54, ‐0.16] |
Comparison 2. Procedural information versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 12 | 1051 | Std. Mean Difference (Random, 95% CI) | ‐0.08 [‐0.26, 0.09] |
| 1.1 Procedural information only | 2 | 186 | Std. Mean Difference (Random, 95% CI) | ‐0.13 [‐0.42, 0.16] |
| 1.2 Procedural information plus other intervention(s) | 10 | 865 | Std. Mean Difference (Random, 95% CI) | ‐0.08 [‐0.29, 0.13] |
| 2 Length of stay (days) | 19 | 1983 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.08, ‐0.18] |
| 2.1 Procedural information only | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐9.95, ‐2.05] |
| 2.2 Procedural information plus other intervention(s) | 18 | 1907 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.01, ‐0.13] |
| 3 Negative affect | 17 | 1334 | Std. Mean Difference (Random, 95% CI) | ‐0.45 [‐0.75, ‐0.16] |
| 3.1 Procedural information only | 3 | 269 | Std. Mean Difference (Random, 95% CI) | ‐0.54 [‐1.25, 0.16] |
| 3.2 Procedural information plus other intervention(s) | 14 | 1065 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.77, ‐0.10] |
Comparison 3. Sensory information versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 11 | 881 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.47, 0.02] |
| 1.1 Sensory information plus other intervention(s) | 11 | 881 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.47, 0.02] |
| 2 Length of stay (days) | 14 | 1236 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.15, ‐0.27] |
| 2.1 Sensory information plus other intervention(s) | 14 | 1236 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.15, ‐0.27] |
| 3 Negative affect | 12 | 919 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐0.90, ‐0.19] |
| 3.1 Sensory information plus other intervention(s) | 12 | 919 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐0.90, ‐0.19] |
Comparison 4. Behavioural instruction versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 21 | 1241 | Std. Mean Difference (Random, 95% CI) | ‐0.14 [‐0.33, 0.05] |
| 1.1 Behavioural instruction only | 9 | 523 | Std. Mean Difference (Random, 95% CI) | 0.01 [‐0.19, 0.21] |
| 1.2 Behavioural instruction plus other intervention(s) | 12 | 718 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.57, 0.01] |
| 2 Length of stay (days) | 25 | 2338 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.84, ‐0.19] |
| 2.1 Behavioural instruction only | 8 | 445 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.55, 0.03] |
| 2.2 Behavioural instruction plus other intervention(s) | 17 | 1893 | Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.12, ‐0.16] |
| 3 Negative affect | 13 | 1183 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.46, 0.02] |
| 3.1 Behavioural instruction only | 3 | 472 | Std. Mean Difference (Random, 95% CI) | 0.18 [‐0.19, 0.55] |
| 3.2 Behavioural instruction plus other intervention(s) | 10 | 711 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.65, ‐0.09] |
Comparison 5. Cognitive interventions versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 6 | 355 | Std. Mean Difference (Random, 95% CI) | ‐0.02 [‐0.29, 0.25] |
| 1.1 Cognitive intervention only | 2 | 136 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.68, ‐0.01] |
| 1.2 Cognitive intervention plus other intervention(s) | 4 | 219 | Std. Mean Difference (Random, 95% CI) | 0.17 [‐0.09, 0.44] |
| 2 Length of stay (days) | 9 | 1074 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.07, 0.22] |
| 2.1 Cognitive intervention only | 2 | 77 | Mean Difference (IV, Random, 95% CI) | 0.62 [‐0.74, 1.99] |
| 2.2 Cognitive intervention plus other intervention(s) | 7 | 997 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐1.27, 0.08] |
| 3 Negative affect | 5 | 251 | Std. Mean Difference (Random, 95% CI) | ‐0.20 [‐0.52, 0.12] |
| 3.1 Cognitive intervention only | 3 | 173 | Std. Mean Difference (Random, 95% CI) | ‐0.08 [‐0.58, 0.42] |
| 3.2 Cognitive intervention plus other intervention(s) | 2 | 78 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.83, 0.05] |
Comparison 6. Relaxation versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 13 | 891 | Std. Mean Difference (Random, 95% CI) | ‐0.46 [‐0.81, ‐0.11] |
| 1.1 Relaxation only | 7 | 417 | Std. Mean Difference (Random, 95% CI) | ‐0.71 [‐1.29, ‐0.13] |
| 1.2 Relaxation plus other intervention(s) | 6 | 474 | Std. Mean Difference (Random, 95% CI) | ‐0.19 [‐0.58, 0.21] |
| 2 Length of stay (days) | 7 | 473 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.94, ‐0.00] |
| 2.1 Relaxation only | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.25, 0.64] |
| 2.2 Relaxation plus other intervention(s) | 5 | 413 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐2.39, 0.24] |
| 3 Negative affect | 11 | 687 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.56, ‐0.12] |
| 3.1 Relaxation only | 4 | 256 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.57, 0.04] |
| 3.2 Relaxation plus other intervention(s) | 7 | 431 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.73, ‐0.08] |
Comparison 7. Hypnosis versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Negative affect | 2 | 72 | Std. Mean Difference (Random, 95% CI) | ‐0.77 [‐1.25, ‐0.30] |
| 1.1 Hypnosis plus other intervention(s) | 2 | 72 | Std. Mean Difference (Random, 95% CI) | ‐0.77 [‐1.25, ‐0.30] |
Comparison 8. Emotion‐focused interventions versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 3 | 180 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐0.85, 0.00] |
| 1.1 Emotion‐focused only | 1 | 18 | Std. Mean Difference (Random, 95% CI) | 0.12 [‐0.76, 1.00] |
| 1.2 Emotion‐focused plus other intervention(s) | 2 | 162 | Std. Mean Difference (Random, 95% CI) | ‐0.54 [‐0.97, ‐0.10] |
| 2 Length of stay (days) | 3 | 212 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.67, 0.94] |
| 2.1 Emotion‐focused plus other intervention(s) | 3 | 212 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.67, 0.94] |
| 3 Negative affect | 4 | 201 | Std. Mean Difference (Random, 95% CI) | ‐0.24 [‐0.55, 0.07] |
| 3.1 Emotion‐focused only | 1 | 18 | Std. Mean Difference (Random, 95% CI) | 0.12 [‐0.76, 1.00] |
| 3.2 Emotion‐focused plus other intervention(s) | 3 | 183 | Std. Mean Difference (Random, 95% CI) | ‐0.30 [‐0.66, 0.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ali 1989.
| Methods | Randomized controlled trial | |
| Participants | 30 patients undergoing planned urinary diversion surgery for bladder cancer. Control group: mean age 45.86 (SD = 4.4); intervention mean age: 45.33 years (SD = 5.9) (overall mean = 45.60). Control group: 12 male, 3 female; intervention group 11 male, 4 female. Overall, 76.7% male. Setting: The National Cancer Institute, Cairo, Egypt. Dates of data collection not provided. | |
| Interventions |
Control: "routine physical pre‐operative care" Intervention: explanation of surgical procedure, appearance of stoma and postoperative device, reasons for wearing device, visit from a previous patient; encouraged to "express fears and anxieties regarding social aspects of living with a stoma". Emotion‐focused; procedural information |
|
| Outcomes | Negative affect: anxiety (STAI A‐state, Arabic translation) on 3rd postoperative day and before discharge (approximately 12 days post‐surgery) | |
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p238: "After patients consented, they were assigned randomly to two groups of 15 each (control and experimental)" |
| Allocation concealment (selection bias) | Unclear risk | No further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but unlikely participants blinded due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information – numbers only reported for 30 people with complete data |
| Selective reporting (reporting bias) | Unclear risk | Consistent throughout paper but no reference to a protocol document to check this |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided related to `intention‐to‐treat' |
| Other bias | Low risk | No obvious other biases |
Ashton 1997.
| Methods | Randomized controlled trial | |
| Participants | 32 patients undergoing elective coronary artery bypass surgery at a large tertiary care teaching institution, New York, USA. Recruitment following approval in July 1994. Control group age "62 ± 3"; intervention "64 ± 3"; assume mean ± SEM from other tables. Control group: 11 male, 1 female; intervention: 17 male, 3 female. Overall, 87.5% male | |
| Interventions |
Control: no intervention Intervention: "self‐hypnosis relaxation techniques": single session, night before surgery. Instructed to take deep breaths, relax muscles and to focus on thoughts e.g. minimize bleeding, reducing pain. Asked to practise hourly the night before surgery and as often as possible postoperatively. Relaxation and hypnosis |
|
| Outcomes |
Negative affect: Profile of Mood States Scale (POMS ‐ tension, depression, anger, vigour, fatigue, confusion) (day 5 post‐surgery) Length of stay |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p70: "randomized to control versus study group based on age (less than or greater than 65), sex, and HIP score (0‐5, 6‐10, 11‐16)" – i.e. no information about the actual process of randomization |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p71: "only the patients and the individuals teaching the self‐hypnosis techniques were not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | p71: "All individuals involved in patient care were blinded to the randomization. Only the patients and the individuals teaching the self‐hypnosis techniques were not blinded". Does not explicitly state whether those taking the outcome measures were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition is reported, but not clearly stated that there was no attrition. 4 participants declined participation – it appears that this was pre‐randomization but not clear (the reasons given for declining on p71 seem to do with hypnosis – so possible that they were allocated to hypnosis condition?). Sample sizes not provided in Results except that 100% (20) intervention participants reported following protocol preoperatively – which suggests that, at least for the intervention group, all were participating at day 5 |
| Selective reporting (reporting bias) | Unclear risk | Did not find evidence of selective reporting but no protocol is referred to |
| ‘Intention‐to‐treat’ | Unclear risk | From the results data, it appears that all intervention participants carried out the intervention and were analysed in the intervention group. It is not clear whether the authors intended to carry out intention‐to‐treat analysis (rather than per‐protocol) but as all participants carried out the intervention this seems to be what has happened. However, as not clearly specified, recorded as `unclear' |
| Other bias | Unclear risk | Small, uneven sample size (12 in control and 20 in intervention group); appear to have made error in analysis ‐ report having used Wilcoxon when Mann‐Whitney more appropriate (independent samples) ‐ caution needed when interpreting their findings in narrative synthesis |
Barbalho‐Moulim 2011.
| Methods | Randomized controlled trial | |
| Participants | 32 women undergoing open Rou‐en‐Y gastric bypass surgery at Meridional Hospital, Cariacica, ES, Brazil (dates not provided). Control group mean age 34.8 (SD = 9.47, n = 17); intervention group mean age 36.13 (SD = 8.12, n = 15) | |
| Interventions |
Control: instructions about post‐surgery care, coughing and ambulation. Behavioural instruction Intervention: As Control group plus: inspiratory muscle training (IMT), starting 2 to 4 weeks before surgery – 6 x 15‐minute sessions a week, 2 supervised by physiotherapist, 4 unsupervised. Behavioural instruction (beyond control group) |
|
| Outcomes |
Pain: visual analogue scale (VAS), 0 = no pain, 10 =intense pain, first postoperative day Length of stay: hospital stay (days) |
|
| Notes | As this study was identified late (and analysis was commencing), authors were not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p1722: "patients were informed about the research protocol, requested to sign the Informed Consent Term, and then randomly assigned to the IMT group or the control group by opening a sealed envelope" |
| Allocation concealment (selection bias) | Unclear risk | p1722: "patients were informed about the research protocol, requested to sign the Informed Consent Term, and then randomly assigned to the IMT group or the control group by opening a sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given the nature of the intervention, it would not have been possible for either the participants or those delivering the intervention to be blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | According to the flow chart (p1725), it appears that there was no attrition after allocation to intervention group. However, 2 participants were excluded for being "unable to perform the tests" and it's not clear at what stage they were excluded |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are reported for all reported measures, and in sufficient detail for inclusion in meta‐analysis. However, no reference to a protocol document |
| ‘Intention‐to‐treat’ | Unclear risk | The flow chart (p1725) suggests that all participants randomized received the allocated intervention and were analysed as such. However, 2 participants were excluded for being "unable to perform the tests" – according to the Methods, this would seem to have been after randomization |
| Other bias | Low risk | No other concerns |
Barlési 2008.
| Methods | Randomized controlled trial | |
| Participants | 102 patients undergoing thoracic surgery for non‐small cell lung cancer randomized, University Teaching Hospital, Marseille, France. Data were collected over 2 years. Control group (n = 34) mean age 63.7 (SE = 7.7); intervention (n = 41) mean age 63.4 (SE = 9.6). Mean for all 75 for whom data analysed: 63.5 (SE = 8.7) | |
| Interventions | Both groups: "individualized oral information" (some procedural information apparent). Control: no further information. Intervention: written document containing information including regarding lung cancer, symptoms, "pretherapeutic work‐up", surgery, postoperative treatments. Procedural information; could include sensory information and behavioural instruction, but insufficient information | |
| Outcomes | Negative affect: used Psychologic Global Well‐being Scale; components include Anxiety, Depressed Mood and Positive Well‐being (also self control, general health, vitality). Timing unclear: either at time of surgery (postoperative period) or 1 month postoperatively | |
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p1147: "patients were randomized…" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information. As the intervention is a leaflet, it is not impossible that it may have been delivered in such a way that the participant was blind to intervention, but no information is provided as to how it was given to the participant |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusion for each group is reported. 10: early postoperative death; 4: loss of follow‐up; 13: incomplete satisfaction or quality of life data at 1 or 3 months (p1147). 15 in control and 12 in intervention group (p1149). Further information would be helpful – a breakdown of reasons for attrition is provided for the overall sample but not by each group so difficult to tell whether this could have led to any bias |
| Selective reporting (reporting bias) | High risk | No evidence of selective reporting (no measures in Methods not reported in Results) but no reference to any protocol that could be checked. Cannot enter into meta‐analysis: no data provided for subscales of measure – only overall measure, which includes non‐negative affect components |
| ‘Intention‐to‐treat’ | Unclear risk | No information. Seems likely as the intervention is giving a leaflet after randomization (i.e. unlikely to not receive the treatment to which randomized) but no information regarding fidelity/ITT |
| Other bias | Low risk | No other concerns |
Beaupre 2004.
| Methods | Randomized controlled trial | |
| Participants | 131 patients undergoing total knee arthroplasty, University of Alberta Hospital, Alberta, Canada. Recruitment dates not provided. Intervention mean age: 67 (SD = 7, n = 65). Control mean age 67 (SD = 6, n = 66). Overall mean: 67. Intervention: 39 (60%) female; control: 33 (50%) female | |
| Interventions |
Control: regular activities (usual care) Intervention: 12 sessions (3 per week, 4 weeks): instruction regarding activities e.g. crutch walking, bed mobility; exercise programme: simple strengthening exercises. Behavioural instruction |
|
| Outcomes | Length of stay | |
| Notes | Three length of stay outcomes: acute care, transfer and readmission. Reporting acute care here as comparable with other studies Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p1167: "Patients were randomized, in blocks of 20 patients, to one of 2 groups, treatment or control, following the enrolment visit. Randomization was performed using consecutively numbered opaque envelopes." Does not state how the random component was introduced |
| Allocation concealment (selection bias) | Low risk | p1167: "Randomization was performed using consecutively numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided in paper but highly unlikely given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p1167: "blinded assessment of outcomes by a physical therapist not involved with the intervention" Given that the outcome of interest is length of stay, seems likely that blinding would be effective (i.e. patient would not be able to mention intervention) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Clear and careful reporting of attrition and exclusions. 16 cancelled surgery (6 control group, 10 intervention). Compared differences for attrition (surgery being cancelled) between groups (none found). Rated as `unclear' as there is some differential in dropout between groups |
| Selective reporting (reporting bias) | Unclear risk | Did not find variables mentioned in methods that were not reported in Results, but no published protocol mentioned |
| ‘Intention‐to‐treat’ | Low risk | No other concerns |
| Other bias | Low risk | p1168: "All analyses were performed on an ‘intent to treat’ basis" |
Bergin 2014a.
| Methods | Randomized controlled trial | |
| Participants | Randomized 140 patients undergoing knee or hip joint replacement surgery (data reported for 106, hip n = 39, knee n = 67) at a 182‐bed community, not‐for‐profit hospital in the mid‐Atlantic region of the United States (dates not given). Age range 61 to 64 (mean 63.8, SD 8.7). Control group mean age 65.7 (SD 8.7); intervention group mean age 61.6 (SD 8.3). Most (57.5%) were female (58.9% of intervention group and 56% of control group) | |
| Interventions |
Control: 1 to 4 weeks before surgery, attended class providing incentive spirometry (IS) device and informal education on use. Behavioural instruction Intervention: stayed on for 15 minutes after class, detailed, structured information on IS provided by researcher – including how to use, how many times, how to keep record on chart. Given daily diary to use for 7 days before operation. Behavioural instruction (beyond Control group) |
|
| Outcomes |
Length of stay Pain: from 0 (no pain) to 10 (worst pain) daily for first 7 days after surgery |
|
| Notes | Author provided unpublished numerical data and information regarding risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p22: "Patients who met eligibility criteria were randomized in a 1:1 ratio to Group 1 (POISE intervention) or to Group 2 (no POISE/no intervention). Randomization was stratified by type of total joint replacement, knee or hip" Author: "A computer‐generated random allocation was used to assign randomization. A randomization log was used was with a 1:1 ratio is blocks of four. For stratification, one log was used for hip patients and one log was used for knee patients. Logs were generated prior to the initiation of the study. The allocation sequence was generated prior to any enrolment" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper Author: "No method was used to conceal the allocation sequence for the investigators as the study randomization logs provided the sequence allocation. The study participants were also not told of the allocation sequence prior to providing consent" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention involved staying behind for 15 minutes for session delivered by researchers – neither participants nor staff could have been blind Author: "Correct, the researchers were not blind to the allocation group" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Researchers reviewed completion of the patient's postoperative study diaries [including pain measure] during daily rounds therefore could not be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported in high level of detail: No./% participants lost to follow‐up: 106 completed study – so 34 (24%) lost to follow‐up. Enrolled: intervention group: 50; control n = 56. For the 34 not completing (group intervention = 21, control = 13), 7 did not continue to meet eligibility criteria (intervention n = 4, control n = 3), 11 surgery cancelled (intervention n = 7, control n = 4), 9 missing incentive spirometry data (intervention n = 6, control n = 3), 7 withdrew (intervention = 4, control = 3). In addition, for first pain outcome, 1 pain value not recorded for intervention group; two not recorded for control group While a high proportion were lost, there does not appear to be indication of the preoperative group allocation having an impact, therefore risk of bias seems low |
| Selective reporting (reporting bias) | Low risk | Authors do report their clearly stated outcomes, but not always clear what was intended (e.g. do not report pain by day – just summary of data of pain when returned to baseline IS volume – no indication as to whether or not this was planned a priori, and no mention of protocol). Pain data are not presented in such a way as can be used for review. However, author has sent us all the pain data we need to include in meta‐analysis. Author: "There were no other outcomes that were not reported" |
| ‘Intention‐to‐treat’ | Low risk | Author: "Yes, patients were kept in the intervention group to which they were randomized. Of the 140 enrolled, 106 completed the study. Data are reported for the 106 completed and as such, technically this is not considered then `intent‐to‐treat'." [This meets the standard for intention‐to‐treat defined in this review] |
| Other bias | Low risk | No other concerns |
Bergmann 2001.
| Methods | Randomized controlled trial | |
| Participants | 60 patients undergoing cardiac surgery (CABG or heart valve operation), Graz, Austria. Data collection dates not provided. Control group mean age 59 (range 55 to 62). Intervention mean age 62 (range 58 to 62). Controls, 14 female, 16 male; Intervention 12 female, 18 male. Overall, 26 female, 34 male (56.67% male) | |
| Interventions |
Control: routine medical information, 5‐page pamphlet, 3 days before surgery. Information on: preoperative course and preparation for operation, surgery technique postoperative course, complications (procedural information likely) Intervention: leaflet as per Control group. Also extensive oral information from a surgeon – more emphasis on perioperative problems and concerns ‐ possibly `emotion‐focused' but insufficient information provided for this categorization; additional procedural information |
|
| Outcomes |
Negative affect. STAI state and trait anxiety [only state reported in Results], day 6 after surgery Negative affect. Well‐being Scale, day 6 after surgery |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p1094: "Subjects were randomly assigned to one of the groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blinded: p1093: "The patients were kept blinded to the actual purpose of the study (not informed that our study involved two different groups)". However, the researcher who delivered the oral intervention was also lead author and is highly likely to not be blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p1094: "The psychologist and the person administering the psychological tests were the same individual, who was strictly blinded with reference to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | p1094: "Two patients in Group I [control group] who asked for additional information were excluded from the study" – clear potential cause of bias – no information‐seekers in control group |
| Selective reporting (reporting bias) | Unclear risk | Some lack of clarity as to what were outcome variables (e.g. trait anxiety appears to have been measured but not reported – but it is state anxiety that would be expected to be used as an outcome so trait anxiety may never have been intended as an outcome). No protocol to refer to |
| ‘Intention‐to‐treat’ | High risk | Not intention‐to‐treat – control participants who sought additional information were excluded ‐ p1094: "Two patients in Group I [control group] who asked for additional information were excluded from the study" |
| Other bias | Low risk | No other concerns |
Bitterli 2011.
| Methods | Randomized controlled trial | |
| Participants | 80 patients undergoing total hip replacement at Cantonal Hospital, Liestal, Switzerland between June 2004 and March 2007. Overall: mean age 66.8 years (SD 10.3). Intervention group mean age 65.37 (10.77); control group 68.42 (9.74). Overall: 31 female, 49 (61%) male. Intervention group 19 female, 22 male; control group 12 female, 27 male | |
| Interventions |
Control: standard care: day before surgery, verbal information about events after surgery and instruction in standing and walking.Procedural information, behavioural instruction Intervention: as Control plus: 2 verbal and written instructions giving exercises designed to promote awareness of position and movement of hip. Period of training ranged from 2 to 6 weeks; participants recorded training in logbook. Behavioural instruction |
|
| Outcomes |
Length of stay Negative affect: Mental health (SF‐36); 8 to 10 days post‐surgery Pain: pain magnitude item from German SF‐36 |
|
| Notes | Author provided some unpublished numerical data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p727: "patients…were assigned to either one of the 2 groups with the aid of a randomisation table" |
| Allocation concealment (selection bias) | High risk | No information in paper. Author: as written consent arrived, author and colleague allocated using randomization table. This would appear to be the use of an "open random allocation schedule", so judged `high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p727: "A person blinded for group allocation anonymised the data. None of the hospital staff was aware of the status (TR or CO) of the participants. The participants were requested not to reveal their allocation to any of the staff members" (TR = training group; CO = control group). But participants and staff delivering the intervention could not have been blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p727: "Two physiotherapists…gave the participants the necessary instructions and performed the follow‐up measurements…the questionnaires were sent to the participants a few days before each appointment". The first part of this would suggest NOT blind. However, author reported outcomes taken by a 3rd, blind person |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition is described in detail: flow chart p726. 39 randomized to control group, 41 to intervention. After randomization, before surgery and intervention, 2 discontinued in control group (1 voluntary basis, 1 "inclusion criteria not met") and 2 discontinued in intervention group (both: "no appointment"). Further loss in intervention group after instruction and training: n = 3 (1 on voluntary basis, 2 surgery brought forward). After surgery, 1 in each group discontinued before 10‐day follow‐up ("voluntary basis"). Total loss control group: n = 3; total loss intervention group: n = 6 (9 of 80 = 11%) Concern: the 2 missing from intervention group (because surgery was brought forward) – would they also have been excluded if in control group, or was the problem that they did not have time to do the intervention? |
| Selective reporting (reporting bias) | High risk | Data were not reported in sufficient detail for meta‐analysis in the paper but the authors have provided us with detailed tables and additional information. However, this also confirmed that outcomes were measured that were not reported in the paper |
| ‘Intention‐to‐treat’ | High risk | p728: "An intention‐to‐treat analytical approach was pursued". However, flow chart p726: 2 patients in intervention group were excluded because their surgery was brought forward. Author confirmed that excluded because less than 15 days of training, and would have not had reason to exclude if had been in control group. Therefore does not meet our criteria for intention‐to‐treat |
| Other bias | Low risk | No other concerns |
Broadbent 2012.
| Methods | Randomized controlled trial | |
| Participants | 75 patients undergoing laparoscopic cholecystectomy at Manukau Surgical Centre, South Auckland, New Zealand (April 2008 to May 2010) were randomized (60 analysed). Control group: age 50.5 (15.5); intervention age: 52.1 (18.0) (assume mean (SD) ‐ not stated in Table 1, p215). 45 female (75%); 15 male | |
| Interventions |
Control: "standard care" Intervention: "standard care" plus relaxation intervention. 45‐minute session with psychologist at least 3 days pre‐surgery (deep breathing, progressive muscle relaxation, guided imagery); given 20‐minute CD recording to practise with daily at home before surgery; CD contained a second recording for them to use each day after surgery for 7 days. Relaxation |
|
| Outcomes | Negative affect: stress – Perceived Stress Scale, day 7 post‐surgery | |
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p213: "Participants were randomized using a random sequence generator and allocation was sealed in consecutively numbered envelopes by EB" (EB = Elizabeth Broadbent, a study author) |
| Allocation concealment (selection bias) | Unclear risk | p213: "Participants were randomized using a random sequence generator and allocation was sealed in consecutively numbered envelopes by EB". Previous paragraph: "invited to participate by the surgical research fellow…following informed consent, patients were randomized by the health psychologist" – need clarification: who took consent? Were envelopes opaque? |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p213: intervention patients met with health psychologist for 45 minutes so neither participants nor person providing intervention blind – also, "Patients were asked not to reveal their group allocation". Other staff were blind to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The surgeons and surgical research fellows conducting the surgical procedure and follow‐up assessments, as well as the laboratory technician performing the hydroxyproline tests, were all blind to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No./% participants lost to follow‐up: 15 (20% of 75) Control group: 8 excluded post‐randomization: 1 declined to have operation; 4 declined to participate post‐randomization; 1 "altered mental state"; 2 had operation at another facility. Remaining N = 30. Intervention group: 7 excluded post‐randomization: 2 declined to participate post randomization; 1 excluded by surgeon (intra‐operative complications); 3 not fit, operation cancelled; 1 had operation at another facility. Remaining N = 30 Attrition is clearly reported and is even across the 2 groups. Of concern: for control group: 4 (over 10% of group) dropped out after randomization; only 2 for intervention group. As sample size is small, this may have had an impact |
| Selective reporting (reporting bias) | High risk | Checked with registered trial – a number of outcomes, some of interest to the review (pain and anxiety), are not reported in this paper. This paper does report that one outcome (fatigue) is being reported in another paper |
| ‘Intention‐to‐treat’ | Unclear risk | From flow chart (p214) no evidence of participants switching groups – and as intervention straight after consent not clear how could receive other intervention (unless in intervention and drop out). However, not clearly stated ‐ check with authors |
| Other bias | Low risk | No other concerns |
Burton 1995.
| Methods | Randomized controlled trial | |
| Participants | 215 patients undergoing mastectomy/sector mastectomy for breast cancer were randomized; 15 later excluded from analysis (found to have benign conditions). 100% female; general anaesthesia assumed. Overall mean age 62.3; range 28 to 37. (Control mean age: 57, "interview only" 64, "interview and chat" 62; "interview and psychotherapeutic intervention" 61). Setting: district general hospital of the NHS (UK); data collection dates not provided | |
| Interventions |
Control: routine care. No follow‐up data within review’s time frame Intervention 1. Preoperative interview. Essentially a research interview but also aspects of emotion‐focused and cognitive intervention Intervention 2. Preoperative interview + 30‐minute chat: as for 2 plus "chat" on unrelated matters Intervention 3. Preoperative interview + 30‐minute brief psychotherapeutic intervention: as for 2 plus emotion‐focused therapeutic intervention |
|
| Outcomes |
Negative affect. HADS Anxiety and Depression at day 4 post‐surgery At 4 days, also appear to have used General Health Questionnaire‐28 and, in a structured interview: modified Present State Examination schedule and the Diagnostic and Statistical Manual of Mental Disorders, 3rd Ed (DSM‐III) but results are not reported |
|
| Notes | Focus of study: 3‐ and 12‐month outcomes. No data for `control' group at 4 days after surgery; HADS data provided for overall sample, not broken down by participant group Burton 1994 paper: same data set as 1995 but 1‐year follow‐up so out of time frame Unsuccessful in locating author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p2: "patients were randomised to experimental groups using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper. However, p3: "A fifth non‐random group of 80 women emerged, those who declined to be interviewed" – so looks as if some participants opted out because of the condition they were placed in. This may not have had much impact on 4‐day postoperative results, however: there are no data for non‐interviewed participants prior to 3 months |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Control participants were not informed they were in a study until 3 months postoperatively so would have been blind. However, no information regarding blinding for the other participants (who would have data at 4 days). High risk as believe staff would have known |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on sample size is provided for the outcome at 4 days postoperation No. eligible patients: 295; No. randomized: 215 (295?). Of the 215, 15 later excluded from outcome study as found to have histologically benign conditions. 80 participants declined to be interviewed (unclear: these 80 must have been initially randomized as "control" participants did not have an interview and did not know were in study until 3 months) So, no. randomized may actually be all 295, with 80 participants then dropping out of interview groups). p3. Number for whom they report outcome data: 200. Table 3, HADS results: 86 cases with complete data (but this includes 3 months and 1 year postoperation) |
| Selective reporting (reporting bias) | High risk | For 4 days, although measures of anxiety and depression were taken (including HADS), data are not provided by experimental group |
| ‘Intention‐to‐treat’ | High risk | It seems very unlikely that results were analysed by intention‐to‐treat – participants who declined to take part because they did not want to have an interview were followed up as a separate group rather than in the group to which they were allocated (p3) |
| Other bias | Low risk | No obvious other sources of bias |
Chaudhri 2005.
| Methods | Randomized controlled trial | |
| Participants | 42 individuals undergoing colorectal resections requiring formation of stoma at an NHS hospital, Newcastle‐upon‐Tyne, dates not provided. 18 female, 24 male; median age: 64 years (range 36 to 82) | |
| Interventions |
Control: usual care: preoperation: 1‐hour meeting with colorectal nurse specialist where received information about stomas; knowledge re‐enforced at admission Intervention: as for control plus additional preoperative education including behavioural instruction: taught to manage stoma system in 2 x 45‐minute home visits by community colorectal nurse specialist. Also: at time of admission, asked to empty and change stoma pouching system |
|
| Outcomes | Postoperative length of stay in hospital | |
| Notes | 42 patients randomized; length of stay data available for 36 (attrition: 3/each group) Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomised to the study or control group by means of a sealed envelope after an initial assessment in the clinic by the hospital colorectal nurse specialist" (p505) |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomised to the study or control group by means of a sealed envelope after an initial assessment in the clinic by the hospital colorectal nurse specialist" (p505) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p505 – "All members of the treating surgical teams and the ward nursing staff were blinded to the patient groups in the study". Unlikely that patients were blind, however, given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | For outcome length of stay: p505 – Patients were discharged by the surgical team and "All members of the treating surgical teams and the ward nursing staff were blinded to the patient groups in the study". Does not state whether blinding was effective though, and it seems likely that the nurses would have been able to guess whether or not patients had had the extra instruction on stoma management. Does not state who took outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition/exclusions were reported for each group; from control group 3 lost: 1 prolonged stay on HDU; 2: reoperation. Intervention: also 3: 1 wound complication, 1 stoma‐related complication, 1 reoperation for time to stoma proficiency. Seems unlikely to lead to bias for the outcome of interest here |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in Methods were reported in Results but no protocol referred to |
| ‘Intention‐to‐treat’ | Unclear risk | Insufficient information is provided in paper |
| Other bias | Low risk | No obvious other bias, although authors have not reported how many patients were approached about the study. However, although the sample might be highly selected, it would not seem that this would bias findings after randomization |
Cheung 2003.
| Methods | Randomized controlled trial | |
| Participants | 96 women undergoing abdominal hysterectomy at a hospital in China; dates not given. Mean age: 41.72 years (range: 30 to 55) | |
| Interventions |
Control: preoperative information booklet (included procedural information and behavioural instruction re. breathing, coughing and leg exercises) Intervention: as Control. Plus Cognitive intervention focusing on cognitive distraction and re‐appraisal – to distract from threatening aspects and re‐evaluate as challenge rather than threat. Patients were asked to express feelings and write down anything that made them feel anxious as part of the cognitive intervention |
|
| Outcomes |
Negative affect: state anxiety (Chinese STAI). Probably day 1 and day 3 post‐surgery but some confusion about timing Pain (VAS). A lot of confusion about timing. According to table 3’s version: on day of operation (postoperative), day 1 and day 2 postoperative |
|
| Notes | Data provided suitable for meta‐analyses (means and SDs) but unclear which data go with which time point Author responded to stage 1 email and provided some risk of bias information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | p209: "the selection of interventions was done at random each month. An equal number of papers were marked either ‘Control’ or ‘Experimental’. There were five of each which represented the 10‐month period over which the study was carried out. These papers were then folded and placed in a box. Every month, one paper was drawn out at random and the number of hysterectomy patients for that month was placed in the group written on the paper" This method of sequence generation would yield a random allocation (with the randomization occurring by month, rather than by individual), but would be clustered by month |
| Allocation concealment (selection bias) | Unclear risk | Equal number of papers marked either "Control" or "Experimental", 5 of each represented the 10‐month period of the study; folded and placed in a box; every month, one paper was drawn out at random and the number of hysterectomy patients for that months was placed in the group written on the paper (p209) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not aware that they were receiving different forms of information (randomized by month to ensure this) (p209) but no mention of personnel being blinded ‐ as intervention was administered in person by a research nurse it is difficult to see how this would be possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p210: recruited 128 women; excluded 32 (16 cancer, 9 outside age range of 30 to 55, 4 unable to read Chinese or speak Cantonese; 3 had diabetes/hypertension). This leaves 96. But then also states that 7 refused to participate and 5 withdrew during the study. The groups of the 5 who withdrew are not stated; also unclear whether the 7 who declined did so after learning which condition they would receive |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting but no reference to a protocol. Contacted authors: "We did not measure any other outcome than those reported" |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Unclear risk | A lot of confusion about time points of anxiety and pain measures. This could lead to a bias if this study’s findings were compared with others unless can gain clarification from authors |
Chumbley 2004.
| Methods | Cluster‐randomized controlled trial | |
| Participants | UK‐based study, funded by London Regional NHS Executive, Research and Development Directorate; dates not provided. 246 undergoing surgery where would routinely receive patient‐controlled analgesia (PCA). Gynaecological 87; orthopaedic 77; abdominal 23; urology/renal 19; thoracic: 13; breast: 4; pancreatic/biliary 1; plastic 1. Control mean age 54 (range 19 to 80); Intervention 1: 59 (21 to 83); Intervention 2: 58 (17 to 78). Control: 48 female, 27 male; Intervention 1: 44 female, 31 male; Intervention 2: 45 female; 30 male. Overall: 137 female, 88 male, 60.89% female | |
| Interventions |
Control: "routine information" – brief visit from anaesthetist; information on PCA would be limited. Author: anaesthetist had approximately 30 minutes to see 5 to 6 patients so brief; information not controlled. Intervention 1: Leaflet presented night before surgery. Included behavioural instruction – e.g. drug = morphine, could not overdose/become addicted; side effects; directions to seek help with side effects and how to use PCA; how long it took to work, why bleeped and why lock‐out period. Intervention 2: Interview: content as per leaflet; delivered night before surgery in 20‐minute interview; PCA pump taken to interview (behavioural instruction) |
|
| Outcomes |
Negative affect: anxiety (Hospital Anxiety and Depression Scale) 24 to 72 hours post‐surgery Negative affect: tension/anxiety (Profile of Mood States) 24 to 72 hours post‐surgery Pain: VAS days 1 to 5 post‐surgery Pain: word rating; days 1 to 5 post‐surgery |
|
| Notes | Author provided information regarding risk of bias and study methods | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p355: "Cluster randomisation was used because patients using PCA were often allocated to adjacent beds in the surgical wards…Fifteen patients were allocated to each cluster. Once recruited, there was a ‘clear out’ period to allow patients to be discharged. Recruitment to the next cluster then began. As the type of surgery could influence many of the outcome variables, the randomisation was stratified so that the intervention groups contained patients having similar operations". Author: "Clinstat [a computer program] was used to generate the allocations". |
| Allocation concealment (selection bias) | Unclear risk | No information in paper. Author responded but still unclear. As cluster‐randomized the issues in this paper are different to those using individual‐level randomization |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients appear to have been blinded to the fact that there was a study and that they would be receiving different information – both because of randomization methods to avoid contamination, and by what they were told – "asked to take part in ‘a survey of their opinions of their postoperative pain relief" (p355). However, the person giving the leaflet – and, in particular, giving the interview – would have known which condition they were in |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | p355: "The other researchers were blinded to the intervention that patients received". This suggested that the researcher presenting the questionnaire could be blind, but it does not state that the person giving the intervention was not the same person collecting outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported: 21 excluded for reasons to do with the hospital treatment provided (p355: 10 returned to ward without PCA; 7 admitted to intensive therapy unit; 2 had operations cancelled; 2 returned to ward with pethidine PCA) and 5 were too unwell to complete postoperative anxiety measures (p355). Unfortunately authors do not say how many lost from each group so unclear whether any likely bias. Additional information from authors: for those too unwell to complete HADS, 2 from control group, 3 interview group and 0 in written group. 2 patients failed to complete both pain measures on day 1; 6 on day 2; author uncertain which groups they were from. Given the size of the study (n = 246) we believe attrition at this level is unlikely to have significant impact |
| Selective reporting (reporting bias) | Unclear risk | Some mis‐match between measures reported in Methods and those in Results – e.g. pain word results not presented, only HADS and POMS anxiety measures. However, most reported results ns so this might be more down to word count than selective outcome reporting. No protocol referred to. Authors responded to contact: "All outcomes were published", but kept as `unclear' after discussion between extractors |
| ‘Intention‐to‐treat’ | Unclear risk | No information in paper. Author: "No the data were not analysed on an intention to treat basis. A few patients dropped out after recruitment, some went to ICU unscheduled, some declined PCA, some had the operation cancelled. The patient had to return to the ward on morphine PCA to be included in the analysis." Author uses vigorous standard of intention‐to‐treat rather than our criterion on whether kept in intervention group to which randomized ‐ the issues raised here are covered by our assessment of attrition bias |
| Other bias | Low risk | No other concerns |
Coslow 1998.
| Methods | Randomized controlled trial | |
| Participants | 30 women undergoing tubal ligation at a tertiary hospital in Michigan, USA (dates not given). Mean age: 33.7 (range: 21 to 47; intervention group mean: 33.4; control group mean 33.8 years) | |
| Interventions |
Control: usual care: unstructured education 1 hour before surgery; likely to include some procedural information Intervention: structured 20‐minute educational session 1 to 2 weeks before surgery, including sensory and procedural information and behavioural instruction |
|
| Outcomes | Length of stay in PACU and pain reported during stay in PACU | |
| Notes | The researchers use length of stay in PACU as an outcome. As these are ambulatory patients this seems likely to = length of stay in hospital Pain: little information about how measured, and in Results this outcome is reported as whether reported pain AND requested analgesia Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p8: "The first 30 patients who met the criteria were randomly assigned to one of two groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blind in the intervention group; not clear if control groups received information about the study and no information as to whether personnel were blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Both the intervention and checking of charts were done by "a nurse researcher" (p8) but no information as to whether or not this was the same person |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appear to be no missing data |
| Selective reporting (reporting bias) | High risk | Outcomes are reported for all measures specified in Results, but no published protocol. Insufficient data provided for entry into meta‐analysis (data not provided for length of stay) |
| ‘Intention‐to‐treat’ | Low risk | No missing data, all randomized participants apparently included in analyses, appears that all intervention participants received the intervention |
| Other bias | Low risk | No obvious other cause of bias |
Crowe 2003.
| Methods | Randomized controlled trial | |
| Participants | 132 participants undergoing arthroplasty (knee(s) or hip), Canada (dates not given). All had "complex needs" ‐ not functioning well, limited social support and/or comorbidities. Mean age control group: 70.7 years; mean age intervention group: 66.9. Control: 55 female, 13 male; Intervention: 51 female, 27 male; overall: 79.7% female | |
| Interventions |
Control: standard preoperative visit. Included some information and instruction about preoperative preparation, hospital stay and postoperative phase including temporary limitations (behavioural instruction and procedural information elements) Intervention: as control group plus "education package" including video, booklet (focusing on postoperative phase and use of equipment) plus individualized aspects e.g. meetings with occupational therapist; dietary, pharmacy and social work input as required. Procedural information and behavioural instruction |
|
| Outcomes | Length of stay: from health record | |
| Notes | Author provided information regarding interventions received and risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p90: "Subjects were allocated to one of the two groups by means of a random number table and a system of sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Paper states that sealed envelopes were used; author confirmed these were numbered and opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p91: "while in‐hospital, all clients received regular occupational therapy and physiotherapy services provided by staff from the program. These staff were frequently aware of the allocation to either control or rehabilitation for each client. Blinding was not possible since many clients choose to discuss previous rehabilitation with staff members" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p92: "assessment of these outcomes were collected by an assessor who was blind to the group allocation for each subject" (no information as to methods used, or whether this was effective) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The 1 participant who had no data for analysis was reported; no re‐inclusion – this seems appropriate as the participant did not undergo surgery. "One subject attended multi disciplinary rehabilitation at the day hospital program, was pleased with the improvement in his functional status and cancelled the surgical procedure. His results were not included in the postoperative analysis" p93 |
| Selective reporting (reporting bias) | Low risk | No report of a published protocol. It initially appeared that there may be other outcomes: "secondary outcomes included [reviewer RP's bold] the actual length of hospital stay and the location to which each client was discharged" (p92). However, author reported by email that all outcomes that were measured were reported |
| ‘Intention‐to‐treat’ | Low risk | The authors do not explicitly address this in the paper. However, the numbers of participants in analysed matches the numbers randomized and the authors confirmed analysis was by intention‐to‐treat |
| Other bias | Low risk | No other important concerns |
Cupples 1990.
