Abstract
The sustainability of coronavirus 19 (COVID-19) vaccine-induced immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is critical to be determined to inform public health decisions on vaccination programs and prevention measures against COVID-19. The aim of the present study was to prospectively evaluate the kinetics of neutralizing antibodies (NAbs) and anti-S-receptor binding domain (RBD IgGs) against SARS-CoV-2 after full vaccination with the BNT162b2 mRNA vaccine for up to 9 months in healthy individuals (NCT04743388). The assessments were performed at the following time points after the second vaccination: 2 weeks, 1 month, 3 months, 6 months, and 9 months. The measurements were performed with the GenScript’s cPassTM SARS-CoV-2 NAbs Detection Kit (GenScript, Inc.; Piscataway, NJ) and the Elecsys Anti-SARS-CoV-2 S assay (Roche Diagnostics GmbH; Mannheim, Germany). Three hundred nine participants with a median age of 48 years were included. A gradual decline in both NAbs and anti-S-RBD IgGs became evident from 2 weeks to 9 months postvaccination. Both NAbs and anti-S-RBD IgGs levels were significantly lower at 9 months compared with the previous timepoints. Interestingly, age was found to exert a statistically significant effect on NAbs elimination only during the first-trimester postvaccination, as older age was associated with a more rapid clearance of NAbs. Furthermore, simulation studies predicted that the median NAb value would fall from 66% at 9 months to 59% and 45% at 12 and 18 months postvaccination, respectively. This finding may reflect a declining degree of immune protection against COVID-19 and advocates for the administration of booster vaccine shots especially in areas with emerging outbreaks.
Introduction
The new coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a worldwide pandemic and becomes a serious public health problem on a global scale.1,2 There are 4 different primary structural proteins encoded by the coronavirus genome, referred to as spike (S), envelope, membrane, and nucleocapsid. Angiotensin-converting enzyme 2 receptors are found primarily on oral mucosal epithelial cells and alveolar lung cells II but also in other human tissues. The virus enters the body via the viral S protein and attaches to the angiotensin-converting enzyme 2 receptors.3 Coronavirus 19 (COVID-19) is a systemic disease with short- and long-term symptoms.4–6 The vast majority of patients experience mild or moderate symptoms, with up to 5% to 10% having a severe or life-threatening course of disease according to the literature. Research and development of effective and safe vaccines and drugs, as well as innovative diagnostics and therapeutics, has become a global priority.7
The BNT162b2 vaccine provides protection against COVID-19 infection.8,9 Healthy individuals exhibit significant levels of IgG antibodies and neutralizing antibodies (NAbs) directed against the SARS-CoV-2 spike-receptor binding domain (anti-SARS-CoV-2 S-receptor binding domain [RBD] or anti-S-RBD), as well as a prolonged B-cell response in the germinal center after immunization.10,11 It is important to note that NAbs levels are associated with clinically relevant immune protection against COVID-19.12,13 However, even 1 month after the second BNT162b2 injection, a slight decrease in antibody titers was observed, while the time elapsed since the second vaccine dose was associated with lower NAb activity against SARS-CoV-2 variants and attenuated protection against COVID-19.14–18 The fundamental question now is whether and when a third dose should be administered.
The aim of this study was to investigate the kinetics of NAbs and anti-S-RBD IgGs against SARS-CoV-2 after full vaccination with the BNT162b2 mRNA vaccine for up to 9 months in healthy individuals.
Materials and methods
Clinical study
This is a prospective study that was designed to determine the kinetics of anti-SARS-CoV-2 antibodies after COVID-19 immunization with the BNT162b2 mRNA vaccine (NCT04743388). The ethics committee of the General Hospital Alexandra approved the study protocol (Ref No. 15/23 December 2020). The study was carried out in accordance with the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice standards of care. All participants provided written informed consent at study entry.
The primary inclusion criteria were eligibility for vaccination against COVID-19 according to the national vaccination program, being above the age of 18 years, and being able to sign an informed consent form. Patients with active malignant disease, those on immunosuppressive therapy, and those with end-stage renal disease were excluded from the study. According to the National Immunization Program, the BNT162b2 mRNA vaccine was offered to anyone who was 18 years of age or older at the time of administration. In Greece, vaccination centers for the BNT162b2 have been created in hospitals to provide immediate medical care in case of rare but severe adverse events such as anaphylaxis. Consecutive vaccinated patients were enrolled in this study.
The confidentiality of the subject data was maintained in line with the rules of the General Data Protection Regulation. All of the participants’ identities were kept strictly private. Names were deidentified according to the principles of pseudoanonymization immediately after sample collection.
Analysis of biological samples
In this clinical study, the blood collection schedules were as follows: on day 1 before the first vaccination, on day 8, on day 22 (the day of the second vaccination and just before receiving the injection), and at the following time points after the second vaccination: 2 weeks, 1 month, 3 months, 6 months, and 9 months after the second injection of the vaccine. Blood was drawn, and serum was isolated within 4 hours of collection. The serum was then freezed at –80°C until the day of measurement.
Using an Food and Drug Administration (FDA)-approved methodology, NAbs against SARS-CoV-2 were determined. GenScript’s cPassTM SARS-CoV-2 NAbs Detection Kit (GenScript, Inc.; Piscataway, NJ), which allows for the indirect detection of SARS-CoV-2 NAbs in blood, was implemented in this study. Anti-S-RBD IgG antibodies were measured using an FDA-approved technology, the Elecsys anti-SARS-CoV-2 S assay, which shows response to either prior infection or immunization (Roche Diagnostics GmbH, Mannheim, Germany).
Data analysis
Patients’ demographic information, medical history, and prescriptions were collected during an interview at study entry. Weight and height were used to compute the individual’s body mass index (BMI). All of the individuals were assigned to 1 of 3 groups based on their BMI: BMI ranges from 18.5 to 24.9 for those who are underweight; 25 to 29.9 for those who are overweight; and 30 or more for those who are obese.
Hypercholesterolemia (e.g., dyslipidemia) was found in many of the subjects, as were cardiovascular disease, diabetes, and autoimmune diseases (e.g., psoriasis, atopic dermatitis, irritable bowel syndrome). Other conditions found in the subjects’ medical histories included allergies (e.g., bronchial asthma, prior reaction to drugs, chronic obstructive pulmonary disease), and thyroid issues (e.g., Hashimoto’s depression, migraine, sleep apnea, gastroesophageal reflux disease). Similar to this, all medications received were grouped into the following categories: hypercholesterolemia medications (e.g., statins), cardiovascular disease medications (e.g., beta blockers, angiotensin-converting enzyme inhibitors), insulin, oral antidiabetics, T4, immunomodulators, centrally acting drugs (e.g., antidepressants, benzodiazepines), and other medications.
