Abstract
Diagnostics have proven to be crucial to the COVID-19 pandemic response. There are three major methods for the detection of SARS-CoV-2 infection and their role has evolved during the course of the pandemic. Molecular tests such as PCR are highly sensitive and specific at detecting viral RNA, and are recommended by WHO for confirming diagnosis in individuals who are symptomatic and for activating public health measures. Antigen rapid detection tests detect viral proteins and, although they are less sensitive than molecular tests, have the advantages of being easier to do, giving a faster time to result, of being lower cost, and able to detect infection in those who are most likely to be at risk of transmitting the virus to others. Antigen rapid detection tests can be used as a public health tool for screening individuals at enhanced risk of infection, to protect people who are clinically vulnerable, to ensure safe travel and the resumption of schooling and social activities, and to enable economic recovery. With vaccine roll-out, antibody tests (which detect the host's response to infection or vaccination) can be useful surveillance tools to inform public policy, but should not be used to provide proof of immunity, as the correlates of protection remain unclear. All three types of COVID-19 test continue to have a crucial role in the transition from pandemic response to pandemic control.
Introduction
At the start of the COVID-19 pandemic, Tedros Adhanom Ghebreyesus, Director-General of WHO, urged countries to “test, test, test”.1 He said that testing, isolation, and contact tracing should be the backbone of the global pandemic response.1 The response from the diagnostic industry was overwhelming. Currently, more than 1000 brands of diagnostic test are available commercially, with more in the pipeline.2 However, these numbers mask the common challenges faced by numerous countries, such as building capacity for testing, the global competition for access to diagnostic kits and supplies (including swabs for specimen collection), choosing the right test for the right situation, and assuring that tests have external validation.3, 4, 5 There is also an absence of global consensus on cost-effective testing strategies that, combined with sound public health measures such as quarantine and contact tracing, would enable the control of community infections in a way that minimises disruptions to society and the economy. The emergence of variants of concern (VOCs) that are increasingly easy to transmit has also highlighted the need to speed up vaccine roll-out while scaling up community-based testing with public health measures to slow the spread of such variants.6, 7, 8
The COVID-19 pandemic response has led to the use of testing outside of health-care settings on an unprecedented scale, as a public health tool to ensure a safe environment for schools, workplaces, and mass gatherings for sports, music, religious, and social events. Governments and airlines have mandated testing to allow the resumption of travel in a safe manner. Policy makers are attempting to determine the best tests to deploy in different settings for patient management, case finding, and outbreak control, often with the limitations of an uncertain supply chain, an insufficient workforce, and insufficient research evidence to inform policy.9
These challenges are compounded by the fact that the COVID-19 pandemic has exposed fault lines and inequities in health-care systems, not only in low-income and middle-income countries, but also in high-income countries. In many cases, fragmentation between public health on the one hand, and political and economic priorities on the other, has led to confusion in reaching policy decisions about how to control the pandemic, preserve lives, avoid social disruption, and protect the economy, which has led to insufficiently clear or coherent messages being given to the public. In this Review, we discuss how the role of diagnostics in both clinical medicine and control of the pandemic has evolved over time with increased knowledge about the SARS-CoV-2 virus and its mode of transmission, and the understanding that it is destined to become endemic.
Key diagnostic tests for patient management and pandemic control
An unprecedented number of novel diagnostic tests have been developed for COVID-19 using state-of-the-art technologies. Three types of diagnostic tests are relevant to patient management and pandemic control: molecular or nucleic acid amplification tests (eg, PCR tests) that detect viral RNA; antigen tests that detect viral proteins (eg, nucleocapsid or spike proteins); and serology tests that detect host antibodies in response to infection, or vaccination, or both (table 1 ). The first two types of tests can be used to diagnose acute infection. By contrast, serology tests provide only indirect evidence of infection 1–2 weeks after the onset of symptoms and are best used for surveillance. Until there is better understanding of the correlates of protection, clinical indications for serologic testing in health-care settings are inadequate.
Table 1.
Advantages and disadvantages of diagnostic tests for SARS-CoV-2 infection in patients with COVID-19-like symptoms, according to clinical scenario
| Aim | Advantages | Disadvantages | |
|---|---|---|---|
| Within 2 weeks after symptom onset | |||
| Molecular test (ideally nasopharyngeal or nasal swabs) | To detect viral RNA (preferred test) | Provides the most sensitive and specific means of confirming a clinical diagnosis | Expensive; requires specialised skills and instruments; testing is not at point of need; results can take longer than 24–48 h |
| Antigen rapid detection test (ideally nasopharyngeal or nasal swabs) | To detect viral protein if molecular testing is not available or the results are delayed | Can provide results within 15–20 min; can be done outside of a laboratory setting with minimal training; cheaper and faster to manufacture than molecular tests | Not as sensitive as molecular tests; more difficult to assure quality, especially with self-tests, compared with laboratory-based tests; if a patient tests negative, it is necessary to collect another sample for molecular testing |
| More than 2 weeks after symptom onset | |||
| Molecular test, antigen rapid detection test, and antibody test | To establish a late or retrospective diagnosis by using antibody tests if molecular and antigen rapid tests are both negative | Can provide results in 15–20 min if a rapid antibody test or within 24 h if a laboratory-based assay | Antibody tests can be non-specific and cause false-positive results; can be difficult to determine if seropositivity is vaccine-induced or natural |
| Patient has persistently negative test results but there is a high index of suspicion based on clinical presentation or other criteria (eg, chest CT scan findings) | |||
| Repeat molecular or antigen rapid detection test using a lower respiratory tract specimen (eg, sputum or bronchioalveolar lavage sample, or tracheal aspirate and blood for an antibody test) and antibody test | To confirm a clinical diagnosis | Confirms clinical diagnosis if the lower respiratory tract specimen is positive; enables retrospective diagnosis of past or recent infection if the antibody test is positive | Antibody tests can be non-specific and cause false-positive results |
All three types of test are available as laboratory-based assays and point-of-care or point-of-need tests that can be done by lay health-care providers outside of laboratory settings and with minimal training. No test is perfect. Although sensitivity and specificity are important attributes of a test, achieving a correct diagnosis in a patient also depends on the time of sampling relative to the stage of infection (such as days after symptom onset), the quality of the specimen collection, the proficiency with which the test is done, and correct interpretation of the results. For molecular or antigen tests, the highest sensitivity occurs when viral loads are high, which occurs early on in infection.
Special characteristics of SARS-CoV-2 infection that affect testing strategies
SARS-CoV-2 has several characteristics that make it different to seasonal coronaviruses and SARS-CoV, which has profound implications for testing strategies.
Asymptomatic and presymptomatic populations driving transmission
High concentrations of virus can be detected in the nasal passages of infected individuals regardless of their clinical manifestations.10, 11, 12, 13 SARS-CoV-2 infections are classified as asymptomatic, presymptomatic, or symptomatic. This characteristic means that symptom-based testing alone is not adequate to control the spread of the virus, making community-based testing a priority.14, 15, 16 Of particular concern are health-care workers and people working in residential care homes for people aged 65 years or older, who are at high risk of inadvertently transmitting SARS-CoV-2 infection to their own family members and to those in their care.
Duration of infectiousness
Evidence from 113 studies done in 17 countries shows that SARS-CoV-2 viral RNA can be detected as early as 6 days before symptom onset, concentrations peak around the time of symptom onset or a few days later, and it usually becomes undetectable from upper respiratory tract samples about 2 weeks after symptom onset, and with no substantial differences between adults and children.17 The viral load from lower respiratory tract samples might be higher, peak later, and persist for longer than the load from upper respiratory tract samples.
