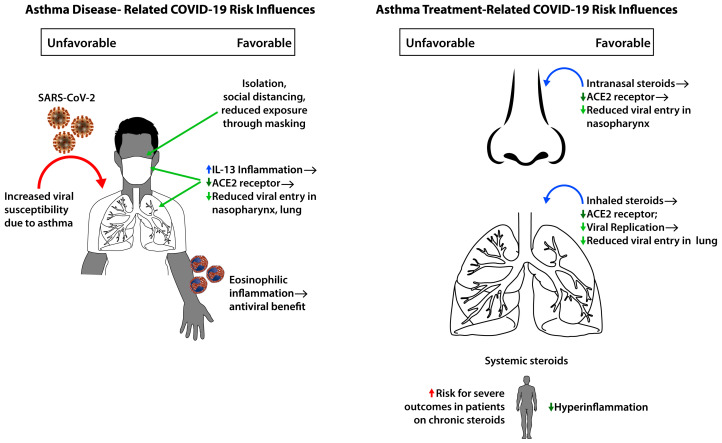
The SARS-CoV-2 pandemic has presented an unprecedented challenge for patients and their health care providers. Asthma is a disease in which patients are susceptible to viral-exacerbated morbidity. Asthma-related complications seemed to decline during pandemic-inflicted isolation and distancing procedures, which was assumed to be a result of less circulation of and exposure to common viruses.1 However, the extent to which asthma or atopy is a risk factor for more severe COVID-19 disease–related outcomes, such as respiratory failure, requirement for intensive care, and death, has been of significant interest. Additionally, the potential impact of therapy for asthma and atopic disease, such as corticosteroids and biologics, on those risks was not clearly understood. Research on this disease, spanning mechanistic to epidemiologic, has started to shed light on the major areas of concern to patients with asthma, their families, and their physicians.
Risk for severe COVID19 disease in asthma/severe asthma
Early descriptive cohort studies of patients with COVID-19 disease reported low rates of asthma or atopic diseases. Bloom et al presented data from all patients admitted to the hospital with COVID-19 disease across England, Scotland, and Wales between January and August of 2020, as captured by the International Severe Acute Respiratory and Emerging Infection Consortium World Health Organization Clinical Characterisation Protocol United Kingdom study.2 The group identified patients with a diagnosis of asthma and/or chronic pulmonary disease and measured mortality with adjustment for demographics, comorbidities, and medications. Patients using an inhaled corticosteroid, long-acting β-agonist, and third maintenance medication were designated as having severe asthma. Patients with asthma were more likely than those without asthma to require critical care; mortality was increased only for those with severe asthma compared with for those with no asthma (adjusted hazard ratio = 1.96 [95% CI = 1.25-3.08] for 16- to 49-year-olds and hazard ratio = 1.24 [95% CI = 1.04-1.49] for patients older than 50 years). In older patients with asthma, inhaled corticosteroid use within 2 weeks before hospital admission was associated with decreased mortality versus for those not using medication for asthma. These data suggest that severe asthma, but not the use of inhaled corticosteroids, might be a risk factor for adverse outcomes with this infection.
In contrast, a retrospective analysis of inpatients and outpatients presenting to the Mount Sinai Health System in New York between March and June 2020 evaluated outcomes between those with and without asthma. After adjustment for COVID-19 disease severity, comorbidities, and therapy, patients with asthma had a lower mortality and rate of hospitalization and intensive care unit admission than did those without asthma.3 The results of studies of additional cohorts continue to be published, with varied methods and outcomes, but asthma does not seem to be a consistent risk factor for COVID-19–related mortality.
Terry et al4 performed a meta-analysis of 150 studies worldwide that examined the prevalence and severity of COVID-19 in patients with asthma, by region. Although the proportion of patients with asthma in the cohorts varied by region, there was no clear relationship between asthma diagnosis and COVID-19 disease diagnosis or severity. In the authors’ experience, however, compared with individuals without a lung disease, many patients with asthma maintain relative hypervigilance against exposure to infectious disease.
