Abstract
Recently identified severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants Mu and C.1.2 have spike proteins with mutations that may confer resistance to natural and vaccine-elicited antibodies. Analysis of neutralizing antibody titers in the sera of vaccinated individuals without previous history of infection and from convalescent individuals show partial resistance of the viruses. In contrast, sera from individuals with a previous history of SARS-CoV-2 infection who were subsequently vaccinated neutralize variants with titers 4- to 11-fold higher, providing a rationale for vaccination of individuals with previous infection. The heavily mutated C.1.2 spike is the most antibody neutralization-resistant spike to date; however, the avidity of C.1.2 spike protein for angiotensin-converting enzyme 2 (ACE2) is low. This finding suggests that the virus evolved to escape the humoral response but has a decrease in fitness, suggesting that it may cause milder disease or be less transmissible. It may be difficult for the spike protein to evolve to escape neutralizing antibodies while maintaining high affinity for ACE2.
Keywords: SARS-CoV-2, Mu, C.1.2, Pfizer BNT162b2, Moderna mRNA-1273, Ad26.COV2.S, antibodies, vaccine, COVID-19, ACE2
Graphical abstract
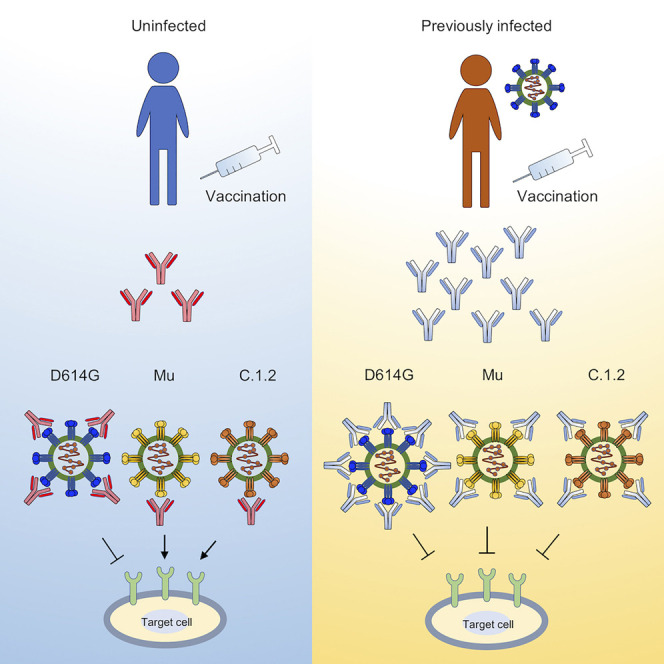
Tada et al. show that infection with SARS-CoV-2 followed by vaccination results in broadly neutralizing antibody. The C.1.2 variant has a highly mutated spike and is the most neutralization-resistant variant; however, its affinity for ACE2 is decreased. Thus, the virus cannot evolve to escape humoral response without becoming less fit.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) isolates have been classified by the World Health Organization (WHO) as variants of concern (VOCs; Alpha [B.1.1.7], Beta [B.1.351], Gamma [B.1.1.248], and Delta [B.1.617.2]) and variants of interest (VOIs) that include Lambda [C.37]) and newly classified Mu (B.1.621) (WHO, 2021). In addition, a yet unclassified C.1.2 variant was identified in South Africa (Scheepers et al., 2021) that appears to be increasing in prevalence and spreading to neighboring countries, and a variant termed Delta+N501S was identified in Japan, currently at low frequency. Mu (Mullen et al., 2021a) and C.1.2 (Mullen et al., 2021b; Scheepers et al., 2021) have mutations in the receptor binding domain (RBD) of the spike protein that could contribute to increased transmissibility and cause resistance to neutralization by convalescent sera and vaccine-elicited and therapeutic monoclonal antibodies (mAbs).
In this study, we measured the infectivity of viruses with the Mu, C.1.2, and Delta+N501S spike proteins and determined their susceptibility to neutralization by convalescent and vaccine-elicited antibodies, both in previously infected and uninfected individuals. Viruses with the variant spikes were partially resistant to neutralization. The C.1.2 variant, which is highly mutated, was the most resistant. Sera from previously infected patients vaccinated with BNT162b2 had high neutralizing titer against all of the variants, providing a strong rationale for the vaccination of previously infected individuals. The resistance of the C.1.2 spike protein to neutralization was largely mediated by the Y449H mutation in the RBD. However, the mutation resulted in a significant decrease in avidity of the spike protein for angiotensin-converting enzyme 2 (ACE2). This encouraging finding suggests that although the viral spike protein has managed to mutate to escape neutralizing antibodies, this resulted in a fitness cost that will limit its spread through the human population.
