Abstract
Background
Advanced age and multiple comorbidities have been established as a risk factor for more severe disease and increased mortality among patients with COVID-19, yet the impact of frailty in patients with cancer 75 years and older who are admitted, remains unclear.
Methods
To better understand the clinical presentation and course of illness for this population, we conducted a chart review of patients with cancer age 75 and older who were admitted to a comprehensive cancer center within 72 h of a confirmed COVID-19 diagnosis over a three-month period (March 1, 2020-May 31, 2020). Frequency and proportion of characteristics were reported. We additionally assessed the association between frailty and 30-day mortality using univariable logistic regression.
Results
Our cohort consisted of 70 patients. We found evidence that increased frailty based on MSK-FI was associated with increased risk of 30-day mortality (OR 1.37, 95% CI 1.00, 1.87; p-value = 0.051), though this did not meet conventional levels of significance.
Conclusion
Our analysis showed evidence of some association between degree of frailty and 30-day survival among older patients with cancer aged ≥75 who were admitted with COVID-19 infection. This finding illustrates the importance of frailty screening in the care management of older patients with cancer and COVID-19.
Keywords: Aging, Frailty, Cancer, COVID-19
1. Introduction
In December 2019, a novel coronavirus (SARS-CoV-2) was identified after a reported cluster of cases of pneumonia in Wuhan, China heralding the start of the COVID-19 pandemic. The COVID-19 pandemic has disproportionally affected older adults, with greater than 81% of deaths being in those >65 [1]. Mortality data among patients with cancer and COVID-19 during the height of the first surge in New York City (March – May 2020) showed that the median ages of patients who died and survived were 76 and 66, respectively [2]. Cancer is a disease of the aging and greater than 50% of older patients with cancer are frail or pre frail and at heightened risk for poor tolerance to cancer treatment and overall mortality [3]. Frail older adults often have atypical disease presentations due to diminished reserves and lack of ability to respond to stressors compared to younger patients. For example, patients with COVID-19 may present with functional decline with the absence of more common symptoms such as fever or cough [4]. Given that many patients with cancer have competing risk factors for complications and death [2,5] the effects of COVID-19 on this population have been a major concern to the oncology community. In this communication we describe our assessment of the relationship between frailty and short-term mortality in older patients with cancer when faced with COVID-19 infection. We conducted a retrospective observational study of patients with cancer age ≥ 75 at a comprehensive cancer center during the initial surge of COVID-19 in the Spring of 2020, with emphasis on clinical presentation and the impact of frailty on 30-day mortality.
2. Methods
This retrospective study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board.
We examined data from our institution's electronic health record (EHR) to identify all patients ≥75 who had a laboratory confirmed diagnosis of COVID-19 within 3 days before or after hospital admission over a three-month period (March 1, 2020-May 31, 2020). COVID-19 was diagnosed based on a positive result of a PCR assay of nasal pharyngeal swabs. Three patients diagnosed with COVID-19 initially admitted to another institution prior to transfer to our hospital were included in our study. Coded data elements extracted from the EHR for analysis included demographics, vital signs, laboratory findings, treatments administered, ICU admission, hospital disposition and mortality. Manual chart review was performed by study staff to abstract presenting symptoms (e.g. fever, cough, etc.) and clinical conditions that developed over the course of hospitalization (e.g. pneumonia, need for mechanical ventilation, etc.). We also abstracted comorbid conditions, cancer diagnosis, stage and recent anticancer treatment (e.g. chemotherapy, radiotherapy) as well as therapies administered for COVID-19 management. Frailty score was determined based on the Memorial Sloan Kettering Frailty Index (MSK-FI) derived at our institution based on the Modified Frailty Index [6]. In brief, the MSK-FI consists of ten comorbidities and one component related to functional assessment (refer to MSK-FI article listed under references for more details). The functional assessment component is based on four patient-reported activities of daily living and one patient reported instrumental activity of daily living. MSK-FI score ranges from 0 to 11, with a higher score indicating greater frailty [7].
Demographic and clinical characteristics, symptoms on presentation or during hospitalization, presence of comorbid conditions prior to admission, conditions arising during hospitalization, presence of elevated laboratory values within 72-h of admission, and type of COVID-19 treatment administered were reported as frequency and proportion across the cohort. To assess the association between frailty and 30-day mortality we used a univariable logistic regression with 30-day mortality as the outcome and continuous MSK-FI as the predictor. This analysis and all 30-day rates excluded one patient who was lost to follow-up 6-days after in-patient admission. All statistical analyses were performed using Stata 15.0 (StataCorp, College Station, TX).