Studies using viral cultures show that, although patients can remain RNA-positive for weeks after symptom onset, live virus cannot be cultured from specimens collected later than 9 days after symptom onset, suggesting that the mean period of infectiousness and risk of transmission could be restricted to the period between 2 and 3 days before and 8 days after symptom onset.16, 17, 18, 19, 20, 21, 22, 23 RNA-positive culture-negative samples could represent the detection of genomic fragments rather than an actively replicating virus.24
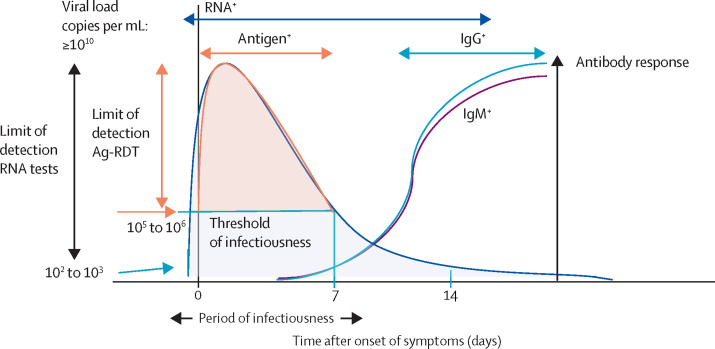
Figure 1 shows a schematic of the viral dynamics of, and antibody response to, SARS-CoV-2 infection in a patient who is symptomatic, and the optimal timeframe for deployment of different types of tests.
Figure 1.
Timelines for optimal use of different diagnostic tests for COVID-19 detection and host response
The optimal timeframe during which molecular and antigen tests can be used for confirming the clinical diagnosis in a patient infected with SARS-CoV-2, based on the lower limits of virus detection for these tests, the dynamics of viral shedding, and the period of infectiousness over the course of infection as reported in the peer-reviewed literature.16, 17, 18, 19, 20, 21, 22, 23, 24 Serology tests to detect host response to infection are usually used 7 days or more after symptom onset to determine exposure or past or recent infection and are primarily used for surveillance. Ag-RDT=antigen rapid detection test.
VOCs and their effect on testing
SARS-CoV-2 is an RNA virus, and RNA viruses are unstable. Mutations arise from errors as viruses replicate in human cells. These mutated viruses are called variants. Although it is believed that most mutations are harmless, occasionally mutation confers a survival advantage to a variant, such as greater transmissibility from host to host. WHO has defined a variant of concern as one that has been shown to be associated with one or more of the following changes at a degree of global public health significance: increase in transmissibility or detrimental change in COVID-19 epidemiology; increase in virulence or change in clinical disease presentation; or decrease in the effectiveness of public health and social measures or available diagnostics, vaccines, and therapeutics.6 The US Centers for Disease Control and Prevention and the European Centre for Disease Prevention and Control have developed similar working definitions for monitoring the emergence of harmful mutations that may be a cause for global or regional concern.7, 8 In vitro studies show that some VOCs are able to escape neutralising antibodies developed from natural infections or vaccination.25, 26, 27
The US Food and Drug Administration (FDA) is monitoring the effectiveness of molecular assays on its Emergency Use Authorization (EUA) list for reduced sensitivity or a negative result owing to VOCs.28 Almost all antigen tests use the SARS-CoV-2 nucleocapsid protein as a target and are therefore less likely to be affected by VOCs. The Program for Technology in Health has a dashboard for tracking whether companies have validated their tests against the VOCs.29
Immune response to SARS-CoV-2 infection
After more than 1 year into the pandemic, our understanding of the immune response to SARS-CoV-2 infection remains incomplete. Current data suggest that both humoral and cellular immune responses occur within 1–2 weeks after onset of symptoms. Humoral immune responses are mediated by antibodies directed to viral surface proteins (mainly the spike and nucleocapsid proteins), whereas the cellular immune responses target a wider repertoire of both structural and non-structural viral proteins.30, 31
Development of both IgM and IgG antibodies after SARS-CoV-2 infection appears to occur earlier than in other viral infections, and peaks at day 11–14 after onset of symptoms.32, 33, 34, 35 IgM and IgG antibodies tend to appear almost simultaneously, unlike many other viral infections, in which IgM antibodies typically appear several weeks earlier than IgG antibodies. The early appearance of antibodies allows the use of IgM antibody tests in combination with molecular testing to increase case detection in people who present late for care and in contact tracing.33, 36
Duration of immunity and the potential for reinfection
Reinfection by a respiratory virus is common, mainly because of waning immunity. COVID-19 reinfection can be defined as the clinical recurrence of symptoms compatible with COVID-19, accompanied by positive PCR test more than 90 days after the onset of the primary infection, supported by close-contact exposure or virus sequencing data to exclude relapse.37 A large population-based study38 in Denmark showed an estimated 80·5% protection against repeat infection over 7 months, with no difference by sex. Among people aged 65 years or older, observed protection against repeat infection decreased to an estimated 47·1% during that period. Until now, there is little evidence that SARS-CoV-2 antibodies confer durable immunity to reinfection. As variants of SARS-CoV-2 emerge, monitoring reinfections is essential.
Neutralising antibodies, vaccines, variants, and correlates of protection
IgG antibodies against the SARS-CoV-2 spike and nucleocapsid proteins are correlated with neutralising activities in vitro.39, 40, 41, 42 However, high amounts of IgG antibodies have been shown in patients who have severe COVID-19 disease, suggesting that a robust IgG response might not be an indicator of protective immunity.39, 40, 41, 42 A neutralising antibody assay is an in vitro measurement of the ability of antibodies to inactivate a pathogen. This assay requires a laboratory in which the pathogen can be safely cultured, and the living organism exposed in vitro to the antibodies detected by the assay. SARS-CoV-2 neutralisation assays can now be done in increasingly safe conditions using pseudo-viruses.43 The development and validation of neutralisation assays to monitor variant strains of SARS-CoV-2, using sera from people who have had natural infection or vaccination, should be a high priority.
A threshold for protective neutralising antibody responses has yet to be defined, and candidate thresholds are likely to be affected by viral variants and viral loads encountered during exposures, among other factors. Furthermore, antibodies are only one component of an effective host response to infection. Cellular immune responses usually have a substantial role in an effective immune response. The correlates of protection against SARS-CoV-2 infection in vivo remain unclear. Hence, antibody testing should not be used to guide decisions about personal or occupational exposures and personal protection.