An examination of the percent inhibition levels was conducted using statistical methods (i.e., NAbs). The first steps in statistical analysis were the estimation of descriptive criteria such as the mean, median, and quartiles, as well as the estimation of dispersion metrics. Before proceed with statistical comparisons between 2 or more groups, a normality test was performed on each group. The Kolmogorov-Smirnov and Shapiro-Wilk tests, as well as QQ plots, were employed to determine whether or not the data distribution was normal. It was found that the data did not conform to normality in all of the instances examined in this study, and therefore nonparametric approaches were employed for the subsequent statistical analysis. The Mann Whitney U test was used for 2 independent group comparisons, such as analyzing the gender effect or the influence of age groups (50 and ≥50 years). If there were any pairwise group comparisons, the Wilcoxon test was used, which included the comparisons of NAbs between 2 successive time points. The Kruskal-Wallis approach was used to assess whether there is a difference in NAbs titers between different age groups, such as those between 20 and 40 years old, 40 to 55 years old, and ≥55 years old. The nonparametric Friedman test was used to discover variations between individuals (e.g., in their NAbs or anti-S-RBDs) over time (i.e., 2 weeks, 1, 3, 6, and 9 months). Each subject’s age, gender, BMI, and medical history (i.e., comorbidities) were all taken into consideration when determining the antibody levels at each time point.
The significance level in this investigation was set at 5%, whereas a result was considered significant if the computed P value was less than the significance level. Python v.3.9.2 was used to carry out the statistical analysis. Data analysis was carried out using the “pandas” and “numpy” libraries, respectively. The “matplotlib” library was used to construct the statistical plots, while the “seaborn” library was used to implement the statistical analysis.
Population modeling and simulations
Individual longitudinal percent inhibition values were explored in terms of population kinetic analysis, utilizing the stochastic approximation expectation maximization methodology for nonlinear mixed effects, followed by importance sampling approaches.19 Because the goal of this study was to determine the pace at which NAbs was eliminated from the body, only the dropping portion of the NAbs levels was modeled. As a result, data from 2 weeks after the second immunization up to 9 months after the second vaccine were used.
A number of structural models, including 1- and 2-compartment designs, were evaluated. Single exponential or linear functions, as well as piecewise linear functions, were used to represent the kinetics of NAbs elimination. The NAbs levels were classified as normal or log-normal, and a variety of residual error models were examined (e.g., constant, proportional, and combined). Following the construction of the final optimal structural model, the impact of the individuals’ characteristics (e.g., age, gender, BMI) on the model parameters was investigated. The Wald test was performed to examine whether or not variables could be utilized to explain variation in the parameters. This was accomplished entirely by writing the appropriate code in Monolix 2020R1 Mlxtran language (Lixoft, Orsay, France). To predict NAbs concentration levels at 12, 15, and 18 months after vaccination, simulations were conducted assuming that the kinetic parameters remained unaltered after the ninth month following the construction of the population model. A number of 1000 individuals were simulated using resampling from the same individual pool with replacement. A sample size of 1000 individuals were used for the simulations, and the individual model parameter values were randomly drawn to allow for robust prediction. However, since the simulated sample size is much larger than the original one, the individual parameter values were drawn from the original set with replacement, that is, each subject can be used more than once. The simulations were carried out using Simulx (Lixoft, Orsay, France).
Results
Baseline characteristics
The BNT162b2 mRNA vaccine was administered in 2 doses to 309 participants in this trial. NAbs and anti-S-RBD levels were measured at days 1, 8, 22 (before the second vaccine), as well as 1, 3, 6, and 9 months later. Table 1 lists the demographic information collected from the study participants. The median age was 48.0 years, whereas women made up nearly two-thirds (202, namely 65.4%) of the group. Almost half of the subjects (47.90%) had normal weight, followed by overweight (33.01%) and obese (14.24%). The underweight subjects referred to the small portion of 4.85%.
Table 1.
Characteristics of the Participants in the Study
| Participant Characteristic | Value * |
|---|---|
| Sample size | 309 |
| Gender | |
| Men | 107 (34.6%) |
| Women | 202 (65.4%) |
| Age (median) | 48.0 |
| Body mass index (median) | 24.8 |
| Underweight (n, %) | 15 (4.85%) |
| Normal weight (n, %) | 148 (47.90%) |
| Overweight (n, %) | 102 (33.01%) |
| Obese (n, %) | 44 (14.24%) |
*Values in parentheses refer to percentages
Neutralizing antibodies and anti-S-RBDs levels
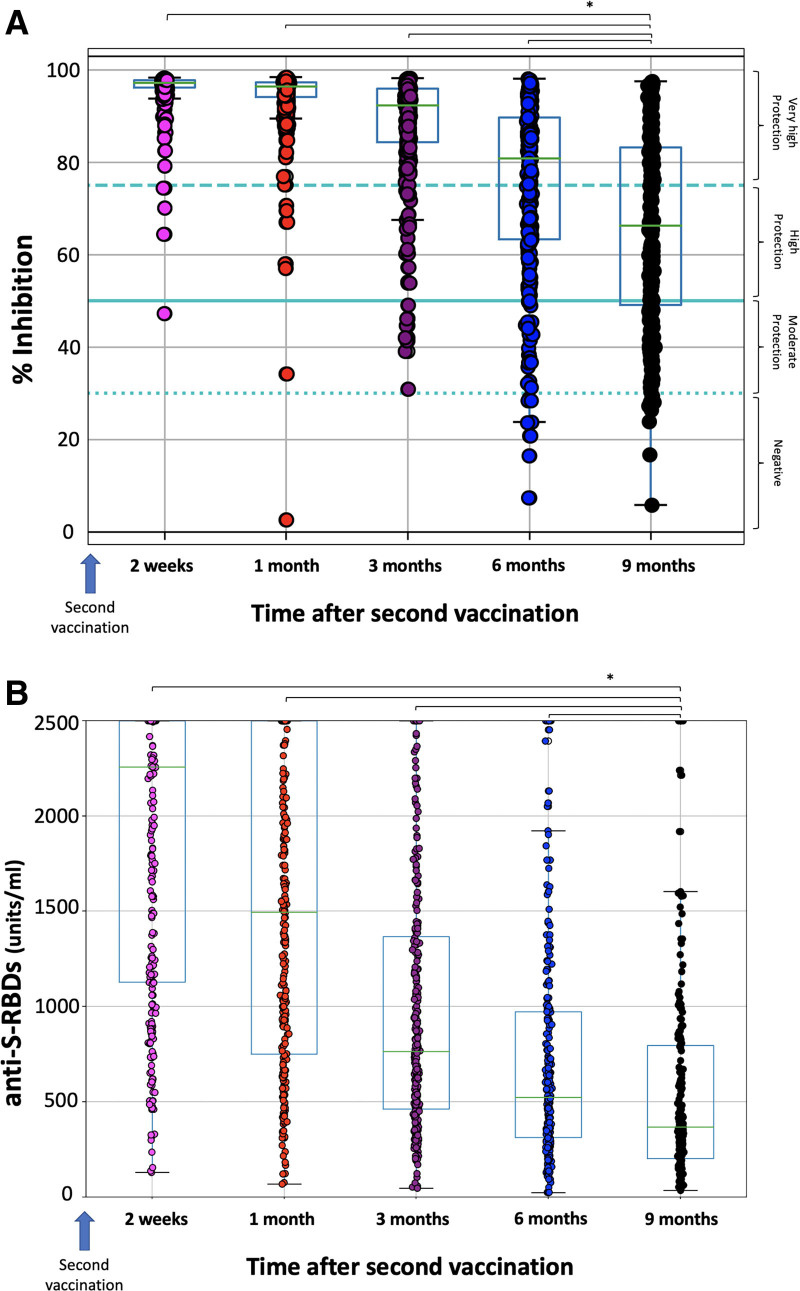
Figure 1 shows the percentage inhibition of NAbs and the anti-S-RBDs levels after 2 weeks, 1 month, 3 months, 6 months, and 9 months of full vaccination with the BNT162b2. Two weeks after the second vaccination, the highest NAbs values are observed (median 97.24%) (Figure 1A). From this point on, a steady, gradual decline is observed, with the following median NAbs titers observed at subsequent measurement time points: 96.35%, 92.27%, 80.79%, and 66.23%, respectively. Paired comparisons between NAbs levels at 9 months with those at 6 months, 3 months, and 1 month resulted in statistically significant differences (P < 0.001, Wilcoxon test). This finding further underlines the significant decrease in NAbs.
