Abstract
The demonstration in model organisms that cellular senescence drives aging and age-related diseases has led to widespread efforts to identify compounds able to selectively kill senescent cells, termed senolytics. Approaches used to identify senolytics include bioinformatic analysis of senescent cell anti-apoptotic pathways (SCAPs) for drug development and screening of drugs libraries on different senescent cell types in culture. Alternatively, cytotoxic compounds can be made specific to senescent cells through a prodrug strategy such as linking the compound to a galactose moiety where toxicity is activated by lysosomal β-galactosidase. Identified senolytics can then be optimized through medicinal chemistry or linking to E3 targeting moieties to facilitate proteolysis of their targets. This review will provide an overview of approaches to identify senolytics and an update of the classes of senolytics identified to date.
Keywords: Senescence, Senolytics, Aging, Drug screening, Prodrugs, PROTACs
1. Introduction
Aging is an important risk factor for most common human diseases. Advancing age significantly increases the risk of developing debilitating chronic diseases. Indeed, over 90 % of individuals over the age of 65 have at least one chronic disease, such as cardiovascular disease, cancer, dementia, diabetes, osteoarthritis and osteoporosis, while more than 70% have at least two such conditions (Barnett et al., 2012; Marengoni et al., 2011). This fact has led to the Geroscience hypothesis, which proposes that targeting fundamental aging mechanisms therapeutically will delay the onset or severity of multiple chronic diseases due to common underlying risk factors (Kennedy et al., 2014). This concept of Geroscience is supported by research in model organisms where multiple longevity pathways can be manipulated to delay aging and its pathological consequences through pharmacological approaches.
Aging is thought to be driven by hallmarks of aging: adaption to stress, epigenetics, inflammation, macromolecular damage, metabolism, proteostasis, and stem cell dysfunction (Kennedy et al., 2014; Lopez-Otin et al., 2013). Many of these hallmarks result in cellular senescence, which has been implicated to play a critical role in driving aging and age-related diseases. Cellular senescence is a state of persistent cell cycle arrest implemented through stress-signaling mechanisms as a safeguard to prevent proliferation of damaged cells and oncogenic neoplasia (Coppe et al., 2010; Munoz-Espin and Serrano, 2014). Despite growth inhibition, senescent cells are metabolically active and exhibit a senescence-associated secretory phenotype (SASP) containing pro-inflammatory chemokines and interleukins, proteases, bioactive lipids, inhibitory molecules, and other factors that stimulate the immune system, but prevent immune clearance by aged immune cells. Importantly, these SASP factors also drive chronic inflammation and age-related diseases (Fig. 1) (Childs et al., 2015; He and Sharpless, 2017). Cellular senescence can be driven by many inducers in different contexts such as replicative, genotoxic, and oxidative stress, as well as oncogene activation, and telomere erosion. Additionally, exposure to ionizing radiation, chemotherapy agents (e.g. etoposide, doxorubicin), reactive metabolites (e.g., ROS, NOS), inflammatory cytokines (e.g., IL-6, IL-1), damage- and pathogen-associated molecular patterns (e.g., HMGB1, LPS, viral RNA), and others have also been shown to induce senescence. (Fig. 1A) (Kennedy et al., 2014; Lopez-Otin et al., 2013; Coppe et al., 2010; Munoz-Espin and Serrano, 2014; Childs et al., 2015; Borghesan et al., 2020; He and Sharpless, 2017). These inducers activate senescence-promoting signaling pathways leading to cell-cycle arrest, release of SASPs, activation of anti-apoptotic and pro-survival pathways, increased SA-β-galactosidase (SA-ß-gal) SA-activity, and other metabolic alterations. Senescence-associated alterations in macromolecules and metabolism provide key markers for the identification of senescent cells including upregulation and activation of proteins involved in DNA damage repair and cell cycle regulation (e.g. p16INK4a, p21CIP1, and p53), SASP factors (e.g. IL-6, TNF, MMP-1, EGF), the formation of different epigenetic alterations associated with DNA damage such as SAHFs, SADFs, TAFs, γH2AX, and others (Fig. 1A). In addition, senescent cells exhibit a change in morphology and experience nuclear membrane dysfunction associated with a reduction of LaminB1. Currently, there is no single universal biomarker of senescence as alterations in signaling pathways and metabolism, as well as relative expression of cell cycle inhibitors and SASP factors also differ between organisms, cell types, and senescence inducers. Therefore, multiple biomarkers of senescence should be utilized to characterize senescent cells.
Fig. 1.
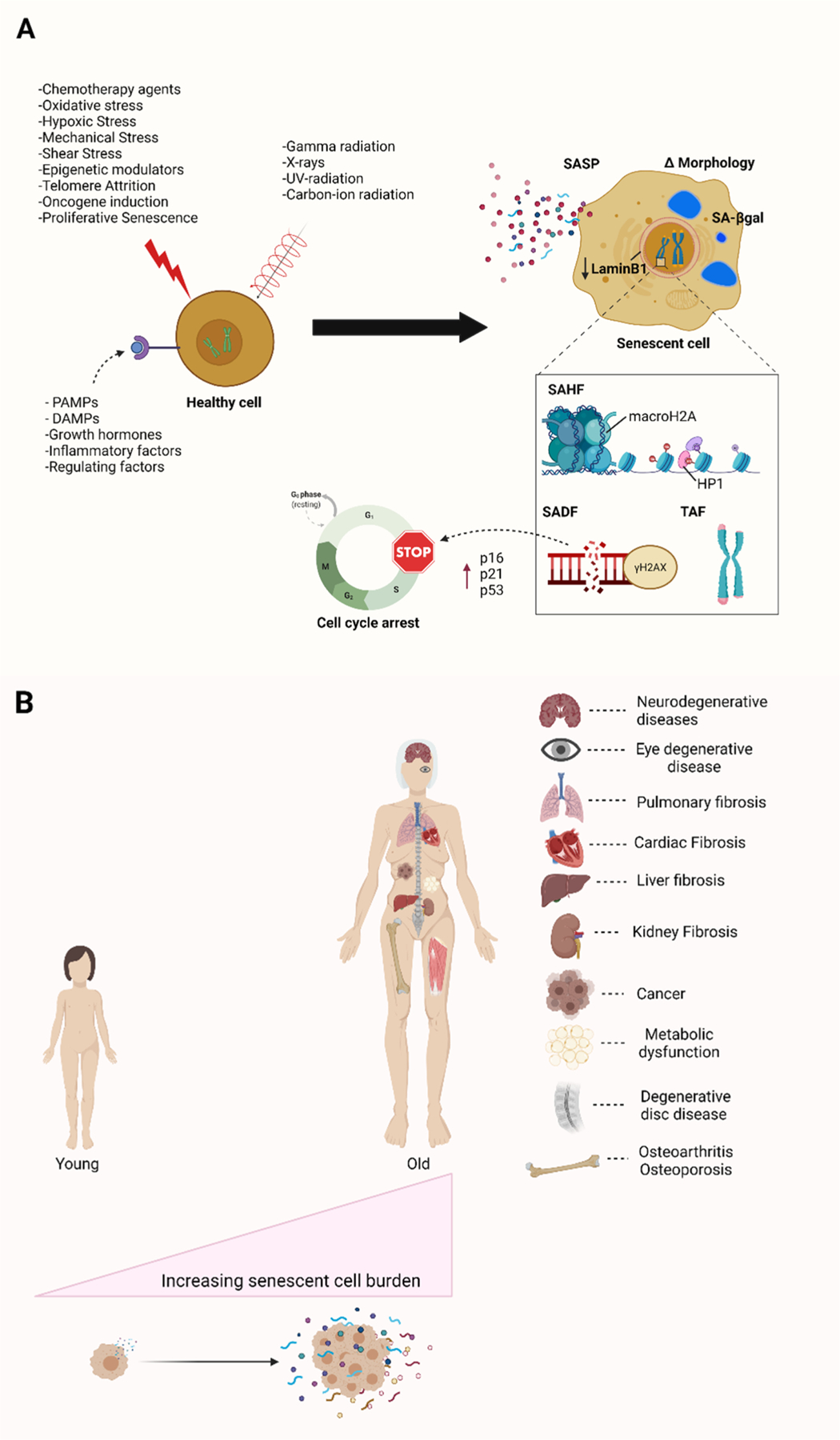
Inducers and biomarkers of cellular senescence as well as its impactions in aging and age-related diseases. (A) Diverse stimuli can induce cellular senescence, including replicative stress, genotoxic stress, oxidative stress, oncogene activation, and inflammatory factors. As a result, senescent cells enter a state of cell cycle arrest characterized by different biomarkers such as enlarged morphology, increased SA-β-galactosidase activity, release of a host of inflammatory SASPs, as well as the formation of different alteration associated with epigenetics and DNA damage such as SAHFs, SADFs, TAFs and γH2AX foci. (B) The accumulation of senescence cells drives and contributes to aging and age-related diseases in organisms. Abbreviations: SASP; senescence-associated secretory phenotype; SAHF, senescence-associated heterochromatin foci; SADF, senescence-associated DNA damage foci; TAF, telomere-associated foci. Created with BioRender.com.