3. Results
Over the study period, we identified 70 patients ≥75 who were admitted to the hospital and had confirmed COVID-19. Median age was 81 (quartiles 77–83) and the oldest patient in our cohort was 97 years old. Half of patients were female, 49% were married. 71% were White, 17% were Black and 11% Asian. The most common comorbidities were Hypertension (70%), Type II Diabetes (24%), Chronic Kidney Disease (16%), and Coronary Artery Disease (16%) and 51% had a history of smoking. Five (7.1%) patients had cognitive impairment or a diagnosis of dementia. The most common cancer diagnoses were Lung and bronchus (20%), Genitourinary (16%) and Lymphoma (13%). Among our cohort of 70 patients, 13 patients (19%; 95% CI 10%, 30%) had hematological malignancies. More than half (53%) of patients had received active anticancer treatment in the past two months. Fevers or chills (51%), cough (49%), weakness (39%), shortness of breath (36%), fatigue (30%), functional decline (26%), and anorexia (26%), were the most common presenting symptoms. Laboratory markers that had the highest elevations on admission were IL-6 (83%), D-dimer (79%), C-reactive protein (57%) and ferritin (51%). Commonly prescribed therapies included hydroxychloroquine (63%), azithromycin (46%), ceftriaxone (39%) and convalescent plasma (10%).
During the course of hospitalization 49% had pneumonia, with 66% reaching levels of hypoxemia requiring oxygen. Nearly one quarter (24%) developed delirium, and 21% experienced acute respiratory distress syndrome. Within 30 days of admission, seventeen patients (25%; 95% CI 15%, 36%) died, eight (11.6%; 95% CI 5.1%, 22%) required ICU admission and five (7.2%; 95% CI 2.4%, 16%) were readmitted to the hospital. Two of the 17 patients who died required ICU admission. MSK-FI scores revealed that 19 patients (27%) were considered non-frail with scores ≤2. Seven patients (10%) had a score of 3, 19% scored 4, 21% scored 5 and the remaining 16 patients (23%) had a score of 6 or higher, indicating severe frailty. In this cohort of patients, increased frailty was associated with increased risk of 30-day mortality (OR 1.37, 95% CI 1.00, 1.87; p-value = 0.051; Fig. 1 ), though this did not meet conventional levels of significance. Patient characteristics for our full cohort are presented in Table 1 . Post-hoc analyses evaluating 30-day mortality or ICU admission based on whether patients were on active cancer treatment in the 2 months prior to COVID-19 infection, yielded non-significant higher rates of outcome in patients not on active treatment compared to those who were (39% vs 27%, respectively; difference = 12%; 95% CI around the difference: −11%, 34%; p-value = 0.3).
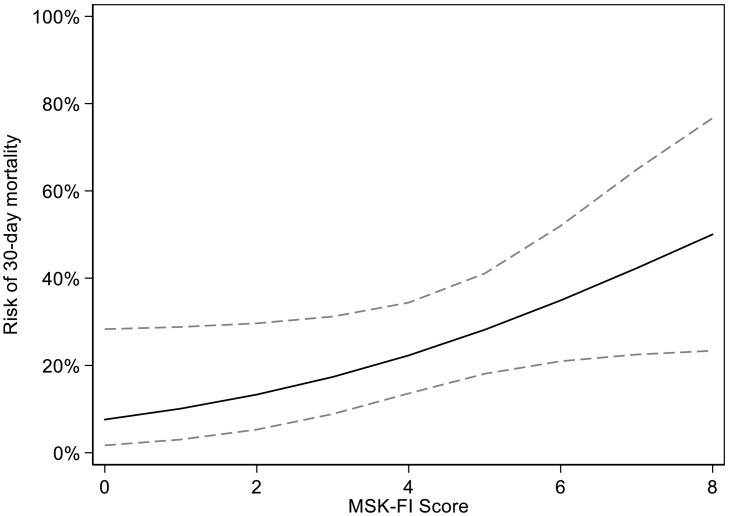
Fig. 1.
Association between MSK-FI and 30-day mortality on univariable logistic regression (p = 0.051), dashed line represents 95% confidence intervals. For example, the risk of 30-day mortality for a patient with an MSK-FI score of 2 is 13% (95% CI 5.3%, 30%) and 22% (95% CI 14%, 34%) for a patient with an MSK-FI score of 4.