Figure 1.
Percentage of SARS-CoV-2 binding inhibition (NAbs) (A) and anti-S-RBD levels (B) for the study population. The asterisk (*) denotes a statistically significant difference between the ninth month levels and the previous timepoints (P < 0.05). The boxplot borders correspond to the distribution’s quartiles. NAbs = neutralizing antibodies; RBD = receptor binding domain.
Similar findings are observed for the anti-S-RBD levels (Figure 1B). Two weeks after the second vaccination, the median anti-S-RBD titers were 2257 units/mL and from this point on, a continuous decline was observed, with median anti-S-RBD values at 1, 3, 6, and 9 months being 1495, 764.15, 523.8, and 367.1 units/mL, respectively. Paired comparisons between anti-S-RBD at 9 months and previous time-points revealed statistically significant differences for all comparisons (P < 0.05).
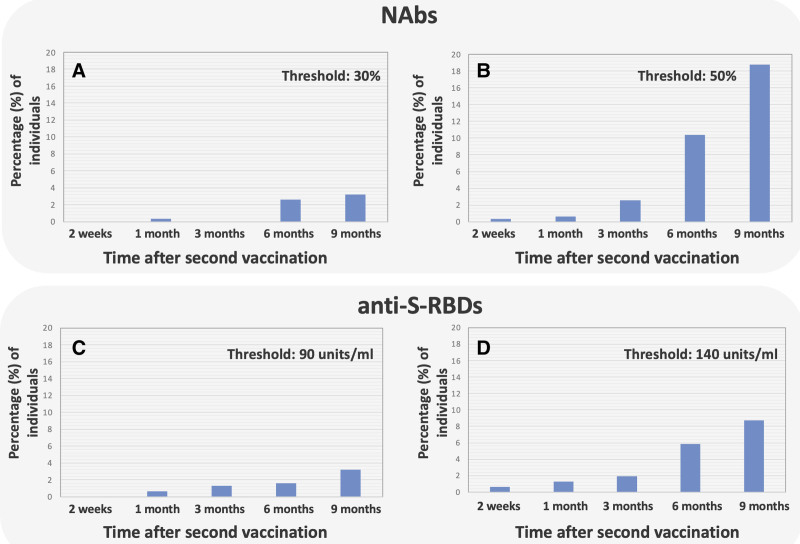
To illustrate the gradual decline of NAbs over time, the proportion of individuals with NAbs below 30% and 50% was recorded and shown in Figure 2A, B, respectively. Figure 2A depicts that there is an almost continuous increase of vaccinated people at risk (i.e., NAbs < 30%). Presumably there would be a continuous increase over time, but because of the limited sample size, minor variations are observed due to chance (e.g., there are none at 3 months). The situation becomes clear in subjects with NAbs < 50% (Figure 2B), where an exponential increase in incidences is observed.
Figure 2.
Percentage of individuals with NAbs and anti-S-RBDs below certain thresholds. For NAbs, thresholds were 30% (A) and 50% (B), while for anti-S-RBDs, the thresholds were 90 units/mL (C) and 140 units/mL (D). NAbs = neutralizing antibodies; RBD = receptor binding domain.
The same calculations were also performed in the case of anti-S-RBDs, and the results are depicted in Figure 2C, D. In this case, 2 thresholds were considered: 90 units/mL and 140 units/mL, which correspond to situations where there is not a high level of protection or where protection is not very high. In both plots, an almost exponential increase in the number of incidences is observed.
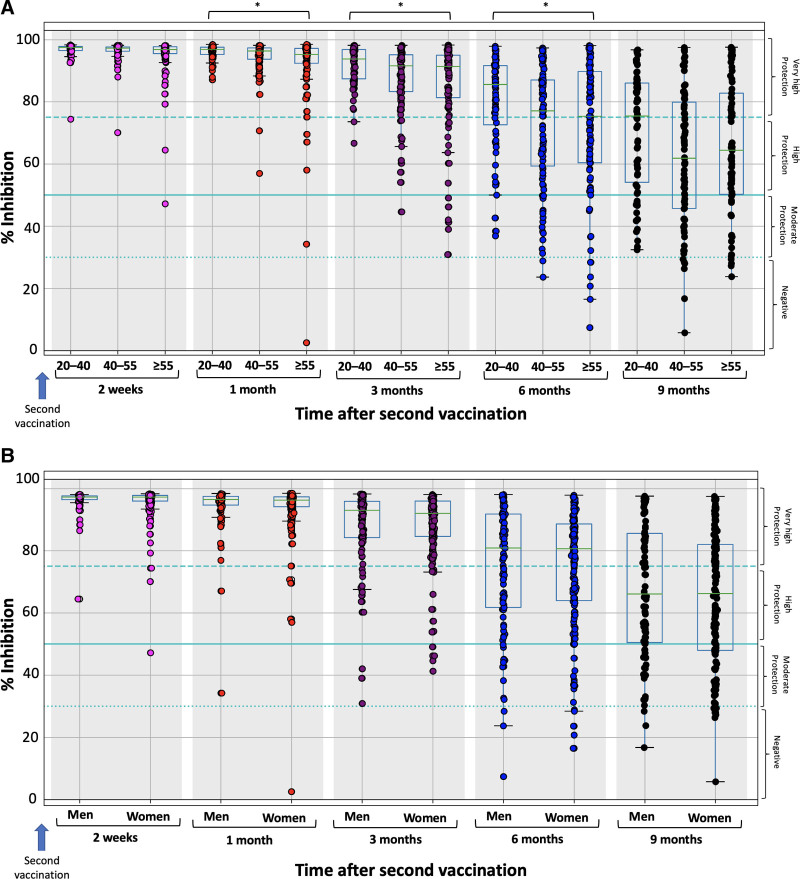
Figure 3 shows the % inhibition of NAbs on each day of measurement for the entire study population, divided by (a) age group: 20–40, 40–55, and ≥55 years (Figure 3A) and (b) gender (Figure 3B). Because the primary goal of this study was to describe the elimination kinetics of NAbs, only the results from 2 weeks after the second immunization, when the maximal inhibition has been achieved, are presented. Statistically significant differences, among the 3 age groups, were observed at 1, 3, and 6 months. At 2 weeks and 9 months, no significant differences were identified in terms of age.