Results
Prevalence and infectivity of Mu, C.1.2, and Delta+N501S variants
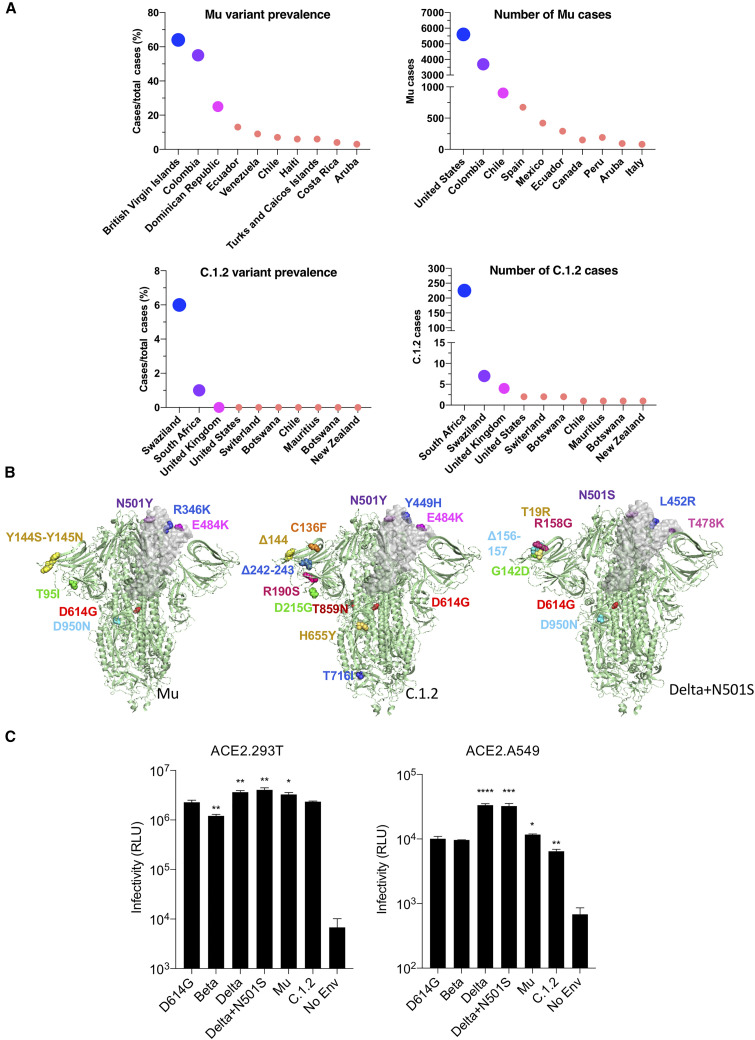
As of October 2021, the prevalence of the Mu variant was highest in the British Virgin Islands and Colombia, where its accounts for 64% and 55% of sequenced cases (Figure 1 A). It is present at low frequency in Central and South America. The virus has also been found in the United States and Europe, although frequencies have not yet been accurately determined. C.1.2 is present with a prevalence rate of 6% in Swaziland and 1% in South Africa, and small numbers of cases have been sequenced in as many as 10 other countries (Figure 1A). In addition, a variant of Delta was recently identified in a handful of cases, termed here Delta+N501S, and has not yet been further characterized.
Figure 1.
Mu (B.1.621), C.1.2, and Delta+501S variant prevalence and spike protein mutations
(A) The global prevalence of Mu and C.1.2 variants is shown for countries with the highest prevalence or cases (extracted from https://outbreak.info/).
(B) Mutations in Mu, C.1.2, and Delta+N501S variant spikes are shown on the three-dimensional spike protein structure. A single RBD in each is shown in gray (side view). The Protein Data Bank (PDB) file of spike protein (PDB: 7BNM) (Benton et al., 2021) was downloaded from the PDB. 3D view of protein was obtained using PyMOL.
(C) The infectivity of Beta, Delta, Delta+N501S, Mu, and C.1.2 (-T716I) variant spikes pseudotyped lentiviruses on ACE2.293T and ACE2.A549 cells is shown. The viruses were normalized for RT activity and measured in triplicate with error bars that indicate the standard deviation. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001. The experiment was done three times with similar results.
The variants have unique mutations in the RBD and N-terminal domain (NTD; Figures 1B and S1A). The Mu spike has RBD mutations R346K, E484K, and N501Y; C.1.2 has Y449H, E484K, and N501Y; and Delta+N501S has L452R and T478K (Figures 1B and S1A). To evaluate the function and sensitivity of the variant spikes to antibody neutralization, we generated lentiviruses pseudotyped with the Mu, C.1.2, and Delta+N501S spike proteins and, in addition, a pseudotype with the C.1.2 RBD mutations (Y449H, E484K, N501Y) and pseudotypes with the individual RBD mutations of each variant spike. The variant spike proteins were similarly expressed and proteolytically processed in transfected cells and were incorporated into lentiviral virions at a level similar to that of the parental D614G spike protein (Figure S1B).
Analysis of the infectivity of viruses with the variant spike proteins on ACE2.293T and ACE2.A549 cells showed a slight decrease for the Beta spike compared with D614G (1.8-fold) on ACE2.293T cells, while Delta, Delta+N501S, and Mu were slightly increased (Figure 1C). The pattern of infectivity was similar on ACE2.A549 cells, except that the infectivity differences were greater, most likely because of the low level of ACE2 on these cells. Analysis of the individual point mutations (Figure S1C) showed the individual Beta and Delta RBD mutations (R346K, Y449H, E484K, N501Y, N501S) did not significantly increase infectivity. A spike protein with the NTD C.1.2 mutations (P9L-C136F-Δ144-190S-D215G-Δ242-243) also had wild-type infectivity, while a spike with the C.1.2 RBD mutations had a significant increase in infectivity (1.9-fold). A spike containing the CTD mutations (H655Y, N679K, T716I, T859) of C.1.2 was decreased 15-fold. Similar infectivity ratios were obtained on ACE2.A549 cells.