Senescent cells have been shown to accumulate with age in various tissues and play a causal role in a host of chronic diseases such as diabetes, cancer, osteoarthritis, Alzheimer’s disease, and others (Fig. 1B) (Childs et al., 2015; Borghesan et al., 2020; He and Sharpless, 2017). Moreover, the elimination of senescent cells through genetic approaches has been shown to alleviate many senescence-associated pathologies and delay the onset of age-related diseases (Baker et al., 2011; Baker et al., 2016). Accordingly, pharmacological clearance of senescent cells using a novel class of drugs termed senolytics has been demonstrated to alleviate a wide range of age-associated symptoms, and even extend lifespan in model organisms (Childs et al., 2017; Di Micco et al., 2021; Pignolo et al., 2020; Kirkland and Tchkonia, 2017). Therefore, approaches to induce senolysis are a promising approach for the treatment of many age-related diseases, and for the extension of health and lifespan (Robbins et al., 2021; Niedernhofer and Robbins, 2018). In the past few years, great progress has been made in discovering and developing senolytic compounds and approaches with some already in clinical trials for the treatment of age-related diseases. Herein, we curate and summarize the senolytics identified to date and provide an overview of the approaches used to identify senolytics.
1.1. Overview
The senolytics summarized in Table 1 and discussed in detail below were identified or developed using the approaches outlined in Fig. 2. For example, bioinformatic analysis of senescent cells (e.g., transcriptome, proteome, lipidome and metabolome) can be used to identify SCAPs, similar to those upregulated in tumor cells. Targeted and non-targeted drug library screening on different types of senescent cells also can be used to identify new senolytic compounds, some of which target the identified SCAPs (Fig. 2A). Additionally, we and others have taken a genetic approach to identify longevity pathways through sequence analysis of different centenarian cohorts. Interestingly, preliminary results suggest that the rare variants in centenarians associated with longevity reduce the senescent cell burden with age. Developing drugs that target the pathways altered in centenarians should, in theory, reduce the senescent cell burden (Figs. 3 and 4).
Table 1.
Senolytics reported to date according to class.
| Class | Senolytic Agents |
|---|---|
| BCL-2 family inhibitors | ABT-263 (Navitoclax) A-1331852 A-1155463 ABT-737 |
| HSP90 inhibitors | 17-DMAG (Alvespimycin) Geldanamycin 17-AAG (Tanespimycin) Ganetespib |
| p53 pathway targeting compounds | FOXO4-DRI UBX0101 RG7112 (RO5045337) P5091 P22077 |
| Natural products and their analogues | Fisetin Curcumin O-vanillin EF-24 Piperlongumine and analogues 47–49 GL-V9 |
| Cardiac glycosides | Ouabain Ouabagenin Proscillaridin A Bufalin K-stropanthin Strophanthidin |
| Galactose modified prodrugs | SSK1 JHB75B Nav-Gal 5FURGa |
| PROTACs | PZ15227 ARV825 |
| Miscellaneous | Fenofibrate Azithromycin Roxithromycin Tamatinib (R406) MitoTam Panobinostat AT-406 Galactose-coated nanoparticles (GalNPs) |
| Senolytic combinations | Dasatinib + Quercetin Piperlongumine + ABT-263 P5091 + ABT-263 Tamatinib + ABT-263 |
| Chemotherapeutic and senolytic combinations | Olaparib + ABT-263 Olaparib + A-1155463 Olaparib + Piperlongumine Taxol + Panobinostat |
Fig. 2.
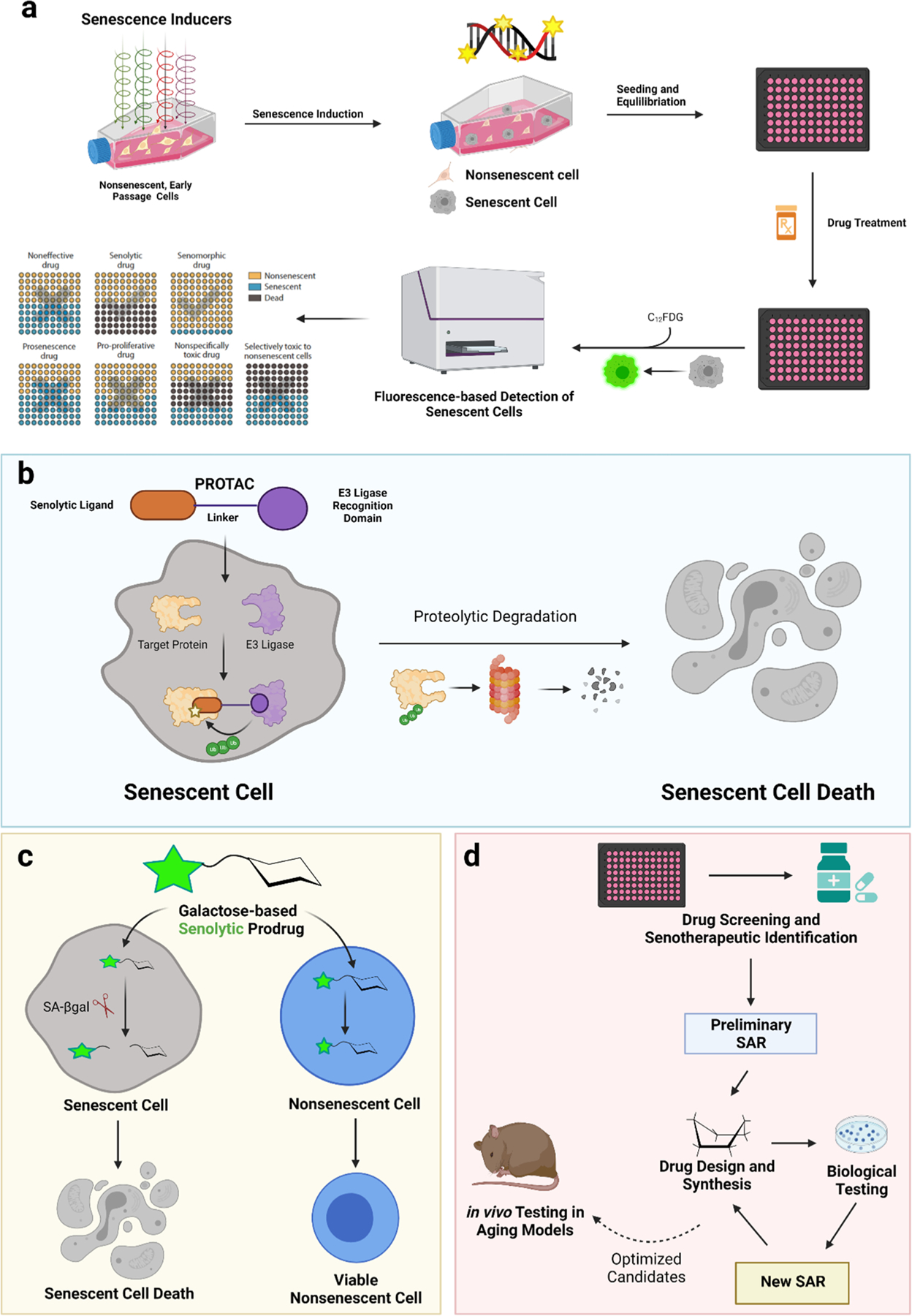
Approaches to identify and optimize senolytics. (A) Schematic diagram of a screening assay for the identification of senolytics. Non-senescent cells were induced to senescence through methods such as oxidative stress or irradiation. This results in a mixed culture of senescent cells (grey) and non-senescent cells (beige). Compounds are tested on these mixed cultures and then stained with fluorogenic C12FDG for the discovery of senolytics. (B) Mechanism of PROTAC senolytics. (C) Mechanism of galactose modified senolytic prodrugs. (D) Structural optimization and development of improved senolytics by medicinal chemistry and drug design. Created with BioRender.com.
Fig. 3.