Figure 3.
Percentage of SARS-CoV-2 binding inhibition for the study population stratified by the age group (A) and gender (B). The study population was split into 3 age groups: 20–40, 40–55, and ≥55 years. The boxplot borders correspond to the quartiles of the distribution. Asterisks (*) indicate statistically significant differences (P < 0.05) between the compared groups. Age was found to significantly influence inhibition levels at the first month (P = 0.00384, Kruskal-Wallis), the third month (P = 0.0027), and the sixth month (P = 0.0316). No statistically significant effect was observed for 2 weeks (P = 0.065) and the ninth month (P = 0.0699), as well as for the impact of gender on inhibition levels.
Regarding the role of gender, no significant differences in NAbs score were found at all examined timepoints. Nine months after vaccination, the median percentage inhibition was 66.11% for males and 66.23% for females. Quite similar percentages between the 2 groups were observed for all examined timepoints (Figure 3B).
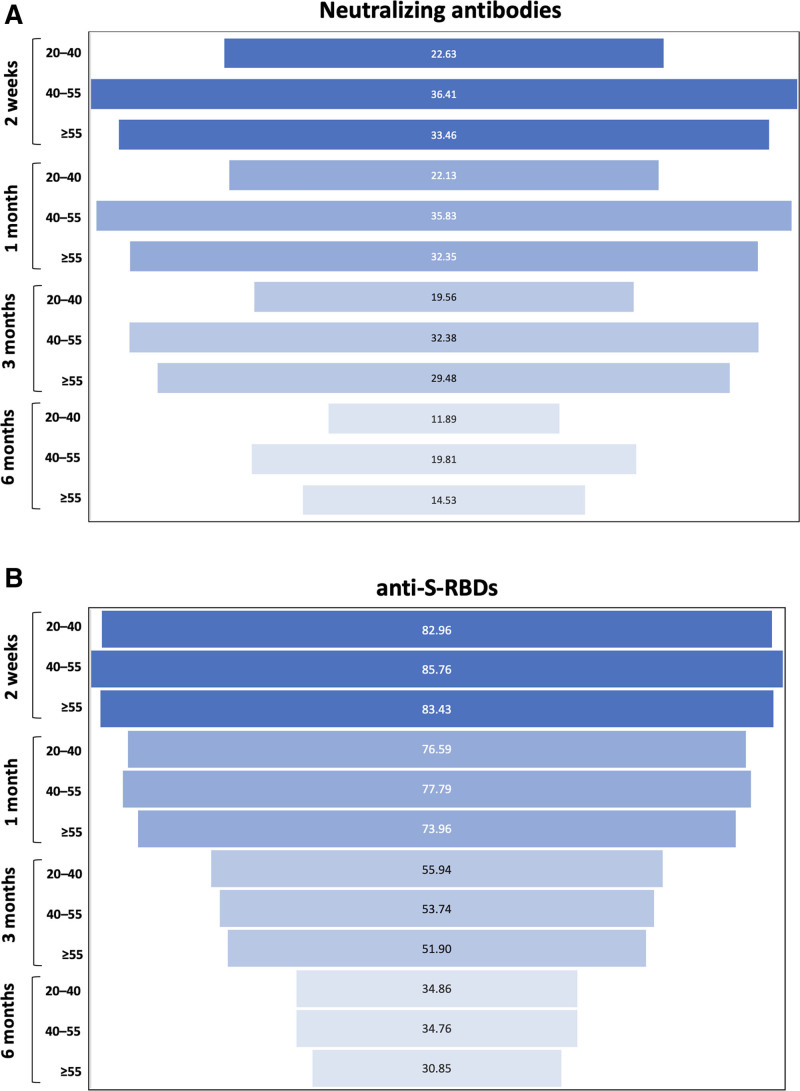
To quantify the decrease in NAbs and anti-S-RBD levels as a function of each subject age group, Figure 4 was constructed to show the percentage decrease in NAbs and anti-S-RBD levels at 9 months postfull vaccination compared with the humoral response at previous timepoints in each age group. As expected, the greatest percentage decrease in NAbs is shown between 9 months and 2 weeks after the second vaccination, when the highest NAbs values are observed (Figure 4A). The decrease is higher in the age group of 40 to 55 years, where a decline of 36.41% is observed, followed by the ≥55-year-old and the 20- to 40-year-old group. A similar pattern is shown for the comparisons between 9 months and 1 month, 3 months, and 6 months, respectively, although the magnitude of the decrease becomes smaller toward the latter time points. Again, the percentage decline is highest in the 40- to 55-year-old group, followed closely by the ≥55-year-old group and then by the 20- to 40-year-old group. Figure 4B shows the decline in anti-S-RBD levels at 9 months compared with previous timepoints. Similar to NAbs, the largest differences are shown between the 9-month and the 2-week comparisons, whereas the magnitude of the decrease becomes smaller toward the latter time points. However, the decline at each time point is almost the same for all age categories. This means that while the NAbs values may be influenced by the subject’s age, no such effect can be suggested for the anti-S-RBDs.
Figure 4.
Percentage decrease in NAbs (A) and anti-S-RBDs (B) at 9 months post vaccination compared with the corresponding values after 2 weeks, 1 month, 3 months, and 6 months. NAbs = neutralizing antibodies; RBD = receptor binding domain.
Modeling the kinetics of neutralizing antibodies
To describe the elimination kinetics of NAbs, a population kinetic model was developed. The final best model obtained from the population study comprised a piecewise function for the elimination constant, as well as a proportional error model (Table 2). The first elimination constant (kel1) was 0.016 NAbs/day (standard error = 0.0014) and corresponded to the early decay period lasting up to 3 months. Following that, the elimination constant was calculated to be kel2 = 0.099 NAbs/day (standard error = 0.0076). This result shows that the NAbs disappear relatively slow at first, but that their removal becomes around 6 times greater from the third to the sixth month, indicating that they are eliminated much more quickly. In the period between the sixth and ninth month, the elimination rate constant is 0.071 NAbs/day (standard error = 0.0065), which is almost 4.5 times higher than in the first trimester and 30% less than in the third to sixth trimester. This finding indicates a decrease in the elimination capacity of NAbs, which is a desirable feature, as individuals retain NAbs for a longer period.
Table 2.