Neutralization of variants by convalescent and vaccine-elicited antibodies
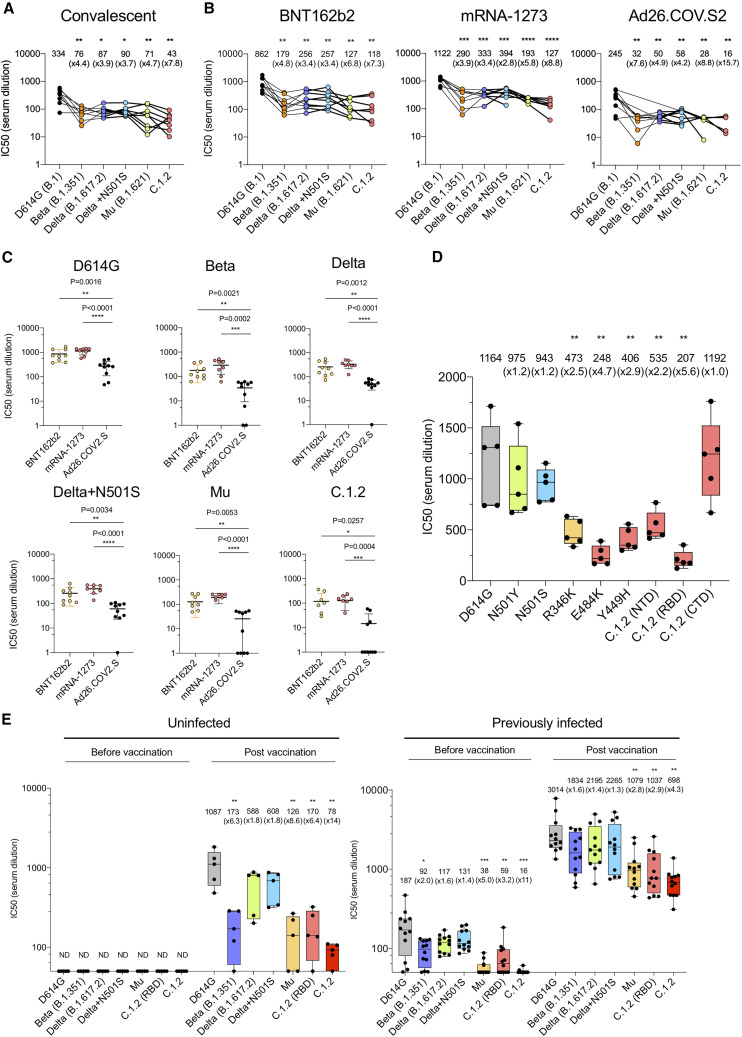
To determine the susceptibility of the viruses with the variant spike proteins to antibody neutralization, we analyzed the neutralizing titers of serum antibodies elicited by the BNT162b2 and mRNA-1273 mRNA vaccines and the Ad26.COV2.S adenoviral vector-based vaccine on the variants. The vaccine sera analyzed were collected from individuals at similar time points after final injection (a mean of 90 days for BNT162b2, 80 for mRNA-1273, and 82 for Ad26.COV2.S; Table S1), and all participants tested negative for antibodies against the SARS-CoV-2 N protein, suggesting no history of SARS-CoV-2 infection (Table S1). Convalescent sera neutralized D614G spike with a mean titer of 334. Neutralization of Beta, Delta, Delta+, and Mu variants showed a modest 3.7- to 4.7-fold decrease in neutralizing titer, while C.1.2 was more resistant to neutralization with a 7.8-fold decrease (Figure 2 A). BNT162b2 sera neutralized virus with the D614G spike with a mean titer of 862, a 2.6-fold increase compared with convalescent sera. The neutralizing titers against Beta, Delta, and Delta+N501S were decreased 4.8-, 3.4-, and 3.4-fold, respectively. Mu and C.1.2 were somewhat more resistant with a 6.8- and 7.3-fold decrease in titer, respectively. mRNA-1273-vaccinated sera showed a similar pattern of neutralization, with C.1.2 being the most resistant (8.8-fold decreased titer). Neutralizing antibody titers of sera from Ad26.COV2.S-immunized individuals neutralized D614G with an average titer of 245 and showed a similar pattern of variant neutralization. Titers against C.1.2 fell into a range below 50, the minimum detectable by the assay (Figure 2B). Presentation of the data grouped by variant shows decreased neutralizing titers against the variants by sera of the Ad26.COV2.S-vaccinated individuals (Figure 2C). Analysis of the spike proteins with individual variant mutations showed that the neutralization resistance of Mu was caused by R346K and E484K, while resistance of C.1.2 was caused by E484K, Y449H, and the NTD (P9L-C136F-Δ144-190S-D215G-Δ242-243) (Figure 2D).
Figure 2.
Neutralization of variant spike pseudotyped viruses by convalescent sera, antibodies elicited by RNA, and adenoviral vector vaccines
(A) Neutralization of pseudotyped viruses with D614G, Beta, Delta, Delta+N501S, Mu, and C.1.2 (-T716I) variant spikes by convalescent serum samples from eight donors was tested. The serum was collected at 32–57 days after infection. Each dot represents the IC50 for a single donor. Neutralization titers of variants were compared with that of D614G.
(B and C) Neutralizing titers of serum samples from BNT162b2-vaccinated individuals (n = 9), mRNA-1273-vaccinated donors (n = 8), and Ad26.COV2.S-vaccinated individuals (n = 10) were measured. Sera were collected at 90, 80, and 82 days on average after the last immunization. IC50 of neutralization of virus from individual donors is shown. Significance was based on two-sided testing.
(D) Neutralization titers of viruses with single point mutations by antibodies elicited by BNT162b2. Neutralizing titers of serum samples from BNT162b2-vaccinated individuals (n = 5). Sera were collected 7 days after second immunization. Each dot represents the IC50 for a single donor.
(E) Neutralizing titers of serum samples from BNT162b2-vaccinated individuals with (n = 5) or without previous SARS-CoV-2 infection (n = 12) was measured. The neutralization IC50 of virus from individual donors is shown. The sera were collected 7 days after the second immunization. The sera were collected prior to February 2021. Thus, the individuals were not infected with Delta and would have been infected with D614G, Alpha, or Iota variant. Significance was based on two-sided testing. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001. The experiment was done twice with similar results.