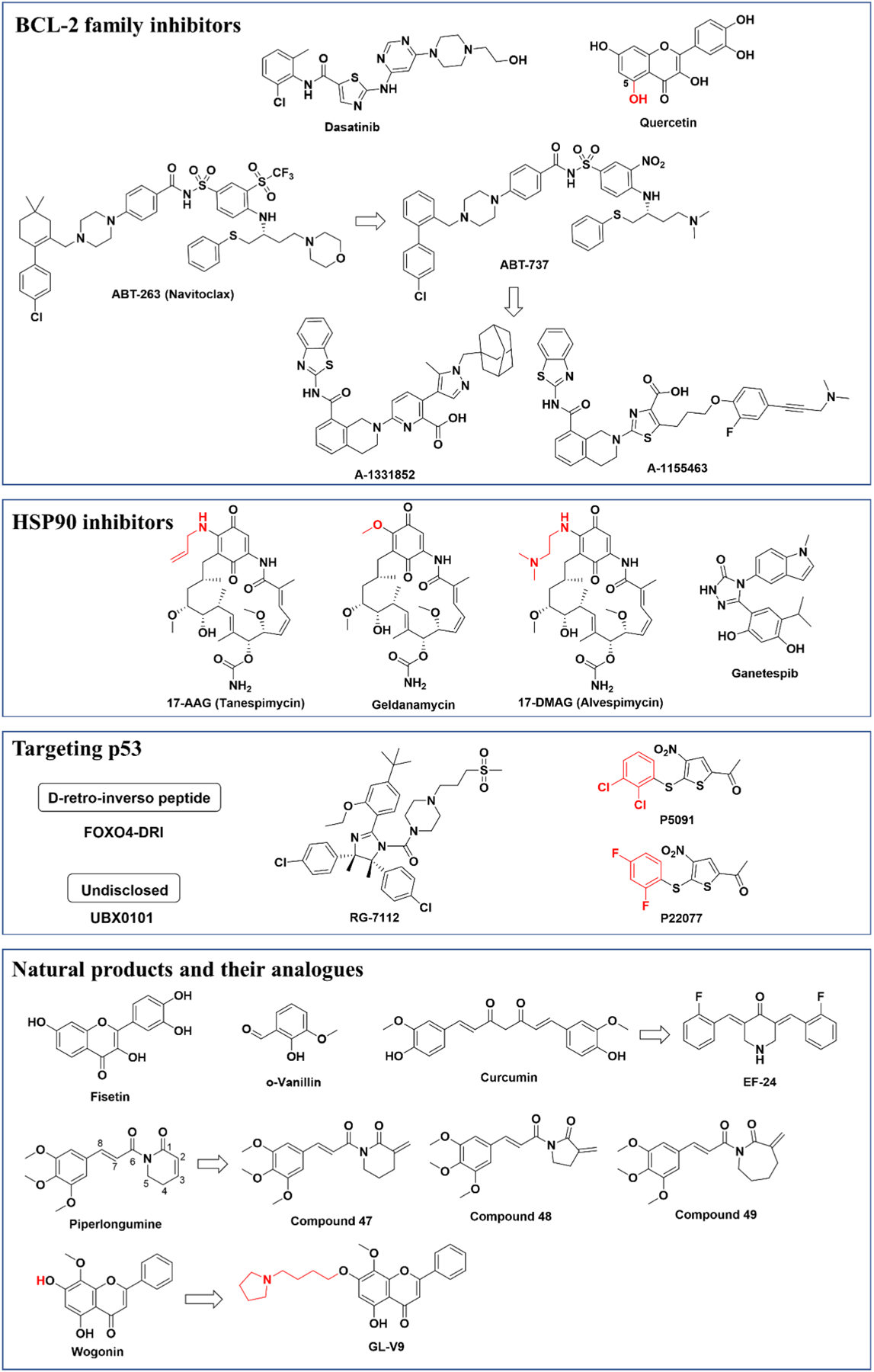
Chemical structures of current senolytics.
Fig. 4.
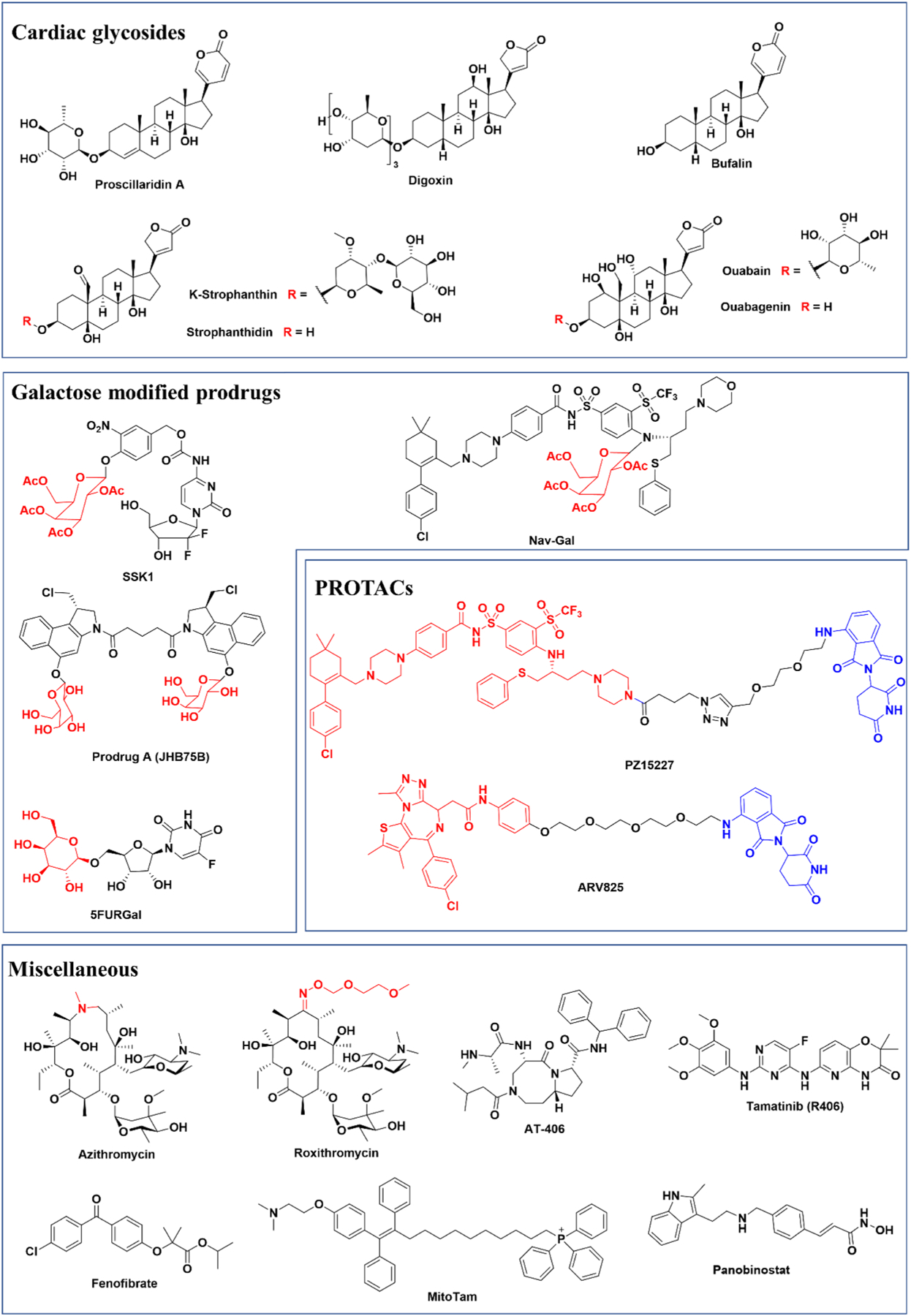
Chemical structures of current senolytics.
The identified senolytics can be improved by medicinal chemistry involving rounds of structure-activity relationship (SAR) studies where small libraries of analogs are screened for activity in senescent cell culture (Fig. 2D). Through SAR studies, critical chemical features involved in potency, specificity, and target affinity can be determined and used to design novel senolytics. Optimized senolytics can be further tested in models of aging in vivo. Also, a prodrug strategy can be used to target senolytic activity to cells expressing high levels of SA-β-gal. Here, the killing activity of a drug is activated only in senescent cells (Fig. 2C). Finally, the activity of identified compounds can also be enhanced using PROTACs where a senolytic drug is linked to an E3 ligase targeting moiety to direct the drug target for proteolysis (Fig. 2B).
