Abstract
Background
The biofilm produced by Cutibacterium acnes is a major infection threat for skin and implanted catheters. Nanoparticles provide a new approach to eradicate biofilms. The present study evaluated the capability of cationic liposomes loaded with DNase I (DNS) and proteinase K (PK) to remove preformed C. acnes biofilms.
Methods
DNS and PK were able to target and disassemble the biofilm by degrading extracellular polymer substances (EPS). Soyaethyl morpholinium ethosulfate (SME) was used to render a positive charge and enhance the antibacterial activity of the liposomes.
Results
The cationic liposomes containing enzymes yielded monodisperse nanovesicles ranging between 95 and 150 nm. The entrapment efficiency of the enzymes in the liposomes achieved a value of 67–83%. All liposomal formulations suppressed planktonic C. acnes growth at a minimum inhibitory concentration (MIC) equal to the free SME in the solution. The enzyme in the liposomal form inhibited biofilm growth much better than that in the free form, with the dual enzyme-loaded liposomes demonstrating the greatest inhibition of 54% based on a crystal violet assay. The biofilm-related virulence genes PA380 and PA1035 were downregulated by the combined enzymes in the liposomes but not the individual DNS or PK. Scanning electron microscopy (SEM) and confocal microscopy displayed reduced C. acnes aggregates and biofilm thickness by the liposomal system. The liposomes could penetrate through about 85% of the biofilm thickness. The in vitro pig skin permeation also showed a facile delivery of liposomes into the epidermis, deeper skin strata, and hair follicles. The liposomes exhibited potent activity to eliminate C. acnes colonization in mouse skin and catheters in vivo. The colony-forming units (CFUs) in the catheter treated with the liposomes were reduced by 2 logs compared to the untreated control.
Conclusion
The data suggested a safe application of the enzyme-loaded cationic liposomes as antibacterial and antibiofilm agents.
Keywords: liposomes, cationic surfactant, DNase I, proteinase K, Cutibacterium acnes, biofilm
Introduction
Bacterial infections are one of the largest causes of human death worldwide. The emergence of bacterial resistance to antibiotics is becoming a major problem due to their broad use and abuse.1 The bacteria in biofilms play a critical role of resistance to antibiotics and host clearance systems, leading to persistent and difficult-to-treat infections. A biofilm is a sessile community of bacteria embedded in a matrix of extracellular polymeric substances (EPS) consisting of proteins, polysaccharides, lipids, and deoxyribonucleic acids (DNAs).2 A high antibiotic dose is required to conquer the biofilm tolerance, which poses a risk of adverse effect. Biofilms are involved in about 80% of microbial infections and cause more than 500,000 deaths each year.3 Cutibacterium acnes is a Gram-positive anaerobic bacterium that forms a biofilm which is more resistant to antibiotics than planktonic cells.4 C. acnes is reported to represent >30% of the facial microbes in acne patients.5 Acne vulgaris is the eighth-most prevalent disorder in the world and affects about 10% of the population, especially adolescents.6 The C. acnes-associated biofilm in hair follicles elicits the symptoms of increased seborrhea, hyperkeratinization, erythema, and comedones in acne patients. C. acnes is also linked to a number of other infections. such as folliculitis, endocarditis, periodontitis, sarcoidosis, and medical catheter-related infections.7 Available drug therapies for treating acne vulgaris are limited, and none of them are regarded as a definite cure because of their side effects.8 New perspectives in the treatment of C. acnes infection focus on biofilm targeting and antibiotic resistance prevention.
Most antibacterial agents are difficult to penetrate through the EPS matrix produced by the biofilm. One of the approaches to conquer this problem is the use of antimicrobial peptides.9,10 The photothermal treatment can produce the heat to disintegrate biofilm for the subsequent drug delivery into the biofilm.11 The polymer-based drug delivery allows improved transport into the biofilm to enhance antibiotic bioavailability.12 Another effort to resolve this drawback is the intervention of nanoparticles.13 Liposomes are the most focused nanoformulations in commercialization and in clinical studies because of their possible industrial scale-up, biocompatibility, low toxicity, and the capacity to entrap both lipophilic and hydrophilic actives.14 The structure of a liposome is a spherical lipid vesicle composed of phospholipid bilayers. Liposomes are reported to preferentially adsorb onto the biofilm surface and then penetrate into the EPS to eradicate bacterial growth.15,16 Liposomes have also been demonstrated to be helpful for targeting hair follicles after the increase of flexibility.17 Cationic surfactants can be coated on the nanoparticulate surface to kill the bacteria in biofilms by disintegrating the cell membrane through electrostatic and hydrophobic interactions.18,19 Cationic nanoparticles can simultaneously encapsulate with antibiotics to synergistically suppress bacterial growth.
Extracellular DNA and proteins are components of biofilms that create a rigid structure. Enzymes that can degrade these components are expected to be useful to disrupt biofilms and improve the delivery of antibacterial agents. Both DNase I (DNS) and proteinase K (PK) have been proved to inhibit biofilm development and detach preformed biofilms to promote bacterial killing.20,21 The combination of both enzymes may be presented as a promising approach for the management of biofilm-related infection. Biofilms exhibit the threat of increased bacterial virulence for infection. There has been no biofilm-specific therapy until now. We aimed to develop cationic liposomes loaded with DNS and PK to assess the combinatorial effect of cationic surfactants and enzymes on C. acnes biofilm eradication. The possible applicability of the liposomal system to cutaneous and catheter infections was explored. The cationic surfactant employed in this study was soyaethyl morpholinium ethosulfate (SME), which is a quaternary ammonium compound with a C18 alkyl chain and morpholin six-membered ring. SME has revealed strong inhibition of methicillin-resistant Staphylococcus aureus (MRSA) growth with a strong fusion with bacterial membrane.22 A major concern of the application of cationic surfactants is the significant cytotoxicity against mammalian cells. This previous study22 also reported a milder toxicity of SME than the other cationic surfactants. SME at 5 μg/mL showed the higher viability of human keratinocytes (>90%) than benzalkonium chloride (about 40%) and cetylpyridinium chloride (about 30%). We generated the already established biofilm to test the inhibitory effect of the liposomes against biofilm-embedded C. acnes in vitro and in vivo.
Materials and Methods
Materials
C. acnes (ATCC6919) was purchased from American Type Culture Collection (VA, USA). Soybean phosphatidylcholine (SPC, Phospholipon 80H) was purchased from American Lecithin Company (CT, USA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and cholesterol were purchased from Sigma-Aldrich (MO, USA). SME was supplied by Croda (East Yorkshire, UK). DNS and PK were purchased from BioTools (Taipei, Taiwan). Alexa Fluor 647-conjugated dextran, SYTO9 and Bodipy FL C16 were purchased from Thermo Fischer Scientific (MA, USA). C. acnes culture medium reinforced clostridial medium (RCM) was purchased from Merck (Darmstadt, Germany), Cell culture medium DMEM and RPMI-1640 were purchased from Thermo Fischer Scientific.
Fabrication of Liposomes
Cationic liposomes loaded with enzymes were prepared by the thin-film method. SPC (250 mg), cholesterol (70 mg), and SME (30 mg) were dissolved in a chloroform-methanol mixture (2:1). This mixture was then deposited as a thin film in a round bottom flask using a rotary evaporator at 50°C. The residual solvent was removed under a vacuum. The film was then hydrated by the addition of double-distilled water containing 5 mg DNS (from bovine pancreas, BioTools, Taipei, Taiwan) and/or 2 mg PK (from Tritirachium album, BioTools) to give a total volume of 10 mL. Both enzymes are proved to be stable up to 50 °C.23,24 Further sonication was performed by a probe-type sonicator at 35 W for 30 min to produce the final product. The scheme of the liposome preparation procedures is illustrated in Suppl. Figure 1.
Vesicle Size and Zeta Potential
The mean diameter (z-average) and zeta potential of the developed liposomes were estimated using the laser scattering method (Nano ZS90, Malvern). Detection was carried out after a 100-fold dilution by double-distilled water. The measurement was repeated three times per sample for three batches.
Encapsulation Efficiency of the Enzymes in the Liposomes
The loading efficiency of DNS and PK was evaluated by using the ultracentrifugation technique to separate the entrapped enzymes from the free forms. The liposomes were centrifuged at 48,000 xg and 4°C for 40 min. The free proteins in the supernatant and the entrapped proteins in the precipitate were quantified using a detergent-compatible protein assay kit (Bio-Rad). The encapsulation efficiency (%) was calculated as: protein amount in precipitate/(protein amount in precipitate+protein amount in supernatant).
Determination of Planktonic Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
C. acnes was cultured in RCM at 37°C under anaerobic conditions for three days. The MIC was considered to be the lowest concentration of SME required to inhibit the growth of C. acnes by serial dilution. The C. acnes was diluted in RCM to achieve an optical density at 600 nm (OD600) of 0.01. The bacterial population was exposed to several dilutions of liposomes, from 0.36 to 750 μg/mL, and then incubated at 37°C for 24 h. An ELISA reader was used to detect the MIC at 595 nm. For the MBC assay, the colony-forming units (CFUs) of C. acnes were counted after treatment of liposomes for 24 h. The highest dilution resulting in a 99.9% reduction of bacterial number was recognized as the MBC.
Time-Response C. acnes Growth Inhibition
The growth inhibition of C. acnes by liposomes over four days was assessed in 96-well plates. The cationic liposomes with an SME at 0.37−5.86 μg/mL were inoculated with bacteria (OD600=0.01) at 37°C. The liposomes were diluted with RCM if necessary. The absorbance of each well was estimated at 600 nm to calculate the growth of C. acnes in a real-time mode.