Parameter Estimates for the Final Best Model Describing the Kinetics of NAb
| Parameter | Value | Standard Error | % Relative Standard Error |
|---|---|---|---|
| Fixed effects | |||
| N0 | 96.05 | 1.690 | 1.76 |
| kel1 | 0.017 | 0.001 | 8.48 |
| beta_kel1_Age* | 0.020 | 0.002 | 9.27 |
| kel2 | 0.111 | 0.008 | 7.63 |
| kel3 | 0.071 | 0.007 | 9.16 |
| Standard deviation of the random effects | |||
| omega_N0 | 0.016 | 0.002 | 12.8 |
| omega_kel1 | 1.37 | 0.099 | 7.25 |
| omega_kel2 | 0.98 | 0.067 | 6.86 |
| omega_kel3 | 1.10 | 0.081 | 7.36 |
| Correlations | |||
| corr_kel2_kel1 | 0.78 | 0.037 | 4.68 |
| Error model parameters | |||
| b | 0.043 | 0.001 | 2.79 |
*Indicates a statistically significant (P = 0.0037 < 0.05) contribution of “age” as a covariate to the first-phase elimination rate constant.
b = the proportional error term of the model residual variability; beta_kel1_Age = constant for the contribution of “age” on kel1; corr_kel2_kel1 = the correlation coefficient between kel1 and kel2; kel1 = elimination rate constant of the first trimester (zero to third month); kel2 = elimination rate constant of the second trimester (third to sixth month); kel3 = elimination rate constant of the third trimester (sixth to ninth month); NAbs = neutralizing antibodies; N0 = average initial NAbs value; omega = between-subject variability estimate for each parameter.
According to the findings, age has a significant impact only on the elimination ability of the first trimester (beta kel1 = 0.019, P = 0.0037) implying that age does not contribute significantly in the elimination of NAbs after the third month. This relationship, on the other hand, was statistically significant (P < 0.001) only during the first phase of the study (up to 3 months). The mathematical model describing the influence of age on the elimination constant is as following: log(kel1) = log(0.016) + eta_kel1, where eta_kel1 refers to the random effect for intersubject variability of the elimination constant. Other variables such as gender, BMI were not shown to have a statistically significant impact on antibody kinetics. Between kel1 and kel2, it was discovered that there was a positive association (coefficient = 0.78). No correlation was found between the third elimination constant and any of kel1 and kel2.
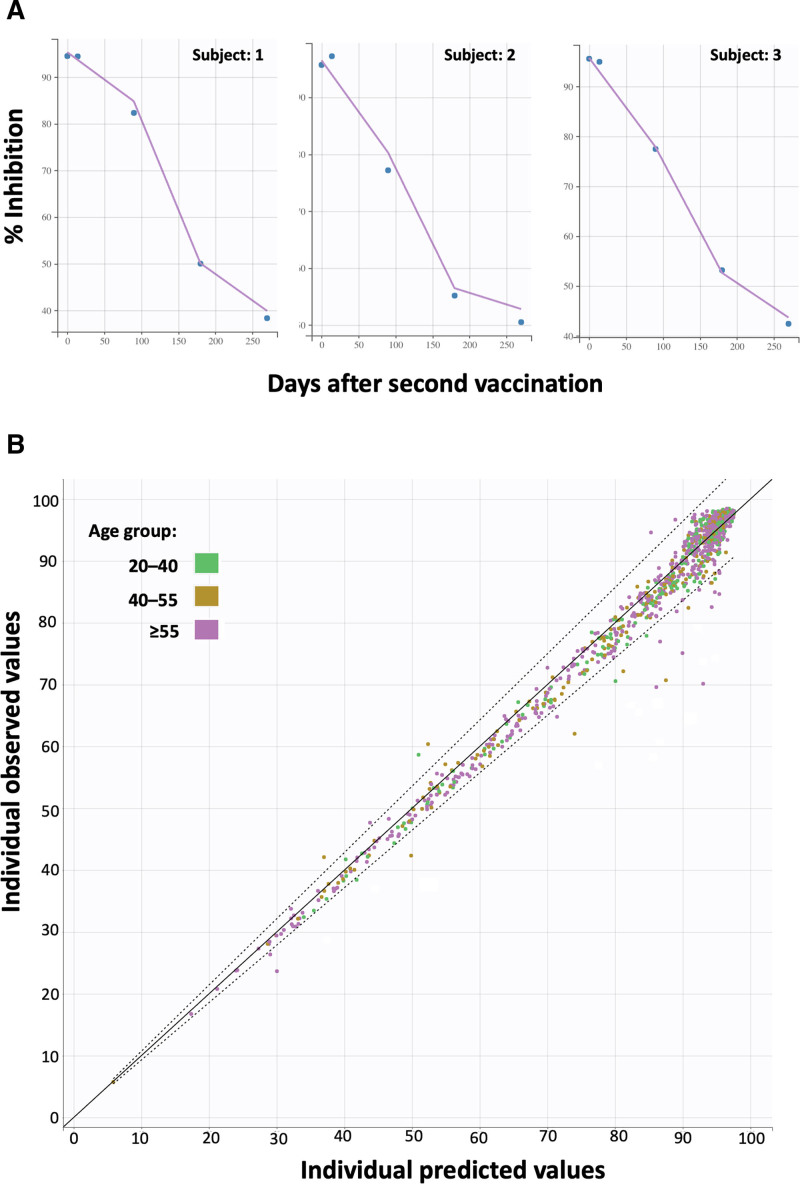
The goodness of fit and validation plots, of the final model, are depicted in Figure 5. Figure 5A shows 3 representative individual profiles of NAbs values (% inhibition) versus time. The solid lines indicate the model’s anticipated values, whereas the circles show the observed NAbs levels. The close proximity of the model’s predicted line to the actual points demonstrates the model’s high descriptive ability. In Figure 5B, the individual observed versus predicted NAbs values are depicted for the 3 age groups, namely, 20 to 40, 40 to 55, and ≥55-year-old category. The nice predictive performance can be validated by the fact that the vast majority of values are lying within the 90% prediction interval (dashed lines in Figure 5B).
Figure 5.
Indicative individual profiles of neutralizing antibodies (% inhibition) over time (A) and predicted vs observed (B). In plot A, the solid lines refer to the values predicted by the model, while the circles show the experimental values. The close transition of the model predicted line to the actual points shows the good predictive ability of the model. Plot B shows the overall fitting results for the 3 age groups, which are indicated as points with different colors. The solid line represents the optimal prediction performance, while the dotted line represents the 90% prediction interval.