| Methods | Randomized controlled trial | |
| Participants | 40 patients undergoing coronary artery bypass graft at a 650‐bed community hospital in a metropolitan area of the East Coast (country not stated – assume USA; dates of data collection also not provided). Most (38, 95%) were male, 2 female. Mean age: 59.4 years, age range 43 to 70 | |
| Interventions |
Control: "routine post‐admission preoperative education provided by hospital personnel" Intervention: 5 to 14 days before admission, 45 to 60‐minute 1‐to‐1 session, covering anatomy, physiology, hospital routines, possible complications. Information included pain, intensive care unit (ICU) experiences, coughing and deep‐breathing exercises. Follow‐up phone call 4 days before operation for questions and answers.Procedural and sensory information; behavioural instruction |
|
| Outcomes | Negative affect 4 days post‐surgery: 1. Total Mood Disturbance score of Profile of Mood States 2. STAI state anxiety |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomly assigned to experimental or control groups until 20 subjects accrued in each group". Sounds suspiciously as if allocation was carried out alternatively, but not clear – could have been randomized in pairs |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both participant and investigator delivering intervention would have been aware if received additional preoperative teaching |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition reported, but sample size for postoperative data collection is also not reported (and degrees of freedom appear to be adjusted so cannot use to verify) |
| Selective reporting (reporting bias) | Unclear risk | Appear to have included all intended outcome measures, but no reference to a protocol document to check this. Data are presented for outcomes. SDs are not available but should be able to calculate from t‐values |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Cuñado Barrio 1999.
| Methods | Randomized controlled trial | |
| Participants | 84 patients undergoing hip or knee arthroplasty at the University Hospital, Comunidad de Madrid, Spain, October 1996 to March 1997 and October to December 1997. Control group: 19 men (45%), 23 women; intervention 9 men (21%), 33 women. Control mean age 64 years (SD 10); intervention mean age 66 (SD 11) | |
| Interventions |
Control: routine care. Visit from nurse – 10 minutes, 2 days before surgery. Conversation about general and arbitrary themes; no structured programme about process of surgery Intervention: nurse visit with individual information structured by before, during and after the surgery. Tried to reply to questions; psychological support to reduce preoperative state anxiety. 2 days pre‐surgery, 20 minutes. Procedural information |
|
| Outcomes |
Negative affect: Spanish version of STAI‐State, 4 or 5 days after surgery Postoperative length of stay |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 2 computer‐generated randomizations ‐ one for knee and one for hip replacement group. Variable blocks |
| Allocation concealment (selection bias) | Unclear risk | Numbered envelopes ‐ sealed and "correlative" ‐ rating unclear because we do not know if these were also opaque, or what happened with these envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor personnel blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Many more lost to follow‐up in Control group (7 versus 1). (2 did not have surgery due to fear, 2 refused to answer postoperative questionnaires, 1 did not consent to postoperative transfusion, 2 had epidurals and 1 had surgery deferred) Gender balance different in 2 groups (19 versus 9 men) – problem as women have higher anxiety |
| Selective reporting (reporting bias) | Unclear risk | No clear evidence of this but no reference to a protocol document |
| ‘Intention‐to‐treat’ | Unclear risk | Seems likely but not stated |
| Other bias | Low risk | No other concerns |
D'Lima 1996.
| Methods | Randomized controlled trial | |
| Participants | 30 patients undergoing total knee replacement surgery in California, USA (dates not provided). Control group mean age 69.5 (SD 6.5); Intervention 1 (physical therapy) mean age 68.5 (SD 4.6); Intervention 2 (cardiovascular conditioning) mean age 71.6 (SD 6.6). Control: 5 male, 5 female; Intervention 1: 3 male, 7 female; Intervention 2: 8 male 2 female. Overall: 16 (53.3%) male; 14 female | |
| Interventions |
Control: 45‐minute preoperative meeting with physical therapist; given information about postoperative exercise regimen, including straight leg raises, knee strengthening and range of motion exercises.Behavioural instruction Intervention 1: 18 physical therapy sessions: 3 x 45‐minute sessions/week. Programme to strengthen extremities and improve knee range of motionBehavioural instruction (beyond Control group) Intervention 2: Cardiovascular conditioning. 18 sessions, 3 x 45‐minute sessions/week. Cardiovascular conditioning programme designed to improve fitness.Behavioural instruction |
|
| Outcomes |
Pain: pain scale from Hospital for Special Surgery Knee Rating (high scores = less pain). 3 weeks post‐surgery Behavioural recovery: function scale from Hospital for Special Surgery Knee Rating. High score = better function Length of stay |
|
| Notes | Authors provided some information regarding risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p175: "A computer generated randomisation list was used to assign patients to 1 of the following treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unless not given full information before consent, patients would have known; the staff implementing the intervention would certainly have known |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition reported – at all – which seems odd as followed up to 48 weeks |
| Selective reporting (reporting bias) | Low risk | No mention of protocol but author confirmed no outcomes measured that were not reported. Unclear why reported subscale scores for the Hospital for Special Surgery Knee Rating but not other measures (Arthritis Impact Measurement Scale and Quality of Well Being) |
| ‘Intention‐to‐treat’ | Unclear risk | No information |
| Other bias | Low risk | No other concerns |
Daltroy 1998.
| Methods | Randomized controlled trial | |
| Participants | 112 patients undergoing total hip or knee arthroplasty at a "large university teaching hospital" in Boston, USA. Recruited March 1985 to December 1987. Full sample: mean age 64 (SD 12, range 20 to 88); 66% female | |
| Interventions |
Control: appears receive usual preoperative preparation, "included instructions in coughing etc" (behavioural instruction) Intervention: as controls (behavioural instruction) and information intervention (procedural and sensory information) the day before surgery, 12‐minute "audiotape slide program", which included hospital processes, surgery and rehabilitation, postoperative pain and immobility, rehabilitation, lights and noises, dietary and smoking restrictions. Leaflet regarding postoperative milestones |
|
| Outcomes |
Length of stay until discharge or second surgery Negative affect: state anxiety (STAI), day 4 after surgery Pain: measure not clearly described, assume same as pre‐surgery: took mean of 3 5‐point scales assessing pain at night, resting and when active. Day 4 after surgery |
|
| Notes | Also had a relaxation condition in a 2 x 2 design (no intervention, information, relaxation, information and relaxation, n = 222) but included relaxation intervention included a postoperative component so not included in review Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p471: "assigned randomly to one of the treatment groups. Randomization was stratified by joint (hip or knee) and patient age" – no information on how allocation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information about blinding of participants. p471: "all questionnaires and the intervention itself were administered by either of two research nurses" – very unlikely that either participants or those administering the intervention were blind. Hospital staff do, however, seem to have been blind – apparently successfully (p473) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "all questionnaires and the intervention itself were administered by either of two research nurses" (p471) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition is reported but not broken down by group. Overall study: no. randomized: 222, but only 2 (of 4) groups fit our inclusion criteria so we are interested in 112. Of whole sample, 1 outlier excluded (42‐day stay); 5 participants had incomplete follow‐up questionnaire data and were excluded from all analyses except length of stay – leaving 216 participants for most analyses (p473). Unfortunately, no information as to which groups these participants are from |
| Selective reporting (reporting bias) | High risk | Researchers decided not to do all analyses with Relaxation groups as the patients did not use the intervention as much as they would have liked (p474), suggesting that they may have selectively reported outcomes. However, as the Relaxation intervention did not fit our criteria it is not clear whether other selective reporting occurred, which would have biased the findings of interest to this review. High risk because data needed for meta‐analysis are not provided |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
DeLong 1970.
| Methods | Randomized controlled trial | |
| Participants | Recruited (and randomized?) 70 individuals undergoing removal of gall bladder or uterus at the Kaiser Foundation Hospital, Los Angeles, USA (dates not provided). All were female; mean age 44.33 (SD 10.71), age range 23 to 64 | |
| Interventions |
Control: General information. 12‐minute tape, day before surgery. General information about hospital and its facilities, procedures of clinic visits, physical examinations, admission procedure, what patients need to bring and what happens on the ward, hospital routines, how to travel home Procedural information Intervention: Specific information. 12‐minute tape: Information about aetiology and reasons for surgery, preoperative preparation and postoperative information. Includes information on procedures e.g. shaving, enema; that might not sleep well and should ask for medications if think needed. Told about preoperative procedures e.g. not eating or drinking, needing to remove makeup, that will be given a sedative that causes dry mouth and an injection causing them to sleep. Postoperative information includes where they will wake, when return to room; that will experience pain and can ask for pain medication; that moving and coughing will be uncomfortable but essential – provided with advice on how to make coughing more comfortable. Also information re. expected timescale for recovery of activities. Procedural information (different to Control); sensory information; behavioural recovery |
|
| Outcomes |
Negative affect: STAI (state and trait), day 5 or 6 post‐surgery Length of stay |
|
| Notes |
Note: classified participants by coping style using Coper‐Avoider Sentence Completion Test – copers/avoiders/non‐specific defenders Unpublished data: PhD thesis Could not locate author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p39: "Ss were assigned randomly to the Specific or General Information condition according to their coping style" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Seems likely that participants were blind as were given the same information about the study and the tape recordings. However, it would appear that the researcher was present and played the tape and so the researcher would have known the grouping |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It does not state by whom the outcome STAI measure was presented but there is no mention of blinding (it seems likely that it would have been the researcher but cannot assume this) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No./% participants lost to follow‐up: p33: "Data from six subjects were discarded for the following reasons: 1) three had a common duct exploration, 2) one sustained a bladder perforation during surgery, and 3) two planned surgeries were not completed when ovarian malignancies were discovered" So, 6 lost from overall sample (6 of 70 = 8.6%). In addition, postoperative anxiety measures obtained on 57 participants (p72) – 2 discharged before day 5; 2 had prolonged recoveries and did not feel able to complete forms; 3 discharged on day 6 before researcher’s visit. States that "The subjects appeared to be equally distributed among groups" but does not give breakdowns by group. Total loss (anxiety outcome) = 13/70 = 18.6% Rating high risk as attrition not provided by group and reaches a large proportion for anxiety outcome |
| Selective reporting (reporting bias) | High risk | No evidence of selective reporting of outcomes but no protocol document to refer to. There may be sufficient details for length of stay to include in meta‐analysis but insufficient details for negative affect (anxiety) |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Dewar 2003.
| Methods | Randomized controlled trial | |
| Participants | 254 patients undergoing following surgeries at a large urban hospital in British Columbia, Canada (all those undergoing particular procedures in 5‐month period): hernia surgery (32), mammary reduction/enhancement (36), arthroscopies (69), anal surgery (85) (data of these 222 analysed). Control mean age: 41.4, intervention group: 42.5. Control, 70 male, 48 female. Intervention: 65 male, 39 female. Overall, 135 male, 87 female, 60.8% male | |
| Interventions |
Control: no information Intervention: preoperative teaching session "about post‐operative pain control" + pamphlet on pain management after surgery. Precise content unclear but states based on Agency for Health Care Policy and Research guidelines. From guidelines, likely content: information about what pain is and that it can indicate a problem; discusses drug and non‐drug options, including relaxation exercises before surgery, and after surgery, e.g. relaxation, hot or cold packs, distraction, positive thinking. Before surgery: instructs to ask about what to expect of the pain, to discuss pain control options, to talk about schedule for pain medications (and describes likely procedures for administering medications); recommends making pain control plan. After surgery: recommends e.g. taking medication when pain first starts; helping staff measure pain (describes possible scales); telling staff about pain that does not go away and reassuring not to worry about being a "bother"; describes a rhythmic breathing method for pain control by relaxation. Procedural information, behavioural instruction, cognitive intervention, relaxation |
|
| Outcomes | Pain: Brief Pain Inventory: numerical rating scale from 0 to 10; evening after surgery | |
| Notes | Attempted to contact authors; no reply received. Study includes a postoperative component to the intervention, but this is administered after the first postoperative pain measurement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p82: "the nurse researcher checked a pre‐determined list of random numbers to determine if the patient was randomly assigned to the control or intervention group. The random numbers were selected using a randomised block design to ensure that equal numbers of control and intervention participants were scheduled for each of the four main surgical types" |
| Allocation concealment (selection bias) | High risk | p82: "the nurse researcher checked a pre‐determined list of random numbers to determine if the patient was randomly assigned to the control or intervention group." – looks like would be possible to foresee allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None stated. It would appear that nurse researcher delivered intervention (p82) and would have known. By nature of the intervention, if patients fully informed, they would have known whether or not received intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | p83: "The researchers did not see the patients after surgery" – so unlikely could have affected patients record in diary in evening after surgery. However, not stated whether those involved in taking outcome assessment were blinded. This may be over‐cautious as would have been discharged from hospital |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition is reported but not by group (p83) so not possible to determine if could have resulted in bias. 34 lost to follow‐up. 16: "protocol failures" (stayed overnight in hospital postoperatively or subsequently admitted elsewhere). Of remaining 238, 22 "mailed completed pain diaries to the researchers" ‐ this is the number for which all data appear to be reported |
| Selective reporting (reporting bias) | High risk | No evidence of outcomes not being reported, but description of measures is fairly vague, and no reference to a protocol document. High risk because insufficient data provided to enter into meta‐analysis |
| ‘Intention‐to‐treat’ | Unclear risk | Participants who did not follow protocol in terms of staying overnight/leaving diaries at hospital/being admitted elsewhere were excluded from analysis (p83). However, p85: asked participants if read instructions – this does not appear to have affected whether included in analysis. No clear statement – suggest ‘unclear’ |
| Other bias | Low risk | No other concerns |
Doering 2000.
| Methods | Randomized controlled trial | |
| Participants | 100 participants undergoing total hip replacement in Department of Orthopedics, Innsbruck University Hospital, Innsbruck, Austria; ethical approval received November 1996. Intervention age 58.7 ± 10.8; control group 60.4 ± 8.7 (assume mean and SDs as paper refers to means and SDs elsewhere for another variable, but not actually stated). Intervention group 21 female, 25 male; control group 17 female 37 male. Overall, 62 (65%) male | |
| Interventions |
Control: no information Intervention: 12‐minute video shown evening before surgery from perspective of a patient who underwent surgery. Includes showing arrival at hospital, talking with nurse, preoperative procedures, procedures of going to theatre, can hear noises which are explained. Also shows postoperative events – blood transfusion, return to ward, staff visits, ambulation with help from physiotherapist and discharge.Procedural information, sensory information |
|
| Outcomes |
Negative affect: STAI state anxiety, first 3 postoperative days Pain: 100 mm VAS actual pain, first 3 postoperative days Length of stay |
|
| Notes | Author provided details on intervention content (including sending video clips) and risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p366: "Patients who agreed to take part were randomly assigned to the preparation or control group". Author: "We used lists of random numbers" |
| Allocation concealment (selection bias) | High risk | No information. Author: allocation concealment not carried out |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No attention control so, if knew possibility of listening to tape, participants were not blind. Investigator listed to tape with intervention participants – so not blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p367: "Questionnaires…were also distributed to the patients" (i.e. does not state how); for length of stay, "Chart records were evaluated by the first author only" – unclear whether 1st author was the investigator who enrolled participants/sat with them when they listened to tape. Author reported that researchers collecting outcome measures were blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition reported. Unclear whether none occurred or not reported. Author: "No participant of the study was lost" |
| Selective reporting (reporting bias) | Low risk | No evidence of measured outcomes not being reported, but no reference of protocol/registration with which to check this. However, author reported that no outcome measures were not reported |
| ‘Intention‐to‐treat’ | Low risk | No information. Author: "Yes, these were ITT analyses" |
| Other bias | Unclear risk | Concern: of 145 eligible participants, 45 declined, many because they thought viewing the videotape would be threatening/distressing (n = 26) or because they did not want information (n = 7). It seems likely that consent occurred prior to randomization (p366) but this is not entirely clear. If participants were randomized before consent then this is a limitation of generalization rather than bias (i.e. have lost a group of patients that may be more anxious/have different coping styles). However, if this occurred after randomization then bias is clearly an issue. Sample size of groups: 46 for intervention, 54 for control – suspicious that intervention group smaller – further details on randomization procedure would also help |
Done 1998.
| Methods | Randomized controlled trial | |
| Participants | 130 patients were randomized (127 analysed) from John James Memorial Hospital and Lidia Perin Memorial Hospital, New South Wales, Australia (dates not provided). Intervention group mean age 35, control mean age 34 years. Intervention: 28 male, 35 female. Control group: 28 male, 36 female. Overall, 71 (56%) female, 56 male | |
| Interventions |
Control: standard care Intervention: 7‐minute video between recruitment and going to admissions area. Included information about the processes IV cannulation, monitoring, observation by anaesthesiologist, follow‐up care and treatment of pain and nausea; also risks of nausea, vomiting, sore throat, memory of extubation, shivering, awareness, anaphylaxis, dental damage. Procedural and sensory information |
|
| Outcomes | Negative affect: 8‐item version of STAI (state and trait versions) at discharge. Maximum score: 32 | |
| Notes | Author was not contacted as insufficient time prior to analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p532: "Patients were then randomly allocated to the video or nonvideo group using a computerized random number table" |
| Allocation concealment (selection bias) | Unclear risk | p532: "To maintain blinding, the envelopes containing the allocation of the intervention were developed by a third party. The researcher was blinded to the allocation of the intervention until after the completion of the first STAI" Need more information – were the envelopes numbered opaque, sealed, numbered? |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p532 – researcher was in room with patient watching video; patient would know whether or not received video |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated (seems unlikely) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow chart p532 states 127 recruited, but p533: "one patient did not wish to be recruited, two patients dropped out because they were transferred to the ward rather than discharged, one could not read the STAI, and one was inadequately prepared for day surgery. The final sample size analysed was 127 patients". Hence appears 132 eligible and 131 randomized. No information as to which patients were from which group |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting, but no mention of protocol to which we could refer. Data for meta‐analysis provided, albeit in figure rather than table format |
| ‘Intention‐to‐treat’ | High risk | p532: "There was no discussion about the video or the pending procedure with the patient unless the patient wished to stop the video. If this discussion was required, the patient was excluded from the study" – suggests not included in analysis if did not receive the intervention as intended – and therefore not intention‐to‐treat |
| Other bias | Low risk | No other concerns |
Elsass 1987.
| Methods | Randomized controlled trial | |
| Participants | Included 81 people undergoing minor surgery in Denmark (inguinal hernia (n = 54) and varicose vein surgery (n = 27), but appears likely that 90 were randomized (dates not given). Age range: 31 to 62; median 46 years. 45 (55.6%) female; 36 male | |
| Interventions |
Control: "routine information" 5‐minute visit at bedside by anaesthesiologist. Included where and how anaesthesia administered, that would induce sleep with no pain or sensation.Procedural and sensory information Intervention: 20‐minute session in private room with anaesthesiologist. More detailed information given – "a thorough account of the various stages of the anaesthetic/surgical procedure".Procedural information (more than controls) |
|
| Outcomes | Negative affect: anxiety: STAI state. At 1 ½ hours after surgery and day after surgery | |
| Notes | Also analysed data by whether experienced/inexperienced in receiving anaesthesia Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p579: "once a patient was admitted to the study, he or she was allocated at random to one of the two groups, receiving either routine or detailed information about the anaesthetic‐surgical procedure" |
| Allocation concealment (selection bias) | Unclear risk | p579: "once a patient was admitted to the study, he or she was allocated at random to one of the two groups, receiving either routine or detailed information about the anaesthetic‐surgical procedure" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Three physicians and authors provided all the information – seems highly unlikely they would be blind. The control condition information was given in 5 minutes at patients' beds, whereas information: 20 minutes in private room, so seems very unlikely patients blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided as to who took outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No./% participants lost to follow‐up: p579: "Eighty‐one patients were included in the study" [of 90]. 5 patients excluded: "lack of cooperation and incomplete answering of the questionnaire. Four patients were excluded because they later gave information that they had taken tranquillizers during their hospitalisation". Therefore 9/90 lost to follow‐up: 10% No information provided as to how many from each group – or which reason by which group |
| Selective reporting (reporting bias) | High risk | No mention of a protocol, and data not provided in a form that we could use in meta‐analysis at present – unclear even whether means or medians presented; no SD/SE (report age in medians and use Mann‐Whitney in analysis) |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper. Given that participants were excluded if "lack of co‐operation" this seems unlikely |
| Other bias | Unclear risk | No other concerns |
Enqvist 1997.
| Methods | Randomized controlled trial | |
| Participants | 50 participants randomized; results presented for 48. Women undergoing breast reduction surgery in Södersjukhuset Hospital, Stockholm, Sweden (dates not provided). Control group mean age: 41.5; intervention group mean age 39 | |
| Interventions |
Control: no information; assume usual care Intervention: Relaxation and self‐hypnosis provided via an audio tape given 6‐8 days pre‐surgery; daily listening recommended. Hypnosis instructions focused on minimising nausea and vomiting; also included reduction of pain, stress and anxiety |
|
| Outcomes | Pain: "day 1‐5"; measured with "10‐degree VAS" | |
| Notes | Unclear exactly what the pain measure asked, and when it was presented Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p1029: "patients were assigned randomly to a control group or to a hypnosis group using the envelope technique" |
| Allocation concealment (selection bias) | Unclear risk | No further information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | p1029: "The groups were blinded to the surgeons, anaesthetists and the other personnel involved". It does not say how they were blinded but, as the intervention was a tape that seems to have been sent to women (it just says they "received" the tape 6 to 8 days pre‐surgery), it would not have been challenging to blind other staff. However, it seems likely that participants would not have been blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | If the outcome assessors are included in "groups were blinded to...other personnel involved" then they were blinded, but no specific information related to this |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants were excluded from intervention group (p1030); as 1 was excluded for not listening to the tape this could be a biasing factor (would not have been excluded from the control group) (the other was excluded for not completing the outcome measures). As the exclusion for not listening to the tape is detailed in `Intention‐to‐treat', the overall bias caused by 2 participants being missing from one group is here denoted as `unclear' |
| Selective reporting (reporting bias) | High risk | Some concern: measured "memories from the operation and dreams from the anaesthesia" but findings were not reported in Results. It may be that these were qualitative findings and were never intended to be included in quantitative analysis, but this is unclear. High risk: pain data for meta‐analysis not provided |
| ‘Intention‐to‐treat’ | High risk | Not intention‐to‐treat: the hypnosis group participant who did not listen to the tape was excluded from analysis |
| Other bias | Low risk | No other concerns |
Felton 1976.
| Methods | Randomized controlled trial | |
| Participants | 62 adults aged 19‐71 undergoing major surgery in two large medical centres, Eastern US, January to December 1974. 49 male, 13 female (79% male) | |
| Interventions |
Control: formalized version of routine preparation: information re. preoperative and postoperative procedures; need to move about postoperatively 15 minutes Procedural information and behavioural instruction Intervention1: ‘Experimental’: average 88 minute meeting with nurse – information provided in response to patient questions; information on procedures and equipment to be used; book of photos to generate discussion. Description of procedures, postoperative discomfort, expectations of care. 2 films re. preventing pneumonia and circulatory complications followed by demonstration and practice of techniques for behaviours e.g. breathing, moving. Procedural and sensory information; behavioural instruction Intervention 2: ‘Communication’: average 62‐minute meeting with nurse; nurse elicited thoughts and feelings re. surgery, non‐judgemental open questions. Asked to talk re. past stressful experience, which might help in dealing with present to improve problem solving. Did not provide information; aimed to help participant decide how might obtain information. Cognitive intervention |
|
| Outcomes |
Negative affect: anxiety: Multiple Affect Adjective Check List. Day 4 or 5 post‐surgery Length of stay |
|
| Notes | Could not locate author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p87: "Subjects were distributed into three groups (experimental, communication, and control) by stratified random assignment, holding constant factors of sex, age, and site of surgery". Does not state how random sequence generated |
| Allocation concealment (selection bias) | High risk | "One nurse, Huss, assigned the subjects to one of three groups, documented the preparation received by the control subjects, obtained patient and physician written consent and physician philosophy, patient record and social data regarding each subject and administered tests of vital capacity and the POI and MAACL". Appears to be high risk as same person made group assignment and consented patients |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p87: "A second nurse conducted the experimental nursing protocol, while a third nurse, Payne, carried out the communications nursing protocol" – the personnel delivering interventions were not blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "One nurse, Huss, assigned the subjects to one of three groups, documented the preparation received by the control subjects… and administered tests of vital capacity and the POI and MAACL" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition reported so unclear – could have been no attrition – or just not reported. Odd: uneven sample size across groups (control n = 25, experimental n = 25, communication n = 12) |
| Selective reporting (reporting bias) | Unclear risk | No reference to protocol. Measures mentioned in Methods were reported in Findings but various measures not reported in Methods were also reported in Findings (e.g. length of stay) so low confidence in reporting |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Unclear risk | Surprising: N for Control and Experimental groups = 25; N for Communication Group = 12. Suggests EITHER excess attrition in Communication Group OR not randomly allocated |
Ferrara 2008.
| Methods | Randomized controlled trial | |
| Participants | 23 people with end‐stage osteoarthritis undergoing total hip replacement surgery in the Orthopaedic Department of the University Hospital `Agostino Gemelli' of Rome, Italy, January 2006 to January 2007. Intervention group mean age 63.82 (SD 9.01); control 63.08 (SD 6.89). Intervention: 7 female, 4 male; control group: 7 female, 5 male. Overall, 60.87% female | |
| Interventions |
Control: appears to be standard care Intervention: from 1 month pre‐surgery, group and individual exercises 5 days/week, admin by physiotherapist for 60 minutes/day. Included strength and flexibility, cardiovascular exercise, posture, advice on movements to avoid, use of devices, correct posture and daily tasks (behavioural instruction) |
|
| Outcomes |
Behavioural recovery: range of motion at hip abduction and external rotation; disability (Barthel Index); functional status (from WOMAC) Pain: WOMAC subscale; VAS. All: 15 days and 4 weeks postoperative |
|
| Notes | Some information from authors regarding risk of bias (selective reporting ‐ stage 1 contact response) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p978: "The patients were randomised using a table of random numbers. The even numbers were allocated to the control group and the odd numbers to the study group" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p978: "pre‐operative and post‐operative treatments were performed by the same physical therapist, who was not blinded" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p978: "outcome measures were administered by two research assistants and two physicians, blinded, who had previously been trained in all the outcome tools" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear description of attrition and exclusions (flow chart p980). 2 dropped out from control group but unlikely due to study condition as they dropped out after 1 month postoperatively (although removed from all analyses reported in paper) |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol, and many of the outcome measures findings were not reported in detail at postoperative time points – in particular, the 15‐day and 4‐week time points of interest to review. However, the study’s endpoint was 3 months so may have been removed to save space rather than as a result of selective reporting, so not clear whether high risk of bias as a result. Absolute scores even at 3 months not reported – change scores only. Email from authors: "We reported in the article all outcome measures analysed" |
| ‘Intention‐to‐treat’ | Unclear risk | By the numbers of participants in the data reported, would appear that participants were analysed according to group to which randomized, but not clearly stated |
| Other bias | Low risk | No other concerns |
Field 1974.
| Methods | Randomized controlled trial | |
| Participants | 60 patients undergoing orthopaedic surgery in US (probably the Veterans Administration Hospital, Brooklyn but not clearly stated; dates not given). Mixed: major and minor surgery, procedures included laminectomies, excisions, skin grafts, amputations. 58 (97.7%) male; 2 female. Age details not provided | |
| Interventions |
Control: 15‐minute tape recording describing facilities available in hospital, day before surgery Intervention: 20‐minute tape recording, day before surgery. Suggestions of relaxation, sleep, eye closure, comfort, freedom from pain during/after operation, quick recovery and confidence; description of operative procedures. Hypnosis, procedural information |
|
| Outcomes |
Length of stay Pain: between 2 and 7 days postoperative, no further information provided |
|
| Notes | Could not locate author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p55 "were randomly assigned to an experimental or control condition" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants appear to have been blind – "tape recordings were presented as a part of the usual ward routine". As tapes, possible that staff delivering them were blind too – but as staff rated extent to which participants followed the experimental instructions this seems extremely unlikely p56: "The surgeons and other ward personnel were blind as to which recording each patient had heard" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information as to who collected length of stay data from records. The postoperative interview was conducted "by the assistant who played the recording" (p56) – so would not have been blind for postoperative pain outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition reported and in results mentioned Ns of 30 for both groups (i.e. a full response rate). However, N is not provided for each outcome so unclear as to whether or not there were any missing data |
| Selective reporting (reporting bias) | High risk | A number of variables were mentioned in Results that were not mentioned in Methods so unclear as to whether other constructs were also measured. Data sufficient for meta‐analysis are not provided (neither means not SDs presented) |
| ‘Intention‐to‐treat’ | Unclear risk | Not explicitly stated. As the intervention was delivered as if it were part of the usual ward routine, it is unlikely that participants were switched by participant choice – but not impossible researcher mistakes could be made |
| Other bias | Low risk | None |
Fortin 1976.
| Methods | Randomized controlled trial | |
| Participants | 69 patients, mean age 41 years (control group mean age 40.5; intervention group 41.8), 87% female, 13% male. A large community hospital, Montreal, Canada, October 1973 to August 1974. All underwent general anaesthesia. Surgery types: herniorrhaphy, cholecystectomy, intra‐pelvic surgery (primarily hysterectomies) | |
| Interventions |
Control: "all preadmission procedures except the education component" (no further information). Preadmission procedures: 15 to 20 days pre‐surgery Intervention: intervention designed to "accelerate…return to usual activities" e.g. respiratory and muscular exercises and techniques to change position – behavioural instruction; procedural information also likely (includes "orientation" to surgical experience, respiratory and muscular exercises for preoperative and postoperative periods; other information. Conducted as part of pre‐admission procedures |
|
| Outcomes |
Behavioural recovery: 2 days: "inpatient ambulatory activity" (IAA). Ability to do physical activities at hospital in immediate postoperative period – e.g. movements in bed, get up, walk. 10 days: ‘activities of daily living’ (ADL). Capacity to perform tasks appropriate to normal life at home Length of stay in hospital |
|
| Notes | Authors sent information on intervention, numerical data and `Risk of bias' table | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p14, matched pairs by surgery type, age and sex – "Within pairs, using random number tables, one member was assigned to participate in the PEPCE programme…" Small no. unmatched patients were randomly assigned to either intervention or control group |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely to have been possible to blind if fully informed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p15: "interviewers were kept unaware of the specific objectives of the study…were not told whether in the experimental or control groups, although the status of some respondents was occasionally deduced". Although "occasionally deduced", this bias seems to have been minimized overall |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete data at 2 days, 2 missing (1 from each group) at 10 days for primary outcome (behavioural recovery). Not stated for other outcomes (can deduce overall sample size for length of hospital stay as t‐test df = 67) |
| Selective reporting (reporting bias) | Low risk | Details of outcomes lacking (e.g. just "ns" (non‐significant)) but no obvious omissions Information from author: "we did not study other variables than those mentioned in the article" |
| ‘Intention‐to‐treat’ | Low risk | Numbers fit intention‐to‐treat but not clearly stated. Response from authors, however, indicated that no participant changed grouping |
| Other bias | Low risk | None |
Fortin 1983.
| Methods | Randomized controlled trial | |
| Participants | Recruited 61 patients undergoing routine elective cholecystectomy or cholecystectomy with intraoperative cholangram at 2 "nonfederal, short term" hospitals, Rhode Island USA mid‐November 1982 to July 1983. Data reported for 52 who continued to fit criteria as progressed through study. Age ranged from 21 to 71, mean 45.42, SD 14.3. Intervention mean age 42 (SD 14.22), control group mean age: 49 years (SD 13.62). Overall, 41 female, 11 male (78.8% female). Intervention group: 6 male, 21 female; control group 5 male, 20 female | |
| Interventions |
Control: day prior to surgery, taped message ‐ 3‐minute message followed by 7‐minute narrated exercise – instructions in exercises to be practised postoperation.Behavioural instruction Intervention: As controls, plus second tape: 5 minutes describing postoperative sensations at incision site; 5‐minute guided practice in muscle relaxation and rhythmic breathing; remained in relaxed state for 5 minutes and procedure then reviewed (total: 30 minutes). Also: instruction: to request pain medications when desired.Sensory information, relaxation, behavioural instruction |
|
| Outcomes |
Pain: Pain Rating Index – Rank of McGill Pain Questionnaire Negative affect: STAI state anxiety Both measures: day 3 post‐surgery |
|
| Notes | Baseline trait (but not state) anxiety difference: control group: higher trait anxiety. However, reported comparing adjusted and unadjusted means ‐ indicated little impact of higher baseline A‐Trait scores for controls on outcomes (their analysis: ANCOVA, controlling for baseline state and trait anxiety) Author is deceased |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p50: "A table of random numbers was used to assign potential subjects to group 1 and group 2 and to randomly assign them to the experimental and control condition" |
| Allocation concealment (selection bias) | High risk | p59: "Each subject was given a brief verbal overview of the study objectives and the condition to which they would be exposed" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Seems unlikely – participants were told what intervention they would receive but may not know whether that was the control or intervention group. However, given time difference, the person administering the intervention would have known |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | p61 – "the researcher visited each subject" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No./% participants lost to follow‐up: p61: if met preliminary criteria, were kept in study, but data only analysed if scored at least 18 on Shipley‐Hartford Vocabulary Test and did not undergo more extensive surgery/have postoperative complications p64: of the 61, 3 scored too low on Shipley‐Hartford vocabulary test, 2: more extensive surgery, 3 discharged before 3rd postoperative day, 1 had extensive psychiatric history that was not noted prior to surgery. These (6 intervention, 3 control) "were excluded from final sample as did not meet criteria for inclusion". Remaining sample size: n = 52 So, no attrition after consenting so long as continued to fit inclusion criteria |
| Selective reporting (reporting bias) | Unclear risk | Report 2 dependent variables with means and SDs. Appear to have conducted analysis according to plan, but no protocol document. Room for selective outcome reporting – e.g. pain VAS taken as secondary outcome and only reported as correlations (appears to be treated as planned); mentioned comparisons of vital signs in Results but did not mention plans for this in Methods |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Furze 2009.
| Methods | Randomized controlled trial | |
| Participants | 204 patients undergoing coronary artery bypass graft surgery. Control group: mean age (SD) 65.29 (8.51); intervention group: 64.8 (8.51). Control group: 85% male. Intervention group: 76% male. Overall: 80.4% male; 19.6% female. Setting: UK NHS hospital trust (Hull and East Yorkshire). Recruitment phase: 1 October 2003 to 31 December 2014 | |
| Interventions | Both groups: 1st interview of 45 to 60 minutes followed by regular follow‐up phone calls until admission Control: participant described illness experience, given verbal advice on risk factors; description of operation and after‐care (procedural information?) Intervention: aimed to dispel misconceptions, worked with patient to agree and set goals to reduce risk factors; relaxation programme. Also information about what to expect during hospital stay and recovery period (cognitive intervention, relaxation, procedural information) |
|
| Outcomes | Length of stay in hospital | |
| Notes | Other outcome measures were taken but outside study's time frame Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p53 – Done by researcher not otherwise involved: "computer‐based random‐sequence generation, stratified by 4 surgeons. Remote randomization to groups was via a remote telephone service manned by staff not otherwise involved" |
| Allocation concealment (selection bias) | Low risk | p53: Those involved in randomization – not otherwise involved. Remote telephone service |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No measures reported of blinding participants. Possible that participants did not know, but seems staff providing intervention would have known |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p53: "Interventions were delivered by a nurse not involved in collecting follow‐up data. All data entry and analysis were blind to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and reasons reported; similar reasons in both groups: Intervention: 11 (1 withdrawn (had MI), 10 – no reason). Plus 3 not operated on. Control: 11 (2 withdrawn with MI, 9 – no reason). Plus 3 – not operated on. BUT in analysis: n = 204: linear interpolation of missing data (all details in flowchart p54) |
| Selective reporting (reporting bias) | Unclear risk | Authors are clear as to which are primary and secondary outcomes. At later time points, details of each outcome are not provided, but a summary of findings are (p56). However, protocol not mentioned |
| ‘Intention‐to‐treat’ | Low risk | p54 – clear that followed intention‐to‐treat |
| Other bias | Low risk | Considered whether contamination weakening findings: p52 – "it was accepted that there was a possibility of some contamination in the delivery of the interventions. For example, smokers in both arms of the study were advised to attend NHS smoking cessation groups". However, "In order to keep contamination between the interventions to a minimum, a prompt sheet was used to structure the interviews and a checklist of questions for the telephone follow‐up was used for each intervention. The written materials were different for each intervention". As the researchers state that both groups had the same advice re. smoking, and in comparison with other studies with similar methods, it was felt that, overall, risk of bias was low |
Gilbey 2003.
| Methods | Randomized controlled trial | |
| Participants | Setting: Sir Charles Gairdner Hospital, Western Australia, participants recruited over 24 months from January 1997. 76 patients undergoing total knee arthroplasty were randomized (data reported for n = 57). Intervention (n = 37): mean age = 66.73 (SD 10.19); control (n = 31) mean = 63.29, (SD 12.01). Total (n = 68) mean = 65.16 (SD = 11.11). Intervention (n = 37) 21 female, 16 male. Control (n = 31) 21 female, 10 male. Total: 42 female, 26 male (61.76% female) | |
| Interventions |
Control: no information Intervention: for the 8 weeks before surgery, 2 x clinic sessions and 2 x home sessions/week. Clinic session: 1 hour; 30‐minute aerobic and strength session, then 30‐minute mobility and gait training session in hydrotherapy pool. Home‐based sessions: tailored for participant's level of mobility, pain and help available and instruction provided during first clinic session. Provided with instruction booklet and home exercise log book. Behavioural instruction |
|
| Outcomes |
Pain: pain domain of Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), 3 weeks after surgery Behavioural recovery: physical function domain of WOMAC, 3 weeks after surgery |
|
| Notes |
N ote: also postoperative components to intervention but delivered after the 3‐week outcome measure Author replied to first email; confirmed general anaesthesia so could include paper in review (no author input into data extracted) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p194: "Patients became familiar with test procedures before random allocation was made to the exercise or control group" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information. As intervention group had quite significant intervention from staff, and required to carry out home practice, very unlikely either patients or intervention providers were blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | p199: "the preoperative and postoperative assessments were made without the assessor being blinded as to the treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition reported quite well, but overall it is fairly high (25%), and does not specify which group patients whose surgeries were cancelled were from. Very interesting that 2 of intervention group withdrew from surgery because of reduced pain and improved function – suggests intervention is effective but also means that groups analysed could be biased – if best‐functioning patients from intervention group have withdrawn then may be underestimating effect p196: 8 (11% of 76) withdrew pre‐surgery. 6 surgeries cancelled for medical reasons (2 x stroke, 2 x infection, 2 x other illness; groups not stated). Intervention group: 2 patients postponed surgery because of reduced pain and improved function after completing intervention. ‐> n = 68 p197: 11 (5 intervention, 6 control) not assessed postoperatively "because of social (vacation) or clinical (superficial wound infection, thrombosis) reasons". N reported: 57 (32 intervention, 25 control) Overall loss to follow‐up: 19/76 = 25% |
| Selective reporting (reporting bias) | High risk | No evidence of selective reporting, but no reference to a protocol document to check whether other outcomes were measured. Authors do not provide enough information for us to include any outcomes in meta‐analysis (means/SDs of pain and behavioural recovery not presented separately) |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Giraudet 2003.
| Methods | Randomized controlled trial | |
| Participants | 100 patients undergoing total hip arthroplasty at a teaching hospital, Paris, France, September 1997 to December 1999. Mean age intervention group: 62.7 years (SD = 8.8, n = 48); mean age control group: 64.3 (SD = 9.5, n = 52). Overall mean (calculated for review): 63.5. Intervention group: 24 male, 24 female; control roup: 20 male, 32 female. Overall: 44 male, 56 female | |
| Interventions |
Control: "usual procedure" – verbal information and leaflet (seems mostly procedural information, with some behavioural instruction and sensory information (re. pain) Intervention: 1/2 day session by multi‐disciplinary team (1/2‐hour slots to each of rheumatologist, surgeon, anaesthetist, physio and psychiatrist. Includes procedural information, behavioural instruction, sensory information and emotion‐focused |
|
| Outcomes |
Negative affect (STAI state anxiety), 1 and 7 days postoperative Pain (VAS) ‐ after surgery, ?1 day post‐surgery Length of hospital stay |
|
| Notes | Significant baseline differences in anxiety and depression Author provided additional information about intervention content and risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p113: "The allocation sequence was generated by the random placement of thoroughly shuffled marked cards into sequentially numbered sealed, opaque envelopes by the outpatient clinic assistant involved in the trial" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated. Unlikely given nature of trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition and exclusion are reported; only 1 person (of 52 in control group) was reported to have withdrawn from follow‐up (p115). The authors state data were analysed according to intention‐to‐treat (116) |
| Selective reporting (reporting bias) | Unclear risk | Discrepancies between measures described in Methods and results BUT more that reported outcomes in Results rather than listing in Methods but not reporting in Results (except for Trait Anxiety – not clear whether or not this was completed at follow‐up – but makes sense to only analyse state at those time points). Did not report multiple regression findings for length of stay even though stated would do this in Analysis. Correspondence with authors: no outcomes measured that were not reported |
| ‘Intention‐to‐treat’ | Low risk | p114 "analysis was done on an intention to treat basis". Looks as though this was the case – 1 dropped out but full sample sizes reported in Results (although this could be due to error rather than imputation) |
| Other bias | High risk | Major problem: differences between groups at baseline on key variables. Authors use change scores rather than comparing means. If use absolute means, not sure what impact of intervention is |
Gocen 2004.
| Methods | Randomized controlled trial | |
| Participants | 59 patients undergoing total hip replacement at a hospital in Turkey (dates not provided). Overall mean age: 51.3 (intervention mean: 46.93 (SD 11.48); control mean: 55.50 (SD 14.44). Gender distribution unclear: p354: 21 male and 36 (64.4%) female. However, p355 suggests other way round, with intervention group: 16 male, 13 female; control group 22 male, 8 female | |
| Interventions |
Control: no treatment reported Intervention: behavioural instruction. Instructed in exercises each to be performed x 3 daily (10 repetitions) for 8 weeks before operation. Also "education programme" including advice on movements to avoid, use of devices, posture, lifting/carrying, washing/bathing |
|
| Outcomes |
Pain: VAS at rest and activity at discharge Length of stay |
|
| Notes | Intervention group significantly younger ‐ may lead to bias in meta‐analysis Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p354: "randomly divided into two groups by using a table of random numbers of a computer programme (Excel 2000). Even numbers were allocated to the control group and odd numbers to the study group" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided in paper. Unlikely given nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All measurements were performed by a staff physical therapist who was blinded to the study" (p354) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | p354: "one patient in the study group was not operated on because of cardiovascular problems". It would seem appropriate to exclude this individual |
| Selective reporting (reporting bias) | High risk | There is no reference to a study protocol. Pain VAS measures are reported to have been measured at 3 months and 2 years after surgery but results are not presented. All measures reported at time point relevant for review, except for length of stay ‐ only P value reported so cannot enter into meta‐analysis (so high risk) |
| ‘Intention‐to‐treat’ | Unclear risk | No information |
| Other bias | Low risk | No other concerns |
Goldsmith 1999.