Analysis of neutralization by the sera of donors who had a history of COVID-19 pre-BNT162b2 vaccination showed an overall higher neutralizing titer against all of the variants. The neutralizing titer of sera from uninfected donors against D614G was 1,087 on average (Figure 2E), with Beta, Delta, Delta+N501S, Mu, and C.1.2 having a 1.8- to 14-fold decrease in titer. In contrast, previously infected and then vaccinated donor sera were significantly increased in titer against D614G (2.8-fold), and the titers remained high for all of the variants (Figure 2E). After two doses of vaccination, previously infected donors had a 10.6-fold increase in neutralizing titer against the Beta variant compared with uninfected donors. Titers were increased 3.7-fold for Delta and Delta+N501S. Overall, the neutralization titers of sera from previously infected donors were 8.5- to 8.9-fold greater against Mu and C.1.2 variants compared with uninfected individuals (Table S2).
Decreased avidity of C.1.2 spike protein for ACE2
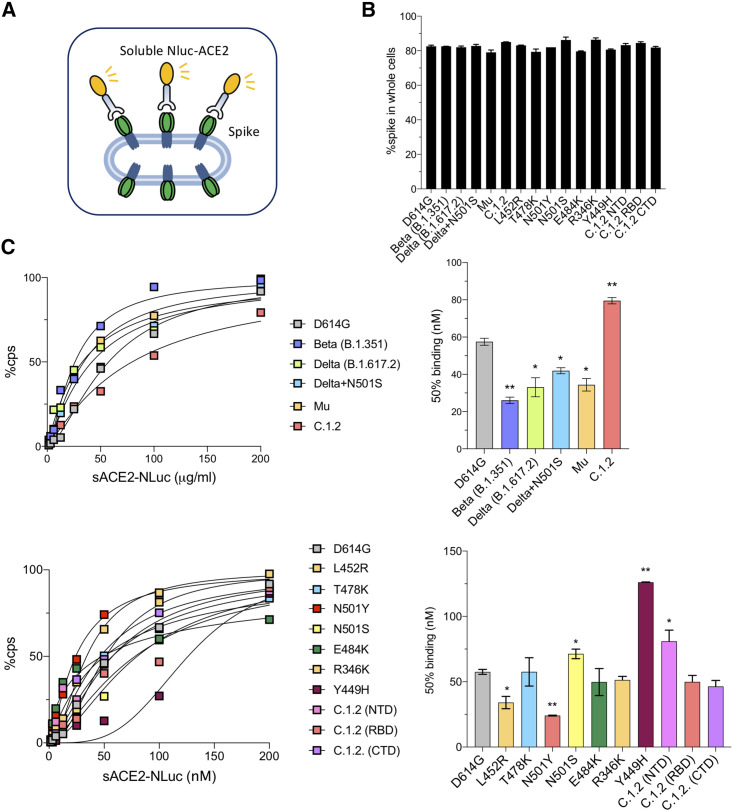
To measure the ACE2 avidity of the variant spikes, we established an ACE2 avidity assay in which the variant spike proteins were expressed in 293T cells and then incubated with a serially diluted soluble ACE2:nanoluciferase fusion protein (sACE2-NLuc) (Figure 3 A). Similar cell surface spike protein expression levels on the transfected 293T cells were confirmed by flow cytometry (Figure 3B). The analysis showed increased ACE2 avidity of the Beta, Delta, Delta+N501S, and Mu spikes (2.2-, 1.7-, 1.4-, and 1.7-fold, respectively), as indicated by a decrease in the concentration required to achieve 50% occupancy of the spike protein. In contrast, C.1.2 bound ACE2 with decreased avidity, requiring 1.4-fold higher concentration of ACE2 for 50% relative light unit (RLU) compared with D614G and 2.4-fold decrease as compared with the avidity of Beta variant spike protein (Figure 3C). Analysis of the point-mutated spike proteins showed that the increased avidity of Beta, Delta, and Mu with ACE2 was attributed to N501Y and L452R (Figure 3C). The decreased avidity C.1.2 for ACE2 was due to the combination of the Y449H in the RBD and the mutated NTD. These results were confirmed in a virion avidity assay in which pseudotyped virions were incubated with sACE2 and then added to ACE2.293T cells, and the amount of bound virions was then measured (Figure S2). In this assay, virions with D614G, Beta, Delta, Delta+N501S, C.1.2 (RBD), and Mu spikes bound similarly to ACE2, while C.1.2 avidity was decreased. These findings suggest that the C.1.2 spike protein binds ACE2 with a relatively lower avidity than the other spike protein variants.
Figure 3.
Avidity and fusion of variant spikes to ACE2
(A) The diagram shows the principle of the ACE2 avidity assay in which 293T cells transfected with variant spike protein expression vector are incubated with serially diluted sACE2-NLuc protein. Following a 30-min incubation, the unbound fusion protein is removed and the bound protein measured by luciferase assay.
(B) The expression level of variant spike proteins in transfected cells was analyzed by flow cytometry.
(C) ACE2 avidity of the indicated variant spike proteins is shown as curves with maximal avidity defined as luciferase activity upon avidity of the ACE2.NLuc fusion protein at 50 mg/mL set to 100% (left two panels). The histogram on the right shows 50% of maximal avidity.
Therapeutic antibodies neutralize Mu and C.1.2
Regeneron mAbs maintained their ability to neutralize Delta, Delta+N501S, Mu, and C.1.2. REGN10933 showed a 50-fold decrease in titer against virus with the Beta spike (Figure S3A), as previously reported (Chen et al., 2021; Tada et al., 2021; Wang et al., 2021a), but maintained neutralizing activity against the others, while REGN10987 maintained activity against all of the variants (Figure S3B). The combination of the two mAbs was highly active against all of the variants (Figure S3C).