1.2. Identification of senolytics using bioinformatics
The first senolytics were identified by a hypothesis-driven, bioinformatics-based approach (Zhu et al., 2015). Through transcriptomic analysis comparing senescent to proliferating human preadipocytes, it was found that senescent cells heavily rely on Senescent Cell Anti-Apoptotic Pathway (SCAP) networks to protect them from pro-apoptotic stimuli. Several key SCAPs identified include tyrosine kinases/ephrins, BCL-2/BCL-XL family, P13 K/AKT, p53/p21/PAI-1&2, and HIF-1α (Zhu et al., 2015). Pharmaceutical targeting of these SCAP pathways can facilitate selective killing of senescent cells without affecting normal cells. This led to the identification of the first senolytics: the combination of dasatinib (D) and quercetin (Q). Dasatinib, an inhibitor of multiple tyrosine kinases used clinically for cancer therapy, is effective at killing senescent preadipocytes. Quercetin, a naturally occurring flavonoid that targets the BCL-2, PI3K/AKT pathway, insulin/IGF-1, HIF-1α and other SCAPs, preferentially kills senescent endothelial cells. The combination of these two compounds (D + Q) more efficiently induces apoptosis in both types of senescent cells. This likely occurs through additional mechanisms that promote targeting of multiple SCAPs (Zhu et al., 2015). To date, the senolytic effects of D + Q have been tested in dozens of in vivo models where intermittent treatment alleviated many age-related diseases and pathologies, such as progeroid symptoms, physical dysfunction, osteoporosis, hepatic steatosis, insulin resistance, neurodegeneration, vasomotor dysfunction, pulmonary fibrosis, chronic kidney disease, and skeletal muscle dysfunction (Wissler Gerdes et al., 2020). Based on these early proof of concept studies, the combination of D + Q has advanced to human clinical trials for idiopathic pulmonary fibrosis (NCT02874989), chronic kidney disease (NCT02848131), skeletal health (NCT04313634), hematopoietic stem cell transplant survivors (NCT02652052), Alzheimer’s disease (NCT04063124) and for adult cancer survivors (NCT04733534).
1.3. Inhibitors of BCL-2 family
The BCL-2 family proteins are key regulators of cell survival and death and BCL-2, BCL-W, and BCL-XL have been found to be upregulated in different types of senescent cells. Therefore, these BCL-2 family member SCAPs pose an excellent senolytic target for pharmacological intervention. ABT-263 (Navitoclax) is a pan-BCL-2 family inhibitor that targets BCL-2, BCL-XL and BCL-W by binding to the BH3 binding groove of BCL-2 proteins, disrupting their interactions with the pro-death protein BIM initiating apoptosis (Tse et al., 2008; Mohamad Anuar et al., 2020; Mérino et al., 2012; Souers et al., 2013). BCL-2, BCL-XL and BCL-W are upregulated in senescent human umbilical vein endothelial cells (HUVECs), human IMR-90 fibroblasts, and murine embryonic fibroblasts (MEFs), but not primary human preadipocytes (Zhu et al., 2016; Chang et al., 2016). Consistent with this, ABT-263 is a cell type-specific senolytic that induces cell death in senescent HUVECs, IMR-90 and MEFs, but not in preadipocytes (Zhu et al., 2016). However, severe side effects such as thrombocytopenia and neutropenia have been observed with ABT-263 treatment in patients, which may impede its clinical application (Schoenwaelder et al., 2011). The undesirable toxicity of ABT-263 towards platelets and neutrophils may be a result of its pan-BCL-2 protein inhibition.
It is proposed that more specific inhibition of BCL-XL, but not pan inhibition of the BCL-2 family, may maintain the desired efficacy while sparing platelets and neutrophils reducing toxicity (Leverson et al., 2015). Indeed, the more specific BCL-XL inhibitors A-1331852 and A-1155463 function as senolytics in senescent HUVECs and human lung fibroblasts (Zhu et al., 2017). However, it is still unknown whether A-1331852 and A-1155463 have fewer side effects in comparison to ABT-263.
ABT-737, a precursor of ABT-263 (Oltersdorf et al., 2005), is also reported to be a senolytic. Treatment of mice with ABT-737 efficiently eliminated senescent cells in the lungs of irradiated mice and p53-induced senescent cells in the epidermis (Yosef et al., 2016). However, ABT-737 is not orally bioavailable and has low aqueous solubility. Therefore, structural optimization was performed on ABT-737 with its derivative ABT-263 developed as an orally bioavailable and potent BCL-2 inhibitor (Tse et al., 2008). Overall, inhibiting BCL-2 family proteins provides a mechanism-based approach for the discovery of senolytics, however, their side effects pose a challenge for clinical use.
1.4. HSP90 inhibitors
The 90-kDa heat shock protein (Hsp90) is an ATP-dependent molecular chaperone that participates in folding, stabilization, and degradation of over 400 proteins. Many of these proteins are critical in a variety of cellular processes, including cell survival and responses to cellular stress (Taipale et al., 2010). Since Hsp90 clients are oncogenic proteins involved in cell apoptosis, proliferation, and angiogenesis, inhibiting Hsp90 has been an active approach for cancer chemotherapy (Dutta Gupta et al., 2019).
Recently, we identified several HSP90 inhibitors as a novel class of senolytics by screening a library of autophagy regulators on oxidatively stressed senescent ERCC1-deficient −MEFs (Fig. 2A). These HSP90 inhibitors, including 17-DMAG (alvespimycin), geldanamycin, 17-AAG (tanespimycin), and ganetespib, display senolytic activity in multiple senescent cells in a cell-type specific manner. For example, 17-DMAG is senolytic in senescent human fibroblasts (IMR90 and WI-38) and mouse stem cells, whereas ganetespib only showed senolytic activity in senescent HUVECs, but not in preadipocytes (Fuhrmann-Stroissnigg et al., 2017). In senescent −MEFs, 17-DMAG promoted apoptotic cell death by disrupting the HSP90-AKT interaction to destabilize the active form of AKT. Treatment of the Ercc1−/Δmouse model of accelerated aging with 17-DMAG reduced tissue senescence, extended healthspan, delayed the onset of multiple age-related symptoms. Thus it is likely that other natural and synthetic HSP90 inhibitors have senolytic activity (Fuhrmann-Stroissnigg et al., 2018; Dutta Gupta and Pan, 2020).
1.5. Targeting p53
The p53 network poses another senolytic target as it is a key regulator of apoptosis and senescence (Mijit et al., 2020). Baar et al. reported that elevated forkhead box protein O4 (FOXO4) contributes to senescence in radiation-induced IMR90 fibroblasts through interactions with p53 to prevent senescent cell death (Baar et al., 2017). They designed a FOXO4-D-retro-inverso (FOXO4-DRI) peptide to mimic the interaction surface of FOXO4 and p53 to disrupt their interaction. This binding disruption allows p53 to translocate to the cytosol and induce senescent IMR-90 cells to undergo caspase-3/7-dependent apoptosis. When administered in vivo, FOXO4-DRI ameliorated frailty and loss of renal function in both the XpdTTD/TTD model of accelerated aging and naturally aged mice. Unlike natural L-peptides, DRI peptides are made up of D-amino acids and cannot be recognized by natural proteases and thus are resistant to proteolytic degradation (Liu et al., 2016). FOXO4-DRI was shown to alleviate age-related onset of hypogonadism in aged male mice likely through selectively induction of apoptosis in senescent Leydig cells as a result of FOXO4/p53 interaction disruption (Zhang et al., 2020). These findings suggest the potential usefulness of modulating cellular senescence by targeting the protein-protein interaction of FOXO4-p53.
Murine double minute 2 (MDM2) E3 ligase is a major negative regulator of p53 which shuttles p53 from the nucleus to the cytoplasm for proteasomal degradation (Moll and Petrenko, 2003). UBX0101 is an inhibitor of MDM2/p53 interaction and was reported to induce apoptosis of senescent chondrocytes in an osteoarthritis mouse model (Jeon et al., 2017). However, the phase 2 clinical trial of intra-articular injection of UBX0101 to treat osteoarthritis did not meet its clinical endpoints (https://clinicaltrials.gov/ct2/show/NCT04229225, 2021). RG7112 (RO5045337) is another small molecule inhibitor of the MDM2/p53 interaction that acts to restore p53 activity. RG7112 has been tested in clinical trials for the treatment of advanced solid tumors and hematologic cancers (Vu et al., 2013). It was recently reported to selectively kill senescent intervertebral disc (IVD) cells through apoptosis and decrease expression of SASP factors in culture including IFN-γ, IL-6 and CCL24 (Cherif et al., 2020). Similarly, ex vivo treatment with RG7112 of intact human discs improved disc matrix homeostasis likely through its senolytic effects on senescent IVD cells (Cherif et al., 2020).
Another similar strategy to stabilize p53 is to target ubiquitin-specific peptidase 7 (USP7). USP7 deubiquitinates MDM2 and prevent its degradation by the ubiquitin-proteasome system, thereby stabilizing and upregulating p53 for apoptosis (Li et al., 2002). Senescent WI-38, IMR-90 fibroblasts, renal epithelial cells (RECs), and HUVECs all express a lower basal level of p53 (He et al., 2020a). Inhibiting USP7 activity with the small molecules P5091 or P22077 selectively induced apoptosis in senescent cells by reducing MDM2 expression and upregulating p53, leading to induction of pro-apoptotic proteins PUMA, NOXA, and FAS and inhibition of BCL-XL/BAK (He et al., 2020a). Furthermore, treatment with USP7 inhibitor P5091 eliminated doxorubicin-induced senescent cells and suppressed SASPs in mice (He et al., 2020a).