Vaccine-induced immunity is mainly targeted at the SARS-CoV-2 spike protein. Analyses of sera from individuals with antibodies (either from vaccination with one or two doses of mRNA vaccines or from natural infection) show low neutralisation against the beta (B.1.351) and gamma (P.1) VOCs. This low neutralisation was found to be mediated largely by mutations in the receptor-binding domain of SARS-CoV-2 spike.27 Thus, a positive antibody test result should not be used as proof of immunity, because in addition to the absence of consensus on how to quantify protection from natural or vaccine-induced immunity, there could be reduced protection against some VOCs that have become the dominant circulating strain in many countries. This fact places in doubt the value of commercially available immunity passports.44, 45
The evolving role of diagnostic testing in the COVID-19 pandemic response
At the start of the pandemic, testing of symptomatic patients was crucial in refining the clinical case definition, confirming clinical diagnosis for patient management, and doing epidemiological studies to understand the speed and extent of the transmission to inform public health control measures. Identification of COVID-19 cases permits contact tracing and research into SARS-CoV-2 modes of transmission. Because molecular tests are based on genome sequences of SARS-CoV-2, they are highly sensitive and specific and are used as the reference standard for the diagnosis of active SARS-CoV-2 infection.46, 47, 48
The second phase in the evolution of the SARS-CoV-2 testing strategy came several months into the pandemic, when it was recognised that more than 20% of virus transmission could be attributed to individuals who were asymptomatic or presymptomatic.12, 13, 14, 15, 16 Control programmes began to develop strategies to interrupt the chains of transmission within communities by scaling up testing, contact tracing, and isolation.49, 50, 51, 52, 53, 54 However, most countries found it more difficult than anticipated to scale up laboratory molecular testing, owing to shortages of trained staff, global competition for reagents, and high costs. It was hoped that point-of-care molecular technology platforms developed for HIV and tuberculosis and widely deployed outside of laboratory settings could be rapidly adapted to test for SARS-CoV-2. Although such point-of-care molecular tests were rapidly developed and validated, their swift deployment never became a reality because the rate-limiting step was the speed of manufacturing of both test instruments and test cartridges.
Antigen tests are available as high-throughput laboratory-based assays or as rapid detection tests (Ag-RDTs) that can be visually read or instrument-read.2, 4, 5 Ag-RDTs are more affordable and easier to use than molecular tests and can provide results within 15–20 min. Unlike molecular tests, Ag-RDTs can be manufactured as single-use lateral-flow tests in the millions per month, making it easier for companies to meet global demand.
Although Ag-RDTs have a lower limit of detection of only 105–106 genome copies per mL compared with 102–103 copies per mL for molecular tests, studies have shown that individuals with a viral load less than 106 genome copies per mL are unlikely to transmit the virus, making Ag-RDTs a useful rapid triage tool to identify those most likely to transmit infection.55, 56, 57, 58
To qualify for WHO Emergency Use Authorization listing, WHO recommends, as a minimum, test sensitivity of 80% and specificity of 97% for Ag-RDTs when compared with a molecular test.52 For patients presenting with COVID-19-like symptoms in settings in which molecular testing is unavailable or results are delayed by more than 48–72 h owing to a high volume of testing, WHO and US Centres for Disease Control and Prevention agencies recommend the use of Ag-RDTs as an alternative means of case detection, enabling public health measures such as isolation and contact tracing to be implemented without delay and the interruption of further disease transmission.52, 59, 60, 61
The third phase in the role of diagnostics in the pandemic response came as the waves of SARS-CoV-2 infection came under increased control and countries began to try to find ways to reopen schools, businesses, and workplaces, and to reintroduce religious gatherings and social, cultural, and sports events. Tedros Adhanom Ghebreyesus, Director-General of WHO, said, “High-quality rapid tests…are key to quickly tracing and isolating contacts and breaking the chains of transmission. The tests are a critical tool for governments as they look to reopen economies and ultimately save both lives and livelihoods.”62 This statement calls for testing to be used as both a public health and a clinical diagnostic tool.
The FDA has approved several home-based or self-tests for use along with molecular tests and Ag-RDTs.5 Self-testing can be used to help to ensure a safe environment in settings where patrons or clients are likely to be unmasked, such as gyms and fitness studios, food and beverage establishments, and beauty and wellness salons. Such tests could help to protect patients and residents from infection when there are visitors to public hospital wards or residential care homes.
Countries that are able to roll out COVID-19 vaccinations are now entering a fourth phase, transitioning from a pandemic response mode to living with the virus, whereby the main role of testing will shift from diagnosis and case detection to surveillance.
Use of diagnostics in clinical practice and public health
Diagnostic tests are useful to confirm the clinical diagnosis in patients presenting with symptoms consistent with COVID-19, regardless of their vaccination status.46 Infected individuals can present with mild to severe symptoms of the infection, such as fever or chills, a persistent cough, shortness of breath, and headaches, and these symptoms typically occur 2–14 days after initial exposure.
For individuals presenting within the first 2 weeks of onset of symptoms that are consistent with COVID-19, a specimen should be collected for molecular testing to confirm the clinical diagnosis.46 Although the primary site of infection is in the lungs, different specimen types have been used with molecular and antigen tests, depending on viral load at the site of collection, the ease and feasibility of specimen collection with regard to the setting, and the age of the patient. A systematic review63 showed that the best specimens are nasopharyngeal or nasal swabs; oral fluid or oropharyngeal swabs have been validated for some tests, but they can be less sensitive. Some studies report prolonged shedding from stool samples, but SARS-Cov-2 is rarely found in blood or urine.64
Given the high sensitivity and high specificity of molecular tests, false-positive or false-negative test results are rare. A positive result confirms the diagnosis and should trigger patient management procedures and public health measures such as self-isolation and contact tracing. Although studies have shown that a positive molecular test cannot be interpreted to mean that the patient is infectious, public health measures should be carried out nevertheless.46 However, for patients in whom the clinical suspicion of COVID-19 is high but the test results are negative, there is a possibility that the specimen was inadequate or an error was made in the testing, in which case the test should be repeated either with another nasopharyngeal swab or a sample from the lower respiratory tract (eg, sputum, bronchoalveolar lavage, or tracheal aspirate), if possible.46, 47, 48 While waiting for test results, the patient should be kept in isolation as a precaution. A positive result on repeat testing confirms COVID-19 and should be managed accordingly.
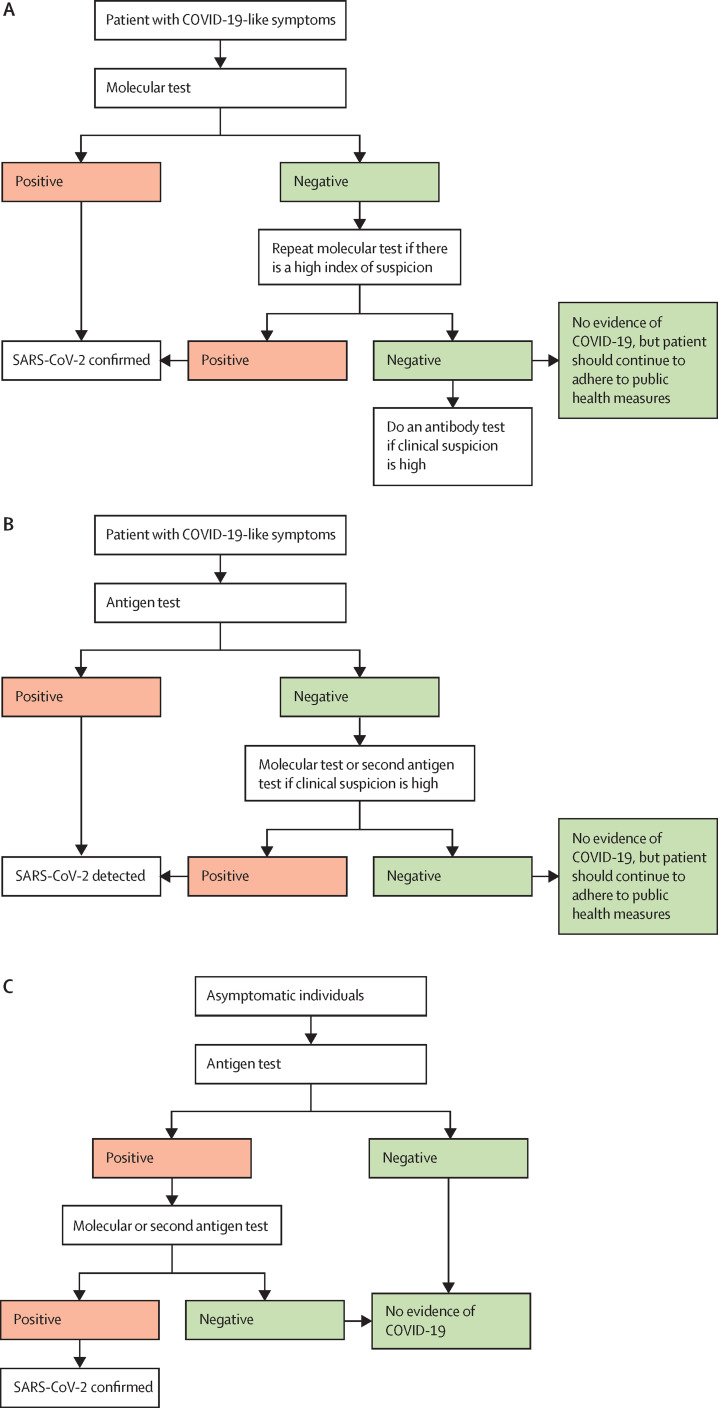
If there is sustained clinical suspicion owing to an epidemiological link and other clinical or radiological findings, but the repeat molecular testing is negative, the patient could be further evaluated using an antibody test, but only for the purpose of documenting retrospectively a recent infection with SARS-CoV-2. Figure 2A shows a diagnostic algorithm for the use of tests in a patient presenting with COVID-19-like symptoms.