Table 1.
Patient characteristics for our full cohort and based on MSK-FI.
| All Patients N = 70 |
|
|---|---|
| Age during inpatient admission | 81 (77, 83) |
| Male | 35 (50%) |
| Marital Status | |
| Single | 11 (16%) |
| Married | 34 (49%) |
| Widowed | 17 (24%) |
| Divorced | 7 (10%) |
| Unknown | 1 (1.4%) |
| Race | |
| White | 50 (71%) |
| Black | 12 (17%) |
| Asian | 8 (11%) |
| Residence | |
| New York County | 19 (27%) |
| Kings County | 18 (26%) |
| Queens County | 14 (20%) |
| Bronx County | 8 (11%) |
| Rest of NY | 6 (8.6%) |
| New Jersey | 4 (5.7%) |
| Florida | 1 (1.4%) |
| Functional Status⁎ | 46 (66%) |
| MSK-FI Score | |
| ≤1 | 4 (5.7%) |
| 2 | 15 (21%) |
| 3 | 7 (10%) |
| 4 | 13 (19%) |
| 5 | 15 (21%) |
| ≥6 | 16 (23%) |
Functional status as used in the MSK-FI is based on limitation with bathing, dressing, grooming, walking outside the home, or preparing meals.
4. Discussion
According to the CDC, the rate of hospitalization and death increases by age among patients with COVID-19. Their analysis found that, with a reference group age 18–29, the hospitalization rate was 4 times higher in age 50–64, 6 times higher in age 65–74, 9 times higher in age 75–84, and 15 times higher in those aged ≥85 compared to the reference group. Similarly, death rate was 35 times higher in ages 50–64, 95 times higher in ages 65–74, 230 times higher in ages 75–84, and 610 times higher in those aged ≥85 [8]. However, these findings were not limited to patients with cancer. Thus, for the purposes of our study, we focused on patients aged ≥75 who would typically be considered the most vulnerable risk group. MSK-FI has been validated in patients aged ≥75, which is also generally the age we use for referral to Geriatrics in our institution.
Our study found that within the oldest population of patients, there was evidence that greater frailty was associated with increased risk of 30-day mortality. These findings are consistent with findings from another multicenter cohort study which found that among older adults with COVID-19 who were admitted, the degree of frailty increased risk of in hospital mortality [9].
The presence of comorbidities and frailty often occur concomitantly in older adults and result in diminished functional status, quality of life and many times worsened prognosis [4]. Our cohort was notably frail and 90% of patients had two or more comorbid conditions, maximum of 7 comorbidities. Additionally, we observed that atypical presentation of disease such as functional decline and anorexia were common among older patients with cancer admitted with COVID-19. That being said, 30-day mortality in our cohort was 25% (95% CI 15%, 36%), which was comparable to other institutions around the similar time frame (13%- 38%), even though the median age in those studies (66–78) was younger compared to our cohort (median age 81) [2,5,9,10]. It is also important to note that the COVID-19 vaccine was not yet developed during our study period.
Caring for the older patient with cancer at increased risk of COVID-19 infection may be more challenging as the needed precautions to minimize exposure to COVID-19 may also become the barrier to timely anti-cancer therapy [11]. During the COVID-19 pandemic, there has been delay in many treatments and follow up with providers including oncology visits. Fewer patients were presenting for care, which may also have increased the risk for poorer outcomes, and mortality in general [ 12,13]. Patients have been isolated and unable to access needed care due to barriers such as transportation or lack of social support among possible causes. Additionally, some institutions developed protocols and algorithms that were highly influenced by the age of the patient. For example, some early emergency guidance for allocation of scarce resources such as ventilators included use of chronologic age as a “tie-breaker,” spurring the American Geriatric Society to issue a position paper in May 2020 urging a more comprehensive consideration of other risk factors in clinical decision-making [14].
The approach to the care of patients with cancer has evolved, also evoking the question on the appropriate intensity of cancer treatments to offer in times of a pandemic [5,11]. Under emergency situations, more than ever, cancer treatment-related clinical decisions and advance care planning should take into consideration patients' level of fitness, life expectancy, and patients and families' preferences.
The COVID-19 pandemic has also resulted in swift expansion of telemedicine to broaden the reach and communication between patients and providers bringing care to where patients are and allowing for the ability to evaluate patients in their home setting [15]. This has allowed for risk stratification, earlier intervention and better follow up for the most vulnerable populations. We hypothesize that, comparable, if not lower mortality rate despite high prevalence of frailty in our cohort may be attributable to close follow up by providers following hospital discharge. A COVID Care Team contacted patients daily for two weeks post-discharge. Excluding one patient who was lost to follow up, all patients received some form of post discharge follow up by a health care provider either through telephone symptom checks or telemedicine visits among other points of contact with the medical team.