| Methods | Randomized controlled trial | |
| Participants | 195 participants undergoing ambulatory surgery at the Ambulatory Surgery Center, Beth Israel Deaconess Medical Center, Boston, USA (80 responded to outcome questionnaire). Dates not provided. Overall mean age: 44.8 years, range 18 to 82. Intervention mean 45.2, range 19 to 82. Control mean 44.5, range 18 to 74. Overall, 56 male, 139 female (71% female). Intervention group: 31 male, 67 female (68% female); control group 25 male, 75 female (74% female) | |
| Interventions |
Control: usual care. Access to website containing information e.g. when to arrive, what to eat, medication to take, what happens at surgery time (procedural information and behavioural instruction). Also face‐to‐face or phone interview with nurse, reviewed information and answered questions Intervention: usual care plus access to additional web area with advice on managing pain. Further, but pain‐specific,behavioural instruction |
|
| Outcomes | Pain: 5‐item verbal response scale from McGill pain questionnaire. Asked about 3 time points: on arrival home, night after surgery, day after surgery. The questionnaire was sent home with patients on discharge | |
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p781: "Patients…were randomized into an intervention or control group"; p782: "patients who did consent to participate were randomized into a study arm" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information. As this intervention was by access to different information on a website, there is a possibility that participants and personnel may have been blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Response rate for postal outcome questionnaire is clearly reported, with 51% control group and 67% control group lost to follow‐up (p782). From Table 7 (p783) it appears that there are further missing data – it appears that the pain on arrival home figure is missing for 2 people from the intervention group (so displaying data for 48 control and 30 intervention participants) (although these figures are obtained by reading data from bar chart). Concern: difference in follow‐up rate between intervention and control groups |
| Selective reporting (reporting bias) | Unclear risk | No reference to a study protocol, but all outcomes mentioned are reported |
| ‘Intention‐to‐treat’ | Unclear risk | Not clearly stated. Numbers in Figure 7 suggests that the authors are not excluding those who did not report using the website |
| Other bias | Low risk | No additional concerns |
Gonzales 2010.
| Methods | Randomized controlled trial | |
| Participants | 44 participants undergoing outpatient surgery of head or neck at Wright‐Patterson Medical Center, Wright‐Patterson Air Force Base, Ohio, USA (dates not provided). Overall mean age: 34.6 years (SD 13, range: 18 to 71). Control group mean age 33.32 (SD 10.76); intervention group mean age 35.91 (SD 15.13). Overall, 26 male, 18 female (59.1% male). Control group: 13 male, 9 female; intervention group: 13 male, 9 female | |
| Interventions |
Control group: 28 minutes of privacy in preoperative holding area Intervention: Relaxation. 28‐minute CD in preoperative holding area containing "a progressive relaxation and guided imagery exercise", plus second "guided imagery" CD immediately prior to induction up to before first cut "soothing biorhythmic music…with positive, encouraging statements" |
|
| Outcomes | Pain: rated at 1 hour and 2 hours after leaving operating room; vertical visual analogue scales regarding pain over previous hour | |
| Notes | Another outcome measure was "discharge time" from postoperative anaesthesia care unit and ambulatory procedure unit. Not included in review because rather than record actual time of discharge, discharge time was "based on the time the patient actually met discharge criteria" in order "to control for multiple factors that could delay actual discharge time" (p183) Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p183: "With the use of computerized random number generation, the patients were assigned to either the guided imagery or control group" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This is reported as a "single blind study". Outcome assessment blind, but no report of other blinding (and, as no placebo CD, it seems likely that the participants were aware of condition) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p183: "All postoperative data was collected…by a blinded investigator". No information as to how this was done or whether effective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that all randomized participants gave full outcome data. No attrition was reported and the sample size in outcome tables matches the sample size randomized |
| Selective reporting (reporting bias) | Unclear risk | No apparent missing of outcome reporting but also no protocol to refer to |
| ‘Intention‐to‐treat’ | Unclear risk | Sample sizes of outcome measures suggests that participants were analysed by intervention groups however this is not stated so there could have been cross‐over |
| Other bias | Low risk | No other concerns |
Goodman 2008.
| Methods | Randomized controlled trial | |
| Participants | 188 patients undergoing cardiac bypass surgery at a London NHS Trust (UK), dates not provided. Overall mean age: 64.8 (intervention mean 63.7, n = 94; control mean 65.9, n = 94). Intervention group: 72 male, 22 female. Control: 80 male, 13 female. Overall: 81.3% male | |
| Interventions |
Control: "standard care" – hospital helpline numbers and preoperative information day (details not given) Intervention: monthly preoperative home appointments with nurse – for patients to ask questions, voice concerns and be counselled regarding anxieties, undergo cardiac risk assessment (nurse ensured appropriate medication/referral to GP), and "counselling" regarding lifestyle change – motivational interviewing techniques, based on Stages of Change model. Copy of manual, guided through sections covering risk factors, preparation for surgery and what to do if chest pain. Manual (sent by author) includes behavioural instruction (e.g. diet, weight and blood pressure control, fitness, smoking); also includes section on relaxation, with specific instructions for learning to breathe deeply. Emotion‐focused, behavioural instruction, relaxation |
|
| Outcomes | Length of stay | |
| Notes | Medians, IQRs, ranges provided rather than means/SDs because data skewed Author provided additional information regarding intervention and risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p192: "Computerised random number allocation by a third party was used to allocate patients to the intervention or control group in a 1:1 ratio" |
| Allocation concealment (selection bias) | Low risk | A central allocation procedure was used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given that intervention involved series of home visits highly unlikely participants were blind; personnel delivering intervention could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Author: "The actual length of stay would have been taken from the hospital PAS system so the collector would not have been blind but there would have been little room for bias" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No./% participants lost to follow‐up: for review outcome of LoS, data reported for n = 90 in control group and n = 91 in intervention group. Odd: flowchart p199 describes 94 allocated to each condition. For Intervention, 4 "removed from list" before surgery and 2 died after surgery; for Control group, 1 removed from list before surgery and 4 died afterwards. Would be good to chase this with Author and find out what numbers are correct – but attrition low and seems to be due to either removal from lists – or death – seem unlikely to be associated with intervention |
| Selective reporting (reporting bias) | Low risk | Author responded that all outcomes were reported. Unfortunately, medians/IQRs presented for includable outcome length of stay rather than means/SDs because the measure was skewed. So, cannot include the data in the meta‐analysis – but this is not because of incomplete reporting |
| ‘Intention‐to‐treat’ | Low risk | p193: "The intention to treat principle was used" |
| Other bias | Low risk | No other concerns |
Greenleaf 1992.
| Methods | Randomized controlled trial | |
| Participants | 32 patients, USA, Dept of Cardiothoracic Surgery and Dept of Nursing, Jack D Weiner Hospital of the Albert Einstein College of Medicine in Bronx, New York, and Ferkauf School of Psychology, Yesiva University. Dates not provided Mean age: 58.75 years (SD = 9). 81% female; 19% male Surgery type: coronary artery bypass surgery |
|
| Interventions |
Control: Routine care. Nurses were trained to teach patients about their surgery and recovery "to improve attitudes and outcome" (procedural information/behavioural instruction likely but not explicit) Intervention 1: Taught self hypnosis with imagery for muscle relaxation Intervention 2: Taught self hypnosis with specific suggestions related to optimal surgical outcome (e.g. letting defence system stay alert, minimal bleeding) Both intervention 1 and 2: 1 x 45‐minute session with psychologist, 1 to 2 days before surgery |
|
| Outcomes | Total length of stay in hospital | |
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a random stratification chart, patients were assigned to one of three experimental groups matched for age, number of predicted bypasses, and degree of hypnotizability" (p121) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information is provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded (neither were those delivering interventions). No information as to whether hospital staff were blind. However, all patients were asked to keep their group assignment to themselves (p121) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data reported. As outcomes are short‐term, medical outcomes, this is believable. 32 participants are reported to have been randomized, and findings are reported for 32. |
| Selective reporting (reporting bias) | High risk | As many findings were non‐significant selective reporting seems unlikely. However, findings by group for one outcome measure, "cumulative stability", were not reported (they may not have been conducted for this categorical variable). Does not provide data to enter into meta‐analysis (no mean/SD) |
| ‘Intention‐to‐treat’ | Low risk | "After six months of recruiting...surgeons began to request hypnosis for anxious patients which would have meant breaking the protocol. Later, when it was discovered that the chief anaesthesiologist was inspired...to use hypnosis with...patients, some who were in the experimental control group, the study protocol had to be terminated" (p125). This suggests that patients were analysed in the groups to which they were assigned |
| Other bias | Unclear risk | There may have been some contamination across groups (see `intention‐to‐treat') |
Griffin 1998.
| Methods | Randomized controlled trial | |
| Participants | 85 patients at a teaching hospital, Ireland, dates not provided Mean age `education group' = 48 years (SD 15.6, N = 42); mean age `controls' = 47 (SD = 17.4, N = 43). 29% female; 71% male. Surgery type: major procedures suitable for postoperative patient‐controlled analgesia. General: 38; gynaecological: 20; urological: 9; orthopaedic: 14; miscellaneous: 4 |
|
| Interventions |
Control: routine preoperative anaesthetic assessment and visit Intervention: Behavioural instruction. 20‐minute tutorial the evening prior to surgery administered by an investigator; information sheet outlining main points given at end of session. The intervention stressed that patients are responsible for their own pain relief; strategies for maximising pain control were suggested e.g. prevent anticipated discomfort; use before sleep; use on wakening. Aimed to reduce fears about safety and the possibility of reduced contact with nursing staff. Side effects and the treatment for side effects were outlined |
|
| Outcomes | Pain at 6, 24 and 48 hours after discharge from recovery room: 100 mm VAS | |
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information is provided on sequence generation method |
| Allocation concealment (selection bias) | Unclear risk | No information is provided on allocation concealment method |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As intervention participants only received a 20‐minute tutorial, blinding would not be possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All patients were assessed by a single investigator unaware of their randomisation status" (p944). No information is provided as to how this was achieved or whether it was effective |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information is provided on attrition or missing data |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting but no reference to a protocol document |
| ‘Intention‐to‐treat’ | Unclear risk | Insufficient information is provided |
| Other bias | High risk | It is not clear how many people were randomly allocated to one or both groups who did not agree to participate. Given that people were only offered one intervention (apparently), and it is not stated how many agreed, it is possible that the people who agreed to each of the groups were people to whom the group appealed, and that others did not consent (i.e. there is no clinical equipoise in the minds of participants) |
Gräwe 2010.
| Methods | Randomized controlled trial | |
| Participants | 96 patients (48 male, 48 female) undergoing mixed surgery (abdominal or vascular surgery) at University Hospital Schleswig‐Holstein, Lübeck, Germany (dates not provided). Age range: 19 to 75, mean 56.7 years (SD 12.2) | |
| Interventions |
Control: attention placebo – individual sessions providing information about background of study and use of numerical rating scale (for study use) Intervention: information about postoperative pain and cognitive methods to cope with pain (distraction, positive thought rehearsal/verbalization). Presented preoperatively by 1st author, conversation and written summary. Duration: 25 minutes. Sensory information, cognitive intervention |
|
| Outcomes |
Pain: pain intensity (numerical rating scale, NRS) – on resting, on average and maximum and pain intensity of affective and sensory components (SES, der Schmerzempfindungsskala, Geissner 1996). Both: days 1 to 3 post‐surgery Negative affect: BSKE (EWE) (Befindlichkeitsskalierung durch Kategorien und Eigenschaftswörter, Janke 1994) – general psychological well‐being; STAI state anxiety (both days 1 to 3 postoperative) |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used a published randomization algorithm |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blind to group but the person carrying out the intervention was not blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information as to who collected or analysed outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition is reported |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis (negative affect outcomes ‐ STAI and BSKE ‐ no means or SDs) |
| ‘Intention‐to‐treat’ | Unclear risk | No mention of participants changing groups but not clearly stated |
| Other bias | Low risk | No other concerns |
Guo 2012.
| Methods | Randomized controlled trial | |
| Participants | 153 patients randomized, undergoing cardiac surgery (including coronary artery bypass grafting, valve surgery, congenital and other open heart surgeries ‐ not heart transplants) in 2 public hospitals in Luoyang, China. Recruitment: 1 December 2009 to 17 March 2010. Control mean age 52.3 (SD 15.99, N = 77); intervention mean age 52.0 (SD 16.12; N = 76) | |
| Interventions |
Control: usual care – visits from surgeon and anaesthetist one day before surgery, providing information re. "general process and risks of their surgery and anesthesia, the use of analgesia and/or pain management".Procedural information Intervention: usual care (as Controls) plus: 2 to 3 days before surgery, 15 to 20 minutes with author, going through information leaflet. Content included preoperative tests and preparation, stay in ICU, returning to ward, recovery at home. Furtherprocedural information |
|
| Outcomes | All measured day 7 after surgery – paper focus: change from baseline Negative affect: anxiety (primary outcome) anddepression – HADS Pain – Brief Pain Inventory Short Form – pain severity in 4 domains (worst, least, average and right now; only analysed average and current) Length of stay |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p131: "Allocation was determined by a stratified block randomization, with random block size and stratified by the two study hospitals. The randomization list was prepared by AA [an author] using the ‘ralloc’ command in Stata version 9.2" |
| Allocation concealment (selection bias) | Low risk | p131: "AA had not contact with study participants. Randomization was implemented by PG an author] using a series of consecutively numbered, opaque, sealed envelopes. The envelope was opened in the presence of the participant after baseline assessment was completed" [baseline assessment took place after consent] |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p131: Intervention group Ps were asked not to inform clinical staff of their allocation. Also: to minimize contamination, leaflet put in envelope to take away, and participants were asked to not share it with other patients on the ward p135: "Due to the nature of the intervention we could not blind participants to study group intervention" Also, intervention was delivered by PG – knew intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p131: "Outcome measures were assessed on the seventh day after surgery by a cardiac nurse who was blinded to group assignment" p135: "the nurse collecting follow‐up self‐completion measures was not the nurse (PG) who delivered the preoperative education intervention" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No./% participants lost to follow‐up: Flowchart p133. Intervention group: loss to follow‐up n = 8 (6 discharged without surgery, 2 transferred to another hospital). Control group: loss to follow‐up n = 10 (8 discharged without surgery; 2 died after surgery). Overall loss to follow‐up n = 18 11.8% p132: "Of the 135 who completed the trial, complete data were available for all outcomes with 100% item response for outcome scales" High quality reporting of attrition. Apparently discharge without surgery is not uncommon in China, so I believe attrition bias risk is low |
| Selective reporting (reporting bias) | Unclear risk | No reference to a study protocol; report only analysing 6 domains of pain to limit number of statistical tests. Outcomes are reported with means and SDs, although change scores for pain |
| ‘Intention‐to‐treat’ | Low risk | p132: "The use of a strict intention to treat analysis was impossible in cases of missing data such as loss to follow‐up (Abraha and Montedori, 2010 [Abraha 2010]). All participants who completed follow‐up were analysed as a part of the group to which they were randomised and those lost to follow‐up were excluded from analyses" |
| Other bias | Unclear risk | Potential risk of contamination. p135: "the possibility of contamination between the two groups cannot be excluded" – did not have resources to cluster. Did take some measures ‐ putting leaflet in envelope for the participant to take away, and asking them not to share it with others (p131) |
Hart 1980.
| Methods | Randomized controlled trial | |
| Participants | 40 participants (33, 92.5% male) undergoing cardiopulmonary bypass surgery at Methodist Hospital, Lubbock, Texas, USA, September 1973 to February 1974 | |
| Interventions |
Control: on admission to hospital – general information covering orientation, information re. surgery ("anatomical" and "corrective" information), and discharge plans – including activity and diet information. Procedural information, behavioural instruction Intervention: as Control group. Plus: listened to tape‐recordings in 5 sessions (2 on day of admission; 3 the next day (the day before surgery)). First session: 10‐minute tape: introduction to benefits of hypnotic relaxation. Then 20‐minute hypnotic induction procedure. 4 other sessions: 20‐minute recording only. Hypnosis; relaxation |
|
| Outcomes | Negative affect: State and Trait Anxiety (STAI); 2 days prior to discharge | |
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p325: "Random assignment of 20 surgery patients (17 males and 3 females) to the control group and 20 surgery patients (16 males and 4 females) to the experimental group was achieved" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No suggestion that participants or those delivering intervention blind – seems very unlikely given nature of intervention – and as nurse required to prepare equipment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p327: "none of the nurses who distributed test materials were completely aware of the exact nature of the study or of the assignment of Ss [participants] to treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | p325: "No patient or patient data was excluded from final analysis". p328: "No S refused the tests". Therefore 0 patients lost to follow‐up and outcome data are complete |
| Selective reporting (reporting bias) | Unclear risk | All measures reported in Methods are fully reported in Results. However, no mention of a protocol so cannot check this |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Hawkins 1993.
| Methods | Randomized controlled trial | |
| Participants | 60 women undergoing various gynaecological surgical procedures (total abdominal hysterectomy (18, TAH), TAH, bilateral salphingoopherectomy (6), vaginal hysterectomy (5), posterior repair (3), vaginoplasty (2), reastonosis (2), cholecystectomy (2), laparoscopy, oophorectomy (2),and a range of other procedures) at Flinders Medical Centre, Adelaide, South Australia (dates not provided) | |
| Interventions |
Control 1: "normal hospital practices" Control 2: an attention control: as 1, and shown video with "public relation style information about the hospital" Intervention: shown video, day before surgery: advice re. how to deal with pain, showing techniques for pain control, encouraged to request pain relief Behavioural instruction |
|
| Outcomes |
Pain: 48 hours after surgery: VAS of average pain; categorical scale (5 categories from no pain to unbearable pain); nurse ratings of pain (collected hourly pain reports when not sleeping for first 48 hours after surgery) Negative affect: anxiety. Hospital Anxiety Scale (Lucente 1972) |
|
| Notes | Findings not reported for negative affect Author confirmed study met inclusion criteria (use of general anaesthesia) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p34: "participants were randomly assigned to the control, pain video or neutral video group" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Made efforts to blind ward staff, but researcher who administered videos would have known; likely that patients also knew |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Researcher was on ward while patients completed the questionnaires, to answer questions and collect questionnaires. No mention of blinding. Seems likely that same researcher who administered videos, but not impossible that blinding was carried out |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data are reported, and sample size for reported outcomes matches the sample size reportedly randomized |
| Selective reporting (reporting bias) | High risk | A number of outcomes that were measured were not reported (including the anxiety measure). States that focus of this paper was on pain but suggests should be cautious. Data are not presented by group so even reported outcomes cannot be included in meta‐analysis |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Heidarnia 2005.
| Methods | Randomized controlled trial | |
| Participants | 80 patients randomized, mean age 53.15 years (53.5 years in experimental group, 52.8 years in control group). Undergoing coronary artery bypass surgery at a specialist heart hospital in Tehran, Iran, April 2002 to August 2002 | |
| Interventions |
Control: completed 118‐item structured baseline questionnaire, administered face‐to‐face, 3 to 5 days before surgery Intervention: completed structured questionnaire as Control group. Also: 3 x 20‐ to 25‐minute face‐to‐face education sessions, both booklet. Focus included: exercise, diet, sexual function, deep breathing, anatomy, procedure of surgery, travel and drug use. Behavioural instruction and procedural information |
|
| Outcomes | All outcomes at 1 month post‐surgery Pain (SF‐36 Bodily Pain, Nottingham Health Profile (NHP) Pain) Behavioural recovery (SF‐36 Physical Function; NHP Physical Mobility) Negative affect (SF‐36 Mental Health; NHP Emotional Reaction) |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p320: "seventy male patients were selected by random sampling"; "80 male patients were selected and assigned to either experimental or control groups. Initially we selected the experimental group, then the control group" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants not blind to intervention; no information regarding personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition reported p320: "nine patients were lost to follow‐up and one died". No information re. groups |
| Selective reporting (reporting bias) | Unclear risk | There is no evidence of outcomes being measured but not reported (but no reference to a protocol document) |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | High risk | The experimental group was run before the control group, so other factors could have influenced the groups differently |
Hoogeboom 2010.
| Methods | Randomized controlled trial | |
| Participants | 21 frail elderly adults undergoing primary total hip replacement due to osteoarthritis. Intervention group: mean age = 77 (SD 3, range 71 to 82); control group mean age 75 (SD 5, range 69 to 90). Overall mean age = 76 years (SD 4). Intervention: 7 female, 3 male, control: 7 female, 4 male. Overall, 66.67% female. Setting: outpatient physiotherapy department, Netherlands. Recruitment: July 2007 to November 2008 | |
| Interventions |
Control: usual care (received by both groups) – "education session about early mobilization, surgery and anaesthesia techniques, restricted movements, benefits of activity and proper use of crutches" – procedural information and behavioural instruction Intervention: supervised sessions at least x 2/week for 3 to 6 weeks pre‐surgery: warm‐up, lower extremity training with leg press, aerobic exercise, individualized physio training. Also encouraged to exercise at home. Behavioural instruction (within sessions and re. home exercise) |
|
| Outcomes |
Length of stay Behavioural recovery: Iowa Level of Assistance Scale ‐ taken each postoperative day in hospital; authors used to measure "time needed to reach functional independence" |
|
| Notes | Small sample ‐ feasibility/pilot study Author provided additional information for `Risk of bias' assessment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p902: "Participants were randomly allocated by use of a sealed envelope method by an independent person to either the intervention or usual care group, stratified for gender" – no information as to how randomization achieved. Author: "The envelopes were opaque, not numbered. With every pick, an unrelated, random bypasser was asked to select one of the envelopes after the baseline assessment. That envelope was opened by the bypasser". Suggests random element |
| Allocation concealment (selection bias) | Low risk | p902: "Participants were randomly allocated by use of a sealed envelope method by an independent person to either the intervention or usual care group, stratified for gender". The independent person would ensure that others did not know the allocation, but not clear that the person conducting the allocation did not have the opportunity to influence allocation for an individual. Author: "The envelopes were opaque, not numbered. With every pick, an unrelated, random bypasser was asked to select one of the envelopes after the baseline assessment. That envelope was opened by the bypasser". Suggests would not have been possible to influence allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None stated, but given nature of intervention blinding of participants and those delivering the intervention would not seem possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p902: "The measurements during the hospital stay were also blindly administered by experienced and trained physiotherapists. The therapeutic intervention was provided by three other physiotherapists |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was reported, although it is not clear what the sample size was for the behavioural recovery outcome. We contacted the authors for clarity However, it is possible that attrition in the intervention group could have led to an under‐estimate of effect: with only 10 patients per group, 2 intervention group participants experienced complications that seem unlikely to be associated with the intervention (in addition, a 3rd was excluded because of "an early transfer to another institute"). It seems that the data for the 2 with complications were not included in the data for time to functional independence as they did not reach this before discharge. Given the small sample (10 per group) this may have had an impact on findings. Author: no longer has access to data but thinks it likely these 2 were excluded |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective reporting, but also no reference to a study protocol document to confirm this. Initially rated `unclear' but authors responded `no' when asked whether any other outcomes were measured but not reported |
| ‘Intention‐to‐treat’ | Low risk | p904: "analysis was performed according to the intention‐to‐treat principle" |
| Other bias | Low risk | No further risk in addition to concerns reported under "incomplete outcome data" |
Hulzebos 2006a.
| Methods | Randomized controlled trial (pilot/feasibility study) | |
| Participants | 26 patients undergoing coronary artery bypass surgery, October to December 2002 (only those at high risk of developing a postoperative pulmonary complication). Research team located in Utrecht, Netherlands. 13 male, 13 female. Intervention group (n = 14), mean age 70.1 (SD 9.9), control group (n = 12) mean age 70.5 (10.1) | |
| Interventions |
Control: usual care: included "education about early mobilization, and coughing with wound support", 1 day preoperative. Behavioural instruction Intervention: as Control, plus: inspiratory muscle training for 2 to 4 weeks pre‐operation Daily training at home, 20‐minute sessions, 1/week supervised by a physical therapist. Instructed to keep a daily diary and trained to use inspiratory threshold‐loading device. Behavioural instruction (beyond that received by control group) |
|
| Outcomes | Length of stay | |
| Notes | Information from author used in assessing risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p953: "randomly assigned using a computer‐generated randomised block design (block of four people)" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As involved significant preoperative training, provided by a physical therapist, neither participants nor therapist would have been blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All measurements were taken by an experienced physical therapist (EH) who was blinded for the group allocation of the patients" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow chart p952: 0 participants excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Means and SDs provided for the outcome relevant to review. No evidence of selective reporting, and authors responded to our queries stating that every outcome measured was reported |
| ‘Intention‐to‐treat’ | Low risk | Flow chart p952: in each group, all received allocated intervention |
| Other bias | Low risk | No other concerns |
Hulzebos 2006b.
| Methods | Randomized controlled trial | |
| Participants | Patients undergoing coronary artery bypass graft surgery (CABG) at high risk of postoperative pulmonary complications at University Medical Center Utrecht, Netherlands, enrolment from July 2002 to August 2005. 279 randomized ‐ intervention group mean age 66.5 (SD 9.0), control mean age 67.3 (SD 9.2). Intervention: 108 male, 31 female. Control group 107 male, 30 female. Overall, 215 (77.9%) male, 61 female | |
| Interventions |
Control: usual care, 1 day pre‐surgery, instruction on deep breathing, coughing and early mobilization.Behavioural instruction Intervention: daily training for at least 2 weeks before surgery, 1/week supervised by a physical therapist, 6/week independently. Each session: 20 minutes of inspiratory muscle training (IMT), instructed to record IMT progression, complaints, adverse events. Trained to breathe with an inspiratory threshold‐loading advice. Received detailed preoperative instruction in active cycle of breathing techniques (with incentive spirometer) and forced expiration technique. At baseline, received information about surgery and schedule of hospital events. Behavioural instruction, procedural information |
|
| Outcomes | Length of stay (duration of postoperative hospitalization) | |
| Notes | Information from author used in assessing risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p1852: "A computer‐generated randomization table was used, and individual allocations were placed in sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | p1852: "A computer‐generated randomization table was used, and individual allocations were placed in sealed envelopes. An external investigator blinded to the allocation sequence picked consecutive allocation envelopes for consecutive participants." Need to know – were they opaque? |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intensive preoperative training delivered by member of research team – not possible to blind either participants or trainer to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p1853: "A microbiologist, who was independent and blinded to patients’ allocation, collected data from the medical charge and clinical records…admission and discharge dates were retrieved by the microbiologist from the patients’ records and used to calculate duration of postoperative hospitalization" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Clear flow chart p1854. Only lost 3 patients to follow‐up – they died before surgery – 1 in intervention group and 2 in control group. So, 140 assigned to intervention; data for 139. 139 assigned to control, data for 137. However, in addition, 4 patients in control group died after surgery. Timing is not stated so for our outcome (length of stay), it is not stated whether n = 137 or 133 for the control group |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting and trial is registered. On checking trial registration, it was registered retrospectively. However, author confirmed that all measured outcomes were reported. Data are reported for outcome of interest but not possible to use in meta‐analysis (median and range) |
| ‘Intention‐to‐treat’ | Unclear risk | Appears to be ITT in that no participant dropped out of intervention group (p1854) and only excluded data from participants who died before surgery. However, not clearly stated |
| Other bias | Low risk | No other concerns |
Johnson 1978b.
| Methods | Randomized controlled trial | |
| Participants | Sample 1: 81 patients undergoing cholecystectomy; mean age 44 years; 82.7% female. Sample 2: 68 patients undergoing inguinal hernia repair; mean age 48; 88.2% male. All: 500‐bed hospital serving middle class community, Detroit, Michigan, dates not provided | |
| Interventions | 2 x 3 design (instruction x information) Control: no information or instructions Intervention 1 ‐ Instruction: Behavioural instruction in e.g. deep breathing, coughing, leg exercises Intervention 2 ‐ Information (Procedure): focus on procedural information: e.g. "things that the staff would do"; some sensory information and behavioural instruction (told to ask nurse for pain medications) Intervention 3 ‐ Information (Sensation): focus: sensory information e.g. how would feel taking premeds, wound sensations); also some procedural information and behavioural instruction All delivered afternoon before surgery using taped recordings delivered by research nurse. Nurse also helped Instruction participants practise exercises |
|
| Outcomes |
Pain: days 1, 2, 3 post‐surgery: intensity of sensations on 10‐point scale Negative affect: Mood Adjective Checklist (well‐being, happiness, fear, helplessness, anger); day 1, 2, 3 post‐surgery (scores totaled over the 3 days) Length of stay |
|
| Notes | 2 samples but identical study designs; analysed separately Author provided information for `Risk of bias' assessment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p8: "Patients were randomly assigned to one of the six experimental conditions" Author: "We used a randomly generated number table. The study groups were assigned a number, i.e., 1,2,3 or4 as appropriate for the study. With eyes closed we placed a pencil tip on the table, and starting from that point we moved down the column until we came to one of the numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper. Author: "A slip of paper with the group assignment was sealed into an envelope. This continued until a significant number of envelopes had been prepared. The envelopes were kept in the order they were prepared. The researcher opened the envelope at the time an intervention was to be delivered. This procedure was used for both studies." Kept as `unclear' as not mentioned if opaque/numbered |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Staff providing intervention would know (research nurses helped participants to practise exercises if appropriate), and likely that participants were also aware of condition |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p8: "the surgeons, nursing staff, and patients interviewers were not informed of the details of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exact numbers of participants excluded are not provided, and no breakdown provided by study group. Sample 1: 3 participants: p8: postoperative interview missing as refused to be interviewed on 1st postoperative day; 4: data for hospitalization length missing because extended by additional operation/diagnostic procedure. But also states that data "discarded if an atypical cholecystectomy was performed, a tube was placed in the bile duct at time of surgery, or a physical complication occurred" – no information on how many this affected. As we are not told how many participants were in each group at the start, and due to the factorial design, it is difficult to establish fully how many participants were lost to follow‐up Sample 2: p15: 10 participants’ data excluded from length of hospitalization as returned for surgery for repair on other side. Also states data discarded if bilateral herniorrhaphy/postoperative complication – no data on sample size for this. No results tables presented so not possible to establish sample size at follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Complex design, high number of analyses and not systematically presented – do not always present means; the reader is informed of significant/trend effects rather than all findings; no information regarding protocol. However, author: "To the best of my memory, all outcomes that we measured were reported in the articles" |
| ‘Intention‐to‐treat’ | High risk | No information. Author: "The idea of analysing data using the notion of intention‐to‐treat came into use after the studies had been conducted, so no we did not do those analyses" |
| Other bias | Unclear risk | Concern: whether the tests the authors ran are sufficient to control for family‐wise error given the high numbers of tests |
Johnson 1985.
| Methods | Randomized controlled trial | |
| Participants | 199 women undergoing abdominal hysterectomy were randomized (31 lost to follow‐up). Mean age: 38; range: 24 to 61. Setting: "387‐bed, inner‐city hospital affiliated with a university medical centre in the great lakes region" (USA), over an 18‐month period | |
| Interventions |
Control 1: usual care as received by all (including some procedural information and behavioural instruction) Control 2: attention control procedural information – general information about hospital and services. Control groups combined in analysis Intervention 1: "concrete sensory information": procedural and sensory information. Tape‐recorded message re. what could experience during hospitalization Intervention 2: "cognitive‐coping technique" – cognitive intervention. Recording instructing patient to distract from negative aspects and focus on positive Intervention 3: "Behavioural‐coping technique" – behavioural instruction. Recording instructing ways to move to minimise pull on incision and reduce pain All interventions delivered evening before surgery by research nurse who also answered questions and helped to practise coping techniques |
|
| Outcomes |
Pain – scale from 1 to 10, day 3 post‐surgery Negative affect: Profile of Mood States (POMS: anxiety, confusion, anger, depression, fatigue, vigour). Day 3 post‐surgery and 1st and 4th week post‐discharge Length of stay |
|
| Notes | Complex factorial design. Also randomized again to postoperative intervention: discharge information/no discharge information. This occurred on day 4 postoperation (after day 3 results day) and method allowed to look at main effects of other interventions. Length of stay seems to have been measured as a co‐variate rather than as an outcome Author provided information regarding `Risk of bias' assessment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p134: "Patients were randomly assigned to study conditions as their names appeared on the operating room schedule. An exception to random assignment occurred when two subjects occupied the same room. In such cases the second subject was assigned to the same condition as the first subject to prevent contamination. Patients lost from the study during the hospital phase were replaced, thus, an equal number of subjects per condition was achieved". Seems unlikely that patients replacing lost patients were randomized – otherwise unclear. Author: "We used a randomly generated number table. The study groups were assigned a number, i.e. 1, 2, 3 or 4 as appropriate for the study. With eyes closed we placed a pencil tip on the table, and starting from that point we moved down the column until we came to one of the numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information provided. Author: "A slip of paper with the group assignment was sealed into an envelope. This continued until a significant number of envelopes had been prepared. The envelopes were keep in the order they were prepared. The researcher opened the envelope at the time an intervention was to be delivered. This procedure was used for both studies." Unclear as may have been successful but would not appear envelopes numbered/opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated, but seems unlikely that participants would be blind if fully informed; research nurses delivering tapes and answering questions would not be blind as sometimes helped participants with interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p136: "on the third postoperative day, a nurse who was not informed of patients’ assignment to study conditions collected data…Finally, the open‐ended questions were asked…thus, the data collector elicited information that could have revealed patients’ study condition only after all other data were obtained". Later time points obtained by postal survey so effectively blind outcome assessor (although some phone reminders) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 31 lost to follow‐up (p133): 19: complication during surgery/major postoperative complication/additional diagnostic/treatment procedure after surgery; 8: too ill/declined for other reasons to continue participating during hospitalization; 4: failure to contact patient during hospitalization. Attrition data are not broken down by study condition |
| Selective reporting (reporting bias) | Unclear risk | Authors did not use all outcomes in analyses on basis of judgements of redundancy. Not known what techniques were specified a priori. p137: "The precision of the experiment was increased by eliminating outcome indicators with minimal variance and those that were redundant. Depression and anger scores from the POMS were eliminated because 30 to 45% of the scores were zero at each measurement…fatigue and pain distress scores were also eliminated as indicators". Emailed authors about 2 papers together: "To the best of my memory, all outcomes that we measured were reported in the articles" |
| ‘Intention‐to‐treat’ | High risk | Appears to be by intention‐to‐treat – p142: report that only 17% of patients in cognitive coping group reported using the technique but the sample sizes suggest that clearly not only 17% of that group used in analysis (e.g. table 2). From procedure, seems unlikely that participant would be able to withdraw between consenting and completing allocated treatment as all done in same session. However, email from author: "The idea of analyzing data using the notion of intention‐to‐treat came into use after the studies had been conducted, so no we did not do those analyses" |
| Other bias | Unclear risk | Coping conditions: 94% behavioural‐coping group reported using techniques cf only 17% in cognitive‐coping group. As result, not clear how to interpret finding where cognitive coping associated with longer stay in hospital Authors discussed allocating patients who were seen together to the same allocation to reduce risk of contamination |
Klos 1980.
| Methods | Randomized controlled trial (2 x 2 design) | |
| Participants | 50 patients undergoing cholecystectomy at a 500‐bed hospital, St Joseph Mercy Hospital, Ann Arbor, Michigan, USA (dates not provided). Mean age: 43.6 years (range: 20 to 72). 38 (76%) female; 12 male | |
| Interventions |
Control: no treatment control Intervention 1: pamphlet containing procedural information: information about operative events; also behavioural instruction e.g. instructions in deep breathing, leg exercises Intervention 2: nurse visit providing same content as 1; also assisted patient in practising exercises until mastery achieved Intervention 3: both pamphlet and nurse visit |
|
| Outcomes |
Length of stay: number of days between surgery and discharge Negative affect: day 2 post‐surgery. Mood Adjective Checklist: 15 adjectives describing the 5 mood dimensions: well‐being, happiness, fear, helplessness, anger |
|
| Notes | Data only presented grouped by preoperative fear level, adjusted for age Behavioural recovery and negative affect were measured, but using scales without published psychometric information Author provided some information regarding risk of bias (stage 1 response only) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p7: "Patients were randomly assigned to one of four experimental groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p7: "surgeons and the nursing staff were uninformed as to the specific dependent variables being studied and the assignment of patients to the experimental groups". But the participants would have known and all interventions were provided by the same nurses |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | p8: "Patients…were visited both pre‐and postoperatively by the same nurse who visited the patients in the other conditions. The purpose of the visits was to explain the study, secure patient consent and collect data. The data collection and preoperative interventions were carried out by the same two nurses over a period of 2 ½ months." However, as the outcome of interest to us is length of stay it is not clear how lack of blinding could influence outcome assessment. Therefore rating of `unclear' rather than `high' risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition and exclusions were reported (p7): 1 had an atypical cholecystectomy; for 5 patients the nurse‐experimenter was unable to collect all observations; 1 patient in the pamphlet‐only group did not read the pamphlet. The authors do not report how many participants were lost from each group, so the impact in terms of bias is unknown. However, the participant who was excluded for not reading the pamphlet is unlikely to have been excluded if they were in a different group |
| Selective reporting (reporting bias) | High risk | The analyses did not allow the authors’ primary aims to be addressed. The stated purposes were (p7): "1) to test the effects of providing instruction about the usual events of surgery and instructions in leg exercises, turning in bed, getting out of bed, and coughing and deep breathing; and 2) to compare the relative impact of two information‐delivery methods on various indicators of postoperative recovery, using as delivery methods a preoperative nurse visit and/or pamphlet". However, data addressing these aims are not provided; all data are split according to low or high preoperative fear. This splitting was not only not pre‐specified, but seems likely to have resulted in unreliably small sample sizes. Data appropriate for meta‐analysis are not provided for either length of stay or negative affect outcome |
| ‘Intention‐to‐treat’ | High risk | Not intention‐to‐treat: p7: the patient who reported not having read the pamphlet in the pamphlet‐only condition was excluded from analysis |
| Other bias | Unclear risk | Potential for contamination across groups (e.g. pamphlets could be passed around) |
Kulkarni 2010.
| Methods | Randomized controlled trial | |
| Participants | 80 patients undergoing major abdominal surgery at a hospital in the UK (dates not provided). Age: Control: 65, Intervention 1: 64, Intervention 2: 65, Intervention 3: 60 (not clear whether median or mean). Gender: Control: 12 male, 8 female; Intervention 1: 10 male, 10 female; Intervention 2: 5 male, 15 female; Intervention 3: 11 male, 9 female. Overall: 38 male, 42 female, 52.5% female | |
| Interventions |
Control: no training Intervention 1: deep breathing training Intervention 2: incentive spirometry Intervention 3: specific inspiratory muscle training All interventions: asked to train x 2 per day for at least 2 weeks prior to surgery (behavioural instruction, each session 15 minutes) |
|
| Outcomes |
Length of stay Postoperative pain (no information on how/when measured) |
|
| Notes | Only Intervention 1 is purely behavioural instruction; Intervention 2 and 3 also involved a device Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p701: "Patients were allocated to four groups by computer‐generated, random numbers placed in sequentially numbered sealed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | No further information to above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely that participant was blind; all patients were "assessed and trained by the researcher" (p701), indicating that the person administering interventions was also not blind across conditions |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | p701: patients were "assessed and trained by the researcher" – implies not blind at assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No./% participants lost to follow‐up: 14 (17.5%). By group: Control n = 3, Intervention 1 n = 3, Intervention 2 n = 5, Intervention 3 n = 3. Consort flow chart (p702) clearly accounts for participants for primary outcomes; loss to follow‐up is similar across conditions and seems unlikely to be a cause of bias, although given the size of the sample impact is not impossible. However, it is not clear whether this also applies to the assessments of secondary outcomes (those outcomes of interest in this review) |
| Selective reporting (reporting bias) | Unclear risk | No protocol mentioned, but clearly state primary outcomes and these are all accounted for. However, less consistent with secondary outcomes |
| ‘Intention‐to‐treat’ | Low risk | The Consort diagram (p702) reports that, for Group D (Intervention 3), 2 participants were lost to follow‐up because they discontinued intervention – but also states that these patients did not have surgery (which seems likely to explain why they discontinued intervention) |
| Other bias | Unclear risk | The authors do not state whether the groups were comparable in terms of baseline demographics. Given the small sample size this could be important. From the demographics table (Table 1), group D (Intervention 3) seems young (60 years, compared with 65, 64, 65 for A (Control), B (Intervention 1), C (Intervention 2)); also different patterns with gender (12:8; 10:10; 5:15; 11:9) |
Lam 2001.
| Methods | Randomized controlled trial | |
| Participants | 60 patients undergoing major gynaecological surgery at a hospital in Shatin, Hong Kong (dates not provided). Control group mean age: 40; intervention group 43. 100% female | |
| Interventions |
Control: "standard information" Intervention: education about patient‐controlled analgesia (PCA). 15‐minute session (verbal instruction), demonstration of PCA device and pamphlet. Includes sensory information and behavioural instruction |
|
| Outcomes |
Pain severity (before discharge from recovery room and 24 and 48 hours post‐surgery) Length of stay |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided in paper. p466: "half of the patients (n=30) were also randomly selected to receive additional structured preoperative education" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information about blinding of study participants – unlikely given the design. The intervention was administered by the first author (p466) so also not blinded. Anaesthesiologists who provided general anaesthesia "were unaware of the purpose of the study" (p466) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p466: "all patient interviews were conducted in a standardized fashion by investigators who were blinded as to study group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | p467: "All patients completed the study" |
| Selective reporting (reporting bias) | Unclear risk | It is not apparent that any outcomes are not reported, but there is no reference of a protocol to which to refer |
| ‘Intention‐to‐treat’ | Low risk | All participants are reported to have completed the study; the same numbers of participants who were randomized were reported per group in Results so unlikely that groupings could have changed in analysis |
| Other bias | Low risk | No other concerns |
Lamarche 1998.