1.6. Natural products and their analogues
From a cell-based screening of a panel of flavonoids, we recently identified fisetin as novel senolytic able to selectively kill oxidative-stress induced senescent MEFs and genotoxin-induced senescent human fibroblasts. Fisetin also reduced senescence in human adipose tissue explants (Zhu et al., 2017; Yousefzadeh et al., 2018). Intermittent treatment of accelerated aged Ercc1−/Δ mice with fisetin reduced senescent cell burden and senescence markers in multiple organs. Administration of fisetin either acutely or chronically to wild-type old mice reduced cellular senescence in multiple tissues, restored tissue homeostasis, alleviated frailty, and extended median and maximum lifespan (Yousefzadeh et al., 2018). Based on these pre-clinical results, fisetin is now in clinical trials for chronic kidney disease (NCT03325322), skeletal health (NCT04313634), osteoarthritis (NCT04210986), COVID-19 (NCT04476953, NCT04537299, NCT04771611) frailty (NCT03675724) and for adult cancer survivors (NCT04733534). Fisetin is a naturally occurring flavonoid present in many fruits, vegetables, flowers and tea. It also has a variety of other pharmacological effects including antioxidant, anti-inflammatory, anti-carcinogenic, anti-diabetic, antibacterial, antiviral, and neuroprotective activities (Khan et al., 2013; Sundarraj et al., 2018). The wide range of biological activities of fisetin may contribute to its diverse and complex mechanisms of action on multiple molecular targets and signaling pathways (Syed et al., 2016). Therefore, the therapeutic effects of fisetin may not be exclusively attributed to its senolytic activity. Interestingly, fisetin bears a very similar chemical structure with quercetin, differing only in the 5-hydroxy group, making it more senolytic than the latter, suggesting that more effective analogues may be developed through structural optimization.
Curcumin is a natural product with many bioactivities (Bielak-Zmijewska et al., 2019). Recently, curcumin and its metabolite o-vanillin were found to have senolytic activities towards senescent human intervertebral disc cells. They were shown to reduce SASP factors associated with inflammation and back pain by downregulating the Nrf2 and NF-κB pathways (Cherif et al., 2019). However, curcumin suffers from low bioavailability due to poor aqueous solubility and chemical instability due to the keto-enol tautomerism. Therefore, EF-24, a synthetic analog of curcumin, was developed as a potent and broad-spectrum senolytic agent in a variety of senescent cells by inducing apoptosis and increasing proteasomal degradation of the BCL-2 anti-apoptotic family proteins (Li et al., 2019).
Piperlongumine, a natural amide alkaloid isolated from long pepper, was shown to have senolytic activity on senescent WI-38 fibroblasts (Wang et al., 2016). Piperlongumine induced cell death in a senescent cell-specific manner through increased ROS production and inhibition of the PI3K/Akt/mTOR pathway (Zhang et al., 2018). Further, SAR studies examining the α,β-unsaturated δ-valerolactam rings of piperlongumine revealed that saturation of the two electrophilic sites (C2-C3 and C7-C8) leads to loss of senolytic activity (Liu et al., 2018). Moreover, it was determined that the Michael acceptor on the lactam ring is critical for senolytic activity, whereas modification on the trimethoxyphenyl ring is well tolerated. By replacing the endocyclic C2-C3 olefin with an exocyclic methylene on C2 of the lactam ring, several piperlongumine analogues (compounds 47–49) were developed with increased senolytic activity that share a similar mechanism of action as piperlongumine (Liu et al., 2018).
GL-V9, a synthetic flavonoid analog of wogonin, has pro-apoptotic, anti-invasive, anti-metastatic, anti-tumor, and autophagic effects in various cancer cells (Li et al., 2011a; Li et al., 2011b; Guo et al., 2020; Zhu et al., 2020). Recently, GL-V9 was shown to be a potential senolytic as it preferentially killed senescent breast cancer cells as well as replication-induced senescent MEFs in a ROS-dependent manner (Yang et al., 2021). In senescent MDA-MB-231 cells, GL-V9 at 10 μM was able to alkalize the lysosome by increasing its pH from 4.95 to 5.51 and elevated ROS levels aggravating mitophagy deficiency to induce apoptosis. When injected intravenously in MMTV-PyMT mice, GL-V9 decreased cellular senescence in epirubicin-induced tumors (Yang et al., 2021). Though wogonin is the parent compound of GL-V9, no senolytic effects were observed. Structurally, a tertiary amine group present in GL-V9 distinguishes these two compounds, and may facilitate its selective localization to acidic lysosomes, contributing to the ROS elevation, and apoptotic killing of senescent cells (Zhang et al., 2015). In contrast to its senolytic activity, a nonlethal dose of GL-V9 (2 μM) was found to promote cellular senescence in malignant T-cells including Jurkat, HuT-102, and HuT-78 (Li et al., 2020). More mechanistic studies are needed to identify the targets of GL-V9 and understand its dose-dependent role in the induction of either senescence or senolysis.
1.7. Cardiac glycosides
Recently, a family of cardiac glycosides (CGs) was reported as potent senolytics (Triana-Martinez et al., 2019; Guerrero et al., 2019). Through high-throughput screening of the Prestwick chemical library, proscillaridin A, a CGs family compound, was identified as having senolytic activity on senescent tumor A549 cells (Triana-Martinez et al., 2019). Further CGs screening identified ouabain and digoxin as having comparable senolytic effects on different senescent tumor and primary cell lines (Triana-Martinez et al., 2019). Ouabain was also identified as a senolytic in the screening of the LOPAC library using cell models of both therapy-induced senescence (TIS) and oncogene-induced senescence (OIS) (Guerrero et al., 2019). A common feature of these CGs is their glycoside chain. Senescent cells have increased enzymatic activity of lysosomal glycosidases, such as β-galactosidase, which can selectively remove glycoside groups. Interestingly, bufalin, a structurally similar cardiac steroid lacking the glycoside chain, also showed senolytic activity. Moreover, other CGs and their structurally related aglycones have comparable senolytic properties, including ouabain vs ouabagenin, and k-stropanthin vs strophanthidin (Guerrero et al., 2019). These results suggest that the senolytic activities of these CGs are independent of the glycoside moieties. Mechanistically, the senolytic effects of these CGs can be partially attributed to their inhibition of the Na+/K+-ATPase, leading to depolarization of the plasma membrane which sensitizing senescent cells to death (Triana-Martinez et al., 2019). Another proposed mechanism of action of these CGs is to trigger apoptosis by upregulating pro-apoptotic BCL2-family protein NOXA in senescent cells (Guerrero et al., 2019).
1.8. Galactose modified senolytic prodrugs
A prodrug refers to a molecule with little or no activity that can be acted on chemically or enzymatically to produce a bioactive substance. A common characteristic of senescent cells is increased lysosomal β-galactosidase (SA-β-gal) activity. Lysosomal β-galactosidases hydrolyze the β-glycosidic bond between a galactose and its organic moiety. As such, novel senolytic prodrugs can be developed by attaching a galactose moiety to different cytotoxic compounds. Upon cellular up-take, the galactose-modified prodrugs can be preferentially processed via enzymatic cleavage by SA-β-gal in senescent cells, resulting in release of the active cytotoxic compounds and selective killing of senescent cells (Fig. 2C).
For example, gemcitabine, a clinically used chemotherapeutic, was modified with an acetyl galactose moiety and a non-toxic aromatic spacer to yield the prodrug SSK1 (Cai et al., 2020). A seco-duocarmycin analogue dimer was linked to two galactose groups generating the senolytic prodrug A (JHB75B) (Guerrero et al., 2020). Nav-Gal was designed by conjugating the BCL-2 family inhibitor navitoclax to an acetylated galactose group (Gonzalez-Gualda et al., 2020). An anti-cancer drug, 5-fluorouracil, was modified with a β-D-galactosyl moiety to afford the senolytic prodrug 5FURGa (Lai and Crews, 2017). All of these prodrugs preferentially kill senescent cells with high selectivity in vitro and in vivo. Notably, when compared to navitoclax, Nav-Gal reduced platelet toxicity, highlighting the success of the prodrug strategy to decrease the occurrence of thrombocytopenia often seen with navitoclax treatment (Gonzalez-Gualda et al., 2020). It is foreseeable that more senolytic prodrugs will be developed with this novel strategy.
1.9. PROteolysis TArgeting Chimera (PROTAC) senolytics
An alternative approach to designing safer senolytics is based on the innovative proteolysis-targeting chimera (PROTAC) technology. PROTACs provide an endogenous method of targeted protein degradation, comprised of a ligand specific to a target protein and an E3 ubiquitin ligase recruiting ligand connected with an optimized linker. PROTACs bring the target protein and the E3 ligase in proximity, leading to the ubiquitination of the target protein and its subsequent degradation through the ubiquitin proteasome system (Fig. 2B). Due to its catalytical mechanism, PROTACs require less drug exposure and usually display prolonged activity and reduced toxicity in comparison to classic inhibitors. Many PROTACs have been developed as effective antitumor therapeutics (He et al., 2020b).