Taken together, the data indicate that the relationship between asthma and COVID-19 disease risk is complex and is likely to be influenced by the presence of asthma, the severity of underlying lung disease, the therapies utilized, and possibly the presence of type 2 inflammation.
Relationship between COVID-19 and type 2 inflammation
The angiotensin-converting enzyme 2 (ACE2) receptor is the target for SARS-CoV-2 spike protein binding, facilitated by transmembrane protease, serine 2 (TMPRSS2) for entry into cells. These molecules may be influenced by type 2 inflammation. Interestingly, our group showed that ACE2 expression in nasal and airway epithelial cells from patients with atopic rhinitis or asthma is negatively associated with type 2 cytokine expression, whereas TMPRSS2 expression is positively associated with it.5 Ex vivo treatment of primary airway epithelial cells from patients with asthma with IL-13 can reduce ACE2 and increase TMPRSS2 expression. Jackson et al6 showed that in a pediatric cohort, allergic sensitization and type 2 biomarkers—including fractional exhaled nitric oxide, IgE, and nasal IL-13 expression—were inversely related to ACE2 expression in the nasal epithelium regardless of asthma status; in an adult cohort, ACE2 expression decreased after exposure to relevant allergens. However, there was no reduction in ACE2 expression in those with nonatopic asthma, suggesting that the protection is limited to those with IL-13–mediated inflammation and allergy.
A commonly used biomarker of type 2 asthma, the eosinophil, is known to play a role in immunity against pathogens. Low eosinophil levels in COVID-19 may portend poorer outcomes or more severe illness, perhaps related to the cytokine storm and hyperinflammation of severe illness characterized by IL-6, IL-1β, and TNF-α. In the aforementioned Mount Sinai study, patients with blood eosinophils of 200 cells/μL or higher had lower mortality, irrespective of asthma status.
Impact of nasal, inhaled, and systemic steroids
Most, if not all, of our patients with asthma and atopy are being treated with some form of corticosteroid. As corticosteroids suppress inflammation, including the possibly protective type 2 inflammation, the concern about safety of these therapeutics in the face of the pandemic is not without merit. However, data suggest that topical and systemic corticosteroids may indeed show benefit as a treatment strategy for moderate-to-severe COVID-19 thanks to their anti-inflammatory effects. However, topical steroids administered intranasally or by inhalation may have other protective benefits against COVID-19 disease. For example, patients with asthma who were using inhaled corticosteroids were shown to have ACE2 levels expressed by sputum cells that were lower than the levels in those asthma patients who were not using corticosteroids,7 suggesting the possibility that steroid-mediated downregulation of the epithelial receptor for SARS-CoV-2 may be a strategy to reduce severity of infection. Providing clinical evidence in support of this hypothesis, Strauss et al8 observed that in the Cleveland Clinic COVID-19 Research Registry, among adult patients with confirmed infection, use of an intranasal steroid before hospitalization was associated with lower risk for hospitalization, intensive care unit admission, and in-hospital mortality; these findings were replicated in sensitivity analyses excluding those patients with documented allergic rhinitis or use of inhaled steroids. The aforementioned International Severe Acute Respiratory and Emerging Infection Consortium World Health Organization Clinical Characterisation Protocol United Kingdom study identified use of inhaled corticosteroids as possibly protective against mortality in patients hospitalized for COVID-19. Interventional studies on inhaled corticosteroids and systemic steroids are as yet inconclusive as to the optimal dose, timing, and duration of treatment or expected benefit.
Further, ciclesonide and mometasone were shown to inhibit in vitro replication of SARS-CoV-2,9 providing an additional possible therapeutic benefit to use of corticosteroids as prevention or treatment for COVID-19 disease. Clinical trials are needed to quantify the benefit of these and other corticosteroid preparations.
Impact of biologics
Adir et al published data from 80,602 patients at Clalit Health Services, the largest health care provider in Israel, to identify all adult patients with asthma (N = 8242) who underwent SARS-CoV-2 PCR testing between March and December 2020.10 In this cohort, neither use of asthma biologics, which target type 2 pathways, nor use of systemic corticosteroids was associated with increased risk of infection. However, systemic corticosteroid use was associated with increased risk of moderate-to-severe COVID-19 disease or all-cause mortality.