| Methods | Randomized controlled trial | |
| Participants | 54 patients undergoing first‐time cardiac surgery (CABG) at "a large metropolitan tertiary care teaching hospital in Canada" (dates not provided). Control group: 63.7 years; intervention: 63.5 (assuming means). Control group: 20 male, 6 female; intervention group: 25 male, 3 female. Overall, 83% male | |
| Interventions |
Control: preadmission teaching session, "cognitive and affective information about hospitalization, along with information about coronary artery disease and lifestyle adjustment". Procedural information, behavioural instruction Intervention: also received phone call 1 week after teaching session to give additional, personalized information and to discuss feelings. Emotion‐focused and procedural information |
|
| Outcomes | Negative affect: anxiety prior to discharge (10 cm VAS) | |
| Notes | Author confirmed suitable for inclusion (general anaesthesia received) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated – p394: "Fifty‐four patients were randomly assigned to the experimental or control group" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated if participants blind but unlikely given nature of intervention. The person delivering the intervention was not blind (p394) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition is reported, but no flow‐chart to clearly view this process – nor any statement that there was no attrition. However, on p398 it is reported that 1 control participant died during the postoperative period ‐ it is not clear whether these data were included |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in Methods were reported in Results, but no mention of a protocol with which to examine this in detail |
| ‘Intention‐to‐treat’ | Unclear risk | As no attrition or problems with fidelity are mentioned this is possible, but it is not clearly stated |
| Other bias | High risk | Unintended postoperative impact of intervention (p401): the investigator who delivered the telephone intervention "observed that during hospitalization, patients in the experimental group asked for her by name, and they reported finding comfort in speaking with a nurse with whom they had already established a link through the telephone contact" – so not clear whether any effects on anxiety at discharge due to phone intervention or this established relationship post‐surgery |
Langer 1975.
| Methods | Randomized controlled trial ‐ analysed as 2 x 2 factorial design | |
| Participants | 60 adults undergoing mixed surgery ‐ including hysterectomy, hernia repair, cholecystectomy, transurethral resection, tubal ligation, D and C ‐ at Yale ‐ New Haven Hospital, USA (dates not provided). No information on age or gender | |
| Interventions |
Control: attention control; administered as all conditions: 20‐minute interview a short time after admission Intervention 1: "Coping device" – trained in cognitive reframing – focusing on positive aspects of a situation. Cognitive intervention Intervention 2: "Preparatory information" – discussed practices e.g. skin preparation, anaesthesia and what could expect after surgery, e.g. nausea, pain; reassured re. high quality of staff. Procedural and sensory information Intervention 3: "Combination" – combined components of Intervention 1 and Intervention 2, in briefer format so still approximately 20 minutes long. Cognitive intervention, procedural and sensory information |
|
| Outcomes | Length of stay | |
| Notes | Author provided details regarding risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p158: "Subjects were assigned to conditions on a stratified random basis, so that the experimental groups were equated on five relevant background factors: type of operation, seriousness of operation, sex, age, and religious affiliation" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is possible that participants were blind, as all had interview of same length. However, all delivered by same investigator – investigator could not be blind p160: "All physicians, nurses, and others on the hospital staff were kept blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information as to who collected length of stay data from patient record. Author confirmed outcome assessor blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost 1 participant from Coping Device group (p161), so it would appear that attrition is being reported – and that the data set was almost complete |
| Selective reporting (reporting bias) | Low risk | All measures reportedly taken were reported in Results, but no mention of a protocol document we can refer to. Author confirmed no measures used that were not reported in Results |
| ‘Intention‐to‐treat’ | Unclear risk | It seems unlikely that patients could have changed groups, but it is not stated that intention‐to‐treat was followed |
| Other bias | Low risk | No other concerns |
Lauder 1995.
| Methods | Randomized controlled trial | |
| Participants | 226 participants randomized ‐ undergoing total abdominal hysterectomy, Southampton, UK (dates not provided). Control group: mean age 43.0, range 29 to 70; intervention mean age 43.8, range 28 to 77 100% female | |
| Interventions |
Control: no discussion re. perioperative nausea and vomiting Intervention: "positive suggestion" but from description seems like procedural and sensory information During a preoperative interview, participants were informed of use of anti‐emetics and told about the expected effect of this |
|
| Outcomes | Pain: 0 to 10‐point scale, in recovery room and on ward 4, 8 and 24 hours post‐surgery | |
| Notes |
Note: primary endpoint: symptoms of nausea and vomiting; pain scores taken as one variable which might impact nausea/vomiting scores Author responded to emails but was unable to provide additional details ‐ no current access to records |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p266: "Patients were allocated to a study (positive suggestion) or control group by means of random numbers generated by a computer program" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p266: control participants not aware that they are missing intervention – "the control group was informed that this was a study of postoperative well being". However, this interview was conducted by the study authors so they would have been aware of the intervention the participant received |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | p267: "Postoperative emetic symptoms were assessed by the patient and documented (blindly) by the nursing staff…pain score…also noted". Unclear rather than `low risk' because not specified that the pain score was documented blindly |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors do report attrition and exclusions (p268) – 10.7% of control group and 16.7% of intervention group. However the numbers of individuals excluded from control group for "asking spontaneous questions about perioperative nausea, vomiting or antiemetics" is not stated |
| Selective reporting (reporting bias) | Unclear risk | No obvious selective outcome reporting but no reference to study protocol |
| ‘Intention‐to‐treat’ | Low risk | No other concerns |
| Other bias | High risk | Very unlikely to be intention‐to‐treat – participants were excluded from control group if they asked questions about nausea, vomiting or antiemetics (p266), which suggests per‐protocol analysis |
Leserman 1989.
| Methods | Randomized controlled trial | |
| Participants | 27 patients undergoing cardiac surgery (21 bypass, 4 valvular, 2 both types, 3 withdrew). Participants were admitted to hospital during summer and autumn 1986 ‐ Boston Beth Israel Hospital, USA. Overall mean age: 68 years (range 47 to 80); intervention mean 65.3 (SD 7.1); control mean 69.6 (SD 9.7). 18 male, 9 female (66.67% male) | |
| Interventions |
Control: both groups received preoperative information and written handouts and visited by nurse to answer questions and give emotional support Intervention: as control; also relaxation training on day of admission to study (2 to 7 days pre‐surgery); nurse helped to practise this. Asked to practise x 2/day, pre‐ and post‐surgery |
|
| Outcomes |
Length of stay Pain: "incisional sensation", scale of 0 to 10. Daily ratings; ratings averaged, excluding day of surgery and postoperative day Negative affect: POMS tension, depression, anger, vigour, fatigue, confusion and total score – administered at discharge |
|
| Notes | Authors provided information regarding: intervention content, risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p112: "We assigned patients randomly into treatment groups, stratified by type of surgery, so that approximately equal numbers of valve and bypass patients were represented in each group". Does not state how randomness introduced. Response from authors: "We block randomized subjects by procedure so that we had representation from subjects who had bypass surgery or valve surgery. Our research director used a table of random numbers to do this and put the randomization number on cards in sealed envelopes. Thus the first CABG patient got the first envelope in that pile and the first valve replacement got the first envelope in that pile" |
| Allocation concealment (selection bias) | Low risk | No information in paper. Information from authors: "Thus the first CABG patient got the first envelope in that pile and the first valve replacement got the first envelope in that pile. We could not forsee the assignment in advance of opening the envelope which was opened at the time of randomization (after the assessment)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p113: "Both groups were visited daily by a nurse who could answer any questions and give emotional support…the nurse also collected questionnaire information and helped experimental patients in practicing their relaxation response". So nurse not blind and unlikely patients were unless ignorant of 2 groupings |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | p113: "Both groups were visited daily by a nurse who could answer any questions and give emotional support…the nurse also collected questionnaire information and helped experimental patients in practicing their relaxation response" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were lost to follow‐up, but from which groups not stated (given small sample size this could be important). Uncertain as to whether/how this would have biased results. Information from authors: this drop‐out occurred before randomization, so there was no loss between randomization and outcome measurement |
| Selective reporting (reporting bias) | Low risk | No apparent selective reporting (intended outcomes appear to be reported) but no reference to a protocol document. Authors confirmed that outcomes were not measured that were not reported: "we did not get additional measures" |
| ‘Intention‐to‐treat’ | Low risk | It does not appear that analysis was per‐protocol, but intention‐to‐treat is not explicitly stated in the paper. Authors were asked: "For analysis, were participants kept in the intervention groups to which they were randomised, regardless of the intervention they received?" Authors reply: "Yes" |
| Other bias | Low risk | No other concerns |
Letterstål 2004.
| Methods | Randomized controlled trial | |
| Participants | 52 patients receiving open repair of abdominal aortic aneurysm at a university clinic department in Sweden (dates not provided). Intervention (n = 18) median age 71.5 (range 56 to 81); control (n = 19) median age = 74.5, range 70 to 83. Intervention: 4 female, 14 male; control 2 female 17 male. Overall: 83.78% male | |
| Interventions |
Control: verbal information from surgeon and nurse; re. disease, treatment, risks Intervention: as control. Also: booklet provided 4 days pre‐surgery, "procedural and sensory information relating to the whole surgical procedure and postoperative course" |
|
| Outcomes | Length of hospital stay | |
| Notes | Of 52 randomized, final sample = 37 Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | p562: "Participants were consecutively randomized into two groups" |
| Allocation concealment (selection bias) | High risk | Reads as if alternative allocation to groups based upon consecutive admission. Thus allocation is predictable and open to manipulation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | States that there is a risk of contamination if participants admitted during the same time period ‐ may have discussed booklets with each other (p566) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p562: 7 excluded from intervention group, 8 from control. Breakdown of reasons only given for overall group – e.g. 4 withdrew consent, but does not state which group(s) these were from. Thus, difficult to determine what impact this could have had on findings |
| Selective reporting (reporting bias) | High risk | Evidence of selective reporting ‐ have omitted to report all days of measurements as captured outcomes on days 1 to 7, but only report days 1, 3 and 7 |
| ‘Intention‐to‐treat’ | Unclear risk | Seems unlikely because information provided on excluded participants and remaining numbers match an ITT analysis, but not clearly stated |
| Other bias | Low risk | No other concerns |
Levesque 1977.
| Methods | Randomized controlled trial | |
| Participants | 140 patients undergoing cholecystectomy (n = 82) or hysterectomy (n = 54) at Montreal University Hospital, Canada (dates not provided). 22 men, 114 (84%) women | |
| Interventions |
Control: "treatment as usual" Intervention: 15 days before admission, 1‐hour meeting with nurse. Gave info re. perioperative activities, demonstrated changes in position, covered breathing and muscle exercises, importance of early ambulation, causes of incisional pain, methods of relaxation and availability of analgesics. Given brochure with information and exercises after group. Also: night before operation – exercises performed and corrected individually. Procedural information, behavioural instruction, relaxation |
|
| Outcomes |
Negative affect: STAI state anxiety, days 2, 3, 5 post‐surgery Length of stay |
|
| Notes | Age information not provided Author replied to email contact, but did not provide details in time for review analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information: participants of each type of surgery were equally and randomly shared between an experimental and a control group. They checked for age, sex and smoking – controlled by randomization |
| Allocation concealment (selection bias) | High risk | Intervention group invited by telephone prior to first measure |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Staff and participants would have known due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lack of information e.g. numbers in groups not reported at any point; group means of some outcomes were not reported |
| Selective reporting (reporting bias) | High risk | Do not provide enough information for meta‐analysis (no sample size by group; no information for length of stay). Also no mention of a protocol document |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Unclear risk | Generally low amount of information |
Levin 1987.
| Methods | Randomized controlled trial | |
| Participants | 40 women undergoing cholecystectomy at a "large, suburban medical centre in the New York metropolitan area", USA (dates not provided). All participants were between 21 and 65 years old | |
| Interventions |
Control: standard care (`CB') (includes procedural information and behavioural instruction – received by all participants) Attention control: (`CA') taped recording of history of medical centre Intervention 1: rhythmic breathing (`RB') (relaxation) – evening prior to surgery, taped instructions, requested to demonstrate to researcher Intervention 2: "Benson’s Relaxation Technique" (`BRT'),relaxation technique without muscle tension. Delivered as per intervention 1 |
|
| Outcomes |
Pain: VAS, evening of surgery and twice (morning and evening) on 2nd and 3rd postoperative days Length of stay |
|
| Notes | Unable to contact author (no longer works at last institution found) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p466: "Using a table of random numbers, participants were randomly assigned to one of four groups prior to the start of data collection. The groups were randomly assigned to one of four treatment conditions" |
| Allocation concealment (selection bias) | Unclear risk | p467: "A research assistant who was a registered nurse obtained the names of potential participants from the operating schedule on the evening prior to surgery and approached those who met the inclusion criteria. From the women who agreed to participate, informed consent was obtained and demographic data collected at this time. The research assistant played the appropriate tape…" – no information about allocation concealment. Seems likely that the research assistant may have had opportunity to foresee – but not clear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | From comments in Discussion, seems likely that participants were blind, at least in the 3 conditions with tapes – p470: "the expectation of participants in the CA group that listening to the taped message would help decrease their pain may have been violated". However, the research assistant administering the intervention would have known |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information – "visited...by one of two data collectors" (p467) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up is reported: p467 states that data were missing for 6 participants for at least 1 data collection point, and then states that resulting sample sizes were CA = 7, CB = 10, RB = 7, BRT = 9. However, not clearly stated from which groups people were lost, and these numbers suggest 7 participants lost to follow‐up, not 6 (40 patients in study) |
| Selective reporting (reporting bias) | Unclear risk | None apparent (all apparent outcomes reported) – but no mention of a protocol document |
| ‘Intention‐to‐treat’ | Unclear risk | Unlikely that did not receive allocated intervention given timing, but no information |
| Other bias | Low risk | No other concerns |
Lilja 1998.
| Methods | Randomized controlled trial | |
| Participants | 46 female patients undergoing surgery for breast cancer (BC) and 55 patients undergoing surgery for total hip replacement surgery (THR) during an 18‐month period. THR patients: intervention group: 13 male, 9 female; control group: 20 male, 8 female; overall 86% male. BC group: median age 53; median age THR group: 65. Setting: 400‐bed hospital in South West Sweden | |
| Interventions |
Control: standard care; information about pre‐ and postoperative routines (procedural information) Intervention: as control plus additional information given day before surgery, ½ hour, by anaesthetic nurse – including importance of patient participation in planning, anaesthesia and surgical procedure, to support patient and attend to their needs, describe operating theatre, care, observation procedures, premedication, training in mobilization after surgery. Also continuity: saw same nurse in operating theatre. Procedural information, behavioural instruction |
|
| Outcomes | Pain: VAS, first 3 post‐surgery days | |
| Notes | Author provided information relevant to `Risk of bias' judgements | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p278: "Patients were randomized on the day before the operation into either an intervention group or a control group and were stratified according to diagnoses". Author: "I randomly picked up sealed envelopes from a pile and distinguished between control‐ and study group" |
| Allocation concealment (selection bias) | High risk | No information in paper. Asked author whether any method was used to conceal allocation concealment. Response: "no" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p278: "The patients were only informed that a study was in progress, but were not informed about the aim and the design of the study. The anaesthetic nurses participating in the study were the only ones who were informed about the aim and design of the trial" So, patients blind, but those delivering intervention not |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No information in paper. Does not state who presented the outcome questionnaires. Asked author whether outcome assessor was blind to intervention allocation ‐ response = "yes" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition data are reported, but not clear exactly how many from each group. Breast cancer: 2 lost to follow‐up (4.3%, no information re. groups): 1 excluded on medical grounds, no information for the other. Total hip replacement: 5 lost to follow‐up (9.09%) (4 withdrew for medical reasons, 3 intervention 1 control); 1 refused post‐randomization Given small sample size, it is possible this led to bias but unclear |
| Selective reporting (reporting bias) | Unclear risk | All stated outcomes were reported, but no reference to a protocol for this to be checked. Authors: "No more outcomes were measured". Only present modal values so cannot enter data into meta‐analysis, and this is not a standard way to present data (would expect medians if means not appropriate) |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Unclear risk | No other specific concerns, but generally poor quality reporting reducing confidence in results – a missing reference, a wrong reference for HADS, errors in sample size numbers and apparent error in placing of VAS data in Table 4 |
Lim 2011.
| Methods | Randomized controlled trial | |
| Participants | 230 participants undergoing abdominal or breast surgery in Southeast Asia (all authors are from Singapore; dates not provided). Overall, mean age 49.0 (SD 9.6). Intervention: 49.34 (8.98); control: 48.70 (10.30). Intervention group: 27 male, 87 female. Control: 30 male, 86 female. Overall: 57 male, 173 female (73.9% female) | |
| Interventions |
Control: "usual information" about "admission procedures"; explanations on indications, nature and postoperative care. Procedural information Intervention: as control group, plus shown list of question prompts and then encouraged to use it when met surgeon 1 day before operation. List of common questions to use to gain clarification – including "what will happen to me during surgery?", "How long do I have to remain in hospital?"; "How much pain will I experience?". Behavioural instruction |
|
| Outcomes | Negative affect: STAI anxiety at Time 3 (1 to 4 days postoperative) and Time 4 (postoperative follow‐up clinic – timing not stated). Unclear: whether trait or state anxiety or some combination reported | |
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p176: "patients meeting the inclusion criteria were randomly assigned to either the experimental (QPL group) or the control group. The participants were asked to select one out of 10 envelopes. Five envelopes contained slips of paper stating “test” and the other five contained slips of paper stating “control” (QPL = question prompt lists)" |
| Allocation concealment (selection bias) | Unclear risk | Unclear as to at what stage during enrolment randomization occurred |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Seems pretty open and participants would have known. Clear that the research co‐ordinator would have known – Encounter Time 2 (p177) states that those with the QPL were "encouraged" to use them – does not say by whom – but someone must have known which group they were in |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | p176: appears that outcomes were taken by the Research Co‐ordinator who met with participants on all 4 occasions. A psychiatrist checked 1 in 5 and was initially blind to [baseline?] anxiety scores – but it is not stated whether he was also blinded to intervention group – and was only checking 1 in 5 to ensure forms were correctly filled, not taking participant responses |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p177: 226 completed "at least the first two interviews" (112 intervention, 114 controls). 207 completed all 4 interviews (101 intervention, 106 control) Interviews 1 and 2: preoperative; interview 3: 1 to 4 days postoperatively; interview 4: "when patients returned to the outpatient clinics for their first postoperative follow‐up appointment" So, interview 3 of primary interest; interview 4 potentially also within remit. In absence of precise data for interview 3, take as for interview 4: loss to follow‐up for overall sample n = 23 (10%). For intervention group, loss at T4 (time 4) = 13 (11.4%); for control group loss at T4 n = 10 (8.6%) Attrition is clearly described and seems fairly even across the 2 groups. However, reasons for attrition were not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol to refer to; unclear which STAI score was reported (both state and trait were described, but only one score mentioned per time point in results). In Table 4 it becomes apparent pain was measured but there is no information on this (included only as a factor that was controlled for) |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Unclear risk | Did surgeons behaviour change across study, in response to patients asking better questions? (i.e. did they start to answer questions more fully if they did not have the prompts?) Also: p176 "We initially intended to recruit patients scheduled for head and neck, abdomen, and breast operations, but decided to concentrate on abdomen and breast patients as these two groups yielded the highest number of patients." Would like more information on this ‐ at what point was this decision made ‐ before or after recruitment started/data collected? |
Lin 2005.
| Methods | Randomized controlled trial | |
| Participants | 62 people undergoing abdominal surgery (stomach, bowel, liver or spleen) at a medical centre in southern Taiwan. Data were collected January to August 2001 | |
| Interventions |
Control: "routine care": included "preoperative physical preparation and education about postoperative breathing and coughing" (behavioural instruction) Intervention: 20 to 30‐minute session, 1 to 3 days before operation. Explained causes of pain, importance of pain management and early out‐of‐bed activities, taught how to decrease pain with non‐medicinal methods, encouraged to request analgesics, discussed setting pain control goal, encouraged expression of feelings and concerns, questions answered. Sensory information, behavioural instruction, emotion focused |
|
| Outcomes |
Pain: VAS (Brief Pain Inventory): intensity at 4 hours and, measured at 24 hours: highest, lowest and average within first 24 hours postoperatively Length of stay |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p253: "Permuted block randomization was used to allocate patients to either the experimental or control group. Those eligible for inclusion were allocated into four sub‐groups according to their gender and whether their surgery area was to be the upper or lower abdomen. A research assistant prepared an envelope containing slips of paper stating ‘experimental group’ or ‘control group’. Patients of the same sub‐group were asked to take a slip of paper from the envelope to determine whether they would belong to the experimental or control group. This method ensured that there was a random distribution of patients and that the number in each group would be fairly evenly distributed in terms of gender and surgery area" |
| Allocation concealment (selection bias) | Unclear risk | See above. Possible for slips of paper to be placed on top to increase allocation to intervention group? |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None. Patients knew group as they drew their slip of paper; the researcher administering intervention would also have known allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "To avoid bias caused by internal validity during data collection, two nurses from another unit of the study hospital were trained as data collectors" (p256) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It would appear that outcome data are complete – no attrition is apparent (the number reported as randomized matches the sample sizes in reported findings) |
| Selective reporting (reporting bias) | Unclear risk | Within the paper, there is no evidence of outcomes being measured but not reported. However, there is some confusion with outcomes being reported that were not reported as being measured, and no protocol is referred to in order to check what was intended |
| ‘Intention‐to‐treat’ | Unclear risk | No information. Reported sample sizes in Results matches those reported allocated, but not clearly stated |
| Other bias | Unclear risk | It would seem that there is a risk of contamination: the intervention was administered on the ward, "If there was another patient in the adjacent bed, curtains were closed to avoid disturbance" (p255) – if a control group participant was in a nearby bed, they would have heard |
Lindeman 1973.
| Methods | Randomized control trial | |
| Participants | 176 patients undergoing any non‐emergency surgery where expected to remain in hospital a minimum of 48 hours at the Luther Hospital, Eau Claire, Wisconsin, USA ‐ a private, non‐profit, community‐owned hospital. Included patients admitted for surgery from 6 February 1972 to 29 March 1972. 73 female, 103 (58.5%) male; aged 16+ (in group 16 to 44 years, mean = 32.03; in 35 to 59 group mean = 53.33; in 60+ group, mean age = 72.03) | |
| Interventions |
Control: visits from anaesthetist and physician/surgeon eve before surgery – described procedures; group class run by nurse – taught deep breathing, coughing, bed exercise. Procedural information; behavioural instruction Intervention: as controls, plus visit from operating room nurse shortly after admission. 2 goals: improve continuity of care and prepare patient. Visit included reviewing charts, confirming information, answering questions, if appropriate, mention might experience discomfort and should request medication if needed; give time to express feelings but not to probe deeply; determine knowledge of surgery and nursing care; explain aspects of care. As controls, plus additional procedural information and behavioural instruction and sensory information |
|
| Outcomes | Length of stay: from day before surgery; day of discharge not counted | |
| Notes | Interested in value of preoperative visit by operating room nurse on both patient anxiety and quality of care Could not locate author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p8: "The investigator randomly assigned patients as their names appeared on the final typed copy of the surgery schedule to one of the two treatment groups" |
| Allocation concealment (selection bias) | Unclear risk | p8: "A listing of the patients to be visited was sent to the head nurse in the operating room. The head nurse then assigned nurses to make preoperative visit. A daily listing of patients included in the study was sent to the nursing units. Unit nursing personnel obtained verbal consent to participate in the study from those patients whose names appeared on the list. Patients were not told whether they would be visited". Unclear: patients would not have foreseen allocation, but does not state whether unit staff who consented participants would have known |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both patients and staff would have known who received the intervention – the nurse visit |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition mentioned. Odd: states 90 randomized into intervention group, but when discussing how the group find the visit, states 96 participants – unclear – could be typo but could reflect varying sample sizes |
| Selective reporting (reporting bias) | Unclear risk | No evidence of measures being taken but not reported, but no reference to a protocol document |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Liu 2004.
| Methods | Randomized controlled trial | |
| Participants | 74 patients undergoing mixed orthopaedic surgery: 39 knee or hip arthroplasty, 12 knee arthroscopy, 15 incision fixation operation for wounds of tibia or fibula; 8 ankle fusion. 39 male, 35 female (52.7% male); mean age = 53.8 years. Authors are based in Shandong Province, China (no other information regarding setting; surgery conducted June to December 2002) | |
| Interventions |
Control: "Traditional model": nurses as experts who decide education and needs, solve problems and are in change. Goal: increase compliance Intervention: "Empowerment model": both nurses and patients are experts; patients decide preoperative education and needs and solve problems supported by nurse. Goal: increase patients' knowledge and encourage to choose and achieve care plan through getting feedback, modifying plan and carrying out plan. Cognitive intervention |
|
| Outcomes | Pain: 0 to 10 VAS; timing not stated | |
| Notes | Extraction from translation from Chinese (translator: Chuan Gao) Confusingly, authors state in discussion "patients from the experimental group…had…low scores on pain compared to the control group with statistical significance" (p5); however, the mean scores reported in results section indicate that the score for the intervention group was higher than control group. However, the difference is very small indeed (2.85 versus 2.50) Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Section 1.2: "All 74 patients were randomised into trial or control groups by drawing ballots" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided as to who provided the intervention, but as it embodied a different approach to preparation highly unlikely individual providing intervention could be blind. Possible that participants were blind but no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers in groups with reported data match numbers reported for pain outcome. No attrition is reported |
| Selective reporting (reporting bias) | Unclear risk | No evidence of not reporting variables measured, but no reference to a protocol document |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Lévesque 1984.
| Methods | Cluster‐randomized control trial | |
| Participants | 125 patients undergoing cholecystectomy at a 750‐bed hospital in French‐speaking community, Montreal, Canada (dates not provided). 25 male, 100 female (80% female); all were between 18 and 65 years (not clear if this is the range); mean age 41.2 | |
| Interventions |
Control: no information Intervention 1: 60 to 70‐minute session, administered by nurse. Included encouragement to express feelings. Concerns, information about pre‐ and postoperative routines, demonstration of respiratory and muscular exercises, change of position and practice of exercises; description of sensation of pain and demonstration of methods to relieve it. Also "tried to help patients become aware of their capacity to influence recovery". Also given booklet containing information and instructions for exercises, including illustrations. Emotion‐focused, procedural information, sensory information, behavioural instruction. Administered at pre‐admission, 15 days before surgery Intervention 2: same as Intervention 1 but administered the afternoon before surgery |
|
| Outcomes |
Negative affect: STAI (French version) State Anxiety, first 3 days after surgery Behavioural recovery: "physical functional ability", first 2 days after surgery Length of stay |
|
| Notes | Author provided some information relevant to risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Unusual method, but reported to include an element of randomization: p228: "Patients were selected from the preadmission list of the hospital and assigned to one of three groups: For the first 2 weeks subjects were assigned to the preadmission experimental group, during the second 2 weeks to the eve‐experimental group, and during a third 2 weeks to the control group. The order of this rotating assignment was randomly chosen and was repeated 13 times". However, no details of whether patients were randomly selected from the list ‐ so potential selection bias into groups |
| Allocation concealment (selection bias) | High risk | No information. However, as the same order was maintained throughout study it would seem likely that it would be possible to foresee group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The nurse delivering the intervention would not be blind; if fully informed, patients also would not be blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p229: "No one in the hospital knew the nature of the dependent variables. Those responsible for administering tests did not know to which group the patient belonged" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no mention of attrition (not clear if this is because of no loss, or simply not reported) |
| Selective reporting (reporting bias) | Low risk | Data for all outcome measures were provided (although no mention of a protocol document). However, no analysis was reported for length of stay – not clear why – most of the other findings reported are not significant so seems unlikely they would be withholding data on the basis of significance. Stage 1 email response: "There were any outcomes measured not reported in the article." We think this suggests that they have reported outcomes |
| ‘Intention‐to‐treat’ | Unclear risk | No information |
| Other bias | Unclear risk | Risk of contamination: Discussion (p234): "The unit staff, who took care of both groups of patients could easily have had access to the booklet given to the experimental patients and could have incorporated certain aspects of the program into their nursing approach" – aspects of this under blinding of other personnel – but also risk of contamination other than blinding influence |
Ma 1996.
| Methods | Randomized controlled trial | |
| Participants | Patients undergoing abdominal surgery April to May 1995. Sample size is unclear ‐ states 52 but likely an error as gender breakdown and no. in each group both give total of 51. 36 male, 15 female; 70.6% male (assuming n = 51). No age information. Setting not described but according to PubMed, lead author is based in a hospital in Beijing, China | |
| Interventions |
Control: normal routine perioperative guidance (insufficient info to categorize) Intervention: as control group. Also: relaxation training 4 days pre‐operation: progressive muscle relaxation. Asked to do this x 3 each day, for 30 minutes each time.Relaxation |
|
| Outcomes |
Negative affect: State anxiety – STAI. 1 and 4 days post‐surgery. Also report measuring Trait Anxiety but no findings reported Pain: measure designed by authors, 1 and 4 days post‐surgery |
|
| Notes | Extraction from translation by Chuan Gao Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Based on the date order of operations, stratified randomisation was used to divide patients into experimental and control groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Insufficient information provided on administration of relaxation training intervention. However, nature of intervention is such that it would be highly unlikely participants or the person administering the intervention would be blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition reported but sample sizes not reported with results so cannot check this. Inconsistent reporting as to whether 51 or 52 participants randomized |
| Selective reporting (reporting bias) | High risk | No reference to a protocol document so cannot check intentions, and reporting poor – pain not reported from pre‐operation/day of operation – only postoperative pain – but Methods state it was measured at all time points. State anxiety – presents what is likely to be means in Figure 1 (not stated). No mention of trait anxiety. Therefore confidence low |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No concerns other than those already noted |
Mahler 1995.
| Methods | Randomized controlled trial | |
| Participants | 30 women undergoing coronary artery bypass graft surgery at Scripps Memorial Hospital, La Jolla, California, February 1992 to October 1994. Mean age: 65.24 (SD 8.21, range 42 to 78) | |
| Interventions |
Control: "standard preoperative preparation"; includes encouragement to ambulate, deep breathe and cough, instructions on how to use incentive spirometer and procedural info, e.g. how long would be in ICU. Behavioural instruction and procedural information Intervention 1: Mastery tape. As control, plus 40‐minute video containing information about procedures and sensations patients could expect. Features narration by nurse and interviews with patients. Edited patient extracts to depict as calm preoperatively with steady progress in recovery, positive and inspiring comments. Procedural and sensory information, cognitive intervention. Intervention 2: Coping tape. As control, and same as Mastery tape except that patient extracts so that they mention concerns preoperatively and recovery as having ups and downs.Procedural and sensory information, cognitive intervention |
|
| Outcomes | Length of stay: number of postoperative days spent in hospital | |
| Notes | Author provided information about control condition and risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p124: "Those who agreed were randomly assigned to view one of the videotapes or to a non‐video control condition". Author: in order to randomize participants to condition, one of the principal investigators (who was not involved in recruiting participants) utilized a block randomization procedure (block sizes of 20). A random number table was used |
| Allocation concealment (selection bias) | Low risk | Author: condition assignment was concealed from researchers in consecutively numbered, sealed, opaque envelopes. Once a participant had been enrolled and initial measures/questionnaires were completed, the researcher opened the envelope to reveal the condition letter (A, B or control) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Author: "The videotapes were marked only with a letter, researchers were not aware of which letter was associated with which condition, and the researcher did not remain in the room when participants viewed the video. Thus, researchers were blind to particular video condition (but not to whether the participant was in a video vs the control condition) throughout their contact with each participant." So, researchers were blind between coping and mastery conditions, but not possible for both participants and researchers to be blind in terms of whether or not video was seen/provided (i.e. for intervention 1/intervention 2 versus control) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Extracted from notes (length of stay) – "blind to condition" (p124) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No./% participants lost to follow‐up: 30 patients recruited. 1 (3.3%) in coping tape condition was excluded because of "severe postoperative complications" (p125). No others lost for length of stay outcome |
| Selective reporting (reporting bias) | Unclear risk | All means/SDs provided; no evidence of measures being taken but not reported (but no reference to a protocol document) |
| ‘Intention‐to‐treat’ | Low risk | Given that 2 patients were kept in Coping group even though discharged early this seems highly likely, but does not report that videos were viewed as intended. Author: "All participants remained in the condition to which randomized" |
| Other bias | Low risk | No other concerns |
Mahler 1998.
| Methods | Randomized controlled trial: 1 control group; 3 intervention groups | |
| Participants | 268 men undergoing 1st time coronary artery bypass graft surgery (without associated procedures) at 2 hospitals in California, USA: Scripps Memorial Hospital (SMH), San Diego Veterans Affairs Medical Centre (SDVAMC) (dates not provided). Mean age: 62.52 years, SD 8.80, range 40 to 80 | |
| Interventions |
Control: standard preoperative preparation, including information on how surgery is performed, length of typical stay in ICU and hospital, instructions regarding e.g. deep breathing, coughing, ambulation (procedural information, behavioural recovery) Intervention 1: "nurse tape": 15‐minute video presented evening before surgery. Nurse narration including procedures prior to surgery, anxiety, surgical procedure, intensive care phase, coming out of anaesthesia, intubation. Emphasises need for deep breathing and coughing, incentive spirometer use and ambulation. Pain, fatigue and emotional experiences discussed. Procedural and sensory information; behavioural information; emotion‐focused Intervention 2: "mastery tape": as Intervention 1, but interspersed with clips of interviews with patients as "mastery" models – "steadily improving without setbacks". 39 minutes. Procedural and sensory information; behavioural information; emotion‐focused Intervention 3: "coping tape": as Intervention 1, interspersed with clips of "coping" models – attention to setbacks, but coming through them. 39 minutes. Procedural and sensory information; behavioural information; emotion‐focused |
|
| Outcomes |
Behavioural recovery: monitoring of ambulation with device that counts movements using mercury tilt switch. Worn on days 2, 3 and 4 post‐surgery at one hospital (SMH); days 3, 4, 5 post‐surgery at other hospital (SDVAMC). Worn from morning to late afternoon/early evening Length of stay: postoperative days in hospital, medical chart |
|
| Notes | Author provided information related to `Risk of bias' assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p40: "randomly assigned to view one of the three videotapes or to a control condition". Author: "In order to randomize participants to condition, one of the principal investigators (who was not involved in recruiting participants) utilized a block randomization procedure (block sizes of 20)." and "A random numbers table was used to generate the randomization sequence" |
| Allocation concealment (selection bias) | Low risk | No information in paper. Author: "Condition assignment was concealed from researchers in consecutively numbered, sealed envelopes. Once a participant had been enrolled and initial measures/questionnaires were completed, the researcher opened the envelope to reveal the condition letter (A, B, C, or control). The envelopes were opaque and the paper inside was folded so that there was no way for the researcher to see the condition until opening the sealed envelope" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information in paper. Given nature of the intervention, it is possible that participant and personnel were blind – at least within the 3 videos (unlikely blind between control‐video conditions). Author: "The videotapes were marked only with a letter, researchers were not aware of which letter was associated with which condition, and the researcher did not remain in the room when participants viewed the video. Thus, researchers were blind to particular video condition (but not to whether the participant was in a video vs the control condition) throughout their contact with each participant. Participants were not aware that the study involved different conditions (at enrolment they were told simply that the study was concerned with examining some of the best methods of preparing patients for surgery, and they were told what participation would involve [e.g., completing questionnaires at 5 time points, abstraction of some information from their medical charts, etc.], but there was no mention of different conditions") |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome extracted was taken from patient notes by someone who was unaware of the allocation status of the patient “indices of preoperative physical status and speed of recovery were abstracted (unaware of condition) from participants' medical charts.” (p40) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | p40: "Ten participants were eliminated from the experiment due to death or debilitating postoperative complications (e.g. stroke), leaving a final sample of 258". No information as to how the attrition distributed across groups. Author: "Four participants were lost from the Coping tape condition, 2 were lost from the Mastery Tape condition, 3 were lost from the Nurse Tape condition, and 1 was lost from the control condition (none withdrew from the study ‐ all were lost due to serious medical complications, e.g., death during surgery, debilitating stroke during the peri‐operative period)" |
| Selective reporting (reporting bias) | Low risk | No suggestion that measures were taken that were not reported, and analyses clearly stated prior to reporting of findings. No reference to a protocol document to check this but email from authors: "There were no major outcomes that were not reported in the paper" |
| ‘Intention‐to‐treat’ | Low risk | No information in paper. Author: "Intention to treat was used for all who were followed‐up. However, we did not impute data for those 10 patients who were entirely lost to follow‐up due to death or debilitating surgical complications" ‐ meets our criterion for intention‐to‐treat |
| Other bias | Low risk | No other concerns |
McDonald 2001.
| Methods | Randomized controlled trial | |
| Participants | 40 patients randomized; undergoing knee/hip replacement/revision at a large urban medical centre in Connecticut, USA (31 followed up). Data were collected June 1998 to January 199. Mean age 74 (SD 6.16), range 65 to 83; 8 were men, 23 (74.2%) women | |
| Interventions |
Both groups: "preoperative joint replacement class" (attended by 24/31 participants followed up): included pre‐surgical preparation, general routine to expect postoperatively; exercises and activities, discharge planning, brief discussion of pain management: informed of importance of pain medications, that patient‐controlled analgesia (PCA) might be an option, and that should tell nurses about pain (procedural information, behavioural instruction) Control: either after this session or at home: 10‐minute narrated slide show describing use of pain rating scales Intervention: either after session or at home: 30‐minute narrated slide show and handout. Addressed pain management – understanding pain, pharmacologic‐ and non‐pharmacologic management. Also pain communication education, included: participant as expert, responsibility to report pain, ways of communicating pain e.g. using scales, checking if health professional understood, strategies for introducing topic and managing discussion. Behavioural instruction |
|
| Outcomes |
Pain: sensory dimension of MPQ‐short form (Melzack 1987) Pain: intensity: present pain index (6‐point scale) Pain: affective dimension of pain: MPQ‐short form All: evening on day of surgery, post‐surgery days 1 and 2. Asked to describe average pain for day |
|
| Notes | Author provided some risk of bias information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p405: "randomly assigned" (by coin toss) |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | States "double blind" (p404). There is a control intervention so "double" seems to imply participants were blind and outcome assessment blind. p406: "The first author administered the intervention and narrated the slides for both groups" so could not have been blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Trained data collectors, blind to the subject condition, measured the elders’ postoperative pain with the MPQ‐SF" (p406) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reports attrition of 9 of the original 40 participants – "unable to complete all their data for the 3 days because of factors such as nausea and vomiting". Given that 13 participants in intervention and 18 in control remained, it seems likely that the 9 were not evenly distributed across groups |
| Selective reporting (reporting bias) | Low risk | The only measure taken but not reported was day 3 postoperatively – explanation – that many participants were discharged – would seem sensible and not indicative of selective outcome reporting. However, no protocol to refer to. Author: "We reported all of our outcomes. There were no additional outcomes" |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
McDonald 2004.
| Methods | Randomized controlled trial | |
| Participants | Recruited 102 patients undergoing total hip or knee replacement surgery at 2 medical centres in Connecticut, USA; data collected January 2000 to August 2001. Data provided for only the 41 with full data, enrolled before change in routines, who had knee surgery only. Over this sample, mean age = 71.8 (SD 5.41, range 65 to 88). Control (n = 9) mean age = 72.2 (7.33); Intervention 1 (n = 15) mean age = 70.5 (3.80); Intervention 2 (n = 17), mean age = 72.8 (5.57). Percentage female: control: 77.8%; Intervention 1: 73.3%; Intervention 2: 47.1% | |
| Interventions |
Control: "standard preoperative teaching" Intervention 1: pain management group. As Control plus 10‐minute film with handout, included: defining pain, understanding causes, pain assessment and use of rating scales; preventive approach to control; drug management; fears of addiction and dependence; controlling side effects; non‐drug modalities; description of imagery, distraction, massage, relaxation; demonstration of relaxation and imagery; behavioural instruction Intervention 2: pain communication skills. As Intervention 1 (so relaxation and behavioural instruction) plus 4‐minute film and handout for both films. Derived from Communication Accommodation Theory strategies: interpersonal control – patient as expert, responsible for pain report and treatment response; team work. Interpretive competence: strategies to describe pain e.g. using scales; discourse management: introducing pain topic, managing discussion with health professional; approximation strategies: how people communicate and adjusting how talk in response to others. Additional behavioural instruction Across all groups: mean time between intervention and surgery: 15.6 days (SD = 13.10) |
|
| Outcomes |
Pain: sensory dimension of MPQ‐short form Pain: intensity: present pain index (6‐point scale) Pain: VAS Pain: affective dimension of pain: MPQ‐short form Measured in person on postoperative days 1 and 2 and over the phone on 1st and 7th day after discharge from hospital; late afternoon to early evening. (VAS omitted from phone interviews – visualization not possible) |
|
| Notes | While they provided their analysis findings for their originally planned analysis, they only provided data for the smaller sample of 41 patients who underwent knee replacement surgery Author provided some risk of bias information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p839: "Random assignment to group was accomplished through use of a table of random numbers". But p840: "Analysis with the sensory and intensity pain measures for older adults who had only total knee replacements and had not attended the new preoperative class at Site 1 indicated that differences in pain neared significance on postoperative Day 1 for the communication group and the pain management only group…further sampling was continued for preoperative total knee replacement older adults at Site 2 with random assignment to the communication group or pain management only group", so these latter participants could not be allocated to control group |
| Allocation concealment (selection bias) | Unclear risk | p844: "After determining which group the older adult had been randomly assigned to, the first author showed the appropriate film or films" – no mention is made of measures to conceal allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p844: the first author knew the groupings and administered interventions so not blind; seems unlikely that participants were blind as would know whether or not had an additional part to training – as randomization happened after the standard session |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p844: "The second author, who was blind to the older adults’ conditions, obtained the postoperative pain measures" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study deviated significantly from protocol in terms of which participants’ data were analysed by the authors. A full analysis, following original plans, is detailed, but this would be after the later recruitment only randomized to the 2 intervention conditions. Means and SDs for the full sample are not provided – only following the revised approach Final sample: 41 (of 102). 22: withdrawn during study: delirium (9), surgery cancelled/rescheduled (7), postoperative complications (4), severe pain requiring immediate intervention by data collector (2). 8: removed from sample because Hospital 1 revised their preoperative class and postoperative protocol – physical rehabilitation during hospitalization differed. 31: patients having hip replacements – in preoperative class, informed that pain would be less of a problem for them – information may have affected their interest and motivation to learn pain information. 2 of these – also in new preoperative programme; but 2 additional adults had incomplete data, so final sample 41. Attrition not detailed by group |
| Selective reporting (reporting bias) | High risk | The reasons provided for not including the affective dimension of pain in the revised analysis were are unconvincing (p846): "Affective pain was removed as a dependent variable because of the low internal consistency of the scale and the difficultly participants expressed when responding to the measure". However, authors: "We reported all of our outcomes. There were no additional outcomes." This analysis does not impact on the review's findings/analysis. However, also did not report pain intensity findings for postoperative days 1 and 2, therefore high risk |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
McDonald 2005.