Discussion
Analysis using pseudotyped lentiviruses showed that viruses with the heavily mutated C.1.2 spike protein were the most resistant to neutralization by convalescent sera and mRNA and adenoviral vector-based vaccine-elicited antibodies of any of the variant spike proteins tested. The Mu spike was also relatively resistant to neutralization. The resistance of virus with the C.1.2 spike to neutralization was caused by a combination of the RBD mutations N501Y, Y449H, and E484K and the NTD mutations. In contrast, the sera of individuals with a previous history of SARS-CoV-2 infection were much more broadly neutralizing than those without a prior history; they neutralized viruses with the variant spike proteins, with a 4- to 11-fold increase in titer (10.6-fold for Beta, 8.6-fold for Mu, and 8.8-fold for C.1.2). The Regeneron therapeutic mAbs retained their ability to neutralize Mu and C.1.2 variants. While the C.1.2 spike protein evaded antibody neutralization, its avidity for ACE2 was decreased compared with that of the other variants, suggesting that the virus may be less fit and less transmissible.
Although neutralizing antibody titers against viruses with the Mu and C.1.2 spike proteins elicited by vaccination of individuals without previous infection were decreased compared with the parental D614G, mathematical modeling predicts that the titers are sufficient to provide a high degree of protection from infection and serious disease. The model proposed by Khoury et al., (2021) predicts that an antibody titer that is 20% that of the convalescent titer will provide 50% protection from SARS-CoV-2 infection. In our study, the mean convalescent titer was 334 (Table S1), predicting that a half-maximal inhibitory concentration (IC50) of 67 would correspond to 50% protection. The model further predicts that an antibody titer that is 3% that of the mean titer of convalescent sera titer is required to protect against severe disease, corresponding to a titer of 10 in our study and suggesting that vaccination with either of the mRNA vaccines will remain protective against severe disease caused by infection with the Mu or C.1.2 variants.
The neutralizing titers of sera from previously infected BNT162b2-vaccinated individuals, those with a history of previous SARS-CoV-2 infection, were from 4- to 11-fold higher than those of vaccinated individuals without previous infection and effectively neutralized all of the variants. These findings are consistent with those previously reported by Wang et al. (2021b) and Urbanowicz et al. (2021), who showed similar increases in the neutralizing titer against the VOCs by sera from individuals with previous infection. The findings demonstrate that individuals can raise a broadly neutralizing humoral response by generating a polyclonal response to multiple spike protein epitopes (Robbiani et al., 2020; Wu et al., 2021). The high titer and breadth of the antibody response suggest that it will protect against current variants and is likely to protect against future variants that may arise.
Our analyses of the mutations in the variant spike protein using lentiviral pseudotyped virions showed an unexpected and apparently artifactual finding with one-point mutation. Viruses pseudotyped by C.1.2 were 18-fold decreased compared with the D614G control virus. The C.1.2 spike contains a T716I mutation, a mutation also present in the Alpha spike protein, where it similarly causes a marked decrease in the infectivity of lentiviral pseudotyped virus. Omission of the mutation from the C.1.2 spike restored infectivity of virus with the variant spike (Figure S1D). The viruses used in this study had the modified C.1.2 (-T716I) spike protein to ensure confidence in the results. The cause of the decreased infectivity of spike proteins with the T716I mutation is unclear. It may be a function of the 293T producer cells because it is clear that viruses such as Alpha produced in vivo are highly infectious. T716 lies in the proximity of a potential glycosylation site at position 717 and thus could interfere with or cause aberrant spike protein glycosylation. The mutation had no effect on expression of the spike protein in transfected cells, packaging into virions, or avidity for ACE2. The mutation also had no effect on the antibody neutralization profile (Figure S4). Although the T716I mutation did not influence the results in our study, its effect should be considered in studies with lentiviral pseudotypes with spike proteins that have the mutation.
In this study, we measured spike protein avidity for ACE2 using a novel assay in which we incubated an ACE2:luciferase fusion protein with spike protein-expressing cells and then determined the amount of bound fusion protein by luciferase assay. A comparison of the avidities measured by this method with those published for the spike:ACE2 interaction correspond closely, validating our method. We measured a KD of 60 nM for the D614G spike:ACE2 interaction, a value very close to that of Barton et al. (2021), who reported a KD of 74 mM by surface plasmon resonance. A survey of other published reports shows a KD of 6–133 nM for the spike:ACE2 interaction (Laffeber et al., 2021; Liu et al., 2021a, 2021b; Supasa et al., 2021; Wrapp et al., 2020; Zhang et al., 2020, 2021). In our assay, the avidities of the variant spike proteins relative to the parental D614G are similar to the values reported by others; Gong et al. (2021) reported a 3.5- and 2.3-fold higher ACE2 avidity for the Beta and Delta variants, respectively, which is close to the 2.2-fold increase for Beta and 1.7-fold increase for Delta measured in our assay.
The emergence of the Mu and C.1.2 variants does not appear to raise new public health concerns. Mu is nearly identical to Delta with the exception of the conservative R346K mutation. As of October 2021, the variant has been found in Europe and North and South America with a total of 3,689 cases in Colombia, 5,604 cases in North America, and 12,762 cases worldwide, and there is no evidence for increasing prevalence (Mullen et al., 2021a). The C.1.2 variant is currently at low prevalence with a highly restricted geographic distribution. The high titers of antibody against all of the variants in individuals with a history of previous infection is encouraging and demonstrates the ability of individuals to mount a broadly neutralizing antibody response that will protect against these variants. Moreover, the breadth of the vaccine response in such individuals suggests that it will protect against future variants that may emerge.