Biofilm Formation and the Detection by Crystal Violet
C. acnes was cultured anaerobically using RCM in a DG250 anaerobic workstation (Don Whitney Scientific) at 37°C for three days. The cultures were diluted to OD600=0.1 using the RCM broth supplemented with 1% glucose. An aliquot of the C. acnes suspension (1 mL) was added to the 24-well plates and incubated anaerobically at 37°C for three days. After the removal of the supernatant, the biofilm formed on the bottom of the well. Next, 500-μL crystal violet (0.1%) was added to the well for 10 min and then washed twice with PBS at a volume of 1 mL. Acetic acid (0.5 mL) was added into the well to extract the crystal violet. The OD555 was determined using a microplate reader.
C. acnes Survival Inside and Outside the Biofilm
The biofilm was established in the microplate by incubating C. acnes (OD600=0.1) in RCM containing 0.1% glucose for three days. The liposomes with an SME of 150 μg/mL and/or DNS/PK (25 and 10 μg/mL) were treated to the biofilm for 24 h. The liposomes were diluted with RCM to obtain the definitive dose. The recovered microbes inside and outside the biofilm were loaded on an agar plate for 48 h to detect CFU.
qPCR Assay of Biofilm-Related Virulence Factors
After three days of biofilm growth, the liposomes containing 150 μg/mL SME and/or DNS/PK (25 and 10 μg/mL) were added to the microplate for 24 h. The samples were centrifuged at 12,000 rpm at 4°C for 10 min. The supernatant was then withdrawn. The C. acnes pellet was extracted by EasyPrep Total RNA kit (BioTools). The total RNA was determined using a microplate reader. The generation of cDNA was carried out using the ToolsQuant II Fast RT kit (BioTools) according to the manufacturer’s protocol. The synthesized cDNA was used for qPCR following the protocol from the Tools SYBR qPCR kit (BioTools). The RecA gene was employed as the housekeeping gene for standardization. The primer sequences used for RecA, PPA380, and PPA1035 were: RecA: 5ʹ-CAGCACGGCAAGGGCTC-3ʹ (forward) and 5ʹ-GGAGGATTCGGGACATAG-3ʹ (reverse); PPA380: 5ʹ-CGCTCTGAAGGATTCGTC-3ʹ (forward) and 5ʹ-GTCGTGCAGGATACACATGA-3ʹ (reverse); PPA1035: 5ʹ-TATCGATCATGGGTGATTCC-3ʹ (forward) and 5ʹ-ATAGAGGGCGAGGACTGACT-3ʹ (reverse).
Scanning Electron Microscopy (SEM)
The biofilm was grown for three days and then treated with liposomes containing 150 μg/mL SME and/or DNS/PK (25 and 10 μg/mL) for 24 h. The liposomes were diluted with RCM to obtain the definitive dose. The samples were fixed with 3% glutaraldehyde and 2% paraformaldehyde in a cacodylate buffer. After dehydration in an ascending series of ethanol, the samples were coated with gold and monitored under a Hitachi SU8220 SEM to examine the biofilm morphology.
Confocal Laser Scanning Microscopy (CLSM)
The biofilm inhibition caused by the liposomes was analyzed under CLSM. C. acnes biofilm was grown in a CELLview dish by incubating microbes (OD600=0.1) in glucose-containing RCM for three days. The biofilm was treated with 150 μg/mL SME and/or DNS/PK (25 and 10 μg/mL) in either the free form or the liposomal form for 24 h. The biofilm was treated with SYTO9 (1.5 μL/mL) and Alexa Fluor 647-conjugated dextran (1.5 μL/mL) for 15 min to stain the cells (green) and biofilm matrix (red), respectively. The biofilm structure and thickness were visualized under confocal microscopy (Leica TCS SP8).
Microfluidic Chamber Assay
The biofilm was grown in a flow cell chamber (Ibidi μ-Slide I0.2 Luer) for three days in a DG250 anaerobic workstation at 37°C. The liposomes and SYTO9 (1.5 μL/mL) were present in the flow-through medium in a syringe (5 mL) and throughout the biofilm growth by a syringe pump (Chemyx Fusion 100). The flow rate was set at 10 μL/min during the first 180 min and then increased to 50 μL/min from 180 to 240 min. The biofilm attached on the chamber surface was observed by CLSM.
The Penetration of Liposomes Through the Biofilm
The C. acnes biofilm was treated with liposomes for 24 h and then rinsed by PBS to remove the loosely adherent planktonic bacteria. The liposomes were stained with Bodipy FL C16 before treatment. Bodipy FL C16 is a green fluorescent fatty acid used to stain phospholipids. The biofilm was labeled with Alexa Fluor 647-conjugated dextran (1.5 μL/mL) for 15 min post-treatment of liposomes. The fluorescence of the biofilm and the penetration depth of liposomes were visualized by confocal microscopy.
Animals
One-week-old pigs were supplied by PigModel Animal Technology (Miaoli, Taiwan). Eight-week-old BALB/c mice were supplied by the National Laboratory Animal Center (Taipei, Taiwan). All animal experiments were approved by the Institutional Animal Care and Use Committee of Chang Gung University and complied with Directive 86/109/EEC from the European Commission.
The Penetration of Liposomes Through Pig Skin
The in vitro liposome absorption was conducted by Franz diffusion assembly. The dorsal skin of the pig was excised and mounted between the donor and receptor compartments, with the stratum corneum facing up toward the donor. The donor and receptor compartments were filled with the prepared liposomes (0.5 mL) and a pH 7.4 citrate-phosphate buffer (5 mL), respectively. Rhodamine 800 (0.5 mg) as a fluorescence marker was added to the lipid-organic solvent mixture before the drying process in the liposomal preparation began. After 24 h of liposomal application, the skin was removed and extracted by methanol in a MagNA Lyser (Roche). The homogenate was centrifuged at 10,000 xg for 10 min to obtain the supernatant. The rhodamine amount in the skin after extraction was analyzed by a fluorescence spectrophotometer (Hitachi F2500) at an excitation and emission wavelength of 553 and 627 nm, respectively. The liposome distribution in the skin was visualized by fluorescence microscopy (Leica DMi8) for a vertical view. After washing the skin removed from the Franz cell, the sample was sectioned in a cryostat microtome at a thickness of 5 μm and then mounted using glycerin and gelatin. The slice was observed with a fluorescence microscope using a filter set at 541−551 and 565−605 nm for excitation and emission, respectively.
In vivo Cutaneous C. acnes Infection Model
The mice were shaved at the dorsal area and then subcutaneously injected with C. acnes (1 x 107 CFU) in PBS. The liposomes, with a volume of 0.2 mL, were topically applied to the injection area every 24 h for three days. The control group was the topical administration of PBS. On day three, 10 μL of XenoLight Bacterial Detection Probe 750 (PerkinElmer) was subcutaneously delivered into the infection region to monitor the C. acnes growth. The bacteria burden was observed using a Lumina in vivo imaging system (Caliper Life Sciences). The lesional skin was then excised to measure the CFU.
In vivo Catheter C. acnes Infection Model
The animals were shaved at the abdominal site. A 0.5-cm long sterile silicone catheter with an inner diameter of 1.6 mm (Ibidi) was incubated with C. acnes (1 x 107 CFU/mL) for 24 h. A 1-cm incision in each flank was created and the catheter segment was implanted subcutaneously. The wound was closed with an absorbable suture and wound clip. The liposomes were subcutaneously injected into the transplantation region. On day three, the XenoLight Detection Probe was subcutaneously delivered to the infection area for imaging. After the animal was sacrificed, the catheter was removed and cut into small pieces. The catheter pieces were incubated with PBS for sonication and vortexing. The supernatant was collected to count the CFU.
Cytotoxicity of Liposomes Against Mammalian Cells
The cytotoxicity of the free and liposomal SME on keratinocytes (HaCaT) and monocytes (THP-1) was assessed by a MTT assay, as described earlier.25 Briefly, HaCaT and THP-1 were seeded in 96-well plates with 2×104 cells/well at 37°C for 24 h. The liposomes were diluted with DMEM if necessary. Each well was treated with SME, DNS, or PK, either in free form or liposomal form, for 24 h. MTT (0.5 mg/mL) was added into the wells and then incubated for three hours. A microplate reader at 550 nm was utilized to measure the cell viability.
In vivo Skin Tolerance
The liposomes, with a volume of 0.15 mL, were applied daily to the mouse back skin for three days. The transepidermal water loss (TEWL) and cutaneous surface pH value were estimated by a Cutometer MPA580 (Courage and Khazaka). The erythema level (a*) was quantified using a CD100 spectrocolorimeter (Yokogawa). Hematoxylin and eosin (H&E) staining was performed for a histological examination of the skin under optical microscopy, as described previously.24
Statistical Analysis
Statistical differences in the data of different treatment groups were measured using the Kruskal–Wallis test, while a post-hoc test to check the individual differences was performed using Dunn’s test. A 0.05, 0.01, or 0.001 level of probability was considered significant.
Results
The Physicochemical Properties of Liposomes
The average diameter, polydispersity index (PDI), and zeta potential of the different liposome formulations were the physicochemical properties examined in this study. The vesicle size of the cationic liposomes (LP) was determined to be 95 nm, as measured by laser scattering (Table 1). The size increased to 148, 154, and 150 nm after the incorporation of DNS, PK, and combined DNS/PK, respectively. This could be due to the fact that more components had been loaded into the liposomes to increase the size. The PDI of all nanoformulations was below 0.3, indicating the narrow distribution of the size. The zeta potential of the SME-loaded liposomes showed a positive value of 17 mV, corroborating the cationic nature of SME. Functionalization with the enzymes decreased the surface charge but remained at a positive level. The surface tension of water is about 72 mN/m, but the presence of nanovesicles in water could decrease this surface tension to about 25 mN/m. The surface tension was not further reduced by enzyme incorporation. We further examined the entrapment percentage of the enzymes in the liposomes by determining the protein concentration. The encapsulation efficiency of DNS and PK in the liposomes was calculated as 67% and 83%, respectively (Table 1). A 70% entrapment of total enzymes was calculated for the combined DNS and PK.