| Methods | Randomized controlled trial | |
| Participants | 50 patients undergoing total knee replacement surgery at one of 2 medical centres in Connecticut, USA. Data collection: February to November 2002. Mean age 73.9 years (SD 5.36, range 65 to 82). Most (68.4%) of the 38 patients whose data were analysed were women | |
| Interventions |
Control: standard preoperative teaching and 10‐minute video (and handout) – general pain management. Content tested during McDonald 2001 and McDonald 2004: defining pain, understanding causes, pain assessment and use of rating scales; preventive approach to control; drug management; fears of addiction and dependence; controlling side effects; non‐drug modalities; description of imagery, distraction, massage, relaxation; demonstration of relaxation and imagery. Behavioural instruction likely Intervention: as control (so included relaxation), but additional 5 minutes to video: pain communication. Derived from Communication Accommodation Theory strategies: interpersonal control – patient as expert, responsible for pain report and treatment response; team work. Interpretive competence: strategies to describe pain e.g. using scales; discourse management: introducing pain topic, managing discussion with health care professional. Behavioural instruction (beyond any that might have been received by control group) Across both groups: mean of 19.3 days between intervention and surgery |
|
| Outcomes |
Pain severity: Brief Pain Inventory Short Form ("the average pain, pain at the time of measurement, worst pain and least pain in the past 24 hours were combined for a mean pain severity score") Post‐surgery days 1 and 2, post‐discharge days 1 and 7 |
|
| Notes | Author provided some information about risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p115: "random assignment to group by a computerized coin toss" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As the principal investigator watched the videos with the participant (p116), the researcher would have known which intervention was received, even if the participant was blind (no information on whether participant was blind) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The postoperative pain measures were gathered…by the fourth author, who was blind to the participants’ conditions" (p116) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition is detailed, but not by group, so difficult to say whether different reasons for attrition (surgery cancelled for 6; 2: postoperative complications; 2 screened positive for delirium; 2 had incomplete data for analysis. Of the 38 who stayed in the study, there were 19 in each group, which suggests attrition did not differ by group, but may have differed by reason for attrition (or "randomisation" may have been manipulated to achieve this) |
| Selective reporting (reporting bias) | Low risk | No evidence of outcome measures not being reported, but no reference to a protocol document. Authors: "We reported all of our outcomes. There were no additional outcomes." |
| ‘Intention‐to‐treat’ | High risk | p112: "The final sample consisted of 38 (of 50) older adults who fulfilled the criteria for on‐treatment analysis (Moher 2001) by meeting the eligibility criteria and completing the intervention and all outcome measures". This would suggest not intention‐to‐treat |
| Other bias | Low risk | No other concerns |
McGregor 2004.
| Methods | Randomized controlled trial (a "pilot" study) | |
| Participants | 39 adults undergoing total hip arthroplasty (data for 35) ‐ at Charing Cross Hospital, London, dates not provided. Overall mean age 71.9 (SD 9.3), range 51 to 92 years. Intervention mean age 70.8 (SD 9.3), control mean age 72.8 (SD 10.1). Most (25, 71.4%) female, 10 male | |
| Interventions |
Control: standard care, included description of surgery, its risks and approximate length of stay. Procedural information Intervention: seem to have received standard care. Also: information booklet – information on surgery, pre‐ and postoperation stages, rehabilitation including exercise regimes, answers to frequently asked questions. Class 2 to 4 weeks pre‐operation – enforced booklet, checked could do exercises and understood how to use walking aids postoperation and how to make adaptations needed in homes.Procedural information (beyond controls), behavioural instruction |
|
| Outcomes |
Pain: VAS and WOMAC pain Behavioural recovery: WOMAC function, Barthel Index Negative affect: PANAS Length of stay: days in hospital |
|
| Notes | Author was not contacted as insufficient time prior to analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p465: "at preadmission, patients were allocated randomly into either group A or group B…patients were randomized by age and not by functional status" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated but very unlikely that either patients or staff administering intervention could be blind – involved attending a preoperative hip class |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four of 35 lost to follow‐up, all from the Intervention group (reducing from 19 to 15). Appear to have retained all 20 in Control group. Concerning that 21% of intervention group dropped out – no information provided as to why |
| Selective reporting (reporting bias) | High risk | All outcomes were mentioned in Results, although very briefly and only positive findings reported (assume not significant otherwise). However, data were not provided for the negative affect outcome of the review |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Miró 1999.
| Methods | Randomized controlled trial (2 x 2 design: control/intervention x high/low monitoring style) | |
| Participants | 93 women undergoing hysterectomy with double oophorectomy in Spain (dates not provided). Mean age: 55 years (range 29 to 59) | |
| Interventions |
Control: attention control, 30 minutes, 1 week before surgery Intervention: 30‐minute session, 1 week before surgery, plus handout: relaxation with instructions of deep breathing and guided imagery |
|
| Outcomes |
Pain: used numerical rating scales (0 to 100) to rate: 24 hours and 72 hours post‐surgery: pain at standing, walking, moving in bed "Follow‐up" (timing not specified – possibly 15 days post‐surgery): "overall estimation of the pain level" |
|
| Notes | Used Spanish version of Miller Behavioural Style Scale (Miller 1987) to assess information‐seeking style of patients. Scale gives 2 main scores: monitoring score and blunting score. Used monitoring subscale (justification p472); used the mean score of the subscale as obtained during Spanish translation and validation as the cut‐off point to allocate participants into high/low monitoring groups (mean = 9.64). Scores on the subscale: low monitoring group mean = 6.04 (SD 0.89), n = 43; high monitoring: mean = 10.04 (SD = 2.56), n = 49 Author provided some information regarding risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p473: "randomly assigned to one of two groups; a random digits table was used to assign individuals to the groups" |
| Allocation concealment (selection bias) | Unclear risk | p473: "Subjects were allocated into the groups before any personal contact had taken place" – not sure if this refers to allocation concealment? |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As there is an attention control, the participant may have been blind, but the clinical psychologist was not – "All interviews and interventions were conducted individually by the same clinical psychologist" (this psychologist was blind to informational coping style) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated who the outcome assessors were. If outcome assessment is included in the statement "all interviews and interventions were conducted individually by the same clinical psychologist" then the outcome assessors were not blind. However it is not clear how the outcome questionnaire was presented |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was excluded from analyses "because of inadequate data" (does not state from which group). While it is not known which group this individual is from, it seems unlikely that such low attrition (1.08% of 93) would impact on findings |
| Selective reporting (reporting bias) | Unclear risk | No appearance of outcomes outline in Methods not being reported in Results (although there is some lack of clarity over activity measures). However, no mention of a protocol to check this. Email from author – in response to question as to whether there were outcome measures that were not reported: "probably yes, but I cannot remember for sure at this moment" |
| ‘Intention‐to‐treat’ | Unclear risk | Not explicitly stated. However, seems unlikely that would have received a different treatment to that to which allocated given that both conditions implemented directly by the psychologist (and groups allocated before this meeting). Also, reported that all participants in Relaxation group reported having practised the relaxation (p473) |
| Other bias | Low risk | No other concerns |
Neary 2010.
| Methods | Randomized controlled trial | |
| Participants | 64 patients undergoing Minimally Invasive Radioguided Parathyroidectomy for primary hyperparathyroidism at a university teaching hospital in Ireland, July 2007 to December 2008. Overall (n = 51) mean age: 61.4 years (SD 13.6); control (n = 21) mean age = 61.5 (SD 16.0); intervention (n = 30) mean age = 61.4 (SD 11.9). Control group: 4 male 17 female; intervention 7 male, 23 female; overall: 11 male, 40 female ‐ 78.4% female | |
| Interventions |
Control: access to "standard" website with limited information – e.g. patient detailed, background information of surgeon Intervention: access to "enhanced" website – information of full patient pathway, "stepwise description" of "clinical course, from initial diagnosis…to eventual discharge". Could also request more information and email staff for additional information. Option of completing quiz. Procedural information |
|
| Outcomes | Pain: VAS, maximum score 10. Postoperative but precise timing unclear | |
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p237: "Patients were then assigned to either a standard Web site or enhanced Web site by permuted block randomization" – does not stated how random element introduced |
| Allocation concealment (selection bias) | Low risk | p237: "to minimize any potential bias…randomization was performed by a person not involved in recruitment or data collection and the recruiter and interviewer were not aware that the study was block randomized prior to its completion" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | p237: "patients and all study personnel were blinded as to which group patients were allotted. Patients were informed only that the Web site they accessed would give them basic details about the surgery and were provided with a username and password that allowed access to their allotted Web site without any researcher knowing to which group they had been randomized" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p237: "the recruiter and interviewer were not aware that the study was block randomized prior to its completion" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p238: 67 met inclusion criteria; 3 declined so 64 randomized in equal numbers to control and intervention group. Of these, 13 did not access website (11 control, 2 intervention) and were not included in analysis. 51 did access website (21 control, 30 intervention) – no further loss to follow‐up. So, attrition was well reported but a large difference in attrition between the groups so may be bias as a result |
| Selective reporting (reporting bias) | Unclear risk | Did not find evidence of outcome measures being mentioned in Methods but not reported in Results. However, no access to protocol (and preoperative depression was not mentioned in Methods but reported in Results) |
| ‘Intention‐to‐treat’ | High risk | Not intention‐to‐treat: "Patients who were randomized who did not subsequently access their relevant Web site were excluded from statistical analysis" (p237) |
| Other bias | Low risk | No other concerns |
O'Connor 2014.
| Methods | Randomized controlled trials | |
| Participants | 85 patients with rectal cancer diagnoses due to undergo surgery were randomized. 6 sites in 4 healthcare Trusts in Northern Ireland; recruited January 2009 to May 2010. Age range: 42 to 84; mean age Intervention group: 63.12 (SD 10.69); mean age Controls 68.29 (SD 9.34). 49 (64.5%) male; 27 female | |
| Interventions |
Control: usual care (demonstration with stoma appliances, discussion of condition, options and concerns with specialist nurse). Also: "generic colorectal cancer and stoma information leaflets". Behavioural instruction Intervention: usual care as Controls. Also, "guided tour" of pack of 14 leaflets, including "surgery for cancer of the rectum" and "coming into hospital"; specialist nurse’s approach based on Knowles’ Process Model (an approach to education). Procedural information |
|
| Outcomes | Negative affect: Hospital Anxiety and Depression Scale (anxiety and depression) after surgery, prior to discharge | |
| Notes | Author provided some information about risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p184: "Randomisation was provided by an independent research secretary, using a computer generated list of random numbers" |
| Allocation concealment (selection bias) | Low risk | p184: "The SCNS [stoma care nurse specialist] telephoned the randomisation service for an allocation code and assignment to either intervention or control group. Blinding of the researcher as to the random allocation group of participants was used to reduce bias" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Difficult to see how the SCNS delivering the interventions could have been blind – p184: "the SCNS who delivered either the new information pack intervention) or the information currently provided in usual care (control)" – so the same person did either |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p187: at time 2 (post‐surgery, prior to discharge), analysed data for 43 of 47 for primary outcome (satisfaction). The excluded 4 did not receive the intervention (1 withdrew, 3 "randomised too soon"). Control group: analysed data for 33 of 38 for primary outcome. The excluded 5 did not receive the allocation (3 withdrew, 2 "randomised too soon"). In addition, 4 participants "felt unable or unwilling to complete the secondary outcome measures" (p186) – groups not specified. So, in total, 13 participants did not provide data for the outcomes of interest to us – 13 of 85 = 15.3% |
| Selective reporting (reporting bias) | High risk | Primary and secondary outcomes clearly stated and all outcomes are mentioned in results. Author: also completed the Miller Behavioural Style Scale (but this is unlikely to be an outcome measure). However, no details (e.g. mean, SD) provided for outcomes relevant to review |
| ‘Intention‐to‐treat’ | High risk | Flow chart p187: 4 in intervention group and 5 in control group did not receive their allocated intervention and are excluded from analysis. This would suggest carrying out per‐protocol analysis |
| Other bias | Unclear risk | Found a significant difference for age between groups – mean age in intervention group significantly lower (intervention group mean = 63.12 (SD 10.69); controls: 68.29 (SD 9.34). Also, odd that group size so different after randomization (intervention n = 47; control n = 38) ‐ unclear if randomization was effective in generating comparable groups Also, the SCNS delivering the intervention in each site would become increasingly aware of the essence of the intervention protocol (the tailoring of information using the Knowles Process Model) during the study. There is a risk of cross‐contamination of the intervention into the control condition as the only differences seemed to be in the type of information leaflets (we are not aware how different these were) and the tailoring of information by the SCNS. There is no assessment of intervention fidelity to assess for this potential bias |
Oetker‐Black 2003.
| Methods | Randomized controlled trial | |
| Participants | 108 women undergoing total abdominal hysterectomy at a 486‐bed teaching hospital in the Midwest, USA (dates not provided). Mean age: 41 years (SD 5.87), range 25 to 52 | |
| Interventions |
Control: usual care: information in 4 areas: mobility, turning, deep breathing, pain reduction through relaxation – explanation about importance and how to do the behaviour. Behavioural instruction, relaxation Intervention: using model of self efficacy. Same explanation re. importance and how to do behaviours, also oral persuasion, vicarious learning activities and modelled behaviours; participants demonstrated the behaviour until correct. Behavioural instruction, relaxation, cognitive intervention |
|
| Outcomes |
Pain: VAS, day 1 post‐surgery; bodily pain (Health Status Questionnaire) at discharge Negative affect: State anxiety (STAI), day 1 post‐surgery and at discharge Behavioural recovery: time able to ambulate (day 1 post‐surgery) and physical functioning subscale of Health Status Questionnaire (at discharge) Length of stay |
|
| Notes | Very limited in terms of data provided on outcomes, but these measures were taken. Authors provided some information about risk of bias but could not provide much information additional to that in the paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p1221: "assigned via a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not clear what information participants were given, but the data collectors who implemented the intervention would have known (each implemented both protocols, p1225) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p1224: "Two different data collectors, blind to the experimental condition of participants, collected data on the postoperative measures of anxiety, pain, ambulation, vital capacity, preventable complication rates, length of stay, and health status" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall attrition = 17% (p1226). No information on loss to follow‐up (or n) for each group or by outcome; attrition can be deduced from degrees of freedom (table 2) but not clear |
| Selective reporting (reporting bias) | High risk | Hypotheses very clearly stated and findings by hypothesis are provided. However, do not report differences in anxiety at discharge (this was included in the hypothesis) and no reference to a protocol document to refer to. Details of analyses (mean, SD) were only provided for the statistically significant result ‐ hence `high risk' decision. Author response: "No additional outcomes" |
| ‘Intention‐to‐treat’ | Low risk | No information in paper; authors state: "Yes groups were kept same" |
| Other bias | Low risk | No other concerns |
Oliphant 2013.
| Methods | Randomized controlled trial | |
| Participants | 199 women undergoing pelvic reconstructive and/or urinary incontinence repair at the Women’s Center for Bladder and Pelvic Health, Magee‐Womens Hospital of the University of Pittsburgh Medical Center (USA) (recruited March 2008 to March 2011). Intervention mean age 59.7 (SD 11.7, N = 99); control mean age 57.7 (SD 9.8; N = 100) | |
| Interventions |
Control: routine discussion of risks and potential for need of catheterization with surgeon Intervention: preoperative video instruction in clean intermittent self catheterization.Behavioural instruction |
|
| Outcomes |
Negative affect: State anxiety (STAI) at time of postoperative voiding time failure (following surgery – specific timing not stated) and at time of discharge Length of stay is reported (as a characteristic on which the 2 groups were compared rather than as an outcome. Median stay for both groups = 1, IQR 0 to 2, so it might be a standard stay length with little variation and never intended to use as DV) |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p420: "Randomization to either preoperative video teaching or usual care was performed in blocks of ten using a computerized random sequence generation program" |
| Allocation concealment (selection bias) | Unclear risk | p420: "Randomization assignments were kept in a log and assigned following completion of consent and enrolment." This could be adequate but it is not clear who kept the log and whether the person consenting would have known |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p420: "Study coordinators, patients, and clinical providers were not masked to assignment following randomization" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | p420: "Study coordinators, patients, and clinical providers were not masked to assignment following randomization" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 99 randomized to Intervention, 100 to Control. Flow chart p421 – states these are the numbers analysed, but also states that 5 withdrew from Intervention and 10 withdrew from Control (reasons not provided). N is not presented in table with anxiety outcomes. Length of stay data: n = 93 for Control and 93 for Intervention – does not explain this difference |
| Selective reporting (reporting bias) | Unclear risk | The trial is registered with ClinicalTrials.gov – the 2 stated outcomes – primary anxiety, secondary satisfaction were reported (although only anxiety in full detail at each time point). Means and SDs provided for anxiety (although Ns unclear); if include length of stay also, medians and IQRs reported so cannot include in meta‐analysis, but this is completely reported – and paper states that reported in this way where not normally distributed |
| ‘Intention‐to‐treat’ | Unclear risk | Need more information. Flow chart (p421) states number analysed per group = number randomized to each group. However, also reports patients who withdrew without indicating how/if imputed data. So, potentially intention‐to‐treat but unclear |
| Other bias | Unclear risk | 379 declined to participate; 111 missed/incomplete research contact; 1 withdrew pre‐randomization. Are those who took part representative? |
Omlor 2000.
| Methods | Randomized controlled trial | |
| Participants | Patients undergoing inguinal hernia surgery or thyroidectomy in Essen, Germany, January 1997 to June 1998. Participants randomized: 211; final sample 208: 107 (51.4%) male; mean age 55.7 years | |
| Interventions |
Control: treatment as usual Intervention: 45‐minute intervention – standardized text ready by psychologist. Included relaxation techniques (guided imagery, breathing exercise, components of autogenic training (warmth, heaviness), progressive muscle relaxation) and familiarization with route from ward to theatre. Relaxation, procedural information |
|
| Outcomes |
Pain: VAS post‐surgery day 1, day of discharge (how much pain at moment) Length of stay |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization procedure not specified |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The individual providing the intervention would have known the condition |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided on any blinding procedure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are provided for full sample |
| Selective reporting (reporting bias) | Unclear risk | No obvious selective reporting but a protocol not referenced and no response from authors |
| ‘Intention‐to‐treat’ | High risk | "Zitronentest" (`lemon test') used to exclude people from intervention group after randomization for not being sufficiently susceptible to intervention methods. It was not also applied to the control group |
| Other bias | Unclear risk | No other concerns |
Oosting 2012.
| Methods | Randomized controlled trial: a pilot/feasibility study | |
| Participants | 30 frail older adults undergoing total hip arthroplasty in orthopaedic department of the Gelderse Vallej Hospital, Ede, Netherlands (dates not provided). Intervention group mean age 76.9 (SD 6.3); control group mean age 75.0 (SD 6.3). Intervention group: 14 female, 1 male. Control: 10 female, 5 male. Overall: 24 female, 6 male ‐ 80% female | |
| Interventions |
Control: usual care – group session 3 weeks preoperative; including information about the operation, walking with crutches and exercises for postoperative phase. Procedural information Intervention: as control group. Also: physical therapists supervised exercise sessions at home 30 minutes/session, twice/week for 3 to 6 weeks. Also exercised by selves (or with help) 4 times/week and aimed to walk a minimum of 30 minutes/day. Training tailored to participant and home environment; intensity and no. repetitions increased over time and functional activities made more challenging. Given pedometer and kept diary. Behavioural instruction. (Note: relaxation was offered in intervention, but only as a treatment option for patients who reported symptoms/pain after training) |
|
| Outcomes |
Length of stay Behavioural recovery: Iowa Level of Assistance Scale (ILAS), day 4 post‐surgery – ability to function in daily life |
|
| Notes | Author provided some information about measures and risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p611: "Participants were randomly assigned to the intervention or control group by a research assistant not associated with the study. Randomization took place after stratification by age…, using prepared envelopes per stratum. Within each stratum a permuted block randomization with a block size of 10 was used" |
| Allocation concealment (selection bias) | Low risk | Participants were randomized after informed consent, by someone who was not associated with the study (p611) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p611: "The 2 physical therapists (RHN and CMD) who performed the training and the patients were not blinded to treatment allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p611: "outcome assessors (EO and SMA) were" (blinded to treatment allocation) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Generally, attrition is well reported but reasons for this are not given for time 2 (T2) – the time of interest to us, but I think not of primary interest to the researchers Intervention group: 1 participant: no surgery (because of co‐morbidities). N for length of stay outcome: 29. Functional mobility score (ILAS) at T2: intervention n = 12; control n = 13. Attrition is low, but sample size is small, and difficult to assess risk of bias without knowing reason for attrition |
| Selective reporting (reporting bias) | Low risk | No obvious selective reporting but no reference to a protocol document. Data are provided for outcomes of interest to the review. Author: "We had no other outcomes" |
| ‘Intention‐to‐treat’ | Low risk | p612: "intention‐to‐treat analyses were used" |
| Other bias | Low risk | No other concerns |
Osinowo 2003.
| Methods | Randomized controlled trial | |
| Participants | 33 patients recruited from surgical or gynaecological wards of hospitals in Ibaan and Ogbomosho, South West Nigeria (dates not provided). Mean age: 32.72, SD (assumed) 15.83, range 17 to 61 (in‐text range given as 5 to 61 but believe error ‐ 2 abstracts (English and French give 17). 18 (54.5%) male; 15 female | |
| Interventions |
Control: no treatment Intervention 1: Rational Emotive Therapy: objectives included to "determine extent to which able to dispute irrational beliefs"; "inform the patients they are responsible for …psychological stress…"; "abolish illogical reasoning and thought processes".Cognitive intervention Intervention 2: Self‐Instructional Training. Objectives included to "make the patients aware of their thoughts, feelings and consequent physiological reactions"; "know the patient’s inner thoughts about the proposed operation"; "explore the thoughts expressed".Cognitive intervention Both interventions: 2 sessions pre‐surgery |
|
| Outcomes | Negative affect: STAI state anxiety; HADS Anxiety; HADS Depression | |
| Notes | Quality of reporting unclear and muddled throughout paper. Used standardized measures with published psychometrics. However, these were translated into Yoruba ‐ no information provided on this process/validation of translated questionnaires Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p339:"Thirty‐three (33) patients awaiting surgery were randomly selected for the study. Eleven (11) patients each were distributed into self‐instructional training, control and Rational Emotive Therapy groups with each subject having equal chances of being selected into any of the three groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information – but highly unlikely either patients or person delivering intervention would be blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition is reported. However, from degrees of freedom, the no. participants analysed = no. participants randomized |
| Selective reporting (reporting bias) | High risk | Comparisons by group were not reported for STAI anxiety. Reporting generally unclear; no reference to a protocol document. While means and SDs are provided for each outcome, there is an error for STAI control group scores such that STAI findings cannot be entered into meta‐analysis |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | High risk | Reporting unclear and muddled throughout such that no confidence can be placed in the data extracted |
Parthum 2006.
| Methods | Cluster‐randomized controlled trial | |
| Participants | Patients undergoing elective cardiac surgery (bypass/heart valve/combined) at University Hospital Erlangen‐Nuremberg, Germany, February to August 2004. Age and gender details not provided | |
| Interventions |
Control: treatment as usual Intervention: evening 1 day preoperative; 20‐minute conversation with information brochure. Information about postoperative pain (factors affecting experience, postoperative course of pain, pain intensity assessment using VAS, information about addiction non‐risk); information about the surgery procedure; instruction as to what can do to help process. Sensory information, procedural information, behavioural instruction |
|
| Outcomes |
Pain:
1. Pain intensity: VAS as part of modified MPQ, day 1 post‐surgery and retrospective rating of pain while on Intensive Care Unit 2. Proportion of patients in pain postoperatively (cut off: VAS >3 on above measures) |
|
| Notes | Not described as cluster‐randomized but review authors CV and RP deduced this was effectively what was done ‐ randomized by day not individually Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | High risk | All patients admitted on the same day were allocated to the same group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information but very unlikely the person administering the intervention could be blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Person assessing outcome was blind to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20 patients were excluded postoperatively because of longer intubation time and longer ICU stay required ‐ 12 control group; 8 intervention. Unclear what impact this may have had |
| Selective reporting (reporting bias) | Unclear risk | Continuous data reported are medians rather than means so would not be able to enter in meta‐analysis. However, as cluster‐randomized we did not meta‐analyse the data from this paper. No mention of protocol |
| ‘Intention‐to‐treat’ | Low risk | It is stated that everyone randomized to the intervention received it |
| Other bias | Unclear risk | Poor quality of reporting makes this difficult to determine |
Pellino 1998.
| Methods | Randomized controlled trial | |
| Participants | 90 participants were randomized, but only 83 of these consented to take part (consent taken after randomization. Underwent various elective orthopaedic procedures ‐ majority (54) hip/knee total joint arthroplasty. Mean age: 53.85 years (SD 17.66, range 18 to 83). Or the 74 with complete data, 35 male, 39 (52.7%) female (inconsistency: Table 2: 36 male, 37 female). University of Wisconsin Hospitals and Clinics, USA, surgery dates October 1995 to July 1996 | |
| Interventions |
Both groups: received booklets (on preparing for surgery and a surgery‐specific booklet) before preoperative visit, included nil by mouth status, instructions for day of surgery, pain management, coughing, deep breathing, coping with surgery, postoperative expectations and restrictions. Teaching session: reviewed information from booklet; option to view procedure‐specific video "if appropriate". Additional procedure‐specific teaching in clinic, then anaesthesia clinic for assessment and reinforcement of teaching. Key aspect of difference: how teaching was delivered, but also some additional elements to intervention Controls: teaching at clinic: teaching in addition to other obligations, around visits of other providers to patient, nurses may focus on priority items not allowing patient choice in options, often little time to discuss patient concerns. Behavioural instruction, procedural information Intervention: teaching at "Learning Centre", followed an empowerment model. Staff had dedicated time for teaching, trained in empowerment techniques, fewer distractions. Empowerment model: assist patients in gaining knowledge, skills, resources they require – partnership in process. Participants could observe use of incentive spirometer and practice use of patient‐controlled analgesia (PCA) and pain scale, and discuss concerns re. surgery.Behavioural instruction, procedural information |
|
| Outcomes | Length of stay | |
| Notes | Problem: clinical practice changed part way through study (expected stay reduced from 6 to 7 to 4 days) therefore data reported: difference between expected and actual LoS, not absolute length of stay. Author provided some information about risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p51: "each patient scheduled for a preoperative clinic visit was randomly assigned to receive teaching in the clinic (control group) or in the Learning Center (experimental group). After arriving at the clinic or Learning Center, the study was described to the patient and informed consent was obtained prior to the educational session. We were unable to obtain consent and then assign patients to groups due to scheduling concerns, i.e. the clinic and learning center staff needed to prepare for the patient’s visit prior to the patient’s arrival" |
| Allocation concealment (selection bias) | High risk | p51: "…all patients in the experimental group had surgery between October 1995 and February 1996, yet only 37% of patients in the comparison group had surgery during that time frame. The remainder (63%) of the comparison group had surgery between March and July 1996". Therefore probably not done (appears recruited after allocated) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As the different groups were sent to different locations, seems highly likely that participants were aware of intervention group; it is certainly clear that the staff delivering the intervention were aware of the intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 90 randomized, more in comparison group refused vs experimental group (5 versus 2, p50). Of the 83 who consented, 2 had surgery cancelled; 7 did not complete the preoperative questionnaire, leaving 39 in experimental group and 35 in intervention group who were included in analysis (n = 74; 82% of 90). Of the 7 who did not complete the preoperative questionnaire, group not stated Problem in that randomized prior to consent – decided whether or not to take part on basis of intervention – 5 declined in intervention group versus 2 in control group |
| Selective reporting (reporting bias) | High risk | No evidence of measured outcomes not being reported, but no reference to a protocol document to check this. Response from 2nd author when asked if other outcomes measured: "no". High risk because report difference between expected and actual length of stay, not absolute, so cannot enter into meta‐analysis |
| ‘Intention‐to‐treat’ | Unclear risk | Not stated, but as intervention is, essentially, whether received teaching in the Clinic or in the Learning Centre, and patients were only informed of the study when at the relevant location, unlikely to have received treatment other than that to which they were allocated |
| Other bias | High risk | Difference in timing for the 2 groups – p51: "due to staffing and clinic flow issues, the patients in the experimental group were recruited more quickly than in the comparison group (one person was responsible for teaching the experimental subjects; multiple individuals were involved in teaching comparison subjects). Therefore, all patients in the experimental group had surgery between October 1995 and February 1996, yet only 37% of patients in the comparison group had surgery during that time frame. The remainder (63%) of the comparison group had surgery between March and July 1996". Problem: may have been other differences over time. As the authors note, the clinical pathways and expected length of stay changed during this period, leading to the length of stay outcome being provided as the difference between expected and actual stay rather than as an absolute length. However, there could have been other, additional differences in experience due to timing that have not been measured |
Pellino 2005.
| Methods | Randomized controlled trial | |
| Participants | No. randomized not stated; 65 completed study ‐ undergoing total hip or knee arthroplasty in Madison, USA (dates not provided). Controls (n = 32), mean age = 63.25 (SD 10.30). intervention (n = 33) mean age = 59.56 (SD 15.41). Overall, 41 female, 24 male (63.1% female). Controls: 12 male, 20 female; intervention: 12 male, 21 female | |
| Interventions |
Control: standard pharmacological intervention Intervention: "kit" of non‐pharmacological interventions: cassette tape player and tape of relaxing music, tape guiding through progressive muscle relaxation; handheld massager with instructions; stress ball; brief booklet with information about use of various forms of relaxation (including music, progressive muscle relaxation, rhythmic breathing, imagery) and descriptions of use of massage, heat and cold. Relaxation; somebehavioural instruction |
|
| Outcomes |
Pain: a modified Brief Pain Inventory (Daut 1983). Rated pain now, and worst and least in last 24 hours; also rated how often in moderate‐severe pain Negative affect: state anxiety (STAI) Both: first 3 days after surgery |
|
| Notes | 2nd author confirmed inclusion (general anaesthesia) but as first author deceased stage 2 email was not sent | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p184: "after consent was obtained, the subjects were randomly assigned to one of two groups using a sealed‐envelope technique" |
| Allocation concealment (selection bias) | Unclear risk | p184: "after consent was obtained, the subjects were randomly assigned to one of two groups using a sealed‐envelope technique". Does not state if envelope was opaque – or if they were sequentially numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information. However, as randomized after consent, if fully informed, as no attention control, seems likely participants would know which condition they were in. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p185: "patients were included if at least 1 day of postoperative data were collected (incomplete survey data for day 1 = 1 patient in kit group; day 2 = 6 patients, 3 in each group; day 3 = 9 patients, 3 in kit group and 6 in control group)." It seems unlikely that attrition would bias findings on day 1 or day 2, but by day 3 have twice as many lost in control group compared with intervention group |
| Selective reporting (reporting bias) | Unclear risk | Means and SDs are not presented for all outcome measures, but findings seem to be summarized for all measures. No reference to a study protocol to check this. Means and SDs for worst and least pain and anxiety; not for pain "now" or how often in moderate‐severe pain ‐ but it is the `worst pain' measure that we used in meta‐analysis. |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Perri 1979.
| Methods | Randomized controlled trial | |
| Participants | 26 women aged 30 to 62 undergoing vaginal hysterectomy, USA (dates not provided) | |
| Interventions |
Control: no contact with experimenters; no other information Intervention: 2 x 90‐minute training sessions in progressive muscle relaxation. Encouraged to practise 2 x day and use with postoperative pain. Relaxation |
|
| Outcomes |
Pain: self report. Days 1 and 3 post‐surgery; "McGill‐Melzack Pain Questionnaire" Pain: observed. Days 1 and 3 post‐surgery; observed pain behaviour – Chambers‐Price Rating Scale for Pain |
|
| Notes | Very brief report ‐ contacted correspondence author to request the "extended report" or the Masters thesis on which this brief report is based but did not receive this | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | As had to attend 2 individual training sessions this is highly unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition stated – but very brief report so not clear that there was none |
| Selective reporting (reporting bias) | High risk | No appearance of outcomes not being reported but very brief report – could have measured other constructs. No means or SDs so insufficient data for meta‐analysis |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Unclear risk | Report is so brief it is very difficult to determine |
Postlethwaite 1986.
| Methods | Randomized controlled trial | |
| Participants | 27 male patients undergoing coronary artery bypass surgery at a public hospital in Australia (dates not provided). Mean age: 52.2 years | |
| Interventions |
Control: no intervention Intervention 1: "attention‐education" – attention control. 2 x 90 minute preoperative sessions with researcher. Discussions related to previous pain experience, including anxiety, "work of worrying", effect of paying attention to pain, influence of locus of control on pain experience. Encouraged to discuss whether concerned about aspects of surgery, given "supportive counseling" Emotion‐focused Intervention 2: "stress inoculation" ‐ 2 x 90 minute preoperative sessions. 3 phases: "education" – explanation of pain experience given and used to provide rationale. "Skills training": progressive muscle relaxation session, and discussion about attention‐diversion strategies (relaxation and cognitive intervention) "Rehearsal phase": further relaxation training, then used guided imagery to demonstrate coping skills |
|
| Outcomes |
Pain: 24‐hour average pain rating; physical therapy pain rating (pain during physical therapy) Negative affect: State Anxiety (STAI); depression (Depression Adjective Checklist) All: measures taken daily for 14 days post‐surgery |
|
| Notes | Author provided some information about risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p221: "Twenty‐seven male patients admitted to a public hospital for elective coronary artery graft surgery were randomly assigned to one of three groups" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blind – p221‐2 "The stress inoculation and attention‐education subjects were told that the study was assessing a program for teaching pain control skills…Control subjects were told merely that the experimenter was collecting data on postsurgical pain, anxiety and depression…" However, all participants were seen by "the same therapist during both the intervention sessions and the data collection" so the person administering intervention was not blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | p222: "Each subject was seen individually by the same therapist during both the intervention sessions and the data collection" – so not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information on attrition/exclusion not provided |
| Selective reporting (reporting bias) | Unclear risk | Some discrepancies were found between methods and results – in Results the outcomes of frequency of using stress inoculation techniques and perceived effectiveness of procedure were reported (p224) – not mentioned in Methods. No measures detailed in Methods but not reported – but no reference to a protocol to check this. Email from author: "In addition the the information reported in the paper I also measured total analgesic intake over the post surgical period for the three groups in my study and also an assessment/analysis of those who reported utilising the stress inoculation strategies and those who didn't or didn't find them useful. I can provide that information to you if you wish." These analyses actually do seem to be reported (p224) ‐ so no apparent selective reporting Table 1, p224: provides means and SDs for all 4 measures – 24‐hour pain, physical therapy pain, anxiety and depression. However, only provide single score for each – would appear that data from all 14 readings combined |
| ‘Intention‐to‐treat’ | Low risk | Author: "In addition the the information reported in the paper … also an assessment/analysis of those who reported utilising the stress inoculation strategies and those who didn't or didn't find them useful." This implies the primary analyses were by intention‐to‐treat |
| Other bias | Unclear risk | As participants appear to have been given different instructions as to what taking part in the study would involve (see `Blinding of participants'), this may have affected who agreed to take part in each group. However, as no numbers are provided re. those who declined to take part in each group, not possible to ascertain if this had an effect |
Rajendran 1998.
| Methods | Randomized controlled trial | |
| Participants | Patients with COPD undergoing CABG December 1992 to September 1994 at the Institute of Cardiovascular Diseases, Chennai, India. Intervention group mean age 55.4 years (SD 6.9); control group mean age 58.7 years (SD 7.0). No information on gender | |
| Interventions |
Control: no information Intervention: training package delivered in week before surgery by multidisciplinary team – education, muscle training and relaxation. Included instruction about respiratory disease and treatment, nutritional counselling, energy conservation, work simplification techniques and stress management (relaxation training). Supervised daily in exercises, told to practise for 10 minutes every waking hour. Included: diaphragmatic and pursed lip breathing exercises. Family support included. Behavioural instruction, relaxation |
|
| Outcomes | Length of stay: hospital stay (in days) calculated from day of surgery until day of discharge | |
| Notes | As this study was identified late (and analysis was commencing), authors were not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract: "Forty‐five patients…were randomised to receive either short‐term pulmonary rehabilitation (group I) or no such programme (group II) p532: "Two groups of patients matched with respect to age, height, weight, duration and severity of COPD and IHD and initial PEFR were identified" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given nature of intervention, neither the patient nor the person administering the intervention could have been blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition is reported – states that 45 patients were selected to take part and results are presented for 45. However, no flow chart is provided. Also, as states that 2 groups were matched, seems odd that groups are not the same size |
| Selective reporting (reporting bias) | Unclear risk | Outcome measures mentioned in Methods are reported in Results, and details provided for length of stay outcome for meta‐analysis. However, no reference to any protocol‐type document so cannot verify this |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Reading 1982.
| Methods | Randomized controlled trial | |
| Participants | 59 women undergoing minor (laparoscopic) gynaecological surgery on a gynaecological ward, setting and dates not known (authors in USA but research possibly conducted in the UK). 3 groups: Control: N = 20, mean age 30.8 (SD 8.9); `Placebo' N = 18, mean age 31.7 (SD 7.1); `Preparation': N = 21, mean age = 30.4 (SD 6.2) | |
| Interventions |
Control: routine care only (not described) Attention control: 15‐minute interview, day before operation. Discussion where relatively neutral questions about surgical experience asked Intervention: 15‐minute interview, day before operation. Information presented in reassuring way – about what will happen during surgery, sensations e.g. feeling sick, pain due to gas from laparoscopy; also some instruction – can ease pain by lying flat. Sensory information; procedural information; some behavioural instruction |
|
| Outcomes |
Pain: post‐surgery and 3 weeks: verbal rating scale (non/mild/moderate/severe) VAS – post‐surgery: at worst and "now" Card‐sort method – post‐surgery Negative affect: STAI state anxiety post‐surgery All post‐surgery measures: 8 to 12 hours postoperatively |
|
| Notes | There seems to be an error in sample size – Methods state that there were 21 in preparation group and 20 in control group but this seems to be reversed in Results where frequencies are reported. It is not clear whether the error is in Methods or Results Attempted to contact authors; no reply received (uncertain whether wrote to correct institution ‐ could not be sure of current location) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p505: "Women were allocated randomly to three conditions" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants appear to be blind – Controls were not seen by research team until after operation; placebo and preparation groups: same information about study purposes. However, due to nature of intervention, and people giving intervention (gynaecology research fellow – placebo and preparation; author: preparation) blinding of person implementing intervention is highly unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p506: "All patients were interviewed by an independent assessor who was unaware as to which of the three conditions each patient belonged" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on attrition is provided, but reported sizes of the 3 groups and the frequencies reported in Results do not add up (do not state sample sizes for measures with continuous outcomes). p508 and 509, Table 3 and 5 – numbers of participants providing responses to verbal pain rating scales = 20 for Preparation (i.e. 1 less than original 21). The reported 18 for placebo remains constant. However, for Control, stated that 20 allocated to this group, but while data for 20 reported at 3‐week follow‐up for verbal rating scale (Table 5 p509), 21 reported postoperatively (table 3 p508). It seems likely there is an error, but not clear where the error lies. 3‐week analgesic use – Control group has 21 responses |
| Selective reporting (reporting bias) | Unclear risk | Measure reporting was very vague in Methods so it was difficult to tell what should have been reported in Results, and no reference to a protocol. We did expect to see more pain measures reported at 3 weeks but Methods is vague about this – "patients were sent a questionnaire that asked about subsequent experience of pain" (p506) |
| ‘Intention‐to‐treat’ | Low risk | No information provided, but numbers suggest intention‐to‐treat followed |
| Other bias | Unclear risk |
|
Ridgeway 1982.
| Methods | Randomized controlled trial. Had an additional 4th group containing 10 participants who expressed a preference to receive no information ‐ this group is not detailed here | |
| Participants | Women undergoing abdominal hysterectomy at St George's Hospital, London, July 1980 to June 1981. 60 patients were randomized. Mean age (including the 10 non‐randomized patients): 42, range 27 to 61 | |
| Interventions |
Attention control: manual describing ward and hospital routines(some procedural information) Intervention 1: information; manual describing procedures and sensations before and after the operation(procedural and sensory information) Intervention 2: cognitive coping. Manual aimed at helping reader provide positive thoughts in response to worry – cognitive reappraisal(cognitive intervention) All: given manual at initial visit; at 2nd visit day before operation, author checked had read it and discussed to ensure fully understood |
|
| Outcomes |
Pain ‐ day 3 post‐surgery: 1. Analogue scale; 2. Questions about intensity and frequency. 3. Scaled checklist of 30 pain descriptors Pain – no. times mentioned in nursing record Negative affect: POMS to record mood – day 3 post‐surgery and 3 weeks post‐discharge. Used subscales tension/anxiety, depression, fatigue and vigour Behavioural recovery – diary record – days when undertook 10 household activities – summed across tasks and no. days each was performed Length of stay |
|
| Notes | Authors provided additional information about measures, results and risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p274: "proceeded to the next stage, determined by opening a sealed envelope containing the randomly allocated manual". Author: "All booklets were prepared in equal numbers and placed in envelopes...They were shuffled thoroughly. The top five envelopes were carried with me to patient visits. The top booklet in the stack was allocated to a subject during the appropriate visit" |
| Allocation concealment (selection bias) | Low risk | p274: "proceeded to the next stage, determined by opening a sealed envelope containing the randomly allocated manual". "the manuals were outwardly identical" Author: "no allocations were changed once assigned". Agreed low risk because even though do not specify that envelopes were opaque, the manuals contained were outwardly identical so it would not be possible to determine contents. Should e.g. the envelopes have been dropped and picked up in a different order, this would effectively be a further re‐shuffling ‐ the original randomization process |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Seems likely that participants were blind – all received same rationale "that reading them would help to set patients at their ease" (p274). However, personnel not blind – "all interviews were carried out by one of the authors (VR)" – this included making sure that each participant understood their manual. Possible that VR was blind when presenting the manual, but not at the 2nd preoperative interview when the manual was discussed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | p273 "all interviews were carried out by one of the authors (VR)". Preoperative interview was part of intervention, so would not be blind to condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition was reported. As the authors were careful in reporting those who agreed to be randomized, seems likely that attrition was not reported because it did not occur – but need this to be stated to be certain. Author: "We had no drop‐outs, but four subjects were excluded because it was decided that they would have vaginal hysterectomies at the time of surgery or both ovaries were removed during the abdominal surgery. We thought that both excluded conditions would be too different, given that others with abdominal incisions would perhaps have more abdominal pain. We also thought there could be mood differences when both ovaries were removed. We included those women who had one ovary removed" |
| Selective reporting (reporting bias) | Low risk | With help from authors and checking of original thesis, obtained mean outcome values, but SDs (or SEs) were not available so would need to impute to enter data into meta‐analysis |
| ‘Intention‐to‐treat’ | Low risk | So long as no attrition, intention‐to‐treat is likely because it is unlikely that participants would not have received the intervention to which they were allocated. However, this is not clearly stated. Author: "That [intention‐to‐treat] was not the standard at that time, unfortunately, but was not an issue, I guess, as we had no drop outs and all subjects stayed in the group to which they were randomly assigned" |
| Other bias | Low risk | No other concerns |
Roman 2012.