It is interesting that the C.1.2 spike protein has a decreased avidity for ACE2 compared with that of the other variant spikes. Its RBD has the N501Y mutation that confers increased avidity for ACE2 (Gu et al., 2020; Starr et al., 2020), but this is counteracted by the novel Y449H mutation that decreases ACE2 avidity. The decreased avidity for ACE2 is unexpected because previous RBD mutations, such as N501Y and L452R, increase ACE2 avidity resulting in increased transmissibility of the variant viruses. Although the Y449 mutation in the C.1.2 RBD decreases its avidity for ACE2, it provides a significant degree of neutralization resistance to convalescent and vaccine-elicited antibodies, suggesting that the mutation was selected as an antibody escape mutation. The decrease in ACE2 avidity of the C.1.2 spike protein is likely to decrease transmissibility of the variant, limiting its spread despite its resistance to antibody neutralization. This finding suggests that it may be difficult for SARS-CoV-2 to mutate to escape the humoral immune response without suffering a decrease in fitness, mitigating fears about the emergence of a highly pathogenic, neutralization-resistant variant. This prediction could lessen concerns regarding the newly identified, highly mutated Omicron variant, if found to be the case for its spike protein.
Limitations of the study
The sample numbers were limited by the subject numbers enrolled in the clinical studies at the NYU Langone Health (NYULH) Vaccine Center. The sample number analyzed in this study was sufficient to provide statistically significant values but would have achieved a higher confidence level with the analysis of a larger number of samples. The donors analyzed were recruited from a single hospital, which could introduce a sampling bias and could result in differences with similar studies that analyze donors from different geographic locations, ethnicities, medical histories, or ages.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-GAPDH | Life Technologies | Cat# AM4300, RRID:AB_437392 |
| anti-p24 antibody (AG3.0) | AIDS respository | Cat# ARP4121 |
| Anti-spike antibody | GeneTex | Cat# GTX632604, RRID:AB_2864418 |
| goat anti-mouse HRP-conjugated second antibody | Sigma | Cat# A4416, RRID:AB_258167 |
| Alexa-fluor 594-conjugated goat anti-mouse IgG | Biolegend | Cat# 405326, RRID:AB_2563308 |
| Bacterial and virus strains | ||
| SARS-CoV-2 Δ19 S (D614G, Beta, Delta, Delta+N501S, Mu, C.1.2) pseudotyped reporter virus | This paper | N/A |
| Biological samples | ||
| Convalescent sera | NYU Vaccine Center with written consent under I.R.B. approval | IRB 20-00595 and IRB 18-02037 |
| Sera from BNT162b2, mRNA-1273, AD26.COV2.S vaccinated donors | NYU Vaccine Center with written consent under I.R.B. approval | IRB 20-00595 and IRB 18-02037 |
| Sera from BNT162b2 vaccinated donors (Previously infected) | NYU Vaccine Center with written consent under I.R.B. approval | IRB 20-00595 and IRB 18-02037 |
| Chemicals, peptides, and recombinant proteins | ||
| Soluble ACE2-NLuc | This paper | N/A |
| Soluble ACE2 | Tada et al., 2020 | N/A |
| REGN10933 | Tada et al., 2021 | N/A |
| REGN10987 | Tada et al., 2021 | N/A |
| Critical commercial assays | ||
| Nano-Glo® Luciferase Assay System | Promega | Cat# N1120 |
| Galacto-Light™ Reaction Buffer Diluent with Galacton-Plus™ Substrate | Applied Biosystems | Cat# T1056 |
| Experimental models: Cell lines | ||
| 293T | ATCC | N/A |
| ACE2.293T | Tada et al., 2020 | N/A |
| ACE2.A549 | Tada et al., 2021 | N/A |
| Software and algorithms | ||
| MacVector ver 17.0.0 (27) | MacVector Inc | N/A |
| GraphPad Prism 8 Software | GraphPad Prism Software, Inc. | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Nathaniel R. Landau (nathaniel.landau@med.nyu.edu).
Materials availability
All unique DNA constructs, proteins and pseudotyped virus generated in this study are available from the Lead Contact upon request.
Experimental model and subject details
Human sera, monoclonal antibodies and recombinant protein
Convalescent sera were collected from healthcare workers at NYU Langone who were symptomatic and had tested positive for SARS-CoV-2 by PCR. Sera were collected 32 to 57 days post-symptom onset. Donor age and gender were not reported. BNT162b2-vaccinated sera were collected 90 days (mean) post-second immunization and mRNA-1273-vaccinated sera were collected at a mean 80 days post-second immunization. Ad26.COV2.S-vaccinated sera were collected at mean 82 days post-immunization. Serum samples from previously infected donors were collected 7 days post-second immunization with BNT162b2. Participants who reported experiencing COVID symptoms were confirmed as previously infected by direct PCR or antibody testing. Age and sex of the vaccinated donors is shown in Tables S1 and S2. Donors participated in clinical studies at the NYU Vaccine Center and sera were obtained with written consent under IRB-approved protocols (18-02035 and 18-02037).
sACE2 protein was produced in transfected CHO cells and purified by nickel chelate chromatography as previously described (Tada et al., 2020). REGN10933 and REGN10987 monoclonal antibodies were produced in transfected Freestyle 293 cells (Invitrogen) as previously described (Tada et al., 2021).
Cells
293T cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (P/S) at 37°C in 5% CO2.