Table 1.
Physicochemical Properties of the Liposomes
| Formulation | Size | PDI | Zeta Potential (mV) | Encapsulation Efficiency (%) | Surface Tension (mN/m) |
|---|---|---|---|---|---|
| LP | 94.96±4.76 | 0.22±0.01 | 16.97±2.58 | − | 25.87±1.00 |
| LP DNS | 148.30±12.32 | 0.27±0.02 | 10.08±3.49 | 66.66±3.05 | 26.23±0.74 |
| LP PK | 154.43±22.58 | 0.29±0.02 | 5.72±0.45 | 83.33±2.88 | 25.17±0.54 |
| LP DNS/PK | 150.37±0.01 | 0.29±0.01 | 12.03±0.87 | 70.00±2.64 | 23.80±0.53 |
Notes: All data are presented as the mean of three experiments±S.E.M.
Abbreviations: DNS, DNase I; LP, liposomes; PK, proteinase K.
Inhibitory Activity of Liposomes Against Planktonic C. acnes
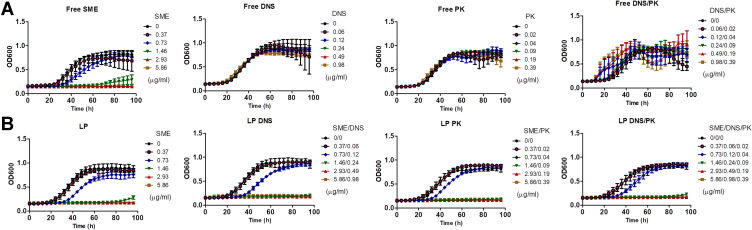
the free and liposomal forms of SME, DNS, and PK were evaluated for their antibacterial activity against planktonic C. acnes. The MIC and MBC of the free SME were approximately equal to 0.73−1.47 and 46.88−93.75 μg/mL, respectively (Suppl. Table 1). Minocycline HCl (MH) is an antibiotic used for treating acne-related infection. The low MIC (0.11 μg/mL) and MBC (3.9 μg/mL) for MH confirmed the great anti-C. acnes activity of this antibiotic. The free enzymes revealed no anti-C. acnes effect. The SME encapsulation in the liposomes maintained the antibacterial potential against planktonic microbes. There was no clear difference between MIC and MBC regardless of the liposomal formulations. DNS and PK could be successfully entrapped in the liposomes without affecting the antibacterial activity of SME. We next examined the growth curve of the planktonic microbes treated with SME, DNS, and PK, either as pure compounds or loaded with liposomes. For the control group with the exposure of PBS, the bacterial amount was gradually increased following the increase of time, with a leveling-off at 60 h (Figure 1A). The free SME markedly suppressed C. acnes growth. This suppression increased with the increase of the SME concentration. A complete killing during 96 h was found for the free SME at ≥2.93 μg/mL. The presence of SME in the liposomes without enzymes did not alter this tendency of bacterial growth inhibition (Figure 1B). Treatment with DNS and/or PK in the free form had no significant influence on C. acnes growth compared to the untreated control. There still remained some C. acnes at the late stage of treatment by liposomal SME at 1.46 μg/mL. The enzyme-loaded liposomes containing 1.46 μg/mL SME demonstrated prolonged and complete inhibition of microbial growth throughout 96 h.
Figure 1.
Determination of the antibacterial activity of SME (0−5.86 μg/mL) and/or DNS/PK (0−0.98 and 0−0.39 μg/mL) in free or liposomal form against planktonic Cutibacterium acnes during 96 h: (A) the time-killing curves of SME and enzymes in free form; and (B) the time-killing curves of SME and enzymes in liposomal form. All data are presented as the mean of three experiments±S.E.M.
The Liposomes Eradicate C. acnes Biofilm
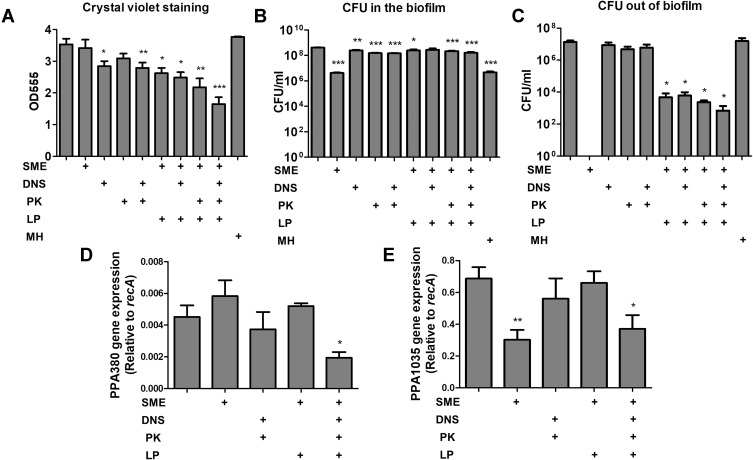
The antibiofilm activity of the liposomes was investigated by two methods: the biofilm mass detected by a crystal violet assay, and the bacterial amount in the biofilm analyzed by CFU. Either the free SME or PK were unable to affect the biofilm mass (Figure 2A). There was a significant reduction of biomass after free DNS treatment for 24 h. The combined DNS and PK in the free form showed a comparable biomass elimination to free DNS alone. We found a marked biomass inhibition by liposomal SME compared to free SME. The level of biofilm inhibition was estimated to be 26% for the liposomal SME as compared to the untreated control. This inhibition could be increased to 36% and 39% after the incorporation of DNS and PK into the cationic liposomes, respectively. The combined DNS and PK in cationic liposomes offered the maximum biomass suppression on the biofilm, with an inhibition percentage of 54%. MH at 7.8 μg/mL (2 x MBC) as the antibiotic control showed a negligible effect to destroy biofilm structure. Although this antibiotic showed strong inhibitory efficacy to eliminate planktonic C. acnes as found in MIC and MBC, the antibiofilm activity was limited. Next, we assessed the C. acnes amount inside and outside the biofilm after antibacterial treatment. The corresponding number of bacteria measured as CFU was log-transformed (Figure 2B and C). Although the free SME could not eliminate the biofilm mass, a significant inhibition of C. acnes amount inside the biofilm (by a 2-log reduction) was observed (Figure 2B). This indicated that bacterial killing was not responsible for the inhibited biofilm formation. The free enzymes also displayed a significant reduction of biofilm C. acnes, although this effect was minor compared to that of the cationic surfactant. All liposomal formulations except the cationic liposomes loaded with DNS alone showed a CFU lessening inside the biofilm. MH could inhibit bacterial burden inside the biofilm, indicating the facile penetration of MH into the biofilm without the disintegration of biofilm architecture. A complete eradication of C. acnes outside the biofilm by free SME was found (Figure 2C). The exposure to free enzymes, either alone or in combination, did not cause substantial killing of the microbes outside the biofilm. When the biofilm was treated with the liposomes, a 3-log reduction in the CFU outside the biomass was detected. This reduction was more significant in the case of the dual enzyme-loaded liposomes than in the other formulations. MH did not exhibit the capability to eliminate C. acnes outside the biofilm. This may suggest that most of MH molecules permeated into the biofilm with minimal residence outside the biofilm for killing the bacteria.
Figure 2.
Determination of the antibacterial activity of SME (150 μg/mL) and/or DNS/PK (25 and 10 μg/mL) in free or liposomal form against Cutibacterium acnes biofilm after a 24-h treatment: (A) the biofilm mass determined by crystal violet staining; (B) C. acnes CFU inside the biofilm; (C) C. acnes CFU outside the biofilm; (D) PPA380 gene expression in C. acnes biofilm; and (E) PPA1035 gene expression in C. acnes biofilm. All data are presented as the mean of three experiments±S.E.M. ***p < 0.001; **p < 0.01; *p < 0.05.
The biofilm-associated virulence genes PPA380 and PPA1035 were examined using qPCR analysis. In terms of the biofilm susceptibility evaluation, we investigated only the SME and dual enzymes for this qPCR assay. PPA380 was unaffected by the treatment of free SME or enzymes (Figure 2D). The level of PPA380 production was reduced to 42% in the biofilm by the cationic liposomes with dual enzymes when compared to the untreated control. After incubation with free SME, the mRNA level of the lipase PPA1035 was decreased to 44% (Figure 2E). However, this reduction was not found in the case of liposomal SME. The incorporation of dual enzymes was more efficient than that of cationic liposomes alone at inhibiting the PPA1035 gene. These results about biofilm eradication suggested that the combination of DNS and PK yielded the cumulative effect of antibiofilm activity.