This PROTAC technology now is being utilized for the development of novel senolytics. For example, a PROTAC-based senolytic PZ15227 was generated by tethering ABT-263 to a pomalidomide moiety, which targets it for cereblon (CRBN) E3 ligase ubiquitination, with an empirically optimized linker (Wakita et al., 2020). Thus, PZ15227 targets Bcl-XL and subjects it to the cereblon (CRBN) E3 ligase for proteasomal degradation. The CRBN E3 ligase, in this study, was chosen because it is minimally expressed in normal platelets. PZ15227 was found to be a more potent at clearing senescent cells than ABT-263 and demonstrated less platelet cytotoxicity in aged mice (Wakita et al., 2020). Thus, PZ15227 achieves greater potency than conventional inhibitor ABT-263 in a manner that relies on equilibrium occupancy.
A second PROTAC senolytic ARV825 was identified by an unbiased high-throughput screening of chemical libraries (Burslem and Crews, 2020). Bifunctional ARV825 is comprised of the BET inhibitor OTX015 and an E3 ligase binder pomalidomide. In senescent cells, ARV825 promoted the degradation of BRD4, which protects senescent cells from autophagy-induced cell death, leading to exacerbation of DNA double-strand breaks and eventually autophagy-induced apoptosis. Furthermore, ARV825 treatment eliminated chemotherapy-induced senescent cells and increased the efficacy of chemotherapy against xenograft tumors in mice (Burslem and Crews, 2020).
PROTACs are comprised of three elements: a ligand of a target protein, a linker, and an E3 ligase recruiting ligand (Fig. 2B). Given the many targetable proteins or protein-protein interactions involved in the regulation of senescence, the increasing number of E3 ligase binding moieties, and linker diversity, it is conceivable that more PROTAC senolytics will be developed in the future (Nogueira-Recalde et al., 2019). However, due to the high molecular weights associated with PROTACs, more pharmacokinetic and pharmacodynamic studies of PROTAC senolytics are needed.
1.10. Miscellaneous
Fenofibrate was recently identified as a novel class of senolytic and pro-autophagy agent from a cell-based screening of the Prestwick Chemical Library using senescent human chondrocytes induced by IL-6 followed by a secondary screening for LC3-mediated autophagic flux (Zhang et al., 2021). Fenofibrate is an activator of peroxisome proliferator-activated receptor alpha (PPARα) which regulates lipid metabolism. In multiple senescent cells, fenofibrate was demonstrated to selectively induce apoptosis, increase PPARα expression, regulate the transcriptional program of fatty acid β-oxidation, and increase autophagic flux (Zhang et al., 2021). In natural aging and surgically induced osteoarthritic mouse models, PPARα is downregulated and fenofibrate treatment protected against cartilage degradation possibly through its senolytic and pro-autophagic effects on chondrocytes (Zhang et al., 2021). In a retrospective study examining cohorts of human OA patients, fibrate use showed promise in alleviating osteoarthritic symptoms (Zhang et al., 2021). Interestingly, the senolytic digitoxigenin was also identified from the library screening, which was reported later by other groups (Triana-Martinez et al., 2019; Guerrero et al., 2019).
Two macrolide antibiotics, azithromycin and roxithromycin, also were reported to be senolytics in human fibroblast MCR-5 and BJ cells (Ozsvari et al., 2018). Erythromycin shares a very similar structure, but did not show any senolytic effect in these cells (Cho et al., 2020). Metabolic analysis found that azithromycin can induce autophagy and glycolysis, possibly driving senescent cell death. Roxithromycin was shown to attenuate pulmonary fibrosis in bleomycin-induced mice by targeting senescent cells mediated by the NOX4 pathway (Ozsvari et al., 2018).
A Syk inhibitor, Tamatinib (R406), was recently identified from a high-throughput screening using senescent human dermal fibroblasts (HDFs) as a novel senolytic with medium potency (Chapman et al., 2019). Tamatinib induced apoptosis in senescent cells by inhibiting cell survival processes including the focal adhesion kinase (FAK) and p38 mitogen-activated protein kinase (MAPK) pathways (Chapman et al., 2019). The senolytic effect of R406 has yet to be verified in other cell types and in vivo.
Senescent cells also are characterized by an altered bioenergetic state including increased oxygen consumption, mitochondrial membrane potential, and high levels of reactive oxygen species (ROS) in mitochondria (Hubackova et al., 2019). A mitochondria-targeted tamoxifen (MitoTam) was reported to selectively kill senescent cells both in vitro and in vivo (Samaraweera et al., 2017). The senolytic effect of MitoTam is facilitated partially through decreasing mitochondrial membrane potential, inhibiting mitochondrial oxidative phosphorylation (OXPHOS), and reducing expression of adenine nucleotide translocase-2 (ANT2) in senescent cells. Thus, by exacerbating mitochondrial dysfunction, MitoTam sensitizes senescent cells to death (Samaraweera et al., 2017).
Panobinostat, an FDA-approved histone deacetylase inhibitor used to treat various cancers, was recently repurposed as a post-chemotherapy senolytic (Peilin et al., 2019). It effectively killed the senescent cells which accumulated following cisplatin or taxol treatment in non-small cell lung cancer (NSCLC) as well as head and neck squamous cell carcinoma (HNSCC) cell lines. The senolytic effect of panobinostat was found to be associated with an increase in the histone H3 acetylation and a reduction of the BCL-XL expression. These results suggest the potential application of senolytics as a post-chemotherapy treatment option that targets senescent cancer cells.
Antiapoptotic proteins c-IAP1, c-IAP2 and XIAP are upregulated in senescent cells and confer resistance to apoptosis. AT-406, an inhibitor of these IAPs, was able to induce apoptosis in senescent cells, reduce SASP secretion, while creating a pro regenerative environment in vitro. When examined in rats, AT-406 attenuated tibial subchondral bone reconstruction of post-traumatic osteoarthritis (Munoz-Espin et al., 2018).
Though not the focus of this review, another emerging approach to selectively clear senescent cells is the use of a galactose-coated nanoparticle (GalNP) delivery system. In this system the nanoparticles are coated with galacto-oligosaccharides which encapsulate senolytics or non-senolytic toxins. They are preferentially delivered to SA-β-gal+ senescent cells, facilitating an enhanced and specific release of nanosenolytics that results in the elimination of senescent cells (Galiana et al., 2020; Amor et al., 2020). More recently, a cell-based therapy was reported as another novel strategy to clear senescent cells (Wang et al., 2017). Given that urokinase-type plasminogen activator receptor (uPAR) is primarily a senescent cell-specific antigen, anti-uPAR chimeric antigen receptor (CAR) T cell were engineered to kill uPAR positive senescent cells. The anti-uPAR CAR-T cells can ablate senescent tumor cells when combined with MEK and CDK4/6 inhibition in a mouse model of lung adenocarcinoma. These CAR-T cells also efficiently clear senescent cells, reduce fibrosis, and improve liver function in mouse models of tetrachloride-induced liver fibrosis and nonalcoholic steatohepatitis. The uPAR-targeting CAR T cells represents an exciting senolytic strategy, yet further work is needed to investigate the dosage, safety, and toxicity profile before its clinical use.
1.11. Senolytic combinations
Senescent cells are heterogeneous, therefore the cell-type specificity of certain senolytics may limit their clinical efficacy. Senolytic drug combinations may promote increased removal of a wider range of senescent cell subpopulations by complementarily or synergistically targeting multiple senescence-associated pathways involved in survival and apoptosis resistance. Moreover, low dose combinations of senolytics may reduce cytotoxic side effects while increasing their efficacy. The most renowned example is the first published senolytic, the combination of dasatinib and quercetin (Zhu et al., 2015). Other senolytic combinations have also been reported. For example, piperlongumine showed synergistic senolytic effects when combined with ABT-263, suggesting mechanistic differences (Wang et al., 2016). Similarly, the combination of the USP7 inhibitor P5091 and BCL-2 inhibitor ABT-263 was more effective in killing senescent cells than either alone (He et al., 2020a). ABT-263 was also found to potentiate the senolytic activity of tamatinib (Chapman et al., 2019).
Chemotherapeutic agents often induce senescence of not only tumor, but also normal cells during standard cancer treatment. Thus senolytics can be paired with pro-senescence anticancer agents to efficiently kill senescent cancer cells and reduce the toxicity. This combination of chemotherapy and senotherapy is termed the one-two punch strategy (Fleury et al., 2019). This two-step approach can potentiate chemotherapeutic efficiency, limit cancer resistance, and relapse. For example, senolytic agent ABT-263 showed little effect on melanoma cancer cells, however, after senescence induction by aurora kinase inhibition, these cancer cells become sensitized to ABT-263 (Fleury et al., 2019). Also, the PARP inhibitor, olaparib (Lynparza), has been used as a form of maintenance therapy for ovarian cancer patients. However, drug resistance and tumor recurrence upon drug withdrawal have been observed. A combination therapy of olaparib with different senolytics (e.g. ABT-263, A-1155463, piperlongumine) provided synergistic killing of ovarian cancer cells (Sieben et al., 2018). Similarly, the combination treatment of panobinostat and the anti-cancer drug Taxol was more efficacious at killing HNSCC and NSCLC cancer cells (Peilin et al., 2019). These results highlight the potential of senolytics for not only the treatment of aging and age-related diseases, but also in cancer chemotherapy (Demaria et al., 2014).