| Methods | Randomized controlled trial | |
| Participants | 52 adults undergoing periodontal surgery. Intervention group: n = 21 (13 female, 9 male (does not add up to 21), mean age 33.63 (SD 6.91). Control group: n = 31 (23 female, n male not specified), mean age 38.86, SD 13.33. Overall: mean age 36.91 (SD 11.58). Setting: Department of Periodontology, Iuliu Haţieganu University of Medicine and Pharmacy, Cluj‐Napoca, Romania, recruited May 2009 to May 2011 | |
| Interventions |
Control: "standard care"; no information about the "support associated" Intervention: describes as "CBT" but seems based on Montgomery’s (Montgomery 2007) "hypnosis". Includes imagery for relaxation, suggestions for visual imagery, relaxation and peace, symptom‐focused suggestions, "a depending procedure" and instructions on using procedure on their own. Relaxation |
|
| Outcomes | Pain: VAS after surgery (time not specified) | |
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p4: "participants were successively and evenly allocated to the Intervention (N = 21) and Control (N = 31) groups by a pairwise randomization procedure" However, does not state how sequence generated and sample size odd – such a procedure would be expected to yield even groups |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p6: "Patients were not blind to group assignment". The 4 clinical psychologists who delivered the intervention must have been aware. "The surgical interventions were performed by an experienced medical doctor (AR) who was blind to the patients’ treatment condition" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Post‐surgical measures were taken by someone who did not select the patients or carry out the pre‐surgical assessment, but it does not state whether that person was blind (p6) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No./% participants lost to follow‐up: 4 for intervention group; 5 for control group (9 of 52 = 17.3%) (p7). No reason given for drop‐out but, "Using their pre‐treatment and post‐treatment scores we did not find any systematic relationship between their missing values and any other measured variable". However, not sure how would have post‐treatment scores if dropped out. Confusing missing items with drop‐out? Odd: report 9 dropping out, but findings reported for 52. So, did these participants drop out after randomization rather than before randomization? |
| Selective reporting (reporting bias) | Unclear risk | Means and SDs provided for outcomes relevant to review; no evidence of unreported outcomes, but no reference to a protocol document |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Rosenfeldt 2011.
| Methods | Randomized controlled trial | |
| Participants | 119 patients undergoing surgery at the Alfred Hospital (a major public hospital in Melbourne Australia), November 2004 to June 2006 were randomized (2 excluded for not receiving allocated intervention). Intervention group: median age 62.5 (IQR 59 to 68.5); control group: median 68 years (IQR 58 to 77). 78% of intervention group (n = 60) were male; 70% of control group (n = 57) were male | |
| Interventions |
Control: usual care Intervention: physical exercise programme: 1st 2 weeks on waiting list: 2 x 60 minute sessions/week with physiotherapist (and physician for first session): exercising to 60% of expected maximum heart rate. Encouraged to also complete 30 to 60‐minute aerobic exercise on at least 2 more occasions/week; after 1st 2 weeks, encouraged to exercise for a minimum of 30 minutes, 4 days/week until surgery. Provided with heart rate monitor Mental stress reduction: 1st 2 weeks on waiting list: 4 x 60‐minute sessions with occupational therapist and family members. Education about effects and management of stress, relaxing techniques e.g. deep breathing exercises and meditation; taught to recognise stressful situations and develop ways to accept or avoid. Given homework and handouts after sessions, encouraged to practise relaxation daily until surgery. Were given relaxing music CD to listen to for 20 minutes/day. Behavioural instruction, cognitive intervention, relaxation |
|
| Outcomes | Length of stay ("hospital length of stay" from medical records) | |
| Notes | As this study was identified late (and analysis was commencing), authors were not contacted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p2: "Patients were randomised, using a computer‐generated code, to receive either usual care (UC) or holistic therapy (HT)" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Given nature of intervention, patients and those delivering the intervention could not have been blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors state that none were lost to follow‐up. However, 2 were excluded because they "did not receive the allocated treatment" – so this exclusion seems to have occurred post‐randomization, and would seem logical that would have been excluded from the treatment group |
| Selective reporting (reporting bias) | Unclear risk | Measures mentioned in Methods were mentioned in Results, and present appropriate statistics (although median and IQR for outcome of interest). However, no mention of a protocol document |
| ‘Intention‐to‐treat’ | Unclear risk | It appears that 2 participants were excluded because they did not receive the allocated treatment (p2), so this seems unlikely to have been conducted according to intention‐to‐treat. However, possible that "treatment" refers to medical treatment and were excluded very early on, pre‐randomization – hence ‘unclear’ |
| Other bias | Low risk | No other concerns |
Schmitt 1973.
| Methods | Randomized controlled trial | |
| Participants | 50 male patients scheduled for surgery (range of types) at a Veterans Administration Hospital in a Midwestern city, USA (dates not provided). Age range not stated, but lowest band minimum = 20; highest band maximum = 70 | |
| Interventions |
Control: routine care. Could include instructions for coughing and deep breathing(behavioural instruction) Intervention: discussion group evening before surgery, approximately 60 minutes. Areas discussed included: "need for orientation‐type information"; "request for knowledge"; "discussion of feelings about surgery"; "health teaching" (techniques e.g. deep breathing, coughing; participants encouraged to practise). Also individualized session on morning of surgery focused on anxiety. Procedural information; behavioural information; emotion‐focused |
|
| Outcomes | Length of stay | |
| Notes | Could not locate author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p109: "subjects were matched according to surgical procedure and ‘level of threat…From each matched pair of patients, one patient was assigned to an experimental treatment group and the other to a control group by use of a random selection procedure" (but does not say what the random selection procedure was) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigator running group would know; participants would also know if fully informed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition is reported – and data are presented for all 50 participants – so it would appear that there was no attrition (rather than just not reporting it) |
| Selective reporting (reporting bias) | Unclear risk | No evidence of not reporting outcomes but no reference to a protocol document |
| ‘Intention‐to‐treat’ | Low risk | p110: 5 patients who participated in the group sessions were not seen individually; 1 participant was only seen individually. States participants who only had group sessions were combined with those who had both. States found no differences between group only, both and individual only. It would suggest that all participants were combined, whether had 1 or both, but not explicitly stating that the one who had the individual session only was included. However, in Results, all tables: 25 in each group, so would appear intention‐to‐treat followed |
| Other bias | Low risk | No other concerns |
Schwartz‐B'tt 1994.
| Methods | Randomized controlled trial | |
| Participants | 111 participants undergoing cholecystectomy at 2 university‐affiliated, university hospitals in USA (dates not provided). 20 lost to follow‐up; for the 91 analysed, mean age = 46 (SD 12.1), range 21 to 65. 28 male, 63 (69%) female | |
| Interventions | All: day before surgery Control: "routine treatment" – taped message containingbehavioural instruction, followed by researcher‐guided practice e.g. coughing, turning, getting out of bed Intervention 1: "informational model" – as per Controls (behavioural instruction) then 2nd tape containingsensory information re. sensations could anticipate after surgery andrelaxation technique including progressive muscle relaxation – followed by researcher‐guided practice. Written instruction: practice evening and morning preoperatively, at least once/day from 1 to 3 days postoperatively Intervention 2: "facilitator model": 10‐minute interaction with nurse – elicit and respond to concerns re. surgery, resolve "underlying perceptual conflicts", bring "objective and emotionally based" perceptions into line with informational model (cognitive intervention). Followed by same intervention as Intervention 1 (behavioural instruction (as controls), sensory information, relaxation) |
|
| Outcomes | DAy 3 post‐surgery: Negative affect: state anxiety (STAI) Pain: Pain Rating Index‐Rank of McGill Pain Questionnaire (ranked sum score of selected pain descriptors) |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p30: "Potential subjects, identified in advance from the operating room schedules of the two participating hospitals, were pre‐randomly assigned to one of three randomly ordered conditions" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information as to whether or not participants were blind – would depend on information given – but would know what they had received. A "researcher" was involved in all 3 conditions (see e.g. p31) – highly unlikely blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper. Does not state who took outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 20 (18%) lost to follow‐up – 12 had more extensive surgeries; could not collect postoperative data for 8 (p28 – does not state why). Numbers not provided by intervention group |
| Selective reporting (reporting bias) | Unclear risk | No measures were included in Methods that were not reported in Results. However, it is not explained why chose the PRI‐R from the MPQ rather than e.g. the pain intensity measure. No reference to a protocol for checking |
| ‘Intention‐to‐treat’ | Unclear risk | Randomization process is unclear. Seems participants were randomized before consent – did this effect the information they were given? If so, from which groups were the 8 participants who declined to take part? (p28) |
| Other bias | Unclear risk | Given the procedure whereby the intervention took place straight after consent, and controlled by researcher, unlikely that received intervention other than that to which randomized. However, not impossible, and intention‐to‐treat is not stated |
Seers 2008.
| Methods | Randomized controlled trial; 4 groups including both usual care and attention controls | |
| Participants | Patients undergoing total knee or hip joint surgery at an orthopaedic hospital in the UK. 200 randomized (data for 118 analysed) ‐ of the 118, mean age = 65.6 years (SD11.4), 50 male, 68 female ‐ 57.6% female. Data collected August 2002 to December 2003 | |
| Interventions |
Control: usual care (resting 15 to 20 minutes) Attention control: 15 to 20 minutes, asked to describe what do, feel, think when in pain Intervention 1: total body relaxation: 15 to 20 minutes, tensing and relaxing each muscle group; concentrating on feelings while doing this. Received audio cassette of instructions Intervention 2: jaw relaxation: 15 to 20 minutes, lower jaw drops slightly, tongue rests quietly, lips soft; slow, deep breaths All interventions: taught at pre‐admission clinic; asked to practise once/day for 1 week pre‐admission; given written instructions and letter 1 week before admission, reminding participants to practise |
|
| Outcomes |
Pain: VAS for pain at rest and at movement (only one score reported – not clear if reported score = one of these or combination) Negative affect: state anxiety (short form STAI) Both: 2 to 3 days after surgery |
|
| Notes | Point made in Discussion: this group: pain scores reduced by a fairly large amount from before surgery to postoperatively – so the main source of pain had been reduced by the surgery, and pain seen as less of a problem than before. Potential for interventions to reduce postoperative pain may depend on level of pain before Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p683: "Random allocation was concealed by using a system of sequentially numbered, opaque, sealed envelopes containing the computer generated randomly [sic] allocation, which was drawn up by a statistician" |
| Allocation concealment (selection bias) | Low risk | p683: "Random allocation was concealed by using a system of sequentially numbered, opaque, sealed envelopes containing the computer generated randomly [sic] allocation, which was drawn up by a statistician. These envelopes had to be used in order so that the allocation could not be altered; thus the allocation was secure" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None reported. It may be that participants were unaware of intervention (especially attention control and the 2 relaxation groups) but it seems that the same researchers were delivering all interventions, and seems unlikely they would not know what they were delivering, so they would have known (p683: "The two researchers teaching the intervention were trained...with a researcher who had extensive experience of teaching relaxation and the techniques used in the attention control") |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | For postoperative, post‐intervention outcomes: "post‐intervention data were collected using a self‐administered questionnaire completed by participants and put in a sealed envelope whilst the researcher was out of the room" (p684) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p683: No. lost from each group: usual care: 20, attention control: 20; jaw relaxation: 18; total body relaxation: 24 Reasons for loss: surgical date changed (n = 25); researcher unavailable (15); surgery postponed to after study closure (4), surgical plan changed so not eligible (10); withdrew because too ill/too much pain (18); did not wish to continue (10). Reasons not provided by group |
| Selective reporting (reporting bias) | Unclear risk | It is not stated whether pain measure reported in results is at rest, on movement, or combination of both (so not clear whether one is unreported) Anxiety VAS – findings not reported – it may be that they used the VAS for post‐intervention outcomes rather than pre‐intervention but unclear |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Unclear risk | Pre‐admission data may have been collected after random allocation. This might affect the baseline for change scores |
Shelley 2007.
| Methods | Randomized controlled trial + tested effects of the moderators External Health Locus of Control and Self‐Efficacy | |
| Participants | 90 participants undergoing coronary artery bypass surgery in Queensland, Australia were randomized (data analysed for 80) (dates not provided). Overall sample: mean age 65.5 (SD 9.2, range 41 to 85); 64 (80% male), 16 female. Intervention mean age 65.1 (SD 9.8); control mean age 66.1 (SD 8.5). Intervention: 22 (of 37) male; control: 31 (of 43) male | |
| Interventions |
Control: standard care Intervention: day before surgery, 30‐minute semi‐structured interview: building rapport, eliciting patient concerns, posting questions about surgery and linking the questions with patient concerns. Intended to address cognitive coping strategies, reframing where appropriate (cognitive intervention) |
|
| Outcomes |
Negative affect: distress (Depression, Anxiety and Stress Scales) Pain: 10 cm VAS Both: at discharge (4 days post‐surgery) |
|
| Notes | Could not locate author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p186: "Participants were randomized to preparation or standard care conditions" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p186: "In keeping with the single‐blind control group design, the RA administered all inventories, and the data produced and assignments were not revealed to patients, the psychologists, or other hospital staff until the conclusion of the study. The RA advised the psychologist only of the number of consenting patients who completed baseline measures and were assigned to the preparation condition for purposes of preparation arrangement." (RA = research assistant). However, the psychologist would have known when they were providing the intervention – for the patient, this would depend on the information provided (and whether they talked to other patients) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The RA who did the outcome assessments also did the randomization |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | p185: "10 patients did not complete questionnaires and/or provide sufficient blood (1 patient was deceased before posttest)" Attrition not provided by group unfortunately p186: "male patients missed more responses than female patients…and there were fewer missing response among patients who received the preparation". p185: "missing data did not exceed 5% and were replaced by group means" |
| Selective reporting (reporting bias) | High risk | It was not clear which measures were assessed at baseline, and which at discharge, however results reported for those measures one would expect to have been measured as outcomes only. Very little detail presented for some outcomes (e.g. ns direct effects). However, no clear evidence of selective reporting – but no reference of a protocol to which to refer. Means and SDs for outcomes not provided by group so cannot enter into meta‐analysis |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Unclear risk | No other concerns |
Shuldham 2002.
| Methods | Randomized controlled trial | |
| Participants | 356 patients at a UK hospital were randomized; 27 were lost to follow‐up; final n = 329 (dates not provided) All underwent general anaesthesia Mean age experimental group: 62.7 (SD = 7.46, n = 173). Mean age control group: 62.3 (SD = 8.46, n = 156). Overall mean: 62.5 (calculated for review). 12% female; 88% male Surgery type: coronary artery bypass surgery |
|
| Interventions |
Control group: standard care. From a few days before to the day of surgery, patients received education as inpatients involving nurse, doctor, physiotherapist, occupational therapist, pharmacist and dietitian. Also regular sessions on wards to which patients were invited Intervention group: included procedural information and behavioural instruction. 1 x 4‐hour hospital visit, early in preoperative waiting period, in groups of 10 to 15 people. Relatives were permitted to join the groups. `Educational intervention' including information on coronary artery disease and surgery; hospital stay (process of admission, preoperative procedure, postoperative procedure, expected stay); medical care (revascularization, possible complications, medication and health promotion); rehabilitation (including physiotherapy, breathing exercises, physical activity, diet) |
|
| Outcomes |
Negative affect: Anxiety ‐ Hospital Anxiety and Depression Scale Negative affect: Depression ‐ Hospital Anxiety and Depression Scale Negative affect: "tense and uptight" ‐ General Well‐being Questionnaire Negative affect: "worn out" ‐ General Well‐being Questionnaire Pain: a new composite questionnaire including VAS, body map and categorical rating scale for pain intensity; VAS only used in analysis Length of hospital stay (from day of surgery, including day of discharge). Questionnaires were presented on 3rd postoperative day (or 3rd day after transfer to ward if still in ICU on 3rd postoperative day) |
|
| Notes | Author provided additional information regarding control and intervention conditions, measures and risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by another member of the department using computer‐generated random numbers (p667) |
| Allocation concealment (selection bias) | Low risk | Randomization by another member of the department meant that researchers and staff should be blind to intervention but no information was given on concealment. However, additional information from author: "There was no way for those concerned to predict the allocation which was done was done by a third party with computer generated random numbers, and the person then told the research assistant that the patient had joined the study and not which group they were in" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Research and hospital staff were blind but the nature of the intervention meant that participants could not be blind (they attended an educational event) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff were blind to group (p667) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information about attrition is not provided for the 3‐day outcome. For trial completion: Control group: 168 allocated to group initially; 12 withdrawn (2 died, 4 did not have operation, 6 had operation and another hospital too late for follow‐up). 156 completed trial. Intervention group: 188 allocated to group. 15 withdrawn: 3 died before admission, 2 died following operation, 1 too ill post‐surgery, 1 heart transplant, 3 did not have operation, 1 did not want to continue, 1: operation at another hospital. 173 completed the trial |
| Selective reporting (reporting bias) | High risk | Author: all outcomes were reported. Only present mean/SD for length of stay so cannot enter other outcomes into meta‐analysis |
| ‘Intention‐to‐treat’ | Low risk | From the flow chart on p670, intention‐to‐treat appears to have been followed; authors confirmed this |
| Other bias | Low risk | There are no apparent other sources of bias |
Vukomanović 2008.
| Methods | Cluster‐randomized, controlled trial | |
| Participants | 45 adults undergoing total hip arthoplasty at the Military Medical Academy, Belgrade, Serbia (dates not provided). Intervention group (n = 23): mean age 60.05 (SD 11.01, median 62.5, range 30 to 70). Controls (n = 22): mean age 56.2. (SD: 18.45, median 66.5, range 19 to 70). Intervention, 14 female; control: 16 female. Unclear whether these figures relate to no. analysed (20 in each group) or numbers randomized (23 and 22 respectively). See Table 1, p294 | |
| Interventions |
Control: Author: both groups: "standard preparatory information" from surgeons and anaesthesiologists Intervention: 1 appointment with "physiatrist" (conversation and brochure: information re. operation and rehabilitation); 2 practical sessions with physiotherapists (instructed to perform exercises and basic activities e.g. bed mobility, standing and walking with crutches, toilet use, sitting on chair, walking). Behavioural instruction; procedural information |
|
| Outcomes |
Pain at rest and movement: VAS at discharge Length of stay |
|
| Notes | Some behavioural recovery measures were taken but not included as judgements required and no psychometric information Author provided additional information regarding control/intervention conditions, measures, data and risk of bias |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p292: "patients were randomly divided into two groups". Author: "Orthopedics department has three units. Most hospital rooms are three ‐ bed rooms. We thought that the patients from the control and study group should not reside in one room at the same time. Also, the patients might have met in the dining room and the hallway of the unit. There was the possibility that the patients from the study and control groups exchange knowledge of preoperative physical therapy and education. So, in order to perform this study, we had to separate the groups spatially. The research team could not influence the distribution of patients admitted to the three units. The patient was admitted to the unit in which an orthopedic surgeon, who planned the operation and who performed arthroplasty worked. So, randomization was performed by drawing the unit where patients will be included in the preoperative physiotherapy and education." So, cluster‐randomized |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding reported. Very unlikely intervention blinded – as involved attending/giving 1 appointment and 2 practical classes (p293) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information in paper. Author: "One therapist was conducting preoperative physical therapy, and a physiatrist conducted the education. They did not participate in the evaluation of outcome. Therapists who were carrying out post ‐ operative physical therapy in all three units were not introduced to a random in its units. They performed the outcome assessment. But I'm not sure whether a double blind study was provided. Specifically, during rehabilitation, the patient from the study group and therapist could talk about the preoperative physical therapy" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition detailed (e.g. flow chart p293) – low numbers lost, reasons given – unlikely to bias findings No./% participants lost to follow‐up: 5 (11.11%). 3 from intervention group; 2 from control group – because of "intraoperative and postoperative complications" (p293). Further detail p204: during operation: 1 fracture of proximal femur; 1 fracture of acetabulum cavity) postoperative complications: 1 participant hip dislocation; 1 epileptic seizure; 1 gastrointestinal disorder) |
| Selective reporting (reporting bias) | Low risk | A lot of measures are reported ‐ however, the 10 behaviours were assessed daily but only reported at day 3 and at discharge – not stated why those particular days. When contacted, the authors provided a high level of detail on some measure that were not included in the report. The level of openness, especially as some of the non‐reported findings were highly significant, suggests that the authors were not selectively reporting significant findings |
| ‘Intention‐to‐treat’ | Low risk | No other concerns |
| Other bias | Low risk | Intention‐to‐treat seems likely as numbers consistent throughout study – but not stated. However, taking author information that allocated and treated by unit seems unlikely patients would have changed groups. Author: "Statistical analyses did not include data of the patients who were excluded due to intra‐and postoperative complications" |
Watt‐Watson 2000.
| Methods | Randomized controlled trial (a pilot study) | |
| Participants | 50 participants randomized ‐ undergoing first CABG (data collected for 45) at a university‐affiliated teaching hospital, Toronto, Canada (dates not provided). `Average' age 61. Control group mean (SD) age: 60.13 (11.0); Intervention 1: 64.18 (7.44); Intervention 2: 57.06 (9.86). Most: male (unclear whether 5 or 11 female, p50) | |
| Interventions |
Control: routine "education" – booklet and videotape 2 to 7 days pre‐operation; general information about surgery, postoperative care, recovery (procedural information) Intervention 1: as control plus additional booklet: importance of pain relief, how and when to ask for help; pain‐relief methods; addresses common concerns that prevent asking for help (procedural information (as controls);behavioural instruction, cognitive intervention Intervention 2: as Intervention 1 plus interview with research nurse – discussed points in booklet, answered questions (components as for Intervention 1) |
|
| Outcomes |
Pain: MPQ‐SF, present pain intensity and numerical rating scale (worst pain last 24 hours). 3 and 5 days post‐surgery Length of Stay |
|
| Notes | Author provided some information about risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p46: "using a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Research staff were blinded but seems likely participants would have known – and the research nurse who carried out the interviews in the second intervention group would know. So, the blinding would have affected outcome assessment rather than knowledge of intervention for participants and those providing intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p46: "Data were collected by a blinded research assistant"; p47: "to maintain blinding of the research assistant and staff, all patients received an envelope…the two intervention groups also received the booklet Pain Relief After Surgery" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States that 50 participants enrolled; data from all 3 time points (including baseline for 45 – "five of the fifty consenting patients who completed baseline measures were too ill or tired after surgery to complete all measures" (p47). Then confusing as states control n = 16, intervention 1 n = 15, intervention 2 n = 16; data from 45 because 2 patients in intervention 1 were too ill to participate after surgery. Therefore, unclear what happened with the other 3 of the 50 participants who did not complete data – which groups were they in? |
| Selective reporting (reporting bias) | High risk | Did not report detail of all measures described in Methods and not explained why; some of these relevant to review (NRS means and SDs not presented) ‐ hence high risk. However, for NRS scores did report findings being non‐significant, which suggests this was either error/word space limits rather than selective reporting (p50/51). Author: reported no outcomes measured that were not reported |
| ‘Intention‐to‐treat’ | Low risk | p49: "The intention‐to‐treat principle (Newell 1992) was maintained so that individuals randomized to the intervention group were included in this group even if they did not read the booklet" |
| Other bias | Low risk | No other concerns |
Watt‐Watson 2004.
| Methods | Randomized controlled trial | |
| Participants | 406 patients undergoing first CABG at a university‐affiliated hospital in Toronto, Canada randomized (July 2000 to July 2001). Control mean age 61.9 (SD 9.4); intervention mean age 61.7 (9.3). 346 (85% men); 60 women | |
| Interventions |
Control: "standard cardiovascular education" – booklet and video at pre‐admission appointment, 2 to 7 days before surgery. Content: general information about surgery, postoperative care and recovery, half‐page pain management guidelines(behavioural instruction, procedural information) Intervention: as controls plus 8‐page booklet about importance of pain relief, how/when to ask for help; information on pain relief; addresses concerns some patients have re. requesting help; research nurse discussed points and answered questions.Behavioural instruction, cognitive intervention (in addition to control material) |
|
| Outcomes |
Behavioural recovery: pain interference with general activities, sleep, walking, deep breathing and coughing (days 3 and 5 post‐surgery) (interference subscale of Brief Pain Inventory) Negative affect: pain interference with mood (days 3 and 5 post‐surgery) Pain: McGill Short‐form; days 1 to 5 post‐surgery; scores: Pain Rating Index (sensory, affective and total); numerical rating scale (on moving and worst pain in previous 24 hours) Present pain intensity: most severe pain in previous 24 hours Length of stay |
|
| Notes | Author provided some information about risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p74: "randomized using a computer‐generated randomization table" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Most staff blind but participants not blind, neither was the research nurse who went over the booklet with them (p75) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "To maintain blinding of the RA and health professional staff, all patients received a brown envelope containing a folder with a copy of the consent and a letter of appreciation…patients assigned to the intervention group received the pain education intervention booklet in a similar folder in their envelope" (p75) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition clearly reported, except not clear which postoperative data are incomplete: No./% participants lost to follow‐up: After surgery: 16: Control: 10 (surgical date changed 5; too ill 4; died 1); intervention: 6 (surgical date changed 2, too ill 3, died 1). This leaves 194 analysed in control (says 192 on flow chart p77 but think must by typo); 196 in intervention; but of these, partial data from 30 to 17 in control group (complete data: 177); 13 in intervention group (complete data: 183). Given sample size, attrition bias seems unlikely |
| Selective reporting (reporting bias) | High risk | Only report details of outcomes that were significant – although do report that other outcomes were non‐significant – suspect more down to word limit than intention to bias findings but this does mean that must be entered as high risk as cannot enter data in meta‐analysis. Very clear about what was the primary outcome but no protocol document mentioned. Author: reported no outcomes measured that were not reported |
| ‘Intention‐to‐treat’ | Low risk | "The intention‐to‐treat principle was maintained so that individuals randomized to the intervention group were included in this group even if they did not participate in the intervention (e.g. reading the booklet or completing the measures postoperatively)". Note: no mention of imputation so not sure if this means that they were included in analyses where they did have data rather than being deleted from the data set? |
| Other bias | Low risk | No other concerns |
Wells 1982.
| Methods | Randomized controlled trial; Note: no `control' group ‐ appear to be comparing 2 interventions | |
| Participants | 12 patients undergoing cholecystectomy at 2 hospitals at different locations in the USA (dates not provided). Mean age: 53.5 (range 30 to 70 years); 6 male and 6 female | |
| Interventions |
Control: preoperative "instruction" including "objective information", description of sensations and practice of deep breathing, coughing, moving in bed. 45 to 70 minutes (both groups: same time distribution. Sensory information; behavioural instruction Intervention: do not seem to receive control preparation. Preoperative relaxation training session including focusing on breathing and contraction and relaxation of abdominal muscles while receiving EMG feedback from muscles. Also a "5‐minute exercise adapted from Jacobson (1938)" (Jacobsen 1938) – no further information. Goal: reduce both physiological and psychological factors that contribute to pain. Relaxation |
|
| Outcomes | Pain – rated on 10 cm line on evening on day of surgery, and days 1 and 2 post‐surgery | |
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p237: "The subjects were randomly assigned to one of the two conditions" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It appears that someone was present with the intervention conditions at least, so blinding of the person conducting the intervention unlikely (high risk). Unclear for participants – depends on information given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome measures were collected "by an uninformed assistant" (p237) – this seems to imply blinded but not entirely clear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition reported and no. reported in analysis matches no. randomized |
| Selective reporting (reporting bias) | Unclear risk | Means and SDs for relevant outcome measures reported. No measures mentioned in introduction or methods that were not reported, but no reference to a protocol document, and looked at some outcomes that were not mentioned in Methods |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Unclear risk | Some concern about selection – "Purposive and convenience sampling were used" (p237) – there is therefore potential for biases related to selection but insufficient information to determine whether likely to be a problem |
Wijgman 1994.
| Methods | Randomized controlled trial (note: actually compared 2 interventions rather than having attention or no treatment control) | |
| Participants | 64 patients undergoing total knee arthroplasty at an academic hospital, Maastricht, Netherlands (January 1991 to March 1992). Mean age 65 (range 42 to 85); 48 (75%) female; 16 male | |
| Interventions |
No control group Intervention 1: 30‐minute group session; "preoperative instruction" – explained peri‐ and postoperative phase; information about inserting hip replacement and clinical and post‐clinical course of the operation. Procedural information Intervention 2: exercise: strengthening exercises, exercises to prevent thrombosis and gait training with crutches. Behavioural instruction |
|
| Outcomes | Pain: VAS where 100 = worst pain. Timing: 2, 5, 7, 10, 14 days post‐surgery and discharge | |
| Notes | Could not locate author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information: "The patients were divided without pre‐stratification at random into two groups. Matching took place with respect to the group size" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Instruction groups ‐ 2 physiotherapists (who were also present in exercise group) so personnel were not blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided in paper |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some attrition ‐ from 64 to 57. N at each time point is not always clear; allocation to each group (31 to instruction; 33 to exercise) is given only in Abstract At day 7 – 63 patients? At day 10 – 62; 30 instructed, 32 non‐instructed. At day 14 – 57; 28 instructed, 29 non‐instructed |
| Selective reporting (reporting bias) | High risk | Mean (SD) not reported for pain outcome (in general, in paper, only reported for significant findings) |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Wilson 1981.
| Methods | Randomized controlled trial, also looked for interaction effects by levels of patient "fear", "denial" and "aggressiveness" | |
| Participants | 78 patients undergoing hysterectomy (analysed n = 37) or cholecystectomy (analysed n = 33, 26 (79%) female) at a 550‐bed community hospital serving suburban and rural population in Michigan, USA (dates not provided) | |
| Interventions |
Control: usual care; visit by anaesthesiologist and surgeon; discussion by nurses re. deep breathing and coughing after surgery. Hysterectomy patients: offered lecture on hospital procedures (behavioural instruction; procedural information (hysterectomy only)) Intervention 1: Information: 9‐minute taped message "describing sensations and procedures likely to be experienced". Procedures e.g. skin preparation, IV infusion, postoperative diet. Sensations e.g. feeling in incision. Also usual care (sensory information, procedural information, usual care components) Intervention 2: Relaxation: 25‐minute tape, used once evening before surgery and could use as often as wished postoperatively. Focused attention on individual muscle groups and relaxed those muscles. Counting task. Relaxation (and usual care components) Intervention 3: Information and relaxation – components as usual care, information and relaxation All interventions: night before surgery |
|
| Outcomes | Length of stay | |
| Notes | Author provided additional details regarding interventions, outcome data and risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p82: "Patients were randomly assigned to one of four groups, stratified according to type of operation". Author: "I used the random number table in the back of the Guilford statistics book to create the sequence" |
| Allocation concealment (selection bias) | Unclear risk | No information in paper. Author: "Only after consent would the treatment condition be known by the interviewers" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Physicians and nursing personnel were not informed of the distribution of patients among treatment conditions" (p83) but seems likely patients and those delivering interventions would not be blind. Author: "relaxation patients would have a tape recorder by their bed. Controls and Information only patients would not have a recorder. Controls received a tape describing only procedural information and not sensory information" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Physicians and nursing personnel were not informed of the distribution of patients among treatment conditions" (p83). However, does not state whether the interviewer collecting outcome data was blind. RP: "Were the staff recording outcomes (e.g. length of stay) blind to the group allocation of participants?" Author: "yes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No./% participants lost to follow‐up: 8 (10.3%). p82: 7 had more intensive surgery than planned or hospitalized for further diagnostic tests – not included because recovery pattern would be atypical. 1 withdrew because she did not wish to complete the questionnaires about recovery. (Assuming she withdrew after randomization). Attrition not stated by group. However, group sizes: 18, 17, 17, 18 suggest that attrition is likely to be evenly spread. Authors confirmed attrition across group: 2 controls, 3 information, 2 relaxation, 1 information and relaxation |
| Selective reporting (reporting bias) | Low risk | No outcomes stated in Methods that were not reported in Results but no reference to a protocol. When asked whether any outcomes were measured but not reported, author response: "no". Length of stay means and SDs provided but adjusted for age, type of operation and coping ability score; unclear re. how would use in meta‐analysis. Author later sent all means and SDs for length of stay so now possible to include in meta‐analysis |
| ‘Intention‐to‐treat’ | Low risk | No information in paper. RP: "Were participants kept in the intervention groups to which they were randomised, regardless of the intervention they received? (i.e. were data analysed according to intention‐to‐treat?)" Author: "yes" |
| Other bias | Low risk | No other concerns |
Yang 2012.
| Methods | Randomized controlled trial | |
| Participants | 120 participants undergoing carotid endarterectomy, Liaocheng People's Hospital, China, April 2008 to March 2010. Intervention group: mean age 57.2 years (SD 10.4, range 35 to 85), 28 of 60 (46.7%) male. Control group mean age: 56.4 (SD 11.4, range 37 to 82), 31/60 (51.7%) male. Overall: mean age 56.8, range 35 to 85; 61/120 (50.8%) female | |
| Interventions |
Control: preoperative preparation by a surgeon at clinic or after admission, standard departmental protocol including procedures, complications, care, nutrition, pain management, rehabilitation. Procedural information Intervention: As controls plus additional preparation by ICU nurses after admission: included more details on e.g. surgery processes, health care, pain management and medication use, tour of ICU, explained procedures and purposes mechanical ventilation and blood pressure monitoring; information on discomforts e.g. throat irritation and symptoms of urinary catheter; discussed postoperative pain management. Further procedural information; sensory information |
|
| Outcomes | Negative affect: anxiety. Zung Self‐rating Anxiety Scale, on day of discharge from ICU to wards | |
| Notes | Anxiety presented with means and SDs, also as no. patients scoring > 40 Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p285: "the selected patients were randomly divided into study group (n = 60) and control group (n = 60). The randomization was conducted by a designated nursing consultant (XXJ) by randomly drawing a number from a container. Within the container, there were 120 odd or even numbers, which were in folded paper balls. For each patient, the investigator drew a paper ball from the container. If it was an even number then the patient was assigned to the study group. By the same token, if it was an odd number the patient was assigned to the control group" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information. If XXJ mentioned above was independent to the researchers consenting patients it is possible that allocation was concealed but need more information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | p286: "Study participants and the investigators were not blinded to patient’s group assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | p286: "Study participants and the investigators were not blinded to patient’s group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors report data for all participants randomized |
| Selective reporting (reporting bias) | Unclear risk | No evidence of outcomes being measured but not reported, but no reference to a protocol document |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Zhang 2012.
| Methods | Randomized controlled trial | |
| Participants | 40 patients undergoing CABG at Liaocheng People's Hospital, Shandong, China, October 2007 to December 2009. Intervention group mean age: 63.6 (SD 6.8); control group 60.2 (SD 8.2). Intervention group: 7 male, 13 female; control group: 5 male, 15 female. Total: 12 male, 28 (70%) female | |
| Interventions |
Control: standard care: preoperative counselling on ward, by ward nurses, including processes in hospitalization, surgery and postoperative care. Procedural information Intervention: standard care. Admitted extra day before surgery (3 days preoperative); received standard care plus course provided by nurse educators over 2 to 3 days. Included: pulmonary care techniques (abdominal breathing, effective coughing); postoperative rehabilitation (including diet, medications, mobility); "counselling" – issues related to surgery sights and sounds, insertion of lines, operation length, pain control, information re. expectations of intensive care and postoperative activities. Behavioural instruction, procedural information, sensory information |
|
| Outcomes |
Negative affect: anxiety. Zung’s Self‐rating Anxiety Scale (Zung 1971), 2 to 3 days after surgery Length of stay |
|
| Notes | Attempted to contact authors; no reply received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | p85: "Patients were divided into study (n = 20) and control groups (n = 20) by randomly drawing a number from a container" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The intervention group was admitted 1 day earlier and received extra education from specialist nurses. Therefore, staff could not have been blind; patients would not have been blind if they gave informed consent |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p86: "The investigators who conducted outcome assessment were blinded to patients’ groupings" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent attrition: 40 enrolled; data reported for 40 |
| Selective reporting (reporting bias) | Unclear risk | Data are reported in a format appropriate for review, but description of measures vague in Methods "These measured included…." Also, there was no reference to a protocol |
| ‘Intention‐to‐treat’ | Unclear risk | No information provided in paper |
| Other bias | Low risk | No other concerns |
Ziemer 1982.
| Methods | Randomized controlled trial | |
| Participants | 122 patients undergoing abdominal surgery; data presented for 111 (gynaecologic (81) or gastrointestinal (30) surgery) at a 700‐bed general hospital in a large metropolitan area, USA, May to October 1981. Mean age 35.8; range 18 to 65. Most (104, 93.7%) were female | |
| Interventions | All: tapes given evening before surgery Control: Procedural information; 5 ½ minute tape: description of procedures around surgical period including, preoperative medication, IV catheter insertion, positioning for spinal anaesthesia and awakening from anaesthesia Intervention 1: Sensory information: 9 ½ minute tape. Procedural information as for Control, plus sensory information e.g. how feel on preoperative medication; how incision will feel Intervention 2: Coping strategies. 22 minute tape and instruction booklet. Procedural information as Control,and sensory information as Intervention 1. Also cognitive intervention including cognitive reappraisal (calming self talk and intentional cognitive control through selective attention), behavioural instruction (including breathing, coughing, turning instructions) and relaxation (progressive muscle relaxation and concentration on breathing) |
|
| Outcomes |
Pain intensity: 5‐point intensity rating scale. 2 to 4 days post‐surgery Length of stay |
|
| Notes | Data are not provided for pain ‐ was not treated as an outcome variable ‐ used it to look at correlations with other variables (this appears to be the stated, a priori intention) No `control' group as such ‐ effectively 3 interventions. However, as all groups had "procedural information" of the first group, this is effectively a control and is treated as such here Could not locate author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p283: "If they agree, they signed an informed consent and were provided with a tape‐recorded message corresponding to a randomly assigned information condition" |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Seems possible: handed a tape‐recorded nurse by a nurse investigator (p283) p286: "It may be that the tape‐recorded messages used to control for experimenter bias were inadequate to attract the careful attention of patients on the evening before surgery" – this implies some degree of blinding. However, different length of tapes, and one had an instruction booklet so overall seems unlikely that the experimenter was blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | p283: "Two to four days following surgery, patients were visited by the research investigator, who was unaware of which message patients received" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No./% participants lost to follow‐up: Not stated. p285: "Some subjects did not complete all items on each scale. Incomplete scale scores were eliminated from the data analysis". In table 4, N for those analyses from 94 to 98 |
| Selective reporting (reporting bias) | High risk | p287: "in addition to the information gathered to test the hypotheses, other outcomes of surgery such as the number of doses of analgesics, sedatives, hypnotics, or the length of hospitalization were considered, but no differences among the groups were found". Ziemer 1982 (dissertation) reported `averages' for length of stay but not standard deviations (these were imputed). In all outputs, means for pain are not provided by group (so cannot meta‐analyse) but the apparently a priori intention was to look at the correlations between pain and other variables |
| ‘Intention‐to‐treat’ | Unclear risk | Not explicitly stated, but does not seem possible to switch groups in this design – they might not listen to the tape, but unless they had a friend undergoing surgery it is difficult to see how they might change groups. However, as not explicitly stated, agreed `unclear' with co‐extractor |
| Other bias | Low risk | No other concerns |
Zieren 2007.