Figure 2.
Algorithms for testing to detect SARS-CoV-2 in symptomatic and asymptomatic individuals
(A) Preferred testing algorithm for individuals with COVID-19-like symptoms. (B) Testing algorithm for individuals with COVID-19-like symptoms when molecular testing is not available or results are delayed. (C) Testing algorithm for COVID-19 case finding among asymptomatic individuals.
If individuals with symptoms of COVID-19 present late (ie, 7–14 days after symptom onset), combining the use of molecular, antigen-detection, and antibody tests should be considered. Studies in China33 suggest that combining molecular and antibody testing in the second week after symptom onset can increase the rate of COVID-19 case detection by as much as 40%.
If molecular testing is not feasible, or if there are delays in reporting the results, rapid antigen testing is recommended by WHO,46 Africa Centres for Disease Control and Prevention,60 and the European Centre for Disease Prevention and Control guidelines.59 Because an individual with symptoms consistent with COVID-19 has a high pretest probability of testing positive, a confirmatory test would not be required for an Ag-RDT-positive result (figure 2B). In countries in which there are adequate resources, and time to result is not an issue, positive antigen test results are confirmed using a molecular test. Table 2 summarises possible interpretations of test results.65
Table 2.
Possible interpretations of SARS-CoV-2 diagnostic test results in patients with COVID-19-like symptoms
| Actions required | |
|---|---|
| Molecular test (eg, PCR) result is positive | |
| True-positive test result | Manage patient and initiate contact tracing and isolation of patient |
| Indeterminate test result, because not all gene targets are positive | Repeat the test or use a different assay to confirm whether a variant of concern is involved |
| False-positive test result, caused by laboratory contamination, or incorrect interpretation | If infection is considered to be unlikely, check the proficiency of the testing personnel and the quality management of the laboratory |
| Molecular test (eg, PCR) result is negative | |
| True-negative test result | No action needed |
| False-negative test result, caused by a past viremic period | If clinical suspicion is high, use an antibody test to check for previous exposure to SARS-CoV-2 |
| False-negative test result, caused by low viral load, specimen not being collected properly, or test not being done correctly | If clinical suspicion is high, check collection technique, quality of test, and retest |
| False-negative test result, caused by the test not detecting a virus variant owing to gene target mutations in the target region | If clinical suspicion is high and the virus variant is widespread, use a test that targets multiple genes |
| Antigen rapid diagnostic test result is positive | |
| True-positive test result | Manage patient and initiate contact tracing and isolation of patient |
| False-positive result, caused by test result being read incorrectly or low pretest probability (disease prevalence) | If infection is considered to be unlikely, confirm test results with a molecular test or a repeat antigen rapid diagnostic test |
| Antigen rapid diagnostic test result is negative | |
| True-negative test result | No action needed |
| False-negative test result, caused by low sensitivity, specimen not being collected properly, or test not being done correctly | Check for quality of specimen collection and rectify; check the sensitivity, or quality, or both, of the test; if there is a high suspicion of infection, retest using another antigenic rapid diagnostic test of higher specificity or a molecular test |
However, it is possible that an individual could have COVID-19, but has an antigen-negative test result because of low viral load, inadequate sample collection, or errors during the testing process. If there is high clinical suspicion of COVID-19 or known exposure, and the antigen test is negative, a second sample should carefully be collected and either sent for molecular testing or tested with an antigen test of higher sensitivity. The patient should be kept in isolation until the test results are available. The European Centre for Disease Prevention and Control has issued guidance for discharge and the ending of isolation of patients with COVID-19.66
This algorithm has been implemented in Cameroon to mitigate the risk of missing cases in patients who have low viral loads, because symptomatic patients are first tested with Ag-RDTs.67 Of 80 000 COVID-19 cases reported up to now in Cameroon, 60% were diagnosed using Ag-RDTs. Such a national algorithm has made widespread testing possible, with substantial savings compared with a programme that relies solely on molecular tests.
Use of diagnostics in containment of pandemics and outbreaks
Case finding in symptomatic or asymptomatic individuals
Identification of people in the community who are infected is an integral element of outbreak management. Case finding can be passive or active. Passive case finding relies on individuals with signs and symptoms recognising these as being associated with infection, and self-reporting to health workers. Active case finding is a systematic search for individuals with infection and, of those found, all those who have signs and symptoms are proactively tested. Other forms of active case finding include mass population screening and targeted screening of people living or working in an area in which there is known transmission and, if linked with geographical information, increase understanding of the extent of virus spread and of the locations with a high risk of transmission.
Households and other contacts of confirmed cases
A meta-analysis of 54 studies (77 758 people infected with SARS-CoV-2)67 estimated an overall secondary-attack rate of 16·6% in households, which is higher than for household transmission rates observed for SARS-CoV and MERS-CoV. Secondary-attack rates were higher in households with symptomatic index cases than asymptomatic ones, higher in adult contacts than in children, and higher in spouses than in non-spouses. A comprehensive contact-tracing programme with testing and effective isolation or quarantine is crucial for successful outbreak control.68, 69
Screening of populations at increased risk of acquisition and transmission
Health-care workers, and workers in residential care homes for people aged 65 years or older, essential front-line workers (including first responders), and public transport and aviation transport operators are at increased risk of COVID-19 acquisition and transmission. Routine asymptomatic testing for COVID-19 in these populations is a crucial component of effective targeted control strategies. Frequent testing and rapid turnaround times of test results could yield a high probability of the early detection of infections, and hence the prevention of outbreaks, in at-risk settings.70, 71, 72, 73, 74
Epidemiological modelling75, 76, 77 suggested that the effectiveness of outbreak control depends largely on the frequency of testing and the speed of reporting, and is only marginally improved by high test sensitivity. Results from a modelling study78 to assess the effects of test sensitivity, testing frequency, and speed of reporting on SARS-Cov-2 case detection suggested that testing frequency might need to be twice a week to ensure a safe workplace in high-risk health environments. In the authors' sensitivity analysis, only small changes in results were observed with variations in test sensitivity, but large changes were seen with variations in the delay in reporting test results. With a reproductive rate of 1·5, reducing test sensitivity by 20% reduced the effectiveness of daily testing from 85·3% to 80·7%. Test result delays of 3 days reduced daily testing effectiveness from 85·3% to 56·5%, and delays of 5 days reduced it to 25·9%. Rapid antigen tests should therefore be considered if time to reporting the results of molecular tests is suboptimal.