1.12. Caveats and challenges
Senescent cells are not always pathological as they have been shown to play important roles in processes like wound healing and parturition (Demaria et al., 2014). In theory, senolytics can be administered intermittently to clear senescent cells as they arise with age or disease (e.g., bi-weekly or monthly). The intermittent administration of senolytics provides a level of safety as treatment may be stopped during pregnancy, wound repair, and other conditions. Also, no senolytic approach should interfere with pathways controlling the cell-cycle or mechanisms that drive senescence as this may promote cancer development. Similarly, senolytics should not interfere with senescent cell generation, allowing senescent cells to form when needed.
Senescent cells are diverse in nature exhibiting variation in transcriptional, metabolic, and SASP profiles in a species, cell type, and even individual cell basis. Because of this diversity, many senolytics have been shown to demonstrate specificity towards different types and subgroups of senescent cells. Currently reported senolytics target critical components involved in unique alterations in senescent cell metabolism including cell cycle arrest, activation of pathways associated with survival, evasion of apoptosis, alterations in energy metabolism, as well as production and secretion of their SASPs. For instance, ABT-263, the pan BCL-2 family inhibitor, selectively induces apoptosis in human IMR-90 fibroblasts and HUVECs, but not primary human preadipocytes. This is likely due to variations in their predominant pro-survival and anti-apoptotic pathways. In addition, methods of senescence induction utilized such as genotoxic stress or oncogenic alterations also contribute to the molecular diversification of senescent cells by differentially activating stress response pathways further contributing to the selectivity of certain senolytics. Thus, studies aimed at characterizing a variety of senescent cells in culure and especially in vivo will be required for the proper application of senolytics.
Although the therapeutic benefit of senolytics has accelerated their journey from discovery to clinical trials, not all senolytics are perfect in their current state. While cell-type dependent selectivity can be addressed through combinatorial applications, lack of bioavailability, off target effects and side effects associated with their application pose barriers to clinical application. Metabolic variations associated with senescent cells are not senescent cell specific. Other cells, such as terminally differentiated and post mitotic adipocytes, neurons, cardiomyocytes and others, exhibit increased SA-β-gal activity and expression of p16INK4a. Thus, galactose-modified senolytic prodrugs, which take advantage of the SA-β-galactosidase activity, will likely produce off target effects. Indeed, off-target effects have been observed in a variety of senolytics, such as Nav-Gal, PZ15227 and navitoclax, demonstrating signs of platelet toxicity. However, due to the intermittent dosing scheme required for senolytics, toxicity associated with senotherapies may be negligible.
Studies investigating the molecular consequences associated with chemical structures and the identification of critical moieties have been shown to be advantageous in the production of safer and more potent senolytic compounds. A notable example of this can be seen in studies leading to the development of piperlongumine analogues bearing lactam ring alterations that improved senolytic activity. However, studies examining the relationship between compound-target selectivity and affinity remain largely unexplored, potentially resulting from mechanistic ambiguity. PROTACs may serve as a solution to complications associated with mechanistic uncertainties by permitting selective targeting of specific proteins for E3 ligase mediated degradation. However, PROTAC technology is still in its infancy and requires further optimization. Thus, studies focused on chemical characterization and mechanistic action of both current and future senolytic compounds would advance current methods of senolytic discovery and their clinical application.
It is important to mention that it is not easy to demonstrate senolytic activity of a compound, especially in vivo. The ability of a compound to reduce the number of SA-β-gal positive cells or the level of p16INK4a or p21CIP1 expression does not necessarily demonstrate selective senescent cell killing. Instead, it could reflect the prevention of senescent cell accumulation or suppression of senescence markers. However, short term treatment with a compound able to yield a sustained reduction in senescence and health benefits over a prolonged period of time are consistent with senolytic activity. In contrast, a requirement for more frequent treatment is consistent with the activity of senomorphics, compounds that suppress markers of senescence and SASPs without causing senescent cell death.
Another approach to demonstrate senolytic activity in vivo is to test the ability of a compound to reduce markers of senescence by inducing senescent cell apoptosis in human tissue explants harboring senescent cells. This approach clearly documents the senolytic activity of a compound, although it does not identify the specific types or subsets of cells undergoing cell death. Additional methods, including single cell analysis, are needed to define the specific cell types within a tissue that are targeted by senolytic drugs. A further complication in proving senolytic activity in vivo is immune system competition. Certain functional immune cell types like NK and T cells are able to remove senescent cells. However, the ability of these immune cells to clear senescent cells wanes with age, leading to the accumulation of senescent cells. Drugs that improve immune function, but don’t target senescent cells directly, can also act to reduce senescent cell burden. Thus, it is important to apply caution when classifying certain drugs as senolytics.
2. Conclusion
Demonstrating the connection between senescent cell accumulation and age, as well as their contribution to many age-related diseases has spurred significant efforts to identify senolytic compounds. Many studies have showcased the potential of senolytics to alleviate age-related disorders and even extend lifespan in model organisms. Despite the complexity of cellular senescence and translational challenges, senolytic discovery is flourishing. We envision that more novel and efficient senolytics will be discovered in the following years with the aid of new technologies and approaches that are likely to advance from discovery to preclinical trials for the treatment and prevention of age-related frailties and diseases.
Funding
This work was supported by NIH grants RO1 AG063543-02S1, P01 AG043376, U19 AG056278, RO1 AG063543, P01 AG062413 and the Glenn Foundation for Medical Research Postdoctoral Fellowships in Aging Research.
References
- Amor C, et al. , 2020. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 583 (7814), 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baar MP, et al. , 2017. Targeted apoptosis of senescent cells restores tissue homeostasis in response to Chemotoxicity and aging. Cell 169 (1), 132–147 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, et al. , 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479 (7372), 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, et al. , 2016. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530 (7589), 184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett K, et al. , 2012. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 380 (9836), 37–43. [DOI] [PubMed] [Google Scholar]
- Bielak-Zmijewska A, et al. , 2019. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci 20 (5), 1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesan M, et al. , 2020. A senescence-centric view of aging: implications for longevity and disease. Trends Cell Biol 30 (10), 777–791. [DOI] [PubMed] [Google Scholar]
- Burslem GM, Crews CM, 2020. Proteolysis-targeting chimeras as therapeutics and tools for biological discovery. Cell 181 (1), 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, et al. , 2020. Elimination of senescent cells by beta-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res 30 (7), 574–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, et al. , 2016. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med 22 (1), 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J, Fielder E, Passos JF, 2019. Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett 593 (13), 1566–1579. [DOI] [PubMed] [Google Scholar]
- Cherif H, et al. , 2019. Curcumin and o-vanillin exhibit evidence of senolytic activity in human IVD cells in vitro. J. Clin. Med 8 (4), 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherif H, et al. , 2020. Senotherapeutic drugs for human intervertebral disc degeneration and low back pain. Elife 9 p. e54693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, et al. , 2015. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med 21 (12), 1424–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs BG, et al. , 2017. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov 16 (10), 718–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, et al. , 2020. Identification of SYK inhibitor, R406 as a novel senolytic agent. Aging (Albany NY) 12 (9), 8221–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, et al. , 2010. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol 5 (1), 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, et al. , 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31 (6), 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco R, et al. , 2021. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol 22 (2), 75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta Gupta S, Pan CH, 2020. Recent update on discovery and development of Hsp90 inhibitors as senolytic agents. Int. J. Biol. Macromol 161, 1086–1098. [DOI] [PubMed] [Google Scholar]
- Dutta Gupta S, Bommaka MK, Banerjee A, 2019. Inhibiting protein-protein interactions of Hsp90 as a novel approach for targeting cancer. Eur. J. Med. Chem 178, 48–63. [DOI] [PubMed] [Google Scholar]