| Methods | Randomized controlled trial | |
| Participants | Setting not stated, but authors from Berlin, Germany. Data collected January 2004 to January 2005. Age information not provided. Intervention group: 48 male, 2 female. Control group: 46 male, 4 female. Overall: 94 (94%) female (n = 100) | |
| Interventions |
Control: preoperatively, informed verbally and with written information re. the operation, possible complications and "expected postoperative course" Procedural information Intervention: as controls. Plus 22‐minute video shown after admission. With actor, shows symptoms of hernia, all the phases of hospitalization (including admission, preoperative procedures, the operation, complications). Postoperative parts included information about nutrition, toileting, analgesics and advice about behaviour after discharge. Were asked to resume activities in "symptoms‐adapted way" Procedural information (beyond control), behavioural instruction |
|
| Outcomes | On first postoperative day: Pain (SF‐36 Pain) Behavioural recovery (SF‐36 physical functioning) Negative affect (SF‐36 mental health) Length of stay. (reported as a group characteristic rather than an outcome) |
|
| Notes | NOTE: Discussion: "If we take into account that patients of both groups received actually the same preoperative information". From information provided in Methods, it appears that intervention group received more information and behavioural instruction. However, if this Discussion statement is accurate then this is a test of format not content. As we were not successful in contacting the authors, the Methods are assumed to be correct and the study is included Attempted to contact authors; no reply received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | p726: "half of them were randomly chosen either to watch the video or not" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information – as intervention group were shown a video, likely that neither patients nor those delivering intervention were blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not given for first postoperative day (the time point of relevance to review). p726: data collected for 100% preoperatively (n = 100), for 97 at 3 months and 92 at 6 months, 89 at 12 months |
| Selective reporting (reporting bias) | Unclear risk | No evidence that selective reporting occurred, but no reference to a protocol document. Means and SDs presented for all outcomes |
| ‘Intention‐to‐treat’ | Unclear risk | No information |
| Other bias | Low risk | No other concerns |
ADL = activities of daily living; ANCOVA = Analysis of Co‐variance; BC = breast cancer; BRT = Benson’s Relaxation Technique; BSKE (EWE) (Befindlichkeitsskalierung durch Kategorien und Eigenschaftswörter, Janke 1994) – a measure of general psychological well‐being; CA and CB = author's differentiation between attention control (CA) and standard care control (CB) groups (Levin 1987); CABG = coronary artery bypass graft; CBT = cognitive behavioural therapy; CD = compact disk; CO = control group (Bitterli 2011); COPD = chronic obstructive pulmonary disease; CV = Claus Vögele (review author); D and C = dilatation and curettage; df = degrees of freedom; DSM = Diagnostic and Statistical Manual (of Mental Disorders); DV = dependent variable; EMG = electromyographic; F = F statistic (ANOVA); GP = general practitioner; HADS = Hospital Anxiety and Depression Scale (Zigmond 1983); HDU = high dependency unit; HIP = Hypnotic Induction Profile (Spiegal 1977); HT = holistic therapy; IAA = inpatient ambulatory activity; ICU = intensive care unit; IHD = ischaemic heart disease; ILAS = Iowa Level of Assistance Scale; IMT = inspiratory muscle training; IQR = interquartile range; IS = incentive spirometry; ITT = intention‐to‐treat; IV = independent variable; LoS = length of stay; MAACL = Multiple Affect Adjective Check List (Zuckerman 1965); MI = myocardial infarction; min = minute; mm = millimetre; MPQ = McGill Pain Questionnaire (Melzack 1975); MPQ‐SF = McGill Pain Questionnaire‐short form (Melzack 1987); N = number of participants in sample; NHP = Nottingham Health Profile (Hunt 1983); NHS = National Health Service; NRS = numeric rating scale; ns = non‐significant; PACU = post‐anaesthesia care unit; PAS = patient administration system; PANAS = Positive and Negative Affect Scale (Watson 1988); PEFR = peak expiratory flow rate; PCA = patient‐controlled analgesia; PEPCE = an intervention name ‐ `Programme d'enseignment preoperatoire dispense a des patients de chirurgie elective'; PhD = doctor of philosophy; POI = Personal Orientation Inventory; POISE = an intervention name ‐ `preoperative incentive spirometry education'; POMS = Profile of Mood States (McNair 1971); PRI‐R = Pain Rating Index‐Rank (of McGill Pain Questionnaire); QPL = an intervention name ‐ question prompt lists; RA = research assistant; RB = rhythmic breathing; RP = Rachael Powell (review author); SCNS = stoma care nurse specialist; SD = standard deviation; SDVAMC = San Diego Veterans Affairs Medical Centre; SE = standard error; SEM = standard error of the mean; SES = der Schmerzempfindungsskala (Geissner 1996); SF‐36 = Short Form‐36 (Ware 2000); SMH = Scripps Memorial Hospital; STAI = State Trait Anxiety Inventory (Spielberger 1983), STAI A‐State = state anxiety measure of STAI; Ss = study author's abbreviation of participants (subjects); T2 = time 2; T4 = time 4; TAH = total abdominal hysterectomy; THR = total hip replacement surgery; TR = training group (Bitterli 2011); UC = usual care; VAS = visual analogue scale; WOMAC = Western Ontario and McMaster Osteoarthritis Index (WOMAC) (Bellamy 1988).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 1987 | From author's email: allocated sequentially, not randomized |
| Blay 2005 | Sample included 14‐year olds |
| Boore 1978 | Method of `randomization' not robust: (p45) "initially, subjects were paired on the basis of three criteria, namely, sex, operation, and consultant under whose care the patient was admitted. ..the first patient admitted was allocated randomly to either the experimental or control group, and the next similar patient was assigned to the other group…towards the end of the study a frequency matching procedure was used (Billewicz 1964)…the identity of each pair of patients is not preserved but allocation of individuals to the control or experimental group is manipulated so that the distribution of significant characteristics is the same in each group. The experiment is then regarded as having been performed on comparable groups, rather than on matched individuals" |
| Burton 1991 | Data from same study as Burton 1995 ‐ but reports contents of psychotherapeutic intervention interviews and attention control "chats" that were an aspect of the intervention rather than the outcomes of interest in this review. This study is listed as `excluded' rather than included as a sub‐study of Burton 1995 as it does not meet the review's inclusion criteria: studies were only included in the review if they collected one of our 4 outcome measure types within 1 month after surgery |
| Burton 1994 | Data from same study as Burton 1995 ‐ Burton 1994 present 1‐year follow‐up data, which is outside of the 1‐month postoperative timeframe of interest in this review. This study is listed as `excluded' rather than included as a sub‐study of Burton 1995 as it does not meet the review's inclusion criteria: studies were only included in the review if they collected one of our 4 outcome measure types within one month after surgery |
| Croog 1994 | Email from the author confirmed that the procedures was not conducted under general anaesthesia ‐ local anaesthesia was used |
| Domar 1987 | We believe surgery was not conducted using general anaesthesia |
| Enqvist 1995 | Randomized to 1 of 3 intervention groups ‐ only 1 preoperative. Then compared with matched controls. So, not randomized to intervention versus control |
| Eremin 2009 | No relevant outcomes (immuno‐modulatory effects of intervention only) |
| Huang 2012 | Believe not randomized ‐ used a rule based on chart number |
| Johnson 1978a | Postoperative components to intervention |
| Lengacher 2008 | Only immune outcomes |
| Liu 2013 | Postoperative component to intervention |
| Manyande 1995 | Method of allocation ‐ not allocated at random. Authors reported to us that "Alternate patients were allocated to the two groups. The alternation was monitored and reversed for four pairs in order to ensure matching of the groups on mean age, number of previous operations, distribution of sex, diagnosis and type of surgery" |
| Manyande 1998 | Method of allocation: "each group was assigned a participant alternatively" (information from authors) |
| Mitchell 2000 | No relevant outcome measures. Information from authors (sent original thesis) confirmed this ‐ no published psychometrics for negative affect outcome |
| Montgomery 2002 | Local anaesthesia with sedation, not general anaesthesia |
| Montgomery 2007 | Local anaesthesia with sedation, not general anaesthesia |
| Sheard 2006 | Intervention = commercially‐produced booklets. Insufficient information was available to determine whether the content (rather than format) differed to the information provided to the control group |
| Shelley 2009 | From same study as Shelley 2007 but no outcomes relevant to review (only cortisol levels are reported within the time frame of the review). This study is listed as `excluded' rather than included as a sub‐study of Shelley 2007 as it does not meet the review's inclusion criteria: studies were only included in the review if they collected one of our 4 outcome measure types within 1 month after surgery |
| Stergiopoulou 2006 | Focus: comparing format rather than content |
| Sugai 2013 | Email from authors: no patients underwent general anaesthesia ‐ "ALL patients had surgery under IV sedation supplemented by local anaesthesia" ‐ so exclude |
| Surman 1974 | Postoperative aspect to intervention. Intervention group only visited by researcher daily when in intensive care, less often afterwards – lasted 10 to 15 minutes ‐ assessed for delirium and rated anxiety, depression, pain |
| Timmons 1993 | No outcomes meeting inclusion criteria (management of pain, but not perceived pain included) |
| Voshall 1980 | Only the first patient was randomized ‐ the sequence was not determined at random. Author: "the first patient to be scheduled for a cholecystectomy was assigned to the experimental group by a flip of the coin. Each person thereafter was assigned to either the experimental or control group on an alternate basis. The assumption was made that patients entered the hospital and were scheduled for surgery in a random order" |
| Wang 2002 | Outcomes were not relevant to review (two `behavioural recovery' outcomes but one: no published psychometric properties; the other: outside of time frame |
| Wells 1986 | Included participants under the age of 16 |
IV: intravenous
Characteristics of studies awaiting assessment [ordered by study ID]
Akinci 2015.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Angioli 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Attias 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Bergin 2014b.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Calsinski Assis 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Chevillon 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Chow 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Dathatri 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Eckhouse 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
El Azem 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Ellett 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Foji 2015.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Fraval 2015.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Furuya 2015.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Gade 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Gillis 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Gyulaházi 2015.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Hansen 2015.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Henney 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Heras 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Hoppe 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Huber 2015.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Johansson 2007.
| Methods | Randomized controlled trial |
| Participants | Of 165 eligible patients undergoing hip arthroscopy at a hospital in Finland, 123 were randomized to intervention or control groups. Intervention group: 32 (52%) female, mean age 59.7 years; control group: 31 (51%) female, mean age 65.2 years |
| Interventions | Control group: `written educational materials. Intervention group: as control group plus `education using the concept map method' |
| Outcomes | Length of stay |
| Notes | Insufficient information was provided to allow us to determine into which of our categories the information provided with the concept map method could be classified (procedural and/or sensory information seemed likely, but we were not certain). The research team (JB) attempted to contact the author but was not successful |
Kol 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Lai Ngor 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Lookinland 1998.
| Methods | Randomized controlled trial |
| Participants | 39 female patients undergoing open abdominal gynaecologic, urologic or general surgery procedures with a minimum postoperative stay of 2 days, at a California community medical centre. Mean age 39.7 years (SD 9.8) |
| Interventions | "The structured content was based on patient education theories and included pertinent perioperative information" (p206) |
| Outcomes | Behavioural recovery and negative affect: "Functional status questionnaire" (Jette 1987). 6 subscales and 6 single‐item questions evaluating "physical, psychological, social and role function in ambulatory patients". Data collected days 1 and 2 after surgery and 1 month after discharge |
| Notes | Insufficient information to determine whether intervention fits review categories (or which information category/ies would be appropriate).The research team (RP) attempted to contact the author but was not successful |
Louw 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Mohammadi 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Novick 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Paul 2015.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Rolving 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Saleh 2015.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Shahmansouri 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Umpierres 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Van Acker 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
West 2014.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Würtzen 2015.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Xin 2015.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | This study was identified in the latest search (7 July 2015) and has not yet been fully assessed for inclusion (screened at Title/Abstract stage only) |
Characteristics of ongoing studies [ordered by study ID]
Hansen 2013.
| Trial name or title | Impact of complementary therapies via mobile technologies on Icelandic same day surgical patients' reports of anxiety, pain and self‐efficacy in healing: a randomized controlled trial in process |
| Methods | Randomized controlled trial |
| Participants | Details not known |
| Interventions | Details not known |
| Outcomes | Details not known |
| Starting date | Not known |
| Contact information | — |
| Notes | This source is a conference abstract. At the time of finalising the review, from correspondence with authors, the research was complete but authors were reluctant to share study details with us prior to publication |
Jong 2012.
| Trial name or title | The effects of guided imagery on preoperative anxiety and pain management in patients undergoing laparoscopic cholecystectomy in a multi‐centre RCT study |
| Methods | Randomized controlled trial |
| Participants | Patients undergoing laparoscopic cholecystectomy ‐ details not known |
| Interventions | Details not known |
| Outcomes | Appear to include postoperative pain but full details not known |
| Starting date | Not known |
| Contact information | — |
| Notes | This source is a conference abstract. At the time of finalising the review, from correspondence with authors, the research was complete but authors were reluctant to share study details with us prior to publication |
Differences between protocol and review
The definitions for cognitive and emotion‐focused interventions are clearer in the review than in the protocol ‐ when extracting data we found that the original definitions were insufficiently detailed to make good judgements. We have also clarified that, rather than `cognitive behavioural intervention', this should have read `cognitive interventions'. We clarified what we meant by `psychological preparation' ‐ indicating that this had to be provided before surgery, and that we were interested in intervention content, not format or timing ‐ and also clarified that where the control group received content that fit one of our psychological intervention categories, the intervention had to receive additional content in that element for that type of psychological preparation to be recorded. We also enhanced the data extraction form as the study progressed (latest version included as Appendix 6) ‐ there were no changes in the data extracted, but the form amendments made it easier for reviewers to provide complete extractions.
An inclusion criterion was that at least some patients in a study underwent general anaesthesia. Unfortunately, many studies did not report the anaesthesia type used, so in the review we first contacted authors to ask about the anaesthesia used and, if no response was received, asked a clinician colleague (either a surgeon or consultant anaesthesiologist) about what the typical procedure would be for that type of surgery.
In the protocol, we stated that outcome measures would not form part of the inclusion criteria, to allow for the inclusion of studies that identified unanticipated benefits or harm, and we also included a range of pre‐specified measures. However, because of the size of the review and team resources, this was not manageable. We therefore limited the review to only include studies with the key outcomes of postoperative pain, negative affect, length of stay and behavioural recovery, and only included postoperative outcomes measured within 30 days or one month after surgery. We also removed the commitment to analyse economic data. We did note on extraction forms when economic data were available, however (information on cost per outcome and resource use), in case a future researcher might find this information useful. Economic data were rarely provided so we do not believe excluding this data has limited the review findings.
We refined our search criteria such that, instead of searching the reference and citation lists of all relevant papers, we only searched the reference lists of relevant papers for additional sources where the papers being searched were in English. We also refined our approach to contacting authors. Rather than sending a single email asking for all additional data (which was highly time consuming and rarely resulted in a response), we followed a two‐stage approach. The first email asked for the key information of whether general anaesthesia was used, whether any outcomes were measured that were not reported and whether they knew of other studies that might be suitable for inclusion in the review. If a response was received to this first email, a second email was sent to request any further details.
We did not carry out subgroup analysis by the way people respond to information (e.g. `monitors' versus `blunters' ‐ information seekers versus avoiders, Miller 1983) because only three included studies fitted these criteria. We also did not carry out subgroup analysis according to whether interventions were classified as `general' versus `specific' because agreement between extractors was low, and it became clear that this is not a dichotomous category ‐ there are varying degrees of the extent to which an intervention could be given to any patient undergoing surgery as opposed to only suiting patients undergoing a specific type of surgery. We had planned to compare studies that differed in the timing of the outcome measure (e.g. comparing acute and chronic postoperative pain). As we have since limited the review to only include outcomes measured within one month of surgery, we have not carried out this subgroup analysis. There were differences in timings of outcome measures on a smaller timescale, and valuable secondary analyses could be conducted to explore this, but it is outside the scope of this review. We also have not carried out planned subgroup comparisons to address different surgical procedures, the use of different measures to assess the same outcome, and differing focuses of interventions within each category type. These would be valuable analyses to carry out but given the size of this review, and the complexity of intervention combinations within and across studies, we decided to focus on the primary questions and outcomes. We also did not conduct subgroup or sensitivity analyses by study quality as so few `low risk' studies were identified (see Risk of bias in included studies).
In the protocol, we anticipated that studies might use multiple measures of pain within a study and pre‐specified the order in which we would use pain measurements. In conducting extraction, we identified additional use of multiple measures and so had to decide which to prioritize in analysis. For pain, we kept the order 1 to 4 as specified in the protocol under pain continuous measures (1a). On carrying out the review we added the further decisions under pain 1a, and also the specifications for multiple measures of behavioural recovery and negative affect. To minimize bias, the lead author (RP) presented the authorship team with the measurement options, with only RP able to view the data extracted. The other team members then discussed and decided on the order of priority, according to the extent to which measures were found to be psychometrically sound and frequency of use in research.
In the protocol, we stated that we would seek English translations of non‐English studies that had the potential to be included. For practical reasons, we amended this procedure slightly, following the procedure outlined in the review Methods.
We did not plan, in the protocol, to assess reporting biases because of the probable heterogenous nature of the studies and probable small number of studies appropriate for comparison using, for example, funnel plots. However, there were sufficient studies to create funnel plots for the overall `omnibus' analyses so we examined these.
We made the following additional analysis decisions: some studies only reported mean (SD) change from baseline (rather than absolute mean (SD)); for these studies we used the difference in mean change scores as the effect size. If no continuous pain data were available but dichotomous data were presented, we used the log odds ratio as the effect size.
Contributions of authors
Conceiving the review: Rachael Powell (RP), Marie Johnston (MJ), Julie Bruce (JB)
Co‐ordinating the review: RP
Undertaking searches of electronic databases: Karen Hovhanisyan
Screening search results: RP, Mary Unsworth (MU)
Organizing retrieval of papers: RP, MU
Screening retrieved papers against inclusion criteria: RP, MU, Anne Manyande (AM), Claus Vögele (CV), Julie Bruce (JB), Neil Scott (NS), MJ, Lucie Byrne‐Davis (LBD)
Appraising quality of papers: RP, JB, Claus Vögele (CV), AM, LBD, MU, NS, MJ
Abstracting data from papers: RP, JB, CV, AM, LBD, MU, NS, MJ
Providing clinical advice: Christian Osmer
Writing to authors of papers for additional information: RP
Obtaining and screening data on unpublished studies: RP, JB, screening and extraction team as per published studies
Data management for the review: RP, JB, NS
Entering data into Review Manager (RevMan 5.3): NS (numerical data) and RP (study characteristics, risk of bias)
Analysis of RevMan statistical data: NS
Other statistical analysis not using RevMan: NS
Interpretation of data: NS and RP
Statistical inferences: NS and RP
Writing the review: RP with support from all other authors
Securing funding for the review: RP with support from all other authors
Performing previous work that was the foundation of the present study: MJ, CV
Guarantor for the review (one author): RP
Person responsible for reading and checking review before submission: RP
Christian Osmer died in February 2015. He provided essential guidance from a clinical perspective. Review data were meta‐analysed and narratively synthesized after this date.
Sources of support
Internal sources
-
Manchester Centre for Health Psychology, University of Manchester, UK.
An award of £2000 was received to support research assistant costs.
External sources
-
British Academy, UK.
We received a small research grant of £7480 to support research assistant costs.
Declarations of interest
Rachael Powell designed a study, whilst she was a post‐doctoral researcher at the University of Auckland, that would have been eligible for inclusion in this review had it been completed. However, the study did not progress due to recruitment problems (very few data sets were completed and the study was halted). As noted in Sources of support funding from two sources was received to support research assistants working on the review.
Neil W Scott's institution received National Health Service (NHS) Grampian Endowment Research Grants for statistical analysis.
Anne Manyande was the first author on two studies that we considered for inclusion in this review (Manyande 1995; Manyande 1998). We excluded these studies because participants were not randomly allocated to condition.
Julie Bruce: none known.
Marie Johnston and Claus Vögele carried out a systematic review and meta‐analysis in this area (Johnston 1993), but searching techniques have since become more sophisticated due to technological developments.
Lucie Byrne‐Davis: none known.
Mary Unsworth had financial support as a Research Assistant at Aston University and the University of Manchester for part of the submitted work.
Christian Osmer is deceased; no declarations of interest available.
Deceased
New
References
References to studies included in this review
Ali 1989 {published data only}
- Ali NS, Khalil HZ. Effect of psychoeducational intervention on anxiety among Egyptian bladder cancer patients. Cancer Nursing 1989;12(4):236‐42. [PubMed] [Google Scholar]
Ashton 1997 {published data only}
- Ashton C, Whitworth GC, Seldomridge JA, Shapiro PA, Weinberg AD, Michler RE, et al. Self‐hypnosis reduces anxiety following coronary artery bypass surgery: a prospective randomized trial. Journal of Cardiovascular Surgery 1997;38:69‐75. [PubMed] [Google Scholar]
- Ashton RC, Whitworth GC, Seldomridge JA, Shapiro PA, Michler RE, Smith CR, et al. The effects of self‐hypnosis on quality of life following coronary artery bypass surgery: preliminary results of a prospective, randomized trial. Journal of Alternative and Complementary Medicine 1995;1(3):285‐90. [DOI] [PubMed] [Google Scholar]
Barbalho‐Moulim 2011 {published data only}
- Barbalho‐Moulim MC, Miguel GPS, Forti EMP, Campos FdA, Costa D. Effects of preoperative inspiratory muscle training in obese women undergoing open bariatric surgery: respiratory muscle strength, lung volumes, and diaphragmatic excursion. Clinics 2011;66(10):1721‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barlési 2008 {published data only}
- Barlési F, Barrau K, Loundou A, Doddoli C, Simeoni M‐C, Auquier P, et al. Impact of information on quality of life an satisfaction of non‐small cell lung cancer patients: a randomized study of standardized versus individualized information before thoracic surgery. Journal of Thoracic Oncology 2008;3(10):1146‐52. [DOI] [PubMed] [Google Scholar]
Beaupre 2004 {published data only}
- Beaupre L A, Lier D, Davies DM, Johnston DBC . The effect of a preoperative exercise and education program on functional recovery, health related quality of life, and health service utilization following primary total knee arthroplasty. Journal of Rheumatology 2004;31(6):1166‐73. [PubMed] [Google Scholar]
Bergin 2014a {published and unpublished data}
- Bergin C, Kelly K, Travis T, Speroni KG. Interim analysis of the pre‐operative incentive spirometry education (POISE) intervention. Journal of PeriAnesthesia Nursing (ASPAN National Conference Abstracts) 2010;25(3):196. [Google Scholar]
- Bergin C, Speroni KG, Travis T, Bergin J, Sheridan MJ, Kelly K, et al. Effect of preoperative incentive spirometry patient education on patient outcomes in the knee and hip joint replacement population. Journal of PeriAnesthesia Nursing 2014;29(1):20‐7. [DOI] [PubMed] [Google Scholar]
Bergmann 2001 {published data only}
- Bergmann P, Huber S, Mächler H, Lieble E, Hinghofer‐Szalkay H, Rehak P, et al. The influence of medical information on the perioperative course of stress in cardiac surgery patients. Cardiovascular Anesthesia 2001;93:1093‐9. [DOI] [PubMed] [Google Scholar]
Bitterli 2011 {published and unpublished data}
- Bitterli R, Sieben JM, Hartmann M, Bruin ED. Pre‐surgical sensorimotor training for patients undergoing total hip replacement: a randomised controlled trial. International Journal of Sports Medicine 2011;32(9):725‐32. [DOI] [PubMed] [Google Scholar]
Broadbent 2012 {published data only}
- Broadbent E, Kahokehr A, Booth R, Thomas J, Windsor JA, Buchanan C, et al. Relaxation improves wound healing following surgery: a randomised trial. Psychology and Health 2011;26(S2):13‐4. [Google Scholar]
- Broadbent E, Kahokehr A, Booth RJ, Thomas J, Windsor JA, Buchanan CM, et al. A brief relaxation intervention reduces stress and improves surgical wound healing response: a randomised trial. Brain, Behaviour and Immunity 2012;26(2):212‐7. [DOI] [PubMed] [Google Scholar]
Burton 1995 {published data only}
- Burton MV, Parker RW, Farrell A, Bailey D, Conneely J, Booth S, et al. A randomized controlled trial of preoperative psychological preparation for mastectomy. Psycho‐oncology 1995;4:1‐19. [Google Scholar]
- Burton MV, Parker VW. A randomized controlled trial of preoperative psychological preparation for mastectomy: a preliminary report. In: Watson M, Greer S, Thomas C editor(s). Psychosocial Oncology: Proceedings of the second and third meetings of the British Psychosocial Oncology Group, London & Leicester, 1985 and 1986. Oxford: Pergamon, 1988:133‐58. [Google Scholar]
Chaudhri 2005 {published data only}
- Chaudhri S, Brown L, Hassan I, Horgan AF. Preoperative intensive, community‐based vs. traditional stoma education: a randomized, controlled trial. Diseases of the Colon and Rectum 2005;48:504‐9. [DOI] [PubMed] [Google Scholar]
Cheung 2003 {published and unpublished data}
- Cheung LH, Callaghan P, Chang AM. A controlled trial of psycho‐educational interventions in preparing Chinese women for elective hysterectomy. International Journal of Nursing Studies 2003;40:207‐16. [DOI] [PubMed] [Google Scholar]
Chumbley 2004 {published and unpublished data}
- Chumbley GM, Ward L, Hall GM, Salmon P. Pre‐operative information and patient‐controlled analgesia: much ado about nothing. Anaesthesia 2004;59:354‐8. [DOI] [PubMed] [Google Scholar]
Coslow 1998 {published data only}
- Coslow BIF, Eddy ME. Effects of preoperative ambulatory gynecological education: clinical outcomes and patient satisfaction. Journal of PeriAnesthesia nursing 1998;13(1):4‐10. [DOI] [PubMed] [Google Scholar]
Crowe 2003 {published and unpublished data}
- Crowe J, Henderson J. Pre‐arthroplasty rehabilitation is effective in reducing hospital stay. Canadian Journal of Occupational Therapy 2003;70(2):88‐96. [DOI] [PubMed] [Google Scholar]
Cuñado Barrio 1999 {published data only}
- Cuñado Barrio A, Legarre Gil MJ, Ruiz Castón J, Silveira de la Torre J, Cabellero Martínez L, García López F. The effect of a structured, individualized "nursing visit" on the anxiety of surgical patients. A randomized clinical study [Efecto de una “visita enfermera” estructurada e individualizada en la ansiedad de los pacientes quirúrgicos. Ensayo clínico aleatorizado]. Enfermería Clínica 1999;9(3):98‐104. [Google Scholar]
Cupples 1990 {published data only}
- Cupples SA. Effects of preadmission teaching on knowledge and postoperative recovery of coronary artery bypass graft patients. In: Funk S, Tornquist E, Champagne M, Copp L, Weise R editor(s). Key Aspects of Recovery: Improving Nutrition, Rest and Mobility. New York: Springer, 1990:299‐307. [Google Scholar]
D'Lima 1996 {published and unpublished data}
- D’Lima DD, Colwell CW, Morris BA, Hardwick ME, Kozin F. The effect of preoperative exercise on total knee replacement outcomes. Clinical Orthopaedics and Related Research 1996;326:174‐82. [DOI] [PubMed] [Google Scholar]
Daltroy 1998 {published data only}
- Daltroy LH, Morlino CI, Eaton HM, Poss R, Liang MH. Preoperative education for total hip and knee replacement patients. Arthritis Care and Research 1998;11(6):469‐78. [DOI] [PubMed] [Google Scholar]
DeLong 1970 {unpublished data only}
- DeLong RD. Individual Differences in Patterns of Anxiety Arousal, Stress‐relevant Information and Recovery from Surgery [Dissertation submitted for PhD in Psychology]. Los Angeles: University of California, 1970. [Google Scholar]
Dewar 2003 {published data only}
- Dewar A, Craig K, Muir J, Cole C. Testing the effectiveness of a nursing intervention in relieving pain following day surgery. Journal of Ambulatory Surgery 2003;10:81‐8. [Google Scholar]
Doering 2000 {published and unpublished data}
- Doering S, Katzlberger F, Rumpold G, Roessler S, Hofstoetter B, Schatz DS, et al. Videotape preparation of patients before hip replacement surgery reduces stress. Psychosomatic Medicine 2000;62(3):365‐73. [DOI] [PubMed] [Google Scholar]
Done 1998 {published data only}
- Done LM, Lee A. The use of a video to convey preanesthetic information to patients undergoing ambulatory surgery. Ambulatory Anesthesia 1998;87:531‐6. [DOI] [PubMed] [Google Scholar]
Elsass 1987 {published data only}
- Elsass P, Eikard B, Junge J, Lykke J, Staun P, Feldt‐Rasmussen M. Psychological effect of detailed preanesthetic information. Acta Anaesthesiologica Scandinavica 1987;31:579‐83. [4041] [DOI] [PubMed] [Google Scholar]
Enqvist 1997 {published data only}
- Enqvist B, Bjöklund C, Engman M, Jakobsson J. Preoperative hypnosis reduces postoperative vomiting after surgery of the breasts: a prospective, randomized and blinded study. Acta Anaesthesiologica Scandinavica 1997;41:1028‐32. [DOI] [PubMed] [Google Scholar]
Felton 1976 {published data only}
- Felton G, Huss K, Payne EA, Srsic K. Preoperative nursing intervention with the patient for surgery: outcomes of three alternative approaches. International Journal of Nursing Studies 1974;13:83‐96. [DOI] [PubMed] [Google Scholar]
Ferrara 2008 {published and unpublished data}
- Ferrara PE, Rabini A, Maggi L, Piazzini DB, Amabile E, Tancredi G, et al. Effect of pre‐operative physiotherapy in patients with end‐stage osteoarthritis undergoing hip arthroplasty. Clinical Rehabilitation 2008;22:977‐86. [DOI] [PubMed] [Google Scholar]
Field 1974 {published data only}
- Field PB. Effects of tape‐recorded hypnotic preparation for surgery. International Journal of Clinical and Experimental Hypnosis 1974;22(1):54‐61. [DOI] [PubMed] [Google Scholar]
- Field PB. Recordings ease fear of surgery. Hospital Topics 1968;46(9):69‐71. [DOI] [PubMed] [Google Scholar]
Fortin 1976 {published and unpublished data}
- Fortin F, Kirouac S. A randomized controlled trial of preoperative patient education. International Journal of Nursing Studies 1976;13:11‐24. [DOI] [PubMed] [Google Scholar]
Fortin 1983 {published data only}
- Fortin JD. An Investigation of the Effects of a Selected Coping Intervention on Pain and Anxiety in Adult Surgical Patients [PhD dissertation]. Boston, USA: Boston University, 1983. [Google Scholar]
Furze 2009 {published data only}
- Furze G, Dumville JC, Miles JNV, Irvine K, Thompson DR, Lewien RJP. "Prehabilitation" prior to CABG surgery improves physical functioning and depression. International Journal of Cardiology 2009;132:51‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbey 2003 {published data only}
- Gilbey HG, Ackland TR, Wang AW, Morton AR, Trouchet T, Tapper J. Exercise improves early functional recovery after total hip arthroplasty. Clinical Orthopaedics and Related Research 2003;408:193‐200. [DOI] [PubMed] [Google Scholar]
- Gilbey HJ, Ackland TR, Wang AW, Tapper J, Wang AW. Perioperative exercise improves function following total hip arthroplasty: a randomized controlled trial. Journal of Musculoskeletal Research 2003;7(2):111‐23. [Google Scholar]
Giraudet 2003 {published and unpublished data}
- Giraudet‐Le Quintrec J‐S, Coste J, Vastel L Pacault V, Jeanne L, Lamas J‐P, et al. Positive effect of patient education for hip surgery. Clinical Orthopaedics and Related Research 2003;414:112‐20. [DOI] [PubMed] [Google Scholar]
Gocen 2004 {published data only}
- Gocen Z, Sen A, Unver B, Karatosun V, Guna I. The effect of preoperative physiotherapy and education on the outcome of total hip replacement: a prospective randomized controlled trial. Clinical Rehabilitation 2004;18:353‐8. [DOI] [PubMed] [Google Scholar]
Goldsmith 1999 {published data only}
- Goldsmith DM, Safran C . Using the web to reduce postoperative pain following ambulatory surgery. Proceedings/AMIA Annual Symposium 1999:780‐4. [PMC free article] [PubMed] [Google Scholar]
Gonzales 2010 {published data only}
- Gonzales EA, Ledesma RJA, McAllister DJ, Eslinger MR, Perry SM, Dyer CA, et al. The effects of guided imagery on postoperative outcomes in patients undergoing same‐day surgical procedures: a randomized, single‐blind study. Journal of the American Association of Nurse Anesthetists 2010;78(3):181‐8. [PubMed] [Google Scholar]
Goodman 2008 {published and unpublished data}
- Goodman H, Parsons A, Davison J, Preedy M, Peters E, Shuldham C, et al. A randomised controlled trial to evaluate a nurse‐led programme of support and lifestyle management for patients awaiting cardiac surgery. European Journal of Cardiovascular Nursing 2008;7:189‐95. [DOI] [PubMed] [Google Scholar]
Gräwe 2010 {published data only}
- Gräwe JS, Mirow L, Bouchard R, Lindig M, Hüppe M. Impact of preoperative patient education on postoperative pain in consideration of the individual coping style [Einfluss präoperativer Patienteninformationen auf postoperative Schmerzen unter Berücksichtigung individueller Stressverarbeitung]. Der Schmerz 2010;24:575‐86. [DOI] [PubMed] [Google Scholar]
Greenleaf 1992 {published data only (unpublished sought but not used)}
- Greenleaf M, Miaskowski, C, DuHamel K. Hypnotizability and recovery from cardiac surgery. American Journal of Clinical Hypnosis 1992;35(2):119‐28. [DOI] [PubMed] [Google Scholar]
Griffin 1998 {published data only}
- Griffin MJ, Brennan L, McShane AJ. Preoperative education and outcome of patient controlled analgesia. Canadian Journal of Anaesthesia 1998;45(10):943‐8. [DOI] [PubMed] [Google Scholar]
Guo 2012 {published data only}
- Guo P, East L, Arthur A. A preoperative education intervention to reduce anxiety and improve recovery among Chinese cardiac patients: a randomized controlled trial. International Journal of Nursing Studies 2012;49:129‐37. [DOI] [PubMed] [Google Scholar]
Hart 1980 {published data only}
- Hart RR. The influence of a taped hypnotic induction treatment procedure on the recovery of surgery patients. International Journal of Clinical and Experimental Hypnosis 1980;28(4):324‐32. [DOI] [PubMed] [Google Scholar]
Hawkins 1993 {published data only}
- Hawkins R, Price K. The effects of an education video on patients’ requests for postoperative pain relief. Australian Journal of Advanced Nursing 1993;10(4):32‐40. [PubMed] [Google Scholar]
Heidarnia 2005 {published data only}
- Babaee G, Keshavarz M, Hidarnia A, Shayegan M. Evaluation of quality of life in patients with coronary artery bypass surgery using controlled clinical trial. Acta Medica Iranica 2007;45(1):69‐75. [Google Scholar]
- Heidarnia A, Dehdari T, Ghofranipour F, Kazemnejad A, Heidarnia M. The effect of health education on health related quality of life in patients with coronary artery bypass surgery. Medical Journal of the Islamic Republic of Iran 2005;18(4):319‐26. [Google Scholar]
Hoogeboom 2010 {published and unpublished data}
- Hoogeboom TJ, Dronkers JJ, Ende CHM, Oosting E, Meeteren NLU. Preoperative therapeutic exercise in frail elderly scheduled for total hip replacement: a randomized pilot trial. Clinical Rehabilitation 2010;24:901‐10. [DOI] [PubMed] [Google Scholar]
Hulzebos 2006a {published and unpublished data}
- Hulzebos EHJ, Meeteren NLU, Buijs BJWM, Bie RA, Rivière AB, Helders PJM. Feasibility of preoperative inspiratory muscle training in patients undergoing coronary artery bypass surgery with a high risk of postoperative pulmonary complications: a randomized controlled pilot study. Clinical Rehabilitation 2006;20:949‐59. [DOI] [PubMed] [Google Scholar]
Hulzebos 2006b {published and unpublished data}
- Hulzebos EHJ, Helders PJM, Favié NJ, Bie RA, Riviere AB, Meeteren NLU. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high‐risk patients undergoing CABG surgery. JAMA 2006;296(15):1851‐7. [DOI] [PubMed] [Google Scholar]
Johnson 1978b {published and unpublished data}
- Johnson JE, Rice VH, Fuller SS, Endress MP. Sensory information, instruction in a coping strategy, and recovery from surgery. Research in Nursing and Health 1978;1(1):4‐17. [DOI] [PubMed] [Google Scholar]
Johnson 1985 {published and unpublished data}
- Johnson JE, Christman NJ, Stitt C. Personal control interventions: short‐ and long‐term effects on surgical patients. Research in Nursing and Health 1985;8:131‐45. [DOI] [PubMed] [Google Scholar]
Klos 1980 {published and unpublished data}
- Klos D, Cummings KM, Joyce J, Graichen J, Quigley A. A comparison of two methods of delivering presurgical instructions. Patient Counselling and Health Education 1980;2(1):6‐13. [DOI] [PubMed] [Google Scholar]
Kulkarni 2010 {published data only}
- Kulkarni SR, Fletcher E, McConnell AK, Poskitt KR, Whyman MR. Pre‐operative inspiratory muscle training preserves postoperative inspiratory muscle strength following major abdominal surgery – a randomised pilot study. Annals of the Royal College of Surgeons of England 2010;92:700‐5 (+supplementary online files). [DOI] [PMC free article] [PubMed] [Google Scholar]
Lam 2001 {published data only}
- Lam KK, Chan MTV, Chen PP, Kee, WDN. Structured preoperative patient education for patient‐controlled analgesia. Journal of Clinical Anaesthesia 2001;13:465‐9. [DOI] [PubMed] [Google Scholar]
Lamarche 1998 {published data only}
- Lamarche D, Taddeo R, Pepler C. The preparation of patients for cardiac surgery. Clinical Nursing Research 1998;7(4):390‐405. [DOI] [PubMed] [Google Scholar]
Langer 1975 {published and unpublished data}
- Langer EJ, Janis IL, Wolfer JA. Reduction of psychological stress in surgical patients. Journal of Experimental Social Psychology 1975;11:155‐65. [Google Scholar]
Lauder 1995 {published data only}
- Lauder GR, Mcquillan PJ, Pickering RM. Psychological adjunct to perioperative antiemesis. British Journal of Anaesthesia 1995;74:266‐70. [DOI] [PubMed] [Google Scholar]
Leserman 1989 {published and unpublished data}
- Leserman J, Stuart EM, Mamish ME, Benson H. The efficacy of the relaxation response in preparing for cardiac surgery. Behavioral Medicine 1989;15(3):111‐7. [DOI] [PubMed] [Google Scholar]
Letterstål 2004 {published data only}
- Letterstål A, Sandström V, Olafsson P, Forsberg C. Postoperative mobilization of patients with abdominal aortic aneurysm. Journal of Advanced Nursing 2004;48(6):560‐8. [DOI] [PubMed] [Google Scholar]
Levesque 1977 {published data only}
- Levesque L, Charlebois M. [Anxiété, foyer de contrôle et les effets d’un enseignement sur l’état physique et émotionel des opérés]. Nursing Papers. Perspectives en Nursing 1977;8(4):11‐26. [PubMed] [Google Scholar]
Lévesque 1984 {published and unpublished data}
- Lévesque L, Grenier R, Kérouac S, Reidy M. Evaluation of a presurgical group program given at two different times. Research in Nursing and Health 1984;7:227‐36. [DOI] [PubMed] [Google Scholar]
Levin 1987 {published data only}
- Levin RF, Malloy GB, Hayman RB. Nursing management of postoperative pain: use of relaxation techniques with female cholecystectomy patients. Journal of Advanced Nursing 1987;12:463‐72. [DOI] [PubMed] [Google Scholar]
Lilja 1998 {published and unpublished data}
- Lilja Y, Rydén S, Fridlund B. Effects of extended preoperative information on perioperative stress: an anaesthetic nurse intervention for patients with breast cancer and total hip replacement. Intensive and Critical Care Nursing 1998;14:276‐82. [DOI] [PubMed] [Google Scholar]
Lim 2011 {published data only}