Testing as a public health tool to ensure safe environments and enable economic recovery
Lockdowns and border closures impose mental, social, and financial hardships in many societies, especially in informal urban settlements.79 Testing could enable resumption of social activities and economic recovery, and many strategies are being piloted and evaluated at present.80, 81, 82 One strategy is to target people attending schools or workplaces in which prolonged daily indoor contact occurs. Another is to target people attending large gatherings in indoor spaces (eg, nightclubs, bars, and karaoke lounges) and indoor or outdoor mass gatherings for religious, sports, music, or other purposes. The objective of screening in these settings is to ensure a safe environment, so it is desirable to use a test that can give almost immediate results, with a high negative predictive value.82, 83, 84, 85, 86 Decisions about easing mass gathering restrictions should be based on the extent of infection in the community and the degree of vaccination coverage in the population. Pre-event testing is usually done in combination with other public health measures as layered interventions to reduce risk of transmission (eg, the wearing of face masks, physical distancing, and adequate air ventilation in the venue; table 3 ).
Table 3.
Examples of testing for SARS-CoV-2 infection as a public health tool, by setting
| Public health measure | Who to screen | Frequency of screening | Test to use | Action to take in response to test results | |
|---|---|---|---|---|---|
| Community testing of fixed cohorts of people in regular or daily contact with each other | |||||
| Health-care facility48, 51, 59, 66, 87, 88 | Face masks, ventilation, and (if possible) physical distancing; implement flexible, non-punitive, paid sick leave and supportive employment policies and practices | Health-care workers and workers in residential care homes for people aged 65 years or older | Twice a week | Molecular test if possible, otherwise an Ag-RDT | If the test is positive, isolate and initiate contact tracing |
| School6, 46, 70 | Face masks, physical distancing, ventilation, and, if possible, moving activities outdoors | Teachers, students, other school staff, and ancillary workers | Frequency depends on COVID-19 prevalence within the community | Ag-RDT; confirm positive test results using a molecular test | If the test is positive, initiate contact tracing and send close contacts home for self-isolation |
| Workplace59, 60, 80 | .. | All staff | Once or twice a week, depending on community prevalence | Ag-RDT; confirm positive test results using a molecular test | Allow entry if the test result is negative; stay at or work from home if possible |
| Mass gatherings of random cohorts who gather at sporadic events | |||||
| Music event84 | Face masks and ventilation | All at entry | Once, at entry | Ag-RDT | Allow entry into event if the test result is negative; confirm positive results using a molecular test |
| Religious gathering85 | Face masks, physical distancing, and limit event size to small groups of 20 accompanied by a health worker | All at entry and on departure | Twice a week for multiday events | Molecular test pre-entry and Ag-RDT at event | Allow entry into event if the test result is negative; confirm positive results using a molecular test |
| Sports event86 | Face masks and physical distancing | Players, staff, and (at entry) spectators | Once at entry; no screening during event | Molecular test (if possible, otherwise an Ag-RDT) for players; Ag-RDT for staff and spectators; confirm positive test results using a molecular test | Stop event if players test positive and initiate contact tracing; if staff or spectators test positive, do not allow them to enter the venue and initiate contact tracing |
Ag-RDT=antigen rapid diagnostic test.
With the low pre-test probability of COVID-19 in the general population, a screening test with 80% sensitivity and 97% specificity will probably generate more false-positive than true-positive results compared with a molecular test. It is important to confirm any positive results (table 3, figure 2B, C).59, 60, 61, 65, 82 The confirmatory test can be a molecular test, if available, or an antigen test of higher specificity.
Some countries have issued guidelines for the prevention of SARS-CoV-2 transmission in school children; testing is generally not recommended unless COVID-19 incidence in the community is high.89, 90 For college campuses, an analytical model91 comparing the effectiveness and cost of using different types of testing for screening on a college campus suggested that screening every 2 days using a rapid, inexpensive, and even low-sensitivity test (>70%), coupled with strict behavioural interventions to keep the reproductive rate less than 2·5, could maintain a controllable number of COVID-19 infections and permit the safe return of students to campus.
Testing travellers to reduce the risk of importing COVID-19
The WHO International Health Regulations urge countries to ensure that measures affecting international traffic (including targeted use of diagnostics and quarantine) are risk-based, evidence-based, coherent, proportionate, and time-limited.87
Many countries require travellers to present proof of a negative antigenic or molecular test result 72 h ahead of a flight, to quarantine after arrival, and to test negative with either a molecular test or Ag-RDT for release.92 Although models of strategies to mitigate the importation risk have been published,93, 94, 95 there is no global or regional consensus on any one risk-reduction strategy.
The latest guidelines from the European Centre for Disease Prevention and Control recommend that fully vaccinated individuals, or those who have recovered from the disease in the past 180 days, should not need to test or quarantine, unless they are coming from an area of very high risk or in which a VOC is circulating.88
As VOCs continue to emerge, it is anticipated that testing strategies for travel will continue to change on the basis of the transmissbility and virulence of VOCs, vaccine coverage, and risk tolerance in different countries.
Transition from pandemic response to living with the virus
As countries move from pandemic response to the control of COVID-19, vaccines will have a big role in protecting individuals and populations at risk of infection. Vaccines were studied in clinical trials for their efficacy in protecting against severe disease and death, and information in post-vaccination surveillance activities suggests that breakthrough infections are occurring as a result of waning immunity or inadequate protective response.96, 97, 98 Although some countries have adopted strategies to live with the virus, testing will continue to be a crucial tool in identifying the breakthrough infections and informing patient care and public policy through surveillance.99, 100
Vaccine passports (or immunity passports) to help ensure safer travel, workplaces, schools, and mass gatherings are being considered by some countries, US states, and businesses, but inadequate understanding about the ability of vaccines to decrease transmission, and about the duration and correlates of immunity, make it difficult to understand the effectiveness of such passports. The development and validation of neutralisation assays to monitor variant strains of SARS-CoV-2, using sera from people who have had natural infection or vaccination, should be a high priority.
Enabling factors to maximise the impact of testing
Clear and coherent messaging to the public about testing
Communication to the public about evolving testing strategies needs to be clear, science-based, systematic, and easily understandable enough to be able to penetrate equitably to all people, including migrant workers, displaced people and refugees, people with minority ethnic backgrounds, and Indigenous people (providing translations as necessary). Likewise, clear communication is needed about symptoms associated with COVID-19, the importance of being tested if those symptoms develop, where to call or to go if testing is required, what to do if the test result is positive or if the person is still waiting for the results, what to expect from activities such as contact tracing, and what it means to have a negative test result, depending on the current epidemiological situation.