Simulations and predictions
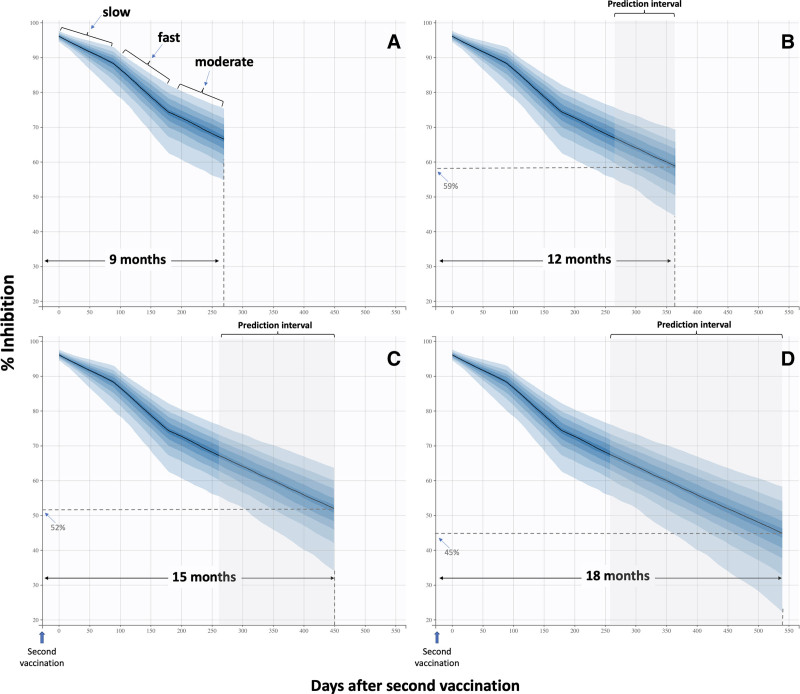
Aiming to predict NAbs levels at 12, 15, and 18 months following the second vaccination, simulations were carried out using the final population model, assuming that the removal of NAbs would be the same as that found in the last trimester (i.e., from sixth to ninth month) after the second vaccination. The simulation results are depicted in Figure 6.
Figure 6.
Simulated neutralizing antibody levels over a period of 9 months (A), 12 months (B), 15 months (C), and 18 months (D). During the first trimester, a relatively slow elimination of antibodies occurs, whereas the antibody elimination tends to increase during the next trimester (third to sixth month). In the last trimester of the study (sixth to ninth month), the elimination ability slows down by 30% compared with the second phase. Predictions for 12, 15, and 18 months assumed a similar elimination ability as the one observed in the last trimester.
Figure 6A shows the simulation prediction for 9 months for which we already have experimental data. The predicted average value of NAbs is 67.1%, which is quite close to the observed value of 65.7% (Figure 1). This result confirms the predictive ability of the model, at least for time points close to 9 months. To address the current fundamental global question of whether and when a third dose should be administered, our simulations were extended to 12, 15, and 18 months. Presumably, the accuracy of the predictions might decrease with increasing duration, as currently there is no information about the elimination ability of NAbs from the human body in long term. Nevertheless, it was worthwhile to make the predictions for longer time periods to gain insight into what we can expect in the future. In this context, Figure 6B predicts that the average percent inhibition of NAbs would be 59% 1 year after the second vaccination. This value will further decrease to 52% after 15 months (Figure 6C) and reach a value of 45% 18 months after the second vaccination (Figure 6D). These results suggest that at 1 year after full vaccination with BNT162b2, a large portion of people (around 41%) would not be highly protected. At 15 and 18 months, the risk would be even higher, as the percentage of individuals who are not highly protected might be as high as 48% and 55%, respectively.
Discussion
The goal of this study was to examine the kinetics of NAbs and anti-S-RBDs against SARS-CoV-2 in 309 healthy individuals, after full vaccination with the BNT162b2 mRNA vaccine for up to 9 months. The maximum levels of NAbs were observed 2 weeks after the second vaccination, whereas there was a statistically significant decline thereafter up to 9 months.
At 9 months, the median % inhibition was found to be 66.23%, while the number of subjects with NAbs levels <50% was 58 (18.77%). In other words, almost one-fifth of individuals is not highly protected against SARS-CoV-2 at 9 months after vaccination. Furthermore, 10 vaccinated individuals (3.24%) had a NAb titer below 30% and, therefore, they were considered at particular risk for COVID-19. A continuous increase (almost exponential in time) of vaccinated people being not highly protected was observed. The proportions of participants with NAbs < 50% were 0.32%, 0.65%, 2.59%, 10.36%, and 18.77% at 2 weeks, 1 month, 3 months, 6 months, and 9 months after the second vaccination, respectively. The results for the anti-S-RBD antibodies, where a stable decline in the anti-S-RBD levels was observed, are in line with the above-mentioned findings. It is worth noting that the anti-S-RBDs at 9 months were around 25% (i.e., 367.1/1495) of those observed at 2 weeks after vaccination.
Our findings are consistent with previous research on the persistence of antibody responses after 2 doses of this mRNA vaccine.14,18,20–22 Although SARS-CoV-2 antibody neutralization ability decreased over time, it remained adequately higher than the positive threshold values. Similar to BNT162b2, a declining but detectable humoral response has been also reported at 6 months following vaccination with the mRNA-1273 COVID-19 vaccine.23–26 Clinical trials have shown that BNT162b2 offers a declining but robust protection from COVID-19 at 6 months postfull vaccination.27 Real-world data from Israel showed that the protective immunity against the delta variant of SARS-CoV-2 waned after a few months following full vaccination with the BNT162b2.16 However, the protection against COVID-19, and especially against severe disease, remain as compared with unvaccinated individuals.16,27 Accumulating data have shown that mRNA COVID-19 vaccines induce humoral and cellular memory against both the wild-type SARS-CoV-2 and variants of concern, including the delta variant, and may offer a declining degree of protection against the infection that persist for at least 6 months postvaccination.11,21,28 Therefore, maintaining a high level of immune response would be essential for persisting protection against COVID-19.21 In this context, the administration of a third vaccine shot with the BNT162b2 at least 5 months post the completion of the 2-dose BNT162b2 vaccination offered a significant protection against severe COVID-19-related outcomes including hospital admission, severe disease, and death due to COVID-19.29
The kinetics of antibody response following mRNA-based vaccine immunization are similar with the kinetics of antibodies against SARS-CoV-2 in convalescent persons. Anti-SARS-CoV-2 S, S-RBD, nucleocapsid (N), N-RBD antibodies, and NAbs persist but decline in time up to 8 months post COVID-19 onset.30–32 Although more severe COVID-19 cases may have a superior antibody response, humoral and cellular activity against SARS-CoV-2 remains evident even among asymptomatic and mild cases at 9 months postsymptom onset.30,31,33–35 This pattern of humoral response is accompanied by persistent changes in the cellular response, as well.36 The decrease in antibody titers is more rapid during the first 6 months from COVID-19 diagnosis compared with the subsequent time period.30 Interestingly, a recent study including 2653 fully vaccinated individuals with the BNT162b2 and 4361 convalescent patients with prior COVID-19 showed that the latter had a lower elimination rate of anti-SARS-CoV-2 IgG antibodies in time (5%/month versus 40%/month for convalescent and vaccinated individuals, respectively).37
A population kinetic model was developed to describe the elimination kinetics of NAbs. The best model consists of a compartment (the whole body), linear kinetics, and a piecewise function for the elimination constant. It is worth noting that 3 different kinetic phases were identified depending on the time period after vaccination. It appears that after the second vaccination, there is a relatively slow elimination of antibodies up to 3 months. It should be mentioned that this apparent slow elimination during the first trimester after vaccination may be due to the fact that NAbs levels are quite high (>90%) and in some cases may exceed the linear phase of the assay. Therefore, this part of the analysis may not be absolutely quantitative. From that point on, elimination increases >6-fold, which means that the NAbs disappear from the body very quickly. Hopefully, this rapid elimination slows down after 6 months and during the last trimester of the study (i.e., from month 6 to month 9), the rate of elimination decreased by about 30% compared with that observed in the second trimester.