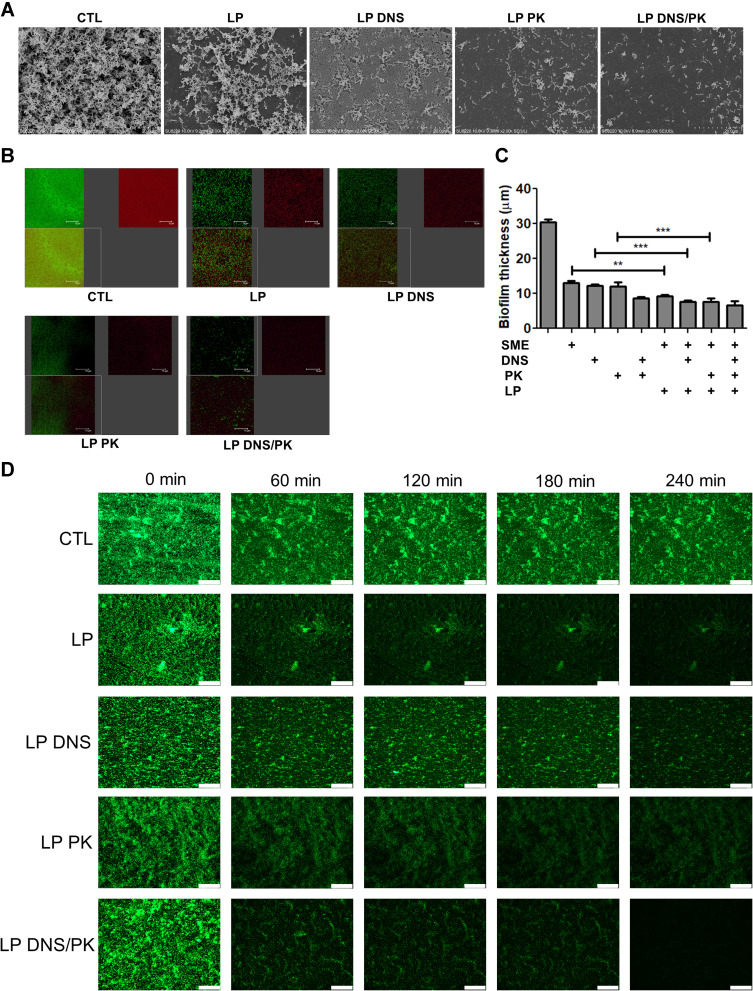
The SEM micrograph of the untreated biofilm clearly displayed a well-structured matrix of the recalcitrant network with numerous embedded C. acnes colonies (Figure 3A). The free SME manifested a limited effect to disrupt the biofilm architecture (Suppl. Figure 2). In contrast, the biofilm treated with SME-loaded liposomes without enzymes exhibited disintegrated clusters with disaggregated bacteria. A further biomass loosening was observed after treatment of the enzyme-loaded liposomes, with the dual enzymes showing the greatest destruction. With respect to the SEM images with a larger magnification, we found morphological damage of the microbes after treatment of the dual enzyme-loaded cationic liposomes, indicating C. acnes killing by SME (Suppl. Figure 3). This phenomenon was not observed in the treatment group of free DNS/PK because of the maintenance of the C. acne membrane. Additional experiments using CLSM were conducted to visualize the biofilm structure. The biofilm was stained by SYTO9 and dextran. The C. acnes, including live and dead cells, were stained green, while the polysaccharides produced by the biofilm were stained red by dextran. A dense colonization of microbes (green signal) was immersed in the massive biofilm (red signal) without liposomal intervention (Figure 3B). The green, red, and merged images of the biofilm are displayed in the upper left, upper right, and lower left panel, respectively. A significant removal of live bacteria and biomass was seen after treatment with liposomes, with the cationic liposomes loaded with dual enzymes demonstrating the greatest reduction. This biofilm elimination was less significant in the free form groups (Suppl. Figure 4). The free SME, DNS, or PK suppressed the biofilm thickness as measured from the confocal imaging (Figure 3C). A further reduction was observed for the combined free DNS and PK. The liposomal SME, DNS, and PK showed a greater biofilm thickness decrease than the free form. The dual enzyme-loaded liposomes lessened the thickness from 30 to 7 μm as compared to the untreated control. The SEM and CLSM images supported the notion that the liposomal systems eliminated the biofilm matrix.
Figure 3.
The antibacterial activity of SME (150 μg/mL) and/or DNS/PK (25 and 10 μg/mL) in free or liposomal form against Cutibacterium acnes biofilm determined by imaging after a 24-h treatment: (A) the biofilm structure observed by scanning electron microscopy; (B) the biofilm structure observed by confocal microscopy; (C) the biofilm thickness quantified from confocal microscopy; and (D) the biofilm mass attached on the surface of microfluidic chamber observed by confocal microscopy. All data are presented as the mean of three experiments±S.E.M. ***p < 0.001; **p < 0.01.
We investigated the formation and adherence of the C. acnes biofilm on the bottom of the microfluidic channel. This represented an in vitro model of catheter infection. A bacterial suspension was injected into the channel. To determine the effect of the liposomal insult, the biofilm was exposed to the controlled passage of nanovesicles. CLSM was employed to observe the C. acnes adherence at different periods, from 0 to 240 min. The confocal images revealed that the control biofilm in the catheter was firmly adhered on the channel, even after the increase of flow rate from 10 to 50 μL/min at 180 min (Figure 3D). Under the passage of liposomes, the biofilm became detached following the increase of time. The combination of DNS and PK in the cationic liposomes was superior to the liposomes with a single enzyme to disorganize and detach the biofilm. The biofilm architecture was almost diminished by this liposomal system at 240 min. This indicates that the enzyme-containing liposomes were capable to loose the biofilm structure and adherence. In the condition of high flow rate, this force could easily remove the biofilm treated by the liposomes. There was no observable green signal after a 240-min passage of the dual enzyme-loaded nanovesicles.
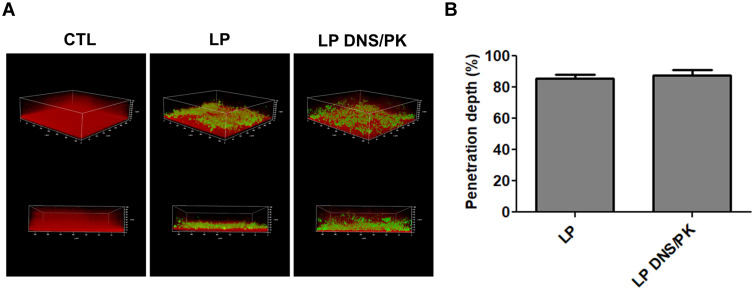
Facile Penetration of Liposomes Through the Biofilm
The penetration of liposomes into the biofilm was monitored by CLSM in the static condition. The biofilm matrix and liposomes were stained by dextran and Bodipy FL C16, respectively. The control biofilm without liposomal application displayed an intact and thick biomass (Figure 4A). The cationic liposomes with or without dual enzymes demonstrated an inhibitory effect on the density and thickness of the biofilm. Both liposomal formulations penetrated deep into the biofilm matrix during the treatment, indicating the capacity of the liposome delivery into the biofilm. The green signal derived from the enzyme-incorporated nanovesicles nearly reached the bottom of biofilm, with a broad dispersal in the matrix. The liposomes could transport deep through biofilm to about 85% of the total thickness (Figure 4B). Thus the permeated enzyme-loaded liposomes disintegrated biofilm structure, resulting in the ease biofilm removal as observed in the CLSM and microfluidic chamber assays.
Figure 4.
The penetration of liposomes containing SME (150 μg/mL) and/or DNS/PK (25 and 10 μg/mL) through Cutibacterium acnes biofilm: (A) the penetration of Bodipy FL C16-stained liposomes into the dextran-stained biofilm observed by confocal microscopy; and (B) the penetration depth of Bodipy FL C16-stained liposomes quantified from confocal microscopy. All data are presented as the mean of three experiments±S.E.M.
Facile Penetration of Liposomes Through Pig Skin
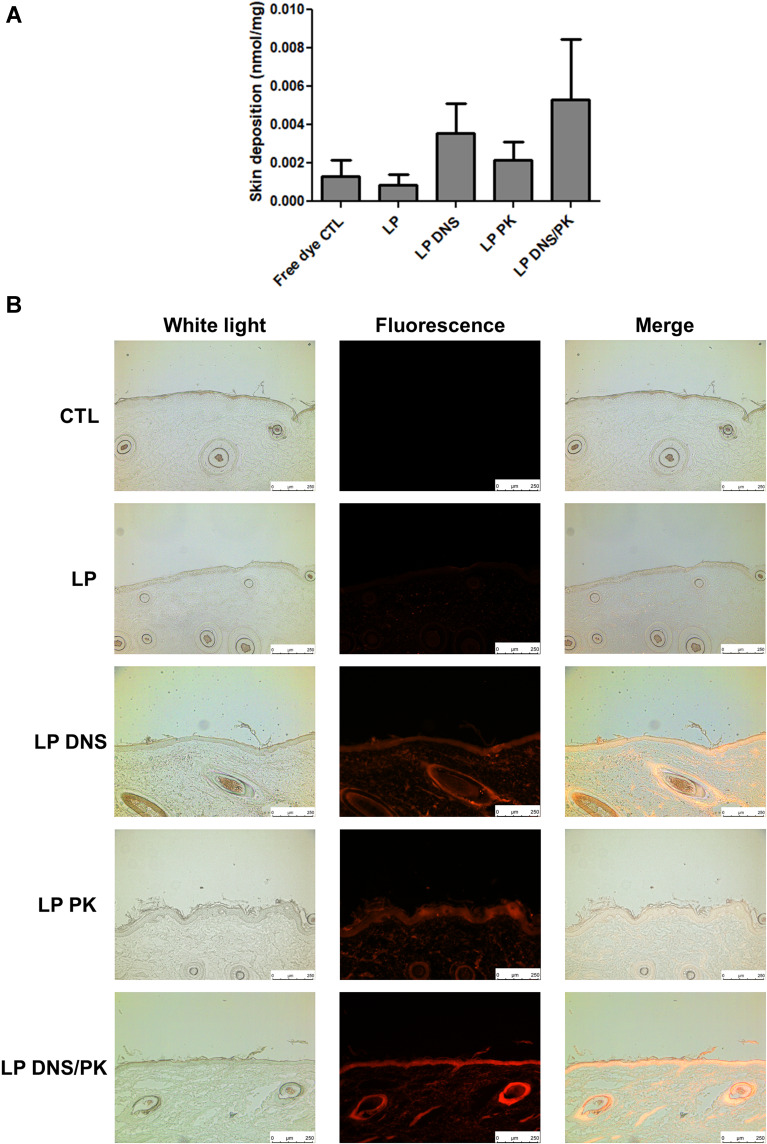
The cutaneous penetration of the liposomes was evaluated using pig skin in vitro. Rhodamine 800 was employed as a fluorescence dye to label the liposomes. The rhodamine 800 in the solution (control) showed a low deposition in the pig skin (Figure 5A). The incorporation of rhodamine in the enzyme-loaded liposomes elevated the skin deposition, although this increase achieved no statistically significant difference as compared to the control. The liposomal system containing dual enzymes showed a higher skin deposition than that without enzymes, by 5-fold. The representative fluorescence photograph of the pig skin treated with free rhodamine represented no red fluorescence throughout the skin layers (Figure 5B), demonstrating a negligible delivery. A mild fluorescence distribution was presented in the epidermis treated with topical liposomes without DNS and PK, with some fluorescence dots in the dermis. A stronger and deeper fluorescence was observed for the enzyme-loaded liposomes. The nanovesicles were mainly detected in the epidermis and hair follicles. When DNS and PK were used in combination with cationic liposomes, we could observe that the fluorescence signal in epidermis and hair follicles was more intense than that obtained when the enzymes were tested individually. We employed ZEN 2.0 software (Zeiss) to quantify the fluorescence intensity in Figure 5B. The mean value of red intensity of the skin treated by blank liposomes, DNS liposomes, PK liposomes, and DNS/PK liposomes was 7.7, 20.4, 17.6, and 24.7, respectively.