- Lim L, Chow P, Wong C‐Y, Chung A, Chan Y‐H, Wong W‐K, et al. Doctor‐patient communication, knowledge, and question prompt lists in reducing preoperative anxiety – a randomized control study. Asian Journal of Surgery 2011;34:175‐80. [DOI] [PubMed] [Google Scholar]
Lin 2005 {published data only}
- Lin L‐Y, Wang R‐H . Abdominal surgery, pain and anxiety: preoperative nursing intervention. Journal of Advanced Nursing 2005;51(3):252‐60. [DOI] [PubMed] [Google Scholar]
Lindeman 1973 {published data only}
- Lindeman CA, Stetzer SL. Effect of preoperative visits by operating room nurses. Nursing Research 1973;22(1):4‐16. [PubMed] [Google Scholar]
Liu 2004 {published data only}
- Liu W, Lü M. Empowerment model of preoperative education for orthopaedic patients [[Chinese script]]. Chinese Journal of Clinical Rehabilitation 2004;8(5):840‐1. [Google Scholar]
Ma 1996 {published data only}
- Ma YI, Qin LJ, Han ZF. Relaxation training on stress response to abdominal surgery [[original title is in Chinese script]]. Chinese Journal of Nursing (Zhonghua Hu Li Za Zhi) 1996;31(7):377‐80. [PubMed] [Google Scholar]
Mahler 1995 {published and unpublished data}
- Mahler HIM, Kulik JA, Hill MR. Effects of videotape preparations on recovery of female coronary bypass surgery patients. Mind/Body Medicine 1995;1(3):121‐9. [Google Scholar]
Mahler 1998 {published and unpublished data}
- Mahler HIM, Kulik JA. Effects of preparatory videotapes on self‐efficacy beliefs and recovery from coronary bypass surgery. Annals of Behavioral Medicine 1998;20(1):39‐46. [DOI] [PubMed] [Google Scholar]
McDonald 2001 {published and unpublished data}
- McDonald DD, Freeland M, Thomas G, Moore J. Testing a preoperative pain management intervention for elders. Research in Nursing and Health 2001;24:402‐9. [DOI] [PubMed] [Google Scholar]
McDonald 2004 {published and unpublished data}
- McDonald DD, Molony SL. Postoperative pain communication skills for older adults. Western Journal of Nursing Research 2004;26(8):836‐52. [2583] [DOI] [PubMed] [Google Scholar]
McDonald 2005 {published and unpublished data}
- McDonald DD, Thomas GJ, Livingston KE, Severson JS. Assisting older adults to communicate their postoperative pain. Clinical Nursing Research 2005;14(2):109‐26. [DOI] [PubMed] [Google Scholar]
McGregor 2004 {published data only}
- McGregor AH, Rylands H, Owen A, Doré CJ, Hughes SPF. Does preoperative hip rehabilitation advice improve recovery and patient satisfaction?. Journal of Arthroplasty 2004;19(4):464‐8. [DOI] [PubMed] [Google Scholar]
Miró 1999 {published and unpublished data}
- Miró J, Raich RM. Effects of a brief and economical intervention in preparing patients for surgery: does coping style matter?. Pain 1999;83:471‐5. [DOI] [PubMed] [Google Scholar]
Neary 2010 {published data only}
- Neary PM, Sung R, Corrigan M, O’Donovan M, Cahill RA, Redmond HP. The benefits of an interactive, individualized online patient pathway for patients undergoing minimally invasive radioguided parathyroidectomy: a prospective, double‐blinded, randomized clinical trial. Surgical Innovation 2010;17(3):236‐41. [DOI] [PubMed] [Google Scholar]
O'Connor 2014 {published and unpublished data}
- O’Connor G, Coates V, O’Neill S. Randomised controlled trial of a tailored information pack for patients undergoing surgery and treatment for rectal cancer. European Journal of Oncology Nursing 2014;18:183‐91. [DOI] [PubMed] [Google Scholar]
Oetker‐Black 2003 {published and unpublished data}
- Oetker‐Black SL, Jones S, Estok P, Ryan M, Gale N, Parker C. Preoperative teaching and hysterectomy outcomes. AORN Journal 2003;77(6):1215‐31. [DOI] [PubMed] [Google Scholar]
Oliphant 2013 {published data only}
- Oliphant SS, Lowder JL, Ghetti C, Zyczynski HM. Effect of a preoperative self‐catheterization video on anxiety: a randomised controlled trial. International Urogynecology Journal 2013;24:419‐24. [DOI] [PubMed] [Google Scholar]
Omlor 2000 {published data only}
- Omlor G, Kiewitz S, Pietschmann S, Roesler S. The benefit of preoperative psychotherapy on surgical outcome after inguinal hernia repair and thyroid gland surgery [Einfluss einer präoperativen Visualisierungstherapie auf die postoperativen Ergebnisse nach Leistenherniotomie und Strumaresektion]. Zentralblatt für Chirurgie 2000;125:380‐6. [PubMed] [Google Scholar]
Oosting 2012 {published and unpublished data}
- Oosting E, Jans MP, Dronkers JJ, Naber RH, Dronkers‐Landman CM, Appelman‐de Vries SM, et al. Preoperative home‐based physical therapy versus usual care to improve functional health of frail older adults scheduled for elective total hip arthroplasty: a pilot randomised controlled trial. Archives of Physical Medicine and Rehabilitation 2012;93:610‐6. [DOI] [PubMed] [Google Scholar]
Osinowo 2003 {published data only}
- Osinowo HO, Olley BO, Adejumo AO. Evaluation of the effect of cognitive therapy on perioperative anxiety and depression among Nigerian surgical patients. West African Journal of Medicine 2003;22(4):338‐42. [DOI] [PubMed] [Google Scholar]
Parthum 2006 {published data only}
- Parthum A, Weinzierl A, Gräßel E, Koppert W. Preoperative pain training. No influence on postoperative pain perception in patients undergoing cardiac surgery [Präoperative Schmerzschulung: Fehlender Einfluss auf postoperative erlebtes Schmerzempfinden kardiochirurgischer Patienten]. Der Schmerz 2006;20:301‐26. [DOI] [PubMed] [Google Scholar]
Pellino 1998 {published and unpublished data}
- Pellino T, Tluczek A, Collins M, Trimborn S, Norwick H, Engelke ZK, et al. Increasing self‐efficacy through empowerment: preoperative education for orthopaedic patients. Orthopaedic Nursing 1998;17(4):48‐59. [PubMed] [Google Scholar]
Pellino 2005 {published data only}
- Pellino TA, Gordon DB, Engelke ZK, Busse KL, Collins MA. Use of nonpharmacologic interventions for pain and anxiety after total hip and total knee arthroplasty. Orthopaedic Nursing 2005;24(3):182‐90. [DOI] [PubMed] [Google Scholar]
Perri 1979 {published data only}
- Perri KD, Perri MG. Use of relaxation training to reduce pain following vaginal hysterectomy. Perceptual and Motor Skills 1979;48:478. [DOI] [PubMed] [Google Scholar]
Postlethwaite 1986 {published and unpublished data}
- Postlethwaite R, Stirling G, Peck CL . Stress inoculation for acute pain: a clinical trial. Journal of Behavioral Medicine 1986;9(2):219‐27. [DOI] [PubMed] [Google Scholar]
Rajendran 1998 {published data only}
- Ranjendran AJ, Pandurangi UM, Murali R, Gomathi S, Vijayan VK, Cherian KM. Pre‐operative short‐term pulmonary rehabilitation for patients of chronic obstructive pulmonary disease undergoing coronary artery bypass graft surgery. Indian Heart Journal 1998;50:531‐4. [PubMed] [Google Scholar]
Reading 1982 {published data only}
- Reading AE. The effects of psychological preparation for pain and recovery after minor gynaecological surgery: a preliminary report. Journal of Clinical Psychology 1982;38(3):504‐12. [DOI] [PubMed] [Google Scholar]
Ridgeway 1982 {published and unpublished data}
- Ridgeway V, Mathews A. Psychological preparation for surgery: a comparison of methods. British Journal of Clinical Psychology 1982;21:271‐80. [DOI] [PubMed] [Google Scholar]
Roman 2012 {published data only}
- Roman A, Moldovan R, Balázsi R, Câmpian R, Soanca A, Stratul S, et al. The role of cognitive behavioural therapy in the periodontal surgical treatment: a randomized clinical trial. Journal of Cognitive and Behavioral Psychotherapies 2012;12(1):1‐14. [Google Scholar]
Rosenfeldt 2011 {published data only}
- Rosenfeldt F, Braun L, Spitzer O, Bradley S, Shepherd J, Bailey M, et al. Physical conditioning and mental stress reduction – a randomised trial in patients undergoing cardiac surgery. BMC Complementary and Alternative Medicine 2011;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmitt 1973 {published data only}
- Schmitt EF, Wooldridge PJ. Psychological preparation of surgical patients. Nursing Research 1973;22(2):108‐16. [PubMed] [Google Scholar]
Schwartz‐B'tt 1994 {published data only}
- Schwartz‐Barcott D, Fortin JD, Kim HS . Client‐nurse interaction: testing for its impact in preoperative instruction. International Journal of Nursing Studies 1994;31(1):23‐35. [DOI] [PubMed] [Google Scholar]
Seers 2008 {published data only}
- Seers K, Crichton N, Tutton L, Smith L, Saunders T. Effectiveness of relaxation for postoperative pain and anxiety: randomized controlled trial. Journal of Advanced Nursing 2008;62(6):681‐8. [DOI] [PubMed] [Google Scholar]
Shelley 2007 {published data only}
- Shelley M, Pakenham K. The effects of preoperative preparation on postoperative outcomes: the moderating role of control appraisals. Health Psychology 2007;26(2):183‐91. [DOI] [PubMed] [Google Scholar]
Shuldham 2002 {published and unpublished data}
- Shuldham CM, Fleming S, Goodman H. The impact of pre‐operative education on recovery following coronary artery bypass surgery: a randomized controlled clinical trial. European Heart Journal 2002;23(8):666‐74. [DOI] [PubMed] [Google Scholar]
Vukomanović 2008 {published and unpublished data}
- Vukomanović A, Popović Z, Đurović A, Krstić L. The effects of short‐term preoperative physical therapy and education on early functional recovery of patients younger than 70 undergoing total hip arthroplasty [Alternative title: Efekti kratkotrayne preoperativne fizikalne terapije I edukacije na rani funkcijski oporavak bolesnika mlađih od 70 godina sa totalnom artroplastikom kuka]. Vojnosanitetski Pregled 2008;65(4):291‐7. [DOI] [PubMed] [Google Scholar]
Watt‐Watson 2000 {published and unpublished data}
- Watt‐Watson J, Stevens B, Costello J, Katz J, Reid G. Impact of preoperative education on pain management outcomes after coronary artery bypass graft surgery: a pilot. Canadian Journal of Nursing Research 2000;31(4):41‐56. [PubMed] [Google Scholar]
Watt‐Watson 2004 {published and unpublished data}
- Watt‐Watson J, Stevens B, Katz J, Costello J, Reid GJ, David T. Impact of preoperative education on pain outcomes after coronary artery bypass graft surgery. Pain 2004;109:73‐85. [DOI] [PubMed] [Google Scholar]
Wells 1982 {published data only}
- Wells N. The effect of relaxation on postoperative muscle tension and pain. Nursing Research 1982;31(4):236‐8. [PubMed] [Google Scholar]
Wijgman 1994 {published data only}
- Wijgman AJ, Dekkers GHG, Waltje E, KrekelsT, Arens HJ. No positive effect of preoperative physical therapy and instruction of patients to be subjected to hip arthroplasty [Geen positief effect van properatieve oefentherapie en instructive bij patiënten die heupartroplastiek zullen ondergaan]. Ned Tijdschr Geneeskd 1994;138(19):949‐52. [PubMed] [Google Scholar]
Wilson 1981 {published and unpublished data}
- Wilson JF. Behavioural preparation for surgery: benefit or harm?. Journal of Behavioral Medicine 1981;4(1):79‐101. [DOI] [PubMed] [Google Scholar]
Yang 2012 {published data only}
- Yang C‐L, Tan Y‐H, Jiang X‐X, Meng F‐Y, Wu Y‐L, Chen, Q‐L, et al. Pre‐operative education and counselling are associated with reduced anxiety symptoms following carotid endarterectomy: a randomized and open‐label study. European Journal of Cardiovascular Nursing 2012;11(3):284‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang C‐Y, Jiang Y, Yin Q‐Y, Chen F‐J, Ma L‐L, Wang L‐X. Impact of nurse‐initiated preoperative education on postoperative anxiety symptoms and complications after coronary artery bypass grafting. Journal of Cardiovascular Nursing 2012;27(1):84‐8. [DOI] [PubMed] [Google Scholar]
Ziemer 1982 {published data only}
- Ziemer M. Providing patients with information prior to surgery and the reported frequency of coping behaviors and development of symptoms following surgery. Dissertation Abstracts International 1983;43:2465B. [Google Scholar]
- Ziemer MM. Providing Patients with Information Prior to Surgery and the Reported Frequency of Coping Behaviors and Development of Symptoms Following Surgery [Dissertation for Degree of Doctor of Nursing Science]. School of Nursing, University of Pennsylvania, 1982. [Google Scholar]
- Ziemer MM. Effects of information on postsurgical coping. Nursing Research 1982;32(5):282‐7. [PubMed] [Google Scholar]
Zieren 2007 {published data only}
- Zieren J, Menenakos C, Mueller JM. Does an informative video before inguinal hernia surgical repair influence postoperative quality of life? Results of a prospective randomized study. Quality of Life Research 2007;16:725‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 1987 {published data only}
- Anderson EA. Preoperative preparation for cardiac surgery facilitates recovery, reduces psychological distress, and reduces the incidence of acute postoperative hypertension. Journal of Consulting and Clinical Psychology 1987;55(4):513‐20. [DOI] [PubMed] [Google Scholar]
Blay 2005 {published data only}
- Blay N, Donoghue J. The effect of pre‐admission education on domiciliary recovery following laparoscopic cholecystectomy. Australian Journal of Advanced Nursing 2005;22(4):14‐9. [PubMed] [Google Scholar]
Boore 1978 {published data only}
- Boore JRP. An Investigation Into the Effect of Some Aspects of Pre‐operative Preparation of Patients on Post‐operative Stress and Recovery [PhD thesis]. University of Manchester, 1976. [Google Scholar]
- Boore JRP. Prescription for Recovery: The Effect of Pre‐Operative Preparation of Surgical Patients on Post‐Operative Stress, Recovery and Infection. Royal College of Nursing Research Series. London: Royal College of Nursing, 1978. [Google Scholar]
Burton 1991 {published data only}
- Burton MV, Parker RW, Wollner JM. The psychotherapeutic value of a "chat": a verbal response modes study of a placebo attention control with breast cancer patients. Psychotherapy Research 1991;1(1):39‐61. [Google Scholar]
Burton 1994 {published data only}
- Burton MV, Parker, RW. Satisfaction of breast cancer patients with their medical and psychological care. Journal of Psychosocial Oncology 1994;12(1/2):41‐63. [Google Scholar]
Croog 1994 {published data only}
- Croog SH, Baume RM, Nalbandian J. Pain response after psychological preparation for repeated periodontal surgery. Journal of the American Dental Association 1994;125:1353‐60. [DOI] [PubMed] [Google Scholar]
Domar 1987 {published data only}
- Domar AD, Noe JM, Ransil B, Benson H. The preoperative use of the relaxation response with ambulatory surgery patients. Journal of Human Stress 1987;13(3):101‐7. [DOI] [PubMed] [Google Scholar]
Enqvist 1995 {published data only}
- Enqvist B, Konow L, Bystedt H . Pre‐ and perioperative suggestion in maxillofacial surgery: effects on blood loss and recovery. International Journal of Clinical and Experimental Hypnosis 1995;43(3):284‐94. [DOI] [PubMed] [Google Scholar]
Eremin 2009 {published data only}
- Eremin O, Walker MB, Simpson E, Heys SD, Ah‐See AK, Hutcheon AW, et al. Immuno‐modulatory effects of relaxation training and guided imagery in women with locally advanced breast cancer undergoing multimodality therapy: a randomised controlled trial. The Breast 2000;18(1):17‐25. [DOI] [PubMed] [Google Scholar]
Huang 2012 {published data only}
- Huang S‐W, Chen P‐H, Chou Y‐H. Effects of a preoperative simplified home rehabilitation education program on length of stay for total knee arthroplasty patients. Orthopaedics & Traumatology: Surgery & Research 2012;98:259‐64. [DOI] [PubMed] [Google Scholar]
Johnson 1978a {published data only}
- Johnson JE, Fuller SS, Endress MP, Rice VH. Altering patients’ responses to surgery: an extension and replication. Research in Nursing and Health 1978;1(3):111‐21. [DOI] [PubMed] [Google Scholar]
Lengacher 2008 {published data only}
- Lengacher CA, Bennett MP, Gonzalez L, Gilvary D, Cox CE, Cantor A, et al. Immune responses to guided imagery during breast cancer treatment. Biological Research Nursing 2008;9(3):205‐14. [DOI] [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu L, Li J. Observation of the postoperative analgesic effect of Lornoxicam preemptive analgesia combined with psychotherapy for elderly cancer patients. Chinese Journal of Clinical Oncology 2013;40(11):664‐7. [Google Scholar]
Manyande 1995 {published data only}
- Manyande A, Berg S, Gettins D, Stanford SC, Mazhero S, Marks DF, et al. Preoperative rehearsal of active coping imagery influences subjective and hormonal responses to abdominal surgery. Psychosomatic Medicine 1995;57:177‐82. [DOI] [PubMed] [Google Scholar]
Manyande 1998 {published data only}
- Manyande A, Salmon P. Effects of pre‐operative relaxation on post‐operative analgesia: immediate increase and delayed reduction. British Journal of Health Psychology 1998;3(3):215‐24. [Google Scholar]
Mitchell 2000 {published data only}
- Mitchell M. Psychological preparation for patients undergoing day surgery. Ambulatory Surgery 2000;8:19‐29. [Google Scholar]
Montgomery 2002 {published data only}
- Montgomery GH, Weltz CR, Seltz M, Bovbjerg DH. Brief presurgical hypnosis reduces distress and pain in excisional breast biopsy patients. International Journal of Clinical and Experimental Hypnosis 2002;50(1):17‐32. [DOI] [PubMed] [Google Scholar]
Montgomery 2007 {published data only}
- Montgomery GH, Bovbjerg DH, Schnur JB, David D, Goldfarb A, Weltz CR, et al. A randomized clinical trial of a brief hypnosis intervention to control side effects in breast surgery patients. Journal of the National Cancer Institute 2007;99(17):1304‐12. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, Hallquist MN, Schnur JB, David, D, Silverstein JH, Bovbjerg DH. Mediators of a brief hypnosis intervention to control side effects in breast surgery patients: response expectancies and emotional distress. Journal of Consulting and Clinical Psychology 2010;78(1):80‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheard 2006 {published data only}
- Sheard C, Garrud P. Evaluation of generic patient information: effects on health outcomes, knowledge and satisfaction. Patient Education and Counselling 2006;64:43‐7. [DOI] [PubMed] [Google Scholar]
Shelley 2009 {published data only}
- Shelley M, Pakenham KI, Frazer I. Cortisol changes interact with the effects of a cognitive behavioural psychological preparation for surgery on 12‐month outcomes for surgical heart patients. Psychology and Health 2009;24(10):1139‐52. [DOI] [PubMed] [Google Scholar]
Stergiopoulou 2006 {published data only}
- Stergiopoulou A, Birbas K, Katostaras T, Diomidous M, Mantas J. The effect of a multimedia health educational program on the postoperative recovery of patients undergoing laparoscopic cholecystectomy. Studies in Health Technology & Informatics 2006;124:920‐5. [PubMed] [Google Scholar]
Sugai 2013 {published data only}
- Sugai DY, Deptula PL, Parsa AA, Don Parsa, F. The importance of communication in the management of postoperative pain. Hawai’i Journal of Medicine and Public Health 2013;72(6):180‐4. [PMC free article] [PubMed] [Google Scholar]
Surman 1974 {published data only}
- Surman OS, Hackett TP, Silverberg EL, Behrendt DM. Usefulness of psychiatric intervention in patients undergoing cardiac surgery. Archives of General Psychiatry 1974;30:830‐5. [DOI] [PubMed] [Google Scholar]
Timmons 1993 {published data only}
- Timmons ME, Bower FL. The effect of structured preoperative teaching on patients' use of patient‐controlled analgesia (PCA) and their management of pain. Orthopaedic Nursing 1993;12(1):23‐31. [DOI] [PubMed] [Google Scholar]
Voshall 1980 {published data only}
- Voshall B. The effects of preoperative teaching on postoperative pain. Topics in Clinical Nursing 1980;2:39‐43. [PubMed] [Google Scholar]
Wang 2002 {published data only}
- Wang AW, Gilbey HJ, Ackland TR. Perioperative exercise programs improve early return of ambulatory function after total hip arthroplasty. A randomized, controlled trial. American Journal of Physical Medicine and Rehabilitation 2002;81(11):801‐6. [DOI] [PubMed] [Google Scholar]
Wells 1986 {published data only}
- Wells JK, Howard GS, Nowlin WF, Vargas MJ. Presurgical anxiety and postsurgical pain and adjustment: effects of a stress inoculation procedure. Journal of Consulting and Clinical Psychology 1986;54(6):831‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Akinci 2015 {published data only}
- Akinci B, Yeldan I, Bayramoglu Z, Akpinar TB. The effects of posture and relaxation training on sleep, dyspnoea, pain and, quality of life in the short‐term after cardiac surgery: a pilot study. European Journal of Preventive Cardiology 2015;22(S1):S171‐2. [Google Scholar]
Angioli 2014 {published data only}
- Angioli R, Plotti F, Capriglione S, Aloisi A, Aloisi ME, Luvero D, et al. The effects of giving patients verbal or written pre‐operative information in gynecologic oncology surgery: a randomized study and the medical‐legal point of view. European Journal of Obstetrics & Gynecology and Reproductive Biology 2014;177:67‐71. [DOI] [PubMed] [Google Scholar]
Attias 2014 {published data only}
- Attias S, Keinan‐Boker L, Sroka G, Matter I, Arnon Z, Schiff E. The effectiveness of a generic guided imagery CD compared with individualized complementary therapies and standard of care for reducing preoperative anxiety: preliminary results. Journal of Alternative and Complementary Medicine 2014;20(5):A137. [Google Scholar]
Bergin 2014b {published data only}
- Bergin C, Speroni KG, Travis T, Bergin J, Sheridan MJ, Kelly K, et al. Effect of preoperative incentive spirometry patient education on patient outcomes in the knee and hip joint replacement population. Journal of Perianesthesia Nursing 2014;29(1):20‐7. [DOI] [PubMed] [Google Scholar]
Calsinski Assis 2014 {published data only}
- Calsinski Assis C, Lima Lopes J, Nogueira‐Martins L A, Bottura Leite de Barros AL. Embracement and anxiety symptoms in patients before cardiac surgery [Acolhimento e sintomas de ansiedade em pacientes no pré‐operatório de cirurgia cardíaca]. Revista Brasileira de Enfermagem 2014;67(3):401‐7. [DOI] [PubMed] [Google Scholar]
Chevillon 2014 {published data only}
- Chevillon C, Hellyar M, Madani C, Kerr K, Son Chae K. Preoperative education on postoperative delirium, anxiety, and knowledge in pulmonary thromboendarterectomy patients. American Journal of Critical Care 2015;24(2):164‐71. [DOI] [PubMed] [Google Scholar]
Chow 2014 {published data only}
- Chow KM, Chan CWH, Chan JCY, Choi KKC, Siu KY. A feasibility study of a psychoeducational intervention program for gynecological cancer patients. European Journal of Oncology Nursing 2014;18(4):385‐92. [DOI] [PubMed] [Google Scholar]
Dathatri 2014 {published data only}
- Dathatri S, Gruberg L, Anand J, Romeiser J, Sharma S, Finnin E, et al. Informed consent for cardiac procedures: deficiencies in patient comprehension with current methods. Annals of Thoracic Surgery 2014;97(5):1505‐12. [DOI] [PubMed] [Google Scholar]
Eckhouse 2014 {published data only}
- Eckhouse DR, Hurd M, Cotter‐Schaufele S, Sulo S, Sokolowski M, Barbour L. A randomized controlled trial to determine the effects of music and relaxation interventions on perceived anxiety in hospitalized patients receiving orthopaedic or cancer treatment. Orthopaedic Nursing 2014;33(6):342‐51. [DOI] [PubMed] [Google Scholar]
El Azem 2014 {published data only}
- Azem A, Benington PCM, Khambay BS, Ayoub AF. Evaluation of an interactive multi‐media device for delivering information on Le Fort I osteotomy. Journal of Cranio‐Maxillofacial Surgery 2014;42(6):885‐9. [DOI] [PubMed] [Google Scholar]
Ellett 2014 {published data only}
- Ellett L, Villegas R, Beischer A, Ong N, Maher P. Use of a multimedia module to aid the informed consent process in patients undergoing gynecologic laparoscopy for pelvic pain: randomized controlled trial. Journal of Minimally Invasive Gynecology 2014;21(4):602‐11. [DOI] [PubMed] [Google Scholar]
Foji 2015 {published data only}
- Foji S, Tadayonfar MA, Mohsenpour M, Rakhshani MH. The study of the effect of guided imagery on pain, anxiety and some other hemodynamic factors in patients undergoing coronary angiography. Complementary Therapies in Clinical Practice 2015;21(2):119‐23. [DOI] [PubMed] [Google Scholar]
Fraval 2015 {published data only}
- Fraval A, Chandrananth J, Chong YM, Tran P, Coventry LS. Internet based patient education improves informed consent for elective orthopaedic surgery: a randomized controlled trial. BMC Musculoskeletal Disorders 2015;16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Furuya 2015 {published data only}
- Furuya RK, Arantes EC, Dessotte CAM, Ciol MA, Hoffman JM, Schmidt A, et al. A randomized controlled trial of an educational programme to improve self‐care in Brazilian patients following percutaneous coronary intervention. Journal of Advanced Nursing 2015;71(4):895‐908. [DOI] [PubMed] [Google Scholar]
Gade 2014 {published data only}
- Gade H, Hjelmesæth J, Rosenvinge JH, Friborg O. Effectiveness of a cognitive behavioral therapy for dysfunctional eating among patients admitted for bariatric surgery: a randomized controlled trial. Journal of Obesity 2014;2014:127936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillis 2014 {published data only}
- Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 2014;121(5):937‐47. [DOI] [PubMed] [Google Scholar]
Gyulaházi 2015 {published data only}
- Gyulaházi J, Varga K, Iglói E, Redl P, Kormos J, Fülesdi B. The effect of preoperative suggestions on perioperative dreams and dream recalls after administration of different general anesthetic combinations: a randomized trial in maxillofacial surgery. BMC Anesthesiology 2015;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2015 {published data only}
- Hansen MM. A feasibility pilot study on the use of complementary therapies delivered via mobile technologies on Icelandic surgical patients' reports of anxiety, pain, and self‐efficacy in healing. BMC Complementary & Alternative Medicine 2015;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henney 2014 {published data only}
- Henney S, Irving R. Prospective, randomised, controlled trial comparing delivery of patient information for functional endoscopic sinus surgery via website versus printed leaflet. Journal of Laryngology & Otology 2014;128(3):249‐54. [DOI] [PubMed] [Google Scholar]
Heras 2014 {published data only}
- Heras P, Natsis V, Sirbilantze T. Preventing anxiety and depression in breast cancer: a randomized controlled trial. Supportive Care in Cancer 2014;22:S175‐6. [Google Scholar]
Hoppe 2014 {published data only}
- Hoppe DJ, Denkers M, Hoppe FM, Wong IH. The use of video before arthroscopic shoulder surgery to enhance patient recall and satisfaction: a randomized‐controlled study. Journal of Shoulder and Elbow Surgery 2014;23(6):e134‐9. [DOI] [PubMed] [Google Scholar]
Huber 2015 {published data only}
- Huber EO, Roos EM, Meichtry A, Bie RA, Bischoff‐Ferrari HA. Effect of preoperative neuromuscular training (NEMEX‐TJR) on functional outcome after total knee replacement: an assessor‐blinded randomized controlled trial. BMC Musculoskeletal Disorders 2015;16:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansson 2007 {published data only}
- Johansson K, Salanterä S, Katajisto J. Empowering orthopaedic patients through preadmission education: results from a clinical study. Patient Education and Counseling 2007;66:84‐91. [DOI] [PubMed] [Google Scholar]
Kol 2014 {published data only}
- Kol E, Alpar SE, Erdoğan A. Preoperative education and use of analgesic before onset of pain routinely for post‐thoracotomy pain control can reduce pain effect and total amount of analgesics administered postoperatively. Pain Management Nursing 2014;15(1):331‐9. [DOI] [PubMed] [Google Scholar]
Lai Ngor 2014 {published data only}
- Lai Ngor C, Lai CKY. The effect of patient education with telephone follow‐up on wound healing in adult patients with clean wounds: a randomized controlled trial. Journal of Wound, Ostomy & Continence Nursing 2014;41(4):345‐55. [DOI] [PubMed] [Google Scholar]
Lookinland 1998 {published data only}
- Lookinland S, Pool M . Study on effect of methods of preoperative education in women. Association of Operating Room Nurses Journal 1998;67(1):203‐13. [DOI] [PubMed] [Google Scholar]
Louw 2014 {published data only}
- Louw A, Diener I, Landers MR, Puentedura EJ. Preoperative pain neuroscience education for lumbar radiculopathy: a multicenter randomized controlled trial with 1‐year follow‐up. Spine 2014;39(18):1449‐57. [DOI] [PubMed] [Google Scholar]
Mohammadi 2014 {published data only}
- Mohammadi A, Mirbagher Ajorpaz N, Torabi M, Mirsane A, Moradi F. Effects of music listening on preoperative state anxiety and physiological parameters in patients undergoing general surgery: a randomized quasi‐experimental trial. Central European Journal of Nursing & Midwifery 2014;5(4):156‐60. [Google Scholar]
Novick 2014 {published data only}
- Novick BJ, Angie M, Walker E, Kitay R, Monday K, Albert NM. The effect of intensive education on urinary incontinence following radical prostatectomy: a randomized control trial. Urologic Nursing 2014;34(5):246‐51. [PubMed] [Google Scholar]
Paul 2015 {published data only}
- Paul L, Rongen S, Hoeken D, Deen M, Klaassen R, Biter LU, et al. Does cognitive behavioral therapy strengthen the effect of bariatric surgery for obesity? Design and methods of a randomized and controlled study. Contemporary Clinical Trials 2015;42:252‐6. [DOI] [PubMed] [Google Scholar]
Rolving 2014 {published data only}
- Rolving N, Nielsen CV, Christensen FB, Holm R, Bunger C, Østergaard L. Does a preoperative cognitive‐behavioural intervention affect postsurgical pain, mobilisation and length of hospitalisation in lumbar spinal fusion patients?. European Spine Journal 2014;23(5 (Suppl)):S572. [Google Scholar]
Saleh 2015 {published data only}
- Saleh AJ, Tang GX, Hadi SM, Yan L, Chen MH, Duan KM, et al. Preoperative cognitive intervention reduces cognitive dysfunction in elderly patients after gastrointestinal surgery: a randomized controlled trial. Medical Science Monitor 2015;21:798‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shahmansouri 2014 {published data only}
- Shahmansouri N, Janghorbani M, Salehi Omran A, Karimi AA, Noorbala AA, Arjmandi A, et al. Effects of a psychoeducation intervention on fear and anxiety about surgery: randomized trial in patients undergoing coronary artery bypass grafting. Psychology, Health & Medicine 2014;19(4):375‐83. [DOI] [PubMed] [Google Scholar]
Umpierres 2014 {published data only}
- Umpierres CS, Ribeiro TA, Marchisio ÂE, Galvão L, Borges ÍNK, Souza Macedo CA, et al. Rehabilitation following total hip arthroplasty evaluation over short follow‐up time: randomized clinical trial. Journal of Rehabilitation Research & Development 2014;51(10):1567‐78. [DOI] [PubMed] [Google Scholar]
Van Acker 2014 {published data only}
- Acker MM, Kuriata MA. Video education provides effective wound care instruction pre‐ or post‐Mohs micrographic surgery. Journal of Clinical and Aesthetic Dermatology 2014;7(4):43‐7. [PMC free article] [PubMed] [Google Scholar]
West 2014 {published data only}
- West AM, Bittner EA, Ortiz VE. The effects of preoperative, video‐assisted anesthesia education in Spanish on Spanish‐speaking patients' anxiety, knowledge, and satisfaction: a pilot study. Journal of Clinical Anesthesia 2014;26(4):325‐9. [DOI] [PubMed] [Google Scholar]
Würtzen 2015 {published data only}
- Würtzen H, Dalton SO, Christensen J, Andersen KK, Elsass P, Flyger HL, et al. Effect of mindfulness‐based stress reduction on somatic symptoms, distress, mindfulness and spiritual wellbeing in women with breast cancer: results of a randomized controlled trial. Acta Oncologica 2015;54(5):712‐9. [DOI] [PubMed] [Google Scholar]
Xin 2015 {published data only}
- Xin YH, Xiao CR. Effect of holistic health education prescription in colorectal cancer patients undergoing surgery. World Chinese Journal of Digestology 2015;23(2):338‐42. [Google Scholar]
References to ongoing studies
Hansen 2013 {published data only}
- Hansen MM. Impact of complementary therapies via mobile technologies on Icelandic same day surgical patients' reports of anxiety, pain and self‐efficacy in healing: A randomized controlled trial in process. Studies in Health Technology & Informatics 2013;192:1165. [PubMed] [Google Scholar]
Jong 2012 {published data only}
- Jong M, Pijl A, Gast H, Sjoling M. The effects of guided imagery on preoperative anxiety and pain management in patients undergoing laparoscopic cholecystectomy in a multi‐centre RCT study. BMC Complementary and Alternative Medicine 2012;12(Suppl 1):184. [Google Scholar]
Additional references
Abraha 2010
- Abraha I, Montedori A. Modified intention to treat reporting in randomised controlled trials: systematic review. BMJ 2010;340:c2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C.: American Psychiatric Association, 1980. [Google Scholar]
APA 2005
- Executive Committee of the American Psychological Association, Division of Psychological Hypnosis. New Definition: Hypnosis. The Division 30 Definition and Description of Hypnosis. http://www.apa.org/divisions/div30/define_hypnosis.html (accessed 21 October 2009) 2005.
Arpino 2004
- Arpino L, Iavarone A, Parlato C, Moraci A. Prognostic role of depression after lumbar disc surgery. Neurological Sciences 2004;3:145‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bellamy 1988
- Bellamy N, Buchanan W, Goldsmith C, Campbell J, Sitt L. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy inpatients with osteoarthritis of the hip or knee. Journal of Rheumatology 1988;15:1833‐40. [PubMed] [Google Scholar]
Billewicz 1964
- Billewicz WZ. Matched samples in medical investigation. British Journal of Preventive and Social Medicine 1964;18:167‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruce 2012
- Bruce J, Thornton AJ, Scott NW, Marfizo S, Powell R, Johnston M, et al. on behalf of the Recovery Study Group. Chronic preoperative pain and psychological robustness predict acute postoperative pain outcomes after surgery for breast cancer. British Journal of Cancer 2012;107:937‐46. [DOI: 10.1038/bjc.2012.341] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1983
- Cohen S Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behaviour 1983;24:385‐96. [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Second Edition. Lawrence Erlbaum Associates, 1988. [Google Scholar]
Daut 1983
- Daut R, Cleeland C, Flanery R. Development of the Wisconsin Brief Pain Inventory to assess pain in cancer and other diseases. Pain 1983;1:197‐210. [DOI] [PubMed] [Google Scholar]
de Bruin 2009
- Bruin M, Viechtbauer W, Hospers HJ, Schaalma HP, Kok G. Standard care quality determines treatment outcomes in control groups of HAART‐adherence intervention studies: implications for the interpretation and comparison of intervention effects. Health Psychology 2009;28(6):668‐74. [DOI: 10.1037/a0015989] [DOI] [PubMed] [Google Scholar]
Dupuy 1984
- Dupuy HJ. The psychological general well‐being (PGWB) index. In: Wenger NK, Matson MI, Furberg CD, Elinson J editor(s). Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. New York: Le Jacq Publ Inc, 1984:170‐83. [DOI] [PubMed] [Google Scholar]
Geissner 1996
- Geissner E. Die Schmerzempfindungs‐Skala. Test und Handanweisung. Göttingen: Hogrefe, 1996. [Google Scholar]
Goldberg 1978
- Goldberg D. Manual of the General Health Questionnaire. Windsor: NEFR‐Nelson, 1978. [Google Scholar]
Granot 2005
- Granot M, Ferber SG. The roles of pain catastrophizing and anxiety in the prediction of postoperative pain intensity: a prospective study. Clinical Journal of Pain 2005;21(5):439‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gurusamy 2014
- Gurusamy KS, Vaughan J, Davidson BR. Formal education of patients about to undergo laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD009933.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hulzebos 2012
- Hulzebos EHJ, Smit Y, Helders PPJM, Meeteren NLU. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010118.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunt 1983
- Hunt SM, McEwen J. The development of a subjective health indicator. Sociology of Health and Illness 1983;2:231‐45. [DOI] [PubMed] [Google Scholar]
Jacobsen 1938
- Jacobsen E. Progressive Relaxation. Chicago: University of Chicago Press, 1938. [Google Scholar]
Janke 1994
- Janke W, Hüppe M. [Befindlichkeitsskalierung durch Kategorien und Eigenschaftswörter: BSKE (EWL)]. In: Janke W, Debus G, Erdman G, Hüppe M editor(s). Test und Handanweisung. Würzburg: Unveröffentlichter Institutsbericht. Institut für Psychologie I, 1994. [Google Scholar]
Jette 1987
- Jette AM, Cleary PD. Functional Disability Assessment. Physical Therapy 1987;67:1854‐9. [DOI] [PubMed] [Google Scholar]
Johnson 1973
- Johnson JE. Effects of accurate expectations about sensations on the sensory and distress components of pain. Journal of Personality and Social Psychology 1973;27(2):261‐75. [DOI] [PubMed] [Google Scholar]
Johnston 1980
- Johnston M. Anxiety in surgery patients. Psychological Medicine 1980;10:145‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnston 1993
- Johnston M, Vögele C. Benefits of psychological preparation for surgery: a meta‐analysis. Annals of Behavioral Medicine 1993;15(4):245‐56. [Google Scholar]
Jüni 2001
- Jüni P, Douglas GA, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323:42‐6. [PUBMED: 11440947] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kekecs 2014
- Kekecs Z, Nagy T, Varga K. The effectiveness of suggestive techniques in reducing postoperative side effects: a meta‐analysis of randomized controlled trials. Anesthesia & Analgesia 2014;119(6):1407‐19. [DOI] [PubMed] [Google Scholar]
Kiecolt‐Glaser 1998
- Kiecolt‐Glaser JK, Page GG, Marucha PT, MacCallum RC, Glaser R. Psychological influences on surgical recovery. Perspectives from psychoneuroimmunology. American Psychologist 1998;53(11):1209‐18. [DOI: 10.1037/0003-066X.53.11.1209] [DOI] [PubMed] [Google Scholar]
Louw 2013
- Louw A, Diener I, Butler DS, Puentedura EJ. Preoperative education addressing postoperative pain in total joint arthroplasty: review of content and educational delivery methods. Physiotherapy Theory and Practice 2013;29(3):175‐94. [DOI] [PubMed] [Google Scholar]
Lucente 1972
- Lucente FE, Fleck MD. A study of hospitalization anxiety in 408 medical and surgical patients. Psychosomatic Medicine 1972;34(4):304‐12. [DOI] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Maryland State Medical Journal 1965;14:61‐5. [PubMed] [Google Scholar]
Maple 2015
- Maple H, Chilcot J, Lee V, Simmonds S, Weinman J, Mamode N. Stress predicts the trajectory of wound healing in living kidney donors as measured by high‐resolution ultrasound. Brain, Behavior and Immunity 2015;43:19‐26. [10.1016/j.bbi.2014.06.012] [DOI] [PubMed] [Google Scholar]
Marucha 1998
- Marucha PT, Kiecolt‐Glaser JK, Favegehi M. Mucosal wound healing is impaired by examination stress. Psychosomatic Medicine 1998;60:362‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mathews 1981
- Mathews A, Ridgeway V. Personality and surgical recovery: a review. British Journal of Clinical Psychology 1981;2:243‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mathews 1984
- Mathews A, Ridgeway V. Psychological preparation for surgery. In: Steptoe A, Mathews A editor(s). Health Care and Human Behaviour. London: Academic Press, 1984:231‐59. [Google Scholar]
McDonald 2014
- McDonald S, Page MJ, Beringer K, Wasiak J, Sprowson A. Preoperative education for hip or knee replacement. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD003526.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
McNair 1971
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States Manual. San Diego: Educational and Industrial Testing Service, 1971. [Google Scholar]
Melzack 1975
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1:277‐99. [PUBMED: 1235985] [DOI] [PubMed] [Google Scholar]
Melzack 1987
- Melzack R. The short‐form McGill Pain Questionnaire. Pain 1987;30:191‐7. [DOI] [PubMed] [Google Scholar]
Michie 2008
- Michie S, Johnston M, Francis JJ, Hardeman W, Eccles M. From theory to intervention: mapping theoretically derived behavioural determinants to behaviour change techniques. Applied Psychology: An International Review 2008;57:660‐80. [Google Scholar]
Michie 2013
- Michie S, Richardson M, Johnston M, Hardeman W, Eccles MP, Cane J, et al. The Behavior Change Technique Taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of Behavioral Medicine 2013;46(1):81‐95. [DOI] [PubMed] [Google Scholar]
Miller 1983
- Miller SM, Manan CE. Interacting effects of information and coping style in adapting to gynecologic stress: should the doctor tell all?. Journal of Personality and Social Psychology 1983;45(1):223‐36. [PUBMED: 6886967] [DOI] [PubMed] [Google Scholar]
Miller 1987
- Miller SM. Monitoring and blunting: validation of a questionnaire to assess styles of information seeking under threat. Journal of Personality and Social Psychology 1987;52:345‐53. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz K, Altman D. Revised recommendations for improving the quality of reports of parallel group randomized trials. http://www.consort‐statement.org/statement/revisedstatement.htm (accessed 6 July 2004) 2001.
Munafó 2001
- Munafó MR, Stevenson J. Anxiety and surgical recovery: reinterpreting the literature. Journal of Psychosomatic Research 2001;51:589‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21:837‐41. [DOI] [PubMed] [Google Scholar]
Nicholson 2013
- Nicholson A, Lewis SR, Lee A, Smith AF, Coldwell CH. Different formats and timing of educational interventions for surgical patients. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD010889] [DOI] [Google Scholar]
Radloff 1968
- Radloff R, Helmreich R. Groups Under Stress. New York: Appleton‐Century‐Crofts, 1968. [Google Scholar]
Rainville 2005
- Rainville P, Bao QVH, Chrétien P. Pain‐related emotions modulate experimental pain perception and autonomic responses. Pain 2005;118:306‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royal College of Surgeons 2016
- Royal College of Surgeons. Surgery and the NHS in numbers. Surgery in numbers: England. https://www.rcseng.ac.uk/media/media‐background‐briefings‐and‐statistics/surgery‐and‐the‐nhs‐in‐numbers (accessed 11 March 2016). Data from Health and Social Care Information Centre (HSCIC), Hospital Episode Statistics 2013/14. London: The Royal College of Surgeons of England, 2016. [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher DG, for the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010;340(c332):698‐702. [DOI: 10.1136/bmj.c332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shields 1995
- Shields RK, Enloe LJ, Evans RE, Smith KB, Steckel SD. Reliability, validity, and responsiveness of functional tests in patients with total joint replacement. Physical Therapy 1995;75(3):169‐76. [DOI] [PubMed] [Google Scholar]
Spiegal 1977
- Spiegal H. The Hypnotic Induction Profile (HIP): a review of its development. Annals of the New York Academy of Science 1977;296:129‐42. [DOI] [PubMed] [Google Scholar]
Spielberger 1983
- Spielberger C. Manual for the State Trait Anxiety Inventory STAI (form Y). Palo Alto, CA: Consulting Psychologists Press, 1983. [Google Scholar]
Sullivan 1995
- Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological Assessment 1995;7(4):524‐32. [Google Scholar]
Tait 1982
- Tait A, Maguire P, Faulkner A, Brooke M, Wilkinson S, Thomson L, et al. Improving communication skills: standardised assessments may help nurses to detect psychiatric problems as they develop in mastectomy patients. Nursing Times 1982;78:2181‐4. [PubMed] [Google Scholar]
Tefikow 2013
- Tefikow S, Barth J, Maichrowitz S, Beelmann A, Strauss B, Rosendahl J. Efficacy of hypnosis in adults undergoing surgery or medical procedures: a meta‐analysis of randomized controlled trials. Clinical Psychology Review 2013;33:623‐36. [DOI] [PubMed] [Google Scholar]
van Middendorp 2010
- Middendorp H, Lumley MA, Jacobs JWG, Bijlsma JWJ, Geenen R. The effects of anger and sadness on clinical pain reports and experimentally‐induced pain thresholds in women with and without fibromyalgia. Arthritis Care and Research 2010;62(10):1370‐6. [DOI: 10.1002/acr.20230] [DOI] [PubMed] [Google Scholar]
Walburn 2009
- Walburn J, Vedhara K, Hankins M, Rixon L, Weinman J. Psychological stress and wound healing in humans: a systematic review and meta‐analysis. Journal of Psychosomatic Research 2009;67:253‐41. [DOI: 10.1016/j.jpsychores.2009.04.002] [DOI] [PubMed] [Google Scholar]
Ware 2000
- Ware JE, Koskinski M, Dewey JE. How to Score Version 2 of the SF‐36 (R) Health Survey. Lincoln, RI: Quality Metric Incorporated, 2000. [Google Scholar]
Watson 1988
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology 1988;47:1063‐70. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavia 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]
Zuckerman 1965
- Zuckerman M, Lubin B. The development of an affect adjective check list for the measurement of anxiety. Manual for the Multiple Affect Adjective Check List. Carolina: Educational and Industrial Testing Service, 1965. [Google Scholar]
Zung 1971
- Zung WWK. A rating instrument for anxiety disorders. Psychosomatics 1971;12:371‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Powell 2010
- Powell R, Bruce J, Johnston M, Vögele C, Scott N, Shehmar M, et al. Psychological preparation and postoperative outcomes for adults undergoing surgery under general anaesthesia. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD008646] [DOI] [PMC free article] [PubMed] [Google Scholar]