ExpiCHO-S were purchase from Thermo Fisher Scientific and cultured in ExpiCHO expression medium at 37 °C in 8% CO2. ACE2.293T and ACE2.A549 cells were established by lipofection of 293T cells with pLenti.ACE2-HA using lipofectamine 2000 (Invitrogen). Single cell clones were selected in 1 μg/ml puromycin.
Method details
Plasmids
Plasmids used in the production of lentiviral pseudotyped virus have been previously described (Tada et al., 2020). Spike protein variant mutations were introduced into pcCOV2.Δ19.D614G by overlap extension PCR and confirmed by DNA sequencing. To construct the plasmid expression vector psACE2-Nluc encoding an ACE2:nanoluciferase fusion protein, DNA sequences corresponding to the extracellualar domain of human ACE2 (amino acids 1-741) and nanoluciferase were amplified by PCR with overlapping oligonucleotide primers. The amplicons were joined by overlap extension PCR using external primers containing Kpn-I and Xho-I sites and the resulting fragment was cloned into pcDNA6.
SARS-CoV-2 spike lentiviral pseudotypes
Lentivirus pseudotyped by variant SARS-CoV-2 spikes were produced as previously reported (Tada et al., 2020). Viruses were concentrated by ultracentrifugation and normalized for reverse transcriptase (RT) activity. Sera and monoclonal antibodies were serially diluted and then incubated with pseudotyped virus (approximately 2.5 X 107 cps) for 30 minutes at room temperature and then added to target cells. Luciferase activity was measured 2 days post-infection.
Protein purification
CHO cells at a density of 2.4 X 109 cells (6 X 106 cells/ml) were transfected with 400μg of sACE2-NLuc expression vector plasmid DNA using ExpiFectamine CHO Reagent (Thermo Fisher Scientific). The following day, ExpiCHOTM Enhancer was added and culture was continued at 32°C for another 5 days after which the culture medium was collected and passed through a 2.5 μM pore size filter. The fusion protein was purified by metal chelate chromatography on an AKTA prime FPLC (GE Healthcare).
ACE2 avidity assay
293T cells were transfected with mutated spike variant expression vectors using lipofectamine 2000 and seeded in a 96-well plate at 1 x 104 / well. Serially diluted sACE2 protein fused with nano-Luciferase was added to the cells. Following incubation for 30 minutes at 37oC, the unbound proteins were washed and luciferase activity was measured using Nano-Glo substrate (Nanolight) in an Envision 2103 microplate luminometer (PerkinElmer).
Neutralization assay by soluble ACE2
Briefly, pseudotyped virus was incubated with serially diluted recombinant soluble ACE2 protein for 1 hour at room temperature and subsequently added to 1 X 104 ACE2.293T cells. After 2 days, the cell medium was removed and 50 μl Nano-Glo luciferase substrate (Nanolight) was added. The luminescence signal was read in an Envision 2103 microplate luminometer.
Flow cytometry
Variant spikes overexpressing 293T cells were stained for 30 min at 4°C with anti-spike mAb and then stained with Alexa-fluor 594-conjugated goat anti-mouse IgG for 30 min at 4°C. The cells were analyzed by flow cytometry on a BD Biosciences LSR-II (Franklin Lakes, NJ) and the data analyzed with FlowJo software.
Immunoblot analysis
Proteins were analyzed on immunoblots probed with mouse anti-spike monoclonal antibody (1A9) (GeneTex), anti-p24 monoclonal antibody (AG3.0) and anti-GAPDH monoclonal antibody (Life Technologies) followed by goat anti-mouse HRP-conjugated secondary antibody (Sigma).
Quantification and statistical analysis
The data shown in Figure 1A were extracted from https://outbreak.info/ (Mullen et al., 2021a, 2021b; Scheepers et al., 2021) and analyzed using GraphPad Prism 8. All experiments were performed in technical duplicates or triplicates and the data were analyzed using GraphPad Prism 8. Statistical significance was determined by the two-tailed unpaired t-test or Nonparametric ANOVA test. The p values are corrected for multiple comparison testing using Welch’s t-test. Significance was based on two-sided testing and attributed to p< 0.05. Confidence intervals are shown as the mean ± SD or SEM (∗P≤0.05, ∗∗P≤0.01, ∗∗∗P≤0.001, ∗∗∗∗P≤0.0001).
Acknowledgments
The work was supported by grants from the National Institutes of Health (NIH) (DA046100, AI122390, and AI120898 to N.R.L.; UM1AI148574 to M.J.M.; U19AI082630 and R01AI158617 to R.S.H.).
Author contributions
T.T., H.Z., and N.R.L. designed the experiments. T.T., H.Z., and B.M.D. carried out the experiments and analyzed data. T.T., H.Z., B.M.D., and N.R.L. wrote the manuscript. M.I.S., A.C., R.S.H., and M.J.M. supervised specimen selection and the collection of clinical information, did the ELISAs, and provided reagents and key insights. All authors provided critical comments on the manuscript.
Declaration of interests
M.J.M. received research grants from Lilly, Pfizer, and Sanofi and serves on advisory boards for Pfizer, Merck, and Meissa Vaccines. The other authors declare no competing interests.
Published: December 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2021.110237.