Figure 5.
The penetration of liposomes containing SME (0.3%, w/v) and/or DNS/PK (0.05% and 0.02%) through pig skin in vitro after a 24-h treatment: (A) the skin deposition of rhodamine 800 in free or liposomal form; and (B) the penetration of rhodamine 800 in free or liposomal form observed by fluorescence microscopy with a vertical view. All data are presented as the mean of four experiments±S.E.M.
The Liposomes Eradicate C. acnes Colonization in Skin and Catheter in vivo
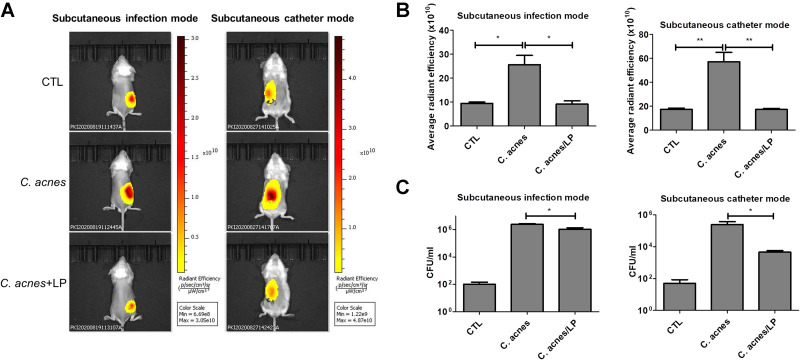
An in vivo mouse study was carried out by subcutaneously injecting C. acnes followed by topical application of dual-enzyme liposomes for three days. Topical delivery can provide a noninvasive approach to treat the infection. The skin permeation data demonstrated a facile absorption of liposomes into the skin. The XenoLight Bacterial kit was injected into the infection site to stain the C. acnes aggregates. The administration of a control vehicle (normal saline) in the infected mice displayed some fluorescence signals at the injection site (the left panel of Figure 6A). These signals could be derived from the resident bacteria flora. Increased fluorescence was visualized in the injection site of the C. acnes-infected mice, indicating the successful colonization of C. acnes subcutaneously. The topical delivery of the liposomes led to the inhibition of the C. acnes burden, with reduced fluorescence area and intensity. We also established a model of a subcutaneous catheter infected by C. acnes. The subcutaneous injection was used to administer liposomes for ensuring the approximate delivery to C. acnes-infected site. Same as in skin infection model, the liposomes effectively mitigated C. acnes colonization in a catheter implanted in the subcutaneous region (the right panel of Figure 6A). The fluorescence intensity was estimated three days after infection in mice. The C. acnes injection produced a 3-fold increase in fluorescence intensity as compared to the control group without infection for both the skin and catheter models (Figure 6B). The liposomal treatment on the infected mice was found to be free of C. acnes because of the fluorescence recovery to the baseline control level. The bacterial count could increase to 4 logs in terms of the CFU after C. acnes injection in the skin (the left panel of Figure 6C). The liposomes with enzymes had the minor but statistically significant effect of decreasing the viable bacteria number recovered from the skin of infected animals. In the case of the catheter infection, the liposomes reduced the bacterial CFU by about 2 logs during three days of incubation (the right panel of Figure 6C).
Figure 6.
In vivo antibacterial activity of liposomes containing SME (0.3%, w/v) and DNS/PK (0.05% and 0.02%) with a volume of 0.2 mL against Cutibacterium acnes in skin or catheter after a 3-day treatment: (A) the C. acnes burden (labeled with XenoLight Bacterial Detection Probe 750) in skin or implanted catheter of the mice observed by in vivo imaging system; (B) the average radiance intensity of C. acnes burden in skin or implanted catheter of the mice quantified by in vivo imaging system; and (C) C. acnes CFU in skin or implanted catheter of the mice. All data are presented as the mean of six experiments±S.E.M. **p < 0.01; *p < 0.05.
The Liposomes Show Minimal Toxicity on Mammalian Cells and Skin
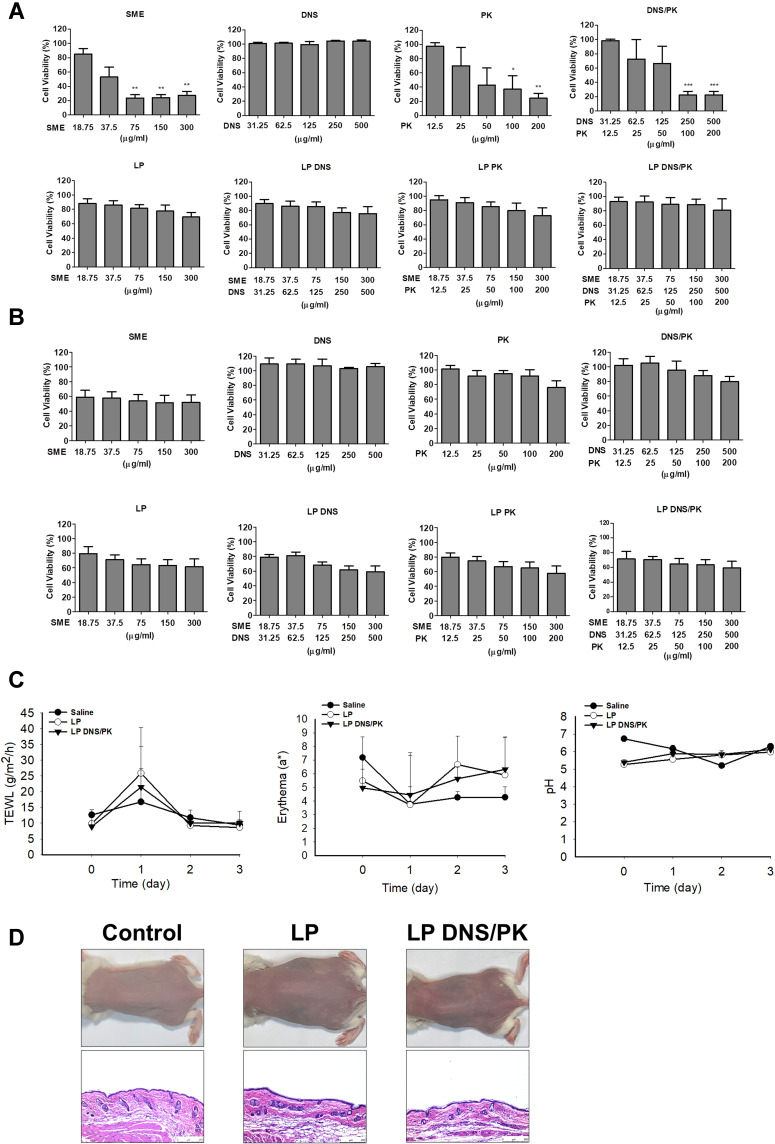
The biocompatibility of the SME and enzymes in the free or liposomal form was tested on keratinocyte (HaCaT) and monocyte (THP-1) cell lines using an MTT assay. The keratinocyte viability was decreased following the increase of the SME and PK concentration (Figure 7A). The keratinocyte viability was decreased to 33% and 25% after the treatment of free SME and PK at the highest concentration tested (300 and 200 μg/mL), respectively. On the other hand, the treatment of HaCaT with free DNS did not cause a significant reduction in viability until 500 μg/mL. The HaCaT viability was reduced by the combined DNS and PK, which could be induced by the cytotoxicity of PK but not DNS. The result clearly indicated the absence of cytotoxicity by all liposomal formulations tested on the HaCaT viability. The inclusion of SME and enzymes into the liposomes could raise the biocompatibility. The free SME manifested mild cytotoxicity against the monocyte cell line (Figure 7B). No cytotoxicity was shown for the free enzyme treatment on the monocytes. SME incorporation in liposomes could improve the biocompatibility on monocytes.