Our study has several limitations given limited sample size, retrospective nature of this study, and short observation period. Additionally, ECOG performance status scores were not available for all patients.
To conclude, in this small study we have found some evidence of an association between degree of frailty and 30-day survival among older patients with cancer aged ≥75 who were admitted with COVID-19 infection, underscoring the importance of risk stratification of older adults based on their frailty level and not on their chronological age alone. This work adds evidence to recommendations to incorporate frailty screening into the care of older adults with cancer to better stratify risk-based interventions, especially during a global health crisis.
Author Contributions
Conception and design: Soo Jung Kim, Armin Shahrokni, Beatriz Korc-Grodzicki, Kristen Fessele.
Collection and assembly of data: Soo Jung Kim, Kristen Fessele, Charlotte Malling, Hayley Litchfield, Beatriz Korc-Grodzicki, Armin Shahrokni.
Data analysis and interpretation: Amy L. Tin.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
Accountable for all aspects of the work: All authors.
Financial Support
The study was supported in part, by the Beatriz and Samuel Seaver Foundation, the Memorial Sloan Kettering Cancer and Aging Program, and grant No. P30 CA008748 from the National Institutes of Health National Cancer Institute Cancer Center Support. Any opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funding organizations.
Conflicts of Interest
The authors declare no conflicts of interest and have nothing to disclose.
References
- 1.Centers for Disease Control People with certain medical conditions. May 13, 2021. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
- 2.Mehta V., Goel S., Kabarriti R., et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 07 2020;10(7):935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Handforth C., Clegg A., Young C., et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. Jun 2015;26(6):1091–1101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 4.Poco P.C.E., Aliberti M.J.R., Dias M.B., et al. Divergent: age, frailty, and atypical presentations of COVID-19 in hospitalized patients. J Gerontol A Biol Sci Med Sci. 02 2021;76(3) doi: 10.1093/gerona/glaa280. e46-e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 06 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsiouris A., Hammoud Z.T., Velanovich V., Hodari A., Borgi J., Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res. Jul 2013;183(1):40–46. doi: 10.1016/j.jss.2012.11.059. [DOI] [PubMed] [Google Scholar]
- 7.Shahrokni A., Tin A., Alexander K., et al. Development and evaluation of a new frailty index for older surgical patients with cancer. JAMA Netw Open. 05 2019;2(5) doi: 10.1001/jamanetworkopen.2019.3545. e193545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control Risk for COVID-19 infection, hospitalization, and death by age group. June 24, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html
- 9.Blomaard L.C., van der Linden C.M.J., van der Bol J.M., et al. Frailty is associated with in-hospital mortality in older hospitalised COVID-19 patients in the Netherlands: the COVID-OLD study. Age Ageing. 05 2021;50(3):631–640. doi: 10.1093/ageing/afab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garassino M.C., Whisenant J.G., Huang L.C., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 07 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunello A., Galiano A., Finotto S., et al. Older cancer patients and COVID-19 outbreak: practical considerations and recommendations. Cancer Med. 12 2020;9(24):9193–9204. doi: 10.1002/cam4.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartnett K.P., Kite-Powell A., DeVies J., et al. Impact of the COVID-19 pandemic on emergency department visits - United States, January 1, 2019-May 30, 2020. MMWR Morb Mortal Wkly Rep. Jun 2020;69(23):699–704. doi: 10.15585/mmwr.mm6923e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum L. The untold toll - the pandemic's effects on patients without Covid-19. N Engl J Med. 06 2020;382(24):2368–2371. doi: 10.1056/NEJMms2009984. [DOI] [PubMed] [Google Scholar]
- 14.Farrell T.W., Ferrante L.E., Brown T., et al. AGS position statement: resource allocation strategies and age-related considerations in the COVID-19 era and beyond. J Am Geriatr Soc. Jun 2020;68(6):1136–1142. doi: 10.1111/jgs.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saldivar R.T., Tew W.P., Shahrokni A., Nelson J. Goals of care conversations and telemedicine. J Geriatr Oncol. Feb 2021 doi: 10.1016/j.jgo.2021.02.016. [DOI] [PubMed] [Google Scholar]