Previous studies have shown that older age is associated with an inferior humoral response after either exposure to SARS-CoV-2 or vaccination.30,38–40 Older individuals may present an impaired ability of antibody production compared with younger persons.38 A study has suggested that more than one-third of older vaccinated individuals may lose their NAb activity against the delta variant of concern at 6 months postfull vaccination with the BNT162b2 compared with <1% among younger adults.41 Even though the age of the subjects has a statistically significant impact on NAbs production, which is reflected on the NAbs values 2 weeks after completion of vaccination, this effect fades with time in our study. Age was found to exert a statistically significant influence only in the first trimester, as older age was associated with a more rapid clearance of NAbs. Furthermore, the percentage decrease in NAbs levels at a given timepoint and age group compared with the maximum NAbs levels observed 2 weeks after vaccination was found to be the highest for the 40 to 55 group and the ≥55 group, while the decrease is lower for the younger people (<40-year-old). However, no statistically significant effect of “age” on elimination ability can be demonstrated, as both using the kinetic modeling approach and the classical statistical comparisons, the P value is above the 5% significance threshold. Regarding anti-S-RBDs, there was no signal suggesting an age-dependent effect on antibody kinetics over time. Age may be one of the several factors, including also prior COVID-19 infection, immune deficiency, and vaccination schedule, that differentiate the magnitude and the kinetics of humoral response following COVID-19 vaccination between individuals.15,17,42–46
It is known that comorbidities can reduce the anticipated immunogenicity after COVID-19 vaccination, but such an association was not found in the period between 2 weeks and 9 months. A larger number of subjects in each category may be needed to detect further significant changes in antibody kinetics. However, it should be noted that we excluded individuals with major comorbidities such as cancer from the present analysis. Active treatment with immunosuppressive drugs has been identified as a key factor for an inferior humoral response following COVID-19 vaccination.47–51
To predict NAbs levels at 9, 12, 15, and 18 months after the second vaccine shot, simulations were conducted using the final population model, assuming that NAbs elimination would be the same as found in the last trimester (i.e., from the sixth to ninth month) after the second vaccine. In the future, similar studies involving time points after 9 months (at least one more) would provide a more accurate assessment of the elimination ability of NAbs. In this study, the simulations revealed that the average value of percent inhibition of NAbs would be 59% 1 year after the second vaccination. This value would further decrease to 52% after 15 months and reach a value of 45% 18 months after the second vaccination. These results mean that at 1 year after completion of vaccination, 41% of individuals would not be highly protected. This percentage would be even higher and reach 55% at 18 months.
The significance of our findings stems from the prognostic value of NAbs levels in terms of immunological protection against symptomatic COVID-19.12 As a result, the described NAbs reduction in time may inform public health policy for the use of booster doses. Despite the fact that emerging SARS-CoV-2 variants pose a threat to the high rate of immune protection following vaccination,16,52 a booster vaccine dose combined with transmission-reducing behaviors remains effective in preventing COVID-19.53,54 It should be noted that in our simulations, we used the identical kinetic parameter values for the extrapolation period after 9 months that we discovered in the 6- to 9-month interval. This option was chosen because it is considered the safest for forecasts, as there are no robust observations on kinetics after 9 months in either the data or the literature. As a result, this option would avoid significant over- or underestimation of NAbs values.
One limitation of this study is the relatively small sample size which can hamper the investigation of specific pathophysiological disorders, such as autoimmune illnesses. Therefore, subgroup analyses should be considered rather exploratory. However, the sample size was adequate enough to reveal significant differences in antibody titers among the examined timepoints. Concerning the role of gender in the numerous comparisons in this analysis, it should be emphasized that men and women were given unequal sample sizes. Women outnumber men by nearly a factor of 2. In general, unequal sample sizes can lead to biased comparisons, but in our case, this is not a problem because the imbalance is not extreme but rather reasonable (34.6% versus 65.4%), and the Mann-Whitney test, in particular, can perform well with unequal sample sizes. Taking also into consideration that a larger study including 3808 individuals showed that male gender was associated with a more rapid decline of NAb titers during the first 6 months following vaccination,22 the null effect of gender in our study should be interpretated with caution. In addition to the earlier, we did not perform laboratory evaluations for asymptomatic SARS-CoV-2 infections by means of either polymerase chain reaction or antigen-based assays at the examined timepoints. In this context, we cannot not rule out the occurrence of asymptomatic breakthrough infections that would increase the durability of anti-SARS-CoV-2 humoral response. A possible approach would have been to evaluate the presence of anti-N antibodies in individuals with substantial increase in antibody titers between consecutive measurements.22 However, none of the participants in our study showed a >4-fold increase in antibody titers during the study period; thus, the potential impact of unrecognized asymptomatic breakthrough infections on our results is low. It should also be mentioned that simulations use the information provided by the experimental data to make predictions. The more representative the original data is of the entire population, the more reliable the simulations will be. A larger sample with more men or patients with several comorbidities would allow for a more accurate investigation and thus more robust simulations. Furthermore, we did not evaluate the kinetics of T-cell responses over time. The sustainability of cellular response against SARS-CoV-2 following vaccination may impact both humoral response and protection against COVID-19.18,55–57 Future research could investigate the neutralizing efficacy of anti-SARS-CoV-2 antibodies against variants of concern as well as the role of anti-S-RBDs toward COVID-19 infection.
In conclusion, our prospective study showed a sustained but declining humoral immunity against SARS-CoV-2 at 9 months postvaccination with BNT162b2 among 309 healthy individuals. This effect may reflect a declining degree of immune protection against COVID-19 and advocates for the administration of booster vaccine shots especially in areas with emerging outbreaks.
Acknowledgments
We thank Mrs Ioanna Charitaki, RN; Mrs Tina Bagratuni, PhD; Mrs Niko-letta-Aikaterini Kokkali, RN; and Christine Ivy Liacos, PhD for administrative, technical, and material support. We also thank the study participants for donating their time and samples.
Disclosures
ET is a HemaSphere Associate Editor. The authors have no conflicts of interest to disclose.
Funding
We thank SYN-ENOSIS (Greece), IEMBITHEK (Greece), and Roche (Greece) for partially funding this study.