Information systems for recording and accessing diagnostic test results
Use of diagnostic tests to facilitate the return to social and economic activity requires an information system that allows capture of test results and access to them. Some Ag-RDT and home tests come with apps that allow automated reading and digitisation of test results as QR codes for display if proof of a negative test is required. Such systems should verify their legitimacy by accepting results only from accredited testing facilities, and must ensure trust, security, and confidentiality.
On a population level, investment in information technology has enabled electronic contact tracing, which ideally produces data that are directly interoperable with electronic dashboards designed to display real-time data and rapid geographical summaries of infection caseloads.101, 102 Visual images of the geographical distribution of cases and VOCs, the speed and extent of transmission, and the location of hotspots have been crucial in allowing clinicians to interpret test results on the basis of local epidemiology, policy makers to track the effectiveness of control strategies over time, and the public to participate in the public health response.
Post pandemic investments to increase capacity for diagnostic testing, coupled with information systems, should be used to build sustainable diagnostic and surveillance systems. Such systems will serve as the backbone of a health system, with data connectivity and appropriate technologies at every level, from ultra-sophisticated detection and sequencing technology at the top, to point-of-care diagnostics in the community. Diagnostic tests serve as the eyes and ears of the health-care system, by sounding alarms about unusual disease patterns or sending early outbreak alerts, and provide the capacity to respond rapidly. No one is safe until we are all safe needs to be a nationally and internationally accepted slogan that leads to the development and sustainability of actions necessary to detect and respond to outbreaks.
Global surveillance and genotyping
VOCs will continue to develop as SARS-CoV-2 continues to reproduce in human populations. Hence, scaling up testing and public health measures to slow viral replication and the rapid spread of VOCs should be a major public health goal. Strengthening global collaboration in both surveillance and building laboratory genomic-sequencing capacity is urgently needed to track the spread of variants and understand the resulting risks.103, 104, 105
A global surveillance network for SARS-CoV-2 and other coronaviruses, similar to the one established for influenza, would provide the information necessary to assess escape from protection and also provide early alerts should other coronaviruses enter human populations.
Conclusion
The COVID-19 pandemic has spurred the development of a wide selection of diagnostic tests. The choice of which test to use in what setting requires careful consideration of the purpose of testing and the resources available, while also balancing test characteristics of accuracy, accessibility, affordability, and the rapidity with which results are needed. For COVID-19 case detection, molecular tests with their high sensitivity and high specificity are the test of choice. For screening of asymptomatic infections in communities to interrupt the chain of transmission, test sensitivity might be a secondary consideration to frequency of testing and time to result. Testing strategies have evolved with different phases of the pandemic. Although a mainstay for patient diagnosis and management, testing has also been used at an unprecedented scale in settings outside health care, for screening to protect the clinically vulnerable, at border crossings to release people from quarantine, and in communities to enable safe environments for the resumption of economic recovery and social and cultural activities. The pandemic should be a wake-up call for countries to invest in a diagnostic and surveillance system that is the backbone of a health-care system with appropriate technologies at every level, and also invest in data connectivity, so that clinicians and policy makers have increased tools at their disposal to practise precision medicine and so that early alerts of possible outbreaks are rapidly investigated. Strategies for testing should also be developed to contribute to global surveillance of SARS-CoV-2 genetic sequences, and to assure linkages that will rapidly identify changes in transmissibility, or virulence, or both, on a global scale.
Search strategy and selection criteria
We searched MEDLINE on Dec 20, 2020, Current Contents on March 31, 2021, and PubMed on June 30, 2021, and references from relevant articles on Sept 22, 2021 for clinical diagnostic tests for COVID-19 using the search terms “SARS-CoV-2”, “COVID”, “tests”, and “diagnostics” for articles published in English between Jan 1, 2019 and Sept 30, 2021. We included abstracts and reports from meetings only if they related directly to previously published work. We found 1828 articles related to clinical diagnostic tests for COVID-19. We included articles if they provided evidence to support the use of COVID-19 tests in the pandemic response to confirm clinical diagnosis in patients who were symptomatic or for screening asymptomatic individuals for SARS-CoV-2 to interrupt the chain of transmission in communities. We also included articles on how test performance can be affected by variants of concern or vaccinations.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
The authors thank Vanessa Tran (Public Health Ontario, Canada) for reviewing the manuscript and giving very helpful advice about clinical algorithms and explaining COVID-19 test results to patients.
Contributors
RWP conceived the paper and drafted the outline, tables, figures, and section on test characteristics and usage cases, DLH drafted the introduction and the section on key diagnostic tests for patient management and pandemic control, Y-YT drafted the section on the use of diagnostics in the containment of pandemics and outbreaks, PJG drafted the section on the use of diagnostics in clinical practice and public health, and RWP collated the sections. All authors contributed to the revisions and finalisation of the manuscript.
References
- 1.WHO WHO Director-General's opening remarks at the media briefing on COVID-19—16 March 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—16-march-2020
- 2.FIND Rapid diagnostic tests for COVID-19. May 18, 2020. https://www.finddx.org/wp-content/uploads/2020/05/FIND_COVID-19_RDTs_18.05.2020.pdf
- 3.Nkengasong J. Let Africa into the market for COVID-19 diagnostics. Nature. 2020;580:565. doi: 10.1038/d41586-020-01265-0. [DOI] [PubMed] [Google Scholar]
- 4.WHO Emergency use listing of in-vitro diagnostics. 2020. https://www.who.int/teams/regulation-prequalification/eul/in-vitro-emergency-use-listing-procedure
- 5.US Food and Drug Administration In vitro diagnostics EUAs. Nov 29, 2021. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas
- 6.WHO Tracking SARS-CoV-2 variants. Nov 30, 2021. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 7.European Centre for Disease Prevention and Control SARS-CoV-2 variants of concern as of Aug 26 2021. Aug 26, 2021. https://www.ecdc.europa.eu/en/covid-19/variants-concern
- 8.US Centers for Disease Control and Prevention SARS-CoV-2 variant classifications and definitions. Aug 31, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html
- 9.Berger L, Berger N, Bosetti V, et al. Rational policymaking during a pandemic. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2012704118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beale S, Hayward A, Shallcross L, Aldridge RW, Fragaszy E. A rapid review and meta-analysis of the asymptomatic proportion of PCR-confirmed SARS-CoV-2 infections in community settings. medRxiv. 2020 doi: 10.1101/2020.05.20.20108183. published online Oct 14. (preprint). [DOI] [Google Scholar]
- 11.Yanes-Lane M, Winters N, Fregonese F, et al. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almadhi MA, Abdulrahman A, Sharaf SA, et al. The high prevalence of asymptomatic SARS-CoV-2 infection reveals the silent spread of COVID-19. Int J Infect Dis. 2021;105:656–661. doi: 10.1016/j.ijid.2021.02.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furukawa NW, Brooks JT, Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2607.201595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles' heel of current strategies to control COVID-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health Information and Quality Authority Evidence summary for the duration of infectiousness in those that test positive for SARS-CoV-2 RNA. Sept 15, 2020. https://www.hiqa.ie/sites/default/files/2020-04/Evidence-Summary_COVID-19_duration-of-infectivity-viral-load.pdf