- Fleury H, et al. , 2019. Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat. Commun 10 (1), 2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann-Stroissnigg H, et al. , 2017. Identification of HSP90 inhibitors as a novel class of senolytics. Nat. Commun 8 (1), 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD, 2018. Hsp90 inhibitors as senolytic drugs to extend healthy aging. Cell Cycle 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiana I, et al. , 2020. Preclinical antitumor efficacy of senescence-inducing chemotherapy combined with a nanoSenolytic. J. Control. Release 323, 624–634. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gualda E, et al. , 2020. Galacto-conjugation of Navitoclax as an efficient strategy to increase senolytic specificity and reduce platelet toxicity. Aging Cell 19 (4) p. e13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero A, et al. , 2019. Cardiac glycosides are broad-spectrum senolytics. Nat Metab 1 (11), 1074–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero A, et al. , 2020. Galactose-modified duocarmycin prodrugs as senolytics. Aging Cell 19 (4) p. e13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, et al. , 2020. Flavonoid GL-V9 induces apoptosis and inhibits glycolysis of breast cancer via disrupting GSK-3beta-modulated mitochondrial binding of HKII. Free Radic. Biol. Med 146, 119–129. [DOI] [PubMed] [Google Scholar]
- He S, Sharpless NE, 2017. Senescence in health and disease. Cell 169 (6), 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, et al. , 2020a. Inhibition of USP7 activity selectively eliminates senescent cells in part via restoration of p53 activity. Aging Cell 19 (3) p. e13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, et al. , 2020b. Using proteolysis-targeting chimera technology to reduce navitoclax platelet toxicity and improve its senolytic activity. Nat. Commun 11 (1), 1996. https://clinicaltrials.gov/ct2/show/NCT04229225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubackova S, et al. , 2019. Selective elimination of senescent cells by mitochondrial targeting is regulated by ANT2. Cell Death Differ 26 (2), 276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon OH, et al. , 2017. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med 23 (6), 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy BK, et al. , 2014. Geroscience: linking aging to chronic disease. Cell 159 (4), 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, et al. , 2013. Fisetin: a dietary antioxidant for health promotion. Antioxid. Redox Signal 19 (2), 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, 2017. Cellular senescence: a translational perspective. EBioMedicine 21 (Supplement C), 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AC, Crews CM, 2017. Induced protein degradation: an emerging drug discovery paradigm. Nat. Rev. Drug Discov 16 (2), 101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverson JD, et al. , 2015. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci. Transl. Med 7 (279), p. 279ra40. [DOI] [PubMed] [Google Scholar]
- Li M, et al. , 2002. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 416 (6881), 648–653. [DOI] [PubMed] [Google Scholar]
- Li L, et al. , 2011a. GL-V9, a newly synthetic flavonoid derivative, induces mitochondrial-mediated apoptosis and G2/M cell cycle arrest in human hepatocellular carcinoma HepG2 cells. Eur. J. Pharmacol 670 (1), 13–21. [DOI] [PubMed] [Google Scholar]
- Li L, et al. , 2011b. Inhibitory effects of GL-V9 on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-2/9. Eur. J. Pharm. Sci 43 (5), 393–399. [DOI] [PubMed] [Google Scholar]
- Li W, et al. , 2019. The curcumin analog EF24 is a novel senolytic agent. Aging (Albany NY) 11 (2), 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, et al. , 2020. Mitotic catastrophe and p53-dependent senescence induction in T-cell malignancies exposed to nonlethal dosage of GL-V9. Arch. Toxicol 94 (1), 305–323. [DOI] [PubMed] [Google Scholar]
- Liu M, et al. , 2016. D-peptides as recognition molecules and therapeutic agents. Chem. Rec 16 (4), 1772–1786. [DOI] [PubMed] [Google Scholar]
- Liu X, et al. , 2018. Senolytic activity of piperlongumine analogues: synthesis and biological evaluation. Bioorg. Med. Chem 26 (14), 3925–3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, et al. , 2013. The hallmarks of aging. Cell 153 (6), 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengoni A, et al. , 2011. Aging with multimorbidity: a systematic review of the literature. Ageing Res. Rev 10 (4), 430–439. [DOI] [PubMed] [Google Scholar]
- Mérino D, et al. , 2012. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood 119 (24), 5807–5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijit M, et al. , 2020. Role of p53 in the regulation of cellular senescence. Biomolecules 10 (3), 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad Anuar NN, et al. , 2020. Clinical review: navitoclax as a pro-apoptotic and anti-fibrotic agent. Front. Pharmacol 11 (1817). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll UM, Petrenko O, 2003. The MDM2-p53 interaction. Mol. Cancer Res 1 (14), 1001–1008. [PubMed] [Google Scholar]
- Munoz-Espin D, Serrano M, 2014. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol 15 (7), 482–496. [DOI] [PubMed] [Google Scholar]
- Munoz-Espin D, et al. , 2018. A versatile drug delivery system targeting senescent cells. EMBO Mol. Med 10 (9), e9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer LJ, Robbins PD, 2018. Senotherapeutics for healthy ageing. Nat. Rev. Drug Discov 17 (5), 377. [DOI] [PubMed] [Google Scholar]
- Nogueira-Recalde U, et al. , 2019. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine 45, 588–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, et al. , 2005. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435 (7042), 677–681. [DOI] [PubMed] [Google Scholar]
- Ozsvari B, et al. , 2018. Azithromycin and Roxithromycin define a new family of “senolytic” drugs that target senescent human fibroblasts. Aging (Albany NY) 10 (11), 3294–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peilin W, et al. , 2019. Directed elimination of senescent cells attenuates development of osteoarthritis by inhibition of c-IAP and XIAP. Biochim. Biophys. Acta Mol. Basis Dis 1865 (10), 2618–2632. [DOI] [PubMed] [Google Scholar]
- Pignolo RJ, et al. , 2020. Reducing senescent cell burden in aging and disease. Trends Mol. Med 26 (7), 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PD, et al. , 2021. Senolytic drugs: reducing senescent cell viability to extend health span. Annu. Rev. Pharmacol. Toxicol 61 (1), 779–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaraweera L, et al. , 2017. A novel indication for Panobinostat as a senolytic drug in NSCLC and HNSCC. Sci. Rep 7 (1), 1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwaelder SM, et al. , 2011. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 118 (6), 1663–1674. [DOI] [PubMed] [Google Scholar]
- Sieben CJ, et al. , 2018. Two-step senescence-focused cancer therapies. Trends Cell Biol 28 (9), 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souers AJ, et al. , 2013. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med 19 (2), 202–208. [DOI] [PubMed] [Google Scholar]
- Sundarraj K, Raghunath A, Perumal E, 2018. A review on the chemotherapeutic potential of fisetin: in vitro evidences. Biomed. Pharmacother 97 (Supplement C), 928–940. [DOI] [PubMed] [Google Scholar]
- Syed DN, et al. , 2016. Exploring the molecular targets of dietary flavonoid fisetin in cancer. Semin. Cancer Biol 40–41, p. 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M, Jarosz DF, Lindquist S, 2010. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol 11 (7), 515–528. [DOI] [PubMed] [Google Scholar]
- Triana-Martinez F, et al. , 2019. Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat. Commun 10 (1), 4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse C, et al. , 2008. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68 (9), 3421–3428. [DOI] [PubMed] [Google Scholar]
- Vu B, et al. , 2013. Discovery of RG7112: a small-molecule MDM2 inhibitor in clinical development. ACS Med. Chem. Lett 4 (5), 466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakita M, et al. , 2020. A BET family protein degrader provokes senolysis by targeting NHEJ and autophagy in senescent cells. Nat. Commun 11 (1), 1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, et al. , 2016. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY) 8 (11), 2915–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. , 2017. High-throughput functional genetic and compound screens identify targets for senescence induction in cancer. Cell Rep 21 (3), 773–783. [DOI] [PubMed] [Google Scholar]
- Wissler Gerdes EO, et al. , 2020. Discovery, development, and future application of senolytics: theories and predictions. FEBS J 287 (12), 2418–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, et al. , 2021. Identification of GL-V9 as a novel senolytic agent against senescent breast cancer cells. Life Sci 272, p. 119196. [DOI] [PubMed] [Google Scholar]
- Yosef R, et al. , 2016. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun 7, 11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, et al. , 2018. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. , 2015. Monitoring lipid peroxidation within foam cells by lysosome-targetable and ratiometric probe. Anal. Chem 87 (16), 8292–8300. [DOI] [PubMed] [Google Scholar]
- Zhang X, et al. , 2018. Oxidation resistance 1 is a novel senolytic target. Aging Cell 17 (4) p. e12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, et al. , 2020. FOXO4-DRI alleviates age-related testosterone secretion insufficiency by targeting senescent Leydig cells in aged mice. Aging (Albany NY) 12 (2), 1272–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. , 2021. Roxithromycin attenuates bleomycin-induced pulmonary fibrosis by targeting senescent cells. Acta Pharmacol. Sin [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. , 2015. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14 (4), 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. , 2016. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15 (3), 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. , 2017. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 9 (3), 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. , 2020. The synthetic flavonoid derivative GL-V9 induces apoptosis and autophagy in cutaneous squamous cell carcinoma via suppressing AKT-Regulated HK2 and mTOR signals. Molecules 25 (21), 5033. [DOI] [PMC free article] [PubMed] [Google Scholar]