References
- 1.Tsang HF, Chan LWC, Cho WCS, et al. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev Anti Infect Ther. 2021;19:877–888. [DOI] [PubMed] [Google Scholar]
- 2.Tentolouris A, Ntanasis-Stathopoulos I, Vlachakis PK, et al. COVID-19: time to flatten the infodemic curve. Clin Exp Med. 2021;21:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyerstedt S, Casaro EB, Rangel ÉB. COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis. 2021;40:905–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korompoki E, Gavriatopoulou M, Fotiou D, et al. Late onset hematological complications post COVID-19: an emerging medical problem for the hematologist. Am J Hematol. 2021 October 23. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavriatopoulou M, Korompoki E, Fotiou D, et al. Organ-specific manifestations of COVID-19 infection. Clin Exp Med. 2020;20:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korompoki E, Gavriatopoulou M, Hicklen RS, et al. Epidemiology and organ specific sequelae of post-acute COVID19: a narrative review. J Infect. 2021;83:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Lancet Infectious D. COVID-19 vaccine equity and booster doses. Lancet Infect Dis. 2021;21:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jalkanen P, Kolehmainen P, Häkkinen HK, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021;12:3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosati M, Terpos E, Agarwal M, et al. Distinct neutralization profile of spike variants by antibodies induced upon SARS-CoV-2 infection or vaccination. Am J Hematol. 2021 October 21. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Beltran WF, Lam EC, Astudillo MG, et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476–488 e11.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wall EC, Wu M, Harvey R, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terpos E, Trougakos IP, Karalis V, et al. Kinetics of anti-SARS-CoV-2 antibody responses 3 months post complete vaccination with BNT162b2; A Prospective Study in 283 health workers. Cells. 2021;10:1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terpos E, Karalis V, Ntanasis-Stathopoulos I, et al. Robust neutralizing antibody responses 6 months post vaccination with BNT162b2: A Prospective Study in 308 healthy individuals. Life (Basel). 2021;11:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonate PL. Pharmacokinetic-Pharmacodynamic Modeling and Simulation. 2nd ed. New York: Springer; 2011 [Google Scholar]
- 20.Campo F, Venuti A, Pimpinelli F, et al. Antibody persistence 6 months post-vaccination with BNT162b2 among health care workers. Vaccines (Basel). 2021;9:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pegu A, O’Connell SE, Schmidt SD, et al. Durability of mRNA-1273 vaccine-induced antibodies against SARS-CoV-2 variants. Science. 2021;373:1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doria-Rose N, Suthar MS, Makowski M, et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384:2259–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tre-Hardy M, Cupaiolo R, Wilmet A, et al. Six-month interim analysis of ongoing immunogenicity surveillance of the mRNA-1273 vaccine in healthcare workers: A third dose is expected. J Infect. 2021;83:381–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tre-Hardy M, Cupaiolo R, Wilmet A, et al. Waning antibodies in SARS-CoV-2 naive vaccinees: Results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers. J Infect 2021;83:381–412.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas SJ, Moreira ED, Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398:2093–2100.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terpos E, Stellas D, Rosati M, et al. SARS-CoV-2 antibody kinetics eight months from COVID-19 onset: persistence of spike antibodies but loss of neutralizing antibodies in 24% of convalescent plasma donors. Eur J Intern Med. 2021;89:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bylicki O, Delarbre D, Mayet A, et al. Neutralizing antibody response to SARS-CoV-2 persists 9 months post symptom onset in mild and asymptomatic patients. Int J Infect Dis. 2021;112:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figueiredo-Campos P, Blankenhaus B, Mota C, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur J Immunol. 2020;50:2025–2040.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terpos E, Politou M, Sergentanis TN, et al. Anti-SARS-CoV-2 antibody responses in convalescent plasma donors are increased in hospitalized patients; Subanalyses of a Phase 2 Clinical Study. Microorganisms. 2020;8:E1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Havervall S, Ng H, Jernbom Falk A, et al. Robust humoral and cellular immune responses and low risk for reinfection at least 8 months following asymptomatic to mild COVID-19. J Intern Med. 2021 August 30. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Zuiani A, Fischinger S, et al. Quick COVID-19 healers sustain anti-SARS-CoV-2 antibody production. Cell. 2020;183:1496–1507.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostopoulos IV, Orologas-Stavrou N, Rousakis P, et al. Recovery of innate immune cells and persisting alterations in adaptive immunity in the peripheral blood of convalescent plasma donors at eight months post SARS-CoV-2 infection. Microorganisms. 2021;9:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Israel A, Shenhar Y, Green I, et al. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. medRxiv. Preprint posted online August 22, 2021. doi:10.1101/2021.08.19.21262111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collier DA, Ferreira IATM, Kotagiri P, et al. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature. 2021;596:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller L, Andree M, Moskorz W, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021;73:2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terpos E, Trougakos IP, Apostolakou F, et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am J Hematol. 2021;96:E257–E259.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tober-Lau P, Schwarz T, Vanshylla K, et al. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respir Med. 2021;9:e104–e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gavriatopoulou M, Terpos E, Malandrakis P, et al. Myeloma patients with COVID-19 have superior antibody responses compared to patients fully vaccinated with the BNT162b2 vaccine. Br J Haematol. 2021 September 16. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terpos E, Trougakos IP, Karalis V, et al. Comparison of neutralizing antibody responses against SARS-CoV-2 in healthy volunteers who received the BNT162b2 mRNA or the AZD1222 vaccine: Should the second AZD1222 vaccine dose be given earlier? Am J Hematol. 2021;96:E321–E324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terpos E, Trougakos IP, Gavriatopoulou M, et al. Low neutralizing antibody responses against SARS-CoV-2 in older patients with myeloma after the first BNT162b2 vaccine dose. Blood. 2021;137:3674–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markewitz R, Pauli D, Dargvainiene J, et al. The temporal course of T- and B-cell responses to vaccination with BNT162b2 and mRNA-1273. Clin Microbiol Infect. 2021 September 20. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gavriatopoulou M, Terpos E, Ntanasis-Stathopoulos I, et al. Poor neutralizing antibody responses in 106 patients with WM after vaccination against SARS-CoV-2; a prospective study. Blood Adv. 2021;5:4398–4405.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terpos E, Gavriatopoulou M, Fotiou D, et al. Poor neutralizing antibody responses in 132 patients with CLL, NHL and HL after vaccination against SARS-CoV-2: a prospective study. Cancers (Basel). 2021;13:4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021;11:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gavriatopoulou M, Terpos E, Kastritis E, et al. Low neutralizing antibody responses in WM, CLL and NHL patients after the first dose of the BNT162b2 and AZD1222 vaccine. Clin Exp Med. 2021 July 20. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corti C, Antonarelli G, Scotté F, et al. Seroconversion rate after vaccination against COVID-19 in cancer patients—a systematic review. Ann Oncol. 2021 October 28. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kustin T, Harel N, Finkel U, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2-mRNA-vaccinated individuals. Nat Med. 2021;27:1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rella SA, Kulikova YA, Dermitzakis ET, et al. Rates of SARS-CoV-2 transmission and vaccination impact the fate of vaccine-resistant strains. Sci Rep. 2021;11:15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agrati C, Castilletti C, Goletti D, et al. Coordinate induction of humoral and spike specific T-cell response in a cohort of Italian health care workers receiving BNT162b2 mRNA vaccine. Microorganisms. 2021;9:1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cassaniti I, Bergami F, Percivalle E, et al. Humoral and cell-mediated response elicited by SARS-CoV-2 mRNA vaccine BNT162b2 e in healthcare workers: a longitudinal observational study. Clin Microbiol Infect. 2021 September 25. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mateus J, Dan JM, Zhang Z, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science. 2021;374:eabj9853. [DOI] [PMC free article] [PubMed] [Google Scholar]