- 18.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 19.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 20.Bullard J, Dust K, Funk D, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis. 2020;71:2663–2666. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh KA, Jordan K, Clyne B, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81:357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. 2020;371 doi: 10.1136/bmj.m3862. [DOI] [PubMed] [Google Scholar]
- 23.Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2021;174:69–79. doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexandersen S, Chamings A, Bhatta TR. SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nat Commun. 2020;11 doi: 10.1038/s41467-020-19883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell F, Archer B, Laurenson-Schafer H, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Beltran WF, Lam EC, St Denis K, et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2372. doi: 10.1016/j.cell.2021.03.013. 83.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration SARS-CoV-2 viral mutations: impact on COVID-19 tests. Sept 23, 2021. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests
- 29.Agarwal N, Leader T, Lillis L. Do COVID-19 tests still work against delta and other variants? April 1, 2021. https://www.path.org/articles/new-variants-will-covid-19-tests-still-work/
- 30.Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Röltgen K, Boyd SD. Antibody and B cell responses to SARS-CoV-2 infection and vaccination. Cell Host Microbe. 2021;29:1063–1075. doi: 10.1016/j.chom.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Health Information and Quality Authority Evidence summary of the immune response following infection with SARSCoV-2 or other human coronaviruses. Aug 6, 2020. https://www.hiqa.ie/sites/default/files/2020-08/Evidence-summary_SARS-CoV-2-immune-response.pdf
- 33.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71:2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long Q-X, Liu B-Z, Deng H-J, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 35.Qu J, Wu C, Li X, et al. Profile of immunoglobulin G and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:2255–2258. doi: 10.1093/cid/ciaa489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peeling RW, Wedderburn CJ, Garcia PJ, et al. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. 2020;20:e245–e249. doi: 10.1016/S1473-3099(20)30517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yahav D, Yelin D, Eckerle I, et al. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin Microbiol Infect. 2021;27:315–318. doi: 10.1016/j.cmi.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11 doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Caeseele P, Bailey D, Forgie SE, et al. SARS-CoV-2 (COVID-19) serology: implications for clinical practice, laboratory medicine and public health. CMAJ. 2020;192:E973–E979. doi: 10.1503/cmaj.201588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nie J, Li Q, Wu J, et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat Protoc. 2020;15:3699–3715. doi: 10.1038/s41596-020-0394-5. [DOI] [PubMed] [Google Scholar]
- 44.Altmann DM, Douek DC, Boyton RJ. What policy makers need to know about COVID-19 protective immunity. Lancet. 2020;395:1527–1529. doi: 10.1016/S0140-6736(20)30985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phelan AL. COVID-19 immunity passports and vaccination certificates: scientific, equitable, and legal challenges. Lancet. 2020;395:1595–1598. doi: 10.1016/S0140-6736(20)31034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO Recommendations for national SARS-CoV-2 testing strategies and diagnostic capacities. Interim guidance. June 25, 2021. https://apps.who.int/iris/bitstream/handle/10665/342002/WHO-2019-nCoV-lab-testing-2021.1-eng.pdf?sequence=1&isAllowed=yoV-lab-testing-2021.1
- 47.Infectious Disease Society of America IDSA guidelines on the diagnosis of COVID-19: molecular diagnostic testing. Dec 23, 2020. https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/
- 48.European Centre for Disease Prevention and Control Diagnostic testing and screening for SARS-CoV-2. May 21, 2021. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/diagnostic-testing
- 49.Girum T, Lentiro K, Geremew M, Migora B, Shewamare S. Global strategies and effectiveness for COVID-19 prevention through contact tracing, screening, quarantine, and isolation: a systematic review. Trop Med Health. 2020;48:91. doi: 10.1186/s41182-020-00285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nussbaumer-Streit B, Mayr V, Dobrescu AI, et al. Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review. Cochrane Database Syst Rev. 2020;4 doi: 10.1002/14651858.CD013574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.European Centre for Disease Prevention and Control Guidelines for the implementation of non-pharmaceutical interventions against COVID-19. Sept 24, 2020. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-guidelines-non-pharmaceutical-interventions-september-2020.pdf
- 52.Contreras S, Dehning J, Loidolt M, et al. The challenges of containing SARS-CoV-2 via test-trace-and-isolate. Nat Commun. 2021;12:378. doi: 10.1038/s41467-020-20699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kucharski AJ, Klepac P, Conlan AJK, et al. Effectiveness of isolation, testing, contact tracing, and physical distancing on reducing transmission of SARS-CoV-2 in different settings: a mathematical modelling study. Lancet Infect Dis. 2020;20:1151–1160. doi: 10.1016/S1473-3099(20)30457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.European Data Portal Widespread testing: differing strategies across Europe. https://www.europeandataportal.eu/en/impact-studies/covid-19/widespread-testing-differing-strategies-across-europe
- 55.Leber W, Lammel O, Siebenhofer A, Redlberger-Fritz M, Panovska-Griffiths J, Czypionka T. Comparing the diagnostic accuracy of point-of-care lateral flow antigen testing for SARS-CoV-2 with RT-PCR in primary care (REAP-2) EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohmer N, Toptan T, Pallas C, et al. The comparative clinical performance of four SARS-CoV-2 rapid antigen tests and their correlation to infectivity in vitro. J Clin Med. 2021;10:328. doi: 10.3390/jcm10020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanser L, BellmannWeiler R, Öttl K, et al. Evaluating the clinical utility and sensitivity of SARSCoV2 antigen testing in relation to RTPCR Ct values. Infection. 2021;49:555–557. doi: 10.1007/s15010-020-01542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porte L, Legarraga P, Vollrath V, et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int J Infect Dis. 2020;99:328–333. doi: 10.1016/j.ijid.2020.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.European Centre for Disease Prevention and Control Options for the use of rapid antigen tests for COVID-19 in the EU/EEA - first update. Oct 26, 2021. https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-first-update
- 60.African Union and Africa Centres for Disease Control and Prevention Interim guidance on the use of rapid antigen tests for COVID-19 response. Dec 16, 2020. https://africacdc.org/download/interim-guidance-on-the-use-of-rapid-antigen-tests-for-covid-19-response/
- 61.Canadian Public Health Ontario Molecular point-of-care testing guidance (eg, ID NOW™, GeneXpert®**) Aug 25, 2021. https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/point_of_care_testing_use_case_guidance.pdf
- 62.WHO Global partnership to make available 120 million affordable, quality COVID-19 rapid tests for low- and middle-income countries. Sept 28, 2020. https://www.who.int/news/item/28-09-2020-global-partnership-to-make-available-120-million-affordable-quality-covid-19-rapid-tests-for-low--and-middle-income-countries
- 63.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bastos ML, Perlman-Arrow S, Menzies D, Campbell JR. The sensitivity and costs of testing for SARS-CoV-2 infection with saliva versus nasopharyngeal swabs: a systematic review and meta-analysis. Ann Intern Med. 2021;174:501–510. doi: 10.7326/M20-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watson J, Whiting PF, Brush JE. Interpreting a COVID-19 test result. BMJ. 2020;369 doi: 10.1136/bmj.m1808. [DOI] [PubMed] [Google Scholar]