Supplemental information
Data and code availability
-
•
Excel spreadsheets containing the data used in this study are available upon request from the lead contact.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Barton M.I., MacGowan S.A., Kutuzov M.A., Dushek O., Barton G.J., van der Merwe P.A. Effects of common mutations in the SARS-CoV-2 Spike RBD and its ligand, the human ACE2 receptor on binding affinity and kinetics. eLife. 2021;10:e70658. doi: 10.7554/eLife.70658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D.J., Wrobel A.G., Roustan C., Borg A., Xu P., Martin S.R., Rosenthal P.B., Skehel J.J., Gamblin S.J. The effect of the D614G substitution on the structure of the spike glycoprotein of SARS-CoV-2. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2022586118. e2022586118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.E., Winkler E.S., Case J.B., Aziati I.D., Bricker T.L., Joshi A., Darling T.L., Ying B., Errico J.M., Shrihari S., et al. In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains. Nature. 2021;596:103–108. doi: 10.1038/s41586-021-03720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S.Y., Chatterjee D., Richard J., Prévost J., Tauzin A., Gasser R., Bo Y., Vézina D., Goyette G., Gendron-Lepage G., et al. Contribution of single mutations to selected SARS-CoV-2 emerging variants Spike antigenicity. bioRxiv. 2021 doi: 10.1016/j.virol.2021.09.001. 2021.2008.2004.455140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H., Chen Q., Yang G., He L., Fan H., Deng Y.-Q., Wang Y., Teng Y., Zhao Z., Cui Y., et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 2020;369:1603–1607. doi: 10.1126/science.abc4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Laffeber C., de Koning K., Kanaar R., Lebbink J.H.G. Experimental evidence for enhanced receptor binding by rapidly spreading SARS-CoV-2 variants. J. Mol. Biol. 2021;433:167058. doi: 10.1016/j.jmb.2021.167058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang Q., Wei P., Chen Z., Aviszus K., Yang J., Downing W., Jiang C., Liang B., Reynoso L., et al. The basis of a more contagious 501Y.V1 variant of SARS-CoV-2. Cell Res. 2021;31:720–722. doi: 10.1038/s41422-021-00496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang Q., Wei P., Chen Z., Aviszus K., Yang J., Downing W., Jiang C., Liang B., Reynoso L., et al. The basis of a more contagious 501Y.V1 variant of SARS-CoV-2. Cell Res. 2021;31:720–722. doi: 10.1038/s41422-021-00496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen J.L., Tsueng G., Abdel Latif A., Alkuzweny M., Cano M., Haag E., Zhou J., Zeller M., Hufbauer E., Matteson N., et al. Center for Viral Systems Biology outbreak.info. Mu lineage report. Accessed September 1, 2021. 2020. https://outbreak.info/
- Mullen J.L., Tsueng G., Abdel Latif A., Alkuzweny M., Cano M., Haag E., Zhou J., Zeller M., Hufbauer E., Matteson N., et al. Center for Viral Systems Biology outbreak.info. C.1.2 lineage report. 2020. https://outbreak.info/ Accessed September 1, 2021.
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepers C., Everatt J., Amoako D.G., Tegally H., Wibmer C.K., Mnguni A., Ismail A., Mahlangu B., Lambson B.E., Richardson S.I., et al. Emergence and phenotypic characterization of C.1.2, a globally detected lineage that rapidly accumulated mutations of concern. medRxiv. 2021 2021.2008.2020.21262342. [Google Scholar]
- Starr T.N., Greaney A.J., Hilton S.K., Ellis D., Crawford K.H.D., Dingens A.S., Navarro M.J., Bowen J.E., Tortorici M.A., Walls A.C., et al. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell. 2020;182:1295–1310.e20. doi: 10.1016/j.cell.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supasa P., Zhou D., Dejnirattisai W., Liu C., Mentzer A.J., Ginn H.M., Zhao Y., Duyvesteyn H.M.E., Nutalai R., Tuekprakhon A., et al. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell. 2021;184:2201–2211.e7. doi: 10.1016/j.cell.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Dcosta B.M., Samanovic M.I., Herati R.S., Cornelius A., Zhou H., Vaill A., Kazmierski W., Mulligan M.J., Landau N.R. Convalescent-phase sera and vaccine-elicited antibodies largely maintain neutralizing titer against global SARS-CoV-2 variant spikes. mBio. 2021;12:e0069621. doi: 10.1128/mBio.00696-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Fan C., Chen J.S., Kaur R., Stapleford K.A., Gristick H., Dcosta B.M., Wilen C.B., Nimigean C.M., Landau N.R. An ACE2 microbody containing a single immunoglobulin Fc domain is a potent inhibitor of SARS-CoV-2. Cell Rep. 2020;33:108528. doi: 10.1016/j.celrep.2020.108528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowicz R.A., Tsoleridis T., Jackson H.J., Cusin L., Duncan J.D., Chappell J.G., Tarr A.W., Nightingale J., Norrish A.R., Ikram A., et al. Two doses of the SARS-CoV-2 BNT162b2 vaccine enhance antibody responses to variants in individuals with prior SARS-CoV-2 infection. Sci. Transl Med. 2021;13:eabj0847. doi: 10.1126/scitranslmed.abj0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- Wang Z., Muecksch F., Schaefer-Babajew D., Finkin S., Viant C., Gaebler C., Hoffmann H.-H., Barnes C.O., Cipolla M., Ramos V., et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2021) https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Liang B., Chen C., Wang H., Fang Y., Shen S., Yang X., Wang B., Chen L., Chen Q., et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat. Commun. 2021;12:1813. doi: 10.1038/s41467-021-22034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Cai Y., Xiao T., Lu J., Peng H., Sterling S.M., Walsh R.M., Jr., Rits-Volloch S., Zhu H., Woosley A.N., et al. Structural impact on SARS-CoV-2 spike protein by D614G substitution. Science. 2021;372:525–530. doi: 10.1126/science.abf2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Jackson C.B., Mou H., Ojha A., Peng H., Quinlan B.D., Rangarajan E.S., Pan A., Vanderheiden A., Suthar M.S., et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat. Commun. 2020;11:6013. doi: 10.1038/s41467-020-19808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Excel spreadsheets containing the data used in this study are available upon request from the lead contact.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.