Figure 7.
The safety of SME and enzymes in free or liposomal form in vitro and in vivo: (A) the cell viability of keratinocytes (HaCaT) after treatment of SME and enzymes in free or liposomal form for 24 h; (B) the cell viability of monocytes (THP-1) after treatment of SME and enzymes in free or liposomal form for 24 h; (C) the physiological parameters (TEWL, erythema, skin pH value) of the mouse skin after in vivo topical administration of liposomes; and (D) the H&E-stained histology of mouse skin treated by liposomes. All data are presented as the mean of three and six experiments±S.E.M. for cell-based and animal-based studies, respectively. ***p < 0.001; **p < 0.01; *p < 0.05.
The skin physiology, including the barrier function, erythema, and cutaneous surface pH, was measured after a three-day treatment of topically applied liposomes with and without DNS/PK. The quantification of the skin physiology was comparable between the saline control and liposomes (Figure 7C). The data indicated no barrier dysfunction (determined by TEWL), skin rash (determined by colorimetry), or skin pH change in the mice administered with liposomes. When the mouse skin was smeared with liposomes, no histological change was observed as compared to the untreated control (Figure 7D). The inflammation and immune cell infiltration in the skin was recognized as negligible after the topical treatment of liposomes. Both the physiological and histopathological profiles verified that the enzyme-loaded liposomes did not irritate the skin.
Discussion
Antibacterial therapy usually exhibits an incomplete response. A major reason is biofilm-mediated tolerance, which prompts the failure of antibiotic management. C. acnes is an opportunistic microbe that is notorious owing to its biofilm formation, which reduces the susceptibility to antibacterial treatment. Currently there is no clinically approved agent targeting bacterial biofilms. The elaboration of delivery carriers that target biofilms and disassemble the EPS is of importance. The present study showed that cationic liposomes incorporated with DNS and PK are advantageous to eradicate both planktonic and biofilm C. acnes. A sufficient enzyme entrapment was demonstrated in our liposomal system. The biofilm structure could be destroyed by the enzymes for increased liposome penetration. The skin absorption was also enhanced by the enzyme loading in the liposomes. The nanovesicles could eliminate C. acnes burden in skin and catheter in vivo. The therapeutic dose of the liposomes was tolerable and induced no cutaneous toxicity.
The cell wall of Gram-positive microbes such as C. acnes comprises a layer of peptidoglycan that is attached to anionic teichoic acid.26 Cationic surfactants can easily adsorb to the surface of Gram-positive bacteria and distort the membrane integrity, thereby permeabilizing the membrane and leaking the internal materials.27 SME has been verified to kill the Gram-positive S. aureus via the direct cell surface rupture and induction of the Fenton reaction and oxidative stress.22 SME incorporation in liposomes could maintain the inhibitory activity against planktonic C. acnes as compared to the free form. It has been accepted that teichoic acid is a common binding site for nanoparticles and liposomes.28 The predominant feature of liposomes is the similarity to the cellular membrane, allowing fusion with the bacterial surface.29 Cationic liposomes show facile fusion with the membrane of both Gram-positive and -negative bacteria.30 The level of fusion is 44% and 66% for Peudomonas aeruginosa and Escherichia coli, respectively. It is proved that cationic liposomes are able to kill both Gram-positive and -negative bacteria with the antimicrobial capability greater than neutral and anionic liposomes.31 Both electrostatic attraction and fusion are responsible for facilitating liposomal interaction with C. acnes. The increase of liposomal fluidity can enhance the bacterial membrane interaction.32 The intercalation of DNS and/or PK in the liposomal bilayers might increase the fluidity to promote fusion and the following bacterial killing. The time-killing curve of planktonic C. acnes showed further growth inhibition after enzyme incorporation into the cationic liposomes, although this effect was not very significant. When the cationic liposomes fused with the bacterial surface, the entrapped SME would be released into the membrane or the intracellular environment, resulting in the planktonic C. acnes death.
Antimicrobial susceptibility is markedly lowered in biofilms where the pathogens are more tolerant to antibacterial agents than the planktonic type. This has resulted in the difficulty or curing biofilm-associated infections. Our data demonstrated a decrease of C. acnes biofilm mass and thickness upon the treatment of liposomes loaded with SME and enzymes. The antibiofilm activity of the liposomal SME and enzymes was greater than that of the free forms. The liposomes could penetrate deeply and extensively into the biofilm matrix, contributing to the conceivable capacity of biofilm distortion. The first step of liposomal penetration into the biofilm should be the partitioning of the matrix surface. The extremely non-wetting surface feature of biofilms has led to the restricted diffusion of antibacterial liquids into the biomass.33 Liquids with high surface tension are repelled by the biofilm surface.34 The low surface tension of the liposomes due to the incorporation of surfactants and enzymes facilitated the entrance into the biofilm. After the biomass entrance, the liposomes would meet an environment rich of negative charge. The extracellular DNA in biofilms exhibits a negative charge and acts as the chelator of cationic antibacterial nanoparticles.35,36 Cationic liposomes could be ideal antimicrobial agents for targeting biofilms.
There is a complex channel network inside the biofilm established by C. acnes.37 These holes allow the diffusion of nanoparticles with the size of several hundred nm.38 In addition to electrostatic attraction, the phospholipid coat on the nanoparticulate surface can interact with the biofilm EPS, leading to matrix disruption.39 This could explain why the cationic liposomes could inhibit biofilm development to some degree in the absence of DNS and PK. The deep and uniform distribution of the liposomes inside the C. acnes biofilm was proved by CLSM. We hypothesized that once the cationic liposomes were facilely deposited on the biofilm surface, they diffused through the EPS via electrostatic and lipophilic interactions, followed by biomass degradation. The dispersion of C. acnes biofilms could result in the release of matrix-degrading enzymes to break down some EPS, allowing the biofilm bacteria to escape into the bulk fluid.40 The shedding of bacteria from the biofilm usually occurs in the late stage of EPS development.18 Although free SME demonstrated limited capacity to eliminate biofilm structure, it could efficiently kill biofilm-embedded C. acnes and the planktonic bacteria released from the biofilm. The small size of the free surfactant molecules was beneficial for diffusion into the biofilm mesh and allowed it to easily interact with the bacteria. The liposomes could eliminate C. acnes inside and outside the biofilm, although this effect was minor compared to the free SME. The nanovesicles facilely penetrated into the biofilm and increased the close interaction with C. acnes. The reduction of biofilm C. acnes colonies diminished the subsequent bacteria release from the matrix.
Extracellular DNA is the main component of the biofilm responsible for initiating bacterial attachment to the surface, bacterial aggregation, and cell-cell interaction.41,42 It is important for the maintenance of biofilm stability. The enzymatic degradation of DNA by DNase has been shown to efficiently scavenge biofilm growth.43 Our data showed that the DNS treatment prevented C. acnes biofilm development and prompted bacterial exposure to the antimicrobial agent for eliminating the colonization. A mature biofilm contains components other than extracellular DNA, including proteins, polysaccharides, and lipids. These components render DNase alone ineffective to disassemble biofilm.44,45 Proteins are a key part of EPS. Biofilm-associated proteins can be another potential target for antibiofilm management.46 PK is a serine protease that cleaves the C-terminal peptide bond for protein digestion. The proteinaceous adhesion during the EPS adherence on surface can be inhibited by PK.47 Our result indicated that PK degraded the protein adhesion and biofilm mass of C. acnes. The combined treatment of DNS and PK in liposomes appeared to show a cumulative effect on biofilm distortion, supporting the role of both DNA and proteins on C. acnes biofilm cohesion. We hypothesized that the liposomes could release the enzymes via concentration gradient of diffusion when penetrating into the biofilm, resulting in the biofilm damage. Then the SME liposomes further fused with biofilm bacteria to cause the elimination of C. acnes. Only the cationic liposomes containing dual enzymes were able to inhibit the expression of virulence factors PPA380 and PPA1035 in C. acnes biofilm. Both virulence genes are largely upregulated in the pathogenesis induced by C. acnes.40,48
A major concern of enzyme-based antibiofilm intervention is the dispersal of bacteria from the biofilm to increase the risk of distant infection.20 A combination of antibacterial agents is warranted for enzyme-based therapy. The co-encapsulation of SME and enzymes in liposomes could display a synergistic effect to eradicate biofilm C. acnes and its structure. The cationic surfactant could be a chelator that binds with extracellular DNA.18 This interaction would approximate the enzymes to the matrix for EPS degradation. On the other hand, DNS or PK has proved to be efficacious in ameliorating antibiotic susceptibility against biofilm infection.46,49 The enzymes weakened the biofilm matrix, rendering the decrease of the diffusion distance of the liposomes. This enabled the liposomes to permeate deep inside the biomass and expose the C. acnes for subsequent killing. In addition to acne, C. acnes is an opportunistic pathogen involving in implant catheter infections through biofilm formation.37 C. acnes can vigorously adhere on catheter surfaces, which is the initial step of the biofilm life cycle. From the in vitro experimental result, the enzyme-loaded cationic liposomes were operative in inhibiting biofilm adhesion on microfluidic chambers.