- 66.European Centre for Disease Prevention and Control Guidance for discharge and ending isolation of people with COVID-19. Oct 16, 2020. https://www.ecdc.europa.eu/en/publications-data/covid-19-guidance-discharge-and-ending-isolation
- 67.Boum Y, Fai KN, Nikolay B, et al. Performance and operational feasibility of antigen and antibody rapid diagnostic tests for COVID-19 in symptomatic and asymptomatic patients in Cameroon: a clinical, prospective, diagnostic accuracy study. Lancet Infect Dis. 2021;21:1089–1096. doi: 10.1016/S1473-3099(21)00132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yong SEF, Anderson DE, Wei WE, et al. Connecting clusters of COVID-19: an epidemiological and serological investigation. Lancet Infect Dis. 2020;20:809–815. doi: 10.1016/S1473-3099(20)30273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Madewell ZJ, Yang Y, Longini IM, Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.WHO . WHO; Sept 17, 2020. Keep health workers safe to keep patients safe.https://www.who.int/news/item/17-09-2020-keep-health-workers-safe-to-keep-patients-safe-who [Google Scholar]
- 71.Lynch P. ECCVID 2020: study shows 40% of healthcare workers with COVID-19 were asymptomatic. Sep 23, 2020. https://www.univadis.co.uk/viewarticle/eccvid-2020-study-shows-40-of-healthcare-workers-with-covid-19-were-asymptomatic-729667
- 72.McGarry BE, SteelFisher GK, Grabowski DC, Barnett ML. COVID-19 test result turnaround time for residents and staff in US nursing homes. JAMA Intern Med. 2021;181:556–559. doi: 10.1001/jamainternmed.2020.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bryant KA, Isaacs P. Rapid testing of healthcare employees for COVID-19: what can we learn from the Seattle experience? Clin Infect Dis. 2020;71:2708–2709. doi: 10.1093/cid/ciaa909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet. 2020;395:1418–1420. doi: 10.1016/S0140-6736(20)30917-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hellewell J, Russell TW, Beale R, et al. Estimating the effectiveness of routine asymptomatic PCR testing at different frequencies for the detection of SARS-CoV-2 infections. BMC Med. 2021;19:106. doi: 10.1186/s12916-021-01982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7 doi: 10.1126/sciadv.abd5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mina MJ, Parker R, Larremore DB. Rethinking COVID-19 test sensitivity—a strategy for containment. N Eng J Med. 2020;383:e120. doi: 10.1056/NEJMp2025631. [DOI] [PubMed] [Google Scholar]
- 78.Chin ET, Huynh BQ, Chapman LAC, Murrill M, Basu S, Lo NC. Frequency of routine testing for coronavirus disease 2019 (COVID-19) in high-risk healthcare environments to reduce outbreaks. Clin Infect Dis. 2021;73:e3127–e3129. doi: 10.1093/cid/ciaa1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wamoyi J, Ranganathan M, Stöckl H. COVID-19 social distancing measures and informal urban settlements. Bull World Health Organ. 2021;99:475–476. doi: 10.2471/BLT.20.265942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Organisation for Economic Cooperation and Development Testing for COVID-19: a way to lift confinement restrictions. May 4, 2020. https://www.oecd.org/coronavirus/policy-responses/testing-for-covid-19-a-way-to-lift-confinement-restrictions-89756248/
- 81.Han E, Tan MMJ, Turk E, et al. Lessons learnt from easing COVID-19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. Lancet. 2020;396:1525–1534. doi: 10.1016/S0140-6736(20)32007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peeling RW, Olliaro PL, Boeras DI, Fongwen N. Scaling up COVD-19 rapid antigen tests: promises and challenges. Lancet Infect Dis. 2021;21:e290–e295. doi: 10.1016/S1473-3099(21)00048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fearon E, Buchan IE, Das R, et al. SARS-CoV-2 antigen testing: weighing the false positives against the costs of failing to control transmission. Lancet Respir Med. 2021;9:685–687. doi: 10.1016/S2213-2600(21)00234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Revollo B, Blanco I, Soler P, et al. Same-day SARS-CoV-2 antigen test screening in an indoor mass-gathering live music event: a randomised controlled trial. Lancet Infect Dis. 2021;21:1365–1372. doi: 10.1016/S1473-3099(21)00268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashim HT, Babar MS, Essar MY, Ramadhan MA, Ahmad S. The Hajj and COVID-19: how the pandemic shaped the world's largest religious gathering. Am J Trop Med Hyg. 2021;104:797–799. doi: 10.4269/ajtmh.20-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dergaa I, Varma A, Tabben M, et al. Organising football matches with spectators during the COVID-19 pandemic: what can we learn from the Amir Cup football final of Qatar 2020? A call for action. Biol Sport. 2021;38:677–681. doi: 10.5114/biolsport.2021.103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.WHO WHO's work in health emergencies—strengthening preparedness for health emergencies: implementation of the International Health Regulations (2005) May 5, 2021. https://apps.who.int/gb/ebwha/pdf_files/WHA74/A74_9Add1-en.pdf
- 88.European Centre for Disease Prevention and Control EASA/ECDC update air travel guidelines to factor in vaccination and latest scientific evidence. June 17, 2021. https://www.ecdc.europa.eu/en/news-events/covid-19-updated-air-travel-guidelines
- 89.US Centers for Disease Control and Prevention COVID-19—schools and child care programs. Nov 1, 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/index.html#print
- 90.Kriemler S, Ulyte A, Ammann P, et al. Surveillance of acute SARS-CoV-2 infections in school children and point-prevalence during a time of high community transmission in Switzerland. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.645577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paltiel AD, Zheng A, Walensky RP. Assessment of SARS-CoV-2 screening strategies to permit the safe reopening of college campuses in the United States. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.US Centers for Disease Control and Prevention COVID-19 travel recommendations by destination. Nov 1, 2021. https://www.cdc.gov/coronavirus/2019-ncov/travelers/map-and-travel-notices.html
- 93.Dickens BL, Koo JR, Lim JT, et al. Strategies at points of entry to reduce importation risk of COVID-19 cases and reopen travel. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Quilty BJ, Russel TW, Clifford S, et al. Quarantine and testing strategies to reduce transmission risk from imported SARS-CoV-2 infections: a global modelling study. medRxiv. 2021 doi: 10.1101/2021.06.11.21258735. published online June 14. (preprint). [DOI] [Google Scholar]
- 95.International Air Transport Association COVID-19: air travel, public health measures and risk—a brief summary of current medical evidence. Version 7. Sept 1, 2021. https://www.iata.org/globalassets/iata/programs/covid/restart/covid-public-health-meausures-evidence-doc.pdf
- 96.Hacisuleyman E, Hale C, Saito Y, et al. Vaccine breakthrough infections with SARS-CoV-2 variants. N Engl J Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.06.036. published online July 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bergwerk M, Gonen T, Lustig Y, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Telenti A, Arvin A, Corey L, et al. After the pandemic: perspectives on the future trajectory of COVID-19. Nature. 2021;596:495–504. doi: 10.1038/s41586-021-03792-w. [DOI] [PubMed] [Google Scholar]
- 100.Iftekhar EN, Priesemanna V, Balling R, et al. A look into the future of the COVID-19 pandemic in Europe: an expert consultation. Lancet Reg Health Eur. 2021;8 doi: 10.1016/j.lanepe.2021.100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.The Lancet Digital Health Contact tracing: digital health on the frontline. Lancet Digit Health. 2020;2:e561. doi: 10.1016/S2589-7500(20)30251-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferretti L, Wymant C, Kendall M, et al. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368 doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fongwen N, Boeras D, Peeling RW, Amukele T. Connected diagnostics systems: the future of disease control in Africa. Afr J Lab Med. 2020;9 doi: 10.4102/ajlm.v9i2.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gardy JL, Loman NJ. Towards a genomics-informed, real-time, global pathogen surveillance system. Nat Rev Genet. 2018;19:9–20. doi: 10.1038/nrg.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.African Union and Africa Centres for Disease Control and Prevention Institute for Pathogen Genomics (IPG) https://africacdc.org/institutes/africa-pathogen-genomics-initiative/