Conclusions
Despite an as-yet undefined risk of our patients with asthma and atopy developing symptomatic disease, severe disease, or mortality from infection with SARS-CoV-2, current data support the idea that the usual therapy for asthma, including inhaled corticosteroids, may actually be protective against adverse outcomes (Fig 1 ). The data presented herein cannot be considered definitive, however, owing to various limitations related to study design. Despite this, the relationship between atopic inflammation, corticosteroids, and pathways related to COVID-19 disease points to complementary downregulation of viral receptors, inhibition of viral replication, and reduction of hyperinflammation. Ongoing mechanistic work with live virus will further elucidate these relationships, including that of the impact of non–type 2 inflammation such as IFN-γ. Further studies will be needed to carefully evaluate the risk among multiple ethnic groups, efficacy of vaccination across patients with asthma who are undergoing therapy, potential benefits or risks of asthma biologics, and risk mitigation strategies for those with severe asthma.
Fig 1.
Influence of asthma disease characteristics and therapies on COVID-19 risk.
Footnotes
Supported by grants from the National Institutes of Health and the American Lung Association.
Disclosure of potential conflict of interest: T. F. Carr reports consulting fees from AstraZeneca, Genentech, Novartis, GSK; speaker fees from Regeneron, AstraZeneca, and Novartis; and editorial fees from UpToDate. M. Kraft reports research grants from AstraZeneca and Sanofi-Regeneron, NIH and American Lung Association paid to the University of Arizona; consulting fees from Chiesi, Astra-Zeneca, Genentech, and Sanofi-Regeneron; speaker fees from Chiesi; patents for RaeSedo, LLC, and is company founder and Chief Medical Officer; and editorial fees from UpToDate.
References
- 1.Olsen S.J., Winn A.K., Budd A.P., Prill M.M., Steel J., Midgley C.M., et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic - United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70:1013–1019. doi: 10.15585/mmwr.mm7029a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloom C.I., Drake T.M., Docherty A.B., Lipworth B.J., Johnston S.L., Nguyen-Van-Tam J.S., et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. 2021;9:699–711. doi: 10.1016/S2213-2600(21)00013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho K.S., Howell D., Rogers L., Narasimhan B., Verma H., Steiger D. The relationship between asthma, eosinophilia, and outcomes in coronavirus disease 2019 infection. Ann Allergy Asthma Immunol. 2021;127:42–48. doi: 10.1016/j.anai.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terry P.D., Heidel R.E., Dhand R. Asthma in adult patients with COVID-19. Prevalence and risk of severe disease. Am J Respir Crit Care Med. 2021;203:893–905. doi: 10.1164/rccm.202008-3266OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura H., Francisco D., Conway M., Martinez F.D., Vercelli D., Polverino F., et al. Type 2 inflammation modulates ACE2 and TMPRSS2 in airway epithelial cells. J Allergy Clin Immunol. 2020;146:80–88.e8. doi: 10.1016/j.jaci.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O'Connor G.T., Wood R.A., et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146:203–206.e3. doi: 10.1016/j.jaci.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters M.C., Sajuthi S., Deford P., Christenson S., Rios C.L., Montgomery M.T., et al. COVID-19-related genes in sputum cells in asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202:83–90. doi: 10.1164/rccm.202003-0821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strauss R., Jawhari N., Attaway A.H., Hu B., Jehi L., Milinovich A., et al. Intranasal corticosteroids are associated with better outcomes in coronavirus disease 2019 (COVID-19) J Allergy Clin Immunol Pract. 2021;9:3934–3940.e9. doi: 10.1016/j.jaip.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuyama S., Kawase M., Nao N., Shirato K., Ujike M., Kamitani W., et al. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol. 2020;95:e01648–20. doi: 10.1128/JVI.01648-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adir Y., Humbert M., Saliba W. COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: nationwide real-world evidence. J Allergy Clin Immunol. 2021;148:361–367.e13. doi: 10.1016/j.jaci.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]