Antibacterial agents can be administered topically for acne treatment because of their ability to escape systemic adverse effects, localize agents near the infection site, and noninvasive management. It is necessary to conquer the barrier property of skin for the enhanced skin penetration of antibacterial agents. Conventional liposomes with their rigid structures have little value as a topical delivery system, as they are difficult to deliver into the deeper skin strata and tend to be retained in superficial layers.50 The nanovesicle-skin interaction can be greatly influenced by the elasticity of liposomes. Some surfactants embedded in the lipid bilayers of liposomes can destabilize the bilayers to offer a deformable membrane, resulting in the enhanced skin transport by squeezing through the intercellular route.51 Previous studies52,53 have reported a higher skin penetration of cationic elastic liposomes than their anionic counterparts due to the inherent negative charge of the skin. Our cationic liposomes containing rhodamine showed higher skin delivery as compared to the free dye. However, the cationic liposomes were mainly confined in the epidermis, with a limited diffusion to deeper layers. The intercalation of DNS and PK within the phospholipid bilayers could further increase the flexibility of the liposomal membrane, thereby facilitating the penetration into the skin, especially the deeper skin strata. It was also expected that these enzymes will degrade the cutaneous structure to expand the permeation pathways for liposomes. Hair follicles have been acknowledged as a common pathway for nanoparticle penetration into the skin.54 A positive liposome charge is favorable for follicular permeation, as the epithelium and the hair shaft are negatively charged on the surface.55 Nanoparticles are easily transported into the deeper skin strata via the hair follicles because of the low barrier characteristic of the follicular epithelium.17 We found a facile delivery of enzyme-containing liposomes to hair follicles and deeper skin layers. A significant delivery to hair follicles is beneficial for treating acne.
In addition to acne, C. acnes biofilm is often detected in catheter implantations. The catheter surface is bioreceptive and can form an environment where C. acnes attaches on the surface.56 Implantation patients have a high risk for latent instrumentation infection and complication raised by C. acnes biofilms.57 Implant-associated infections require a prolonged treatment course and have an increased cost for antibacterial therapy. Our in vitro data validated the efficiency of liposomes for reducing the biofilm adherence on microfluidic chamber surfaces. An animal model of a subcutaneous catheter infection in mice was established to examine the in vivo effect. An elimination of the C. acnes burden in the catheter was achieved by the liposomes loaded with dual enzymes. Cationic liposomes are known to cause cytotoxicity against mammalian cells.58 Quaternary ammonium surfactants are often employed as antimicrobial ingredients; however, their cytotoxicity has hampered their application.32 Quaternary ammonium compounds also exhibit skin irritation after topical administration.59 The significant cytotoxicity of free SME against keratinocytes and monocytes proved the concern of using quaternary ammonium surfactants. Our result demonstrated the minimal cytotoxicity after encapsulating SME into the liposomes. It could be due to the SME intercalation in phospholipid bilayers preventing the direct contact with the cells. The liposomes could be used for eradicating biofilms at certain concentrations without causing toxicity to mammalian cells. The liposomes induced no skin irritation and barrier disintegration according to physiological and histological examinations. The biocompatible properties of the liposomes provided satisfactory skin tolerability. Currently, topical acne treatments using azelaic acid and benzoyl peroxide have led to the adverse responses of cutaneous burning, redness, and scaling. The use of the developed liposomes may be effective for providing C. acnes treatments with few side effects. Alternatives to antibiotics are urgently required to decrease the risk of antibiotic resistance. We designed a liposomal system with innate antibacterial and antibiofilm activities in the absence of antibiotics.
Conclusions
Our liposomes successfully entrapped SME and enzymes to develop a nanocarrier for C. acnes biofilm treatment. The cationic liposomes loaded with enzymes were effective in inhibiting planktonic C. acnes growth and correlated with the affinity of SME to the bacterial surface. The liposomes manifested improved efficacy in disassembling biofilm structures compared to the free control, on account of their facile penetration and the subsequent extracellular DNA and protein degradation. The co-treatment of the dual enzymes DNS and PK further enhanced the capability of preventing biofilm formation and virulence activation. Eventually, we found the excellent in vivo inhibition activity of the liposomes against the C. acnes burden in the skin and in catheters. The dose used for biofilm eradication was nontoxic for mammalian cells and skin. The dual enzyme-loaded cationic liposomes represented an efficient carrier for antimicrobial and antibiofilm properties against C. acnes, without the use of antibiotics.
Acknowledgments
The authors are grateful for the financial support from Ministry of Science and Technology of Taiwan (MOST-107-2320-B-182-016-MY3) and Chang Gung Memorial Hospital (CMRPG2K0371).
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Luepke KH, Suda KJ, Boucher H, et al. Past, present, and future of antibacterial economics: increasing bacterial resistance, limited antibiotic pipeline, and societal implications. Pharmacotherapy. 2017;37(1):71–84. doi: 10.1002/phar.1868 [DOI] [PubMed] [Google Scholar]
- 2.Van Dyck K, Pinto RM, Pully D, Van Dijck P. Microbial interkingdom biofilms and the quest for novel therapeutic strategies. Microorganisms. 2021;9:412. doi: 10.3390/microorganisms9020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abouelhassan Y, Zhang Y, Jin S, Huigens RW. Transcript profiling of MRSA biofilms treated with a halogenated phenazine eradicating agent: a platform for defining cellular targets and pathways critical to biofilm survival. Angew Chem Int Ed. 2018;57:1–7. doi: 10.1002/anie.201809785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dréno B, Pécastaings S, Corvec S, Veraldi S, Khammari A, Roques C. Cutibacterium acnes (Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol. 2018;32 Suppl. 2:5–14. doi: 10.1111/jdv.15043 [DOI] [PubMed] [Google Scholar]
- 5.Platsidaki E, Dessinioti C. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research. 2018;7:1953. doi: 10.12688/f1000research.15659.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754. doi: 10.1038/s41598-020-62715-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLaughlin J, Watterson S, Layton AM, Bjourson AJ, Barnard E, McDowell A. Propionibacterium acnes and acne vulgaris: new insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms. 2019;7:128. doi: 10.3390/microorganisms7050128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodarzi A, Mozafarpoor S, Bodaghabadi M, Mohamadi M. The potential of probiotics for treating acne vulgaris: a review of literature on acne and microbiota. Dermatol Ther. 2020;33:e13279. doi: 10.1111/dth.13279 [DOI] [PubMed] [Google Scholar]
- 9.Hancock REW, Alford MA, Haney EF. Antibiofilm activity of host defense peptides: complexity provides opportunities. Nat Rev Microbiol. 2021;19:786–797. doi: 10.1038/s41579-021-00585-w [DOI] [PubMed] [Google Scholar]
- 10.Li W, Separovic F, O’Brien-Simpson NM, Wade JD. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem Soc Rev. 2021;50(8):4932–4973. doi: 10.1039/d0cs01026j [DOI] [PubMed] [Google Scholar]
- 11.Ibelli T, Templeton S, Levi-Polyachenko N. Progress on utilizing hyperthermia for mitigating bacterial infections. Int J Hyperthermia. 2018;34(2):144–156. doi: 10.1080/02656736.2017.1369173 [DOI] [PubMed] [Google Scholar]
- 12.Kasza K, Gurnani P, Hardie KR, Cámara M, Alexander C. Challenges and solutions in polymer drug delivery for bacterial biofilm treatment: a tissue-by-tissue account. Adv Drug Deliv Rev. 2021;178:113973. doi: 10.1016/j.addr.2021.113973 [DOI] [PubMed] [Google Scholar]
- 13.Fulaz S, Vitale S, Quinn L, Casey E. Nanoparticle-biofilm interactions: the role of the EPS matrix. Trends Microbiol. 2019;27:915–926. doi: 10.1016/j.tim.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 14.Filipczak N, Pan J, Yalamarty SSK, Torchilin VP. Recent advancements in liposome technology. Adv Drug Deliv Rev. 2020;156:4–22. doi: 10.1016/j.addr.2020.06.022 [DOI] [PubMed] [Google Scholar]
- 15.Vyas SP, Sihorkar V, Jain S. Mannosylated liposomes for biofilm targeting. Int J Pharm. 2007;330:6–13. doi: 10.1016/j.ijpharm.2006.08.034 [DOI] [PubMed] [Google Scholar]
- 16.Wang Y. Liposome as a delivery system for the treatment of biofilm-mediated infections. J Appl Microbiol. 2021. doi: 10.1111/jam.15053 [DOI] [PubMed] [Google Scholar]
- 17.Hsu CY, Yang SC, Sung CT, Weng YH, Fang JY. Anti-MRSA malleable liposomes carrying chloramphenicol for ameliorating hair follicle targeting. Int J Nanomed. 2017;12:8227–8238. doi: 10.2147/IJN.S147226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang JY, Lin YK, Wang PW, Alalaiwe A, Yang YC, Yang SC. The droplet-size effect of squalene@cetylpyridinium chloride nanoemulsions on antimicrobial potency against planktonic and biofilm MRSA. Int J Nanomed. 2019;14:8133–8147. doi: 10.2147/IJN.S221663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labena A, Hegazy MA, Kamel WM, Elkelish A, Hozzein WN. Enhancement of a cationic surfactant by capping nanoparticles: synthesis, characterization and multiple applications. Molecules. 2020;25:2007. doi: 10.3390/molecules25092007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan JB, LoVetri K, Cardona ST, et al. Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci. J Antibiot. 2012;65(2):73–77. doi: 10.1038/ja.2011.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu WS, Nam DM, Kim JS, Koo OK. Synergistic anti-biofilm effects of Brassicaceae plant extracts in combination with proteinase K against Escherichia coli O157:H7. Sci Rep. 2020;10:21090. doi: 10.1038/s41598-020-77868-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang SC, Aljuffali IA, Sung CT, Lin CF, Fang JY. Antimicrobial activity of topically-applied soyaethyl morpholinium ethosulfate micelles against Staphylococcus species. Nanomedicine. 2016;11:657–671. doi: 10.2217/nnm.15.217 [DOI] [PubMed] [Google Scholar]
- 23.Bajorath J, Saenger W, Pal GP. Autolysis and inhibition of proteinase K, a subtilisin-related serine proteinase isolated from the fungus Tritirachium album Limber. Biochim Biophys Acta. 1988;854:176–182. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Gany S, El-Badry MO, Fahmy AS, Mohamed SA. Purification and characterization of deoxyribonuclease from small intestine of camel Camelus dromedaries. J Genet Eng Biotechnol. 2017;15:463–467. doi: 10.1016/j.jgeb.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan TL, Wang PW, Aljuffali IA, Huang CT, Lee CW, Fang JY. The impact of urban particulate pollution on skin barrier function and the subsequent drug absorption. J Dermatol Sci. 2015;78:51–60. doi: 10.1016/j.jdermsci.2015.01.011 [DOI] [PubMed] [Google Scholar]
- 26.Dagnelie MA, Corvec S, Saint-Jean M, Nguyen JM, Khammari A, Dréno B. Cutibacterium acnes phylotypes diversity loss: a trigger for skin inflammatory process. J Eur Acad Dermatol Venereol. 2019;33:2340–2348. doi: 10.1111/jdv.15795 [DOI] [PubMed] [Google Scholar]
- 27.Colomer A, Perez L, Pons R, et al. Mixed monolayer of DPPC and lysine-based cationic surfactants: an investigation into the antimicrobial activity. Langmuir. 2013;29:7912–7921. doi: 10.1021/la401092j [DOI] [PubMed] [Google Scholar]
- 28.Kannan S, Solomon A, Krishnamoorthy G, Marudhamuthu M. Liposome encapsulated surfactant abetted copper nanoparticles alleviates biofilm mediated virulence in pathologic Pseudomonas aeruginosa and MRSA. Sci Rep. 2021;11:1102. doi: 10.1038/s41598-020-79976-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rukavina Z, Klarić MŠ, Filipović-Grčić J, Lovrić J, Vanić Ž. Azithromycin-loaded liposomes for enhanced topical treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections. Int J Pharm. 2018;553:109–119. doi: 10.1016/j.ijpharm.2018.10.024 [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Ma Y, Khali H, et al. Fusion between fluid liposomes and intact bacteria: study of driving parameters and in vitro bacterial efficacy. Int J Nanomed. 2016;11:4025–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drulis-Kawa Z, Gubernator J, Dorotkiewicz-Jach A, Doroszkiewicz W, Kozubek A. A comparison of the in vitro antimicrobial activity of liposomes containing meropenem and gentamicin. Cell Mol Biol Lett. 2006;11:360. doi: 10.2478/s11658-006-0030-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montefusco-Pereira CV, Formicola B, Goes A, et al. Coupling quaternary ammonium surfactants to the surface of liposomes improves both antibacterial efficacy and host cell biocompatibility. Eur J Pharm Biopharm. 2020;149:12–20. doi: 10.1016/j.ejpb.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 33.Zhang C, Li B, Tang JY, Wang XL, Qin Z, Feng XQ. Experimental and theoretical studies on the morphogenesis of bacterial biofilms. Soft Matter. 2017;13:7389–7397. doi: 10.1039/C7SM01593C [DOI] [PubMed] [Google Scholar]
- 34.Sun LM, Zhang CL, Li P. Characterization, antibiofilm, and mechanism of action of novel PEG-stabilized lipid nanoparticles loaded with terpinen-4-ol. J Agric Food Chem. 2012;60:6150–6156. doi: 10.1021/jf3010405 [DOI] [PubMed] [Google Scholar]
- 35.Hajipour MJ, Fromm KM, Ashkarran AA, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30:499–511. doi: 10.1016/j.tibtech.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 36.Anjum MM, Patel KK, Dehari D, et al. Anacardic acid encapsulated solid lipid nanoparticles for Staphylococcus aureus biofilm therapy: chitosan and DNase coating improves antimicrobial activity. Drug Deliv Transl Res. 2021;11(1):305–317. doi: 10.1007/s13346-020-00795-4 [DOI] [PubMed] [Google Scholar]
- 37.Achermann Y, Goldstein EJ, Coenye T, Shirtliff ME. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27:419–440. doi: 10.1128/CMR.00092-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richter K, Facal P, Thomas N, et al. Taking the silver bullet colloidal silver particles for the topical treatment of biofilm-related infections. ACS Appl Mater Interf. 2017;9(26):21631–21638. doi: 10.1021/acsami.7b03672 [DOI] [PubMed] [Google Scholar]
- 39.Mu H, Tang J, Liu Q, Sun C, Wang T, Duan J. Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci Rep. 2016;6:18877. doi: 10.1038/srep18877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanter BB, Davies DG. Propionibacterium acnes recovered from atherosclerotic human carotid arteries undergoes biofilm dispersion and release lipolytic and proteolytic enzymes in response to norepinephrine challenge in vitro. Infect Immun. 2015;83:3960–3971. doi: 10.1128/IAI.00510-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sivasankar C, Maruthupandiyan S, Balamurugan K, Bhaskar JP, Krishnan V, Pandian SK. A combination of ellagic acid and tetracycline inhibits biofilm formation and the associated virulence of Propionibacterium acnes in vitro and in vivo. Biofouling. 2016;32:397–410. doi: 10.1080/08927014.2016.1148141 [DOI] [PubMed] [Google Scholar]
- 42.Kavanaugh JS, Flack CE, Lister J, et al. Identification of extracellular DNA-binding proteins in the biofilm matrix. mBio. 2019;10:e01137–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlafer S, Garcia J, Meyer RL, Vaeth M, Neuhaus KW. Effect of DNase treatment on adhesion and early biofilm formation of Enterococcus faecalis. Eur Endod J. 2018;2:82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li W, Liu H, Xu Q. Extracellular dextran and DNA affect the formation of Enterococcus faecalis biofilms and their susceptibility to 2% chlorhexidine. J Endod. 2012;38:894–898. doi: 10.1016/j.joen.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 45.Sharma K, Singh AP. Antibiofilm effect of DNase against single and mixed species biofilm. Foods. 2018;7:42. doi: 10.3390/foods7030042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shukla SK, Rao TS. Dispersal of bap-mediated Staphylococcus aureus biofilm by proteinase K. J Antibiot. 2013;66:55–60. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen UT, Burrows LL. DNase I and proteinase K impair Listeria monocytogenes biofilm formation and induce dispersal of pre-existing biofilms. Int J Food Microbiol. 2014;187:26–32. doi: 10.1016/j.ijfoodmicro.2014.06.025 [DOI] [PubMed] [Google Scholar]
- 48.Kang D, Shi B, Erfe MC, Craft N, Li H. Vitamin B12 modulates the transcriptome of the skin microbiota in acne pathogenesis. Sci Transl Med. 2015;7:293ra103. doi: 10.1126/scitranslmed.aab2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel KK, Surekha DB, Tripathi M, et al. Antibiofilm potential of silver sulfadiazine-loaded nanoparticle formulations: a study on the effect of DNase-1 on microbial biofilm and wound healing activity. Mol Pharm. 2019;16:3916–3925. doi: 10.1021/acs.molpharmaceut.9b00527 [DOI] [PubMed] [Google Scholar]
- 50.Elmoslemany RM, Abdallah OY, El-Khordagui LK, Khalafallah NM. Propylene glycol liposomes as a topical delivery system for miconazole nitrate: comparison with conventional liposomes. AAPS PharmSciTech. 2012;1:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bnyan R, Khan I, Ehtezazi T, et al. Surfactant effects on lipid-based vesicles properties. J Pharm Sci. 2018;107(5):1237–1246. doi: 10.1016/j.xphs.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 52.Lin H, Xie Q, Huang X, et al. Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery. Int J Nanomed. 2018;13:831–842. doi: 10.2147/IJN.S150086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang ZJ, Michniak-Kohn B. Flavosomes, novel deformable liposomes for the co-delivery of anti-inflammatory compounds to skin. Int J Pharm. 2020;585:119500. doi: 10.1016/j.ijpharm.2020.119500 [DOI] [PubMed] [Google Scholar]
- 54.Patzelt A, Lademann J. Recent advances in follicular drug delivery of nanoparticles. Expert Opin Drug Deliv. 2020;17(1):49–60. doi: 10.1080/17425247.2020.1700226 [DOI] [PubMed] [Google Scholar]
- 55.Jung S, Otberg N, Thiede G, et al. Innovative liposomes as a transfollicular drug delivery system: penetration into porcine hair follicles. J Invest Dermatol. 2006;126:1728–1732. doi: 10.1038/sj.jid.5700323 [DOI] [PubMed] [Google Scholar]
- 56.Mongaret C, Velard F, Reffuveille F, Freitag NE. Cutibacterium acnes: the urgent need to identify diagnosis markers. Infect Immun. 2021;89(4):e00753–20. doi: 10.1128/IAI.00753-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia D, Mayfield CK, Leong J, et al. Early adherence and biofilm formation of Cutibacterium acnes (formerly Propionibacterium acnes) on spinal implant materials. Spine J. 2020;20:981–987. doi: 10.1016/j.spinee.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 58.Cui S, Wang Y, Gong Y, et al. Correlation of the cytotoxic effects of cationic lipids with their headgroups. Toxicol Res. 2018;7:473. doi: 10.1039/C8TX00005K [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alalaiwe A, Wang PW, Lu PL, Chen YP, Fang JY, Yang SC. Synergistic anti-MRSA activity of cationic nanostructured lipid carriers in combination with oxacillin for cutaneous application. Front Microbiol. 2018;9:1493. doi: 10.3389/fmicb.2018.01493 [DOI] [PMC free article] [PubMed] [Google Scholar]